Phineas Gage: His Accident and Impact on Psychology
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
On This Page:

Key Takeaways
- In 1848, 25-year-old Phineas Gage survived an accident where an iron rod was propelled through his left cheek and skull. He made an improbable recovery and lived for 12 more years.
Examination of Gage’s exhumed skull in 1867 revealed the probable trajectory of the tamping iron through left frontal lobe structures, offering insight into his improbable survival and selective changes in behavior following this massive traumatic brain injury.
- Gage’s case is famous in psychology as it shows the resilience of the human brain and profoundly influenced early understanding of cerebral localization.
What happened to Phineas Gage?
Phineas Gage was an American railroad construction foreman born in 1823 near Lebanon, New Hampshire.
On September 13, 1848, when Gage was 25 years old, he was working in Cavendish, Vermont, leading a crew preparing a railroad bed for the Rutland and Burlington Railroad by blasting away rock using explosives.
Around 4:30 pm, as Gage was using a 43-inch-long, 13-pound iron tamping rod to pack the explosive powder into a hole in the rock, the powder detonated unexpectedly.
The tamping iron launched from the hole and entered the left side of Gage’s face from the bottom up.
The iron rod entered Gage’s left cheek near the lower jaw hinge, passing behind his left eye socket, penetrating the base of his skull, traversing the left frontal lobe upwards at an angle, and exiting through the top frontal portion of his skull before landing about 25-30 yards behind him.
After the incident, Gage was thrown onto his back from the force of the iron rod and had some brief convulsions of the arms and legs.
Within minutes, however, assisted by his crew, Gage could stand, speak, and walk to an oxcart to be transported nearly a mile to the inn where he resided in Cavendish village.
Dr. Edward H. Williams arrived about an hour later to examine Gage. In his 1848 report, Williams noted visible pulsations of Gage’s exposed brain through an inverted funnel-shaped opening at the top of his skull from which brain tissue protruded.
Williams claimed that Gage was recounting his injuries to bystanders, and he did not initially believe the story, thinking that Gage was ‘deceived.’
Apparently, Gage had greeted Williams by angling his head at him and saying, ‘Here’s business enough for you.’
During repeated episodic vomiting, Williams observed additional small amounts of Gage’s brain matter expelled onto the floor through the frontal exit wound, as the cerebral tissue had likely detached from the skull during the passage of the tamping iron.
From Harlow’s written account, Gage was considered to be fully recovered and felt fit enough to reapply for his previous role as a foreman.
After an arduous early recovery, Gage eventually regained physical health, though his personality was markedly altered. He lived another 11 years before dying from severe epilepsy in 1860 at age 36.
How Did Phineas Gage’s Personality Change?
The descriptions of Gage’s personality and behavior before the accident are limited.
Before his accident, 25-year-old Gage was described by his railroad employers as a capable and efficient foreman, displaying a strong work ethic, drive, and dependability in overseeing his crews.
However, after surviving passage of the tamping iron through his frontal lobe in 1848, significant changes in Gage’s personality emerged during his physical recovery.
The contractors, who had regarded Gage as ‘efficient and capable’ before the accident, could no longer offer him work due to considerable changes in Gage’s personality.
In medical reports by Dr. John Martyn Harlow in 1848 and 1868, Gage is depicted as struggling with volatility, profanity, little deference for others, impatience, obstinance, unpredictability, and devising plans hastily abandoned.
Harlow wrote that Gage’s equilibrium between intellectual faculties and animal propensities was destroyed, reverting to childlike mental capacity regarding self-restraint and social appropriateness.
Though the specific neuroanatomical links were unclear at the time, Friends and colleagues felt Gage was “no longer Gage” after the traumatic brain injury, unable to process emotions or control impulsive behavior like his pre-accident self.
The shocking changes aligned with emerging localization theories that the frontal lobes regulate personality.
Marlow (1868) described Gage as follows:
“The equilibrium or balance, so to speak, between his intellectual faculties and animal propensities, seems to have been destroyed. He is fitful, irreverent, indulging at times in the grossest profanity (which was not previously his custom), manifesting but little deference for his fellows, impatient of restraint or advice when it conflicts with his desires, at times pertinaciously obstinate, yet capricious and vacillating, devising many plans of future operations, which are no sooner arranged than they are abandoned in turn for others appearing more feasible. A child in his intellectual capacity and manifestations, he has the animal passions of a strong man.”
“Previous to his injury, though untrained in the schools, he possessed a well-balanced mind, and was looked upon by those who knew him as a shrewd, smart business man, very energetic and persistent in executing all his plans of operation. In this regard his mind was radically changed, so decidedly that his friends and acquaintances said he was ‘no longer Gage.”
Through Harlow’s reports, it can be suggested that Gage’s personality changed due to the accident he endured.
The accounts imply that the injury led to a loss of social inhibition, meaning that Gage would behave in ways that were considered inappropriate.
Accuracy of Sources
In his 1848 and 1868 reports, Dr. Harlow provides a limited description of Gage’s pre-accident, stating he was “temperate inhabit, of great energy of character, possessed of considerable stamina of both brain and body” and was “a great favorite” with his men (Harlow, 1848, 1868).
However, later accounts add exaggerated positive traits not found in Harlow’s description. For example, Suinn (1970) describes Gage as enjoying “the respect as well as the favor of his men,” while Myers (1998) calls him “soft-spoken,” and Lahey (1992) says he was “polite and reasonable.”
Other sources paint him as friendly, affable, dependable, conscientious, and happy (Macmillan, 2000).
Similarly, post-accident descriptions often emphasize Gage’s negative qualities while ignoring any positive traits he retained.
Harlow documents personality changes but notes Gage remained employable for a period as a long-distance stagecoach driver in Chile (Harlow, 1868).
However, many accounts focus solely on traits like aggression, unreliability, or aimlessness (Macmillan, 2000). Damasio goes so far as to describe him as behaving violently with no self-control (Blakeslee, 1994).
In this way, later accounts tend to polish Gage’s pre-accident image as an upstanding citizen while presenting an almost cartoonishly perturbed version post-injury – neither in keeping with Harlow’s more nuanced clinical descriptions.
This likely reflects enthusiasm for fitting Gage’s case to localization theories. Macmillan (2000) argues that we must cautiously analyze such embellished personality descriptions when assessing Phineas Gage’s legacy.
Severity of Gage’s Brain Damage
When Gage died in 1861, no autopsies were performed until his skull was later recovered by Harlow years later. The brain damage that caused the significant personality changes was presumed to have involved the left frontal region of the brain.
It was not until 1994 that complex computer-based methods to examine brain damage could be used to investigate whether other areas of the brain were affected.
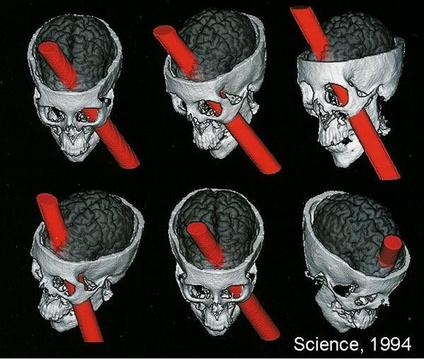
Damasio et al. (1994) used measurements from Gage’s skull and neuroimaging techniques to determine the exact placement of the entry and exit point of the iron rod on a replica model (see Fig. 1).
They found that the damage caused by the rod involved both the left and right prefrontal cortices.
The left and right cortices are responsible for emotional processing and rational decision-making; therefore, it can be assumed that Gage had deficits in these areas.
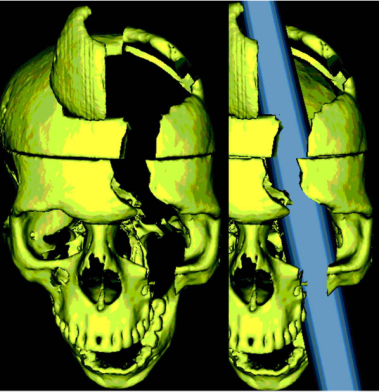
A later study by Ratiu et al. (2004) also investigated Gage’s injury and the location of where the iron rod entered and exited the head. They used Gage’s actual skull rather than a model of it, as Damasio et al. (1994) had used.
Ratiu et al. (2004) generated three-dimensional reconstructions of the skull using computed tomography scans (CAT) and found that the extent of the brain injury was limited to the left frontal lobe only and did not extend to the right lobe (see Fig. 2).
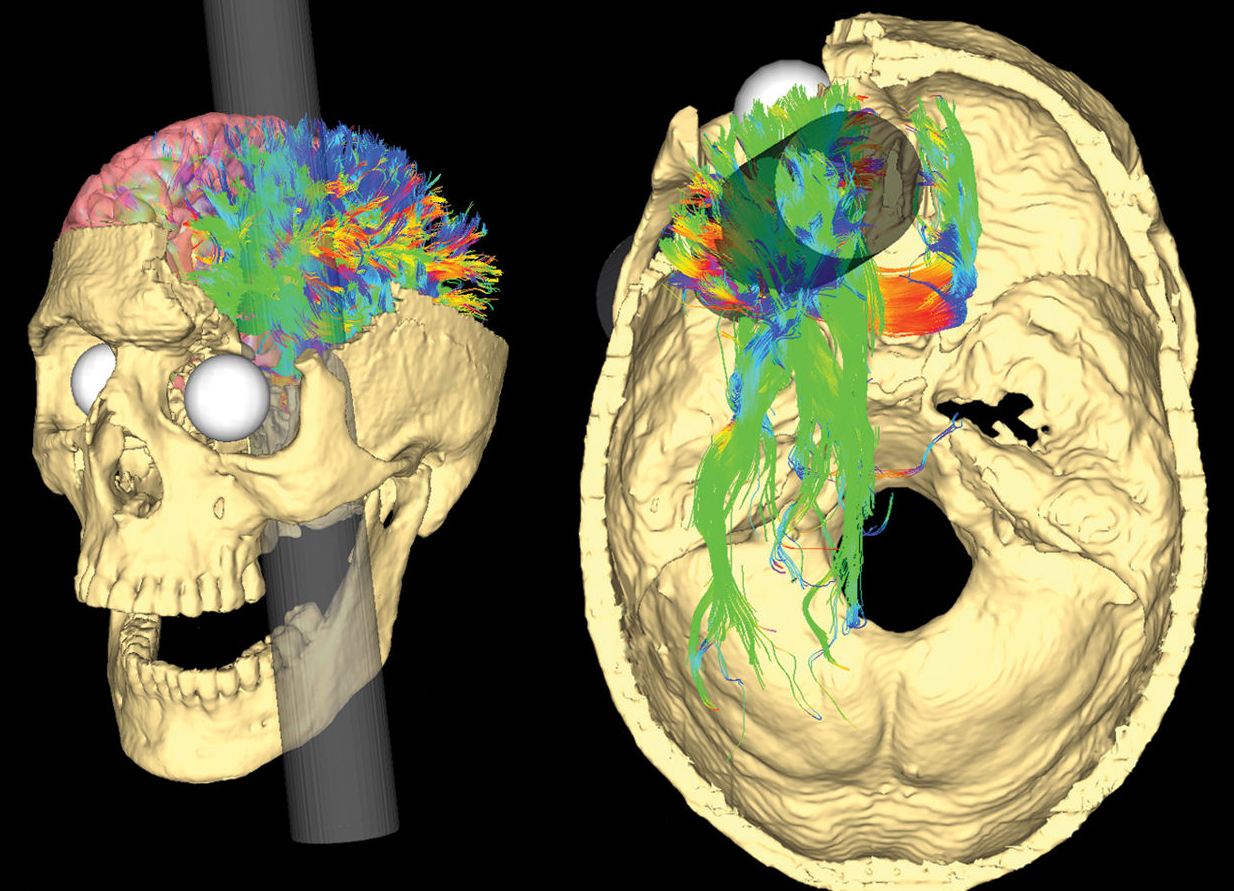
More recently, Van Horn et al. (2012) used a CAT scan of Gage’s skull as well as magnetic resonance imaging (MRI) data obtained from male participants of a similar age to Gage at the time (aged 25-36).
Their results supported Ratiu et al. (2004) in that they always concluded that the rod only damaged the left lobe and not the right.
Van Horn, however, went a step further in their research and investigated the potential levels of white and grey matter damaged due to Gage’s injury. White matter is deep in the brain and provides vital connections around the brain, essential to normal motor and sensory function.
Grey matter in the brain is essential to many areas of higher learning, including attention, memory, and thought.
The research by Van Horn proposed that Gage lost about 11% of his white matter and about 4% of his grey matter. White matter has the ability to regenerate, so this could explain why Gage recovered as well as he did.
Van Horn et al. (2012) compared Gage’s white matter damage to the damage that is caused by neurogenerative diseases such as Alzheimer’s.
This is supported by other studies that have found that changes in white matter is significantly associated with Alzheimer’s disease (Nasrabady, Rizvi, Goldman & Brickman, 2018; Kao, Chou, Chen & Yang, 2019).
It could be suggested that Gage’s apparent change in personality could have been the result of an early onset of Alzheimer’s.
However, as Dr. Harlow, who examined Gage, only reported on Gage’s behaviors shortly after his accident, rather than months or years later when Alzheimer’s symptoms may have emerged, we cannot be certain whether Gage actually had this condition.
All studies investigating the brain damage suffered by Gage is essentially all speculation as we cannot know for certain the extent of the accident’s effects.
We know that some brain tissue got destroyed, but any infections Gage may have suffered after the accident may have further destroyed more brain tissue.
We also cannot determine the exact location where the iron rod entered Gage’s skull to the millimeter. As brain structure varies from person to person, researchers cannot ever know for certain what areas of Gage’s brain were destroyed.
What Happened to Phineas Gage After the Brain Damage?
Dr. John Martyn Harlow took over Gage’s case soon after. Harlow (1848) reported that Gage was fully conscious and recognized Harlow immediately but was tired from the bleeding.
In the next couple of days, Harlow observed that Gage spoke with some difficulty but could name his friends, and the bleeding ceased. Gage then spent September 23rd to October 3rd in a semi-comatose state but was able to take steps out of bed by October 7th.
By October 11th, Harlow claimed Gage’s intellectual functioning began to improve. He recognized how much time had passed since the accident and could describe the accident clearly.
Four years after his injury, Gage moved to Chile and worked taking care of horses and being a stagecoach driver.
Harlow noted emerging personality changes in this period, with Gage becoming more erratic in behavior and responsibility.
In 1860, Gage moved to San Francisco to live near family but began suffering epileptic seizures – likely related to scar tissue and injury sequelae.
The convulsions worsened over months, and on May 21, 1861, almost 13 years after his shocking accident, Gage died at age 38 from complications of severe epilepsy.
How did Phineas Gage die?
On May 21st, 1861, twelve years after his accident, Gage died after having a series of repeated epileptic convulsions.
In 1867, Harlow arranged an exhumation of Gage’s body, claiming his skull and tamping iron for medical study.
These historic artifacts remain on display at the Harvard School of Medicine.
Though Gage initially survived, it was the secondary long-term effects of this massive brain injury that ultimately led to his premature death over a decade later.
Why Is Phineas Gage Important to Psychology?
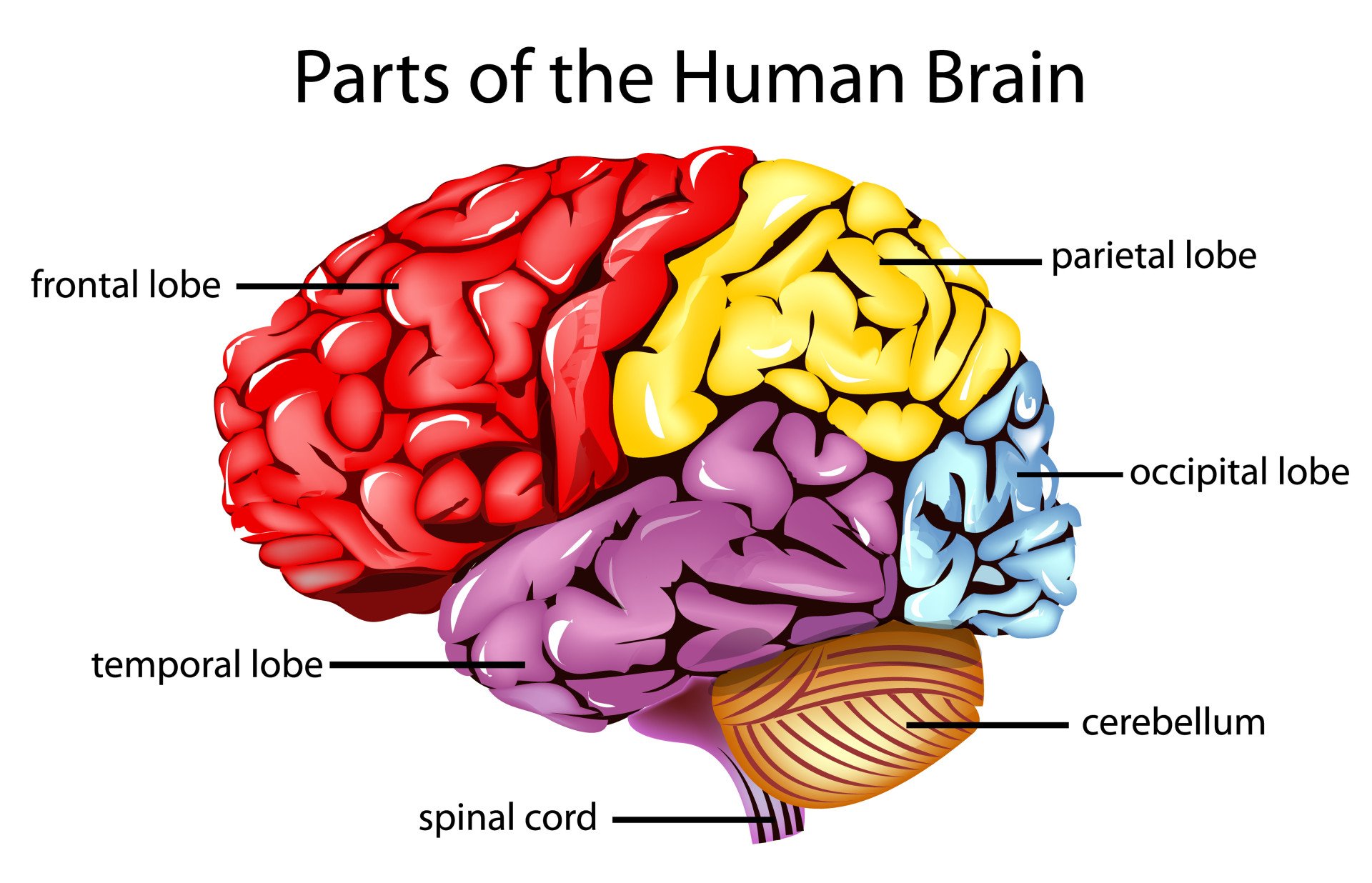
Gage’s case is important in the field of neuroscience . The reported changes in his behavior post-accident are strong evidence for the localization of brain function , meaning that specific brain areas are associated with certain functions.
Neuroscientists have a better understanding of the function of the frontal cortex today. They understand that the frontal cortex is associated with language, decision-making, intelligence, and reasoning functions. Gage’s case became one of the first pieces of evidence suggesting that the frontal lobe was directly involved in personality.
It was believed that brain lesions caused permanent deficits in a person. However, Gage was proven to have recovered remarkably and lived a mostly normal life despite his injury. It was even suggested by a psychologist called Malcolm Macmillan that Gage may have relearned lost skills.
People with damage to their frontal lobes tend to have trouble completing tasks, get easily distracted, and have trouble planning.
Despite this damage to his frontal lobe, Gage was reported to have worked as a coach driver which would have involved Gage being focused and having a routine, as well as knowing his routes and multitasking.
Macmillan (2002), therefore, suggests that Gage’s damage to the frontal lobe could have somewhat repaired itself and recovered lost functions. The ability of the brain to change in this way is called brain plasticity .
Over time, Gage’s story has been retold, and this has sometimes led to a lot of exaggeration as to the personality changes of Gage.
Some popular reports described him as a hard-working, kind man prior to the accident and then described him as an aggressive, dishonest, and drunk man who could not hold down a job and died pennilessly.
Gage’s story seemed to take on a life of its own, and some even went as far as to say that Gage became a psychopath after his accident, without any facts behind this.
From the actual reports from the people in contact with Gage at the time, it appears that his personality change was nowhere near as extreme and that Gage was far more functional than some reports would have us believe (Macmillan, 2002).
Blakeslee, S. (1994, July 6). A miraculous recovery that went wrong . New York Times.
Damasio, H., Grabowski, T., Frank, R., Galaburda, A. M., & Damasio, A. R. (1994). The return of Phineas Gage: clues about the brain from the skull of a famous patient . Science, 264 (5162), 1102-1105.Harlow J. M. (1848). Passage of an iron rod through the head. Boston Medical and Surgical Journal, 39 , 389–393.
Harlow, J. M. (1868). Recovery from the Passage of an Iron Bar through the Head . Publications of the Massachusetts Medical Society. 2 (3), 327-347.
Kao, Y. H., Chou, M. C., Chen, C. H., & Yang, Y. H. (2019). White matter changes in patients with Alzheimer’s disease and associated factors . Journal of Clinical Medicine, 8 (2), 167.
Lahey, B. B. (1992). Psychology: An introduction . Wm. C. Brown Publishers.
Macmillan, M. (2000). Restoring Phineas Gage: A 150th retrospective. Journal of the History of the Neurosciences, 9 (1), 46-66.
Macmillan, M. (2002). An odd kind of fame: Stories of Phineas Gage. MIT Press.
Myers, D. G. (1998). Psychology (5th ed.). Worth Publishers.
Nasrabady, S. E., Rizvi, B., Goldman, J. E., & Brickman, A. M. (2018). White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta neuropathologica communications, 6 (1), 1-10.
Ratiu, P., Talos, I. F., Haker, S., Lieberman, D., & Everett, P. (2004). The tale of Phineas Gage, digitally remastered . Journal of neurotrauma, 21 (5), 637-643.
Suinn, R. M. (1970). Fundamentals of behavior pathology. Wiley.
Van Horn, J. D., Irimia, A., Torgerson, C. M., Chambers, M. C., Kikinis, R., & Toga, A. W. (2012). Mapping connectivity damage in the case of Phineas Gage . PloS one, 7(5) , e37454.
Further Reading
- Griggs, R. A. (2015). Coverage of the Phineas Gage Story in Introductory Psychology Textbooks: Was Gage No Longer Gage?. Teaching of Psychology, 42(3), 195-202.
- Wilgus, J., & Wilgus, B. (2009). Face to face with Phineas Gage. Journal of the History of the Neurosciences, 18(3), 340-345.
- Macmillan, M., & Lena, M. L. (2010). Rehabilitating Phineas Gage. Neuropsychological Rehabilitation, 20, 641–658.
- Macmillan, M. (2000). Restoring phineas gage: a 150th retrospective. Journal of the History of the Neurosciences, 9(1), 46-66.
- Kotowicz, Z. (2007). The strange case of Phineas Gage. History of the Human Sciences, 20(1), 115-131.
- O”driscoll K, Leach JP. “No longer Gage”: an iron bar through the head. Early observations of personality change after injury to the prefrontal cortex. BMJ. 1998;317(7174):1673-4. doi:10.1136/bmj.317.7174.1673a
If a person suffers from a traumatic brain injury in the prefrontal cortex, similar to that of Phineas Gage, what changes might occur?
A traumatic brain injury to the prefrontal cortex could result in significant changes in personality, emotional regulation, and executive function. This region is vital for impulse control, decision-making, and moderating social behavior.
A person may exhibit increased impulsivity, poor judgment, and reduced ability to plan or organize. Emotional volatility and difficulty in interpersonal relationships may also occur.
Just like the case of Phineas Gage, who became more impulsive and less dependable, the injury could dramatically alter one’s character and abilities.
Related Articles

Soft Determinism In Psychology

Branches of Psychology

Social Action Theory (Weber): Definition & Examples
Biopsychology
Summary of the Cranial Nerves
Parts of the Brain: Anatomy, Structure & Functions
Amygdala: What It Is & Its Functions
- Brain Development
- Childhood & Adolescence
- Diet & Lifestyle
- Emotions, Stress & Anxiety
- Learning & Memory
- Thinking & Awareness
- Alzheimer's & Dementia
- Childhood Disorders
- Immune System Disorders
- Mental Health
- Neurodegenerative Disorders
- Infectious Disease
- Neurological Disorders A-Z
- Body Systems
- Cells & Circuits
- Genes & Molecules
- The Arts & the Brain
- Law, Economics & Ethics
Neuroscience in the News
- Supporting Research
- Tech & the Brain
- Animals in Research
- BRAIN Initiative
- Meet the Researcher
- Neuro-technologies
- Tools & Techniques
- Core Concepts
- For Educators
- Ask an Expert
- The Brain Facts Book

The Curious Case of Patient H.M.
- Reviewed 28 Aug 2018
- Author Deborah Halber
- Source BrainFacts/SfN
On September 1, 1953, time stopped for Henry Molaison. For roughly 10 years, the 27-year-old had suffered severe seizures. By 1953, they were so debilitating he could no longer hold down his job as a motor winder on an assembly line. On September 1, Molaison allowed surgeons to remove a thumb-sized section of tissue from each side of his brain. It was an experimental procedure that he and his surgeons hoped would quell the seizures wracking his brain.
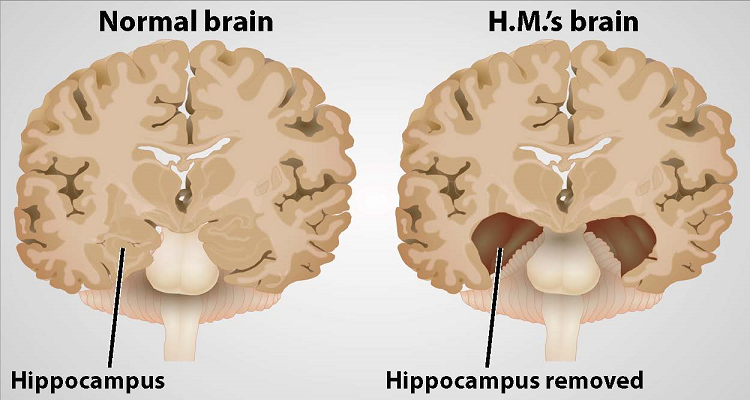
And, it worked. The seizures abated, but afterwards Molaison was left with permanent amnesia. He could remember some things — scenes from his childhood, some facts about his parents, and historical events that occurred before his surgery — but he was unable to form new memories. If he met someone who then left the room, within minutes he had no recollection of the person or their meeting.
What was a tragedy for Molaison led to one of the most significant turning points in 20th century brain science: the understanding that complex functions such as learning and memory are tied to discrete regions of the brain.
In 1955, scientists William Beecher Scoville and Brenda Milner began studying Molaison — referred to as H.M. to protect his privacy — and nine other patients who had undergone similar surgery. Only patients who had specific portions of their medial temporal lobes removed experienced memory problems. And, the more tissue removed, the more severe the memory impairment. The researchers noted patients’ amnesia was “curiously specific to the domain of recent memory.”
Scoville and Milner’s observations pointed to a particular structure within the medial temporal lobe that was necessary for normal memory — the hippocampus. Over the next five decades, neuroscientists studying Molaison learned that the hippocampus and adjacent regions transform our transient perceptions and awareness into memories that can last a lifetime.
For Molaison, this transformation could no longer take place. He experienced every aspect of his daily life — eating a meal, taking a walk — as a first. Yet his intellect, personality, and perception were intact, and he was able to acquire new motor skills. Over time, he became more proficient at tasks such as tracing patterns while watching his hand movements in a mirror, despite the fact that he could never recall performing the task before.
Studies of Molaison paved the way for further exploration of the brain networks encoding conscious and unconscious memories. Even after his death in 2008 at the age of 82, neuroscientists continue to learn from him.
This article was adapted from the 8th edition of Brain Facts by Deborah Halber.
About the Author

Deborah Halber
Deborah Halber is a Boston-based author, science writer and journalist. Her work has appeared in The Atlantic, Time.com, The Boston Globe, MIT Technology Review, Boston magazine, and university publications.
CONTENT PROVIDED BY
BrainFacts/SfN
What to Read Next

Also In Tools & Techniques

Popular articles on BrainFacts.org

Check out the latest news from the field.
Research & Discoveries
See how discoveries in the lab have improved human health.
BrainFacts Book
Download a copy of the newest edition of the book, Brain Facts: A Primer on the Brain and Nervous System.

SUPPORTING PARTNERS
- Privacy Policy
- Accessibility Policy
- Terms and Conditions
- Manage Cookies
Some pages on this website provide links that require Adobe Reader to view.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Spring 2024 | VOL. 36, NO. 2 CURRENT ISSUE pp.A4-174
- Winter 2024 | VOL. 36, NO. 1 pp.A5-81
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Case Study 1: A 55-Year-Old Woman With Progressive Cognitive, Perceptual, and Motor Impairments
- Scott M. McGinnis , M.D. ,
- Andrew M. Stern , M.D., Ph.D. ,
- Jared K. Woods , M.D., Ph.D. ,
- Matthew Torre , M.D. ,
- Mel B. Feany , M.D., Ph.D. ,
- Michael B. Miller , M.D., Ph.D. ,
- David A. Silbersweig , M.D. ,
- Seth A. Gale , M.D. ,
- Kirk R. Daffner , M.D.
Search for more papers by this author
CASE PRESENTATION
A 55-year-old right-handed woman presented with a 3-year history of cognitive changes. Early symptoms included mild forgetfulness—for example, forgetting where she left her purse or failing to remember to retrieve a take-out order her family placed—and word-finding difficulties. Problems with depth perception affected her ability to back her car out of the driveway. When descending stairs, she had to locate her feet visually in order to place them correctly, such that when she carried her dog and her view was obscured, she had difficulty managing this activity. She struggled to execute relatively simple tasks, such as inserting a plug into an outlet. She lost the ability to type on a keyboard, despite being able to move her fingers quickly. Her symptoms worsened progressively for 3 years, over which time she developed a sad mood and anxiety. She was laid off from work as a nurse administrator. Her family members assumed responsibility for paying her bills, and she ceased driving.
Her past medical history included high blood pressure, Hashimoto’s thyroiditis with thyroid peroxidase antibodies, remote history of migraine, and anxiety. Medications included mirtazapine, levothyroxine, calcium, and vitamin D. She had no history of smoking, drinking alcohol, or recreational drug use. There was no known family history of neurologic diseases.
What Are Diagnostic Considerations Based on the History? How Might a Clinical Examination Help to Narrow the Differential Diagnosis?
Insidious onset and gradual progression of cognitive symptoms over the course of several years raise concern for a neurodegenerative disorder. It is helpful to consider whether or not the presentation fits with a recognized neurodegenerative clinical syndrome, a judgment based principally on familiarity with syndromes and pattern recognition. Onset of symptoms before age 65 should prompt consideration of syndromes in the spectrum of frontotemporal dementia (FTD) and atypical (nonamnesic) presentations of Alzheimer’s disease (AD) ( 1 , 2 ). This patient’s symptoms reflect relatively prominent early dysfunction in visual-spatial processing and body schema, as might be observed in posterior cortical atrophy (PCA), although the history also includes mention of forgetfulness and word-retrieval difficulties. A chief goal of the cognitive examination would be to survey major domains of cognition—attention, executive functioning, memory, language, visual-spatial functioning, and higher somatosensory and motor functioning—to determine whether any domains stand out as more prominently affected. In addition to screening for evidence of focal signs, a neurological examination in this context should assess for evidence of parkinsonism or motor neuron disease, which can coexist with cognitive changes in neurodegenerative presentations.
The patient’s young age and history of Hashimoto’s thyroiditis might also prompt consideration of Hashimoto’s encephalopathy (HE; also known as steroid-responsive encephalopathy), associated with autoimmune thyroiditis. This syndrome is most likely attributable to an autoimmune or inflammatory process affecting the central nervous system. The time course of HE is usually more subacute and rapidly progressive or relapsing-remitting, as opposed to the gradual progression over months to years observed in the present case ( 3 ).
The patient’s mental status examination included the Montreal Cognitive Assessment (MoCA), a brief global screen of cognition ( 4 ), on which she scored 12/30. There was evidence of dysfunction across multiple cognitive domains ( Figure 1 ). She was fully oriented to location, day, month, year, and exact date. When asked to describe a complex scene from a picture in a magazine, she had great difficulty doing so, focusing on different details but having trouble directing her saccades to pertinent visual information. She likewise had problems directing her gaze to specified objects in the room and problems reaching in front of her to touch target objects in either visual field. In terms of other symptoms of higher order motor and somatosensory functioning, she had difficulty demonstrating previously learned actions—for example, positioning her hand correctly to pantomime holding a brush and combing her hair. She was confused about which side of her body was the left and which was the right. She had difficulty with mental calculations, even relatively simple ones such as “18 minus 12.” In addition, she had problems writing a sentence in terms of both grammar and the appropriate spacing of words and letters on the page.
FIGURE 1. Selected elements of a 55-year-old patient’s cognitive examination at presentation a
a BNT-15=Boston Naming Test (15-Item); MoCA=Montreal Cognitive Assessment.
On elementary neurologic examination she had symmetrically brisk reflexes, with spread. She walked steadily with a narrow base, but when asked to pass through a doorway she had difficulty finding her way through it and bumped into the door jamb. Her elemental neurological examination was otherwise normal, including but not limited to brisk, full-amplitude vertical eye movements, normal visual fields, no evidence of peripheral neuropathy, and no parkinsonian signs such as slowness of movement, tremor, or rigidity.
How Does the Examination Contribute to Our Understanding of Diagnostic Considerations? What Additional Tests or Studies Are Indicated?
The most prominent early symptoms and signs localize predominantly to the parietal association cortex: The patient has impairments in visual construction, ability to judge spatial relationships, ability to synthesize component parts of a visual scene into a coherent whole (simultanagnosia or asimultagnosia), impaired visually guided reaching for objects (optic ataxia), and most likely impaired ability to shift her visual attention so as to direct saccades to targets in her field of view (oculomotor apraxia or ocular apraxia). The last three signs constitute Bálint syndrome, which localizes to disruption of dorsal visual networks (i.e., dorsal stream) with key nodes in the posterior parietal and prefrontal cortices bilaterally ( 5 ). She has additional salient symptoms and signs suggesting left inferior parietal dysfunction, including ideomotor limb apraxia and elements of Gerstmann syndrome, which comprises dysgraphia, acalculia, left-right confusion, and finger agnosia ( 6 ). Information was not included about whether she was explicitly examined for finger agnosia, but elements of her presentation suggested a more generalized disruption of body schema (i.e., her representation of the position and configuration of her body in space). Her less prominent impairment in lexical-semantic retrieval evidenced by impaired confrontation naming and category fluency likely localizes to the language network in the left hemisphere. Her impairments in attention and executive functions have less localizing value but would plausibly arise in the context of frontoparietal network dysfunction. At this point, it is unclear whether her impairment in episodic memory mostly reflects encoding and activation versus a rapid rate of forgetting (storage), as occurs in temporolimbic amnesia. Regardless, it does not appear to be the most salient feature of her presentation.
This localization, presenting with insidious onset and gradual progression, is characteristic of a PCA syndrome. If we apply consensus clinical diagnostic criteria proposed by a working group of experts, we find that our patient has many of the representative features of early disturbance of visual functions plus or minus other cognitive functions mediated by the posterior cerebral cortex ( Table 1 ) ( 7 ). Some functions such as limb apraxia also occur in corticobasal syndrome (CBS), a clinical syndrome defined initially in association with corticobasal degeneration (CBD) neuropathology, a 4-repeat tauopathy characterized by achromatic ballooned neurons, neuropil threads, and astrocytic plaques. However, our patient lacks other suggestive features of CBS, including extrapyramidal motor dysfunction (e.g., limb rigidity, bradykinesia, dystonia), myoclonus, and alien limb phenomenon ( Table 1 ) ( 8 ).
| Feature | Posterior cortical atrophy | Corticobasal syndrome |
|---|---|---|
| Cognitive and motor features | Visual-perceptual: space perception deficit, simultanagnosia, object perception deficit, environmental agnosia, alexia, apperceptive prosopagnosia, and homonymous visual field defect | Motor: limb rigidity or akinesia, limb dystonia, and limb myoclonus |
| Visual-motor: constructional dyspraxia, oculomotor apraxia, optic ataxia, and dressing apraxia | ||
| Other: left/right disorientation, acalculia, limb apraxia, agraphia, and finger agnosia | Higher cortical features: limb or orobuccal apraxia, cortical sensory deficit, and alien limb phenomena | |
| Imaging features (MRI, FDG-PET, SPECT) | Predominant occipito-parietal or occipito-temporal atrophy, and hypometabolism or hypoperfusion | Asymmetric perirolandic, posterior frontal, parietal atrophy, and hypometabolism or hypoperfusion |
| Neuropathological associations | AD>CBD, LBD, TDP, JCD | CBD>PSP, AD, TDP |
a Consensus diagnostic criteria for posterior cortical atrophy per Crutch et al. ( 7 ) require at least three cognitive features and relative sparing of anterograde memory, speech-nonvisual language functions, executive functions, behavior, and personality. Diagnostic criteria for probable corticobasal syndrome per Armstrong et al. ( 8 ) require asymmetric presentation of at least two motor features and at least two higher cortical features. AD=Alzheimer’s disease; CBD=corticobasal degeneration; FDG-PET=[ 18 ]F-fluorodexoxyglucose positron emission tomography; JCD=Jakob-Creutzfeldt disease; LBD=Lewy body disease; PSP=progressive supranuclear palsy; SPECT=single-photon emission computed tomography; TDP=TDP–43 proteinopathy.
TABLE 1. Clinical features and neuropathological associations of posterior cortical atrophy and corticobasal syndrome a
In addition to a standard laboratory work-up for cognitive impairment, it is important to determine whether imaging of the brain provides evidence of neurodegeneration in a topographical distribution consistent with the clinical presentation. A first step in most cases would be to obtain an MRI of the brain that includes a high-resolution T 1 -weighted MRI sequence to assess potential atrophy, a T 2 /fluid-attenuated inversion recovery (FLAIR) sequence to assess the burden of vascular disease and rule out less likely etiological considerations (e.g., infection, autoimmune-inflammatory, neoplasm), a diffusion-weighted sequence to rule out subacute infarcts and prion disease (more pertinent to subacute or rapidly progressive cases), and a T 2 *-gradient echo or susceptibility weighted sequence to examine for microhemorrhages and superficial siderosis.
A lumbar puncture would serve two purposes. First, it would allow for the assessment of inflammation that might occur in HE, as approximately 80% of cases have some abnormality of CSF (i.e., elevated protein, lymphocytic pleiocytosis, or oligoclonal bands) ( 9 ). Second, in selected circumstances—particularly in cases with atypical nonamnesic clinical presentations or early-onset dementia in which AD is in the neuropathological differential diagnosis—we frequently pursue AD biomarkers of molecular neuropathology ( 10 , 11 ). This is most frequently accomplished with CSF analysis of amyloid-β-42, total tau, and phosphorylated tau levels. Amyloid positron emission tomography (PET) imaging, and most recently tau PET imaging, represent additional options that are approved by the U.S. Food and Drug Administration for clinical use. However, insurance often does not cover amyloid PET and currently does not reimburse tau PET imaging. [ 18 ]-F-fluorodeoxyglucose (FDG) PET and perfusion single-photon emission computed tomography imaging may provide indirect evidence for AD neuropathology via a pattern of hypometabolism or hypoperfusion involving the temporoparietal and posterior cingulate regions, though without molecular specificity. Pertinent to this case, a syndromic diagnosis of PCA is most commonly associated with underlying AD neuropathology—that is, plaques containing amyloid-β and neurofibrillary tangles containing tau ( 12 – 15 ).
The patient underwent MRI, demonstrating a minimal burden of T 2 /FLAIR hyperintensities and some degree of bilateral parietal volume loss with a left greater than right predominance ( Figure 2A ). There was relatively minimal medial temporal volume loss. Her basic laboratory work-up, including thyroid function, vitamin B 12 level, and treponemal antibody, was normal. She underwent a lumbar puncture; CSF studies revealed normal cell counts, protein, and glucose levels and low amyloid-β-42 levels at 165.9 pg/ml [>500 pg/ml] and elevated total and phosphorylated tau levels at 1,553 pg/ml [<350 pg/ml] and 200.4 pg/ml [<61 pg/ml], respectively.
FIGURE 2. MRI brain scan of the patient at presentation and 4 years later a
a Arrows denote regions of significant atrophy.
Considering This Additional Data, What Would Be an Appropriate Diagnostic Formulation?
For optimal clarity, we aim to provide a three-tiered approach to diagnosis comprising neurodegenerative clinical syndrome (e.g., primary amnesic, mixed amnesic and dysexecutive, primary progressive aphasia), level of severity (i.e., mild cognitive impairment; mild, moderate or severe dementia), and predicted underlying neuropathology (e.g., AD, Lewy body disease [LBD], frontotemporal lobar degeneration) ( 16 ). This approach avoids problematic conflations that cause confusion, for example when people equate AD with memory loss or dementia, whereas AD most strictly describes the neuropathology of plaques and tangles, regardless of the patient’s clinical symptoms and severity. This framework is important because there is never an exclusive, one-to-one correspondence between syndromic and neuropathological diagnosis. Syndromes arise from neurodegeneration that starts focally and progresses along the anatomical lines of large-scale brain networks that can be defined on the basis of both structural and functional connectivity, a concept detailed in the network degeneration hypothesis ( 17 ). It is important to note that neuropathologies defined on the basis of specific misfolded protein inclusions can target more than one large-scale network, and any given large-scale network can degenerate in association with more than one neuropathology.
The MRI results in this case support a syndromic diagnosis of PCA, with a posteriorly predominant pattern of atrophy. Given the patient’s loss of independent functioning in instrumental activities of daily living (ADLs), including driving and managing her finances, the patient would be characterized as having a dementia (also known as major neurocognitive disorder). The preservation of basic ADLs would suggest that the dementia was of mild severity. The CSF results provide supportive evidence for AD amyloid plaque and tau neurofibrillary tangle (NFT) neuropathology over other pathologies potentially associated with PCA syndrome (i.e., CBD, LBD, TDP-43 proteinopathy, and Jakob-Creutzfeldt disease) ( 13 , 14 ). The patient’s formulation would thus be best summarized as PCA at a level of mild dementia, likely associated with underlying AD neuropathology.
The patient’s symptoms progressed. One year after initial presentation, she had difficulty locating the buttons on her clothing or the food on her plate. Her word-finding difficulties worsened. Others observed stiffness of her right arm, a new symptom that was not present initially. She also had decreased ability using her right hand to hold everyday objects such as a comb, a brush, or a pen. On exam, she was noted to have rigidity of her right arm, impaired dexterity with her right hand for fine motor tasks, and a symmetrical tremor of the arms, apparent when holding objects or reaching. Her right hand would also intermittently assume a flexed, dystonic posture and would sometime move in complex ways without her having a sense of volitional control.
Four to 5 years after initial presentation, her functional status declined to the point where she was unable to feed, bathe, or dress herself. She was unable to follow simple instructions. She developed neuropsychiatric symptoms, including compulsive behaviors, anxiety, and apathy. Her right-sided motor symptoms progressed; she spent much of the time with her right arm flexed in abnormal postures or moving abnormally. She developed myoclonus of both arms. Her speech became slurred and monosyllabic. Her gait became less steady. She underwent a second MRI of the brain, demonstrating progressive bilateral atrophy involving the frontal and occipital lobes in addition to the parietal lobes and with more left > right asymmetry than was previously apparent ( Figure 2B ). Over time, she exhibited increasing weight loss. She was enrolled in hospice and ultimately passed away 8 years from the onset of symptoms.
Does Information About the Longitudinal Course of Her Illness Alter the Formulation About the Most Likely Underlying Neuropathological Process?
This patient developed clinical features characteristic of corticobasal syndrome over the longitudinal course of her disease. With time, it became apparent that she had lost volitional control over her right arm (characteristic of an alien limb phenomenon), and she developed signs more suggestive of basal ganglionic involvement (i.e., limb rigidity and possible dystonia). This presentation highlights the frequent overlap between neurodegenerative clinical syndromes; any given person may have elements of more than one syndrome, especially later in the course of a disease. In many instances, symptomatic features that are less prominent at presentation but evolve and progress can provide clues regarding the underlying neuropathological diagnosis. For example, a patient with primary progressive apraxia of speech or nonfluent-agrammatic primary progressive aphasia could develop the motor features of a progressive supranuclear palsy (PSP) clinical syndrome (e.g., supranuclear gaze impairment, axial rigidity, postural instability), which would suggest underlying PSP neuropathology (4-repeat tauopathy characterized by globose neurofibrillary tangles, tufted astrocytes, and oligodendroglial coiled bodies).
If CSF biomarker data were not suggestive of AD, the secondary elements of CBS would substantially increase the likelihood of underlying CBD neuropathology presenting with a PCA syndrome and evolving to a mixed PCA-CBS. But the CSF amyloid and tau levels are unambiguously suggestive of AD (i.e., very low amyloid-β-42 and very high p-tau levels), the neuropathology of which accounts for not only a vast majority of PCA presentations but also roughly a quarter of cases presenting with CBS ( 18 , 19 ). Thus, underlying AD appears most likely.
NEUROPATHOLOGY
On gross examination, the brain weighed 1,150 g, slightly less than the lower end of normal at 1,200 g. External examination demonstrated mild cortical atrophy with widening of the sulci, relatively symmetrical and uniform throughout the brain ( Figure 3A ). There was no evidence of atrophy of the brainstem or cerebellum. On cut sections, the hippocampus was mildly atrophic. The substantia nigra in the midbrain was intact, showing appropriate dark pigmentation as would be seen in a relatively normal brain. The remainder of the gross examination was unremarkable.
FIGURE 3. Mild cortical atrophy with posterior predominance and neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body a
a Panel A shows the gross view of the brain, demonstrating mild cortical atrophy with posterior predominance (arrow). Panel B shows the hematoxylin and eosin of the hippocampus at high power, demonstrating neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body.
Histological examination confirmed that the neurons in the substantia nigra were appropriately pigmented, with occasional extraneuronal neuromelanin and moderate neuronal loss. In the nucleus basalis of Meynert, NFTs were apparent on hematoxylin and eosin staining as dense fibrillar eosinophilic structures in the neuronal cytoplasm, confirmed by tau immunohistochemistry (IHC; Figure 4 ). Low-power examination of the hippocampus revealed neuronal loss in the subiculum and in Ammon’s horn, most pronounced in the cornu ammonis 1 (CA1) subfield, with a relatively intact neuronal population in the dentate gyrus. Higher power examination with hematoxylin and eosin demonstrated numerous NFTs, neurons exhibiting granulovacuolar degeneration, and Hirano bodies ( Figure 3B ). Tau IHC confirmed numerous NFTs in the CA1 region and the subiculum. Amyloid-β IHC demonstrated occasional amyloid plaques in this region, less abundant than tau pathology. An α-synuclein stain revealed scattered Lewy bodies in the hippocampus and in the amygdala.
FIGURE 4. Tau immunohistochemistry demonstrating neurofibrillary tangles (staining brown) in the nucleus basalis of Meynert, in the hippocampus, and in the cerebral cortex of the frontal, temporal, parietal, and occipital lobes
In the neocortex, tau IHC highlighted the extent of the NFTs, which were very prominent in all of the lobes from which sections were taken: frontal, temporal, parietal and occipital. Numerous plaques on amyloid-β stain were likewise present in all cortical regions examined. The tau pathology was confined to the gray matter, sparing white matter. There were no ballooned neurons and no astrocytic plaques—two findings one would expect to see in CBD ( Table 2 ).
| Feature | Case of PCA/CBS due to AD | Exemplar case of CBD |
|---|---|---|
| Macroscopic findings | Cortical atrophy: symmetric, mild | Cortical atrophy: often asymmetric, predominantly affecting perirolandic cortex |
| Substantia nigra: appropriately pigmented | Substantia nigra: severely depigmented | |
| Microscopic findings | Tau neurofibrillary tangles and beta-amyloid plaques | Primary tauopathy |
| No tau pathology in white matter | Tau pathology involves white matter | |
| Hirano bodies, granulovacuolar degeneration | Ballooned neurons, astrocytic plaques, and oligodendroglial coiled bodies | |
| (Lewy bodies, limbic) |
a AD=Alzheimer’s disease; CBD=corticobasal degeneration; CBS=corticobasal syndrome; PCA=posterior cortical atrophy.
TABLE 2. Neuropathological features of this case compared with a case of corticobasal degeneration a
The case was designated by the neuropathology division as Alzheimer’s-type pathology, Braak stage V–VI (of VI), due to the widespread neocortical tau pathology, with LBD primarily in the limbic areas.
Our patient had AD neuropathology presenting atypically with a young age at onset (52 years old) and a predominantly visual-spatial and corticobasal syndrome as opposed to prominent amnesia. Syndromic diversity is a well-recognized phenomenon in AD. Nonamnesic presentations include not only PCA and CBS but also the logopenic variant of primary progressive aphasia and a behavioral-dysexecutive syndrome ( 20 ). Converging lines of evidence link the topographical distribution of NFTs with syndromic presentations and the pattern of hypometabolism and cortical atrophy. Neuropathological case reports and case series suggest that atypical AD syndromes arise in the setting of higher than normal densities of NFTs in networks subserving the functions compromised, including visual association areas in PCA-AD ( 21 ), the language network in PPA-AD ( 22 ), and frontal regions in behavioral-dysexecutive AD ( 23 ). In a large sample of close to 900 cases of pathologically diagnosed AD employing quantitative assessment of NFT density and distribution in selected neocortical and hippocampal regions, 25% of cases did not conform to a typical distribution of NFTs characterized in the Braak staging scheme ( 24 ). A subset of cases classified as hippocampal sparing with higher density of NFTs in the neocortex and lower density of NFTs in the hippocampus had a younger mean age at onset, higher frequency of atypical (nonamnesic) presentations, and more rapid rate of longitudinal decline than subsets defined as typical or limbic-predominant.
Tau PET, which detects the spatial distribution of fibrillary tau present in NFTs, has corroborated postmortem work in demonstrating distinct patterns of tracer uptake in different subtypes of AD defined by clinical symptoms and topographical distributions of atrophy ( 25 – 28 ). Amyloid PET, which detects the spatial distribution of fibrillar amyloid- β found in amyloid plaques, does not distinguish between typical and atypical AD ( 29 , 30 ). In a longitudinal study of 32 patients at early symptomatic stages of AD, the baseline topography of tau PET signal predicted subsequent atrophy on MRI at the single patient level, independent of baseline cortical thickness ( 31 ). This correlation was strongest in early-onset AD patients, who also tended to have higher tau signal and more rapid progression of atrophy than late-onset AD patients.
Differential vulnerability of selected large-scale brain networks in AD and in neurodegenerative disease more broadly remains poorly understood. There is evidence to support multiple mechanisms that are not mutually exclusive, including metabolic stress to key network nodes, trophic failure, transneuronal spread of pathological proteins (i.e., prion-like mechanisms), and shared vulnerability within network regions based on genetic or developmental factors ( 32 ). In the case of AD, cortical hub regions with high intrinsic functional connectivity to other regions across the brain appear to have high metabolic rates across the lifespan and to be foci of convergence of amyloid-β and tau accumulation ( 33 , 34 ). Tau NFT pathology appears to spread temporally along connected networks within the brain ( 35 ). Patients with primary progressive aphasia are more likely to have a personal or family history of developmental language-based learning disability ( 36 ), and patients with PCA are more likely to have a personal history of mathematical or visuospatial learning disability ( 37 ).
This case highlights the symptomatic heterogeneity in AD and the value of a three-tiered approach to diagnostic formulation in neurodegenerative presentations. It is important to remember that not all AD presents with amnesia and that early-onset AD tends to be more atypical and to progress more rapidly than late-onset AD. Multiple lines of evidence support a relationship between the burden and topographical distribution of tau NFT neuropathology and clinical symptomatology in AD, instantiating network-based neurodegeneration via mechanisms under ongoing investigation.
The authors report no financial relationships with commercial interests.
Supported by NIH grants K08 AG065502 (to Dr. Miller) and T32 HL007627 (to Dr. Miller).
The authors have confirmed that details of the case have been disguised to protect patient privacy.
1 Balasa M, Gelpi E, Antonell A, et al. : Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease . Neurology 2011 ; 76:1720–1725 Crossref , Medline , Google Scholar
2 Mercy L, Hodges JR, Dawson K, et al. : Incidence of early-onset dementias in Cambridgeshire, United Kingdom . Neurology 2008 ; 71:1496–1499 Crossref , Medline , Google Scholar
3 Kothbauer-Margreiter I, Sturzenegger M, Komor J, et al. : Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment . J Neurol 1996 ; 243:585–593 Crossref , Medline , Google Scholar
4 Nasreddine ZS, Phillips NA, Bédirian V, et al. : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment . J Am Geriatr Soc 2005 ; 53:695–699 Crossref , Medline , Google Scholar
5 Rizzo M, Vecera SP : Psychoanatomical substrates of Bálint’s syndrome . J Neurol Neurosurg Psychiatry 2002 ; 72:162–178 Crossref , Medline , Google Scholar
6 Rusconi E : Gerstmann syndrome: historic and current perspectives . Handb Clin Neurol 2018 ; 151:395–411 Crossref , Medline , Google Scholar
7 Crutch SJ, Schott JM, Rabinovici GD, et al. : Consensus classification of posterior cortical atrophy . Alzheimers Dement 2017 ; 13:870–884 Crossref , Medline , Google Scholar
8 Armstrong MJ, Litvan I, Lang AE, et al. : Criteria for the diagnosis of corticobasal degeneration . Neurology 2013 ; 80:496–503 Crossref , Medline , Google Scholar
9 Marshall GA, Doyle JJ : Long-term treatment of Hashimoto’s encephalopathy . J Neuropsychiatry Clin Neurosci 2006 ; 18:14–20 Link , Google Scholar
10 Johnson KA, Minoshima S, Bohnen NI, et al. : Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association . Alzheimers Dement 2013 ; 9:e-1–e-16 Crossref , Medline , Google Scholar
11 Shaw LM, Arias J, Blennow K, et al. : Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease . Alzheimers Dement 2018 ; 14:1505–1521 Crossref , Medline , Google Scholar
12 Alladi S, Xuereb J, Bak T, et al. : Focal cortical presentations of Alzheimer’s disease . Brain 2007 ; 130:2636–2645 Crossref , Medline , Google Scholar
13 Renner JA, Burns JM, Hou CE, et al. : Progressive posterior cortical dysfunction: a clinicopathologic series . Neurology 2004 ; 63:1175–1180 Crossref , Medline , Google Scholar
14 Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. : Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy . Neurology 2004 ; 63:1168–1174 Crossref , Medline , Google Scholar
15 Victoroff J, Ross GW, Benson DF, et al. : Posterior cortical atrophy: neuropathologic correlations . Arch Neurol 1994 ; 51:269–274 Crossref , Medline , Google Scholar
16 Dickerson BC, McGinnis SM, Xia C, et al. : Approach to atypical Alzheimer’s disease and case studies of the major subtypes . CNS Spectr 2017 ; 22:439–449 Crossref , Medline , Google Scholar
17 Seeley WW, Crawford RK, Zhou J, et al. : Neurodegenerative diseases target large-scale human brain networks . Neuron 2009 ; 62:42–52 Crossref , Medline , Google Scholar
18 Lee SE, Rabinovici GD, Mayo MC, et al. : Clinicopathological correlations in corticobasal degeneration . Ann Neurol 2011 ; 70:327–340 Crossref , Medline , Google Scholar
19 Whitwell JL, Jack CR Jr, Boeve BF, et al. : Imaging correlates of pathology in corticobasal syndrome . Neurology 2010 ; 75:1879–1887 Crossref , Medline , Google Scholar
20 Warren JD, Fletcher PD, Golden HL : The paradox of syndromic diversity in Alzheimer disease . Nat Rev Neurol 2012 ; 8:451–464 Crossref , Medline , Google Scholar
21 Hof PR, Archin N, Osmand AP, et al. : Posterior cortical atrophy in Alzheimer’s disease: analysis of a new case and re-evaluation of a historical report . Acta Neuropathol 1993 ; 86:215–223 Crossref , Medline , Google Scholar
22 Mesulam MM, Weintraub S, Rogalski EJ, et al. : Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia . Brain 2014 ; 137:1176–1192 Crossref , Medline , Google Scholar
23 Blennerhassett R, Lillo P, Halliday GM, et al. : Distribution of pathology in frontal variant Alzheimer’s disease . J Alzheimers Dis 2014 ; 39:63–70 Crossref , Medline , Google Scholar
24 Murray ME, Graff-Radford NR, Ross OA, et al. : Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study . Lancet Neurol 2011 ; 10:785–796 Crossref , Medline , Google Scholar
25 Ossenkoppele R, Lyoo CH, Sudre CH, et al. : Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer’s disease . Alzheimers Dement 2020 ; 16:335–344 Crossref , Medline , Google Scholar
26 Phillips JS, Das SR, McMillan CT, et al. : Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease . Hum Brain Mapp 2018 ; 39:691–708 Crossref , Medline , Google Scholar
27 Tetzloff KA, Graff-Radford J, Martin PR, et al. : Regional distribution, asymmetry, and clinical correlates of tau uptake on [18F]AV-1451 PET in atypical Alzheimer’s disease . J Alzheimers Dis 2018 ; 62:1713–1724 Crossref , Medline , Google Scholar
28 Xia C, Makaretz SJ, Caso C, et al. : Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease . JAMA Neurol 2017 ; 74:427–436 Crossref , Medline , Google Scholar
29 Formaglio M, Costes N, Seguin J, et al. : In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings . J Neurol 2011 ; 258:1841–1851 Crossref , Medline , Google Scholar
30 Lehmann M, Ghosh PM, Madison C, et al. : Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease . Brain 2013 ; 136:844–858 Crossref , Medline , Google Scholar
31 La Joie R, Visani AV, Baker SL, et al. : Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET . Sci Transl Med 2020 ; 12:12 Crossref , Google Scholar
32 Zhou J, Gennatas ED, Kramer JH, et al. : Predicting regional neurodegeneration from the healthy brain functional connectome . Neuron 2012 ; 73:1216–1227 Crossref , Medline , Google Scholar
33 Buckner RL, Sepulcre J, Talukdar T, et al. : Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease . J Neurosci 2009 ; 29:1860–1873 Crossref , Medline , Google Scholar
34 Hoenig MC, Bischof GN, Seemiller J, et al. : Networks of tau distribution in Alzheimer’s disease . Brain 2018 ; 141:568–581 Crossref , Medline , Google Scholar
35 Liu L, Drouet V, Wu JW, et al. : Trans-synaptic spread of tau pathology in vivo . PLoS One 2012 ; 7:e31302 Crossref , Medline , Google Scholar
36 Rogalski E, Johnson N, Weintraub S, et al. : Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives . Arch Neurol 2008 ; 65:244–248 Crossref , Medline , Google Scholar
37 Miller ZA, Rosenberg L, Santos-Santos MA, et al. : Prevalence of mathematical and visuospatial learning disabilities in patients with posterior cortical atrophy . JAMA Neurol 2018 ; 75:728–737 Crossref , Medline , Google Scholar
- Jeffrey Maneval , M.D. ,
- Kirk R. Daffner , M.D. ,
- Scott M. McGinnis , M.D.
- Seth A. Gale , M.A., M.D. ,
- C. Alan Anderson , M.D. ,
- David B. Arciniegas , M.D.

- Posterior Cortical Atrophy
- Corticobasal Syndrome
- Atypical Alzheimer Disease
- Network Degeneration
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Bringing back a long extinct bird

‘The scientist is not in the business of following instructions.’

Glimpse of next-generation internet
Video: Editor Kai-Jae Wang; videographers Joe Sherman and Kai-Jae Wang; interviews by Ned Brown
Lessons of the brain: The Phineas Gage story
Harvard Correspondent
In 1848, an iron bar pierced his brain, his case providing new insights on both trauma and recovery
Imagine the modern-day reaction to a news story about a man surviving a three-foot, 7-inch, 13½-pound iron bar being blown through his skull — taking a chunk of his brain with it.
Then imagine that this happened in 1848, long before modern medicine and neuroscience. That was the case of Phineas Gage.
Whether the Vermont construction foreman, who was laying railroad track and using explosives at the time of the industrial accident, was lucky or unlucky is a judgment that Warren Anatomical Museum curator Dominic Hall puzzles over to this day.
“It is an impossible question, because he was extraordinarily unlucky to have an iron bar borne through his skull, but equally lucky to have survived, on such a low level of care,” said Hall. “We are lucky, to have him.”
Gage’s skull, along with the tamping iron that bore through it, are two of the approximately 15,000 artifacts and case objects conserved at the Warren, which is a part of the Center for the History of Medicine in Harvard’s Francis A. Countway Library of Medicine .
The resultant change in Gage’s personality — when he went from being well-liked and professionally successful to being “fitful, irreverent, and grossly profane, showing little deference for his fellows” and unable to keep his job — is widely cited in modern psychology as the textbook case for post-traumatic social disinhibition.
But as the years have gone by and we’ve learned more about his life, argued Hall, the teachings have changed.
“In 1848, he was seen as a triumph of human survival. Then, he becomes the textbook case for post-traumatic personality change. Recently, people interpret him as having found a form of independence and social recovery, which he didn’t get credit for 15 years ago.”
When Gage died 12 years after the accident, following epileptic seizures, his body was exhumed, while his skull and tamping iron were sent to the physician who had cared for him since the accident, John Harlow. Harlow later donated the items to the Warren, where they have remained for 160 years.
“In many ways, I see Gage similarly to how you would see a portrait of one of the famous professors hanging on the wall — he’s an important part of Harvard Medical School’s identity,” said Hall. “By continually reflecting on his case, it allows us to change how we reflect on the human brain and how we interact with our historical understanding of neuroscience.”
Share this article
You might like.
Scientists sequence complete genome of bush moa, offering insights into its natural history, possible clues to evolution of flightless birds

George Whitesides became a giant of chemistry by keeping it simple

Physicists demo first metro-area quantum computer network in Boston
Women who follow Mediterranean diet live longer
Large study shows benefits against cancer, cardiovascular mortality, also identifies likely biological drivers of better health
Harvard-led study IDs statin that may block pathway to some cancers
Cholesterol-lowering drug suppresses chronic inflammation that creates dangerous cascade
- The Big Think Interview
- Your Brain on Money
- Explore the Library
- Will true AI turn against us?
- Do we have free will?
- Why are there conspiracy theories?
- Is religion helping or hurting us?
- Are we alone in the universe?
- Should we trust science?
- Michio Kaku
- Neil deGrasse Tyson
- Michelle Thaller
- Steven Pinker
- Ray Kurzweil
- Cornel West
- Helen Fisher
- Smart Skills
- High Culture
- The Present
- Hard Science
- Special Issues
- Starts With A Bang
- Perception Box
- Strange Maps
- The Learning Curve
- Everyday Philosophy
- Free Newsletters
- Memberships
Psychology’s 10 greatest case studies – digested

These ten characters have all had a huge influence on psychology and their stories continue to intrigue each new generation of students. What’s particularly fascinating is that many of their stories continue to evolve – new evidence comes to light, or new technologies are brought to bear, changing how the cases are interpreted and understood. What many of these 10 also have in common is that they speak to some of the perennial debates in psychology, about personality and identity, nature and nurture, and the links between mind and body.
Phineas Gage
One day in 1848 in Central Vermont, Phineas Gage was tamping explosives into the ground to prepare the way for a new railway line when he had a terrible accident. The detonation went off prematurely, and his tamping iron shot into his face, through his brain, and out the top of his head.
Remarkably Gage survived, although his friends and family reportedly felt he was changed so profoundly (becoming listless and aggressive) that “he was no longer Gage.” There the story used to rest – a classic example of frontal brain damage affecting personality. However, recent years have seen a drastic reevaluation of Gage’s story in light of new evidence. It’s now believed that he underwent significant rehabilitation and in fact began work as a horse carriage driver in Chile. A simulation of his injuries suggested much of his right frontal cortex was likely spared, and photographic evidence has been unearthed showing a post-accident dapper Gage. Not that you’ll find this revised account in many psychology textbooks: a recent analysis showed that few of them have kept up to date with the new evidence.
Henry Gustav Molaison (known for years as H.M. in the literature to protect his privacy), who died in 2008, developed severe amnesia at age 27 after undergoing brain surgery as a form of treatment for the epilepsy he’d suffered since childhood. He was subsequently the focus of study by over 100 psychologists and neuroscientists and he’s been mentioned in over 12,000 journal articles! Molaison’s surgery involved the removal of large parts of the hippocampus on both sides of his brain and the result was that he was almost entirely unable to store any new information in long-term memory (there were some exceptions – for example, after 1963 he was aware that a US president had been assassinated in Dallas). The extremity of Molaison’s deficits was a surprise to experts of the day because many of them believed that memory was distributed throughout the cerebral cortex. Today, Molaison’s legacy lives on: his brain was carefully sliced and preserved and turned into a 3D digital atlas and his life story is reportedly due to be turned into a feature film based on the book researcher Suzanne Corkin wrote about him: Permanent Present Tense, The Man With No Memory and What He Taught The World .
Victor Leborgne (nickname “Tan”)
The fact that, in most people, language function is served predominantly by the left frontal cortex has today almost become common knowledge, at least among psych students. However, back in the early nineteenth century, the consensus view was that language function (like memory, see entry for H.M.) was distributed through the brain. An eighteenth century patient who helped change that was Victor Leborgne, a Frenchman who was nicknamed “Tan” because that was the only sound he could utter (besides the expletive phrase “sacre nom de Dieu”). In 1861, aged 51, Leborgne was referred to the renowned neurologist Paul Broca, but died soon after. Broca examined Leborgne’s brain and noticed a lesion in his left frontal lobe – a segment of tissue now known as Broca’s area. Given Leborgne’s impaired speech but intact comprehension, Broca concluded that this area of the brain was responsible for speech production and he set about persuading his peers of this fact – now recognised as a key moment in psychology’s history. For decades little was known about Leborgne, besides his important contribution to science. However, in a paper published in 2013, Cezary Domanski at Maria Curie-Sklodowska University in Poland uncovered new biographical details, including the possibility that Leborgne muttered the word “Tan” because his birthplace of Moret, home to several tanneries.
Wild Boy of Aveyron
The “Wild boy of Aveyron” – named Victor by the physician Jean-Marc Itard – was found emerging from Aveyron forest in South West France in 1800, aged 11 or 12, where’s it’s thought he had been living in the wild for several years. For psychologists and philosophers, Victor became a kind of “natural experiment” into the question of nature and nurture. How would he be affected by the lack of human input early in his life? Those who hoped Victor would support the notion of the “noble savage” uncorrupted by modern civilisation were largely disappointed: the boy was dirty and dishevelled, defecated where he stood and apparently motivated largely by hunger. Victor acquired celebrity status after he was transported to Paris and Itard began a mission to teach and socialise the “feral child”. This programme met with mixed success: Victor never learned to speak fluently, but he dressed, learned civil toilet habits, could write a few letters and acquired some very basic language comprehension. Autism expert Uta Frith believes Victor may have been abandoned because he was autistic, but she acknowledges we will never know the truth of his background. Victor’s story inspired the 2004 novel The Wild Boy and was dramatised in the 1970 French film The Wild Child .
Nicknamed ‘Kim-puter’ by his friends, Peek who died in 2010 aged 58, was the inspiration for Dustin Hoffman’s autistic savant character in the multi-Oscar-winning film Rain Man . Before that movie, which was released in 1988, few people had heard of autism, so Peek via the film can be credited with helping to raise the profile of the condition. Arguably though, the film also helped spread the popular misconception that giftedness is a hallmark of autism (in one notable scene, Hoffman’s character deduces in an instant the precise number of cocktail sticks – 246 – that a waitress drops on the floor). Peek himself was actually a non-autistic savant, born with brain abnormalities including a malformed cerebellum and an absent corpus callosum (the massive bundle of tissue that usually connects the two hemispheres). His savant skills were astonishing and included calendar calculation, as well as an encyclopaedic knowledge of history, literature, classical music, US zip codes and travel routes. It was estimated that he read more than 12,000 books in his life time, all of them committed to flawless memory. Although outgoing and sociable, Peek had coordination problems and struggled with abstract or conceptual thinking.
“Anna O.” is the pseudonym for Bertha Pappenheim, a pioneering German Jewish feminist and social worker who died in 1936 aged 77. As Anna O. she is known as one of the first ever patients to undergo psychoanalysis and her case inspired much of Freud’s thinking on mental illness. Pappenheim first came to the attention of another psychoanalyst, Joseph Breuer, in 1880 when he was called to her house in Vienna where she was lying in bed, almost entirely paralysed. Her other symptoms include hallucinations, personality changes and rambling speech, but doctors could find no physical cause. For 18 months, Breuer visited her almost daily and talked to her about her thoughts and feelings, including her grief for her father, and the more she talked, the more her symptoms seemed to fade – this was apparently one of the first ever instances of psychoanalysis or “the talking cure”, although the degree of Breuer’s success has been disputed and some historians allege that Pappenheim did have an organic illness, such as epilepsy. Although Freud never met Pappenheim, he wrote about her case, including the notion that she had a hysterical pregnancy, although this too is disputed. The latter part of Pappenheim’s life in Germany post 1888 is as remarkable as her time as Anna O. She became a prolific writer and social pioneer, including authoring stories, plays, and translating seminal texts, and she founded social clubs for Jewish women, worked in orphanages and founded the German Federation of Jewish Women.
Kitty Genovese
Sadly, it is not really Kitty Genovese the person who has become one of psychology’s classic case studies, but rather the terrible fate that befell her. In 1964 in New York, Genovese was returning home from her job as a bar maid when she was attacked and eventually murdered by Winston Mosely. What made this tragedy so influential to psychology was that it inspired research into what became known as the Bystander Phenomenon – the now well-established finding that our sense of individual responsibility is diluted by the presence of other people. According to folklore, 38 people watched Genovese’s demise yet not one of them did anything to help, apparently a terrible real life instance of the Bystander Effect. However, the story doesn’t end there because historians have since established the reality was much more complicated – at least two people did try to summon help, and actually there was only one witness the second and fatal attack. While the main principle of the Bystander Effect has stood the test of time, modern psychology’s understanding of the way it works has become a lot more nuanced. For example, there’s evidence that in some situations people are more likely to act when they’re part of a larger group, such as when they and the other group members all belong to the same social category (such as all being women) as the victim.
Little Albert
“Little Albert” was the nickname that the pioneering behaviourist psychologist John Watson gave to an 11-month-old baby, in whom, with his colleague and future wife Rosalind Rayner, he deliberately attempted to instill certain fears through a process of conditioning. The research, which was of dubious scientific quality, was conducted in 1920 and has become notorious for being so unethical (such a procedure would never be given approval in modern university settings). Interest in Little Albert has reignited in recent years as an academic quarrel has erupted over his true identity. A group led by Hall Beck at Appalachian University announced in 2011 that they thought Little Albert was actually Douglas Merritte, the son of a wet nurse at John Hopkins University where Watson and Rayner were based. According to this sad account, Little Albert was neurologically impaired, compounding the unethical nature of the Watson/Rayner research, and he died aged six of hydrocephalus (fluid on the brain). However, this account was challenged by a different group of scholars led by Russell Powell at MacEwan University in 2014. They established that Little Albert was more likely William A Barger (recorded in his medical file as Albert Barger), the son of a different wet nurse. Earlier this year, textbook writer Richard Griggs weighed up all the evidence and concluded that the Barger story is the more credible, which would mean that Little Albert in fact died 2007 aged 87.
Chris Sizemore
Chris Costner Sizemore is one of the most famous patients to be given the controversial diagnosis of multiple personality disorder, known today as dissociative identity disorder. Sizemore’s alter egos apparently included Eve White, Eve Black, Jane and many others. By some accounts, Sizemore expressed these personalities as a coping mechanism in the face of traumas she experienced in childhood, including seeing her mother badly injured and a man sawn in half at a lumber mill. In recent years, Sizemore has described how her alter egos have been combined into one united personality for many decades, but she still sees different aspects of her past as belonging to her different personalities. For example, she has stated that her husband was married to Eve White (not her), and that Eve White is the mother of her first daughter. Her story was turned into a movie in 1957 called The Three Faces of Eve (based on a book of the same name written by her psychiatrists). Joanne Woodward won the best actress Oscar for portraying Sizemore and her various personalities in this film. Sizemore published her autobiography in 1977 called I’m Eve . In 2009, she appeared on the BBC’s Hard Talk interview show.
David Reimer
One of the most famous patients in psychology, Reimer lost his penis in a botched circumcision operation when he was just 8 months old. His parents were subsequently advised by psychologist John Money to raise Reimer as a girl, “Brenda”, and for him to undergo further surgery and hormone treatment to assist his gender reassignment.
Money initially described the experiment (no one had tried anything like this before) as a huge success that appeared to support his belief in the important role of socialisation, rather than innate factors, in children’s gender identity. In fact, the reassignment was seriously problematic and Reimer’s boyishness was never far beneath the surface. When he was aged 14, Reimer was told the truth about his past and set about reversing the gender reassignment process to become male again. He later campaigned against other children with genital injuries being gender reassigned in the way that he had been. His story was turned into the book As Nature Made Him, The Boy Who Was Raised As A Girl by John Colapinto, and he is the subject of two BBC Horizon documentaries. Tragically, Reimer took his own life in 2004, aged just 38.
Christian Jarrett ( @Psych_Writer ) is Editor of BPS Research Digest
This article was originally published on BPS Research Digest . Read the original article .

- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
Phineas Gage: His Accident and Impact on Psychology
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Emily is a board-certified science editor who has worked with top digital publishing brands like Voices for Biodiversity, Study.com, GoodTherapy, Vox, and Verywell.
:max_bytes(150000):strip_icc():format(webp)/Emily-Swaim-1000-0f3197de18f74329aeffb690a177160c.jpg)
Author unknown / Wikimedia Commons
- Phineas Gage's Accident
- Change in Personality
- Severity of Brain Damage
- Impact on Psychology
What Happened to Phineas Gage After the Brain Damage?
Phineas Gage is often referred to as the "man who began neuroscience." He experienced a traumatic brain injury when an iron rod was driven through his skull, destroying much of his frontal lobe .
Gage miraculously survived the accident. However, his personality and behavior were so changed as a result of the frontal lobe damage that many of his friends described him as an almost different person entirely. The impact that the accident had has helped us better understand what the frontal lobe does, especially in relation to personality .
At a Glance
In 1848, Phineas Gage had a workplace accident in which an iron tamping rod entered and exited his skull. He survived but it is said that his personality changed as a result, leading to a greater understanding of the brain regions involved in personality, namely the frontal lobe.
Phineas Gage's Accident
On September 13, 1848, 25-year-old Gage was working as the foreman of a crew preparing a railroad bed near Cavendish, Vermont. He was using an iron tamping rod to pack explosive powder into a hole.
Unfortunately, the powder detonated, sending the 43-inch-long, 1.25-inch-diameter rod hurling upward. The rod penetrated Gage's left cheek, tore through his brain , and exited his skull before landing 80 feet away.
Gage not only survived the initial injury but was able to speak and walk to a nearby cart so he could be taken into town to be seen by a doctor. He was still conscious later that evening and able to recount the names of his co-workers. Gage even suggested that he didn't wish to see his friends since he would be back to work in "a day or two" anyway.
The Recovery Process
After developing an infection, Gage spent September 23 to October 3 in a semi-comatose state. On October 7, he took his first steps out of bed, and, by October 11, his intellectual functioning began to improve.
Descriptions of Gage's injury and mental changes were made by Dr. John Martyn Harlow. Much of what researchers know about the case is based on Harlow's observations.
Harlow noted that Gage knew how much time had passed since the accident and remembered clearly how the accident occurred, but had difficulty estimating the size and amounts of money. Within a month, Gage was well enough to leave the house.
In the months that followed, Gage returned to his parent's home in New Hampshire to recuperate. When Harlow saw Gage again the following year, the doctor noted that while Gage had lost vision in his eye and was left with obvious scars from the accident, he was in good physical health and appeared recovered.
Theories About Gage's Survival and Recovery
The type of injury sustained by Phineas Gage could have easily been fatal. While it cannot be said with certainty why Gage was able to survive the accident, let alone recover from the injury and still function, several theories exist. They include:
- The rod's path . Some researchers suggest that the rod's path likely played a role in Gage's survival in that if it had penetrated other areas of the head—such as the pterygoid plexuses or cavernous sinus—Gage may have bled to death.
- The brain's selective recruitment . In a 2022 study of another individual who also had an iron rod go through his skull—whom the researchers referred to as a "modern-day Phineas Gage"—it was found that the brain is able to selectively recruit non-injured areas to help perform functions previously assigned to the injured portion.
- Work structure . Others theorize that Gage's work provided him structure, positively contributing to his recovery and aiding in his rehabilitation.
How Did Phineas Gage's Personality Change?
Popular reports of Gage often depict him as a hardworking, pleasant man before the accident. Post-accident, these reports describe him as a changed man, suggesting that the injury had transformed him into a surly, aggressive heavy drinker who was unable to hold down a job.
Harlow presented the first account of the changes in Gage's behavior following the accident. Where Gage had been described as energetic, motivated, and shrewd prior to the accident, many of his acquaintances explained that after the injury, he was "no longer Gage."
Severity of Gage's Brain Damage
Since there is little direct evidence of the exact extent of Gage's injuries aside from Harlow's report, it is difficult to know exactly how severely his brain was damaged. Harlow's accounts suggest that the injury did lead to a loss of social inhibition, leading Gage to behave in ways that were seen as inappropriate.
In a 1994 study, researchers utilized neuroimaging techniques to reconstruct Phineas Gage's skull and determine the exact placement of the injury. Their findings indicate that he suffered injuries to both the left and right prefrontal cortices, which would result in problems with emotional processing and rational decision-making .
Another study conducted in 2004 used three-dimensional, computer-aided reconstruction to analyze the extent of Gage's injury. It found that the effects were limited to the left frontal lobe.
In 2012, new research estimated that the iron rod destroyed approximately 11% of the white matter in Gage's frontal lobe and 4% of his cerebral cortex.
Some evidence suggests that many of the supposed effects of the accident may have been exaggerated and that Gage was actually far more functional than previously reported.
Why Is Phineas Gage Important to Psychology?
Gage's case had a tremendous influence on early neurology. The specific changes observed in his behavior pointed to emerging theories about the localization of brain function, or the idea that certain functions are associated with specific areas of the brain.
In those years, neurology was in its infancy. Gage's extraordinary story served as one of the first sources of evidence that the frontal lobe was involved in personality.
Today, scientists better understand the role that the frontal cortex has to play in important higher-order functions such as reasoning , language, and social cognition .
After the accident, Gage was unable to continue his previous job. According to Harlow, Gage spent some time traveling through New England and Europe with his tamping iron to earn money, supposedly even appearing in the Barnum American Museum in New York.
He also worked briefly at a livery stable in New Hampshire and then spent seven years as a stagecoach driver in Chile. He eventually moved to San Francisco to live with his mother as his health deteriorated.
After a series of epileptic seizures, Gage died on May 21, 1860, almost 12 years after his accident. Seven years after his death, Gage's body was exhumed. His brother gave his skull and the tamping rod to Dr. Harlow, who subsequently donated them to the Harvard University School of Medicine. They are still exhibited in its museum today.
Bottom Line
Gage's accident and subsequent experiences serve as a historical example of how case studies can be used to look at unique situations that could not be replicated in a lab. What researchers learned from Phineas Gage's skull and brain injury played an important role in the early days of neurology and helped scientists gain a better understanding of the human brain and the impact that damage could have on both functioning and behavior.
Sevmez F, Adanir S, Ince R. Legendary name of neuroscience: Phineas Gage (1823-1860) . Child's Nervous System . 2020. doi:10.1007/s00381-020-04595-6
Twomey S. Phineas Gage: Neuroscience's most famous patient . Smithsonian Magazine.
Harlow JM. Recovery after severe injury to the head . Bull Massachus Med Soc . 1848. Reprinted in Hist Psychiat. 1993;4(14):274-281. doi:10.1177/0957154X9300401407
Harlow JM. Passage of an iron rod through the head . 1848. J Neuropsychiatry Clin Neurosci . 1999;11(2):281-3. doi:10.1176/jnp.11.2.281
Itkin A, Sehgal T. Review of Phineas Gage's oral and maxillofacial injuries . J Oral Biol . 2017;4(1):3.
de Freitas P, Monteiro R, Bertani R, et al. E.L., a modern-day Phineas Gage: Revisiting frontal lobe injury . The Lancet Regional Health - Americas . 2022;14:100340. doi:10.1016/j.lana.2022.100340
Macmillan M, Lena ML. Rehabilitating Phineas Gage . Neuropsycholog Rehab . 2010;20(5):641-658. doi:10.1080/09602011003760527
O'Driscoll K, Leach JP. "No longer Gage": An iron bar through the head. Early observations of personality change after injury to the prefrontal cortex . BMJ . 1998;317(7174):1673-4. doi:10.1136/bmj.317.7174.1673a
Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: Clues about the brain from the skull of a famous patient . Science . 1994;264(5162):1102-5. doi:10.1126/science.8178168
Ratiu P, Talos IF. Images in clinical medicine. The tale of Phineas Gage, digitally remastered . N Engl J Med . 2004;351(23):e21. doi:10.1056/NEJMicm031024
Van Horn JD, Irimia A, Torgerson CM, Chambers MC, Kikinis R, Toga AW. Mapping connectivity damage in the case of Phineas Gage . PLoS One . 2012;7(5):e37454. doi: 10.1371/journal.pone.0037454
Macmillan M. An Odd Kind of Fame: Stories of Phineas Gage . MIT Press.
Shelley B. Footprints of Phineas Gage: Historical beginnings on the origins of brain and behavior and the birth of cerebral localizationism . Archives Med Health Sci . 2016;4(2):280-6. doi:10.4103/2321-4848.196182
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
CASE REPORT article
Case report: an mri traumatic brain injury longitudinal case study at 7 tesla: pre- and post-injury structural network and volumetric reorganization and recovery.

- 1 Cambridge Intellectual and Developmental Disabilities Research Group, Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom
- 2 Department of Rehabilitation and Human Performance, Brain Injury Research Center, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3 Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 4 Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 5 Department of Computer Science, Mathematics, Physics, and Statistics University of British Columbia, Kelowna, BC, Canada
Importance: A significant limitation of many neuroimaging studies examining mild traumatic brain injury (mTBI) is the unavailability of pre-injury data.
Objective: We therefore aimed to utilize pre-injury ultra-high field brain MRI and compare a collection of neuroimaging metrics pre- and post-injury to determine mTBI related changes and evaluate the enhanced sensitivity of high-resolution MRI.
Design: In the present case study, we leveraged multi-modal 7 Tesla MRI data acquired at two timepoints prior to mTBI (23 and 12 months prior to injury), and at two timepoints post-injury (2 weeks and 8 months after injury) to examine how a right parietal bone impact affects gross brain structure, subcortical volumetrics, microstructural order, and connectivity.
Setting: This research was carried out as a case investigation at a single primary care site.
Participants: The case participant was a 38-year-old female selected for inclusion based on a mTBI where a right parietal impact was sustained.
Main outcomes: The main outcome measurements of this investigation were high spatial resolution structural brain metrics including volumetric assessment and connection density of the white matter connectome.
Results: At the first scan timepoint post-injury, the cortical gray matter and cerebral white matter in both hemispheres appeared to be volumetrically reduced compared to the pre-injury and subsequent post-injury scans. Connectomes produced from whole-brain diffusion-weighted probabilistic tractography showed a widespread decrease in connectivity after trauma when comparing mean post-injury and mean pre-injury connection densities. Findings of reduced fractional anisotropy in the cerebral white matter of both hemispheres at post-injury time point 1 supports reduced connection density at a microstructural level. Trauma-related alterations to whole-brain connection density were markedly reduced at the final scan timepoint, consistent with symptom resolution.
Conclusions and Relevance: This case study investigates the structural effects of traumatic brain injury for the first time using pre-injury and post-injury 7 Tesla MRI longitudinal data. We report findings of initial volumetric changes, decreased structural connectivity and reduced microstructural order that appear to return to baseline 8 months post-injury, demonstrating in-depth metrics of physiological recovery. Default mode, salience, occipital, and executive function network alterations reflect patient-reported hypersomnolence, reduced cognitive processing speed and dizziness.
Introduction
Traumatic brain injury (TBI) is a leading cause of disability worldwide, particularly in young and military populations, with well-documented links to psychiatric and neurodegenerative pathology ( 1 ). Patients who experience mild traumatic brain injury (mTBI) typically report vestibular, sensory, cognitive or emotional symptoms that persist for several months after injury ( 2 ). Patients experiencing mTBI typically show low frequency of positive MRI findings at 6 months post-injury, highlighting the need for more sophisticated and sensitive imaging techniques in the clinical investigation of mTBI ( 3 , 4 ). Previous studies have reported the utility of diffusion-weighted imaging and tractography methodology in determining the presence of neuronal injury in cases where conventional neuroimaging findings are negative ( 5 , 6 ), as they allow examination of fiber- and tract-related pathology. Tracking of the spinothalamic tract in a mTBI case demonstrated thinning and discontinuation of fibers at the subcortical white matter in mTBI patients with no conventional radiological abnormalities ( 6 ), and the corticobulbar tract and fornix exhibited similar narrowing and discontinuations in an mTBI case study caused by violence ( 5 ). It is probable that white matter damage, common in TBI due to both indirect shearing forces and direct damage, may be a pertinent but often undetected pathophysiology in this population ( 7 ). Moreover, it is generally appreciated that injury to the brain resulting from trauma often arises globally, as axons crossing areas of differing tissue density react differently to the mechanical force of the trauma ( 8 ). This can cause widespread damage, which may be explored using network and connectivity analyses that draw directly upon anatomically accurate estimations of white matter connection density. A connectomic network approach allows data integration of distinct regions of brain anatomy and connection strength, which makes it a useful methodology for examining both localized and global effects of mTBI on white matter ( 9 ).
In this case study, unique due to the rare availability of pre- and post-injury 7 Tesla high-resolution data, we investigated the trajectory of structural changes attributed to mTBI. The multiple time-point pre-injury data is an uncommon strength to the present research, as longitudinal data can be examined in both healthy and post-injury settings. Moreover, we aimed to investigate and characterize disparities between conventional structural MRI and diffusion-weighted connectomic findings. In this report, we hope to illustrate how recent developments in the field of computational neuroimaging, such as morphometric subcortical segmentations and network theory, may aid in the identification of suitably sensitive biomarkers of brain injury.
Case Description
A 38-year-old female was involved in a motor vehicle accident in which she was a pedestrian hit by a car turning into the intersection she was crossing. She was thrown across the road where her head hit the curb. She was transported to the nearby hospital where acutely, the patient was dizzy, faint and mildly confused. Head CT revealed subcutaneous soft tissue swelling over the right parietal bone. There was no evidence of acute territorial infarction or intracranial hemorrhage. Ventricles and sulci appeared normal in size and configuration for the patient's age. There was no midline shift or other mass effect, and gray-white matter differentiation was maintained throughout the brain. The patient received surgical staples to close a laceration over the right parietal bone and was discharged home. The patient reported minimal headaches or nausea, but dizziness, daytime fatigue, hypersomnolence, reduced problem-solving skills and slowed cognitive processing persisted for several weeks following the injury. She returned to work the day after the injury, working slightly reduced hours to accommodate fatigue. Full recovery, defined as full symptom resolution and return to baseline function, was achieved ~6 months post-injury. The patient gave fully informed consent for participation in the presented research. Institutional Review Board (IRB) approval for human research was obtained for this experiment from the Program for Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai.
Longitudinal Data Acquisition
The patient had undergone two scanning sessions at 7 Tesla prior to the head injury as a healthy control. Two more scans were acquired post-injury. The scan times in relation to injury were as follows: 23 months prior to injury, 12 months prior to injury, 2 weeks post-injury, and 8 months post-injury. All MRI scanning was performed using the same Siemens 7T scanner. Clinical assessment and an initial head CT were carried out immediately after injury, and neurocognitive testing was administered by a trained clinician 18 months post-injury.
Clinical Neurocognitive Data Acquisition
A brief battery of performance-based neurocognitive tests was administered to estimate premorbid intellectual functioning and quantitatively confirm cognitive recovery 18 months post-injury ( 10 ).
MRI Acquisition
Included in each MRI protocol was a T 1 -weighted Magnetization Prepared 2 Rapid Acquisition Gradient Echo (MP2RAGE) ( 11 ), a T2-weighted Turbo Spin Echo (TSE), and a diffusion MRI.
The MP2RAGE sequence obtains improved gray-white contrast at high field compared to the classic MPRAGE acquisition ( 11 ). High spatial resolution voxel size was 0.8 mm isotropic, TR/TE = 6,000/3.2 ms, TI1(θ 1 )/TI2(θ 2 ) = 1,050(5°)/ 3,000(4°) ms and total acquisition time was 7:26 minutes. From the MP2RAGE dataset, a total of four images were reconstructed from (a) data acquired after inversion time (TI) of 1,050 ms, (b) data acquired after TI of 3,000 ms, (c) T1 relaxation maps calculated from (a) and (b), and (d) uniform-denoised (UNIDEN) images calculated from (a) and (b). An in-plane acceleration factor of 3 was used.
Two TSE structural images were obtained at high in-plane resolution (0.4 × 0.4 mm 2 ), a slice thickness of 2 mm, TR/TE = 6,900/69 ms and θ = 150°. An in-plane acceleration factor of 2 was used. The first T2-TSE was obtained with a 6:14 min acquisition time in a coronal-oblique orientation where the imaging plane was aligned perpendicular to the long axis of the hippocampus. The second T2-TSE was obtained in an axial orientation; the imaging plane alighted along the axis connecting the anterior commissure and the posterior commissure (AC-PC). The acquisition time for the second T2-TSE scan was 6:50 min.
Diffusion MRI data were collected using a single-shot spin-EPI sequence aligned axially with an isotropic resolution of 1.05 mm, an in-plane acceleration factor of 3, a multi-band acceleration factor of 2 and TR/TE = 6,900/67 ms. The diffusion sequence was a paired acquisition with reversed phase encoding in the AP/PA direction, and each pair had 64 diffusion encoding directions ( b = 1,200 s/mm 2 ) and 4 unweighted scans ( b = 0 s/mm 2 ). Total scan time for the paired acquisition was 20 min.
Structural MRI Analysis
The FreeSurfer “recon-all” pipeline (version 6.0) ( 12 ) was used to carry out the following processing steps on T1-weighted structural data: motion correction, intensity correction, transform to Talairach space, intensity normalization, skull strip, subcortical segmentation, neck remove, subcortical labeling, segmentation statistics, a second intensity correction using brain only (after skull strip), white matter segmentation, subcortical mass creation, brain surface creation, surface inflation, automatic topology fixer, cortical thickness/pial surfaces, cortical ribbon mask, spherical inflation of the brain surface, ipsilateral surface registration, contralateral surface registration, resampling of the average atlas curvature to subject, cortical parcellation, and creation of summary table for parcellation statistics. As the T1-weighted data had a submillimeter isotropic voxel size, the “-hires” flag was used to preserve enhanced spatial resolution ( 13 ).
Hippocampal subfield ( 14 ) and amygdala subnuclei segmentation ( 15 ) was carried out using FreeSurfer 6.0 development version. A multi-spectral approach was used, utilizing both the T1-weighted and T2-weighted images, leveraging the enhanced resolution of the T2-weighted image to provide additional anatomical information. This subcortical segmentation is visualized in Figure 1 .
Figure 1. (a) Multi-spectral hippocampal subfield segmentation (CA1, CA3, CA4, dentate gyrus, and subicular complex) with underlay of axial T1-weighted data. (b) Hippocampal subfield segmentation with underlay of axial T2-weighted data. (c) Multi-spectral amygdala subnuclei segmentation (lateral, basal, accessory basal, central, cortical, medial nuclei, and corticoamygdaloid transition area (CATA) with underlay of axial T1-weighted data. (d) Amygdala subnuclei segmentation with underlay of axial T2-weighted data.
To investigate test–rest variability, blinded re-runs of the imaging processing were carried out, and pairwise coefficients of variation were calculated per measure by dividing the standard deviation by the mean and multiplying by 100 to produce a percentage.
Diffusion MRI Analysis
Denoising of the diffusion weighted data was performed using MRTrix two-shell phase-reversed processing ( 16 , 17 ). Segmented and parcellated structural images from the FreeSurfer “recon-all” pipeline were used for whole brain masking ( 12 ). B 1 field inhomogeneity correction was carried out ( 18 ) and the fiber orientation distributions (FODs) were created from the diffusion data using constrained super-resolved spherical deconvolution ( 19 ). Estimation of the diffusion tensor was done using iteratively reweighted linear least squares methodology ( 20 ). The tensor image was used to create a whole brain map of fractional anisotropy (FA) ( 21 ). Mean FA was extracted from cerebral white matter hemispheric masks created by the FreeSurfer pipeline.
Co-registration of anatomical images into diffusion space was then carried out using Statistical Parametric Mapping software (SPM12). Degree of spline interpolation was 4. The MRTrix command “5ttgen” was used to generate a five tissue-type segmentation image, utilizing the FreeSurfer outputs, to use in anatomically constrained tractography ( 22 ). A segmented mask image was then created for the seeding of tractography streamlines at the gray-white matter interface ( 22 ). The fiber orientation distributions were then used to create whole brain tractograms for each participant ( 23 ). Ten million streamlines were generated from the probabilistic tractography per brain. Individual step size for the streamlines was 0.1 mm × voxel size, the fiber orientation distribution amplitude cut-off was 0.05 and the maximum angle between successive steps was 90° × step size × voxel size. Seeds were placed in the gray white matter interface. Spherical deconvolution informed filtering (SIFT2) was applied to the tractograms, the purpose of which was to weight streamlines based on likelihood of anatomical accuracy, remove spurious streamlines from further analysis and ensure data that is highly representative of ground-truth biology ( 24 ). A structural connectome, based on node-to-node connection density, was created using MRTrix ( 25 ).
Structural connectomes at different timepoints were compared by custom functions that performed elementwise subtraction of the matrices in MATLAB. Similarly, variability of the connectomes was assessed by stacking matrices into a 3-dimensional array and computing the mean and standard deviation along the z -axis for each network edge. To investigate variability between scan timepoints, specifically to examine test–retest variation, the two pre-injury connectomes were compared using co-efficients of variation, calculated elementwise for each edge of the connectivity matrix by dividing the standard deviation by the mean and multiplying by 100 to produce a percentage. The mean co-efficient of variation was calculated by averaging the co-efficients of variation across the whole matrix. To determine a streamline threshold of the connectome with an acceptable level of variability, mean matrix co-efficients of variation were calculated for the following streamline thresholds: 25, 50, 100, 200, 400, 800, 1,600, 3,200, 6,400, 12,800, and 25,600. Actual streamline thresholding was subsequently set at 15,000, discarding edges consisting of streamline bundles with less density than the threshold.
Clinical Neurocognitive Data
This high-achieving woman with a history of academic excellence throughout 20 years of formal education had an estimated premorbid intellectual ability in the high average-superior range ( 10 ). At the time of testing her performance-based intellectual quotient (IQ) was in the superior range, consistent with expectation. She demonstrated a relative strength in verbal comprehension (96th percentile) as compared to perceptual reasoning (>99th percentile). Performance on tests of contextual and non-contextual verbal memory was consistently above the 98th percentile, while visual memory performance was at the 34th percentile (Average range). Tests of complex attention and working memory were generally above the 85th percentile (High Average range), and tests of verbal fluency were variable (semantic fluency 38th percentile; phonemic fluency 96th percentile). Performance on timed tests of sequencing and task-switching were below expectation (<1st percentile – 62nd percentile) while untimed tests of these higher order executive functions were well within expectation (>96th percentile). Overall, performance on neurocognitive tests indicate superior intellectual ability with performance 18 months post-TBI largely consistent with expectations; impaired performance on select timed tests suggest a tendency to sacrifice speed to ensure accuracy which may reflect a compensatory strategy.
The subcortical segmentation of the amygdala nuclei and hippocampal subfields did not reveal any clear changes between the scanning timepoints. The average test-retest coefficient of variation for the hippocampal subfield segmentation was 1.4%, and 5.2% for the amygdala nuclei. Considering this estimation of variability within the image processing, the data did not reveal evidence of volumetric change to the hippocampus or amygdala post-injury, either at a whole or substructure level.
At post-injury timepoint 1, the right and left hemispheric brain segmentation revealed lower cortical gray matter and cerebral white matter volume compared to other scanning timepoints. No change was apparent in ventricle volume ( Figure 2 ). The average test-retest variation co-efficient for each of these variables was <0.001%, significantly less than the observed change post-TBI.
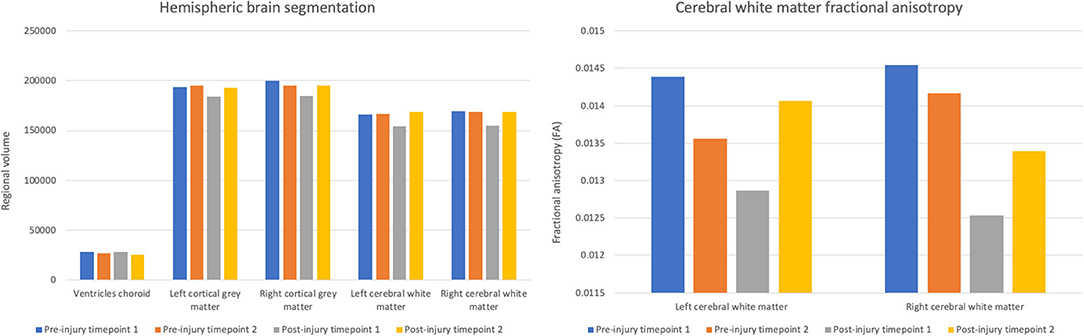
Figure 2 . Volumes of choroid ventricles, hemispheric gray matter, and hemispheric white matter and fractional anisotropy of the hemispheric cerebral white matter across scanning timepoints.
Concurrent with the changes in the structural volumetrics, FA of the cerebral white matter was markedly reduced in both hemispheres in the first scan following the head trauma. In both the left and right hemispheres, the final timepoint scan revealed a subsequent increase of FA to levels similar to those pre-injury ( Figure 2 ).
Averaging of the structural diffusion MRI connectomes pre- and post-injury revealed a widespread decrease in connectivity after the patient’s head trauma, mainly involving connections between cortical regions ( Figure 3A ). To a lesser degree, mean pre- to post-injury comparison also revealed some increased connectivity, primarily in subcortical areas and the forebrain ( Figure 3B ). A comparison between the first and second post-injury connectome matrices was then carried out, to investigate if changes to the patient's structural connectivity post-TBI were consistent over time. The results showed that at post-injury timepoint 1, connection density was extensively reduced, but this decrease in connectivity was partially reduced by post-injury timepoint 2.
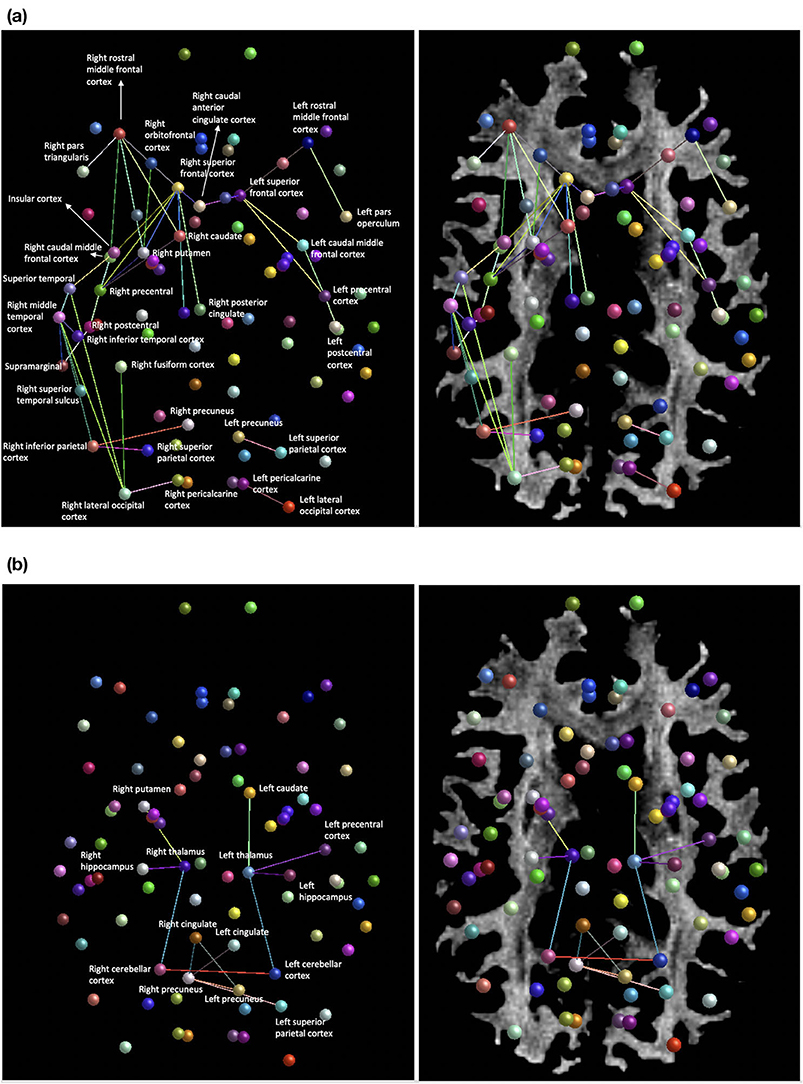
Figure 3. (a) Areas of decreased connection density of the structural network mean post-injury compared to mean pre-injury. (b) Regions of increased connection density of the structural network mean post-injury compared to mean-pre-injury.
Structural high-resolution neuroimaging data in this mTBI case study revealed reduced cerebral white matter and cortical gray matter volumes post-injury that appeared to restore to pre-injury quantifications by the 8-month post-TBI MRI acquisition. Whole brain white matter fractional anisotropy demonstrated a concurrent pattern of change, with marked short-term reductions post-injury returning to a baseline level by the 8-month following TBI. The structural connectome, derived from tractography-based connection density metrics, showed that post-injury connectivity was reduced extensively between cortical nodes, in particular in the right parietal injury site. To a lesser degree, limbic, and forebrain regions were intra-hyperconnected. The final post-injury scanning timepoint showed globalized increases in connection density compared to the primary post-injury data, suggestive of recovery of the network. We highlight here the rare availability of pre-injury data, which gives significant benefits to interpretability compared to post-injury only research into mTBI.
Interestingly, subcortical segmentation and analysis of regional brain volumetrics did not reveal changes post-TBI, indicating a robustness of the limbic structures in this case. This is in contrast to previous reports of the hippocampal and amygdala structures being promising predictors of outcome when analyzed at a gross level ( 26 ); however, severity and type of TBI are significant contributors to heterogeneity. Our results suggest that subcortical volumetrics may not be a sensitive measure of mTBI pathology in all cases. At a whole brain level, it appeared that quantification of hemispheric white and gray matter volumes was a more effective metric of brain changes post-TBI in this case, especially when considering the minimal test–retest variability.
The mechanical properties of the white matter make it particularly vulnerable to injury in TBI ( 5 ), which was a prominent motivation for the use of high spatial resolution diffusion-weighted MRI in this case investigation. Our findings show that primary post-injury connectivity is reduced in a widespread manner, mainly between cortical nodes. Similarly, a study of mTBI patients and matched controls revealed decreased fractional anisotropy in the association, commissural and projection white matter tracts, indicative of reduced connectivity, which partially resolved 6 months post-injury ( 27 ). In addition, our results identified increased white matter connectivity in the limbic and forebrain regions post-injury compared to pre-injury data. Resting-state investigation into mTBI has shown comparable hyperconnectivity in the limbic system post-injury ( 28 ), and thalamic circuitry, in particular, has been shown to be a key underlying factor in mTBI recovery ( 29 ). Alterations of the connectome post-mTBI in the present case were further substantiated by widespread corroborative changes in fractional anisotropy, suggesting that after injury, white matter microstructure was changed in a way highly indicative of axonal damage ( 30 ).
The involvement of the posterior cingulate, precuneus and prefrontal cortices in the decline of structural network density implicates decreased cohesiveness of the default mode system. Decreased functional coupling of the default mode network, in particular in the frontal regions, has been shown to occur during sleep in both humans and primates, suggesting that default mode cohesiveness may be required to maintain conscious states ( 31 ). The default mode white matter damage detected in the present study; therefore, may be a contributory factor in the patient's reported hypersomnolence and fatigue post-injury. Similarly, the superior frontal and orbitofrontal regions are integral sites for executive processing ( 32 ), and the observed decreases in network connection density here tally closely with the patient's symptomology of delayed cognitive processing post-injury. Decreased white matter connectivity of the insular cortex implicates decreased salience network integration, which may be another possible physiological correlate of cognitive slowing via diminished attentional regulation ( 33 ). Alterations to the thalamic-occipital lobe circuitry, which forms the posterior portion of the primary visual pathway ( 34 ), may also underlie symptomatic dizziness post-TBI. Localized increases in the mean connection density of the thalamus post-injury, a factor previously reported as a protective feature against long-term pathological effects in mTBI ( 29 ), is also seen in this patient, and may reflect compensatory plasticity promoting sensory relay and upstream network integration ( 35 ).
Additionally, consistent with previous studies of diffusion-weighted imaging in TBI ( 27 ), the current data show recovery of the brain white matter, which is consistent with symptomatic recovery per subjective report at ~6 months post-injury. However, the structural network at post-injury timepoint 2 exhibits some evidence for enduring TBI-related change, given that compared to pre-injury data, the final timepoint connectivity shows some disorganization, albeit markedly improved compared to the initial post-injury network. The functional implications of long-term reorganization of the network appear to be minimal, although may account for the few isolated areas of cognitive performance that were lower than expected in patient at 18 months post-injury. Taken together, our findings show that diffusion MRI connectomics and microstructural measurements may be sensitive to clinical status.
This 7 Tesla case report demonstrates novel evidence of widespread connectivity and microstructural changes at a highly granular level after mTBI, where conventional neuroimaging at a clinical level showed no radiological abnormalities. Moreover, we demonstrate the disparity between T1- and T2-weighted acquisition-derived information and diffusion MRI and suggest that diffusion-weighted investigation of TBI symptomology may be of significant use in clinical practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Regional ethics committee, Icahn School of Medicine at Mount Sinai. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SB, KD-O'C, PB, and RF contributed substantially to the study conception and design, drafted, and revised the article for important intellectual content and gave final approval of the version to be published. EW provided guidance for cognitive assessment. SB carried out all data processing and neuroimage analyses. All authors contributed to the article and approved the submitted version.
This work was funded by DOD-IDA W81XWH-19-1-0616 and NIH R01 MH109544.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. DeKosky ST, Asken BM. Injury cascades in TBI-related neurodegeneration. Brain Inj. (2017) 31:−82. doi: 10.1080/02699052.2017.1312528
CrossRef Full Text | Google Scholar
2. Biagianti B, Stocchetti N, Brambilla P, Van Vleet T. Brain dysfunction underlying prolonged post-concussive syndrome: a systematic review. J Affect Disord. (2020) 262:71–6. doi: 10.1016/j.jad.2019.10.058
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Ellis MJ, Leiter J, Hall T, McDonald PJ, Sawyer S, Silver N, et al. Neuroimaging findings in pediatric sports-related concussion. J Neurosurg Pediatr. (2015) 16:241–7. doi: 10.3171/2015.1.PEDS14510
4. Bigler ED, Abildskov TJ, Goodrich-Hunsaker NJ, Black G, Christensen ZP, Huff T, et al. Structural neuroimaging findings in mild traumatic brain injury. Sports Med Arthrosc Rev. (2016) 24:e42–52. doi: 10.1097/JSA.0000000000000119
5. Jang SH, Kim SH, Kwon YH. Extensive traumatic axonal injury of brain due to violence: a case report. Medicine (Baltimore). (2018) 97:e13315. doi: 10.1097/MD.0000000000013315
6. Jang SH, Seo YS. Headache due to spinothalamic tract injury in patients with mild traumatic brain injury: Two case reports. Medicine (Baltimore). (2019) 98:e14306. doi: 10.1097/MD.0000000000014306
7. Schouten JW, Fulp CT, Royo NC, Saatman KE, Watson DJ, Snyder EY, et al. A review and rationale for the use of cellular transplantation as a therapeutic strategy for traumatic brain injury. J Neurotrauma. (2004) 21:1501–38. doi: 10.1089/neu.2004.21.1501
8. Goriely A, Weickenmeier J, Kuhl E. Stress singularities in swelling soft solids. Phys Rev Lett. (2016) 117:138001. doi: 10.1103/PhysRevLett.117.138001
9. Sotiropoulos SN, Zalesky A. Building connectomes using diffusion MRI: why, how and but. NMR Biomed. (2019) 32:e3752. doi: 10.1002/nbm.3752
PubMed Abstract | CrossRef Full Text
10. Pearson NCS. Advanced Clinical Solutions for WAIS-IV and WMS-IV: Administration and Scoring Manual . San Antonio, TX: The Psychological Corporation (2009).
Google Scholar
11. Marques JP, Gruetter R. New developments and applications of the MP2RAGE sequence–focusing the contrast and high spatial resolution R1 mapping. PLoS One. (2013) 8:e69294. doi: 10.1371/journal.pone.0069294
12. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. (2002) 33:341–55. doi: 10.1016/S0896-6273(02)00569-X
13. Zaretskaya N, Fischl B, Reuter M, Renvall V, Polimeni JR. Advantages of cortical surface reconstruction using submillimeter 7 T MEMPRAGE. Neuroimage. (2018) 165:11–26. doi: 10.1016/j.neuroimage.2017.09.060
14. Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. (2015) 115:117–37. doi: 10.1016/j.neuroimage.2015.04.042
15. Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. (2017) 155:370–82. doi: 10.1016/j.neuroimage.2017.04.046
16. Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. (2016) 142:394–406. doi: 10.1016/j.neuroimage.2016.08.016
17. Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory. Magn Reson Med. (2016) 76:1582–93. doi: 10.1002/mrm.26059
18. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. (2010) 29:1310–20. doi: 10.1109/TMI.2010.2046908
19. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. (2007) 35:1459–72. doi: 10.1016/j.neuroimage.2007.02.016
20. Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. Neuroimage. (2013) 81:335–46. doi: 10.1016/j.neuroimage.2013.05.028
21. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. (1994) 66:259–67. doi: 10.1016/S0006-3495(94)80775-1
22. Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. (2012) 62:1924–38. doi: 10.1016/j.neuroimage.2012.06.005
23. Tournier J. D., Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proc Int Soc Magn Reson Med. (2010) 18:1670.
24. Smith RE, Tournier JD, Calamante F, Connelly A. SIFT2: enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage. (2015) 119:338–51. doi: 10.1016/j.neuroimage.2015.06.092
25. Smith RE, Tournier JD, Calamante F, Connelly A. The effects of SIFT on the reproducibility and biological accuracy of the structural connectome. Neuroimage. (2015) 104:253–65. doi: 10.1016/j.neuroimage.2014.10.004
26. Ledig C, Kamnitsas K, Koikkalainen J, Posti JP, Takala RSK, Katila A, et al. Regional brain morphometry in patients with traumatic brain injury based on acute- and chronic-phase magnetic resonance imaging. PLoS One. (2017) 12:e0188152. doi: 10.1371/journal.pone.0188152
27. Messe A, Caplain S, Pelegrini-Issac M, Blancho S, Montreuil M, Lévy R, et al. Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging Behav. (2012) 6:283–92. doi: 10.1007/s11682-012-9159-2
28. Messe A, Caplain S, Pelegrini-Issac M, Blancho S, Lévy R, Aghakhani N, et al. Correction: specific and evolving resting-state network alterations in post-concussion syndrome following mild traumatic brain injury. PLoS One. (2013) 8(10). doi: 10.1371/annotation/fd9f9796-b42d-480d-b9f4-0adfbb919148
29. Banks SD, Coronado RA, Clemons LR, Abraham CM, Pruthi S, Conrad BN, et al. Thalamic functional connectivity in mild traumatic brain injury: longitudinal associations with patient-reported outcomes and neuropsychological tests. Arch Phys Med Rehabil. (2016) 97:1254–61. doi: 10.1016/j.apmr.2016.03.013
30. Li L, Chopp M, Ding G, Davoodi-Bojd E, Li Q, Mahmood A, et al. Diffuse white matter response in trauma-injured brain to bone marrow stromal cell treatment detected by diffusional kurtosis imaging. Brain Res. (2019) 1717:127–35. doi: 10.1016/j.brainres.2019.04.020
31. Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, et al. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci U S A. (2009) 106:11376–81. doi: 10.1073/pnas.0901435106
32. Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. (2008) 86:216–44. doi: 10.1016/j.pneurobio.2008.09.001
33. Seeley WW. The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci. (2019) 39:9878–82. doi: 10.1523/JNEUROSCI.1138-17.2019
34. Warntges S, Michelson G. Detailed illustration of the visual field representation along the visual pathway to the primary visual cortex: a graphical summary. Ophthalmic Res. (2014) 51:37–41. doi: 10.1159/000355464
35. Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. (2002) 111:761–73. doi: 10.1016/S0306-4522(02)00025-8
Keywords: 7T MRI, diffusion MRI, traumatic brain injury, structural connectivity, case study
Citation: Brown SSG, Dams-O’Connor K, Watson E, Balchandani P and Feldman RE (2021) Case Report: An MRI Traumatic Brain Injury Longitudinal Case Study at 7 Tesla: Pre- and Post-injury Structural Network and Volumetric Reorganization and Recovery. Front. Neurol. 12:631330. doi: 10.3389/fneur.2021.631330
Received: 19 November 2020; Accepted: 15 April 2021; Published: 17 May 2021.
Reviewed by:
Copyright © 2021 Brown, Dams-O'Connor, Watson, Balchandani and Feldman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie S. G. Brown, sb2403@medschl.cam.ac.uk
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

H.M.’s Contributions to Neuroscience: A Review and Autopsy Studies
Jean c. augustinack.
1 Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, Massachusetts
André J.W. van der Kouwe
David h. salat, thomas benner, allison a. stevens, jacopo annese.
2 The Brain Observatory, San Diego, California 92101, USA and Department of Radiology, University of California San Diego, San Diego, California 92093, USA
Bruce Fischl
3 CSAIL, Massachusetts Institute of Technology, Cambridge, Massachusetts
Matthew P. Frosch
4 C.S. Kubik Laboratory for Neuropathology, Massachusetts General Hospital, Boston, Massachusetts
Suzanne Corkin
5 Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, Massachusetts
H.M., Henry Molaison, was one of the world’s most famous amnesic patients. His amnesia was caused by an experimental brain operation, bilateral medial temporal lobe resection, carried out in 1953 to relieve intractable epilepsy. He died on December 2, 2008, and that night we conducted a wide variety of in situ MRI scans in a 3 T scanner at the Massachusetts General Hospital (Mass General) Athinoula A. Martinos Center for Biomedical Imaging. For the in situ experiments, we acquired a full set of standard clinical scans, 1 mm isotropic anatomical scans, and multiple averages of 440 µm isotropic anatomical scans. The next morning, H.M.’s body was transported to the Mass General Morgue for autopsy. The photographs taken at that time provided the first documentation of H.M.’s lesions in his physical brain. After tissue fixation, we obtained ex vivo structural data at ultra-high resolution using 3 T and 7 T magnets. For the ex vivo acquisitions, the highest resolution images were 210 µm isotropic. Based on the MRI data, the anatomical areas removed during H.M.’s experimental operation were the medial temporopolar cortex, piriform cortex, virtually all of the entorhinal cortex, most of the perirhinal cortex and subiculum, the amygdala (except parts of the dorsal-most nuclei—central and medial), anterior half of the hippocampus, and the dentate gyrus (posterior head and body). The posterior parahippocampal gyrus and medial temporal stem were partially damaged. Spared medial temporal lobe tissue included the dorsal-most amygdala, the hippocampal-amygdalotransition- area, ~2 cm of the tail of the hippocampus, a small part of perirhinal cortex, a small portion of medial hippocampal tissue, and ~2 cm of posterior parahippocampal gyrus. H.M.’s impact on the field of memory has been remarkable, and his contributions to neuroscience continue with a unique dataset that includes in vivo, in situ, and ex vivo high-resolution MRI.
INTRODUCTION
This review chronicles H.M.’s history, his contributions to the neuroscience of memory, neuroimaging studies past and present, and his autopsy. In the following paragraphs, we walk through the anatomical details of the medial temporal lobe, describe the specific structures removed and spared in H.M., and provide the only glimpse of his intact, fresh brain. We recount the critical discoveries that made him one of the most famous amnesic patients in the world, and illustrate, with high-resolution imaging, the age-related white matter disease that likely accounts for his dementia in the final part of his life. We also identify key questions to be addressed in the forthcoming neuropathological examination and in future histological studies.
On August 25, 1953, the neurosurgeon William Beecher Scoville performed an experimental operation in a 27-year-old man, Henry Gustave Molaison (H.M.), in the hope of curing his medically intractable epilepsy ( Scoville, 1954 ; Scoville and Milner, 1957 ). H.M. had experienced petit mal seizures from the age of 10 and grand mal seizures that began on his 15th birthday. The etiology of his seizures was unclear—as a young boy, he had sustained a minor head injury and, in addition, several of his father’s relatives had epilepsy. H.M. graduated from high school when he was 21 and later repaired electric motors and worked on a typewriter assembly line. He took large doses of anti-epilepsy drugs, but they did not quell his attacks. Because numerous EEG studies failed to reveal a precise surgical target for seizure control, Scoville proposed a psychosurgical procedure that he had devised, bilateral medial temporal lobotomy ( Scoville and Milner, 1957 ). He had previously performed the operation in patients with psychiatric disorders, mainly schizophrenia, with mixed results. H.M. was the first patient to undergo this procedure for intractable epilepsy. Scoville later renamed the operation bilateral medial temporal lobe resection.
Postoperatively, H.M.’s petit and grand mal attacks continued, and although their frequency decreased markedly, he required anti-epilepsy drugs for the rest of his life. His seizure control, however, was accompanied by a devastating loss. For the next 55 years, H.M. was trapped in the moment because of profound anterograde amnesia. His amnesia was pure—unconfounded by other cognitive deficits. His IQ was above average, and his language, reasoning, and perceptual capacities were normal. The exceptions were impaired olfactory function, caused by the operation, and cerebellar symptoms, a side effect of his anti-seizure medication, Dilantin.
The discrete nature and severity of H.M.’s amnesia made him the topic of scientific scrutiny for the remainder of his life and even after his death. Over 100 researchers participated in collaborative projects to study him, integrating behavioral testing, standardized interviews, and structural and functional imaging. In 1955, Brenda Milner conducted the first postoperative psychological testing of H.M., providing quantitative evidence of profound memory loss with preserved intelligence and immediate memory ( Scoville and Milner, 1957 ). She and Scoville concluded, “The findings reported herein have led us to attribute a special importance to the anterior hippocampus and hippocampal gyrus in the retention of new experience” (p. 21). Milner later introduced the idea that some memory processes were not hippocampus dependent by showing that H.M.’s error scores decreased across three days of testing on a motor skill-learning task, mirror tracing ( Milner, 1962 ). This discovery constituted the first experimental demonstration of preserved learning in amnesia.
Dissociable Memory Processes
Subsequent research with H.M. extended Milner’s pioneering work and established several firm conclusions. The evidence supported the dual process theory of memory proposed by James (1890) and Hebb (1949) . They viewed short-term and long-term memory as separate processes. Accordingly, H.M.’s short-term memory was preserved, while his long-term memory was impaired. His episodic and semantic learning were both deficient, indicating overlapping neural substrates ( Gabrieli et al., 1988 ; Steinvorth et al., 2005 ). Tests that distinguished two forms of recognition memory—recollection and familiarity—revealed that H.M. could make familiarity-based judgments to recognize complex pictures, even six months after encoding. This surprising result showed that recollection depends on the hippocampus, but familiarity does not. Examinations of H.M.’s retrograde amnesia led to the discovery that he could remember only two preoperatively experienced autobiographical episodes, whereas his semantic memory for the same time period was normal ( Steinvorth et al., 2005 ; Corkin, 2013 ). This dissociation implicates the hippocampus as necessary for the retrieval of premorbid autobiographical but not semantic information. At the same time, certain kinds of nondeclarative learning—motor skill learning, classical conditioning, and repetition priming—were preserved.
The issues that motivated decades of research with H.M. were to understand the scope of his amnesia, to elucidate the kinds of learning and memory that were spared, and to establish a causal link between his amnesia and specific brain circuits. Some information about the integrity of his brain was available even before his operation. H.M. had a pneumoencephalogram in 1946 and another in 1953, both of which were read as normal. At the time of his operation in 1953, information about the damage to his brain came exclusively from Scoville’s account of what he had removed. His notes and drawings formed the basis of a set of detailed drawings by another neurosurgeon, Lamar Roberts, which accompanied Scoville and Milner’s 1957 paper ( Scoville and Milner, 1957 ). Scoville estimated that the medial temporal lobe resection extended 8 cm back from the tip of each temporal lobe, but subsequent MRI scans indicated that the removal was much less extensive.
Postoperative In Vivo Imaging
CT scans carried out in 1977 and 1984 showed metallic clips from the operation, minimal atrophic change in the anterior temporal region bilaterally, cerebellar atrophy, and, in the 1984 scan when he was 58, mild to moderate cortical atrophy ( Corkin, 1984 ). Specific brain structures were not visualized. A SPECT scan conducted in 1992 at Brigham and Women’s Hospital in Boston confirmed his bilateral medial temporal lobe resection and cerebellar atrophy.
H.M.’s first MRI scans occurred in 1992 at Brigham and Women’s Hospital and in 1993 at Mass General, when he was 66 and 67 years old, respectively ( Corkin et al., 1997 ). These images showed that the removal extended back about 5.4 cm from the tip of the temporal lobe on the left and about 5.1 cm on the right. The bilaterally symmetrical lesion damaged most of the amygdaloid complex, the entorhinal cortex, part of perirhinal cortex, the uncal and rostral portions of the hippocampal complex, and part of parahippocampal cortex. Some of the ventral perirhinal and posterior parahippocampal cortices were intact. Approximately 2 cm of caudal hippocampal tissue was also spared, but it appeared atrophic and was likely deafferented due to removal of the entorhinal cortex. The subcortical white matter associated with the most anterior portions of the superior, middle, and inferior temporal gyri may have been compromised by the resection. The cerebellar atrophy was dramatic, but the cortical surface appeared normal for H.M.’s age ( Corkin et al., 1997 ).
A decade later, in 2002 to 2004, Salat and colleagues scanned H.M. at the Mass General Martinos Center, using improved MRI data acquisition and analysis tools—higher resolution, quantitative measures of tissue morphometry, and indices of tissue integrity ( Salat et al., 2004 , 2006 ; van der Kouwe et al., 2005 , 2006 ; Wiggins et al., 2006 ). By then, H.M. was 74 to 77 years old, and we uncovered new age-related abnormalities that were not connected to his 1953 resection—cortical thinning and abnormal signal in white matter and deep gray matter. H.M.’s T1 morphometry images showed significant atrophy of the cerebral ribbon, ranging from ~0.3 mm to ~0.7 mm relative to control participants. The atrophy that occurred between 1998 and 2003 was greater than that between 1993 and 1998, suggesting an aging-related degenerative process ( Salat et al., 2006 ). T1-weighted images also revealed infarcts in a number of subcortical gray matter structures, including the thalamus and putamen. Consistent with earlier imaging studies, H.M.’s cerebellum was severely atrophied. In T2-weighted images, Salat et al. noted significant white matter hyperintensities throughout H.M.’s brain that were especially pronounced in the inferior frontal gyrus near the corpus callosum. These new abnormalities appeared to be the result of high blood pressure and small vessel disease. We also collected the first diffusion MRI scans of H.M.’s brain, allowing the examination of fractional anisotropy (FA) maps to quantify the microstructural integrity of the white matter. Overall, H.M. had decreased FA compared to matched controls, and the focal areas of white matter damage had reduced FA. We never found any abnormality that would account for his original seizure disorder.
Current Study
H.M. died on December 2, 2008. That night, we conducted a wide variety of in situ MRI scans in a 3 T scanner at the Mass General Athinoula A. Martinos Center for Biomedical Imaging. The next morning, H.M.’s body was transported to the Mass General Morgue where Matthew Frosch, Director of the Neuropathology Unit, performed an autopsy. Jacopo Annese assisted with the autopsy. Photographs taken immediately afterward provided the first documentation of H.M.’s lesions in his physical brain. After ~10 weeks of tissue fixation, we obtained ex vivo structural data at ultra-high resolution using 3 T and 7 T magnets. This postmortem research had two goals—to document the specific structures that were removed and spared in H.M.’s brain, based on the gross examination of the fresh brain and analysis of the MRI images, and to relate the behavioral dissociations documented during H.M.’s life to the precisely established sparing and loss of brain tissue. An additional motivation for the ex vivo imaging was to provide an MRI-based method to later register the histological sections in 3D. The Partners Human Research Committee approved all studies described here. In this report, we first introduce the anatomical structures that define the medial temporal lobe region and then describe H.M.’s autopsy and MRI results.
MATERIALS AND METHODS
Participant, h.m.
At the time of his death, H.M. was 82 years old. The cause of death was arteriosclerotic cardiovascular disease. In 1992, H.M. and his court-appointed conservator had signed a brain donation form authorizing Mass General and MIT to perform a postmortem examination upon his death, and the conservator gave consent for the autopsy the evening H.M. died.
Neuroanatomy of the Intact Adult Medial Temporal Lobe—Terminology
To establish the terminology used in this report, we first describe pertinent structures in an intact adult brain, focusing on the medial temporal lobe region ( Rosene and Van Hoesen, 1987 ; Gloor, 1995 ; Insausti et al., 1995 ; Van Hoesen, 1995 ; Insausti et al., 1998 ). To educate the reader on the relevant structures, we selected nine blockface images from a control case (60 year old, male) in our MGH brain collection and labeled them ( Fig. 1 ). The areas include the piriform cortex (primary olfactory cortex), mesocortices of the parahippocampal gyrus (entorhinal and perirhinal cortices), and temporal polar cortex; the hippocampal formation—hippocampus, subiculum, and dentate gyrus; and the subcortical collection of nuclei that comprise the amygdala ( Fig. 1 ). The structures and slices are described from anterior to posterior.

Blockface images of medial temporal lobe structures from a control case (60-year-old, male). Nine levels represent the anterior-posterior extent of the medial temporal lobe. Level (A) temporal pole, (B) pyriform (olfactory) cortex, anterior-most entorhinal cortex, (C) anterior amygdala, (D) mid-amygdala, (E) anterior hippocampal head (pes), (F) posterior hippocampal head, (G) posterior hippocampal head and anterior hippocampal body, (H) hippocampal body, (I) posterior hippocampal body, hippocampal tail (not illustrated). Numerical labels correspond to Brodmann areas, and letter abbreviations are defined as: AAA = anterior amygdala area, AB = accessory basal nucleus of amygdala, B = basal nucleus of amygdala, CA = cornu ammonis (1–4), CAT = cortical amygdala transition area, Ce = central nucleus of amygdala, Co = cortical nucleus of amygdala, CS = collateral sulcus, DG = dentate gyrus, ES = endorhinal sulcus, fm = fimbria, HATA = hippocampal amygdala transition area, HF = hippocampal fissure, L = lateral nucleus of amygdala, LV = lateral ventricle, M = medial nucleus of amygdala, Pre = presubiculum, Par = parasubiculum, SUB = subiculum, TP = temporal pole, and Un = uncus. Magnification bar = 1 cm.
The temporal pole is divided into four regions: dorsal, ventral, medial, and lateral. Anteriorly, the temporal polar cortex hangs unattached to other brain tissue for ~1.5 cm ( Fig. 1A ), after which the temporal lobe joins the frontal cortex at the level of the limen insula. At this level, several features are noteworthy. The frontal insula and temporal insula merge together, the temporal polar cortex ends, and the piriform cortex begins to occupy the medial temporal area ( Fig. 1B ). The sulcal configuration in this region is complicated because the rhinal sulcus is incipient in humans and not present in all brains. The main sulci that outline the parahippocampal gyrus include the rhinal sulcus anteriorly, albeit variably, and the collateral sulcus laterally. The control case illustrated in Figure 1 does not exhibit a rhinal sulcus. If a rhinal sulcus were present, it would reside approximately at level B, at the level of the piriform cortex. Deep to the parahippocampal gyrus, the endorhinal sulcus dorsally separates the corticomedial nuclei of the amygdala from the optic tract area, the sulcus semi-annularis separates the medial parahippocampal cortex from the corticoamygdala-transition-area, and the hippocampal fissure separates the parahippocampal gyrus from the hippocampus ( Figs. 1C–F ). At the point where the frontal and temporal lobar regions connect, the temporal stem, one of the major white matter conduits for the temporal lobe, appears.
The amygdala lies posterior and slightly dorsal to the piriform cortex, which is situated deep beneath the parahippocampal cortex between the olfactory cortex anteriorly and the hippocampus posteriorly. The key amygdala nuclei are (from lateral to medial) the lateral nucleus, basal nucleus, accessory basal nucleus, paralaminar nucleus, medial nucleus, cortical nucleus, and corticoamygdala-transition-area ( Figs. 1C,D ). At the medial most edge of the amygdala, three nuclei line up from superior to inferior: medial nucleus, cortical nucleus, and corticoamygdala-transition area ( Figs. 1C,D ).
At the amygdala’s broadest part, immediately anterior and slightly dorsal to the hippocampus, the parahippocampal gyrus is also at its largest width. The anterior parahippocampal gyrus contains two Brodmann areas, area 34 medially and area 28 laterally ( Brodmann, 1909 ; Lorente de No, 1934 ). Brodmann area 34 corresponds to the gyrus ambiens and sometimes has a bulbar configuration that is often mistaken for the uncus of the hippocampus, but the uncus resides deep to the gyrus ambiens and slightly posterior ( Fig. 1D ). The hippocampal fissure borders the uncus and lower bank of the parahippocampal gyrus where the subicular cortices are located ( Fig. 1E ). Brodmann area 28 makes up a substantial component of the parahippocampal territory and occupies the entire crown of the anterior parahippocampal gyrus, commonly referred to as the entorhinal cortex. Equally prominent within the parahippocampal cortex is the entorhinal cortex’s neighbor laterally, the perirhinal cortex ( Figs. 1B–I ). The perirhinal cortex (Brodmann area 35, Braak’s transentorhinal) ( Braak and Braak, 1985 ) is sometimes slightly larger than the entorhinal cortex and surrounds it anteriorly, laterally, and posteriorly ( Insausti et al., 1998 ; Van Hoesen et al., 2000 ; Ding and Van Hoesen, 2010 ). The perirhinal cortex lies lateral to the rhinal sulcus but medial relative to the collateral sulcus, once the collateral sulcus has begun ( Van Hoesen et al., 2000 ; Ding et al., 2009 ). The ectorhinal cortex (Brodmann area 36) is temporal isocortex; we classify it separately from perirhinal cortex based on the fact that perirhinal area 35 is agranular and dysgranular (area 35a periallocortex and 35b proisocortex, respectively), whereas temporal isocortex (area 36) contains a granular layer. On the crown of the posterior parahippocampal gyrus, areas TH and TF ( von Economo and Koskinas, 1925 ; von Bonin and Bailey, 1947 ) make up the remaining parahippocampal cortex as it ends caudally at the retrosplenial cortex and calcarine sulcus (TH-TF, not illustrated).
The hippocampal formation comprises the hippocampus proper, subicular cortices (subiculum, presubiculum, and parasubiculum), and dentate gyrus ( Rosene and van Hoesen, 1987 ). The hippocampus proper contains subfields CA1, CA2, CA3, and CA4, named for cornu ammonis because it resembles a ram’s horn. The hippocampus, which contains four main structural parts, genu ( Fig. 1D ), head ( Figs. 1E–G ), body ( Figs. 1G–I ), and tail (not illustrated), sits deep beneath the parahippocampal cortex. Its structure changes significantly from anterior to posterior, with the head being disproportionately larger than the body and tail. The head of the hippocampus is made up of several convolutions, the pes hippocampi, where the medial-most convolution defines the uncus ( Figs. 1E,F ). The inferior horn of the lateral ventricle makes its first appearance at the level of the amygdala and hippocampal head.
Autopsy and Fixation
On December 4, 2008, when the postmortem interval was ~19 hrs, Matthew Frosch conducted the autopsy. H.M.’s brain was fixed in standard 10% formalin for several hours and was then transferred to buffered 4% paraformaldehyde. The fixative solution was changed twice during the two months that it remained in the Mass General Department of Pathology, allowing the brain tissue to fix thoroughly. On February 12, 2009, when it was transferred to the Martinos Center for ex vivo scanning, it remained in 4% paraformaldehyde.
In Situ MRI Acquisition
On the evening of December 2, 2008, just under 4 hr after H.M.’s death in Windsor Locks, Connecticut, we collected in situ scans at the Mass General Martinos Center. In situ refers here to postmortem imaging of the brain in the head. Images were collected in a 3 T Siemens (Erlangen, Germany) TIM Trio MRI scanner with a 32-channel head coil. We determined beforehand that the configuration of this system (in particular, the imaging gradient switching and RF energy deposition) would not damage the brain by heating the tissue or by heating or vibrating the surgical clips.
Because subject fatigue and motion were not an issue and scanning could continue for several hours, we collected high-quality, high-resolution images that would not be possible in a living subject. The in situ 3 T session lasted 9 hr. We obtained a wide range of contrasts of the unfixed brain with different scan types and high-resolution anatomical images.
We first acquired a full set of standard clinical scans for comparison with antemortem images. In addition, we obtained a 2 mm isotropic diffusion scan, 1 mm isotropic single and multiecho MPRAGEs ( Mugler and Brookeman, 1990 ; van der Kouwe et al., 2008 ), 1 mm isotropic multi-flip angle multiecho FLASH scans with 2 mm isotropic B1± maps, and multiple averages of a 440 µm isotropic single-echo multi-flip angle FLASH scan. All scans were automatically localized for acquisition using AutoAlign ( van der Kouwe et al., 2005 ; Benner et al., 2006 ). The synthetic images presented in this report were generated using estimates of intrinsic tissue parameters derived from a combination of acquired multiecho FLASH images with native contrast ( Fischl et al., 2004 ).
Clinical scans
We obtained five standard clinical scans: (1) sagittal T1-weighted spin-echo, (2) axial T2-weighted turbo-spin-echo, (3) axial FLAIR, (4) susceptibility weighted imaging (SWI), and (5) diffusion with matching B0 field map. Prescan intensity normalization was applied to all scans.
- T1-weighted 2D-encoded spin-echo ( T acq 4 m 20 s); 24 sagittal slices of 5 mm with 1 mm gap, 240 mm field of view with 256 matrix and 75% phase resolution, phase encode AP (1.3 × 0.9 × 5 mm). TE 7.1 ms, TR 550 ms, BW 201 Hz/px, FA 120°, two concatenations.
- T2-weighted 2D-encoded turbo-spin-echo ( T acq 5 m 27 s); 23 axial slices of 5 mm with 1 mm gap, 230 × 172.5 mm field of view with 512 matrix and 75% phase resolution, phase encode RL (0.6 × 0.4 × 5 mm). TE 91 ms, TR 5 s, BW 100 Hz/px, FA 134°, two averages, flow compensation in slice direction.
- FLAIR 2D-encoded turbo-spin-echo ( T acq 6 m 52 s); 23 axial slices of 5 mm with 1 mm gap, 230 × 172.5 mm field of view with 384 matrix and 75% phase resolution, phase encode RL (0.8 × 0.6 × 5 mm). TE 71 ms, TR 10 s, TI 2,500 ms, BW 130 Hz/px, FA 150°, two averages, flow compensation in slice direction.
- Susceptibility-weighted 3D-encoded gradient echo ( T acq 6 m 59 s). 96 axial partitions (k-space slices) of 1.5 mm, 220 × 171.9 mm field of view with 448 × 350 matrix, phase encode RL (0.5 × 0.5 × 1.5 mm). TE 20 ms, TR 28 ms, BW 120 Hz/px, FA 15° (slab-selective), flow compensation.
Standard anatomical scans for morphometry
We collected a standard set of 1 mm isotropic anatomical scans designed to elucidate brain morphometry using FreeSurfer ( http://surfer.nmr.mgh.harvard.edu ) ( Dale et al., 1999 ; Fischl et al., 2002 , 2004 ). The set consisted of T1-weighted multiecho MPRAGE (MEMPR) for cortical modeling and subcortical segmentation, multiecho FLASH (MEF) for tissue parameter quantification, and T2-SPACE and FLAIR T2-SPACE for T2 contrast. Prescan normalization was applied to all scans, and, for accurate alignment, scans were matched with respect to geometry and bandwidth (thus, degree of distortion).
- T1-weighted 3D-encoded 4-echo MEMPR ( van der Kouwe et al., 2008 ) ( T acq 6 m 3 s); 176 sagittal partitions of 1 mm, 256 mm field of view with 256 × 256 matrix, phase encode AP (1 mm isotropic), 2× GRAPPA with 32 reference lines. TE 1.64/3.5/5.36/7.22 ms, TR 2,530 ms, TI 1,200 ms (non-selective), BW 651 Hz/px, FA 7° (nonselective).
- T1-weighted 3D-encoded 8-echo FLASH ( T acq 13 m 25 s at each of three flip angles); 176 sagittal partitions of 1 mm, 256 mm field of view with 256 × 256 matrix, phase encode AP, 50% phase oversampling (1 mm isotropic), 2× GRAPPA with 32 reference lines. TE (1.85 + n × 2.0 + n × ( n −1)/2×0.1) ms ( n = 0,…,7) (uneven spacing for phase unwrapping), TR 22 ms, BW 651 Hz/px, FA 5/20/30° (nonselective), magnitude and phase images.
- T2-weighted variable flip angle 3D-encoded turbo-spin-echo (T2-SPACE) ( Mugler et al., 2000 ) (T acq 4 m 43 s); 176 sagittal partitions of 1 mm, 192 mm field of view with 192 × 192 matrix, phase encode AP (1 mm isotropic), 2× GRAPPA with 24 reference lines. TE 368 ms, TR 3,200 ms, BW 651 Hz/px, FA variable (nonselective).
- T2-weighted variable flip angle 3D-encoded turbo-spin-echo with fluid attenuation (FLAIR T2-SPACE) ( Mugler et al., 2000 ) ( T acq 7 m 22 s). 176 sagittal partitions of 1 mm, 192 mm field of view with 192 × 192 matrix, phase encode AP (1 mm isotropic), 2× GRAPPA with 24 reference lines. TE 352 ms, TR 5 s, TI 1,800 ms, BW 789 Hz/px, FA variable (nonselective).
T2*-weighted anatomical scans
To quantify tissue T2*, we carried out a multiecho FLASH scan according to a protocol that had a longer TR and more widely spaced echoes than the 8-echo FLASH described above. We also repeated the 8-echo FLASH protocol at 2 mm isotropic resolution (two repetitions of T acq 6 m 42 s with 128 × 128 matrix).
- 3D-encoded 8-echo FLASH ( T acq 14 m 15 s). 128 sagittal partitions of 1.33 mm, 256 mm field of view with 192 × 192 matrix, phase encode AP (1.33 mm isotropic), 2× GRAPPA with 32 reference lines. TE (3.41 + n × 6.9 + n × ( n − 1)/2 × 0.1) ms ( n = 0,․,7) (uneven spacing for phase unwrapping), TR 60 ms, BW 202 Hz/px, FA 20° (nonselective), magnitude and phase images.
High-resolution anatomical scans
We dedicated ~5 hr of the in situ scanning time to obtaining high-resolution anatomical scans, using a single-echo FLASH protocol with an isotropic resolution of 440 µm.
- T1-weighted 3D-encoded FLASH ( T acq 24 m 19 s at each of 6 flip angles); 384 sagittal partitions of 0.44 mm, 225 mm field of view with 512 × 512 matrix, phase encode AP, 50% phase oversampling (1 mm isotropic), 2× GRAPPA with 32 reference lines. TE 4.09 ms, TR 9.5 ms, BW 199 Hz/px, FA 3/5/8/10/15/18° (non-selective), magnitude and phase images.
Quality control scans
We obtained additional scans to correct artifacts. For the diffusion scans, matching B0 field maps were acquired. We collected data for 1 mm isotropic DESPOT1, DESPOT2, and HiFi analyses ( Deoni et al., 2003 , 2005 ; Deoni, 2007 ). Based on these results, we chose the optimal range of flip angles for the subsequent FLASH scans. We obtained a B1 transmit map using Actual Flip Angle Imaging ( Yarnykh, 2007 ) at 2.5 mm isotropic resolution and a B1 transmit/receive map by imaging at 2 mm isotropic resolution with the body coil and with each element of the 32-channel array. In addition, we acquired a gradient echo EPI series of volumes (TR 3 s, 192 measurements, 3 mm resolution with 0.6 mm gap between slices, 42 slices). Two additional runs used an experimental radial and Cartesian-encoded gradient echo protocol with an ultrashort echo (FLUSTER) ( Van der Kouwe, 2008 ) (data not shown).
Ex Vivo Imaging
Ex vivo imaging occurred after H.M.’s brain had been stored in 10% formalin for ~10 weeks. The fixed brain was placed in a custom-made Plexiglas chamber and imaged at high field ( Annese et al., 2014 ). We repeated the in situ scans ex vivo, and as expected the images confirmed the observations made in in situ.
High-resolution scans (7 T)
We used a 7 T Siemens scanner based on the Avanto platform for high-resolution imaging with a 31-channel custom-built head coil. The highest resolution images were 210 µm isotropic single-echo multi-flip angle FLASH scans. We measured coil covariance for image reconstruction and obtained 1.68 mm isotropic B1 transmit/receive maps and a two-echo gradient echo field map (2 mm × 2 mm × 3 mm resolution) for image correction.
We dedicated 15.5 hr of scan time to collecting high-resolution images of the entire fixed brain. Encoding at 210 µm isotropic resolution with the 31-channel head coil required offline image reconstruction because the k-space data volumes were larger than the 32 GB of RAM available on the scanner image reconstruction computer. The k -space data were streamed to an external storage site during acquisition because the total amount of data per scan exceeded the 320 GB of disk space available on the scanner RAID. We used the coil covariance matrix to combine the signals from the 31 head coil channels to form a single image volume ( Roemer et al., 1990 ).
- T1-weighted 3D-encoded FLASH ( T acq 4 h 50 m at each of three flip angles); 704 partitions of 210 µm, 175 × 153 mm field of view with 832 × 728 matrix (210 µm isotropic). TE 15.1 ms, TR 34 ms, BW 40 Hz/px, FA 10/20/30° (nonselective).
Temperature Monitoring
From the time of H.M.’s death until his body reached the Martinos Center, his head was enclosed in a Cryopak Ice Blanket to keep his brain cool. During the in situ scanning, room temperature was maintained at ~18°C. During ex vivo scanning, we monitored temperature carefully, keeping it below 19°C on the outer surface of the chamber at 3 T and 7 T. Temperature was a concern because RF energy deposition during MR imaging can heat the sample. To ensure that temperature fluctuations due to imaging would be well below normal fluctuations in room temperature, we previously monitored a test sample (whole brain) during imaging with the highest specific absorption rate sequences. A multichannel fiber optic temperature sensor (Neoptix, Inc., Quebec, Canada) recorded the temperature in four areas—deep in the tissue, close to the inner wall of the chamber, on the outer surface of the chamber, and in the room air. As expected, even during high (100%) specific absorption rate protocols, the temperature inside the tissue never increased more than 4°C relative to the inside and outside of the container. The specific absorption rate of RF energy for the 3 T diffusion sequence was close to 100% of the clinically safe value imposed by the scanner hardware, and it was substantially lower for the other protocols. The room temperature was at the minimum thermostat setting (18°C), and an additional fan was used to dissipate heat from around the chamber. The temperatures of the outer surface of the chamber and the surrounding air were monitored with the fiber optic system. Because these temperatures never exceeded 19°C, we reasoned that the temperature of the brain tissue never went above 23°C during imaging. We believe that the temperature differentials were even smaller during in situ imaging because the imaging session was shorter, the protocols were less energy intensive, and the energy could dissipate throughout the body.
Lesions in the Fresh
Brain The unfixed brain weighed 1,100 g, ~200 g less than one would expect for a 6-foot-tall, healthy man. Photographs of the brain taken immediately after its removal from the skull showed the overall topography of the gross brain to be relatively preserved. Notably, the olfactory bulbs and tracts were intact, and we saw no explicit damage, with the obvious exceptions of the medial temporal lobe excisions and a shriveled cerebellum ( Fig. 2 ). The dashed white lines drawn laterally and posteriorly illustrate where a normal sized cerebellum would be. The blood vessels appeared mildly atherosclerotic. In the right ventral temporal lobe, we identified a black surgical clip, which we believe was intentionally left behind by Scoville to prevent bleeding ( Scoville and Milner, 1957 ). A second clip was located in the left temporal lobe.

Photograph of the whole fresh brain (inferior surface) taken at H.M.’s autopsy. In the ventral view, the white arrows on both sides of the brain indicate the lines of cut in the coronal MRI slices in Figure 5 . These slices (white arrows) correspond to the in situ MRI ( Figs. 5A–L ). Note the area of excision and additional fibrous tissue (i.e., scar tissue) bilaterally, and the residual medial most tissue (bilaterally but larger on the left) in the medial temporal area, next to cranial nerve III. Abbreviations: B = basilar artery, M = medulla, MB = mammillary bodies, OB = olfactory bulb, OC = optic chiasm, ON = optic nerve, TP = temporal pole, V = vertebral artery, and III = cranial nerve III (oculomotor nerve). White arrowheads point to posterior temporal artery on both right and left. Dashed white lines illustrate atrophy in the cerebellum. Note the black surgical clip on right temporal lobe. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com .]
Figure 3 magnifies the ventromedial view to reveal a close-up of the right (A) and left (B) medial temporal lobe lesions. The photographs show slightly different views, a consequence of the unfixed state. (An unfixed brain collapses or widens slightly and does not retain the classical formation until fixed.) The medial temporal lobe lesions began immediately to the left and right of the optic nerves, which had been cut during the autopsy so the brain could be pulled out of the skull, leaving the eyes intact. On both sides, the lesion extended posteriorly from the temporal polar cortex through the parahippocampal gyrus until about the level of the halfway point of the basilar artery. In a normal case, the gyrus ambiens would reside immediately and slightly anterior to the uncus. In H.M., medial tissue remained on both sides but to a greater extent on the left ( Figs. 3A,B ). We could not determine definitively whether the remaining medial tissue on the left was uncus or gyrus ambiens because the banks of the hippocampal fissure and other landmarks had been surgically removed. The histological analysis, which will be the topic of a later report, will reveal whether the cytoarchitecture of this tissue is hippocampal (allocortical) or cortical (periallocortical). Continuing posteriorly, significant scar tissue occupied the medial temporal areas bilaterally.

Close-up photographs of H.M.’s medial temporal lobe showing a ventral view of the right and left temporal regions (A and B). Tissue was splayed out (due to being unfixed) to reveal a slightly different viewpoint of the extent of the lesion. In A and B, the lesion extends from the temporal pole to the midparahippocampal gyrus. Note the absence of tissue and the abundant scar tissue bilaterally. The basilar and vertebral arteries contain several atherosclerotic plaques. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com .]
In Situ MRI
The several different contrasts acquired in situ provided an MEMPR (T1-weighted) image ( Fig. 4A ) with the classic MRI contrast that shows CSF as dark and white matter as white. In the T2-SPACE acquisition ( Fig. 4B ), white matter was relatively dark, gray matter was brighter, and CSF was brightest. Because the lesion void was filled with CSF, it appeared bright in the image. In the T2-SPACE acquisition with fluid attenuation (FLAIR) ( Fig. 4C ), the CSF in the region of the lesion was dark, but the remaining tissue fragments were relatively bright and clearest compared to other contrasts. The bottom panels of Figure 4 show quantitative proton density (PD) ( Fig. 4D ) and T1 estimates ( Fig. 4E ) together with a synthetic image constructed from the corresponding 440 µm volumes ( Fig. 4F ) ( Fischl et al., 2004 ). We estimated the T1 and PD volumes from a combination of multiecho FLASH scans with different flip angles. Figure 4D shows only PD contrast (arbitrary units, but proportional to spin density); Figure 4E shows estimated T1 relaxation time (sec). PD reflected only the proton (or spin) density and was a relatively flat contrast. The lesions appeared fairly bright because CSF is relatively dense in free water, while the PD of white matter is slightly less than that of gray matter. Both appeared darker than CSF in the PD image. Because higher T1 produces darker voxels in T1-weighted images, the image showing the absolute T1 value had the opposite contrast of the MEMPR (i.e., white matter is darker than gray matter and CSF in the quantitative image because white matter has a shorter T1 relaxation time). To optimize contrast and increase signal-to-noise, we created a synthetic image from the FLASH scans ( Deoni et al., 2003 ; Fischl et al., 2004 ). Figure 4F shows the synthetic FLASH image that would result if the TR had been 22 ms, flip angle had been 20°, and TE had been 0. This image was synthesized from the PD and T1 estimates by applying the steady-state FLASH model, in the reverse of the estimation procedure. We chose the TR and flip angle to achieve optimal discrimination between the structures segmented by FreeSurfer ( http://www.surfer.nmr.mgh.harvard.edu/ ), based on contrast (TE 0 implies no signal decay due to T2* relaxation). The synthetic image in Figure 4F has a T1-weighted contrast similar to the MPRAGE.

Various MRI contrasts acquired at 3.0 T in situ. (A) multiecho MPRAGE (MEMPR), (B) T2-SPACE, (C) T2-SPACE FLAIR, (D) quantitative PD, (E) quantitative T1, (F) synthetic FLASH. Note the lesion in all contrasts, with the borders especially clear in the multiecho MPRAGE and synthetic FLASH images. T1 and T2-SPACE FLAIR revealed scar tissue faintly in addition to the lesion.
Lesions in medial temporal lobe structures
Here we describe the specific medial temporal lobe areas that were explicitly removed and identify other structures that remained. The 12 coronal MR images ( Fig. 5 ) highlight the lesion extent and illustrate remaining anatomical structures, based on remaining landmarks ( Figs. 5A–L ). Levels are spaced 4 mm apart. The first image shows the temporal pole where the anterior-most portion of the lesion began ( Fig. 5A ). The medial temporopolar cortex, mainly dysgranular area 38 and area 36, were removed. The temporal polar sulcus, located dorsally, was partially destroyed.

Twelve coronal MR images showing the anterior and posterior extent of H.M.’s medial temporal lobe lesion. Images were synthesized from multi-echo FLASH scans acquired in situ and are ordered from anterior (A) to posterior (L). In all images, note the enlarged ventricles, general atrophy, and a plethora of regional white matter signal abnormalities. We identify particular structures (present or absent) in each panel. A, B, C: medial temporal pole removed; D: anterior entorhinal cortex removed (i.e. piriform cortex), E: cortical amygdala and possibly central nucleus remained; F: gyrus ambiens or uncus remained; G and H: perirhinal cortex and damaged parahippocampal cortex; I: the body of the hippocampus visible; J: first full observation of parahippocampal cortex (right side) and fimbria; K: posterior tip of the lesion (right side is past the lesion), and L: the undamaged parahippocampal cortex posterior to the lesion. Magnification bar = 1 cm.
In the second image ( Fig. 5B ), the lesion entered prime temporal polar cortex territory, where we observed the optic nerves (slightly off the midline) and the caudate nucleus. Here, the limen insula had not yet connected to the frontal and temporal lobes. The temporal polar areas, 38 and area 36, were still absent at this level. The olfactory tract, seen inferiorly to the orbitofrontal gyrus, appeared normal. The third image ( Fig. 5C ) was approximately at the level of the anterior amygdala.
The fourth image ( Fig. 5D ) fell at the level of the optic chiasm. Presumptive medial structures were the posterior piriform cortex, part of perirhinal cortex (area 35), and temporal isocortical area 36. In all slices described thus far, the medial temporal stem was partially damaged bilaterally, and the white matter quality appeared compromised, with the lesion in the left hemisphere extending farther medially than in the right. The collateral sulcus appeared in images four through eight ( Figs. 5D–H ), and perirhinal cortex occupied its medial bank.
A small part of the entorhinal cortex appeared in the fifth and sixth images ( Figs. 5E,F ), but only on the extreme lateral convexity of the gyrus. The border between the entorhinal and perirhinal cortices typically falls on the corner of the parahippocampal gyrus near the collateral sulcus. The fifth image captured the emerging optic tracts, anterior commissure (midline), and caudate/putamen ( Fig. 5E ). The anterior-most amygdala—specifically parts of the endopiriform nucleus and the corticoamygdalo-transition-area—might have been present in this image. Noticeably, the anterior-amygdala-area was lacking at this level. The landmarks in the sixth image included the optic tract, anterior commissure laterally (very subtle), caudate/putamen/anterior limb of the internal capsule, globus pallidus, hypothalamus, and columns of the fornix, indicating that this slice was at the level of the mid-amygdala ( Fig. 5F ). In a healthy brain, this level would represent the amygdala at its largest extent along with the gyrus ambiens (Brodmann area 34 in humans). The left side may have contained a portion of the semilunar gyrus slightly dorsal to gyrus ambiens; this possibility will be examined in the histological analysis. Figure 5F shows the best MRI example of the medial tissue that remained. The hypothalamus appeared atrophied, likely due to the lack of hippocampal input, and the fornix and hippocampal commissure showed significant atrophy.
The seventh image is where one would expect to see the posterior amygdala, subiculum, and anterior-most hippocampus, given that other landmarks usually present at this level are visible—the mammillary bodies of the hypothalamus, the thalamus, putamen, globus pallidus, and the optic tract (tucked in medially) ( Fig. 5G ). The spared posterior hippocampal tissue first appeared in this slice, with the right side showing more hippocampal head than the left ( Fig. 5G ).
The eighth image ( Fig. 5H ) embodied the presumptive uncal hippocampus and anterior dentate gyrus level, where neighboring anatomical landmarks were the cerebral peduncles, red nucleus, substantia nigra in the brainstem, anterior nucleus of the thalamus and posterior putamen. In the ninth image ( Fig. 5I ), the right hippocampus began to resemble the classic hippocampal shape, and this slice showed the body of the hippocampus for the first time. The tenth image exposed the body of the hippocampus where the lateral geniculate nucleus of the thalamus made its first appearance, defining the end of the entorhinal cortex ( Fig. 5J ). Images ten and eleven revealed badly damaged parahippocampal gyri, especially on the left side ( Figs. 5J,K ). Images eleven and twelve showed the shrunken posterior body and tail of the hippocampus ( Figs. 5K,L ). In the twelfth image, the posterior thalamus (pulvinar), fimbria-fornix, and posterior commissure all came into view ( Fig. 5L ).
In summary, the high-resolution, high-contrast images reported here indicated that the areas removed bilaterally by suction during H.M.’s experimental operation were the medial temporopolar cortex, piriform cortex, virtually all of the entorhinal cortex, most of the perirhinal cortex (area 35), a large amount of subiculum, amygdala, except the dorsal most nuclei (i.e., central and medial), most of the hippocampus (head and body), and the dentate gyrus (posterior head and body). Parts of posterior parahippocampal gyrus (roughly equivalent to TH and TF) remained but were damaged. Other medial temporal lobe areas spared bilaterally were: the dorsal-most amygdala, part of the hippocampal-amygdalo-transition-area, a portion of perirhinal area 35, a medial portion of the hippocampus or gyrus ambiens, and the posterior body and tail of the hippocampus. The major sulci in the medial temporal lobe were remarkably well preserved bilaterally, with the exception of the hippocampal fissure. The collateral sulci were partially present, but shallow. The endorhinal sulci were present dorsally and medially, the sulci semi-annularis were spared medially, but the rhinal sulci were absent rostrally.
Ex Vivo MRI
At higher field strength (7.0 T), we acquired ultra-high-resolution images at 210 µm for the whole brain. Because the brain was suspended in 4% paraformaldehyde during scanning, the background contained some MRI signal from the water molecules in the solution. The contrast to noise ratio (CNR) and signal to noise ratio (SNR) were compromised for two reasons—the tissue contrast and solution contrast were similar, and the resolution was high. Due to the preciousness of this brain, we chose not to subject it to a proton-free liquid, such as Fomblin. Had we used a proton-free liquid, we would have avoided the unsatisfactory background observed in the 7.0 T images ( Fig. 6 ). Even with the high background, however, the ex vivo scans showed new details, and we delineated specific landmarks, such as the endorhinal sulcus and collateral sulcus, which helped determine the exact boundaries of the lesion. We outlined the lesion with dotted lines at three pertinent anterior-posterior levels: the anterior commissure, columns of fornix, and mammillary bodies. The high-resolution ex vivo scans provided additional information about the exact shape of H.M.’s lesions.

Ex vivo images of medial temporal lobe areas acquired at 7 T. Images at 210 µm isotropic show the extent of the lesion and remaining medial structures. For level-of-cut cross reference, see Figure 5 . Panels A, B, and C correspond to the MRI slices in Figures 5E–G . A shows H.M.’s lesion approximately at the level of the amygdala. The lesion shape is relatively uniform at this level. Note the square corners at the dorsal-most part of the lesion. B demonstrates the lesion where the posterior amygdala-anterior hippocampal level would have been. Note the fornix columns medially in the hypothalamus and the irregular lesion shape. The large round black structures in C are regions of susceptibility surrounding air bubbles. C illustrates the lesion at the level of the mammillary bodies where the head of the hippocampus (i.e., pes hippocampus) would normally reside. At level C the lesion narrows. The shape of the lesion changes considerably from anterior to posterior, scar tissue is visible. The white arrowheads point to the collateral sulcus. Numbers represent Brodmann areas. Abbreviations: AC = anterior commissure, ES = endorhinal sulcus, FX = fornix, MB = mammillary bodies, OC = optic chiasm, RN = red nucleus, and SN = substantia nigra.
At the level of the anterior commissure, the anterior-most levels of the entorhinal cortex (i.e., the piriform cortex) and the extreme anterior parts of amygdala were removed ( Fig. 6A ). It is noteworthy that at this level, the lesion represented a rectangular shape and showed sharp 90° angles at the innermost (and superior) borders.
The second level is at the columns of the fornix ( Fig. 6B ). At this mid-amygdala level, the lesion shape, indicated by the dotted white lines, became irregular, especially on the left ( Fig. 6B ). The white arrow points to tissue that was likely the anterior hippocampus.
The third medial temporal lobe level is at the mammillary bodies of the hypothalamus, where the hippocampal head is largest. The right lesion appeared significantly larger than the left in the medial-lateral direction, consistent with our lesion measurements. Based on the quantitative data and these qualitative data, the right-sided lesion was slightly shorter than the left-sided in the anterior-posterior plane but slightly wider in the medial-lateral plane.
Lesion Size
We defined the overall length and width of H.M.’s lesion as the distance parallel to the anterior-posterior direction and left-right direction, respectively. We considered the lesion measurements in two ways, the total ablation and the total ablation plus damaged cortex. For the left-sided lesion, the length from the tip of the temporal lobe was 4.6 cm for the ablation and 6.0 cm for the ablation and damaged cortex ( Fig. 7 ). For the right-sided lesion, the length from the tip of the temporal lobe was 4.2 cm for the ablation and 5.5 cm for the ablation and damaged cortex ( Fig. 7 ). These dimensions indicated that the left medial temporal lobe lesion was larger than the right. The width was 2.04 cm on the left and 1.89 cm on the right at the temporal pole, 1.54 cm on the left and 1.63 cm on the right at the amygdala/uncus, and 1.02 cm on the left and 0.95 cm on the right at the hippocampal tail. The overall shape of the lesion matched a truncated cone with the wide base anterior and the narrow end posterior.

Axial view of the multiecho FLASH images. Line measurements were acquired in the axial plane to assess the anterior-posterior lesion size. The ablation on the right side measured 5.5 cm while the left measured 6.0 cm. The left temporal lobe lost considerably more cortex during surgery. Note the cerebellar atrophy due to Dilantin.
We also obtained two high-resolution anatomical volumes—the 440 µm in situ volume and the 210 µm ex vivo volume. Both were synthesized from multiple FLASH scans collected at different flip angles. The in situ 440 µm volume had near in vivo contrast, with gray matter appearing darker than white matter and CSF that was dark, but not black (visible also in the vicinity of the lesion).
The ex vivo 210 µm volume had a different contrast because the tissue was fixed (tissue classes are better distinguished by T2* contrast in fixed ex vivo brain tissue) ( Tovi and Ericsson, 1992 ). In these images, gray matter was brighter than white matter and exhibited distinct layers, such as the stria Gennari , which were not visible in the in situ scans. Because the brain was packed in paraformaldehyde, which generated a relatively strong signal that was comparable to white matter, the ventricles and region of the lesion were not extremely distinct from the adjacent structures. The cotton packing and the brain contained small bubbles, and the latter gave rise to spin dephasing artifacts, which were exaggerated by the choice of a long TE, a consequence of the low bandwidth needed to obtain reasonable SNR and gradient strengths at the required high resolution.
The white matter was noticeably inhomogeneous in both the in situ and ex vivo images, presumably a consequence of aging-related white matter disease. In the ex vivo images, B1 + (RF transmit field) inhomogeneities due to the dielectric resonance effect at high field contributed further to overall image non-uniformity. Despite the fact that we invested 15.5 hrs in the 210 µm volumes vs. 2.5 hrs for the 440 µm, SNR was higher for the 440 µm volume because SNR falls dramatically with increasing isotropic resolution. SNR was roughly 20 to 50 and 130 to 150 for brain tissue in the 210 µm and 440 µm volumes, respectively. These values are approximate because SNR varies spatially, and the noise distribution is not precisely Gaussian. We selected protocols that would give reasonable SNR in the time available. The CNR (gray/white) for the 440 µm data was 14.7. In the 210 µm data, CNR measures for gray/white matter were 15.1 for high signal areas and 5.7 for medium signal areas. The SNR measures in these regions were 4.24 and 3.39, respectively ( Fig. 4 ).

Structures Outside the Medial Temporal Lobes
The cerebellum was severely atrophic and at autopsy appeared nearly half the size of a healthy cerebellum. The cerebellar atrophy seemed uniform with the flocculonodulus, vermis, and lateral hemispheres all reduced in size. In the in situ MRI images, we observed the dentate nucleus, the largest of the deep cerebellar nuclei, in its usual location, but we could not detect the other nuclei (fastigial, globose, emboliform), given the resolution (440 um) 3 .
Aging-related changes
At the end of his life, H.M. became demented. Multiple small strokes due to untreated hypertension and white matter atrophy likely contributed to his mental deterioration. The black spots in the MRI images indicate hypertensive disease and were particularly notable in the brainstem in Figure 4A . As a consequence of the untreated hypertension and perhaps other aging processes, we observed extensive isocortical and subcortical atrophy in all structures. Limbic structures such as the fornix, hippocampal commissure, and mammillary bodies, which were connected to the hippocampus before the surgery, were markedly degenerated ( Fig. 5G ). Similarly, the extended amygdala (centromedial amygdala and bed nucleus of stria terminalis) and other structures (i.e., the substantia inominata, including the nucleus basalis of Meynert and the diagonal band of Broca) showed noteworthy deterioration, as did the ventral striatopallidal areas. In the basal forebrain, the atrophy was so extensive that it was difficult to discern the location of nuclei. Degeneration went beyond the limbic cortices and limbic-related areas. We observed severe atrophy in the striatopallidum (caudate, putamen, globus pallidus) and thalamus (anterior nucleus, ventral anterior nucleus, mediodorsal nucleus, lateral posterior, lateral dorsal nucleus, pulvinar nucleus, medial geniculate nucleus and lateral geniculate nucleus) as demonstrated by the large size of the third ventricle ( Figs. 5H–J ). We noted atrophy of the anterior commissure and all parts of the corpus callosum. The corpus callosum appeared so thin that it resembled the thickness of a healthy anterior commissure. We found isocortical thinning in all areas, including primary and secondary sensory and motor cortices. Shrinkage was especially pronounced in prefrontal, temporal, parietal, and occipital association areas.
White Matter Integrity
The diffusion imaging data acquired as part of the imaging sessions will be reported at a later time. The structural images, however, demonstrated a clear and substantial deterioration of the connective integrity of H.M.’s brain, with overt macrostructural white matter lesions throughout several portions of the cerebrum ( Fig. 5 ). We previously described this damage and noted that hints of white matter deterioration were apparent as far back as 1998 when he was 72 ( Corkin et al., 1997 ; Salat et al., 2006 ). In the 2008 imaging sessions, dark bands of hypointense white matter in T1 images were obvious throughout the periventricular regions. Such changes are commonly observed in older adults, but the degree of alterations here was well beyond what could be considered a benign consequence of aging.
Blood Supply
We did not explicitly trace the blood vessels in H.M.’s brain, but based on the anatomical areas that were surgically removed, it is likely that the blood supply was also interrupted. The arterial origins of the vascular supply to the medial temporal lobe include the middle cerebral artery, internal carotid artery, anterior choroidal artery, and posterior cerebral artery. From the fresh brain, it appeared that the anterior temporal artery, a branch of the posterior cerebral artery was removed bilaterally. The anterior choroidal artery, known to supply the anterior hippocampus, was absent on both sides, as was the anteroinferior parahippocampal artery, which supplies the anterior parahippocampal gyrus (i.e., entorhinal cortex). The posterior temporal artery was spared on both sides ( Figs. 2 and and3) 3 ) as well as the posterior parahippocampal artery, which supplies the posterior parahippocampal gyrus (i.e., TH and TF). Further tissue damage suggested that the posterior hippocampal artery, which supplies the posterior hippocampus, was compromised bilaterally.
The onset of H.M.’s profound memory impairment immediately after the operation established for the first time that removal of the hippocampus and surrounding structures causes amnesia ( Scoville and Milner, 1957 ). The postmortem studies reported here are part of an ongoing effort to characterize the damage as precisely as possible. Our first goal was to document in detail the medial temporal lobe lesions, and the second was to examine the integrity of other structures that likely supported his intact intellect and preserved learning capacities. Bearing this anatomical information in mind, we associate specific cognitive processes that were impaired or spared in H.M. to particular circuits inside and outside the medial temporal lobe. Many of the findings with H.M. informed ongoing controversies in cognitive neuroscience concerning dissociations of function.
This report extends previous anatomical findings in MRI by revealing more detail and specific anatomy about what structures were removed or spared. The autopsy photographs showed the dense scar tissue in H.M.’s lesion and the marked cerebellar atrophy, while the MRI data, including in situ and ex vivo images, capitalized on improved contrast and resolution to reveal the lesion shape (or remaining tissue shape) and the precise measurements for lesion size. For example, the improved contrast highlighted tissue that may be the gyrus ambiens or a sliver of the anterior uncus. It is difficult to estimate whether this tissue is gyrus ambiens (medial part of entorhinal cortex) or uncal hippocampal tissue (i.e., medial CA1) and this new finding must be evaluated histologically. The high-resolution MRI also showed contrast differences between damaged and undamaged tissue that allowed more precise measurement of overall lesion size and shape. In this report, the lesion, measured from 440 µm 3 MRI, was 6.0 cm on the right and 5.5 cm on the left, suggesting that Scoville ( Scoville and Milner, 1957 ) overestimated the lesion at 8 cm while Corkin and colleagues ( Corkin et al., 1997 ) slightly underestimated the right-sided lesion at 5.1 cm. The high-resolution MRI also revealed the shape of the lesions, which exactly followed the contour of the parahippocampal gyrus, wider anteriorly and narrower posteriorly. Previous in vivo MRI images showed that H.M. developed significant white matter damage and cortical thinning as he aged ( Salat et al., 2006 ). The new in situ MRI findings provide evidence of white matter disease progression, showing that at the time of H.M.’s death, his white matter was riddled with white matter signal abnormalities and extensive cortical thinning. Cortical thinning and white matter signal abnormalities were widespread, and no structure was spared. The following paragraphs review how H.M.’s lesions relate to the rich body of behavioral data collected over 55 years.
Lesion Dimensions
Overall lesion size.
At the time of H.M.’s operation, Scoville estimated that the lesion extended 8 cm back from the tip of the temporal lobe. If this had been the case, then the damage would have invaded visual cortex, which it did not. With the advent of MRI, we were able to get a more accurate idea of lesion size. The in vivo MRI estimates in the rostrocaudal extent, based on 1 mm MRI data, were ~5.4 cm on the left and ~5.1 cm on the right ( Corkin et al., 1997 ). The comparable postmortem MRI dimensions were slightly greater, showing that H.M.’s lesion was 6.0 cm on the left and 5.5 cm on the right ( Fig. 7 ). The high-resolution in situ data allowed us to measure the excision and the damaged tissue more precisely. In the left temporal lobe, the excised region measured 4.6 cm, the damaged area an additional ~1.5 cm, and the entire lesion 6.0 cm. On the right side, the excision measured 4.2 cm, the damaged region an additional ~1.4 cm, and the whole lesion 5.5 cm. The ex vivo measures were more accurate because of the superior resolution we could obtain in long scan sessions at 3 T and 7 T that would not have been feasible in vivo. We hypothesize that the greater lesion size was due in part to age- and disease-related degeneration. The in situ MRI findings reported here are the most accurate measures of H.M.’s lesion size because the brain had not yet been distorted by removal and fixation procedures.
Correlation between lesion size, distribution, and severity of amnesia
H.M. had both extensive amnesia and large medial temporal lesions, suggesting that amnesia severity is related to lesion size. His amnesia was more profound than that of Penfield and Milner’s patients F.C. and P.B., whose excisions for epilepsy and pre-existing damage spared a considerable amount of medial temporal tissue ( Penfield and Milner, 1958 ; Milner, 1959 ). More recent findings from Squire’s laboratory extend the evidence concerning correlations with lesion size and support the view that lesions restricted to the hippocampus produce less severe memory loss than lesions of the hippocampus plus other temporal lobe areas. R.B.’s lesion, limited to the CA1 field, resulted in moderately severe amnesia ( Zola-Morgan and Squire, 1986 ), whereas two other patients, G.P. and E.P., with damage to their entire medial temporal lobes bilaterally, were profoundly amnesic ( Stefanacci et al., 2000 ; Bayley and Squire, 2005 ). Notably, E.P.’s lesion included the temporal pole, amygdala, entorhinal cortex, hippocampus, perirhinal cortex, and rostral parahippocampal cortex and also extended into lateral temporal neocortex; his declarative memory was even more impaired than H.M.’s ( Insausti et al., 2013 ).
Medial Temporal Lobe Structures Excised and Spared
After H.M.’s death in 2008, we assessed his lesion using high-resolution in situ and ex vivo MRI. These images showed that the following areas were removed or damaged: substantial portions of the medial temporopolar, piriform, entorhinal, perirhinal, and parahippocampal cortices, as well as the subiculum, presubiculum, parasubiculum, amygdala, hippocampal fields CA1, CA2, CA3, and CA4 (in the hippocampal head and body), and dentate gyrus (posterior head and body). Further, our analyses suggested that a few noteworthy structures survived the medial temporal lobe surgery and may have been more difficult to discern with in vivo MRI scans due to lower resolution ( Corkin et al., 1997 ; Salat et al., 2006 ). Notably, several areas within the amygdala were spared—parts of the amygdalar medial nucleus, cortical nucleus, cortical amygdaloid transition area, amygdala-striatal zone, endopiriform nucleus, and a portion of the central nucleus. Also visible were the hippocampal-amygdalo-transition-area (HATA), a small portion of the uncus, the tail of the hippocampus (~2 cm), a small part of the perirhinal cortex (Brodmann area 35), the entire ectorhinal cortex (Brodmann area 36), and ~2 cm of the posterior parahippocampal gyrus. Although the residual hippocampal, perirhinal, and parahippocampal tissue was first documented in the 1992 and 1993 MRI images ( Corkin et al., 1997 ), the present findings further specify the locus and extent of the spared tissue.
Neural Substrate for H.M.’s Amnesia
Parahippocampal cortices and hippocampal formation.
After his operation in 1953, H.M. could not consolidate and retrieve new facts and events, documenting for the first time that circuits within the medial temporal lobe are necessary for the establishment of long-term declarative memory ( Scoville and Milner, 1957 ). Memory experiments carried out over the next five decades showed that H.M.’s deficits were severe and extensive, affecting the acquisition of verbal and nonverbal material presented via four sensory modalities ( Milner, 1968 ; Corkin, 1984 , 2002 ). He could not learn new episodic or semantic information ( Gabrieli et al., 1988 ; O’Kane et al., 2004 ), highlighting the critical role of medial temporal lobe structures in all kinds of declarative memory. Further support for this brain-behavior correlation came from MRI studies carried out in the 1990s, which gave a more accurate picture of the specific structures that were excised and spared in H.M.’s brain. The postmortem studies reported here provide new details of his lesions that could not be gleaned from the in vivo imaging studies.
By far the greatest territory removed on the day of surgery was the parahippocampal gyrus, in particular, the anterior parts of the perirhinal cortex and the entire entorhinal cortex. In a normal brain, the entorhinal cortex receives input from several secondary and tertiary association cortices and multimodal areas (in prefrontal cortex and superior temporal association cortex) and acts as the ultimate end station before extrinsic sensory afferents converge prior to entering the hippocampus ( Van Hoesen, 1997 ; Van Hoesen et al., 1972 ). After this convergence on the entorhinal cortex, entorhinal layer II and superficial layer III then project to the hippocampus via the perforant pathway ( Van Hoesen and Pandya, 1975 ).
Scoville’s resection included the mesocortices of the anterior parahippocampal gyrus (entorhinal and perirhinal cortices (perirhinal area 35)) and also the hippocampal head and body. As a result, H.M.’s perforant pathway was destroyed at its origin and termination, thereby eliminating the entire circuitry necessary for long-term declarative memory. The remaining ~2 cm of hippocampal tissue was deafferented, and, therefore, not able to support long-term memory formation, storage, and retrieval. A preliminary histological study showed that the remaining hippocampal tissue contained substantial gliosis, which would further compromise the residual tissue ( Annese et al., 2014 ).
H.M.’s pervasive memory impairment resulted from the removal of a significant portion of his hippocampi, including all CA subfields, the anterior dentate gyrus, anterior subiculum, anterior presubiculum, and prosubiculum. The hippocampal remnants included the hippocampal-amygdala-transition-area (HATA), the tail of the hippocampus, and a small portion of medial hippocampal tissue. Subsequent histological studies will clarify whether the small medial remnant is uncus or gyrus ambiens. Also spared were the posterior portions of the subiculum, dentate gyrus, and hippocampal body and tail. The posterior portion of the subiculum appeared to be intact, possibly leaving the subicular projections to the anterior, lateral dorsal, reuniens, and paraventricular nuclei of the thalamus untouched ( Aggleton et al., 1986 ). It is clear, however, that this modest input, if it existed, was not sufficient to support normal memory function in H.M.
A new finding in the postmortem scans concerned the medial temporal stem. In 1997, we reported that the temporal stem was intact ( Corkin et al., 1997 ), although Gaffan disagreed ( Gaffan, 2001 ). In the current study, major advances in technology allowed us to examine the temporal stem with greater precision in the in situ and ex vivo images. We noted that this structure was markedly deteriorated in H.M. in situ and showed decreased contrast in MPRAGE (see Figs. 5C–E ). It is unclear whether these lesions dated back to the surgical excision or were caused by degenerative disease late in life, but we favor the view that this damage was due to aging and white matter deterioration as noted in previous MRIs ( Salat et al., 2006 ) and not to Scoville’s original resection. The role of the temporal stem in amnesia has been somewhat controversial ( Horel, 1978 ; Gaffan et al., 2001 ; Gaffan, 2001 ), but a study by Zola-Morgan, Squire, and Mishkin ( Zola-Morgan et al., 1982 ) appeared to be irrefutable. They found that monkeys in whom the temporal stem white matter had been cut bilaterally were unimpaired on a delayed nonmatching-to-sample task, whereas animals with bilateral lesions of the amygdala, hippocampus, and parahippocampal gyrus showed severe impairment. A later study by Gaffan et al. was consistent with this view ( Gaffan et al., 2001 ). On a delayed matching-to-sample task, the performance of monkeys with transection of the anterior temporal stem alone did not differ significantly from their preoperative levels. It is, therefore, unlikely that H.M.’s temporal stem lesions contributed to his declarative memory impairment. Rather, his profound amnesia was caused by the excision of the parahippocampal cortices and hippocampal formation. H.M.’s performance on delayed-match-to-sample and delayed-nonmatch-to-sample tasks was comparable to that of control participants 6 months after learning ( Freed et al., 1987 ; Freed and Corkin, 1988 ), suggesting that his partially intact perirhinal cortex may have been engaged to carry out these tasks.
Aging, Cortical Thinning, and White Matter Damage
Previous in vivo MRIs characterized H.M.’s cortical and white matter damage ( Salat et al., 2006 ). At the time of his death, he was demented, and his entire brain was severely atrophied, with no structure escaping degeneration. As noted previously ( Annese et al., 2014 ), we observed a small lesion in the left orbitofrontal region in the in situ and ex vivo MRI scans that was not described in Scoville’s original report ( Scoville and Milner, 1957 ). The etiology of this lesion is unclear, but we are confident that planned histological studies will reveal the cause. The possibilities include damage by the retractor used to elevate H.M.’s left frontal lobe, deafferentation of a medial temporal lobe projection, or white matter disease, possibly due to small vessel ischemic disease, such as untreated hypertension. The white matter damage throughout H.M.’s brain was severe in the in situ and ex vivo images, and it was far worse than in other untreated hypertensive cases at his age (JCA and DHS, personal observation) ( Fazekas et al., 1993 ; Young et al., 2008 ). Substantial contrast changes in older adults may be due to significant dysfunction of vascular regulatory mechanisms ( Braffman et al., 1988 ; Breteler et al., 1994b ; Longstreth et al., 1996 ; Erkinjuntti, 2007 ), and given the substantial white matter abnormalities that we observed in H.M.’s brain ex vivo, it is likely that white matter damage caused his dementia.
Neural Substrates for H.M.’s Preserved Memory Capacities
H.M.’s deep and lasting amnesia attests to the fact that the spared medial temporal lobe structures were unable to support normal memory function or anything approaching it. Still, over the years, he occasionally surprised his examiners by retrieving episodic and semantic information that he encountered after his operation. The most astonishing example came from a picture recognition experiment in which we asked him to look at and remember complex colorful pictures for 20 sec each. Not only did he achieve normal recognition at 10 min, 24 hrs, 72 hrs, and 1 wk after encoding, but he also scored within 1 SD of the control mean 6 months later ( Freed et al., 1987 ; Freed and Corkin, 1988 ).
We attribute H.M.’s ability to recognize the complex pictures to the engagement of familiarity–based processes supported by his residual perirhinal and parahippocampal cortices in communication with his preserved cortical circuitry. Our MRI data obtained in vivo and in situ showed some remnants of perirhinal and parahippocampal cortices bilaterally ( Figs. 5E–G ) ( Corkin et al., 1997 ). Evidence accumulated over the last 20 years strongly suggests that recollection and familiarity engage different medial temporal lobe areas, with recollection mediated by the hippocampus and familiarity by perirhinal and parahippocampal cortices ( Aggleton and Brown, 1999 , 2005 ; Yonelinas and Jacoby, 2012 ). H.M.’s ability to recognize complex visual stimuli underscores the point that the hippocampus is not necessary for recognition memory based on familiarity.
H.M. was also able to recognize and provide a few distinguishing details about celebrities and politicians who rose to fame after his operation, such as JFK, Ray Charles, and Liza Minnelli ( Gabrieli et al., 1988 ; O’Kane et al., 2004 ). We attribute these glimmers of memory formation in part to processing in preserved medial temporal lobe structures seen in the in situ and ex vivo MRI images: part of perirhinal cortex, posterior parahippocampal cortex, dorsal-most amygdala, and the medial-most uncus. It is possible that these small pieces of medial temporal lobe that remained, especially posteriorly, helped support declarative memory formation on rare occasions. H.M. spent a lot of time watching television and leafing through magazines, which exposed him to a wealth of information about celebrities. This repeated stimulation over months and years enabled him to build up meager representations of a handful of famous people; he acquired this knowledge slowly over time and not via the fast declarative memory processes that healthy individuals would employ.
H.M.’s preserved memory capacities also included those now classified as nondeclarative. Milner’s 1962 groundbreaking report that he showed procedural learning over three days introduced the idea that the human brain houses dissociable memory circuits ( Milner, 1970 ). Subsequent experiments extended this result, showing that H.M. could acquire a variety of motor skills ( Corkin, 1968 ). Studies in patients with Parkinson disease, Huntington disease, and cerebellar degeneration later indicated that the striatum and cerebellum mediate motor skill learning ( Sanes et al., 1990 ; Knopman and Nissen, 1991 ; Pascual-Leone et al., 1993 ; Breteler et al., 1994a , b ; Corkin, 2013 ). Our in vivo MRI results confirmed that the striatum was not damaged in H.M.’s operation, and although his cerebellum was atrophied, it did not appear grossly abnormal.
Subsequent studies evaluated H.M.’s performance on other kinds of nondeclarative memory tasks. In a series of eyeblink classical conditioning experiments, he acquired conditioned responses in both the delay and trace paradigms ( Woodruff-Pak, 1993 ). Although he required more trials to reach criterion than his control, the fact that he showed any conditioned responses is a challenge to explain because previous research has established that the cerebellum, hippocampus, and amygdala play a major role in eyeblink conditioning (Woodruff-Pak et al., 1985; Weisz et al., 1992 ; Thompson and Kim, 1996 ; Clark et al., 2002 ; Christian and Thompson, 2003; Thompson and Steinmetz, 2009). In H.M.’s brain, the hippocampus and amygdala were extirpated, and the cerebellum was markedly atrophied. Still, it is possible that his cerebellum and deep cerebellar nuclei supported the learning, and we will examine these structures microscopically in hope of uncovering clues about his ability to acquire conditioned responses.
Repetition priming refers to a kind of learning in which recent incidental exposure to test stimuli, such as words, pictures, and patterns, facilitates subsequent processing of that information. Priming is evidence that past experience can influence memory unconsciously, that is, when participants are not trying intentionally to recall the past, and it is mediated by cortical pathways undamaged in H.M. In several experiments, he demonstrated intact performance on perceptual and conceptual priming tasks ( Gabrieli et al., 1990 , 1995 ; Keane et al., 1995 ). Companion studies in patients with cortical lesions indicated that conceptual priming is mediated by lateral temporal and parietal circuits, while perceptual priming depends on occipital circuits ( Keane et al., 1991 , 1994 , 1995 ; Gabrieli et al., 1994 ). These cortical networks were intact in H.M. and likely the underpinnings of his intact priming performance. This finding of preserved nondeclarative memory considered side by side with his impoverished declarative memory, measured by tests of recall and recognition, established the validity of cognitive and neural dissociations among memory processes.
Neural Substrates for H.M.’s Nonmnemonic Behavioral Deficits
Examination of H.M.’s fresh brain at autopsy indicated that his olfactory bulbs and tract were undamaged, but the surgical removal did include primary olfactory cortex in the temporal lobe (piriform cortex and periamygdaloid cortex). As a result, H.M. was anosmic. Extensive behavioral testing uncovered limited preserved function and severe deficits on several olfactory tasks ( Eichenbaum et al., 1983 ). His detection of weak odorants was normal as was his threshold for discrimination of intensity differences, and he showed normal adaptation to a strong odor. In contrast, his ability to discriminate odor quality was completely absent on three different measures: signal-detection testing, the triangle match-to-sample task, and a common-odor-matching task, likely due to the absence of piriform and periamygdaloid cortices ( Eichenbaum et al., 1983 ). This striking dissociation of olfactory perceptual capacities established that odor quality discrimination and recognition are not necessary for detection, intensity discrimination, or adaptation.
The in situ and ex vivo MRI studies described here brought to light the details of H.M.’s extensive amygdala resection, which included the lateral, basolateral, accessory basal, and paralaminar nuclei, a portion or all of the central nucleus, and the anterior amygdala area. The result was that most of H.M.’s amygdala output was silenced, and it is likely that the amygdala resection explains a cluster of behavioral abnormalities. H.M.’s perception of pain was diminished in the laboratory and in daily life, he showed no change in his ratings of hunger and thirst from before to after a meal, he was asexual, and he was not fearful of anything ( Hebben et al., 1985 ; Corkin, 2013 ). Nevertheless, he could experience and display a range of emotions, such as happiness, friendliness, sadness, worry, guilt, and aggression, and he was able to label the emotion in various facial expressions ( Corkin, 2013 ). The emotional response system is complex, and the underlying brain mechanisms connecting inputs and outputs engage cortical and subcortical circuits beyond the amygdala ( Price, 2003 ). A goal of future histological studies will be to examine the integrity of these connections and remnants of the amygdala in H.M.’s brain.
Neural Substrate for H.M.’s Preserved Cognitive Capacities
The MR images collected from H.M. in 1992 and 1993, four decades after his operation, showed that his frontal, parietal, and occipital cortices were normal for his age, as was the lateral temporal neocortex. At that time, it was unclear whether the subcortical white matter associated with the most anterior portions of the superior, middle, and inferior temporal gyri was damaged, but our in situ and ex vivo images confirmed that these tracts were abnormal. Nevertheless, the vast expanse of cortex on both sides of H.M.’s brain likely functioned near optimally, allowing the engagement of multiple specialized circuits to support his performance on a broad spectrum of cognitive tasks.
Milner conducted H.M.’s first postoperative psychological examination in 1955, 2 years after his operation ( Scoville and Milner, 1957 ). On the Wechsler-Bellevue Intelligence Scale, he achieved an IQ of 112, but on the Wechsler Memory Scale, his MQ was only 67, indicating normal intelligence coupled with markedly impaired long-term memory ( Wechsler, 1945 ). On subsequent testing with different forms of the Intelligence Scale and Memory Scale, he maintained this pattern of performance through 2000 ( Kensinger et al., 2001 ). This longitudinal analysis firmly established that medial temporal lobe structures are not necessary for optimal performance on IQ tests, indicating a clear dissociation between high-order cognition and long-term declarative memory.
The high-resolution, high-contrast MRI methods highlighted here confirmed that H.M.’s cortical and subcortical language areas remained intact following his surgery, and the results from numerous experimental measures and standardized tests showed that his language functions were largely spared ( Kensinger et al., 2001 ). He successfully completed seven lexical memory tasks: spelling; picture naming, name recognition, and information retrieval; Boston naming test; picture naming; picture judgment; category identification; and landmark identification, and on tests of morphology, he was able to produce and judge regular and irregular inflectional or derivational forms, including plural production, past-tense production, past tense judgment, and derivational morphology production. Two additional tasks measured his syntax processing, and he performed them normally. In general, H.M. maintained his preoperative lexical knowledge without explicit retraining, indicating that medial temporal lobe structures are not necessary for the retention of already learned lexical information. They are, however, critical for the acquisition of new lexical information (e.g., new vocabulary, celebrities) ( Gabrieli et al., 1988 ; Postle and Corkin, 1998 ). Notable exceptions in the language domain were H.M.’s impaired performance on fluency tasks and uneven success in detecting linguistic ambiguities ( Lackner, 1974 ; Corkin, 2013 ). These deficits likely stemmed from a combination of factors: minimal surgical damage to anterolateral temporal cortex, slow responding, substandard education, and lower socioeconomic background ( Kensinger et al., 2001 ; Schmolck et al., 2002 ).
Evidence of preserved problem solving and working memory processes came from H.M.’s consistently excellent performance on the Wisconsin Card Sorting Test ( Milner, 1968 ). During each administration of the task over years of testing, he quickly changed to a new sorting category as needed and had very few perseverative errors, but he was always unaware that he had done the test before. To perform this complex task, H.M. had to recruit multiple cognitive processes and engage circuits in prefrontal cortex and posterior parietal cortex, areas that were spared in the 1953 surgery ( Corkin et al., 1997 ; Salat et al., 2006 ).
H.M.’s bilateral medial temporal lobe resection was circumscribed, and the resulting amnesia was pure. He revolutionized the science of memory through his participation in numerous behavioral and imaging studies, and he continues to illuminate the science of memory. During his lifetime, neuroimaging advanced with specialized sequences and sophisticated multichannel array coils that enabled high resolution MRI. These tools allowed a final and riveting inspection of H.M.’s lesions and remaining anatomy. This postmortem research is consistent with his wishes: He knew he was contributing to science and gladly donated his brain for future study. His mantra was, “Whatever is beneficial.” It is fitting that the field of neuroscience continues to benefit from his contributions, even after his death.
Acknowledgments
The authors are grateful to H.M. and his conservator for the generous tissue donation. The authors also thank M. Dylan Tisdall, Jonathan R. Polimeni, and Thomas Witzel for technical assistance in developing protocols to accommodate extremely large data files and Kristen Huber for photographing the blockface images and laboratory preparations.
Grant sponsor: National Center for Research Resources; Grant number: P41-RR14075; Grant sponsor: NCRR BIRN Morphometric Project; Grant numbers: BIRN002 and U24 RR021382; Grant sponsor: National Institute for Biomedical Imaging and Bioengineering; Grant number: R01EB006758; Grant sponsor: National Institute on Aging; Grant numbers: AG022381 and 5R01AG008122-22; Grant sponsor: NIH Blueprint for Neuroscience Research; Grant number: 5U01-MH093765; Grant sponsors: Part of the Multi-Institutional Human Connectome Project and National Science Foundation; Grant number: NSF-SGER0714660; Grant sponsor: Dana Foundation; Grant number: 2007-4234.
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999; 22 :425–444. discussion 444–489. [ PubMed ] [ Google Scholar ]
- Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005; 58 :218–233. [ PubMed ] [ Google Scholar ]
- Aggleton JP, Desimone R, Mishkin M. The origin, course, and termination of the hippocampothalamic projections in the macaque. J Comp Neurol. 1986; 243 :409–421. [ PubMed ] [ Google Scholar ]
- Annese J, Schenker-Ahmed NM, Bartsch H, Maechler P, Sheh C, Thomas N, Kayano J, Ghatan A, Bresler N, Frosch MP, Klaming R, Corkin S. Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nat Commun. 2014; 5 :3122. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bayley PJ, Squire LR. Failure to acquire new semantic knowledge in patients with large medial temporal lobe lesions. Hippocampus. 2005; 15 :273–280. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Benner T, Wisco JJ, van der Kouwe AJ, Fischl B, Vangel MG, Hochberg FH, Sorensen AG. Comparison of manual and automatic section positioning of brain MR images. Radiology. 2006; 239 :246–254. [ PubMed ] [ Google Scholar ]
- Braak H, Braak E. On areas of transition between entorhinal allocortex and temporal isocortex in the human brain. Normal morphology and lamina-specific pathology in Alzheimer’s disease. Acta Neuropathol. 1985; 68 :325–332. [ PubMed ] [ Google Scholar ]
- Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: Pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR Am J Roentgenol. 1988; 151 :559–566. [ PubMed ] [ Google Scholar ]
- Breteler MM, van Amerongen NM, van Swieten JC, Claus JJ, Grobbee DE, van Gijn J, Hofman A, van Harskamp F. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke. 1994a; 25 :1109–1115. [ PubMed ] [ Google Scholar ]
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, van Harskamp F, Tanghe HL, de Jong PT, van Gijn J, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam Study. Neurology. 1994b; 44 :1246–1252. [ PubMed ] [ Google Scholar ]
- Brodmann K. English translation by Laurence Garey of the German book, translator. London, UK: Smith-Gordon; 1909. Brodmann’s ’Localisation in the Cerebral Cortex. [ Google Scholar ]
- Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci. 2002; 6 :524–531. [ PubMed ] [ Google Scholar ]
- Corkin S. Acquisition of motor skill after bilateral medial temporal lobe excision. Neuropsychologia. 1968; 6 :255–265. [ Google Scholar ]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in H.M. Semin Neurol. 1984; 4 :249–259. [ Google Scholar ]
- Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002; 3 :153–160. [ PubMed ] [ Google Scholar ]
- Corkin S. Permanent Present Tense: The Unforgettable Life of the Amnesic Patient, H. M. New York: Basic Books; 2013. p. 400. [ Google Scholar ]
- Corkin S, Amaral DG, Gonzalez RG, Johnson KA, Hyman BT. H. M.’s medial temporal lobe lesion: Findings from magnetic resonance imaging. J Neurosci. 1997; 17 :3964–3979. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999; 9 :179–194. [ PubMed ] [ Google Scholar ]
- Deoni SC. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI) J Magn Reson Imaging. 2007; 26 :1106–1111. [ PubMed ] [ Google Scholar ]
- Deoni SC, Peters TM, Rutt BK. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med. 2005; 53 :237–241. [ PubMed ] [ Google Scholar ]
- Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med. 2003; 49 :515–526. [ PubMed ] [ Google Scholar ]
- Ding SL, Van Hoesen GW. Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Hum Brain Mapp. 2010; 31 :1359–1379. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: A combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol. 2009; 514 :595–623. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eichenbaum H, Morton TH, Potter H, Corkin S. Selective olfactory deficits in case H.M. Brain. 1983; 106 :459–472. [ PubMed ] [ Google Scholar ]
- Erkinjuntti T. Vascular cognitive deterioration and stroke. Cerebrovasc Dis. 2007; 24 (Suppl 1):189–194. [ PubMed ] [ Google Scholar ]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993; 43 :1683–1689. [ PubMed ] [ Google Scholar ]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33 :341–355. [ PubMed ] [ Google Scholar ]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004; 23 (Suppl 1):S69–S84. [ PubMed ] [ Google Scholar ]
- Freed DM, Corkin S. Rate of forgetting in H.M.: 6-month recognition. Behav Neurosci. 1988; 102 :823–827. [ PubMed ] [ Google Scholar ]
- Freed DM, Corkin S, Cohen NJ. Forgetting in H.M.: A second look. Neuropsychologia. 1987; 25 :461–471. [ PubMed ] [ Google Scholar ]
- Gabrieli JD, Cohen NJ, Corkin S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain Cogn. 1988; 7 :157–177. [ PubMed ] [ Google Scholar ]
- Gabrieli JD, Milberg W, Keane MM, Corkin S. Intact priming of patterns despite impaired memory. Neuropsychologia. 1990; 28 :417–427. [ PubMed ] [ Google Scholar ]
- Gabrieli JD, Keane MM, Stanger BZ, Kjelgaard MM, Corkin S, Growdon JH. Dissociations among structural-perceptual, lexical-semantic, and event-fact memory systems in Alzheimer, amnesic, and normal subjects. Cortex. 1994; 30 :75–103. [ PubMed ] [ Google Scholar ]
- Gabrieli JD, McGlinchey-Berroth R, Carrillo MC, Gluck MA, Cermak LS, Disterhoft JF. Intact delay-eyeblink classical conditioning in amnesia. Behav Neurosci. 1995; 109 :819–827. [ PubMed ] [ Google Scholar ]
- Gaffan D. What is a memory system? Horel’s critique revisited. Behav Brain Res. 2001; 127 :5–11. [ PubMed ] [ Google Scholar ]
- Gaffan D, Parker A, Easton A. Dense amnesia in the monkey after transection of fornix, amygdala and anterior temporal stem. Neuropsychologia. 2001; 39 :51–70. [ PubMed ] [ Google Scholar ]
- Gloor P. The Temporal Lobe. Oxford University Press; New York and Oxford: 1995. p. 1997. [ Google Scholar ]
- Hebb DO. The organization of behavior. New York, NY: Wiley; 1949. [ Google Scholar ]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: Case H.M. Behav Neurosci. 1985; 99 :1031–1039. [ PubMed ] [ Google Scholar ]
- Horel JA. The neuroanatomy of amnesia. A critique of the hippocampal memory hypothesis. Brain. 1978; 101 :403–445. [ PubMed ] [ Google Scholar ]
- Insausti R, Tunon T, Sobreviela T, Insausti AM, Gonzalo LM. The human entorhinal cortex: A cytoarchitectonic analysis. J Comp Neurol. 1995; 355 :171–198. [ PubMed ] [ Google Scholar ]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998; 19 :659–671. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Insausti R, Annese J, Amaral DG, Squire LR. Human amnesia and the medial temporal lobe illuminated by neuropsychological and neurohistological findings for patient E.P. Proc Natl Acad Sci USA. 2013; 110 :E1953–E1962. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- James W. The Principles of Psychology: Dover Publications. New York: 1890. p. 1950. [ Google Scholar ]
- Keane MM, Gabrieli JD, Fennema AC, Growdon JH, Corkin S. Evidence for a dissociation between perceptual and conceptual priming in Alzheimer’s disease. Behav Neurosci. 1991; 105 :326–342. [ PubMed ] [ Google Scholar ]
- Keane MM, Gabrieli JD, Growdon JH, Corkin S. Priming in perceptual identification of pseudowords is normal in Alzheimer’s disease. Neuropsychologia. 1994; 32 :343–356. [ PubMed ] [ Google Scholar ]
- Keane MM, Gabrieli JD, Mapstone HC, Johnson KA, Corkin S. Double dissociation of memory capacities after bilateral occipital-lobe or medial temporal-lobe lesions. Brain. 1995; 118 :1129–1148. [ PubMed ] [ Google Scholar ]
- Kensinger EA, Ullman MT, Corkin S. Bilateral medial temporal lobe damage does not affect lexical or grammatical processing: Evidence from amnesic patient H.M. Hippocampus. 2001; 11 :347–360. [ PubMed ] [ Google Scholar ]
- Knopman D, Nissen MJ. Procedural learning is impaired in Huntington’s disease: Evidence from the serial reaction time task. Neuropsychologia. 1991; 29 :245–254. [ PubMed ] [ Google Scholar ]
- Lackner JR. Observations on the speech processing capabilities of an amnesic patient: Several aspects of H.M.’s language function. Neuropsychologia. 1974; 12 :199–207. [ PubMed ] [ Google Scholar ]
- Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996; 27 :1274–1282. [ PubMed ] [ Google Scholar ]
- Lorente de No R. Studies on the structure of the cerebral cortex II Continuation of the study of ammonic system. J Fur Psychol Neurol. 1934; 46 :113–177. [ Google Scholar ]
- Milner B. The memory defect in bilateral hippocampal lesions. Psychiatr Res Rep Am Psychiatr Assoc. 1959; 11 :43–58. [ PubMed ] [ Google Scholar ]
- Milner B. Les troubles de la memorie accompagnant des lesions hippocampiques bilaterales. In: Passouant P, editor. Physiologie de l’hippocampe. Paris: Centre National de la Recherche Scientifique; 1962. pp. 257–272. [ Google Scholar ]
- Milner B. Biology of Memory. New York: Academic Press, Inc; 1970. Memory and the medial temporal regions of the brain; pp. 29–50. [ Google Scholar ]
- Milner B, Corkin S, Teuber H-L. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia. 1968; 6 :215–234. [ Google Scholar ]
- Mugler JP, III, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990; 15 :152–157. [ PubMed ] [ Google Scholar ]
- Mugler JP, III, Bao S, Mulkern RV, Guttmann CR, Robertson RL, Jolesz FA, Brookeman JR. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology. 2000; 216 :891–899. [ PubMed ] [ Google Scholar ]
- O’Kane G, Kensinger EA, Corkin S. Evidence for semantic learning in profound amnesia: An investigation with patient H.M. Hippocampus. 2004; 14 :417–425. [ PubMed ] [ Google Scholar ]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. Procedural learning in Parkinson’s disease and cerebellar degeneration. Ann Neurol. 1993; 34 :594–602. [ PubMed ] [ Google Scholar ]
- Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch Neurol Psychiatry. 1958; 79 :475–497. [ PubMed ] [ Google Scholar ]
- Postle BR, Corkin S. Impaired word-stem completion priming but intact perceptual identification priming with novel words: Evidence from the amnesic patient H.M. Neuropsychologia. 1998; 36 :421–440. [ PubMed ] [ Google Scholar ]
- Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003; 985 :50–58. [ PubMed ] [ Google Scholar ]
- Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990; 16 :192–225. [ PubMed ] [ Google Scholar ]
- Rosene DL, Van Hoesen GW. The hippocampal formation of the primate brain, a review of some comparative aspects of cytoarchitecture and connections. In: Jones EG, Peters A, editors. Cerebral Cortex. Further Aspects of Cortical Function, Including Hippocampus. New York: Plenum Press; 1987. pp. 345–456. [ Google Scholar ]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004; 14 :721–730. [ PubMed ] [ Google Scholar ]
- Salat DH, van der Kouwe AJ, Tuch DS, Quinn BT, Fischl B, Dale AM, Corkin S. Neuroimaging H.M.: A 10-year follow-up examination. Hippocampus. 2006; 16 :936–945. [ PubMed ] [ Google Scholar ]
- Sanes JN, Dimitrov B, Hallett M. Motor learning in patients with cerebellar dysfunction. Brain. 1990; 113 :103–120. [ PubMed ] [ Google Scholar ]
- Schmolck H, Kensinger EA, Corkin S, Squire LR. Semantic knowledge in patient H.M. other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus. 2002; 12 :520–533. [ PubMed ] [ Google Scholar ]
- Scoville WB. The Limbic Lobe in Man. J Neurosurg. 1954; 11 :64–66. [ PubMed ] [ Google Scholar ]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957; 20 :11–21. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stefanacci L, Buffalo EA, Schmolck H, Squire LR. Profound amnesia after damage to the medial temporal lobe: A neuroanatomical and neuropsychological profile of patient E. P. J Neurosci. 2000; 20 :7024–7036. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: Evidence from H.M. and W.R. Neuropsychologia. 2005; 43 :479–496. [ PubMed ] [ Google Scholar ]
- Thompson RF, Kim JJ. Memory systems in the brain and localization of a memory. Proc Natl Acad Sci USA. 1996; 93 :13438–13444. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tovi M, Ericsson A. Measurements of T1 and T2 over time in formalin-fixed human whole-brain specimens. Acta Radiol. 1992; 33 :400–404. [ PubMed ] [ Google Scholar ]
- Van der Kouwe A, Benner T. Combined brain morphometry and skull imaging with FLUSTER. Sydney; Australia: 2008. [ Google Scholar ]
- van der Kouwe AJ, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorensen AG, Dale AM. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005; 27 :222–230. [ PubMed ] [ Google Scholar ]
- van der Kouwe AJ, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magn Reson Med. 2006; 56 :1019–1032. [ PubMed ] [ Google Scholar ]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008; 40 :559–569. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Hoesen GW. Anatomy of the medial temporal lobe. Magnet Reson Imaging. 1995; 13 :1047–1055. [ PubMed ] [ Google Scholar ]
- Van Hoesen GW. Cortical feedforward and cortical feedback neural systems in Alzheimer’s disease. In: Hyman BT, Duychaerts C, Christen Y, editors. Connections, Cognition and Alzheimer’s Disease. Berlin, Heidelberg: Springer-Verlag; 1997. [ Google Scholar ]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res. 1975; 95 :39–59. [ PubMed ] [ Google Scholar ]
- Van Hoesen GW, Pandya DN, Butters N. Cortical afferents to the entorhinal cortex of the Rhesus monkey. Science. 1972; 175 :1471–1473. [ PubMed ] [ Google Scholar ]
- Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer’s disease. Clinical and preclinical neuroanatomical correlates. In: Scharfman W, Schwarcz, editors. The Parahippocampal Region, Implications for Neurological and Psychiatric Diseases. Ann N Y Acad Sci. Vol. 911. 2000. pp. 254–274. [ PubMed ] [ Google Scholar ]
- von Bonin G, Bailey P. The Neocortex of Macaca Mulatta. Urbana, IL: University of Illinois Press; 1947. [ Google Scholar ]
- von Economo C, Koskinas GN. Atlas of Cytoarchitectonics of the Adult Human Cerebral Cortex. Karger Publications; Berlin: 1925. [ Google Scholar ]
- Wechsler D. A standardized memory scale for clinical use. J Psychol. 1945; 19 :87–95. [ Google Scholar ]
- Weisz DJ, Harden DG, Xiang Z. Effects of amygdala lesions on reflex facilitation and conditioned response acquisition during nictitating membrane response conditioning in rabbit. Behav Neurosci. 1992; 106 :262–273. [ PubMed ] [ Google Scholar ]
- Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 T receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006; 56 :216–223. [ PubMed ] [ Google Scholar ]
- Woodruff-Pak DS. Eyeblink classical conditioning in H.M.: Delay and trace paradigms. Behav Neurosci. 1993; 107 :911–925. [ PubMed ] [ Google Scholar ]
- Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007; 57 :192–200. [ PubMed ] [ Google Scholar ]
- Yonelinas AP, Jacoby LL. The process-dissociation approach two decades later: Convergence, boundary conditions, and new directions. Mem Cognit. 2012; 40 :663–680. [ PubMed ] [ Google Scholar ]
- Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008; 71 :804–811. [ PubMed ] [ Google Scholar ]
- Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behav Neurosci. 1986; 100 :155–160. [ PubMed ] [ Google Scholar ]
- Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: Amygdala-hippocampus versus temporal stem. Science. 1982; 218 :1337–1339. [ PubMed ] [ Google Scholar ]

Normal brain MRI of a 40-year-old male: A case study
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Reprints and Permissions
- Cite Icon Cite
- Search Site
Mohammed Erkhawan Hameed Rasheed , Mansour Youseffi , Luca Parisi , Saeed Afshin Javid , Farideh Javid; Normal brain MRI of a 40-year-old male: A case study. AIP Conf. Proc. 28 September 2023; 2872 (1): 120019. https://doi.org/10.1063/5.0162994
Download citation file:
- Ris (Zotero)
- Reference Manager
Magnetic resonance imaging (MRI) is one of the medical imaging modalities that is most often used to examine the brain, especially considering that it is a non-ionising technique [1]. With the aid of various MRI sequences, such as T1-weighted, T2-weighted, or fluid attenuated inversion recovery (FLAIR), brain MRI can provide detailed three-dimensional images of the brain used to analyse the anatomy and pathology of the various regions of the brain [2]. Thus, MRI is a safe, painless, and non-invasive medical imaging technique [3]; however, it may take longer to perform a brain scan, as compared to other medical imaging modalities, such as Computed Tomography (CT) [4], although CT is a technique that leverages ionising radiation. This case study describes the case of a 40-year-old male with migraine, including an analysis of his medical history and MRI images. The MRI images showed a physiological brain with no suspicious intracranial lesions.
Sign in via your Institution
Citing articles via, publish with us - request a quote.

Sign up for alerts
- Online ISSN 1551-7616
- Print ISSN 0094-243X
- For Researchers
- For Librarians
- For Advertisers
- Our Publishing Partners
- Physics Today
- Conference Proceedings
- Special Topics
pubs.aip.org
- Privacy Policy
- Terms of Use
Connect with AIP Publishing
This feature is available to subscribers only.
Sign In or Create an Account
Neuroimaging: Visualizing Brain Structure and Function
Case studies.

7.1. Case Study 1
7.2. case study 2.
Susan Shin, a 24-year-old healthy graduate student is crossing the street to attend class when a delivery truck runs a red light and hits her. She is thrown several feet, hits her head on the curb, and loses consciousness. EMTs have difficulty obtaining blood pressure and her oxygen saturation is below normal. In the Emergency Department (ED) she is still unconscious and is intubated. She is found to have multiple rib fractures, a collapsed lung, and is markedly hypotensive from internal bleeding. A non-contrast CT scan of her brain shows diffuse subarachnoid blood and contusions of her frontal and temporal lobes. Neck CT shows no fractures.
A chest tube is inserted, helping Susan's lung reinflate. She is attached to a ventilator. She is transfused and rushed to emergency surgery which normalizes her blood pressure. After surgery she enters the ICU. Forty hours later, well after the anesthesia was worn off, she still has not regained consciousness. A neurologist is called.
Imaging is appropriate at this point for diagnostic purposes. Further structural imaging can help identify the cause of Susan's coma. Although a repeat CT scan would probably also have been done to follow up on the blood in the brain, MRI will show more detail of which structures are injured. MRI of the spinal cord would be done to exclude a cord injury from the trauma.
The neurologist recommends MRIs of the brain and its vasculature and of the cervical spine. Overnight the ICU nurse notices some quick jerks of the fingers that could represent seizure, so the resident physician obtains an electroencephalogram (EEG). The study shows diffuse slowing of the brain's normal electrical activity as is often seen in comatose patients, but no evidence of seizures.
Susan does not have an Advance Directive in the form of a designated health care proxy or a living will. She also has no spouse and no children, so her parents are the next in line as her surrogates to make medical decisions for her. They say that she was a competitive athlete and active in her church and would want "to fight this out."
What it means to have positive outcome in this setting is not well defined overall, but one attempt is the Glasgow Outcome Scale, which defines "moderate disability" as independence in daily living with physical or mental limitations preventing return to one's previous level of function. For traumatic and non- traumatic coma, detailed tables based on studies with large sample sizes exist, correlating the different features seen on neurological exams with the percentages of patients who go on to recover neurological function to various extents (ranging from none to resumption of former activities). , These numbers can be cited to families who want to know overall odds and to prepare them for the possibility of severe disability if expected. However, except for some scenarios, the percentages cannot foretell the outcome for any one individual patient.
Susan has two different types of injury, both traumatic and anoxic/ischemic, making her prognosis more difficult and complex, because it is not clear which is more severe or contributing most to her current condition.
She is currently in a vegetative state (VS), "awake but unaware." It is too early to comment on its permanence. Data on the likelihood of recovery from the vegetative state collected by the Multi-Society Task Force described outcomes beginning from one month of ongoing VS, after which the term persistent may apply. This patient could remain vegetative or could go on to recover to a higher level of awareness such as minimal consciousness.
Although they are not required for diagnosis of a vegetative state, electrodiagnostic studies can sometimes aid in prognosis. EEG can exclude seizures or demonstrate other patterns known to be associated with poor outcome. Somatosensory evoked potentials (SSEPs) and Brainstem auditory- evoked responses (BAERs) can test the integrity of different circuits in the cortex and brainstem, respectively.
The parents want to know if Susan knows that they are there at her bedside, if she can hear them talking to her, and if she is in pain. The neurologist explains that it is not known how much of what healthy people would recognize as conscious awareness is present in minimally conscious individuals. It is probably not the case that she is living an active mental life inside her severely limited body, the way a person with a neuro-degenerative disease might.
The neurologist further explains that patients who recover from MCS do not recall the period of minimal consciousness. Rather, it is thought, and imaging has supported the idea, that MCS involves a fluctuating limited ability to interact, and that these patients have limited activation of selected areas of cortex permitting some interaction without the full integration required for complete awareness. What exactly is intact is highly individual and dependent on the injury each patient sustains. Large areas of pain networks may be preserved, so it is reasonable to ensure patients' comfort, including pain medication. Several studies have shows preservation of auditory networks and at least one has shown evidence of auditory processing and cognitive command following, so although it is unlikely that the patient has total awareness of her family's presence, her brain could be processing their speech rudimentarily.
The neurologist reassures the Shins that Susan will continue to be examined at regular intervals for evidence of neurological recovery. He also provides them with a realistic explanation of her likely severe degree of permanent disability.
The proposed neuroimaging studies will be experimental and descriptive. They are not validated for prognosis in Susan's case. Currently, there are many research studies but no large, validated set of prognostic data using fMRI or PET for patients in MCS, so even if it were performed, the test's results would be of uncertain significance. The results might enter a database which in aggregate data could be used to prospectively or retrospectively correlate eventual outcome with features seen on such imaging, and thus might eventually help scientists form prognostic schemes such as those currently in existence for coma. The benefit will not be for this patient or family, but for others in the future. Eventually, physicians may be able to construct a functional, neuroimaging profile of a particular injured patient that gives good information about likely recovery. However, that is a future direction, not a current reality.
- Great Diseases
- Pre-College Programs
- Undergraduate Internships
- Resources for Building Inclusivity in Science
- Publications
Center for Science Education at Tufts University
- The Great Diseases
- Request Access
- Student Portal
- Online Courses
- Partnerships
- Infectious Diseases (ID)
- Neurological Disorders (ND)
- Metabolic Disease (MD)
- Cancer (CA)
- Order Printing
- - Online Courses
- - Webinars
- - Workshops
- - Partnerships
- - Community
- - COVID-19
- - Infectious Diseases (ID)
- - Neurological Disorders (ND)
- - Metabolic Disease (MD)
- - Cancer (CA)
- - Order Printing
Neurological Disorders Case Studies
Case study 1 – the man with no memory.

Henry Molaison, known by thousands as “H.M.”, is probably the best known single patient in the history of neuroscience. His severe memory impairment, which resulted from experimental brain surgery to control seizures, was the subject of study for five decades until his death in December 2008. Work with H.M. established fundamental principles about how memory functions are organized in the brain. In this case study, students predict H.M.’s performance in some of Milner’s most famous experiments with H.M.
Download Case Study 1 here .
Case Study 2 — What causes Alzheimer’s Disease?

Axonal transport systems are crucial to maintain neuronal viability and differentiation. Considerable evidence suggests that failure of axonal transport may play a role in the development and progression of neurological diseases such as Alzheimer’s disease. The goal of the research study this lesson focuses on investigates the role of axonal transport in the pathology of Alzheimer’s disease.
Download Case Study 2 here .
Case Study 3 — How do placebos work?

The placebo effect occurs when we perceive that an inactive substance is having the effect of an active substance. In this case study, students analyze real data from an original research paper investigating subjects’ reaction to pain when given a placebo ‘pain reliever’ or a control. Both control and ‘pain reliever’ were actually the same. Students are asked to consider the design of the experiment and then predict outcomes based on stated hypotheses. They then analyze and interpret the actual data and compare to their own predictions.
Download Case Study 3 here .
Case Study 4 — What causes narcolepsy?

In this study, students synthesize information from different studies to arrive at a model to explain the neuronal basis for narcolepsy. In this study the authors actually removed the gene candidate for causing narcolepsy and were able to show that removing the gene caused narcolepsy symptoms to develop in comparison with controls. This evidence is indicative of causation. In a second set of experiments the authors showed that humans with narcolepsy had fewer neurons expressing this gene, further evidence that the gene in question plays a causative role in narcolepsy.
Download Case Study 4 here .
Case Study 5 – What role do cues play in addiction?

In this study, students analyze and interpret data from different studies then synthesize this information to arrive at a model to explain the role that contextual cues play in self-stimulation with alcohol or sugar. In this study the authors trained rats to anticipate a self-reinforcer (alcohol or sugar) and then measured the time frame of the rats’ response and levels of dopamine in the rats’ nucleus accumbens. They were able to show that rats were able to learn to anticipate self-stimulation when presented with a contextual cue and that their locomotion increased during the anticipatory phase. When the self-reinforcer was alcohol, dopamine levels in the nucleus accumbens rose during the anticipatory phase as well as during the consummatory phase showing that the rise is not simply a consequence of self-stimulation. In contrast although rats could learn to self-stimulate for sugar in response to a contextual cue it neither increased locomotion during the anticipatory phase nor increased dopamine levels in the nucleus accumbens during either phase. The results are evidence that a contextual cue can activate the reward pathway in advance of a self-reinforcing stimulus.
Download Case Study 5 here .

Disclaimer | Non-Discrimination | Privacy | Terms for Creating and Maintaining Sites
Meet The Man Who Lives Normally With Damage to 90% of His Brain
A French man who lives a relatively normal, healthy life - despite damaging 90 percent of his brain - is causing scientists to rethink what it is from a biological perspective that makes us conscious.
Despite decades of research, our understanding of consciousness - being aware of one's existence - is still pretty thin . Many scientists think that the physical source of consciousness is based in the brain, but then how can someone lose the majority of their neurons and still be aware of themselves and their surroundings?
First described in The Lancet in 2007, the case of the man who appears to be missing most of his brain has been puzzling scientists for almost 10 years.
The French man was 44 years old at the time the journal article came out, and although his identity was kept confidential, the researchers explained how he'd lived most of his life without realising anything was wrong with him.
He only went to the doctor complaining of mild weakness in his left leg, when brain scans revealed that his skull was mostly filled with fluid, leaving just a thin outer layer of actual brain tissue, with the internal part of his brain almost totally eroded away.
You can see his scans below:
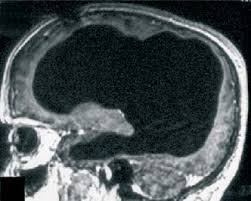
Doctors think the majority of the man's brain was slowly destroyed over the course of 30 years by the build-up of fluid in the brain, a condition known as hydrocephalus. He'd been diagnosed with it as an infant and treated with a stent, but it was removed when he was 14 years old, and since then, the majority of his brain seems to have been eroded.
But despite his minimal remaining brain tissue, the man wasn't mentally disabled - he had a low IQ of 75, but was working as a civil servant. He was also married with two children, and was relatively healthy.
Not only did his case study cause scientists to question what it takes to survive, it also challenges our understanding of consciousness.
In the past, researchers have suggested that consciousness might be linked to various specific brain regions - such as the claustrum , a thin sheet of neurons running between major brain regions, or the visual cortex .
But if those hypotheses were correct, then the French man shouldn't be conscious, with the majority of his brain damaged.
"Any theory of consciousness has to be able to explain why a person like that, who's missing 90 percent of his neurons, still exhibits normal behaviour," Axel Cleeremans, a cognitive psychologist from the Université Libre de Bruxelles in Belgium, told Quartz.
In other words, it's unlikely that one specific region on its own is going to be responsible for consciousness.
Cleeremans has instead come up with a hypothesis that's based on the brain learning consciousness over and over again, rather than being born with it. Which means its location can be flexible and learnt by different brain regions.
"Consciousness is the brain's non-conceptual theory about itself, gained through experience - that is learning, interacting with itself, the world, and with other people," he explains.
He first published this idea back in 2011 , and has now given a lecture on the subject at the 2016 Association for the Scientific Study of Consciousness conference in Buenos Aires in June.
He calls his hypothesis the ' radical plasticity thesis ', and it fits in pretty well with recent research that suggests the adult brain is more adaptable than we previously thought - and capable of taking on new roles in case of injury.
As Olivia Goldhill reports for Quartz :
"Cleeremans argues that in order to be aware, it's necessary not simply to know information, but to know that one knows information. In other words, unlike a thermostat that simply records temperature, conscious humans both know and care that they know. Cleeremans claims that the brain is continually and unconsciously learning to re-describe its own activity to itself, and these descriptions form the basis of conscious experience."
But what does all that have to do with a man surviving with only 10 percent of his brain? According to Cleeremans, even though his remaining brain was only tiny, the neurons left over were able to still generate a theory about themselves, which means the man remained conscious of his actions.
In itself, the concept isn't new - we're discovering more and more each day just how flexible and adaptable our brains really are. Just this week, scientists were able to trigger brain cells to start growing again in order to restore vision in blind mice.
But it's a striking reminder of what our brains can learn to achieve, even when they're incredibly damaged, and provides hope that we might one day learn how to reverse some of the illnesses that cause neurodegeneration.
Update 3 Jan 2017: This man has a specific type of hydrocephalus known as chronic non-communicating hydrocephalus , which is where fluid slowly builds up in the brain. Rather than 90 percent of this man's brain being missing, it's more likely that it's simply been compressed into the thin layer you can see in the images above. We've corrected the story to reflect this.
Breadcrumbs Section. Click here to navigate to respective pages.

Cases of Amnesia
DOI link for Cases of Amnesia
Get Citation
In all cognitive domains, neuropsychological research has advanced through the study of individual patients , and detailed observations and descriptions of their cases have been the backbone of medical and scientific reports for centuries. Cases of Amnesia describes some of the most important single case studies in the history of memory, as well as new case studies of amnesic patients. It highlights the major contribution they make to our understanding of human memory and neuropsychology.
Written by world-leading researchers and considering the latest theory and techniques in the field, each case study provides a description of the patient's history, how their memory was assessed and what conclusions can be made in relation to cognitive models of memory.
Edited by Sarah E. MacPherson and Sergio Della Sala, Cases of Amnesia is a must read for researchers and clinicians in neuropsychology, cognitive psychology and cognitive neuroscience.
TABLE OF CONTENTS
Chapter 1 | 15 pages, the single case study of memory, chapter 2 | 24 pages, the earthquake that reshaped the intellectual landscape of memory, mind and brain, chapter 3 | 25 pages, the case of yr, chapter 4 | 27 pages, amnesic patient vc, chapter 5 | 18 pages, what did amnesic actor ab teach us about learning his lines, chapter 6 | 21 pages, cases of hippocampal memory loss, chapter 7 | 25 pages, persistent déjà vu, recollective confabulation and the case of patient akp, chapter 8 | 31 pages, case kc (kent cochrane) and his contributions to research and theory on memory and related, non-memory functions, chapter 9 | 16 pages, right is right for episodic memories in two contrasting case studies, ch and jrcases ch and jr, chapter 10 | 17 pages, sensory-specific visual amnesia (cases 1 and 2), chapter 11 | 20 pages, ‘yes, i remember’—apparent consolidation under conditions of minimal sensory input in a case of severe anterograde amnesia, chapter 12 | 21 pages, chapter 13 | 31 pages, a “purest” impairment of verbal short-term memory. the case of pv and the phonological short-term input store, chapter 14 | 23 pages, semantic short-term memory and its role in sentence processing and long-term memory, chapter 15 | 21 pages, interrelationship between semantic memory and personal experience, chapter 16 | 18 pages, iris murdoch, chapter 17 | 11 pages, the wealth of evidence from brain lesions affecting memory, chapter 18 | 12 pages, biases and concerns with the single case approach in the neuropsychology of memory, chapter 19 | 12 pages, the case for single case studies in memory research, chapter 20 | 9 pages, comments on the single case approach to the study of memory and other domains of cognition.
- Privacy Policy
- Terms & Conditions
- Cookie Policy
- Taylor & Francis Online
- Taylor & Francis Group
- Students/Researchers
- Librarians/Institutions
Connect with us
Registered in England & Wales No. 3099067 5 Howick Place | London | SW1P 1WG © 2024 Informa UK Limited
Module 13: The Nervous System
Case study: the brain, interactive link.
Click on the link below to access the case study created by Tangi Mitchell and Cheryl L. Watson of Central Connecticut State University
Biological Sciences Central Connecticut State University
- Study Protocol
- Open access
- Published: 08 April 2024
Rational and design of prophylactic cranial irradiation (PCI) and brain MRI surveillance versus brain MRI surveillance alone in patients with limited-stage small cell lung cancer achieving complete remission (CR) of tumor after chemoradiotherapy: a multicenter prospective randomized study
- Mengyuan Chen 1 ,
- Runhua Li 1 ,
- Yue Kong 1 ,
- Lei Shi 1 ,
- Jing Wang 1 ,
- Yuezhen Wang 1 ,
- Yujin Xu 1 ,
- Yongling Ji 1 &
- Xiao Hu 1
BMC Cancer volume 24 , Article number: 429 ( 2024 ) Cite this article
650 Accesses
1 Citations
1 Altmetric
Metrics details
Prophylactic cranial irradiation (PCI) is part of standard care in limited-stage small cell lung cancer (SCLC) at present. As evidence from retrospective studies increases, the benefits of PCI for limited-stage SCLC are being challenged.
A multicenter, prospective, randomized controlled study was designed. The key inclusion criteria were: histologically or cytologically confirmed small cell carcinoma, age ≥ 18 years, KPS ≥ 80, limited-stage is defined as tumor confined to one side of the chest including ipsilateral hilar, bilateral mediastinum and supraclavicular lymph nodes, patients have received definitive thoracic radiotherapy (regardless of the dose-fractionation of radiotherapy used) and chemotherapy, evaluated as complete remission (CR) of tumor 4–6 weeks after the completion of chemo-radiotherapy. Eligible patients will be randomly assigned to two arms: (1) PCI and brain MRI surveillance arm, receiving PCI (2.5 Gy qd to a total dose of 25 Gy in two weeks) followed by brain MRI surveillance once every three months for two years; (2) brain MRI surveillance alone arm, undergoing brain MRI surveillance once every three months for two years. The primary objective is to compare the 2-year brain metastasis-free survival (BMFS) rates between the two arms. Secondary objectives include 2-year overall survival (OS) rates, intra-cranial failure patterns, 2-year progression-free survival rates and neurotoxicity. In case of brain metastasis (BM) detect during follow-up, stereotactic radiosurgery (SRS) will be recommended if patients meet the eligibility criteria.
Based on our post-hoc analysis of a prospective study, we hypothesize that in limited-stage SCLC patients with CR after definitive chemoradiotherapy, and ruling out of BM by MRI, it would be feasible to use brain MRI surveillance and omit PCI in these patients. If BM is detected during follow-up, treatment with SRS or whole brain radiotherapy does not appear to have a detrimental effect on OS. Additionally, this approach may reduce potential neurotoxicity associated with PCI.
Peer Review reports
Small cell lung cancer (SCLC) accounts for approximately 15-20% of all cases of bronchopulmonary carcinoma. It is characterized by high malignancy, a tendency to early metastasis. At the time of diagnosis, about one-third of cases are of limited-stage [ 1 ]. The standard treatment is chemoradiotherapy for the majority of limited-stage SCLC patients [ 2 ].
A meta-analysis showed that prophylactic cranial irradiation (PCI) significantly reduce the incidence of BM by 25.3% compared to the control group (33.3% vs. 58.6%, P < 0.001), in patients who achieved complete remission (CR) after chemoradiotherapy, and it also improved the 3-year overall survival (OS) rate by 5.4% (20.7% vs. 15.3%, P = 0.01) [ 3 ]. Based on this study, PCI has been recommended for patients with limited-stage SCLC who have achieved a good response to chemoradiotherapy [ 2 , 4 ].
However, the meta-analysis [ 3 ] has several limitations. The most important one is that no routine brain MRI was performed before PCI. A study conducted in the era of MRI demonstrated that 25% of newly diagnosed SCLC patients had brain metastasis (BM), and the cumulative incidence of BM after initial treatment could exceed 50% [ 5 ]. Our retrospective study showed that among all limited-stage SCLC patients who did not receive PCI and developed BM, 30.2% of them were detected before planned PCI with brain MRI [ 6 ]. Therefore, it can be speculated that some of the patients included in this meta-analysis actually received “treatment” rather than “prophylaxis”.
We have completed a prospective randomized study on the target volume of thoracic radiotherapy in limited-stage SCLC [ 7 ]. Our post-hoc analysis showed that, among 300 patients who received definitive chemoradiotherapy in this study, 134 (44.7%) achieved CR, 105 patients received PCI. All patients underwent baseline brain MRI, and 85.6% of them received brain MRI before PCI. The median follow-up time for the entire group was 22.3 months. Although PCI significantly reduced the incidence of BM (16.2% vs. 37.9%, P = 0.02), the median survival time for the PCI and the non-PCI arm were 30.2 months and 30.5 months, respectively, with 3-year OS rates of 39.9% and 43.0% ( P = 0.93). PCI did not significantly improve the OS of patients who achieved CR after chemoradiotherapy. Among the 300 patients, 143 (47.7%) patients achieved partial remission (PR), of whom, 90 patients received PCI. Before PCI, 90.9% of patients received brain MRI. The median survival time for the PCI and the non-PCI arm were 27.4 months and 18.6 months, respectively, with 3-year OS rates of 42.6% and 17.4% ( P <0.0001). PCI significantly improved the OS of patients who achieved PR after chemoradiotherapy (unpublished data).
Therefore, in the era of routine brain MRI surveillance, the role of PCI in limited-stage SCLC warrants further study. Based on above preliminary results, we speculate that PCI could be spared in patients who achieve CR after definitive chemoradiotherapy and instead, receive brain MRI surveillance. Even if BM is detected during follow-up, subsequent salvage treatment with SRS or whole-brain radiotherapy (WBRT) would not have a detrimental effect on OS.
Study design and objective
This is a prospective, randomized controlled study that includes patients with limited-stage SCLC who achieve CR after definitive chemoradiotherapy. Eligible patients will be randomly assigned to two arms:
Control arm: Patients receive PCI and regular brain MRI surveillance.
Study arm: Patients receive regular brain MRI surveillance alone.
If BM is detected during follow-up, SRS is recommended when appropriate.
The primary endpoint is to compare the 2-year brain metastasis -free survival (BMFS) rates between the two arms. Secondary endpoints include 2-year OS, intra-cranial failure patterns, 2-year progression-free survival (PFS), cognitive functions(Hopkins Verbal Learning Test will be used to assess cognitive function).
Key eligibility criteria
Inclusion criteria:
Histologically or cytologically confirmed small cell carcinoma.
Age ≥ 18 years.
Karnofsky Performance Status (KPS) ≥ 80.
Limited-stage disease, defined as tumor confined to one side of the chest including ipsilateral lung, bilateral mediastinal lymph nodes, and bilateral supraclavicular lymph nodes (metastatic lymph nodes defined as short-axis diameter ≥ 1 cm or showing increased metabolic activity on PET-CT, or confirmed by mediastinoscopy / EBUS / TBNA biopsy). Pleural effusion thickness on chest CT is less than 1 cm (unless cytologically confirmed as malignant pleural effusion). Staging is determined according to the 8th edition of the AJCC staging system (2017), specifically stages I-IIIC without intrapulmonary metastasis.
Have received curative-intent thoracic radiotherapy and chemotherapy.
Assessment of treatment response within 4–6 weeks after completion of curative-intent thoracic chemoradiotherapy shows CR (evaluation includes contrast-enhanced chest and abdominal CT, contrast-enhanced brain MRI, whole-body bone scan, and lung cancer biomarkers such as NSE and ProGRP).
Willingness and ability to comply with the follow-up schedule.
Full understanding of the study and voluntary signing of an informed consent form.
Exclusion criteria:
History of malignant tumors in other parts of the body (previous or concurrent), excluding non-malignant melanoma, papillary carcinoma of the thyroid and cervical carcinoma in situ.
Patients who have undergone curative surgery (excluding biopsy).
Patients with a history of mental illness, in pregnancy or lactation.
Uncontrolled diabetes, hypertension, or severe active infections.
Manifested chronic central nervous system disorders.
Contraindications for brain MRI examination.
Other situations deemed unsuitable for enrollment by doctors in charge.
Pre-treatment evaluation
Baseline staging include enhanced MRI of the brain, enhanced CT of the chest and upper abdomen, ultrasonography of the supraclavicular lymph nodes and bone scan. PET-CT is recommended, bone scan could be omitted if PET-CT is available. Laboratory tests include routine blood tests, routine liver and kidney function tests, lung cancer biomarker tests, electrocardiogram, echocardiography and pulmonary function test.
Statistical analysis & sample size considerations
The study is designed as a prospective, randomized controlled non-inferiority trial. Based on previous study results, we hypothesize that the 2-year BMFS rate in the control group is 83%, and the 2-year BMFS in the study group is 68%, which is deemed acceptable as non-inferior. With a power of 80% and a one-sided significance level of 0.025, considering a shedding rate of 10%, 110 patients will be required in each group.
Radiotherapy
Positioning and ct/mri simulation.
All patients will be placed in supine position with thermoplastic mask for whole-brain immobilization. Contrast-enhanced CT simulations are recommended. The slice thickness of the scan should be ≤ 5 mm, and the scanning range should extend from 2 cm above the skull to the lower margin of the 7th cervical vertebra, including the entire cranial and neck. When PCI with hippocampal avoidance is required, a MRI simulation of the brain is strongly recommended and the thickness of the brain MRI scanning should be 1 mm.
Definition of radiation target volume
The entire brain is contoured as the clinical target volume (CTV), and the CTV is expanded by 0.3 cm to create the planning target volume (PTV) which is named PTV-Brain. The PTV of hippocampal avoidance PCI is named PTV-HA. PTV-HA is PTV-Brain minus hippocampi. Critical organs at risk (OAR) include the bilateral lenses and eyeballs. For patients who are eligible for brain MRI simulation, bilateral hippocampi should be contoured.
Radiation dose and planning evaluation
The prescribed dose for PTV is 2.5 Gy per fraction, once daily, 5 days a week for two weeks. The minimum requirement for the radiation technique is three-dimensional conformal radiotherapy. If PCI with hippocampal avoidance is required, the minimum requirement for irradiation method is stated to be IMRT. Helical tomotherapy is recommended if available.After completing the radiation treatment planning, the dose distribution in the target volume and the dose to OAR are evaluated. The dose-volume histogram (DVH) is used as a fundamental tool, and the dose distribution in the PTV and OAR is assessed based on the distribution of dose curves in three-dimensional space. The prescribed dose is determined based on the dose received by 95% of the PTV, with dose uniformity ranging from 94 to 106%. The dose constraints for OAR are as follows: lenses: Dmax < 9 Gy, Dmean < 5 Gy; hippocampi: Dmax < 16 Gy, Dmean < 9 Gy.
Toxicity evaluation
Treatment related toxicities will be evaluated in accordance with Common Terminology Criteria for Adverse Events (CTCAE) 5.0 criteria. (1) Complete blood counts and physical examinations will be performed at least once a week during radiation therapy. (2) Adverse events related to the nervous system will be documented at least once a week during radiation therapy and during follow-up. Hopkins Verbal Learning Test will be used to assess cognitive function.
Tumor response evaluation criteria and definition of survival time
Tumor response will be evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
Brain metastasis-free survival (BMFS) is defined as the time from the start of treatment to BM or death from any cause. Progression-free survival (PFS) is defined as the time from the start of treatment to disease progression or death from any cause. Overall survival (OS) is defined as the time from the start of treatment to death from any cause.
All enrolled patients will be followed up starting from the start of PCI and continuing for 2 years or until death. Follow-up will be conducted once every three months. At each follow-up, patients routinely received examination of brain enhanced MRI, chest and abdomen enhanced CT, and lung tumor markers. After two years, the frequency of follow-up is left to the discretion of the doctor in charge.
Radiotherapy for brain metastasis
For patients with ≤ 5 intracranial metastases and a maximum diameter of ≤ 3 cm for a single metastasis, SRS or Cyber Knife radiotherapy will be recommended. Otherwise, WBRT will be administered, especially for patients who have not yet received PCI. For patients who have received PCI and developed BM, SRS or Cyber Knife will be recommended.
SCLC tends to develop early distant metastasis. Brain is a common site of metastasis in SCLC patients, with approximately 25% of patients present with brain metastases at the time of initial diagnosis [ 5 ]. Due to the presence of the blood-brain barrier, conventional chemotherapy drugs have limited efficacy in penetrating brain tissue, making it a potential “sanctuary” for small brain metastatic lesions. Therefore, PCI does not truly “prevent” the development of BM, it plays a role of eliminating potentially but undetectable small metastasis in brain.
With the advances in comprehensive treatment, the prognosis of limited-stage SCLC patients has improved, the probability of BM has also increased correspondingly. Among patients who achieved CR after treatment, the incidence of BM within 2 years was 67%, with the brain being the first site of metastasis in 45% of cases [ 8 ].
A meta-analysis demonstrated that in patients who achieved a complete response (CR) after chemoradiotherapy, PCI could significantly reduce the incidence of BM by 25.3% ( P < 0.001) compared to the control group. Additionally, it was associated with a 5.4% improvement in 3-year overall survival rate ( P = 0.01) [ 3 ].
However, due to the limitations of the meta-analysis, and with the availability of MRI for regular surveillance of BM, the role of PCI in improving OS in SCLC patients is being challenged.
A prospective randomized study conducted by EORTC demonstrated that extensive-stage SCLC patients who received PCI had significantly better outcomes compared to the observation group (1-year incidence of symptomatic BM: 14.6% vs. 40.4%; median survival time: 6.7 months vs. 5.4 months; 1-year overall survival rate: 27.1% vs. 13.3%) [ 9 ]. However, one of the major limitations of this study was the absence of pre-PCI brain MRI. In contrast, a phase III randomized study showed that in extensive-stage SCLC patients who have ruled out BM with MRI, PCI did not have a positive impact on OS, compared to the observation group [ 10 ].
Traditionally, WBRT is recommended for SCLC patients with BM. A recent large cohort study [ 11 ] compared the outcomes of SCLC patients with BM who received SRS or WBRT. The results showed that those treated with SRS had a median survival time of 8.5 months and a time to central nervous system progression (TTCP) of 8.1 months, while patients with a solitary BM treated with SRS had a median survival time of 11.0 months, and those treated with WBRT had a median survival time of 5.2 months. Although WBRT improved TTCP, it did not improve OS. Furthermore, after adjusting for prognostic factors, the OS results favored SRS.
Another study showed that in limited-stage SCLC patients who underwent MRI surveillance after definitive chemoradiotherapy, the occurrence of BM and survival rates did not significantly differ between patients who received SRS for detected BM and those who received PCI [ 12 ]. The survival benefit was attributed at least partially to SRS, reducing the contribution of PCI to survival. Although PCI is still recommended based on previous studies, these benefits may be diminished if MRI and SRS are available for diagnosis and treatment.
These findings challenge the traditional approach of using WBRT or PCI for all SCLC patients with BM and suggest that SRS guided by MRI surveillance could be a viable alternative in selected cases. However, it is important to consider individual patient factors, tumor characteristics, and treatment goals when determining the most appropriate management strategy.
Pezzi et al. [ 13 ] reported that in patients with limited-stage SCLC who had BM excluded by MRI were matched using a propensity score, although the 3-year incidence of BM was higher in the non-PCI group than in the PCI group, the difference was not statistically significant (20.4% vs. 11.2%, P = 0.10). Also, whether PCI was performed or not did not affect the overall survival (HR: 0.84, 95% CI: 0.604–1.180, P = 0.32).
Similarly, Qi et al. [ 14 ] conducted a retrospective matched analysis of 150 patients with limited-stage SCLC. The results showed a significantly lower 3-year cumulative incidence of BM in the PCI group compared to the non-PCI group (14.7% vs. 22.7%, P = 0.007), but there was no significant difference in median survival time between the two groups (35 months vs. 28 months, P = 0.128).
Of note, PCI potentially leads to acute and late neuro-toxicities. A pooled analysis of the RTOG 0212 and 0214 showed that patients who received PCI had reduced cognitive function as measured by the Hopkins Verbal Learning Test at the 6-month and 12-month follow-up, compared to baseline ( P = 0.002). Patient-reported cognitive decline was even three times higher ( P < 0.0001) [ 15 ]. A survey indicated that 38% of limited-stage SCLC patients who did not receive PCI had concerns about the side effects [ 16 ].
At present, there is no prospective randomized controlled study on PCI versus regular MRI follow-up after definitive radio-chemotherapy for limited-stage SCLC available. However, several similar studies are underway. SWOG S1827 [ 17 ] is a phase III prospective randomized controlled study that aims to enroll patients with limited-stage SCLC who have received radical therapy and patients with extensive-stage SCLC who have responded to systemic therapy. The study will randomize patients into two groups: one group will receive PCI plus regular brain MRI follow-up, while the other group will receive only regular brain MRI follow-up. The primary objective of the study is to compare the two-year survival rates between the two groups. The PRIMA Lung Study [ 18 ] is conducted by EORTC, has similar study design, aims to investigate whether brain MRI surveillance alone is non-inferior in terms of OS compare to PCI followed by brain MRI surveillance in both limited and extensive-stage SCLC patients. Another Chinese study [ 19 ] recruits and randomize limited-stage SCLC patients who achieve remission after first-line chemoradiotherapy to PCI or MRI surveillance. The primary end point is OS at two years.
In conclusion, with the widespread use of brain MRI, the favorable prognosis of SRS for treating SCLC BM, and the insights gained from the Japanese prospective study on PCI in extensive-stage SCLC, as well as the emphasis on the quality of life of long-term survivors, it is worthwhile to conduct a prospective randomized study comparing active brain MRI surveillance alone with PCI after chemoradiotherapy for limited-stage SCLC. The results of this study are highly likely to change current clinical practices.
Data availability
Not applicable.
Govindan R, Page N, Morgensztern D, William R, Ryan T, Anna V, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–44.
Article PubMed Google Scholar
https:// www.nccn.org/professionals/physician_gls/pdf/sclc.pdf .
Aupérin A, Arriagada R, Pignon JP, Le PC, Gregor A, Stephenset RJ. Prophylactic cranial irradiation for patients with small cell lung cancer in complete remission. N Engl J Med. 1999;341:476–84.
Clinical practice guideline for. Radiation therapy in small cell lung cancer (2020 version) Chinese Journal of Radiation Oncology|. Chin J Radiat Oncol. 2020;29(8):608–14.
Google Scholar
Seute T, Leffers P, ten Velde GP, Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer. 2008;112(8):1827–34.
Wu Q, Chen M, Peng F, et al. A study of the prognosis of patients with limited-stage small cell lung cancer who did or did not receive prophylactic cranial irradiation after effective chemoradiotherapy. Front Oncol. 2023;13:1118371.
Article PubMed PubMed Central Google Scholar
Hu X, Bao Y, Xu YJ, Zhu H, Chen M, et al. Final report of a prospective randomized study on thoracic radiotherapy target volume for limited-stage small cell lung cancer with radiation dosimetric analyses. Cancer. 2020;126(4):840–9.
Article CAS PubMed Google Scholar
Arriagada R, Le Chevalier T, Borie F, Santos-Miranda JA, Bardec E, Laplanche A, et al. Prophylactic cranial irradiation for patients with small–cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87(3):183–90.
Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–72.
Takahashi T, Yamanaka T, Seto T, Nokihara H, Saka H, Nishio M, Kaneda H, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663–71.
Rusthoven CG, Yamamoto M, Bernhardt D, Smith DE, Robin TP. Evaluation of first-line radiosurgery vs whole-brain radiotherapy for small cell lung cancer brain metastases: the FIRE-SCLC Cohort Study. JAMA Oncol. 2020;6(7):1028–37.
Ozawa Y, Omae M, Fujii M, Takashi M, Masato K, Shinyaet S, et al. Management of brain metastasis with magnetic resonance imaging and stereotactic irradiation attenuated benefits of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. BMC Cancer. 2015;15:15:589.
Pezzi TA, Fang P, Gjyshi O, Feng L, Liu S, Komaki R, et al. Rates of overall survival and Intracranial Control in the magnetic resonance imaging era for patients with Limited-Stage Small Cell Lung Cancer with and without prophylactic cranial irradiation. JAMA Netw Open. 2020;3(4):e201929.
Qi C, Li W, Li H, Patel A, Beriwal S, et al. Benefits of prophylactic cranial irradiation in the MRI era for patients with Limited Stage Small Cell Lung Cancer. Front Oncol. 2022;12:833478.
Gondi V, Paulus R, Bruner DW, Christina A, Elizabeth M, Aaron W, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86(4):656–64.
Lok BH, Ma J, Foster A, Perez A, Abraham J. Factors influencing the utilization of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. Adv Radiat Oncol. 2017;2(4):548–54.
SWOG S1827 (MAVERICK). Testing whether the use of brain scans alone instead of brain scans plus preventive brain radiation affects lifespan in patients with small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT04155034 .
PRophylactic Cerebral Irradiation or Active MAgnetic. Resonance imaging surveillance in small-cell lung Cancer patients (PRIMALung Study). https://clinicaltrials.gov/study/NCT04790253?cond=NCT04790253&rank=1
Efficacy and Safety of Prophylactic Cranial Irradiation Versus MRI Surveillance in Patients. With limited-stage small cell Lung Cancer who Achieved Remission after First-line Chemoradiotherapy: a Multicenter Randomized Controlled Phase III Clinical Trial. https://clinicaltrials.gov/study/NCT04829708?cond=NCT04829708&rank=1
Download references
Acknowledgements
All authors have read and approved the manuscript.
This work was funded by the National Natural Science Foundation of China (grant numbers 81402540), Zhejiang Science and Technology Plan on Medicine and Health (grant number 2019KY046, 2022KY618 and 2023KY610), Science and Technology Project of Wenling City (2019S0180018) and Zhejiang Cancer Hospital Special Program of Investigator-Initiated Clinical Trial (IIT2022ZA005).
Author information
Authors and affiliations.
Zhejiang Cancer Hospital, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, Zhejiang, 310022, China
Mengyuan Chen, Runhua Li, Yue Kong, Lei Shi, Jing Wang, Yuezhen Wang, Yujin Xu, Yongling Ji & Xiao Hu
You can also search for this author in PubMed Google Scholar
Contributions
Mengyuan Chen wrote the main manuscript text, Runhua Li counted the sample size, Lei Shi, Yuezhen Wang and Jin Wang Yue Kong participated in the design of the research scheme,and Xiao Hu, Yongling Ji and Yujin Xu examined and revised the manuscript, other authors participated in reviewing and revising the language.
Corresponding author
Correspondence to Xiao Hu .
Ethics declarations
Ethics approval and consent to participate.
All experimental protocols were approved by the Medical Ethics Committee of Zhejiang Cancer Hospital(The Ethics is IRB-2022-758, the work will be obtained from all subjects and/or their legal guardian(s). The corresponding author has read the journal policies and submit this manuscript in accordance with those policies, we confirmed that informed consent will be obtained from all subjects and/or their legal guardian(s).
Consent to publication
Conflict of interest.
The authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper. The manuscript had not been published elsewhere, nor are they under consideration by another publisher.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chen, M., Li, R., Kong, Y. et al. Rational and design of prophylactic cranial irradiation (PCI) and brain MRI surveillance versus brain MRI surveillance alone in patients with limited-stage small cell lung cancer achieving complete remission (CR) of tumor after chemoradiotherapy: a multicenter prospective randomized study. BMC Cancer 24 , 429 (2024). https://doi.org/10.1186/s12885-024-12123-x
Download citation
Received : 06 September 2023
Accepted : 14 March 2024
Published : 08 April 2024
DOI : https://doi.org/10.1186/s12885-024-12123-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Small cell lung cancer
- Limited-stage
- Prophylactic cranial irradiation
- MRI surveillance
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Author Guidelines
- Submission Site
- Why publish with this journal?
- Open Access
- Self-Archive Policy
- About Brain Communications
- Editorial Board
- Advertising & Corporate Services
- Observers Programme
- Reviewer Academy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Statin use and risk of parkinson’s disease among older adults in japan: a nested case-control study using the longevity improvement and fair evidence study.
- Article contents
- Figures & tables
- Supplementary Data
Sanyu Ge, Ling Zha, Yasuyoshi Kimura, Yoshimitsu Shimomura, Masayo Komatsu, Yasufumi Gon, Sho Komukai, Fumiko Murata, Megumi Maeda, Kosuke Kiyohara, Tomotaka Sobue, Tetsuhisa Kitamura, Haruhisa Fukuda, Statin use and risk of Parkinson’s disease among older adults in Japan: a nested case-control study using the longevity improvement and fair evidence study, Brain Communications , 2024;, fcae195, https://doi.org/10.1093/braincomms/fcae195
- Permissions Icon Permissions
The association between statin use and the risk of Parkinson’s disease remains inconclusive, particularly in Japan’s super-aging society. This study aimed to investigate the potential association between statin use and the risk of Parkinson’s disease among Japanese participants aged ≥65 years. We used data from the Longevity Improvement and Fair Evidence Study, which included medical and long-term care claims data from April 2014 to December 2020 across 17 municipalities. Using a nested case-control design, we matched one case to five controls based on age, sex, municipality, and cohort entry year. A conditional logistic regression model was used to estimate the odds ratios with 95% confidence intervals. Among the 56,186 participants (9,397 cases and 46, 789 controls), 53.6% were women. The inverse association between statin use and Parkinson’s disease risk was significant after adjusting for multiple variables (odds ratio: 0.61; 95% confidence interval: 0.56–0.66). Compared with non-users, the dose analysis revealed varying odds ratios: 1.30 (1.12–1.52) for 1–30 total standard daily doses, 0.77 (0.64–0.92) for 31-90 total standard daily doses, 0.62 (0.52–0.75) for 91–180 total standard daily doses, and 0.30 (0.25–0.35) for >180 total standard daily doses. Statin use among older Japanese adults was associated with a decreased risk of Parkinson’s disease. Notably, lower cumulative statin doses were associated with an elevated risk of Parkinson’s disease, whereas higher cumulative doses exhibited protective effects against Parkinson’s disease development.

- parkinson disease
- older adult
Supplementary data
| Month: | Total Views: |
|---|---|
| June 2024 | 6 |
Email alerts
Citing articles via.
- Guarantors of Brain
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 2632-1297
- Copyright © 2024 Guarantors of Brain
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Appointments at Mayo Clinic
Vitamin D is a nutrient your body needs for building and maintaining healthy bones. That's because your body can only absorb calcium, the primary component of bone, when vitamin D is present. Vitamin D also regulates many other cellular functions in your body. Its anti-inflammatory, antioxidant and neuroprotective properties support immune health, muscle function and brain cell activity.
Vitamin D isn't naturally found in many foods, but you can get it from fortified milk, fortified cereal, and fatty fish such as salmon, mackerel and sardines. Your body also makes vitamin D when direct sunlight converts a chemical in your skin into an active form of the vitamin (calciferol).
The amount of vitamin D your skin makes depends on many factors, including the time of day, season, latitude and your skin pigmentation. Depending on where you live and your lifestyle, vitamin D production might decrease or be completely absent during the winter months. Sunscreen, while important to prevent skin cancer, also can decrease vitamin D production.
Many older adults don't get regular exposure to sunlight and have trouble absorbing vitamin D. If your doctor suspects you're not getting enough vitamin D, a simple blood test can check the levels of this vitamin in your blood.
Taking a multivitamin with vitamin D may help improve bone health. The recommended daily amount of vitamin D is 400 international units (IU) for children up to age 12 months, 600 IU for people ages 1 to 70 years, and 800 IU for people over 70 years.
What the research says
Research on vitamin D use for specific conditions shows:
- Cancer. Findings on the benefits of vitamin D for cancer prevention are mixed. More studies are needed to determine whether vitamin D supplementation may reduce the risk of certain cancers.
- Cognitive health. Research shows that low levels of vitamin D in the blood are associated with cognitive decline. However, more studies are needed to determine the benefits of vitamin D supplementation for cognitive health.
- Inherited bone disorders. Vitamin D supplements can be used to help treat inherited disorders resulting from an inability to absorb or process vitamin D, such as familial hypophosphatemia.
- Multiple sclerosis. Research suggests that long-term vitamin D supplementation reduces the risk of multiple sclerosis.
- Osteomalacia. Vitamin D supplements are used to treat adults with severe vitamin D deficiency, resulting in loss of bone mineral content, bone pain, muscle weakness and soft bones (osteomalacia).
- Osteoporosis. Studies suggest that people who get enough vitamin D and calcium in their diets can slow bone mineral loss, help prevent osteoporosis and reduce bone fractures. Ask your doctor if you need a calcium and vitamin D supplement to prevent or treat osteoporosis.
- Psoriasis. Applying vitamin D or a topical preparation that contains a vitamin D compound called calcipotriene to the skin can treat plaque-type psoriasis in some people.
- Rickets. This rare condition develops in children with vitamin D deficiency. Supplementing with vitamin D can prevent and treat the problem.
Generally safe
Without vitamin D your bones can become soft, thin and brittle. Insufficient vitamin D is also connected to osteoporosis. If you don't get enough vitamin D through sunlight or dietary sources, you might need vitamin D supplements.
Safety and side effects
Taken in appropriate doses, vitamin D is generally considered safe.
However, taking too much vitamin D in the form of supplements can be harmful. Children age 9 years and older, adults, and pregnant and breastfeeding women who take more than 4,000 IU a day of vitamin D might experience:
- Nausea and vomiting
- Poor appetite and weight loss
- Constipation
- Confusion and disorientation
- Heart rhythm problems
- Kidney stones and kidney damage
Interactions
Possible interactions include:
- Aluminum. Taking vitamin D and aluminum-containing phosphate binders, which may be used to treat high serum phosphate levels in people with chronic kidney disease, might cause harmful levels of aluminum in people with kidney failure in the long term.
- Anticonvulsants. The anticonvulsants phenobarbital and phenytoin (Dilantin, Phenytek) increase the breakdown of vitamin D and reduce calcium absorption.
- Atorvastatin (Lipitor). Taking vitamin D might affect the way your body processes this cholesterol drug.
- Calcipotriene (Dovonex, Sorilux). Don't take vitamin D with this psoriasis drug. The combination might increase the risk of too much calcium in the blood (hypercalcemia).
- Cholestyramine (Prevalite). Taking vitamin D with this cholesterol-lowering drug can reduce your absorption of vitamin D.
- Cytochrome P-450 3A4 (CYP3A4) substrates. Use vitamin D cautiously if you're taking drugs processed by these enzymes.
- Digoxin (Lanoxin). Avoid taking high doses of vitamin D with this heart medication. High doses of vitamin D can cause hypercalcemia, which increases the risk of fatal heart problems with digoxin.
- Diltiazem (Cardizem, Tiazac, others). Avoid taking high doses of vitamin D with this blood pressure drug. High doses of vitamin D can cause hypercalcemia, which might reduce the drug's effectiveness.
- Orlistat (Xenical, Alli). Taking this weight-loss drug can reduce your absorption of vitamin D.
- Thiazide diuretics. Taking these blood pressure drugs with vitamin D increases your risk of hypercalcemia.
- Steroids. Taking steroid mediations such as prednisone can reduce calcium absorption and impair your body's processing of vitamin D.
- Stimulant laxatives. Long-term use of high doses of stimulant laxatives can reduce vitamin D and calcium absorption.
- Verapamil (Verelan, Calan SR). Taking high doses of vitamin D with this blood pressure drug can cause hypercalcemia, and might also reduce the effectiveness of verapamil.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Vitamin D: Fact sheet for health professionals. Office of Dietary Supplements. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/. Accessed Dec. 6, 2020.
- Vitamin D: Fact sheet for consumers. Office of Dietary Supplements. https://ods.od.nih.gov/factsheets/VitaminD-Consumer/. Accessed Dec. 6, 2020.
- Vitamin D. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Dec. 6, 2020.
- AskMayoExpert. Vitamin D deficiency. Mayo Clinic; 2017.
- Cholecalciferol. IBM Microdemex. https://www.microdemexsolutions.com. Accessed Dec. 11, 2020.
- Gold J, et al. The role of vitamin D in cognitive disorders in older adults. US Neurology. 2018; doi:10.17925/USN.2018.14.1.41.
- Sultan S, et al. Low vitamin D and its association with cognitive impairment and dementia. Journal of Aging Research. 2020; doi:10.1155/2020/6097820.
- Pazirandeh S, et al. Overview of vitamin D. https://www.uptodate.com/contents/search. Accessed Dec. 13, 2020.
- Hassan-Smith ZK, et al. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exerct distinct effects on human skeletal muscle function and gene expression. PLOS One. 2017; doi:10.1371/journal.pone.0170665.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Long COVID or Post-COVID Conditions
Some people who have been infected with the virus that causes COVID-19 can experience long-term effects from their infection, known as Long COVID or Post-COVID Conditions (PCC). Long COVID is broadly defined as signs, symptoms, and conditions that continue or develop after acute COVID-19 infection. This definition of Long COVID was developed by the Department of Health and Human Services (HHS) in collaboration with CDC and other partners.
People call Long COVID by many names, including Post-COVID Conditions, long-haul COVID, post-acute COVID-19, long-term effects of COVID, and chronic COVID. The term post-acute sequelae of SARS CoV-2 infection (PASC) is also used to refer to a subset of Long COVID.
What You Need to Know
- Long COVID is a real illness and can result in chronic conditions that require comprehensive care. There are resources available .
- Long COVID can include a wide range of ongoing health problems; these conditions can last weeks, months, or years.
- Long COVID occurs more often in people who had severe COVID-19 illness, but anyone who has been infected with the virus that causes COVID-19 can experience it.
- People who are not vaccinated against COVID-19 and become infected may have a higher risk of developing Long COVID compared to people who have been vaccinated.
- People can be reinfected with SARS-CoV-2, the virus that causes COVID-19, multiple times. Each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing Long COVID.
- While most people with Long COVID have evidence of infection or COVID-19 illness, in some cases, a person with Long COVID may not have tested positive for the virus or known they were infected.
- CDC and partners are working to understand more about who experiences Long COVID and why, including whether groups disproportionately impacted by COVID-19 are at higher risk.
In July 2021, Long COVID was added as a recognized condition that could result in a disability under the Americans with Disabilities Act (ADA). Learn more: Guidance on “Long COVID” as a Disability Under the ADA .
About Long COVID
Long COVID is a wide range of new, returning, or ongoing health problems that people experience after being infected with the virus that causes COVID-19. Most people with COVID-19 get better within a few days to a few weeks after infection, so at least 4 weeks after infection is the start of when Long COVID could first be identified. Anyone who was infected can experience Long COVID. Most people with Long COVID experienced symptoms days after first learning they had COVID-19, but some people who later experienced Long COVID did not know when they got infected.
There is no test that determines if your symptoms or condition is due to COVID-19. Long COVID is not one illness. Your healthcare provider considers a diagnosis of Long COVID based on your health history, including if you had a diagnosis of COVID-19 either by a positive test or by symptoms or exposure, as well as based on a health examination.
Science behind Long COVID
RECOVER: Researching COVID to Enhance Recovery
People with Long COVID may experience many symptoms.
People with Long COVID can have a wide range of symptoms that can last weeks, months, or even years after infection. Sometimes the symptoms can even go away and come back again. For some people, Long COVID can last weeks, months, or years after COVID-19 illness and can sometimes result in disability.
Long COVID may not affect everyone the same way. People with Long COVID may experience health problems from different types and combinations of symptoms that may emerge, persist, resolve, and reemerge over different lengths of time. Though most patients’ symptoms slowly improve with time, speaking with your healthcare provider about the symptoms you are experiencing after having COVID-19 could help determine if you might have Long COVID.
People who experience Long COVID most commonly report:
General symptoms ( Not a Comprehensive List)
- Tiredness or fatigue that interferes with daily life
- Symptoms that get worse after physical or mental effort (also known as “ post-exertional malaise ”)
Respiratory and heart symptoms
- Difficulty breathing or shortness of breath
- Fast-beating or pounding heart (also known as heart palpitations)
Neurological symptoms
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”)
- Sleep problems
- Dizziness when you stand up (lightheadedness)
- Pins-and-needles feelings
- Change in smell or taste
- Depression or anxiety
Digestive symptoms
- Stomach pain
Other symptoms
- Joint or muscle pain
- Changes in menstrual cycles
Symptoms that are hard to explain and manage
Some people with Long COVID have symptoms that are not explained by tests or easy to manage.
People with Long COVID may develop or continue to have symptoms that are hard to explain and manage. Clinical evaluations and results of routine blood tests, chest X-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and other poorly understood chronic illnesses that may occur after other infections. People with these unexplained symptoms may be misunderstood by their healthcare providers, which can result in a delay in diagnosis and receiving the appropriate care or treatment.
Review these tips to help prepare for a healthcare provider appointment for Long COVID.
Health conditions
Some people experience new health conditions after COVID-19 illness.
Some people, especially those who had severe COVID-19, experience multiorgan effects or autoimmune conditions with symptoms lasting weeks, months, or even years after COVID-19 illness. Multi-organ effects can involve many body systems, including the heart, lung, kidney, skin, and brain. As a result of these effects, people who have had COVID-19 may be more likely to develop new health conditions such as diabetes, heart conditions, blood clots, or neurological conditions compared with people who have not had COVID-19.
People experiencing any severe illness may develop health problems
People experiencing any severe illness, hospitalization, or treatment may develop problems such as post-intensive care syndrome (PICS).
PICS refers to the health effects that may begin when a person is in an intensive care unit (ICU), and which may persist after a person returns home. These effects can include muscle weakness, problems with thinking and judgment, and symptoms of post-traumatic stress disorder (PTSD), a long-term reaction to a very stressful event. While PICS is not specific to infection with SARS-CoV-2, it may occur and contribute to the person’s experience of Long COVID. For people who experience PICS following a COVID-19 diagnosis, it is difficult to determine whether these health problems are caused by a severe illness, the virus itself, or a combination of both.
People More Likely to Develop Long COVID
Some people may be more at risk for developing Long COVID.
Researchers are working to understand which people or groups of people are more likely to have Long COVID, and why. Studies have shown that some groups of people may be affected more by Long COVID. These are examples and not a comprehensive list of people or groups who might be more at risk than other groups for developing Long COVID:
- People who have experienced more severe COVID-19 illness, especially those who were hospitalized or needed intensive care.
- People who had underlying health conditions prior to COVID-19.
- People who did not get a COVID-19 vaccine.
Health Inequities May Affect Populations at Risk for Long COVID
Some people are at increased risk of getting sick from COVID-19 because of where they live or work, or because they can’t get health care. Health inequities may put some people from racial or ethnic minority groups and some people with disabilities at greater risk for developing Long COVID. Scientists are researching some of those factors that may place these communities at higher risk of getting infected or developing Long COVID.
Preventing Long COVID
The best way to prevent Long COVID is to protect yourself and others from becoming infected. For people who are eligible, CDC recommends staying up to date on COVID-19 vaccination , along with improving ventilation, getting tested for COVID-19 if needed, and seeking treatment for COVID-19 if eligible. Additional preventative measures include avoiding close contact with people who have a confirmed or suspected COVID-19 illness and washing hands or using alcohol-based hand sanitizer.
Research suggests that people who get a COVID-19 infection after vaccination are less likely to report Long COVID, compared to people who are unvaccinated.
CDC, other federal agencies, and non-federal partners are working to identify further measures to lessen a person’s risk of developing Long COVID. Learn more about protecting yourself and others from COVID-19 .
Living with Long COVID
Living with Long COVID can be hard, especially when there are no immediate answers or solutions.
People experiencing Long COVID can seek care from a healthcare provider to come up with a personal medical management plan that can help improve their symptoms and quality of life. Review these tips to help prepare for a healthcare provider appointment for Long COVID. In addition, there are many support groups being organized that can help patients and their caregivers.
Although Long COVID appears to be less common in children and adolescents than in adults, long-term effects after COVID-19 do occur in children and adolescents .
Talk to your doctor if you think you or your child has Long COVID. Learn more: Tips for Talking to Your Healthcare Provider about Post-COVID Conditions
Data for Long COVID
Studies are in progress to better understand Long COVID and how many people experience them.
CDC is using multiple approaches to estimate how many people experience Long COVID. Each approach can provide a piece of the puzzle to give us a better picture of who is experiencing Long COVID. For example, some studies look for the presence of Long COVID based on self-reported symptoms, while others collect symptoms and conditions recorded in medical records. Some studies focus only on people who have been hospitalized, while others include people who were not hospitalized. The estimates for how many people experience Long COVID can be quite different depending on who was included in the study, as well as how and when the study collected information. Estimates of the proportion of people who had COVID-19 that go on to experience Long COVID can vary.
CDC posts data on Long COVID and provides analyses, the most recent of which can be found on the U.S. Census Bureau’s Household Pulse Survey .
CDC and other federal agencies, as well as academic institutions and research organizations, are working to learn more about the short- and long-term health effects associated with COVID-19 , who gets them and why.
Scientists are also learning more about how new variants could potentially affect Long COVID. We are still learning to what extent certain groups are at higher risk, and if different groups of people tend to experience different types of Long COVID. CDC has several studies that will help us better understand Long COVID and how healthcare providers can treat or support patients with these long-term effects. CDC will continue to share information with healthcare providers to help them evaluate and manage these conditions.
CDC is working to:
- Better identify the most frequent symptoms and diagnoses experienced by patients with Long COVID.
- Better understand how many people are affected by Long COVID, and how often people who are infected with COVID-19 develop Long COVID
- Better understand risk factors and protective factors, including which groups might be more at risk, and if different groups experience different symptoms.
- Help understand how Long COVID limit or restrict people’s daily activity.
- Help identify groups that have been more affected by Long COVID, lack access to care and treatment for Long COVID, or experience stigma.
- Better understand the role vaccination plays in preventing Long COVID.
- Collaborate with professional medical groups to develop and offer clinical guidance and other educational materials for healthcare providers, patients, and the public.
Related Pages
- Caring for People with Post-COVID Conditions
- Preparing for Appointments for Post-COVID Conditions
- Researching COVID to Enhance Recovery
- Guidance on “Long COVID” as a Disability Under the ADA
For Healthcare Professionals
- Post-COVID Conditions: Healthcare Providers
Search for and find historical COVID-19 pages and files. Please note the content on these pages and files is no longer being updated and may be out of date.
- Visit archive.cdc.gov for a historical snapshot of the COVID-19 website, capturing the end of the Federal Public Health Emergency on June 28, 2023.
- Visit the dynamic COVID-19 collection to search the COVID-19 website as far back as July 30, 2021.
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Settlement is at the end—common trauma scores require a critical reassessment due to the possible dynamics of traumatic brain injuries in patients’ clinical course.

1. Introduction
2. materials and methods, 3.1. demographic data, 3.2. mechanism of injury, 3.3. classification of injury severity according to the glasgow coma scale, 3.4. ais and iss changes, 3.5. influence of the altered ais scores on the triss, 3.6. influence of the altered ais/iss values on clinical outcome, 4. discussion, 5. limitations, 6. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Andriessen, T.M.; Horn, J.; Franschman, G.; van der Naalt, J.; Haitsma, I.; Jacobs, B.; Steyerberg, E.W.; Vos, P.E. Epidemiology, Severity Classification, and Outcome of Moderate and Severe Traumatic Brain Injury: A Prospective Multicenter Study. J. Neurotrauma 2011 , 28 , 2019–2031. [ Google Scholar ] [ CrossRef ]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019 , 130 , 1080–1097. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teasdale, G.; Jennett, B. Assessment of Coma and Impaired Consciousness. Lancet 1974 , 304 , 81–84. [ Google Scholar ] [ CrossRef ]
- Maegele, M.; Lefering, R.; Sakowitz, O.; Kopp, M.A.; Schwab, J.M.; Steudel, W.-I.; Unterberg, A.; Hoffmann, R.; Uhl, E.; Marzi, I. The Incidence and Management of Moderate to Severe Head Injury. Dtsch. Arztebl. Int. 2019 , 116 , 167–173. [ Google Scholar ] [ CrossRef ]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974 , 14 , 187–196. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gennarelli, T.A.; Wodzin, E. AIS 2005: A contemporary injury scale. Injury 2006 , 37 , 1083–1091. [ Google Scholar ] [ CrossRef ]
- Maeda, Y.; Ichikawa, R.; Misawa, J.; Shibuya, A.; Hishiki, T.; Maeda, T.; Yoshino, A.; Kondo, Y. External validation of the TRISS, CRASH, and IMPACT prognostic models in severe traumatic brain injury in Japan. PLoS ONE 2019 , 14 , e0221791. [ Google Scholar ] [ CrossRef ]
- Domingues, C.D.A.; Coimbra, R.; Poggetti, R.S.; Nogueira, L.D.S.; De Sousa, R.M.C. New Trauma and Injury Severity Score (TRISS) adjustments for survival prediction. World J. Emerg. Surg. 2018 , 13 , 12. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hosseinpour, R.; Barghi, A.; Mehrabi, S.; Salaminia, S.; Tobeh, P. Prognosis of the Trauma Patients According to the Trauma and Injury Severity Score (TRISS); A Diagnostic Accuracy Study. Bull. Emerg. Trauma 2020 , 8 , 148–155. [ Google Scholar ]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients with post traumatic brain injury using National Trauma Registry and Machine Learning Approach. Scand. J. Trauma Resusc. Emerg. Med. 2020 , 28 , 44. [ Google Scholar ] [ CrossRef ]
- TraumaRegister DGU ® . 20 years TraumaRegister DGU ® : Development, aims and structure. Injury 2014 , 45 , S6–S13. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maegele, M.; Schöchl, H.; Menovsky, T.; Maréchal, H.; Marklund, N.; Buki, A.; Stanworth, S. Coagulopathy and haemorrhagic progression in traumatic brain injury: Advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017 , 16 , 630–647. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kurland, D.; Hong, C.; Aarabi, B.; Gerzanich, V.; Simard, J.M. Hemorrhagic Progression of a Contusion after Traumatic Brain Injury: A Review. J. Neurotrauma 2012 , 29 , 19–31. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chauny, J.-M.; Marquis, M.; Bernard, F.; Williamson, D.; Albert, M.; Laroche, M.; Daoust, R. Risk of Delayed Intracranial Hemorrhage in Anticoagulated Patients with Mild Traumatic Brain Injury: Systematic Review and Meta-Analysis. J. Emerg. Med. 2016 , 51 , 519–528. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Champion, H.R.; Sacco, W.J.; Copes, W.S.; Gann, D.S.; Gennarelli, T.A.; Flanagan, M.E. A Revision of the Trauma Score. J. Trauma Inj. Infect. Crit. Care 1989 , 29 , 623–629. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Boyd, C.R.; Tolson, M.A.; Copes, W.S. Evaluating trauma care: The TRISS method. Trauma Score and the Injury Severity Score. J. Trauma 1987 , 27 , 370–378. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008 , 61 , 344–349. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M.; RECORD Working Committee. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015 , 12 , e1001885. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lefering, R. Trauma scoring systems. Curr. Opin. Crit. Care 2012 , 18 , 637–640. [ Google Scholar ] [ CrossRef ]
- Lefering, R.; Huber-Wagner, S.; Nienaber, U.; Maegele, M.; Bouillon, B. Update of the trauma risk adjustment model of the TraumaRegister DGUTM: The Revised Injury Severity Classification, version II. Crit. Care 2014 , 18 , 476. [ Google Scholar ] [ CrossRef ]
- Paffrath, T.; Lefering, R.; Flohé, S. How to define severely injured patients?—An Injury Severity Score (ISS) based approach alone is not sufficient. Injury 2014 , 45 , S64–S69. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Huber-Wagner, S.; Stegmaier, J.; Mathonia, P.; Paffrath, T.; Euler, E.; Mutschler, W.; Kanz, K.G.; Lefering, R.; Working Group on Polytrauma (NIS) of the German Trauma Society (DGU). The sequential trauma score—A new instrument for the sequential mortality prediction in major trauma. Eur. J. Med. Res. 2010 , 15 , 185. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018 , 16 , 1224–1238. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grote, S.; Böcker, W.; Mutschler, W.; Bouillon, B.; Lefering, R. Diagnostic Value of the Glasgow Coma Scale for Traumatic Brain Injury in 18,002 Patients with Severe Multiple Injuries. J. Neurotrauma 2011 , 28 , 527–534. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Reith, F.C.; Synnot, A.; van den Brande, R.; Gruen, R.L.; Maas, A.I. Factors Influencing the Reliability of the Glasgow Coma Scale: A Systematic Review. Neurosurgery 2017 , 80 , 829–839. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Clark, M.v.B.; Eisenberg, H.M.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991 , 75 , S14–S20. [ Google Scholar ] [ CrossRef ]
- Maas, A.I.R.; Hukkelhoven, C.W.P.M.; Marshall, L.F.; Steyerberg, E.W. Prediction of Outcome in Traumatic Brain Injury with Computed Tomographic Characteristics: A Comparison between the Computed Tomographic Classification and Combinations of Computed Tomographic Predictors. Neurosurgery 2005 , 57 , 1173–1182. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maas, A.I.R.; Lingsma, H.F.; Roozenbeek, B. Predicting outcome after traumatic brain injury. Handb. Clin. Neurol. 2015 , 128 , 455–474. [ Google Scholar ] [ PubMed ]
- Savitsky, B.; Givon, A.; Rozenfeld, M.; Radomislensky, I.; Peleg, K. Traumatic brain injury: It is all about definition. Brain Inj. 2016 , 30 , 1194–1200. [ Google Scholar ] [ CrossRef ]
- Pape, H.C.; Lefering, R.; Butcher, N.; Peitzman, A.; Leenen, L.; Marzi, I.; Lichte, P.; Josten, C.; Bouillon, B.; Schmucker, U.; et al. The definition of polytrauma revisited: An international consensus process and proposal of the new ‘Berlin definition’. J. Trauma Acute Care Surg. 2014 , 77 , 780–786. [ Google Scholar ] [ CrossRef ]
- Palmer, C. Major trauma and the injury severity score--where should we set the bar? Annu. Proc. Assoc. Adv. Automot. Med. 2007 , 51 , 13–29. [ Google Scholar ] [ PubMed ]
- Bendinelli, C.; Ku, D.; King, K.L.; Nebauer, S.; Balogh, Z.J. Trauma patients with prehospital Glasgow Coma Scale less than nine: Not a homogenous group. Eur. J. Trauma Emerg. Surg. 2020 , 46 , 873–878. [ Google Scholar ]
- Bossers, S.M.; Pol, K.M.; Ophuis, E.P.A.O.; Jacobs, B.; Visser, M.C.; Loer, S.A.; Boer, C.; van der Naalt, J.; Schober, P. Discrepancy between the initial assessment of injury severity and post hoc determination of injury severity in patients with apparently mild traumatic brain injury: A retrospective multicenter cohort analysis. Eur. J. Trauma Emerg. Surg. 2018 , 44 , 889–896. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Relja, B.; Huber-Lang, M.; van Griensven, M.; Hildebrand, F.; Maegele, M.; Nienaber, U.; Brucker, D.P.; Sturm, R.; Marzi, I. A nationwide fluidics biobank of polytraumatized patients: Implemented by the Network “Trauma Research” (NTF) as an expansion to the TraumaRegister DGU ® of the German Trauma Society (DGU). Eur. J. Trauma Emerg. Surg. 2020 , 46 , 499–504. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Deutsche Gesellschaft für Neurochirurgie, e.V. DGNC, S2e-Leitlinie Schädel-Hirn-Trauma im Erwachsenenalter, Version: 3.0, State: 2 December 2015. Available online: https://register.awmf.org/de/leitlinien/detail/008-001 (accessed on 1 December 2023).
- Polytrauma Guideline Update Group. Level 3 guideline on the treatment of patients with severe/multiple injuries: AWMF Register-Nr. 012/019. Eur. J. Trauma Emerg. Surg. 2018 , 44 , 3–271. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Picetti, E.; Maier, R.V.; Rossi, S.; Kirkpatrick, A.W.; Biffl, W.L.; Stahel, P.F.; Moore, E.E.; Kluger, Y.; Baiocchi, G.L.; Ansaloni, L.; et al. Preserve encephalus in surgery of trauma: Online survey. (P.E.S.T.O). World J. Emerg. Surg. 2019 , 14 , 9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Picetti, E.; Rossi, S.; Abu-Zidan, F.M.; Ansaloni, L.; Armonda, R.; Baiocchi, G.L.; Bala, M.; Balogh, Z.J.; Berardino, M.; Biffl, W.L.; et al. WSES consensus conference guidelines: Monitoring and management of severe adult traumatic brain injury patients with polytrauma in the first 24 hours. World J. Emerg. Surg. 2019 , 14 , 53. [ Google Scholar ] [ CrossRef ]
- Giannoudi, M.; Harwood, P. Damage control resuscitation: Lessons learned. Eur. J. Trauma Emerg. Surg. 2016 , 42 , 273–282. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pape, H.C.; Giannoudis, P.; Krettek, C. The timing of fracture treatment in polytrauma patients: Relevance of damage control orthopedic surgery. Am. J. Surg. 2002 , 183 , 622–629. [ Google Scholar ]
- Gebhard, F.; Huber-Lang, M. Polytrauma—Pathophysiology and management principles. Langenbecks Arch. Surg. 2008 , 393 , 825–831. [ Google Scholar ] [ CrossRef ]
- Van Wessem, K.J.P.; Leenen, L.P.H.; Hietbrink, F. Physiology dictated treatment after severe trauma: Timing is everything. Eur. J. Trauma Emerg. Surg. 2022 , 48 , 3969–3979. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pfeifer, R.; Kalbas, Y.; Coimbra, R.; Leenen, L.; Komadina, R.; Hildebrand, F.; Halvachizadeh, S.; Akhtar, M.; Peralta, R.; Fattori, L.; et al. Indications and interventions of damage control orthopedic surgeries: An expert opinion survey. Eur. J. Trauma Emerg. Surg. 2021 , 47 , 2081–2092. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Younsi, A.; Unterberg, A.; Marzi, I.; Steudel, W.-I.; Uhl, E.; Lemcke, J.; Berg, F.; Woschek, M.; Friedrich, M.; Clusmann, H.; et al. Development and first results of a national databank on care and treatment outcome after traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2023 , 49 , 1171–1181. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| n = 80 Patients | |
|---|---|
| age (years) ± SD | 54.6 ± 20.9 |
| sex (male) | 49 (61.3%) |
| GCS (pts.) ± SD | 7.9 ± 5.1 |
| ICU (days) ± SD | 8.6 ± 7.4 |
| AIS (pts.) ± SD | 3.66 ± 0.93 |
| AIS (pts.) ± SD | 4.11 ± 0.91 |
| ISS (pts.) ± SD | 22.89 ± 14.73 |
| ISS (pts.) ± SD | 26.68 ± 14.89 |
| TRISS (%) ± SD | 74.82 ± 29.07 |
| TRISS (%) ± SD | 66.25 ± 33.3 |
| mortality (n) | 9 (11.3%) |
| Injury mechanism (n) | |
| fall below 3 m | 25 (31.3%) |
| traffic accident | 18 (22.5%) |
| bicycle fall | 14 (17.5%) |
| fall over 3 m | 14 (17.5%) |
| assault | 8 (10%) |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Hörauf, J.-A.; Woschek, M.; Schindler, C.R.; Verboket, R.D.; Lustenberger, T.; Marzi, I.; Störmann, P. Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course. J. Clin. Med. 2024 , 13 , 3333. https://doi.org/10.3390/jcm13113333
Hörauf J-A, Woschek M, Schindler CR, Verboket RD, Lustenberger T, Marzi I, Störmann P. Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course. Journal of Clinical Medicine . 2024; 13(11):3333. https://doi.org/10.3390/jcm13113333
Hörauf, Jason-Alexander, Mathias Woschek, Cora Rebecca Schindler, Rene Danilo Verboket, Thomas Lustenberger, Ingo Marzi, and Philipp Störmann. 2024. "Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course" Journal of Clinical Medicine 13, no. 11: 3333. https://doi.org/10.3390/jcm13113333
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

- Neuroscience
The Neuroscience of Behavior: Five Famous Cases
Five patients who shaped our understanding of behavior and the brain..
Posted January 16, 2020 | Reviewed by Lybi Ma
“Considering everything, it seems we are dealing here with a special illness… There are certainly more psychiatric illnesses than are listed in our textbooks.” —Alois Alzheimer (In: Benjamin, 2018)
Once thought to be the product of demonic possession, immorality, or imbalanced humors, we now know that psychiatric symptoms are often caused by changes in the brain. Read on to learn about the people who helped us understand the brain as the driving force behind our behaviors.
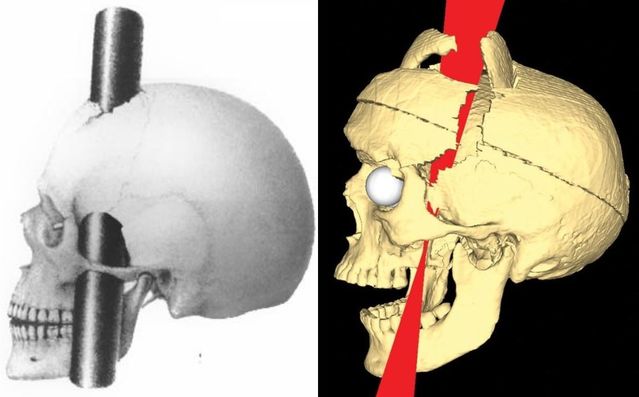
Phineas Gage
In 1848, John Harlow first described the case of a 25-year-old railroad foreman named Phineas Gage. Gage was a "temperate" man: hardworking, polite, and well-liked by all those around him. One day, Gage was struck through the skull by an iron rod launched in an accidental explosion. The rod traveled through the prefrontal cortex of his brain. Remarkably, he survived with no deficits in his motor function or memory . However, his family and friends noticed major changes in his personality . He became impatient, unreliable, vulgar, and was even described as developing the "animal passions of a strong man." This was the first glimpse into the important role of the prefrontal cortex in personality and social behavior (David, 2009; Thiebaut de Schotten, 2015; Benjamin, 2018).
Louis Victor Leborgne
Pierre Broca first published the case of 50-year-old Louis Victor Leborgne in 1861. Despite normal intelligence , Leborgne inexplicably lost the ability to speak. His nickname was Tan, after this became the only word he ever uttered. He was otherwise unaffected and seemed to follow directions and understand others without difficulty. After he died, Broca examined his brain, finding an abnormal area of brain tissue only in the left anterior frontal lobe. This suggested that the left and right sides of the brain were not always symmetric in their functions, as previously thought. Broca later went on to describe several other similar cases, cementing the role of the left anterior frontal lobe (now called Broca’s area) as a crucial region for producing (but not understanding) language (Dronkers, 2007; David, 2009; Thiebaut de Schotten, 2015).

Auguste Deter
Psychiatrist and neuropathologist Aloysius Alzheimer described the case of Auguste Deter, a 56-year-old woman who passed away in 1906 after she developed strange behaviors, hallucinations, and memory loss. When Alzheimer looked at her brain under the microscope, he described amyloid plaques and neurofibrillary tangles that we now know are a hallmark of the disease that bears his name. This significant discovery was the first time that a biological molecule such as a protein was linked to a psychiatric illness (Shorter, 1997; David, 2009; Kalia & Costa e Silva, 2015).
In 1933, Spafford Ackerly described the case of "JP” who, beginning at a very young age, would do crude things like defecate on others' belongings, expose himself, and masturbate in front of other children at school. These behaviors worsened as he aged, leading to his arrest as a teenager . He was examined by Ackerly who found that the boy had a large cyst, likely present from birth, that caused severe damage to his prefrontal cortices. Like the case of Phineas Gage, JP helped us understand the crucial role that the prefrontal cortex plays in judgment, decision-making , social behaviors, and personality (Benjamin, 2018).
HM (Henry Gustav Molaison)
William Scoville first described the case of HM, a 29-year-old man whom he had treated two years earlier with an experimental surgery to remove his medial temporal lobes (including the hippocampus and amygdala on both sides). The hope was that the surgery would control his severe epilepsy, and it did seem to help. But with that improvement came a very unexpected side effect: HM completely lost the ability to form certain kinds of new memories. While he was still able to form new implicit or procedural memories (like tying shoes or playing the piano), he was no longer able to form new semantic or declarative memories (like someone’s name or major life events). This taught us that memories were localized to a specific brain region, not distributed throughout the whole brain as previously thought (David, 2009; Thiebaut de Schotten, 2015; Benjamin, 2018).
Facebook /LinkedIn image: Gorodenkoff/Shutterstock
Benjamin, S., MacGillivray, L., Schildkrout, B., Cohen-Oram, A., Lauterbach, M.D., & Levin, L.L. (2018). Six landmark case reports essential for neuropsychiatric literacy. J Neuropsychiatry Clin Neurosci, 30 , 279-290.
Shorter, E., (1997). A history of psychiatry: From the era of the asylum to the age of Prozac. New York: John Wiley & Sons, Inc.
Thiebaut de Schotten, M., Dell'Acqua, F., Ratiu, P. Leslie, A., Howells, H., Cabanis, E., Iba-Zizen, M.T., Plaisant, O., Simmons, A, Dronkers, N.F., Corkin, S., & Catani, M. (2015). From Phineas Gage and Monsieur Leborgne to H.M.: Revisiting disconnection syndromes. Cerebral Cortex, 25 , 4812-4827.
David, A.S., Fleminger, S., Kopelman, M.D., Lovestone, S., & Mellers, J. (2009). Lishman's organic psychiatry: A textbook of neuropsychiatry. Hoboken, NJ: Wiley-Blackwell.
Kalia, M., & Costa e Silva, J. (2015). Biomarkers of psychiatric diseases: Current status and future prospects. Metabolism, 64, S11-S15.
Dronkers, N.F., Plaisant, O., Iba-Zizen, M.T., & Cabanis, E.A. (2007). Paul Broca's historic cases: High resolution MR Imaging of the brains of Leborgne and Lelong. Brain , 130, 1432–1441.
Scoville, W.B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiat., 20, 11-21.

Melissa Shepard, MD , is an assistant professor of psychiatry at the Johns Hopkins School of Medicine.
- Find Counselling
- Find a Support Group
- Find Online Therapy
- United Kingdom
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting

Health | Judge strikes down North Carolina restrictions…
Share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
e-Pilot Evening Edition
- Latest Headlines
- Environment
Health | Judge strikes down North Carolina restrictions on abortion pills. Here’s what it means for access.

A federal judge issued her final ruling Monday in a lawsuit by an OB-GYN challenging state regulations on medication abortion.
The ruling marks the potential end of a months-long lawsuit and means:
- Mifepristone does not need to be taken in a clinical setting and can be taken in one’s own home.
- The pill can be provided by pharmacies and not solely by licensed physicians.
- A follow-up appointment is not required, but an advance consultation is.
Here’s a look at what else the ruling means.
What was the lawsuit about?
The lawsuit was filed by Dr. Amy Bryant of UNC Health in January 2023. This is the same month that the U.S. Food and Drug Administration approved a protocol for certified pharmacies to provide mifepristone directly to patients.
This approval opened access to the drug for people beyond clinical settings and for delivery by a mail-order pharmacy with a valid prescription. Mifepristone is the first part of a two-pill regimen for the termination of a pregnancy within the first 10 weeks of gestation.
Bryant challenged the state’s regulations on mifepristone. More specifically, she challenged state laws in place around prescribing the pill, such as requiring doctors to provide the pill in-person at certified facilities, and after a 72-hour waiting period.
She argued that the FDA’s usage requirements, which are more lenient than state laws, preempt North Carolina’s restrictions.
Who were the parties in the case?
Bryant filed the lawsuit against the state’s top lawyer, Attorney General Josh Stein, a Democrat. She also named Kody Kinsley, the secretary of North Carolina’s Department of Health and Human Services, as well as a district attorney and members of the state medical board as defendants in this case.
After Stein recused himself from the lawsuit — opting instead to argue against the state laws in a legal brief — House Speaker Tim Moore and Senate leader Phil Berger were allowed to join the lawsuit in defense of the laws.
In a court filing, attorneys for Berger and Moore said that the lawsuit “seeks to eradicate important state-law protections for unborn children and their mothers’ health and welfare,” and that the FDA does not have the final say over “one of the most divisive and consequential social and political issues of our day and the past fifty years.”
What did the judge decide?
U.S. District Judge Catherine Eagles, tasked with reviewing the case, had previously issued a detailed opinion in early May granting a partial victory to Bryant.
In that opinion, Eagles argued that several state abortion laws on medication abortion went against a congressional mandate that the FDA create the regulatory framework for safe drug distribution and use.
On Monday, Eagles issued a judgment and permanent injunction, essentially a final court order requiring compliance by the parties involved.
In this determination, Eagles ruled that the following provisions are preempted by federal law:
- Prohibitions on health care providers other than physicians providing mifepristone.
- Requirements that mifepristone be provided in-person.
- Requirements that an in-person follow-up appointment be scheduled, or that efforts be made to ensure such a follow-up appointment.
- Requirements on reporting non-fatal complications caused by the drug to the FDA.
- Eagles ruled that not only were the state laws specifically cited in the lawsuit preempted by the FDA, but also any other similar provisions in state law.
Eagles wrote that enforcement, penalization, or requiring compliance with any of the provisions is prohibited.
She also held that requirements not preempted by the FDA, such as requirements for an in-person, 72-hour advance consultation and blood type testing, were allowed to stand.
NC Reality Check is an N&O series holding those in power accountable and shining a light on public issues that affect the Triangle or North Carolina. Have a suggestion for a future story? Email [email protected]
©2024 The Charlotte Observer. Visit charlotteobserver.com. Distributed by Tribune Content Agency, LLC.
More in Health

Education | EVMS, ODU celebrate long-awaited merger to create Virginia’s largest health sciences center

Health | Safety-net health clinics cut services and staff amid Medicaid ‘unwinding’

National Politics | Wins at the ballot box for abortion rights still mean court battles for access

Health | Push for defibrillators in schools becomes personal
Trending nationally.
- Is California the worst state for fast food operators?
- Sick checklist linked to Rex Heuermann, newly accused of 2 more slayings in Gilgo Beach case
- 3 moms sue Florida, saying state only helps parents who want books banned
- What’s up with the canceled tours and slow ticket sales for arena concerts?
- Giant Joro spiders could arrive in Massachusetts this year: ‘There’s no stopping them’

IMAGES
VIDEO
COMMENTS
Source: By Henry Jacob Bigelow; Ratiu et al. Phineas Gage. In 1848, John Harlow first described the case of a 25-year-old railroad foreman named Phineas Gage. Gage was a "temperate" man ...
In the United State alone, there are approximately 1.5 million traumatic brain injuries (TBI) per year, and TBI is the leading cause of death among individuals under the age of 45 [ 1, 2 ]. Annually, these injuries result in approximately 50 000 deaths and about 80 000-90 000 cases of debilitating head injuries [ 2 ].
The case of Phineas Gage has been of huge interest in the field of psychology and is a largely speculated phenomenon. Gage suffered a severe brain injury from an iron rod penetrating his skull, which he miraculously survived. After the accident, Gage's personality was said to have changed as a result of the damage to the frontal lobe of his brain.
On September 1, 1953, time stopped for Henry Molaison. For roughly 10 years, the 27-year-old had suffered severe seizures. By 1953, they were so debilitating he could no longer hold down his job as a motor winder on an assembly line. On September 1, Molaison allowed surgeons to remove a thumb-sized section of tissue from each side of his brain.
In the case of AD, cortical hub regions with high intrinsic functional connectivity to other regions across the brain appear to have high metabolic rates across the lifespan and to be foci of convergence of amyloid-β and tau accumulation (33, 34). Tau NFT pathology appears to spread temporally along connected networks within the brain .
In 1848, an iron bar pierced his brain, his case providing new insights on both trauma and recovery. Imagine the modern-day reaction to a news story about a man surviving a three-foot, 7-inch, 13½-pound iron bar being blown through his skull — taking a chunk of his brain with it. Then imagine that this happened in 1848, long before modern ...
Phineas Gage. One day in 1848 in Central Vermont, Phineas Gage was tamping explosives into the ground to prepare the way for a new railway line when he had a terrible accident. The detonation went ...
The brain's selective recruitment. In a 2022 study of another individual who also had an iron rod go through his skull—whom the researchers referred to as a "modern-day Phineas Gage"—it was found that the brain is able to selectively recruit non-injured areas to help perform functions previously assigned to the injured portion. Work ...
Background. Traumatic brain injury (TBI) is a key cause of death and disability in young adults and is becoming more prevalent. 1 While advances in prehospital care have reduced all-cause mortality following major trauma, a reduction in mortality following TBI has not occurred since 1994. 1 Intracranial pressure (ICP) and cerebral perfusion ...
Conclusions and Relevance: This case study investigates the structural effects of traumatic brain injury for the first time using pre-injury and post-injury 7 Tesla MRI longitudinal data. We report findings of initial volumetric changes, decreased structural connectivity and reduced microstructural order that appear to return to baseline 8 ...
Post mortem examination showed injury to the same area of the brain as seen in other case studies of a similar type of aphasia. Autism. Jebediah Buxton: Man with savant skills for mathematical calculations. Brain Injury. Phineas Gage: Man who survived after a large metal rod was sent flying through his skull and brain. Following the injury, he ...
Further support for this brain-behavior correlation came from MRI studies carried out in the 1990s, which gave a more accurate picture of the specific structures that were excised and spared in H.M.'s brain. The postmortem studies reported here provide new details of his lesions that could not be gleaned from the in vivo imaging studies.
Thus, MRI is a safe, painless, and non-invasive medical imaging technique [3]; however, it may take longer to perform a brain scan, as compared to other medical imaging modalities, such as Computed Tomography (CT) [4], although CT is a technique that leverages ionising radiation. This case study describes the case of a 40-year-old male with ...
7.1. Case Study 1. Jason, an undergraduate student at a large state university, is walking back to his apartment after a late night of studying chemistry in the library when he is stopped by the local (non-university) police. They tell him they discovered a deliberately broken window off-campus near the library, and as the only person they ...
Case Study 1 - The man with no memory Henry Molaison, known by thousands as "H.M.", is probably the best known single patient in the history of neuroscience. His severe memory impairment, which resulted from experimental brain surgery to control seizures, was the subject of study for five decades until his death in December 2008.Read More
Health 13 July 2016. By Fiona MacDonald. (Michael Horton/Flickr) A French man who lives a relatively normal, healthy life - despite damaging 90 percent of his brain - is causing scientists to rethink what it is from a biological perspective that makes us conscious. Despite decades of research, our understanding of consciousness - being aware of ...
Written by world-leading researchers and considering the latest theory and techniques in the field, each case study provides a description of the patient's history, how their memory was assessed and what conclusions can be made in relation to cognitive models of memory. Edited by Sarah E. MacPherson and Sergio Della Sala, Cases of Amnesia is a ...
Case Study: The Brain. Interactive Link. Click on the link below to access the case study created by Tangi Mitchell and Cheryl L. Watson of Central Connecticut State University. Biological Sciences Central Connecticut State University.
This case study explores the current trends in MRI neonatal brain imaging and advancements being made in this field. Introduction Magnetic resonance imaging (MRI) for premature neonatal brain injuries (PNBI) is a recurrent request that we face at many clinical centres in Australia and New Zealand in my experience.
ORLANDO, Fla., June 04, 2024 (GLOBE NEWSWIRE) -- Aviv Clinics, one of the world's most advanced brain clinics, published the results of a new case study which show a unique hyperbaric oxygen ...
The new report says that some health effects of Long COVID, including chronic fatigue and post-exertional malaise, cognitive impairment (sometimes referred to as "brain fog"), and autonomic dysfunction, can impair an individual's ability to work or attend school for six months to two years or more after COVID-19 infection.
In case of brain metastasis (BM) detect during follow-up, stereotactic radiosurgery (SRS) will be recommended if patients meet the eligibility criteria. Based on our post-hoc analysis of a prospective study, we hypothesize that in limited-stage SCLC patients with CR after definitive chemoradiotherapy, and ruling out of BM by MRI, it would be ...
We used data from the Longevity Improvement and Fair Evidence Study, which included medical and long-term care claims data from April 2014 to December 2020 across 17 municipalities. Using a nested case-control design, we matched one case to five controls based on age, sex, municipality, and cohort entry year.
More studies are needed to determine whether vitamin D supplementation may reduce the risk of certain cancers. Cognitive health. Research shows that low levels of vitamin D in the blood are associated with cognitive decline. However, more studies are needed to determine the benefits of vitamin D supplementation for cognitive health.
Researchers are working to understand which people or groups of people are more likely to have Long COVID, and why. Studies have shown that some groups of people may be affected more by Long COVID. These are examples and not a comprehensive list of people or groups who might be more at risk than other groups for developing Long COVID:
Background: Scientific studies on severely injured patients commonly utilize the Abbreviated Injury Scale (AIS) and the Injury Severity Score (ISS) for injury assessment and to characterize trauma cohorts. However, due to potential deterioration (e.g., in the case of an increasing hemorrhage) during the clinical course, the assessment of injury severity in traumatic brain injury (TBI) can be ...
Source: By Henry Jacob Bigelow; Ratiu et al. Phineas Gage. In 1848, John Harlow first described the case of a 25-year-old railroad foreman named Phineas Gage. Gage was a "temperate" man ...
The laws for North Carolinians seeking to take a pill to end a pregnancy are changing. A federal judge issued her final ruling Monday in a lawsuit by an OB-GYN challenging state regulations on medi…