

How To Get Clinical Research Associate (CRA) Experience
- by Kunal Sampat
- July 5, 2018
- in Clinical Operations

Do you want to start a career in clinical research? Do you want to get a Clinical Research Associate (CRA) job in ANY organization?
Well, you’ve landed in the right place.
There are a ton of CRA jobs out there. But there is one major concern most employers have with entry-level candidates.
Employers want applicants to have relevant CRA experience. Your resume doesn’t even make it to the hiring manager’s desk because you don’t have two years of CRA experience.
How are you able to get CRA experience when they don’t really teach you to become a CRA in college?
- You’ve gone through four years of college education and have a degree engineering, science or management
- You’ve spent years working as a foreign doctor or even had your own private practice as a clinician
- You’ve successfully completed a certification in clinical trial management
You certainly have the right skills for a CRA role. But you’re finding it almost impossible to get your foot in the door.
And let’s be honest. Most people in clinical research learn how to be a CRA on the job. Through some mysterious way, they get hired and trained to become a CRA.
In this article, I’ll share with you everything I know that will help you build your clinical research resume. Pick 1-2 opportunities from this list below and follow all the steps in my BEAVER Method – Get A Clinical Research Job and you’re golden!
Ultimately, my goal is for you to get a paid CRA job in any organization of your choice.
So let’s get started
- Hospital or Clinic Volunteer

This is my favorite strategy to get CRA experience. It’s also exactly how I got my first break in the industry.
Believe it or not, most large hospital or clinics have an official volunteering program. The volunteer program mainly geared towards retired individuals who want to give back to the community. But that doesn’t mean you can’t qualify.
There are two ways you can volunteer at a local hospital or clinic. I’ll cover each one in-depth.
Traditional Volunteer
This is the type of volunteering role where you’re cleaning hospital beds and putting new sheets after the patient leaves.
Or you may be tasked to move patients from the emergency room to the radiology department for a CT scan or X-Ray.
Or you may be simply stuffing envelopes with letters to hospital donors.
There is no research involved. However, these internships are equally for someone who has close to zero experience in the medical field.
Such volunteer roles are quite easy to secure. All you have to do is fill out the volunteering application form to indicate your interested and availability to serve as a volunteer. Make sure you fill the forms accurately.
Generally speaking, you’ll want to commit at least 4-6 hours each week and be willing to serve the community and patients. It’s not about you or your desire to get CRA experience. It’s about the patients.
You volunteering application will be accepted in a few days. Next, you’ll be asked to complete simple medical exams to ensure you’re a safe human being with no diseases that pose a threat to the patients.
Once your test results are in and you’re all clear, you’ll get volunteer badge and become an official volunteer.
It’s really that simple.
Now you’re wondering, “How does being a hospital volunteer help me become a CRA?”
My short answer: The foundation of any clinical research job is to serve patients.
By working closely with patients, physicians and nurses, you’re unknowingly absorbing clinical knowledge that you otherwise wouldn’t be able to do so.
You’re also building a valuable skill of being able to work with doctors and nurses. These are the same individuals that serve as site investigators and research coordinators on clinical studies.
Once you’ve been a traditional volunteer for 3-4 months, you’ll have relevant clinical experience that you can list on your resume.
You want the recruiter and hiring managers to get a sense of how your volunteering work had an impact on the clinical practice, the things you learned or observed and the influence you had on patient’s lives.
Clinical Research Volunteer
I consider this as a special and lesser known volunteer path.
Many practicing physicians have a deep desire to conduct research. But they don’t end up publishing research papers or presenting at conferences.
Because seeing patients is a full-time job. For instance, a family practice physician in the United States has to see a minimum of 18-22 patients in an 8-hour day. This leaves little or no time to conduct research.
The doctors don’t have enough time on their hands to write a protocol, make an IRB submission, review medical records or charts, or perform analysis on the clinical data.
But this is where you come into rescue. Follow these five simple steps and get relevant clinical research experience.
Step 1: Determine which therapeutic areas you’re interested in. If you’re not sure, I’d recommend cardiology, oncology or diabetes.
Step 2: Next, you want to identify doctors working in those specialties. You can find these doctors by looking at the local hospital website.
Step 3: After identifying potential doctors you want to work with, send each of them a personalized email an expressing your interest in being a research volunteer.
Step 4: After a few email exchanges and probably an in-person interview with the doctor, you’ll land with you dream clinical research volunteer role.
I’ve actually followed this exact process and was able to get one publication and one presentation on neonatal care.
Step 5: Once your research paper or presentation is complete, you can add it to your resume and brag about it to recruiters and hiring managers.
- Intern at a Fortune 500 Company

Most of us understand the value of an internship. It’s a great way to get relevant clinical experience and probably making a bit of money on the side.
Internship at a Fortune 500 healthcare company such as Pfizer, Abbott, Merck, Apple and even Google (yes, Apple and Google have healthcare products) can be a very rewarding experience.
These are also most sought-after internships. But that doesn’t mean you can’t get in.
Pros/Cons of Internship Roles at Fortune 500 Companies
- Excellent clinical training opportunity. You get to learn and implement standard operating procedures, application of GCP in real-world studies, working with cross-functional teams and more
- You will most likely get paid for your time as an intern
- A structured application process requires advanced planning. You will generally to apply 9-12 months prior to the target internship start date
- The internship program is mostly geared towards full time or part-time students
- Highly competitive (but hey, competition can make things better)
Resources: Fortune 500 Healthcare Company Internship and Coop Programs
Here is the current list of Fortune 500 companies .
You can apply filters such as “Industry – Pharmaceuticals” to identify healthcare companies.
Links to a few Co-op and Internship Programs at Pharmaceutical, Biotechnology, and Medical Device Companies
Johnson & Johnson
Pfizer’s US Summer Student Intern Program
Merck’s career program for interns, coops and recent grads
AbbVie’s US Student Internship Program
Gilead Sciences Internships
Eli-Lilly Student Opportunities
Amgen Internships and Coops
Bristol-Myers Squibb Internship Programs
Biogen Internship and Coop Programs
Abbott Internships and Development Programs
- Work at a Healthcare Start-up

This strategy is an extension of securing an internship role at Fortune 500 healthcare companies.
When most people think of jobs or internships, multinational corporations come to the top of our mind. However, the Fortune 500 list is limited to the top companies.
There are thousands of other companies that operate in the healthcare space but do not make it to the Fortune 500 list.
A quick search on Angel.co revels that there are at least 18,956 healthcare startups . This list doesn’t even account for established companies that don’t make it to the Fortune 500 list.
A position at a healthcare startup that sells medical products or services is an excellent opportunity to not only learn more about healthcare space but have a greater impact in society.
Here is why I think a healthcare startup will love you:
- Most start-up companies are resource strapped. Resources could be money, time, or people. Therefore, if a startup can acquire an extraordinary talent such as yourself, they can save one or more of these resources
- Most start-ups are trying to grow and spread their message. If you can contribute towards the company’s goals and help them get one step closer to the finish line, they have no choice but to fall in love with your passion and commitment towards their organization.
Pros/cons of working at a healthcare startup
- You get to wear different hats at a healthcare start-up. One day you’ll be working on a new clinical trial and the next day you’ll be packing boxes to ship medical products to a clinical trial site. Such experience is of much value to companies hiring for CRA positions
- You’ll be presented with opportunities to work on important problems. If you can jump on these opportunities and show that you can execute and deliver results, you can accelerate your clinical research career
- Unlike Fortune 500 healthcare companies, start-ups are lesser known to job seekers. This makes it relatively easy to secure an internship, full-time or part-time position
- Your work objectives may not be as structured as a Fortune 500 company. You will probably be on your own and will need to figure things out by asking questions to people or finding clinical resources on government websites such as FDA.gov .
- There won’t be an established internship application process. You’ll need to email and call the CEO or head of clinical or R&D to get your foot in the door
- Your role may not be limited to clinical research. You’ll be expected to work on non-clinical tasks or goals
- You may or may not get paid. It depends on what you can negotiate
Resources: Working for Healthcare Startups
My favorite resource to research healthcare companies is biospace.com . It is a comprehensive list of biotechnology, pharmaceutical, and medical device companies. Similarly, you can look up healthcare companies on angel.co .
Just start going through the company websites one-by-one, find out the CEO or vice president of clinical and begin contacting them for potential internship opportunities.
I know it’s a tedious process.
Plus there is a fear-factor of sending cold emails to people you don’t know. That’s precisely why very few people are doing this.
But if you want to become a CRA, this is your best shot to become one.
- Work for a Clinical Research Site or Site Network

Similar to a volunteer at a hospital or clinic, you can apply to work at a clinical research site or a clinical research site network.
To begin, let’s get some definitions out of the way.
A clinical research site is a location where a clinical trial is conducted.
It is generally a place where the site investigator and research coordinators see potential and current clinical trial patients, store regulatory binders, maintain patient medical records, place where CRA performs monitoring activities and more.
A research site can be independent, standalone office or part of a larger hospital system.
Many current clinical research professionals, which includes CRAs, started their careers as a clinical research coordinator (CRC) at a trial site.
Hospitals and clinics are looking for CRCs to enroll clinical trial patients, perform medical chart review or perform tasks such as data entry in clinical databases. This experience is in many ways the foundation of many CRAs in the industry.
It is important to note that although many CRCs are Registered Nurses (RN), you don’t need to be an RN to become a CRC.
On the other hand, a clinical research site network, is a network of sites and it means just that.
Sites will form networks or belong to paid networks to reduce fixed costs such as marketing and business development, provide a consistent experience to trial sponsors and CROs, and/or meet the highest level of clinical research standards.
Pros/cons of working at clinical research sites or site networks
- One of the best ways to understand the inner workings of a clinical research site
- Opportunity to work with multiples sponsors, CROs and patients. You’ll not only gain valuable experience but also grow your network, which can ultimately help you secure a full-time CRA job
- Highly sought-after experience for companies looking for CRA or CRA assistant candidates
- Given the academic and research-driven focus, internship stipends or salaries may be lower than industry jobs (sponsor/ CRO)
- If you’re an intern, you may not be able to work directly with patients due to privacy reasons or site policy. Instead, you’ll be assigned menial jobs such as filing regulatory documents or subject case report forms, all of which you should undertake with pride
Resources: How do you find a clinical research site?
Finding clinical research sites is a bit tricky.
For independent clinical research sites, you’ll have to rely on Google search or word of mouth.
Search of the terms “clinical research site + [your city/state/country].” If the site has a web presence, you will likely find them.
Large hospitals, particularly medical schools, are involved in clinical trials and have a clinical research department.
You basically contact the director of their clinical research or human resources (HR) department and ask them about career opportunities in their department.
Resources: How do you find a clinical research site network?
There are quite a few site networks but the sites that participate in these networks are sometimes not publicly available.
Well, because some site networks charge sites a flat annual fee to belong to their site research network or the site network charges sponsors get access to their sites. Someone has to pay for this information, it’s either the sites or sponsors.
Clinical Research Network has a public listing of sites in their network. You can reach out to some or all of these sites and ask them about potential career opportunities in research.
Center Watch also has a list of sites by geographic location
Research Match is an NIH funded initiative that connects patients and researchers. There are some big institutional names here. You can reach out to any of these sites and see if they are looking to hire or get volunteer help.
Platinum Research is another website where you can find a list of site research networks. Click on the “company websites” to get information on research sites in your area.
- Work at a Site Management Organization (SMO)
Similar to other career options we’ve discussed so far, working at a SMO is a great way to secure CRA experience.
A SMO is responsible for managing day-to-day trial management activities at a research site. Simply stated, the role of the SMO is to fill the gaps at a site.
For instance, a site may not have a dedicated research coordinator. This is where the SMO comes in play. Some sites have their own preferred SMO whereas other sites may be assigned a SMO by the clinical trial sponsor or clinical research organization (CRO).
Working at an SMO is in many ways similar to working at the site as described earlier. 12 – 24 months of SMO experience will generally be sufficient to meet the “two-year” CRA experience most sponsors or CROs require.
Pros/cons of working at a SMO
- Lesser known career opportunities, so your chances of getting hired are quite good
- Opportunity to truly understand the challenges faced by clinical research sites. This experience is valuable for anyone interested in becoming a CRA
- SMOs are more common in Asian countries such as Japan, India, Taiwan, and China. If you’re looking for international clinical research experience, this might be one the best ways to get it
- US-based SMOs are not as common and it can be challenging to come across a SMO closer to where you currently live
- Many SMOs are regional as they serve sites in their area
Resources: How to find a Site Management Organization?
Below are a few U.S. based SMOs
CMX Research Partner
PMG Research
CSSi Lifesciences
Consolidated Clinical Trials
- Work at a Clinical Research Organization (CRO)

A CRO provides clinical research services to sponsors. They are also known as Contract Research Organizations.
Today CROs are more popular than ever. Many Sponsor companies, large and small, don’t have the in-house clinical expertise to conduct a study. This is where a CRO comes into the picture.
A CRO provides the Sponsor the clinical resources i.e. people needed to execute on a clinical strategy. By hiring a CRO, the Sponsor isn’t stuck with permanent headcount, should they decide to abandon a medical product or terminate a clinical trial.
CRO size and specialty can vary. For instance, there are niche CROs that only serve certain therapies, regions, or types medical products such as a CRO specializing in medical device research.
Pros/cons of working at a CRO
- More likely to hire individuals with little or no clinical research experience
- Opportunity to work on multiple therapeutic areas, medical products, and Sponsor companies
- Many CROs offer on the job training programs to help you with your professional development
- Service-based nature of CROs can lead to a stressful work environment when working with demanding Sponsors
- CRO personnel assignments are generally reviewed and approved by Sponsors. You may not get to work on your dream project if a Sponsor things you are not qualified, especially if you’re a newbie
- Pay for entry-level positions will likely be low. But if you truly care about CRA experience, the pay shouldn’t matter when you’re starting out. Build CRA relevant experience and then take on another opportunity within or outside the CRO
- Job descriptions may list a minimum two-year experience requirement, but I encourage you to apply to these junior level positions such as CRA assistant or clinical site coordinator/associate
Resources: How to find a Clinical Research Organizations?
Below are a few global CROs for you to consider:
PRA Health Sciences
Another option for finding CROs is First Clinical’s Supplier Directory , that lists several the companies that provide contract clinical research services.
- Intern at a Government (Regulatory) Agency

Government agencies play a crucial role in clinical research. They review and approve medical products. Their primary concern is patient safety.
Such organizations include the US Food and Drug Administration , competent authorities in the European Union such as Germany’s BfArM or China FDA .
Government laws and regulations form the foundation for any company wanting to obtain medical product approval for commercialization. The laws and regulations also apply to service-based companies such as CROs and SMOs and clinical trial sites conducting research.
Every clinical organization must follow the law and stay compliant. This is what makes working for government agencies exciting. You can learn things that truly matter when it comes to clinical research. You can then apply these learning as you advance in your clinical research career.
Pros/cons of an internship at a Government (Regulatory) Agency
- You’ll learn a lot about health care regulations, patient safety and how decisions are made inside government agencies. This is an incredibly valuable experience to have early on in your career
- Highly regarded among CRA hiring managers and recruiters
- Many opportunities will be unpaid or may come with a small stipend. If your primary objective is to gain valuable research experience, taking on volunteer opportunities within government organization is a great way to build your resume
- Certain organizations may require you to be a citizen of the country you’re interested in working for
Resources: Government (Regulatory) Agencies
Check your local country, state, county or city website to find out which department or division of the government is responsible for overseeing healthcare projects such as clinical trials.
Food and Drug Administration has an unpaid student volunteer program . I was pleasantly surprised to learn that this program is open for non-US citizens including permanent residents and visa holders.
You can also volunteer at FDA field offices, so you don’t need to relocate to Maryland. But if you can work at the FDA headquarters, that is probably the best as you’ll have access to more people and resources.
Other notable government or government-funded organizations are as follows:
- National Institutes of Health (NIH) has a program for students and recent graduates
- Centers for Disease Control and Prevention has an amazing program for students and college graduates
- U.S. National Library of Medicine has career opportunities for you to consider
- World Health Organization (WHO) , an agency part of the United Nation also has an internship program
- Work For A Nonprofit Focused On Healthcare Initiatives

Nonprofit organizations existing to fill gaps that the private and public sector do not or are unable to address. Nonprofits are mission-driven organizations.
There are thousands of nonprofits in the United States that are focused on healthcare initiatives and medical research. Such nonprofits include clinical research associations such as Association of Clinical Research Professionals , and American Heart Association .
Many people looking for CRA experienced are focused on CROs and Sponsor companies. But there are many lesser-known nonprofits that are looking for people like to you to work in the healthcare sector.
Your first job at these nonprofits will probably not a CRA position but you’ll gain valuable experience that will allow you to transition to a CRA position in the future.
Pros/cons of working at a nonprofit
- Nonprofits are generally looking for motivated individuals to support their mission
- Prior clinical research experience won’t be required in most organizations
- Excellent opportunity to take on meaningful work that isn’t focused on the financial bottom line. For-profit organizations are primarily driven by money and the work itself may not be as fulfilling
- A nonprofit may not be able to afford high salaries. You may be given a stipend or just enough money to survive
Resources: How to find Nonprofits with Healthcare Initiatives?
- First Clinical has one of the most comprehensive clinical research association directory listing . Many of these industry associations are probably nonprofits. Just start going through these associations one by one, reach out the Executive Director of each of these organizations and inquire about volunteering, full-time or part-time opportunities
- Guidestar is the world’s most comprehensive database for nonprofits. You can search for nonprofits in “Medical Research” or “Health” category and sort by “Gross Receipts”. If you start with organizations that have funding, they are more likely to hire you for a paid position
- Similar to Guidestar, there is Charity Navigator , where you can perform an Advanced Search for nonprofits in the Health → Medical Research category
- Health Finder , which was developed by the Department of Health and Human Services has a listing of nonprofit organizations in the healthcare field
- Invest In Your Learning

One of the best ways to get CRA experience is to invest in your learning. You can take on a certification program with organizations such as ACRP or SOCRA .
There are also paid and free online courses that you can take with ACRP , free GCP certification with NDAT CTN Training, or clinical research courses on Coursera .
However, knowledge through certifications or courses in and by itself is not sufficient to get a CRA role. You need to “learn” through practical experience.
You can also consider attending or volunteering at clinical research conferences hosted by companies such as ExL events , CBI events , ACRP or SOCRA . Such conferences allow you to network with like-minded people, which in turn creates opportunities for you and everyone else attending these events.
Finally, I would do a disservice to this article if I did not mention the important soft skills you need to master on your way to becoming a CRA. Some soft skills include negotiation, active listening, not complaining, empathy and self-awareness.
We’ve covered 9 different ways you can gain CRA experience. Pick 1-2 opportunities from this list, and pursue them with your full focus and energy.
Apply the foundational principles I share in the BEAVER Method – How to Get A Clinical Research Job and you’ll be on your way to getting your dream clinical research position in any organization.
What are you planning to do next? Let me know if the comments section below.
The World of Program Management with Stephen Smith
Become an outstanding clinical researcher with dr. jeff kingsley, 69 thoughts on "how to get clinical research associate (cra) experience", overview of clinical research | clinical trial podcast, how directors can attract and develop clinical research associates, can you do clinical research with a phd – online phd program, how to prepare for the ccrp exam – zksnyder.com.
Extreme Night Owl
Very good information. Thank you!
Kunal Sampat
Thank you@E@extremenightowl:disqus Let me know if you have any specific questions. Happy to help.
Gunturu .Neeharika
Hi kunal, I’ve been reading articles about volunteer research work online and I just got ended up here. Firstly, thanks for this simple and detailed information . I’m an ent surgeon from India but settled in Sydney as my husband is an Australian citizen. Im on my visitors visa now for next 5 months and I do want to utilize this time gaining experience in / learning research work while waiting for my medical registration here in Australia. I want to enrol as a volunteer in clinical trials or research work and I’m new to this area so, it would be great if you could help me with this ( any idea about opportunities in Sydney) .Thanks neeharika .
Thanks for taking the time to read and comment. Glad to hear you find the information useful. I’m happy to help you and will send you an email with details.
kunal i have questiion. I am base sas certified, currently living in atlanta. last ten years i was busy looking after my kids and family. now i want to work as clinical sas progrmmer. i want to know the job prospects especially in atlanta. can you help me.
Hi Asma, Have you tried to apply the BEAVER Method towards your job search efforts? https://clinicaltrialpodcast.com/get-a-clinical-research-job/ What is your #1 challenge with identifying potential opportunities? – Kunal
Hi Kunal, Thank you for your great information. I am a medical school applicant doing my msc in medical physics. I was thinking of part time joining a clinical research group as well. I also would love to publish some papers on clinical research area as well. I am very skilled in data analysis and working with people. My background is biophysics and math. Do you have any idea how I could go about that? Thank you,
Hi Adina, Thank you for reaching out. Yes, I think there are many ways for you publish papers in clinical research. One recommendation I have for you is that you reach out to some medical doctors that are actively conducting clinical trials at a teaching hospital near your home. You can then collaborate with them on a research project as volunteer. Since you’re proficient at data analysis, you can help the medical doctor collect and analyze clinical data. This can be retrospective data. The doctor will know where to publish etc. and he/she will add you as an author on the paper. This is how I got my very first paper in clinical research. Hope this helps. Let me know if I can answer any additional questions.
Hi Kunal, I am research professional with experience of 8 yrs in clinical trials in laboratory, quality assurance and recent role which was in data management /administration. (volunteer).Despite of such a strong professional experience can’t get a break through in the field. Because of recent volunteer role get some interview calls but don’t have ethics application experience holds me back. I am desperately looking and applying for clinical research assistant role but could not get it.Have a good references too but don’t know where I’m lagging behind as feedback of interviewers says they have more experienced candidates who has recently done the program or role. Kindly share some advice. Thanks . Pradeep
Hi Pradeep,
Have you checked out my other blog on “How to Get A Clinical Research Job” Here is the link to the blog post. https://clinicaltrialpodcast.com/get-a-clinical-research-job/
Please give this method a try and let me know if you have any additional questions.
Hi kunal, Thanks for these important tips. I am graduate as dentist from India and then i completed my post graduate diploma in clinical research from Institute of Clinical Research India. Post completion of this course, I had 6 months training as clinical research manager at a CRO and then worked as CRA at PGIMER, Chandigarh, India on an oncology trial for 6 months. Currently I am on visitor visa in Melbourne, Australia with my husband who is PR here. I want to utilize this time in some training or education for 3 months as i cant work on visitor visa. Kindly guide me with best of your knowledge and experience. Email is [email address removed]
Wasim Kamate
Hii Neha, we too have completed MDS and postgraduate diploma course in clinical research in Maharashtra India..Please guide me further as to what can I do to get experience in clinical research and post of clinical research manager in India, more comfortably in Maharashtra. Please guide us on email: [email protected]
Hi Kunal,,, Please guide me….I am Dr. Wasim Kamate, MDS with postgraduate diploma course in clinical research in Maharashtra India..Please guide me further as to what can I do to get experience in clinical research and post of clinical research manager in India, more comfortably in Maharashtra
thanks for sharing great article with us keep doing great work
Thank you, Preeti
Hi Kunal! Thank you for the valuable information.Prior to reading this article, I graduated with a bachelors degree in science and have been trying to get clinical research coordinator positions but to no avail. Hence I took up a clinical research internship with a Therapeutics company that focuses on immunotherapy for cancer.I figured getting experience from this internship would help me with attaining a CRC position in the future. I have thought long about whether what I did was right as I know I won’t be fully focusing on research after this internship. Your article has truly given me hope 🙂 Thank you!
Glad to hear Anisha. Don’t give up hope but take action towards your goal. Good luck and keep me posted on your progress.
Wow what an incredible resource and well written article. I thank you very much Kunal!
Hi Kunal, I am a Commerce graduate and I did MBA in Finance and Supply chain management in 2011. I have changed my area of expertise a couple of times ranging from Banking and Finance, Retail, HR (compliance) and then ventured into data science in 2016 with a certificate in Big Data. I have been literally struggling to get a break in clinical SAS programming since this is my area of interest for a Full-time career option. I don’t know where to start. I appeared for an interview at PPD in March but did not clear it. I am due to appear for Clinical SAS programmer exam in the month of November but I am finding it extremely difficult to study the subject matter. I want a break in a CRO asap but dunno how and where to start. PPD had said that they are going to come up with a fresher opening where adequate training would be given but there has been no response. Please guide me. Many thanks. KG
Thanks for reaching out. A couple of things to consider: 1. I would reach out to the hiring managers at the various companies and jobs you’re interested in and feel qualified. You’ll need to use LinkedIn to find hiring managers. It is important that you contact people who are responsible for making hiring decisions. Don’t contact recruiters. 2. Get super organized around your job search. Focus on 10 job openings at a given time. You should consider creating a spreadsheet that lists this jobs and the name and phone number of the hiring managers. Then call those hiring managers and let them know you want to work for them. Get their advice and listen to their feedback. If you hear “no’s” change your job search strategy based on the hiring manager’s feedback.
To summarize, you need to focus on 10 positions at a given time and talk to the hiring managers. Your goal should be to get a “Yes” or a “No” from them. Don’t rely on PPD only.
Koyel Ghosh
Hi Kunal, I am writing to you to inform you that I am struggling to get a break in CRO for the last six months. I am BCom graduate and I have done MBA in Finance. I have experience in varied fields ranging from Finance, Banking, Retail, and finally Data science. I worked as a data scientist in a startup for 3months and I decided to pursue Clinical SAS as a career option. I have taken various training online Edex and Course-Era. I also completed certificate training from NIH and secured 88%. I am due to appear for clinical SAS exam in November. I am finding it extremely hard to secure a job in clinical SAS given the fact that I don’t have a life sciences background. Getting an internship is also so difficult. Please guide me.
Hi Kunal, Thank you very much for this incredible article, I’m a Foreing Medical Doctor and I have more than 2 years working as a CRC, I want to became a monitor,what do you advise to me to do.??… I would appreciate it very much!!..
AbeerSarwar
Hi, This article is so well written , i am a foreign medical graduate in usa , and i tried many of the resources mentioned in this article , all the websites i went to mostly required enrolled students in usa universities, what would be the best bet in this scenario , i live in florida and i asked around in hospitals near me where i couldn’t find anyone to be in research. thank you
biospherecro
Thanks for sharing it
Hi Kunal, thanks a lot for the tips and Information. I am a German. I have a masters degree in pharmaceutical biotechnology in Germany. Had to stay at home after that because of my kids. Because I wanted to work on my career, I decided to do the CRA course in Germany which I comleted in June 2018 in addition to a GCP-ICH Training. I just moved to Canada last December. I don’t really know how to go about job search here in Canada in the CRA field. Will be grateful if you can be of help. Sincerely yours
Hi Kunal, Thanks for this informative article regarding clinical research industry. I’m an international medical graduate in Sydney and l’m looking for a clinical research experience so that i can make an entry into this field, can you please guide me the options i can avail here in Sydney? Thanks!
Hi, I Would like to thank you for useful information before addressing my questions. The information which was mentioned is really helpful and informative. Coming to my queries, I need a guidance for getting into the Clinical research training. I have visited the sites who are providing training but unfortunately few applications are closed for summer. I stay in USA , I have a Post Graduate Degree in Pharmaceutical’s and I have a 2 years experience in India. But when I’m looking for opportunities here, I’m unable to get into the right path. Please guide me in choosing a right platform. Thanks & Regards, Anu
SRILAXMI KADAMBALA
Hi Kunal, Thanks a lot for useful information. I have done Masters in organic chemistry from kakatiya University , India.Here in Texas ,have worked as research assistant at Texas University for very short period. Drug chemistry and clinical trials are main major subjects in my studies. I am looking for CRA position and also attended 2 interviews, but all are asking for experience with patients and doctors. This article is very helpful to me. I will try to follow this. Thank you. Regards, Srilaxmi.
Hi Srilaxmi – great! keep me posted on your progress. Thanks! Kunal
Hi Kunal, Great article. Can you let me know your thoughts on the CRA online training provided by ccrpcourse dot com/? I am a research scientist and my company is giving me the opportunity to work on our upcoming trials. They will pay for the training course and I am trying to find one that is most inclusive, instead of 7 or 8 courses that will give me the experience. Please let me know your thoughts!
It is great and excellent article by you now I’ll chose some tips n tricks from this article that will help me out to get a cra job in india. Thank you so much. I have some query see I’m in india right now and want to wark in UAE as clinical research (crc/cra) if you have any idea about that how i go there and get an opportunity in good cro smo or hospitals and any article related it please share by mail. Thank you so much
Priyanka Thalluri
Thank you so much kunal
Ashwin Krishnamoorthy
Hi I am a medical graduate from India. I am looking for a medical field related job while I’m studying for the USMLE. What are the possibilities?
Hi Ashwin, You have multiple options to choose from. Check out this blog on the various roles in clinical research https://clinicaltrialpodcast.com/clinical-research/ . Find companies/ organizations that are hiring for the role you’re most interested in and then apply for the jobs.
Purval Yele
Hi Kunal This is very nice article which would certainly help all the people out there. I am thankful to you for the guidance. I am pursuing 2nd year Msc Clinical Research. Pls guide me whether to go for Phd? Which University would be good?
I’m a CVICU RN with a BSN and 5 years experience in healthcare plus previous 8 years of accounting experience. What would you suggest as the best route to break into the CRA profession. Every position I’m seeing is requiring 2 years of experience which I don’t have. Thank you.
Hi Liz, Your experience in the CVICU is SUPER-VALUABLE! And knowing about accounting (finance) is an equally valuable skill in clinical research. My question to you, “Why do you want to be a CRA?” I would recommend other roles such as clinical educator at a sponsor company where you’ll explain how a medical product works, clinical research scientist, clinical safety associate. These would be relatively easier opportunities for you to get your foot in the door. Hope this helps. Kunal
I just want to know how to initiate a clinical research? I am not a doctor or nurse or have any desire to become one I just think I may have discovered something and know that it if I am correct in my theory would benefit countless scores of women! Is there any way for me to suggest something be conducted or can I just throw my idea out to social media and get ppl to volunteer that way? It can b done at home no need for any exams or preemptive measures just a commitment to make one simple lifestyle change.
Hi Kelly, Starting a research study is an involved process. At a minimum, you will need IRB approval at the medical center where you plan to conduct research. You will likely also need a medical doctor as a site investigator to ensure there is patient safety and adequate study oversight. You can read more about clinical trials at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trials-guidance-documents . Take care, Kunal
Denise A MarulloCook
Great informative article…I now have a place to begin for Clinical Research Assistant. I have 35 yrs experience as a Psychiatric Nurse ( LVN) and a Certified Nursing Assistant. My licences have since expired, but my background is still relevant. I have done research for doctors for many years. Do I need a certificate for this? Will my medical background help me up the ladder? My age (61) seems to be a deterrent as it’s always part of an application. I am very organized, great at details, love research and earned a BA degree in English; I am excelent at written tasks. I’m also a published author. I really want this career and know I’m perfect for it. I do live in a small town which is dominated by Military bases and affiliations. I live in NC, but it’s far from the more prominent towns where big hospitals and universities prevail. Any suggestions??
palak patel
Hi, I m doing a job as a clinical research coordinator from India’s reputed hospital now i will move in USA so what should i do ? kindly request you to guide me. in India i did my B.pham. so please guide me.
Hi Palak, I’ve just sent you an email. Talk soon!
Paradzai C Tirivashoma
Hi. This article was a true eye opener. I’m a 36 year old Zimbabwean trained physiotherapist living in South Africa. I have tried in vain to obtain registration as a physiotherapist. It is government policy here to exclude recruitment from developing countries. I have developed an interest in clinical research and my prospects look promising once I get my foot in the door. What is the best way available to me to enter into this field?
Paradzai Tirivashoma
Hi Paradzai – Just start implementing one or more strategies I share in this post.
Hi..This article is more informative for students who are looking career in Clinical Research Industry. Thank You!
You are welcome!
Hi Kunal, thank you for such detailed information. I am a dental graduate from India and have recently moved to Canada few months back. I am looking for a medical/dental field research related job while I’m studying for my equilvalency exams. If you could suggest some possibilities?
Dear Kunal, Thank you so much for a great and very helpful article. It is awesome! I have MS degree in Biochemistry and Molecular Biology from Saint-Petersburg State University, Russia. I have been in States since 2006 (I am a citizen). I worked as biochemist at university, QC technician and R&D scientist/supervisor for a small biotech company, my last job was R/D scientist in start up pharma company (unfortunately they ran out of money). I am really interested in becoming CRC or CRA. I always was inspired by this field. Should I join a training program first? I am just trying to do my research and find the best way to start my carrier in Clinical Research. Please advice. Thank you again and best regards, Anna
Hema Ramaswamy
Very informative! I need some advice / confirmation if I’m in the right path. I have done MS in (OB/GYN) from India and have close to 10 yrs break due to family commitments. I’m not interested in USMLE. I’m looking to restart my career. My plan is to do a certification in clinical research, followed by internship or volunteering, thereafter do a CRA certification course. Does this sound ok? Any advice would be great. Thanks
Mehar Sulthana
I am a physician from India,with MD anatomy post graduation.At present residing in USA for 4yrs.Due to family circumstances unable to pursue my career here.Now planning to do CRA certification course.Have you heard of fast track clinical research in Arizona.Through google I learnt about their course and contacted them.Is it reliable to join.Any adivce plz?
Dear Kunal, Very informative article. thank you for sharing all this information. I am Ph.D. in Organic chemistry from India and currently working as an Assistant professor of Chemistry at a US University, but interested in making a transition to full-time research jobs. Clinical research seems a very interesting research field, as I am doing computer-aided drug designing research, so transitioning from the preliminary step to the final step of the drug discovery spectrum is fascinating. After relocating to Pittsburgh, I was just searching for volunteer opportunities and came across your blog. Very helpful information. Any personal advice to step foot in with my background will be highly appreciated. Thank you!!
Hi Kunal , My name is Gabriel from Ghana. I have a bachelor’s degree in Nursing. I am very interested in becoming a clinical research professional. I read your article recently about CRA and I am very interested . I am currently unemployed. I would be very glad to know you more and to contact you. Kindly give me the opportunity to get in touch with you and learn more from you. I need information on clinical research and I have some pressing issues. I received an admission to enroll in a course, but I am still not very clear about it. Please help me 🙏. Thank you in advance for your kindness. I hope to hear from you soon.
Hi Gabby, What specific questions do you have for me?
Hi Kunal, This is a very thorough article and I tend to revisit it whenever I’m in doubt. However, I needed some more guidance/ expert opinion with respect to my career path ,It’d be great if we could get in touch to discuss this. On a side note, what do you think of Masters programs in Clinical Research ( e.g. Master of applied clinical research St.cloud state university) ? How effective are they (in the US/ worldwide) in terms of clinical research education and for a clinical research job profile (specifically CRA) ?
If you invest your hard earned cash on any program and put in the work, you’ll see the results.
Salunke Ganesh
Hi Kunal I m MBBS dch md in pediatric from Mumbai I m practicing since 2002 I want to make my own CRO how much investment I need where can get complete information in mumbai for making CRO I do ve investors too is it lucrative than making hospital set up of 50 beded in mumbai please guide me Thanking you
Hi, i am a psychology major who just graduated this august 2021. How do I get my foot in the door? this was very helpful but for someone like me what is my best bet?
Wow. This is most comprehensive, yet simplistic and very-well explained. I feel like I am in a unique position, but after reading this I have so much more confidence in going forward with my educational plans and pursuing my dream career. I am a student again, after receiving a long-awaited kidney transplant. I was asymptomatic when diagnosed so for this reason, over time, I became extremely interested in learning how kidney disease and other life-altering diseases are diagnosed and treated. The week following my transplant, I enrolled in a AS degreed program for Clinical Research Professional., which comes with a CRC certificate as well. I am nervous that I won’t start my career with a BS degree, but with so many contacts in healthcare, I am quite confident that between my physicians and The Nat’l Kidney Foundation, which I have been working with and for whom I have been fundraising over the last few years, will help me in the area of experience while volunteering. After years of working in Customer Service and management, I am so excited to finally find a career that is a true fit for me. My question is will I be able to eventually transition into a CRA position after a certain amount of years of experience, or do I absolutely need to go back to school for a BS? I am planning to get into as many Professional Associations as I can and get certified wherever possible as well. Again, I am saving this article and I am looking forward to reading all of your insight on Clinical Research Careers. Sincerely, K. Araya
Getting a bachelors degree in your safest bet to being able to considered for the CRA role. It will open up many opportunities for you. Just look through some of the open opportunities for CRA roles, I bet many require a Bachelors degree. If that is the case, consider getting a Bachelors degree sooner than later.
Nethia Mohana Kumaran
Hi Kunal, I am in Perth, Australia and eagerly looking for either CTA or CTC positions. I am also open to volunteering. Could you please help with any opportunities? Thank you
Thanks for this wonderful Article, I have read and expanded my learning skills, but still find it difficult to get a CRA job. I am open for volunteers. I live in Atlanta Georgia. I will be glad if you can be of help. Thanks
This is so helpful. I call this the ABC of entry level to any profession, Well appreciated
You’re welcome, Chris!
Thank you for this wonderful resource into the field of clinical research. Current MD candidate looking for CRC/CRA roles.
IS THERE ANY AGE LIMIT TO START A CAREERIN CLINICAL RESEARCH?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
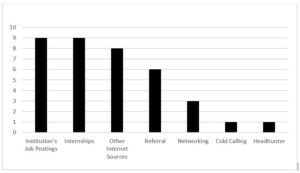
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
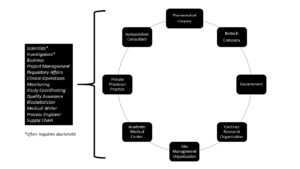
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
| Clinical Research Patient Recruiter | PPD | Bachelor’s degree and related experience |
| Clinical Research Assistant | Duke University | Associate degree |
| Clinical Trials Assistant | Guardian Research Network | Bachelor’s degree and knowledge of clinical trials |
| Clinical Trials Coordinator | Advarra Health Analytics | Bachelor’s degree |
| Clinical Research Specialist | Castle Branch | Bachelor’s degree and six months in a similar role |
| Clinical Research Technician | Rose Research Center, LLC | Knowledge of Good Clinical Practice and experience working with patients |
| Clinical Research Lab Coordinator | Coastal Carolina Research Center | One year of phlebotomy experience |
| Project Specialist | WCG | Bachelor’s degree and six months of related experience |
| Data Coder | WCG | Bachelor’s degree or currently enrolled in an undergraduate program |
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

Improving Cardiovascular Research for Transgender and Gender Diverse Populations

Disruptive Technologies Redefining the Path of Clinical Trials

How an Innovative Statistical Methodology Enables More Patient-Centric Design and Analysis of Clinical Trials
How to Get Research Experience
New section.
Working in a research setting can help make you a competitive medical school applicant and help you to determine if a career in medicine or medical research is right for you

How do I find a research position?
If you’re currently in college, check with your institution’s science or undergraduate research websites for opportunities to assist with faculty research projects. You can also review faculty bio pages and lab websites for more information. Next, reach out to your immediate network: express your interest in assisting with a research project to your science professors, academic advisor, and your pre-health advisor.
Try exchanging ideas with your peers and upper-classmen for advice on research opportunities at your institution. You can also ask peer advisors, resident advisors, or any fellow premedical students for introductions to principal investigators (PIs). You might even try the “Undergrad-Grad-PI” method. This is where you first reach out to undergraduate students in research labs to learn about their responsibilities; they oftentimes are more responsive. Then, reach out to the graduate or post-doc students to learn about the research question being investigated. After this, read the most recent paper or abstract the lab published. Once you complete these steps, you can approach the PI more confidently and more effectively demonstrate your commitment to and understanding of their project.
Your school’s career center or student employment office may know about research job openings, and they can also offer resume help and go over interview tips and techniques. Remember, opportunities may be on or off campus, full- or part-time, paid or unpaid, or part of a summer program. Once you find a position, you can connect with your school’s fellowships or awards office to inquire about research funding opportunities.
If you’ve already graduated, consider looking into open positions. Research hospitals, universities, and biotech companies are always looking for lab technicians or clinical research coordinators (CRC). Job opportunities are typically posted on the career pages of their websites.
When should I begin gaining research experience in college?
Some premedical students begin their research experiences during their first year of college, and others begin research positions after they have already graduated. On average, most students secure a research position junior or senior year. There are three big factors that will impact this:
- Your level of interest in pursuing research. If you are really excited to investigate a question under a mentor, you might find yourself reaching out to professors early and often. Other students may focus on gaining clinical experience, and therefore wait later in their academic career to start research.
- Readiness for the research project. Different PIs will have different expectations for preparation. A research project might require you to first take coursework in basic lab sciences, statistics, or another advanced topic specific to the project. Other PIs may prefer to train you “on-the-job” through their graduate or post-doc students. This will impact when you are ready to join a project.
- Finding the right research project. There is a process of reviewing different PIs and research projects to find the right fit for you. What subject do you want to investigate? Do you want your research project to take place in a lab or non-lab setting? Is there an independent question you want to investigate with the help of a mentor?
When is the best time to look for a position?
According to Kate Stutz, Ph.D., Director of Pre-Health Advising at Brandeis University, if you’re interested a research position during the academic year, the best time to look for positions is at the very beginning of the semester. There also tend to be a lot of research opportunities in the summer, both paid and volunteer, through set programs like the National Science Foundation’s Research Experience for Undergraduates (REUs). It’s best to start applying for summer research positions in December-February for the upcoming summer. Remember, typically there are more applicants than available spots so get your applications in early. Each undergraduate institution will be different, therefore make sure to connect with your advisors and peers for feedback on when to start looking.
What’s the best way to apply?
The outreach email message that you send to potential research faculty is very important. This message should include a formal introduction of yourself, evidence that you are familiar with their research project(s), and a clear, specific ask. Identify what you hope to contribute to the project. Do you want to clean the glassware or analyze lab findings? Consider attaching your resume as well. Dr. Stutz stresses that networking and persistence are crucial to finding a position. Make sure you’re using all of your network, including your peers and professors, to find open positions. Don’t be afraid to send follow up emails; faculty are very busy and often overlook emails. Sometimes, it can be even more effective to stop by a professor’s office hours to hand deliver your materials and indicate your interest in person.
How should I prepare for an interview?
With any interview, it’s important to make a good impression. Be sure to dress appropriately. Come prepared with a resume. Use your campus career center for advice on proper attire and resume best practices.
Often during interviews, you’ll be asked about your career goals. It’s helpful to be able to speak about the steps you plan to take to meet those goals. Talk about classes you’ve taken, especially upper-level science courses. Speak about your skills, your knowledge of techniques, and the equipment you’ve used throughout your coursework. Be prepared to discuss the lab experiments you’ve completed. If you’ve done any sort of research—even in your coursework—keep track of it. This shows you have experience. Lastly, interviewers often ask candidates if they have any questions. Dr. Stutz suggests asking something that indicates you’ve done your own research into their project. You could ask where they see their research going in the next three years or what challenges they anticipate. You could also ask about expectations for undergraduate researchers; do they expect you to work 20+ hours a week? Full time over the summer? Do they require you to have work study or to sign up for research credits? Asking these questions ahead of time can help you plan ahead and determine if this position is the best fit for you. Check out these interview resources for more tips.
Does research experience have to be in a wet lab?
No! Research can be performed in any field or subject. We’ve had successful applicants with research in classics, sociology, history, and policy, as well as applicants with research in biology, biochemistry, and neuroscience. Medical schools value all types of research. Research can take place in a scientific lab that requires advanced devices and procedures to obtain data for analysis. Research can also take place in the humanities or social sciences where participant interviews or surveys are needed to obtain an individual's life perspective. The clinical research field is constantly investigating patient outcomes and how to improve care through clinical trials or analysis of patient data. As a premedical student, consider what question you want to investigate further. Do you want to learn more about how health inequities impact disadvantaged communities in your area, or perhaps you want to know more about the protein channels involved in memory cognition? Once you choose a direction, you can then partner with a research PI for guidance on how to navigate your question. Sierra Perez, Pre-Health Advisor at Brandeis University, shares not to be afraid to get creative with your research question. She has been impressed by the medical school applicants who have created independent questions that address the community needs. “Applicants are recognizing the critical needs of specific populations, such as homelessness, LGBTQ+, veterans, youth with disabilities, etc.,” she stated. “There is also a demand for translational researchers, or individuals who can take complicated bench topics and apply it to the clinical world.”
Is research experience required to be accepted to medical school?
It depends. Some medical schools are very research focused; they may require a research thesis or have research time built into the curriculum. Other schools are more community or clinically focused; they would rather have an applicant work in a healthcare setting or volunteer at their local soup kitchen than be at the bench moving clear liquids from one test tube to another. Research experience (in whatever discipline) is helpful for developing some of the Premed Competencies , such as critical thinking, quantitative reasoning, scientific reasoning, as well as teamwork and oral communication skills. How much you should engage in research depends on how much you enjoy it once you try it!
The majority of accepted medical school applicants have some form of academic or clinical research at the time they apply. Competence in research has become increasingly important in the medical field to improve patient care outcomes.
You can also review medical school mission statements to see if research is a focus at a particular school. You can read each school’s mission, and the number of accepted students in their most recent class who had research experience, in the Medical School Admission Requirements . Remember, it’s best to pursue experiences that you’re genuinely interested in, rather than just to check a box, but you may not know if research is for you until you give it a try.

A Day in the Life of a Clinical Research Associate
For the 2023-2024 academic year, we have 112 schools in our MHAOnline.com database and those that advertise with us are labeled “sponsor”. When you click on a sponsoring school or program, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Every pill, cream, tonic, and capsule a pharmacist passes over the counter to an individual has taken a long journey from concept to the point of sale. In fact, many medications that do not require a prescription, such as those found in the allergy, cold, and flu aisle, also go through the same arduous process.
Before a drug can be prescribed and sold, it must be proven safe and effective. Pharmaceutical companies, universities, and health organizations put drugs through clinical trials or clinical study processes to obtain this proof. In the clinical trials world, the company that provides financial support to a research group selected to put the drug through clinical trials is known as a contract research organization or CRO. Primarily, CRO organizations offer financial aid for pharmaceutical development and biotechnology for agricultural and medical device industries.
In the case of pharmaceuticals, drugs that have not yet been approved for sale are administered to vetted participants in a controlled manner during a clinical trial. Clinical trials involve a great deal of documentation, analysis, observation, and organization. A team of professionals is engaged in administering a clinical trial, including a clinical research associate (CRA).
The CRA acts as a liaison between the study’s sponsor CRO (e.g., pharmaceutical company) and the clinics where the study occurs. Because the clinical trial results must be kept entirely transparent and not influenced by the sponsor’s interests, this is a critical role. Therefore, a successful CRA will be detail-oriented, highly educated, and communicate clearly with sponsors and clinical representatives.
| Featured Clinical Research Administration Programs | ||
|---|---|---|
| Arizona State University | Clinical Research Management (MS) | |
| Arizona State University | Clinical Research Management - Regulatory Science (MS) | |
| Arizona State University | Regulatory Science (MS) | |
| Arizona State University | Regulatory Science (MS) - Food Safety | |
| Johns Hopkins University - Advanced Academic Programs | MS Regulatory Science | |
| For the 2023-2024 academic year, we have 112 schools in our MHAOnline.com database and those that advertise with us are labeled “sponsor”. When you click on a sponsoring school or program, or fill out a form to request information from a sponsoring school, we may earn a commission. View our for more details. | ||
THANK YOU FOR YOUR INTEREST IN Southern New Hampshire University Online MS - Construction Management
Work environment of clinical research associates.
A clinical research associate works both at clinical sites and sponsor locations. During a trial, the CRA conducts regular site visits—virtually and physically—to ensure good progress and record-keeping on the clinical site.
CRAs are often responsible for multiple trials at one time, meaning significant amounts of travel between these sites. Sometimes, a CRA may be assigned to a specific geographical region, limiting travel.
Clinical Team of a Clinical Research Associate
Typically, a clinical research associate needs to have direct contact with the participants involved in the study. However, CRAs must work in a collaborative environment, coming into frequent contact with the clinical team at the study site and the supervisors from the study sponsor. Thus, clinical research associates work in the middle of the chain of command, which begins at the top with:
- Contract research organization (CRO) or sponsor
- Principal investigator (PI)
- Clinical research associate (CRA)
- Clinical research coordinators (CRC)
In short, a CRA is focused more on data, accuracy, and quality control while a CRC collects data and interacts with patients. Dan Sfera , in his YouTube channel titled The Clinical Trials Guru, summarizes the CRA profession with this description: “CROs select various PIs across the country, and PIs make sure that sites follow good clinical practices and protocols. They ensure this by hiring CRAs who look at all the recorded data and do not interact with patients when they are completing a site visit.”
Typical Daily Responsibilities of Clinical Research Associates
The daily responsibilities of a CRA are mainly dependent on the stage of the trial they are supervising. As such, below is a breakdown of the typical duties of a CRA during the beginning, middle, and end of a clinical study.
Before a Study Begins
Every clinical study must take place in an appropriately equipped clinical location. The CRA plays a critical role in selecting a site for a clinical study and may even be asked to suggest sites based on their previous experiences. CRAs may also evaluate the applications of sites that self-select as eligible for a particular study. A pool of potential study sites is narrowed down by having sites complete and submit a feasibility survey.
When the pool of sites has been narrowed down, the clinical research associate conducts site selection visits with the chosen locations. During these visits, the CRA spends up to half a day confirming the validity of the feasibility study, meeting with the team (mainly the assigned coordinator), and observing the capabilities and equipment at the facility.
Upon completion of the site visit, the CRA compiles a report for the study sponsor and presents their findings and recommendations for proceeding with the study.
During a Study
Once a site has been selected, the CRA is now responsible for ensuring that the site knows the sponsor’s required protocol and is appropriately set up to conduct the study. In addition, the clinical research associate conducts site visits at regular intervals throughout the study to ensure that protocols are followed and data is effectively collected.
Depending on the study, CRAs may conduct in-person site visits as well as virtual visits. In recent years, the use of remote visit technology has allowed CRAs to review paperwork online, for instance, and reserve in-person visits for necessary personal interactions. During site visits, the CRA ensures that the study is always proceeding with good clinical practices. More details on the specific methods are available below.
Ultimately, since the CRA is a liaison, developing and maintaining positive relationships is an essential part of the job. During the trial, the CRA must communicate effectively and help the clinical staff appropriately to ensure the study progresses smoothly.
When a Study Ends
The CRA typically conducts a closeout visit after a study or when it becomes necessary to end a study—e.g. when enrollment is too low.
During the closeout visit, the CRA verifies that all paperwork is in order and that all obligations have been met on both sides. For example, the verification process may mean ensuring the trial drugs are returned or destroyed, completing and adequately filing all documentation, and compiling all information necessary to complete a final report for the study sponsor.
Required Skills & Knowledge of Clinical Research Associates
Based on the responsibilities outlined above, it should be no surprise that CRAs must have a knack for detail. Proper documentation, filing, and storage are all critical parts of the job description. It is up to the CRA to ensure that the sponsor and clinic understand their obligations at every point of the study. All policies and procedures are being followed to ensure successful data collection.
In addition to being detail-oriented, a good CRA also pays heed to the ethics of the position. For example, clinical trials and studies can have extensive consequences for the organizations that sponsor them, as well as for the trial participants, and eventually, for consumers at large. Therefore, CRAs act as vital monitors for ethical issues and should be able to stand up to any perceived transgressions.
Above all, it is essential that a CRA understands and can implement good clinical practices (GCP) successfully. The GCP guidelines are an internationally developed set of standards from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) that details ethically and effectively conducting drug trials with human subjects.
The ICH topics are divided into four categories: quality, efficacy, safety, and multidisciplinary. CRAs can find the guidelines in more detail on the ICH website. The YouTube Channel, GCP Mindset , outlines the different roles and responsibilities of CRAs. In short, upholding the GCP standards is the most critical part of the CRA job, meaning that any CRA must be familiar with GCP and recognize and enforce these practices in a clinical setting.
Certification for Clinical Research Associates
While certification is not a legal requirement to work as a clinical research associate, it can provide an important differentiator when looking for employment or career advancement in many professions.
Two main bodies offer certification for CRAs: the Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SOCRA).
Association of Clinical Research Professionals (ACRP) – Certified Clinical Research Associate (CCRA) Certification
To earn the Certified Clinical Research Associate (CCRA) credential from ACRP, applicants have two available pathways:
- Pathway 1 – Clinical research professionals with 3,000 hours of verifiable work experience are eligible to sit for the CCRA Exam.
- Pathway 2 – Clinical research professionals with 1,500 hours of verifiable work experience and a clinical research degree are eligible to sit for the CCRA Exam.
Applicants also have to submit proof of their current job description and resume and pass the CCRA exam. In addition, CCRAs must complete at least 24 hours of continuing education credits and be recertified every two years to maintain certification. Beyond the CCRA certification, ACRP also offers certifications for project managers, research coordinators, and principal investigators, and an umbrella certification for clinical professionals.
Society of Clinical Research Associates (SOCRA) – Certified Clinical Research Professional (CCRP) Certification
To earn the Certified Clinical Research Professional (CCRP) certification from SOCRA, applicants must either:
- Have at least two years of full-time experience as clinical research professionals or 3,500 hours of part-time experience in the past five years
- Have a degree in clinical research plus at least one year of full-time experience (or 1,750 hours part-time during the previous two years)
- Have a certificate in clinical research (undergraduate or graduate), a degree in science, health science, pharmacy, or a related field (associate’s or bachelor’s), plus at least one year of full-time experience (or 1,750 hours part-time during the previous two years)
Applicants must also pass the CCRP exam and be recertified every three years. Recertification requires 45 total hours of continuing education credits.
The critical difference between the SOCRA and ACRP certifications is that ACRP expressly certifies CRAs, while the SOCRA certification can apply to other clinical research professionals.
Matt Zbrog is a writer and researcher from Southern California. Since 2018, he’s written extensively about emerging issues in healthcare administration and public health, with a particular focus on progressive policies that empower communities and reduce health disparities. His work centers around detailed interviews with researchers, professors, and practitioners, as well as with subject matter experts from professional associations such as the American Health Care Association / National Center for Assisted Living (AHCA/NCAL) and the American College of Health Care Executives (ACHCA).
Related Programs
- 1 Online Master’s in Clinical Research Administration Programs
- 2 Online Doctor of Health Sciences (DHS)
- 3 Online Master’s Degrees in Food Safety – Regulatory Affairs & Quality Assurance
- 4 Online Master’s in Bioinformatics Programs
- 5 Online Master’s in Biotechnology Programs
Related FAQs
- 1 Clinical Significance vs. Statistical Significance
- 2 How Do I Become a Clinical Trials Research Nurse?
- 3 How Do You Become a Clinical Research Coordinator (CRC)?
- 4 What Can You Do with a Degree in Clinical Research Administration?
- 5 What is Clinical Data Management (CDM)?
Related Posts
A day in the life of a clinical data analyst.
Every day, health organizations like hospitals, clinics, and physician offices collect data about their patients. This information is used to make data-driven decisions in order to provide the highest level of care to their patients as well as reduce expenses and errors. From the outcome of a particular treatment to a large-scale clinical trial, health data is a critical part of the modern healthcare process. Clinical data analysts help to make sense of the extensive data that is at their fingertips, creating stories that turn numbers into actionable intelligence to improve healthcare outcomes.
Healthcare Debates: Does Costly US Healthcare Fund R&D and Medical Research?
This article explores research and development in the context of greater healthcare expenditures, including looking into the main drivers of healthcare spending, influences on pricing, and where R&D fits into the whole ecosystem.
Pharmaceutical Quality Director – A Day in the Life
Consumers and corporations need to know that the drugs dispensed by pharmaceutical companies are manufactured safely and efficiently. Pharmaceutical quality directors ensure that quality control protocols are followed within the industry’s manufacturing, testing, and inspection procedures.
Closing the Gap: Women Leading Healthcare
Women drive healthcare. They are the industry's biggest consumers and workers. They serve as caregivers in their homes and make most decisions for their family's health. Why is it, then, that of the 40 Fortune 500 healthcare companies, not a single one is helmed by a woman?
Clinical Application Analyst – A Day in the Life
Electronic health records (EHR) and other clinical software systems are the new industry standard in healthcare. However, plenty of clinics, hospitals, and private practices have yet to make the full migration. That’s where clinical application analysts come in.
- MTS Health Sciences How to Become a Clinical Research Associate
- Anesthesia Technician & Technologist
- Audiologist & SLP
- Cardiovascular Technologist
- Dental Assistant
- Dental Hygienist
- Diagnostic Medical Sonographer
- Dialysis Technician
- EKG Technician
- EMT & Paramedic
- Kinesiologist
- Mammography Technologist
- Medical Assistant
- MRI Technologist
- Neurodiagnostic Technologist
- Nuclear Medicine Technologist
- Ophthalmic Technician
- Pharmacy Technician
- Phlebotomist
- Physical Therapist Assistant & Aide
- Psychiatric & Mental Health Technician
- Radiation Therapist
- Radiologic Technologist
- Respiratory Therapist
- Surgical Technologist
- Cytologist (Cytotechnologist)
- Dental Lab Technician
- Histotechnologist
- Medical Lab Assistant
- Medical Lab Technician
- Biological Sciences
- Biomedical Science
- Biotechnology
- Health Sciences
- Infection Preventionist
- Medical Laboratory Scientist
- Nutritionist & Dietitian
- Pathologists' Assistant (PathA)
- Pre-Vet (Veterinarian)
- Biomedical Equipment Technician
- Biomedical Informatics
- Health Informatics
- Health Information Management
- Health Information Technology
- Healthcare Administration
- Medical Billing & Coding
- Nursing Informatics
- Sterile Processing Technician
- Patient-Facing Technology Programs
- Laboratory Technology programs
- Natural & Clinical Lab Science
- Medical IT & Administrative
Certification Guides
Career guides, interviews & features, how to become a clinical research associate (cra), search for schools.
When you click on a sponsoring school or program advertised on our site, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Scientists, researchers, and doctors make discoveries about drugs, surgical procedures, behavioral therapies, or medical devices through their work in laboratories and healthcare settings. This is only the beginning of the journey for pharmaceuticals, therapies, and devices, as bringing the findings from the lab to the street requires a vigorous scientific process known as a clinical trial. Clinical research associates (CRAs) are the professionals responsible for ensuring that clinical trials move forward following established guidelines and regulations for ethics, safety, and reporting.
Clinical research associates, also known as “monitors,” work on behalf of sponsors funding clinical trials for the new or existing drug, device, surgery, or behavioral intervention. Working directly for the sponsor or through a contract research organization, the main task of a CRA is to monitor the progress of an ongoing clinical trial.
Through in-person site visits or remote monitoring systems, a CRA serves as the central point of contact between a sponsor and testing sites; ensures that the trial is being administered per approved protocols; verifies that the clinical trial is being conducted ethically at all sites; and confirms the validity and accuracy of all data being collected and reported at test sites.
In addition to reading, interpreting, and understanding medical technology, clinical research associates must have excellent interpersonal and communication skills. The ability to understand best clinical practices, design protocols, and data standards requires CRAs to have outstanding attention to detail, analytical skills, and the capacity to deliver constructive feedback to participating research sites on their performance.
Although not a requirement, many CRAs travel between multiple research sites for study oversight, which may require a valid driver’s license, the physical capacity to travel, and/or willingness to fly or drive regularly.
This detailed guide explores the education and credentials required to become a clinical research associate (CRA).
Arizona State University
Johns hopkins university (aap), university of west florida, steps to become a clinical research associate (cra).
The pathways to becoming a clinical research associate are numerous and available to anyone with a high school diploma or higher. While formal education is not technically required to enter the field, having a bachelor’s degree or higher can make potential candidates much more competitive.
Certification in the field is also not required, but obtaining certification from the Society of Clinical Research Associates (SOCRA) or the Association of Clinical Research Professionals (ACRP) can result in more opportunities and even more competitive salaries.
Finally, all aspiring CRAs are advised to check out the International Conference on Harmonisation’s (ICH) guidelines for Good Clinical Practice (GCP) to get a feel for the professional expectations and responsibilities.
Here is how to become a CRA depending on one’s level of education. Please note that in the United States, there are two major certification bodies for CRAs: the Society of Clinical Research Associates (SOCRA) and the Association of Clinical Research Professionals (ACRP). Each pathway includes the eligibility requirements to pursue credentialing through either of these entities.
PATH 1: Earn a High School Diploma and Gain Experience
Perhaps the most strenuous route to this career is becoming a certified CRA with a high school diploma and between 3,000 and 3,500 hours of qualifying work experience (depending on the certification entity).
These candidates often start out in support positions assisting a more experienced or certified CRA with mundane tasks. An entry-level worker can earn increased responsibilities through a demonstrated capacity to learn the regulations, protocols, and ethical considerations. To qualify for the following CRA certification exams, high school graduates must:
SOCRA Category 1
- Complete two full-time years of CRA work within five years, or 3,500 hours of part-time work
ACRP CCRA (Certified Clinical Research Associate)
- Complete 3,000 hours performing essential duties
- Submit a resume documenting and demonstrating job performance
Please note that in some cases, additional education can be used to substitute for work experience hours. Please see credentialing websites for details.
PATH 2: Earn an Associate Degree and Gain Experience
Depending on the program, an associate’s degree of applied science (AAS) in clinical research can be a standalone degree or a stepping-stone to a bachelor’s or master’s. Licensed vocational or practical nurse (LVN or LPN) programs are designed specifically for practical, job-ready skills and may qualify aspiring CRAs for the ACRP certification.
Similar to the path taken by those with a high school diploma, having an associate degree, LPN, or LVN can open the door to some entry-level jobs in the industry. At this level, some prospective CRAs assist more experienced CRAs or some engage independently in entry-level tasks related to study monitoring. Those working as CRAs with an associate’s degree, LPN, or LVN can qualify for certification after working a certain number of hours in the field.
To qualify for the following CRA certification exams, associate degree graduates must:
SOCRA Category 2
- Hold a “clinical research” degree
- Complete one full-time year as a CRA or 1,750 hours part-time
ACRP Option 2 (Also for LVN, LPN)
- Hold a “clinical research degree” or complete 1,500 hours performing essential duties
PATH 3: Earn a Bachelor’s Degree and Gain Experience
Most entry-level clinical research associate positions require candidates to have a bachelor’s of science (BS) in a health-related field from an accredited four-year university. In some cases, programs are designed to add practical hours needed to qualify for certification tests.
Those interested in becoming a CRA can study nursing, health sciences, biological sciences, clinical research, clinical research administration, clinical research management, medical technology, or life sciences, among many other subjects. Because many entry-level positions are looking for those with previous work in the field, those earning a BS should seek internships, part-time work, and/or fellowships involving participation in research, if possible.
To qualify for the following CRA certification exams, bachelor’s degree graduates must:
SOCRA Category 3
- Hold a “clinical research” undergraduate degree
ACRP Options 1 & 2
- Complete 3,000 hours performing essential job duties or 1,500 hours of equivalent work experience requirements through ACRP certifications or approved clinical research degree programs accredited by the Council for Higher Education
PATH 4: Earn a Master’s Degree for Opportunities in Management
A master’s program in clinical research is generally designed for those already working as CRAs to expand their skills or to advance into management or supervisory roles within the field. However, for those with non-health science bachelor’s degrees who want to become CRAs, seeking a master’s of science in clinical research or a master’s of science in clinical research management could be a pathway to breaking into the field.
Because many of these programs are offered online, earning a degree is possible for even those students who need full flexibility of schedule to complete the degree. Although requirements for admission into master’s programs vary, those looking to gain admission into a master’s of science for clinical research commonly need the following:
- A bachelor’s degree
- Official transcripts demonstrating specific coursework in science
- A statement of purpose
- Letters of Recommendation or Reference
- A resume or CV
- An application fee
- TOEFL or IELTS scores (international students only)
Clinical Research Associate (CRA) Degree Programs
There is a range of formalized training programs that prepare professionals for this key role in ensuring the safe, and ethical development of medical technologies. Below you will find examples of programs at a range of educational levels available to those interested in a career as a CRA.
Durham Tech – AAS Program
Durham tech, located in Durham, North Carolina, offers a 71-credit hybrid on-campus and online clinical trials research associate (CTRA) associate of applied science (AAS) program. Durham’s CTRA AAS prepares graduates to work on any side of clinical research in an assistant’s role.
While most programs require the student to attend on-campus courses, there are several courses that are offered completely online. The program takes 20 to 21 months and includes coursework in research site management; clinical research management; research protocol design; an introduction to ethics; anatomy and physiology; an introduction to clinical data; pathophysiology; and clinical research terminology.
Graduates of the program may be eligible to sit for national certification examinations and will be prepared for opportunities at medical centers, pharmaceutical industries, hospitals, research facilities, clinics, physicians’ offices, and device companies.
- Location: Durham, NC
- Accreditation: Southern Association of Colleges and Schools Commission on Colleges (SACSCOC); Commission on Accreditation of Allied Health Education Programs (CAAHEP)
- Expected Time to Completion: 20 to 21 months
- Estimated Tuition: $5,396
Washington University in St.Louis University College – BS, MS, Certificates
Washington University in St. Louis, Missouri, has various degree options for CRAs at all stages of their career to work as monitors. Students can enhance their current skills and knowledge in clinical research management, as well as gain a deep mastery regarding how to best move clinical research forward in an ethical, compliant, and safe way.
Those with at least six units of transferable coursework qualify to apply to the 120-credit-hour bachelor of science in clinical research management to start their careers. Anyone with any educational background can pursue University College’s 21-credit undergraduate certificate in clinical research management to enhance career skills or make a resume more competitive.
Students who already have a BA or BS also have options at Washington University. Experienced professionals in the clinical research field who wish to seek formalized training can earn a 21-credit advanced certificate in clinical research management or a 30-credit master of science (MS) in clinical research management. Those with a non-healthcare bachelor’s degree who wish to become high-level CRAs can up their skills and knowledge by choosing the combined bachelor’s and master’s degree options.
Although the coursework in each program varies to suit the level of education, themes across all the programs include the fundamentals of clinical research management; research ethics and regulatory affairs; compliance, legal and regulatory issues; and data and information management in health sciences.
- Location: St. Louis, MO
- Accreditation: Higher Learning Commission (HLC)
- Expected Time to Completion: BS (up to 48 months); certificate (12 months); MS (24 months)
- Estimated Tuition: Undergraduate courses ($695 to 895 per credit); Graduate courses ($665 to 995 per credit)
Barnett International – Online Seminar
Designed for CRAs with two years of experience or less, this online clinical research associate onboarding program by Barnett International prepares entry-level employees to monitor clinical trials at high levels appropriate to industry standards.
Over ten weeks of synchronous online coursework lasting three hours per week, participants will learn topics including informed consent, investigational product accountability, safety definitions and reporting requirements, and regulatory compliance and quality assurance: audits and inspections. Participants receive 30 hours (3.0 CEUs) of continuing education credits.
- Location: Needham, MA
- Accreditation: Accreditation Council for Pharmacy Education
- Expected Time to Completion: Ten weeks
- Estimated Tuition: By Early Bird Deadline ($1,795); After Early Bird Deadline ($1,995); June 10 is the early bird deadline
Continuing Education for Clinical Research Associates (CRAs)
Both CRA certification bodies require continuing education to maintain active certification status.
SOCRA requires recertification every three years. It calls for 45 hours of CE to be completed over the course of the first three years beyond passing the initial test. Twenty-two CE units must be related to clinical research; the remainder can be in the professional or therapeutic area in which one works or specializes. In addition, those looking to maintain or renew certification must complete a “recertification continuing competence learning module.”
The ACRP expects certified CRAs to engage in continuing education (CE) and continuing involvement (CI) to maintain certifications. Continuing education should include coursework in research and healthcare, and continuing involvement requires candidates to engage in activities such as authorship, participating in investigator meetings, or working as a peer reviewer, among other opportunities. Notably, ACRP utilizes an ongoing point system for professionals to maintain their certifications.
CRA Career and Salary
Clinical trials and the objectivity they bring to advances in treatment are extremely important. In an increasingly globalized society, diseases spread across borders, and in an age of increased antibiotic resistance, new ways to fight bacteria will be needed. Furthermore, with an aging U.S. population comes increased rates of chronic conditions and the subsequent reliance on pharmaceuticals to improve people’s quality of life.
It’s not surprising that the Bureau of Labor Statistics (2022) predicted a 7 percent increase in openings for medical and clinical laboratory technicians between 2021 and 2031, much more than the average growth anticipated across all U.S. occupations during that same decade (5 percent). As far as the salaries are concerned, here are the salary percentiles for clinical laboratory technologists and technicians in the US ( BLS May 2022):
| United States | |
|---|---|
| Number of professionals employed | 333,600 |
| Annual mean wage | $59,130 |
| 10th percentile | $35,220 |
| 25th percentile | $40,440 |
| 50th percentile (median) | $57,380 |
| 75th percentile | $74,920 |
| 90th percentile | $84,670 |
Lastly, while the BLS doesn’t track salaries for CRAs, PayScale.com (June 2023)—a site that relies on self-reported data—found that the median annual salary for a CRA was $72,393. Among the 1,391 CRAs reporting their annual salaries, Payscale found these percentiles:
- 10th percentile: $48,000
- 50th percentile (median): $72,393
- 90th percentile: $101,000
Specialized skills in CRA that increased salaries included medical devices (37 percent pay increase over average), team leadership (35 percent), and writing procedures & documentation (20 percent).
Years of experience, predictably, also have an impact on salary. Entry-level CRAs earn 15 percent below the average, while experienced CRAs (ten to 19 years) earn 16 percent above the average and late-career CRAs (20+ years) earn 25 percent above the average.
It is important to note that these figures also vary based on the data source. For illustration, Indeed.com (June 2023) found an average annual salary of $80,957 among United States clinical research associates.

Becca Brewer is building a better future on a thriving earth by healing herself into wholeness, divesting from separation, and walking the path of the loving heart. Previously to her journey as an adventurer for a just, meaningful, and regenerative world, Becca was a formally trained sexuality educator with a master of education.
Related Articles
- Upskilling in the Allied Health Professions
- Single-use Plastics in Medicine Raise Concerns About Sustainability
- Clinical Research Certification - CCRA, CCRC, CPI
- Online Master’s in Clinical and Translational Research Programs
- Online Bachelor’s in Health Science Programs (BSHS Degree)
- Online Master's Programs in Clinical Management and Leadership
- Clinical Lab Science vs. Clinical Research
- BSHS (Health Sciences) vs BSHA (Health Administration) Programs
- Clinical vs Translational Research
- Guide to Health Science Careers
Related Programs
Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)

Thomas Boothby, MS, CCRP CRA II, Boston Scientific
Abstract : Research coordinators may transition to clinical research associates/monitors during their careers. This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process. A roadmap in the form of a checklist is provided to help make the transition from research coordinator to clinical research associate (CRA) a smooth one.
Disclosure: The author has a relevant financial relationship with respect to this article with Boston Scientific, where he is employed as a monitoring CRA.
Introduction
A research coordinator is a person at the clinical research site who is involved in the daily tasks of enrollment, data entry, and all other aspects of clinical trials at the site level. A clinical research associate (CRA), or monitor, is the individual who visits clinical research sites to review their medical records and do the standard monitoring visits. Before the author was a CRA, he was a research coordinator for fourteen years. This article describes how the author made the transition from clinical research coordinator to CRA/clinical research monitor and includes some suggestions for those looking to make a similar career change.
When to Transition from Research Coordinator to CRA
While people naturally want to progress their careers as fast as possible, it is important to only make thetransition from research coordinator to CRA when the time is right. The grass is not always greener on the other side of the work fence.
The author knew that he was ready to make the transition from research coordinator to CRA because he felt that he had mastered all the tasks of a research coordinator. His job became stagnant, and he was looking for something better. Fatigue in the current work environment is another reason for why individuals may be looking to make this transition. Of all members of the clinical research team, research coordinators have the most difficult job. In the author’s opinion, they are often overworked and underpaid, and their contributions to the overall study are sometimes overlooked. Other reasons to make the transition from research coordinator to CRA include potential career progression and the opportunity to try something new. Some individuals may find that the travel component that goes along with being a monitor is a positive as well.
There are five stages of change according to a behavioral change model: pre-contemplation, contemplation, determination, action, and maintenance. In the pre-contemplation phase, people are not thinking about transitioning yet or may have obstacles in their daily lives that are preventing them from exploring new opportunities. When people are becoming serious about change, they are in the determination or action phases. During these phases, research coordinators who want to transition to CRAs might apply for new positions or become certified clinical research professionals (SOCRA CCRP®) as they try to gain new skills for the job market. When considering a transition from research coordinator to CRA, it is important to identify one’s place in the behavioral change model.
Qualifications and Background of CRAs
When the author was applying for CRA positions in 2015, he always saw a requirement for at least two years of experience as a monitor. This requirement is often a barrier to those looking to make this career transition. In 2010, ClinicalTrials.gov listed more than 100,000 clinical studies. By 2019, that number has increased to more than 300,000 clinical studies. The clinical research market has exploded over the last decade. More people are needed to monitor and to run clinical studies now than ever before. While some companies are less likely to require two years of monitoring experience now due to a depleted pool of candidates, these same companies may be more open to supplemental forms of experience such as certifications, course work, and on-the-job experience.
Thus, this is a great time to act on the decision to transition from research coordinator to CRA. From 2014 to 2024, the United States Bureau of Labor Statistics estimates that CRA positions will increase 14% annually. This increase in the job market, coupled with the high level of CRA turnover, could lead to a very strong job market in the future. At Boston Scientific, turnover among CRAs is fairly low due to the strong structure and principles. Many CRAs within Boston Scientific have been with the company for 10 to 20 years or longer.
Table 1 highlights the typical background of CRAs. Most CRAs are current or former nurses who have experience as a research coordinator or a research assistant. Many universities now offer bachelor’s, master’s, and certificate programs in clinical research as another form of training for these research related roles. In Michigan, where the author is from, Eastern Michigan University has a two-year master’s degree program in clinical research. Like the author, CRAs can often be a former research coordinator.
When the author was transitioning from research coordinator to CRA, he got his foot in the door by working closely with a monitor who still works for Boston Scientific. Relationships between research coordinators and CRAs can be contentious due to the nature of monitoring. Research coordinators should treat monitors and sponsor staff well and with respect, and they should treat monitoring visits as a learning opportunity and not a criticism of the coordinator’s work. These relationships do not need to be contentious. A good working relationship with a clinical research site’s CRAs can serve as a potential audition for a monitoring position.
CRAs typically have a clinical research certification, either SOCRA’s certified clinical research professional (CCRP®) or the Association of Clinical Research Professionals-Certified Professional (ACRP-CP). Some companies provide tuition reimbursement for programs and certifications such as these as a way of employee enhancement. Research coordinators can participate in enrichment programs such as these and obtain certifications to help boost their resume and become more marketable to CROs and sponsors. When researching these programs, individuals must do their due diligence to ensure that the program or certification is offered by a legitimate organization and is accredited. Hiring managers know where to find the gold standards in clinical research programs and certifications, and those that do not fit this standard can even be viewed as a negative on ones resume.
The author is a SOCRA CCRP®, Certified Clinical Research Professional, which is an excellent indicator of knowledge for a monitoring position. The test includes knowledge of the regulations and the role of the monitor. There are also some CRO-development programs such as SOCRA’s Clinical Research Monitoring Conference and one-year certificate programs such as the Harvard Medical School global clinical scholar’s research training program.
Networking through the clinical research site’s CRAs and professional forums and groups such as SOCRA is a great way to find CRA positions and interact with other research professionals. At conferences, CROs often have booths in the exhibit hall where research coordinators can meet CRO staff, learn more about opportunities, and leave their resume with CRO staff.
A Typical Day in the Life of a CRA
The life of a CRA has its positives and negatives (Table 2). There are many things that the author wishes he knew before he became a monitor. The author works from home a great deal of the time. If he is not on the road visiting a clinical research site, he is working at home either preparing for a visit, writing follow-up visit letters, or performing other administrative work. Visit preparation and follow up is a crucial part of the home office work. CRAs have very strict compliance guidelines for completing monitoring visits and monitoring reports in a timely manner. Since recently becoming a lead CRA, the author has also been doing a great deal of administrative and compliance work with more of a global view of a clinical trial.
Some clinical research organizations (CROs) and sponsors have onsite monitors who can do remote visits and activities. Whether visits are onsite or remote, monitors are constantly in contact with clinical research sites to follow up on action items from monitoring visits or to answer protocol specific questions the site coordinators may have.
At most companies, about 60-80% of the monitor’s time is spent traveling to sites. The author currently covers all of Michigan, and he has covered other areas, including Wisconsin, New York, Pennsylvania, and Ohio. CRAs are often away for several days at a time depending on the current workload. This can be difficult on families and personal relationships. While the author travels extensively, there are some times when he travels more than others. Sometimes he does back-to-back visits and may be gone for several days at a time. After that, he may be home for several days. The extensive travel required of CRAs is a key consideration when exploring this career transition.
Being a CRA takes a great deal of self-discipline. Monitoring offers a flexible work arrangement, so monitors can work later in the day or take time off during the normal workday. However, if the CRA does not accomplish what he/she should accomplish, this will be glaringly obvious. Management and co-workers will immediately know if the CRA does not show up to meetings or has difficulty answering questions about his/her monitoring activities or their monitor role in general.
Starting a Monitoring Job
Boston Scientific has a rigorous onboarding process comprised of four to six months of training. After the author was hired as a CRA, he spent months learning the work instructions and going out on preceptor visits. In the beginning, the new CRA observes a senior CRA. Over time, the new CRA does more of the monitoring. By the end of the training, the new CRA is doing the monitoring visit, and the senior CRA is observing and making suggestions to the new hire on how the new hire can improve.
There are various levels of monitors at Boston Scientific: CRA I (for new hires), CRA II, and senior CRA. More experienced CRAs often mentor new CRAs. It is extremely helpful to find CRAs who can serve as mentors and answer questions.
CROs and sponsors have many systems that CRAs must learn. At Boston Scientific, these systems include electronic data capture, clinical trial management, auxiliary programs to help remote employees, and cloud-based filing systems. Being a CRA might be very difficult for people who are resistant to change or have difficulty with technology.
There are several types of monitoring (Table 3). The author would be considered a traditional CRA or monitor. By this, he does traditional onsite monitoring via annual or semi-annual visits to clinical research sites based upon the study’s monitoring plan. At smaller organizations, monitors may travel more often or may have an expanded territory to cover. It is important to ask how much travel is involved and how many monitors are on the team during the interview process. If a company has fewer monitors, more travel will be involved.
Many Boston Scientific protocols require annual monitoring visits. The author visits his clinical research sites at minimum once a year but generally 2-3 times per year. Some of the more difficult sites, high enrollers, and those that are more likely to be inspected by the U.S. Food and Drug Administration are monitored more often. Many sites are participating in more than one Boston Scientific study. For example, the author monitors a site in New York that is conducting several studies. He will monitor two studies during one visit. This saves him time and travel and saves the company money by reducing travel costs. Boston Scientific also uses a risk-based monitoring strategy.
In-house regulatory CRAs at Boston Scientific, called trial management CRAs, interact with the sites on regulatory matters, study startup, and study closure. They work primarily by email and lean on traditional CRAs such as the monitor to be the face of the company with the research coordinators and help ensure that tasks are completed on time. Many hospitals also run their own clinical studies and may have in-house monitors.
Boston Scientific does use remote monitoring in certain studies and circumstances. Remote monitoring takes a great deal of work and technological experience at both the sponsor and site level. It involves a great deal of scanning and correspondence by the research coordinators, which can take a lot of their time and resources.
Sponsor CRAs generally deal with one indication, while CRO CRAs can work on studies for different indications or therapies. In one month, for example, CRO CRAs may be doing four indications at four sites for four sponsors. This requires understanding a great deal of information and being able to use different systems. Good organization is key when working as a CRA, whether for a sponsor or a CRO.
Recently, the author progressed from a CRA II to a senior CRA. As a senior CRA, the author has a larger leadership role and is expected to participate more in training and mentoring other CRAs. Boston Scientific has some centralized monitoring that will look at certain metrics and internal documents to guide monitors in their daily monitoring activities. Monitors are closely linked to the trial managers who actually run the studies. They also deal with safety and data managers as well as their CRA manager and the director of operations. Boston Scientific recently created an associate clinical trial manager position as a way to slowly transition some staff members into clinical trial managers, and the author is also transitioning into this role.
One common drawback about this transition process from research coordinator to CRA is that a CRA is one step removed from patient care. Working directly with patients as a research coordinator is something that the author misses. It is important to remember that CRAs help protect patients who are participating in clinical studies at more of an indirect level. This ideology helps prevent burnout, especially when monitors are swamped with the many reports that are necessary as part of the monitoring process.
Checklist for Transitioning from Research Coordinator to CRA
Table 4 has a checklist for determining whether one is ready to make the transition from research coordinator to CRA. Prior to applying for positions, the research coordinator must consider his/her stage in the behavior change model. Unless the research coordinator is ready to transition to a CRA position, he/she should not do it. Becoming a CRA can be difficult without two to five years of research experience in medical devices, pharma, or academia in some capacity. A research coordinator who wants to transition to a CRA should work closely with current CRAs who can provide mentoring and networking opportunities as well as exploring other networking avenues such as SOCRA and ACRP forums, LinkedIn, and also attending the annual events or local events put on by these organizations.
It is important for research coordinators to bolster their resumes by completing supplemental training or certifications. Resumes should be up-to-date and attractive to potential employers. This means including details about accomplishments along with basic information such as job titles and education.
The research coordinator must also consider the travel demands of a CRA position, the types of monitoring to pursue, and his/her stage in the behavior change model. Travel is a major part of a CRA position and should be a focal point of your conversation with a hiring representative. Finally, the types of monitoring including central monitoring, remote monitoring, and regional monitoring should be considered.
Monitoring is a great job. It allows a lot of freedom. However, CRAs also have a great deal of responsibility. CRAs must be driven, willing to put in the time, and have the necessary work ethic while maintaining vigilance and holding others accountable for good clinical practices.
Typical Background of a CRA
- Nursing degree with a clinical research background
- Bachelor’s or master’s degree in clinical research
- Former/current research coordinator
- Clinical experience (medical assistant, registered nurse, or nurse practitioner)
- Clinical research certified ( SOCRA CCRP ® or ACRP-CP)
- Research experience/background
- Science/academic research background
The Life of a CRA
- This requires being self-motivated and driven
- Sometimes performing a combination of onsite and remote monitoring
- Email, etc.
- At times, CRAs are gone for several days at a time depending on current workload
- Visit preparation and follow-up is a crucial part of work at home
Types of Monitoring
- Annual or semi-annual visits based upon the monitoring plan
- Risk-based monitoring/central monitoring
- Remote monitoring
- In-house CRAs and regulatory CRAs
- Sponsor CRA/monitor
- CRO CRA/monitor
Checklist for Transitioning from a Clinical Research Coordinator
to a Monitoring CRA (Clinical Research Associate)
- 2-5 years of research experience as a research coordinator or research assistant
- Able to work with current CRAs as part of a mentorship or network with CRAs
- Completion of supplemental training or certifications to support career goals and bolster resume
- Explore networking avenues
- Up-to-date resume that is attractive to potential employers
- Able to meet travel demands of a CRA position
- Consideration of types of monitoring to pursue
- Stage in the behavior change model
7 thoughts on “Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)”
Your articles are always helpful and I always get something new to learn from them.
Research Update Organization
you are always giving something new. thank you for that.
Very clear and helpful article! The tables listing the different types of monitoring roles and the items to consider whether this is right transition are a great summary, too.
- Pingback: An overview of a career in clinical research: What jobs are available in this field? - Rebiz Zield
This is a very clear and collective article. The tables are the best helpful tips and resources for anyone interested for career advancement in clinical research. I really appreciate the author’s time and effort to sharing this article.
- Pingback: Career Growth Opportunities in Clinical Research
- Pingback: Introduction to Clinical Research: What Every Student Should Know - Drug Safety and Pharmacovigilance Course
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
20 Clinical Research Associate Interview Questions and Answers
Common Clinical Research Associate interview questions, how to answer them, and sample answers from a certified career coach.

Clinical research associates are essential to the success of any clinical trial. As a CRA, you’ll be responsible for managing the day-to-day operations of a study and ensuring that it adheres to all regulations.
But before you can start making sure trials run smoothly, you have to get through your job interview. To help you prepare for this important step in the hiring process, we’ve put together some common questions you might encounter during a clinical research associate interview. Read on—and ace your next job interview!
- What experience do you have with clinical trial design and protocol development?
- Describe a time when you had to manage multiple research projects simultaneously.
- How do you ensure that all data collected is accurate and compliant with regulations?
- Explain the concept of Good Clinical Practice (GCP) and how it applies to your role as a Clinical Research Associate.
- Are you familiar with the different types of clinical trials and their purpose?
- What strategies do you use to stay organized while managing multiple research sites?
- How do you handle difficult conversations with research participants or sponsors?
- Describe a situation in which you had to troubleshoot an issue with a research site.
- What are the most important considerations for ensuring patient safety during a clinical trial?
- Have you ever worked on a project where there were language barriers between yourself and the research participants? If so, what did you do to overcome them?
- What strategies do you use to ensure that research protocols are followed correctly at each research site?
- Describe your experience working with Institutional Review Boards (IRBs).
- What steps do you take to ensure that informed consent forms are properly completed by research participants?
- How do you handle conflicts between research staff and investigators?
- What methods do you use to monitor research progress and identify potential issues before they become serious problems?
- Do you have any experience with developing recruitment materials for clinical trials?
- How do you approach training new research staff members?
- What strategies do you use to maintain relationships with research sponsors?
- Describe a time when you had to present complex research results to a non-technical audience.
- What challenges have you faced while conducting remote clinical trials?
1. What experience do you have with clinical trial design and protocol development?
Clinical research associates, or CRAs, are responsible for managing clinical trials for pharmaceutical companies and other organizations. This means they need to be well-versed in clinical trial design and protocol development, as these are the two main tasks they will be responsible for. The interviewer is looking for candidates who have experience in these areas, so they can be sure the candidate is capable of handling the job.
How to Answer:
When answering this question, you should provide a detailed explanation of your experience with clinical trial design and protocol development. Talk about the types of trials you have designed or developed protocols for, as well as any special techniques or methods you used. You can also mention any challenges you faced during these processes and how you overcame them. Finally, be sure to emphasize any successes you’ve had in designing successful clinical trials or developing effective protocols.
Example: “I have extensive experience with clinical trial design and protocol development. I’ve designed dozens of trials for pharmaceutical companies, ranging from phase 1 to phase 3 studies. My work has included developing protocols for randomized controlled trials, observational studies, and crossover studies. I have a strong understanding of the principles of good clinical practice and am well-versed in FDA regulations and guidelines. Additionally, I’m familiar with various statistical methods used in clinical research and have developed data analysis plans for my studies. I take great pride in my ability to develop effective protocols that lead to successful clinical trials.”
2. Describe a time when you had to manage multiple research projects simultaneously.
Clinical research associates often have a lot of tasks to juggle, from recruiting and screening participants to organizing and maintaining data. This question helps the interviewer get an understanding of how well you can multitask and manage your workload, as well as how you handle the stress of working on multiple projects at the same time.
To answer this question, you should focus on any experience you have in clinical trial design and protocol development. Talk about how you’ve worked with a team to develop protocols for trials, what processes you used, and the results of those protocols. You can also discuss other tasks that you may have completed related to clinical research such as data analysis or report writing. Finally, be sure to mention any tools or software you have used to help manage your workload.
Example: “In my previous role as a clinical research associate, I was responsible for managing multiple research projects simultaneously. I worked with the team to develop protocols for trials and then monitored their progress. To help me manage all of the tasks associated with each project, I used software such as Microsoft Project and Excel to track timelines, budgets, and other key data points. In addition, I regularly communicated with the participants and stakeholders involved in the trial to ensure that everything stayed on schedule. As a result of my work, we were able to successfully complete all of our research projects on time and within budget.”
3. How do you ensure that all data collected is accurate and compliant with regulations?
Clinical research is a highly regulated field, and it’s important for a Clinical Research Associate to remain compliant with all applicable laws and regulations. By asking this question, the interviewer is trying to get a sense of how you will be able to ensure accuracy and compliance when collecting data. They want to know that you understand the importance of accuracy and compliance, and that you will take the necessary steps to ensure that data is collected in a way that meets all applicable regulations.
You should emphasize your understanding of the importance of accuracy and compliance when collecting data. Explain that you are familiar with all applicable regulations, and that you will take the necessary steps to ensure accuracy and compliance. These steps may include double-checking data entry for accuracy, or verifying data against source documents. You can also explain that you have experience using software tools designed to help maintain accuracy and compliance, such as electronic data capture systems or digital forms.
Example: “I understand the importance of accuracy and compliance when collecting data, and I always take the necessary steps to ensure that all data collected is accurate and compliant with regulations. To do this, I double-check all data entry for accuracy and verify it against source documents. I also have experience using software tools designed to help maintain accuracy and compliance, such as electronic data capture systems or digital forms.”
4. Explain the concept of Good Clinical Practice (GCP) and how it applies to your role as a Clinical Research Associate.
Clinical research associates (CRAs) are responsible for ensuring that clinical trials are conducted according to the highest ethical and scientific standards. GCP is a set of ethical and scientific quality standards for the design, conduct, monitoring, and reporting of clinical trials. By asking this question, the interviewer is looking to assess your knowledge and understanding of GCP and how it applies to your role.
Start by explaining what Good Clinical Practice is and why it’s important. You can mention that GCP provides an international ethical and scientific quality standard for the design, conduct, monitoring, recording, auditing, analysis, and reporting of clinical trials. Then explain how you ensure compliance with GCP as a CRA. Talk about your experience in developing protocols, reviewing case report forms, collecting data, and conducting site visits to ensure compliance with applicable standards. Finally, discuss any additional measures you take to ensure the highest ethical and scientific standards are met.
Example: “Good Clinical Practice (GCP) is a set of international ethical and scientific quality standards for the design, conduct, monitoring, recording, auditing, analysis, and reporting of clinical trials. As a Clinical Research Associate, it’s my responsibility to ensure that all studies I am involved in adhere to GCP guidelines. This includes developing protocols, reviewing case report forms, collecting data, and conducting site visits to ensure compliance with applicable standards. Additionally, I always make sure to document any deviations from GCP and take corrective action when needed. My experience has taught me that following these guidelines is essential for the success of clinical trials.”
5. Are you familiar with the different types of clinical trials and their purpose?
Clinical research associates are responsible for a variety of tasks, from designing and implementing clinical studies to monitoring and analyzing results. To make sure you have the necessary knowledge and experience to handle these tasks, interviewers want to know if you’re familiar with the different types of clinical trials and their purpose. Being able to answer this question can demonstrate that you understand the basics of clinical research and can help the company design, execute, and analyze clinical trials.
To answer this question, you should be familiar with the types of clinical trials and their purpose. Common types of clinical trials include observational studies, interventional trials, randomized controlled trials, and crossover trials. You should also understand the different phases of a clinical trial (Phase I-IV) and what each phase entails. Finally, explain why these trials are important for advancing medical research and helping to develop new treatments and therapies.
Example: “Yes, I am familiar with the different types of clinical trials and their purpose. Observational studies are used to collect data on a particular group or population in order to identify any correlations between variables. Interventional trials involve giving participants either a drug or placebo and measuring the outcome. Randomized controlled trials compare two or more treatments by randomly assigning participants to one of the groups. Crossover trials measure the effects of multiple interventions over time. Finally, Phase I-IV clinical trials are conducted to evaluate safety, efficacy, and dosage of new drugs or treatments. These trials are essential for advancing medical research and helping to develop new treatments and therapies.”
6. What strategies do you use to stay organized while managing multiple research sites?
Clinical research associates have a lot of responsibility, and they need to be able to stay organized while they’re managing multiple research sites. This question gives the interviewer an opportunity to understand what strategies you use to keep track of all your tasks and make sure they’re completed in a timely manner.
Talk about the strategies you use to stay organized while managing multiple research sites. This could include things like using a calendar or scheduling system, setting up reminders for yourself, breaking projects down into smaller tasks, and delegating when needed. You can also discuss how you prioritize tasks and make sure all your deadlines are met. Additionally, talk about any specific tools or techniques that you’ve used in the past that have been successful.
Example: “I use a combination of strategies to stay organized while managing multiple research sites. I always have a calendar or scheduling system set up, and I make sure to break down larger projects into smaller tasks that are easier to manage. Additionally, I prioritize my tasks based on urgency and importance, so I can focus on the most important ones first. To ensure that I don’t miss any deadlines, I also set reminders for myself ahead of time. Finally, when needed, I’ll delegate tasks to other members of the team in order to complete projects quickly and efficiently. All these strategies help me stay organized and on top of all my responsibilities.”
7. How do you handle difficult conversations with research participants or sponsors?
Clinical research associates are responsible for collecting and documenting data and keeping track of records. This requires a lot of interaction with research participants, sponsors and other stakeholders, which can often be difficult conversations. An interviewer will want to know that you are capable of handling these conversations in a professional and effective manner.
To answer this question, you should discuss your approach to difficult conversations. For example, you could talk about how you maintain an open and honest dialogue with research participants or sponsors, while still being respectful of their time and opinions. You can also share examples of how you have successfully dealt with challenging conversations in the past, such as providing solutions or alternatives that satisfied both parties. Additionally, emphasize any communication skills you possess that help you navigate these types of conversations, such as active listening or problem-solving.
Example: “I understand that difficult conversations can be a part of my role as a clinical research associate, and I always strive to handle them with respect and professionalism. For example, when working with research participants or sponsors, I make sure to maintain an open dialogue and listen actively to their concerns. I also try to provide solutions or alternatives that are agreeable to both parties, while still staying on track with the project objectives. My strong communication skills help me to navigate these conversations effectively, ensuring that all stakeholders feel heard and respected.”
8. Describe a situation in which you had to troubleshoot an issue with a research site.
Clinical research associates are expected to be able to handle any and all problems that arise during a clinical trial. They must be able to quickly identify and address issues, from simple paperwork errors to more complex data discrepancies. This question allows the interviewer to get a sense of how you might handle any potential problems that may arise in the course of a research project.
Start by explaining the issue you faced, and then explain how you identified it. Talk about any steps you took to troubleshoot the problem and the methods you used to find a solution. If possible, provide an example of how your efforts resulted in a successful outcome. Finally, discuss what you learned from the experience and how it will help you in future projects.
Example: “During a recent clinical trial, I noticed a discrepancy between the data collected from one of the research sites and the data from the rest of the sites. I quickly identified the issue and identified the source of the discrepancy. I worked with the research site to troubleshoot the problem, and we were able to resolve the issue without having to halt the trial. This experience taught me the importance of being proactive in identifying and addressing potential issues, and I now have a better understanding of how to effectively troubleshoot any problems that may arise in a research project.”
9. What are the most important considerations for ensuring patient safety during a clinical trial?
Patient safety is paramount in clinical research, so it’s important for potential employers to make sure you understand the importance of safeguarding the participants in any clinical trial. This question is a great chance for you to demonstrate your knowledge of the regulations and protocols that govern clinical research and how to ensure that the trial is conducted in a safe and ethical manner.
Start by talking about the importance of patient safety in clinical research and how you prioritize it in your work. You can then discuss any protocols or regulations that you are aware of, such as those outlined by the FDA or other governing bodies. Talk about specific steps that you take to ensure patient safety, such as monitoring for adverse events, reporting any incidents, and following up with participants throughout the trial. Finally, mention any experience you have had with handling patient safety issues.
Example: “Patient safety is always my top priority when I am conducting clinical research. I always ensure that I am familiar with the relevant regulations and protocols, such as those outlined by the FDA, that govern clinical trials. I also take a number of steps to ensure patient safety, such as monitoring for adverse events and responding quickly if any are reported. I report any incidents to the appropriate parties and follow up with participants throughout the trial to ensure that they are comfortable and informed. I have also had experience in the past dealing with patient safety issues and I understand the importance of addressing them quickly and thoroughly.”
10. Have you ever worked on a project where there were language barriers between yourself and the research participants? If so, what did you do to overcome them?
Clinical research associates often have to work with people from different cultures and backgrounds, and they need to be able to communicate effectively with them. This means working with people who may have limited English proficiency or who use language that is unfamiliar to the interviewer. By asking this question, the interviewer is looking to find out if the candidate has the skills necessary to work in a multicultural environment and to find creative solutions to language barriers.
This question is designed to assess your ability to work with people from different cultural backgrounds and language abilities. You should be prepared to explain how you have handled language barriers in the past, such as utilizing translators or other resources, learning some basic phrases of the language, or using visual aids to communicate. Be sure to emphasize any successes that you’ve had in overcoming these challenges. Additionally, if you don’t have direct experience with this, discuss how you would go about tackling a similar challenge in the future.
Example: “I have worked on several projects where language barriers have been present. In these cases, I have employed a variety of strategies to ensure that I am able to communicate effectively with research participants. For instance, I have used translators to bridge the language gap, learned some basic phrases of the language, and utilized visual aids to communicate important research information. I have also found that having a friendly and open attitude towards the research participants helps to make them feel more comfortable and willing to communicate. Overall, I am confident that I have the skills necessary to effectively overcome language barriers in order to achieve successful research outcomes.”
11. What strategies do you use to ensure that research protocols are followed correctly at each research site?
Clinical research associates are responsible for ensuring the research protocols are followed correctly and that the data collected is accurate and reliable. They must be able to develop and implement strategies to ensure research sites are following the protocols established by the study sponsor. This question allows the interviewer to understand how the candidate approaches this responsibility and assess their level of experience in this area.
To answer this question, you should explain the strategies you use to ensure that research protocols are followed correctly at each research site. This may include conducting regular on-site monitoring visits, providing training and support to study staff, developing standard operating procedures (SOPs) for each research site, and ensuring all data collected is accurate and complete. Additionally, you can discuss any additional strategies you have used in the past such as establishing a system of checks and balances or implementing quality assurance processes.
Example: “I use a variety of strategies to ensure that research protocols are followed correctly at each research site. This includes conducting regular on-site monitoring visits to ensure that all study staff are following the protocols and procedures established by the study sponsor. I also provide training and support to study staff to ensure that all data collected is accurate and complete. In addition, I have developed standard operating procedures (SOPs) for each research site and established a system of checks and balances to ensure that all protocols are followed correctly. I have also implemented quality assurance processes to ensure that all data collected is accurate and reliable.”
12. Describe your experience working with Institutional Review Boards (IRBs).
Clinical research associates must have a strong understanding of the regulations and ethical considerations that surround the medical research field. Working with IRBs is a critical part of ensuring that a research study is conducted in a safe, ethical, and legal manner. This question is designed to ensure you have the knowledge and experience necessary to understand and comply with the applicable regulations.
To answer this question, you should provide specific examples of how you have worked with IRBs in the past. Talk about any protocols or procedures that you have followed to ensure compliance with regulations and ethical considerations. You can also mention any experience you have had submitting applications for approval, as well as any feedback you have received from the IRB on a research study. Additionally, it is important to demonstrate your understanding of the importance of working with an IRB and why their role is so critical in medical research.
Example: “I have extensive experience working with Institutional Review Boards (IRBs). I have prepared and submitted applications for approval, as well as developed protocols and procedures to ensure compliance with regulations and ethical considerations. I have also interacted with IRBs to discuss any changes or modifications that need to be made to the study. I understand the importance of working with an IRB and the role they play in ensuring that a research study is conducted in a safe, ethical, and legal manner. I am confident that I have the knowledge and experience necessary to work with IRBs effectively.”
13. What steps do you take to ensure that informed consent forms are properly completed by research participants?
Clinical research associates are responsible for ensuring that research participants are properly informed and have given consent before taking part in any study. This is a critical part of the job, and the interviewer wants to know that you understand the importance of this and are capable of following the necessary steps to ensure compliance with the applicable regulations.
Start by outlining the general steps you take when obtaining informed consent from research participants. Explain that you clearly explain the study to the participant and review any relevant documents or materials with them, such as the protocol and the informed consent form. You should emphasize that you always ensure that the participant fully understands what they are agreeing to before they sign the form. Additionally, discuss any additional steps you take to ensure compliance, such as having a witness present during the consent process or providing additional resources for the participant to review after signing the form.
Example: “When obtaining informed consent from research participants, I make sure to clearly explain the study and all relevant documents and materials, such as the protocol and the informed consent form, to the participant. I also ensure that they understand what they are agreeing to before they sign the form. Additionally, I always have a witness present during the consent process and provide additional resources for the participant to review after signing the form. This helps to ensure that the participant has been fully informed and is comfortable with the study before agreeing to participate.”
14. How do you handle conflicts between research staff and investigators?
Clinical research associates are responsible for managing the research process from start to finish, which includes managing any conflicts that may arise between research staff and investigators. The interviewer wants to ensure that the candidate can handle these conflicts in a professional, effective manner, and that they have the necessary skills to do so. This includes the ability to listen to both sides of the issue and identify potential solutions that work for everyone.
Start by describing a situation in which you had to manage a conflict between research staff and investigators. Explain the steps that you took to resolve the issue, such as listening to both sides of the story, understanding each person’s perspective, and identifying potential solutions that worked for everyone involved. Be sure to emphasize your ability to remain calm and professional throughout the process. Additionally, mention any strategies or techniques that you use to prevent conflicts from arising in the first place, such as proactively communicating expectations and keeping all parties informed about progress.
Example: “When I encounter conflicts between research staff and investigators, I always try to remain calm and professional. My top priority is to listen to both sides of the issue and understand all perspectives. I then identify potential solutions that work for everyone, and I make sure that all parties are informed about the outcome. I also take proactive steps to prevent conflicts from arising in the first place, such as setting clear expectations and timelines at the outset of a project, and keeping all parties informed about progress throughout the process.”
15. What methods do you use to monitor research progress and identify potential issues before they become serious problems?
Clinical research associates are responsible for overseeing research projects from start to finish, and they must be able to identify potential issues before they become serious problems. This question is designed to help the interviewer understand how you plan, monitor, and evaluate research projects and how you handle unexpected issues that might arise.
You should explain the methods you use to monitor research progress, such as tracking timelines and milestones, reviewing data regularly, and communicating with key stakeholders. You should also discuss how you identify potential issues before they become serious problems, such as proactively addressing any deviations from protocol or analyzing data for trends that may indicate a problem. Finally, you should describe how you handle unexpected issues, such as by quickly assessing the situation and taking appropriate action.
Example: “I use a combination of methods to monitor research progress and identify potential issues. I track timelines and milestones closely to make sure that the research is progressing on schedule, and I review data regularly to ensure accuracy and integrity. I also communicate regularly with key stakeholders to ensure that everyone is on the same page and that any potential issues are addressed quickly. Furthermore, I proactively analyze data for trends that may indicate a potential problem, and I address any deviations from protocol immediately. When unexpected issues arise, I quickly assess the situation and take appropriate action to ensure that the research progresses smoothly and that any potential issues are addressed in a timely manner.”
16. Do you have any experience with developing recruitment materials for clinical trials?
Clinical research associates are responsible for the day-to-day management of clinical trials, including patient recruitment and enrollment. Developing recruitment materials is a key part of the job, and interviewers will want to know if you have the experience to do it effectively. This question also serves as a way to gauge your understanding of the clinical research process and your overall knowledge of the industry.
If you have experience developing recruitment materials, this is a great opportunity to discuss your successes and the strategies you used. Talk about how you developed creative and effective messaging that resonated with potential participants and drove high-quality enrollments. If you don’t have any direct experience in this area, talk about transferable skills from past roles such as copywriting or marketing, and explain why they make you a strong candidate for this role.
Example: “I have extensive experience developing recruitment materials for clinical trials. In my previous role as a clinical research associate, I was responsible for designing and executing a comprehensive recruitment strategy for a high-profile clinical trial. I designed a series of print and digital materials targeting potential participants, which resulted in a high-quality enrollment of more than 500 participants. My approach was creative and data-driven, and I was able to use insights from our research to craft effective messaging that resonated with our target audience. I also have experience in copywriting and marketing, which I believe makes me well-suited for this role.”
17. How do you approach training new research staff members?
Clinical research associates are responsible for training and managing staff, as well as providing guidance to ensure research is conducted according to protocol. This question allows an interviewer to gauge your ability to lead and mentor others, as well as the level of detail you provide when providing instruction.
When answering this question, it’s important to demonstrate your ability to lead and guide others. Talk about the steps you take when training new staff members, such as providing a detailed overview of the protocol and expectations, offering hands-on demonstrations, and giving feedback throughout the process. You should also discuss the importance of remaining patient and understanding during the training process, especially if the individual is unfamiliar with the research field. Lastly, be sure to mention any additional techniques or methods you use to ensure new research staff understand the protocols and procedures they are expected to follow.
Example: “When training new research staff members, I make sure to provide a comprehensive overview of the protocol and expectations, and then I walk them through each step of the process. I also make sure to provide hands-on demonstrations of the tasks they will be expected to complete, and I give them feedback throughout the process. I understand that some research staff are new to the field and may not have the same level of experience as others, so I always make sure to remain patient and understanding. Additionally, I make sure to provide additional resources and materials they can refer to as needed, so they can continue to learn and grow in their role.”
18. What strategies do you use to maintain relationships with research sponsors?
Clinical research is a collaborative process, and it’s important for research associates to develop strong relationships with the sponsors they’re working with. Interviewers want to know that you’re able to maintain these relationships and keep them in good standing. By asking this question, they’re looking for an understanding of how you approach communication with sponsors and build trust with them over time.
The best way to answer this question is by providing concrete examples of strategies you’ve used in the past. You can talk about how you keep sponsors updated on progress and timelines, provide clear communication around any changes or delays, and show appreciation for their support. Additionally, you should emphasize your ability to be proactive and anticipate potential needs or issues that could arise. By demonstrating a strong understanding of how to maintain relationships with research sponsors, you’ll demonstrate that you have the skills necessary to be successful in the role.
Example: “I believe that strong relationships are the foundation of successful clinical research. I strive to maintain positive relationships with sponsors by being organized, reliable, and proactive. I always keep sponsors updated on progress and timelines, and I make sure to be clear and transparent in my communication around any changes or delays. I also make sure to show my appreciation for their support. Additionally, I’m always looking for opportunities to anticipate potential needs or issues that could arise and proactively address them. I believe that my commitment to communication and relationship building has been an essential part of my success as a clinical research associate.”
19. Describe a time when you had to present complex research results to a non-technical audience.
Clinical research associates are often responsible for communicating complex research results to a variety of stakeholders, from scientists and physicians to government representatives and the public. It’s important to be able to effectively communicate the results of your research in an understandable and accessible way. This question helps determine if you have the ability to do that.
To answer this question, you should provide an example from your past experience of a time when you had to present complex research results to a non-technical audience. Talk about the challenges you faced in terms of understanding their level of knowledge and how you overcame them. Describe how you were able to explain the research results in a way that was easy for them to understand. Finally, discuss what you learned from the experience and how it has helped you with similar presentations since then.
Example: “I once had to present a complex study on cancer genetics to a group of local government representatives who weren’t experts in the field. I was nervous at first, because I knew they wouldn’t understand the technical details of the research. But I was able to break down the results into simple language and visuals that made it easier for them to comprehend. I also made sure to answer any questions they had in a way that was easy for them to understand. After the presentation, I received positive feedback from the representatives, and I felt confident that I had communicated the study results effectively. From this experience, I learned the importance of tailoring my message to the audience, and I now make sure to do this for all of my presentations.”
20. What challenges have you faced while conducting remote clinical trials?
Clinical research associates often work remotely, and this question is designed to assess your understanding of the challenges associated with that type of work. It’s important to be able to communicate effectively with colleagues and stakeholders, as well as to be able to troubleshoot any issues that may arise while working remotely. This question also assesses how well you handle the unique challenges of remote clinical trials.
Be prepared to discuss the challenges you’ve faced while working remotely, such as difficulty in communication and coordination with colleagues, or problems with data collection. Additionally, talk about how you have overcome these challenges, such as using video conferencing tools or other technology platforms to stay connected with colleagues. You can also mention any strategies you use to remain organized and efficient while conducting remote clinical trials.
Example: “I’ve had a lot of experience conducting remote clinical trials and I’m well aware of the challenges that come with it. One of the biggest challenges I’ve faced is staying organized and on track with data collection. To overcome this, I use project management software and task tracking tools to ensure that data is collected on time and accurately. I also communicate regularly with my colleagues via video conferencing and other online collaboration tools to ensure that everyone is on the same page. I’ve found that these strategies have been effective in helping me to stay organized and efficient when conducting remote clinical trials.”
20 Continuous Improvement Specialist Interview Questions and Answers
20 most asked ulta beauty advisor interview questions (with answers), you may also be interested in..., 30 subcontract administrator interview questions and answers, 20 long term care administrator interview questions and answers, 30 customer assistant interview questions and answers, 30 tenant coordinator interview questions and answers.
Resume Worded | Proven Resume Examples
- Resume Examples
- Research & Science Resumes
10 Clinical Research Resume Examples - Here's What Works In 2024
Clinical research is an important part of the pharmaceutical industry and helps us understand illnesses and create effective drugs to improve our health. this guide will show clinical researchers how to create winning resumes and progress in their clinical research careers..

Being a clinical researcher requires a very specific set of skills and extended research experience. Educationally, recruiters will be looking to see at least a bachelor’s degree in a life science subject. Beyond that, you need to show that you can use all industry standards research tools and that you understand clinical research procedures and regulations.
If you manage to get a job in this profession, you will be joining just over 7 thousand researchers (in the US), 61.7% of whom are women. You will most likely work in the education sector or the private sector, with those two sectors taking up around 73% of all clinical researchers.
Beyond your education and industry standards tools, your resume should indicate your great communication and teamwork skills as well as dedication to your work and critical thinking skills. This guide includes successful resume samples approved by recruiters, as well as some skills and action verb options that you could use to elevate your resume today.
Clinical Research Resume Templates
Jump to a template:
- Clinical Research Associate
- Clinical Research Coordinator
- Research Coordinator
- Clinical Pharmacist
- Clinical Manager
- Credentialing Specialist
- Clinical Data Manager
- Medical Science Liaison
- Clinical Trial Manager
Jump to a resource:
- Keywords for Clinical Research Resumes
Clinical Research Resume Tips
- Action Verbs to Use
- Related Medical Resumes
Get advice on each section of your resume:
Template 1 of 10: Clinical Research Associate Resume Example
A clinical research associate (CRA) is in charge of planning and coordinating the execution of clinical trials aimed at testing products. You will also serve as the contact person for doctors, patients, pharmacists, etc. This means you will need to have excellent skills and a strong clinical research background. Take a look at this recruiter-approved resume.

We're just getting the template ready for you, just a second left.
Tips to help you write your Clinical Research Associate resume in 2024
show your career progression in medical research..
Show recruiters that you are a hardworking and successful researcher by showing an upwards trajectory of your medical research career. It will also show recruiters your dedication to your profession. This applicant started as a medical researcher and moved their way up to a clinical research associate.

Include information about some of the medical trials you have conducted.
Conducting medical trials is a key part of your job. Give recruiters some insight into the kind of trials you have conducted. For example, this applicant has conducted trials on chest pain.
Skills you can include on your Clinical Research Associate resume
Template 2 of 10: clinical research coordinator resume example.
In this position, you will not be conducting the trials themselves but rather coordinating to ensure everything that needs to be done for the trial is done. This may include ensuring the finances and personnel needed are available, and that all related regulations and laws are followed. You will be reporting to a clinical principal investigator.

Tips to help you write your Clinical Research Coordinator resume in 2024
include your strongest and most relevant abilities in the introduction..
Start your resume strong by including your strongest and most relevant skills and abilities in the introduction section. It sets a strong and impressive tone for the rest of your resume.
Include relevant awards or recognition.
Awards show that not only do you do excellent work but that your work is good enough to receive recognition over others. This applicant has effectively added 2 relevant awards.

Skills you can include on your Clinical Research Coordinator resume
Template 3 of 10: research coordinator resume example.
In this position, you will be the support system for clinical trials and other lab investigations. You will not work directly on the trials but you will assist in any administrative work that needs to be done and ensure that the trials have everything that is needed and that they follow all guidelines. In essence, you will be coordinating all the moving parts that must work together to make a trial successful. Take a look at this successful resume sample.

Tips to help you write your Research Coordinator resume in 2024
use coordinator keywords throughout your resume..
Using keywords related to coordinator functions is a great way to ensure that your resume highlights your coordinator experience. Some keywords this applicant uses include ‘spreadsheets’, ‘organizing’, and ‘WCAG’.

Include student research experience.
If you do not have a lot of professional research and coordinator experience since graduating from school, you can include any student research experience you had. The skills used and experience gained would largely be the same as you would get from professional experience.

Skills you can include on your Research Coordinator resume
Template 4 of 10: clinical pharmacist resume example.
As a clinical pharmacist, your resume needs to convey your unique blend of patient care and technical knowledge. With recent industry trends leaning towards an increased involvement in patient management and care, clinical pharmacists are being called upon to be more hands-on. This makes it critical to showcase your experience and skills in direct patient care and health management on your resume. Keep in mind the increasing use of electronic health records (EHRs) across companies and facilities. A clinical pharmacist who is proficient in the latest EHRs systems is an asset, so don't forget to include your technical skills as well.

Tips to help you write your Clinical Pharmacist resume in 2024
emphasize your patient care skills.
Clinical pharmacist roles have evolved to become more patient-oriented. This means when you're creating your resume, you should emphasize how you've directly contributed to patient care: adjusting medication plans, educating patients, or collaborating with other healthcare professionals for optimal patient outcomes.

Showcase your proficiency in EHRs
As digital transformation sweeps through the healthcare sector, being tech-savvy is no longer optional for a clinical pharmacist. In your resume, it's crucial to highlight your proficiency in using electronic health records (EHRs). If you have experience using specific systems, list them.
Skills you can include on your Clinical Pharmacist resume
Template 5 of 10: clinical manager resume example.
Thinking of stepping into a Clinical Manager role? Well, it's a multi-faceted job that requires not just medical expertise, but solid management skills as well. The healthcare industry has seen a push for more efficiency and better patient care, and Clinical Managers play a crucial role in achieving these goals. As such, recruiters are on the lookout for those who can balance administrative tasks while ensuring top-notch medical service. When crafting your resume, it's important to demonstrate this balance clearly. Clinical Managers are now often expected to lead initiatives like electronic health record implementation or new healthcare regulations. This has made experience with project management and change leadership highly desirable. So, besides your clinical experience and skills, it's essential to showcase these aspects on your resume to stand out in today's market.

Tips to help you write your Clinical Manager resume in 2024
show off your project management experience.
Clinical Managers are expected to lead major initiatives, so it's important to incorporate your project management experience on your resume. You should discuss specific projects you've managed, the teams involved, and the results achieved.

Prove your proficiency in healthcare regulations
Given the rapidly changing landscape, compliance with healthcare regulations is crucial. Your resume should indicate your understanding of current healthcare laws, policies, and procedures. Mention any audit or compliance activities you've been involved in, and the outcomes.
Skills you can include on your Clinical Manager resume
Template 6 of 10: clinical manager resume example.
Clinical Managers navigate the busy intersection of healthcare administration and patient care, often working behind the scenes to keep hospitals or clinics running smoothly. The demand for these professionals is on the rise, with changes in healthcare policy and an increasingly aging population requiring efficient management. When writing your resume, it’s crucial to convey your capability to handle the administrative side of healthcare, while not losing sight of patient satisfaction and staff morale. As a Clinical Manager, you're expected to wear many hats, so your resume must reflect your multifaceted skill set, from project management to your understanding of healthcare regulations.
Detail your understanding of healthcare policy
A Clinical Manager role often entails making decisions based on healthcare policy. Therefore, you should include any experience you have in interpreting healthcare policy changes and implementing policies in a clinical setting. This can illustrate your ability to navigate the complexities of the healthcare system effectively.
Quantify your achievements in patient care quality
As a Clinical Manager, one of your key responsibilities is to manage and improve patient care quality. On your resume, go beyond simply listing this responsibility. Include metrics or quantifiable evidence of how your management improved patient care quality or increased patient satisfaction during your previous roles.

Template 7 of 10: Credentialing Specialist Resume Example
As a Credentialing Specialist, you're the gatekeeper of quality and competency within a healthcare setting. This role is critical and unique, seeing that you are the one verifying the qualifications and backgrounds of medical professionals. These are sensitive tasks, governed by specific rules and regulations. Recently, we've noticed a trend for Credentialing Specialists to have a more prominent role in healthcare compliance, so it's essential to emphasize your understanding of these aspects. When developing your resume, remember that you're marketing your unique skills and knowledge within this niche field. It's not just about listing job duties but showing how you've excelled in this role.

Tips to help you write your Credentialing Specialist resume in 2024
show knowledge of compliance and regulations.
In your resume, it's essential you demonstrate your understanding of healthcare industry compliance and regulations. Mention specific laws, standards, or regulations you've worked with and how you ensured adherence within your organization.

Experience with credentialing software
Credentialing Specialists often use specific software to track and manage information. If you're familiar with any of these systems, ensure you mention them. This shows you can hit the ground running without needing much training.

Skills you can include on your Credentialing Specialist resume
Template 8 of 10: clinical data manager resume example.
Clinical Data Managers oversee the complete lifecycle of clinical trials, from design and setup to data collection and analysis. Their role is pivotal in ensuring the accuracy and reliability of data used in healthcare decisions. Recently, there has been a surge in demand for this job, especially with the increase in remote clinical trials and focus on digital health. When crafting your resume for such a role, remember it's not just about showcasing your technical abilities, but also demonstrating your grasp of regulatory guidelines, data management best practices, and problem-solving skills. In the ever-evolving world of healthcare, staying updated with new technologies and methodologies is integral. Potential employers are on the lookout for Clinical Data Managers who keep up with industry trends and standard regulations. When preparing your resume, emphasize your continuously updated industry knowledge, understanding of the latest software, and ability to adapt and learn quickly.
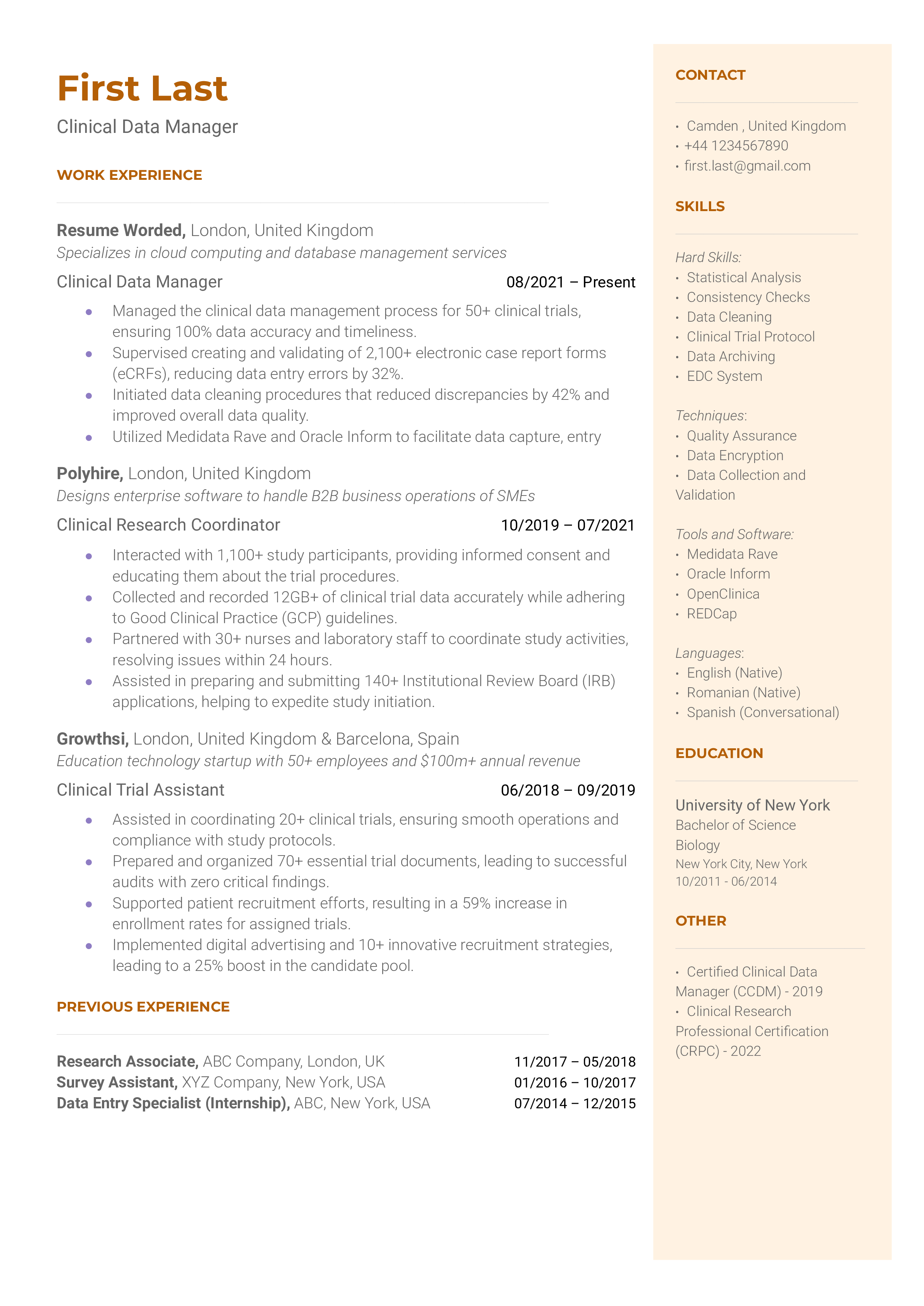
Tips to help you write your Clinical Data Manager resume in 2024
showcase your technical proficiency.
You should list and elaborate on the platforms and software you've used in data collection and management. Know your way around Oracle Clinical, Medidata RAVE, or SAS? Include these. It's essential to show that you're equipped with the necessary technical skills.
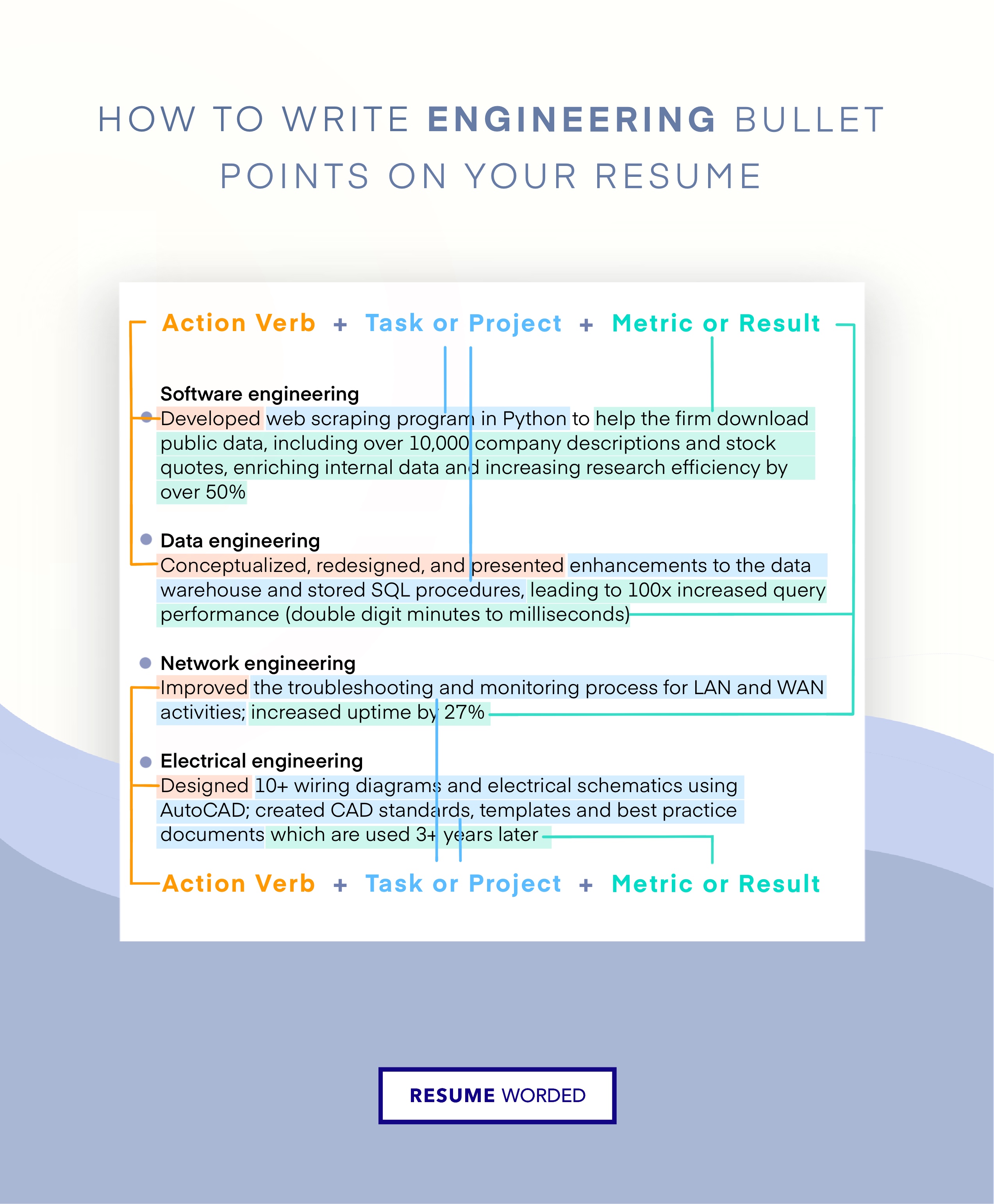
Highlight Regulatory Knowledge
A Clinical Data Manager needs a solid understanding of regulations like HIPAA and FDA requirements. Make sure your resume reflects your familiarity with these regulations and any successful audits or inspections you've handled.
Skills you can include on your Clinical Data Manager resume
Template 9 of 10: medical science liaison resume example.
As a Medical Science Liaison (MSL), you're a bridge between pharmaceutical companies and healthcare professionals. This role is all about translating complex scientific information into digestible insights that help improve patient care. The MSL industry has evolved recently, with an emphasis on demonstrating value to healthcare providers beyond just providing data. Your resume needs to show hiring managers that you're not only a capable mediator, but also that you’re up-to-date on the latest industry trends. Further, it's pivotal to illustrate your ability to build and maintain relationships, lead educational trainings, and ensure compliance with industry regulations.

Tips to help you write your Medical Science Liaison resume in 2024
showcase your scientific expertise.
Your resume should detail your deep understanding of the therapeutic area you'll be working in. While your degree(s) will show your academic qualifications, use your experience section to highlight specific projects or initiatives where you've applied this knowledge.
Evidence of relationship-building skills
MSL roles require the ability to build and maintain relationships with key opinion leaders in the healthcare field. Use your resume to show how you've successfully built these relationships in the past, whether through network development, clinical trial collaboration, or peer-to-peer education.

Skills you can include on your Medical Science Liaison resume
Template 10 of 10: clinical trial manager resume example.
As a Clinical Trial Manager, you're in the exciting and challenging world of managing clinical research activities. This role often involves collaborating with different stakeholders and ensuring clinical trials are executed properly, safely, and ethically. Over the past few years, the industry has seen an increase in the use of technology and data analysis, so understanding these trends can give your resume an edge. When crafting your resume, it's crucial to show not only your knowledge in managing clinical trials but also your ability to adapt to new technologies and relevant regulations. Your resume is your opportunity to showcase your experiences and achievements. It should tell the story of how you've successfully managed trials and navigated regulatory requirements. It's not just about listing your previous roles, but about showing how your unique contributions have made a difference in those positions. In doing so, remember to quantify your achievements where possible.
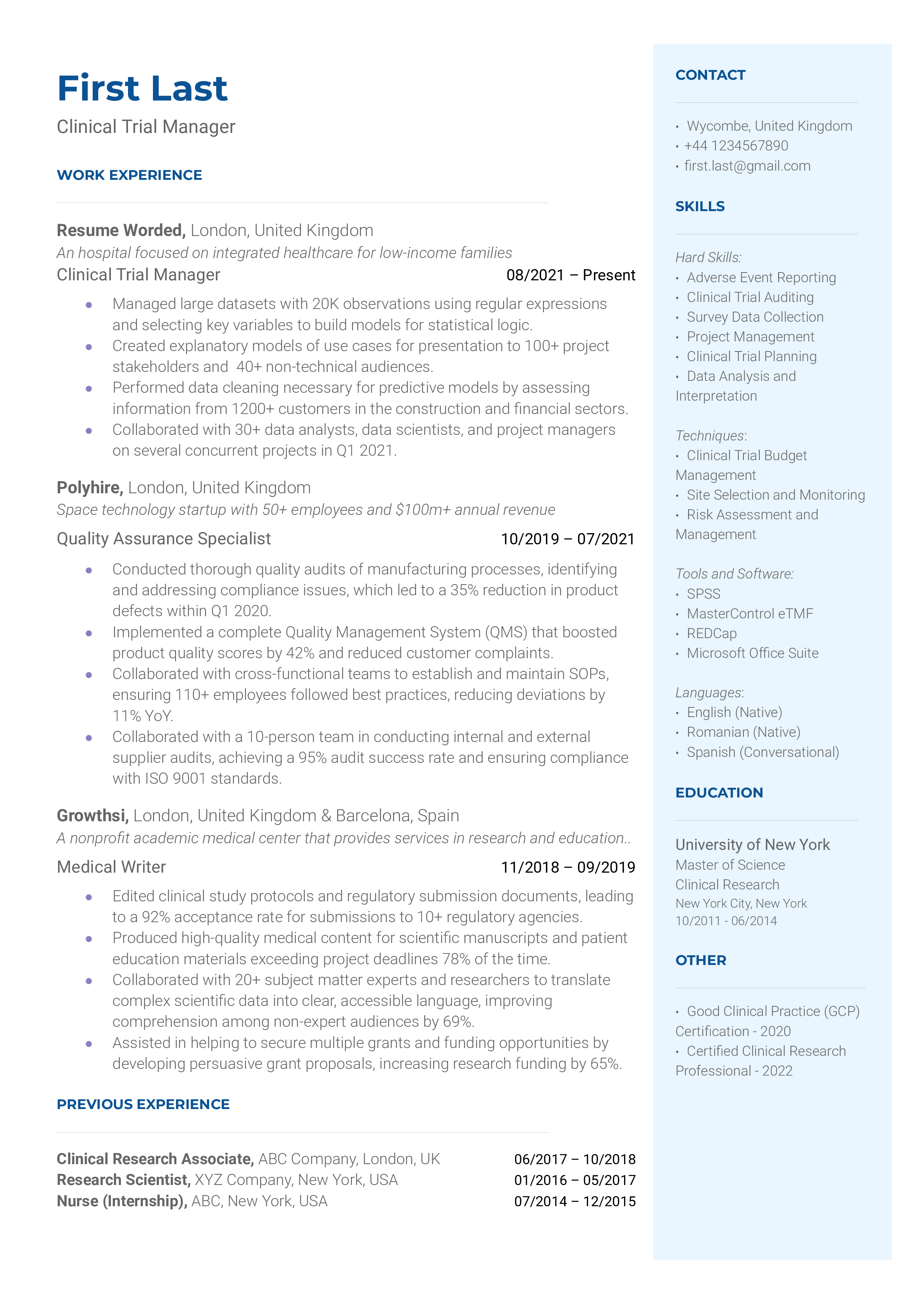
Tips to help you write your Clinical Trial Manager resume in 2024
include certifications and relevant qualifications.
Beyond your educational background, it's crucial to include any certifications or qualifications related to clinical trial management. Certifications like Certified Clinical Research Professional (CCRP) or Certified Clinical Research Associate (CCRA) show you're well-qualified and committed to staying current in the field.

Detail your experience with technology and data analysis
Given the rise of technology in clinical trials, it's wise to detail any experience you have with relevant software, databases, and data analysis. Showing your proficiency in using electronic data capture systems or statistical software can make your resume stand out to a hiring manager.

Skills you can include on your Clinical Trial Manager resume
We spoke with hiring managers at leading clinical research organizations, including Pfizer, Johnson & Johnson, and Novartis, to gather their insights on what makes a strong clinical research resume. Based on their feedback and our analysis of successful resumes in this field, we've compiled a list of essential tips to help you craft a compelling clinical research resume that will catch the attention of employers and increase your chances of landing an interview.
Highlight your clinical research experience
Employers want to see that you have hands-on experience in clinical research. When describing your work history, focus on your specific roles and responsibilities in clinical trials.
- Managed a Phase III clinical trial for a novel cancer treatment, overseeing a team of 12 research associates and ensuring compliance with FDA regulations
- Conducted patient recruitment and enrollment for a 500-participant cardiovascular study, exceeding target enrollment by 15%
Avoid vague or general descriptions that don't showcase your unique contributions:
- Worked on various clinical trials
- Assisted with patient recruitment and data collection

Showcase your therapeutic area expertise
Many clinical research positions require specialized knowledge in specific therapeutic areas, such as oncology, neurology, or infectious diseases. If you have experience or training in a particular area, make sure to highlight this on your resume.
- Conducted clinical trials for novel immunotherapies in the treatment of melanoma and lung cancer
- Collaborated with neurology specialists to develop protocols for a Phase II study on Alzheimer's disease
If you lack direct experience in a therapeutic area, consider emphasizing any relevant coursework, certifications, or personal research you've conducted to demonstrate your interest and knowledge.
Demonstrate your knowledge of regulatory requirements
Clinical research is heavily regulated, and employers value candidates who have a strong understanding of relevant guidelines and regulations, such as Good Clinical Practice (GCP) and FDA requirements.
- Ensured compliance with ICH-GCP guidelines and local regulatory requirements for a global Phase III trial
- Collaborated with Quality Assurance to prepare for FDA audits and maintain inspection-readiness
If you have completed any training or certifications related to regulatory compliance, such as NIH Human Subjects Protection or HIPAA training, make sure to include these on your resume as well.
Quantify your achievements
Whenever possible, use specific metrics to quantify your accomplishments and demonstrate the impact of your work. This helps employers better understand the scope and significance of your contributions.
- Managed a $5 million budget for a global Phase III clinical trial, ensuring all milestones were met on time and within budget
- Implemented a new electronic data capture system, reducing data entry errors by 30% and saving an estimated 200 hours per month
Avoid using vague or subjective terms to describe your achievements:
Successfully managed large budgets and improved data quality for several clinical trials
Tailor your resume to the job description
Customize your resume for each job application to highlight the skills and experiences that are most relevant to the position. Carefully review the job description and incorporate keywords and phrases that align with the employer's requirements.
For example, if a job posting emphasizes experience with oncology clinical trials, make sure to prioritize any relevant oncology experience on your resume:
- Managed a Phase II clinical trial for a novel targeted therapy in advanced non-small cell lung cancer
- Collaborated with oncologists to develop study protocols and ensure patient safety in a first-in-human solid tumor trial
Include relevant certifications and professional development
In addition to your formal education, include any relevant certifications or professional development courses you have completed. These can demonstrate your commitment to staying current in the field and expanding your skill set.
- Certified Clinical Research Professional (CCRP) - Association of Clinical Research Professionals
- Completed NIH Good Clinical Practice (GCP) and HIPAA Privacy Rule Training
If you have attended any conferences, workshops, or seminars related to clinical research, consider including these as well, especially if you presented or contributed to the event in some way.
Writing Your Clinical Research Resume: Section By Section
header, 1. include your full name and key contact details.
Your header should include your full name, phone number, and email address. If you have a professional website or LinkedIn profile, you can include those links as well. Make sure your email address is professional and avoid using nicknames or aliases.
- John Doe, Ph.D. | 123-456-7890 | [email protected] | linkedin.com/in/johndoe
- Jane Smith, M.S. | 987-654-3210 | [email protected] | janesmith.com
Avoid cluttering your header with unnecessary details or unprofessional email addresses like the following:
- John "Johnny" Doe | 123-456-7890 | [email protected] | 123 Main St, Anytown USA
2. Highlight relevant credentials and certifications
If you have relevant credentials or certifications for clinical research, such as a CCRP (Certified Clinical Research Professional) or a SOCRA certification, include them after your name. This helps establish your credibility and expertise right from the start.
- John Doe, Ph.D., CCRP | 123-456-7890 | [email protected]
- Jane Smith, M.S., SOCRA-certified | 987-654-3210 | [email protected]
However, avoid listing irrelevant or outdated certifications that don't directly apply to clinical research roles, such as:
- John Doe, CPR-certified, OSHA 30 | 123-456-7890 | [email protected]
3. Keep it simple and easy to read
Your header should be clean, concise, and easy to read at a glance. Use a clear font and simple formatting. Avoid using multiple colors, graphics, or complex layouts that can distract from the key information.
John Doe, Ph.D. Clinical Research Associate 123-456-7890 | [email protected] | linkedin.com/in/johndoe
Compare that to an overly complex header like this:
Dr. John Doe, Ph.D., CCRP, ACRP Senior Clinical Research Associate | Data Manager | Project Leader 123-456-7890 | [email protected] 123 Main St, Anytown, USA 12345 linkedin.com/in/johndoe | facebook.com/johndoe | twitter.com/johndoe
Keep it simple and focused on the most important details.
Summary
A resume summary, also known as a professional summary or summary statement, is an optional section that sits at the top of your resume, just below your contact information. It provides a brief overview of your professional background, skills, and accomplishments, giving hiring managers a quick snapshot of what you bring to the table.
While a summary is not strictly necessary, it can be particularly useful if you are a career changer or have a diverse background that may not be immediately apparent from your work history. It allows you to provide context and connect the dots for the reader. However, avoid using an objective statement, which focuses on your goals rather than what you can offer the employer.

To learn how to write an effective resume summary for your Clinical Research resume, or figure out if you need one, please read Clinical Research Resume Summary Examples , or Clinical Research Resume Objective Examples .

1. Tailor your summary to the clinical research role
When crafting your summary for a clinical research position, it's essential to align your skills and experience with the requirements of the role. Research the position and the company to identify the key qualifications they are seeking.
For example, if the job emphasizes experience with clinical trial protocols, you might write:
- Clinical Research Associate with 5+ years of experience in managing and executing clinical trial protocols for Phase I-III studies.
In contrast, a generic summary like the following doesn't provide any specific information relevant to clinical research:
- Experienced professional seeking a challenging position in a reputable organization where I can utilize my skills and knowledge.
2. Highlight your clinical research skills and achievements
Your summary is the perfect place to showcase your most impressive clinical research skills and accomplishments. Focus on the specific expertise and experiences that make you a strong candidate for the role.
Instead of simply listing your skills, quantify your achievements to make them more impactful. For instance:
- Managed a portfolio of 10+ clinical trials, ensuring compliance with FDA regulations and GCP guidelines.
- Collaborated with cross-functional teams to develop and implement study protocols, leading to a 20% reduction in study start-up time.
Avoid using vague or generic phrases that don't provide any concrete information, such as:
- Skilled in clinical research.
- Proven track record of success.
3. Keep your summary concise and objective
Your resume summary should be a brief, high-level overview of your professional profile. Aim for a few concise sentences or bullet points that encapsulate your most relevant qualifications and experiences. A good rule of thumb is to keep your summary to around 50-100 words.
Clinical Research Coordinator with 3+ years of experience in oncology clinical trials. Skilled in patient recruitment, informed consent, and data management. Collaborated with investigators to ensure protocol compliance and patient safety. Seeking to leverage expertise to contribute to groundbreaking research at XYZ Pharmaceuticals.
Avoid writing a lengthy or overly detailed summary that reads like a dense paragraph. Also, steer clear of subjective statements or self-assessments, such as:
Highly motivated and hardworking individual with a passion for clinical research. I am a team player with excellent communication skills and a strong work ethic. My goal is to secure a challenging position where I can grow and make a difference.
Experience
The work experience section is the heart of your resume. It's where you show potential employers what you've accomplished in previous roles and how you can add value in the position you're applying for. When writing your work experience section for a clinical research role, focus on highlighting your relevant skills, achievements, and impact.
1. Tailor your bullet points to the job description
When applying for a clinical research role, it's crucial to align your work experience with the requirements and responsibilities listed in the job description. This demonstrates to the hiring manager that you have the relevant skills and experience they're looking for.
For example, if the job description mentions experience with clinical trial protocols, you could include a bullet point like:
- Developed and implemented clinical trial protocols for a Phase III study, ensuring adherence to FDA regulations and GCP guidelines
In contrast, a generic bullet point that doesn't relate to the job description might look like:
- Assisted with various tasks in the clinical research department
2. Quantify your achievements with metrics
Numbers and metrics help to contextualize your achievements and make them more impactful. They give the hiring manager a clear picture of the scale and scope of your work. When possible, include specific numbers and percentages to quantify your accomplishments.
Here are some examples of how to incorporate metrics:
- Managed a clinical trial with 150+ participants across 5 sites, resulting in a 95% retention rate
- Reduced data query resolution time by 30% through implementing a new workflow process
- Co-authored 3 peer-reviewed publications in top-tier medical journals
3. Highlight relevant tools and technologies
Clinical research often involves using various tools and technologies, such as electronic data capture (EDC) systems, clinical trial management systems (CTMS), and statistical analysis software. Showcasing your proficiency with these tools demonstrates your technical skills and ability to work efficiently in a clinical research setting.
For example:
- Proficient in using EDC systems such as Medidata Rave and Oracle Clinical
- Experienced in managing clinical trial data using CTMS platforms like Veeva Vault and Oracle Siebel
- Skilled in conducting statistical analyses using SAS and R
4. Showcase your career progression
Highlighting your career growth within the clinical research field shows your dedication, adaptability, and potential for future growth. If you've been promoted or taken on increasing responsibilities in your previous roles, make sure to emphasize this in your work experience section.
Clinical Research Associate, XYZ Pharmaceuticals Jan 2018 - Present - Promoted from Clinical Research Coordinator to Clinical Research Associate within 2 years due to outstanding performance and leadership skills - Manage a portfolio of 8 clinical trials, overseeing all aspects of study conduct from start-up to close-out - Serve as a mentor to junior CRCs, providing training and guidance on study protocols and GCP compliance
Education
Your education section is a key part of your clinical research resume. It shows hiring managers that you have the necessary qualifications and background to excel in the role. Follow these tips to make sure your education section is effective and compelling.
1. Put education at the top if you're a recent grad
If you graduated within the last few years, place your education section above your work experience. This highlights your most relevant qualification first. For example:
Bachelor of Science in Biology University of California, Los Angeles Graduated: May 2022 Relevant Coursework: Clinical Research Methods Biostatistics Pharmacology
Avoid this mistake:
- Listing your high school diploma when you have a college degree
- Placing education at the bottom if it's your strongest qualification
2. Keep it concise if you're experienced
If you have several years of clinical research experience, keep your education section brief. Place it below your work history and simply list your degree, school, and graduation year. For example:
M.S. Biomedical Sciences, University of Pennsylvania B.S. Biology, Boston University
Seasoned professionals should avoid:
- Listing graduation dates, which may lead to ageism
- Including coursework or irrelevant details that distract from work accomplishments
3. Highlight clinical research certifications
Clinical research certifications demonstrate your expertise and qualifications. You can list them in your education section or a separate certifications section. For example:
- Certified Clinical Research Professional (CCRP), SOCRA
- Certified Clinical Research Coordinator (CCRC), ACRP
Certifications to avoid listing:
- Online course completion certificates
- Expired or irrelevant certifications
- Memberships or affiliations not directly related to clinical research
Action Verbs For Clinical Research Resumes
Action verbs are a powerful way to frame your achievements and experience effectively. They can be used to make your abilities look diverse and your achievements look impressive. For a clinical researcher, you need your action verbs to show that you are skilled in multiple stages of the research process. They also need to highlight your successes and show recruiters that you work well in teams and that your work has borne fruit.
This is a sample of action verbs that can be used to elevate your resume. Use those that apply most to your experience.
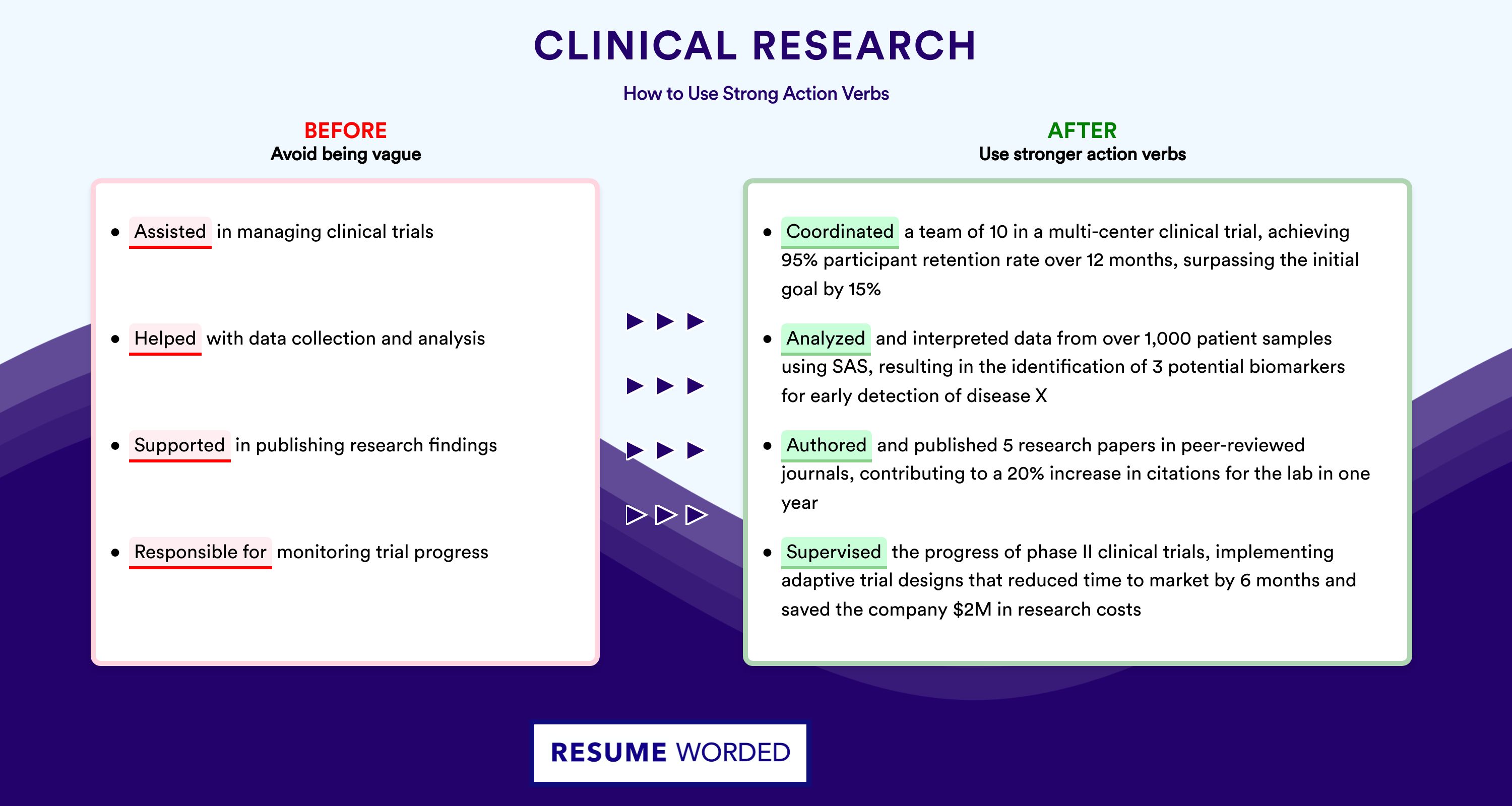
- Coordinated
- Collaborated
For more related action verbs, visit Medical Action Verbs .
For a full list of effective resume action verbs, visit Resume Action Verbs .
Action Verbs for Clinical Research Resumes
Skills for clinical research resumes.
This profession is very technical and as such your skills section should reflect that technical nature. For one, you should show that you are well-versed in the regulations and industry expectations of your research so include things like ‘Good Clinical Practice (GCP)’.
You also need to show that you are experienced in using the industry standard tools for clinical research. If you can find out the tools used by the company you are applying to, add all those tools to your list. Ensure you do a crash course in the tools you are not familiar with, before adding them to your tools section.
It would also be beneficial to include the areas of healthcare that you have done clinical research in. For example, specify ‘Oncology clinical research’ instead of only listing ‘clinical research’. It helps give recruiters a more clear understanding of your research background.
Here is a list of skills that would be beneficial to add to your skills section.
- Good Clinical Practice (GCP)
- Clinical Monitoring
- Clinical Trial Management System (CTMS)
- Electronic Data Capture (EDC)
- Clinical Trials
- Clinical Research
- Clinical Development
- CRO Management
- Clinical Research Associates
- Clinical Data Management
- Standard Operating Procedure (SOP)
- Therapeutic Areas
- Clinical Operations
- Oncology Clinical Research
- Regulatory Submissions
- U.S. Food and Drug Administration (FDA)
- Clinical Site Monitoring
- Biotechnology
How To Write Your Skills Section On a Clinical Research Resumes
You can include the above skills in a dedicated Skills section on your resume, or weave them in your experience. Here's how you might create your dedicated skills section:
Skills Word Cloud For Clinical Research Resumes
This word cloud highlights the important keywords that appear on Clinical Research job descriptions and resumes. The bigger the word, the more frequently it appears on job postings, and the more 'important' it is.
How to use these skills?
Other medical resumes, equity research.

Microbiologist

Research Assistant

- Nursing Resume Guide
- Dental Assistant Resume Guide
- Case Manager Resume Guide
- Respiratory Therapist Resume Guide
- Medical Billing Resume Guide
- Therapist Resume Guide
- Quality Control Resume Guide
- Care Coordinator Resume Guide
- Occupational Therapist Resume Guide
Clinical Research Resume Guide
- Radiologic Technologist Resume Guide
- Pharmacy Technician Resume Guide
- Medical Technologist Resume Guide
- Microbiologist Resume Guide
- SLP Resume Guide
- Clinical Research Associate Resume Example
- Clinical Research Coordinator Resume Example
- Research Coordinator Resume Example
- Clinical Pharmacist Resume Example
- Clinical Manager Resume Example
- Credentialing Specialist Resume Example
- Clinical Data Manager Resume Example
- Medical Science Liaison Resume Example
- Clinical Trial Manager Resume Example
- Tips for Clinical Research Resumes
- Skills and Keywords to Add
- All Resume Examples
- Clinical Research CV Examples
- Clinical Research Cover Letter
- Clinical Research Interview Guide
- Explore Alternative and Similar Careers
Download this PDF template.
Creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option..
- Have an account? Sign in
E-mail Please enter a valid email address This email address hasn't been signed up yet, or it has already been signed up with Facebook or Google login.
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number. It looks like your password is incorrect.
Remember me
Forgot your password?
Sign up to get access to Resume Worded's Career Coaching platform in less than 2 minutes
Name Please enter your name correctly
E-mail Remember to use a real email address that you have access to. You will need to confirm your email address before you get access to our features, so please enter it correctly. Please enter a valid email address, or another email address to sign up. We unfortunately can't accept that email domain right now. This email address has already been taken, or you've already signed up via Google or Facebook login. We currently are experiencing a very high server load so Email signup is currently disabled for the next 24 hours. Please sign up with Google or Facebook to continue! We apologize for the inconvenience!
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number.
Receive resume templates, real resume samples, and updates monthly via email
By continuing, you agree to our Terms and Conditions and Privacy Policy .
Lost your password? Please enter the email address you used when you signed up. We'll send you a link to create a new password.
E-mail This email address either hasn't been signed up yet, or you signed up with Facebook or Google. This email address doesn't look valid.
Back to log-in
These professional templates are optimized to beat resume screeners (i.e. the Applicant Tracking System). You can download the templates in Word, Google Docs, or PDF. For free (limited time).
access samples from top resumes, get inspired by real bullet points that helped candidates get into top companies., get a resume score., find out how effective your resume really is. you'll get access to our confidential resume review tool which will tell you how recruiters see your resume..

Writing an effective resume has never been easier .
Upgrade to resume worded pro to unlock your full resume review., get this resume template (+ 9 others), plus proven bullet points., for a small one-time fee, you'll get everything you need to write a winning resume in your industry., here's what you'll get:.
- 📄 Get the editable resume template in Google Docs + Word . Plus, you'll also get all 9 other templates .
- ✍️ Get sample bullet points that worked for others in your industry . Copy proven lines and tailor them to your resume.
- 🎯 Optimized to pass all resume screeners (i.e. ATS) . All templates have been professionally designed by recruiters and 100% readable by ATS.
Buy now. Instant delivery via email.
instant access. one-time only., what's your email address.

I had a clear uptick in responses after using your template. I got many compliments on it from senior hiring staff, and my resume scored way higher when I ran it through ATS resume scanners because it was more readable. Thank you!

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.

Investing in the next generation of medical and research pioneers
Masks strongly recommended but not required in maryland, starting immediately.
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.
Clinical trials study the safety and effectiveness of interventions and procedures on people’s health. Interventions may include medications, radiation, foods or behaviors, such as exercise. Usually, the treatments in clinical trials are studied in a laboratory and sometimes in animals before they are studied in humans. The goal of clinical trials is to find new and better ways of preventing, diagnosing and treating disease. They are used to test:
Drugs or medicines

New types of surgery

Medical devices

New ways of using current treatments

New ways of changing health behaviors

New ways to improve quality of life for sick patients

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story

Interview Questions for Clinical Research: A Comprehensive Guide
Preparing for a clinical research interview can be a daunting task. You want to make sure you are well-prepared and able to showcase your knowledge and skills in this field. To help you in your preparation, we have compiled a list of common interview questions that you may encounter during the hiring process. Whether you are a seasoned professional or just starting out in clinical research, these questions will give you an idea of what to expect and how to best answer them. Read on to learn more!
Why Should You Prepare for Clinical Research Interviews?
Preparing for clinical research interviews is crucial for several reasons. First and foremost, it shows your dedication and commitment to the field. By taking the time to research and understand the types of questions you may be asked, you demonstrate your enthusiasm and willingness to go above and beyond. Additionally, being prepared allows you to confidently articulate your qualifications and experiences, making a strong impression on the interviewer. Lastly, preparation helps calm your nerves and reduces anxiety, allowing you to perform at your best during the interview.
15 Common Interview Questions for Clinical Research
1. what is your experience in clinical research.
When answering this question, provide a brief overview of your experience in clinical research, including the types of studies you have worked on, any specific therapeutic areas you are familiar with, and your role in these studies. Highlight any relevant certifications or training you have completed.
2. Can you explain the different phases of clinical trials?
Discuss the four phases of clinical trials: Phase I, Phase II, Phase III, and Phase IV. Explain the purpose of each phase and the key objectives. Provide examples if possible to demonstrate your understanding.
3. How do you ensure patient safety in clinical trials?
Explain the importance of patient safety in clinical trials and discuss the measures you take to ensure it. Mention the regulatory guidelines you follow, the informed consent process, and the monitoring and reporting of adverse events.
4. What are the key elements of a clinical research protocol?
Discuss the essential components of a clinical research protocol, such as the study objectives, design, inclusion/exclusion criteria, endpoints, and statistical analysis plan. Emphasize the importance of a well-designed protocol in ensuring the success of a clinical trial.
5. How do you handle data management in clinical research?
Explain your approach to data management in clinical research, including data collection, validation, and analysis. Discuss the importance of data integrity, confidentiality, and compliance with regulatory requirements.
6. Can you describe your experience with regulatory compliance in clinical research?
Highlight your knowledge and experience with regulatory requirements in clinical research, such as Good Clinical Practice (GCP) guidelines, Institutional Review Board (IRB) approvals, and local regulatory authority submissions. Provide examples of how you have ensured compliance in previous studies.
7. How do you handle challenges or conflicts in clinical research?
Describe your approach to resolving challenges or conflicts that may arise during a clinical trial. Discuss your communication and problem-solving skills, as well as your ability to collaborate with team members to find effective solutions.
8. How do you ensure the quality of clinical trial data?
Explain your methods for ensuring the quality of clinical trial data, including data monitoring, source data verification, and query resolution. Discuss your attention to detail and your ability to identify and address data discrepancies.
9. What are the ethical considerations in clinical research?
Discuss the ethical principles that guide clinical research, such as respect for autonomy, beneficence, and justice. Explain the importance of obtaining informed consent from study participants and maintaining confidentiality and privacy.
10. How do you stay updated with the latest developments in clinical research?
Share your strategies for staying informed about the latest advancements in clinical research. Mention any professional organizations you are a part of, conferences or workshops you attend, and scientific journals or publications you regularly read.
11. Can you describe a challenging situation you encountered in a clinical trial and how you resolved it?
Provide an example of a challenging situation you faced during a clinical trial, such as a protocol deviation or a participant dropout. Explain how you identified the issue, collaborated with the team to find a solution, and implemented corrective measures.
12. How do you handle the documentation and reporting requirements in clinical research?
Discuss your approach to documentation and reporting in clinical research, including study progress reports, adverse event reporting, and study closeout activities. Emphasize your attention to detail and your ability to meet deadlines.
13. Can you explain the role of a clinical research coordinator?
Describe the responsibilities of a clinical research coordinator, such as participant recruitment and enrollment, data collection and management, regulatory compliance, and study coordination. Highlight your experience in these areas.
14. How do you ensure compliance with Good Clinical Practice (GCP) guidelines?
Explain your knowledge and understanding of GCP guidelines and how you ensure compliance with them in your work. Discuss your experience with GCP audits and inspections.
15. What do you consider the most important qualities of a clinical researcher?
Discuss the qualities that you believe are essential for a successful clinical researcher, such as attention to detail, critical thinking, problem-solving skills, and effective communication. Provide examples of how you have demonstrated these qualities in your previous work.
Additional Tips for Clinical Research Interviews
- Research the company: Familiarize yourself with the company’s background, current projects, and recent achievements. This will demonstrate your interest and enthusiasm during the interview.
- Review your resume and qualifications: Be prepared to discuss your previous experiences, education, and qualifications in detail. Highlight relevant skills and accomplishments.
- Practice your answers: Rehearse your responses to common interview questions, focusing on concise and clear explanations. Use the STAR method (Situation, Task, Action, Result) to structure your answers.
- Ask questions: Prepare a list of thoughtful questions to ask the interviewer about the company, the role, and the team. This shows your interest and engagement in the opportunity.
- Dress professionally: Dress appropriately for the interview, opting for business attire unless otherwise specified. First impressions matter, so make sure you present yourself professionally.
- Follow up: Send a thank-you email or note to the interviewer after the interview to express your gratitude for the opportunity and to reiterate your interest in the position.
Preparing for a clinical research interview is essential to showcase your knowledge, skills, and qualifications. By familiarizing yourself with common interview questions and practicing your responses, you can confidently navigate the hiring process and increase your chances of securing the job. Remember to remain calm, be yourself, and demonstrate your passion for clinical research. Good luck!
Related Posts:
- Clinical Research Associate Interview Questions (Ace Your Job) Preparing for a clinical research associate (CRA) interview can be both exciting and nerve-wracking. As a CRA, you play a crucial role in ensuring the…
- Clinical Data Management Interview Questions Are you preparing for a job interview in clinical data management? Whether you are a fresh graduate or an experienced professional, it's essential to be…
- Interview Questions for Clinical Research Coordinator: How to Prepare and Succeed When applying for a position as a clinical research coordinator, it's important to be well-prepared for the interview process. The role of a clinical research…
- Master the Clinical Research Interview: 15 Common Questions and How to Answer Them Preparing for a clinical research interview can be a daunting task, but with the right guidance and preparation, you can ace it. Whether you're a…
- Clinical Coordinator Interview Questions: Tips and Examples Preparing for a clinical coordinator interview can be nerve-wracking, but with the right preparation, you can ace the interview and land your dream job. In…
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.

Clinical Research Experience: What It Is and How to Find It
The COMLEX Level 1 and USMLE Step 1 exams move to pass/fail scoring takes some pressure off the initial medical licensure exams for medical students. However, with it, it has taken away the ability to use those Level 1 or Step 1 exams as a way to stand out to residency directors. This has left medical students looking for new ways to stand out on the Match. Clinical research experience has been an early, but popular, option.
Keep reading as we dive into what makes clinical research so valuable and how you can secure your own research experience.
What is Clinical Research Experience?
During a clinical research experience, you can expect to work with patient medical data, medications, medical devices, procedures, or other patient care topics to solve or formulate a hypothesis laid out by the precepting physician or research team. Participating in a clinical research experience can often lead to co-authorship or being involved with the publication process of a research paper.
Unlike laboratory research, clinical research involves working with human subjects. This means clinical research projects will look different from one project to another. Even day-to-day work may be constantly changing. However, because clinical researchers are also often physicians, research projects are typically focused on a specialty. This means that when you are looking for clinical research experience, you are able to find a project that matches your interests.
How Does it Help?
Clinical research can improve your residency application in a number of ways. First, it gives you experience to add to your C.V. If you already graduated from medical school or already finished clinicals, this shows residency directors your dedication to staying involved with medicine. If you are still in medical school, research diversifies your C.V. outside of standard school activities.
As mentioned above, clinical research can lead to publishing opportunities. This can boost your credibility with residency directors.
Thirdly, research experience helps build your medical knowledge. Some aspects of research may reinforce concepts you learned in school. Other aspects may expose you to new ideas or understanding of medicine.
How Do I Find Research Experience?
If your school does not offer clinical experiences in the location or specialties you want, there are a few ways to go about finding research experience. One way is to reach out to research sites individually and ask if there are any openings. This is a traditional way of finding clinical experience, so it is not an unusual approach. However, it is often difficult, especially for international medical students trying to plan travel to the U.S.
A more straightforward way to find clinical experience is through a clinical experience provider, like AMO. AMO hosts clinical training sites through its partners on its platform, letting you know where and when availability is. Even better, the platform allows you to filter by specialty and experience type, including a research filter to help you find available clinical research experiences.
Interested in securing clinical research experience. Reach out to an AMO by email at advisor at [email protected] or via WhatsApp by sending a message to +1-312-241-0640
Related Posts

March Means Match Week: How AMO is Supporting Trainees’ Match Dreams

Program Highlight: Florida International University

Future Physicians for Change: Dare to Blaze the Path Forward

Enhance Your Trip to AMSA’s Future Physicians for Change Conference
Leave a comment cancel reply.
EXPLORE ROTATIONS
Create an Account
How It Works
GET UPDATES

Clinical Research Careers
Clinical research offers a dynamic and fulfilling career path for individuals passionate about advancing medical knowledge and improving patient outcomes. As the demand for groundbreaking treatments and therapies continues to grow, so does the need for skilled professionals in this field. In this article, we will delve into the various roles within clinical research, shed light on the day-to-day responsibilities, discuss the career trajectory from entry-level to seasoned professional, and present exciting opportunities in the field and at Velocity Clinical Research.
Clinical Research Associate (CRA)
Clinical research associates play a pivotal role in ensuring the smooth operation of clinical trials. Their responsibilities include monitoring trial progress, collecting and analyzing data, ensuring compliance with regulations, and maintaining effective communication between research sites, sponsors, and investigators . CRAs serve as the backbone of clinical research teams, ensuring the highest standards of quality and patient safety.
As an entry-level position, most CRA openings require a high school diploma and some experience in the life sciences, clinical research, or healthcare industry.
Clinical Research Coordinator (CRC)
Clinical research coordinators work closely with investigators, participants, and Sponsors to manage the administrative and logistical aspects of clinical trials. They oversee patient recruitment and enrollment, obtain informed consent , maintain accurate records, and coordinate study visits and procedures.
CRCs are the linchpin of clinical trial operations, ensuring adherence to protocols and maintaining ethical standards. As such, most openings for clinical research coordinators require a combination of a degree (bachelor’s or associate’s) and previous work experience in the life sciences, clinical research, or healthcare industry.
Site Director or Site Manager
Site directors or managers oversee the operations of clinical trial sites. They coordinate activities among various departments, ensuring smooth workflow and adherence to study protocols. Site directors manage resources, budgeting, and personnel to ensure effective recruitment, enrollment, and timely completion of trials. They collaborate with Sponsors, regulatory authorities, and research teams to maintain high standards of quality and compliance.
As the overall manager of the clinical research site, a site director or manager must have several years’ experience working in the field of clinical research, especially overseeing trials, along with at least a bachelor’s degree, with preference towards those with advanced degrees.
Clinical Research Nurse
Clinical research nurses combine their clinical expertise with research skills to provide direct participant care during clinical trials. They administer investigational products, monitor participants for adverse events, collect samples, and educate participants about the study. These dedicated professionals bridge the gap between patient care and research, helping ensure participant safety and well-being.
Those with nursing degrees, like LPNs and RNs, can also serve other roles in clinical research, including as coordinators and sub-investigators. At a minimum, an associate degree in nursing is required for these positions.
Sub-Investigator
Sub-investigators work closely with the primary investigator and provide support in conducting clinical trials . They assist with patient assessments, perform medical procedures, collect data, and collaborate with other members of the research team. Sub-Investigators often include resident physicians, fellows, or other healthcare professionals seeking to gain experience and contribute to clinical research studies under the guidance of the lead investigator. Nurses and nurse practitioners can also serve as sub-investigators with the right experience.
As sub-investigators, an advanced degree in life sciences is necessary to help ensure participant safety and protocol adherence.
Principal Investigator
Principal investigators are experienced medical professionals who lead and oversee clinical trials. These individuals, often physicians or specialized researchers, follow study protocols, ensure ethical and regulatory compliance, and supervise the research team. Investigators are responsible for participant safety, accurate data collection, and the delivery of study results. Their expertise and leadership guide the entire research process, contributing to the advancement of medical knowledge and the development of new therapies.
Most principal investigators in clinical trials are physicians, though those with specialized degrees with a thorough knowledge of clinical research can also be investigators in certain therapeutic areas.
Remote Clinical Research Jobs
With technological advancements, remote and hybrid clinical research jobs have gained prominence. Professionals in this role perform their duties from a remote location, utilizing virtual tools to collaborate with study sites and stakeholders. Remote positions offer flexibility and enable talented individuals to contribute to clinical research projects irrespective of geographical constraints. These positions tend to be in the following departments and do not require extensive medical training:
- Business Development
- Operations
- Data Analysis
- Quality Assurance
- Information Technology
- Marketing
- Patient Recruitment
- Human Resources
Career Path
Entering the clinical research field typically starts with an entry-level position, such as a clinical research assistant or data coordinator. With experience and professional development, individuals can progress to roles such as clinical research associate or clinical research coordinator. Further advancement may lead to leadership positions, such as clinical research manager or director. Pursuing certifications, such as the certified clinical research coordinator (CRCC) certification, enhances career prospects and demonstrates expertise in the field.
Start Your Career in Clinical Research
If you are intrigued by the prospects of a clinical research career, Velocity Clinical Research invites you to explore exciting opportunities with us. With a commitment to innovation and excellence, Velocity offers a wide range of roles across various therapeutic areas. Join a remarkable team doing remarkable work at velocityclinical.com/careers .
- “Clinical Research Careers” – Clinical Research Pathways. Retrieved from https://www.clinicalresearchpathways.org/careers/
- “Clinical Research Coordinator (CRC)” – Association of Clinical Research Professionals (ACRP). Retrieved from https://acrpnet.org/certify/certifications/certified-clinical-research-coordinator-crc/
- “Career Path” – Society of Clinical Research Associates (SoCRA). Retrieved from https://www.socra.org/career-development/career-path/
- “Patient Recruitment Specialist” – Applied Clinical Trials. Retrieved from https://www.appliedclinicaltrialsonline.com/view/role-patient-recruitment-specialist
- “Investigator” – National Institutes of Health. Retrieved from https://clinicalcenter.nih.gov/participate/ethics/investigator.html
- “Sub-Investigator” – Clinical Research Society. Retrieved from https://clinicalresearchsociety.org/the-role-of-the-sub-investigator-in-clinical-research/
- “Site Director/Manager” – Society of Clinical Research Associates (SoCRA). Retrieved from https://www.socra.org/about-socra/job-descriptions/
- “Quality Assurance Specialist” – Association of Clinical Research Professionals (ACRP). Retrieved from https://acrpnet.org/clinical-research-careers/career-resources/career-descriptions/quality-assurance-specialist/

Recent articles
- Metabolic Syndrome and Heart Health June 28, 2024
- The Latest in Migraine Research: New Potential Treatments for Migraines June 7, 2024
- All About Liver Disease March 8, 2024
- The Latest in Gastrointestinal Disease Research: New Potential Treatments For IBS, Constipation, Crohn’s Disease, Celiac Disease, Ulcerative Colitis, and More March 4, 2024
- The Latest in Asthma Research: New Potential Treatments for Asthma February 6, 2024
It all starts with people like you.
Without clinical trial participants, it would not be possible to create new medicines, treatments, and cures..
Health Professions Advising
Questions about clinical experience, the big picture.
It helps to think about what professional schools want to know about you based on your clinical experience. As they read your application, they will ask questions like:
- Does this applicant know what they’re getting into before committing to an expensive, time-intensive career path? Is their perspective sufficiently concrete and gained from personal experience?
- Has this applicant started to develop a good "bedside manner" and ability to care for people from diverse backgrounds who have medical needs?
- What is this applicant’s career vision? Where do they see themselves longterm?
- Is this applicant ready to navigate in hospital and clinical environments and work successfully as a team member within them?
No single experience is likely to cover all four of these aspects of clinical experience, so most students do some of each. Here are a few common ways that students gain clinical experience.
- Shadowing : short-term, passive opportunity to get a glimpse into a certain specialty by following a doctor in their day to day work. You may have a chance to see how a physician interacts with their patients, discuss rewards and challenges of the profession, and gain insight on what you might want in your career.
- Hospital / Clinic / Hospice volunteering : longer-term, active opportunity in which you provide a service to the clinical setting. This can give you a sense of the culture within a unit of the hospital or other care facility over time, allow you to interact with the team within the unit (nurses, techs, physicians, etc.), and, in some units, you may have the opportunity to interact directly with patients and their families.
- Volunteering with patient populations: Opportunities outside of the hospital/clinic setting interacting with individuals with medical needs, such as working at a summer camp with kids with health issues; spending time with elderly individuals who are navigating dementia, Alzheimer's, and other conditions; assisting with health screenings for at-risk populations.
- Working / volunteering as an Emergency Medical Technician : after a course and certification test, Emergency Medical Technicians respond to emergency situations. Great opportunity to gain hands-on skills, but does not provide familiarity with the hospital setting or work of physicians. Some Princeton students train with Princeton First Aid and Rescue Squad .
- Working as a Certified Nurse Assistant (CNA) : after a course and certification test, CNAs work alongside nurses to provide direct care to patients. Training is available through community colleges and through care facilities like nursing homes. Check your state's Department of Health for a list of training facilities.
- Scribing : paid position in which you follow doctors as they visit with patients and take notes for them, so that they can focus on the patient.
- Clinical research: students may be able to assist in enrolling patients or administering tests, which can help develop interpersonal skills and provide better understanding of the patient experience. Research also gives students access to mentors in the field and a sense of what it's like to work within an academic medical/research environment.
- Volunteering through Hotline/Counseling opportunities: many students value the opportunity to develop active listening and counseling skills in these helping roles.
Don't limit yourself to one type of experience or one setting - the broader and more diverse the exposure to health and health care, the better. In addition to active experience, reading books about medicine, attending talks by medical professionals and med students, watching documentaries, keeping up with articles posted on our Facebook page about medicine, and taking health-related courses will further prepare students for medical school and their future careers.
A few medical schools will specify a minimum number of hours in patient-facing roles or shadowing. A few will require a letter of recommendation from a physician who can speak to your readiness for the profession. Most, if not all of them, are seeking to enroll students who show commitment to the investment required to become a physician. Medicine is an evidence-based profession, so it's unsurprising that admissions committees will seek evidence of your motivation that is grounded in experience, not in the abstract.
Every year, we have a few medical school applicants with very high GPAs and MCATs who have relatively weak/limited experience in and knowledge of health and healthcare settings. They often struggle to articulate their motivation for medicine in their application and interviews, and are unsuccessful in the admissions process. Most of them spend a year gaining additional experience and then gain admission in a future cycle, but they would have saved a lot of time and emotional stress gaining the clinical experience prior to their first application.
Beyond medical school, there are some health professions programs that do have more specific, explicit requirements. Many Physician Assistant programs require a minimum number of hours spent directly caring for patients (often about 1,000 hours minimum with a recommendation for more). Many veterinary schools similarly seek minimum hours of animal care under the supervision of veterinarians. Explore the requirements for careers of interest in the Exploring the Health Professions section of our website.
Our applicants tend to maintain some relevant experience during the academic year, and maximize vacation and glide year time. Admissions committees are looking at your collective experiences over many years to determine whether you have a realistic understanding of what you’re getting yourself into, and how you have developed the competencies that they seek in entering medical students. Think about both the activities you choose, and more importantly the lessons you can learn and articulate in your application materials about those activities. What will be most meaningful to you when it comes to learning about the profession, demonstrating your cultural competency, service orientation, and other competencies, and look for those experiences. No single experience is going to do everything for you—it’s about the big picture when you add all of the experiences together and tell the story in your application. Pragmatically speaking, according to the latest survey of entering medical students , 94% have shadowed health professionals and 92% have volunteered in the healthcare field, and a survey of admissions officers shows that they place the highest importance on service in medical settings, service in non-medical settings, physician shadowing, and leadership, so those activities are worth targeting. Use small pockets of time during breaks to shadow (single days add up!), volunteer alongside classes and internships, and prioritize clinical opportunities post-grad if you haven’t accrued enough experience during your high school and college years to feel confident in your preparation.
It’s great that you had such an inspiring summer experience! Now that you’ve clarified your interest, turn your focus to thinking more about the kind of physician you want to be and refining the skills you’ll need to hit the ground running in medical school. We don’t mean that you need to narrow down your medical specialty (although it’s great to shadow physicians to learn about areas of interest). We mean that you should start to think about how and where you want to spend your time as a physician.
- Interested in urban medicine with diverse patient populations? Volunteer in areas that will help you better understand the needs of underserved and under-resourced individuals.
- Thinking about an academic medicine career? Look for research internships that will immerse you in that setting so that you see how it works.
- Hoping to pair work as a veterinarian with policy or administration work? Get involved in student groups or leadership opportunities where you help mobilize operations behind the scenes and can contibute to improving systems.
- Wondering how your passion for visual arts / engineering / whatever you love will make you a better dentist? Talk to some dentists who have similar passions and see if they’ve seen a connection.
As you think about these interests, talk with alumni health professionals and other mentors who have a similar background to yours or who are doing the kinds of things that you hope to do. The more that you hear from others about how and why they do what they do, and how they got there, the better you’ll be at telling your own story of where you’re coming from and where you want to go, which is the crux of the medical school application process. And no matter what, direct experience helping others who are navigating medical needs is critical, even if that’s virtual for the time being. Think about this in the context of other activities – once you know you like it, seek ways to get better at it – if you wanted to be a professional athlete, you wouldn’t rely on one summer of hard training to get you through. You would keep refining your training and preparation, from technical skill to mindset to learning from other professionals and coaches. The same is true of how to think about preparing for your medical career. If you need ideas on who to shadow or what kinds of experience to gain, we’re happy to help you brainstorm.
Your best bet is to return to an organization that already knows you: one week really isn't enough time to deeply engage in a clinical setting. Many healthcare settings require training and documentation, and they may prioritize investing in longer-term volunteers. Spring break is a great time to gain shadowing experience, though! Spend a few days observing and having conversations with health professionals. You might also check with local organizations that have short-term volunteer opportunities--these are often shared through Idealist.org or local non-profits (like NYCares in NYC). Keep an eye on residential college newsletters for Princeton-sponsored service-oriented spring break trips, as well.
Volunteering
Generally, your clinical experiences in college will probably include volunteering because you likely don't have the training required for a paid position where you're interacting with patients, but we cannot quantify the amount: how much of your health-related experience is for pay, or through an internship, or shadowing, or true volunteer work is all up to the individual.
In addition to clinical experience, civic engagement in other settings -- tutoring, coaching kids, helping the elderly -- are also valued, so don't turn down those opportunities just because you don't think med schools will be interested in them. It's critical to get outside of the "Orange Bubble" and prepare yourself to care for the diverse patient population.
There is no magical formula here (and we know how much easier it would be if there was!)—the more time that you spend ensuring that you know what you’re getting yourself into before applying to medical school, the better. We recommend quality engagement over quantity, but there is also value to showing long-term commitment. One semester spent volunteering in a hospital is more likely to come across as “just checking a box” than as meaningful clinical engagement (especially if you start right before you’re about to apply). Think holistically about what medical schools are looking for from time spent with patients. This can include assessing whether you’ve developed empathy for people with medical needs from diverse backgrounds; your familiarity with health care settings and ability to work as part of the health care team; your service orientation; your “bedside manner” in communicating with patients and their families. Think about ways that you demonstrate some of these qualities drawing from other cocurricular activities, classes, reading, shadowing, etc., alongside your direct work with patients (but don’t use other activities as an excuse to neglect clinical experience altogether).
You probably want to focus on the things you do enjoy about being in the clinical setting and not the things that are tedious or uninteresting. Medical schools do not “insist” on hospital volunteering per se, but they do value applicants with experience interacting with patients, and before one goes to medical school and obtains proper qualifications and skills, one is left with volunteering as the main means for gaining patient contact. A few things to remember:
- If your frustration comes from a lack of contact with physicians, and the patients you see are asleep, you might be volunteering at a less than optimal time of day. When scheduling your volunteer work, think beyond what is best for your schedule. Volunteer in the mornings or afternoons, possibly on weekends if you have to, not late in the evening when the docs have gone home and the patients are sleeping. And remember: we have a list of local physicians who have volunteered to have you shadow them--it's on our website. You might try contact some of these physicians in order to have more interaction with doctors.
- Students often experience more than they realize when serving as a volunteer. Write down your experiences. Spend a little time recording conversations you've had with patients or conversations you've overheard between doctors and staff. The more detailed you are with your note-taking, the better equipped/informed you'll be when asked to discuss your volunteer work. (It may even help you better understand why you're volunteering in the first place.)
- Lastly, remember this isn't about your doing something you find interesting as much as it is about backing up your desire to work in a profession where serving others is at the heart of all you do. To be blunt, it's not about you, it's about what is needed to be done in order to help a hospital help its patients. Tasks like comforting patients, talking with them and their families, transporting them, etc. are essential experiences in your development as a caregiver.
If you want a more active role, consider spending the summer gaining a certification as an EMT or Certified Nursing Assistant, which will give you hands-on skills that you can use to care for patients. We've had students who pursued certification and then worked part-time during the school year or full-time post-graduation in these positions.
When you apply to medical school, you will categorize each of your activities--two of the possible categories are Community Service: Medical/Clinical and Community Service: Not Medical/Clinical. We’ve seen applicants classify text/chat lines under both categories—there are no hard and fast rules. If someone asked you at an interview about how you classified an activity, you should be able to justify your choice (the same is true for how you categorize each class you’ve taken).
Regardless of the category they chose, applicants often tell us they’ve gained insights and developed competencies that have helped them consider their future work with patients. Do be careful not to overstate how much you can gain from texting versus talking with patients and spending time with them face-to-face, and be sure to expand beyond this activity in preparing to show evidence of your knowledge of and experience in health and healthcare settings.
Shadowing can an excellent way to gain exposure to and become informed about the everyday practice of medicine. Working closely with a healthcare provider and his/her staff in an office helps you to learn about expectations in the field and the challenges and rewards of practicing medicine. A shadowing experience also allows you to build a relationship with a mentor in the field and ask questions, ultimately helping you to decide if this path is the right one for you.
- Local: email HPA and request our list of local physicians in the Princeton area who are open to having students shadow them.
- Away from Princeton: Reach out to a Princeton alumni via the Tigernet Alumni Directory and LinkedIn’s Princeton alumni community . In LinkedIn, you can enter keywords like “ physician ” (1900 results), “ physician assistant ” (650 results), “ public health ” (9500 results). Cast a wide net with a concise, professional yet friendly message and see if you receive responses. If you live in a more isolated area, you might also just search for your hometown and use Princeton as your initial means of connection, then see if they can connect you to health professionals in the area.
- The Center for Career Development offers " Princeternships " with pre-identified alums, and you can apply to participate in these shadowing opportunities through HandShake . The deadline to apply for January Princeternships is in November, and the deadline for Spring Break Princeternships is in February.
When you contact a physician, tell the person where you found them, give a brief introduction of yourself, and what in particular interests you about their background, position, or organization. Let the doctor know that you'd be interested in any shadowing opportunities that they can provide. If your first contact is by email, attach a copy of your resume and let them know that you're happy to connect by phone if it's helpful. If your first contact is by phone, have your calendar available in case the physician wants to schedule something right away. Try to have an idea of what you're looking for when you shadow in case you are asked. You also might want to check out your peers’ Princeternship blogs to get a sense of what they have gained from shadowing experiences in the health professions.
If the doctor can't accommodate you for shadowing, you might see if they would just be willing to talk with you for an hour or so, and then put together a list of questions you might like to know more about in pursuing your interests in medicine (this is often called an "informational interview"). Career Development has a great list of starting questions for informational interviews . For more ideas, read through our Shadowing Guide .
You sign a statement of integrity when you apply to medical school stating that you are portraying your experiences honestly, and this will suffice. You will be asked to provide contact information for each activity that you report in your application so that schools could follow up on the experiences if desired, but for the most part, they will trust that you are being truthful in your application.
We do recommend logging your hours and your personal reflections on each shadowing experience for yourself. That way, you'll be able to easily calculate your total hours when you apply and you'll have a record of how you've grown through each experience.
Excellent question! We want shadowing experiences to be positive for students and for the physicians who are providing this valuable opportunity. A group of medical school personnel, prehealth advisers, ethicists, and others have collaborated to developed Guidelines for Clinical Shadowing Experiences for Pre-medical Students . We encourage you to read through them so that you can provide a summary for the physicians, and you can also provide them with a copy of the guidelines, or a link to the document.
It depends on what you’ll be doing as you shadow and what you want to get out of it. Generally, if you’re just observing and asking a few questions, about 3-4 days full time will give you a good sense of what’s going on. Spread your time out across different shadowing opportunities with physicians or other health professionals in diverse specialties, types of practice (e.g., private practice vs. hospital, in-patient vs. outpatient) to maximize the learning experience. The more that you can shadow physicians with whom you have something in common, the more it may help you think about yourself in the role later. If you're a humanities concentrator, look for physicians with a similar academic background; if you're interested in MD/PhD, try to shadow physician-scientists or MDs who run their own labs. A couple of the goals behind shadowing are to see enough that you understand the rewards and challenges of day-to-day doctoring, and that you gain insight from folks who are doing the kinds of things you want to be doing.
Shadowing is a valuable way to gain exposure to how a doctor thinks and what their work looks like day to day. You spend time learning from watching a physician interact with patients. Generally, shadowing is a shorter-term experience; after a few days, you’ve gained a fair amount of insight on how that doctor does her work. To complement the insights gained from shadowing, we recommend seeking experience where you have an active role and personal responsibility for being a part of the health care team, even if your role is as simple as making sure that beds are made and patients have water. Additionally, shadowing is largely a passive experience and is mainly for your benefit. We encourage you to supplement your shadowing experience with something more hands-on that helps others. It may be that the physician you plan to shadow already has this in mind and will allow you to sit with patients or their families as they wait for the physician or assist administrative staff with tasks – pure shadowing is a good first step, but something where you are able to contribute to and not just benefit from the clinical experience is recommended. After all, part of the reason you want to be a doctor is to help others – demonstrate that interest by finding ways that you can help!
The Princeternship program through the Center for Career Development is a great place to start. The alumni physicians offering Princeternships are eager to have you, and many of them have hosted many “Princeterns” and will help to make the experience more comfortable. Here are a couple of stories from past Princeterns: Alice Vinogradsky learned more about private practice with Dr. Ganchi; Ava Torjani spent time with Richmond ENT; and Jasmine Hao and four other Princeterns had a virtual opportunity with Children’s Hospital of the King’s Daughters. Read through the Princeternship position listings to get a feel for the hosts’ work and what they hope to learn, then write a detailed, specific application about your “fit” for the positions you’re interested in. Applications are due next week, so take some time to apply now!
Here are a few ways to start the search online:
- Simple google search: Using a search term like physician Alabama “Princeton University” (without the full university name in quotation marks, you’ll get a lot of physicians affiliated with an Alabama hospital with Princeton in its name).
- Search on med school websites: Go to the Alabama medical schools and type Princeton or Princeton University into their search bars.
- LinkedIn: Start with Princeton’s LinkedIn page and use keyword searches like Alabama physician .
- TigerNet : Log into the Princeton based alumni directory and search. It can take a while to find the right search terms, but using the state and the “healthcare” field/specialty sometimes yields better results than “occupation”.
- Start with the state/regional Alumni Association and reach out to officers who may be able to help connect you.
In addition to Princeton-affiliated health professionals, use your local network: your own pediatrician/physician, those who care for family members, high school guidance counselors/teachers, and other organizations you might’ve been involved in could help you find connections. For those of you from areas with fewer Princeton connections, we may also be able to connect you with alums—feel free to email us and ask! See the HPA “Making Connections” guides for more recommendations.
International Experience
If you plan to practice in the United States, it would benefit you to have familiarity with the way that healthcare is organized and delivered in the US. You can gain some of this through GHP classes, reading on your own, following the news, etc., but your own expectations for what it means to be a doctor or work with a healthcare team are often best informed by what you have seen/experienced in shadowing and volunteering. If you have an interest in global health, try reaching out to physicians in the US who work in that sphere. If you worked with US-trained physicians abroad (e.g., attended a mission where US-based doctors go abroad to work with patients), see if there’s a way to shadow them when they’re back in the US—it could be particularly interesting to observe them in both settings. It will also benefit you to gain familiarity with the diversity of patients that you’ll see in the US. Many students don’t have significant exposure to certain patient populations: elderly individuals, those from lower socio-economic backgrounds, others who are underserved in the US system. Gain cultural competency in the US as well as abroad—volunteering, medical or not, can be a great way to do this—so that you’ll be better able to serve all patients. Volunteering and shadowing abroad can add valuable perspective, of course. Do be careful that you’re not overstepping what you should be doing as an individual without medical training. It makes us nervous when students talk about doing more abroad than they can here—you do not want to perform any procedures or otherwise interact with patients in ways that are beyond your training. Before heading off to gain clinical experience abroad, take a look at the guidelines provided by the Association of American Medical Colleges for providing patient care outside of the US .
It’s hard to quantify the benefits of these programs relative to the expense. There are many ways to shadow without paying a high price for it, such as reaching out to alumni physicians or participating in a program like the Summer Health Professions Enrichment Program (which provides a stipend) or the Premed Volunteer Program at St. Mary Medical Center . There are also funded IIP internships and GHP Health Grand Challenges internships that provide exposure to health care in international settings. There is no expectation that premed students participate in international health experiences—there are many ways to develop cultural competence in the US and abroad that don’t involve an expensive price tag.
We would also caution you against programs that seem more like “voluntourism,” where you may be asked to perform tasks that you aren’t qualified to perform, or which may negatively affect the local culture ( see links on our website for details ). We recommend looking for programs that follow the AAMC guidelines for students during clinical experiences abroad (and being sure that you follow them yourself). Atlantis is one of the programs that does adhere to these guidelines, and we’ve had students who had positive experiences with the program. The program outlines suggestions on how to fundraise; to our knowledge, Princeton students have not received university funding for the program.
There has been a recent proliferation of companies that coordinate this kind of abroad experience, and they have pros and cons (as outlined in this NPR podcast: The Risk (and Unexpected Benefits) of Sending Health Students Abroad ). We at HPA feel most comfortable with programs that are partnering with campus programs like IIP and PICS – that way, we know that you have support from Princeton and that the programs have been vetted.
We maintain a list of summer programs in which Princeton prehealth students have participated on the Clinical Experience page of our website, but we do not endorse any particular programs; we recommend considering what these programs offer critically, and using the AAMC and ADEA guidelines for providing patient care internationally as you evaluate organizations. Also keep in mind that there are many vulnerable and underserved populations in the US who would benefit greatly from your assistance as a volunteer for the summer – do not feel that you can only make a difference by traveling abroad. As for funding, the Student Activities Funding Engine (SAFE) is the best place to start.
Q: I have some specific questions about a global health internship that I found in Vitals . How do I know if it’s legitimate or recommended by HPA? Who do I contact about the opportunity to learn more? A: We advertise internships that sponsoring programs ask us to share with our students, but we do not necessarily endorse/recommend them. There are some abroad programs that can do more harm than good (see “ Additional perspectives about international opportunities ” on our website), so we try to screen those out of our listings, but it can be hard to judge from their websites. And if students reach out and tell us that they had a negative experience with an internship sponsor, we will often remove that internship from Vitals . In terms of abroad opportunities, we have the most faith in the programs that are affiliated with the Office of International Programs , Global Health Programs/Center for Health and Wellbeing , and other Princeton offices that do more closely screen their sites, and provide support while you’re abroad. We list the sponsoring office/program with all of our Vitals links, and you should reach out to them directly with questions.
Activities for Predental Students
Just like premeds, focusing on your passions and pursuing them in depth and over time is key. Shadowing dentists in different settings so that you can gain perspective on the career opportunities is valuable. A couple of areas that are emphasized more for predental students than premeds are business skills and manual dexterity. Many dentists go into private or group practice, and if that’s a route that you’re considering, some early preparation in business/management can be valuable. Manual dexterity, developed though activities like ceramics, hand crafts, video games, etc., is also beneficial. It’s also important to be able to connect with others—both patients and colleagues—in close proximity. Dentists are working side by side with their assistants and techs, often in close spaces, and have to be attuned to patients’ feelings via verbal and especially non-verbal cues in ways that physicians in many specialties do not. Activities that develop this team mentality and ability to put others at ease will prepare you well for the career. For more perspectives on predental preparation, read through the information on the ADEA GoDental website .
Local Clinical Opportunities
Work with individuals with healthcare needs in the Princeton area.
Summer Clinical Opportunities
Clinical opps webinar.
A discussion around the value of clinical experience, and how to find it with HPA peer leaders.
Princeton login required
- Mayo Clinic Careers
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Internal Medicine
- Lung Transplant
- Psychiatry & Psychology
- Nurse Practitioner & Physician Assistant
- Ambulance Service
- Clinical Labs
- Med Surg RN
- Radiology Imaging
- Clinical Research Coordinator
- Respiratory Care
- Senior Care
- Surgical Services
- Travel Surgical Tech
- Practice Operations
- Administrative Fellowship Program
- Administrative Internship Program
- Career Exploration
- Nurse Residency and Training Program
- Nursing Intern/Extern Programs
- Residencies & Fellowships (Allied Health)
- Residencies & Fellowships (Medical)
- SkillBridge Internship Program
- Training Programs & Internships
- Diversity, Equity & Inclusion
- Employees with Disabilities
You're using Internet Explorer - therefore, some pages or features may not display properly. We recommend switching to a modern browser such as Chrome, Microsoft Edge, or Firefox for a smoother experience.
Search life-changing careers..
Search by Role or Keyword
Enter Location
- United States Applicants
- United Kingdom Applicants
- United Arab Emirate Applicants
- Current Employees
Associate Clinical Research Coordinator
- Phoenix, AZ
Not ready to apply? Join our talent community
Receives direction from principal investigator, supervisor, or other staff involved in research protocol(s). Interacts with various departments within the institution. Works cooperatively with other investigators and personnel at all levels. Interacts with research participants, other research centers, and sponsoring companies to resolve problems and ensure efficient completion of research studies. Position Overview: (Major Functions and Non-Essential Functions): Coordinates non-therapeutic (i.e. minimal risk, survey, chart review) clinical research protocols with direction from the principal investigator and/or supervisor in compliance with regulatory laws and institutional guidelines. May assist in complex (i.e. interventional, therapeutic, greater than minimal risk) studies with direction but does not have overall responsibility for these studies. Screens, enrolls, and recruits research participants. Coordinates schedules and monitors research activities and subject participation. Recognizes adverse events, protocol deviations, and other unanticipated problems and reports appropriately. Collects, abstracts, and enters research data. Performs administrative and regulatory duties related to the study as assigned. Some travel may be required. ADDENDUM (if applicable) Protocol Development and Maintenance Activities Responsibilities may include, but are not limited to: ongoing management of the protocol document and process through editing, amendments, proofing, coordination of study logistics (i.e. data collection booklets, use of CRU, etc.), and verification of content to meet institutional and federal standards; Institutional Review Board (IRB) submission; and communication with study sites and/or federal agencies regarding study status changes. Participates in other protocol development activities and executes other assignments as warranted and assigned.
Additional Information:
This position is onsite and may be eligible for telework 1-2 days per week after a patient and study assessment has been completed and deemed acceptable. While working remotely, staff are expected to be onsite for essential study tasks. Staff must also successfully complete a 6-month training assessment, demonstrate ability to work independently, be reliable, and exhibit efficiency and accuracy of completing tasks.
Minimum Education and/or Experience Required: (Education Requirements and Experience): HS Diploma with at least 3 years of experience OR Associate's degree/college Diploma/Certificate Program with at least 1 year of experience, Associate’s in Clinical Research from an accredited academic institution without experience OR Bachelor's degree. Experience should be in the clinical setting or related experience.
Additional Experience and/or Qualifications: (Has Achieved Competency in the Following Areas, Job Knowledge and Additional Considerations): Graduate or diploma from a study coordinator training program is preferred. One year of clinical research experience is preferred. Medical terminology course is preferred. Licensure/Certification Required: N/A
About our location
Phoenix, Arizona
We would love to connect with you.
Click the button for a list of our upcoming events.
Join Our Talent Community
Sign up, stay connected and get opportunities that match your skills sent right to your inbox
Email Address
Phone Number
Upload Resume/CV (Must be under 1MB) Remove
Job Category* Select One Advanced Practice Providers Business Education Engineering Executive Facilities Support Global Security Housekeeping Information Technology Internship Laboratory Nursing Office Support Patient Care - Other Pharmacy Phlebotomy Physician Post Doctoral Radiology Imaging Research Scientist Surgical Services Therapy
Location Select Location Albert Lea, Minnesota Arcadia, Wisconsin Austin, Minnesota Barron, Wisconsin Bloomer, Wisconsin Caledonia, Minnesota Cannon Falls, Minnesota Chandler, Arizona Decorah, Iowa Duluth, Minnesota Eau Claire, Wisconsin Fairmont, Minnesota Holmen, Wisconsin Jacksonville, Florida La Crosse, Wisconsin Lake City, Minnesota London, England Mankato, Minnesota Menomonie, Wisconsin Minneapolis-St. Paul-Bloomington, Minnesota New Prague, Minnesota Onalaska, Wisconsin Osseo, Wisconsin Owatonna, Minnesota Phoenix, Arizona Prairie du Chien, Wisconsin Red Wing, Minnesota Rice Lake, Wisconsin Rochester, Minnesota Saint Cloud, Minnesota Saint James, Minnesota Scottsdale, Arizona Sparta, Wisconsin Tomah, Wisconsin Waseca, Minnesota Zumbrota, Minnesota
Area of Interest Select One Nursing Research Laboratory Medicine & Pathology Radiology Finance Surgery Facilities Physical Medicine & Rehabilitation Licensed Practical Nurse (LPN) Pharmacy Neurology Cardiovascular Medicine Emergency Medicine Respiratory Therapy Surgical Technician Psychiatry & Psychology Anesthesiology & Perioperative Medicine Radiation Oncology Environmental Services Ambulance Services Social Work Administration Orthopedics General Services Information Technology Mayo Collaborative Services Hematology Hospital Internal Medicine Gastroenterology & Hepatology Housekeeping Patient Scheduling Pediatrics Family Medicine Global Security Mayo Clinic Laboratories Medical Oncology General Internal Medicine Obstetrics & Gynecology Transplant International Office Support Oncology Senior Care Cardiovascular Surgery Desk Operations Healthcare Technology Management Community Internal Medicine Dermatology Education Engineering Ophthalmology Critical Care Rheumatology Biochemistry & Molecular Biology Business Development Hospice & Palliative Care Linen & Central Services Nephrology & Hypertension Clinical Genomics Endocrinology Neurologic Surgery Neurosciences Regenerative Biotherapeutics Sports Medicine Urology Artificial Intelligence & Informatics Digital Health Care Delivery Research Otolaryngology (ENT) Physiology & Biomedical Engineering Pulmonary/Sleep Medicine Immunology Infectious Diseases Legal Molecular Medicine Spiritual Care Cancer Biology Cancer Center Comparative Medicine Molecular Pharmacology & Experimental Therapeutics Pain Medicine Primary Care Quality Travel Allergic Diseases Bariatric Medicine Dental Specialities Development/Philanthropy Epidemiology Geriatric Medicine & Gerontology Health Information Management Services Information Security Marketing Media Support Services Occupational/Preventative Medicine Spine Center Surgical Assistant Urgent Care
- Research, Phoenix, Arizona, United States Remove
Confirm Email
By submitting your information, you consent to receive email communication from Mayo Clinic.
Join our talent community.
Join our global talent community to receive alerts when new life-changing opportunities become available.

If you want to know what it's really like at Mayo Clinic, just ask. You'll find that our pride–in where we work, and in what we do–is a common trait. You will also find a lot of inspiring stories about lives changed for the better.
Nurse Residency Program
The Nurse Residency Program (NRP) is for all nurses with less than 12 months of experience, to be completed within the first year. NRP provides a framework for a successful transtion to a professional nurse by promoting educational and personal advancement.

As your career evolves, our compensation and benefits packages are designed to change with you — meeting needs now, and anticipating what comes next. We know that when Mayo Clinic takes care of you, you can take better care of our patients.
Equal opportunity
All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, gender identity, sexual orientation, national origin, protected veteran status, or disability status. Learn more about "EEO is the Law." Mayo Clinic participates in E-Verify and may provide the Social Security Administration and, if necessary, the Department of Homeland Security with information from each new employee's Form I-9 to confirm work authorization.
Reasonable accommodations
Mayo Clinic provides reasonable accommodations to individuals with disabilities to increase opportunities and eliminate barriers to employment. If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440.
Job offers are contingent upon successful completion of a post offer placement assessment including a urine drug screen, immunization review and tuberculin (TB) skin testing, if applicable.
Recruitment Fraud
Learn more about recruitment fraud and job scams
Advertising
Mayo Clinic is a not-for-profit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
Advertising and sponsorship policy | Advertising and sponsorship opportunities
Reprint permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
Terms and Conditions | Privacy Policy | Notice of Privacy Practices | Notice of Nondiscrimination
© 1998-2024 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved.
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

Student placements

Gain valuable experience and training while developing your scientific or clinical research experience in a supportive world-class research environment.
Student placements: what you can expect
What student placements do you offer?
Our 12 month full-time student placements are for motivated life sciences or chemistry students. We have office-based placements across the Centre for Drug Development (CDD) and lab-based placements within Cancer Research Horizons. Our placements provide the opportunity for you to develop your scientific / clinical research experience in a supportive environment.
Who can apply?
Our student placements are designed for current life sciences or chemistry students whose degree has a placement year. You don't need any previous experience of working or volunteering in the charity sector. The skills and requirements for each stream are listed on the role profile.
When can I apply?
Placement roles are advertised annually in October/November with negotiable start dates between July and September.
How much can I earn?
Our placement students earn a competitive salary of £23,000 per annum plus benefits.
What happens at the end of my placement?
At the end of your placement you'll return to your place of study where you'll continue your life sciences or chemistry degree.
Hear from placement students
I thoroughly enjoyed learning on the job, undertaking such a wide variety of tasks and seeing the direct impact of my work on the department’s decisions.
I gained confidence and developed key skills required to tackle my final year at university, such as self-awareness, perseverance, and the ability to take initiative.
The placement improved my soft skills and gave me greater confidence in using online software. I strongly believe my final year grade at university was a direct result of taking this placement.
The combination of formal training and hands-on experience supporting clinical trial projects during this placement motivated me to pursue a career in the pharmaceutical industry.
For more information about the experience please refer to our placement student blog .
Find the business area that most interests you
About the centre for drug development.
The Centre for Drug Development consists of a diverse and experienced office-based team who sponsor and manage high quality early phase clinical trials. We work in collaboration with leading scientists and clinicians around the UK to develop effective new anti-cancer treatments.
You will be provided with training, mentorship, and exposure to key roles in drug development and pharmaceutical research.
Student Portfolio Analyst (SPA)
Assist with gathering, analysing, and presenting project information to support the effective review and management of Cancer Research UK's CDD portfolio of projects. Support resource forecasting across CDD and developing data visualisation methods and tools for CDD portfolio information. Expand and learn new skills with Excel, PowerPoint and data visualisation software.
Student Medical Writing and Pharmacovigilance Assistant (SMWPV)
Provide administrative support to the Medical Writing (MW) and Pharmacovigilance (PV) teams in accordance with Cancer Research UK Standard Operating Procedures (SOPs) and policies, International Conference on Harmonisation (ICH) and Good Clinical Practice (GCP) guidelines and requirements in order to facilitate the successful execution of MW and PV clinical trials related activities.
Student Clinical Study Coordinator (SCSC)
Aid in the development of new cancer therapies by supporting the exploratory, pre-clinical, set-up, monitoring and archiving aspects of clinical trials in accordance with Cancer Research UK Standard Operating Procedures (SOPs) and policies, ICH GCP guidelines, UK legislations and other regulatory requirements.
About Cancer Research Horizons
Cancer Research Horizons is an innovation engine built to complement Cancer Research UK’s network of exceptional researchers. We take cutting-edge innovations from the lab bench to the bedside, translating them into effective treatments and diagnostics for cancer patients.
Biosciences Student
Join our Bioscience team at Cancer Research Horizons, Cancer Research UK’s in-house drug discovery engine, to learn in-vitro laboratory techniques and develop a broad understanding of cancer cell biology and its application within oncology drug discovery
Molecular Sciences Project Student
Support and develop capabilities within the cellular mechanistic pharmacology group, involving cell-based assays using cell lines in both 2D and 3D culture for the identification, profiling and characterisation of small molecules.
Medicinal Chemistry Student
Prosecute a laboratory-based project that is designed to expand the Cancer Research Horizon's Medicinal Chemistry team’s technical capabilities. Apply an understanding of organic synthesis in our chemistry labs and work alongside an expert team of medicinal chemists.
Become a Cancer Research UK placement student

Stage 1: Online application
Complete an online application form. Fill out your details, answer 4 eligibility and competency based questions and upload your CV. Please note that in our commitment to equality, diversity and inclusion, our application process is anonymised, meaning we will not be able to view your CV until later, so please fill out all required fields even if the information is provided in your CV.

Stage 2: Interview
Successful applicants will be invited to attend a final interview. You will be interviewed by representatives from the department you are applying to, who will ask you competency-based questions relating to the role.

Stage 3: Offer
We will get in touch to make successful applicants an offer. We’ll arrange the induction week for you and other new starters, a diverse and talented cohort, launching their careers with us.
Sometimes, applicants may be unsuccessful in their desired department but have shown qualities of a great intern. We will do our best to match them to another role that suits their interests and skillset.
Please contact us if you have any questions or should you require any adjustment due to a disability or a long-term health condition.
Thanks for starting your application to {{companyName}}.
To complete your application you must do one of the following:
Forward an email from your mobile device with your resume attached to {{fromEmail}}
Reply to this email from your laptop or desktop computer with your resume attached.
Thank you for your interest, The Recruiting Team
Reply to this email from your laptop or desktop computer with your cover letter attached.
In order to create an account with us and submit applications for positions with our company you must read the following Terms and Agreements and select to agree before registering.
In the event that you do not accept our Terms and Agreements you will not be able to submit applications for positions with our company.
You agree to the storage of all personal information, applications, attachments and draft applications within our system. Your personal and application data and any attached text or documentation are retained by Jibe Apply in accordance with our record retention policy and applicable laws.
You agree that all personal information, applications, attachments and draft applications created by you may be used by us for our recruitment purposes, including for automated job matching. It is specifically agreed that we will make use of all personal information, applications, attachments and draft applications for recruitment purposes only and will not make this information available to any third party unconnected with the our recruitment processes.
Your registration and access to our Careers Web Site indicates your acceptance of these Terms and Agreements.
Dear ${user.firstName},
Thanks for choosing to apply for a job with ${client.display.name}! Please verify ownership of your email address by clicking this link .
Alternatively, you can verify your account by pasting this URL into your browser: ${page.url}?id=${user.id}&ptoken=${user.token}
Please note that your job application will not be submitted to ${client.display.name} until you have successfully verified ownership of your email address.
The ${client.display.name} Recruiting Team
- Vertical moves : Get promoted to a higher level of your current position.
- Horizontal moves : Move to a new role at the same level of responsibility.
- Leadership position : Take on additional responsibilities and manage a team.
- Gain new expertise : Gain expertise and experience not necessarily related to your current position.
This will help the HR Team to recommend you the right position.
Nice to meet you. 👋
Lets quickly set up your profile
to start having tailored recommendations.
You agree to the storage of all personal information, applications, attachments and draft applications within our system. Your personal and application data and any attached text or documentation are retained by Sequoia Apply in accordance with our record retention policy and applicable laws.
This career site protects your privacy by adhering to the European Union General Data Protection Regulation (GDPR). We will not use your data for any purpose to which you do not consent.
We store anonymized interaction data in an aggregated form about visitors and their experiences on our site using cookies and tracking mechanisms. We use this data to fix site defects and improve the general user experience.
We request use of your data for the following purposes:
Job Application Data
This site may collect sensitive personal information as a necessary part of a job application. The data is collected to support one or more job applications, or to match you to future job opportunities. This data is stored and retained for a default period of 12 months to support job matching or improve the user experience for additional job applications. The data for each application is transferred to the Applicant Tracking System in order to move the application through the hiring process. \nYou have the right to view, update, delete, export, or restrict further processing of your job application data. To exercise these rights, you can e-mail us at [email protected] . \nConversion Tracking \nWe store anonymized data on redirects to the career site that is used to measure the effectiveness of other vendors in sourcing job candidates.
Consent and Data Privacy
This application protects your privacy by adhering to the European Union General Data Protection Regulation (GDPR). Jibe will not use your data for any purpose to which you do not consent.\n
We request use of your data for the following purposes:\n \n User Authentication \n
\n This site retains personally identifiable information, specifically e-mail addresses, as a necessary part of user login. This data is retained for the duration of the user profile lifecycle and enables user authentication.\n
\n \n Usage Analytics \n
We store anonymized usage data to measure and improve the effectiveness of this CRM application in filling job requisitions and managing talent communities.\n
\n \n E-mails to Candidates \n
We collect your personal information such as name and email address. This information is used when you send marketing or contact emails to candidates.\n\n
Enter your email address to continue. You'll be asked to either log in or create a new account.
There was an error verifying your account. Please click here to return home and try again.
You are about to enter an assessment system which is proprietary software developed and produced by Kenexa Technology, Inc. The content in this questionnaire has been developed by Kenexa Technology, Inc., Kenexa’s Suppliers and/or Yum Restaurant Services Group, Inc.’s (“Company”) third party content providers and is protected by International Copyright Law. Under no condition may the content be copied, transmitted, reproduced or reconstructed, in whole or in part, in any form whatsoever, without express written consent by Kenexa Technology, Inc. or the applicable third party content provider. Under no circumstances will Kenexa Technology, Inc. be responsible for content created or provided by Company’s third party content providers.
IN NO EVENT SHALL KENEXA, AN IBM COMPANY, KENEXA’S SUPPLIERS OR THE COMPANY’S THIRD PARTY CONTENT PROVIDERS, BE LIABLE FOR ANY DAMANGES WHATSOEVER INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF VOCATIONAL OPPORTUNITY ARISING OUT OF THE USE OF, THE PERFORMANCE OF, OR THE INABILITY TO USE THIS KENEXA ASSESSMENT SYSTEM OR THE CONTENT, REGARDLESS OF WHETHER OR NOT THEY HAVE BEEN ADVISED ABOUT THE POSSIBILITY OF SUCH DAMAGES.
By clicking below, you are also confirming your identity for purposes of the questionnaire. You may not receive assistance, refer to any written material, or use a calculator (or similar device) while completing the questionnaire.
Unless otherwise directed by the Questionnaire Administrator, you are only authorized to take each requested questionnaire once. Failure to comply may result in disqualification. All Kenexa SelectorTM questionnaires are monitored.
A web browser is a piece of software on your computer. It lets you visit webpages and use web applications.
It's important to have the latest version of a browser. Newer browsers save you time, keep you safer, and let you do more online.
Try a different browser - all are free and easy to install. Visit whatbrowser.org for more information.
If you are using a later version of Internet Explorer, please make sure you are not in compatibility mode of an older version of the browser.
- Medpace.com Home
- Search Jobs
- About Medpace
- Explore Careers
- Life at Medpace
- My Career Portal
Cookies are used on this site to assist in continually improving the candidate experience and all the interaction data we store of our visitors is anonymous. Learn more about your rights on our Privacy Policy page.
Research Scientist, Grayken Center for Addiction
Research-clinical data.
- 1 Boston Medical Center Place, Boston, Massachusetts
Position: Research Scientist, Grayken Center for Addiction
Location: Boston, MA
Schedule: Per Diem, Remote
At Boston Medical Center (BMC), our diverse staff works together for one goal — to provide exceptional and equitable care to improve the health of the people of Boston. Our bold vision to transform health care is powered by our respect for our patients and our commitment to ensure everyone who comes through our doors has a positive experience.
You’ll find a supportive work environment at BMC, with rich opportunities throughout your career for training, development, and growth and where you’ll have the tools you need to take charge of your own practice environment.
POSITION SUMMARY:
The Research Scientist will work on multiple research studies including those funded by major foundations, as well as unfunded studies. The research scientist will design and conduct statistical analyses, assist with grant preparation, be responsible for data management, prepare data visuals, and contribute to manuscripts and reports. The research scientist will supervisor more junior data analysts and students on the team.
JOB RESPONSIBILITIES :
- Design and develop relational database, including management of data for clinical research including clinical trials, health services research, survey studies, qualitative research, and quality improvement projects.
- Management and oversight of data analysts and students
- Plans, coordinates and supports complex data analyses, including data cleaning, management, coding and regression modeling for mixed effects and multilevel modeling from survey data, electronic medical record (EMR) data), claims data and clinical trial databases.
- Implements methods and techniques for new activities involved in extracting, coding, and using data for modeling analysis and epidemiologic studies. Uses STATA (statistical software), and other analytic software tools as necessary.
- Works on a day-to-day basis with the leadership team and evaluation team members to implement research protocols, including data cleaning, maintenance and analysis.
- Meticulously organizes and stores data and research protocols and ensures that all study details are documented and protected in accordance with established procedures and requirements.
- Writes custom scripts and software programs, using statistical programming languages (e.g. SAS) to aid in data analysis and management.
- Prepares monthly data reports for internal stakeholders and assists with writing for publication using a variety of formats for peer-reviewed journals that summarize study results.
- Works in partnership with PIs and senior staff for new grant proposal development, and preparation of research reports and presentations. Conduct pre-to-research exploratory data analyses using descriptive statistics and data visualization.
- Assist with power analyses and preliminary data generation for grant proposals. Participates in the preparation of manuscripts, reports for funders, reports for the public/key stakeholders and national presentations
(The above statements in this job description are intended to depict the general nature and level of work assigned to the employee(s) in this job. The above is not intended to represent an exhaustive list of accountable duties and responsibilities required).
JOB REQUIREMENTS:
- Requires PhD Degree, preferably in a relevant health-related field (e.g. nutrition or public health)
EXPERIENCE:
- Minimum of three years of experience in related work required.
- Past supervisory experience preferred
- Strong STATA or SAS, and/or other programming experience required.
- Experience working in complex environments with a high degree of organizational effectiveness.
- Experience working with community and policy leaders preferred.
- Experience with teaching and/or academic mentorship
KNOWLEDGE AND SKILLS:
- Advanced proficiency in use of Stata, SAS, or other statistical programs
- Experience with survey and qualitative data, EMR data, clinical trial data
- Demonstrated ability to work with a wide variety of data structures, coding schemes, and data sources.
- In depth knowledge of study design and analytic methods. General familiarity with grant writing and submission.
- Demonstrated leadership capacity applicable to design and implementation of scientific research, including applications for funding and other external support.
- Excellent written and oral skills with a proven ability to write for a variety of audiences and interact with all levels of employees.
ABOUT THE DEPARTMENT:
As the primary teaching hospital for Boston University Chobanian & Avedisian School of Medicine and BU schools of public health and dentistry, intellectual rigor shapes our inquiries. Our research is led by a belief that skin color, zip code, and financial circumstances shouldn’t dictate health.
Boston Medical Center is an Equal Opportunity/Affirmative Action Employer. If you need accommodation for any part of the application process because of a medical condition or disability, please send an e-mail to [email protected] or call 617-638-8582 to let us know the nature of your request.
Equal Opportunity Employer/Disabled/Veterans
Privacy policy
By clicking Submit, you acknowledge that you are willing to receive messages from Boston Medical Center relating to the status of your application or other recruitment related information to any mobile / cell phone number entered in the form above. You can easily opt-out of receiving messages by replying STOP to any messages you receive.
Not the right fit? Check out these other jobs
Occupational therapist - brockton behavioral health center (per diem), psychiatric registered nurse, brockton behavioral health center, per diem, psychiatric registered nurse, brockton behavioral health center, per diem, public safety officer, refer a friend.
Your information:
Apply for this job now
EEO & Accommodation Statement Boston Medical Center is an equal employment/affirmative action employer. We ensure equal employment opportunities for all, without regard to race, color, religion, sex, national origin, age, disability, veteran status, sexual orientation, gender identity and/or expression or any other non-job-related characteristic. If you need accommodation for any part of the application process because of a medical condition or disability, please send an e-mail to [email protected] or call 617-638-8582 to let us know the nature of your request
E-Verify Program Boston Medical Center participates in the Electronic Employment Verification Program. As an E-Verify employer, prospective employees of BMC must complete a background check and receive medical clearance before beginning their employment at the hospital.
Federal Trade Commission Statement: According to the FTC, there has been a rise in employment offer scams. Our current job openings are listed on our website and applications are received only through our website. We do not ask or require downloads of any applications, or “apps” job offers are not extended over text messages or social media platforms. We do not ask individuals to purchase equipment for or prior to employment. To avoid becoming a victim of an employment offer scam, please follow these tips from the FTC: FTC Tips
Join the BMC Talent Community
Before you go, don't forget to join our talent community!

We use cookies to make your interactions with our website more meaningful. They help us better understand how our websites are used, so we can tailor content for you. For more information about the different cookies we are using, read the Privacy Statement . By continuing to navigate the site, you agree to the use of cookies on our behalf.
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Clinical Research Coordinator - Cardiology (CARE)
- Columbia University Medical Center
- Opening on: Jul 12 2024
- Job Type: Officer of Administration
- Bargaining Unit: N/A
- Regular/Temporary: Regular
- End Date if Temporary: N/A
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $62,400 Annual - $68,000 Annual
Position Summary
The Clinical Research Coordinator provides research coordination support for multiple clinical research projects. The primary focus of this role is to assist with the coordination of studies rather than independently managing clinical trials. The position involves coordinating various clinical research projects, such as registries, retrospective data reviews, long-term follow-up studies, and other non-interventional studies.
Responsibilities
Clinical Research Responsibilities:
- Screen participants for study eligibility and accurately enroll them in various databases.
- Perform simple study procedures with accuracy.
- Understand the structure of study protocols and interpret study requirements to ensure compliance.
- Follow proper documentation techniques as outlined in the ICH-GCP guidelines.
- Process subject reimbursement using pcards.
- Retrieve and utilize information from electronic medical records (EMR) and databases/CTMS/EDC.
- Maintain essential regulatory documents as required.
- Assist the research coordinator in the conduct of Site Initiation Visits (SIV) and attend monitor visits and audits.
Data Coordination Responsibilities:
- Collect basic demographic information during study visits.
- Enter data into forms (CRFs) on paper, databases, or electronic data capture systems (EDCs).
- Assist in collecting external medical records and radiology CDs as assigned.
- Administer minimal risk consents independently or complex consents under supervision.
- Conduct surveys and questionnaires.
- Verify the accuracy of own work and resolve simple queries.
- Perform concomitant medications abstraction.
- Build patient research study charts.
- Assist in quality control efforts, such as reviewing consents for signatures.
Regulatory Coordination Responsibilities:
- Collaborate with regulatory support to collect essential documents and maintain the regulatory binder (e.g., CVs, MD licenses, lab certifications, IRB rosters, lab norms).
- Assist with adverse events (AEs) and serious adverse events (SAEs).
Administrative Responsibilities:
- Demonstrate an understanding of the clinical research objectives associated with the program.
- Communicate with study participants by sending study correspondence via mail or email.
- Schedule subjects for research visits and follow-up appointments.
- Monitor study calendar for completion of study procedures.
- Manage study supply inventory.
- Utilize documents and systems to track recruitment and retention of participants.
- Work with regulatory support to maintain the regulatory binder.
- Gain appropriate training and knowledge of electronic medical records (EMR), clinical trial management systems (CTMS), electronic data capture (EDC), databases, and other relevant systems.
- Willingly learn and utilize available technology and systems to fulfill job requirements.
- Understand the disease process associated with the program.
- Attend and actively participate in all assigned training classes.
- Perform other responsibilities as assigned.
**Responsibilities may vary based on the specific needs of the unit or team. Some units/teams may require a proportionate focus on clinical, data, regulatory, or other specific needs. The Clinical Research Coordinator position will primarily support task-oriented needs.
Minimum Qualifications
- Bachelor's degree in Health Science or equivalent in education, training and experience.
Preferred Qualifications
- At least two years of related experience.
- Knowledge of university policies and procedures.
- Previous experience working in a large and complex healthcare setting.
Other Requirements
- Familiarity with medical terminology.
- Ability to communicate effectively with staff and faculty members at all levels.
- Contact with patients and/or human research subjects
- Successful completion of applicable compliance and systems training requirements.
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Program Manager - CCHD
Administrative coordinator, assistant dean, specialized degree programs.
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .
Health Equity
Sharing Patient Narratives With Clinical Staff is Linked to Better Patient Experience
Ldi researchers highlight the beneficial impacts of sharing patient narratives, miles meline, mbe.
- Share this page on Twitter
- Share this page on Facebook
- Share this page on LinkedIn

How can providers, policymakers, and health care leaders ensure that patients are having good health care experiences? Research from Wharton LDI Fellows points toward one solution: sharing patients’ narratives about their care experiences with clinical staff to inform health care operations. Narratives are stories about care experiences in patients’ own words.
Dr. Ingrid Nembhard , Wharton Professor of Health Care Management and LDI Senior Fellow, alongside Associate Fellow Sasmira Matta and colleagues, found that organizations that often share patient narratives with their staff receive higher patient experience scores .
In clinical settings, a patient’s experiences provide integral information for understanding the quality of their interactions with health systems and providers. Patient experience scores, provided by the Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys from the Agency for Healthcare Research and Quality and the Centers for Medicare and Medicaid Services , have become popular metrics for quantifying how patients perceive their experiences of care.
Patient experience scores are important for improving health care delivery, as they help indicate the degree to which treatment is patient-centered.
The investigators studied survey data from 5,751 patients across nine adult primary care clinics that adopted measures of patient experience derived from the CAHPS Clinician and Group Survey . The survey included a series of open-ended questions that elicited patient narratives about their experiences; questions such as, “What do you like best about our office? What do you like least about our office? Is there anything else that you would like to share about your experience?” Investigators also reviewed patient narrative responses to five questions from the CAHPS Narrative Item Set (NIS) . They then assessed the correlation between clinical staff’s narrative exposure and their clinic’s patient experience score performance.
Study findings revealed that sharing narratives was linked to better patient experience scores across the clinics, although this depended on the specific domain of patient experience measured and the staff’s confidence in their knowledge of their patients and practice.
According to Dr. Nembhard, “Patient narratives provide additional insights about organizational functioning. In their narratives, patients often elaborate on why they chose their closed-ended responses, provide information about aspects of their experiences not captured by closed-ended questions in patient surveys, and often offer their own creative ideas for how patient care experiences can be improved. Organizations that use this additional insight to guide their care practices and improvement efforts should deliver more patient-centered care, and thus have better patient experience survey scores. Of course, as our study shows, that linkage also depends on organizational attributes.”
For example, clinical staff that considered themselves more confident in their own knowledge had higher rates of using patients’ narratives, which correlated to higher scores for operational measures of patient experience—measures that capture the structural components of a health system that shape a patient’s perceptions. However, findings also showed lower scores for more confident staff for relational measures , which are measures that capture the quality of interpersonal components of health care delivery, when they had higher rates of narrative exposure. This highlights the need to further address how a patient’s narrative interacts with staff confidence in the future.
In the realm of clinical medicine, acknowledging experience through narrative has become popular over the past few decades. Lessons from the fields of narrative medicine and ethics show that narrative competence is a model for humane and effective care and policymaking —the ability to recognize, listen to, interpret, and act on the stories of others. Although patients’ narratives provide a voice to their experiences, the power of narrative has been difficult to interpret in research settings , creating gaps between research and clinical practice. Nembhard and colleagues research shows that it is possible to bridge this gap.
The importance of narrative and measures of patient experience are hard to overstate. The incorporation of narrative-informed practices in medicine and policymaking will make lived experiences central, not incidental, to deliberations in practice.
For example, regulators are making a concerted effort to augment the experiences of consumers during critical moments in their lives, including those of patients when receiving clinical care. This is evidenced by the 2023 announcement of nine Life Experience Projects by the Office of Management and Budget, which are federal efforts to put people at the center of all government-supported endeavors. The Life Experience Projects were part of the 2021 Executive Order that called for more accountability of government leaders for how they are perceived by the public during times of critical need and crisis.
Contemporary efforts for social justice will require medicine, policy, and law to become humanized and democratized , which the incorporation of narrative use into practice can help facilitate. By giving patients a voice in reporting how they perceive their care, providers, policymakers, and health care leaders can help inspire more substantive change that uplifts the positions of those who are impacted by their decision-making the most.
The study, “ Learning from Patients: The Impact of Using Patients’ Narratives on Patient Experience Scores ,” was published on January 3, 2024, in Health Care Management Review . Authors include Ingrid M. Nembhard , Sasmira Matta , Dale Shaller, Yuna S. H. Lee, Rachel Grob, and Mark Schlesinger.

Policy Coordinator
More on health equity.

News | Video
How the Hidden Mechanics of Genetic Medicines Can Disadvantage Non-White Individuals
Penn LDI’s Carmen Guerra Warns a White House Forum
- Hoag Levins

Penn LDI at AcademyHealth 2024 – The Photo Story
A Widespread Presence at the Country’s Largest Gathering of Health Services Researchers

Photo Report: Poster Hall at AcademyHealth ARM 2024, Baltimore
Penn LDI Health Services Researchers Spotlight and Network Around Their Latest Work

Young Scholars Arrive at 2024 AcademyHealth Research Meeting
Baltimore Event Immerses Them in the National Health Services Research Community

New Study Documents Racial and Ethnic Disparities in Veterans’ Experiences With VA-Funded Community Care
Disparities Were Modest but Persisted
- Eric T. Roberts, PhD

Amanda Kreider Receives ASHEcon Chair Award
Cited for “Exceptional Abstract” on Adverse Selection in Medicaid Networks
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- The patients bringing...
The patients bringing lived experience to research teams
- Related content
- Peer review
- Eva Amsen , freelance journalist
- eva.amsen{at}gmail.com
Patients are increasingly acting as co-leads on research projects, but the infrastructure to support these collaborations is still in its infancy. Eva Amsen reports
In 2015, when the Cambridge Clinical Trials Unit launched its Patient Led Research Hub (PLRH) it noticed that patients were interested in research topics that weren’t covered by most published studies. “Most patient priorities were around quality of life and symptom management, whereas a lot of funded projects were about interventions and drugs,” says Laura Cowley, research lead at PLRH.
A similar discrepancy had come up in the Netherlands in 2006, when the Dutch Burns Foundation invited patients to help set their research agenda. 1 It learnt that this group’s main concern was itching, which wasn’t a research priority at that time. “It’s such a good example to show that people with lived experience ask very different questions,” says Sanne Steenhuisen, programme secretary of the participation and citizen science initiative at the Netherlands Organisation for Health Research and Development (ZonMW).
Many organisations and patient groups around the world, including PLRH and ZonMW, are now creating better ways for patients and researchers to collaborate.
Beyond box ticking
“Patient led” now means that research is driven by patients or by people with lived experience. This might be in consulting roles or as co-researchers. It goes further than merely encouraging researchers to incorporate patient and public involvement (PPI) in their work—something that is already becoming common practice at many medical research funders.
Funders that ask for PPI plans include Australia’s National Health and Medical Research Council, the Canadian Institutes of Health Research (CIHR), the UK’s National Institute for Health Research, the National Institutes of Health in the US, ZonMW, and many others, including charitable research funders.
While asking funding applicants about their PPI plans encourages researchers to think about patient involvement in the early stages, there is often very little follow-up 2 and no guarantee that researchers will actually involve patients. “It’s often seen as a box ticking exercise and we want to change that,” says Steenhuisen. “Although funders say they want a PPI plan or evidence of PPI, it’s still very tokenistic,” agrees Cowley.
PLRH is taking a more active approach to getting patients involved. Its current focus is on delivering clinical studies in collaboration with rare disease patient groups.
“Originally, the ethos was to take on any research idea from any patient group,” says Cowley. “But that got too big too quickly.” Since most of the feasible research ideas came from rare disease groups, it decided to limit the scope. PLRH now takes research suggestions from rare disease patient support groups and facilitates the formation of equal partnerships between patients and research groups if the idea is feasible.
Patient groups are becoming research partners
Organisations involved in patient led research are exploring different ways of giving patients an active role. Some initiatives are led by patient communities, such as the Patient-Led Research Collaborative for Long Covid 3 (see box 1 ). Others are spearheaded by research or funding organisations that take research topic suggestions from community and patient groups, involve them as equal research partners, or both.
Long covid research has been patient led from the beginning.
People who did not fully recover after a covid infection found each other online and created communities to drive awareness. One of these online forums branched out into the Patient-Led Research Collaborative, which facilitates patient led research into long covid by working closely with a group of researchers.
Other members of the long covid community also recently organised the Unite to Fight 5 conference, where researchers and others shared updates on long covid and myalgic encephalomyelitis/chronic fatigue syndrome research.
One of the speakers was Alison Cohen, epidemiologist at the University of California, San Francisco. Cohen worked with the Patient-Led Research Collaborative on a report documenting 13 case studies of people who tried nirmatrelvir/ritonavir (Paxlovid) as a long covid treatment, currently available as preprint. 6 “I’m particularly proud of this work as a model of patient driven research—many of the people whose experiences are documented were also involved in the paper as co-authors,” says Cohen. “When patients and other people with relevant lived experiences are involved in research, it makes the research stronger.”
ZonMW, for example, is currently running a process to connect researchers on youth matters with community members who have suggestions for research topics. “One of our goals is to expand this to other topics,” says Steenhuisen. Other funders are taking similar steps. Diabetes UK, which is already flexible about including patients as co-applicants on some of their funded projects, is broadening its scope to include more people with lived experience.
Meanwhile, the Strategy for Patient-Oriented Research (SPOR) Evidence Alliance in Canada routinely includes patient partners in knowledge synthesis projects, but has recently also selected several new research topics that were submitted by patients or community members who are co-leading their chosen project with a research team.
Becoming equal partners in a research team is currently more common in areas with tangible and applicable outcomes, such as knowledge synthesis at the SPOR Evidence Alliance or clinical effectiveness research, the focus of the Patient Centred Outcomes Research Institute (PCORI) in the US.
PCORI includes patients as advisers in study design and implementation; on some projects it also involves community organisations as co-leaders. “It represents a model that we may be able to repeat over time,” says Harold Feldman, deputy executive director for patient centred research programmes at PCORI.
But while all these organisations and projects are exploring new ways of collaborating and co-creating with patient communities, they’re also identifying new challenges.
A system unfriendly to patient led research
Patients can be involved as advisers in setting research directions, in providing feedback when approached, or even in co-leading research projects. That level of involvement deserves compensation, but this is not a straightforward process. Paying patients for their involvement may affect any government benefits they are receiving. That’s why some organisations give patients the choice between remuneration or gift cards, for example.
“We try to find different options to pay people for their time, and we ask what their preference is,” says Andrea Tricco, principal investigator for the SPOR Evidence Alliance and scientist at St Michael’s Hospital, Toronto.
Problems also arise when patients are co-applicants on grant proposals, because existing funding systems aren’t set up for applicants without institutional affiliations. “When patient partners apply as co-applicants, they need to submit an academic CV, which is just not appropriate,” says Cowley of UK funding systems. “And there’s no box for PPI. If you don’t have a medical background, you select ‘other.’ It’s such an unwelcoming first interaction for a patient.”
The situation in Canada is similar. “Right now, patients can’t necessarily hold funding unless they’re part of a formal affiliation or group,” says Tricco. “But I do know that that’s something CIHR is looking into.”
Another roadblock to further implementation of patient led research is that the research community is only gradually becoming aware of how it can collaborate with patients. “It has to become more normalised,” says Steenhuisen. “Why would you do research without involving the people that the research is about?”
“Unfortunately, a lot of research groups are still new to the idea of partnering with a patient or having patients involved at all,” says Dana Lewis. Lewis, who has type 1 diabetes, has worked closely with researchers for the past decade to develop and test a closed loop artificial pancreas system (see box 2 ). But when she was also diagnosed with exocrine pancreatic insufficiency, a digestive condition, she found it much harder to collaborate with gastroenterology researchers. “It’s like a different universe,” she says.
In 2013, Dana Lewis was not able to hear the alarms on the continuous glucose monitor that she used to manage her type 1 diabetes.
Together with Scott Leibrand she found a way to pull data from the device and build a better alarm system. Further modifications connected the system to an insulin pump to create a fully functional closed loop automated insulin delivery system.
“We spent a lot of time thinking about safety as it evolved to a closed loop system,” says Lewis. “We wanted people to have the advantage of building from the safety based design that we had.”
That was one of the reasons Lewis made everything openly available through the OpenAPS project, where anyone can download the software they need to run the system on either a small hardware device or through an Android phone. Even now that commercial closed loop systems are available, thousands of people are still using OpenAPS because it’s more accessible and affordable.
Lewis connected with researchers and clinicians at conferences where she was invited to speak. This network made it possible to fund and carry out a clinical trial with Lewis as co-investigator. The results of this trial further validated the effectiveness of the new Android based OpenAPS system.
This lack of familiarity among the research community is something that the SPOR Evidence Alliance is hoping to tackle. “We’re trying to change the research community to be more accepting of patient engagement,” says Tricco.
Organisations and funders are learning where the bottlenecks are in managing patient led research. They’re working on building fair compensation systems, supporting researchers, and evaluating best practices.
PCORI is also funding research into the science of engagement, 4 to learn more about the best ways researchers and funders can work together with patients in research planning and execution. “We need to take the evidence and the findings from those programmes,” says Feldman. “Then we can weave that back in so that we can do an even better job of facilitating and promoting patient engaged research.”
It’s a learning process for everyone and the next few years will likely see further progress. Cowley is optimistic, and says, “In general, people are much more aware and are at least having conversations around the challenges of patient led research.”
I have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
- Broerse JEW ,
- Zweekhorst MBM ,
- van Rensen AJML ,
- de Haan MJM
- Jenkins G ,
- Patient-Led Research Collaborative
- ↵ PCORI. Measuring what matters for advancing the science and practice of engagement. www.pcori.org/resources/measuring-what-matters-advancing-science-and-practice-engagement
- ↵ Unite to Fight. https://unitetofight2024.world
- ↵ Cohen AK, Jaudon TW, Schurman EM, et al. Impact of extended course oral nirmatrelvir/ritonavir (Paxlovid) in established long covid: case series and research considerations. www.researchsquare.com/article/rs-3359429/v1
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
Expand community-based research to make clinical trials more diverse
By Robert Metcalf and Jeffrey Francer July 10, 2024

I nnovations in clinical trial designs and tools have the potential to unlock a new era of research that is more convenient for patients, more reflective of real-world treatment conditions, and more likely to enable participation of a diverse set of individuals. But a recent study reveals how far the U.S. is from realizing this potential: regions of the country with the worst social drivers of health are the least likely to host clinical trials.
The disconnect between need and where clinical trials are conducted is a longstanding one. But it was recently highlighted by University of Michigan researchers through an examination of demographic data for people enrolled in clinical trials for new cancer medicines. The most socially vulnerable counties were far less likely to have any nearby trial, a disparity that has worsened over time.
advertisement
Research sponsors and the Food and Drug Administration can respond to this challenge by continuing to support community-based clinical trials. But the regulatory framework that governs these and other modern approaches has not kept pace with innovations in clinical trials and must be updated to enable expansion of trials into more communities.
Clinical trials are essential for establishing the safety and effectiveness of new medicines. Trial results have a greater impact when participants reflect the demographic diversity of those who could potentially benefit from the treatments being evaluated. The University of Michigan research is one more confirmation that the U.S.’s existing clinical trial infrastructure often fails to meet these goals.
Designing and implementing clinical trials is hard work. Reports over time suggest that up to 85% of clinical trials don’t meet their recruitment goals and up to 80% are delayed due to recruitment challenges.
Large-scale clinical trials are typically hosted by large research hospitals and academic institutions, most of which are in big cities. This can exclude people in rural communities from participating in clinical trials, and can present logistical challenges even for individuals who live relatively close to these centers because they may not have the economic means or schedule flexibility to attend multiple appointments.
Today’s clinical trial regulations were created for a different era, when the technology of the time necessitated that studies be conducted at a single location under the direct supervision of an investigator and staff who carried out all aspects of the trial. Participants were required to come to that location. Clinical research still largely relies on this outmoded approach, which frequently requires participants to organize their lives around the trial, and often includes traveling, finding a place to stay, and taking time off from work.
New tools and approaches developed by clinical trial sponsors, working with the FDA, should help make trials more representative. The FDA has signaled an openness to supporting trial designs that make them more accessible for participants, more reflective of real-world conditions, and enable more diverse participation. This modernization of the regulatory framework is critically needed and will contribute to healthier communities by speeding the development of new and better treatments that address unmet medical needs.
Community-based trials, also known as decentralized trials, have the potential to significantly increase participation and diversity in clinical research. By forcing a shift to this model, the Covid-19 pandemic showed just how successful these types of studies can be. To help ensure studies could continue during the pandemic, investigators, trial sponsors, and regulators worked collaboratively during the nationwide shutdown to reverse the process, bringing trials to participants rather than participants to trials.
Lilly, the company we work for, partnered with a leading decentralized research organization to bring our Covid-19 research to at-risk patients in long-term care facilities. An innovative cloud-based system helped recruit participants across multiple sites and make adjustments as needed in real time.
This model allowed Lilly to move quickly, reach more people who were traditionally underrepresented in clinical trials, and protect the health of participants and trial staff during the pandemic, all while maintaining the highest standards of scientific research, patient safety, and data integrity. To be sure, Lilly wasn’t alone in doing this: companies across the biopharmaceutical industry can share similar stories of leveraging innovative, community-based approaches to keep clinical trials running during the Covid-19 crisis.
These updated approaches shouldn’t fade away with the pandemic. Drug developers, investigators, and regulators must build on what was learned. Several key updates to the U.S. clinical trial regulatory framework will be crucial to supporting this progress:
Ease the burden on clinical trial investigators. Enabling better support from sponsor staff can create efficiencies and fill resource needs for community-based providers. Local health care professionals are essential to the success of community-based trials, but most of them do not have the resources or infrastructure to manage many of the demands of clinical studies, such as recruiting participants, providing them with logistical support, and shipping investigational products to them. Trial sponsor staff have the capability to perform tasks like these that involve limited or no contact with participants to avoid conflict of interest. Current regulatory rules, however, provide little guidance on what types of sponsor roles are appropriate, which creates uncertainty for sponsors that can discourage such support.
Update the role of investigators. The shift in clinical trial services to multiple care settings, such as community clinics, mobile medical units, and participants’ homes, must be accompanied by updating how clinical trial investigators provide oversight of these settings. Current regulations state that an investigator must personally conduct or supervise a trial. This requirement can create confusion for a community-based study that includes multiple care settings in numerous communities.
To better accommodate community-based trials without compromising patient safety or data integrity, FDA regulations should be updated to clarify that trial investigators may provide oversight by ensuring that study staff such as local health care providers are appropriately qualified and trained for the trial-related activities they will perform. Such assurance could include confirming proper education and qualifications and meeting state licensing requirements.
Current regulations also state that investigators may administer an investigational product only to study participants they personally supervise. Such regulations do not lend themselves to the flexibility needed to enable community-based research, where patients can receive clinical trial services in many types of settings.
Consistently support the use of digital health technologies. Wearable devices and other advances can help make trials more convenient for participants by enabling remote collection of data from them in real time as they go about their daily lives. This convenience can promote diversity by reducing the number of clinic visits needed, making it possible for people to participate in trials whose income, work, or travel issues would prevent multiple in-person visits. Yet current FDA guidance lacks clarity on what evidence is needed to validate the use of digital health technologies. A modernized approach for qualifying digital health technologies is needed. Sponsors of new drug trials are currently encouraged to use the drug development tools pathway , which was not designed for digital health technologies and can be cumbersome and complicated for this use.
It also is not clear how digital health technologies will be reviewed when multiple FDA divisions or offices are involved. Providing greater clarity on the evidence required for validation and on cross-agency standards will support acceleration of the application of digital health technologies, further enabling community-based clinical trials.
By the end of this decade, we believe that community-based clinical trials will become the norm, not the outlier. To achieve this, all clinical trial stakeholders — including the FDA, drug developers, and investigators — must work together to foster a patient-centric clinical trial culture that embraces innovation and brings trials closer to potential participants. The result will be a win for everyone.
Robert Metcalf, Ph.D., is group vice president for clinical design, delivery and analytics, China and Japan medical, for Lilly. Jeffrey Francer, J.D., is Lilly’s vice president, head of global regulatory policy and strategy.
LETTER TO THE EDITOR
Have an opinion on this essay submit a letter to the editor here ., about the authors reprints, robert metcalf, jeffrey francer.
Clinical trials
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

Adaptive trial designs will increase clinical trial speed, safety, and effectiveness

Unsettling truths about maternal mortality in the U.S.

STAT Plus: Troubled for-profit chains are stealthily operating dozens of psychiatric hospitals under nonprofits’ names

STAT Plus: Biopharma layoffs are leaving the industry’s scientists reeling — and in search of scarce opportunities

STAT Plus: A faster, simpler, cheaper cancer cell therapy is about to be tested in humans

IMAGES
VIDEO
COMMENTS
Step 3: After identifying potential doctors you want to work with, send each of them a personalized email an expressing your interest in being a research volunteer. Step 4: After a few email exchanges and probably an in-person interview with the doctor, you'll land with you dream clinical research volunteer role.
7—Have the courage to hear "No." Remember that you will eventually hear "Yes.". Many entry-level clinical research applicants lack the courage to hear that, "No, we cannot hire you for this job" from potential employers. It is painful to hear a "No" and rightfully so. Furthermore, most employers do a poor job of providing ...
Here are four steps you can take to become a researcher: 1. Take relevant classes. Clinical researchers typically pursue an undergraduate degree in biology, chemistry, medicine, psychology or a related field. Many also earn a master's, especially if they hope to work at a university or pharmaceutical company.
Here are the top 6 factors to support a successful career as a CRA at IQVIA. 1. Communication. As a CRA, you are the key liaison between management, the study site, and the sponsor. With so many moving parts in clinical research, strong interpersonal skills and good command of English are important. It is also critical to have fluency in the ...
The ACRP offers the Certified Clinical Research Associate credential. To earn this certification, you must have one of the following: A bachelor's degree and at least 3,000 hours of experience as a CRA. A current CCRC, CPI or ACRP-CP certification and be able to substitute 1,500 hours of work experience.
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job. Respondents stressed the importance of flexibility and persistence ...
Building on Healthcare Experience to Advance Clinical Research Dr. Barkoudah points out that while patient care continues to be the main focus for many physicians, a growing number of them are also building on their experiences in the exam room by broadening out into the investigative side as well to deepen their understanding of disease states ...
A research project might require you to first take coursework in basic lab sciences, statistics, or another advanced topic specific to the project. Other PIs may prefer to train you "on-the-job" through their graduate or post-doc students. This will impact when you are ready to join a project. Finding the right research project.
To work in clinical research, you need to hold a degree and have a certain amount of experience. You can pursue certifications and graduate qualifications to further your chances of a position. Bachelor's degree. A bachelor's degree is generally step one for anyone who wants to work as a clinical research coordinator.
Pathway 2 - Clinical research professionals with 1,500 hours of verifiable work experience and a clinical research degree are eligible to sit for the CCRA Exam. Applicants also have to submit proof of their current job description and resume and pass the CCRA exam. In addition, CCRAs must complete at least 24 hours of continuing education ...
Hold a "clinical research degree" or complete 1,500 hours performing essential duties. Submit a resume documenting and demonstrating job performance. Please note that in some cases, additional education can be used to substitute for work experience hours. Please see credentialing websites for details.
Remote monitoring takes a great deal of work and technological experience at both the sponsor and site level. It involves a great deal of scanning and correspondence by the research coordinators, which can take a lot of their time and resources. ... Former/current research coordinator; Clinical experience (medical assistant, registered nurse ...
Example: "I have extensive experience with clinical trial design and protocol development. I've designed dozens of trials for pharmaceutical companies, ranging from phase 1 to phase 3 studies. ... Clinical research associates often work remotely, and this question is designed to assess your understanding of the challenges associated with ...
Highlighting your career growth within the clinical research field shows your dedication, adaptability, and potential for future growth. If you've been promoted or taken on increasing responsibilities in your previous roles, make sure to emphasize this in your work experience section. Clinical Research Associate, XYZ Pharmaceuticals Jan 2018 ...
Here are seven steps for writing a clinical research associate resume: 1. Study the job description. Study the job description carefully to check if you fulfill all the job requirements, as it may require specific certifications or a certain number of years of experience.
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
15 Common Interview Questions for Clinical Research. 1. What is your experience in clinical research? When answering this question, provide a brief overview of your experience in clinical research, including the types of studies you have worked on, any specific therapeutic areas you are familiar with, and your role in these studies.
Research Accountant 1. Stanford University. Remote in Redwood City, CA. $60,000 - $96,000 a year. Full-time. Weekends as needed + 1. Four-year college degree and three years of relevant experience or combination of education and relevant experience. Strong working knowledge of Excel. Posted 3 days ago ·.
Clinical research experience has been an early, but popular, option. ... During a clinical research experience, you can expect to work with patient medical data, medications, medical devices, procedures, or other patient care topics to solve or formulate a hypothesis laid out by the precepting physician or research team. Participating in a ...
Clinical research offers a dynamic and fulfilling career path for individuals passionate about advancing medical knowledge and improving patient outcomes. As the demand for groundbreaking treatments and therapies continues to grow, so does the need for skilled professionals in this field. In this article, we will delve into the various roles ...
Clinical research: students may be able to assist in enrolling patients or administering tests, which can help develop interpersonal skills and provide better understanding of the patient experience. Research also gives students access to mentors in the field and a sense of what it's like to work within an academic medical/research environment.
Experience should be in the clinical setting or related experience. Additional Experience and/or Qualifications: (Has Achieved Competency in the Following Areas, Job Knowledge and Additional Considerations): Graduate or diploma from a study coordinator training program is preferred. One year of clinical research experience is preferred.
Our 12 month full-time student placements are for motivated life sciences or chemistry students. We have office-based placements across the Centre for Drug Development (CDD) and lab-based placements within Cancer Research Horizons. Our placements provide the opportunity for you to develop your scientific / clinical research experience in a ...
This is an exciting opportunity for clinical research professionals with at least one year of Clinical Research Coordinator experience to fill Clinical Research Associate (CRA) openings with Medpace. Through our PACE ® Training Program, you will receive the training needed to become a fully functional CRA with the opportunity to work home ...
The Research Scientist will work on multiple research studies including those funded by major foundations, as well as unfunded studies. The research scientist will design and conduct statistical analyses, assist with grant preparation, be responsible for data management, prepare data visuals, and contribute to manuscripts and reports.
Job Type: Officer of Administration Bargaining Unit: N/A Regular/Temporary: Regular End Date if Temporary: N/A Hours Per Week: 35 Standard Work Schedule: Building: Salary Range: $62,400 Annual - $68,000 Annual The salary of the finalist selected for this role will be set based on a variety of factors, including but not limited to departmental budgets, qualifications, experience, education ...
The CRC must be able to work in a multidisciplinary clinical team, display a high degree of initiative, and have excellent interpersonal, organizational, and time management skills. Competencies Required. Experience and knowledge of regulatory aspects of clinical research.
In the realm of clinical medicine, acknowledging experience through narrative has become popular over the past few decades. Lessons from the fields of narrative medicine and ethics show that narrative competence is a model for humane and effective care and policymaking —the ability to recognize, listen to, interpret, and act on the stories of ...
Patients are increasingly acting as co-leads on research projects, but the infrastructure to support these collaborations is still in its infancy. Eva Amsen reports In 2015, when the Cambridge Clinical Trials Unit launched its Patient Led Research Hub (PLRH) it noticed that patients were interested in research topics that weren't covered by most published studies.
Lilly, the company we work for, partnered with a leading decentralized research organization to bring our Covid-19 research to at-risk patients in long-term care facilities.