
- Previous Article
- Next Article

Case Presentation
Case study: a patient with uncontrolled type 2 diabetes and complex comorbidities whose diabetes care is managed by an advanced practice nurse.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Geralyn Spollett; Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse. Diabetes Spectr 1 January 2003; 16 (1): 32–36. https://doi.org/10.2337/diaspect.16.1.32
Download citation file:
- Ris (Zotero)
- Reference Manager
The specialized role of nursing in the care and education of people with diabetes has been in existence for more than 30 years. Diabetes education carried out by nurses has moved beyond the hospital bedside into a variety of health care settings. Among the disciplines involved in diabetes education, nursing has played a pivotal role in the diabetes team management concept. This was well illustrated in the Diabetes Control and Complications Trial (DCCT) by the effectiveness of nurse managers in coordinating and delivering diabetes self-management education. These nurse managers not only performed administrative tasks crucial to the outcomes of the DCCT, but also participated directly in patient care. 1
The emergence and subsequent growth of advanced practice in nursing during the past 20 years has expanded the direct care component, incorporating aspects of both nursing and medical care while maintaining the teaching and counseling roles. Both the clinical nurse specialist (CNS) and nurse practitioner (NP) models, when applied to chronic disease management, create enhanced patient-provider relationships in which self-care education and counseling is provided within the context of disease state management. Clement 2 commented in a review of diabetes self-management education issues that unless ongoing management is part of an education program, knowledge may increase but most clinical outcomes only minimally improve. Advanced practice nurses by the very nature of their scope of practice effectively combine both education and management into their delivery of care.
Operating beyond the role of educator, advanced practice nurses holistically assess patients’ needs with the understanding of patients’ primary role in the improvement and maintenance of their own health and wellness. In conducting assessments, advanced practice nurses carefully explore patients’ medical history and perform focused physical exams. At the completion of assessments, advanced practice nurses, in conjunction with patients, identify management goals and determine appropriate plans of care. A review of patients’ self-care management skills and application/adaptation to lifestyle is incorporated in initial histories, physical exams, and plans of care.
Many advanced practice nurses (NPs, CNSs, nurse midwives, and nurse anesthetists) may prescribe and adjust medication through prescriptive authority granted to them by their state nursing regulatory body. Currently, all 50 states have some form of prescriptive authority for advanced practice nurses. 3 The ability to prescribe and adjust medication is a valuable asset in caring for individuals with diabetes. It is a crucial component in the care of people with type 1 diabetes, and it becomes increasingly important in the care of patients with type 2 diabetes who have a constellation of comorbidities, all of which must be managed for successful disease outcomes.
Many studies have documented the effectiveness of advanced practice nurses in managing common primary care issues. 4 NP care has been associated with a high level of satisfaction among health services consumers. In diabetes, the role of advanced practice nurses has significantly contributed to improved outcomes in the management of type 2 diabetes, 5 in specialized diabetes foot care programs, 6 in the management of diabetes in pregnancy, 7 and in the care of pediatric type 1 diabetic patients and their parents. 8 , 9 Furthermore, NPs have also been effective providers of diabetes care among disadvantaged urban African-American patients. 10 Primary management of these patients by NPs led to improved metabolic control regardless of whether weight loss was achieved.
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes.
A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes. Although he was diagnosed in 1997, he had symptoms indicating hyperglycemia for 2 years before diagnosis. He had fasting blood glucose records indicating values of 118–127 mg/dl, which were described to him as indicative of “borderline diabetes.” He also remembered past episodes of nocturia associated with large pasta meals and Italian pastries. At the time of initial diagnosis, he was advised to lose weight (“at least 10 lb.”), but no further action was taken.
Referred by his family physician to the diabetes specialty clinic, A.B. presents with recent weight gain, suboptimal diabetes control, and foot pain. He has been trying to lose weight and increase his exercise for the past 6 months without success. He had been started on glyburide (Diabeta), 2.5 mg every morning, but had stopped taking it because of dizziness, often accompanied by sweating and a feeling of mild agitation, in the late afternoon.
A.B. also takes atorvastatin (Lipitor), 10 mg daily, for hypercholesterolemia (elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides). He has tolerated this medication and adheres to the daily schedule. During the past 6 months, he has also taken chromium picolinate, gymnema sylvestre, and a “pancreas elixir” in an attempt to improve his diabetes control. He stopped these supplements when he did not see any positive results.
He does not test his blood glucose levels at home and expresses doubt that this procedure would help him improve his diabetes control. “What would knowing the numbers do for me?,” he asks. “The doctor already knows the sugars are high.”
A.B. states that he has “never been sick a day in my life.” He recently sold his business and has become very active in a variety of volunteer organizations. He lives with his wife of 48 years and has two married children. Although both his mother and father had type 2 diabetes, A.B. has limited knowledge regarding diabetes self-care management and states that he does not understand why he has diabetes since he never eats sugar. In the past, his wife has encouraged him to treat his diabetes with herbal remedies and weight-loss supplements, and she frequently scans the Internet for the latest diabetes remedies.
During the past year, A.B. has gained 22 lb. Since retiring, he has been more physically active, playing golf once a week and gardening, but he has been unable to lose more than 2–3 lb. He has never seen a dietitian and has not been instructed in self-monitoring of blood glucose (SMBG).
A.B.’s diet history reveals excessive carbohydrate intake in the form of bread and pasta. His normal dinners consist of 2 cups of cooked pasta with homemade sauce and three to four slices of Italian bread. During the day, he often has “a slice or two” of bread with butter or olive oil. He also eats eight to ten pieces of fresh fruit per day at meals and as snacks. He prefers chicken and fish, but it is usually served with a tomato or cream sauce accompanied by pasta. His wife has offered to make him plain grilled meats, but he finds them “tasteless.” He drinks 8 oz. of red wine with dinner each evening. He stopped smoking more than 10 years ago, he reports, “when the cost of cigarettes topped a buck-fifty.”
The medical documents that A.B. brings to this appointment indicate that his hemoglobin A 1c (A1C) has never been <8%. His blood pressure has been measured at 150/70, 148/92, and 166/88 mmHg on separate occasions during the past year at the local senior center screening clinic. Although he was told that his blood pressure was “up a little,” he was not aware of the need to keep his blood pressure ≤130/80 mmHg for both cardiovascular and renal health. 11
A.B. has never had a foot exam as part of his primary care exams, nor has he been instructed in preventive foot care. However, his medical records also indicate that he has had no surgeries or hospitalizations, his immunizations are up to date, and, in general, he has been remarkably healthy for many years.
Physical Exam
A physical examination reveals the following:
Weight: 178 lb; height: 5′2″; body mass index (BMI): 32.6 kg/m 2
Fasting capillary glucose: 166 mg/dl
Blood pressure: lying, right arm 154/96 mmHg; sitting, right arm 140/90 mmHg
Pulse: 88 bpm; respirations 20 per minute
Eyes: corrective lenses, pupils equal and reactive to light and accommodation, Fundi-clear, no arteriolovenous nicking, no retinopathy
Thyroid: nonpalpable
Lungs: clear to auscultation
Heart: Rate and rhythm regular, no murmurs or gallops
Vascular assessment: no carotid bruits; femoral, popliteal, and dorsalis pedis pulses 2+ bilaterally
Neurological assessment: diminished vibratory sense to the forefoot, absent ankle reflexes, monofilament (5.07 Semmes-Weinstein) felt only above the ankle
Lab Results
Results of laboratory tests (drawn 5 days before the office visit) are as follows:
Glucose (fasting): 178 mg/dl (normal range: 65–109 mg/dl)
Creatinine: 1.0 mg/dl (normal range: 0.5–1.4 mg/dl)
Blood urea nitrogen: 18 mg/dl (normal range: 7–30 mg/dl)
Sodium: 141 mg/dl (normal range: 135–146 mg/dl)
Potassium: 4.3 mg/dl (normal range: 3.5–5.3 mg/dl)
Lipid panel
• Total cholesterol: 162 mg/dl (normal: <200 mg/dl)
• HDL cholesterol: 43 mg/dl (normal: ≥40 mg/dl)
• LDL cholesterol (calculated): 84 mg/dl (normal: <100 mg/dl)
• Triglycerides: 177 mg/dl (normal: <150 mg/dl)
• Cholesterol-to-HDL ratio: 3.8 (normal: <5.0)
AST: 14 IU/l (normal: 0–40 IU/l)
ALT: 19 IU/l (normal: 5–40 IU/l)
Alkaline phosphotase: 56 IU/l (normal: 35–125 IU/l)
A1C: 8.1% (normal: 4–6%)
Urine microalbumin: 45 mg (normal: <30 mg)
Based on A.B.’s medical history, records, physical exam, and lab results, he is assessed as follows:
Uncontrolled type 2 diabetes (A1C >7%)
Obesity (BMI 32.4 kg/m 2 )
Hyperlipidemia (controlled with atorvastatin)
Peripheral neuropathy (distal and symmetrical by exam)
Hypertension (by previous chart data and exam)
Elevated urine microalbumin level
Self-care management/lifestyle deficits
• Limited exercise
• High carbohydrate intake
• No SMBG program
Poor understanding of diabetes
A.B. presented with uncontrolled type 2 diabetes and a complex set of comorbidities, all of which needed treatment. The first task of the NP who provided his care was to select the most pressing health care issues and prioritize his medical care to address them. Although A.B. stated that his need to lose weight was his chief reason for seeking diabetes specialty care, his elevated glucose levels and his hypertension also needed to be addressed at the initial visit.
The patient and his wife agreed that a referral to a dietitian was their first priority. A.B. acknowledged that he had little dietary information to help him achieve weight loss and that his current weight was unhealthy and “embarrassing.” He recognized that his glucose control was affected by large portions of bread and pasta and agreed to start improving dietary control by reducing his portion size by one-third during the week before his dietary consultation. Weight loss would also be an important first step in reducing his blood pressure.
The NP contacted the registered dietitian (RD) by telephone and referred the patient for a medical nutrition therapy assessment with a focus on weight loss and improved diabetes control. A.B.’s appointment was scheduled for the following week. The RD requested that during the intervening week, the patient keep a food journal recording his food intake at meals and snacks. She asked that the patient also try to estimate portion sizes.
Although his physical activity had increased since his retirement, it was fairly sporadic and weather-dependent. After further discussion, he realized that a week or more would often pass without any significant form of exercise and that most of his exercise was seasonal. Whatever weight he had lost during the summer was regained in the winter, when he was again quite sedentary.
A.B.’s wife suggested that the two of them could walk each morning after breakfast. She also felt that a treadmill at home would be the best solution for getting sufficient exercise in inclement weather. After a short discussion about the positive effect exercise can have on glucose control, the patient and his wife agreed to walk 15–20 minutes each day between 9:00 and 10:00 a.m.
A first-line medication for this patient had to be targeted to improving glucose control without contributing to weight gain. Thiazolidinediones (i.e., rosiglitizone [Avandia] or pioglitizone [Actos]) effectively address insulin resistance but have been associated with weight gain. 12 A sulfonylurea or meglitinide (i.e., repaglinide [Prandin]) can reduce postprandial elevations caused by increased carbohydrate intake, but they are also associated with some weight gain. 12 When glyburide was previously prescribed, the patient exhibited signs and symptoms of hypoglycemia (unconfirmed by SMBG). α-Glucosidase inhibitors (i.e., acarbose [Precose]) can help with postprandial hyperglycemia rise by blunting the effect of the entry of carbohydrate-related glucose into the system. However, acarbose requires slow titration, has multiple gastrointestinal (GI) side effects, and reduces A1C by only 0.5–0.9%. 13 Acarbose may be considered as a second-line therapy for A.B. but would not fully address his elevated A1C results. Metformin (Glucophage), which reduces hepatic glucose production and improves insulin resistance, is not associated with hypoglycemia and can lower A1C results by 1%. Although GI side effects can occur, they are usually self-limiting and can be further reduced by slow titration to dose efficacy. 14
After reviewing these options and discussing the need for improved glycemic control, the NP prescribed metformin, 500 mg twice a day. Possible GI side effects and the need to avoid alcohol were of concern to A.B., but he agreed that medication was necessary and that metformin was his best option. The NP advised him to take the medication with food to reduce GI side effects.
The NP also discussed with the patient a titration schedule that increased the dosage to 1,000 mg twice a day over a 4-week period. She wrote out this plan, including a date and time for telephone contact and medication evaluation, and gave it to the patient.
During the visit, A.B. and his wife learned to use a glucose meter that features a simple two-step procedure. The patient agreed to use the meter twice a day, at breakfast and dinner, while the metformin dose was being titrated. He understood the need for glucose readings to guide the choice of medication and to evaluate the effects of his dietary changes, but he felt that it would not be “a forever thing.”
The NP reviewed glycemic goals with the patient and his wife and assisted them in deciding on initial short-term goals for weight loss, exercise, and medication. Glucose monitoring would serve as a guide and assist the patient in modifying his lifestyle.
A.B. drew the line at starting an antihypertensive medication—the angiotensin-converting enzyme (ACE) inhibitor enalapril (Vasotec), 5 mg daily. He stated that one new medication at a time was enough and that “too many medications would make a sick man out of me.” His perception of the state of his health as being represented by the number of medications prescribed for him gave the advanced practice nurse an important insight into the patient’s health belief system. The patient’s wife also believed that a “natural solution” was better than medication for treating blood pressure.
Although the use of an ACE inhibitor was indicated both by the level of hypertension and by the presence of microalbuminuria, the decision to wait until the next office visit to further evaluate the need for antihypertensive medication afforded the patient and his wife time to consider the importance of adding this pharmacotherapy. They were quite willing to read any materials that addressed the prevention of diabetes complications. However, both the patient and his wife voiced a strong desire to focus their energies on changes in food and physical activity. The NP expressed support for their decision. Because A.B. was obese, weight loss would be beneficial for many of his health issues.
Because he has a sedentary lifestyle, is >35 years old, has hypertension and peripheral neuropathy, and is being treated for hypercholestrolemia, the NP performed an electrocardiogram in the office and referred the patient for an exercise tolerance test. 11 In doing this, the NP acknowledged and respected the mutually set goals, but also provided appropriate pre-exercise screening for the patient’s protection and safety.
In her role as diabetes educator, the NP taught A.B. and his wife the importance of foot care, demonstrating to the patient his inability to feel the light touch of the monofilament. She explained that the loss of protective sensation from peripheral neuropathy means that he will need to be more vigilant in checking his feet for any skin lesions caused by poorly fitting footwear worn during exercise.
At the conclusion of the visit, the NP assured A.B. that she would share the plan of care they had developed with his primary care physician, collaborating with him and discussing the findings of any diagnostic tests and procedures. She would also work in partnership with the RD to reinforce medical nutrition therapies and improve his glucose control. In this way, the NP would facilitate the continuity of care and keep vital pathways of communication open.
Advanced practice nurses are ideally suited to play an integral role in the education and medical management of people with diabetes. 15 The combination of clinical skills and expertise in teaching and counseling enhances the delivery of care in a manner that is both cost-reducing and effective. Inherent in the role of advanced practice nurses is the understanding of shared responsibility for health care outcomes. This partnering of nurse with patient not only improves care but strengthens the patient’s role as self-manager.
Geralyn Spollett, MSN, C-ANP, CDE, is associate director and an adult nurse practitioner at the Yale Diabetes Center, Department of Endocrinology and Metabolism, at Yale University in New Haven, Conn. She is an associate editor of Diabetes Spectrum.
Note of disclosure: Ms. Spollett has received honoraria for speaking engagements from Novo Nordisk Pharmaceuticals, Inc., and Aventis and has been a paid consultant for Aventis. Both companies produce products and devices for the treatment of diabetes.
Email alerts
- Advanced Practice Care: Advanced Practice Care in Diabetes: Epilogue
- Advanced Practice Care: Advanced Practice Care in Diabetes: Preface
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Diabetes Care for Children & Young People
Vol:05 | No:01
Children and young people’s diabetes care: Case study
- 12 Jul 2016
This case study demonstrates the physical and psychological difficulties faced by many young people with type 1 diabetes. Over the year following her diagnosis, Max had a deterioration in glycaemic control despite reporting that little had changed in her management. Detailed assessment revealed a number of psychosocial factors that were preventing her from achieving good control. However, working with her multidisciplinary team, she was able to address these issues and improve her blood glucose levels. This article outlines these issues and the action plan that Max and her diabetes team drew up to overcome them.
Share this article + Add to reading list – Remove from reading list ↓ Download pdf
This case study represents the challenges and issues, both physical and psychological, faced by a young person with type 1 diabetes and the support given by her diabetes multidisciplinary team (MDT). Implications for practice are addressed using current evidence-based research. The names of the child and family have been anonymised to protect their identity.
Case study Max (a pseudonym) is a 17-year-old girl who was diagnosed with type 1 diabetes 4 years ago at the age of 13 years. She and her mother were shocked and upset by the diagnosis, and both felt its management would be too great a task to take on by themselves.
Max is an only child and lives with her mother, a single parent. She attends the local state comprehensive school and is popular with her peer group. Her mother was very involved in her care and diabetes management from the onset. Despite this, her diabetes control deteriorated over time ( Table 1 ). In October 2012, her HbA 1c was 56 mmol/mol (7.3%); however, over the next year, this increased to 84 mmol/mol (9.8%) in July 2013. She found it difficult to count the carbohydrate portions in her food and her injections were hurting much more than when she was first diagnosed. She also expressed a fear of hypoglycaemia and of “looking stupid” in front of her friends.
Max and her MDT discussed treatment options to improve her glycaemic control. She refused insulin pump therapy but agreed to a blood glucose monitor and bolus advisor to assist with her regimen of multiple daily insulin injections (MDI). She is now using the bolus advisor confidently and has had regular one-to-one sessions with a psychologist. She is having fewer hypoglycaemic episodes and her HbA 1c has improved; in January 2016 it was 69 mmol/mol (8.5%) and in April 2016 it was 58 mmol/mol (7.5%).
Discussion Diagnosis Max and her mother were extremely shocked and upset by the diagnosis of type 1 diabetes and the potential severity of the condition and intense management required. Both felt it would be too great a task to take on by themselves.
Kübler-Ross and Kessler (2005) suggested that a diagnosis of diabetes is a life-changing event comparable to the experience of loss, and that children and families will often go through the five stages of grief defined by Kübler-Ross (1970) and outlined in Box 1 . They use this as a coping strategy to enable them to eventually acknowledge the condition. However, many families never reach the fifth stage of acceptance and many will fluctuate between the stages.
Although Max and her mum did accept the diagnosis eventually, at times both of them reverted to the earlier stages of grief. The diabetes MDT supported the family from diagnosis and will continue to support them throughout their time within the paediatric diabetes service, through the transition period with both paediatric and young people’s teams, until discharged to adult diabetes care.
The diabetes MDT was established after the Best Practice Tariff was introduced in 2012. It consists of doctors, nurses, dietitians, a psychologist and a personal assistant. It is well recognised that the MDT needs to work together in close cooperation to achieve good practice, and this can be strengthened by using written protocols, guidelines and targets (Brink, 2010). Logic would suggest that centres with MDTs and the same approaches and treatment regimens would have similar outcomes, yet the Hvidøre Childhood Diabetes Study Group has shown this is not the case (de Beaufort et al, 2013). In terms of glycaemic control, there were notable differences in patient outcomes across 21 diabetes clinics, all of which were committed to MDT-based practice. Although factors such as age, type of insulin regimen and socioeconomic status were shown to have some influence over specific outcomes, they did not explain the apparent differences between these clinics.
Family/social history Max is an only child and lives with her mother, a single parent. East et al (2006) suggested that rapid social change over the past 20 years has seen a marked increase in the number of mother-headed single-parent families. Max attends the local state comprehensive school, where she is generally doing well. She is popular with her peer group. La Greca et al (1995) suggested that peer relationships are important in diabetes management, as children and young people (CYP) may receive considerable emotional support from their friends. However, on occasions, Max’s peer relationships have had a counterproductive effect on her, and she feels she is different from her friends as the only one who has diabetes. This at times affects her self-esteem and impacts her diabetes control.
Max’s mother was very involved in her care and diabetes management from the onset. Anderson and Brackett (2005) suggested that parents typically take on most of the responsibility for management of diabetes when children are young or newly diagnosed.
Deterioration in diabetes control Max’s diabetes control had deteriorated since her diagnosis ( Table 1 ). In October 2012, her HbA 1c was 56 mmol/mol (7.3%), which indicated a good level of diabetes control and a reduced risk of diabetes complications, as suggested by the DCCT (Diabetes Control and Complications Trial; DCCT Research Group, 1994). At her subsequent diabetes clinic appointments up to July 2013, she reported that “nothing had really changed,” except she “didn’t have time to think about her diabetes,” although she felt guilty because she knew she could make herself ill and her mum would get upset. She stated that it was hard counting the carbohydrate portions in her food and her injections were hurting much more than when she was first diagnosed. Her height and weight remained static.
Diabetes care is greatly influenced by psychosocial factors when they obstruct people’s ability to manage their diabetes and achieve good metabolic control. A team-based approach to addressing an individual’s ability to cope is critical (Kent et al, 2010). It is important for healthcare professionals to be aware of how CYP think at the different stages of their development, as their understanding of illness and chronic health conditions is often greater than that of their peers. Jean Piaget (1896–1980) investigated cognitive processes in children, calling them “schemas”. By the time children reach around 12 years of age, they can describe illness in terms of non-functioning or malfunctioning of an internal organ or process. Later in development they can appreciate that a person’s thoughts or feelings can affect the way the body functions, which demonstrates an awareness of psychological factors (Taylor et al, 1999).
Spear (2013) proposed that we can begin to understand how young people with type 1 diabetes think, feel and behave if we consider the cognitive and biological changes that occur during adolescence. Glasper and Richardson (2005) suggested there is now a growing awareness that CYP are able to make their own decisions if given information in an age-appropriate manner. Gillick competence identifies children aged under 16 years as having the capacity to consent to their own treatment if they understand the consequences (NSPCC, 2016).
Butler et al (2007) suggest that adolescence is a time of upheaval when young people have to deal with the influence of peers, school life and developing their own identity, as well as all the physiological changes that occur. Young people with type 1 diabetes have the added responsibility of developing autonomy regarding the self-management of their condition. Hanas (2006) suggests that parents should continue to take part in their child’s diabetes care into adolescence and not hand the responsibility to the young person too early. Snoek and Skinner (2002) suggest that intensive self-management of diabetes is complex and time-consuming, and creates a significant psychosocial burden on children and their families.
There are significant challenges for CYP to engage in effective diabetes self-management. Several of these were identified with Max and her mother:
- Deterioration in diabetes control.
- Difficulty with carbohydrate counting.
- Insulin omission.
- Fear of hypoglycaemia.
- Painful injections.
Action plan An action plan was discussed between Max and the MDT. As she was on an MDI regimen (a long-acting insulin at bedtime and rapid-acting insulin with meals), a bolus advisor/blood glucose monitor was demonstrated and discussed with her and her mum. Max felt she would be able to use this to help eliminate the calculations which, although she was capable of doing them, she often lacked time to do so. With further discussion, Max said she was “scared of getting it wrong and having a hypo”. Insulin pump therapy was discussed but she did not want to “have a device attached to my body because it would remind me all the time that I have diabetes”. Insulin pump therapy is recommended as a treatment option for adults and children over 12 years of age with type 1 diabetes whose HbA 1c levels remain above 69 mmol/mol (8.5%) on MDI therapy despite a high level of care (NICE, 2015a).
The National Service Framework standard 3 (Department of Health, 2001) recommends empowering people with diabetes and encourages them and their carers to gain the knowledge and skills to be partners in decision-making, and giving them more personal control over the day-to-day management of their diabetes, ensuring the best possible quality of life. However, if a diabetes management plan is discussed in partnership with a (Gillick-competent) young person but they elect not to comply with the plan despite full awareness of the implications of their actions, then the diabetes team should support them whilst trying to encourage them to maintain the treatment plan. This can be very difficult and frustrating at times, as a healthcare professional is an advocate for the patient, and promotion of the best interests of the patient is paramount.
Psychology involvement Max was reviewed by the psychologist to assess her psychological health and wellbeing. The psychologist used the Wellbeing in Diabetes questionnaire (available from the Yorkshire and Humber Paediatric Diabetes Network) to assess her and identify an optimal plan of care.
The psychology sessions were focussed on her issues around the following:
- Worry about deterioration in control.
- The consequences of insulin omission.
Max had a series of one-to-one appointments and some joint sessions with the paediatric diabetes specialist nurse and/or dietitian, so this linked into other team members’ specialities.
Carbohydrate counting and use of a bolus advisor The dietitian assessed Max and her mother’s ability to carbohydrate count using a calculator, food diagrams and portion sizes, and both of them were able to demonstrate competency in this task. Garg et al (2008) have shown that the use of automated bolus advisors is safe and effective in reducing postprandial glucose excursions and improving overall glycaemic control. However, this can only be true if the bolus advisor is being used correctly and is confirmed as such by comparing blood glucose and HbA 1c results before and after initiation of the bolus advisor, and observing the patient using the device to ensure it is being used safely and correctly.
Barnard and Parkin (2012) propose that, as long as safety and lifestyle are taken into consideration, advanced technology will benefit CYP, as inaccurate bolus calculation can lead to persistent poor diabetes control. These tools can help with removing the burden of such complex maths and have the potential to significantly improve glycaemic control.
Insulin omission and fear of hypoglycaemia Max also expressed her fear of hypoglycaemia and of “looking stupid” in front of her friends. She admitted to missing some of her injections, especially at school. Wild et al (2007) suggest that a debilitating fear of hypoglycaemia can result in poor adherence to insulin regimens and subsequent poor metabolic control. Crow et al (1998) describe the deliberate omission or reduced administration of insulin, which results in hyperglycaemia and subsequent rapid reduction in body weight. Type 1 diabetes predisposes a person to a high BMI. Adolescent girls and adult women with type 1 diabetes generally have higher BMI values than their peers without the condition (Domargård et al, 1999). Affenito et al (1998) observed that insulin misuse was the most common method of weight control used by young women with type 1 diabetes. However, Max’s weight remained stable and there was no clinical indication that she was missing insulin to lose weight; rather, it was her fear of hypoglycaemia that drove her to omitting insulin at school. With the use of the bolus calculator, she was reassured about her calculations for insulin-to-carbohydrate ratios, but it was reinforced with her that the device would only work efficiently if she used it correctly with each meal.
Painful injections Max also highlighted that her injections were now more painful than when she was first diagnosed, and this was causing her distress each time she had to inject. Injection technique was discussed with her and demonstrated using an injection model, and her injection technique was observed and appeared satisfactory. She was using 5-mm insulin needles and so was switched to 4-mm needles, as recommended by Forum for Injection Technique (2015) guidelines.
Appropriate technique when giving injections is key to optimal blood glucose control; however, evidence suggests that injection technique is often imperfect. Studies by Strauss et al (2002) and Frid et al (2010) revealed disturbing practices in relation to injection technique, with little improvement over the years. Current diabetes guidelines do not include detailed advice on injection technique, and only the guidance on type 2 diabetes in adults (NICE, 2015b) makes any reference to providing education about injectable devices for people with diabetes. However, the older Quality Standard for diabetes in adults (NICE, 2011) recommends a structured programme of education, including injection site selection and care (Diggle, 2014).
Conclusion The issues and concerns this young girl had were identified and addressed by the diabetes MDT. She was assessed by several members of the team, and a credible, evidence-based action plan was put into place to assist her and her mother to manage her diabetes at this difficult time. Max is now using the bolus advisor confidently and having fewer hypoglycaemic episodes, and her HbA 1c has improved. She prefers using the 4-mm injection pen needles, although she remains hesitant when giving injections; she will still not consider insulin pump therapy. Her one-to-one sessions with the psychologist have now ceased, but she is aware she can access a psychologist at clinic on request, or if the MDT assesses that her psychological health has deteriorated.
When a child in a family develops a chronic condition such as type 1 diabetes, effective communication is vitally important to address issues with the family at the earliest stage so that problems can be discussed and, hopefully, resolved before they escalate out of control. Upon reflection, the team could have become more intensely involved at an earlier stage to prevent Max’s diabetes management issues and stop her HbA 1c from reaching such a high level. Furthermore, the new NICE (2015a) guideline has set the target HbA 1c at ≤48 mmol/mol (6.5%), so there is still some work to be done. However, the outcome of this case appears to be favourable at present.
Affenito SG, Rodriguez NR, Backstrand JR et al (1998) Insulin misuse by women with type 1 diabetes mellitus complicated by eating disorders does not favorably change body weight, body composition, or body fat distribution. J Am Diet Assoc 98 : 686–8 Anderson BJ, Brackett J (2005) Diabetes in children. In: Snoek FJ, Skinner TC (eds). Psychology in Diabetes Care (2nd edition). John Wiley & Sons, Chichester Barnard K, Parkin C (2012) Can automated bolus advisors help alleviate the burden of complex maths and lead to optimised diabetes health outcomes? Diabetes Care for Children & Young People 1 : 6–9 Brink SJ (2010) Pediatric and adolescent multidisciplinary diabetes team care. Pediatr Diabetes 11 : 289–91 Butler JM, Skinner M, Gelfand D et al (2007) Maternal parenting style and adjustment in adolescents with type I diabetes. J Pediatr Psychol 32 : 1227–37 Crow SJ, Keel PK, Kendall D (1998) Eating disorders and insulin-dependent diabetes mellitus. Psychosomatics 39 : 233–43 de Beaufort CE, Lange K, Swift PG et al (2013) Metabolic outcomes in young children with type 1 diabetes differ between treatment centers: the Hvidoere Study in Young Children 2009. Pediatr Diabetes 14 : 422–8 Department of Health (2001) National Service Framework: Diabetes . DH, London. Available at: http://bit.ly/18OpAzL (accessed 24.02.16) Diabetes Control and Complications Trial Research Group (1994) Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 125 : 177–88 Diggle J (2014) Are you FIT for purpose? The importance of getting injection technique right . Journal of Diabetes Nursing 18 : 50–7 Domargård A, Särnblad S, Kroon M et al (1999) Increased prevalence of overweight in adolescent girls with type 1 diabetes mellitus. Acta Paediatr 88 : 1223–8 East L, Jackson D, O’Brien L (2006) Father absence and adolescent development: a review of the literature. J Child Health Care 10 : 283–95 Forum for Injection Technique (2015) The UK Injection Technique Recommendations (3rd edition). Available at: http://bit.ly/1QeZU2E (accessed 24.02.16) Frid A, Hirsch L, Gaspar R et al (2010) The Third Injection Technique Workshop in Athens (TITAN). Diabetes Metab 36 (Suppl 2): 19–29 Garg SK, Bookout TR, McFann KK et al (2008) Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software. Diabetes Technol Ther 10 : 369–75 Glasper EA, Richardson J (2005) A Textbook of Children’s and Young People’s Nursing . Churchill Livingston, London Hanas R (2006) Type 1 Diabetes in Children, Adolescents and Young Adults (3rd edition). Class Publishing, London: 329, 349–50 Kent D, Haas L, Randal D et al (2010) Healthy coping: issues and implications in diabetes education and care. Popul Health Manag 13 : 227–33 Kübler-Ross E (1970) On Death and Dying: What the Dying Have to Teach Doctors, Nurses, Clergy and Their Own Families . Tavistock Publications, London Kübler-Ross E, Kessler D (2005) On Grief and Grieving: Finding the Meaning of Grief Through the Five Stages of Loss . Simon & Schuster UK, London La Greca AM, Auslander WF, Greco P et al (1995) I get by with a little help from my family and friends: adolescents’ support for diabetes care. J Pediatr Psychol 20 : 449–76 NICE (2011) Diabetes in adults (QS6). NICE, London. Available at: www.nice.org.uk/guidance/qs6 (accessed 24.02.16) NICE (2015a) Diabetes (type 1 and type 2) in children and young people: diagnosis and management (NG18). NICE, London. Available at: www.nice.org.uk/guidance/ng18 (accessed 24.02.16) NICE (2015b) Type 2 diabetes in adults: management (NG28). NICE, London. Available at: www.nice.org.uk/guidance/ng28 (accessed 24.02.16) NSPCC (2016) A Child’s Legal Rights: Gillick Competency and Fraser Guidelines . NSPCC, London. Available at: http://bit.ly/1Tj6DcF (accessed 24.02.16) Snoek FJ, Skinner TC (2002) Psychological counselling in problematic diabetes: does it help? Diabet Med 19 : 265–73 Spear LP (2013) Adolescent neurodevelopment. J Adolesc Health 52 (Suppl 2): 7–13 Strauss K, De Gols H, Hannat I et al (2002) A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Practical Diabetes International 19 : 71–76 Taylor J, Müller D, Wattley L, Harris P (1999) The development of children’s understanding. In: Nursing Children: Psychology, Research and Practice . Stanley Thornes, Cheltenham Wild D, von Maltzahn R, Brohan E et al (2007) A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 68 : 10–5
Do youth workers have a role in improving diabetes transition services?
Cgm for children and young people with type 1 diabetes: nice criteria and effects of decision fatigue and alarm fatigue , improving paediatric diabetes in england: areas of focus, delays in accessing continuous glucose monitoring in people with type 1 diabetes, celebrating may ng: the woman behind the obe, fiona campbell awarded an obe for services to paediatric diabetes, diabetes transition: a time to act.

Can the involvement of youth workers improve diabetes care for young people transitioning to adult diabetes services?
The impact of decision fatigue and alarm fatigue in children and young people using continuous glucose monitoring

NHSEI National Clinical Lead for Diabetes in Children and Young People, Fulya Mehta, outlines the areas of focus for improving paediatric diabetes care.
16 Nov 2022

NICE guidance urges local trusts to improve processes and advocate for CGM use in children and young people.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Diabetes & Primary Care
- Journal of Diabetes Nursing
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Type 2 diabetes: a case study
Affiliation.
- 1 Queen's University Belfast, Belfast, Northern Ireland.
- PMID: 25270482
- DOI: 10.7748/ns.29.5.37.e9142
Increased prevalence of diabetes in the community has been accompanied by an increase in diabetes in hospitalised patients. About a quarter of these patients experience a hypoglycaemic episode during their admission, which is associated with increased risk of mortality and length of stay. This article examines the aetiology, pathophysiology, diagnosis and treatment of type 2 diabetes using a case study approach. The psychosocial implications for the patient are also discussed. The case study is based on a patient with diabetes who was admitted to hospital following a hypoglycaemic episode and cared for during a practice placement. The importance of early diagnosis of diabetes and the adverse effects of delayed diagnosis are discussed.
Keywords: Blood glucose; case study; diabetes; glucose testing; hyperglycaemia; hypoglycaemia; insulin resistance; sulfonylureas; type 2 diabetes.
PubMed Disclaimer
Similar articles
- The early treatment of type 2 diabetes. Pratley RE. Pratley RE. Am J Med. 2013 Sep;126(9 Suppl 1):S2-9. doi: 10.1016/j.amjmed.2013.06.007. Am J Med. 2013. PMID: 23953075
- Clinical characterisation of severe hypoglycaemia--a prospective population-based study. Holstein A, Plaschke A, Egberts EH. Holstein A, et al. Exp Clin Endocrinol Diabetes. 2003 Sep;111(6):364-9. doi: 10.1055/s-2003-42728. Exp Clin Endocrinol Diabetes. 2003. PMID: 14520604
- [Identification of factors predicting early evolution of secondary oral hypoglycaemic agent failure and evaluation of clinical standards applied by primary care physicians during qualification to insulin therapy of patients with type 2 diabetes]. Machoy M. Machoy M. Ann Acad Med Stetin. 2004;50(2):29-39. Ann Acad Med Stetin. 2004. PMID: 16529163 Polish.
- [Diabetes, sport and exercise]. Fischer H. Fischer H. Clin Res Cardiol Suppl. 2011 May;6:6-9. doi: 10.1007/s11789-011-0029-z. Clin Res Cardiol Suppl. 2011. PMID: 22528172 Review. German.
- Early use of insulin in type 2 diabetes. Eldor R, Stern E, Milicevic Z, Raz I. Eldor R, et al. Diabetes Res Clin Pract. 2005 Jun;68 Suppl1:S30-5. doi: 10.1016/j.diabres.2005.03.004. Epub 2005 Mar 31. Diabetes Res Clin Pract. 2005. PMID: 15955372 Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- scite Smart Citations
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Case Study: Uncontrolled Type 2 Diabetes
—this patient’s hba1c level is improving, but not at goal after starting metformin. how should we think about the issues that will determine next steps.
By Kevin O. Hwang, MD, MPH Reviewed by Clifton Jackness, MD, Assistant Professor, Hofstra Northwell School of Medicine, New York, NY
A 45-year-old woman with type 2 diabetes arrives for a follow-up visit 1 week after her HbA1c was determined. She has been compliant with metformin 1000 mg twice daily. She reports that her home blood sugar readings have improved slightly but are still high. She admits to a few dietary indiscretions, such as having multiple servings of dessert when going out with friends. For exercise, she has been walking 10 to 15 minutes a day.
She denies polyuria, polydipsia, or blurry vision. The review of systems is unremarkable.

- Type 2 diabetes, diagnosed 6 months ago when she presented with polyuria, blurry vision, and a random glucose level of 276 mg/dL. Her HbA1c at that time was 8.0%. She was started on metformin 500 mg twice daily, and within 3 months her HbA1c dropped to 7.6%. The metformin was increased to 1000 mg twice daily at that time. She has not had significant hypoglycemic episodes.
- Hypertension, treated with lisinopril 40 mg daily.
- Dyslipidemia, treated with atorvastatin 20 mg daily.
- Esophageal reflux treated with omeprazole 20 mg daily.
Vital signs are blood pressure 122/76 mm Hg, heart rate 82, respiratory rate 18, temperature 98.1 °F, height 5’5”, weight 196 pounds, and BMI 32.6. She has not gained or lost significant weight since she started treatment for diabetes.
On exam, the lungs are clear to auscultation, the heart has a regular rate and rhythm without murmurs, and the abdomen is nontender. Peripheral pulses are normal, and there is no lower extremity edema. The foot exam shows normal sensation to light touch and no skin or toenail lesions.
- HbA1c level, determined last week, is 7.3%.
- Patient’s blood glucose log shows morning fasting glucose ranging from 120 mg/dL to 150 mg/dL, and postprandial readings at 190 mg/dL to 220 mg/dL.
Targets for Diabetes Control
The American Diabetes Association (ADA) recommends a target HbA1c of less than 7.0%, fasting glucose less than 130 mg/dL, and postprandial glucose less than 180 mg/dL for most patients. 1 A more ambitious HbA1c target of 6.0% to 6.5% may be appropriate for patients with a long life expectancy and no cardiovascular disease, provided that this can be achieved without adverse effects, such as severe hypoglycemia. On the other hand, a target HbA1c of 7.5% to 8.0% may be suitable for patients with significant comorbidities, limited life expectancy, and a history of severe hypoglycemia. This goal is also reasonable for patients who have not been able to reach lower HbA1c levels with multiple diabetes medications and extensive education about diabetes self-management. Given our patient’s overall health profile, her target is an HbA1c level of less than 7.0%, or eventually even 6.0% to 6.5%.
The patient’s HbA1c has improved since starting metformin, but is still not at target. Her fasting and postprandial glucose levels are also too high. The underlying causes for hyperglycemia in this patient include dietary factors, inadequate exercise, and obesity. She has no signs or symptoms of an acute illness that could cause hyperglycemia.
The maximum recommended dose of metformin for adults is 2000 to 2500 mg daily, depending on the formulation. Her current total daily dose is 2000 mg, and it is unlikely that her glycemic control will improve significantly just by adding another 500 mg of metformin.
The patient is referred to a diabetes education and support class. She is briefly counseled on lifestyle changes to improve her diet and increase her physical activity. Diabetic individuals in the intensive lifestyle intervention arm of the Look AHEAD study lost 8.6% of their weight in the first year, with an average reduction in fasting glucose from 152 mg/dL to 130 mg/dL and reduction in HbA1c from 7.3% to 6.6%. 2 If a similarly intensive program is available, this patient should be referred to it.
• Sulfonylurea
• Thiazolidinedione
• Glucagon-like peptide (GLP)-1 agonist
• Dipeptidyl peptidase (DPP)-4 inhibitor
• Patient preference for route of administration and other factors
• Efficacy in reducing HbA1c
• Potential to cause hypoglycemia
• Potential to induce weight gain
• Side effects
Our patient is agreeable to adding another diabetes medication but does not want to use an injectable medication. Since she is obese and has not been losing weight, an important consideration would be to avoid inducing further weight gain. After starting the second medication and working on lifestyle changes, a repeat HbA1c test and follow-up appointment is arranged for 3 months.
Published: March 01, 2017
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care . 2012;35:1364-1379.
- 2. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care . 2007;30:1374-1383.
Significant weight loss associated with long-term improvement in psoriasis, the effects of obesity on end-of-life care, new pediatric obesity guidelines: what you should know, executive functioning linked to eating behavior in adults with overweight and obesity, stopping prediabetes in its tracks, study finds binge eating and food cravings are common in women with pcos, new behavioral approach to weight loss: timely guidance for new year’s resolutions, weight management and related news.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Diabetes Spectr
- v.29(1); 2016 Feb

Case Study: Remission of Type 2 Diabetes After Outpatient Basal Insulin Therapy
Sierra c. schmidt.
1 Auburn University Harrison School of Pharmacy, Auburn, AL
Martha Ann Huey
Heather p. whitley.
2 Baptist Health System, Montgomery Family Medicine Residency Program, Montgomery, AL
Diabetes is a chronic, progressive disease with potentially serious sequelae. Treatment for type 2 diabetes often begins with oral agents and eventually requires insulin therapy. As the disease progresses, drug therapies are often intensified and rarely reduced to control glycemia. Conversely, in type 1 diabetes, some patients experience a “honeymoon period” shortly after diagnosis, wherein insulin needs decrease significantly before intensification is needed ( 1 ). No comparable honeymoon period has been widely described for type 2 diabetes. However, a few studies have demonstrated that drug-free glycemic control can be achieved in type 2 diabetes for 12 months on average after a 2-week continuous insulin infusion ( 2 – 4 ). Here, we describe an unusual case of a 26-month drug holiday induced with outpatient basal insulin in a patient newly diagnosed with type 2 diabetes.
Case Presentation
A 69-year-old white woman (weight 72.7 kg, height 59 inches, BMI 32.3 kg/m 2 ) was diagnosed with type 2 diabetes in June 2011. She presented with an A1C of 17.6% (target <7%) and a fasting blood glucose (FBG) of 452 mg/dL (target 70–130 mg/dL). Before diagnosis, the patient had not used any oral or parenteral steroids nor had she experienced any traumatic physical or emotional event or illness that could have abruptly increased her blood glucose. Metformin 500 mg twice daily was initiated at diagnosis, but was discontinued 9 days later to avoid risk of lactic acidosis, as her serum creatinine was 1.5 mg/dL. At that time, her fasting self-monitoring of blood glucose (SMBG) values ranged from 185 to 337 mg/dL. Treatment with 25 units of insulin detemir daily (0.34 units/kg/day) was initiated in place of metformin. The patient was counseled on diet modifications and encouraged to exercise.
One month later (July 2011), the patient’s fasting SMBG values had improved to a range of 71–212 mg/dL with a single hypoglycemic episode (58 mg/dL); her weight and BMI increased slightly to 74.1 kg and 32.9 kg/m 2 , respectively. Hypoglycemia education was reinforced, and insulin therapy was switched from 25 units of detemir delivered with the Levemir FlexPen to 28 units (0.38 units/kg/day) of insulin glargine delivered with the Lantus SoloStar due to the patient’s preference for this device. Two weeks later, the patient reported continued improvements in fasting SMBG (70–175 mg/dL) with one hypoglycemic episode (67 mg/dL). In response to the hypoglycemic episode, her insulin glargine dose was decreased to 25 units daily.
In September, the patient reported fasting SMBG values ranging between 71 and 149 mg/dL, and her A1C was 7.9%. On days when the patient skipped lunch, her midday blood glucose level would drop to <70 mg/dL (54–60 mg/dL). She was counseled not to skip meals, and her insulin glargine dose was maintained.
In October, the patient’s weight was 71.4 kg, and her BMI was 31.7 kg/m 2 . She reported recently initiating a cinnamon supplement and switching her beverage intake from sugar-sweetened products to water and diet soda. Although the majority of her fasting SMBG values were controlled (80–110 mg/dL), she had experienced six hypoglycemic episodes (FBG 13–64 mg/dL). All values were objectively confirmed in the patient’s glucose meter, and the meter was replaced in case of device error. Her daily insulin glargine dose was decreased to 20 units (0.28 units/kg/day).
In December, her SMBG values ranged between 70 and 106 mg/dL preprandially and 111 and 207 mg/dL postprandially, and she had had six additional hypoglycemic episodes (42–66 mg/dL). The patient’s weight remained stable at 71.4 kg (BMI 31.7 kg/m 2 ). At this follow-up visit, her daily insulin glargine dose was decreased further to 15 units (0.21 units/kg/day).
The patient self-discontinued daily insulin glargine in March 2012 but continued using the cinnamon supplements. She continued to perform SMBG 1–3 times/day, anticipating loss of glycemic control. During the next 2 years, her A1C remained stable (from 6.3% in January 2012 to 6.9% in May 2014) ( Figure 1 ).

Daily basal insulin dose and A1C over time. Black triangle = insulin units; black square = A1C.
At a follow-up visit in May 2014, the patient’s SMBG indicated a need for resumed drug therapy (FBG 107–169 mg/dL, postprandial blood glucose 108–328 mg/dL). Her weight at this time was 65.5 kg (BMI 29.1 kg/m 2 ). Insulin glargine was reinitiated at 5 units daily (0.08 units/kg/day).
During the drug-free period of March 2012 to May 2014, the patient maintained her lack of sugar-sweetened beverage consumption. However, she reported having difficulties purchasing healthy food options because of financial constraints. In August 2013, she was specifically encouraged to incorporate physical activity (walking) into her daily routine. The patient’s weight during the drug-free interval declined from 70 kg in March 2012 to 65.5 kg in May 2014.
Hyperglycemia causes pancreatic β-cell toxicity, leading to decreased insulin release ( 3 ). In type 1 diabetes, the honeymoon period occurs when residual pancreatic β-cell function is partially restored for an average of 7.2 months, as hyperglycemic stress is removed before the β-cells are ultimately destroyed ( 1 , 3 ).
Past studies demonstrated induction of a drug-free period when patients newly diagnosed with type 2 diabetes were treated with 2–3 weeks of intensive insulin therapy ( 2 – 5 ). Ilkova et al. ( 2 ) induced a 12-month drug-free period in 46.2% ( n = 6) of patients using an insulin infusion averaging 0.61 units/kg/day. Three patients maintained glycemic control for 37–59 months. Li et al. ( 3 ) also induced a 12-month drug-free period in 47.1% ( n = 32) of patients with an insulin infusion of 0.7 units/kg. Additional studies indicate that basal-bolus insulin therapy (0.37–0.74 units/kg/day) using NPH and regular insulin can also induce a 12-month drug-free period in a similar percentage of patients (43.8–44.9%) ( 4 , 5 ).
The mechanism of remission appears to be related to resumption of endogenous insulin production after glucotoxicity is resolved. Glucotoxicity has been shown to inhibit first-phase insulin secretion from the pancreatic β-cells ( 3 ). Li et al. ( 3 ) theorized that an insulin infusion corrects hyperglycemia and removes stress from the β-cells, allowing them to produce insulin, resulting in euglycemia. Their study quantified an increase in secretion of endogenous insulin (44%) and C-peptide (26%) after 2 weeks of continuous insulin infusion. The mechanism through which insulin induces a period of drug-free glycemic control in type 2 diabetes appears to be similar to that causing the honeymoon period in type 1 diabetes.
To our knowledge, this is the first report of basal insulin monotherapy–induced remission of type 2 diabetes. Previous studies required multiple daily injections in a basal-bolus therapy regimen using NPH and regular insulin or hospitalization of patients administered a continuous insulin infusion ( 2 – 5 ).
Basal-only insulin therapy may be a slower method of achieving remission compared to more intensive insulin regimens. In this case, basal insulin was maintained for 9 months. However, according to the FBG trend, discontinuation could have occurred sooner. This report suggests that a trial of basal insulin dosed at 0.2–0.3 units/kg/day, with follow-up every 2–4 weeks in severely hyperglycemic patients with newly diagnosed type 2 diabetes, may be an alternative method to achieving temporary remission. Although this insulin regimen requires a longer timeframe compared to remission induced by basal-bolus therapy or continuous insulin infusion, it provides a more convenient outpatient therapeutic option at a lower cost.
Limitations of this case study include the patient’s use of cinnamon supplementation, which was continued throughout the drug-free period. Although reports are conflicting regarding its efficacy in type 2 diabetes, it is possible that cinnamon may have exerted a mild antidiabetic effect. Positive cinnamon studies have demonstrated a 0.36% A1C reduction after 3 months of use ( 6 ). Additionally, the patient’s weight declined by 3.75% during the 9 months of basal insulin therapy, which was likely in response to introducing dietary modifications related to beverage consumption. Most studies suggest that an A1C reduction of 0.36% ( 7 ) to 0.66% ( 8 ) can be achieved with intensive lifestyle interventions. Therefore, it is unlikely that cinnamon in combination with the mild lifestyle modifications accounted for a nearly 11% A1C reduction from baseline.
Eliminating the consumption of sugar-rich beverages alters the postprandial glycemic curve. In clinical practice, suppressing postprandial blood glucose excursions by adopting significant dietary improvements may postpone or obviate the need for bolus insulin therapy. Likewise, the remission of diabetes potentially may be achieved, as seen in this case, with monotherapy basal insulin when dietary modifications significantly alter the postprandial glycemic curve. However, it is unknown whether remission can be achieved using basal insulin administration alone in patients who choose not to incorporate lifestyle modifications or in patients with baseline healthy eating and exercise habits.
Although weight changes did not appear to contribute to disease remission, the moderate weight loss (6.5%) achieved during the drug-free interval and continued SMBG both may have contributed to maintaining and extending the remission period. The Diabetes Prevention Program ( 9 ) showed that lifestyle modifications aimed at achieving a 7% reduction of weight significantly delay the onset of diabetes compared to placebo and metformin. Finally, performing SMBG through the drug-free period may have empowered the patient by providing objective criteria necessary to validate the benefits of lifestyle modifications.
Based on this case, it is possible that initial type 2 diabetes management with basal insulin can temporarily restore β-cell function to a degree to which blood glucose control can be maintained without drug therapy. Although previous studies conducted with intensive insulin regimens have reported response rates nearing 50% for ∼12 months ( 2 – 5 ), future studies should investigate the ideal basal dose, percentage of patient responders, duration of drug-free glycemic control, and mechanism through which this phenomenon occurs. This case further highlights the need to educate every newly diagnosed patient about the treatment of hypoglycemic events.
The purposeful remission of diabetes is not widely attempted or generally considered possible. Although literature exists regarding the temporary honeymoon period experienced after insulin initiation in some people with type 1 diabetes ( 1 ), comparatively little research is available regarding the influence of insulin on the remission of type 2 diabetes. Current literature suggests benefit in nearly 50% of patients newly diagnosed with type 2 diabetes using one of the following strategies: a 2-week inpatient insulin infusion or multiple daily injections of basal-bolus therapy ( 2 – 5 ). However, there are disadvantages to these methods. A continuous insulin infusion requires inpatient admission, whereas a basal-bolus insulin regimen requires purchase of two products and administration of multiple subcutaneous injections daily. Unfortunately, both methods may be impractical, costly, and inconvenient for many patients newly diagnosed with type 2 diabetes.
This case outlines a third potential option for inducing remission of type 2 diabetes: basal insulin monotherapy. Using this approach avoids the costly and inconvenient hospital admission required for the continuous insulin infusion strategy. Furthermore, the cost of drug therapy is reduced with the purchase of one rather than two insulin products, as needed in a basal-bolus insulin regimen. Additionally, using basal insulin alone reduces the risk of hypoglycemic events that may occur with stacking of multiple insulin products. Finally, requiring only one injection of insulin each day offers a more manageable alternative for newly diagnosed patients compared to the multiple daily injections required with a basal-bolus insulin regimen.
By using this basal insulin strategy, the patient in this case was able to achieve drug-free glycemic control for 26 months. Early initiation of basal insulin monotherapy in patients newly diagnosed with type 2 diabetes is a more convenient and cost-effective approach than methods previously described and could potentially induce remission of type 2 diabetes in other patients.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
- News and Features
- Conferences
- Clinical Tools
- Special Collections
Case Study: Hyperglycemia, concern for diabetic ketoacidosis, and type 1 diabetes
History of present illness.
The patient is a 36-year-old man who has had type 1 diabetes for 15 years. He presents to the emergency room with hyperglycemia and concern for possible diabetic ketoacidosis after not taking his insulin for 3 days. The patient reports that he is currently homeless and has lost his supply of insulin, syringes, glucose meter, and related glucose testing supplies.
Diabetes-related comorbidities/complications
Hypertension, hyperlipidemia, retinopathy, and bipolar disorder
Diabetes related history
The patient states that at the time of his initial diagnosis with type 1 diabetes , he was hospitalized, with a glucose value >1000 mg/dL, and he was experiencing polyuria, polydipsia, and polyphagia. He reports that he has been on insulin since the time of his diagnosis, and he has never been prescribed oral agents for diabetes management. He recalls that glutamic acid decarboxylase (GAD) antibodies and a C-peptide level have been previously evaluated. GAD antibodies were positive, and C-peptide value was low, helping to confirm the diagnosis of type 1 diabetes.
Most recently, he has been using insulin glargine 55 units once daily, and insulin aspart per correction doses 3 times daily. There was an imbalance when comparing his basal and bolus insulin doses. When asked about meal doses of insulin aspart, the patient relates that he is currently homeless and eats when food is available, often snacking on bits of food throughout the day. He was not using a meal dose of insulin aspart, but he would use this insulin to correct for hyperglycemia.
The patient has had previous episodes of diabetic ketoacidosis, for which he was hospitalized. With this episode of hyperglycemia, he is not experiencing any nausea, vomiting, or abdominal discomfort, and he appears well. The patient has no recent concerns for hypoglycemia. He reports that with past episodes of hypoglycemia, he experienced sweatiness and shakiness, for which he treated with juice or food.
Laboratory values on admission
- Creatinine: 0.9 mg/dL with eGFR >60 mL/min
- Aspartate aminotransferase (AST): 17 U/L
- Alanine aminotransferase (ALT): 14 U/L
- Beta-hydroxybutyrate: 0.1 mmol/L
- Bicarbonate: 25 mEq/L
- Anion Gap: 14 mEq/L
Latest News Your top articles for Tuesday
Haymarket Medical Network Top Picks
Continuing Medical Education (CME/CE) Courses
Want to read more?
Please login or register first to view this content.
Login Register
Medicine Matters Diabetes
Patient case studies, clinical practice highlights that underscore important best practice messages, report on unusual adverse effects or describe presentations of diabetes and its complications., diabetes treatment in the elderly: kathryn.
Primary care physician Jay Shubrook shares his recommendations for diabetes management in a 72-year-old, multi-morbid patient.
10-01-2019 | Diet | Case study | Article
A physician’s introduction to therapeutic fasting
Is this increasingly popular weight management tactic an option for your patients with type 2 diabetes? Primary care physician Jay Shubrook and Jamie Katuna explain in this illustrative case study.
01-30-2019 | Cardiovascular outcomes | Case study | Article
Cardiovascular risk reduction in type 2 diabetes
Pharmacological management of comorbid cardiovascular risk and type 2 diabetes is undergoing a paradigm shift. General practitioner Kevin Fernando puts recommendations from the ADA/EASD into practice in this patient case study.
09-05-2018 | Diagnosis | Case study | Article
Acute illness while on a SGLT2 inhibitor and ketogenic diet
Advisory Board member Theresa Smyth tackles an emergency case in a type 2 diabetes patient treated with empagliflozin. What went wrong and how should he be managed?
05-09-2018 | Diagnosis | Case study | Article
Atypical diabetes: Case study 4
12-13-2017 | Diagnosis | Case study | Article
Atypical diabetes: Case study 3
11-01-2017 | Diagnosis | Case study | Article
Atypical diabetes: Case study 2
10-04-2017 | Diagnosis | Case study | Article
Atypical diabetes: Case study 1
Interactive patient case studies, renal complications in diabetic ketoacidosis.
Develop and test your knowledge through this practical, 15-minute interactive case study on renal complications and treatment in diabetic ketoacidosis, authored by Professor Francesco Chiarelli.
Maturity-onset diabetes of the young (MODY)
This 15-minute case presents the characterization, diagnosis and management of maturity-onset diabetes of the young (MODY) and discusses the pros and cons of predictive genetic testing for MODY.
Hypoglycemia and technology
This 15-minute case presents the identification of problematic hypoglycemia and the appropriate use and monitoring of different technologies in the context of reducing the occurrence and risk of hypoglycemia.
Patient case reports
08-09-2018 | Neonatal diabetes | Case report | Article
Successful treatment of diabetic ketoacidosis and hyperglycemic hyperosmolar status in an infant with KCNJ11-related neonatal diabetes mellitus via continuous renal replacement therapy
Chen T et al. Diabetes Ther 2018. doi: 10.1007/s13300-018-0484-3
06-06-2018 | Type 1 diabetes | Case report | Article
Sudden onset of immune-mediated type 1 diabetes mellitus in a 93-year-old woman: A case report
Oriot P et al. Acta Diabetol 2018. doi: 10.1007/s00592-018-1170-7
05-28-2018 | Lifestyle interventions | Case report | Article
Successful management of poorly controlled type 2 diabetes with multidisciplinary neurobehavioral rehabilitation: A case report and review
Deng Z et al. Diabetes Ther 2018. doi: 10.1007/s13300-018-0448-7
Flash glucose monitoring technology
Browse slide sets summarizing results from real-world studies.
- » Real-world study: Determining the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes
- » Clinical trial meta-analysis and real-world study: Impact of flash glucose monitoring on HbA1c
- » Funded by an educational grant from Abbott Diabetes Care
- Latest news
- Cardiovascular disorders
- Chronic kidney disease
- Diabetes devices & technology
- GLP-1 receptor agonists
- Hyperglycemia
- Hypoglycemia
- Obesity, diet, & lifestyle interventions
- SGLT2 inhibitors
- Type 1 diabetes
- Type 2 diabetes
- Browse all topics
- Novel clinical evidence in continuous glucose monitoring
- Fasting for religion and health in people with diabetes
- The challenge of type 2 diabetes in children
- HFpEF & diabetes: Diagnosis and management in the wake of EMPEROR-Preserved
- Addressing suicide risk in diabetes: Rates and risk factors, HCP fears, and practical advice
- The SURPASS trials: Summarizing efficacy data on tirzepatide
- Semaglutide for weight loss: STEP and SELECT trials: Results from the STEP and SELECT trials
- Why psychosocial care matters
- DiRECT: Type 2 diabetes remission through weight management
- EASD 58th Annual Meeting 2022
- The American Diabetes Association 82nd Scientific Sessions
- DOI: 10.2337/DIASPECT.16.1.32
- Corpus ID: 73083750
Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse
- G. Spollett
- Published 2003
- Diabetes Spectrum
5 Citations
Management of ketosis-prone type 2 diabetes mellitus., integrating a pico clinical questioning to the ql4pomr framework for building evidence-based clinical case reports, nursing practice guideline for foot care for patients with diabetes in thailand, goal-driven structured argumentation for patient management in a multimorbidity setting, logic and argumentation: third international conference, clar 2020, hangzhou, china, april 6–9, 2020, proceedings, 18 references, using a primary nurse manager to implement dcct recommendations in a large pediatric program, diabetes in urban african americans. iii. management of type ii diabetes in a municipal hospital setting., primary care outcomes in patients treated by nurse practitioners or physicians: a randomized trial., caring for a child with diabetes: the effect of specialist nurse care on parents' needs and concerns., standards of medical care for patients with diabetes mellitus, management of patients with diabetes by nurses with support of subspecialists., a practical approach to type 2 diabetes., the diabetes control and complications trial (dcct): the trial coordinator perspective, oral antihyperglycemic therapy for type 2 diabetes: scientific review., caring for feet: patients and nurse practitioners working together., related papers.
Showing 1 through 3 of 0 Related Papers
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Pathophysiology

https://slideplayer.nl/slide/2005829/
According to McCance and Huether (2019), 9.3 % of the adult population in the United States is affected by Type 2 diabetes mellitus. Risk factors for developing Type 2 diabetes are family history, hypertension, obesity, and increased age. Lifestyle choices, genetic factors, and environmental factors combined can all contribute to the development of Type 2 diabetes mellitus. One main issue leading to Type 2 diabetes is insulin resistance in peripheral tissues specifically the muscle, liver, and adipose tissue (McCance & Huether, 2019).
Alpha cells and beta cells are islet cells that are found in the pancreas. The beta cells are responsible for creating insulin and the alpha cells are responsible for creating glucagon. The increasingly high glucagon levels cause blood glucose levels to increase leading to the stimulation of gluconeogenesis and glycogenolysis (McCance & Huether, 2019). Due to the decreased reactiveness of the alpha cells to glucose, the glucagon secretion begins increasing as well. Amylin which is a beta-cell hormone is responsible for repressing the alpha cells release of glucagon (McCance & Huether, 2019). In Type 2 diabetes the cells begin to become insulin resistant. This means the needed glucose is unable to get inside of the cells which causes it to accumulate in the blood. In this case, the insulin receptors are abnormal or missing causing glucose to be locked out of the cells.
The beta cells attempt to keep up with the increased demand for insulin but eventually lose the ability to produce enough. The beta cells begin to decrease in number and size and eventually fail due to exhaustion (McCance & Huether, 2019). This leads to hyperglycemia which is the buildup of glucose in the bloodstream. In an attempt to compensate for hyperglycemia, the pancreas will produce more insulin. The pancreas will eventually reach exhaustion and no longer be able to compete with the body’s increased demand for insulin.
Our GI hormones (gut hormones) contribute to diabetes & insulin resistance as well. Ghrelin is a hormone made in the stomach and pancreatic islets that control food intake. Insulin resistance has been associated with reduced levels of ghrelin. Incretins are released from the GI tract to increase insulin release, regenerate the beta-cell and provide a barrier to beta-cell damage (McCance & Huether, 2019). Studies show the incretin glucagon-like peptide 1, (GLP-1) depicts a decrease in beta-cell responsiveness in type 2 diabetes (McCance & Huether, 2019).
Due to hyperglycemia and the current lack of insulin polyphagia, polydipsia and polyuria are classic signs that appear while recurrent infections and visual changes occur later on. If hyperglycemia continues to progress without treatment microvascular complications such as nephropathy, neuropathy, and retinopathy can occur along with macrovascular complications: cerebrovascular disease, coronary artery disease, and peripheral artery disease (McCance & Huether, 2019).
According to the American Diabetes Association (2015), there are four ways to diagnose Type 2 diabetes
- Glycated hemoglobin (A1C) test: Diabetics diagnosed using this test will have an A1C of 6.5% or higher
- Random blood sugar test: Diabetics diagnosed using this test will have a blood sugar of > 200 mg/dL
- Fasting plasma glucose (FPG): Diabetics diagnosed using this test will have a FPG of 126 mg/dL or higher
- Oral glucose tolerance test (OGTT): Diabetics diagnosed using this test will have an OGTT of 200 mg/dL or higher.
American Diabetes Association. (2015, January 1). 2. Classification and Diagnosis of Diabetes. Retrieved from https://care.diabetesjournals.org/content/38/Supplement_1/S8.
McCance, K. L., Huether, S. E., Brashers, V. L., & Rote, N. S. (2019). Pathophysiology: the biologic basis for disease in adults and children (8th ed.). St. Louis, MO: Elsevier.
- Study Guides
- Homework Questions
Diabetes and CVD Case Study finished
- Health Science
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals

You are here
- Online First
- Inpatient case characteristics of SGLT2 inhibitor-associated diabetic ketoacidosis: a retrospective study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0009-0008-4991-7190 Zhongpei Yang 1 ,
- Weixia Zhang 2 ,
- Hefeng Chen 2 ,
- Qianwen Peng 2
- 1 Department of Pharmacy , The People's Hospital of QianNan , Duyun , China
- 2 Department of Pharmacy , Shanghai Jiao Tong University Medical School Affiliated Ruijin Hospital , Shanghai , China
- Correspondence to Dr Weixia Zhang, Department of Pharmacy, Shanghai Jiao Tong University Medical School Affiliated Ruijin Hospital, Shanghai, China; wxzhang2001{at}163.com
Objectives Diabetic ketoacidosis (DKA) is a serious complication in patients treated with sodium-glucose co-transporter 2 inhibitors (SGLT2i). The aim of this study was to investigate the relationship between SGLT2i and the risk of DKA, and to identify high-risk groups and characteristics that should be emphasised.
Methods A retrospective case series study was conducted to collect medical records of inpatients diagnosed with DKA and using SGLT2i before the onset of the disease from September 2022 to September 2023 in a tertiary hospital in Shanghai. Cases that met the inclusion criteria were retrieved through the electronic medical record system. Information was collected to compare the risk of DKA in patients with different characteristics.
Results A total of 21 patients (12 men and 9 women) met the criteria for SGLT2i-associated DKA. The mean diabetes duration was 10.4 years, with 47.6% (10/21) of patients diagnosed with euglycaemic DKA. The drug treatment regimen most commonly used was the combination of SGLT2i and metformin, representing 52.4% (11/21) of cases. The most common clinical symptoms were nausea, vomiting, abdominal pain and malaise. Common predisposing factors were acute infections, acute pancreatitis (predominantly hyperlipidaemic type), dietary inappropriateness, acute cardiovascular and cerebrovascular events and surgery. 71.4% of patients (15/21) had multiple risk factors.
Conclusion The use of SGLT2i in diabetic patients is associated with an increased risk of DKA, particularly in the presence of predisposing factors such as infection. Furthermore, long diabetes duration, decreased pancreatic β-cell function and the combined use of metformin may also contribute to the risk of DKA in patients treated with SGLT2i. The findings of this study provide valuable insights for better identification and management of DKA risks associated with SGLT2i in clinical practice.
- Diabetes Mellitus
- DRUG-RELATED SIDE EFFECTS AND ADVERSE REACTIONS
- HEALTH SERVICES ADMINISTRATION
- Pharmacovigilance
- Substance-Related Disorders
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
https://doi.org/10.1136/ejhpharm-2024-004124
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Read the full text or download the PDF:
- Open access
- Published: 01 April 2021
Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era
- Ammira Al-Shabeeb Akil ORCID: orcid.org/0000-0001-5381-070X 1 ,
- Esraa Yassin 1 ,
- Aljazi Al-Maraghi 1 ,
- Elbay Aliyev 1 ,
- Khulod Al-Malki 1 &
- Khalid A. Fakhro 1 , 2 , 3
Journal of Translational Medicine volume 19 , Article number: 137 ( 2021 ) Cite this article
39k Accesses
43 Citations
2 Altmetric
Metrics details
Type 1 diabetes affects millions of people globally and requires careful management to avoid serious long-term complications, including heart and kidney disease, stroke, and loss of sight. The type 1 diabetes patient cohort is highly heterogeneous, with individuals presenting with disease at different stages and severities, arising from distinct etiologies, and overlaying varied genetic backgrounds. At present, the “one-size-fits-all” treatment for type 1 diabetes is exogenic insulin substitution therapy, but this approach fails to achieve optimal blood glucose control in many individuals. With advances in our understanding of early-stage diabetes development, diabetes stratification, and the role of genetics, type 1 diabetes is a promising candidate for a personalized medicine approach, which aims to apply “the right therapy at the right time, to the right patient”. In the case of type 1 diabetes, great efforts are now being focused on risk stratification for diabetes development to enable pre-clinical detection, and the application of treatments such as gene therapy, to prevent pancreatic destruction in a sub-set of patients. Alongside this, breakthroughs in stem cell therapies hold great promise for the regeneration of pancreatic tissues in some individuals. Here we review the recent initiatives in the field of personalized medicine for type 1 diabetes, including the latest discoveries in stem cell and gene therapy for the disease, and current obstacles that must be overcome before the dream of personalized medicine for all type 1 diabetes patients can be realized.
Introduction
Type 1 Diabetes (T1D) is a potentially life-threatening multifactorial autoimmune disorder characterized by T-cell-mediated destruction of pancreatic β cells, resulting in a deficiency of insulin synthesis and secretion [ 1 ]. The incidence of T1D has been rising globally since the 1950s, with an average annual increase of 3–4% over the past three decades [ 2 ]. In particular, the incidence of childhood T1D is increasing, most rapidly in populations that previously had low incidence [ 3 , 4 , 5 ], and varying by ethnicity and race [ 4 ].
This worrying growth in T1D incidence has driven concerted research efforts to better understand the underlying risk factors, etiology, and pathology of the disease.
T1D has a largely heritable element, supported by a twin concordance rate of up to 70% [ 6 ] and of 8–10% sibling risk [ 7 ]. The bulk of risk is explained by difference at a several but strongly associated loci involving the HLA region “HLA class II, DQ and DR loci and HLA class I region” on chromosome 6p21 that account for ~ 50% of familial T1D [ 8 , 9 ]. Genome‐wide association (GWAS) and candidate gene association studies have produced an abundance body of evidence and provided convincing support about other genes and loci external to the HLA region that protect or confer the risk for T1D [ 8 , 10 ]. Single nucleotide polymorphisms (SNPs) comprising insulin gene ( INS ) presents ~ 10% of genetic predisposition of T1D [ 8 , 11 ], cytotoxic T-lymphocyte–associated antigen ( CTLA )-4 gene [ 12 ], protein tyrosine phosphatase non-receptor type 22 ( PTPN22) [ 8 , 13 ], nterferon induced with helicase C domain 1 ( IFIH1 ) genes [ 14 ] and Interleukin-2 receptor alpha chain ( IL2RA ) [ 11 ]. This great genetic heritability generates the capacity for effective diagnostic discrimination if the most of genetic risk for T1D can be allocated [ 15 , 16 ].
Prospective birth cohorts studies have facilitated the identification of potential triggers of islet autoimmunity (IA) and the natural history of progression to T1D [ 17 , 18 , 19 , 20 ]. Candidate triggers such as infections [ 21 ], early life diet [ 22 ], vitamin D levels [ 23 ], gut microbiota composition [ 24 ], vaccinations [ 25 ], pollutants and toxins [ 26 ], and geographic variation [ 27 ] when combine with genetic susceptibility [ 28 ] and specific epigenetic modifications [ 29 , 30 , 31 ], the perfect storm occurs and autoimmune destruction of pancreatic β cells is initiated (Fig. 1 ). These triggers required to be logged prospectively in well-designed studies instead of recollected retrospectively at the time of T1D diagnosis, couple of years later.
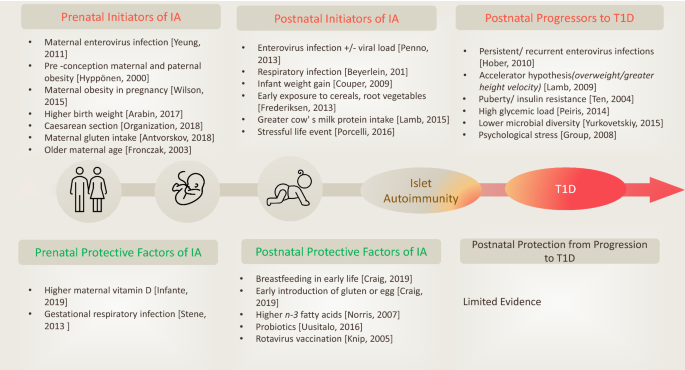
Environmental factors associated with initiation of, or protection from islet autoimmunity (IA) and progression to T1D. Adopted with permission from (Craig et al. 2019)
The plethora of factors that can lead to development and expression of T1D underpin the clinical heterogeneity of the disease. The gene polymorphisms and environmental triggers combinations that impact the risk of T1D and lead to the disease development are tremendously high [ 32 ]. Until now, this heterogeneity has not been taken into account and almost all T1D patients are treated with the standard approach of regular blood glucose monitoring combined with exogenous insulin replacement. However, the rising social and healthcare costs globally associated with T1D and its complications are providing the impetus for prioritizing more tailored approaches [ 33 , 34 , 35 ]. There is now increasing recognition of the opportunity to identify specific patient subgroups at different stages or with different driving factors of their early disease and prevent or even reverse their emerging T1D: this is the concept of personalized medicine. Personalized medicine is characterized by the mantra of "offering the right therapy at the right time for the right affected individual"; as an idea it is not new, but only recently has scientific and clinical research provided us with the necessary information and the means with which to apply it to novel treatment strategies for T1D.
In this review, we bring together the latest knowledge of the factors underpinning T1D heterogeneity in distinct patient groups and how these differences are being used to design personalized medicine approaches to diagnose, prevent, and hopefully treat the disease. We will discuss recent advances in gene therapy and stem cell-based treatments for specific groups of T1D patients, and will highlight key obstacles that must be overcome if further progress towards the goal of personalized medicine for all T1D patients is to be achieved.
Personalized diagnosis of T1D
Although all patients with overt T1D exhibit pancreatic destruction and consequent dysregulation of blood glucose levels, not all cases of the disease are driven by the same factors or along the same timeline. Many patients experience a sometimes prolonged clinically silent phase in which it might have been possible to intervene and prevent or even reverse the course of disease. This knowledge has led to development of a staging classification system for T1D. Even once T1D is clinically evident, we are now beginning to appreciate that not all cases are the same, and that particular sub-types of the disease would benefit from distinct treatment strategies. We discuss both of these important advances within the field below.
Staging classification system for T1D
By dissecting population- and individual-level risk factors for developing T1D, we now know that the disorder exists across developmental spectrum that can be categorized into distinct stages, and the likelihood of an individual developing clinically symptomatic status can be foreseen with considerable accuracy.
All cases are proposed to start with a period of "incubation" where exposure to defined and undefined driving factors creates the conditions for β-cell autoimmunity to emerge. When the process of ß-cell autoimmunity begins, the development towards clinical T1D can be classified into three distinct main stages: (I) asymptomatic ß-cell autoimmunity, defined by the presence of ≥ 2 types of autoantibodies such as GAD65 (GADA), zinc transporter 8 (ZnT8A), insulin (IAA), islet cell antibodies (ICA), insulinoma-associated proteins (IA-2A and IA-2β), with normoglycemia; (II) asymptomatic ß-cell autoimmunity, characterized by the presence of ≥ 2 types of autoantibodies but with dysglycemia, indicating functional damage to ß-cells; and (III) symptomatic T1D recognized by the symptoms of dysglycemia including polyuria or diabetic ketoacidosis (DKA) (Fig. 2 ). The sequence of events from emerging autoimmunity to dysglycemia and then to overt diabetes occurs along this predictable course, but the length of each stage may vary broadly between different individuals [ 36 , 37 , 38 ].
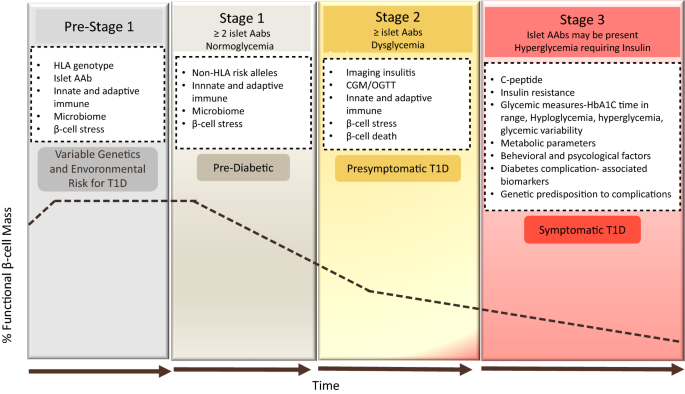
adapted from the same publication on addition to [ 36 ]© 2015 The American Diabetes Association
Development and staging of type 1 diabetes. T1D is characterized by a gradual loss of β-cell function (black dashed-dotted line) over time. As the disease progresses, beta cell function falls below the threshold required to maintain glucose control creating a requirement for insulin replacement therapy. Genetic and environmental risk are both included in the disease etiology. In stage 1, β-cell autoantibodies are persistent, but normoglycemia remains and there are no clinical symptoms. Throughout stage 2, the number of β-cell autoantibodies may induce dysglycemia but still without any diabetes symptoms. In stage 3, β-cell autoantibodies are predominant and clear symptoms of diabetes have emerged. In the white boxes are categories of biomarkers which could be leveraged to refine the staging paradigm, improve prognostic predictions, or subset individuals within a given stage of disease [ 38 ]. The specifics of these biomarkers are discussed in the text related to the relevant stage. The staging of T1D pathogenesis was proposed by Insel et al. [ 36 ] and the figure explanation was
There are several valuable clinical outcomes for children monitored across prospective longitudinal natural history studies such as. Notably, those children have better metabolic markers at and soon after the clinical diagnosis stage, making the disease management relatively easier, reduce hypoglycemic incidents and delay the progress of the associated long-term complications. Rigorous diabetes management commenced afterward the diagnosis of symptomatic T1D increases the chance of a honeymoon phase [ 39 ], assists patients to preserve greater C-peptide ranges [ 40 , 41 ], and reduce mortality rate [ 42 ], indicating that patients who are treated earlier will have improved long-term outcomes. In addition, genetically at risk children of DAISY (Diabetes Autoimmunity Study in the Young) cohort had lower HbA 1C levels maintained within the normal range, a figure much lower than the average HbA 1C levels of T1D children in the community [ 43 , 44 ]. Also, only 3% of the DAISY children were hospitalized at T1D diagnosis compared to 44% of matched children in the community [ 44 ]. The DKA levels was detected in around 30% of the participants of the SEARCH for diabetes in youth study [ 45 ], while the same marker observed in lower prevalence in children screened positive for islet autoantibodies followed by German BABYDIAB and Munich family study [ 46 ].
Children followed by Diabetes Prediction in Skåne (DiPiS) study experienced decreased HbA 1C up to 24 months after the diagnosis against similar daily insulin dose requirements [ 47 ].
The predictable progression of T1D from early stages of autoimmunity to dysglycemia ahead of the symptomatic clinical disease could ease the design of reliable clinical trials using intermediate endpoint that require ~ 50% smaller sample size that those using T1D as the endpoint. In TrialNet natural history study, diabetes- related autoantibodies were analyzed in relatives of T1D patients in respect to elevated HbA 1C, decreased C-peptide following oral glucose tolerance test (OGTT) value as intermediate markers of T1D progression [ 48 ]. Also, the TrialNet CTLA4-Ig (abatacept) ongoing trial designed to test whether intervention with Abatacept could prevent or delay the development of abnormal glucose tolerance (AGT) in at-risk relatives of T1D patients [ 49 ]. Combined predictive risk score for an improved prediction of disease progression by incorporating fixed and variable factors (genetic, immunologic and metabolic markers) in newborn screening to prevent DKA and to enhance personalized risk predication for better T1D prevention trial selection [ 50 , 51 ]. The crucial benefit of utilizing this staging system is to aid in development of innovative, stage-specific diagnostic and predictive biomarkers, support the design of clinical trials that utilizing the available data on risk profiles and individuals’ pre-symptomatic classification to design therapies specifically targeted to each phase of disease and ultimately, practice of personalized medicine approaches to avert symptomatic T1D. Future research will be needed to identify the main drivers of the transitions between stages in order to identify novel therapeutic targets to prevent the emergence of T1D in high-risk populations.
Diagnostic sub-groups within symptomatic T1D
Diagnosis of T1D has historically been made on the basis of detecting blood glucose dysregulation; however, this has led to patients with diverse underlying pathologies being grouped, and treated, together. Evidence of β-cell destruction via the presence of anti-islet-autoantibodies (which may recognize insulin, Glutamic Acid Decarboxylase 65(GAD65), zinc transporter isoform 8 (ZnT8), or islet cell antigen (ICA512) and the age at which initial autoantibodies were detected are important factors that characterize the “classical” etiological subtype of T1D. However, less frequently, hypoglycemia might be caused by loss of function or de novo mutation in a sporadic gene, giving rise to monogenic diabetes, which represents 3% of all diabetes cases in children and adults [ 52 ]. The heterozygous activation of genes encoding the ATP-sensitive potassium-channel subunit Kir6.2 reported to cause permanent neonatal diabetes in addition to some neurological abnormalities in some affected individuals. Distinguishing monogenic diabetes from T1D is crucial for accurate diagnosis, applying the correct treatment “such as sulfonylureas in Kir6.2 mutation”, and in the future, stratifying these patients into a group most likely to benefit from gene therapy targeting the mutation.
The aim of increasing correct diagnosis of classical versus monogenic T1D has been assisted by the introduction of the genomic risk score (GRS), which assesses an individual’s risk of T1D based on their possession of a collection of multiple (10–40) T1D risk variants [ 53 , 54 ]. The GRS also effectively identifies those individuals with early-onset or pre-clinical T1D who show more autoimmunity and fewer syndromic features in comparison with those of monogenic diabetes [ 55 ]. The sensitivity and specificity of the T1D-GRS exceeds 80% [ 55 ], but this figure might reasonably expect to be increased when the GRS is combined with the available clinical data and autoantibody results. Accordingly, incorporating the T1D-GRS into strategies aimed at intervening in the pre-symptomatic T1D stages noted above (Fig. 1 , [ 31 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 ]) is likely to prove productive in the development of personalized diabetes-preventative therapies targeting either mutational correction or prevention of overt autoimmunity.
Somewhat surprisingly, T1D and type 2 diabetes (T2D) are often distinguished based on whether the person exhibiting blood glucose dysregulation is young and a healthy weight (T1D-typical), or instead an older adult with obesity (T2D-typical). However, these two manifestations have different causes and medication requirements [ 80 ]. Research in 2017 found that approximately 40% of people who developed T1D after the age of 30 were initially diagnosed and treated for T2D [ 81 ]. Given the potentially life-threatening nature of insulin-deficiency status [ 81 , 82 ], these findings call for increased use of autoantibody testing to discriminate T1D and T2D, and widespread recognition of the fact that clinical features alone cannot reliably distinguish these two conditions.
Current advances in affordable high-throughput genomic and molecular deep phenotyping technologies have pushed the rise of “next-generation epidemiology” with a more systematic focus than before. In particular, deep phenotyping can be described as the precise and broad analysis of phenotypic data to aid in identifying disease biomarkers that assist the prediction, prevention and disease monitoring [ 83 ]. Recently, an integrative multi-omics approaches were used on the Environmental Determinants of Diabetes in the Young (TEDDY) children, a prospective longitudinal birth cohort created to study T1D by following children with high genetic risk [ 84 ]. The analysis identified a multi-omics signature that able to predict the IA before seroconversion in one year, in addition, defects in lipid metabolism, problems with nutrient absorption, reactive oxygen species (ROS) detected prior to the IA progression.
In conclusion, identification of high risk for T1D genetic groups in the pre-symptomatic stages, coupled with the use of autoantibody testing, GRS and molecular deep phenotyping through utilizing the advanced integrative data analysis, could support the development of approaches for early diagnosis and treatment of T1D in both symptomatic and pre-symptomatic patients. This strategy could form the mainstay of accurate “personalized diagnoses” moving forward. Understanding the genetic etiology and specific pathophysiology of these distinct patient groups within the T1D family will be necessary for the rationale design and application of personalized therapies in the future.
Personalized treatment of T1D
Progress in recognition of the need for personalized diagnosis in T1D has been accompanied by intense research efforts towards personalized therapies. Before the discovery of insulin in 1921, it was remarkable for T1D patients to live more than one or two years after disease onset: one of the twentieth century’s utmost medical breakthroughs, insulin replacement, is still the mainstay of treatment for the vast majority of T1D patients today. That said, innovative ways of achieving improved insulin-mediated glycemic control are becoming accessible to patients, while tissue transplants, genetic modification and stem-cell therapies are showing promise in pre-clinical models and human trials in specific sub-groups of patients. In this section we will discuss the “old and new” of T1D therapies and moves towards personalization to increase treatment efficacy.
Insulin and combination drug therapies
By far, the most common T1D treatment approach is manual testing of blood sugar levels followed by sub-cutaneous injections of insulin, repeated throughout the day. Insulin pumps may be used in place of traditional injections [ 85 ]; these have the advantage of being able to continuously infuse small amounts of insulin sub-cutaneously, helping those patients with difficult-to-control glucose levels to better treat their disease. This is especially the case when coupled with continuous glucose monitoring (CGM) technology, which has been shown to improve control of blood glucose, thereby reducing long-term risks of diabetic complications [ 86 , 87 ].
Taking the combination of CGM and continuous insulin infusion to the next level is the advent of the artificial pancreas. By utilizing a CGM coupled via a control algorithm to an implanted insulin pump, people with T1D can achieve improved glycemic outcomes while reducing the burden of self-management [ 88 , 89 , 90 ]. A closed-loop artificial pancreas approach removes the need for the patients to manage their dosages at all, and some models also incorporate the pancreatic hormone glucagon, enabling glucose-responsive hormone delivery guided by real-time glucose sensor readings. This approach has the potential to accommodate highly variable day-to-day insulin/glucagon requirements. There will be a shift toward systems that offer more personalization, and individualization of adjusting parameters, glucose set algorithm aggressiveness proposed to be individualized including the daily targets [ 91 ] that can ensure tight glycemic control in affected patients [ 92 , 93 ]. Despite these advantages, still relatively few T1D patients are using an artificial pancreas, with the main obstacles being cost of the equipment, the need for a training infrastructure for users and clinicians, and a lack of clarity around which patient groups would benefit most from this technology (reviewed in [ 92 ]). In this case the technology has preceded the clinical sub-group analysis required to identify the patient groups who are most suited to the approach, calling for urgent research in order to fully exploit this important advance in insulin-replacement therapy.
Alongside developments in insulin replacement therapy, there has been a focus on identifying other drugs that can be combined with insulin to reduce hyper/hypoglycemia and improve metabolic variables without increasing adverse events (reviewed in [ 94 ]). Obese/T1D patients who predisposed to hypoglycemia and others with residual β-cell function could benefit from non-insulin antidiabetic drugs for future clinical trials [ 94 , 95 ]. Of these, promising candidates include metformin [ 96 ] and pramlintide, which have a role in glycemic control in both T1D and T2D and can modestly reduce triglyceride levels in T1D patients, as well as lowering hemoglobin A1c (HbA1 c ) and supporting weight loss [ 97 ]. In addition, glucagon-like peptide-1 receptor agonists (GLP-RAs) combined with insulin can reduce the daily bolus insulin dose required and improve glucose control and weight loss [ 98 ]. The incretins glucagon-like peptide 1 (GLP-1) is gut-derived hormone secreted upon food ingestion. The key physiological actions of GLP-1 are to accelerate nutrient-induced insulin release and inhibit glucagon secretion, in that way contributing to regulate postprandial glucose excursions [ 99 ]. In addition, other functions represented by inhibition of gastrointestinal motility and therefore works as “enterogastrone”, a hormone released by the lower gastrointestinal tract in reaction to lipids intake that constrains the caudal motion of the guts of chyme [ 100 ]. GLP-RAs used peripherally or centrally reduce food intake and escalate glucose-stimulated insulin secretion. The enzyme dipeptidyl peptidase-4 inhibitors (DPP-4) prevents the inactivation of GLP-1 and an adjunct therapy in a closed loop-system that can reduce postprandial blood glucose levels [ 101 ] and can significantly reduce the daily insulin dose but not the HbA1c level or the risk of hypoglycemia [ 102 ]. The DPP-4 enzyme is widely released in multiple organs and acts by cleavage of the two NH 2 -terminal amino acids of bioactive peptides if the second amino acid is alanine or proline [ 103 ]. It functions through affixed transmembrane fragment and a soluble protein. Both transmembrane fragment and soluble DPP-4 apply catalytic cleavage which alternatively inactivates peptides or generates new bioactive moieties that may exert competing or unique functions. Finally, sodium-glucose co-transporter inhibitors (SGLTi) are associated with improved glycemic control and a reduced insulin dosage leading to lower rate of hypoglycemic episodes [ 104 ]. In non-diabetics, approximately, 180 g of glucose is filtered diurnal through the renal glomeruli and is then re reabsorbed in the proximal convoluted tubule (PCT). This mechanism attained by inactive transporters, specifically, facilitated glucose transporters (GLUTs), and by active co-transporters, precisely, sodium-glucose co-transporters (SGLTs). SGLT1 and SGLT2 are considered most important out of the six identified SGLTs [ 105 ]. SGLTi acts by inhibiting SGLT2 in the PCT to block glucose reabsorption and ease its secretion in urine. The plasma glucose levels drop resulting in an improvement in the entire glycemic parameters [ 106 ].
In summary, traditional and combined approaches to insulin therapy remain important tools in the treatment of T1D, but they do not represent a cure and may not be able to achieve the level of glucose control necessary to avoid long-term complications arising from diabetes. Automated full closed-loop systems that can be programed to automatically manage meals may substantially benefit from faster acting insulins with a shorter duration of action. Proposing automatic flexibility to the individual’s changes not only daily patterns of insulin sensitivity but also to mechanically adjust to changes developing from illness, workout practices, eating routines and menstrual cycles. With the applications of machine learning (artificial intelligence), (AI), the future devices with the AI technologies could achieve the above relationship and to provide treatment suggestions and decisions based on the available data input. A unique and individualized predictive and decision support models using complex machine learning software and algorithms developed for insulin pumps for easier use and much more spontaneous daily life. Recently, Tyler et al. (reviewed in [ 107 ]) reported an algorithm for early recognition of unsafe insulin regimens which could be useful for improvement the glycemic results and minimize the dangerous complications of T1D [ 107 ]. Briefly, the algorithm offers weekly insulin dosage recommendations for adult patients with T1D using multiple daily injections protocol of long-acting basal and short-acting bolus insulin [ 108 ]. The hyperglycemia or hypoglycemia causes identification performed through validated single and dual hormone mathematical models that demonstrate a virtual platform of T1D patients [ 109 ]. The novel “virtual platform” employed to generate glucose observations used to train “decision making system”, which appeared to be in agreement with the endocrinologists’ decision of 67.9% when confirmed on actual human data [ 107 , 110 ]. In conclusion, such data provides guidance to physicians and T1D patients in effective use of insulin pumps data including but not limited to insulin dosing adjustments and other treatment decisions. It’s worth to mention how crucial that both physicians and diabetic patients understand the usefulness and limitations of insulin pumps and related treatment technologies. Sustaining the relationship between both will remain a critical factor in safe, thriving T1D treatment technology use.
- Gene therapy
Given the strong genetic component of T1D development, gene therapy offers a promising alternative to insulin injection for T1D treatment. Gene therapy is the procedure of transporting or manipulating genetic substances inside the cell as a therapeutic technique to cure disease [ 111 ]; it aims to modify faulty genes that are accountable for disease progression and thereby prevent disease onset or reverse its development (Fig. 3 ). The three key methodologies in gene therapy are: (I) introducing a new gene into the body (II) substituting defective genes with functional genes, and (III) deactivating the faulty genes triggering the disease [ 112 ]. Pre-clinical trials of gene therapy have now been tested with the aims of preventing or delaying onset of T1D, correcting insulin deficiency, promoting β-cell proliferation and survival, modulating the immune/inflammatory response or inducing insulin secretion by non-β cells (reviewed in [ 113 ]).
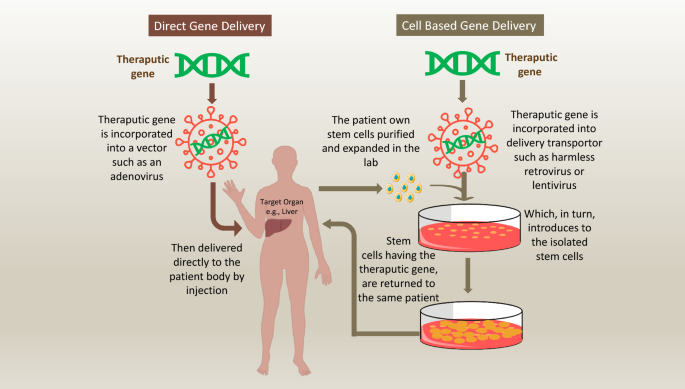
How genes are delivered to the human body during gene therapy approaches. Gene therapy have utilized two major approaches for transferring therapeutic transgenes into recipients 'body. First approach, is by direct infusion of the therapeutic gene into human body through a vehicle. Altered viruses often used for delivering the gene into specific human cell types. This method is inexact as it is limited to specific cell types that the viral vehicle can infect. Nonviral vehicles for directly delivering genes into cells are also being explored, including the use of plain DNA and DNA wrapped in a coat of fatty molecules known as liposomes. Th second approach utilize a living cells to transfer the therapeutic transgenes into recipients 'body. The transferring cells often a type of stem cell that removed from the body, and the therapeutic transgene is presented to them through direct transfer method. The genetically altered cells then grow and multiply before infused back to the recipient
Over the last few decades, gene transfer trials for the treatment of inherited or acquired diseases have mainly been performed in mice models. Non-obese diabetic (NOD) mouse has been the main animal model for studying autoimmune T1D. A key element of NOD model is the presence of spontaneous autoimmunity and T1D. The incidence of T1D is higher in females in NOD mice, [ 114 , 115 ], and is stated to have a minor prevalence in males in humans [ 116 , 117 ]. Like human, NOD mice develop autoantibodies and show elevated levels of autoreactive T-Cells ahead of disease onset [ 118 , 119 , 120 ]. The targeted antigens of β cell are also similar of both species, however, in the NOD mouse, the insulin seems to be the initiating antigen, while in human T1D, several antigens thought to be involved in this stage [ 118 ]. Gradual β cell death or malfunction, and autoimmune phenotypes shadowed by the onset of hyperglycemia exist in both human and NOD mouse [ 121 ], however, the appearance of pathogenic T cells have been noticed at 5-week-old NOD mice followed by insulitis throughout the pancreas by 12 weeks, reflecting the very aggressive nature of disease onset hits in shortened timeline (weeks only), compared to slower onset in humans (years after the autoantibodies appearance) [ 122 , 123 ].
The paradoxical assumption is that preventing T1D in NOD mice does not certainly convey what triggered the disease nor how to converse it. The NOD mouse model could be suitable to understand the genetic and immunologic features and causes of T1D including reversing the hyperglycemia when occurs. The model could serve as an approach to identify causative gene variants that can be tailored to discover novel therapeutic approaches for reversing new-onset T1D.
One particularly interesting strategy is the induced over-expression of insulin-like growth factor 1 (IGF1), which regulates immune functions and enhances the survival and proliferation of β-cells. Non-obese diabetic (NOD) mice spontaneously develop diabetes from around 10 weeks-of-age; however, when 4-week-old NOD mice underwent intra-ductal injection of an adeno-associated virus (AAV) encoding IGF1 to specifically transduce pancreatic cells, normoglycemia remained in 80% of these mice at week 28 [ 124 ]. Importantly, the same study also showed that treating NOD mice with the IGF1-encoding virus at 11 weeks-of-age, by which time significant β-cell destruction was evident, was able to re-establish lasting normoglycemia in 75% of mice [ 124 ].
In other animal studies, induced expression of regenerating islet-derived protein 3 gamma (Reg3g) has been reported to be able to regenerate β cells and preserve the cells despite autoimmune attacks [ 125 , 126 ]. Alongside, another study demonstrated the dynamic regulation of blood glucose levels in a model of T1D by stimulating the expression of glucose 6-phosphatase (G6Pase) in the liver of diabetic rats [ 127 ]. Here, expression of the G6Pase gene was induced by rising glucose levels and inhibited by insulin expression; in addition to achieving normoglycemia within a few hours of eating, no hypoglycemia was observed in the tested animals [ 127 ].
Gene therapy can also be used to induce insulin production in non-β-cells. Initial studies conducted on genetically engineered intestinal K cells [ 128 ] and hepatocytes showed that these cells were sensitive to glucose and could be induced to produce insulin. More recently, Jaen et al. demonstrated that a single injection of an AAV encoding insulin and glucokinase genes into skeletal muscle of diabetic dogs was able to induce metabolic normalization and normoglycemia lasting 8 years [ 129 ]. This study represents an important safety and efficacy step forwards for diabetes gene therapy, as although AAV vectors have been trialed in humans, their therapeutic use for gene transduction has yet to be tested clinically. There are concerns that transduced cells might be susceptible to recurring autoimmune attack, so enduring autoimmune protection must be demonstrated [ 130 , 131 ]. It is also possible that the viral vectors themselves might trigger an immune response that could worsen the disease condition [ 132 ], though Jaen et al. did not report any evidence of this in their study [ 129 ]. Modifications to the AAV vectors might hold some of the answers: in response to concerns that constitutive over-expression of insulin might risk hypo-glycaemia, one group has developed a Tet-off regulatable AAV vector for insulin expression that was able to both induce the expression of human insulin in diabetic mice, and be reversibly switched off to reduce insulin levels [ 133 ]. Thus, fine tuning of viral vectors combined with more long-term studies will be required to move towards vector-mediated reinstatement of insulin production in human patients.
In addition to induced insulin expression, several studies have looked at other targets implicated in T1D pathogenesis. For example, Klotho is an anti-aging gene that is expressed in pancreatic islets in mice [ 134 ] and humans [ 135 ]; a Klotho deficiency is linked with β-cell apoptosis, and reinstating its expression in mice under the control of a β-cell-specific promoter led to protection of β-cell function [ 134 ]. In human islet cells, treatment with the T1D drug gamma-aminobutyric acid in vitro significantly increased Klotho expression [ 136 ], indicating the possible clinical potential for this approach. A study by Flotyńska et al. demonstrated the relationship between fibroblast growth factor 23 (FGF23)/ Klotho system as a player in the human body metabolism, in addition to promoting longevity [ 137 ]. Despite the improvements in diabetes treatment, the long-term complications remain a big problem. The interesting correlation between the FGF23/Klotho system concentration and T1D management, duration, insulin resistance, and complications development require further attention and could be a predictor of cardiovascular risk in diabetic patients [ 138 ]. Combining gene therapy with immune modulation may also be promising. When NOD mice were pre-treated with anti-T-cell receptor β chain monoclonal antibody followed by hepatic gene therapy with Neurogenin-3 (which determines islet lineage) and the islet growth factor betacellulin, the researchers observed sustained induction of insulin-producing cells in the liver that achieved enduring reversal of new-onset or overt diabetes [ 139 ].
The discovery of β-cell mitogenic effects of ANGPTL8 (Angiopoietin Like 8), which was renamed “Betatrophin” to underline its effect on β cell replication, initially, created large interest but consequently, have been subjected to substantial debate regarding its anticipated mitogenic effects [ 140 ]. The initial findings proposed that the over expression of ANGPTL8 in mice model stimulated a 17-fold increase in pancreatic β-cell proliferation [ 140 , 141 ]. Consequent research studies in mice disputed this statement as no substantial evidence could be observed to support the direct effects of ANGPTL8 on beta-cell proliferation [ 140 , 142 , 143 ], Therefore, ANGPTL8 is not considered as a potential agent for diabetes intervention although some reports supported the initial observations in rats [ 144 ]. In a study performed by Chen et al. (reviewed by [ 144 ]), targeted gene delivery approach has been used to deliver human ANGPTL8 gene plasmids to different organs of normal adult rats including the pancreas, liver and skeletal muscles and compared the efficiency of beta β cell replication induced by ANGPTL8 gene using the rat model of streptozotocin (STZ)-induced diabetes. The improvement in glucose tolerance plus the elevated fasting plasma insulin levels were directly associated with β cell proliferation. A novel gene therapy technique used here through targeting the transfer of non-viral DNA to the pancreatic islet by using ultrasound-targeted microbubble destruction (UTMD) beside an altered insulin promoter [ 140 , 145 ]. UTMD considered as promising method for target-specific gene delivery, and it has been successfully investigated for the treatment of many diseases in the past decade including cardiovascular disorders and cancer.
A novel approach to gene therapy for T1D involves targeting post-transcriptional modifications that give rise to pathogenic splice variants. Cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4) is an immune-modulatory protein where expression of different forms has been linked to T1D susceptibility or resistance in T1D patients [ 146 ] and some other autoimmune diseases [ 147 ]. To modulate the immune response leading to T1D onset, Mourich et al. employed an antisense-targeted splice-switching approach to produce CTLA-4 splice forms in NOD mouse T-cells [ 148 ]. In this study, when the antisense approach was used to mask pre-mRNA splice recognition sites and redirect the splicing machinery to skip selected exons, induced over-expression of the protective ligand-independent form of CTLA-4 protected NOD mice from disease [ 148 ].
Lastly, while these studies clearly indicate the exciting potential of in vivo gene therapy, the process remains complex, in addition, the possible toxicity of the viral vectors and the improvements needed to the delivery systems to achieve the maximum levels of gene expression still under development [ 125 ]. That said, twenty gene and cell-based gene therapy products have now been licensed for the treatment of human cancers and monogenic disorders “e.g., Neovasculgen (Vascular endothelial growth factor, VEGF), Glybera (lipoprotein lipase, LPL S447X gene), Defitelio (single-stranded oligonucleotides-VOD), Rexin-G (Retroviral vector encoding cyclin G1 inhibitor), Onpattro (RNAi-transthyretin gene)” and clinical trials in these diseases are ongoing [ 149 ]. There is real hope that effective approaches to direct gene therapy for T1D patients, particularly those with monogenic T1D, will be developed in the near future, building on its success in other conditions.
Stem cell therapies
Perhaps the most promising innovation in T1D therapy has been the exploration of the potential of stem cells. This unique population is able to self-renew indefinitely, form single cell-derived clonal cell populations, and differentiate into various cell types [ 150 ]. Stem cells from diverse sources have now been investigated for their potential in β-cell regeneration, as discussed below.
Embryonic stem cells
Embryonic Stem Cells (ESCs) are derived from the undifferentiated inner cell mass of human embryos and have the advantage of being completely pluripotent. Several different approaches to generating insulin-producing cells (IPCs) from ESCs have been explored. Human Embryonic Stem Cells ESCs (hESCs) in feeder-free cultures avoid the risk of animal pathogen transfer and are readily scalable, making this approach best-suited to clinical use [ 151 ].
Kroon et al. instructed the differentiation of hESCs by directly overexpressing essential β-cell transcription factors (TFs) including Pancreatic and Duodenal Homeobox 1 (PDX1), SRY-Box Transcription Factor 9 (SOX9), Homeobox protein Nkx-6.1 (NKX6.1) and Neurogenin 3 (NGN3; following engraftment into diabetic mice, the resulting cells recapitulated key features of pancreatic β-cells and protected against hyperglycemia [ 152 ]. Subsequently, an important step forwards in the use of hESCs for T1D therapy occurred when scientists from the University of British Columbia developed a seven-stage protocol that efficiently converted hESCs into IPCs. This protocol generated endocrine cells with insulin content similar to that of human islet cells and that were capable of glucose-stimulated insulin secretion in vitro as well as rapid reversal of diabetes in vivo in mice [ 153 ]. Additional studies have highlighted the possible roles of other growth and extracellular matrix factors, including laminin, nicotinamide, insulin [ 154 ], and retinoic acid [ 155 ] in the generation of IPCs from ESCs, but these findings have yet to be integrated into a combined approach suitable for clinical use.
hESCs also have the potential to generate cells uniquely tailored for the recipient. Recently, Sui et al. showed that transferring the nucleus of skin fibroblasts from T1D patients into hESCs gave rise to differentiated β-cells with comparable performance to naturally occurring β-cells when transplanted into mice [ 156 ].
Despite the promise of hESCs, great concern around their potential to initiate teratomas has largely limited their clinical exploration in T1D. However, Qadir et al. recently demonstrated a means of overcoming this risk: the authors modified hESCs to include two suicide gene cassettes, whose expression results in cell death in the presence of specific pro-drugs [ 157 ]. Their method is designed to provide a double fail-safe control, such that I) only IPCs survive selection; and II) cells that may de-differentiate after transplantation can be eliminated. Furthermore, ensuring that undifferentiated cells are sensitive to two pro-drugs makes it less likely than any tumorigenic cells would survive or became resistant [ 158 ].
Human pluripotent stem cells
Naturally, Human Pluripotent Stem Cells (hPSCs) are immature cells that have the capacity to become nearly any cell type in the body. Accordingly, there has been much research interest in using them to regenerate a wide range of tissues, including the pancreas. Under the control of specific growth factors, signaling pathways and activating/inhibitory molecules [ 159 , 160 ] the steps of pancreatic cell differentiation have been successfully recreated in vitro.
The importance of this approach is its potential to generate a ready supply of in vitro-differentiated β-cells for transplantation into T1D patients. Recent studies have reported the successful differentiation of β-like cells with enhanced function from pancreatic progenitors through modulating Epidermal growth factor beta (EGF-β) signaling and cellular cluster size, giving rise to stem cell-derived β-cells with the ability to express key β-cell markers and insulin [ 161 , 162 ]. What remains unclear is how well these in vitro-derived cells will function in vivo , but this is nonetheless a promising first step.
Hematopoietic stem cells
Taking a different approach, myeloablation coupled with autologous Hematopoietic Stem Cells (HSCs) transplantation aims to halt the autoimmune destruction of the pancreas and reestablish tolerance. The first autologous HSCs transplantation in a T1D patient was executed by the Voltarelli’ group in 2007: 15 patients aged between 14 add 31 years, and with recent T1D onset (previous 6 weeks) diagnosed by clinical findings, hyperglycemia and GAD65 autoantibodies were involved in the study [ 163 ]. When these patients were treated with autologous HSCs, most achieved insulin independence with good glycemic control lasting until the final 29.8-month follow-up, together with a notable increase in β-cell function [ 164 ]. Autologous HSC transplantation has also been used successfully to treat diabetic sequelae, including vascular complications [ 165 ] and retinopathy [ 166 ]. Other studies have focused on understanding the mechanisms underlying successful HSCs transplantation in T1D: for example, Ye et al., found that autologous HSC treatment was associated with the inhibition of T-cell proliferation and pro-inflammatory cytokine production [ 167 ]; while Xiang et al. uncovered a critical role for the remaining functional β-cells on the autologous transplant of HSCs [ 168 ].
Despite the evident successes of autologous HSCs transplantation for T1D, various complications can occur, ranging from relatively mild symptoms such as febrile neutropenia, nausea, and alopecia to more severe complications such as de novo autoimmunity and systemic infections, which in one case resulted in death [ 169 , 170 ]. The development of new strategies involving autologous HSCs therapy for newly-diagnosed T1D patients coupled with appropriate and effective use of immunosuppressive drugs will be crucial to maximize the frequency and function of T and B regulatory cells, while minimizing the activity of autoreactive islet-specific T and B memory cells. In this way, we should be able to improve treatment outcomes in T1D patients undergoing transplantation.
Mesenchymal stem cells
Mesenchymal Stem Cells (MSCs) are multi-potent stromal cells able to differentiate in vitro into a range of cell types; characteristically adipocytes, chondrocytes, myocytes, and osteoblasts [ 171 ]. MSCs are relatively easy to isolate from different sources in the body and numerous studies have assessed their use in T1D therapy.
Historically, the bone marrow has been the main source of MSCs [ 172 ]. Xie et al. first trialed generating IPCs from T1D patients’ bone marrow MSCs (BM-MSCs) and showed the co-expression of insulin and C-peptide in cells injected into diabetic mice, leading to attenuated hyperglycemia [ 173 ]. Alongside, genetically-modified human BM-MSCs expressing VEGF and PDX1 reversed hyperglycemia in more than half of diabetic mice and enabled survival and weight maintenance in all animals [ 174 ]. These promising pre-clinical results led to human trials: when BM-MSCs were injected into the splenic artery of T1D patients, they induced an increase in C-peptide levels that was maintained for 3 years; unfortunately, this had no significant effects on glycemic control due to insufficient production of insulin by the grafted cells [ 175 ]. Since then, new methods have been developed aiming to improve in vivo outcomes. For example, Zhang et al. co-cultured BM-MSCs with pancreatic stem cells which led the MSCs to adopt a pancreatic islet morphology; when these cells were injected into diabetic rats they attenuated glycated albumin levels and significantly increased serum insulin and C-peptide [ 176 ].
The main disadvantage of BM-MSCs is the difficulty in isolating the cells and the morbidity associated with the procedure. These issues led to interest in the use of Muscle-Derived Stem/Progenitor Cells (MDSPCs), which exist in skeletal muscle and have the capacity for long-term proliferation, are resistant to oxidative and inflammatory stress, and show multi-lineage differentiation potential [ 177 ]. To investigate the therapeutic potential of autologous MDSPCs transplantation for T1D, Lan et al. applied a four-stage MDSPCs differentiation protocol to generate IPCs in vitro and injected them into diabetic mice: these β-cell-like-cells effectively improved hyperglycemia and glucose intolerance and increased the survival rate in diabetic mice without the use of immunosuppressants [ 178 ].
Building on the promise of BM-MSCs and MDSPCs, researchers sought an equally potent but more abundant and easily accessed source of stem cells. Adipose-Derived Stem Cells (ADSCs) have recently been explored for T1D treatment, and have the advantage over MDSPCs of being readily accessible and harvested, even in older patients [ 179 ]. IPCs differentiated from ADSCs show significant expression of β-cell markers, insulin and c-peptide following transfer into diabetic mice [ 180 ]. In 2019, IPCs derived from ADSCs using a novel three-dimensional (3D) xenoantigen-free protocol were shown to exhibit key features of pancreatic β cells in vitro and differentiated into IPCs in diabetic nude mice in vivo [ 181 ]. Another study showed the potential for combining ADSCs treatment with gene therapy by transducing ADSCs with a furin-cleavable insulin gene (INS-FUR), which led to enhanced insulin expression in the differentiated adipocytes, and alleviated hyperglycemia in diabetic mice [ 182 ].
Removing the need for adult stem cell donors completely, the umbilical cord is now used as a successful alternative stem cell source for regenerative medicine. Umbilical cord blood (UCB) is rich in HSCs, can be easily harvested without the need for interventions, and also contains a large number of naive functioning T-regulatory cells (Treg) with the potential to reduce autoimmunity [ 183 , 184 ]. Moreover, the MSCs within UCB (UCB-MSCs) have high proliferative capacity, are easily bankable and have low tumorigenicity [ 185 ]. Together these features are making UCB-MSCs the preferred option for potential T1D cell-based therapies. Studies in animal models have showed encouraging results: when Prabakar et al. adapted an ESC protocol for IPC culture and applied it to UCB-MSCs they generated expanded populations of undifferentiated IPCs expressing the key pancreatic TFs PDX1, NGN3, Neuronal Differentiation 1 (NEUROD1), NKX6.1, and Insulin Gene Enhancer Protein ISL-1 “ISL LIM Homeobox 1” (ISL1) [ 186 ]. Following transplantation into mice, these cells subsequently differentiated into glucose-responsive IPCs [ 186 ]. Zhao et al. took a different approach to exploiting stem cells for T1D treatment, instead focusing on their capacity to downregulate immune responses. The authors achieved reversal of the autoimmune response in NOD mice by transferring autologous Tregs that had been co-cultured with human UCB-MSCs; this led to increased insulin secretion, reduced hyperglycemia and preservation of islet architecture [ 187 , 188 , 189 ].
Despite promising signs in rodent studies, the potential of UCB-MSCs treatment for T1D in humans has yet to be fully realized. Haller et al. attempted the first autologous UCB-MSCs transplantation in recently-diagnosed T1D patients in 2008: early indications were encouraging, with transplanted patients showing slowed loss of endogenous insulin production and an increase in peripheral blood Treg cells after 6 months [ 190 ]. However, a subsequent study by the same group found no significant difference in C-peptide levels after autologous transfusion of UCB-MSCs combined with oral docosahexaenoic acid and vitamin D supplementation [ 191 ]. Similarly, in a non-randomized controlled trial in seven new-onset T1D children who underwent autologous UCB-MSCs infusion, there was no evidence of improvements in metabolic regulation or immune function at the one-year follow-up [ 192 ].
The possible reasons for the failure of UCB-MSCs to effectively halt the autoimmune progression in human subjects’ trials, could be the inadequate number of cells with immunomodulation capacity being transferred to T1D patients, or due to the ongoing autoimmune reactions especially in new-onset T1D patients that may comprise memory T-cells, refractive to regulation by Tregs, that enhance the autoimmune destruction of β-cells [ 193 ]. Merging transient immune depletion agents with consequent infusion of expanded UCB Tregs may effectively balance the environment of Tregs and effector T cells in T1D patients. Finally, more controlled and randomized clinical trials are crucial to further improve the transplantation process and to investigate the mechanism of UB-MSC survival and behavior in live bodies overtime. Further investigations with larger sample sizes will be important to understand how to translate the successful application of UCB-MSCs infusion from mouse to human.
Cord blood is not the only source of stem cells within the human umbilical cord; Wharton’s jelly is a mucoid connective tissue in the umbilical cord that can also serve as a source of clinically-relevant MSCs (Wharton’s jelly-derived mesenchymal stem cells, WJ-MSCs) for both IPC derivation and immunosuppression [ 194 ]. Briefly, WJ-MSCs collection occurs at the time of delivery and avoids the known adverse effects associated with adult stem cell collection from the bone marrow or adipose tissue. Furthermore, features including a high WJ-MSCs proliferation rate, an immune privileged status, minimal associated ethical concerns, and non-tumorigenic capacity render these cells an excellent option to be used in regenerative medicine applications [ 195 ].
One of the first studies to use β-cell-like cells derived from WJ-MSCs tested their effects following transplantation into patients with new-onset T1D [ 196 ]. Interestingly, a concurrent study suggested that the WJ-MSCs might restore the function of β-cell in T1D patients but it could be affected by the patient’s ketoacidosis history [ 197 ], though the underlying mechanism to support this has not yet been tested. A genetically and chemically combined approach for WJ-MSCs induction into IPCs has also been shown to improve the cells’ homing efficiency to the pancreatic gland of diabetic rats [ 198 ]; taken together with a growing body of clinical data, these findings may help optimize the use of differentiated WJ-MSCs in T1D.
Undifferentiated WJ-MSCs also have the capacity to induce a protective immune-suppressive state in animal models of T1D and in patients. A study in mice performed by Tsai et al. showed that undifferentiated WJ-MSCs implanted into NOD mice both differentiated into IPCs in vivo, leading to islet repair and maintaining levels of C-peptide and insulin production, and induced beneficial immunosuppression [ 199 ]. Such evidence in rodents has since led to the initiation of human trials. A safety and dose-escalation trial is ongoing: in the first stage, Carlsson et al. are carrying out WJ-MSCs allotransplantation into newly-diagnosed (< 2 years) T1D adult men with dose-escalation to establish safety parameters; in the second double-blinded, parallel, placebo-controlled stage, a cohort of T1D patients (men and women) will undergo WJ-MSCs allotransplantation aiming to achieve immunosuppression and preserve endogenous insulin production [ 200 ]. Altogether, comparing WJ-MSCs, UCB-MSCs [ 201 ] and BM-MSCs [ 202 ], it seems that WJ-MSCs are the better anti-diabetic agents, being more homogenous and having greater potential to initiate pancreatic regeneration.
Medical nutrition therapy in managing T1D
A healthy lifestyle including eating pattern beside pharmacotherapy are major components of managing T1D. For many diabetic patients, determining what to eat is the most challenging part of the treatment plan. Effectual nutrition therapy interventions may be an element of a comprehensive T1D education package or an individualized session [ 203 ]. Furthermore, T1D individuals on multiple daily insulin doses, the main focus for nutrition therapy must be on how to adjust insulin doses based on scheduled carbohydrate intake [ 204 , 205 ]. Reported HbA1 C from medical nutrition therapy (MNT) decreases are similar or greater than what would be expected with currently available pharmacologic therapies for T1D [ 206 ]. Rigorous insulin management education programs that include MNT have been shown to reduce HbA1 C up to 1.9% at 3–6 months, in addition to significant improvement in quality of life over the time [ 203 , 207 ]. There is no “one-size-fits-all” eating pattern that could work collectively for all T1D individuals, nutritional therapy should be individualized and supervised under the care of a dietitian based on the heath goals, personal favorites and access to healthy options should be considered [ 208 , 209 ].
Remaining obstacles and future directions
Marked progress has been made in the past decade towards both personalized diagnosis and treatment for T1D, but significant obstacles and research gaps remain between the current state of knowledge and its translation into widespread clinical benefit. As in many other diseases, the precision medicine for T1D is a new and growing field. Increases ethical, social and legal issues and the necessity to find precise ways to protect subjects’ privacy and confidentiality of their health data. In addition, patients need to know and understand the associated risks and expected benefits of being part of precision medicine research, which requires researchers to create a meticulous approach of obtaining informed consent to recruit participants to research studies. Furthermore, cost-effectiveness of precision medicine approaches comparing to the current standard of care is a gap that needs to be resolved. The impact of diabetes on healthcare systems has been evaluated as the largest contributor to entire healthcare costs. For example, in a study performed by Stedman et al. (reviewed in [ 34 ]), the differences between T1D/T2D and non-diabetes subjects in connection to hospital and associated costs in in England. In summary, T1D individuals demanded five times additional secondary care support than non-diabetes subjects. The analysis shows that extra cost of running of hospital services due to their diabetes comorbidities is £3 billion over that for non-diabetes, within this figure, T1D has three times as much cost impact as T2D, suggesting that supporting patients in diabetes management may considerably decrease hospital activity, in addition, the possibility and potential for precision treatment in diabetes is massive, yet profound understanding is missing. It will be vital to decide when and how the application of therapeutics in precision diabetes medicine improves outcomes in a cost-effective style.
Much of our current knowledge of personalized therapeutic approaches to treat T1D comes from experiments in animal models; but a recurring theme in the T1D therapy field is the lack of translation between promising results in mice and the same outcome in humans. Mice are most commonly used for these experiments but exhibit both macroscopic and microscopic differences in pancreatic physiology and T1D pathophysiology. For example, rodents islets have a distinct core structure comprising 60–80% β-cells, 15–20% α- cells, < 10% δ-cells and < 1% PP cells [ 210 , 211 , 212 ]; while human islets tend to have ~ 50% β-cells, ~ 40% α- cells, ~ 10% δ-cells and < 5% P-cells [ 213 , 214 ]. In addition, notable differences in the repertoire of receptors and long non-coding RNAs between mouse and human beta cells have been identified [ 215 ]. In terms of modeling T1D, the NOD mouse has long been the approach of choice for majority of pre-clinical and translational invasive studies [ 216 ]. The main strength of the NOD mouse is the presence of spontaneous autoimmunity leading to T1D [ 118 , 216 ] however, in the mice, this is triggered by the insulin antigen, while in humans this phenomenon is more complex, involving several inducing antigens followed by hyperglycemia [ 217 , 218 ]. Taken together, extreme caution must be exercised when attempting to draw conclusions from animal models and apply them to the human situation [ 219 ].
Despite advances in the various therapies discussed above, an ongoing challenge in T1D treatment is the extreme heterogeneity in patients’ disease triggers, prognosis, pathological pathways and thus the response to treatment [ 220 , 221 , 222 , 223 ]. Important research in human populations has revealed previously unappreciated heterogeneity within the T1D patient population. This has two major implications: firstly, that we are unlikely to discover a “one-size-fits-all” therapy able to cure every case; and secondly that personalized diagnosis is a necessary pre-requisite for personalized treatment. The first step towards this will be the routine assessment of T1D subtype in newly diagnosed patients, including screening for monogenic T1D as well as autoantibody testing to distinguish idiopathic T1D, and, in future, genetic profiling to inform potential gene therapy or stem cell approaches.
In diabetes, the precision medicine approach has been inspired by work including that of Zhao et al., who first developed stem cell educator therapy where T1D patients’ lymphocytes are briefly separated from the blood and co-cultured with UC-MSCs within a closed-loop-system, before being returned to the patient; this treatment dramatically improved metabolic control, reversed autoimmunity and promoted β-cell regeneration [ 143 ]. Al-Anazi et al. used a similar approach to try and treat multiple myeloma in 45 adults with T1D who had undergone autologous HSCs; surprisingly the patients were also cured of their diabetes and became insulin-independent [ 144 ].
In fact, the next step towards stem-cell-mediated precision medicine for T1D is likely to involve the incorporation of gene therapeutic approaches, synergizing existing stem cell knowledge with advances in cellular and genetic engineering techniques, such as nuclear transfer and genome editing. Moreover, an emerging understanding of the TFs and epigenetic processes that control pancreatic islet lineage-commitment [ 224 ], as well as the role of microRNAs in driving cell lineage differentiation [ 225 ] are beginning to unlock new knowledge on T1D pathogenesis [ 226 , 227 ], and are opening fresh possibilities in β-cell generation [ 228 , 229 , 230 ].
Together these factors can all be used towards designing a successful protocol for precision medicine in T1D. Alongside, the reframing of T1D as primarily a metabolic disorder (rather than an autoimmune condition) that reflects the combined genomic and environmental landscape of the patient, has facilitated the discovery of new therapeutic targets and diagnostic/prognostic biomarkers [ 231 , 232 ]. Finally, the ongoing discovery of new and important influences on diabetic pathology, such as the role of gut microbiota [ 233 ], and the latest perceptions into the mechanism of T1D and the accumulated recent data that being translated into prospects for tissue-specific prevention trials toward eliminating progressive β-cell loss [ 234 ], continues to add to our understanding of this important disease, and thereby our ability to rationally design and test novel interventions with the promise of the future eradication of T1D.
Availability of data and materials
Not applicable.
Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clin North Am. 2005;52(6):1553–78.
Article PubMed Google Scholar
Patterson CC, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408–17.
Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes–the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–403.
Article CAS PubMed Google Scholar
Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23(10):1516.
Saeedi P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:48.
Article Google Scholar
Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med. 2008;359(26):2849–50.
Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–9.
Barrett JC, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–7.
Article CAS PubMed PubMed Central Google Scholar
Sharp SA, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care. 2019;42(2):200.
Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90(1):7–24.
Steck AK, et al. Association of Non-HLA Genes With Type 1 Diabetes Autoimmunity. Diabetes. 2005;54(8):2482.
Nisticò L, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Hum Mol Genet. 1996;5(7):1075–80.
Bottini N, Vang T, Cucca F, Mustelin T. Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol. 2006;18(4):207–13.
Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57(2):176–85.
Clayton DG. Prediction and interaction in complex disease genetics: experience in type 1 diabetes. PLoS Genet. 2009;5(7):e1000540.
Article PubMed PubMed Central CAS Google Scholar
Wray NR, Yang J, Goddard ME, Visscher PM. The genetic interpretation of area under the ROC curve in genomic profiling. PLoS Genet. 2010;6(2):e1000864.
Ilonen J, Hammais A, Laine AP, Lempainen J, Vaarala O, Veijola R, Simell O, Knip M. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62(10):3636–40.
Krischer JP, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–7.
Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290(13):1713–20.
Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A. Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia. 2015;58(6):1188–97.
Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet (London, England). 2016;387(10035):2340–8.
Article CAS Google Scholar
Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318(7):647–56.
Grammatiki M, Rapti E, Karras S, Ajjan R, Kotsa K. Vitamin D and diabetes mellitus: Causal or casual association? Rev Endocr Metab Disord. 2017;18(2):227–41.
Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12(3):154.
Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination and type 1 diabetes. N Engl J Med. 2004;350(14):1398–404.
Butalia S, Kaplan GG, Khokhar B, Rabi DM. Environmental risk factors and type 1 diabetes: past, present, and future. Canad J Diabetes. 2016;40(6):586–93.
Kolb H, Elliott R. Increasing incidence of IDDM a consequence of improved hygiene? Diabetologia. 1994;37(7):729–729.
Baschal EE, Eisenbarth GS. Extreme genetic risk for type 1A diabetes in the post-genome era. J Autoimmun. 2008;31(1):1–6.
Wang Z, Xie Z, Lu Q, Chang C, Zhou Z. Beyond genetics: what causes type 1 diabetes. Clin Rev Allergy Immunol. 2017;52(2):273–86.
Mehers KL, Gillespie KM. The genetic basis for type 1 diabetes. Br Med Bull. 2008;88(1):115–29.
Craig ME, Kim KW, Isaacs SR, Penno MA, Hamilton-Williams EE, Couper JJ, Rawlinson WD. Early-life factors contributing to type 1 diabetes. Diabetologia. 2019;62(10):1823–34.
Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15(11):635–50.
Chua K-P, Lee JM, Conti RM. Out-of-pocket spending for insulin, diabetes-related supplies, and other health care services among privately insured US patients with type 1 diabetes. JAMA Internal Med. 2020;180(7):1012–4.
Stedman M, et al. Cost of hospital treatment of type 1 diabetes (T1DM) and type 2 diabetes (T2DM) compared to the non-diabetes population: a detailed economic evaluation. BMJ Open. 2020;10(5):e033231.
Article PubMed PubMed Central Google Scholar
Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the US: a propensity score matching method. PloS one. 2010;5(7):e11501–e11501.
Insel RA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–74.
Ziegler AG, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–9.
Insel R, Dutta S, Hedrick J. Type 1 diabetes: disease stratification. Biomedicine Hub. 2017;2:1–16.
Ludvigsson J, Heding L, Larsson Y, Leander E. C-peptide in juvenile diabetics beyond the postinitial remission period relation to clinical manifestations at onset of diabetes remission and diabetic control. Acta Pædiatrica. 1977;66(2):177–84.
Control, D. and C.T.R. Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–23.
Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832–6.
Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund J-YC, Lachin JM. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45–53.
Larsson HE, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347–52.
Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–404.
Dabelea D, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133(4):e938–45.
Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308–13.
Lundgren M, et al. Reduced morbidity at diagnosis and improved glycemic control in children previously enrolled in DiPiS follow-up. Pediatr Diabetes. 2014;15(7):494–501.
Krischer JP. The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia. 2013;56(9):1919–24.
Orban T, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–9.
Ferrat LA, et al. A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med. 2020;26(8):1247–55.
Sosenko JM, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2008;31(3):528–33.
Rubio-Cabezas O, Hattersley AT, Njølstad PR, Mlynarski W, Ellard S, White N, Chi DV, Craig ME. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2014;15(S20):47–64.
Bonifacio E, et al. Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med. 2018;15(4):e1002548.
Redondo MJ, et al. A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care. 2018;41(9):1887–94.
Patel KA, Oram RA, Flanagan SE, De Franco E, Colclough K, Shepherd M, Ellard S, Weedon MN, Hattersley AT. Type 1 diabetes genetic risk score: a novel tool to discriminate monogenic and type 1 diabetes. Diabetes. 2016;65(7):2094–9.
Wilson RM, Messaoudi I. The impact of maternal obesity during pregnancy on offspring immunity. Mol Cell Endocrinol. 2015;418:134–42.
Arabin B, Baschat AA. Pregnancy: An Underutilized Window Of Opportunity To Improve Long-Term Maternal And Infant Health—An Appeal For Continuous Family Care And Interdisciplinary Communication. Front Pediat. 2017;5:69.
Google Scholar
Organization WH. WHO recommendations non-clinical interventions to reduce unnecessary caesarean sections. Berlin: World Health Organization; 2018.
Antvorskov JC, et al. Association between maternal gluten intake and type 1 diabetes in offspring: national prospective cohort study in Denmark. BMJ (Clinical research ed). 2018;362:k3547–k3547.
Fronczak CM, Barón AE, Chase HP, Ross C, Brady HL, Hoffman M, Eisenbarth GS, Rewers M, Norris JM. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26(12):3237–42.
Penno, M.A.S., J.J. Couper, M.E. Craig, P.G. Colman, W.D. Rawlinson, A.M. Cotterill, T.W. Jones, L.C. Harrison, and E.S. Group. Environmental determinants of islet autoimmunity (ENDIA): a pregnancy to early life cohort study in children at-risk of type 1 diabetes. BMC Pediatr. 2013;13(1):124.
Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167(9):800–7.
Couper JJ, Beresford S, Hirte C, Baghurst PA, Pollard A, Tait BD, Harrison LC, Colman PG. Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care. 2009;32(1):94–9.
Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, Rewers M, Norris JM. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediat. 2013;167(9):808–15.
Lamb MM, Miller M, Seifert JA, Frederiksen B, Kroehl M, Rewers M, Norris JM. The effect of childhood cow’s milk intake and HLA-DR genotype on risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young. Pediatr Diabetes. 2015;16(1):31–8.
Porcelli B, Pozza A, Bizzaro N, Fagiolini A, Costantini MC, Terzuoli L, Ferretti F. Association between stressful life events and autoimmune diseases: a systematic review and meta-analysis of retrospective case-control studies. Autoimmun Rev. 2016;15(4):325–34.
Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol. 2010;6(5):279.
Lamb MM, Yin X, Zerbe GO, Klingensmith GJ, Dabelea D, Fingerlin TE, Rewers M, Norris JM. Height growth velocity, islet autoimmunity and type 1 diabetes development: the Diabetes Autoimmunity Study in the Young. Diabetologia. 2009;52(10):2064–71.
Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89(6):2526–39.
Peiris H, Bonder CS, Coates PTH, Keating DJ, Jessup CF. The β-cell/EC axis: how do islet cells talk to each other? Diabetes. 2014;63(1):3.
Yurkovetskiy LA, Pickard JM, Chervonsky AV. Microbiota and autoimmunity: exploring new avenues. Cell Host Microbe. 2015;17(5):548–52.
Group, T.S. The environmental determinants of diabetes in the young (TEDDY) Study. Ann N Y Acad Sci. 2008;1150:1–13.
Chakhtoura M, Azar ST. The role of vitamin D deficiency in the incidence, progression, and complications of type 1 diabetes mellitus. Int J Endocrinol. 2013;2013:148673.
Pereira PF, Alfenas R, Araújo RMA. Does breastfeeding influence the risk of developing diabetes mellitus in children? A review of current evidence. J Pediat (English Edition). 2014;90(1):7–15.
Norris JM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298(12):1420–8.
Uusitalo U, et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY Study. JAMA Pediatr. 2016;170(1):20–8.
Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Åkerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(suppl 2):S125.
Infante M, et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients. 2019;11(9):2185.
Article CAS PubMed Central Google Scholar
Stene LC, Gale EAM. The prenatal environment and type 1 diabetes. Diabetologia. 2013;56(9):1888–97.
Oram RA, Patel K, Hill A, Shields B, McDonald TJ, Jones A, Hattersley AT, Weedon MN. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337–44.
Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6(2):122–9.
Thomas NJ, Lynam AL, Hill AV, Weedon MN, Shields BM, Oram RA, McDonald TJ, Hattersley AT, Jones AG. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia. 2019;62(7):1167–72.
Franks PW, Pomares-Millan H. Next-generation epidemiology: the role of high-resolution molecular phenotyping in diabetes research. Diabetologia. 2020;63(12):2521–32.
Elding Larsson H, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15(2):118–26.
McAdams BH, Rizvi AA. An overview of insulin pumps and glucose sensors for the generalist. J Clin Med. 2016;5(1):5.
Article PubMed Central CAS Google Scholar
Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371–8.
Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, Longo M, Giugliano D, Esposito K. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(5):1146.
Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41(8):1579–89.
Sherr JL, Tauschmann M, Battelino T, de Bock M, Forlenza G, Roman R, Hood KK, Maahs DM. ISPAD clinical practice consensus guidelines 2018: diabetes technologies. Pediatr Diabetes. 2018;19:302–25.
DeSalvo DJ, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271–5.
Lal RA, Ekhlaspour L, Hood K, Buckingham B. Realizing a closed-loop (artificial pancreas) system for the treatment of type 1 diabetes. Endocr Rev. 2019;40(6):1521–46.
Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet Med. 2019;36(3):279–86.
Choi SB, Hong ES, Noh YH. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes. 2018;67:964.
Frandsen CS, Dejgaard TF, Madsbad S. Non-insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus. Lancet Diabetes Endocrinol. 2016;4(9):766–80.
Ahrén B, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. 2016;39(10):1693–701.
Article PubMed CAS Google Scholar
Meng H, Zhang A, Liang Y, Hao J, Zhang X, Lu J. Effect of metformin on glycaemic control in patients with type 1 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2018;34(4):e2983.
Hoogwerf BJ, Doshi KB, Diab D. Pramlintide, the synthetic analogue of amylin: physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vasc Health Risk Manag. 2008;4(2):355–62.
Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2017;8(4):727–38.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39.
Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283–212283.
Underland LJ, Ilkowitz JT, Katikaneni R, Dowd A, Heptulla RA. Use of sitagliptin with closed-loop technology to decrease postprandial blood glucose in type 1 diabetes. J Diabetes Sci Technol. 2017;11(3):602–10.
Guo H, Fang C, Huang Y, Pei Y, Chen L, Hu J. The efficacy and safety of DPP4 inhibitors in patients with type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;121:184–91.
Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35(6):992–1019.
Dellepiane S, BenNasr M, Assi E, Usuelli V, Letizia T, Addio F, Zuccotti GV, Fiorina P. Sodium glucose cotransporters inhibitors in type 1 diabetes. Pharmacol Res. 2018;133:1–8.
Whalen K, Miller S, Onge ES. the role of sodium-glucose co-transporter 2 inhibitors in the treatment of type 2 diabetes. Clin Ther. 2015;37(6):1150–66.
Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Therapy. 2014;5(2):355–66.
Tyler NS, et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat Metabol. 2020;2(7):612–9.
Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV. Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8.
Resalat N, El Youssef J, Tyler N, Castle J, Jacobs PG. A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. PLoS ONE. 2019;14(7):e0217301.
Cover T, Hart p. Nearest Neighbor Pattern Classification (1967) journal= The Edison Foundation Institute for Electric Efficiency. The Edison Foundation Institute for Electric Efficiency, p. 21–27.
Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: As good as it gets? Nat Med. 1999;5(6):601–4.
Kaufmann KB, Büning H, Galy A, Schambach A, Grez M. Gene therapy on the move. EMBO Mol Med. 2013;5(11):1642–61.
Chellappan DK, et al. Gene therapy and type 1 diabetes mellitus. Biomed Pharmacother. 2018;108:1188–200.
Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Exp Anim. 1980;29(1):1–13.
Makino S, Muraoka Y, Kishimoto Y, Hayashi Y. Genetic analysis for insulitis in NOD mice. Exp Anim. 1985;34(4):425–31.
Group, E.A.S. Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355(9207):873–6.
Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harbor perspectives in medicine. 2012;2(11):7641.
Melanitou E, Devendra D, Liu E, Miao D, Eisenbarth GS. Early and quantal (by litter) expression of insulin autoantibodies in the nonobese diabetic mice predict early diabetes onset. J Immunol. 2004;173(11):6603–10.
You S, et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54(5):1415–22.
Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171(8):4040–7.
DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor α chain gene rearrangement. Proc Natl Acad Sci. 1998;95(21):12538–43.
Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–31.
Leete P, Willcox A, Krogvold L, Dahl-Jørgensen K, Foulis AK, Richardson SJ, Morgan NG. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65(5):1362–9.
Mallol C, et al. AAV-mediated pancreatic overexpression of Igf1 counteracts progression to autoimmune diabetes in mice. Mol Metab. 2017;6(7):664–80.
Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7(1):31–4.
Parikh A, Stephan A-F, Tzanakakis ES. Regenerating proteins and their expression, regulation, and signaling. Biomol Concepts. 2012;3(1):57–70.
Chen R, Meseck ML, Woo SL. Auto-regulated hepatic insulin gene expression in type 1 diabetic rats. Mol Ther. 2001;3(4):584–90.
Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science. 2000;290(5498):1959–62.
Jaen ML, et al. Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol Ther Methods Clin Dev. 2017;6:1–7.
Ramshur EB, Rull TR, Wice BM. Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function in vivo. J Cell Physiol. 2002;192(3):339–50.
Ren B, O’Brien BA, Swan MA, Koina ME, Nassif N, Wei MQ, Simpson AM. Long-term correction of diabetes in rats after lentiviral hepatic insulin gene therapy. Diabetologia. 2007;50(9):1910–20.
Touchefeu Y, Harrington KJ, Galmiche JP, Vassaux G. Review article: gene therapy, recent developments and future prospects in gastrointestinal oncology. Aliment Pharmacol Ther. 2010;32(8):953–68.
Gan SU, Fu Z, Sia KC, Kon OL, Calne R, Lee KO. Development of a liver-specific Tet-off AAV8 vector for improved safety of insulin gene therapy for diabetes. J Gene Med. 2019;21(1):3067.
Lin Y, Sun Z. Antiaging Gene klotho attenuates pancreatic β-Cell apoptosis in type 1 diabetes. Diabetes. 2015;64(12):4298–311.
Lim K, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100(10):E1308–18.
Prud’homme GJ, Glinka Y, Kurt M, Liu W, Wang Q. The anti-aging protein Klotho is induced by GABA therapy and exerts protective and stimulatory effects on pancreatic beta cells. Biochem Biophys Res Commun. 2017;493(4):1542–7.
Flotyńska J, Uruska A, Araszkiewicz A, Zozulińska-Ziółkiewicz D. Klotho protein function among patients with type 1 diabetes. Endokrynol Pol. 2018;69(6):696–704.
Berezin AE, Berezin AA. Impaired function of fibroblast growth factor 23 / Klotho protein axis in prediabetes and diabetes mellitus: promising predictor of cardiovascular risk. Diabetes Metabol Syndr. 2019;13(4):2549–56.
Xie A, et al. Anti-TCRbeta mAb in combination with neurogenin3 gene therapy reverses established overt type 1 diabetes in female NOD mice. Endocrinology. 2017;158(10):3140–51.
Cox AR, et al. Resolving discrepant findings on ANGPTL8 in β-cell proliferation: a collaborative approach to resolving the betatrophin controversy. PLoS ONE. 2016;11(7):159276.
Yi P, Park J-S, Melton DA. Retraction notice to: betatrophin: A hormone that controls pancreatic β cell proliferation. Cell. 2017;168(1–2):326.
Gusarova V, et al. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell. 2014;159(3):691–6.
Cox AR, Lam CJ, Bonnyman CW, Chavez J, Rios JS, Kushner JA. Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase beta cell proliferation in mice. Diabetologia. 2015;58(7):1523–31.
Chen J, Chen S, Huang P, Meng X-L, Clayton S, Shen J-S, Grayburn PA. In vivo targeted delivery of ANGPTL8 gene for beta cell regeneration in rats. Diabetologia. 2015;58(5):1036–44.
Chen S, Shimoda M, Wang M-Y, Ding J, Noguchi H, Matsumoto S, Grayburn PA. Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene Ther. 2010;17(11):1411–20.
Chen Y, et al. CTLA-4 +49 G/A, a functional T1D risk SNP, affects CTLA-4 level in Treg subsets and IA-2A positivity, but not beta-cell function. Sci Rep. 2018;8(1):10074.
Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–11.
Mourich DV, Oda SK, Schnell FJ, Crumley SL, Hauck LL, Moentenich CA, Marshall NB, Hinrichs DJ, Iversen PL. Alternative splice forms of CTLA-4 induced by antisense mediated splice-switching influences autoimmune diabetes susceptibility in NOD mice. Nucleic Acid Ther. 2014;24(2):114–26.
Shahryari A, Saghaeian Jazi M, Mohammadi S, Razavi Nikoo H, Nazari Z, Hosseini ES, Burtscher I, Mowla SJ, Lickert H. Development and clinical translation of approved gene therapy products for genetic disorders. Front Genetics. 2019;10:868.
Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82–83:1–11.
Lee JB, Lee JE, Park JH, Kim SJ, Kim MK, Roh SI, Yoon HS. Establishment and maintenance of human embryonic stem cell lines on human feeder cells derived from uterine endometrium under serum-free condition1. Biol Reprod. 2005;72(1):42–9.
Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121.
Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1(2):495–507.
Cai J, Yu C, Liu Y, Chen S, Guo Y, Yong J, Lu W, Ding M, Deng H. Generation of homogeneous PDX1(+) pancreatic progenitors from human ES cell-derived endoderm cells. J Mol Cell Biol. 2010;2(1):50–60.
Sui L, et al. beta-Cell replacement in mice using human type 1 diabetes nuclear transfer embryonic stem cells. Diabetes. 2018;67(1):26–35.
Qadir MMF, et al. A double fail-safe approach to prevent tumorigenesis and select pancreatic β cells from human embryonic stem cells. Stem Cell Reports. 2019;12(3):611–23.
Kotini AG, de Stanchina E, Themeli M, Sadelain M, Papapetrou EP. Escape mutations, ganciclovir resistance, and teratoma formation in human iPSCs expressing an HSVtk suicide gene. Molecular Therapy. 2016;5:284.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):7–1920.
Millman JR, Pagliuca FW. Autologous pluripotent stem cell-derived β-like cells for diabetes cellular therapy. Diabetes. 2017;66(5):1111.
Velazco-Cruz L, Song J, Maxwell KG, Goedegebuure MM, Augsornworawat P, Hogrebe NJ, Millman JR. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Reports. 2019;12(2):351–65.
Voltarelli JC, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297(14):1568–76.
Couri CE, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301(15):1573–9.
Penaforte-Saboia JG, et al. Microvascular complications in type 1 diabetes: a comparative analysis of patients treated with autologous nonmyeloablative hematopoietic stem-cell transplantation and conventional medical therapy. Front Endocrinol (Lausanne). 2017;8:331.
Bhatwadekar AD, et al. Hematopoietic stem/progenitor involvement in retinal microvascular repair during diabetes: Implications for bone marrow rejuvenation. Vision Res. 2017;139:211–20.
Ye L, Li L, Wan B, Yang M, Hong J, Gu W, Wang W, Ning G. Immune response after autologous hematopoietic stem cell transplantation in type 1 diabetes mellitus. Stem Cell Res Therapy. 2017;8(1):90.
Xiang H, et al. Residual β-cell function predicts clinical response after autologous hematopoietic stem cell transplantation. Stem Cells Transl Med. 2016;5(5):651–7.
Snarski E, et al. Immunoablation and autologous hematopoietic stem cell transplantation in the treatment of new-onset type 1 diabetes mellitus: long-term observations. Bone Marrow Transplant. 2016;51(3):398–402.
Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res. 2012;71(2):439–44.
Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Investig. 2003;111(6):843–50.
Xie Q-P, Huang H, Xu B, Dong X, Gao S-L, Zhang B, Wu Y-L. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation. 2009;77(5):483–91.
Milanesi A, Lee J-W, Li Z, Da Sacco S, Villani V, Cervantes V, Perin L, Yu JS. β-Cell regeneration mediated by human bone marrow mesenchymal stem cells. PLoS ONE. 2012;7(8):42177.
Ghodsi M, Heshmat R, Amoli M, Keshtkar AA, Arjmand B, Aghayan H, Hosseini P, Sharifi AM, Larijani B. The effect of fetal liver-derived cell suspension allotransplantation on patients with diabetes: first year of follow-up. Acta Med Iran. 2012;50(8):541–6.
PubMed Google Scholar
Zhang J, Mao R, Wang X, Liu K, Geng Q, Yu Y, Li Y, Qi J. Targeted induction of bone marrow mesenchymal stem cells to have effectiveness on diabetic pancreatic restoration. Vitro Cell Dev Biol. 2019;55(6):453–61.
Qu-Petersen Z, et al. Identification of a novel population of muscle stem cells in mice. Potent Muscle Regener. 2002;157(5):851–64.
CAS Google Scholar
Lan KC, Wang CC, Yen YP, Yang RS, Liu SH, Chan DC. Islet-like clusters derived from skeletal muscle-derived stem/progenitor cells for autologous transplantation to control type 1 diabetes in mice. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S328-s335.
Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63(11):1886–92.
Amer MG, Embaby AS, Karam RA, Amer MG. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene. 2018;654:87–94.
Ikemoto T, Feng R, Iwahashi SI, Yamada S, Saito Y, Morine Y, Imura S, Matsuhisa M, Shimada M. In vitro and in vivo effects of insulin-producing cells generated by xeno-antigen free 3D culture with RCP piece. Sci Rep. 2019;9(1):10759.
Fang Q, Zhai M, Wu S, Hu X, Hua Z, Sun H, Guo J, Zhang W, Wang Z. Adipocyte-derived stem cell-based gene therapy upon adipogenic differentiation on microcarriers attenuates type 1 diabetes in mice. Stem Cell Res Ther. 2019;10(1):36.
Dessels C, Alessandrini M, Pepper MS. Factors influencing the umbilical cord blood stem cell industry: an evolving treatment landscape. Stem Cells Transl Med. 2018;7(9):643–50.
Kim Y-J, Broxmeyer HE. Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance. Crit Rev Oncol Hematol. 2011;79(2):112–26.
Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:6901286.
Prabakar KR, Domínguez-Bendala J, Molano RD, Pileggi A, Villate S, Ricordi C, Inverardi L. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell Transplant. 2012;21(6):1321–39.
Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS ONE. 2009;4(1):4226.
Zhao Y, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10(1):1–11.
Cai J, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39(1):149–57.
Haller MJ, Viener H-L, Wasserfall C, Brusko T, Atkinson MA, Schatz DA. Autologous umbilical cord blood infusion for type 1 diabetes. Exp Hematol. 2008;36(6):710–5.
Haller MJ, et al. Autologous umbilical cord blood infusion followed by oral docosahexaenoic acid and vitamin D supplementation for C-peptide preservation in children with Type 1 diabetes. Biol Blood Marrow Transplant. 2013;19(7):1126–9.
Giannopoulou EZ, et al. Effect of a single autologous cord blood infusion on beta-cell and immune function in children with new onset type 1 diabetes: a non-randomized, controlled trial. Pediatr Diabetes. 2014;15(2):100–9.
Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181(10):7350–5.
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem cells. 2004;22(7):1330–7.
Kalaszczynska I, Ferdyn K. Wharton’s Jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. BioMed Res Int. 2015;2015:430847.
Hu J, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocrine J. 2012;5:89.
Li L, Lu J, Shen S, Jia X, Zhu D. Wharton’s jelly-derived mesenchymal stem cell therapy to improve β-cell function in patients with type 1 diabetes and ketoacidosis: a single-centre, single-group, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2016;4:S17.
Hong-Wu Wang PN. Han-Hua Yang, Li-Chun Xie, Li-Min Lin, Xiu-Lan Lai, Tian-You Wang, Lian Ma, Partially repair damaged Islets of diabetic rat model via insulin-producing cells differentiated from human umbilical cord mesenchymal stem cells infusion. Int J Clin Exp Med. 2018;11(5):4520–9.
Tsai P-J, et al. Undifferentiated Wharton’s jelly mesenchymal stem cell transplantation induces insulin-producing cell differentiation and suppression of T-cell-mediated autoimmunity in nonobese diabetic mice. Cell Transplant. 2015;24(8):1555–70.
Carlsson P-O, Svahn M. Wharton’s jelly derived allogeneic mesenchymal stromal cells for treatment of type 1 diabetes: Study protocol for a double-blinded, randomized, parallel, placebo-controlled trial. Clin Trials Degener Dis. 2018;3(2):32–7.
El-Demerdash RF, Hammad LN, Kamal MM, El Mesallamy HO. A comparison of Wharton’s jelly and cord blood as a source of mesenchymal stem cells for diabetes cell therapy. Regen Med. 2015;10(7):841–55.
Som C, Venkataramana NK. Evaluation of efficacy and regenerative potential of Wharton’s jelly and bone marrow derived mesenchymal stem cells in diabetic rats. J Pre-Clin Clin Res. 2018;12(1):30–5.
Gray A, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK279012/ .
Scott SN, Anderson L, Morton JP, Wagenmakers AJM, Riddell MC. Carbohydrate restriction in type 1 diabetes: a realistic therapy for improved glycaemic control and athletic performance? Nutrients. 2019;11(5):1022.
Laurenzi A, et al. Effects of carbohydrate counting on glucose control and quality of life over 24 weeks in adult patients with type 1 diabetes on continuous subcutaneous insulin infusion: a randomized, prospective clinical trial (GIOCAR). Diabetes Care. 2011;34(4):823–7.
Evert AB, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731.
Rossi MC, et al. Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes Care. 2010;33(1):109–15.
Kattelmann KK, Conti K, Ren C. The medicine wheel nutrition intervention: a diabetes education study with the Cheyenne River Sioux Tribe. J Am Diet Assoc. 2010;110(5):S44–51.
Cavanaugh K, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148(10):737–46.
Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087–97.
Quesada I, Tudurí E, Ripoll C, Nadal A. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199(1):5–19.
Wieczorek G, Pospischil A, Perentes E. A comparative immunohistochemical study of pancreatic islets inlaboratory animals (rats, dogs, minipigs, nonhuman primates). Exp Toxicol Pathol. 1998;50(3):151–72.
Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci. 2006;103(7):2334–9.
Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–45.
Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15(1):620.
Chen Y-G, Mathews CE, Driver JP. The role of NOD mice in type 1 diabetes research: lessons from the past and recommendations for the future. Front Endocrinol. 2018;9:51.
Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017;60(8):1370–81.
Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Prim. 2017;3(1):1–17.
Shanks N, Greek R, Greek J. Are animal models predictive for humans? PEHM. 2009;4:2–2.
PubMed PubMed Central Google Scholar
Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult β-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology. 2009;150(4):1627–35.
Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia. 2006;49(8):1838–44.
Sims EK, et al. Proinsulin Secretion Is a Persistent Feature of Type 1 Diabetes. Diabetes Care. 2019;42(2):258.
Wasserfall C, et al. Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab. 2017;26(3):568-575.e3.
Rehan M. Epigenetics and diabetes mellitus. Egyp J Int Med. 2016;28(2):39–51.
Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, Liu ZM. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract. 2011;91(1):94–100.
Garcia-Contreras M, Shah SH, Tamayo A, Robbins PD, Golberg RB, Mendez AJ, Ricordi C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep. 2017;7(1):5998.
Jerram ST, Dang MN, Leslie RD. The role of epigenetics in type 1 diabetes. Curr DiabRep. 2017;17(10):89–89.
Chakravarthy H, et al. Converting adult pancreatic islet alpha cells into beta cells by targeting Both Dnmt1 and Arx. Cell Metab. 2017;25(3):622–34.
Fontcuberta-PiSunyer M, Cervantes S, Miquel E, Mora-Castilla S, Laurent LC, Raya A, Gomis R, Gasa R. Modulation of the endocrine transcriptional program by targeting histone modifiers of the H3K27me3 mark. Biochim Biophys Acta Gene Regul Mech. 2018;1861(5):473–80.
Akil A-SA-S, et al. Reading between the (Genetic) lines: How epigenetics is unlocking novel therapies for type 1 diabetes. Cells 2020;9(11):2403.
Beger RD, et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective.” Metabolomics. 2016;12(9):149.
Cooper-Dehoff RM, et al. Is diabetes mellitus-linked amino acid signature associated with beta-blocker-induced impaired fasting glucose? Circ Cardiovasc Genet. 2014;7(2):199–205.
Jobin C. Precision medicine using microbiota. Science. 2018;359(6371):32–4.
Bart O. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol. 2019;7(1):65–74.
Download references
Acknowledgements
We wish to thank Lucy Robinson of Insight Editing London for assistance with editing support and critical reading of the manuscript prior to submission.
This research was funded by Sidra Medicine through its Precision Medicine Program Grant—SDR#400149, Doha, Qatar.
Author information
Authors and affiliations.
Department of Human Genetics-Precision Medicine Program, Sidra Medicine, P.O. Box 26999, Doha, Qatar
Ammira Al-Shabeeb Akil, Esraa Yassin, Aljazi Al-Maraghi, Elbay Aliyev, Khulod Al-Malki & Khalid A. Fakhro
Department of Genetic Medicine, Weill Cornell Medicine, P.O. Box 24144, Doha, Qatar
Khalid A. Fakhro
College of Health and Life Sciences, Hamad Bin Khalifa University, P.O. Box 34110, Doha, Qatar
You can also search for this author in PubMed Google Scholar
Contributions
Original draft preparation, ASA; review and editing, AAM; KAM; EY, EA and KF; visualization, ASA; supervision, ASA; KF; project administration, ASA; funding acquisition, ASA. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ammira Al-Shabeeb Akil .
Ethics declarations
Ethics approval and consent to participate.
Not applicable
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Akil, A.AS., Yassin, E., Al-Maraghi, A. et al. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med 19 , 137 (2021). https://doi.org/10.1186/s12967-021-02778-6
Download citation
Received : 18 December 2020
Accepted : 08 March 2021
Published : 01 April 2021
DOI : https://doi.org/10.1186/s12967-021-02778-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 1 diabetes
- Autoimmunity
- Personalized medicine
- Personalized treatment
- Genomic Risk Score
- Insulin therapy
- Gene polymorphism
- Pancreatic β cells
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Endocrine Diseases
Hyperthyroidism (Overactive Thyroid)
- Español
On this page:
What is hyperthyroidism?
How common is hyperthyroidism, who is more likely to develop hyperthyroidism, is hyperthyroidism during pregnancy a problem, what are the complications of hyperthyroidism, what are the symptoms of hyperthyroidism, what causes hyperthyroidism, how do doctors diagnose hyperthyroidism, how do doctors treat hyperthyroidism, how does eating, diet, and nutrition affect hyperthyroidism, clinical trials for hyperthyroidism.
Hyperthyroidism, also called overactive thyroid, is when the thyroid gland makes more thyroid hormones than your body needs. The thyroid is a small, butterfly-shaped gland in the front of your neck. Thyroid hormones control the way the body uses energy, so they affect nearly every organ in your body, even the way your heart beats. With too much thyroid hormone, many of your body’s functions speed up.

About 1 out of 100 Americans ages 12 years and older have hyperthyroidism. 1
Hyperthyroidism is more common in women and people older than 60. 2 You are more likely to have hyperthyroidism if you
- have a family history of thyroid disease
- pernicious anemia , a condition caused by a vitamin B12 deficiency
- type 1 or type 2 diabetes
- primary adrenal insufficiency , a disorder of hormones
- eat large amounts of food containing iodine , such as kelp
- use medicines that contain iodine
- use nicotine products 3
- were pregnant within the past 6 months
Mild hyperthyroidism during pregnancy is usually not a problem. But severe hyperthyroidism during pregnancy , when untreated, can affect both the mother and the baby. If you have hyperthyroidism and plan to get pregnant or become pregnant, work with your doctor to get the disease under control.

Untreated, hyperthyroidism can cause serious health problems, including
- an irregular heartbeat that can lead to blood clots, stroke , heart failure , and other heart-related problems
- an eye disease called Graves’ ophthalmopathy
- thinning bones, osteoporosis , and muscle problems
- menstrual cycle and fertility issues
Symptoms of hyperthyroidism can vary from person to person and may include 4
- weight loss despite an increased appetite
- rapid or irregular heartbeat
- nervousness, irritability, trouble sleeping, fatigue
- shaky hands, muscle weakness
- sweating or trouble tolerating heat
- frequent bowel movements
- an enlargement in the neck, called a goiter
In older adults, hyperthyroidism is sometimes mistaken for depression or dementia . Older adults may have different symptoms, such as loss of appetite or withdrawal from people, than younger adults with hyperthyroidism. You may want to ask your doctor about hyperthyroidism if you or your loved one shows these symptoms.
Hyperthyroidism has several causes, including
Graves’ disease
- overactive thyroid nodules
- inflammation of the thyroid gland, called thyroiditis
- too much iodine
- too much thyroid hormone medicine
- a noncancerous tumor of the pituitary gland
Graves’ disease, the most common cause of hyperthyroidism, is an autoimmune disorder . With this disease, your immune system attacks the thyroid and causes it to make too much thyroid hormone.
Overactive thyroid nodules
Overactive thyroid nodules, or lumps in your thyroid, are common and usually not cancerous. However, one or more nodules may become overactive and produce too much thyroid hormone. Overactive nodules are found most often in older adults.
Thyroiditis
Thyroiditis is inflammation of your thyroid gland. Some types of thyroiditis can cause thyroid hormone to leak out of your thyroid gland into your bloodstream. As a result, you may develop symptoms of hyperthyroidism.
The types of thyroiditis that can cause hyperthyroidism include
- subacute thyroiditis, which involves a painfully inflamed and enlarged thyroid.
- postpartum thyroiditis , which can develop after a woman gives birth.
- painless thyroiditis, which is similar to postpartum thyroiditis, but occurs in the absence of pregnancy. Your thyroid may be enlarged. Experts think painless thyroiditis is probably an autoimmune condition.
Thyroiditis can also cause symptoms of hypothyroidism , or underactive thyroid. In some cases, after your thyroid is overactive for a period of time, it may become underactive.
Too much iodine
Your thyroid uses iodine to make thyroid hormone . How much iodine you consume affects how much thyroid hormone your thyroid makes. In some people, consuming large amounts of iodine may cause the thyroid to make too much thyroid hormone.
Some cough syrups and medicines, including some heart medicines, may contain a lot of iodine. Seaweed and seaweed-based supplements also contain a lot of iodine.
Too much thyroid hormone medicine
Some people who take thyroid hormone medicine for hypothyroidism may take too much. If you take thyroid hormone medicine, see your doctor at least once a year to have your thyroid hormone levels checked . You may need to adjust your dose if your doctor finds your thyroid hormone level is too high.
Some other medicines may also interact with thyroid hormone medicine and raise hormone levels. If you take thyroid hormone medicine, ask your doctor about interactions when starting new medicines.
Noncancerous tumor
In some rare cases, a noncancerous tumor of the pituitary gland , located at the base of the brain, can cause hyperthyroidism.

Your doctor will take a medical history and perform a physical exam. A hyperthyroidism diagnosis can’t be based on symptoms alone because many of its symptoms are the same as those of other diseases. That’s why your doctor may use several thyroid blood tests and imaging tests to confirm the diagnosis and find its cause.
Because hyperthyroidism can cause fertility problems, women who have trouble getting pregnant often get tested for thyroid problems.
Your doctor will treat your hyperthyroidism to bring your thyroid hormone levels back to normal. Treating the disease will prevent long-term health problems, and it will relieve uncomfortable symptoms. No single treatment works for everyone.
Your treatment depends on what’s causing your hyperthyroidism and how severe it is. When recommending a treatment, your doctor will consider
- possible allergies to or side effects of the medicines
- other conditions, such as pregnancy or heart disease
- whether you have access to an experienced thyroid surgeon
Treatment options
Hyperthyroidism is usually treated with medicines, radioiodine therapy, or thyroid surgery.
Beta-blockers
Beta-blockers are drugs that block the action of substances, such as adrenaline, on nerve cells. They cause blood vessels to relax and widen.
- They can reduce symptoms—such as tremors, rapid heartbeat, and nervousness—until other treatments start working.
- They can make you feel better within hours.
- They don’t stop thyroid hormone production.
Antithyroid medicines
Antithyroid therapy is the simplest way to treat hyperthyroidism. Methimazole is used most often. Propylthiouracil is often used for women during the first 3 months of pregnancy because methimazole can, on rare occasions, harm the fetus.
- They cause the thyroid to make less thyroid hormone.
- Some patients’ symptoms from Graves’ disease may go away temporarily after taking antithyroid drugs.
- allergic reactions, such as rashes and itching
- a decrease in the number of white blood cells in your body, which can lower resistance to infection
- liver failure , in rare cases
- don’t provide a permanent cure, but they may allow symptoms to go away temporarily in the case of Graves’ disease
- may take several weeks or months for thyroid hormone levels to move into the normal range
- take about 1–2 years of total average treatment time, but can continue for many years
- are not used to treat hyperthyroidism caused by thyroiditis
Seek care right away

Call your doctor right away if you have any of the following symptoms
- fatigue or weakness
- dull pain in your abdomen
- loss of appetite
- skin rash, itching, or easy bruising
- yellowing of your skin or whites of your eyes, called jaundice
- constant sore throat
- fever, chills, or constant sore throat
Radioiodine therapy
Radioiodine therapy is a common and effective treatment. You can take radioactive iodine-131 by mouth as a capsule or liquid.

- Radioiodine therapy slowly destroys the cells of the thyroid gland that produce thyroid hormone.
- Radioiodine therapy does not affect other body tissues.
- You might need more than one treatment to bring thyroid hormone levels into the normal range, but beta-blockers can control symptoms between treatments.
- Radioiodine therapy isn’t used for women who are pregnant or breastfeeding. It can harm the fetus’ thyroid and can be passed from mother to child in breast milk.
Almost everyone who gets radioiodine therapy later develops hypothyroidism. But hypothyroidism is easier to treat than hyperthyroidism by using a daily thyroid hormone medicine, and it causes fewer long-term health problems.
Thyroid surgery
Surgery to remove part or most of the thyroid gland is used less often to treat hyperthyroidism. Sometimes doctors use surgery to treat people with large goiters or pregnant women who cannot take antithyroid medicines.

- When part of the thyroid is removed, your thyroid hormone levels may return to normal.
- Thyroid surgery requires general anesthesia , which can cause a condition called thyroid storm—a sudden, severe worsening of symptoms. Taking antithyroid medicines before surgery can help prevent this problem.
When part of your thyroid is removed, you may develop hypothyroidism after surgery and need to take thyroid hormone medicine. If your whole thyroid is removed, you will need to take thyroid hormone medicine for life. After surgery, your doctor will continue to check your thyroid hormone levels.
Researchers are looking into new ways to treat hyperthyroidism. An example is radiofrequency ablation (RFA) , a new approach to treating thyroid nodules that cause hyperthyroidism. 5,6 RFA is used mainly in cases where medicines or surgery won’t help, and is not yet widely available.
Your thyroid uses iodine to make thyroid hormones. If you have Graves’ disease or another autoimmune thyroid disorder, you may be sensitive to harmful side effects from iodine. Eating foods that have large amounts of iodine—such as kelp, dulse, or other kinds of seaweed—may cause or worsen hyperthyroidism. Taking iodine supplements can have the same effect. Talk with members of your health care team about
- what foods to limit or avoid
- any iodine supplements you take
- any cough syrups or multivitamins you take because they may contain iodine
The NIDDK conducts and supports clinical trials in many diseases and conditions, including endocrine diseases. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for hyperthyroidism?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help doctors and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of hyperthyroidism, such as its natural history, clinical presentation, and genetics.
Find out if clinical studies are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical studies for hyperthyroidism are looking for participants?
You can view a filtered list of clinical studies on hyperthyroidism that are open and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the NIH does not review these studies and cannot ensure they are safe. Always talk with your health care provider before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank: Thanh D. Hoang, D.O., FACP, FACE, CAPTAIN (select), Walter Reed National Military Medical Center

Popular Keywords

Proactive Health Risk Management: A Success Story from the Egyptian Energy Sector
- Case Studies
- Current Page
In 2022, International SOS embarked on a strategic collaboration with a leading energy contractor in Egypt. The contractor was constructing a large-scale project in a remote location. The partnership aimed to provide the contractor's 6,000-strong workforce with comprehensive health and wellbeing services . This included medical staffing, an on-site clinic, multiple first aid stations, and ambulance services.
Shortly after the mobilisation of our medical professionals , a critical incident occurred. An employee on the site collapsed and lost consciousness. His colleagues, who lacked first aid skills, attempted interventions that could have been harmful. Fortunately, they also promptly alerted our on-site medical staff for support.
After our on-site medical team swiftly intervened, it was quickly determined that the individual was suffering from hypoglycemia, a condition associated with diabetes. The team administered necessary first aid, including vital signs assessment, IV cannula insertion, and the provision of 10% glucose fluid.
Following this incident, our medical staff collaborated with the organisation's HSE management team. Together, they launched a diabetes awareness and prevention campaign , introduced pre-employment fitness assessments , and initiated first aid training for the site workforce.
The diabetes awareness campaign identified six workforce members with previously undiagnosed conditions . Introducing pre-employment fitness assessments enhanced the safety measures during workforce deployment on the site. Furthermore, over 800 workforce members received training in essential first aid skills .

IMAGES
VIDEO
COMMENTS
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes. Case Presentation A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes.
CASE. A 47-year-old woman was found to have hyperglycemia at a health fair when a random blood glucose level was 227 mg/dL (12.6 mmol/L). Several days later, a fasting blood glucose value was 147 mg/dL (8.2 mmol/L). She has no previous history of diabetes, is alarmed by the possibility of having this disorder, and seeks your advice.
PRESENTATION OF CASE. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia.. Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit.
12 Jul 2016. Page: 29. This case study demonstrates the physical and psychological difficulties faced by many young people with type 1 diabetes. Over the year following her diagnosis, Max had a deterioration in glycaemic control despite reporting that little had changed in her management. Detailed assessment revealed a number of psychosocial ...
This article examines the aetiology, pathophysiology, diagnosis and treatment of type 2 diabetes using a case study approach. The psychosocial implications for the patient are also discussed. The case study is based on a patient with diabetes who was admitted to hospital following a hypoglycaemic episode and cared for during a practice placement.
SUMMARY: Further assessment of this case study shows that the patient has poorly controlled hypertension, despite multiple medications. The patient also has metabolic syndrome complicated by diabetes, microalbuminuria and peripheral arterial disease. The patient's hypertensive treatment options must be evaluated in light of the fact that ...
The review of systems is unremarkable. Her medical history is significant for: Type 2 diabetes, diagnosed 6 months ago when she presented with polyuria, blurry vision, and a random glucose level ...
However, a few studies have demonstrated that drug-free glycemic control can be achieved in type 2 diabetes for 12 months on average after a 2-week continuous insulin infusion ( 2 - 4 ). Here, we describe an unusual case of a 26-month drug holiday induced with outpatient basal insulin in a patient newly diagnosed with type 2 diabetes.
Presentation of Case. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood ...
Case Study: Hyperglycemia, concern for diabetic ketoacidosis, and type 1 diabetes History of present illness The patient is a 36-year-old man who has had type 1 diabetes for 15 years.
Cardiovascular risk reduction in type 2 diabetes. Pharmacological management of comorbid cardiovascular risk and type 2 diabetes is undergoing a paradigm shift. General practitioner Kevin Fernando puts recommendations from the ADA/EASD into practice in this patient case study. 09-05-2018 | Diagnosis | Case study | Article.
Case Studies /. Hyperglycemia in a 56-Year-Old Woman. Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge.
Diabetes education carried out by nurses has moved beyond the hospital bedside into a variety of health care settings. Among the disciplines involved in diabetes education, nursing has played a pivotal role in the diabetes team management concept. ... {Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose ...
Yazzie's mother had type 2 diabetes mellitus and well-controlled hypertension. Ms. Yazzie's sister died at age 64, the specific cause is unknown. She had been diagnosed with obesity, hypertension, diabetes, and suspected coronary artery disease. Ms. Yazzie's other sister is alive at age 59. She was diagnosed with obesity at age 37 and ...
Studies show the incretin glucagon-like peptide 1, (GLP-1) depicts a decrease in beta-cell responsiveness in type 2 diabetes (McCance & Huether, 2019). Due to hyperglycemia and the current lack of insulin polyphagia, polydipsia and polyuria are classic signs that appear while recurrent infections and visual changes occur later on.
7. Page 1 of 7. Health-science document from Pensacola State College, 7 pages, Case Study - Diabetes and Cardiovascular Disease (100 points) Name _Haley McGinnas_ When you finish this case study you should be able to: 1. Compare the similarities and differences in risk factors for the development of diabetes and cardiovascular disea.
Objectives Diabetic ketoacidosis (DKA) is a serious complication in patients treated with sodium-glucose co-transporter 2 inhibitors (SGLT2i). The aim of this study was to investigate the relationship between SGLT2i and the risk of DKA, and to identify high-risk groups and characteristics that should be emphasised. Methods A retrospective case series study was conducted to collect medical ...
Diabetes is a condition that happens when your blood sugar is too high. It develops when your pancreas doesn't make any insulin, or your body isn't using it properly. ... Several studies have shown that untreated chronic high blood sugar shortens your lifespan and worsens your quality of life. In the United States, diabetes is the eighth ...
In the case of type 1 diabetes, great efforts are now being focused on risk stratification for diabetes development to enable pre-clinical detection, and the application of treatments such as gene therapy, to prevent pancreatic destruction in a sub-set of patients. ... In TrialNet natural history study, diabetes- related autoantibodies were ...
rapid or irregular heartbeat. nervousness, irritability, trouble sleeping, fatigue. shaky hands, muscle weakness. sweating or trouble tolerating heat. frequent bowel movements. an enlargement in the neck, called a goiter. In older adults, hyperthyroidism is sometimes mistaken for depression or dementia.
Discover how proactive health risk management in the Egyptian energy sector led to improved workforce health and safety, business continuity, and cost savings. Learn about the comprehensive health and wellbeing services provided by International SOS, including a diabetes awareness campaign, pre-employment fitness assessments, and first aid training