Advertisement

Zirconia: A Unique Multifunctional Ceramic Material
- Technical Paper
- Published: 11 June 2019
- Volume 72 , pages 1981–1998, ( 2019 )
Cite this article

- Pradyut Sengupta 1 ,
- Arjak Bhattacharjee 2 nAff3 &
- Himadri Sekhar Maiti 2
962 Accesses
18 Citations
3 Altmetric
Explore all metrics
Zirconia ceramics possess the unique combination of multi-functionality. There have been a number of landmark discoveries in the areas of its mechanical, electrical and thermal properties. The material is known to have a number of polymorphs like monoclinic at room temperature, tetragonal normally above around 1170 °C and cubic above around 2300 °C. The high-temperature cubic phase can be stabilized at room temperature by forming a solid solution with di-, tri- or tetravalent metal oxides. Its fracture toughness can be enhanced significantly by taking advantage of the stress-induced polymorphic transformation. Partially stabilized zirconia is known to have the highest toughness among all the monolithic ceramic materials. The addition of zirconia to other ceramics like alumina also toughens the ceramic composite. Stabilization of zirconia to its cubic form generates very large amount of oxygen ion vacancy, thereby enhancing the oxygen diffusivity and oxygen ion conductivity, which makes this material one of the most important solid-state high-temperature electrolytes suitable for electrochemical devices like solid oxide fuel cell, oxygen sensor and oxygen pump. Low thermal conductivity of the material makes it useful as thermal barrier coating for gas turbine blades in order to increase the operating temperature and therefore the efficiency of the turbines. High mechanical strength and high toughness together with excellent resistant to toxicity have made it emerge as a new material for biomedical prosthesis, particularly dental implants. Reinforcing with carbon nano-tube or graphene oxide enhances the mechanical properties and changes the nature of electrical conduction significantly.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
[Reproduced with permission of the publisher of Ref. 23 ]
[Reproduced with permission of the publisher of Ref. 34 ]
[Reproduced with permission of the publisher of Ref. [ 62 ]

[Reproduced with permission of the publisher of Ref. 62 ]

[Reproduced with permission of the publisher of Ref. 87 ]
[Reproduced with permission of the publisher of Ref. 105 ]
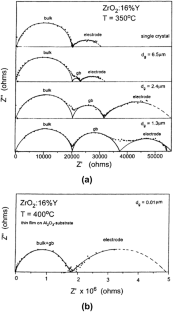
[Reproduced with permission of the publisher of Ref. 113 ]

Similar content being viewed by others
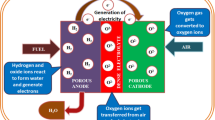
Review of solid oxide fuel cell materials: cathode, anode, and electrolyte
A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications

Hardening and Strengthening Effects Induced by Incorporation of Titanium in Hexagonal Boron Nitride Ceramics
Choudhary C B, Maiti H S, and Subbarao E C, in Solid Electrolytes and Their Applications , (ed) Subbarao E C, Plenum Press, New York (1980), p 1.
Subbarao E C, and Maiti H S, Solid State Ion 11 (1984) 317.
Google Scholar
Subbarao E C, Maiti H S, and Srivastava K K, Phys Stat Solidi 21 (1974) 9.
Garvie R, Hannink R H, and Pascoe R T, Nature 258 (1975) 703.
Cao X Q, Vassenb R, and Stoever D, J Eur Ceram Soc 24 (2004) 1.
Srikanth V, Subbarao E C, Agrawal D K, Huang C Y, and Roy R, J Am Ceram Soc 74 (1991) 365.
Wagner C, Naturwissenschaften , 31 (1943) 265.
Kiukola K, and Wagner C, J Electrochem Soc 104 (1957) 379.
Kingery W D Jr, Pappis J, Doty M E, and Hill D C, J Am Ceram Soc 42 (1959) 393.
Heuer A H, and Hobbs LW, (eds) Science and Technology of Zirconia - I (Advances in Ceramics) , American Ceramic Society (1981).
Rühle M, Heuer A H, and Claussen N, (eds) Science and Technology of Zirconia - II (Advances in Ceramics) , American Ceramic Society (1984).
Somiya S, Yamamoto N, and Yanagida H, (eds) Science and Technology of Zirconia - III (Advances in Ceramics) , American Ceramic Society (1988).
Meriani S, and Palmonari C, (eds) Zirconia - 88 (Advances in Zirconia Science and Technology) , Europian Ceramic Society (1989).
Badwal S P S, Bannister M J, and Hannink R H J, (eds) Science and Technology of Zirconia — V , CRC Press (1993).
Subbarao E C, Adv Ceram 3 (1981) 1.
Tien T Y, and Subbarao E C, J Chem Phys 39 (1963) 1014.
Tien T Y, and Subbarao E C, J Am. Ceram. Soc. 46 (1963) 489.
Adam J, and Rogers M D, Acta Cryst 12 (1959) 951.
Smith D K, and Newkirk H W, Acta Cryst 18 (1965) 983.
Ruff O, and Ebert F, Z Anorg Ch 180 (1929) 19.
Teufer G, Acta Cryst 15 (1962) 1187.
Liao Y, ZrO 2 - Practical Electron Microscopy and Data Base - An online book , http://www.globalsino.com/EM/ .
Hannink R H J, Kelly P M, and Muddle B C, J Am Ceram Soc 87 (2000) 461.
Kelly J R, Denry I, Dent Mater 24 (2008) 289.
Kelly P M, and Ball C J, J Am Ceram Soc 69 (1986) 259.
Kelly P M, Wauchope C J, Key Eng Mater 153–154 (1998) 97.
Kelly P M, Rose L R F, Prog Mater Sci 47 (2002) 463.
Zhang Y L, Jin X J, Rong Y H, Hsu T Y, Jiang D Y, and Shi J L, Acta Mater 54 (2006) 1289.
Jin X J, Curr Opin Solid State Mater Sci 9 (2005) 313.
Becher P F, Acta Metall 34 (1986) 1885.
Zhang Y L, Jin X J, and Hsu T Y, J Eur Ceram Soc 23 (2003) 685.
Baun W L, Science 140 (1963) 1330.
Wolten G M, J Am Ceram Soc 46 (1963) 418.
Patil R N, and Subbarao E C, Acta Crystallogr Sect A 26 (1970) 535.
Patil R N, and Subbarao E C, J Appl Crystallogr 2 (1969) 281.
Maiti H S, Gokhale K V G K, Subbarao E C, J Am Ceram Soc 55 (1972) 317.
Fehrenbacher L L, and Jacobson L A, J Am Ceram Soc 48 (1965) 157.
Ruh R, Garrett H J, Domagala R F, and Tallan N M, J Am Ceram Soc 51 (1968) 23.
Vest R W, and Tallan N M. J Am Ceram Soc 48 (1965) 472.
Wolten G M, J Am Chem Soc 80 (1958) 4772.
Tien T Y, J Am Ceram Soc 47 (1964) 430.
Garvie R C, J Phys Chem 69 (1965) 1238.
Djurado E, Bouvier P, and Lucazeau G, J Solid State Chem 149 (2000) 399.
Chevalier J, Gremillard L, Virkar A V, and Clarke D R, J Am Ceram Soc 92 (2009) 1901.
Evans A G, and Heuer A H, J Am Ceram Soc 63 (1980) 241.
Bansal G K, and Heuer A H, Acta Metall 20 (1972) 1281.
Yoshimura M, Am Ceram Soc Bull 67 (1988) 1950.
Chevalier J, Cales B, and Drouin J M, J Am Ceram Soc 82 (1999) 2150.
Scott H G, J Mater Sci 10 (1975) 1527.
Sheu T S, TienT Y, and Chen I W, J Am Ceram Soc 75 (1992) 1108.
Weber B C, Technical Report ARL 64-205, Aerospace Research Laboratories , U.S.A.F. (1964)—reproduced in refs. 3 and 34.
Evans A G, J Am Ceram Soc 73 (1990) 187.
Kisi E H, and Howard C J, Key Eng Mater 153 – 154 (1998) 1.
Shukla S, and Seal S, Int Mater Rev 50 (2005) 45.
Mazdiyasni K S, Lynch C T, and Smith J S, J Am Ceram Soc 49 (1966) 286.
Mcmeeking R M, Evans A G, J Am Ceram Soc 65 (1981) 242.
Lange F F, J Mater Sci 17 (1982) 240.
Lange F F, J Mater Sci 17 (1982) 225.
Porter D L, and Heuer A H, J Am Ceram Soc 60 (1977) 183.
Budiansky B, Hutchinson J W, and Lambropoulos J C, Int J Solids Struct 19 (1983) 337.
Eichler J, Eisele U, and Rodel J, J Am Ceram Soc 87 (2004) 1401.
Basu B, Int Mater Rev 50 (2005) 239.
Ruhle M, and Evans A G, Prog Mater Sci 33 (1989) 85.
Li P, Chen I -W, and Penner-Hahn J, J Am Ceram Soc 77 (1994) 118.
Li P, Chen I -W, and Penner-Hahn J E, J Am Ceram Soc 77 (1994) 1281.
Li P, Chen I -W, and Penner-Hahn J E, J Am Ceram Soc 77 (1994) 1289.
Hannink R H J, Johnston K A, Pascoe R T, and Garvie R C, in Advanced Ceramics Science Technology Zirconia , American Ceramic Society, Ohio (1990) p. 116.
Garvie R C, Hannink R H J, and Urbani C, Ceramurg Int 8 (1980) 19.
Drennan J, and Hannink R H J, J Am Ceram Soc 69 (1986) 541.
Hughan R R, and Hannink R H J, J Am Ceram Soc 69 (1986) 556.
Farmer S C, Heuer A H, and Hannink R H J E, J Am Ceram Soc 70 (1987) 431.
Hannink R H J, and Garvie R C, J Mater Sci 17 (1982) 2637.
Hannink R H J, J Mater Sci 18 (1983) 457.
Basu B, Vleugels J, and Van Der Biest O, J Mater Res 16 (2001) 2158.
Vleugels J, Yuan Z X, and Van Der Biest O, J Eur Ceram Soc 22 (2002) 873.
Swain M V, J Mater Sci Lett 5 (1986) 1159.
Chung T, Song H, Kim G, and Kim D, J Am Ceram Soc 80 (1997) 2607.
Vasylkiv O, Sakka Y, and Skorokhod V , J Am Ceram Soc 86 (2003) 299.
Basu B, Vleugels J, and Van Der Biest O, Mater SciEng A 380 (2004) 215.
Basu B, Vleugels J, and Van Der Biest O, Mater Sci Eng A 366 (2004) 338.
Hutchinson J W, Acta Metall 35 (1987) 1605.
Heuer A H, and Ruhle M, Acta Metall , 33 (1985) 2101.
Porter D L, and Heuer A H, Adv Ceram 12 (1984) 653.
Rauchs G, Fett T, and Munz D, Eng Fract Mech 69 (2002) 389.
Srinivasan G V, Jue J-F, Kuo S-Y, and Virkar A V, J Am Ceram Soc 72 (1989) 2098.
Chan C, Lunge F F, and Ruhle M, J Am Ceram Soc 74 (1991) 807.
Subbarao E C, and Maiti H S in Proceedings of the Conference on High Temperature Solid Oxide Electrolytes , (ed) Salzano F J, Associated Universities Inc., New York (1983), p 151.
Subbarao E C, Trans Ind Ceram Soc 46 (1987) 65.
Subbarao E C Sutter P H, and Hrizo J, J Am Ceram Soc 48 (1965) 443.
Subbarao E C, Ferroelecrrics 102 (1990) 267.
Kröger F A, and Vink H J, in Solid State Physics, Vol. 3, (eds) Seitz F, and Turnbull D, Academic Press, New York (1956), p. 307.
Brook R J, in Electrical Conductivity in Ceramics and Glass , (ed) Tallan N M, Marcel Dekker, New York (1974), p 179.
Van Gool W, Principles of Defect Chemistry of Crystalline Solids, Academic Press, New York (1966).
Eyring L, and O’Keefe M (eds) The Chemistry of Extended Defects in Non - Metallic Solids , North-Holland, Amsterdam (1970).
Kofstad P, Nonstoichiemetry, Diffusion and Electrical Conductivity in Binary Metal Oxides , Wiley, New York (1972).
Kröger F A, Chemistry of Imperfect Crystals , Vol. 2, North Holland, Amsterdam (1974).
Singhal S C (ed) Proceedings of the First International Symposium on Solid Oxide Fuel Cells , The electrochemical Society Inc. NJ, USA (1989).
Devi P S, Sharma A D, and Maiti H S, Trans Ind Ceram Soc 63 (2004) 75.
Mahato N, Banerjee A, Gupta A, Omar S, and Balani K, Prog Mater Sci 72 (2015) 141.
Chen K, Li N, Ai N, Li M, Cheng Y, Rickard W D A, Li J, and Jiang S P, J Mater Chem A 4 (2016) 17678.
Singh B, Ghosh S, Aich S, and Roy B, J Power Sources 339 (2017) 103.
Zhuiykov S, and Miura N, Sens Actuators B 121 (2007) 639.
Pham A Q, and Glass R S, Electrochim Acta 43 (1998) 2699.
Gunduz S, Dogu D, Deka D J, Meyer K E, Fuller A, Co A C, and Ozkan U S, Catal Today 323 (2019) 3.
Ikeda S, Sakurai O, Uematsu K, Mizutani N, and Kato M, J Mater Sci 20 (1985) 4593.
Catlow C R A, Chadwick A V, Greaves G N, and Moroney L M, Nature 312 (1984) 601.
Catlow C R A, Chadwick A V, Greaves G N, and Moroney L M, J Am Ceram Soc 69 (1986) 131 272.
Roth W L, Wong R, Goldman A I, Canova E, Kao Y H, and Dunn B, Solid State lon 18 & 19 (1986) 1115.
Yugami H, Koike A, and Ishigame M, Phys Rev B 44 (1991) 9214.
Li X, and Hafskjold B, J. Phys. Condens. Matter 7 (1995) 1255.
Yamamura Y, Kawasaki S, and Sakai H, Solid State Ion 126 (1999) 181.
Ahamer C, Opitz A K, Rupp G M, and Fleig J, J Electrochem Soc 164 (2017) F790.
Tuller H L, Solid State Ion 131 (2000) 143.
Bauerle J E, J Phys Chem Solids 30 (1969) 2657.
Benítez-Rico A, García-Sánchez M F, Picquart M, Monroy-Peláez B M, and Santana-Rodríguez G, J Nanomater. (Hindawi) 2015 (2015) 1.
Kosacki I, and Anderson H U, Ioni cs 6 (2000) 294.
Yamamoto O, Arati Y, Takeda asuo, Imanishi N, Mizutani Y, Kawai M, and Nakamura Y, Solid State Ion 79 (1995) 137.
Xu G, Zhang Y-W, Liao C-S, and Yan C-H, Solid State Ion 166 (2004) 391.
Chakrapani V, Chetan J, Christina C, and Kumar B, J Power Sources 147 (2005) 128.
Okamoto M, Akimune Y, Furuya K, Hatano M, Yamanaka M, and Uchiyama M, Solid State Ion 176 (2005) 675.
Jaisa A A, Muhammed Ali S A, Anwar M, Rao Somalu M, Muchtar A, Roslam W N, Isahak W, Tan C Y, Singh R, and Brandon N P, Ceram Int 43 (2017) 8119.
Robson L G, Reis S L, Muccillo E N S, Ceram Int 43 (2017) 10934.
Souza J P, Grosso R L, Muccillo R, and Muccillo E N S, Mater Lett 229 (2018) 53.
Raghvendra, and Prabhakar S, J Eur Ceram Soc 35 (2015) 1485.
Faryna M, Bobrowski P, Pędzich Z, and Bućko M M, Mater Lett 161 (2015), 352.
Cordier A, El Khal H, Siebert E, and Steil M C, J Eur Ceram Soc 39 (2019) 2518.
Xavier V, Devinder Y, Raj R, and West Anthony R, J Eur Ceram Soc 39 (2019) 1352.
Lughi V, and Clarke D R, Surf Coat Technol 200 (2005) 1287.
Raghavan S, Wang H, Porter W D, Dinwiddie R B, and Mayo M J, Acta Mater 49 (2001) 169.
Sun J, Hu Z, Li J, Zhang H, and Sun C, Ceram Int 40 (2014) 11787.
Brandon J R, and Taylor R, Surf Coat Technol 39 (1989) 143.
Padture N P, Gell M, and Jordan E H, Science 12 (2002) 296.
Liu Y, Vida V, Le Roux S, Blas F, Ansart F, and Lours P, J Eur Ceram Soc 35 (2015) 4269.
Hirvonen A, Nowak R, Yamamoto Y, Sekino T, and Niihara K, J Eur Ceram Soc 26 (2006) 1497.
Nakonieczny D S, Ziębowicz A, Paszenda Z K, and Krawczyk C, Biocybern Biomed Eng 37 (2017) 229.
Sabaliauskas V, Juciute R, Bukelskiene V, Rutkunas V, Trumpaite-Vanagiene R, and Puriene A, Stomatologija 13 (2011) 75.
Wang F, Ph.D. thesis (2011), https://www.escholar.manchester.ac.uk/uk-ac-man-scw:128436 .
Roufosse M, and Klemens P G, Phys Rev B 7 (1973) 5379.
Naumann M, Ernst J, Reich S, Weißhaupt P, and Beuer F, Clin Oral Investig 15 (2011) 657.
Federlin M, Männer T, Hiller K A, Schmidt S, and Schmalz G, Clin Oral Investig 10 (2006) 126.
Brackett M G, Lockwood P E, Messer R L W, Lewis J B, Bouillaguet S, and Wataha J C, Dent Mater 24 (2008) 450.
Tsitrou E A, Northeast S E, and van Noort R, J Dent 35 (2007) 68.
Huang X, Zheng X, Zhao G, Zhong B, Zhang X, and Wen G, Mater Chem Phys 143 (2014) 845.
Kelly J R, J Evid Based Dent Pract 11 (2011) 203.
Beuer F, Schweiger J, Eichberger M, Kappert H F, Gernet W, and Edelhoff D, Dent Mater 25 (2009) 121.
Panwar S S, Umasankar P T, Balasubramanian K, and Venkataraman B, Bull MaterSci 39 (2016) 321.
Derelioglu Z, Carabat A L, Song G M, Van der Zwaag S, and Sloof W G, J Eur Ceram Soc 35 (2015) 4507.
Subhasis N, Indranil M, and Jyotsna D M, Ceram Int 41 (2015) 5247.
Liu B, Liu Y, Zhu C, Xiang H, Chen H, Sun L, Gao Y, and Zhou Y, J Mater Sci Technol 35 (2019) 833.
Kirubaharan A M K, Kuppusami P, Chakravarty S, Ramachandran D, and Singh A, J Alloys Compd 722 (2017) 585.
Pilathadka S, Vahalová D, and Vosáhlo T, Prague Med Rep 108 (2007) 5.
Güngör B M, Aydın C, Yılmaz H, Gül E B, J Oral Implantol 40 (2014) 485.
Osman R B, and Swain M V, Materials 8 (2015) 932.
Manicone P F, Iommetti P R, and Raffaelli L, J Dent 35 (2007) 819.
Torricelli P, Verne E, Brovarone C V, Appendino P, Rustichelli F, Krajewski A, Ravaglioli A, Pierini G, Fini M, and Giavaresi G, Biomaterials 22 (2001) 2535.
Dion I, Bordenave L, Lefebvre F, Bareille R, Baquey C, Monties J R, and Havlik P, J Mater Sci Mater Med 5 (1994) 18.
Suárez M J, Lozano J F L, Salido M P, and Martínez F, Int J Prosthodont 17 (2004) 35.
Aboushelib M N, De Jager N, and Kleverlaan C J, Feilzer A J, Dent Mater 21 (2005) 984.
Chen Z, Li Z, Li J, Liu C, Lao C, Fu Y, and Liu C, Li Y, Wang P, and He Y, J Eur Ceram Soc 39 (2019) 661.
Mota Y A, Cotes C, Carvalho R F, Machado J P B, Leite F P P, Souza Rodrigo O A, and Ozccan M, J Biomed Mater Res B Appl Biomater 105B (2017) 1972.
Ramesh S, Sara L KY, and Tan C Y, Ceram Int 44 (2018) 20620.
Ling Y, Nakanishib Y, Alaoa A-R, Song X-F, Abduo J, and Zhang Y, Procedia CIRP 65 (2017) 284.
Schünemann F H, Galárraga-Vinueza M E, Magini R, Fredel M, Silva F, Souza J C M, Yu Z, Henriques B, Mater Sci Eng C 98 (2019) 1294.
Suveen K, Saurabh K, Sachchidanand T, Saurabh S, Manish S, Birendra Kumar Y, Saro K, Toan T T, Ajay Kumar D, Ashok M, Gopal S J, Sagar M, and Dhar M B, Adv Sci 2 (2015) 1500048.
Sinnott S B, and Andrews R, Crit Rev Solid State Mater Sci 26 (2001) 145.
Zhu Y, Murali S, Cai W, Li X, Won S J, Potts J R, and Ruoff R S, Adv Mater 22 (2010) 3906.
Pratyasha M, Siddharth R, Neelima M, and Kantesh B, Metall Mater Trans A , 46A (2015) 2965.
Rodríguez A M, Poyato R, Gutiérrez–Mora F, Muñoz A, Gallardo–López A, Ceram Int 44 (2018) 17716.
Carmen M-F, Ana M-R, Cristina R T, Emilio J-P, Cristina L-P, Rosalía P, and Angela G-L, J Alloys Compd 777 (2019) 213.
Nina O, and Frank K, Ceram Int 44 (2018) 16931.
Kurapovaa O Y, Glumova O V, Lomakina I V, Sergey N G, Mikhail M, Pivovarov K, Julia V, and Konakova V G, Ceram Int 44 (2018) 15464.
Gutiérrez-Mora F, Morales-Rodríguez A, Gallardo-López A, and Poyato R, J Eur Ceram Soc 39 (2019) 1381.
Liu J, Yan H, Reece M J, and Kyle J, J Eur Ceram Soc 32 (2012) 4185.
Rafael C-C, Malmal M B, Diego G-G, Rodrigo M, and Arturo D-R, J Eur Ceram Soc 38 (2018) 3994.
Marinha D, and Manue B, J Eur Ceram Soc 39 (2019) 389.
Download references
Acknowledgements
The authors acknowledge the support provided by their respective organizations in preparing this manuscript.
Author information
Arjak Bhattacharjee
Present address: School of Mechanical and Materials Engineering, Washington State University, Pullman, USA
Authors and Affiliations
Department of Advanced Materials Technology, CSIR–Institute of Minerals and Materials Technology, Bhubaneswar, 751013, India
Pradyut Sengupta
Department of Ceramic Technology, Government College of Engineering and Ceramic Technology, Kolkata, 700010, India
Arjak Bhattacharjee & Himadri Sekhar Maiti
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Himadri Sekhar Maiti .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Himadri Sekhar Maiti was formerly in CSIR-Central Glass and Ceramic Research Institute, Kolkata, 700032 India
Rights and permissions
Reprints and permissions
About this article
Sengupta, P., Bhattacharjee, A. & Maiti, H.S. Zirconia: A Unique Multifunctional Ceramic Material. Trans Indian Inst Met 72 , 1981–1998 (2019). https://doi.org/10.1007/s12666-019-01742-9
Download citation
Received : 20 April 2019
Accepted : 21 May 2019
Published : 11 June 2019
Issue Date : 01 August 2019
DOI : https://doi.org/10.1007/s12666-019-01742-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Polymorphic transformation
- Stabilization
- Transformation toughening
- Ionic conductivity
- Solid oxide fuel cell
- Biomedical implant
- CNT- and GO-reinforced composite
- Find a journal
- Publish with us
- Track your research
Conventional, Speed Sintering and High-Speed Sintering of Zirconia: A Systematic Review of the Current Status of Applications in Dentistry with a Focus on Precision, Mechanical and Optical Parameters
Affiliations.
- 1 Department of Reconstructive Dentistry and Gerodontology, School of Dental Medicine, University of Bern, 3010 Bern, Switzerland.
- 2 Division of Dental Biomaterials, Clinic of Reconstructive Dentistry, Center of Dental Medicine, University of Zurich, 8032 Zurich, Switzerland.
- 3 Department of Prosthetic Dentistry, Danylo Halytsky Lviv National Medical Universtiy, 79010 Lviv, Ukraine.
- 4 Clinic of Reconstructive Dentistry, Center of Dental Medicine, University of Zurich, 8032 Zurich, Switzerland.
- 5 Department of Reconstructive Dentistry, University Center for Dental Medicine Basel, University of Basel, 4058 Basel, Switzerland.
- PMID: 36013131
- PMCID: PMC9409711
- DOI: 10.3390/jcm11164892
The aim of this systematic review was to provide an overview of the technical and clinical outcomes of conventional, speed sintering and high-speed sintering protocols of zirconia in the dental field. Data on precision, mechanical and optical parameters were evaluated and related to the clinical performance of zirconia ceramic. The PICOS search strategy was applied using MEDLINE to search for in vitro and in vivo studies using MeSH Terms by two reviewers. Of 66 potentially relevant studies, 5 full text articles were selected and 10 were further retrieved through a manual search. All 15 studies included in the systematic review were in vitro studies. Mechanical, precision and optical properties (marginal and internal fit, fracture strength and modulus, wear, translucency and opalescence, aging resistance/hydrothermal aging) were evaluated regarding 3-, 4- and 5-YTZP zirconia material and conventional, high- and high-speed sintering protocols. Mechanical and precision results were similar or better when speed or high-speed sintering methods were used for 3-, 4- and 5-YTZP zirconia. Translucency is usually reduced when 3 Y-TZP is used with speed sintering methods. All types of zirconia using the sintering procedures performed mechanically better compared to lithium disilicate glass ceramics but glass ceramics showed better results regarding translucency.
Keywords: aesthetics; all-ceramic; clinical outcome; cost efficacy; dental; dental materials; firing; mechanical; post-processing; pre-processing; prosthetic dentistry; sintering; speed sintering; systematic review; technical; time efficacy; zirconia; zirconium dioxide.
Publication types
Grants and funding.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Materials (Basel)
- PMC10004380

Recent Advances on 3D-Printed Zirconia-Based Dental Materials: A Review
Ana catarina branco.
1 Centro de Química Estrutural (CQE), Departamento de Engenharia Química, Institute of Molecular Sciences, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon, Portugal
2 Centro de Desenvolvimento de Produto e Transferência de Tecnologia, Department of Mechanical Engineering, Escola Superior de Tecnologia de Setúbal, Instituto Politécnico de Setúbal, Estefanilha, 2910-761 Setúbal, Portugal
3 Centro de Investigação Interdisciplinar Egas Moniz, Instituto Universitário Egas Moniz, Quinta da Granja, Monte de Caparica, 2829-511 Caparica, Portugal
Rogério Colaço
4 Departamento de Engenharia Mecânica, Instituto de Engenharia Mecânica, Instituto Superior Técnico, Universidade de Lisboa, 1049-001 Lisboa, Portugal
Célio Gabriel Figueiredo-Pina
5 Center of Physics and Engineering of Advanced Materials, Instituto Superior Técnico, University of Lisbon, Av. Rovisco Pais, 1049-001 Lisboa, Portugal
Ana Paula Serro
Associated data.
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Zirconia-based materials are widely used in dentistry due to their biocompatibility and suitable mechanical and tribological behavior. Although commonly processed by subtractive manufacturing (SM), alternative techniques are being explored to reduce material waste, energy consumption and production time. 3D printing has received increasing interest for this purpose. This systematic review intends to gather information on the state of the art of additive manufacturing (AM) of zirconia-based materials for dental applications. As far as the authors know, this is the first time that a comparative analysis of these materials’ properties has been performed. It was performed following the PRISMA guidelines and using PubMed, Scopus and Web of Science databases to select studies that met the defined criteria without restrictions on publication year. Stereolithography (SLA) and digital light processing (DLP) were the techniques most focused on in the literature and the ones that led to most promising outcomes. However, other techniques, such as robocasting (RC) and material jetting (MJ), have also led to good results. In all cases, the main concerns are centered on dimensional accuracy, resolution, and insufficient mechanical strength of the pieces. Despite the struggles inherent to the different 3D printing techniques, the commitment to adapt materials, procedures and workflows to these digital technologies is remarkable. Overall, the research on this topic can be seen as a disruptive technological progress with a wide range of application possibilities.
1. Introduction
Teeth damage/loss has strong implications in phonetics, aesthetics, and mastication processes [ 1 ]. The repair/replacement of the damaged/lost tissues is carried out using artificial materials, which should be able to withstand the severe mechanical, chemical and thermal oral requirements. Currently, there are different materials and techniques that allow researchers to restore the function of dental tissues. Ceramic materials are quite widely used for this purpose, mainly due to their aesthetic properties. However, they raise concerns related to the risk of failure induced by fatigue processes that decrease their ability to support the high loads endured during biting and mastication. Furthermore, the wear induced on the antagonist dental surface, mainly due to microfracture-based wear mechanisms, is also an issue [ 2 ]. Ceramics used in dentistry can be divided into two main groups: low- and high-toughness ceramics. The first type corresponds to vitroceramic materials, such as leucite or lithium disilicate, and is essentially used in the restoration of dental crowns (inlays/onlays, facets and veneers—see Figure 1 C,D). However, the brittle behavior of these materials impairs their performance. They suffer wear, which is generally associated with fracture/chipping wear mechanisms. The formation of third-body particles dramatically increases dental wear. Contrarily, in high-toughness ceramics such as zirconia, the prosthetic material wear is negligible, and the wear on the opponent teeth is much lower than that observed against vitroceramic materials. In this case, the main wear mechanism of the teeth results from the penetration of ceramic harder-surface asperities in the enamel/dentine that cut/plough these softer tissues (two-body abrasion) [ 2 , 3 , 4 ]. Thus, in this case, the wear is highly affected by the prosthetic material surface roughness [ 5 ]. In dentistry, zirconia is commonly applied in the production of dental crowns, bridges, implants and abutments ( Figure 1 A,B). More, this material can be used to substitute or repair both anterior and posterior teeth since it is able to resist the different types of efforts associated with the mastication process. It should be stressed that, even for the same type of prosthesis, the requirements may vary, depending on the retention method [ 6 , 7 ]. For example, according to Saverio et al. [ 8 ], cement-retained configurations are generally considered to be more fracture-resistant than screw-retained ones since they better distribute the occlusal forces evenly across the implant.

Types of dental restorations (adapted from [ 2 ]). ( A ) Crown and abutment; ( B ) Bridge and implant; ( C ) Facet/veneer; ( D ) Onlay and inlay.
Several strategies have been investigated to improve the mechanical resistance of ceramics. For vitroceramics, the control of the size and morphology of the crystallites within the vitreous matrix is crucial, since it delays or suppresses the propagation of cracks through the absorption of the fracture energy [ 9 ]. In addition, mechanical resistance can be improved by the reinforcement of the vitroceramic with ceramic particles (e.g., zirconia and alumina) [ 10 , 11 , 12 , 13 , 14 , 15 , 16 ]. For alumina, this is achieved by a high level of control of the chemical composition, porosity, density and grain size (≤4.5 µm) [ 17 ]. Finally, in zirconia, the control of the phase transformation by the addition of stabilizing oxides (e.g., Y 2 O 3 ) leads to an increased fracture toughness [ 18 , 19 ]. In fact, it is known that the addition of 3 mol.% of yttria to tetragonal polycrystalline zirconia (3Y-TZP) results in the retention of this phase at room temperature [ 20 ]. The application of an external load induces a stress concentration at the crack’s tips, leading to the local transformation of the metastable tetragonal phase to a stable monoclinic phase with a consequent volume expansion (approximately 4.5%) [ 21 ]. This transformation toughening mechanism sets the cracks into compression, retarding their growth which subsequently improves strength [ 20 ].
Besides the high fracture toughness, 3Y-TZP presents excellent properties such as high flexural strength, excellent ionic conductivity, thermal and chemical stability, good biocompatibility, and corrosion resistance. Additionally, it does not induce allergic reactions. 3Y-TZP restorations are usually coated with a glaze (glass veneer) in order to achieve optical properties similar to those of the adjacent teeth (e.g., color, translucency) [ 4 , 22 ]. However, these restorations often suffer adhesive chipping of the coating, which results from the difference in materials’ coefficient of thermal expansion (CTE) [ 22 ]. In addition, several in vitro studies have demonstrated that these coatings usually lead to abnormal wear of the antagonist teeth due to the interlocking of third-body particles between the sliding surfaces [ 1 , 4 ].
In the dentistry industry, ceramic-based materials are usually processed by subtractive manufacturing (SM) techniques, namely through milling, diamond grinding, laser ablation or ultrasonic machining. All of them involve the removal of material from ceramic blocks, differing in terms of the cutting tools used (drill, diamond disk, laser beam and high-frequency vibrations/abrasives, respectively). The mostly used method for the production of zirconia pieces is milling, which is based on computer-aided design (CAD) and computer-aided manufacturing (CAM) technologies. Pre-sintered or fully sintered blocks are processed through a computer numeric controlled machine to obtain pieces of a specific size and shape. The use of this methodology entails several drawbacks: high material waste; limited accuracy in the production of parts with intricate internal details which impairs the reproduction of fine details; high processing time; and the cost of fabrication of complex pieces [ 23 ]. Additive manufacturing (AM), also known as 3D printing, has emerged as a promising technique to produce long-term dental structures. AM uses a computerized 3D model to produce pieces following a layer-by-layer approach (each layer is deposited one on top of the previous, consecutively, until achieving a 3D part). Its innumerous advantages when applied to this field have been highly reported in the literature [ 24 ]. Dental restorations are usually expensive, not only due to the price of the raw material, but also because they are customized products and their production involves a high workload. This restricts their accessibility to the general population, especially to the most disadvantaged sectors of society. The use of AM in the dental products’ digital production system (industry 4.0) presents innumerous economic, environmental, and social profits, such as low energy consumption, low production time and material waste, high efficiency, and the possibility of implementing a decentralized production, allowing mass production. Due to a rising life expectancy, higher awareness about oral health problems and the increasing importance of aesthetic issues, there is a high demand for dental restoration products. It is predictable that the generalization of AM use in dentistry will have a quite positive impact on the healthcare sector, with consequent benefits to the population. Therefore, it is important to understand how the current dental materials and novel materials can be processed by this technology so that they can be integrated into the production systems.
There are several AM techniques that can be used to produce 3D-printed dental structures. As shown in Figure 2 , they can be divided into two main groups: the indirect and direct methods. In the first case, after printing, the part undergoes debinding and sintering, while in the latter technique no further processing after printing is required.

Indirect and direct AM methods. In some cases, equivalent nomenclatures are referred.
Indirect methods are preferable since they lead to a higher degree of consolidation of the parts and reduce the risk of cracking. In fact, contrarily to direct methods, where the material is melted by a focused energy source (e.g., plasma arc, laser, or electron beam) and simultaneously deposited in non-controlled temperature conditions, and which tend to suffer from fast cooling, indirect methods involve gradual heating protocols, reducing thermal shock. It should be stressed that the final results, namely the accuracy/resolution and the mechanical and aesthetic properties of the parts produced by a given technique, are influenced by a number of factors such as the raw materials and binders used, printing parameters (e.g., layer height, printing velocity and orientation, nozzle/light source characteristics), and post-printing treatments such as debinding and sintering [ 25 , 26 ]. Debinding consists of the elimination of the organic compounds that are mixed with the ceramic powders in order to allow printing. This is typically performed by heating the printed part in an oven at a temperature above the glass transition temperature of those compounds. The temperature required for removing the resin can vary depending on the specific type of resin, but usually ranges between 80 and 120 °C. Subsequent sintering is performed at higher temperatures, typically at 1500–1600 °C [ 20 ], but these temperatures can be lower if zirconia is mixed with vitroceramic materials [ 16 ].
There are few studies in the literature addressing the use of direct methods to process zirconia-based materials. Several authors state that, since zirconia presents low thermal conductivity, high melting temperature, and low thermal shock resistance, it is difficult to obtain pieces without defects (e.g., cracks and large open pores) [ 27 ].
This review will focus on the most widely used methods for the 3D printing of zirconia-based materials for dental applications, which fall in the indirect methods’ group. Below, we explain the working principle of vat polymerization, which includes stereolithography (SLA) and direct light processing (DLP), robocasting (RC), material jetting (MJ) and binder jetting (BJ) (see Figure 2 ) [ 27 , 28 , 29 , 30 ].
1.1. Vat Photopolymerization
In vat polymerization technology, a photopolymerizable liquid is placed inside a vat and cured by light action (UV light or UV laser, typically 380–405 nm) in accordance with a pre-defined design (CAD file) of the piece. The oligomers/monomers (epoxy or acrylic and methacrylic) present in the liquid are crosslinked in the presence of photo-initiators in thin layers, on/under a submersed platform, depending on if it is a top-down/bottom-up approach. After building each layer, the platform is re-submerged in the leftover resin to allow its spreading over the vat. The process is repeated until all the layers that constitute the piece are stacked and cured. There are two types of vat photopolymerization technologies: stereolithography (SLA) and direct light processing (DLP), which basically differ in the type of light used and in the way the light reaches the 3D-printed object [ 23 ].
- ▪ Stereolithography (SLA)
Stereolithography (SLA) uses a laser beam that moves across the forming layer surface, leading to a localized polymerization of the photosensitive resin. It allows for the production of complexly shaped pieces with high dimensional accuracy and surface quality since the curing of the resin is performed spot-by-spot. Generally, the laser is not directly focused onto the resin, being deflected by a non-fixed mirror galvanometer that directs the beam to a specific point ( Figure 3 A). There are two main types of SLA printers: one where the laser is located above the vat and points down into the resin, and another where the laser is placed below the vat and points upwards into the resin. SLA is the technique most widely used to produce pieces for dental applications since it provides the highest accuracy and resolution, as well as flawless surface finishing.

Scheme of AM indirect methods used for dental applications (adapted from [ 31 , 32 ]). ( A ) Stereolithography (SLA), ( B ) Digital light processing (DLP), ( C ) Robocasting (RC), ( D ) Material Jetting (MJ), ( E ) Binder Jetting (BJ).
- ▪ Direct light processing (DLP)
Digital light processing (DLP) (referred to by some authors as mask projection stereolithography (MPSL), maskless projection slurry stereolithography (MPSS) or 3D slurry printing (3DSP)) use a stationary digital micromirror device that reflects and focuses UV light, curing a complete layer of resin at once. Contrarily to SLA, layer curing in DLP is not performed spot-by-spot but rather through a single projection in a plane where photopolymerization occurs, resulting in a faster printing rate ( Figure 3 B) [ 33 ]. Since the projector is a digital screen, each layer is made up of a number of pixels and may be described as a set of little rectangular bricks known as voxels. The intensity of the UV light can be adjusted, allowing us to control its effect on the resin. DLP offers good feature resolution (down to several micrometers) and accuracy, being suitable to build larger and more intricate parts at higher speeds than SLA. Regarding the building configuration, DLP can produce pieces in a bottom-up or top-down setup. In the first case, the piece is cured under an inverted platform and dipped into a thin slurry layer deposited in the vat rather than entirely immersed in the liquid resin, as happens in the second case. Bottom-up setup is less expensive since it requires a lower amount of slurry to produce the desired piece. On the other hand, printers with a top-down setup allow the production of larger parts.
1.2. Robocasting (RC)
Robocasting (RC), also known as material extrusion (ME) or direct ink writing (DIW), involves the use of a stable slurry/paste/ink with high solid loading that is extruded through a nozzle ( Figure 3 C). This moves over a platform to directly “write” the desired shape in a layer-by-layer manner until the piece is complete. It should be noted that after the extrusion of one layer, it is not necessary to wait for the material’s solidification or drying before depositing the next layer. RC may be challenging due to issues related to the printability of pastes. The paste optimization process is crucial since an ink with adequate composition, rheological properties (relatively low viscosity under stress) and excellent shape retention capacity (high elastic/storage modulus, high yield stress) should be obtained to ensure its adequate extrusion and that, during deposition, each layer supports its own weight without collapsing. Pastes with high solid loading allow the production of bulk samples with high densities.
1.3. Material Jetting (MJ)
Material jetting (MJ), also known as direct inkjet printing (DIP), is a process that operates in a similar way to the 2D ink-jetting process. However, instead of a filament, the material is deposited in the form of droplets. In this technique, a printhead moves horizontally ( x – y axis), and droplets of a photosensitive material are deposited onto the building platform, at that point being directly cured under UV light. The piece is built in a layer-by-layer approach. In brief, first the liquid resin is heated 30–60 °C to achieve a suitable viscosity for printing. Then, the print head travels over the building platform and hundreds of tiny droplets of photopolymer are jetted/deposited inside a support (see Figure 3 D). A UV light source that is attached to the print head cures the deposited material, solidifying it and creating the first layer of the part. After each layer is complete, the building platform moves downwards one in layer height, and the process is repeated until the whole part is completed. After printing, the support material (which is soluble in specific solvents) is removed. MJ is a fast and economical AM technique with minimal waste and high flexibility which allows for a high level of accuracy in the deposition process.
1.4. Binder Jetting (BJ)
In binder jetting (BJ) ( Figure 3 E), a recoating blade spreads a thin layer of powder over a building platform. Then, the printhead selectively deposits droplets of a liquid binding agent that bonds the powder particles together, according to the CAD project. After the application of the binder, the building platform moves down, and another layer is built over the previous, following the same procedure.
This process is fast, simple, and cheap, requiring the use of materials in the powder form. Additionally, it prevents the formation of residual stresses in the produced parts since there are no thermal inputs (no light source is used). However, it leads to pieces with low mechanical resistance and is not suitable for use to produce structural parts.

2. Materials and Methods
To conduct this review, the authors formulated the following question: “What is the state-of-the-art regarding the use of 3D printing technologies to produce zirconia-based materials for dental applications?”
2.1. Protocol
This systematic literature review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [ 34 ].
2.2. Eligibility Criteria
The works considered in this study were gathered based on the following inclusion criteria:
- - Studies that include zirconia based materials;
- - Studies that use additive manufacturing techniques/3D printing technologies;
- - Studies that evaluate the properties of the printed materials;
- - Studies that focus on materials used for dental applications;
- - Articles published in English;
- - Articles published in peer-reviewed journals;
- - In vitro studies.
Literature reviews, systematic reviews, case series, manufacturer reports, and conference abstracts were not considered.
2.3. Information Sources and Search Strategy
The search for this review article was conducted on three different online databases: PubMed, Scopus and Web of Science. The last search was carried out on 14 December 2022. Regarding the year of publication, no restriction was applied. The keywords used for searching were: (Additive Manufacturing OR 3D printing) AND (robocasting OR direct ink writing OR material extrusion OR stereolithography OR digital light processing OR material jetting OR direct inkjet printing OR binder jetting) AND (dental materials OR dental applications OR dentistry) AND (zirconia OR zirconia composites OR ceramic composites) AND (leucite OR lithium disilicate OR vitroceramics).
This search was conducted using the Mendeley software (version 1.19.8). Two independent researchers (A.C.B. and A.P.S.) started by analyzing titles and abstracts identified in the initial search for their relevance and fulfilment of eligibility criteria. Firstly, articles were classified as “include”, “exclude”, or “uncertain”. Then, the full-text articles of the “include” and “uncertain” records were analyzed for further eligibility screening by the same researchers, who worked independently. When there were discrepancies in screening of titles/abstracts and full-text papers, the two researchers discussed the issue. In case of disagreement, the opinion of a third researcher (C.F.P.) was obtained, and a decision was reached. Finally, after full-text review, some articles were excluded and the reasons for rejecting the studies were highlighted (see Figure 4 ).

PRISMA flow chart diagram.
2.4. Data Extraction and Results Achievement
This review article is focused on 3D printing technologies used to produce zirconia-based materials for dental applications. In order to summarize the retrieved information, a table with the following information was built: author (publication year), manufacturing technology, ceramic material, dental application, studied properties, and main results. Then, a descriptive analysis was performed to compare and point out similarities and differences among the studies.
The initial search yielded 932 potentially relevant articles, of which 685 were excluded as duplicates. Following a title/abstract review and subsequent full-text review, 160 additional articles were excluded as they were considered irrelevant to the main aim of this review. Of the 87 remaining articles assessed for eligibility, after full-text screening, 39 more studies were excluded due to the following reasons: 11 were studies whose application was not for dental applications, 10 did not fulfil the aim of the review, 4 were articles published in not peer-reviewed journals, 4 did not mention zirconia as one of the studied materials, 4 were review papers, 3 did not mention the additive manufacturing technique used and 3 were not available in the English language. This screening process led to the inclusion of 48 studies in the review. The PRISMA flow chart diagram below ( Figure 4 ) depicts the selection process.
The considered papers were published between 2010 and 2022, despite no restrictions being imposed regarding the publication dates. This reveals the innovative character of the subject, whose interest has increased even more in the last four years: 8 articles were from 2019, 9 from 2020, 15 from 2021 and 9 from 2022. Most of the studies are published in materials or dental materials journals and are focused on yttria-stabilized tetragonal zirconia. Most of the studies were performed in Asia (total 28), mostly from China (21), but also from South Korea (5) and Saudi Arabia (2). In Europe (total 13), studies from Portugal (4), Belgium (3) and the Netherlands, Germany and Italy (2 in each case) were found. From North America only 7 studies were found (United States (6) and Canada (1)). The most productive group was that of Revilla-León et al. which has published 5 papers [ 29 , 35 , 36 , 37 , 38 ] since 2020 that, at present, have received 57 citations. Concerning the additive manufacturing technique, 24 used digital light processing (DLP), 16 used stereolithography (SLA), 6 used robocasting (RC)/material extrusion (ME)/direct ink writing (DIW), 5 used material jetting (MJ)/direct inkjet printing (DIP) and 1 used 3D gel deposition ( Figure 5 A). In terms of the dental application, some works centered on the production of materials for crowns (16), bridges (3), implants and abutments (9) and copings (2) ( Figure 5 B). However, some studies did not report any specific dental application (19). Several properties were studied among the selected studies: cure depth, density, shrinkage, dimensional accuracy, trueness and precision, internal fit and marginal adaptation, translucency, surface roughness, microstructure, mechanical properties (hardness, fracture toughness, flexural strength, elastic modulus, fracture load), and wear (antagonist teeth and prosthetic materials).

( A ) Most studied AM techniques; ( B ) Dental applications.
Table 1 , Table 2 , Table 3 , Table 4 and Table 5 précis the main findings of studies involving the 3D printing of zirconia-based materials.
Summary of the main results obtained for ZrO 2 -based samples produced by digital light processing (DLP).
Summary of the main results obtained for ZrO 2 -based samples produced by stereolithography (SLA).
Summary of the main results obtained for ZrO 2 -based samples produced by robocasting (RC).
Summary of the main results obtained for ZrO 2 -based samples produced by material jetting (MJ).
Summary of the main results obtained for ZrO 2 -based samples produced by different 3D printing techniques.
4. Discussion
Most of the studies reported in the literature concerning the production of zirconia-based dental pieces by 3D printing are focused on vat polymerization techniques, namely digital light processing (DLP) and stereolithography (SLA) [ 23 , 81 , 82 ], which allow the curing of consecutive layers of a photosensitive polymeric material mixed with ceramic particles. Both techniques usually lead to the production of parts with high accuracy and resolution, smooth surface finishing and fine building details [ 83 , 84 , 85 ]. Although there are far fewer studies addressing robocasting (RC)/direct ink writing (DIW) and material jetting (MJ)/direct inkjet printing (DIP), these techniques have also been reported as potentially suitable processing methods to be used in dentistry. Below, the main properties of the materials processed by the different techniques will be addressed, as well as some aspects affecting them.
4.1. Cure Depth
DLP and SLA are similar technologies that use light (UV or laser), and therefore the nature and size of the ceramic particles and the pastes’ solid loading are key factors in the curing process of the layers. Since zirconia presents a high refractive index (2.1, which is 20–27% higher than silica and alumina [ 86 ]), it usually induces significant scattering of the incident light during polymerization of the resin that contains it, decreasing the cure depth [ 42 , 43 , 87 , 88 ]. As for the size, smaller particles also tend to increase light scattering [ 89 ], threatening the final result. A high solid load (≥60 vol.%) usually leads to low cure depth, resulting in extra ceramic material loosely adhering to the final printed piece [ 42 , 90 ]. Besides, it translates into a high viscosity, which may impair the resin flow during each layer formation and the cleaning of the resin tank. Ideally, pastes should present a viscosity lower than 5 Pa·s [ 91 ], comparable to unloaded resins. However, solid loading must be enough to ensure the obtention of pieces with high density after sintering.
The ceramic suspension used for DLP/SLA must be stable, i.e., the ceramic particles must be homogeneously and effectively dispersed in the photocurable resin for a reasonable period (e.g., hours to days) and not suffering from sedimentation. To achieve suspensions’ stability/homogeneity, several approaches can be followed such as the addition of dispersants and other additives to the slurry, particles’ coating, ultrasonication, vacuum drying, ball milling, and acid treatment [ 88 ].
4.2. Density and Mechanical Properties
For DLP and SLA, light curing slurries have been prepared with ceramic powder loadings that range from 34.5 to 58.0 vol% [ 42 , 43 , 44 , 45 , 46 , 48 , 49 , 51 , 52 , 53 , 54 , 56 , 57 , 59 , 60 , 63 , 65 , 66 , 67 , 77 ]. Values between 98 and 99.8% were obtained for the materials’ theoretical density (TD) [ 40 , 41 , 44 , 45 , 48 , 49 , 52 , 53 , 57 , 60 , 67 ]. As expected, higher solid loadings led to a higher density of the sintered pieces [ 42 , 59 , 90 ]. Similarly, in general, the mechanical properties improved for higher solid amounts [ 42 , 57 , 59 ]. One of the most commonly evaluated mechanical properties was flexural strength (see Figure 6 A). This was found to be, in most of the cases, lower than that of zirconia pieces produced by conventional manufacturing techniques (200–831 MPa [ 35 , 39 , 40 , 41 , 42 , 45 , 46 , 48 , 49 , 51 , 53 , 57 , 60 , 63 , 77 ] vs. 900–1200 MPa [ 20 , 46 , 92 , 93 ]). However, pieces with a higher flexural strength (943–1519 MPa) [ 36 , 41 , 49 , 77 ] were also obtained by some authors using DLP or SLA. These higher values may be a result of the improvement of slurry composition, which leads to more adequate viscosity, as well as the optimization of the parameters for debinding and sintering processes. Hardness and fracture toughness were also widely characterized: their values are reported to encompass 1038–1556 HV and 3.43–6.42 MPa.m 1/2 , respectively (see Figure 6 B,C) [ 35 , 39 , 40 , 43 , 44 , 46 , 47 , 49 , 52 , 53 , 57 , 60 ]. These values are similar to those found for zirconia parts obtained by conventional manufacturing methods [ 1 , 2 ]. Regarding zirconia composites, there are still only few studies where its production by 3D printing is addressed. Wu et al. [ 44 ] and Coppola et al. [ 51 ] used DLP in the manufacturing of Al 2 O 3 -ZrO 2 (ATZ) composites and observed that the mechanical properties improved significantly compared to full zirconia materials, achieving hardness values in the range of 1290–2141 HV. More, Coppola et al. [ 51 ] verified that the values decrease with the increasing zirconia content.

Dispersion charts of retrieved data regarding flexural strength, hardness and fracture toughness from the selected studies presented on Table 1 , Table 2 , Table 3 and Table 4 . ( A ) Flexural strength; ( B ) Hardness; ( C ) Fracture toughness.
Other techniques, like robocasting (RC) and material jetting (MJ), also present high potential in the dentistry field. However, information available in the literature related to their use in processing zirconia for those applications is limited. As referred to in Table 3 and Table 4 , the TD of the materials produced by these techniques fall in the range of 94.0–98.1% for RC samples [ 1 , 69 , 70 , 72 ] and 96.0–99.7% for MJ samples [ 73 , 74 , 75 , 76 ]. Their mechanical properties, namely flexural strength, hardness and fracture toughness are, in most cases, comparable to those found when vat polymerization methods (DLP and SLA) are used (see Figure 6 A–C) [ 1 , 69 , 72 , 73 , 74 , 75 , 76 , 94 ].
4.3. Defects
Concerning the defects of 3D-printed samples, some studies reported the presence of pores and cracks/fractures on the samples’ surface. Li et al. [ 61 ] obtained ZrO 2 pieces for bridges and implants by SLA and found cracks on the outer surface that suffered propagation. Besides, they observed the presence of pores (200–400 nm) distributed all over the surface. In the work of Osman et al. [ 41 ], cracks, microporosities and interconnected pores with sizes ranging from 196 nm to 3.3 µm were also observed. Revilla-León et al. [ 36 ] produced ZrO 2 pieces by SLA but SEM images revealed that there was no evidence of cracks, fracture surfaces, or flaws. Instead, an irregular surface with pits of 10–40 µm was detected. Marsico et al. [ 47 ] showed that DLP-sintered pieces presented fractures that began at layer lines and surface defects (pores). Jang et al. [ 42 ] concluded that surface cracks decreased with the increasing content of ZrO 2 , leading to the formation pieces with higher mechanical resistance. Xiang et al. [ 65 ] produced pieces using SLA and observed a weak bonding strength among the successive layers as well as surface defects resultant from the process of separating the piece from the building platform. Apart from impairing the aesthetic properties of the final piece (e.g., decreasing the translucency), these surface defects (internal flaws such as pores and agglomerations) also increase the failure probability of the restorations. These authors performed three-point bending tests and observed two types of fracture modes after testing: fractures due to stress concentration and splintering due to crack deflection. In another work where pieces for crowns and fixed prosthesis were produced by SLA [ 38 ], it was found that, as expected, the higher the porosity was, the lower the fracture load, flexural strength and flexural modulus would be: samples with 0% porosity showed fracture loads of 1132.7 N, flexural strength of 755.1 MPa and flexural modulus of 41.273 GPa, while samples with 40% porosity showed a fracture load of 72.13 N, flexural strength of 48.09 MPa and flexural modulus of 7.177 GPa. Willems et al. [ 76 ], produced pieces by MJ from a suspension with low solid loading (12.5 vol%), observing delamination, cracks, agglomerates and spherical pores. Finally, Zang et al. [ 80 ] produced restorations by 3D gel deposition and cold isostatic pressing and determined the fracture force before and after fatigue tests (5,000,000 cycles ≈ 20 years of clinical service). They observed that the first samples led to a higher fracture force (≈8000 N) than the latter (≈7000 N) (both before and after fatigue testing without statistically significant difference) due to the fine-grained microstructure without visible microscopic voids.
4.4. Aesthetic Features
Since 3D printing manufacturing techniques involve layer-by-layer deposition, and aesthetic requirements are quite relevant in dental applications, it is critical to ensure that the interfaces between layers, in the final product, are barely defined. Revilla-León et al. [ 36 ] printed parts by SLA and found a layer strand texture with a smooth depression between the layers (no more than 5 and 10 µm). Silva et al. [ 68 ] produced fixed partial dental structures by RC and observed a surface with a “stair stepped” appearance and the presence of cracks derived from the drying step. Contrarily, Özkol et al. [ 73 ] produced dental bridges by MJ and observed a smooth surface without “stair steps”. In turn, Li et al. [ 43 ] and Kim et al. [ 48 ] produced dental crowns via DLP and verified that the interlayered structure disappeared after sintering. It should be stressed that the application of glass veneers over the zirconia restorations, which is a common procedure to improve aesthetics, generally hides the layered structure, minimizing the importance of this issue.
4.5. Dimensional Accuracy and Resolution
Other important aspects that must be evaluated after the printing process are the accuracy and resolution of the produced pieces. These are influenced by a wide range of factors, e.g., technology used, post-treatment procedures, particle size and layer thickness [ 95 ]. Accuracy evaluates two parameters: trueness and precision. While trueness is defined by the deviation of the produced printed piece from its desired dimensions, precision measures the consistency between repeated prints (i.e., the ability to produce the same part with the same dimensions in consecutive prints) [ 83 ]. A high precision in the production of dental restorations ensures an appropriate fit and an adequate biological response. Resolution concerns the detail level achieved in three dimensions and depends on the number of points that a 3D printer can effectively reproduce. Although the accuracy and resolution of the process is crucial for the success of a dental restoration, the number of studies on this topic is still quite limited in the literature. Lerner et al. [ 50 ] produced ZrO 2 crowns by DLP and SM and evaluated their trueness and precision. They found that crowns produced by SM showed higher trueness than DLP crowns. Regarding precision, both techniques led to similar results in terms of the quality of interproximal contact points and marginal closure. Lüchtenborg et al. [ 79 ] compared the accuracy of fixed dental prostheses produced by SLA, DLP, MJ and SM and concluded that SM led to the most accurate pieces. More, a prototype piece of DLP equipment resulted in surface deviations higher than 100 µm, representing the least accurate method. However, this depended on the DLP printer/characteristics of the material used, since the authors reached better results with a commercial DLP printer that uses a specific material optimized for that printer. Also, Moon et al. [ 55 ] observed that DLP pieces produced for dental copings suffered higher thermal shrinkage and presented lower accuracy than those produced by SM. In another study performed by Wang et al. [ 62 ], ZrO 2 crowns were produced by SLA and SM. They verified that the trueness of the external surface, intaglio (interior) surface, marginal area, and occlusal surface were similar in both cases ( Figure 7 A). Additionally, Kim et al. [ 78 ] used DLP, SLA and SM to produce ZrO 2 crowns and did not observe statistically significant differences at the inner surface area, concluding that the trueness of intaglio crown surface was similar regardless of the manufacturing method. However, differences regarding the trueness of the occlusal, margin and axial areas were found: at the occlusal area, DLP samples showed the lowest mean values, being statistically significantly different from SLA and SM-4YZ samples; at the marginal area, both DLP and SLA samples presented significantly higher values than SM groups; finally, at the axial area, a significantly lower value was found for SLA samples when compared to SM samples (4YZ and 5YZ). Accuracy is crucial to ensure a suitable internal fit and the marginal adaptation of provisional crowns and fixed-dental prosthesis ( Figure 7 B). A suitable internal fit (≤300 µm) improves the retention, resistance, and durability of the restorations, while an adequate marginal adaptation (≤120 µm) impairs microleakage, the dissolution of fixation cement, bacterial plaque accumulation, secondary caries and periodontal inflammation [ 56 ]. The last is the principal measurement metric used for dentistry since it has a significant impact on the longevity of dental restorations [ 46 ]. Meng et al. [ 56 ] produced ZrO 2 crowns by DLP and observed that the internal fit and marginal adaptation of fixed crowns were 239.3 ± 7.9 µm and 128.1 ± 7.1 µm, respectively, being close to clinical standards. However, crowns produced by SM led to values of 68.5 ± 3.9 µm for internal fit and 71.6 ± 2.8 µm for marginal adaptation, being more reliable than those produced by DLP. In another study, Hsu et al. [ 46 ] measured the marginal adaptation of premolar teeth produced by DLP and SM and observed that DLP led to higher values (98.9 µm) than SM (72 µm). However, they were lower than 120 µm, which is the maximum value acceptable for clinical use. Li et al. [ 63 ] produced crowns by SLA. Although they presented suitable mechanical properties, they were considered non-ideal for dental application since it was observed a cement space of 169.58 µm in the marginal area. Finally, Abualsaud et al. [ 25 ] studied the internal fit, marginal adaptation, precision and trueness of zirconia crowns produced by SLA and SM and reported similar internal fit and marginal adaptation between pieces produced by both methods. Regarding trueness, SLA crowns revealed better occlusal (8.77 ± 0.89 µm) and axial (14.77 ± 2.03 µm) trueness than SM crowns (14.78 ± 2.23 µm and 20.37 ± 4.49 µm, respectively), while SM crowns showed better intaglio trueness (20.29 ± 3.82 µm) than SLA crowns (23.90 ± 1.60 µm). Furthermore, SLA led to more precise crowns (9.59 ± 0.75 µm) than SM crowns (17.31 ± 3.39 µm).

( A ) Different areas of prosthetic crowns relevant for the evaluation of trueness and precision; ( B ) Internal fit and marginal adaptation (adapted from [ 25 ]).
4.6. Bonding Strength between Materials
When ZrO 2 crowns are damaged and/or need to be refurbished, the application of glass ceramic copings can avoid their replacement. This procedure allows us to improve aesthetic properties like translucency and color, is less harmful to patients, leading to lower recovery times, and involves lower costs. Its success is determined, among other factors, by the bonding strength between the two materials. Moon et al. [ 55 ] produced pieces of porcelain-fused zirconia, the being later obtained by DLP or SM. They observed adhesive failure (debonding) between the two interfaces in both cases. However, the bond strength to zirconia produced by DLP was significantly higher than the strength of the bond to zirconia processed by SM (35.12 ± 4.09 MPa vs. 30.26 ± 5.20 MPa).
4.7. Tribological Behavior
Finally, although wear is a subject of great relevance for dental materials, few studies address the tribological behavior of prosthetic materials obtained by 3D printing and antagonist teeth. Kim et al. [ 78 ] produced 3YZ pieces for full-contour monolithic crowns via DLP and SLA and performed chewing simulation tests against human molars. They observed that the antagonist’s wear volume loss was 2.06 ± 1.24 mm 3 (DLP) and 1.74 ± 1.20 mm 3 (SLA), similar to the values observed with samples produced by SM (2.51 ± 2.13 mm 3 and 2.40 ± 1.66 mm 3 for SM-4YZ and SM-5YZ, respectively). Branco et al. [ 1 ] conducted a study where ZrO 2 pieces were produced by RC methods and compared with those produced by SM. In chewing simulation studies carried out in artificial saliva against natural human teeth cusps, it was found that any of the prosthetic materials suffered wear. Contrarily, all ZrO 2 pieces induced wear on the cusps, this being significantly higher in the case of SM samples. These authors also applied a glaze finishing over RC and SM pieces and observed that, contrarily to the uncoated surfaces, both glazed surfaces and dental cusps suffered wear. The wear of the cusps was higher than that found on unglazed specimens. Moreover, RC glazed pieces induced less wear on the antagonist cusps than SM glazed pieces.
4.8. Printing Orientation
One important factor that must be considered when producing pieces by 3D printing is the printing orientation since it will strongly influence the printed piece quality, especially in terms of accuracy, surface roughness, translucency, and mechanical properties. Depending on the printing parameters, the adhesion between layers may be different from the adhesion between lines in the same layer. Xiang et al. [ 65 ] concluded that samples printed by SLA in an upright way led to higher density and translucency than when printed horizontally, while samples printed horizontally led to excellent accuracy and mechanical properties. In another study, Coppola et al. [ 57 ] produced samples via the DLP method and found that plane they showed higher flexural strength when tested perpendicularly to the printing (≈751 MPa) than when tested parallelly (≈675 MPa) to the printing plane. Similarly, Zhao et al. [ 53 ] observed that the flexural strength of samples printed by DLP in the horizontal direction was higher than those printed in the vertical direction (597 MPa vs. 89 MPa). Marsico et al. [ 47 ], in a study where zirconia was printed by DLP in three different orientations (0°, 45° and 90°), found that 45° orientation presented the highest indentation fracture resistance. In turn, 0° orientation showed the highest flexural strength (657 MPa) since it minimized the impact of layer line defects, these values being comparable to those reported in the literature for monolithic zirconia produced by conventional methods. Additionally, Osman et al. [ 41 ] printed ZrO 2 implants by DLP and found that pieces printed in 0° orientation led to the highest flexure strength values (943 MPa), while the lowest values were observed for 45° printing orientation (822 MPa).
4.9. Ageing
The effect of ageing treatments on 3D-printed zirconia-based materials has also been scarcely addressed in the literature. Tan et al. [ 54 ] produced 3Y-TZP for implant abutments by DLP and SM and evaluated the effect of ageing (134 °C with 100% humidity, 0.2 MPa) on their physical and biological properties. They found that DLP samples presented higher initial cubic phase content and a greater rate of phase transformation than SM samples. Concerning their biological performance, the ageing treatment almost did not affect cellular behavior in any zirconia type. Only minor changes in adhered cell numbers, recorded in the function of the aging time/culturing time, were found. In another study, Léon et al. [ 35 ] investigated the effect of artificial ageing (8000 cycles between 5 °C and 55 °C) on the mechanical properties of 3Y-TZP produced by SLA and SM and observed that the flexural strength decreased ≈12% after such treatment for SLA samples and ≈37% for SM samples. Zhai et al. [ 77 ] produced ZrO 2 samples by SLA, DLP and SM methods. They submitted the samples to ageing (134 °C, 0.2 MPa for 5 h, 10 h and 15 h) and observed that ageing times until 15 h only affected SLA samples’ properties: the flexural strength increased from 776.7 MPa in non-treated samples to 1010.3 MPa after 5 h of ageing. A decrease in the flexural strength was observed after 10 h and 15 h (913.1 MPa and 814.28 MPa, respectively). Regarding DLP and SM samples, before and after ageing, this value was around 800 MPa and higher than 1200 MPa, respectively. These authors also found that DLP samples showed zirconia grain fragments, while SLA samples presented grain pullout. Moreover, the monoclinic phase content increased with the aging time, both for DLP and SLA samples. Lastly, Wu et al. [ 44 ] produced alumina-toughened zirconia (ATZ) implants by DLP and hydrothermally treated them with steam at 134 °C for 5 h, 20 h and 40 h. After, they evaluated the aging rate and tetragonal-monoclinic phase transformation and, as expected, observed that it was lower than that of 3Y-TZP samples.
Overall, this review allowed us to summarize the most recent advances on 3D printing of zirconia-based dental materials, highlighting the main issues associated with the production that impact the performance of the materials. This task revealed to be challenging since it was found a great variability regarding, e.g.,:
- − The characteristics of the raw materials used (including the ceramic powders and the resins), such as their concentrations, the solids content, the size distribution and shape of the particles, that determine the rheological properties of the suspension;
- − The printing parameters (e.g., velocity, layer height/line width, orientation, nozzle/light source characteristics);
- − The post-printing treatments (e.g., debinding and sintering thermocycles) and the surface finishing;
- − The experimental procedure used for specimen characterization.
It should be stressed that the number of studies that were considered, based on the defined inclusion criteria, was relatively low, reflecting the need for more studies in this area.
5. Conclusions
This review aimed to present the current state of the art of additive manufacturing (AM) of zirconia-based materials for dental applications, highlighting the main outcomes and advances reached in the last few years, as well as challenges. To the authors’ best knowledge, a comparative analysis of these materials’ properties was never carried out previously. Studies on this topic are relatively scarce and quite recent (all published after 2010). It is a consensus that AM has great potential to produce dental devices, mainly due to the possibility of customization, with a lower cost compared to the conventional techniques of subtractive manufacturing (SM), such as milling, uniaxial compression and cold isostatic pressure. However, the final products still lag behind those obtained by SM methods.
Vat polymerization, namely stereolithography (SLA) and digital light processing (DLP), leads to pieces with high accuracy and resolution, revealing itself to be promising for the production of dental crowns, bridges, copings, implants and abutments. Although material jetting (MJ) and robocasting (RC) also have led to interesting results, the parts produced through these technologies usually present lower resolution/accuracy and worse mechanical resistance compared to SLA and DLP, limiting their applicability to the restoration of anterior teeth.
Despite significant progress, AM of ceramic materials is still taking its first steps since it falls short in some points, e.g., the control of the process, the preparation of suitable feedstock materials, the development of dedicated ceramic printers and the obtention of materials with adequate properties that match the dentistry requirements (e.g., aesthetics, dimensional accuracy, thermal shock resistance, chemical stability, mechanical and tribological resistance). A great effort has been mounted to adapt materials, methods, and workflows to improve the mechanical performance (by reducing surface defects such as cracks and porosity), dimensional accuracy (internal fit and marginal adaptation) and aesthetics of the restorations.
In conclusion, the research on zirconia-based materials produced by AM techniques is expanding and represents serious technological progress. However, there is still a long way to go in order to include AM techniques in the dentistry industry in a way that ensures the production of safe and durable prosthesis. Further work must be done, in particular regarding the 3D printing of vitroceramic materials reinforced with zirconia, about which there are no insights. Additionally, 3D printing of dental restorations with a multilayer approach that mimics the complex properties of the natural tooth is an important topic that has not been addressed in the literature yet.
Funding Statement
To Fundação para a Ciência e a Tecnologia (FCT) for funding through the unit projects UIDB/00100/2020 (CQE), UIDB/04585/2020 (CiiEM), UID/CTM/04540/2020 (CeFEMA) and UIDB/50022/2020 (IDMEC/LAETA), and for the PhD grant of A.C. Branco (SFRH/BD/145423/2019).
Author Contributions
Conceptualization: A.C.B., R.C., C.G.F.-P. and A.P.S.; Methodology: A.C.B.; Software: A.C.B., C.G.F.-P. and A.P.S.; Validation: A.C.B., C.G.F.-P. and A.P.S.; Formal analysis: A.C.B., C.G.F.-P. and A.P.S.; Investigation, A.C.B., R.C., C.G.F.-P. and A.P.S.; Resources: A.C.B.; Data curation: A.C.B.; Writing—original draft preparation: A.C.B.; Writing—review and editing: A.C.B., R.C., C.G.F.-P. and A.P.S.; Visualization: A.C.B.; Supervision: R.C., C.G.F.-P. and A.P.S.; Project administration: R.C., C.G.F.-P. and A.P.S.; Funding acquisition: A.C.B., R.C., C.G.F.-P. and A.P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

Zirconia-based nanomaterials: recent developments in synthesis and applications

First published on 23rd August 2022
In the last decade, the whole scientific community has witnessed great advances and progress in the various fields of nanoscience. Among the different nanomaterials, zirconia nanomaterials have found numerous applications as nanocatalysts, nanosensors, adsorbents, etc. Additionally, their exceptional biomedical applications in dentistry and drug delivery, and interesting biological properties, viz. anti-microbial, antioxidant, and anti-cancer activity, have further motivated the researchers to explore their physico-chemical properties using different synthetic pathways. With such an interest in zirconia-based nanomaterials, the present review focuses systematically on different synthesis approaches and their impact on the structure, size, shape, and morphology of these nanomaterials. Broadly, there are two approaches, viz. , chemical synthesis which includes hydrothermal, solvothermal, sol–gel, microwave, solution combustion, and co-precipitation methods, and a greener approach which employs bacteria, fungus, and plant parts for the preparation of zirconia nanoparticles. In this review article, the aforementioned methods have been critically analyzed for obtaining specific phases and shapes. The review also incorporates a detailed survey of the applications of zirconia-based nanomaterials. Furthermore, the influence of specific phases, morphology, and the comparison with their counterpart composites for different applications have also been included. Finally, the concluding remarks, prospects and possible scope are given in the last section.
1 Introduction
Zirconia exists in three crystal phases, cubic, tetragonal, and monoclinic, in a typical air atmosphere and at distinct temperature ranges. The pure monoclinic phase is stable up to 1100 °C, the tetragonal phase is stable in the range of 1100–2370 °C and the cubic phase exists at temperatures over 2370 °C. 6 Among the different phases, the cubic and tetragonal crystals are found to be unstable in bulk forms at ambient temperature. Therefore, considerable efforts have been dedicated to stabilizing the unstable cubic and tetragonal crystal phases by doping with several divalent or trivalent cationic stabilizers such as Ca 2+ , Mg 2+ , Sc 3+ , and Y 3+ or by the reduction of particle/grain size to the nanometre scale. 7–10 Moreover, the interconversion of phases such as cubic to tetragonal and subsequently tetragonal to monoclinic takes place at higher temperatures and also affects the volume of zirconia nanomaterials significantly which ultimately limits their applicability in different fields such as catalysis, coatings etc. Another limitation of zirconia nanoparticles has been their tendency to undergo agglomeration during their synthesis. Furthermore, limited methods have been reported for the synthesis of anisotropic zirconia nanoparticles with the desired size range. In this regard, it appears quite worthwhile to critically analyze the various methods, routes, and reaction conditions for the synthesis of such versatile nanomaterials. Therefore, the present review article contributes toward a comprehensive analysis of the state-of-art specific synthetic methods for the preparation of zirconia nanomaterials for the desired applications and provides a database for novel future research perspectives. There are two major sections in this review paper; the first section involves details about different synthesis methods of zirconia-based nanomaterials and a critical analysis of the effect of various reaction parameters and methods on size, shape, and morphology, and in the second part, a variety of applications of these materials have been included. Finally, perspectives and scope of further developments of such materials have been given.
1.1 Synthesis of zirconia nanomaterials
Recently, Yousaf et al. 17 demonstrated the microwave synthesis of zirconia nanoparticles by using honey as a capping agent, for optical applications. The bandgap of the prepared tetragonal zirconia was found to be in the range of 4.7–4.82 eV. The structural characterization revealed the formation of single-phase tetragonal zirconium oxide with high purity under optimized conditions.
Similarly, Bukhari et al. 18 prepared zirconia nanofibers by a microwave-assisted sol–gel approach. In this study, honey was used as a structure-directing and capping agent to enhance the stability, reduce the crystallite size of nanofibers, and prevent the hard agglomeration of nanoparticles. Consequently, soft or less agglomeration resulted in a stabilized tetragonal phase of zirconia by inhibiting its interphase transformation from tetragonal into monoclinic ( Fig. 1 ).
The microwave wattage (100–900 W) also significantly affected the stabilization of zirconia nanoparticles. Powder X-ray diffraction study revealed the formation of highly pure tetragonal zirconia at low microwave power ∼100 W with a smaller crystallite size of approximately 26 nm due to the presence of an optimum quantity of honey to coat the zirconia particles.
In another interesting report by Batool et al. , 19 the preparation of zirconia nanoparticles and the dilemma of long-term stability were addressed. The obtained tetragonal zirconia nanoparticles were found to be stable even after a period of 6 to 12 months at room temperature.
Recently, Mishra et al. 20 demonstrated the synthesis of tetragonal zirconia nanoparticles having an average crystal size of 10 nm using a microwave-assisted solvothermal route. In this study, the bandgap of the prepared nanoparticles was found to be 3.67 eV.
Usually, attempting the synthesis of t-ZrO 2 nanoparticles by using an inorganic precursor without any surfactant results in the agglomeration of particles. Under such circumstances, the hydrothermal corrosion method, which involves a corroding medium such as H 2 SO 4 , HCl etc. to break down hard aggregates into dispersed fine nanoparticles during the hydrothermal treatment, could be employed. Zhou et al. 21 demonstrated the hydrothermal–corrosion method to produce highly dispersed tetragonal zirconia nanoparticles (less than 10 nm) without using any surfactants. It was stated that the dispersity and crystallinity of the prepared tetragonal ZrO 2 powders can be modified by using reaction conditions such as calcination of the precipitates followed by hydrothermal HCl corrosion at different temperatures. In the follow-up study, Sagadevan et al. 22 investigated the synthesis of spherical zirconia nanoparticles by the hydrothermal technique. The optical band gap of 5.02 eV and photoluminescence measurements confirmed the presence of oxygen vacancies accompanied by intrinsic defects in such NPs.
Ahmad et al. 23 successfully prepared ZrO 2 nanoparticles in an alkaline medium by employing the hydrothermal technique. Investigation of the structure and phase composition of the ZrO 2 nanoparticles by powder X-ray diffraction indicated the presence of pure monoclinic zirconia. The dielectric investigations suggested that ZrO 2 nanoparticles could be used for storage and electronic devices owing to the values of dielectric constant and dielectric loss, which were 7.5 and 0.0094, respectively.
Meetei et al. 24 demonstrated the synthesis of white light-emitting nanocrystals composed of cubic ZrO 2 doped with Eu 3+ via the hydrothermal method. In this study, the dopant Eu 3+ was employed to stabilize the cubic phase with simultaneous production of the red counterpart from the white light. The wide range of photoluminescence emission arose due to the oxygen vacancies which could coalesce with a dopant to produce white light within the sample. The prolonged lifetime of the red counterpart of the sample could efficiently rectify the issue of the brief lifetime of traditional phosphors. Such materials could be beneficial in simulating the daylight of the sun by preparing light-emitting diodes, electronic flash, and lights for theatres and operas.
Noh et al. 25 showcased the synthesis of anisotropic-shaped zirconia nanocrystals by using amorphous zirconium hydroxide powder and tetragonal zirconia powder at 150–250 °C along with varying concentrations of sodium hydroxide as an additive. The higher temperature facilitated the transformation of nanocrystals from spherical into spindle/rod-like structures with increase in the monoclinic phase fraction. Additionally, the fraction of the monoclinic phase was also found to increase with increasing sodium hydroxide concentration.
Abdelaal et al. 26 reported a one-pot method for the fabrication of hollow sub-microspheres of zirconia having a diameter of 300 nm and a wall of thickness of 8 nm. In the first step, in situ preparation of the core@shell composite was achieved via autoclaving of glucose monohydrate as a core and sacrificial template, and zirconyl chloride octahydrate as the oxide precursor. Subsequently, calcination at 550 °C for 4 h resulted in the formation of a hollow sphere of ZrO 2 due to the removal of the template.
In another study, Nath et al. 27 reported the synthesis of highly thermally stable zirconia nanoparticles by a surfactant-assisted hydrothermal route ( Fig. 2 ). Different types of surfactants such as cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), and Triton X-100 were used in the synthesis. Consequently, different morphologies of nanocrystals, for example, rod and mortar–pestle-shaped particles with different sizes were obtained. The distinct modifications in phase composition and morphology can also be achieved by tuning the reaction conditions during the preparation of the nanoparticles.
Stolzenberg et al. 28 demonstrated the fractal growth of ZrO 2 nanocrystals by varying the temperature and concentrations of the zirconia precursor and benzyl alcohol during their synthesis. A possible reaction mechanism for the non-aqueous preparation of ZrO 2 NPs under solvothermal conditions was proposed. The growth mechanism involved two steps, in the first step, the precursor reacted with benzyl alcohol (solvent) through a ligand exchange to convert it into an intermediate which finally reacts in a subsequent ether condensation reaction to form zirconia nanoparticles ( Fig. 3 ). During the growth of NPs, the size increases from 2.7 nm to 7 nm along with phase transformation from tetragonal into monoclinic. Transmission electron microscopic images revealed that the nanoparticles grew from a round shape to a dendritic form along with the transformation of phase. In contrast to the supposition that only thermodynamics could control phase transformation, the reported study proposed that phase transition could be controlled by kinetics as well.
Zirconia nanotubes in the range of 60–90 nm were also prepared by hydrothermal treatment. For this, 50 mL aqueous solution of each of ZrO(NO 3 ) 2 · x H 2 O (0.5 M) and NaOH (5 M) were uniformly mixed followed by the addition of 2 mL of absolute ethanol as a buffering agent. The final mixture was taken in a hydrothermal vessel which was heated at 250 °C for 14 h. 29
Reddy et al. 31 demonstrated that hydrothermal treatment of a zirconyl chloride octahydrate precursor and NH 4 OH at 200 °C for 12 h could result in pure tetragonal zirconia NPs without using any template. The prepared nanoparticles were spherical.
Madhusudhana et al. 32 reported the synthesis of monoclinic zirconia nanoparticles by a facile sol–gel technique using acetic acid as a chelating agent. The resultant nanoparticles were found to be suitable for thermal applications due to their high resistance behavior to crack propagation. Lim et al. 33 developed a synthesis of tetragonal phase zirconia nanoparticles. Different concentrations of NH 3 solution and nitric acid were used with zirconium isopropoxide to analyze the impact on the crystallinity and particle size of ZrO 2 . In contrast, smaller sizes of nanoparticles were obtained when nitric acid was used instead of ammonia in the reaction mixture.
Bahari et al. 34 investigated the preparation of ZrO 2 at a relatively lower temperature. It was observed that some amount of the monoclinic phase gets converted into metastable tetragonal ZrO 2 during heating. Heshmatpour et al. 13 reported a simple sol–gel synthesis of zirconia nanoparticles of uniform size. Glucose and fructose were used as organic additives which could prevent the phase transition from the monoclinic to the tetragonal phase.
Likewise, the synthesis of zirconia thin films having a pure tetragonal phase was reported by Majedi et al. 35 via a sucrose-mediated sol–gel method. Initially, for preparing the sol, the zirconium( IV ) acetylacetonate precursor was stirred in ethanol at 70 °C for 30 min followed by the addition of an ammoniacal solution as the hydrolyzing agent. Subsequently, sucrose was added to the solution as a gelation agent under stirring at 110 °C which results in the formation of the sol. The as-obtained sol was transformed into a xerogel by drying in an oven at 80 °C for 24 h. Furthermore, calcination at 350–500 °C results in t-zirconia, and calcination at 650 °C results in the formation of a mixture of the tetragonal and monoclinic phases. Such findings were expected as the post-calcination temperature could influence phase formation as well as phase transition.
Sponchia et al. 36 demonstrated a simple and efficient neutral surfactant-assisted sol–gel method for the synthesis of spherical mesoporous zirconia nanoparticles by using a zirconium propoxide precursor, surfactant, ethanol, and alkali halide, followed by solvothermal thermal treatment using ethanol and water. The obtained dried powder was subjected to heat treatment under vacuum conditions to extract the surfactant without any structural modification. The prepared material exhibited a high surface area in comparison to literature reports. The effect of the addition of different alkali halides on the shape and size of the nanoparticle was also investigated. The salt's solubility plays a crucial role in governing the particle size. Biological experiments demonstrated that mesoporous zirconia nanoparticles were biocompatible, degradable, and cell-permeable, therefore they could be employed for theranostic applications.
Ramachandran et al. 38 reported a co-precipitation method for the synthesis of zirconia nanoparticles using zirconium nitrate as the precursor and KOH as the precipitating agent. The ratio of zirconium nitrate and KOH (0.5, 1, and 1.5 M) was optimized to obtain crystalline zirconium oxide with spherical morphology. At the same line, Foo et al. 39 demonstrated the preparation of uniformly shaped zirconia nanoparticles having crystal sizes in the range of 6 to 35 nm. In this study, it was demonstrated that the calcination temperature in the reaction procedure could significantly impact the surface morphology, crystal size, and purity of the resulting ZrO 2 NPs. A higher calcination temperature increased the degree of agglomeration and size of crystals. Similarly, Huang et al. 40 also demonstrated that calcination temperature and time could drastically influence the crystallite size of tetragonal ZrO 2 . In this study, when the freeze-dried precursor powder was calcined at 1173 K, crystallites of size 3.3 nm were obtained; however, comparatively bigger crystallites of 20 nm were observed upon calcination at 1473 K.
Dwivedi et al. 42 prepared ZrO 2 NPs by a microwave-driven citrate sol–gel technique. In this study, the prepared zirconia nanoparticles exhibited a smaller size due to gelation and rapid combustion.
Prakash Babu et al. 46 also introduced a simple low-temperature solution combustion method without any stabilizing agent and calcination. In this method, zirconyl nitrate as the metal precursor and oxlayl di-hydrazide as the fuel were dissolved in water. The aqueous solution was taken in a dish and subjected to heat treatment at 400 °C in a preheated muffle furnace. Zirconia nano-powder having a cubic phase and crystallite size in the range from 6 to 12 nm was obtained.
Similarly, Tang et al. 47 reported the synthesis of hollow mesoporous zirconia nanocapsules with a porous shell structure. In this study, first, monodispersed silica nanoparticles were prepared via Stober's method using concentrated ammonia, water, ethanol, and TEOS followed by the formation of the SiO 2 @ZrO 2 composite using Zr(OBu) 4 . Finally, the composite was treated with NaOH (5 M) to remove silica which resulted in the formation of hexagonal mesoporous ZrO 2 .
Though different methods for the formation of specific phases have been reported, microwave-assisted sol–gel and solution combustion methods can be identified as promising choices for the formation of specific phases. Particularly, with the solution combustion method, formation of the cubic phase has been observed predominantly. This could be due to the attainment of high temperature that favors the formation of the cubic phase which is also a high-temperature stable form of zirconia. Interestingly, the use of microwave treatment in the case of the sol–gel has also resulted in the formation of selective phases, particularly the tetragonal phase. Apart from that, employing a corrosive solvent (HCl, H 2 SO 4 ) during the hydrothermal treatment of zirconia nanoparticles could be a useful approach for obtaining monodisperse nanoparticles.
In most of the published literature studies on zirconia nanomaterials, the formation of spherical particles has been reported. However, in some studies, the formation of unique shapes has also been reported using unusual reaction conditions and precursors. For instance, the formation of nanosheets has been reported via the sol–gel method using zirconium acetylacetonate as the metal precursor, and the formation of cubic and star-like shapes has been reported via the hydrothermal method at a comparatively higher temperature of 240–250 °C. 29,30,48 Interestingly, comparatively smaller particle sizes have been observed along with the pure tetragonal phase in all types of chemical synthesis methods.
Nowadays, extracts of different parts of plants such as roots, leaves, flowers, and fruits are also being used for the preparation of nanomaterials. Various phytochemicals, viz. flavones, terpenoids, sugars, ketones, aldehydes, carboxylic acids, and amides, are found to be responsible for the reduction of metal precursors. These species act as both stabilizing and reducing agents. In general, the plant-mediated synthesis of zirconia NPs is a simple and facile process, in which a zirconia salt, such as zirconium oxychloride or zirconia nitrate is mixed and stirred with an appropriate plant extract at a suitable temperature followed by heat treatment. However, there are limited reports on the mechanism of formation and stabilization of zirconia NPs from such biomolecules. Different reaction parameters, and resultantly different findings in terms of crystal structure and size distribution from recent reports on plant-mediated synthesis have been summarized in Table 3 .
Silva et al. 57 demonstrated a green approach for the preparation of zirconia nanoparticles, exhibiting monoclinic and tetragonal phases by using Euclea natalensis plant extract. In this study, factorial design suggested that a higher concentration of precursor, lower amount of extract, and higher calcination temperature supported the synthesis of the tetragonal phase. Moreover, a possible mechanism ( Fig. 5 ) for the preparation of ZrO 2 NPs using phytochemicals of Euclea natalensis such as pentacyclic terpenoids (lupeol, betulin, and β-sitosterol) and naphthoquinones (diospirine, shinanolone, and 7-methyl juglone) is also given in this report. However, there is ambiguity in the mechanism of formation of ZrO 2 NPs. It involves three steps: the reduction of zirconyl ions, the grouping of nanoparticles, and the growth of nanoparticles. But, only the reduction of zirconium ions could not lead to the formation of ZrO 2 as oxidation is also a necessary step for its formation. In another report, 58 bioreduction of zirconium ions, followed by complexation with water molecules, chelation with phytochemicals and finally oxidation were the major suggested steps in the plant-mediated synthesis of zirconia. As per our recent investigation on the plant-mediated synthesis of ZrO 2 , which is in the process of publication, a change in the oxidation state of zirconium from +4 to +2 followed by complexation with phytocomponents was observed. The complex upon heat treatment regains its oxidation state of +4 in zirconia as that of the precursor (ZrOCl 2 ). These findings were supported by DFT calculations.
Another study on the green fabrication of ZrO 2 NPs was reported by Jalill et al. 59 and the prepared nanoparticles exhibited excellent antifungal and antibacterial activity. Shanthi et al. 60 synthesized ZrO 2 nanoparticles by employing aqueous leaf extracts of Acalypha indica as a reducing agent. Encouraged by previous studies, Padma et al. 61 fabricated zirconium dioxide nanoparticles using Azadirachta indica leaves. Similarly, Sathish et al. 62 synthesized zirconia nanoparticles using Curcuma longa tuber. In this study, a higher pH was found to be favorable for the fabrication of zirconia NPs. Similarly, Gowri et al. 63 investigated the greener synthesis of spherical zirconia nanoparticles using Aloe vera plant extract. The nanoparticles were found to have sizes in the range of 50–100 nm. Majedi et al. 64 reported the synthesis of cubic phase zirconia nanoparticles using zirconium acetate as the precursor and lemon juice as the reducing agent. The impacts of sucrose addition on enhancing the particle size and the yield of the product were examined. A mixture of 20 mL lemon juice and sucrose was found to show a positive effect on uniform morphology having an average particle size of 21 nm. The as-prepared nanoparticles were employed in electrolyte materials for intermediate or low-temperature solid oxide fuel cells. Saraswathi et al. 65 demonstrated the fabrication of tetragonal zirconia nanoparticles (2 nm) using Lagerstroemia speciosa leaves. The authors investigated the photocatalytic activity of the nanoparticles by monitoring the degradation of an azo dye using sunlight and reported excellent photocatalytic degradation of up to 94.58%.
It can be pointed out from the data listed in Tables 2 and 3 that zirconia nanomaterials prepared via greener routes (plant and microorganism-mediated methods) were found to have mainly pure phases either monoclinic or tetragonal. However, small particles having sizes in the range of 6–9 nm can be observed in all kinds of crystal systems known for zirconia. Interestingly, in almost all the instances of microorganism-mediated synthesis, heat treatment was not given which is surprising as merely the use of enzymes and proteins for the transformation of the zirconium precursor into zirconia could not yield fruitful results under ex situ conditions. However, in most of the plant-mediated synthesis, heat treatment in the temperature range of 60–120 °C of the reaction mixtures as well as in the range of 300–400 °C of the reaction product was given to obtain ZrO 2 .
1.2 Applications of zirconia and its nanocomposites
Metal organic frameworks (MOFs) constitute a new emerging class of porous materials. However, these materials are still in early stages of development and gradually progressing towards diverse applications such as catalysis, drug delivery, fluorescence sensing, etc. Zr based metal organic frameworks (Zr-MOFs) are most likely considered as encouraging MOF materials owing to their excellent physico-chemical properties and outstanding chemical stability. In 2008, an extraordinary discovery was made by Cavka et al. , 84 wherein 12-coordinated Zr 6 (μ 3 -O) 4 (μ 3 -OH) 4 (CO 2 ) 12 clusters were synthesized, commonly referred to as UiO-66. It was found that the chemical and hydrothermal stability of UiO-66 was much higher than that of the other reported MOFs. 85–88 Since then, extensive efforts have been dedicated for the synthesis of Zr based MOFs, and the investigation of their properties, structures, functions and applications has been carried out intensively.
Schaate et al. 89 described the very first example of employing a modulated fabrication approach to prepare Zr based MOFs and studied the effects of different modulators such as acetic acid, water and benzoic acid on the formation of Zr-MOFs. Interestingly, it was found out that among all modulators, only benzoic acid affected the synthesis process; as its concentration was increased, bigger and quite distinct crystals were expected to form from the intermediates of UiO-66. Hence, the first large single crystals of Zr-MOF were obtained by this strategy, as confirmed by the single-crystal XRD technique. An interesting study by Zhou et al. 90 revealed the catalytic activity of UiO-66 prepared by using terephthalic acid and ZrCl 4 . The catalyst demonstrated excellent recyclability for the preparation of biodiesel using tributyrin and soybean oil with methanol. The performance of the catalyst was significantly dependent upon linker defects under different reaction parameters. Similarly, Abou-Elyazed et al. 91 reported the use of UiO-66(Zr)-green, UiO-66(Zr)-NO 2 -green, and UiO-66(Zr)-NH 2 green for the preparation of biodiesel. Among all these MOFs, UiO-66(Zr)-NH 2 is found to be an efficient catalyst with an activation energy of 15.13 kJ mol −1 . Xie et al. 92 demonstrated a one-pot preparation of biodiesel from low-cost acidic vegetable oils by using the AILs/POM/UiO-66-2COOH catalyst with 95.8% conversion of oil under optimized conditions. The obtained catalyst exhibited various merits such as good activity, higher surface area, reusability up to 5 cycles, and tolerance to high amounts of free fatty acid (9 wt%) and water (3 wt%) in the feedstock. Lu et al. 93 synthesized sulphated Zr-based MOFs which had flower-like mesoporous nanosheets of zirconium and sulphate. In this study, the catalyst exhibited excellent activity, larger surface area (186.1 m 2 g −1 ), and recyclability for the transesterification reaction. Recently Zhang et al. 94 prepared a ZrSiW/UiO-66 metal–organic framework by a hydrothermal method and used it for the biodiesel production. The prepared catalyst exhibited good activity, high surface area, and showcased heterogeneous nature as per the mechanism depicted in Fig. 7 .
It is manifested from the literature cited in Table 4 that zirconia-based nanomaterials with enhanced acidity (sulphated zirconia) have been investigated extensively as a catalyst for simultaneous transesterification and esterification. However, a comparatively higher temperature and molar ratio of methanol to oil is required for the high yield of the reaction in contrast to the catalyst with enhanced basic sites (zirconia modified with KOH). Therefore, exploring Zr-based MOF and composites with basic sites could result in an efficient catalyst for transesterification.
As can be seen in Table 5 , different sizes of ZrO 2 NPs and their nanocomposites have been used for the degradation of different dyes. Therefore, a clear conclusion about their photocatalytic activity can't be drawn directly. However, a generalization on the band-gap which is responsible for photocatalysis can be made. The lowest band gap of ZrO 2 was observed in its composites with reduced graphene oxide followed by that for the pure ZrO 2 NPs having a spherical shape and an average size of 17 nm. Thus, composites of small size ZrO 2 could further result in promising photocatalytic activities.
Gowri et al. 67 synthesized zirconia nanoparticles of 50–100 nm using leaves of aloe vera and investigated their antimicrobial and antifungal properties. Abdul et al. 59 demonstrated excellent antifungal and antibacterial properties of ZrO 2 NPs against F. moniliforme , F. graminearum , E. coli , and S. aureus . Similarly, Imran et al. 104 prepared Fe 3 O 4 -doped ZrO 2 nanoparticles of average size ∼23 nm by the microwave method and investigated their antimicrobial activity. These nanoparticles (NPs) were used as dental fillers. Kumaresan et al. 105 demonstrated the antibacterial activity of zirconia NPs against Bacillus subtilis , Escherichia coli , and Salmonella typhi . The authors used marine brown alga, Sargassum wightii for the synthesis of zirconia nanoparticles having the size of ∼4.8 nm. Masim et al. 106 prepared a polyaniline zirconia (PANI-ZrO 2 ) composite and employed it for the treatment of wastewater. The obtained composite showed antibacterial and anti-corrosion activity and acted as a phosphate adsorbent material.
Fathima et al. 107 reported the fabrication of zirconia nanoparticles having sizes in the range of 15–21 nm and investigated their antimicrobial activity. In this study, they identified antimicrobial activity against Gram-positive ( Bacillus subtilis and Staphylococcus aureus ) and Gram-negative bacteria ( Escherichia coli and Pseudomonas aeruginosa ), and found superior inhibitory activity against Pseudomonas aeruginosa at the concentration of 100 μg mL −1 . The antimicrobial activity of ZrO 2 NPs for different bacterial species is listed in Table 6 . In another study, ZrO 2 –ZnO nanoparticles were prepared by the sol–gel method and further evaluated for their antibacterial activity. 108 The mechanism of antibacterial activity by ZrO 2 NPs was proposed by Asha et al. 43 against Pseudomonas aeruginosa due to the rapid attraction between the negatively charged cell walls of Pseudomonas aeruginosa and positively charged zirconia NPs. Khan et al. 109 prepared glutamic acid-functionalized ZrO 2 NPs by using a solvothermal method and studied their antimicrobial activity against four strains of oral bacteria, viz. , Streptococcus mutans , Rothia dentocariosa , Streptococcus mitis , and Rothia mucilaginosa . The alteration of the surface of ZO 2 with glutamic acid increases the dispersion characteristics of nanoparticles in an aqueous medium which in turn possibly increases interactions between bacteria and nanoparticles.
Derbalah et al. 110 reported antifungal activity against Rhizoctonia solani which is known to be responsible for root rot in cucumber. The induction of resistance was examined using a real-time polymerase chain reaction. A concentration of 100 μg L −1 of zirconia nanoparticles showed better inhibition to improve the growth of the cucumber plant than the untreated cucumber plant. Ahmed et al. 53 reported the synthesis of biogenic ZrO 2 NPs by using Enterobacter sp. and studied their antifungal activity against P. versicolor ( Fig. 9 ). In this study, nanoparticles ruptured the cells of pathogens by getting absorbed on the cell membrane and demonstrated a significant inhibition zone at the concentration level of 20 μg mL −1 . Similarly, Balaji et al. 68 demonstrated the synthesis of spherical zirconia nanoparticles (∼9 to 11 nm) by using the leaf extract of Eucalyptus globulus . In this study, the antioxidant activity of the prepared material was also investigated using human colon carcinoma (HCT-116) and human lung carcinoma (A-549) cell lines by DPPH assay. The antioxidant studies of the prepared zirconia NPs displayed significant inhibition (85.6%) at the concentration level of 158.15 μg mL −1 . The cytotoxicity of the prepared ZrO 2 was also investigated against HCT-116 colon cancer and A-549 lung cancer cell lines. A mechanism for the action of ZrO 2 towards cytotoxicity is also given in this report where ROS have been identified in key roles ( Fig. 10 ). Furthermore, the anticancer activity of zirconia-based nanomaterials against various cell lines, and their % reduction in cell viability are summarized in Table 7 .
Based upon data listed in Tables 3 and 5 , it can't be concretely concluded that a particular phase of zirconia would be highly efficient for antibacterial and anticancer activity as most of the studies have been carried out on different microorganisms and cell lines by different methods. However, prima facie , the tetragonal phase seems to be more efficient as it had shown antibacterial activity on a range of microorganisms and significant anticancer activity on a range of cell lines. But, composites of zirconia with silver or yttria or iron oxide have shown promising results for both antibacterial as well as anti-cancer activities. Probably, composites of the tetragonal phase of zirconia could show enhanced superior activities.
It can be suggested from the literature summarized in Table 8 that both bare ZrO 2 and its composites could be employed for the adsorption of pollutants from effluents effectively. Specifically, designing composites of ZrO 2 NPs with graphene oxide could be a useful approach for fabricating economical and efficient adsorbents for a range of pollutants and industrial-by-products.
Recently, an yttria-stabilized t-zirconia polycrystal material has been used for implants and dental applications due to its excellent mechanical properties. 7 Interestingly, zirconia-based nanomaterials can also be used to improve the physical and mechanical properties of polymethyl methacrylate (PMMA) which is a denture repair material. Gad et al. 126 also demonstrated that the tensile strength of PMMA could be increased using zirconia nanoparticles and can be used as a denture base with reduced translucency. Lohbauer et al. 127 demonstrated the preparation of spherical ZrO 2 NPs in the range of 20–50 nm using laser vaporization. These nanomaterials were used as fillers in the dental adhesive.
Fathima et al. 107 synthesized zirconia nanoparticles and demonstrated their excellent antibacterial activity and anti-decay application on teeth. Bacteria may generate acids during their metabolic activities which result in the demineralization of teeth ( Fig. 12 ). However, coating with zirconia NPs has shown resistance to acid attacks as well as remarkable activity against oral pathogens bearing negatively charged cell walls. The electrostatic interactions between positively charged nanoparticles and charged microorganisms inhibit the activities of microbes. Gad et al. 128 reported a novel method for denture stomatitis inhibition by using ZrO 2 nanoparticles owing to their antifungal activity. The authors investigated the inhibitory effect of ZrO 2 nanoparticles on Candida albicans adhesion to restore polymethyl methacrylate denture bases. Similarly, in another investigation by Oshima et al. , 129 a zirconia/alumina composite nanomaterial (Ce-TZP/Al 2 O 3 ) was found to increase the osseointegration capability and osteogenic response of implants, where the surface of the nanostructured Ce-TZP/Al 2 O 3 was modified by HF treatment.
Yamada et al. 130 prepared silver NP-coated yttria-stabilized ZrO 2 and investigated its antibacterial activity against oral pathogens such as Staphylococcus aureus , Streptococcus mutans , Escherichia coli , and Aggregatibacter actinomycetemcomitans ( Fig. 13 ). The composite shows antimicrobial activity, particularly against Escherichia coli . The efficacy of the prepared material was tested via an accelerated aging test. In this test, the release profile of silver NPs was investigated which was found to be less than 1 percent, hence the prepared material was found to be thermally stable. Therefore, it was suggested that the bactericidal action of the composite was due to its contact with the bacteria and not owing to the release of silver NPs. Thus, the prepared material could be further investigated for the treatment of dental caries and periodontal disease.
Garadkar et al. 132 fabricated thick film resistors using nanostructured ZrO 2 (25 nm) via the screen printing technique. The as-prepared resistor was used for the selective detection of H 2 gas and showed a significant signal at room temperature even in the presence of a smaller amount of H 2 . It was reported that films exhibited a clear response to 50 ppm of H 2 in contrast to other gases at a higher amount of 1000 ppm. Chikere et al. 133 fabricated a zirconia nanoparticle modified carbon paste electrode and employed it for the electrochemical detection of gallic acid in a concentration range of 1 × 10 −6 to 1 × 10 −3 mol L −1 . The weak ionic interactions between the oxygen anions of zirconia nanoparticles (monoclinic) and positively charged graphite sheets, as depicted in Fig. 14 , were found to stabilize the sensor. The designed sensor generated a signal via electrochemical oxidation of gallic acid and was employed for detection of the latter in red and white wine.
Wang et al. 134 prepared zirconia nanoparticles having an average size of 20 nm and employed them to make a thick film humidity sensor using a substrate. The prepared sensor showed a change in impedance from 10 6 to 10 2 Ω with an increase in relative humidity (RH) from 11 to 98%. In this report, the impact of temperature on the impedance of the sensor was evaluated and the conduction process of the sensor was explained using an equivalent circuit with resistors and capacitors. Gong et al. 135 fabricated a nanostructured composite of zirconia and graphene by solid-phase extraction. The prepared material afforded high recognition and enrichment for phosphoric moieties due to the NPs of ZrO 2 , large surface area, and high conductivity owing to graphene nanosheets. The prepared material was successfully employed for the detection of methyl parathion via square-wave voltammetry.
Trinadh et al. 136 prepared an electrochemical sensor using graphene quantum dots (GQDs) and lanthanum doped zirconia nanoparticles for the detection of flutamide in urine samples as per the scheme depicted in Fig. 15 . For designing this sensor, La 3+ @ZrO 2 was prepared by the coprecipitation method using a solution of zirconyl chloride and lanthanum nitrate as metal precursors and NH 4 OH as the precipitating agent. The addition of NH 4 OH decreases the pH of the solution which leads to the formation of precipitates. The obtained precipitates were washed with methanol and water, dried, and calcined at 600 °C. Finally, the glass carbon electrode-based sensor was fabricated by drop casting a suspension of 1.0 mg of GQDs and 1.0 mg of La 3+ @ZrO 2 on a pre-treated GCE. In this study, the signal was generated due to the electrochemical reduction of flutamide (12 μM) in phosphate buffer solution (pH = 7).
Lee et al. 137 demonstrated the preparation of Au nanoparticle coated zirconia nanofibers and employed them as a surface-enhanced Raman scattering sensor for the detection of a variety of pesticide (carbaryl, phosmet, cypermethrin, and permethrin) residues in fruits or vegetables. In this study, nanofibers of zirconia were fabricated on a silicon wafer via the spin coating technique using a solution of ZrCl 4 in isopropanol. Afterward, gold NPs were deposited on ZrO 2 nanofibers via an electron beam evaporator. The prepared sensor shows excellent selectivity to organophosphates even in the presence of multiple pesticides. It was successfully employed for a practical application using diluted juice containing sliced pesticide-containing apple peels and could differentiate different pesticides.
The literature summarized in Table 9 revealed that ZrO 2 or its composites could be employed for designing a range of sensors based upon different techniques such as electrochemical, luminescence etc. However, significant LODs have been displayed by them via electrochemical signals. Specifically, a cyclic voltammetry-based signal from such sensors seems to be a more promising one owing to the electrical properties of ZrO 2 .
2 Outlook and futuristic scope
Experimentally, two approaches have been employed to enhance the stability and applications of zirconia nanoparticles. The first one involves in situ modification with stabilizers and other metal oxides such as CaO, MgO, and Y 2 O 3 during the preparation of the nanomaterials. Modification with another metal oxide could generate vacancies stimulating changes in the electronic and geometric environment that can further enhance the optical and electrical properties which is also supported by several theoretical studies. 143–145 The second strategy involves exploring greener methods with novel additives that could be employed. Although the synthesis of zirconia nanomaterials using plant extracts and microbes is considered to be clean, nontoxic, simple, and safe, there are only a few reports in this regard and sufficient efforts have not been made with diverse varieties of plants and microorganisms with different possible reaction pathways. Moreover, the combination of the sol–gel method and greener reactants is rarely reported. Specifically, the sol–gel method offers unique advantages such as good control over the structure and kinetics of the process. Moreover, fine control of the composition of a multicomponent nanomaterial could be achieved via this method as small quantities of dopants can be introduced in the sol and uniformly dispersed in the final product. Therefore, further improvising this method using greener ingredients by keeping in view the motive of increasing the strength and biocompatibility could result in the invention of new materials suitable for various applications such as dentistry, catalysis, sensors, etc.
Conflicts of interest
Acknowledgements.
- J. L. Gole, S. M. Prokes, J. D. Stout, O. J. Glembocki and R. Yang, Adv. Mater. , 2006, 18 , 664–667 CrossRef CAS .
- S. Zhuiykov and N. Miura, Sens. Actuators, B , 2007, 121 , 639–651 CrossRef CAS .
- G. D. Wilk and R. M. Wallace, Appl. Phys. Lett. , 2000, 76 , 112–114 CrossRef CAS .
- X. J. Jin, Curr. Opin. Solid State Mater. Sci. , 2005, 9 , 313–318 CrossRef CAS .
- S. Gupta, S. Noumbissi and M. F. Kunrath, Med. Devices Sens. , 2020, 3 , 1–8 Search PubMed .
- B. Tyagi, K. Sidhpuria, B. Shaik and R. V. Jasra, Ind. Eng. Chem. Res. , 2006, 45 , 8643–8650 CrossRef CAS .
- E. Camposilvan, F. G. Marro, A. Mestra and M. Anglada, Acta Biomater. , 2015, 17 , 36–46 CrossRef CAS PubMed .
- L. Renuka, K. S. Anantharaju, S. C. Sharma, H. P. Nagaswarupa, S. C. Prashantha, H. Nagabhushana and Y. S. Vidya, J. Alloys Compd. , 2016, 672 , 609–622 CrossRef CAS .
- E. Mustafa, M. Wilhelm and W. Wruss, Ceram. Int. , 2003, 29 , 189–194 CrossRef CAS .
- K. Anandan, K. Rajesh, K. Gayathri, S. Vinoth Sharma, S. G. Mohammed Hussain and V. Rajendran, Phys. E , 2020, 124 , 114342 CrossRef CAS .
- J. Liang, Z. Deng, X. Jiang, F. Li and Y. Li, Inorg. Chem. , 2002, 41 , 3602–3604 CrossRef CAS PubMed .
- A. Behbahani, S. Rowshanzamir and A. Esmaeilifar, Procedia Eng. , 2012, 42 , 908–917 CrossRef .
- F. Heshmatpour and R. B. Aghakhanpour, Powder Technol. , 2011, 205 , 193–200 CrossRef CAS .
- E. Geuzens, G. Vanhoyland, J. D'Haen, M. K. Van Bael, H. Van Den Rul, J. Mullens and L. C. Van Poucke, Key Eng. Mater. , 2004, 264–268 , 343–346 CAS .
- C. O. Kappe, Angew. Chem., Int. Ed. , 2004, 43 , 6250–6284 CrossRef CAS PubMed .
- A. K. Singh and U. T. Nakate, Sci. World J. , 2014, 2014 , 349457 CAS .
- H. Yousaf, M. Azhar, M. Bashir, S. Riaz, Z. N. Kayani and S. Naseem, Optik , 2020, 222 , 165297 CrossRef CAS .
- B. S. Bukhari, M. Imran, M. Bashir, S. Riaz and S. Naseem, J. Sol-Gel Sci. Technol. , 2018, 87 , 554–567 CrossRef CAS .
- T. B. Riaz, K. B. Hadi and S. Naseem, J. Mech. Behav. Biomed. Mater. , 2020, 112 , 104012 CrossRef PubMed .
- S. Mishra, A. K. Debnath, K. P. Muthe, N. Das and P. Parhi, Colloids Surf., A , 2021, 608 , 125551 CrossRef CAS .
- H. Zhou, S. Pu, J. Huo, W. Cao, B. Wang and J. Li, Ceram. Int. , 2016, 42 , 15005–15011 CrossRef CAS .
- S. Sagadevan, J. Podder and I. Das, J. Mater. Sci.: Mater. Electron. , 2016, 27 , 5622–5627 CrossRef CAS .
- T. Ahmad, Mater. Sci. Eng. Int. , 2017, 1 , 4–8 Search PubMed .
- S. D. Meetei and S. D. Singh, J. Alloys Compd. , 2014, 587 , 143–147 CrossRef CAS .
- H. J. Noh, D. S. Seo, H. Kim and J. K. Lee, Mater. Lett. , 2003, 57 , 2425–2431 CrossRef CAS .
- H. M. Abdelaal, Mater. Lett. , 2018, 212 , 218–220 CrossRef CAS .
- S. Nath, A. Biswas, P. P. Kour, L. S. Sarma, U. K. Sur and B. G. Ankamwar, J. Nanosci. Nanotechnol. , 2018, 18 , 5390–5396 CrossRef CAS PubMed .
- P. Stolzenburg, A. Freytag, N. C. Bigall and G. Garnweitner, CrystEngComm , 2016, 18 , 8396–8405 RSC .
- G. Bhanjana, N. Dilbaghi, S. Chaudhary, K. H. Kim and S. Kumar, Analyst , 2016, 141 , 4211–4218 RSC .
- Z. Shu, X. Jiao and D. Chen, CrystEngComm , 2013, 15 , 4288–4294 RSC .
- C. V. Reddy, B. Babu, I. N. Reddy and J. Shim, Ceram. Int. , 2018, 44 , 6940–6948 CrossRef CAS .
- R. Madhusudhana, M. A. Sangamesha, R. G. Krishne Urs, L. Krishnamurthy and G. L. Shekar, Int. J. Adv. Res. , 2014, 2 , 433–436 Search PubMed .
- H. S. Lim, A. Ahmad and H. Hamzah, AIP Conf. Proc. , 2013, 1571 , 812–816 CrossRef CAS .
- A. Bahari and M. Ghanbari, Int. J. ChemTech Res. , 2011, 3 , 1686–1691 CAS .
- A. Majedi, F. Davar, A. Abbasi and A. Ashrafi, J. Inorg. Organomet. Polym. Mater. , 2016, 26 , 932–942 CrossRef CAS .
- G. Sponchia, E. Ambrosi, F. Rizzolio, M. Hadla, A. Del Tedesco, C. R. Spena, G. Toffoli, P. Riello and A. Benedetti, J. Mater. Chem. B , 2015, 3 , 7300–7306 RSC .
- F. Davar and M. R. Loghman-Estarki, Ceram. Int. , 2014, 40 , 8427–8433 CrossRef CAS .
- M. Ramachandran, R. Subadevi, W. R. Liu and M. Sivakumar, J. Nanosci. Nanotechnol. , 2018, 18 , 368–373 CrossRef CAS PubMed .
- Y. T. Foo, A. Z. Abdullah, B. Amini Horri and B. Salamatinia, Ceram. Int. , 2019, 45 , 22930–22939 CrossRef CAS .
- H. J. Huang and M. C. Wang, Ceram. Int. , 2013, 39 , 1729–1739 CrossRef CAS .
- A. K. Singh and U. T. Nakate, Sci. World J. , 2014, 2014 Search PubMed .
- R. Dwivedi, A. Maurya, A. Verma, R. Prasad and K. S. Bartwal, J. Alloys Compd. , 2011, 509 , 6848–6851 CrossRef CAS .
- S. Baby Asha, D. Muthuraj, E. Kumar and V. Veeraputhiran, J. Nanosci. Nanotechnol. , 2019, 5 , 642–644 Search PubMed .
- S. Manjunatha and M. S. Dharmaprakash, J. Lumin. , 2016, 180 , 20–24 CrossRef CAS .
- M. R. H. Siddiqui, A. I. Al-Wassil, A. M. Al-Otaibi and R. M. Mahfouz, Mater. Res. , 2012, 15 , 986–989 CrossRef CAS .
- D. Prakash babu, R. Hari Krishna, B. M. Nagabhushana, H. Nagabhushana, C. Shivakumara, R. P. S. Chakradar, H. B. Ramalingam, S. C. Sharma and R. Chandramohan, Spectrochim. Acta, Part A , 2014, 122 , 216–222 CrossRef CAS PubMed .
- S. Tang, X. Huang, X. Chen and N. Zheng, Adv. Funct. Mater. , 2010, 20 , 2442–2447 CrossRef CAS .
- F. Davar, A. Hassankhani and M. R. Loghman-Estarki, Ceram. Int. , 2013, 39 , 2933–2941 CrossRef CAS .
- S. Iravani, H. Korbekandi, S. V. Mirmohammadi and B. Zolfaghari, Res. Pharm. Sci. , 2014, 9 , 385–406 CAS .
- R. K. Das, V. L. Pachapur, L. Lonappan, M. Naghdi, R. Pulicharla, S. Maiti, M. Cledon, L. M. A. Dalila, S. J. Sarma and S. K. Brar, Nanotechnol. Environ. Eng. , 2017, 2 , 1–21 CrossRef CAS .
- K. N. Thakkar, S. S. Mhatre and R. Y. Parikh, Nanomedicine , 2010, 6 , 257–262 CrossRef CAS PubMed .
- N. I. Hulkoti and T. C. Taranath, Colloids Surf., B , 2014, 121 , 474–483 CrossRef CAS PubMed .
- T. Ahmed, H. Ren, M. Noman, M. Shahid, M. Liu, M. A. Ali, J. Zhang, Y. Tian, X. Qi and B. Li, NanoImpact , 2021, 21 , 100281 CrossRef CAS PubMed .
- I. Uddin and A. Ahmad, J. Mater. Environ. Sci. , 2016, 7 , 3068–3075 CAS .
- V. Bansal, D. Rautaray, A. Ahmad and M. Sastry, J. Mater. Chem. , 2004, 14 , 3303–3305 RSC .
- S. P. Suriyaraj, G. Ramadoss, K. Chandraraj and R. Selvakumar, Mater. Sci. Eng., C , 2019, 105 , 110021 CrossRef PubMed .
- A. F. V. da Silva, A. P. Fagundes, D. L. P. Macuvele, E. F. U. de Carvalho, M. Durazzo, N. Padoin, C. Soares and H. G. Riella, Colloids Surf., A , 2019, 583 , 123915 CrossRef CAS .
- T. Van Tran, D. T. C. Nguyen, P. S. Kumar, A. T. M. Din, A. A. Jalil and D. V. N. Vo, Environ. Chem. Lett. , 2022, 20 , 1309–1331 CrossRef PubMed .
- R. D. Abdul Jalill, M. M. H. M. Jawad and A. N. Abd, J. Genet. Environ. Resour. Conserv. , 2017, 5 , 6–23 Search PubMed .
- S. Shanthi and S. Sri Nisha Tharani, Int. J. Eng. Appl. Sci. , 2016, 3 , 257689 Search PubMed .
- P. Nimare and A. A. Koser, Int. J. Eng. Technol. , 2016, 3 , 1910–1912 Search PubMed .
- M. Sathishkumar, K. Sneha and Y. S. Yun, Mater. Lett. , 2013, 98 , 242–245 CrossRef CAS .
- S. Gowri, R. R. Gandhi and M. Sundrarajan, J. Mater. Sci. Technol. , 2014, 30 (8), 782–790 CrossRef CAS .
- A. Majedi, A. Abbasi and F. Davar, J. Sol-Gel Sci. Technol. , 2016, 77 , 542–552 CrossRef CAS .
- V. S. Saraswathi and K. Santhakumar, J. Photochem. Photobiol., B , 2017, 169 , 47–55 CrossRef PubMed .
- B. Debnath, M. Majumdar, M. Bhowmik, K. L. Bhowmik, A. Debnath and D. N. Roy, J. Environ. Manage. , 2020, 261 , 110235 CrossRef CAS PubMed .
- S. Gowri, R. Rajiv Gandhi and M. Sundrarajan, J. Mater. Sci. Technol. , 2014, 30 , 782–790 CrossRef CAS .
- S. Balaji, B. K. Mandal, S. Ranjan, N. Dasgupta and R. Chidambaram, J. Photochem. Photobiol., B , 2017, 170 , 125–133 CrossRef CAS PubMed .
- W. N. N. Wan Omar and N. A. S. Amin, Fuel Process. Technol. , 2011, 92 , 2397–2405 CrossRef CAS .
- F. Qiu, Y. Li, D. Yang, X. Li and P. Sun, Bioresour. Technol. , 2011, 102 , 4150–4156 CrossRef CAS .
- S. K. Das and S. A. El-Safty, ChemCatChem , 2013, 5 , 3050–3059 CrossRef CAS .
- Y. Zhang, W. T. Wong and K. F. Yung, Appl. Energy , 2014, 116 , 191–198 CrossRef CAS .
- M. Takase, M. Zhang, W. Feng, Y. Chen, T. Zhao, S. J. Cobbina, L. Yang and X. Wu, Energy Convers. Manage. , 2014, 80 , 117–125 CrossRef CAS .
- P. Patil and A. Pratap, J. Oleo Sci. , 2016, 65 , 331–337 CrossRef CAS PubMed .
- V. K. Booramurthy, R. Kasimani, S. Pandian and B. Ragunathan, Environ. Sci. Pollut. Res. , 2020, 27 , 20598–20605 CrossRef CAS PubMed .
- Y. F. Lin, J. H. Chen, S. H. Hsu, H. C. Hsiao, T. W. Chung and K. L. Tung, J. Colloid Interface Sci. , 2012, 368 , 660–662 CrossRef CAS PubMed .
- S. Gopinath, P. S. M. Kumar, K. A. Y. Arafath, K. V. Thiruvengadaravi, S. Sivanesan and P. Baskaralingam, Fuel , 2017, 203 , 488–500 CrossRef CAS .
- Y. Luo, Z. Mei, N. Liu, H. Wang, C. Han and S. He, Catal. Today , 2017, 298 , 99–108 CrossRef CAS .
- G. L. Shi, F. Yu, X. L. Yan and R. F. Li, J. Fuel Chem. Technol. , 2017, 45 , 311–316 CrossRef CAS .
- S. Dehghani and M. Haghighi, Ultrason. Sonochem. , 2017, 35 , 142–151 CrossRef CAS .
- I. Fatimah, D. Rubiyanto, A. Taushiyah, F. B. Najah, U. Azmi and Y. L. Sim, Sustainable Chem. Pharm. , 2019, 12 , 100129 CrossRef .
- N. J. A. Rahman, A. Ramli, K. Jumbri and Y. Uemura, Sci. Rep. , 2019, 9 , 1–12 CrossRef .
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc. , 2008, 130 , 13850–13851 CrossRef PubMed .
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater. , 2010, 22 , 6632–6640 CrossRef CAS .
- J. B. Decoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y. G. Huang and K. S. Walton, J. Mater. Chem. A , 2013, 1 , 5642–5650 RSC .
- X. Liu, N. K. Demir, Z. Wu and K. Li, J. Am. Chem. Soc. , 2015, 137 , 6999–7002 CrossRef CAS PubMed .
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater. , 2011, 23 , 1700–1718 CrossRef CAS .
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J. , 2011, 17 , 6643–6651 CrossRef CAS .
- F. Zhou, N. Lu, B. Fan, H. Wang and R. Li, J. Energy Chem. , 2016, 25 , 874–879 CrossRef .
- A. S. Abou-Elyazed, G. Ye, Y. Sun and A. M. El-Nahas, Ind. Eng. Chem. Res. , 2019, 58 , 21961–21971 CrossRef CAS .
- W. Xie and F. Wan, Chem. Eng. J. , 2019, 365 , 40–50 CrossRef CAS .
- N. Lu, X. Zhang, X. Yan, D. Pan, B. Fan and R. Li, CrystEngComm , 2019, 22 , 44–51 RSC .
- Q. Zhang, D. Lei, Q. Luo, J. Wang, T. Deng, Y. Zhang and P. Ma, RSC Adv. , 2020, 10 , 8766–8772 RSC .
- Y. Zhou, I. Noshadi, H. Ding, J. Liu, R. S. Parnas, A. Clearfield, M. Xiao, Y. Meng and L. Sun, Catalysts , 2018, 8 (1), 17 CrossRef .
- S. Zinatloo-Ajabshir and M. Salavati-Niasari, J. Mol. Liq. , 2016, 216 , 545–551 CrossRef CAS .
- J. Zhang, Y. Gao, X. Jia, J. Wang, Z. Chen and Y. Xu, Sol. Energy Mater. Sol. Cells , 2018, 182 , 113–120 CrossRef CAS .
- S. N. Basahel, T. T. Ali, M. Mokhtar and K. Narasimharao, Nanoscale Res. Lett. , 2015, 10 (1), 1–13 CrossRef CAS .
- N. Al-Zaqri, A. Muthuvel, M. Jothibas, A. Alsalme, F. A. Alharthi and V. Mohana, Inorg. Chem. Commun. , 2021, 127 , 108507 CrossRef CAS .
- H. M. Shinde, T. T. Bhosale, N. L. Gavade, S. B. Babar, R. J. Kamble, B. S. Shirke and K. M. Garadkar, J. Mater. Sci.: Mater. Electron. , 2018, 29 , 14055–14064 CrossRef CAS .
- S. Haq, H. Afsar, I. U. Din, P. Ahmad, M. U. Khandaker, H. Osman, S. Alamri, M. I. Shahzad, N. Shahzad, W. Rehman and M. Waseem, Catalysts , 2021, 11 (12), 1481 CrossRef CAS .
- K. Gurushantha, K. S. Anantharaju, L. Renuka, S. C. Sharma, H. P. Nagaswarupa, S. C. Prashantha, Y. S. Vidya and H. Nagabhushana, RSC Adv. , 2017, 7 , 12690–12703 RSC .
- P. Rasheed, S. Haq, M. Waseem, S. U. Rehman, W. Rehman, N. Bibi and S. A. A. Shah, Mater. Res. Express , 2020, 7 (2), 025011 CrossRef CAS .
- M. Imran, S. Riaz, I. Sanaullah, U. Khan, A. N. Sabri and S. Naseem, Ceram. Int. , 2019, 45 , 10106–10113 CrossRef CAS .
- M. Kumaresan, K. Vijai Anand, K. Govindaraju, S. Tamilselvan and V. Ganesh Kumar, Microb. Pathog. , 2018, 124 , 311–315 CrossRef CAS .
- F. C. P. Masim, C. H. Tsai, Y. F. Lin, M. L. Fu, M. Liu, F. Kang and Y. F. Wang, Environ. Technol. , 2019, 40 , 226–238 CrossRef CAS PubMed .
- J. B. Fathima, A. Pugazhendhi and R. Venis, Microb. Pathog. , 2017, 110 , 245–251 CrossRef CAS PubMed .
- A. Precious Ayanwale and S. Y. Reyes-López, ACS Omega , 2019, 4 , 19216–19224 CrossRef CAS PubMed .
- M. Khan, M. R. Shaik, S. T. Khan, S. F. Adil, M. Kuniyil, M. Khan, A. A. Al-Warthan, M. R. H. Siddiqui and M. Nawaz Tahir, ACS Omega , 2020, 5 , 1987–1996 CrossRef CAS PubMed .
- A. Derbalah, M. M. Elsharkawy, A. Hamza and A. El-Shaer, Pestic. Biochem. Physiol. , 2019, 157 , 230–236 CrossRef CAS PubMed .
- L. Chen, H. Zhong, X. Qi, H. Shao and K. Xu, RSC Adv. , 2019, 9 , 13220–13233 RSC .
- C. Han, J. Yang and J. Gu, J. Nanopart. Res. , 2018, 20 (3), 1–11 CrossRef CAS .
- F. M. Alzahrani, K. M. S. Katubi, D. Ali and S. Alarifi, Int. J. Nanomed. , 2019, 14 , 7003–7016 CrossRef CAS PubMed .
- A. Mftah, F. H. Alhassan, M. S. Al-Qubaisi, M. E. El Zowalaty, T. J. Webster, M. Sh-Eldin, A. Rasedee, Y. H. Taufiq-Yap and S. S. Rashid, Int. J. Nanomed. , 2015, 10 , 765–774 CrossRef PubMed .
- A. Ghosh, S. Kanrar, D. Nandi, P. Sasikumar, K. Biswas and U. C. Ghosh, J. Chem. Eng. Data , 2020, 65 , 885–895 CrossRef CAS .
- Y. Sun, M. Chen, H. Liu, Y. Zhu, D. Wang and M. Yan, Appl. Surf. Sci. , 2020, 525 , 146614 CrossRef CAS .
- R. Bhatt, V. Ageetha, S. B. Rathod and P. Padmaja, Carbohydr. Polym. , 2019, 208 , 441–450 CrossRef CAS PubMed .
- S. Salehi, S. Alijani and M. Anbia, Int. J. Biol. Macromol. , 2020, 164 , 105–120 CrossRef CAS PubMed .
- J. Wang, X. Shao, J. Liu, Q. Zhang, J. Ma and G. Tian, Mater. Chem. Phys. , 2020, 249 , 123024 CrossRef CAS .
- X. Fang, S. Wu, Y. Wu, W. Yang, Y. Li, J. He, P. Hong, M. Nie, C. Xie, Z. Wu, K. Zhang, L. Kong and J. Liu, Appl. Surf. Sci. , 2020, 518 , 146226 CrossRef CAS .
- K. Shehzad, M. Ahmad, C. Xie, D. Zhan, W. Wang, Z. Li, W. Xu and J. Liu, J. Hazard. Mater. , 2019, 373 , 75–84 CrossRef CAS PubMed .
- A. Hafezeqoran and R. Koodaryan, BioMed Res. Int. , 2017, 2017 , 9246721 Search PubMed .
- M. Aivazi, M. Fathi, F. Nejatidanesh, V. Mortazavi, B. H. Beni and J. P. Matinlinna, J. Lasers Med. Sci. , 2018, 9 , 87–91 CrossRef PubMed .
- T. Tahsili, W. Park, M. Hirota, N. M. Rezaei, M. Hasegawa, M. Ishijima, K. Nakhaei, T. Okubo, T. Taniyama, A. Ghassemi, T. Tahsili, W. Park, M. Hirota and T. Ogawa, Int. J. Nanomed. , 2018, 13 , 3381–3395 CrossRef .
- P. Yu, C. Wang, J. Zhou, L. Jiang, J. Xue and W. Li, BioMed Res. Int. , 2016, 2016 , 8901253 Search PubMed .
- M. M. Gad, R. Abualsaud, A. Rahoma, A. M. Al-Thobity, K. S. Al-Abidi and S. Akhtar, Int. J. Nanomed. , 2018, 13 , 283–292 CrossRef CAS .
- U. Lohbauer, A. Wagner, R. Belli, C. Stoetzel, A. Hilpert, H. D. Kurland, J. Grabow and F. A. Müller, Acta Biomater. , 2010, 6 , 4539–4546 CrossRef CAS .
- M. M. Gad, A. M. Al-Thobity, S. Y. Shahin, B. T. Alsaqer and A. A. Ali, Int. J. Nanomed. , 2017, 12 , 5409–5419 CrossRef CAS PubMed .
- Y. Oshima, F. Iwasa, K. Tachi and K. Baba, Int. J. Oral. Maxillofac. Implants , 2017, 32 , 81–91 CrossRef PubMed .
- R. Yamada, K. Nozaki, N. Horiuchi, R. Nemoto, H. Miura and A. Nagai, Mater. Sci. Eng., C , 2017, 78 , 1054–1060 CrossRef CAS PubMed .
- Z. Zhang, C. Zhang and X. Zhang, Analyst , 2002, 127 , 792–796 RSC .
- K. M. Garadkar, B. S. Shirke, Y. B. Patil, D. R. Patil, Sensors and transducers , 2009, Vol. 110, Iss. 11, pp. 17–25 Search PubMed .
- C. O. Chikere, N. H. Faisal, P. Kong-Thoo-Lin and C. Fernandez, Nanomaterials , 2020, 10 (3), 537 CrossRef CAS PubMed .
- J. Wang, M. Y. Su, J. Q. Qi and L. Q. Chang, Sens. Actuators, B , 2009, 139 , 418–424 CrossRef CAS .
- J. Gong, X. Miao, H. Wan and D. Song, Sens. Actuators, B , 2012, 162 , 341–347 CrossRef CAS .
- T. Trinadh, H. Khuntia, T. Anusha, K. S. Bhavani, J. V. S. Kumar and P. K. Brahman, Diamond Relat. Mater. , 2020, 110 , 108143 CrossRef CAS .
- J. Der Liao, K. Sivashanmugan, B. H. Liu, W. E. Fu, C. C. Chen, G. D. Chen and Y. Der Juang, Nanomaterials , 2018, 8 (6), 402 CrossRef .
- C. Yu, G. Liu, B. Zuo and R. Tang, Luminescence , 2009, 24 , 282–289 CrossRef CAS PubMed .
- A. V. Borhade, D. R. Tope and J. A. Agashe, J. Mater. Sci.: Mater. Electron. , 2018, 29 , 7551–7561 CrossRef CAS .
- R. Jain, D. C. Tiwari and S. Shrivastava, J. Electrochem. Soc. , 2014, 161 , B39–B44 CrossRef CAS .
- T. N. Myasoedova, T. S. Mikhailova, G. E. Yalovega and N. K. Plugotarenko, Chemosensors , 2018, 6 (4), 67 CrossRef CAS .
- F. Maleki and G. Pacchioni, Top. Catal. , 2020, 63 , 1717–1730 CrossRef CAS .
- A. R. Puigdollers, F. Illas and G. Pacchioni, J. Phys. Chem. C , 2016, 120 , 4392–4402 CrossRef CAS .
- L. Liu, X. Su, H. Zhang, N. Gao, F. Xue, Y. Ma, Z. Jiang and T. Fang, Appl. Surf. Sci. , 2020, 528 , 146900 CrossRef CAS .
- A. Ruiz Puigdollers, F. Illas and G. Pacchioni, Rend. Lincei. , 2017, 28 , 19–27 CrossRef .
- N. Kumari, M. K. Aulakh, S. Sareen, A. Sharma, H. S. Sohal, M. Verma, S. K. Mehta and V. Mutreja, Top. Catal. , 2022 DOI: 10.1007/s11244-022-01652-z .

IMAGES
VIDEO
COMMENTS
1. Introduction. Monolithic zirconia restorations, manufactured exclusively by the CAD/CAM technology, have considerable advantages: they exhibit high flexural strength, require more conservative dental preparation, minimize wear on the antagonists, exhibit satisfactory aesthetics, require less laboratory time and fewer dental sessions, and as monolithic, they lack the unwanted complication of ...
monolithic zirconia [1,74,85-92] and others compared the wear properties of monolithic zirconia following different ways of surfac e treat ment [77,78,80,81,84,92-97].
Zirconia is a kind of ceramic material, this short name stands for zirconium oxide (ZrO 2 ), hydrous zirconia (ZrO 2 .nH 2 O) is also a ceramic material, and its properties are similar to zirconia ...
A Thesis submitted to the Faculty of the Graduate School, Marquette University, in Partial Fulfillment of the Requirements for ... Zirconia has been introduced to dentistry as an alternative material due to its excellent mechanical properties. Zirconia exhibits a tetragonal-to-monoclinic phase transformation on cooling, which is
No other specimens demonstrated marginal integrity issued at 30X. None of the specimens demonstrated any micro crack at 100X and 150X. Due to its fracture resistance, 3Y-zirconia may be considered as a desirable material for partial coverage restorations. Following a strict bonding protocol, bonding zirconia seems to be promising and durable.
Introduction. Zirconia (ZrO 2) is a crystalline oxide of zirconium and it holds good mechanical, optical, and biological properties (Bapat et al., 2022).This biomaterial has three basic chemical forms; monoclinic, tetragonal, and cubic (Saridag, Tak & Alniacik, 2013; Bocanegra-Bernal & dela Torre, 2002).The metal-free restorations, mostly consisting of all-ceramic/zirconia restorations, are ...
the presence of water trigger slow t- m. transitions on the surface of zirconia, which eventually spread into the material's. mass. Extreme microcracking, grain. pullout, and s urface roughening ...
MONOLITHIC ZIRCONIA PARTIAL COVERAGE RESTORATIONS: AN IN-VITRO CLINICAL SIMULATION . Savita Gupta . A thesis submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Master of Science in the Division of Comprehensive Oral Health in the Adams School of Dentistry ...
The purpose of this paper was to update the knowledge concerning the wear, translucency, as well as clinical performance of monolithic zirconia ceramics, aiming at highlighting their advantages and weaknesses through data presented in recent literature. New ultra-translucent and multicolor monolithic zirconia ceramics present considerably improved aesthetics and translucency, which, according ...
nano-scale zirconia and hafnia dielectrics grown by atomic layer deposition: crystallinity, interface structures and electrical properties a dissertation submitted to the department of materials science and engineering and the committee on graduate studies of stanford university in partial fulfillment of the requirements for the degree of
Zirconia ceramics possess the unique combination of multi-functionality. There have been a number of landmark discoveries in the areas of its mechanical, electrical and thermal properties. The material is known to have a number of polymorphs like monoclinic at room temperature, tetragonal normally above around 1170 °C and cubic above around 2300 °C. The high-temperature cubic phase can be ...
The aim of this systematic review was to provide an overview of the technical and clinical outcomes of conventional, speed sintering and high-speed sintering protocols of zirconia in the dental field. Data on precision, mechanical and optical parameters were evaluated and related to the clinical per …
stabilized tetragonal zirconia polycrystal" (3y-TZP), is a mono-phasic material. 3Y-TZP: yttria partially stabilized tetragonal zirconia polycrystal (3y-TZP) is the most popular and frequently used form of zirconia commercially avail-able for dental applications. It consists of an array of transformable t-Zr grains stabilized by the addition
Correspondence: [email protected]; Tel.: +966-50754-4691. Abstract: The aim of this article is to comprehensively review the revolution of dental zirconia (Zir), including its types, properties, applications, and cementation procedures. A comprehensive search of PubMed and Embase was conducted.
The use of zirconia in dental ceramics is evidently becoming quite established, with major advancements in the last decade especially on 3Y-TZP. This can be attributed to its capabilities of meeting all key patient satisfaction criteria (comfort, functionality, social aspects, appearance). 3Y-TZP prosthetics are commonly formed and machined ...
Zirconia exists in three crystal phases, cubic, tetragonal, and monoclinic, in a typical air atmosphere and at distinct temperature ranges. ... The title of his Ph.D. thesis is "Development of Heterogeneous Catalysts for the Formation of Biodiesel". After completing his Ph.D., he served as Assistant Professor at Maharishi Markandeshwar ...
Electrical and thermal conductivities of the yttria-stabilised zirconia/alumina (YSZ/Al2O3) composites and the yttria-zirconia-ceria (YSZ-CeO2) solid solutions are studied in this thesis. The electrical conductivity of the YSZ/Al2O3 composites decreases with an increase in the volume fraction of Al2O3 and exhibits typical percolation behaviour ...
The zirconia powder was also of high purity commercially available 3 mol% Y-TZP manufactured by the co-precipitation method. The researchers employed a sintering method in which the green bodies were placed in a powder bed consisting of a mixture of ZrO 2 and Al 2 O 3. The sintering process involved an initial temperature increase to 1200 °C ...
The effect of ageing treatments on 3D-printed zirconia-based materials has also been scarcely addressed in the literature. Tan et al. [ 54] produced 3Y-TZP for implant abutments by DLP and SM and evaluated the effect of ageing (134 °C with 100% humidity, 0.2 MPa) on their physical and biological properties.
Statistical analysis showed that glazing decreased the flexural strength of the highly translucent zirconia. This finding was supported by the results from another study [ 7 ], reporting that glazing decreased the biaxial flexural strength of Zirkonzahn, Cercon, and Ceramill zirconia. We conducted this study on heat-treated groups to determine ...
The title of his Ph.D. thesis is "Development of Heterogeneous Catalysts for the Formation of Biodiesel". After completing his Ph.D., he served as Assistant Professor at Maharishi Markandeshwar University, Mullana-Ambala, India for 3 years (2014-2017). ... known as zirconia or zirconium oxide. Zirconia is also known as ceramic steel owing ...
The martensitic transformation in zirconia. Ph.D. Thesis. The metastable t-ZrO2 particles in both Y-TZP and Mg-PSZ can transform to m-ZrO2, and in the PSZ also to o-ZrO2, via a martensitic transformation. This research has demonstrated that the transformation is controlled by the thermally activated, stress-assisted nucleation of the martensite ...
Zirconia toughened alumina (ZTA) are among those materials which have high demand in the manufacturing industries, due to its application as cutting tool material for machining of high strength steel alloys. The properties such as high hardness, high wear resistance, chemical inertness at room temperature, high hot hardness, rapture strength ...