Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.

Search life-sciences literature (45,050,293 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists.
Author information, affiliations.
Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences , 01 Sep 2012 , 367(1601): 2475-2484 https://doi.org/10.1098/rstb.2011.0357 PMID: 22826346 PMCID: PMC3405673
Review Free full text in Europe PMC
Abstract
Free full text , a neurotrophic hypothesis of depression: role of synaptogenesis in the actions of nmda receptor antagonists.
Molecular and cellular studies have demonstrated opposing actions of stress and antidepressant treatment on the expression of neurotrophic factors, particularly brain-derived neurotrophic factor, in limbic structures of the brain. These changes in neurotrophic factor expression and function result in structural alterations, including regulation of neurogenesis, dendrite length and spine density in hippocampus and prefrontal cortex (PFC). The deleterious effects of stress could contribute to the reduced volume of these brain regions in depressed patients. Conversely, the actions of antidepressant treatment could be mediated in part by blocking or reversing the atrophy caused by stress and depression. Recent studies have identified a novel, rapid-acting antidepressant, ketamine, in treatment-resistant depressed patients that addresses the limitations of currently available agents (i.e. delayed onset of action and low response rates). We have found that ketamine, an N -methyl- d -aspartate (NMDA) receptor antagonist, causes a rapid induction of synaptogenesis and spine formation in the PFC via stimulation of the mammalian target of the rapamycin signalling pathway and increased synthesis of synaptic proteins. These effects of ketamine rapidly reverse the atrophy of PFC neurons caused by chronic stress and correspond to rapid behavioural actions of ketamine in models of depression. Characterization of a novel signalling pathway also identifies new cellular targets that could result in rapid and efficacious antidepressant actions without the side effects of ketamine.
- 1. Introduction
Depression is a widespread illness, affecting approximately 17 per cent of the population at some point in life, with tremendous personal and socioeconomic consequences [ 1 ]. The underlying causes of this heterogeneous illness as well as other mood disorders remain poorly understood. Moreover, the available pharmacological treatments for depression have significant limitations, including relatively low efficacy (i.e. approximately one-third of patients respond to the first agent prescribed), and time lag for treatment response (i.e. therapeutic effects are observed only after two to three weeks, and in many cases months of treatment) [ 2 ]. These limitations highlight a major unmet need for more efficacious and fast-acting antidepressant agents, particularly with the high rates of suicide in depressed subjects.
Despite these problems, recent studies have begun to elucidate the neurobiology of depression as well as treatment response, and have identified novel agents that have the potential to provide more efficacious and rapid response rates. In this review, we provide a brief update on the role of neurotrophic factors in the aetiology and treatment of depression- and stress-related illnesses. Then, we discuss the cellular and behavioural consequences of altered neurotrophic factor signalling in response to stress and antidepressant treatments. In particular, new evidence demonstrating that novel, rapid-acting N -methyl- d -aspartate (NMDA) receptor antagonists increase synaptogenesis, and the mechanisms underlying this effect are discussed.
- 2. Neurobiology of depression: atrophy and loss of neurons
Recent studies have begun to elucidate the pathophysiology of mood disorders, providing evidence for cell atrophy and loss in relevant limbic brain structures. Brain imaging studies demonstrate a reduction in the volume of limbic brain regions implicated in depression, notably the hippocampus and prefrontal cortex (PFC) [ 3 , 4 ]. Post-mortem studies report a reduction in the size of neurons and loss of glia [ 3 , 5 ], and preclinical studies show that exposure to repeated stress causes atrophy of neurons in the hippocampus and PFC, as well as loss of glia [ 6 , 7 ]. These studies provide strong evidence that atrophy and loss of neurons and glia are contributing factors to depression- and stress-related disorders.
A role for neurotrophic factors in cell atrophy and loss is supported by evidence that stress or depression decreases the expression of certain factors in limbic brain regions. One of the most highly studied factors is brain-derived neurotrophic factor (BDNF). Exposure to different types of physical or social stress decreases levels of BDNF in the hippocampus and PFC in rodent models [ 6 – 8 ]. Post-mortem studies also demonstrate a reduction of BDNF in these regions in post-mortem brains of depressed subjects [ 6 ]. This work has led to studies of growth factors in blood, which demonstrate decreased levels of BDNF in serum of depressed patients and reversal with antidepressant treatment, suggesting that BDNF is a biomarker of depression and treatment response [ 9 , 10 ]. In contrast to stress and depression, antidepressant treatment increases the expression of BDNF in the hippocampus and PFC [ 6 , 8 ]. Upregulation of BDNF is observed after chronic, but not acute, administration of different classes of antidepressants, including 5-hydroxytryptamine (5-HT) and norepinephrine-selective reuptake inhibitors. There is also evidence that antidepressant treatment increases BDNF in post-mortem brains of subjects on antidepressants at the time of death, as well as increasing blood levels of patients as discussed earlier [ 6 , 9 , 10 ].
In addition to BDNF, other neurotrophic/growth factors have been implicated in depression, including vascular endothelial growth factor (VEGF), fibroblast growth factor 2 and insulin-like growth factor 1 (IGF-1). Some of these factors have been best known for their effects on peripheral tissues (e.g. VEGF and IGF-1), but they are also expressed in neurons and glia and influence brain function [ 6 , 11 , 12 ]. Stress and antidepressant treatments have opposing effects on the expression of these factors. Moreover, functional studies demonstrate that altered levels of these neurotrophic/growth factors result in consequences in behavioural models of depression. However, this review will focus primarily on BDNF.
- 3. A neurotrophic hypothesis of depression and treatment response
Together, the preclinical and clinical gene expression and imaging studies support a neurotrophic hypothesis of depression and antidepressant response. This hypothesis proposes that depression results from decreased neurotrophic support, leading to neuronal atrophy, decreased hippocampal neurogenesis and loss of glia, and that antidepressant treatment blocks or reverses this neurotrophic factor deficit, and thereby reverses the atrophy and cell loss [ 6 , 13 ].
The neurotrophic hypothesis has been tested using various strategies for over-expression or knockdown of BDNF. These studies provide strong evidence that BDNF infusion is sufficient to produce an antidepressant response in behavioural models, and that BDNF is required for a response to antidepressant treatments [ 6 , 8 ]. However, there is much less evidence that BDNF depletion causes depressive behaviours. Most studies of BDNF-deletion mutant mice report normal behaviour in models of depression, with the exception that female conditional mutant mice show increased immobility in the forced swim test (FST) [ 14 , 15 ]. However, a recent study using RNA interference to knockdown BDNF expression in subregions of the hippocampus reports depressive behaviours in the forced swim and sucrose preference tests [ 16 ]. The discrepancy between these studies could be due to different knockdown approaches as well as behavioural methodology [ 16 ]. In addition, region-specific effects of BDNF (antidepressant effect in the hippocampus, but a pro-depressive effect in the nucleus accumbens) could influence behavioural outcomes particularly in mutant mouse models where knockout is global and not localized to a particular brain region [ 6 , 7 ].
(a) Brain-derived neurotrophic factor gene × environment interactions
It is also important to consider the possibility that although BDNF depletion may not be sufficient to cause depressive behaviour it may result in a state of increased susceptibility. Recent basic research and clinical studies provide evidence for a BDNF gene × environment interaction. Heterozygous deletion mutant mice, which express approximately half the normal levels of BDNF, display normal behaviour under baseline conditions, but exhibit a depressive phenotype upon exposure to stress ([ 17 ] but see [ 18 ]).
Advances in human genetics also provide a means to examine the influence of BDNF on susceptibility and resilience. A BDNF single nucleotide polymorphism, Val66Met, which decreases the processing and activity-dependent release of BDNF has been identified [ 19 ]. The BDNF Met allele is associated with reduced episodic memory and executive function, and decreased hippocampal volume in normal and depressed patients [ 19 ]. Although there is no direct association with depression, the BDNF Met allele increases vulnerability to develop depression in subjects exposed to early life stress or trauma [ 20 – 22 ]. Mutant mice with a knockin of the BDNF Met allele display increased anxiety in behavioural models and are unresponsive to antidepressant treatment [ 23 ].
- 4. Regulation of neurogenesis by stress and antidepressant treatment
Alterations of BDNF, as well as other neurotrophic factors, indicate that stress and antidepressant treatment result in cellular changes, notably regulation of neurogenesis and complexity of neuronal processes (see below). A brief review of neurogenesis and a more extensive overview of recent studies of synaptogenic responses are provided.
Birth of new neurons or neurogenesis continues to occur in selected neurogenic zones in the adult brain. This includes the subventricular zone that gives rise to olfactory bulb neurons, and the subgranular zone that generates granule cells of the hippocampal dentate gyrus. Similar to regulation of BDNF in the dentate gyrus, stress and antidepressant treatments exert opposing effects on neurogenesis in the adult hippocampus ( figure 1 ). Different types of acute or chronic physical and social stress decrease neurogenesis, while chronic antidepressant treatments, including serotonin-selective reuptake inhibitors (SSRIs) and norepinephrine-selective reuptake inhibitors (NSRIs), increase neurogenesis [ 6 , 24 ]. The relevance of neurogenesis to depression and antidepressant responses in humans has been examined, although the evidence is limited to a few post-mortem studies. A recent report shows that the rate of new cell birth is significantly increased in depressed subjects receiving antidepressant treatment prior to and at the time of death [ 25 ]. However, cell birth was not decreased in untreated depressed subjects.

Opposing actions of stress and antidepressants on brain-derived neurotrophic factor (BDNF) and neurogenesis. Stress decreases and antidepressant treatment increases the expression of BDNF, as well as vascular endothelial growth factor (VEGF) in the dentate gyrus granule cell layer of the hippocampus. These changes in growth factor expression contribute to the regulation of neurogenesis by stress and antidepressants. The negative effects of stress are also mediated in part by interleukin-1 (IL-1). This model shows the proliferation of neural progenitor cells giving rise to new neurons in the adult hippocampus. Antidepressants influence both the proliferation and survival of new neurons via effects on BDNF and VEGF. See text for details.
The role of BDNF in the regulation of neurogenesis has been examined using a number of different approaches. Studies in BDNF deletion mice have been mixed, whereas deletion of TrkB in neural progenitor cells is reported to block the proliferation of newborn neurons [ 26 , 27 ]. Localized BDNF knockdown using RNA interference is reported to block the differentiation but not proliferation of newborn neurons [ 16 ]. Blockade of TrkB by the expression of dominant negative TrkB or deletion of TrkB in progenitor cells also blocks antidepressant-induction of neurogenesis [ 27 , 28 ]. Together, these studies provide evidence that alterations of BDNF contribute to the regulation of neurogenesis by stress and antidepressant treatments.
- 5. Regulation of neuronal processes and synaptogenesis by stress
In addition to regulation of neurogenesis, the complexity of the dendritic arbour of neurons is altered by stress and antidepressant treatments. The formation of spine synapses or synaptogenesis is a key form of neuroplasticity, and represents a fundamental characteristic of neurons. Synaptogenesis is a structural change at a subcellular level that takes place in response to synaptic activity, and provides a mechanism for processing and incorporating new information that can be used to make the appropriate, future adaptive response ( figure 2 ). Cellular models of learning and memory, such as long-term potentiation (LTP), have been used to study the mechanisms underlying synaptogenesis. Increased neuronal activity leads to insertion of glutamate receptors and maturation of spine synapses [ 29 ].

Model for activity-dependent stimulation of synaptogenesis and spine formation. Synaptic activity and increased glutamate transmission can lead to increased synapse formation and spine density. This occurs through insertion of glutamate-AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors into the postsynaptic membrane. The mechanisms underlying the regulation of synaptogenesis and spine formation have been studied using a cellular model of learning and memory, known as long-term potentiation (LTP). See text for details.
The dendrite branches of neurons can be visualized by using a number of approaches, including Golgi impregnation or by filling cells with a dye that diffuses throughout the processes. These approaches allow for analysis of the number and length of dendrite branch points, and even the number of spines, the points of synaptic contact between neuronal processes.
The complexity of neuronal dendrites and number of spine synapses is markedly decreased by chronic stress exposure. This includes decreased number and length of apical dendrites in the CA3 pyramidal cell layer of the hippocampus and layer V pyramidal neurons of the PFC [ 30 , 31 ]. Reductions of dendrites and spines are observed in the PFC after as little as 7 days of restraint stress [ 32 ], and have been associated with depressive behaviours after exposure to chronic unpredictable stress (CUS) [ 33 ]. These studies support the possibility that decreased dendrite complexity contributes to the reduced volume of hippocampus and PFC reported in depressed patients.
The role of BDNF in the regulation of dendrite complexity and spine formation has also been examined in mutant mice. A knockin of the BDNF Met polymorphism has been developed, and studies show that expression of even a single copy of this human variant decreases the number and length of apical dendrites of CA3 pyramidal neurons, similar to the effects of chronic stress on dendrites [ 23 ]. BDNF heterozygous deletion mutant mice also have reduced CA3 apical dendrites [ 23 ]. Further studies will be required to determine whether reduced BDNF is responsible for the dendritic atrophy caused by chronic stress, but the current findings are consistent with this hypothesis.
Although antidepressant medications increase the expression of BDNF, there is little evidence that these agents reverse the dendrite atrophy caused by chronic stress. There is one study demonstrating that an atypical antidepressant, tianeptine, reverses the effects of chronic stress on atrophy of CA3 pyramidal neurons [ 34 ]. The lack of evidence for antidepressant reversal could reflect the technical challenges and time commitment required to conduct these difficult studies. In addition, it is possible that although BDNF expression is increased, typical antidepressant treatments do not increase BDNF release, which is required for increased synaptogenesis [ 23 , 35 , 36 ].
- 6. Cellular actions of the rapid-acting antidepressant, ketamine
Recent studies demonstrate that a non-selective NMDA receptor antagonist, ketamine, could address the limited efficacy and time lag for therapeutic response to typical antidepressants. Ketamine is a psychotomimetic at low doses and a dissociative anaesthetic at high doses. The rapid antidepressant actions of ketamine were first reported by Krystal and co-workers [ 37 ], who found that a low dose of ketamine (0.5 mg kg −1 , i.v.) produced a rapid antidepressant response, observed after only 4 h and that lasted for 3 days. Subsequent studies have confirmed and extended this finding, reporting rapid antidepressant responses to ketamine within 2 h with sustained effects for up to 7 days [ 37 – 41 ]. Moreover, these rapid effects of ketamine are observed in patients resistant to two or more typical antidepressants and considered treatment resistant.
These findings represent one of the most significant advances in the field of depression over the past 50 years: a novel, rapid-acting, efficacious antidepressant agent with a mechanism that is completely different from currently available medications.
(a) Ketamine rapidly increases synaptogenesis
The cellular and molecular mechanisms underlying the rapid antidepressant effects of ketamine are more complex than simple blockade of NMDA receptors. The fast actions of ketamine also indicate that the effects occur via regulation of synaptic transmission and/or neuronal plasticity. This could include increased synaptogenesis and spine density that could oppose the neuronal atrophy caused by chronic stress in PFC and hippocampus.
To address this possibility, we examined the influence of ketamine on the number and function of spine synapses on PFC neurons. We found that a single dose of ketamine increased the number of spines on the apical dendrites of layer V pyramidal neurons [ 42 ]. Ketamine administration also increased spine function, demonstrated by an increase in neurotransmitter-induced excitatory post-synaptic currents (EPSCs) of neurons. This included an increase in the frequency and amplitude of 5-HT- and hypocretin-induced EPSCs of layer V pyramidal neurons. Further analysis of spine morphology shows that ketamine treatment increases the number of ‘mushroom’-shaped spines, which are the mature and most functionally active spines [ 42 ] ( figure 2 ). The increase in number of mushroom spines is consistent with the increase of synaptic function resulting from ketamine administration, as the glutamate receptors are incorporated into mature spines and underlie the increase in EPSC amplitude.
The initial studies of spine density and function were conducted 24 h after ketamine administration, but preliminary studies indicate that synaptogenesis may occur even faster, within hours after treatment. This possibility is supported by analysis of synaptic proteins that are required for synaptogenesis and new spine formation (figures (figures2 2 and and3). 3 ). Levels of the synaptic proteins PSD95, GluR1 and synapsin I were measured in synaptoneurosome preparations of the PFC. Ketamine administration rapidly increased levels of these synaptic proteins, with significant increases observed after 2 h and sustained induction for up to 7 days [ 42 ]. This rapid time course for induction of synaptic proteins is consistent with the time course for the therapeutic actions of ketamine.

Regulation of mammalian target of rapamycin (mTOR) signalling by NMDA receptor antagonists. Ketamine increases extracellular glutamate, possibly via NMDA receptors on GABAergic interneurons resulting in disinhibition of glutamate transmission. This leads to activity-dependent release of BDNF and stimulation of signalling cascades, including Akt, that activate the mTOR translational system in dendrites of neurons. Induction of translation results in increased levels of GluR1 and other synaptic proteins, providing the machinery required for increased synaptogensis and spine formation. These effects contribute to the rapid and sustained antidepressant actions of ketamine. See text for further details.
(b) Ketamine rapidly reverses neuronal atrophy caused by chronic stress
The ability of ketamine to increase spine number and function observed in normal animals suggests that the atrophy of dendrites caused by chronic stress might be reversed by ketamine administration. To test this hypothesis, we used a CUS model of depression. This is considered one of the better animal models of depression because CUS exposure results in anhedonia or the inability to experience pleasure, a hallmark symptom of depression. Moreover, chronic stress exposure causes atrophy of apical dendrites and decreases spine density of PFC neurons [ 32 ].
The results of these studies demonstrate that CUS exposure for three weeks significantly decreases the number and function of spines on layer V pyramidal neurons in the PFC [ 33 ]. This includes a decrease in the number of spines in the distal and proximal tufts of layer V neurons, and a decrease in 5-HT- and hypocretin-induced EPSCs. There was also a significant decrease in levels of synaptic proteins, including reduced levels of PSD95, GluR1 and synapsin I, consistent with the downregulation of synaptogenesis. Surprisingly, a single dose of ketamine caused a rapid and complete reversal of the deficit in spine number and function caused by three weeks of CUS exposure [ 33 ]. Ketamine administration also completely reversed the deficit in synaptic proteins, including PSD95, GluR1 and synapsin I, consistent with the reversal of synaptogenesis.
These findings demonstrate that ketamine rapidly reverses the deficits in spine number and function in the PFC resulting from chronic stress exposure. Ketamine may also cause a similar reversal of atrophy of hippocampal neurons resulting from stress, although further studies will be required to test this hypothesis. Nevertheless, the results suggest that the therapeutic actions of ketamine may result, at least in part, from reversal of neuronal atrophy caused by depression, and reinstatement of the limbic circuitry required for control of emotion and mood.
(c) Ketamine produces rapid antidepressant behavioural actions
To examine the possibility that the induction of synaptogenesis and synaptic proteins could contribute to the therapeutic actions of ketamine, studies of rodent behavioural models of depression were conducted. Previous studies have demonstrated that a single low dose of ketamine produces a rapid antidepressant response in behavioural models of depression, including the FST and learned helplessness (LH) paradigm [ 43 ]. In addition, a selective NMDA NR2B antagonist, Ro 25-6981, also produced an antidepressant effect in the FST and LH model similar to the effects of ketamine. The significance of these findings is supported by a clinical study demonstrating that another NR2B antagonist, CP101,606 also produces an antidepressant response in depressed patients [ 44 ].
We have confirmed these findings in the FST and LH paradigm, and also demonstrate that ketamine and Ro 25-6981 produce a rapid antidepressant response in a novelty suppressed feeding test (NSFT) [ 42 ]. The NSFT, which measures the latency to feed in an open field, is considered a model of anxiety but is responsive to chronic, but not acute administration of SSRI antidepressants. Because of the requirement for chronic, three-week, antidepressant treatment, NSFT provides a measure of the rapid actions of ketamine that cannot be determined with the FST and LH models, which are responsive to acute or subchronic (1 or 6 days, respectively) administration of a typical antidepressant.
We have also examined the influence of ketamine in the CUS model of depression and resulting anhedonic behaviour, measured by preference for a sweetened solution. Exposure to CUS for three weeks decreases sucrose preference and this effect is reversed by chronic administration (three weeks) of a typical antidepressant, another reason why the CUS paradigm is considered one of the better rodent models of depression. The CUS model thereby provides a rigorous test in rodents of the ability of ketamine to produce rapid antidepressant actions. The results demonstrate that a single dose of ketamine completely reverses the deficit in sucrose consumption caused by CUS exposure [ 33 ]. This rapid action of ketamine parallels the rapid reversal of the atrophy of PFC pyramidal neuron spine density caused by CUS exposure. Together, the results are consistent with the hypothesis that the rapid synaptogenic effects of ketamine underlie the therapeutic response to this agent.
- 7. Role of mammalian target of rapamycin signalling in the actions of ketamine
Studies of protein synthesis-dependent long-term memory have demonstrated that the induction of synaptogenesis requires protein synthesis and activation of the mammalian target of rapamycin (mTOR) [ 35 ]. The mTOR complex and translational machinery have been localized in dendrites and spines, as well as cell bodies, and are therefore available for regulation of new synaptic protein synthesis as needed [ 35 , 45 ]. Activation occurs via phosphorylation of specific residues in the kinase domain of mTOR. An adjacent domain, the FKBP12-rapamycin binding region, is also critical for rapamycin inhibition ( figure 3 ). Activation of the mTOR complex 1, the rapamycin-sensitive complex, regulates two key components of translation initiation, p70 ribosomal S6 kinase (p70S6K) and eIF4E-binding proteins. Activation occurs via a number of pathways, most notably release of BDNF, stimulation of its receptor TrkB and downstream signalling cascades PI-3K-Akt and MEK-ERK [ 34 , 35 ].
Studies were conducted to determine whether ketamine increases the phosphorylated and activated forms of mTOR signalling proteins, including mTOR, p70S6K and 4E-BP1 ( figure 3 ). The results demonstrate that a single dose of ketamine stimulates the mTOR cascade, increasing levels of phospho-mTOR, phospho-p70S6K and phospho-4E-BP1 [ 42 ]. Ketamine-induction of mTOR signalling is rapid, with induction of phosphorylated proteins observed at 30 and 60 min, but transient, as levels return to baseline by 2 h. A similar rapid and transient increase in mTOR was observed with the NR2 selective antagonist, Ro 25-6981. Although transient, the activation of mTOR signalling precedes the induction of synaptic proteins and could thereby underlie the increase in protein synthesis.
In contrast to ketamine, acute or chronic administration of fluoxetine or imipramine, two typical antidepressants that block the reuptake of 5-HT, did not influence levels of mTOR signalling in the PFC [ 42 ]. We also examined the influence of electroconvulsive seizures (ECSs), a model for one of the most effective therapies for treatment-resistant depressed patients that also has a slightly faster onset of action than typical antidepressant medications. However, ECS did not increase mTOR signalling in the PFC [ 42 ] . Together, these results indicate that the rapid induction of mTOR signalling and synaptic proteins is specific to ketamine.
(a) Synaptogenic and behavioural actions of ketamine are blocked by rapamycin
The induction of mTOR signalling suggests that the ability of ketamine to increase synaptogenesis is mediated by stimulation of this protein synthesis regulatory pathway. To directly test this hypothesis, the influence of rapamycin, a selective inhibitor of mTOR ( figure 3 ), on synaptogenesis was examined. Rapamycin pretreatment completely blocked ketamine-induction of spine number and function of layer V pyramidal neurons in the PFC [ 42 ]. In addition, rapamycin pretreatment completely blocked the induction of the synaptic proteins PSD95, GluR1 and synapsin I, resulting from ketamine administration. These findings provide direct evidence that ketamine-induction of synaptogenesis requires mTOR signalling and synaptic protein synthesis.
Next, studies were conducted to determine whether the behavioural actions of ketamine are also dependent on mTOR signalling. Pretreatment with rapamycin completely blocked the antidepressant effects of ketamine in the FST, LH and NSF test [ 42 ]. Moreover, the rapid antidepressant effects of ketamine on the deficit in sucrose preference caused by CUS were completely blocked by pretreatment with rapamycin [ 33 ]. The behavioural actions of the selective NR2B antagonist, Ro 25-6981, were also blocked by rapamycin pretreatment [ 33 , 42 ]. Together, these studies demonstrate that the rapid synaptogenic and antidepressant behavioural actions of ketamine are dependent on stimulation of mTOR signalling, induction of synaptic protein synthesis and increased synaptogenesis.
A recent study has reported that the behavioural actions of ketamine require BDNF protein synthesis, and that this effect is mediated by activation of eukaryotic elongation factor 2 (eEF2) [ 46 ]. The induction of eEF2, which plays an important role in the translocation of ribosomes during protein synthesis, is dependent on inhibition of eEF2 kinase. The activation of eEF2 could also synergize with the actions of ketamine on mTOR signalling. The requirement for BDNF and protein synthesis is consistent with the results of our study, although Monteggia and co-workers were unable to detect ketamine-induction of mTOR signalling or rapamycin blockade of the behavioural actions of ketamine [ 46 ]. There are several technical reasons that could explain these discrepancies. First, mTOR signalling was measured in crude homogenates of hippocampus, not synaptoneurosome-enriched fractions of PFC as previously reported by Li and co-workers [ 42 ]. This is critical as mTOR is expressed in neuronal and glial cell bodies, which could mask changes in the smaller dendritic compartment. Second, the behavioural analysis was conducted 30 min after ketamine administration, when ketamine levels are still high in brain and corresponds to the time when patients experience mild psychotomimetic and dissociative effects of ketamine [ 36 , 40 ]. This would also correspond to the time when ketamine increases levels of glutamate [ 47 ], which could underlie the increased activity observed in the FST. As pointed out by the authors [ 46 ], rapamycin would not be expected to block the effects of ketamine at this time point since the induction of synaptic proteins and synaptogenesis is delayed by approximately 2 h. This later time point corresponds more closely to the initial antidepressant response observed in depressed patients [ 36 , 40 ].
- 8. Potential synaptogenic targets
The rapid and efficacious actions of ketamine in treatment-resistant-depressed patients represent major advances for the treatment of depression. There are also reports that ketamine is effective for treatment of bipolar depression [ 48 ] and suicide [ 49 , 50 ], and it is possible that it will also be effective for other psychiatric illnesses (e.g. post-traumatic stress disorder). Despite this promise, the use of ketamine also has limitations. Ketamine is a street drug with abuse potential, and preclinical studies report that repeated daily administration of ketamine may have neurotoxic effects, particularly on the function of GABAergic interneurons [ 51 , 52 ]. However, characterization of the mechanisms underlying the actions of ketamine suggests potential targets that could lead to the development of medications with ketamine-like effects. It may also be possible that activation of certain targets could sustain the actions of ketamine so that repeated dosing is not needed.
One possibility that has already been discussed is a selective NMDA receptor antagonist. Basic and clinical studies have demonstrated that NR2B selective agents increase mTOR signalling and synaptic protein synthesis, and have antidepressant effects in rodent models and depressed subjects. Further studies in depressed patients will be needed to confirm the clinical results with CP101,606, in particular to determine whether this or other NR2B selective agents produce rapid and efficacious therapeutic responses in treatment-resistant depressed patients.
Other leads have come from basic research reports that the behavioural actions of ketamine are dependent on glutamate–AMPA receptor activation [ 42 , 43 ]. Ketamine is reported to increase levels of extracellular glutamate in the PFC, possibly via blockade of NMDA receptors on GABAergic interneurons resulting in disinhibition of glutamate transmission [ 47 , 53 ]. We have also found that ketamine induction of mTOR signalling and synaptic protein levels are dependent on AMPA receptor activation [ 42 ]. These studies suggest that pharmacological agents that increase glutamate–AMPA receptor transmission should produce a ketamine-like effect, or sustain the actions of ketamine. Two targets that influence glutamate–AMPA activity have already received attention: metabotropic glutamate receptor type II antagonists and AMPA receptor potentiating agents.
(a) Enhancing glutamate–AMPA receptor transmission
The mGlu II receptors, mGlu2/3, are located on presynaptic glutamate terminals and provide inhibitory control of glutamate release. Previous studies demonstrate that mGlu2/3 antagonists, notably MGS0039 and LY341,495, have antidepressant actions in standard behavioural models [ 54 , 55 ]. In addition, the antidepressant actions of mGlu2/3 antagonists are blocked by pretreatment with an AMPA receptor antagonist, similar to the blockade of ketamine [ 54 , 56 ]. These findings support the possibility that mGlu2/3 receptor antagonist would increase glutamate transmission and thereby stimulate mTOR signalling and synaptogenesis, effects which could underlie the antidepressant behavioural actions that have been reported. Studies are currently underway to determine whether mGlu2/3 antagonists stimulate mTOR and synaptic protein synthesis.
Positive AMPA receptor modulators increase AMPA receptor function by altering receptor kinetics (e.g. decrease receptor desensitization or deactivation) [ 57 , 58 ]. AMPA receptor potentiating drugs increase the size of EPSPs, enhance LTP, enhance learning and memory, and increase BDNF [ 59 , 60 ]. One AMPA receptor potentiating drug, CX614, is reported to stimulate mTOR signalling and dendritic protein synthesis in cultured neurons in a BDNF-dependent manner [ 36 ]. These findings are consistent with the possibility that AMPA receptor potentiating drugs could produce rapid ketamine-like effects. There are several AMPA receptor potentiating drugs available, including high- (CX614, LY451646) and low-impact (CX1739, Org 26576) agents, based on efficacy to increase current flow of AMPA receptors [ 57 , 58 ]. Moreover, these AMPA receptor potentiating drugs have shown promise in biochemical and behavioural studies, including induction of BDNF and antidepressant responses in the FST [ 61 , 62 ].
One potential caveat is that the function of these glutamate-modulating agents may be dependent on basal glutamate transmission. If synaptic glutamate levels are very low, then a presynaptic mGlu2/3 antagonist would not be expected to enhance glutamate transmission since there would be no negative tone to block. Similarly, the actions of an AMPA receptor potentiating agent would be dependent on the presence of sufficient synaptic glutamate to cause low levels of receptor activation that could be potentiated. Another potential problem is that these agents may produce global effects on glutamate transmission that could lead to toxicity or unwanted side effects. Direct tests will be required to determine whether these approaches rapidly stimulate mTOR and synaptogenesis and produce antidepressant actions without side effects. Alternatively, mGlu2/3 antagonists or AMPA receptor potentiating drugs could sustain the response to ketamine and still provide a critical unmet therapeutic need.
- 9. Summary and conclusions
Exposure to chronic stress and/or depression results in neuronal atrophy and decreased neurogenesis in limbic brain regions involved in regulation of mood and emotion. The mechanisms underlying the actions of stress have not been identified. Based on studies demonstrating that stress decreases BDNF, and that BDNF and other neurotrophic factors stimulate mTOR signalling, it will be interesting to determine whether downregulation of mTOR contributes to the reduction in synaptic proteins, spine number and dendrite branching in PFC. The ability of ketamine and NMDA receptor antagonists to rapidly increase synaptogenesis represents a fundamental shift in our understanding of the mechanisms underlying rapid acting antidepressants. Studies are underway to further characterize the mechanisms that lead to stimulation of mTOR signalling by ketamine, including experiments to determine the role of BDNF in activation of mTOR.
The advances made in our understanding of rapid-acting NMDA receptor antagonists could also lead to identification of novel drug targets for the treatment of depression. However, these novel agents do not come without risk, particularly when using approaches that enhance glutamate signalling that could lead to neurotoxic effects when over-activated. Investigating mechanisms and treatment strategies to optimize therapeutic response while limiting toxicity will be critical to the success of these approaches. Nevertheless, these exciting findings raise optimism for a new generation of rapid-acting agents with superior therapeutic efficacy.
- Acknowledgements
This work is supported by US Public Health Service (grant nos MH93897 and MH45481), and the state of Connecticut, Department of Mental Health and Addiction Services.
Full text links
Read article at publisher's site: https://doi.org/10.1098/rstb.2011.0357
Citations & impact
Impact metrics, citations of article over time, alternative metrics.

Smart citations by scite.ai Smart citations by scite.ai include citation statements extracted from the full text of the citing article. The number of the statements may be higher than the number of citations provided by EuropePMC if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. Explore citation contexts and check if this article has been supported or disputed. https://scite.ai/reports/10.1098/rstb.2011.0357
Article citations, challenges and rewards of in vivo synaptic density imaging, and its application to the study of depression..
Asch RH , Abdallah CG , Carson RE , Esterlis I
Neuropsychopharmacology , 50(1):153-163, 22 Jul 2024
Cited by: 0 articles | PMID: 39039139
The association between levels of brain-derived neurotrophic factor and comorbid depression in patients with cardiovascular disease: The Framingham Heart Study.
Medved S , Salinas J , Kojis D , Weinstein G , Vasan RS , Beiser A , Seshadri S
Psychiatry Clin Neurosci , 78(8):438-445, 06 Jun 2024
Cited by: 0 articles | PMID: 38842141
Serotonin effects on human iPSC-derived neural cell functions: from mitochondria to depression.
Cardon I , Grobecker S , Jenne F , Jahner T , Rupprecht R , Milenkovic VM , Wetzel CH
Mol Psychiatry , 29(9):2689-2700, 26 Mar 2024
Cited by: 3 articles | PMID: 38532010 | PMCID: PMC11420088
eIF4E phosphorylation mediated LPS induced depressive-like behaviors via ameliorated neuroinflammation and dendritic loss.
Gong Q , Li W , Ali T , Hu Y , Mou S , Liu Z , Zheng C , Gao R , Li A , Li T , Li N , Yu Z , Li S
Transl Psychiatry , 13(1):352, 17 Nov 2023
Cited by: 4 articles | PMID: 37978167 | PMCID: PMC10656522
Effect of acute and long-term exercise on leptin levels in depressed outpatients.
Heinen D , Heissel A , Heinzel S , Fydrich T , Ströhle A , Rapp MA , Vogel H
BMC Public Health , 23(1):2509, 14 Dec 2023
Cited by: 1 article | PMID: 38098007 | PMCID: PMC10722655
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists.
Li N , Lee B , Liu RJ , Banasr M , Dwyer JM , Iwata M , Li XY , Aghajanian G , Duman RS
Science , 329(5994):959-964, 01 Aug 2010
Cited by: 1581 articles | PMID: 20724638 | PMCID: PMC3116441
Free full text in Europe PMC
Signaling pathways underlying the rapid antidepressant actions of ketamine.
Duman RS , Li N , Liu RJ , Duric V , Aghajanian G
Neuropharmacology , 62(1):35-41, 02 Sep 2011
Cited by: 289 articles | PMID: 21907221 | PMCID: PMC3195863
Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine.
Deyama S , Duman RS
Pharmacol Biochem Behav , 188:172837, 09 Dec 2019
Cited by: 57 articles | PMID: 31830487 | PMCID: PMC6997025
Essential roles of neuropeptide VGF regulated TrkB/mTOR/BICC1 signaling and phosphorylation of AMPA receptor subunit GluA1 in the rapid antidepressant-like actions of ketamine in mice.
Shen M , Lv D , Liu X , Li S , Chen Y , Zhang Y , Wang Z , Wang C
Brain Res Bull , 143:58-65, 11 Oct 2018
Cited by: 11 articles | PMID: 30316917
Funding
Funders who supported this work.
NIMH NIH HHS (5)
Grant ID: MH45481
113 publication s
Grant ID: R37 MH045481
76 publication s
Grant ID: MH93897
1 publication
Grant ID: R01 MH045481
65 publication s
Grant ID: R01 MH093897
77 publication s
Partnerships & funding
Europe PMC is developed by EMBL-EBI with support from the Europe PMC Funders' Group , in collaboration with the National Library of Medicine (NLM) , as part of the PubMed Central International archive network.

Europe PMC is an ELIXIR Core Data Resource , Global Core Biodata Resource , and conforms with EMBL-EBI’s long term data preservation policies .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 09 February 2024
Major depressive disorder: hypothesis, mechanism, prevention and treatment
- Lulu Cui 1 , 2 , 3 ,
- Shu Li 1 , 2 , 3 ,
- Siman Wang 1 , 2 , 3 ,
- Xiafang Wu 1 , 2 , 3 ,
- Yingyu Liu 1 , 2 , 3 ,
- Weiyang Yu 1 , 2 , 3 ,
- Yijun Wang 1 , 2 , 3 ,
- Yong Tang ORCID: orcid.org/0000-0002-2543-066X 4 ,
- Maosheng Xia ORCID: orcid.org/0000-0003-4829-0812 5 &
- Baoman Li ORCID: orcid.org/0000-0002-3959-9570 1 , 2 , 3
Signal Transduction and Targeted Therapy volume 9 , Article number: 30 ( 2024 ) Cite this article
64k Accesses
64 Citations
457 Altmetric
Metrics details
- Cellular neuroscience
- Diseases of the nervous system
Worldwide, the incidence of major depressive disorder (MDD) is increasing annually, resulting in greater economic and social burdens. Moreover, the pathological mechanisms of MDD and the mechanisms underlying the effects of pharmacological treatments for MDD are complex and unclear, and additional diagnostic and therapeutic strategies for MDD still are needed. The currently widely accepted theories of MDD pathogenesis include the neurotransmitter and receptor hypothesis, hypothalamic-pituitary-adrenal (HPA) axis hypothesis, cytokine hypothesis, neuroplasticity hypothesis and systemic influence hypothesis, but these hypothesis cannot completely explain the pathological mechanism of MDD. Even it is still hard to adopt only one hypothesis to completely reveal the pathogenesis of MDD, thus in recent years, great progress has been made in elucidating the roles of multiple organ interactions in the pathogenesis MDD and identifying novel therapeutic approaches and multitarget modulatory strategies, further revealing the disease features of MDD. Furthermore, some newly discovered potential pharmacological targets and newly studied antidepressants have attracted widespread attention, some reagents have even been approved for clinical treatment and some novel therapeutic methods such as phototherapy and acupuncture have been discovered to have effective improvement for the depressive symptoms. In this work, we comprehensively summarize the latest research on the pathogenesis and diagnosis of MDD, preventive approaches and therapeutic medicines, as well as the related clinical trials.
Similar content being viewed by others

Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis
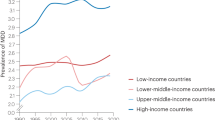
Major depressive disorder

Brain connectivity in major depressive disorder: a precision component of treatment modalities?
Introduction.
Major depressive disorder (MDD), a main cause of disability worldwide, is characterized by physical changes such as tiredness, weight loss, and appetite loss. Anhedonia is a classic feature of MDD, and MDD is also accompanied by a lack of drive, sleep issues, cognitive challenges, and emotional symptoms such as guilt. 1 The prevalence of depression is increasing yearly. About 300 million people in the world are affected by MDD, which has become one of the main causes of disability. 2 In 2018, MDD ranked third in terms of disease burden according to the WHO, and it is predicted to rank first by 2030. 3 Pregnant women, elderly people, children, and others have a higher incidence rate of MDD, which may be related to genetic, psychological, and social factors. 4 Depression can be accompanied by recurrent seizures, which may occur even during remission or persist for longer than the disease itself. 5 Pharmacological therapies for MDD can effectively control symptoms; thus, patients may experience recurrence within a short time after discontinuing medication. 6 During recurrence, the patient experiences symptoms of low mood, loss of interest in life, fatigue, delayed thinking, and repeated fluctuations in mental state. 7
There is a certain correlation between the occurrence of MDD and social development. 8 A survey reported that with the development of the economy and increased life pressure, MDD has begun to emerge at a younger age, and the incidence of MDD in women is approximately twice that in men. 9 Specifically, women are more likely to develop depressive symptoms when they encounter social emergencies or are under significant stress. 8 Additionally, autumn and winter have been reported to be associated with a high incidence of MDD, namely, seasonal depression. 10
The clinical symptoms of MDD include a depressed mood, loss of interest, changes in weight or appetite, and increased likelihood of committing suicide. 11 These symptoms are also listed as the criteria for MDD in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 12 In addition to the criteria listed in the DSM-5, the criteria reported in the International Classification of Diseases (ICD-10) are also used to guide clinical diagnosis. 13 However, due to the lack of characteristic symptoms and objective diagnostic evidence for MDD, identification and early prevention are difficult in the clinic. 14
Due to the complexity of the pathological mechanism of MDD, accurate diagnostic approaches and pharmacological therapeutic strategies are relatively limited. Several hypothesis were developed to explain MDD pathogenesis pathogenic including (i) the hypothalamic‒pituitary‒adrenal (HPA) axis dysfunction hypothesis, (ii) the monoamine hypothesis, (iii) the inflammatory hypothesis, (iv) the genetic and epigenetic anomaly hypothesis, (v) the structural and functional brain remodeling hypothesis, and (vi) the social psychological hypothesis 3 , 15 , 16 (Fig. 1 ). However, none of these hypotheses alone can fully explain the pathological basis of MDD, while many mechanisms proposed by these hypotheses interact with each other. In recent years, great progress has been made in identifying novel pharmacological therapies, diagnostic criteria, and nonpharmacological preventive measures for MDD, initiating related clinical trials. Specifically, increasing evidence suggests that astrocytic dysfunction plays a substantial role in MDD. 17 Pharmacological ablation of astrocytes in the medial prefrontal cortex (mPFC) causes depressive-like symptoms in experimental animals, 18 and postmortem studies of patients with MDD have shown reduced densities of glial cells in the prefrontal cortex (PFC), hippocampus and amygdala. 19 In addition, glial fibrillary acidic protein (GFAP), one of the markers of astrocytes, is expressed at various levels, 20 and the levels of connexins, 21 glutamine synthase (GS), glutamate transporter-1 (GLT-1), 21 , 22 and aquaporin-4 (AQP4) 23 are reduced in patients with MDD.
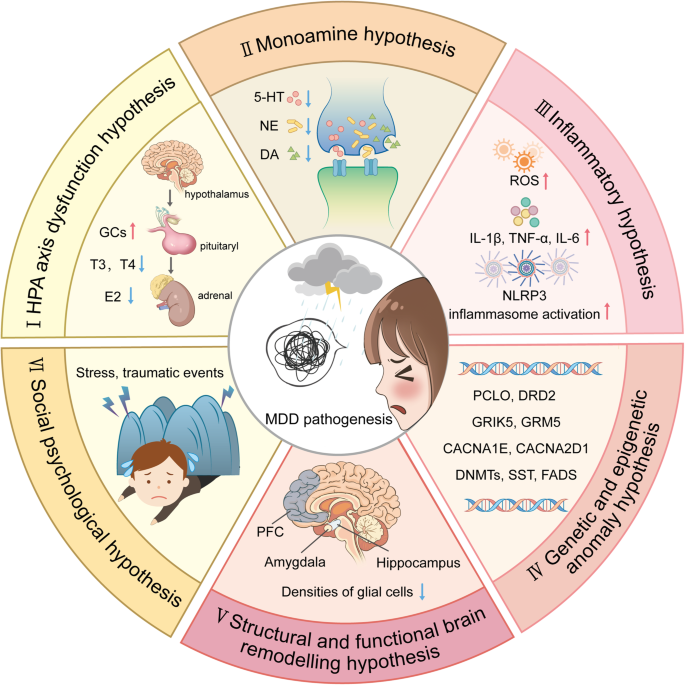
An outline map of the hypotheses to explain MDD pathogenesis. (I) HPA axis dysfunction hypothesis: high levels of glucocorticoids (GCs) play a core role in the pathogenesis of MDD, and thyroid hormone (TH) and estrogen are also involved in functions of the HPA axis; (II) the monoamine hypothesis: the functional deficiency of serotonin (5-HT), dopamine (DA) and norepinephrine (NE) are the main pathogenesis of MDD; (III) the inflammatory hypothesis: the neuro-inflammation induced by reactive oxygen species (ROS), inflammatory cytokines and inflammasomes activation is suggested to promote the occurrence of MDD; (IV) the genetic and epigenetic anomaly hypothesis: some genes are susceptible in the patients with MDD, including presynaptic vesicle trafficking (PCLO), D2 subtype of the dopamine receptor (DRD2), glutamate ionotropic receptor kainate type subunit 5 (GRIK5), metabotropic glutamate receptor 5 (GRM5), calcium voltage-gated channel subunit alpha1 E (CACNA1E), calcium voltage-gated channel auxiliary subunit alpha2 delta1(CACNA2D1), DNA methyltransferases (DNMTs), transcription levels of somatostatin (SST), fatty acid desaturase (FADS); (V) the structural and functional brain remodeling hypothesis: the postmortem results of patients with MDD are mostly associated with the reduced densities of glial cells in the prefrontal cortex (PFC), hippocampus, and amygdala; (VI) the social psychological hypothesis: the traumatic or stressful life events are the high risks of the occurrence of MDD. Adobe Illustrator was used to generate this figure
In this review, we summarize the latest research on the etiology, pathogenesis, diagnosis, prevention, mechanism, and pharmacological and nonpharmacological treatment of MDD as well as related clinical experiments.
Potential etiologies and pathogenic hypotheses
The common pathogenic factors.
Although the etiology of MDD is still unclear, it is widely accepted that MDD is associated with multiple pathogenic factor. In addition to well-known mental factors, MDD is also related to genetic factors, social stress, and even other common chronic diseases. Therefore, the etiology of MDD cannot be described from the perspective of a single factor.
Genetic factors
Although the etiology of MDD is still unclear, numerous studies have been performed and various models have been employed to explore the genetic factors, environmental factors and gene-environment interactions related to the disease. 24 Recent family, twin, and adoption studies suggests that genetic factors play a crucial role in the occurrence of MDD. 25 As a genetically diverse illness, MDD has a heritability of 30–50%. 26 Over 100 gene loci, including those associated with presynaptic vesicle trafficking (PCLO), dopaminergic neurotransmission (a primary target of antipsychotics), glutamate ionotropic receptor kainate type subunit 5 (GRIK5), and metabotropic glutamate receptor 5 (GRM5), and neuronal calcium signaling such as calcium voltage-gated channel subunit alpha1 E (CACNA1E) and calcium voltage-gated channel auxiliary subunit alpha2 delta1 (CACNA2D1), are found to be associated with an increased risk of MDD by genome-wide association studies. 19 , 27 , 28 In addition, rare copy number variants are also identified to be related to MDD risk, there may be three copy number variants (CNV) loci associated with Prader-Willis syndrome: 1q21.1 duplication, 15q11-13, and 16p11.2. However, no single genetic variation has been found to increase the risk of MDD thus far. 26 Genome Wide Association Studies (GWAS) identified 178 genetic risk loci and proposed over 200 candidate gene, using of biobank data, novel imputation methods, combined with clinical cases improved the ability to identify MDD specific pathways. 29 In the study of human MDD transcriptome, there are defects in the transcription levels of somatostatin (SST) in the subgenus anterior cingulate cortex and amygdala of MDD patients, 30 , 31 and SST levels are directly involved in the cellular processes that affect the synaptic output of intermediate neuronal circuits. 32 Recent studies revealed that gender specific genomic differences in MDD patients, the downregulation of the MDD-related gene Dusp6 in females leads to an increased susceptibility to stress, but this expression is not present in male mice. 33 In addition, studies of drug gene interactions, transcriptional genes associated with the risk of MDD are also reported, such as D2 subtype of the dopamine receptor (DRD2) and fatty acid desaturase (FADS), 34 which may serve as promising new targets for therapeutic intervention points. Thus, genetic variants are expected to have only minor effects on the overall risk of disease, and various hereditary factors combined with environmental factors such as stress are likely more essential for the development of MDD. 35
Stress factors
In addition to heritable factors, environmental influences such as stress also significantly contribute to the development of MDD, both independently and in conjunction with genetic factors. 26 Numerous studies have suggested that adverse life events can lead to the development of MDD. 18 A major depressive episode always follows a traumatic or stressful life event. In particular, severe events such as job loss, extramarital affairs and divorce are known to provoke the onset of the disease. 36 The exact pathological mechanism by which social stress results in the development of MDD is still not known, mainly due to the difficulty of separating social factors from genetic factors in patients and the impracticality of exposing disease model animals to relevant environmental factors. It has been proved that the changes in the structure and function of neurons may occur under the chronic stress and lead to the occurrence of MDD. 37 , 38 In some MDD patients, stress leads to long-term elevated glucocorticoids, resulting in synaptic structural changes and remodeling, and the stress-induced hyperactivity of the HPA axis leads to negative feedback imbalance of the HPA axis, which is also related to depression. 39 Studies on damage to microglia and astrocytes suggest the significance of glial cells in the development of environmental factor-induced depression-like behaviors in mice. 40 In addition, our previous studies proved that chronic environmental stress-induced depressive-like behaviors in mice can be dependent on purinergic ligand-gated ion channel 7 receptor (P2X 7 R) activation in astrocytes. 41
Comorbidity factors
The existence of various physiological and psychological comorbidities in patients with depression reveals a clear link between physical and mental health, which has given us a better understanding of MDD. The presence of MDD is a risk factor for a variety of complications, including neurodegenerative diseases (such as dementia, Alzheimer’s disease, and Parkinson’s disease), cardiovascular diseases (such as ischemic coronary artery disease and myocardial infarction), metabolic and endocrine diseases (such as obesity in females and diabetes in males), and some autoimmune diseases. 42 , 43 The relationship between the onset of MDD and several diseases is complex and potentially bidirectional in nature. 44 The impact of depression on society and the economy is increased by the existence of comorbidities. 45 Specifically, in 2018, comorbid disorders rather than MDD itself were responsible for 63% of all costs related to MDD in the United States. 46 , 47 Furthermore, compared to people without depression, patients with MDD have been demonstrated to have a shorter life expectancy. 48 Additionally, the worsening of comorbidities could be a factor in the premature mortality of MDD patients. 44
Neurotransmitter and receptor hypothesis
The traditional monoamine theory contends that in addition to common pathogenic factors, deficiencies in monoamine neurotransmitters, such as serotonin (5-HT), dopamine (DA) and norepinephrine (NE), are the root cause of clinical depression. 49 Selective serotonin reuptake inhibitors (SSRIs), a class of antidepressants that have been proven to successfully treat clinical depression, were developed in response to this hypothesis, which was derived primarily on the basis of the pharmacological mechanism of drug that were accidentally discovered to act as antidepressants. It is also crucial to note that astrocytes express NE transporter (NETT) and 5-HT transporter (SERT), which are the targets of some traditional antidepressants. 50 A previous study suggested that the function of astrocytes can be directly regulated by SSRIs. 51 Monoamine oxidase (MAO) activates the metabolism of adrenaline and triggers calcium signaling in astrocytes, 52 which suggests that antidepressants may directly affect astrocytes by preventing them from reabsorbing monoamines.
Serotonin (5-HT)
An essential neuromodulatory transmitter with specific neuroplastic properties is serotonin. Numerous investigations have demonstrated that 5-HT is intimately related to the pathophysiological process of major depression. The 5-HT hypothesis primarily asserts that a decrease in the 5-HT level is a risk factor for depression. 53 In addition, low levels of 5-HT and L-tryptophan, which is a precursor of 5-HT, 54 in blood platelets are also found in depressed people. Additionally, long-term treatment with fluoxetine, a typical SSRIs, reverses the stress-induced reduction in the quantity of astrocytic cells in the hippocampus in a tree shrew model of depression. 55
5-HT receptors, which are mostly found on the bodies and dendrites of neurons, play a role in the pathogenesis of MDD. 56 To date, 5-HT receptor subfamilies comprising 14 different receptor subunits expressed in various brain regions, namely, 5-HT 1A , 5-HT 1B , 5-HT 1D , 5-HT 1E , 5-HT 1F , 5-HT 2A , 5-HT 2B , 5-HT 2C , 5-HT 3 , 5-HT 4 , 5-HT 5A , 5-HT 5B , 5-HT 6 and 5-HT 7 , have been reported. Among these 5-HT receptor subtypes, the 5-HT 1 , 5-HT 2 , 5-HT 6 , and 5-HT 7 subtypes are expressed on brain and spinal astrocytes in humans and rodents. Numerous 5-HT receptors expressed on astrocytes are G-coupled proteins that are associated with changes in the concentration of free cytosolic calcium ([Ca 2+ ] i ). These changes may trigger the release of a variety of astrocyte-derived signaling modulators, which may control neuronal activity. 57 In astrocytes, 5-HT has a strong effect on the 5-HT 2B receptor. 58 5-HT receptors have been extensively studied to determine the pharmacological mechanism of antidepressants, and many novel pharmaceutical preparations are being investigated. For example, some novel antidepressants function as agonists of the 5-HT 1A , 5-HT 2B , or 5-HT 4 receptor or antagonists of the 5-HT 1B , 5-HT 2A , 5-HT 2C , 5-HT 3 , 5-HT 6 , or 5-HT 7 receptor. 59
Administration of fluoxetine in different concentrations to astrocytes expressing the 5-HT 2B receptor may activate distinct signaling pathways to control gene expression. Fluoxetine reduces the mRNA expression of c-Fos through the PI3K/AKT signaling pathway after acute application at concentrations below 1 μM, while the treatments with the higher doses (above 5 μM), it increases the gene expression of c-Fos via the MAPK/ERK signaling pathway in astrocytes. 60 Then, in the nucleus, the altered transcription factor c-Fos can further biphasic change the expression of caveoline under the chronic treatments, thus the alteration levels of caveoline on cellular membrane can finally affect the downstream activation of PTEN/PI3K/AKT/GSK3β 60 . The GSK3β polymorphisms are associated with the high risk of MDD in Chinese Han Population. 61 In our recent reports, the activation of GSK3β is also increased in the sorted astrocytes from the MDD-related stress-treated mice model and MDD clinic patients’ plasma. 62 In addition, after fluoxetine-mediated stimulation of the 5-HT 2B receptor in astrocytes, epidermal growth factor receptor (EGFR) is transactivated and subsequently activates the MAPK/ERK and PI3K/AKT signaling cascades, which control the expression of mRNA or proteins that may be linked to mood disorders, such as SERT. Ca 2+ -dependent phospholipase A2 (cPLA 2 ), adenosine deaminase acting on RNA 2 (ADAR2), and kainate receptor subtype 2 (GluK2) are all involved in kainate receptor signaling. 63 , 64 These discoveries promise astrocytic 5-HT 2B receptors can be the potential pharmacological target of SSRIs (Fig. 2 ).
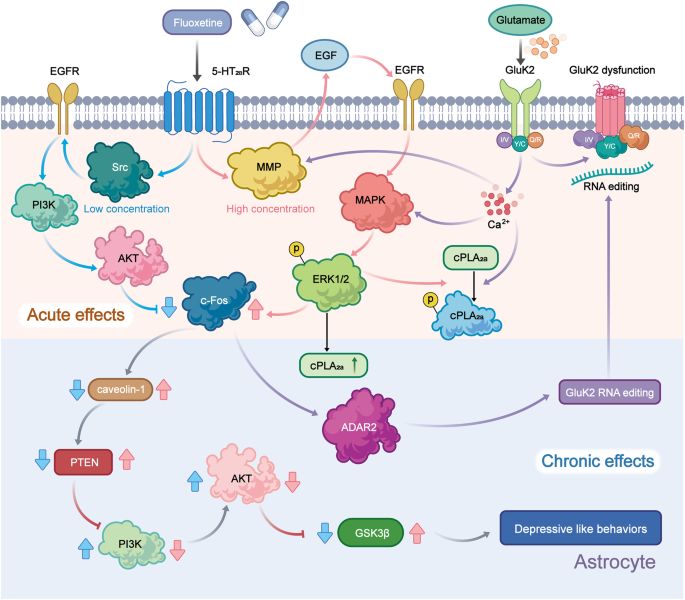
Schematic illustration of the pharmacological mechanism of fluoxetine in astrocytes. Acute treatment with fluoxetine at low concentrations (green arrows) stimulates Src, which phosphorylates EGF receptors by activating 5-HT 2B receptors (5-HT 2B R) and activates the PI3K/AKT signaling pathway. AKT phosphorylation induced by fluoxetine at low concentrations inhibits the expression of cFos and subsequently decreases the expression of caveolin-1 expression (chronic effects), which in turn decreases the membrane content of PTEN, induces phosphorylation and stimulation of PI3K and increases the phosphorylation of GSK3β, thus suppressing its activity. At higher concentrations, fluoxetine (red arrows) stimulates metalloproteinases (MMP) by activating 5-HT 2B R and induces the release of growth factors, which stimulates EGF receptors and activates the mitogen-activated protein kinases (MAPK)/ERK 1/2 signaling pathway. ERK 1/2 phosphorylation induced by fluoxetine at high concentrations stimulates the expression of cFos and subsequently increases the expression of caveolin-1 (chronic effects), which inhibits PTEN/PI3K/AKT/GSK3β, 60 ultimately leading to MDD like behavior. At high concentration, fluoxetine can also stimulate the activation of cPLA 2a by the transactivation of EGFR/MAPK/ERK 1/2 pathway, and the activated ERK 1/2 can also increases the expression of cPLA 2a at chronic treatments. 61 In addition, the increased expression of cFos induced by fluoxetine can further increases the RNA editing of GluK2 by increasing the expression of ADAR2 at the chronic treatments, the function of the edited GluK2 by fluoxetine is down-regulated, which causes the acute glutamated induced Ca 2+ -dependent ERK phosphorylation is suppressed. 63 Adobe Illustrator was used to generate this figure
Norepinephrine (NE)
NE released by the locus coeruleus (LC) can participate in regulating various neural functions, such as smell, movement, and sensation. 65 It is significant to note that after being released, noradrenaline (NA) is not restricted to the area around the synaptic cleft and can reach nearby glial cells. 66 Atomoxetine is a norepinephrine reuptake inhibitor (NRI) clinically used for the treatment of MDD. After systemic inflammatory attack with bacterial lipopolysaccharide (LPS), atomoxetine can decrease neuroinflammation in the rat cerebral cortex. 67
The bioavailability of 5-HT and NE are increased by antidepressants called serotonin/norepinephrine reuptake inhibitors (SNRIs), which belong to antidepressants. Currently, new SNRIs, including duloxetine (DXT), 68 desvenlafaxine (DVS), 69 and venlafaxine, 70 are widely used in MDD patients resistant to other treatments. Chronic treatment with DXT increases the expression of connexin 43 (Cx43), a crucial component of astrocyte gap junctions, in the rat PFC, preventing chronic unpredictable stress-induced dysfunction of astrocyte gap junctions and reversing the depressive-like behaviors caused by gap junction inhibition. 71 A novel therapeutic target for MDD is transforming growth factor β1 (TGF-β1), the expression of which is controlled by antidepressants. Venlafaxine has also been found to exert neuroprotection by boosting the production of type 2 fibroblast growth factor (FGF-2) and transforming growth factor 1 TGF-β1 in astrocytes following stroke. 72 However, the expression of protein markers of astrocytes and neurons is unaffected by DVS, and the chronic unpredictable mild stress (CUMS)-induced reduction in the levels of myelin- and oligodendrocyte-related proteins can be prevented by DVS. 69 DVS may reduce oligodendrocyte dysfunction in the CUMS mouse model by altering cholesterol production and reducing depression-like phenotypes. 69
Dopamine (DA)
There is increasing evidence that people with depression have reduced dopamine neurotransmission. 73 Astrocytes in the lateral habeula are involved in regulating depressive-like behavior, 74 whereas the reward circuit is mediated by the striatum. 75 The dorsolateral part of the striatum is linked to the drug-seeking behavior and drug addiction associated with psychiatric disorders. As the major input to the basal ganglia, the striatum and related nuclei are linked to psychiatric morbidity, while the chronic stress reduces dopamine levels in areas such as the striatum and hippocampus. 76 Due to processes involving dopamine D2 receptor signaling, 77 the glutamine level increases in the presence of dopaminergic lesions and decreases in the presence of a high DA level. 78 DA signaling is considered to play a key role in astrocyte-neuron crosstalk in the striatum. 79 Sulpiride is an antidepressant that blocks the ability of the GLT-1 inhibitor TFB-TBOA to induce synaptic depression 80 and partly attenuates the impact of fluorocitrate (a metabolic uncoupler that blocks aconitase in the tricarboxylic acid (TCA) cycle) on synaptic output. According to these results, astrocyte dysfunction results in an increase in DA levels, which decreases neuronal activity resulting from the binding of DA to dopamine D2 receptors, 80 which generates neuronal depolarization, reducing DA selectivity at dopamine D1-like receptors and promoting DA inhibition through dopamine D2 receptors, which may contribute to increasing extracellular glutamate levels. 81 An increase in DA signaling brought on by compromised astrocyte activity may induce a long-lasting change in striatal neurotransmission 80 since DA signaling is crucial for both structural and synaptic plasticity. 82
Glutamate is the main excitatory neurotransmitter in the central nervous system (CNS) 83 and can be released by neurons through exocytosis, which in turn activates extracellular N-methyl-D-aspartate receptors (eNMDARs) in neurons, leading to synaptic loss. 84 Exosynaptic glutamate also contributes to metabolism in neurons and astrocytes. When exosynaptic glutamate is taken up by astrocytes, it can become a substrate for glutamine synthesis or be metabolized by astrocytes and neurons. 85 In addition, extracellular glutamate can also promote glucose uptake by astrocytes and inhibit glucose uptake by neurons. Therefore, glutamate is an important signal that mediates the interaction between central neurons and astrocytes, and its normal release and transport are the result of the functional cooperation between neurons and astrocytes. Glutamate homeostasis and neurotransmission play a major role in the onset of depression and anxiety. Studies have shown that glutamate levels in frontal cortex samples from autopsied patients with severe depression are increased, and antidepressants can restore normal glutamate levels. 86 It has been observed in animal models that sustained glucocorticoid stimulation can increase the excitability of glutamatergic neurons and simultaneously decrease the number and plasticity of astrocytes, in addition to decreasing neuronal dendrite connectivity in the hippocampus and frontal cortex, leading to depression. 87
It is well-documented that astrocytes have a wide range of modulatory functions that may either increase or decrease the release of many different neurotransmitters. Specifically, astrocytes are essential regulators of glutamatergic neurotransmission, and reuptake of glutamate by astrocytes regulates excitatory synaptic activity. 85 When a large amount of glutamate is released from neuronal vesicles, glutamate clearance is mainly achieved by glutamate transporters (EAATs) on the membrane of astrocytes, which transport excess glutamate into astrocytes, where it is converted to glutamylamine through the action of glutamine synthase, reducing damage to neurons. 88 , 89 In the classic glutamate-glutamine cycle, astrocytes and neurons convert glutamate to the nonexcitatory amino acid glutamine, which is then released back into the extracellular space and absorbed by neurons. Alterations in astrocytic glutamate clearance are known to occur in schizophrenia and other psychiatric illnesses, and mice with glutamate/aspartate transporter (GLAST) deletion show phenotypic abnormalities such as mental and behavioral deficits. 90 , 91
Adenosine triphosphate (ATP)
Ectonucleotidases that are found in synapses can catabolize extracellular ATP to produce adenosine, and synapses also contain bidirectional nucleoside transporters that can release adenosine. 92 Adenosine primarily stimulates inhibitory A1 and facilitatory adenosine receptors (A 2A R) to play function. 93 Notably, depressive behavior is linked to purinergic signaling. Depressive-like symptoms are exacerbated by activation of P2X 7 R in glial cells. 94 Polimorphisms at P2X 7 R increase vulnerability to mood disorders whereas P2X 2 R-mediated neuronal activity is decreased in mice exposed to chronic stress due to insufficient ATP release from astrocytes. 95 According to our earlier studies, chronic sleep deprivation (SD) can cause depressive-like behaviors by increasing extracellular ATP levels in vivo. 41 Acting through P2X 7 R and FoxO3a cascade ATP inhibits expression of the 5-HT 2B receptor, the decrease in extracellular ATP levels caused by chronic stress and an increase in ATP levels caused by SD are both linked to depressive-like behaviors. 41 In detail, the elevated extracellular ATP induced by SD stress stimulates P2× 7 R and down-regulates the expression of 5-HT 2B R by suppressing the activation of AKT, which inhibits the phosphorylation of FoxO3a and promotes its transportation into the nucleus, the reduced 5-HT 2B R alleviates the inhibition of STAT3 to cPLA 2 , the activated cPLA 2 further increases the release of AA and PGE2, these indicators have high relationship with the depressive-like behaviors, because in P2X 7 R knockout mice, the above changes of these indicators and behavioral performance are all eliminated. 41 This increased activation of cPLA 2 and the elevated levels of AA and PGE2 in astrocytes are supported by our discoveries in MDD patients’ plasma. 62
After building a stress injury model in rats through maternal separation (MS), it is found that MS obviously reduces the total length of apical dendrites, however, the use of A 2A R antagonists could prevent synaptic loss 96 and reverse behavioral, electrophysiological, and morphological damage caused by MS, 97 this is related to the activity reconstruction of the HPA axis. In another study, the abnormally increased A 2A R in the lateral septum(LS) is a key factor in recurrent stress for leading to depressive-like behaviors. This function is mainly achieved by the increased activity of A 2A R-positive neurons and the inhibited activity of ambient neurons, associating with the neural circuits of dorsomedial hypothalamus(DMH) and lateral habenular(LHb). 98
Caffeine is an adenosine receptor antagonist, and epidemiological studies have shown that the intake of caffeine is closely related to the occurrence of suicide 99 and depression. 100 Since A 2A R polymorphisms are associated with emotional problems, adenosine A 2A R overexpression leads to emotional dysfunction, and A 2A R blockade protects against the persistent emotional disturbance brought on by stress. 101 Moreover, animal experiments have demonstrated that A 2A R are upregulated in chronic stress animal models. 102 Additionally, neuronal A1 receptors exhibit hypofunction caused by a decrease in astrocyte-derived adenosine levels; 103 this decrease, as well as depressive-like behavior, can be reversed by certain antidepressants. 104 , 105
HPA axis hypothesis
Stress and MDD are closely related, and stressful life events can often lead to depressive episodes. The activation of the HPA axis by stress can cause cognitive and emotional changes. 106 An increase in HPA activity is one of the most common neurobiological alterations in depressed people. Studies have shown that the main factor contributing to the elevation of hypothalamic-pituitary activity is the increased production of corticotropin-releasing hormone (CRH). In addition, pituitary adrenal corticotropic hormone (ACTH) is released in response to CRH, which in turn triggers the adrenal cortex to release glucocorticoids (GCs).
Glucocorticoids
The HPA axis, a component of the neuroendocrine system, is commonly associated with the stress response. Hyperactivity of the HPA axis is thought to be an important pathophysiological mechanism underlying depression. High HPA activity is among the most typical neurobiological alterations in depressed individuals. The HPA axis is the primary stress response system that produces GCs, which are a class of steroid hormones. There is evidence that GCs, which are released in response to stress, are harmful to neurons in various brain regions. The hypothalamic paraventricular nucleus (PVN) rapidly secretes CRH and arginine vasopressin (AVP) 107 when the HPA axis is activated by stress. The anterior pituitary is stimulated by CRH and AVP to produce ACTH, which in turn increases the release of GCs into the bloodstream. 108
The GC and mineralocorticoid (MC) receptors GR and MR are members of the nuclear receptor (NR) superfamily. Both NRs can be triggered by binding to either MCs (such as aldosterone) or GCs (such as cortisol). However, the affinity of MR for its ligands is 10 times higher than that of GR for its ligands. 109 , 110 GRs are expressesd at higher levels and particularly concentrated in the pituitary and hypothalamus, as well as a variety of regions of the limbic system (including the amygdala, hippocampus, and PFC), which are important for cognitive and psychological functions.
To prevent loss of control over the HPA axis, GCs exert negative feedback on the axis in all regions involved (the limbic system, hypothalamus, and pituitary). Some data suggest that HPA axis imbalance and high levels of GCs play a core role in the pathogenesis of MDD and suggest that GR may serve as an important target for treating depression. 111
Thyroid hormone
Thyroxine (T4) and triiodothyronine (T3) are the two primary Thyroid hormones (THs) that regulate metabolism, protein synthesis, the growth of bones, and nervous system development. Thyrotropin-releasing hormone (TRH), which regulates the synthesis of thyroid-stimulating hormone (TSH) by the anterior pituitary gland, is mostly produced by neurons in the PVN. TSH stimulates the thyroid gland to produce T3 and T4. The levels of serum-free T4 and free T3 are regulated by negative feedback from pituitary TSH release. Tissue deiodinase mostly transforms T4 into the less physiologically active metabolite reverse T3 and the more biologically active metabolite T4. 112
Overactivity of the HPA axis may be caused by damaged astrocytes and aberrant GR function. The HPA and hypothalamic-pituitary-thyroid (HPT) axes are inextricably linked. The most important related finding is that cortisol directly affects TRH secretion (which regulates TSH release), potentially through the response of GCs to TRH mRNA expression in neurons. According to research, hypercortisolemia may result in a reduction in TRH mRNA levels in the mid-caudal PVN. 113 TRH expression in the PVN is lower in nonpsychiatric patients treated with corticosteroids, and the mRNA levels of TRH are lower in the PVN of depressed patients who have recurrent suicidal thoughts. This suggests that the effect of hypothalamic TRH is weaker in these individuals.
THs are required for neuronal growth and function not only in the periphery but also in the CNS, 114 where they promote the formation of microglia, astrocytes, including radial glial cells, and oligodendrocytes. The role of THs in glial cells is becoming clear because of new discoveries in the field of glial cell biology. THs affect the shape and proliferation of astrocytes, as well as the organization and expression of GFAP/vimentin, and boost GS activity. 115 T3 has an effect on glial morphology and hence on glial function in the adult brain; therefore, it also has an effect on neuron-glia interactions. 115 , 116 It has been shown that T3 induces astrocyte proliferation by autocrine production of growth factors such as epidermal growth factor (EGF) and FGF-2. Apart from their proliferation-promoting impact, these growth factors increase and modify the pattern of deposition of the extracellular matrix components laminin and fibronectin, therefore boosting cell adherence and attachment to the substratum. Together with the discovery that animals with hypothyroidism and mice with TH receptor mutations display significant defects in glial development, these findings indicate that astrocytes are TH targets and that TH can protect neurons and astrocytes from glutamate toxicity. 115
The hippocampus is closely related to memory and learning, and estrogen plays an important role in these processes. Estrogen increases the proliferation, migration, and differentiation of neurons in the dentate gyrus to maintain hippocampal function and is also important for controlling the HPA axis. 117
Estrone (E1), estradiol (E2), and estriol (E3) are the three physiological estrogens; among these estrogens, E2 is the most active, and its level quickly decreases throughout menopause. 118 E2 has been demonstrated in numerous studies to alter systems involved in the pathophysiology of depression, including the serotonin and norepinephrine systems, and to considerably alleviate depressive symptoms in animal models. Estrogen therapy can decrease the quantity of 5-HT 1 and β-adrenergic receptors while increasing the quantity of 5-HT 1 receptors. 119 In addition, estradiol may influence the pathogenesis of male MDD patients. 120 In animal models, E2 has been shown to alleviate depressive-like behavior. 121 , 122 Estrogen receptor 1 (ER1) and estrogen receptor 2 (ER2) are transcription factors that are members of the NR family. Activating ER2 with a range of ER2 agonists has been reported to reduce stress-induced HPA activity and anxiety-like behaviors. 123 , 124
Astrocytes are estrogen targets, 125 as both ER1 and ER2 receptors are present on the astrocyte membrane or intracellularly in astrocytes. The transmembrane receptors ER and GPR30 have been shown to facilitate nongenomic and fast estrogen signaling in astrocytes, contributing to the neuroprotective effects of E2. In mature astrocytes differentiated from human induced pluripotent stem cells (iPSC)-derived astrocyte progenitors, ketamine can exert rapid antidepressant effects through the activation of amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) glutamate receptors, and estrogen enhances this effect of ketamine by increasing the gene expression of AMPA receptor subunits. 126
The obese gene (OB) encodes the hormone leptin, which is derived from adipocytes and the stomach and exerts its function through a specific receptor (OB-R). Leptin controls the function of the HPA axis 127 via its receptor in the hypothalamus. The cerebral cortex, hippocampus, hypothalamus, dorsal raphe (DR) nucleus, arcuate nucleus, and solitary tract nucleus are some regions of the brain that can express leptin receptors. Increasing experimental data have recently shown that leptin is linked to the pathological and physiological processes of numerous mental illnesses and plays a vital regulatory role in the CNS. 128 , 129 According to our previous reports, leptin can enhance the pharmacological effects of fluoxetine in astrocytes sorting from GFAP-GFP transgenic mice. 130 Leptin selectively increases the expression of the astrocytic 5-HT 2B receptor by activating the JAK2/STAT3 pathway, and fluoxetine in turn stimulates the 5-HT 2B receptor and increases the secretion of brain-derived neurotrophic factor (BDNF) from astrocytes in vivo, thus ameliorating depressive-like behaviors. 130 All of these findings indicate leptin’s potential to boost protein expression and functionally stimulate SERT.
Cytokine hypothesis
MDD is accompanied by changes in the levels of proinflammatory cytokines and trophic factors, including BDNF, interleukins (IL-1β, IL-6), and tumor necrosis factor alpha (TNF-α). Increasing data suggest that the production of certain cytokines by brain astrocytes plays a significant role in the pathogenesis of MDD.
Oxidative stress
Oxidative stress (OS), which is caused by an imbalance between antioxidants and reactive oxygen species (ROS), can harm proteins, lipids, or DNA. The activity of monoamine oxidase, the enzymes that break down monoamines such as DA, 5-HT and NE, is influenced by ROS and in turn can increase ROS production in mitochondria. The brain is more vulnerable to OS than other organs. In depression, OS plays a crucial role. 131 , 132 The brain is particularly sensitive to OS due to numerous variables, including rapid oxidative energy metabolism (a process through which ROS, which are harmful molecules, are constantly produced), high levels of unsaturated fatty acids (which are vulnerable to lipid peroxidation), and relatively low intrinsic antioxidant capability. 133 Adults with MDD exhibit ROS-mediated reductions in nitric oxide (NO)-dependent dilation. 134
Thioredoxin reductase, heme-oxygenase 1, glutathione, and glutathione peroxidase are only a few of the ROS-detoxifying enzymes that are abundant in astrocytes. 135 Astrocytes are the major producers of glutathione in the brain because they express a system xc-cyttine/glutamate antiporter, which does not exist in neurons; hence, neurons cannot synthesize glutathione. Notably, astrocytes can protect nearby neurons against toxic dosages of NO, H 2 O 2 , and superoxide anion in combination with NO, iron, or 6-hydroxydopamine in coculture systems, 135 indicating that neurons rely on the strong antioxidant capacity of astrocytes for protection against OS. Nuclear factor erythroid 2 (Nrf2), a redox-sensitive transcription factor required for coordinating the cellular antioxidant response, can be activated by astrocytes. In our recent study, lithium salt (Li + ) was found to effectively alleviate ischemia-induced anhedonia in mice by suppressing the production of mitochondrial ROS in glial cells. 136
Recent investigations have indicated that MDD is caused by increased ROS production and promotes inflammation. 137 The brain has weak antioxidative defenses and a high oxygen consumption rate, making it particularly susceptible to OS. Inflammasomes in microglia can be activated by ROS, which causes inflammatory cytokines, including TNF-α, IL-1β, and IFN-γ, to be produced. 138 Neuroendocrine-immune activities can be compromised by inflammation, which can also result in numerous disorders, such as MDD. Proinflammatory cytokines have become pathological indicators of MDD, and using the right antioxidants to combat ROS may be a useful method for treating MDD.
Proinflammatory cytokines
Higher levels of inflammation increase the chance of developing new-onset depression. 138 , 139 Although depression can cause inflammation, its cause is still unclear and may be influenced and regulated by immune cells, inflammatory cytokines, and the nervous system. In addition to contributing to the etiology of depression, activation of proinflammatory signaling pathways occurs as a result of elevated OS. 140 Evidence suggests that MDD is associated with the immune response, as shown by increased levels of IL-1β, TNF-α, and IL-6. 141 LPS-induced astrocyte activation also contributes to the symptoms of MDD. Systemic treatment with LPS induces depressive-like behaviors and increases the production of inducible nitric oxide synthase (iNOS), IL-1β, TNF-α, and GFAP in the hippocampus and cortex. Inhibition of activated astrocytes reduces neuroinflammation. These alterations are followed by amelioration of LPS-induced depressive-like behaviors. 142
Neurotrophic factors
In the vast majority of patients with severe depression, antidepressants affect the levels of neurotrophic factors. For example, the primary regultaory factor of neuronal survival, growth, and differentiation during development is BDNF. For the treatment of depression, targeting signaling transduction by BDNF and its receptor, tropomycin receptor kinase B (TrkB), is essential. 143 , 144 Recent research has shown a link between decreased hippocampal neurogenesis and low levels of BDNF and glial-derived neurotrophic factor (GDNF) in the brains of depressed individuals. 145 Under normal conditions, astrocytes release various nutrients and cytokines. After cell reactivation, the secretion of these factors is further increased. 146 According to previous studies, fluoxetine stimulates c-Fos expression and ERK 1/2 phosphorylation, which in turn promotes BDNF production in astrocytes sorting from GFAP-GFP transgenic mice. 147 Imipramine acts as an antidepressant by increasing the mRNA expression of BDNF in astrocytes. Fluoxetine also induces BDNF expression by activating cAMP-response element binding protein(CREB) through the PKA and/or ERK pathways. 148
BDNF is an essential molecule for neural plasticity and development and is related to several CNS diseases. Currently, it is known that BDNF can regulate the activity of neurons and that it is produced not only by neurons but also by astrocytes. 149 SSRIs and tricyclic antidepressants increase BDNF expression in cultured primary astrocytes, and BDNF overexpression in mouse hippocampal astrocytes is sufficient to promote neurogenesis and causes anxiolytic behavior. 149 By promoting neurotransmitter release, facilitating vesicle docking, and upregulating the expression of synaptic vesicle proteins, BDNF, which is released by astrocytes in response to long-term antidepressant therapy, may assist in increasing synaptic plasticity at presynaptic terminals. 150 In addition, astrocyte-secreted BDNF can stimulate adult hippocampal neurogenesis and may contribute to synaptic and structural plasticity that underlies the long-lasting behavioral effects of antidepressants. 150 Astrocytes can secrete numerous nerve growth factors. Vascular endothelial growth factor (VEGF) is a member of the vasoactive growth factor family. It exerts its unique molecular effects by binding and activating endothelial cell tyrosine kinase receptors. VEGF is traditionally associated with angiogenesis and its stimulation. Recent evidence indicates, however, that it also influences nerve cells and plays a crucial role in hippocampal neurogenesis and neuroprotection. 151
Inflammasomes
Neuroinflammation is a central pathophysiological mechanism and defining characteristic of MDD. Numerous elements in the periphery and CNS interact to generate neuroinflammation, thereby stimulating astrocytes. The nucleotide-binding domain and leucine-rich repeat protein-3 (NLRP3) inflammasome is one of the largest typical inflammasomes discovered thus far. It is composed of pro-Casp-1 protein, NLRP3, and apoptosis-associated speck-like protein (ASC). 152 The sensitization of the NLRP3 inflammasome and the suppression of BDNF synthesis result in MDD. 153 In our research, SD is found to reduce BDNF levels and induce depressive-like behaviors in the sorted astrocytes from GFAP-GFP transgenic mice by activating the NLRP3 inflammasome. 130 NLRP3 inflammasome activation causes astrocytes to produce more IL-1β and IL-18. 154 , 155
The release of proinflammatory cytokines is the primary consequence of the activation of caspase-1, a component of the NLRP3 inflammasome. In addition, it has been observed that stimulating NLRP3 inflammasome assembly can induce depression-like behaviors in rodents exposed to LPS or CUMS. 156 , 157 Research on the effect of astrocyte-specific NLRP3 knockout suggests that the astrocytic NLRP3 inflammasome exerts a significant effect on astrocytic pyroptosis via the Casp-1/GSDMD pathway in depression. 156 , 157 Therefore, efficient NLRP3 inflammasome inhibitors are novel therapeutic agents for MDD. As we previously reported, chronic SD can specifically activate the NLRP3 inflammasome and decrease the level of BDNF in astrocytes to ameliorate depressive-like behaviors. Fluoxetine can suppress the effects of SD on astrocytes by stimulating astrocytic 5-HT 2B receptors directly. 147 Additionally, in the middle cerebral artery occlusion (MCAO) stroke model of mice, Li + can significantly attenuate GSDMD-mediated glial pyroptosis by regulating the AKT/GSK3β/TCF4/β-catenin signaling pathway, in which, the activation of AKT induced by Li + can also increase the phosphorylation of FoxO3a and promote the transportation of FoxO3a from nucleus into cytoplasm, the reduced FoxO3a in nucleus dissolves its competition with TCF4 in order to confirm more β-catenin/TCF4 complex. The increased latter complex further up-regulates the expression and activation of STAT3 in nucleus, the latter further inhibits the activation of the NLRP3 inflammasome by increase UCP2 which can decrease the production of ROS from mitochondrion. 136 This neuroprotective mechanism of Li + after ischemia-reperfusion injuries contributes to the improved depressive-like behaviors, besides of motor and cognitive capacities. 136
In conclusion, there have been so many hypothesis to explain the pathogenesis of MDD associating with many booming researches (Fig. 3 ). However, it is still hard to adopt only one above hypothesis to completely reveal pathophysiology of MDD. The main problem may contribute to the limitations of the theoretical perspective and the limitations of detection methods. Some key scientific problems in the neurobiology of neurological and psychiatric disorders are still unclear, such as how to identify the pathological characteristic changes for mood disorders, how to metabolize the cerebral metabolic waste under the pathological condition,how to observe the instant interactions of neural cells and the real-time changes of intracellular organelles in the patients of MDD? In the pathological conditions, conducting research from the perspective of comprehensive collaboration of the whole body and increasing the proportion of new technological applications in research will open up the new paths to reveal the pathogenesis of MDD in the future.
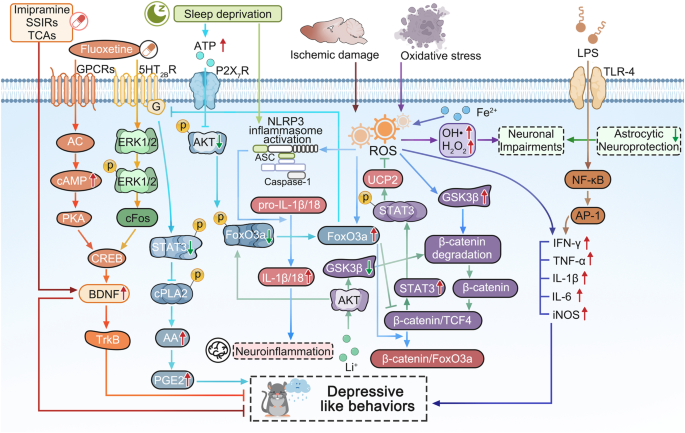
The molecular signaling schematic of cytokine hypothesis in the pathogenesis of MDD. The rodent performed the depressive like behaviors are impaired by some widely accepted risk factors, such as long-term sleep deprivation (SD), oxidative stress, lipopolysaccharide (LPS), ischemic damage and so on. Long-term SD can increase the extracellular ATP level, the latter inhibits the activation of AKT and the followed phosphorylation of FoxO3a by stimulating P2X7 receptors (P2X7R), the dephosphorylated FoxO3a translocates into the astrocytic nucleus, then the increased FoxO3a decreases the expression of 5-HT 2B R expression, which results the reduced phosphorylation of STAT3 which increases the activation of cPLA2 and the followed release of arachidonic acid (AA) and prostaglandin E2 (PGE2), finally causing the depressive-like behaviors. 41 Thus, antidepressant fluoxetine activates ERK 1/2 /cFos pathway by stimulating 5-HT 2B R and AC/cAMP/PKA pathway by activating GPCRs in order to increase the activation of CREB and the level of BDNF and TrkB, which can alleviate the depressive like behaviors induced by long-term SD. 147 , 148 As well as, imipramine, other SSIRs, and TCAs can also play antidepressive roles by increasing BDNF mRNA expression in astrocytes. 148 Ischemic stroke can trigger the increase of reactive oxygen species (ROS) which can induce the activation of NLRP3 inflammasome and the release of IL-1β/18, resulting in the neuroinflammation, however, Li + salt inhibits the activation of GSK3β and increases the phosphorylation of FoxO3a by activating AKT, which promotes the more FoxO3a transportation from nucleus into cytoplasm, and the reduced FoxO3a in nucleus lacks the competition with TCF4, the increased complex level of β-catenin and TCF4 further stimulates the expression and the phosphorylation of STAT3, which further induce the mRNA and protein expression of UCP2, then in mitochondrion, the increased UCP2 suppresses the production of ROS and results in the deactivation of NLRP3 inflammasomeincreases. 136 Superoxidation of Fe 2+ stimulates an increase in ROS, resulting in the production of inflammatory cytokines (including IFN-γ, TNF-α, IL-1β, IL-6) and inducible nitric oxide synthase (iNOS). 138 While, the treatments of oxidative stress (OS) can produce a large number of ROS, such as OH• and H 2 O 2 , resulting in neuronal impairments, while astrocytes can play their neuroprotective role by antioxidation. 135 Additionally, LPS can also increase TNF-α, IL-1β, and IL-6 by TLR-4/NFkB/AP-1 pathway and cause depressive-like behavior. 142 Adobe Illustrator was used to generate this figure
Interactions of multi-cells and multi-organs
Recently, increasing evidence has shown that pathological changes in a single cell type or brain region limited are insufficient explain the pathogenesis of MDD. This section mainly introduces the latest research on the pathogenesis of MDD, discussing the multiple interactions among neural cells and the multiple regulatory mechanisms between the brain and peripheral organs in detail.
The interaction between neuron and glial cell
Over the past few decades, studies on MDD have identified decreased PFC activity and excitatory/inhibitory (E/I) imbalance as probable mechanisms underlying depression. 158 Astrocytes are recognized to be essential for controlling neural network activity and to take part in higher brain activities. 159 To explore efficient treatments for MDD, it is important to focus on how to regulate the E/I balance and neuronal remodeling. 160
MDD-related marker proteins in neural cells
Astrocytes in the CNS form the neurovascular unit with neurons and blood vessels. The neurovascular unit mediates the exchange of nutrients and other functional substances between its components. 161 The blood-brain barrier (BBB) consists of endothelial cells tight junctions, a continuous basement membrane and astrocytic end-feet. Two proteins expressed on astrocytes, connexin 30 (Cx30) and Cx43, have been linked to the pathogenesis of depression. 162 Gap junctions that enable communication between astrocytes are formed by the membrane proteins Cx30 and Cx43. 163 Chronic unpredictable stress (CUS) and acute stress both specifically reduce the expression of the gap junction-forming proteins Cx30 and Cx43, 164 and the integrity of the BBB is weakened in mice lacking Cx30 and Cx43. 165
In addition to being an essential component of the developing astrocyte cytoskeleton, GFAP serves as the main intermediate filament protein in adult astrocytes. Although increased expression of GFAP is commonly observed in reactive astrogliosis, postmortem results suggest that the frequency and intensity of reactive astrogliosis are decreased in the brains of patients with MDD. 166 Accompanied by a decreased astrocyte density, the levels of GFAP and the GFAP intermediate filament domain are also reduced in brain samples from patients with MDD. 167 Researchers have even proposed that the GFAP content in serum can be used to determine the severity of MDD, 168 but this point is controversial.
AQP4, a kind of water channel, is mainly expressed on astrocytic end-feet in contact with blood vessels. The water channel AQP4 regulates the equilibrium of ions and water in the brain and is an essential part of the neurovascular unit. The vascular coverage of AQP4-immunopositive astrocytes in the orbitofrontal cortex (OFC) is lower in people with clinically significant depression than in psychiatrically healthy control patients. 169 In another postmortem study, it was found that the coverage of blood vessels by AQP4-positive astrocyte terminals was reduced in the OFC of MDD patients. 170 In addition, the K + -buffering capacity and presumably synaptic transmission are impaired in mice lacking AQP4, and impairment of these processes is associated with depressive-like behaviors. 171 In our previous study, we reported that the expression of AQP4 was decreased by exposure to CUMS, which contributed to dysfunction of glymphatic circulation and depressive-like behaviors in mice. 172 Additionally, the coverage of blood vessels by AQP4-positive astrocytic endfeet is decreased by 50% in MDD patients, indicating that decreased levels or mislocalization of AQP4 may contribute to the pathogenesis of MDD. 169 , 173
S100B is produced and secreted by astrocytes in the gray matter, 174 and changes in the levels of S100B in the blood and cerebrospinal fluid (CSF) of patients with MDD can cause glial cell dysfunction and damage. 175 , 176 In individuals with MDD, the number of S100B-immunopositive astrocytes in the pyramidal layer of the bilateral hippocampal CA1 region is decreased. 177 S100B secreted by damaged astrocytes can enter the extracellular space and CSF, 178 and the level of S100B is increased in the dorsolateral prefrontal cortex (dlPFC) of patients with MDD. 179 S100B levels are elevated in the CSF or serum of patients with MDD, 180 which suggests that S100B is a potential diagnostic biomarker for depressive episodes associated with MDD.
Communication between neurons and microglia plays an important role in the pathogenesis of depression. C-X3-C Motif Chemokine Ligand 1 (CX3CL1)- C-X3-C Motif Chemokine Ligand 1 receptor (CX3CR1) and OX-2 membrane glycoprotein (CD200)-OX-2 membrane glycoprotein receptor (CD200R) form ligand-receptor pairs, and these molecules are the most important chemokines and clusters of differentiation in maintaining CNS homeostasis. 181 CX3CL1 and CD200 are mainly expressed in neurons, and their receptors CX3CR1 and CD200R are expressed on microglia. 182 Activated microglia and decreased expression of CX3CL1 in the hippocampus were observed in an LPS-induced depression model. 183 CX3CR1-deficient mice show a temporary decrease in the number of microglia and a resulting deficiency of synaptic pruning, which may be related to neurodevelopmental and neuropsychiatric disorders. 184 However, CX3CR1-deficient mice show significant resistance to stress-induced depressive-like behaviors. 185 The level of CX3CL1 in the serum is increased in patients with moderate-severe depression compared with healthy subjects; thus, CX3CL1 could be used as a target for depression treatment. 186 Patients diagnosed with MDD with comorbid cocaine addiction show higher serum levels of CX3CL1. 187 Additionally, in a rat early-life social isolation (ESI) model, the expression of CD200 receptors in microglia is significantly reduced. 188 Exposure to unavoidable tail shock causes a decrease in CD200R expression in the hippocampus and amygdala, 189 and stress was also discovered to suppress CD200R expression in the hippocampus of rats. 190
Synaptic plasticity
Long-term potentiation (LTP) serves as the physiological basis for learning and conditioned responses. 191 Ketamine has a quick antidepressant effect, as it is a noncompetitive channel blocker of N-methyl-D-aspartate receptors (NMDARs). 192 Excessive glutamate in the synaptic cleft activates synaptic metabotropic glutamate receptors (mGluRs), which lead to neural excitotoxicity. 193 In a mouse model of chronic social defeat stress (CSDS), which causes depression, mGluR5 was shown to induce long-term depression (LTD). The major process responsible for synaptic plasticity is the mGluR-mediated LTD, which likely plays a significant role in the pathophysiological changes underlying depressive-like behaviors in the CSDS-induced depression paradigm. 194
ATP can mediate the activity of the astrocyte-neuron network, and ATP is a signaling molecule that also controls synaptic plasticity. 195 ATP can increase the expression of amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) by stimulating P2X 7 R and increasing the amplitude of miniature excitatory postsynaptic currents. 196 Stress exposure is a major pathogenic factor in disease models and can increase Ca 2+ -dependent release of ATP from neurons, which causes excitotoxicity. 197 , 198
Regulated in development and DNA damage response-1 (REDD1) is a stress response gene that can regulate development and the response to DNA damage. Virus-mediated overexpression of REDD1 in the rat PFC is sufficient to cause anxiety- and depressive-like behaviors and neuronal atrophy. 199 According to postmortem studies, the volume of the dlPFC is smaller and the density of neurons in the dlPFC is lower in MDD. 200 BDNF can modulate synaptic plasticity in the brain. TrkB is a functional receptor of BDNF. 201 BDNF produces antidepressant-like effects by increasing synaptic plasticity in a mouse model of CUMS. 202
Neuron-glia integrity
The term “tripartite synapse” was initially used to describe the intimate relationship between astrocytes and neurons at glutamatergic synapses, similar to the glutamate-glutamine cycle described above. 203 Moreover, glutamic acid decarboxylase, an enzyme that transforms glutamate into γ-aminobutyric acid (GABA), also exists in inhibitory GABAergic neurons. Increased inhibitory neurotransmission, glutamatergic/GABAergic E/I imbalance, and chronic stress-related emotional dysfunction reduce PFC activity. 204 , 205 In local circuits, various glutamatergic and GABAergic neurons interact in complicated ways to achieve E/I balance. 206 A meta-regression analysis indicated that glutamine and glutamate levels are decreased in the PFC, which is correlated with the therapies to MDD. 207 Global topological E/I imbalance in MDD is discovered through gene and protein expression of molecules related to inhibitory GABAergic and excitatory glutamatergic signaling in the postmortem MDD brains. 22 , 208 , 209 It shows the imbalance in cortical-subcortical limbic regions with decreased GABAergic signaling and increased glutamatergic signaling. 210 , 211 Meanwhile, GABAergic signaling is decreased in regions comprising the default mode network (DMN), while it is increased in the lateral prefrontal cortex (LPFC). 212 , 213 Stimulating P2X 7 R in neocortical nerve terminals can block the reuptake of GABA and glutamate by the presynaptic membrane and promote the release of these two neurotransmitters in the cerebral cortex of rats and humans, 214 , 215 and activation of P2X 7 R reduces the expression of GLAST. 216 This results in neuronal damage, a reduced number of synapses, decreased neurogenesis, and even impairment of key cerebral circuits that regulate mood.
Astrocytes are fundamental elements in synapses, participate in synaptogenesis and maturation, and maintain synaptic homeostasis. Ionic homeostasis in the extracellular space is critical for central nervous system function. 217 Astrocytes play an important role in maintaining extracellular K + homeostasis in the CNS, as well as H + , Cl - , and Ca 2+ homeostassis. 218 In addition, it also plays an important role in maintaining transmitter homeostasis, in which glutamate and GABA play particularly important roles. 219
In addition to the tripartite synapse, the more recent concepts of the four-part extracellular matrix and the microglial five-part synapse 220 also support the idea that glial dysfunction plays key roles in the early pathological features common to psychiatric disorders. 221 , 222 Under physiological conditions, microglia can play a neuroprotective role by producing cytokines. However, under pathological conditions, microglia can also affect the balance between excitatory and inhibitory synapses by phagocytosing synapses 223 and activating inflammatory factors in microglia. 224 In addition, the extracellular matrix (ECM) plays a significant role in maintaining normal communication in mature neural networks, which can limit the synaptic restriction of glutamate. 225 The components of the ECM are mainly produced by neurons and astrocytes, and microglia can also regulate the remodeling of the ECM. 226
Interaction mechanism in multi-organs
Abnormalities in cytokine levels in the brain and peripheral organs, disruption of the brain/immune system balance, and dysfunction of communication between the peripheral organs and the brain can cause neuroinflammation and depressive symptoms. For instance, cirrhosis and depression have been linked to intestinal dysbiosis, which results in intestinal barrier disruption, increasing bacterial translocation. Increased bacterial translocation then activates circulating immune cells, which produce cytokines and induce systemic inflammation. 227 In comparison with the healthy population, MDD patients have a much higher incidence and prevalence of chronic liver disease. 228 Inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) with increased intestinal permeability, which may have both inflammatory and autoimmune sources, are common comorbidities of MDD and anxiety. 229 , 230
Neuroendocrine-immune axis
Microglia secrete chemokines that disrupt the integrity of the BBB and increase the ability of immune cells to enter the brain parenchyma. 231 The stress response is a complex array of behavioral, neuroendocrine, autonomic, and immunological responses that enable adaptation to unpleasant psychological and physiological stimuli. 232 The HPA axis is a crucial endocrine system that orchestrates this response. 233 Stress can activate microglia, which are considered important immunocytes of the CNS. Mediators released by activated microglia can stimulate the HPA axis and induce GC production. 39 Similarly, high levels of GCs can also activate microglia, creating a vicious cycle. 234
Tryptophan (TRP) can be converted into a variety of biologically active molecules, and more than 95% of TRP is metabolized to kynurenine (KYN) and its breakdown products, with only a small portion of TRP being converted to 5-HT. 235 Indoleamine 2,3 dioxygenase (IDO) is an immune inducible enzyme that metabolizes TRP through the KYN pathway and plays an important role in the immune response. 236 In the brain, KYN is metabolized to the neurotoxic substance quinolinic acid (QUIN). 237
The primary GC in the HPA axis, corticosterone, plays a role in regulating the stress response in rodents. Stress, high GC levels, and serious depression are all linked. Analysis of transcriptomic changes associated with corticosterone-induced cytotoxicity revealed an association of neurite outgrowth-related genes with depression. Therapies for MDD may target the expression of genes involved in neurite formation, such as calpain 2 (Capn2), vesicle-associated membrane protein (Vamp7), and c-type natriuretic peptide (Cnp). 238
Consumption of a high-fat diet (HFD; for approximately 16 weeks) results in anxiety and anhedonic behaviors, and 4 months of HFD consumption results in increased levels of corticosterone and blood glucose, which also activate the innate immune system, increasing the release of inflammatory cytokines (i.e., IL-6, IL-1β, TNF-α). The behavioral abnormalities that arise from long-term consumption of a HFD are quickly reversed by ketamine. Additionally, giving HFD-fed rats a P2X 7 R antagonist greatly alleviates their anxiety. 239
Microbiota-gut–brain axis
In recent years, the microbiota-gut-brain axis has been reported to be disrupted in MDD. Stress stimulation can affect the gut microbiota, which in turn induces the production of inflammatory mediators (mainly IL-6 and IFN-γ) and a reduction in short-chain fatty acid levels. 240 The increased level of inflammatory cytokines may be caused by disturbance of the gut microbiota, which may also disrupt the gut barrier. 241 Alterations in the gut microbiota and inflammatory agents have an impact on the KYN pathway, metabolism, and toxin metabolism in the periphery. 242 Proinflammatory cytokines or toxic byproducts resulting from microbiota alterations may pass through the BBB and enter the brain. 243 This increases the levels of cytokines such as IL-1β and IL-6 and NLRP3 inflammasome activation in brain-resident cells. 244 In particular, microglia and astrocytes are activated and undergo atrophy, respectively. These glial cell changes, which affect the brain networks involved in learning and memory, mood regulation, and emotional regulation, may cause depressive symptoms or anxiety episodes. 245
According to clinical research, TRP and tryptophan catabolites (TRYCATs) may play a crucial role in psychiatric illnesses, including MDD. Peripheral and central inflammation can both stimulate the KYN pathway and trigger TRP metabolism and subsequent synthesis of various TRYCATs, including the toxic NMDAR activator QUIN, 246 which influences glutamate transmission, has a variety of immunomodulatory effects and has both neurotoxic and neuroprotective effects on the CNS. 141 Studies have proven that peripherally injected LPS increases the central and peripheral metabolism of TRP via the KYN pathway by exerting neurotoxic effects, inducing reactivation of microglia and astrocytes in the CNS. 247 Excessive production of QUIN, an NMDAR agonist, stimulates the release of glutamate and inhibits reuptake, leading to neuronal excitotoxicity. 248
Liver-brain axis
Patients with liver diseases often struggle with depression. According to one study on the frequency of liver disease and major depression in the United States, liver disease is linked to both major depression and suicidal thoughts. 249 A further population-based cohort study discovered that patients with MDD had much higher prevalence and incidence rates of chronic liver disease than the general population. 228 The incidence of depression is high in cirrhosis patients; moreover, depression is an independent predictor of mortality from cirrhosis. 250
An internal metabolic mechanism regulated by the liver can control depressive-like behavior. A crucial enzyme in epoxyeicosatrienoic acid (EET) signaling in the liver is epoxide hydrolase (sEH). Chronic stress selectively exacerbates sEH-induced depression-related changes in the liver while dramatically lowering the plasma levels of 14,15-EET. Deletion of hepatic epoxide hydrolase 2 (Ephx2) (which encodes sEH) rescues the chronic mild stress (CMS)-induced decrease in 14,15-EET plasma levels. 251 In a rat model of CUMS, electroacupuncture (EA) was found to downregulate P2X 7 R, NLRP3, and IL-1β expression in the prefrontal cortex and liver and relieved depression-like behavior. 252
In summary, as shown in Fig. 4 , although the etiology of MDD is still unclear, it is widely accepted that the common pathogenic factors of MDD are genetic, stress, and comorbidity. 3 The levels of monoamine neurotransmitters (5-HT, NE, and DA) are insufficient in the synaptic cleft of MDD patients, correspondingly, the explored antidepressants such as tricyclic antidepressants(TCAs), SSRIs and SNRIs almostly act on the channels responsible for inhibiting reuptake of these neurotransmitters. 51 Thus, according to these traditional pharmacological theories, these antidepressants always have the delayed clinical efficacy, this promises the potential new pharmacological mechanism still requires further study. As the well-known glutamate-glutamine cycle, astrocytes play key roles in resolving neuronal glutamate toxicity. However, under the MDD pathological condition, due to the decreased expression of EAATs in astrocytes, excessive glutamate in the synaptic cleft activates synaptic mGluRs, which leads to neuronal excitotoxicity. 194 In addition, the overdose glutamate can also be decarboxylated by glutamate decarboxylase (GAD) to GABA and activates the GABA receptors on the postsynaptic membranes. 206 In our previous studies, the expression of 5-HT 2B is selectively decreased in the sorting astrocytes from MDD model mice. 64 The antidepressants SSRIs and leptin can increase the expression of the astrocytic 5-HT 2B receptor. 147 Furthermore, OS plays a crucial role in the emergence of depression, including by elevating the levels of ROS and NO in the mitochondrion of astrocytes. 253 Proinflammatory signaling pathways are activated as a result of elevated OS, the mitochondrial dysfunction results in an increased generation of ROS and NO. 137 As well as, the pathogenesis of MDD are associated with the inflammatory-immune response, as shown by elevated levels of proinflammatory cytokines, mainly IL-1β, TNF-α, and IL-6. 141 The expression of neural cell marker proteins in neural cells, including Cx30/43, 162 GFAP, 167 AQP4, 172 and S100B, 177 are all decreased under MDD pathological conditions. In brain, KYN is metabolized by microglia to the neurotoxic metabolite QUIN and by astrocytes to the beneficial metabolite kynurenic acid (KynA), thus, QUIN is increased and KynA is decreased in MDD patients’ brain. 141 , 254 , 255 Recently, growing evidence support that the occurrence of MDD are the results of the correlational disorders from multiple systems or organs, not only limiting in brain. 227 , 228 The comorbidities of MDD have attracted widespread attention, the intestinal gut microbial dysbiosis, liver dysfunction, immune system disorders all play important roles in the pathogenesis of MDD. Stressful conditions can affect the gut microbiota, which in turn induces the production of inflammatory mediators (mainly IL-6 and IFN-γ). 256 Proinflammatory cytokines or toxic QUIN resulting from alterations in the microbiota may pass through the BBB and activate NMDARs. 243 Under the dysfunction of liver, the level of ammonia is increased in the brain. 257 The pathogenic factors of various organs at the body level and the pathological changes of glial cells at the cellular level should attract more attention to explain the pathogenesis of MDD.
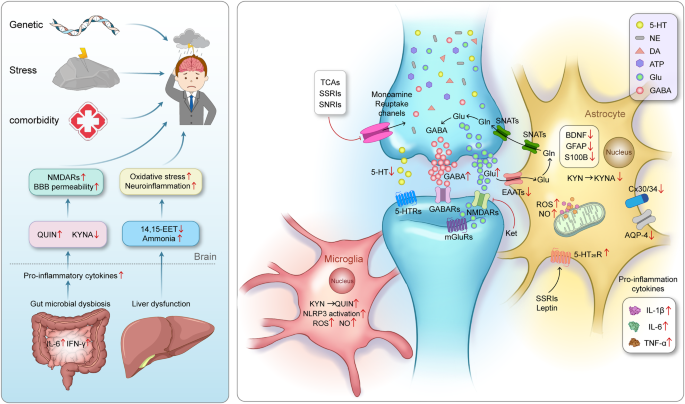
The pathogenesis of MDD is closely related to synapses, astrocytes, microglia, and their interactions as well as interactions among organ. Genetic factors, stress and comorbidities are considered the most common pathogenic factors of MDD 3 . The traditional monoamine theory contends that MDD may cause by the deficits in monoamine neurotransmitters. 49 Moreover, the other abnormal increase of neurotransmitters in the synaptic cleft, such as glutamate, GABA and ATP, has the high relationship with the pathogenesis of MDD. 41 , 496 The interaction between neurons and glial cells can induce the oxidative stress, pro-inflammatory cytokines released, the reduction of neurotrophic factors. The microbiota-gut-brain axis is clearly disrupted in MDD. 243 , 248 When liver dysfunction occurs and causes OS and neuroinflammation in the brain, which also contribute to the pathophysiology of MDD. 497 Adobe Illustrator was used to generate this figure
New diagnostic approaches
MDD is a prevalent psychiatric disorder worldwide and is expected to become one of top disease in terms of burden by 2030. 258 However, the current clinical diagnostic criteria for MDD are subjective, and diagnoses are mainly based on clinical symptoms, leading to high rates of missed and incorrect diagnoses. This section summarizes the newest research on diagnostic approaches for MDD, including serum indicators, neuroimaging indicators and multimodality scales. Research on new diagnostic approaches for MDD has the potential to improve our understanding of MDD pathogenesis and the accuracy of clinical diagnosis.
Potential serum indicators
The pathological mechanism of MDD can be studied in two ways: by exploring the pathophysiology of the disease and by identifying MDD-related neurobiological indicators4. Hence, identifying potential biomarkers for MDD could allow accurate diagnosis, faster treatment and effective monitoring of the disease. Recently, an increasing number of studies have confirmed the involvement of OS and neuroinflammation in MDD pathology. 259 , 260 Two novel biomarkers, serum nicotinamide adenine dinucleotide phosphate oxidase 1 (NOX1) and Raftlin, are reported to have good diagnostic value in MDD patients. The effectiveness of elevated NOX1 and Raftlin levels in diagnosing MDD has been evaluated in clinical trials; the related mechanism is that NOX1 can regulate the ROS-antioxidant balance in patients with MDD through OS and the inflammatory repsonse. 261 The serum level of the chemokine-like protein TAFA-5 (FAM19A5) has also been reported to be increased in patients with MDD, and increased serum FAM19A5 levels are associated with reactive astrogliosis, neuroinflammation, and neurodegeneration. 262 In addition, the level of serum FAM19A5 was shown to have a negative correlation with cortical thickness in specific brain regions. These findings suggest that serum FAM19A5 could be a potential biomarker for neurodegenerative changes in MDD.
Functional magnetic resonance imaging indicators
In addition to serum indicators, neuroimaging metrics are potential objective tools for improving the accuracy of MDD diagnosis and must be studied in death. In recent years, many researchers have tried to diagnose MDD using MRI by identifying disease-specific functional and/or structural abnormalities in patients with MDD compared with healthy subjects. 263 Structural MRI techniques, such as voxel-based morphometry (VBM), can be used to detect volume changes in gray matter. 264 It has been reported that abnormal gray matter volume (GMV) in several brain regions is positively correlated with MDD. 265 , 266 Regarding functional MRI, recent studies have revealed that cerebral functional abnormalities are not limited to specific brain regions in patients with MDD. These differences are also associated with hypoconnectivity within the frontoparietal network (FN), the DMN, and midline cortical regions. 267 , 268 Furthermore, resting-state functional magnetic resonance imaging (R-fMRI) is an emerging neuroimaging technique used to study functional connectivity in the brain and holds great potential in aiding clinical diagnosis. 269 It has the benefits of being noninvasive and easy to perform and offering high temporal and spatial resolution. 270 As a result, it has played a significant role in MDD research and is a superior technique for researching MDD pathogenesis and identifying neuroimaging markers for MDD. 271 Thus, indicators such as amplitude of low-frequency fluctuation (ALFF), fractional amplitude of low-frequency fluctuation (fALFF), regional homogeneity and functional connectivity (FC) have shown promise as neuroimaging markers for MDD. Recently, a study reported that increased average values of ALFF and fALFF in the right caudate and corpus callosum may serve as potential markers for diagnosing MDD. 272 Another study based on the largest R-fMRI database of MDD patients confirmed that the DMN plays a crucial role in MDD diagnosis, as DMN FC is reduced in patients with recurrent MDD. 273 These findings also suggest that the DMN should continue to be a prominent focus of MDD research.
New multi-modal evaluation scales
Given that structural and functional abnormalities are associated with MDD, 274 using multimodal approaches is more appropriate than relying on a single feature for the diagnosis of MDD. However, research results related to the effectiveness of neuroimaging techniques in diagnosing MDD remain inconsistent. 275 This may be attributed to variations in the types of structural and functional features examined; however, more importantly, very few studies have used multimodal approaches to diagnose MDD. 276 Recently, in a study utilizing multimodal MRI data, patients with MDD were successfully distinguished from healthy controls by radiomics analysis. 276 Radiomics is a rapidly developing field involving the extraction of quantitative information from diagnostic images, and it can be mainly divided into three steps: image acquisition, analysis and model building. 277 Additionally, omics and neuroimaging techniques can be combined to construct models for diagnosing MDD; specifically, 5-hydroxytryptamine receptor 1 A/1B methylation data can be integrated with resting-state functional connectivity (rsFC) data. It was shown that this combination could be used to more accurately distinguish patients with MDD from healthy subjects than R-fMRI data or DNA methylation data alone. 278
By now, the widely accepted objective diagnostic indicators or methods for MDD are still deficient. In addition to the unclear pathogenesis of MDD, insufficient sensitivity and accuracy of detection instruments are also the main reasons, especially the correlation between imaging characterization and disease-specific changes that need to be discussed.
Preventing the occurrence and recurrence of MDD
MDD is a disease with a high prevalence worldwide, 279 and preventing its occurrence and recurrence is crucial. Lifestyle medicine is an evolving medical specialty that aims to prevent chronic, noncommunicable diseases through lifestyle interventions. The goal of lifestyle medicine is to prevent the occurrence and recurrence of disease by improving sleep hygiene and diet, increasing physical exercise, avoiding sedentary behavior, increasing social support, and improving mood. In recent years, an increasing number of studies have demonstrated that the occurrence and recurrence of MDD can be prevented by means of lifestyle medicine; 280 we summarize these reports in this section.
Sleep improvements
Improving sleep is an important strategy to prevent the occurrence of depression. Insomnia is included in the diagnostic criteria for MDD. 281 However, few studies have examined whether treating insomnia can prevent the exacerbation of depressive symptoms. Treating insomnia can prevent the worsening of depressive symptoms, and cognitive behavioral therapy for insomnia (CBT-I) is a recommended intervention for treating insomnia to improve sleep and mood. 282 , 283 , 284 As a first-line treatment for insomnia, CBT-I includes cognitive therapy, stimulus management, sleep restriction, improved sleep hygiene, and relaxation. 282 , 285 CBT-I can also lead to sustained remission of insomnia-related disorders, and continuous treatment of insomnia with CBT-I can also reduce the occurrence and recurrence of MDD. 286 Circadian rhythm support (CRS) can strengthen the circadian rhythm by means of scheduled bright light exposure, physical activity, and body warming. 287 Although CRS has been reported to have only an indirect effect in alleviating sleep disturbance and depressive symptoms, 288 treatment with CRS may help maintain the beneficial effects of CBT-I. 288 , 289 In one study, 44% of untreated patients but 38%, 28% and 9% of patients treated with CRS, CBT-I, and CBT-I + CRS, respectively, experienced clinically significant worsening of depressive symptoms during a 1-year follow-up period. Between-group comparisons showed that the percentage of patients who experienced worsening of depressive symptoms was significantly different between the CBT-I + CRS group and the nontreated and CRS groups. 289 In a randomized controlled trial, exacerbation of depressive symptoms over one year was decreased in insomia patients with an increased risk of depression and insomnia patients treated by therapist-guided CBT-I combined with CRS; however, untreated insomnia patients with a high risk of depression experienced clinically significant worsening of depressive symptoms. 288 , 289
Disrupted sleep is a common symptom of depressive episodes and increases the risk of MDD, 290 but the correlation between the onset of sleep disturbance and MDD is still unclear. Additionally, patients with symptoms of sleep disturbance have a greater risk of MDD occurrence and recurrence. 290 , 291 One study suggests that disrupted sleep may affect monoamine function and the HPA axis, 292 even causing hyperarousal and inflammation. 293 Additional studies on the pathological mechanism of depression have suggested that the HPA axis is hyperactive in MDD patients and that sensitivity to negative feedback is decreased. 15 Additionally, one prospective cohort study reported that a history of sleep disorders can increase the risk of depression later in life and that subjective sleep problems are associated with clinically significant depressive symptoms. 294
Dietary adjustment
Dietary adjustment is an effective, safe, and widely applicable method for preventing MDD, especially by inhibiting MDD-related pathological inflammation. 295 Various nutrients can possess different anti-inflammatory properties; in contrast, there are many proinflammatory foods, such as those high in refined starch, sugar, and saturated fat and low in fiber and omega-3 fatty acids, 296 which can promote the occurrence of inflammation to increase the risk of MDD. 297 One study reported that the chance of being diagnosed with depression is higher among individuals who consume a proinflammtory diet than among those who consume an anti-inflammatory diet. 295 Stimulation of the innate immune system by proinflammatory foods can result in mild inflammation and chronic illness, which may contribute to an increased risk of MDD. 298 Furthermore, an increasing number of studies suggest that at the molecular and cellular levels, dietary factors have effects on neuronal function and synaptic plasticity, which may be implicated in the etiology of MDD. 299 , 300 Therefore, adherence to a healthier diet can reduce the incidence of MDD, which is of great significance for the clinical treatment and prevention of depression. 295
In addition, an increasing number of studies have identified the importance of the interaction among the microbiota, gut permeability, and immune-inflammatory processes in the pathophysiology of MDD. 301 Because the interaction of bacteria of some taxa in the gut with peripheral inflammation with the brain may be related to depression pathophysiology, 302 , 303 regulating the gut-microbe-brain axis may be a therapeutic and preventive strategy for psychiatric disorders. 304 Restoration of the gut eubiosis can prevent the occurrence of MDD, and probiotics can normalize the gut ecosystem. Additionally, by altering the microbiota and regulating gut permeability, a gluten-free diet can alter the activity of the gut-microbe-brain axis, which has been discovered to be related to the pathogenesis of MDD. 305 , 306 , 307 Other studies report that consuming a gluten-free diet and probiotic supplements together may inhibit the immune-inflammatory cascade in MDD patients, and decreased inflammation can improve the integrity of the gut barrier and alleviate depressive symptoms. 307 Similarly, dietary fiber can also improve immune function by regulating the gut microbiota to prevent the occurrence of MDD, 308 which is attributed to the inhibition of OS and inflammation.
Increasing evidence suggests that physical exercise can prevent some mental disorders in addition to cardiovascular disease. 280 , 309 This finding suggests that physical exercise may be able to prevent MDD. As reported in some studies, physical exercise can effectively prevent depression by affecting many molecular and cellular pathways; for instance, physical exercise can stimulate VEGF expression, 310 , 311 leading to cellular level changes, such as stimulation of angiogenesis, increased delivery of neurotrophic factors and oxygen by the vascular system, 312 an increase in the neurogenesis rate and induction of synaptogenesis. 312 , 313 Ultimately, VEGF improves function in the hippocampus, which is one of the brain regions related to depression and stress regulation. 314 , 315 , 316 Exercise also reduces the levels of proinflammatory factors (e.g., IL-6) and increases the levels of anti-inflammatory factors (e.g., IL-10), which is beneficial for preventing the occurrence of MDD. 317 , 318 , 319 Furthermore, physical exercise for approximately 45 minutes per day can significantly reduce the risk of MDD. 320 , 321 High-intensity activity, such as aerobic exercise, dancing, and the usage of exercise machines, and low-intensity exercises, including yoga and stretching, can all reduce the occurrence of MDD. 322 Specifically, the combination of aerobic exercise and stretching as a multimodal therapeutic strategies has a significant antidepressant effect in depressed inpatients. 323
Patients with MDD have significantly more sedentary than ordinary people, and they engage in less physical activity than what is recommended, i.e., an average of 150 min of moderate- to high-intensity physical activity weekly. 324 This finding suggests that decreasing sedentary behavior or increasing physical activity levels should be a priority to prevent the occurrence of disease. In psychiatric centers, aerobic exercise has received increasing attention as a valuable method of prevention. 324 Studies report that reduced depressive symptoms in MDD patients can be observed after increasing aerobic exercise and stretching exercise, with more significant alleviation of depressive symptoms after 8 weeks of aerobic exercise. 325 Reward positivity (RewP) and error-related negativity (ERN) were identified as potential biomarkers of the exercise treatment response in depression. 325 In individuals with MDD, aerobic exercise was found to be beneficial in ameliorating depressive symptoms, particularly in those with more severe depressive symptoms and a higher baseline RewP. 325 , 326 RewP may be useful for identifying those who will benefit from exercise as a treatment for depression. 325
Social intervention
Social support refers to the help provided by social relations and transactions. 327 Social support may be obtained from a variety of individuals, including family members, friends, coworkers, and community members. 328 Furthermore, a variety of factors, including the quantity and quality of support as well as subjectively perceived social support by individuals, impact the level of social support. 329 It has been reported that MDD patients often lack social support, and receiving adequate social support can confer greater resistance to stress and prevent the occurrence and recurrence of MDD. 330 , 331 Low-functioning social support or self-perceived poor social support causes worse symptoms and treatment outcomes in depressed patients. 332 , 333 , 334 A previous study also reported that patients who lack adequate social support are more likely to experience MDD. 335 Social support may have an influence on depression through neuroendocrine pathways, 336 , 337 and social support can improve a person’s psychological wellbeing and make the individual more resistant to stress. 337
Studies on structural social support, social network size, and mental health disorders have shown that less social contact and loneliness can cause more severe depressive symptoms. 338 For individuals with MDD, it is necessary not only to increase the frequency of social contact but also to improve self-awareness and foster close functional supporttive relationships. 335 , 339 Studies have reported that when controlling for all other variables, each aspect of social support is clearly associated with MDD, and to some extent, the occurrence of panic disorder in patients with MDD is more strongly associated with poor functional support. This finding suggests that functional support may be an important protective factor against MDD. 331 , 335 Social support itself, especially emotional support, 340 may alleviate and prevent depressive symptoms, and support from family members or friends can replace formal health care. 341
In general, the pathological development of MDD is a gradual transition from subclinical state to clinical pathological changes. It is crucial to identify the core targets that lead to pathological changes from quantitative to qualitative changes during this process, and the above preventive interventions, sleep improvement, physical exercise, dietary regulation, and social intervention, may prolong or reverse the subclinical pathological stage (Fig. 5 ).
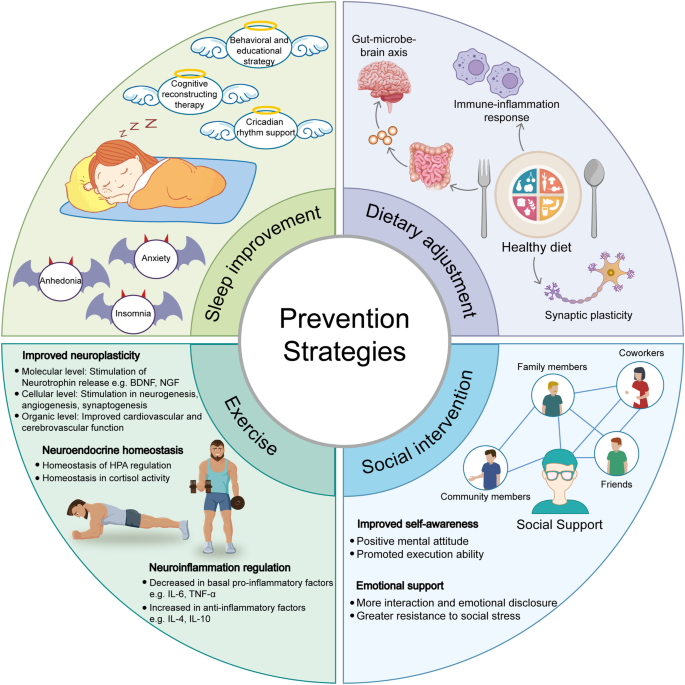
Schematic of prevention strategies for the occurrence and reoccurrence of MDD. An outline of various prevention strategies for MDD includes sleep improvement, dietary adjustment, exercise, and social intervention. Sleep disturbances have the high relationship with the occurrence of MDD, the anhedonia, anxiety and insomnia are the main symptoms of patients with MDD. The behavioral and educational strategies, cognitive reconstructing therapy and circadian rhythm support can be applied to improve sleep quality. 281 , 289 Dietary adjustments are also suggested to have the potential effects to prevent the occurrence or re-occurrence of MDD, the improvement mechanism of diet may involve in the regulated immune-inflammatory responses, the improved gut-microbe-brain axis and synaptic plasticity. 295 , 299 , 304 In addition, xxercise is an effective way to improve neuroplasticity, to maintain neuroendocrine homeostasis, and to regulate neuroinflammation, in order to effectively prevent the occurrence or re-occurrence of MDD. 280 , 309 Importantly, getting social support from family members, friends, coworkers and community members can be helpful for the MDD patients’ recovery, these social interventions can let patients get emotional support and improve their self-awareness. 328 , 340 Adobe Illustrator was used to generate this figure
Therapeutic drugs and strategies
This section summarizes new advances in research on the pharmacological mechanisms of common antidepressants and novel therapeutic strategies. Moreover, as laboratory animal models of MDD and other mental diseases are lacking, hindering the development of strategies for evaluating pharmacological effects and studying pathological mechanisms, we also discuss recent research on animal models.
The molecular mechanism of antidepressants
Tricyclic antidepressants.
In the late 1950s, the first TCAs were approved and used for the treatment of depression. 342 TCAs have a common three-ring chemical structure, and the main TCAs are imipramine, amitriptyline, clomipramine, desipramine and doxepin. The pharmacological mechanism of TCAs mainly involves its interaction with neurotransmitters in the brain, resulting in changes in neurotransmitter levels and an antidepressant effect. First, TCAs can inhibit the reuptake of neurotransmitters, leading to antidepressant effects. For example, they can influence the levels of 5-HT, NE, and to a lesser degree, DA, causing an increase in neurotransmitter concentrations in the synaptic gap and increasing neurotransmitter signaling to exert pharmacological effects. 343 However, different TCAs inhibit 5-HT and NE reuptake to varying degrees. For instance, amitriptyline, imipramine, and desipramine strongly inhibit 5-HT reuptake, 344 clomipramine specifically inhibits NE reuptake, and nortriptyline can inhibit both NE and 5-HT reuptake while also exerting central anticholinergic effects. 345 , 346 , 347 Additionally, TCAs can antagonize 5-HT 2A and 5-HT 2C , thereby increasing the release of NE and DA in cortical areas. 348 , 349 , 350 TCAs can bind to histamine receptors, especially H1 receptors, as well. 351 By blocking H1 receptors, they can induce sedation and drowsiness, which may benefit depressed patients with sleep disorders. 352 Furthermore, TCAs can also block muscarinic acetylcholine receptors, exerting anticholinergic effects and resulting in side effects such as dry mouth and constipation. 353
In addition to the above-known pharmacological mechanisms, some recent studies have reported that amitriptyline can induce the activation of fibroblast growth factor receptor (FGFR), leading to the production of GDNF. 354 In addition, amitriptyline can increase the expression of Cx43 to promote gap junction intercellular communication (GJIC) between astrocytes, thereby relieving depressive symptoms. 355 This suggests that TCAs may also ameliorate severe depression through additional mechanisms involving astrocytes that are independent of the monoamine system to some extent. Further exploration is needed to fully understand the specific mechanism. Another study demonstrated that FKBP51, a crucial modulator of the glucocorticoid receptor (GR) pathway, can bind to clomipramine and impede its interaction with PIAS4. Inhibition of this interaction subsequently hinders sumoylation; this alteration represents a newly discovered mechanism by which the antidepressant drug exerts its effect. 356
Selective serotonin reuptake inhibitor
According to a study, most severe depression patients are still advised to consider SSRIs as the initial choice for treatment. 350 The main representative SSRIs drugs include fluoxetine, sertraline, paroxetine, and escitalopram. The mechanisms of action of SSRIs are commonly known as follows: first, SSRIs can selectively inhibit SERT, inhibiting the reuptake of 5-HT in the synaptic cleft and thereby exerting pharmacological effects. 357 Second, SSRIs can impact the 5-HT signaling pathway, activating 5-HT 1A. 358 , 359 In addition, studies have shown that antagonism of 5-HT 2A/2C receptors can enhance the effects of SSRIs such as fluoxetine. 360 , 361 Third, long-term use of SSRIs can increase 5-HT transmission in the LC, 362 thereby increasing the release of GABA to exert inhibitory effects on NA neurons. 363 Fourth, long-term use of SSRIs is associated with neuroplasticity and neurogenesis in certain brain regions. 364 SSRIs have been found to increase the expression of BDNF, a protein crucial for neuronal growth and survival, by acting on TrkB, 365 which may contribute to the long-term therapeutic effects of SSRIs. Thus, our previous reports and others researches all suggested that astrocytic 5-HT 2B receptors may be the potential pharmacological target of SSIRs. 59 , 60 , 366 , 367 , 368
According to previous studies by our group, in the absence of SERT, SSRIs such as fluoxetine can act as direct agonists of astrocytic 5-HT 2B receptors to exert antidepressant-like effects. 60 , 64 , 179 , 366 , 369 In astroglia isolated from mice exposed to CUMS, fluoxetine activates the 5-HT 2B receptor, promoting ERK 1/2 phosphorylation. This increases downstream c-Fos expression, which in turn boosts BDNF synthesis. 147 Furthermore, administration of fluoxetine effectively inhibits SD-induced stimulation of the NLRP3 inflammasome by the AKT/STAT3 and ERK/STAT3 pathways in vivo, and SD dramatically triggers depressive-like behaviors by stimulating astrocytic P2X 7 Rs. 41 , 155 As previously mentioned, leptin may increase the expression of the 5-HT 2B receptor in astrocytes via the LepR/JAK2/STAT3 pathway, and fluoxetine may be more effective in increasing BDNF levels and alleviating depressive-like behaviors due to the leptin-mediated increase in 5-HT 2B receptor expression. 130 Both in vivo and in vitro, fluoxetine’s inhibitory actions on A1 reactive astrocytes depend on astrocytic 5-HT 2B R. 55 Recently, fluoxetine was shown to act as a 5-HT 2B agonist, and this finding is also supported by research by other groups. Fluoxetine has been reported to suppress the activation of A1 reactive astrocytes and decrease unusual behaviors in CMS-exposed mice. In vitro, Gq protein and b-arrestin1 are not necessary for fluoxetine’s effects on A1 astrocyte activation, and downstream signaling through astrocytic 5-HT 2B R is responsible for fluoxetine’s inhibitory effects on A1 astrocyte activation in primary culture. 55
Serotonin/norepinephrine reuptake inhibitors
SNRIs are often recommended as the initial choice for the treatment of MDD. Representative SNRIs include milnacipran, DXT, DVS, and venlafaxine. The molecular mechanisms of SNRIs can be summarized as follows: First, SNRIs inhibit the norepinephrine transporter (NET), which prevents the reuptake of NE into presynaptic neurons, leading to an increased concentration of NE in the synaptic cleft. 370 Second, similar to SSRIs, SNRIs also inhibit SERT, resulting in an increased concentration of 5-HT in the synaptic cleft. 371 For example, paroxetine and venlafaxine can inhibit SERT and, to a lesser extent, NET. 372 Third, SNRIs inhibit the reuptake of both NE and 5-HT; thus, they have a dual mechanism of action. This dual inhibitory effect is believed to contribute to the broader therapeutic effects of SNRIs compared to SSRIs. 373 Chronic treatment with fluoxetine has been shown to increase the expression of Cx43 in the rat PFC, which further prevents the dysfunction of astrocytic gap junctions induced by CUS and reverses the depressive-like behaviors caused by gap junction blockade. 71
In a randomized controlled trial, MRI scan were taken after treatment with duloxetine and desvenlafaxine, and the results showed that the thalamo-cortico-periaqueductal network, which is associated with the experience of pain, may be an important target of action of antidepressant drugs. 374

New potential pharmacological targets
The abovementioned antidepressants have been utilized as clinical therapies for MDD, but it is difficult to elucidate the exact pharmacological mechanisms of every medicine due to delayed clinical efficacy, poor treatment response to some patients, and difficulty in effectively controlling the incidence of suicide. Recently, several pharmacological agents have been discovered as potential antidepressants.
Ketamine, a noncompetitive antagonist of the NMDAR, has been shown to induce rapid and significant antidepressant effects within a few hours. 375 Due to the rapid antidepressant effects of ketamine, unlike the delayed effects of traditional antidepressant drugs, 376 research on this drug has continued and has revealed its mechanisms of action and potential drug targets. Ketamine can increase the level of BDNF in the prefrontal cortex, especially in the hippocampus, to exert antidepressant-like effects. 377 Studies have suggested that ketamine can increase the synthesis of synaptic proteins through BDNF signaling dependent on the activate protein kinase B (Akt) and mammalian target of rapamycin complex 1 (mTORC1) signaling cascades. 378 , 379 Ketamine may induce the activation of mTOR by the upstream kinase Akt, regulate the phosphorylation of GSK-3β, and exert antidepressant effects. 380 Ketamine can block NMDARs in postsynaptic principal neurons in the PFC and hippocampus, increase synaptic function through homeostatic mechanisms, and reverse synaptic defects caused by chronic stress. 381 , 382 Furthermore, by inhibiting NMDARs, ketamine can reduce the excitation of specific cortical GABAergic interneurons, resulting in a temporary increase in glutamate release that stimulates postsynaptic AMPA glutamate receptors. This, in turn, leads to the release of BDNF, activation of the TrkB receptor, and subsequent activation of the Akt/mTORC1 signaling pathway. These molecular events ultimately contribute to an increase in the number and functionality of synapses, leading to amelioration of depressive symptoms. 383
Similar as ketamine, some other psychedelics can also produce fast and persistent antidepressant effects. 384 Psilocybin, a classical psychedelic, can play its antidepressant roles by activating 5-HT 2A receptors (5-HT 2A R). 385 Thus, to block the 5-HT 2A R can not produce the antidepressant effects of psilocybin, only induce the hallucinogenic-like behaviors in mice. 386 This proposes 5-HT 2A R may not be the real pharmacological target for its antidepressant effects. Another study reports that the combination of lysergic acid diethylamide (LSD) and psilocybin may exert long-term antidepressant effects by promoting neural plasticity, which dose not involve in the hallucinogenic effects. 384 Additionally, to target 5-HT 2A R, the combination of LSD and psilocybin can lead to biased activation of the mediated signaling pathway and produce antidepressant effects without the side effects of hallucinations. 387 Thus, the administration of psilocybin can rapidly and persistently induce neuronal dendritic remodeling in the medial frontal cortex of mice, and the psilocybin-induced newly formed dendritic spines can successfully transform functional synapses, suggesting that synaptic rewiring may also be one pharmacological mechanism of the rapid antidepressant effects of psilocybin. 388 To further dissociate the hallucinogens effects from the psychedelics can be beneficial to develop more specific antidepressants with better therapeutic capacities.
Additionally, some novel potential therapeutic targets for MDD have also been reported, such as TGF-β1 389 and growth-associated protein 43 (GAP-43). 390 Multiple studies have shown that antidepressants may cause changes in TGF-β1 expression. Fluoxetine, paroxetine, venlafaxine, and sertraline have been shown to have the potential to increase the levels of TGF-β1, which may contribute to their antidepressant effects. 391 , 392 Venlafaxine has also been reported to exert neuroprotection by increasing the production of FGF-2 and TGF-β1 in astrocytes following stroke. 72 Then, chronic administration of desipramine has been shown to upregulate the expression of GAP-43 in the hippocampus of rats, potentially influencing neuronal plasticity in the CNS. 390 GAP-43 has been suggested as a relevant target for the pharmacological effects of antidepressants. 393 , 394
The most of above antidepressants have been widely used for the MDD patients according to the respective potential pharmacological actions (Fig. 6 ). Thus, the exactly neuromolecular mechanisms require deep studied and the new potential therapeutic targets and strategies still need further exploration.
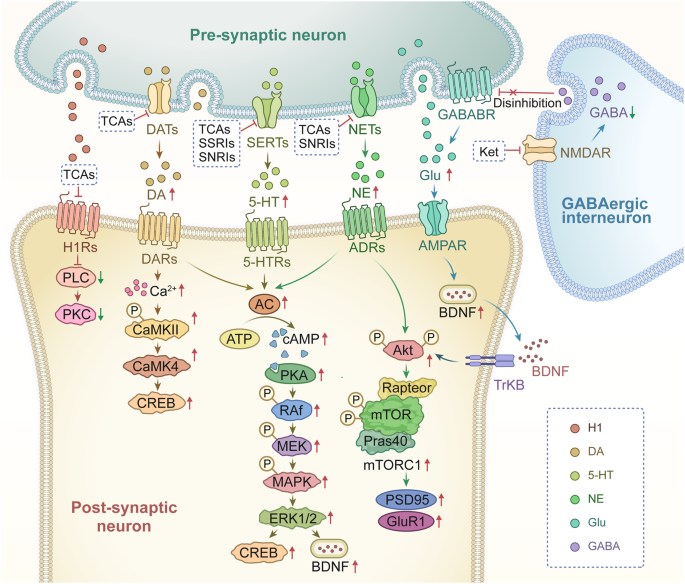
The molecular mechanisms of tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin/norepinephrine reuptake inhibitors (SNRIs) and ketamine. TCAs can inhibit the protein kinase C (PKC) pathway by blocking the H1 receptors (H1Rs), 351 , 352 TCAs decreases the reuptake of dopamine (DA) by inhibiting dopamine transporters (DATs) in the presynaptic membrane, and increases the DA concentration in the synaptic gap, increase the effect of DA on dopamine receptors (DARs) of postsynaptic membrane. 343 The activated DARs increase Ca 2+ dependent CaMKII and CaMK4, as well as, the secretion of CREB. 498 , 499 In another way, the stimulated DARs by DA can also activate the cAMP-PKA pathway, which in turn activates the levels of CREB and BDNF by stimulating MAPK/ERK 1/2 pathway. 500 TCAs, SSRIs, and SNRIs can all inhibit the reuptake of 5-HT by SERTs, specially SSRIs have the selective inhibition on SERTs, which increase the concentration of 5-HT in the synaptic gap and play antidepressive roles by effecting on 5-HTRs in postsynaptic membrane, 343 , 344 which also activate the cAMP-PKA pathway. 49 , 501 Moreover, TCAs and SNRIs can also inhibit the reuptake of NE by NETs, which also increase the concentration of NE in the synaptic gap, and in turn activate the effect of NE on adrenoceptors (ADRs) and activate the cAMP-PKA pathway in postsynaptic membrane. 502 Besides of the AC/cAMP/PKA pathway, the effect of NE on ADRs can also activate protein kinase B (Akt) phosphorylation and mammalian target of rapamycin complex 1 (mTORC1) by stimulating TrkB, in order to promote the secretion of postsynaptic density 95 (PSD95) and glutamate receptor 1 (GluR1). 502 Ketamine works as the antagonist of NMDAR on GABAergic interneurons, it suppresses the excitation of subsets of GABAergic interneurons, which reduces the gamma aminobutyric acid (GABA) effects on gamma aminobutyric acid type B receptor (GABABR), and relieves the inhibition of GABAergic interneurons on the release of glutamate, the latter further stimulates AMPAR on postsynaptic membrane and increases the level of BDNF, even the release of BDNF stimulates the above TrkB/AKT/mTORC1 pathway. 503 , 504 Adobe Illustrator was used to generate this figure
Novel therapeutic strategies
New animal models.
Establishing animal models with pathological features representative of those seen in humans is key for advancing MDD research. Currently, the widely utilized animal models of MDD include CUMS, behavioral despair (BD), learned helplessness (LH), and CSDS, drug withdrawal, and transgenic animal models. 395 The CUMS model, one of the most commonly used animal models for MDD, 64 , 172 exhibits depressive-like behaviors. 396 , 397 According to a meta-analysis of 408 papers involving stress protocols, the most commonly used stressors for CUMS models are food and water deprivation, light cycle modification, wet bedding, cage tilting, social stress, and forced swimming. 398 Recently, we constructed an improved depression model named the chronic unpredictable mild restraint (CUMR) model by using environmental interference. 62 The stressors used to construct this CUMR mouse model included activity restriction, damp bedding, cage shaking, tail suspension, forced swimming, and 45° cage tilting. These stressors all restrict the activity of the mice; moreover, stressors that disturb physiological rhythms, chronic unpredictable rhythm disturbance (CURD), can cause manic-like behaviors in mice (Fig. 7 ). The disease-related pathological changes and serum indicators in the CUMR and CURD models are highly similar to those in patients in the clinic, and therapeutic medicines can effectively improve brain function and behavior in these models. 62
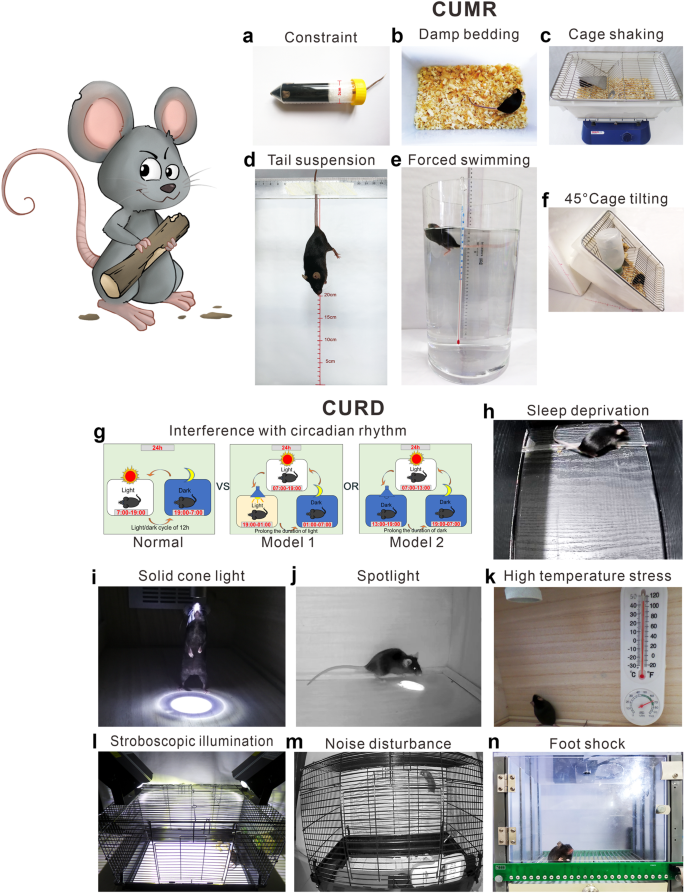
The protocol and stressors used for CURD and CUMR. In order to establish the CUMR model, a combination of various stressors includes interference of constraint ( a ), damp bedding ( b ), cage shaking ( c ), tail suspension ( d ), forced swimming ( e ), and cage tilting ( f ). Among these six stressors, two were randomly selected and administered daily for a duration of 3 weeks. On the other hand, to establish the CUMR model, a set of behavioral constraints includes circadian rhythm ( g ), sleep deprivation ( h ), interference of cone light ( i ), interference of followed spotlight ( j ), high temperature stress ( k ), stroboscopic illumination ( l ), noise disturbance ( m ), and foot shock ( n ). Similarly, two out of these eight constraints were randomly chosen and applied daily for a period of 3 weeks 62
Phototherapy
Phototherapy plays a significant role in regulating emotional behavior 399 and can have strong and rapid effects on mood and alertness. 400 , 401 , 402 There is increasing evidence for the therapeutic efficacy of phototherapy for MDD. 403 , 404 The combination of phototherapy and antidepressants has better effects than antidepressants alone. 402 , 405 Phototherapy utilizes bright light with a specific wavelength to stimulate the retina and affect the production of 5-HT and hormones in the brain. 406 Furthermore, phototherapy can alleviate depressive-like behavior by targeting the retinal-thalamic ventral lateral geniculate nucleus/intergeniculate leaflet-lateral habenula (retinal-vlGN/IGL-LHb) circuit; this mechanism may explain how phototherapy alleviates MDD. 407
Repetitive transcranial magnetic stimulation
Repetitive transcranial magnetic stimulation (rTMS) is an effective method used in clinical practice for treating patients with MDD. 408 Multiple evaluations and analyses have shown that rTMS can effectively treat MDD in patients from different age groups, including children and adolescents, 409 , 410 adults, 411 , 412 and elderly patients. 413 , 414 It is suggested that early use of rTMS in the treatment of depression in elderly patients may yield better results. 415 Furthermore, research has indicated that rTMS can effectively treat perinatal depression. 416 Increasing evidence suggests that rTMS of the anterior stimulation site of the left dlPFC can yield optimal treatment outcomes. 417 , 418 , 419 A randomized controlled trial demonstrated that the efficacy of rTMS in treating depression is linked to precise targeting of the dlPFC, the activity of which exhibits a negative correlation with subgenual cingulate cortex activity. 420 Identifying the optimal site for stimulation may further enhance the ability of rTMS to treat depression. 421 Recently, a retrospective study was conducted, which included 29 systematic evaluations and reanalyzed 15 meta-analyses to assess the effectiveness and safety of transcranial magnetic stimulation (TMS) for treating MDD in adults. 422 The results of the study indicated significant variations in the efficacy of TMS for MDD across different settings and revealed poor tolerability in certain populations, the further research is necessary to identify specific beneficiary populations for TMS in treating MDD and to personalize treatment based on comprehensive and detailed information. 422
Psychological intervention
MDD is characterized by a gradual onset and a high risk of relapse. 421 The American Medical Association recommends psychological interventions for individuals who are at a high risk of MDD. Some of the interventions commonly used for depression treatment include acceptance and commitment therapy, cognitive therapy, cognitive behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapies. 423 Specifically, the combination of psychological interventions and antidepressants effectively decreases the risk of relapse in cases of MDD. 424 , 425 , 426
Acupuncture
Acupuncture, which mainly includes traditional body needling, moxibustion, EA, and laser acupuncture, is a traditional Chinese treatment modality used to treat various diseases. 427 Compared with pharmacological therapies, acupuncture is more cost-effective and has fewer side effects. 428 EA stimulation can effectively treat MDD; 429 , 430 , 431 however, the specific mechanism by which acupuncture treats depression remains unclear. In previous research, EA at the ST36 acupoint was shown to prevent shrinkage of the prefrontal cortical astrocytes and alleviate depressive-like behavior in mice exposed to CUMS. 432 The results of an 8-week clinical study involving 46 female patients with severe depression suggested that acupuncture may achieve therapeutic effects by modulating the corticostriatal reward/motivation circuit in patients with severe depression. 433 Additionally, studies indicate that EA may have the potential to promote neuronal regeneration and exert antidepressant effects by elevating the phosphorylation of cyclic adenosine monophosphate response element binding protein and the levels of BDNF. 434 Acupuncture at the GV20 and GV24 acupoints may alleviate depression symptoms by regulating the calmodulin-dependent protein kinase (CaMK) signaling pathway. 435 The antidepressant effect of EA may also be associated with increased synaptic transmission in the ventromedial prefrontal cortex (vmPFC). 436 A recent meta-analysis of 43 randomized controlled trials involving adult subjects with acupuncture for MDD demonstrated that acupuncture, either alone or in combination with antidepressants, significantly reduced the hamilton depression scal scores and had fewer adverse effects compared to antidepressants, however, further rigorous experiments are still required to determine the optimal frequency of acupuncture for MDD in order to achieve better efficacy. 437
In conclusion, the common antidepressants can improve some depressive symptoms in some patients with depression, but are always associated with the risk of adverse effects or recurrence. Although some new developed treatment methods can improve depression symptoms in a certain program, the compatibility between potential treatment mechanisms and pathological mechanisms still needs further research. In particular, the therapeutic principle of acupuncture still needs to be explored in depth, and the accompanied therapeutic mechanism and application potential of traditional Chinese medicine in depression deserve to be explored in depth.
Clinical research progress
In summary, the pathological features of MDD and pharmacological mechanism of antidepressants have been widely studied. Furthermore, there have been many clinical studies on MDD, and studies of human postmortem tissues and clinical medical images, multomics studies, and preclinical/clinical trials of new therapeutic drugs have improved our understanding of the disease mechanism.
Transcriptional studies of human postmortem tissue
A recent meta-analysis of eight transcriptome datasets identified 566 disease-related genes that are consistently up- or downregulated in patients with MDD. The brain regions in which these genes are expressed include the amygdala, subgenual anterior cingulate, and dorsolateral prefrontal cortex, and the associated molecular pathways include reduced neurotrophic support, neural signaling, and GABA function. 438 Through the discovery of nonoverlapping proteins that bind to calcium parvalbumin, calretinin, and the neural peptide somatostatin, subgroups of GABA interneurons that govern main pyramidal neurons differently were identified. 439 Decreased cortical levels of GABA and specific populations of GABA neurons have been reported in investigations of postmortem MDD patient tissues, 440 and the SST mRNA level is specifically decreased in patients with MDD. 213
The DR nucleus is the largest and most significant conduit of forebrain serotonergic input. 441 In postmortem samples of the human brain, several transcriptional regulators are dysregulated within the DR, including transcription-related elements (such as EGR1, TOB1, and CSDA), which bind to genes to stimulate their expression directly or in response to environmental cues, and NRs (NR4A2, NR4A3, THRA, and THRB), which are activated by ligands and regulate translation by targeting genes. 442 In addition, transporters for GRs generally regulate the activity of the HPA axis by negative feedback. 443 According to studies of postmortem brain tissues, hyperactivity of the HPA axis in MDD patients could be caused by methylation-mediated changes in GR transcription. 444 The expression of nerve growth factor-inducible protein A (NGFI-A), an enzyme that bindss exon 1 F of GR, is reduced in the hippocampus of patients with MDD, which may contribute to low methylation levels in the brain. 444 Moreover, in postmortem MDD patients, total GR levels are unchanged, while level of GRα in the amygdala and cingulate gyrus is decreased.
Sex-related molecular markers of MDD
Women are more likely than males to experience recurring MDD 445 and are twice as likely to experience MDD throughout their lifetimes. 446 Compared with male patients, female patients with MDD have symptoms that manifest sooner in the disease course, last longer, and are more severe; in addition, they experience hunger changes, weight fluctuations, and sleep difficulties more frequently. 447 , 448
In postmortem samples of patients who committed suicide due to MDD, the expression of DNA methyltransferases (DNMTs) in the frontopolar cortex was found to be more significantly increased in women than in men; elevated methylation is associated with decreased levels of the GABA A receptor alpha-1 subunit in men, which supports sex-related epigenetic alterations in transcription. 449 A gene array meta-analysis also revealed sex differences in MDD, with depressed females being more likely than depressed men to have lower production of somatostatin, a GABA neuron biomarker in corticolimbic brain regions according to postmortem analysis. 450 X-linked chromosomal polymorphisms affect the expression of the GABA-synthesizing enzyme and somatostatin. 450 Analyses of postmortem brain tissues showed an increase in the transcription of numerous glutamate-related genes in the prefrontal cortex in depressed women but not in depressed men; depressed women exhibited more alterations in glutamate receptor expression, while depressed men showed only GRM5 downregulation. 451
In postmortem brain specimens, there were no transcription differences between MDD men and controls, and the levels of 5-HT 1D receptors and the transcription factors NUDR and REST, which regulate 5-HT activity, in 5-HT-containing neurons in the ventral raphe nuclei were found to be higher in MDD females. 452 5-HT receptors and regulators were shown to exhibit sex-specific alterations in expression at the protein level, and postmortem investigations have largely focused on female subjects. The protein levels of 5-HT 1A R and NUDR, which regulate 5-HT signaling, in the prefrontal cortex were found to be lower in MDD women than in control subjects; however, this difference was not observed in MDD males compared with controls. 453 The NA/NE system, especially in the LC, is another monoaminergic system that exhibits sex-related variations and influences MDD risk. In fact, some researchers have found that the levels of microRNAs (miRNAs), short RNA molecules that control the expression of genes and play roles in psychological disorders, 454 are higher in the LC of suicidal female subjects than in the LC of suicidal male subjects. MiR-1179 is associated with GRIA3 and MAOA, which are involved in neuropsychiatric diseases. 455
OS is commonly linked to the onset of MDD. A study found that whereas cysteine and 1-methylinosine levels were much higher in males with MDD, they were significantly lower in females with MDD. 456 These metabolites are related to OS. Furthermore, several studies found a significant link between MDD and lipid metabolism; 457 for example, as 1-Oalkyl-2-acyl-PEs levels are decreased in MDD, showing a negative correlation with the extent of depression, lysophospholipid (LPC) and phospholipid (PC) levels are increased in MDD, exhibiting a substantial positive correlation with depression severity. 458 Similarly, a study found that men and women had different lipid concentrations. 456 These clinical data suggest that sex differences in MDD may result from differences in OS and lipid metabolism, but further research is required to make this connection.
Multiomics studies
Transcriptome studies, which explore relationships among the expression of genes and diseases, are regarded as an essential for investigating disease-causing mutations in genes, the mechanisms of disease development and progression, and disease-related target genes. 459 Dorsolateral prefrontal cortex tissues have been employed to identify genes and miRNAs that show changes in expression and biological processes that are altered in patients with MDD. 460 Serpin Family H Member 1 (SERPINH1), IL-8, humanin like-8 (MTRNRL8), and chemokine ligand 4 (CCL4) are among the genes whose expression is altered in MDD. 460 , 461 According to Gene Ontology (GO) enrichment analysis, MDD is related to decreased expression of genes related to oligodendrocyte development, glutamatergic neurotransmission modulation, and oxytocin receptor expression. These findings confirm that impairment of the blood-brain barrier and microglial, endothelial cell, ATPase, and astrocyte function exacerbate MDD; the involvement of these cells, molecules, and structures in MDD should be further investigated. 460
The field of study known as genomics focuses on the transcription of genes, the precise interactions among genes, and the control of gene activity. MDD has been linked to numerous biological processes, including energy metabolism. When the transcription of genes involved in glycolysis and glycogen synthesis was examined in the hippocampus of depressed rats, it was found that the mRNA expression of Slc2a3, which codes for GLUT3, is considerably increased. 462 Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and lactate dehydrogenase B (LDHB) mRNA levels were found to be substantially decreased in MDD. 462 The transcription of genes in the brain tissues of IL18 - / - mice was examined with the use of genome-wide microarrays, and the results revealed that urocortin 3 (Ucn3) expression was increased. 463 Ucn3 controls how the body processes glucose; 464 therefore, a change in Ucn3 expression will result in energy imbalance. Gene comethylation analysis was performed in the brains of individuals with MDD. The findings revealed that the methylation of genes associated with mitochondria was dramatically decreased, indicating impaired mitochondrial function. 465
Metabolomics has recently emerged as a useful technique for identifying markers and pathways associated with a wide range of diseases. 466 It is often used to analyze the mechanisms underlying disease occurrence and progression and the effects of small-molecule compounds. In one study, targeted metabolomic analysis of the CSF of 14 MDD patients who were not taking medication, 14 MDD patients in remission, and 18 healthy controls was performed. 467 An analysis of the tryptophan, tyrosine, purine pathways, and associated pathways revealed that in patients in remission, methionine levels were higher, while tryptophan and tyrosine levels were lower. The same group of patients also showed changes in the methionine-to-glutathione ratio, indicating alterations in OS and methylation. The levels of these same metabolites were altered in MDD patients who were not taking medication, although not to a significant degree. 467
Clinical medical imaging studies
MRI has been widely employed in research in recent years to pinpoint patterns of brain alterations linked to MDD. Many studies have demonstrated that structural and fMRI has outstanding potential as trustworthy imaging modalities for monitoring MDD treatment responses. A study indicated that MDD patients had large volume decreases in various frontal areas, such as the anterior cingulate cortex and OFC, which were linked to problems with stress management and emotional processing. 468 People with MDD also exhibited structural changes in their parietal lobes. 469 Alterations in the total gray matter volume and an increase in cortical thickness are the two findings that are most consistent. 470
The functional changes in the frontal lobe in MDD are hotly contested. A study discovered lower precuneus, supragenual anterior cingulate cortex, dorsomedial PFC, and dorsomedial thalamus lower activity when processing pleasant stimuli in MDD patients. 471 Another study found that during the processing of favorable self-indulgent information, individuals with MDD displayed higher activity in the medial PFC and anterior cingulate cortex. 472 The right hippocampus, parahippocampal gyrus, left amygdala, and the whole caudate nucleus all had functional changes in activity in MDD patients compared to healthy controls, indicating that the temporal lobe might be involved in the pathogenesis of MDD. 473
Although it is not feasible to evaluate synapse density directly in people in vivo, positron emission tomography (PET) can be utilized to gather useful information. It is thought that impairments of functional connections and synaptic atrophy are two factors that contribute to the symptoms of MDD. An indirect method of estimating synaptic density is to count the number of nerve terminals using synaptic vesicle glycoprotein 2 A (SV2A). The researchers examined synaptic density in MDD patients who were not taking any medication using positron emission PET with the SV2A radioligand [ 11 C] UCB-J. 474 The results revealed that reductions in the synapse density in areas connected with various processes, such as emotion control and thought (the dorsolateral prefrontal cortex, anterior cingulate cortex, and hippocampus), are related to to the severity of depressive disorders. Additionally, it was shown that compared with healthy subjects, subjects with MDD had reduced dlPFC resting-state connectivity throughout the brain. It was found that the dlPFC-posterior cingulate cortex connection was inversely negatively linked to the severity of depression symptoms and connected with synapse activity in the dlPFC, indicating that synaptic loss may impair antagonistism within the centers of both networks, which are typically at odds. 474
Preclinical and clinical trials of new therapeutic drugs
Esmethadone is a new, noncompetitive NMDAR antagonist 475 that exhibits fast antidepressant-like action by improving performance of rats in the forced swim test. 476 Esmethadone can also alleviate neural dysfunction linked to symptoms of depression by boosting the synapse and spine density and restoring spinogenesis, in addition to correcting depressive-like behaviors in animal models of depression. 378 , 477 Esmethadone was found to reduce cognitive symptoms in individuals with MDD in a stage II clinical study 478 and to increase the levels of circulating BDNF in normal individuals in a stage I clinical investigation. 479 In a phase II study involving patients who had received insufficient benefit from conventional antidepressants, esmethadone demonstrated immediate, strong, and long-lasting antidepressant benefits. 478
Ketamine is the most well-known rapid-acting antidepressant and an NMDAR antagonist. 383 GluN1, GluN2, and GluN3 are NMDAR subunits. 480 Ketamine exerts a quick and effective antidepressant effect by binding to the asparagine 616 residue of GluN1 and the leucine 642 residue of GluN2A. 192 In a clinical experiment, the effect of supplementary injection of subanesthetic doses of ketamine on thoughts of suicide in MDD patients was evaluated, and the results showed that the reduction in thoughts of suicide among MDD patients receiving ketamine was mostly sustained. 481 In several studies, a single dose of ketamine reduced immobility in the forced swim test immediately after injection and had effects similar to those of an antidepressant. 482 , 483
The S-enantiomer of ketamine, esketamine, has been approved by the U.S. Food and Drug Administration (FDA) for depression treatment. 383 Moreover, formulations of ketamine are also being developed, and intranasal esketamine spray has shown high efficacy in treating MDD. 484 Additionally, hydroxynorketamine (HNK), a metabolite of ketamine, can exert its anti-depressive effects by an NMDAR-independent mechanism. 377 One of these mechanisms involves increasing BDNF levels; an increasing number of studies have shown that BDNF signaling is an important target of antidepressants. 377 Thus, ketamine can also exert anti-inflammatory effects, a large amount of evidence suggests a tight relationship between neuroinflammation and the pathogenesis of MDD. 485 , 486 , 487 A summary of clinical trials related to new therapeutic drugs for MDD is shown in Table 1 .
The development of the present therapeutic medicines in clinic mainly targets the discovered pharmacological targets, mainly focusing on the key receptors or enzymes. However, at the organelle level of neural cells, the disturbed energy metabolism of mitochondria and the related RNA drugs, as well as the dysfunctions of lipid and glucose metabolism in psychopathological condition, still need deep exploration. Totally, the research on the mechanism of therapeutic drugs always requires the development of pathological mechanisms as support.
Conclusions and future perspectives
MDD is a heterogeneous disease, its pathological and pharmacological mechanisms are still unclear, and diagnostic and therapeutic methods for MDD are limited. SSRIs and SNRIs are the first-line treatments for MDD in the clinic; however, a sizable portion of MDD patients do not respond well to the currently available antidepressants. According to research on real-world sequential therapies, even after numerous treatment attempts, almost 30% of MDD patients do not experience remission. This suggests that the existing theories and hypotheses cannot completely explain the pathogenesis of MDD and that more research on the pharmacological mechanisms of currently available antidepressants is still needed. We mainly discussed the potential etiology and pathogenesis of MDD from the perspective of widely accepted theories, including the neurotransmitter and receptor hypothesis, HPA axis hypothesis, cytokine hypothesis, neuroplasticity hypothesis and systemic influence hypothesis. A more comprehensive understanding of the pathophysiological mechanisms of MDD might significantly improve our capacity to develop preventive and more effective therapeutic methods that can help reduce the burden of and pain caused by major depression. Knowledge of the cellular processes that drive these alterations and the symptoms they cause may offer crucial will provide insight for new treatments.
MDD is connected with several cellular and structural modifications in the nervous system. Nonetheless, in the majority of these alterations cannot be consistently observed in vivo. Therefore, several issues need to be considered in future research: (i) Studies of animal models have made important contributions to our understanding of the pathophysiology of major depression, and more representative animal models of MDD should be developed. (ii) Because of our incomplete understanding of the disease and the disease’s intrinsic intricacy, there is an urgent need to develop updated imaging technologies and imaging software to allow advances in our understanding of the disease. (iii) The therapeutic shortcomings of traditional antidepressants have prompted the need for further drug discovery and development. (iv) MDD is strongly associated with many systems, and it will be important to further elucidate the mechanisms associated with MDD and other pathological conditions.
Disease, G. B. D., Injury, I. & Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1789–1858 (2018).
Article Google Scholar
Nagy, C. et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci. 23 , 771–781 (2020).
Article CAS PubMed Google Scholar
Malhi, G. S. & Mann, J. J. Depression Lancet 392 , 2299–2312 (2018).
Article PubMed Google Scholar
Fellinger, M. et al. Seasonality in major depressive disorder: effect of sex and age. J. Affect Disord. 296 , 111–116 (2022).
Liu, C. H. et al. Role of inflammation in depression relapse. J. Neuroinflammation 16 , 90 (2019).
Article PubMed PubMed Central Google Scholar
Burcusa, S. L. & Iacono, W. G. Risk for recurrence in depression. Clin. Psychol. Rev. 27 , 959–985 (2007).
Monroe, S. M. & Harkness, K. L. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol. Rev. 112 , 417–445 (2005).
Albert, K. M. & Newhouse, P. A. Estrogen, stress, and depression: cognitive and biological interactions. Annu. Rev. Clin. Psychol. 15 , 399–423 (2019).
Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R. & Walters, E. E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62 , 617–627 (2005).
Monteleone, P. & Maj, M. The circadian basis of mood disorders: recent developments and treatment implications. Eur. Neuropsychopharmacol. 18 , 701–711 (2008).
Rice, F. et al. Adolescent and adult differences in major depression symptom profiles. J. Affect Disord. 243 , 175–181 (2019).
Hasin, D. S. et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75 , 336–346 (2018).
Howard, D. M. et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 9 , 1470 (2018).
Kovacs, M. & Lopez-Duran, N. Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: implications for prevention. J. Child Psychol. Psychiatry 51 , 472–496 (2010).
Stetler, C. & Miller, G. E. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 73 , 114–126 (2011).
Milaneschi, Y., Lamers, F., Berk, M. & Penninx, B. Depression heterogeneity and its biological underpinnings: toward immunometabolic depression. Biol. Psychiatry 88 , 369–380 (2020).
Zhou, X. et al. Astrocyte, a promising target for mood disorder interventions. Front. Mol. Neurosci. 12 , 136 (2019).
Article CAS PubMed PubMed Central Google Scholar
Monroe, S. M. & Harkness, K. L. Major depression and its recurrences: life course matters. Annu. Rev. Clin. Psychol. 18 , 329–357 (2022).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50 , 668–681 (2018).
Gittins, R. A. & Harrison, P. J. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J. Affect. Disord. 133 , 328–332 (2011).
Miguel-Hidalgo, J. J. et al. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J. Affect. Disord. 127 , 230–240 (2010).
Sequeira, A. et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE 4 , e6585 (2009).
Rajkowska, G. & Stockmeier, C. A. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 14 , 1225–1236 (2013).
Tsuang, M. T., Taylor, L. & Faraone, S. V. An overview of the genetics of psychotic mood disorders. J. Psychiatr. Res 38 , 3–15 (2004).
Lohoff, F. W. Overview of the genetics of major depressive disorder. Curr. Psychiatry Rep. 12 , 539–546 (2010).
Kendall, K. M. et al. The genetic basis of major depression. Psychol. Med. 51 , 2217–2230 (2021).
Power, R. A. et al. Genome-wide association for major depression through age at onset stratification: Major Depressive Disorder Working Group of the psychiatric genomics consortium. Biol. Psychiatry 81 , 325–335 (2017).
Huang, C. et al. Proteomic analysis of olfactory bulb suggests CACNA1E as a promoter of CREB signaling in microbiota-induced depression. J. Proteom. 194 , 132–147 (2019).
Article CAS Google Scholar
Flint, J. The genetic basis of major depressive disorder. Mol. Psychiatry 28 , 2254–2265 (2023).
Tripp, A., Kota, R. S., Lewis, D. A. & Sibille, E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 42 , 116–124 (2011).
Seney, M. L., Tripp, A., McCune, S., Lewis, D. A. & Sibille, E. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 73 , 213–219 (2015).
Hicks, E. M. et al. Integrating genetics and transcriptomics to study major depressive disorder: a conceptual framework, bioinformatic approaches, and recent findings. Transl. Psychiatry 13 , 129 (2023).
Labonte, B. et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 23 , 1102–1111 (2017).
Levey, D. F. et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 24 , 954–963 (2021).
Cohen-Woods, S., Craig, I. W. & McGuffin, P. The current state of play on the molecular genetics of depression. Psychol. Med. 43 , 673–687 (2013).
Slavich, G. M. & Sacher, J. Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology 236 , 3063–3079 (2019).
McEwen, B. S., Nasca, C. & Gray, J. D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41 , 3–23 (2016).
Fries, G. R., Saldana, V. A., Finnstein, J. & Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 28 , 284–297 (2023).
Keller, J. et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 22 , 527–536 (2017).
Dong, L., Li, B., Verkhratsky, A. & Peng, L. Cell type-specific in vivo expression of genes encoding signalling molecules in the brain in response to chronic mild stress and chronic treatment with fluoxetine. Psychopharmacology 232 , 2827–2835 (2015).
Xia, M. et al. Sleep deprivation selectively down-regulates astrocytic 5-HT(2B) receptors and triggers depressive-like behaviors via stimulating P2X(7) receptors in mice. Neurosci. Bull. 36 , 1259–1270 (2020).
Hare, D. L., Toukhsati, S. R., Johansson, P. & Jaarsma, T. Depression and cardiovascular disease: a clinical review. Eur. Heart J. 35 , 1365–1372 (2014).
Dunbar, J. A. et al. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care 31 , 2368–2373 (2008).
Arnaud, A. M. et al. Impact of major depressive disorder on comorbidities: a systematic literature review. J. Clin. Psychiatry 83 , 21r14328 (2022).
Sato, S. & Yeh, T. L. Challenges in treating patients with major depressive disorder: the impact of biological and social factors. CNS Drugs 27 , S5–10 (2013).
Greenberg, P. E., Fournier, A. A., Sisitsky, T., Pike, C. T. & Kessler, R. C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 76 , 155–162 (2015).
Greenberg, P. E. et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics 39 , 653–665 (2021).
Dean, J. & Keshavan, M. The neurobiology of depression: an integrated view. Asian J. Psychiatr. 27 , 101–111 (2017).
Bhatt, S., Devadoss, T., Manjula, S. N. & Rajangam, J. 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr. Neuropharmacol. 19 , 1545–1559 (2021).
Inazu, M., Takeda, H. & Matsumiya, T. Functional expression of the norepinephrine transporter in cultured rat astrocytes. J. Neurochem. 84 , 136–144 (2003).
Schipke, C. G., Heuser, I. & Peters, O. Antidepressants act on glial cells: SSRIs and serotonin elicit astrocyte calcium signaling in the mouse prefrontal cortex. J. Psychiatr. Res. 45 , 242–248 (2011).
Novikova, I. N. et al. Adrenaline induces calcium signal in astrocytes and vasoconstriction via activation of monoamine oxidase. Free Radic. Biol. Med. 159 , 15–22 (2020).
Martin, H. et al. Insulin modulates emotional behavior through a serotonin-dependent mechanism. Mol. Psychiatry (2022). [Oline ahead of print].
Ogawa, S. et al. Plasma L-tryptophan concentration in major depressive disorder: new data and meta-analysis. J. Clin. Psychiatry 75 , e906–915 (2014).
Fang, Y. et al. Fluoxetine inhibited the activation of A1 reactive astrocyte in a mouse model of major depressive disorder through astrocytic 5-HT(2B)R/beta-arrestin2 pathway. J. Neuroinflammation 19 , 23 (2022).
Savitz, J., Lucki, I. & Drevets, W. C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 88 , 17–31 (2009).
Verkhratsky, A., Rodriguez, J. J. & Parpura, V. Astroglia in neurological diseases. Future Neurol. 8 , 149–158 (2013).
Zhang, S. et al. 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors. Neuron Glia Biol. 6 , 113–125 (2010).
Verkhratsky, A., Parpura, V., Scuderi, C. & Li, B. Astroglial serotonin receptors as the central target of classic antidepressants. Adv. Neurobiol. 26 , 317–347 (2021).
Li, B. et al. Biphasic regulation of caveolin-1 gene expression by fluoxetine in astrocytes: opposite effects of PI3K/AKT and MAPK/ERK signaling pathways on c-fos. Front. Cell. Neurosci. 11 , 335 (2017).
Li, B., Zhang, S., Li, M., Hertz, L. & Peng, L. Chronic treatment of astrocytes with therapeutically relevant fluoxetine concentrations enhances cPLA2 expression secondary to 5-HT 2B -induced, transactivation-mediated ERK 1/2 phosphorylation. Psychopharmacology 207 , 1–12 (2009).
Li, X. et al. A novel murine model of mania. Mol. Psychiatry 28 , 3044–3054 (2023).
Li, B., Zhang, S., Zhang, H., Hertz, L. & Peng, L. Fluoxetine affects GluK2 editing, glutamate-evoked Ca(2+) influx and extracellular signal-regulated kinase phosphorylation in mouse astrocytes. J. Psychiatry Neurosci. 36 , 322–338 (2011).
Li, B. et al. Cell type-specific gene expression and editing responses to chronic fluoxetine treatment in the in vivo mouse brain and their relevance for stress-induced anhedonia. Neurochem. Res. 37 , 2480–2495 (2012).
Breton-Provencher, V., Drummond, G. T., Feng, J., Li, Y. & Sur, M. Spatiotemporal dynamics of noradrenaline during learned behaviour. Nature 606 , 732–738 (2022).
Mori, K. et al. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 43 , 1026–1034 (2002).
O’Sullivan, J. B., Ryan, K. M., Curtin, N. M., Harkin, A. & Connor, T. J. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int. J. Neuropsychopharmacol. 12 , 687–699 (2009).
Choi, H. S. et al. The anti-inflammatory activity of duloxetine, a serotonin/norepinephrine reuptake inhibitor, prevents kainic acid-induced hippocampal neuronal death in mice. J. Neurol. Sci. 358 , 390–397 (2015).
Wang, J. et al. Desvenlafaxine prevents white matter injury and improves the decreased phosphorylation of the rate-limiting enzyme of cholesterol synthesis in a chronic mouse model of depression. J. Neurochem. 131 , 229–238 (2014).
Luo, Y., Kataoka, Y., Ostinelli, E. G., Cipriani, A. & Furukawa, T. A. National prescription patterns of antidepressants in the treatment of adults with major depression in the US Between 1996 and 2015: a population representative survey based analysis. Front. Psychiatry 11 , 35 (2020).
Sun, J. D., Liu, Y., Yuan, Y. H., Li, J. & Chen, N. H. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 37 , 1305–1320 (2012).
Zepeda, R. et al. Venlafaxine treatment after endothelin-1-induced cortical stroke modulates growth factor expression and reduces tissue damage in rats. Neuropharmacology 107 , 131–145 (2016).
Bekhbat, M. et al. Correction to: functional connectivity in reward circuitry and symptoms of anhedonia as therapeutic targets in depression with high inflammation: evidence from a dopamine challenge study. Mol. Psychiatry 27 , 4122 (2022).
Cui, Y. et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554 , 323–327 (2018).
Skupio, U. et al. Astrocytes determine conditioned response to morphine via glucocorticoid receptor-dependent regulation of lactate release. Neuropsychopharmacology 45 , 404–415 (2020).
Rasheed, N. et al. Differential response of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem. Res. 35 , 22–32 (2010).
Morales, I., Fuentes, A., Ballaz, S., Obeso, J. A. & Rodriguez, M. Striatal interaction among dopamine, glutamate and ascorbate. Neuropharmacology 63 , 1308–1314 (2012).
Solis, O., Garcia-Sanz, P., Herranz, A. S., Asensio, M. J. & Moratalla, R. L-DOPA reverses the increased free amino acids tissue levels induced by dopamine depletion and rises GABA and tyrosine in the striatum. Neurotox. Res. 30 , 67–75 (2016).
Corkrum, M. et al. Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron 105 , 1036–1047.e1035 (2020).
Adermark, L. et al. Astrocytes modulate extracellular neurotransmitter levels and excitatory neurotransmission in dorsolateral striatum via dopamine D2 receptor signaling. Neuropsychopharmacology 47 , 1493–1502 (2022).
Agren, R. & Sahlholm, K. Voltage-dependent dopamine potency at D(1)-like dopamine receptors. Front Pharmacol. 11 , 581151 (2020).
Fasano, C. et al. Dopamine facilitates dendritic spine formation by cultured striatal medium spiny neurons through both D1 and D2 dopamine receptors. Neuropharmacology 67 , 432–443 (2013).
Hao, Y. & Plested, A. J. R. Seeing glutamate at central synapses. J. Neurosci. Methods 375 , 109531 (2022).
Trudler, D. et al. alpha-synuclein oligomers induce glutamate release from astrocytes and excessive extrasynaptic NMDAR activity in neurons, thus contributing to synapse loss. J. Neurosci. 41 , 2264–2273 (2021).
Schmitz, F. et al. Methylphenidate decreases ATP levels and impairs glutamate uptake and Na(+),K(+)-ATPase activity in juvenile rat hippocampus. Mol. Neurobiol. 54 , 7796–7807 (2017).
Kucukibrahimoglu, E. et al. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur. J. Clin. Pharmacol. 65 , 571–577 (2009).
Chiba, S. et al. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 39 , 112–119 (2012).
Sullivan, R. et al. Cloning, transport properties, and differential localization of two splice variants of GLT-1 in the rat CNS: implications for CNS glutamate homeostasis. Glia 45 , 155–169 (2004).
Zou, J. et al. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem. Int. 56 , 577–584 (2010).
Karlsson, R. M. et al. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology 34 , 1578–1589 (2009).
Karlsson, R. M. et al. Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology 63 , 181–189 (2012).
Di Castro, M. A. et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14 , 1276–1284 (2011).
Matos, M. et al. Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol. Psychiatry 78 , 763–774 (2015).
Yue, N. et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J. Neuroinflammation 14 , 102 (2017).
Cao, X. et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 19 , 773–777 (2013).
Cunha, G. M., Canas, P. M., Oliveira, C. R. & Cunha, R. A. Increased density and synapto-protective effect of adenosine A2A receptors upon sub-chronic restraint stress. Neuroscience 141 , 1775–1781 (2006).
Batalha, V. L. et al. Adenosine A(2A) receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol. Psychiatry 18 , 320–331 (2013).
Wang, M. et al. Lateral septum adenosine A(2A) receptors control stress-induced depressive-like behaviors via signaling to the hypothalamus and habenula. Nat. Commun. 14 , 1880 (2023).
Lucas, M. et al. Coffee, caffeine, and risk of completed suicide: results from three prospective cohorts of American adults. World J. Biol. Psychiatry 15 , 377–386 (2014).
Lucas, M. et al. Coffee, caffeine, and risk of depression among women. Arch. Intern. Med. 171 , 1571–1578 (2011).
Coelho, J. E. et al. Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Front. Psychiatry 5 , 67 (2014).
Kaster, M. P. et al. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc. Natl Acad. Sci. USA 112 , 7833–7838 (2015).
Hines, D. J., Schmitt, L. I., Hines, R. M., Moss, S. J. & Haydon, P. G. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatry 3 , e212 (2013).
Etievant, A. et al. Astroglial control of the antidepressant-like effects of prefrontal cortex deep brain stimulation. EBioMedicine 2 , 898–908 (2015).
Serchov, T. et al. Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 87 , 549–562 (2015).
Dostal, C. R., Carson Sulzer, M., Kelley, K. W., Freund, G. G. & McCusker, R. H. Glial and tissue-specific regulation of Kynurenine Pathway dioxygenases by acute stress of mice. Neurobiol. Stress 7 , 1–15 (2017).
Yang, W. et al. Dex modulates the balance of water-electrolyte metabolism by depressing the expression of AVP in PVN. Front. Pharmacol. 13 , 919032 (2022).
Jacobson, L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res. 1583 , 109–121 (2014).
Perez, P. Glucocorticoid receptors, epidermal homeostasis and hair follicle differentiation. Dermatoendocrinol 3 , 166–174 (2011).
Reichrath, J. Ancient friends, revisited: new aspects on the important role of nuclear receptor signalling for skin physiology and for the treatment of skin diseases. Dermatoendocrinol 3 , 121–124 (2011).
Herbert, J. & Lucassen, P. J. Depression as a risk factor for Alzheimer’s disease: genes, steroids, cytokines and neurogenesis - What do we need to know? Front. Neuroendocrinol. 41 , 153–171 (2016).
Rosenthal, L. J., Goldner, W. S. & O’Reardon, J. P. T3 augmentation in major depressive disorder: safety considerations. Am. J. Psychiatry 168 , 1035–1040 (2011).
Mokrani, M. C., Duval, F., Erb, A., Gonzalez Lopera, F. & Danila, V. Are the thyroid and adrenal system alterations linked in depression? Psychoneuroendocrinology 122 , 104831 (2020).
Stenzel, D. & Huttner, W. B. Role of maternal thyroid hormones in the developing neocortex and during human evolution. Front. Neuroanat. 7 , 19 (2013).
Mendes-de-Aguiar, C. B. et al. Thyroid hormone increases astrocytic glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J. Neurosci. Res. 86 , 3117–3125 (2008).
Rastogi, L., Godbole, M. M., Sinha, R. A. & Pradhan, S. Reverse triiodothyronine (rT3) attenuates ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 506 , 597–603 (2018).
Isgor, C. & Watson, S. J. Estrogen receptor alpha and beta mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience 134 , 847–856 (2005).
Russell, J. K., Jones, C. K. & Newhouse, P. A. The role of estrogen in brain and cognitive aging. Neurotherapeutics 16 , 649–665 (2019).
Biegon, A., Reches, A., Snyder, L. & McEwen, B. S. Serotonergic and noradrenergic receptors in the rat brain: modulation by chronic exposure to ovarian hormones. Life Sci. 32 , 2015–2021 (1983).
Arinami, H., Suzuki, Y., Tajiri, M., Tsuneyama, N. & Someya, T. Role of insulin-like growth factor 1, sex and corticosteroid hormones in male major depressive disorder. BMC Psychiatry 21 , 157 (2021).
Walf, A. A., Rhodes, M. E. & Frye, C. A. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharm. Biochem Behav. 78 , 523–529 (2004).
Frye, C. A. & Walf, A. A. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav. Neurosci. 118 , 306–313 (2004).
Hughes, Z. A. et al. WAY-200070, a selective agonist of estrogen receptor beta as a potential novel anxiolytic/antidepressant agent. Neuropharmacology 54 , 1136–1142 (2008).
Weiser, M. J., Wu, T. J. & Handa, R. J. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology 150 , 1817–1825 (2009).
Irwin, R. W. et al. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J. Neuroendocrinol. 24 , 236–248 (2012).
Ho, M. F. et al. Ketamine and ketamine metabolites as novel estrogen receptor ligands: Induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem. Pharmacol. 152 , 279–292 (2018).
Hochol, A. et al. Effects of neuropeptides B and W on the rat pituitary-adrenocortical axis: in vivo and in vitro studies. Int J. Mol. Med. 19 , 207–211 (2007).
CAS PubMed Google Scholar
Kurosawa, N., Shimizu, K. & Seki, K. The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1beta-induced elevated leptin levels in mice. Psychopharmacology 233 , 1725–1737 (2016).
Lee, J. Y. et al. The association between serum leptin levels and post-stroke depression: a retrospective clinical study. Ann. Rehabil. Med. 39 , 786–792 (2015).
Li, Z. et al. Fluoxetine improves behavioural deficits induced by chronic alcohol treatment by alleviating RNA editing of 5-HT(2C) receptors. Neurochem. Int. 134 , 104689 (2020).
de Morais, H. et al. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav. Brain Res. 258 , 52–64 (2014).
Rawdin, B. J. et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 31 , 143–152 (2013).
Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62 , 649–671 (2000).
Greaney, J. L., Saunders, E. F. H. & Alexander, L. M. Short-term salicylate treatment improves microvascular endothelium-dependent dilation in young adults with major depressive disorder. Am. J. Physiol. Heart Circ. Physiol. 322 , H880–H889 (2022).
Belanger, M. & Magistretti, P. J. The role of astroglia in neuroprotection. Dialogues Clin. Neurosci. 11 , 281–295 (2009).
Chen, B. et al. The neuroprotective mechanism of lithium after ischaemic stroke. Commun. Biol. 5 , 105 (2022).
Maes, M. et al. Major differences in neurooxidative and neuronitrosative stress pathways between major depressive disorder and types I and II bipolar disorder. Mol. Neurobiol. 56 , 141–156 (2019).
Early, J. O. et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc. Natl Acad. Sci. USA 115 , E8460–E8468 (2018).
Pasco, J. A. et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry 197 , 372–377 (2010).
Bakunina, N., Pariante, C. M. & Zunszain, P. A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144 , 365–373 (2015).
Paul, E. R. et al. Peripheral and central kynurenine pathway abnormalities in major depression. Brain Behav. Immun. 101 , 136–145 (2022).
Wang, Y. et al. Inhibition of activated astrocyte ameliorates lipopolysaccharide- induced depressive-like behaviors. J. Affect Disord. 242 , 52–59 (2019).
Lenk, T., Rabet, S., Sprick, M., Raabe, G. & Schroder, U. Insight into the interaction of furfural with metallic surfaces in the electrochemical hydrogenation process. Chemphyschem 24 , e202200614 (2023).
Zhang, J. C., Yao, W. & Hashimoto, K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 14 , 721–731 (2016).
Kennis, M. et al. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol. Psychiatry 25 , 321–338 (2020).
Norden, D. M., Trojanowski, P. J., Villanueva, E., Navarro, E. & Godbout, J. P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 64 , 300–316 (2016).
Li, X. et al. Leptin increases expression of 5-HT(2B) receptors in astrocytes thus enhancing action of fluoxetine on the depressive behavior induced by sleep deprivation. Front. Psychiatry 9 , 734 (2018).
Takano, K., Yamasaki, H., Kawabe, K., Moriyama, M. & Nakamura, Y. Imipramine induces brain-derived neurotrophic factor mRNA expression in cultured astrocytes. J. Pharmacol. Sci. 120 , 176–186 (2012).
Zhang, Y. et al. BDNF enhances electrophysiological activity and excitatory synaptic transmission of RA projection neurons in adult male zebra finches. Brain Res. 1801 , 148208 (2023).
Quesseveur, G. et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry 3 , e253 (2013).
Zor, F. et al. Effect of VEGF gene therapy and hyaluronic acid film sheath on peripheral nerve regeneration. Microsurgery 34 , 209–216 (2014).
Sun, L. et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. 10 , 542 (2019).
Alcocer-Gomez, E. & Cordero, M. D. NLRP3 inflammasome: a new target in major depressive disorder. CNS Neurosci. Ther. 20 , 294–295 (2014).
Beckwith, K. S. et al. Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat. Commun. 11 , 2270 (2020).
Xia, M. et al. The ameliorative effect of fluoxetine on neuroinflammation induced by sleep deprivation. J. Neurochem. 146 , 63–75 (2018).
Jeon, S. A. et al. NLRP3 inflammasome contributes to lipopolysaccharide-induced depressive-like behaviors via indoleamine 2,3-dioxygenase induction. Int. J. Neuropsychopharmacol. 20 , 896–906 (2017).
Wong, M. L. et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 21 , 797–805 (2016).
Page, C. E. & Coutellier, L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neurosci. Biobehav. Rev. 105 , 39–51 (2019).
Santello, M., Toni, N. & Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 22 , 154–166 (2019).
Castillo-Gomez, E., Perez-Rando, M., Vidueira, S. & Nacher, J. Polysialic acid acute depletion induces structural plasticity in interneurons and impairs the excitation/inhibition balance in medial prefrontal cortex organotypic cultures. Front. Cell. Neurosci. 10 , 170 (2016).
Haydon, P. G. & Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 86 , 1009–1031 (2006).
Huang, D. et al. Dysfunction of astrocytic connexins 30 and 43 in the medial prefrontal cortex and hippocampus mediates depressive-like behaviours. Behav. Brain Res. 372 , 111950 (2019).
Nagy, C., Torres-Platas, S. G., Mechawar, N. & Turecki, G. Repression of astrocytic connexins in cortical and subcortical brain regions and prefrontal enrichment of H3K9me3 in depression and suicide. Int J. Neuropsychopharmacol. 20 , 50–57 (2017).
PubMed Google Scholar
Murphy-Royal, C. et al. Stress gates an astrocytic energy reservoir to impair synaptic plasticity. Nat. Commun. 11 , 2014 (2020).
Ezan, P. et al. Deletion of astroglial connexins weakens the blood-brain barrier. J. Cereb. Blood Flow Metab. 32 , 1457–1467 (2012).
Torres-Platas, S. G., Nagy, C., Wakid, M., Turecki, G. & Mechawar, N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol. Psychiatry 21 , 509–515 (2016).
Cobb, J. A. et al. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 316 , 209–220 (2016).
Steinacker, P. et al. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J. Psychiatr. Res. 144 , 54–58 (2021).
Rajkowska, G., Hughes, J., Stockmeier, C. A., Javier Miguel-Hidalgo, J. & Maciag, D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol. Psychiatry 73 , 613–621 (2013).
Rajkowska, G. & Miguel-Hidalgo, J. J. Glial pathology in major depressive disorder: an approach to investigate the coverage of blood vessels by astrocyte endfeet in human postmortem brain. Methods Mol. Biol. 1938 , 247–254 (2019).
Kong, H. et al. Aquaporin-4 knockout exacerbates corticosterone-induced depression by inhibiting astrocyte function and hippocampal neurogenesis. CNS Neurosci. Ther. 20 , 391–402 (2014).
Xia, M., Yang, L., Sun, G., Qi, S. & Li, B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology 234 , 365–379 (2017).
Di Benedetto, B. et al. Fluoxetine requires the endfeet protein aquaporin-4 to enhance plasticity of astrocyte processes. Front. Cell. Neurosci. 10 , 8 (2016).
Du, J. et al. S100B is selectively expressed by gray matter protoplasmic astrocytes and myelinating oligodendrocytes in the developing CNS. Mol. Brain 14 , 154 (2021).
Schroeter, M. L., Abdul-Khaliq, H., Krebs, M., Diefenbacher, A. & Blasig, I. E. Serum markers support disease-specific glial pathology in major depression. J. Affect Disord. 111 , 271–280 (2008).
Michel, M. et al. Increased GFAP concentrations in the cerebrospinal fluid of patients with unipolar depression. Transl. Psychiatry 11 , 308 (2021).
Gos, T. et al. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J. Psychiatr. Res. 47 , 1694–1699 (2013).
Michetti, F. et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J. Neurochem. 120 , 644–659 (2012).
Zhang, L. et al. Changes in glial gene expression in the prefrontal cortex in relation to major depressive disorder, suicide and psychotic features. J. Affect Disord. 295 , 893–903 (2021).
Gules, E., Iosifescu, D. V. & Tural, U. Plasma neuronal and glial markers and anterior cingulate metabolite levels in major depressive disorder: a pilot study. Neuropsychobiology 79 , 214–221 (2020).
Chamera, K., Szuster-Gluszczak, M., Trojan, E. & Basta-Kaim, A. Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200-CD200R and CX3CL1-CX3CR1 systems. Cells 9 , 1676 (2020).
Jiang, X. et al. Asperosaponin VI ameliorates the CMS-induced depressive-like behaviors by inducing a neuroprotective microglial phenotype in hippocampus via PPAR-gamma pathway. J. Neuroinflammation 19 , 115 (2022).
Tang, M. M., Lin, W. J., Pan, Y. Q. & Li, Y. C. Fibroblast growth factor 2 modulates hippocampal microglia activation in a neuroinflammation induced model of depression. Front. Cell. Neurosci. 12 , 255 (2018).
Zhan, Y. et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17 , 400–406 (2014).
Hellwig, S. et al. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav. Immun. 55 , 126–137 (2016).
Merendino, R. A. et al. Involvement of fractalkine and macrophage inflammatory protein-1 alpha in moderate-severe depression. Mediat. Inflamm. 13 , 205–207 (2004).
Garcia-Marchena, N. et al. Inflammatory mediators and dual depression: Potential biomarkers in plasma of primary and substance-induced major depression in cocaine and alcohol use disorders. PLoS ONE 14 , e0213791 (2019).
Wang, H. T. et al. Early-Life Social Isolation-Induced Depressive-Like Behavior in Rats Results in Microglial Activation and Neuronal Histone Methylation that Are Mitigated by Minocycline. Neurotox. Res 31 , 505–520 (2017).
Frank, M. G., Fonken, L. K., Annis, J. L., Watkins, L. R. & Maier, S. F. Stress disinhibits microglia via down-regulation of CD200R: A mechanism of neuroinflammatory priming. Brain Behav. Immun. 69 , 62–73 (2018).
Fonken, L. K. et al. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav. Immun. 70 , 257–267 (2018).
Popov, A. et al. Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 20 , e13334 (2021).
Zhang, Y. et al. Structural basis of ketamine action on human NMDA receptors. Nature 596 , 301–305 (2021).
Wang, Q., Jie, W., Liu, J. H., Yang, J. M. & Gao, T. M. An astroglial basis of major depressive disorder? An overview. Glia 65 , 1227–1250 (2017).
Jiang, X., Lin, W., Cheng, Y. & Wang, D. mGluR5 facilitates long-term synaptic depression in a stress-induced depressive mouse model. Can. J. Psychiatry 65 , 347–355 (2020).
Guo, W. et al. Iptakalim alleviates synaptic damages via targeting mitochondrial ATP-sensitive potassium channel in depression. FASEB J. 35 , e21581 (2021).
Curro, D., Navarra, P., Samengo, I. & Martire, M. P2X7 receptors exert a permissive effect on the activation of presynaptic AMPA receptors in rat trigeminal caudal nucleus glutamatergic nerve terminals. J. Headache Pain 21 , 83 (2020).
Iwata, M. et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol. Psychiatry 80 , 12–22 (2016).
He, Y., Taylor, N., Fourgeaud, L. & Bhattacharya, A. The role of microglial P2X7: modulation of cell death and cytokine release. J. Neuroinflammation 14 , 135 (2017).
Ota, K. T. et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med 20 , 531–535 (2014).
Kang, H. J. et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med 18 , 1413–1417 (2012).
Tomassoni-Ardori, F. et al. Rbfox1 up-regulation impairs BDNF-dependent hippocampal LTP by dysregulating TrkB isoform expression levels. Elife 8 , e49673 (2019).
Wang, G. et al. Antidepressant-like effect of ginsenoside Rb1 on potentiating synaptic plasticity via the miR-134-mediated BDNF signaling pathway in a mouse model of chronic stress-induced depression. J. Ginseng Res. 46 , 376–386 (2022).
Lalo, U., Koh, W., Lee, C. J. & Pankratov, Y. The tripartite glutamatergic synapse. Neuropharmacology 199 , 108758 (2021).
Shepard, R., Page, C. E. & Coutellier, L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience 332 , 1–12 (2016).
Shepard, R. & Coutellier, L. Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Mol. Neurobiol. 55 , 2591–2602 (2018).
Bouamrane, L. et al. Reelin-haploinsufficiency disrupts the developmental trajectory of the E/I Balance in the Prefrontal Cortex. Front. Cell. Neurosci. 10 , 308 (2016).
Arnone, D., Mumuni, A. N., Jauhar, S., Condon, B. & Cavanagh, J. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur. Neuropsychopharmacol. 25 , 1109–1117 (2015).
Hu, Y. T., Tan, Z. L., Hirjak, D. & Northoff, G. Brain-wide changes in excitation-inhibition balance of major depressive disorder: a systematic review of topographic patterns of GABA- and glutamatergic alterations. Mol. Psychiatry 28 , 3257–3266 (2023).
Zhao, J. et al. Prefrontal alterations in GABAergic and glutamatergic gene expression in relation to depression and suicide. J. Psychiatr. Res. 102 , 261–274 (2018).
Douillard-Guilloux, G., Lewis, D., Seney, M. L. & Sibille, E. Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety 34 , 68–78 (2017).
Scifo, E. et al. Sustained molecular pathology across episodes and remission in major depressive disorder. Biol. Psychiatry 83 , 81–89 (2018).
Oh, D. H., Son, H., Hwang, S. & Kim, S. H. Neuropathological abnormalities of astrocytes, GABAergic neurons, and pyramidal neurons in the dorsolateral prefrontal cortices of patients with major depressive disorder. Eur. Neuropsychopharmacol. 22 , 330–338 (2012).
Sibille, E., Morris, H. M., Kota, R. S. & Lewis, D. A. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J. Neuropsychopharmacol. 14 , 721–734 (2011).
Barros-Barbosa, A. R., Lobo, M. G., Ferreirinha, F., Correia-de-Sa, P. & Cordeiro, J. M. P2X7 receptor activation downmodulates Na(+)-dependent high-affinity GABA and glutamate transport into rat brain cortex synaptosomes. Neuroscience 306 , 74–90 (2015).
Barros-Barbosa, A. R. et al. Up-regulation of P2X7 receptor-mediated inhibition of GABA uptake by nerve terminals of the human epileptic neocortex. Epilepsia 57 , 99–110 (2016).
Liu, Y. P., Yang, C. S., Chen, M. C., Sun, S. H. & Tzeng, S. F. Ca(2+)-dependent reduction of glutamate aspartate transporter GLAST expression in astrocytes by P2X(7) receptor-mediated phosphoinositide 3-kinase signaling. J. Neurochem. 113 , 213–227 (2010).
Macaulay, N. & Zeuthen, T. Glial K(+) clearance and cell swelling: key roles for cotransporters and pumps. Neurochem. Res. 37 , 2299–2309 (2012).
Larsen, B. R. et al. Contributions of the Na(+)/K(+)-ATPase, NKCC1, and Kir4.1 to hippocampal K(+) clearance and volume responses. Glia 62 , 608–622 (2014).
Verkhratsky, A. & Nedergaard, M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369 , 20130595 (2014).
Aramideh, J. A., Vidal-Itriago, A., Morsch, M. & Graeber, M. B. Cytokine signalling at the microglial penta-partite synapse. Int J. Mol. Sci. 22 , 13186 (2021).
Chung, W. S. et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504 , 394–400 (2013).
Yang, J. et al. Astrocytes contribute to synapse elimination via type 2 inositol 1,4,5-trisphosphate receptor-dependent release of ATP. Elife 5 , e15043 (2016).
Petrache, A. L. et al. Aberrant excitatory-inhibitory synaptic mechanisms in entorhinal cortex microcircuits during the pathogenesis of Alzheimer’s disease. Cereb. Cortex 29 , 1834–1850 (2019).
Arioz, B. I. et al. Melatonin attenuates LPS-Induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 10 , 1511 (2019).
Bikbaev, A., Frischknecht, R. & Heine, M. Brain extracellular matrix retains connectivity in neuronal networks. Sci. Rep. 5 , 14527 (2015).
Strackeljan, L. et al. Microglia depletion-induced remodeling of extracellular matrix and excitatory synapses in the hippocampus of adult mice. Cells 10 , 1862 (2021).
Leclercq, S., De Saeger, C., Delzenne, N., de Timary, P. & Starkel, P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol. Psychiatry 76 , 725–733 (2014).
Hsu, J. H., Chien, I. C. & Lin, C. H. Increased risk of chronic liver disease in patients with major depressive disorder: a population-based study. J. Affect Disord. 251 , 180–185 (2019).
Chen, D., Zhang, Y., Huang, T. & Jia, J. Depression and risk of gastrointestinal disorders: a comprehensive two-sample Mendelian randomization study of European ancestry. Psychol. Med . 53 , 7309–7321 (2023).
Facanali, C. B. G. et al. The relationship of major depressive disorder with Crohn’s disease activity. Clinics (Sao Paulo) 78 , 100188 (2023).
Ronaldson, P. T. & Davis, T. P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow. Metab. 40 , S6–S24 (2020).
Qing, H. et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell 182 , 1660 (2020).
Labad, J. et al. Hypothalamic-pituitary-adrenal axis function and exposure to stress factors and cannabis use in recent-onset psychosis. World J. Biol. Psychiatry 21 , 564–571 (2020).
Frank, M. G., Miguel, Z. D., Watkins, L. R. & Maier, S. F. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav. Immun. 24 , 19–30 (2010).
Deng, Y. et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 13 , 1–16 (2021).
Munn, D. H. & Mellor, A. L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34 , 137–143 (2013).
Gos, T. et al. Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain Behav. Immun. 41 , 59–64 (2014).
Li, M. et al. Transcriptome profiles of corticosterone-induced cytotoxicity reveals the involvement of neurite growth-related genes in depression. Psychiatry Res. 276 , 79–86 (2019).
Dutheil, S., Ota, K. T., Wohleb, E. S., Rasmussen, K. & Duman, R. S. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology 41 , 1874–1887 (2016).
Skonieczna-Zydecka, K. et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients 10 , 1939 (2018).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167 , 1469–1480.e1412 (2016).
Fan, L. et al. Total glycosides from stems of Cistanche tubulosa alleviate depression-like behaviors: bidirectional interaction of the phytochemicals and gut microbiota. Phytomedicine 83 , 153471 (2021).
Zhang, J. C. et al. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl. Psychiatry 7 , e1138 (2017).
Pan, Y., Chen, X. Y., Zhang, Q. Y. & Kong, L. D. Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav. Immun. 41 , 90–100 (2014).
Woodburn, S. C., Bollinger, J. L. & Wohleb, E. S. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol. Stress 14 , 100312 (2021).
Zhang, K., Liu, R., Gao, Y., Ma, W. & Shen, W. Electroacupuncture relieves LPS-induced depression-like behaviour in rats through IDO-Mediated tryptophan-degrading pathway. Neuropsychiatr. Dis. Treat. 16 , 2257–2266 (2020).
Parrott, J. M., Redus, L. & O’Connor, J. C. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflammation 13 , 124 (2016).
Alzarea, S., Abbas, M., Ronan, P. J., Lutfy, K. & Rahman, S. The Effect of an alpha-7 nicotinic allosteric modulator PNU120596 and NMDA receptor antagonist memantine on depressive-like behavior induced by lps in mice: the involvement of brain microglia. Brain Sci. 12 , 1493 (2022).
Le Strat, Y., Le Foll, B. & Dubertret, C. Major depression and suicide attempts in patients with liver disease in the United States. Liver Int. 35 , 1910–1916 (2015).
Buganza-Torio, E. et al. Depression in cirrhosis - a prospective evaluation of the prevalence, predictors and development of a screening nomogram. Aliment. Pharmacol. Ther. 49 , 194–201 (2019).
Qin, X. H. et al. Liver soluble epoxide hydrolase regulates behavioral and cellular effects of chronic stress. Cell Rep. 29 , 3223–3234.e3226 (2019).
Pang, F. et al. Electroacupuncture alleviates depressive-like behavior by modulating the expression of P2X7/NLRP3/IL-1beta of prefrontal cortex and liver in rats exposed to chronic unpredictable mild stress. Brain Sci. 13 , 436 (2023).
Zhang, Y. et al. Effects of hydrogen-rich water on depressive-like behavior in mice. Sci. Rep. 6 , 23742 (2016).
Brown, S. J. et al. Sex- and suicide-specific alterations in the kynurenine pathway in the anterior cingulate cortex in major depression. Neuropsychopharmacology (2023). Online ahead of print.
Guillemin, G. J. et al. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 5 , 108–111 (2000).
Park, H. J., Kim, S. A., Kang, W. S. & Kim, J. W. Early-life stress modulates gut microbiota and peripheral and central inflammation in a sex-dependent manner. Int J. Mol. Sci. 22 , 1899 (2021).
Ninan, J. & Feldman, L. Ammonia levels and hepatic encephalopathy in patients with known chronic liver disease. J. Hosp. Med. 12 , 659–661 (2017).
Kautzky, A. et al. The influence of the rs6295 gene polymorphism on serotonin-1A receptor distribution investigated with PET in patients with major depression applying machine learning. Transl. Psychiatry 7 , e1150 (2017).
Das, R. et al. Higher levels of serum IL-1beta and TNF-alpha are associated with an increased probability of major depressive disorder. Psychiatry Res. 295 , 113568 (2021).
Lu, Z. et al. Oxidative stress and psychiatric disorders: evidence from the bidirectional mendelian randomization study. Antioxidants (Basel) 11 , 1386 (2022).
Hursitoglu, O. et al. Serum NOX1 and Raftlin as new potential biomarkers of Major Depressive Disorder: a study in treatment-naive first episode patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 121 , 110670 (2023).
Han, K. M. et al. Serum FAM19A5 levels: A novel biomarker for neuroinflammation and neurodegeneration in major depressive disorder. Brain Behav. Immun. 87 , 852–859 (2020).
Winter, N. R. et al. Quantifying deviations of brain structure and function in major depressive disorder across neuroimaging modalities. JAMA Psychiatry 79 , 879–888 (2022).
Zhao, Y. et al. Gray matter abnormalities in non-comorbid medication-naive patients with major depressive disorder or social anxiety disorder. EBioMedicine 21 , 228–235 (2017).
Wise, T. et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatry 22 , 1455–1463 (2017).
Liu, Y. et al. Abnormal brain gray matter volume in patients with major depressive disorder: Associated with childhood trauma? J. Affect Disord. 308 , 562–568 (2022).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72 , 603–611 (2015).
Wang, Y. et al. Topologically convergent and divergent functional connectivity patterns in unmedicated unipolar depression and bipolar disorder. Transl. Psychiatry 7 , e1165 (2017).
Kremneva, E. I., Sinitsyn, D. O., Dobrynina, L. A., Suslina, A. D. & Krotenkova, M. V. [Resting state functional MRI in neurology and psychiatry]. Zh. Nevrol. Psikhiatr Im. S S Korsakova 122 , 5–14 (2022).
Luo, Z. et al. Shared and specific dynamics of brain segregation and integration in bipolar disorder and major depressive disorder: A resting-state functional magnetic resonance imaging study. J. Affect. Disord. 280 , 279–286 (2021).
Drysdale, A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23 , 28–38 (2017).
Chen, Q. et al. Regional amplitude abnormities in the major depressive disorder: A resting-state fMRI study and support vector machine analysis. J. Affect. Disord. 308 , 1–9 (2022).
Yan, C. G. et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl Acad. Sci. USA 116 , 9078–9083 (2019).
Pizzagalli, D. A. & Roberts, A. C. Prefrontal cortex and depression. Neuropsychopharmacology 47 , 225–246 (2022).
Muller, V. I. et al. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry 74 , 47–55 (2017).
Sun, K. et al. A two-center radiomic analysis for differentiating major depressive disorder using multi-modality MRI data under different parcellation methods. J. Affect. Disord. 300 , 1–9 (2022).
Mayerhoefer, M. E. et al. Introduction to radiomics. J. Nucl. Med. 61 , 488–495 (2020).
Xu, Z. et al. Combined HTR1A/1B methylation and human functional connectome to recognize patients with MDD. Psychiatry Res. 317 , 114842 (2022).
Verduijn, J. et al. Reconsidering the prognosis of major depressive disorder across diagnostic boundaries: full recovery is the exception rather than the rule. BMC Med. 15 , 215 (2017).
Blumenthal, J. A. & Rozanski, A. Exercise as a therapeutic modality for the prevention and treatment of depression. Prog. Cardiovasc. Dis. 77 , 50–58 (2023).
Baglioni, C., Spiegelhalder, K., Nissen, C. & Riemann, D. Clinical implications of the causal relationship between insomnia and depression: how individually tailored treatment of sleeping difficulties could prevent the onset of depression. EPMA J. 2 , 287–293 (2011).
Riemann, D. et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep. Res. 26 , 675–700 (2017).
Soh, H. L., Ho, R. C., Ho, C. S. & Tam, W. W. Efficacy of digital cognitive behavioural therapy for insomnia: a meta-analysis of randomised controlled trials. Sleep. Med. 75 , 315–325 (2020).
Ho, F. Y., Chan, C. S., Lo, W. Y. & Leung, J. C. The effect of self-help cognitive behavioral therapy for insomnia on depressive symptoms: an updated meta-analysis of randomized controlled trials. J. Affect. Disord. 265 , 287–304 (2020).
Qaseem, A. et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the american college of physicians. Ann. Intern. Med. 165 , 125–133 (2016).
Irwin, M. R. et al. Prevention of incident and recurrent major depression in older adults with insomnia: a randomized clinical trial. JAMA Psychiatry 79 , 33–41 (2022).
Scheer, F. A., Pirovano, C., Van Someren, E. J. & Buijs, R. M. Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience 132 , 465–477 (2005).
Dekker, K. et al. Combined internet-based cognitive-behavioral and chronobiological intervention for insomnia: a randomized controlled trial. Psychother. Psychosom. 89 , 117–118 (2020).
Leerssen, J. et al. Treating insomnia with high risk of depression using therapist-guided digital cognitive, behavioral, and circadian rhythm support interventions to prevent worsening of depressive symptoms: a randomized controlled trial. Psychother. Psychosom. 91 , 168–179 (2022).
Zhai, L., Zhang, H. & Zhang, D. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress. Anxiety 32 , 664–670 (2015).
Baglioni, C. et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 135 , 10–19 (2011).
Utge, S. J. et al. Systematic analysis of circadian genes in a population-based sample reveals association of TIMELESS with depression and sleep disturbance. PLoS ONE 5 , e9259 (2010).
Irwin, M. R. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol. 19 , 702–715 (2019).
Hill Almeida, L. M. et al. Disrupted sleep and risk of depression in later life: A prospective cohort study with extended follow up and a systematic review and meta-analysis. J. Affect. Disord. 309 , 314–323 (2022).
Tolkien, K., Bradburn, S. & Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 38 , 2045–2052 (2019).
Galland, L. Diet and inflammation. Nutr. Clin. Pr. 25 , 634–640 (2010).
Oddy, W. H. et al. Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain Behav. Immun. 69 , 428–439 (2018).
Bosma-den Boer, M. M., van Wetten, M. L. & Pruimboom, L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr. Metab. (Lond.) 9 , 32 (2012).
Sanchez-Villegas, A. et al. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: the PREDIMED-NAVARRA randomized trial. Nutr. Neurosci. 14 , 195–201 (2011).
Jiang, C. & Salton, S. R. The role of neurotrophins in major depressive disorder. Transl. Neurosci. 4 , 46–58 (2013).
Ogbonnaya, E. S. et al. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry 78 , e7–9 (2015).
Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21 , 786–796 (2016).
Jiang, H. et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48 , 186–194 (2015).
Burokas, A. et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 82 , 472–487 (2017).
Bonder, M. J. et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 8 , 45 (2016).
Mohan, M. et al. Dietary gluten-induced gut dysbiosis is accompanied by selective upregulation of microRNAs with intestinal tight junction and bacteria-binding motifs in rhesus macaque model of celiac disease. Nutrients 8 , 684 (2016).
Karakula-Juchnowicz, H. et al. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr. J. 18 , 50 (2019).
Khosravi, M. et al. The relationship between dietary patterns and depression mediated by serum levels of Folate and vitamin B12. BMC Psychiatry 20 , 63 (2020).
Schuch, F. B. et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 77 , 42–51 (2016).
Morland, C. et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8 , 15557 (2017).
Coelho, F. G. et al. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 56 , 10–15 (2013).
Stimpson, N. J., Davison, G. & Javadi, A. H. Joggin’ the noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci. Biobehav Rev. 88 , 177–186 (2018).
De Rossi, P. et al. A critical role for VEGF and VEGFR2 in NMDA receptor synaptic function and fear-related behavior. Mol. Psychiatry 21 , 1768–1780 (2016).
Bugg, J. M. & Head, D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging 32 , 506–514 (2011).
Cole, R. C. et al. Cardiorespiratory fitness and hippocampal volume predict faster episodic associative learning in older adults. Hippocampus 30 , 143–155 (2020).
Zotcheva, E. et al. Associations of changes in cardiorespiratory fitness and symptoms of anxiety and depression with brain volumes: The HUNT Study. Front Behav. Neurosci. 13 , 53 (2019).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67 , 446–457 (2010).
Valkanova, V., Ebmeier, K. P. & Allan, C. L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect Disord. 150 , 736–744 (2013).
Kohler, C. A. et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 135 , 373–387 (2017).
Choi, K. W. et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample mendelian randomization study. JAMA Psychiatry 76 , 399–408 (2019).
Schuch, F. B. et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am. J. Psychiatry 175 , 631–648 (2018).
Choi, K. W. et al. Physical activity offsets genetic risk for incident depression assessed via electronic health records in a biobank cohort study. Depress Anxiety 37 , 106–114 (2020).
Imboden, C. et al. Aerobic exercise or stretching as add-on to inpatient treatment of depression: Similar antidepressant effects on depressive symptoms and larger effects on working memory for aerobic exercise alone. J. Affect Disord. 276 , 866–876 (2020).
Vancampfort, D. et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry 16 , 308–315 (2017).
Brush, C. J. et al. A randomized trial of aerobic exercise for major depression: examining neural indicators of reward and cognitive control as predictors and treatment targets. Psychol. Med. 52 , 893–903 (2022).
Kujawa, A. et al. Reduced reward responsiveness predicts change in depressive symptoms in anxious children and adolescents following treatment. J. Child Adolesc. Psychopharmacol. 29 , 378–385 (2019).
Kandola, A., Ashdown-Franks, G., Hendrikse, J., Sabiston, C. M. & Stubbs, B. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav Rev. 107 , 525–539 (2019).
Yildirim, Y. & Kocabiyik, S. The relationship between social support and loneliness in Turkish patients with cancer. J. Clin. Nurs. 19 , 832–839 (2010).
Lin, J. et al. Perceived stressfulness mediates the effects of subjective social support and negative coping style on suicide risk in Chinese patients with major depressive disorder. J. Affect. Disord. 265 , 32–38 (2020).
Smith, L., Hill, N. & Kokanovic, R. Experiences of depression, the role of social support and its impact on health outcomes. J. Ment. Health 24 , 342–346 (2015).
Wang, X., Cai, L., Qian, J. & Peng, J. Social support moderates stress effects on depression. Int. J. Ment. Health Syst. 8 , 41 (2014).
Hybels, C. F., Pieper, C. F., Blazer, D. G. & Steffens, D. C. Heterogeneity in the three-year course of major depression among older adults. Int. J. Geriatr. Psychiatry 31 , 775–782 (2016).
Backs-Dermott, B. J., Dobson, K. S. & Jones, S. L. An evaluation of an integrated model of relapse in depression. J. Affect. Disord. 124 , 60–67 (2010).
Holma, I. A., Holma, K. M., Melartin, T. K., Rytsala, H. J. & Isometsa, E. T. A 5-year prospective study of predictors for disability pension among patients with major depressive disorder. Acta Psychiatr. Scand. 125 , 325–334 (2012).
Stewart, R. A., Patel, T. A., McDermott, K. A. & Cougle, J. R. Functional and structural social support in DSM-5 mood and anxiety disorders: a population-based study. J. Affect Disord. 308 , 528–534 (2022).
Ouakinin, S. R. S., Barreira, D. P. & Gois, C. J. Depression and obesity: integrating the role of stress, neuroendocrine dysfunction and inflammatory pathways. Front. Endocrinol. (Lausanne) 9 , 431 (2018).
Hallgren, M., Lundin, A., Tee, F. Y., Burstrom, B. & Forsell, Y. Somebody to lean on: Social relationships predict post-treatment depression severity in adults. Psychiatry Res. 249 , 261–267 (2017).
van Beljouw, I. M., Verhaak, P. F., Cuijpers, P., van Marwijk, H. W. & Penninx, B. W. The course of untreated anxiety and depression, and determinants of poor one-year outcome: a one-year cohort study. BMC Psychiatry 10 , 86 (2010).
Dour, H. J. et al. Perceived social support mediates anxiety and depressive symptom changes following primary care intervention. Depress Anxiety 31 , 436–442 (2014).
Teo, A. R., Choi, H. & Valenstein, M. Social relationships and depression: ten-year follow-up from a nationally representative study. PLoS ONE 8 , e62396 (2013).
Griffiths, K. M., Crisp, D. A., Barney, L. & Reid, R. Seeking help for depression from family and friends: a qualitative analysis of perceived advantages and disadvantages. BMC Psychiatry 11 , 196 (2011).
Hillhouse, T. M. & Porter, J. H. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp. Clin. Psychopharmacol. 23 , 1–21 (2015).
Sulser, F., Vetulani, J. & Mobley, P. L. Mode of action of antidepressant drugs. Biochem. Pharmacol. 27 , 257–261 (1978).
Gillman, P. K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 151 , 737–748 (2007).
Taylor, C., Fricker, A. D., Devi, L. A. & Gomes, I. Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal. 17 , 549–557 (2005).
Jann, M. W. & Slade, J. H. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy 27 , 1571–1587 (2007).
Shultz, E. & Malone, D. A. Jr A practical approach to prescribing antidepressants. Cleve Clin. J. Med. 80 , 625–631 (2013).
Croom, K. F., Perry, C. M. & Plosker, G. L. Mirtazapine: a review of its use in major depression and other psychiatric disorders. CNS Drugs 23 , 427–452 (2009).
Benjamin, S. & Doraiswamy, P. M. Review of the use of mirtazapine in the treatment of depression. Expert Opin. Pharmacother. 12 , 1623–1632 (2011).
Cleare, A. et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J. Psychopharmacol. 29 , 459–525 (2015).
Guloglu, C., Orak, M., Ustundag, M. & Altunci, Y. A. Analysis of amitriptyline overdose in emergency medicine. Emerg. Med. J. 28 , 296–299 (2011).
David, D. J. & Gourion, D. [Antidepressant and tolerance: determinants and management of major side effects]. Encephale 42 , 553–561 (2016).
Richelson, E. Antimuscarinic and other receptor-blocking properties of antidepressants. Mayo Clin. Proc. 58 , 40–46 (1983).
Hisaoka, K. et al. Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. J. Biol. Chem. 286 , 21118–21128 (2011).
Morioka, N. et al. Amitriptyline up-regulates connexin43-gap junction in rat cultured cortical astrocytes via activation of the p38 and c-Fos/AP-1 signalling pathway. Br. J. Pharmacol. 171 , 2854–2867 (2014).
Budzinski, M. L. et al. Tricyclic antidepressants target FKBP51 SUMOylation to restore glucocorticoid receptor activity. Mol. Psychiatry 27 , 2533–2545 (2022).
Malison, R. T. et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol. Psychiatry 44 , 1090–1098 (1998).
Samuels, B. A. et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat. Neurosci. 18 , 1606–1616 (2015).
Scorza, M. C. et al. Preclinical and clinical characterization of the selective 5-HT(1A) receptor antagonist DU-125530 for antidepressant treatment. Br. J. Pharmacol. 167 , 1021–1034 (2012).
Cremers, T. I. et al. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology 29 , 1782–1789 (2004).
Boothman, L. J., Mitchell, S. N. & Sharp, T. Investigation of the SSRI augmentation properties of 5-HT(2) receptor antagonists using in vivo microdialysis. Neuropharmacology 50 , 726–732 (2006).
Haddjeri, N., de Montigny, C. & Blier, P. Modulation of the firing activity of noradrenergic neurones in the rat locus coeruleus by the 5-hydroxtryptamine system. Br. J. Pharmacol. 120 , 865–875 (1997).
Szabo, S. T., de Montigny, C. & Blier, P. Modulation of noradrenergic neuronal firing by selective serotonin reuptake blockers. Br. J. Pharmacol. 126 , 568–571 (1999).
Jang, S. W. et al. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem. Biol. 16 , 644–656 (2009).
Casarotto, P. C. et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 184 , 1299–1313.e1219 (2021).
Li, B. et al. Down-regulation of GluK2 kainate receptor expression by chronic treatment with mood-stabilizing anti-convulsants or lithium in cultured astrocytes and brain, but not in neurons. Neuropharmacology 57 , 375–385 (2009).
Diaz, S. L., Narboux-Neme, N., Boutourlinsky, K., Doly, S. & Maroteaux, L. Mice lacking the serotonin 5-HT2B receptor as an animal model of resistance to selective serotonin reuptake inhibitors antidepressants. Eur. Neuropsychopharmacol. 26 , 265–279 (2016).
Sviridova, A. et al. The role of 5-HT(2B)-receptors in fluoxetine-mediated modulation of Th17- and Th1-cells in multiple sclerosis. J. Neuroimmunol. 356 , 577608 (2021).
Hertz, L. Isotope-based quantitation of uptake, release, and metabolism of glutamate and glucose in cultured astrocytes. Methods Mol. Biol. 814 , 305–323 (2012).
Arakawa, R. et al. Venlafaxine ER blocks the norepinephrine transporter in the brain of patients with major depressive disorder: a PET study using [18F]FMeNER-D2. Int J. Neuropsychopharmacol. 22 , 278–285 (2019).
Gould, G. G., Javors, M. A. & Frazer, A. Effect of chronic administration of duloxetine on serotonin and norepinephrine transporter binding sites in rat brain. Biol. Psychiatry 61 , 210–215 (2007).
Owens, M. J. et al. Estimates of serotonin and norepinephrine transporter inhibition in depressed patients treated with paroxetine or venlafaxine. Neuropsychopharmacology 33 , 3201–3212 (2008).
Sramek, J. J. et al. Exploratory biomarker study of the triple reuptake inhibitor sep-432 compared to the dual reuptake inhibitor duloxetine in healthy normal subjects. CNS Neurosci. Ther. 22 , 404–412 (2016).
Wang, Y. et al. The association between antidepressant treatment and brain connectivity in two double-blind, placebo-controlled clinical trials: a treatment mechanism study. Lancet Psychiatry 6 , 667–674 (2019).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47 , 351–354 (2000).
Murrough, J. W. et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170 , 1134–1142 (2013).
Zanos, P. et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533 , 481–486 (2016).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 , 959–964 (2010).
Li, N. et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 69 , 754–761 (2011).
Beurel, E., Grieco, S. F., Amadei, C., Downey, K. & Jope, R. S. Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling. Bipolar Disord. 18 , 473–480 (2016).
Miller, O. H., Moran, J. T. & Hall, B. J. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 100 , 17–26 (2016).
Mingardi, J. et al. miR-9-5p is involved in the rescue of stress-dependent dendritic shortening of hippocampal pyramidal neurons induced by acute antidepressant treatment with ketamine. Neurobiol. Stress 15 , 100381 (2021).
Krystal, J. H., Charney, D. S. & Duman, R. S. A new rapid-acting antidepressant. Cell 181 , 7 (2020).
Moliner, R. et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 26 , 1032–1041 (2023).
Halberstadt, A. L., Koedood, L., Powell, S. B. & Geyer, M. A. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 25 , 1548–1561 (2011).
Hesselgrave, N., Troppoli, T. A., Wulff, A. B., Cole, A. B. & Thompson, S. M. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl Acad. Sci. USA 118 , e2022489118 (2021).
Cao, D. et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375 , 403–411 (2022).
Shao, L. X. et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109 , 2535–2544 e2534 (2021).
Caraci, F. et al. Neurobiological links between depression and AD: The role of TGF-beta1 signaling as a new pharmacological target. Pharmacol. Res 130 , 374–384 (2018).
Chen, B., Wang, J. F., Sun, X. & Young, L. T. Regulation of GAP-43 expression by chronic desipramine treatment in rat cultured hippocampal cells. Biol. Psychiatry 53 , 530–537 (2003).
Lee, K. M. & Kim, Y. K. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int. Immunopharmacol. 6 , 1298–1304 (2006).
Sutcigil, L. et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin. Dev. Immunol. 2007 , 76396 (2007).
Zhao, Y. N. et al. Nelumbo nucifera gaertn stems (Hegeng) improved depression Behavior in CUMS mice by regulating NCAM and GAP-43 expression. Evid. Based Complement Altern. Med 2020 , 3056954 (2020).
Zavvari, F., Nahavandi, A. & Goudarzi, M. Fluoxetine attenuates stress-induced depressive-like behavior through modulation of hippocampal GAP43 and neurogenesis in male rats. J. Chem. Neuroanat. 103 , 101711 (2020).
Wang, Q., Timberlake, M. A. 2nd, Prall, K. & Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 77 , 99–109 (2017).
Aten, S. et al. Chronic stress impairs the structure and function of astrocyte networks in an animal model of depression. Neurochem. Res. 48 , 1191–1210 (2023).
Liang, S. et al. Iron aggravates the depressive phenotype of stressed mice by compromising the glymphatic system. Neurosci. Bull. 36 , 1542–1546 (2020).
Antoniuk, S., Bijata, M., Ponimaskin, E. & Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci. Biobehav Rev. 99 , 101–116 (2019).
Fernandez, D. C. et al. Light affects mood and learning through distinct retina-brain pathways. Cell 175 , 71–84.e18 (2018).
Smolders, K. C., de Kort, Y. A. & Cluitmans, P. J. A higher illuminance induces alertness even during office hours: findings on subjective measures, task performance and heart rate measures. Physiol. Behav. 107 , 7–16 (2012).
Liu, A. et al. Encoding of environmental illumination by primate melanopsin neurons. Science 379 , 376–381 (2023).
Lam, R. W. et al. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry 73 , 56–63 (2016).
Rutten, S. et al. Bright light therapy for depression in Parkinson disease: a randomized controlled trial. Neurology 92 , e1145–e1156 (2019).
Li, V. W. et al. Functional outcomes with bright light in monotherapy and combined with fluoxetine in patients with major depressive disorder: results from the LIFE-D trial. J. Affect. Disord. 297 , 396–400 (2022).
Guzel Ozdemir, P. et al. Comparison of venlafaxine alone versus venlafaxine plus bright light therapy combination for severe major depressive disorder. J. Clin. Psychiatry 76 , e645–654 (2015).
Lambert, G. W., Reid, C., Kaye, D. M., Jennings, G. L. & Esler, M. D. Effect of sunlight and season on serotonin turnover in the brain. Lancet 360 , 1840–1842 (2002).
Huang, L. et al. A visual circuit related to habenula underlies the antidepressive effects of light therapy. Neuron 102 , 128–142.e128 (2019).
McClintock, S. M. et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatry 79 , 16cs10905 (2018).
Wall, C. A. et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J. Clin. Psychiatry 72 , 1263–1269 (2011).
Qiu, H. et al. Efficacy and safety of repetitive transcranial magnetic stimulation in children and adolescents with depression: a systematic review and preliminary meta-analysis. J. Affect. Disord. 320 , 305–312 (2023).
Mutz, J. et al. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ 364 , l1079 (2019).
Faller, J. et al. Daily prefrontal closed-loop repetitive transcranial magnetic stimulation (rTMS) produces progressive EEG quasi-alpha phase entrainment in depressed adults. Brain Stimul. 15 , 458–471 (2022).
Kaster, T. S. et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology 43 , 2231–2238 (2018).
Desbeaumes Jodoin, V., Miron, J. P. & Lesperance, P. Safety and efficacy of accelerated repetitive transcranial magnetic stimulation protocol in elderly depressed unipolar and bipolar patients. Am. J. Geriatr. Psychiatry 27 , 548–558 (2019).
Wathra, R. A. et al. Effect of prior pharmacotherapy on remission with sequential bilateral theta-burst versus standard bilateral repetitive transcranial magnetic stimulation in treatment-resistant late-life depression. Br. J. Psychiatry 223 , 504–506 (2023).
Cox, E. Q. et al. Repetitive transcranial magnetic stimulation for the treatment of postpartum depression. J. Affect. Disord. 264 , 193–200 (2020).
Pascual-Leone, A., Rubio, B., Pallardo, F. & Catala, M. D. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 348 , 233–237 (1996).
Zrenner, B. et al. Brain oscillation-synchronized stimulation of the left dorsolateral prefrontal cortex in depression using real-time EEG-triggered TMS. Brain Stimul. 13 , 197–205 (2020).
Fitzgerald, P. B. Targeting repetitive transcranial magnetic stimulation in depression: do we really know what we are stimulating and how best to do it? Brain Stimul. 14 , 730–736 (2021).
Rosen, A. C. et al. Targeting location relates to treatment response in active but not sham rTMS stimulation. Brain Stimul. 14 , 703–709 (2021).
Moffitt, T. E. et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol. Med. 40 , 899–909 (2010).
Brini, S. et al. Efficacy and safety of transcranial magnetic stimulation for treating major depressive disorder: An umbrella review and re-analysis of published meta-analyses of randomised controlled trials. Clin. Psychol. Rev. 100 , 102236 (2023).
Qaseem, A., Barry, M. J. & Kansagara, D. Clinical Guidelines Committee of the American College of PhysiciansNonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the american college of physicians. Ann. Intern. Med. 164 , 350–359 (2016).
Biesheuvel-Leliefeld, K. E. et al. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: meta-analysis and meta-regression. J. Affect. Disord. 174 , 400–410 (2015).
Breedvelt, J. J. F. et al. Psychological interventions as an alternative and add-on to antidepressant medication to prevent depressive relapse: systematic review and meta-analysis. Br. J. Psychiatry 219 , 538–545 (2021).
Guidi, J. & Fava, G. A. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: a systematic review and meta-analysis. JAMA Psychiatry 78 , 261–269 (2021).
Tang, Y., Yin, H. Y., Rubini, P., Illes, P. & Acupuncture-Induced Analgesia: a neurobiological basis in purinergic signaling. Neuroscientist 22 , 563–578 (2016).
Chan, Y. Y., Lo, W. Y., Yang, S. N., Chen, Y. H. & Lin, J. G. The benefit of combined acupuncture and antidepressant medication for depression: a systematic review and meta-analysis. J. Affect Disord. 176 , 106–117 (2015).
Mischoulon, D., Brill, C. D., Ameral, V. E., Fava, M. & Yeung, A. S. A pilot study of acupuncture monotherapy in patients with major depressive disorder. J. Affect Disord. 141 , 469–473 (2012).
Yue, N. et al. Electro-acupuncture alleviates chronic unpredictable stress-induced depressive- and anxiety-like behavior and hippocampal neuroinflammation in rat model of depression. Front. Mol. Neurosci. 11 , 149 (2018).
Jung, J. et al. Lipidomics reveals that acupuncture modulates the lipid metabolism and inflammatory interaction in a mouse model of depression. Brain Behav. Immun. 94 , 424–436 (2021).
Lin, S. S. et al. Electroacupuncture prevents astrocyte atrophy to alleviate depression. Cell Death Dis. 14 , 343 (2023).
Wang, Z. et al. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J. Psychiatr. Res. 84 , 18–26 (2017).
Duan, D. M., Tu, Y., Liu, P. & Jiao, S. Antidepressant effect of electroacupuncture regulates signal targeting in the brain and increases brain-derived neurotrophic factor levels. Neural Regen. Res. 11 , 1595–1602 (2016).
Bai, L. et al. Mechanisms underlying the antidepressant effect of acupuncture via the CaMK signaling pathway. Front Behav. Neurosci. 14 , 563698 (2020).
Cai, X. et al. Electroacupuncture alleviated depression-like behaviors in ventromedial prefrontal cortex of chronic unpredictable mild stress-induced rats: Increasing synaptic transmission and phosphorylating dopamine transporter. CNS Neurosci. Ther. 29 , 2608–2620 (2023).
Xu, G. et al. Clinical evidence for association of acupuncture with improved major depressive disorder: a systematic review and meta-analysis of randomized control trials. Neuropsychobiology 82 , 1–13 (2023).
Ding, Y. et al. Molecular and genetic characterization of depression: overlap with other psychiatric disorders and aging. Mol. Neuropsychiatry 1 , 1–12 (2015).
PubMed PubMed Central Google Scholar
Kay, R. B. & Brunjes, P. C. Diversity among principal and GABAergic neurons of the anterior olfactory nucleus. Front. Cell. Neurosci. 8 , 111 (2014).
Karolewicz, B. et al. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int. J. Neuropsychopharmacol. 13 , 411–420 (2010).
Azmitia, E. C. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology 21 , 33S–45S (1999).
Kerman, I. A. et al. Evidence for transcriptional factor dysregulation in the dorsal raphe nucleus of patients with major depressive disorder. Front. Neurosci. 6 , 135 (2012).
Keller-Wood, M. Hypothalamic-pituitary–adrenal axis-feedback control. Compr. Physiol. 5 , 1161–1182 (2015).
Alt, S. R. et al. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology 35 , 544–556 (2010).
van Loo, H. M., Aggen, S. H., Gardner, C. O. & Kendler, K. S. Sex similarities and differences in risk factors for recurrence of major depression. Psychol. Med. 48 , 1685–1693 (2018).
Salk, R. H., Hyde, J. S. & Abramson, L. Y. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 143 , 783–822 (2017).
Cavanagh, A., Wilson, C. J., Kavanagh, D. J. & Caputi, P. Differences in the expression of symptoms in men versus women with depression: a systematic review and meta-analysis. Harv. Rev. Psychiatry 25 , 29–38 (2017).
Marcus, S. M. et al. Gender differences in depression: findings from the STAR*D study. J. Affect Disord. 87 , 141–150 (2005).
Poulter, M. O. et al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol. Psychiatry 64 , 645–652 (2008).
Seney, M. L. et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front. Psychiatry 4 , 104 (2013).
Gray, A. L., Hyde, T. M., Deep-Soboslay, A., Kleinman, J. E. & Sodhi, M. S. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol. Psychiatry 20 , 1057–1068 (2015).
Goswami, D. B., May, W. L., Stockmeier, C. A. & Austin, M. C. Transcriptional expression of serotonergic regulators in laser-captured microdissected dorsal raphe neurons of subjects with major depressive disorder: sex-specific differences. J. Neurochem. 112 , 397–409 (2010).
Szewczyk, B. et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int. J. Neuropsychopharmacol. 12 , 155–168 (2009).
Fiori, L. M. et al. miR-323a regulates ERBB4 and is involved in depression. Mol. Psychiatry 26 , 4191–4204 (2021).
Roy, B., Wang, Q., Palkovits, M., Faludi, G. & Dwivedi, Y. Altered miRNA expression network in locus coeruleus of depressed suicide subjects. Sci. Rep. 7 , 4387 (2017).
Zheng, P. et al. Identification of sex-specific urinary biomarkers for major depressive disorder by combined application of NMR- and GC-MS-based metabonomics. Transl. Psychiatry 6 , e955 (2016).
Liu, X. et al. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J. Proteome Res. 14 , 2322–2330 (2015).
Liu, X. et al. Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal. Bioanal. Chem. 408 , 6497–6507 (2016).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet 10 , 57–63 (2009).
Xu, Y. et al. Serum cytokines-based biomarkers in the diagnosis and monitoring of therapeutic response in patients with major depressive disorder. Int. Immunopharmacol. 118 , 110108 (2023).
Pantazatos, S. P. et al. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatry 22 , 760–773 (2017).
Herbet, M. et al. Altered expression of genes involved in brain energy metabolism as adaptive responses in rats exposed to chronic variable stress; changes in cortical level of glucogenic and neuroactive amino acids. Mol. Med. Rep. 19 , 2386–2396 (2019).
Yamanishi, K. et al. Analysis of genes linked to depressive-like behaviors in interleukin-18-deficient mice: gene expression profiles in the brain. Biomed. Rep. 12 , 3–10 (2020).
Kuperman, Y. et al. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc. Natl Acad. Sci. USA 107 , 8393–8398 (2010).
Bustamante, A. C., Armstrong, D. L. & Uddin, M. Epigenetic profiles associated with major depression in the human brain. Psychiatry Res. 260 , 439–442 (2018).
Beger, R. D. et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics 12 , 149 (2016).
Kaddurah-Daouk, R. et al. Cerebrospinal fluid metabolome in mood disorders-remission state has a unique metabolic profile. Sci. Rep. 2 , 667 (2012).
Schmaal, L. et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22 , 900–909 (2017).
Schmitgen, M. M. et al. Aberrant cortical neurodevelopment in major depressive disorder. J. Affect. Disord. 243 , 340–347 (2019).
Chen, Z. et al. High-field magnetic resonance imaging of structural alterations in first-episode, drug-naive patients with major depressive disorder. Transl. Psychiatry 6 , e942 (2016).
Grimm, S. et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum. Brain Mapp. 30 , 2617–2627 (2009).
Yoshimura, S. et al. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Soc. Cogn. Affect. Neurosci. 9 , 487–493 (2014).
Yu, H. et al. Functional brain abnormalities in major depressive disorder using the Hilbert-Huang transform. Brain Imaging Behav. 12 , 1556–1568 (2018).
Holmes, S. E. et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 10 , 1529 (2019).
Bettini, E. et al. Pharmacological comparative characterization of REL-1017 (Esmethadone-HCl) and Other NMDAR channel blockers in human heterodimeric N-methyl-D-aspartate receptors. Pharmaceuticals (Basel) 15 , 997 (2022).
Hanania, T., Manfredi, P., Inturrisi, C. & Vitolo, O. V. The N-methyl-D-aspartate receptor antagonist d-methadone acutely improves depressive-like behavior in the forced swim test performance of rats. Exp. Clin. Psychopharmacol. 28 , 196–201 (2020).
Fogaca, M. V. et al. N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology 44 , 2230–2238 (2019).
Fava, M. et al. REL-1017 (esmethadone) as adjunctive treatment in patients with major depressive disorder: a phase 2a randomized double-blind trial. Am. J. Psychiatry 179 , 122–131 (2022).
De Martin, S. et al. REL-1017 (Esmethadone) increases circulating BDNF levels in healthy subjects of a phase 1 clinical study. Front. Pharmacol. 12 , 671859 (2021).
Chou, T. H., Kang, H., Simorowski, N., Traynelis, S. F. & Furukawa, H. Structural insights into assembly and function of GluN1-2C, GluN1-2A-2C, and GluN1-2D NMDARs. Mol. Cell 82 , 4548–4563.e4544 (2022).
Hochschild, A. et al. Ketamine vs midazolam: Mood improvement reduces suicidal ideation in depression. J. Affect Disord. 300 , 10–16 (2022).
Burgdorf, J. et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38 , 729–742 (2013).
Gigliucci, V. et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl.) 228 , 157–166 (2013).
Daly, E. J. et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75 , 139–148 (2018).
Zhao, J. et al. Low-dose ketamine inhibits neuronal apoptosis and neuroinflammation in PC12 cells via alpha7nAChR mediated TLR4/MAPK/NF-kappaB signaling pathway. Int. Immunopharmacol. 117 , 109880 (2023).
Yang, Y. et al. Ketamine relieves depression-like behaviors induced by chronic postsurgical pain in rats through anti-inflammatory, anti-oxidant effects and regulating BDNF expression. Psychopharmacology (Berl.) 237 , 1657–1669 (2020).
Shibakawa, Y. S. et al. Effects of ketamine and propofol on inflammatory responses of primary glial cell cultures stimulated with lipopolysaccharide. Br. J. Anaesth. 95 , 803–810 (2005).
Abbasi, S., Hosseini, F., Modabbernia, A., Ashrafi, M. & Akhondzadeh, S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J. Affect. Disord. 141 , 308–314 (2012).
Akhondzadeh, S. et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety 26 , 607–611 (2009).
Nettis, M. A. et al. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology 46 , 939–948 (2021).
Hasebe, K. et al. Exploring interleukin-6, lipopolysaccharide-binding protein and brain-derived neurotrophic factor following 12 weeks of adjunctive minocycline treatment for depression. Acta Neuropsychiatr. 34 , 220–227 (2022).
Su, K. et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from arandomized, controlled trial. Biol. Psychiatry 76 , 559–566 (2014).
Berk, M. et al. Youth Depression Alleviation with Anti-inflammatory Agents (YoDA-A): a randomised clinical trial of rosuvastatin and aspirin. BMC Med . 18 , 16 (2020).
Meltzer-Brody, S. et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392 , 1058–1070 (2018).
Leal, G. C. et al. Arketamine as adjunctive therapy for treatment-resistant depression: A placebo-controlled pilot study. J Affect. Disord. 330 , 7–15 (2023).
Kuga, N., Sasaki, T., Takahara, Y., Matsuki, N. & Ikegaya, Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 31 , 2607–2614 (2011).
Shawcross, D. L. et al. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J. Hepatol. 54 , 640–649 (2011).
Page, G. et al. The up-regulation of the striatal dopamine transporter’s activity by cAMP is PKA-, CaMK II- and phosphatase-dependent. Neurochem. Int. 45 , 627–632 (2004).
França, A. S. et al. D2 dopamine receptor regulation of learning, sleep and plasticity. Eur. Neuropsychopharmacol. 25 , 493–504 (2015).
Beaulieu, J. M. & Gainetdinov, R. R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 63 , 182–217 (2011).
Opal, M. D. et al. Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol. Psychiatry 19 , 1106–1114 (2014).
Seki, K., Yoshida, S. & Jaiswal, M. K. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regen. Res. 13 , 1159–1169 (2018).
Duman, R. S., Aghajanian, G. K., Sanacora, G. & Krystal, J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22 , 238–249 (2016).
Yang, B. et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology 233 , 3647–3657 (2016).
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China, MX [grant number 32271038] and BL [grant number 81871852]; Shenyang Science and Technology Innovation Talents Project, BL [grant number RC210251]; ‘ChunHui’ Program of Education Ministry, BL [grant number 2020703]; National Natural Science Foundation of China-Russian Science Foundation (NSFC-RSF), YT [grant number 82261138557]; Sichuan Provincial Administration of Traditional Chinese Medicine, YT [grant number 2023zd024].
Author information
Authors and affiliations.
Department of Forensic Analytical Toxicology, School of Forensic Medicine, China Medical University, Shenyang, China
Lulu Cui, Shu Li, Siman Wang, Xiafang Wu, Yingyu Liu, Weiyang Yu, Yijun Wang & Baoman Li
Liaoning Province Key Laboratory of Forensic Bio-evidence Sciences, Shenyang, China
China Medical University Centre of Forensic Investigation, Shenyang, China
International Joint Research Centre on Purinergic Signalling/Key Laboratory of Acupuncture for Senile Disease (Chengdu University of TCM), Ministry of Education/School of Health and Rehabilitation, Chengdu University of Traditional Chinese Medicine/Acupuncture and Chronobiology Key Laboratory of Sichuan Province, Chengdu, China
Department of Orthopaedics, The First Hospital, China Medical University, Shenyang, China
Maosheng Xia
You can also search for this author in PubMed Google Scholar
Contributions
L.C., X.W., and B.L. provided direction and guidance throughout the preparation of this manuscript. L.C., X.W., and B.L. wrote and edited the manuscript. L.C., S.L., S.W., M.X., and B.L. reviewed and made significant revisions to the manuscript. L.C., S.L., S.W., X.W., Y.L., W.Y., Y.W., Y.T., M.X., and B.L. collected and prepared the related papers. All authors have read and approved the article.
Corresponding authors
Correspondence to Maosheng Xia or Baoman Li .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cui, L., Li, S., Wang, S. et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Sig Transduct Target Ther 9 , 30 (2024). https://doi.org/10.1038/s41392-024-01738-y
Download citation
Received : 20 September 2023
Revised : 24 December 2023
Accepted : 28 December 2023
Published : 09 February 2024
DOI : https://doi.org/10.1038/s41392-024-01738-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Association between neutrophil-to-lymphocyte ratio and specific depressive symptoms: an analysis of a population-based cross-sectional survey.
- Moshui Shan
- Shuhua Wang
BMC Psychiatry (2024)
The influence of an emotion regulation intervention on challenges in emotion regulation and cognitive strategies in patients with depression
- Mohamed Hussein Ramadan Atta
- Mervat Mostafa El-Gueneidy
- Ola Ahmed Rashad Lachine
BMC Psychology (2024)
Peer-led intervention for individuals with major depression: study protocol for a randomized controlled trial (SUPEERMood)
- Xandra Gonzalez-Garcia
- M. Lucia Moreno-Sancho
- Aina M. Yañez
Association between depression and infertility risk among American women aged 18–45 years: the mediating effect of the NHHR
- QiaoRui Yang
- ZhenLiang Fan
Lipids in Health and Disease (2024)
Investigating the modulatory effects of lactoferrin on depressed rats through 16S rDNA gene sequencing and LC–MS metabolomics analysis
- Hongmei Xin
- Rilebagen Hu
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
New hypothesis and treatment targets of depression: an integrated view of key findings
Shangli cai, shucai huang.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2014 Jul 10; Accepted 2014 Oct 5; Collection date 2015 Feb.
Major depressive disorder (MDD) is a common and devastating psychiatric disorder characterized by persistent low mood, cognitive disorder, and impaired social function. Despite its complex mechanisms, increasing evidence has identified the involvement of neurotrophic factors, inflammatory cytokines, the hypothalamus-pituitary-adrenal axis, and glutamate receptors in the pathophysiology of this illness. The present review synthesizes recent research achievements to define the network between different hypotheses of MDD and to understand which part is most pivotal for its pathogenesis. By integrating MDD-related signal pathways, we highlight brain-derived neurotrophic factor (BDNF) dysfunction and increased apoptosis as the final common cascades, and new therapeutic strategies aiming to enhance BDNF function have been shown to exert a rapid and effective antidepressant action.
Keywords: depression, BDNF, cytokines, hypothalamus-pituitary-adrenal axis, glutamate receptor
- [1]. Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- [2]. Mathers C, Fat DM, Boerma JT. The global burden of disease: 2004 update. 2008. [ Google Scholar ]
- [3]. Brundtland GH. From the World Health Organization. Mental health: new understanding, new hope. JAMA. 2001;286:2391. doi: 10.1001/jama.286.19.2391. [ DOI ] [ PubMed ] [ Google Scholar ]
- [4]. Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, et al. Innovative approaches for the development of antidepressant drugs: current and future strategies. NeuroRx. 2005;2:590–611. doi: 10.1602/neurorx.2.4.590. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [5]. Block SG, Nemeroff CB. Emerging antidepressants to treat major depressive disorder. Asian J Psychiatr. 2014;12C:7–16. doi: 10.1016/j.ajp.2014.09.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- [6]. Montes JM, Ferrando L, Saiz-Ruiz J. Remission in major depression with two antidepressant mechanisms: results from a naturalistic study. J Affect Disord. 2004;79:229–234. doi: 10.1016/S0165-0327(02)00353-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- [7]. Bortolozzi A, Castane A, Semakova J, Santana N, Alvarado G, Cortes R, et al. New antidepressant strategy based on acute siRNA silencing of 5-HT1A autoreceptors. Mol Psychiatry. 2012;17:567. doi: 10.1038/mp.2012.52. [ DOI ] [ PubMed ] [ Google Scholar ]
- [8]. Assie MB, Lomenech H, Ravailhe V, Faucillon V, Newman-Tancredi A. Rapid desensitization of somatodendritic 5-HT1A receptors by chronic administration of the high-efficacy 5-HT1A agonist, F13714: a microdialysis study in the rat. Br J Pharmacol. 2006;149:170–178. doi: 10.1038/sj.bjp.0706859. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [9]. Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012;367:2475–2484. doi: 10.1098/rstb.2011.0357. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [10]. Neto FL, Borges G, Torres-Sanchez S, Mico JA, Berrocoso E. Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol. 2011;9:530–552. doi: 10.2174/157015911798376262. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [11]. Holtzman DM, Mobley WC. Neurotrophic factors and neurologic disease. West J Med. 1994;161:246–254. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [12]. Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120:87–95. doi: 10.1016/S0166-4328(00)00364-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- [13]. Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/S0165-1781(02)00005-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- [14]. Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/S0006-3223(00)00935-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- [15]. Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [16]. Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- [17]. Sarchiapone M, Carli V, Roy A, Iacoviello L, Cuomo C, Latella MC, et al. Association of polymorphism (Val66Met) of brain-derived neurotrophic factor with suicide attempts in depressed patients. Neuropsychobiology. 2008;57:139–145. doi: 10.1159/000142361. [ DOI ] [ PubMed ] [ Google Scholar ]
- [18]. Dwivedi Y. Brain-derived neurotrophic factor in suicide pathophysiology. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press; 2012. [ PubMed ] [ Google Scholar ]
- [19]. Kanellopoulos D, Gunning FM, Morimoto SS, Hoptman MJ, Murphy CF, Kelly RE, et al. Hippocampal volumes and the brain-derived neurotrophic factor val66met polymorphism in geriatric major depression. Am J Geriatr Psychiatry. 2011;19:13–22. doi: 10.1097/JGP.0b013e3181f61d62. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [20]. Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153(Suppl1):S310–324. doi: 10.1038/sj.bjp.0707509. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [21]. Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/S0165-6147(99)01309-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- [22]. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- [23]. Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [ DOI ] [ PubMed ] [ Google Scholar ]
- [24]. Lu B. BDNF an d activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [25]. Duncan WC, Jr., Zarate CA., Jr. Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep. 2013;15:394. doi: 10.1007/s11920-013-0394-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [26]. Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [27]. Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–139. doi: 10.1093/sleep/30.2.129. [ DOI ] [ PubMed ] [ Google Scholar ]
- [28]. Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–28. doi: 10.1016/j.biopsych.2012.05.031. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [29]. Bachmann V, Klein C, Bodenmann S, Schafer N, Berger W, Brugger P, et al. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep. 2012;35:335–344. doi: 10.5665/sleep.1690. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [30]. Barker PA. Whither proBDNF? Nat Neurosci. 2009;12:105–106. doi: 10.1038/nn0209-105. [ DOI ] [ PubMed ] [ Google Scholar ]
- [31]. Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- [32]. Kikuchi-Utsumi K, Nakaki T. Chronic treatment with a selective ligand for the sigma-1 receptor chaperone, SA4503, up-regulates BDNF protein levels in the rat hippocampus. Neurosci Lett. 2008;440:19–22. doi: 10.1016/j.neulet.2008.05.055. [ DOI ] [ PubMed ] [ Google Scholar ]
- [33]. Fujimoto M, Hayashi T, Urfer R, Mita S, Su TP. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse. 2012;66:630–639. doi: 10.1002/syn.21549. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [34]. Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [35]. Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [36]. Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav. 1998;61:247–252. doi: 10.1016/S0091-3057(98)00093-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- [37]. Kobayashi T, Matsuno K, Murai M, Mita S. Sigma 1 receptor subtype is involved in the facilitation of cortical dopaminergic transmission in the rat brain. Neurochem Res. 1997;22:1105–1109. doi: 10.1023/A:1027361101419. [ DOI ] [ PubMed ] [ Google Scholar ]
- [38]. Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/S0306-4522(97)00268-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- [39]. Kulkarni SK, Dhir A. sigma-1 receptors in major depression and anxiety. Expert Rev Neurother. 2009;9:1021–1034. doi: 10.1586/ern.09.40. [ DOI ] [ PubMed ] [ Google Scholar ]
- [40]. Han QQ, Yu J. Inflammation: a mechanism of depression? Neurosci Bull. 2014;30:515–523. doi: 10.1007/s12264-013-1439-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [41]. Hashmi AM, Butt Z, Umair M. Is depression an inflammatory condition? A review of available evidence. J Pak Med Assoc. 2013;63:899–906. [ PubMed ] [ Google Scholar ]
- [42]. Anisman H, Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Sci Signal. 2012;5:pe45. doi: 10.1126/scisignal.2003579. [ DOI ] [ PubMed ] [ Google Scholar ]
- [43]. Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102–111. doi: 10.1016/j.pnpbp.2013.09.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- [44]. Spedding M, Gressens P. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp. 2008;289:222–233. doi: 10.1002/9780470751251.ch18. [ DOI ] [ PubMed ] [ Google Scholar ]
- [45]. Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [ DOI ] [ PubMed ] [ Google Scholar ]
- [46]. Na KS, Lee KJ, Lee JS, Cho YS, Jung HY. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:79–85. doi: 10.1016/j.pnpbp.2013.09.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- [47]. Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [48]. Li WZ, Li WP, Yao YY, Zhang W, Yin YY, Wu GC, et al. Glucocorticoids increase impairments in learning and memory due to elevated amyloid precursor protein expression and neuronal apoptosis in 12-month old mice. Eur J Pharmacol. 2010;628:108–115. doi: 10.1016/j.ejphar.2009.11.045. [ DOI ] [ PubMed ] [ Google Scholar ]
- [49]. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [50]. Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett. 2008;432:232–236. doi: 10.1016/j.neulet.2007.12.047. [ DOI ] [ PubMed ] [ Google Scholar ]
- [51]. Maciejewski PK, Rothman DL. Proposed cycles for functional glutamate trafficking in synaptic neurotransmission. Neurochem Int. 2008;52:809–825. doi: 10.1016/j.neuint.2007.09.015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [52]. Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant’Anna M, Cunha AB, Post RM. Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Rev Bras Psiquiatr. 2008;30:243–245. doi: 10.1590/S1516-44462008000300011. [ DOI ] [ PubMed ] [ Google Scholar ]
- [53]. Lee B, Cao R, Choi YS, Cho HY, Rhee AD, Hah CK, et al. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J Neurochem. 2009;108:1251–1265. doi: 10.1111/j.1471-4159.2008.05864.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [54]. Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [ DOI ] [ PubMed ] [ Google Scholar ]
- [55]. Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [56]. Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [ DOI ] [ PubMed ] [ Google Scholar ]
- [57]. Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- [58]. Koike H, Fukumoto K, Iijima M, Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res. 2013;238:48–52. doi: 10.1016/j.bbr.2012.10.023. [ DOI ] [ PubMed ] [ Google Scholar ]
- [59]. Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev Neurother. 2011;11:33–36. doi: 10.1586/ern.10.176. [ DOI ] [ PubMed ] [ Google Scholar ]
- [60]. Monteggia LM, Gideons E, Kavalali ET. The role o f eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry. 2013;73:1199–1203. doi: 10.1016/j.biopsych.2012.09.006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [61]. Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [62]. Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW, Luo ZJ. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/beta-catenin signaling pathway. J Neurosci Res. 2013;91:30–41. doi: 10.1002/jnr.23138. [ DOI ] [ PubMed ] [ Google Scholar ]
- [63]. Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [ DOI ] [ PubMed ] [ Google Scholar ]
- [64]. Zugno AI, Juliao RF, Budni J, Volpato AM, Fraga DB, Pacheco FD, et al. Rivastigmine reverses cognitive deficit and acetylcholinesterase activity induced by ketamine in an animal model of schizophrenia. Metab Brain Dis. 2013;28:501–508. doi: 10.1007/s11011-013-9417-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- [65]. Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T, et al. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology. 2013;65:29–38. doi: 10.1016/j.neuropharm.2012.09.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- [66]. Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol. 2012;15:429–434. doi: 10.1017/S1461145711001702. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [67]. Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacol. 2013;38:729–742. doi: 10.1038/npp.2012.246. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [68]. Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014;23:243–254. doi: 10.1517/13543784.2014.852536. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [69]. Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/S0889-1591(02)00021-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- [70]. Kumamaru E, Numakawa T, Adachi N, Kunugi H. Glucocorticoid suppresses BDNF-stimulated MAPK/ERK pathway via inhibiting interaction of Shp2 with TrkB. FEBS Lett. 2011;585:3224–3228. doi: 10.1016/j.febslet.2011.09.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- [71]. Wosiski-Kuhn M, Erion JR, Gomez-Sanchez EP, Gomez-Sanchez CE, Stranahan AM. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology. 2014;42:165–177. doi: 10.1016/j.psyneuen.2014.01.020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [72]. Zhang J, Fan Y, Li Y, Zhu H, Wang L, Zhu MY. Chronic social defeat up-regulates expression of the serotonin transporter in rat dorsal raphe nucleus and projection regions in a glucocorticoid-dependent manner. J Neurochem. 2012;123:1054–1068. doi: 10.1111/jnc.12055. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [73]. Kling MA, Coleman VH, Schulkin J. Glucocorticoid inhibition in the treatment of depression: can we think outside the endocrine hypothalamus? Depress Anxiety. 2009;26:641–649. doi: 10.1002/da.20546. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [74]. Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- [75]. Jing L, Bu M. Role of macrophage migration inhibitory factor in glucocorticoid release and glucocorticoid receptor function in rats. Ann Clin Lab Sci. 2011;41:14–19. [ PubMed ] [ Google Scholar ]
- [76]. Edwards KM, Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Elevated macrophage migration inhibitory factor (MIF) is associated with depressive symptoms, blunted cortisol reactivity to acute stress, and lowered morning cortisol. Brain Behav Immun. 2010;24:1202–1208. doi: 10.1016/j.bbi.2010.03.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- [77]. Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison K J, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacol. 2013;38:377–385. doi: 10.1038/npp.2012.191. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [78]. Conboy L, Varea E, Castro JE, Sakouhi-Ouertatani H, Calandra T, Lashuel HA, et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol Psychiatry. 2011;16:533–547. doi: 10.1038/mp.2010.15. [ DOI ] [ PubMed ] [ Google Scholar ]
- [79]. Moon HY, Kim SH, Yang YR, Song P, Yu HS, Park HG, et al. Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise. Proc Natl Acad Sci U S A. 2012;109:13094–13099. doi: 10.1073/pnas.1205535109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [80]. Quera Salva MA, Hartley S, Barbot F, Alvarez JC, Lofaso F, Guilleminault C. Circadian rhythms, melatonin and depression. Curr Pharm Des. 2011;17:1459–1470. doi: 10.2174/138161211796197188. [ DOI ] [ PubMed ] [ Google Scholar ]
- [81]. Murray G. Diurnal mood variation in depression: a signal of disturb ed circadian function? J Affect Disord. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- [82]. Suchecki D, Tiba PA, Machado RB. REM Sleep Rebound as an Adaptive Response to Stressful Situations. Front Neurol. 2012;3:41. doi: 10.3389/fneur.2012.00041. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [83]. Beck-Friis J, Kjellman BF, Aperia B, Unden F, von Rosen D, Ljunggren JG, et al. Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome. Acta Psychiatr Scand. 1985;71:319–330. doi: 10.1111/j.1600-0447.1985.tb02531.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- [84]. Khaleghipour S, Masjedi M, Ahade H, Enayate M, Pasha G, Nadery F, et al. Morning and nocturnal serum melatonin rhythm levels in patients with major depressive disorder: an analytical cross-sectional study. Sao Paulo Med J. 2012;130:167–172. doi: 10.1590/S1516-31802012000300006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- [85]. Monteleone P, Martiadis V, Maj M. Circadian rhythms and treatment implications in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1569–1574. doi: 10.1016/j.pnpbp.2010.07.028. [ DOI ] [ PubMed ] [ Google Scholar ]
- [86]. Gulec M, Selvi Y, Boysan M, Aydin A, Besiroglu L, Agargun MY. Ongoing or re-emerging subjective insomnia symptoms after full/partial remission or recovery of major depressive disorder mainly with the selective serotonin reuptake inhibitors and risk of relapse or recurrence: a 52-week follow-up study. J Affect Disord. 2011;134:257–265. doi: 10.1016/j.jad.2011.05.056. [ DOI ] [ PubMed ] [ Google Scholar ]
- [87]. Campino C, Valenzuela FJ, Torres-Farfan C, Reynolds HE, Abarzua-Catalan L, Arteaga E, et al. Melatonin exerts direct inhibitory actions on ACTH responses in the human adrenal gland. Horm Metab Res. 2011;43:337–342. doi: 10.1055/s-0031-1271693. [ DOI ] [ PubMed ] [ Google Scholar ]
- [88]. Konakchieva R, Mitev Y, Almeida OF, Patchev VK. Chronic melatonin treatment counteracts glucocorticoid-induced dysregulation of the hypothalamic-pituitary-adrenal axis in the rat. Neuroendocrinology. 1998;67:171–180. doi: 10.1159/000054312. [ DOI ] [ PubMed ] [ Google Scholar ]
- [89]. Avery D, Lenz M, Landis C. Guidelines for prescribing melatonin. Ann Med. 1998;30:122–130. doi: 10.3109/07853899808999394. [ DOI ] [ PubMed ] [ Google Scholar ]
- [90]. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–585. doi: 10.1002/hup.964. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (765.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet?
Affiliations.
- 1 Paramedicine Discipline, Charles Sturt University, Bathurst Campus, NSW Australia. Electronic address: [email protected].
- 2 Paramedicine Discipline, Charles Sturt University, Bathurst Campus, NSW Australia.
- 3 Brain Injury Rehabilitation Service, Westmead Hospital, Hawkesbury Rd, Wentworthville, NSW Australia.
- PMID: 29284108
- DOI: 10.1016/j.bbr.2017.12.025
A number of factors (biogenic amine deficiency, genetic, environmental, immunologic, endocrine factors and neurogenesis) have been identified as mechanisms which provide unitary explanations for the pathophysiology of depression. Rather than a unitary construct, the combination and linkage of these factors have been implicated in the pathogenesis of depression. That is, environmental stressors and heritable genetic factors acting through immunologic and endocrine responses initiate structural and functional changes in many brain regions, resulting in dysfunctional neurogenesis and neurotransmission which then manifest as a constellation of symptoms which present as depression.
Keywords: Depression; Endocrine factors; Environmental factor; Genetic factor; Immunologic factor; Monoamine hypothesis; Neurogenesis; Pathophysiology.
Copyright © 2017 Elsevier B.V. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Biogenic Monoamines / metabolism
- Brain / physiopathology
- Depressive Disorder / physiopathology*
- Models, Neurological
- Neurogenesis / physiology
- Biogenic Monoamines
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Activation of mTOR and Synaptogenesis: Role in the Actions of Rapid-Acting Antidepressants
Jason m dwyer, ronald s duman.
- Author information
- Article notes
- Copyright and License information
Correspondence: Ronald S. Duman 34 Park Street New Haven, CT 06508 Tel: 203-974-7726 [email protected]
Issue date 2013 Jun 15.
Antidepressants that produce rapid and robust effects, particularly for severely ill patients, represent one of the largest unmet medical needs for the treatment of depression. Currently available drugs that modulate monoamine neurotransmission provide relief for only a subset of patients and this minimal efficacy requires several weeks of chronic treatment. The recent discovery that the glutamatergic agent ketamine produces rapid antidepressant effects within hours has opened a new area of research to explore the molecular mechanisms through which ketamine produces these surprising effects. Clinical and preclinical findings have exposed some of ketamine's unique actions and identified a cell-signaling pathway known as the mammalian target of rapamycin (mTOR). Activation of mTOR and increased synaptogenesis in the prefrontal cortex appear to be crucial in mediating the antidepressant effects of ketamine. Importantly, the synaptic actions of ketamine allow rapid recovery from the insults produced by exposure to repeated stress that cause neuronal atrophy and loss of synaptic connections. In the following review, we explore some of the clinical and preclinical findings that have thrust ketamine to the forefront of rapid antidepressant research and unveiled some of its unique molecular and cellular actions.
Keywords: GABA, glutamate, BDNF, lithium, scopolamine, GSK-3
Introduction
Depression affects the lives of over 30 million adults in the US ( 1 ). While currently available antidepressants may provide roughly 60% of these patients with some relief, achieving this clinical response may require 6-12 weeks of chronic pharmacotherapy, and even longer before an effective treatment is identified. Particularly in the case of acutely suicidal patients, this poses a serious concern to patient welfare and presents a critical need for antidepressants that are more efficacious and rapid-acting. Therapeutic drugs currently approved to treat depressive symptoms target the monoaminergic neurotransmitter systems, however, the discovery that the glutamatergic drug ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, produces rapid (within 2 hrs) and long-lasting (~1 week) antidepressant effects in patients that have previously not responded to several courses of typical antidepressants ( 2 , 3 ) has sparked a flurry of research to understand how ketamine produces surprisingly fast and efficacious actions.
Findings in post-mortem tissue of depressed patients as well as in rodent stress models designed to mimic depressive-like symptoms suggest that depression is characterized by neuronal atrophy in the prefrontal cortex (PFC) and hippocampus. Interestingly, ketamine has the unique ability to rapidly reverse these neuronal deficits, an effect that typical antidepressants lack, suggesting that recovery of synaptic connections is critical for a rapid antidepressant response. Recent evidence points toward a signaling cascade involved in regulating protein translation and synaptic plasticity known as the mammalian target of rapamycin (mTOR) in mediating these rapid and robust effects of ketamine, which may represent a mechanism common to other putative rapid antidepressants.
In the following review, we describe clinical and preclinical neuronal alterations observed in depression as well as discuss research demonstrating the molecular signaling changes produced by ketamine that mediate the rapid reversal of neuronal deficits and antidepressant responses. Finally, we will discuss novel treatment strategies that may allow ketamine-like rapid antidepressant effects by targeting other receptor systems.
Chronic stress produces neuronal atrophy and maladaptive impairments of neuroplasticity
Theories of depression suggest that exposure to chronic stress, and the neuronal changes that follow, produce susceptibility to mood disorders by impairing synaptic number and function ( 4 - 6 ). Studies of post-mortem human tissue report decreases in neuronal size in the dorsolateral PFC (dlPFC) ( 7 ), anterior cingulate cortex ( 8 , 9 ), orbitofrontal cortex ( 10 - 12 ), and hippocampus ( 13 ). Alterations in glial density in the PFC and hippocampus have also been observed ( 7 , 9 , 12 ). A recent electron microscopy study demonstrates a significant reduction in the number of synapses in the dlPFC of depressed patients ( 14 ) providing direct evidence for alterations in synaptic structure.
Rodent models using chronic stress exposure to produce depressive-like behavioral states also provide clear evidence of stress-induced neuronal atrophy. Stress or chronic glucocorticoid treatment decreases the number and length of dendritic branches of CA3 pyramidal cells of the hippocampus ( 15 - 17 ). Similar atrophy of glutamatergic pyramidal neuron apical dendrites and decreased spine number are also observed in layers II/III and V of the rodent medial PFC (mPFC) following stress or glucocorticoid treatment ( 18 - 20 ) ( 21 , 22 and Figure 1 ). Similarly, the loss of dendritic spines following chronic stress is accompanied by a loss of key synaptic proteins such as post-synaptic density 95 (PSD-95), the GluR1 subunit of -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and the presynaptic protein Synapsin I ( 23 ). Other brain regions involved in emotional behavior also demonstrate stress-induced changes. For example, dendritic hypertrophy is observed in the amygdala following chronic immobilization stress ( 24 ) and a recent report demonstrates that the manifestation of stress-induced anhedonic behavior is mediated by decreased synaptic excitation in the nucleus accumbens ( 25 ).
Figure 1. Pyramidal neurons in the PFC with spine synapses and influence of stress and ketamine treatments.
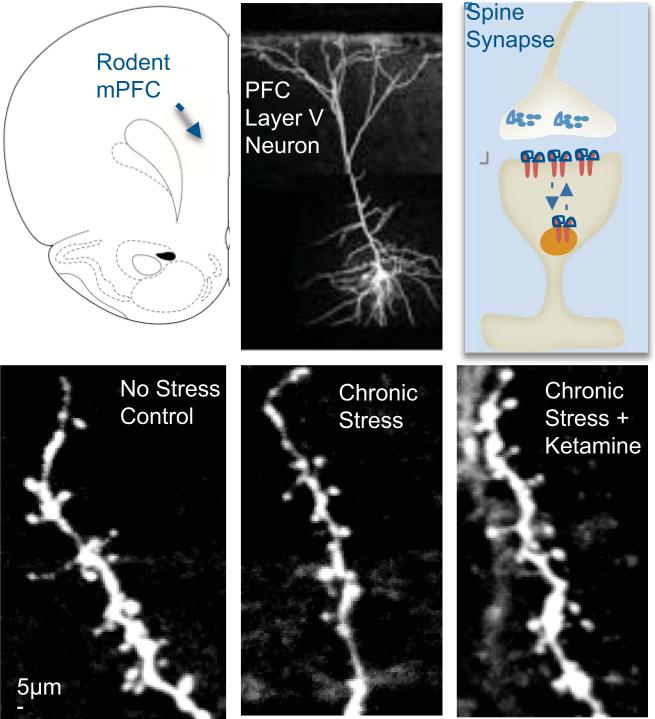
Top panel: shown on the left is a schematic displaying the rat mPFC. In the middle is an image of a neurobiotin-labeled layer V pyramidal neuron from rat mPFC; on the right is a diagram of a spine, with pre- and postsynaptic elements. Bottom panel: the influence of chronic stress exposure (21 d) without or with ketamine administration, compared to non-stressed controls, on apical dendrite spines in layer V pyramidal neurons of rat mPFC. (Adapted from 23).
In addition to neuronal atrophy, chronic stress impairs normal plasticity and some forms of learning and memory. The hippocampus, a brain region that is important for declarative memory, is particularly vulnerable to stress and stress hormones because of the high levels of glucocorticoid receptors in this region. For example, reduced hippocampal volume and reduced neurogenesis are observed following chronic stress (reviewed in 26 ), which also reduces hippocampal long-term potentiation (LTP), a cellular model of memory ( 27 - 29 ). These alterations in synaptic plasticity are accompanied by impairments of memory in rodent models ( 30 - 34 ). The neuronal and behavioral deficits produced by stress strongly implicate impaired plasticity in the etiology of depression. Recent evidence indicates that mTOR, an important kinase that regulates long-term, protein synthesis-dependent forms of synaptic plasticity may be a key mediator capable of reversing these neuronal and behavioral deficits induced by stress exposure.
mTOR regulates protein translation and synaptic plasticity
mTOR is a ubiquitously expressed serine-threonine protein kinase that integrates signals from neuronal activity, growth factors, energy and nutrient levels to regulate rates of protein translation and synaptic plasticity, as well as other cellular functions. mTOR exists in two complexes known as mTORC1 and mTORC2, which are bound to distinct accessory proteins Raptor and Rictor, respectively, and have different substrates (for an extensive review of mTOR signaling see 35 ). Downstream of mTORC1 lie two major substrates, p70S6 kinase (S6K) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1). Activation of S6K by mTOR regulates a number of downstream targets, including the S6 ribosomal subunit, leading to induction of mRNA translation. Phosphorylation of 4E-BP1 by mTOR results in its separation from an inhibitory complex with eukaryotic initiation factor 4E (eIF4E), which allows complete formation of the eIF4F complex and cap-dependent translation.
mTOR activity is regulated by a number of upstream mechanisms that converge on the tuberous sclerosis complex 1/2 (TSC1/2) consisting of hamartin (TSC1) and tuberin (TSC2) proteins. This complex has GTPase activating protein (GAP) activity toward the GTPase Ras homologue enriched in brain (RHEB), shifting RHEB toward the GDP-bound form and reduced mTOR signaling. Upstream phosphorylation of TSC1/2 by extracellular signal-related kinase (ERK) and Protein Kinase B (PKB/Akt) inhibit the TSC1/2 complex and therefore activate mTOR. Conversely, activating phosphorylation by glycogen synthase kinase 3 (GSK-3) leads to increased GAP activity of TSC1/2 and subsequent suppression of mTOR signaling. Thus, mTOR has multiple points of upstream regulation that allow it to integrate signals from a variety of stimuli ( Figure 2 ).
Figure 2. Increases in mTOR signaling and synapse formation in response to treatment with rapid-acting antidepressants.
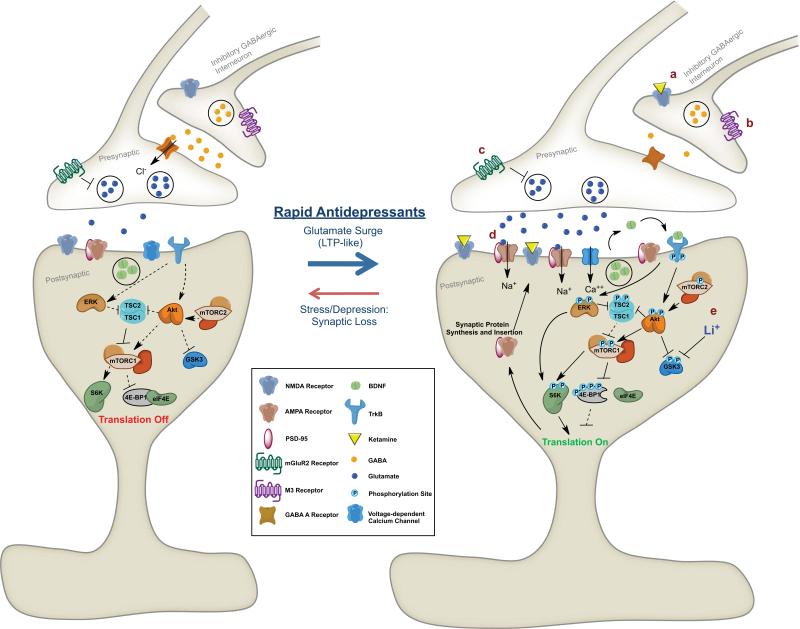
Excitatory synapse before (Left) and after (Right) treatment with rapid-acting antidepressants. Decreases in inhibitory GABAergic signaling from interneurons induced by (a) NMDA receptor blockade or (b) M3 muscarinic receptor blockade lead to increased glutamate release from pyramidal neurons. Blockade of pre-terminal mGluR2 receptors (c) also leads to increased glutamate release. Activation of postsynaptic AMPA receptors by increased glutamate transmission or (d) direct acting Ampakines leads to depolarization, activation of voltage-dependent calcium channels and release of BDNF. BDNF binds TrkB receptors, leading to transphosphorylation and downstream activation of the ERK and Akt pathways and suppression of GSK-3, which can be augmented by (e) Lithium. These signaling events activate mTOR, leading to downstream phosphorylation of mTOR substrates, S6K and 4E-BP1. mTOR signaling activation leads to increase protein translation and synaptogenesis, mediating rapid antidepressant effects. See text for additional details of pathways leading to regulation of mTOR signaling, including the TSC1/2 complex.
Protein translation, especially translation mediated by mTOR signaling plays an important role in long-term synaptic plasticity. mTOR is activated by signals from growth factors such as Brain-Derived Neurotrophic Factor (BDNF) to regulate protein translation and plasticity locally in the synapse ( 36 ). BDNF binds TrkB receptors to activate Ras-MAP Kinase and phophoinositide 3-kinase (PI3K)-Akt pathways, which converge on TSC1/2 to increase mTOR signaling. Treatment of hippocampal slices with the mTORC1 inhibitor rapamycin prevents late-phase, protein synthesis-dependent LTP ( 37 ). Tsc1 heterozygous (+/-) deletion mutant mice exhibit enhanced hippocampal LTP, owing to increased activation of mTOR, and have deficits in spatial and context discrimination learning (most likely as a result of aberrant synaptic activity) that can be reversed by inhibiting mTOR with rapamycin ( 38 ).
Aberrant mTOR signaling is observed in neurological and psychiatric disorders
A large body of evidence implicates mTOR dysregulation in the etiology of various neurological and psychiatric disorders ( 39 ). For example, mTOR signaling is increased in Alzheimer's disease ( 40 ) and is involved in learning deficits observed in tuberous sclerosis ( 38 ). mTOR signaling is altered in patients with Fragile X syndrome ( 41 ), an autism spectrum disorder that is caused by the silencing of the FMR1 gene ( 42 ). The product of FMR1 is the mRNA binding protein Fragile X Mental Retardation Protein (FMRP), which controls the expression of hundreds of mRNAs ( 43 ) and can be regulated by the downstream effector of mTOR, S6K ( 44 ). In a mouse model of Fragile X syndrome, mTOR signaling is increased in the hippocampus and may be an important mediator of this disorder ( 45 ). A recent study reports that mTOR signaling proteins are decreased in postmortem PFC of depressed subjects ( 46 ).
An interesting feature shared by these disorders is that they are due, in part, to impairments of synaptic plasticity. Given it's important role in regulating synaptic protein translation and LTP, mTOR is perfectly positioned to a mediate rapid reversal of synaptic deficits. Indeed, mTOR activation can regulate rapid translation of both PSD-95 ( 47 ) and GluR1 ( 48 ), proteins critical for synaptic function that are decreased by chronic stress ( 23 ). Novel drugs that can regulate mTOR activity could have utility in the treatment of some of these psychiatric and neurological disorders associated with aberrant mTOR signaling, especially depression.
NMDA receptor antagonists activate mTOR signaling, produce mTOR-dependent behavioral responses, and rapidly reverse the effects of stress
Ketamine produces rapid and acute antidepressant-like effects in the rodent forced swim and learned helplessness tests ( 49 - 51 ). Similarly, in non-stressed, naïve rats, systemic treatment with ketamine rapidly increases levels of synaptic proteins (i.e., GluR1, PSD95) and the number and function of excitatory glutamatergic synapses in the PFC ( 51 ). Biochemical studies have discovered that ketamine rapidly activates signaling through the mTOR pathway, as well as downstream substrates of mTOR including, S6K and 4E-BP1 ( 51 ). The NR2B selective compound, Ro 25-6981 produces similar activation of mTOR and its substrates ( 51 ). Ketamine also increases signaling through ERK and Akt ( 51 ), two upstream mTOR regulators that are activated by neurotrophic factors such as BDNF. Importantly, signaling through the mTOR pathway is required for the effects of ketamine as pretreatment with the mTOR inhibitor rapamycin completely abolishes the rapid antidepressant-like activity of ketamine and induction of spine-synapses ( 51 ). Rapamycin specifically inhibits mTORC1 ( 52 ) indicating a primary role for this complex, although mTORC2 may play a role since ketamine induces phosphorylation of Akt at Ser473 ( 51 ), a substrate for mTORC2 ( 53 ).
Interestingly, there is a lack of evidence suggesting that some NMDA receptor antagonists, such as PCP and memantine, produce rapid antidepressant effects in humans. The reason for this discrepancy requires further study, but may be due to the doses tested or off-target effects of these compounds. It is also noteworthy that high, subchronic doses of systemic rapamycin, but not acute treatment, produces antidepressant-like effects in rodents ( 54 ), which may be due to mTORC2 inhibition by prolonged, but not acute rapamycin ( 55 ) or other non-specific pharmacological targets. At present, only systemic ketamine administration has been tested, and future local infusion studies are necessary to determine the specific brain regions that mediate the rapid effects of ketamine.
More recently, studies have focused on the use of rodent chronic stress models that require several weeks of treatment with typical antidepressants to reverse behavioral deficits, recapitulating the therapeutic lag observed in depressed patients. Chronic stress exposure results in anhedonia, a core symptom of depression that is measured by preference for a sweetened solution. In the chronic unpredictable stress (CUS) model, ketamine, as well as the NR2B selective antagonist, Ro 25-6981 produces rapid antidepressant-like behavioral responses within 24 hours of a single treatment, compared to 3 weeks administration of a typical antidepressant ( 23 ).
In addition to producing mTOR-dependent antidepressant-like behavioral responses, ketamine reverses the neuronal atrophy produced by stress. Rats exposed to CUS demonstrate reduced levels of synaptic proteins and number and function of glutamatergic synapses in layer V pyramidal cells in the mPFC ( 21 ). Ketamine rapidly reverses these synaptic deficits within 24 hours in an mTOR-dependent manner ( 23 ). Together, these data demonstrate that NMDA receptor antagonists have the unique ability to rapidly activate mTOR signaling and reverse the impairment of synapses produced by chronic stress exposure.
In contrast to NMDA receptor antagonists, typical antidepressants (e.g., SSRIs or tricyclics) that require several weeks to produce antidepressant effects fail to activate mTOR signaling ( 51 ), indicating that these agents produce antidepressant effects through a different mechanism. There are reports that typical antidepressants can influence synaptic plasticity and related proteins. For example, chronic fluoxetine administration is reported to increase dendritic spine density in the retrosplenial granular and prelimbic cortical regions ( 56 ). Chronic treatment with typical antidepressants can also reverse the neuronal atrophy and decrease in spine density in the hippocampus and PFC produced by chronic stress ( 57 ). Interestingly, other reports found no reversal of stress-induced neuronal atrophy in the hippocampus by typical antidepressants but did find prevention by the atypical antidepressant tianeptine ( 58 ). In ovariectomized female rats, chronic fluoxetine increases synaptic levels of PSD-95 and GluR1, as well as phospho-synapsin ( 59 ). However, these changes in AMPA receptor subunit expression may be due to trafficking of receptors to the synaptic membrane induced by typical antidepressants and not via protein translation ( 60 , 61 ). These data highlight ketamine's unique ability to rapidly activate mTOR signaling, increase synaptic protein synthesis and synaptogenesis, and rapidly reverse the synaptic deficits caused by chronic stress.
Several studies provide insight into the mechanisms through which NMDA receptor antagonists activate mTOR signaling and increase synaptogenesis. Low, sub-anesthetic doses of ketamine preferentially decrease the firing rate of fast-spiking GABAergic interneurons, decreasing inhibitory tone, and increasing extracellular levels of glutamate in the rat PFC ( 62 ). Although acute stress is also reported to increase extracellular glutamate in the PFC ( 63 ) and treatment with typical antidepressants prevents glutamate release induced by acute stress (reviewed in 64 ), the effects of stress may be more long-lasting and widespread than ketamine, which produces rapid but transient effects. Additionally, synaptic and extrasynaptic NMDA receptors can have different effects on signaling pathways. For example, activation of synaptic NMDA receptors can activate ERK whereas extrasynaptic NMDA receptors suppress ERK signaling (for review see 65 ). Thus, it is also possible that the detrimental effects of prolonged glutamate elevation produced by chronic stress are mediated by extrasynaptic NMDA receptors, whereas ketamine's primary effects may be mediated by synaptic NMDA receptors, although this requires further study.
Activation of AMPA receptors is required for the antidepressant-like effects of ketamine ( 50 ) and blocking AMPA receptors prevents the ketamine-induced increase in mTOR signaling ( 51 ). Studies in cultured cells demonstrate that activation of AMPA receptors causes post-synaptic depolarization that opens voltage-dependent calcium channels (VDCCs) leading to calcium influx and release of neurotrophins, including BDNF ( 66 ) ( Figure 2 ).
BDNF is required for the actions of ketamine
Reduced BDNF expression and signaling have long been linked to actions of stress and conversely induction of this factor has been implicated in the response to antidepressants. Electroconvulsive shock and chronic antidepressants increase BDNF expression in the hippocampus and PFC ( 67 ). Furthermore, infusion of BDNF is sufficient to produce antidepressant-like effects in rodents ( 68 , 69 ).
Recent studies have demonstrated the importance of BDNF signaling in the rapid antidepressant effects of ketamine. A human single-nucleotide polymorphism (SNP) that substitutes a methionine for valine (Vall66Met) in the gene coding for BDNF produces impaired trafficking and activity-dependent secretion of BDNF ( 70 ). Val66Met is associated with memory impairments and hippocampal dysfunction in humans ( 70 ). Homozygous mice carrying the Met allele exhibit impaired BDNF secretion and increased anxiety-related behaviors ( 71 ), as well as increased susceptibility to stress and altered response to typical antidepressants ( 72 ). Interestingly, ketamine fails to produce rapid synaptogenic and behavioral responses in Met mice ( 73 ). Importantly, a new study reports that the therapeutic response to ketamine is significantly decreased in depressed patients carrying the Val66Met SNP ( 74 ). Ketamine also rapidly increases BDNF translation and ketamine fails to produce antidepressant-like effects in BDNF conditional deletion mutant mice ( 75 ). Together, these studies suggest an important role for BDNF signaling in the rapid antidepressant effects of ketamine. Studies are needed to determine if BDNF is sufficient to produce a rapid antidepressant-like response in rodent CUS models.
GSK-3 and inhibition of long-term depression
GSK-3 is important for regulating gene expression and synaptic plasticity and is thought to play an important role in depression, as well as other psychiatric illnesses such as schizophrenia ( 76 ). Genetic analyses suggest that SNPs in the GSK-3β gene are associated with occurrence of depression and structural brain changes ( 77 , 78 ). GSK-3 acts on numerous downstream effectors including TSC1/2. Phosphorylation of TSC2 by GSK-3 enhances the suppression of mTOR signaling in cultured cells ( 79 ) and GSK-3 suppresses mTOR signaling in hippocampal tissue ( 80 ). GSK-3 can be controlled by several upstream regulators (e.g., Akt and protein phosphatases), which in turn are controlled by NMDA receptor activation. Phosphorylation by Akt suppresses the activity of GSK-3 and leads to activation of mTOR. A recent study provides evidence that suppression of GSK-3 is required for the rapid antidepressant-like effects of ketamine. Treatment of mice with ketamine rapidly increases phosphorylation of both α and β isoforms of GSK-3, leading to suppressed GSK-3 activity ( 81 ). Mice carrying a mutant form of GSK-3 that prevents phosphorylation are resistant to the antidepressant-like effects of ketamine ( 81 ). The mechanisms through which ketamine inhibits GSK-3 could occur via AMPA receptor activation, BDNF release and activation of Akt signaling leading to decreased GSK-3 activity and activation of mTOR.
In addition, inhibition of GSK-3 could occur via blockade of postsynaptic NMDA receptors that mediate LTD-like effects. LTP and LTD are cellular models for the two major forms of plasticity in the mammalian nervous system ( Figure 3 ). Maintaining the proper balance of these neuronal plasticity processes is important for regulation of neuronal function, and disruption of this delicate balance may contribute to psychiatric disorders. Ketamine increases the number of mature, mushroom-type spines in the PFC, an indication of increased synaptic stabilization and function, and enhances post-synaptic excitatory responses to serotonin and hypocretin ( 51 ). These effects of ketamine are suggestive of an LTP-like mechanism. Conversely, signaling through GSK-3 contributes to LTD mediated by NMDA receptors (NMDA-LTD). During NMDA-LTD, low levels of NMDA receptor stimulation lead to calcium influx and binding of calcium to calmodulin. Calcium/calmodulin activates the phosphatase calcineurin (also known as PP2B) leading to dephosphorylation of Inhibitor-1 (I-1), which suppresses I-1 inhibition of PP1 (i.e., increased PP1 activity) ( 82 ). Dephosphorylation of GSK-3 by PP1 may lead to GSK-3 activation and subsequent induction of LTD. Blockade of NMDA receptors with low doses of ketamine may prevent low-level NMDA receptor signaling and LTD, leading to synaptic bias toward LTP and could contribute to enhanced formation and maturation of excitatory spine synapses ( Figure 3 ).
Figure 3. Signaling mechanisms for the regulation of LTD and LTP: potential site of action for ketamine.
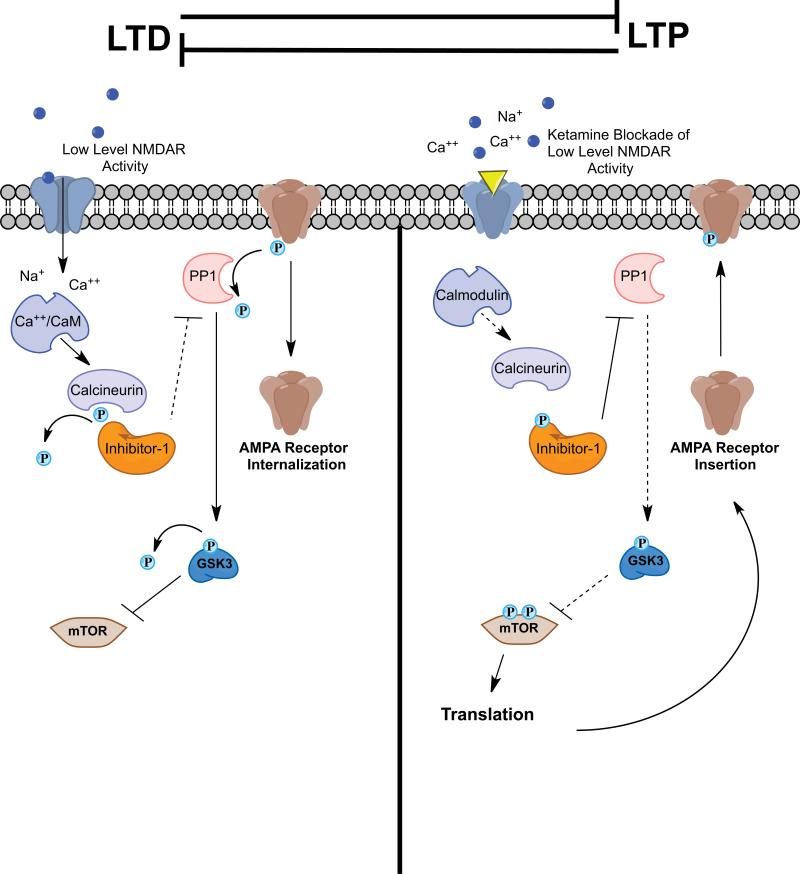
LTP and LTD are distinct and opposing processes. During some forms of LTD, low levels of NMDA receptor activation lead to calcium influx and binding to calmodulin. Calcium/Calmodulin activates calcineurin (PP2B), which dephosphorylates and inactivates Inhibitor-1 leading to loss of PP1 suppression. PP1 dephosphorylates AMPA receptor subunits and can lead to receptor internalization. PP1 also dephosphorylates GSK-3 leading to its activation and suppression of mTOR. Blockade of NMDA receptors by ketamine may prevent low-level NMDA receptor activity and subsequent inhibition of mTOR, ultimately leading to activation of mTOR signaling and a synaptic bias toward LTP.
Novel targets for rapid-acting antidepressants
There is an unmet need for novel antidepressant agents that produce more efficacious and rapid antidepressant actions. Recent studies have demonstrated that putative rapid-acting antidepressants may share ketamine's ability to increase mTOR signaling and rapid reversal of the effects of stress. Some of these mechanisms are discussed below and shown in Figure 2 .
Combination/Continuation Therapies
Ketamine represents a major advance for the treatment of depression, but the psychotomimetic effects and abuse potential limit its widespread use. Therapies that allow the use of lower and more tolerable doses of ketamine combined with a safer antidepressant or a single dose of ketamine followed by another agent may have promise for improvements. One example of a putative combination therapy may be a low-dose ketamine plus lithium. Lithium inhibits GSK-3 activity and in combination with low-dose ketamine may act synergistically to increase mTOR signaling and produce rapid antidepressant effects. Lithium can take several weeks of treatment to be clinically effective, but when combined with ketamine rapidly decreases immobility time in the forced swim test (FST) in mice, similar to a higher dose of ketamine ( 83 ). Given the widespread clinical use of lithium, a ketamine/lithium combination treatment strategy could be quickly evaluated in depressed patients.
As of yet, studies have failed to demonstrate successful prevention of relapse following ketamine and subsequent chronic continuation therapy with safer and better-tolerated agents. For example, treatment with riluzole, a compound that can alter glutamatergic signaling by blocking NMDA receptors ( 84 , 85 ), facilitating glutamate uptake ( 86 ) and increasing membrane AMPA receptor expression ( 87 ), failed to delay relapse when administered following a single dose of ketamine ( 88 , 89 ). Further studies are needed to evaluate additional drugs that could potentially prevent relapse when given following ketamine. It is worth noting, however, that ketamine continues to be effective after repeated treatments (3 × per week for two weeks) in depressed patients ( 90 ), indicating that safer agents could prove effective for long-term care.
AMPA Receptors
Based on the evidence that the actions of ketamine require glutamate-induced AMPA receptor activation, it is possible that agents that directly activate or modulate AMPA receptors could produce rapid, ketamine-like antidepressant actions. Ampakines are a unique class of drugs that positively modulate AMPA receptors by altering AMPA receptor kinetics ( 91 ). Recently, Ampakines have been developed as nootropic agents due to their ability to positively modulate synaptic plasticity and enhance memory ( 92 , 93 ). These compounds likely produce their effects, in part by enhancing neurotrophin signaling. For example, the Ampakine CX614, in cultured neurons, increases BDNF release, TrkB receptor activation, and stimulation of mTOR signaling and local protein synthesis ( 66 ). Recent studies have demonstrated that Ampakines produce antidepressant-like effects more rapidly than fluoxetine in a rat model of submissive behavior ( 94 ). Given these characteristics, Ampakines are good candidates for novel rapid-acting antidepressants, although studies will be required to determine the clinical efficacy and safety of such agents.
mGluR2/3 Receptors
The antidepressant actions of NMDA receptor antagonists may occur via increases in extracellular glutamate ( 62 ), which can also be regulated by glutamate terminal autoreceptors. mGluR2 and mGluR3 receptors are Group II metabotropic glutamate receptors that are expressed in limbic brain regions associated with depression including the hippocampus and PFC ( 95 - 97 ). mGluR2 receptors are located in the pre-terminal region of presynaptic neurons ( 98 ), while mGluR3 receptors are located post-synaptically and on glia ( 97 ). These receptors function through a G i -coupled mechanism to negatively regulate adenylyl cyclase activity, and presynaptic mGluR2 receptors inhibit the release of glutamate and other neurotransmitters. Selective mGluR2/3 receptor antagonists are reported to produce antidepressant-like effects ( 99 ). Similar to the actions of ketamine, blockade of mGluR2/3 receptors with the selective compound LY341495 increases glutamate outflow in limbic regions and the PFC ( 100 , 101 ). In addition, blockade of post-synaptic AMPA receptors blocks the antidepressant-like effects of mGluR2/3 antagonists as observed with ketamine ( 99 ). Recent data also demonstrate that LY341495 increases signaling through the mTOR pathway and increases the expression of the synaptic proteins GluR1, PSD-95 and Synapsin I ( 102 ). Indeed, the antidepressant-like behavioral actions of mGluR2/3 antagonists require mTOR signaling ( 102 , 103 ). Given these similarities to ketamine, compounds that block mGluR2/3 receptors are strong candidates for novel rapid-acting antidepressants. A recent study demonstrates that an mGluR2-selective potentiator also produces antidepressant-like effects in rodents ( 104 ), and studies are needed to determine if this is a characteristic of this particular agent or of a class of mGluR2-selective agonists.
Muscarinic Receptors
In the early 1970s, the discovery that the acetylcholinesterase inhibitor, physostigmine, produces symptoms of depression led to the hypothesis that hyperactivity of the cholinergic system contributes to the etiology of depression ( 105 ). Early preclinical evaluation of the non-selective muscarinic receptor antagonist, scopolamine, identified antidepressant-like effects in mice, however, these effects were prematurely regarded as non-specific and unrelated to antidepressant activity ( 106 ). Recent clinical work demonstrates that intravenous infusion of scopolamine produces rapid antidepressant effects within 3 to 5 days following treatment, with anecdotal reports of improvement after only 1 day ( 107 , 108 ). Since scopolamine non-selectively blocks all muscarinic receptor subtypes (M1-M5), further studies are necessary to determine which subtype(s) mediate the rapid antidepressant effects. There is evidence that muscarinic receptors modulate glutamate neurotransmission in the cortico-striatal circuit ( 109 ). Scopolamine increases the release of glutamate in the striatum ( 110 ) and increases excitatory neurotransmission in the medial enthorhinal cortex ( 111 ). In the rat visual cortex M3 receptors are located on GABAergic interneurons and antagonists of these receptors lead to decreased inhibitory signaling ( 112 ). There is also evidence that postsynaptic M1 receptor agonists produce LTD and decrease postsynaptic glutamate activity in the hippocampus and PFC ( 113 , 114 ). It is possible that blockade of these M1 and M3 receptor-mediated actions by scopolamine could lead to increased excitatory signaling, glutamate release and subsequent mTOR activation.
Summary and Conclusions
The discovery that ketamine produces rapid and efficacious antidepressant responses in treatment-resistant patients has had a profound impact on research to understand and develop additional rapid-acting agents. Unlike typical antidepressants, ketamine has the unique ability to rapidly reverse the neuronal deficits and impaired plasticity produced by chronic stress. The mTOR pathway plays a critical role in these effects by activating synaptic translational machinery and increasing mature excitatory synapse number and function in the PFC. Future studies aimed at identifying novel targets that increase mTOR signaling could lead to the generation of new antidepressant agents with rapid onset and hold promise for developing more efficacious treatments for depression. Caution must be used when developing compounds that activate mTOR given the role of these cell growth and translation pathways in cancer biology ( 35 ). Further studies are also needed to determine the mechanisms underlying the loss of synaptic connections and neuronal atrophy caused by chronic stress (e.g., inhibition of mTOR function). In addition, the neuronal circuits and connections to and from the PFC that underlie the antidepressant actions of ketamine will require further studies. Characterizing the activity of ketamine in other brain regions sensitive to chronic stress will also enhance our understanding of the actions of rapid-acting antidepressants. Finally, it may be possible to develop strategies for preventing, as well treating stress-related mood disorders, such as behaviors that reduce the damaging effects of stress (e.g., stress reduction and management) and approaches that enhance synaptic flexibility and function (e.g., diet, exercise, enriched environments). Combined pharmacological and behavioral therapies that target growth and stabilization of the appropriate cortical and limbic synaptic connections will provide benefits for improved and sustained mental health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Duman reports having received honorarium fees from Lilly, Pfizer, Bristol Myers Squibb, Johnson & Johnson, Forest, and Lundbeck, consulting fees Taisho, and research support from Lilly, Lundbeck, Johnson & Johnson, and Forest. Mr. Dwyer reported no biomedical financial interests or potential conflicts of interest.
- 1. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7:358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Kang H-J, Voleti B, Hajszan T, Rajkowska G, Stockmeier C, Licznerski P, et al. Decreased expression of synapse related genes and loss of synapses in major depression. Nat Med. 2012 doi: 10.1038/nm.2886. In Press. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Krugers HJ, Douma BR, Andringa G, Bohus B, Korf J, Luiten PG. Exposure to chronic psychosocial stress and corticosterone in the rat: effects on spatial discrimination learning and hippocampal protein kinase Cgamma immunoreactivity. Hippocampus. 1997;7:427–436. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychiatry. 2001;50:994–1004. [ Google Scholar ]
- 34. Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Hoeffer CA, Sanchez E, Hagerman RJ, Mu Y, Nguyen DV, Wong H, et al. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 2012;11:332–341. doi: 10.1111/j.1601-183X.2012.00768.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, et al. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Cleary C, Linde JA, Hiscock KM, Hadas I, Belmaker RH, Agam G, et al. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull. 2008;76:469–473. doi: 10.1016/j.brainresbull.2008.03.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Ampuero E, Rubio FJ, Falcon R, Sandoval M, Diaz-Veliz G, Gonzalez RE, et al. Chronic fluoxetine treatment induces structural plasticity and selective changes in glutamate receptor subunits in the rat cerebral cortex. Neuroscience. 2010;169:98–108. doi: 10.1016/j.neuroscience.2010.04.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharmacol. 1997;7(Suppl 3):S323–328. doi: 10.1016/s0924-977x(97)00064-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. O'Leary OF, Wu X, Castren E. Chronic fluoxetine treatment increases expression of synaptic proteins in the hippocampus of the ovariectomized rat: role of BDNF signalling. Psychoneuroendocrinology. 2009;34:367–381. doi: 10.1016/j.psyneuen.2008.09.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Martinez-Turrillas R, Frechilla D, Del Rio J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43:1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Martinez-Turrillas R, Del Rio J, Frechilla D. Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology. 2005;49:1178–1188. doi: 10.1016/j.neuropharm.2005.07.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Musazzi L, Racagni G, Popoli M. Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int. 2011;59:138–149. doi: 10.1016/j.neuint.2011.05.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Antidepressant Efficacy of Ketamine in Depressed Patients. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.031. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, et al. Association of GSK3beta polymorphisms with brain structural changes in major depressive disorder. Arch Gen Psychiatry. 2009;66:721–728. doi: 10.1001/archgenpsychiatry.2009.70. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Saus E, Soria V, Escaramis G, Crespo JM, Valero J, Gutierrez-Zotes A, et al. A haplotype of glycogen synthase kinase 3beta is associated with early onset of unipolar major depression. Genes Brain Behav. 2010;9:799–807. doi: 10.1111/j.1601-183X.2010.00617.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Ma T, Tzavaras N, Tsokas P, Landau EM, Blitzer RD. Synaptic stimulation of mTOR is mediated by Wnt signaling and regulation of glycogen synthetase kinase-3. J Neurosci. 2011;31:17537–17546. doi: 10.1523/JNEUROSCI.4761-11.2011. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol. 2010;24:585–594. doi: 10.1177/0269881109104845. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235:283–289. doi: 10.1016/0014-2999(93)90147-a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Hubert JP, Delumeau JC, Glowinski J, Premont J, Doble A. Antagonism by riluzole of entry of calcium evoked by NMDA and veratridine in rat cultured granule cells: evidence for a dual mechanism of action. Br J Pharmacol. 1994;113:261–267. doi: 10.1111/j.1476-5381.1994.tb16203.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Granger R, Staubli U, Davis M, Perez Y, Nilsson L, Rogers GA, et al. A drug that facilitates glutamatergic transmission reduces exploratory activity and improves performance in a learning-dependent task. Synapse. 1993;15:326–329. doi: 10.1002/syn.890150409. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, et al. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, et al. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacol. 2002;440:27–35. doi: 10.1016/s0014-2999(02)01338-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Ohishi H, Ogawa-Meguro R, Shigemoto R, Kaneko T, Nakanishi S, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron. 1994;13:55–66. doi: 10.1016/0896-6273(94)90459-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–98. doi: 10.1016/j.brainres.2005.02.032. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A, et al. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol. 2012;15:429–434. doi: 10.1017/S1461145711001702. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011;61:1419–1423. doi: 10.1016/j.neuropharm.2011.08.034. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Fell MJ, Witkin JM, Falcone JF, Katner JS, Perry KW, Hart J, et al. N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methy l-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther. 2011;336:165–177. doi: 10.1124/jpet.110.172957. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Browne RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58:331–334. doi: 10.1016/0014-2999(79)90483-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Niittykoski M, Ruotsalainen S, Haapalinna A, Larson J, Sirvio J. Activation of muscarinic M3-like receptors and beta-adrenoceptors, but not M2-like muscarinic receptors or alpha-adrenoceptors, directly modulates corticostriatal neurotransmission in vitro. Neuroscience. 1999;90:95–105. doi: 10.1016/s0306-4522(98)00447-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Rawls SM, McGinty JF. Muscarinic receptors regulate extracellular glutamate levels in the rat striatum: an in vivo microdialysis study. J Pharmacol Exp Ther. 1998;286:91–98. [ PubMed ] [ Google Scholar ]
- 111. Hamam BN, Sinai M, Poirier G, Chapman CA. Cholinergic suppression of excitatory synaptic responses in layer II of the medial entorhinal cortex. Hippocampus. 2007;17:103–113. doi: 10.1002/hipo.20249. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Amar M, Lucas-Meunier E, Baux G, Fossier P. Blockade of different muscarinic receptor subtypes changes the equilibrium between excitation and inhibition in rat visual cortex. Neuroscience. 2010;169:1610–1620. doi: 10.1016/j.neuroscience.2010.06.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–9902. doi: 10.1523/JNEUROSCI.1366-09.2009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Jo J, Son GH, Winters BL, Kim MJ, Whitcomb DJ, Dickinson BA, et al. Muscarinic receptors induce LTD of NMDAR EPSCs via a mechanism involving hippocalcin, AP2 and PSD-95. Nat Neurosci. 2010;13:1216–1224. doi: 10.1038/nn.2636. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.8 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
REVIEW article
From serotonin to neuroplasticity: evolvement of theories for major depressive disorder.

- Key Laboratory of Psychiatry and Mental Health of Hunan Province, Mental Health Institute, The Second Xiangya Hospital of Central South University, National Clinical Research Center for Mental Disorder, National Technology Institute of Psychiatry, Changsha, China
The serotonin (5-HT) hypothesis of depression has played an important role in the history of psychiatry, yet it has also been criticized for the delayed onset and inadequate efficacy of selective serotonin reuptake inhibitors (SSRIs). With evolvement of neuroscience, the neuroplasticity hypothesis of major depressive disorder (MDD) has been proposed and may provide a better framework for clarification the pathogenesis of MDD and antidepressant efficacy. In this article, we first summarized the evidence challenging the monoamine hypothesis and proposed that the antidepressant efficacy of SSRIs is not derived from elevated monoamine (5-HT, noradrenaline (NE), or dopamine (DA)) concentration or monoamine neurotransmission. Second, we reviewed the role of stress in the pathogenesis of MDD and gave a brief introduction to the neuroplasticity hypothesis of MDD. Third, we explored the possible mechanisms underlying the antidepressant efficacy of typical antidepressants in the context of neuroplasticity theory. Fourth, we tried to provide an explanatory framework for the significant difference in onset of efficacy between typical antidepressants and ketamine. Finally, we provided a brief summarization about this review article and some perspectives for future studies.
Introduction
Major depressive disorder (MDD) is a highly prevalent and highly debilitating psychiatric disorder. MDD is the leading cause of disability worldwide with approximately 350 million people around the world suffering from this disorder, and the disease burden of depression has been considered to become the second highest among all diseases by 2020 ( World Health Organization, 2016 ). However, despite the devastating burden of MDD, the pathogenesis of this complex disorder still remains unclear and the current available treatment for depression is also far from optimal ( Collins et al., 2011 ). Specifically, clinical diagnosis of depression is still suffering from lack of objective diagnostic biomarker ( Jentsch et al., 2015 ) and the overall remission rate of sequenced first-line antidepressant treatments (including drugs and cognitive behavioral therapy) for MDD is only about 60%–70% ( Rush et al., 2006 ). Besides, the first-line drugs recommended for MDD in authentic MDD guidelines (most are selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-noradrenaline reuptake inhibitors (SNRIs)) are often criticized by the delayed onset of efficacy, namely, it takes 2 weeks or longer on average for these drugs to work ( Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Depression, 2004 ; National Institute for Health and Care Excellence, 2009 ; American Psychiatric Association Work Group on Major Depressive Disorder, 2010 ; Bauer et al., 2013 ; Kennedy et al., 2016 ).
The suboptimal clinical practice of MDD calls for deep understanding of the pathogenesis of MDD and development of more potent and fast-acting antidepressants. Although several hypotheses have been proposed for MDD, the monoamine (serotonin (5-HT), noradrenaline (NE) and dopamine (DA)) hypothesis is still the most prevailing hypothesis of MDD since most of the currently available antidepressants work on monoamine transporters or receptors. This hypothesis, initially based on the unintentional findings that chemical compounds inhibiting reuptake (imipramine) or metabolism (iproniazid) of monoamine neurotransmitters (5-HT and NE) would demonstrate antidepressant efficacy ( Hirschfeld, 2000 ; Mulinari, 2012 ), claims that MDD is derived from deficiency of 5-HT and/or NE in the synaptic cleft, and antidepressant efficacy would be achieved by increasing 5-HT and/or NE in synaptic cleft through inhibiting clearance or promoting synthesis and release of these monoamines.
The monoamine hypothesis satiates the intense needs of interpretation for the mechanism of pathogenesis of MDD from academy, pharmacies and public population and has guided the development of new antidepressants in 1980s–2000s. Nevertheless, accounting the complicated and heterogeneous clinical manifestations of MDD to deficiency of a molecule is too simplistic and may misguide our understanding of the complexity of this disorder. Indeed as expected, numerous findings inconsistent with this hypothesis have arisen from daily clinical observations, clinical researches and preclinical studies since the proposal of this hypothesis, among which the most prominent findings are the delayed onset of efficacy and inadequate response/remission rate of typical antidepressants as illustrated above. These findings challenged the monoamine hypothesis on one hand, and promoted the evolvement of theories about depression on the other hand. Specifically, to make up for the shortage of monoamine hypothesis, researchers have proposed monoaminergic receptor hypothesis, signaling hypothesis, neuroplasticity hypothesis, etc. ( Racagni and Popoli, 2008 ). These hypotheses evolved towards a more comprehensive and reasonable understanding of MDD and antidepressant efficacy, and the succeeding hypothesis may be totally different from the initial monoamine hypotheses.
Increased Synaptic Serotonin (or NE, DA) Concentration Does Not Account for The Antidepressant Efficacy of Antidepressants
Several published reviews have casted doubt on the low 5-HT hypothesis of MDD and summarized the evidence inconsistent with this hypothesis ( Lacasse and Leo, 2005 ; Racagni and Popoli, 2008 ; Fischer et al., 2014 ; Andrews et al., 2015 ). One article even hypothesized that depression is a result of elevated 5-HT concentration rather than deficiency of 5-HT ( Andrews et al., 2015 ). The evidence challenging the low 5-HT hypothesis may be summarized as the following three categories: first, the rapid increase of 5-HT concentration in the synaptic cleft of neurons is inconsistent with the clinical delayed onset of antidepressant efficacy; second, lowering the concentration of 5-HT in synaptic cleft through tryptophan depletion ( Ruhé et al., 2007 ) or serotonin transporter (SERT) enhancer (i.e., Tinaptine; Kasper and McEwen, 2008 ) failed to induce depression in healthy subjects, actually long-term antidepressants treatment had been detected to downregulating the total 5-HT concentration in the brain ( Marsteller et al., 2007 ; Bosker et al., 2010 ; Siesser et al., 2013 ), which was contrary to the common sense of low 5-HT in depression; and third, genetic variants associated with potentiated SERT function ( l allele of 5-HTTLPR) have been repeatedly found to be related with reduced risk of depression or better prognosis than variants associated with decreased SERT function ( s allele of 5-HTTLPR; Karg et al., 2011 ). A timeline of historical publications or events supporting or opposing the monoamine hypothesis is shown in Figure 1 .
Figure 1 . Timeline of historical events or publications supporting or opposing the monoamine hypothesis of depression. The blue boxes are events or publications supporting monoamine hypothesis and the yellow boxes are those opposing monoamine hypothesis. The following are the publications: 1. Selikoff et al. (1952) , 2. Davies and Shepherd (1955) , 3. Kuhn (1958) , 4. Schildkraut (1965) , 5. Coppen (1967) , 6. Schildkraut and Kety (1967) , 7. Lapin and Oxenkrug (1969) , 8. Oswald et al. (1972) , 9. Stahl (1984) , 10. Caspi et al. (2003) , 11. Andrews et al. (2015) .
The above findings together put sand in the wheels of low 5-HT hypothesis and indicate that it may not be reasonable to account the antidepressant efficacy of SSRIs to elevated 5-HT concentration or increased 5-HT neurotransmission in the brain. Thus the presumption that depression is caused by deficiency of 5-HT is also lack of solid basis. Actually, as stated in the Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications , “there is no clear and convincing evidence that monoamine deficiency accounts for depression, i.e., there is no “real” monoamine deficit” ( Stahl, 2013 ). Similar opinions or comments from other authentic researchers or publications had been summarized in the impressive article of Lacasse and Leo (2005) . Therefore, the low 5-HT hypothesis, although intriguing, are too simplistic and arbitrary for interpretation of the mechanisms underlying the complex manifestations of MDD.
To address the delayed onset of antidepressant efficacy, scientists further proposed the monoamine receptor hypothesis, which asserts that downregulation or desensitization of somatodendritic monoamine autoreceptor (such as 5-HT 1A ), rather than the elevation of monoamine concentration itself, is the key mechanism of antidepressant efficacy ( Stahl, 2013 ). Since the somatodendritic 5-HT 1A autoreceptor inhibits impulse flow of 5-HT neurons, the downregulation or desensitization of this somatodendritic receptor induced by elevated concentration of 5-HT resulted from antidepressant intake would turn on neuronal impulse flow and bring about increased 5-HT in axonal terminals. The enhanced axonal 5-HT transmission and its subsequent neurobiochemical events, like regulation of gene transcription and protein synthesis, are deemed as the final mediators of antidepressant efficacy. As it takes several days to 2 weeks for the downregulation of 5-HT 1A autoreceptor to happen, the monoamine receptor hypothesis perfectly explained the delayed onset of antidepressant efficacy. However, both the clinical molecular imaging and postmortem studies failed to find consistent evidence supporting alterations of 5-HT 1A in patients with MDD ( Ruhé et al., 2014 ). Besides, 5-HT 1A antagonists also failed to achieve consistent antidepressant efficacy in clinical trials. These research findings all casted doubts on the monoamine receptor hypothesis and calls for better hypothesis for the pathogenesis of depression.
Considering the antidepressant efficacy of electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), transcranial direct-current stimulation (tDCS) and new antidepressant ketamine and its derivatives, a legitimate inference might be that these therapies, although differed in forms and styles, would work on a final common pathway which underlies the pathogenesis of or vulnerability to MDD, and the antidepressant efficacy of these therapies is found on reversing or repairing the alteration of this final common pathway. Since no direct evidence about the association between 5-HT and depression and indirect evidence is highly inconsistent, there is no reason to claim that deficiency of 5-HT may serve as the “final common pathway” of depression. Then what else mechanism would be competent for the “final common pathway” of these diverse therapies? As has been repeated confirmed by preclinical and clinical studies, the relationship between stress and depression is robust and steady-going ( Biegler, 2008 ; Risch et al., 2009 ; Binder and Nemeroff, 2010 ; Young and Korszun, 2010 ; Pizzagalli, 2014 ), thus it is legitimate to deduce that revealing the neurobiological sequelae of stress on the brain and its association with depression might provide insight in exploring the “final common pathway” of depression and antidepressant efficacy. Here we would like to take a brief look at the effect of stress on the brain and its role in the pathogenesis of depression at first.
The Role of Stress in The Pathogenesis of MDD
In the framework of gene X environment for psychiatric disorders, stress is the validated environmental factor accounting to increased risk of development, exacerbation, chronicity and relapse of MDD. Generally, major depressive episodes (MDEs) are associated with about 2.5 times more frequent stressful life events in the period before episode as compared with comparable time period in controls ( Hammen, 2005 ), and one stressful life event would lead to about 1.41-fold increased risk of MDE ( Risch et al., 2009 ). In addition, stress is suggested to be linked with treatment resistance ( Amital et al., 2008 ), poorer prognosis ( Gilman et al., 2013 ) and higher rate of relapse and recurrence ( Monroe and Harkness, 2005 ; Harkness et al., 2014 ) of MDD.
How should stress and depression be linked? Numerous theories has been proposed for interpretation of this phenomenon, among which the vicious circle between the dysregulation of hypothalamic-pituitary-adrenocortical (HPA) axis and morphological and functional deficits of hippocampal formation is considered as the key route between stress and depression. Specifically, the elevation of circulating cortisol during chronic stress response would exert neurotoxic effect on hippocampal neurons through glucocorticoid receptor and its downstream effects, which would result in decreased neurogenesis, synaptogenesis and dendritic spines and increased apoptosis of neurons ( Holsboer and Barden, 1996 ; Holsboer, 2000 ; de Kloet et al., 2005 ). The morphological loss of neurons further leads to functional deficits loss of long-term potentiation (LTP) or long-term depression (LTD) of hippocampus, which gives rise to decreased GABAergic control of the HPA axis from the bed nucleus of stria terminalis (BNST) normally driven by the action of hippocampus ( Holsboer, 2000 ; Egeland et al., 2015 ), and the the disinhibition of HPA axis would inversely exacerbate the morphological and functional loss of hippocampus. Thus, a vicious circle is formed and the hippocampal formation gradually goes to structural atrophy and functional deficit, which are commonly seen in depression.
Apart from the hypercortisolemia and deficits of hippocampal formation, the effect of stress on the biochemical metabolism and neurotransmission is also deemed to partly mediate the link between stress and depression. Biochemically, chronic stress would induce increased release of glutamate ( Sanacora et al., 2012 ) in the hippocampus and prefrontal cortex (PFC), and blunted neurotransmission of 5-HT ( Mahar et al., 2014 ) and DA ( Pizzagalli, 2014 ) in mesocortical monoaminergic circuits. Specifically, chronic stress would downregulate the firing rate of dorsal raphe (DR) 5-HT neurons projecting to PFC and 5-HT 1A receptor sensitivity in PFC, which may be mediated by hypercortisolemia ( Mahar et al., 2014 ). Similarly, diminished basal DA neuron firing in striatum is also observed in rodents exposed to chronic mild stress ( Bekris et al., 2005 ). And, elevated release of glutamate in PFC is repeatedly observed after chronic stress, which is deemed to exert neurotoxic efficacy on the PFC and hippocampus neurons ( Sanacora et al., 2012 ). These neurochemical changes would together result in negative influence on neuroplasticity through blunted neurogenesis, disrupted synaptogenesis, diminished dendritic spines and reduced synaptic connections. Besides, stress would diminish the cell proliferation and promote apoptosis of glial cells ( Rial et al., 2015 ), which is the primary cell responsible for clearance of glutamate in the brain and may be responsible for the atrophy of hippocampus in MDD ( Duman, 2004 ).
The functional and morphological changes of the brain induced by hypercortisolemia resulted from chronic stress are roughly consistent with the neuroimaging findings of abnormalities in MDD, i.e., atrophy and hypofunction of hippocampus and PFC, and hypertrophy and hyperfunction of amygdala ( Andrade and Rao, 2010 ). Interestingly, the alterations in different brain regions may underlie different symptoms of MDD. Specifically, structural and functional alterations in the PFC-amygdala/hippocampus circuit may underlie depressive emotions; abnormalities in the PFC-nucleus accumbens (NAc) circuit may serve as the neural substrate of anhedonia ( Phillips et al., 2015 ); and alterations of medial and dorsolateral PFC may mediate the cognitive dysfunction of MDD ( Thomas and Elliott, 2009 ).
With the accumulated evidence supporting the strong correlation between stress and depression, and findings revealing the efficacy of stress on brain in line with the abnormalities found in MDD, the term “stress-induced depression” or at least “stress-correlated depression” would seem reasonable. As the case stands, the most frequently used and research validated depression animal model is the chronic stress induced depression model ( Czéh et al., 2016 ). Thus, exploring the pathogenesis of MDD in the framework of stress-induced depression may be reasonable and necessary for our comprehending of this complex and heterogeneous psychiatric disorder.
The routes through which stress exert neurobiological effect on the brain as discussed above are all correlated with the growth, maturation, apoptosis and function of neurons. These processes, usually conceptualized as “neuroplasticity”, are of key significance in the pathogenesis of MDD. Therefore, they may also be competent for the role of “final common pathway” of antidepressant efficacy achieved by diverse treatment strategies. Below, we will give a brief introduction to the main contents of the neuroplasticity hypothesis of depression and take typical antidepressants and ketamine as examples to illustrate how neuroplasticity would serve as the “final common pathway” of antidepressant efficacy.
Neuroplasticity Hypothesis of Depression: Main Contents
Although proposed for a long time and has won a lot of attention in academy, there is still no validated definition about the term “neuroplasticity”. Generally, neuroplasticity refers to the ability of neural system to adapt itself to the internal and external stimuli and to respond adaptively to future stimuli ( Cramer et al., 2011 ). The processes of neuroplasticity are complex and the underlying mechanisms have not yet been fully understood, while it is widely accepted that the “neuroplasticity” includes both morphological and functional adaptation. Generally, the morphological neuroplasticity usually refers to neurogenesis, synaptogenesis, dendritic length and branching, spine density etc ( Cramer et al., 2011 ; Egeland et al., 2015 ) and the functional neuroplasticity includes at least four forms: homologous area adaptation, cross-modal reassignment, map expansion and compensatory masquerade ( Grafman, 2000 ). Neuroplasticity is of key significance in brain’s adaptation to stress, and maladaptive neuroplasticity may underlie various psychiatric disorders, such as depression, post-traumatic stress disorder, etc. Usually, the neuroplasticity theory of depression is usually supported by evidence from three domains ( Serafini, 2012 ): (1) decreased neuroplasticity in hippocampus and PFC in depressed patients; (2) decreased concentration of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), in subjects with depression; and (3) antidepressants would elevate the concentration of neurotrophic factors and improve the neuroplasticity in hippocampus and PFC.
In addition, what deserves to be mentioned is the role of “metaplasticity” (a term coined by Abraham and Bear, 1996 , meaning “plasticity of neuroplasticity”) in explaining stress-induced neural plasticity. The “metaplasticity”, or “activity-dependent and persistent change in neuronal state that shapes the direction, duration or magnitude of future synaptic change ( Abraham and Bear, 1996 )” in another way of saying, includes some key functions like preparing synapses for plasticity and learning and regulating synaptic plasticity homeostatically ( Hulme et al., 2013 ). These functions may be achieved through actions on NMDA and metabtropic glutamate receptors (mGluRs) or heterosynaptic metaplasticity mechanisms like synaptic tagging and capture ( Abraham, 2008 ; Hulme et al., 2013 ). Metaplasticity is sensitive to environmental stimuli, like environment enrichment or stress and dysregulation of metaplasticity induced by chronic stress may contribute to induction of depression ( Vose and Stanton, 2017 ). For a detailed description of mechanisms underlying metaplasticity and their clinical relevance, the impressive articles of Abraham and Bear (1996) , Abraham (2008) and Hulme et al. (2013) may be valuable.
As discussed above, with the establishment of stress-induced depression conceptual framework and the key role of neuroplasticity as mediator between stress and depression, neuroplasticity theory would be an optimal choice for understanding the pathogenesis of depression and antidepressant efficacy. Since we have illustrated the role of stress in the pathogenesis of MDD and the changes of brain induced by stress hereinbefore, next we will discuss how the antidepressants work on neuroplasticity.
How Typical Antidepressants Work on Neuroplasticity?
The possible mechanisms of typical antidepressants on neuroplasticity have been reviewed in several articles ( Racagni and Popoli, 2008 ; Andrade and Rao, 2010 ; Serafini, 2012 ; Harmer and Cowen, 2013 ; Hayley and Litteljohn, 2013 ). Briefly, antidepressants may improve neuroplasticity through the following pathways.
First, antidepressants improve neuroplasticity through monoamine neurotransmitters’ stimulation of the postsynaptic monoamine receptors. These receptors are mostly G-protein coupled receptor (GPCR) and would initiate subsequent signaling after stimulation. Specifically, stimulation of these receptors would activate the adenylate cyclase (AC), which would catalyze the ATP to cyclic adenosine monophosphate (cAMP), and cAMP would further activate the cAMP-response element binding protein (CREB) through activation of protein kinase A (PKA; Carlezon et al., 2005 ). The transcription factor CREB is responsible for gene expression of many proteins involved in the neuroplasticity of hippocampus, such as BDNF, glutamate receptor unit 1 (GluR1), etc ( Pittenger and Duman, 2008 ). Since the atrophy of hippocampus has been consistently found to play a key role in the vulnerability, chronicity, and treatment-resistance of MDD ( MacQueen and Frodl, 2011 ), improving the neurogenesis of hippocampus through activation of postsynaptic monoamine receptors may effectively promote depression recovery. This pathway may be abbreviated as the “GPCR-cAMP” pathway. While the “GPCR-cAMP” pathway is commonly seen in other organs or tissues, it is not the major pathway regulating the function of CREB in the brain ( Carlezon et al., 2005 ).
Second, antidepressant would regulate neuroplasticity through reducing release of presynaptic glutamate, especially the depolarization-evoked release of glutamate, in PFC ( Bonanno et al., 2005 ). The possible molecular mechanism of antidepressants on the release of glutamate had been reviewed in the article of Sanacora et al. (2012) . The reduced glutamate release may imply decreased neurotoxic efficacy and strengthened synaptogenesis, synaptic connections and neurogenesis. To be mentioned, chronic antidepressant would also prevent the stress-induced glutamate release, which may underlie the clinical prophylaxic efficacy of maintenance antidepressant treatment for relapse or recurrence of MDE.
Third, antidepressant may work on neuroplasticity through enhancing AMPA to NMDA throughput ( Du et al., 2006 ). Antidepressants may binding to the glycine-binding site of NMDA receptor and inactivate this site ( Paul and Skolnick, 2003 ). The inactivation of NMDA receptor activity would result in inhibition of eukaryotic elongation factor 2 (eEF2) and enhance the expression of BDNF through subsequent signaling ( Monteggia et al., 2013 ). Besides, antidepressant would upregulate the expression of AMPA subunits GluR1 and potentiate the function of AMPA ( Martinez-Turrillas et al., 2002 ). The depolarization of AMPA receptor would activate the voltage-dependent calcium channels (VDCCs) and induce influx of Ca 2+ into cytoplasm, which would further trigger the exocytosis of BDNF. Then the extracellular BDNF would further stimulate its membrane receptor—TrkB and regulate gene expression and neuroplasticity through subsequent signaling ( Yoshii and Constantine-Paton, 2010 ). Thus stimulation of AMPA and inactivation of NMDA would work synergistically to improve neuroplasticity in the brain.
Fourth, antidepressant may improve neuroplasticity directly through LTP-like process. It has been repeatedly revealed that hippocampal synaptic plasticity was suppressed by stress through diminished amount of LTP, while antidepressant would reverse the negative efficacy of stress and potentiate synaptogenesis and synaptic connectivity through inducing LTP-like processes ( Popoli et al., 2002 ; Shakesby et al., 2002 ).
Last but not least, antidepressant may also improve neurogenesis in the hippocampus through activation of the 5-HT 1A receptor ( Santarelli et al., 2003 ).
Despite the role of BDNF in promoting neuroplasticity and neurogenesis in the hippocampus and PFC and mediating the antidepressant efficacy as mentioned above, what needs special attention is that BDNF may also promote neuroplasticity and neurogenesis in the amygdala, ventral tegmental area (VTA) and NAc, which is assumed to provoke depressive-like behaviors or exacerbate depressive symptoms ( Racagni and Popoli, 2008 ; Harmer and Cowen, 2013 ; Hayley and Litteljohn, 2013 ). Thus, the antidepressant efficacy is not totally opposite to the site-specific neurophysiological and neurochemical efficacy of stress on different brain regions, which inhibits neuroplasticity, induces atrophy in hippocampus and PFC and promotes maladaptive neuroplasticity and induces hypertrophy in amygdala. The hypertrophy and elevated activation of amygdala may underline the heightened risk of relapse in recurrent MDD.
Delayed Efficacy of SSRI and Fast Responding Ketamine: Clinical Trial Findings and Possible Interpretations of Discrepancy in Onset
The rapid onset of antidepressant efficacy of ketamine and delayed onset of efficacy in SSRIs treatment is of special interest. A meta-analysis revealed that overall response rate of single dose ketamine after 24 h is about 52.6%, and this efficacy would last about 3 days and decreased gradually with 10.9% of response rate remained at the end of week two after injection ( Newport et al., 2015 ). Repeated ketamine infusions are associated with a relatively higher overall response rate (70.8%), and the efficacy lasts about 18 days on average after the last ketamine injection ( Murrough et al., 2013 ). Although the clinical application of ketamine for depression is limited by its potential of abuse, the significant difference in time of efficacy onset between ketamine and typical antidepressants is of special clinical significance, since rapid onset of efficacy is urgently needed for MDD patients, particularly for those with suicidal ideation. Clarification the mechanisms underlying the discrepancy of efficacy onset between the two genre drugs may be helpful for the development of new antidepressants with rapid onset of efficacy.
The possible mechanism of antidepressant efficacy of ketamine has been summarized in several reviews ( Browne and Lucki, 2013 ; Zunszain et al., 2013 ; Kavalali and Monteggia, 2015 ; Scheuing et al., 2015 ), which all stated that the blockade of NMDA receptor and potentiation of AMPA receptor is of key significance in ketamine’s antidepressant efficacy. NMDA and AMPA are two ionotropic glutamate receptors distributed widely in the brain. Their physiological ligand, glutamate, is the only excitatory neurotransmitter and innervates the majority of neurons in the brain. Neurohistological studies found that 85% of the brain mass are composed of neocortex, and glutamate is the primary neurotransmitter of 80% neocortex neurons and 85% neocortex synapses ( Douglas and Martin, 2007 ). It is not difficult to infer from the above data that glutamate neurons account for so high proportion of the whole brain neurons that some researchers believe that the brain is largely a “glutamatergic excitatory machine” and all brain functions, particularly cognition and emotion are “ultimately mediated by the changes in excitatory transmission (glutamate) and its counterbalance of the inhibitory component (GABA)” ( Sanacora et al., 2012 ).
As discussed above, glutamate is closely related to neuroplasticity in the brain. Release of glutamate may induce rapid LTP and promote synaptogenesis and synaptoconnectomes. Blocking NMDA receptor and activating AMPA receptor may promote the expression of BDNF gene and promote neuroplasticity synergistically. Thus glutamate is the primary system regulating neuroplasticity in the brain. With these arguments, we believe that the fast onset of antidepressant efficacy of ketamine may be explained by the following two reasons: (1) ketamine acts directly on NMDA receptor and indirectly on AMPA receptor, while SSRIs mainly act on SERT and indirectly regulate efficacy of glutamate receptors; although activating the postsynaptic monoamine receptors also plays a role in the neuroplasticity, this pathway is much slower and weaker than direct working on ionotropic glutamate receptors, i.e., NMDA and AMPA, as discussed above; and (2) the glutamate neurons and neurotransmitters account for much higher proportion in number of neurons and synapses than 5-HT neurons (and other monoamine receptors), drugs work on the glutamate system would exert much greater efficacy on the brain than drugs work on the 5-HT system. Namely, ketamine takes a faster speed and shorter route to regulate neuroplasticity than SSRIs, and this is why the fast responding of ketamine and delayed onset of SSRIs would occur.
Summary and Future Perspectives
The monoamine theory of depression originated from the interpretation of the phenomenon observed in clinical practice, and has served as the primary hypothesis of MDD for more than 50 years. The prosperity of low 5-HT hypothesis is contributed to multilateral force coming from public, academy, industry, history, etc, as illustrated in the wonderful article of Mulinari (2012) . However, with new observations and research evidence constantly emerging, this simplistic hypothesis has been intensely challenged and modifications or even totally new hypothesis are needed. Although SSRIs are currently first-line antidepressants in psychiatry practice, new efficacious drugs with rapid onset of efficacy are emerging. And, in theory research area, a paradigm shift has occurred from monoamine hypothesis to glutamate and neuroplasticity theory, which provides a more mature interpretation framework for the complicated psychiatric disorder.
Neuroplasticity hypothesis of MDD evolves from the monoamine hypothesis and tries to address the problems of monoamine hypothesis. This theory starts from the key role of stress in the pathogenesis of MDD, and provides a reasonable framework for the interpretation of the relationship between stress, brain, depression and antidepressant efficacy. Although the molecular mechanisms underlying neuroplasticity are not fully clarified, this hypothesis provides the most promising framework for understanding the pathogenesis of depression and antidepressant efficacy. However, there are some major themes urgently needed for clarification in future studies.
First, the relationship between stress and MDD has been extensively explored, while gene also plays a key role in the pathogenesis of MDD, how the interaction between gene and stress work on neuroplasticity and its relationship with depression pathogenesis and antidepressant efficacy is of special interest for scientists and clinicians.
Second, more comprehensive and detailed understanding of the molecular mechanisms, particularly the interaction between the neurotransmitter receptors and their subsequent signaling pathways, underlying neuroplasticity, depression and antidepressant efficacy is needed. Targets in these signaling pathways may be of special value in new antidepressant development.
Third, the neuroplasticity theory is not exclusive for MDD, it may also account for the pathogenesis of other psychiatric disorders, such as schizophrenia and bipolar disorder. Thus an interesting question is how the alterations in neuroplasticity account for the significantly different symptomatology of these disorders? Exploring answers to this question may help delineating the boundaries of MDD and searching for objective diagnostic biomarkers for MDD.
Author Contributions
LL, YZ and BL co-designed the topic and contributed substantially to the conception of the article. BL performed the literature work, drafted the manuscript and approved the final version of the article. JL, MW and YZ contributed valuable suggestions to the conception of the article and partial literature analysis. LL and YZ critically revised the manuscript and have approved the final version of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by grant from the National Science and Technologic Program of China (2015BAI13B02 to LL), National Basic Research Program of China (+ 2013CB835100 to LL) and National Natural Science Foundation of China (81171286 and 91232714 to LL; 81671353 to YZ).
Abraham, W. C. (2008). Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9:387. doi: 10.1038/nrn2356
PubMed Abstract | CrossRef Full Text | Google Scholar
Abraham, W. C., and Bear, M. F. (1996). Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19, 126–130. doi: 10.1016/s0166-2236(96)80018-x
American Psychiatric Association Work Group on Major Depressive Disorder. (2010). Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd Edn. Washington, DC: American Psychiatric Association Press.
Google Scholar
Amital, D., Fostick, L., Silberman, A., Beckman, M., and Spivak, B. (2008). Serious life events among resistant and non-resistant MDD patients. J. Affect. Disord. 110, 260–264. doi: 10.1016/j.jad.2008.01.006
Andrade, C., and Rao, N. S. (2010). How antidepressant drugs act: a primer on neuroplasticity as the eventual mediator of antidepressant efficacy. Indian J. Psychiatry 52, 378–386. doi: 10.4103/0019-5545.74318
Andrews, P. W., Bharwani, A., Lee, K. R., Fox, M., and Thomson, J. A. Jr. (2015). Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neurosci. Biobehav. Rev. 51, 164–188. doi: 10.1016/j.neubiorev.2015.01.018
Bauer, M., Pfennig, A., Severus, E., Whybrow, P. C., Angst, J., Möller, H. J., et al. (2013). World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry 14, 334–385. doi: 10.3109/15622975.2013.804195
Bekris, S., Antoniou, K., Daskas, S., and Papadopoulou-Daifoti, Z. (2005). Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav. Brain Res. 161, 45–59. doi: 10.1016/j.bbr.2005.01.005
Biegler, P. (2008). Autonomy, stress, and treatment of depression. BMJ 336, 1046–1048. doi: 10.1136/bmj.39541.470023.ad
Binder, E. B., and Nemeroff, C. B. (2010). The CRF system, stress, depression and anxiety—insights from human genetic studies. Mol. Psychiatry 15, 574–588. doi: 10.1038/mp.2009.141
Bonanno, G., Giambelli, R., Raiteri, L., Tiraboschi, E., Zappettini, S., Musazzi, L., et al. (2005). Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J. Neurosci. 25, 3270–3279. doi: 10.1523/jneurosci.5033-04.2005
Bosker, F. J., Tanke, M. A., Jongsma, M. E., Cremers, T. I., Jagtman, E., Pietersen, C. Y., et al. (2010). Biochemical and behavioral effects of long-term citalopram administration and discontinuation in rats: role of serotonin synthesis. Neurochem. Int. 57, 948–957. doi: 10.1016/j.neuint.2010.10.001
Browne, C. A., and Lucki, I. (2013). Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front. Pharmacol. 4:161. doi: 10.3389/fphar.2013.00161
Carlezon, W. A. Jr., Duman, R. S., and Nestler, E. J. (2005). The many faces of CREB. Trends Neurosci. 28, 436–445. doi: 10.1016/j.tins.2005.06.005
Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., et al. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389. doi: 10.1126/science.1083968
Collins, P. Y., Patel, V., Joestl, S. S., March, D., Insel, T. R., Daar, A. S., et al. (2011). Grand challenges in global mental health. Nature 475, 27–30. doi: 10.1038/475027a
Coppen, A. (1967). The biochemistry of affective disorders. Br. J. Psychiatry 113, 1237–1264. doi: 10.1192/bjp.113.504.1237
Cramer, S. C., Sur, M., Dobkin, B. H., O’Brien, C., Sanger, T. D., Trojanowski, J. Q., et al. (2011). Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609. doi: 10.1093/brain/awr039
Czéh, B., Fuchs, E., Wiborg, O., and Simon, M. (2016). Animal models of major depression and their clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 293–310. doi: 10.1016/j.pnpbp.2015.04.004
Davies, D. L., and Shepherd, M. (1955). Reserpine in the treatment of anxious and depressed patients. Lancet 266, 117–120. doi: 10.1016/s0140-6736(55)92118-8
de Kloet, E. R., Joëls, M., and Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475. doi: 10.1038/nrn1683
Douglas, R. J., and Martin, K. A. (2007). Mapping the matrix: the ways of neocortex. Neuron 56, 226–238. doi: 10.1016/j.neuron.2007.10.017
Du, J., Machado-Vieira, R., Maeng, S., Martinowich, K., Manji, H. K., Zarate, C. A. Jr. (2006). Enhancing AMPA to NMDA throughput as a convergent mechanism for antidepressant action. Drug Discov. Today Ther. Strateg. 3, 519–526. doi: 10.1016/j.ddstr.2006.11.012
Duman, R. S. (2004). Depression: a case of neuronal life and death? Biol. Psychiatry 56, 140–145. doi: 10.1016/j.biopsych.2004.02.033
Egeland, M., Zunszain, P. A., and Pariante, C. M. (2015). Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat. Rev. Neurosci. 16, 189–200. doi: 10.1038/nrn3855
Fischer, A. G., Jocham, G., and Ullsperger, M. (2014). Dual serotonergic signals: a key to understanding paradoxical effects? Trends Cogn. Sci. doi: 10.1016/j.tics.2014.11.004 [Epub ahead of print].
Gilman, S. E., Trinh, N. H., Smoller, J. W., Fava, M., Murphy, J. M., and Breslau, J. (2013). Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychol. Med. 43, 303–316. doi: 10.1017/s0033291712001080
Grafman, J. (2000). Conceptualizing functional neuroplasticity. J. Commun. Disord. 33, 345–355; quiz 355–346. doi: 10.1016/s0021-9924(00)00030-7
Hammen, C. (2005). Stress and depression. Annu. Rev. Clin. Psychol. 1, 293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938
Harkness, K. L., Theriault, J. E., Stewart, J. G., and Bagby, R. M. (2014). Acute and chronic stress exposure predicts 1-year recurrence in adult outpatients with residual depression symptoms following response to treatment. Depress. Anxiety 31, 1–8. doi: 10.1002/da.22177
Harmer, C. J., and Cowen, P. J. (2013). ‘It’s the way that you look at it’—a cognitive neuropsychological account of SSRI action in depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368:20120407. doi: 10.1098/rstb.2012.0407
Hayley, S., and Litteljohn, D. (2013). Neuroplasticity and the next wave of antidepressant strategies. Front. Cell. Neurosci. 7:218. doi: 10.3389/fncel.2013.00218
Hirschfeld, R. M. A. (2000). History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 61, 4–6.
PubMed Abstract | Google Scholar
Holsboer, F. (2000). The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23, 477–501. doi: 10.1016/s0893-133x(00)00159-7
Holsboer, F., and Barden, N. (1996). Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr. Rev. 17, 187–205. doi: 10.1210/er.17.2.187
Hulme, S. R., Jones, O. D., and Abraham, W. C. (2013). Emerging roles of metaplasticity in behaviour and disease. Trends Neurosci. 36, 353–362. doi: 10.1016/j.tins.2013.03.007
Jentsch, M. C., Van Buel, E. M., Bosker, F. J., Gladkevich, A. V., Klein, H. C., Oude Voshaar, R. C., et al. (2015). Biomarker approaches in major depressive disorder evaluated in the context of current hypotheses. Biomark. Med. 9, 277–297. doi: 10.2217/bmm.14.114
Karg, K., Burmeister, M., Shedden, K., and Sen, S. (2011). The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry 68, 444–454. doi: 10.1001/archgenpsychiatry.2010.189
Kasper, S., and McEwen, B. S. (2008). Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs 22, 15–26. doi: 10.2165/00023210-200822010-00002
Kavalali, E. T., and Monteggia, L. M. (2015). How does ketamine elicit a rapid antidepressant response? Curr. Opin. Pharmacol. 20, 35–39. doi: 10.1016/j.coph.2014.11.005
Kennedy, S. H., Lam, R. W., McIntyre, R. S., Tourjman, S. V., Bhat, V., Blier, P., et al. (2016). Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can. J. Psychiatry 61, 540–560. doi: 10.1177/0706743716659417
Kuhn, R. (1958). The treatment of depressive states with G 22355 (imipramine hydrochloride). Am. J. Psychiatry 115, 459–464. doi: 10.1176/ajp.115.5.459
Lacasse, J. R., and Leo, J. (2005). Serotonin and depression: a disconnect between the advertisements and the scientific literature. PLoS Med. 2:e392. doi: 10.1371/journal.pmed.0020392
Lapin, I. P., and Oxenkrug, G. F. (1969). Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1, 132–136. doi: 10.1016/s0140-6736(69)91140-4
MacQueen, G., and Frodl, T. (2011). The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol. Psychiatry 16, 252–264. doi: 10.1038/mp.2010.80
Mahar, I., Bambico, F. R., Mechawar, N., and Nobrega, J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 38, 173–192. doi: 10.1016/j.neubiorev.2013.11.009
Marsteller, D. A., Barbarich-Marsteller, N. C., Patel, V. D., and Dewey, S. L. (2007). Brain metabolic changes following 4-week citalopram infusion: increased 18 FDG uptake and γ-amino butyric acid levels. Synapse 61, 877–881. doi: 10.1002/syn.20428
Martinez-Turrillas, R., Frechilla, D., and Del Río, J. (2002). Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology 43, 1230–1237. doi: 10.1016/s0028-3908(02)00299-x
Monroe, S. M., and Harkness, K. L. (2005). Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol. Rev. 112, 417–445. doi: 10.1037/0033-295x.112.2.417
Monteggia, L. M., Gideons, E., and Kavalali, E. T. (2013). The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol. Psychiatry 73, 1199–1203. doi: 10.1016/j.biopsych.2012.09.006
Mulinari, S. (2012). Monoamine theories of depression: historical impact on biomedical research. J. Hist. Neurosci. 21, 366–392. doi: 10.1080/0964704X.2011.623917
Murrough, J. W., Perez, A. M., Pillemer, S., Stern, J., Parides, M. K., aan het Rot, M., et al. (2013). Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74, 250–256. doi: 10.1016/j.biopsych.2012.06.022
National Institute for Health and Care Excellence. (2009). Depression in adults: recognition and management. Available online at: https://www.nice.org.uk/guidance/cg90/chapter/1-Guidance-stepped-care . [Accessed on January 12, 2017].
Newport, D. J., Carpenter, L. L., McDonald, W. M., Potash, J. B., Tohen, M., Nemeroff, C. B., et al. (2015). Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 172, 950–966. doi: 10.1176/appi.ajp.2015.15040465
Oswald, I., Brezinova, V., and Dunleavy, D. L. F. (1972). On the slowness of action of tricyclic antidepressant drugs. Br. J. Psychiatry 120, 673–677. doi: 10.1192/bjp.120.559.673
Paul, I. A., and Skolnick, P. (2003). Glutamate and depression: clinical and preclinical studies. Ann. N Y Acad. Sci. 1003, 250–272. doi: 10.1196/annals.1300.016
Phillips, M. L., Chase, H. W., Sheline, Y. I., Etkin, A., Almeida, J. R., Deckersbach, T., et al. (2015). Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am. J. Psychiatry 172, 124–138. doi: 10.1176/appi.ajp.2014.14010076
Pittenger, C., and Duman, R. S. (2008). Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33, 88–109. doi: 10.1038/sj.npp.1301574
Pizzagalli, D. A. (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 10, 393–423. doi: 10.1146/annurev-clinpsy-050212-185606
Popoli, M., Gennarelli, M., and Racagni, G. (2002). Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord. 4, 166–182. doi: 10.1034/j.1399-5618.2002.01159.x
Racagni, G., and Popoli, M. (2008). Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin. Neurosci. 10, 385–400.
Rial, D., Lemos, C., Pinheiro, H., Duarte, J. M., Gonçalves, F. Q., Real, J. I., et al. (2015). Depression as a glial-based synaptic dysfunction. Front. Cell. Neurosci. 9:521. doi: 10.3389/fncel.2015.00521
Risch, N., Herrell, R., Lehner, T., Liang, K. Y., Eaves, L., Hoh, J., et al. (2009). Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA 301, 2462–2471. doi: 10.1001/jama.2009.878
Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Depression. (2004). Australian and New Zealand clinical practice guidelines for the treatment of depression. Aust. N Z J. Psychiatry 38, 389–407. doi: 10.1111/j.1440-1614.2004.01377.x
Ruhé, H. G., Mason, N. S., and Schene, A. H. (2007). Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatry 12, 331–359. doi: 10.1038/sj.mp.4001949
Ruhé, H. G., Visser, A. K. D., Frokjaer, V. G., Haarman, B. C. M., Klein, H. C., and Booij, J. (2014). “Molecular imaging of depressive disorders,” in PET and SPECT in Psychiatry , eds R. A. J. O. Dierckx, A. Otte, E. F. J. de Vries, A. van Waarde, and J. A. den Boer (Berlin, Heidelberg: Springer), 93–172.
Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., et al. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917. doi: 10.1176/appi.ajp.163.11.1905
Sanacora, G., Treccani, G., and Popoli, M. (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77. doi: 10.1016/j.neuropharm.2011.07.036
Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809. doi: 10.1126/science.1083328
Scheuing, L., Chiu, C. T., Liao, H. M., and Chuang, D. M. (2015). Antidepressant mechanism of ketamine: perspective from preclinical studies. Front. Neurosci. 9:249. doi: 10.3389/fnins.2015.00249
Schildkraut, J. J. (1965). The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry 122, 509–522. doi: 10.1176/ajp.122.5.509
Schildkraut, J. J., and Kety, S. S. (1967). Biogenic amines and emotion. Science 156, 21–37. doi: 10.1126/science.156.3771.21
Selikoff, I. J., Robitzek, E. H., and Ornstein, G. G. (1952). Treatment of pulmonary tuberculosis with hydrazine derivatives of isonicotinic acid. J. Am. Med. Assoc. 150, 973–980.
Serafini, G. (2012). Neuroplasticity and major depression, the role of modern antidepressant drugs. World J. Psychiatry 2, 49–57. doi: 10.5498/wjp.v2.i3.49
Shakesby, A. C., Anwyl, R., and Rowan, M. J. (2002). Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J. Neurosci. 22, 3638–3644.
Siesser, W. B., Sachs, B. D., Ramsey, A. J., Sotnikova, T. D., Beaulieu, J. M., Zhang, X., et al. (2013). Chronic SSRI treatment exacerbates serotonin deficiency in humanized Tph2 mutant mice. ACS Chem. Neurosci. 4, 84–88. doi: 10.1021/cn300127h
Stahl, S. M. (1984). Regulation of neurotransmitter receptors by desipramine and other antidepressant drugs: the neurotransmitter receptor hypothesis of antidepressant action. J. Clin. Psychiatry 45, 37–45.
Stahl, S. M. (2013). Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 4th Edn. Cambridge, MA: Cambridge University Press.
Thomas, E. J., and Elliott, R. (2009). Brain imaging correlates of cognitive impairment in depression. Front. Hum. Neurosci. 3:30. doi: 10.3389/neuro.09.030.2009
Vose, L. R., and Stanton, P. K. (2017). Synaptic plasticity, metaplasticity and depression. Curr. Neuropharmacol. 15, 71–86. doi: 10.2174/1570159x14666160202121111
World Health Organization. (2016). Depression: fact sheet. Available online at: http://www.who.int/mediacentre/factsheets/fs369/en/
Yoshii, A., and Constantine-Paton, M. (2010). Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 70, 304–322. doi: 10.1002/dneu.20765
Young, E., and Korszun, A. (2010). Sex, trauma, stress hormones and depression. Mol. Psychiatry 15, 23–28. doi: 10.1038/mp.2009.94
Zunszain, P. A., Horowitz, M. A., Cattaneo, A., Lupi, M. M., and Pariante, C. M. (2013). Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol. Psychiatry 18, 1236–1241. doi: 10.1038/mp.2013.87
Keywords: major depressive disorder, serotonin, neuroplasticity, final common pathway, antidepressant efficacy
Citation: Liu B, Liu J, Wang M, Zhang Y and Li L (2017) From Serotonin to Neuroplasticity: Evolvement of Theories for Major Depressive Disorder. Front. Cell. Neurosci. 11:305. doi: 10.3389/fncel.2017.00305
Received: 05 March 2017; Accepted: 13 September 2017; Published: 28 September 2017.
Reviewed by:
Copyright © 2017 Liu, Liu, Wang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, [email protected] Lingjiang Li, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- About / Top Posts

- Bryan Caplan
- David Friedman
- Pseudoerasmus
- Scott Sumner
- Tyler Cowen
Effective Altruism
- 80000 Hours Blog
- Effective Altruism Forum
- GiveWell Blog
Rationality
- Alyssa Vance
- Elizabeth Van Nostrand
- Gwern Branwen
- Jacob Falkovich
- Jeff Kaufman
- Katja Grace
- Kelsey Piper
- Paul Christiano
- Robin Hanson
- Sarah Constantin
- Zvi Mowshowitz
- Andrew Gelman
- Greg Cochran
- Michael Caton
- Scott Aaronson
- Stephan Guyenet
SSC Elsewhere
- SSC Discord Server
- SSC Podcast
- SSC Subreddit
- January 2021
- September 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- February 2013
- Full Archives
What Is Depression, Anyway?: The Synapse Hypothesis
The problem with depression research isn’t that we don’t have any leads on what causes depression. It’s that we have so many leads on what causes depression that we don’t know what to do with all of them. For example:
1. Life adversity, like getting fired or breaking up with a partner, can make people depressed. The biological correlate of this seems to be the hypothalamic–pituitary–adrenal axis (HPA), where your brain tells your adrenal glands to produce glucocorticoid stress hormones like cortisol and this does something to your brain that increases the risk of depression.
2. Inflammation and immune overactivity can make people depressed. The classic examples of this are cancer-related depression (which exceeds what you would expect just from cancer being stressful) and depression induced by administration of the immunomodulator interferon-a. Antiinflammatory drugs have a small but clinically relevant antidepressant effect. Some of the relevant chemicals here seem to be TNF-A and IL-1; these do something to your brain that increases the risk of depression.
3. Serotonin and other monoamines seem to be involved. Most existing antidepressants, like SSRIs and MAOIs, seem to work by increasing monoamine levels. There are some conditions which affect monoamine levels and also increase risk of depression, though it’s nothing like a perfect correlation.
4. The glutamate system (eg NMDA and AMPA receptors) seem to be involved. Ketamine acts on both of these receptors in different ways, and one of those actions is the source of its rapid and unprecedented antidepressant effects.
5. There’s some kind of important link between depression and folate balance. Various folate-related chemicals (eg l-methylfolate and s-adenosylmethionine) are effective antidepressants . Some studies show that people with depression sometimes have disrupted folate cycles, for example elevated homocysteine levels .
6. Electroconvulsive therapy (“shock therapy”) is very effective at treating depression if it induces a seizure in the patient, so the increased activity from seizures must be helpful somehow.
So if we wanted to know what depression really was, it might be promising to look for some process that seems to match depressive symptoms and affects/is affected by life adversity, inflammation, monoamines, glutamate, folate, and electricity.
Recently some people think they’ve found one. According to Duman’s Neurobiology of Stress, Depression, and Rapid Acting Antidepressants , it’s decreased synaptogenesis, and it’s regulated by a protein complex called mTORC1.
Neurons communicate with other neurons through branches called dendrites and connections called synapses. Healthy neurons often create new dendrites and synapses to expand their network of connections and adjust to new information. The process of making new synapses is called “synaptogenesis”, and it’s common throughout the adult brain.
As mentioned above, depressed people have decreased volume in some brain areas. But in postmortem studies, they don’t actually have fewer cells in those areas. So it looks like maybe these neurons just have less synaptogenesis going on.
Synaptogenesis is partly controlled by a protein complex called mechanistic target of rapamycin complex 1 (mTORC1 to its friends). Like every other protein, mTORC is controlled by a giant mess of receptors and second messengers and intracellular signals with names like VDCC and GSK3.
People try to make this seem simple by displaying it as a system of billiard balls and tubes in a cute cartoon, but don’t be fooled – no human being has ever remembered any of it for more than two seconds.
The factors that affect synaptogenesis and mTORC are many of the same factors that affect depression. Let me count the ways:
1. Life adversity causes chronic stress, biologically represented by upregulation of the HPA axis and increased corticosteroid production. A 2008 study finds that rats who are subjected to chronic stress develop atrophy of dendrites in their prefrontal cortex. Administering glucocorticoids directly mimicked some of these effects, suggesting that stress is a whole cocktail of things including glucocorticoids and other things. When humans take glucocorticoids (they’re a useful medicine for various diseases) they tend to develop hippocampal atrophy and “simplification of dendrites” there, which I think is the same as decreased synaptogenesis. They also tend to get depressed – in some studies of Cushing’s Syndrome (the medical name for the collection of bad things that happen when you take too much glucocorticoid medication), up to 90% of patients are depressed .
2. I didn’t find the linked paper’s attempt to link inflammation to synaptogenesis very convincing, but it looks like there’s a little bit of research that has found that systemic inflammation decreases synaptogenesis. “Morphometric analysis of dendritic spines identified a period of vulnerability, manifested as a decrease in [dendritic] spine density in response to inflammation. The density of presynaptic excitatory terminals was similarly affected. When the systemic inflammation was extended from 24h to 8 days, the negative effects on the excitatory terminals were more pronounced and suggested a reduced excitatory drive.” This seems pretty relevant.
3. Everyone used to think that traditional antidepressants like SSRIs worked by increasing serotonin (and so by extension depression must have something to do with low serotonin levels). But SSRIs increase serotonin very quickly (within hours) yet take months to work. Something longer-term must happen when serotonin levels have been increased for long enough. That something has now been pretty conclusively identified as an increase in brain-derived neurotrophic factor (BDNF) – although I can’t find any good explanation of why increased serotonin should cause increased BDNF after a month. BDNF is a nerve growth factor – its main action is activating mTORC and telling nerve cells to grow more dendrites and synapses. And it’s most active in the cortex and hippocampus.
4. Ketamine affects the brain by either blocking NMDA receptors (boring traditional explanation), activating AMPA receptors ( exciting new explanation ), or possibly both (wishy-washy neoliberal compromise explanation). Duman et al are kind of ambiguous about which explanation they accept, but I think they present a theory where NMDA blockade causes AMPA activation, or something, which I’d never heard before. In any case, they present ample evidence that AMPA rapidly affects BDMF and dendritogenesis – for example, Positive AMPA Receptor Modulation Rapidly Stimulates BDNF Release And Increases Dendritic MRNA Translation . The “rapidly” part is important – the surprising thing about ketamine is how quickly it works compared to other antidepressants, so it’s exciting to find a theory that predicts this should happen.
5. I haven’t seen much attempt to fit folate into this theory, which is a shame. A quick Google search brings up a few people talking about how folate deficiency decreases neurogenesis in the hippocampus , which is sort of related.
6. Studies show that ECT increases BDNF levels and increases hippocampal volume , though I’m not sure exactly how or why giving someone a seizure should do that.
So the synapse hypothesis can unify at least five of the six lines of research into the causes of depression.
My remaining skepticism is mostly based on a worry that anyone can do this with anything. The body is so interconnected, and there’s so much bad biology research out there, that I worry that if I said that the real cause of depression was, uh, thickness of the blood, I could find some way that all of those lines of research above affected blood thickness.
A quick demonstration: glucocorticoids can cause thicker blood , inflammation can cause thicker blood , SSRIs cause thinner blood , folate causes thinner blood . Huh, actually that’s kind of creepy.
My point isn’t that the (very respectable) academic research on depression is anywhere near this silly. It’s just to explain why I can hear a theory that seems to explain everything beautifully and my only reaction is “Eh, sounds like it has potential, let’s see what happens.”
Here are some of the things that confuse me, or that I hope get researched more in the future:
1. Why should decreased synaptogenesis cause depression, of all things? If you asked me, a non-neuroscientist, to guess what happens if the brain can’t create new synapses very well and loses hippocampal volume, I would say “your memory gets worse and you stop being able to learn new things”. But this doesn’t really happen in depression – even the subset of depressed people who get cognitive problems usually just have “pseudo-dementia” – they’re too depressed to put any effort into answering questions or doing intelligence tests. Why should decreased synaptogenesis in the hippocampus and prefrontal cortex cause poor mood, tiredness, and even suicidality? All that the Duman et al paper has to say about this is:
This reduction in dendrite complexity and synaptic connections could contribute to the decreased volume of PFC and hippocampus observed in depressed patients. Moreover, loss of synaptic connections could contribute to a functional disconnection and loss of normal control of mood and emotion in depression (Fig. 1). In particular, the medial PFC exerts top down control over other brain regions that regulate emotion and mood, most notably the amygdala, and loss of synaptic connections from PFC to this and other brain regions could thereby result in more labile mood and emotion, as well as cognitive deficits.
…which sounds more like an IOU for a theory than anything really fleshed out.
2. Why can’t we just give people BDNF for depression? I’ve been looking into this and it seems like the answer is something like “this works great if you cut open someone’s skull and inject it directly into their brain, but most people aren’t up for it” (the relevant studies were done in rats). But why can’t it be given peripherally? Some studies suggest it’s stable on injection and crosses the blood-brain barrier . Some people tried this in mice and got modest results , but why aren’t people looking into it more?
3. Why does the body have so many “decrease synaptogenesis” knobs? That is, why go through the trouble to evolve all these chemicals and systems whose job is to tell your brain to decrease synapse formation so much that you end up depressed? Is there some huge problem with having too much synapse formation which the brain is desperately trying to avoid? For that matter, what is it like to have too much synapse formation? If it’s the opposite of depression, it sounds kind of fun. If I got someone to open up my skull and inject a lot of BDNF, could I be really happy and energetic all the time? How come all the good stuff is always reserved for rats?
4. Why is depression an episodic disease? That is, how come so many people get depressed for no reason, stay depressed for a few months to a few years, and then get better – only to relapse back into depression a few years later? If people get depressed because of some life stressor like a divorce, how come they don’t get un-depressed once the life stressor goes away? Is depression some kind of attractor state? If so, why?
5. Why doesn’t rapamycin cause depression? Remember, mTORC is “mechanistic target of rapamycin”, so named because the drug rapamycin inhibits it. But we give people rapamycin for various things all the time, and depression isn’t really known as a major side effect (even though IIRC it crosses the blood-brain barrier). If depression is really under the immediate control of mTORC, rapamycin should be the most depressive thing. Instead it’s not obviously depressive at all.
6. How does bipolar disorder fit into all of this? Is mania the answer to my “what is it like to have too many synapses?” question from point (3)? If so, why do some people go back and forth between that and depression?
A lot of these questions could be answered in one stroke if we had a good evolutionary theory of depression. I’m skeptical that this exists – depression just seems too fitness-decreasing, and the various just-so stories people have come up with for why it might increase fitness in certain weird situations seem a little too convoluted. So it’s not that I’m expecting some sort of evolutionary story to work out. Just noticing that, even if the synapse theory of pathophysiology turns out to be right, there’s still a lot more that needs to be explained.
147 Responses to What Is Depression, Anyway?: The Synapse Hypothesis
“A lot of these questions could be answered in one stroke if we had a good evolutionary theory of depression. I’m skeptical that this exists – depression just seems too fitness-decreasing, and the various just-so stories people have come up with for why it might increase fitness in certain weird situations seem a little too convoluted. So it’s not that I’m expecting some sort of evolutionary story to work out. Just noticing that, even if the synapse theory of pathophysiology turns out to be right, there’s still a lot more that needs to be explained.”
I think at the basic level there is a good evolutionary base for why depression exists. An organisms optimal activity level depends heavily on its environment. You don’t want to spend your winters wandering around looking for scarce food when you should be hibernating, you don’t want to fight with other members of your clan/tribe after challenging and alpha and losing and you don’t want to flower at the wrong time of year. Having multiple energy states that your body cycles through makes a lot of sense.
I don’t know if fleshing this theory out will help anything though. It would if depression is an important function, but it won’t if depression is the symptom of an important function getting messed up for one reason or another, and it doesn’t generate clear pathways to investigate for depression if the body is as complicated as you say (which it is).
Depression may exist because it can amplify sexual selection pressures. If you get dumped, but you stay upbeat about it, find another mate, and go on with life, maybe your tribe’s gene pool is worse off than if you had gotten depressed about it and dropped out of the race all together.
I think this is interesting. I was about to suggest that stress itself, broadly defined, might work similarly, as an amplifier of minor differences in fitness. Populations with large degrees of genetic similarity may trend towards a Malthusian situation in which every individual is on the margins of survival, leaving the entire population vulnerable to small normal fluctuations in resource availability. Some mechanism for avoiding that situation seems adaptive in populations. Though it’s not precisely the same thing, consider how many species have adapted so that the young eliminate and in many cases eat their siblings, usually as a result of the ‘fitness’ of higher birth order. The problem this is overcoming is insufficient resources for offspring to survive. In the absence of birth order, it would seem that some biologically primed mechanism for eliminating the less successful (though possibly genetically identical) individual at little cost to the more successful individual helps assure the survival of the genetic legacy of both, and certainly the genetic legacy of the parents.
I believe in the Selfish Gene a group of birds are discussed in which after the mating season the birds who failed to procure a mate go off on their own and typically die before the next mating season. These birds passively observe other birds and, if a mate opens up, they can then procure a mate for the season. This strategy isn’t maladaptive as typically the behavioural costs of struggling to find a new mate (Competing with another male) are higher.
This sounds like a variant of the mechanism you have proposed. Such a strategy makes the group better adapted for dealing with the problem of having a surplus of unfit males and reduces competition for resources in malthusian conditions. However, such a gene isn’t actively selected against as if you are a carrier the gene is also beneficial. (Reduces probability of you dying wasting energy trying to procure a mate someone else has already procured)
Sounds wrong. Any maladaptive trait that’s likely to take you out of the gene pool has to have some pretty hefty selective pressure on it for it to become so common. From what I remember the rule of thumb is double your siblings chance of reproduction, then double that for cousins, and so on.
If I understand it right, it doesn’t have to double the sibling’s fitness, only increase it at least 2x as much as it decreases the gene bearer’s own fitness. So if a gene decreases it’s bearer’s offspring by 1%, and increases his sibling’s offspring by 2%, then it spreads right?
If only a small effect was needed, maybe depression was supposed to function in small doses, tempered with lots of exercise.
Depression may exist because it can amplify sexual selection pressures. If you get dumped, but you stay upbeat about it, find another mate, and go on with life, maybe your tribe’s gene pool is worse off than if you had gotten depressed about it and dropped out of the race all together.
This is exceedingly unlikely, as others have pointed out. It is basically impossible for you to have an adaptation that kills you without leaving offspring but improves your overall group fitness by helping some distant relative.
Additionally most depressed people don’t commit suicide, many (most?) people will undergo at least one depressive episode in their lives, but they generally don’t kill themselves.
maybe your tribe’s gene pool is worse off than if you had gotten depressed about it and dropped out of the race all together.
Remember that a gene coding for this behavior wouldn’t know or care about the purity of your tribe’s gene pool. Genes exist to make more of themselves, and people with suboptimal genetics (cripples, retards, etc) strive to reproduce just like everyone else.
The idea that depression is s specific response to being dumped reminds me of reminds me of Kevin Simler’s theory that tears evolved as a specific response to bullying.
http://www.meltingasphalt.com/tears/
The being-dumped theory could be generalised into a theory that depression is way of responding to status-lowering events, a way of saying “I get the message, I will be low-statussy from now one”.
That doesn’t entirely contradict the idea that depression is a energy-saving adaptation, since signals have to come from somewhere. Simler thinks that crying evolved as a signal from the physiological response to being punched on the nose
Signalling theory also explain its apparent maladaptiveness: that’s the cost of the costly signal.
(But it’s less adaptive in modern societies, where stressors tend to be impersonal forces).
My awful, unrigorous, anecdotal, biased theory of depression (my source: many years of it) is: a) (my) depression seemingly WAS some combination of social factors: not being taken seriously, lacking power , not having things to be proud of, or not being able to model where future self-esteem/pride/being-taken-seriously were going to come from. I don’t really mean to assert those things caused the depression, or that depression caused me to think those things, though I think the former is true.
And b) depression was incompatible with actual, practical survival instincts. When money got short, depression left me and was replaced by something else, stress about finding a job, cheap food, etc. This stress WAS my body knowing it needed to locate resources for the future.
That’s my impression at this point, anyway. Perhaps this is useless or trivial. Depression left me (mostly for good) when I found a way to take some control of my life, moved cities, switched fields & greatly increased income (to tech, so basically like cheating). Did I do those things because depression departing allowed me to? Not sure.
Probably I’m ignorant. I do think it’s important (and probably hard) to distinguish between what depression IS and what’s causing it.
This makes a whole lot of sense to me as an ecologist/evolutionary biologist. At least at first blush, depression looks an awful lot like ‘energy-saving mode’ for an organism. Sleep a ton–check. Don’t move around a lot–check. Don’t leave your home/den/cave–check. It also nicely ties in a seventh common trigger for depression that Scott doesn’t touch on: seasonality. I’m from Alaska originally, and it’s common knowledge that during the winters you need to maximize use of limited daylight or get one of those UV happy lamps to stave off Seasonal Affective Disorder (SAD).
If you think about populations of animals that are resource limited in environments with variable resource availability, you’d sure as shit expect an evolutionarily stable strategy to come about that looked an awful lot like ‘energy-saver mode’. And you’d want it to be potentially triggerable in a variety of ways, including general stress, seasonality, and potentially things like traumatic events (if I’m getting the shit kicked out of me in life, it probably means I don’t have the best access to resources, and I should turn on energy-saver mode). It looks like the connection between animal metabolic depression (i.e. torpor/aestivation/hibernation) and clinical major depression has been made before in the medical literature .
As far as why this might be so seemingly maladaptive in humans, it could just have something to do with all the downtime spent in pseudo-hibernation/torpor opens up opportunity for existential dread to set in (I don’t imagine this is a problem for, say, our pro-simian ancestors). 21st century humans have a lot of evolutionary baggage.
It’s a clever idea, but I wonder about the side effects. We have a couple of other energy-saving modes that point toward hanging around and not doing very much. Tiredness, for example, or the malaise that comes with flu-like illness. Both pretty much do what it says on the box and not much more. If we’ve got these, why would we evolve a completely different mechanism that comes with all these random dysfunctions?
One possibility is time-scale. You’d want sleepiness to be pretty well synced to your circadian rhythm, but torpor/hibernation to be a more seasonal, stickier state.
There are situations where tiredness doesn’t cut it. Take fighting over mates, this is often ritualized in a lot of animals. Big horn sheep, as an example, butt heads until one just sort of wanders off aimlessly, this is a great result evolutionary for both participants. They could easily continue on until one dies from injuries/exhaustion but then not only does that one not reproduce, but the opposition is also likely to be exhausted/injured by the end and will be greatly reduced in his ability to mate.
A mechanism that allows the loser to signal (and credibility is key, the winner doesn’t want to have to battle another challenger and have the initial loser comeback refreshed for another shot) that they are done allows both to gain.
So we’re basically saying that depression is hibernation gone haywire?
If that’s the case, we should find it more prevalent in human populations that evolved in colder areas, and probably close to non-existent in Africans. Do we find that?
(Then again, maybe there are relevant seasons there, too. But surely we should find some sort of correlation with ancestral climate.)
Apparently, it is rampant in Scandinavia, but this is attributed to vitamin D deficiency.
To the extent to which suicide rates are a proxy for depression…
Black people have a lower suicide rate than white people
http://students.com.miami.edu/netreporting/?page_id=1285
I suspect there is more directly on point data somewhere
‘Hibernation gone haywire’ is a catchy handle, but a bit different from what I would contend or the author of the paper I cited would probably contend (caveat: haven’t had a chance to read the main text yet). What I’m saying is that metabolic depression (which underpins hibernation, aestivation, and other forms of extended torpor in animals) in humans may be what we identify as major depression.
That’s still a long way from saying that depression = hibernation. I can’t think of any primates that undergo hibernation or aestivation, so we’re talking about physiological pathways that long ago would have been evolutionarily co-opted from creating a true hibernation state, to a softer, torpor-like ‘energy-saver mode’ (alternatively, there could have been an independent evolutionary origin of bistable mood/activity states). To give just one example, I know white-faced capuchins in dry forests of Central America face extreme seasonality in food availability, and researchers who study them have a hundred different ways of quantifying just how lethargic they are during the dry season.
All that’s to say that even if the evolutionary origin of major depression in humans is the same physiological/neurological state as hibernation, tens of millions of years of evolutionary history would separate the two and one would expect that they would look pretty different. It could even be vestigial physiology (i.e. evolutionary baggage that hasn’t been selected out of the gene pool yet), and certainly to the degree that it’s maladaptive in modern humans, it is. If that’s the case, you might even expect less clinical features of depression (e.g. suicidal thoughts) in polar human populations that have existed in those regions for a sufficient number of generations (since selection would have a chance to weed out some of the problematic aspects of human ‘energy saver mode’). A stronger prediction of this theory would be high levels of major depression in populations of humans from seasonal mid-latitudes translocated to higher-latitudes during winter.
I think the more immediate questions derived from the theory to test at this point would be things along the lines of ‘do we see similar physiological pathways in animals (especially primates) that experience strongly seasonal resource availability as we do in hibernating animals (especially mammals)?’ or ‘is there genetic evidence of shared evolutionary origin of pathways underpinning metabolic depression in primates and hibernation in other mammals?’ If it is shared ancestral genetic architecture, it would be really interesting to figure out at what point in our evolutionary tree the state became triggerable by things like traumatic events, low social standing, etc. Also it would be interesting to see if apes in very seasonal habitats have clear depressed and elevated metabolic equilibria, and if any of the characteristics of the former look like symptoms of major depression. I bet there’s clear bistable metabolic equilibria that have a lot of behavior manifestations for orangutans (Southeast Asia’s crazy dipterocarp forests are crazy variable in resource availability–basically its a bumper ‘mast’ crop of fruit every few years, then very little in between).
This also is my go-to hypothesis.The decrease in synaptogenesis also fits with this. The brain uses about a fifth of the calories we expend, and growing stuff always costs a lot of energy. It makes sense to me that if you go into forced hibernation mode you not only stop building stuff but you slack on the upkeep too. This would also explain the atrophy seen in existing structures.
I’d agree as well, with the caveat that this explanation might be even more applicable to bipolar disorder than it is to depression. If depression is the energy-saving response to low resource conditions, then the obvious explanation for mania/hypomania is that it is the inverse effect – upregulation of energy expenditure in response to a perceived increase in resources. If that’s correct, then the first hypothesis that comes to mind for bipolar disorder is that people with it have lower-than-usual thresholds for activating the “low resources, save energy” and “high resources, go nuts” modes.
That doesn’t explain depression quite as well, though. The best hypotheses I can think of are depression involving missing signals to come out of energy-saving mode (or not those signals just not being there?) and/or the depression energy-saving mode running headlong into some other effect (Durkheim’s original work on anomie comes to mind here).
This makes a whole lot of sense to me as an ecologist/evolutionary biologist. At least at first blush, depression looks an awful lot like ‘energy-saving mode’ for an organism. Sleep a ton–check. Don’t move around a lot–check. Don’t leave your home/den/cave–check. It also nicely ties in a seventh common trigger for depression that Scott doesn’t touch on: seasonality. I’m from Alaska originally, and it’s common knowledge that during the winters you need to maximize use of limited daylight or get one of those UV happy lamps to stave off Seasonal Affective Disorder (SAD).
Also muscle loss and fat gain, which occur in depression and are specific side effects of corticosteroid therapies.
Synapses are metabolically expensive: an adult brain consumes 20% of the body energy intake, the brain of a young child, around the age of peak synapses, consumes over 40% of the body energy intake. Therefore, decreased synaptogenesis in the brain is probably another energy saving mechanism. Maybe it does not cause depression per se, but it is a correlate of it.
Humans wouldn’t have evolved such a system in tropical Africa, and it’s unlikely there was enough time to evolve this complex of a system in the few tens of thousands of years they’ve been in cold climates. It couldn’t have come from Neanderthals or anything because we find it in lots of different racial groups in pretty much equal amounts.
More to the point, depression only correlates a little with the season. And it has some features – like suicidality – which don’t make sense in a hibernation context. And early humans don’t seem to have had a tendency to store up enough food for proper hibernation. And we see hunter-gatherers all the time, including some far-northern ones, and none of them do anything like hibernating.
The one about not wasting your time in losing situations makes a little more sense, but still not much. Why become suicidal and insomniac after you lose something? How do you know it’s not better to work extra hard to maintain your place in the group and make up for what you lost? If you’re already in people’s bad books, isn’t stopping all work pretty much the worst response? Don’t people already know how to lay low without being told by a mental state which most of the time misfires and results in your death or impoverishment?
These are exactly the kinds of just-so story that make me very suspicious of the whole field.
Why become suicidal and insomniac after you lose something?
I think of it more as driven to ignore pain and make a change. Much like a wolf will (allegedly) gnaw off its own leg to escape a trap. For hunter-gatherers, perhaps this manifests itself as fleeing the current tribe with the possible result of being adopted by another tribe. In today’s culture that could look like going to Tibet to “find yourself”, running off and joining the circus, or committing suicide.
I think the much more compelling version of this just-so story involves inherited, rather than de novo genetic architecture (more mammalian dive reflex than… I dunno, speech?). Bistability (or adjustable set points, to use a control theory framework) of metabolic state is almost certainly an ancestral characteristic. I bet there was an early mammal/mammal precursor somewhere along the line that hibernated, and from first principles that physiology would be super useful to maintain or tweak in any environment with seasonal/interannual cycles in resource availability.
To get even more just-so, in primates you might even predict that that system would evolve toward triggerability by low social status. If you’re the omega of a troop of monkeys, you probably get last pick of food resources, and you’d want to start drifting toward ‘low-activity equilibrium’ sooner than if you’re alpha.
A few hundreds of thousands of years of hominid evolution probably isn’t enough to scrub out such a deeply ingrained physiology, I’d think. Plus, if ‘human depression = vestigial torpor’ is right, my hunch is that it wouldn’t be that maladaptive in hunter-gather humans, both because it allows them to appropriately respond to resource availability, and because you’re probably going to be forced into being functional anyway, or else starve/get left behind by your clan. It was only super relatively recently in evolutionary time that humans encountered situations where we’re capable of storing up enough food to last long enough for depression to get so severe you commit suicide.
Humans wouldn’t have evolved such a system in tropical Africa
The oldest human remains we have don’t come from tropical Africa (I don’t even concede that point as food scarcity isn’t the only reason it could be adaptive), they come from Ethiopia and (now) Morroco.
Why not? All that’s needed for the hypothesis is alternating times of dearth and times of plenty. A dry season would work as well for the hypothesis as a cold season.
and it’s unlikely there was enough time to evolve this complex of a system in the few tens of thousands of years they’ve been in cold climates.
It seems like an amplification of the “bad mood”/”lethargy”/”learned helplessness” systems which many animals have for various reasons. A whole new system doesn’t need to be evolved; you just need to turn an existing system up to 11, which is an easier evolutionary challenge. I’ve read somewhere – sorry for forgetting where – that the personality of North American wolves was altered toward excessive shyness after only a few generations of hunting which sought to wipe them out completely, and that they’re getting bolder now that hunting has decreased. Foxes were domesticated in half a century in a classic Soviet/Russian experiment, presumably by neoteny. With an existing system and strong selection pressure, personality traits can be altered fairly rapidly.
I’m not saying that it did happen, but it’s not a ridiculous hypothesis.
There’s a reason I framed it as regulating energy expenditure in response to perceived changes in resources (and apparently did a bad job of it). I’d expect access to food and mates to be more relevant to those systems overall (if you don’t have current access to resources, conserve what resources you have so that you are more likely to live to the next point resources are abundant), with hibernation and estivation are the extreme form of the general effect appearing when you get severe seasonal changes in resource availability, and food/mate access (and, by proxy, status) were definitely important to human ancestors!
I remember some research a few years back about some fans of sports teams seeing a testosterone boost/drop when their team wins/loses, respectively. If I’m right then the systems responsible for that are also responsible for bipolar/depression. (PPE: Actually, I’m slow. Forget sports fan blood testosterone, the obvious modern example of upregulating in response to resource/status/mate availability is the Baby Boom .)
Which, now that I actually think about it, offers an obvious test for the up/down regulation hypothesis: is there an inverse correlation between blood testosterone levels and bipolar/depression? If there is no such correlation then that’s a hit to this energy regulation hypothesis, though it could also be due to varying sensitivity to whichever molecule or molecules are responsible.
(That doesn’t explain suicide, but I suspect suicide is another matter entirely and just piggybacking on depression mechanisms.)
Clinical depression may be just an extreme and maladaptive form of a normal and adaptive behavioral and physiological mechanism: sadness, melancholia, being pissed off, whatever you want to call it.
The Discovery of France has an interesting historical example of human semi-hibernation . As thoramboinensis mentions in their example from Alaska, seasonality was the driver in France, too. If growing seasons are such that a big expenditure of energy during some times of the year will yield minimal returns, it would be idiotic for most people not to semi-hibernate. It’s going to increase your chance of death, and reduce the resources you have available to feed your children.
What’s weird is the modern era, in which fossil fuels mean that there is endless-ish energy available all the time. People who are “on” all the time are suited to this new environment, but they would’ve been at a disadvantage through most of human history.
The cognitive features of depression are interesting, too. Last I looked at the research, cognitive approaches to the treatment of depression worked as well as any of biochemical approaches. Animals which can’t speak can suffer obvious analogues of human depression, so it’s not purely a cognitive thing, but our internal monologues have an amplifying effect. It’s a reminder that, while DNA and genes hold a lot of information and decision-making power, they have ceded some of that power to neural networks which can learn and adapt more quickly. We use narratives to guide some of our decision-making, including decisions about whether or not to get out of bed in the morning. How do you map that to the physical, chemical and electrical events happening in the brain? I have no idea, but the relative effectiveness of cognitive therapies suggests that a mapping exists, even though it’s too complicated for us to map at this point.
Wait. “Life adversity” can affect synapto-genesis? But, but, that can’t be.
We just had a 300-comment post that took as an assumption that inter-group differences in intelligence must be mostly genetic, despite clear differences between groups in the levels of “life adversity” which seem to correlate pretty well with intelligence.
Much of the discussion was based on a 10-year old paper positing the primacy of beneficial synaptic effects (well, dendritic, but you also equate synaptic and dendritic effects) of otherwise deleterious mutations.
You should have told us about this adverse life issue earlier, Scott. It might have improved the quality of the discussion. A lot of commenters look rather ridiculous now.
Before I started jumping to conclusions here, I would want to know what “life adversity” means specifically and how it cashes out in inter-group differences. Most minority groups in the US have lower rates of depression than whites (although women have higher rates than men, IIRC), so if we’re using that as a proxy it doesn’t seem to fit the model of oppression -> stress -> lower synaptogenesis -> poor outcomes.
Doesn’t entirely matter. Scott’s gloss of the study is that life adversity reduces synaptogenesis. What Cochran and Harpending argued was that it was increased “dendritogenesis” in people with Tay Sachs and Niemann-Pick genes that led to increased intelligence. The fact that depression can be another expression of reduced synaptogenesis, an alternate pathway for the pathology when circumstances are different, doesn’t really change the devastating implications for Cochran and Harpending, since the demographics of “life adversity” are much more consistent with population intelligence than the demographics of Tay Sachs or Niemann Pick.
I’m editing to add that I agree with you about jumping to conclusions. I don’t think Scott’s reference to a 2008 study of rats is proof positive that stress is the primary cause of inter-population intelligence differentials. That would be jumping to conclusions.
What I believe pretty strongly is that Cochran and Harpending jumped even farther. And that Scott’s reference is a very big strike against them. I find it bizarre that so many commenters two weeks ago clung to such a poorly proven theory. Or rather, I find it self-serving.
since the demographics of “life adversity” are much more consistent with population intelligence than the demographics of Tay Sachs or Niemann Pick.
The point is more that we don’t have the data for statements like this. The examples of life adversity given in the OP are divorce and losing a job; these are rare, severe, acute events. I don’t think we even have a good model for what the stress of oppression looks like, but I’ll bet it isn’t much like the stress of divorce.
Might have to get even more specific. Neither divorce nor job loss have to be stressful events. Things like “loss of security”, “animosity”, “interpersonal conflict” would be better terms to focus on.
Divorce is tricky though, happy marriages don’t end in divorce, so while the actual event is a one off, the build up is often about years of stress. This is often (but less often) the case with job loss as well.
I think you raise interesting complications.
I wasn’t actually thinking of “oppression”, whether as an abstraction or in the post-modern “micro-aggression” mode.
I was thinking more of direct causes, like high rates of violence or a parental reliance on negative reinforcement. (I cut most of my comment b/c I feel I’ve hijacked an interesting post on depression.)
@alwhite Stress doesn’t really have to do with whether something is good or bad though. It mostly has to do with whether something is different. Both getting married and getting divorced are very stressful events. Anything that means you have to adapt to a new circumstance, whether that circumstance is good or bad for you, is going to be stressful.
Is the the theory of eustress and distress disproven? If not maybe getting married counts as eustress while divorce as distress.
Yet divorce has been used to compare adaptive and non-adaptive theories of depression in this study . Granted, antidepressant purchases might not be the best proxy for depression, but the best models here are the adaptive and stress-relief
the demographics of “life adversity” are much more consistent with population intelligence than the demographics of Tay Sachs or Niemann Pick.
Yep, early 20th century Jews sure had remarkably little life adversity.
You’re saying that sarcastically, but it’s true of most of the remarkable Jews who formed our impression of Central European Jewish genius. They were mostly raised in well-off homes, and experienced only brief adversity.
If you were able to get intelligence scores which included all the Jews who experienced long-term, grinding childhood suffering, anxiety and malnutrition as a result of pogroms and prejudice, I suspect that the average IQ would come down a bit.
This assumes there is a base standard for “depression”. If people respond relative to their expectations (which could or could not be true) you would have people with high levels of perpetual stress not identify their depression as it would be within their normal range of emotions. Just one hypothetical where this line of reasoning wouldn’t work.
Sure. Could be other reasons for demographic differences in reporting, too; to take men vs. women as an example, men might be acculturated to minimize their symptoms and stay out of the doctor’s office as long as possible. This would look in the medical stats like rarer but more severe depression.
These are all pretty standard issues in medical surveys, though, and we can figure them out.
A quick search turns up a study which suggests that African Americans have a higher rate of depression, but a lower rate than what might be predicted after an attempt to control for confounding variables.
“Results. African Americans … exhibited elevated rates of major depression relative to Whites. After control for confounders, Hispanics and Whites exhibited similar rates, and African Americans exhibited significantly lower rates than Whites.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1199525/
Sunlight, exercise, and coffee also increase synaptogenesis. I don’t think it means what you think it means, or implies what you think it implies. And depression has no effect on intelligence (as far as we know).
This comment is the kind of combination of mean, misunderstanding, and bringing-culture-wars-into-a-previously-unsullied-thread that may get people banned in the future.
That’s unfair. I admitted it isn’t definitive, but pointed out that synaptogenesis is absolutely critical to Cochran and Harpending, whose study was the basis of dozens of replies to your Hungarian piece, most of which were actually mean and culture-war oriented.
On reflection, I think it fair to describe my initial post as mean. It was troll-like. I want to apologize to the host. I did offer caveats and balance in my replies to others.
I am not a neuroscientist or anything close, so apologies if this is totally off base. But re: the question of why decreased synaptogenesis leads to depression instead of memory/learning effects – is it possible that there’s a control system-y answer here? I can tell a vague sort of story where having fewer connections available means the brain has to adapt its behavior to ensure that its fewer connections doesn’t cripple it. Thus a tendency to avoid high-complexity tasks, etc, so as to preserve the availability of what connections it does have for pre-existing memories or tasks of extremely high import.
Is there any feasibility to a theory like this, or am I glossing over too many important details?
YES. Very long time and very varied depression sufferer here. Have done lots of thinking about this and I suspect it is something like a drop below some minimal level of structural complexity that drops mechanism of consciousness below some threshold of irreducible complexity–I’ve long thought the best description of chronic and or severe depression is “sub human”. Sadly my guess is that it will be a long time before our models both in the neuroscience and control-complexity theory are sophisticated enough to characterize these effects in a way that would offer more insight than the hand-wavy hypothesis you described
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2743873/
That study requires what I would consider relatively large amounts of caffeine for the results to show up. From Wentz & Magavi , the study you linked:
A low dose of caffeine, 10 mg/kg/day, had no effect on proliferation. Moderate doses, between 20 and 25 mg/kg/day, depressed proliferation in the dentate gyrus by 20 to 25% (Figure 2a, Table 1).
While extrapolating effects from mice to humans presents a number of challenges, 25 mg/kg of caffeine in rodent models is generally assumed to be equivalent to 5–7 cups of coffee, or 625 mg of caffeine, in adult humans (Fredholm et al., 1999).
To investigate, as I didn’t feel comfortable assuming linearity in dose equivalence (i.e. assuming that 25 mg/kg in mice being equivalent to 625mg in humans means 10 mg/kg is equivalent to 250mg in humans), I dug up the Fredholm article , and it confirmed that this is indeed what the authors were assuming:
A similar dose-concentration relationship is found in many species, including rodents and primates (Hirsh, 1984). However, because the metabolism of caffeine differs between rodents and humans and the half-life of the methylxanthine is much shorter in rats (0.7–1.2 h) than in humans (2.5–4.5 h) (Morgan et al., 1982), it seems reasonable to correct for the metabolic body weight when comparing animal and human doses. Thus, it is generally assumed that 10 mg/kg in a rat represents about 250 mg of caffeine in a human weighing 70 kg (3.5 mg/kg) , and that this would correspond to about 2 to 3 cups of coffee [emphasis added].
I personally do not drink more than 250mg of caffeine per day and I weigh more than 70kg. I wonder how many people consume beyond 7 mg/kg/day of caffeine, the equivalent level at which significant effects were found in mice — it seems like quite a lot!
It’s not much actually. A strong medium coffee from, say, Starbucks, will hit somewhere in the 200 range. (Too lazy to provide a link, but google it)
Starbucks is known for having large amounts of caffeine, larger than other comparable coffees. It’s such that CaffeineInformer , which has copious stats on caffeine content in various beverages, suggests:
This chain has some of the highest caffeinated retail brewed coffee available!… Those new to Starbucks should use caution when drinking their coffee as it’s probably much higher in caffeine than the coffee they are used to.
I wanted to find data on how much coffee people are drinking, to see if my intuitions were correct, so I found a study that surveyed caffeine intake in Americans: the mean was 2.2 mg/kg/day, and even the 90th percentile consumed only 5.0 mg/kg/day (350mg for a 70kg individual, less than 2 medium coffees at Starbucks).
Another paper from the FDA summarizes multiple surveys. One particularly interesting study was from the National Coffee Association, who surveyed “regular coffee drinkers,” and found the mean caffeine intake (at time of survey in Winter, 2009) to be 374.7mg/day: “Each annual NCA survey since 1974 has found daily consumption by regular coffee drinkers to be 3 to 3.6 cup of coffee.” I expect this is on the high side relative to other surveys.
Using data from the Wentz & Magavi study, for 70kg individuals, 250mg represents a lower bound and 500mg represents an upper bound on amount one needs to drink to witness the demonstrated effect.
Now, I’m not trying to imply that there aren’t people out there drinking far more than 500mg of coffee per day, but most people are not consuming that much caffeine regularly. Many seem to be in the ambiguous region (250mg-500mg) where it’s unclear whether it’s safe or not, but many are also below 250mg.
I also wonder whether the study’s methodology makes a difference: they spaced the two doses of caffeine at 12 hours apart, whereas most coffee drinkers are unlikely to space their drinks such. Most I know have a morning cup followed by an afternoon cup 4-6 hours later. I wonder if this dosage pattern would affect the results of the Wentz & Magavi study.
Bipolar depression shares genetic correlations with high B5 Openness: http://www.nature.com/ng/journal/v49/n1/full/ng.3736.html
Schizophrenia also has genetic correlations with high B5 Openness. ADHD has genetic correlations with high Extraversion. And in the not-terribly-surprising category, anxiety disorders and such have genetic correlations with high Neuroticism.
Is there some huge problem with having too much synapse formation which the brain is desperately trying to avoid?
In my field, aberrant synapse formation in the hippocampus is a good candidate for the pathogenesis of temporal lobe epilepsy. The purported cycle is : seizure damages hippocampus, abnormal neurons proliferate and synapse inappropriately, these neurons cause seizures, which damage the hippocampus, etc.
Autism, too:
http://newsroom.cumc.columbia.edu/blog/2014/08/21/children-autism-extra-synapses-brain/
That’s weird because autism is linked to a higher rate of mood disorders .
Ozy Frantz,
People with epilepsy have a higher rate of mood disorders, too .
And “low synaptogenesis causes depression” (even if it is found to be true) does not have to mean/imply “high synaptogenesis prevents depression.” There’s a lot wrapped up within “synaptogenesis.” Synaptogenesis could have different effects based on synapse types, dose, micro-/macroscopic localization, and if its source is genetic, the other effects of the causative gene.
Probably, as Deiseach suggests , depressive symptomatology acts as a funnel for multiple causes (just as with dizziness, or sedation, or fever).
There is so much left to learn. “Synaptogenesis” is yet a placeholder for verified pathophysiology.
Re: people with epilepsy having more mood disorders – I thought I heard that ECT was invented by a guy who noticed that people with seizures had less depression. I wonder why the contradiction.
I agree with Ozy that a low-synapse model of depression plus a high-synapse model of autism is at least suspicious.
I may have overinterpreted/misread. Maybe the common ground is overall suspicion of the abundance-of-synapses model of depression. That’s definitely sufficient.
A possible other hypothesis is that some degree of depressability is an outcome of the overall synaptogenesis plan, and that it is a normal and necessary adaptive reaction that can become maladaptive if excessive or if it occurs with no cause. Like anxiety : a little tiny bit of anxiety could be described as “attention to detail,” (and there are situations in which it is very weird not to be anxious, no matter your brain microstructure), but turn up the volume and you have “generalized anxiety disorder.” Some people are tuned to be a little more anxious – and some people are tuned a little more depressable.
That would unite the “something about your brain makes you easily depressable,” and “depression is a reaction to your circumstances” ideas. It follows then that : maybe people with epilepsy and people with autism have higher rates of depression because those things just plain suck . We don’t have to second-guess their depression – being depressed is a plausible reaction to the suckiness of epilepsy or autism.
So maybe what’s weird is that some people with autism don’t get depressed. Perhaps every person with autism[1] would be depressed, if not for their plenitude of synapses[1]?
Any or all of that would fall to the same reasoning I described earlier. Much is unknown and I am just spitballin’.
[1] mumble mumble phenotypic variability mumble mumble
Regarding the interface between seizures and psychiatric disease, the rabbit hole is deeper yet : – Sometimes, a kid with autism will have a seizure and postictally their behavior and language will temporarily improve. Sometimes people (autistic and otherwise) will have a seizure and have postictal psychosis. – There is the somewhat controversial phenomenon of forced normalization, wherein abruptly gaining control of a patient’s seizures is linked to the development of psychosis. I always wondered if this is the-other-side-of-the-coin from ECT – Many of the antiseizure medications can cause a mood disturbance as part of their sedative effects, which is more likely the worse your seizures are and/or the more affected your substrate. Some of the antiseizure medications stabilize mood. Neither of these is necessarily predictable by the drugs’ known mechanisms.
A quick demonstration: glucocorticoids can cause thicker blood, inflammation can cause thicker blood, SSRIs cause thinner blood, folate causes thinner blood. This only draws in four of the six lines, and they’re pointing in opposite directions,
It sounds like those are all pointing in the same direction. Things that cause depression cause thicker blood, things that treat depression cause thinner blood.
A lot of these questions could be answered in one stroke if we had a good evolutionary theory of depression.
Isn’t depression one of those things that basically doesn’t exist in the ancestral environment?
My prior is, it would be really hard to get clinically depressed if you’re getting frequent social interaction and high levels of physical activity (which should have been the case for almost everyone until recently). If that’s not true, I’d be very interested to hear.
Isn’t depression one of those things that basically doesn’t exist in the ancestral environment?
Since we can’t hop into a handy time machine and go back 100,000 years to check, we don’t know.
My prior is, it would be really hard to get clinically depressed if you’re getting frequent social interaction and high levels of physical activity
This is the “all you need is a nice cup of tea and a chat” mindset which really pisses me off. You know when I really wanted to/was thinking about the best way to throw myself off a bridge? When I was getting all that nice healthy physical activity out in the sunshine and fresh air, and daily interacting with people at my place of employment.
Oddly enough, I do better in winter where I can stay indoors and not have to talk to or see people. Sunshine and getting outside and mingling with humans drives me down, not raises me up.
I’m not trying to trivialize depression. But I suspect that, like diabetes (and cancer? and Alzheimers?), it’s a disease that occurs primarily due to the extreme lifestyle shifts made possible by modernity. And if that’s true, it’s worth saying.
You know when I really wanted to/was thinking about the best way to throw myself off a bridge? When I was getting all that nice healthy physical activity out in the sunshine and fresh air, and daily interacting with people at my place of employment.
I think these would still qualify as extraordinarily low levels of social interaction and physical activity, by historical standards.
I don’t want to eat the face off you because I think you are trying to offer what you think are genuinely successful interventions.
But consider: if there was One Weird Trick to cure depression where all that was needed was “go to the gym five nights a week and make lots of friends”, then Scott’s profession could roll up its tents and the pharma companies could concentrate on the search for the female viagra.
Can you see why your advice does not strike me as hugely helpful? You don’t know my particular levels of exercise and social interaction apart from what you can pick up in my comments, yet your stance is:
You: Exercise and social interaction, just like the Good Old Days! That’s what’ll knock depression for six!
Me: Exercise does not have the magic “ah, endorphins!” result for me
You: Plainly the trouble here is YOU ARE NOT DOING IT RIGHT, YOU NEED TO EXERCISE MOAR!
Me: I don’t like being around people, interacting with humans gives me headaches, makes me feel light-headed and nauseous, and if prolonged for too long a period makes me think that running amok is a reasonable way of dealing with social requirements
You: Ah, I see the problem! What is lacking is MOAR PEOPLE ALL THE TIME LESS ALONE TIME!
Me: I prefer winter to summer, unlike some I don’t find that SAD sets in and indeed the brighter, hotter weather does not agree with me (also, I sunburn terribly )
You: See, here’s the snag! What you need is MOAR UV EXPOSURE FOR LONGER!
As far as I’m concerned, your advice – though well-meant – falls on the spectrum of “the beatings will continue until morale improves” 🙂
Both of these things could be true.
Consider that exercising is a great way to reduce your risk of heart disease. Consider also that, once you HAVE heart disease, exercise is a great way to cause a heart attack.
Maybe proper diet/exercise/socialization when done from infancy could help prevent depression, but once a few fuses blow they don’t help and can hurt by disrupting the body’s habitual patterns even more.
But it’s also true that that is a fairly unsubstantiated, even if intuitive, theory at this point.
Thank you for being civil, my comment was definitely kind of dismissive and I apologize.
Really I don’t mean to argue that high levels of socialization and exercise can heal major depression; certainly not that they can heal major depression quickly . (And even if they could, it’s hard for a depressed person to exercise a lot, and extremely hard for a depressed person to socialize a lot.)
What I do believe is that these factors are strongly preventive before an individual gets depression. As Forge the Sky says.
It’s also highly subject to survivorship bias. Social ostracism is a pretty real and dramatic thing in forager groups; we’ve seen it happen and it will kill you, or make you permanently and miserably alone. Even if we can prove that there are no depressive people among foragers (and I insist we don’t know if that’s true), that may be simply because they all die. Postagricultural societies may have more depressives because they can afford to keep them around.
Further, when I look at the list of suicides by country, I can’t see obvious correlates of exercise, social isolation and suicide. What’s the common cultural or lifestyle trend between Sri Lanka, Lithuania, South Korea, Bolivia and India (high-suicide) that opposes all of them to Brunei, Albania, Myanmar, Guatemala and Pakistan (low-suicide)? Exactly.
Postagricultural societies may have more depressives because they can afford to keep them around.
On the “going on strike” theory, depression would make more sense in agricultural societies,where most people are under someone’s thumb.
> Since we can’t hop into a handy time machine and go back 100,000 years to check,
But we could check indio tribes still living in the rain forest and other very rural cultures?
Are they sufficiently isolated and are there great enough numbers of them to draw useful conclusions?
Also, modern hunter-gatherers have as much time behind them as we do, and aren’t on the best land. They may be different from earlier hunter-gatherers.
My prior is, it would be really hard to get clinically depressed if you’re getting frequent social interaction and high levels of physical activity (which should have been the case for almost everyone until recently).
Although entries involving interaction or activity are rated among the higher effective interventions here , the historical record is less clear. During ~1200—1900 in Europe, there was an abundance of both, but it did not prevent the shift towards depression in the general population that is seen in (among others) the whole “we are all doomed sinners”, “repent”, “vale of tears” mood and coloration of art and the predominant religion (please read the book below for the detailed argumentation, no “but there were no psychiatrists then so we can’t really know”, ‘K?).
Cause was a colder climate (the ‘little ice age’) – reflected e.g. in the clothing of people in pictures from that times: light flowing dresses, cleavages before 1200 vs. furs, high (anti-draft) collars, velvet after 1300.
Among the discussed mechanisms are cloudier skies (less light, SAD longer/stronger/all-year), hard rains and storms or too little sun, leading to staying indoors more, crammed together with fellow humans (more agression, easier spread of infections) or rats that moved in (the plague), not to mention frequent crop failures and stocks rotting. Down the causal chains, distribution conflicts, social upheaval and witch hunts added to life stress.
I recommend Behringer’s “A Cultural History of Climate”, it’s easy reading and very informative.
Don’t mistake lack of documentation for lack of physical reality.
There are few records from WW1 of PTSD. There are however plenty of records of people being shot for “cowardice” and later they started getting a handle on the idea of “shell shock” which was still often viewed as a “lack of moral fiber”.
Do you expect them to have called depression depression? Up until quite recently in real terms a large fraction of the population were living in abject poverty and surviving pretty much on a knife edge.
“It sounds like those are all pointing in the same direction. Things that cause depression cause thicker blood, things that treat depression cause thinner blood.”
Oooh, you’re right, thanks.
There is no way of knowing. What we know is that since writing was invented, we have accounts of people being sad and inactive for long times (depressed, as we would say in modern parlance), people committing suicide, and people becoming alcoholics.
Depression certainly existed in ancient times, though we don’t know if it was as frequent as it is now. Probably there was stronger selection against it: the few wealthy people could stay depressed for long times, write poems about it and maybe even eventually kill themselves, while the depressed peasants either pulled themselves up by their bootstraps or starved to death.
>Is there some huge problem with having too much synapse formation …
Isn’t one mainstream theory of autism …
Edited because Azure said it better with a link.
Surprise, surprise! Psychedelics also increase BDNF and mitigate depressive symptoms.
As for how it could be problematic to have too many synaptic connections, I have one word for you: tripping. If there were any subjective experience that just screamed “too many synaptic connections being made, too many thoughts, not enough pruning and logical organizing,” it is the subjective experience of tripping on psychedelics. While some people enjoy visiting this state from time to time, I imagine that few people would want to (or find it adaptive to) feel like this all the time, whether in an ancestral environment or in a modern context.
Still, habitual micro-dosing and/or occasional supervised tripping sessions could be promising for treating depression and…dare I say it, even becoming mentally sharper all around and bumping one’s IQ up a few points in a lasting way. One of the nicest things about going on a trip every few years is the renewed feeling of mental crispness that I’m left with for several months afterwards. The best way I can describe it is, it feels like learning new things and recalling things becomes as easy once again as when I was 10 or 11 years old. So, it’s like I get to return to that fluid intelligence while also keeping my accumulated crystallized, domain-specific intelligence as an adult. It’s pretty rad. And it makes sense if the stuff really is helping to grow new synaptic connections.
If there were a pill that gave this feeling without having to go through a trip first, I’d be very interested. “Ask your doctor about orally active BDNF!” One can dream….
Which psychedelics?
My views align with citizencokane’s – my experience was with LSD.
Ones that influence 5-HT2A receptors, at the very least.
When you release that new pill, I think “one can dream” should be the tagline of your ad campaign.
What is the argument against “software” causes of depression and/or software cures?
CBT is claimed as the most effective non-medication treatment for depression and it’s even claimed as equally effective as medication. CBT is all about changing thought patterns, ie changing software.
Can we even describe or define depression as a biological/hardware problem? It seems like all of our diagnostic criteria, whether DSM or Beck Depression Inventory, rely on self-reported software like symptoms (“I feel down”, etc).
I’d say CBT works for some people/some forms of depression, but not for others. And depression is a lot more than simply “I feel down”, but that’s where symptoms get murky. It’s very hard to communicate how it feels to constantly be “not there”, when you’re not sure exactly what there should be anyway, and all you have are phrases like “I feel down, I have no energy, I’ve lost my enthusiasm, I don’t enjoy the things I used to anymore” etc.
This is the best explanation of what I felt when I was depressed as well. The most important intentional factor in not being depressed anymore for me was getting a dog. Something about the whole package really worked for me.
I think that there isn’t one over-all illness called “depression”, there are various depressive illnesses that get lumped in under the one umbrella. So that’s why different treatments seem to work in different fashions.
I do think biology has something to do with it, but who the hell knows what. Some people may be pre-disposed to be depressives, so that you get whomped with the life stressors or something like “whoops your auto-immune system is attacking itself with the inflammatory response” and as an added bonus just for you, we’ll throw in depression as part of the package!
Other people have it from birth, practically, so even if “life is okay, I’m not sick, things are pretty normal”, they still suffer from depression (and I think treatment-resistant depression falls into this side of the balance).
I have a family member who is depressed and on top of that she has hypothyroidism, so when her doctor got that sorted out, it helped with the depression. If that gets out of balance again, the depression gets worse, so see the biological explanation.
I think there is also “normal” depression, in that if your life goes to hell in a handbasket, it’s normal to react with depression. But once you get things sorted out to an acceptable or functional level, the depression clears up. Maybe in the biologically pre-disposed people, this is what triggers the depressive reaction: “how come so many people get depressed for no reason, stay depressed for a few months to a few years, and then get better – only to relapse back into depression a few years later?”
The ‘normal’ people get normally depressed but once the stressors are removed, or they’ve learned how to deal with them, that knocks the depression on the head (I think this is why CBT works for some people). The ‘biological’ people get depressed for the same reasons in the same situation, once the situation improves the depression goes away – but it’s like getting shingles or a cold sore virus; once you’re run-down again or something triggers it, it flares back up. The depression hasn’t really gone away, it’s just gone into remission after it’s been activated and your system has been sensitised to it.
The really weird thing about CBT is how it works. Much of the time the client is asked to keep a thought record, a practice in learning awareness about your own thoughts. Evidence is almost always demanded for thoughts. Depression says “everybody hates me”, therapist “what is the evidence for that thought?”, “Does literally EVERYBODY hate you?” And it continues on this way.
I think there’s a very real phenomenon that if you think the wrong kind of thoughts for a long enough period, you will give yourself depression, and I think most of CBT relies on this idea. At the very least, I think we can say this is true for some people.
From this perspective, when we talk about depression in remission, it seems off. It’s like I have a practice of smashing my leg with a hammer, which results in repeated broken legs. After stopping the hammer beatings we then say my broken leg has “gone into remission”. Just doesn’t seem right.
Then there’s loneliness . The research shows that loneliness can cause all of the symptoms represented by depression and that increasing social connection both cures and protects against depression.
Sure, I can totally understand how things like hyperthyroidism causes depression, but when trying to tackle the giant umbrella of depression, it seems like we need software-like solutions held in equal regard as hardware-like solutions, unless we can effectively detect and segregate the different types.
From this perspective, when we talk about depression in remission, it seems off. It’s like I have a practice of smashing my leg with a hammer, which results in repeated broken legs. After stopping the hammer beatings we then say my broken leg has “gone into remission”. Just doesn’t seem right.
Which is why I said CBT works for some people and some forms of depression. If you’re complaining about crippling leg pains, and a neutral third party points out mildly that this might have something to do with you hitting your shins with a hammer, then you can ‘put your depression into remission’ by stopping doing that .
However, if your leg pains are caused by a bear gnawing on your ankle, a third party telling you “now just ignore the bear, don’t entertain its presence, negative thoughts reinforce negative behaviour” is not going to help. You need to get the bear to let go.
Or maybe it’s not a bear, maybe you have leg pains because both your legs have been cut off below the knees. Again, talking about ways to feel upbeat and ignore the missing halves of your lower limbs are not going to be much use.
In other words, CBT is not going to help as much when it comes to “your negative thoughts are exaggerating and distorting the reality of the situation and things are not, objectively, that bad” if you are in a situation where objectively things are gone to hell and are indeed shitty.
But what I meant by depression going into remission was meant to parallel an allergic or viral condition; you suffer an attack of depression for a good reason (you lose your job unexpectedly and it’s hard to find a new one; your house burns down with all your goods the day after your insurance lapsed), you get over that (maybe by gritting your teeth and bootstrapping your way out, maybe with treatment) and then we have two possibilities: you are not someone genetically predisposed to depression, so further down the road you are not likely to lapse back into depression for a minor set-back, or you are someone who is so disposed, so now your system is ‘ sensitised ‘ and when a lesser stressor or some other situation occurs, or maybe just out of the blue, you relapse into depression.
I think that there isn’t one over-all illness called “depression”, there are various depressive illnesses that get lumped in under the one umbrella. So that’s why different treatments seem to work in different fashions.
If this is true – it might be why it’s so hard to develop an evolutionary theory of depression. We have a bunch of subsystems which we need to develop evolutionary theories for but due to the interactions between these subsystems its really hard to identify the function of each subsystem. In a simple case imagine you have three subsystems and subsystem one – due to genetic load – isn’t performing in an evolutionary optimal manner. Subsystems two and three will also be performing differently than expected. This gets worse if there is feedback between the subsystems.
So as you say – if life goes to hell in a handbasket it might be normal to react with depression but the other cases are abnormal as they are primarily maladaptive. I’m unsure if people have developed extensive vocabularies to describe the different types of depression but it seems like it would be necessary to begin to understand depression. There’s also the problem of developing vocabularies to describe the range of symptoms people exhibit so maybe it’s all a wash.
One might quibble with the details, but this is essentially right: we don’t know yet if this is one disease or different diseases with similar symptoms. Kinda like flu and cold wouldn’t have been distinguished from each other back in the day.
I… I wasn’t aware there was a difference between flu and cold.
https://en.wikipedia.org/wiki/Influenza
https://en.wikipedia.org/wiki/Common_cold
The more you know!
There’s been a suggested evolutionary theory of depression focusing around how it happens in humans and baboons but not other primates, which postulates that depression happens to creatures capable of changing their environment and it causes the individual to spend time trying to figure out what they should do. This seems to dovetail reasonably with the sort of situations which trigger depression in the first place (including just plain living in the modern world) and an easy just so story for why people pull out of it is that their brain eventually gives up on changing anything and goes back to being content. If this is the case, then maybe trying to stop depression directly while it’s happening is a fool’s errand and what should really be done is fixing the mechanism for pulling out of a funk which seems to be broken in depressive people. If existing drugs do that, then that might explain why they take so long to kick in. Using drugs to pull the person directly out might have the ill effect of forcing the person to stay on very strong medications the rest of their lives or rapidly go into a tailspin of depression if there’s any hiccup in their medication. It also might cause people to living what are fundamentally unfulfilling lives and not do anything about it.
Stop giving people choices?
You mean like in “Choiceless mode” , which the author argues is the natural way for humans to live?
Naaaaa… Can’t follow that, because Unfortunately, the choiceless mode depends on ignorance of alternatives. It’s usually impossible for nearly everyone in the developed world, and survives mostly only in remote areas in the most “backward” countries.
EDIT: Maybe a temporary relief from having to manage all of life could work, say, in a clinic. With an additional re-evaluation of life priorities and lifestyle (and if necessary, training required skills) afterwards (do it too early and the patients are just pushed back into overwhelmed mode). Would require clinics to accept that it’s not always the dreaded “facilitating regressive development” for the first part.
THIS. Longtime depression sufferer here, both chronic and or severe at times. I have a working hypothesis, suggested by my own and others (low n) experience, that being on antidepressants then going through a breakup, say; or starting antidepressants right after a breakup, IS EXACTLY WHAT YOU DO NOT WANT TO DO. not being able to ” prune” the old memories, always being bathed in a flood of BDNF to protect those synapses that really really need to wither, just makes things worse, more obsessive, and in the long term, makes the person less able to grow and move on.
Being on semi effective antidepressants, then going through a sudden and traumatic breakup for which the tesponse was to increase dosage, resulted in over a year of horroific pathologocial obsessive grief…in my mined, caused by “unpruned” synapses
I’ve always been attracted to the bargaining model of depression:
http://anthro.vancouver.wsu.edu/media/PDF/Hagen_2003_The_bargaining_model_of_depression.pdf
I wouldn’t say that this accounts for all forms of depression, but it seems to have quite a lot of explanatory power. The idea is that depression is inherently social. It’s a form of going on strike, but not a voluntary one. And it’s a costly signal — the depressed person neglects their duties, neglects their own interests even their personal hygiene. True depressive behavior of this kind is very hard to fake (sort of like being head-over-heels in love is very hard to fake — any non affected person would find it terribly embarrassing to do the silly things that an infatuated person does. Which is the evolutionary point of it). Depression is a way for a low-status person to involuntarily demand better treatment from their friends and loved ones.
Compelling. This would suggest that allowing sick leave for depression would only enhance the bargain, thereby further incentivizing depression.
Compelling because it fits the evidence well, or because you like the moral implications?
This is also just from low n observation but don’t depressed people often try to hide their depression for as long as they can?
Even from themselves, and then they beging acknowledging with the term “burnout”.
Scott, I tried Semax a while back on the basis of one of your nootropics surveys – and I found it to be very effective. It’s mechanism of action is also supposed to be an acute, immediate release of BDNF.
If this model is essentially correct, it is yet another reason why the iron curtain of psychopharmacology is so tragic.
I didn’t know Semax was a direct BDNF releaser. The only thing like that I’d ever heard about was Lion’s Mane, which doesn’t really do anything. Anyone know of anything else in this class?
7,8-dihydroxyflavone supposedly is an agonist at the TRKb receptor, target of BDNF
My first thought experiment not mentioned in your post, was exercise.
Exercise appears to increase synaptogenesis ( https://scholar.google.com/scholar?q=exercise+synaptogenesis ).
Thus, one would predict that exercise would improve depression, which it appears to do ( http://bjsm.bmj.com/content/35/2/114.short ).
Well, one theory is that depression (or at least bipolar disorder) is a way of acting out to force other tribe members to pay attention to you and thus raise one’s status.
Sometime ago in my wild and reckless youth that hopefully isn’t over yet, a certain ex-girlfriend took to harassing me with suicide threats. (So making her stay alive was presumably our common interest in this variable-sum game.) As soon as I got around to looking at the situation through Schelling goggles, it became clear that ignoring the threats just leads to escalation. The correct solution was making myself unavailable for threats. Blacklist the phone number, block the email, spend a lot of time out of home. If any messages get through, pretend I didn’t receive them anyway. It worked. It felt kinda bad, but it worked.
Why does the body have so many “decrease synaptogenesis” knobs? That is, why go through the trouble to evolve all these chemicals and systems whose job is to tell your brain to decrease synapse formation so much that you end up depressed?
Hand-wavy thick-blood* hypothesis (along the lines of the optimal activity theory of depression “baconbacon” introduces in a top-level comment):
Sometimes an organism might find itself in a non-ideal situation where it has very few options. Maybe the safest immediate thing to do is reduce activity, and correspondingly reducing risk. Once you’ve correctly recognized the situation as one in which you have few options, it isn’t necessarily a good idea to learn from the experience. That is, the sustained state of reduced options might be relatively harmless, but isn’t a fruitful way to exercise interesting synaptic connections. Perhaps, besides being the safest thing to do, curling up and sleeping is a way to avoid developing an entirely new set of habits that will be inappropriately once the depression somehow passes.
Major flaws in this vague hypothesis include: * At the very least, anecdotal evidence suggests that depressed people probably do form more bad habits, while depressed. Similarly, as Scott hinted, I don’t get the impression that depressed people actually fail to learn new things (say, from a book) if they actually engage in the right activities. On the other hand, anecdotally, the most depressed people I know do seem to fail to retain certain kinds of information, including personal insights about their own depression and mental state. * The situation I described where an organism has few options, in nature is probably either escapable or fatal. If it’s the former, then “curl up and wait it out and repress it” sounds a lot less helpful. If it’s a certain doom, then there’s no selection pressure to favor a particular cognitive strategy. * Learning and neuroplasticity entail a much more complicated correspondence than “new synapses = new behaviors”.
In any case, it seems clear that humans have a lot of ways to create “depressing” situations that are somewhat decoupled from transient or periodic environmental conditions. And, again, anecdotally, it seems obvious that depression in humans is rife with feedback loops (a classic one being depression-induced inactivity leading to a lack of new, fulfilling experiences, and, presumably, more depression). I’d be interested in re-examining the factors Scott discussed in his post, with an eye towards the extent to which depression causally feeds back into stimulating the intensification of the (purportedly causal) factors preceding it. Presumably, depressed people are more prone to inflammation (as a correlation). But which way does causality go, and is there a feedback loop?
* Comparing to Scott’s cherry picking is generous, since I didn’t even cherry pick. But I claim that all of the bold assertions here could probably be justified with a literature search, adding absolutely zero clarity to the discussion.
Why should decreased synaptogenesis cause depression, of all things? If you asked me, a non-neuroscientist, to guess what happens if the brain can’t create new synapses very well and loses hippocampal volume, I would say “your memory gets worse and you stop being able to learn new things”. But this doesn’t really happen in depression – even the subset of depressed people who get cognitive problems usually just have “pseudo-dementia” – they’re too depressed to put any effort into answering questions or doing intelligence tests. Why should decreased synaptogenesis in the hippocampus and prefrontal cortex cause poor mood, tiredness, and even suicidality?
My intuition here is that depression may be tied to lower-than-normal synaptogenesis somewhere , but not necessarily the hippocampus. So increasing synaptogenesis everywhere increases it in a whole bunch of places that don’t matter (including the hippocampus) as well as the mystery target area that’s causing the problems. Given how strongly depression seems to affect motivation, I’m surprised there aren’t stronger links with the reward/dopamine system.
There is an interesting one: retinal contrast processing neurons are influenced by dopamine level. Depressed patients have less of their activity, literally seeing more gray-in-gray than bright wight besides dark black. It can be measured easily with a thin thread electrode in the lower lid of the eye (and a counterpart somewhere else) – think of a one-point EEG. Eyes of depressed patients give readings of <2µV when presented with strong b/w checkboard patterns, eyes of healthy probands are 3µV. Voltages even correlate with the timing of depressive episodes. Media speculations say you could go to your ophthalmologist for a quick depression check in a few years.
Thank you for explaining why lights seem brighter to me when I’m not feeling as depressed! This explanation is making me wonder if there’s actually some correlation between the literal contrast sensations and the feeling that life is “greyer” under depression. Maybe that metaphor develops out of the sensory changes.
* wight => white
Also, the opposite may also be true. Some hypersensitivity to what patients say is “light” in other mental diseases may be more a sensitivity to sharp contrast. I don’t know enough to say which are correlated with *increased* dopamine levels or effects.
EDIT: Original paper , voltages here were even more different than I remembered. They used b/w patterns, maybe there is a similar effect for colors, also possible at a later stage of processing (related to this ?)?
I think that MRIs and BDNF injection experiments have pretty conclusively found the hippocampus and parts of the cortex are the relevant area, but I’m not sure how strong those results are.
Just shooting from the hip, but my approach would be to understand depression by first looking at the mild non-clinical depression that most people suffer now and then.
From introspection, what seems to cause mild depression are (1) lack of sunlight; (2) insufficient exercise; (3) social rejection; and (4) other bad news where I have little control. So I would guess that depression (at least mild depression) is an evolutionary response to put you into an energy-saving state. Perhaps clinical depression is a situation where the normal depression response goes overboard. Kind of like clinical anxiety.
Just shooting from the hip, I’d look at Darwin’s Expression of the Emotions in Animals and Man – the six basic emotions of anger, fear, appetite, disgust, happiness, unhappiness. What is depression? Unhappiness. Is it noticeable in lower animals, plants, bacterial colonies? Yes, but you kind of have to want to see it and have read Darwin recently. Can you see it in dogs, pigs, chimps, the enlisted swine, lawyers? Sure.
Given the long history of depression/melancholia I think it is unhelpful to link it too closely with modern influences such as caffeine or artificial light, other than to speculate about why it is more common. It also seems to be present in simple societies, even the !Kung.
It seems more like a homeostatic state change that can be brought on by multiple factors, similar to the idea of a fever being a general physiological state in response to all sorts of infections or hormones or other causes. As such it probably has an adaptive advantage under the right circumstances. I see the parallel with obesity here- in precivilisational and even preindustrial societies the ability to eat as much as possible and put on weight during good times would be adaptive for unreliable food supplies. Under industrial conditions depression can be maladaptive, in that we demand people to be constantly engaged and “productive”. Even non-depressive introverted people find this burdensome.
If depression is designed to realign behaviour to minimise energy output or distractions to ruminate on a problem with a view to solving it sooner, then in modern societies people may be so highly constrained by their circumstances that they get stuck in that state longer than they should, or even worse are simply denied the “luxury” of going through the necessary process of being depressed. Kind of similar to people taking pain killers and fever reducing medication because life demands they keep work-work-working and cannot rest and recover instead.
This brings up the disturbing possibility that modern science may indeed find a way to switch off depression (more consistently and safely than current medications at least) so people can pop a pill and remain at least serene if not blissful in their allotted work station in the ant-hill we call civilisation.
How about the good old stability-plasticity dilemna ? The unbridled creation of new synapses would lead to catastrophic forgetting just as well as the loss of old ones, by altering what each neuron responds to.
Maybe all these fast depression therapies act by temporarily shifting the stability-plasticity balance a tiny, tiny bit towards more plasticity, knocking the brain out of its stable but miserable thought patterns. But massive, constant rewiring would essentially scramble your brain.
Which is of course what ECT does…blank out all memories from months to a year before the treatment series
There’s a possible evolutionary explanation for depression even if it is maladaptive. The anti-depressive state is so maladaptive that avoiding it is worth risking depression. Think sickle cell disease. The resistance to malaria is so valuable that sickle cell disease exists despite even stronger selection pressures.
What could be wrong with anti-depression? Rather than lethargy, needless activity. This increases starvation risk. This makes getting proper rest and healing for injuries less likely. Rather than avoiding useful risks, taking needless risks. This increases predation and conflict between humans. Rather than sticking to a bad routine, excessive innovation. Most innovations don’t work in the evolutionary environment. This wastes resources and increases risks. Rather than social isolation, increased social contact. If depression is linked to immune response, depression may slow the spread of disease, especially STDs.
I’ve read somewhere that depressed people have a more accurate view of the world than non-depressed people. Which is plausible, since the latter group is more likely to be unjustifiably optimistic than the former. Maybe “depression” is piggy-backing on “accurate perception”, like the Jewish genetic illnesses are piggy-backing on intelligence?
Depressive realism has gained almost mythical status around here, so I went and looked it up. I’m not very impressed with the evidence given.
What you are calling anti-depressive is pretty much mania, but it is quite possible to be neither manic nor depressed.
Worth noting there are some other interesting drugs that have strong effects on growth factors eg BDNF and NGF. One that I have looked at in particular is apomorphine, which seems to in some case have protective effects in Parkinson’s and actively regenerative effects on dopaminergic varicosities. It had an interesting history in the treatment of addiction (initially as an aversion therapy, but then at subemetic doses). Arvid Carlsson is still convinced to this day it works.
I would be very interested to see a test of apomorphine for depression, actually, as it’s anxiolytic to boot.
I might be able to suggest an explanation for your last question. At least, this seems like a regime in which control theory may be applicable.
Control systems that try to maintain homeostasis tend to fall into one of three stereotypes, depending on whether or not they’re correctly tuned.
– Stable. If everything works right then the error signal will stick close to zero, and any excursions will last only a short time (on the timescale of the system) before being corrected. The system won’t overcorrect, or overcorrections will be small relative to the original excursion.
– Cyclic. Stability is fleeting and unreliable; the system will tend towards a cyclic form of metastability where it constantly overcorrects. A plot of this may look roughly like a sine curve. The error graph is limited to a maximum amplitude, though the exact value of that may vary.
Of course there are states in-between the two above to consider as well, but… Did that sound a bit like bipolar disorder? I can certainly see why it would be attractive to think so.
You can get this behavior with about three analog components in a PID controller, though, and the brain is vastly more complicated. Though that doesn’t mean it can’t be what’s happening.
And for completeness:
– Unstable. Errors are magnified rather than corrected, or alternately there are cyclic errors whose magnitude grows over time up to the physical limit of the system. These get bundles together because both conditions are generally fatal, as the control system usually has a purpose for existence. (The usual fix is to start from scratch.)
… Does *that* sound like anything?
The problem with using any cycling theory to explain bipolar disorder is that most sufferers have long periods of normal mood in between their depressive and manic episodes.
What about ciclotimia? Besides, with Fourier analysis you could theoretically decompose different rhytms, ultradian and infradian.
The interesting implication of one hypothesis for the evolutionary adaptiveness of depression has to do with treatment: the idea that people become depressed as a way to credibly signal that they need more support from their group than they are getting.
Sister Y speaking on this topic 9 years ago. Surely the field has advanced since then, and I couldn’t predict how, but this “social motivation function” seems interesting.
I’m extremely dubious about depression being adaptive, especially the more severe sort of depression. If depression were adaptive, I think it would have a better off switch.
My model is that broken bones aren’t adaptive. Having bones is adaptive, and we live in a world where sometimes bones are subjected to more stress than they can take.
Also, evolutionary theories seem to just address the lethargy part of depression and ignore the misery part.
If I’m right, the thing to research might be trying to get better understanding of how healthy people work.
For example, speaking as a person with some serious problems with akrasia, how does a person go from having an intention to doing something to achieve it? I can do that much more easily at some times than others, but what’s the difference? Sometimes, enacting an intention seems like having to haul myself over a high threshold. This might be mostly physiological, but I’m not sure.
My model is that broken bones aren’t adaptive. Having bones is adaptive, and we live in a world where sometimes bones are subjected to more stress than they can take.
That’s a good point. Another model for you: Lack of oxygen to a baby’s brain causes brain damage. But if you cool the brain just after the injury , the amount of injury is greatly reduced. Without cooling, there’s a cascade of biochemical events which makes the injury much *worse* than what was strictly caused by the lack of oxygen. This natural response is clearly maladaptive; it increases the chance of mental retardation by about 50%. Without intervention, the system is pushed beyond what it can handle, and it amplifies the injury instead of limiting it.
The misery part seems like it fits well with your broken bone example, in support of the evolutionary Just So story: The pain is there to enforce inaction. It says: Don’t move your broken leg, or you will be punished with physical pain; don’t get your lethargic self out of bed, or you will be punished with emotional pain. (Although… it doesn’t always work that way with depression, does it?)
I’m extremely dubious about depression being adaptive, especially the more severe sort of depression. If depression were adaptive, I think it would have a better off switch.
The evolutionary discussion isn’t about X’s current experiences with depression, they are about understanding how basic, short term depression could be beneficial. There is a big difference between looking at clinical depression as a screw up of a system that would normally produce a base level of depression at times, and a screw up of a system that constantly prevents any kind of depression from occurring when it is working well.
For at least some people the initial stages of depression don’t include misery, link for one person’s description .
While broken bones aren’t adaptive, having portions of your bone that are easily broken at times of your life is adaptive. See growth plates.
Think of anxiety.
If a person has a tiny bit of anxiety, it exists as a little worry in the back of their brains. “Did I double-check my calculations?” “Have I considered all potential causes for my patient’s symptoms?” “Is there something I could be doing better to help my child succeed?” Etc.
It is adaptive in small doses, in which case we call it something like “attention to detail” or “being driven.” Turn up the volume and you get so anxious that it starts to affect your life adversely, and we call it something like “generalized anxiety disorder” or “this is why you have migraines.” Being anxious isn’t being broken. Being anxious is having an excess dose of worry.
Depressability could exhibit a similar dose-response.
I get that it is hard to say exactly what a tiny dose of depressability would do for a person, but if we are going to entertain the notion that it could be adaptive in small doses, we first have to discard the “depressed = broken” model. Depression isn’t being broken. It may be a normal response. People who are depressed all the time or for no reason may just have an excess dose of depressability.
A tiny dose of depressability might be ordinary responses of guilt/shame. What depression looks like for a lot of people is thoughts like “I’m a burden on those around me,” “I’m a bad person,” “I don’t do worthwhile things with my life.” It’s not inconceivable that these thoughts could be adaptive in tiny doses – as an incentive to repair social relationships, make amends, work hard. (Haven’t we all met people who seem completely unfazed by any kind of guilt or social rejection, immune to any kind of shame or guilt response, who seem to breeze through life until they realize they’ve burned one too many bridge?)
The trouble with full-scale depression, of course, is that it often drives one to socially isolate oneself and do less, so that one ends up “proving” to oneself all those depressed ideas about being a burden and insufficiently productive and so on.
Fascinating stuff. I would take issue with the encaptioned claim that nobody is capable of memorizing the mTOR pathway in all its terrible majesty; I’ve met such folks, they walk among us. I’m not one of them, but I do have a few points to add.
Rapamycin/sirolimus is commonly referred to as an mTORC1 inhibitor, but it’s sort of the odd man out among that class of drugs (despite being the founding member). Unlike most of the synthetic mTOR inhibitors ground out by big pharma med-chem teams, rapamycin doesn’t directly inhibit the kinase activity of either mTOR complex. Instead, it works through a complex mechanism involving a bank shot off of an accessory protein (FKBP12), which seems to inhibit mTORC1 and have some more complex, tissue-specific effect on mTORC2. Even the mTORC1 inhibition is only partial: not for nothing did David Sabatini, perhaps the world’s preeminent mTOR expert (who definitely has the whole pathway stored in RAM at all times) published a paper bluntly titled “ Rapamycin inhibits mTORC1, but not completely “.
All that’s simply to say that rapamycin’s failure to induce depression may only count as weak evidence against mTORC1’s involvement, depending on exactly what mTORC1 is doing to regulate synaptogenesis. The second-generation mTOR inhibitors coming out of pharma, which completely block the signaling activity of mTORC1 and/or mTORC2, might make a better test of the hypothesis if someone took a close look at the Phase I trial data. All I could find with a quick dive is this slide deck claiming that 18% of the patients in Novartis’ BKM-120 trial reported depression.
Also, I wouldn’t necessarily count the folate connection out just yet. There may not be any research directly linking folate metabolism to synaptogenesis, but it looks like mTOR can probably sense folate levels, and folate deficiency probably suppresses mTOR signaling: paper . Not terribly surprising, since mTOR’s generally thought to be the “nutrient availability” signal integrator.
Is there anything that does inhibit mTOR really well, and do we know if it causes depression?
Pharma’s cranked out a boatload of mTOR inhibitors in the past decade or two, a gold rush sparked by the observation that the mTOR pathway is frequently dysregulated in cancer. However, unlike rapamycin these compounds pretty much all hit both mTORC1 and mTORC2, which govern somewhat different pathways (so if synaptogenesis is truly mTORC1-driven, as the article suggests, they may be imperfect tools). There’s been a lot of interest in specific mTORC1 inhibitors but I don’t think anyone’s cracked that nut; looks like Sabatini’s taking a swing at it, though.
A lot of the mTORC1/2 inhibitors have made it into clinical trials for cancer, but to my knowledge none of them have made it back out – looks like the FDA-approved mTOR inhibitors are all still “rapalogs”. These compounds tend to fail on efficacy, as most tumors seem to be able to weasel their way around an mTOR blockade pretty quickly (for an interesting and non-obvious reason). What this means is that we – or rather, some Big Pharma datavaults – have a lot of Phase I/II safety data, but we may never get the chance to see what long-term dosing in a large population looks like.
That said, that PhI/II safety data might be instructive if anyone could get a close look. A lot of those compounds were brain penetrant, and could get up to high enough levels to hit brain tumors in animal models.
This is obviously something we don’t know nearly enough about yet, and I can’t provide much past reiterating the questions in the OP. But two things that might be interesting:
First (and correct me if I’ve misremembered) it seems like whenever we look at the neurology involved in depression, the forebrain is much more implicated than mid- or hindbrain structures. This is interesting because human forebrain structures are in a sense fairly primitive; they only evolved fairly recently, and have therefore had little time to become strong or optimized compared with more ancient hindbrain structures. In addition to the concerns Scott has with evo-bio explanations above, this makes me think that an adaptationist explanation for depression is less likely. Possibly there is some sort of regulatory function at play, but that regulatory function may have ‘unintended’ side effects when a pre-frontal cortex is in the warpath in addition to the original intended targets.
This is kind of just saying evolution is blind in a lot of words, but it suggests that maybe the solution isn’t turning on or off a single trigger or imbalance, but in trying to find an optimal balance of many systems that properly nurtures the more delicate parts of our neurology. Hard problem.
Second: this is anecdotal but maybe weird enough to be interesting. I’ve never been diagnosed with clinical depression but have had subclinical depressive symptoms before. Supplementing with s-AMe has tended to help a good deal, and would work pretty quickly – in about 5 days, and it also seemed to have a mild stronger effect for a few hours after taking it, starting about an hour after ingestion. That seems like an awful fast effect for it to be doing things with synaptic development in the brain, but I’m not a neurologist so I’m not sure. At any rate, after about 3 weeks I’d have to half the dose because I would become manic – unable to sleep, having more ideas about things I wanted to do than I could do, pacing, etc. I’ve heard of others getting the same effect, it subsides very quickly once you decrease the dose. Making people manic if they have pre-existing manic spells is a known side-effect of s-AMe, but I haven’t heard it reported scientifically in people who usually do not suffer from mania.
But that’s interesting because you don’t hear about mania from SSRI’s….maybe the ‘manic/depressive’ dipole is only relevant in certain aspects of the depression dynamic. I did subjectively notice that s-AMe seems to give motivation more than it dispells dysphoric mood.
Finally, since I’m somewhat rambling – does anyone know of diseases other than cancer that create greater risk of becoming depressed? I work in health optimization and am trying to figure out how much optimizing lifestyle might have to do with avoiding depression, and the sorts of things it’s co-morbid with could strongly inform that.
Check http://curetogether.com/ . You should be able to find correlated conditions for depression with a few clicks.
Supplementing with s-AMe has tended to help a good deal, and would work pretty quickly…
Because of the proven power of placebo in depression, there’s unfortunately little-to-nothing that we can learn about treatment for it (and any mechanisms that a treatment might suggest) from individual cases. You really do need a blinded, placebo-controlled trial. Even those present challenges, since the side effects of treatments often pierce the blind, and depressive symptoms lift faster when people are convinced that they’re getting the real drug.
Edit: Did the comment I was responding to disappear?
Looks like you were responding to my comment, which has disappeared. I have no idea why, don’t think there was anything that would have made it mod-worthy.
At any rate, I’m not trying to make a case for anecdata here. But in studies s-AMe does seem to work for depression; given that I found it interesting that a few people without prompting mentioned getting mania from it and never, say, euphoria. And also that I experienced a state of mind I never had before, without being told that was a possibility.
These are the sorts of things that don’t give us any power to speculate about mechanisms with any rigor, but can be the starting-point to actual research being done.
This seems silly for a variety of reasons. First, it would silly be to notice that many things can decrease someone’s chance of partnering or living to 100, and then posit some type of “decrease pairing” and “anti-centenarian” knobs. We understand these are complex, difficult outcomes, so of course many things affect the final outcome. Building a brain seems plausibly to be such an outcome.
Second, if synaptogenesis is expensive, then many things should affect the optimal amount of it to do, and a proper feedback system should involve tuning up or down the amount in response to a variety of inputs. (This holds even if the first point is false, and the body can easily choose an exact degree of synaptogensis) We don’t wonder why the body has so many “how much / what should I eat” knobs, because it’s obvious that past & predicted exercise, food availability, weather/season, sleep, etc. should all affect how much / what a forager eats.
And finally, as the “eat” example alludes to, all of these control systems evolved in a different environment with a different parameter space than we have now. It is not in the slightest bit surprising that if we take a complex control system optimized for a particular range of parameters (some shape in multi-dimensional space of possible inputs to control system) and feed it parameters from a different part of the space, it will do the wrong thing. Or to put another way the performance of any learning system must degrade towards zero as the test set diverges from the training set. Any performance demonstrates (perhaps tautologically) similarity in structure between test and training sets.
How does the effectiveness of active placebo in the treatment of depression tie into the synapse hypothesis?
The classic examples of this are cancer-related depression (which exceeds what you would expect just from cancer being stressful)
I wonder how did they work that one out. How did they decide how many cancer sufferers you’d expect to be depressed just from cancer been stressful?
Did somebody say something like 52% of people with cancer suffering depression is what you’d expect so if 70% suffer from depression is way above what you’d expect?!
Seems totally arbitrary (which means I must be missing something).
1) Develop a fairly objective measure of stress 2) Measure the correlation between stress and depression 3) Notice that cancer patients have higher depression than can be explained by the normal correlation between stress and depression
Hello; this being my first comment ever on a SSC post, allow me to thank you for having created a highly interesting blog.
Thanks! ^__^
Now, not having read all SSC posts, it could be that you’ve already seen this. But on that evolutionary theory: http://www.sciencedirect.com/science/article/pii/S0149763415000287
There are some very intriguing ideas in that article, perhaps the most relevant to this article being the hypothesis that the serotonergic system evolved to regulate energy metabolism (in a sense).
Here is the simplest most obvious evolutionary theory of depression and I feel we need to eliminate it before we feel any need to look for alternatives.
At any time how happy you are is a combination of some innate happiness set point and what is going on in your life.
For obvious reasons evolution balances our happiness set point to best avoid both the harms of mania and depression. Being very unhappy much of the time is an obviously shitty thing so is itself a negative life event explaining why one can get stuck being depressed and why it can disappear and reappear. Having a lower happiness set point makes one disposed to this state.
The obvious maladaptive nature of depression can be accounted for by noting that mistakes on the manic side were probably even more dangerous as they encouraged excessive risk taking. In other words given the tools available to it evolution struck a balance…not sure what more is wanted from an evolutionary explanation.
Maybe special kinds of depression like anhedonic depression do require more explanation but doesn’t this suffice for the basic phenomena?
Here’s a theory that I find promising: http://psych-networks.com/challenges-to-the-network-approach/
” For that matter, what is it like to have too much synapse formation? If it’s the opposite of depression, it sounds kind of fun.”
I think cannabis increases connectivity in the brain and it is kind of fun ;-).
“The results suggest increases in connectivity, both structural and functional that may be compensating for grey matter losses. Eventually, however, the structural connectivity or ‘wiring’ of the brain starts degrading with prolonged marijuana use.”
https://www.theguardian.com/society/2014/nov/10/cannabis-smoking-brain-shrinks-increases-connectivity-study-texas “How does bipolar disorder fit into all of this? Is mania the answer to my “what is it like to have too many synapses?” question from point (3)? If so, why do some people go back and forth between that and depression?” Anecdote: a friend of mine got something like manic episodes when he was using cannabis and was depressed when he was not using. So the down side of too many synapses might be, that you get too many crazy ideas and magical thinking and perhaps even act upon them. The behavior in turn could get you into trouble. The negative feedback could force you to think about your own behavior and rewire your brain. From an evolutionary point this could have resulted in an exclusion from the tribe or group that goes in hand with deprivation of resources. So the “energy-save-mode” as discussed earlier might work for this scenario as well.
- Entries feed
- Comments feed
- WordPress.org
AISafety.com hosts a Skype reading group Wednesdays at 19:45 UTC, reading new and old articles on different aspects of AI Safety. We start with a presentation of a summary of the article, and then discuss in a friendly atmosphere.
B4X is a free and open source developer tool that allows users to write apps for Android, iOS, and more.
Substack is a blogging site that helps writers earn money and readers discover articles they'll like.
The Effective Altruism newsletter provides monthly updates on the highest-impact ways to do good and help others.
80,000 Hours researches different problems and professions to help you figure out how to do as much good as possible. Their free career guide show you how to choose a career that's fulfilling and maximises your contribution to solving the world's most pressing problems.
The COVID-19 Forecasting Project at the University of Oxford is making advanced pandemic simulations of 150+ countries available to the public, and also offer pro-bono forecasting services to decision-makers.
Altruisto is a browser extension so that when you shop online, a portion of the money you pay goes to effective charities (no extra cost to you). Just install an extension and when you buy something, people in poverty will get medicines, bed nets, or financial aid.
Giving What We Can is a charitable movement promoting giving some of your money to the developing world or other worthy causes. If you're interested in this, consider taking their Pledge as a formal and public declaration of intent.
Support Slate Star Codex on Patreon . I have a day job and SSC gets free hosting, so don't feel pressured to contribute. But extra cash helps pay for contest prizes, meetup expenses, and me spending extra time blogging instead of working.
Norwegian founders with an international team on a mission to offer the equivalent of a Norwegian social safety net globally available as a membership. Currently offering travel medical insurance for nomads , and global health insurance for remote teams .
Metaculus is a platform for generating crowd-sourced predictions about the future, especially science and technology. If you're interested in testing yourself and contributing to their project, check out their questions page

Jane Street is a quantitative trading firm with a focus on technology and collaborative problem solving. We're always hiring talented programmers, traders, and researchers and have internships and fulltime positions in New York, London, and Hong Kong. No background in finance required.
Beeminder's an evidence-based willpower augmention tool that collects quantifiable data about your life, then helps you organize it into commitment mechanisms so you can keep resolutions. They've also got a blog about what they're doing here
MealSquares is a "nutritionally complete" food that contains a balanced diet worth of nutrients in a few tasty easily measurable units. Think Soylent, except zero preparation, made with natural ingredients, and looks/tastes a lot like an ordinary scone.
Dr. Laura Baur is a psychiatrist with interests in literature review, reproductive psychiatry, and relational psychotherapy; see her website for more. Note that due to conflict of interest she doesn't treat people in the NYC rationalist social scene.
Seattle Anxiety Specialists are a therapy practice helping people overcome anxiety and related mental health issues (eg GAD, OCD, PTSD) through evidence based interventions and self-exploration. Check out their free anti-anxiety guide here

IMAGES
VIDEO
COMMENTS
Yasenyavskaya A, Tsibizova A and Samotrueva M (2024) Neurotrophic Hypothesis of the Development of Depression, Human Physiology, 10.1134/S0362119723700664, 50:2, (194-200), Online publication date: 1-Apr-2024.
Basic studies show that ketamine rapidly induces synaptogenesis and reverses the synaptic deficits caused by chronic stress. These findings highlight the central importance of homeostatic control of mood circuit connections and form the basis of a synaptogenic hypothesis of depression and treatment response.
A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists ... actions of antidepressant treatment could be mediated in part by blocking or reversing the atrophy caused by stress and depression. Recent studies have identified a novel, rapid-acting antidepressant, ketamine, in treatment-resistant ...
Here, we revisit the neurotrophic hypothesis of depression more than 20 years later, restricting our focus to BDNF as the prototypical example best studied in this regard, and discuss evidence in support, as well as challenges to this hypothesis. ... A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor ...
Abstract. The neurogenesis hypothesis of depression posits (1) that neurogenesis in the subgranular zone of the dentate gyrus is regulated negatively by stressful experiences and positively by treatment with antidepressant drugs and (2) that alterations in the rate of neurogenesis play a fundamental role in the pathology and treatment of major ...
Abstract. Depression is a common, devastating illness. Current pharmacotherapies help many patients, but there are high rates of partial- or non-response and the delayed onset of the effects of antidepressant leave many patients inadequately treated. However, new insights into the neurobiology of stress and human mood disorders have shed light ...
To directly test this hypothesis, the influence of rapamycin, a selective inhibitor of mTOR (figure 3), on synaptogenesis was examined. Rapamycin pretreatment completely blocked ketamine-induction of spine number and function of layer V pyramidal neurons in the PFC [ 42 ].
Changing paradigms include thinking about the neural circuits involved in depression, the waxing and waning of neurogenesis and synaptogenesis, and realising that there may be important abnormalities of glial cells rather than just abnormalities of neuron to neuron synapses. These shifts in paradigm have occurred within broader developments in ...
A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists ... synapses or synaptogenesis is a key form of neuro-plasticity, and represents a ...
A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists September 2012 Philosophical Transactions of The Royal Society B Biological Sciences 367 ...
We propose a hypothesis that depression is caused by disruption of homeostatic mechanisms that control synaptic plasticity, resulting in de-stabilization and loss of synaptic connections in mood and emotion circuitry. We compare and contrast the mechanisms underlying typical anti-depressantsandketamine,particularlytheinduction of synaptogenesis.
The neural plasticity theory of depression: assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus Neural Plast. ... migration, and integration of new neurons to neurite outgrowth, synaptogenesis, and the modulation of mature synapses. This review critically assesses the role of adult hippocampal neurogenesis and the cell ...
More recently, however, there has been a paradigm shift toward a neuroplasticity hypothesis of depression in which downstream effects of antidepressants, such as increased neurogenesis, contribute ...
Molecular and cellular studies have demonstrated opposing actions of stress and antidepressant treatment on the expression of neurotrophic factors, particularly brain-derived neurotrophic factor, in limbic structures of the brain. These changes in ...
Numerous investigations have demonstrated that 5-HT is intimately related to the pathophysiological process of major depression. The 5-HT hypothesis primarily asserts that a decrease in the 5-HT ...
A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012; 367:2475-2484. doi: 10.1098/rstb.2011.0357. ... et al. Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome.
A number of factors (biogenic amine deficiency, genetic, environmental, immunologic, endocrine factors and neurogenesis) have been identified as mechanisms which provide unitary explanations for the pathophysiology of depression. Rather than a unitary construct, the combination and linkage of these …
Activation of mTOR and increased synaptogenesis in the prefrontal cortex appear to be crucial in mediating the antidepressant effects of ketamine. ... In the early 1970s, the discovery that the acetylcholinesterase inhibitor, physostigmine, produces symptoms of depression led to the hypothesis that hyperactivity of the cholinergic system ...
The serotonin (5-HT) hypothesis of depression has played an important role in the history of psychiatry, yet it has also been criticized for the delayed onset and inadequate efficacy of selective serotonin reuptake inhibitors (SSRIs). With evolvement of neuroscience, the neuroplasticity hypothesis of major depressive disorder (MDD) has been ...
According to Duman's Neurobiology of Stress, Depression, and Rapid Acting Antidepressants, it's decreased synaptogenesis, and it's regulated by a protein complex called mTORC1. Neurons communicate with other neurons through branches called dendrites and connections called synapses. Healthy neurons often create new dendrites and synapses ...