WAEC chemistry past questions and answers (PDF) free
Free download waec chemistry past questions, join waec whatsapp group (sciences) 2021.
February 02 2021

WhatsApp Group for WAEC Arts students 2021
February 03 2021
May/June 2023 WAEC expo/runs (also, join WhatsApp Group)
February 10 2021
Add a Comment
Notice: Posting irresponsibily can get your account banned!
Comments, Page 1/1
I can click on answer
i need 2023 physics
I need past question for 2023 wace
I really love the past questions and answer because it help to no how to answer the weac questions
I need 2003 past questions and answers
Past question and answer on chemistry 2022
i need 2021 question on chemistry
I need an answers
I need past paers for 2021
I need 2019 past question
I really like past questions andanswers good done
nice one this is good
Featured Posts
Latest posts.

WASSCE / WAEC Elective Chemistry Past Questions
Welcome to our WASSCE / WAEC Elective Chemistry past questions page.
Larnedu has the largest WASSCE past questions collection on the web and this is not an exaggeration.
We’re not perfect but we have been working towards improving every day and achieving our mission , which includes helping every student that accesses our learning resources and is ready to work hard, excel academically.
All the WAEC Elective Chemistry past questions on this page have been100% free since day one and would likely remain so in the foreseeable future.
We actually spend our financial resources to have some of our WASSCE past questions sent to us, so please:
- DO NOT download any of our WASSCE Elective Chemistry past papers to post on other forums or websites without giving us credit.
- DO NOT sell any WAEC Elective Chemistry past paper you downloaded from Larnedu.
- DO NOT post any WASSCE Elective Chemistry past question paper you downloaded from Larnedu on websites that ask for membership payment or request users to complete tasks (surveys, etc) before they download the paper.
Remember, we started this page to help students who don’t have the budget for hardcopy WASSCE Elective Chemistry past question papers or who want easy access to the past question papers on their mobile devices or computers.
| Go directly to the WASSCE / WAEC Elective Chemistry past questions section on this page (skip the introductory text). |
The West African Senior School Certificate Examination (WASSCE) is a type of standardized test taken in West Africa, mostly by students who wish to proceed to the higher institution. It is administered by the West African Examination Council (WAEC).
It is only offered to candidates residing in Anglophone West African countries namely: Ghana, Gambia, Nigeria, Liberia and Sierra Leone and is written 2 times a year ( May/June and Nov/Dec) .
The WASSCE tests candidates according to the topics on the WAEC syllabus.
The contents in each WASSCE Elective Chemistry question paper (for a specific year) is usually similar from one country to another. Questions on the WASSCE Elective Chemistry theory section may be specified to be answered by candidates from a particular country and this happens mostly in the theory section.
A WASSCE question paper on a particular subject may be entirely cancelled and changed in a region when the West African Examination Council (WAEC) heading that region suspects a leakage of examination papers before the start of the exam.
Benefits of regular WAEC past questions practice
Speed: Regular practice of our WASSCE Elective Chemistry past questions makes you faster on the exam day. It’s no secret that questions on the WASSCE for each particular subject are usually similar to questions in previous years since they’re from the same WAEC syllabus . WAEC also sometimes repeats questions word-for-word.
Exposure: Regular practice exposes you to your weaknesses and gives you a chance to better yourself before the exam.
Decreases chances of anxiety: Regular and efficient practice improves your confidence before the exam.
These and many more are some of the beautiful benefits of practising WASSCE Elective Chemistry past questions.
So it’s important you make it a habit to regularly practice with the past question papers. There’s no doubt that this would help you achieve the grades you desire on the WASSCE on the long run.
Don’t just focus only on the WAEC past questions we provide on this page. We also have other WASSCE related resources that will be of great help to you.
Below this section are the Elective Chemistry WASSCE / WAEC past questions we have for now. Feel free to use them in accordance to the rules stated on this page and our Terms of Service .
Download (pdf) or view online-WASSCE / WAEC Chemistry past questions
| 2013 | NG | |
| 2013 | NG | |
| 2011 | NG | |
| 2011 | GH | |
| 2006 | NG | |
| 2006 | NG | |
| 2006 | NG | |
| 2000 | NG | |
Make a difference now
We’re always striving to provide a better user experience on Larnedu and this includes providing high quality resources to help every student out there. The best part is that we do this without requesting for a dime most of the time.
Join other helpful users that support what we do by taking a few minutes of your day to do any of the following:
- Send a digital copy of any WASSCE Chemistry past question paper in your collection that is absent from our collection (rewards available). If you don’t have access to a scanner, you can take clear pictures of the pages on a particular paper with your mobile phone/tablet, and send it to us with the appropriate title-we’ll do the rest.
- Share our content on social media or spread the word through word of mouth. This would enable us reach more people to potentially help (in accordance to our mission).
- Donate some funds (no matter how little) to help support our rising costs (hosting fees, etc).
- Feature us on your blog/site to create more awareness of what we do.
- Give recommendations or feedback on what we do.
Feel free to choose whatever option that’s not in the list above that you feel would also support our work.
Recommended posts or pages
We go through great lengths to provide the best resources to every student preparing for the WASSCE and here are some of the ones we recommend:
- LinkedIn 31
Subscribe now to get summarised alerts of new posts by email.
Subscribe to new posts by email:, recent posts.
- The 2020 US Election Uncertainty Cure For Investors November 2, 2020
- The COVID-19 ‘Coronomic’ Impact: Short-and Long-Term Risks March 15, 2020
- Happy New Year! January 1, 2020
- List of Universities or Colleges in UK That Accept WASSCE (WAEC) Results November 13, 2019
- List of Universities or Colleges in Canada That Accept WASSCE (WAEC) Results November 13, 2019
- List of Universities or Colleges in Us That Accept WASSCE (WAEC) Results November 13, 2019
- Capitalism Without Capital (2018) Book Summary and Insights November 12, 2019
- AI Superpowers (2018) Book Summary and Insights November 12, 2019
- The Curse of Bigness (2018) Book Summary and Insights November 12, 2019
- The Road to Character (2015) Book Summary and Insights November 12, 2019

WASSCE(WAEC)
Steps to Take if You Have A Poor WASSCE / WAEC Result
16 Sep, 2014

6 Ways: How to Upgrade Your WASSCE / WAEC Result
13 Aug, 2015

List of Universities or Colleges in Canada That Accept WASSCE (WAEC) Results
13 Nov, 2019

Interviews & Memoir
Interview: Shree Bose–Google Science Fair Winner And More
30 Mar, 2015
Online Education/Distance Learning
How to Get Free Certificates on Coursera (Financial Aid)
8 Sep, 2015

QNA: How to Check Your WAEC Result Without Scratch Card
5 Nov, 2015

REVEALED: How to Check WASSCE / WAEC Results Online For Free

50+ Tips: How to Pass the WASSCE / WAEC Examination
1 Sep, 2015

Study Abroad & International Students Experience
16 Best Reasons To Study In Finland
4 Apr, 2015

International Students Guide: How To Study In Finland For Free
8 Apr, 2015
10 Steps: How to Check Your WASSCE / WAEC Result Online
11 Aug, 2015

Studying Abroad In Qatar: What Will Your Experience Entail?
15 Jul, 2015

Scholarships, Grants & Awards
Top 2017 Scholarship Opportunities For International Students
15 Feb, 2017

Uncategorised
Can Your Idea Change The World? Google Will “Pay” You
25 Feb, 2015
Why Facebook Should Implement MOOCs To Internet.org
23 Feb, 2015

Chemistry Paper 2 (Practical), WASSCE (SC), 2018
- Subject Home
| Menu |
General Comments
The standard of the paper was high and compared favourably with those of the previous years. The questions were unambiguous and within the scope of the examination syllabus. The marking scheme was fair to all candidates and was unambiguous. It was reported that the performance of the candidates was below average and was worse than WASSCE for School Candidates, 2017 with a raw mean score of 47.0 and standard deviation of 16.0 compared to a raw mean score of 29.0 and standard deviation of 13.78 for WASSCE for School Candidates, 2018. The total number of candidates that sat the examination was 728,998.
Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here
WAEC Chemistry Questions and Answers for 2023/2024 (Theory and Objectives)
2023 WAEC Chemistry Answers, WAEC Chemistry questions and answers, WAEC Chemistry questions and everything you need to know about 2023 WAEC Chemistry Examination will be provided in this article. You will find Chemistry Paper 1 (Objective) and Chemistry 2 (Essay) here
Scroll to the bottom for today’s Chemistry Answers
Below are the WAEC Chemistry questions. Read them and get ready to score high in your WAEC Chemistry exam.
The West Africa Examination Council (WAEC) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in May/June and November/December respectively.
Table of Contents
WAEC Chemistry Questions and Answers 2023
WAEC Chemistry Questions and Answers 2023 Loading…
The 2023 answers will be posted here on 24th May during the exam
WAEC Chemistry OBJ Answers Loading…
1-10: ACADABBDAC
11-20: BCAABBCCBB
21-30: ACBABDACBB
31-40: ABBDAADCDD
41-50: BDCDDCDDCD
A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
(i) Element B (2:8:2)
(ii) Element C (2:8:1)
The increase in the first ionization energies across a period is mainly due to the increasing effective nuclear charge and decreasing atomic radius. The stronger pull from the increasing nuclear charge and the closer proximity of the electrons to the nucleus make it harder to remove an electron, leading to higher ionization energies.
Number 1di)
Two examples of aliphatic compounds are:
A. Ethane (C2H6): Ethane is a saturated hydrocarbon belonging to the alkane family. It consists of a straight chain of two carbon atoms bonded to each other, with six hydrogen atoms attached to the carbons.
B. Butanol (C4H10O): Butanol is an alcohol with a four-carbon straight chain. It has the chemical formula C4H10O and exists in four isomeric forms: n-butanol (normal butanol), sec-butanol (secondary butanol), isobutanol (isopropyl alcohol), and tert-butanol (tert-butyl alcohol).

*Reaction between Iron (Fe) and Dilute H2SO4:*
Iron reacts with dilute sulfuric acid to form iron sulfate (FeSO4) and hydrogen gas (H2). The balanced chemical equation for this reaction is:
3Fe(s) + 4H2SO4(aq) -> 3FeSO4(aq) + 2H2(g) + 2H2O(l)
In this reaction, iron displaces hydrogen from the acid, resulting in the production of iron sulfate and hydrogen gas. The iron sulfate is soluble in water and remains in solution, while the hydrogen gas is released as a gas.
*Reaction between Aluminum (Al) and Dilute H2SO4:*
Aluminum reacts differently with dilute sulfuric acid due to the protective oxide layer that forms on its surface. The oxide layer prevents further reaction by acting as a barrier between the aluminum metal and the acid. However, the reaction can occur if the protective layer is disrupted or removed
2Al(s) + 3H2SO4(aq) -> Al2(SO4)3(aq) + 3H2(g)
In this reaction, aluminum sulfate (Al2(SO4)3) and hydrogen gas are produced. The aluminum sulfate formed is soluble in water and remains in solution, while the hydrogen gas is liberated as a gas.

More Answers Loading…
—————————————————————-
—————————————————–
The questions below are the WAEC Chemistry Questions. Go through them and be ready to score high in your WAEC 2023 Chemistry Examination.
1. What condition favours the formation of the product for the endothermic reaction, N2O4(g) —><—– 2NO2(g)
A. Decrease in pressure
B. A decrease in volume
C. An increase in pressure
D. A constant volume
ANSWER: A ( Decrease in pressure)
2. How many alkoxy alkanes can be obtained from the molecular formula C 4 H 4 O 4
ANSWER: C (3)
3. Element Y has two isotopes Y and Y present in the ratio 1:3. The relative atomic mass of Y would be
ANSWER: C (21.5)
4. 100.0g of KClO 3 was added to 40.0 cm^3 of water to give a saturated solution at 298K. If the solubility of the salt is 20.0 moldm^-3 at 298K, what percentage of the salt is left undissolved? {K= 39, Cl = 35.5, O = 16}
5. Elements X and Y have electronic configuration 1S 2 2S 2 2P 4 and 1S 2 2S 2 2P 6 3S 2 3P 1 respectively. When they combine the formula of the compound formed is
ANSWER: B (Y 2 X 3 )
Recommended: WAEC Timetable
6. A solution of 0.20 mole of NaBr and 0.20 mole of MgBr 2 in 2.0 dm 3 of water is to be analysed. How many moles of Pb(NO 3 ) 2 must be added to precipitate all the bromide as insoluble PbBr 2
A. 0.30 mol
B. 0.10 mol
C. 0.20 mol
D. 0.40 mol
ANSWER: A (0.30 mol)
7. Na 2 CO 3 + HCl —-> NaHCO 3 + NaCl. The indicator most suitable for this reaction should have pH equal to.
ANSWER: D (9)
8. A satuarted solution of silver trioxocarbonate (IV), was found to have concentration of 1.30 x 10 -5 moldm -3 . The solubility product of the trioxocarbonate (IV) is
A. 8.79 x 10 -15
B. 1.69 x 10 -10
C. 1.82 x 10 -11
D. 9.84 x 10 -10
ANSWER: A (8.79 x 10 -15 )
9. When platinum electodes are used during the electrolysis of copper (II) tetraoxosulphate (IV) solution, the solution gets progressive
D. Atmospheric
ANSWER: A (Acidic)
10. Tetraoxosulphate (VI) ions are final test using
A. acidified silver nitrate
B. acidic barium chloride
C. lime water
D. dilute hydrochloric acid
ANSWER: D (dilute hydrochloric acid)
- WAEC Physics Questions and Answers
- WAEC Biology Questions and Answers
- WAEC English Questions and Answers
WAEC 2020 Chemistry Theory Questions
Paper 2 (essay).
1. Identify the solid remaining when each of the following is heated. (a) lithium trioxonitrate (V) (b) potassium trioxonitrate (V) (c) calcium trioxonitrate (V)
2. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
5. The first ionization energy of chlorine is +1260KJmol-1. (a) Define the term first ionization energy. (b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
7. State two factors other than a change in temperature or the use of a catalyst that influence the rate of a chemical reaction.
1. (a) Two elements represented by the letters Q and R have atomic numbers 9 and 12 respectively. (i) Write the election configuration of R. (ii) To what group does Q belong in the periodic table. (iii) Write the formula of the compound formed when Q combines with R. (iv) Explain briefly, why Q is a good oxidizing agent. (v) State whether R would be expected to form acidic or basic oxide. (b) (i) State two assumptions of the kinetic theory of gases. (ii) When some solids are heated, they change directly into the gaseous state. What name is given to this phenomenon? (iii) List two substances which exhibit the phenomenon mentioned in (ii). (iv) Write an expression to show the mathematical relationship between the rate of diffusion of a gas and its vapour.
2. An aqueous solution has a pH of 4.0. (a) (i) What is the hydrogen ion concentration of the solution? (ii) What effect will it have on litmus paper? (iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq). (b) (i) Differentiate between a fine chemical and a heavy chemical. (ii) Name two sources of air pollution. (iii) Suggest one way of reducing air pollution in cities
3. (a) (i) Explain briefly the fermentation process. (ii) Write a balanced equation for the fermentation of glucose. (iii) What substance must be added to glucose solution to ferment it? (iv) Explain briefly why tightly corked glass filled to the brim with palm wine shatters on standing. (b) State one industrial application of each of the following methods of separation: (i) Crystallization; (ii) Fractional distillation. (c) Explain the following terms: (i) Saponification; (ii) Esterification. (d) Write a balanced equation to illustrate each of the terms in (c). (e) i) What is hydrocarbon compound? (ii) Name two principal sources of hydrocarbons.
WAEC Chemistry Essay and Objective 2023 (EXPO)
The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Chemistry repeated questions and answers.
These questions are for practice. The 2023 WAEC Chemistry expo will be posted on this page during the WAEC Chemistry examination. Keep checking and refreshing this page for the answers.
Keep reloading this page for more answers
Tips to Pass WAEC Chemistry Questions
1. study hard.
It is known to everyone that hard work brings success. Ensure to read the chemistry textbooks recommended by The West Africa Examination Council (WAEC).
This will help you to take a look at most of the questions you will see on the day of WAEC Chemistry examination.
Let me clearly state that questions are not imported from heaven but from those textbooks recommended by WAEC.
2. Read WAEC Chemistry Past Questions
WAEC usually picks questions from the previous years to form fresh questions. Studying WAEC Chemistry past questions would give you an idea of how WAEC Chemistry questions are set.
3. Start Preparing for WAEC 2023 :
Stop wasting your time. Now that you have gotten textbooks and past questions, the next thing is to begin your reading. Early reading and practice is good for you; you will pass well on WAEC 2023 Chemistry
4. Solve and try to understand every example and exercise you see in textbooks
It is very unfortunate that Secondary school students are fond of skipping exercises and even examples while studying textbooks.
They loved notebooks so much that they could ask, “can I read my Chemistry notebook and pass WAEC 2023?”. Don’t be scared of attempting exercises; they are there to help you. Face it and overcome it!
5. Practise Regularly
Don’t get discouraged when certain topics are annoying, keep on practicing until you master everything. Never give up and never say never. Keep pushing….
If you have any questions about WAEC Chemistry Questions and Answers for 2023, kindly drop your question in the comment box and please, remember to share this information by clicking the Facebook share icon or any of the social media share icons below.
Last Updated on May 24, 2023 by Admin
Related posts:

144 thoughts on “WAEC Chemistry Questions and Answers for 2023/2024 (Theory and Objectives)”
Can we get waec questions for 2024 pls?
Sir pls send me the past question and answer i beg Sir pls send me the past question and answer i beg sir
I need number four question please
Pls upload the waec chemistry questions
Pls this this are correct very correct this is what came out in chemistry
Pls sir, i need the questions on civic education tomorrow
Pls sir send me waec question and answer for this year
Please sir i will like You to send waec 2023 chemistry theory and essay
Thanks u alot sir But I need waec chemistry around 9am Please update me soon
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

St Charles Edu Services
Genuine Exam Past Questions and Answers Online Bookshop – PDF and MS Word Download
WAEC Chemistry Past Questions and Answers in 2023 PDF Download Objective & Theory
Are you writing the West Africa Examination Council WAEC Internal or External examination, if yes you need the WAEC Past Questions on Chemistry
we at stcharlesedu.com has compiled a good number of Chemistry WAEC Past Questions and Answers in Pdf Chemistry 2 – Theory/Essay Questions. Chemistry 1 – Objective Test Questions.
Our research has confirm that candidate that uses WASSCE Chemistry past questions to prepare is ten times better than those who do not.
Table of Contents
- 1.1 Chemistry WAEC Objective Questions
- 2 SSCE WAEC Chemistry Theory Questions
- 3 Chemistry WAEC Essay Questions
- 4 Free WAEC Chemistry Exam Past Questions Download
- 5 How to Get WASSCE Chemistry Exam Past Questions and Answers
SSCE WAEC Chemistry Objective Questions and Answers
CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout.
Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen. Give only one answer to each question. An example is given below
What others are downloading WAEC Past Questions for all Subjects
Chemistry WAEC Objective Questions
Which of the following elements reacts with water? A. Carbon B. Iodine C. Sodium D. Sulphur
The correct answer is Sodium, which is lettered C and therefore answer space C would be shaded. [A] [ B ] [C] [ D ]
Think carefully before you shade the answer spaces; erase completely any answer you wish to change.
Which of the following raw materials is used in the plastic industry? A. Ethene B. Methane C. Sulphur D. Hydrogen
Which of the following organic compounds can undergo both addition and substitution reactions? A. Petane B. Benzene C. Propane D. Hexane
Which of the following equations represents a redox reaction? A. AgNO 3 (aq) + KCl(ag)->AgCl(s)+ KNO 3 (aq) B. HNO 3 (aq)+ NaOH(aq) -> NaNO 3 (aq) + H 2 O(l) C. CaCO 3 (s) -> CaO(s) + CO 2 (g) D. 2H 2 S(g) + SO 2 (g) -> 2H 2 O(I) + 3S(g)
T he process of extraction of iron from its ore is A. decomposition. B. oxidation. C. reduction. D. sublimation.
What is the solubility of a salt if 0.4 g of it is obtained on evaporating 200 cm3 of its saturated solution to dryness? A. 0.08 gdm -3 B. 2.00 gdm -3 C. 8.00 gdm -3 D. 80.00 gdm -3
An acidic salt has A. double anions in its aqueous solution. B. a single cation in its aqueous solution. C. hydrogen ions in its aqueous solution. D. hydrogen atoms in its aqueous solution.
A reaction is endothermic if the A. reaction vessel feels cool during the reaction. B. enthalpy change is negative. C. bond forming energy exceeds bond breaking energy. D. heat of formation of reactants exceeds heat of formation of products.
In which of the following compounds does hydrogen form ionic compounds? A. CH 4 B. HCl C. NH 3 D. NaH
Consider the following reaction equation: Br 2 + 2KI -> 2KBr + I 2 . Bromine is acting as A. an oxidizing agent. B. a reducing agent. C. an acid. D. a base.
An organic compound has the empirical formula CH 2 . If its molar mass is 42 gmol-1 what is its molecular formula? [H = 1.0, C = 12.0] A. C 2 H 4 B. C 3 H 4 C. C 3 H 6 D. C 4 H 8
Ethene is produced from ethanol by A. decomposition. B. hydrolysis. C. ozonolysis. D. dehydration.
Consider the following equilibrium reaction: 2 AB(g) + B 2 (g) -><- 2AB 3 (g) AH = -XkJmol -1 The backward reaction will be favored by A. a decrease in pressure. B. an increase in pressure. C. a decrease in temperature. D. an introduction of a positive catalyst.
What is the mass of solute in 500 cm 3 of 0.005 moldm -3 H 2 SO 4 ? [H =1.0, O = 16.0, S = 32.0] A. 0.490 g B. 0.049 g C. 0.245 g D. 0.0245 g
Pure water can be made to boil at a temperature lower than 100 °C by A. reducing its quantity. B. decreasing the external pressure. C. distilling it. D. increasing the external pressure.
Consider the following sketch of the solubility curve of some substances. Note: scroll down to download the free chemistry waec questions in pdf copy to view the sketch
At what temperature does the solubility of KNO 3, equal that of NaNO 3 ? A. 0°C B. 20 °C C. 30 °C D. 40 °C
When a salt is added to its saturated solution, the salt A. dissolves and the solution becomes super saturated. B. dissolves and the solution becomes unsaturated. C. precipitates and the solution remains unchanged. D. dissolves and crystals are formed.
When substance X was added to a solution of bromine water, the solution became colorless. X is likely to be A. propane. B. propanoic acid. C. propyne. D. propanol.
The preferential discharge of ions during electrolysis is influenced by the A. mechanism of electrolysis. B. electrolytic reactions. C. nature of the electrode. D. type of electrolytic cell.
The valence electrons of 12 Mg are in the A. 3s orbital. B. 2px orbital. C. 2s orbital. D. 1s orbital.
Stainless Steel is an alloy comprising of A. Fe and C. B. Fe and Ni. C. Fe, C and Ni. D. Fe, C and Al.
The number of hydrogen ions in 1.0 dm 3 of 0.02 moldm -3 tetraoxosulphate(VI) acid is [NA = 6.02 x 1023] A. 1.2 x 10 22 B. 1.2 x 10 23 . C. 2.4 x 10 22 . D. 2.4 x 10 23 .
The most suitable substance for putting out petrol fire is A. water. B. carbon(IV)oxide. C. fire blanket. D. sand.
The following factors would contribute to environmental pollution except A. production of ammonia. B. manufacture of cement. C. photosynthesis. D. combustion.
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants. B. vigorous stirring of the reaction mixture. C. presence of a catalyst. D. change in concentration of the reactants.
The diagram below illustrates a conical flask containing water and ice.
NOTE: scroll down and download the free chemistry pdf past questions to see the diagram
Which of the following statements about the diagram is correct? A. The water is at a lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point
Which of the following statements best explains the differences between a gas and a vapor? A. Unlike gases, vapors are liquids at room temperature B. Unlike gases, vapor can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases
Consider the following reaction equation: 2HCl + Ca(OH) 2 –> CaCl 2 + H 2 O. What is the volume of 0.1 moldrn -3 HCl that would completely neutralize 25cm 3 of 0.3 moldm -3 Ca(OH) 2 ? A. 150 cm 3 B. 75 cm 3 C. 30 cm 3 D. 25 cm 3
Cu and HNO 3 are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively. B. conductivity and corrosiveness respectively. C. melting point and reduction respectively. D. electro negativity and solubility respectively.
Which of the following formulae cannot be an empirical formula? A. CH B. CH2 C. P2O5 D. N204
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity. B. boiling point. C. mass. D. colour.
Want more Chemistry Objective Test Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answers (Obj and Essay) in PDF Format from us.
SSCE WAEC Chemistry Theory Questions
Chemistry Paper 2 Paper 2 will last for 2 hours This paper consists of two sections A and B. Answer one questions from Section A and three questions from Section B.
Credit will be given for clarity of expression and orderly presentation of material.
SECTION A (1ai) Define the term fermentation. (1aii) Name the catalyst that can be used for this process.
(b) Name two factors which determines the choice of an indicator for an acid-base titration. (c) Consider the following reaction equation: [Fe + H2S04 ] FeS04 + H2. Calculate the mass of unreacted iron when 5.0g of iron reacts with 10cm3 of 1.0 moldrrv3 H SO [Fe = 56.0] (d) Name one: (di) Heavy chemical used in electrolytic cells; (dii) Fine chemical used in textile industries.
(e) Explain briefly how a catalyst increases the rate of a chemical reaction. (f) (i) Write the chemical formula for the product formed when ethanoic acid reacts with ammonia. (ii) Give the name of the product formed in 1 (f) (i)..
(g) List three properties of aluminum that makes it suitable for the manufacture of drink can. (h) State two industrial uses of alkylalkanoates. (i) List two effects of global warming. (j) Name two steps involved in the crystallization of a salt from its solution.
Chemistry WAEC Essay Questions
SECTION B. 2ai. State the collision theory of reaction rates. 2aii.Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction.
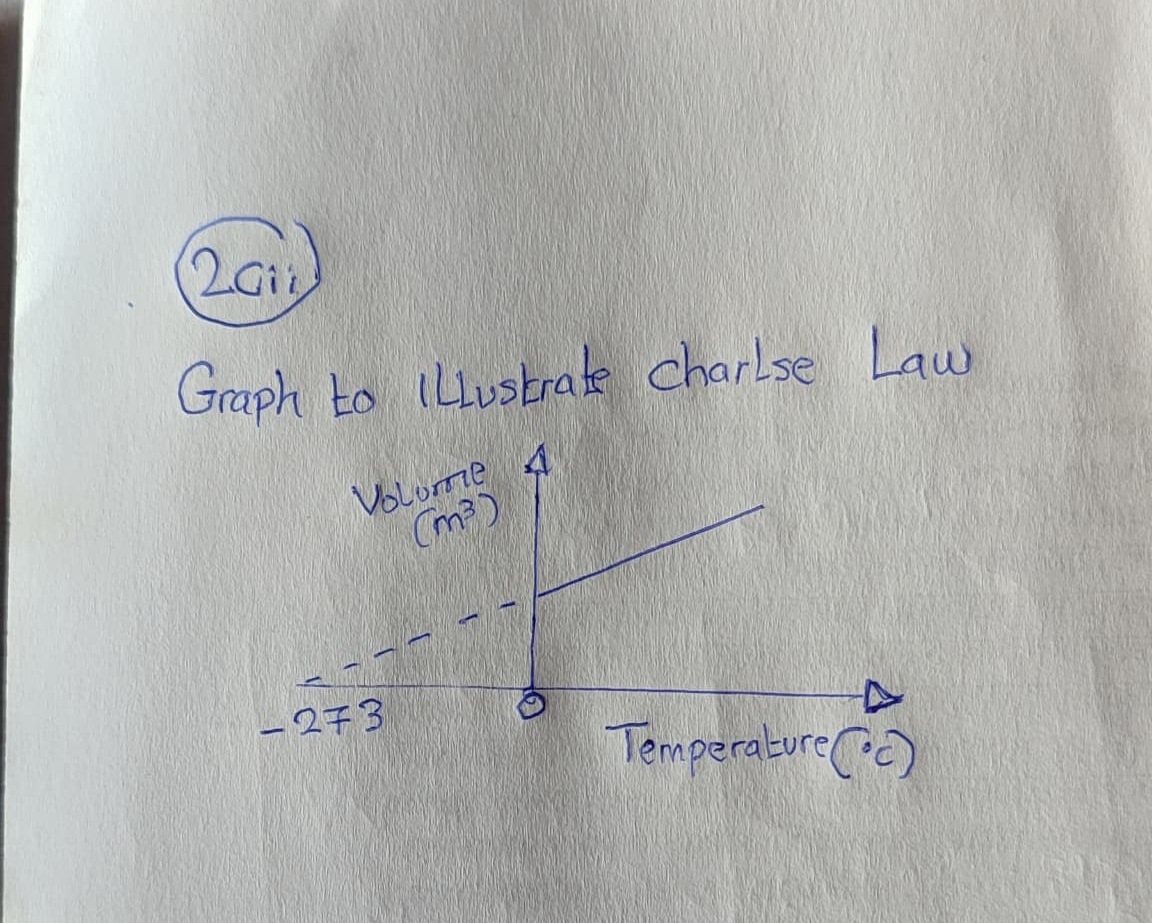
bi. Sketch a graphical representation of Charles’s law. bii. Calculate the volume of oxygen that would be required for the complete combustion of 2.5moles of ethanol at s.t.p. [ molar volume at s.t.p = 22.4dm3]
ci. Define esterification. cii. Give two uses of alkanoates. ciii. Give the products of the alkaline hydrolysis of ethyl ethanoate.
d. A tin coated plate and a galvanized plate were exposed for the same length of time. di. Which of the two plates corrodes faster? dii. Explain briefly your answer in 2 (d) (i).
Want more Chemistry Theory Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answer (Obj and Essay) in PDF Format from us.
Free WAEC Chemistry Exam Past Questions Download
Click to Download your free NECO Past Question on Painting and Decorating Paper 2 and 3
Link 1: WASSCE Chemistry Questions Booklet Link 2: WASSCE Chemistry Questions Booklet
How to Get WASSCE Chemistry Exam Past Questions and Answers
To get the complete and more recent copy of the West Africa Examination Council WAEC Past Questions and answer
Take Note of the following step
Make a Call Call or whatsapp us on 08051311885 for the account number to make payment and how to received your complete copy of the past questions to be sent directly to your email address or whatsapp number.
Mode of Payment. Mobile Transfer or Direct Bank Deposit.
After Payment send us the following Depositor Name: Name of Product Paid for: Valid email address.
DELIVERY ASSURANCE We will deliver the past question to you 10 mins after confirmation of payment to the email you will send to us.
Related Posts:
- WAEC Technical Drawing Past Questions PDF Download – Objective, Essay, Building Plan/Practical Drawing
- WAEC Government Past Questions and Answers in 2023 PDF Download Objective & Theory
- WAEC Financial Accounting Past Questions and Answer 2023 – Objective & Essay
- WAEC Visual Art Past Questions and Answers – Objective, Theory in 2023
- WASSCE/WAEC Electrical Installation & Maintenance Past Questions PDF – Objective/Essay

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
No 1 Nigeria Education News
WAEC Chemistry Past Question & Answer (2008 – 2024) | SSCE & GCE
July 16, 2024 by A.Y 16 Comments
Hey Readers, Here is the WAEC Chemistry Past Question and Answer for 2008 till 2024. However, this article will be updated once the latest Chemistry Past question is available. So if you are getting prepared for the Senior Secondary School Certificate examination then I will advise you to bookmark this page.

The importance of these past questions is to know how prepared you are for the SSCE and GCE examination. It will help you to know how WAEC sets their question and how they want it to be answered. WAEC itself releases answers to all the past questions available on the platform. Meanwhile, If you treat the past question very well you might come across some questions which will be asked in the WAEC Examination you are preparing to write (Repeated Questions).
Recommended Link
- WAEC Chemistry Syllabus
- WAEC Past Question for all Subject
How to Access & Use WAEC Past Question
- After using the WAEC Syllabus and Recommended Textbook to Read and Meditate
- Click on the link below to access the WAEC Chemistry past questions
- Once you land on the new page you will see (General Comment) click on Next to see the Weakness/Remedies and Strength
- To access the WAEC Maths Question and Answer click on the number in the Question Column at the top of the page.
WAEC Chemistry Past Question & Answer 2008 – 2024
The resources below on Chemistry have been provided by WAEC to assist the student to understand the required standards expected in the Chemistry final Examination. Students’ performance in the examination under review was done by the Chief examiner, this you will see while exploring links like General Comment, Performance, Weaknesses, Strength, and Observation to respective Questions.
- WASSCE Chemistry Paper 1, May/June. 2008
- WASSCE Chemistry Paper 2, May/June. 2008
- WASSCE Chemistry Paper 2, Nov/Dec. 2008 (Private)
- WASSCE Chemistry Paper 3, Nov/Dec. 2008 (Private)
- WASSCE Chemistry Paper 2, Nov/Dec. 2009 (Private)
- WASSCE Chemistry Paper 3, Nov/Dec. 2009 (Private)
- WASSCE Chemistry Paper 2, May/June. 2010
- WASSCE Chemistry Paper 2, Nov/Dec. 2010 (Private)
- WASSCE Chemistry Paper 3, Nov/Dec. 2010 (Private)
- WASSCE Chemistry Paper 1, May/June. 2011
- WASSCE Chemistry Paper 2, May/June. 2011
- WASSCE Chemistry Paper 2, Nov/Dec. 2011 (Private)
- WASSCE Chemistry Paper 3, Nov/Dec. 2011 (Private)
- WASSCE Chemistry Paper 1, May/June. 2012
- WASSCE Chemistry Paper 2, May/June. 2012
- WASSCE Chemistry Paper 2, Nov/Dec. 2012 (Private)
- WASSCE Chemistry Paper 3, Nov/Dec. 2012 (Private)
- WASSCE Chemistry Paper 1, May/June. 2013
- WASSCE Chemistry Paper 2, May/June. 2013
- WASSCE Chemsitry Paper 3, Nov/Dec. 2013 (Private)
- WASSCE Chemistry Paper 2, May/June. 2014
- WASSCE Chemistry Paper 3, Nov/Dec. 2014 (Private)
- WASSCE Chemistry Paper 3, May/June. 2015
- WASSCE Chemistry Paper 2, Nov/Dec. 2015 (Private)
- WASSCE Chemistry Paper 3, Nov/Dec. 2015 (Private)
- WASSCE Chemistry Paper 2 for School Candidate 2016
- WASSCE Chemistry Paper 3 for School Candidate 2016
- WASSCE Chemistry Paper 2 for Private Candidate 2016
- WASSCE Chemistry Paper 3 for Private Candidate 2016
- WASSCE Chemistry Paper 2 for School Candidate 2017
- WASSCE Chemistry Paper 3 for School Candidate 2017
- WASSCE Chemistry Paper 2 for Private Candidate 2017
- WASSCE Chemistry Paper 3 for Private Candidate 2017
- WASSCE Chemistry Paper 2 for Private Candidate 2018 (1st Series)
- WASSCE Chemistry Paper 3 for Private Candidate 2018 (1st Series)
- WASSCE Chemistry Paper 2 for School Candidate 2018
- WASSCE Chemistry Paper 3 for School Candidate 2018
- WASSCE Chemistry Paper 2 for Private Candidate 2018 (2nd Series)
- WASSCE Chemistry Paper 3 for Private Candidate 2018 (2nd Series)
- WASSCE Chemistry Paper 2 for Private Candidate 2019 (1st Series)
- WASSCE Chemistry Paper 3 for Private Candidate 2019 (1st Series)
- WASSCE Chemistry Paper 2 for School Candidate 2019
- WASSCE Chemistry Paper 3 for School Candidate 2019
- WASSCE Chemistry Paper 2 for Private Candidate 2019 (2nd Series)
IF YOU FIND THIS ARTICLE HELPFUL SOMEONE MIGHT ALSO NEED IT SO DON’T HESITATE TO SHARE.
THANKS FOR VISITING NEWSEDUNG, LEAVE A COMMENT BEFORE CLOSING THE TAB.
January 14, 2023 at 10:31 PM
how much for mathematics text book
November 28, 2022 at 10:41 PM
November 27, 2022 at 4:55 PM
August 23, 2022 at 10:16 PM
Bravo, very good
August 16, 2022 at 3:47 PM
I want to join
August 16, 2022 at 7:23 AM
It is so helpful
August 8, 2022 at 7:56 AM
more better than i thought
June 7, 2022 at 7:34 PM
I want to be part of the team
June 7, 2022 at 8:25 AM
it seems to be helpful
April 28, 2022 at 11:19 PM
Success is my first legal priority
April 28, 2022 at 11:15 PM
March 2, 2022 at 5:12 PM
Please what is the difference between school candidate and private candidate? Also, is it possible to get the paper1 past questions of the years posted above?
March 2, 2022 at 5:01 PM
This sacrifice of time and resources is great. Thank you so much I’ve bookmarked the page
February 5, 2022 at 12:23 PM
Yes is helpful
February 3, 2022 at 11:16 AM
Success is my
February 5, 2022 at 12:24 PM
Yes is true
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
SAY GOODBYE TO JAMB,GAIN DIRECT ENTRY ÀDMISSION INTO 200LEVEL TO STUDY YOUR DESIRED COURSE IN ANY UNIVERSITY OF YOUR CHOICE.LOW FEES. REGISTRATION IS IN PROGRESS . CALL / WHATSAPP 09059908384.
15 Common Topics In Chemistry Questions In WAEC, NECO & JAMB Examination
Chemistry is one of the most feared subjects by science candidates sitting for the West African Senior Secondary Certificate Examination (WASSCE), NECO (National Examination Council) or the Unified Tertiary Matriculation Examination (UTME) because they fail to prepare properly for this subject. One strategy of passing Chemistry in any of the above listed examinations is studying hard and most importantly, knowing the compulsory questions that the body (WAEC, NECO or JAMB) test students on that is why we have listed the 15 most common WAEC topics in Chemistry for you to test yourself on. Out of the topics on Chemistry syllabus , at least more than 80% of the questions must come from the 15 of the listed topics because they are the most common questions set by examiners every year.
The importance of Chemistry as a requirement for admission into various higher institution cannot be underestimated. According to various higher institutions, one of the numerous admission requirements for studying any science or engineering course is that candidate passes Chemistry, Physics, Mathematics, and English language. However, there are some courses offered in higher institutions that do not require Chemistry or a Credit pass as a requirement but they are just a handful.
Passing Chemistry in WAEC, NECO or JAMB is very simple so long as you are prepared for the questions ahead. Getting prepared as a result of being aware of the most likely Chemistry questions in WAEC makes it even more interesting.
The most likely topics listed below are common topics in both Objective (Obj) and theory. To further break it down, we highlighted the most common topics under objective and theory for ease of understanding. Knowing the most likely topics in Chemistry for WAEC is not enough as adequate preparedness is key to passing Chemistry in your exam that is why you are advised to get past questions and answer papers and test yourself on the under-listed topics below to ascertain your strengths and weaknesses.
Always remember that Examiners are very smart, they know it is very possible to attempt all questions adequately given the allotted time so they present you with time wasting questions like that carries equal marks with other simpler and direct ones. In this case, you are advised to skip such questions on and come back to it after being done with the others. However, strengths and weaknesses differ so if you know you are very good at the so called time wasters , you could go ahead with it when confronted with one.
One last thing to note is that different marks are allotted to different questions. In the West African Senior Secondary School Certificate Examination (WASSCE), the National Examination Council or UTME, some questions carry higher mark than the others though they could be time wasting too so you must find a balance when solving questions on such questions.
Much has been said not just about the 15 WAEC or NECO compulsory topics to expect in Chemistry examination but how to attempt them and which question must be given priority. Now I will be listing the 15 most common WAEC and NECO topics in Chemistry you must come across in both Objective and theory section. These topics when mastered MUST guarantee you a distinction in Chemistry in any of the examination you are tested at.
15 Most Common WAEC Topics in Chemistry Examination (Theory and Obj)
See the list of topics below:
1) Atoms Moles Formulae and Equation and their properties (isotopes of an atom, atomic structures and related atomic questions,
2) Periodic Table (Properties and the likes)
3) Organic compounds like methane, propane butane etc (their properties)
4) Acid Base and Salts
5) Atomic Structures: Chemistry Combination
6) Physical Properties of Elements and Their Variations
7) Electrolysis
8) Chemical Equilibrium
9) Chemical Thermodynamics
10) Kinetic Theory of Matter
11) Carbon and Its Compounds
12) Sulphur and Its Compounds
13) Organic Chemistry
14) Proactivity and Nuclear Chemistry
Please take the above topics very seriously. If you are willing and determined to pass Chemistry in WAEC or NECO or JAMB your mastery of the 15 compulsory topics with their approved text book for senior secondary schools and attempts on various past questions would do you a lot of positives in passing this subject.
Do you have any questions as regards the above? Please reach out to us via the comment section below and we shall respond immediately. Note that the most likely UTME, NECO or WAEC topics for Chemistry examination listed above does not mean you should avoid other topics, remember that the examiner isn’t testing you for Chemistry topics in SS 3 classes only but on all Chemistry topics ranging from SS 1 first term to the completion of your SS 3. It is as a result of its cumbersomeness that I thought it necessary to list the exact topics that must come out yearly after thorough research.
I wish you the very best of luck in your forthcoming examination.
Share this:
Say Goodbye to JAMB, Enter 200-Level Directly! Gain University Admission via JUPEB/IJMB . Enjoy Low Fees! Call 08033006849 Now!

HOME » Past Questions » Free WAEC Past Questions and Answers for All Subjects
Free WAEC Past Questions and Answers for All Subjects
Free WAEC past questions and answers are available here for download!

Are you in your last stage of Secondary School Education (May/June) or not in the School system (GCE)? If yes, you can now download West African Senior School Certificate Examination (WASSCE) past papers to assist you with your studies.
The importance of using past questions in preparing for your West African Senior School Certificate Examination (WASSCE), cannot be over emphasised. By using past exam papers as part of your preparation, you can find out what you already know and at the same time also find out what you do not know well enough or don’t know at all.
See: WAEC Timetable for May/June Candidates and WAEC Timetable for GCE Candidates .
WAEC Past Questions
- WAEC Agricultural Science Past Questions
- WAEC Biology Past Questions
- WAEC Chemistry Past Questions
- WAEC Commerce Past Questions
- WAEC CRK Past Questions
- WAEC Economics Past Questions
- WAEC English Past Questions
- WAEC Financial Accounting Past Questions
- WAEC Further Maths Past Questions
- WAEC Geography Past Questions
- WAEC Literature in English Past Questions
- WAEC Mathematics Past Questions
- WAEC Physics Past Questions
- WAEC Technical Drawing Past Questions
- WAEC Visual Arts Past Questions
- WAEC Yoruba Past Questions
See also: WAEC Latest Syllabus for all subjects and WAEC Sample Questions and Scheme for All Subjects .
Do you have any other past question(s) other than the ones listed here? If yes, don’t hesitate to share them with others by sending it to [email protected] .
Practice WAEC Past Questions and Answers Online – All Subjects.
WAEC recently launched a portal called WAEC e-learning to curb the number of failures in the WAEC May/June SSCE by creating a portal that contains the resources for all WAEC approved subjects that will students understand the standards required for success in respective examinations.
WAEC e-learning contains past questions and solutions of all subjects.
WAEC e-learning portal is accessible from http://waeconline.org.ng/e-learning/index.htm.
Don’t forget to share this with your friends if you want their success.
Similar Posts:
- Price of WAEC GCE Scratch Cards & Selling Points
- WAEC GCE Registration Form Template
- WAEC GCE ‘Walk-in Candidates’ Registration Process
- 2024 WAEC GCE Syllabus Available Here : All Subjects
- Joint Universities Preliminary Examinations Board (JUPEB) Past Questions
- WAEC Christian Religious Knowledge (CRK) Past Questions | FREE DOWNLOAD
- WAEC Economics Past Questions | FREE DOWNLOAD
- WAEC English Language Past Questions | FREE DOWNLOAD
2024 WAEC CHEMISTRY: Chemistry (Chems) WAEC Authentic Questions and Answer 2024 (1531)
Notice board, with examplaza a1 is sure in 2024 neco.
Account Number: 7040209000
Bank Name: Wema
Account Name: Monify Onuwa Mobile
Note: After payment upload your proof of payment to prnt.sc and send the link and subject(s) name to 08106996452 as TEXT MESSAGE to get your pin and whatsapp group link. Pos, Transfer, Airtime are allowed. If you want to pay using recharge card, send it to the number as text message. Do not subscribe on Whatsapp, we reply faster via text message.
OBJ & NOTICE SECTION
Chemistry (chems) waec authentic questions and answer 2023 password/pin/code: 1531 ..
CHEMISTRY OBJ
1- 10: ACADABBDAC
11-20: BCACBBCCBA
21-30: ACBABDCCCB
31-40: CBBDAABDAD
41-50: ACCDABDDCD
Welcome to official 2024 Chemistry WAEC answer page. We provide 2024 Chemistry WAEC Questions and Answers on Essay, Theory, OBJ midnight before the exam, this is verified & correct WAEC Chems Expo. WAEC Chemistry Questions and Answers 2024. WAEC Chems Expo for Theory & Objective (OBJ) PDF: verified & correct expo Solved Solutions, Chemistry (Chems) WAEC Authentic Questions and Answer 2024. 2024 WAEC EXAM Chemistry Questions and Answers
CLICK HERE TO VIEW ANSWER No. 1 (V1) on Chemistry

CLICK HERE TO VIEW ANSWER No. 1 (V2) on Chemistry

CLICK HERE TO VIEW ANSWER No. 2 (V2) on Chemistry

CLICK HERE TO VIEW ANSWER No. 2 (V1) on Chemistry

CLICK HERE TO VIEW ANSWER No. 3 on Chemistry

CLICK HERE TO VIEW ANSWER No. 5 on Chemistry

GENERAL & QUESTIONS SECTION

Welcome to official 2024 Chemistry WAEC answer page. We provide 2024 Chemistry WAEC Questions and Answers on Essay, Theory, OBJ midnight before the exam, this is verified & correct WAEC Chems Expo
Name: examplaza.com
Founded: 2010 (14 years)
Founder: Mr. Onuwa
Headquarters: Borno, Nigeria
Official Website: https://examplaza.com/
Official Contact: +2348108515604
READ THIS. CLICK HERE NOW

RELATED ANSWERS
ITS MYSCHOOL LIBRARY
Your classroom in a blink, 2024 waec: chemistry essay and objectives questions with solution.

🔒 Unlock this
JOIN 2024 NECO MIDNIGHT SOLUTIONS
Share this:
Leave a reply cancel reply, discover more from its myschool library.
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Waec Chemistry 2024 Answers, waec Chemistry answers 2024, Waec Chemistry 2024 question, Waec 2024 Chemistry essay and obj Answers, Chemistry waec 2024, Here is the only legitimate website where can get the 100% Verified Waec 2024 Chemistry Answers. and also have the chance to score A's , B's and C's in this ongoing Waec 2024 Chemistry Examination. We Assure you of getting the waec Chemistry questions and answers 2024 on time, only those that subscribed. WE DONT SCAM, ONLY A TRIAL WILL CONVINCE YOU
NOTE THAT SUBSCRIBERS GET ANSWERS FIRST BEFORE BEING POSTED HERE, ALWAYS SUBSCRIBE TO GET ANSWERS EARLY.
ALWAYS SUBSCRIBE TO AVOID DISAPPIONTEMENT
Wednesday, 22nd May 2024 Chemistry 2 (Essay) 9:30am – 11:30am Chemistry 1 (Objective) 11:30am – 12:30pm
CHEMISTRY OBJ:
1-10: BCBDBACCCB
11-20: CAACDCBCAB
21-30: BBCCBDDDCD
31-40: BCADDAACAC
41-50: BCBCDBCADA
NOTE: YOU’RE TO ANSWER FOUR(4) QUESTIONS IN ALL.
NUMBER ONE(1) IN SECTION A AND ANY OTHER THREE(3) FROM SECTION B.
ALSO, NUMBER TWO(2) HAS TWO VERSIONS FOR VARIETIES SAKE... CHOOSE ANY

(I)Deliquescence is the process by which a substance absorbs moisture from the atmosphere until it dissolves in the absorbed water and forms a solution. This typically occurs with hygroscopic substances that have a high affinity for water.
(II)Efflorescence is the process by which a substance loses water to the atmosphere, resulting in the formation of a powdery surface deposit. This occurs when the vapor pressure of the water in the hydrated salt is greater than the partial pressure of water vapor in the air.
(I)Deliquescence: Calcium chloride (CaCl 2 )
(II)Efflorescence: Sodium carbonate decahydrate (Na 2 CO 2 ·10H 2 O)
Ionization energy is the amount of energy required to remove an electron from an isolated gaseous atom or ion in its ground state. The first ionization energy refers to the energy needed to remove the first electron, while subsequent ionization energies refer to the removal of additional electrons.
The second ionization energy of sodium is greater than the first because, after the first electron is removed, the remaining electrons are closer to the nucleus and experience a stronger attractive force. Removing a second electron requires more energy as it disrupts the stable, noble gas configuration left behind.
Charles’ Law states that the volume of a given mass of gas is directly proportional to its absolute temperature, provided the pressure remains constant. Mathematically, it is expressed as:
Answers Loading...


- Username Password Remember me Sign in New here ? Join Us

Chemistry 2021 WAEC Past Questions
The hydrolysis of proteins by diluting mineral acids produces
- C. amino acids
- D. fatty acids
Which of the following oxide causes acid rain?
- C. H\(_{2}\)O\(_{2}\)
- D. NO\(_{2}\)
The ratio of carbon atoms of hydrogen atoms in a hydrocarbon is 1:2. If its molecular mass is 56, what is its molecular formula?
- A. C\(_{3}\)H\(_{6}\)
- B. C\(_{4}\)H\(_{8}\)
- C. C\(_{2}\)H\(_{4}\)
- D. CH\(_{2}\)
What is the relative molecular mass of the compound below?
[H = 1.0; C = 12.0; O = 16.0]
Cathodic protection of metals is based on
- A. standard electrode potential of hydrogen
- B. its electrical conductivity
- C. nature of oxides formed
- D. relative tendencies of oxidation
- Mathematics
- English Language
- Animal Husbandry
- Literature in English
- Accounts - Principles of Accounts
- Christian Religious Knowledge (CRK)
- Agricultural Science
- Islamic Religious Knowledge (IRK)
- Civic Education
- Further Mathematics
- Home Economics
- Book Keeping
- Data Processing
- Catering Craft Practice
- Computer Studies
- Physical Education
- Office Practice
- Technical Drawing
- Food and Nutrition
- Home Management
Journal of Materials Chemistry A
Band and microstructure engineering toward high thermoelectric performance in snte.
SnTe based compounds have long been considered to be competitive in thermoelectric power generation. However, its intrinsically high hole concentration due to Sn vacancies and inferior band structure featured by large energy offset in two valence bands at band edges largely limit the electrical performance. Meanwhile, the relatively high lattice thermal conductivity of SnTe compared to other IV-VI based thermoelectrics makes the overall thermoelectric performance promotion challenging. In this study, we prove the spontaneous optimization in both carrier concentration and band structure can be realized in SnTe by a small amount of AgBiS 2 alloying, giving rise to a high power factor of 2.25 mW m –1 K –2 . It is further elaborated that AgBiS 2 is a proper choice for band structure engineering by comparing to others dopants and alloying substances that facilitate band convergence through first-principles calculations. Moreover, the substitution of Ge on Sn sites generates plenty of nano-precipitates and grain boundaries in SnTe matrix, which leads to the reduction of lattice thermal conductivity to amorphous limit of 0.3 W/m K, finally resulting in a peak zT of 1.6 at 903 K and exceeding most of the values reported in SnTe-based compounds.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers
Supplementary files
- Supplementary information PDF (734K)
Transparent peer review
To support increased transparency, we offer authors the option to publish the peer review history alongside their article.
View this article’s peer review history
Article information
Download citation, permissions.
J. Xu, Z. Zhou, K. Zhang, T. Zhao, Y. Wei, B. Zhang, H. Wang, X. Lu and X. Zhou, J. Mater. Chem. A , 2024, Accepted Manuscript , DOI: 10.1039/D4TA03729D
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

IMAGES
VIDEO
COMMENTS
SUBJECT: WAEC 2024 CHEMISTRY (ESSAY & OBJ) ANSWERS - WAECAFRICA.COM AND THE PIN IS "019"
Chemistry. The resources below on Chemistry have been provided by WAEC to assist you understand the required standards expected in Chemistry final Examination. Students performance in examination under review was done by the Chief examiner, this you will see while exploring links like General Comment, Performance, Weaknesses, Strength and ...
Equilibrium is said to be attained in reversible reaction when. A. all the reactants have been used up. B. all the products have been formed. C. there is no further change in temperature. D. the rates of the forward and backward reactions are equal. E. the rate of formation of the products decreases with time. View Answer & Discuss (7) WAEC 1988.
You can practise for your Chemistry WAEC Exam by answering real questions from past papers. This will give you a better chance of passing. WAEC Past Questions for Chemistry. Click on the year you want to start your revision. Chemistry Paper 2 (Objective Test and Essay) - November 2000; Chemistry Paper 2 (Objective Test and Essay) - November ...
View Answer & Discuss WAEC 2022. 3. (a)i. Define an acid according to the Lewis concept. ii. Give one example of a Lewis acid. (b) Explain salting out in soap preparation. (c) State the reagent and condition necessary for the following conversion. H−C≡C−H→Ag−C≡C−Ag.
February 10 2021. We've made available some WAEC chemistry past questions and answers to help the students that will take chemistry during their West African Examinations Council. The free copies of the chemistry past questions can be downloaded from the link provided here. We hope to.
Speed: Regular practice of our WASSCE Elective Chemistry past questions makes you faster on the exam day. It's no secret that questions on the WASSCE for each particular subject are usually similar to questions in previous years since they're from the same WAEC syllabus. WAEC also sometimes repeats questions word-for-word.
It was reported that the performance of the candidates was below average and was worse than WASSCE for School Candidates, 2017 with a raw mean score of 47.0 and standard deviation of 16.0 compared to a raw mean score of 29.0 and standard deviation of 13.78 for WASSCE for School Candidates, 2018. The total number of candidates that sat the ...
The 2023 answers will be posted here on 24th May during the exam. WAEC Chemistry OBJ Answers Loading…. 1-10: ACADABBDAC. 11-20: BCAABBCCBB. 21-30: ACBABDACBB. 31-40: ABBDAADCDD. 41-50: BDCDDCDDCD. (1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
Chemistry WAEC Essay Questions. SECTION B. 2ai. State the collision theory of reaction rates. 2aii.Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction. bi. Sketch a graphical representation of Charles's law. bii. Calculate the volume of oxygen that would be required for the complete ...
After using the WAEC Syllabus and Recommended Textbook to Read and Meditate. Click on the link below to access the WAEC Chemistry past questions. Once you land on the new page you will see (General Comment) click on Next to see the Weakness/Remedies and Strength. To access the WAEC Maths Question and Answer click on the number in the Question ...
2024 WAEC CHEMISTRY (CHEM) THEORY (ESSAY) ANSWERS: (2a) (I)Deliquescence is the process by which a substance absorbs moisture from the atmosphere until it dissolves in the absorbed water and forms a solution. This typically occurs with hygroscopic substances that have a high affinity for water. (II)Efflorescence is the process by which a ...
This is a video of our top 10 commonly repeated topics in WAEC Chemistry to aid students who are preparing to write the 2023 WASSCE Chemistry. It is a summar...
15 Most Common WAEC Topics in Chemistry Examination (Theory and Obj) See the list of topics below: 1) Atoms Moles Formulae and Equation and their properties (isotopes of an atom, atomic structures and related atomic questions, 2) Periodic Table (Properties and the likes) 3) Organic compounds like methane, propane butane etc (their properties)
Chemistry 2023 WAEC Past Questions. A. energy is released when liquids change to solids. B. carbon atoms in gaseous methane are further apart than those in solid diamond. C. there is large decrease in the volume of a solid metal when pressure is applied to it. D. particles move faster in the gaseous state than in the liquid state.
Call +2348033006849 Right Away! Spread the Word: If you found this post useful, help others discover it too! Just click and share using the buttons below! Free WAEC past questions and answers are available here for download! Are you in your last stage of Secondary School Education (May/June) or not in the School s.
Welcome to official 2024 Chemistry WAEC answer page. We provide 2024 Chemistry WAEC Questions and Answers on Essay, Theory, OBJ midnight before the exam, this is verified & correct WAEC Chems Expo. WAEC Chemistry Questions and Answers 2024.
Published by Myschoollibrary. I am an educator who believes teaching and learning should go beyond the classroom hence the need to host my esteemed scholars in my global citadel of learning. Thanks for stopping by. I do hope my contents meet your search. You can connect with me via my contact page. View all posts by Myschoollibrary.
Waec Chemistry 2024 Answers, waec Chemistry answers 2024, Waec Chemistry 2024 question, Waec 2024 Chemistry essay and obj Answers, Chemistry waec 2024, Here is the only legitimate website where can get the 100% Verified Waec 2024 Chemistry Answers. and also have the chance to score A's , B's and C's in this ongoing Waec 2024 Chemistry Examination.
Due to the urgent environmental concerns and the energy crisis, the pursuit of sustainable clean energy as an alternative to conventional fossil fuels has become a focal point for industry scholars. Electrochemical water splitting is an environment friendly and efficient method for high-purity hydrogen generation. Journal of Materials Chemistry A HOT Papers Journal of Materials Chemistry A ...
Electrochemical CO2 reduction reaction (CO2RR) driven by clean electricity to valuable chemicals provides a feasible way to carbon neutrality and thus attracts increasing attention. Among different transition-metal based CO2RR catalysts, Pd is the unique one because of the potential-dependent 2e− reduction p Journal of Materials Chemistry A HOT Papers Journal of Materials Chemistry A Recent ...
The atomic radii of metals are usually. A. greater than their ionic radii. B. equal to their ionic radii. C. less than their ionic radii. D. less than those of non-metals in the same period. View Answer & Discuss (2) WAEC 2012. 5.
Metal-organic compounds derived composites are promising multi-functional materials due to their alterable composition, tunable shape, and porous structure. However, it is very attractive and challenging to design light and thin composites derived from metal-organic compounds with broad and strong electromag Journal of Materials Chemistry C HOT Papers
C. H 2 2 O 2 2. D. NO 2 2. View Answer & Discuss (3) WAEC 2021. 3. The ratio of carbon atoms of hydrogen atoms in a hydrocarbon is 1:2. If its molecular mass is 56, what is its molecular formula? A. C 3 3 H 6 6.
SnTe based compounds have long been considered to be competitive in thermoelectric power generation. However, its intrinsically high hole concentration due to Sn vacancies and inferior band structure featured by large energy offset in two valence bands at band edges largely limit the electrical performance. Journal of Materials Chemistry A HOT Papers