- Advanced search

Deposit your research
- Open Access
- About UCL Discovery
- UCL Discovery Plus
- REF and open access
- UCL e-theses guidelines
- Notices and policies
UCL Discovery download statistics are currently being regenerated.
We estimate that this process will complete on or before Mon 06-Jul-2020. Until then, reported statistics will be incomplete.
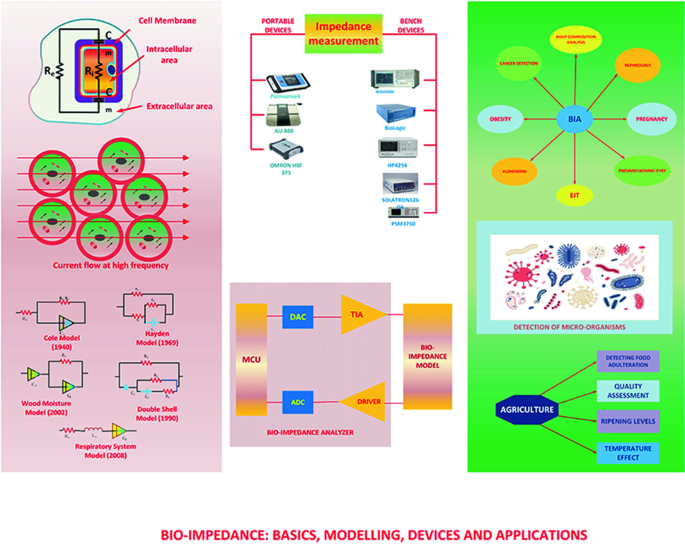
Study of bioimpedance measurement systems and development of bioimpedance amplifiers
]. Doctoral thesis , UCL (University College London).
This thesis is prepared for the examination of the Master of Philosophy degree from University College London. It provides fundamental knowledge in the field of bio-impedance, including basic concepts, measurement techniques and derived various applications for improving health care options for the wide community of human being. The thesis is organized into six chapters. Chapter One starts the thesis by introducing the concept of bio-impedance. Some representative designs of measurement cells and probes are briefly presented and classified into two categories for in vivo and in vitro application; so as to provide the parameter reference for designing an integrated version of bio-impedance measurement system (BMS). In addition, a brief mathematic foundation is included to explain the signal path of bio-impedance measurement system. A few applications of bio- impedance techniques, including the Electrical Impedance Tomography (EIT) are discussed. This chapter concludes with the limitation of BMS implemented using discrete components and proposes an integrated version of BMS to be applicable for increasingly challenging needs of health care service. This chapter also defines the system specification of BMS, such as operating frequencies, input dynamic range, and safety voltage/current requirement. Chapter Two investigates the origins of bio-impedance by studying the biological structure of a cell and its passive electrical parameter, such as at macro level impedance, and at the micro level, permitivity and conductivity. Chapter Three reviews various macro level electrical models of biological tissue, such as finger, leg, nerve etc, proposed by previous researchers. The disagreement of various electrical modelling presents a big challenge for fair comparison of various BMS performance. Chapter four reviews four categories of fundamental techniques underlying various BMS structures. The current/voltage technique is preferred by most current designs. Chapter five focuses the design of a monolithic instrumentation amplifier (in-amps) for BMS. Formulas of various critical system parameters, such as Gain/Bandwidth, Noise, Input/output impedance are derived. Schematics of amplifier are implemented in Cadence Virtuoso custom design platform with AMS 0.35μm technology. Simulation results, which proves the design meets specification are summarised in the end. Chapter Six ends the entire research thesis by summarising achievements of this work. In addition, some possible future work for the project are suggested and discussed.
| Type: | Thesis (Doctoral) |
|---|---|
| Title: | Study of bioimpedance measurement systems and development of bioimpedance amplifiers |
| Language: | English |
| Additional information: | Permission for digitisation not received |
| UCL classification: | > > > > |
| URI: |

Archive Staff Only
| View Item |
- Freedom of Information
- Accessibility
- Advanced Search
Advertisement
A review of bio-impedance devices
- Review Article
- Published: 13 January 2023
- Volume 61 , pages 927–950, ( 2023 )
Cite this article

- Insha Showkat 1 ,
- Farooq A. Khanday ORCID: orcid.org/0000-0002-2514-5703 1 &
- M. Rafiq Beigh 2
1895 Accesses
8 Citations
Explore all metrics
Bio-impedance measurement analysis primarily refers to a safe and a non-invasive technique to analyze the electrical changes in living tissues on the application of low-value alternating current. It finds applications both in the biomedical and the agricultural fields. This paper concisely reviews the origin and measurement approaches for concepts and fundamentals of bio-impedance followed by a critical review on bio-impedance portable devices with main emphasis on the embedded system approach which is in demand due to its miniature size and present lifestyle preference of monitoring health in real time. The paper also provides a comprehensive review of various bio-impedance circuits with emphasis on the measurement and calibration techniques.
Graphical Abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Wearable Bioimpedance Measuring Devices
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring

RF energy harvesters for wireless sensors, state of the art, future prospects and challenges: a review
Grimnes S, Martinsen O (2014) Bioimpedance and bioelectricity basics, 3rd edn. Academic Press, London, UK
Google Scholar
Borelli E, Paolini G, Antoniazzi F, Barbiroli M, Benassi F, Chesani F, Costanzo A et al (2019) Habitat: an IoT solution for independent elderly. Sensors 19(5):1258
Punj R, Kumar R (2019) Technological aspects of WBANs for health monitoring: a comprehensive review. Wireless Netw 25(3):1125–1157
Article Google Scholar
Rapin M, Braun F, Adler A et al (2019) Wearable sensors for frequency-multiplexed EIT and multilead ECG data acquisition. IEEE Trans Biomed Eng 66(3):810–820
Article PubMed Google Scholar
Piuzzi E, Pisa S, Pittella E, Podesta L, Sangiovanni S (2019) Low-cost and portable impedance plethysmography system for the simultaneous detection of respiratory and heart activities. IEEE Sens J 19(7):2735–2746
Summers RL, Shoemaker WC, Peacock WF, Ander DS, Coleman TG (2003) Bench to bedside: electrophysiologic and clinical principles of noninvasive hemodynamic monitoring using impedance cardiography. Acad Emerg Med 10(6):669–680
Majumder S, Mondal T, Deen MJ (2019) A simple, low-cost and efficient gait analyzer for wearable healthcare applications. IEEE Sens J 19(6):2320–2329
Thomasset MA (1962) Proprietes bioelectrique des tissuş, Mesures de l’impedance en clinique" [Bioelectric properties of tissue. Impedance measurement in clinical medicine. Significance of curves obtained]. Lyon Med 94:107–18
CAS PubMed Google Scholar
Kyle UG, Bosaeus I, De Lorenzo AD et al (2004) Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 23(5):1226–1243
Cole KS, Cole RH (1941) Dispersion and absorption in dielectrics I. Alternating current characteristics. J Chem Phys 9(4):341–351
Article CAS Google Scholar
Cole KS (1940) Permeability and impermeability of cell membranes for ions. Proc Cold Spring Harbor Symp Quant Biol 8:110–122
De Lorenzo A, Candeloro N, Andreoli A, Deurenberg P (1995) Determination of intracellular water by multifrequency bioelectrical impedance. Ann Nutr Metab 39(3):164–176
Freeborn TJ, Maundy B, Elwakil AS (2014) Extracting the parameters of the double-dispersion Cole bioimpedance model from magnitude response measurements. Med Biol Eng Compu 52(9):749–758
Hayden RI, Moyse CA, Calder FW, Crawford DP, Fensom DS (1969) Electrical impedance studies on potato and alfalfa tissue. J Experim Botany 20(2):177–200
Zhang MIN, Stout DG, Willison JHM (1990) Electrical impedance analysis in plant Tissues3. J. Experim Bot 41(3):371–380
Zhang MIN, Willison JHM (1991) Electrical impedance analysis in plant Tissues11. J Experim Bot 42(11):1465–1475
Zhang MIN, Willison JHM (1992) Electrical impedance analysis in plant tissues: in vivo detection of freezing injury. Can J Bot 70(11):2254–2258
Ionescu CM, De Keyser R (2008) Time domain validation of a fractional order model for human respiratory system. In MELECON 2008-The 14th IEEE Mediterranean Electrotechnical Conference. IEEE, pp 89–95
Tiitta M, Olkkonen H (2002) Electrical impedance spectroscopy device for measurement of moisture gradients in wood. Rev Sci Instrum 73(8):3093–3100
Repo T, Laukkanen J, Silvennoinen R (2005) Measurement of the tree root growth using electrical impedance spectroscopy. Silva Fennica 39(2):159–166
Magin RL, Ovadia M (2008) Modeling the cardiac tissue electrode interface using fractional calculus. J Vib Control 14(9–10):1431–1442
Huang WH, Chui CK, Teoh SH, Chang SK (2012) A multiscale model for bioimpedance dispersion of liver tissue. IEEE Trans Biomed Eng 59(6):1593–1597
Article CAS PubMed Google Scholar
Lizhi H, Toyoda K, Ihara I (2008) Dielectric properties of edible oils and fatty acids as a function of frequency, temperature, moisture and composition. J Food Eng 88:151–158
Yang J, Zhao KS, He YJ (2016) Quality evaluation of frying oil deterioration by dielectric spectroscopy. J Food Eng 180:69–76
Ragni L, Iaccheri E, Cevoli C, Berardinelli A, Bendini A, GallinaToschi T (2013) A capacitive technique to assess water content in extra virgin olive oils. J Food Eng 116:246–252
Yang Y, Li Q, Yu X, Chen X, Wang Y (2014) A novel method for determining peroxide value of edible oils using electrical conductivity. Food Control 39:198–203
Roa LM, Naranjo D, Reina-Tosina J et al (2013) Applications of bioimpedance to end stage renal disease (ESRD). Stud Comput Intell 404:689–769
Aberg P, Nicander I, Hansson J, Geladi P, Holmgren U, Ollmar S (2004) Skin cancer identification using multifrequency electrical impedance-a potential screening tool. IEEE Trans Biomed Eng 51:2097–2102
Chowdhury D, Chattopadhyay M (2021) Study and classification of cell bio-impedance signature for identification of malignancy using artificial neural network. IEEE Trans Instrum Meas 70:1–8
Penza V, Cheng Z, Koskinopoulou M, Acemoglu A, Caldwell DG, Mattos LS (2021) Vision-guided autonomous robotic electrical bio-impedance scanning system for abnormal tissue detection. IEEE Trans Med Robot Bionics 3(4):866–877
Baghbani R, Shadmehr MB, Ashoorirad M, Molaeezadeh SF, Moradi MH (2021) Bioimpedance spectroscopy measurement and classification of lung tissue to identify pulmonary nodules. IEEE Trans Instrum Meas 70:1–7
Cheng Zhuoqi et al (2022) Active search of subsurface lymph nodes using robot-assisted electrical impedance scanning. IEEE Trans Instrum Meas 71:1–11
Harker FR, Maindonald JH (1994) Ripening of nectarine fruit. Plant Physiol 106:165–171
Article CAS PubMed PubMed Central Google Scholar
AboBakr A, Mohsen M, Said LA, Madian AH, Elwakil AS, Radwan AG (2019) Banana ripening and corresponding variations in bioimpedance and glucose levels, vol 1. In 2019 Novel Intelligent and Leading Emerging Sciences Conference (NILES). IEEE, pp 130–133
Aboalnaga BM, Said LA, Madian AH, Elwakil AS, Radwan AG (2019) Cole bio-impedance model variations in daucus carota sativus under heating and freezing conditions. IEEE Access 7:113254–113263
Mbezi MT, Fouda H, Tabi CB (2015) Estimated photosynthetic activity from its electrical impedance spectroscopy. Amer Sci Res J Eng Technol Sci 13(1):178–193
Harker FR, Forbes SK (1997) Ripening and development of chilling injury in persimmon fruit: an electrical impedance study. New Zeal J Crop Hort 25:149–157
Bauchot AD, Harker FR, Arnold WM (2000) The use of electrical impedance spectroscopy to assess the physiological condition of kiwifruit. Postharvest Biol Tec 18:9–18
Jackson PJ, Harker FR (2000) Apple bruise detection by electrical impedance measurement. HortSci 35:104–107
Rehman M, Abu Izneid AJA, Abdullah MZ, Arshad MR (2011) Assessment of quality of fruits using impedance spectroscopy. Int J Food Sci Tech 46:1303–1309
Juansah J, Budiastra IW, Dahlan K, Seminar KB (2012) Electrical behavior of garut citrus fruit during ripening changes in resistance and capacitance models of internal fruits. Int J Eng Tech 12:1–8
Chowdhury A, KantiBera T, Ghoshal D, Chakraborty B (2017) Electrical impedance variations in banana ripening: an analytical study with electrical impedance spectroscopy. J Food Process Eng 40(2):12387
Chowdhury A, Singh P, Bera TK, Ghoshal D, Chakraborty B (2017) Electrical impedance spectroscopic study of mandarin orange during ripening. J Food Meas Charact 11(4):1654–1664
Kriˇzaj D (2018) Basics of numerical simulations of bioimpedance phenomena. in Bioimpedance in Biomedical Applications and Research. Springer, Cham, pp 101–116
Xu K, Lu Y, Takei K (2019) Multifunctional skin-inspired flexible sensor systems for wearable electronics. Adv Mater Technol 4(3):1800628
Lukaski H (1996) Biological indexes considered in the derivation of the bioelectrical impedance analysis. Am J Clin Nutr 64(3):397S–404S
Hanai T (1968) Electrical properties of emulsions. Academic Press, London, UK, Sherman PH Emulsion Science, pp 354–477
Matthie JR (2005) Second generation mixture theory equation for estimating intracellular water using bioimpedance spectroscopy. J Appl Physiol 99(2):780–781
Jaffrin MY, Morel H (2008) Body fluid volume measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Engin Physics 30(10):1257–1269
Barnett A, Bagno S (1936) The physiological mechanisms involved in the clinical measure of phase angle. Am J Physiol 114(2):366–382
Buchholz AC, Bartok C, Schoeller DA (2004) The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract 19(5):433–446
Gabriel C, Gabriel S, Corthout E (1996) The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol 41(11):2231–2249
Gabriel S, Lau RW, Gabriel C (1996) The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol 41(11):2271–2293
Van Loan MD, Withers P, Matthie J, Mayclin PL (1993) Use of bioimpedance spectroscopy to determine extracellular fluid, intracellular fluid, total body water, and fat-free mass. Human body composition, Springer, US, pp 67–70
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Scharfetter H (2004) Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 23(5):1226–1243
Zhang M, Willison J (1992) Electrical impedance analysis in plant tissues: the effect of freeze-thaw injury on the electrical properties of potato tuber and carrot root tissues. Can J Plant Sci 72(2):545–553
Bhardwaj P, Rai DV, Garg ML, Mohanty BP (2018) Potential of electrical impedance spectroscopy to differentiate between healthy and osteopenic bone. Clin Biomech 57:81–88
De Lorenzo A, Andreoli A, Matthie J, Withers P (1997) Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol 82(5):1542–1558
Kassanos P, Constantinou L, Triantis IF, Demosthenous A (2014) An integrated analog readout for multi-frequency bioimpedance measurements. IEEE Sens J 14(8):2792–2800
Chester CJ, Gaynor PT, Jones RD, Huckabee M (2014) Electrical bioimpedance measurement as a tool for dysphagia visualisation. Healthc Technol Lett 1(3):115–118
Article PubMed PubMed Central Google Scholar
Y´ufera A, Rueda A (2010) Design of a CMOS closed-loop system with applications to bio-impedance measurements. Microelectron J 41(4):231–239
Kassanos P, Triantis IF, Demosthenous A (2013) A CMOS magnitude/phase measurement chip for impedance spectroscopy. IEEE Sens J 13(6):2229–2236
Kweon S-J, Park J-H, Shin S, Yoo S-S, Yoo H-J (2017) A reconfigurable time-to-digital converter based on time stretcher and chain-delay-line for electrical bioimpedance spectroscopy. Proc 60th IEEE Int Midwest Symp Circ Syst MWSCAS 2017 2017:1037–1040
Hersek S, Toreyin H, Inan OT (2016) A robust system for longitudinal knee joint edema and blood flow assessment based on vector bio-impedance measurements. IEEE Trans Biomed Circuits Syst 10(3):545–555
Hersek S, Toreyin H, Teague CN et al (2017) Wearable vector electrical bio-impedance system to assess knee joint health. IEEE Trans Biomed Eng 64(10):2353–2360
Sanchez B, Vandersteen G, Bragos R, Schoukens J (2012) Basics of broadband impedance spectroscopy measurements using periodic excitations. Meas Sci Technol 23(10):105501
Min M, Parve T, Ronk A, Annus P, Paavle T (2007) Synchronous sampling and demodulation in an instrument for multifrequency bioimpedance measurement. IEEE Trans Instrum Meas 56(4):1365–1372
Huertas G, Maldonado A, Yufera A, Rueda A, Huertas JL (2015) The bio-oscillator: a circuit for cell-culture assays. IEEE Trans Circuits Syst II Express Briefs 62(2):164–168
Parente FR, Di Giovanni S, Ferri G, Stornelli V, Pennazza G, Santonico M (2018) An analog bootstrapped bio-signal read-out circuit with common-mode impedance two-electrode compensation. IEEE Sens J 18(7):2861–2869
Liu Y, Qiao X, Li G, Lin L (2016) An improved device for bio-impedance deviation measurements based on 4-electrode half bridge. Rev Sci Instrum 87(10):105107
Li N, Xu H, Wang W, Zhou Z, Qiao G, D-U Li D (2013) A high-speed bioelectrical impedance spectroscopy system based on the digital auto-balancing bridge method. Meas Sci Technol 24(6):065701.
Bag JC, Wi H, Oh TI, McEwan AL, Woo EJ (2013) An amplitude-to-time conversion technique suitable for multichannel data acquisition and bio-impedance imaging. IEEE Trans Biomed Circuits Syst 7(3):349–354
Sanchez B, Vandersteen G, Bragos R, Schoukens J (2011) Optimal multisine excitation design for broadband electrical impedance spectroscopy. Meas Sci Technol 22(11):115601
Sanchez B, Schoukens J, Bragos R, Vandersteen G (2011) Novel estimation of the electrical bioimpedance using the local polynomial method application to in vivo real-time myocardium tissue impedance characterization during the cardiac cycle. IEEE Transactions on Biomedical Engineering 58(12):3376–3385
Shi X, You F, Ji Z, Fu F, Liu R, Dong X (2010) Digital demodulation in data acquisition system for multi-frequency electrical impedance tomography. In 2010 4th International Conference on Bioinformatics and Biomedical Engineering. IEEE, pp 1–3
Gracia J, Seppa VP, Viik J, Hyttinen J (2012) Multilead measurement system for the time-domain analysis of bio-impedance magnitude. IEEE Trans Biomed Eng 59(8):2273–2280
Morgan H, Sun T, Holmes D, Gawad S, Green NG (2007) Single cell dielectric spectroscopy. J Phys D Appl Phys 40:61–70
Zhang X, Hatamie A, Ewing AG (2020) Nanoelectrochemical analysis inside a single living cell. Curr Opin Electrochem 22:94–101
Gawad S, Schild L, Renaud PH (2001) Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 1:76–82
Cheung KC, Di Berardino M, Schade-Kampmann G, Hebeisen M, Pierzchalski A, Bocsi J, Mittag A, Tarnok A (2010) Microfluidic impedance-based flow cytometry. Cytometry part A 77(7):648–666
Xu Y, Xie X, Duan Y, Wang L, Cheng Z, Cheng J (2016) A review of impedance measurements of whole cells. Biosens Bioelectron 77:824–836
Chen J, Zheng Y, Tan Q, Shojaei-Baghini E, Zhang YL, Li J, Prasad P, You L, Wu XY, Sun Y (2011) Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells. Lab Chip 11:3174–3181
Sriphutkiat Y, Zhou Y (2017) Particle accumulation in a microchannel and its reduction by a standing surface acoustic wave (SSAW). Sensors 17:106
Malleo D, Nevill JT, Lee LP, Morgan H (2010) Continuous differential impedance spectroscopy of single cells. Microfluid Nanofluid 9:191–198
Younghak C, Hyun Soo K, Frazier AB, Chen ZG, Dong Moon S, Han A (2009) Whole-cell impedance analysis for highly and poorly metastatic cancer cells. J Microelectromech Syst 18:808–817
Lan KC, Jang LS (2011) Integration of single-cell trapping and impedance measurement utilizing microwell electrodes. Biosens Bioelectron 26:2025–2031
Xu B, Shi Y, Lao Z, Ni J, Li G, Hu Y, Li J, Chu J, Wu D, Sugioka K (2018) Real-time two-photon lithography in controlled flow to create a single-microparticle array and particle-cluster array for optofluidic imaging. Lab Chip 18:442–450
Guo X, Zhu R (2016) Controllable in-situ cell electroporation with cell positioning and impedance monitoring using micro electrode array. Sci Rep 6:31392
Asphahani F, Zhang M (2007) Cellular impedance biosensors for drug screening and toxin detection. Analyst 132:835–841
Kovacs GTA (2003) Electronic sensors with living cellular components. Proc IEEE 91:915–929
Geng Y, Zhu Z, Zhang Z, Xu F, Marchisio MA, Wang Z, Pan D, Zhao X, Huang QA (2021) Design and 3D modelling investigation of a microfluidic electrode array for electrical impedance measurement of single yeast cells. Electrophor 42:1996–2009
Nguyen TA, Yin T-I, Reyes D, Urban GA (2013) Microfluidic chip with integrated electrical cell-Impedance sensing for monitoring single cancer cell migration in three-dimensional matrixes. Anal Chem 85:11068–11076
Tsai SL, Wang MH (2016) 24 h observation of a single HeLa cell by impedance measurement and numerical modeling. Sens Actuators B Chem 229:225–231
AboBakr A, Mohsen M, Said LA, Madian AH, Elwakil AS, Radwan AG (2019) Toward portable bio-impedance devices. In 2019 Fourth International Conference on Advances in Computational Tools for Engineering Applications (ACTEA). IEEE, pp 1–4
Chabowski K, Piasecki T, Dzierka A, Nitsch K (2015) Simple wide frequency range impedance meter based on ad5933 integrated circuit. Metrol Meas Syst 22(1):13–24
Ibba P et al (2021) Design and validation of a portable AD5933–based impedance analyzer for smart agriculture. IEEE Access 9:63656–63675. https://doi.org/10.1109/ACCESS.2021.3074269
Breniuc L, David V, Haba C-G (2014) Wearable impedance analyzer based on AD5933. In 2014 International Conference and Exposition on Electrical and Power Engineering (EPE). IEEE, pp 585–590
Harder R et al (2016) Smart multi-frequency bioelectrical impedance spectrometer for BIA and BIVA applications. IEEE Trans Biomed Circ Syst 10(4):912–919
Teague NC et al (2020) A wearable, multimodal sensing system to monitor knee joint health. IEEE Sensors J 20(18):10323–10334
Qiu C et al (2022) A wearable bioimpedance chest patch for real-time ambulatory respiratory monitoring. IEEE Trans Biomed Eng 69(9):2970–2981. https://doi.org/10.1109/TBME.2022.3158544
Istanbullu M, Avci M (2020) An ANN-based single calibration impedance measurement system for skin impedance range. IEEE Sens J 21(3):3776–3783
Ibrahim B, Jafari R (2022) Cuffless blood pressure monitoring from a wristband with calibration-free algorithms for sensing location based on bio-impedance sensor array and autoencoder. Sci Rep 12(1):1–14
Dutt AG, Verling M, Karlen W (2020) Wearable bioimpedance for continuous and context-aware clinical monitoring. In 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE, pp 3985–3988
Van Steenkiste T et al (2020) Portable detection of apnea and hypopnea events using bio-impedance of the chest and deep learning. IEEE J Biomed Health Informa 24(9):2589–2598
Songkakul T et al (2021) Wearable bioimpedance hydration monitoring system using conformable AgNW electrodes. 2021 IEEE Sensors, IEEE.
Critcher S, Freeborn T (2022) System performance and user feedback regarding wearable bioimpedance system for multi-site knee tissue monitoring: free-living pilot study with healthy adults. Frontiers in Electronics. https://doi.org/10.3389/felec.2022.824981
Freeborn TJ, Maundy BJ, Elwakil AS (2013) Cole impedance extractions from the step-response of a current excited fruit sample. Comput Electron Agric 98:100–108
Al-Ali AA,. Maundy BJ, Elwakil A (2018) “Design and implementation of a bio-impedance analyzer based on the kramers-kronig transform. In 2018 IEEE International Symposium on Circuits and Systems (ISCAS). IEEE, pp 1–5
Al-Ali AA, Elwakil AS, Maundy BJ (2018) Bio-impedance measurements with phase extraction using the kramers-kronig transform: application to strawberry aging. In 2018 IEEE 61st International Midwest Symposium on Circuits and Systems (MWSCAS). IEEE, pp 468–471
Vastarouchas C, Psychalinos C, Elwakil AS, Al-Ali AA (2019) Novel two-measurements-only cole-cole bio-impedance parameters extraction technique. Meas 131:394–399
Mohsen M, Said LA, Madian AH, Elwakil AS, Radwan AG (2019) Using meta-heuristic optimization to extract bio-impedance parameters from an oscillator circuit. In 2019 17th IEEE International New Circuits and Systems Conference (NEWCAS). IEEE, pp 1–4
Mohsen M, Said LA, Elwakil AS, Madian AH, Radwan AG (2020) Extracting optimized bio-impedance model parameters using different topologies of oscillators. IEEE Sensors J 20(17):9947–9954
Meade ML (1983) Lock-in amplifiers: principles and applications. IEE Electrical Measurement Series, ark:/13960/t23c2hb4f
Wenn D (2007) Implementing digital lock-in amplifiers using the dsPIC®DSC. Appl Note AN1115, pp 1–12
Aguirre J, Medrano N, Calvo B, Celma S (2011) Lock-in amplifier for portable sensing systems. Electron Lett 47(21):1172
Li N, Wang W, Xu H, Yu H, Diao J, Li DDU (2013) Wide-bandwidth biological impedance spectroscopy system based on the digital lock-in technique. Spectrosc Lett 46(7):476–482
Caplan LC, Stern R (1971) Inexpensive lock-in amplifier. Rev Sci Instrum 42:689
Gervasoni G, Carminati M, Ferrari G (2017) Switched ratiometric lock in amplifier enabling sub-ppm measurements in a wide frequency range. Rev Sci Instrum 88(10):104704
Giaconia G, Greco G, Mistretta L, Rizzo R (2017) Exploring FPGA based lock-in techniques for brain monitoring applications. Electron 6(1):18
Divakar D, Mahesh K, Varma MM, Sen P (2018) FPGA-based lock-in amplifier for measuring the electrical properties of individual cells. In 2018 IEEE 13th Annual International Conference on Nano/Micro Engineered and Molecular Systems. IEEE, pp 1–5
De Marcellis A et al (2007) An integrated analog lock-in amplifier for low voltage low-frequency sensor interface. In 2007 2nd International Workshop on Advances in Sensors and Interface. IEEE, pp 1–5
Webster JG (ed) (1995) Design of Cardiac Pacemakers. IEEE Press, Piscataway, New Jersey
Maya-Hernández PM, Sanz-Pascual MT, Calvo B (2014) CMOS low-power lock-in amplifiers with signal rectification in current domain. IEEE Trans Instrum Meas 64(7):1858–1867
Cho YC, Kim MS, Yoon JO (2013) A study on the electrical difference for the limbs and thoracic impedance using real-time bio-impedance measurement system. J Korea Ind Inf Syst Res 18(6):9–16
Moe AE, Marx SR, Bhinderwala I, Wilson DM (2004) A miniaturized lock-in amplifier design suitable for impedance measurements in cells [biological cells]. In SENSORS, 2004 IEEE. IEEE, pp 215–218
Min M, Parve T (2007) Improvement of lock-in electrical bio-impedance analyzer for implantable medical devices. IEEE Trans Instrum Meas 56(3):968–974
Stewart GN (1899) The changes produced by the growth of bacteria in the molecular concentration and electrical conductivity of culture media. J Exp Med 4:235–243
Fistenberg-Eden R (1983) Rapid estimation of the number of microorganisms in raw meat by impedance measurement. Food Technol 37:64–70
Grossi M, Lanzoni M, Pompei A, Lazzarini R, Matteuzzi D, Riccò B (2008) Detection of microbial concentration in ice-cream using the impedance technique. Biosens Bioelectron 23:1616–1623
Grossi M, Lazzarini R, Lanzoni M, Riccò B (2011) A novel technique to control ice-cream freezing by electrical characteristics analysis. J Food Eng 106:347–354
Grossi M, Lanzoni M, Pompei A, Lazzarini R, Matteuzzi D, Riccò B (2011) A portable biosensor system for bacterial concentration measurements in cow’s raw milk. In 2011 4th IEEE International Workshop on Advances in Sensors and Interfaces (IWASI). IEEE, pp 132–137
Hardy D, Kraeger SJ, Dufour SW, Cady P (1977) Rapid Detection of Microbial Contamination in Frozen Vegetables by Automated Impedance Measurements. Appl Environ Microb 34:14–17
Settu K, Chen CJ, Liu JT, Chen CL, Tsai JZ (2015) Impedimetric method for measuring ultra-low E. coli concentrations in human urine. Biosens Bioelectron 66:s244-250
Kumar G, Kasiviswanathan U, Mukherjee S, Mahto SK, Sharma N, Patnaik R (2019) Changes in electrolyte concentrations alter the impedance during ischemia-reperfusion injury in rat brain. Physiol Meas 40(10):105004
Bora DJ, Dasgupta R (2020) Various skin impedance models based on physiological strati_cation. IET Syst Biol 14(3):147–159
Simi¢-Krsti¢ JB, Kalauzi AJ, Ribar SN, Matija LR, Misevic GN (2014) Electrical properties of human skin as aging biomarkers. Experim. Gerontol 57:163–167
Fu B, Freeborn T (2019) Electrical equivalent network modeling of forearm tissue bioimpedance. n 2019 SoutheastCon. IEEE, pp 1–7
Sanchez B, Li J, Geisbush T, Bardia RB, Rutkove SB (2016) Impedance alterations in healthy and diseased mice during electrically induced muscle contraction. IEEE Trans Biomed Eng 63(8):1602–1612
Khalil S, Mohktar M, Ibrahim F (2014) The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 14(6):10895–10928
Gupta PP, Fonarow GC, Horwich TB (2015) Obesity and the obesity paradox in heart failure. Can J Cardiol 31(2):195–202
Hocking S, Samocha-Bonet D, Milner K-L, Greenfield JR, Chisholm DJ (2013) Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev 34(4):463–500
Widen EM, Gallagher D (2014) Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr 68(6):643–652
Shuster A, Patlas M, Pinthus JH, Mourtzakis M (2012) The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol 85(1009):1–10
Toomey CM, Cremona A, Hughes K, Norton C, Jakeman P (2015) A review of body composition measurement in the assessment of health. Top Clin Nutr 30(1):16–32
Ackland TR, Lohman TG, Sundgot-Borgen J et al (2012) Current status of body composition assessment in sport: review and position statement on behalf of the Ad Hoc research working group on body composition health and performance, under the auspices of the I.O.C. medical commission. Sports Med 42(3):227–249
SherwoodChair A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Doornen LJ (1990) Methodological guidelines for impedance cardiography. Psychophysiol 27(1):1–23
Mły´nczak M, Niewiadomski W, ˙Zyli´nski M, Cybulski G (2015) Verification of the respiratory parameters derived from impedance pneumography during normal and deep breathing in three body postures. In 6th European Conference of the International Federation for Medical and Biological Engineering. Springer, Cham, pp 881–884
Li N, Xu H, Zhou Z, Xin J, Sun Z, Xu X (2013) Reconfigurable bioimpedance emulation system for electrical impedance tomography system validation. IEEE Trans Biomed Circuits Syst 7(4):460–468
Zhang J, Yang B, Li H et al (2017) Anovel 3D-printedheadphantom with anatomically realistic geometry and continuously varying skull resistivity distribution for electrical impedance tomography. Sci Rep 7(1):4608
Frerichs I, Amato M, van Kaam AH et al (2017) Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the translational EIT development study group. Thorax 72(1):83–93
Richard JC, Pouzot C, Gros A et al (2009) Electrical impedance tomography compared to positron emission tomography for the measurement of regional lung ventilation: an experimental study. Critical Care 13(3):1–9
Critchley H, Nagai Y (2013) Electrodermal activity (EDA). Encyclopedia of behavioral medicine 78:666–669
Surowiec A, Stanislaw SS, Barr JR, Swarup A (1988) Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans Biomed Eng 35:257–263
Morimoto T, Kinouchi Y, Iritani T et al (1990) Measurement of the electrical bioimpedance of breast tumors. Eur Surg Res 22:86–92
Chauveau N, Hamzaoui L, Rochaix P, Rigaud B, Voigt JJ, Morucci JP (1999) Ex vivo discrimination between normal and pathological tissues in human breast surgical biopsies using bioimpedance spectroscopy. Ann N Y Acad Sci 873:42–50
Ohmine Y, Morimoto T, Kinouchi Y et al (2004) Basic study of new diagnostic modality according to non-invasive measurement of the electrical conductivity of tissues. J Med Invest 51:218–225
Download references
Acknowledgements
The research work is supported by University Grants Commission, Government of India in the form of Maulana Azad National Fellowship (MANF) (210510145558).
Author information
Authors and affiliations.
Department of Electronics and Instrumentation Technology, University of Kashmir, Hazratbal, Srinagar, Jammu and Kashmir, India
Insha Showkat & Farooq A. Khanday
Department of Electronics, Govt. Degree College Sumbal, Sumbal, J&K, India
M. Rafiq Beigh
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Farooq A. Khanday .
Ethics declarations
Ethics approval.
We declare that the manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Showkat, I., Khanday, F.A. & Beigh, M.R. A review of bio-impedance devices. Med Biol Eng Comput 61 , 927–950 (2023). https://doi.org/10.1007/s11517-022-02763-1
Download citation
Received : 19 September 2022
Accepted : 27 December 2022
Published : 13 January 2023
Issue Date : May 2023
DOI : https://doi.org/10.1007/s11517-022-02763-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bio-impedance
- Health monitoring
- Impedance analyzer
- Find a journal
- Publish with us
- Track your research
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Sensors (Basel)

The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases
Sami f. khalil.
1 Department of Biomedical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia; E-Mails: ym.ude.mu.awsis@ihtafimas (S.F.K.); ym.ude.mu@anayad_sam (M.S.M.)
2 Centre for Innovation in Medical Engineering (CIME), Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia
3 Department of Biomedical Engineering, College of Engineering, Sudan University of Science and Technology, 407, Khartoum, Sudan
Mas S. Mohktar
Fatimah ibrahim.
Bioimpedance analysis is a noninvasive, low cost and a commonly used approach for body composition measurements and assessment of clinical condition. There are a variety of methods applied for interpretation of measured bioimpedance data and a wide range of utilizations of bioimpedance in body composition estimation and evaluation of clinical status. This paper reviews the main concepts of bioimpedance measurement techniques including the frequency based, the allocation based, bioimpedance vector analysis and the real time bioimpedance analysis systems. Commonly used prediction equations for body composition assessment and influence of anthropometric measurements, gender, ethnic groups, postures, measurements protocols and electrode artifacts in estimated values are also discussed. In addition, this paper also contributes to the deliberations of bioimpedance analysis assessment of abnormal loss in lean body mass and unbalanced shift in body fluids and to the summary of diagnostic usage in different kinds of conditions such as cardiac, pulmonary, renal, and neural and infection diseases.
1. Introduction
Bioimpedance analysis is a broadly applied approach used in body composition measurements and healthcare assessment systems. The essential fundamentals of bioimpedance measurement in the human body and a variety of methods are used to interpret the obtained information. In addition there is a wide spectrum of utilization of bioimpedance in healthcare facilities such as disease prognosis and monitoring of body vital status. Thus, with such a broad utilization, we feel that this warrants a review of the most fundamental aspects and healthcare applications of bioimpedance analysis.
Studies on the electrical properties of biological tissues have been going on since the late 18th century [ 1 ]. Thomasset [ 2 ] explored the utilization of bioimpedance measurement in total body water estimation using needle electrodes. Nyboer [ 3 ] applied quad surface electrode readings for bioimpedance measurements to estimate the fat free mass of the human body. Hoffer [ 4 ] introduced the association between total body impedance and total body water content in reference to tritium dilution techniques.
The electrical properties of biological tissues are currently categorized based on the source of the electricity, i.e. , active and passive response. Active response (bioelectricity) occurs when biological tissue provokes electricity from ionic activities inside cells, as in electrocardiograph (ECG) signals from the heart and electroencephalograph (EEG) signals from the brain. Passive response occurs when biological tissues are simulated through an external electrical current source [ 5 ]. Bioimpedance or biological impedance is defined as the ability of biological tissue to impede electric current [ 6 ].
Due to the noninvasiveness, the low cost and the portability of bioimpedance analysis systems, numerous researchers have conducted studies on bioimpedance analysis and its applications in body composition estimation and evaluation of clinical conditions. Recently, Mialich et al. [ 7 ] reviewed the applications of bioimpedance analysis in body composition assessment and monitoring of chronic diseases with a comprehensive listing of the most used equations, however, recent techniques such as real time multi-sine bioimpedance analysis and bioimpedance vector analysis methods were not discussed. Lukaski [ 8 ] has revised the conceptual modules of bioimpedance analysis for physiological activities assessment and diseases prognosis. The study states that the applied multiple regression approaches and physical modules in bioimpedance analysis have limited utilization in individuals' measurement. This paper is a review of the basic fundamentals and the applications of bioimpedance analysis. The first section highlights the main bioimpedance measurement approaches using single frequency, multiple frequencies and broadband frequency spectrum signals, in addition to applied bioimpedance measurements method across the whole body, through body segments and other alternative analysis method such as vector bioimpedance analysis and real time bioimpedance methods. Body composition parameters, which include lean mass and fluid volumes estimation using bioimpedance measurements, are discussed in the second section. Basic factors in bioimpedance measurements, including anthropometric measurements, age, race, protocols and postures, and shape and artifacts of electrode are discussed in the third section. Finally, applications of bioimpedance analysis in diseases prognosis and clinical monitoring systems are outlined in the fourth section.
2. Fundamentals of Bioimpedance Measurement Techniques
Impedance (Z), from an electrical point of view, is the obstruction to the flow of an alternating current and, hence, is dependent on the frequency of the applied current, defined in impedance magnitude (|Z|) and phase angle (φ) as shown in Equations (1) – (3) [ 9 ]. Bioimpedance is a complex quantity composed of resistance (R) which is caused by total body water and reactance (X c ) that is caused by the capacitance of the cell membrane [ 5 ]:
Resistance of an object is determined by a shape, that is described as length (L) and surface area (A), and material type, that is described by resistivity (ρ), as shown in Equation (4) , [ 9 ]. Reactance (X c ) of an object as shown in Equation (5) , is defined as resistance to voltage variation across the object and is inversely related with signal frequency (f) and capacitance (C) [ 9 ]. In biological systems resistance is caused by total water across the body, and reactance occurs due to the capacitance of the cell membrane [ 5 , 10 ]:
Capacitance (C) is defined as the ability of the non-conducting object to save electrical charges, that is equal to the ratio between differentiation in voltage across object (dV/dt) and current that is passed through the object (I(t)), as shown in Equation (7) . In the parallel capacitor module, capacitance is in direct proportion to the surface area (A) in meters square and inversely proportional to distance (d) in meters between the charged plates, and is dependent on the permittivity constant of vacuum (ε 0 ≈ 8.854 × 10 −12 F·m −1 ) and the relative dielectric permittivity constant (ε r ) that is defined based on the material between the plates (for a vacuum space, ε r = 1), as shown in Equation (6) [ 9 ]:
Body composition estimation using bioimpedance measurements is based on determination of body volume (V b ) through the basic means of resistance measurement. From Equation (4) that gives the relation between resistance and ratio of length (L) to surface area (A), body volume (V b ) can be obtained by substituting the surface area (A) with the numerator and denominator of the length (L), as in Equation (8) :
The human body as a volume is composed generally of fat mass (FM) which is considered as a non-conductor of electric charge and is equal to the difference between body weight (Wt Body ) and fat free mass (FFM), as shown in Equation (9) ; and FFM, which is considered as the conducting volume that helps the passing of electric current due to conductivity of electrolytes dissolved in body water. Studies show that water, known as total body water (TBW) is the major compound of FFM and is equal to 73.2% in normal hydration subjects, as in Equation (10) [ 11 ]:
In bioimpedance measurements, the human body is divided into five inhomogeneous segments, two for upper limbs, two for lower limbs and one for the trunk. In the five compartment module, the human body is composed of FM and FFM which consists of bone minerals and body cell mass (BCM) that include protein and total body water that consists of extracellular fluid (ECF) and intracellular fluid (ICF) [ 5 ]. Figure 1 , shows the five segments and compartments of human body.

Main body segments and compartments.
Most of the known prediction methods rely on the relation between water volume and the ratio between square length to resistance (L 2 /R) [ 12 ], however the alternation in anatomical and anthropometric features of the whole human body and segments cause variations in estimated volumes. Jaffrin and Morel reviewed that most TBW estimation equations between 1985 and 1994 were based on values predicted using the H 2 /R 50 that was introduced by Kyle et al. [ 13 , 14 ] and Houtkouper et al. [ 15 ].
Measurement of bioimpedance is obtained from the whole body and body segments separately, using single frequency, multiple frequencies and bioimpedance spectroscopy analysis. In addition to several alternative assessments method such as bioimpedance vector analysis and real time bioimpedance analysis.
2.1. Single Frequency Bioimpedance Analysis (SF-BIA)
Analysis of bioimpedance information obtained at 50 KHz electric current is known as single-frequency bioimpedance analysis (SF-BIA). SF-BIA is the most used and is one of the earliest proposed methods for the estimation of body compartments, It is based on the inverse proportion between assessed impedance and TBW, that represents the conductive path of the electric current [ 5 , 16 ].
SF-BIA predicts the volume of TBW that is composed of fluctuating percentages of extra cellular fluid (ECF) which is almost equal to 75% of TBW, and ICF that represent the rest [ 5 ]. SF-BIA instruments have been used to assess TBW and FFM using the derived Equations (2) and (3) , respectively, for normal hydrated subjects, although SF-BIA is not valid for body conditions with significantly altered hydration [ 17 ]. Studies by Hanai [ 18 ] on mixture theory report that body tissue conductivity is diverse [ 5 ], and SF-BIA shows limitations in ICF variance prediction, however many of studies show an acceptable correlation in ICF estimation [ 19 ].
2.2. Multiple Frequency Bioimpedance Analysis (MF-BIA)
Analysis of bioimpedance that is obtained at more than two frequencies is known as multiple-frequency bioimpedance analysis (MF-BIA). MF-BIA is based on the finding that the ECF and TBW can be assessed by exposing it to low and high frequency electric currents, respectively. Thomasset [ 2 ] has proposed TBW and ECF estimation using 100 and 1 kHz based on the Cole model [ 20 ]. However, in later years, Jaffrin et al. [ 21 ] stated that technically a bioimpedance analyzer should use frequency range between 5–1000 kHz. Simpson et al. [ 22 ] state that low frequency in MF-BIA is generally less than 20 KHz and high frequency is more than 50 KHz. Hannan et al. [ 23 ] report that parameters measured using a frequency of less than 5 KHz and more than 200 KHz fluctuate around the actual value and conclude that estimated TBW is more accurate using the MF-BIA than the BIS method with the same predicted values of ECF for both methods. Patel et al. [ 24 ] reported that in diseased subjects, TBW prediction using SF-BIA gave more precise results than MF-BIA. In general, the MF-BIA method predicts ECF more precisely than the SF-BIA method; however in elderly diseased subjects the MF-BIA method shows less sensitivity in detecting fluid shifts between ECF and ICF [ 19 ].
2.3. Bioimpedance Spectroscopy (BIS)
Analysis of bioimpedance data obtained using a broad band of frequencies is known as bioimpedance spectroscopy (BIS). The BIS method is based on the determination of resistance at zero frequency (R 0 ) and resistance at infinity frequency (R inf ) that is then used to predict ECF and TBW, respectively. The use of 100 and 1 kHz, respectively, was earlier proposed by Thomasset [ 25 ] who applied the basics of Hanai's mixture theory [ 18 ] and Cole's module [ 26 , 27 ] as explained by the Cole-Cole plot ( Figure 2 ), however it is complicated to directly measure these values because of the relaxation phenomena of living tissue [ 20 ].

Cole-Cole module plot and Cole module parameters.
Reference methods for estimating TBW are based on radioisotopic dilution of deuterium, and for ECF estimation they are based on the dilution of bromide [ 28 ] and for ICF they are based on the radioactive potassium isotope, 40 K, both elements which are readily diffused in the human body [ 29 , 30 ]. Reference techniques are invasive, expensive and complicated when compared to bioimpedance methods, although the precision is dependent on the electrical module and body parameter variation [ 21 ].
Estimation of TBW, ECF and ICF using BIS techniques can be performed using either an equation modules approach [ 10 , 31 – 33 ] or an analytically derived equations approach [ 27 ]. Hanai's mixture theory shows limitations in some studies [ 14 , 15 , 34 ], however it showed advantages in other studies [ 35 , 36 ]. Ward et al. [ 37 ] stated that the differences in biological construction among subjects may limit mixture theory as noted in some studies [ 38 , 39 ]. Scharfetter et al. [ 40 ] report that an accurate module for body fluid allocation and trusted fitting methods are most crucial factors in the BIS method.
The determination of Cole module parameters (R 0 , R inf , α, F c ), in Figure 2 is done using the BIS method which is based on the argument that the human body is composed of a mixture containing conducting and non-conducting compartments [ 18 ].
In Equation (4) , the reference method is based on the assumption that the measured resistance (R) represents the total conducting volume of the lean body mass. However in the BIS method, the measured resistance represents the total conducting and non-conducting part of the lean body mass, so that the non-conducting part is included by multiplying the obtained resistance by body shape factor (K b ) and substituting the surface area (A) by body volume (V b ). Ayllon et al. [ 41 ] reports that the estimation of Cole module parameters (R 0 , R inf , α, F c ) that is obtained by using only resistance achieves slightly better results and there is less standard error based on the Non-Linear Least Squares technique as compared to the capacitive and impedance complex components. Ward et al. [ 42 ] concludes that the Cole parameters can be obtained by using four selected frequencies and substituting a fitting technique based on amplitude impedance values at these frequencies:
where, R is resistance, ρ is resistivity, Ht is the human height, V b is the body volume and K b is a dimensionless shape factor calculated from the length and perimeters of the upper and lower limbs, and the trunk, taken into consideration the body shape composed of the five cylinders.; Van Loan et al. [ 43 ] calculated the shape factor (K b ) from statistical anatomical measurements in adults to be equal to 4.3.
2.4. Whole Body Bioimpedance Measurement
Measurement of total body bioimpedance is the most commonly used method for estimating whole body compartments. Many of the whole body bioimpedance instruments apply three approaches for impedance measurement: hand to foot method [ 14 , 17 ], foot to foot [ 44 – 46 ] method and hand to hand method [ 47 , 48 ]. The hand to foot ( Figure 3a ) one is the most commonly used method. It was introduced by Hoofer [ 4 ] and later revised by Nyboer [ 3 ] to decrease the contact impedance between skin and electrodes, and validated by Lukaski [ 17 ] in 140 normal adults. Tetrapolar hand to foot measurements are performed on a supine subject for 15 min, placing electrodes filled with gel to minimize gap impedance on the dorsal surfaces of the right hand and foot, distal (current) ones being respectively proximal to the metacarpal and metatarsal phalangeal joints, in accordance with standard tetrapolar electrode placement [ 49 ]. Foot to foot measurements ( Figure 3b ) were introduced by Nuñez et al. [ 50 ] through the use of a pressure-contact foot-pad electrode. In leg to leg bioimpedance measurements, the subject stands vertically, with uncovered feet, on four stainless steel footpads electrodes and divided for each foot into frontal and back portion for current injecting and voltage measurement [ 46 ]. Hand to hand bioimpedance measurements were introduced by Ghosh et al. [ 48 ] by performing body composition analyses using a handheld impedance meter in subjects with malnutrition. The device was held while both arms were stretched out horizontally in front of the body. Deurenberg et al. [ 47 ] validated the hand to hand method on 298 Singaporean subjects and reported that readings obtained using a handheld impedance meter were significantly acceptable for those subjects.

Whole body bioimpedance measurement techniques, ( a ) hand to foot and ( b ) foot to foot electrodes positioning.
2.5. Body Segment Bioimpedance Measurement
Segmental bioimpedance analysis achieves better estimation of skeletal muscle mass (SMM) than whole body bioimpedance analysis, with a reported standard error of 6.1% in reference to MRI measurements among 30 male subjects [ 51 ]. Baumgartner et al. [ 52 ] stated that multi-frequency segmental bioimpedance analysis enhances and elucidates the relationship between bioimpedance analysis and body compartment estimation after examining the impact of phase angle on body composition prediction among 116 normal subjects.
Segmental bioimpedance analysis detects the fluctuation in ECF due to differences in posture and is more precise than the ankle foot method [ 53 ], and gives a better estimation of TBW than total body measurements with reference to dilution method [ 54 ].
Segmental or perpendicular bioimpedance analysis defines the measurement method of body segments that is mostly treated as five cylinders as in Figure 1 [ 5 ], and was introduced to overcome the disagreement between trunk resistance to upper limbs ratio and trunk resistance to lower limbs ratio of 0.72 and 0.66 respectively [ 52 ]; Earthman et al. stated that the trunk represents 50% of the body mass [ 55 ]. Kyle et al. pointed out that total bioimpedance measurement assesses mainly the upper and lower limb compartments, and shows some limitation to predict water compartments of the trunk [ 13 ].
Measurement of segmental bioimpedance can be achieved through four types of protocols. The first approach, as suggested by Scheltinga et al. [ 56 ], uses dual current injection electrodes on the proximal area of the right forearm and lower leg, and quad voltage electrodes placed on the right proximal forearm, shoulder, upper thigh and lower leg ( Figure 4a ). The second approach is suggested by Zhu et al. [ 57 ], through the sum of segments technique, that uses dual current injection electrodes on the right wrist and foot, and quad voltage electrodes placed on the right wrist, shoulder, upper iliac spine and foot ( Figure 4b ). A third approach was presented by Organ et al. [ 58 ], who suggested the use of dual current injection electrodes on the right wrist and foot, and quad voltage electrodes, two placed on the right wrist and foot, and two on the left wrist and foot ( Figure 4c ). The fourth approach as suggested by Jaffrin et al. [ 16 , 59 , 60 ], is through the use of quad current injection electrodes located on the right and left wrist and foot, and quad voltage electrodes located at the same place ( Figure 4d ).

Segmental bioimpedance analysis techniques, ( a ) right side dual current and quad voltage electrodes, ( b ) right side dual current and quad voltage electrodes, ( c ) double sides dual current and quad voltage electrodes and ( d ) double sides quad current and quad voltage electrodes.
Limitations of whole body bioimpedance measurement in evaluating body segment compartments have given rise to the demand for segment localized bioimpedance analysis applications. Scharfetter et al. [ 40 ], reported that using segmental (across the waist) localized bioimpedance analysis can significantly estimate abdominal fat with a correlation coefficient of R 2 = 0.99; furthermore Seward et al. [ 61 ], introduced localized bioimpedance analysis as a trending diagnostic tool for neuromuscular disorders. The study was applied on 25 neuromuscular patients and 45 normal subjects for control.
Studies report that the segmental bioimpedance analysis method shows some limitations in the estimation of FFM [ 62 , 63 ], with estimation power not significantly different from whole body bioimpedance method [ 44 ]. However, Kyle et al. [ 13 ] concluded that enhancement can be achieved through applying the MF-BIA method and further studies on electrode types and allocation.
2.6. Alternative Bioimpedance Analysis Method
Bioimpedance analysis, as an independent method for the assessment of the human health status from absolute bioimpedance measurements, has triggered a new path of data analysis and interpretation. The bioimpedance vector analysis method (BIVA) is a novel approach established essentially by Piccoli et al. [ 64 , 65 ] to estimate the hydration status using height indexed resistance and reactance data (R-X c graph) from bioimpedance measurements. Using 8,022 normal subjects (3796 female and 4226 male) Piccoli et al. [ 66 ] formulated 50%, 75%, and 95% tolerance ellipses that determine increasing and decreasing body mass if the minor vector falls in the left and right half of the 50% ellipse, along with increasing and decreasing hydration ratio if the major vector falls in the lower and upper half of the 50% ellipse ( Figure 5 ).

Bioimpedance vector analysis (BIVA) and tolerance ellipses.
Evaluation study of the BIVA method by Cox-Reijven et al. [ 67 ], on 70 diseased subjects with gastrointestinal disorders, conclude the high specificity and low sensitivity of BIVA method in classifying patients with extraordinarily rates of body fluids. Low values (Xc/H < 27.7 O/m and R/H < 563.6 O/m) in the BIVA method can be considered as predictors of severity among diseased children, as shown in a study conducted on 332 precarious pediatric patients with multiple organ dysfunction (MODS), acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) [ 68 ].
In [ 69 ] the BIVA method successfully monitored rapid increases in ECF during short term recovery (3 weeks) and a dramatic increase in BCM during long term recovery (3 months) among 47% of 57 diseased women with anorexia nervosa [ 5 ].
The BIVA method is also considered as a valid tool for the estimation of dry weight in 24 haemodialysis patients' with reference to the Bilbrey Index based on different allocation of values before and after obtrusion [ 70 ].
Kyle et al. reported that the BIVA method is affected by differences in biological factors and measurement artifacts [ 5 ]. Ward and Heithmann state that BIVA is affected by body size and influenced by the cross-sectional area of the body [ 71 ].
A specific BIVA method has been proposed by Marini et al. [ 72 ] to neutralize the bias due to body size. The specific BIVA method used a resistivity-reactivity graph that is constructed using information and results collected from multiplication of resistance and reactance by ratio of cross section area and length (L/A) from Ohm's law ( Equation (1) ). The cross section area (A) and length (L) were estimated as follows: A = (0.45(arm area) + 0.45(calf area) + 0.10(waist area)) in square meter [ 73 , 74 ], where segment area = c 2 /4π and (c) is the circumference in meter of the arm, waist and calf, respectively; L = 1.1 (Ht), where Ht is body height in meters.
Another alternative method for analysis is real time processing of bioimpedance data which is currently introduced as a key feature for body health monitoring applications. A logarithmic analysis carried out between 0.01 and 10 Hz with five frequencies needs 276 s to be completed, this includes the calculation time [ 75 ]. Sanchez et al. [ 76 ] stated that real time processing, accuracy and the ability of data retrieval and throughput of a BIS system were the most important features to be applied in health monitoring systems, and Sanchez et al. [ 77 ] introduced a local polynomial based method for impedance-frequency-response estimation. Comparison studies between four different multi-sine periodic broadband excitations broadband for EIS measurements in term of accuracy and speed in frequency and time domain concluded that multi-sine and discrete interval binary sequences (DIBS) enhance SNRZ and have better accuracy than chirp and maximum length binary sequences (MLBS) [ 75 ].
Use of multi-sine excitation signals in bioimpedance measurements that is proposed in [ 78 , 79 ] helped increase the accuracy of the measured bioimpedance parameters. It has been validated using a set of optimal multi-sine measurements on 2R-1C equivalent electrical circuits, then applied on healthy myocardium tissue. The multi-sine excitation method was introduced as a parametric-in-time identification method for electrical bioimpedance measurements with inclusion of harmonic impedance spectra (HIS). HIS directly identified from noisy current and voltage myocardium measurements at the multi-sine measurement frequencies to express periodic changes of impedance, rather than the commonly used method that assumed the measurement changing over time [ 80 ].
3. Body Composition Prediction Using Bioimpedance Analysis
Body composition assessment is considered a key factor for the evaluation of general health status of humans. Several methods use different assumptions to estimate body composition based on the number of compartments. This review considers that the human body is composed of two main compartments, FM and body lean mass or FFM. FFM is composed of bone minerals and body cell mass (BCM) that includes skeletal muscle mass (SMM). BCM contains proteins and TBW that represents 73% of lean mass in normal hydrated subjects. TBW is composed of ICF and ECF as illustrated in Figure 1 . In this section, several predictive equations for both lean and fat mass, in addition to body fluids, will be discussed.

3.1. Fat Mass (FM) and Fat Free Mass (FFM)
FM and FFM estimations are considered one of the main objectives of body composition assessment techniques. Variations in FM among the reference population are due to several factors, but are believed to follow aging factors in addition to gradual changes in lifestyle [ 81 ].
Anthropometric and skin fold thickness measurements are traditional, simple and inexpensive methods for body fat estimation to assess the size of specific subcutaneous fat depots [ 82 ] compared with other methods such as underwater weighing, dilution method and dual-energy x-ray absorptiometry [DXA] that requires a trained practitioner to perform it.
Bioimpedance analysis has been shown in recent studies to be more precise for determining lean or fat mass in humans [ 83 ]. In comparison with BMI, anthropometric and skin fold methods, BIA offers trustable results in the estimation of fatness across human tissues [ 84 ]. Several studies conducted to establish reference values for FFM are based on bioimpedance measurements.
Kyle et al. [ 13 ] developed a single Equation (12) for the prediction of FFM, using 343 normal subjects aged from 22 to 94 years old, with body mass indexes between 17.0 and 33.8 kg/m 2 in reference to DXA method:
where (Ht) is body height, (R 50 ) and (X C, 50 ) is resistance and reactance at 50 KHz, and (Wt) is body weight. The developed equation achieved a correlation coefficient (R) that is equal to 0.986, standard error of the estimate (SEE) is equal to 1.72 kg and technical error is 1.74 kg.
In [ 81 , 85 ], FFM was assessed in a population of 5,225 white subjects aged from 15 to 98 years old using bioimpedance measurements and it was concluded that mean FFM was 8.9 kg or 14.8% lower in men older than 85 years than in men 35 to 44 years old and 6.2 kg or 14.3% lower in women older than 85 years than in women 45 to 54 years old.
Sun et al. [ 86 ], used a multi-component model based on densitometry, isotope dilution, and dual-energy X-ray absorptiometry to build Equations (13) and (14) for FFM estimation:
The mean FFM prediction equations achieved a correlation coefficient R 2 = 0.90 and 0.83 and root mean square errors of 3.9 and 2.9 kg for males and females, respectively.
Deurenberg et al. [ 87 ], used densitometry, anthropometry and bioelectrical impedance to formulate FFM prediction Equation (15) using 661 normal adult subjects aged from 7 to 83 years old:
The FFM prediction equations achieved a correlation coefficient R 2 = 0.93 and standard estimation error (SEE) = 2.63 kg.
Pichard et al. [ 88 ], assessed FFM and FM in a 3,393 white subject population aged from 15 to 64 years old using bioimpedance measurements and performed a comparison of %FM as determined by BIA with %FM determined by calculations using BMI developed by Deurenberg et al. [ 89 ], and concluded that the mean FFM ranged of 59.1–61.0 kg for men and 43.3–44.1 kg for women which is 38% greater in men.
Heitmann [ 90 ] compared three body composition methods (BMI, skin folds and BIA) using 139 healthy subjects aged from 35 to 65 years old:
The multiple regression Equation (16) for impedance had a higher correlation coefficient (R 2 = 0.89) and lower standard estimation error (SEE = 3.32 kg) than the multiple regression equations for skin fold (R 2 = 0.81, SEE = 3.91 kg) or body mass index (R 2 = 0.85, SEE = 3.94 kg).
Heitmann [ 88 ] assessed FFM and FM in 2987 out of a 3608 subject Danish population aged from 35 to 65 years old. The obtained data, which are estimated from measurements of electrical impedance, concluded that men have a FM of 4.5 kg, an increase by 30%, when compared to women that have a 6.9 kg increase of 36% for evaluated sample.
Recently, Pichler et al. [ 91 ] assessed FM in 116 subjects (32 healthy subjects and 84 patients) and concluded that the following prediction equation overestimated FM by 6.55 ± 3.86 kg:
where R ecf and R tbw represents resistance of extracellular fluids and total body water extracted using the Cole module [ 26 ]. In conclusion, all studies state that the men have higher estimated FM as compared to women. Moreover, FFM for both genders decreases progressively with increasing age [ 81 , 88 ].
3.2. Body Fluids
Body fluid is the total volume of fluids inside a human body that represents the majority of the FFM volume percentage. TBW includes the fluids inside the cellular mass that is known as ICF; and the fluid located outside the cell body which is composed of plasma and interstitial fluid which is known as ECF. ECF and ICF fluids that are incorporated under TBW, contain several ion types with different concentrations, however the main ions in ECF are Na + and Cl − , and for ICF are K + and PO −4 [ 92 ].
Body fluids estimation using bioimpedance measurements are based on the inversely proportional between body resistance and the total amount of body water [ 93 ]. There are varieties of methods for estimating body fluid volumes based on bioimpedance analysis approach.
Sun et al. [ 86 ] developed prediction Equations (19) and (20) of the TBW reference to dilution method using SF-BIA from a multi-ethnic pool of 1830 people aged from 12 to 94 years old:
The developed equation achieved a correlation coefficient (R 2 ) and mean square error equal to 0.84 and 3.8 L in men, and 0.79 and 2.6 L in women.
For ECF and ICF estimation using SF-BIA, a few studies performed were based on measurement of bioimpedance in 50 KHz frequency, Sergi et al. [ 94 ], predict ECF using two frequencies (1 and 50 KHz):
After measurements performed using bioimpedance and bromide dilution methods on 40 subjects (19 males and 21 females) aged 21–81 years, of which 22 were healthy subjects, 12 were affected by chronic heart failure and 6 by chronic renal failure, the best estimation results at 1 KHz achieved a correlation coefficient (R 2 ) and standard estimation error equal to 0.89 and 1.7 L.
Due to incomplete conduction of the intracellular fluid at 50 kHz [ 2 ], MF-BIA was proposed to increase accuracy of estimation of TBW, ECF and ICF. Deurenberg et al. [ 95 ] used MF-BIA (1, 5, 50, 100 KHz) to predict TBW using Z 100KHz and Z 50KHz ; and ECF using Z 1KHz and Z 5KHz among 139 normal adult subjects with reference to deuterium oxide dilution and bromide dilution:
The prediction equation of TBW achieved a correlation coefficient (R 2 ) and standard error of estimate (SEE) equal to 0.95 and 1.73 L using Z 100KHz , and 0.95 and 1.74 L using Z 50KHz :
The prediction equation of ECF achieved a correlation coefficient (R 2 ) and standard error of estimate (SEE) equal to 0.87 and 0.98 L using Z 1KHz , and 0.86 and 1.02 L using Z 5KHz .
Prediction of body fluids using the BIS method in three steps involves firstly determination using the values of R e from R 0 and R inf , secondly, inclusion of the body shape factor K b due to the variation of body segments, and thirdly, inclusion of apparent resistivity ρ a instead of the general resistivity ρ as stated by Hanai in mixture theory [ 18 ]:
where ( c ) is volume fraction of non-conducting tissue. Based on Hanai's mixture method [ 18 ], tissue resistance (R) is measured based on conductive tissue, so it should exclude non-conducting tissue. Thus, by substituting Equation (27) in Equation (11) , the apparent resistance (R a ) can be calculated using the following Equation (28) :
At low frequencies the current will pass through extracellular fluids only without intracellular fluid due to the high capacitance of cell membranes [ 96 ]. In that case the conducting volume is equal to the ratio between ECF volume (V ecf ) and TBW volume (V b ). The volume fraction of non-conducting tissues at low frequencies calculated as in Equation (29) :
Based on the mixture theory [ 18 ], apparent resistivity (ρ a ) at low frequency represents the extracellular fluid resistivity (ρ Aecf ), thus the resistance of ECF (R ecf ) can be recalculated in Equation (31) , by substituting Equation (29) in Equation (28) and including the outcome of apparent resistivity (ρ aecf ) from Equation (30) :
Hanai [ 18 ], calculated ρ ecf to be equal to 40.3 Ω ·cm for men and 42.3 Ω ·cm for women, which is close to that achieved by saline, and is about 40 Ω ·cm t for the ECF composed of plasma and interstitial water [ 49 ].
To reform the equation to evaluate the variance in ECF volume (V ecf ) caused by changes in estimated ECF resistance (R ecf ), that is achieved by replacing body volume (V b ), that is equal to the ratio between body weight ( Wt ) in Kg and body density ( D b ) in Kg/L from Equation (32) in Equation (33) :
Body factor (K b ), extracellular fluid resistivity (ρ aecf ) and body density ( D b ) are constant values that can be included in one factor defined as extracellular fluid factor (K e ) as in Equation (34) , and for extracellular fluid volume (V ecf ) as in Equation (35) :
Van Loan et al. [ 43 ], calculated K e using the bromide dilution method to be equal 0.306 for men and 0.316 for women; and the ratio between ρ icf and ρ ecf to be equal to 3.82 for men and 3.40 for women. De Lorenzo et al. [ 10 ] calculated K e to be equal to 0.229 in women; and ρ ecf to be equal to 40.5 Ω ·cm and 39.0 Ω ·cm for men and women, respectively; and the ratio between ρ icf and ρ ecf to be equal to 6.76 for men and 6.79 for women.
Ellis and Wong [ 30 ], analyzed the BIS method as introduced by Van Loan et al. [ 43 ], with reference to the H 2 O and Br dilution technique in 469 multi-ethnic healthy subjects. The study suggested that the ratio between ρ icf and ρ ecf is equal to 3.032 for men and 2.694 for women, due to underestimation of TBW caused by misprediction of ICF measurements. Biasing factors and different regression module approaches caused slight differences in the ratios obtained by these researchers [ 30 ].
Moissl et al. [ 97 ], suggested a body composition spectroscopy method through recalculating K ecf , using different assumptions through inclusion of body mass index (BMI) and taking the module of non-conducting tissue factor (c) in Equation (14) as a valid assumption, as in Equation (36) , and then determining the (V ecf ) using the same equation as Equation (20) :
From [ 97 ], (a) and (b) were calculated to be equal to 0.188 and 0.2883 based on measurements using the Br dilution method as a reference method on dialyzed patients and 120 healthy subjects. At high frequencies, the current will pass through the whole TBW which is composed of ECF and ICF [ 96 ], so the conducting volume is equal to the ratio between TBW and total body volume.
Jaffrin et al. [ 31 ] suggested calculating the TBW directly from R inf using the same assumption of mixture theory [ 96 ], and assuming uniformity of water compartments inside human body. Thus, using the same assumption as in Equation (29) , the volume fraction of non-conducting tissue (c) at high frequencies can be calculated using Equation (37) :
To determine the apparent resistivity of total body water (ρ a_tbw ) from actual total body water resistivity (ρ tbw ), the parameters in (c) from Equation (37) , was included into Equation (38) :
By replacing the actual resistivity by apparent resistivity for total body water in Equation (11) , and restoring the value of (V b ) from Equation (32) , Equation (40) to determine the total body water factor (K tbw ) and total body water volume (V tbw ) is recalculated by using Equation (39) :
Considering that total body water is equal to the accumulation of ECF and ICF, Jaffrin et al. [ 31 ] calculated ρ tbw to be equal to 104.3 Ω ·cm in men and 100.5 Ω ·cm. A validation study conducted in 28 dialysed patients [ 31 ], concluded that ρ tbw was equal to 108.1 Ω·cm in men and 100.2 Ω·cm, which predicted 91% of mean water loss when compared with 39% for Cole method [ 43 ], but overestimated TBW compared to the original BIS method in 21 healthy subjects with the same ρ tbw and hydration rate values.
For ICF prediction using a BIS method, Matthie et al. [ 32 ] introduced a second generation mixture theory to overcome the limitations of the first generation in predicting intracellular fluid volume (V icf ) using a new assumption for TBW resistivity (ρ tbw ), as in Equation (32) :
In the second version of mixture theory, total body water volume is considered to be equal to the summation of ECF and ICF, for ECF estimation, the relation in Equation (35) is considered as a valid method, and for ICF estimation, the method uses Equation (42) ; note that the ratio (R tbw /R ecf ) is opposite and proportional to (V tbw /V ecf ):
Moissl et al. [ 97 ] calculated ρ icf to be equal to 273.9 Ω·cm and ρ ecf = 40.5 Ω·cm in men and 264.9 Ω·cm and 39.0 Ω·cm, respectively in women. De Lorenzo et al. [ 10 ] suggest the formula in Equation (34) to determine intracellular volume (V icf ):
Jaffrin and Morel [ 21 ] claim that the prediction of ECF by Hanai [ 18 ] mixture theory is valid and direct, however the ICF prediction by De Lorenzo et al. [ 10 ], who state that the determination of R i is less accurate than for R e in parallel module because it sums up the errors on R e and R inf , is not.
Moissl et al. [ 97 ] introduced a different method for calculation of intracellular fluid volume (V icf ), taking into consideration that the non-conducting tissue factor (c) is as given in Equation (44) :
Then the recalculated intracellular fluid factor (K icf ) and intracellular fluid volume (V icf ) are added as in Equations (45) and (46) , respectively, and it is concluded that total body water factor (K tbw ) and total body water volume (V tbw ) is equal to the summation of ECF and ICF volumes as in Equation (47) and recalculated (V tbw ) using different assumption of (K tbw ) and (ρ tbw ) from Jaffrin et al. [ 31 ], and Matthie et al. [ 32 ], as given in Equations (48) and (49) :
where (c) and (d) are calculated to be equal to 5.8758 and 0.4194 in [ 97 ], when using the 40 K isotope [ 98 ] as a reference method on dialyzed patients and 120 healthy subjects.
Fenech and Jaffrin [ 2 ] state that ECF prediction using segmental bioimpedance analysis in supine position (0.79 liter) is less than Watson anthropomorphic method [ 3 ] (1.12 liter) and for ICF is reduced by 3.4% for segmental bioimpedance and 3.8% for the Watson anthropomorphic method [ 3 ]:
Pichler et al. [ 91 ] examined the BIS method using an Impedimed device (SFB7) in TBW, ECF and FFM with reference to the deuterium space method, sodium bromide space method and DXA method, respectively. The study was applied on 32 healthy subjects and 84 patients with different types of diseases (congestive heart failure, coronary heart disease, essential hypertension, atherosclerosis, kidney disease, chronic renal failure, gastrointestinal diseases, type II diabetes, morbid obesity, osteoporosis, cancer, chronic polyarthritis and anorexia nervosa):
Pichler's equations for TBW achieved a correlation coefficient 0.91 and 0.89 for men and women, respectively, as in Equations (51) and (52) . For ECF it achieved 0.87 and 0.89 for men and women, respectively, as in Equations (53) and (54) [ 91 ]. Hanai mixture equations [ 18 ], when applied in SFB7 give ECF measurements higher than the sodium bromide space method by mean ± SD (0.93 ± 2.62 Liter) however it is noted that the Hanai mixture equations applied in SFB7 detect ECF excess in 9 patients, and TBW measurements higher than the deuterium space method by mean ± SD (3.82 ± 3.37 Liter), and FFM measurements lower than the DXA method by mean ± SD (6.55 ± 3.86 kg).
4. Bioimpedance Measurement Biasing Factors
4.1. anthropometric measurements.
Anthropometric measurements such as weight, height, skin fold thickness, lengths, diameters and circumferences that involves mathematical modules are the main contributors in the estimation of body compartments [ 5 , 99 ].
Bioimpedance parameters only without body dimension measurements are considered poor estimators for body composition [ 91 , 100 ]. Diaz et al. [ 101 ] concluded that in FM and FFM prediction, resistance and capacitance measurements contribute by 0%–20%. In contrast, the percentages increase to 11%–53% after height inclusion, and 22%–68% after inclusion of Ht 2 /R ratio.
Ward et al. [ 102 ] presented a validation study to predict BCM and ECF as a portion of TBW without measuring height and using BIA device, the Soft Tissue Analyzer STA TM (Akern Sri, Florence, Italy) with a correlation coefficient referenced to the total body potassium counting method is equal to 0.91, 0.82 and 0.89, and a standard estimation error equal of 5.6 kg, 6.3 kg and 1.3 kg for FFM, BCM and ECF, respectively.
4.2. Gender
Variations in body composition between male and female were proven in several studies [ 103 ]. In body composition prediction, methods based on bioimpedance analysis, and most equations tend to include gender as one of the main determining factors for body compartment assessment [ 13 , 86 , 87 ].
FFM or lean mass studies show that males have greater FFM than females with different ranges. Kyle et al. [ 81 ] state that mean FFM for male is 8.9 kg and 6.2 kg for female and fat mass index FMI increases based on age, in females from 5.6 to 9.4 and from 3.7 to 7.4 in males. In a recent study [ 104 ] on 1649 healthy children-adults (6–18 years) and 925 adult-elders (19–92 years) using BIA and DXA it was concluded that for all age ranges, males have less fat mass and more fat free mass than females.
TBW averaged 73.2% of fat free mass in the healthy population; however several studies show that males have less TBW than females [ 11 ]. Sun et al. [ 86 ], stated that in a mixed ethnic groups prediction equation, TBW volume for males start from 1.2 L compared with 3.75 L for females. Jaffrin et al. [ 31 ] state that determined TBW resistivity (ρ) is on average 104.3 ± 7.9 Ω·cm for men and 100.5 ± 7.8 Ω·cm for women. The values are smaller in men are due to their larger limb cross section.
Due to the different body composition between males and females, gender considerations have a strong impact in estimating body compartments.
Aging is defined as a multi-factor changing in the physical and biological activities of the human body that leads to differences in body composition among age groups. When the human body becomes older it leads to a gradual increase in fat mass and spontaneous decrease in lean mass. Fat free mass to fat mass ratio increases gradually in response to increase of age, and a noticeable increment in average weight is seen among the elder population compared with adults associated with increment in fat mass [ 81 ]. In some studies [ 58 ], the general body composition prediction equations were unsatisfactory in elderly men over 75 years of age, especially in TBW estimation.
Several studies were conducted using the BIA method on children [ 68 , 105 ] adults [ 13 ], and elders [ 106 , 107 ]. In children, the BIA method using the Deurenberg equation [ 87 ], underestimates body fat as determined by DXA. It however achieved a better correlation than the skin fold method [ 108 ]. Muscle mass loss among the elderly reduces the fat free mass at a certain age, followed by decreases in total body water and bone mass [ 109 ]. Marja et al. [ 107 ] reported that in 75-year-old Swedes, average fat free mass index was 15.6 and 18.3; and body fat index was 11.0 and 8.6 for women and men, respectively, compared to the DXA method.
4.4. Ethnic Groups
Body composition varies among different races and ethnic groups due to the environment, nutrition factors, culture and anthropometric measurements that include body conformation [ 110 ]. There is also difference in limb length [ 111 ], body structure [ 112 ], body size [ 89 ] and that lead to variation in body fat percentages among different ethnic groups which may lead to prediction errors (3%) [ 111 ].
The majority of bioimpedance measurement studies have been done on Caucasian subjects [ 5 ], Kotler et al. [ 113 ] and Sun et al. [ 86 ] have included African American and Hispanic subjects in their studies. Kim et al. assessed the segmental lean mass among Koreans [ 106 ], Schulz et al. assessed the fat free mass among Germans and compared it to the American and Swiss population [ 114 ]. Siváková et al. studied the clinical applications of BIVA on Slovaks [ 115 ]. Nigam et al. had performed a comparative study among two different Indian races [ 116 ], whereas Saragat et al. obtained specific BIVA reference values for the Italian healthy elderly population in order to construct the specific tolerance ellipses to be used for reference purposes for assessing body composition in gerontological practice and for epidemiological purposes [ 117 ]. Validation of bioimpedance measurements among different ethnicities is thus needed due to differences in body composition among certain populations.
4.5. Measurements Protocols and Posture
Simplicity and the economic acceptance of bioimpedance analysis method for body composition estimation have increased the need to unify the protocols and procedures of bioimpedance measurements in order to retrieve robust data.
For the foot to ankle measurement method, bioimpedance measurements performed in a supine position with abduction of the upper limbs to 30 degrees and lower limbs to 45 degrees for 5 to 10 min. studies show that when the posture changes from a standing to a supine body position, the ECV decreased in the arms by 2.51% and legs by 3.02%, but increased in the trunk by 3.2% [ 118 ]. Fasting for at least 8 hours and bladder voiding before measurements are recommended as consumption of food and beverages may decrease impedance by 4–15 O over a 2–4 h period after meals and that causes an error (<3%) [ 84 , 119 , 120 ]. Body anthropometric measurements should be retrieved prior of the test and for scale or foot to foot bioimpedance analyzer weight retrieved automatically [ 1 ].
Electrodes should be placed on the pre-cleaned metacarpal and metatarsal phalangeal joints with a distance in between of at least 5 cm without skin lesions at the location of the electrodes. In some studies skin temperature should be counted [ 84 , 120 ]. Subjects under test should not perform any exercise activities before measurements that could lead to errors in assessed resistance and reactance equal to 3% and 8% respectively [ 121 ]. Roos et al. concluded that the error in total body water prediction range from 1 to 1.5 L figured out after laying at rest for one hour [ 122 ].
4.6. Electrode Shape and Measurement Error
In bioimpedance analysis, the geometrical structure of electrode has a strong impact on elementary data retrieved during the measurement process. In bioimpedance analysis electrodes are defined as isoelectric materials with a negligible voltage drop along the connectors. The minimum numbers of electrodes required to perform the bioimpedance measurements are two, one for current injection with the assumption of zero potential difference and the other for collecting the voltage drop with a negligible current flow and is more affected by position.
The tetrapolar electrode approach become widely used for whole bioimpedance measurements because of the uniformity of current distribution compared to monopolar electrodes [ 6 ], and the usage of more than two potential collecting electrodes or octapolar electrode method were used for segmental bioimpedance studies to assess compartments in different body segments [ 73 ].
Ag-AgCl electrodes are now used in most bioimpedance measurements because it has a well-defined DC potential with electrolyte gel to minimize the gap impedance between skin and electrodes. Circular and rectangular electrode shapes with a contact area greater than 4 cm 2 are the most commonly used shapes [ 1 ].
Buendía et al. investigated the impact of electrode discrepancy on BIS measurements and concluded that mismatched potential electrode causes 4% overestimated measurements in resistance at zero and infinite frequency because of an imbalanced electrical field distribution [ 123 ]. Shiffman [ 124 ] addressed the artifacts caused by inaccurate distance between electrodes in four electrode measurement methods performed on a 17.5 cm segment of the thigh area. That study reported that the values of resistance and reactance were four times larger when the current injecting electrodes were placed 2.5 cm from the sensing electrodes. Scharfetter et al. stated that capacitance between different body segments and earth, and capacitance between the signal ground of the device and earth cause a significant false dispersion in the measured impedance spectra at frequencies >500 kHz [ 125 ].
Errors in bioimpedance measurements are caused by many factors such as motion, miss-positioning, connector length and fabrication errors. Moreover, the diversity of the commercially available bioimpedance analyzers cause a wide range of fluctuations in measurements between the devices. Thus the calibration of the components inside a bioimpedance analyzer such as signal generator, sensing apparatus, scales of weight and height and electrical interference should be conducted to ensure the reliability of the bioimpedance analyzers [ 1 ].
5. Applications of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases
Bioimpedance analysis in healthcare practice contributes to the estimation of body compartments to assess the regular change in nutrition status in in-patients and to monitor nutritional risk in out-patients [ 126 ]. Most of the body composition assessment methods like BMI techniques, skin fold method and underwater weight measurements is used to estimate fat mass and fat free mass, however bioimpedance analysis can estimate FM and FFM in addition to total and particular body fluids which is very helpful for disease prognosis [ 127 ]. The National Health and Nutrition Examination Survey program in United States included bioimpedance analysis in the third NHANES program between 1999 and 2004 to assess the health and nutritional status of adults and children because of a general frustration with the dependability of the skin fold thickness method to estimate FM and FFM, especially in subjects with higher amount of segmented fat [ 128 ].
Observation of body compartment fluctuations like fat free mass, fat mass and total body water from normal limits are considered as key factors to be used in bioimpedance analysis in healthcare applications. Abnormal loss in lean body mass and unbalanced shifts in body fluids are the most measured parameters to be used to assess the healthiness of the human body. Analysis of bioimpedance parameters has bern used in several studies to estimate and analyze the changes in disorders of different kind of diseases.
Norman et al. [ 70 ] stated that phase angle is an essential predictor of clinical status. Pichler et al. [ 91 ] stated that estimation of body fluids using BIS was slightly better than anthropometric methods among healthy and diseased.
Table 1 contains some of the applications of bioimpedance analysis in disease diagnosis that are organized according to the organ systems of human body, diseases or abnormalities diagnosed based on bioimpedance parameters, and comments on how these factors are applied to determine the health condition. Bioimpedance analysis is a common method used for estimating body composition among healthy and diseased subjects in research and clinical trials. This review has focused on the theoretical and the fundamentals of bioimpedance analysis. Thus it may have some limitations, where possible important studies on the applications of bioimpedance analysis in diagnostic of diseases and the related shifts in bioimpedance parameters may have been missed.
Applications of bioimpedance analysis in clinical status monitoring and diagnosis of diseases.
| Organ Systems | Diseases | BIA Parameters | Remarks | Authors |
|---|---|---|---|---|
| Pulmonary system | Lung cancer, stages IIIB and IV | R and X (BIVA) | Reactance components decrease in patients (phase angle <4.5). | Toso , 2000 [ ] |
| Pulmonary edema monitoring | R (SFBIA) | Mean resistivity for left and right lung (1205 ± 163, 1200 ± 165 Ω·cm) and system reproducibility (2%). | Zlochiver , 2007 [ ] | |
| Cardio-vascular system | Fluid accumulation after cardiac surgery. | Ht /Z (MFBIA) | Significant increase in segmental trunk bioimpedance after surgery due to fluid accumulation. | Bracco , 1998 [ ] |
| Circulatory system | Volaemic status and hyponatraemia | TBW (SFBIA) | In elderly hyponatraemic patients, TBW assessment using BIA method was correlated with dilution of deuterium oxide (R = 0.68). | Hoyle , 2011 [ ] |
| Hydration status and hyponatraemia in elderly | TBW (SFBIA) | Assessment of hydration status in elderly hyponatraemic patients using BIA method was more accurate than clinical procedures (Cohen's kappa coefficient = 0.52). | Cumming , 2014 [ ] | |
| Renal system | Chronic hemodialysis | ECF (BIS) | ECF to weight ratio of hypertensive patient's increase from that of normal patients (24.29 ± 3.56% 21.50 ± 2.38). | Chen , 2002 [ ] |
| Dry weight in kidney failure. | ECF (BIS) | ECF/Wt is 0.239 and 0.214 L/kg for male and female healthy subjects. | Chamney , 2002 [ ] | |
| Hydration states monitoring in hemodialysis patients | Calf-BIS (BIS) | Normalized resistivity (μ = ρ /BMI) increased from 17.9 ± 3 to 19.1 ± 2.3 × 10 Ω ·Kg , and weight was reduced from 78.3 ± 28 to 77.1 ± 27 kg in Post-dialysis. | Zhu , 2007 [ ], 2008 [ ] | |
| Dry weight assessment hemodialysis patients | Calf-BIS (BIS) | Dry weight assessed by cBIS underestimate left ventricular mass and blood pressure while antihypertensive medication remains unchanged. | Seibert , 2013 [ ] | |
| Body fluids estimation in hemodialysis patients | ECF, ICF and TBW (BIS) | Correlation between proposed equation corrected for BMI and the references (mean ± SD) was −0.4 ± 1.4 L for ECF, 0.2 ± 2.0 L for ICF and −0.2 ± 2.3 L for TBW. | Moissl , 2006 [ ] | |
| Dry weight assessment HD patients | R and X (BIVA) | BIVA method shows significant different in vectors in post dialysed patients. | Atilano , 2012 [ ] | |
| Neural system | Alzheimer's disease | R and X (BIVA) | BCM decreased in patients for men, T (Hotelling's statistic) = 12.8 and for women, T = 34.9. . | Buffa , 2010 [ ] |
| Anorexia nervosa (eating disorder) | FM, FFM, TBW and ECF (BIS) | The BCM to Ht ratio was found to be significantly changed between diseased and controls subjects. | Moreno , 2008 [ ] | |
| Anorexia nervosa (eating disorder) | R and X (BIVA) | Gradually increasing in BCM and decreasing in ECF during treatments. | Haas , 2012 [ ] | |
| Muscular system | Body composition changes monitoring during exercise training | FFM and FM (MFBIA) | BIA method underestimates FM (−3.42 kg) and overestimated FFM (3.18 kg); and undetected small shift in body composition due to exercise training. | Sillanpää , 2013 [ ] |
| Immunology system | Comparison between SFBIA and MFBIA in HIV patients | ECF and TBW (BIS) | Insignificant differences in TBW and ECF estimation using SFBIA, MFBIA and BIS methods. | Paton , 1998 [ ] |
| Dengue haemorrhagic fever estimation in children | ECF and ICF (BIS) | (ECF/ICF) increase with increasing dengue virus infections severity in children. | Libraty , 2002 [ ] | |
| Cancer patients | TBW (SFBIA) | Change in TBW using BIA method (Ht /R ) correlate with deuterium dilution in underweight and normal-weight cancer patients (R = 0.43 and SEE = 1.22 L). | Simons , 1999 [ ] | |
| Early diagnosis and risk analysis of dengue | R, C, φ and Xc (SFBIA) | Reactance variations among dengue patients during defervescence of feverintervalis an indicator for classifying risk category in the DHF patients. | Ibrahim , [ ] | |
| Other diseases | Critically ill subjects | FM, TBW and ECF (BIS) | Body composition using BIS method show slightly more significant in estimation of FM, TBW and ECF among healthy and diseased subjects. | Pichler , 2013 [ ] |
| Gastrointestinal disease | R, Xc, Fc, FFM, TBW, ECF and ICF (BIS) | In critically diseased subjects, Fc and ECF increased, Xc decreased, and TBW and ICF remain the same. | Cox-Reijven , 2003 [ ] | |
6. Conclusions
Increasing demands for accurate, cost effective and non-invasive systems for clinical status monitoring and diagnosis of diseases in healthcare, has accelerated the research endeavors to provide new methods and technologies to evaluate the health condition of human body. Body composition assessment tools has been considered a promising approach for the quantitative measurement of tissues characteristic over time, in addition to direct relativity between fluctuations in body composition equivalences and survival rate, clinical condition, illness and quality of life. Bioimpedance analysis is a growing method for body compartments estimation in nutrition studies, sport medicine and evaluation of hydration rate, fat mass and fat free mass between healthy and diseased populations. Fat mass, fat free mass including skeletal muscle mass, bone minerals, and total body water, which is composed of intercellular fluid and extracellular fluid, are compartments that can be predicted and analyzed using suitable bioimpedance measurements techniques, procedures and population, age, ethnic groups or disease-dedicated bioimpedance analysis equations. Further studies are needed to evaluate the correlations between variations in bioimpedance parameters, especially in ECF and ICF, and the deviation from health to disease.
Acknowledgments
This research is supported by UM High Impact Research Grant UM-MOHE UM.C/625/1/HIR/MOHE/05 from the Ministry of Higher Education Malaysia, Fundamental Research Grant Scheme (FRGS: FP042-2013B) and University of Malaya Research Grant (UMRG: RP009C-13AET).
Author Contributions All authors contributed extensively to the work presented in this paper.
Conflict of Interest
The authors declare no conflict of interest.
- Architecture and Design
- Asian and Pacific Studies
- Business and Economics
- Classical and Ancient Near Eastern Studies
- Computer Sciences
- Cultural Studies
- Engineering
- General Interest
- Geosciences
- Industrial Chemistry
- Islamic and Middle Eastern Studies
- Jewish Studies
- Library and Information Science, Book Studies
- Life Sciences
- Linguistics and Semiotics
- Literary Studies
- Materials Sciences
- Mathematics
- Social Sciences
- Sports and Recreation
- Theology and Religion
- Publish your article
- The role of authors
- Promoting your article
- Abstracting & indexing
- Publishing Ethics
- Why publish with De Gruyter
- How to publish with De Gruyter
- Our book series
- Our subject areas
- Your digital product at De Gruyter
- Contribute to our reference works
- Product information
- Tools & resources
- Product Information
- Promotional Materials
- Orders and Inquiries
- FAQ for Library Suppliers and Book Sellers
- Repository Policy
- Free access policy
- Open Access agreements
- Database portals
- For Authors
- Customer service
- People + Culture
- Journal Management
- How to join us
- Working at De Gruyter
- Mission & Vision
- De Gruyter Foundation
- De Gruyter Ebound
- Our Responsibility
- Partner publishers

Your purchase has been completed. Your documents are now available to view.
The emerging role of biomarkers and bio-impedance in evaluating hydration status in patients with acute heart failure
Prof. Salvatore Di Somma (07/06/1953) graduated in Medicine cum laude (University of Naples, 1978) and postgraduated cum laude both in Internal Medicine and in Cardiology (University of Naples, 1983 and 1987). He began his university career at the Department of Clinical and Experimental Medicine and as Assistant Professor – Emergency Medicine and Cardioangiology – at the University Federico II of Naples (1978–2001). He was research fellow in cardiology at the University of Pavia (1989–1992) and was Assistant Professor in the Cardiovascular Research Institute of the Department of Medicine, New York Medical College, USA (1994–1996). Now Prof. Di Somma is Associate Professor of Medicine and Chairman of the Postgraduate School of Emergency Medicine, II Medical School, La Sapienza University of Rome. He is the Director of Emergency Medicine in Sant’Andrea Hospital, Rome. Prof. Di Somma is author to more than 400 papers in the field of Cardiology and Emergency Medicine. He is the director of an international cooperation team and reviewer for international medical journals.
Silvia Navarin born on (01/08/1987) was born in Rome and graduated from Liceo Classico E. Montale also in Rome (100/100). She graduated from the Second School of Medicine, La Sapienza, University of Rome. She has been a member of the Italian Red Cross since 2006 as a first aid instructor, ambulance operator, and she was the Italian Red Cross delegate for the international cooperation work between Italy and Bosnia Herzegovina (2008) and also at the international meeting in Solferino (2009). She has been involved in medical practise and research programs in the Emergency Department of Sant’Andrea Hospital in Rome since 2009. She participated in a professional exchange program of the International Federation of Medical Students’ Association at the Department of Emergency Medicine in the University Clinical Centre Ljubljana in 2010. She won a 3 months international scholarship at the University of California, San Diego and took part in a research project for her graduation thesis in the Veterans Affair San Diego Healthcare System.
Stefania Giordano (23/07/1987) was born in Rome. She graduated from Liceo Classico-Linguistico Gaio Valerio Catullo in 2006, and she also graduated in the second School of Medicine at La Sapienza University of Rome. She has been involved in medical practise and research programs at the Emergency Department of Sant’Andrea Hospital in Rome since 2008. In 2011 she received a certificate of “Echography in the emergency department”. She won a 3 months international scholarship at the University of California, San Diego where she took part in a research project for her graduation thesis at the Veterans Affairs San Diego Healthcare System.
Francesco Spadini (07/01/1976) was born in Rome. He graduated from Liceo Scientifico Statale Louis Pasteur in 1994. From 1994 to 1997 he worked at the Physics Department at La Sapienza Rome University. From 1997 to 2005 he worked in the IT industry. He is currently studying at the Second School of Medicine at La Sapienza Rome University, where he is also collaborating on various research projects.
Prof. Giuseppe Lippi is the Director of the Clinical Chemistry and Hematology Laboratory of the Academic Hospital of Parma and Associate Professor of Clinical Biochemistry and Molecular Biology. He has a degree in Medicine and a specialization in Clinical Biochemistry and Laboratory Medicine. He currently serves as Associate Editor of Clinical Chemistry and Laboratory Medicine and Seminars in Thrombosis of Hemostasis . He is also chairman of the scientific division of the Italian Society of Clinical Biochemistry and Molecular Biology (SIBioC), and member of the board of the European Association of Clinical Chemistry (EFCC). Prof. Lippi is author of more than 750 publications on international journals indexed in PubMed and Thomson, and in more than 90% of them as main or senior author. His main area of research includes preanalytical variability, analytical and clinical validation of novel biomarkers, metabolism of lipoproteins and relevant assay methods, diagnosis and management of disorders of hemostasis.
Prof. Cervellin Gianfranco (30/06/1955) graduated in Medicine in 1980. He completed his postgratuate studies in Geriatrics in 1984 and in Cardiology in 1988 at the University of Parma, Italy). He has been the Director of Emergency Medicine in Azienda Ospedaliero, University of Parma, Italy since October 2005. In April 2006 he became the Professor of Emergency Medicine at the University of Parma, Italy. He is author of approximately100 publications (66 available on PubMed), and some book chapters.
Bryan Dieffenbach is currently a medical student at the University of California, San Diego (UCSD) School of Medicine. Prior to medical school, he completed a Master of Science degree at UCSD studying the innate immune response in rheumatoid arthritis. As an undergraduate, he graduated with honors from UCSD with a degree in Biochemistry and Cell Biology. He was also the captain of the UCSD Men’s Track and Field Team.
Dr. Alan Maisel attended University of Michigan Medical School and completed his cardiology training at the University of California, San Diego. He is currently Professor of Medicine at the University and Director of the Coronary Care Unit and the Heart Failure Program at the affiliated Veterans Affairs Medical Center. He is considered one of the world’s experts on cardiac biomarkers, and is given credit for ushering in the use of BNP levels in clinical practice around the world. He has over 300 articles in print and a large clinical and basic science laboratory. Dr. Maisel is also a fixture at the medical school, where he has won countless teaching awards from medical students as well as interns and residents. His yearly San Diego Biomarker meeting is considered the most prestigious of its kind. He is currently an Associate Editor of the Journal of American College of Cardiology.
The quantitative and qualitative estimation of total body fluid content has proven to be crucial for both diagnosis and prognosis assessment in patients with heart failure. The aim of this review is to summarize the current techniques for assessing body hydration status as well as the principal biomarkers associated with acute heart failure (AHF). Although clinical history, physical examination and classical imaging techniques (e.g., standard radiography and echocardiography) still represent the cornerstones, novel and promising tools, such as biomarkers and bio-electrical impedance are achieving an emerging role in clinical practice for the assessment of total body fluid content. In the acute setting, the leading advantages of these innovative methods over device are represented by the much lower invasiveness and the reasonable costs, coupled with an easier and faster application. This article is mainly focused on AHF patients, not only because the overall prevalence of this disease is dramatically increasing worldwide, but also because it is well-known that their fluid overload has a remarkable diagnostic and prognostic significance. It is thereby conceivable that the bio-electrical vector analysis (BIVA) coupled with laboratory biomarkers might achieve much success in AHF patient management in the future, especially for assisting diagnosis, risk stratification, and therapeutic decision-making.
Introduction
The total body water (TBW) in the healthy population is estimated to be approximately 60% of the body weight, and is supposed to change during life because it is influenced by age, amount of fat tissues as well as hormonal homeostasis. Although there is a continuous shift of body fluids throughout the cells, they are operatively divided into two compartments, intracellular and extracellular. The intracellular fluid is defined as the water contained within the cell membranes and represents nearly two-thirds of TBW. The remaining one-third is the extracellular compartment that is further divided into interstitial fluid and intravascular fluid (1) ( Figure 1 ). The TBW is constantly adjusted by some important homeostatic mechanisms including the balance between water intake and water loss through renal and gastrointestinal output, breathing and sweating (2) . The estimation of TBW content is important in the prognostic assessment of critically ill patients and should be considered a vital parameter coupled with blood pressure, heart and respiratory rates, oxygen saturation and temperature.

Non-invasive methods to assess hydration status.
The accurate and fast assessment of total fluid balance in critical patients, along with a standard and reliable means for its evaluation, has always been challenging for physicians working in the acute care setting. The current gold standard (i.e., isotope dilution) is not used in everyday clinical practice and is even more difficult to apply when an emergency clinical decision is required (3) . Several approaches have been used to estimate the hydration status of patients, including history and physical examination, laboratory testing and imaging techniques (3) . The clinical experience has provided unquestionable evidence that fluid overload reflects the worsening of clinical conditions in a variety of severe disorders, such as heart failure (HF), chronic kidney diseases (CKD), and liver cirrhosis (4) . Moreover, the invasive catheterization of heart and great vessels only reflects the circulating volume of total fluid content and not the TBW.
In this important scenario, several studies have investigated and have further confirmed the emerging role of biomarkers, such as natriuretic peptides (NPs) (5) , assisted by bio-impedance vector analysis (BIVA) in the management of congestion due to increased TBW content in the setting of chronic heart failure (CHF) or acute heart failure (AHF) patients (6, 7).
The aim of this review is thereby to provide some insights on the currently used non-invasive methods for hydration assessment and to describe the appealing results obtained with the various bio-electrical impedance methods coupled with some laboratory parameters in AHF patients, since they represent the most common and evident example of hyperhydration status among critical diseases (7) .
Classical methods
In this section, we aim to examine the classic, non-invasive techniques and biomarkers for hydration assessment in patients primarily affected by AHF.
History assessment
The patient’s history at presentation has been proven useful in the assessment of hydration status in a variety of clinical scenarios. The presence of orthopnea and paroxysmal nocturnal dyspnea (PND) are two hallmark symptoms associated with the diagnosis of fluid overload secondary to AHF. When evaluated more closely in a study of 52 patients with established left ventricular dysfunction, Butman et al. (8) showed that only 50% presented with orthopnea and 35% with PND. In a meta-analysis of over 22 studies on the diagnosis of CHF performed by Wang et al. (9) it was shown that the pooled sensitivities and specificities of common symptoms between the studies yielded only modest values for the diagnosis or exclusion of HF. The presence of PND or orthopnea in the history yielded sensitivities of 41% and 50%, respectively, while providing more useful specificities of 84% and 77%, respectively. These data suggest that when used as criteria for the diagnosis of body fluid congestion secondary to AHF, classic elements of the history may be unable to provide meaningful information to the physician.
The physical examination
The physical examination provides important information on the clinical status of the patients. Several studies have assessed the efficacy of the physical exam in diagnosis of volume overload, using decompensated HF as a positive control for the disease. Elevations in jugular venous pressure (JVP) and rales have been proven to be inconsistent diagnostic tests for HF, displaying sensitivities between 37% and 70%, and 24% and 66%, respectively, yet may still provide useful information regarding the severity of disease (8–11). In patients with congestive HF studied in the RESOLVD trial, peripheral edema ( Figure 1 ) was present in 21% of patients with AHF and in only 10% of patients who were free of events (12) . This suggests that clinically evident peripheral edema is only present in a minority of patients that have decompensated, and thereby does not sufficiently reflect the hydration status of the patient. Other studies have assessed the presence of a third heart sound (S3) as an informative finding in the diagnosis of acutely decompensated HF. Unfortunately, the presence of an S3 also fails to adequately support a diagnosis of HF and fluid overload, with prevalence between 36% and 55% in decompensated patients (9–12). Overall, the reliability of the physical exam in detecting volume overload is questionable (9, 13). Stevenson et al. (14) showed that the evaluation of all volume overload characteristics by means of the presence of a positive physical exam (including evidence of JVD, rales and/or edema) had a rather limited sensitivity (i.e., 58%) in patients with diagnosed HF and volume overload (PCWP >22 mm Hg).
Standard radiography
Chest radiography is also frequently used to identify the presence of volume overload in the acutely ill patient ( Figure 1 ). Gao et al. (15) recently showed that chest radiography can be a useful tool for identifying elevated intravascular volumes in peritoneal dialysis patients before treatment, by using the cardiothoracic ratio and the vascular pedicle width. However, chest radiography lacks accuracy in the diagnosis of decompensated HF. In a study analyzing hospitalization rates in 86,376 HF patients from the Acute Decompensated Heart Failure Registry (ADHERE), it was found that the frequency of patient admission with a negative chest radiography was greater than that of patients with a positive chest radiography, 23.3% vs. 13.0% (16) . Although radiography is performed on many patients presenting to the ED with decompensated HF, it is still unclear whether the test would provide correct and appropriate diagnostic information. An important chest radiography parameter is, however, the vascular pedicle width, since its evaluation shows a statistically significant difference (p<0.0004) in AHF patients as compared with healthy controls (6) .
Echocardiography
The European Society of Cardiology (ESC) (17) and the American College of Cardiology/American Heart Association (ACC/AHA) guidelines (18) clearly state that echocardiography represents “ the single most useful diagnostic test in the evaluation of patients with HF ”. Echocardiography is a non-invasive surrogate which can provide hemodynamic data, such as stroke volume and cardiac output. The estimation of pulmonary artery pressure requires the presence of tricuspid valve regurgitation for mean and diastolic pressure as well as an accurate estimate of right atrial pressure and thus of circulatory volume content. Echocardiography also evaluates the diastolic function, and its dysfunction is classified according to severity. Mild diastolic dysfunction is characterized by a decrease in early diastolic flow velocity (E wave) and a greater reliance on atrial contraction (A wave) to fill the left ventricle (E/A<1). Moderate diastolic dysfunction presents as increasing left atrial pressure at the onset of diastole and increases in early diastolic flow velocity to a level near that of normal filling (E/A 1 to 1.5). Severe diastolic dysfunction occurs when left atrial pressure is further elevated, so that early diastolic flow is very rapid and left atrial and left ventricle pressures equalize quickly during early diastole (E/A>2). According to the American Society of Echocardiography (ASE) guidelines, the ejection fraction (EF) measured with the 2D method is considered abnormal if <55% (18) . An important part of echocardiography is the evaluation of Inferior Vena Cava (IVC), because it is considered as a surrogate circulatory volume ( Figure 1 ). The use of IVC sonography has increased markedly as a method for determining volume status, especially with the advent of portable, handheld ultrasound devices. The assessment is based on the measurement of the changes in vena cava diameter with inspiration and expiration, with the maximum IVC diameter occurring with increases in thoracic pressure during expiration. In a study of 35 CKD patients before and after hemodialysis, the IVC diameter decreased in parallel with reductions of blood volume after hemodialysis (19) . A similar study on blood donors before and after donation of 450 mL of blood also showed changes in IVC diameter measurements after volume removal. Mean changes in diameter ranged from 17.4 to 11.9 mm on expiration (p<0.0001), and from 13.3 to 8.13 mm on inspiration (p<0.0001) (20) . These findings demonstrate an effective role for IVC sonography in the detection of mild changes in volume state. It is noteworthy, however, that echocardiographic assessment is of great utility in the evaluation of vascular fluid content in patients with AHF, but cannot provide reliable information on TBW content.
Thoracic ultrasound
Thoracic ultrasound (TUS) is a relatively new imaging technique used for identifying interstitial and/or alveolar edema in volume overloaded patients, especially those with CHF ( Figure 1 ). TUS depends on the identification of sonographic artifact called “B-lines” or lung comets. These findings were first described in 1997 by Lichtenstein and colleagues (21) . Liteplo et al. (22) found similar results in a study of 100 patients in the Emergency Thoracic Ultrasound in the Differentiation of the Etiology of Shortness of Breath Study (ETUDES), where it was shown that a positive TUS displayed a likelihood ratio for the diagnosis of CHF of 3.88 (99% CI 1.55–9.73), whereas a negative TUS had a negative likelihood ratio of 0.5 (99% CI 0.30–0.82). These results suggest that TUS alone is effective for diagnosing volume overload secondary to CHF in patients presenting to the ED with shortness of breath.
Isotopic tracers of water – the gold standard for TBW measurement
Isotopic tracers of water represent the “gold standard” for TBW measurement (23, 24). These radioisotope assays utilize structural similarity between radioisotope (D 2 O, 3 H 2 O, H 2 18 O) and water to estimate the TBW after sufficient time for balance. After equilibration, samples are collected and radioisotopes are measured by mass spectrometry. TBW estimation can then be accurately performed based on the atom percentage of the radioisotope compared with water in the sample (25) . Although the test is very effective for assessing the fluid volume in a patient with abnormal homeostasis, it is unfortunately impractical given the time (often more than 6 h) required for equilibration (26, 27). Therefore, accurate, and even more importantly, practical measures of hydration status are still necessary for the evaluation of patients in a timely manner.
Laboratory biomarkers
The Biomarkers Definitions Working Group of the National Institute of Health (NIH) has reliably defined a biological marker, better known as “biomarker”, as a “… characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention ” (28) . According to this definition, biomarkers are now used in a kaleidoscope of clinical conditions in the ED, including diagnosis (29) and prognostic assessment (30) of acute myocardial infarction, diagnosis of acute renal injury (31) , acute pancreatitis (32) , pre-eclampsia (33) , stroke (34) , CHF assessment (35) and AHF ( Table 1 ).
Summary of the main utility biomarkers in HF.
| Biomarkers | Diagnosis and physiopathology | Adverse prognosis |
|---|---|---|
| Serum sodium | (n.v.: 136–145 mmol/L) AHF- RAA activation | <120 mmol/L |
| Serum creatinine | (n.v.: 0.5–1.5 mg/dL) Cardiorenal syndrome | >150 μmol/L >1.5 mg/dL |
| Serum uric acid | (n.v.: m 3.2–8.1 mg/100 mL; 2.2–7.1 mg/100 mL) AHF- oxidative stress | >9.8 mg/dL |
| NPs (blood and serum): • BNP • NT-proBNP • MR-proANP | v.n.: HF cut-off: <100 pg/mL; >400 pg/mL <400 pg/mL; >2000 pg/mL <120 pmol/L; >120 pmol/L AHF: left ventricle volume and pressure overload (heart muscle stretching) | >1000 pg/mL >5000 pg/mL – |
| ST2 (serum) | (n.v.: 1.75–34.3 U/mL) AHF-overload | >10 ng/mL |
| PCT (serum) | (cut-off: 0.05 ng/mL) Infection in AHF | 0.05–0.5 ng/mL local infection 0.5–2 ng/mL systemic infection 2–10 ng/mL SIRS – sepsis >10 ng/mL sepsis |
| NGAL (serum and urine) | (cut-off: 150 ng/mL serum ; 130 ng/mL urine ) Cardiorenal syndrome | >100 ng/mL |
| Copeptin (serum) | (median 3.7 pmol/L ) AHF-vascular alterations | >54.2 pmol/L |
| MR-proADM (serum) | AHF-vascular alterations | >2.15 nmol/L |
| ADMA (serum) | AHF-oxidative stress |
Serum blood indices are commonly used for assessment of hydration status abnormalities in AHF patients. Changes in serum sodium concentrations and their associations with diseases including abnormal fluid homeostasis are accurate tools for assessing hydration status. Decreased serum osmolarity is a common finding in patients with CHF. In these patients, the heart becomes unable to pump blood forward effectively, thus leading to activation of neurohormonal mechanisms that result in increased serum renin activity and downstream aldosterone secretion (36) . The resultant effect is that these patients develop hyponatremia secondary to unequal and pathologic retention of water compared to salt. Hyponatremia can also be used as a metric for disease status. A study of 66 chronic CHF patients with hyponatremia showed that those with neurohormonal hyperactivation required more diuretic (i.e, furosemide) than those without the electrolyte abnormality in order to recompensate (37) . Severe hyponatremia is also associated with worse prognosis in HF patients, and this parameter is currently included in the guidelines as an indicator of high mortality outcome for these patients (38) .
Changes in hematocrit may also be effective measures of hydration status, especially elevations with decreased blood volume. Although postural variations can affect the hematocrit value, changes in hematocrit are expected in the context of net water gain or loss (39) . However, mild to moderate decreases in the water volume are not easily detected by blood indices (40) .
Uric acid levels >9.8 mg/dL (>585 μmol/L) have been associated with a worse outcome in HF patients. Hyperuricemia reflects increased activity of the xanthine oxidase pathway that causes oxidative stress and impair nitric oxide (NO) production, thus worsening cardiovascular condition (41) .
The accuracy of brain natriuretic peptide (BNP) in the diagnosis, monitoring, and prognostic stratification of AHF has been unquestionably established in a variety of international trials (42, 43). BNP is the active hormone, composed of 32 amino acids, while N-Terminal pro-brain natriuretic peptide (NT-Pro BNP) is the inactive form, composed of 76 amino acids. They are both produced by the heart in response to volume and pressure overload, and their increase is proportional to systolic and diastolic dysfunction. In patients with acute dyspnea the cut-off value for BNP (1-32) are traditionally established at <100 pg/mL (NT-Pro BNP <400 pg/mL) to rule out HF, and >400 pg/mL (NT-Pro BNP>2000 pg/mL) to confirm HF. Values between 100 and 400 pg/mL (between 400 and 2000 pg/mL for NT-Pro BNP) are considered in the so-called “grey zone”, and require further scrutiny (42) . Limitations in using these NPs emerge in certain conditions including renal dysfunction, obesity and atrial fibrillation. Pro-B-type natriuretic peptide 1-108 (proBNP 1-108) is the 108-amino acid prohormone that is cleaved to the 32-amino acids, biologically active brain natriuretic peptide (BNP 1-32), also known as B-type natriuretic peptide, and to the 76-amino acids, biologically inactive N-terminal pro-B-type natriuretic peptide (NTproBNP1-76). Recently, it has been shown that ProBNP (1-108) circulates in the majority of healthy humans in the general population and is a sensitive and specific biomarker for the detection of systolic dysfunction. The proBNP (1-108) to NT-proBNP (1-76) ratio may provide insights into altered proBNP (1-108) processing during HF progression, providing important new insights into the biology of the BNP system (44, 45).
The application of NPs in everyday practice carries, however, undisputed advantages for improving the clinical management of patients with AHF, in that they are useful aids for stratifying the risk in the ED, predicting death and rehospitalization, and guiding therapy (46, 47).
The role of mid-region pro-atrial natriuretic peptide (MR-proANP) has also been investigated. In the diagnosis of AHF a cut-off of 120 pmol/L has been proven to be as accurate as BNP (1-32) (negligible accuracy difference of 0.9%). MR-proANP is considered particularly useful not only in obese and intermediate BNP (1-32) levels patients (48) but also in patients with impaired renal function as compared with BNP (1-32) and NT-ProBNP (1-76) (49) .
Recently, in addition to NPs, other interesting and promising biomarkers have proven useful in the management of AHF, and a multimarker panel approach has been suggested to detect different causes of acute dyspnea (50) .
Latest results from the biomarkers in acute heart failure (BACH) trial support the role of several innovative biomarkers, such as procalcitonin (PCT). PCT has been investigated for the diagnosis of pneumonia in patients with AHF. Elevated PCT (>0.21 ng/mL) is associated with a worse outcome when antibiotic therapy is not established (p=0.046), while a low PCT value (i.e., <0.05 ng/mL) is associated with a better outcome if not treated with antibiotics (p=0.049) (51) .
Another interesting result from the 15-center BACH trial defines the mid-region pro-adrenomedullin (MR-proADM), the precursor of the hypotensive adrenomedullin, an accurate 14-day mortality predictor. This is also confirmed by the area under the curve (AUC) in receiver operating characteristics (ROC) curve of MR-proADM (0.742) which is higher than that of BNP (0.484) and NT-proBNP (0.586) (52) . Another promising biomarker is copeptin, the C-terminal part of the vasopressin pro-hormone. It is an independent predictor for short-term (30 days) mortality, especially in patients with AHF (p<0.0001). The prognostic value of copeptin (>54.2 pmol/L) has been evaluated alone and in association with NT-proBNP and BNP, and their AUC were 0.83, 0.76 and 0.63, respectively (53) . Finally, a subanalysis of the BACH trial confirmed that MR-proADM and copeptin in combination have the best 14-day mortality prediction (AUC=0.818), compared with all other markers (52) .
A new and still not adequately investigated biomarker is soluble ST2, which is involved in cardiac remodelling and overload (54) . An increase in ST2 above 10 ng/mL is considered a predictor of mortality in patients with AHF (p<0.001) (55) .
Due to the known, complex and multifaceted interplay between heart and kidneys, AHF is often complicated by acute kidney injury (AKI) in critical patients. This circumstance defines a high mortality condition, called cardiorenal syndrome (56) . Classically, an increase of serum creatinine (>150 μmol/L) (16) is used to define renal injury, but it is only marginally associated with the outcome (AUC of 0.57) (57) . Neutrophil gelatinase-associated lipocalin (NGAL) is a new biomarker that might help risk stratification in AHF patients. Although not absolutely specific for AKI (it is also produced and released by neutrophils) (58) , it is still an early marker of AKI. A discharge value of NGAL >100 ng/mL, combined with BNP values or even measured alone, has been proven to be a powerful predictor of 30-day adverse outcomes (57) . Finally, a preliminary study has shown that increased plasma levels of asymmetric dimethylarginine (ADMA) are strong and independent predictor of short- and long-term mortality in AHF patients (NYHA Class III/IV) with reduced EF (59) .
Bio-impedance analysis (BIA)
Bio-electrical impedance is the term used to describe the response of a living organism to an externally applied electric current by surface electrodes. It measures the opposition to the flow of electric current throughout the tissues. Electric current is administered by surface electrodes that need high current (800 µA) and high voltage to decrease the instability related to cutaneous impedance (10,000 Ω/cm 2 ) (60) . This impedance value, termed electrical impedance (Z), consists of two components, resistance (R) and reactance (Xc). In terms of impedance, the human body can be schematically considered as a system composed of several different conductors in parallel, each of which opposes the passage of an alternating current, which passes through two pathways: extracellular tissue and intracellular membranes. Since extracellular (ECW) and intracellular water (ICW) compartments contain ions, they are electrically conductive. Thus, estimations of fluid volume can be based on their impedance to electrical flow as cell membranes may act as capacitors. The resistivity of ECW ionic composition is close to that of saline, but the ICW ionic composition depends on the type of cell and, as a result, resistivity cannot be measured directly. For technical reasons, impedance meters using surface electrodes are limited to a frequency range of 5–1000 kHz, and the ECW and the TBW) resistance must be calculated by extrapolation as proposed by the Cole-Cole model (61) .
In bio-impedance analysis (BIA) the angular component of the polar coordinate representation, called the phase angle (PA), is assessed. The principle of measurement is based upon the fact that the condensers in the alternating current circuit lead to a time delay Δt: the current maximum is in advance of the voltage maximum. PA represents the measurement of this time delay between the periodic signals of current and voltage, which vary sinusoidally at the same frequency. It is calculated from resistance and reactance according to the formula: PA=arc-tangent reactance/resistance×180°/π. The PA might be an indicator of cell membrane integrity as well as distribution of ICW and ECW, and it can also be used to assess total cell mass (62) . Cox multivariate models have also been used to establish that PA may be considered a significant and independent predictor of mortality in patients with liver cirrhosis (p<0.01) (63) .
However, it is important to consider that impedance measurements are rooted in an approximation of the human body as a sum of five interconnected cylinders (limbs and trunk). This approximation is calculated from the length and perimeters of the limbs and the trunk through a dimensionless shape factor (Kb) in the resistance–volume relationship for a single cylinder. A value of 4.3 for Kb has been established from statistical anatomical measurements in adults (64) . It is also noteworthy that there are several factors impacting BIA including height, weight, position of the body and limbs, intense physical activity before BIA measurements, infection, dermatological conditions, ambient temperature and non-adherence of electrodes (65–68).
In 1871, Thomasset et al. (69) performed the initial study assessing the electrical properties of tissues to measure impedance using two frequencies of 1 kHz and 100 kHz through two subcutaneously inserted needles. Using the Cole-Cole model, he could then estimate the ECW and TBW volumes. Since then, tetra-polar BIA has been widely investigated in medical research as a tool for assessing body composition. Its use has gradually expanded from estimation of body composition in healthy individuals to embrace the assessment of fluid volume and distribution in various patient populations.
Single-frequency whole-body BIA (SF-WBIA) and bio-electrical impedance spectroscopy (BIS)
The whole-body BIA (WBIA) uses four surface electrodes, two placed on the wrist and two on the ankle, at the distal metacarpals and metatarsals, respectively, with the subject supine. A low-level alternating current is administered at either single (50 kHz) or multiple frequencies (e.g., 1, 5, 50, 200, 500 and 2000 kHz), and then whole-body Z, R and Xc are measured. These variables are adjusted for height and then combined with various physical and demographic variables (body weight, age, gender, etc), into regression models to predict volume status.
In 1983, Nyboer et al. (70) used the four-surface electrode WBIA technique, initially presented by Hoffer in 1969, to assess body composition in 144 subjects. A significant correlation was observed between body fat mass (r=0.860), TBW (r=0.947) and lean body weight (r=0.934) with the whole-body impedance and the hydrostatic weighing, which is another method for measuring the body mass density. It is based on Archimedes’ principle and is performed during forced exhalation with residual lung volume (70) . By 1986, the WBIA was accurately described and validated by Kushner (71) , for assessing TBW by BIA and deuterium-isotope dilution in 58 subjects. He found a significant correlation (r=0.99) between BIA, weight and deuterium-dilution space.
In 1990, BIS was introduced by Xitron as a new method to measure both ECW and ICW volumes using Hanai’s mixture conductivity theory for concentrated disperse systems in evaluating dielectric dispersion due to interfacial polarization (72, 73).
The BIS device, which is able to assess TBW and differentiate between ECW and ICW (TBW-ICW), uses low and high frequencies of 5 kHz–2 MHz and extrapolates resistance values of extracellular and intracellular fluids, respectively, with the Cole-Cole model. From these resistance values, extra- and intra-cellular resistivities are derived with the Hanai model.
There is now consolidated evidence that the whole-body technique, as compared with reference methods, can lead to inaccurate assessment of body composition in some circumstances. In 54 patients with end stage renal disease, TBW measurements derived by deuterium dilution and WBIA showed no significant difference (mean difference=–1.221 L, p=0.12) between the two methods in estimating fluid status. Unfortunately, WBIA lacked consistency across all patient populations and significantly overestimated fluid status in obese patients (mean=–6.789 L, p=0.001) (74) . In 2003, Sun et al. developed a gender-specific, age- and race-combined equation with the use of a multi-component model including densitometry, isotope dilution and dual-X-ray absorptiometry (DXA). A sample of 1829 patients aged from 12 to 94 years old were studied and their body composition was assessed by SF-WBIA. The final equation, validated by prediction of sum of squares (PRESS) statistics, showed the utility of WBIA in epidemiologic studies (75) .
Segmental BIA (SBIA)
Segmental BIA (SBIA) has been developed as a practical alternative to WBIA. It is a four-electrode bio-impedance device with contact (tactile) electrodes that measure impedance in the upper (arm-to-arm) or the lower (leg-to-leg) body of a standing subject (76–79). The main assumptions of this measurement technique are that conductor volume is equally distributed in the upper and lower body, segmental impedance values are proportional to whole-body impedance and whole-body resistance can be derived by the sum of segmental resistances (80, 81). Interestingly, the segmental impedance technique may be used for personal monitoring of body composition because it does not require the presence of an operator. Another type of segmental bio-electrical impedance uses eight electrodes, four of which are placed in the handles of the machine (in contact with the thumbs and palms) and the other four in the foot scale pads, in contact with the balls of feet and heels. These machines operate at either single or multiple frequencies while the subject stands. A recent study on 112 AHF patients used a segmental multi-frequency bio-electrical analysis to estimate the 6-month prognostic value of pre-discharge edema index, a derived surrogate for hydration status, and established that an edema index >0.390 (i.e., the cut-off value) represents a significant HF-related predictor of re-hospitalization (p=0.04) (82) .
WBIA vs. SBIA
The clinical application of segmental BIA was assessed for determining fluid accumulation in 30 patients undergoing abdominal surgery. ECW distribution changes (ΔECW) were monitored preoperatively (before the induction of anesthesia) and postoperatively (after recovery from anesthesia). The ΔECW was estimated by the multi-frequency whole-body device and as a sum of five body segments. The most significant resistance decrease was found in the trunk where the fluid composition contributes minimally to the whole body resistance. It was thus concluded that segmental multi-frequency bio-electrical impedance analysis provides ΔECW assessment better then the whole-body technique in patients with non-homogeneous fluid distribution (83) . Although BIA cannot be considered as an individual reference tool, newer studies have warranted its broader application in combination with other techniques.
Bio-electrical impedance vector analysis (BIVA)
BIVA can be considered an integrated component of BIA measurement, and is a simple and quick method for assessing fluid status and body cell mass (84) . It can also be used as a quality control measure for correct analysis of BIA results (85) .
BIVA is a non-invasive technique to estimate body composition by bio-electrical impedance measurements, R, Xc and Z (86, 87). All biological structures have a specific resistance, defined as the strength of opposition by a tissue to the electric current flow (7) . Fat-free tissues and fluids are good conductors because they offer a low resistance to the electric current flow, while bone and fat tissues are bad conductors because they are electrically resistant. Therefore, the resistance is inversely related with the TBW, thus representing an indirect measure of the amount of body fluid. The measured reactance is dependent upon the presence of inductors and/or electrolytic capacitors. Since all cell membranes act like a small capacitor, reactance can be considered as an indirect measure of cell membrane activity and integrity, and is proportional to body mass (66, 88).
The BIVA technique was developed at the University of Padua (Italy) (7) . The machine uses an alternating current flux of 800 μA and an operating frequency of 50 kHz. The results are visualized in two ways, as a vector or as a BIVA-derived hydration percentage. The first method includes a direct impedance plot which measures R and Xc, as a bi-variate vector in a normogram (85) . Reference values adjusted for age, body mass index (BMI), and gender are plotted as so-called tolerance ellipses in the same coordinate system. Three tolerance ellipses are distinguished, corresponding to the 50th, 75th and 95th vector percentile of the healthy reference population (86) . The major axis of this ellipse indexes hydration status and the minor axis reflects tissue mass. The second method involves a scale called the Hydrograph (or Hydrogram), which expresses the state of hydration as a percentage. This value is calculated by an independently determined equation that uses the two components of BIVA, R and Xc. A normal value is 73.3% with tolerance between 72.7% and 74.3%, corresponding to the 50th percentile (7) ( Figure 1 ).
Regarding interpretation of values, the length of Z vector is inversely related to fluid volume, whereas the PA offers insight into the relative distribution of fluids. A fundamental outcome of several studies is the delineation of the 75% tolerance ellipse as the indicator of the boundary of normal tissue hydration. Vectors outside the upper pole of the 75% ellipse indicate dehydration, whereas others outside the 75% confidence ellipse of the lower pole are characteristic of fluid overload or overhydration. Thus, short vectors with a small PA are associated with edema, whereas longer ones with an increased PA indicate dehydration (86, 89, 90). Moreover, vectors above or below the minor axis (meaning upper-left or lower right half of ellipses) are associated with more or less cell mass in soft tissue, respectively, with extremes along the minor axis. As previously discussed, the normal BIVA-derived hydration values for the hydrograph are comprised between 72.7% and 74.3%, values above or below such relative thresholds indicate a state of hyperhydration (wet) and dehydration (dry) (91) . These two classes can be further subdivided into mild, moderate or severe volume abnormalities (92) .
The BIVA technique is very handy and can be used at the bedside, with the patient supine with inferior limbs at 45° and superior limbs abducted at 30° to avoid skin contacts with the trunk (66) . Four skin electrodes are applied, two on the wrist and two on the ipsilateral ankle. A minimal inter-electrode distance of 5 cm has been recommended to prevent interaction between electrodes. The subject is laid recumbent on a non-conductive surface. Free fluid in thorax and abdomen (lung congestion, pleural effusion, ascites, urine, food) does not affect the impedance values measured by this technique (6) .
Despite being relatively new, BIVA is becoming recognized as a superior method for TBW content assessment (93) . Clinical studies have been carried out in hospitalized patients with severe renal diseases to assess the utility of BIVA in assessing volume status. Studies involving uremic patients, compared to healthy controls, showed significantly shorter vectors with smaller PAs. As discussed above, shorter vectors (low impedance) are associated with hyperhydration. These vectors then lengthened after dialysis, showing the ability of BIVA to detect significant changes of fluid status after dialysis. Changes in the volume of fluid removed significantly correlated with changes in vector components (p<0.001 in men, p=0.03 in women). Vectors of unstable (e.g., adverse outcomes) compared to stable hemodialysis patients were significantly different. It was also found that vectors of unstable patients were longer with smaller PAs, and these differences persisted after hemodialysis (89) . Similar findings were found in patients treated with peritoneal dialysis, before and after fluid removal (86) .
New application fields are emerging in critical care. In intensive care unit patients, the central venous pressure (CVP) was correlated with impedance measured by BIVA. CVP values are significantly and inversely correlated with individual impedance vector components (r 2 =0.28 and 0.27 with resistance and reactance, respectively), and with both vector components together (r 2 =0.31). Specifically, CVP values>12 mm Hg were associated with shorter impedance vectors (outside the lower pole of the 75% reference ellipse) in 93% of patients, thus indicating fluid overload. Conversely, CVP values <3 mm Hg were associated with long impedance vectors (outside the upper pole of the 75% reference ellipse) in only 10% of patients, indicating tissue dehydration. The progressive increase of CVP values was associated with shorter and down-sloping impedance vectors on the R-Xc graph (94) .
The role of BIVA coupled with TUS has shown its effectiveness in discriminating cardiac and non-cardiac acute dyspnea patients presenting in ED (69% sensitivity, 79% specificity) (95) .
Future directions in managing AHF patients using biomarkers plus BIVA
The BIVA evaluation is an appealing perspective when applied in patients in the acute setting with congestive HF because of specific fluid overload. A study by Di Somma et al. (6) , shows that BIVA data in decompensated AHF patients at admission to the ED are statistically different as compared with controls (p<0.0007). AHF patients had a significantly higher value of hydration status (77±4) as compared with controls (73±2). Sequential BIVA measurements in AHF patients showed reduction of congestion due to diuretic treatment. A significant correlation with events (death or re-hospitalization) at 3 months was also observed in patients with average hydration values >80%. It was also demonstrated that combined use of BIVA and BNP may improve the management of AHF patients in ED when compared to BNP alone, thus allowing a faster and much more accurate triage. BIVA helped distinguish cardiogenic dyspnea from non-cardiogenic causes and – in combination with BNP – was also useful for management of AHF patients, since both measures were helpful to guide emergency physician’s decisions about diuretic therapy (e.g., preventing overuse).
Valle et al. (91) found that the combination of BNP and BIVA measurements could prevent unnecessary aggressive diuretic therapy, thereby reducing the level of renal complications. BIVA-BNP guided management during hospitalization for HF was associated with lower events after discharge, independent of other prognostic variables (91) .
The evaluation of total body fluid has a great utility also in patients with cardiorenal syndrome, and its use in combination with other parameters (e.g., in a multimarker approach) has been proposed as a new model of management of ED patients with cardiorenal syndrome (96) .
According to the large number of studies available in the scientific literature, the role of impedance is becoming more and more predominant for the assessment of hydration status. Characteristics, such as quick and simple use, non-invasiveness and low cost would make this device appealing and potentially useful in a kaleidoscope of medical fields. BIVA seems to be more accurate and more reliable when compared with other impedance techniques ( Figure 1 ). Further trials with larger patient populations are obviously needed to express a definitive consensus on the clinical effectiveness of BIVA, as well as for identifying those clinical settings where it can be more advantageous.
The use of BIVA in guiding the treatment of various disease states has not been adequately studied. It would be interesting to standardize BIVA employment in AHF patients, in combination with biomarkers, to guide a proper and correct diuretic therapy. Preliminary data from studies that have monitored the variations of BIVA values in volume overloaded patients has already shown its utility in guiding diuretic treatment, but more clinical evidence is necessary to create BIVA/diuretic guidelines. Another promising application is related to the emerging role of ultrafiltration in unloading CHF patients that do not benefit from diuretic therapy. Regular use of BIVA plus NPs might assist physicians to decide the amount of water to remove, thus avoiding harmful consequences. Due to its quick and easy use, BIVA should also be tested for its efficacy in primary care setting as a means for monitoring congested patients. This application could help primary care physicians in the management of these patients, since exacerbations may be detected sooner, avoiding severe worsening that may require hospitalization.
BIVA alone is probably not the definitive answer to all challenging questions about hydration status, but it seems to be a useful and promising device for everyday use, especially when coupled with biomarker measurement, because it is accurate, non-invasive, cheap and easy to use in combination with other techniques.
Conflict of interest statement
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
About the authors

1. Di Somma S, Lukaski HC, Codognotto M, Peacock WF, Fiorini F, Aspromonte N, et al. Consensus paper on the use of BIVA (bioelectrical impedance vector analisis) in medicine for the management of body hydration. Emerg Care J 2011;4:6–14. 10.4081/ecj.2011.4.6 Search in Google Scholar
2. Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 2003;17:472–503. 10.1016/S1521-690X(03)00049-6 Search in Google Scholar
3. Peacock FW, Karina M, Soto MS. Current technique of fluid status assessment. Congest Heart Fail 2010;16:45–51. 10.1111/j.1751-7133.2010.00166.x Search in Google Scholar
4. Di Somma S, Gori CS, Grandi T, Risicato M, Salvatori E. Fluid assessment and management in emergency department. Contrib Nephrol 2010;164:227–36. 10.1159/000313734 Search in Google Scholar
5. Clerico A, Vittorini S, Passino C. Measurement of the pro- hormone of brain type natriuretic peptide (proBNP): methodological considerations and pathophysiological relevance. Clin Chem Lab Med 2011;49:1949–54. 10.1515/CCLM.2011.686 Search in Google Scholar
6. Di Somma S, De Berardinis B, Bongiovanni C, Marino R, Ferri E, Alfei B. Use of BNP and bioimpedance to drive therapy in heart failure patients. Congest Heart Fail 2010;(Suppl 1):S56–61. 10.1111/j.1751-7133.2010.00162.x Search in Google Scholar
7. Marino R, Magrini L, Ferri E, Gagliano G, Di Somma S. B-Type natriuretic peptide and non-invasive haemodynamics and hydration assessment in the management of patients with acute heart failure in emergency department. High Blood Press Cardiovasc Prev 2010;17:219–25. 10.2165/11587990-000000000-00000 Search in Google Scholar
8. Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovasculer examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distention. J Am Coll Cardiol 1993;22:968–74. 10.1016/0735-1097(93)90405-P Search in Google Scholar
9. Wang CS, Fitzgerald JM, Shulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? J Am Med Assoc 2005;294:1944–56. 10.1001/jama.294.15.1944 Search in Google Scholar
10. Chakko S, Woska D, Martinez H, De Marchena E, Futterman L, Kessler KM, et al. Clinical, radiographic, and haemodynamic correlations in chronic congestive heart failure. Am J Med 1991;90:353–9. 10.1016/0002-9343(91)90576-J Search in Google Scholar
11. Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance ef elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med 2001;345: 574–81. 10.1056/NEJMoa010641 Search in Google Scholar
12. Tsuyuki RT, McKelvie RS, Arnold JM, Avezum AJ, Barretto AC, Carvalho AC, et al. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med 2001;161:2337–42. 10.1001/archinte.161.19.2337 Search in Google Scholar
13. Marantz PR, Kaplan MC, Alderman MH. Clinical diagnosis of congestive heart failure in patients with acute dyspnea. Chest 1990;97:776–81. 10.1378/chest.97.4.776 Search in Google Scholar
14. Stevenson LW, Perloff JK. The limited reliability of physical sign for estimating hemodynamics in chronic heart failure. J Am Med Assoc 1989;261:884–8. 10.1001/jama.1989.03420060100040 Search in Google Scholar
15. Gao N, Kwan BC, Chow KM, Chung KY, Leung CB, Li PK, et al. Measurements on the routine chest radiograph as prognostic markers in Chinese peritoneal dialysis patients. Clin Nephrol 2011;76:16–22. 10.5414/CN106896 Search in Google Scholar
16. Collins SP, Lindsell CJ, Storrow AB, Abraham WT. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med 2006;47:13–8. 10.1016/j.annemergmed.2005.04.003 Search in Google Scholar
17. Dickstein K, Choen-Solal A, Philippatos G, McMurray J, Ponikowski P, Poole-Wilson PA. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur J Heart Fail 2008;10:933–89. 10.1016/j.ejheart.2008.08.005 Search in Google Scholar
18. Kirkpatrick JN, Vannan MA, Narula J, Lang RM. Echocardigraphy in heart failure: applications, utility and new horizons. J Am Coll Cardiol 2007;50:381–96. 10.1016/j.jacc.2007.03.048 Search in Google Scholar
19. Katzarski KS, Nisell J, Randmaa I, Danielsson A, Freyschuss U, Bergström J. A critical evaluation of ultrasound measurement of inferior vena cava diameter in assessing dry weight in normotensive and hypertensive hemodialysis patients. Am J Kidney Dis 1997;30:459–65. 10.1016/S0272-6386(97)90302-4 Search in Google Scholar
20. Lyon M, Blaivas M, Brannam L. Sonographic measurement of inferior vena cava as a marker of blood loss. Am J Emerg Med 2005;23:45–50. 10.1016/j.ajem.2004.01.004 Search in Google Scholar PubMed
21. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 1997;156:1640–6. 10.1164/ajrccm.156.5.96-07096 Search in Google Scholar PubMed
22. Liteplo AS, Marill KA, Villen T, Miller RM, Murray AF, Croft PE, et al. Emergency thoracic ultrasound in the differentiaion of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal Pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med 2009;16:201–10. 10.1111/j.1553-2712.2008.00347.x Search in Google Scholar PubMed
23. Ritz P. Bioelectrical impedance analysis estimation of water compartments in elderly disease patients: the source study. J Gerontol A Biol Sci Med Sci 2001;56:M344–8. 10.1093/gerona/56.6.M344 Search in Google Scholar PubMed
24. Cheuvront S. Hydration assessment of athletes. Sports Sci Exchange 2005;18. Search in Google Scholar
25. Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 1980;33:2686–93. 10.1093/ajcn/33.12.2686 Search in Google Scholar PubMed
26. Armstrong LE. Assessing hydration status: the elusive gold standard. J Am Coll Nutr 2007;26:575S–84S. 10.1080/07315724.2007.10719661 Search in Google Scholar PubMed
27. Colley RC, Byrne NM, Hills AP. Implications of the variability in time to isotopic equilibrium in the deuterium dilution technique. Eur J Clin Nutr 2007;61:1250–5. 10.1038/sj.ejcn.1602653 Search in Google Scholar PubMed
28. Biomarkers-Definitions-Working-Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. 10.1067/mcp.2001.113989 Search in Google Scholar PubMed
29. Christ M, Bertsch T, Popp S, Bahrmann P, Heppner HJ, Müller C. High-sensitivity troponin assays in the evaluation of patients with acute chest pain in the emergency department. Clin Chem Lab Med 2011;49:1955–63. 10.1515/CCLM.2011.695 Search in Google Scholar PubMed
30. Lippi G, Plebani M, Di Somma S, Monzani V, Tubaro M, Volpe M. Considerations for early acute myocardial infarction rule-out for emergency department chest pain patients: the case of copeptin. Clin Chem Lab Med 2012;50:243–53. 10.1515/cclm-2011-0845 Search in Google Scholar PubMed
31. Soni SS, Pophale R, Ronco C. New biomarkers for acute renal injury. Clin Chem Lab Med 2011;49:1257–63. 10.1515/CCLM.2011.664 Search in Google Scholar PubMed
32. Lippi G, Valentino M, Cervellin G. Laboratory diagnosis of acute pancreatitis: in search of the Holy Grail. Crit Rev Clin Lab Sci 2012;49:18–31. 10.3109/10408363.2012.658354 Search in Google Scholar PubMed
33. Can M, Sancar E, Harma M, Guven B, Mungan G, Acikgoz S. Inflammatory markers in preeclamptic patients. Clin Chem Lab Med 2011;49:1469–72. 10.1515/CCLM.2011.232 Search in Google Scholar PubMed
34. Cho SY, Park TS, Lee HJ, Lee WI, Suh JT. Functional assay or antigen test for protein C and protein S in ischemic stroke: which shows the greatest change? Clin Chem Lab Med 2011;49:1919–20. 10.1515/cclm.2011.660 Search in Google Scholar PubMed
35. Jungbauer C, Riedlinger J, Buchner S, Birner C, Resch M, Lubnow M. High-sensitive troponin T in chronic heart failure correlates with severity of symptoms, left ventricular dysfunction and prognosis independently from N-terminal pro-B-type natrinatriuretic peptide. Clin Chem Lab Med 2011;49:1899–906. 10.1515/cclm.2011.251 Search in Google Scholar
36. Lilly LS, Dzau VJ, Williams GH, Rydstedt L, Hollenberg NK. Hyponatremia in congestive heart failure: implication for neurohumoral activation and responses to orthostasis. J Clin Endocrinol Metab 1984;59:924–30. 10.1210/jcem-59-5-924 Search in Google Scholar PubMed
37. Mettauer B, Rouleau JL, Bichet D, Juneau C, Kortas C, Barjon JN, et al. Sodium and water excretion abnomalities in congestive heart failure. Ann Intern Med 1986;105:161–7. 10.7326/0003-4819-105-2-161 Search in Google Scholar PubMed
38. Packer M, Lee WH, Kessler PD, Gottlieb SS, Bernstein JL, Kukin ML. Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation 1987;75:IV80–92. Search in Google Scholar
39. Harrison MH. Effects of thermal stress and exercise on blood volume in humans. Physiol Rev 1985;65:149–209. 10.1152/physrev.1985.65.1.149 Search in Google Scholar PubMed
40. Armstrong LE, Soto JA, Hacker FT, Casa DJ, Kavouras SA, Maresh CM. Urinary indices during dehydration, exercise and rehydration. Int J Sport Nutr 1998;8:345–55. 10.1123/ijsn.8.4.345 Search in Google Scholar PubMed
41. Hare JM, Johnson RJ. Uric acid predicts clinical outcomes in heart failure. Circulation 2003;107:1951–3. 10.1161/01.CIR.0000066420.36123.35 Search in Google Scholar PubMed
42. Maisel AS, Nakao K, Ponikowski P, Peacock WF, Yoshimura M, Suzuki T, et al. Japanese-Western consensus meeting on biomarkers. Int Heart J 2011;53:253–65. 10.1536/ihj.52.253 Search in Google Scholar PubMed
43. Brunetti M, Pregno S, Schünemann H, Plebani M, Trenti T. Economic evidence in decision-making process in laboratory medicine. Clin Chem Lab Med 2011;49:617–21. 10.1515/CCLM.2011.119 Search in Google Scholar PubMed
44. Macheret F, Boerrigter G, McKie P. Pro-B-type natriuretic peptide 1-108 circulates in the general community. Plasma determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 2011;57:1386–95. 10.1016/j.jacc.2011.01.005 Search in Google Scholar PubMed PubMed Central
45. Emdin M, Passino C, Clerico A. Natriuretic peptide assays revisited. Do we need pro-B-type natriuretic peptide? J Am Coll Cardiol 2011;57:1396–8. 10.1016/j.jacc.2010.09.075 Search in Google Scholar PubMed
46. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;18;50:2357–68. 10.1016/j.jacc.2007.09.021 Search in Google Scholar PubMed
47. Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 2008;10:824–9. 10.1016/j.ejheart.2008.07.014 Search in Google Scholar PubMed
48. Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (biomarkers in acute heart failure) trial. J Am Coll Cardiol 2010;55:2062–76. 10.1016/j.jacc.2010.02.025 Search in Google Scholar PubMed
49. Daniels LB, Clopton P, Potocki M, Mueller C, McCors J, Richhards M, et al. Influence of age, race, sex, and body mass index on interpretation of midregional pro atrial natriuretic peptide for the diagnosis of acute heart failure: results from the BACH multinational study. Eur J Heart Fail 2010;14:22–31. 10.1093/eurjhf/hfr157 Search in Google Scholar PubMed
50. Gori CS, Magrini L, Travaglino F, Di Somma S. Role of biomarkers in patients with dyspnea. Eur Rev Med Pharmacol Sci 2011;15:229–40. Search in Google Scholar
51. Maisel AS, Neath SX, Landsberg J, Mueller C, Nowak RM, Peacock WF, et al. Use of procalcitonin for the diagnosis of pneumonia in patients with a chief complaint of dyspnoea: results from the BACH (biomarkers in acute heart failure) trial. Eur J Heart Fail 2012;14:278–86. 10.1093/eurjhf/hfr177 Search in Google Scholar PubMed PubMed Central
52. Peacock WF, Nowak R, Christenson R, Di Somma S, Neath SX, Hartmann O, et al. Short-term mortality risk in emergency department acute heart failure. Acad Emerg Med 2011;18:947–58. 10.1111/j.1553-2712.2011.01150.x Search in Google Scholar PubMed
53. Potocki M, Breidthardt T, Mueller A, Reichlin T, Socrates T, Arenja N, et al. Copeptin and risk stratification in patients with acute dyspnea. Crit Care 2010;14:R213. 10.1186/cc9336 Search in Google Scholar PubMed PubMed Central
54. Alsous SJ, Richards AM, Troughton R, Than M. ST2 as diagnostic and prognostic utility for all cause mortality and heart failure patients presenting to emergency department with chest pain. J Cardiac Fail 2012;18:304–10. 10.1016/j.cardfail.2012.01.008 Search in Google Scholar PubMed
55. Pascual-Figal DA, Manzano-Fernández S, Boronat M, Casas T, Garrido IP, Bonaque JC, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail 2011;13:718–25. 10.1093/eurjhf/hfr047 Search in Google Scholar PubMed
56. Cruz DN, Fard A, Clementi A, Ronco C, Maisel A. Role of biomarkers in the diagnosis and management of cardio-renal syndromes. Semin Nephrol 2012;32:79–92. 10.1016/j.semnephrol.2011.11.011 Search in Google Scholar PubMed
57. Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, et al. Prognostic utility of plasma neutrophil gelatinaseassociated lipocalin in patients with acute heart failure: The NGAL evaluation along with B-type natriuretic peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail 2011;13:846–51. 10.1093/eurjhf/hfr087 Search in Google Scholar PubMed PubMed Central
58. Lippi G, Cervellin G. Neutrophil gelatinase-associated lipocalin: a more specific assay is needed for diagnosing renal injury. Clin Chim Acta 2012;413:1160–1. 10.1016/j.cca.2012.03.013 Search in Google Scholar PubMed
59. Zairis MN, Patsourakos NG, Tsiaousis GZ, Theodossis-Georgilas A, Melidonis A, Makrygiannis SS, et al. Plasma asymmetric dimethylarginine and mortality in patients with acute decompensation of chronic heart failure. Heart 2012;98:860–4. 10.1136/heartjnl-2011-301372 Search in Google Scholar PubMed
60. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis part I: review of principles and methods. Clin Nutr 2004;23:1226–43. 10.1016/j.clnu.2004.06.004 Search in Google Scholar PubMed
61. Cole KS. Membranes, ions and impulses: a chapter of classical biophysics. Berkeley, CA: University of California Press, 1972:1. Search in Google Scholar
62. Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr 2005;82:49–52. 10.1093/ajcn/82.1.49 Search in Google Scholar
63. Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol 2002;86:509–16. 10.1007/s00421-001-0570-4 Search in Google Scholar PubMed
64. Van Loan MD, Withers PO, Matthie JR, Mayclin PL. Use of bioimpedance spectroscopy to determine extracellular fluid, intracellular fluid, total body water and fat-free mass. Basic Life Sci 1993;60:67–70. 10.1007/978-1-4899-1268-8_13 Search in Google Scholar PubMed
65. Matthie R. Bioimpedence measurements of human body composition: critical analysis and outlook. Expert Rev Med Devices 2008;5:239–61. 10.1586/17434440.5.2.239 Search in Google Scholar PubMed
66. Kushner RF. Bioelectrical impedance analysis: a review of principles and applications. J Am Coll Nutr 1992;11:119–209. 10.1080/07315724.1992.12098245 Search in Google Scholar
67. Dehghar M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J 2008;7:26. 10.1186/1475-2891-7-26 Search in Google Scholar PubMed PubMed Central
68. Buchholz AC, Bartok C, Shoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract 2004;19:433–46. 10.1177/0115426504019005433 Search in Google Scholar PubMed
69. Thomasset A. Bio-electrical properties of tissue impedance measurements. Lyon Med 1963;209:1325–52. Search in Google Scholar
70. Nyboer J, Liedtke RJ, Reid KA, Gessert WA. Nontraumatic electrical detection of total body water and density in man. Med Jadertina 1983;15:381–4. Search in Google Scholar
71. Kushner RF, Schoeller DA. Estimation of total body water by bioelectrical impedance analysis. Am J Clin Nutr 1986;44:417–24. 10.1093/ajcn/44.3.417 Search in Google Scholar PubMed
72. Matthie JR, Withers PO, Van Loan MD, Mayclin PL. Development of commercial complex bio-impedance spectroscopic system for determining intracellular and extracellular water volumes. Proceedings of the 8th international conference on electrical bio-impedance 1992;203–5. Search in Google Scholar
73. Hanai T. Electrical properties of emulsions. In: Sherman PH, editor. Emulsion Science. London: Academic, 1968:354–477. Search in Google Scholar
74. Cooper BA, Aslani A, Ryan M. Comparing different methods of assessing body composition in end-stage renal failure. Kidney Int 2000;58:408–16. 10.1046/j.1523-1755.2000.00180.x Search in Google Scholar PubMed
75. Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, et al. Development of bioelectrical impedance prediction equations for body composition with the use of a multi-component model for use in epidemiologic surveys. Am J Clin Nutr 2003;77:331–40. 10.1093/ajcn/77.2.331 Search in Google Scholar PubMed
76. Nunez C, Gallagher D, Grammes J, Baumgartner RN, Ross R, Wang Z, et al. Bioimpedance analysis: potential for measuring lower limb skeletal muscle mass. J Parenter Enteral Nutr 1999;23:96–103. 10.1177/014860719902300296 Search in Google Scholar PubMed
77. Utter AC, Nieman DC, Ward AG, Butterworth DE. Use of the leg-to-leg bioelectrical impedance method in assessing body-composition change in obese women. Am J Clin Nutr 1999;69:603–7. 10.1093/ajcn/69.4.603 Search in Google Scholar PubMed
78. Mitayani M, Kanehisa H, Fukunaga T. Validity of bioelectrical impedance and ultrasonographic methods for estimating muscle volume of the upper arm. Eur J Appl Physiol 2000;82:391–6. 10.1007/s004210000213 Search in Google Scholar PubMed
79. Biggs J, Cha K, Horch K. Electrical resistivity of the upper arm and leg yields good estimates of whole body fat. Physiol Meas 2001;22:365–76. 10.1088/0967-3334/22/2/308 Search in Google Scholar PubMed
80. Baumgartner RN, Chumlea WC, Roche AF. Estimation of body composition from bioelectric impedance of body segment. Am J Clin Nutr 1989;50:221–6. 10.1093/ajcn/50.2.221 Search in Google Scholar PubMed
81. Lukaski HC, Scheltinga MR. Improved sensitivity of tetrapolar bioelectrical impedance estimates of fluid change and fat-free mass with proximal placements of electrodes. Age Nutr 1994;5:123–9. Search in Google Scholar
82. Liu MH, Wang CH, Huang YY, Tung TH, Lee CM, Yang NI, et al. Edema index-guided disease management improves 6-month outcomes of patients with acute heart failure. Int Heart J 2012;53:11–7. 10.1536/ihj.53.11 Search in Google Scholar PubMed
83. Tatara T, Tsuzaki K. Segmental bioelectrical impedance analysis improves the prediction for extracellular water volume changes during abdominal surgery. Crit Care Med 1998;26:470–6. 10.1097/00003246-199803000-00017 Search in Google Scholar PubMed
84. Walter-Kroger A, Kroker A, Mattiucci-Guehlke M, Glaab T. A practical guide to bioelectrical impedence analysis using the example of chronic obstructing pulmonary disease. Nutr J 2011;10:35. 10.1186/1475-2891-10-35 Search in Google Scholar PubMed PubMed Central
85. Bosy-Westphal A, Danielzyck S, Dorhofer RP, Piccoli A, Muller MJ. Pattern of bioelectrical impedance vector distribution by body mass index and age: implication for body-composition analysis. Am J Clin Nutr 2005;82:60–8. 10.1093/ajcn/82.1.60 Search in Google Scholar
86. Piccoli A. Bioeletric impedance vector distribution in peritoneal dialysis patient with different hydration status. Kidney Int 2004;65:1050–63. 10.1111/j.1523-1755.2004.00467.x Search in Google Scholar PubMed
87. Lukasky HC, Johnson PE, Bolonchuk WW. Assesment of fat-free mass using bioelectrical impedance measurements of human body. Am J Clin Nutr 1985;41:810–7. 10.1093/ajcn/41.4.810 Search in Google Scholar PubMed
88. Talluri T, Lietdke RJ, Evangelisti A, Talluri J, Maggia G. Fat-free mass qualitative assessment with bioelectrical impedance analysis (BIA). Ann N Y Acad Sci 1999;20:873–94. 10.1111/j.1749-6632.1999.tb09454.x Search in Google Scholar
89. Piccoli A. Identification of operational clues to dry weight prescription in hemodialysis using bioimpedance vector analysis. Kidney Int 1998;53:1036–43. 10.1111/j.1523-1755.1998.00843.x Search in Google Scholar PubMed
90. Bozzetto S, Piccoli A, Montini G. Bioelectrical impedence vector analysis to evaluate relative hydration status. Pediatr Nephrol 2010;25:329–34. 10.1007/s00467-009-1326-3 Search in Google Scholar PubMed
91. Valle R, Aspromonte N, Milani L, Peacock FW, Maisel AS, Santini M, et al. Optimizing fluid management in patient with acute decompensated heart failure (ADHF): the emerging role of combined measurement of body hydration status and brain natriuretic peptide (BNP) levels. Heart Fail Rev 2011;16:519–29. 10.1007/s10741-011-9244-4 Search in Google Scholar PubMed PubMed Central
92. Piccoli A, Rossi B, Pilon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedence analysis: the R/Xc graph. Kidney Int 1994;46:534–9. 10.1038/ki.1994.305 Search in Google Scholar PubMed
93. Tuy T, Peacok F. Noninvasive volume assessment in the emergency department: a look at B-type natriuretic peptide and bioimpedence vector analysis. Contrib Nephrol 2011;171: 187–93. 10.1159/000327205 Search in Google Scholar PubMed
94. Piccoli A, Pittoni G, Facco E. Relationship between central venous pressure and bioimpedance vector analysis in critically ill patients. Crit Care Med 2000;28:132–7. 10.1097/00003246-200001000-00022 Search in Google Scholar PubMed
95. Piccoli A, Codognotto M, Chanchi V, Vettore G, Zaninotto M, Plebani M, et al. Differentation of cardiac and non cardiac dyspnea using bio-electrical impedance vector analysis. J Card Fail 2012;18:226–32. 10.1016/j.cardfail.2011.11.001 Search in Google Scholar PubMed
96. Di Somma S, Gori CS, Salvatori E. How to manage cardiorenal syndrome in the emergency room. Contrib Nephrol 2010;165:93–100. 10.1159/000313748 Search in Google Scholar PubMed
©2012 by Walter de Gruyter Berlin Boston
- X / Twitter
Supplementary Materials
Please login or register with De Gruyter to order this product.
Journal and Issue
Articles in the same issue.

- People Directory
- Safety at UD

Category: Animal and Food Sciences
Graduate student Joe Patria applies his molecular biology expertise to combat the viral evolution of Marek’s disease
July 09, 2024 Written by Nya Wynn | Photos by Jeremy Wayman
When Joe Patria first came to UD for his Ph.D. in molecular biology, he never thought he would end up doing research involving chickens. But, when he came across Mark Parcells , professor of molecular virology, and his lab, Patria ended up doing just that.
His main interests were molecular and cell biology — how changes within them affect the overall physiology of an entire system.
“Those contributions of everything that goes on down on a molecular level,” said Patria, who was named a Hiram Lasher Fellow. “When I met Prof. Parcells and did a rotation in his lab, I saw how it all came together by studying viruses and pathogens and how they kind of affect those pathways to cause systemic disease.”

As a molecular biology student doing research in CANR, Patria researches the mechanisms behind Marek’s disease virus. For his work in the field, Patria won top research poster at a graduate symposium at the Wistar Institute this past February.
Marek’s disease virus primarily causes cancer in immune cells, which usually results in fatality in chickens. It is highly contagious and transmissive, so it poses a large threat to the poultry industry.
“With Marek’s, you put it into chickens, and if they're not vaccinated, like 95 percent of them will die of cancer within four to six weeks, so it’s an incredibly penetrant virus,” Parcells said. “Every chicken that is exposed to Marek’s in the field, carries that virus for their entire life, just like if you got chickenpox as a kid, you have that virus for your whole life.”
To combat this extremely infectious virus, scientists developed an effective vaccine to protect chickens against it; however, the virus has evolved.
“Over the course of several decades, Marek’s disease virus has shown to break through the protection afforded by those vaccines,” Patria said. “So that is the focus of my work, and studying how the virus actually overcomes the protection from these vaccines.”
As the viruses evolve to evade vaccines, they also evolve with their hosts and learn to target areas that would be most detrimental to the host, such as DNA repair and damage response.
“By studying how viruses evolve, to evade the host immune responses and overcome protection from vaccines, it gives us a better understanding of how we can develop improved vaccines to target those pathways that the viruses have evolved to be dependent on,” Patria said.

Not only can Patria’s work impact the poultry industry, there is also an application to human health. Patria explained that Marek’s disease virus causes cancer of the lymphocytes, which can be compared to Hodgkins and non-Hodgkin's lymphoma, a type of cancer that affects humans.
“There are a lot of parallels between chickens and humans, which you might not expect. On the basis of immunology, we are very similar,” Patria explained. “There’s some parallels there.”
According to Parcells, more than 95 percent of all humans are positive for Epstein Barr virus, another herpes virus like Marek’s disease virus.
“When that goes latent, which it does, a small percentage of people within 20 years develop Hodgkin's lymphoma or another type of cancer associated with that virus,” Parcells said. “It’s not a high frequency, and you can’t really study it in humans.”
This is where our similarities with chickens really come into play.
“Because Marek’s disease virus’ natural host is a chicken, we’re able to study it in its natural reservoir host, which is a unique thing that you can't really do with other viruses,” Patria said. “Especially for viruses that infect humans.”
“It's a way in which you can kind of study these different interactions with the virus in the context of something where it normally causes cancer,” Parcells added. “That may inform how things are working with the development in Hodgkin’s lymphoma.”
Patria continues to work alongside Parcells, other graduate students, and undergraduate students in the Parcells Lab to make more discoveries in the field of molecular biology and with Marek’s disease virus specifically.
In addition to his own development as a researcher, Patria values his opportunities to work with other graduate students and mentor UD undergraduates.
“I hold the worth of science advocacy in high regard,” Patria said. “It was great to see that their contribution to this work has made an impact.”
Related News
Ud’s non-thesis master’s in animal science prepares alena brown for a career in animal care, hands-on veterinary experience.
- Cooperative Extension
- Plant and Soil Sciences
- Entomology and Wildlife Ecology
- Animal and Food Sciences
- Undergraduate Students
- Applied Economics and Statistics
- Graduate Students
- CANR Facilities
- Risk Management
- Agriculture and Natural Resources
- Graduate Programs
- Undergraduate Majors
- Nutrition, Food Safety and Wellness
- Center for Experimental and Applied Economics
- UDairy Creamery
- Equine Science
- 4-H Youth Development
- Master Gardeners
- Pest Management
- Sustainable Production Systems
- Doug Tallamy
- Lawn and Garden
- Environmental Stewardship
- Health and Wellbeing
- Internships
- Rodrigo Vargas
- Nutrient Management
- Master Naturalist Program
- Master Wellness
- Carvel-Research
- Chris Williams
- Health Insurance
- UD Waterfowl
- UD Fresh to You
- Forest Fragments in Managed Ecosystems
- Rice Paddies
- Master Gardener Book Reviews
- MS Soil Science
- Greenhouse Gases
- Dining with Diabetes
- Delaware FitBiz
- Well Connected Community
- Spanish Language
- Financial Management
- Charles C. Allen Biotechnology Laboratory
- LEADelaware
College of Agriculture & Natural Resources
Additional Links
- Faculty & Staff Resources
531 South College Avenue Newark, DE 19716 (302) 831-2501

IMAGES
VIDEO
COMMENTS
Measurement and analysis of bio-impedance for non-invasive, continuous sensing of urinary bladder volume
In this chapter, a revision of the basic principles of bio-impedance and its different measurement techniques is presented. Also, different portable impedance analyzer designs from the literature and the market are discussed. Finally, some of the main bio-impedance applications are reviewed.
Determination of Bioimpedance Using Multi-Frequency Bio-electrical Impedance Analysis by Satyanarayana Shashank Brahmandlapalli A thesis submitted to the Faculty of Graduate and
Abstract: Bioimpedance analysis is a noninvasive, low cost and a commonly used approach for body composition measurements and assessment of clinical condition. There are a variety of methods applied for interpretation of measured bioimpedance data and a wide range of utilizations of bioimpedance in body composition estimation and evaluation of clinical status. This paper reviews the main ...
This extensive list of studies demonstrates the promise of bio-impedance measurement and analysis as an alternative approach to the time-tested visual-observation based analysis.
It provides fundamental knowledge in the field of bio-impedance, including basic concepts, measurement techniques and derived various applications for improving health care options for the wide community of human being. The thesis is organized into six chapters. Chapter One starts the thesis by introducing the concept of bio-impedance.
Bio-impedance measurement analysis primarily refers to a safe and a non-invasive technique to analyze the electrical changes in living tissues on the application of low-value alternating current. It finds applications both in the biomedical and the agricultural fields. This paper concisely reviews the origin and measurement approaches for concepts and fundamentals of bio-impedance followed by ...
This article describes the materials and methodology for an individual with little background in designing bio-impedance instrumentation to build and operate a sys-tem that measures tissue impedance, specifically in the brain.
A method for non-invasive, unobtrusive, artefact-free and con-tinuous bladder monitoring using bio-impedance measurements is presented in thesis. Bio-impedance is a measure of the body's ability to impede injected current due to its composition of tis-sue and fluid.
Bioimpedance analysis has found utility in many fields of medical research, yet instrumentation can be expensive and/or complicated to build. Advancements in electronic component design and equipment allow for simple bioimpedance analysis using equipment now commonly found in an engineering lab, combined with a few components exclusive to impedance analysis.
Figure 1: Bio-impedance model of a tissue This thesis work focuses on the development of an implantable ASIC from excitation source to the receiver and is capable of measuring both the real and imaginary components of the complex bio-impedance.
Bio-impedance spectroscopy has been increasingly used for medical and food industrial applications as it provides information about cellular structure, composition and integrity of cell membranes of biological samples.
The use of bioelectrical impedance analysis (BIA) is widespread both in healthy subjects and patients, but suffers from a lack of standardized method and quality control procedures.
Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of
Bioimpedance refers to the electrical properties of a biological tissue, measured when current flows through it. This impedance varies with frequency and different tissue types, and varies sensitively with the underlying histology. This appendix is a brief summary of its principles; we hope it will be useful for any non-technical readers new to EIT. The section is unreferenced; a suggested ...
PDF | On Jan 1, 2010, Alberto Olmo and others published Computer Simulation of Microelectrode based Bio-Impedance Measurements with COMSOL. | Find, read and cite all the research you need on ...
Alternatives to the traditional sine wave excitation are studied in the paper. Impedance measurements can be performed much faster by using a broad bandwidth signal for excitation. Using of square ...
Electrical bioimpedance entails the measurement of the electrical properties of tissues as a function of frequency. It is thus a spectroscopic technique. It has been applied in a plethora of biomedical applications for diagnostic and monitoring purposes. In this tutorial, the basics of electrical bioimpedance sensor design will be discussed. The electrode/electrolyte interface is thoroughly ...
Bio-impedance spectroscopy has been increasingly used for medical and food industrial applications as it provides information about cellular structure,
Bioimpedance analysis is a noninvasive, low cost and a commonly used approach for body composition measurements and assessment of clinical condition. There are a variety of methods applied for interpretation of measured bioimpedance data and a wide range of utilizations of bioimpedance in body composition estimation and evaluation of clinical status. This paper reviews the main concepts of ...
Although clinical history, physical examination and classical imaging techniques (e.g., standard radiography and echocardiography) still represent the cornerstones, novel and promising tools, such as biomarkers and bio-electrical impedance are achieving an emerging role in clinical practice for the assessment of total body fluid content.
This thesis introduces the concept of multilayer impedance pump, a novel pumping mechanism inspired from the embryonic heart structure. The multilayer impedance pump is a composite two-layer fluid-filled elastic tube featuring a thick, gelatin-like internal layer similar in nature to the embryonic cardiac jelly, and that is used to amplify ...
Finally, current trends on bioimpedance sensors are discussed followed by an overview of instrumentation methods for bioimpedance measurements, covering aspects of voltage signal excitations ...
Joe Patria, a molecular biology Ph.D. student at UD, unexpectedly pursued poultry research under Professor Mark Parcells, focusing on Marek's disease virus (MDV). He is exploring the molecular virology of MDV and its evolution to become more virulent over time. His work has implications for both poultry and human health, as the virus causes a lymphoma that is similar to some human lymphomas.