
- My Library Account
- Articles, Books & More
- Course Reserves
- Site Search
- Advanced Search
- Sac State Library
- Research Guides

APA Style Guide
- Numbers, Statistics, Mathematics, and Equations
- Student Paper Format
- In-Text Citation Style
- Paraphrasing and Quotations
- Tables and Figures
- Periodicals (Journals, Newspapers, Magazines, Blogs, Etc.)
- Reference Works (Dictionaries, Encyclopedias, Etc.)
- Webpages & Online Media
- Audiovisual Media
- Reports and Gray Literature
- Mental Measurements Yearbook
- Class Material
- Punctuation
- Avoiding Biased Language
- Numbers Expressed in Words
- Numbers Expressed in Numerals
- << Previous: Spelling
- Next: Lists >>
- Last Updated: Jan 17, 2024 10:39 AM
- URL: https://csus.libguides.com/APAstyle

- Walden University
- Faculty Portal
Other APA Guidelines: Numbers
Basics of numbers.
Per APA 7, Section 6.32, use numerals to express numbers 10 or above (e.g., 11, 23, 256). Per Section 6.33, write out numbers as words to express numbers up to nine (e.g., three, seven, eight).
Take the APA Style Diagnostic Quiz to test your knowledge.
Numbers Expressed as Words
Use words to express numbers in these situations:
Seventeen computer programmers went out to dinner last night
The principal presented awards to three fourths of the student body.
(This is a new rule in APA 7. APA 6 recommended using numerals in the abstract.)
Numbers Expressed as Numerals
Use numerals to express numbers in these situations:
She had been a nurse for 3 years.
Chapter 4 was considered required reading.
The student scored a perfect 7.
Each post was roughly 2.45 ft apart.
Teachers gave students ice cream if they scored in the top 5%.
You owe me $5.
Numbers Video
- APA Formatting & Style: Numbers (video transcript)
Related Resources
Knowledge Check: Numbers
Didn't find what you need? Email us at [email protected] .
- Previous Page: Latin Abbreviations
- Next Page: Serial Commas
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.
Free All-in-One Office Suite with PDF Editor
Edit Word, Excel, and PPT for FREE.
Read, edit, and convert PDFs with the powerful PDF toolkit.
Microsoft-like interface, easy to use.
Windows • MacOS • Linux • iOS • Android
Select areas that need to improve
- Didn't match my interface
- Too technical or incomprehensible
- Incorrect operation instructions
- Incomplete instructions on this function
Fields marked * are required please
Please leave your suggestions below
- Quick Tutorials
- Practical Skills
How to Write a Paper in APA Format | For Students
When I was a student, I was told to submit my essays in APA format. At the time, I had no idea what that even meant or how to do it. If this sounds familiar to you, don’t worry—I’ve been there, too. In this guide, I’ll show you the easiest way to understand APA format and a simple hack to help you comfortably write your essays and then format them in APA style.
When is APA format used?
APA format is commonly used in the social and behavioral sciences, such as psychology, sociology, anthropology, education, and economics, as well as in fields like business and nursing. This standardized format is adopted by professionals, researchers, and students to structure and present research papers, essays, and other academic documents.
It ensures consistency and clarity in communication within these disciplines by providing specific guidelines for nearly all aspects of manuscript formatting, from font choice to margins and punctuation. By adhering to paper APA format 7th edition style, writers in these fields can effectively share their findings and ideas in a clear and organized manner.
General Guidelines/ Rules of APA Formatting
Understanding the guidelines is key when learning how to write a paper in APA format for students. However, there's one important point that is often missed by many: the APA 7th edition now has different guidelines for students and professionals. So, if you notice a few extra details that might be missing in the guidelines below, it is because we have skipped the APA 7th edition guidelines for professionals to avoid any confusion. Let's review the guidelines:
General Formatting:
Margins: Set 1-inch margins on all sides.
Font: Use a readable font such as Times New Roman (12 pt.).
Line Spacing: Double-space throughout the document, including the title page, abstract, references, and any other sections.
Indentation: Indent the first line of each paragraph by 0.5 inches (use the tab key or the paragraph formatting function).
Alignment: Left-align all text except for headings, which follow specific formats.
Page Numbers: Include page numbers in the top right corner of every page, starting on the title page (which is considered page 1).
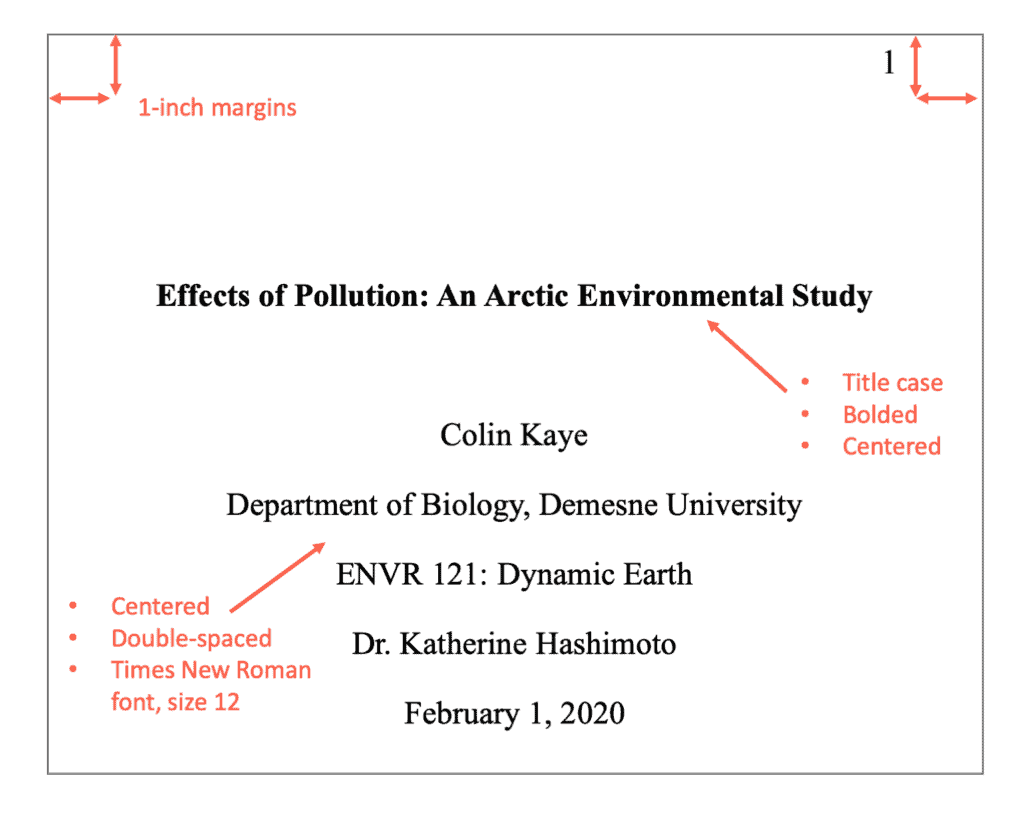
Title Page: Follow guidelines for the placement of the title, author information, affiliation (your school), course information, and instructor's name.
Abstract: Include a brief summary of your paper on a separate page after the title page.
Body Text: Write in clear and concise language, avoiding jargon. Use headings to organize your content.
In-text Citations: Cite your sources within the text using the author’s name and publication year in parentheses. There are specific formats for different types of sources.
Reference List: Start a new page for your references, listed alphabetically by the first author's last name. Follow specific formatting guidelines for different types of sources (books, articles, websites, etc.).
Here's what a title page of a reference paper template looks like in APA format:
How to Set up APA Format Paper [Step-by-Step]
After understanding the guidelines, the next step is to apply them effectively to format your paper in APA 7th edition style. To achieve this, we need an efficient writing tool that provides all the necessary formatting tools. Since we're just starting our journey to format essays in APA style, the tool should be easy to use. For these reasons, I'll be using a professional writing tool— WPS Office .
WPS Office not only provides all the necessary tools but also has a major benefit—it's completely free to use. I recommend downloading WPS Office on your system to ensure you can follow the steps smoothly. So, let's begin. I have an example paper that I will format in APA style using WPS Office.
1.Page Margins
Before you begin formatting your essay, let's set the page margins according to APA 7th edition guidelines, which require 1-inch margins on all sides.
Step 1: To set the page margins in WPS Writer, simply go to the Page Layout tab.
Step 2: In the Page Layout ribbon, locate the Margin fields on the left end of the ribbon.
Step 3: Here, set all margins—top, bottom, left, and right—to 1 inch.
Once you've adjusted the margins, we can proceed with formatting the rest of the document.
2.Font Settings and Line Spacing
Next, let's adjust the font and line spacing according to APA style requirements.
Step 1: Go to the Home tab in WPS Writer and change the font to “Times New Roman” in the “Fonts” field.
Step 2: To change the font size, enter "12" in the "Font size" field.
Step 3: For adjusting line spacing, simply click on the "Line spacing" icon in the Home ribbon and select "2.0" to apply double spacing in your essay.
Once we've completed setting the general formatting of our entire essay according to APA style, we now need to prepare the header.
Step 1: To set the header, double-click on the header area to enter the header in WPS Writer.
The header in APA style for students includes only the page number on the top right.
Step 2: To insert the page number, click on the "Page Number" button in the Header/Footer ribbon.
Step 3: From the Page Number drop-down menu, select the "Header right" option to insert the page number on the top right.
Step 4: Next, we need to set the header height to "0.5 in" in the "Header height" field.
4.Title Page
Sure! Let's start formatting each page of your essay, beginning with the title page. The title page should include the title of your paper, your name (as the author), the professor's name, course details, university name, and the due date. Each of these headings should start on a new line with 3-4 blank lines at the top of the page. This formatting ensures that your essay's title page follows APA style guidelines accurately.
Step 1: Press the "Enter" key on the keyboard to leave 3-4 blank lines at the top of the page.
Step 2: Type the title of your essay and center align it by clicking on the "Center" icon in the Home ribbon.
Step 3: Make the title bold by selecting the title text and clicking on the "Bold" icon in the Home ribbon.
Step 4: Press the "Enter" key twice to create a blank line between the title and the essay details. Then, enter the essay details in the following order, each on a separate line:
Your name (Author)
Department, University
Course Name, Course code
Professor's name
Step 5: After entering the essay details as described, ensure that each detail is centered on the page by selecting all the text with your mouse. Then, click on the "Center" icon in the Home ribbon to center-align the selected text.
Step 1: To insert a new blank page after the title page, place the cursor at the end of the due date on the title page and go to the Insert tab.
Step 2: In the Insert ribbon, click on "Breaks" and then select "Page Break" from the drop-down menu. This will create a new blank page where we will enter our abstract.
Step 3: Enter the heading "Abstract" in bold font style and center align it.
Step 4: Type the body of the abstract with no indentation. Simply start typing the abstract text.
After completing the abstract, insert another page break to start the next section of your essay.
6.Headings and Subheadings
To ensure your paper adheres to APA style guidelines for headings and paragraph indentation, here's how you can format them:
Step 1: On a new blank page, enter the Level 1 heading and ensure it is centered and in bold.
Step 2: For the body of the headings, indent the first line of each new paragraph by “0.5 in” by pressing the “Tab” key on your keyboard
Level 1 Heading: Centered and bold. It is used for main sections, like "Methods" or "Results".
Level 2 Heading: Left-aligned and bold. It is used to divide the main sections into subsections.
Level 3 Heading: Left-aligned, bold, and italicized. It further divides subsections into smaller parts.
Level 4 Heading: Indented, bold, and ends with a period. Text immediately follows this period, and it continues with lowercase text.
Level 5 Heading: Indented, bold, and in italics. Similar to Level 4, it also continues with lowercase text..
7.Table of contents
Essays can be lengthy, so including a table of contents can help make navigation easier. Let's take a look at how we can add a table of contents in WPS Writer.
Step 1: The Table of Contents is placed right after the title page, so the first step is to create a blank space after the title page using a Page Break.
Step 2: Now, on the blank page, go to the References tab and click on the Table of Contents button.
Step 3: From the Table of Contents drop-down menu, select any of the default templates available. I prefer using the 3rd template as it allows coverage of 3 levels of headings.
Step 4: Once the Table of Contents has been added, ensure that its heading is set to "Table of Contents", and it is formatted in bold and centered alignment.
Step 5: Additionally, ensure that the font settings of the Table of Contents are set to Times New Roman and 12-point font size.
8.Reference page
Before completing our essay, it's important to insert references that were helpful during the research process. For this, the end of your essay will include a separate References page.
Step 1: On a blank page at the end of your essay, enter the heading "References". Center align the heading and make it bold.
Step 2: List all the works cited in your essay. You can use the free Scribbr citation generator to generate APA 7th edition citations, which makes the process easier and ensures accuracy.
Step 3: Ensure the references are formatted with hanging indents using the Ruler in WPS Writer. To access the ruler, go to the View tab and check the "Ruler" checkbox in the ribbon.
Step 4: Drag the arrow on the ruler to half an inch to set the hanging indent .
Step 5: Then, drag the rectangle (below the ruler) back to 0 to reset the left indent for the subsequent lines of each reference.
And here is our APA 7th edition formatted essay from scratch. As you may have noticed, the whole process can be lengthy without an outline, but formatting your essay step by step makes the process clearer and easier to complete. I've used a few other writing tools for formatting, but I recommend WPS Writer because of its ease of navigation—all formatting tools are readily available in the tab, with no need to navigate through extra menus or open additional guides to learn additional steps. Try using WPS Writer for your essay assignments and experience the difference.
Bonus Tips: How to Convert Word to PDF without losing Format
WPS Office not only provides the necessary tools for students to efficiently format their essays according to APA 7th edition, but it also offers tools to easily convert these papers to PDF format within the WPS Writer application. Therefore, because submitting your work promptly is the next step after writing, ensure that your submission doesn't cost you any marks due to formatting issues after putting in so much effort.
To convert your essay documents to PDF using WPS PDF without quality loss, simply follow these steps:
Step 1: Open your document in WPS Writer.
Step 2: Click on the Menu button at the top left corner of the screen.
Step 3: Select "Export to PDF" from the menu that appears.
Step 4: Adjust any settings, such as the output path, in the Export to PDF window.
Step 5: After configuring the settings, click on "Export to PDF" to save your essay document as a PDF.
FAQs about writing a paper in APA format
1. how should i format tables and figures in apa style.
To correctly format a table in APA style, follow these guidelines:
Boldly label the table number above the table.
Provide a brief, italicized title in the title case just below the table number.
Avoid using vertical lines in the table design.
Use horizontal lines sparingly, only where necessary for clarity.
Ensure column and row headings are clearly labeled and concise.
Maintain consistent number formatting, such as decimal places.
Include any necessary notes below the table to explain details or sources.
To correctly format a figure in APA style, follow these guidelines:
Place the figure number in bold above the figure.
Provide a brief, italicized title in the title case beneath the figure number.
Include clear labels and legends within the image if needed.
Add any pertinent notes below the figure.
2. How to cite a Journal article in APA Style?
An APA Style citation for a journal article includes the author's name(s), the year of publication (in round brackets), the title of the article, the name of the journal in italics, the volume (in italics) and issue number, the page range of the article, and a DOI (if available).
APA format:
Author's last name, First name initial. (Year of publication). Title of article. Journal Title, Volume(Issue), Page range. DOI or URL
Johnson, M. (2023). Explore with us. Journal of random discoveries, 5(2), 123-135. https://doi.org/10.1234/jes.2023.5.2.123
3. How to cite a website in APA style?
APA website citations include the author's name, publication date, the title of the page or article in italics, the website name, and the URL. If no author is known, begin with the title of the article. If updates to the content are possible, include a retrieval date.
Author's Last Name, First initial. (Year, Month Date of publication). Title of the page. Name of the Website. URL
Johnson, M. (2024, March 12). Explore with us. Random Discoveries. https://www.randomdiscoveries.com/explore-with-us
Master APA Format Easily with WPS Office
I personally find APA format to be the most complex of all formats, but doing it on WPS Office sure makes it easy. The fact that it’s so user-friendly, with every feature readily available, is a huge advantage. The best part is that WPS Office is completely free. As a student, finding a good office suite that is cost-effective can be a challenge, and I’ve been there. Download WPS Office and spare yourself the hassle of hunting for an office suite— WPS Office is the answer to all your problems.
- 1. How to Write a Research Paper [Steps & Examples]
- 2. How to Find and Replace in Word for Your Paper? [For Students]
- 3. 10 PowerPoint presentation in APA Format example templates
- 4. 10 Top APA Format PowerPoint Presentation Example
- 5. APA Format in PowerPoint Presentation Example Templates
- 6. How to Create a Table of Contents in Word for Your Paper? [For Students]
15 years of office industry experience, tech lover and copywriter. Follow me for product reviews, comparisons, and recommendations for new apps and software.
- Free Tools for Students
- APA Citation Generator
Free APA Citation Generator
Generate citations in APA format quickly and automatically, with MyBib!

🤔 What is an APA Citation Generator?
An APA citation generator is a software tool that will automatically format academic citations in the American Psychological Association (APA) style.
It will usually request vital details about a source -- like the authors, title, and publish date -- and will output these details with the correct punctuation and layout required by the official APA style guide.
Formatted citations created by a generator can be copied into the bibliography of an academic paper as a way to give credit to the sources referenced in the main body of the paper.
👩🎓 Who uses an APA Citation Generator?
College-level and post-graduate students are most likely to use an APA citation generator, because APA style is the most favored style at these learning levels. Before college, in middle and high school, MLA style is more likely to be used. In other parts of the world styles such as Harvard (UK and Australia) and DIN 1505 (Europe) are used more often.
🙌 Why should I use a Citation Generator?
Like almost every other citation style, APA style can be cryptic and hard to understand when formatting citations. Citations can take an unreasonable amount of time to format manually, and it is easy to accidentally include errors. By using a citation generator to do this work you will:
- Save a considerable amount of time
- Ensure that your citations are consistent and formatted correctly
- Be rewarded with a higher grade
In academia, bibliographies are graded on their accuracy against the official APA rulebook, so it is important for students to ensure their citations are formatted correctly. Special attention should also be given to ensure the entire document (including main body) is structured according to the APA guidelines. Our complete APA format guide has everything you need know to make sure you get it right (including examples and diagrams).
⚙️ How do I use MyBib's APA Citation Generator?
Our APA generator was built with a focus on simplicity and speed. To generate a formatted reference list or bibliography just follow these steps:
- Start by searching for the source you want to cite in the search box at the top of the page.
- MyBib will automatically locate all the required information. If any is missing you can add it yourself.
- Your citation will be generated correctly with the information provided and added to your bibliography.
- Repeat for each citation, then download the formatted list and append it to the end of your paper.
MyBib supports the following for APA style:
| ⚙️ Styles | APA 6 & APA 7 |
|---|---|
| 📚 Sources | Websites, books, journals, newspapers |
| 🔎 Autocite | Yes |
| 📥 Download to | Microsoft Word, Google Docs |

Daniel is a qualified librarian, former teacher, and citation expert. He has been contributing to MyBib since 2018.
Home / Guides / Citation Guides / APA Format / How to format APA page numbers
How to format APA page numbers
In an APA style paper, page numbers generally appear in three places:
- On every page in the upper right corner (pagination for the paper)
- APA in-text citations
- The reference list
Let’s review all three.
1. Pagination for the paper
Every page written in APA style needs to have the page number listed at the top right corner of the paper . It also needs to appear on every page. It should also appear on the title page of the paper, as well as every page of the appendices, footnotes, and other supplemental sections.
The page number should be in the same font and size as the rest of your paper. APA provides different font point sizes depending on the font. For example, 12-point for Times New Roman or 11-point for Arial.
To summarize, your APA page number needs to be:
- At the top of every page (including the title page, body, appendices, etc.)
- Placed in the header
- Flush against the right margin
- In the same font and size as the rest of your paper
It’s recommended that you use autogenerated page numbers in the “header” section of your paper. These features are available in most popular word processors.
2. In-text citations
APA style, page number are recommended (but optional) for paraphrasing, and required for direct quotations from sources with page numbers. When citing a website in APA , or other sources without page numbers, you can use paragraph numbers to mark the quote’s location instead.
In-text citation structure and example for one page:
Text (Author Last Name, Year Published, p. #)
“And in our heart—strange are the ways of evil!—in our heart there is the first peace we have known in twenty years.” (Rand, 2019, p. 32)
In-text citation structure and example for a page range:
Text (Author Last Name, Year Published, pp. #-#)
“It is not good to be different from our brothers, but it is evil to be superior to them” (Rand, 2019, pp. 12-13)
Reference list entry for both examples:
Rand, A. (2019). Anthem . Project Gutenberg. https://www.gutenberg.org/ebooks/1250 (Original work published in 1938)
Notice that unlike the in-text citations, the example reference list entry does NOT include page numbers. Whether a reference includes page numbers is not dependent on the in-text citation; it depends on the source type.
3. Reference list
Page numbers are only included in reference list entries when the following happens:
- The source has page numbers.
- The cited source is a smaller, complete work within a bigger work.
Common example sources:
- A journal article (smaller work) from a journal (bigger work)
- A newspaper article (smaller work) that was printed in a newspaper (bigger work)
- A magazine article (smaller work) in a printed magazine (bigger work)
- A chapter (smaller work) in an edited book (bigger work) where each chapter has a different author
Periodical/Article page numbers
Articles in periodicals (e.g., journals, newspapers, magazines, etc.) include page numbers in their references. The page number or page number range are formatted as the following:
Template and examples:
Notice that unlike in-text citations, there is no “p.” or “pp.” preceding the page numbers.
Example reference (journal article):
Gunn, R., Whear, R., & Douglas, L. (2012, June). A second recent canine burial from the Arnhem Land Plateau. Australian Archaeology , (74), 103-105. http://www.jstor.org/stable/23621527
Chapter in an edited book page numbers
Similar to in-text citations, page numbers are indicated by “p. #” or “pp. #-#” in the reference.
Example reference (chapter in an edited book):
Lisi, G. (2012). Uncalculated risk. In J. Brockman (Ed.), This will make you smarter (pp. 68-73). Harper Perennial.
Published October 28, 2020.
APA Formatting Guide
APA Formatting
- Annotated Bibliography
- Block Quotes
- et al Usage
- In-text Citations
- Multiple Authors
- Paraphrasing
- Page Numbers
- Parenthetical Citations
- Reference Page
- Sample Paper
- APA 7 Updates
- View APA Guide
Citation Examples
- Book Chapter
- Journal Article
- Magazine Article
- Newspaper Article
- Website (no author)
- View all APA Examples
You need not include page numbers in in-text citations unless you want to cite a particular page or page ranges of the source being cited. In such cases, you need to include the page information after the publication year.
If you want to cite a direct quotation, you do need to include the page information. To indicate you are quoting directly from a single page, use the abbreviation “p.” To indicate you are quoting from a continuous page range, use the abbreviation “pp.” and use an en dash between the page range (e.g., pp. 1-2). If the pages are discontinuous, use “pp.” but separate the page numbers with a comma, not an en dash (e.g., pp. 1, 3).
Below are examples of how to include page numbers in in-text citations when using direct quotations:
Narrative:
Jones (1999) states, “It is important to study all children” (p. 47).
Neer et al. (2014) agree with his argument that “the behavior of working women changes drastically” (pp. 47, 49).
Blake and Garger (2002) assert “Humans fight for rights” (pp. 32–34).
Parenthetical:
The study performed in Alaska showed that “it is important to study all children” (Jones, 1999, p. 47).
According to the study, “The behavior of working women changes drastically” (Neer et al., 2014, pp. 47, 49).
“Humans fight for rights,” says the study (Blake & Garger, 2002, pp. 32–34).
The abbreviation “p.” refers to a single page, and “pp.” denotes multiple pages. When you want to cite a single page, use “p.” You can use “pp.” if you want to include a page range (e.g., pp. 45–57) or multiple pages that are not in a range (e.g., pp. 37, 39).
APA Citation Examples
Writing Tools
Citation Generators
Other Citation Styles
Plagiarism Checker
Upload a paper to check for plagiarism against billions of sources and get advanced writing suggestions for clarity and style.
Get Started
Scribbr APA Citation Generator
Accurate APA citations, verified by experts, trusted by millions.
Scribbr for Chrome: Your shortcut to APA citations
Cite any page or article with a single click right from your browser. The extension does the hard work for you by automatically grabbing the title, author(s), publication date, and everything else needed to whip up the perfect APA citation.
Add to Chrome. It's free!
| ⚙️ Styles | APA 7 & APA 6 |
|---|---|
| 📚 Source types | Websites, books, articles |
| 🔎 Autocite | Search by title, URL, DOI, or ISBN |

Rely on accurate APA citations, verified by experts.
You don’t want points taken off for incorrect citations. That’s why our APA citation experts have invested countless hours perfecting our algorithms. As a result, we’re proud to be recommended by teachers worldwide.
Enjoy the APA Citation Generator with minimal distraction.
Staying focused is already challenging enough. You don’t need video pop-ups and flickering banner ads slowing you down. At Scribbr, we keep distractions to a minimum while also keeping the APA Citation Generator free for everyone.

Citation Generator features you'll love
Search for your source by title, URL, DOI, ISBN, and more to retrieve the relevant information automatically.
APA 6th & 7th edition
Scribbr's Citation Generator supports both APA 6 and APA 7 (as well as MLA and Harvard ). No matter what edition you're using, we’ve got you covered!
Export to Bib(La)TeX
Easily export in BibTeX format and continue working in your favorite LaTeX editor.
Export to Word
Reference list finished? Export to Word with perfect indentation and spacing set up for you.
Sorting, grouping, and filtering
Organize the reference list the way you want: from A to Z, new to old, or grouped by source type.
Save multiple lists
Stay organized by creating a separate reference list for each of your assignments.
Choose between Times New Roman, Arial, Calibri, and more options to match your style.
Industry-standard technology
The Scribbr Citation Generator is built using the same citation software (CSL) as Mendeley and Zotero, but with an added layer for improved accuracy.
Annotations
Create perfectly formatted annotated bibliographies with just a few clicks.
Explanatory tips help you get the details right to ensure accurate citations.
Citation guides
Getting to grips with citation is simple with the help of our highly rated APA citation guides and videos .
Secure backup
Your work is saved automatically after every change and stored securely in your Scribbr account.
- Introduction
- Parenthetical vs. narrative
- Multiple authors
Missing information
- Sources to include
Tools and resources
- Scroll to top

How to create APA citations
APA Style is widely used by students, researchers, and professionals in the social and behavioral sciences. Scribbr’s free citation generator automatically generates accurate references and in-text citations.
This citation guide outlines the most important citation guidelines from the 7th edition APA Publication Manual (2020).
- Cite a webpage
- Cite a book
- Cite a journal article
- Cite a YouTube video
APA in-text citations
APA in-text citations include the author’s last name, publication date, and, if relevant, a locator such as a page number or timestamp. For example, (Smith, 2021, p. 170) . See it as a shorter version of the entry in the reference list .
You should include in-text citations every time you’re quoting or paraphrasing someone else’s ideas or words. In doing so, you give credit to the original author and avoid plagiarism .
Parenthetical vs. narrative citation
The in-text citation can take two forms: parenthetical and narrative. Both types are generated automatically when citing a source with Scribbr’s APA Citation Generator.
- Parenthetical citation: According to new research … (Smith, 2020) .
- Narrative citation: Smith (2020) notes that …
Multiple authors and corporate authors
The in-text citation changes slightly when a source has multiple authors or an organization as an author. Pay attention to punctuation and the use of the ampersand (&) symbol.
| Author type | Parenthetical citation | Narrative citation |
|---|---|---|
| One author | (Smith, 2020) | Smith (2020) |
| Two authors | (Smith & Jones, 2020) | Smith and Jones (2020) |
| Three or more authors | (Smith et al., 2020) | Smith et al. (2020) |
| Organization | (Scribbr, 2020) | Scribbr (2020) |
When the author, publication date or locator is unknown, take the steps outlined below.
| Missing element | What to do | Parenthetical citation |
|---|---|---|
| Author | Use the source title.* | ( , 2020) |
| Date | Write “n.d.” for “no date.” | (Smith, n.d.) |
| Page number | Either use an or omit the page number. | (Smith, 2020, Chapter 3) or (Smith, 2020) |
APA Citation Generator
Generate accurate APA citations in seconds
Get started
APA references
APA references generally include information about the author , publication date , title , and source . Depending on the type of source, you may have to include extra information that helps your reader locate the source.
It is not uncommon for certain information to be unknown or missing, especially with sources found online. In these cases, the reference is slightly adjusted.
| Missing element | What to do | Reference format |
|---|---|---|
| Author | Start the reference entry with the source title. | Title. (Date). Source. |
| Date | Write “n.d.” for “no date”. | Author. (n.d.). Title. Source. |
| Title | Describe the work in square brackets. | Author. (Date). [Description]. Source. |
Formatting the APA reference page
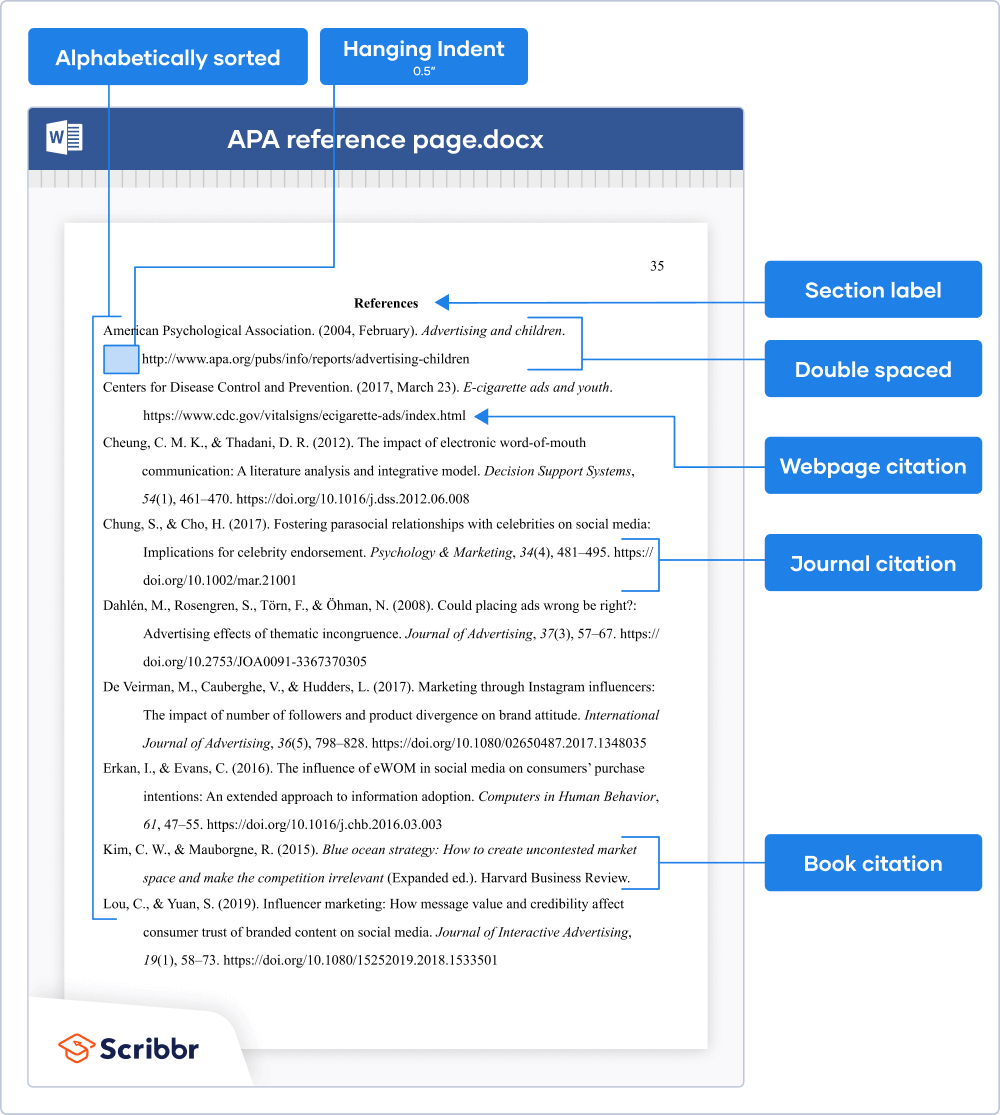
On the first line of the page, write the section label “References” (in bold and centered). On the second line, start listing your references in alphabetical order .
Apply these formatting guidelines to the APA reference page:
- Double spacing (within and between references)
- Hanging indent of ½ inch
- Legible font (e.g. Times New Roman 12 or Arial 11)
- Page number in the top right header
Which sources to include
On the reference page, you only include sources that you have cited in the text (with an in-text citation ). You should not include references to personal communications that your reader can’t access (e.g. emails, phone conversations or private online material).
In addition to the APA Citation Generator, Scribbr provides many more tools and resources that help millions of students and academics every month.
- Citation Generator : Generate flawless citations in APA, MLA , and Harvard style .
- Citation Checker : Upload your paper and have artificial intelligence check your citations for errors and inconsistencies.
- Free plagiarism checker : Detect plagiarism with unparalleled accuracy with Scribbr’s free plagiarism checker.
- AI Proofreader : Upload and improve unlimited documents and earn higher grades on your assignments. Try it for free!
- Paraphrasing tool: Avoid accidental plagiarism and make your text sound better.
- Grammar checker : Eliminate pesky spelling and grammar mistakes.
- Summarizer: Read more in less time. Distill lengthy and complex texts down to their key points.
- AI detector: Find out if your text was written with ChatGPT or any other AI writing tool. ChatGPT 2 & ChatGPT 3 supported.
- Proofreading services : Have a professional editor (or team of editors) improve your writing so you can submit your paper with pride and confidence. Scribbr offers admission essay editing , paper editing , and academic editing .
- Guides and videos : Explore hundreds of articles, bite-sized videos, time-saving templates, and handy checklists that guide you through the process of research, writing, and citation.
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
In-Text Citations: The Basics

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
Note: This page reflects the latest version of the APA Publication Manual (i.e., APA 7), which released in October 2019. The equivalent resource for the older APA 6 style can be found here .
Reference citations in text are covered on pages 261-268 of the Publication Manual. What follows are some general guidelines for referring to the works of others in your essay.
Note: On pages 117-118, the Publication Manual suggests that authors of research papers should use the past tense or present perfect tense for signal phrases that occur in the literature review and procedure descriptions (for example, Jones (1998) found or Jones (1998) has found ...). Contexts other than traditionally-structured research writing may permit the simple present tense (for example, Jones (1998) finds ).
APA Citation Basics
When using APA format, follow the author-date method of in-text citation. This means that the author's last name and the year of publication for the source should appear in the text, like, for example, (Jones, 1998). One complete reference for each source should appear in the reference list at the end of the paper.
If you are referring to an idea from another work but NOT directly quoting the material, or making reference to an entire book, article or other work, you only have to make reference to the author and year of publication and not the page number in your in-text reference.
On the other hand, if you are directly quoting or borrowing from another work, you should include the page number at the end of the parenthetical citation. Use the abbreviation “p.” (for one page) or “pp.” (for multiple pages) before listing the page number(s). Use an en dash for page ranges. For example, you might write (Jones, 1998, p. 199) or (Jones, 1998, pp. 199–201). This information is reiterated below.
Regardless of how they are referenced, all sources that are cited in the text must appear in the reference list at the end of the paper.
In-text citation capitalization, quotes, and italics/underlining
- Always capitalize proper nouns, including author names and initials: D. Jones.
- If you refer to the title of a source within your paper, capitalize all words that are four letters long or greater within the title of a source: Permanence and Change . Exceptions apply to short words that are verbs, nouns, pronouns, adjectives, and adverbs: Writing New Media , There Is Nothing Left to Lose .
( Note: in your References list, only the first word of a title will be capitalized: Writing new media .)
- When capitalizing titles, capitalize both words in a hyphenated compound word: Natural-Born Cyborgs .
- Capitalize the first word after a dash or colon: "Defining Film Rhetoric: The Case of Hitchcock's Vertigo ."
- If the title of the work is italicized in your reference list, italicize it and use title case capitalization in the text: The Closing of the American Mind ; The Wizard of Oz ; Friends .
- If the title of the work is not italicized in your reference list, use double quotation marks and title case capitalization (even though the reference list uses sentence case): "Multimedia Narration: Constructing Possible Worlds;" "The One Where Chandler Can't Cry."
Short quotations
If you are directly quoting from a work, you will need to include the author, year of publication, and page number for the reference (preceded by "p." for a single page and “pp.” for a span of multiple pages, with the page numbers separated by an en dash).
You can introduce the quotation with a signal phrase that includes the author's last name followed by the date of publication in parentheses.
If you do not include the author’s name in the text of the sentence, place the author's last name, the year of publication, and the page number in parentheses after the quotation.
Long quotations
Place direct quotations that are 40 words or longer in a free-standing block of typewritten lines and omit quotation marks. Start the quotation on a new line, indented 1/2 inch from the left margin, i.e., in the same place you would begin a new paragraph. Type the entire quotation on the new margin, and indent the first line of any subsequent paragraph within the quotation 1/2 inch from the new margin. Maintain double-spacing throughout, but do not add an extra blank line before or after it. The parenthetical citation should come after the closing punctuation mark.
Because block quotation formatting is difficult for us to replicate in the OWL's content management system, we have simply provided a screenshot of a generic example below.

Formatting example for block quotations in APA 7 style.
Quotations from sources without pages
Direct quotations from sources that do not contain pages should not reference a page number. Instead, you may reference another logical identifying element: a paragraph, a chapter number, a section number, a table number, or something else. Older works (like religious texts) can also incorporate special location identifiers like verse numbers. In short: pick a substitute for page numbers that makes sense for your source.
Summary or paraphrase
If you are paraphrasing an idea from another work, you only have to make reference to the author and year of publication in your in-text reference and may omit the page numbers. APA guidelines, however, do encourage including a page range for a summary or paraphrase when it will help the reader find the information in a longer work.
- Environment
- Science & Technology
- Business & Industry
- Health & Public Welfare
- Topics (CFR Indexing Terms)
- Public Inspection
- Presidential Documents
- Document Search
- Advanced Document Search
- Public Inspection Search
- Reader Aids Home
- Office of the Federal Register Announcements
- Using FederalRegister.Gov
- Understanding the Federal Register
- Recent Site Updates
- Federal Register & CFR Statistics
- Videos & Tutorials
- Developer Resources
- Government Policy and OFR Procedures
- Congressional Review
- My Clipboard
- My Comments
- My Subscriptions
- Sign In / Sign Up
- Site Feedback
- Search the Federal Register
The Federal Register
The daily journal of the united states government.
- Legal Status
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
Addressing Misinformation About Medical Devices and Prescription Drugs: Questions and Answers; Draft Guidance for Industry; Availability; Agency Information Collection Activities; Proposed Collection; Comment Request
A Notice by the Food and Drug Administration on 07/09/2024
This document has a comment period that ends in 59 days. (09/09/2024) Submit a formal comment
Thank you for taking the time to create a comment. Your input is important.
Once you have filled in the required fields below you can preview and/or submit your comment to the Health and Human Services Department for review. All comments are considered public and will be posted online once the Health and Human Services Department has reviewed them.
You can view alternative ways to comment or you may also comment via Regulations.gov at /documents/2024/07/09/2024-15009/addressing-misinformation-about-medical-devices-and-prescription-drugs-questions-and-answers-draft .
- What is your comment about?
Note: You can attach your comment as a file and/or attach supporting documents to your comment. Attachment Requirements .
this will NOT be posted on regulations.gov
- Opt to receive email confirmation of submission and tracking number?
- Tell us about yourself! I am... *
- First Name *
- Last Name *
- State Alabama Alaska American Samoa Arizona Arkansas California Colorado Connecticut Delaware District of Columbia Florida Georgia Guam Hawaii Idaho Illinois Indiana Iowa Kansas Kentucky Louisiana Maine Maryland Massachusetts Michigan Minnesota Mississippi Missouri Montana Nebraska Nevada New Hampshire New Jersey New Mexico New York North Carolina North Dakota Ohio Oklahoma Oregon Pennsylvania Puerto Rico Rhode Island South Carolina South Dakota Tennessee Texas Utah Vermont Virgin Islands Virginia Washington West Virginia Wisconsin Wyoming
- Country Afghanistan Åland Islands Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia, Plurinational State of Bonaire, Sint Eustatius and Saba Bosnia and Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory Brunei Darussalam Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos (Keeling) Islands Colombia Comoros Congo Congo, the Democratic Republic of the Cook Islands Costa Rica Côte d'Ivoire Croatia Cuba Curaçao Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Falkland Islands (Malvinas) Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard Island and McDonald Islands Holy See (Vatican City State) Honduras Hong Kong Hungary Iceland India Indonesia Iran, Islamic Republic of Iraq Ireland Isle of Man Israel Italy Jamaica Japan Jersey Jordan Kazakhstan Kenya Kiribati Korea, Democratic People's Republic of Korea, Republic of Kuwait Kyrgyzstan Lao People's Democratic Republic Latvia Lebanon Lesotho Liberia Libya Liechtenstein Lithuania Luxembourg Macao Macedonia, the Former Yugoslav Republic of Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia, Federated States of Moldova, Republic of Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Palestine, State of Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Poland Portugal Puerto Rico Qatar Réunion Romania Russian Federation Rwanda Saint Barthélemy Saint Helena, Ascension and Tristan da Cunha Saint Kitts and Nevis Saint Lucia Saint Martin (French part) Saint Pierre and Miquelon Saint Vincent and the Grenadines Samoa San Marino Sao Tome and Principe Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Sint Maarten (Dutch part) Slovakia Slovenia Solomon Islands Somalia South Africa South Georgia and the South Sandwich Islands South Sudan Spain Sri Lanka Sudan Suriname Svalbard and Jan Mayen Swaziland Sweden Switzerland Syrian Arab Republic Taiwan, Province of China Tajikistan Tanzania, United Republic of Thailand Timor-Leste Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom United States United States Minor Outlying Islands Uruguay Uzbekistan Vanuatu Venezuela, Bolivarian Republic of Viet Nam Virgin Islands, British Virgin Islands, U.S. Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe
- Organization Type * Company Organization Federal State Local Tribal Regional Foreign U.S. House of Representatives U.S. Senate
- Organization Name *
- You are filing a document into an official docket. Any personal information included in your comment text and/or uploaded attachment(s) may be publicly viewable on the web.
- I read and understand the statement above.
- Preview Comment
Document Details
Information about this document as published in the Federal Register .
Document Statistics
Enhanced content.
Relevant information about this document from Regulations.gov provides additional context. This information is not part of the official Federal Register document.
Published Document
This document has been published in the Federal Register . Use the PDF linked in the document sidebar for the official electronic format.
Enhanced Content - Table of Contents
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
Electronic Submissions
Written/paper submissions, for further information contact:, supplementary information:, i. background, ii. paperwork reduction act of 1995, disclosures for tailored responsive communications addressing misinformation about medical devices and prescription drugs, omb control number 0910-new, iii. electronic access, enhanced content - submit public comment.
- Submit a public comment on this document
Enhanced Content - Read Public Comments
1 comment has been received at regulations.gov, across 1 docket.
Agencies review all submissions and may choose to redact, or withhold, certain submissions (or portions thereof). Submitted comments may not be available to be read until the agency has approved them.
| Docket Title | Document ID | Comments | |
|---|---|---|---|
| Internet/Social Media Platforms: Correcting Independent Third-Party Misinformation About Prescription Drugs and Medical Devices | 1 |
Enhanced Content - Sharing
- Email this document to a friend
Enhanced Content - Document Print View
- Print this document
Enhanced Content - Document Tools
These tools are designed to help you understand the official document better and aid in comparing the online edition to the print edition.
These markup elements allow the user to see how the document follows the Document Drafting Handbook that agencies use to create their documents. These can be useful for better understanding how a document is structured but are not part of the published document itself.
Enhanced Content - Developer Tools
This document is available in the following developer friendly formats:.
- JSON: Normalized attributes and metadata
- XML: Original full text XML
- MODS: Government Publishing Office metadata
More information and documentation can be found in our developer tools pages .
Official Content
- View printed version (PDF)
This PDF is the current document as it appeared on Public Inspection on 07/08/2024 at 8:45 am. It was viewed 0 times while on Public Inspection.
If you are using public inspection listings for legal research, you should verify the contents of the documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 . Learn more here .
Food and Drug Administration, HHS.
Notice of availability.
The Food and Drug Administration (FDA or Agency) is announcing the availability of a revised draft guidance for industry entitled “Addressing Misinformation About Medical Devices and Prescription Drugs: Questions and Answers.” This revised draft guidance, when finalized, will describe FDA's current thinking on common questions firms may have when voluntarily addressing misinformation about or related to their approved/cleared medical products. This guidance revises and replaces the draft guidance for industry entitled “Internet/Social Media Platforms: Correcting Independent Third-Party Misinformation About Prescription Drugs and Medical Devices” issued in June 2014. This revised draft guidance is not final nor is it in effect at this time.
Submit either electronic or written comments on the draft guidance by September 9, 2024 to ensure that the Agency considers your comment on this draft guidance before it begins work on the final version of the guidance. Submit electronic or written comments on the proposed collection of information in the draft guidance by September 9, 2024.
You may submit comments on any guidance at any time as follows:
Submit electronic comments in the following way:
- Federal eRulemaking Portal: https://www.regulations.gov . Follow the instructions for submitting comments. Comments submitted electronically, including attachments, to https://www.regulations.gov will be posted to the docket unchanged. Because your comment will be made public, you are solely responsible for ensuring that your comment does not include any confidential information that you or a third party may not wish to be posted, such as medical information, your or anyone else's Social Security number, or confidential business information, such as a manufacturing process. Please note that if you include your name, contact information, or other information that identifies you in the body of your comments, that information will be posted on https://www.regulations.gov .
- If you want to submit a comment with confidential information that you do not wish to be made available to the public, submit the comment as a written/paper submission and in the manner detailed (see “Written/Paper Submissions” and “Instructions”).
Submit written/paper submissions as follows:
- Mail/Hand Delivery/Courier (for written/paper submissions): Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
- For written/paper comments submitted to the Dockets Management Staff, FDA will post your comment, as well as any attachments, except for information submitted, marked and identified, as confidential, if submitted as detailed in “Instructions.”
Instructions: All submissions received must include the Docket No. FDA-2014-D-0447 for “Addressing Misinformation About Medical Devices and Prescription Drugs: Questions and Answers.” Received comments will be placed in the docket and, except for those submitted as “Confidential Submissions,” publicly viewable at https://www.regulations.gov or at the Dockets Management Staff between 9 a.m. and 4 p.m., Monday through Friday, 240-402-7500.
- Confidential Submissions—To submit a comment with confidential information that you do not wish to be made publicly available, submit your comments only as a written/paper submission. You should submit two copies total. One copy will include the information you claim to be confidential with a heading or cover note that states “THIS DOCUMENT CONTAINS CONFIDENTIAL INFORMATION.” The Agency will review this copy, including the claimed confidential information, in its consideration of comments. The second copy, which will have the claimed confidential information redacted/blacked out, will be available for public viewing and posted on https://www.regulations.gov . Submit both copies to the Dockets Management Staff. If you do not wish your name and contact information to be made publicly available, you can provide this information on the cover sheet and not in the body of your comments and you must identify this information as “confidential.” Any information marked as “confidential” will not be disclosed except in accordance with 21 CFR 10.20 and other applicable disclosure law. For more information about FDA's posting of comments to public dockets, see 80 FR 56469 , September 18, 2015, or access the information at: https://www.govinfo.gov/content/pkg/FR-2015-09-18/pdf/2015-23389.pdf .
Docket: For access to the docket to read background documents or the electronic and written/paper comments received, go to https://www.regulations.gov and insert the docket number, found in brackets in the heading of this document, into the “Search” box and follow the prompts and/or go to the Dockets Management Staff, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852, 240-402-7500.
You may submit comments on any guidance at any time (see 21 CFR 10.115(g)(5) ).
Submit written requests for single copies of the draft guidance to the Division of Drug Information, Center for Drug Evaluation and Research, Food and Drug Administration, 10001 New Hampshire Ave., Hillandale Building, 4th Floor, Silver Spring, MD 20993-0002; the Office of Communication, Outreach and Development, Center for Biologics Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 71, Rm. 3128, Silver Spring, MD 20993-0002; the Office of Policy, Center for Devices and Radiological Health, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 66, Rm. 5431, Silver Spring, MD 20993-0002; or the Policy and Regulations Staff, Center for Veterinary Medicine, Food and Drug Administration, 7500 Standish Pl., Rockville, MD 20855. Send one self-addressed adhesive label to assist that office in processing your request or include a Fax number to which the draft guidance may be sent. See the SUPPLEMENTARY INFORMATION section for information on electronic access to the draft guidance.
With regard to the draft guidance: Samantha Bryant, Center for Drug Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 51, Room 3203, Silver Spring, MD 20993-0002, 301-796-1200; James Myers, Center for Biologics Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 71, Rm. 7301, Silver Spring, MD 20993-0002, 240-402-7911; Stephanie Philbin, Center for Devices and Radiological Health, Food and Drug Administration, Start Printed Page 56388 10903 New Hampshire Ave., Bldg. 66, Rm. 5456, Silver Spring, MD 20993-0002, 301-837-7151; Kathryn Dennehy, Center for Veterinary Medicine (HFV-245), Food and Drug Administration, 7519 Standish Pl., Rockville, MD 20855, 240-402-7082, [email protected] ; or Julie Finegan, Office of Policy, Office of the Commissioner, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 32, Rm. 4252, Silver Spring, MD 20993-0002, 301-827-4830.
With regard to the proposed collection of information: Domini Bean, Office of Operations, Food and Drug Administration, Three White Flint North, 10A-12M, 11601 Landsdown St., North Bethesda, MD 20852, 301-796-5733 , P RA S [email protected] .
FDA is announcing the availability of a revised draft guidance for industry entitled “Addressing Misinformation About Medical Devices and Prescription Drugs: Questions and Answers.” In addition to describing already existing avenues for communications by firms, the guidance sets out an enforcement policy for certain kinds of internet-based communications that firms might choose to use to address internet-based misinformation about or related to the firm's approved/cleared medical product when that misinformation is created or disseminated by an independent third party. This guidance is not intended to address a firm's correction of its own false or misleading representations about its medical products. For the purposes of this guidance, the term firms refers to the persons or entities legally responsible for the labeling of approved/cleared medical products, which includes applicants, sponsors, manufacturers, packers, distributors, and any persons communicating on behalf of these entities. The term medical product refers to a medical device for human use (including one that is a biological product), a prescription human drug (including one that is a biological product), or a prescription animal drug. The term approved/cleared medical product refers to medical products (as that term is defined in this guidance) that may be introduced into interstate commerce for at least one use under the Federal Food, Drug, and Cosmetic Act (FD&C Act), the Public Health Service Act, and their implementing regulations (collectively, the FDA Authorities) as a result of having satisfied applicable premarket requirements. For ease of reference, when approval and clearance (and similar terms) are used in discussing devices, the terms refer to FDA permitting the marketing of a device via the premarket approval, premarket notification under section 510(k) of the FD&C Act ( 21 U.S.C. 360(k) ), De Novo classification, or Humanitarian Device Exemption pathways and to devices that are exempt from premarket notification.
For the purposes of this guidance and as further described in section II of the guidance, the term misinformation refers to implicit or explicit false, inaccurate, or misleading representations of fact about or related to the firm's approved/cleared medical product.
Misinformation about a firm's approved/cleared medical product can cause harm to both individuals and the public health in general. Basing medical decisions on misinformation can lead patients and healthcare providers to choose treatments that are not safe and effective, or forgo treatments that are, which can have adverse consequences. While misinformation can appear in many forms of communication and be shared in many different ways, internet-based forms of communication have enabled misinformation to travel quickly and reach more people who otherwise might not be exposed to that misinformation. Additionally, misinformation about or related to medical products that treat or prevent serious or life-threatening diseases is especially concerning and represents a significant public health concern.
This guidance revises and replaces the draft guidance for industry entitled “Internet/Social Media Platforms: Correcting Independent Third-Party Misinformation About Prescription Drugs and Medical Devices,” issued in June 2014 (2014 draft guidance). The revised draft guidance reflects the Agency's consideration of feedback from interested parties, including comments received on the 2014 draft guidance. Changes include a revised title, a question-and-answer format, and certain changes in scope. For example, the enforcement policy now extends to a firm's voluntary “tailored responsive communications” that address misinformation that suggests that the firm's cleared/approved medical product be used for an unapproved use. Additionally, new content has been added to reflect changes in technology and functionality of internet-based platforms, as well as changes in the way information is shared online to help a firm to have greater flexibility and control over the timing of the firm's communication when the firm chooses to address certain internet-based third-party misinformation with “tailored responsive communications.” This guidance also now includes a subsection on “general medical product communications” that describes many existing avenues available to firms for communicating information about or related to their approved/cleared medical products. New examples were also added to illustrate the new considerations and recommendations outlined in the guidance and to provide additional clarity to firms.
This revised draft guidance, when finalized, is intended to advance FDA's mission to help members of the public get the accurate, up-to-date, science-based information they need to inform their decisions about medical products to maintain and improve their health. More specifically, the guidance describes two categories of communications firms might choose to use to address misinformation: tailored responsive communications and general medical product communications.
As described in the guidance, a “tailored responsive communication” is a firm's voluntary, internet-based communication that identifies and addresses internet-based misinformation about or related to the firm's approved/cleared medical product when that misinformation is created or disseminated by an independent third party.
For the purposes of this guidance, communications through existing avenues are collectively referred to as “general medical product communications.” Unlike the tailored responsive communications described in the guidance, general medical product communications are not necessarily internet-based or prompted by or tailored to address specific identified internet-based misinformation. General medical product communications can include, among other things, content and messaging that address misinformation about a firm's approved/cleared medical product. Inclusion in a general medical product communication of content that addresses misinformation creates no special considerations regarding the application of the FDA Authorities or other FDA enforcement policies.
FDA recognizes that misinformation about or related to medical products authorized for emergency use is a public health concern. The Agency continues to evaluate the unique considerations that can apply to communications by firms addressing misinformation about or related to such products. As such, this revised draft guidance does not apply to communications by firms that address misinformation about or related Start Printed Page 56389 to an emergency use authorized for the firm's medical product under section 564 of the FD&C Act ( 21 U.S.C. 360bbb-3 ), whether that be an emergency use authorized for an “unapproved use of an approved product”, or an emergency use authorized for an “unapproved product”, as those terms are used in section 564(a) of the FD&C Act. See section 564 of the FD&C Act for more information on the authorities for emergency use authorizations.
This revised draft guidance is being issued consistent with FDA's good guidance practices regulation ( 21 CFR 10.115 ). The revised draft guidance, when finalized, will represent the current thinking of FDA on “Addressing Misinformation About Medical Devices and Prescription Drugs: Questions and Answers.” It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations.
Under the Paperwork Reduction Act of 1995 (PRA) ( 44 U.S.C. 3501-3521 ), Federal Agencies must obtain approval from the Office of Management and Budget (OMB) for each collection of information they conduct or sponsor. “Collection of information” is defined in 44 U.S.C. 3502(3) and 5 CFR 1320.3(c) and includes Agency requests or requirements that members of the public submit reports, keep records, or provide information to a third party. Section 3506(c)(2)(A) of the PRA ( 44 U.S.C. 3506(c)(2)(A) ) requires Federal Agencies to provide a 60-day notice in the Federal Register concerning each proposed collection of information before submitting the collection to OMB for approval. To comply with this requirement, FDA is publishing notice of the proposed collection of information set forth in this document.
With respect to the following collection of information, FDA invites comments on these topics: (1) whether the proposed collection of information is necessary for the proper performance of FDA's functions, including whether the information will have practical utility; (2) the accuracy of FDA's estimate of the burden of the proposed collection of information, including the validity of the methodology and assumptions used; (3) ways to enhance the quality, utility, and clarity of the information to be collected; and (4) ways to minimize the burden of the collection of information on respondents, including through the use of automated collection techniques, when appropriate, and other forms of information technology.
The revised draft guidance document, “Addressing Misinformation About Medical Devices and Prescription Drugs: Questions and Answers,” describes two categories of communications firms might choose to use to address misinformation: tailored responsive communications and general medical product communications. As explained in the guidance, general medical product communications are already existing avenues for communication and are subject to approved information collections, summarized below. The revised draft guidance recommends that a firm's tailored responsive communication clearly identify both the specific misinformation that the firm is addressing and a specific internet-based, independent third-party communication in which that misinformation appears. Additionally, the revised draft guidance discusses disclosures that we recommend firms include when choosing to share tailored responsive communications.
Specifically, the guidance recommends that firms include (1) a mechanism for obtaining a copy of the current FDA-required labeling (including FDA-approved patient labeling, if any), (2) the date the firm's tailored responsive communication is posted (if a date is not automatically generated), and (3) a disclosure that the tailored responsive communication is being shared by the medical product firm or that the person addressing the misinformation is affiliated with the firm and is authorized to provide information on behalf of the firm about the medical product. The guidance also provides recommendations for firms that wish to use a tailored responsive communication to address misinformation about or related to an unapproved use of the firm's approved/cleared medical product. Specifically, the guidance recommends including an additional disclosure identifying the unapproved use and noting that the unapproved use of the medical product has not been approved by FDA and that the safety and effectiveness of the medical product for the unapproved use has not been established.
We estimate the burden of the information collection as follows:
| Recommended disclosure activity; guidance section | Number of respondents | Number of disclosures per respondent | Total annual disclosures | Average burden per disclosure | Total hours |
|---|---|---|---|---|---|
| Clearly identify both the specific misinformation that the firm is addressing and a specific internet-based, independent third-party communication in which that misinformation appears; Section IV.A. Q3 | 958 | 50 | 47,900 | 0.4 (24 minutes) | 19,160 |
| A mechanism for obtaining a copy of the current FDA-required labeling (including FDA-approved patient labeling, if any); Section IV.A. Q5 | 958 | 50 | 47,900 | 0.1 (6 minutes) | 4,790 |
| The date the firm's tailored responsive communication is posted (if a date is not automatically generated); Section IV.A. Q5 | 958 | 50 | 47,900 | 0.05 (3 minutes) | 2,395 |
| A disclosure that the tailored responsive communication is being shared by the medical product firm or that the person addressing the misinformation is affiliated with the firm and is authorized to provide information on behalf of the firm about the medical product; Section IV.A. Q5 | 958 | 50 | 47,900 | 0.1 (6 minutes) | 4,790 |
| In the case of a tailored responsive communication that addresses misinformation about an unapproved use of the firm's approved/cleared medical product, a disclosure identifying the unapproved use and noting that the unapproved use of the medical product has not been approved by FDA and that the safety and effectiveness of the medical product for the unapproved use has not been established; Section IV.A. Q5 | 958 | 5 | 4,790 | 0.1 (6 minutes) | 479 |
| Total | 196,390 | 31,614 | |||
| There are no capital costs or operating and maintenance costs associated with this collection of information. | |||||
Based on data currently available to FDA on the number of firms disseminating promotional communications about prescription drugs (697) combined with an estimated number of device firms marketing products (261), we assume that approximately 958 firms (“number of respondents” in table 1) might each choose to disseminate 50 tailored responsive communications annually. Our estimate of the burden per disclosure reflects what we believe is the average burden based on the number and content and complexity of disclosures as recommended in the guidance.
This draft guidance also refers to previously approved FDA collections of information. The collections of information in 21 CFR part 314 are approved under OMB control number 0910-0001. The collections of information in 21 CFR part 201 regarding content and format of labeling for human drug and biological products are approved under OMB control number 0910-0572. The collections of information in 21 CFR part 801 are approved under OMB control number 0910-0485. The collections of information in 21 CFR 202.1 regarding prescription drug advertising are approved under OMB control number 0910-0686. The collections of information in 21 CFR part 601 regarding marketing approval of biological products are approved under OMB control number 0910-0338; and the collections of information regarding marketing approval of animal drug products in 21 CFR part 514 are approved under OMB control number 0910-0032.
Persons with access to the internet may obtain an electronic version of the draft guidance at https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm , https://www.fda.gov/vaccines-blood-biologics/guidance-compliance-regulatory-information-biologics/biologics-guidances , https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/default.htm , https://www.fda.gov/animal-veterinary/guidance-regulations/guidance-industry , https://www.fda.gov/regulatory-information/search-fda-guidance-documents , or https://www.regulations.gov .
Dated: July 3, 2024.
Lauren K. Roth,
Associate Commissioner for Policy.
[ FR Doc. 2024-15009 Filed 7-8-24; 8:45 am]
BILLING CODE 4164-01-P
- Executive Orders
Reader Aids
Information.
- About This Site
- Accessibility
- No Fear Act
- Continuity Information

Numbered Lists
Use a numbered list to display complete sentences or paragraphs in a series (e.g., itemized conclusions, steps in a procedure).
Use a lettered list or bulleted list rather than a numbered list if the items are phrases.
To create a numbered list, use the numbered list function of your word-processing program. This will automatically indent the list as well. Select the option for an Arabic numeral followed by a period but not enclosed in or followed by parentheses.
This is an example of a numbered list:
Our hypotheses were as follows:
- Social media use would be associated with lower mood.
- Active participation in social media would be associated with higher mood than passive participation.
- Perceived meaningfulness of online activity would mediate the relationship between online activity and mood.
Numbered lists are covered in the seventh edition APA Style manuals in the Publication Manual Section 6.51 and the Concise Guide Section 4.13
From the APA Style blog

Navigating the not-so-hidden treasures of the APA Style website
This post links directly to APA Style topics of interest that users may not even know exist on the website.

IMAGES
VIDEO
COMMENTS
Numbers are used in all sorts of scholarly works. For example, writers may report numerical information about participants (number of participants, demographic information such as age, etc.) as well as the results of statistical analyses. Even writers who are not conducting empirical research often use statistical information to support key points.
More information on APA Style can be found in the Publication Manual of the American Psychological Association (7th ed.) and the Concise Guide to APA Style (7th ed.).
In general, use numerals to express numbers 10 and above, as well as cases such as numbers preceding a unit of measurement, most fractions, statistics, ratios, percentages and times.
Here are a few more rules concerning numbers to adhere to as you follow APA style: If you are using two modifiers against a noun, use a combination of both numerals and words. Three 5-point scales. If you're unsure which modifier to write and which to express numerically, try it both ways.
Numbers & Statistics. Writers often need to discuss numbers and statistics in their manuscripts, and it can be a challenge to determine how to represent these in the most readable way. APA 7 contains detailed guidelines for how to write numbers and statistics, and the most common are listed below. These guidelines, however, are not exhaustive ...
In this article we follow the guidelines of APA Style, one of the most common style guides used in academic writing. In general, words should be used for numbers from zero through nine, and numerals should be used from 10 onwards. This is true for both cardinal numbers (e.g., two, 11) and ordinal numbers (e.g., second, 11 th ).
The APA Publication Manual is commonly used for reporting research results in the social and natural sciences. This article walks you through APA Style standards for reporting statistics in academic writing.
APA Stylistics: Basics. APA Stylistics: Avoiding Bias. Footnotes & Appendices. Numbers & Statistics. Additional Resources. APA Headings and Seriation. APA PowerPoint Slide Presentation. APA Sample Paper. Tables and Figures.
Based on Publication Manual of the American Psychological Association, 7th ed. (2020)
Learn how to set up APA format for your paper. From the title page and headings to references and citations.
Review some of APA style's guidelines on scholarly writing for topics such as abbreviations, active versus passive voice, anthropomorphism, capitalization, numbers, and more.
Numbers Expressed in Words. In general, use words to express numbers zero through nine, and use numerals to express numbers 10 and above. there were five nurses on duty. the study had three conditions. students were in the third, sixth, eighth, 10th, and 12th grades. However, there are exceptions to this general guideline for number usage.
Note: The APA Publication Manual, 7th Edition specifies different formatting conventions for student and professional papers (i.e., papers written for credit in a course and papers intended for scholarly publication). These differences mostly extend to the title page and running head. Crucially, citation practices do not differ between the two styles of paper.
For full information on punctuation, refer to sections 6.1-6.10, the APA Publication Manual. Use a single space after punctuation marks that end a sentence. Use hyphens for compound words. Use an em dash to distinguish part of a sentence that either amplifies or digresses from the point. Microsoft Word usually converts two hyphens that are ...
When you include a paraphrase in a paper, you are required to include only the author and date in the citation. You are encouraged (but not required) to also provide the page number (or other location information) for a paraphrased citation when it would help the reader locate the relevant passage in a long or complex text (such as when you use ...
The American Psychological Association (APA) published the 7th edition of its style manual in 2019. As well as rules for citation and paper formatting, the manual provides various language guidelines to help you present your ideas in a clear, concise, and inclusive manner.
Here's what a title page of a reference paper template looks like in APA format: Title Page of APA Paper format template. How to Set up APA Format Paper [Step-by-Step] After understanding the guidelines, the next step is to apply them effectively to format your paper in APA 7th edition style.
This guide will help you set up an APA Style student paper. The basic setup directions apply to the entire paper. Annotated diagrams illustrate how to set up the major sections of a student paper: the title page or cover page, the text, tables and figures, and the reference list.
General APA Guidelines. Your essay should be typed and double-spaced on standard-sized paper (8.5" x 11"), with 1" margins on all sides. Include a page header (also known as the "running head") at the top of every page. For a professional paper, this includes your paper title and the page number. For a student paper, this only includes the ...
Generate APA style citations quickly and accurately with our FREE APA citation generator. Enter a website URL, book ISBN, or search with keywords, and we do the rest! Updated with APA 7th Edition!
Learn how to format page numbers in APA style for a paper, in-text citations, and references in a reference list. Examples are included.
7th edition Anatomy of a Journal Article Scientific journal articles share a common anatomy, or structure. Each part of an article serves a purpose, and if you know
Scribbr's free APA Citation Generator lets you generate perfect APA Style citations in seconds. Now even easier with a Chrome extension.
APA Citation Basics. When using APA format, follow the author-date method of in-text citation. This means that the author's last name and the year of publication for the source should appear in the text, like, for example, (Jones, 1998). One complete reference for each source should appear in the reference list at the end of the paper.
The Food and Drug Administration (FDA or Agency) is announcing the availability of a revised draft guidance for industry entitled "Addressing Misinformation About Medical Devices and Prescription Drugs: Questions and Answers." This revised draft guidance, when finalized, will describe FDA's...
Numbered Lists. Use a numbered list to display complete sentences or paragraphs in a series (e.g., itemized conclusions, steps in a procedure). Use a lettered list or bulleted list rather than a numbered list if the items are phrases. To create a numbered list, use the numbered list function of your word-processing program.