Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 November 2023

Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract
- Manogar Priya 1 na1 ,
- Raja Venkatesan 2 ,
- Simon Deepa 1 ,
- Siva Sankar Sana 2 na1 ,
- Soundhar Arumugam 3 ,
- Abdulnasser M. Karami 4 ,
- Alexandre A. Vetcher 5 &
- Seong-Cheol Kim 2
Scientific Reports volume 13 , Article number: 18838 ( 2023 ) Cite this article
7318 Accesses
29 Altmetric
Metrics details
- Materials science
- Nanoscience and technology
The green methodologies of nanoparticles with plant extracts have received an increase of interest. Copper oxide nanoparticles (CuO NPs) have been utilized in a many of applications in the last few decades. The current study presents the synthesis of CuO NPs with aqueous extract of Morinda citrifolia as a stabilizing agent. The leaf extract of Morinda citrifolia was mixed with a solution of copper sulphate (CuSO 4 ·5H 2 O) and sodium hydroxide as a catalyst. UV–visible spectroscopy, FTIR, XRD, SEM, TEM, and EDAX analysis were performed to study the synthesized CuO NPs. Particle size distribution of the synthesized CuO NPs have been measured with dynamic light scattering. The CuO NPs synthesized were highly stable, sphere-like, and have size of particles from 20 to 50 nm. Furthermore, as-formed CuO NPs shown strong antibacterial activity against the Gram-positive bacteria ( Bacillus subtilis, and Staphylococcus aureus ), and Gram-negative bacteria ( Escherichia coli ). CuO NPs revealed a similar trend was analysed for antifungal activity. The zone of inhibition for the fungi evaluated for Aspergillus flavus (13.0 ± 1.1), Aspergillus niger (14.3 ± 0.7), and Penicillium frequentans (16.8 ± 1.4). According to the results of this investigation, green synthesized CuO NPs with Morinda citrifolia leaf extract may be used in biomedicine as a replacement agent for biological applications.
Similar content being viewed by others
Biosynthesis of copper nanoparticles using Alstonia scholaris leaves and its antimicrobial studies
Green synthesis of copper oxide nanoparticles and its efficiency in degradation of rifampicin antibiotic

Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential
Introduction.
Nanotechnology is growing as an essential area with enormous potential for many applications due to the distinctive characteristics of nanoparticles (NPs) 1 . In comparison with their bulk substitutes, these nanoscale materials have enhanced optical, magnetic, catalytic, and electrical capacities 2 , 3 . As a result, there is more interest in producing sustainable and effective methods for synthesizing nanoparticles. Traditional methods of synthesizing nanoparticles often involve the use of hazardous chemicals, high temperatures, and energy-intensive processes, leading to environmental concerns and potential toxicity. To address these issues, green synthesis has gained considerable attention as a promising alternative. Green synthesis, also known as environmentally friendly or sustainable synthesis, involves the use of natural resources, biomolecules, or environmentally benign materials to fabricate nanoparticles 4 , 5 , 6 . It offers several advantages over conventional methods, including reduced energy consumption, minimal use of toxic chemicals, biodegradability, and the potential for large-scale production 7 , 8 .
Metal oxide nanoparticles attract the attention of researchers due to the connect bulk and atomic structures. NPs have unique physicochemical characteristics which include significant reactivity, huge surface area, pore size, and particles shape 9 . Introduction to novel nanoparticles might put immunological in nature and inflammation responses to the challenge 10 . The most rapid adopters of nanotechnology are the areas of information and communication (such as electrical and optoelectronic sectors), food technology, energy technology, and medical products (including a number of pharmaceuticals and drug delivery systems, diagnostics, and medical technology). Toxicity arising from nanomaterials might present new problems. These situations may involve nanoparticle which have been introduced into the environment or which were given to individuals via nanotechnology products. Nanoparticles are can be synthesized by physical, chemical, biological, and hybrid procedures 11 , 12 , 13 . Toxic materials render the production of physical and chemical nanoparticles more difficult. Effective eco-friendly biogenetic methods of production have become more common due to their ease of use and flexibility 14 , 15 . Before, nanoparticles that needed to be produced via chemical and physical methods. Nanoparticles and nanotechnology deal with small materials. Nanoparticles were extensively studied in recent years due to their many potential uses in chemistry, drug delivery, biomedical, and other areas 16 , 17 , 18 , 19 .
As a result of their biocidal characteristics, copper nanoparticles are now attractive wounds treatment. With its cheap price and excellent physical and chemical attributes, copper NPs can be utilized in process bandage. The method for the production of nanomaterials is dependent on their small dimensions and high surface-to-volume ratio 20 . Metal and metal oxide nanoparticles have been employed in a wide range of applications. Several distinctive methods for adjusting shape and size includes metal vapour co-deposition, electrochemical reduction, gas phase evaporation, thermal decomposition, radiolytic reduction, and chemical reduction 21 , 22 , 23 , 24 . Nanosized particles can be produced with chemical and physical methods like micro-emulsion are immersed. For instance, flame-based aerosol techniques, Sono chemical hydrothermal techniques, solid-state techniques, and the system for producing nanoparticles. Nanoparticles cannot be used in healthcare due to there are generated with toxic materials. Clean, biocompatible, nontoxic, and sustainable nanoparticle processing is thus advantageous 25 , 26 . This field is currently concentrating on “green” chemistry and bio-processors.
Plants are used in “green synthesis” for the production of metal nanoparticles. Green synthesis in biotechnology and nanotechnology has an opportunity for advantages for the economy and the environment 27 . Green chemistry synthesizes in an environmentally friendly and efficient method. Nanoparticles have been proposed to be synthesized in plants, algae, bacteria, yeast, and fungi 28 . The nanoparticles of copper can be produced from plant extracts using eco-friendly, low-cost, and biocompatible reducing agents 29 , 30 . Copper oxide nanoparticles development is enhanced with ascorbic acid in Morinda citrifolia leaf extract. In addition to their distinctive characteristics, such as a large surface area, catalytic activity, and antibacterial capabilities, CuO NPs have attracted interest in many other fields. Bioengineered CuO NPs are those that undergo CuO nanoparticle synthesis or modification with biological processes like bacteria, fungi, or plant extracts. The significance of using bioengineered CuO NPs lies in their potential to provide more sustainable, efficient, and biocompatible solutions across various fields, from healthcare and environmental protection to materials science and energy.
In this work, we developed an efficient method to synthesize CuO NPs and studied the crystalline nature, chemical composition, and interactions between NPs and the reducing agent. Morinda citrifolia leaf extract was used as a stabilizing agent in the green synthesis of CuO NPs. The copper oxide nanoparticles with functional components, structure, and particle size were studied with UV–vis, FTIR, XRD, SEM, TEM, and DLS analysis. Furthermore, the antibacterial effects of the CuO NPs were investigated by Gram-positive bacteria ( Bacillus subtilis, and Staphylococcus aureus ), and Gram-negative bacteria ( Escherichia coli ) with the agar diffusion method.
Materials and methods
Copper sulphate (CuSO 4 ·5H 2 O) was purchased from Sigma-Aldrich (98%). Hydrochloric acid (HCl) (35%), sodium hydroxide (98%) was used to monitoring the pH, were received from Merck. The leaves of Morinda citrifolia have been collected in Chennai, Tamil Nadu. The dissolution of 2.5 g of CuSO 4 ·5H 2 O in 100 mL deionized water yielded a 1 × 10 –2 M stock solution of copper sulphate. Bacterial and fungal cultures were grown in the medium, including Bacillus subtilis , Staphylococcus aureus , Escherichia coli , Aspergillus flavus , Aspergillus niger , and Penicillium frequentans . All of the chemical and solvents utilized were of analytical grade.
Preparation of Morinda citrifolia leaf extract
The Morinda citrifolia leaf extract can be seen in Fig. 1 A. Morinda citrifolia , a plant of the Rubiaceae family, had its leaves collected from a garden in Chennai. We weighed and cleaned Morinda citrifolia leaves several times with tap water and deionized water after collecting to get rid of any extra dust or contaminants. After that, a slice the leaf in small pieces, add 100 mL of distilled water, and immerse the mixture in a water bath heated to 60 °C for 1 h. The green extract can be processed in a burette and used as a reducing or capping agent. The extract was kept at 4 °C for further studies.
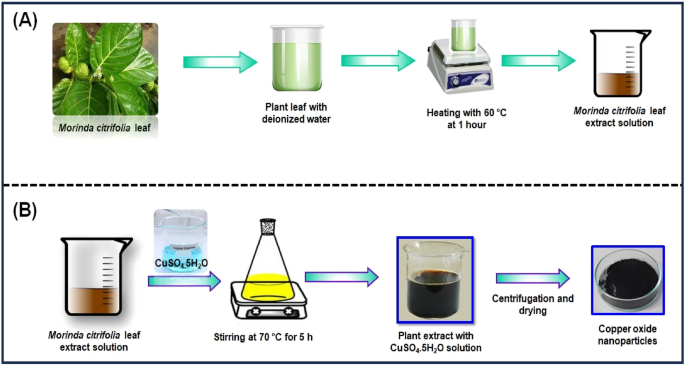
( A ) Schematic representation of eco-friendly synthesis of copper oxide nanoparticles using Morinda citrifolia leaf extract; ( B ) Schematic diagram of CuO NPs from leaf extract of Morinda citrifolia.
Synthesis of CuO NPs from Morinda citrifolia leaf extract
Figure 1 B shows the synthesis of CuO NPs from Morinda citrifolia leaf extract solution. 2.5 g of CuSO 4 ·5H 2 O was dissolved in 100 mL of Deionized water (DI) to initiate the green synthesis process for CuO NPs. After, 50 mL of Morinda citrifolia extract solution to 100 mL of 1 × 10 –2 M CuSO 4 ·5H 2 O solution, the pH was kept at 7.0 with NaOH. The solution then underwent to a reflux at a magnetic stirrer. The colour of the solution changed as it was stirring with a from pale-green to a deep-brown while maintaining for 5 h at 70 °C. After centrifuging the solution for 24 h, it was filtered. The solid precipitate was washed three times with deionised water, followed by an 100% ethanol wash for CuO NPs separation, dried at 60 °C for 4 h, and kept at 4 °C for further application.
The following equations explain the synthesis mechanism for CuO NPs;
Characterization of synthesized CuO NPs
The UV–Visible spectrum of effectively obtained CuO NPs was collected with an ( Oceian optics JAZ, USA ) spectrophotometer. The UV spectrum of copper oxide nanoparticle synthesis in colloidal solution was observed at wavelengths ranging from 200 to 800 nm. The FTIR spectrometer ( Perkin Elmer, Spectrum-2, USA ) with KBr pellet was used for collecting functional group data in the region of 4000–400 cm −1 . The FTIR spectrum of obtained CuO NPs was examined. Different modes of vibration in the CuO NPs have been identified and assigned to evaluate the presence of different functional groups that aid the extract of the Morinda citrifolia plant. XRD measurement of the CuO NPs, where only 5.0 ml of the extract was added, was done on a Shimadzu XRD-6000 diffractometer operating at a voltage of 40 kV and current of 20 mA with Cu-Kα radiation (λ = 1.54 Å). The XRD spectrum has been examined and acquired with scanning range values of 20° and 80°.SEM study of the surface morphology of CuO NPs was performed ( CARL ZEISS, Jena, Germany ). The inner morphology of the CuO nanoparticles was studied with Morinda citrifolia extract, and images were captured using a TEM ( JEOL, JEM-2100, Japan ). For descriptive purposes, a 5.0 ml of the materials were sonicated in ethanol, and a drop of it was cast in a copper grid with a 300-mesh carbon layer by layer for magnetic measurements. The particle size distribution (PSD) of the synthesized CuO NPs have been measured with the Dynamic Light Scattering (DLS) measurements instrument's standard operating procedure.
Antibacterial and antifungal studies
Methodology.
Bacillus subtilis (MTCC6133), Escherichia coli (MTCC6133), and Staphylococcus aureus (MTCC96) were collected from the Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology, Chandigarh, India. Standard cultures of bacteria have been sub-cultured into newly prepared nutrient agar and incubated at 37 °C for 24 h for the production of fresh cultures of bacteria. Marina Labs Research and Development offers fungal cultures of Aspergillus flavus (MLAC1101), Aspergillus niger (MLAC1201), and Penicillium frequentans (MLAC 2101). The fungi were sub-cultured for 72 h to produce the sporulation process and the developing spore were examined for antifungal activity.
Assay for antibacterial activity by well diffusion
The zone of inhabitation method was employed to evaluate the antibacterial activity of the offered materials 31 , 32 , 33 . Mueller–Hinton agar plates were applied to test the samples. The agar plate was streaked with the different cultures (bacterial strains). Then, using a sterile cork-borer, 5 mm diameter wells were cut into the agar medium. For 20 min, the plates are allowed to dry to remove all remaining moisture. The compounds of 15 µL, 20 µL, and 25 µL were administered into each well. As a positive control, a well containing 15 µL of streptomycin antibiotic was used. The plates were incubated at 37 °C. The tests were performed in duplicates. Every plate was evaluated for zones of inhibition 24 h after incubation. The diameter of the inhibitory zone was calculated in millimetres (mm).
Assay for antifungal activity by well diffusion
For testing the antibacterial activity of the offered sample, the agar well diffusion method was employed. Sabouraud’s Dextrose agar plates were employed for testing the specimens. The agar plate’s surface was streaked with the different cultures (fungal strains). The agar medium was then cut into 5 mm diameter wells with a sterile cork-borer. For 20 min, the plates are allowed to dry to remove additional moisture. Compounds of 15 µL, 20 µL, and 25 µL were dispensed into each well, with 5.0 mg of Fluconazole serve as a positive control. At 37 °C, the plates were incubated. The tests have been carried out in duplicate. After 24 h of incubation, each plate was examined for zones of inhibition. The zone of inhibition was recorded as the diameter of inhibition zone in mm.
Leaves collection permission
The Morinda citrifolia leaves have been obtained from Chennai, Tamil Nadu in India, and all of the national guidelines, legislation and/or protocols have adhered appropriately. Morinda citrifolia is a flora species found predominantly in India. In Tamil Nadu, this species is a very common tree seen in road sides and in every gardens. Hence, the usage of this plant needs no permission and licensing.
Ethical approval
We comply with relevant guidelines and legislation regarding the sample collection in the present study. The plant leaves ( Morinda citrifolia ), in the present study is not endangered. In 2023, leaves of the Morinda citrifolia plant were collected in Chennai, Tamil Nadu, India. There are no plant material samples for the current study.
Consent to participate
All person named as author in this manuscript have participated in the planning, design and performance of the research and in the interpretation of the result.
Result and discussion
The change in the colour of the reaction solution suggests the synthesis of CuO NPs by the reduction of CuSO 4 ·5H 2 O during treatment with extracts of Morinda citrifolia leaf. The change in color of the reaction solution after 2 h reveals the synthesis of CuO NPs. The result indicates that the Cu-Extract 2+ ions in the reaction mixture have changed to copper oxide with nanometric size. In the synthesis of CuO NPs, different types of plant extracts are used as reducing and stabilizing agents. The resultant nanoparticles have no surface instead of encased in a medium or gel, and their catalytic and other characteristics can be restricted, while particle stabilized and microgel stabilized nanoparticles characteristics may be altered by modifying the temperature and pH. Table 1 presents the green synthesis of CuO NPs with different plants.
UV–Visible spectroscopy
The CuO NPs were investigated with UV–Visible spectroscopy to identify the optical band gap. A distinctive peak was found at 256 nm, which might be assigned to surface plasmon resonance (SPR), and it was revealed. The SPR at 256 nm indicates the synthesis of CuO NPs. SPR occurred as a result of an oscillation of surface electron of nanoparticles, so this result agreed with earlier research 34 . In accordance with Mie's theory, the quantity of SPR bands is mainly determined by the shape of nanoparticles that are produced. The spherical form of the nanoparticle is mostly because of a single SPR band. With equation, the band gap energy similar to the wavelength of peak absorption was calculated. The band gap energy can be calculated with the formulas below.
where h is the plank constant, C is the velocity of light, Eg is the energy gap, and g is the measured absorption wavelength.
The synthesized Cu nanoparticle’s strongest and most sharp absorption peak appears at 256 nm, and it shows the blue shift absorption observed in Fig. 2 A. The calculated band gap energy from the UV–visible absorption spectrum is 1.006 eV 56 , 57 . The decrease in particle size has been triggered with a shift in absorption towards smaller wavelengths.
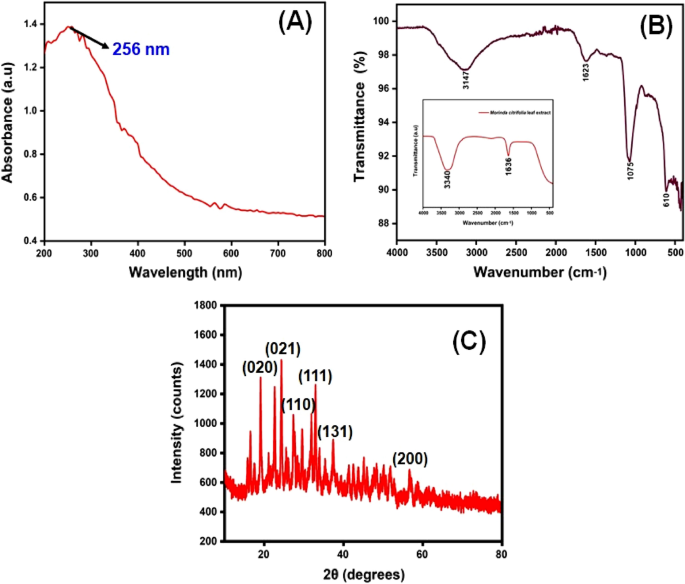
( A ) UV–visible spectrophotometer results of synthesized CuO NPs; ( B ) FTIR spectra and ( C ) XRD pattern.
FTIR spectral analysis
The FTIR spectrum of ecofriendly obtained plants extracts and CuO NPs were studied. The spectra were collected between 4000 and 400 cm −1 . A type of vibrations in the CuO materials have been determined and assigned to identify the existence of different functional groups that aid with the chemical reduction. The FTIR spectra of the plant extract Morinda citrifolia are shown in (Fig. 2 B inset), consistent with the earlier research 42 . The absorption bands at 3340 cm −1 correspond to the absorption band of –OH functional group. The significant peaks at 1636 cm −1 suggest the existence of a functional group denoted as –NO 2 in the plant extract The Morinda citrifolia leaf plant served in the reduction of copper ions as well as the capping of CuO. Figure 2 B presents the results of the research study performed on the peaks. For O–H stretching of water and –C=O stretching of aldehydes and ketones, the major peaks are observed at 3147 cm −1 and 1623 cm −1 , respectively 58 , 59 . The stretching vibration peak for the C=H and H–C–H functional groups is at 495 cm −1 , and the stretching vibration peak for the C=H and H–C–H functional groups is at 2923 cm −1 , confirmation the presence of synthesized CuO nanoparticles in the materials 60 .
XRD analysis
XRD measurements revealed the crystalline characteristics of the obtained copper nanoparticles. The XRD spectrum of the synthesized copper nanoparticles is presented in Fig. 2 C. The CuO NPs exhibited crystalline XRD peaks at 2θ values of 19.03°, 24.36°, 27.39°, 32.99°, 37.55°, and 56.74° which correspond to the planes of crystals of (020), (021), (110), (111), (131) and (200), respectively. The plane alignments of the synthesized CuO NPs were in excellent accordance with the standard CuO nanoparticles obtained for the International Centre of Diffraction Data Card (JCPDS No.: 00-041-0254). The XRD pattern suggested that the synthesized CuO nanoparticles are polycrystalline in characteristic and resembled the monoclinic tenorite phase of the CuO structure. Lattice parameters are α = 4.79 Å, the intensities and positions of peaks are in moral promise with the stated standards 61 , 62 . Additionally, the well- distinct and sharp CuO images detected from XRD patterns approves the moral crystalline nature of the green synthesized CuO NPs. Comparable results were also stated in earlier like works 63 , 64 . Strong orientation and broad diffraction bands in the XRD spectrum can be attributed to the nano dimensional conditions of the obtained nanoparticles. In addition, the XRD pattern indicated the newly synthesised nanoparticles are nanocrystalline. The average crystallite size of CuO nanoparticles was calculated using a Debye–Scherrer formula (Eq. 5 ).
where D is the average diameter of the nanoparticles, K is the Sherrer constant, λ is the wavelength of x-ray diffraction (015,406 nm), β is the full width at half maximum, and θ is Bragg angle (degree).
The average crystalline size of synthesized CuO nanoparticles has been estimated to be in the range of 25–30 nm using the Debye Scherrer formula, and the crystal structure of synthesised CuO nanoparticles has been shown to be face-cantered cubic structure.
SEM analysis
The scanning electron microscope (SEM) confirmed the size and structure of the nanoparticles that were synthesized. The images from SEM suggest that the green synthesized CuO NPs have a major distribution and spherical shapes 65 , and have an average size of 29.2 nm. As predicted, agglomerations decreased as the size of particles increased, due to size of particles increased gain size linear. When the agglomeration of particles can be attributed to an effort to decrease surface free energy, SEM images of CuO nanoparticles are showed in Fig. 3 A–C. The surface alternatives are clearly shown, paying special attention to the fact that nanoparticles were synthesized.
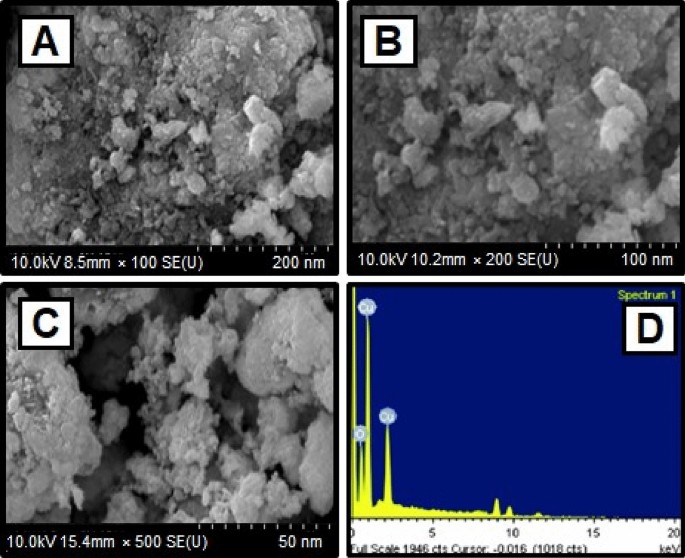
( A )–( C ) SEM image of CuO NPs synthesized using CuSO 4 ·5H 2 O and Morinda citrifolia leaf extract ( D ) EDX analysis of CuO NPs.
The elemental composition of CuO NPs produced with green synthesis method have been identified using a EDAX device. The elemental composition of CuO nanoparticles can be seen in Fig. 3 D. The elements are copper (65%), oxygen (23%), and carbon (12%) shown the Table 2 . The high concentration of copper metal in the advanced levels indicates the synthesis of CuO NPs via a green methodology.
TEM analysis
The TEM images of synthesized CuO nanoparticles are shown in Fig. 4 A–C. TEM was employed to study the particle size and surface morphology of Morinda citrifolia -mediated CuO NPs, and the results suggested that the CuO were polydisperse and cylindrical in structure. The SAED pattern confirmed the crystal structure of CuO NPs. SAED patterns suggest that CuO NPs have distinctive lattice fringes which are similar with the normal CuO structures and have excellent crystalline quality. Padmavathi et. al., observed that produced CuO NPs are surface elements and can serve as a successful reducing agent of CuO ions to CuO NPs in Morinda citrifolia extract 66 . Sodium hydroxide as a catalyst agent, inhibiting CuO NPs aggregation. The TEM results of CuO NPs were fully consistent with the XRD pattern of obtained CuO NPs. This study was aided by the results of Fardood et al., which noticed the FCC structure of CuO NPs using the TEM and SAED patterns of CuO NPs synthesized from Morinda citrifolia leaf extract 67 . The corresponding SAED pattern (inset in Fig. 4 C) indicates that the copper particles given among the CuO NPs are highly crystalline and have the predicted alignment. The Cu, O, and C elements are seen in the element mapping images of the synthesized CuO NPs (Fig. 4 D–F). The presence of nanoparticles in the material is evident as Cu, O, and C are confirmed with synthesized CuO NPs.
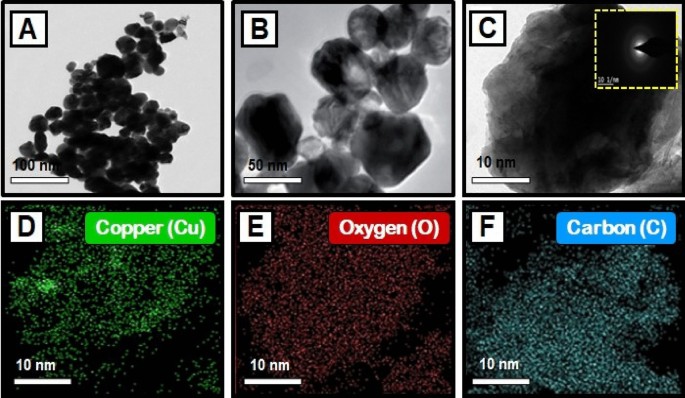
( A – C ) TEM images of copper oxide nanoparticles, and SAED image of CuO NPs [insets Fig. ( C )]; Elemental mapping analysis of CuO NPs from Morinda citrifolia leaf extract, ( D ) Copper, ( E ) Oxygen, and ( F ) Carbon elements.
The particle size distribution of CuO NPs
This method is utilized for synthesizing particles with colloidal structure. The particle size distribution (PSD) for colloids produced at different concentrations of CuSO 4 ·5H 2 O and constant Morinda citrifolia content, as measured with the dynamic light scattering (DLS) method, is shown in Fig. 5 . For the three samples, the types of distribution and average diameters changed. The sample prepared with 1 × 10 –2 M CuSO 4 ·5H 2 O is the most monodisperse and has an average diameter of about 100.0 nm, however the samples obtained with 15, 20 and 25 µL, despite having average dimensions of 49.1 nm, 37.0 nm and 29.2 nm, respectively, have more polydispersity suggested that 15 µL given the best performance. The results reported in previous articles 68 , 69 , comparing Fig. 5 A–C indicates the concentration of the copper sulphate greatly impacts the size distribution of the nanoparticles. Aside from the formation of smaller particles, it was expected that a lower CuSO 4 .5H 2 O concentration would result in a narrower size distribution since the ratio of Morinda citrifolia :Cu 2+ ions would be greater in this case. However, the DLS data shown a trend toward the reverse site. As the concentration of CuSO 4 ·5H 2 O decreased, the size distribution expanded. The result can be addressed if we understand that the average diameter measured with DLS results from nanoparticles surrounded by Morinda citrifolia rather than “naked” CuO NPs. In addition Morinda citrifolia molecules may attach to the surface of particles at lower concentrations of copper sulphate due to the higher Morinda citrifolia :Cu 2+ ions ratio 70 , 71 . Morinda citrifolia molecules may form more than one layer on the nanoparticles. The outermost layer can absorb water, producing tumescence of the composite nanoparticles and, as a result, increasing particle sizes.
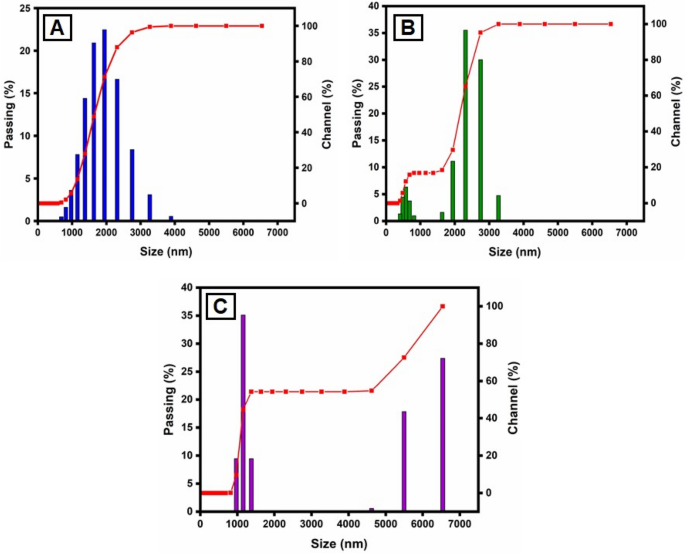
Particle size distribution (PSD) of the synthesized CuO NPs by the DLS methods, for varying CuSO 4 ·5H 2 O concentration: ( A ) 15 µL; ( B ) 20 µL and ( C ) 25 µL.
Antibacterial activity
The disk diffusion method was used to study the antibacterial activity of CuO NPs against gram-positive and gram-negative pathogenic bacteria such as B. subtilis, S. aureus , and E. coli (Fig. 6 a). In laboratories, nutritional broth has been commonly utilized for sustaining live pathogens of bacteria (as subcultures with 0.5 Mc turbidity) cultivated overnight at 37 °C 72 .
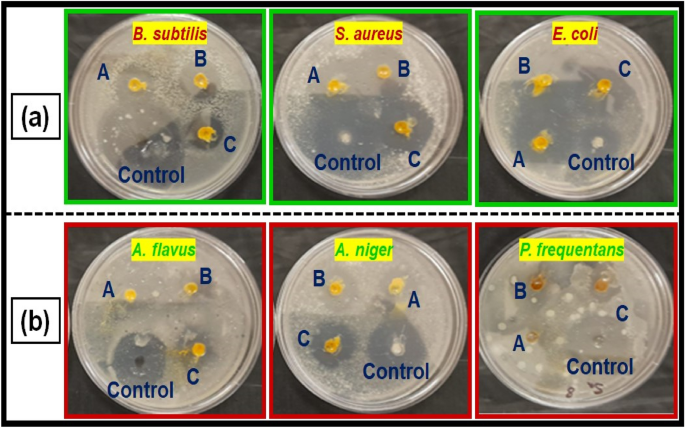
( a ) Antibacterial activity, and ( b ) antifungal activity of copper oxide nanoparticles from Morinda citrifolia leaf extracts; (A) 15 µL, (B) 20 µL and (C) 25 µL; and control of CuO NPs.
The fresh bacterial culture was swiped evenly on sterilized Petri dishes with nutrient agar. On the clean disks, synthesized CuO NPs (15, 20 and 25 µL) and an aqueous ( Morinda citrifolia ) leaf extract (25 µL) was poured. As a positive control, 25 mL of chloramphenicol disks were maintained, and all plates were incubated overnight at 37 °C for 24–48 h to identify the development of bacterial inhibition zone surrounding the surface of the disk. The results revealed that the CuO NPs has showed antibacterial activity against the bacteria, Bacillus subtilis. It has recorded 13.0 mm zone of inhibition at the concentration of 25 µl . However, there was no zone recorded for the bacteria, Escherichia coli. The compound showed less activity against the bacteria, Staphylococcus aureus. The zone of inhibition recorded for the bacteria, Bacillus subtilis (13.6 ± 1.1), Staphylococcus aureus (13.2 ± 0.2), and Escherichia coli (13.1 ± 1.2) respectively. The antibacterial activity mechanism of green synthesized CuO NPs is shown in Fig. 7 . The antibacterial activity mechanism of copper oxide nanoparticles is dependent on the size, structure, and concentration of copper oxide. The three major ways that antibacterial activity follows are as follows. (1) Degeneration of the cell wall and membrane, (2) Infiltration and cellular disruption, and (3) Oxidation stress 73 , 74 , 75 . The antibacterial activity recorded against each individual bacteria for the CuO nanoparticles is presented in Table 3 .
Schematic representation of green synthesis of copper oxide nanoparticles using Morinda citrifolia leaf extract.
Antifungal activity
Figure 6 b shows a similar pattern for CuO nanoparticle’s antifungal activity. The zone of inhibition recorded for the fungi, Aspergillus flavus (13.1 ± 1.1), Aspergillus niger (14.7 ± 0.7), and Penicillium frequentans (16.2 ± 1.4) respectively. However antifungal activity for the fungus, A . niger was similar to that of control ( Flucanazole ) 76 , 77 . The antifungal activity recorded for the CuO NPs against each individual fungal species is presented in Table 3 .
Conclusions
The copper oxide nanoparticles were synthesized with an eco-friendly methodology obtained from plant extracts such as Morinda citrifolia . The size, shape, elemental composition, and structure of the synthesized CuO NPs were characterized by UV–visible spectroscopy, FTIR, XRD, SEM, TEM and DLS. Within the process of synthesis, the UV–visible absorption spectrum reveals a blue shift as the percentage of plant extract in the resultant mixture rises. XRD patterns suggest that the crystallites of the CuO NPs that developed have a centre cubic structure. The SEM image of the synthesized CuO NPs suggests that these particles exhibit a spherical structure with an average size of the NPs was 29.2 nm. In addition, structural and size studies reveal that CuO NPs synthesized by Morinda citrifolia have a high surface-to-volume ratio. In the EDAX spectrum, the elemental percentage of copper in the CuO NPs was found to be highly uniform. However, the results of bacterial activity showed that the CuO NPs acted well. The synthesized CuO NPs has antibacterial activity against Bacillus subtilis , Escherichia coli , and Staphylococcus aureus . According to the results, CuO NPs is more effective than the other two against Bacillus subtilis . CuO NPs has been proven to be efficient against three distinct types of fungi: Aspergillus flavus , Aspergillus niger , and Penicillium frequentans . Copper oxide nanoparticles has been shown to be most effective against all three kinds of fungi based on the data: Aspergillus flavus , Aspergillus niger , and Penicillium frequentans . As a result, the data show that the antifungal activity of the green synthesised CuO NPs has a higher than its antibacterial activity. This study suggests that the synthesized CuO NPs could be employed in the biomedical, fuel cell, battery and food storage industries. However, more study should be done on minimize the toxicity of CuO NPs though maintaining and improving their biological efficiency in order to promote the biomedical uses of CuO NPs.
Data availability
All data generated or analysed during this study are included in this published article.
Khan, I., Saeed, K. & Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12 (7), 908–931. https://doi.org/10.1016/j.arabjc.2017.05.011 (2019).
Article CAS Google Scholar
Baig, N., Kammakakam, I. & Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2 (6), 1821–1871. https://doi.org/10.1039/D0MA00807A (2021).
Article Google Scholar
Chandrakala, V., Aruna, V. & Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emerg. Mater. 5 , 1593–1615. https://doi.org/10.1007/s42247-021-00335-x (2022).
Noah, N. M. & Ndangili, P. M. Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sens. Int. 3 , 100166. https://doi.org/10.1016/j.sintl.2022.100166 (2022).
Aswathi, V. P., Meera, S., Maria, C. G. A. & Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 8 , 377–397. https://doi.org/10.1007/s41204-022-00276-8 (2023).
Madani, M. et al. Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes. Nanotechnol. Rev. 11 (1), 731–759. https://doi.org/10.1515/ntrev-2022-0034 (2022).
Harish, V. et al. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 12 (3), 457. https://doi.org/10.3390/nano12030457 (2022).
Article CAS PubMed PubMed Central Google Scholar
Szczyglewska, P., Feliczak-Guzik, A. & Nowak, I. Nanotechnology-general aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules 28 (13), 4932. https://doi.org/10.3390/molecules28134932 (2023).
Chavali, M. S. & Nikolova, M. P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 1 , 607. https://doi.org/10.1007/s42452-019-0592-3 (2019).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20 , 101–124. https://doi.org/10.1038/s41573-020-0090-8 (2021).
Article CAS PubMed Google Scholar
Ying, S. et al. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 26 , 102336. https://doi.org/10.1016/j.eti.2022.102336 (2022).
Vijayaram, S. et al. Applications of green synthesized metal nanoparticles—a review. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-023-03645-9 (2023).
Article PubMed PubMed Central Google Scholar
Sharma, N. K. et al. Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega 7 (31), 27004–27020. https://doi.org/10.1021/acsomega.2c01400 (2022).
Sharma, D., Kanchi, S. & Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 12 (8), 3576–3600. https://doi.org/10.1016/j.arabjc.2015.11.002 (2019).
Gudikandula, K., Vadapally, P. & Charya, M. A. S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano. 2 , 64–78. https://doi.org/10.1016/j.onano.2017.07.002 (2017).
Haleem, A., Javaid, M., Singh, R. P., Rab, S. & Suman, R. Applications of nanotechnology in medical field: A brief review. J. Glob. Health. 7 (2), 70–77. https://doi.org/10.1016/j.glohj.2023.02.008 (2023).
Ray, S. S. & Bandyopadhyay, J. Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives. Nanotechnol. Rev. 10 (1), 728–743. https://doi.org/10.1515/ntrev-2021-0052 (2021).
McNamara, K. & Tofail, S. A. M. Nanoparticles in biomedical applications. Adv. Phys.-X 2 (1), 54–88. https://doi.org/10.1080/23746149.2016.1254570 (2017).
Patra, J. K. et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 16 , 71 (2018).
Joudeh, N. & Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 20 , 262. https://doi.org/10.1186/s12951-022-01477-8 (2022).
Theerthagiri, J. et al. Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo-and electrocatalytic applications. Light Sci. Appl. 11 , 250. https://doi.org/10.1038/s41377-022-00904-7 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Thanh, N. T. K., Maclean, N. & Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 114 (15), 7610–7630. https://doi.org/10.1021/cr400544s (2014).
Hui, S. et al. Three-dimensional cathodes for electrochemical reduction of CO 2 : From macro- to nano-engineering. Nanomaterials 10 (9), 1884. https://doi.org/10.3390/nano10091884 (2020).
Ahmed, S. F. et al. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 204 , 111967. https://doi.org/10.1016/j.envres.2021.111967 (2022).
Nath, D. & Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Phar. 36 (3), 997–1014. https://doi.org/10.1016/j.etap.2013.09.002 (2013).
Venkatesan, R. et al. Biodegradable composites from poly(butylene adipate- co -terephthalate) with carbon nanoparticles: Preparation, characterization and performances. Environ. Res. 235 , 116634. https://doi.org/10.1016/j.envres.2023.116634 (2023).
Singh, J. et al. Green synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 16 , 84. https://doi.org/10.1186/s12951-018-0408-4 (2018).
Pandit, C. et al. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ. Sci. 34 (3), 101869. https://doi.org/10.1016/j.jksus.2022.101869 (2022).
Murugappan, G. & Sreeram, K. J. Nano-biocatalyst: Bi-functionalization of protease and amylase on copper oxide nanoparticles. Colloids Surf. B. 197 , 111386. https://doi.org/10.1016/j.colsurfb.2020.111386 (2021).
Manzoor, M. A. et al. Environmental sustainable: Biogenic copper oxide nanoparticles as nano-pesticides for investigating bioactivities against phytopathogens. Environ. Res. 231 (1), 119451. https://doi.org/10.1016/j.envres.2023.115941 (2023).
Venkatesan, R., Rajeswari, N. & Tamilselvi, A. Antimicrobial, mechanical, barrier, and thermal properties of bio-based poly (butylene adipate- co -terephthalate) (PBAT)/Ag 2 O nanocomposite films for packaging application. Polym. Adv. Technol. 29 (1), 61–68. https://doi.org/10.1002/pat.4089 (2018).
Ngamsurach, P. & Praipipat, P. Antibacterial activities against Staphylococcus aureus and Escherichia coli of extracted Piper betle leaf materials by disc diffusion assay and batch experiments. RSC Adv. 12 (40), 26435–26454. https://doi.org/10.1039/D2RA04611C (2022).
Venkatesan, R., Zhang, Y. & Chen, G. Preparation of poly(butylene adipate-co-terephthalate)/ZnSnO 3 composites with enhanced antimicrobial activity. Compos. Commun. 22 , 100469. https://doi.org/10.1016/j.coco.2020.100469 (2020).
Alao, I. I., Oyekunle, I. P., Iwuozor, K. O. & Emenike, E. C. Green synthesis of copper nanoparticles and investigation of its antimicrobial properties. Adv. J. Chem. B. 4 (1), 39–52. https://doi.org/10.22034/ajcb.2022.323779.1106 (2022).
Yallappa, S. et al. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim. Acta A 110 , 108–115. https://doi.org/10.1016/j.saa.2013.03.005 (2013).
Article ADS CAS Google Scholar
Manimaran, K. et al. Eco-friendly approaches of mycosynthesized copper oxide nanoparticles (CuONPs) using Pleurotus citrinopileatus mushroom extracts and their biological applications. Environ. Res. 232 , 116319. https://doi.org/10.1016/j.envres.2023.116319 (2023).
Ismail, M. I. M. Green synthesis and characterizations of copper nanoparticles. Mater. Chem. Phys. 240 , 122283. https://doi.org/10.1016/j.matchemphys.2019.122283 (2020).
Mohamed, E. A. Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates . Heliyon 6 (1), e03123. https://doi.org/10.1016/j.heliyon.2019.e03123 (2020).
Naika, H. R. et al. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 9 (1), 7–12. https://doi.org/10.1016/j.jtusci.2014.04.006 (2015).
Sivaraj, R., Rahman, P. K. S. M., Rajiv, P., Narendhran, S. & Venckatesh, R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim. Acta A 129 , 255–258. https://doi.org/10.1016/j.saa.2014.03.027 (2014).
Abbas, A. H. & Fairouz, N. Y. Characterization, biosynthesis of copper nanoparticles using ginger roots extract and investigation of its antibacterial activity. Mater. Today Proc. 61 (3), 908–913. https://doi.org/10.1016/j.matpr.2021.09.551 (2022).
Dayana, K. S., Mani, R. J. & Durai, S. C. V. Morinda citrifolia leaf extract mediated green synthesis of copper oxide nanoparticles and it’s potential and antibacterial studies. Rasayan J. Chem. 14 (2), 897–904. https://doi.org/10.31788/RJC.2021.1426264 (2021).
Sharma, M., Sharma, S. K., Mathur, M. & Choudhary, M. K. A novel approach towards eco-friendly green synthesis of copper nanoparticles from Bunium persicum and their biomedical applications. Int. J. Health Sci. 6 (5), 3099–3119. https://doi.org/10.53730/ijhs.v6nS5.9155 (2022).
Aheri, H. R., Han, S. H., Vikhe, A. S. & Kuchekar, S. R. Green synthesis of copper nanoparticles using Syzygium Cumin leaf extract, characterization and antimicrobial activity. Chem. Sci. Trans. 8 (1), 1–6. https://doi.org/10.7598/cst2019.1552 (2019).
Wu, S., Rajeshkumar, S., Madasamy, M. & Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechn. 48 (1), 1153–1158. https://doi.org/10.1080/21691401.2020.1817053 (2020).
Suárez-Cerda, J. et al. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J. Saudi Chem. Soc. 21 (3), 341–348. https://doi.org/10.1016/j.jscs.2016.10.005 (2017).
Hassanien, R., Husein, D. Z. & Al-Hakkani, M. F. Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon. 4 (12), e01077. https://doi.org/10.1016/j.heliyon.2018.e01077 (2018).
Mali, S. C., Dhaka, A., Githala, C. K. & Trivedi, R. Green synthesis of copper nanoparticles using Celastrus paniculatus . Willd leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 27 , e00518. https://doi.org/10.1016/j.btre.2020.e00518 (2020).
Tahir, A. et al. Green synthesis, characterization and antibacterial, antifungal, larvicidal and anti-termite activities of copper nanoparticles derived from Grewia asiatica L. Bull. Natl. Res. Cent. 46 , 188. https://doi.org/10.1186/s42269-022-00877-y (2022).
Heydari, R., Koudehi, M. F. & Pourmortazavi, S. M. Antibacterial activity of Fe 3 O 4 /Cu nanocomposite: Green synthesis using Carum carvi L. seeds aqueous extract. ChemistrySelect 17 (2), 531–535. https://doi.org/10.1002/slct.201803431 (2019).
Salas, Z. H. et al. Green synthesis of copper nanoparticles and their formulation into face masks: An antibacterial study. Polym. Compos. 44 (2), 907–916. https://doi.org/10.1002/pc.27142 (2023).
Thandapani, G. et al. Green synthesis of copper oxide nanoparticles using Spinacia oleracea leaf extract and evaluation of biological applications: Antioxidant, antibacterial, larvicidal and biosafety assay. Mater. Today Commun. 34 , 105248. https://doi.org/10.1016/j.mtcomm.2022.105248 (2023).
Kalaiyan, G., Prabu, K. M., Suresh, S. & Suresh, N. Green synthesis of CuO nanostructures with bactericidal activities using Simarouba glauca leaf extract. Chem. Phys. Lett. 761 , 138062. https://doi.org/10.1016/j.cplett.2020.138062 (2020).
Lafmejani, Z. N., Jafari, A. A., Moradi, P. & Moghadam, A. L. Impact of foliar application of copper sulphate and copper nanoparticles on some morpho-physiological traits and essential oil composition of peppermint ( Mentha piperita L.). Herba Pol. 64 (2), 13–24. https://doi.org/10.2478/hepo-2018-0006 (2018).
Benassai, E. et al. Green and cost-effective synthesis of copper nanoparticles by extracts of non-edible and waste plant materials from Vaccinium species: Characterization and antimicrobial activity. Mater. Sci. Eng. C 119 , 111453. https://doi.org/10.1016/j.msec.2020.111453 (2021).
Ramasubbu, K. et al. Green synthesis of copper oxide nanoparticles using sesbania grandiflora leaf extract and their evaluation of anti-diabetic, cytotoxic, anti-microbial, and anti-inflammatory properties in an in-vitro approach. Fermentation 9 (4), 332. https://doi.org/10.3390/fermentation9040332 (2023).
Majeed, S., Shukhairi, A. N. B. & Danish, M. Green Approach for the synthesis of copper oxide nanoparticles and its antibacterial effect against methicillin-resistant staphylococcus aureus (MRSA). J. Pure Appl. Microbiol. 16 (1), 708–716. https://doi.org/10.22207/JPAM.16.1.74 (2022).
Amin, F. et al. Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. Evid. Based Complement. Altern. Med. , https://doi.org/10.1155/2021/5589703 (2021).
Raul, P. K. et al. CuO nanorods: A potential and efficient adsorbent in water purification. RSC Adv. 4 , 40580–40587. https://doi.org/10.1039/C4RA04619F (2014).
Ethiraj, A. S. & Kang, D. J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 7 , 70. https://doi.org/10.1186/1556-276X-7-70 (2012).
Arockiasamy, J. S. K. & Irudayaraj, J. Natural dye sensitized CuO nanorods for luminescence applications. Ceram. Int. 42 (5), 6198–6205. https://doi.org/10.1016/j.ceramint.2015.12.180 (2016).
Kamble, S. P. & Mote, V. D. Structural, optical and magnetic properties of Co doped CuO nano-particles by sol-gel auto combustion technique. Solid State Sci. 95 , 105936. https://doi.org/10.1016/j.solidstatesciences.2019.105936 (2019).
Kannan, K., Radhika, D., Nesaraj, A. S., Sadasivuni, K. K. & Krishna, L. S. Facile synthesis of NiO-CYSO nanocomposite for photocatalytic and antibacterial applications. Inorg. Chem. Commun. 122 , 108307. https://doi.org/10.1016/j.inoche.2020.108307 (2020).
Karthik, K., Dhanuskodi, S., Gobinath, C., Prabukumar, S. & Sivaramakrishnan, S. Dielectric and antibacterial studies of microwave assisted calcium hydroxide nanoparticles. J. Mater. Sci. Mater. Electron. 28 , 16509–16518. https://doi.org/10.1007/s10854-017-7563-5 (2017).
Kannan, K. et al. Facile fabrication of novel ceria-based nanocomposite (CYO-CSO) via co-precipitation: Electrochemical, photocatalytic and antibacterial performance. J. Mol. Struct. 1256 , 132519. https://doi.org/10.1016/j.molstruc.2022.132519 (2022).
Padmavathi, A. R. et al. Copper oxide nanoparticles as an effective anti-biofilm agent against a copper tolerant marine bacterium, Staphylococcus lentus . Biofouling 35 (9), 1007–1025. https://doi.org/10.1080/08927014.2019.1687689 (2019).
Fardood, S. T., Ramazani, A., Asiabi, P. A. & Joo, S. W. A novel green synthesis of copper oxide nanoparticles using a Henna extract powder. J. Struct. Chem. 59 , 1737–1743. https://doi.org/10.1134/S0022476618070302 (2018).
Yugandhar, P., Vasavi, T., Uma Maheswari Devi, P. & Savithramma, N. Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: Characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl. Nanosci. 7 , 417–427. https://doi.org/10.1007/s13204-017-0584-9 (2017).
Dagher, S., Haik, Y., Ayesh, A. I. & Tit, N. Synthesis and optical properties of colloidal CuO nanoparticles. J. Lumin. 151 , 149–154. https://doi.org/10.1016/j.jlumin.2014.02.015 (2014).
Sondors, R. et al. Size distribution, mechanical and electrical properties of CuO nanowires grown by modified thermal oxidation methods. Nanomaterials 10 , 1051. https://doi.org/10.3390/nano10061051 (2020).
Santhoshkumar, J., Agarwal, H., Menon, S., Rajeshkumar, S. & Venkat Kumar, S. A biological synthesis of copper nanoparticles and its potential applications. In Green Synthesis, Characterization and Applications of Nanoparticles (ed. Shukala, A.K.; Iravani, S.) 2019, 199–221. https://doi.org/10.1016/B978-0-08-102579-6.00009-5 .
Kannan, K., Radhika, D., Gnanasangeetha, D., Krishna, L. S. & Gurushankar, K. Y 3+ and Sm 3+ co-doped mixed metal oxide nanocomposite: Structural, electrochemical, photocatalytic, and antibacterial properties. Appl. Surf. Sci. Adv. 4 , 100085. https://doi.org/10.1016/j.apsadv.2021.100085 (2021).
Naz, S., Gul, A., Zia, M. & Javed, R. Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Appl. Microbiol. Biotechnol. 107 , 1039–1061. https://doi.org/10.1007/s00253-023-12364-z (2023).
Shkodenko, L., Kassirov, I. & Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 8 , 1545. https://doi.org/10.3390/microorganisms8101545 (2020).
Slavin, Y. N., Asnis, J., Häfeli, U. O. & Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15 , 65. https://doi.org/10.1186/s12951-017-0308-z (2017).
Chinnaiah, K., Kannan, K., Krishnamoorthy, R. & Gurushankar, K. Datura metal L. leaf extract mediated sodium alginate polymer membrane for supercapacitor and food packaging applications. Int. J. Biol. Macromol. 242 , 125112. https://doi.org/10.1016/j.ijbiomac.2023.125112 (2023).
Rangayasami, A., Kannan, K., Joshi, S. & Subban, M. Bioengineered silver nanoparticles using Elytraria acaulis (L.F.) Lindau leaf extract and its biological applications. Biocatal. Agric. Biotech. 27 , 101690. https://doi.org/10.1016/j.bcab.2020.101690 (2020).
Download references
Acknowledgements
The authors, R.V. and S.-C. Kim, would like to thank their gratitude to the National Research Foundation of Korea (NRF), which was funded supported a by the Ministry of Education (2020R1I1A3052258). This paper has also been supported by the RUDN University Strategic Academic Leadership Program (recipient A.V.). This work was funded by the Researchers Supporting Project Number (RSPD2023R764), King Saud University, Riyadh, Saudi Arabia.
Author information
These authors contributed equally: Manogar Priya and Siva Sankar Sana.
Authors and Affiliations
Department of Chemistry, School of Basic Sciences, Vels Institute of Science, Technology and Advanced Studies, Chennai, Tamil Nadu, 600117, India
Manogar Priya & Simon Deepa
School of Chemical Engineering, Yeungnam University, Gyeongsan, 38541, Republic of Korea
Raja Venkatesan, Siva Sankar Sana & Seong-Cheol Kim
Department of Mechanical Engineering, Indian Institute of Technology Guwahati, Guwahati, Assam, 781039, India
Soundhar Arumugam
Department of Chemistry, College of Science, King Saud University, 11451, Riyadh, Saudi Arabia
Abdulnasser M. Karami
Institute of Biochemical Technology and Nanotechnology, Peoples’ Friendship, University of Russia (RUDN), 6 Miklukho‐Maklaya St., Moscow, Russia, 117198
Alexandre A. Vetcher
You can also search for this author in PubMed Google Scholar
Contributions
M.P.: Formal analysis, Writing—original draft; R.V.: Investigation, Conceptualization, Writing—original draft; S.D.: Data curation; S.S.S.: Formal analysis, Writing—original draft; S.A.: Investigation; A.M.K.: Formal analysis; A.A.V.: Formal analysis, Data curation; S.-C.K.: Supervision, Project administration, Funding acquisition, Writing—review & editing. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Manogar Priya , Raja Venkatesan or Seong-Cheol Kim .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Priya, M., Venkatesan, R., Deepa, S. et al. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci Rep 13 , 18838 (2023). https://doi.org/10.1038/s41598-023-46002-5
Download citation
Received : 08 September 2023
Accepted : 26 October 2023
Published : 01 November 2023
DOI : https://doi.org/10.1038/s41598-023-46002-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmaceuticals (Basel)

Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles
Madalina elena grigore.
1 Department of Biomaterials and Medical Devices, Faculty of Medical Engineering, University Politehnica of Bucharest, Bucharest 060042, Romania; [email protected] (M.E.G.); [email protected] (E.R.B.)
Elena Ramona Biscu
Alina maria holban.
2 Division of Earth, Environmental and Life Sciences, Research Institute of the University of Bucharest (ICUB), Bucharest 060042, Romania; moc.oohay@h_m_anila (A.M.H.); moc.oohay@ucsezemurg (A.M.G.)
3 Department of Science and Engineering of Oxide Materials and Nanomaterials, Faculty of Applied Chemistry and Materials Science, University Politehnica of Bucharest, Bucharest 060042, Romania
Monica Cartelle Gestal
4 Department of Infectious Diseases, College of Veterinary Medicine, University of Georgia Athens, Athens, GA 30602, USA
Alexandru Mihai Grumezescu
This study aims to provide an updated survey of the main synthesis methods of copper oxide (CuO) nanoparticles in order to obtain tailored nanosystems for various biomedical applications. The synthesis approach significantly impacts the properties of such nanoparticles and these properties in turn have a significant impact on their biomedical applications. Although not widely investigated as an efficient drug delivery system, CuO nanoparticles have great biological properties including effective antimicrobial action against a wide range of pathogens and also drug resistant bacteria. These properties have led to the development of various approaches with direct applications to the biomedical field, such as tailored surfaces with antimicrobial effect, wound dressings and modified textiles. It is also believed that these nanosystems could represent efficient alternatives in the development of smart systems utilized both for the detection of pathogens and for the treatment of infections.
1. Introduction
Research interest in nanomaterials has increased exponentially thanks to their unique chemical and physical features, different of those of their bulk materials, including but not limited to diffusivity, electrical resistivity, electrical conductivity, strength and hardness, chemical reactivity and diverse and versatile biological activity [ 1 , 2 ].
Interest has especially increased in the case of metal oxide nanoparticles, because these particles are widely used as industrial catalysts, chemical sensing devices, in medical applications, disinfection, as antimicrobials, fillers, opacifiers, catalysts, semiconductors and they are also useful in the development of cosmetics and microelectronics [ 1 , 2 , 3 , 4 , 5 ].
Metal oxide nanoparticles, such as copper oxide (CuO), have attracted attention mostly because of their antimicrobial and biocide properties and they may be used in many biomedical applications [ 6 , 7 ]. Copper oxide is a semiconductor metal with unique optical, electrical and magnetic properties and it has been used for various applications, such as the development of supercapacitors, near-infrared filters, in magnetic storage media, sensors, catalysis, semiconductors, etc. [ 8 , 9 , 10 ].
One of the most important parameters in the synthesis of these nanoparticles is the control of particle size, morphology and crystallinity and in order to achieve this goal, different synthesis methods were developed; some of the most investigated approaches include the sonochemical method, the sol-gel method, laser ablation, the electrochemical method, chemical precipitation and surfactant-based techniques [ 2 , 5 , 11 , 12 , 13 ].
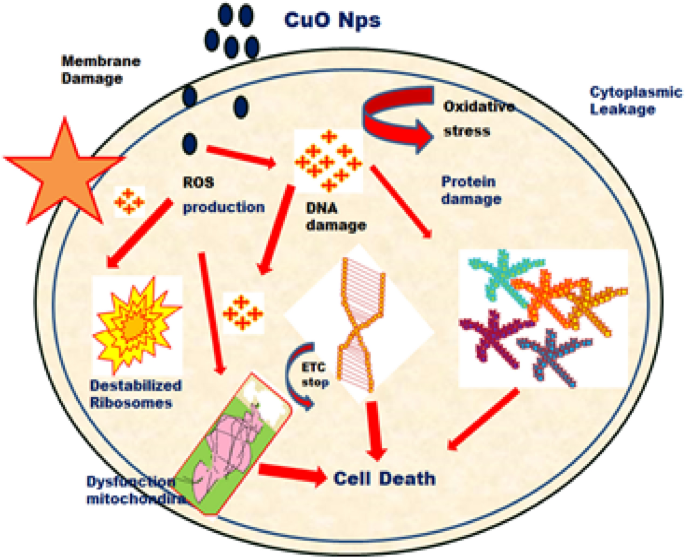
Even though CuO nanoparticles (CuO NPs) have proved their use in biomedical applications; the major disadvantage for their use on the medical field is due to their potentially toxic effects [ 7 , 14 , 15 , 16 ]. CuO NPS may be toxic for mammalian cells as well as for vertebrates and invertebrates. The main toxicity process relies on the increased production of reactive oxygen species [ 17 ]. These nanoparticles thus induce oxidative stress in human pulmonary epithelial cells, promote toxicity and can damage DNA and mitochondria [ 7 , 15 , 18 ].
2. Methods of Synthesis for Biomedical CuO Nanoparticles
The synthesis approaches of CuO NPs have advanced significantly in the last ten years because of their important biomedical and industrial applications [ 19 ]. The synthesis technique is important for the properties of the final nanosystem, since it may control the size and morphology of the nanoparticles. Also, these nanoparticles present various optical and magnetic properties, mechanical strengths and electrical resistivity, which differ from the characteristics of bulk solid material. Several methods for the synthesis of CuO NPs have been used, and the most relevant approaches, along with the typical resulting particle sizes, are listed in Table 1 .
The synthesis of CuO NPs with different methods results in different sizes [ 10 ].
| Preparation Method | Size (nm) |
|---|---|
| Electrochemical method | 4 |
| Sonochemical synthesis | 20–30 |
| Sol-gel techniques | 7–9 |
| Microemulsion system | 5–25 |
| Precipitation synthesis | 4 |
| Microwave irradiation | 3–5 |
2.1. Electrochemical Method
The electrochemical method was invented by Switzer as a way to synthesize ceramic films. Since then this method has been continuously used for the preparation of nano-metal oxides such as ZnO, CuO, etc. The first reported CuO nanocrystals were prepared by using Cu as a sacrificial anode [ 20 , 21 ].
The electrochemical method is based on reactions occurring between the electrode and the electrolyte. With this approach electrodeposition occurs on a small portion of the electrode, because chemical potentials are developed on its surface.
The electrochemical method is included in the group of soft chemical techniques that produce copper oxide nanoparticles [ 5 ]. One of the most notable advantages of this method is the ability to control the morphology and size of the resulting CuO NPs by modifying the temperature, time, current density, composition or voltage. Zhang et al. synthesized CuO nanospindles and nanorods by varying the density from 5 mA·cm −2 to 10 mA·cm −2 and then to 20 mA·cm −2 . By changing the electrolytic solvent, these authors obtained CuO nanorods with diameters between 20 nm and 50 nm and with lengths of 200 nm to 300 nm [ 8 ]. Jadhav et al. also synthesized CuO NPS by applying the electrochemical method using a copper sheet as anode and a platinum sheet as a cathode [ 22 ].
Katwal et al. also described a CuO NPs manufacturing process using the electrochemical method under different reaction conditions. In this experiment the usual procedure was used, where the electrodes, copper plate and inert platinum were fixed at 1 cm. Also, the approach included a supporting electrolyte, which was added to acetonitrile and to a water to methanol solution in 12:1 molar ratio at room temperature. The dark brown precipitate could be centrifuged, washed, dried and finally the material was be easily calcined and characterized by different methods. This approach also revealed that physic and chemical properties of the nanostructures (including size) might be modified by controlling various parameters of the reaction and molar ratios of the utilized chemicals [ 5 ].

2.2. PEG–Dependent Synthesis
Polyethylene glycol (PEG) is a cheap non-ionic surfactant that is used for the synthesis of metal oxides [ 13 ]. It is also used in many biomedical applications, especially drug delivery, since it offers a good biocompatibility to the whole structure which contains it [ 9 ]. PEG 400 is the most commonly utilized variant due to its lower toxic [ 23 ]. For example, Ranjbar-Karimi et al. used this surfactant to study the effects induced by its presence on the dimension and morphology of CuO NPs. CuO NPs were prepared with the addition of sodium hydroxide solution at various concentrations and solutions of copper acetate in ethanol/water. The sample, which consisted of Cu(OAc) 2 ·2H 2 O 50 mL (0.05 M) and NaOH 100 mL (0.1 M) that were sonicated for 1 h with 30 W ultrasound power. The method led to the production of nanoparticles with relatively homogenous dimensions (with an average diameter of 70 nm) [ 24 ].
Also, it was demonstrated by different studies that PEG has a significant effect on the size of CuO NPs. Vidyasagar et al. synthesized CuO NPs by mixing copper chloride, sodium hydroxide and PEG 400. The product was washed with ethyl alcohol to remove the PEG 400 and then it was dried. The resulting solid was calcined at 400 °C, 600 °C and 800 °C.
Samples calcined at 800 °C formed uniform particles, with sizes between 400 and 454 nm, while the samples calcined at 400 °C presented a nanoparticle size of approximately 65 nm. It was observed that the increase in temperature was proportional to the particle agglomeration [ 23 ]. In comparison, when Lashanizadegan and Erfaninia synthesized Ag/CuO nanoparticles and CuO nanorods by using PEG 400 and PEG 6000 it was observed that Ag/CuO nanoparticles synthesized with 10 mL PEG 400 or 20 mL PEG 400 have uniform morphology. However, the best morphology and distribution was obtained with PEG 6000 [ 25 ].
2.3. Sonochemical Method
The sonochemical method is a simple process that follows three steps: (1) formation, (2) development, (3) the implosive collapse of the obtained microcavities. The method involves the application of ultrasound during the synthesis of the product [ 8 , 26 , 27 ].
Suleiman et al. obtained CuO NPs of different morphology by using cupric acetate as a precursor and poly(vinylpyrrolidone) (PVP), acting as a reducing agent by applying a sonochemical method [ 28 ]. In comparison, Karunakaran et al. synthesized two sets of samples by applying the sonochemical method. The first set was formed from CuO NPs and cetyltrimethylammonium bromide (CTAB) and the second from CuO NPs without CTAB, after sonication and calcination. The results revealed that in the absence of CTAB, the nanoparticles have irregular shape and particle agglomeration was favored. Karunakaran et al. reported that the presence of CTAB promotes the crystal formation of CuO [ 29 ]. Also, Wongpisutpaisan et al. synthesized CuO NPs using the sonochemical method and then the product was calcined at various temperatures between 400 and 700 °C for 2 h. It was observed that at 400 and 500 °C the formation of CuO NPs was proved to be incomplete.
However, at 600 °C and 700 °C the authors observed the crystallization and the formation of uniform nanoparticles [ 30 ]. This method was used for the design of CuO NPs intended for medical applications. For example, Abramov et al. synthesized CuO NPs, which were used to coat medical cotton wound dressings and bandages by using the sonochemical method. It was reported that this combination was able to avoid microbial colonization and even kill various clinically relevant microorganisms such as Escherichia coli , which are reported to colonize the wounds of patients with skin lesions and require the usage of cotton dressings [ 31 ]. Also, Perelshtein et al. used the sonochemical method for the synthesis of CuO NPs to obtain coated textiles. These nanocoatings have shown an efficient antibacterial activity [ 32 ], therefore they are considered for the development of further biomedical applications, especially in the design of anti-infective surfaces, medical devices and anti-biofilm approaches.
2.4. Sol-Gel Method
The sol-gel technique is a simple and relatively fast method and therefore it is widely used in the design of nanoparticles [ 33 ]. This method is applied often as it ensures the rigorous control of the nanoparticle size. The method was optimized in order to obtain nanoparticles with dimensions ranging between 10 and 40 nm. Karthik et al. synthesized CuO NPs with dimensions of 25 nm by a sol-gel method [ 34 ]. The physical properties of CuO NPs also depend on the applied sol-gel method and the calcination time [ 28 ].
Moreover, in the case of sol-gel method, the size of nanoparticles is proportionally related with the temperature, physical conditions are very important for the design of functional nanoparticles using this approach [ 35 ]. Also by a sol-gel method, Jayaprakash et al. synthesized uncapped and capped CuO NPs by using ethylene diaminetetraacetic acid (EDTA). The capping agent was used to control the dimensions of CuONPs. Uncapped CuO NPs were synthesized with Cu(CH 3 COO) 2 ·H 2 O and urea. It was reported that this method allows the fine control of morphology and shape of the nanoparticles [ 33 ].
2.5. Other Synthetic Methods
Other methods for the synthesis of CuO NPs have been developed; such as hydrothermal approach [ 31 ], thermal oxidation method [ 8 ], alcohol-thermal synthesis [ 36 ], liquid ammonia [ 37 ] and microwave-assisted synthesis [ 29 ].
Direct thermal decomposition method is broadly used for the synthesis of CuO NPs. One approach consists in adding sodium carbonate to copper sulfate and by calcination spherical CuO NPs are formed [ 28 ].
CuO NPs obtained by using the thermal plasma technique have been proved to display improved properties which may be helpful in their biomedical applications. For example, such nanostructures seem to have enhanced antimicrobial activity against drug resistant bacteria, while maintaining an acceptable biocompatibility and small dimensions [ 38 ].
In the last years, green syntheses of nanoparticles, including of CuO NPs with biomedical purpose were intensively studied. Green synthesis is a preferred alternative of synthesis since is safer for the biological systems, environmental friendly and physical and chemical characteristics of nanoparticles are still suitable for biomedical use ( Figure 1 ) [ 39 ].

Microscopic images of CuO NPs synthesized by a green method: ( a ) scanning electron microscopy (SEM) image; ( b ) EDAX spectrometry of the CuO NPs; inset: elemental mapping of oxygen and copper; ( c – e ) transmission electron microscopy (TEM) images at different magnifications; ( f ) high magnification view of the CuO NPs; and ( g ) Selected Area Electron Diffraction (SAED) pattern of the CuO NPs [ 39 ].
3. Properties
The properties of the CuO NPs depend on the synthesis method selected and they are very important for their applications in various areas, such as biomedical research, which is the most predominant. The most important feature is the size of the nanoparticles (which may be controlled during the synthesis) because it allows the tailored modeling of their optical, catalytic, electrical, and biological properties [ 5 ]. These properties make them useful for multiple applications such as the development of cosmetics, pharmacological alternatives, paints, coatings, etc. [ 17 ]. Therefore, the applied synthesis method, the modulation of the reaction parameters and the composition of bulk material represent key aspects in the direct control of size and direct or indirect control of other important physical, chemical and biological properties.
3.1. Optical Properties
The optical properties of CuO NPs are significantly influenced by the temperature, size and morphology [ 8 , 13 , 23 ].
For example, one useful technique is UV–Vis absorption spectroscopy, which is very important as it provides essential information about the optical properties of the material. El Sayed et al. studied the optical properties of thin films made of carboxymethyl cellulose and polyvinyl alcohol (PVA) doped with CuO nanoparticles. The optical properties of carboxymethyl cellulose (CMC), PVA/carboxymethyl cellulose and CuO/PVA/carboxymethyl cellulose films were found to be different, depending on their composition. It was observed than the optical transmittance (T%) of carboxymethyl cellulose increased to approximately 87% by adding PVA, but decreased to approximately 77% after doping with 0.5 wt % CuO NPs. The refractive index of CMC has reached 1.576 by adding PVA and 1.852 by doping with CuO NPs [ 40 ].
Kayani et al. reported an analysis of the transmission spectra of CuO NPs: at 400 °C the average size of the nanoparticles was 350 nm and at 1000 °C, 367 nm, corresponding to 3.38 eV and 3.54 eV, respectively [ 11 ].
El-Trass et al. also synthesized CuO NPs by using the alcohol-thermal method and it was observed by UV-Vis that the absorption bandwidth of bulk CuO (1.85 eV) is narrower than the bandwidth of the CuO NPs (2.36 eV) [ 41 ].
3.2. Magnetic Properties
The magnetic properties of CuO NPs are also influenced by their dimensions [ 8 ]. Moreover, the magnetic properties of CuO NPs strictly depend on their morphology [ 42 ]. In a study focused on the properties of nanoparticles, the authors have obtained CuO NPs with dimensions from 13 nm to 33 nm and they confirmed a weak ferromagnetic interaction, the process being slightly influenced by the size of the particles [ 8 ]. On the contrary, Bisht et al. reported that in the case of CuO NPs with dimensions between 9 and 16 nm, the peak present in zero field cooled magnetization is absent. Also, there was a bifurcation between the zero field cooled and field cooled systems and it was observed that the system shows hysteresis at room temperature. The authors reported that the associated peak in magnetic viscosity and the relaxation of magnetization are similar to other nanoparticle systems [ 43 ]. These studies indicate that the magnetic properties of CuO NPs may be influenced by the particle size, but are definitely also controlled by other aspects, possibly their synthesis method, composition and ratio of bulk material and other physico-chemical properties.
3.3. Electrical Conductivity
It was reported in the literature that the synthesis temperature might control the electrical conductivity of CuO NPs. Zhang et al. reported that when temperature increases from 300 °C to 700 °C during the synthesis, the electrical conductivity of the CuO NPs increases from 10 −6 (Ω cm) −1 to 10 −5 (Ω cm) −1 , due to the removal of H 2 O vapor from the air [ 8 ].
Azimi and Taheri performed an assessment in order to investigate the electrical conductivity of CuO NPs. They used an aqueous solution of CuO NPs at different concentrations (0.12 g/L, 0.14 g/L, 0.16 g/L and 0.18 g/L), various temperatures of nanofluids, different particle sizes (89 nm, 95 nm, 100 nm and 112 nm) and concentrations of nanofluids obtained at the following temperatures: 25 °C, 35 °C, 45 °C and 50 °C. The authors revealed that electrical conductivity grew with the increase of temperature and nanoparticle concentration. It was also noted that the electrical conductivity increased until the dimensions of the nanoparticles reached 95 nm in diameter. This study demonstrated that there is a correlation between the values of electrical conductivity and nanoparticle size [ 44 ].
4. Medical Applications
CuO nanoparticles may have different applications depending on the various properties they manifest, which are highly influenced by their size, surface properties, optical and magnetic traits, the synthesis method being an important parameter for controlling all these and thus, their biological properties. Some of these applications include doping materials in semiconductors, such as chemical sensors, antimicrobial agents, catalyst for different cross coupling reactions, anti-cancer formulations, coating materials etc. Future biomedical applications of CuO NPs are focused intensively on disease detection and could present potential applications in many other areas, for example, in the detection of viruses in the human body [ 45 ]. In a recent study, Li et al. developed a highly sensitive and selective method for the detection of H1N1 flu virus. The principle of this method is based on labeling of antibodies by using CuO NPs. This approach was designed a sandwich complex made of CuO NPs labeled polyclonal antibody, able to detect and bind antigens represented by the H1N1 virus [ 46 ]. The method is an enzymatic chromogenic approach, belonging to the so-called enzyme linked immunosorbent assay (ELISA) methods, and proved to be highly sensitive and faster, as compared with other related methods.
4.1. Antibacterial Activity
Although the specific mechanism of the antimicrobial effect related with the use of CuO nanoparticles is not known, several of their mechanisms of action on bacterial cells have been discussed. Even if not specific to CuO nanoparticles, but for most oxide nanoparticles, Zhang et al. reported that the generation of reactive oxygen species (ROS) within bacterial cells is enhanced when using CuO-water suspensions [ 8 ].
The antibacterial activity of CuO NPs seems to be different depending on the particularities of bacteria cells. For examples, their cellular walls seem to impact the antimicrobial effect of CuO NPs, Gram character being a key aspect. It was reported that 100% of E. coli cells, which are Gram negative, were killed when a concentrations of CuO NPs higher than 9.5% was used, while for the Gram positive species Staphylococcus aureus the killing ability was lower [ 47 ]. It was also reported that CuO nanoparticles inhibit the growth of E. coli , Pseudomonas aeruginosa , and S. aureus in a time dependent manner, the utilized dose being, of course, the most important factor [ 48 ].
Goyal et al. also reported that the antimicrobial properties depend on the surface properties and size of nanoparticles. It seems that small particles with a large surface area have better antibacterial activity, as compared with larger ones. CuO NPs showed a major antimicrobial activity also against Bacillus subtilis [ 49 ]. El-Nahhal et al. tested the antibacterial activity of CuO NP-coated cotton dressings and CuS nanoparticle-coated cotton dressings. Both were inoculated with E. coli and S. aureus in order to compare the antimicrobial effect of the two coating systems in a Gram negative and Gram positive model, respectively. The results showed that the sample with CuO NPs presented higher antibacterial activity than the sample coated with CuS nanoparticles which showed no reduction in the viability of tested bacteria [ 50 ]. Devi et al. studied the antimicrobial activity of bulk, as-prepared and annealed CuO NPs against E. coli , Proteus mirabilis , Klebsiella spp., and their effect was comparable with the antimicrobial activity of gentamycin on these strains [ 9 , 51 ].
4.2. Toxicity of CuO Nanoparticles
CuO nanoparticles reveal different toxic activities in vitro and in vivo, when were tested on mammalian cells and on various animal models [ 52 , 53 ]. A study published in 1995 demonstrated that bioavailability of copper is the primary factor to determine the toxicity, a similar situation to what happens with toxic heavy metals [ 54 ]. Some features that can be modified to influence the toxicity of CuO NPs are:
- (a) Size: small nanoparticles are more toxic than larger ones.
- (b) Surface charge: the toxicity of nanoparticles is enhanced by a positive charge. This positive charge facilitates interactions between cells and nanoparticles.
- (c) Dissolution: the dissolution of CuO NPs depends on the temperature and pH of the Solution and this has a major influence on their toxicity [ 55 ].
CuO NPs appear to be twenty times more toxic to the protozoan Tetrahymena thermophila as compared with their bulk material. It has been shown that the toxicity depends on the exposure time, the efficiency being maintained at maximum level between 4 and 24 h of exposure [ 4 , 56 ]. Also, in many studies the toxicity of nanoparticles of CuO was compared with their bulk form. Franklin et al. performed the first study on CuO NPs toxicity on algae. They used algal cultures exposed to various concentrations of the test substance. The experiment proved that CuO NPs were more toxic for algae than their bulk material. Their study revealed that toxic effects of CuO NPs were maintained for at least 72 h [ 54 ]. Another study, confirmed that the solubility and the toxicity of CuO NPs in artificial freshwater is higher than compared with their bulk form, their solubility being proposed to strictly influence the biological effect of these nanoparticles and thus, toxicity [ 57 ].
Saison et al. studied the toxicity of a core-shell formed by nanoparticles coated with polystyrene on Chlamydomonas reinhardtii . It was observed that this nanosystem was toxic for this algae and it was concluded that toxicity was caused not only by the type of nanoparticle, but also by the surface properties of these nanoparticles [ 58 ].
Another study was performed to determine the effects of CuO NPs on Xenopus laevis tadpoles through metamorphosis. CuO NPs with size ranging to 23–37 nm and surface area of 24–40 m 2 ·g −1 were tested. The results revealed that the exposure to this nanoparticles for 5 days induced at least 40% mortality in all test groups [ 6 ]. Also, a high toxicity of these nanoparticles on Cyprinus carpio has been reported [ 59 ].
Even though the molecular toxicity mechanisms of CuO NPs on eukaryote models are not fully understood, many studies have shown that these nanoparticles promote mitochondrial damage, DNA damage and oxidative DNA damage [ 8 ].
In vitro assays revealed that toxicity of CuO NPs depended on the size and shape of the particles. For example, it was reported in literature that treatment with CuO NPs caused a significant decrease in cell proliferation, resulting in little cellular coverage of the culturing substrate after up to 6 days after the treatment. Moreover, the toxicity of CuO NPs is different depending on the differentiation state of the cells. CuO NPs toxicity seems to be significantly increased in differentiated cells as compared to non-differentiated cells.
In a recent study, Thit et al. aimed to determine the role of ROS release and establish the sequence of events during CuO NP toxicity. Their research utilized N -acetylcysteine to examine if increasing the cellular oxidative defense can mitigate DNA cytotoxicity and damage. The cells treated with Cu 2+ did not show a significant increase in the death of the cells 48 h after experiment initiation.
Results showed that CuO NPs were more toxic than Cu 2+ , due to the increase in the generation of ROS, DNA damage and decrease levels of reduced glutathione (GSH) compared to control [ 60 , 61 ].
The exposure of different vertebrate embryos to CuO NPs containing 10 mg·Cu/L did not produce a significant decrease of embryo survival. However, the ionic form seemed to be the most toxic, altering all the analyzed parameters, including survival and malformation, especially when embryos were exposed to 5–10 mg·Cu/L [ 62 ].
CuO NPs showed relatively high toxicity for human lung cultured cells and for human skin organ cultures, when compared with their micron-sized alternatives [ 63 , 64 ]. Rafiei et al. reported that CuO NPs induced oxidative stress in various cultured cells [ 65 ]. Also, Karlsson et al. reported that the oxidative stress induced by CuO ions could also support its genotoxicity [ 66 ]. In another study, Sun et al. compared the cytotoxicity of CuO NPs, ZnO nanoparticles, Fe 2 O 3 nanoparticles, Fe 2 O 4 nanoparticles and Al 2 O 3 nanoparticles in human cardiac microvascular endothelial cells (HCMECs). Cells were exposed to concentrations from 0.001 to 100 μg/mL of these nanoparticles for 12 to 24 h and the proliferation rates of these cells were analyzed by the reduction of tetrazolium salt (MTT) assay. Results showed that CuO and ZnO nanoparticles had a high degree of cytotoxicity against endothelial cells at all time points [ 67 ]. Dai et al. also investigated if the toxicity and bioaccumulation of sediment-associated Cu depends on the form of nanoparticles (CuO NPs vs. aqueous Cu, micron-sized particles) and nanoparticles shape (sphere-, rod-, or platelet-shaped CuO NPs) in a Capitella teleta worm model.
The results revealed no significant differences between measured sediment Cu concentrations; mortality rates were lower than 10% in worms grown in the presence of CuO NPs for up to 7 days. The results also revealed that worms were growing faster during the uptake period and slower during the depuration period, in all growth conditions and applied treatments [ 68 ].
Karlsson et al. studied the damage of cell membranes after treatment with CuO NPs and Cu metal nanoparticles. The authors observed that CuO NPs had caused very limited damage to the cell membrane, in comparison with Cu metal nanoparticles, which caused significant damage [ 63 ]. Also, Isani et al. reported that cell membrane damage caused by CuO NPs also increased hemolysis [ 15 ].
Fahmy and Cormier performed a study to analyze whether CuO NPs induce oxidative stress and cytotoxicity in airway epithelial cells. In this study, they were trying to compare the in vitro responses of respiratory epithelial cells after exposure to commercially available CuO NPs. They examined the ability of CuO NPs to generate ROS in HEP-2 cells, and compared the results with the ability of CuO NPs to induce oxidative stress in human epithelial cells. The authors observed that CuO NPs generated cytotoxicity, even at low doses, and were capable of inducing cell death [ 69 ]. In another study, Ahir et al. investigated the cytotoxicity of CuO NPs in HEP G2 cells and their results showed that these particles inhibit the growth of melanoma cells. CuO NPs fabricated with folic acid proved to be an efficient therapeutic option against triple negative breast cancer cells [ 70 ].
Chibber et al. assessed the neuronal toxicity of CuO NPs. After 24 h of exposure, some of the treated neurons partially detached from the dish and were found in suspension in the culture media. Detachment of the cells indicates the loss of the membrane integrity of neurons [ 71 ].
The mechanism of toxicity of CuO NPs in the cellular membrane was suggested to follow a Trojan horse-type mechanism ( Figure 2 ). If these nanoparticles are soluble they can penetrate the membrane cancelling the barrier function of the membrane. After entering the cells, nanoparticles are able to dissolve at the acidic intracellular pH (4.5), and metal ions produce pores in the membrane [ 72 , 73 ].

Toxicity mechanism of CuO nanoparticles in eukaryotic cells.
4.3. Current Applications of CuO NPs
CuO nanoparticles are mainly utilized as antimicrobial agents. They are used in hospitals due to their antimicrobial ability to kill more than 99.9% of Gram-positive and negative bacteria within 2 h of exposure, if a suitable dose is applied. Studies reported that the utilization of CuO reduces the occurrence of hospital-acquired infections and the costs associated with health care in health care facilities. Bed sheets containing CuO NPs are considered one of the most interesting innovations in medical care, since they reduce microbial attachment and thus microbial infections within hospitals [ 74 ].
Previous work had demonstrated that CuO NPs also have beneficial effects on the skin. Studies conducted on women who utilized pillowcases and beddings containing CuO NPs revealed an improved aspect of the facial skin and an increase in the foot skin elasticity using socks impregnated with copper oxide nanoparticles [ 75 ]. Some advantages of using copper-oxide in hospital textiles are:
- (1) It is effective against both, susceptible and antibiotic resistant microorganisms involved in nosocomial infections;
- (2) It has wide antifungal spectrum and antibacterial properties;
- (3) It inhibits biofilm or the development of microorganisms in attached communities on the surface of materials coated with CuO NPs;
- (4) It does not cause skin irritation or sensitization;
- (5) It is safe for humans if used externally and in low amounts [ 76 ].
Another application of these nanoparticles relies in their wound healing activity. Various wound dressings and textiles have been developed to treat burns and other skin injuries. The healing activity is proved to be strictly correlated with the capacity of CuO NPs to limit microbial colonization of the treated areas as well to avoid infection, while promoting regeneration of damaged tissue [ 77 ].
5. Conclusions and Perspectives
CuO nanoparticles can be synthesized by various methods, and by using various bulk materials and coating agents to obtain different types of nanosystems with various applications. All these aspects may significantly impact on their physico-chemical and biological properties and may affect their biomedical applications. The use of CuO nanoparticles in drug delivery formulations is still limited due to the enhanced toxicity, however other applications, such as topic formulations, dressings and coated textiles are of a great interest among the medical environments and others (i.e., cosmetics, textile industry etc.). The main application of such nanoformulations relies on their antimicrobial ability that allows for the development of multiple products, from antimicrobial solutions utilized to disinfect the surfaces and medical devices to antimicrobial wound dressings, textiles and coatings. For improving their applications on the biomedical field, researchers are striving to find optimal synthesis approaches in order to decrease the toxicity of CuO NPs but at the same time to keep or even improve their efficiency in diagnosis, therapy and, maybe even prophylaxis.
Acknowledgments
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS–UEFISCDI, project number PN-II-RU-TE 2014-4-2269.
Author Contributions
Authors equally contributed to this review paper.
Conflicts of Interest
The authors declare no conflict of interest.
Particle morphology and soil properties affect the retention of copper oxide nanoparticles in agricultural soils
- Original Paper
- Published: 04 July 2024
- Volume 46 , article number 281 , ( 2024 )
Cite this article

- Minghui Chang 1 ,
- Yinghao Liu 1 ,
- Meilan Xu 1 ,
- Helian Li 1 &
- Shi–Wei Li 1
10 Accesses
Explore all metrics
The interaction between nanoscale copper oxides (nano-CuOs) and soil matrix significantly affects their fate and transport in soils. This study investigates the retention of nano-CuOs and Cu 2+ ions in ten typical agricultural soils by employing the Freundlich adsorption model. Retention of nano-CuOs and Cu 2+ in soils was well fitted by the Freundlich model. The retention parameters (K D , K F , and N) followed an order of CuO NTs > CuO NPs > Cu 2+ , highlighting significant impact of nano-CuOs morphology. The K F and N values of CuO NPs/Cu 2+ were positively correlated with soil pH and electrical conductivity (EC), but exhibited a weaker correlation for CuO NTs. Soil pH and/or EC could be used to predict K F and N values of CuO NPs or CuO NTs, with additional clay content should be included for Cu 2+ .The different relationship between retention parameters and soil properties may suggest that CuO NTs retention mainly caused by agglomeration, whereas adsorption and agglomeration were of equal importance to CuO NPs. The amendment of Ca 2+ at low and medium concentration promoted retention of nano-CuOs in alkaline soils, but reduced at high concentration. These findings provided critical insights into the fate of nano-CuOs in soil environments, with significant implications for environmental risk assessment and soil remediation strategies.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Biochar for the removal of contaminants from soil and water: a review

Impact of microplastics on soil (physical and chemical) properties, soil biological properties/soil biota, and response of plants to it: a review

Biochar and its importance on nutrient dynamics in soil and plant
Data availability.
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Adisa, I. O., Pullagurala, V. L. R., Peralta-Videa, J. R., Dimkpa, C. O., Elmer, W. H., Gardea-Torresdey, J. L., & White, J. C. (2019). Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environmental Science: Nano, 6 , 2002–2030.
CAS Google Scholar
Auffan, M., Liu, W., Brousset, L., Scifo, L., Pariat, A., Sanles, M., Chaurand, P., Angeletti, B., Thiéry, A., Masion, A., & Rose, J. (2018). Environmental exposure of a simulated pond ecosystem to a CuO nanoparticle-based wood stain throughout its life cycle. Environmental Science: Nano, 5 , 2579–2589.
Baddar, Z. E., Matocha, C. J., & Unrine, J. M. (2019). Surface coating effects on the sorption and dissolution of ZnO nanoparticles in soil. Environmental Science: Nano, 6 , 2495–2507.
Google Scholar
Bae, S., Hwang, Y. S., Lee, Y. J., & Lee, S. K. (2013). Effects of water chemistry on aggregation and soil adsorption of silver nanoparticles. Environmental Health and Toxicology, 28 , e2013006.
Article Google Scholar
Chung, H., Kim, S. H., & Nam, K. (2020). Inhibition of urea hydrolysis by free Cu concentration of soil solution in microbially induced calcium carbonate precipitation. Science of the Total Environment, 740 , 140194.
Article CAS Google Scholar
Deng, C., Wang, Y., Cota-Ruiz, K., Reyes, A., Sun, Y., Peralta-Videa, J., Hernandez-Viezcas, J. A., Turley, R. S., Niu, G., Li, C., & Gardea-Torresdey, J. (2020). Bok choy (Brassica rapa) grown in copper oxide nanoparticles-amended soils exhibits toxicity in a phenotype-dependent manner: Translocation, biodistribution and nutritional disturbance. Journal of Hazardous Materials, 398 , 122978.
Elbana, T. A., Selim, H. M., Akrami, N., Newman, A., Shaheen, S. M., & Rinklebe, J. (2018). Freundlich sorption parameters for cadmium, copper, nickel, lead, and zinc for different soils: Influence of kinetics. Geoderma, 324 , 80–88.
Fang, J., Shan, X.-Q., Wen, B., Lin, J.-M., Owens, G., & Zhou, S.-R. (2011). Transport of copper as affected by titania nanoparticles in soil columns. Environmental Pollution, 159 , 1248–1256.
Fedorenko, A. G., Minkina, T. M., Chernikova, N. P., Fedorenko, G. M., Mandzhieva, S. S., Rajput, V. D., Burachevskaya, M. V., Chaplygin, V. A., Bauer, T. V., Sushkova, S. N., & Soldatov, A. V. (2021). The toxic effect of CuO of different dispersion degrees on the structure and ultrastructure of spring barley cells (Hordeum sativum distichum). Environmental Geochemistry and Health, 43 , 1673–1687.
Fischer, J., Evlanova, A., Philippe, A., & Filser, J. (2020). Soil properties can evoke toxicity of copper oxide nanoparticles towards springtails at low concentrations. Environmental Pollution, 270 , 116084.
Gao, X., Avellan, A., Laughton, S., Vaidya, R., Rodrigues, S. M., Casman, E. A., & Lowry, G. V. (2018). CuO nanoparticle dissolution and toxicity to wheat ( Triticum aestivum) in rhizosphere soil. Environmental Science & Technology, 52 , 2888–2897.
Gao, X., Rodrigues, S. M., Spielman-Sun, E., Lopes, S., Rodrigues, S., Zhang, Y., Avellan, A., Duarte, R., Duarte, A., Casman, E. A., & Lowry, G. V. (2019). Effect of soil organic matter, soil pH, and moisture content on solubility and dissolution rate of CuO NPs in soil. Environmental Science & Technology, 53 , 4959–4967.
Glad, X., Profili, J., Cha, M.S., Hamdan, A., 2020. Synthesis of copper and copper oxide nanomaterials by electrical discharges in water with various electrical conductivities. Journal of Applied Physics, 127 , 023302.
Guan, X., Gao, X., Avellan, A., Spielman-Sun, E., Xu, J., Laughton, S., Yun, J., Zhang, Y., Bland, G. D., Zhang, Y., Zhang, R., Wang, X., Casman, E. A., & Lowry, G. V. (2020). CuO nanoparticles alter the rhizospheric bacterial community and local nitrogen cycling for wheat grown in a calcareous soil. Environmental Science & Technology, 54 , 8699–8709.
Julich, D., & Gäth, S. (2014). Sorption behavior of copper nanoparticles in soils compared to copper ions. Geoderma, 235–236 , 127–132.
Komárek, M., Vaněk, A., Chrastný, V., Száková, J., Kubová, K., Drahota, P., & Balík, J. (2009). Retention of copper originating from different fungicides in contrasting soil types. Journal of Hazardous Materials, 166 (2–3), 1395–1402.
Kool, P. L., Ortiz, M. D., & van Gestel, C. A. M. (2011). Chronic toxicity of ZnO nanoparticles, non-nano ZnO and ZnCl 2 to Folsomia candida (Collembola) in relation to bioavailability in soil. Environmental Pollution, 159 , 2713–2719.
Liu, Y., Li, Y., Pan, B., Zhang, X., Zhang, H., Steinberg, C. E. W., Qiu, H., Vijver, M. G., & Peijnenburg, W. (2020). Application of low dosage of copper oxide and zinc oxide nanoparticles boosts bacterial and fungal communities in soil. Science of the Total Environment, 757 , 143807.
Mahdavi, S., Jalali, M., & Afkhami, A. (2012). Removal of heavy metals from aqueous solutions using Fe 3 O 4 , ZnO, and CuO nanoparticles. Journal of Nanoparticle Research, 14 , 846.
Pu, S., Yan, C., Huang, H., Liu, S., & Deng, D. (2019). Toxicity of nano-CuO particles to maize and microbial community largely depends on its bioavailable fractions. Environmental Pollution, 255 , 113248.
Qiu, Y., Zuting, M., Wang, N., Wang, X., Meilan, X., & Li, H. (2020). The aggregation and sedimentation of two different sized copper oxide nanoparticles in soil solutions: Dependence on pH and dissolved organic matter. Science of the Total Environment, 731 , 139215. https://doi.org/10.1016/j.scitotenv.2020.139215
Rahmatpour, S., Shirvani, M., Mosaddeghi, M. R., & Bazarganipour, M. (2017). Retention of silver nano-particles and silver ions in calcareous soils: Influence of soil properties. Journal of Environmental Management, 193 , 136–145.
Rodrigues, S. M., Trindade, T., Duarte, A. C., Pereira, E., Koopmans, G. F., & Römkens, P. F. A. M. (2016). A framework to measure the availability of engineered nanoparticles in soils: Trends in soil tests and analytical tools. Trends in Analytical Chemistry, 75 , 129–140.
Shah, V., Luxton, T. P., Walker, V. K., Brumfield, T., Yost, J., Shah, S., Wilkinson, J. E., & Kambhampati, M. (2016). Fate and impact of zero-valent copper nanoparticles on geographically-distinct soils. Science of the Total Environment, 573 , 661–670.
Shaheen, S. M., Tsadilas, C. D., & Rinklebe, J. (2013). A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Advances in Colloid & Interface Science, 201–202 , 43–56.
Sun, L., Xue, Y., Peng, C., Xu, C., & Shi, J. (2020). Influence of sulfur fertilization on CuO nanoparticles migration and transformation in soil pore water from the rice ( Oryza sativa L.) rhizosphere. Environmental Pollution, 257 , 113608.
Usman, A. R. A. (2008). The relative adsorption selectivities of Pb, Cu, Zn, Cd and Ni by soils developed on shale in New Valley. Egypt. Geoderma, 144 , 334–343.
VandeVoort, A. R., & Arai, Y. (2012). Effect of silver nanoparticles on soil denitrification kinetics. Industrial Biotechnology, 8 , 358–364.
Wu, H., Fang, H., Xu, C., Ye, J., Cai, Q., & Shi, J. (2020). Transport and retention of copper oxide nanoparticles under unfavorable deposition conditions caused by repulsive van der Waals force in saturated porous media. Environmental Pollution, 256 , 113400.
Xu, M., Wang, Y., Mu, Z., Li, S., & Li, H. (2021). Dissolution of copper oxide nanoparticles is controlled by soil solution pH, dissolved organic matter, and particle specific surface area. Science of the Total Environment, 772 , 145477.
Zong, X., Wu, D., Zhang, J., Tong, X., Yin, Y., Sun, Y., & Guo, H. (2022). Size-dependent biological effect of copper oxide nanoparticles exposure on cucumber (Cucumis sativus). Environmental Science and Pollution Research, 29 , 69517–69526.
Download references
This work was financially supported by the National Natural Science Foundation of China (Grant number 41771524); Shandong Provincial Natural Science Foundation, China (ZR2023MD026); Development Plan of Youth Innovation Team in Colleges and Universities of Shandong Province (2022KJ099).
Author information
Authors and affiliations.
School of Water Conservancy and Environment, University of Jinan, Jinan, 250022, China
Minghui Chang, Yinghao Liu, Meilan Xu, Helian Li & Shi–Wei Li
You can also search for this author in PubMed Google Scholar
Contributions
MHC: investigation, writing—original draft. YHL: investigation. MLX: investigation. HLL: conceptualization, supervision, writing—review and editing, funding acquisition. SWL: review and editing, methodology, review and editing, funding acquisition.
Corresponding authors
Correspondence to Helian Li or Shi–Wei Li .
Ethics declarations
Conflict of interest.
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The manuscript did not contain any reporting studies involving human data.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that all research participants provided informed consent for publication of all the data in this study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chang, M., Liu, Y., Xu, M. et al. Particle morphology and soil properties affect the retention of copper oxide nanoparticles in agricultural soils. Environ Geochem Health 46 , 281 (2024). https://doi.org/10.1007/s10653-024-02057-5
Download citation
Received : 26 March 2024
Accepted : 28 May 2024
Published : 04 July 2024
DOI : https://doi.org/10.1007/s10653-024-02057-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Copper oxide nanoparticles
- Agricultural soils
- Soil properties
- Find a journal
- Publish with us
- Track your research
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Synthesis and superficial modification “in situ” of copper selenide (cu 2-x se) nanoparticles and their antibacterial activity.

1. Introduction
2. materials and methods, 2.1. materials, 2.2. synthesis of copper selenide (cu 2-x se) nanoparticles, 2.3. antimicrobial analysis, 2.4. characterization techniques, 3. results and discussion, 3.1. x-ray diffraction analysis of copper selenide (cu 2-x se) nanoparticles, 3.2. fourier transformed infrared spectroscopy (ftir), 3.3. thermogravimetric analysis (tga), 3.4. differential scanning calorimetry (dsc), 3.5. transmission electronic microscopy (tem), 3.6. antimicrobial sensitivity, 4. conclusions, author contributions, data availability statement, acknowledgments, conflicts of interest.
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Lugo-Uribe, L.E.; Cabello-Alvarado, C.; Mata-Padilla, J.M.; Barriga-Castro, E.D. Synthesis of Copper Nanoparticles Stabilized with Organic Ligands and Their Antimicrobial Properties. Polymers 2021 , 13 , 2846. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cota-Ungson, D.; González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Graphene–Cu Nanocomposites Induce Tolerance against Fusarium oxysporum , Increase Antioxidant Activity, and Decrease Stress in Tomato Plants. Plants 2023 , 12 , 2270. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jardón-Maximino, N.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; Comparán-Padilla, V.E.; Lugo-Uribe, L.E.; Pérez-Alvarez, M.; Tavizón, S.F.; Santillán, G.d.J.S. Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers 2021 , 13 , 1694. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch Toxicol. 2014 , 88 , 1929. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Harishchandra, B.D.; Pappuswamy, M.; Antony, P.U.; Shama, G.; Pragatheesh, A.; Arumugam, V.A.; Periyaswamy, T.; Sundaram, R. Copper Nanoparticles: A Review on Synthesis, Characterization and Applications. Asian Pac. J. Cancer Biol. 2020 , 5 , 201–210. [ Google Scholar ] [ CrossRef ]
- Pulkkinen, P.; Shan, J.; Leppänen, K.; Känsäkoski, A.; Laiho, A.; Järn, M.; Tenhu, H. Poly (ethylene imine) and Tetraethylenepentamine as Protecting Agents for Metallic Copper Nanoparticles. ACS Appl. Mater. Interfaces 2009 , 1 , 519–525. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review synthesis and biomedical applications. Mater. Adv. 2022 , 3 , 1415–1431. [ Google Scholar ] [ CrossRef ]
- Zhu, M.; Niu, G.; Tang, J. Elemental Se: Fundamentals and its optoelectronic applications. J. Mater. Chem. C 2019 , 7 , 2199–2206. [ Google Scholar ] [ CrossRef ]
- Mbewana-Ntshanka, N.G.; Moloto, M.J.; Mubiayi, P.K. Antimicrobial activity of the synthesized of copper chalcogenide nanoparticles. J. Nanotechnol. 2021 , 2021 , 6675145. [ Google Scholar ] [ CrossRef ]
- Khan, M.M. Introduction and fundamentals of chalcogenides and chalcogenides-based nanomaterials. In Chalcogenide-Based Nanomaterials as Photocatalysts, Micro and Nano Technologies ; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–6. [ Google Scholar ] [ CrossRef ]
- Hao, X.; Jia, J.; Chang, Y.; Jia, M.; Wen, Z. Monodisperse copper selenide nanoparticles for ultrasensitive and selective non-enzymatic glucose biosensor, Electrochim . Acta 2019 , 327 , 135020. [ Google Scholar ] [ CrossRef ]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper Selenide Nanocrystals for Photothermal Therapy. Nano Lett. 2011 , 11 , 2560–2566. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dong, H.; Quintilla, A.; Cemernjak, M.; Popescu, R.; Gerthsen, D.; Ahlswede, E.; Feldmann, C. Colloidally stable selenium@copper selenide core@shell nanoparticles as selenium source for manufacturing of copper–indium–selenide solar cells. J. Colloid Interface Sci. 2014 , 415 , 103–110. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, S.C.; Li, H.; Yao, C.; Zhan, Z.; Yu, W.; Yu, Z.; Guo, C. Structural and compositional control in copper selenide nanocrystals for light-induced self-repairable electrodes. Nano Energy 2018 , 51 , 774–785. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, X.; Law, W.-C.; Jeon, M.; Wang, X.; Liu, M.; Kim, C.; Prasad, P.N.; Swihart, M.T. Cu 2–x Se Nanocrystals with Localized Surface Plasmon Resonance as Sensitive Contrast Agents for in Vivo Photoacoustic Imaging: Demonstration of Sentinel Lymph Node Mapping. Adv. Healthc. Mater. 2013 , 2 , 952–957. [ Google Scholar ] [ CrossRef ]
- Patel, S.R.; Chaki, S.H.; Giri, R.K.; Khimani, A.J.; Vaidya, Y.H.; Thakor, P.; Thakkar, A.B.; Deshpande, M.P. Pristine, Ni- and Zn—Doped CuSe Nanoparticles: An Antimicrobial, Antioxidant, and Cytotoxicity Sutdy. ACS Appl. Bio. Mater. 2023 , 6 , 2211. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shi, Y.; Li, Y.; Huang, C.; Xu, Y.; Xu, Y. Electrogenerated copper selenide with positive charge to efficiently capture and combat drug-resistant bacterial for wound healing. J. Colloid Interface Sci. 2023 , 634 , 852–863. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019 , 10 , 677. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology control and electronic tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023 , 460 , 141674. [ Google Scholar ] [ CrossRef ]
- Lie, S.Q.; Wang, D.M.; Gao, M.X.; Huang, C.Z. Controllable copper deficiency in Cu 2-x Se nanocrystals with tunable localized surface plasmon resonance and enhanced chemiluminescence. Nanoscale 2014 , 6 , 10289–10296. [ Google Scholar ] [ CrossRef ]
- Rasheed, M.; Saira, F.; Batool, Z.; Yaseen, J.; Arshad, M.; Kalsoom, A.; Ahmed, H.E.; Ashiq, M.N. Facile synthesis of a CuSe/PVP nanocomposite for ultrasensitive non-enzymatic glucose biosensing. RSC Adv. 2023 , 13 , 26755–26765. [ Google Scholar ] [ CrossRef ]
- Iqbal, M.; Bhatti, H.N.; Younis, S.; Rehmat, S.; Alwadai, N.; Almuqrin, A.H.; Iqbal, M. Graphene oxide nanocomposite with CuSe and photocatalytic removal of methyl green dye under visible light irradiation. Diam. Relat. Mater. 2021 , 113 , 108254. [ Google Scholar ] [ CrossRef ]
- Alharbi, F.F.; Ahmad, Z.; Chughtai, A.H.; Khosa, R.Y.; Farid, H.M.T. Designing CuSe-gCN nanocomposite as an active electrocatalyst for water oxidation. Korean J. Chem. Eng. 2023 , 40 , 2303–2311. [ Google Scholar ] [ CrossRef ]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Nguyen, T.T.; Van Tran, T. Formation, antimicrobial activity, and biomedical performance of plant-based nanoparticles: A review. Environ. Chem. Lett. 2022 , 20 , 2531–2571. [ Google Scholar ] [ CrossRef ]
- Donald-Heyding, R.; MacLaren Murray, R. The crystal structures of Cu 1.8 Se, Cu 3 Se 2 , α- and γCuSe, CuSe, and CuSe 2 II. Can. J. Chem. 1976 , 54 , 841–848. [ Google Scholar ] [ CrossRef ]
- Gosavi, S.R.; Deshpande, N.G.; Gudage, Y.G.; Sharma, R. Physical, optical and electrical properties of copper selenide (CuSe) thin films deposited by solution growth technique at room temperature. J. Alloys Compd. 2008 , 448 , 344–348. [ Google Scholar ] [ CrossRef ]
- Shafizade, R.B.; Ivanova, I.V.; Kazinets, M.M. Electron diffraction study of phase transformations of the compound CuSe. Thin Solid Films 1978 , 55 , 211–220. [ Google Scholar ] [ CrossRef ]
- Bhuse, V.M.; Hankare, P.P.; Garadkar, K.M.; Khomane, A.S. A simple, convenient, low temperature route to grow polycrystalline copper selenide thin films. Mater. Chem. Phys. 2003 , 80 , 82–88. [ Google Scholar ] [ CrossRef ]
- Senthilkumar, M.; Imla Mary, C.; Moorthy Babu, S. Morphological controlled synthesis of hierarchical copper selenide nanocrystals by Oleic acid, 1-Dodecanethiol and 1-Octadecene as surfactants. J. Cryst. Growth 2017 , 468 , 169–174. [ Google Scholar ] [ CrossRef ]
- Abraham, A.; Tarachand; Okram, G.S.; Jacob, R.; Sreenivasan, P.V.; Reena Philip, R. Low temperature electrical conductivity and seebeck coefficient of nanostructured CuSe thin films with Ga addition. Vacuum 2016 , 129 , 74–78. [ Google Scholar ] [ CrossRef ]
- Chen, X.; Li, Z.; Yang, J.; Sun, Q.; Dou, S. Aqueous preparation of surfactant-free copper selenide nanowires. J. Colloid Interface Sci. 2015 , 442 , 140–146. [ Google Scholar ] [ CrossRef ]
- Gurin, V.S.; Alexeenko, A.A.; Zolotovskaya, S.A.; Yumashev, K.V. Copper and copper selenide nanoparticles in the sol-gel metrices: Structural and optical. Mater. Sci. Eng. C 2006 , 26 , 952–955. [ Google Scholar ] [ CrossRef ]
- Liu, K.; Liu, H.; Wang, J.; Shi, L. Synthesis and characterization of Cu2Se prepared by hydrothermal co-reduction. J. Alloys Compd. 2009 , 484 , 674–676. [ Google Scholar ] [ CrossRef ]
- Hu, H.; Ge, X.; Deng, C.; Sun, M.; Xuan, H.; Zhang, K. Copper selenide (CuSe and Cu 2 Se) nanocrystals: Controllable synthesis through a facile ultrasonic chemical route. Asian J. Chem. 2013 , 25 , 5516–5518. [ Google Scholar ] [ CrossRef ]
- Liew, J.Y.C.; Talib, Z.A.; Zainal, Z.; Ahmad Kamarudin, M.; Osman, N.H.; Kee Lee, H. Structural and transport mechanism studies of copper selenide nanoparticles. Semicond. Sci. Technol. 2019 , 34 , 125017. [ Google Scholar ] [ CrossRef ]
- Huang, P.; Kong, Y.; Li, Z.; Gao, F.; Cui, D. Copper Selenide Nanosnakes: Bovine Serum Albumin-Assisted Room Temperature Controllable Synthesis and Characterization. Nanoscale Res Lett. 2010 , 5 , 949–956. [ Google Scholar ] [ CrossRef ]
- Patidar, D.; Saxena, N.S. Characterization of single phase copper selenide nanoparticles and their growth mechanism. J. Cryst. Growth 2012 , 343 , 68–72. [ Google Scholar ] [ CrossRef ]
- Mancillas-Salas, S.; Ledón-Smith, J.Á.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; Mata-Padilla, J.M.; Andrade-Guel, M.; Esparza-González, S.C.; Vargas-Gutiérrez, G.; Sierra-Gómez, U.A.; Saucedo-Salazar, E.M. Nanostructured Copper Selenide Coatings for Antifouling Applications. Polymers 2024 , 16 , 489. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016 , 6 , 71–79. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Clinical and Laboratory Standards Institute (CLSI). CLSI Standard M07 ; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed, Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1-56238-837-1. [ Google Scholar ]
- Xie, Y.; Zheng, X.; Jiang, X.; Lu, J.; Zhu, L. Sonochemical Synthesis and Mechanistic Study of Copper Selenides Cu 2-x Se, β-CuSe, and Cu 3 Se 2 . Inorg. Chem. 2002 , 41 , 387–392. [ Google Scholar ] [ CrossRef ]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Veerabhadram, G. Synthesis of Stable Silver Nanoparticles Using Gum Acacia as Reducing and Stabilizing Agent and Study of Its Microbial Properties: A Novel Green Approach. Int. J. Green Nanotechnol. 2012 , 4 , 199–206. [ Google Scholar ] [ CrossRef ]
- Mazhar, M.; Bakhtawar, S.; Rana, A.; Usmani, M.; Akhtar, N.; Abbas, W.; Khan, K.; Ahmad, J. Insight into the Structural Characterization of Pure and Zr-Doped Hydrothermally Synthesized Cerium Oxide Nanoparticles. Mater. Res. Express 2019 , 6 , 105022. [ Google Scholar ] [ CrossRef ]
- Vieira, A.P.; Stein, E.M.; Andregietti, D.X.; Cebrián-Torrejón, G.; Doménech-Carbó, A.; Colepicolo, P.; Ferreira, A.M.D.C. “Sweet chemistry”: A green way for obtaining selenium nanoparticles active against cancer cells. J. Braz. Chem. Soc. 2017 , 28 , 2021–2027. [ Google Scholar ] [ CrossRef ]
- Souza, L.M.D.S.; Dibo, M.; Sarmiento, J.J.P.; Seabra, A.B.; Medeiros, L.P.; Lourenço, I.M.; Kobayashi, R.K.T.; Nakazato, G. Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. Curr. Res. Green Sustain. Chem. 2022 , 5 , 100303. [ Google Scholar ] [ CrossRef ]
- Jiang, F.; Cai, W.; Tan, G. Facile Synthesis and Optical Properties of Small Selenium Nanocrystals and Nanorods. Nanoscale Res. Lett. 2017 , 12 , 401. [ Google Scholar ] [ CrossRef ]
- Pérez-Alvarez, M.; Cadenas-Pliego, G.; Pérez-Camacho, O.; Comparán-Padilla, V.E.; Cabello-Alvarado, C.J.; Saucedo-Salazar, E. Green Synthesis of Copper Nanoparticles Using Cotton. Polymers 2021 , 13 , 1906. [ Google Scholar ] [ CrossRef ]
- Velayati, M.; Hassani, H.; Sabouri, Z.; Mostafapour, A.; Darroudi, M. Biosynthesis of Se-Nanorods using Gum Arabig (GA) and Investigation of Their Photocatalytic and Cytotoxicity Effects. Inorg. Chem. Commun. 2021 , 128 , 108589. [ Google Scholar ] [ CrossRef ]
- Shitu, I.G.; Talib, Z.A.; Chi, J.L.Y.; Kechick, M.M.A.; Baqiah, H. Influence of Tartaric Acid Concentration on Structural and Optical Properties of CuSe Nanoparticles Synthesized Via Microwave Assisted Method. Results Phys. 2020 , 17 , 103041. [ Google Scholar ] [ CrossRef ]
- Daoub, R.M.A.; Elmubarak, A.H.; Misran, M.; Hassan, E.A.; Osman, M.E. Characterization and Functional Properties of Some Natural Acacia Gums. J. Saudi Soc. Agric. Sci. 2018 , 17 , 241–249. [ Google Scholar ] [ CrossRef ]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal Studies on Natural and Modified Gums. Polym. Test. 2004 , 23 , 575–579. [ Google Scholar ] [ CrossRef ]
- Jamaludin, J.; Adam, F.; Rasid, R.A.; Hassan, Z. Thermal Studies on Arabic Gum—Carrageenan Polysaccharides Film. Chem. Eng. Res. Bull. 2017 , 19 , 80–89. [ Google Scholar ] [ CrossRef ]
- Nawaz, M.; Shakoor, R.; Kahraman, R.; Montemor, M. Cerium Oxide Loaded with Gum Arabic as Environmentally Friendly Anti-Corrosion Additive for Protection of Coated Steel. Mater. Des. 2021 , 198 , 109361. [ Google Scholar ] [ CrossRef ]
- Amir, M.; Farooq, M.; Ambreen, J.; Ahmad, N.; Iqbal, M.; Haleem, A.; Saeed, S.; Shah, A.; Siddiq, M. Synthesis and Characterization of Gum Arabic Microgels Stabilizing Metal Based Nanocatalysts for Ultrafast Catalytic Reduction of 4-Nitrophenol at Ambient Conditions. J. Environ. Chem. Eng. 2019 , 7 , 103280. [ Google Scholar ] [ CrossRef ]
- Farooq, M.; Sagbas, S.; Mildiz, M.; Meral, K.; Siddiq, M.; Aktas, N.; Sahiner, N. Gum Arabic Microgels as Template for in Situ Metal-Sulfide Based Quantum Dots Preparation and Their Thermal, Spectroscopic, Optical, and Magnetic Characterization. J. Electron. Mater. 2017 , 46 , 4373–4383. [ Google Scholar ] [ CrossRef ]
- Liu, Q.; Wu, J.; Wang, P.; Lu, Y.; Ban, X. Neutral Polysaccharides From Hohenbuehelia serotina With Hypoglycemic Effects in a Type 2 Diabetic Mouse Model. Front. Pharmacol. 2022 , 13 , 883653. [ Google Scholar ] [ CrossRef ]
- Jadhav, A.A.; Khanna, P.K. Impact of Microwave Irradiation on Cyclo-Octeno1,2,3-Selenadiazole: Formation of Selenium Nanoparticles and Their Polymorphs. RSC Adv. 2015 , 5 , 44756–44763. [ Google Scholar ] [ CrossRef ]
- Saltana, F.; Hakan, A. Synthesis and thermal degradation Kinetics of D-(+)-galacotse containing polymers. Polímeros 2013 , 23 , 697–704. [ Google Scholar ] [ CrossRef ]
- Chen, Z.; Shen, Y.; Xie, A.; Zhu, J.; Wu, Z.; Huang, F. L-Cysteine-Assisted Controlled Synthesis of Selenium Nanospheres and Nanorods. Cryst. Growth Des. 2009 , 9 , 1327. [ Google Scholar ] [ CrossRef ]
- Zhang, S.; Huang, Q.; Zhang, L.; Zhang, H.; Han, Y.; Sun, Q.; Cheng, Z.; Qin, H.; Dou, S.; Li, Z. Vacancy Engineering of Cu 2-x Se Nanoparticles with Tunable LSPR and Magnetism for Dual-Modal Imaging Guided Photothermal Therapy of Cancer. Nanoscale 2018 , 10 , 3130–3143. [ Google Scholar ] [ CrossRef ]
- Gobeaut, A.; Laffont, L.; Tarascon, J.M.; Parissi, L.; Kerrec, O. Influence of secondary phases during annealing on re-crystallization of CuInSe 2 electrodeposited films. Thin Solid Films 2009 , 517 , 4436–4442. [ Google Scholar ] [ CrossRef ]
- Wolf, D.; Müller, G. Kinetics of CIS-formation studied in situ by thin film calorimetry. Thin Solid Film 2000 , 361 , 155–160. [ Google Scholar ] [ CrossRef ]
- Venegas del Castillo, A.; Vásquez-Valles, M.N. Efecto Del Aceite Esencial De Lantana Camara Sobre El Crecimiento De Staphylococcus Aureus y Escherichia coli . REBIOL 2020 , 36 , 29–37. Available online: https://revistas.unitru.edu.pe/index.php/facccbiol/article/view/1311 (accessed on 16 June 2024).
Click here to enlarge figure
| Sample | Concentration (% wt.) | Microorganism | Average Inhibition Diameter (mm) and Standard Deviation |
|---|---|---|---|
| CL S.A. | 0 | Staphylococcus aureus | 0 |
| 1.0% S.A. | 1.0 | Staphylococcus aureus | 15.1 ± 1.2 |
| 2.0% S.A. | 2.0 | Staphylococcus aureus | 21.1 ± 2.9 |
| 3.0% S.A. | 3.0 | Staphylococcus aureus | 23.9 ± 3.3 |
| 4.0% S.A. | 4.0 | Staphylococcus aureus | 24.0 ± 2.2 |
| 8% S.A. | 8.0 | Staphylococcus aureus | 29.2 ± 1.8 |
| CL C. | 0 | Candida albicans | 0 |
| 1.0% C. | 1.0 | Candida albicans | 8.1 ±1.8 |
| 2.0% C. | 2.0 | Candida albicans | 15.2 ± 1.0 |
| 3.0% C. | 3.0 | Candida albicans | 17.3 ± 1.0 |
| 4.0% C. | 4.0 | Candida albicans | 18.3 ± 2.2 |
| 8.0% C. | 8.0 | Candida albicans | 21.3 ± 1.2 |
| CL E.C. | 0 | Escherichia coli | 0 |
| 1.0% E.C. | 1.0 | Escherichia coli | 8.2 ± 0.70 |
| 2.0% E.C. | 2.0 | Escherichia coli | 11.9 ± 2.3 |
| 3.0% E.C. | 3.0 | Escherichia coli | 13.8 ± 2.0 |
| 4.0% E.C. | 4.0 | Escherichia coli | 15.6 ± 1.2 |
| 8.0% E.C. | 8.0 | Escherichia coli | 17.2 ± 0.40 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Mata-Padilla, J.M.; Ledón-Smith, J.Á.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Barriga-Castro, E.D.; Pérez-Camacho, O.; Cabello-Alvarado, C.J.; Silva, R. Synthesis and Superficial Modification “In Situ” of Copper Selenide (Cu 2-x Se) Nanoparticles and Their Antibacterial Activity. Nanomaterials 2024 , 14 , 1151. https://doi.org/10.3390/nano14131151
Mata-Padilla JM, Ledón-Smith JÁ, Pérez-Alvarez M, Cadenas-Pliego G, Barriga-Castro ED, Pérez-Camacho O, Cabello-Alvarado CJ, Silva R. Synthesis and Superficial Modification “In Situ” of Copper Selenide (Cu 2-x Se) Nanoparticles and Their Antibacterial Activity. Nanomaterials . 2024; 14(13):1151. https://doi.org/10.3390/nano14131151
Mata-Padilla, José Manuel, José Ángel Ledón-Smith, Marissa Pérez-Alvarez, Gregorio Cadenas-Pliego, Enrique Díaz Barriga-Castro, Odilia Pérez-Camacho, Christian Javier Cabello-Alvarado, and Rodolfo Silva. 2024. "Synthesis and Superficial Modification “In Situ” of Copper Selenide (Cu 2-x Se) Nanoparticles and Their Antibacterial Activity" Nanomaterials 14, no. 13: 1151. https://doi.org/10.3390/nano14131151
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Zinc oxide and copper oxide nanoparticles as a potential solution for controlling Phytophthora infestans, the late blight disease of potatoes
- AlHarethi, Amira A.
- Abdullah, Qais Y.
- AlJobory, Hala J.
- Anam, AbdulRahman M.
- Arafa, Ramadan A.
- Farroh, Khaled Y.
Late blight, caused by Phytophthora infestans, is a major potato disease globally, leading to significant economic losses of $6.7 billion. To address this issue, we evaluated the antifungal activity of ZnO and CuO nanoparticles (NPs) against P. infestans for the first time in laboratory and greenhouse conditions. Nanoparticles were synthesized via a chemical precipitation method and characterized using various techniques. The XRD results revealed that the synthesized ZnO nanoparticles had a pure hexagonal wurtzite crystalline structure, whereas the CuO NPs had a monoclinic crystalline structure. TEM images confirmed the synthesis of quasi-spherical nanoparticles with an average size of 11.5 nm for ZnO NPs and 24.5 nm for CuO NPs. The UV-Vis Spectral Report showed peaks corresponding to ZnO NPs at 364 nm and 252 nm for CuO NPs.In an in vitro study, both ZnO and CuO NPs significantly (p < 0.05) inhibited the radial growth of P. infestans at all tested concentrations compared to the untreated control. The highest inhibitory effect of 100% was observed with ZnO and CuO NPs at 30 mg/L. A lower inhibition of 60.4% was observed with 10 mg/L CuO NPs. Under greenhouse conditions, 100 mg/L ZnO NPs was the most effective treatment for controlling potato late blight, with an efficacy of 71%. CuO NPs at 100 mg/L followed closely, with an efficacy of 69%. Based on these results, ZnO and CuO NPs are recommended as promising eco-friendly fungicides for the management and control of potato late blight after further research.
- P. infestans;
- Zinc oxide (ZnO) and Copper oxide (CuO) nanoparticles (NPs);
- Synthesis and characterization;
- Greenhouse conditions;
- Antifungal activity
- DOI: 10.1002/pc.28752
- Corpus ID: 271031855
Effect of synthesized copper oxide nanorods on electrical and thermal properties of compatibilized high‐density polyethylene/carbon nanofiber nanocomposite films
- Sameer Ahmad , Shivani Tyagi , +1 author Syed Wazed Ali
- Published in Polymer Composites 3 July 2024
- Materials Science, Engineering
41 References
Enhanced mechanical, electrical, thermal, and optical properties of poly(methyl methacrylate)/copper oxide nanocomposites for flexible optoelectronic devices via in‐situ polymerization technique, a comparative study of polycarbonate nanocomposites respectively containing graphene nanoplatelets, carbon nanotubes and carbon nanofibers, a thermochromic poplar-based tetradecyl ester/methyl composite encapsulated with peg400–sio2 (ps-r-ptc) for improving thermal energy storage, structure, properties, and antibacterial behavior of nickel oxide reinforced natural rubber nanocomposites for flexible electronic applications, role of copper alumina nanoparticles on the performance of polyvinylchloride nanocomposites, fabrication and determination of the sun protection factor and ultraviolet protection factor for piscean collagen/bischalcone derivative (b1) composite films with wide-range uv shielding, analytical study to determine the optical properties of gold nanoparticles in the visible solar spectrum, compliant materials based on nickel oxide/chlorinated natural rubber nanocomposites, structure, thermal, optical and dielectric properties of sno2 nanoparticles-filled hdpe polymer, the production of carbon nanofiber on rubber fruit shell-derived activated carbon by chemical activation and hydrothermal process with low temperature, related papers.
Showing 1 through 3 of 0 Related Papers

COMMENTS
2.1. Plant mediated synthesis of copper oxide nanoparticles. Plant extracts have been widely used for the production of copper oxide nanoparticles [42], [43], [44].Even with the different advantages of the fabrication of copper oxide nanoparticles from bacteria, algae, and fungi, there are some limitations to using these organisms [45], [46].The toxicity of bacteria, isolation of the ...
Copper oxide nanoparticles (CuO NPs) are often made using the sol-gel procedure (Bokov et al., 2021, Parashar et al., 2020, Feinle et al., 2016). A metal precursor is dissolved in a solvent, hydrolyzed, then condensed to create a gel. The gel is dried and calcinated to make copper oxide nanoparticles. Cost-effective and accurate nanoparticle ...
Copper oxide (CuO) nanoparticles synthesized by green Synthesis method using Curcuma plant extract and Cu nitrate. This gives a large-scale production of CuO nanoparticles simply. X-ray diffraction pattern (XRD) reveals single-phase monoclinic structure. The transmission electron microscopy (TEM) showed 12-110 nm size of as prepared CuO ...
Copper oxide nanoparticles (CuO-NPs) were synthesized using two different methods (chemical and biosynthesis) to study the influence of the preparation method on the structural, optical, morphological, photocatalyst, antibacterial and in vitro antioxidant of these nanoparticles. The synthesized nanoparticles were analysed by XRD, UV-Vis, HR-TEM, DLS, ZE, PL and FT-IR spectroscopy. The X-ray ...
The probable applications of copper oxide nanoparticles (CuONPs) in gas sensors, waste treatment, catalysis, batteries, food preservation, high temperature superconductors, solar energy conversion, photovoltaic devices, dye removal, field emission emitters and in agriculture have been established [11, 12, 13, 14].
2. Copper Nanoparticles . In recent years, the development of metal and metal oxide NPs has greatly enhanced the biomedical field in terms of biosensing, imaging, diagnosis, and therapy [31,32,33,34].The most commonly used metals and their oxides are gold (Au), silver (Ag), and copper (Cu).
Synthesis of copper oxide nanoparticles utilized copper (II) sulphate as a metal precursor. 0.01 M CuSO 4 solution was prepared by dissolving 1.59 g in 100 mL of distilled water. The metal ...
Development of green technology is generating interest of researchers towards ecofriendly and low-cost methods for biosynthesis of nanoparticles (NPs). In this study, copper oxide (CuO) NPs were synthesized using a copper nitrate trihydrate precursor and Catha edulis leaves extract as a reducing and capping agent during the synthesis. The ...
Green methods of synthesizing nanoparticles are safer than chemical and physical methods, as well as being eco-friendly and cost-efficient. In this study, we use copper oxide nanoparticles (CuO NPs) fabricated with Sesbania grandiflora (Sg) (Hummingbird tree) leaves to test the effectiveness of green synthesizing methods. The attained Sg-CuO NPs physical and optical nature is characterized by ...
Abstract and Figures. Copper and Copper oxide nanoparticles have garnered a lot of attention among the metal oxide nanoparticles, especially because of their many characteristics and applications ...
During the green synthesis, the biomolecules present in the sample extract reduce the Cu 2+ ion to Cu 0 state and simultaneously oxidize it to form CuO nanoparticles. Certain biomolecules found in the sample extract serve as a capping agent and also aid in the stabilization of the produced nanoparticles ( 43 ). 2.2.
A novel green synthesis of copper oxide nanoparticles using a henna extract powder. J. Struct. Chem. 2018, 59, 1737-1743. [Google Scholar] Santhosh Kumar, J.; Shanmugam, V. Green synthesis of copper oxide nanoparticles from magnolia champaca floral extract and its antioxidant & toxicity assay using Danio Rerio. Int. J.
We report the synthesis of copper oxide (CuO) nanoparticles from Cu (NO3)2 through wet chemical precipitation method. Characterization was done by X-ray diffraction (XRD) analysis, scanning ...
The applications of copper (Cu) and Cu-based nanoparticles, which are based on the earth-abundant and inexpensive copper metal, have generated a great deal of interest in recent years, especially in the field of catalysis. The possible modification of the chemical and physical properties of these nanoparticles using different synthetic strategies and conditions and/or via postsynthetic ...
Copper oxide nanoparticles (CuONPs) have attracted much attention because of appropriat e redox potential, high speci fi c surface area, and excellent stab ility in different solutions ( Nagajyothi
Copper oxide nanoparticles (CuO NPs) have been utilized in a many of applications in the last few decades. The current study presents the synthesis of CuO NPs with aqueous extract of Morinda ...
Abstract In this paper, we study the synthesis dependence of structural, optical and antimicrobial properties for copper oxide nanoparticles on, synthesized using microwave irradiation CuO(M), co-precipitation CuO(P) and hydrothermal CuO(H) protocols. Structural and morphological properties were studied using XRD, SEM, TEM and SAED techniques. XPS studies confirmed the presence of copper ions ...
Copper oxide (CuO) nanoparticles were synthesised from Ginkgo biloba L. leaf extract and several parameters that affected their formation were adjusted. The optimal synthesis system was ascertained as 10 ml leaf filtrate, 1 mM copper sulfate, and 80°C. The scanning electron microscope image showed that the synthesised CuO nanoparticles were ...
Abstract. This study aims to provide an updated survey of the main synthesis methods of copper oxide (CuO) nanoparticles in order to obtain tailored nanosystems for various biomedical applications. The synthesis approach significantly impacts the properties of such nanoparticles and these properties in turn have a significant impact on their ...
Copper oxide nanoparticles (CuO NPs) use has exponentially increased in various applications (such as industrial catalyst, gas sensors, electronic materials, biomedicines, environmental remediation) due to their flexible properties, i.e. large surface area to volume ratio. These broad applications, however, have increased human exposure and ...
@article{Jabeen2024BiogenicSO, title={Biogenic Synthesis of Copper Oxide Nanoparticles from Aloe vera: Antibacterial Activity, Molecular Docking, and Photocatalytic Dye Degradation}, author={Sabeeha Jabeen and Vasi Uddin Siddiqui and Shashi Bala and Nidhi Mishra and Anamika Mishra and Rubina Lawrence and Pratibha Bansal and Abdul Rahman Khan ...
Thus, FTIR result suggests that the formation of copper oxide compound is obtained after annealing process in this method accompanying the presence of Cu O bonds corresponding to Cu O stretching modes. Figure 3(a-d) are SEM images illustrating the morphologies of CuO-nanoparticles prepared with different precursors via the precipitation method.
@article{Pavlikov2024SynthesisOC, title={Synthesis of Copper(II) Oxide Nanoparticles by Anion-Exchange Resin-Assisted Precipitation and Production of Their Stable Hydrosols}, author={Alexander Pavlikov and S. V. Saikova and Alexander S. Samoilo and D.V. Karpov and S. A. Novikova}, journal={Russian Journal of Inorganic Chemistry}, year={2024 ...
The interaction between nanoscale copper oxides (nano-CuOs) and soil matrix significantly affects their fate and transport in soils. This study investigates the retention of nano-CuOs and Cu2+ ions in ten typical agricultural soils by employing the Freundlich adsorption model. Retention of nano-CuOs and Cu2+ in soils was well fitted by the Freundlich model. The retention parameters (KD, KF ...
Mbewana-Ntshanka et al. reported the synthesis of copper selenide, copper sulfide and copper oxide nanoparticles via the hot-injection method, where oleylamine was used as a stabilizer agent and CuCl 2, selenium and sulfur powders were used as precursors . They obtained spherical nanoparticles in the ranges of 1.0-27.0 nm for CuSe, 1.0-18.0 ...
- Writing a thesis on copper oxide nanoparticles can be challenging due to the vast amount of information to sift through and understand regarding synthesis methods, properties, applications, and environmental impacts of copper oxide nanoparticles. - Effectively communicating complex scientific concepts and research findings in a thesis requires not only a deep understanding of the topic but ...
Late blight, caused by Phytophthora infestans, is a major potato disease globally, leading to significant economic losses of $6.7 billion. To address this issue, we evaluated the antifungal activity of ZnO and CuO nanoparticles (NPs) against P. infestans for the first time in laboratory and greenhouse conditions. Nanoparticles were synthesized via a chemical precipitation method and ...
Copper oxide nanoparticles (CuO-NPs) are receiving more attention owing to their availability and lower cost when compared to more costly and noble metals like gold and silver, as well as their effective potential for application as microbial agents (Sankar et al., 2014).
High‐density polyethylene (HDPE) nanocomposites containing different optimized fractions of carbon nanofiber (CNF) at 6 wt.%, polyethylene glycol (PEG) at 4 wt.%, and copper oxide (CuO) nanorods in concentration ranges of 0.15, 0.5, and 2 wt.% were successfully melt processed using corotating twin extrusion and compression molding technique. The effect of PEG and CuO nanofiller on the HDPE ...
Copper Oxide Nanoparticles Thesis - Free download as PDF File (.pdf), Text File (.txt) or read online for free. The document discusses the challenges of writing a thesis on Copper Oxide Nanoparticles, including extensive research requirements, navigating vast literature, conducting experiments, and adhering to academic standards. It notes that seeking professional writing assistance from ...