Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center

Human Evolution
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Last »
- Palaeolithic Archaeology Follow Following
- Paleoanthropology Follow Following
- Neanderthals (Palaeolithic Archaeology) Follow Following
- Biological Anthropology Follow Following
- Lithic Technology Follow Following
- Prehistoric Archaeology Follow Following
- Middle Palaeolithic Follow Following
- Middle to Upper Paleolithic Transition Follow Following
- Lithics Follow Following
- Evolutionary Anthropology Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Journals
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The Evolution of the Human Genome
Corinne n simonti, john a capra.
- Author information
- Article notes
- Copyright and License information
Corresponding author: [email protected]
Issue date 2015 Dec.
Human genomes hold a record of the evolutionary forces that have shaped our species. Advances in DNA sequencing, functional genomics, and population genetic modeling have deepened our understanding of human demographic history, natural selection, and many other long-studied topics. These advances have also revealed many previously underappreciated factors that influence the evolution of the human genome, including functional modifications to DNA and histones, conserved 3D topological chromatin domains, structural variation, and heterogeneous mutation patterns along the genome. Using evolutionary theory as a lens to study these phenomena will lead to significant breakthroughs in understanding what makes us human and why we get sick.
Keywords: human evolution, population genetics, evolution of gene regulation, evolutionary medicine
Introduction
Understanding the evolution of the human genome is a tantalizing goal. Accurately decoding the biological programs encoded in the human genome would reveal molecular answers to fundamental questions about human origins and the genetic basis for human-specific traits. Studying the evolutionary and demographic history of our species also has great promise to reveal how and why modern humans get sick. The human genome has been shaped by evolutionary pressures that, in many cases, no longer reflect the circumstances of most humans, and this mismatch between our genes and our environment can lead to disease [ 1 , 2 ].
In spite of immense progress since the sequencing of the first human genome more than 10 years ago, there is still much we do not understand about the evolution of the human genome. Recent statistical and experimental advances and the sequencing of thousands of human genomes from diverse populations have revealed significant complexity in classical topics in human population genetics, including the dynamics of selection across human populations and closely related species [ 3 – 6 ], the determinants of variation in mutation rates [ 7 , 8 ], inference of ancient human population histories [ 9 , 10 ], and how variants, in particular rare variants, contribute to phenotypes [ 11 – 13 ]. Perhaps the most dramatic result in this field over the past five years has been the sequencing of ancient DNA from archaic hominins, like Neanderthals and Denisovans [ 14 , 15 ], and the comprehensive demonstration of admixture between the ancestors of modern humans and several archaic hominin groups [ 16 – 20 ]. Each of these topics has been covered in recent comprehensive reviews (referenced above) and a recent issue of this journal [ 21 ], so here we highlight several additional genetic, environmental, and demographic factors influencing human genome evolution that we believe deserve further attention ( Figure 1 ).
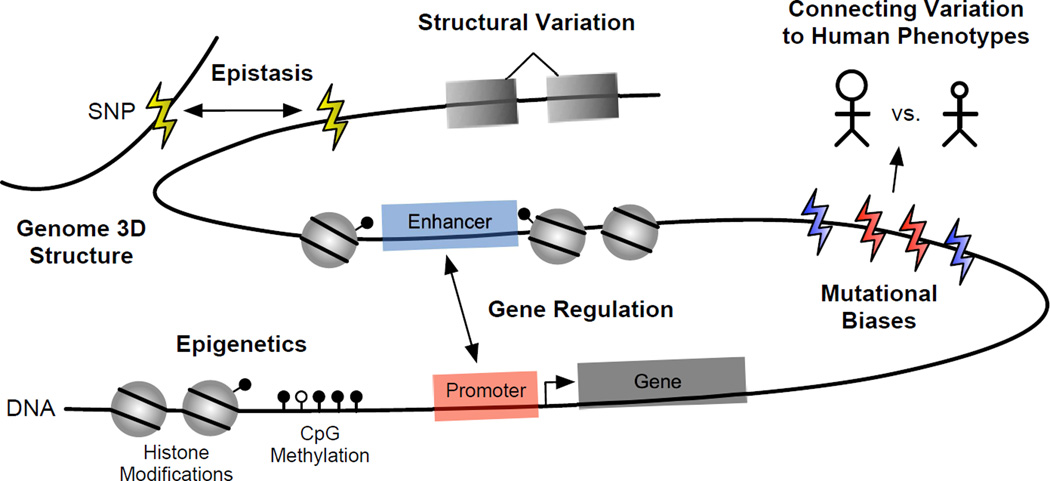
Many areas of ongoing research in genomics have not been fully integrated into models of genome evolution. In this article, we discuss how study of several emerging topics (bold) in an evolutionary context will enrich our understanding of human evolution.
How do gene regulatory processes influence human genome evolution?
In the 40 years since King and Wilson hypothesized that phenotypic differences between closely related species were driven by gene regulatory changes, considerable support has been found for the importance of cis -regulatory elements (CREs), such as promoters and enhancers, in human evolution [ 22 , 23 ] and disease [ 24 , 25 ]. The recent ability to map gene expression, transcription factor (TF) binding sites, and histone modifications genome-wide in many tissues and species [ 26 – 28 ] has revealed that, while gene expression is generally conserved within similar tissues across species [ 29 ], CREs experience rapid turnover [ 30 – 32 ]. For example, a recent study of liver promoters and enhancers across 20 mammalian species found that 25% of a species’ enhancers and 10% of its promoters were unique, even when the underlying sequence was deeply conserved [ 33 ]; similar results were found for limb CREs across human, macaque, and mouse [ 34 ].
There is still much to be learned about the evolution of regulatory sequence, in particular about its dynamics across tissues, species, and different classes of CREs, and how selection acts on these elements. For example, transposable elements (TEs) have helped reprogram gene regulatory networks in tissues relevant to pregnancy in humans and other mammals [ 35 ]. In contrast, TEs have made only a modest contribution to the evolution of new CREs in the liver [ 33 ]. This suggests that, while fast turnover of CREs and conserved gene expression are common features of mammalian genome evolution, different evolutionary dynamics and pressures act on CREs active in different tissues. It is likely that the regulatory landscapes of some tissues are more conducive to turnover than others; for example, tissues with greater phenotypic diversity across species, like those involved in pregnancy, may be more susceptible to TE-based rewiring. The maintenance and modification of these regulatory processes and their influence on genome evolution requires further investigation.
Integrating genome-wide maps of CREs, TF binding, and expression with recent advances in techniques for determining in vivo chromatin conformation of DNA [ 36 ] may provide a promising framework for modeling the influence of gene regulation on genome evolution. A recent study of chromatin looping in multiple human and mouse tissues found significant conservation of gene activity within local topological domains across cells and species [ 37 ]. These results suggest that, as is true for proteins, the 3D structure of regulatory neighborhoods maybe more deeply conserved and important for function than the sequence-level conservation of individual CREs. Integrating data about genome structure and CREs across many individuals will likely lead to better models of regulatory sequence evolution and how selection acts on gene expression across evolutionary time and tissues.
How do chemical modifications to DNA and histones constrain human genome evolution?
The human body contains hundreds of different cell types with diverse forms and functions, yet each cell contains (essentially) the same genome. The past decade has seen increasing appreciation for the role of DNA and histone modifications, such as methylation and acetylation, in the diverse gene expression programs observed across different cell types within complex organisms [ 38 – 40 ]. These modifications can be influenced by environmental factors [ 41 ] and in some cases inherited across generations, though the extent of trans-generational inheritance in humans is still unclear [ 42 ].
In spite of extensive work linking these modifications to nearly all processes of development, aging, and disease [ 39 , 43 – 45 ], the influence of these modifications on patterns of genome sequence evolution has received comparatively little attention. For example, the extent to which the potential for chemical modification places constraint on DNA sequence patterns, e.g, CpG sites, is not resolved. Several recent studies have explored the degree of conservation of DNA and histone modifications across humans and closely related species [ 31 , 33 , 34 , 46 – 48 ]; changes to the modification status of orthologous regions are common between closely related species and, for DNA methylation, there is a positive correlation between sequence variation and promoter methylation changes. However, even in the presence of deep sequence conservation, many sites show differential modification. Much work remains to model the evolution of these modifications between individuals and species and to identify associated sequence constraints (or lack thereof). Understanding the evolution of these modifications may help resolve debates about whether specific modifications are causal or are the result of other processes like TF binding and transcription [ 49 ].
How should interactions between multiple genetic variants and phenotypes be modeled?
The majority of human phenotypes of clinical and evolutionary interest are specified by multiple loci across the human genome. Developing models that account for relationships between multiple genetic variants and phenotypes will be critical to fully dissecting the evolution and complex genetic architecture of most human traits. For example, pleiotropy—when a locus influences multiple independent traits—is found throughout the human genome; however, there is still considerable uncertainty about its prevalence and influence on genome evolution [ 50 – 53 ]. Similarly, epistasis—a non-additive interaction between genetic variants—is common in model organisms, but its influence on human traits has been controversial due to a number of technical and biological factors that can confound current tests for interactions between variants [ 54 , 55 ]. Each of these areas is in need of new statistical approaches that update existing models to make full use of the wealth of genotype and phenotype data that have become available in the last five years.
What are the causes and effects of mutational biases along the human genome?
There is considerable variation in the rate and pattern of substitution along the human genome. Failure to account for these biases can confound tests for selection, complicate demographic inference, and weaken power in association tests [ 7 ]. One of the most potentially influential mutational biases is a recombination-associated process called GC-biased gene conversion (gBGC). gBGC results from a slight preference for G/C alleles in the mismatch repair machinery that has the potential to promote the maintenance of deleterious alleles [ 57 ]. The action of gBGC is widespread in human populations and across diverse species [ 58 – 60 ]. Genome-wide modeling of gBGC has demonstrated differences in its strength across the human and chimpanzee lineages [ 59 ] and between different human populations [ 61 , 62 ].
The evolution and effects of gBGC are intimately tied to the dynamics of recombination, which vary considerably in rate along the genome within human populations and between closely related species [ 63 ]. Recombination patterns influence many drivers of genome evolution, including the efficacy of selection, mutation rates, and the accumulation of deleterious mutations [ 64 , 65 ]. In humans, the fast evolving PRDM9 protein directs recombination to specific hotspots based on the occurrence of a GC-rich motif [ 66 ]. Using modern and archaic genome sequences, modeling suggests that gBGC degrades the PRDM9 motif over time and that this may drive the rapid turnover of the recombination landscape in human populations [ 67 ].
gBGC is only one of several sources of mutation rate variation that are not well understood [ 7 ]. Recent direct estimates from trios indicate that the human germline mutation rate is only half of what is expected from phylogenetic estimates [ 68 ], and analysis of whole genome sequence data suggests the evolution of population-specific mutation rates since the divergence of Europeans and Asians [ 69 ]. These results underscore the need for further study of the dynamics and causes of human mutation rate variation across evolutionary time and genomic space. We need to develop high-resolution maps of mutation rates in different populations, better models of how it interacts with selection and recombination, and most importantly, a deeper understanding of its effects on organismal fitness.
What are appropriate models for the evolution and functional impact of structural variation?
The initial comparison of the draft human and chimpanzee genomes identified approximately 35 million single nucleotide polymorphisms (SNPs), 5 million small insertions and deletions (indels), and hundreds of larger structural variants (SVs). Indels and large SVs account for far more nucleotide differences between the human and chimpanzee genomes than SNPs [ 70 ] and have restructured the genomes of great apes [ 71 ]. Recent work on de novo rates of indels and SVs in human populations found that structural changes are much more rare and occur at lower frequency than SNPs; nonetheless, they influence an average of 4.1 kilobases per generation, which is 91 times more than de novo substitutions [ 72 ]. Indels and large SVs (including copy number variants, inversions, and other genomic rearrangements) are more likely to cause disease and have been hypothesized to have a greater influence on recent human evolution than SNPs [ 73 , 74 ]. Indeed, many human- and population-specific SVs have been tied to human-specific phenotypes [ 75 ], e.g., human-specific deletion of a conserved enhancer of the androgen receptor gene may be responsible for the lack of penile spines in humans [ 76 ]. It has also been suggested that de novo creation of new genes is more common than previously appreciated; tens of new human-specific genes have been detected, with particular enrichment for expression in the brain and testes [ 77 , 78 ].
In spite of the potential importance of indels, large SVs, and new genes to phenotypic differences between human individuals and between closely related species, they have received considerably less attention in evolutionary modeling and testing for association with disease than SNPs. Developing appropriate models for the evolution of indels and SVs faces several challenges including the difficulty of accurately identifying them in short read sequencing data, the diversity of mechanisms that generate them, and their highly heterogeneous mutation rates and distributions along the genome [ 72 , 79 ]. Nevertheless, it is essential to develop evolutionary models akin to those in common use for testing hypotheses about patterns of single nucleotide variant evolution and association with disease for indels and SVs. Sufficiently accurate maps of these events across hundreds of humans are now becoming available [ 79 , 80 ]; these data should facilitate the development of new modeling approaches.
How can we efficiently connect human-specific genomic changes to phenotypes?
Sequencing the genomes of thousands of humans, several archaic humans, and our closest great ape relatives has revealed thousands of loci in the human genome that have experienced accelerated evolution on the human lineage and hundreds more with signatures of recent positive selection [ 81 – 83 ]. These loci hold the promise of explaining much of human-specific biology, and many hypotheses have been proposed about their effects [ 75 ]. However, beyond a handful of successes that involved detailed experimental validation [ 76 , 84 – 88 ], connecting these mutations to effects on human phenotypes has been difficult. The first obstacle comes from the fact that the vast majority of these regions are non-coding and have minimal functional annotation. Furthermore, most human-specific traits have complex genetic architectures in which many coding and non-coding loci influence the phenotype [ 89 ]. Finally, appropriate model systems in which to test potential effects of mutations are not available for many phenotypes, and it is challenging to test variants in a high throughput manner in available systems.
Algorithmic and experimental innovations paired with increases in available phenotype and functional genomic data will significantly increase the pace with which human-specific variants can be characterized. For example, algorithms that integrate diverse functional, evolutionary, and DNA sequence data have shown that many human accelerated regions are developmental gene regulatory enhancers, with particular enrichment for brain activity [ 81 , 88 , 90 ]. As our understanding of how non-coding mutations influence gene expression and function improves [ 91 ], so will the accuracy and specificity of hypotheses about the effects of these regions on human-specific phenotypes.
Over the past ten years, genome-wide association studies (GWAS) have identified hundreds of variants associated with complex diseases [ 92 , 93 ]. These studies provide insight into the functions encoded in specific regions of the genome that can inform evolutionary questions. However, the majority of human- and population-specific variants have not been associated with functions. The recent integration of large databases of electronic health records (EHRs) linked to patient genotypes [ 94 ] provides a new approach to this problem. Thousands of phenotypes can be algorithmically derived from EHRs and then simultaneously tested for association with the loci of interest across thousands of individuals in a phenome-wide association study (PheWAS) [ 95 ]. As EHR databases grow and sequencing decreases in price, the PheWAS approach will enable efficient testing of hypotheses about the effects of mutations of evolutionary interest.
Finally, new technologies, including directed stem cell differentiation, massively parallel reporter assays [ 96 ], and CRISPR gene editing [ 97 ], will facilitate faster exploration of the mechanisms driving phenotypic associations in models that closely resemble the in vivo human context.
Understanding how evolutionary processes produced the human species and how developmental programs are encoded in the human genome is of great importance to basic and clinical science. The evolutionary history of the human genome is directly relevant to our ability to anticipate and treat human disease [ 1 ]. In this review, we have highlighted several research areas that have potential to significantly deepen our knowledge of human genome evolution over the next few years, but our list is not exhaustive. Many other areas, including the evolutionary study of human–microbe interactions [ 98 , 99 ] and experimental evolution [ 100 ], are poised for breakthroughs. We are also eager to see how recent technical advances in long-read genome sequencing and single cell analysis will change our understanding of evolutionary processes. Ultimately, continued analysis of the human genome in an evolutionary framework will further reveal the genetic origins of human-specific biology and improve our understanding of the etiology of human disease.
Acknowledgments
C.N.S. was supported by NIH training grant 5T32EY021453. J.A.C. was supported by institutional funds from Vanderbilt University and a March of Dimes Innovation Catalyst award. We are grateful to Seth Bordenstein, Alexandra Fish, Emily Hodges, Dennis Kostka, Katherine Pollard, and Scott Williams for discussions related to topics covered in this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY AND REFERENCES CITED
- 1. Rodríguez JA, Marigorta UM, Navarro A. Integrating genomics into evolutionary medicine. Current Opinion in Genetics & Development. 2014;29:97–102. doi: 10.1016/j.gde.2014.08.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Gluckman PD, Beedle A, Hanson MA. Principles of evolutionary medicine. Oxford: Oxford University Press; 2009. [ Google Scholar ]
- 3. Fu W, Akey JM. Selection and Adaptation in the Human Genome. Annual Review of Genomics and Human Genetics. 2013;14:467–489. doi: 10.1146/annurev-genom-091212-153509. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Hernandez RD, Kelley JL, Elyashiv E, Melton S, Auton A, McVean G, Sella G, Przeworski M. Classic selective sweeps were rare in recent human evolution. Science. 2011;331:920–924. doi: 10.1126/science.1198878. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Jensen JD. On the unfounded enthusiasm for soft selective sweeps. Nat Commun. 2014;5 doi: 10.1038/ncomms6281. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Prado-Martinez J, Sudmant PH, Kidd JM, Li H, Kelley JL, Lorente-Galdos B, Veeramah KR, Woerner AE, O'Connor TD, Santpere G, et al. Great ape genetic diversity and population history. Nature. 2013;499:471–475. doi: 10.1038/nature12228. * This study reported the sequencing of 79 wild- and captive-born individuals from all six great ape species. These data revealed radical fluctuations in effective population size and genetic diversity and provide a broader context in which to interpret human evolution.
- 7. Ségurel L, Wyman MJ, Przeworski M. Determinants of Mutation Rate Variation in the Human Germline. Annual Review of Genomics and Human Genetics. 2014;15:47–70. doi: 10.1146/annurev-genom-031714-125740. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Henn BM, Botigue LR, Bustamante CD, Clark AG, Gravel S. Estimating the mutation load in human genomes. Nat Rev Genet. 2015 doi: 10.1038/nrg3931. advance online publication. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Gronau I, Hubisz MJ, Gulko B, Danko CG, Siepel A. Bayesian inference of ancient human demography from individual genome sequences. Nature genetics. 2011;43:1031–1034. doi: 10.1038/ng.937. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Li H, Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493–496. doi: 10.1038/nature10231. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Lee S, Abecasis GR, Boehnke M, Lin X. Rare-variant association analysis: study designs and statistical tests. Am J Hum Genet. 2014;95:5–23. doi: 10.1016/j.ajhg.2014.06.009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Gibson G. Rare and common variants: twenty arguments. Nat Rev Genet. 2012;13:135–145. doi: 10.1038/nrg3118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Sulem P, Helgason H, Oddson A, Stefansson H, Gudjonsson SA, Zink F, Hjartarson E, Sigurdsson GT, Jonasdottir A, Jonasdottir A. Identification of a large set of rare complete human knockouts. Nature genetics. 2015;47:448–452. doi: 10.1038/ng.3243. * This study revealed the patterns and prevalence of loss-of-function mutations in the Icelandic population by sequencing the whole genomes of 2,636 Icelanders and imputing the sequence variants identified into more than 100,000 more with genotyping data.
- 14. Fu Q, Li H, Moorjani P, Jay F, Slepchenko SM, Bondarev AA, Johnson PL, Aximu-Petri A, Prüfer K, de Filippo C. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. ** This paper presented the genome sequence of the oldest anatomically modern human sequenced to date. It provides insights into the timing of Neanderthal interbreeding and estimates of the human mutation rate.
- 15. Prüfer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, Heinze A, Renaud G, Sudmant PH, de Filippo C. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43–49. doi: 10.1038/nature12886. ** This study presented a high quality Neanderthal genome sequence and demonstrated several gene flow events between archaic hominins and anatomically modern humans.
- 16. Kelso J, Prüfer K. Ancient humans and the origin of modern humans. Current opinion in genetics & development. 2014;29:133–138. doi: 10.1016/j.gde.2014.09.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Pääbo S. The Human Condition—A Molecular Approach. Cell. 2014;157:216–226. doi: 10.1016/j.cell.2013.12.036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Racimo F, Sankararaman S, Nielsen R, Huerta-Sanchez E. Evidence for archaic adaptive introgression in humans. Nat Rev Genet. 2015 doi: 10.1038/nrg3936. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Sankararaman S, Mallick S, Dannemann M, Prüfer K, Kelso J, Pääbo S, Patterson N, Reich D. The genomic landscape of Neanderthal ancestry in present-day humans. Nature. 2014;507:354–357. doi: 10.1038/nature12961. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Vernot B, Akey JM. Resurrecting Surviving Neandertal Lineages from Modern Human Genomes. Science. 2014;343:1017–1021. doi: 10.1126/science.1245938. * This study presented an approach for identifying introgressed Neanderthal DNA in modern human genomes and mapped the presence and frequency of Neanderthal DNA in the genomes of non-African individuals.
- 21. Andrés AM, Nowick K. Editorial overview: Genetics of human evolution: The genetics of human origins. Current Opinion in Genetics & Development. 2014;29:v–vii. doi: 10.1016/j.gde.2014.11.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Siepel A, Arbiza L. Cis-regulatory elements and human evolution. Current opinion in genetics & development. 2014;29:81–89. doi: 10.1016/j.gde.2014.08.011. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Villar D, Flicek P, Odom DT. Evolution of transcription factor binding in metazoans [mdash] mechanisms and functional implications. Nature Reviews Genetics. 2014;15:221–233. doi: 10.1038/nrg3481. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Edwards Stacey L, Beesley J, French Juliet D, Dunning Alison M. Beyond GWASs: Illuminating the Dark Road from Association to Function. American Journal of Human Genetics. 2013;93:779–797. doi: 10.1016/j.ajhg.2013.10.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Sakabe NJ, Savic D, Nobrega MA. Transcriptional enhancers in development and disease. Genome Biol. 2012;13:238. doi: 10.1186/gb-2012-13-1-238. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. GTEx Consortium. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. * This study presented pilot RNA sequencing data from 1641 samples across 43 tissues from 175 human individuals. This is the largest analysis of the relationship between genetic variation and gene expression to date
- 27. Melé M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, Young TR, Goldmann JM, Pervouchine DD, Sullivan TJ, et al. The human transcriptome across tissues and individuals. Science. 2015;348:660–665. doi: 10.1126/science.aaa0355. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Brawand D, Soumillon M, Necsulea A, Julien P, Csardi G, Harrigan P, Weier M, Liechti A, Aximu-Petri A, Kircher M, et al. The evolution of gene expression levels in mammalian organs. Nature. 2011;478:343–348. doi: 10.1038/nature10532. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Weirauch MT, Hughes TR. Conserved expression without conserved regulatory sequence: the more things change, the more they stay the same. Trends in genetics. 2010;26:66–74. doi: 10.1016/j.tig.2009.12.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. ** This study determined gene transcription, DNase I hypersensitivity, transcription factor binding, and chromatin modifications across the mouse genome in diverse cell and tissue types. Comparing these profiles to human data they highlighted significant similarities and differences between different levels of the regulatory landscape of mouse and human.
- 32. Necsulea A, Kaessmann H. Evolutionary dynamics of coding and non-coding transcriptomes. Nature Reviews Genetics. 2014;15:734–748. doi: 10.1038/nrg3802. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Villar D, Berthelot C, Aldridge S, Rayner TF, Lukk M, Pignatelli M, Park TJ, Deaville R, Erichsen JT, Jasinska AJ, et al. Enhancer evolution across-20 mammalian species. Cell. 2015;160:554–566. doi: 10.1016/j.cell.2015.01.006. * This study characterized the conservation of enhancer and promoter activity across 20 diverse mammalian species. The authors demonstrated rapid turnover in enhancer activity and greater stability of promoters across mammals.
- 34. Cotney J, Leng J, Yin J, Reilly SK, DeMare LE, Emera D, Ayoub AE, Rakic P, Noonan JP. The evolution of lineage-specific regulatory activities in the human embryonic limb. Cell. 2013;154:185–196. doi: 10.1016/j.cell.2013.05.056. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Lynch VJ, Nnamani MC, Kapusta A, Brayer K, Plaza SL, Mazur EC, Emera D, Sheikh SZ, Grützner F, Bauersachs S. Ancient Transposable Elements Transformed the Uterine Regulatory Landscape and Transcriptome during the Evolution of Mammalian Pregnancy. Cell reports. 2015;10:551–561. doi: 10.1016/j.celrep.2014.12.052. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Pombo A, Dillon N. Three-dimensional genome architecture: players and mechanisms. Nat Rev Mol Cell Biol. 2015;16:245–257. doi: 10.1038/nrm3965. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Rao Suhas SP, Huntley Miriam H, Durand Neva C, Stamenova Elena K, Bochkov Ivan D, Robinson James T, Sanborn Adrian L, Machol I, Omer Arina D, Lander Eric S, et al. A 3D Map of the Human Genome at Kilobase Resolution Reveals Principles of Chromatin Looping. Cell. 159:1665–1680. doi: 10.1016/j.cell.2014.11.021. ** This paper used in situ Hi-C to determine high resolution (1–5 kb) maps of the chromatin conformation in nine cell types. These maps demonstrated chromatin contact domains with coherent gene expression and functional patterns that are maintained across cell types and species.
- 38. Dixon JR, Jung I, Selvaraj S, Shen Y, Antosiewicz-Bourget JE, Lee AY, Ye Z, Kim A, Rajagopal N, Xie W. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Roadmap Epigenomics C, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 2011;12:7–18. doi: 10.1038/nrg2905. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13:97–109. doi: 10.1038/nrg3142. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Heard E Martienssen Robert A. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell. 157:95–109. doi: 10.1016/j.cell.2014.02.045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20:282–289. doi: 10.1038/nsmb.2489. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Brunet A, Berger SL. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S17–S20. doi: 10.1093/gerona/glu042. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Hernando-Herraez I, Prado-Martinez J, Garg P, Fernandez-Callejo M, Heyn H, Hvilsom C, Navarro A, Esteller M, Sharp AJ, Marques-Bonet T. Dynamics of DNA Methylation in Recent Human and Great Ape Evolution. PLoS Genet. 2013;9:e1003763. doi: 10.1371/journal.pgen.1003763. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Pai AA, Gilad Y. Comparative studies of gene regulatory mechanisms. Current opinion in genetics & development. 2014;29:68–74. doi: 10.1016/j.gde.2014.08.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Zhou X, Cain CE, Myrthil M, Lewellen N, Michelini K, Davenport ER, Stephens M, Pritchard JK, Gilad Y. Epigenetic modifications are associated with inter-species gene expression variation in primates. Genome Biol. 2014;15:547. doi: 10.1186/s13059-014-0547-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Ptashne M. Epigenetics: Core misconcept. Proceedings of the National Academy of Sciences. 2013;110:7101–7103. doi: 10.1073/pnas.1305399110. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013;14:483–495. doi: 10.1038/nrg3461. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Pavlicev M, Wagner GP. A model of developmental evolution: selection, pleiotropy and compensation. Trends in Ecology & Evolution. 2012;27:316–322. doi: 10.1016/j.tree.2012.01.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Sivakumaran S, Agakov F, Theodoratou E, Prendergast James G, Zgaga L, Manolio T, Rudan I, McKeigue P, Wilson James F, Campbell H. Abundant Pleiotropy in Human Complex Diseases and Traits. American Journal of Human Genetics. 2011;89:607–618. doi: 10.1016/j.ajhg.2011.10.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Wagner GP, Zhang J. The pleiotropic structure of the genotype–phenotype map: the evolvability of complex organisms. Nat Rev Genet. 2011;12:204–213. doi: 10.1038/nrg2949. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Hemani G, Shakhbazov K, Westra H-J, Esko T, Henders AK, McRae AF, Yang J, Gibson G, Martin NG, Metspalu A, et al. Detection and replication of epistasis influencing transcription in humans. Nature. 2014;508:249–253. doi: 10.1038/nature13005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] [ Retracted ]
- 55. Wood AR, Tuke MA, Nalls MA, Hernandez DG, Bandinelli S, Singleton AB, Melzer D, Ferrucci L, Frayling TM, Weedon MN. Another explanation for apparent epistasis. Nature. 2014;514:E3–E5. doi: 10.1038/nature13691. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Galtier N, Duret L. Adaptation or biased gene conversion? Extending the null hypothesis of molecular evolution. TRENDS in Genetics. 2007;23:273–277. doi: 10.1016/j.tig.2007.03.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Duret L, Galtier N. Biased gene conversion and the evolution of mammalian genomic landscapes. Annual review of genomics and human genetics. 2009;10:285–311. doi: 10.1146/annurev-genom-082908-150001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Lartillot N. Phylogenetic Patterns of GC-Biased Gene Conversion in Placental Mammals and the Evolutionary Dynamics of Recombination Landscapes. Molecular Biology and Evolution. 2013;30:489–502. doi: 10.1093/molbev/mss239. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Capra JA, Hubisz MJ, Kostka D, Pollard KS, Siepel A. A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes. PLoS Genet. 2013;9:e1003684. doi: 10.1371/journal.pgen.1003684. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Katzman S, Capra JA, Haussler D, Pollard KS. Ongoing GC-Biased Evolution Is Widespread in the Human Genome and Enriched Near Recombination Hot Spots. Genome Biology and Evolution. 2011;3:614–626. doi: 10.1093/gbe/evr058. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Lachance J, Tishkoff SA. Biased Gene Conversion Skews Allele Frequencies in Human Populations, Increasing the Disease Burden of Recessive Alleles. The American Journal of Human Genetics. 2014;95:408–420. doi: 10.1016/j.ajhg.2014.09.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Glemin S, Arndt PF, Messer PW, Petrov D, Galtier N, Duret L. Quantification of GC-biased gene conversion in the human genome. 2014 doi: 10.1101/gr.185488.114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Auton A, Fledel-Alon A, Pfeifer S, Venn O, Ségurel L, Street T, Leffler EM, Bowden R, Aneas I, Broxholme J, et al. A Fine-Scale Chimpanzee Genetic Map from Population Sequencing. Science. 2012;336:193–198. doi: 10.1126/science.1216872. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Coop G, Przeworski M. An evolutionary view of human recombination. Nat Rev Genet. 2007;8:23–34. doi: 10.1038/nrg1947. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Hussin JG, Hodgkinson A, Idaghdour Y, Grenier J-C, Goulet J-P, Gbeha E, Hip-Ki E, Awadalla P. Recombination affects accumulation of damaging and disease-associated mutations in human populations. Nat Genet. 2015;47:400–404. doi: 10.1038/ng.3216. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet. 2013;14:794–806. doi: 10.1038/nrg3573. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Lesecque Y, Glémin S, Lartillot N, Mouchiroud D, Duret L. The Red Queen Model of Recombination Hotspots Evolution in the Light of Archaic and Modern Human Genomes. PLoS Genet. 2014;10:e1004790. doi: 10.1371/journal.pgen.1004790. * This paper used modern human and archaic hominin genome sequences to provide support for the Red Queen hypothesis that there is a destructive interaction between gBGC and PRDM9 binding preferences that drives the turnover of recombination hotspots.
- 68. Scally A, Durbin R. Revising the human mutation rate: implications for understanding human evolution. Nature Reviews Genetics. 2012;13:745–753. doi: 10.1038/nrg3295. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Harris K. Evidence for recent, population-specific evolution of the human mutation rate. Proceedings of the National Academy of Sciences. 2015;112:3439–3444. doi: 10.1073/pnas.1418652112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. and Analysis Consortium The Chimpanzee S. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Sudmant PH, Huddleston J, Catacchio CR, Malig M, Hillier LW, Baker C, Mohajeri K, Kondova I, Bontrop RE, Persengiev S. Evolution and diversity of copy number variation in the great ape lineage. Genome research. 2013;23:1373–1382. doi: 10.1101/gr.158543.113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Kloosterman WP, Francioli LC, Hormozdiari F, Marschall T, Hehir-Kwa JY, Abdellaoui A, Lameijer E-W, Moed MH, Koval V, Renkens I, et al. Characteristics of de novo structural changes in the human genome. Genome Research. 2015 doi: 10.1101/gr.185041.114. * This study provided a large-scale analysis of de novo rates of indels and structural variation in the human genome. They found that de novo structural changes influence far more nucleotides per generation than de novo substitutions.
- 73. Stankiewicz P, Lupski JR. Structural Variation in the Human Genome and its Role in Disease. Annual Review of Medicine. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Chuzhanova NA, Anassis EJ, Ball EV, Krawczak M, Cooper DN. Meta-analysis of indels causing human genetic disease: mechanisms of mutagenesis and the role of local DNA sequence complexity. Human mutation. 2003;21:28–44. doi: 10.1002/humu.10146. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. O'Bleness M, Searles VB, Varki A, Gagneux P, Sikela JM. Evolution of genetic and genomic features unique to the human lineage. Nat Rev Genet. 2012;13:853–866. doi: 10.1038/nrg3336. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. McLean CY, Reno PL, Pollen AA, Bassan AI, Capellini TD, Guenther C, Indjeian VB, Lim X, Menke DB, Schaar BT. Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature. 2011;471:216–219. doi: 10.1038/nature09774. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Knowles DG, McLysaght A. Recent de novo origin of human protein-coding genes. Genome research. 2009;19:1752–1759. doi: 10.1101/gr.095026.109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Zhang YE, Long M. New genes contribute to genetic and phenotypic novelties in human evolution. Current Opinion in Genetics & Development. 2014;29:90–96. doi: 10.1016/j.gde.2014.08.013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Montgomery SB, Goode DL, Kvikstad E, Albers CA, Zhang ZD, Mu XJ, Ananda G, Howie B, Karczewski KJ, Smith KS, et al. The origin, evolution, and functional impact of short insertion–deletion variants identified in 179 human genomes. Genome Research. 2013;23:749–761. doi: 10.1101/gr.148718.112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Capra JA, Erwin GD, McKinsey G, Rubenstein JL, Pollard KS. Many human accelerated regions are developmental enhancers. Philosophical Transactions of the Royal Society B. Biological Sciences. 2013;368 doi: 10.1098/rstb.2013.0025. 20130025. * This largest study of the functions of human accelerated regions demonstrated that many of them act as developmental gene regulatory enhancers with enrichment for activity in the brain.
- 82. Grossman SR, Andersen KG, Shlyakhter I, Tabrizi S, Winnicki S, Yen A, Park DJ, Griesemer D, Karlsson EK, Wong SH. Identifying recent adaptations in large-scale genomic data. Cell. 2013;152:703–713. doi: 10.1016/j.cell.2013.01.035. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Colonna V, Ayub Q, Chen Y, Pagani L, Luisi P, Pybus M, Garrison E, Xue Y. Human genomic regions with exceptionally high levels of population differentiation identified from 911 whole-genome sequences. 2014 doi: 10.1186/gb-2014-15-6-r88. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Prabhakar S, Visel A, Akiyama JA, Shoukry M, Lewis KD, Holt A, Plajzer-Frick I, Morrison H, Fitzpatrick DR, Afzal V, et al. Human-specific gain of function in a developmental enhancer. Science. 2008;321:1346–1350. doi: 10.1126/science.1159974. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Pollard KS, Salama SR, Lambert N, Lambot M-A, Coppens S, Pedersen JS, Katzman S, King B, Onodera C, Siepel A. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172. doi: 10.1038/nature05113. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Kamberov YG, Wang S, Tan J, Gerbault P, Wark A, Tan L, Yang Y, Li S, Tang K, Chen H. Modeling recent human evolution in mice by expression of a selected EDAR variant. Cell. 2013;152:691–702. doi: 10.1016/j.cell.2013.01.016. * This study provided one of the most comprehensive experimental characterizations of a region that has experienced recent positive selection in a human population. Using mouse models, it demonstrated that EDAR variants were likely adaptive in Chinese populations and that they influence relevant hair, mammary, and eccrine gland phenotypes.
- 87. Boyd JL, Skove Stephanie L, Rouanet Jeremy P, Pilaz L-J, Bepler T, Gordân R, Wray Gregory A, Silver Debra L. Human-Chimpanzee Differences in a FZD8 Enhancer Alter Cell-Cycle Dynamics in the Developing Neocortex. Current Biology. 25:772–779. doi: 10.1016/j.cub.2015.01.041. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Boyd JL, Skove SL, Rouanet JP, Pilaz L-J, Bepler T, Gordân R, Wray GA, Silver DL. Human-Chimpanzee Differences in a FZD8 Enhancer Alter Cell-Cycle Dynamics in the Developing Neocortex. Current Biology. 2015;25:772–779. doi: 10.1016/j.cub.2015.01.041. ** This study demonstrated that enhancer sequence changes may contribute to unique features of the human brain. It showed that the human version of a human accelerated enhancer region produces a faster progenitor cell cycle and increased neocortical size.
- 89. Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Rev Genet. 2015;16:197–212. doi: 10.1038/nrg3891. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Erwin GD, Oksenberg N, Truty RM, Kostka D, Murphy KK, Ahituv N, Pollard KS, Capra JA. Integrating diverse datasets improves developmental enhancer prediction. PLoS computational biology. 2014;10:e1003677. doi: 10.1371/journal.pcbi.1003677. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Battle A, Khan Z, Wang SH, Mitrano A, Ford MJ, Pritchard JK, Gilad Y. Impact of regulatory variation from RNA to protein. Science. 2014 doi: 10.1126/science.1260793. 1260793. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Stranger BE, Stahl EA, Raj T. Progress and Promise of Genome-Wide Association Studies for Human Complex Trait Genetics. Genetics. 2011;187:367–383. doi: 10.1534/genetics.110.120907. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Bush WS, Moore JH. Chapter 11: Genome-wide association studies. PLoS Comput Biol. 2012;8:e1002822. doi: 10.1371/journal.pcbi.1002822. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik GP, Larson EB, Li R, Masys DR, Ritchie MD, Roden DM. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC medical genomics. 2011;4:13. doi: 10.1186/1755-8794-4-13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, Field JR, Pulley JM, Ramirez AH, Bowton E, et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotech. 2013;31:1102–1111. doi: 10.1038/nbt.2749. * This study established the feasiblity of the phenome-wide association study (PheWAS) using eletronic health records, by demonstrating that PheWAS replicates the majority of known associations tested and identifies novel gene-phenotype associations.
- 96. Patwardhan RP, Hiatt JB, Witten DM, Kim MJ, Smith RP, May D, Lee C, Andrie JM, Lee S-I, Cooper GM, et al. Massively parallel functional dissection of mammalian enhancers in vivo. Nat Biotech. 2012;30:265–270. doi: 10.1038/nbt.2136. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotech. 2014;32:347–355. doi: 10.1038/nbt.2842. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Brucker RM, Bordenstein SR. The capacious hologenome. Zoology. 2013;116:260–261. doi: 10.1016/j.zool.2013.08.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nature Reviews Genetics. 2014;15:379–393. doi: 10.1038/nrg3734. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Barrick JE, Lenski RE. Genome dynamics during experimental evolution. Nature Reviews Genetics. 2013;14:827–839. doi: 10.1038/nrg3564. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (283.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- BOOKS AND ARTS
- 14 February 2018
The past, present and future of human evolution
- María Martinón-Torres 0
María Martinón-Torres is a palaeoanthropologist, director of the National Research Centre on Human Evolution (CENIEH) in Burgos, Spain, and an honorary reader at University College London.
You can also search for this author in PubMed Google Scholar
You have full access to this article via your institution.
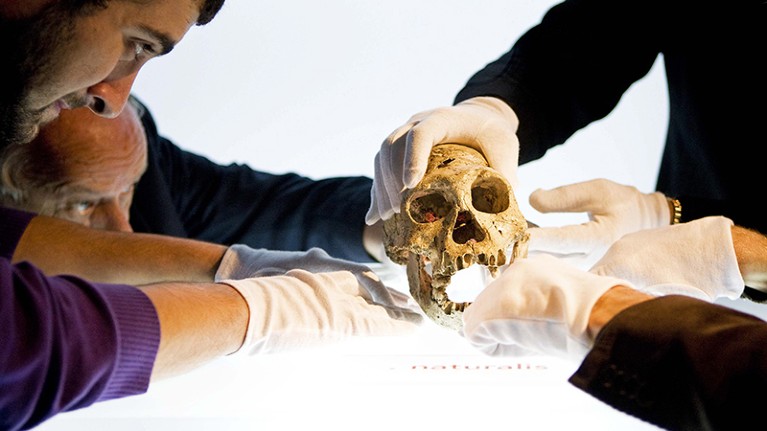
A 1.8-million-year-old skull discovered in Dmanisi, Georgia, is one of the oldest hominin fossils found outside Africa. Credit: Valerie Kuypers/Epa/REX/Shutterstock
Close Encounters with Humankind: A Paleoanthropologist Investigates Our Evolving Species Sang-Hee Lee W. W. Norton: 2018.
Many people assume that palaeoanthropology deals only with the past. The thinking goes that, beyond a curious, somehow romantic interest in the early accounts of our ancestors, there is not much that this discipline can add to the understanding of present-day humans. South Korean palaeoanthropologist Sang-Hee Lee disputes that view in Close Encounters with Humankind . She shows us to ourselves as the living (and, importantly, still changing) outcome of a wonderful interplay between biology and natural selection over the roughly 6 million years since hominins diverged from the chimpanzee lineage.
Avoiding the usual narrative, from bipedal ape-like creature to complex behaviour, Lee offers an original journey along our singular evolutionary path. When did our ancestors lose their fur? Did the taste for meat change our destiny? Was farming a blessing or a curse? Is altruism unique to us? Succinctly and engagingly, Lee revisits these and other key questions about the story of our evolving species — and gives some unconventional answers.
Notably, she supports multiregionalism. This is the theory that modern humans originated in many places simultaneously, in contrast to the ‘out of Africa’ model that posits a single origin for our species. Thus, she counters the sometimes rigid interpretations of the fossil record propounded in a literature dominated by the English language and the Western scientific community. In her book, Asia makes a comeback as a birthplace of modern humans and their ancestors. Lee reminds us that the Dmanisi hominin fossils from the republic of Georgia are as old as the earliest Homo fossils found in Africa; and that Homo erectus might have originated in Asia and migrated “back into Africa” to give rise to later Homo species. She also discusses the Denisovans, the mysterious hominins that coexisted with modern humans and left behind extensive DNA, but few fossils. She refers to them as “Asian Neanderthals” to highlight how the reconstruction of European hominins’ evolutionary story should not be disconnected from that of their Asian cousins.

The site near Beijing where the 750,000-year-old ‘Peking Man’ Homo erectus fossils were found. Credit: Granger/REX/Shutterstock
Not everything in Close Encounters with Humankind is about the past. Are humans still evolving? It’s commonly thought that our interaction with the world through culture and technology (such as clothes, tools or medicines) has buffered the pressure on our bodies to adapt biologically to the environment. Lee challenges this view and traces a cascade of other evidence for ongoing human evolution. She points to studies on skin colour as evidence.
Dark skin is thought to have evolved in the first furless hominins in Africa, to protect against the ultraviolet radiation in intense direct sunlight. Hominins living in higher latitudes, went this line of reasoning, would be exposed to less UV radiation, and so would need less-active melanocytes (the cells that produce the pigment melanin). That might largely explain the lighter skin of populations in regions farther from the Equator. However, studies by geneticist Iain Mathieson, now at the University of Pennsylvania in Philadelphia, and his colleagues on a large ancient-DNA sample from western Eurasian populations revealed that the light skin of Europeans is due to a new gene variant that emerged no more than 4,000 years ago ( I. Mathieson et al . Nature 528, 499–503; 2015 ). They link these populations’ lighter skin to the rise of agriculture and sedentary communal lifestyles, a view Lee favours.
As she shows, the shift to agriculture led to a diet based on processed grains and starches, which is deficient in many nutrients, including vitamin D. This deficiency forces the body itself to synthesize the vitamin — a metabolic process requiring the absorption of UV through the skin. The mutation for paler skin in Europeans pinpointed by Mathieson would maximize the UV absorption in populations facing low vitamin D intake. With this example, Lee emphasizes how culture — in this case, agriculture and a change in diet — might even have accelerated evolution.
Farming also led to a population explosion, despite increased vulnerability to infectious disease in settled communities. The availability of cereals allowed earlier weaning of infants, and meant women could give birth at shorter intervals. The resulting population increase brought higher genetic diversity, “the raw material of evolution”. Another demonstration of how our biology is still subject to change is the lactase mutation that has allowed some humans over at least the past 5,000 years to digest milk into adulthood. This eccentricity, less common in East Asia (predominantly China), became a key advantage for pastoralists and might represent an additional mechanism for overcoming the scarcity of vitamin D, because cow’s milk is rich in the nutrient.
Moreover, living in communities is central to our species’ success. As Lee notes, large groups became essential to survival because they offer assistance, to offset the difficulties of giving birth to big-brained babies and caring for them through a long infancy. Modern humans are also the longest-living primate species: three generations can overlap in time. Individuals stay ‘useful’ beyond their reproductive period by taking care of their children’s offspring and even unrelated infants. As Lee states, the concept of “fictive kin” (close bonds with those outside family or marriage) is unique to humans. She notes the remains of an elderly hominin in Dmanisi, dated to 1.8 million years ago, that evidently survived for some time without teeth, at a time without sophisticated tools or the knowledge of how to control fire. That could indicate that the hominin was treated with compassion by the group: the fossil could be the earliest evidence of human altruistic behaviour.
Lee’s style is breezy. A chapter entitled ‘King Kong’ discusses Gigantopithecus , the puzzling gigantic ape found in China that might have coexisted with Homo erectus from 1.2 million to 300,000 years ago. ‘Breaking Back’ looks at back pain as a trade-off of bipedalism. That accessibility sometimes risks over-simplifying, and occasionally strays into territory where every trait seems to have a function or to have evolved for a use.
Yet, ultimately, Lee will inspire even experts with her efforts at elucidating a field often seen as arid and inscrutable. Close Encounters with Humankind emphasizes how much the past matters. Our 6-million‑year story has been massively shaped by chance and a changing environment. Lee shows that, now more than ever, our decisions can shape the future of Earth and its inhabitants, including ourselves.
Nature 554 , 296-297 (2018)
doi: https://doi.org/10.1038/d41586-018-01807-7

Related Articles

- Palaeontology
How human brains got so big: our cells learned to handle the stress that comes with size
News 15 NOV 24

Huge carnivorous ‘terror bird’ rivalled the giant panda in size
Research Highlight 14 NOV 24
Growing up slowed down for an early Homo individual
News & Views 13 NOV 24
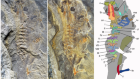
The oldest tadpole reveals evolutionary stability of the anuran life cycle
Article 30 OCT 24

US trust in scientists plunged during the pandemic — but it’s starting to recover
News 14 NOV 24
Devastating Spanish floods expose an urgent need for more flood-risk professionals
Correspondence 12 NOV 24

‘I get paid for my outputs, not because I am Māori’: why Indigenous researchers often face double duty
Career Q&A 12 NOV 24
Group leader positions at the IMP
We invite applications for group leader positions at the Research Institute of Molecular Pathology (IMP) in Vienna, Austria.
Vienna (Landbezirke) (AT)
Research Institute of Molecular Pathology (IMP)
Postdoctoral fellow
The laboratory of Prof. Ylva Ivarsson is seeking a highly motived candidate for a two-year postdoctoral fellowship on a project focused on short li...
Uppsala (Stad) (SE)
Uppsala University, Department of Chemistry - BMC
Call for Participation at Forum of Young Scientists Shenzhen University of Advanced Technology
Shenzhen University of Advanced Technology (SUAT) invites interested individuals to its Forum of Young Scientists.
Shenzhen, Guangdong, China
Shenzhen University of Advanced Technology
Director of the Institute of Cancer Research and Robert and Janet Perro Endowed Chair
Northwell Health has formed a strategic partnership with the NCI-designated Cancer Center at Cold Spring Harbor Laboratory (CSHL). The institutions...
Manhasset, New York
Head of the Translational Cancer Research Laboratory
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Support Our Work
The Smithsonian Institution's Human Origins Program
Human evolution research.

Smithsonian Human Origins Program & Field Research
The Smithsonian’s Human Origins Program conducts field and lab research on the evolution of early human adaptations. Our key research partners are in East Africa and East Asia – especially in Kenya, China, and Indonesia. Our digs and studies in these regions, along with investigations by associates working in Ethiopia, Tanzania, India, Mozambique, among other countries, help generate scientific data on the long-term interaction of human ancestors with their surroundings. Curiosity about human origins drives our research. The research supports our effort to bring the latest findings to public audiences worldwide.

Climate and Human Evolution
Explore how environment change influenced evolution, and how dramatic climate instability over the past 6 million years may have shaped human adaptations.

East African Research Projects
The Human Origins Program has been conducting research in East Africa for over 25 years. Read about some of our current field projects, focused in Kenya, here.

Asian Research Projects
The Human Origins Program conducts research at a variety of sites in Asia, currently with a focus in China and Indonesia. Read about the earliest humans in China, 'hobbits' of Indonesia, and other research projects here.

The Age of Humans: Evolutionary Perspectives on the Anthropocene

- Fossil Forensics: Interactive
Scientists can study fossil bones to find clues about how early humans lived and died, and the variety of dangers they faced. Fossil animal bones can also reveal evidence of the meat-eating habits of early humans - and their competitors for animal prey, the large carnivores.
Choose a fossil bone specimen to see what scientists have learned about it by examining it up close, sometimes even under a microscope.

- What's Hot in Human Origins?
Check out the latest human origins discoveries and research findings!

Digital Archive of Ungulate and Carnivore Dentition
Members of the Human Origins Program team describe how they use cutting-edge technology in their scientific investigations.
- Climate Effects on Human Evolution
- Survival of the Adaptable
- Human Evolution Timeline Interactive
- 2011 Olorgesailie Dispatches
- 2004 Olorgesailie Dispatches
- 1999 Olorgesailie Dispatches
- Olorgesailie Drilling Project
- Kanam, Kenya
- Kanjera, Kenya
- Ol Pejeta, Kenya
- Olorgesailie, Kenya
- Evolution of Human Innovation
- Adventures in the Rift Valley: Interactive
- 'Hobbits' on Flores, Indonesia
- Earliest Humans in China
- Bose, China
- Anthropocene: The Age of Humans
- Instructions
- Carnivore Dentition
- Ungulate Dentition
- Primate Behavior
- Footprints from Koobi Fora, Kenya
- Laetoli Footprint Trails
- Footprints from Engare Sero, Tanzania
- Hammerstone from Majuangou, China
- Handaxe and Tektites from Bose, China
- Handaxe from Europe
- Handaxe from India
- Oldowan Tools from Lokalalei, Kenya
- Olduvai Chopper
- Stone Tools from Majuangou, China
- Middle Stone Age Tools
- Burin from Laugerie Haute & Basse, Dordogne, France
- La Madeleine, Dordogne, France
- Butchered Animal Bones from Gona, Ethiopia
- Katanda Bone Harpoon Point
- Oldest Wooden Spear
- Punctured Horse Shoulder Blade
- Stone Sickle Blades
- Projectile Point
- Oldest Pottery
- Pottery Fragment
- Fire-Altered Stone Tools
- Terra Amata Shelter
- Qafzeh: Oldest Intentional Burial
- Assyrian Cylinder Seal
- Blombos Ocher Plaque
- Ishango Bone
- Bone and Ivory Needles
- Carved Ivory Running Lion
- Female torso in ivory
- Ivory Horse Figurine
- Ivory Horse Sculpture
- Lady of Brassempouy
- Lion-Man Figurine
- Willendorf Venus
- Ancient Shell Beads
- Carved Bone Disc
- Cro-Magnon Shell Bead Necklace
- Oldest Known Shell Beads
- Ancient Flute
- Ancient Pigments
- Apollo 11 Plaque
- Carved antler baton with horses
- Geometric incised bone rectangle
- Tata Plaque
- Mystery Skull Interactive
- Shanidar 3 - Neanderthal Skeleton
- One Species, Living Worldwide
- Human Skin Color Variation
- Ancient DNA and Neanderthals
- Human Family Tree
- Swartkrans, South Africa
- Shanidar, Iraq
- Walking Upright
- Tools & Food
- Social Life
- Language & Symbols
- Humans Change the World
- Introduction to Human Evolution
- Nuts and bolts classification: Arbitrary or not? (Grades 6-8)
- The Origins and Evolution of Human Bipedality (Grades 9-12)
- Comparison of Human and Chimp Chromosomes (Grades 9-12)
- Hominid Cranial Comparison: The "Skulls" Lab (Grades 9-12)
- Investigating Common Descent: Formulating Explanations and Models (Grades 9-12)
- Fossil and Migration Patterns in Early Hominids (Grades 9-12)
- For College Students
- Why do we get goose bumps?
- Chickens, chimpanzees, and you - what do they have in common?
- Grandparents are unique to humans
- How strong are we?
- Humans are handy!
- Humans: the running ape
- Our big hungry brain!
- Our eyes say it!
- The early human tool kit
- The short-haired human!
- The “Nutcracker”
- What can lice tell us about human evolution?
- What does gut got to do with it?
- Why do paleoanthropologists love Lucy?
- Why do we have wisdom teeth?
- Human Origins Glossary
- Learning Unity and Diversity in Alabama
- Teaching Evolution through Human Examples
- Frequently Asked Questions
- Recommended Books
- Exhibit Floorplan Interactive
- Print Floorplan PDF
- Reconstructions of Early Humans
- Chesterfield County Public Library
- Orange County Library
- Andover Public Library
- Ephrata Public Library
- Oelwein Public Library
- Cedar City Public Library
- Milpitas Library
- Spokane County Library
- Cottage Grove Public Library
- Pueblo City-County Library
- Springfield-Greene County Library
- Peoria Public Library
- Orion Township Public Library
- Skokie Public Library
- Wyckoff Free Public Library
- Tompkins County Public Library
- Otis Library
- Fletcher Free Library
- Bangor Public Library
- Payne Theological Seminary
- Columbia Theological Seminary
- Yuma County Library
- Hood Theological Seminary & Livingstone College
- Broward County Public Library
- Human Origins Do it Yourself Exhibit
- Exhibit Field Trip Guide
- Acknowledgments
- Human Origins Program Team
- Connie Bertka
- Betty Holley
- Nancy Howell
- Lee Meadows
- Jamie L. Jensen
- David Orenstein
- Michael Tenneson
- Leonisa Ardizzone
- Fatimah Jackson
- Shai Cherry
- David Haberman (Emeritus)
- Fred Edwords (Emeritus)
- Elliot Dorff (Emeritus)
- Francisca Cho (Emeritus)
- Peter F. Ryan (Emeritus)
- Mustansir Mir (Emeritus)
- Randy Isaac (Emeritus)
- Mary Evelyn Tucker (Emeritus)
- Wentzel van Huyssteen (Emeritus)
- Joe Watkins (Emeritus)
- Tom Weinandy (Emeritus)
- Members Thoughts on Science, Religion & Human Origins (video)
- Science, Religion, Evolution and Creationism: Primer
- The Evolution of Religious Belief: Seeking Deep Evolutionary Roots
- Laboring for Science, Laboring for Souls: Obstacles and Approaches to Teaching and Learning Evolution in the Southeastern United States
- Public Event : Religious Audiences and the Topic of Evolution: Lessons from the Classroom (video)
- Evolution and the Anthropocene: Science, Religion, and the Human Future
- Imagining the Human Future: Ethics for the Anthropocene
- Human Evolution and Religion: Questions and Conversations from the Hall of Human Origins
- I Came from Where? Approaching the Science of Human Origins from Religious Perspectives
- Religious Perspectives on the Science of Human Origins
- Submit Your Response to "What Does It Mean To Be Human?"
- Volunteer Opportunities
- Submit Question
- "Shaping Humanity: How Science, Art, and Imagination Help Us Understand Our Origins" (book by John Gurche)
- What Does It Mean To Be Human? (book by Richard Potts and Chris Sloan)
- Bronze Statues
- Reconstructed Faces

IMAGES
VIDEO
COMMENTS
In one of the most remarkable understatements in the history of science, Charles Darwin wrote about his theory of evolution by natural selection that "light will be thrown on the origin of man ...
Human evolution is a topic that interests not just researchers specialized in paleoanthropology, but also other scientists and the general public. ... Invitation to participate in the survey was sent by email to the authors of articles and review papers that had been published in a scientific journal of a relevant field during the three ...
The new findings and their implications to the late stage of human evolution have been explored by different studies recently, all dedicated to some extent to understanding the trajectory of human evolution from the Chibanian to the present, focusing on specific topics like the origins of our species (Mounier and Mirazón Lahr, 2016, Mounier and Mirazón Lahr, 2019), the evolution of the human ...
Keywords: Chimpanzee, Pan troglodytes, human evolution, Gombe National Park Abstract. Sixty years of research on chimpanzees (Pan troglodytes) at Gombe National Park, Tanzania have revealed many similarities with human behaviour, including hunting, tool use and coalitionary killing.The close phylogenetic relationship between chimpanzees and humans suggests that these traits were present in the ...
Population-level right-handedness is a defining characteristic of humans. Despite extensive research, we still do not know the conditions or timing of its emergence in human evolution. We present a review of research into the origins of handedness, based on fossil and archaeological data for hand preference and great ape hand-use.
In this review, we have highlighted several research areas that have potential to significantly deepen our knowledge of human genome evolution over the next few years, but our list is not exhaustive. Many other areas, including the evolutionary study of human-microbe interactions [ 98 , 99 ] and experimental evolution [ 100 ], are poised for ...
The Journal of Human Evolution concentrates on publishing the highest quality papers covering all aspects of human evolution.The central focus is aimed jointly at paleoanthropological work, covering human and primate fossils, and at comparative studies of living species, including both morphological and molecular evidence.These include descriptions of new discoveries, interpretative analyses ...
María Martinón-Torres. María Martinón-Torres is a palaeoanthropologist, director of the National Research Centre on Human Evolution (CENIEH) in Burgos, Spain, and an honorary reader at ...
The Smithsonian's Human Origins Program conducts field and lab research on the evolution of early human adaptations. Our key research partners are in East Africa and East Asia - especially in Kenya, China, and Indonesia. Our digs and studies in these regions, along with investigations by associates working in Ethiopia, Tanzania, India ...
A review of positive selection and human trait evolution since the common ancestors with chimpanzees and other great apes. Wang, X., W. E. Grus, and J. Zhang. 2006. Gene losses during human origins. PLoS Biology 4:e52. An investigation of the impact of gene loss on human evolution, providing case studies of its adaptive ef fects.