
ERIN HENDRIKS, MD, RACHEL ROSENBERG, MD, AND LINDA PRINE, MD
Am Fam Physician. 2020;101(10):599-606
Author disclosure: No relevant financial affiliations.
Ectopic pregnancy occurs when a fertilized ovum implants outside of the uterine cavity. In the United States, the estimated prevalence of ectopic pregnancy is 1% to 2%, and ruptured ectopic pregnancy accounts for 2.7% of pregnancy-related deaths. Risk factors include a history of pelvic inflammatory disease, cigarette smoking, fallopian tube surgery, previous ectopic pregnancy, and infertility. Ectopic pregnancy should be considered in any patient presenting early in pregnancy with vaginal bleeding or lower abdominal pain in whom intrauterine pregnancy has not yet been established. The definitive diagnosis of ectopic pregnancy can be made with ultrasound visualization of a yolk sac and/or embryo in the adnexa. However, most ectopic pregnancies do not reach this stage. More often, patient symptoms combined with serial ultrasonography and trends in beta human chorionic gonadotropin levels are used to make the diagnosis. Pregnancy of unknown location refers to a transient state in which a pregnancy test is positive but ultrasonography shows neither intrauterine nor ectopic pregnancy. Serial beta human chorionic gonadotropin levels, serial ultrasonography, and, at times, uterine aspiration can be used to arrive at a definitive diagnosis. Treatment of diagnosed ectopic pregnancy includes medical management with intramuscular methotrexate, surgical management via salpingostomy or salpingectomy, and, in rare cases, expectant management. A patient with diagnosed ectopic pregnancy should be immediately transferred for surgery if she has peritoneal signs or hemodynamic instability, if the initial beta human chorionic gonadotropin level is high, if fetal cardiac activity is detected outside of the uterus on ultrasonography, or if there is a contraindication to medical management.
Ectopic pregnancy occurs when a fertilized ovum implants outside of the uterine cavity. The prevalence of ectopic pregnancy in the United States is estimated to be 1% to 2%, but this may be an underestimate because this condition is often treated in the office setting where it is not tracked. 1 , 2 The mortality rate for ruptured ectopic pregnancy has steadily declined over the past three decades, and from 2011 to 2013 accounted for 2.7% of pregnancy-related deaths. 1 , 3 Risk factors for ectopic pregnancy are listed in Table 1 4 , 5 ; however, one-half of women with diagnosed ectopic pregnancy have no identified risk factors. 4 – 6 The overall rate of pregnancy (including ectopic) is less than 1% when a patient has an intrauterine device (IUD). However, in the rare case that a woman does become pregnant while she has an IUD, the prevalence of ectopic pregnancy is as high as 53%. 7 , 8 There is no difference in ectopic pregnancy rates between copper or progestin-releasing IUDs. 9
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| , | Expert opinion and consensus guideline in the absence of clinical trials | |
| Expert opinion and consensus guideline in the absence of clinical trials | ||
| Expert opinion and consensus guideline in the absence of clinical trials | ||
| , | Expert opinion and consensus guideline in the absence of clinical trials |
| Age > 35 years Cigarette smoking Documented fallopian tube pathology Infertility Pelvic inflammatory disease Pregnancy while intrauterine device is in place Previous ectopic pregnancy Previous fallopian tube surgery |

Making the Diagnosis
Signs and symptoms.
Ectopic pregnancy should be considered in any pregnant patient with vaginal bleeding or lower abdominal pain when intrauterine pregnancy has not yet been established ( Table 2 ) . 10 Vaginal bleeding in women with ectopic pregnancy is due to the sloughing of decidual endometrium and can range from spotting to menstruation-equivalent levels. 10 This endometrial decidual reaction occurs even with ectopic implantation, and the passage of a decidual cast may mimic the passage of pregnancy tissue. Thus, a history of bleeding and passage of tissue cannot be relied on to differentiate ectopic pregnancy from early intrauterine pregnancy failure.
| Appendicitis Early pregnancy loss Ectopic pregnancy Ovarian torsion Pelvic inflammatory disease Subchorionic hemorrhage in viable intrauterine pregnancy Trauma Urinary calculi |
The nature, location, and severity of pain in ectopic pregnancy vary. It often begins as a colicky abdominal or pelvic pain that is localized to one side as the pregnancy distends the fallopian tube. The pain may become more generalized once the tube ruptures and hemoperitoneum develops. Other potential symptoms include presyncope, syncope, vomiting, diarrhea, shoulder pain, lower urinary tract symptoms, rectal pressure, or pain with defecation. 11
The physical examination can reveal signs of hemodynamic instability (e.g., hypotension, tachycardia) in women with ruptured ectopic pregnancy and hemoperitoneum. 12 Patients with unruptured ectopic pregnancy often have cervical motion or adnexal tenderness. 13 Sometimes the ectopic pregnancy itself can be palpated as a painful mass lateral to the uterus. There is no evidence that palpation during the pelvic examination leads to an increased risk of rupture. 10
BETA HUMAN CHORIONIC GONADOTROPIN
Beta human chorionic gonadotropin (β-hCG) can be detected in pregnancy as early as eight days after ovulation. 14 The rate of increase in β-hCG levels, typically measured every 48 hours, can aid in distinguishing normal from abnormal early pregnancy. In a viable intrauterine pregnancy with an initial β-hCG level less than 1,500 mIU per mL (1,500 IU per L), there is a 99% chance that the β-hCG level will increase by at least 49% over 48 hours. 15 As the initial β-hCG level increases, the rate of increase over 48 hours slows, with an increase of at least 40% expected for an initial β-hCG level of 1,500 to 3,000 mIU per mL (1,500 to 3,000 IU per L) and 33% for an initial β-hCG level greater than 3,000 mIU per mL. 15 A slower-than-expected rate of increase or a decrease in β-hCG levels suggests early pregnancy loss or ectopic pregnancy. The rate of increase slows as pregnancy progresses and typically plateaus around 100,000 mIU per mL (100,000 IU per L) at 10 weeks' gestation. 16 A decrease in β-hCG of at least 21% over 48 hours suggests a likely failed intrauterine pregnancy, whereas a smaller decrease should raise concern for ectopic pregnancy. 17
The discriminatory level is the β-hCG level above which an intrauterine pregnancy is expected to be seen on transvaginal ultrasonography; it varies with the type of ultrasound machine used, the sonographer, and the number of gestations. A combination of β-hCG level greater than the discriminatory level and ultrasonography that does not show an intrauterine pregnancy should raise concern for early pregnancy loss or an ectopic pregnancy. 5 The discriminatory zone was previously defined as a β-hCG level of 1,000 to 2,000 mIU per mL (1,000 to 2,000 IU per L); however, this cutoff can miss some intrauterine pregnancies that do not become apparent until a slightly higher β-hCG level is achieved. Therefore, in a desired pregnancy, it is recommended that a discriminatory level as high as 3,500 mIU per mL (3,500 IU per L) be used to avoid misdiagnosis and interruption of a viable pregnancy, although most pregnancies will be visualized by the time the β-hCG level reaches 1,500 mIU per mL. 18 , 19
TRANSVAGINAL ULTRASONOGRAPHY
Intrauterine pregnancy visualized on transvaginal ultrasonography essentially rules out ectopic pregnancy except in the exceedingly rare case of heterotopic pregnancy. 5 The definitive diagnosis of ectopic pregnancy can be made with ultrasonography when a yolk sac and/or embryo is seen in the adnexa; however, ultrasonography alone is rarely used to diagnose ectopic pregnancy because most do not progress to this stage. 5 More often, the patient history is combined with serial quantitative β-hCG levels, sequential ultrasonography, and, at times, uterine aspiration to arrive at a final diagnosis of ectopic pregnancy.
PREGNANCY OF UNKNOWN LOCATION
Ultrasonography showing neither intrauterine nor ectopic pregnancy in a patient with a positive pregnancy test is referred to as a pregnancy of unknown location. In a desired pregnancy, β-hCG levels and serial ultrasonography combined with patient reports of pain or bleeding guide management. 20 In an undesired pregnancy or when the possibility of a viable intrauterine pregnancy has been excluded, manual vacuum aspiration of the uterus can evaluate for chorionic villi that differentiate intrauterine pregnancy loss from ectopic pregnancy. If chorionic villi are seen, further workup is unnecessary, and exposure to methotrexate can be avoided ( Figure 1 ) . 5 , 15 – 17 , 21 If chorionic villi are not seen after uterine aspiration, it is imperative to initiate treatment for ectopic pregnancy or repeat β-hCG measurement in 24 hours to ensure at least a 50% decrease. Ectopic precautions and serial β-hCG levels should be continued until the level is undetectable.
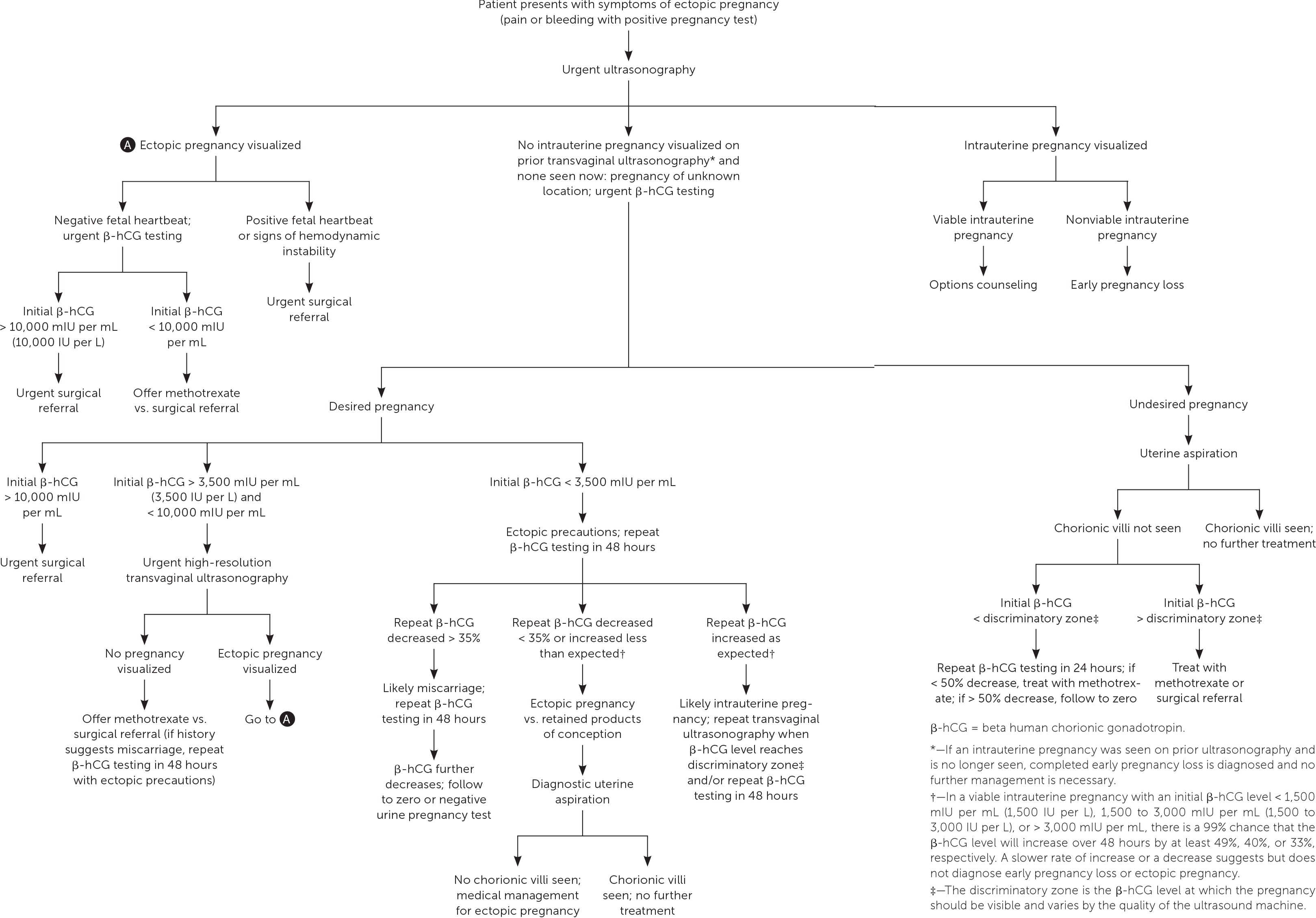
Management of Ectopic Pregnancy
It is appropriate for family physicians to treat hemodynamically stable patients in conjunction with their primary obstetrician. Patients with suspected or confirmed ectopic pregnancy who exhibit signs and symptoms of ruptured ectopic pregnancy should be emergently transferred for surgical intervention. If ectopic pregnancy has been diagnosed, the patient is deemed clinically stable, and the affected fallopian tube has not ruptured, treatment options include medical management with intramuscular methotrexate or surgical management with salpingostomy (removal of the ectopic pregnancy while leaving the fallopian tube in place) or salpingectomy (removal of part or all of the affected fallopian tube). The decision to manage the ectopic pregnancy medically or surgically should be informed by individual patient factors and preferences, clinical findings, ultrasound findings, and β-hCG levels. 12 Expectant management is rare but can be considered with close follow-up for patients with suspected ectopic pregnancy who are asymptomatic and have β-hCG levels that are very low and continue to decrease. 5
MEDICAL MANAGEMENT
Intramuscular methotrexate is the only medication appropriate for the management of ectopic pregnancy. A folate antagonist, it interrupts the rapidly dividing cells of the ectopic pregnancy, which are then resorbed by the body. 22 Its success rate decreases with higher initial β-hCG levels ( Table 3 ) . 23 Contraindications to methotrexate include renal insufficiency; moderate to severe anemia, leukopenia, or thrombocytopenia; liver disease or alcoholism; active peptic ulcer disease; and breastfeeding. 5 Therefore, a complete blood count and comprehensive metabolic panel should be obtained before it is administered.
| < 1,000 | 98 |
| 1,000 to 1,999 | 94 |
| 2,000 to 4,999 | 96 |
| 5,000 to 9,999 | 85 |
| ≥ 10,000 | 81 |
Several methotrexate regimens have been studied, including a single-dose protocol, a two-dose protocol, and a multi-dose protocol ( Table 4 ) . 5 The single-dose protocol carries the lowest risk of adverse effects, whereas the two-dose protocol is more effective than the single-dose protocol in patients with higher initial β-hCG levels. 24 There is no consistent evidence or consensus regarding the cutoff above which a two-dose protocol should be used, so clinicians should choose a regimen based on the initial β-hCG level and ultrasound findings, as well as patient preference regarding effectiveness vs. the risk of adverse effects. In general, the single-dose protocol should be used in patients with β-hCG levels less than 3,600 mIU per mL (3,600 IU per L), and the two-dose protocol should be considered for patients with higher initial β-hCG levels, especially those with levels greater than 5,000 mIU per mL. Multidose protocols carry a higher risk of adverse effects and are not preferred. 25
| 1 | Verify baseline stability of complete blood count and comprehensive metabolic panel; determine β-hCG level Administer single dose of methotrexate, 50 mg per m | Verify baseline stability of complete blood count and comprehensive metabolic panel; determine β-hCG level Administer single dose of methotrexate, 50 mg per m |
| 4 | Measure β-hCG level | Measure β-hCG level Administer second dose of methotrexate, 50 mg per m |
| 7 | Measure β-hCG level If decrease from days 4 to 7 is ≤ 15%, offer choice of readministration of single-dose methotrexate, 50 mg per m , or refer for surgical management; if β-hCG level does not decrease after two doses of methotrexate, refer for surgical management If decrease from days 4 to 7 is > 15%, measure β-hCG levels weekly until they are undetectable | Measure β-hCG level If decrease from days 4 to 7 is ≤ 15%, offer choice of further methotrexate doses or refer for surgical management; further methotrexate doses should be 50 mg per m on day 7 with measurement of β-hCG level on day 11, then another dose of 50 mg per m on day 11 if β-hCG level does not decrease ≤ 15% from days 7 to 11; if β-hCG level does not decrease ≤ 15% from days 11 to 14, refer for surgical management If decrease from days 4 to 7 is > 15%, measure β-hCG levels weekly until they are undetectable |
Before administering methotrexate, β-hCG levels should be measured on days 1, 4, and 7 of treatment. The first measurement helps the clinician decide between the one- and two-dose protocols. Levels commonly increase between days 1 and 4, but should decrease by at least 15% between days 4 and 7. If this decrease does not occur, the clinician should discuss with the patient whether she prefers to repeat the course of methotrexate or pursue surgical treatment. If the β-hCG level does decrease by at least 15% between days 4 and 7, the patient should return for weekly β-hCG measurements until levels become undetectable, which can take up to eight weeks. 26
Close follow-up is critical for the safe use of methotrexate in women with ectopic pregnancies. Patients should be counseled that the risk of rupture persists until β-hCG levels are undetectable, and that they should seek emergency care if signs of ectopic pregnancy occur. It is common for patients to experience some abdominal pain two to three days after administration of methotrexate. This pain can be managed expectantly as long as there are no signs of rupture. 5 Gastrointestinal adverse effects (e.g., abdominal pain, vomiting, nausea) and vaginal spotting are common. Patients should be counseled to avoid taking folic acid supplements and nonsteroidal anti-inflammatory drugs, which can decrease the effectiveness of methotrexate, and to avoid anything that may mask the symptoms of ruptured ectopic pregnancy (e.g., narcotic analgesics, alcohol) and activities that increase the risk of rupture (e.g., vaginal intercourse, vigorous exercise). Sunlight exposure during treatment can cause methotrexate dermatitis and should be avoided. 5 Other adverse effects of methotrexate include alopecia and elevation of liver enzymes. Patients should be counseled to avoid repeat pregnancy until at least one ovulatory cycle after the serum β-hCG level becomes undetectable, although some experts recommend waiting three months so that the methotrexate can be cleared completely. 27 There is no evidence that methotrexate therapy affects future fertility. 28
SURGICAL MANAGEMENT
Overall, surgical management has a higher success rate for ectopic pregnancy than methotrexate. 5 The initial β-hCG level at which to transfer a patient for possible surgical treatment depends on local standards, although a level of 5,000 mIU per mL (5,000 IU per L) is commonly used. 5 , 11 Ultrasound visualization of an embryo with fetal cardiac activity outside of the uterus is an indication for urgent transfer for surgical management. 5 , 25 Additionally, social factors that preclude frequent laboratory testing (e.g., poor telephone access, work and family obligations, lack of transportation) can make surgical management the safer option 5 ( Table 5 5 , 11 ) . In cases where methotrexate is contraindicated or not preferred by the patient, surgical management can usually be performed laparoscopically if the patient is hemodynamically stable. Surgical options include salpingostomy or salpingectomy. Randomized trials have shown no difference in sequelae between methotrexate administration and fallopian tube–sparing laparoscopic surgery, including rates of future intrauterine pregnancy and risk of future ectopic pregnancy. 29 The decision whether to remove the fallopian tube or leave it in place depends on the extent of damage to the tube (evaluated intraoperatively) and the patient's desire for future fertility.
| Hemodynamic instability Peritoneal signs Ultrasonography shows ectopic pregnancy with fetal cardiac activity Ultrasonography shows substantial fluid in the cul-de-sac and/or beyond |
| Barriers to close follow-up or refusal to accept blood transfusion High initial β-hCG levels (> 5,000 to 10,000 mIU per mL [5,000 to 10,000 IU per L]) or ectopic pregnancy > 4 cm Insufficient decline in β-hCG levels after administration of methotrexate Medical conditions that preclude medical management with methotrexate (e.g., active peptic ulcer disease, active pulmonary disease, anemia, breastfeeding, clinically important hepatic or renal disease, immunodeficiency, leukopenia, thrombocytopenia) |
EXPECTANT MANAGEMENT
Expectant management can be considered for patients whose peak β-hCG level is below the discriminatory zone and is decreasing, but has plateaued or is decreasing more slowly than expected for a failed intrauterine pregnancy. 30 In cases where the initial β-hCG level is 200 mIU per mL (200 IU per L) or less, 88% of patients will have successful spontaneous resolution of the pregnancy; however, rates of spontaneous resolution decrease with higher β-hCG levels. 31 Patient counseling must include the risks of spontaneous rupture, hemorrhage, and need for emergency surgery. Patients who choose expectant management should have β-hCG levels monitored every 48 hours, and medical or surgical management should be recommended if β-hCG levels do not decrease sufficiently. 5
This article updates a previous article on this topic by Barash, et al. 12
Data Sources: An evidence summary from Essential Evidence Plus was reviewed and relevant studies referenced. Additionally, a PubMed search was completed in Clinical Queries using the key terms ectopic pregnancy, first trimester bleeding, and pregnancy of unknown location. The search included meta-analyses, guidelines, and reviews. Also searched were the Cochrane database, DynaMed, and the National Guideline Clearinghouse. Search dates: October 26, 2018, through January 14, 2020.
Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980–2007. Obstet Gynecol. 2011;117(4):837-843.
Marion LL, Meeks GR. Ectopic pregnancy: history, incidence, epidemiology, and risk factors. Clin Obstet Gynecol. 2012;55(2):376-386.
Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366-373.
Ankum WM, Mol BW, Van der Veen F, et al. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril. 1996;65(6):1093-1099.
ACOG practice bulletin no. 193: tubal ectopic pregnancy [published correction appears in Obstet Gynecol . 2019;133(5):1059]. Obstet Gynecol. 2018;131(3):e91-e103.
Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190(1):50-54.
Hardeman J, Weiss BD. HardemanJWeissBDIntrauterine devices: an update. Am Fam Physician2014;89(6):445–450. Accessed November 9, 2019. https://www.ncbi.nlm.nih.gov/pubmed/24695563?dopt=Abstract
Bosco-Lévy P, Gouverneur A, Langlade C, et al. Safety of levonorgestrel 52 mg intrauterine system compared to copper intrauterine device: a population-based cohort study. Contraception. 2019;99(6):345-349.
Crochet JR, Bastian LA, Chireau MV. Does this woman have an ectopic pregnancy?: the rational clinical examination systematic review. JAMA. 2013;309(16):1722-1729.
Newbatt E, Beckles Z, Ullman R, et al.; Guideline Development Group. Ectopic pregnancy and miscarriage: summary of NICE guidance. BMJ. 2012;345:e8136.
Barash JH, Buchanan EM, Hillson C. BarashJHBuchananEMHillsonCDiagnosis and management of ectopic pregnancy. Am Fam Physician2014;90(1):34–40. Accessed November 9, 2019. https://www.aafp.org/afp/2014/0701/p34.html
Ramakrishnan K, Scheid DC. Ectopic pregnancy: forget the “classic presentation” if you want to catch it sooner. J Fam Pract. 2006;55(5):388-395.
Stewart BK, Nazar-Stewart V, Toivola B. Biochemical discrimination of pathologic pregnancy from early, normal intrauterine gestation in symptomatic patients. Am J Clin Pathol. 1995;103(4):386-390.
Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128(3):504-511.
Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol. 2004;104(1):50-55.
Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
Doubilet PM, Benson CB, Bourne T, et al.; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443-1451.
Connolly A, Ryan DH, Stuebe AM, et al. Reevaluation of discriminatory and threshold levels for serum β-hCG in early pregnancy. Obstet Gynecol. 2013;121(1):65-70.
Rodgers SK, Chang C, DeBardeleben JT, et al. Normal and abnormal US findings in early first-trimester pregnancy: review of the Society of Radiologists in Ultrasound 2012 consensus panel recommendations. Radiographics. 2015;35(7):2135-2148.
Reproductive Health Access Project. Diagnosis and treatment of ectopic pregnancy algorithm. June 2019. Accessed June 29, 2019. https://www.reproductiveaccess.org/resource/ectopic-algorithm
Stika CS. Methotrexate: the pharmacology behind medical treatment for ectopic pregnancy. Clin Obstet Gynecol. 2012;55(2):433-439.
Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87(3):481-484.
Yang C, Cai J, Geng Y, et al. Multiple-dose and double-dose versus single-dose administration of methotrexate for the treatment of ectopic pregnancy: a systematic review and meta-analysis. Reprod Biomed Online. 2017;34(4):383-391.
Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.
Barnhart KT, Gosman G, Ashby R, et al. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol. 2003;101(4):778-784.
Hospira. Methotrexate injection, USP [package insert]. October 2011. Accessed November 9, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/011719s117lbl.pdf
Ohannessian A, Loundou A, Courbière B, et al. Ovarian responsiveness in women receiving fertility treatment after methotrexate for ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod. 2014;29(9):1949-1956.
Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007(1):CD000324.
van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28(1):60-67.
Korhonen J, Stenman UH, Ylöstalo P. Serum human chorionic gonadotropin dynamics during spontaneous resolution of ectopic pregnancy. Fertil Steril. 1994;61(4):632-636.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2020 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Screening & Prevention
- Sexual Health & Relationships
- Birth Control
- Preparing for Surgery Checklist
- Healthy Teens
- Getting Pregnant
- During Pregnancy
- Labor and Delivery
- After Pregnancy
- Pregnancy Book
- Mental Health
- Prenatal Testing
- Menstrual Health
- Heart Health
- Special Procedures
- Diseases and Conditions
- Browse All Topics
- View All Frequently Asked Questions

Read common questions on the coronavirus and ACOG’s evidence-based answers.
Ectopic Pregnancy
URL has been copied to the clipboard
Frequently Asked Questions Expand All
An ectopic pregnancy occurs when a fertilized egg grows outside of the uterus . Almost all ectopic pregnancies—more than 90%—occur in a fallopian tube . As the pregnancy grows, it can cause the tube to burst (rupture). A rupture can cause major internal bleeding. This can be a life-threatening emergency that needs immediate surgery.
The risk factors for ectopic pregnancy include the following:
Previous ectopic pregnancy
Prior fallopian tube surgery
Previous pelvic or abdominal surgery
Certain sexually transmitted infections (STIs)
Pelvic inflammatory disease
Endometriosis
Other factors that may increase a woman’s risk of ectopic pregnancy include:
Cigarette smoking
Age older than 35 years
History of infertility
Use of assisted reproductive technology , such as in vitro fertilization (IVF)
About one half of all women who have an ectopic pregnancy do not have known risk factors. Sexually active women should be alert to changes in their bodies, especially if they experience symptoms of an ectopic pregnancy.
At first, an ectopic pregnancy may feel like a typical pregnancy with some of the same signs, such as a missed menstrual period, tender breasts, or an upset stomach. Other signs may include:
Abnormal vaginal bleeding
Low back pain
Mild pain in the abdomen or pelvis
Mild cramping on one side of the pelvis
At this stage, it may be hard to know if you are experiencing a typical pregnancy or an ectopic pregnancy. Abnormal bleeding and pelvic pain should be reported to your obstetrician–gynecologist (ob-gyn) or other health care professional.
As an ectopic pregnancy grows, more serious symptoms may develop, especially if a fallopian tube ruptures. Symptoms may include the following:
Sudden, severe pain in the abdomen or pelvis
Shoulder pain
Weakness, dizziness, or fainting
A ruptured fallopian tube can cause life-threatening internal bleeding. If you have sudden, severe pain; shoulder pain; or weakness, you should go to an emergency room.
If you do not have the symptoms of a fallopian tube rupture but your ob-gyn or other health care professional suspects you may have ectopic pregnancy, he or she may:
Perform a pelvic exam
Perform an ultrasound exam to see where the pregnancy is developing
Test your blood for a pregnancy hormone called human chorionic gonadotropin (hCG)
An ectopic pregnancy cannot move or be moved to the uterus, so it always requires treatment. There are two methods used to treat an ectopic pregnancy: 1) medication and 2) surgery. Several weeks of follow-up are required with each treatment.
The most common drug used to treat ectopic pregnancy is methotrexate. This drug stops cells from growing, which ends the pregnancy. The pregnancy then is absorbed by the body over 4–6 weeks. This does not require the removal of the fallopian tube.
Methotrexate may be used if the pregnancy has not ruptured a fallopian tube. Several factors go into the decision to use methotrexate. One of the most important factors is your ability to follow up with blood tests that check your blood levels of hCG. You will not be able to use methotrexate if you are breastfeeding or have certain health problems.
Methotrexate often is given by injection in one dose. Before you take methotrexate, blood tests will be done to measure the level of hCG and the functions of certain organs. If hCG levels have not decreased enough after the first dose, another dose of methotrexate may be recommended. You will have careful follow-up over time until hCG is no longer found in your blood.
Taking methotrexate can have some side effects. Most women have some abdominal pain. Vaginal bleeding or spotting also may occur. Other side effects may include:
It is important to follow up with your ob-gyn or other health care professional until your treatment with methotrexate is complete. The risk of a fallopian tube rupture does not go away until your treatment is over. Seek care right away if you have symptoms of a rupture, including sudden abdominal pain, shoulder pain, or weakness.
Yes, during treatment with methotrexate you should avoid the following:
Heavy exercise
Sexual intercourse
Vitamins and foods that contain folic acid, including fortified cereal, enriched bread and pasta, peanuts, dark green leafy vegetables, orange juice, and beans
Prescription pain medication and nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen. These medications can affect the way methotrexate works in the body.
Foods that produce gas, which can cause discomfort and mask the pain of a possible rupture of a fallopian tube
Prolonged exposure to sunlight. Methotrexate can cause sun sensitivity.
If the ectopic pregnancy has ruptured a tube, emergency surgery is needed. Sometimes surgery is needed even if the fallopian tube has not ruptured. In these cases, the ectopic pregnancy can be removed from the tube, or the entire tube with the pregnancy can be removed.
Surgery typically is done with laparoscopy . This procedure uses a slender, lighted camera that is inserted through small cuts in the abdomen. It is done in a hospital with general anesthesia .
Your ob-gyn or other health care professional will talk with you about the possible side effects and risks of surgery for ectopic pregnancy. These may include pain, fatigue, bleeding, and infection.
Whether you were treated with methotrexate or surgery, you may feel tired for several weeks while you recover. You may feel abdominal discomfort or pain. If you have pain that does not respond to over-the-counter medication, talk with your ob-gyn or other health care professional.
It can take time for the level of hCG in your body to drop after treatment for an ectopic pregnancy. You may continue to feel pregnant for a while. It may take a few cycles for your periods to return to normal.
For some women, ectopic pregnancy can be traumatic. You may be dealing with many emotions after an ectopic pregnancy, even if you were not planning to become pregnant. Take time to work through your feelings. Counseling may be helpful. Ask your ob-gyn or other health care professional to recommend a counselor. Online forums also can be a place to get support from other women who have had ectopic pregnancies.
Once you have had an ectopic pregnancy, you are at higher risk of having another one. During future pregnancies, be alert for signs and symptoms of ectopic pregnancy until your ob-gyn or other health care professional confirms the next pregnancy is growing in the right place.
Assisted Reproductive Technology: A group of infertility treatments in which an egg is fertilized with a sperm outside the body; the fertilized egg then is transferred to the uterus.
Endometriosis: A condition in which tissue that lines the uterus is found outside of the uterus, usually on the ovaries, fallopian tubes, and other pelvic structures.
Fallopian Tube: Tube through which an egg travels from the ovary to the uterus.
General Anesthesia: The use of drugs that produce a sleep-like state to prevent pain during surgery.
Hormone: A substance made in the body by cells or organs that controls the function of cells or organs.
In Vitro Fertilization (IVF): A procedure in which an egg is removed from a woman’s ovary, fertilized in a laboratory with the man’s sperm, and then transferred to the woman’s uterus to achieve a pregnancy.
Laparoscopy: A surgical procedure in which an instrument called a laparoscope is inserted into the pelvic cavity through a small incision. The laparoscope is used to view the pelvic organs. Other instruments can be used with it to perform surgery.
Obstetrician–Gynecologist (Ob-Gyn): A physician with special skills, training, and education in women’s health.
Pelvic Inflammatory Disease: An infection of the uterus, fallopian tubes, and nearby pelvic structures.
Sexually Transmitted Infections (STIs): Infections that are spread by sexual contact, including chlamydia, gonorrhea, human papillomavirus (HPV), herpes, syphilis, and human immunodeficiency virus (HIV, the cause of acquired immunodeficiency syndrome [AIDS]).
Ultrasound Exam: A test in which sound waves are used to examine internal structures. During pregnancy, it can be used to examine the fetus.
Uterus: A muscular organ located in the female pelvis that contains and nourishes the developing fetus during pregnancy.
Article continues below
Advertisement
If you have further questions, contact your ob-gyn.
Don't have an ob-gyn? Learn how to find a doctor near you .
Published: April 2020
Last reviewed: May 2024
Copyright 2024 by the American College of Obstetricians and Gynecologists. All rights reserved. Read copyright and permissions information . This information is designed as an educational aid for the public. It offers current information and opinions related to women's health. It is not intended as a statement of the standard of care. It does not explain all of the proper treatments or methods of care. It is not a substitute for the advice of a physician. Read ACOG’s complete disclaimer .
Clinicians: Subscribe to Digital Pamphlets
Explore ACOG's library of patient education pamphlets.
A Guide to Pregnancy from Ob-Gyns
For trusted, in-depth advice from ob-gyns, turn to Your Pregnancy and Childbirth: Month to Month.
ACOG Explains
A quick and easy way to learn more about your health.
What to Read Next
Laparoscopy
Early Pregnancy Loss
Pelvic Inflammatory Disease (PID)
Repeated Miscarriages

Ectopic Pregnancy
- Author: Vicken P Sepilian, MD, MSc; Chief Editor: Michel E Rivlin, MD more...
- Sections Ectopic Pregnancy
- Practice Essentials
- Epidemiology
- Patient Education
- Physical Examination
- Approach Considerations
- Beta–Human Chorionic Gonadotropin Levels
- Progesterone Levels
- Other Markers
- Ultrasonography
- Dilatation and Curettage
- Culdocentesis
- Laparoscopy
- Expectant Management
- Methotrexate Therapy
- Methotrexate Treatment Protocols
- Investigational Medical Treatments
- Salpingostomy and Salpingectomy
- Medication Summary
- Antineoplastics, Antimetabolite
- Vasopressors
- Media Gallery
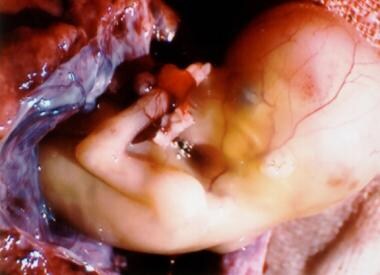
Ectopic pregnancy is the result of a flaw in human reproductive physiology that allows the conceptus to implant and mature outside the endometrial cavity (see the image below), which ultimately ends in the death of the fetus. Without timely diagnosis and treatment, ectopic pregnancy can become a life-threatening situation. [ 1 ]
Signs and symptoms
The classic clinical triad of ectopic pregnancy is as follows:
Abdominal pain
Vaginal bleeding
Unfortunately, only about 50% of patients present with all 3 symptoms.
Patients may present with other symptoms common to early pregnancy (eg, nausea, breast fullness). The following symptoms have also been reported:
Painful fetal movements (in the case of advanced abdominal pregnancy)
Dizziness or weakness
Flulike symptoms
Cardiac arrest
The presence of the following signs suggests a surgical emergency:
Abdominal rigidity
Involuntary guarding
Severe tenderness
Evidence of hypovolemic shock (eg, orthostatic blood pressure changes, tachycardia)
Findings on pelvic examination may include the following:
The uterus may be slightly enlarged and soft
Uterine or cervical motion tenderness may suggest peritoneal inflammation
An adnexal mass may be palpated but is usually difficult to differentiate from the ipsilateral ovary
Uterine contents may be present in the vagina, due to shedding of endometrial lining stimulated by an ectopic pregnancy
See Clinical Presentation for more detail.
Serum β-HCG levels
In a normal pregnancy, the β-HCG level doubles every 48-72 hours until it reaches 10,000-20,000mIU/mL. In ectopic pregnancies, β-HCG levels usually increase less. Mean serum β-HCG levels are lower in ectopic pregnancies than in healthy pregnancies.
No single serum β-HCG level is diagnostic of an ectopic pregnancy. Serial serum β-HCG levels are necessary to differentiate between normal and abnormal pregnancies and to monitor resolution of ectopic pregnancy once therapy has been initiated.
The discriminatory zone of β-HCG (ie, the level above which an imaging scan should reliably visualize a gestational sac within the uterus in a normal intrauterine pregnancy) is as follows:
1500-1800 mIU/mL with transvaginal ultrasonography, but up to 2300 mIU/mL with multiple gestates [ 2 ]
6000-6500 mIU/mL with abdominal ultrasonography
Absence of an intrauterine pregnancy on a scan when the β-HCG level is above the discriminatory zone represents an ectopic pregnancy or a recent abortion.
Ultrasonography is probably the most important tool for diagnosing an extrauterine pregnancy.
Visualization of an intrauterine sac, with or without fetal cardiac activity, is often adequate to exclude ectopic pregnancy. [ 3 ]
Transvaginal ultrasonography, or endovaginal ultrasonography, can be used to visualize an intrauterine pregnancy by 24 days post ovulation or 38 days after the last menstrual period (about 1 week earlier than transabdominal ultrasonography). An empty uterus on endovaginal ultrasonographic images in patients with a serum β-HCG level greater than the discriminatory cut-off value is an ectopic pregnancy until proved otherwise.
Color-flow Doppler ultrasonography improves the diagnostic sensitivity and specificity of transvaginal ultrasonography, especially in cases in which a gestational sac is questionable or absent.
Laparoscopy remains the criterion standard for diagnosis; however, its routine use on all patients suspected of ectopic pregnancy may lead to unnecessary risks, morbidity, and costs. Moreover, laparoscopy can miss up to 4% of early ectopic pregnancies.
Laparoscopy is indicated for patients who are in pain or hemodynamically unstable.
See Workup for more detail.
Therapeutic options in ectopic pregnancy are as follows:
Expectant management
Methotrexate
Candidates for successful expectant management should be asymptomatic and have no evidence of rupture or hemodynamic instability. Candidates should demonstrate objective evidence of resolution (eg, declining β-HCG levels).
Close follow-up and patient compliance are of paramount importance, as tubal rupture may occur despite low and declining serum levels of β-HCG.
Methotrexate is the standard medical treatment for unruptured ectopic pregnancy. A single-dose IM injection is the more popular regimen. The ideal candidate should have the following:
Hemodynamic stability
No severe or persisting abdominal pain
The ability to follow up multiple times
Normal baseline liver and renal function test results
Absolute contraindications to methotrexate therapy include the following:
Existence of an intrauterine pregnancy
Immunodeficiency
Moderate to severe anemia, leukopenia, or thrombocytopenia
Sensitivity to methotrexate
Active pulmonary or peptic ulcer disease
Clinically important hepatic or renal dysfunction
Breastfeeding
Evidence of tubal rupture
Surgical treatment
Laparoscopy has become the recommended surgical approach in most cases. Laparotomy is usually reserved for patients who are hemodynamically unstable or for patients with cornual ectopic pregnancies; it also is a preferred method for surgeons inexperienced in laparoscopy and in patients in whom a laparoscopic approach is difficult.
See Treatment and Medication for more detail.
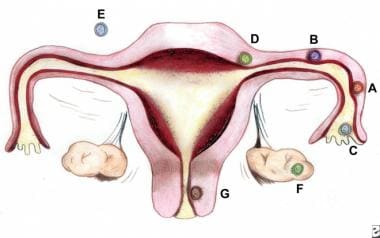
Ectopic pregnancy refers to the implantation of a fertilized egg in a location outside of the uterine cavity, including the fallopian tubes (approximately 97.7%), cervix, ovary, cornual region of the uterus, and abdominal cavity. Of tubal pregnancies, the ampulla is the most common site of implantation (80%), followed by the isthmus (12%), fimbria (5%), cornua (2%), and interstitia (2-3%). (See the image below.)
In ectopic pregnancy (the term ectopic is derived from the Greek word ektopos , meaning out of place), the gestation grows and draws its blood supply from the site of abnormal implantation. As the gestation enlarges, it creates the potential for organ rupture, because only the uterine cavity is designed to expand and accommodate fetal development. Ectopic pregnancy can lead to massive hemorrhage, infertility, or death (see the images below). (See Etiology and Prognosis.)
In 1970, the Centers for Disease Control and Prevention (CDC) began to record statistics regarding ectopic pregnancy, reporting 17,800 cases. By 1992, the number of ectopic pregnancies had increased to 108,800. Concurrently, however, the case-fatality rate decreased from 35.5 deaths per 10,000 cases in 1970 to 2.6 per 10,000 cases in 1992. (See Epidemiology.)
The increased incidence of ectopic pregnancy has been partially attributed to improved ability in making an earlier diagnosis. Ectopic pregnancies that previously would have resulted in tubal abortion or complete, spontaneous reabsorption and remained clinically undiagnosed are now detected. (See Presentation, DDx, and Workup.)
In the 1980s and 1990s, medical therapy for ectopic pregnancy was implemented; it has now replaced surgical therapy in many cases. [ 4 , 5 , 6 ] As the ability to diagnose ectopic pregnancy improves, physicians will be able to intervene sooner, preventing life-threatening sequelae and extensive tubal damage, as well as, it is hoped, preserving future fertility. (See Treatment and Medication.)
Implantation sites
The faulty implantation that occurs in ectopic pregnancy occurs because of a defect in the anatomy or normal function of either the fallopian tube (as can result from surgical or infectious scarring), the ovary (as can occur in women undergoing fertility treatments), or the uterus (as in cases of bicornuate uterus or cesarean delivery scar). Reflecting this, most ectopic pregnancies are located in the fallopian tube; the most common site is the ampullary portion of the tube, where over 80% of ectopic pregnancies occur. (See Etiology.)
Nontubal ectopic pregnancies are a rare occurrence, with abdominal pregnancies accounting for 1.4% of ectopic pregnancies and ovarian and cervical sites accounting for 0.2% each. Some ectopic pregnancies implant in the cervix (< 1%), in previous cesarean delivery scars, [ 7 ] or in a rudimentary uterine horn; although these may be technically in the uterus, they are not considered normal intrauterine pregnancies. [ 8 ]
About 80% of ectopic pregnancies are found on the same side as the corpus luteum (the old, ruptured follicle), when present. [ 9 ] In the absence of modern prenatal care, abdominal pregnancies can present at an advanced stage (>28 wk) and have the potential for catastrophic rupture and bleeding. [ 10 ]
An ectopic pregnancy requires the occurrence of 2 events: fertilization of the ovum and abnormal implantation. Many risk factors affect both events; for example, a history of major tubal infection decreases fertility and increases abnormal implantation.
Multiple factors contribute to the relative risk of ectopic pregnancy. In theory, anything that hampers or delays the migration of the fertilized ovum (blastocyst) to the endometrial cavity can predispose a woman to ectopic gestation. The following risk factors have been linked to ectopic pregnancy:
Tubal damage - Which can be the result of infections such as pelvic inflammatory disease (PID) or salpingitis (whether documented or not) or can result from abdominal surgery or tubal ligation or from maternal in utero diethylstilbestrol (DES) exposure
History of previous ectopic pregnancy
Smoking - A risk factor in about one third of ectopic pregnancies; smoking may contribute to decreased tubal motility by damage to the ciliated cells in the fallopian tubes
Altered tubal motility - As mentioned, this can result from smoking, but it can also occur as the result of hormonal contraception; progesterone-only contraception and progesterone intrauterine devices (IUDs) have been associated with an increased risk of ectopic pregnancy
History of 2 or more years of infertility (whether treated or not) [ 11 ] - Women using assisted reproduction seem to have a doubled risk of ectopic pregnancy (to 4%), although this is mostly due to the underlying infertility [ 12 ]
History of multiple sexual partners [ 11 ]
Maternal age - Although this is not an independent risk factor [ 11 ]
The most logical explanation for the increasing frequency of ectopic pregnancy is previous pelvic infection; however, most patients presenting with an ectopic pregnancy have no identifiable risk factor. [ 13 ]
A 2009 literature review found 56 reported cases of ectopic pregnancy (by definition), dating back to 1937, after hysterectomy. [ 14 ]
Pelvic inflammatory disease
The most common cause of PID is an antecedent infection caused by Chlamydia trachomatis. Patients with chlamydial infection have a range of clinical presentations, from asymptomatic cervicitis to salpingitis and florid PID. More than 50% of women who have been infected are unaware of the exposure.
Other organisms that cause PID, such as Neisseria gonorrhoeae , also increase the risk of ectopic pregnancy, and a history of salpingitis increases the risk of ectopic pregnancy 4-fold. The incidence of tubal damage increases after successive episodes of PID (ie, 13% after 1 episode, 35% after 2 episodes, 75% after 3 episodes).
Effective vaccination against Chlamydia trachomatis is under investigation. Once clinically available, it should have a dramatic impact on the frequency of ectopic pregnancy, as well as on the overall health of the female reproductive system.
After 1 ectopic pregnancy, a patient incurs a 7- to 13-fold increase in the likelihood of another ectopic pregnancy. Overall, a patient with a previous ectopic pregnancy has a 50-80% chance of having a subsequent intrauterine gestation and a 10-25% chance of a future tubal pregnancy.
History of tubal surgery and conception after tubal ligation
Previous tubal surgery has been demonstrated to increase the risk of developing ectopic pregnancy. The increase depends on the degree of damage and the extent of anatomic alteration. Surgeries carrying higher risk of subsequent ectopic pregnancy include salpingostomy , neosalpingostomy, fimbrioplasty, tubal reanastomosis, and lysis of peritubal or periovarian adhesions.
Conception after previous tubal ligation also increases a women's risk of having an ectopic pregnancy; 35-50% of patients who conceive after a tubal ligation are reported to experience an ectopic pregnancy. Failure after bipolar tubal cautery is more likely to result in ectopic pregnancy than is occlusion using suture, rings, or clips. This failure is attributed to fistula formation that allows sperm passage. In one study, 33% of pregnancies occurring after tubal ligation were ectopic; those who underwent electrocautery and women younger than 35 years were at higher risk. [ 15 ]
Ectopic pregnancies following tubal sterilizations usually occur 2 or more years after sterilization rather than immediately after. In the first year, only about 6% of sterilization failures result in ectopic pregnancy.
Cigarette smoking has been shown to be a risk factor for ectopic pregnancy development. Studies have demonstrated an elevated risk ranging from 1.6 to 3.5 times that of nonsmokers. A dose-response effect has also been suggested.
Based on laboratory studies in humans and animals, researchers have postulated several mechanisms by which cigarette smoking might play a role in ectopic pregnancies. These mechanisms include one or more of the following: delayed ovulation, altered tubal and uterine motility, and altered immunity. To date, however, no study has supported a specific mechanism by which cigarette smoking affects the occurrence of ectopic pregnancy.
Use of oral contraceptives or an intrauterine device
All contraceptive methods lead to an overall lower risk of pregnancy and therefore to an overall lower risk of ectopic pregnancy. However, among cases of contraceptive failure, women at increased risk of ectopic pregnancy compared with pregnant controls included those using progestin-only oral contraceptives, progestin-only implants, or IUDs and those with a history of tubal ligation. [ 16 ]
The presence of an inert, copper-containing or progesterone IUD traditionally has been thought to be a risk factor for ectopic pregnancy. Data from the Contraceptive CHOICE Project demonstrated a relative risk of 3.16 for ectopic pregnancy in women not using any form of contraception as compared with women using the progesterone IUD. [ 17 ] Nevertheless, if a woman ultimately conceives with an IUD in place, it is more likely to be an ectopic pregnancy. [ 18 ] The incidence of ectopic pregnancy in IUD users is 1 in 1000 over a 5-year period. [ 17 ]
Emergency contraception (levonorgestrel, or Plan B) does not appear to lead to a higher-than-expected rate of ectopic pregnancy. [ 19 ]
Use of fertility drugs or assisted reproductive technology
Ovulation induction with clomiphene citrate or injectable gonadotropin therapy has been linked to a 4-fold increase in the risk of ectopic pregnancy in a case-control study. This finding suggests that multiple eggs and high hormone levels may be significant factors.
One study demonstrated that infertility patients with luteal phase defects have a statistically higher ectopic pregnancy rate than do patients whose infertility is caused by anovulation. In addition, the risk of ectopic pregnancy and heterotopic pregnancy (ie, pregnancies occurring simultaneously in different body sites) dramatically increases when a patient has used assisted reproductive techniques—such as in vitro fertilization (IVF) or gamete intrafallopian transfer (GIFT)—to conceive. [ 20 ]
In a study of 3000 clinical pregnancies achieved through in vitro fertilization, the ectopic pregnancy rate was 4.5%, which is more than double the background incidence. Furthermore, studies have demonstrated that up to 1% of pregnancies achieved through IVF or GIFT can result in a heterotopic gestation, compared with an incidence of 1 in 30,000 pregnancies for spontaneous conceptions. [ 21 ]
In a retrospective (2006-2014) cohort study of 8120 assisted reproduction technology cycles, Rombauts et al found that endometrial combined thickness (ECT) measured prior to embryo transfer was associated with ectopic pregnancy. [ 22 ] The investigators reported that, following IVF, there was a 4-fold increased risk of ectopic pregnancy in women with an ECT of up to 9 mm compared with women with an ECT of at least 12 mm. They noted that increased ECT is a marker for increased fundus-to-cervix uterine peristalsis, which may be a reason for the increased risk for placenta praevia but a decreased risk for ectopic pregnancy. [ 22 ]
Increasing age
The highest rate of ectopic pregnancy occurs in women aged 35-44 years. A 3- to 4-fold increase in the risk of developing an ectopic pregnancy exists compared with women aged 15-24 years. One proposed explanation suggests that aging may result in a progressive loss of myoelectrical activity in the fallopian tube; myoelectrical activity is responsible for tubal motility.
Salpingitis isthmica nodosum
Salpingitis isthmica nodosum is defined as the microscopic presence of tubal epithelium in the myosalpinx or beneath the tubal serosa. These pockets of epithelium protrude through the tube, similar to small diverticula. Studies of serial histopathologic sections of the fallopian tube have revealed that approximately 50% of patients treated with salpingectomy for ectopic pregnancy have evidence of salpingitis isthmica nodosum. The etiology of salpingitis isthmica nodosum is unclear, but proposed mechanisms include postinflammatory and congenital changes, as well as acquired tubal changes, such as those observed with endometriosis. [ 23 ]
DES exposure
Before 1971, several million women were exposed in utero to DES, which was given to their mothers to prevent pregnancy complications. In utero exposure of women to DES is associated with a high lifetime risk of a broad spectrum of adverse health outcomes, including infertility, spontaneous abortion, and ectopic pregnancy. [ 24 ]
Other risk factors associated with increased incidence of ectopic pregnancy include anatomic abnormalities of the uterus such as a T-shaped or bicornuate uterus, fibroids or other uterine tumors, previous abdominal surgery, failure with progestin-only contraception, and ruptured appendix. [ 13 ]
United States statistics
The incidence of ectopic pregnancy is reported most commonly as the number of ectopic pregnancies per 1000 conceptions. Since 1970, when the reported rate in the United States was 4.5 cases per 1000 pregnancies, the frequency of ectopic pregnancy has increased 6-fold, with ectopic pregnancies now accounting for approximately 1-2% of all pregnancies. Consequently, the prevalence is estimated at 1 in 40 pregnancies, or approximately 25 cases per 1000 pregnancies. These statistics are based on data from the US Centers for Disease Control and Prevention (CDC), which used hospitalizations for ectopic pregnancy to determine the total number of ectopic pregnancies.
Looking at raw data, 17,800 hospitalizations for ectopic pregnancies were reported in 1970. This number rose to 88,000 in 1989 [ 25 ] but fell to 30,000 in 1998. An estimated 108,800 ectopic pregnancies in 1992 resulted in 58,200 hospitalizations, with an estimated cost of $1.1 billion.
Changes in the management of ectopic pregnancy, however, have made it difficult to reliably monitor incidence (and therefore mortality rates). [ 26 ] A review of hospital discharges in California found a rate of 15 cases per 1,000 in 1991, declining to a rate of 9.3 cases per 1,000 in 2000, [ 27 ] but a review of electronic medical records (inpatient and outpatient) from a large health maintenance organization (HMO) in northern California found a stable rate of 20.7 cases per 1,000 reported pregnancies from 1997-2000. [ 28 ] This suggests that the incidence of ectopic pregnancy in the United States remained steady at about 2% in the 1990s, despite the shift to outpatient treatment.
The above data raise the question of whether the number of ectopic pregnancies is declining or whether many ectopic pregnancies are now being treated in ambulatory surgical centers or are even being addressed with medical therapy, without admission. Some authors believe the latter is true, but truly accurate statistics are lacking.
Diagnoses of ectopic pregnancy in US emergency departments (ED) may be on the rise. From 2006 to 2013, the overall ratio of ED visits with an ectopic pregnancy diagnosis increased from 11.0 per 1000 live births to 13.7 per 1000 live births. [ 29 ]
Approximately 85-90% of ectopic pregnancies occur in multigravid women. In the United States, rates are nearly twice as high for women of other races compared with white women.
International statistics
The increase in incidence of ectopic pregnancy in the 1970s in the United States was also mirrored in Africa, although data there tend to be hospital based rather than derived from nationwide surveys, with estimates in the range of 1.1-4.6%. [ 30 ]
The United Kingdom estimated the incidence of ectopic pregnancy at about 11.1 per 1,000 reported pregnancies from 1997 to 2005, compared with 9.6 per 1,000 from 1991 to 1993. [ 31 ]
Racial- and age-related demographics
In the United States from 1991 to 1999, ectopic pregnancy was the cause of 8% of all pregnancy-related deaths among black women, compared with 4% among white women. [ 32 ]
Any woman with functioning ovaries can potentially have an ectopic pregnancy, which includes women from the age of menarche until menopause. Women older than 40 years were found to have an adjusted odds ratio of 2.9 for ectopic pregnancy. [ 13 ]
Ectopic pregnancy presents a major health problem for women of childbearing age. It is the result of a flaw in human reproductive physiology that allows the conceptus to implant and mature outside the endometrial cavity, which ultimately ends in the death of the fetus. Without timely diagnosis and treatment, ectopic pregnancy can become a life-threatening situation. [ 1 ]
The evidence in the literature reporting on the treatment of ectopic pregnancy with subsequent reproductive outcome is limited mostly to observational data and a few randomized trials comparing treatment options.
Assessment of successful treatment and future reproductive outcome with various treatment options is often skewed by selection bias. For example, comparing a patient who was managed expectantly with a patient who received methotrexate or with a patient who had a laparoscopic salpingectomy is difficult.
A patient with spotting, no abdominal pain, and a low initial beta–human chorionic gonadotropin (β-HCG) level that is falling may be managed expectantly, whereas a patient who presents with hemodynamic instability, an acute abdomen, and high initial β-HCG levels must be managed surgically. These 2 patients probably represent different degrees of tubal damage; thus, comparing the future reproductive outcomes of the 2 cases would be flawed.
Salpingostomy, salpingectomy, and tubal surgery
Data in the literature have failed to demonstrate substantial and consistent benefit from either salpingostomy or salpingectomy with regard to improving future reproductive outcome. However, despite the risk of persistent ectopic pregnancy, some studies have shown salpingostomy to improve reproductive outcome in patients with contralateral tubal damage. Yao and Tulandi concluded from a literature review that laparoscopic salpingostomy had a reproductive performance that was equal to or slightly better than salpingectomy; however, slightly higher recurrent ectopic pregnancy rates were noted in the salpingostomy group. [ 33 ]
In reporting on 10 years of surgical experience in Paris, Dubuisson et al concluded that, for selected patients who desire future fertility, using salpingectomy, which is simpler and avoids the risk of persistent ectopic pregnancy, is possible and can result in a comparable fertility rate to tubal conservation surgery. [ 34 ] Future fertility rates were no different with either surgical approach when the contralateral tube was either normal or scarred but patent.
Clausen reviewed literature from the previous 40 years and concluded that only a small number of investigators have suggested, indirectly, that conservative tubal surgery increases the rate of subsequent intrauterine pregnancy. He also concluded that the more recent studies may reflect an improvement in surgical technique. [ 35 ]
In an earlier study, Maymon et al, after reviewing 20 years of ectopic pregnancy treatment, concluded that conservative tubal surgery provided no greater risk of recurrent ectopic pregnancy than the more radical salpingectomy. [ 36 ]
The modern pelvic surgeon has been led to believe that the treatment of choice for unruptured ectopic pregnancy is salpingostomy, sparing the affected fallopian tube and thereby improving future reproductive outcome.
However, if the treating surgeon has neither the laparoscopic skill nor the instrumentation necessary to atraumatically remove the trophoblastic tissue via linear salpingostomy, then salpingectomy by laparoscopy or laparotomy is not the wrong surgical choice. Leaving a scarred, charred fallopian tube behind after removing the ectopic pregnancy but requiring extensive cautery to control bleeding does not preserve reproductive outcome.
Fertility following surgery
Previous history of infertility has been found to be the most significant factor affecting postsurgical fertility.
Parker and Bistis concluded that when the contralateral fallopian tube is normal, the subsequent fertility rate is independent of the type of surgery. [ 37 ] Similarly, a prospective study of 88 patients by Ory et al indicated that the surgical method had no effect on subsequent fertility in women with an intact contralateral tube. [ 38 ]
Several other studies reported that the status of the contralateral tube, the presence of adhesions, and the presence of other risk factors, such as endometriosis, have a more significant impact on future fertility than does the choice of surgical procedure.
According to Rulin, salpingectomy should be the treatment of choice in women with intact contralateral tubes, because conservative treatment provides no additional benefit and incurs the additional costs and morbidity associated with persistent ectopic pregnancy and recurrent ectopic pregnancy in the already damaged tube. [ 39 ]
Future fertility rates have been found to be similar in patients who are treated surgically by laparoscopy or laparotomy. Salpingectomy by laparotomy carries a subsequent intrauterine pregnancy rate of 25-70%, compared with laparoscopic salpingectomy rates of 50-60%. Very similar rates exist for laparoscopic salpingostomy versus laparotomy. The rate of persistent ectopic pregnancy between the 2 groups is also similar, ranging from 5-20%.
A slightly higher recurrent ectopic pregnancy rate exists in patients treated by laparotomy (7-28%), regardless of conservative or radical approach, when compared with laparoscopy (6-16%). This surprising finding is believed to be secondary to increased adhesion formation in the group treated by laparotomy.
Comparison of medical and surgical treatment of small, intact extrauterine pregnancies also revealed similar success and subsequent spontaneous pregnancy rates in a prospective, randomized trial. [ 40 ]
A study by Xu et al found that in women undergoing 51,268 fresh in vitro fertilization-intracytoplasmic sperm injection (IVF-ICSI) cycles, previous ectopic pregnancy has no effect on IVF-ICSI outcomes. The study also found that women with a prior history of ectopic pregnancy have a higher recurrence risk of ectopic pregnancy after IVF in comparison with women with no history of ectopic pregnancy. [ 41 ]
Methotrexate versus surgery
The success rates after methotrexate are comparable with laparoscopic salpingostomy, assuming that the previously mentioned selection criteria are observed. The average success rates using the multiple-dosage regimen are in the range of 91-95%, as demonstrated by multiple investigators. One study of 77 patients desiring subsequent pregnancy showed intrauterine pregnancies in 64% of these patients and recurrent ectopic pregnancy in 11% of them. Other studies have demonstrated similar results, with intrauterine pregnancy rates ranging from 20-80%.
The average success rates for the single-dosage methotrexate regimen are reported to be from 88-94%. In a study by Stovall and Ling, 113 patients (94%) were treated successfully, 4 (3.3%) of whom needed a second dose. [ 40 ] No adverse effects were encountered. Furthermore, 87.2% of these patients achieved a subsequent intrauterine pregnancy, whereas 12.8% experienced a subsequent ectopic pregnancy. [ 40 ] Other studies have reported similar results, with some mild adverse effects and lower reproductive outcomes.
A meta-analysis that included data from 26 trials demonstrated a success rate of 88.1% with the single-dose methotrexate regimen and a success rate of 92.7% with the multiple-dose regimen. [ 42 ] A small, randomized clinical trial also demonstrated the single-dose regimen to have a slightly higher failure rate. [ 43 ] A hybrid protocol, involving 2 equal doses of methotrexate (50 mg/m 2 ) given on days 1 and 4 without the use of leucovorin, has been shown to be an effective and convenient alternative to the existing regimens. [ 44 ]
Complications
Complications of ectopic pregnancy can be secondary to misdiagnosis, late diagnosis, or treatment approach. Failure to make the prompt and correct diagnosis of ectopic pregnancy can result in tubal or uterine rupture (depending on the location of the pregnancy), which in turn can lead to massive hemorrhage, shock, disseminated intravascular coagulopathy (DIC), and death. Ectopic pregnancy is the leading cause of maternal death in the first trimester, accounting for 9-13% of all pregnancy-related deaths. In the United States, an estimated 30-40 women die each year from ectopic pregnancy.
Any time a surgical approach is chosen as the treatment of choice, consider the complications attributable to the surgery, whether it is laparotomy or laparoscopy. These include bleeding, infection, and damage to surrounding organs, such as the bowel, bladder, and ureters, and to the major vessels nearby. Infertility may also result secondary to loss of reproductive organs after surgery. Also consider the risks and complications secondary to anesthesia. Make the patient aware of these complications, and obtain the appropriate written consents.
In the United States, ectopic pregnancy is estimated to occur in 1-2% of all pregnancies and accounts for 3-4% of all pregnancy-related deaths. [ 45 ] It is the leading cause of pregnancy-related mortality during the first trimester in the United States. In a review of deaths from ectopic pregnancy in Michigan, 44% of the women who died were either found dead at home or were dead on arrival at the emergency department. [ 46 ]
Virtually all ectopic pregnancies are considered nonviable and are at risk of eventual rupture and resulting hemorrhage. In addition to the immediate morbidity caused by ectopic pregnancy, the woman's future ability to reproduce may be adversely affected as well. However, patients who are diagnosed with ectopic pregnancy before rupture have a low mortality rate and also have a chance at preserved fertility.
From 1970 to 1989, the US mortality rate for ectopic pregnancies dropped from 35.5 deaths to 3.8 deaths per 10,000 ectopic pregnancies. [ 25 ] If the overall incidence of ectopic pregnancy remained stable in the 1990s, then the mortality rate dropped to 3.19 deaths per 10,000 ectopic pregnancies by 1999. [ 47 ]
Surveillance data for pregnancy-related deaths in the United States from 1991-1999 showed that ectopic pregnancy was the cause of 5.6% of 4200 maternal deaths. Of these deaths, 93% occurred via hemorrhage. [ 32 ]
During 1999–2008, the ectopic pregnancy mortality rate in the United States was 0.6 deaths per 100,000 live births. The CDC reported a higher rate in Florida, 2.5 deaths per 100,000 live births during 2009-2010. The 11 ectopic pregnancy deaths in Florida during 2009-2010 contrasted with the total number of deaths (14) identified in national statistics for 2007. There was a high prevalence of illicit drug use among the women who died in Florida. [ 45 ]
The mortality rate reported in African hospital-based studies varied from 50-860 deaths per 10,000 ectopic pregnancies; these were almost certainly underestimates resulting from underreporting of maternal deaths and misclassification of ectopic pregnancies as induced abortions. [ 30 ]
Using data from 1997 to 2002, the World Health Organization (WHO) estimated that ectopic pregnancy was the cause of 4.9% of pregnancy-related deaths in the industrialized world. [ 48 ] Ectopic pregnancy caused 26% of maternal deaths in early pregnancy in the United Kingdom from 2003-2005, second only to venous thromboembolism, despite a relatively low mortality rate of 0.035 per 10,000 estimated ectopic pregnancies. [ 31 ]
Advise patients receiving methotrexate therapy to avoid alcoholic beverages, vitamins containing folic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and sexual intercourse, until advised otherwise. A signed written consent demonstrating the patient's comprehension of the course of treatment must be obtained.
Provide an information pamphlet to all patients receiving methotrexate; the pamphlet should include a list of adverse effects, a schedule of follow-up visits, and a method of contacting the physician or the hospital in case of emergency, as well as the need to return to the emergency department for concerning symptoms.
Patients with risk factors for ectopic pregnancy should be educated regarding their risk of having an ectopic pregnancy. Women who are being discharged with a pregnancy of unknown location should be educated regarding the possibility of ectopic pregnancy and their need for urgent follow-up.
Patients undergoing assisted reproduction technology should be educated regarding their risk of heterotopic pregnancy.
For patient education information, see the Pregnancy Center and the Women's Health Center , as well as Ectopic Pregnancy , Bleeding During Pregnancy , Vaginal Bleeding , Birth Control Overview , and Birth Control Methods .
Farquhar CM. Ectopic pregnancy. Lancet . 2005 Aug 13-19. 366(9485):583-91. [QxMD MEDLINE Link] .
Kadar N, Bohrer M, Kemmann E, Shelden R. The discriminatory human chorionic gonadotropin zone for endovaginal sonography: a prospective, randomized study. Fertil Steril . 1994 Jun. 61(6):1016-20. [QxMD MEDLINE Link] .
Stein JC, Wang R, Adler N, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: a meta-analysis. Ann Emerg Med . 2010 Dec. 56(6):674-83. [QxMD MEDLINE Link] .
Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol . 2010 Mar. 115(3):495-502. [QxMD MEDLINE Link] .
Lipscomb GH. Medical therapy for ectopic pregnancy. Semin Reprod Med . 2007 Mar. 25(2):93-8. [QxMD MEDLINE Link] .
Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol . 1991 May. 77(5):754-7. [QxMD MEDLINE Link] .
Riaz RM, Williams TR, Craig BM, Myers DT. Cesarean scar ectopic pregnancy: imaging features, current treatment options, and clinical outcomes. Abdom Imaging . 2015 Oct. 40 (7):2589-99. [QxMD MEDLINE Link] .
Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod . 2002 Dec. 17(12):3224-30. [QxMD MEDLINE Link] .
Saito M, Koyama T, Yaoi Y, Kumasaka T, Yazawa K. Site of ovulation and ectopic pregnancy. Acta Obstet Gynecol Scand . 1975. 54(3):227-30. [QxMD MEDLINE Link] .
Nkusu Nunyalulendho D, Einterz EM. Advanced abdominal pregnancy: case report and review of 163 cases reported since 1946. Rural and Remote Health 8 (online) . 2008;1087. [Full Text] .
Ankum WM, Mol BW, Van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril . 1996 Jun. 65(6):1093-9. [QxMD MEDLINE Link] .
Strandell A, Thorburn J, Hamberger L. Risk factors for ectopic pregnancy in assisted reproduction. Fertil Steril . 1999 Feb. 71(2):282-6. [QxMD MEDLINE Link] .
Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol . 2003 Feb 1. 157(3):185-94. [QxMD MEDLINE Link] .
Fylstra DL. Ectopic pregnancy after hysterectomy: a review and insight into etiology and prevention. Fertil Steril . 2010 Jul. 94(2):431-5. [QxMD MEDLINE Link] .
Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of ectopic pregnancy after tubal sterilization. U.S. Collaborative Review of Sterilization Working Group. N Engl J Med . 1997 Mar 13. 336(11):762-7. [QxMD MEDLINE Link] .
Furlong LA. Ectopic pregnancy risk when contraception fails. A review. J Reprod Med . 2002 Nov. 47(11):881-5. [QxMD MEDLINE Link] .
Williams S, Peipert J, Buckel C, Zhao Q, Madden T, Secura G. Contraception and the risk of ectopic pregnancy. Contraception . 2014 Sept. 90(3):326.
[Guideline] National Collaborating Centre for Women’s and Children’s Health (UK). Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. National Institute for Health and Care Excellence: Guidance . 2005 Oct. [QxMD MEDLINE Link] . [Full Text] .
Vinson DR. Emergency contraception and risk of ectopic pregnancy: is there need for extra vigilance?. Ann Emerg Med . 2003 Aug. 42(2):306-7. [QxMD MEDLINE Link] .
Dor J, Seidman DS, Levran D, Ben-Rafael Z, Ben-Shlomo I, Mashiach S. The incidence of combined intrauterine and extrauterine pregnancy after in vitro fertilization and embryo transfer. Fertil Steril . 1991 Apr. 55(4):833-4. [QxMD MEDLINE Link] .
Svare JA, Norup PA, Thomsen SG, et al. [Heterotopic pregnancy after in vitro fertilization]. Ugeskr Laeger . 1994 Apr 11. 156(15):2230-3. [QxMD MEDLINE Link] .
Rombauts L, McMaster R, Motteram C, Fernando S. Risk of ectopic pregnancy is linked to endometrial thickness in a retrospective cohort study of 8120 assisted reproduction technology cycles. Hum Reprod . 2015 Dec. 30 (12):2846-52. [QxMD MEDLINE Link] .
Majmudar B, Henderson PH 3rd, Semple E. Salpingitis isthmica nodosa: a high-risk factor for tubal pregnancy. Obstet Gynecol . 1983 Jul. 62(1):73-8. [QxMD MEDLINE Link] .
Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med . 2011 Oct 6. 365(14):1304-14. [QxMD MEDLINE Link] .
Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy--United States, 1970-1989. MMWR CDC Surveill Summ . 1993 Dec 17. 42(6):73-85. [QxMD MEDLINE Link] .
Zane SB, Kieke BA Jr, Kendrick JS, Bruce C. Surveillance in a time of changing health care practices: estimating ectopic pregnancy incidence in the United States. Matern Child Health J . 2002 Dec. 6(4):227-36. [QxMD MEDLINE Link] .
Calderon JL, Shaheen M, Pan D, Teklehaimenot S, Robinson PL, Baker RS. Multi-cultural surveillance for ectopic pregnancy: California 1991-2000. Ethn Dis . 2005 Autumn. 15(4 Suppl 5):S5-20-4. [QxMD MEDLINE Link] .
Van Den Eeden SK, Shan J, Bruce C, Glasser M. Ectopic pregnancy rate and treatment utilization in a large managed care organization. Obstet Gynecol . 2005 May. 105(5 Pt 1):1052-7. [QxMD MEDLINE Link] .
Mann LM, Kreisel K, Llata E, Hong J, Torrone EA. Trends in Ectopic Pregnancy Diagnoses in United States Emergency Departments, 2006-2013. Matern Child Health J . 2020 Feb. 24 (2):213-21. [QxMD MEDLINE Link] . [Full Text] .
Goyaux N, Leke R, Keita N, Thonneau P. Ectopic pregnancy in African developing countries. Acta Obstet Gynecol Scand . 2003 Apr. 82(4):305-12. [QxMD MEDLINE Link] .
Lewis G, ed. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer - 2003-2005. The Seventh Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom . London, UK: The Confidential Enquiry into Maternal and Child Health (CEMACH). 2007:92-3.:
Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance--United States, 1991--1999. MMWR Surveill Summ . 2003 Feb 21. 52(2):1-8. [QxMD MEDLINE Link] .
Yao M, Tulandi T. Current status of surgical and nonsurgical management of ectopic pregnancy. Fertil Steril . 1997 Mar. 67(3):421-33. [QxMD MEDLINE Link] .
Dubuisson JB, Morice P, Chapron C, De Gayffier A, Mouelhi T. Salpingectomy - the laparoscopic surgical choice for ectopic pregnancy. Hum Reprod . 1996 Jun. 11(6):1199-203. [QxMD MEDLINE Link] .
Clausen I. Conservative versus radical surgery for tubal pregnancy. A review. Acta Obstet Gynecol Scand . 1996 Jan. 75(1):8-12. [QxMD MEDLINE Link] .
Maymon R, Shulman A, Halperin R, Michell A, Bukovsky I. Ectopic pregnancy and laparoscopy: review of 1197 patients treated by salpingectomy or salpingotomy. Eur J Obstet Gynecol Reprod Biol . 1995 Sep. 62(1):61-7. [QxMD MEDLINE Link] .
Parker J, Bisits A. Laparoscopic surgical treatment of ectopic pregnancy: salpingectomy or salpingostomy?. Aust N Z J Obstet Gynaecol . 1997 Feb. 37(1):115-7. [QxMD MEDLINE Link] .
Ory SJ, Nnadi E, Herrmann R, O'Brien PS, Melton LJ 3rd. Fertility after ectopic pregnancy. Fertil Steril . 1993 Aug. 60(2):231-5. [QxMD MEDLINE Link] .
Rulin MC. Is salpingostomy the surgical treatment of choice for unruptured tubal pregnancy?. Obstet Gynecol . 1995 Dec. 86(6):1010-3. [QxMD MEDLINE Link] .
Stovall TG, Ling FW, Carson SA, Buster JE. Serum progesterone and uterine curettage in differential diagnosis of ectopic pregnancy. Fertil Steril . 1992 Feb. 57(2):456-7. [QxMD MEDLINE Link] .
Xu Z, Yan L, Liu W, Xu X, Li M, Ding L, et al. Effect of treatment of a previous ectopic pregnancy on in vitro fertilization-intracytoplasmic sperm injection outcomes: a retrospective cohort study. Fertil Steril . 2015 Dec. 104 (6):1446-51.e1-3. [QxMD MEDLINE Link] .
Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing "single dose" and "multidose" regimens. Obstet Gynecol . 2003 Apr. 101(4):778-84. [QxMD MEDLINE Link] .
Alleyassin A, Khademi A, Aghahosseini M, Safdarian L, Badenoosh B, Hamed EA. Comparison of success rates in the medical management of ectopic pregnancy with single-dose and multiple-dose administration of methotrexate: a prospective, randomized clinical trial. Fertil Steril . 2006 Jun. 85(6):1661-6. [QxMD MEDLINE Link] .
Barnhart KT, Sammel MD, Hummel AC, Jain JK, Chakhtoura N, Strauss III J. A novel "two dose" regimen of methotrexate to treat ectopic pregnancy. Fertil Steril . 2005 Sept. 84(Suppl):S1:S130-S131. [Full Text] .
Ectopic pregnancy mortality - Florida, 2009-2010. MMWR Morb Mortal Wkly Rep . 2012 Feb 17. 61(6):106-9. [QxMD MEDLINE Link] .
Anderson FW, Hogan JG, Ansbacher R. Sudden death: ectopic pregnancy mortality. Obstet Gynecol . 2004 Jun. 103(6):1218-23. [QxMD MEDLINE Link] .
Grimes DA. Estimation of pregnancy-related mortality risk by pregnancy outcome, United States, 1991 to 1999. Am J Obstet Gynecol . 2006 Jan. 194(1):92-4. [QxMD MEDLINE Link] .
Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet . 2006 Apr 1. 367(9516):1066-74. [QxMD MEDLINE Link] .
Alsuleiman SA, Grimes EM. Ectopic pregnancy: a review of 147 cases. J Reprod Med . 1982 Feb. 27(2):101-6. [QxMD MEDLINE Link] .
Stovall TG, Kellerman AL, Ling FW, Buster JE. Emergency department diagnosis of ectopic pregnancy. Ann Emerg Med . 1990 Oct. 19(10):1098-103. [QxMD MEDLINE Link] .
Barnhart KT, Sammel MD, Gracia CR, Chittams J, Hummel AC, Shaunik A. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril . 2006 Jul. 86(1):36-43. [QxMD MEDLINE Link] .
Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med . 1996 Jul. 28(1):10-7. [QxMD MEDLINE Link] .
Abbott J, Emmans LS, Lowenstein SR. Ectopic pregnancy: ten common pitfalls in diagnosis. Am J Emerg Med . 1990 Nov. 8(6):515-22. [QxMD MEDLINE Link] .
Dart RG, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med . 1999 Mar. 33(3):283-90. [QxMD MEDLINE Link] .
Huchon C, Panel P, Kayem G, et al. Is a standardized questionnaire useful for tubal rupture screening in patients with ectopic pregnancy?. Acad Emerg Med . 2012 Jan. 19(1):24-30. [QxMD MEDLINE Link] .
Mol F, van den Boogaard E, van Mello NM, et al. Guideline adherence in ectopic pregnancy management. Hum Reprod . 2011 Feb. 26(2):307-15. [QxMD MEDLINE Link] .
Pereira N, Bender JL, Hancock K, et al. Routine monitoring of liver, renal, and hematologic tests after single- or double-dose methotrexate treatment for ectopic pregnancies after in vitro fertilization. J Minim Invasive Gynecol . 2015 Nov-Dec. 22 (7):1266-70. [QxMD MEDLINE Link] .
Shepherd RW, Patton PE, Novy MJ, Burry KA. Serial beta-hCG measurements in the early detection of ectopic pregnancy. Obstet Gynecol . 1990 Mar. 75(3 Pt 1):417-20. [QxMD MEDLINE Link] .
Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol . 2004 Jul. 104(1):50-5. [QxMD MEDLINE Link] .
Condous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet Gynecol . 2005 Dec. 26(7):770-5. [QxMD MEDLINE Link] .
Taran FA, Kagan KO, Hubner M, Hoopmann M, Wallwiener D, Brucker S. The diagnosis and treatment of ectopic pregnancy. Dtsch Arztebl Int . 2015 Oct 9. 112 (41):693-704. [QxMD MEDLINE Link] .
Barclay L. Transvaginal sonogram may be best test for ectopic pregnancy. Medscape News from WebMD. April 23, 2013. Available at http://www.medscape.com/viewarticle/802998 . Accessed: April 29, 2013.
Crochet JR, Bastian LA, Chireau MV. Does this woman have an ectopic pregnancy?: the rational clinical examination systematic review. JAMA . 2013 Apr 24. 309(16):1722-9. [QxMD MEDLINE Link] .
Barnhart K, Mennuti MT, Benjamin I, Jacobson S, Goodman D, Coutifaris C. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol . 1994 Dec. 84(6):1010-5. [QxMD MEDLINE Link] .
Verma U, English D, Brookfield K. Conservative management of nontubal ectopic pregnancies. Fertil Steril . 2011 Dec. 96(6):1391-1395.e1. [QxMD MEDLINE Link] .
Bonin L, Pedreiro C, Moret S, Chene G, Gaucherand P, Lamblin G. Predictive factors for the methotrexate treatment outcome in ectopic pregnancy: A comparative study of 400 cases. Eur J Obstet Gynecol Reprod Biol . 2017 Jan. 208:23-30. [QxMD MEDLINE Link] .
Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril . 2007 Mar. 87(3):481-4. [QxMD MEDLINE Link] .
Thurman AR, Cornelius M, Korte JE, Fylstra DL. An alternative monitoring protocol for single-dose methotrexate therapy in ectopic pregnancy. Am J Obstet Gynecol . 2010 Feb. 202(2):139.e1-6. [QxMD MEDLINE Link] .
Ozcan MCH, Wilson JR, Frishman GN. A Systematic Review and Meta-analysis of Surgical Treatment of Ectopic Pregnancy with Salpingectomy versus Salpingostomy. J Minim Invasive Gynecol . 2021 Mar. 28 (3):656-67. [QxMD MEDLINE Link] .
Medical treatment of ectopic pregnancy. Fertil Steril . 2008 Nov. 90(5 Suppl):S206-12. [QxMD MEDLINE Link] .
Douglas D. Salpingectomy may often have edge in ectopic pregnancy. Medscape News from WebMD. 2014 Feb 27. Available at http://www.medscape.com/viewarticle/821131 . Accessed: February 27, 2014.
Mol F, van Mello NM, Strandell A, et al. Salpingotomy versus salpingectomy in women with tubal pregnancy (ESEP study): an open-label, multicentre, randomised controlled trial. Lancet . 2014 Apr 26. 383(9927):1483-9. [QxMD MEDLINE Link] .
Sivin I. Dose- and age-dependent ectopic pregnancy risks with intrauterine contraception. Obstet Gynecol . 1991 Aug. 78(2):291-8. [QxMD MEDLINE Link] .
ACOG Practice Bulletin No. 193: Tubal Ectopic Pregnancy. Obstet Gynecol . 2018 Mar. 131 (3):e91-e103. [QxMD MEDLINE Link] .
- Sites and frequencies of ectopic pregnancy. By Donna M. Peretin, RN. (A) Ampullary, 80%; (B) Isthmic, 12%; (C) Fimbrial, 5%; (D) Cornual/Interstitial, 2%; (E) Abdominal, 1.4%; (F) Ovarian, 0.2%; and (G) Cervical, 0.2%.
- Laparoscopic picture of an unruptured right ampullary tubal pregnancy; bleeding out of the fimbriated end has resulted in hemoperitoneum.
- A 12-week interstitial gestation, which eventually resulted in a hysterectomy. Courtesy of Deidra Gundy, MD, Department of Obstetrics and Gynecology at Medical College of Pennsylvania and Hahnemann University (MCPHU).
- An endovaginal sonogram reveals an intrauterine pregnancy at approximately 6 weeks. A yolk sac (ys), gestational sac (gs), and fetal pole (fp) are depicted.
- Linear incision being made at the antimesenteric side of the ampullary portion of the fallopian tube.
- Laparoscopic picture of an ampullary ectopic pregnancy protruding out after a linear salpingostomy was performed.
- Schematic of a tubal gestation being teased out after linear salpingostomy.
Contributor Information and Disclosures
Vicken P Sepilian, MD, MSc Medical Director, Reproductive Endocrinology and Infertility, CHA Fertility Center Vicken P Sepilian, MD, MSc is a member of the following medical societies: American College of Obstetricians and Gynecologists , American Society for Reproductive Medicine Disclosure: Nothing to disclose.
Ellen Wood, DO, FACOG Voluntary Assistant Professor, University of Miami, Leonard M Miller School of Medicine Ellen Wood, DO, FACOG is a member of the following medical societies: American Society for Reproductive Medicine Disclosure: Nothing to disclose.
Frances E Casey, MD, MPH Associate Professor, Director of Family Planning Services, Department of Obstetrics and Gynecology, VCU Medical Center Frances E Casey, MD, MPH is a member of the following medical societies: American College of Obstetricians and Gynecologists , Association of Reproductive Health Professionals , National Abortion Federation , Physicians for Reproductive Health , Society of Family Planning Disclosure: Nothing to disclose.
Michel E Rivlin, MD Former Professor, Department of Obstetrics and Gynecology, University of Mississippi School of Medicine Michel E Rivlin, MD is a member of the following medical societies: American College of Obstetricians and Gynecologists , American Medical Association , Mississippi State Medical Association , Royal College of Surgeons of Edinburgh , Royal College of Obstetricians and Gynaecologists Disclosure: Nothing to disclose.
A David Barnes, MD, PhD, MPH, FACOG Consulting Staff, Department of Obstetrics and Gynecology, Mammoth Hospital (Mammoth Lakes, California), Pioneer Valley Hospital (Salt Lake City, Utah), Warren General Hospital (Warren, Pennsylvania), and Mountain West Hospital (Tooele, Utah)
A David Barnes, MD, PhD, MPH, FACOG is a member of the following medical societies: American College of Forensic Examiners , American College of Obstetricians and Gynecologists , American Medical Association , Association of Military Surgeons of the US , and Utah Medical Association
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Robert K Zurawin, MD Associate Professor, Director of Baylor College of Medicine Program for Minimally Invasive Gynecology, Director of Fellowship Program, Minimally Invasive Surgery, Department of Obstetrics and Gynecology, Baylor College of Medicine
Robert K Zurawin, MD is a member of the following medical societies: American Association of Gynecologic Laparoscopists , American College of Obstetricians and Gynecologists , American Society for Reproductive Medicine , Association of Professors of Gynecology and Obstetrics , Central Association of Obstetricians and Gynecologists , Harris County Medical Society , North American Society for Pediatric and Adolescent Gynecology , and Texas Medical Association
Disclosure: Johnson and Johnson Honoraria Speaking and teaching; Conceptus Honoraria Speaking and teaching; ConMed Consulting fee Consulting
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- HIV in Pregnancy
- Anemia and Thrombocytopenia in Pregnancy
- Adrenal Disease and Pregnancy
- Pulmonary Disease and Pregnancy
- Kidney Disease and Pregnancy
- Pregnancy After Transplantation
- Vaccinations/Immunizations During Pregnancy
- Is immunotherapy for cancer safe in pregnancy?
- Labetalol, Nifedipine: Outcome on Pregnancy Hypertension
- Are Nicotine Patches and E-Cigarettes Safe in Pregnancy?

- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2020/viewarticle/immunotherapy-cancer-safe-pregnancy-2024a100083dnews news Is immunotherapy for cancer safe in pregnancy?

- 2002261369-overviewDiseases & Conditions Diseases & Conditions Postterm Pregnancy
- 2002246123-overviewDiseases & Conditions Diseases & Conditions Kidney Disease and Pregnancy
- Ectopic pregnancy
On this page
When to see a doctor, risk factors, complications.
Pregnancy begins with a fertilized egg. Normally, the fertilized egg attaches to the lining of the uterus. An ectopic pregnancy occurs when a fertilized egg implants and grows outside the main cavity of the uterus.
An ectopic pregnancy most often occurs in a fallopian tube, which carries eggs from the ovaries to the uterus. This type of ectopic pregnancy is called a tubal pregnancy. Sometimes, an ectopic pregnancy occurs in other areas of the body, such as the ovary, abdominal cavity or the lower part of the uterus (cervix), which connects to the vagina.
An ectopic pregnancy can't proceed normally. The fertilized egg can't survive, and the growing tissue may cause life-threatening bleeding, if left untreated.

In a healthy pregnancy, the fertilized egg attaches itself to the lining of the uterus. In an ectopic pregnancy, the egg attaches itself somewhere outside the uterus usually to the inside of a fallopian tube.
Products & Services
- A Book: Mayo Clinic Guide to a Healthy Pregnancy
- A Book: Mayo Clinic Guide to Fertility and Conception
You may not notice any symptoms at first. However, some women who have an ectopic pregnancy have the usual early signs or symptoms of pregnancy — a missed period, breast tenderness and nausea.
If you take a pregnancy test, the result will be positive. Still, an ectopic pregnancy can't continue as normal.
As the fertilized egg grows in the improper place, signs and symptoms become more noticeable.
Early warning of ectopic pregnancy
Often, the first warning signs of an ectopic pregnancy are light vaginal bleeding and pelvic pain.
If blood leaks from the fallopian tube, you may feel shoulder pain or an urge to have a bowel movement. Your specific symptoms depend on where the blood collects and which nerves are irritated.
Emergency symptoms
If the fertilized egg continues to grow in the fallopian tube, it can cause the tube to rupture. Heavy bleeding inside the abdomen is likely. Symptoms of this life-threatening event include extreme lightheadedness, fainting and shock.
Seek emergency medical help if you have any signs or symptoms of an ectopic pregnancy, including:
- Severe abdominal or pelvic pain accompanied by vaginal bleeding
- Extreme lightheadedness or fainting
- Shoulder pain
From Mayo Clinic to your inbox
A tubal pregnancy — the most common type of ectopic pregnancy — happens when a fertilized egg gets stuck on its way to the uterus, often because the fallopian tube is damaged by inflammation or is misshapen. Hormonal imbalances or abnormal development of the fertilized egg also might play a role.
Some things that make you more likely to have an ectopic pregnancy are:
- Previous ectopic pregnancy. If you've had this type of pregnancy before, you're more likely to have another.
- Inflammation or infection. Sexually transmitted infections, such as gonorrhea or chlamydia, can cause inflammation in the tubes and other nearby organs, and increase your risk of an ectopic pregnancy.
- Fertility treatments. Some research suggests that women who have in vitro fertilization (IVF) or similar treatments are more likely to have an ectopic pregnancy. Infertility itself may also raise your risk.
- Tubal surgery. Surgery to correct a closed or damaged fallopian tube can increase the risk of an ectopic pregnancy.
- Choice of birth control. The chance of getting pregnant while using an intrauterine device (IUD) is rare. However, if you do get pregnant with an intrauterine device (IUD) in place, it's more likely to be ectopic. Tubal ligation, a permanent method of birth control commonly known as "having your tubes tied," also raises your risk, if you become pregnant after this procedure.
- Smoking. Cigarette smoking just before you get pregnant can increase the risk of an ectopic pregnancy. The more you smoke, the greater the risk.
An ectopic pregnancy can cause your fallopian tube to burst open. Without treatment, the ruptured tube can lead to life-threatening bleeding.
There's no way to prevent an ectopic pregnancy, but here are some ways to decrease your risk:
- Limiting the number of sexual partners and using a condom during sex helps to prevent sexually transmitted infections and may reduce the risk of pelvic inflammatory disease.
- Don't smoke. If you do, quit before you try to get pregnant.
Mar 12, 2022
- Cunningham FG, et al., eds. Implantation and placental development. In: Williams Obstetrics. 25th ed. McGraw-Hill Education; 2018. https://accessmedicine.mhmedical.com. Accessed Dec. 4, 2019.
- Tulandi T. Ectopic pregnancy: Epidemiology, risk factors, and anatomic sites. https://www.uptodate.com/contents/search. Accessed Dec. 4, 2019.
- Cunningham FG, et al., eds. Ectopic pregnancy. In: Williams Obstetrics. 25th ed. McGraw-Hill Education; 2018. https://accessmedicine.mhmedical.com. Accessed Dec. 4, 2019.
- Frequently asked questions. Pregnancy FAQ 155. Ectopic pregnancy. American College of Obstetricians and Gynecologists. https://www.acog.org/Patients/FAQs/Ectopic-Pregnancy. Accessed Dec. 4, 2019.
- Tulandi T. Ectopic pregnancy: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/search. Accessed Dec. 29, 2017.
- Burnett TL (expert opinion). Mayo Clinic. Dec. 4, 2019.
- Diseases & Conditions
- Ectopic pregnancy symptoms & causes

More Information
Associated procedures.
CON-XXXXXXXX
Double your impact on fighting cancer
Make a gift before July 31 and it can go twice as far to fight cancer.
- Share this —

- Watch Full Episodes
- Read With Jenna
- Inspirational
- Relationships
- TODAY Table
- Newsletters
- Start TODAY
- Shop TODAY Awards
- Citi Concert Series
- Listen All Day
Follow today
More Brands
- On The Show
- TODAY Plaza
I blamed myself for my ectopic pregnancy. Here’s what helped me get through it

I’ve always known that I want to be a mother.
Even though, as a Black woman , the statistics say my desire to be one could kill me , the vivid imagery of hugging my babies never faded or even wavered when I pictured what I wanted my life to look like. I prayed and told God my heart’s desire. I did the research. I took and continue to take the vitamins. I practice advocating for myself every time I go in for a routine checkup. I’m quick to send an email with questions if something in my body ever doesn’t feel right, even if I know if it’s probably nothing.
But this time it wasn’t nothing. Something was very wrong.
The first time I ever heard the word “ectopic” was on television. I was watching “Love & Hip Hop” and I found myself crying for rapper Remy Ma and her husband, Papoose, two people I didn’t even know. I watched as their joy in finding out Remy was pregnant was washed away with devastation as it was revealed her pregnancy was ectopic, and I was heartbroken for them.
At that point, I didn’t think I knew anyone who had had an ectopic pregnancy . To be fair, no one rushes to share that news. Why would they? So when I found out that the something that was wrong with me was life-threatening, I felt really alone, despite being surrounded by people who loved me as I sat in the hospital waiting room angry, confused and heartbroken for myself.
As a young woman navigating the ins and outs of managing a career, networking, trying to buy a home, establish new generational traditions, and still hoping to be a mom one day, I started being proactive about my reproductive health in my late 20s. I had a lot I wanted to do and I knew that my timeline for having children might not align.

When I discovered a lump in my breast in 2020, I immediately went to get examined. When the lump turned out to be multiple cysts, I immediately did my research and learned the same hormones that could cause cysts in my breasts could cause uterine fibroids . When I remembered my family history of fibroids, I immediately asked for a screening. When doctors told me I wasn’t old enough, I immediately demanded they put in my chart that they wouldn’t screen me. When they did the exam and sure enough found two fibroids, I immediately started incorporating things like exercise, vitamins for hormone regulation, and regular check-ins with my OB-GYN. So, when I did everything right and the doctor told me I was definitely pregnant but my uterus was empty, I immediately blamed myself for something going so tragically wrong. Had I missed something? Should I have done something differently?
My brain knows that it’s not my fault. Black and brown women have the highest rate of ectopic pregnancies . And also, our bodies sometimes just do things without explanation, i.e. sometimes ish just happens. Even the doctors told me that. But none of that mattered because in my heart this was my pregnancy, this was my fault, and this body I loved so deeply betrayed me.
That hospital visit may have saved my life. Ectopic pregnancies are the leading cause of maternal death in the first trimester . My partner’s insistence that I go more than likely is the reason I was able to avoid an invasive surgery to save my fallopian tube, and although I’ll have to be monitored frequently, I’ll still likely be able to get pregnant in the future. For that I am grateful. You’d think that would mean all was well, but after that, things were very far from well .
The shock of being pregnant for the first time quickly followed by the grief of loss just 24 hours later made me feel like my brain, heart and body were not in sync anymore.
The shock of being pregnant for the first time quickly followed by the grief of loss just 24 hours later made me feel like my brain, heart and body were not in sync anymore. My brain thought logically. My heart had all the feelings. My body felt completely foreign to me. The guilt over my body “malfunctioning,” the sadness that bubbled up whenever I saw babies or pregnant bellies, the imposter’s syndrome I felt in relating to women who went through pregnancy loss, and the ache I had for women who didn’t have access to the reproductive care I did — all of it became too much. I felt like I had no reason to feel so awful when I was, technically, physically, OK. So many women weren’t OK and still aren’t OK, yet I did feel incredibly awful.
Why did this have to happen? What did I do wrong? Why did my body betray me? How am I supposed to move past this? What if something goes wrong again? How do I even catch my breath after this?
My family and friends were all showing up. My partner was supportive and saying all the right things. Still, nothing was enough. I could barely look at myself. None of the tools I had accumulated in therapy were helping this time. I had to accept that I had never felt this kind of loss before and I needed help. I found a new therapist and I finally gave myself permission to feel every single emotion, even the ones that didn’t make sense. And, oh boy, were there a lot!
If I had to describe my grief, it would be like a boat in the middle of the ocean. I can’t see the shore, but according to my compass I’m headed in the right direction. The most powerful thing, though? My anchor — other women.

Back in that hospital waiting room as I cried, terrified that my desire to be a mom was slipping out of my hands, my own mom gifted me an anchor. In her efforts to comfort me, she listed off women I loved and looked up to who had experienced pregnancy loss and still went on to be moms to beautiful, healthy children. Women who had been scared, like I was. Women who were strong, who were loving, who had my same desire to be moms and were damn good at it. Women I’d spent lots of time with, and yet I didn’t know they’d gone through this. That reassurance let me know it’s OK to release my anchor.
I can be both incredibly devastated at losing my pregnancy — allowing myself to sit in my boat, in that ocean of feelings — and also hopeful that I’ll still get to hug my babies one day, just as I pictured. My anchor is a reminder that my grief is real and it’s necessary, but it’s not all I have. I’m not alone in my loss and when I’m ready I can pull that anchor back up, keep heading to the shore and release the self-blame. I wish I could say I never cried again. The truth is on any given day (read: yesterday) I find myself struggling with those complicated emotions the grief of losing a pregnancy brings. But, I’m on the right course and for now that’s enough for me.
If you find yourself grieving a pregnancy loss, I hope you’ll also find your anchor. I hope you’ll realize, just as I did, that nothing is wrong with you and it is not your fault. And I hope that that will be enough for you too.
Alexis Holliday is an associate producer for TODAY. She is a proud HBCU alum and in her free time indulges in all things Beyonce, beauty and TikTok.
I thought my heavy bleeding was normal. Then I wound up in the emergency room

My best friend and I were both diagnosed with cancer before 40. Survivorship brought us closer

I have a painful condition known as the ‘suicide disease.’ This is how I got my life back

I worked with terminal patients for decades. This is what they taught me about life

My lung collapsed 6 times before I was finally diagnosed with a rare form of endometriosis

After my stroke, I didn’t know how to identify as someone with a disability

I started running to lose weight. I never thought I’d fall in love with it

My shame over having an STD is one of the hardest things I’ve ever dealt with

I almost died after my routine C-section. Things got worse from there
I ignored my back pain. 5 days later I almost lost my leg
Revisiting Ectopic Pregnancy: A Pictorial Essay
- Journal of Clinical Imaging Science 4(1):37
- CC BY-NC-SA 3.0
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Winthrop University Hospital

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- ARCH GYNECOL OBSTET

- Sarah Flaieh
- Essa * Hawraa

- Wadea Saeed Bin Ghouth
- Abdulkhaleq Ayedh Bin Nuhaid

- Giacomo Bonito
- Gabriele Masselli
- Silvia Gigli

- Sarah Flaieh Essa
- Miriam Crespo
- Jaione de los Bueis
- Estibaliz Goikoetxea

- REPROD BIOMED ONLINE
- Zallo Adriana
- Hernandez- Pailos Rafael

- J MINIM INVAS GYN
- Lumin Wang BM
- Xiaoming Zhu Postdoc

- Raj Mohan Paspulati

- Kazuya Mimura

- N Job-Spira

- W. Rinehart
- Appl Radiol

- Leslie M. Scoutt
- RADIOGRAPHICS

- Klaus Diedrich

- J.-L. Hwang
- Rajesh Varma
- L Mascarenhas
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Ectopic Pregnancy: Diagnosis and Management
Affiliations.
- 1 University of Michigan Medical School, Ann Arbor, MI, USA.
- 2 Mount Sinai School of Medicine, New York, NY, USA.
- PMID: 32412215
Ectopic pregnancy occurs when a fertilized ovum implants outside of the uterine cavity. In the United States, the estimated prevalence of ectopic pregnancy is 1% to 2%, and ruptured ectopic pregnancy accounts for 2.7% of pregnancy-related deaths. Risk factors include a history of pelvic inflammatory disease, cigarette smoking, fallopian tube surgery, previous ectopic pregnancy, and infertility. Ectopic pregnancy should be considered in any patient presenting early in pregnancy with vaginal bleeding or lower abdominal pain in whom intrauterine pregnancy has not yet been established. The definitive diagnosis of ectopic pregnancy can be made with ultrasound visualization of a yolk sac and/or embryo in the adnexa. However, most ectopic pregnancies do not reach this stage. More often, patient symptoms combined with serial ultrasonography and trends in beta human chorionic gonadotropin levels are used to make the diagnosis. Pregnancy of unknown location refers to a transient state in which a pregnancy test is positive but ultrasonography shows neither intrauterine nor ectopic pregnancy. Serial beta human chorionic gonadotropin levels, serial ultrasonography, and, at times, uterine aspiration can be used to arrive at a definitive diagnosis. Treatment of diagnosed ectopic pregnancy includes medical management with intramuscular methotrexate, surgical management via salpingostomy or salpingectomy, and, in rare cases, expectant management. A patient with diagnosed ectopic pregnancy should be immediately transferred for surgery if she has peritoneal signs or hemodynamic instability, if the initial beta human chorionic gonadotropin level is high, if fetal cardiac activity is detected outside of the uterus on ultrasonography, or if there is a contraindication to medical management.
PubMed Disclaimer
Similar articles
- New Evidence to Guide Ectopic Pregnancy Diagnosis and Management. Brady PC. Brady PC. Obstet Gynecol Surv. 2017 Oct;72(10):618-625. doi: 10.1097/OGX.0000000000000492. Obstet Gynecol Surv. 2017. PMID: 29059454 Review.
- Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Insogna IG, Farland LV, Missmer SA, Ginsburg ES, Brady PC. Insogna IG, et al. Am J Obstet Gynecol. 2017 Aug;217(2):185.e1-185.e9. doi: 10.1016/j.ajog.2017.04.023. Epub 2017 Apr 19. Am J Obstet Gynecol. 2017. PMID: 28433735
- Clinical diagnosis and treatment of ectopic pregnancy. Alkatout I, Honemeyer U, Strauss A, Tinelli A, Malvasi A, Jonat W, Mettler L, Schollmeyer T. Alkatout I, et al. Obstet Gynecol Surv. 2013 Aug;68(8):571-81. doi: 10.1097/OGX.0b013e31829cdbeb. Obstet Gynecol Surv. 2013. PMID: 23921671 Review.
- Diagnosis and management of ectopic pregnancy. Lozeau AM, Potter B. Lozeau AM, et al. Am Fam Physician. 2005 Nov 1;72(9):1707-14. Am Fam Physician. 2005. PMID: 16300032 Review.
- Ectopic pregnancy. DeCherney AH, Jones EE. DeCherney AH, et al. Clin Obstet Gynecol. 1985 Jun;28(2):365-74. doi: 10.1097/00003081-198528020-00014. Clin Obstet Gynecol. 1985. PMID: 2410172
- Multimodality imaging of acute gynecological emergencies-a pictorial essay. Singla V, Dua A, Singh T, Jain V. Singla V, et al. Abdom Radiol (NY). 2024 Jun 5. doi: 10.1007/s00261-024-04399-1. Online ahead of print. Abdom Radiol (NY). 2024. PMID: 38836883 Review.
- Unintended and ectopic pregnancy in woman with iud translocation with history of two times previous cesarean section and history of spontaneous abortion: Case report. Marlina D, Sutrisno, Tjandraprawira K, Aziz MA. Marlina D, et al. SAGE Open Med Case Rep. 2024 May 28;12:2050313X241258840. doi: 10.1177/2050313X241258840. eCollection 2024. SAGE Open Med Case Rep. 2024. PMID: 38812838 Free PMC article.
- Spontaneous Ectopic Tubal Pregnancy After Partial Salpingectomy. Fei H, Yin Y, Guo X, Jin X. Fei H, et al. Int J Womens Health. 2024 May 23;16:917-922. doi: 10.2147/IJWH.S455616. eCollection 2024. Int J Womens Health. 2024. PMID: 38803341 Free PMC article.
- Obstetrical Complications in Venezuelan Refugee and Migrant Women: Analysis of Ecuadorian National Hospital Discharge Data, 2018-2021. Weigel MM, Armijos RX. Weigel MM, et al. J Immigr Minor Health. 2024 May 3. doi: 10.1007/s10903-024-01600-x. Online ahead of print. J Immigr Minor Health. 2024. PMID: 38700574
- The relationship between serum levels of epidermal growth factor and β-human chorionic gonadotropin and the type and prognosis of ectopic pregnancy. Geng D, Liu M, Wu D, Yue B. Geng D, et al. Arch Gynecol Obstet. 2024 Apr 29. doi: 10.1007/s00404-024-07523-0. Online ahead of print. Arch Gynecol Obstet. 2024. PMID: 38683393
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- American Academy of Family Physicians
- Ovid Technologies, Inc.
- Genetic Alliance
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Diagnosis and...
Diagnosis and management of ectopic pregnancy
- Related content
- Peer review
- Davor Jurkovic , consultant gynaecologist 1 ,
- Helen Wilkinson , director 2
- 1 Department of Obstetrics and Gynaecology, University College Hospital, London NW1 2BU, UK
- 2 Ectopic Pregnancy Trust, King’s College Hospital, London, UK
- Correspondence to: D Jurkovic davor.jurkovic{at}uclh.nhs.uk
Summary points
The prevalence of ectopic pregnancy is 1-2% and it is an important cause of maternal morbidity and mortality
Consider ectopic pregnancy in all pregnant women presenting with abdominal pain or vaginal bleeding
Women with a history of ectopic pregnancy, tubal surgery, or tubal pathology are at increased risk, as are those who have had tubal ligation or who have an intrauterine contraceptive device in place
A transvaginal ultrasound scan is the best test to diagnose ectopic pregnancy
Laparoscopic surgery is the main treatment; medical and expectant management is used in a select group
Women who are treated for ectopic pregnancy have significantly lower subsequent spontaneous intrauterine pregnancy rates and higher ectopic pregnancy rates than the general population
The estimated prevalence of ectopic pregnancy is 1-2% worldwide. In the United Kingdom nearly 12 000 ectopic pregnancies are diagnosed each year, which gives a prevalence of 1.1%. 1 Although death after ectopic pregnancy is rare, the burden of disease is high owing to the cost of diagnostic tests and expensive treatment. 2 Serious adverse outcomes in ectopic pregnancies are typically caused by delayed diagnosis; this highlights the need for primary care and secondary care health professionals to be familiar with the risk factors for ectopic pregnancy, its clinical symptoms, and the local facilities that provide care for women with early pregnancy problems. In recent years it has become more common to treat women with ectopic pregnancy conservatively in the outpatient setting. 3 General practitioners increasingly advise women on different management options and support them during follow-up.
Sources and selection criteria
We searched Embase and Medline for articles with titles that included the keywords “ectopic pregnancy” and “extrauterine pregnancy”. We limited the search to meta-analyses, reviews, and randomised controlled trials published in English in the past 10 years. We consulted a systematic review on ectopic pregnancy in the …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £33 / $40 / €36 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Acad Pathol
- v.7; Jan-Dec 2020
Educational Case: Ectopic Pregnancy
Xiomara brioso rubio.
1 Department of Pathology, Immunology and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL, USA
Jesse Kresak
Melanie zona, stacy g. beal, julia a. ross.
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040 . 1
Primary Objective
Objective FDP1.1: Ectopic Pregnancy . Describe risk factors, characteristic morphologic findings, potential outcomes, and the medical/surgical options for management of ectopic pregnancy in relation to the pathogenesis and likelihood of adverse consequences.
Competency 2: Organ System Pathology; Topic: Female Reproductive—Disorders of Pregnancy (FDP); Learning Goal 1: Disorders of Pregnancy.
Patient Presentation
A 19-year-old woman presents with a 1-week history of left lower abdominal pain and vaginal spotting. She is sexually active, and her last menstrual period was 7 weeks ago. She does not use any contraception and has a history of gonorrhea diagnosed 2 years ago.
Diagnostic Findings, Part 1
The patient’s vital signs are normal. On physical examination, she appears uncomfortable and there is left adnexal tenderness, closed cervix, and scant blood in the vaginal vault. The remainder of the physical examination is noncontributory.
Questions/Discussion Points, Part 1
What is the differential diagnosis of an adnexal mass in a reproductive-age woman.
The differential diagnosis (see Table 1 ) includes functional cysts, endometriomas, tubo-ovarian abscesses, and neoplasms. Functional ovarian cysts, such as follicular cysts and corpus luteum cysts, are structures that form following normal ovarian function. Endometriomas are blood-containing cysts that are commonly associated with endometriosis. Tubo-ovarian abscesses are walled-off areas of infection associated with pelvic inflammatory disease. Neoplasms, such as germ cell tumors or yolk sac tumors, can also present as adnexal masses. 2
Differential Diagnosis of an Adnexal Mass in a Reproductive-Age Woman.
| Differential Diagnosis | Pathophysiology | Presentation |
|---|---|---|
| Functional cysts | ||
| Follicular cyst | Ovulation does not occur and ovarian follicle remains | |
| Corpus luteal cyst | Enlarged corpus luteum that remains past 14 days | |
| Endometrioma | Ectopic endometrial tissue forms blood-filled cyst after bleeding | |
| Tubo-ovarian abscess | Abscess formation secondary to pelvic inflammatory disease | |
| Neoplasms | ||
| Mature cystic teratoma | Germ cell tumor that contains differentiated tissue from all germ layers | |
| Others (yolk sac tumor, dysgerminomas, etc) |
Diagnostic Findings, Part 2
The patient had a positive urine pregnancy test and a serum βhCG resulted at 4979 mIU/mL (negative is <5 mIU/mL). The patient’s transvaginal ultrasound (TVUS) is shown in Figure 1 .

Transvaginal ultrasound.
Questions/Discussion Points, Part 2
How would you describe the findings of the transvaginal ultrasound.
Transvaginal ultrasound of the uterus demonstrates an extrauterine gestational sac and yolk sac with a fetal pole in the left fallopian tube ( Figure 2 ). No fetal cardiac activity was noted.

Transvaginal ultrasound (TVUS) showing ectopic pregnancy, characterized by presence of a yolk sac, gestational sac, and fetal pole in the left adnexa with an empty uterine cavity.
What Is Your Working Diagnosis?
The working diagnosis is an ectopic pregnancy. This is based on the patient’s clinical presentation, history of sexual activity without contraception, history of sexually transmitted illness, lack of menstrual period, elevated serum βhCG, and ultrasound finding of a definitive gestational sac, yolk sac, and fetal pole in the left adnexa, outside of the uterine cavity.
The results of the TVUS and βhCG levels in the right clinical setting are the most useful tools for diagnosing an abnormal or ectopic pregnancy. Once the βhCG crosses the discriminatory level of 3500 mIU/mL, a normal intrauterine pregnancy should be visible within the endometrial cavity. Diagnosis is made if a pregnancy is clearly identified in an ectopic location. If neither a clear ectopic nor clear intrauterine pregnancy are visualized, the provider must consider an early abortion or ectopic pregnancy. In these scenarios, βhCG levels are obtained 2 days after initial evaluation. In a normal pregnancy, βhCG is expected to at least double in 2 days. In an early abortion, βhCG decreases during repeat testing, while in an ectopic pregnancy, βhCG does not rise appropriately. 3 However, it has been noted that ectopic pregnancies do not follow a specific trend or curve when compared to the patterns seen with normal pregnancies or spontaneous abortions. 4 The ability to trend βhCG and observe a patient until a definitive diagnosis is made is based on the patient’s stability.
What Is the Clinical Presentation and Potential Outcomes for an Ectopic Pregnancy?
Ectopic pregnancies are defined as pregnancies occurring outside of the uterus. The presentation varies per patient, including absence of symptoms, but most women present for medical evaluation secondary to abdominal pain, vaginal bleeding, or amenorrhea between 6 and 8 weeks of gestation. 5 , 6 The outcomes are also variable but usually fall within 3 categories: spontaneous abortion, tubal abortion, tubal rupture. The latter is the most concerning outcome. In this scenario, the patient presents with intractable abdominal pain and unstable vital signs secondary to internal hemorrhage. If not recognized and adequately treated, a ruptured ectopic pregnancy can result in hemorrhagic shock and death. Generally, there is an increased risk of ectopic rupture with higher βhCG levels and higher gestational ages, particularly greater than 1500 IU/mL and greater than 6 weeks, respectively. 2 , 3 , 6 , 7
What Are Common Locations for an Ectopic Pregnancy and Risk Factors Associated With Them?
The most common location is the fallopian tube, with 98% of ectopic pregnancies found there. Most of these are within the ampulla (80%). 2 Other sites include the abdomen, uterine cesarean delivery scar, ovaries, or cervix. 5
Previous history of an ectopic pregnancy and previous tubal surgery or tubal sterilization are the strongest risk factors for a future episode. A woman with a history of a previous ectopic pregnancy has a 10% chance of recurrence after a single episode. Other risk factors include fallopian tube abnormalities, including scarring from pelvic inflammatory disease, other pelvic surgeries, assisted reproductive techniques, such as multiple embryo transfer and in vitro fertilization, and infertility. 3 , 8 Of note, gonorrhea and chlamydia are 2 common sexually transmitted infections that result in inflammatory damage of the fallopian tubes, leading to scar formation and disruption of their architecture. This physical roadblock interferes with the migration of a fertilized ovum and predisposes women to a tubal pregnancy. 2
What Are the Medical/Surgical Options for Management of an Ectopic Pregnancy?
Management of an ectopic pregnancy is based on patient stability, characteristics of the ectopic mass, desire for future fertility, and understanding of risks and benefits of each therapeutic option. Possibilities include expectant management, medical management, and surgical management, with either a salpingectomy or salpingostomy.
Expectant management is defined as watchful waiting with no medical intervention. Patients qualifying for expectant management are those whom are asymptomatic, have no adnexal mass on imaging, and show signs of resolution, such as a plateaued or decreasing βhCG. Patients’ βhCG must be trended to observe a quantitative decrease. Alternative treatments must be implemented if patients become symptomatic or if βhCG levels rise. 3 , 8
Medical treatment is with 50 mg methotrexate intramuscularly; there are no other medical alternatives or effective routes of administration for this medication. 3 To qualify for medical management, a patient must meet certain criteria: no methotrexate contraindications, including presence of an intrauterine pregnancy or a ruptured ectopic pregnancy, immunodeficiency, bone marrow abnormalities (severe anemia, leukopenia, thrombocytopenia), active pulmonary, renal, liver, or peptic ulcer disease, or currently breastfeeding. The patient must be hemodynamically stable and able to adhere to a strict follow-up surveillance schedule. Relative contraindications include presence of fetal heartbeat, high βhCG levels, or an ectopic mass greater than 4 cm. These have been shown to have an increased risk of treatment failure, especially in the case of a high presenting βhCG level. Currently, there are 3 available dosing schedules: single dose, 2 dose, and multiple fixed dose schedule. They differ by the simplicity of the schedule and side effect profile, but, for the most part, there have been no clinically significant differences found in relation to treatment success. 3 , 8
In hemodynamically unstable patients, surgical management is the only treatment option. It is also the standard of care for those with a ruptured ectopic pregnancy, those who failed methotrexate trial, or those who meet absolute contraindication for methotrexate therapy. Laparoscopy is the least invasive method for surgical management; however, laparotomy is indicated in cases of severe instability, uncontrolled hemorrhage, or inadequate pelvic visualization. Salpingostomy involves the removal of the ectopic mass only, leaving the fallopian tube in place, thus attempting to preserve future fertility. If a salpingostomy is done, follow-up βhCG levels are required to ensure resolution. Salpingectomy is the removal of the affected fallopian tube. There is no consensus on success rates of a salpingectomy versus salpingostomy. Some reports state there is no difference in the frequency of future intrauterine pregnancies or risk of future ectopic ones, while others state the opposite. For this reason, ample discussion with the patient is recommended. 3 , 8
If Salpingectomy Is Pursued, What Is the Histology and Morphology of an Ectopic Pregnancy?
A tubal ectopic pregnancy can be grossly described as a distended fallopian tube with a thin wall, which may be ruptured, with dusky and dark serosa ( Figure 3 ). Sectioning usually reveals hemorrhage with villous-appearing tissue. Fetal parts may be seen occasionally ( Figure 4 ). On histology, chorionic villi associated with the fallopian tube is diagnostic of an ectopic pregnancy ( Figures 5 and and6). 6 ). Hemorrhage within the tubal lumen is often present.

Gross tubal ectopic pregnancy. Serosa is dusky red-purple with prominent vasculature and a small amount of adherent blood clot.

Gross tubal ectopic pregnancy. The dilated portion of the tube contains an intact fetus.

Cross section of fallopian tube diagnostic of an ectopic/tubal pregnancy. H&E-stained section at ×20. Note the fallopian tube epithelium (arrow) and luminal hemorrhage with chorionic villi (arrowhead).

Intraluminal immature chorionic villi with edematous stroma and surrounding trophoblasts. The presence of villi within the fallopian tube is diagnostic of a tubal (ectopic) pregnancy. H&E-stained section at ×100.
Teaching Points
- Ectopic pregnancies are pregnancies that occur outside of the uterus, most commonly in the fallopian tube.
- The clinical presentation varies but includes abdominal pain, bleeding, and amenorrhea.
- The diagnosis involves a positive pregnancy βhCG test and a TVUS showing an empty uterine cavity with a clear ectopic pregnancy. If the ectopic pregnancy is not visualized, βhCG must be trended every 2 days. If it does not double, consideration of an ectopic in an unknown location or an abortion must be made.
- The risk of a ruptured ectopic pregnancy increases with increased gestational age and βhCG levels. Rupture may result in hemorrhage and shock and can be lethal if not properly managed.
- Management of an ectopic pregnancy is based mostly on patient stability. Options include watchful waiting, methotrexate, or surgical removal of the pregnancy via a salpingostomy or salpingectomy.
- Grossly, a tubal ectopic pregnancy appears as a thin-walled fallopian tube with dusky and dark serosa containing a collection of hemorrhagic and villous tissue. Fetal parts may or may not be apparent.
- Histologically, the presence of chorionic villi within the tubal epithelium is diagnostic of a tubal ectopic pregnancy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The article processing fee for this article was funded by an Open Access Award given by the Society of ‘67, which supports the mission of the Association of Pathology Chairs to produce the next generation of outstanding investigators and educational scholars in the field of pathology. This award helps to promote the publication of high-quality original scholarship in Academic Pathology by authors at an early stage of academic development.

- Share full article
Advertisement
Supported by
Guest Essay
In a Post-Roe World, We Can Avoid Pitting Mothers Against Babies

By Leah Libresco Sargeant
Mrs. Sargeant has written books about religion and community building and runs an online community that focuses on the dignity of dependence.
Now that the Supreme Court has overturned Roe v. Wade, states face a new reality about where to draw the line in pregnancy for when abortion is permitted. In these debates, ectopic pregnancy is a key issue.
In an ectopic pregnancy , the baby implants somewhere other than the uterus — usually in a fallopian tube. The situation is fatal for the baby. It’s also dangerous for the mother. The fallopian tube can rupture, and the bleeding can be so fast and so sudden that it puts the mother’s life at risk.
Pro-life doctors and pro-life ethicists agree it is morally licit to save a mother’s life, even at her baby’s expense — but they draw a distinction between the treatment for ectopic pregnancy and an abortion.
From a pro-life perspective, delivering a baby who is ectopic is closer to delivering a baby very prematurely because the mother has life-threatening eclampsia. A baby delivered at 22 weeks may or may not survive. A baby delivered in the first trimester because of an ectopic pregnancy definitely won’t survive. But in both cases, a pro-life doctor sees herself as delivering a child, who is as much a patient as the mother.
A pro-life approach to ectopic pregnancy may countenance similar procedures but still sees it as different from an approach that equates it to abortion. When a mother’s life is threatened by the course of her pregnancy, there is a wide gulf between a culture that assumes she and her baby are pitted against each other and one in which both are valued.
Having gone through ectopic pregnancy, I have firsthand experience of this. And what I have learned is that a pro-life response to ectopic pregnancy isn’t just a matter of what is forbidden and what is permitted, but of what can be offered to parents to make room for their grief and to treat their child with love and dignity.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

IMAGES
VIDEO
COMMENTS
An ectopic pregnancy is the implantation of a fertilized ovum outside the endometrial lining of the uterus. Ectopic pregnancies are estimated to occur in 1.4% of all pregnancies and account for 15% of pregnancy-related deaths. [ 1] The classic presentation of an ectopic pregnancy is the triad of abdominal or pelvic pain, vaginal bleeding, and ...
Ectopic pregnancy is a known complication of pregnancy that can carry a high rate of morbidity and mortality when not recognized and treated promptly. It is essential that providers maintain a high index of suspicion for an ectopic in their pregnant patients as they may present with pain, vaginal bleeding, or more vague complaints such as nausea and vomiting. Fertilization and embryo ...
Ectopic pregnancy occurs when a fertilized ovum implants outside of the uterine cavity. In the United States, the estimated prevalence of ectopic pregnancy is 1% to 2%, and ruptured ectopic ...
An ectopic pregnancy occurs when a fertilized egg implants in the wrong place in a woman's body. So instead of attaching to the lining of the uterus, where it can survive, it grows elsewhere ...
An ectopic pregnancy occurs when a fertilized egg implants and grows in a location that cannot support the pregnancy. Almost all ectopic pregnancies—more than 90%—occur outside of the uterine cavity in a fallopian tube, but they can also implant in the abdomen, cervix, ovary, and cesarean scar. An ectopic pregnancy in any location is life ...
An ectopic pregnancy occurs when a fertilized egg grows outside of the uterus. This can be a life-threatening emergency that needs immediate surgery.
Ectopic pregnancies are the leading cause of maternal mortality in the first trimester, with an incidence of 5%-10% of all pregnancy-related deaths. Diagnosis of ectopic pregnancies is difficult due to clinical mimics and non-specific symptoms ...
Practice Essentials. Ectopic pregnancy is the result of a flaw in human reproductive physiology that allows the conceptus to implant and mature outside the endometrial cavity (see the image below), which ultimately ends in the death of the fetus. Without timely diagnosis and treatment, ectopic pregnancy can become a life-threatening situation.
An ectopic pregnancy involves an embryo settling outside of the uterus, and it can be life threatening. Learn about the symptoms, effects, and treatments.
However, some women who have an ectopic pregnancy have the usual early signs or symptoms of pregnancy — a missed period, breast tenderness and nausea. If you take a pregnancy test, the result will be positive. Still, an ectopic pregnancy can't continue as normal. As the fertilized egg grows in the improper place, signs and symptoms become ...
I'd advocated for myself at doctor's appointments and been proactive about my reproductive health. So when something went wrong, I thought: What did I miss?
An ectopic pregnancy is the implantation of a fertilized. ovum outside the endometrial lining of the uterus. Ectopic pregnancies are estimated to occur in 1.4% of all. pregnancies and account for ...
Ectopic pregnancies occur in approximately 1.4% of all pregnancies and account for 15% of pregnancy-related deaths. Considering the high degree of mortality, recognizing an ectopic pregnancy is important. Signs and symptoms of an ectopic pregnancy are nonspecific and include pain, vaginal bleeding, …
Ectopic pregnancy is a condition of immense gynecological importance, particularly in the developing world, because of the high morbidity and mortality associated with it and the enormous threat to life. When ruptured, ectopic pregnancy is a true medical emergency.
Ectopic pregnancy is a complication of pregnancy in which the embryo attaches outside the uterus. [5] Signs and symptoms classically include abdominal pain and vaginal bleeding, but fewer than 50 percent of affected women have both of these symptoms. [1] The pain may be described as sharp, dull, or crampy. [1] Pain may also spread to the shoulder if bleeding into the abdomen has occurred. [1 ...
An ectopic pregnancy is one that grows outside the uterus (womb). In the UK, 1 in 90 pregnancies (just over 1%) is an ectopic pregnancy. Women who have had a previous ectopic pregnancy are at higher risk. A pregnancy cannot survive in these situations and it can pose a serious risk to you.
Ectopic Pregnancy Essay. In a normal pregnancy, a fertilized egg travels from the location of fertilization (the fallopian tube) to the uterus. Sometimes however, the egg grows in the wrong place, which is known as an ectopic pregnancy. Ectopic pregnancy was first documented as early as 1693 during a routine autopsy performed on a female ...
Treatment of diagnosed ectopic pregnancy includes medical management with intramuscular methotrexate, surgical management via salpingostomy or salpingectomy, and, in rare cases, expectant management. A patient with diagnosed ectopic pregnancy should be immediately transferred for surgery if she has peritoneal signs or hemodynamic instability ...
Opinion: I chose to receive treatment for the ectopic pregnancy. Yet, as a young woman from rural Appalachia, I know others aren't so lucky.
A transvaginal ultrasound scan is the best test to diagnose ectopic pregnancy. Laparoscopic surgery is the main treatment; medical and expectant management is used in a select group. Women who are treated for ectopic pregnancy have significantly lower subsequent spontaneous intrauterine pregnancy rates and higher ectopic pregnancy rates than ...
Objective FDP1.1: Ectopic Pregnancy. Describe risk factors, characteristic morphologic findings, potential outcomes, and the medical/surgical options for management of ectopic pregnancy in relation to the pathogenesis and likelihood of adverse consequences. Competency 2: Organ System Pathology; Topic: Female Reproductive—Disorders of ...
Especially in hard cases like ectopic pregnancy, we can offer more love and material support to mothers and children.
In a July 4 guest essay on ectopic pregnancy for the Times, Leah Libresco Sargeant used language that doesn't meet medical standards for discussion and treatment of such conditions, as a social ...