- Patient Care & Health Information
- Diseases & Conditions
- What is a stroke? A Mayo Clinic expert explains
Learn more from neurologist Robert D. Brown, Jr. M.D., M.P.H.
I'm Dr. Robert Brown, neurologist at Mayo Clinic. In this video, we'll cover the basics of a stroke. What is it, who it happens to, the symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available. You've likely heard the term stroke before. They affect about 800,000 people in the United States each year. Strokes happen in two ways. In the first, a blocked artery can cut off blood to an area of the brain. And this is known as an ischemic stroke. 85% of strokes are of this type. The second type of stroke happens when a blood vessel can leak or burst. So the blood spills into the brain tissue or surrounding the brain. And this is called a hemorrhagic stroke. Prompt treatment can reduce brain damage and the likelihood of death or disability. So if you or someone you know is experiencing a stroke, you should call 911 and seek emergency medical care right away.
Anyone can have a stroke, but some things put you at higher risk. And some things can lower your risk. If you're 55 and older, if you're African-American, if you're a man, or if you have a family history of strokes or heart attacks, your chances of having a stroke are higher. Being overweight, physically inactive, drinking alcohol heavily, recreational drug use. Those who smoke, have high blood pressure or high cholesterol, have poorly controlled diabetes, suffer from obstructive sleep apnea, or have certain forms of heart disease are at greater risk as well.
Look for these signs and symptoms if you think you or someone you know is having a stroke: Sudden trouble speaking and understanding what others are saying. Paralysis or numbness of the face, arm or leg on one side of the body. Problems seeing in one or both eyes, trouble walking, and a loss of balance. Now many strokes are not associated with headache, but a sudden and severe headache can sometimes occur with some types of stroke. If you notice any of these, even if they come and go or disappear completely, seek emergency medical attention or call 911. Don't wait to see if symptoms stop, for every minute counts.
Once you get to the hospital, your emergency team will review your symptoms and complete a physical exam. They will use several tests to help them figure out what type of stroke you're having and determine the best treatment for the stroke. This could include a CT scan or MRI scan, which are pictures of the brain and arteries, a carotid ultrasound, which is a soundwave test of the carotid arteries which provide blood flow to the front parts of the brain, and blood tests.
Once your doctors can determine if you're having an ischemic or hemorrhagic stroke, they'll be able to figure out the best treatment. If you're suffering an ischemic stroke, it's important to restore blood flow to your brain as quickly as possible, providing the oxygen and other nutrients your brain cells need to survive. To do this, doctors may use an intravenous clot buster medicine, dissolving the clot that is obstructing the blood flow or they may perform an emergency endovascular procedure. This involves advancing a tiny plastic tube called a catheter up into the brain arteries, allowing the blockage in the artery to be removed directly. Unlike ischemic strokes, the goal for treating a hemorrhagic stroke is to control the bleeding and reduce pressure in the brain. Doctors may use emergency medicines to lower the blood pressure, prevent blood vessel spasms, encourage clotting and prevent seizures. Or, if the bleeding is severe, surgery may be performed to remove the blood that is in the brain.
Every stroke is different, and so every person's road to recovery is different. Management of a stroke often involves a care team with several specialties. This may include a neurologist and a physical medicine and rehabilitation physician, among others. Now, in the end, our goal is to help you recover as much function as possible so that you can live independently. A stroke is a life-changing event that can affect you emotionally as much as it can physically. You may feel helpless, frustrated, or depressed. So look for help and support from friends and family. Accept that recovery will take hard work and most of all time. Strive for a new normal and remember to celebrate your progress. If you'd like to learn even more about strokes, watch our other related videos or visit mayoclinic.org. We wish you all the best.
An ischemic stroke occurs when the blood supply to part of the brain is blocked or reduced. This prevents brain tissue from getting oxygen and nutrients. Brain cells begin to die in minutes. Another type of stroke is a hemorrhagic stroke. It occurs when a blood vessel in the brain leaks or bursts and causes bleeding in the brain. The blood increases pressure on brain cells and damages them.
A stroke is a medical emergency. It's crucial to get medical treatment right away. Getting emergency medical help quickly can reduce brain damage and other stroke complications.
The good news is that fewer Americans die of stroke now than in the past. Effective treatments also can help prevent disability from stroke.

Products & Services
- A Book: Future Care
- A Book: Mayo Clinic Family Health Book
- Assortment of Products for Independent Living from Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
If you or someone you're with may be having a stroke, pay attention to the time the symptoms began. Some treatments are most effective when given soon after a stroke begins.
Symptoms of stroke include:
- Trouble speaking and understanding what others are saying. A person having a stroke may be confused, slur words or may not be able to understand speech.
- Numbness, weakness or paralysis in the face, arm or leg. This often affects just one side of the body. The person can try to raise both arms over the head. If one arm begins to fall, it may be a sign of a stroke. Also, one side of the mouth may droop when trying to smile.
- Problems seeing in one or both eyes. The person may suddenly have blurred or blackened vision in one or both eyes. Or the person may see double.
- Headache. A sudden, severe headache may be a symptom of a stroke. Vomiting, dizziness and a change in consciousness may occur with the headache.
- Trouble walking. Someone having a stroke may stumble or lose balance or coordination.
When to see a doctor
Seek immediate medical attention if you notice any symptoms of a stroke, even if they seem to come and go or they disappear completely. Think "FAST" and do the following:
- Face. Ask the person to smile. Does one side of the face droop?
- Arms. Ask the person to raise both arms. Does one arm drift downward? Or is one arm unable to rise?
- Speech. Ask the person to repeat a simple phrase. Is the person's speech slurred or different from usual?
- Time. If you see any of these signs, call 911 or emergency medical help right away.
Call 911 or your local emergency number immediately. Don't wait to see if symptoms stop. Every minute counts. The longer a stroke goes untreated, the greater the potential for brain damage and disability.
If you're with someone you suspect is having a stroke, watch the person carefully while waiting for emergency assistance.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
There are two main causes of stroke. An ischemic stroke is caused by a blocked artery in the brain. A hemorrhagic stroke is caused by leaking or bursting of a blood vessel in the brain. Some people may have only a temporary disruption of blood flow to the brain, known as a transient ischemic attack (TIA). A TIA doesn't cause lasting symptoms.
- Ischemic stroke
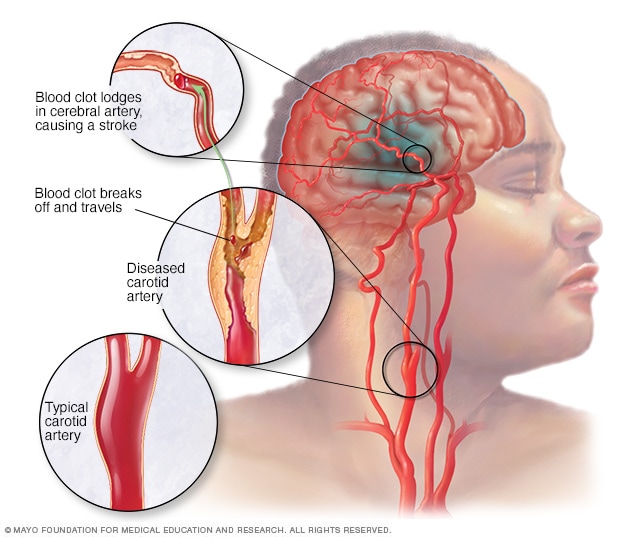
An ischemic stroke occurs when a blood clot, known as a thrombus, blocks or plugs an artery leading to the brain. A blood clot often forms in arteries damaged by a buildup of plaques, known as atherosclerosis. It can occur in the carotid artery of the neck as well as other arteries.
This is the most common type of stroke. It happens when the brain's blood vessels become narrowed or blocked. This causes reduced blood flow, known as ischemia. Blocked or narrowed blood vessels can be caused by fatty deposits that build up in blood vessels. Or they can be caused by blood clots or other debris that travel through the bloodstream, most often from the heart. An ischemic stroke occurs when fatty deposits, blood clots or other debris become lodged in the blood vessels in the brain.
Some early research shows that COVID-19 infection may increase the risk of ischemic stroke, but more study is needed.
Hemorrhagic stroke
Hemorrhagic stroke occurs when a blood vessel in the brain leaks or ruptures. Bleeding inside the brain, known as a brain hemorrhage, can result from many conditions that affect the blood vessels. Factors related to hemorrhagic stroke include:
- High blood pressure that's not under control.
- Overtreatment with blood thinners, also known as anticoagulants.
- Bulges at weak spots in the blood vessel walls, known as aneurysms.
- Head trauma, such as from a car accident.
- Protein deposits in blood vessel walls that lead to weakness in the vessel wall. This is known as cerebral amyloid angiopathy.
- Ischemic stroke that leads to a brain hemorrhage.
A less common cause of bleeding in the brain is the rupture of an arteriovenous malformation (AVM). An AVM is an irregular tangle of thin-walled blood vessels.
Transient ischemic attack
A transient ischemic attack (TIA) is a temporary period of symptoms similar to those of a stroke. But a TIA doesn't cause permanent damage. A TIA is caused by a temporary decrease in blood supply to part of the brain. The decrease may last as little as five minutes. A transient ischemic attack is sometimes known as a ministroke.
A TIA occurs when a blood clot or fatty deposit reduces or blocks blood flow to part of the nervous system.
Seek emergency care even if you think you've had a TIA . It's not possible to tell if you're having a stroke or TIA based only on the symptoms. If you've had a TIA , it means you may have a partially blocked or narrowed artery leading to the brain. Having a TIA increases your risk of having a stroke later.
Risk factors
Many factors can increase the risk of stroke. Potentially treatable stroke risk factors include:
Lifestyle risk factors
- Being overweight or obese.
- Physical inactivity.
- Heavy or binge drinking.
- Use of illegal drugs such as cocaine and methamphetamine.
Medical risk factors
- High blood pressure.
- Cigarette smoking or secondhand smoke exposure.
- High cholesterol.
- Obstructive sleep apnea.
- Cardiovascular disease, including heart failure, heart defects, heart infection or irregular heart rhythm, such as atrial fibrillation.
- Personal or family history of stroke, heart attack or transient ischemic attack.
- COVID-19 infection.
Other factors associated with a higher risk of stroke include:
- Age — People age 55 or older have a higher risk of stroke than do younger people.
- Race or ethnicity — African American and Hispanic people have a higher risk of stroke than do people of other races or ethnicities.
- Sex — Men have a higher risk of stroke than do women. Women are usually older when they have strokes, and they're more likely to die of strokes than are men.
- Hormones — Taking birth control pills or hormone therapies that include estrogen can increase risk.
Complications
A stroke can sometimes cause temporary or permanent disabilities. Complications depend on how long the brain lacks blood flow and which part is affected. Complications may include:
- Loss of muscle movement, known as paralysis. You may become paralyzed on one side of the body. Or you may lose control of certain muscles, such as those on one side of the face or one arm.
- Trouble talking or swallowing. A stroke might affect the muscles in the mouth and throat. This can make it hard to talk clearly, swallow or eat. You also may have trouble with language, including speaking or understanding speech, reading or writing.
- Memory loss or trouble thinking. Many people who have had strokes experience some memory loss. Others may have trouble thinking, reasoning, making judgments and understanding concepts.
- Emotional symptoms. People who have had strokes may have more trouble controlling their emotions. Or they may develop depression.
- Pain. Pain, numbness or other feelings may occur in the parts of the body affected by stroke. If a stroke causes you to lose feeling in the left arm, you may develop a tingling sensation in that arm.
- Changes in behavior and self-care. People who have had strokes may become more withdrawn. They also may need help with grooming and daily chores.
You can take steps to prevent a stroke. It's important to know your stroke risk factors and follow the advice of your healthcare professional about healthy lifestyle strategies. If you've had a stroke, these measures might help prevent another stroke. If you have had a transient ischemic attack (TIA), these steps can help lower your risk of a stroke. The follow-up care you receive in the hospital and afterward also may play a role.
Many stroke prevention strategies are the same as strategies to prevent heart disease. In general, healthy lifestyle recommendations include:
- Control high blood pressure, known as hypertension. This is one of the most important things you can do to reduce your stroke risk. If you've had a stroke, lowering your blood pressure can help prevent a TIA or stroke in the future. Healthy lifestyle changes and medicines often are used to treat high blood pressure.
- Lower the amount of cholesterol and saturated fat in your diet. Eating less cholesterol and fat, especially saturated fats and trans fats, may reduce buildup in the arteries. If you can't control your cholesterol through dietary changes alone, you may need a cholesterol-lowering medicine.
- Quit tobacco use. Smoking raises the risk of stroke for smokers and nonsmokers exposed to secondhand smoke. Quitting lowers your risk of stroke.
- Manage diabetes. Diet, exercise and losing weight can help you keep your blood sugar in a healthy range. If lifestyle factors aren't enough to control blood sugar, you may be prescribed diabetes medicine.
- Maintain a healthy weight. Being overweight contributes to other stroke risk factors, such as high blood pressure, cardiovascular disease and diabetes.
- Eat a diet rich in fruits and vegetables. Eating five or more servings of fruits or vegetables every day may reduce the risk of stroke. The Mediterranean diet, which emphasizes olive oil, fruit, nuts, vegetables and whole grains, may be helpful.
- Exercise regularly. Aerobic exercise reduces the risk of stroke in many ways. Exercise can lower blood pressure, increase the levels of good cholesterol, and improve the overall health of the blood vessels and heart. It also helps you lose weight, control diabetes and reduce stress. Gradually work up to at least 30 minutes of moderate physical activity on most or all days of the week. The American Heart association recommends getting 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous aerobic activity a week. Moderate intensity activities can include walking, jogging, swimming and bicycling.
- Drink alcohol in moderation, if at all. Drinking large amounts of alcohol increases the risk of high blood pressure, ischemic strokes and hemorrhagic strokes. Alcohol also may interact with other medicines you're taking. However, drinking small to moderate amounts of alcohol may help prevent ischemic stroke and decrease the blood's clotting tendency. A small to moderate amount is about one drink a day. Talk to your healthcare professional about what's appropriate for you.
- Treat obstructive sleep apnea (OSA). OSA is a sleep disorder that causes you to stop breathing for short periods several times during sleep. Your healthcare professional may recommend a sleep study if you have symptoms of OSA . Treatment includes a device that delivers positive airway pressure through a mask to keep the airway open while you sleep.
- Don't use illicit drugs. Certain illicit drugs such as cocaine and methamphetamine are established risk factors for a TIA or a stroke.
Preventive medicines
If you have had an ischemic stroke, you may need medicines to help lower your risk of having another stroke. If you have had a TIA , medicines can lower your risk of having a stroke in the future. These medicines may include:
Anti-platelet drugs. Platelets are cells in the blood that form clots. Anti-platelet medicines make these cells less sticky and less likely to clot. The most commonly used anti-platelet medicine is aspirin. Your healthcare professional can recommend the right dose of aspirin for you.
If you've had a TIA or minor stroke, you may take both an aspirin and an anti-platelet medicine such as clopidogrel (Plavix). These medicines may be prescribed for a period of time to reduce the risk of another stroke. If you can't take aspirin, you may be prescribed clopidogrel alone. Ticagrelor (Brilinta) is another anti-platelet medicine that can be used for stroke prevention.
Blooding-thinning medicines, known as anticoagulants. These medicines reduce blood clotting. Heparin is a fast-acting anticoagulant that may be used short-term in the hospital.
Slower acting warfarin (Jantoven) may be used over a longer term. Warfarin is a powerful blood-thinning medicine, so you need to take it exactly as directed and watch for side effects. You also need regular blood tests to monitor warfarin's effects.
Several newer blood-thinning medicines are available to prevent strokes in people who have a high risk. These medicines include dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (Savaysa). They work faster than warfarin and usually don't require regular blood tests or monitoring by your healthcare professional. These medicines also are associated with a lower risk of bleeding complications compared to warfarin.
Stroke care at Mayo Clinic
- Walls RM, et al., eds. Stroke. In: Rosen's Emergency Medicine: Concepts and Clinical Practice. 10th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Sept. 13, 2023.
- Ferri FF. Ferri's Clinical Advisor 2024. Elsevier; 2024. https://www.clinicalkey.com. Accessed Sept. 13, 2023.
- Patients and caregivers. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/public-education/know-stroke/patients-and-caregivers#. Accessed Sept. 13, 2023.
- Stroke. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/stroke. Accessed Sept. 13, 2023.
- Oliveira-Filho J, et al. Initial assessment and management of acute stroke. https://www.uptodate.com/contents/search. Accessed Sept. 13, 2023.
- About stroke. Centers for Disease Control and Prevention. https://www.cdc.gov/stroke/healthy_living.htm. Accessed Sept. 13, 2023.
- Effects of stroke. American Stroke Association. https://www.stroke.org/en/about-stroke/effects-of-stroke. Accessed Sept. 13, 2023.
- Rehab therapy after a stroke. American Stroke Association. https://www.stroke.org/en/life-after-stroke/stroke-rehab/rehab-therapy-after-a-stroke. Accessed Sept. 13, 2023.
- Arteriovenous malformations (AVMs). National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/disorders/arteriovenous-malformations-avms?search-term=arterial#. Accessed Oct. 2, 2023.
- Cerebral aneurysms. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/cerebral-aneurysms-fact-sheet. Accessed Sept. 13, 2023.
- Transient ischemic attack. Merck Manual Professional Version. https://www.merckmanuals.com/professional/neurologic-disorders/stroke/transient-ischemic-attack-tia?query=transient%20ischemic%20attack#. Accessed Sept. 13, 2023.
- Stroke. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Post-Stroke-Rehabilitation-Fact-Sheet. Accessed Sept. 13, 2023.
- Rose NS, et al. Overview of secondary prevention of ischemic stroke. https://www.uptodate.com/contents/search. Accessed Sept. 13, 2023.
- Prevent stroke: What you can do. Centers for Disease Control and Prevention. https://www.cdc.gov/stroke/prevention.htm#print. Accessed Sept. 13, 2023.
- Know your risk for stroke. Centers for Disease Control and Prevention. https://www.cdc.gov/stroke/risk_factors.htm#. Accessed Oct. 2, 2023.
- Powers WJ, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke — A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; doi:10.1161/STR.0000000000000211.
- Papadakis MA, et al., eds. Quick Medical Diagnosis & Treatment 2023. McGraw Hill; 2023. https://accessmedicine.mhmedical.com. Accessed Sept. 13, 2023.
- Tsao CW, et al. Heart disease and stroke statistics — 2023 update: A report from the American Heart Association. Circulation. 2023; doi:10.1161/CIR.0000000000001123.
- Grotta JC, et al., eds. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier, 2022. https://www.clinicalkey.com. Accessed Sept. 15, 2023.
- Suppah M, et al. An evidence-based approach to anticoagulation therapy comparing direct oral anticoagulants and vitamin K antagonists in patients with atrial fibrillation and bioprosthetic valves: A systematic review, meta-analysis and network meta-analysis. American Journal of Cardiology. 2023; doi:10.1016/j.amjcard.2023.07.141.
- Tyagi K, et al. Neurological manifestations of SARS-CoV-2: Complexity, mechanism and associated disorders. European Journal of Medical Research. 2023; doi:10.1186/s40001-023-01293-2.
- Siegler JE, et al. Cerebrovascular disease in COVID-19. Viruses. 2023; doi:10.3390/v15071598.
- Lombardo M, et al. Health effects of red wine consumption: A narrative review of an issue that still deserves debate. Nutrients. 2023; doi:10.3390/nu15081921.
- Jim J. Complications of carotid endarterectomy. https://www.uptodate.com/contents/search. Accessed Oct. 2, 2023.
- Van Nimwegan D, et al. Interventions for improving psychosocial well-being after stroke: A systematic review. International Journal of Nursing Studies. 2023; doi:10.1016/j.ijnurstu. 2023.104492 .
- Hasan TF, et al. Diagnosis and management of acute ischemic stroke. Mayo Clinic Proceedings. 2018; doi:10.1016/j.mayocp.2018.02.013.
- Ami TR. Allscripts EPSi. Mayo Clinic. Sept. 4, 2023.
- Barrett KM, et al. Ambulance-based assessment of NIH stroke scale with telemedicine: A feasibility pilot study. Journal of Telemedicine and Telecare. 2017; doi:10.1177/1357633X16648490.
- Sener U, et al. Ischemic stroke in patients with malignancy. Mayo Clinic Proceedings. 2022; doi:10.1016/j.mayocp.2022.09.003.
- Quality check. The Joint Commission. https://www.qualitycheck.org/search/?keyword=mayo%20clinic. Accessed Oct. 4, 2023.
- Quality care you can trust. American Heart Association. https://www.heart.org/en/professional/quality-improvement/hospital-maps. Accessed Oct. 4, 2023.
- Attig JM. Allscripts EPSi. Mayo Clinic. Oct. 9, 2023.
- How much physical activity do you need? American Heart Association. https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-infographic. Accessed Oct. 12, 2023.
- Graff-Radford J (expert opinion). Mayo Clinic. Oct. 11, 2023.
- Healthcare. DNV Healthcare USA, Inc. https://www.dnvhealthcareportal.com/hospitals?search_type=and&q=mayo+clinic&c=&c=20806&c=&c=&prSubmit=Search. Accessed Nov. 1, 2023.
- Brain hemisphere connections
- Cerebral angiogram
- CT scan of brain tissue damaged by stroke
- Stroke rehabilitation
- Strokes FAQ Neurologist Robert D. Brown, Jr. M.D., M.P.H., answers the most frequently asked questions about strokes.
- Typing with Brain Waves
Associated Procedures
- Carotid angioplasty and stenting
- Carotid endarterectomy
- Carotid ultrasound
- Coronary angioplasty and stents
- Echocardiogram
- Home enteral nutrition
News from Mayo Clinic
- Mayo Clinic Minute: Stroke treatment Oct. 24, 2024, 04:00 p.m. CDT
- Expert alert: Surgical approaches being studied to restore function after stroke Aug. 21, 2024, 02:30 p.m. CDT
- Expert alert: Procedure offers hope to patients living with physical changes from stroke, other conditions July 11, 2024, 02:30 p.m. CDT
- Clot-busting medication helps retiree return to the open road after a stroke June 28, 2024, 04:00 p.m. CDT
- Mayo Clinic Minute: The link between heart disease and stroke June 05, 2024, 03:26 p.m. CDT
- 5 things to know about stroke May 04, 2024, 11:00 a.m. CDT
- Mayo Clinic Minute: Preventing stroke May 02, 2024, 05:00 p.m. CDT
- Mayo Clinic Minute: How extreme temperatures can increase stroke risk July 20, 2023, 04:30 p.m. CDT
- Mayo Clinic Minute: What women need to know about stroke May 30, 2023, 04:15 p.m. CDT
- From lift off to splash down: An update on Mayo Clinic stem cells in space May 26, 2023, 04:39 p.m. CDT
- Mayo Clinic Minute: How to reduce your stroke risk May 10, 2023, 01:30 p.m. CDT
- A cheeseburger's role in one man's stroke recovery Nov. 11, 2022, 05:30 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been ranked among the best Neurology & Neurosurgery hospitals in the nation for 2024-2025 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- NEW: Listen to Health Matters Podcast - Mayo Clinic Press NEW: Listen to Health Matters Podcast
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Ischaemic stroke
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
When viewing this topic in a different language, you may notice some differences in the way the content is structured, but it still reflects the latest evidence-based guidance.
Ischaemic stroke is a leading cause of morbidity and mortality. If you suspect stroke, work rapidly through the initial assessment and aim for quick access to computed tomographic (CT) scan. Early initiation of reperfusion strategies (intravenous thrombolysis or mechanical thrombectomy) within 4.5 hours from onset of symptoms, if not contraindicated, is associated with improved functional outcomes.
Use a validated tool to aid recognition: use ROSIER (Recognition of Stroke in the Emergency Room) in the emergency department; use FAST (Face Arm Speech Test) in the community.
Manage any airway, breathing, and circulatory insufficiencies requiring urgent treatment.
Admit everyone with suspected stroke directly to a hyperacute or acute stroke unit within 4 hours of presentation.
Request non-enhanced CT scan as soon as possible (at most within 1 hour of arrival at hospital). Ischaemic stroke is a clinical diagnosis based on signs and symptoms. A normal CT scan does not rule out a stroke but will rule out intracranial haemorrhage, which must be excluded before starting thrombolysis.
Intravenous alteplase should be given (if not contraindicated) if treatment is started as soon as possible within 4.5 hours of onset of symptoms AND intracranial haemorrhage has been excluded by imaging.
Tenecteplase may be considered as a safe and effective alternative to alteplase within 4.5 hours of ischaemic stroke, but is off-label for this indication in the UK.
Mechanical thrombectomy can be performed in selected patients within 6 to 24 hours of symptoms onset.
The World Health Organization defines stroke as “a clinical syndrome consisting of rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin”. [1] Aho K, Harmsen P, Hatano S, et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58(1):113-30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2395897 http://www.ncbi.nlm.nih.gov/pubmed/6966542?tool=bestpractice.com
Stroke can be further subdivided into ischaemic stroke (caused by vascular occlusion or stenosis) and haemorrhagic stroke (caused by vascular rupture, resulting in intraparenchymal and/or subarachnoid haemorrhage). Central venous sinus thrombosis is a rare form of stroke that occurs due to thrombosis of the dural venous sinuses. This topic focuses on the first 24 hours of acute care of patients with ischaemic stroke.
For information on other types of stroke, see Stroke due to spontaneous intracerebral haemorrhage , Subarachnoid haemorrhage , and Cavernous sinus thrombosis .
History and exam
Key diagnostic factors.
- unilateral weakness or paralysis in the face, arm or leg
- visual disturbance
- risk factors
Other diagnostic factors
- sensory loss (numbness)
- gaze paresis
- arrhythmias, murmurs, or pulmonary oedema
- nausea and/or vomiting
- neck or facial pain
- miosis, ptosis, and facial anhidrosis (hemilateral)
- decreased level of consciousness or coma
Risk factors
- family history of stroke
- history of ischaemic stroke or TIA
- hypertension
- diabetes mellitus
- atrial fibrillation
- comorbid cardiac conditions
- carotid artery stenosis
- sickle cell disease
- dyslipidaemia
- lower levels of education
- black or South Asian ethnic groups
- poor diet and nutrition
- physical inactivity
- alcohol abuse
- oestrogen-containing therapy
- obstructive sleep apnoea
- illicit drug use
- hyperhomocysteinaemia
- elevated lipoprotein(a)
- hypercoagulable states
- elevated C-reactive protein
- aortic arch plaques
Diagnostic investigations
1st investigations to order.
- non-contrast CT head
- serum glucose
- serum electrolytes
- serum urea and creatinine
- cardiac enzymes
- prothrombin time and PTT (with INR)
Investigations to consider
- serum toxicology screen
- CT angiography
- CT or MRI perfusion-weighted imaging
- carotid ultrasound
- echocardiogram
Treatment algorithm
Suspected ischaemic stroke, confirmed ischaemic stroke, contributors, expert advisers, matthew jones, md, frcp.
Consultant Neurologist
Manchester Centre for Clinical Neurosciences
Northern Care Alliance
Honorary Senior Lecturer
University of Manchester
Disclosures
MJ is the chair of the Association of British Neurologists Education Committee (unpaid position). MJ is a faculty member of an MRCP revision course. MJ has received honoraria from Eisai for educational talks.
Rachael Power, MBChB, MRCP
Neurology Registrar
RP has been sponsored by Novartis to attend the International Headache Conference.
Acknowledgements
BMJ Best Practice would like to gratefully acknowledge the previous expert contributor for this topic, whose work has been retained in parts of the content:
George Ntaios, MD, MSc (ESO Stroke Medicine), PhD, FESO
Assistant Professor of Internal Medicine
Medical School
University of Thessaly
GN is on the advisory boards for, and has received honoraria, speaker fees, and research support from: Amgen, Bayer, Boehringer-Ingelheim, BMS/Pfizer, Elpen, Galenica, Medtronic, Sanofi, and Winmedica.
Peer reviewers
Kayvan khadjooi, md, frcp, pgcertmeded.
Consultant in Stroke Medicine
Addenbrooke’s Hospital
Associate Lecturer
School of Clinical Medicine
University of Cambridge
KK has received travel grants for conferences/speaker honoraria from Bayer, Boehringer, Daiichi-Sankyo, Pfizer, and Shire.
Helena Delgado-Cohen
Section Editor, BMJ Best Practice
HDC declares that she has no competing interests.
Tannaz Aliabadi-Oglesby
Lead Section Editor, BMJ Best Practice
TAO declares that she has no competing interests.
Julie Costello
Comorbidities Editor, BMJ Best Practice
JC declares that she has no competing interests.
Adam Mitchell
Drug Editor, BMJ Best Practice
AM declares that he has no competing interests.

Differentials
- Intracerebral haemorrhage
- Transient ischaemic attack (TIA)
- Hypertensive encephalopathy
- National clinical guideline for stroke for the United Kingdom and Ireland
- Stroke and transient ischaemic attack in over 16s: diagnosis and initial management
Calculators
NIH Stroke Score
Glasgow Coma Scale
Tracheal intubation animated demonstration
Bag-valve-mask ventilation animated demonstration
Patient information
Preventing another stroke
Stroke: emergency treatment
Use of this content is subject to our disclaimer
Log in or subscribe to access all of BMJ Best Practice
Log in to access all of bmj best practice, help us improve bmj best practice.
Please complete all fields.
I have some feedback on:
We will respond to all feedback.
For any urgent enquiries please contact our customer services team who are ready to help with any problems.
Phone: +44 (0) 207 111 1105
Email: [email protected]
Your feedback has been submitted successfully.
Stroke: causes and clinical features
Affiliations.
- 1 is a Stroke Fellow at University College Hospital and The National Hospital, Queen Square, London, UK. Competing interests: none declared.
- 2 is Professor of clinical neurology at UCL Institute of Neurology, Queen Square, and Honorary Consultant Neurologist at University College Hospital and The National Hospital, Queen Square, London, UK. He trained in medicine at Guy's Hospital, and in neurology in London including The Maudsley Hospital, King's College Hospital, St Thomas' Hospital and the National Hospital. Competing interests: DJW reports honoraria from Bayer, Alnylam and Portola.
- PMID: 32837228
- PMCID: PMC7409792
- DOI: 10.1016/j.mpmed.2020.06.002
Stroke is a clinically defined syndrome of acute, focal neurological deficit attributed to vascular injury (infarction, haemorrhage) of the central nervous system. Stroke is the second leading cause of death and disability worldwide. Stroke is not a single disease but can be caused by a wide range of risk factors, disease processes and mechanisms. Hypertension is the most important modifiable risk factor for stroke, although its contribution differs for different subtypes. Most (85%) strokes are ischaemic, predominantly caused by small vessel arteriolosclerosis, cardioembolism and large artery athero-thromboembolism. Ischaemic strokes in younger patients can result from a different spectrum of causes such as extracranial dissection. Approximately 15% of strokes worldwide are the result of intracerebral haemorrhage, which can be deep (basal ganglia, brainstem), cerebellar or lobar. Deep haemorrhages usually result from deep perforator (hypertensive) arteriopathy (arteriolosclerosis), while lobar haemorrhages are mainly caused by cerebral amyloid angiopathy or arteriolosclerosis. A minority (about 20%) of intracerebral haemorrhages are caused by macrovascular lesions (vascular malformations, aneurysms, cavernomas), venous sinus thrombosis or rarer causes; these are particularly important in young patients (<50 years). Knowledge of vascular and cerebral anatomy is important in localizing strokes and understanding their mechanisms. This guides rational acute management, investigation, and secondary prevention.
Keywords: Cerebrovascular disease; MRCP; intracerebral haemorrhage; ischaemic stroke; stroke pathogenesis; stroke risk factors; transient ischaemic attack.
© 2020 Published by Elsevier Ltd.
Publication types
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Understanding stroke...
Understanding stroke:Pathophysiology, presentation, and investigation
- Related content
- Peer review
- K A L Carroll , fifth year medical student 1 ,
- J Chataway , consultant neurologist 1
- 1 Imperial College, London
- 2 St Mary's Hospital, London
Every 45 seconds, someone in the United States has an attack of stroke. K A L Carroll and J Chataway discuss the pathology and clinical features of stroke, in the first of a two part series
Stroke is an acute neurological injury in which blood supply to a part of the brain is interrupted. Five and a half million survivors of stroke are living in the world today. 1 In the United States alone, half a million people have their first stroke each year, and 200 000 have a recurrent attack. 2 The World Health Organization esti mates that 15 million people have strokes each year worldwide, 5.5 mil lion of which are fatal. 1 In industri alised countries, stroke is the third most common single cause of death (after ischaemic heart disease and cancer). In the US, someone has an attack every 45 seconds, and there is a stroke related death every three minutes. 2
Even if age specific stroke incidence remains stable or falls slightly because more people live into old age, the annual incidence will continue to rise. This increases mortality, but, because of the direct cost of treatment and the indirect costs of lost productivity, the result is a loss - of $57.9bn (£30.4bn; €45.1bn) a year in the US. 2 A thorough understanding of stroke's pathophysiology, presentation, investigation, and current and future treatments is crucial.
Strokes may either be haemorrhagic or ischaemic. Eighty eight per cent of all strokes are ischaemic, 9% are due to intracerebral haemorrhage, and 3% are due to subarachnoid haemorrhage. 2
Haemorrhagic stroke
Intracranial haemorrhage may occur within the brain parenchyma (intracerebral haemorrhage) or within the surrounding meningeal spaces (including epidural haematoma, subdural haematoma, and subarachnoid haemorrhage).
In intracerebral haemorrhage, bleeding occurs directly into the brain parenchyma. In addition to the area of the brain injured by the haemorrhage, the surrounding brain can be damaged by pressure produced by the mass effect of the haematoma. A general increase in intracranial pressure may occur.
Non-traumatic intracerebral haemorrhage is usually due to hypertensive damage to blood vessel walls. Chronic hypertension causes lipohyalinosis, fibrinoid necrosis, and the development of Charcot-Bouchard aneurysms in arteries throughout the brain, which may then rupture. Non-traumatic intracerebral haemorrhage may also be due to excessive cerebral blood flow (for example, haemorrhagic transformation of an ischaemic infarct); rupture of an aneurysm or an arteriovenous malformation; an arteriopathy (for example, cerebral amyloid angiopathy); a coagulopathy; a vasculitis; haemorrhagic necrosis (for example, due to tumour or infection); or venous outflow obstruction (for example, cerebral venous thrombosis).
Non-penetrating and penetrating cranial trauma are also common causes of intracerebral haemorrhage.
Strokes due to hypertension more commonly occur in sites such as the basal ganglia, thalamus, pons, cerebellum, and other brainstem sites, whereas those due to other causes more commonly occur in lobar regions (particularly the parietal and occipital lobes).
Subarachnoid haemorrhage usually occurs after rupture of a berry aneurysm in the circle of Willis. Other uncommon causes include trauma, hypertensive haemorrhage, vasculitides, tumours, and coagulopathies. This results in blood accumulating in the basal cisterns and around the brainstem.
Ischaemic stroke
An acute vascular occlusion results in ischaemia in the dependent area of the brain. About 80% of ischaemic strokes are due to thromboses and emboli. The most common sites of thrombotic occlusion are cerebral artery branch points, particularly in the distribution of the internal carotid artery. Arterial stenosis precipitated by turbulent blood flow, atherosclerosis, and platelet adherence cause blood clots to form. Less common causes of thromboses, particularly seen in younger stroke patients, include cervical artery dissection, essential thrombocythaemia, polycythemia, sickle cell anaemia, protein C deficiency, fibromuscular dysplasia of the cerebral arteries, and cocaine misuse. 3
Emboli may arise from the heart, the extracranial arteries, or, rarely, the right sided circulation (paradoxical emboli), and can occlude the vasculature. Furthermore, rarely infective causes of emboli, such as subacute bacterial endocarditis, may cause occlusion, as may emboli due to iatrogenic causes, such as a cardiac prosthesis.
Small vessel disease within the brain causes a further 20% of ischaemic strokes. These are usually in patients with generalised small vessel disease - for example, hypertensive and diabetic patients. Multiple small emboli or an in situ process called lipohyalinosis (in which multiple microatheromata occlude the vessels) are thought to be responsible.
A system of categories of subtypes of ischaemic stroke mainly based on cause has been developed for the “Trial of Org” 10 172 in acute stroke treatment (TOAST). 4 This classification denotes five subtypes of ischaemic stroke - large artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined cause, and stroke of undetermined cause.
In all cases, loss of perfusion to a part of the brain results in an “ischaemic cascade” ( fig 1 ). Conse- quently the initial ischaemic insult is locally amplified.

Ischaemic cascade
- Download figure
- Open in new tab
- Download powerpoint
The high intracellular calcium acti- vates various enzymes that cause the destruction of the cell. Free radicals, arachidonic acid, and nitric oxide are generated by this process leading to further neuronal damage. Within hours to days of a stroke occurring, specific genes are activated that cause the formation of cytokines and other factors that in turn cause further inflammation and microcirculatory compromise. The area of damage thus spreads rapidly after the initial ischaemic event.
Risk factors
Stroke has numerous risk factors, some of which (such as increasing age and systolic blood pressure) are risk factors for both ischaemic and haemorrhagic stroke, however, other factors are more specific for type of stroke. The table gives important risk factors and their relative risk.
Risk factors for stroke
- View inline
Clinical presentation
Stroke should be considered in any patient presenting with an acute neu- rological deficit (focal or global) or altered level of consciousness. Patients' symptoms vary depending on the area of the brain affected and the extent of the damage.
Because of the importance of get- ting people who have had a stroke into hospital as rapidly as possible, there has been extensive research into prehospital assessment by patients themselves, family members, and prehospital care personnel, such as emergency medical technicians. The Cincinnati prehospital stroke scale has been developed using the three most important items (facial paresis, arm drift, and abnormal speech) derived from the stroke scale of the National Institutes of Health. 16
The Los Angeles prehospital stroke screen assesses for a unilateral arm drift, handgrip strength, and facial paresis. 17 Regardless of the scale used, it is important to increase public awareness as to the presenta- tion of stroke to decrease the time from onset to presentation in hospi- tal. In the UK, a campaign is cur- rently being run by the Stroke Association called FAST (the face arm speech test), which guides the public to present at hospital immedi- ately in the case of facial weakness, arm weakness, or speech disturbance.
No features of the history can accurately distinguish between ischaemic and haemorrhagic stroke. But haemorrhagic stroke is perhaps more likely if the presentation includes features of raised intracranial pressure (such as nausea, vomiting, and headache). Seizures are also more common in hemor- rhagic stroke than in ischaemic stroke, occurring in up to 28% of hemorrhagic strokes. Meningism, the symptoms of meningeal irritation associated with acute febrile illness or dehydration without actual infection of the meninges, may also result from blood in the ventricles after a haem- orrhagic stroke.
Four important stroke syndromes are caused by disruption of particular cerebrovascular distributions.
Anterior cerebral artery - This prima- rily affects frontal lobe function, which results in altered mental status, con- tralateral lower limb weakness and hypoaesthesia, and gait disturbance.
Middle cerebral artery - This com- monly results in contralateral hemi- paresis, contralateral hypoaesthesia, ipsilateral hemianopia, and gaze preference toward the side of the lesion. Agnosia, a loss in ability to recognise objects, persons, sounds, shapes or smells, in the absence of a specific sensory deficit or memory loss, is common.
Receptive or expressive aphasia may result if the lesion occurs in the dominant (mainly left) hemisphere. Neglect (behaviour as if the contralateral sensory space does not exist) may result when the lesion occurs in the parietal cortex.
Posterior cerebral artery - This affects vision and thought, producing homonymous hemianopia, cortical blindness, visual agnosia, altered mental status, and impaired memory.
Vertebrobasilar artery - causes a wide variety of cranial nerve, cerebellar, and brainstem deficits. These include vertigo, nystagmus, diplopia, visual field deficits, dysphagia, dysarthria, facial hypoaesthesia, syncope, and ataxia. Loss of pain and temperature sensation occurs on the ipsilateral face and contralateral body.
Investigations
After the necessary basic blood tests (including full blood count, biochemistry, and coagulation studies) and cardiac monitoring with electrocardiogram, a non-contrast head computed tomography scan is essential for rapidly distinguishing ischaemic from haemorrhagic stroke and may be able to define the anatomic distribution of the stroke. This is crucial because treatments for each type of stroke differ.
Within six hours of the onset of ischaemic stroke, most patients will have a normal computed tomography scan. After 6-12 hours, sufficient oedema may collect into the area of the stroke so that a region of hypo- density may be seen on the scan.
Radiological clues before this include:
Insular ribbon sign (loss of definition of grey-white interface in the lateral margins of the insula due to oedema in the insular cortex; fig 2 ) 18
Hyperdense middle cerebral artery sign ( fig 3 ) 19
Hypoattenuation in the lentiform nucleus ( fig 4 )
Sulcal obliteration
Shifting due to oedema
Loss of grey-white matter differentiation. 20 21

Computed tomograph after ischaemic stroke, showing oedema in insular cortex, as shown by solid arrows (open arrows show normal side). Reproduced from Chokski et al 27 with permission of Anderson Publishing

(below left) Computed tomograph after ischaemic stroke, showing hyperdense middle cerebral artery sign. Reproduced from Chokski et al 27 with permission of Anderson Publishing

(below right) Computed tomograph after ischaemic stroke, showing hypoattenuation in the lentiform nucleus, as shown by solid arrows (open arrows show normal side). Reproduced from Chokski et al 27 with permission of Anderson Publishing
These are all due to an increasing level of oedema in the brain, however, they rely on a high level of expertise of the radiologist and are often not present. Computed tomography scans also may fail to show some parenchymal haemorrhages smaller than 1 cm as a result of low resolution.
Conventional magnetic resonance imaging is not as sensitive as computed tomography for detecting haemorrhage in the acute setting. But newer techniques, such as perfusion and diffusion weighted magnetic resonance examinations, are more sensitive imaging methods for diagnosis in acute settings. Ischaemic areas can be determined within minutes or hours. But use of these methods has been restricted because they are not generally available and are difficult to employ under emergency conditions, particularly as they involve a patient lying flat for 40 minutes when they may be agitated or have a level of cardiorespiratory compromise.
Perfusion brain computed tomography, conversely, is a new imaging method capable of providing information about ischaemic brain tissue, which can be used in emergency conditions. 22 23 Perfusion is measured by monitoring the passage of contrast material (non-ionic iodine) through the brain using computed tomography. Perfusion examination of the entire brain is not possible yet, and because only a few neighbouring sections can be imaged, the anatomical region must be clinically determined. Various studies have shown that computed tomography perfusion scans yield comparable information to diffusion weighted magnetic resonance imaging scans. 24 25 But the entire brain cannot be analysed using perfusion computed tomography scanning, which is the major drawback, and further research is required to obtain an ideal investigation. Both types of investigation used have a high detection rate for haemorrhagic stroke.
Further investigations may include carotid duplex scanning for patients in whom carotid artery stenosis or occlusion is suspected, and transcranial Doppler ultrasound for evaluating the more proximal vasculature, including the middle cerebral artery, intracranial carotid artery, and vertebrobasilar artery. Echocardiography may be used for patients in whom cardiogenic embolism is suspected, and trans- oesophageal echocardiography may be used to detect a suspected thoracic aortic dissection, or transthoracic echocardiography for suspected acute myocardial infarction.
Computed tomography angiography is useful for patients with acute ischaemic stroke in whom accurate analysis of the cerebrovascular anatomy is required, particularly preoperatively. 26
References 1 – 27 are on Student BMJ.com.
Originally published as: Student BMJ 2006;14:319
- ↵ Atlas of Heart Disease and Stroke, WHO, September 2004
- ↵ A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2006) Heart Disease and Stroke Statistics – 2006 Update Circulation (February 14th 2006)
- ↵ Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, Schroth G, Remonda L, Sturzenegger M, Fischer U – Baumgartner RW (2005) Ischaemic stroke in young adults: predictors of outcome and recurrence J Neurol Neurosurg Psychiatry 76 : 191 – 195
- ↵ Adams Jr HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL & Marsh 3d EE (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment Stroke 24: 35-41
- ↵ Rodgers H, Greenaway J, Davies T, Wood R, Steen N, Thomson R (2004) Risk factors for first-ever stroke in older people in the north East of England: a population-based study Stroke 35 ( 1 ): 7 – 11
- ↵ Kiely DK, Wolf PA, Cupples LA, Beiser AS & Myers RH (1993) Familial Aggregation of stroke. The Framingham Study Stroke 24 : 1366 – 1371
- ↵ Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ & Wolf PA (1994) Independent Risk Factors for Atrial Fibrillation in a population-based cohort. The Framinham Heart Study JAMA 271 : 840 – 844
- ↵ Wolf PA, Abbott RD & Kannel WB (1991) Atrial Fibrillation as an independent risk factor for Stroke: the Framingham Study Stroke 22 : 983 – 988
- ↵ Burchfiel CM, Curb JD, Rodriguez BL, Abbott RD, Chiu D & Yano K (1994) Glucose Intolerance and 22-year stroke incidence. The Honolulu Heart Program Stroke 25 : 951 – 957
- ↵ Shinton R & Beevers G (1989) Meta-Analysis of Relation between cigarette Smoking and Stroke BMJ 298 ( 6676 ): 789 – 94
- ↵ Camargo CA (1989) Moderate Alcohol Consumption and Stroke. The Epidemiologic Evidence Stroke 20 : 1611 – 1626
- ↵ Kasner S, Chimowitz M,. Lynn M, Howlett-Smith H, Stern B, Hertzberg V, Frankel M, Levine S, Chaturvedi S, Benesch C, Sila C, Jovin T, Romano J, & Cloft H, for the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators (2006) Predictors of Ischemic Stroke in the Territory of a Symptomatic Intracranial Arterial Stenosis Circulation 113 : 555 – 563
- ↵ Broderick JP (1994) Intracerebral Haemorrhage. In: Gorelick PB & Alter M Handbook of Neuroepidemiology
- ↵ Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS & Singer DE (2004) Advanced age, anticoagulant intensity, and risk of intracranial hemorrhage among patients taking warfarin for atrial fibrillation Ann. Intern. Med. 141 ( 10 ): 745 – 52
- ↵ Lin CH, Shimizu Y, Kato H, Robertson TL, Furonaka H, Kodama K & Fukunaga Y (1984) Cerebrovascular diseases in a fixed population of Hiroshima and Nagasaki, with special reference to relationship between type and risk factors Stroke 15 : 653 – 660
- ↵ Kothari R, Hall K, Brott T & Broderick J (1997) Early stroke recognition: developing an out-of-hospital NIH Stroke Scale. Acad Emerg Med . 4 : 986 – 990
- ↵ Kidwell CS, Starkman S, Eckstein M, Weems K & Saver JL (2000) Identifying stroke in the field. Stroke . 31 : 71 – 76 .
- ↵ Truwit CL, Barkovich AJ, Gean-Marton A, Hibri N & Norman D (1990) Loss of insular ribbon: another early CT sign of acute middle cerebral artery infarction Radiology 176 : 801 – 806
- ↵ Garg N, Eshkar N, Tanenbaum L, Cohen B & Sen S (2004) Computed Tomography Angiographic Correlates of Early Computed Tomography Signs in Acute Ischaemic Stroke J. Neuroimaging 14 : 242 – 245
- ↵ Von Kummer V, Meyding-Lamade U, Forsting M, et al. (1994) Sensitivity and prognostic value of early CT in occlusion of middle cerebral artery trunk Am J Neuroradiol 15 : 9 – 15 .
- ↵ Moulin T, Cattin F, Crepin-Leblond T, et al. (1996) Early CT signs in acute middle cerebral artery infarction: predictive value for subsequent infarct locations and outcome. Neurology 47 : 366 – 375 .
- ↵ Koenig M, Kraus M, Theek C, Klotz E, Gehlen W & Heuser L. (2001) Quantitative assessment of the ischemic brain by means of perfusion related parameters derived from perfusion CT. Stroke 32 : 431 – 437 .
- ↵ Tomandl BF, Klotz E, Handschu R, et al. (2003) Comprehensive imaging of ischemic stroke with multisection CT. Radiographics 23 : 565 – 592 .
- ↵ Teksam M, Cakir B & Coskun M (2005) CT perfusion imaging in the early diagnosis of acute stroke Diagn Interv Radiol . 11 ( 4 ): 202 – 5
- ↵ Flacke S, Urbach H, Keller E, Traber F, Hartmann A, Textor J, Gieseke J, Block W, Folkers P & Schild HH (2000) Middle Cerebral Artery (MCA) Susceptibility Sign at Susceptibility-based Perfusion MR Imaging: Clinical Importance and Comparison with Hyperdense MCA Sign at CT Radiology 215 : 476 – 482
- ↵ Shrier DA, Tanaka H, Numaguchi Y, Konno S, Patel U & Shibata D (1997) CT angiography in the evaluation of acute stroke AJNR Am J Neuroradiol . 18 ( 6 ): 1011 – 20
- ↵ Choksi V, Quint DJ, Maly-Sundgren P, Hoeffner E. Appl Radiol 2005 ; 34 : 10 – 9 . OpenUrl

EDWIN Y. CHOI, MD, MS, GILBERTO A. NIEVES, MD, AND DARRELL EDWARD JONES, DO
Am Fam Physician. 2022;105(6):616-624
Author disclosure: No relevant financial relationships.
Stroke accounts for significant morbidity and mortality and is the fifth leading cause of death in the United States, with direct and indirect costs of more than $100 billion annually. Expedient recognition of acute neurologic deficits with appropriate history, physical examination, and glucose testing will help diagnose stroke and rule out mimicking presentations. The National Institutes of Health Stroke Scale should be used to determine stroke severity and to monitor for evolving changes in clinical presentation. Initial neuroimaging is used to differentiate between ischemic and hemorrhagic stroke or other pathologic processes. If a stroke is determined to be ischemic within four and a half hours of last known well or baseline state, determining the patient's eligibility for the administration of intravenous recombinant tissue plasminogen activator is necessary to proceed with informed decision-making for diagnostic workup and appropriate treatment options. Additional evaluation with specialized magnetic resonance imaging studies can help determine if patients can receive recombinant tissue plasminogen activator within nine hours of last known well. Subarachnoid hemorrhage should be considered in the differential diagnosis if the patient presents with rapid onset of severe headache. If radiographic imaging is negative for hemorrhage when there is high suspicion for delayed presentation of stroke, a lumbar puncture should be considered for further evaluation. Patients with cerebellar symptoms should be evaluated with a HINTS (head-impulse, nystagmus, test of skew) examination because it is more sensitive for cerebellar stroke than early magnetic resonance imaging. Additional cerebrovascular imaging should be considered in patients with large vessel occlusions presenting within 24 hours of last known well to assess benefits of endovascular interventions. Once initial interventions have been implemented, poststroke evaluations such as telemetry, echocardiography, and carotid imaging should be performed as clinically indicated to determine the etiology of the stroke.
Risk Factors
Classification of strokes.
Stroke classification is based on the underlying pathologic process and vascular distribution. Appropriate classification of a stroke assists in formulating definitive treatment decisions and communicating potential short- and long-term prognoses. In the United States, approximately 87% of all strokes are ischemic, 10% are intracerebral hemorrhages, and 3% are subarachnoid hemorrhages. 1
An ischemic stroke is defined as an episode of neurologic dysfunction secondary to a focal CNS infarction. A silent ischemic infarction is defined as imaging or neuropathologic evidence of an infarction without neurologic dysfunction.
Intracerebral and spontaneous subarachnoid hemorrhages are defined as rapidly developing neurologic dysfunction secondary to the focal accumulation of blood in the brain parenchyma and in the subarachnoid space, respectively, and are not precipitated by trauma. A silent cerebral hemorrhage is similar to a silent ischemic stroke in that both are without clinical symptoms. Imaging shows a focal collection of blood within the brain parenchyma or ventricular system, often presenting in the form of microhemorrhages. 2
Clinical Diagnosis
Standardized stroke scoring systems should be used to determine severity of injury and prognosis. The National Institutes of Health Stroke Scale (NIHSS) is the most widely used clinical tool 7 ( Table 2 8 ) . NIHSS scores generally reflect the degree of stroke severity: mild (8 or less), moderate (9 to 15), and severe (16 or more). 9 , 10 Stroke scales allow for standardization of communication between clinicians, hospitals, and health care systems to provide an objective means of monitoring for evolving changes in clinical presentation.
Initial Evaluation
An appropriate workup should be done immediately following history and physical examination to rule out certain conditions that can present similarly to stroke. Stroke mimics include encephalopathies, infection, electrolyte disturbances, psychogenic conditions, and toxicities 11 ( Table 3 8 , 11 ) . The national standard for identifying stroke mimics is less than 3%; however, they can be identified in a range as low as 1% to as high as 25% based on hospital registries, reflecting that mimics may be difficult to differentiate from acute stroke at presentation. 12 Pending diagnostic studies for stroke mimics should not delay initiation of rtPA in eligible patients because the risk of symptomatic intracranial hemorrhage is low. 8 , 9
Subarachnoid hemorrhage should be considered in patients with sudden onset of a severe atraumatic headache that is described as the worst headache of their life. 19 A lumbar puncture should be considered for further evaluation, especially in patients who present six hours after headache onset, because subarachnoid hemorrhage can be missed by NCCT and MRI. The Ottawa Subarachnoid Hemorrhage Rule for Headache Evaluation can be used to rule out subarachnoid hemorrhage with 100% sensitivity in patients 15 years and older who present with new severe atraumatic headache with maximum intensity within one hour. With any positive findings (i.e., age 40 years and older; neck pain or stiffness; witnessed loss of consciousness; onset during exertion; thunderclap headache [pain peaking within one second]; and limited neck flexion), subarachnoid hemorrhage cannot be ruled out. 19 – 21
In patients with cerebellar symptoms that are concerning for posterior circulation stroke ( Table 6 5 ) , such as gait ataxia, limb ataxia, or vertigo, a HINTS (head-impulse, nystagmus, test of skew) examination should be performed to determine a CNS vs. peripheral cause. It is more sensitive for stroke than early MRI, with a sensitivity of 100% and a specificity of 96%. 22 – 24 A diffusion-weighted MRI is the preferred imaging study, but a negative early MRI may need to be repeated in three to seven days or followed up with a HINTS examination 5 , 17 , 22 ( Table 7 5 , 22 – 25 ) . A video demonstrating the HINTS examination is available at https://www.youtube.com/watch?v=1q-VTKPweuk .
Large Vessel Occlusion
Recent data from multiple randomized trials have shown that patients with large vessel occlusion have significantly better outcomes with endovascular treatment. 9 , 18 , 26 – 28 Patients presenting within 24 hours with severe neurologic deficits thought to be caused by large vessel occlusions of the internal carotid arteries or proximal middle cerebral arteries should undergo noninvasive cerebrovascular imaging with computed tomographic angiography (CTA) or magnetic resonance angiography (MRA). This allows for the evaluation of ischemia and large vessel occlusion and assessment of the ratio of potentially reversible ischemic tissue to the volume of infarcted tissue to determine eligibility for mechanical thrombectomy. 9 , 18 , 29 Evaluation with noninvasive imaging has a sensitivity of 87% to 100% and specificity of more than 95% for detecting large vessel occlusions, with CTA having greater accuracy than MRA. 8 , 9 Magnetic resonance transcranial Doppler ultrasonography is less sensitive than angiography (46% to 60%) but can inform improved treatment outcomes of rtPA therapy. 8 , 9
Digital subtraction angiography (conventional angiography) is the diagnostic standard for determining large vessel involvement. 8 Neurologic complications, including stroke, increase with invasive imaging, but based on a large prospective study the risk is about 1%. 30 Intra-arterial treatments include mechanical thrombectomy, which is performed with a stent retriever or an aspiration catheter, or administration of intra-arterial fibrinolysis. Both approaches may be used in combination. The success of mechanical clot removal depends on determining the length of the clot.

Poststroke Evaluation
When the initial evaluation is completed, whether or not the patient received rtPA therapy, a full evaluation is necessary to determine the etiology of the stroke. If rtPA was administered, diffusion-weighted MRI should be performed before starting anticoagulants or antiplatelet agents. CTA or MRA is an appropriate imaging technique to evaluate for carotid artery stenosis, but digital subtraction angiography is the diagnostic standard. 8 Carotid Doppler ultrasonography has less sensitivity (83% to 86%) than any radiologic evaluation. CTA has more than 90% sensitivity for carotid artery stenosis. Echocardiography should be performed to evaluate for a cardiac source of embolic strokes. In patients 18 to 60 years of age, echocardiography with bubble study should be used to determine if there is an anomalous opening between the right and left side of the heart, such as a patent foramen ovale, for consideration of closure of the abnormality. The RESPECT trial showed a significant reduction in recurrent ischemic strokes with patent foramen ovale closures. 32
Transesophageal echocardiography is more sensitive than transthoracic echocardiography, but it is also more invasive. Transesophageal echocardiography should be performed in patients who have a high probability of a cardiac source of embolism, are younger than 45 years without cardiac disease, have mechanical valves, or have suspected aortic pathology. 33 All patients with stroke should be placed on telemetry while in the hospital to evaluate for paroxysmal atrial fibrillation. Two studies have shown that 10% to 15% of patients with a diagnosis of stroke, not otherwise specified without atrial fibrillation who were on 24 hours of telemetry were diagnosed with paroxysmal atrial fibrillation on a 30-day loop recorder in the following six months after their stroke, further supporting the need for long-term monitoring for arrhythmia in older, high-risk individuals following unspecified strokes. 34 , 35
This article updates previous articles on this topic by Yew and Cheng 5 and Yew and Cheng . 36
Data Sources: A PubMed search was completed in Clinical Queries using the key terms stroke, stroke evaluation, AAN guidelines, stroke imaging, post stroke care, stroke subtypes, stroke differential, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also used search results from the Cochrane database, Essential Evidence Plus, UpToDate, and Dynamed. Search dates: March 15, 2021, and April 10, 2022.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army, Department of Defense, or the U.S. government.
- Virani SS, Alonso A, Aparicio HJ, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743.
- Sacco RL, Kasner SE, Broderick JP, et al.; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke . 2019; 50(8): e239]. Stroke. 2013;44(7):2064-2089.
- Price AJ, Wright FL, Green J, et al. Differences in risk factors for 3 types of stroke: UK prospective study and meta-analyses. Neurology. 2018;90(4):e298-e306.
- Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77(13):1222-1228.
Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91(8):528-536. Accessed January 3, 2022. https://www.aafp.org/afp/2015/0415/p528.html
Shah KH, Edlow JA. Transient ischemic attack: review for the emergency physician. Ann Emerg Med. 2004;43(5):592-604.
- Muir KW, Weir CJ, Murray GD, et al. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996;27(10):1817-1820.
- Jauch EC, Saver JL, Adams HP, et al.; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke . 2019;50(12):e440–e441]. Stroke. 2019;50(12):e344-e418.
- Muchada M, Rubiera M, Rodriguez-Luna D, et al. Baseline National Institutes of Health stroke scale-adjusted time window for intravenous tissue-type plasminogen activator in acute ischemic stroke. Stroke. 2014;45(4):1059-1063.
Hosseininezhad M, Sohrabnejad R. Stroke mimics in patients with clinical signs of stroke. Caspian J Intern Med. 2017;8(3):213-216.
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al.; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke . 2016;47(11):e262]. Stroke. 2016;47(2):581-641.
- Kernan WN, Ovbiagele B, Black HR, et al.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke . 2015;46(2):e54]. Stroke. 2014;45(7):2160-2236.
National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. NICE guideline [NG128]. May 1, 2019. Accessed March 15, 2022. https://www.nice.org.uk/guidance/ng128
- Ramos-Pachón A, López-Cancio E, Bustamante A, et al. d-dimer as predictor of large vessel occlusion in acute ischemic stroke. Stroke. 2021;52(3):852-858.
Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9(11):1085-1096.
- Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: a joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117-E127.
Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. 2021;325(11):1088-1098.
- Perry JJ, Stiell IG, Sivilotti MLA, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA. 2013;310(12):1248-1255.
Suarez JI. Diagnosis and management of subarachnoid hemorrhage. Continuum (Minneap Minn). 2015;21(5 neurocritical care):1263-1287.
Long B, Koyfman A, Runyon MS. Subarachnoid hemorrhage: updates in diagnosis and management. Emerg Med Clin North Am. 2017;35(4):803-824.
- Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20(10):986-996.
Hankey GJ, Blacker DJ. Is it a stroke?. BMJ. 2015;350:h56.
- Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40(11):3504-3510.
Newman-Toker DE. 3-Component H.I.N.T.S. battery. Accessed May 24, 2021. http://content.lib.utah.edu/cdm/singleitem/collection/ehsl-dent/id/6/rec/5
- Berkhemer OA, Fransen PSS, Beumer D, et al.; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke [published correction appears in N Engl J Med . 2015; 372(4): 394]. N Engl J Med. 2015;372(1):11-20.
- Campbell BCV, Mitchell PJ, Kleinig TJ, et al.; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018.
- Goyal M, Demchuk AM, Menon BK, et al.; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030.
- Powers WJ, Derdeyn CP, Biller J, et al.; American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020-3035.
- Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227(2):522-528.
- Mokin M, Primiani CT, Siddiqui AH, et al. ASPECTS (Alberta Stroke Program Early CT Score) measurement using hounsfield unit values when selecting patients for stroke thrombectomy. Stroke. 2017;48(6):1574-1579.
- Collado FMS, Poulin MF, Murphy JJ, et al. Patent foramen ovale closure for stroke prevention and other disorders. J Am Heart Assoc. 2018;7(12):e007146.
- Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45(2):520-526.
- Sanna T, Diener HC, Passman RS, et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-2486.
Thijs V. Atrial fibrillation detection: fishing for an irregular heartbeat before and after stroke. Stroke. 2017;48(10):2671-2677.
- Yew KS, Cheng E. Acute stroke diagnosis. Am Fam Physician. 2009;80(1):33-40. Accessed January 3, 2022. https://www.aafp.org/afp/2009/0701/p33.html
Continue Reading

More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Find in topic
RELATED TOPICS
INTRODUCTION
While more common in older adults, stroke also occurs in neonates, infants, children, and young adults, resulting in significant morbidity and mortality.
An overview of the presentation, evaluation, and diagnosis of arterial ischemic stroke in children one month of age or older is provided here.
Other aspects of ischemic stroke in children and young adults are reviewed elsewhere:
Ischemic stroke in children and young adults: Epidemiology, etiology, and risk factors Ischemic stroke in children: Management and prognosis Hemorrhagic stroke in children Cerebral venous thrombosis: Etiology, clinical features, and diagnosis Stroke in the newborn: Classification, manifestations, and diagnosis
CLINICAL PRESENTATION
Infants and young children with stroke may present with focal weakness, but are more likely than older children to present with seizures and altered mental status [ 1,2 ]. Older children usually have hemiparesis or other focal neurologic signs such as aphasia, visual disturbance, or cerebellar signs, although seizures, headache, and lethargy are more common among children than adults [ 3-7 ].
- Research article
- Open access
- Published: 07 August 2019
Risk factors, clinical presentations and predictors of stroke among adult patients admitted to stroke unit of Jimma university medical center, south west Ethiopia: prospective observational study
- Ginenus Fekadu 1 ,
- Legese Chelkeba 2 &
- Ayantu Kebede 3
BMC Neurology volume 19 , Article number: 187 ( 2019 ) Cite this article
23k Accesses
32 Citations
1 Altmetric
Metrics details
Stroke is the second-leading global cause of death behind heart disease in 2013 and is a major cause of permanent disability. The burden of stroke in terms of mortality, morbidity and disability is increasing across the world. It is currently observed to be one of the commonest reasons of admission in many health care setups and becoming an alarming serious public health problem in our country Ethiopia. Despite the high burden of strokes globally, there is insufficient information on the current clinical profile of stroke in low and middle income countries (LMICs) including Ethiopia. So, this study was aimed to assess risk factors, clinical presentations and predictors of stroke subtypes among adult patients admitted to stroke unit of Jimma university medical center (JUMC).
Prospective observational study design was carried out at stroke unit (SU) of JUMC for 4 consecutive months from March 10–July 10, 2017. A standardized data extraction checklist and patient interview was used to collect data. Data was entered into Epi data version 3.1 and analyzed using SPSS version 20. Multivariable logistic regression was used to identify the predictors of stroke subtypes.
A total of 116 eligible stroke patients were recruited during the study period. The mean age of the patients was 55.1 ± 14.0 years and males comprised 62.9%. According to world health organization (WHO) criteria of stroke diagnosis, 51.7% of patients had ischemic while 48.3% had hemorrhagic stroke. The most common risk factor identified was hypertension (75.9%) followed by family history (33.6%), alcohol intake (22.4%), smoking (17.2%) and heart failure (17.2%). The most common clinical presentation was headache complained by 75.0% of the patients followed by aphasia 60.3% and hemiparesis 53.4%. Atrial fibrillation was the independent predictor of hemorrhagic stroke (AOR: 0.08, 95% CI: 0.01–0.68).
The clinical characteristics of stroke in this set up were similar to other low- and middle-resource countries. As stroke is a high priority chronic disease, large-scale public health campaign should be launched focusing on public education regarding stroke risk factors and necessary interventions.
Peer Review reports
Stroke is acute clinical event of focal or global neurological disturbance related to impairment of cerebral circulation, which lasts longer than 24 h resulting in death with no known cause other than vascular origin. Without blood to supply oxygen and to remove waste products, brain cells quickly begin to die [ 1 , 2 , 3 , 4 ]. Stroke is the second-leading global cause of death behind heart disease in 2013 and is a major cause of permanent disability [ 5 , 6 , 7 ]. Currently, the burden of stroke in terms of mortality, morbidity and disability is increasing across the world [ 8 , 9 ]. Additionally, data from Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) of 2010 revealed that stroke is the leading cardiovascular disease (CVD) which causes mortality and disability in sub-Saharan Africa (SSA) and other low and middle income countries (LMICs) [ 10 ].
Risk factors for stroke can be classified as modifiable and non-modifiable. Age, sex, family history and race/ethnicity are non-modifiable risk factors; while hypertension, smoking, diet, and physical inactivity are among some of identified modifiable risk factors [ 11 ]. Different risk factors apply to an African population in the development of stroke [ 12 ]. Africa might be increasingly affected by high burden of stroke and other vascular diseases due to health transitions in line with ever-changing social, economic and demographic patterns [ 13 ]. Additionally, the poor are increasingly affected by stroke, which can be attributable to the changing population exposures to risk factors and inability to afford the high cost of stroke care [ 14 ]. Yet, only little data about context-specific risk factors for prioritizing interventions to reduce the stroke burden in sub-Saharan Africa is available [ 15 , 16 ].
Compared to developed countries, the percentage of hemorrhagic stroke (HS) mortality rate was higher in SSA and other LMICs [ 10 , 17 , 18 ]. There have been variations in the prevalence of major risk factors among the stroke subtypes, demonstrating that knowledge of pathophysiology is crucial for the right management and care of the patients [ 19 ]. In addition to highest burden of stroke risk factors in LMICs, the racial or genetic factors also plays key roles in the pathogenesis of stroke. For example, hypertension and diabetes mellitus (DM) appear to be more prevalent among black races as compared to white races [ 17 ]. Currently even though several modifiable risk factors are becoming significant, hypertension is still the most common risk factor globally including our country [ 20 ].
Stroke is currently observed to be one of the commonest reasons of admission in many health care setups and becoming an alarming serious public health problem in our country Ethiopia [ 21 , 22 ]. Under-diagnosing of hypertension and other risk factors, delayed presentation to the hospital, poor risk factors control and failure to adhere to the treatments are some of the major challenges that needs to be addressed [ 21 , 23 ]. Etiologic investigation for stroke was infrequently performed due to lack of systematic cardiological examinations and brain imaging, most of the time for economic reasons and unavailability of the instruments [ 24 ]. The findings of the studies done in Ethiopia frequently changed from one another with respect to various demographic profiles, location and risk factors [ 21 ]. Most of the data’s regarding stroke that used in the management, follow-up and prevention of stroke come from studies in developed countries [ 22 ]. Thus, in our country we haven’t pooled data on prevalence, risk factors and outcome of the stroke.
The shortage of data specific to the Ethiopian setting limits the formulation of well-designed response and management of stroke [ 21 ]. So it is imperative that a lot has to be done to overcome the current challenges concerning the risk factors and clinical profile of stroke in Ethiopia [ 22 ]. Hence this study will generate evidences for improving the prevention strategy of stroke and guide health authorities to halt or reduce the devastating effects of stoke at different sectors of our community by having overview knowledge of clinical characteristics of stroke. This study data was part of huge study project done in stroke unit (SU) of Jimma university medical center (JUMC) with novel and extensive findings focusing on stroke. Hence, this study was aimed to assess risk factors, clinical presentation and predictors of stroke subtypes among adult patients admitted to SU of JUMC.
Since this data was part of study previously described by Fekadu etal [ 24 ], we have used similar methods. Additionally, the study participants in this finding share similarity with previously published articles of the same study project. Prospective observational study design was conducted at SU of JUMC located at south-west Ethiopia for 4 consecutive months from March 10–July 10, 2017. All adult patients (> 18 years) diagnosed to had stroke clinically or by brain imaging and admitted to SU of JUMC during the study period were included. Those not willing to give an informed consent, died before evaluation, changed diagnosis of stroke, transformed stroke and with hematomas were excluded [ 23 , 24 ].
Data collection tool and procedure
Data collection was carried out by two trained nurses and one internal medicine resident. Data collectors collect data using interviewer administered questionnaire and standardized data extraction form from the case records of the patients. Data collection tool (Additional file 1 ) was developed based on the previous study findings done at different sites and using the WHO step wise approach to stroke surveillance [ 25 ]. The necessary history used for the study was taken from the patient and/or caregivers by the language they understood. To ensure quality of data, the data abstraction tool was developed in English, translated to local language (Amharic and Afan Oromo) and back translated into English to check its consistency. The data collection form was used to collect data on the sociodemographic characteristics, clinical characteristics of patients such as risk factors, clinical presentation and subtypes of stroke.
Data processing and analysis
The data was entered to Epidata version 3.1 and analyzed using statistical package for the social sciences (SPSS) version 20. Descriptive statistics such as proportions, means, standard deviations, medians and Interquartile ranges were calculated to describe the independent variables. During candidate selection because of adequate significant variables were obtained at P < 0.05, it was considered as cut off point for candidate selection for multivariable logistic regression analysis model with backward stepwise approach to identify the independent predictors of stroke subtypes. The data was summarized using odds ratio (OR) and 95% confidence interval. Confidence interval which doesn’t contain 1 and predictor variables with p value less than 0.05 was considered statistically significant.
Operational definition
Alcohol abuse/ consumption: on average ≥ 2 drinks/day for males and ≥ 1 drinks for females (previous drinker: ex drinker for more than 1 year) [ 26 ].
Diabetes mellitus: If the patient was previously on oral hypoglycemic agents/insulin treatment or had the diagnosis of any type of DM or FBS ≥ 126 mg/dl or had a documented RBS ≥ 200 mg/dl or glycosylated hemoglobin of ≥6.5% [ 7 , 27 , 28 , 29 ]
Dyslipidemia or hyperlipidemia: Previous had history of hyperlipidemia or using lipid lowering medication or total cholesterol ≥200 mg/dl, LDL cholesterol ≥100 mg/dl, and HDL-cholesterol < 40 mg/dl for men or < 50 mg/dl for women, and/or serum triglyceride level ≥ 150 mg/dl [ 27 , 30 ].
Hypertension: Previously receiving antihypertensive medication or when the patient was previously diagnosed with hypertension or detecting blood pressure of ≥ 140/90 mm/Hg for two measurements [ 7 , 27 , 28 , 29 ].
Obesity: According to the WHO, Body Mass Index (BMI) ≥ 30 kg/m 2 [ 28 ].
Central obesity: Waist circumference greater than 102 cm in men and 88 cm in women [ 28 ].
Smoker: On average 2 cigarettes per day in men and 1 per day in women
Former smoker: who abstained from smoking for greater than 1 years [ 31 ].
Current smoker: smoking within 1 year ago [ 31 ].
One hundred twenty five patients were admitted to SU of JUMC with suspected diagnosis of stroke and 9 patients were excluded from the study during the study period. From 116 study participants included in the study; history was obtained solely from 11 patients (9.5%), from the patient and caregiver in 50 cases (43.1%), and solely from caregivers in 55 cases (47.4%). According to WHO criteria 51.7% patients had ischemic type of stroke (IS) while 48.3% had hemorrhagic stroke (HS). Of the total 116 patients, 61 patients evaluated with CT scan of the brain and the rest 55 patients were evaluated clinically to have stroke [ 24 ].
Patient characteristics
The mean age of the patients was 55.1 ± 14.0 years and 65 (56.0%) were in age of group of 45–65 years. Males comprised of 73 (62.9%) with male: female ratio of 1.70:1. Majority of the participants (42.2%) had informal education and 85.3% of patients were independent at home during pre-stroke. Majority of the patients had normal mean body mass index (BMI) (63.8%) and 15.5% of the patients were overweight [ 23 ]. Regarding the food habit of the patients during the pre-stroke, 81.9% were mixed diet users ( Table 1 ).
Risk factors for stroke subtypes
Risk factors were identified in 114 (98.3%) patients; 59 (98.3%) of IS and 55 (98.2%) of HS patients. The most common risk factor identified was hypertension in 88 (75.9%) patients followed by family history in 39 (33.6%), alcohol intake 26 (22.4%) and smoking 20 (17.2%). Thirty six patients (83.7%) of IS and 34 (75.6%) of HS patients had a pre-stroke knowledge of being hypertensive. Twenty eight patients (24.1%) had no current and previous history of hypertension [17 (28.3%) of IS and 11(19.6%) of HS patients] ( Table 2 ).
About 18 (20.5%) of the patients had no prior knowledge of being hypertensive, but diagnosed in hospital during admission for stroke. From 46 patients with no previous history of hypertension including newly diagnosed, 19 (41.3%) were never had their blood pressure measured and the remaining measured but was in normal range.
Among the patients with recorded history of hypertension, the median duration of hypertension prior to stroke diagnosis was 3 years (ranged 0.04 to 25 years). Of 70 patients with pre-existing hypertension, 27 (38.6%) were on anti-hypertensive medications, 24 (34.3%) of the patients were discontinued their antihypertensive medication and 19 (27.1%) hadn’t started antihypertensive medication before stroke occurrence. From 51 patients previously started antihypertensive medication the median duration since the medication started was 3 years. From the 27 patients that were on antihypertensive medication during hospital arrival, 19 (70.4%) of the patients’ blood pressure was not controlled. The median month since discontinuation of their antihypertensive medications before onset of stroke was 2.5 months (ranged 0.5 to 48 months).
Diabetes Mellitus was identified as co-morbidity in 8 patients (4 of them previously diagnosed). It was more prevalent in males and in middle age group, but there was no statistically significant difference between stroke subtypes ( p = 0.178). Among the patients with previous history of diabetes, the mean duration of diabetes prior to stroke was 5.3 years (ranged 3 to 9 years). Although all previously diagnosed patients were on anti-diabetics, only 1 patient’s blood glucose was controlled (RBS ≤ 200 mg/dl) during hospital arrival.
Physical inactivity/ sedentary life was detected in 13 (11.2%) patients, the remaining patients had habit of physical activity. Of those who had physical activity, 101 (98.1%) had work related aerobic physical activity and 2 (1.9%) had aerobic/planned physical activity. From nine patients (7.8%) who gave a history of previous stroke, eight of them were ischemic stroke patients. From those patients who had previous history of stroke, one patient had history of hypertension for 25 years.
Alcohol consumption and cigarette smoking was less prevalent in IS than HS patients, which was statistically significant among smokers ( p = 0.038). Majority of the patients who used alcohol were former drinker before 1 year (84.6%), but no difference in smoking status of the patients between the current and previous smokers. Seventy stoke patients (83.6%) had two or more risk factors for stroke, while 17 (14.7%) had one identified risk factor. In addition 17 (14.7%) patients had more than five identified risk factors. With this, the average risk factor for the patient was 3.38 (ranged 0 to 9) risk factors.
C linical presentation of stroke patients
The most common clinical presentation was headache complained by 87 (75.0%) patients followed by aphasia 70 (60.3%) and hemiparesis 62 (53.4%). Most of ischemic stroke patients presented with headache (71.7%), aphasia (60.0%) and facial palsy (58.3%). Similarly, the common clinical presentations among hemorrhagic stroke patients was headache (78.6%) followed by aphasia (60.7%) and vomiting (57.1%) ( Table 3 ).
Hemorrhagic stroke patients were more likely to be presented with coma ( P = 0.033), vomiting ( P = 0.028) and neck stiffness ( p = 0.015), but ischemic stroke patients were more likely presented with chest pain ( p = 0.016). In other clinical presentations there was no statistical significant difference between stroke subtypes. The average clinical presentation per patient was 6 (ranged from 2 to 12).
Predictors of stroke subtypes
Using P < 0.05 for candidate variable selection for predictors of stroke subtypes on binary logistic regression; atrial fibrillation, heart failure, previous stroke, coronary disease, smoking, migraine/headache and previous situation of hypertension management were selected to be included in multivariable logistic regression. Up on multivariable logistic regression only atrial fibrillation (AOR: 0.08; 95% CI: 0.01–0.68, P: 0.021) was the independent predictor for hemorrhagic stroke. Patients having atrial fibrillation were 0.08 times less likely experience hemorrhagic stroke than ischemic stroke ( Table 4 ).
This study data was drawn from the huge study project done on stroke in SU of JUMC. The study populations participated in this finding share similarity with previously published articles of the same project [ 23 , 24 ]. Even though this study shared similarity and textual overlap in the method and the socio-demographic part with previous findings, this finding provides advance and unique contribution over the previous published studies by exploring the risk factors and clinical presentation of stroke.
The mean age of the patients (55.1 ± 14.0 years), was in line with other studies carried out in developing countries including Ethiopia [ 29 , 32 , 33 , 34 , 35 , 36 ], but lower compared to studies by Tirschwell et al. and Sagui et al. [ 37 , 38 ]. In developing countries like Ethiopia, stroke occurs a few years earlier as compared to developed countries. This disagreement may be due to liability of hospital based studies to selection bias, demographic differences (differences in birth rates and survival into old age) and poor risk factor control. Thus community based studies are required to clearly find out and compare incidence as well as prevalence of stroke by age in our area. Young stroke (< 45 Years) comprised of more than one fifth (22.4%) of all patients similar to study in other part of Ethiopia [ 36 ], but higher than study in Gujarat, Nigeria and other parts of Ethiopia [ 21 , 22 , 32 , 39 ].
The higher percentage of stroke in male patients over females was in line with other previous studies [ 14 , 17 , 29 , 30 , 39 ]. The possible reason may be increased risk factors such as cigarette smoking and alcohol consumption among males. In addition, there is no vascular protection of endogenous estrogens in males. This was unlike to some studies where female patients were dominant [ 13 , 22 ]; may be due high use of contraception, pregnancy related disorders and migraine causing stroke among females in those studies. In our study finding majority of the patients were rural residents. Contrary to this, findings by Gebremariam et al. [ 21 ] and Greffie et al. [ 22 ] showed that majority of the patients were from urban areas. It is clear that hospital-based cohorts differ in the type of persons that come to the hospital. The location and catchment area of the hospital determines category of patients visiting the hospital. Additionally, cities and rural regions may differ in age constituencies. The high burden of stroke in rural population may also be due to reduced awareness and poor control of risk factors.
Majority of the patients were farmers (37.9%) and housewives (35.3%), which correlates with the study in Nigeria [ 40 ], but contrary to studies in Zambia and Vietnam [ 37 , 41 ]. Lack of information, ignorance of the risk factors and inability to manage such risk factors might be responsible to this effect. Even when the patients understand the risk factors, they may not accept them to be the cause for stroke nor be able to afford the cost of medications. Additionally, since managing risk factors of stroke require longer period or may be life time; most patients failed to adhere and follow it properly. The above causes might have contributed in many directions to the high prevalence of stroke among peoples with lower educational level including housewives and farmers.
In this study majority of the patients (63.8%) had normal BMI and only 15.5% of the patients were overweight. Majority of the patients in developing countries had low or normal BMI because of low economic status and have increased labor related physical activities. Compared to normal weight patients, obese and overweight patients are susceptible to develop a stroke. This may be associated with increased risk factors, insulin resistance, pro-thrombotic state, excessive secretion of free fatty acids, release of excitatory amino acids and sympathetic nervous system activation. This directly or indirectly related to thrombotic and coagulation adverse events thereby reducing the functional outcome and may result in catabolic imbalance. At the same time, immobilization in obese patients can impair the post stroke recovery and outcome.
The most common risk factor identified was hypertension in 75.9%, consistent with other findings as uncontrolled hypertension is the most important risk factor for stroke both in developing and developed countries [ 12 , 13 , 29 , 30 , 32 , 36 , 37 ]. This trend may reflect poor community awareness, health practices and access to healthcare including different patient related factors. Even when blacks are treated for hypertension, they are less likely than white races to be adhere with treatments given for them. This leads us to believe that hypertension is underdiagnosed and less treated in our study community due to lack of an active screening program, failure to take routine blood pressure measurements, poor medical history taking and poor follow up of the patients. Additionally, adherence with long-term treatment is a great challenge to achieve the optimum outcome as uncomplicated hypertension is usually asymptomatic and denial of the disease is common.
In this study, 79.5% of the hypertensive patients had a pre-stroke knowledge of being hypertensive and 27(38.6%) were on anti-hypertensive medication prior to the stroke occurrence. This was in line with study by Gebremariam et al. in which 20 (37.0%) of the patients had prescribed anti-hypertensive medication prior to the stroke occurrence [ 21 ]. But it was in contrary to study by Watila et al. [ 32 ] in which more than half of the patients had no prior knowledge of being hypertensive and only small proportion of patients had treatment for hypertension prior to having a stroke. The median duration of hypertension prior to stroke was 3 years, in line with previous study in Ethiopia by Gebremariam et al. [ 21 ].
From patients who were on antihypertensive medication during hospital arrival, in majority of the patients’ blood pressure was not controlled (≥ 140/90 mmHg). Poor control of blood pressure is associated with adherence problem, lack of frequent monitoring, cost issue for medications and transportation for follow up. The proportion of patients that never had their blood pressure measured was lower than finding by Walker etal [ 33 ]. Most patients discontinued their antihypertensive medications by convincing themselves as they were cured or improved, because hypertension is asymptomatic disease until organ damage is evident.
Diabetes mellitus is one of the major risk factor for the development of atherosclerosis and the excess risk of stroke. It was diagnosed as co-morbidity in 8 patients, without statistically significant difference between stroke subtypes. According to study by Alemayehu et al. infarction is the most common type of stroke events in diabetic individuals (57.7%) [ 13 ]. In our study the prevalence of DM was lower compared to study by Sarkar et al. (25.9%) [ 34 ], 46.8% by De Carvalho etal [ 42 ], 23.8% by Desalu etal [ 43 ], 19.5% by Owolabi etal [ 17 ] and 10.1% by Watila etal [ 32 ]. But was closely similar with study by Deresse et al. in Ethiopia which was identified in 7.8% of stroke patients [ 29 ]. This discrepancy could be due to our small sample size, referral bias and single hospital-based design of our study. We recommend well designed multi-centered studies to quantify the risk of diabetes in Ethiopian stroke patients. The mean duration of diabetes prior to stroke was 5.3 year, that was closely correlates with study by Gebremariam etal [ 21 ].
Habituation of alcohol (22.4%) and smoking (17.2%) was higher compared to other previous studies [ 14 , 17 , 32 , 39 , 43 ]. This was mostly associated with the community in catchment area of our hospital were highly abuser of social drugs. The majority of smokers develop stroke due to smoking may predispose blood vessels to thrombosis and facilitates platelets aggregation possibly by causing an imbalance between brain vascular coagulation and abnormal fibrinolysis. This might alter the function of blood brain barrier and disrupt normal endothelial cell function. The relation between alcoholism and risk factor of stroke is more susceptible to aggravating effect which causes cardio embolism and hypertension thereby increases the risk of ischemic stroke.
In this study 12.9% of patients were previous user of diet containing low fruit and vegetable. The relation between risk of stroke and diet may be associated with increased daily total fat intake that greatly increases risk of stroke. But vegetable foods have low saturated fat and are protective for our health and organ function. Similar to previous study by Tirschwell et al. [ 37 ] cardiac disease like atrial fibrillation, coronary disease and heart failure were commonly associated with ischemic stroke than hemorrhagic strokes. Atrial fibrillation which is great source of cardioembolic stroke was diagnosed in 16.4% that was consistent with study by De Carvalho etal 14.95% [ 42 ] and Sagui etal 14.7% [ 38 ].
Up on multivariate logistic regression, atrial fibrillation was the independent predictor for hemorrhagic stroke. Patients having atrial fibrillation were less likely experience hemorrhagic stroke than ischemic stroke. From the pathophysiology of the stroke, atrial fibrillation is the most common reason for cardioembolic stroke that occludes cerebral arteries which favors ischemic stroke over hemorrhagic stroke. This finding complies with study by Atadzhanov etal in Zambia [ 16 , 41 ].
At the onset of stroke, the most common clinical presentation was headache (75.0%) followed by aphasia (60.3%) and hemiparesis (53.4%), similar finding was reported on study by Walker et al. in Gambia [ 33 ]. This finding was unlike to other studies where motor symptoms (hemiplegia/hemiparesis) were the most common clinical presentation among stroke patients [ 13 , 14 , 22 , 35 , 39 , 42 ]. The difference could be due to two major reasons. Primarily we have collected data on motor symptoms separately; hemiparesis and hemiplegia. Thus if we had collected as one category the result complies with those other previous studies, as 82.6% of patients manifest either hemiplegia/hemiparesis. Secondly even though the severity varies in degrees due to the nature of the disease most patients may complain the headache as the study was prospective with face to face interview. Initial presentation of urinary incontinence was higher (37.9%) as compared to other study by Greffie et al. [ 22 ]. Aphasia was one common presentation in this study which was less presentation as compared to other previous studies [ 14 , 22 , 39 ]. Similar to our finding, study by Kuriakose etal [ 7 ] reported that vomiting favors hemorrhagic stroke. This may be one indicator of stroke diagnosis based on clinical where brain imaging is not available. In general average clinical presentation for the patient was 6, which was higher than study in India by Kuriakose et al. in which majority of the patients had 3–4 clinical manifestation during admission [ 7 ].
Strength and limitations of the study
This study attempted to identify different risk factors related to stroke with a prospective clinical follow-up that focused on the need of preventive strategy and improvement of patient care. To ensure a uniform data collection, we ascertained consistently ascertainable risk factor identification and obtained more or less reliable information to achieve the goals of our study. More generable case ascertainment than in earlier studies, in-person health care professional assessment to verify eligibility for inclusion was addressed.
The study was associated with some limitations and drawbacks. First, this study was a hospital-based study rather than longitudinal community based study. Hence it may be subjected to referral bias, as most of the acute stroke patients’ visit our hospital only from the south western part of Ethiopia. These referral bias as well as convenience sampling approach used might not reflect the true prevalence of the stroke in the community. Even though the study was hospital based, having only one referral center might probably reflect the actual magnitude of stroke in our country.
Secondly, about half of the patients were diagnosed clinically alone to have stroke based on clinical presentations, risk profiles, disease course and other supportive investigations. Clinical way of diagnosis based on clinician judgment rather than biological may distort accuracy and reliability of the data. This may cause unintended false positive and false negative association between different variables of the study. Thus caution should be taken for the generalization of the finding for large community.
Thirdly, in our study protocol, the risk factor status was not refined sufficiently enough especially for ischemic stroke patients with cardiac cases. Even simple and inexpensive diagnostic tests like electrocardiograms (ECG) were not routinely performed. Poor risk factor identification and diagnosis may underestimate or overestimates some factors. Finally, the sample size was small hampering the analysis of some prognostic indicators due to the short recruitment period. In addition, we counted on patient reports of some of their risk factors and other patient related histories, which may introduce recall bias.
Majority of the patients were males, middle aged, rural residents, uneducated and farmers with low socioeconomic status. The increasing burden of stroke in LMICs countries like Ethiopia poses a challenge to the health care system and the community as a whole. The most common risk factor identified was hypertension and the level of poor blood pressure control in hypertensive patients we observed in this study was alarming. The most common clinical presentation was headache and motor symptoms (hemiplegia/hemiparesis). Hemorrhagic stroke patients were more likely to have coma, vomiting and neck stiffness but ischemic stroke patients were more likely presented with chest pain.
As stroke is a high priority chronic global case, large-scale community health campaign should be launched focusing on community education regarding risk factors of stroke as well as recognition of stroke-related symptoms, prognosis and outcomes. The importance of early recognition and treatment may help to improve outcomes, facilitate consistent and continuous follow up as well as with available treatment options disability can be minimized. Educational programs for front-line health-care providers, focusing on simple supportive interventions, could improve outcomes in settings where advanced diagnostics and treatment of stroke remain limited.
In addition, there should be influential contribution from every social media and political level of the country with the goal of increasing the awareness of risk factors and making the community to understand the challenging effect of the stroke on human health and economy of the country. Thus, policy makers should put strategies for screening and management of common risk factors like hypertension.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Atrial fibrillation
Body Mass Index
Diabetes Mellitus
Global Burden of Diseases
Hemorrhagic stroke
Ischemic heart Disease
Ischemic stroke
Jimma university medical center
Low and middle income countries
Sub Saharan Africa
Stroke unit
World Health Organization
Investigators WMPP. The world health organization monica project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41(2):105–14.
Article Google Scholar
National Collaborating Centre for Chronic Conditions (UK). Stroke: National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). London: Royal College of Physicians (UK); 2008. (NICE Clinical Guidelines, No. 68 (1), Introduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53302/
Google Scholar
Jowi JO, Mativo PM. Pathological sub-types, risk factors and outcome of stroke at the Nairobi hospital, Kenya. East Afr Med J. 2008;85(12):572–81.
CAS PubMed Google Scholar
Ekeh B, Ogunniyi A, Isamade E, Ekrikpo U. Stroke mortality and its predictors in a Nigerian teaching hospital. Afr Health Sci. 2015;15(1):74–81.
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet (London, England). 2014;383(9913):245–54.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, et al. Heart disease and stroke Statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360.
PubMed Google Scholar
Kuriakose C, Shifafiya MN, Tharakan NS, K S, Kumar RS. A prospective study of clinical profile of stroke in a tertiary care hospital. Asian J Pharm Clin Res. 2016;9(3):1–4.
Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–48.
Article CAS Google Scholar
Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, Barker-Collo S, Moran AE, Sacco RL, Truelsen T, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology. 2015;45(3):161–76.
Moran A, Forouzanfar M, Sampson U, Chugh S, Feigin V, Mensah G. The epidemiology of cardiovascular diseases in sub-Saharan Africa: the global burden of diseases, injuries and risk factors 2010 study. Prog Cardiovasc Dis. 2013;56(3):234–9.
Boehme AK, Esenwa C, Elkind MSV. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–95.
Zewdie A, Debebe F, Kebede S, Azazh A, Laytin A, Pashmforoosh G, Hassen GW. Prospective assessment of patients with stroke in Tikur Anbessa specialised hospital, Addis Ababa, Ethiopia. African J Emerg Med. 2018;8(1):21–4.
Alemayehu CM, Birhanesilasie SK. Assessment of stoke patients: occurrence of unusually high number of haemorrhagic stroke cases in Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Clin Med Res. 2013;2(5):94–100.
Patne SV, Chintale KN. Study of clinical profile of stroke patients in rural tertiary health care Centre. Int J Adv Med. 2016;3(3):666–70.
Owolabi MO, Sarfo F, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A, Wahab K, Obiako R, Owolabi L, Ovbiagele B, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health. 2018;6(4):e436–46.
Namale G, Kamacooko O, Kinengyere A, Yperzeele L, Cras P, Ddumba E, Seeley J, Newton R. Risk factors for hemorrhagic and ischemic stroke in sub-Saharan Africa. J Trop Med. 2018;2018:11.
Owolabi MO, Agunloye AM. Which risk factors are more associated with ischemic rather than hemorrhagic stroke in black Africans? Clin Neurol Neurosurg. 2013;115(10):2069–74.
Owolabi MO, Akarolo-Anthony S, Akinyemi R, Arnett D, Gebregziabher M, Jenkins C, Tiwari H, Arulogun O, Akpalu A, Sarfo FS, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26(2):S27–38.
Al-Hashel JY, Al-Sabah AA, Ahmed SF, Al-Enezi M, Al-Tawheid N, Al Mesailekh Z, Eliwa J, Alroughani R. Risk factors, subtypes, and outcome of ischemic stroke in Kuwait: a National Study. J Stroke Cerebrovasc Dis. 2016;25(9):2145–52.
Abate AT, Bayu N, Mariam TG. Hypertensive Patients’ Knowledge of Risk Factors and Warning Signs of Stroke at Felege Hiwot Referral Hospital, Northwest Ethiopia: A Cross-Sectional Study. Neurol Res Int. 2019;2019:7.
Gebremariam SA, Yang HS. Types, risk profiles, and outcomes of stroke patients in a tertiary teaching hospital in northern Ethiopia. eNeurologicalSci. 2016;3:41–7.
Greffie ES, Mitiku T, Getahun S. Risk factors, clinical pattern and outcome of stroke in a referral hospital, Northwest Ethiopia. Clin Med Res. 2015;4(6):182–8.
Fekadu G, Wakassa H, Tekle F. Stroke Event Factors among Adult Patients Admitted to Stroke Unit of Jimma University Medical Center: Prospective Observational Study. Stroke Res Treat. 2019;2019:4650104 8 pages.
PubMed PubMed Central Google Scholar
Fekadu G, Chelkeba L, Melaku T, Tegene E. Pathological sub types and diagnostic protocols of stroke among adult patients admitted to Jimma University medical center, south West Ethiopia. J Neurol Neurophysiol. 2018;9(4):466.
STEPS-Stroke. The WHO step wise approach to stroke surveillance. Geneva: 2006 [cited 2017 March 28]; Available from: http://www.who.int/ncd_surveillance/en/steps_stroke_manual_v1.2.pdf .
Wu CY, Wu HM, Lee JD, Weng HH. Stroke risk factors and subtypes in different age groups: a hospital-based study. Neurol India. 2010;58(6):863–8.
Jia XY, Huang M, Zou YF, Tang JW, Chen D, Yang GM, Lu CH. Predictors of poor outcomes in first-event ischemic stroke as assessed by magnetic resonance imaging. Clin Invest Med. 2016;39(3):E95–E104.
Manorenj S, Inturi S, Jyotsna B, Sai Savya V, Areli D, Balarami Reddy O. Prevalence, pattern, risk factors and outcome of stroke in women: a clinical study of 100 cases from a tertiary care center in South India. Int J Res Med Sci. 2016;4(6):2388–93.
Deresse B, Shaweno D. Epidemiology and in-hospital outcome of stroke in South Ethiopia. J Neurol Sci. 2015;355(1–2):138–42.
Nkoke C, Lekoubou A, Balti E, Kengne AP. Stroke mortality and its determinants in a resource-limited setting: a prospective cohort study in Yaounde, Cameroon. J Neurol Sci. 2015;358(1–2):113–7.
Gezmu T, Schneider D, Demissie K, Lin Y, Gizzi MS. Risk factors for acute stroke among south Asians compared to other racial/ethnic groups. PLoS One. 2014;9(9):e108901.
Watila MM, Nyandaiti YW, Ibrahim A, Balarabe SA, Gezawa I, Bakki B, Tahir A, Sulaiman MM, Bwala SA. Risk factor profile among black stroke patients in northeastern Nigeria. Neurosci Biobehav Rev. 2012;4(5):50–8.
Walker RW, Rolfe M, Kelly PJ, George MO, James OF. Mortality and recovery after stroke in the Gambia. Stroke. 2003;34(7):1604–9.
Sarkar D, Halder S, Saha BK, Biswas P. A study of stroke patients with respect to their clinical and demographic profile and outcome. Int J Res Med Sci. 2016;4(9):4061–6.
Masood CT, Hussain M, Anis ur R, Abbasi S. Clinical presentation, risk factors and outcome of stroke at a district level teaching hospital. J Ayub Med Coll Abbottabad. 2013;25(1–2):49–51.
Bekele A, Kebede O. Stroke admission to Tikur Anbessa teaching hospital: with emphasis on stroke in the young. Ethiop J Health Dev. 2002;16(3):309–15.
Tirschwell DL, Ton TG, Ly KA, Van Ngo Q, Vo TT, Pham CH, Longstreth WT Jr, Fitzpatrick AL. A prospective cohort study of stroke characteristics, care, and mortality in a hospital stroke registry in Vietnam. BMC Neurol. 2012;12:150.
Sagui E, M'Baye PS, Dubecq C, Ba Fall K, Niang A, Gning S, Bellefleur JP, Sane M, Debonne JM. Ischemic and hemorrhagic strokes in Dakar, Senegal: a hospital-based study. Stroke. 2005;36(9):1844–7.
Vaidya CV, Majmudar DK. A retrospective study of clinical profile of stroke patients from GMERS medical college and hospital, Gandhinagar, Gujarat. Int J Clin Trials. 2014;1(2):62–6.
Njoku CH, Aduloju AB. Stroke in Sokoto, Nigeria: a five year retrospective study. Ann Afr Med. 2004;3(2):73–6.
Atadzhanov M, Mukomena P, ShabirLakhi ROA, Meschia JF. Stroke characteristics and outcomes of adult patients admitted to the university teaching hospital, Lusaka, Zambia. Open Gen Intern Med J. 2012;5:3–8.
De Carvalho JJ, Alves MB, Viana GA, Machado CB, dos Santos BF, Kanamura AH, Lottenberg CL, Neto MC, Silva GS. Stroke epidemiology, patterns of management, and outcomes in Fortaleza, Brazil: a hospital-based multicenter prospective study. Stroke. 2011;42(12):3341–6.
Desalu OO, Wahab KW, Fawale B, Olarenwaju TO, Busari OA, Adekoya AO, Afolayan JO. A review of stroke admissions at a tertiary hospital in rural southwestern Nigeria. Ann Afr Med. 2011;10(2):80–5.
Download references
Acknowledgments
We thank Jimma University for supporting the study. We are grateful to staff members of stroke unit of JUMC, data collectors and study participants for their cooperation in the success of this study.
The only funder for the study was Jimma University . The funding body did not have any role in study design, data collection, data analysis, interpretation of data or in writing the manuscript.
Author information
Authors and affiliations.
Department of Pharmacy, Institute of Health Sciences, Wollega University, P. O Box 395, Nekemte, Ethiopia
Ginenus Fekadu
School of Pharmacy, Institute of Health, Jimma University, Jimma, Ethiopia
Legese Chelkeba
Department of Epidemiology, Institute of Health, Jimma University, Jimma, Ethiopia
Ayantu Kebede
You can also search for this author in PubMed Google Scholar
Contributions
GF contributes in the design of the study, analysis, interpretation and write up of the manuscript. AK made the data analysis and interpretation of the data. LC contributed to the design of the study and edition of the manuscript. All authors critically revised the manuscript and have approved the final manuscript.
Corresponding author
Correspondence to Ginenus Fekadu .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was obtained from the Institutional Review Board (IRB) of Jimma University, Institute of health with reference number of IHRPGC/107/207. Permission was obtained from responsible bodies of the JUMC and stroke unit prior to the interview and review of the patient data. At hospital written informed consent was obtained from the study participants. All patients got the right to opt out of the research. For patients who were not of sound mind to consent; those of altered level of consciousness or severe aphasias, one of the family members or caregivers was given the written consent. This was done by explaining the objective and importance of the study as it is beneficial for patient’s quality service delivery for future encounters. The data from the case records and interview was handled with strong confidentiality. Neither the case records nor the data extracted was used for any other purpose. The confidentiality and privacy of patients was assured throughout by removing identifiers from data collection tools using different codes [ 23 , 24 ].
Consent for publication
Not applicable. No individual person’s personal details, images or videos are being used in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Data abstraction tool. (DOCX 24 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Fekadu, G., Chelkeba, L. & Kebede, A. Risk factors, clinical presentations and predictors of stroke among adult patients admitted to stroke unit of Jimma university medical center, south west Ethiopia: prospective observational study. BMC Neurol 19 , 187 (2019). https://doi.org/10.1186/s12883-019-1409-0
Download citation
Received : 21 December 2018
Accepted : 23 July 2019
Published : 07 August 2019
DOI : https://doi.org/10.1186/s12883-019-1409-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Risk factors
- Clinical presentation
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Hemorrhagic stroke.
Ajaya Kumar A. Unnithan ; Joe M. Das ; Parth Mehta .
Affiliations
Last Update: May 8, 2023 .
- Continuing Education Activity
Hemorrhagic stroke is due to bleeding into the brain by the rupture of a blood vessel. Hemorrhagic stroke may be further subdivided into intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH). Hemorrhagic stroke is associated with severe morbidity and high mortality. Progression of hemorrhagic stroke is associated with worse outcomes. Early diagnosis and treatment are essential given the usual rapid expansion of hemorrhage, causing sudden deterioration of consciousness and neurological dysfunction. This activity highlights the role of the interprofessional team in the evaluation and treatment of hemorrhagic stroke.
- Summarize the pathophysiology of hemorrhagic stroke.
- Identify the most common causes of hemorrhagic stroke and the most common site of the bleeding.
- Review the common presentations of this hemorrhagic stroke.
- CT scan is the initial investigation of choice. Prompt medical, sometimes surgical (in indicated cases), management is needed for recovery from hemorrhagic stroke.
- Introduction
Cerebrovascular accident (CVA) , otherwise called a stroke, is the third major cause of morbidity and mortality in many developed countries. Stroke can be either ischemic or hemorrhagic. Ischemic stroke is due to the loss of blood supply to an area of the brain. It is a common type of stroke.
Hemorrhagic stroke is due to bleeding into the brain by the rupture of a blood vessel. Hemorrhagic stroke may be further subdivided into intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH). ICH is bleeding into the brain parenchyma, and SAH is bleeding into the subarachnoid space. Hemorrhagic stroke is associated with severe morbidity and high mortality. [1] Progression of hemorrhagic stroke is associated with worse outcomes. Early diagnosis and treatment are essential given the usual rapid expansion of hemorrhage, causing sudden deterioration of consciousness and neurological dysfunction.
Hypertension is the most common cause of hemorrhagic stroke. [2]
- Longstanding hypertension produces degeneration of media, breakage of the elastic lamina, and fragmentation of smooth muscles of arteries.
- Lipohyalinosis, fibrinoid necrosis of the subendothelium, microaneurysms, and focal dilatations are seen in the arterioles. The microaneurysms are named as Charcot-Bouchard aneurysms.
- The common sites of hypertension-induced intracerebral hemorrhage are the small penetrating arteries originating from basilar arteries or the anterior, middle, or posterior cerebral arteries.
- Small artery branches of 50 to 700 μm in diameter often have multiple sites of rupture associated with layers of platelet and fibrin aggregates.
- Hypertensive change causes non-lobar intracranial hemorrhage (ICH). As seen in eclampsia, acute hypertension can also cause ICH, known as postpartum ICH.
Cerebral amyloid angiopathy (CAA) is an important cause of primary lobar intracerebral bleeding in older adults. [3]
- It is characterized by the deposition of the amyloid-β peptide in the capillaries, arterioles, and small- and medium-sized arteries in the cerebral cortex, leptomeninges, and cerebellum.
- This causes ICH in older adults, commonly associated with variations in the gene encoding apolipoprotein E.
- A familial syndrome can occur in young patients, typically associated with mutations in the gene encoding amyloid precursor protein.
- The incidence of CAA increases with age to the extent that around 50% of those aged more than 70years have CAA. Recurrent hemorrhages can occur due to CAA.
Other Important Risk Factors
- Cigarette smoking and moderate or heavy alcohol consumption, and chronic alcoholism are significant risk factors.
- Chronic liver disease also increases the chance of ICH due to coagulopathy and thrombocytopenia.
- Decreased low-density lipoprotein cholesterol and low triglycerides are also risk factors.
- Dual antiplatelet therapy has an increased risk of ICH than monotherapy.
- Sympathomimetics such as cocaine, heroin, amphetamine, ephedrine, and phenylpropanolamine carry an increased risk of a cerebral hemorrhage.
- Cerebral microbleeds (CMBs) associated with hypertension, diabetes mellitus, and cigarette smoking increase the risk of ICH.
- Old age and male sex. The incidence of ICH increases after 55 years of age. The relative risk after 70 years is 7.
- The tumors which are more prone to bleed are glioblastoma, lymphoma, metastasis, meningioma, pituitary adenoma, and hemangioblastoma.
The usual causes of spontaneous subarachnoid hemorrhage (SAH) are ruptured aneurysm, arteriovenous malformation, vasculitis, cerebral artery dissection, dural sinus thrombosis, and pituitary apoplexy. The risk factors are hypertension, oral contraceptive pills, substance abuse, and pregnancy.
Intracranial hemorrhage of pregnancy (ICHOP-intracerebral or subarachnoid hemorrhage) occurs with eclampsia. It is due to the loss of cerebrovascular autoregulation.
- Epidemiology
Hemorrhagic stroke contributes to 10% to 20% of strokes annually. [4] [1] [5] The percentage of hemorrhage in stroke is 8-15% in the United States of America, the United Kingdom, and Australia, and 18% to 24% in Japan and Korea. The incidence is around 12% to 15% of cases per 1,00,000 per year. The incidence is high in low and middle-income countries and Asians. The incidence is more common in men and increases with age. The global incidence is increasing, predominantly in African and Asian countries. Japanese data have shown that control of hypertension reduces the incidence of ICH. The case fatality rate is 25% to 30% in high-income countries, while it is 30% to 48% in low- to middle-income countries. The ICH fatality rate depends on the efficacy of critical care.
- Pathophysiology
The common sites of the bleed are the basal ganglia (50%), cerebral lobes (10% to 20%), the thalamus (15%), pons and the brain stem (10% to 20%), and the cerebellum(10%)(fig.1,2,3). [1] The hematoma disrupts the neurons and glia. This results in oligaemia, neuro-transmitter release, mitochondrial dysfunction, and cellular swelling. Thrombin activates microglia and causes inflammation and edema. [5] [6]
The primary injury is due to the compression of brain tissue by the hematoma and an increase in the intracranial pressure(ICP). [7]
Secondary injury is contributed to by inflammation, disruption of the blood-brain barrier (BBB), edema, overproduction of free radicals such as reactive oxygen species (ROS), glutamate-induced excitotoxicity, and release of hemoglobin and iron from the clot.
Usually, the hematoma enlarges in 3 hours to 12 hours. The enlargement of hematoma occurs in 3 hours in one-third of cases. The perihematomal edema increases within 24 hours, peaks around 5 to 6 days, and lasts up to 14 days. There is an area of hypoperfusion around the hematoma. The factors causing deterioration in ICH are an expansion of hematoma, intraventricular hemorrhage, perihematomal edema, and inflammation. [1] Cerebellar hematoma produces hydrocephalus by compression of the fourth ventricle in the early stage.
Non-aneurysmal spontaneous subarachnoid hemorrhage may be either perimesencephalic or non-perimesencephalic SAH. In perimesencephalic SAH, bleeding is mainly in the interpeduncular cistern. Physical exertion, such as the Valsalva maneuver producing increased intrathoracic pressure, and elevated intracranial venous pressure, is a predisposing factor for perimesencephalic nonaneurysmal SAH (PM-SAH). [8] There is diffuse blood distribution in non-perimesencephalic SAH (NPM-SAH). [9]
- History and Physical
The common presentations of stroke are headache, aphasia, hemiparesis, and facial palsy. [10] The presentation of hemorrhagic stroke is usually acute and progressing. Acute onset headache, vomiting, neck stiffness, increases in blood pressure, and the rapidly developing neurological signs are the common clinical manifestations of hemorrhagic stroke. [5] Symptoms can lead to the extent and location of hemorrhage.
- Headache is more common in a large hematoma.
- Vomiting indicates raised intracranial pressure and is common with cerebellar hematoma.
- Coma occurs in the involvement of the reticular activating system of the brainstem.
- Seizure, aphasia, and hemianopia are seen in lobar hemorrhage. A prodrome consisting of numbness, tingling, and weakness may also occur in lobar bleed.
- Contralateral sensorimotor deficits are the features in hemorrhage of the basal ganglia and thalamus.
- Loss of all sensory modalities is the main feature of thalamic hemorrhage.
- Extension of thalamic hematoma into the midbrain can cause vertical gaze palsy, ptosis, and unreactive pupil.
- Cranial nerve dysfunction with contralateral weakness indicates brainstem hematoma. [5]
- Usually, pontine hematoma produces coma and quadriparesis. [11]
Cerebellar hemorrhage produces symptoms of raised ICP, such as lethargy, vomiting, and bradycardia. Progressive neurological deterioration indicates the enlargement of hematoma or an increase in edema.
The clinical features of subarachnoid hemorrhage are severe headache described as a thunderclap, vomiting, syncope, photophobia, nuchal rigidity, seizures, and decreased level of consciousness. [8] [9] Signs of meningismus such as the Kernig sign (pain on straightening the knee when the thigh is flexed to 90 degrees) and Brudzinski sign (involuntary hip flexion on flexing the neck of the patient) may be positive.
Computerized tomography (CT) is usually the initial investigation. [12] The hemorrhage increases in attenuation from 30-60 Hounsfield units (HU) in the hyperacute phase to 80 to 100 HU over hours. [13] The attenuation may be decreased in anemia and coagulopathy. Vasogenic edema around the hematoma may increase for up to 2 weeks. CT is considered the “gold standard” in detecting acute hemorrhage due to its sensitivity. However, gradient echo and T2* susceptibility-weighted magnetic resonance imaging (MRI) has the same sensitivity as CT to detect acute hemorrhage. These sequences are more sensitive than CT for identification of prior hemorrhage.
In the subacute phase, the hematoma may be isodense to brain tissue, and magnetic resonance imaging (MRI) may be necessary. The volume of the hematoma can be measured by the formula AxBxC/2, where A and B are the largest diameter and the diameter perpendicular to that. [14] C is the vertical height of the hematoma. Intracerebral hemorrhage with a volume of more than 60 ml is associated with high mortality. [15] The other poor prognostic factors are hematoma expansion, intraventricular hemorrhage, infra-tentorial location, and contrast extravasation on CT scan (spot sign). [5] The paramagnetic properties of deoxyhemoglobin allow early detection of hemorrhage in MRI. [16] Gradient echo (GRE) imaging is as good as CT in detecting acute bleed. MRI can distinguish between the hemorrhagic transformation of infarct and primary hemorrhage. MRI can detect underlying causes of secondary hemorrhages, such as vascular malformations, including cavernomas, tumors, and cerebral vein thrombosis.
Extravasation of contrast in CT angiogram (CTA) indicates ongoing bleeding associated with fatality. [17] Multidetector CT angiography(MDCTA) helps rule out the causes of secondary hemorrhagic stroke such as arteriovenous malformation (AVM), ruptured aneurysm, dural venous sinus (or cerebral vein) thrombosis (DVST/CVT), vasculitis, and Moya-Moya disease. [18] (fig.4).
Certain imaging characteristics help in the differentiation of the underlying disease. [19]
- Multiple hemorrhages of different ages in parieto-occipital lobes are seen in cerebral amyloid antipathy.
- Hemorrhage in an arterial territory indicates hemorrhagic infarction.
- Multiple stages of bleed in the same hematoma with a fluid level are seen in anticoagulation-induced hemorrhages.
- A combination of small ischemic and hemorrhagic lesions indicates vasculitis.
- Hemorrhage in the presence of occlusion of arteries is the feature of Moyamoya disease.
Four-vessel digital subtraction angiography (DSA) is necessary in the case of SAH. A repeat study is needed to confirm if the DSA is negative for an aneurysm. Repeat angiography is advisable at 1-week and 6-weeks intervals.
Vascular abnormalities need to be suspected if the following findings are present on a plain CT scan:
- Subarachnoid hemorrhage
- Enlarged vessels or calcifications along the margins of the ICH
- Hyperattenuation within a dural venous sinus
- A cortical vein along the presumed venous drainage path
- Unusual hematoma shape
- Presence of edema out of proportion to the time of presumed ICH
- An unusual hemorrhage location
- Presence of other abnormal structures in the brain (like a mass) [20] [21]
An additional MRI scan will be beneficial in the following circumstances to identify a secondary cause for ICH:
- Lobar hemorrhage location
- Age <55 years, and
- No history of hypertension
Magnetic resonance venography or CT venography is indicated based on the following conditions that suggest cerebral venous thrombosis:
- Hemorrhage location
- Relative edema volume
- Abnormal signal in the cerebral sinuses
Blood investigations such as bleeding time, clotting time, platelet count, peripheral smear, prothrombin time (PT), and activated partial thromboplastin time(aPTT) will detect any abnormality of bleeding or coagulation and any hematological disorder which can cause hemorrhage. Liver function tests and renal function tests are also needed to exclude any hepatic or renal dysfunction as a cause. The investigations to rule out vasculitis are the quantitative evaluation of immunoglobulins, thyroid antibodies, rheumatoid factor, antinuclear antibodies (ANA), anti-double-stranded DNA (ds-DNA antibodies), Histon antibodies, complement, anti-Ro [SS-A] and anti-La [SS-B-] antibodies, cytoplasmic staining and perinuclear staining antineutrophil cytoplasmic antibodies (c- and pANCA), and anti-endothelial antibodies. [22]
- Treatment / Management
There are many different opinions on the treatment of hemorrhagic stroke. There are many trials on the optimal management of hemorrhagic stroke - Antihypertensive Treatment in Acute Cerebral Hemorrhage(ATACH), Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT), Factor VIIa for Acute Hemorrhagic Stroke Treatment (FAST), and Surgical Trial in Intracerebral Haemorrhage (STICH). [23] The role of surgery in hemorrhagic stroke is a controversial topic.
Blood pressure (BP) Management
BP should be reduced gradually to 150/90 mmHg using beta-blockers (labetalol, esmolol), ACE inhibitor (enalapril), calcium channel blocker (nicardipine), or hydralazine. [4] BP should be checked every 10-15 minutes. ATACH study observed a nonsignificant relationship between the magnitude of systolic blood pressure (SBP) reduction and hematoma expansion and 3-month outcome. [24] But the INTERACT study showed that early intensive BP-lowering treatment attenuated hematoma growth over 72 hours. [25] It has been found that high SBP is associated with neurological deterioration and death. [20] The American Stroke Association (ASA) recommendation is that for patients presenting with SBP between 150 and 220 mmHg, the acute lowering of SBP to 140 mmHg is safe and can improve functional outcomes. For patients presenting with SBP >220 mmHg, an aggressive reduction of BP with a continuous intravenous infusion is needed.
Management of Raised Intracranial Pressure (ICP)
The initial treatment for raised ICP is elevating the head of the bed to 30 degrees and using osmotic agents (mannitol, hypertonic saline). Mannitol 20% is given at a dose of 1.0 to 1.5 g/kg. [4] Hyperventilation after intubation and sedation to a pCO of 28 to 32 mmHg will be necessary if ICP increases further. ASA recommends monitoring ICP with a parenchymal or ventricular catheter for all patients with Glasgow coma scale (GCS) <8 or those with evidence of transtentorial herniation or hydrocephalus. [20] The ventricular catheter has the advantage of drainage of cerebrospinal fluid (CSF) in the case of hydrocephalus. The aim is to keep cerebral perfusion pressure (CPP) between 50 to 70mmHg.
Hemostatic Therapy
Hemostatic therapy is given to reduce the progression of hematoma. [4] This is especially important to reverse the coagulopathy in patients taking anticoagulants. Vitamin K, prothrombin complex concentrates (PCCs), recombinant activated factor VII (rFVIIa), fresh frozen plasma (FFP), etc., are used. [4] [20] [4] ASA recommends that patients with thrombocytopenia should receive platelet concentrate. [20] Patients with elevated prothrombin time INR should receive intravenous vitamin K and FFP or PCCs. FFP has the risk of allergic transfusion reactions. PCCs are plasma-derived factor concentrates containing factors II, VII, IX, and X. PCCs can be reconstituted and administered rapidly. The FAST trial showed that rFVIIa reduced the growth of the hematoma but did not improve survival or functional outcome. [26] rFVIIa is not recommended in unselected patients since it does not replace all clotting factors. [20]
Antiepileptic Therapy
Around 3 to 17% of patients will have a seizure in the first two weeks, and 30% of patients will show electrical seizure activity on EEG monitoring. [20] Those with clinical seizures or electrographic seizures should be treated with antiepileptic drugs. Lobar hematoma and the enlargement of hematoma produce seizures associated with neurological worsening. Subclinical seizures and non-convulsive status epileptics also can occur. C ontinuous EEG monitoring is indicated in patients with a decreased level of consciousness. Otherwise, prophylactic anticonvulsant medication is not recommended, according to ASA guidelines. [27]
The different types of surgical treatment for hemorrhagic stroke are craniotomy, decompressive craniectomy, stereotactic aspiration, endoscopic aspiration, and catheter aspiration. [4] The STICH trial showed no overall benefit from early surgery for supratentorial intracerebral hemorrhage compared with initial conservative treatment. [28] Those who have lobar hemorrhages within 1 cm of the surface of the brain and milder clinical deficits (GCS>9) may benefit from early surgery. Emergency surgical evacuation is indicated in cerebellar hemorrhage with hydrocephalus or brainstem compression.[21] Patients with cerebellar hemorrhages of >3 cm in diameter will have better outcomes with surgery. Cerebellar hematoma is evacuated by suboccipital craniectomy. Evacuation of brainstem hemorrhages can be harmful and is not recommended. A minimally invasive procedure such as stereotactic aspiration is also on trial. Hattori et al. showed in a randomized study that stereotactic evacuation is of value in patients with spontaneous putaminal hemorrhage, whose eyes will open in response to strong stimuli. [29]
Minimally invasive surgery plus recombinant tissue plasminogen activator(rt-PA) for Intracerebral Hemorrhage Evacuation (MISTIE) was a randomized, prospective trial that tested image-guided catheter-based removal of the blood clot. [30] It showed a reduction in perihematomal edema with clot evacuation.
The Clot Lysis: Evaluating Accelerated Resolution of IntraVentricularr Hemorrhage (CLEAR IVH) trial showed that low-dose rt-PA can be safely administered to stable intraventricular clots and can increase lysis rates. [31] Decompressive craniectomy and hematoma evacuation are now being done more frequently for hemorrhagic stroke. Moussa and Khedr showed the improvement in outcome gained by adding decompressive craniectomy with expansive duraplasty to the evacuation of large hypertensive hemispheric ICH in a randomized controlled trial. [32] Decompressive hemicraniectomy with hematoma evacuation is performed in patients with GCS scores of 8 or less and large hematomas with a volume greater than 60 ml (fig.5). [33] It reduces mortality and may improve functional outcomes.
Cerebroprotection
The secondary injury of hemorrhagic stroke comprises inflammation, oxidative stress, and toxicity of erythrocyte lysates and thrombin. So, strategies to reduce these are being tried. Pioglitazone, misoprostol, and celecoxib are tried to reduce inflammatory damage. Edaravone, flavanoid, and nicotinamide mononucleotide can reduce oxidative stress. The iron chelator deferoxamine is also in the experimental phase. The safety and neuroprotective efficacy of the cell membrane component citicoline (cytidine-5-diphosphocholine) has been shown in some studies. [34] Rosuvastatin, a competitive inhibitor of the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase, was associated with a better outcome in a trial. The calcium channel blocker nimodipine improves outcomes in SAH by a neuroprotective effect. [35] [35]
General Care
Good medical care, nursing care, and rehabilitation are paramount. [20] Common problems include dysphagia, aspiration, cardiac arrhythmias, stress-induced cardiomyopathy, cardiac failure, acute kidney injury, gastrointestinal bleeding, urinary tract infection, etc. Percutaneous endoscopic gastrostomy (PEG) may be needed to prevent aspiration. Screening for myocardial ischemia with electrocardiogram and cardiac enzyme testing is recommended in hemorrhagic stroke. Intermittent pneumatic compression reduces the occurrence of deep vein thrombosis, but the usefulness of elastic stockings is doubtful. Multidisciplinary rehabilitation is advised to reduce disability. Blood glucose should be monitored, and measures should be taken to prevent both hyperglycemia and hypoglycemia. [36]
- Differential Diagnosis
The differential diagnoses of hemorrhagic stroke are:
- Acute hypertensive crisis
- Pituitary apoplexy
- Cerebral venous thrombosis
- Dural sinus thrombosis
- Cervical artery dissection
- Reversible cerebral vasoconstrictive syndrome (RCVS)
- Hemorrhagic neoplasms
- Arterio-venous malformations
- Acute subdural hematoma
- Hemorrhagic infarct
Imaging studies such as CT and MRI can rule out these entities. [37]
The poor prognostic factors are coma, large hematoma with volume greater than 30 ml, intraventricular hemorrhage, posterior fossa hemorrhage, old age greater than 80 years, hyperglycemia, and chronic kidney disease. [5] Early deterioration and death are the major problems with ICH. Coma, at the time of the presentation, indicates a grave prognosis. ASA recommends that the monitoring and management of patients with ICH should be in a dedicated stroke unit. At six months, only 20 percent of patients become independent. The survivors may enter into a persistent vegetative state or locked-in syndrome in case of extensive hemispherical damage or brainstem involvement, respectively.
ICH score introduced by Hemphill et al. predicts mortality. [38] [39] The points are given as 2 points for Glasgow Coma Scale score (GCS) 3-4, 1 point for GCS 5-12, 0 points for GCS 13-15, 1 point for>80 years, 0 points for <80 years, 1 point for infratentorial location, 0 points for supratentorial location, 1 point for ICH volume >30 ml, 0 points for volume <30 ml. 1 point for intraventricular hemorrhage and 0 points for the absence of intraventricular hemorrhage. The 30-day mortality of each score is as: 0% for score 0, 13% for score 1, 26% for score 2, 72% for score 3, 97% for score 4, and 100% for scores 5 and 6.
- Complications
Complications of ICH include cerebral edema, increased intracranial pressure, hydrocephalus, seizures, venous thrombotic events, hyperglycemia, increased blood pressure, fever, and infections. [40] Patients with ICH, especially women, have a risk of thromboembolic disease. [20] Almost one-third of patients with ICH develop pulmonary complications such as pneumonia, aspiration, pulmonary edema, respiratory failure, and respiratory distress. About 4% of patients with ICH suffer cardiac complications such as myocardial infarction, atrial fibrillation, ventricular fibrillation, ventricular tachycardia, stress-induced cardiomyopathy, and acute heart failure. [41]
Vasospasm, ischemia, rebleeding, seizure, hyponatremia, and hydrocephalus are the complications of SAH. Neurogenic pulmonary edema, an increase in interstitial and alveolar fluid, commonly occurs in subarachnoid hemorrhage. [42]
- Deterrence and Patient Education
There is a chance of recurrence of ICH. Hypertension and old age are risk factors. BP should be controlled. Lifestyle modifications should be advised, including avoidance of alcohol, tobacco, and illicit drugs. Continued multidisciplinary rehabilitation should be done.
The following are the possible risk factors for a recurrent ICH:
- Lobar location of the initial ICH
- Presence and number of microbleeds on gradient echo MRI
- Ongoing anticoagulation
- Presence of apolipoprotein E epsilon 2 or epsilon 4 alleles [20]
- Enhancing Healthcare Team Outcomes
Patients with hemorrhagic stroke should be managed in a dedicated stroke unit with emergency and critical care, neurology and neurosurgery, and neuroradiology for the best outcome. The intensivists, physicians, and critical care nurses should be well versed in emergency neurological life support (ENLS). This is especially necessary in low- and middle-income countries. The surgical methods should be done and assessed in a standardized manner. More trials are needed to confirm the role and usefulness of surgical interventions. Cognitive rehabilitation therapy (CRT) also should be given to the survivors.
- Review Questions
- Access free multiple choice questions on this topic.
- Click here for a simplified version.
- Comment on this article.
The illustration shows how a hemorrhagic stroke can occur in the brain. An aneurysm in a cerebral artery breaks open, which causes bleeding in the brain. The pressure of the blood causes brain tissue death. Contributed by National Heart Lung and Blood (more...)
Fig.1. CT scan of lobar hemorrhage Owned by Dr. Ajaya Kumar A.
Fig.2. CT scan of pontine hemorrhage. Owned by Dr. Ajaya Kumar A.
Fig.3. CT scan of cerebellar hemorrhage. Owned by Dr. Ajaya Kumar A.
Fig.4. CT Angiogram of a patient with ICH Owned by Dr. Ajaya Kumar A.
Disclosure: Ajaya Kumar Unnithan declares no relevant financial relationships with ineligible companies.
Disclosure: Joe Das declares no relevant financial relationships with ineligible companies.
Disclosure: Parth Mehta declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Unnithan AKA, Das JM, Mehta P. Hemorrhagic Stroke. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- A troponin study on patients with ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage: Type II myocardial infarction is significantly associated with stroke severity, discharge disposition and mortality. [J Clin Neurosci. 2019] A troponin study on patients with ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage: Type II myocardial infarction is significantly associated with stroke severity, discharge disposition and mortality. Alkhachroum AM, Miller B, Chami T, Tatsuoka C, Sila C. J Clin Neurosci. 2019 Jun; 64:83-88. Epub 2019 Apr 20.
- Acute symptomatic seizures in intracerebral and subarachnoid hemorrhage: A population study of 19,331 patients. [Epilepsy Res. 2020] Acute symptomatic seizures in intracerebral and subarachnoid hemorrhage: A population study of 19,331 patients. Zöllner JP, Konczalla J, Stein M, Roth C, Krakow K, Kaps M, Steinmetz H, Rosenow F, Misselwitz B, Strzelczyk A. Epilepsy Res. 2020 Mar; 161:106286. Epub 2020 Feb 5.
- Review CNS-peripheral immune interactions in hemorrhagic stroke. [J Cereb Blood Flow Metab. 2023] Review CNS-peripheral immune interactions in hemorrhagic stroke. Li X, Chen G. J Cereb Blood Flow Metab. 2023 Feb; 43(2):185-197. Epub 2022 Dec 7.
- Identification of nine genes as novel susceptibility loci for early-onset ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage. [Biomed Rep. 2018] Identification of nine genes as novel susceptibility loci for early-onset ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage. Yamada Y, Kato K, Oguri M, Horibe H, Fujimaki T, Yasukochi Y, Takeuchi I, Sakuma J. Biomed Rep. 2018 Jul; 9(1):8-20. Epub 2018 May 29.
- Review Protective effects of flavonoids against intracerebral and subarachnoid hemorrhage (Review). [Exp Ther Med. 2024] Review Protective effects of flavonoids against intracerebral and subarachnoid hemorrhage (Review). Dong H, Gao X, Li H, Gao J, Zhang L. Exp Ther Med. 2024 Sep; 28(3):350. Epub 2024 Jul 4.
Recent Activity
- Hemorrhagic Stroke - StatPearls Hemorrhagic Stroke - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Release Details
Biolinerx announces oral presentation on data from phase 1 clinical trial evaluating motixafortide for cd34+ hematopoietic stem cell mobilization for gene therapies in sickle cell disease at ash 2024.
- Findings suggest motixafortide alone, and in combination with natalizumab, could support the collection of the large number of stem cells required by gene therapies for sickle cell disease within a single apheresis cycle -
- Data from proof-of-concept study shows that motixafortide was safe and well tolerated -
- Oral presentation at ASH 2024 on Saturday, December 7, 2024 in San Diego, California -
TEL AVIV, Israel and WALTHAM, Mass. , Nov. 5, 2024 /PRNewswire/ -- BioLineRx Ltd. (NASDAQ: BLRX) (TASE: BLRX), a commercial stage biopharmaceutical company pursuing life-changing therapies in oncology and rare diseases, today announced that an abstract including the initial results from a Phase 1 clinical trial evaluating motixafortide as monotherapy and in combination with natalizumab for CD34+ hematopoietic stem cell (HSC) mobilization for gene therapies in sickle cell disease (SCD) was accepted for oral presentation at the 66 th American Society of Hematology (ASH) Annual Meeting & Exposition taking place December 7-10, 2024 in San Diego, California . The proof-of-concept study, conducted in collaboration with Washington University School of Medicine in St. Louis , is exploring alternative HSC mobilization strategies that could significantly improve the treatment journey of patients with sickle cell disease seeking gene therapy.

Zachary Crees , MD, principal investigator for the trial, Division of Oncology , Washington University School of Medicine . "The findings in this trial suggest that patients with sickle cell disease given motixafortide alone, or in combination with natalizumab, could mobilize and potentially collect the number of stem cells required for approved gene therapies in a single apheresis cycle. These are encouraging findings that we look forward to presenting in greater detail at ASH 2024."
"We are encouraged by the initial findings in this Phase 1 study showing that motixafortide is safe and well-tolerated and may hold potential to improve the overall treatment process and access to gene therapy for more people with SCD," said Philip Serlin, Chief Executive Officer of BioLineRx. "We look forward to continued collaboration with Washington University on this important research and our ongoing work to develop motixafortide for the potential benefit of patients with sickle cell disease."
The Phase 1 safety and feasibility study is evaluating motixafortide (CXCR4 inhibitor) as monotherapy and in combination with natalizumab (VLA-4 inhibitor) as novel regimens to mobilize CD34+ hematopoietic stem cells for gene therapies in SCD. As reported in the abstract, five patients completed mobilization and apheresis with motixafortide alone, and four of five with motixafortide in combination with natalizumab.
Motixafortide alone, and in combination with natalizumab, were safe and well-tolerated in the trial. Common adverse events (AEs) were transient and included Grade 1-2 injection site (pruritis, tingling/pain) and systemic reactions (pruritis, hives). No Grade 4 AEs or vaso-occlusive events occurred.
Motixafortide alone, and in combination with natalizumab, resulted in robust CD34+ HSC mobilization to peripheral blood (PB). Motixafortide alone mobilized a median of 198 CD34+ cells/μl (range 77-690) to PB with median 3.49x10 CD34+ cells/kg as part of a single blood volume collection, projecting the collection of 13.9x10 6 HSCs in a normal, single-day four blood volume apheresis collection session. Motixafortide in combination with natalizumab mobilized a median of 231 CD34+ cells/μl (range 117-408), with median 4.64x10 CD34+ cells/kg collected as part of a single blood volume collection, projecting the collection of 18.6x10 6 CD34+ HSCs in a single day four blood volume apheresis collection session.
The two approved gene therapies for sickle cell disease in the U.S. require 16.5 million, and 22 million, total CD34+ HSCs, respectively. i,ii Unfortunately, granulocyte colony-stimulating factor (G-CSF), the most commonly used drug to support the collection of stem cells, is contraindicated in patients with SCD. The use of the mobilization agent plerixafor is the current standard of care for collecting HSCs for SCD gene therapies; however, plerixafor alone requires multiple mobilization attempts and often yields suboptimal HSC numbers. For some, gene therapy may be prohibitive due to the failure to obtain adequate numbers of HSCs.
In the trial, patients who underwent prior mobilization with plerixafor, experienced 2.8- fold greater HSC mobilization with motixafortide alone, and 3.2-fold greater HSC mobilization with motixafortide in combination with natalizumab compared to plerixafor.
Oral Presentation at ASH 2024 San Diego Convention Center , San Diego, California Oral Presentation Details
Session Name : 711. Cell Collection and Manufacturing of HSPCs, CAR-T Cells, and Other Cellular Therapy Products: Innovations in Mobilization, Collection, and Manufacturing for Cellular Therapies
Title: Motixafortide (CXCR4 Inhibition) Alone and in Combination with Natalizumab (VLA-4 Inhibition) As a Novel Regimen to Mobilize Hematopoietic Stem Cells for Gene Therapies in Sickle Cell Disease: A First-in-Human, Proof-of-Principle Safety and Feasibility Study
Presenter: Zachary D. Crees, MD, Division of Oncology , Washington University School of Medicine , Saint Louis , MO
Abstract ID#: 193210
Date: Saturday, December 7, 2024
Time: 12:00 PM
Location: San Diego Convention Center , Room 25
About the Clinical Trial of Motixafortide in Sickle Cell Disease (SCD) The trial ( ClinicalTrials.gov Identifier: NCT05618301 ) is a safety and feasibility study to evaluate motixafortide (CXCR4 inhibitor) as monotherapy and in combination with natalizumab (VLA-4 inhibitor) as novel regimens to mobilize CD34+ hematopoietic stem cells for gene therapies in SCD. The study enrolled five adults with a diagnosis of SCD who are receiving automated red blood cell exchanges via apheresis. The trial's primary objective is to assess the safety and tolerability of motixafortide alone and the combination of motixafortide + natalizumab in SCD patients, defined by dose-limiting toxicities. Secondary objectives include determining the number of CD34+ hematopoietic stem and progenitor cells (HSPCs) mobilized via apheresis; and determining the kinetics of CD34+ HSPC mobilization to peripheral blood in response to motixafortide alone and motixafortide + natalizumab in SCD patients.
About Sickle Cell Disease Sickle cell disease (SCD) is one of the most common genetic diseases globally, affecting millions of people throughout the world and disproportionately impacting persons of color. Sickle cell disease arises from mutations in the hemoglobin gene, ultimately leading to the production of abnormally shaped (sickle) red blood cells that tend to stick within blood vessels causing their occlusion. The clinical manifestations of SCD include anemia and blood vessel occlusion which can lead to both acute and chronic pain, as well as tissue ischemia across multiple organ systems (e.g., stroke, heart attack, respiratory failure), ultimately compromising end organ function. The cumulative impact of these complications significantly impacts morbidity and mortality for patients with SCD.
About BioLineRx BioLineRx Ltd. ( NASDAQ : BLRX ) ( TASE : BLRX ) is a commercial stage biopharmaceutical company pursuing life-changing therapies in oncology and rare diseases. The company's first approved product is APHEXDA ® ( motixafortide ) with an indication in the U.S. for stem cell mobilization for autologous transplantation in multiple myeloma . BioLineRx is advancing a pipeline of investigational medicines for patients with sickle cell disease, pancreatic cancer, and other solid tumors. Headquartered in Israel , and with operations in the U.S. , the company is driving innovative therapeutics with end-to-end expertise in development and commercialization, ensuring life-changing discoveries move beyond the bench to the bedside.
Learn more about who we are, what we do, and how we do it at www.biolinerx.com , or on Twitter and LinkedIn .
Forward Looking Statement Various statements in this release concerning BioLineRx's future expectations constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include words such as "anticipates," "believes," "could," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," and "would," and describe opinions about future events. These include statements regarding management's expectations, beliefs and intentions regarding, among other things, the potential benefits of APHEXDA, the execution of the launch of APHEXDA and the plans and objectives of management for future operations and expectations and commercial potential of motixafortide, as well as its potential investigational uses. These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of BioLineRx to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Factors that could cause BioLineRx's actual results to differ materially from those expressed or implied in such forward-looking statements include, but are not limited to: the initiation, timing, progress and results of BioLineRx's preclinical studies, clinical trials, and other therapeutic candidate development efforts; BioLineRx's ability to advance its therapeutic candidates into clinical trials or to successfully complete its preclinical studies or clinical trials; whether BioLineRx's collaboration partners will be able to execute on collaboration goals in a timely manner; whether the clinical trial results for APHEXDA will be predictive of real-world results; BioLineRx's receipt of regulatory approvals for its therapeutic candidates, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of BioLineRx's therapeutic candidates , including the degree and pace of market uptake of APHEXDA for the mobilization of hematopoietic stem cells for autologous transplantation in multiple myeloma patients; whether access to APHEXDA is achieved in a commercially viable manner and whether APHEXDA receives adequate reimbursement from third-party payors; BioLineRx's ability to establish, operationalize and maintain corporate collaborations; BioLineRx's ability to integrate new therapeutic candidates and new personnel; the interpretation of the properties and characteristics of BioLineRx's therapeutic candidates and of the results obtained with its therapeutic candidates in preclinical studies or clinical trials; the implementation of BioLineRx's business model and strategic plans for its business and therapeutic candidates; the scope of protection BioLineRx is able to establish and maintain for intellectual property rights covering its therapeutic candidates and its ability to operate its business without infringing the intellectual property rights of others; estimates of BioLineRx's expenses, future revenues, capital requirements and its needs for and ability to access sufficient additional financing, including any unexpected costs or delays in the commercial launch of APHEXDA; risks related to changes in healthcare laws, rules and regulations in the United States or elsewhere; competitive companies, technologies and BioLineRx's industry; statements as to the impact of the political and security situation in Israel on BioLineRx's business; and the impact of the COVID-19 pandemic, the Russian invasion of Ukraine , the declared war by Israel against Hamas and the military campaigns against Hamas and other terrorist organizations, which may exacerbate the magnitude of the factors discussed above. These and other factors are more fully discussed in the "Risk Factors" section of BioLineRx's most recent annual report on Form 20-F filed with the Securities and Exchange Commission on March 26, 2024 . In addition, any forward-looking statements represent BioLineRx's views only as of the date of this release and should not be relied upon as representing its views as of any subsequent date. BioLineRx does not assume any obligation to update any forward-looking statements unless required by law.
John Lacey BioLineRx [email protected]
Moran Meir LifeSci Advisors, LLC [email protected]
i LYFGENIA Prescribing Information; December 2023 . ii CASGEVY Prescribing Information; December 2023 .
Logo - https://mma.prnewswire.com/media/2154863/4547338/BioLineRx_Ltd_Logo.jpg
SOURCE BioLineRx Ltd.

Each molecule in our development pipeline is carefully selected for its potential ability to improve upon the standard of care, delivering maximum value to patients
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Atypical painful stroke presentations: A review
Michael bayat, allan bayat, rolf a blauenfeldt.
- Author information
- Article notes
- Copyright and License information
Correspondence , Michael Bayat, Department of Neurology & Centre for Rare Diseases, Aarhus University Hospital, Aarhus, Denmark. Email: [email protected]
Corresponding author.
Revised 2022 Jun 16; Received 2022 Feb 4; Accepted 2022 Jun 17; Issue date 2022 Nov.
This is an open access article under the terms of the http://creativecommons.org/licenses/by-nc-nd/4.0/ License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non‐commercial and no modifications or adaptations are made.
Stroke is a leading cause of death and disability. Some patients may present with atypical symptoms. One of the very rare presentations of stroke is initial neurogenic pain. Rare painful presentations include, amongst others, acute trigeminal neuralgia, atypical facial pain, hemi‐sensory pain, and episodic pain. Based on the available literature, the pain at presentation may be episodic, transient, or persistent, and it may herald other debilitating stroke symptoms such as hemiparesis. Pain quality is often described as burning; less often as sharp. Patients often have accompanying focal symptoms and findings on neurological examination. However, in several of the reviewed cases, these were discrete or non‐existent. In patients with pain located in the trunk and/or extremities, lesions may involve the thalamus, lateral medulla oblongata, insula, or parietal lobe. In patients with atypical facial or orbital pain (including the burning “salt and pepper” sensation), the stroke lesions are typically located in the pons. In this narrative review, we included studies/case series of patients who had pain at the time of onset, shortly before or within 24 h of stroke symptoms (on the day of admission). Cases with pain related to aortic or cervical vessel dissection, cerebral venous sinus thrombosis, subarachnoid hemorrhage, reversible cerebral vasoconstriction syndrome, and CNS vasculitis were excluded. With this review, we aim to summarize the current knowledge on stroke presenting with acute pain.
Keywords: acute pain, atypical facial pain, cerebrovascular diseases, hemi‐sensory pain, neuropathic pain, pain other than headache, strokes, trigeminal neuralgia
1. INTRODUCTION
Stroke is a leading cause of death and disability. Reperfusion therapies with intravenous thrombolysis and mechanical thrombectomy have greatly improved outcomes but the therapeutic time window remains narrow. 1 Prehospital delay and presentation outside this time window is the leading cause of not receiving reperfusion therapy 2 Causes of prehospital delay are multifactorial but impaired stroke symptom awareness and help seeking behavior in patients and missed identification of stroke by healthcare professionals may play an important role. 3 , 4 Some patients may present with atypical symptoms leading to an initial misdiagnosis, increased prehospital delay, and missed treatment with thrombolysis or thrombectomy. Stroke “chameleons” are patients with actual stroke who have atypical or unusual presentations, mimicking a non‐vascular cause. 5 Presenting as a “stroke chameleon” may be associated with a worse outcome. 6 One of the very rare presentations of stroke is initial neurogenic pain. Post‐stroke pain is well‐known amongst neurologists but pain as an initial symptom of stroke is not a well‐known entity. It has been anecdotally described but a review of painful stroke presentations has not been published previously. Acute trigeminal neuralgia, atypical facial pain, hemisensory pain, and episodic pain may be the most frequent encountered syndromes but the incidence of painful presentation in acute stroke is unknown. Awareness and knowledge of this rare stroke presentation may reduce the risk of misdiagnosis and contribute to improved patient care. With this review, we aim to summarize the current knowledge on stroke presenting with acute pain.
This article is a narrative review. We searched PubMed using MESH terms “stroke,” “acute pain,” “neuralgia,” “facial pain,” and non‐MESH terms such as “painful,” “stroke chameleon,” “atypical pain,” “hemisensory pain,” and “episodic pain.” We also identified additional studies by reviewing the bibliographies of relevant articles. We included only studies/case series of patients who had pain at the time of onset, shortly before or within 24 h of stroke symptoms (on the day of admission). Excluded studies included cases/case series of stroke with well‐known non‐neuropathic type pain such as tension type headache or pain secondary to aortic or cervical vessel dissection, cerebral venous sinus thrombosis, subarachnoid hemorrhage, reversible cerebral vasoconstriction syndrome and CNS vasculitis. Wallenberg syndrome or lateral medullary stroke is not included in detail since painful presentation in this syndrome has been previously described. Only cerebral stroke patients are included; therefore, the clinical presentation of spinal cord infarction (which can be associated with truncal pain) is not included. Central post‐stroke pain or thalamic pain syndrome is beyond the scope of this article. Table 1 shows the relevant case series. Figures were created using biorender.com .
Cases and case‐series mentioned in this article
Abbreviations: FP, Facial palsy; HA, Hemiataxia; HP, Hemiparesis; HS, Hemisensory; INO, Internuclear ophthalmoplegia; V1–V3, Branches of the trigeminal nerve.
An initial PubMed search yielded a total of 601 studies for title and abstract screening using the search terms as described above. Out of these, 582 were excluded from abstract and title screen for not meeting inclusion criteria, leaving 19 studies for full‐text screening. The included studies were 6 case series and 13 single case reports (total participants: 36). Figure 1 shows the article selection flowchart. A risk of bias assessment table is included in the Appendix S1 .
Article selection flowchart
3.1. Pain in the trunk and/or extremities
3.1.1. consider stroke in lateral medulla, thalamus, insula or parietal lobe.
Rarely, stroke patients may present with hemi‐sensory neurogenic pain. In a large illustrative case‐series of patients with pure sensory strokes ( n = 135), C.M.Fisher shortly described three patients with neurogenic pain occurring before or shortly after the occurrence of numbness. 7 The pain itself was not well described; in one patient, it was described as a “sharp pain” in the chest and the right arm immediately followed by a hemi‐sensory numbness that lasted 3 days. In another, it was described as “persistent thalamic type of pain” that occurred 10 min after appearance of right‐sided numbness. Treatment and prognosis were not elaborated. Pain as a prodromal symptom was also described in a 44 year old man who had several 1–2 min episodes of pain in the right hand and shoulder before developing a right‐sided lateral medullary stroke 4 days later. Limitations include the low number of cases, sparse clinical information and magnetic resonance imaging (MRI) not being available at the time. Similarly, Bogousslavsky et al. described a stroke patient who presented with sudden right‐sided burning pain in the arm and shoulder and ataxic hemiparesis on examination. CT imaging showed an ischemic lesion in the left thalamus. After 10 days, the pain had nearly disappeared. 8 Acute neurogenic pain can be focal and isolated to one extremity 9 , 10 or involve the entire side of the body. 11 As an example of focal pain are two cases with ischemic parietal lesions. 9 , 10 In these patients, the pain was located in the distal arm 9 and dorsum of the foot, 10 respectively. One patient had left‐sided hemiballismus‐hemichorea, left inferior quadrantanopia, left‐sided sensory deficits/tactile hemi‐extinction, and slight ataxic hemiparesis. 17 The other patient had an accompanying left leg weakness and diminished pinprick, light touch, temperature, and vibratory sensation. 10 In the aforementioned patient, the painful sensation remitted after 48 h. 9 MRI revealed an ischemic lesion in the anterior parietal cortex 9 and right parasagittal parietal cortex, 10 respectively. Complete hemisensory pain has been described in insular stroke in a patient presenting with acute onset of constant burning paresthesias in the left side of the body. The ischemic lesion was located in the right posterior insular region. The pain remitted during the course of a few weeks without medical treatment. The authors hypothesized that the painful state could be caused by a disruption of the parasylvian cortex processing of pain and temperature sensations. 11
When the pain is located in the truncal region/chest and/or the proximal part of the upper extremities, the stroke may mimic myocardial ischemia. Two case‐series have been described. Gorson et al. presented five stroke patients with acute, prominent chest discomfort at the time of presentation. The pain quality was depicted as burning dysesthesia. In four patients, the pain and paresthesias included the left arm. Three patients had accompanying neurological findings such as transient hemiparesis, diplopia, vertigo, or decreased sensory perception. Two patients had no other neurological symptoms. Brain MRI revealed ischemic lesions in the thalamus, corona radiata, and lateral medulla, respectively, in 3 patients. The remaining two patients had nonrevealing CT scans. Three patients had persistent chest dysesthesia at follow‐up (range, 6–12 months). The limitations of the study included its retrospective design, the limited number of patients and MRI not being performed in all patients. 12 In a later case series by Rebordão et al, five stroke patients with initial chest or epigastric pain as the dominant symptom at presentation in all patients. Three patients had focal sensory loss, three had nausea and vertigo, two complained of gait disturbances, and one had a syncopal episode. Neurological examination revealed neurological deficits (such as hemi‐hypoesthesia, facial palsy, hemianopia, hemiparesis, hemiataxia, or Horner syndrome) in all patients but these were underappreciated or unrecognized due to prioritization of the chest pain. Brain lesions included the cerebellar, thalamic, and lateral medullary regions. The chest pain resolved during admission in all patients The limitations of the study included its retrospective design and the limited number of patients. 13
The pain can also be episodic rather than constant. Hashimi et al. described a patient with right‐sided weakness and episodic painful burning paresthesias due to an acute left paramedian pontine infarction. The pain occurred spontaneously one to two times a day, lasted 3–4 h, was located to the medial parts of the right arm and leg and was accompanied by blood pressure surges. Pregabalin and amitriptyline caused only minimal relief but carbamazepine improved the symptoms. At 3 months follow‐up, the pain had improved significantly. 14 Similarly, Chen et al. described a 54 year old patient with a sudden onset of episodic burning pain at both mouth angles and in the left hand, occurring six to eight times a day and lasting 10–15 min which could be provoked by touching the left mouth angle. The painful episodes were accompanied by a rise in blood pressure. MRI showed a T2‐weighted hyperintensity in the left pontine tegmentum involving the left medial lemniscus and the dorsal trigeminal tract. However, the authors did not provide diffusion‐weighted imaging (DWI) images which is an important limitation. The painful episodes subsided after 1 week. 15
3.2. Atypical facial pain or trigeminal neuralgia
3.2.1. consider stroke in the pons.
Facial pain can occur weeks or months after an ischemic stroke in the parietal lobe, the somatosensory cortex, thalamus, or the medial lemniscus. Acute onset of facial pain in stroke is rare but has been previously described in lateral medullary stroke (Wallenberg's syndrome) patients. For instance, in a case series of 33 patients with Wallenberg's syndrome, facial pain was present at the onset of stroke in two patients. 26 However, a delayed post‐stroke pain syndrome remains much more common as illustrated by a series of Wallenberg's syndrome patients with post‐stroke pain syndrome (16 patients) where the mean onset of pain was 4 weeks after the stroke. 27
An atypical variant of facial pain was first described by Caplan in three patients with a burning “salt and pepper” sensation. The patients described a sensation of having salt and pepper thrown into their eyes and/or face. In two patients, the pain was bilateral but more pronounced on one side. In one patient, the feeling lasted seconds to a few minutes, recurred several times during the day and was superseded by a hemiparesis while in another patient, the pain occurred concomitantly with a hemiparesis. In all patients, the unpleasant sensation was only temporary. The location of the lesions was believed to be the paramedian part of the pons, but MRI was not available at the time. Treatment and prognosis were not elaborated. 16 Since then, several other case reports 17 , 18 with similar symptoms have been published. In several of these cases, the painful sensation heralded the subsequent development of neurological symptoms. The MRI showed paramedian pontine infarcts 17 , 18 and in one case series, 17 three out of four patients had basilar artery occlusive disease. Involvement of the trigeminothalamic tract decussation, the reticular formation adjacent to the trigeminal sensory complex or the trigeminal sensory nucleus itself was believed to be the cause of the symptoms. Since the symptoms may serve as a prelude to pontine ischemia, recognizing the symptoms, and evaluating the patient may prevent debilitating stroke symptoms.
Somewhat different transient eye and nose pain has been described in 3 other patients. All patients presented with acute unilateral sharp pain radiating from one eye to the nose. The pain lasted few minutes (5–30 min) and was shortly afterward superseded by focal neurological symptoms and deficits such as numbness and ataxic hemiparesis. In these cases, MRI also showed paramedian pontine infarctions. 19 Acute pain in the orbital region superseded by a left‐sided hemiparesis 30 min later has also been described in a patient with a paramedian pontine hemorrhage. 20 Transient burning nose and mouth pain similarly preceding a pontine infarction and “locked in” syndrome episodes has been described in a different patient. 21
A single case report of a 40 years old patient with acute onset of dental pain due to stroke has been described. The painful sensation (which was not further described) was located diffusely at the right upper and lower teeth, neurological examination showed decreased sensitivity for pain, temperature, and touch sensation in the V2 og V3 dermatomes of the right trigeminal nerve and MRI revealed an acute ischemic lesion in the lateral right side of the pons. The patient responded to carbamazepine treatment. 22 Isolated dental pain can also occur due to internal carotid artery dissections. 28
Isolated secondary trigeminal neuropathy is a rare presentation of stroke. Chronic trigeminal neuropathy 29 , 30 and trigeminal neuralgia 31 , 32 , 33 due to stroke has been described several times but acute onset of trigeminal neuralgia is an extraordinarily rare presentation. Somewhat similar to the previously mentioned case with episodic trigeminal neuralgia‐like pain, 15 a case was described by Peker et al. where a 72 year old patient developed acute trigeminal neuralgic pain in her left chin with a trigger point at the left upper lip. Neurological examination revealed hypoesthesia of the left V2 and V3 dermatomes. MRI showed a presumed ischemic lesion at the left side of the pons close to the trigeminal nucleus. The patient was treated with gabapentin with some effect. 23 In a difference case report, a patient had painful trigeminal neuralgia 9 h after the onset of facial numbness. The clinical description of the pain syndrome, treatment, and prognosis is sparse. The authors simply stated that “the characteristics of the pain were typical of classic trigeminal neuralgia” and they did not mention trigger points. MRI showed a T2 FLAIR positive lesion in the lateral pons which the authors proposed represented a stroke. However, the lesion was only slightly hyperintense on DWI sequences which makes it likely that it could have been radiological T2 shine‐through from a non‐vascular lesion. The pain was treated with carbamazepine. 24 The symptoms were presumably caused by disruption of the central trigeminal pathways and the trigeminal sensory nucleus. Stabbing headache had been described in a patient secondary to a thalamic hemorrhage. The patient had repetitive, sharp, 1–2 s paroxysms of pain in the right frontal and supraorbital region accompanied by slurred speech and gait disturbance and the MRI showed a left‐sided thalamic hemorrhage. No trigger factors were described. The pain abated within the first day of admission. 25
3.3. Pathophysiology
Pain is the unpleasant sensation associated with actual or potential tissue damage. The following is a short outline of the general pathways of pain sensation. After an initial activation of nociceptors, the stimuli are converted into electrical signals and conducted to the central nervous system via the A δ and C fibers. In the dorsal horn of the spinal cord, these fibers synapse with secondary afferent neurons and the nociceptive signals are transmitted via the spinothalamic and spinoreticular tracts after initial decussation. The spinothalamic pathway carries signals to the ventroposterolateral (VPL) nucleus and ventroposteroinferior (VPI) nucleus of the thalamus. From here, third order neurons ascend and terminate in the somatosensory cortex. There are also projections to the cingulate and insular cortices via the amygdala and the parabrachial nuclei in the dorsolateral pons and also projections to the periaqueductal gray (PAG) and rostral ventral medulla (RVM) that are involved in the descending feedback system.
Similarly, primary afferent nerve fibers carry pain signals from the free nerve endings in the face, intra‐oral structures and the dura to the spinal trigeminal nucleus. Here, the secondary axons decussate, form the ventral trigeminal lemniscus, and relay the signal to the periaqueductal gray and the ventroposteromedial (VPM) and intralaminar nuclei parts of the thalamus. Tertiary afferent fibers then relay the signal to the somatosensory cortex. 34
Neurogenic pain is defined as pain related to a dysfunction or lesion involving the peripheral or central nervous system. It is a term that encompasses neuropathic pain (related to disease or damage to the nerves), central pain or deafferentation pain (related to the loss or interruption of sensory nerve fiber transmissions). 35 Central pain is caused by a lesion of the spinal cord, brainstem, and/or brain. Causes include vascular, infectious, demyelination, traumatic, or neoplastic disorders. Common descriptions include burning, pins‐and‐needles, stabbing, shooting, or lancinating pain. It can be continuous or paroxysmal, can be provoked by touch or temperature stimuli and is often moderate to severe in intensity. Central pain is believed to be linked to lesions of the spinothalamocortical tract and a decreased sensitivity to pain and temperature is characteristic. Why some lesions cause pain while others do not, is beyond the scope of this article, but it may be related to incomplete lesion of the spinothalamic tract and residual tract pathways that help maintain the central pain. 36 , 37
4. DISCUSSION
In this article, we have reviewed several case reports and case‐series of stroke patients manifesting initially with different types of pain. Pain may rarely be the first or only symptom of stroke. As we have shown, the number of cases is low, and the incidence is likely to be low but the true incidence is unknown. Based on the available literature, the pain at presentation may be episodic, transient or persistent and it may herald other debilitating stroke symptoms such as hemiparesis. Pain quality is often described as burning; less often as sharp. Patients often have accompanying focal symptoms and findings on neurological examination. However, in several of the reviewed cases, these were discrete or non‐existent. In patients with pain located in the trunk and/or extremities, lesions may involve the thalamus, lateral medulla oblongata, insula, or parietal lobe. In patients with atypical facial or orbital pain (including the burning “salt and pepper” sensation), the stroke lesions are typically located in the pons (Figure 2 ). It seems that pain is more frequent with a lesion involving nuclear structures rather than nerve tracts. This seems reminiscent of C.M Fisher's speculation that “(…) a disturbance acting on nerve tracts is not translated into pain whereas a similar disturbance acting on nuclear masses of neurons might give rise to pain; that is, a pain pattern of impulses might arise at neuronal terminals but not along axons”. 38
Stroke localization and specific pain syndromes
In most described patients with initially persisting pain symptoms, spontaneous remission within days or weeks is typical. In the few patients where medication such as gabapentin or carbamazepine was used, symptoms tend to improve after treatment and, typically, the patients do not have debilitating chronic pain as might be seen in post‐stroke pain syndromes. However, due to the low number of cases and the heterogeneous neuroimaging findings, we cannot derive conclusions in regard to treatment and prognosis. Painful stroke presentations can be a diagnostic challenge for clinicians. Several factors may contribute to misdiagnosis and diagnostic delay in stroke cases with a painful presentation. Firstly, we believe that knowledge and awareness of painful stroke presentations is lacking. Since the vast majority of strokes present in a painless manner, clinicians may view pain as a “red flag,” potentially dissuading them from considering stroke in these situations. Secondly, while the patients often have associated neurological symptoms and/or abnormal findings on neurological examination that may allude to a stroke, this is not always the case. Thirdly, the presence of pain in the chest and epigastric region may distract a clinician and cause diagnostic delay due to the many potentially serious and life‐threatening differential diagnoses that may need ruling out first.
Admittedly, initial pain at presentation is a very rare “stroke chameleon” but awareness is important, so, that the patient may receive the proper treatment. For instance, these patients may still be eligible for thrombolysis and there is a risk that the atypical symptoms may dissuade the clinician from offering this treatment or delay treatment initiation. It seems especially important to recognize that painful symptoms can herald stroke symptoms since it presents a unique opportunity for preventing disabling stroke symptoms through a relevant clinical evaluation and treatment. In our opinion, the episodic “salt and pepper” sensation and transient eye and nose pain may be sufficiently characteristic that clinicians may recognize it as a stroke‐heralding symptom. In these situations, an admission to a stroke department or a clinical work‐up in a stroke clinic seems reasonable. We believe that MRI is a valuable diagnostic tool in patients with atypical stroke symptoms and that the threshold for performing an MRI scan should be low.
A limitation of this review is the nature of the reviewed articles—single cases and small case series—and the inherent risk of publication bias. This is a significant limitation and hinders robust conclusions; because of it we cannot estimate the true incidence of painful presentation in stroke, and we cannot provide evidence‐based recommendations on clinical diagnosis and management. Prospective studies investigating the prevalence of acute pain in stroke patients are needed.
CONFLICT OF INTEREST
Peer review.
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13666 .
Supporting information
Appendix S1
ACKNOWLEDGMENTS
Bayat, M. , Bayat, A. & Blauenfeldt, R. A. (2022). Atypical painful stroke presentations: A review. Acta Neurologica Scandinavica, 146, 465–474. 10.1111/ane.13666
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019. Dec;50(12):e344‐e418. doi: 10.1161/STR.0000000000000211 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Reiff T, Michel P. Reasons and evolution of non‐thrombolysis in acute ischaemic stroke. Emerg Med J. 2017;34(4):219‐226. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Iversen AB, Johnsen SP, Blauenfeldt RA, et al. Help‐seeking behaviour and subsequent patient and system delays in stroke. Acta Neurol Scand. 2021;144(5):524‐534. doi: 10.1111/ane.13484 [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Iversen AB, Blauenfeldt RA, Johnsen SP, et al. Understanding the seriousness of a stroke is essential for appropriate help‐seeking and early arrival at a stroke Centre: a cross‐sectional study of stroke patients and their bystanders. Eur Stroke J. 2020;5(4):351‐361. doi: 10.1177/2396987320945834 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Edlow JA, Selim MH. Atypical presentations of acute cerebrovascular syndromes. Lancet Neurol. 2011. Jun;10(6):550‐560. doi: 10.1016/S1474-4422(11)70069-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Richoz B, Hugli O, Dami F, Carron PN, Faouzi M, Michel P. Acute stroke chameleons in a university hospital: risk factors, circumstances, and outcomes. Neurology. 2015;85(6):505‐511. doi: 10.1212/WNL.0000000000001830 [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Fisher CM. Pure sensory stroke and allied conditions. Stroke. 1982;13(4):434‐447. doi: 10.1161/01.str.13.4.434 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Bogousslavsky J, Regli F. Ghika J Feldmeyer JJ. Painful Ataxic Hemiparesis Arch Neurol. 1984;41(8):892‐893. doi: 10.1001/archneur.1984.04050190102024 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Rossetti AO, Ghika JA, Vingerhoets F, Novy J, Bogousslavsky J. Neurogenic pain and abnormal movements contralateral to an anterior parietal artery stroke. Arch Neurol. 2003;60(7):1004‐1006. doi: 10.1001/archneur.60.7.1004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Saucedo MA, Francesco LD, Chertcoff A, et al. Pain as the first manifestation of an acute ischemic parietal stroke: a case report. Curr J Neurol. 2020;19(1):40‐42. [ Google Scholar ]
- 11. Bayat M, Bayat A. Letter to the editor: insular stroke presenting with acute onset of pain. J Neurol. 2018;265(6):1472‐1473. doi: 10.1007/s00415-018-8867-y [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Gorson KC, Pessin MS, DeWitt LD, Caplan LR. Stroke with sensory symptoms mimicking myocardial ischemia. Neurol. 1996;46(2):548‐551. doi: 10.1212/wnl.46.2.548 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Rebordão L, Nannoni S, Strambo D, Michel P. Stroke chameleons: acute central pain mimicking acute coronary syndrome. Eur J Neurol. 2020;27(11):2312‐2317. doi: 10.1111/ene.14457 [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Al HAM, Aaron S, Pancharatnam D. Acute onset post‐stroke pain from a paramedian pontine infarct. Neurol Asia. 2021;26(1):145‐148. [ Google Scholar ]
- 15. Chen WH, Tseng YL, Lui CC, Liu JS. Episodic pain syndrome restricted cheiro‐oral region associated with pontine lesion. Brain Inj. 2005;19(11):949‐953. doi: 10.1080/02699050500109936 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Caplan L, Gorelick P. "Salt and pepper on the face" pain in acute brainstem ischemia. Ann Neurol. 1983;13(3):344‐345. doi: 10.1002/ana.410130327 [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Conforto AB, Martin Mda G, Ciríaco JG, et al. "Salt and pepper" in the eye and face: a prelude to brainstem ischemia. Am J Ophthalmol. 2007;144(2):322‐325. doi: 10.1016/j.ajo.2007.03.030 [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Lyons LJ, Law SW, Kubie JL. "Salt and pepper" Pontine Infarct. J Neuroophthalmol. 2017;37(3):276‐280. doi: 10.1097/WNO.0000000000000437 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Doi H, Nakamura M, Suenaga T, Hashimoto S, et al. Transient eye and nose pain as an initial symptom of pontine infarction. Neurology. 2003;60(3):521‐523. doi: 10.1212/wnl.60.3.521 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Doi H, Hashimoto S, Kira J. Transient burning pain in the ipsilateral orbit as an initial manifestation of dorsal pontine hemorrhage: case report. Neurol Med Chir (Tokyo). 2009;49:206‐208. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Reutens DC. Burning oral and mid‐facial pain in ventral pontine infarction. Aust N Z J Med. 1990;20(3):249‐250. doi: 10.1111/j.1445-5994.1990.tb01030.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Goel R, Kumar S, Panwar A, Singh AB. Pontine infarct presenting with atypical dental pain: a case report. Open Dent J. 2015;9:337‐339. doi: 10.2174/1874210601509010337 eCollection 2015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Peker S, Akansel G, Sun I, Pamir NM. Trigeminal neuralgia due to pontine infarction. Headache. 2004;44(10):1043‐1045. doi: 10.1111/j.1526-4610.2004.4200_1.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Katsuno M, Teramoto AJ. Secondary trigeminal neuropathy and neuralgia resulting from pontine infarction. Stroke Cerebrovasc Dis. 2010;19(3):251‐252. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.00 [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Robbins MS. Transient stabbing headache from an acute thalamic hemorrhage. J Headache Pain. 2011;12(3):373‐375. doi: 10.1007/s10194-011-0303-y [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Sacco RL, Freddo L, Bello JA, Odel JG, Onesti ST, Mohr JP. Wallenberg's lateral medullary syndrome. Clinical‐magnetic resonance imaging correlations. Arch Neurol. 1993;50(6):609‐614. doi: 10.1001/archneur.1993.00540060049016 [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. MacGowan DJ, Janal MN, Clark WC, et al. Central poststroke pain and Wallenberg's lateral medullary infarction: frequency, character, and determinants in 63 patients. Neurology. 1997;49(1):120‐125. doi: 10.1212/wnl.49.1.120 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Roz TM, Schiffman LE, Schlossberg S. Spontaneous dissection of the internal carotid artery manifesting as pain in an endodontically treated molar. J Am Dent Assoc. 2005;136(11):1556‐1559. doi: 10.14219/jada.archive.2005.0086 [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Fukutake T, Hattori T. Trigeminal root entry zone infarct presenting as isolated trige‐minal sensory neuropathy. J Neurol. 1999;246(10):978‐979. doi: 10.1007/s004150050497 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Holtzman RN, Zablozki V, Yang WC, Leeds NE. Lateral pontine tegmental hemorrhage presenting as isolated trigeminal sensory neuropathy. Neurology. 1987;37(4):704‐706. doi: 10.1212/wnl.37.4.704 [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Balestrino M, Leandri M. Trigeminal neuralgia in pontine ischaemia. J Neurol Neurosurg Psychiatry. 1997;62(3):297‐298. doi: 10.1136/jnnp.62.3.297-a [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Golby AJ, Norbash A, Silverberg GD. Trigeminal neuralgia resulting from infarction of the root entry zone of the trigeminal nerve: case report. Neurosurgery. 1998. Sep;43(3):620‐622; discussion 622‐3. doi: 10.1097/00006123-199809000-00130 [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Kim JS, Kang JH, Lee MC. Trigeminal neuralgia after pontine infarction. Neurology. 1998;51(5):1511‐1512. doi: 10.1212/wnl.51.5.1511 [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 2018;19(8):2164. doi: 10.3390/ijms19082164 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Bowsher D. Neurogenic pain syndromes and their management. Br Med Bull. 1991;47(3):644‐666. doi: 10.1093/oxfordjournals.bmb.a072498 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259‐301. doi: 10.1152/physrev.00045.2019 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Watson JC, Sandroni P. Central neuropathic pain syndromes. Mayo Clin Proc. 2016;91(3):372‐385. doi: 10.1016/j.mayocp.2016.01.017 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Fisher CM. Is pressure on nerves and roots a common cause of pain? Trufant, Samuel a (ed ). Trans Am Neurol Assoc. 1972;97:282‐283. [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (984.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Stroke ranks as the second leading cause of death worldwide and is a major contributor to disability, thereby imposing significant economic burdens. Acute stroke can be categorized as ischemic or hemorrhagic, with some overlap in risk factors and clinical presentations; however, their management approaches differ substantially.
Acute stroke is the acute onset of focal neurological deficits in a vascular territory affecting the brain, retina, or spinal cord due to underlying cerebrovascular diseases.[1] Stroke is prevalent across patient populations and can significantly cause morbidity and mortality. Strokes are categorized as ischemic and hemorrhagic. Hemorrhagic strokes can further be classified as intracerebral ...
Classic symptoms — Knowing the signs and symptoms of a stroke can be lifesaving. Classic stroke symptoms can be recalled with the acronym BE FAST (figure 1). Each letter in the word stands for one of the things you should watch for: B alance - Difficulty standing or walking. E yes - Changes in vision.
An ischemic stroke occurs when fatty deposits, blood clots or other debris become lodged in the blood vessels in the brain. Some early research shows that COVID-19 infection may increase the risk of ischemic stroke, but more study is needed. Hemorrhagic stroke. Hemorrhagic stroke occurs when a blood vessel in the brain leaks or ruptures.
Stroke should be considered in any patient presenting with an acute neurologic deficit (focal or global) or altered level of consciousness. No historical feature distinguishes ischemic from hemorrhagic stroke, although nausea, vomiting, headache, and a sudden change in the patient's level of consciousness are more common in hemorrhagic ...
Overview of the evaluation of stroke. Literature review current through: Aug 2024. This topic last updated: Mar 23, 2023. The symptoms of brain ischemia may be transient, lasting seconds to minutes, or may persist for longer periods of time. Symptoms and signs remain indefinitely if the brain becomes irreversibly damaged and infarction occurs.
Rapid onset of neurologic deficits localized to a single cerebral arterial vascular territory is the archetypal clinical presentation of acute ischemic stroke. The blood glucose level should be ...
Admit everyone with suspected stroke directly to a hyperacute or acute stroke unit within 4 hours of presentation. Request non-enhanced CT scan as soon as possible (at most within 1 hour of arrival at hospital). Ischaemic stroke is a clinical diagnosis based on signs and symptoms.
Clinical Presentation. Clinical presentation of stroke depends on the area of the brain affected by occlusion of the arteries. AHA/ASA (American heart association/American stroke association) have popularised the FAST algorithm to diagnose stroke in the prehospital setting. FAST acronym stands for facial droop, arm weakness, slurred speech and ...
Stroke is a clinically defined syndrome of acute, focal neurological deficit attributed to vascular injury (infarction, haemorrhage) of the central nervous system. Stroke is the second leading cause of death and disability worldwide. Stroke is not a single disease but can be caused by a wide range of risk factors, disease processes and ...
Understanding stroke:Pathophysiology, presentation, and investigation. Every 45 seconds, someone in the United States has an attack of stroke. K A L Carroll and J Chataway discuss the pathology and clinical features of stroke, in the first of a two part series. Stroke is an acute neurological injury in which blood supply to a part of the brain ...
Additional tests may be indicated by clinical presentation and history. 8, 9 Table 4 outlines the appropriate initial workup for a patient with suspected stroke. 8, 13 - 15 Evaluation Tests
Secondly, about half of the patients were diagnosed clinically alone to have stroke based on clinical presentations, risk profiles, disease course and other supportive investigations. Clinical way of diagnosis based on clinician judgment rather than biological may distort accuracy and reliability of the data. This may cause unintended false ...
Clinical Presentations of Posterior Circulation Stroke. In clinical practice, not all PC stroke presentations are classic. Many patients present with signs and symptoms of multifocal PC infarctions. Moreover, the PC is rich in potential collateral support and clinical manifestations of BA ischemia may be highly variable. Symptoms associated ...
CLINICAL PRESENTATION. Infants and young children with stroke may present with focal weakness, but are more likely than older children to present with seizures and altered mental status [1,2]. Older children usually have hemiparesis or other focal neurologic signs such as aphasia, visual disturbance, or cerebellar signs, although seizures ...
Table 3 Clinical presentations of stroke subtypes among adult patients admitted to stroke unit of JUMC from March 10-July 10, 2017 Full size table Hemorrhagic stroke patients were more likely to be presented with coma ( P = 0.033), vomiting ( P = 0.028) and neck stiffness ( p = 0.015), but ischemic stroke patients were more likely presented ...
The common presentations of stroke are headache, aphasia, hemiparesis, and facial palsy. The presentation of hemorrhagic stroke is usually acute and progressing. ... Fekadu G, Chelkeba L, Kebede A. Risk factors, clinical presentations and predictors of stroke among adult patients admitted to stroke unit of Jimma university medical center, south ...
Clinical presentation. Regarding the clinical presentation of VB stroke, in a clinical series of 407 patients, the most common symptoms experienced by patients were dizziness (47%), unilateral limb weakness (41%), dysarthria (31%), headache (28%), vomiting and nausea (27%); as for clinical signs, the most frequent were unilateral limb weakness ...
The clinical manifestations of SCD include anemia and blood vessel occlusion which can lead to both acute and chronic pain, as well as tissue ischemia across multiple organ systems (e.g., stroke, heart attack, respiratory failure), ultimately compromising end organ function.
Abstract. Stroke is a leading cause of death and disability. Some patients may present with atypical symptoms. One of the very rare presentations of stroke is initial neurogenic pain. Rare painful presentations include, amongst others, acute trigeminal neuralgia, atypical facial pain, hemi‐sensory pain, and episodic pain.