
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.

13.1 Formatting a Research Paper
Learning objectives.
- Identify the major components of a research paper written using American Psychological Association (APA) style.
- Apply general APA style and formatting conventions in a research paper.
In this chapter, you will learn how to use APA style , the documentation and formatting style followed by the American Psychological Association, as well as MLA style , from the Modern Language Association. There are a few major formatting styles used in academic texts, including AMA, Chicago, and Turabian:
- AMA (American Medical Association) for medicine, health, and biological sciences
- APA (American Psychological Association) for education, psychology, and the social sciences
- Chicago—a common style used in everyday publications like magazines, newspapers, and books
- MLA (Modern Language Association) for English, literature, arts, and humanities
- Turabian—another common style designed for its universal application across all subjects and disciplines
While all the formatting and citation styles have their own use and applications, in this chapter we focus our attention on the two styles you are most likely to use in your academic studies: APA and MLA.
If you find that the rules of proper source documentation are difficult to keep straight, you are not alone. Writing a good research paper is, in and of itself, a major intellectual challenge. Having to follow detailed citation and formatting guidelines as well may seem like just one more task to add to an already-too-long list of requirements.
Following these guidelines, however, serves several important purposes. First, it signals to your readers that your paper should be taken seriously as a student’s contribution to a given academic or professional field; it is the literary equivalent of wearing a tailored suit to a job interview. Second, it shows that you respect other people’s work enough to give them proper credit for it. Finally, it helps your reader find additional materials if he or she wishes to learn more about your topic.
Furthermore, producing a letter-perfect APA-style paper need not be burdensome. Yes, it requires careful attention to detail. However, you can simplify the process if you keep these broad guidelines in mind:
- Work ahead whenever you can. Chapter 11 “Writing from Research: What Will I Learn?” includes tips for keeping track of your sources early in the research process, which will save time later on.
- Get it right the first time. Apply APA guidelines as you write, so you will not have much to correct during the editing stage. Again, putting in a little extra time early on can save time later.
- Use the resources available to you. In addition to the guidelines provided in this chapter, you may wish to consult the APA website at http://www.apa.org or the Purdue University Online Writing lab at http://owl.english.purdue.edu , which regularly updates its online style guidelines.
General Formatting Guidelines
This chapter provides detailed guidelines for using the citation and formatting conventions developed by the American Psychological Association, or APA. Writers in disciplines as diverse as astrophysics, biology, psychology, and education follow APA style. The major components of a paper written in APA style are listed in the following box.
These are the major components of an APA-style paper:
Body, which includes the following:
- Headings and, if necessary, subheadings to organize the content
- In-text citations of research sources
- References page
All these components must be saved in one document, not as separate documents.
The title page of your paper includes the following information:
- Title of the paper
- Author’s name
- Name of the institution with which the author is affiliated
- Header at the top of the page with the paper title (in capital letters) and the page number (If the title is lengthy, you may use a shortened form of it in the header.)
List the first three elements in the order given in the previous list, centered about one third of the way down from the top of the page. Use the headers and footers tool of your word-processing program to add the header, with the title text at the left and the page number in the upper-right corner. Your title page should look like the following example.

The next page of your paper provides an abstract , or brief summary of your findings. An abstract does not need to be provided in every paper, but an abstract should be used in papers that include a hypothesis. A good abstract is concise—about one hundred fifty to two hundred fifty words—and is written in an objective, impersonal style. Your writing voice will not be as apparent here as in the body of your paper. When writing the abstract, take a just-the-facts approach, and summarize your research question and your findings in a few sentences.
In Chapter 12 “Writing a Research Paper” , you read a paper written by a student named Jorge, who researched the effectiveness of low-carbohydrate diets. Read Jorge’s abstract. Note how it sums up the major ideas in his paper without going into excessive detail.

Write an abstract summarizing your paper. Briefly introduce the topic, state your findings, and sum up what conclusions you can draw from your research. Use the word count feature of your word-processing program to make sure your abstract does not exceed one hundred fifty words.
Depending on your field of study, you may sometimes write research papers that present extensive primary research, such as your own experiment or survey. In your abstract, summarize your research question and your findings, and briefly indicate how your study relates to prior research in the field.
Margins, Pagination, and Headings
APA style requirements also address specific formatting concerns, such as margins, pagination, and heading styles, within the body of the paper. Review the following APA guidelines.
Use these general guidelines to format the paper:
- Set the top, bottom, and side margins of your paper at 1 inch.
- Use double-spaced text throughout your paper.
- Use a standard font, such as Times New Roman or Arial, in a legible size (10- to 12-point).
- Use continuous pagination throughout the paper, including the title page and the references section. Page numbers appear flush right within your header.
- Section headings and subsection headings within the body of your paper use different types of formatting depending on the level of information you are presenting. Additional details from Jorge’s paper are provided.

Begin formatting the final draft of your paper according to APA guidelines. You may work with an existing document or set up a new document if you choose. Include the following:
- Your title page
- The abstract you created in Note 13.8 “Exercise 1”
- Correct headers and page numbers for your title page and abstract
APA style uses section headings to organize information, making it easy for the reader to follow the writer’s train of thought and to know immediately what major topics are covered. Depending on the length and complexity of the paper, its major sections may also be divided into subsections, sub-subsections, and so on. These smaller sections, in turn, use different heading styles to indicate different levels of information. In essence, you are using headings to create a hierarchy of information.
The following heading styles used in APA formatting are listed in order of greatest to least importance:
- Section headings use centered, boldface type. Headings use title case, with important words in the heading capitalized.
- Subsection headings use left-aligned, boldface type. Headings use title case.
- The third level uses left-aligned, indented, boldface type. Headings use a capital letter only for the first word, and they end in a period.
- The fourth level follows the same style used for the previous level, but the headings are boldfaced and italicized.
- The fifth level follows the same style used for the previous level, but the headings are italicized and not boldfaced.
Visually, the hierarchy of information is organized as indicated in Table 13.1 “Section Headings” .
Table 13.1 Section Headings
| Level of Information | Text Example |
|---|---|
| Level 1 | |
| Level 2 | |
| Level 3 | |
| Level 4 | |
| Level 5 |
A college research paper may not use all the heading levels shown in Table 13.1 “Section Headings” , but you are likely to encounter them in academic journal articles that use APA style. For a brief paper, you may find that level 1 headings suffice. Longer or more complex papers may need level 2 headings or other lower-level headings to organize information clearly. Use your outline to craft your major section headings and determine whether any subtopics are substantial enough to require additional levels of headings.
Working with the document you developed in Note 13.11 “Exercise 2” , begin setting up the heading structure of the final draft of your research paper according to APA guidelines. Include your title and at least two to three major section headings, and follow the formatting guidelines provided above. If your major sections should be broken into subsections, add those headings as well. Use your outline to help you.
Because Jorge used only level 1 headings, his Exercise 3 would look like the following:
| Level of Information | Text Example |
|---|---|
| Level 1 | |
| Level 1 | |
| Level 1 | |
| Level 1 |
Citation Guidelines
In-text citations.
Throughout the body of your paper, include a citation whenever you quote or paraphrase material from your research sources. As you learned in Chapter 11 “Writing from Research: What Will I Learn?” , the purpose of citations is twofold: to give credit to others for their ideas and to allow your reader to follow up and learn more about the topic if desired. Your in-text citations provide basic information about your source; each source you cite will have a longer entry in the references section that provides more detailed information.
In-text citations must provide the name of the author or authors and the year the source was published. (When a given source does not list an individual author, you may provide the source title or the name of the organization that published the material instead.) When directly quoting a source, it is also required that you include the page number where the quote appears in your citation.
This information may be included within the sentence or in a parenthetical reference at the end of the sentence, as in these examples.
Epstein (2010) points out that “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive” (p. 137).
Here, the writer names the source author when introducing the quote and provides the publication date in parentheses after the author’s name. The page number appears in parentheses after the closing quotation marks and before the period that ends the sentence.
Addiction researchers caution that “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive” (Epstein, 2010, p. 137).
Here, the writer provides a parenthetical citation at the end of the sentence that includes the author’s name, the year of publication, and the page number separated by commas. Again, the parenthetical citation is placed after the closing quotation marks and before the period at the end of the sentence.
As noted in the book Junk Food, Junk Science (Epstein, 2010, p. 137), “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive.”
Here, the writer chose to mention the source title in the sentence (an optional piece of information to include) and followed the title with a parenthetical citation. Note that the parenthetical citation is placed before the comma that signals the end of the introductory phrase.
David Epstein’s book Junk Food, Junk Science (2010) pointed out that “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive” (p. 137).
Another variation is to introduce the author and the source title in your sentence and include the publication date and page number in parentheses within the sentence or at the end of the sentence. As long as you have included the essential information, you can choose the option that works best for that particular sentence and source.
Citing a book with a single author is usually a straightforward task. Of course, your research may require that you cite many other types of sources, such as books or articles with more than one author or sources with no individual author listed. You may also need to cite sources available in both print and online and nonprint sources, such as websites and personal interviews. Chapter 13 “APA and MLA Documentation and Formatting” , Section 13.2 “Citing and Referencing Techniques” and Section 13.3 “Creating a References Section” provide extensive guidelines for citing a variety of source types.
Writing at Work
APA is just one of several different styles with its own guidelines for documentation, formatting, and language usage. Depending on your field of interest, you may be exposed to additional styles, such as the following:
- MLA style. Determined by the Modern Languages Association and used for papers in literature, languages, and other disciplines in the humanities.
- Chicago style. Outlined in the Chicago Manual of Style and sometimes used for papers in the humanities and the sciences; many professional organizations use this style for publications as well.
- Associated Press (AP) style. Used by professional journalists.
References List
The brief citations included in the body of your paper correspond to the more detailed citations provided at the end of the paper in the references section. In-text citations provide basic information—the author’s name, the publication date, and the page number if necessary—while the references section provides more extensive bibliographical information. Again, this information allows your reader to follow up on the sources you cited and do additional reading about the topic if desired.
The specific format of entries in the list of references varies slightly for different source types, but the entries generally include the following information:
- The name(s) of the author(s) or institution that wrote the source
- The year of publication and, where applicable, the exact date of publication
- The full title of the source
- For books, the city of publication
- For articles or essays, the name of the periodical or book in which the article or essay appears
- For magazine and journal articles, the volume number, issue number, and pages where the article appears
- For sources on the web, the URL where the source is located
The references page is double spaced and lists entries in alphabetical order by the author’s last name. If an entry continues for more than one line, the second line and each subsequent line are indented five spaces. Review the following example. ( Chapter 13 “APA and MLA Documentation and Formatting” , Section 13.3 “Creating a References Section” provides extensive guidelines for formatting reference entries for different types of sources.)

In APA style, book and article titles are formatted in sentence case, not title case. Sentence case means that only the first word is capitalized, along with any proper nouns.
Key Takeaways
- Following proper citation and formatting guidelines helps writers ensure that their work will be taken seriously, give proper credit to other authors for their work, and provide valuable information to readers.
- Working ahead and taking care to cite sources correctly the first time are ways writers can save time during the editing stage of writing a research paper.
- APA papers usually include an abstract that concisely summarizes the paper.
- APA papers use a specific headings structure to provide a clear hierarchy of information.
- In APA papers, in-text citations usually include the name(s) of the author(s) and the year of publication.
- In-text citations correspond to entries in the references section, which provide detailed bibliographical information about a source.
Writing for Success Copyright © 2015 by University of Minnesota is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.

- MJC Library & Learning Center
- Research Guides
Format Your Paper & Cite Your Sources
- Harvard Style
- Citing Sources
- Avoid Plagiarism
- MLA Style (8th/9th ed.)
- APA Style, 7th Edition
- Chicago Style
What is Harvard Style?
What you need to know, harvard style tutorial.
- Other Styles
- Annotated Bibliographies
- How to Create an Attribution
Harvard Style
The Harvard referencing system is known as the Author-Date style . It emphasizes the name of the creator of a piece of information and the date of publication, with the list of references in alphabetical order at the end of your paper.
Unlike other citation styles, there is no single, definitive version of Harvard Style. Therefore, you may see a variation in features such as punctuation, capitalization, abbreviations, and the use of italics.
Always check with your instructor and follow the rules he or she gives you.
- Harvard Style Guidelines Your class handout
- Harvard Referencing Quick Guide From Staffordshire University
Harvard Style will affect your paper in two places:
- In-text citations in the body of your paper, and
- The reference list at the end of your paper
- All in-text citations should be listed in the reference list at the end of your paper.
- Reference list entries need to contain all the information that someone reading your paper would need in order to find your source.
- Reference lists in Harvard Style are arranged alphabetically by first author.
- Begin your Reference list on a new page after your text and number it consecutively.
Sample References List:

Click on the Links Below to See Additional Examples:
- Sample Paper Paper provided by Kurt Olson
- Harvard Citation Examples Document created by The University of Western Australia
Click on the image below to launch this tutorial that was created by the University of Leeds. The section on Citing in Text is especially useful.

- << Previous: Chicago Style
- Next: Other Styles >>
- Last Updated: Jul 10, 2024 5:03 PM
- URL: https://libguides.mjc.edu/citeyoursources
Except where otherwise noted, this work is licensed under CC BY-SA 4.0 and CC BY-NC 4.0 Licenses .
- Privacy Policy

Home » Research Paper Format – Types, Examples and Templates
Research Paper Format – Types, Examples and Templates
Table of Contents

Research paper format is an essential aspect of academic writing that plays a crucial role in the communication of research findings . The format of a research paper depends on various factors such as the discipline, style guide, and purpose of the research. It includes guidelines for the structure, citation style, referencing , and other elements of the paper that contribute to its overall presentation and coherence. Adhering to the appropriate research paper format is vital for ensuring that the research is accurately and effectively communicated to the intended audience. In this era of information, it is essential to understand the different research paper formats and their guidelines to communicate research effectively, accurately, and with the required level of detail. This post aims to provide an overview of some of the common research paper formats used in academic writing.
Research Paper Formats
Research Paper Formats are as follows:
- APA (American Psychological Association) format
- MLA (Modern Language Association) format
- Chicago/Turabian style
- IEEE (Institute of Electrical and Electronics Engineers) format
- AMA (American Medical Association) style
- Harvard style
- Vancouver style
- ACS (American Chemical Society) style
- ASA (American Sociological Association) style
- APSA (American Political Science Association) style
APA (American Psychological Association) Format
Here is a general APA format for a research paper:
- Title Page: The title page should include the title of your paper, your name, and your institutional affiliation. It should also include a running head, which is a shortened version of the title, and a page number in the upper right-hand corner.
- Abstract : The abstract is a brief summary of your paper, typically 150-250 words. It should include the purpose of your research, the main findings, and any implications or conclusions that can be drawn.
- Introduction: The introduction should provide background information on your topic, state the purpose of your research, and present your research question or hypothesis. It should also include a brief literature review that discusses previous research on your topic.
- Methods: The methods section should describe the procedures you used to collect and analyze your data. It should include information on the participants, the materials and instruments used, and the statistical analyses performed.
- Results: The results section should present the findings of your research in a clear and concise manner. Use tables and figures to help illustrate your results.
- Discussion : The discussion section should interpret your results and relate them back to your research question or hypothesis. It should also discuss the implications of your findings and any limitations of your study.
- References : The references section should include a list of all sources cited in your paper. Follow APA formatting guidelines for your citations and references.
Some additional tips for formatting your APA research paper:
- Use 12-point Times New Roman font throughout the paper.
- Double-space all text, including the references.
- Use 1-inch margins on all sides of the page.
- Indent the first line of each paragraph by 0.5 inches.
- Use a hanging indent for the references (the first line should be flush with the left margin, and all subsequent lines should be indented).
- Number all pages, including the title page and references page, in the upper right-hand corner.
APA Research Paper Format Template
APA Research Paper Format Template is as follows:
Title Page:
- Title of the paper
- Author’s name
- Institutional affiliation
- A brief summary of the main points of the paper, including the research question, methods, findings, and conclusions. The abstract should be no more than 250 words.
Introduction:
- Background information on the topic of the research paper
- Research question or hypothesis
- Significance of the study
- Overview of the research methods and design
- Brief summary of the main findings
- Participants: description of the sample population, including the number of participants and their characteristics (age, gender, ethnicity, etc.)
- Materials: description of any materials used in the study (e.g., survey questions, experimental apparatus)
- Procedure: detailed description of the steps taken to conduct the study
- Presentation of the findings of the study, including statistical analyses if applicable
- Tables and figures may be included to illustrate the results
Discussion:
- Interpretation of the results in light of the research question and hypothesis
- Implications of the study for the field
- Limitations of the study
- Suggestions for future research
References:
- A list of all sources cited in the paper, in APA format
Formatting guidelines:
- Double-spaced
- 12-point font (Times New Roman or Arial)
- 1-inch margins on all sides
- Page numbers in the top right corner
- Headings and subheadings should be used to organize the paper
- The first line of each paragraph should be indented
- Quotations of 40 or more words should be set off in a block quote with no quotation marks
- In-text citations should include the author’s last name and year of publication (e.g., Smith, 2019)
APA Research Paper Format Example
APA Research Paper Format Example is as follows:
The Effects of Social Media on Mental Health
University of XYZ
This study examines the relationship between social media use and mental health among college students. Data was collected through a survey of 500 students at the University of XYZ. Results suggest that social media use is significantly related to symptoms of depression and anxiety, and that the negative effects of social media are greater among frequent users.
Social media has become an increasingly important aspect of modern life, especially among young adults. While social media can have many positive effects, such as connecting people across distances and sharing information, there is growing concern about its impact on mental health. This study aims to examine the relationship between social media use and mental health among college students.
Participants: Participants were 500 college students at the University of XYZ, recruited through online advertisements and flyers posted on campus. Participants ranged in age from 18 to 25, with a mean age of 20.5 years. The sample was 60% female, 40% male, and 5% identified as non-binary or gender non-conforming.
Data was collected through an online survey administered through Qualtrics. The survey consisted of several measures, including the Patient Health Questionnaire-9 (PHQ-9) for depression symptoms, the Generalized Anxiety Disorder-7 (GAD-7) for anxiety symptoms, and questions about social media use.
Procedure :
Participants were asked to complete the online survey at their convenience. The survey took approximately 20-30 minutes to complete. Data was analyzed using descriptive statistics, correlations, and multiple regression analysis.
Results indicated that social media use was significantly related to symptoms of depression (r = .32, p < .001) and anxiety (r = .29, p < .001). Regression analysis indicated that frequency of social media use was a significant predictor of both depression symptoms (β = .24, p < .001) and anxiety symptoms (β = .20, p < .001), even when controlling for age, gender, and other relevant factors.
The results of this study suggest that social media use is associated with symptoms of depression and anxiety among college students. The negative effects of social media are greater among frequent users. These findings have important implications for mental health professionals and educators, who should consider addressing the potential negative effects of social media use in their work with young adults.
References :
References should be listed in alphabetical order according to the author’s last name. For example:
- Chou, H. T. G., & Edge, N. (2012). “They are happier and having better lives than I am”: The impact of using Facebook on perceptions of others’ lives. Cyberpsychology, Behavior, and Social Networking, 15(2), 117-121.
- Twenge, J. M., Joiner, T. E., Rogers, M. L., & Martin, G. N. (2018). Increases in depressive symptoms, suicide-related outcomes, and suicide rates among U.S. adolescents after 2010 and links to increased new media screen time. Clinical Psychological Science, 6(1), 3-17.
Note: This is just a sample Example do not use this in your assignment.
MLA (Modern Language Association) Format
MLA (Modern Language Association) Format is as follows:
- Page Layout : Use 8.5 x 11-inch white paper, with 1-inch margins on all sides. The font should be 12-point Times New Roman or a similar serif font.
- Heading and Title : The first page of your research paper should include a heading and a title. The heading should include your name, your instructor’s name, the course title, and the date. The title should be centered and in title case (capitalizing the first letter of each important word).
- In-Text Citations : Use parenthetical citations to indicate the source of your information. The citation should include the author’s last name and the page number(s) of the source. For example: (Smith 23).
- Works Cited Page : At the end of your paper, include a Works Cited page that lists all the sources you used in your research. Each entry should include the author’s name, the title of the work, the publication information, and the medium of publication.
- Formatting Quotations : Use double quotation marks for short quotations and block quotations for longer quotations. Indent the entire quotation five spaces from the left margin.
- Formatting the Body : Use a clear and readable font and double-space your text throughout. The first line of each paragraph should be indented one-half inch from the left margin.
MLA Research Paper Template
MLA Research Paper Format Template is as follows:
- Use 8.5 x 11 inch white paper.
- Use a 12-point font, such as Times New Roman.
- Use double-spacing throughout the entire paper, including the title page and works cited page.
- Set the margins to 1 inch on all sides.
- Use page numbers in the upper right corner, beginning with the first page of text.
- Include a centered title for the research paper, using title case (capitalizing the first letter of each important word).
- Include your name, instructor’s name, course name, and date in the upper left corner, double-spaced.
In-Text Citations
- When quoting or paraphrasing information from sources, include an in-text citation within the text of your paper.
- Use the author’s last name and the page number in parentheses at the end of the sentence, before the punctuation mark.
- If the author’s name is mentioned in the sentence, only include the page number in parentheses.
Works Cited Page
- List all sources cited in alphabetical order by the author’s last name.
- Each entry should include the author’s name, title of the work, publication information, and medium of publication.
- Use italics for book and journal titles, and quotation marks for article and chapter titles.
- For online sources, include the date of access and the URL.
Here is an example of how the first page of a research paper in MLA format should look:
Headings and Subheadings
- Use headings and subheadings to organize your paper and make it easier to read.
- Use numerals to number your headings and subheadings (e.g. 1, 2, 3), and capitalize the first letter of each word.
- The main heading should be centered and in boldface type, while subheadings should be left-aligned and in italics.
- Use only one space after each period or punctuation mark.
- Use quotation marks to indicate direct quotes from a source.
- If the quote is more than four lines, format it as a block quote, indented one inch from the left margin and without quotation marks.
- Use ellipses (…) to indicate omitted words from a quote, and brackets ([…]) to indicate added words.
Works Cited Examples
- Book: Last Name, First Name. Title of Book. Publisher, Publication Year.
- Journal Article: Last Name, First Name. “Title of Article.” Title of Journal, volume number, issue number, publication date, page numbers.
- Website: Last Name, First Name. “Title of Webpage.” Title of Website, publication date, URL. Accessed date.
Here is an example of how a works cited entry for a book should look:
Smith, John. The Art of Writing Research Papers. Penguin, 2021.
MLA Research Paper Example
MLA Research Paper Format Example is as follows:
Your Professor’s Name
Course Name and Number
Date (in Day Month Year format)
Word Count (not including title page or Works Cited)
Title: The Impact of Video Games on Aggression Levels
Video games have become a popular form of entertainment among people of all ages. However, the impact of video games on aggression levels has been a subject of debate among scholars and researchers. While some argue that video games promote aggression and violent behavior, others argue that there is no clear link between video games and aggression levels. This research paper aims to explore the impact of video games on aggression levels among young adults.
Background:
The debate on the impact of video games on aggression levels has been ongoing for several years. According to the American Psychological Association, exposure to violent media, including video games, can increase aggression levels in children and adolescents. However, some researchers argue that there is no clear evidence to support this claim. Several studies have been conducted to examine the impact of video games on aggression levels, but the results have been mixed.
Methodology:
This research paper used a quantitative research approach to examine the impact of video games on aggression levels among young adults. A sample of 100 young adults between the ages of 18 and 25 was selected for the study. The participants were asked to complete a questionnaire that measured their aggression levels and their video game habits.
The results of the study showed that there was a significant correlation between video game habits and aggression levels among young adults. The participants who reported playing violent video games for more than 5 hours per week had higher aggression levels than those who played less than 5 hours per week. The study also found that male participants were more likely to play violent video games and had higher aggression levels than female participants.
The findings of this study support the claim that video games can increase aggression levels among young adults. However, it is important to note that the study only examined the impact of video games on aggression levels and did not take into account other factors that may contribute to aggressive behavior. It is also important to note that not all video games promote violence and aggression, and some games may have a positive impact on cognitive and social skills.
Conclusion :
In conclusion, this research paper provides evidence to support the claim that video games can increase aggression levels among young adults. However, it is important to conduct further research to examine the impact of video games on other aspects of behavior and to explore the potential benefits of video games. Parents and educators should be aware of the potential impact of video games on aggression levels and should encourage young adults to engage in a variety of activities that promote cognitive and social skills.
Works Cited:
- American Psychological Association. (2017). Violent Video Games: Myths, Facts, and Unanswered Questions. Retrieved from https://www.apa.org/news/press/releases/2017/08/violent-video-games
- Ferguson, C. J. (2015). Do Angry Birds make for angry children? A meta-analysis of video game influences on children’s and adolescents’ aggression, mental health, prosocial behavior, and academic performance. Perspectives on Psychological Science, 10(5), 646-666.
- Gentile, D. A., Swing, E. L., Lim, C. G., & Khoo, A. (2012). Video game playing, attention problems, and impulsiveness: Evidence of bidirectional causality. Psychology of Popular Media Culture, 1(1), 62-70.
- Greitemeyer, T. (2014). Effects of prosocial video games on prosocial behavior. Journal of Personality and Social Psychology, 106(4), 530-548.
Chicago/Turabian Style
Chicago/Turabian Formate is as follows:
- Margins : Use 1-inch margins on all sides of the paper.
- Font : Use a readable font such as Times New Roman or Arial, and use a 12-point font size.
- Page numbering : Number all pages in the upper right-hand corner, beginning with the first page of text. Use Arabic numerals.
- Title page: Include a title page with the title of the paper, your name, course title and number, instructor’s name, and the date. The title should be centered on the page and in title case (capitalize the first letter of each word).
- Headings: Use headings to organize your paper. The first level of headings should be centered and in boldface or italics. The second level of headings should be left-aligned and in boldface or italics. Use as many levels of headings as necessary to organize your paper.
- In-text citations : Use footnotes or endnotes to cite sources within the text of your paper. The first citation for each source should be a full citation, and subsequent citations can be shortened. Use superscript numbers to indicate footnotes or endnotes.
- Bibliography : Include a bibliography at the end of your paper, listing all sources cited in your paper. The bibliography should be in alphabetical order by the author’s last name, and each entry should include the author’s name, title of the work, publication information, and date of publication.
- Formatting of quotations: Use block quotations for quotations that are longer than four lines. Indent the entire quotation one inch from the left margin, and do not use quotation marks. Single-space the quotation, and double-space between paragraphs.
- Tables and figures: Use tables and figures to present data and illustrations. Number each table and figure sequentially, and provide a brief title for each. Place tables and figures as close as possible to the text that refers to them.
- Spelling and grammar : Use correct spelling and grammar throughout your paper. Proofread carefully for errors.
Chicago/Turabian Research Paper Template
Chicago/Turabian Research Paper Template is as folows:
Title of Paper
Name of Student
Professor’s Name
I. Introduction
A. Background Information
B. Research Question
C. Thesis Statement
II. Literature Review
A. Overview of Existing Literature
B. Analysis of Key Literature
C. Identification of Gaps in Literature
III. Methodology
A. Research Design
B. Data Collection
C. Data Analysis
IV. Results
A. Presentation of Findings
B. Analysis of Findings
C. Discussion of Implications
V. Conclusion
A. Summary of Findings
B. Implications for Future Research
C. Conclusion
VI. References
A. Bibliography
B. In-Text Citations
VII. Appendices (if necessary)
A. Data Tables
C. Additional Supporting Materials
Chicago/Turabian Research Paper Example
Title: The Impact of Social Media on Political Engagement
Name: John Smith
Class: POLS 101
Professor: Dr. Jane Doe
Date: April 8, 2023
I. Introduction:
Social media has become an integral part of our daily lives. People use social media platforms like Facebook, Twitter, and Instagram to connect with friends and family, share their opinions, and stay informed about current events. With the rise of social media, there has been a growing interest in understanding its impact on various aspects of society, including political engagement. In this paper, I will examine the relationship between social media use and political engagement, specifically focusing on how social media influences political participation and political attitudes.
II. Literature Review:
There is a growing body of literature on the impact of social media on political engagement. Some scholars argue that social media has a positive effect on political participation by providing new channels for political communication and mobilization (Delli Carpini & Keeter, 1996; Putnam, 2000). Others, however, suggest that social media can have a negative impact on political engagement by creating filter bubbles that reinforce existing beliefs and discourage political dialogue (Pariser, 2011; Sunstein, 2001).
III. Methodology:
To examine the relationship between social media use and political engagement, I conducted a survey of 500 college students. The survey included questions about social media use, political participation, and political attitudes. The data was analyzed using descriptive statistics and regression analysis.
Iv. Results:
The results of the survey indicate that social media use is positively associated with political participation. Specifically, respondents who reported using social media to discuss politics were more likely to have participated in a political campaign, attended a political rally, or contacted a political representative. Additionally, social media use was found to be associated with more positive attitudes towards political engagement, such as increased trust in government and belief in the effectiveness of political action.
V. Conclusion:
The findings of this study suggest that social media has a positive impact on political engagement, by providing new opportunities for political communication and mobilization. However, there is also a need for caution, as social media can also create filter bubbles that reinforce existing beliefs and discourage political dialogue. Future research should continue to explore the complex relationship between social media and political engagement, and develop strategies to harness the potential benefits of social media while mitigating its potential negative effects.
Vii. References:
- Delli Carpini, M. X., & Keeter, S. (1996). What Americans know about politics and why it matters. Yale University Press.
- Pariser, E. (2011). The filter bubble: What the Internet is hiding from you. Penguin.
- Putnam, R. D. (2000). Bowling alone: The collapse and revival of American community. Simon & Schuster.
- Sunstein, C. R. (2001). Republic.com. Princeton University Press.
IEEE (Institute of Electrical and Electronics Engineers) Format
IEEE (Institute of Electrical and Electronics Engineers) Research Paper Format is as follows:
- Title : A concise and informative title that accurately reflects the content of the paper.
- Abstract : A brief summary of the paper, typically no more than 250 words, that includes the purpose of the study, the methods used, the key findings, and the main conclusions.
- Introduction : An overview of the background, context, and motivation for the research, including a clear statement of the problem being addressed and the objectives of the study.
- Literature review: A critical analysis of the relevant research and scholarship on the topic, including a discussion of any gaps or limitations in the existing literature.
- Methodology : A detailed description of the methods used to collect and analyze data, including any experiments or simulations, data collection instruments or procedures, and statistical analyses.
- Results : A clear and concise presentation of the findings, including any relevant tables, graphs, or figures.
- Discussion : A detailed interpretation of the results, including a comparison of the findings with previous research, a discussion of the implications of the results, and any recommendations for future research.
- Conclusion : A summary of the key findings and main conclusions of the study.
- References : A list of all sources cited in the paper, formatted according to IEEE guidelines.
In addition to these elements, an IEEE research paper should also follow certain formatting guidelines, including using 12-point font, double-spaced text, and numbered headings and subheadings. Additionally, any tables, figures, or equations should be clearly labeled and referenced in the text.
AMA (American Medical Association) Style
AMA (American Medical Association) Style Research Paper Format:
- Title Page: This page includes the title of the paper, the author’s name, institutional affiliation, and any acknowledgments or disclaimers.
- Abstract: The abstract is a brief summary of the paper that outlines the purpose, methods, results, and conclusions of the study. It is typically limited to 250 words or less.
- Introduction: The introduction provides a background of the research problem, defines the research question, and outlines the objectives and hypotheses of the study.
- Methods: The methods section describes the research design, participants, procedures, and instruments used to collect and analyze data.
- Results: The results section presents the findings of the study in a clear and concise manner, using graphs, tables, and charts where appropriate.
- Discussion: The discussion section interprets the results, explains their significance, and relates them to previous research in the field.
- Conclusion: The conclusion summarizes the main points of the paper, discusses the implications of the findings, and suggests future research directions.
- References: The reference list includes all sources cited in the paper, listed in alphabetical order by author’s last name.
In addition to these sections, the AMA format requires that authors follow specific guidelines for citing sources in the text and formatting their references. The AMA style uses a superscript number system for in-text citations and provides specific formats for different types of sources, such as books, journal articles, and websites.
Harvard Style
Harvard Style Research Paper format is as follows:
- Title page: This should include the title of your paper, your name, the name of your institution, and the date of submission.
- Abstract : This is a brief summary of your paper, usually no more than 250 words. It should outline the main points of your research and highlight your findings.
- Introduction : This section should introduce your research topic, provide background information, and outline your research question or thesis statement.
- Literature review: This section should review the relevant literature on your topic, including previous research studies, academic articles, and other sources.
- Methodology : This section should describe the methods you used to conduct your research, including any data collection methods, research instruments, and sampling techniques.
- Results : This section should present your findings in a clear and concise manner, using tables, graphs, and other visual aids if necessary.
- Discussion : This section should interpret your findings and relate them to the broader research question or thesis statement. You should also discuss the implications of your research and suggest areas for future study.
- Conclusion : This section should summarize your main findings and provide a final statement on the significance of your research.
- References : This is a list of all the sources you cited in your paper, presented in alphabetical order by author name. Each citation should include the author’s name, the title of the source, the publication date, and other relevant information.
In addition to these sections, a Harvard Style research paper may also include a table of contents, appendices, and other supplementary materials as needed. It is important to follow the specific formatting guidelines provided by your instructor or academic institution when preparing your research paper in Harvard Style.
Vancouver Style
Vancouver Style Research Paper format is as follows:
The Vancouver citation style is commonly used in the biomedical sciences and is known for its use of numbered references. Here is a basic format for a research paper using the Vancouver citation style:
- Title page: Include the title of your paper, your name, the name of your institution, and the date.
- Abstract : This is a brief summary of your research paper, usually no more than 250 words.
- Introduction : Provide some background information on your topic and state the purpose of your research.
- Methods : Describe the methods you used to conduct your research, including the study design, data collection, and statistical analysis.
- Results : Present your findings in a clear and concise manner, using tables and figures as needed.
- Discussion : Interpret your results and explain their significance. Also, discuss any limitations of your study and suggest directions for future research.
- References : List all of the sources you cited in your paper in numerical order. Each reference should include the author’s name, the title of the article or book, the name of the journal or publisher, the year of publication, and the page numbers.
ACS (American Chemical Society) Style
ACS (American Chemical Society) Style Research Paper format is as follows:
The American Chemical Society (ACS) Style is a citation style commonly used in chemistry and related fields. When formatting a research paper in ACS Style, here are some guidelines to follow:
- Paper Size and Margins : Use standard 8.5″ x 11″ paper with 1-inch margins on all sides.
- Font: Use a 12-point serif font (such as Times New Roman) for the main text. The title should be in bold and a larger font size.
- Title Page : The title page should include the title of the paper, the authors’ names and affiliations, and the date of submission. The title should be centered on the page and written in bold font. The authors’ names should be centered below the title, followed by their affiliations and the date.
- Abstract : The abstract should be a brief summary of the paper, no more than 250 words. It should be on a separate page and include the title of the paper, the authors’ names and affiliations, and the text of the abstract.
- Main Text : The main text should be organized into sections with headings that clearly indicate the content of each section. The introduction should provide background information and state the research question or hypothesis. The methods section should describe the procedures used in the study. The results section should present the findings of the study, and the discussion section should interpret the results and provide conclusions.
- References: Use the ACS Style guide to format the references cited in the paper. In-text citations should be numbered sequentially throughout the text and listed in numerical order at the end of the paper.
- Figures and Tables: Figures and tables should be numbered sequentially and referenced in the text. Each should have a descriptive caption that explains its content. Figures should be submitted in a high-quality electronic format.
- Supporting Information: Additional information such as data, graphs, and videos may be included as supporting information. This should be included in a separate file and referenced in the main text.
- Acknowledgments : Acknowledge any funding sources or individuals who contributed to the research.
ASA (American Sociological Association) Style
ASA (American Sociological Association) Style Research Paper format is as follows:
- Title Page: The title page of an ASA style research paper should include the title of the paper, the author’s name, and the institutional affiliation. The title should be centered and should be in title case (the first letter of each major word should be capitalized).
- Abstract: An abstract is a brief summary of the paper that should appear on a separate page immediately following the title page. The abstract should be no more than 200 words in length and should summarize the main points of the paper.
- Main Body: The main body of the paper should begin on a new page following the abstract page. The paper should be double-spaced, with 1-inch margins on all sides, and should be written in 12-point Times New Roman font. The main body of the paper should include an introduction, a literature review, a methodology section, results, and a discussion.
- References : The reference section should appear on a separate page at the end of the paper. All sources cited in the paper should be listed in alphabetical order by the author’s last name. Each reference should include the author’s name, the title of the work, the publication information, and the date of publication.
- Appendices : Appendices are optional and should only be included if they contain information that is relevant to the study but too lengthy to be included in the main body of the paper. If you include appendices, each one should be labeled with a letter (e.g., Appendix A, Appendix B, etc.) and should be referenced in the main body of the paper.
APSA (American Political Science Association) Style
APSA (American Political Science Association) Style Research Paper format is as follows:
- Title Page: The title page should include the title of the paper, the author’s name, the name of the course or instructor, and the date.
- Abstract : An abstract is typically not required in APSA style papers, but if one is included, it should be brief and summarize the main points of the paper.
- Introduction : The introduction should provide an overview of the research topic, the research question, and the main argument or thesis of the paper.
- Literature Review : The literature review should summarize the existing research on the topic and provide a context for the research question.
- Methods : The methods section should describe the research methods used in the paper, including data collection and analysis.
- Results : The results section should present the findings of the research.
- Discussion : The discussion section should interpret the results and connect them back to the research question and argument.
- Conclusion : The conclusion should summarize the main findings and implications of the research.
- References : The reference list should include all sources cited in the paper, formatted according to APSA style guidelines.
In-text citations in APSA style use parenthetical citation, which includes the author’s last name, publication year, and page number(s) if applicable. For example, (Smith 2010, 25).
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Research Results Section – Writing Guide and...

Research Paper Introduction – Writing Guide and...

Delimitations in Research – Types, Examples and...

APA Table of Contents – Format and Example

Future Research – Thesis Guide

Research Paper Abstract – Writing Guide and...
APA Research Paper Outline: Examples and Template
Table of contents
- 1 Why Is Research Paper Format Necessary?
- 2.1 Purpose of research paper outline
- 2.2 APA outline example
- 3.1 APA paper outline example
- 3.2 Introduction:
- 3.4 Conclusion:
- 4 The Basic APA Outline Format
- 5 APA Style Outline Template Breakdown
- 6.1 APA Research Paper Outline Example
- 6.2 APA Paper Outline Format Example
- 7.1 First Paragraph: Hook and Thesis
- 7.2 Main Body
- 7.3 Conclusion
- 7.4 Decimal APA outline format example
- 7.5 Decimal APA outline format layout
- 8.1 A definite goal
- 8.2 Division
- 8.3 Parallelism
- 8.4 Coordination
- 8.5 Subordination
- 8.6 Avoid Redundancy
- 8.7 Wrap it up in a good way
- 8.8 Conclusion
Formatting your paper in APA can be daunting if this is your first time. The American Psychological Association (APA) offers a guide or rules to follow when conducting projects in the social sciences or writing papers. The standard APA fromat a research paper outline includes a proper layout from the title page to the final reference pages. There are formatting samples to create outlines before writing a paper. Amongst other strategies, creating an outline is the easiest way to APA format outline template.
Why Is Research Paper Format Necessary?
Consistency in the sequence, structure, and format when writing a research paper encourages readers to concentrate on the substance of a paper rather than how it is presented. The requirements for paper format apply to student assignments and papers submitted for publication in a peer-reviewed publication. APA paper outline template style may be used to create a website, conference poster, or PowerPoint presentation . If you plan to use the style for other types of work like a website, conference poster, or even PowerPoint presentation, you must format your work accordingly to adjust to requirements. For example, you may need different line spacing and font sizes. Follow the formatting rules provided by your institution or publication to ensure its formatting standards are followed as closely as possible. However, to logically structure your document, you need a research paper outline in APA format. You may ask: why is it necessary to create an outline for an APA research paper?
Concept & Purposes of Research Paper Outline
A path, direction, or action plan! Writing short essays without a layout may seem easy, but not for 10,000 or more words. Yet, confusing a table of contents with an outline is a major issue. The table of contents is an orderly list of all the chapters’ front matter, primary, and back matter. It includes sections and, often, figures in your work, labeled by page number. On the other hand, a research APA-style paper outline is a proper structure to follow.
Purpose of research paper outline
An outline is a formalized essay in which you give your own argument to support your point of view. And when you write your apa outline template, you expand on what you already know about the topic. Academic writing papers examine an area of expertise to get the latest and most accurate information to work on that topic. It serves various purposes, including:
- APA paper outline discusses the study’s core concepts.
- The research paper outlines to define the link between your ideas and the thesis.
- It provides you with manageable portions that you can handle.
- The research paper’s APA outline enables the detection of structural faults or gaps.
- As shown in the example, it must clearly comprehend the subject at hand.
APA outline example

This research paper outline example will guide you in formatting the layout for a clear direction to work on. It eliminates the inconsistency along with lacking proper substance in the paper.
Understanding the APA Outline Format
It would not be wrong to say there is no standard outline format. The official publishing handbook does not give precise guidelines for preparing an outline. But, it requires certain basic guidelines to follow regarding typeface, font size, structure, margins, etc.
APA paper outline example
Moreover, the final shape of your work relies on your instructor’s specifications and your particular preferences for APA citation format. Though, it would be better to follow some standards for formatting your outline, for instance:
Times New Roman is a widely accessible standard typeface for an APA essay format in 12-point font. However, serif and sans serif fonts like Arial and Georgia are acceptable in font size 11pt.
The text of your paper format should be double-spaced.
The primary headlines use Roman and Arabic numerals to write an outline.
Headings & Subheadings
While writing an APA essay, there are particular standards for utilizing headings in your outline: I – Main headings are numbered by Roman numerals like I, II, III, IV A – Subheadings are numbered with Capital letters (A, B, C, D) 1 – The APA outline uses Arabic numerals (1-9 type numbers) within those subheadings. a – Below Arabic number subheadings, lower-case letters are used (a, b, a). [1] – Headings below those subheadings use Arabic numbers enclosed in parenthesis.
APA format offers a standard layout for each paper, such as
- 1-inch margins on the top, bottom, left, and right.
- The page number on the upper right corner.
The structure of writing an outline consists of three major sections:
- Introduction
Introduction:
This section highlights crucial background information.
Explain the primary points that support your ideas.
Conclusion:
- Summarize your key arguments.
- Explain how these concepts support your ultimate stance, as shown in APA outline example below.
An outline in APA has three common formats that vary in the numeric sequence of all. To make it easier for you, we have compiled all three templates. You can format your document using these examples for added coherence and structure.
The Basic APA Outline Format

APA Style Outline Template Breakdown
Numbering the APA style format follows five levels of headings that use different alphabets and numbers. For instance, I – Headings use Roman numerals like I, II, and III. A – CAPITAL ALPHABETS”, such as A, B, C, etc. 1 – Headings and subheadings use Arabic numbers (1, 2, 3). a – If there are further headings (the fourth level), use lower-case alphabets. [1] – Headings below that (the fifth level) use Arabic numerals enclosed in parentheses, such as [1], [2], [3].
Full Sentence Outline Format
As the name specifies, the full-sentence style outline format requires every line to be a proper sentence. Full-sentence APA style outline is best recommended for essays and speeches. It gives your writing process an idea or a logical path to follow.
APA Research Paper Outline Example
If you are looking for how to write a research paper outline APA in Full Sentence Format, here is an example:
Full Sentence APA format heading utilizes Roman numerals I, II, and III. Every heading must be a full sentence. Here is an APA style paper outline template for the full-sentence format that will clear all your confusion on how to write an outline in full-sentence format.

APA Paper Outline Format Example
I. Introduction
III. Conclusion
Decimal Outline Format
The decimal outline format for APA research papers differs from other formats. The decimal APA style is simple and uses paragraphs for structure. It contains three main paragraphs, introduction, main body, and conclusion.
First Paragraph: Hook and Thesis
- The first paragraph is a sentence or two that introduces the central concept of your article.
- Introduce your topic or subject of study where your research is applicable as a context for further research.
- Explain why the mentioned issue is essential or relevant to the audience.
- A thesis statement is a claim that you make throughout your whole essay.
- The topic phrase is the first point in any writing to support a thesis statement.
- Give an explanation or provide evidence to support your point.
- Provide verifiable facts, figures, and/or citations from credible sources in your writing. It helps in the substantiating assertion.
- Include as many supporting statements and related evidence in your decimal outline.
Finally, when you write an outline, provide a concluding remark to support your claims.
Decimal APA outline format example
1.0 The main heading 1.1 Subheading under the main heading 1.2 Second digit is represented by subheadings under the main headings 1.2.1 Further division adds another digit in decimal format 1.2.2 You can number them as per the number of paragraphs or points, or lines An easy way to write in decimal APA outline format is to remember the structure, i.e.; 1.1.1 = Heading.Paragraph.Sentence/point under paragraph.”
Decimal APA outline format layout
1.0 Main heading 1.1 First paragraph for first heading. 1.2 Second paragraph for first heading. 1.2.1 First point or sentence for the second paragraph. 2.0 Second heading 2.1 Second heading, first paragraph. 2.2 Second heading, second paragraph. 2.2.1 Second, heading, second paragraph, first sentence, or point. 3.0 Decimal working 3.1 You must remember that each digit represents a segment. 3.2 It is easier to remember the placement of numbers. 3.2.1 First digit represents the heading 3.2.2 Second digit represents the paragraph under the main heading <3.2.3 The third digit represents any point or sentence under the paragraph.
Tips for Writing an Outline: Organize Your Ideas
You may feel it is easier to write without outlines, but once you start writing, organizing your ideas or thoughts becomes hard. Even if you have some fantastic ideas, producing an engaging story is practically hard. If you are not first creating an outline or conceptual guides while writing a research paper, you may lose track. A well-written outline is essential in completing your paper and maintaining quality. Establishing your point in paper writing is easy if you create an outline first. You can find an APA research paper outline template that best suits your requirement. Moreover, these tips can help you polish your writing. These tips and sample papers can help you write outstanding outlines without making any hassle.
A definite goal
For better expression, make a list of primary objectives on a title page in a single phrase or less. Your goal should be specific and measurable. If it is too broad or imprecise, you will not achieve anything. If you are working on a large paper format that covers a variety of themes or topics, you may have a more general purpose in mind. But, if you plan to write an essay, the aim should be as specific and clear as possible to be effective.
Breaking things up rather than allowing them to become verbose is known as the division rule. Make sure that each subsection in the document corresponds to its parent heading. If it doesn’t compare to the section, removing it or moving it to another location is better.
Parallelism
It is mainly related to the consistency and structure of the document. It keeps your paper’s layout tidy and also ensures relevancy. For instance, if you begin one heading with a verb, make sure all other headings and subheadings also start with a verb.
Coordination
Having headings aligned is critical to creating a well-organized outline. This rule also applies to subheadings, which is a good thing. If one title is less important than another, consider changing your layout by incorporating it into a subsection instead.
Subordination
Subordination deals with maintaining a connection between your paper’s headings and subheadings. It helps in the proper sequencing of headings and subheadings. Headings should be broad at the outset. At the same time, the subheadings become more particular as they go further into the document.
Avoid Redundancy
While writing a paper outline, look through it many times and cross out any items that aren’t necessary or have no significance. While outlining, make sure to be specific and concise. It will prevent you from adding information that does not supporting your final essay. Remove all the extra information and points while c that weighs you down while you write.
Wrap it up in a good way
Creating an outline does not only help in writing a coherent term paper, but it also helps in ending with precise understanding. Be considerate of your audience’s time and effort when you write an outline in APA, and ensure it serves its purpose. If you still have any doubts about formatting your paper outline, you can use this APA-style research paper outline template to write your document. We have provided Outline Format Example for every style.
People find it hard to write an outline in APA, but if you are aware of the requirements and structure, it’s no breeze. Sometimes, your instructor may alter your paper format by introducing or removing existing sections. As a result, if you come across any templates for an outline in APA, pay close attention to them. If you are looking for a quick answer to how to outline an APA paper, here’s a standard logical sequence of typical parts to include when writing an outline in APA:
- Thesis statement
- Techniques employed
- Body of paper
- Conclusions section
- List of references
A well-written outline is an excellent tool for presenting an outstanding paper. Including the key components while writing an outline for a research paper is necessary.
Readers also enjoyed

WHY WAIT? PLACE AN ORDER RIGHT NOW!
Just fill out the form, press the button, and have no worries!
We use cookies to give you the best experience possible. By continuing we’ll assume you board with our cookie policy.

Turabian Style Guide: Sample Papers in Turabian
- Turabian (& Chicago)
- Sample Papers in Turabian
- Research Paper Topics
- Research Tools This link opens in a new window
Sample Turabian Papers
- Turabian Style paper (Austin Peay State University) from Austin Peay State University, Academic Support Center
- "Turabian Tutor" (Tennessee Temple University) Tennessee Temple University, Turabian-style paper
- Intro to Chicago-Turabian Style (paper) (University of North Alabama) University of North Alabama, turabian-style paper
- Sample Turabian Bibliography from Carpenter Library, UNF
Turabian formatting in Word
Turabian title page in Word
Simple Formatting Guidelines
- Paper size - 8.5 inch x 11 inch
- 1 inch on all four edges of the page
- Typeface/Font size
- Easily read; preferably serifed, ex. Times New Roman
- 12-point is accepted size
- Spacing/Indentation
- Double-space text with exceptions that are single-spaced:
- Block quotations
- Table elements, ex. titles and figure captions
- Appendices' lists
- Footnotes/endnotes are single-spaced but separated by a space between each item.
- Items in a bibliography/reference list are single-spaced but each item is separated by a space.
- Do not number the title page.
- Start arabic numbers (at 1) on first page of paper that is not considered front matter (front matter = title page).
- Place page numbers consistently in same location throughout paper:
- Options for page numbers include: centered in footer OR right-hand of footer OR centered in header OR right-hand of header
- Title Page elements
- Center all elements
- Use consistent typeface and font size
- font size can increase slightly for title elements
- preferred format is boldface for title
- Employ Headline Capitalization with All Elements (first letter of each noun/pronoun is capitalized) ( definition of Headline Style Capitalization)
- Title is placed approx. 1/3 down the page. A subtitle follow the main title with a colon and starts on a new line.
- Two-thirds down the page, your name, any title page information provided by your professor, and the date should be included.
- Body of Paper (Text)
- Includes
- Introduction
- Quotations, including block quotations, should follow Turabian's standard formatting rules.
- Text Formatting
- Be consistent throughout body of paper with typeface, font size, and other formatting elements.
- Make sure text is aligned left.
- Do not add color in the text (hyperlinked text will automatically become blue; this is unavoidable).
- Block quotations should be set apart by blank space above and below and, internally, should be single-spaced.
- Back Matter elements
- Notes (endnotes section) ( http://writing.wisc.edu/Handbook/DocChicago_Notes_Formatting.html )
- Bibliography OR References OR Works Cited (your choice as to heading)
- Center page titles
- Font size can increase slightly for title elements.
- Preferred format is boldface for title
- Leave two blank spaces between heading and first endnote/work referenced.
- Notes section
- Use standard paragraph indentations for each endnote.
- Single-space each endnote and separate each by a blank space.
- Use standard-size numbers with periods to enumerate your endnote list.
- Bibliography/References/Works Cited section
- Use hanging indentations for works referenced (first line is not indented; second and remaining lines are indented standard tab of five spaces)
- This section is typically arranged alphabetically by author, then alphabetically by title if you list multiple works by one author.
All information comes from
Turabian, Kate L. A Manual for Writers of Research Papers, Theses, and Dissertations : Chicago Style for Students & Researchers . 8th ed. Chicago: University of Chicago Press, 2013.
- << Previous: Turabian (& Chicago)
- Next: Research Paper Topics >>
- Last Updated: Mar 6, 2023 10:35 AM
- URL: https://library.cbc.edu/Turabian
Help | Advanced Search
Computer Science > Computer Vision and Pattern Recognition
Title: styleshot: a snapshot on any style.
Abstract: In this paper, we show that, a good style representation is crucial and sufficient for generalized style transfer without test-time tuning. We achieve this through constructing a style-aware encoder and a well-organized style dataset called StyleGallery. With dedicated design for style learning, this style-aware encoder is trained to extract expressive style representation with decoupling training strategy, and StyleGallery enables the generalization ability. We further employ a content-fusion encoder to enhance image-driven style transfer. We highlight that, our approach, named StyleShot, is simple yet effective in mimicking various desired styles, i.e., 3D, flat, abstract or even fine-grained styles, without test-time tuning. Rigorous experiments validate that, StyleShot achieves superior performance across a wide range of styles compared to existing state-of-the-art methods. The project page is available at: this https URL .
| Comments: | project page: |
| Subjects: | Computer Vision and Pattern Recognition (cs.CV) |
| Cite as: | [cs.CV] |
| (or [cs.CV] for this version) | |
| Focus to learn more arXiv-issued DOI via DataCite |
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
Instructions for Authors
Contact Monica Mungle for help if edits are needed to the top section.
Original Investigation
Caring for the critically ill patient, brief report, research letter, systematic review (without meta-analysis), narrative review, special communication, clinical challenge, diagnostic test interpretation, a piece of my mind, letter to the editor, letter in reply.
- Randomized Clinical Trial
- Parallel-Design Double-blind Trial
- Crossover Trial
- Equivalence and Noninferiority Trial
- Cluster Trial
- Nonrandomized Controlled Trial
Meta-analysis
- Cohort Study
- Case-Control Study
- Cross-sectional Study
- Case Series
- Economic Evaluation
- Decision Analytical Model
- Comparative Effectiveness Research
- Genetic Association Study
- Diagnostic/Prognostic Study
- Quality Improvement Study
- Survey Study
- Qualitative Study
Manuscript Submission
Copies of previous editorial and reviewer comments, cover letter, manuscript style, manuscript components, recommended file sizes, manuscript file formats, abbreviations, units of measure, names of drugs, devices, and other products, gene names, symbols, and accession numbers, reproduced and re-created material, online-only supplements and multimedia.
What to Expect
Editorial and Peer Review
The jama network advantage.
- JAMA-Express
Authorship Form and Publishing Agreement
Publication.
- Postpublication Online Commenting
Reprints/e-Prints
Corrections, previous publication, related manuscripts and reports, and preprints, previous or planned meeting presentation or release of information, embargo policy, research article public access, depositing in repositories, and discoverability.
Editorial Policies for Authors
Authorship and Disclosures
Authorship criteria and contributions, role of the corresponding author, changes in authorship, name change policy, group authorship, conflicts of interest and financial disclosures, funding/support and role of funder/sponsor, data access, responsibility, and analysis, acknowledgment section, equator reporting guidelines, use of causal language, timeliness of data, statistical methods and data presentation, reporting demographic information for study participants, ethical approval of studies and informed consent, patient identification, use of ai in publication and research, personal communications and unpublished data, manuscripts that pose security risks.
Journal Policies, Forms, Resources
Decisions and Management of Editorial Conflicts of Interest
Publishing agreement, unauthorized use.
- Patient Permission Form
- AMA Manual of Style
- EQUATOR Network
- About This Journal
Contact Information
JAMA , Kirsten Bibbins-Domingo, PhD, MD, MAS, Editor in Chief, 330 N Wabash Ave, Chicago, IL 60611-5885; telephone: (312) 464-4444; fax: (312) 464-5824; email: [email protected] . Manuscripts should be submitted online at http://manuscripts.jama.com .
Determine My Article Type
Categories of articles.
Original Investigation full info
Clinical trial Meta-analysis Intervention study Cohort study Case-control study Epidemiologic assessment Survey with high response rate Cost-effectiveness analysis Decision analysis Study of screening and diagnostic tests Other observational study
- ≤5 tables and/or figures
- Structured abstract
Data Sharing Statement
Follow EQUATOR Reporting Guidelines
Caring for the Critically Ill Patient full info
Original research reports, preferably clinical trials or systematic reviews that address virtually any aspect of critical illness, from prevention and triage, through resuscitation and acute treatment, to rehabilitation and palliative care.
- See also requirements for Clinical Trial , Meta-analysis , and Systematic Review
Brief Report full info
Short reports of original studies or evaluations or unique, first-time reports of clinical case series.
It is very rare for this journal to publish case reports.
- 15 references
- ≤3 tables and/or figures
Research Letter full info
Concise, focused reports of original research. Can include any of the study types listed under Original Investigation.
- ≤6 references
- ≤2 small tables and/or figures
- No Abstract or Key Points
Back to top
Clinical Review and Education
Systematic Review (without meta-analysis) full info
This article type requires a presubmission inquiry. See the "full info" below for requirements and contact information.
Critical assessments of the literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention.
Systematic Reviews without meta-analysis are published as Reviews; those with meta-analysis are published as Original Investigations (see Meta-analysis ).
- 50-75 references
- A PRISMA-style flow diagram should be included as an online supplement
- Include a table with ratings of the quality of the studies/evidence
- Subtitle should be "A Systematic Review"
Narrative Review full info
Up-to-date review for clinicians on a topic of general common interest from the perspective of internationally recognized experts in these disciplines.
The focus should be an update on current understanding of the physiology of the disease or condition, diagnostic consideration, and treatment.
These reviews should address a specific question or issue that is relevant for clinical practice.
- 2000-3500 words
- 3-part structured abstract
- No Key Points
- Subtitle should be "A Review"
Special Communication full info
This journal publishes very few of these types of articles.
These manuscripts describe an important issue in clinical medicine, public health, health policy, or medical research in a scholarly, thorough, well-referenced, systematic, and evidence-based manner.
- 50 references
- ≤4 tables and/or figures
- Requires a presubmission inquiry
Clinical Challenge full info
Presents an actual patient case with a specific disease or condition with an accompanying clinical image.
- "What Would You Do Next?" with 4 single-phrase plausible treatment options describing possible courses of action with 1 being preferred
- Case presentation: 250 words
- Discussion: 500-600 words
- ≤10 references
- 1-2 small figures
- Patient permission required
Diagnostic Test Interpretation full info
This article requires a presubmission inquiry.
Presentation of the results of a diagnostic test from a single patient with exploration of the clinical application of the test result; intended to help clinicians understand the underlying rationale in ordering tests, interpreting test results, and acting on the diagnostic test findings.
- How Do You Interpret These Test Results? (or What Would You Do Next?) with 4 plausible responses
- Case presentation: 200 words
- Discussion: 650 words
Viewpoint full info
May address virtually any important topic in medicine, public health, research, discovery, prevention, ethics, health policy, or health law and generally are not linked to a specific article.
- 1200 words (or 1000 words with 1 small table or figure)
- ≤7 references at submission
- ≤3 authors, with no more than 2 affiliations per author
A Piece of My Mind full info
Personal vignettes (eg, exploring the dynamics of the patient-physician relationship) taken from wide-ranging experiences in medicine; occasional pieces express views and opinions on the myriad issues that affect the profession.
- ≤1600 words
- Patient permission may be needed
Poetry full info
Original poems related to the medical experience, whether from the point of view of a health care worker or patient, or simply an observer.
- No longer than 44 lines
Correspondence
Letter to the Editor full info
Letters discussing a recent article in this journal should be submitted within 4 weeks of the article's publication in print.
- ≤5 references (1 of which should be to the recent article)
Letter in Reply full info
Replies by authors of original articles to letters from readers.
Determine My Study Type
Randomized Clinical Trial full info
A trial that prospectively assigns participants to intervention or comparison groups to study the cause-and-effect relationship between an intervention and a health outcome. Interventions include but are not limited to drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, process-of-care changes, and the like.
- ≤5 tables and/or figures, including CONSORT flow diagram
- Subtitle should be "A Randomized Clinical Trial"
- Trial registration and ID
- Trial protocol
- CONSORT checklist
- Follow CONSORT Reporting Guidelines
Parallel-Design Double-blind Trial full info
A randomized trial that prospectively assigns participants to 2 or more groups to receive different interventions. Participants and those administering the interventions are unaware of which intervention individual participants are receiving.
Crossover Trial full info
A trial in which participants receive more than 1 of the treatments under investigation, usually in a randomly determined sequence, and with a prespecified amount of time (washout period) between sequential treatments.
Equivalence and Noninferiority Trial full info
A trial designed to assess whether the treatment or intervention under study (eg, a new intervention) is no worse than an existing alternative (eg, an active control). In these trials, authors must prespecify a margin of noninferiority that is consistent with all relevant studies and within which the new intervention can be assumed to be no worse than the active control.
Cluster Trial full info
A trial that includes random assignment of groups rather than individuals to intervention and control groups.
Nonrandomized Controlled Trial full info
A trial that prospectively assigns groups or populations to study the efficacy or effectiveness of an intervention but in which the assignment to the intervention occurs through self-selection or administrator selection rather than through randomization. Control groups can be historic, concurrent, or both. This design is sometimes called a quasi-experimental design.
- ≤5 tables and/or figures, including a trial flow diagram
- Subtitle should be "A Nonrandomized Controlled Trial"
- TREND checklist
Meta-analysis full info
A systematic review that includes a statistical technique for quantitatively combining the results of multiple studies that measure the same outcome into a single pooled or summary estimate.
- Subtitle should include "A Meta-analysis"
- Follow PRISMA Reporting Guidelines or MOOSE Reporting Guidelines
Cohort Study full info
An observational study that follows a group (cohort) of individuals who are initially free of the outcome of interest. Individuals in the cohort may share some underlying characteristic, such as age, sex, diagnosis, exposure to a risk factor, or treatment.
- Follow STROBE Reporting Guidelines
Case-Control Study full info
An observational study designed to determine the association between an exposure and outcome in which study participants are selected by outcome. Those with the outcome (cases) are compared with those without the outcome (controls) with respect to an exposure or event. Cases and controls may be matched according to specific characteristics (eg, age, sex, or duration of disease).
Cross-sectional Study full info
An observational study of a defined population at a single point in time or during a specific interval, in which exposure and outcome are ascertained simultaneously.
Case Series full info
An observational study that describes a selected group of participants with similar exposure or treatment and without a control group. A case series may also involve observation of larger units such as groups of hospitals or municipalities, as well as smaller units such as laboratory samples.
- Follow Reporting Guidelines
Economic Evaluation full info
A study using formal, quantitative methods to compare 2 or more treatments, programs, or strategies with respect to their resource use and expected outcomes. This includes cost-effectiveness, cost-benefit, and cost-minimization analyses.
- Follow CHEERS Reporting Guidelines
Decision Analytical Model full info
A mathematical modeling study that compares consequences of decision options by synthesizing information from multiple sources and applying mathematical simulation techniques, usually with specific software. Reporting should address the relevant non-cost aspects of the CHEERS guideline.
Comparative Effectiveness Research full info
A study that compares different interventions or strategies to prevent, diagnose, treat, and monitor health conditions to determine which work best for which patients, under what circumstances, and are associated with the greatest benefits and harms.
- Follow ISPOR Reporting Guidelines
Genetic Association Study full info
A study that attempts to identify and characterize genomic variants that may be associated with susceptibility to multifactorial disease.
- Follow STREGA Reporting Guidelines
Diagnostic/Prognostic Study full info
A prospective study designed to develop, validate, or update the diagnostic or prognostic accuracy of a test or model.
- Follow STARD Reporting Guidelines or TRIPOD Reporting Guidelines
Quality Improvement Study full info
A study that uses data to define, measure, and evaluate a health care practice or service to maintain or improve the appropriateness, quality, safety, or value of that practice or service.
- Follow SQUIRE Reporting Guidelines
Survey Study full info
A survey study includes a representative sample of individuals who are asked to describe their opinions, attitudes, or behaviors. Survey studies should have sufficient response rates (generally ≥60%) and appropriate characterization of nonresponders to ensure that nonresponse bias does not threaten the validity of the findings.
- Follow AAPOR Best Practices for Survey Research
- Optional: Survey instrument as supplemental file
Qualitative Study full info
A study based on observation and interview with individuals that uses inductive reasoning and a theoretical sampling model and that focuses on social and interpreted, rather than quantifiable, phenomena and aims to discover, interpret, and describe rather than to test and evaluate. This includes mixed-methods studies that combine quantitative and qualitative designs in a sequential or concurrent manner.
- Follow SRQR Reporting Guidelines or COREQ Reporting Guidelines
These reports typically include randomized trials (see Clinical Trial ), intervention studies, cohort studies, case-control studies, epidemiologic assessments, other observational studies, surveys with high response rates (see Reports of Survey Research ), cost-effectiveness analyses and decision analyses (see Reports of Cost-effectiveness Analyses and Decision Analyses ), and studies of screening and diagnostic tests (see also Reports of Diagnostic Tests ). Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria and/or participation or response rates, or data sources, and how these were selected for the study); the essential features of any interventions; the main outcome measures; the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions and relevant implications for clinical practice or health policy. Data included in research reports must be original and should be as timely and current as possible (see Timeliness of Data ). Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 5 tables and/or figures.
These manuscripts are original research reports, preferably clinical trials, or systematic reviews (see above classifications for manuscript submission requirements by category of article) that address virtually any aspect of critical illness, from prevention and triage, through resuscitation and acute treatment, to rehabilitation and palliative care. Manuscripts that provide new insights into the diagnosis, prognosis, and treatment of critically ill patients, as well as those that explore pathophysiological, technological, ethical, or other related aspects of critical care medicine, are welcome. Follow EQUATOR Reporting Guidelines . For reports of original data and systematic reviews, a structured abstract is required; see instructions for preparing Abstracts for Reports of Original Data or Abstracts for Reviews . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 5 tables and/or figures.
These manuscripts are short reports of original studies or evaluations or unique, first-time reports of clinical case series. Follow EQUATOR Reporting Guidelines . A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Recommended length: 1200 words (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 3 tables and/or figures and no more than 15 references. Note: It is very rare for this journal to publish case reports.
Research Letters are concise, focused reports of original research. These should not exceed 600 words of text and 6 references and may include up to 2 tables or figures. Online supplementary material is only allowed for brief additional and absolutely necessary methods but not for any additional results or discussion. The text should include the full name, academic degrees, and institutional affiliation for each author and the email address for the corresponding author. Other persons who have contributed to the study may be indicated in an Acknowledgment, with their permission, including their academic degrees, affiliation, contribution to the study, and an indication if compensation was received for their role. Letters must not duplicate other material published or submitted for publication. In general, Research Letters should be divided into the following sections: Introduction, Methods, Results, and Discussion. They should not include an abstract or key points, but otherwise should follow all of the guidelines in Manuscript Preparation and Submission Requirements . Letters not meeting these specifications are generally not considered.
This article type requires a presubmission inquiry to [email protected] .
The journal will consider 2 types of review articles:
Systematic Reviews
These types of Review articles differ by the scope and level of analysis of the literature searches and the titles used. Systematic Reviews require a complete systematic search of the literature using multiple databases, covering many years, and grading of the quality of the cited evidence. Narrative Reviews do not require a rigorous literature search but should rely on evidence and should be written by established experts in the field. See below for more detail on each type of Review.
Titles for these Reviews should include a concise description of the main topic. Use specific and not overly broad wording for the title; the type of review should be indicated in the subtitle. For example:
Behavioral Treatment of Obesity: A Systematic Review
Behavioral Treatment of Obesity: A Review (note: the word "narrative" is not included in the subtitle)
Systematic Reviews are critical assessments of the literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention. Systematic Reviews without meta-analysis are published as Reviews; those with meta-analysis are published as Original Investigations (see Meta-analysis ). Systematic Reviews should address a specific question or issue that is relevant for clinical practice and provide an evidence-based, balanced, patient-oriented review on a focused topic. Follow EQUATOR Reporting Guidelines .
The basic structure of manuscripts reporting Systematic Reviews should include the following: Abstract (structured abstract of no more than 350 words); Introduction (150-250 words); Methods (150-250 words); Results (1000-1250 words, with the following subsections, if appropriate, depending on the specific question or issue addressed: Pathophysiology, Clinical Presentation, Assessment and Diagnosis, Treatment, and Prognosis); Discussion (1000 words); and Conclusions (2-3 sentences).
Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material), with no more than a total of 5 tables and/or figures and no more than 50-75 references. For an example of a published Systematic Review, see JAMA . 2014;312(6):631-640 and below for the general structure of a Systematic Review article.
Prospective authors interested in submitting a review manuscript should prepare a detailed outline of the proposed article. There should also be a brief summary of the extent and quality of the literature supporting the proposed review. Alternatively, if a draft of the manuscript has been completed, this can be sent. Prospective authors should also summarize their publication record in the field. Send this information to the editorial office via email to Mary McDermott, MD, at [email protected] .

Specific Components of a Systematic Review
Key Points (75-100 words)
This feature provides a quick structured synopsis of the Review, following 3 key points: Question, Findings, and Meaning. Limit to no more than 100 words. This is different from the Abstract.
Question: What are the most effective medical treatments for adult chronic sinusitis? Findings: In this systematic review, symptoms of chronic sinusitis were improved with saline irrigation and topical corticosteroid therapy compared to no therapy. Compared with placebo, 3-week courses of systemic corticosteroids or oral doxycycline were associated with reduced polyp size, and a 3-month course of macrolide antibiotic was associated with improved symptoms in patients without polyps. Meaning: First-line therapy for chronic sinusitis should begin with daily topical intranasal corticosteroid in conjunction with saline irrigation; subsequent therapies should be based on the patient's polyp status and severity of symptoms.
Abstract (350 words)
A structured abstract is required; Systematic Review articles should include a structured abstract of no more than 350 words using the headings listed below.
Importance: Include 1 or 2 sentences describing the clinical question or issue and its importance in clinical practice or public health. Objective: State the precise primary objective of the review. Indicate whether the review emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being reviewed. Evidence Review: Describe the information sources used, including the search strategies, years searched, and other sources of material, such as subsequent reference searches of retrieved articles. Methods used for inclusion of identified articles and quality assessment should be explained. Findings: Include a brief summary of the number of articles included, numbers of various types of studies (eg, clinical trials, cohort studies), and numbers of patients/participants represented by these studies. Summarize the major findings of the review of the clinical issue or topic in an evidence-based, objective, and balanced fashion, with the highest-quality evidence available receiving the greatest emphasis. Provide quantitative data. Conclusions and Relevance: The conclusions should clearly answer the questions posed if applicable, be based on available evidence, and emphasize how clinicians should apply current knowledge. Conclusions should be based only on results described in the abstract Findings subsection.
Introduction (150-250 words)
The first 2 to 3 sentences of the Introduction should draw in readers such that they want to continue reading the article and should establish the importance of the Review. Reviews should include the clinical question or issue and its importance for general medical practice, specialty practice, or public health. The first paragraph should provide a general summary of the clinical problem (eg, obesity). The next paragraph should focus on the specific aspect of the clinical problem the article will explore (eg, treatments for obesity). The epidemiology of the disease or condition should be briefly summarized and generally should include disease prevalence and incidence. The third paragraph should discuss exactly what material will be covered in the Review (eg, obesity treatments reported in trials with a minimum follow-up of 2 years including 80% of the original cohort).
Methods/Literature Search (150-250 words)
The literature search should be as current as possible, ideally with end dates within a month or two before manuscript submission. A search of the primary literature should be conducted, including multiple bibliographic databases (eg, PubMed/MEDLINE, Embase, CINAHL, PsycINFO). This can be facilitated by collaborating with a medical librarian to help with the search.
Briefly describe characteristics of the literature searched and included in the review, following the PRISMA reporting guidelines , including the bibliographic databases and other sources searched, search terms used, dates included in the search, date the literature search was conducted, screening process, language limitations, and inclusion and exclusion criteria. The rating system used to evaluate the quality of the evidence should be specified (see table below) and the methods used to evaluate quality should be described, including number of quality raters, how agreement on quality ratings was assessed, and how disagreements on quality ratings were resolved.
The highest-quality evidence (eg, randomized clinical trials, meta-analyses, systematic reviews, and high-quality prospective cohort studies) should receive the greatest emphasis. Clinical practice guidelines ordinarily should not be used as a primary component of the evidence base for the systematic review, although relevant guidelines should be addressed in the Discussion section of the article.
The search methods should be described in sufficient detail so the search can be reproduced based on the information provided in the manuscript. A summary of the methods of the literature search including this information should be included in the main article; details can be included in an online-only supplement. A PRISMA-style flow diagram showing this information should also be included as an online-only supplement. In addition, a completed PRISMA checklist should be submitted for the items completed that apply to systematic reviews (the checklist items that apply to meta-analyses do not need to be completed for systematic reviews without meta-analysis). The checklist will be used during review but will not be published.
Results (1000-1250 words)
First, briefly report the results of the literature search, including the number of articles reviewed and included, numbers of various types of studies (eg, clinical trials, cohort studies) included, and the aggregate numbers of patients included in the reviewed studies. Also provide a brief summary of the quality of the evidence. Details of this information can be included in a PRISMA-style flow diagram and table(s).
Next, the subsections listed below should generally appear in the Results sections of most Reviews although all of these subsections may not be necessary for some topics, depending on the specific question or issue addressed. The word counts following each subsection are suggested to assist with keeping the overall Results section limited to 1000-1250 words.
Pathophysiology (150-250 words). Provide a brief overview of the pathophysiology of the disease. The intent is to provide readers with sufficient background information about the underpinnings of a disease to provide context for the rest of the article. Clinical Presentation (150-250 words). Briefly describe the clinical characteristics that result in a patient seeking medical care for the condition or what features of the disease should lead a clinician to evaluate or treat it. Assessment and Diagnosis (250-300 words). Describe the clinical examination for evaluation of the disease and explain the most salient physical examination findings. If laboratory or imaging studies are necessary, provide the sensitivity and specificity and diagnostic accuracy of these tests and consider providing positive and negative likelihood ratios. Sequences of diagnostic tests are best presented as algorithms or in tables. Treatment (250-500 words). Treatments should be based on the most recently available and highest level of evidence. Treatment options should be summarized in the text and presented in detail in tables along with an indication of the strength of evidence supporting the individual treatments. In general, treatment recommendations should be supported by a systematic review of the literature, either performed by the author of the Review or published in the form of a high-quality review or guideline. If possible, the costs for various treatments should be provided. Prognosis (100-150 words). A section outlining the overall prognosis for the condition, once treated, should be included. Discussion (Approximately 1000 words)
Key findings should be summarized in the first paragraph of the Discussion section. All statements made should be supported by evidence. It is very important to not simply list findings from the studies reviewed. This information is best presented in tables. The Discussion should provide a critical synthesis of data and information based on the results of the review, an assessment of the quality of studies summarized, and a description of how studies can be interpreted and used to guide clinical practice. The limitations of the evidence and of the review should be discussed, and gaps in evidence should be addressed. A discussion of controversial or unresolved issues and topics in need of future research also should be included.
Clinical Practice Guidelines: In the Discussion section, describe current clinical practice guidelines, relevant to the topic of the review, if available, and whether the conclusions of this review agree with, or disagree with, the current clinical practice guidelines. If this is done and there is more than 1 guideline, a table should be prepared comparing the major features that differ between the guidelines. Guideline quality should be discussed using the standards outlined for the JAMA Clinical Guidelines Synopsis .
Conclusions
Include a 2- to 3-sentence summary of the major conclusions of the review.
Construct tables that summarize the search results. Tables summarizing treatments should have information organized by category of treatment and then by individual treatments. Columns should include the name of the treatment, strength of evidence supporting the treatment, the treatment's effect (preferably shown as the treatment's effect as compared to control on the measured outcome together with 95% confidence intervals), adverse effects, and very brief comments, if necessary. Lengthy text-based tables should be avoided. Additional or lengthy tables may be published online only, if justified.
Ratings of the quality of the evidence. Tables summarizing evidence should include ratings of the quality of the evidence. Use the rating scheme listed below with ratings of 1-5 for Reviews that include individual studies (modified from the Oxford Centre for Evidence-based Medicine for ratings of individual studies).
| Quality Rating Scheme for Studies and Other Evidence | |
|---|---|
| 1 | Properly powered and conducted randomized clinical trial; systematic review with meta-analysis |
| 2 | Well-designed controlled trial without randomization; prospective comparative cohort trial |
| 3 | Case-control studies; retrospective cohort study |
| 4 | Case series with or without intervention; cross-sectional study |
| 5 | Opinion of respected authorities; case reports |
There are several other preferred systems for rating the quality of evidence in Review articles. For Reviews that synthesize findings from numerous studies into a single summary recommendation, use the rating scale shown above or the Oxford Centre for Evidence-based Medicine's Levels of Evidence and Grades of Recommendation or the recommendations in the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . For reviews that include diagnostic studies, use The Rational Clinical Examination Levels of Evidence table .
Follow additional instructions for preparation and submission of Tables .
A PRISMA-style flow diagram should be included as an online supplement that summarizes the results of the literature search and the numbers of articles/records/studies and patients/participants represented in the studies identified, screened, eligible, and included in the final review.
Additional figures that illustrate pathophysiology or clinical presentation may be considered. Note: All figures will be re-created. For each proposed illustration, the authors should provide a list of the elements to be included in the illustration; 3-4 relevant recent references; example illustrations, if available; a working figure title and legend; and an explanation of how this new illustration would add to the published literature. We encourage videos, if appropriate, to illustrate a point made or process described in the Review.
Follow additional instructions for preparation and submission of Figures and Video .
Narrative Reviews on clinical topics provide an up-to-date review for clinicians on a topic of general common interest from the perspective of internationally recognized experts in these disciplines. The focus of Narrative Reviews will be an update on current understanding of the physiology of the disease or condition, diagnostic consideration, and treatment. These reviews should address a specific question or issue that is relevant for clinical practice. Narrative Reviews do not require (but may include) a systematic review of the literature search. Recommendations should be supported with evidence and should rely on recent systematic reviews and guidelines, if available, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention.
The basic structure of manuscripts reporting Narrative Reviews should include the following: Abstract (structured abstract of no more than 300 words); Introduction (150-250 words); Methods, if included (150-250 words); Discussion/Observations (1000-1250 words, with the following subsections, if appropriate: Pathophysiology, Clinical Presentation, Assessment and Diagnosis, Treatment, and Prognosis); and Conclusions (2-3 sentences).
Typical length: 2000-3500 words (maximum), with no more than a total of 5 tables and/or figures, and no more than 50-75 references. For an example of this type of article, see JAMA . 2015;314(23):2544-2554 .
Specific Components of a Narrative Review
Abstract (300 words)
Narrative Review articles should include a 3-part structured abstract of no more than 300 words using the headings listed below:
Importance: An overview of the topic and discussion of the main objective or reason for this review. Observations: The principal observations and findings of the review. Conclusions and Relevance: The conclusions of the review that are supported by the information, along with clinical applications. How the findings are clinically relevant should be specifically stated.
The first 2 to 3 sentences of the Introduction should draw in readers in such that they want to continue reading the article and should establish the importance of the Review. Reviews should include the clinical question or issue and its importance for general medical practice, specialty practice, or public health. The first paragraph should provide a general summary of the clinical problem (eg, obesity). The next paragraph should focus on the specific aspect of the clinical problem the article will explore (eg, treatments for obesity). Briefly summarize the epidemiology of the disease. This information should include disease prevalence and incidence and perhaps discussion of the presence and frequency of any relevant subpopulations and any geographic or seasonal variations of the disease if these are relevant. The third paragraph should discuss exactly what material will be covered in the Review (eg, obesity treatments).
Methods (150-250 words)
A Methods section is not required for Narrative Reviews, but may be included to summarize a literature search that was conducted for this Review. If included, briefly describe the characteristics of the literature searched and included in the review, including the bibliographic databases and other sources searched, search terms used, dates included in the search, date the literature search was conducted, and any process used to evaluate the literature.
Discussion/Observations (1000-1250 words)
The principal observations of the Narrative Review generally should include the subsections listed below, although each section may not be necessary for some topics. The word counts following each subsection are suggested to assist with keeping the overall Observations section limited to 1000-1250 words.
Pathophysiology (150-250 words). Provide a brief overview of the pathophysiology of the disease. The intent is to provide readers with sufficient background information about the underpinnings of a disease to provide context for the rest of the article. Clinical Presentation (150-250 words). Briefly describe the clinical characteristics that result in a patient seeking medical care for the condition or what features of the disease should lead a physician to evaluate or treat it. Assessment and Diagnosis (250-300 words). Describe the clinical examination for evaluation of the disease and explain the most salient physical examination findings. If laboratory or imaging studies are necessary, provide the sensitivity and specificity and diagnostic accuracy of these tests and consider providing positive and negative likelihood ratios. Sequences of diagnostic tests are best presented as algorithms or in tables. Treatment (250-500 words). Treatments should be based on the most recently available and highest level of evidence. Treatment options should be summarized in the text and presented in detail in tables along with an indication of the strength of evidence supporting the individual treatments. In general, treatment recommendations should be supported by a systematic review or a high-quality guideline. If possible, the costs for various treatments should be provided. Prognosis (100-150 words). A section outlining the overall prognosis for the condition, once treated, should be included.
For most Narrative Reviews, tables should be included that summarize the epidemiology, diagnostic tools, and therapies available for the disease. In some cases, these 3 topics may not all be relevant to the review topic and tables may be appropriately modified to fit the review. Include a fourth table that compares the findings of the review and current clinical practice recommendations or diagnostic and therapeutic uncertainty or controversies.
Table 1: Major epidemiologic and burden of disease facts Table 2: Major diagnostic tools available Table 3: Major therapies available Table 4: Current clinical practice recommendations and/or diagnostic and therapeutic uncertainty, and controversies
Tables summarizing treatments should have information organized by category of treatment and then by individual treatments. Columns may include the treatment, strength of evidence supporting the treatment, the effect of the treatment (preferably shown as the treatment's effect as compared to control on the measured outcome together with 95% confidence intervals), adverse effects, and very brief explanatory comments, if necessary. Lengthy text-based tables should be avoided. Additional or lengthy tables may be published online only, if justified.
Figures that illustrate pathophysiology or clinical presentation may be included. Note: All figures will be re-created. For each proposed illustration, the authors should provide a list of the elements to be included in the illustration; 3-4 relevant recent references; example illustrations, if available; a working figure title and legend; and an explanation of how this new illustration would add to the published literature. We encourage videos, if appropriate, to illustrate a point made or process described in the Review.
Note: This journal publishes very few of these types of articles. These manuscripts describe an important issue in clinical medicine, public health, health policy, or medical research in a scholarly, thorough, well-referenced, systematic, and evidence-based manner.
A structured abstract is required. Maximum length: 3000 words of text (not including tables, figures, or references) with no more than a total of 4 tables and/or figures and no more than 50 references. For a recently published example, see JAMA . 2019;322(20):1996-2016 .
Clinical Challenge presents an actual patient scenario about a specific disease or condition with an accompanying clinical image.
Authors should provide 4 single-phrase plausible treatment options describing possible courses of action with one of these being the most correct response for the question "What Would You Do Next?" Manuscripts should include a brief discussion of the relevant clinical issues and provide well-supported (evidence-based) explanations discussing the 4 potential courses of action. For a recently published example, see JAMA . 2022;327(24):2448-2449. doi:10.1001/jama.2022.8384 .
All diagnostic and treatment recommendations should be supported by referencing recent authoritative texts or journal articles. Preferably, these recommendations should be supported by governmental or multisociety guidelines, clinical trials, meta-analyses, or systematic reviews. The text should have a maximum length of 850 words, consisting of no more than 250 words for the case presentation, question, and 4 one-sentence answers, followed by no more than 600 words that include the diagnosis and a brief discussion. There should be no more than 3 authors. At least 1 of the authors, ideally the corresponding author, should have sufficient expertise and experience with the topic. There should be no more than 10 references, and no more than 2 small figures totaling 3 image components (Figure 1, with no more than 2 components, for the case presentation; and Figure 2, with no more than 1 component, for the diagnosis and discussion).
Provide a short title that briefly describes the disease entity or case presentation and does not include the diagnosis. Do not include the patient's race, ethnicity, or country of origin in the title or the first line of the article. If this information is clinically relevant and necessary, it can be included in the case description.
In addition, the JAMA Network Patient Permission form must be completed and signed by the patient (or a family member if the patient has died, is a minor, or is an adult without decisional capacity) and included at the time of manuscript submission. Please read Patient Identification before submitting your manuscript.
The image and case presentation should be from the same patient and must not have been published previously. In some cases, additional figures may be included to accompany the answer explanations (see description of additional figure(s) above). All images submitted should be high-quality .jpg or .tif files. Submit the original version of all image files at the highest resolution possible without labels. In general, the original image file should have a minimum resolution of 350 dpi at a width of about 5 inches. Do not increase the original resolution, resize, or crop the image; where applicable, we will crop to maintain patient confidentiality. If any labels, arrowheads, or A/B panel indicators are desired, provide a separate labeled version of the figure(s) for reference. All labels will be reformatted to journal style.
For more information on how to submit figures, see Figures.
We would like to receive common problems presenting uncommonly, rather than unusual or rare conditions (ie, "zebras"). These cases should be of interest to clinicians; they should be problems that clinicians are likely to encounter and have an outstanding image that illustrates the disorder and contributes to the diagnostic challenge.
Manuscripts not meeting these guidelines will not be considered.
Diagnostic Test Interpretation presents the results of a diagnostic test from a single patient and explores the clinical application of the test result. The Diagnostic Test Interpretation is intended to help clinicians understand the underlying rationale in ordering tests, interpreting test results, and acting on the diagnostic test findings.
The diagnostic test result must be obtained from the care of an actual patient and must include that patient's written permission. The JAMA Network Patient Permission form should be read and completed and signed by the patient (or a family member if the patient has died, is a minor, or is an adult without decisional capacity) and included at the time of manuscript submission. The results of laboratory, pathologic, or radiographic tests are appropriate but clinical images are not. Results of the diagnostic test of interest (and related tests) and the range of reference values should be included after the case. Authors of manuscripts based on clinical images should consult the instructions for Clinical Challenge .
Provide a short title that briefly describes the disease entity or case presentation and does not include the diagnosis. Do not include the patient's race, ethnicity, or country of origin in the title or first line of the article. If this information is clinically relevant and necessary, it can be included in the case description.
Manuscripts for Diagnostic Test Interpretation should have the following sections:
Case presentation. The case presentation should be brief and focus on the diagnostic test in question. At the end of the case presentation the pertinent diagnostic test results and reference ranges should be provided (200 words). Include: JAMA Exclude: Specialty Journals, JNO Comments: How do you interpret these test results? How do you interpret these test results? (or What would you do next?) Four plausible responses should be provided. While most Diagnostic Test Interpretation articles will pose the question "How do you interpret these results?" a subset may more appropriately focus on the next best step regarding workup of the abnormal test result. In these cases, the question "How do you interpret these test results?" can be replaced with "What would you do next?" Either question should be presented in the format of a multiple choice question with a single correct (or best) answer. The answers may be brief phrases or short sentences, should be similar in length, and should be arranged alphabetically by first word in the answer. Response options should not describe treatments (about 50 words). Include: CAR,ONC Exclude: JAMA, DER, IMD, NEU, OPH, PED, OTO, PSY, SUR, JNO Comments: How do you interpret these test results? Test characteristics. A brief review of the diagnostic test should be provided (approximately 200 words). For biomarkers, this should include a brief description of the related physiology. Test accuracy should be reported using sensitivity and specificity or likelihood ratios, and predictive values should be provided for common clinical scenarios. Please use likelihood ratios whenever possible, since they do not depend on disease prevalence. The prevalence of the disease should be stated so that the pretest probability may be estimated. For example, "For patients with a typical disease prevalence of 10%, the predictive values of positive and negative test results are approximately 50% and 1%, respectively." Discussion of the application and utility of the diagnostic test should be based on a high-quality systematic review or authoritative practice guideline. If a more recent, original study supersedes or adds meaningfully to the prior synthesis of research, that article also should be cited. The approximate fee for the test should be provided. For example, some fees for laboratory tests can be obtained from the Medicare fee schedules . Radiology procedure fees can be found at the Medicare Physician Fee Schedule website . Application of test result to this patient. A brief discussion of how the diagnostic test result will facilitate the next steps in a patient's management should be presented. Please also address the correct answer to the question about test interpretation in this section (200 words). What Are Alternative Diagnostic Testing Approaches? If there are different testing strategies that can be used to evaluate patients to establish a diagnosis, please discuss them (100 words). Patient Outcome. Long-term follow-up (most recent as possible) regarding the patient's condition and outcome of treatment is necessary (100 words). Clinical Bottom Line. Please provide a bulleted list of 3-5 items that reflect the most important message readers should obtain from this article.
The overall text of the manuscript should have a maximum of 850 words, no more than 10 references, and no more than 3 authors. At least 1 of the authors, ideally the corresponding author, should have sufficient expertise and experience with the topic. The case presentation must not have been previously published.
For an example of this article type, see JAMA . 2022;327(13):1284-1285. doi:10.1001/jama.2022.2037 .
If there are questions about patient identifiability, please contact the editorial office. Authors interested in submitting a manuscript for Diagnostic Test Interpretation should contact the editorial office prior to manuscript preparation and submission by sending an email to Kristin Walter at [email protected] .
Viewpoints may address virtually any important topic in medicine, public health, research, discovery, prevention, ethics, health policy, or health law and generally are not linked to a specific article. Viewpoints should be well focused, scholarly, and clearly presented but should not include the findings of new research or data that have not been previously published.
Viewpoints must have no more than 3 authors. Editors encourage diversity of gender, race, ethnicity, geographic location, and discipline for Viewpoint authors, and the first author should have sufficient expertise and experience with the topic to provide an authoritative opinion. The text should include the full name, academic degrees, and no more than 2 institutional affiliations for each author. Maximum length: up to 1200 words of text—or 1000 words of text with 1 small table or figure—and no more than 7 references, which should be as current as possible. Viewpoints not meeting these guidelines will not be considered.
Most essays published in A Piece of My Mind are personal vignettes (eg, exploring the dynamics of the patient-physician relationship) taken from wide-ranging experiences in medicine; occasional pieces express views and opinions on the myriad issues that affect the profession. If the patient(s) described in these manuscripts is identifiable, a Patient Permission form , which provides consent for publication, must be completed and signed by the patient(s) or family member(s) and submitted with the manuscript. Manuscripts that describe identifiable patients that do not have a signed form will not be reviewed. Omitting data or making data less specific to deidentify patients is acceptable, but changing any such data is not acceptable. Fictional or composite accounts are not permitted.
Manuscripts are not published anonymously or pseudonymously and must have no more than 3 authors. All manuscripts must be submitted formally via the journal's manuscript submission system; we do not review drafts or unfinished manuscripts prior to submission. Length limit: 1600 words.
Poems related to the medical experience, whether from the point of view of a health care worker or patient, or simply an observer, will be considered. Poems should be original, not previously published or under consideration elsewhere, no longer than 44 lines, and with individual lines no longer than 55 characters (including spaces). Authors should submit each poem separately (ie, one poem per submission record, and only one author per poem). Submissions containing multiple poems will be returned with instructions to split into individual files. Do not submit artwork, music/audio, or other accompanying materials, which are not considered. All poems must be submitted online via the online manuscript submission and review system . Authors of poems that are accepted for publication are required to complete Authorship Forms and transfer copyright to the publisher as part of a publishing agreement. An email with links to the Authorship Form will be sent to authors for completion before final acceptance. Author requests to republish poems are generally granted by our permissions department following a formal request.
Questions about submitting poems (but not submissions) may be sent to [email protected] .
Letters discussing a recent article in this journal should be submitted within 4 weeks of publication of the article in print. 3 Letters received after 4 weeks will rarely be considered. Letters should not exceed 400 words of text and 5 references, 1 of which should be to the recent article. Letters may have no more than 3 authors. The text should include the full name, academic degrees, and a single institutional affiliation for each author and the email address for the corresponding author. Letters must not duplicate other material published or submitted for publication and should not include unpublished data. Letters not meeting these specifications are generally not considered. Letters being considered for publication ordinarily will be sent to the authors of the original article, who will be given the opportunity to reply. Letters will be published at the discretion of the editors and are subject to abridgement and editing for style and content. To read more about Letters, see the AMA Manual of Style .
Replies by authors should not exceed 500 words of text and 6 references. They should have no more than 3 authors.
Clinical Trial
These manuscripts include reports of Randomized Clinical Trials, Parallel-Design Double-blind Trials, Crossover Trials, Equivalence and Noninferiority Trials, Cluster Trials, and Nonrandomized Controlled Trials.
The ICMJE defines a clinical trial as any research project that prospectively assigns human participants to intervention or comparison groups to study the cause-and-effect relationship between an intervention and a health outcome. 4 Interventions include but are not limited to drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, process-of-care changes, and the like. All manuscripts reporting clinical trials, including those limited to secondary exploratory or post hoc analysis of trial outcomes, must include the following:
- Copy of the original trial protocol, including the complete statistical analysis plan and any amendments. The journal recommends using the SPIRIT reporting guidelines when preparing original protocols (see Protocols ).
- CONSORT flow diagram (see Figure ).
- Completed trial checklist (see Checklist ).
- Registry at an appropriate online public clinical trial registry (see Trial Registration requirements).
- A Data Sharing Statement to indicate if data will be shared or not. Specific questions regarding the sharing of data are included in the manuscript submission system.
For additional guidance on reporting Randomized Clinical Trial, Parallel-Design Double-blind Trial, Crossover Trial, Equivalence and Noninferiority Trial, Cluster Trial, and Nonrandomized Controlled Trial, see Study Types .
Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria, or data sources, and how these were selected for the study); the essential features of any interventions; the primary and secondary outcome measures (consistent with those reported in the trial protocol); the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions.
A structured abstract is required, and trial registration information (registry name, trial ID, and URL) must be listed at the end of the abstract; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Randomized Clinical Trial" or, for Nonrandomized Controlled Trials, "A Nonrandomized Controlled Trial." To read more about clinical trials, see the AMA Manual of Style .
Trial Registration:
In concert with the ICMJE, JAMA Network requires, as a condition of consideration for publication, registration of all trials in a public trials registry that is acceptable to the ICMJE (ie, the registry must be owned by a not-for-profit entity, be publicly accessible, and require the minimum registration data set as described by ICMJE). 4 , 8 , 9
Acceptable trial registries include the following and others listed at http://www.icmje.org :
- anzctr.org.au
- clinicaltrials.gov
- trialregister.nl
- umin.ac.jp/ctr
All clinical trials, regardless of when they were completed, and secondary analyses of original clinical trials must be registered before submission of a manuscript based on the trial. Secondary data analyses of primary (parent) clinical trials should not be registered as separate clinical trials, but instead should reference the trial registration number of the primary trial. Please note: for clinical trials starting patient enrollment after July 2005, trials must have been registered before onset of patient enrollment. For trials that began before July 2005 but that were not registered before September 13, 2005, trials must have been registered before journal submission. Trial registry name, registration identification number, and the URL for the registry should be included at the end of the abstract and also in the space provided on the online manuscript submission form.
Authors of manuscripts reporting clinical trials must submit trial protocols (including the complete statistical analysis plan) along with their manuscripts. Protocols in non-English languages should be translated into English. This should include the original approved protocol and statistical analysis plan, and all subsequent amendments to either document. Do not submit a summary version that was published as an article in another journal. If the manuscript is accepted, the protocol and statistical analysis plan will be published as a supplement.
CONSORT Flow Diagram and Checklist:
Manuscripts reporting the results of randomized trials must include the CONSORT flow diagram showing the progress of patients throughout the trial. The CONSORT checklist also should be completed and submitted with the manuscript. 10
Figure. Profile of a Randomized Clinical Trial

Trial Protocol
These manuscripts are documents that describe the organization and plan for a randomized clinical trial, including the trial's objective(s), design, methodology, all outcomes to be measured, and statistical analysis plan. All trial protocol manuscripts must include a copy of the trial protocol including the complete statistical analysis plan (see Protocols ). All clinical trials that have begun randomization must be registered at an appropriate online public registry (see Trial Registration requirements). Follow SPIRIT Reporting Guidelines .
A structured abstract is required, and trial registration information (registry name, trial ID, and URL) must be listed at the end of the abstract; for more information, see instructions for preparing Abstracts for Trial Protocols . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Trial Protocol."
These manuscripts are systematic, critical assessments of literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention, and that includes a statistical technique for quantitatively combining the results of multiple studies that measure the same outcome into a single pooled or summary estimate. All articles or data sources should be searched for and selected systematically for inclusion and critically evaluated, and the search and selection process should be described in the manuscript. The specific type of study or analysis, population, intervention, exposure, and tests or outcomes should be described for each article or data source. The data sources should be as current as possible, ideally with the search having been conducted within several months of manuscript submission. Authors of reports of meta-analyses of clinical trials should submit the PRISMA flow diagram and checklist . Authors of meta-analyses of observational studies should submit the MOOSE checklist . Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Meta-analysis . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material), with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Meta-analysis." To read more about meta-analyses, see the AMA Manual of Style .
Other Observational Studies
These manuscripts include Cohort Study, Case-Control Study, Cross-sectional Study, Case Series, Economic Evaluation, Decision Analytical Model, Comparative Effectiveness Research, Genetic Association Study, Diagnostic/Prognostic Study, Quality Improvement Study, Survey Study, and Qualitative Study. Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria and/or participation or response rates, or data sources, and how these were selected for the study); the essential features of any interventions or exposures; the main outcome measures; the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions and relevant implications for clinical practice or health policy. Data included in research reports must be original and should be as timely and current as possible (see Timeliness of Data ). Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references.
Format My Manuscript
Manuscript preparation and submission requirements.
All manuscripts must be submitted online via the online manuscript submission and review system .
At the time of submission, complete contact information (affiliation, postal/mail address, email address, and telephone numbers) for the corresponding author is required. First and last names, email addresses, and institutional affiliations of all coauthors are also required. After the manuscript is submitted, the corresponding author will receive an acknowledgment confirming receipt and a manuscript number. Authors will be able to track the status of their manuscripts via the online system. After manuscript submission, all authors of papers under consideration for publication will be sent a link to the Authorship Form to complete and submit. See other details in these instructions for additional requirements. 2 , 4
As recommended by the ICMJE, "if the manuscript has been submitted previously to another journal, it is helpful to include the previous editors' and reviewers' comments with the submitted manuscript, along with the authors' responses to those comments." 4 It is not uncommon for manuscripts to have been submitted to and peer reviewed by other journals and sharing this information will not bias an editor's decision for this journal. Thus, authors are encouraged to submit these previous comments in their entirety and indicate how they have revised the manuscript in response to these comments, which may expedite the review process. In the submission system, there is a file type for Previous Peer Review and Editorial Comments.
Include a cover letter and complete contact information for the corresponding author (affiliation, postal/mail address, email address, and telephone number) and whether the authors have published, posted, or submitted any related papers from the same study (see Previous Publication, Related Manuscripts and Reports, and Preprints ).
Manuscripts should be prepared in accordance with the AMA Manual of Style , 11th edition, 2 and/or the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals . 4
Include in the manuscript file a title page, abstract, text, references, and as appropriate, figure legends and tables. Start each of these sections on a new page, numbered consecutively, beginning with the title page. Figures should be submitted as separate files (1 file per figure) and not included in the manuscript text.
We recommend individual file sizes of no more than 500 kB and not exceeding 1 MB, with the total size for all files not exceeding 5 MB (not including any video files).
For submission and review, please submit the manuscript as a Word document. Do not submit your manuscript in PDF format.
Use 10-, 11-, or 12-point font size, double-space text, and leave right margins unjustified (ragged).
The title page should be the first page of your manuscript file. It should include a manuscript title; the full names, highest academic degrees, and affiliations of all authors (if an author's affiliation has changed since the work was done, the new affiliation also should be listed); name and complete contact information for corresponding author; and manuscript word count (not including title, abstract, acknowledgment, references, tables, and figure legends).
Titles should be concise, specific, and informative. 2(p8) Please limit the length of titles to 100 characters (including spaces) for reports of research and other major articles and 60 characters for shorter article types such as opinion articles and Letters as well as for subtitles to major articles. For scientific manuscripts, do not use overly general titles, declarative titles, titles that include the direction of study results, or questions as titles. For reports of clinical trials, meta-analyses, and systematic reviews, include the type of study as a subtitle (eg, A Randomized Clinical Trial, A Meta-analysis, A Systematic Review). For reports of other types of research, do not include study type or design in the title or subtitle. Depending on the context, avoid inclusion of specific locations (eg, state, province, or country) and specific years. To read more about titles, see the AMA Manual of Style .
In the manuscript, include a separate section called "Key Points" before the Abstract.
This feature provides a quick structured synopsis of the findings of your manuscript (required only for research and review manuscripts), following 3 key points: Question, Findings, and Meaning. Limit this section to 75-100 words or less.
Question: Focused question based on the study hypothesis or goal/purpose. Limit to 1 sentence. Findings: Results of the study/review. Include the design (eg, clinical trial, cohort study, case-control study, meta-analysis). Focus on primary outcome(s) and finding(s). Do not emphasize secondary outcomes. Report basic numbers only but state if results are statistically significant or not significant; do not include results of statistical tests or measures of variance (see example below). Can include 1 to 2 sentences. Meaning: Key conclusion and implication based on the primary finding(s). Limit to 1 sentence. Example of Research Article Question: What is the immunogenicity of an inactivated influenza A vaccine with and without adjuvant? Findings: In this randomized clinical trial that included 980 adults, the proportion achieving an effective antibody response was 84% with adjuvant vs 2% without adjuvant, a significant difference. Meaning: In an influenza pandemic the use of an adjuvant with inactivated influenza A vaccine may be warranted. Include: All Journals except JNO and JHF Exclude: JNO and JHF Comments: Example of Review Article Example of Review Article Question: What are the most effective medical treatments for adult chronic sinusitis? Findings: In this systematic review, symptoms of chronic sinusitis were improved with saline irrigation and topical corticosteroid therapy compared to no therapy. Compared with placebo, 3-week courses of systemic corticosteroids or oral doxycycline were associated with reduced polyp size, and a 3-month course of macrolide antibiotic was associated with improved symptoms in patients without polyps. Meaning: First-line therapy for chronic sinusitis should begin with daily topical intranasal corticosteroid in conjunction with saline irrigation; subsequent therapies should be based on the patient's polyp status and severity of symptoms.
Include a structured abstract for reports of original data, meta-analyses, and systematic reviews. Abstracts should be prepared in JAMA Network style—see instructions for preparing abstracts below. Abstracts are not required for Editorials, Viewpoints, and special features. No information should be reported in the abstract that does not appear in the text of the manuscript. To read more about abstracts, see the AMA Manual of Style .
Abstracts for Reports of Original Data:
Reports of original data should include an abstract of no more than 350 words using the headings listed below. For brevity, parts of the abstract may be written as phrases rather than complete sentences. Each section should include the following content:
Importance: The abstract should begin with a sentence or 2 explaining the clinical (or other) importance of the study question. Objective: State the precise objective or study question addressed in the report (eg, "To determine whether..."). If more than 1 objective is addressed, the main objective should be indicated and only key secondary objectives stated. If an a priori hypothesis was tested, it should be stated. Design: Describe the basic design of the study and include the specific study type (eg, randomized clinical trial, cohort, cross-sectional, case-control, case series, survey, meta-analysis, bibliometric analysis). State the years of the study and the duration of follow-up. For older studies (eg, those completed >3 years ago), add the date of the analysis being reported. If applicable, include the name of the study (eg, the Framingham Heart Study). As relevant, indicate whether observers were blinded to patient groupings, particularly for subjective measurements. Setting: Describe the study setting to assist readers to determine the applicability of the report to other circumstances, for example, multicenter, population-based, primary care or referral center(s), etc. Participants: State the clinical disorders, important eligibility criteria, and key sociodemographic features of patients (or other study participants). The numbers of eligible participants and how they were selected should be provided, including the number approached but who refused or were excluded. For selection procedures, these terms should be used, if appropriate: random sample (where random refers to a formal, randomized selection in which all eligible individuals have a fixed and usually equal chance of selection); population-based sample; referred sample; consecutive sample; volunteer sample; convenience sample. If matching is used for comparison groups, characteristics that are matched should be specified. In follow-up studies, the proportion of participants who completed the study must be indicated.
Note: The preceding 3 sections are usually combined for accepted papers during the editing process as "Design, Setting, and Participants," but for manuscript submission these sections should be kept separate.
Intervention(s) (for clinical trials) or Exposure(s) (for observational studies): The essential features of any interventions, or exposures, should be described, including their method and duration. The intervention, or exposure, should be named by its most common clinical name, and nonproprietary drug names should be used. Main Outcome(s) and Measure(s): Indicate the primary study outcome measurement(s) as planned before data collection began. If the manuscript does not report the main planned outcomes of a study, this fact should be stated and the reason indicated. State clearly if the hypothesis being tested was formulated during or after data collection. Explain outcomes or measurements unfamiliar to a general medical readership. Results: Summary demographic information (eg, characteristics such as sex and age) and the number of study participants should be reported in the first sentence of the Results paragraph. The main outcomes of the study should be reported and quantified, including final included/analyzed sample. When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P =.13). P values should never be presented alone without the data that are being compared. See also Reporting Standards and Data Presentation . Measures of relative risk also may be reported (eg, relative risk, hazard ratios) and should include confidence intervals. Studies of screening and diagnostic tests should report sensitivity, specificity, and likelihood ratio. If predictive value or accuracy is reported, prevalence or pretest likelihood should be given as well. All randomized clinical trials should include the results of intention-to-treat analysis as well. In intervention studies, the number of patients withdrawn because of adverse effects should be given. Approaches such as number needed to treat to achieve a unit of benefit may be included when appropriate. All surveys should include response/participation rates. Conclusions and Relevance: Provide only conclusions of the study that are directly supported by the results. Give equal emphasis to positive and negative findings of equal scientific merit. Also, provide a statement of relevance indicating implications for clinical practice or health policy, avoiding speculation and overgeneralization. The relevance statement may also indicate whether additional study is required before the information should be used in clinical settings. Trial Registration: For clinical trials only (not nontrial observational studies), the name of the trial registry, registration number, and URL of the registry must be included. See Trial Registration .
Abstracts for Meta-analysis:
Manuscripts reporting the results of meta-analyses should include an abstract of no more than 350 words using the headings listed below. The text of the manuscript should also include a section describing the methods used for data sources, study selection, data extraction, and data synthesis. Each heading should be followed by a brief description:
Importance: A sentence or 2 explaining the importance of the systematic review question that is used to justify the meta-analysis. Objective: State the precise primary objective of the meta-analysis. Indicate whether the systematic review for the meta-analysis emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being analyzed. Data Sources: Succinctly summarize data sources, including years searched. The search should include the most current information possible, ideally with the search being conducted within several months before the date of manuscript submission. Potential sources include computerized databases and published indexes, registries, meeting abstracts, conference proceedings, references identified from bibliographies of pertinent articles and books, experts or research institutions active in the field, and companies or manufacturers of tests or agents being reviewed. If a bibliographic database is used, state the exact indexing terms used for article retrieval, including any constraints (for example, English language or human study participants). If abstract space does not permit this level of detail, summarize sources in the abstract including databases and years searched, and place the remainder of the information in the Methods section. Study Selection: Describe inclusion and exclusion criteria used to select studies for detailed review from among studies identified as relevant to the topic. Details of selection should include particular populations, interventions, outcomes, or methodological designs. The method used to apply these criteria should be specified (for example, blinded review, consensus, multiple reviewers). State the proportion of initially identified studies that met selection criteria. Data Extraction and Synthesis: Describe guidelines (eg, PRISMA , MOOSE ) used for abstracting data and assessing data quality and validity. The method by which the guidelines were applied should be stated (for example, independent extraction by multiple observers). Indicate whether data were pooled using a fixed-effect or random-effects model. Main Outcome(s) and Measure(s): Indicate the primary study outcome(s) and measurement(s) as planned before data collection began. If the manuscript does not report the main planned outcomes of a study, this fact should be stated and the reason indicated. State clearly if the hypothesis being tested was formulated during or after data collection. Explain outcomes or measurement unfamiliar to a general medical readership. Results: Provide the number of studies and patients/participants in the analysis and state the main quantitative results of the review. When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P = .13). P values should never be presented alone without the data that are being compared. See also Reporting Standards and Data Presentation . Meta-analyses should state the major outcomes that were pooled and include odds ratios or effect sizes and, if possible, sensitivity analyses. Evaluations of screening and diagnostic tests should include sensitivity, specificity, likelihood ratios, receiver operating characteristic curves, and predictive values. Assessments of prognosis should summarize survival characteristics and related variables. Major identified sources of variation between studies should be stated, including differences in treatment protocols, co-interventions, confounders, outcome measures, length of follow-up, and dropout rates. Conclusions and Relevance: The conclusions and their applications (clinical or otherwise) should be clearly stated, limiting interpretation to the domain of the review.
Abstracts for Systematic Reviews or Special Communications:
Systematic Review articles should include a structured abstract of no more than 350 words using the headings listed below.
Importance: Include 1 or 2 sentences describing the clinical question or issue and its importance in clinical practice or public health. Objective: State the precise primary objective of the review. Indicate whether the review emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being reviewed. Evidence Review: Describe the information sources used, including the search strategies, years searched, and other sources of material, such as subsequent reference searches of retrieved articles. Methods used for inclusion of identified articles and quality assessment should be explained. Findings: Include a brief summary of the number of articles included, numbers of various types of studies (eg, clinical trials, cohort studies), and numbers of patients/participants represented by these studies. Summarize the major findings of the review of the clinical issue or topic in an evidence-based, objective, and balanced fashion, with the highest-quality evidence available receiving the greatest emphasis. Provide quantitative data. Conclusions and Relevance: The conclusions should clearly answer the questions posed if applicable, be based on available evidence, and emphasize how clinicians should apply current knowledge. Conclusions should be based only on results described in the abstract Findings subsection.
Abstracts for Narrative Reviews or Special Communications:
Importance: An overview of the topic and discussion of the main objective or reason for this review. Observations: The principal observations and findings of the review. Conclusions and Relevance: The conclusions of the review that are supported by the information, along with clinical applications. How the findings are clinically relevant should be specifically stated.
Ratings of the quality of the evidence
Tables summarizing evidence should include ratings of the quality of the evidence. Use the rating scheme listed below with ratings of 1-5 for Reviews that include individual studies (modified from the Oxford Centre for Evidence-based Medicine for ratings of individual studies).
Do not use abbreviations in the title or abstract and limit their use in the text. Expand all abbreviations at first mention in the text. To read more about abbreviation use, see the AMA Manual of Style .
Laboratory values are expressed using conventional units of measure, with relevant Système International (SI) conversion factors expressed secondarily (in parentheses) only at first mention. Articles that contain numerous conversion factors may list them together in a paragraph at the end of the Methods section. In tables and figures, a conversion factor to SI should be presented in the footnote or legend. The metric system is preferred for the expression of length, area, mass, and volume. For more details, see the Units of Measure conversion table on the website for the AMA Manual of Style . 2
To read more about units of measure, click here .
Use nonproprietary names of drugs, devices, and other products and services, unless the specific trade name of a drug is essential to the discussion. 2(pp567-569) In such cases, use the trade name once and the generic or descriptive name thereafter. Do not include trademark symbols. To read more about names of drugs, see the AMA Manual of Style .
Authors describing genes or related structures in a manuscript should include the names and official symbols provided by the US National Center for Biotechnology Information (NCBI) or the HUGO Gene Nomenclature Committee . Before submission of a research manuscript reporting on large genomic data sets (eg, protein or DNA sequences), the data sets should be deposited in a publicly available database, such as NCBI's GenBank , and a complete accession number (and version number if appropriate) must be provided in the Methods section or Acknowledgment of the manuscript. To read more about gene nomenclature, see the AMA Manual of Style .
JAMA does not republish text, tables, figures, or other material from other publishers, except under rare circumstances. Please delete any such material and replace with originals.
The submission and publication of content created by artificial intelligence, language models, machine learning, or similar technologies is discouraged, unless part of formal research design or methods, and is not permitted without clear description of the content that was created and the name of the model or tool, version and extension numbers, and manufacturer. Authors must take responsibility for the integrity of the content generated by these models and tools. See also Use of AI in Publication and Research .
Authors are responsible for the accuracy and completeness of their references and for correct text citation. Number references in the order they appear in the text; do not alphabetize. In text, tables, and legends, identify references with superscript arabic numerals. When listing references, follow AMA style and abbreviate names of journals according to the journals list in PubMed . List all authors and/or editors up to 6; if more than 6, list the first 3 followed by "et al." Note: Journal references should include the issue number in parentheses after the volume number.
Examples of reference style:
Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficileinfection. JAMA . 2014;312(17):1772-1778. Murray CJL. Maximizing antiretroviral therapy in developing countries: the dual challenge of efficiency and quality [published online December 1, 2014]. JAMA . doi:10.1001/jama.2014.16376 Centers for Medicare & Medicaid Services. CMS proposals to implement certain disclosure provisions of the Affordable Care Act. http://www.cms.gov/apps/media/press/factsheet.asp?Counter=4221 . Accessed January 30, 2012. McPhee SJ, Winker MA, Rabow MW, Pantilat SZ, Markowitz AJ, eds. Care at the Close of Life: Evidence and Experience . New York, NY: McGraw Hill Medical; 2011.
For more examples of electronic references, click here .
Tables and Figures
Restrict tables and figures to those needed to explain and support the argument of the article and to report all outcomes identified in the Methods section. Number each table and figure and provide a descriptive title for each. Every table and figure should have an in-text citation. Verify that data are consistently reported across text, tables, figures, and supplementary material.
See also Tables and Figures .
Frequency data should be reported as "No. (%)," not as percentages alone (exception, sample sizes exceeding ~10,000). Whenever possible, proportions and percentages should be accompanied by the actual numerator and denominator from which they were derived. This is particularly important when the sample size is less than 100. Do not use decimal places (ie, xx%, not xx.xx%) if the sample size is less than 100. Tables that include results from multivariable regression models should focus on the primary results. Provide the unadjusted and adjusted results for the primary exposure(s) or comparison(s) of interest. If a more detailed description of the model is required, consider providing the additional unadjusted and adjusted results in supplementary tables.
Tables have a minimum of 2 columns. Comparisons must read across the table columns.
Do not duplicate data in figures and tables. For all primary outcomes noted in the Methods section, exact values with measures of uncertainty should be reported in the text or in a table and in the Abstract, and not only represented graphically in figures.
Pie charts and 3-D graphs should not be used and should be revised to alternative graph types.
Bar graphs should be used to present frequency data only (ie, numbers and rates). Avoid stacked bar charts and consider alternative formats (eg, tables or splitting bar segments into side-by-side bars) except for comparisons of distributions of ordinal data.
Summary data (eg, means, odds ratios) should be reported using data markers for point estimates, not bars, and should include error bars indicating measures of uncertainty (eg, SDs, 95% CIs). Actual values (not log-transformed values) of relative data (for example, odds ratios, hazard ratios) should be plotted on log scales.
For survival plots, include the number at risk for each group included in the analysis at intervals along the x-axis scale. For any figures in which color is used, be sure that colors are distinguishable.
All symbols, indicators, line styles, and colors in statistical graphs should be defined in a key or in the figure legend. Axes in statistical graphs must have labels. Units of measure must be provided for continuous data.
Note: All figures are re-created by journal graphics experts according to reporting standards using the JAMA Network style guide and color palette.
- Number all tables in the order of their citation in the text.
- Include a brief title for each table (a descriptive phrase, preferably no longer than 10 to 15 words).
- Include all tables at the end of the manuscript file.
- Refer to Categories of Articles for limits on the number of tables.
- NOTE: Do not embed tables as images in the manuscript file or upload tables in image formats, and do not upload tables as separate files.
Table Creation
Use the table menu in the software program used to prepare the text. Tables can be built de novo using Insert→Table or copied into the text file from another document (eg, Word, Excel, or a statistical spreadsheet).
Avoid using tabs, spaces, and hard returns to set up the table; such tables will have to be retyped, creating delays and opportunities for error.
Tables should be single-spaced and in a 10- or 12-point font (do not shrink the point size to fit the table onto the page). Do not draw extra lines or rules—the table grid will display the outlines of each cell.
Missing data and blank space in the table field (ie, an empty cell) may create ambiguity and should be avoided; use abbreviations such as NA for not applicable or not available. Each piece of data needs to be contained in its own cell. Do not try to align cells with hard returns or tabs; alignment will be imposed in the production system if the manuscript is accepted. To show an indent, add 2 spaces.
When presenting percentages, include numbers (numerator and denominator).
Include statistical variability where applicable (eg, mean [SD], median [IQR]). For additional detail on requirements for data presentation in tables, see Statistical Methods and Data Presentation .
Place each row of data in a separate row of cells, and note that No. (%) and measures of variability are presented in the same cell as in the example Table 1 below:
Table 1. Baseline Values in the Editors' Health Study

SI conversion factors: To convert cholesterol to mmol/L, multiply values by 0.0259.
Note that JAMA Network journals report laboratory values in conventional units. In a table, provide a footnote with the conversion factor to SI units. For a calculator of SI and conventional units, see the AMA Manual of Style . 2
To present data that span more than 1 row, merge the cells vertically. For example, in Table 2 the final column presents the P value for overall age comparisons.
Table 2. Blood Pressure Values Stratified by Age

The table should be constructed such that the primary comparison reads horizontally. For example, see Table 3 (incorrect) and Table 4 (correct).
Table 3. Patient Data by Study Group

Table 4. Patient Data by Study Group

If a table must be continued, repeat the title and column headings on the second page, followed by "(continued)."
Table Footnotes
Footnotes to tables may apply to the entire table, portions (eg, a column), or an individual entry.
The order of the footnotes is determined by the placement in the table of the item to which the footnote refers.
When both a footnote letter and reference number follow data in a table, set the superscript reference number first followed by a comma and the superscript letter.
Use superscript letters (a, b, c) to mark each footnote and be sure each footnote in the table has a corresponding note (and vice versa).
List abbreviations in the footnote section and explain any empty cells.
If relevant, add a footnote to explain why numbers may not sum to group totals or percentages do not add to 100%.
For more detail on the components and recommended structure of tables, see the AMA Manual of Style . 2
Number all figures (graphs, charts, photographs, and illustrations) in the order of their citation in the text. The number of figures should be limited. Avoid complex composite or multipart figures unless justified. See Categories of Articles for limits on the number of figures and/or tables according to article type.
For initial manuscript submissions, figures must be of sufficient quality and may be embedded at the end of the file for editorial assessment and peer review. If a revision is requested and before a manuscript is accepted, authors will be asked to provide figures that meet the requirements described in Figure File Requirements for Publication .
Graphs, charts, some illustrations, titles, legends, keys, and other elements related to figures in accepted manuscripts will be re-created and edited according to JAMA Network style and standards prior to publication. Online-only figures will not be edited or re-created (see Online-Only Supplements and Multimedia ).
Image Integrity
Preparation of scientific images (clinical images, radiographic images, micrographs, gels, etc) for publication must preserve the integrity of the image data. Digital adjustments of brightness, contrast, or color applied uniformly to an entire image are permissible as long as these adjustments do not selectively highlight, misrepresent, obscure, or eliminate specific elements in the original figure, including the background. Selective adjustments applied to individual elements in an image are not permissible. Individual elements may not be moved within an image field, deleted, or inserted from another image. Cropping may be used for efficient image display or to deidentify patients but must not misrepresent or alter interpretation of the image by selectively eliminating relevant visual information. Juxtaposition of elements from different parts of a single image or from different images, as in a composite, must be clearly indicated by the addition of dividing lines, borders, and/or panel labels.
The submission and publication of images created by artificial intelligence, machine learning tools, or similar technologies is discouraged, unless part of formal research design or methods, and is not permitted without clear description of the content that was created and the name of the model or tool, version and extension numbers, and manufacturer. Authors must take responsibility for the integrity of the content generated by these models and tools. See also Use of AI in Publication and Research .
When inappropriate images or image adjustments are detected by the journal staff, authors will be asked for an explanation and will be requested to submit the image as originally captured prior to any adjustment, cropping, or labeling. Authors may be asked to resubmit the image prepared in accordance with the above standards.
Acceptable Figure Files for Initial Submission and Review
Each figure for the main article may be uploaded as a separate file or appended to the end of the manuscript with the figure titles and legends. Online-only figures must be combined into the PDF of the online-only supplement (see Online-Only Supplements and Multimedia ). Note: If a revision is requested and before acceptance, authors must upload each figure for the main article as a separate file and follow the instructions in Figure File Requirements for Publication .
See the Table of Figure Requirements for additional guidance for specific types of figures for suggested resolution and file formats. In general each figure should be no larger than 1 MB.
Figure File Requirements for Publication
Each figure for the main article must be uploaded as a separate file. Online-only figures must be combined into the PDF of the online-only supplement (see Online-Only Supplements and Multimedia ).
See the Table of Figure Requirements for additional guidance and file formats for specific types of figures.
Files created by vector programs are best for accurately plotting and maintaining data points. JAMA Network journals are unable to use file formats native to statistical software applications to prepare figures for publication; most statistical software programs allow users to save or export files in digital vector formats.
Images created digitally (by digital camera or electronically created illustrations) must meet the minimum resolution requirements at the time of creation. Electronically increasing the resolution of an image after creation causes a breakdown of detail and will result in an unacceptable poor-quality image. Each component of a composite image must be uploaded separately at submission and individually meet the minimum resolution requirement.
Color photographs should be submitted in RGB mode using profiles such as Adobe RGB or sRGB. Digital cameras capture images in RGB. Do not change any color settings once the file is on the computer. Black-and-white photographs (eg, radiographs, ultrasound images, CT and MRI scans, and electron micrographs) can be submitted in either RGB or grayscale modes.
Figure Titles and Legends (Captions)
At the end of the manuscript, include a title for each figure. The figure title should be a brief descriptive phrase, preferably no longer than 10 to 15 words. A figure legend (caption) can be used for a brief explanation of the figure or markers if needed and expansion of abbreviations. For photomicrographs, include the type of specimen, original magnification or a scale bar, and stain in the legend. For gross pathology specimens, label any rulers with unit of measure. Digitally enhanced images must be clearly identified in the figure legends as enhanced or manipulated, eg, computed tomographic scans, magnetic resonance images, photographs, photomicrographs, x-ray films.
Figures With Labels, Arrows, or Other Markers
Photographs, clinical images, photomicrographs, gel electrophoresis, and other types that include labels, arrows, or other markers must be submitted in 2 versions: one version with the markers and one without. Provide an explanation for all labels, arrows, or other markers in the figure legend. The Figure field in the File Description tab of the manuscript submission system allows for uploading of 2 versions of the same figure.
Number of Figures
Refer to Categories of Articles because there may be a limit on the number of figures by article type.
General Figure Guidelines
- Primary outcome data should not be presented in figures alone. Exact values with measure of variability should be reported in the text or table as well as in the abstract.
- All symbols, indicators (including error bars), line styles, colors, and abbreviations should be defined in a legend.
- Each axis on a statistical graph must have a label and units of measure should be labeled.
- Do not use pie charts, 3-D graphs, and stacked bar charts as these are not appropriate for accurate statistical presentation of data and should be revised to another figure type or converted to a table.
- Error bars should be included in both directions, unless only 1-sided variability was calculated.
- Values for ratio data—odds ratios, relative risks, hazard ratios—should be plotted on a log scale. Values for ratio data should not be log transformed.
- For footnotes, use letters (a, b, c, etc) not symbols.
- Do not submit figures with more than 4 panels unless otherwise justified.
- See the AMA Manual of Style for more guidance on figure types and components.
For images featuring patients or other identifiable persons, it is not acceptable to use black bars across the eyes in an attempt to deidentify. Cropping may be acceptable as long as the condition under discussion is clearly visible and necessary anatomic landmarks display. If the person in the image is possibly identifiable (not only by others but also by her/himself), permission for publication is required (see Patient Identification ).
Table of Figure Requirements

To present frequency data (numbers or percentages). Each bar represents a category.
Bar graphs are typically vertical but when categories have long titles or there are many of them, they may run horizontally.
The scale on the frequency axis should begin at 0, and the axis should not be broken.
If the data plotted are a percentage or rate, error bars may be used to show statistical variability.
Acceptable File Formats for Initial Submission: .ai, .bmp, .docx, .emf, .eps, .jpg, .pdf, .ppt, .psd, .tif, .wmf, .xls
Acceptable File Formats for Revision and Publication: .ai, .emf, .eps, .pdf, .wmf, .xls

To demonstrate the relationship between 2 or more quantitative variables, such as changes over time.
The dependent variable appears on the vertical axis (y) and the independent variable on the horizontal axis (x); the axes should be continuous, not broken.
Flow diagram

To show participant recruitment and follow-up or inclusions and exclusions (such as in a systematic review).
Acceptable File Formats for Initial Submission: .ai, .docx, .emf, .eps, .jpg, .pdf, .ppt
Acceptable File Formats for Revision and Publication: .ai, .docx, .emf, .eps, .pdf
Survival plot

To display the proportion or percentage of individuals (represented on the y-axis) remaining free of or experiencing a specific outcome over time (represented on the x-axis).
The curve should be drawn as a step function (not smoothed).
The number of individuals followed up for each time interval (number at risk) should be shown underneath the x-axis.
Box-and-whisker plot (box plot)

To show data distribution from 1 or more groups, particularly aggregate/summary data.
Each element should be described (the ends of the boxes, the middle line, and the whiskers). Data points that fall beyond the whiskers are typically shown as circles.
Forest plot

To illustrate summary data, particularly in meta-analyses and systematic reviews.
The data are presented both tabularly and graphically.
The sources (with years and citations, when relevant) should comprise the first column.
Provide indicators of both directions of results at the top of the plot on either side of the vertical line (eg, favors intervention).
Typically, proportionally sized boxes represent the weight of each study and a diamond shows the overall effect at the bottom of the plot.

To display quantitative data other than counts or frequencies on a single scaled axis according to categories on a baseline (horizontal or vertical). Point estimates are represented by discrete data markers, preferably with error bars (in both directions) to designate variability.
Scatterplot

To show individual data points plotted according to coordinate values with continuous, quantitative x- and y-axis scales.
A curve that is generated mathematically may be fitted to the data to summarize the relationship among the variables.
Illustration

To explain physiological mechanisms, describe clinical maneuvers and surgical techniques, or provide orientation to medical imaging.
Required minimum resolution for publication: ≥350 ppi
Acceptable File Formats for Initial Submission: .ai, .docx, .eps, .jpg, .pdf, .ppt, .psd., tif
Acceptable File Formats for Revision and Publication: .ai, .eps, .jpg, .pdf, .psd, .tif
Photographs and other clinical images

To display clinical findings, experimental results, or clinical procedures, including medical imaging, photomicrographs, clinical photographs, and photographs of biopsy specimens.
Legends for photomicrographs should include details about the type of stain used and magnification.
Acceptable File Formats for Initial Submission: .eps, .jpg, .pdf, .ppt, .psd, .tif
Acceptable File Formats for Revision and Publication: .eps, .jpg, .psd, .tif
Line drawings

To illustrate anatomy or procedures.
Line drawings are almost always black and white.
Required minimum resolution for publication: ≥600 ppi
Acceptable File Formats for Initial Submission: .docx, .jpg, .pdf, .ppt, .psd, .tif
Acceptable File Formats for Revision and Publication: .jpg, .psd, .tif
Authors may submit supporting material to accompany their article for online-only publication when there is insufficient space to include the material in the print article. This material should be important to the understanding and interpretation of the report and should not repeat material in the print article. The amount of online-only material should be limited and justified. Online-only material should be original and not previously published.
Online-only material will undergo editorial and peer review with the main manuscript. If the manuscript is accepted for publication and if the online-only material is deemed appropriate for publication by the editors, it will be posted online at the time of publication of the article as additional material provided by the authors. This material will not be edited or formatted; thus, authors are responsible for the accuracy and presentation of all such material.
Online-only material should be submitted in a single Word document with pages numbered consecutively. Each element included in the online-only material should be cited in the text of the main manuscript (eg, eTable in the Supplement) and numbered in order of citation in the text (eg, eTable 1, eTable 2, eFigure 1, eFigure 2, eMethods). The first page of the online-only document should list the number and title of each element included in the document.
Online-Only Text
Online-only text should be set in Times New Roman font, 10 point in size, and single-spaced. The main heading of the online-only text should be in 12 point and boldface; subheadings should be in 10 point and boldface.
Online-Only References
All references cited within the online-only document must be included in a separate reference section, including those that also were cited in the main manuscript. They should be formatted just as in the main manuscript and numbered and cited consecutively in the online-only material.
Online-Only Tables
Online-only tables should be inserted in the document and numbered consecutively according to the order of citation as eTable 1, eTable 2, etc. All online-only tables should be cited in the relevant text of the main manuscript. The text and data in online tables should be Arial font, 10 point in size, and single-spaced. The table title should be set in Arial font, 12 point, and bold. Headings within tables should be set in 10 point and bold. Table footnotes should be set in 8 point and single-spaced. See also instructions for Tables above. If a table runs on to subsequent pages, repeat the column headers at the top of each page. Wide tables may be presented using a landscape orientation.
If data are better displayed in a separate Excel file, this can be submitted, provided that the Excel file is cited as an eTable and is numbered in the order cited in the text. If multiple Excel files of data are submitted, these should be placed in a single Excel file, with multiple tabs (sheets) at the bottom of the file. The first tab (sheet) should include a table of contents with eTable numbers and titles, and the subsequent tabs (sheets) should be labeled as eTable 1, eTable 2, etc. Please note: the journal is not a data repository; large data sets should be deposited into publicly accessible data repositories, and a link should be provided in the Methods or Results section and the Data Sharing Statement .
Online-Only Figures
Online-only figures should be inserted in the document and numbered consecutively according to the order of citation as eFigure 1, eFigure 2, etc. All online-only figures should be cited in the relevant text of the main manuscript. Figure titles should be set in Arial font, 12 point, bold, and single-spaced. Text within figures should be set as Arial font, 10 point. Figure legends should be set in 8 point and single-spaced. Graphs and diagrams should be exported directly out of the software application used to create them in a vector file format, such as .wmf, and then inserted into the Word document. Image file formats such as .jpg, .tif, and .gif are generally not suitable for graphs. Photographs, including all radiological images, should be prepared as .jpg (highest option) or .tif (uncompressed) files at a resolution of 300 dpi and width of 3-5 inches, but the resolution of photographic files with an original resolution <300 dpi should not be increased digitally to achieve a 300-dpi resolution. Photographs should be inserted in the document with the "Link to File" button turned off. Wide figures may be presented using a landscape orientation.
For editorial and review of an initial submission, submit videos according to the following specifications:
- Acceptable file formats: .mov, .wmv, .mpg, .mpeg, .mp4, or .avi
- Maximum file size: ≤25 MB
- Preferred dimensions: 1920x1080 (HD) or greater (4k UHD footage is acceptable)
- Minimum dimensions: 640 pixels wide by 360 pixels deep
- Recommended frame rate: 24 fps (or 23.976 fps), 25 and 30 fps (or 29.97 fps)
- Maximum length: ≤5 minutes
- Desired aspect ratio: 4:3 (standard) or 16:9 (widescreen)
- If compression is required to reduce file size for uploading, please use a minimum bit rate of 10,000 kbit/s – 20,000 kbit/s
- When filming, please use a landscape orientation, not a portrait orientation. This is especially important when filming video or taking photographs with a smartphone or a mobile device.
Verify that the videos are viewable in QuickTime or Windows Media Player before uploading.
For each video, provide an in-text citation (eg, Video 1). At the end of the manuscript file, include a title (a brief phrase, preferably no longer than 10 to 15 words) and a caption that includes the file format and a brief explanation for each video. The same title and caption must be entered in the designated fields in the manuscript submission system when uploading each video. If multiple video files are submitted, number them in the order in which they should be viewed.
If patient(s) are identifiable in the video, authors must submit a Patient Permission form completed and signed by each patient. See also Patient Identification .
If the author does not hold copyright to the video, the author must obtain permission for the video to be published in the journal. This permission must be for unrestricted use in all print, online, and licensed versions of the journal.
NOTE: If your manuscript and accompanying videos are accepted for publication, the video files will be placed into a journal video frame and will be edited by JAMA Network video production staff according to journal style. In addition, a JAMA Network staff person may contact you to resubmit your videos to meet our production specifications. For example, a larger size may be needed, and if your videos were submitted with embedded text such as titles, annotations, labels, or captions, we will ask you to remove the text at this stage and resubmit the video without text, and JAMA Network video production will re-create all text using our house style.
Guidelines for Optimal Video Quality
- Use plenty of diffuse light; avoid shadows.
- Use the appropriate white-balance based on your lighting conditions. Different cameras have different settings, but most have presets for incandescent (yellow) light, fluorescent light, daylight, and tungsten light. Please make sure to select the correct one so that the color of your footage renders accurately.
- Do not overexpose the image; a bit underexposed is preferable.
- Use a tripod. This is especially important in close-ups.
- Avoid excessive zooming. Use the optical zoom only; do not use a digital zoom.
- Turn off all camera special effects.
- Avoid using autofocus. Manual focus is more accurate. Keep the camera at a fixed distance from the subject.
- Instruct people on camera to speak clearly and face the camera when speaking. Try to avoid large movements while speaking or immediately after speaking. Allow pauses before and after speaking for easier editing.
- If the situation permits, ensure that individuals being filmed are not wearing white clothing or clothing with busy patterns or stripes, especially shirts, jackets, and ties. Subdued medium blue, brown, tan, beige, and green colors all work well for shirt and clothing choices.
- Do not include an introduction by the physician as a "talking head" explaining a procedure. All footage should be of the procedure or relevant subject matter only.
- Record a few extra seconds before and after each cut or after changing the camera's position. This allows for easier editing.
Additional Considerations for Filming Surgical Procedures
- Coordinate with the surgical staff to establish a vantage point for the camera that has a clear view of the surgical field.
- Before the procedure, if the situation permits, identify the surgical staff's positions for access into and out of the surgical field to ensure there is no immediate obstruction of the camera.
- During the procedure, avoid typical obstructions of the camera's main view such as arms reaching across the field or soiled surgical sponges. Where possible, keep the heads, hands, and any instruments away from the immediate sightline of the camera. This will ensure that all moments of the procedure are captured in full view and focus.
- If the situation permits a choice of glove type, use brown or tan. White gloves reflect bright light; vividly colored surgical gloves can distract the viewer from the teaching point of the video.
- If the situation permits, avoid rapid movements for procedural steps that should be noticed and understood. To demonstrate a key moment or use of an instrument, movement that is deliberate and steady will allow a standard camera to focus properly.
For editorial and review of an initial submission, submit audio files according to the following minimum requirements:
- Acceptable file formats: .mp3, .wav, or .aiff
- Maximum file size: 25 MB
- To achieve the best quality, use a setting of 256 kbps or higher for stereo or 128 kbps or higher for mono.
- Sampling rate should be either 44.1 kHz or 48 kHz.
- Bit rate should be either 16 or 24 bit.
- To avoid audible clipping noise, please make sure that audio levels do not exceed 0 dBFS.
For each audio file, provide an in-text citation. At the end of the manuscript, include a title (a brief phrase, preferably no longer than 10-15 words) and a caption that includes the file format and a brief explanation for each audio.
NOTE: If your manuscript is accepted for publication, JAMA Network video production staff may contact you to request an original uncompressed audio file in .wav or .aiff format. There is no maximum file size requirement for publication at this stage.
After Submission
Authors will be sent notifications of the receipt of manuscripts and editorial decisions by email. During the review process, authors can check the status of their submitted manuscript via the online manuscript submission and review system . Authors should not disclose the fact that their manuscript has been submitted to anyone, except coauthors and contributors, without permission of the editor.
All submitted manuscripts are reviewed initially by one of the editors. Manuscripts are evaluated according to the following criteria: material is original and timely, writing is clear, study methods are appropriate, data are valid, conclusions are reasonable and supported by the data, information is important, and topic has general interest to readers of this journal. From these basic criteria, the editors assess a paper's eligibility for publication. Manuscripts with insufficient priority for publication are rejected promptly. Other manuscripts are sent to expert consultants for peer review. The journal uses a single-anonymized peer review process: peer reviewer identities are kept confidential (unless reviewers choose to reveal their names in their formal reviews); author identities are made known to reviewers. The existence of a manuscript under review is not revealed to anyone other than peer reviewers and editorial staff. Peer reviewers are required to maintain confidentiality about the manuscripts they review and must not divulge any information about a specific manuscript or its content to any third party without prior permission from the journal editors. Reviewers are instructed to not submit confidential manuscripts, abstracts, or other text into a chatbot, language model, or similar tool. At submission, authors may choose to have manuscripts that are not accepted by the journal referred to one of the JAMA Network specialty journals and/or JAMA Network Open along with reviewers' comments (if available). Information from submitted manuscripts may be systematically collected and analyzed as part of research to improve the quality of the editorial or peer review process. Identifying information remains confidential. Final decisions regarding manuscript publication are made by an editor who does not have any relevant conflicts of interest.
At the time of manuscript submission, authors may preselect the option to have their manuscript and reviewers' comments automatically referred to one of the JAMA Network specialty journals if the manuscript is not accepted by JAMA .
JAMA -EXPRESS
JAMA -EXPRESS provides rapid peer review and publication of major clinical trials and other original research studies that have immediate or public health importance. Authors who wish to have manuscripts considered for JAMA -EXPRESS should send the manuscript file and a request letter to [email protected] or call (312) 464-4444. Authors will be notified promptly whether the manuscript is approved for rapid peer review. Authors of those manuscripts determined not to qualify for rapid review may be invited to submit the manuscript for further consideration under the standard review process.
Authors may appeal decisions. All appeals are reviewed by the editor in chief, on a case-by-case basis, or a designated editor if the editor in chief is recused from the review.
After Revision/Acceptance
All authors are required to complete an Authorship Form and Publishing Agreement. See Authorship Criteria and Contributions .
Accepted manuscripts are edited in accordance with the AMA Manual of Style , 2 and returned to the corresponding author (or her/his designee) for approval. Authors are responsible for all statements made in their work, including changes made during editing and production that are authorized by the corresponding author.
Authors should not disclose the fact that their manuscript has been accepted to anyone, except coauthors and contributors, until it is published without permission of the editor or as described in the guidance on Previous or Planned Meeting Presentaton or Release of Information and Embargo Policy .
If accepted for publication, all articles are published quickly in one of JAMA 's weekly print/online issues; selected articles are published Online First.
After Publication
Postpublication correspondence.
For accepted manuscripts, the corresponding author will be asked to respond to letters to the editor.
Reprints and e-prints may be ordered online when the edited manuscript is sent for approval to the corresponding author.
Requests to publish corrections should be sent to the editorial office. Errors and requests for corrections are reviewed by editors and authors, and, if warranted, a Correction notice summarizing the errors and corrections is published promptly and linked online to the original article, and the original article is corrected online with the date of correction. 15
First and last authors of peer-reviewed articles are eligible to receive CME credit. See CME From the JAMA Network .
About Previous Release of Information, Embargo, and Access
Manuscripts are considered with the understanding that they have not been published previously and are not under consideration by another publication.
Copies of all related or similar manuscripts and reports by the same authors (ie, those containing substantially similar content or using the same, similar, or a subset of data) that have been previously published or posted electronically or are under consideration elsewhere must be provided at the time of manuscript submission. All related previously published articles should be cited as references and described in the submitted manuscript along with explanation of how the submitted manuscript differs from the related previously published article(s).
Manuscripts that have been previously posted on a preprint server may be submitted for consideration for publication. When the manuscript is submitted, authors must provide information about the preprint, including a link to it and a description of whether the submitted manuscript has been revised or differs from the preprint.
See also Previous or Planned Meeting Presentation or Release of Information and Research Article Public Access, Depositing in Repositories, and Discoverability.
Meeting presentation: A complete manuscript submitted to the journal following or prior to presentation at a scientific meeting or publication of preliminary findings elsewhere (ie, as an abstract) is eligible for consideration for publication. Authors considering presenting or planning to present the work at an upcoming scientific meeting should indicate the name and date of the meeting on the manuscript submission form. For accepted papers, the editors may be able to coordinate publication with the meeting presentation. Authors of submitted papers, including those accepted but not yet published, should not disclose the status of such papers during such meeting presentations that occur before the work is published. Authors who present information contained in a manuscript that is under consideration by this journal during scientific or clinical meetings should not distribute complete reports (ie, copies of manuscripts) or full data presented as tables and figures to conference attendees or journalists. Publication of abstracts in print and online conference proceedings, as well as posting of slides or videos from the scientific presentation on the meeting website, is acceptable. However, for manuscripts under consideration by this journal, publication of full reports in meeting proceedings or online, issuing detailed news releases reporting the results of the study that go beyond the meeting abstract, or participation in formal news conferences will ordinarily jeopardize chances for publication of the submitted manuscript in this journal. 5 Media coverage of presentations at scientific meetings will not jeopardize consideration, but direct release of information through press releases or news media briefings may preclude consideration of the manuscript by this journal. 5 Rare instances of papers reporting public health emergencies should be discussed with the editor. Authors submitting manuscripts or letters to the editor regarding adverse drug or medical device reactions, reportable diseases, etc, should also report this information to the relevant government agency.
Authors should not release information about accepted manuscripts via social media until publication.
See also Previous Publication, Related Manuscripts and Reports, and Preprints . For more information, see the AMA Manual of Style .
Authors should not disclose the fact that their manuscript has been accepted to anyone, except coauthors and contributors, without permission of the editor until it is published. All information regarding the content and publication date of accepted manuscripts is strictly confidential. Unauthorized prepublication release of accepted manuscripts and information about planned publication date may result in rescinding the acceptance and rejecting the paper. This policy applies to all categories of articles, including research, review, opinion, correspondence, etc. Information contained in or about accepted articles cannot appear in print, audio, video, or digital form or be released by the news media until the specified embargo release date. 2 , 5 See also Previous or Planned Meeting Presentation or Release of Information .
The journal makes all JAMA research articles free public access 6 months after publication on the journal website.
Authors of research articles may deposit the accepted version (ie, the peer-reviewed manuscript that you submitted on which this decision is based) of the manuscript in a repository of your choice on or after the date of publication provided that it links to the final published version on the journal website. You may not deposit the published article (version of record), which is the final copyedited, formatted, and proofed version published by the journal. The journal will deposit a copy of the published research article into PubMed Central (PMC) at the time of publication, where it will be publicly available 6 months after publication. A few weeks after publication, you may obtain your PMCID on the PMC site at: https://www.ncbi.nlm.nih.gov/pmc/pmctopmid/ . These options apply only to research articles. Non-research articles may not be deposited into repositories.
In addition, the journal will add metadata to all articles to ensure web-based search engine discoverability and will provide publicly discoverable information about your article to PubMed/Medline and numerous other bibliographic databases on the day of publication.
Author Responsibilities
Most of the JAMA Network journals' editorial policies for authors are summarized in these instructions. Citations and links to the AMA Manual of Style: A Guide for Authors and Editors 2 and other publications with additional information are also provided.
Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. 2 One or more authors should take responsibility for the integrity of the work as a whole, from inception to published article. According to the guidelines of the International Committee of Medical Journal Editors (ICMJE), 4 authorship credit should be based on the following 4 criteria:
- substantial contributions to conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; and
- drafting of the work or reviewing it critically for important intellectual content; and
- final approval of the version to be published; and
- agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Each author should be accountable for the parts of the work he or she has done. In addition, each author should be able to identify which coauthors are responsible for specific other parts of the work and should have confidence in the integrity of the contributions of any coauthors.
All those designated as authors should meet all 4 criteria for authorship, and all who meet the 4 criteria should be identified as authors. Those who do not meet all 4 criteria should be acknowledged (see Acknowledgment Section ).
All authors (ie, the corresponding author and each coauthor) must read, complete, and submit an electronic Authorship Form with required statements on Authorship Responsibility, Criteria, and Contributions; Confirmation of Reporting Conflicts of Interest and Funding; and Publishing Agreement. 2(pp128-133) In addition, authors are required to identify their specific contributions to the work described in the manuscript. Requests by authors to designate equal contributions or shared authorship positions (eg, co-first authorship) may be considered if justified and within reason. 6 An email with links to the Authorship Form will be sent to authors for completion after manuscripts have been submitted.
For reports of original data, authors' specific contributions will be published in the Acknowledgment section (see Manuscript Preparation and Submission Requirements , Acknowledgment section ). 2 All other persons who have made substantial contributions to the work reported in this manuscript (eg, data collection, analysis, or writing or editing assistance) but who do not fulfill the authorship criteria should be named with their specific contributions and affiliations in an Acknowledgment in the manuscript. Written permission to include the names of individuals in the Acknowledgment section must be obtained.
Nonhuman artificial intelligence, language models, machine learning, or similar technologies do not qualify for authorship. If these models or tools are used to create content or assist with writing or manuscript preparation, authors must take responsibility for the integrity of the content generated by these tools. Authors should report the use of artificial intelligence, language models, machine learning, or similar technologies to create content or assist with writing or editing of manuscripts in the Acknowledgment section or Methods section if this is part of formal research design or methods. See also Use of AI in Publication and Research , Reproduced and Re-created Material , and Image Integrity .
The authors also must certify that the manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere (see also About Previous Release of Information, Embargo, and Access ). 2 Authors of manuscripts reporting original data or systematic reviews must provide an access to data statement from 1 or 2 named authors, often the corresponding author (see also Data Access, Responsibility, and Analysis ). If requested, authors should be prepared to provide the data and must cooperate fully in obtaining and providing the data on which the manuscript is based for examination by the editors or their assignees.
A single corresponding author (or coauthor designee in the event that the corresponding author is unavailable) will serve on behalf of all coauthors as the primary correspondent with the editorial office during the submission and review process. If the manuscript is accepted, the corresponding author will review an edited manuscript and proof, make decisions regarding release of information in the manuscript to the news media or federal agencies, handle all postpublication communications and inquiries, and will be identified as the corresponding author in the published article.
The corresponding author also is responsible for ensuring that the Acknowledgment section of the manuscript is complete (see Acknowledgment Section ) and that the conflict of interest disclosures reported in the Acknowledgment section of the manuscript are accurate, up-to-date, and consistent with the information provided in each author's potential conflicts of interest section in the Authorship Form (see Conflicts of Interest and Financial Disclosures ).
The corresponding author also must complete the Acknowledgment statement part of the Authorship Form confirming that all persons who have contributed substantially but who are not authors are identified in the Acknowledgment section and that written permission from each person acknowledged has been obtained (see Acknowledgment Section ).
Requests for co-corresponding authors will be considered on a very limited basis if justified, but no more than 2 co-corresponding authors will be permitted. In such cases, a primary corresponding author must be designated as the point of contact responsible for all communication about the manuscript and article, manage the tasks described above, and will be listed first in the corresponding author section. 6 To read more about the role and responsibilities of corresponding authors, see the AMA Manual of Style .
Authors should determine the order of authorship among themselves and should settle any disagreements before submitting their manuscript. Changes in authorship (ie, order, addition, and deletion of authors) should be discussed and approved by all authors. Any requests for such changes in authorship after initial manuscript submission and before publication should be explained in writing to the editor in a letter or email from all authors. 2(pp128-133)
The JAMA Network recognizes that authors may change their names for personal reasons, and the editors respect authors' rights to autonomy and privacy in this regard. Authors who request confidential name changes after publication because of changes in identity, marital status, religion, or other reasons may have their names changed in articles without indication of the reason for the change and without a formal correction notice. If an author prefers this change to be public, a formal Correction notice can be issued, with or without the reason per author preference. The journal will not request the approval of coauthors, but the requesting author may wish to notify coauthors if this change will affect subsequent citations to the article. The requester may be asked to notify the corresponding author about this change to the published article; alternatively, the journal may inform the corresponding author of this change (without explaining the reason for the change). The journal will make this change to the online and PDF versions of the published article and will notify postpublication indexes and databases as a standard process but cannot guarantee when or if the change will be reflected in these indexes and databases.
If authorship is attributed to a group (either solely or in addition to 1 or more individual authors), all members of the group must meet the full criteria and requirements for authorship as described above, and all group member authors must complete Authorship Forms. 6 If all members of a group do not meet all authorship criteria, a group must designate 1 or more individuals as authors or members of a writing group who meet full authorship criteria and requirements and who will take responsibility for the group. 2 , 6 Group names should appear at the end of the byline and should not be interspersed within the list of individually named authors. Group authors may not be included for article types with limited numbers of authors (eg, opinion articles).
For articles with a large number of authors (eg, >50), a long list of authors will not fit in the byline of a print/PDF version of the article. In such cases, a group byline will be recommended with the individual names of each author listed at the end of the article. All author names would still be individually indexed, displayed, and easily searchable in bibliographic records such as PubMed. 6
Nonauthor Collaborators: Other group members who do not meet the criteria for authorship (eg, investigators, advisors, assistants) may be identified. For group author manuscripts, a Nonauthor Collaborator Template (with names, academic degrees, institution, location, role/contribution, and subgroup) must be completed during revision. The template will be available to authors with the request for revision. The collaborators will be published in an online Supplement based on this template and will be deposited to PubMed.
To read more about authorship, click here .
A conflict of interest may exist when an author (or the author's institution or employer) has financial or personal relationships or affiliations that could influence (or bias) the author's decisions, work, or manuscript. All authors are required to report potential conflicts of interest including specific financial interests relevant to the subject of their manuscript in the Acknowledgment section of the manuscript 2 and in the Disclosure of Potential Conflicts of Interest section of the Authorship Form. Note: These forms will be requested after a manuscript has been submitted, but authors should also include conflict of interest disclosures in the Acknowledgment section of the submitted manuscript.
Definitions and Terms of Conflicts of Interest Disclosures:
Authors are expected to provide detailed information about all relevant financial interests, activities, relationships, and affiliations (other than those affiliations listed in the title page of the manuscript) including, but not limited to, employment, affiliation, funding and grants received or pending, consultancies, honoraria or payment, speakers' bureaus, stock ownership or options, expert testimony, royalties, donation of medical equipment, or patents planned, pending, or issued.
Following the guidelines of the ICMJE, 4 the definitions and terms of such disclosures include
Any potential conflicts of interest "involving the work under consideration for publication" (during the time involving the work, from initial conception and planning to present), Any "relevant financial activities outside the submitted work" (over the 3 years prior to submission), and Any "other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing" what is written in the submitted work (based on all relationships that were present during the 3 years prior to submission).
Authors without conflicts of interest, including relevant financial interests, activities, relationships, and affiliations, should indicate such in their disclosures and include a statement of no such interests in the Acknowledgment section of the manuscript. Failure to include this information in the manuscript may delay evaluation and review of the manuscript. Authors should err on the side of full disclosure and should contact the editorial office if they have questions or concerns.
Although many universities and other institutions and organizations have established policies and thresholds for reporting financial interests and other conflicts of interest, the JAMA Network requires complete disclosure of all relevant financial relationships and potential financial conflicts of interest, regardless of amount or value. For example, authors of a manuscript about hypertension should report all financial relationships they have with all manufacturers and owners of products, devices, tests, and services used in the management of hypertension, not only those relationships with entities whose specific products, devices, tests, and services are mentioned in the manuscript. If authors are uncertain about what constitutes a relevant financial interest or relationship, they should contact the editorial office.
For all accepted manuscripts, the corresponding author will have been asked to confirm that each coauthor's disclosures of conflicts of interest and relevant financial interests, activities, relationships, and affiliations and declarations of no such interests are accurate, up-to-date, and consistent with the disclosures reported in the Acknowledgment section of the manuscript because this information will be published in the Acknowledgment section of the article. Decisions about whether such information provided by authors should be published, and thereby disclosed to readers, are usually straightforward. Although editors are willing to discuss disclosure of specific conflicts of interest with authors, JAMA Network policy is one of complete disclosure of all potential conflicts of interest, including relevant financial interests, activities, relationships, and affiliations (other than those affiliations listed in the title page of the manuscript). The policy requiring disclosure of conflicts of interest applies for all manuscript submissions, including letters to the editor. If an author's disclosure of potential conflicts of interest is determined to be inaccurate or incomplete after publication, a correction will be published to rectify the original published disclosure statement, and additional action may be taken as necessary.
All authors must also complete the Disclosure of Potential Conflicts of Interest section of the Authorship Form. 7
All financial and material support for the research and the work should be clearly and completely identified in an Acknowledgment section of the manuscript. At the time of submission, information on the funding source (including grant identification) must also be completed via the online manuscript submission and review system. The specific role of the funding organization or sponsor in each of the following should be specified: "design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication." 7 To read more about reporting funding and other support, see the AMA Manual of Style .
For all reports (regardless of funding source) containing original data, at least 1 named author (eg, the principal investigator), and no more than 2 authors, must indicate that she or he "had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis." 7 This exact statement should be included in the Acknowledgment section at the end of the manuscript. Modified statements or generic statements indicating that all authors had such access are not acceptable. In addition, for all reports containing original data, the names and affiliations of all authors (or other individuals) who conducted and are responsible for the data analysis must be indicated in the Acknowledgment section of the manuscript. If the individual who conducted the analysis is not named as an author, a detailed explanation of his/her contributions and reasons for his/her involvement with the data analysis should be included.
For all reports of research, authors are required to provide a Data Sharing Statement to indicate if data will or will not be shared. Specific questions regarding the sharing of data are included in the manuscript submission system. If authors choose to share or not share data, this information will be published in a Data Sharing Statement in an online supplement linked to the published article. Authors will be asked to identify the data, including individual patient data, a data dictionary that defines each field in the data set, and supporting documentation (eg, statistical/analytic code), that will be made available to others; when, where, and how the data will be available (eg, a link to a data repository); types of analyses that are permitted; and if there will be any restrictions on the use of the data. Authors also have the option to explain why data may not be shared. A list of generalist public repositories that authors may consider using is available from the National Library of Medicine .
The Acknowledgment section is the general term for the list of contributions, disclosures, credits, and other information included at the end of the text of a manuscript but before the references. The Acknowledgment section includes authors' contributions; information on author access to data; disclosure of potential conflicts of interest, including financial interests, activities, relationships, and affiliations; sources of funding and support; an explanation of the role of funder(s)/sponsor(s); names, degrees, and affiliations of participants in a large study or other group (ie, collaborators); any important disclaimers; information on previous presentation of the information reported in the manuscript; and the contributions, names, degrees, affiliations, and indication if compensation has been received for all persons who have made substantial contributions to the work but who are not authors. 2
All other persons who have made substantial contributions to the work reported in the manuscript (eg, data collection, analysis, and writing or editing assistance) but who do not fulfill the authorship criteria should be named with their specific contributions in an Acknowledgment in the manuscript.
Authors must obtain written permission to include the names of all individuals included in the Acknowledgment section, and the corresponding author must confirm that such permission has been obtained in the Authorship Form.
Authors should report the use of artificial intelligence, language models, machine learning, or similar technologies to create content or assist with writing or editing of manuscripts in the Acknowledgment section or the Methods section if this is part of formal research design or methods. This should include a description of the content that was created or edited and the name of the language model or tool, version and extension numbers, manufacturer, date(s) of use, and confirmation that the authors take responsibility for the integrity of the content generated. (Note: this does not include basic tools for checking grammar, spelling, references, etc.) See also Use of AI in Publication and Research and Statistical Analysis Subsection .
Requirements for Reporting
Authors of research articles should follow the EQUATOR Reporting Guidelines . See specific Study Types for detailed guidance on reporting.
Causal language (including use of terms such as effect and efficacy) should be used only for randomized clinical trials. For all other study designs (including meta-analyses of randomized clinical trials), methods and results should be described in terms of association or correlation and should avoid cause-and-effect wording. To read more about use of causal language, see the AMA Manual of Style .
Research reports should be timely and current and should be based on data collected as recently as possible. Manuscripts based on data from randomized clinical trials should be reported as soon as possible after the trial has ended, ideally within 1 year after follow-up has been completed.
For cohort studies, the date of final follow-up should be no more than 5 years before manuscript submission. Likewise, data used in case-control or cross-sectional studies should have been collected as recently as possible, but no more than 5 years before manuscript submission. Manuscripts in which the most recent data have been collected more than 5 years ago ordinarily will receive lower priority for publication; thus, authors of such manuscripts should provide a detailed explanation of the relevance of the information in light of current knowledge and medical practice as well as the most recent date(s) of analysis of the study.
General Considerations
Authors are encouraged to consult "Reporting Statistical Information in Medical Journal Articles." 1 In the Methods section, describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to reproduce the reported results. Such description should include appropriate references to the original literature, particularly for uncommon statistical methods. For more advanced or novel methods, provide a brief explanation of the methods and appropriate use in the text and consider providing a detailed description in an online supplement.
In the reporting of results, when possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty, such as confidence intervals (see Reporting Standards and Data Presentation ). Avoid relying solely on statistical hypothesis testing, such as the use of P values, which fails to convey important quantitative information. For observational studies, provide the numbers of observations. For randomized trials, provide the numbers randomized. Report losses to observation or follow up (see Missing Data ). For multivariable models, report all variables included in models, and report model diagnostics and overall fit of the model when available (see Statistical Procedures ).
Define statistical terms, abbreviations, and symbols, if included. Avoid nontechnical uses of technical terms in statistics, such as correlation, normal, predictor, random, sample, significant, trend. Do not use inappropriate hedge terms such as marginal significance or trend toward significance for results that are not statistically significant. Causal language (including use of terms such as effect and efficacy) should be used only for randomized clinical trials. For all other study designs (including meta-analyses of randomized clinical trials), methods and results should be described in terms of association or correlation and should avoid cause-and-effect wording.
Sample Size Calculations
For randomized trials, a statement of the power or sample size calculation is required (see the EQUATOR Network CONSORT Guidelines ). For observational studies that use an established population, a power calculation is not generally required when the sample size is fixed. However, if the sample size was determined by the researchers, through any type of sampling or matching, then there should be some justification for the number sampled. In any case, describe power and sample size calculations at the beginning of the Statistical Methods section, following the general description of the study population.
Descriptive Statistics
It is generally not necessary to provide a detailed description of the methods used to generate summary statistics, but the tests should be briefly noted in the Methods section (eg, ANOVA or Fisher exact test).
Statistical Procedures
Identify regression models with more than 1 independent variable as multivariable and regression models with more than 1 dependent variable as multivariate. Report all variables included in models, as well as any mathematical transformations of those variables. Provide the scientific rationale (clinical, statistical, or otherwise) for including variables in regression models.
For regression models fit to dependent data (eg, clustered or longitudinal data), the models should account for the correlations that arise from clustering and/or repeated measures. Failure to account for such correlation will result in incorrect estimates of uncertainty (eg, confidence intervals). Describe how the model accounted for correlation. For example, for an analysis based on generalized estimating equations, identify the assumed correlation structure and whether robust (or, sandwich) variance estimators were used. Or, for an analysis based on mixed-effects models, identify the assumed structure for the random effects, such as the level of random intercepts and whether any random slopes were included. Fixed-effects estimation should be described as conditional likelihood. Avoid the term fixed effects for describing covariates.
Missing Data
Report losses to observation, such as dropouts from a clinical trial or those lost to follow-up or unavailable in an observational study. If some participants are excluded from analyses because of missing or incomplete data, provide a supplementary table that compares the observed characteristics between participants with complete and incomplete data. Consider multiple imputation methods to impute missing data and include an assessment of whether data were missing at random. Approaches based on "last observation carried forward" should not be used.
Primary Outcomes, Multiple Comparisons, and Post Hoc Comparisons
Both randomized and observational studies should identify the primary outcome(s) before the study began, as well as any prespecified secondary, subgroup, and/or sensitivity analyses. Comparisons arrived at during the course of the analysis or after the study was completed should be identified as post hoc. For analyses of more than 1 primary outcome, corrections for multiple testing should generally be used. For secondary outcomes, address multiple comparisons or consider such analyses as exploratory and interpret them as hypothesis-generating. The reporting of all outcomes should match that included in study protocols. For randomized clinical trials, protocols with complete statistical analysis plans should be cited in the Methods section and submitted as online supplementary content. Randomized clinical trials should be primarily analyzed according to the intention-to-treat approach. Deviations from strict intention-to-treat analysis should be described as "modified intention-to-treat," with the modifications clearly described.
Statistical Analysis Subsection
At the end of the Methods section, briefly describe the statistical tests used for the analysis. State any a priori levels of significance and whether hypothesis tests were 1- or 2-sided. Also include the statistical software used to perform the analysis, including the version and manufacturer, along with any extension packages (eg, the svy suite of commands in Stata or the survival package in R). Do not describe software commands (eg, SAS proc mixed was used to fit a linear mixed-effects model). If analysis code is included, it should be placed in the online supplementary content.
Reporting Standards and Data Presentation
Analyses should follow EQUATOR Reporting Guidelines and be consistent with the protocol and statistical analysis plan, or described as post hoc.
When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P = .13). P values should never be presented alone without the data that are being compared. If P values are reported, follow standard conventions for decimal places: for P values less than .001, report as " P <.001"; for P values between .001 and .01, report the value to the nearest thousandth; for P values greater than or equal to .01, report the value to the nearest hundredth; and for P values greater than .99, report as " P >.99." For studies with exponentially small P values (eg, genetic association studies), P values may be reported with exponents (eg, P = 1×10 −5 ). In general, there is no need to present the values of test statistics (eg, F statistics or χ² results) and degrees of freedom when reporting results.
For secondary and subgroup analyses, there should be a description of how the potential for type I error due to multiple comparisons was handled, for example, by adjustment of the significance threshold. In the absence of some approach, these analyses should generally be described and interpreted as exploratory, as should all post hoc analyses.
For randomized trials using parallel-group design, there is no validity in conducting hypothesis tests regarding the distribution of baseline covariates between groups; by definition, these differences are due to chance. Because of this, tables of baseline participant characteristics should not include P values or statements of statistical comparisons among randomized groups. Instead, report clinically meaningful imbalances between groups, along with potential adjustments for those imbalances in multivariable models. To read more about statistical tests and data presentation, see the AMA Manual of Style .
Researchers are encouraged to report studies that include diverse and representative participants and to indicate participant inclusion and exclusion criteria and how the findings generalize to the population(s) that are the focus of or are compatible with the research question. Aggregate, deidentified demographic information (eg, age, sex, race and ethnicity, and socioeconomic indicators) should be reported for all research reports along all prespecified outcomes. Demographic variables collected for a specific study should be reported in the Methods section. Demographic information assessed should be reported in the Results section, either in the main article or in an online supplement or both. If any demographic characteristics that were collected are not reported, the reason should be stated. Summary demographic information (eg, baseline characteristics of study participants) should be reported in the first line of the Results section of Abstracts.
Reporting Age
Study inclusion or exclusion criteria by age or age group should be defined in the Methods section. Stratification by age groups should be based on relevance to disease, condition, or population (eg, <5 or >65 years). The ages for study participants should be reported in aggregate (ie, mean and SD or median and IQR or range) in the Results section.
Reporting Sex and Gender
The term sex should be used when reporting biological factors and gender should be used when reporting gender identity or psychosocial/cultural factors. The methods used to obtain information on sex, gender, or both (eg, self-reported, investigator observed or classified, or laboratory test) should be explained in the Methods section. 12 The distribution of study participants or samples should be reported in the Results section, including for studies of humans, tissues, cells, or animals. All participants should be reported, not just the category that represents the majority of the sample. Studies that address pregnancy should follow these recommendations, and if the gender identity of participants was not assessed, use the terms "pregnant participants," "pregnant individuals," "pregnant patients," etc, as appropriate.
In research articles, sex or gender should be reported and defined, and how sex or gender was assessed should be described. Whenever possible, all main outcomes should be reported by sex (or gender if appropriate). In nonresearch reports, choose sex-neutral terms that avoid bias, suit the material under discussion, and do not intrude on the reader's attention.
Reporting Race and Ethnicity
The Methods section should include an explanation of who identified participant race and ethnicity and the source of the classifications used (eg, self-report or selection, investigator observed, database, electronic health record, survey instrument).
If race and ethnicity categories were collected for a study, the reasons that these were assessed also should be described in the Methods section. If collection of data on race and ethnicity was required by the funding agency, that should be noted.
Specific racial and ethnic categories are preferred over collective terms, when possible. Authors should report the specific categories used in their studies and recognize that these categories will differ based on the databases or surveys used, the requirements of funders, and the geographic location of data collection or study participants. Categories included in groups labeled as "other" should be defined.
Categories should be listed in alphabetical order in text and tables.
Race and ethnicity of the study population should be reported in the Results section.
For additional information, see " Updated Guidance on Reporting Race and Ethnicity in Medical and Science Journals " and the Summary Guide for Preferred Terms When Reporting Race and Ethnicity .
For all manuscripts reporting data from studies involving human participants or animals, formal review and approval, or formal review and waiver, by an appropriate institutional review board or ethics committee is required and should be described in the Methods section. 2(p226) For those investigators who do not have formal ethics review committees, the principles outlined in the Declaration of Helsinki should be followed. 13 For investigations of humans, state in the Methods section the manner in which informed consent was obtained from the study participants (ie, oral or written) and whether participants received a stipend. Authors of research studies involving humans should not make independent determinations of exemption or exclusion of IRB or ethical review; they should cite the institutional or regulatory policy for that determination and indicate if the data are deidentified and publicly available or protected by prior consent or privacy safeguards. Editors may request that authors provide documentation of the formal review and recommendation from the institutional review board or ethics committee responsible for oversight of the study.
A signed statement of informed consent to publish patient descriptions, photographs, video, and pedigrees should be obtained from all persons (parents or legal guardians for minors) who can be identified (including by the patients themselves) i/n such written descriptions, photographs, or pedigrees and should be submitted with the manuscript and indicated in the Acknowledgment section of the manuscript. Such persons should be offered the opportunity to see the manuscript before its submission. 2(pp229-232)
Omitting data or making data less specific to deidentify patients is acceptable, but changing any such data is not acceptable. Only those details essential for understanding and interpreting a specific case report or case series should be provided. Although the degree of specificity needed will depend on the context of what is being reported, specific ages, race/ethnicity, and other sociodemographic details should be presented only if clinically or scientifically relevant and important. 2 Cropping of photographs to remove identifiable personal features that are not essential to the clinical message may be permitted as long as the photographs are not otherwise altered. Please do not submit masked photographs of patients. Patients' initials or other personal identifiers must not appear in an image.
Patient Permission Form:
The Patient Permission form for publication of identifying material is available here . Translated versions in Arabic, Chinese, French, German, Hindi, Italian, Japanese, Portuguese, and Spanish are available on request.
AI Used in Manuscript Preparation
When traditional and generative AI technologies are used to create, review, revise, or edit any of the content in a manuscript, authors should report in the Acknowledgment section the following:
- Name of the AI software platform, program, or tool
- Version and extension numbers
- Manufacturer
- Date(s) of use
- A brief description of how the AI was used and on what portions of the manuscript or content
- Confirmation that the author(s) take responsibility for the integrity of the content generated
Note this guidance does not apply to basic tools for checking grammar, spelling, references, and similar.
AI Used in Research
When AI (eg, large language model [LLM] or natural language processing [NLP], supervised or unsupervised machine learning [ML] for predictive/prescriptive or clustering tasks, chatbots, or similar other technologies) is used as part of a scientific study, authors should:
- Follow relevant reporting guidelines for specific study designs when they exist and report each recommended guideline element with sufficient detail to enable reproducibility.
- Avoid inclusion of identifiable patient information in text, tables, and figures.
- Be aware of copyright and intellectual property concerns - if including content (text, images) generated by AI, and indicate rights or permissions to publish that content as determined by the AI service or owner.
Also address the following:
Methods Section
- Include the study design and, if a relevant reporting guideline exists, indicate how it was followed, with sufficient detail to enable reproducibility.
- Describe how AI was used for specific aspects of the study (eg, to generate or refine study hypotheses, assist in the generation of a list of adjustment variables, create graphs to show visual relationships).
- For studies using LLMs, provide the name of the platform or program, tool, version, and manufacturer; specify dates and prompt(s) used and their sequence and any revisions to prompts in response to initial outputs.
- For studies reporting ML and algorithm development, include details about data sets used for development, training, and validation. Clearly state if algorithms were trained and tested only on previously collected or existing data sets or if the study includes prospective deployment. Include the ML model and describe the variables and outcome(s) and selection of the fine-tuning parameters. Describe any assumptions involved (eg, log linearity, proportionality) and how these assumptions were tested.
- Indicate the metric used to evaluate the performance of the algorithms, including bias, discrimination, calibration, reclassification, and others as appropriate.
- Indicate the methods used to address missing data.
- Indicate institutional review board/ethics review, approval, waiver, or exemption.
- Describe methods or analyses included to address and manage AI-related methodologic bias and inaccuracy of AI-generated content.
- Indicate, when appropriate, if sensitivity analyses were performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
- Provide a data sharing statement, including if code will be shared.
Results Section
- When reporting comparisons, provide performance assessments (eg, against standard of care), include effect sizes and measures of uncertainty (eg, 95% CIs) and other measurements such as likelihood ratios, and include information about performance errors, inaccurate or missing data, and sufficient detail for others to reproduce the findings.
- Report the results of analyses to address methodologic bias and population representation.
- If examples of generated text or content are included in tables or figures, be sure to indicate the source and licensing information, as noted above.
Discussion Section
- Discuss the potential for AI-related bias and what was done to identify and mitigate such bias.
- Discuss the potential for inaccuracy of AI-generated content and what was done to identify and manage this.
- Discuss generalizability of findings across populations and results of analyses performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
A signed statement of permission should be included from each individual identified as a source of information in a personal communication or as a source for unpublished data, and the date of communication and whether the communication was written or oral should be specified. 2(p199) Personal communications should not be included in the list of references but added to the text parenthetically.
Authors and reviewers are expected to notify editors if a manuscript could be considered to report dual use research of concern (ie, research that could be misused by others to pose a threat to public health and safety, agriculture, plants, animals, the environment, or material). 14 The editor in chief will evaluate manuscripts that report potential dual use research of concern and, if necessary, consult additional reviewers.
Journal Policies
Final decisions regarding manuscript publication are made by the editor in chief or a designated editor who does not have any relevant conflicts of interest. The journal has a formal recusal process in place to help manage potential conflicts of interest of editors. In the event that an editor has a conflict of interest with a submitted manuscript or with the authors, the manuscript, review, and editorial decisions are managed by another designated editor without a conflict of interest related to the manuscript.
All authors are required to complete and submit a Publishing Agreement that is part of the journal's electronic Authorship Form. In this agreement, authors will transfer copyright or a publication license; or indicate that they are employed by a federal government; or indicate that they are an employee of an institution that considers the work in the manuscript a work for hire, in which case an authorized representative of that institution will assign copyright or a publication license on the author's behalf.
Published articles become the permanent property of the American Medical Association (AMA) and may not be published elsewhere without written permission. Unauthorized use of the journal's name, logo, or any content for commercial purposes or to promote commercial goods and services (in any format, including print, video, audio, and digital) is not permitted by the JAMA Network or the AMA.
1. Cummings P, Rivara FP. Reporting statistical information in medical journal articles. Arch Pediatr Adolesc Med . 2003;157(4):321-324. doi:10.1001/archpedi.157.4.321
2. Iverson C, Christiansen S, Flanagin A, et al. AMA Manual of Style: A Guide for Authors and Editors . 11th ed. Oxford University Press; 2020. http://www.amamanualofstyle.com
3. Golub RM. Correspondence course: tips for getting a letter published in JAMA . JAMA . 2008;300(1):98-99. doi:10.1001/jama.300.1.98
4. International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Updated May 2023. Accessed May 18, 2023. http://www.icmje.org/recommendations/
5. Fontanarosa PB, Flanagin A, DeAngelis CD. Update on JAMA 's policy on release of information to the public. JAMA . 2008;300(13):1585-1587. doi:10.1001/jama.300.13.1585
6. Fontanarosa P, Bauchner H, Flanagin A. Authorship and team science. JAMA . 2017;318(24):2433-2437. doi:10.1001/jama.2017.19341
7. Fontanarosa PB, Flanagin A, DeAngelis CD. Reporting conflicts of interest, financial aspects of research, and role of sponsors in funded studies. JAMA . 2005;294(1):110-111. doi:10.1001/jama.294.1.110
8. DeAngelis CD, Drazen JM, Frizelle FA, et al; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA . 2004;292(11):1363-1364. doi:10.1001/jamainternmed.2014.6933
9. DeAngelis CD, Drazen JM, Frizelle FA, et al; International Committee of Medical Journal Editors. Is this clinical trial fully registered? a statement from the International Committee of Medical Journal Editors. JAMA . 2005;293(23):2927-2929. doi:10.1001/jama.293.23.jed50037
10. The CONSORT Group. The CONSORT statement. Updated 2014. Accessed September 23, 2016. http://www.consort-statement.org/consort-2010
11. American Association for Public Opinion Research. Best practices for survey research. Accessed March 23, 2023. https://aapor.org/standards-and-ethics/best-practices/
12. Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA . 2016;316(18):1863-1864. doi:10.1001/jama.2016.16405
13. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA . 2013;310(20):2191-2194. doi:10.1001/jama.2013.281053
14. Journal Editors and Authors Group. Statement on scientific publication and security. Science . 2003;299(5610):1149. doi:10.1126/science.299.5610.1149 . Published correction appears in Science . 2003;299(5614):1845.
15. Christiansen S, Flanagin A. Correcting the medical literature: "to err is human, to correct divine." JAMA . 2017;318(9):804-805. doi:10.1001/jama.2017.11833
Last Updated: June 13, 2024
Quick Links
- Why Publish in JAMA
- Submit and Track Your Manuscript
- Author Reprints
- Permissions Requests
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- DOI: 10.3390/rel15070801
- Corpus ID: 271030074
“Buddhist-Christian Style”: The Collaboration of Prip-Møller and Reichelt—From Longchang Si to Tao Fong Shan Christian Centre
- Weiqiao Wang , Yan Liu
- Published in Religions 30 June 2024
- Art, History
15 References
Comparative review of ideal layouts in han buddhist and catholic monasteries, transcending history: (re)building longchang monastery of mount baohua in the seventeenth century, a comprehensive survey of catholic churches in hong kong, china's old churches, building st. francis of assisi church and school in hong kong, social and political factors in induction of catholic churches in hong kong, related papers.
Showing 1 through 3 of 0 Related Papers
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
MLA Formatting and Style Guide

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
The following overview should help you better understand how to cite sources using MLA 9 th edition, including how to format the Works Cited page and in-text citations.
Please use the example at the bottom of this page to cite the Purdue OWL in MLA. See also our MLA vidcast series on the Purdue OWL YouTube Channel .
Creating a Works Cited list using the ninth edition
MLA is a style of documentation that may be applied to many different types of writing. Since texts have become increasingly digital, and the same document may often be found in several different sources, following a set of rigid rules no longer suffices.
Thus, the current system is based on a few guiding principles, rather than an extensive list of specific rules. While the handbook still describes how to cite sources, it is organized according to the process of documentation, rather than by the sources themselves. This gives writers a flexible method that is near-universally applicable.
Once you are familiar with the method, you can use it to document any type of source, for any type of paper, in any field.
Here is an overview of the process:
When deciding how to cite your source, start by consulting the list of core elements. These are the general pieces of information that MLA suggests including in each Works Cited entry. In your citation, the elements should be listed in the following order:
- Title of source.
- Title of container,
- Other contributors,
- Publication date,
Each element should be followed by the corresponding punctuation mark shown above. Earlier editions of the handbook included the place of publication and required different punctuation (such as journal editions in parentheses and colons after issue numbers) depending on the type of source. In the current version, punctuation is simpler (only commas and periods separate the elements), and information about the source is kept to the basics.
Begin the entry with the author’s last name, followed by a comma and the rest of the name, as presented in the work. End this element with a period.
Bhabha, Homi K. The Location of Culture. Routledge, 1994.
Title of source
The title of the source should follow the author’s name. Depending upon the type of source, it should be listed in italics or quotation marks.
A book should be in italics:
Henley, Patricia. The Hummingbird House . MacMurray, 1999.
An individual webpage should be in quotation marks. The name of the parent website, which MLA treats as a "container," should follow in italics:
Lundman, Susan. "How to Make Vegetarian Chili." eHow, www.ehow.com/how_10727_make-vegetarian-chili.html.*
A periodical (journal, magazine, newspaper) article should be in quotation marks:
Bagchi, Alaknanda. "Conflicting Nationalisms: The Voice of the Subaltern in Mahasweta Devi's Bashai Tudu." Tulsa Studies in Women's Literature , vol. 15, no. 1, 1996, pp. 41-50.
A song or piece of music on an album should be in quotation marks. The name of the album should then follow in italics:
Beyoncé. "Pray You Catch Me." Lemonade, Parkwood Entertainment, 2016, www.beyonce.com/album/lemonade-visual-album/.
*The MLA handbook recommends including URLs when citing online sources. For more information, see the “Optional Elements” section below.
Title of container
The eighth edition of the MLA handbook introduced what are referred to as "containers," which are the larger wholes in which the source is located. For example, if you want to cite a poem that is listed in a collection of poems, the individual poem is the source, while the larger collection is the container. The title of the container is usually italicized and followed by a comma, since the information that follows next describes the container.
Kincaid, Jamaica. "Girl." The Vintage Book of Contemporary American Short Stories, edited by Tobias Wolff, Vintage, 1994, pp. 306-07.
The container may also be a television series, which is made up of episodes.
“94 Meetings.” Parks and Recreation, created by Greg Daniels and Michael Schur, performance by Amy Poehler, season 2, episode 21, Deedle-Dee Productions and Universal Media Studios, 2010.
The container may also be a website, which contains articles, postings, and other works.
Wise, DeWanda. “Why TV Shows Make Me Feel Less Alone.” NAMI, 31 May 2019, www.nami.org/Blogs/NAMI-Blog/May-2019/How-TV-Shows-Make-Me-Feel-Less-Alone . Accessed 3 June 2019.
In some cases, a container might be within a larger container. You might have read a book of short stories on Google Books , or watched a television series on Netflix . You might have found the electronic version of a journal on JSTOR. It is important to cite these containers within containers so that your readers can find the exact source that you used.
“94 Meetings.” Parks and Recreation , season 2, episode 21, NBC , 29 Apr. 2010. Netflix, www.netflix.com/watch/70152031?trackId=200256157&tctx=0%2C20%2C0974d361-27cd-44de-9c2a-2d9d868b9f64-12120962.
Langhamer, Claire. “Love and Courtship in Mid-Twentieth-Century England.” Historical Journal , vol. 50, no. 1, 2007, pp. 173-96. ProQuest, doi:10.1017/S0018246X06005966. Accessed 27 May 2009.
Other contributors
In addition to the author, there may be other contributors to the source who should be credited, such as editors, illustrators, translators, etc. If their contributions are relevant to your research, or necessary to identify the source, include their names in your documentation.
Foucault, Michel. Madness and Civilization: A History of Insanity in the Age of Reason. Translated by Richard Howard , Vintage-Random House, 1988.
Woolf, Virginia. Jacob’s Room . Annotated and with an introduction by Vara Neverow, Harcourt, Inc., 2008.
If a source is listed as an edition or version of a work, include it in your citation.
The Bible . Authorized King James Version, Oxford UP, 1998.
Crowley, Sharon, and Debra Hawhee. Ancient Rhetorics for Contemporary Students. 3rd ed., Pearson, 2004.
If a source is part of a numbered sequence, such as a multi-volume book or journal with both volume and issue numbers, those numbers must be listed in your citation.
Dolby, Nadine. “Research in Youth Culture and Policy: Current Conditions and Future Directions.” Social Work and Society: The International Online-Only Journal, vol. 6, no. 2, 2008, www.socwork.net/sws/article/view/60/362. Accessed 20 May 2009.
Quintilian. Institutio Oratoria. Translated by H. E. Butler, vol. 2, Loeb-Harvard UP, 1980.
The publisher produces or distributes the source to the public. If there is more than one publisher, and they are all are relevant to your research, list them in your citation, separated by a forward slash (/).
Klee, Paul. Twittering Machine. 1922. Museum of Modern Art, New York. The Artchive, www.artchive.com/artchive/K/klee/twittering_machine.jpg.html. Accessed May 2006.
Women's Health: Problems of the Digestive System . American College of Obstetricians and Gynecologists, 2006.
Daniels, Greg and Michael Schur, creators. Parks and Recreation . Deedle-Dee Productions and Universal Media Studios, 2015.
Note : The publisher’s name need not be included in the following sources: periodicals, works published by their author or editor, websites whose titles are the same name as their publisher, websites that make works available but do not actually publish them (such as YouTube , WordPress , or JSTOR ).
Publication date
The same source may have been published on more than one date, such as an online version of an original source. For example, a television series might have aired on a broadcast network on one date, but released on Netflix on a different date. When the source has more than one date, it is sufficient to use the date that is most relevant to your writing. If you’re unsure about which date to use, go with the date of the source’s original publication.
In the following example, Mutant Enemy is the primary production company, and “Hush” was released in 1999. Below is a general citation for this television episode:
“Hush.” Buffy the Vampire Slayer , created by Joss Whedon, performance by Sarah Michelle Gellar, season 4, Mutant Enemy, 1999 .
However, if you are discussing, for example, the historical context in which the episode originally aired, you should cite the full date. Because you are specifying the date of airing, you would then use WB Television Network (rather than Mutant Enemy), because it was the network (rather than the production company) that aired the episode on the date you’re citing.
“Hush.” Buffy the Vampire Slayer, created by Joss Whedon, performance by Sarah Michelle Gellar, season 4, episode 10, WB Television Network, 14 Dec. 1999 .
You should be as specific as possible in identifying a work’s location.
An essay in a book or an article in a journal should include page numbers.
Adiche, Chimamanda Ngozi. “On Monday of Last Week.” The Thing around Your Neck, Alfred A. Knopf, 2009, pp. 74-94 .
The location of an online work should include a URL. Remove any "http://" or "https://" tag from the beginning of the URL.
Wheelis, Mark. "Investigating Disease Outbreaks Under a Protocol to the Biological and Toxin Weapons Convention." Emerging Infectious Diseases , vol. 6, no. 6, 2000, pp. 595-600, wwwnc.cdc.gov/eid/article/6/6/00-0607_article. Accessed 8 Feb. 2009.
When citing a physical object that you experienced firsthand, identify the place of location.
Matisse, Henri. The Swimming Pool. 1952, Museum of Modern Art, New York .
Optional elements
The ninth edition is designed to be as streamlined as possible. The author should include any information that helps readers easily identify the source, without including unnecessary information that may be distracting. The following is a list of optional elements that can be included in a documented source at the writer’s discretion.
Date of original publication:
If a source has been published on more than one date, the writer may want to include both dates if it will provide the reader with necessary or helpful information.
Erdrich, Louise. Love Medicine. 1984. Perennial-Harper, 1993.
City of publication:
The seventh edition handbook required the city in which a publisher is located, but the eighth edition states that this is only necessary in particular instances, such as in a work published before 1900. Since pre-1900 works were usually associated with the city in which they were published, your documentation may substitute the city name for the publisher’s name.
Thoreau, Henry David. Excursions . Boston, 1863.
Date of access:
When you cite an online source, the MLA Handbook recommends including a date of access on which you accessed the material, since an online work may change or move at any time.
Bernstein, Mark. "10 Tips on Writing the Living Web." A List Apart: For People Who Make Websites, 16 Aug. 2002, alistapart.com/article/writeliving. Accessed 4 May 2009.
As mentioned above, while the MLA handbook recommends including URLs when you cite online sources, you should always check with your instructor or editor and include URLs at their discretion.
A DOI, or digital object identifier, is a series of digits and letters that leads to the location of an online source. Articles in journals are often assigned DOIs to ensure that the source is locatable, even if the URL changes. If your source is listed with a DOI, use that instead of a URL.
Alonso, Alvaro, and Julio A. Camargo. "Toxicity of Nitrite to Three Species of Freshwater Invertebrates." Environmental Toxicology , vol. 21, no. 1, 3 Feb. 2006, pp. 90-94. Wiley Online Library, doi: 10.1002/tox.20155.
Creating in-text citations using the previous (eighth) edition
Although the MLA handbook is currently in its ninth edition, some information about citing in the text using the older (eighth) edition is being retained. The in-text citation is a brief reference within your text that indicates the source you consulted. It should properly attribute any ideas, paraphrases, or direct quotations to your source, and should direct readers to the entry in the Works Cited list. For the most part, an in-text citation is the author’s name and the page number (or just the page number, if the author is named in the sentence) in parentheses :
When creating in-text citations for media that has a runtime, such as a movie or podcast, include the range of hours, minutes and seconds you plan to reference. For example: (00:02:15-00:02:35).
Again, your goal is to attribute your source and provide a reference without interrupting your text. Your readers should be able to follow the flow of your argument without becoming distracted by extra information.
How to Cite the Purdue OWL in MLA
Entire Website
The Purdue OWL . Purdue U Writing Lab, 2019.
Individual Resources
Contributors' names. "Title of Resource." The Purdue OWL , Purdue U Writing Lab, Last edited date.
The new OWL no longer lists most pages' authors or publication dates. Thus, in most cases, citations will begin with the title of the resource, rather than the developer's name.
"MLA Formatting and Style Guide." The Purdue OWL, Purdue U Writing Lab. Accessed 18 Jun. 2018.

Experience Stern | Faculty & Research
Pitch perfect: match the message to the idea's newness, study finds.

Entrepreneurs should tweak their pitches based on how innovative their idea is, researchers from the NYU Stern School of Business, Bayes Business School (formerly Cass) and George Washington University School of Business have found.
In a paper entitled, “ Radically Concrete or Incrementally Abstract? The Contingent Role of Abstract and Concrete Framing in Pitching Novel Ideas ,” NYU Stern Professor Gino Cattani , Professor Simone Ferriani (Bayes) and Professor Denise Falchetti (George Washington University) found that pitches promoting radical ideas are better received when framed in concrete and explanatory ‘how’ terms, while progressive ideas do better with abstract ‘why’ style of pitches.
Previous research found that professional audiences, like investors, prefer concrete pitches with how-style explanations, while lay audiences such as students and crowdfunders respond better to ‘why’ style pitches for abstract ideas.
“We wanted to identify the best way for entrepreneurs to pitch their ideas to get audiences’ attention and investment,” said Ferriani. “Could the way they pitch affect their success? What if they had great ideas but were pitching them in the wrong way? We wanted to explore which styles of pitching work best with differing types of ideas.”
To test this, academics conducted two experiments using an online survey with business students evaluating pitch decks, to see when new ideas were more likely to be viewed positively. The study used entrepreneurial pitches and varied the ideas’ originality and the style of abstract ‘why’ the idea works versus concrete ‘how’ the idea works. They looked at how these factors influenced people's reception of the idea and their willingness to support it.
The results indicate that the pitching strategy should match the idea's novelty to make it more appealing and likely to attract investment.
Professor Ferriani added: “Imagine a tech startup introducing a groundbreaking new virtual reality (VR) gaming platform that revolutionises the gaming experience. Our findings suggest that in their pitch to potential users, they should emphasise concrete usability details such as the advanced feedback technology, the immersive 360-degree visuals and the seamless integration with existing gaming consoles. When ideas have the potential to disrupt the status quo, this explanatory approach is key to offset the puzzlement that novel ideas can cause. Conversely, when ideas are less of a leap and more of a step forward, such as with incremental innovations, abstract language that paints the 'why' can be more effective.”
“This strategy taps into the audience's existing knowledge and expectations, connecting the new idea to familiar concepts and emphasizing its place within a broader vision or goal,” noted Falchetti.
“The research advises a tailored approach: for groundbreaking innovations, detail the practicalities; for incremental improvements, focus on the overarching vision,” concluded Professor Cattani. “As the language of entrepreneurship continues to evolve, this study offers a compass for navigating the intricate dance of persuasion and influence, providing a linguistic toolkit for turning novel concepts into embraced innovations.”
The paper was recently published in Innovation: Organization & Management .
This article was adapted from a piece written by the Bayes Business School (formerly Cass), City, University of London. See the original publication here .
More from Professor Gino Cattani
Generate accurate Chicago citations for free
- Knowledge Base
- Chicago Style
- Chicago Style Format for Papers | Requirements & Examples
Chicago Style Format for Papers | Requirements & Examples
Published on September 25, 2019 by Jack Caulfield . Revised on April 9, 2024.
The information in this article is largely drawn from Turabian style —a version of Chicago style aimed at students and researchers. When writing a paper in Chicago style, these are the guidelines to follow; for the sake of simplicity, the term “Chicago” is used here.
Chicago Citation Generator
To apply Chicago format:
- Use a standard font like 12 pt. Times New Roman.
- Double-space the text.
- Use 1 inch margins or larger.
- Indent new paragraphs by ½ inch.
- Place page numbers in the top right or bottom center.
Note that any specific formatting advice from your instructor or faculty overrules these guidelines. Template documents set up in Chicago style are available to download below. Just select the one with the citation style you’re following.
Author-date Notes and bibliography
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
General formatting, block quotes, numbers and acronyms, in-text citations and notes, bibliography or reference list, frequently asked questions about chicago format.
Chicago doesn’t require a specific font or font size, but recommends using something simple and readable (e.g., 12 pt. Times New Roman). Use margins of at least 1 inch on all sides of the page.
The main text should be double-spaced, and each new paragraph should begin with a ½ inch indent. Text should be left-aligned and not “justified” (meaning that the right margin should look ragged).
Page numbers can be placed either in the top right or the bottom center of the page—one or the other, not both.
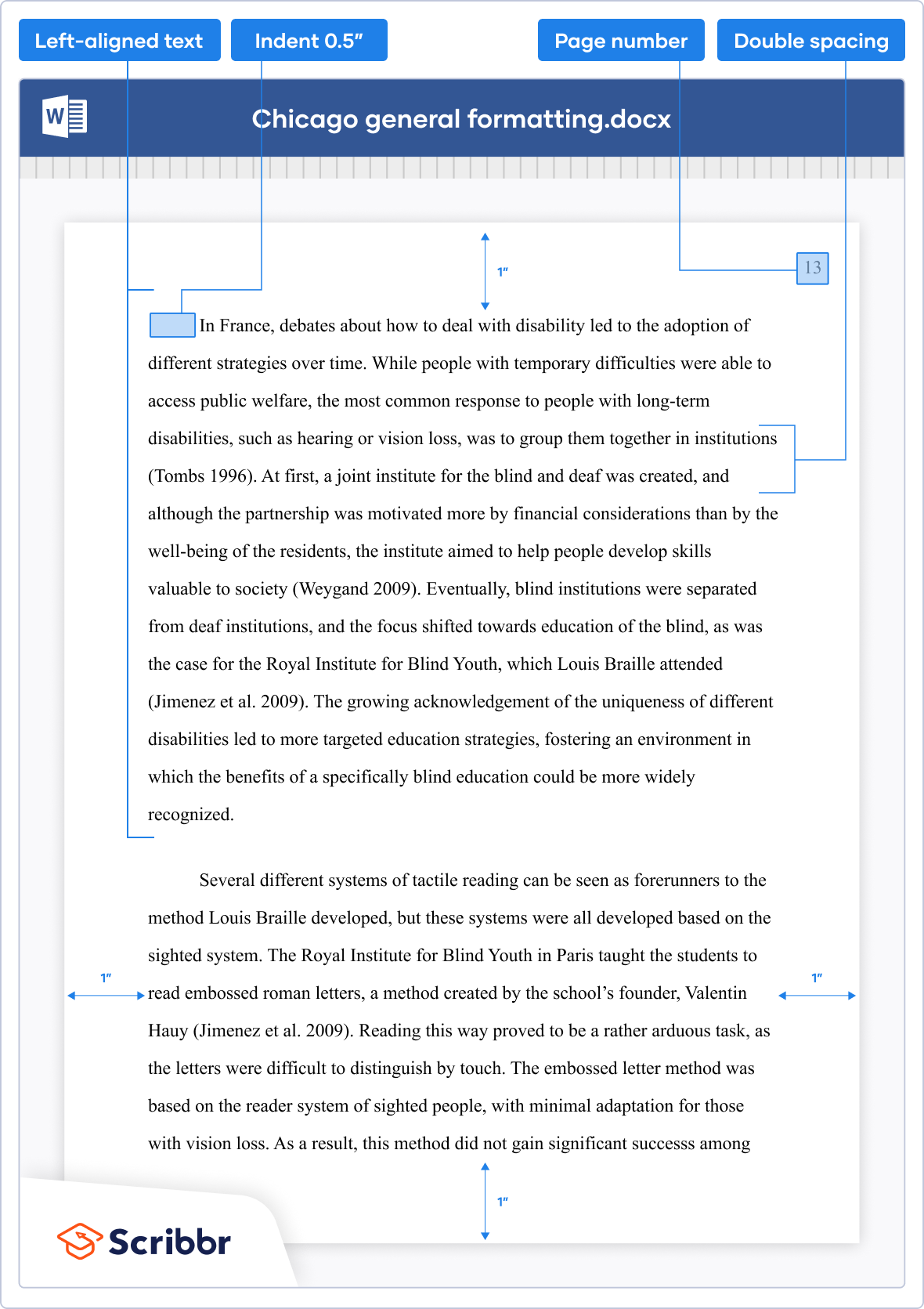
Don't submit your assignments before you do this
The academic proofreading tool has been trained on 1000s of academic texts. Making it the most accurate and reliable proofreading tool for students. Free citation check included.
Try for free
A Chicago title page isn’t required—often it’s sufficient to just include your title at the top of the first page—but if you’re asked to include one, Turabian provides guidelines for how to present it.
All text on the title page should be center-aligned and double-spaced, and written in the same font as the rest of your text. The title should appear about ⅓ of the way down the page, in headline capitalization and in bold.
If you have a subtitle, the main title ends with a colon and the subtitle appears on the following line, also in bold and the same size as the main title.
About ⅔ of the way down the page, add any information your instructor requests you to include—your name, student code, the course name and code, the date, etc. Each new piece of information appears on a new line.
The title page should not have a page number, but should be included in the page count—in other words, the page numbering starts on page 2.
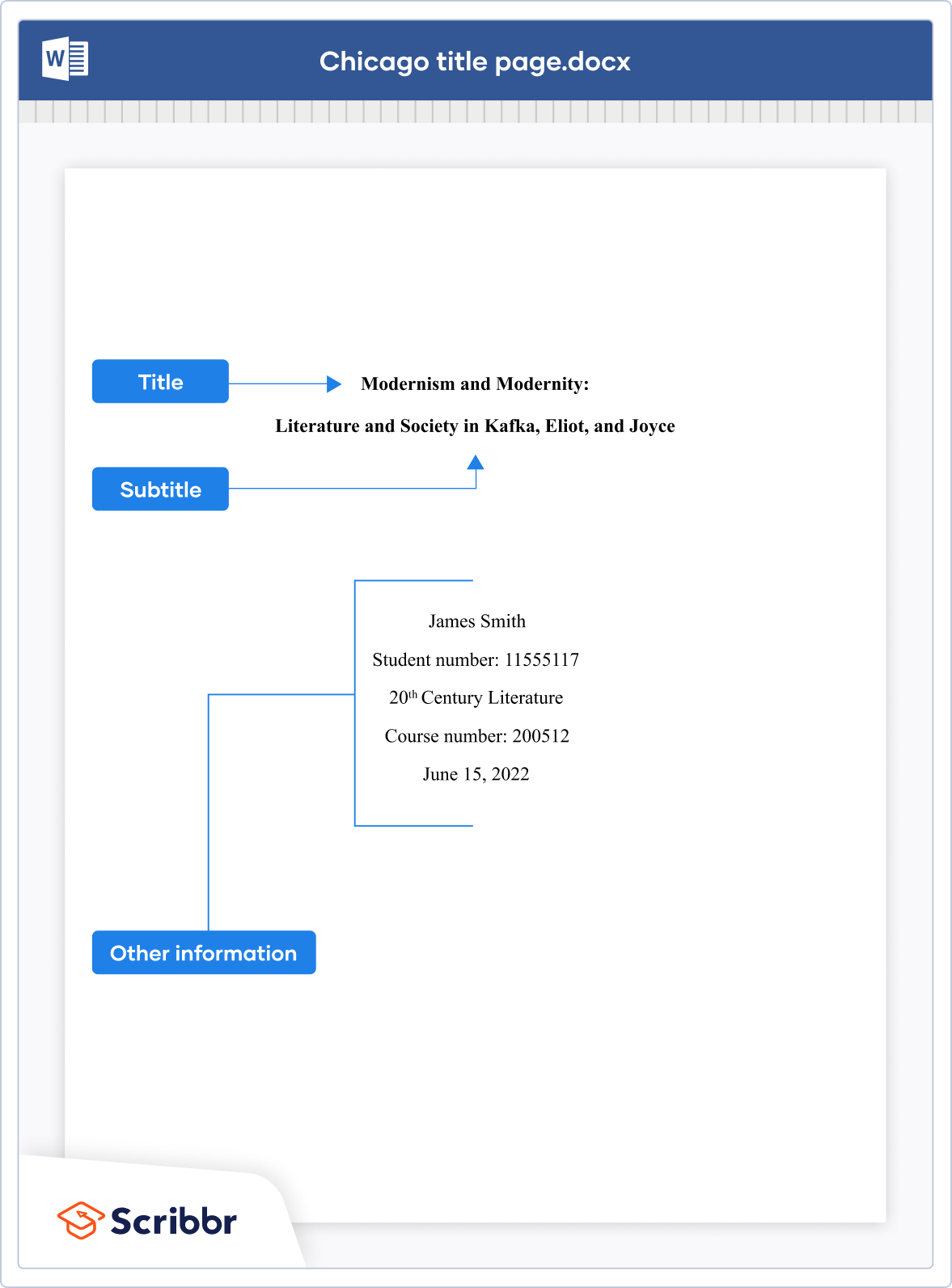
Headings should use headline capitalization:
- Summary of results
- Summary of Results
If you use different levels of heading (e.g., chapters, sections, subheadings), make sure your presentation makes clear which type of heading each one is.
All headings of one level should be presented the same way, and higher-level headings should stand out more from the text. For example, you might use a larger font for chapter headings, bold for section headings, and italics for subheadings:
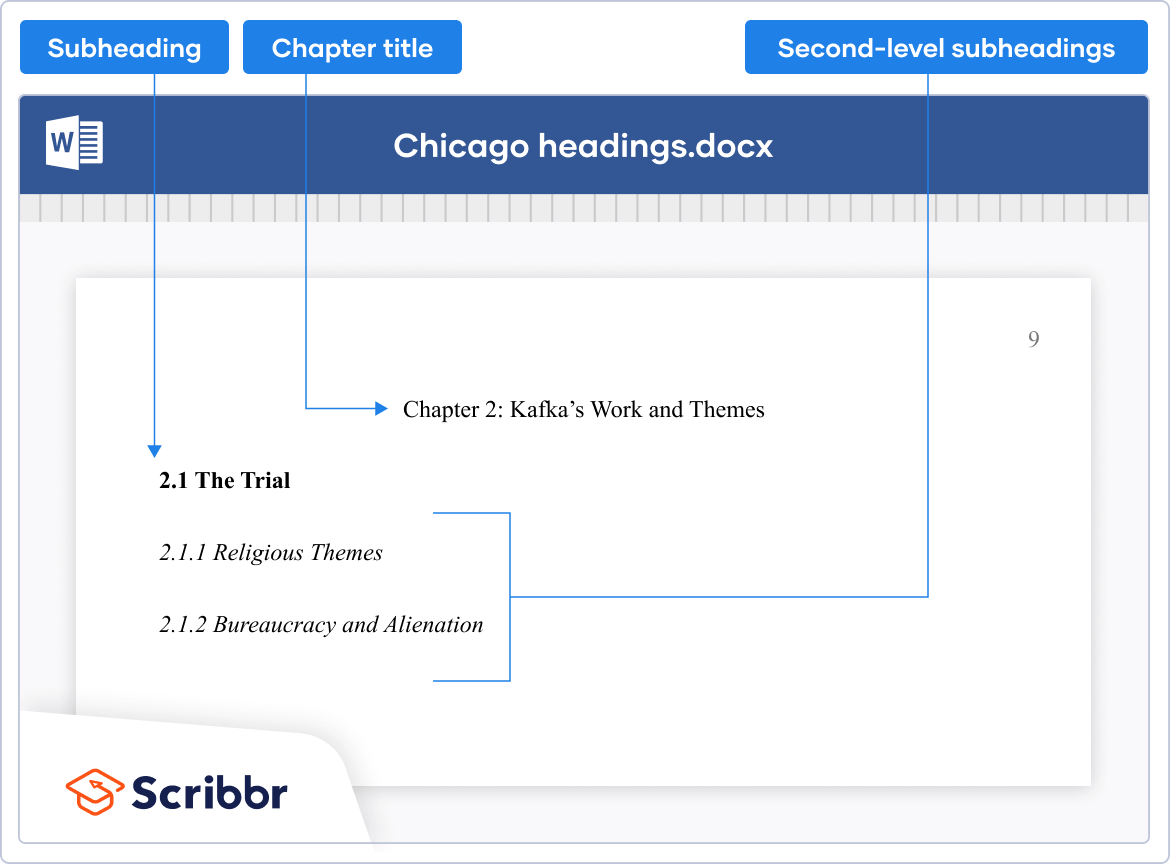
Prose quotations of five or more lines (or more than 100 words), as well as poetry quotations of two or more lines, are presented as block quotes .
Block quotes do not use quotation marks . Instead, a blank line separates them from the surrounding text on both sides and they are indented by an additional ½ inch. Unlike the rest of the text, they are not double-spaced.
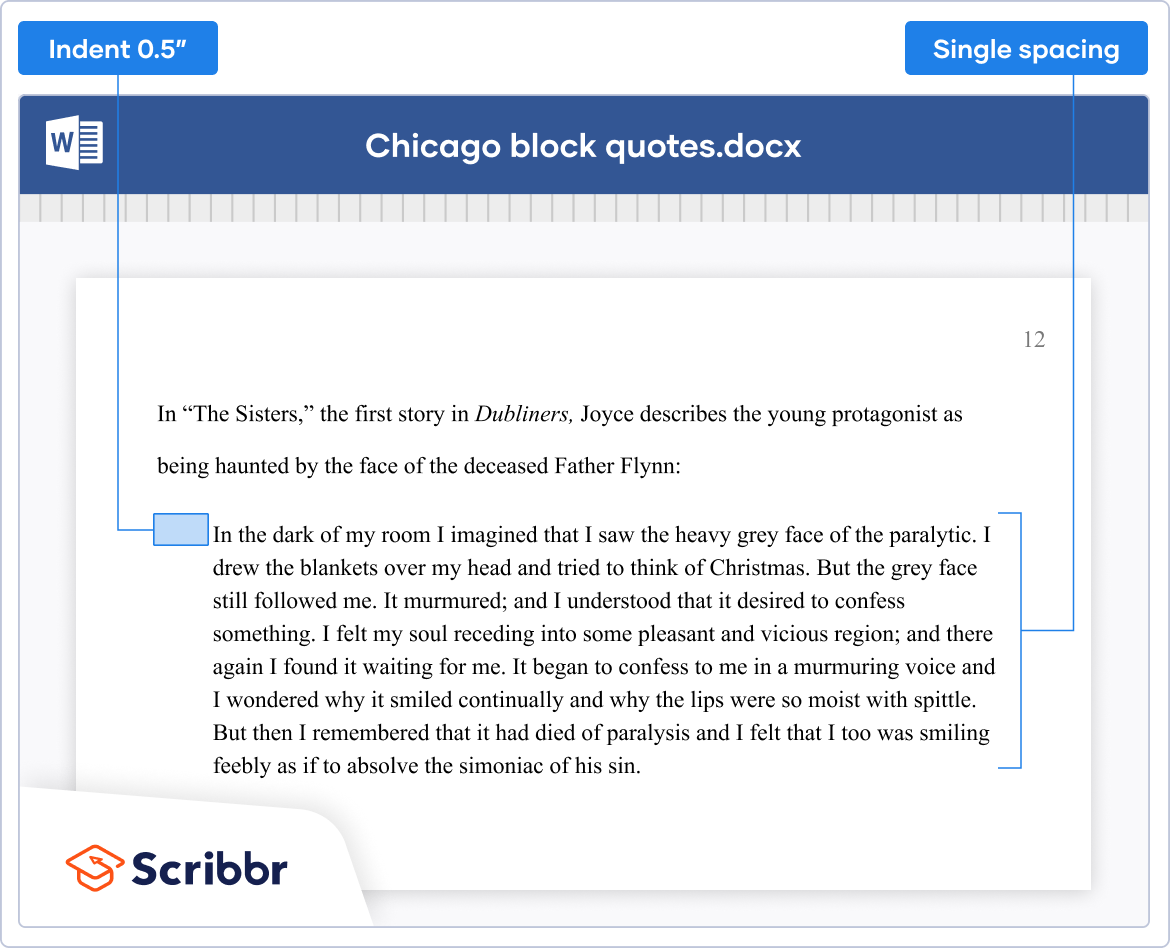
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Chicago recommends using words, not numerals, for numbers lower than 100. For example, you would write “ninety-five,” not “95.” But numerals should still be used when you’re referring to a specific measurement (e.g., “15 cm”) and when using decimals (e.g., “1.5”).
Acronyms should be introduced the first time you refer to the thing they stand for:
After this point, you can use the acronym alone.
Neither numerals nor acronyms should be used at the beginning of a sentence. Either rewrite the sentence so that the numeral or acronym appears elsewhere, or write out the full phrase or number:
- 100 people responded to the survey.
- One hundred people responded to the survey.
- The survey received 100 responses.
Chicago provides guidelines for not one but two citation styles : author-date and notes and bibliography.
In author-date style , citations are placed directly in the text in parentheses . In this style, you have some flexibility about how exactly to integrate the citation:
In notes and bibliography style, citations appear in Chicago footnotes or endnotes (the format is identical either way), and the reader is referred to them by superscript numbers in the text.
Footnote and endnote numbers appear at the end of the relevant clause or sentence, after any punctuation except a dash .
Endnotes appear on their own page just before the bibliography ; footnotes appear at the bottom of each page. Footnotes should be separated from the text by a short rule and be presented in the same font size as the main text, or smaller. Word’s footnote function automatically creates footnotes like this:

At the end of your paper, you’ll likely include a bibliography (for notes and bibliography style) or a reference list (for author-date).
Bibliographies and reference lists are not double-spaced, but leave a blank line between entries.
If an entry extends onto a second line, a ½ inch indent should be applied to all but the first line of the entry.
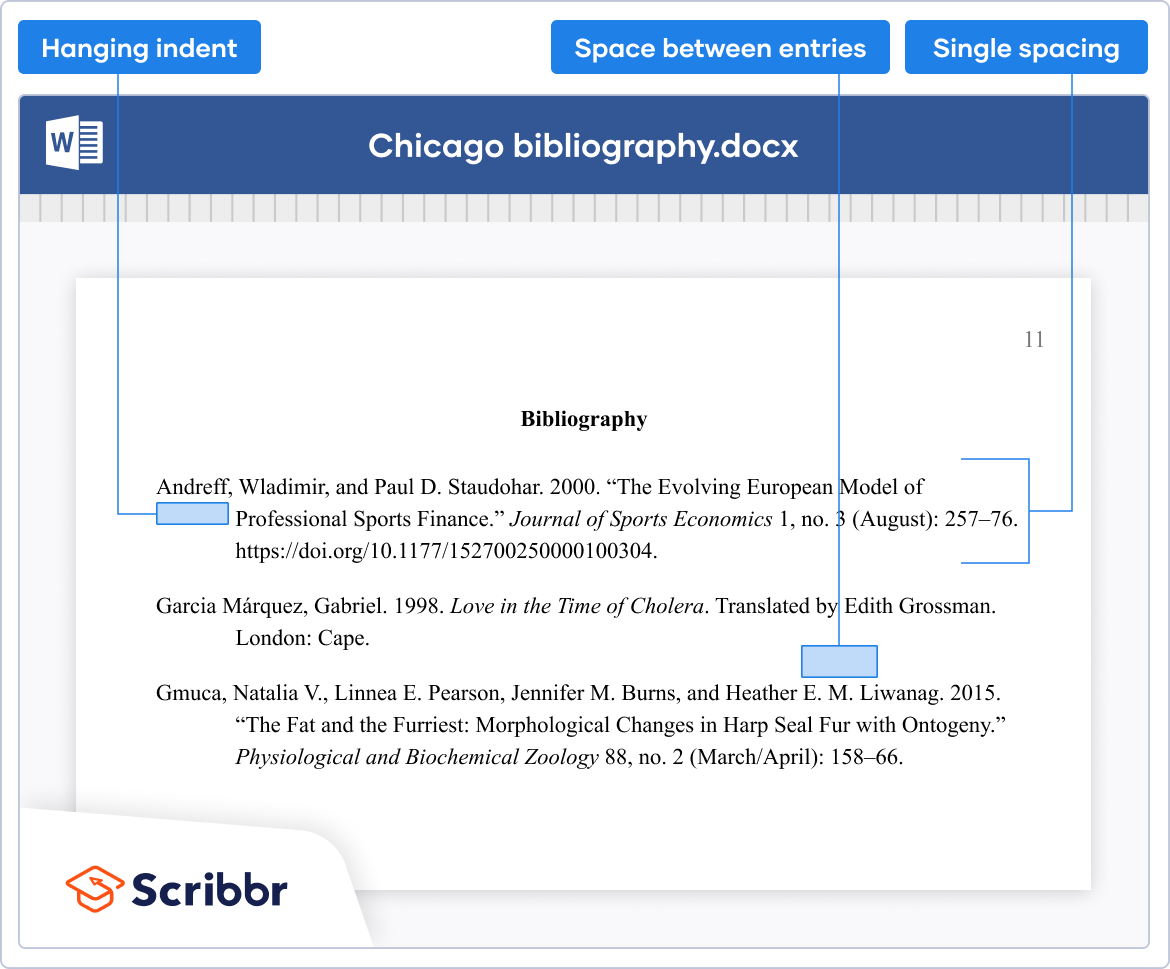
If you have to create a Chicago style annotated bibliography , follow the same format as a normal bibliography, but indent and double-space the annotations under each source reference.
Turabian style is a version of Chicago style designed specifically for students and researchers. It follows most Chicago conventions, but also adds extra guidelines for formatting research papers , theses and dissertations .
More information can be found in A Manual for Writers of Research Papers, Theses, and Dissertations by Kate L. Turabian, now in its ninth edition.
- A reference list is used with Chicago author-date citations .
- A bibliography is used with Chicago footnote citations .
Both present the exact same information; the only difference is the placement of the year in source citations:
- In a reference list entry, the publication year appears directly after the author’s name.
- In a bibliography entry, the year appears near the end of the entry (the exact placement depends on the source type).
There are also other types of bibliography that work as stand-alone texts, such as a Chicago annotated bibliography .
In Chicago author-date style , your text must include a reference list . It appears at the end of your paper and gives full details of every source you cited.
In notes and bibliography style, you use Chicago style footnotes to cite sources; a bibliography is optional but recommended. If you don’t include one, be sure to use a full note for the first citation of each source.
Footnotes appear at the bottom of the relevant page. Endnotes appear in a list at the end of the text, just before the reference list or bibliography. Don’t mix footnotes and endnotes in the same document: choose one or the other and use them consistently.
In Chicago notes and bibliography style , you can use either footnotes or endnotes, and citations follow the same format in either case.
In APA and MLA style , footnotes or endnotes are not used for citations, but they can be used to provide additional information.
Chicago format doesn’t require you to use any specific font, as long as you choose something readable. A good standard choice is 12 pt Times New Roman.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2024, April 09). Chicago Style Format for Papers | Requirements & Examples. Scribbr. Retrieved July 10, 2024, from https://www.scribbr.com/chicago-style/format-paper/
Is this article helpful?

Jack Caulfield
Other students also liked, creating a chicago style bibliography | format & examples, chicago in-text citations | styles, format & examples, chicago style footnotes | citation format & examples, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts

Research Shows That AI-Generated Slop Overuses Specific Words
Wordsmiths, these ais are not., disease control.
AI models may be trained on the entire corpus of humanity's writing , but it turns out their vocabulary can be strikingly limited. A new yet-to-be-peer-reviewed study , spotted by Ars Technica , adds to the general understanding that large language models tend to overuse certain words that can give their origins away .
In a novel approach, these researchers took a cue from epidemiology by measuring "excess word usage" in biomedical papers in the same way doctors gauged COVID-19's impact through "excess deaths." The results are a fascinating insight into AI's impact in the world of academia, suggesting that at least 10 percent of abstracts in 2024 were "processed with LLMs."
"The effect of LLM usage on scientific writing is truly unprecedented and outshines even the drastic changes in vocabulary induced by the COVID-19 pandemic," the researchers wrote in the study.
The work may even provide a boost for methods of detecting AI writing, which have so far proved notoriously unreliable .
Style Over Substance
These findings come from a broad analysis of 14 million biomedical abstracts published between 2010 and 2024 that are available on PubMed. The researchers used papers published before 2023 as a baseline to compare papers that came out during the widespread commercialization of LLMs like ChatGPT.
They found that words that were once considered "less common," like " delves ," are now used 25 more times than they used to, and others, like "showcasing" and "underscores," saw a similarly baffling nine times increase. But some "common" words also saw a boost: "potential," "findings," and "crucial" went up in frequency by up to 4 percent.
Such a marked increase is basically unprecedented without the explanation of some pressing global circumstance. When the researchers looked for excess words between 2013 and 2023, the ones that came up were terms like "ebola," "coronavirus," and "lockdown."
Beyond their obvious ties to real-world events, these are all nouns, or as the researchers put it, "content" words. By contrast, what we see with the excess usage in 2024 is that they're almost entirely "style" words. And in numbers, of the 280 excess "style" words that year, two-thirds of them were verbs, and about a fifth were adjectives.
To see just how saturated AI language is with these tell-tales, have a look at this example from a real 2023 paper (emphasis the researchers'): "By meticulously delving into the intricate web connecting [...] and [...], this comprehensive chapter takes a deep dive into their involvement as significant risk factors for [...].
Language Barriers
Using these excess style words as "markers" of ChatGPT usage, the researchers estimated that around 15 percent of papers published in non-English speaking countries like China, South Korea, and Taiwan are now AI-processed — which is higher than in countries where English is the native tongue, like the United Kingdom, at 3 percent. LLMs, then, may be a genuinely helpful tool for non-native speakers to make it in a field dominated by English .
Still, the researchers admit that native speakers may simply be better at hiding their LLM usage. And of course, the appearance of these words is not a guarantee that the text was AI-generated.
Whether this will serve as a reliable detection method is up in the air — but what is certainly evidence here is just how quickly AI can catalyze changes in written language.
More on AI: AI Researcher Elon Musk Poached From OpenAI Returns to OpenAI
Share This Article
- Mobile Site
- Staff Directory
- Advertise with Ars
Filter by topic
- Biz & IT
- Gaming & Culture
Front page layout
Delving deep —
The telltale words that could identify generative ai text, new paper counts "excess words" that started appearing more often in the post-llm era..
Kyle Orland - Jul 1, 2024 11:30 am UTC

Further Reading
To measure these vocabulary changes, the researchers analyzed 14 million paper abstracts published on PubMed between 2010 and 2024, tracking the relative frequency of each word as it appeared across each year. They then compared the expected frequency of those words (based on the pre-2023 trendline) to the actual frequency of those words in abstracts from 2023 and 2024, when LLMs were in widespread use.
The results found a number of words that were extremely uncommon in these scientific abstracts before 2023 that suddenly surged in popularity after LLMs were introduced. The word "delves," for instance, shows up in 25 times as many 2024 papers as the pre-LLM trend would expect; words like "showcasing" and "underscores" increased in usage by nine times as well. Other previously common words became notably more common in post-LLM abstracts: the frequency of "potential" increased 4.1 percentage points; "findings" by 2.7 percentage points; and "crucial" by 2.6 percentage points, for instance.
These kinds of changes in word use could happen independently of LLM usage, of course—the natural evolution of language means words sometimes go in and out of style. However, the researchers found that, in the pre-LLM era, such massive and sudden year-over-year increases were only seen for words related to major world health events: "ebola" in 2015; "zika" in 2017; and words like "coronavirus," "lockdown" and "pandemic" in the 2020 to 2022 period.
In the post-LLM period, though, the researchers found hundreds of words with sudden, pronounced increases in scientific usage that had no common link to world events. In fact, while the excess words during the COVID pandemic were overwhelmingly nouns, the researchers found that the words with a post-LLM frequency bump were overwhelmingly "style words" like verbs, adjectives, and adverbs (a small sampling: "across, additionally, comprehensive, crucial, enhancing, exhibited, insights, notably, particularly, within").
This isn't a completely new finding—the increased prevalence of "delve" in scientific papers has been widely noted in the recent past , for instance. But previous studies generally relied on comparisons with "ground truth" human writing samples or lists of pre-defined LLM markers obtained from outside the study. Here, the pre-2023 set of abstracts acts as its own effective control group to show how vocabulary choice has changed overall in the post-LLM era.
An intricate interplay
By highlighting hundreds of so-called "marker words" that became significantly more common in the post-LLM era, the telltale signs of LLM use can sometimes be easy to pick out. Take this example abstract line called out by the researchers, with the marker words highlighted: "A comprehensive grasp of the intricate interplay between [...] and [...] is pivotal for effective therapeutic strategies."
After doing some statistical measures of marker word appearance across individual papers, the researchers estimate that at least 10 percent of the post-2022 papers in the PubMed corpus were written with at least some LLM assistance. The number could be even higher, the researchers say, because their set could be missing LLM-assisted abstracts that don't include any of the marker words they identified.
Those measured percentages can vary a lot across different subsets of papers, too. The researchers found that papers authored in countries like China, South Korea, and Taiwan showed LLM marker words 15 percent of the time, suggesting "LLMs might... help non-natives with editing English texts, which could justify their extensive use." On the other hand, the researchers offer that native English speakers "may [just] be better at noticing and actively removing unnatural style words from LLM outputs," thus hiding their LLM usage from this kind of analysis.
Detecting LLM use is important, the researchers note, because "LLMs are infamous for making up references, providing inaccurate summaries, and making false claims that sound authoritative and convincing." But as knowledge of LLMs' telltale marker words starts to spread, human editors may get better at taking those words out of generated text before it's shared with the world.
Who knows, maybe future large language models will do this kind of frequency analysis themselves, lowering the weight of marker words to better mask their outputs as human-like. Before long, we may need to call in some Blade Runners to pick out the generative AI text hiding in our midst.
reader comments
Promoted comments.
Channel Ars Technica
To menu | To main contents
Script can not be used because of invalid.
*Because style sheet is disabled , can not use.
This is the menu from here
- About NITech
- Prospective Students
- Current Students
- International Relations
Home > Topics > A research paper released by the YAMAMURA-MIYAGAWA Lab was published in the International Journal of Biological Macromolecules
A research paper released by the YAMAMURA-MIYAGAWA Lab was published in the International Journal of Biological Macromolecules
Category:News|Publishing : July 8, 2024
YAMAMURA-MIYAGAWA Laboratory's research paper titled "Preparation of β-1,3-glucan mimics via modification of polymer backbone, and evaluation of cytokine production using the polymer library in immune activation," was published in the International Journal of Biological Macromolecules (Vol. 264, 130546, 2024; Impact Factor=8.2). The authors are Associate Professor MIYAGAWA Atsushi (NITech Life Science and Applied Chemistry Group), YAMAMOTO Nami (student in the Master's course, at the time), OHNO Ayane (student in the Master's course, at the time), and Professor YAMAMURA Hatsuo (NITech Life Science and Applied Chemistry Group).
In this study, the researchers successfully developed molecules that exhibit strong cytokine induction, significantly surpassing the immunostimulating activity of natural β-glucan, by chemically synthesizing branched oligosaccharides as the repeating structures of β-glucan and polymerizing them using norbornene. By reconstructing β-glucan via the polymerization of the oligosaccharides and modifying the resulting polymers, the team created a library of β-glucan mimic polymers with diverse backbone structures. The structural elements essential for β-glucan-like immune activation was then identified through structure-activity relationship studies. The resultant artificial polymers have the potential to activate the immune system and induce anti-tumor activity. Through effective use of the biological activity of β-glucan, the polymers are expected to lead to broad applications in the development of safe anti-tumor and antiviral agents.
Link to International Journal of Biological Macromolecules
Preparation of β-1,3-glucan mimics via modification of polymer backbone, and evaluation of cytokine production using the polymer library in immune activation - ScienceDirect
Back to Topics
To page top

IMAGES
VIDEO
COMMENTS
Formatting a Chicago paper. The main guidelines for writing a paper in Chicago style (also known as Turabian style) are: Use a standard font like 12 pt Times New Roman. Use 1 inch margins or larger. Apply double line spacing. Indent every new paragraph ½ inch. Place page numbers in the top right or bottom center.
Paper Format. Consistency in the order, structure, and format of a paper allows readers to focus on a paper's content rather than its presentation. To format a paper in APA Style, writers can typically use the default settings and automatic formatting tools of their word-processing program or make only minor adjustments.
Set the top, bottom, and side margins of your paper at 1 inch. Use double-spaced text throughout your paper. Use a standard font, such as Times New Roman or Arial, in a legible size (10- to 12-point). Use continuous pagination throughout the paper, including the title page and the references section.
These sample papers demonstrate APA Style formatting standards for different student paper types. Students may write the same types of papers as professional authors (e.g., quantitative studies, literature reviews) or other types of papers for course assignments (e.g., reaction or response papers, discussion posts), dissertations, and theses.
Do not use a period after your title or after any heading in the paper (e.g., Works Cited). Begin your text on a new, double-spaced line after the title, indenting the first line of the paragraph half an inch from the left margin. Fig. 1. The top of the first page of a research paper.
Media Files: APA Sample Student Paper , APA Sample Professional Paper This resource is enhanced by Acrobat PDF files. Download the free Acrobat Reader. Note: The APA Publication Manual, 7 th Edition specifies different formatting conventions for student and professional papers (i.e., papers written for credit in a course and papers intended for scholarly publication).
Throughout your paper, you need to apply the following APA format guidelines: Set page margins to 1 inch on all sides. Double-space all text, including headings. Indent the first line of every paragraph 0.5 inches. Use an accessible font (e.g., Times New Roman 12pt., Arial 11pt., or Georgia 11pt.).
The authority on APA Style and the 7th edition of the APA Publication Manual. Find tutorials, the APA Style Blog, how to format papers in APA Style, and other resources to help you improve your writing, master APA Style, and learn the conventions of scholarly publishing.
MLA format is a widely used citation style for academic papers. Learn how to format your title page, header, and Works Cited page with our free template and examples. Watch our 3-minute video to see how easy it is to apply MLA rules to your document.
A research paper is a piece of academic writing that provides analysis, interpretation, and argument based on in-depth independent research. About us; ... Research papers include citations and references to acknowledge the sources of information and ideas used in the paper. They use a specific citation style, such as APA, MLA, or Chicago, to ...
The reference list at the end of your paper; Rules: All in-text citations should be listed in the reference list at the end of your paper. Reference list entries need to contain all the information that someone reading your paper would need in order to find your source. Reference lists in Harvard Style are arranged alphabetically by first author.
MLA Sample Paper. This resource contains a sample MLA paper that adheres to the 2016 updates. To download the MLA sample paper, click this link.
Research paper format is an essential aspect of academic writing that plays a crucial role in the communication of research findings.The format of a research paper depends on various factors such as the discipline, style guide, and purpose of the research. It includes guidelines for the structure, citation style, referencing, and other elements of the paper that contribute to its overall ...
If you are looking for how to write a research paper outline APA in Full Sentence Format, here is an example: A. For subheadings, you use capital alphabets A, B, C. B. Subheadings must complement, lead, or link to the paper's main idea. 1. Arabic numerals are used for headings under subheadings like 1, 2, and 3. 2.
Writing a research paper is an essential aspect of academics and should not be avoided on account of one's anxiety. In fact, the process of writing a research paper can be one of the more rewarding experiences one may encounter in academics. What is more, many students will continue to do research throughout their careers, which is one of the ...
APA Style is primarily used in the behavioral sciences, which are subjects related to people, such as psychology, education, and nursing. It is also used by students in business, engineering, communications, and other classes. Students use it to write academic essays and research papers in high school and college, and professionals use it to ...
Simple Formatting Guidelines. Margins. Paper size - 8.5 inch x 11 inch. 1 inch on all four edges of the page. Typeface/Font size. Easily read; preferably serifed, ex. Times New Roman. 12-point is accepted size. Spacing/Indentation.
A decimal outline is similar in format to the alphanumeric outline, but with a different numbering system: 1, 1.1, 1.2, etc. Text is written as short notes rather than full sentences. Example: 1 Body paragraph one. 1.1 First point. 1.1.1 Sub-point of first point. 1.1.2 Sub-point of first point.
In this paper, we show that, a good style representation is crucial and sufficient for generalized style transfer without test-time tuning. We achieve this through constructing a style-aware encoder and a well-organized style dataset called StyleGallery. With dedicated design for style learning, this style-aware encoder is trained to extract expressive style representation with decoupling ...
A PRISMA-style flow diagram should be included as an online supplement ... Research Letters are concise, focused reports of original research. ... Most essays published in A Piece of My Mind are personal vignettes (eg, exploring the dynamics of the patient-physician relationship) taken from wide-ranging experiences in medicine; occasional ...
The leadership style adopted in an organization considerably reflects on the employees' performances. The paper investigated the effect of leadership styles on employees' performance.
Unlike the "Chinese Roof with Western walls style" or "mixed Easten and Western façade style", Tao Fong Shan represents a "Buddhist-Christian style", with its success rooted in the similarity of life and spatial modes between Buddhist and Christian monasteries. ... Through an in-depth study of Prip-Møller's field research in the ...
Indent the first line of every paragraph of text 0.5 in. using the tab key or the paragraph-formatting function of your word-processing program. Page numbers: Put a page number in the top right corner of every page, including the title page or cover page, which is page 1. Student papers do not require a running head on any page.
MLA (Modern Language Association) style is most commonly used to write papers and cite sources within the liberal arts and humanities. This resource, updated to reflect the MLA Handbook (9th ed.), offers examples for the general format of MLA research papers, in-text citations, endnotes/footnotes, and the Works Cited page.
In a paper entitled, "Radically Concrete or Incrementally Abstract? The Contingent Role of Abstract and Concrete Framing in Pitching Novel Ideas," NYU Stern Professor Gino Cattani, Professor Simone Ferriani (Bayes) and Professor Denise Falchetti (George Washington University) found that pitches promoting radical ideas are better received when framed in concrete and explanatory 'how ...
Chicago doesn't require a specific font or font size, but recommends using something simple and readable (e.g., 12 pt. Times New Roman). Use margins of at least 1 inch on all sides of the page. The main text should be double-spaced, and each new paragraph should begin with a ½ inch indent.
Using these excess style words as "markers" of ChatGPT usage, the researchers estimated that around 15 percent of papers published in non-English speaking countries like China, South Korea, and ...
The word "delves," for instance, shows up in 25 times as many 2024 papers as the pre-LLM trend would expect; words like "showcasing" and "underscores" increased in usage by nine times as well.
Thank you for using the APA Style annotated sample professional paper for guidance when wri ng your paper or assignment. This sample paper PDF contains annota ons that draw aten on to key APA Style content and forma ng such as the tle page, headings, in-text cita ons, references, and more. Relevant sec ons of the seventh edi on of the ...
YAMAMURA-MIYAGAWA Laboratory's research paper titled "Preparation of β-1,3-glucan mimics via modification of polymer backbone, and evaluation of cytokine production using the polymer library in immune activation," was published in the International Journal of Biological Macromolecules (Vol. 264, 130546, 2024; Impact Factor=8.2). The authors are Associate Professor MIYAGAWA Atsushi (NITech ...