
Home > ICSK > IJESAB > Vol. 2 > Iss. 12 (2020)

Case Presentation for Polycystic Ovarian Syndrome
Hannah Lattanzio , University of Illinois at Chicago Follow Vered Arbel , University of Illinois at Chicago Follow Terry Nicola Tal Amasay Follow
CASE HISTORY : The patient is a fourteen-year-old female who presented to the clinic for bilateral hip and lumbar back pain. She stated that the pain has been present for approximately seven months and described it as a deep ache in the low back and both hips anteriorly. The patient said she plays a variety of sports but denies any specific event that could contribute to her pain. She stated her pain is worse with prolonged walking, standing, and sitting. Additionally, the patient mentioned her first menstrual cycle lasted fifty-six days and she has since not had any following menses, indicating secondary amenorrhea. Secondary amenorrhea is characterized by the cessation of irregular menses for six months and is commonly caused by hormonal imbalances. PHYSICAL EXAM : Examination of the hip, abdomen, and back did not demonstrate any deformities. She had tenderness to palpation at the mid-abdomen and at the insertion of the hip flexors, at the ASIS and AIIS bilaterally. Her patellar reflex was normal and 5/5 strength in hip flexion, extension, and abduction was observed along with full range of motion of both hips. FABER and FADIR tests were conducted and resulted in a positive sign of pain for both tests. DIFFERENTIAL DIAGNOSES : Hip dysplasia, Slipped capital femoral epiphysis, Polycystic Ovarian Syndrome, Femoroacetabular impingement, and Snapping hip. TESTS & RESULTS : Patient had an x-ray of both hips that were negative for tissue abnormalities. A pelvic MRI suggested small areas of sub-chondral sclerosis and possible polycystic ovaries. FINAL DIAGNOSIS : Polycystic Ovarian Syndrome (PCOS). DISCUSSION : PCOS is a common endocrine disorder that effects an estimated 10% of women between the ages of fifteen to forty-four, though it is commonly diagnosed in adolescence to early twenties. PCOS is diagnosed when two of the following criteria are evident: menstrual irregularity, polycystic ovaries and/or symptoms of androgen excess. Though pain is not an indicator of PCOS, it is not uncommon, and presentation varies widely to include abdominal, anterior pelvic, and low back pain. PCOS is believed to be caused by genetics but is greatly influenced by lifestyle factors and is associated with many morbidities including obesity, insulin resistance, and depression. Management of PCOS consists of controlling the symptoms of androgen excess and/or the absence of ovulation, and to reduce the chances of long-term complications such as infertility, metabolic syndrome, and type two diabetes. Oral contraceptives are the most common treatment for menstrual irregularity in adolescents. Androgen excess is managed with a combination of cosmetic management, oral contraceptives, and anti-androgen therapy, such as cyproterone acetate. Prevention of long-term complications include diet and lifestyle changes to reduce the risk of developing type two diabetes. Metformin may also be an effective treatment for both type two diabetes and androgen excess. OUTCOME OF THE CASE : Patient was referred to physical therapy to include protective range of motion and exercise of hip flexors. She continued to take Diclofenac for pain. RETURN TO ACTIVITY AND FURTHER FOLLOW-UP : The patient will follow-up with endocrinology and gynecologist for questionable polycystic ovarian syndrome due to polycystic ovaries present on the hip MRI and elevated testosterone levels. An x-ray without contrast of bilateral hips will be obtained to evaluate bony anatomy and she will return to the clinic in 4-6 weeks to follow-up on symptoms and discuss the imaging findings.
Recommended Citation
Lattanzio, Hannah; Arbel, Vered; Nicola, Terry; and Amasay, Tal (2020) "Case Presentation for Polycystic Ovarian Syndrome," International Journal of Exercise Science: Conference Proceedings : Vol. 2: Iss. 12, Article 128. Available at: https://digitalcommons.wku.edu/ijesab/vol2/iss12/128
Since February 17, 2020
To view the content in your browser, please download Adobe Reader or, alternately, you may Download the file to your hard drive.
NOTE: The latest versions of Adobe Reader do not support viewing PDF files within Firefox on Mac OS and if you are using a modern (Intel) Mac, there is no official plugin for viewing PDF files within the browser window.
- Journal Home
- Editorial Board
- Submit Abstract
- Most Popular Papers
- Receive Custom Email Notices or RSS
Advanced Search
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Impact of polycystic ovary syndrome on quality of life of women in correlation to age, basal metabolic index, education and marriage
Roles Investigation
Affiliation Department of Pharmacology, Santosh Medical College, Santosh University, Ghaziabad, Uttar-Pradesh, India
Roles Data curation
Affiliation Department of Gynecology and Obstetrics, All India Institute of Medical Sciences, Patna, Bihar, India
Roles Project administration
* E-mail: [email protected] (KD); [email protected] (MSA)
Roles Formal analysis, Writing – original draft, Writing – review & editing
Affiliation College of Pharmacy, King Khalid University, Abha, Kingdom of Saudi Arabia
- Fauzia Tabassum,
- Chandra Jyoti,
- Hemali Heidi Sinha,
- Kavita Dhar,
- Md Sayeed Akhtar

- Published: March 10, 2021
- https://doi.org/10.1371/journal.pone.0247486
- Reader Comments
Polycystic ovary syndrome (PCOS) is the major endocrine related disorder in young age women. Physical appearance, menstrual irregularity as well as infertility are considered as a sole cause of mental distress affecting health-related quality of life (HRQOL). This prospective case-control study was conducted among 100 PCOS and 200 healthy control cases attending tertiary care set up of AIIMS, Patna during year 2017 and 2018. Pre-validated questionnaires like Short Form Health survey-36 were used for evaluating impact of PCOS in women. Multivariate analysis was applied for statistical analysis. In PCOS cases, socioeconomic status was comparable in comparison to healthy control. But, PCOS cases showed significantly decreased HRQOL. The higher age of menarche, irregular/delayed menstrual history, absence of child, were significantly altered in PCOS cases than control. Number of child, frequency of pregnancy, and miscarriage were also observed higher in PCOS cases. Furthermore, in various category of age, BMI, educational status and marital status, significant differences were observed in the different domain of SF-36 between PCOS and healthy control. Altogether, increased BMI, menstrual irregularities, educational status and marital status play a major role in altering HRQOL in PCOS cases and psychological care must be given during patient care.
Citation: Tabassum F, Jyoti C, Sinha HH, Dhar K, Akhtar MS (2021) Impact of polycystic ovary syndrome on quality of life of women in correlation to age, basal metabolic index, education and marriage. PLoS ONE 16(3): e0247486. https://doi.org/10.1371/journal.pone.0247486
Editor: Antonio Simone Laganà, University of Insubria, ITALY
Received: July 24, 2020; Accepted: January 1, 2021; Published: March 10, 2021
Copyright: © 2021 Tabassum et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data cannot be shared publicly because of confidentiality. Data are available from the Ethics Committee, AIIMS, Patna for researchers who meet the criteria for access to confidential data. Contact information for the ethics committee: (The Chairman, Institutional review board, All India Institute of Medical Sciences, Patna, Bihar (India), PIN-801507).
Funding: This work is supported by the Dean of Scientific Research, King Khalid University for the financial support is greatly appreciated for the general research Project under grant number [GRP/190/42], awarded to MSA.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Polycystic ovary syndrome (PCOS) is a major endocrine disorder in young age women affecting their health-related quality of life (HRQOL) and their mental well-being as well [ 1 , 2 ]. Moreover, this develop into lifelong health condition that continues far beyond the young ages and affects around 5 million young age population in the United States of America [ 3 , 4 ]. In India, PCOS has been reported to vary between racial counterparts with an estimated prevalence of 9.13% in adolescents [ 5 ]. The major changes in physical appearance, obesity, along with menstrual irregularity have been found to be the main contributing factor of psychological dilemma [ 6 – 8 ]. PCOS negative impact is always underestimated and dominates on women’s life and may lead to a risk for serious anxiety and psychological disorder [ 9 , 10 ]. Importantly, the psychological burden greatly varies with the change in geographical areas and societal perceptions (Barnard et al., 2007; Brady et al., 2009). These patients may experience characteristics of PCOS as stressful and may be at higher risk for depression and anxiety disorders and even this may lead towards suicidal tendency [ 9 , 10 ].
Clinically, PCOS is characterized by either oligoovulation or anovulation and hyperandrogenism that may cause infertility, and other related metabolic disorders [ 11 ]. This progresses to increased risk of reproductive issues like infertility endometrial cancer, gestational as well as mental disturbances [ 12 ]. However, novel treatments and therapies can then be targeted toward improving those problems, which are most important for the individual concerned [ 13 , 14 ]. Recently, increased importance has been given on understanding the impact of PCOS symptoms and in particular about the feminine identity and thus their treatment from the patients’ perspective for the better quality of life (QOL). HRQOL is a self-perceived health status as a consequence of any disease that is measured by health status questionnaires [ 15 ]. Therefore, HRQOL questionnaires like Short Form Health Survey-36 (SF-36) for PCOS, was used to understand the impact of PCOS and evaluating individual patients’ health status and monitoring and comparing disease burden [ 16 , 17 ]. The SF-36 scale leaves out important detrimental issues linked to PCOS patients such as physical and emotional symptoms associated with menses [ 16 ]. PCOS questionnaire has reasonable internal reliability, good test-retest reliability, good concurrent and discriminated validity, and a reasonable factor analysis making PCOS questionnaire a useful and promising tool for HRQOL in PCOS cases.
At present, there is a paucity of information related to PCOS among women of the reproductive age group in India, in particular, North India. Thus, considering these factors into account, this prospective study was planned to compare socioeconomic status (SDS) and association of age, body mass index (BMI), education level and marital status between PCOS and healthy control cases among the women in the reproductive age group visiting the department of gynaecology and obstetrics of tertiary care hospital.
Material and methods
Ethical approval.
Ethical approval (SU/2017/1226-3) was obtained from the institutional review board of Santosh medical college, Uttar Pradesh, India. The institutional review board of All India Institute of Medical Sciences, Patna, India, granted study site approval (176/AIIMS/PAT/IEC/2017). Informed consent form was obtained from parents or guardians of the minors (<18 years).
Study design
This prospective, cross sectional, observational study was designed and conducted in the tertiary care teaching hospital of north India.
Study setting
Patients visiting the outpatients’ department of Gynecology and Obstetrics, All India Institute of Medical Sciences, Patna (India) were included in the study.
Participants
Patients diagnosed with PCOS, based on criteria derived from the 2003 ESHRE/ASRM (Rotterdam criteria) were arbitrarily enrolled in the study. PCOS is diagnosed as the presence of at least two of three of the following: 1) Oligo/anovulation, 2) hyperandrogenism, 3) Polycystic ovaries [ 18 ]. A healthy control (HC) was selected from participants of the same population and having regular menses and had no clinical features of hyperandrogenism as well as infertility.
Data sources
Data was collected after describing both written and verbal information about the study. After explaining, the informed consent form was signed by each participant and then they were requested to complete the questionnaires. Face-to-face interviews were conducted by investigators to the subjects meeting the inclusion criteria and consented for the participation into the study in three parts: Part A: Semi-structured, pre-validated questionnaires were used for collecting information on the socio-demographic, economic and reproductive history. Part B: Pre-validated SF-36 questionnaire is a standard diagnostic tool for evaluating various aspects of the HRQOL over the previous 4 weeks [ 19 ]. Its validity, sensitivity, reliability, internal consistency and stability, as well as test-retest reliability have frequently been confirmed in various studies [ 20 – 22 ]. SF-36 contains 8 domains: general health, physical functioning, and role limitations due to physical health, role limitation due to the emotional problem, body pain, social functioning, energy/fatigue and emotional well-being. The scores for each domain range from 0–100, where higher scores indicate better condition.
The sample size was estimated post assuming α-error of 0.05, power of 80%, percentage of controls having a poor quality of life to be 20% based on previous studies and odds of poor QOL among cases to be twice than among controls. Hence, a total of 100 PCOS cases and 200 healthy control cases were enrolled in the study.
Inclusion criteria
We included all diagnosed case of PCOS only, female from menarche to menopausal age between the age of 10–49 years, and those given informed consent.
Exclusion criteria
Patients having cognitive or developmental disabilities/another major illness that substantially influenced the HRQOL of women, confirmed malignancy and deformities, as well as breastfeeding women were excluded from the study.
Statistical analysis
Data were analyzed by using statistical software-Stata Version 14.0 (Stata Corp, Texas, USA). After checking for the normality condition for continuous variables, the appropriate statistical test was applied. Confounders like excessive body weight were taken into consideration. Quantitative data expressed as mean±SD, minimum and maximum followed normal and skewed distribution respectively. Analysis of covariance model (ANCOVA) was used to address potential confounders. Categorical variables expressed as frequency and percentage. Pearson Chi-Square test and Fisher exact test were used to checking the association between qualitative variables and categorical variables. Logistic regression analysis was used to estimate odds (95% CI) and models were robust for PCOS and other variables. Multivariable linear regression analysis was performed to observe the association between the variables. Independent t-test and One Way ANOVA used to compare normally distributed continuous variable between two and three categories respectively. Rank sum/Kruskal Wallis test used for comparing skewed continuous variables among categories and to look association between demographic categories. For all statistical tests, P-value < 0.05 is considered as statistical significant.
The outcome of socioeconomic status (SES) of a woman with PCOS and HC cases are mentioned in Table 1 . The women with PCOS and HC were comparable in respect of marital status and family type. Statistically significant differences were observed between PCOS and HC in terms of age (P<0.020), BMI (P<0.001), educational status (P<0.001), marital status (P>0.05) and work category (P<0.001). Total 97% of PCOS case was below the age of 30 years in comparison to 78% of control. Among all PCOS cases, 60% was student and almost 54% received higher education. Among the HC group, 39% was student and only 15% received higher education (P<0.001). A higher percentage of PCOS cases (16%) belong to greater BMI (>30) in comparison to HC (2%).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0247486.t001
As shown in Table 2 , among the PCOS group, a significant percentage of women (33%) has menarche at age greater than 14 years and there was no any HC cases lies in this category (P<0.001). In respect of menstrual history, PCOS cases have a higher percentage of irregular (45%) and delayed (54%) menses and this comprises a signify`cant difference (P<0.001) in comparison to HC that was 8% irregular and no any delay in menses were observed. Around 64.3% of cases of PCOS women have no child (P<0.001) in contrast to HC cases (9.5%). However, 86.67% PCOS cases have less than ≤ 2 children in comparison to HC where 69.47% have less than ≤2 children (P<0.169). In terms of pregnancy, almost 77.27% PCOS women got pregnant ≤2 times in comparison to HC cases (P>0.05).
https://doi.org/10.1371/journal.pone.0247486.t002
As depicted in Table 3 , Overall differences of mean in PCOS and HC case comparable in respect of BMI (P<0.125).
https://doi.org/10.1371/journal.pone.0247486.t003
However, the highest range of BMI was more in PCOS cases in comparison to HC. Whereas, a statistically, significant differences in mean were observed in respect of age (P<0.009), age at marriage (P<0.001), age of menarche (P<0.001), number of children (P<0.001) and number of pregnancy (P<0.006) between PCOS and HC cases. In case of age, women with PCOS at age ≤19 showed significantly higher score for of general health (P<0.001), role limitation due to physical health (P<0.001), role limitation due to the emotional problem (P<0.022), pain (P<0.025) and social function (P<0.010) in comparison to age >30. However, comparable differences were observed in physical function (P<0.116), energy or fatigue (P<0.087) and emotional well-being (P<0.108). In HC cases, women of age ≤19 showed a statistically higher score in general health (P<0.001), physical health (P<0.001), role limitation due to the emotional problem (P<0.005) and energy/fatigue (P<0.001). Comparable differences were observed for role limitation due to physical health (P<0.818), pain (P<0.424), social functioning (P<0.110) and emotional well-being (P<0.147; Table 4 ).
https://doi.org/10.1371/journal.pone.0247486.t004
As per BMI is concerned, PCOS women scored high and statistically significant differences were observed in case of BMI those who have value <18 in comparison to >30. In addition, significant differences were observed for general health score (P<0.001), physical health (P<0.001), energy and emotion (P<0.001). Whereas, comparable differences observed in role limitation due to physical health (P<0.085), role limitation due to the emotional problem (P<0.565), pain (P<0.189), social function (P<0.549) and emotional well-being (P<0.127). In HC case, there no significant difference was observed in all the eight domains of SF-36 ( Table 5 ). As per the level of education is concerned, HRQOL score was higher in all eight domain of SF-36 in well-educated women in comparison to illiterate or women having education of primary level, but this difference was observed to be statistically non-significant and comparable. In case of HC women, HRQOL score in graduation level was higher and significant differences were observed in relation to the level of education for SF-36 domains like general health (P<0.001), physical health (P<0.039) and energy/fatigue (P<0.003). However, we observed comparable differences among all other domains of SF-36 ( Table 6 ).
https://doi.org/10.1371/journal.pone.0247486.t005
https://doi.org/10.1371/journal.pone.0247486.t006
We observed significant differences between married and unmarried PCOS cases in terms of general health (P<0.001), physical functioning (P<0.027), role limitation due to physical health (P<0.006), role limitations due to emotional problems (P<0.002), pain (P<0.001), social functioning (P<0.001), energy/fatigue (P<0.003) and emotional well-being (P<0.001). Whereas, in HC cases, no differences were observed between married and unmarried cases in regarding SF-36 domain score for role limitation due to physical health (P<0.538), role limitation due to the emotional problem (P<0.105), Pain (P<0.044), social functioning (P<0.225), emotional well-being (P<0.857). However, significant differences were observed for general health (P<0.001), physical health (P<0.002) and energy/fatigue (P<0.001) among married and unmarried HC cases ( Table 7 ).
https://doi.org/10.1371/journal.pone.0247486.t007
The Fig 1 and Table 8 exhibits the regression analysis data plot and we was observed strong association between infertility and menstrual irregularities (P<0.049) as well as emotional well being (P<0.001) of PCOS patients. We also observed infertility (P<0.001) and hirsutism (P<0.05) as a major predictor affecting in all domain scores ( Table 9 ).
https://doi.org/10.1371/journal.pone.0247486.g001
https://doi.org/10.1371/journal.pone.0247486.t008
https://doi.org/10.1371/journal.pone.0247486.t009
PCOS has no any constant treatment due to its multifaceted features. However, lifestyle modification, hormonal contraceptives and some other drugs like inositol, clomiphene, eflornithine, finasteride, flutamide, letrozole, metformin, spironolactone has been reported to ameliorate the PCOS symptoms [Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. American family physician. 2016; 94(2): 106–113; Lagana AS, Garzon S, Casarin J, Franchi M, Ghezzi F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol Metab. 2018;29(11):768–780; Lagana AS, Garzon S, Unfer V. New clinical targets of d-chiro-inositol: rationale and potential applications. Expert Opin Drug Metab Toxicol. 2020;16(8):703–710].
PCOS is an endocrine disorder and its long term complications affect various aspects of HRQOL in women [ 23 , 24 ]. Despite the various evidence about compromised HRQOL in women with PCOS, we further explored the other determinants that may help the clinician in care of the patient well-being [ 17 , 25 , 26 ]. Overall, we demonstrated the drastically compromised HRQOL in young women suffering from PCOS. As earlier reported, the woman with PCOS belongs to a lesser age group in relation to HC indicating a higher prevalence of PCOS cases in young age woman especially in adolescents [ 27 ]. As per SDS is concerned, the major difference between PCOS and control cases was observed in case of age, BMI and level of education [ 28 ]. Thus, all this indicated that PCOS affects HRQOL more in the young woman and the SDS definitely affect the prevalence of PCOS. The age of menarche in the majority of women was > 18 years as earlier reported [ 29 ]. This was in contrast to other reports [ 30 ]. Consistently, increased age of menarche was observed in PCOS cases indicating the impact of first menstruation in young women life and in the development of PCOS and another reproductive as well as metabolic disorder [ 31 , 32 ].
As previously reported, we observed a direct correlation between the PCOS and irregular or delayed menses and having no children that can be taken as a symptom of PCOS diagnosis [ 33 ]. The higher number of women having lesser than two children and lesser number of times get pregnant further supported this compromised HRQOL [ 30 ]. This compromised HRQOL in PCOS case was further supported by our study.
The overall decreased mean of BMI and age at menarche indicated as PCOS symptoms. Concurrently, mean age, age at marriage, number of children and frequency of pregnancy was less in PCOS cases than control. Consistent to the previous study, these indices corroborate above findings and strongly indicated the deterioration of HRQOL in PCOS women [ 28 ]. Altogether, higher fertility disorder in PCOS cases was observed that directly affects their HRQOL due to physical, social as well as emotional issues.
Furthermore, in PCOS cases, physical, social and emotional well-being more affected as evidenced from all eight domains of SF-36 indicating strongly compromised HRQOL than HC cases [ 34 ]. In particular, we also compare the mean score in relation to age, BMI, educational level and marital status. Consistent with the previous report, increasing the age had a more negative impact on different domains of SF-36 in PCOS cases than HC cases. Comparable scores in PCOS women with increasing age for physical health, energy and emotional well-being may be due to improved regular menses with age concurrent to improved PCOS features and loss of societal fear. Whereas, in HC cases, changes in normal life trend in the prospect of HRQOL was seen with increasing age indicating normal HRQOL [ 35 ].
In a similar fashion, with the increase in the BMI, physical activity was not affected in PCOS cases as observed from different domains of SF-36 but the emotional problem was more affected in PCOS cases in comparison to HC. This may be a major reason for compromised HRQOL in PCOS women [ 36 , 37 ]. In HC cases, none of the scores of SF-36 domains was different between BMI groups. Consistent to previous studies, with increasing the level of education, all the domains of SF-36 in PCOS cases have improved HRQOL. Similar to other HRQOL studies in different diseases, where well-qualified patients have better HRQOL than illiterate cases [ 38 ]. We also observed that well-qualified group probably have higher number of PCOS cases that directly support that improved SDS is a major contributing factor in developing PCOS. The differences in scores of all the domains of SF-36 were observed in married PCOS cases in contrast to unmarried. Thus, consistent to the previous report, the HRQOL of the unmarried cases was better in comparison to married women [ 39 ]. Whereas, in HC cases, all physical, social, as well as emotional wellness, were similar in both married and unmarried women. This may be due to their social independency and quality of education in young women with PCOS.
As reported earlier, infertility and hirsutism emerges as the major problem affecting the overall HRQOL and a strong association has been observed between infertility and emotional well-being [ 40 ].
Our data compares the relations between PCOS and HC cases of overall HRQOL. We explored the strong association between PCOS and SES, and suggest that with increasing age and BMI PCOS patients had lower scores on SF-36; opposite association was with education level. However, Infertility emerges as the major predictor affecting overall HRQOL in PCOS cases. The present study does have its limitations of not measuring biochemical assessment and ultrasonography indices.
Acknowledgments
The authors are thankful to Dean of Scientific Research, King Khalid University and the College of Pharmacy, Department of Clinical Pharmacy for providing facilities to carry out our research work. I also want to acknowledge Dr. R. M. Pandey, Professor & Head, Department of Biostatistics, All India Institute of Medical Sciences, Delhi (India) for supporting me to analyse and interpret my data.
- View Article
- Google Scholar
- PubMed/NCBI
- 3. Centers for disease control and prevention, 2019. Accessed on 06/11/2019. https://www.cdc.gov/diabetes/library/spotlights/pcos.html .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Womens Health (Lond)
An update on polycystic ovary syndrome: A review of the current state of knowledge in diagnosis, genetic etiology, and emerging treatment options
1 Biotechnology Program, Department of Mathematics and Natural Sciences, School of Data and Sciences, Brac University, Dhaka, Bangladesh
Jaasia Masud
Yushe nazrul islam, fahim kabir monjurul haque.
2 Microbiology Program, Department of Mathematics and Natural Sciences, School of Data and Sciences, Brac University, Dhaka, Bangladesh
Polycystic ovary syndrome is the most common endocrine disorder in women of reproductive age, which is still incurable. However, the symptoms can be successfully managed with proper medication and lifestyle interventions. Despite its prevalence, little is known about its etiology. In this review article, the up-to-date diagnostic features and parameters recommended on the grounds of evidence-based data and different guidelines are explored. The ambiguity and insufficiency of data when diagnosing adolescent women have been put under special focus. We look at some of the most recent research done to establish relationships between different gene polymorphisms with polycystic ovary syndrome in various populations along with the underestimated impact of environmental factors like endocrine-disrupting chemicals on the reproductive health of these women. Furthermore, the article concludes with existing treatments options and the scopes for advancement in the near future. Various therapies have been considered as potential treatment through multiple randomized controlled studies, and clinical trials conducted over the years are described in this article. Standard therapies ranging from metformin to newly found alternatives based on vitamin D and gut microbiota could shine some light and guidance toward a permanent cure for this female reproductive health issue in the future.
Introduction
Polycystic ovary syndrome (PCOS) is a widely prevalent metabolic and endocrine disorder diagnosed in reproductive-aged females. The disease is distinguished by the presence and degree of three major features: irregular menstruation, hyperandrogenism, and polycystic ovarian morphology (PCOM). 1 The prevalence of PCOS is known to be around 5%–20%, depending on the varying definitions used. 2 Despite many advances and adaptations in developing the diagnostic criteria and interpreting the condition’s pathophysiology, PCOS remains a less-understood disorder in terms of criteria for uniform diagnosis and treatment. 3 The multifaceted effects of the disease are spread across a woman’s lifetime beginning from conception and extending across the years following menopause. 4 A majority of the studies related to PCOS were performed to develop a timely and efficient diagnosis, particularly for adolescents, effective treatment, and management of comorbidities associated with PCOS that gravely impact the quality of life, and a homogeneous protocol that can be implemented by healthcare officials. 5 In this review, the diagnostic procedures and other screening protocols mentioned were centered around the most recent international evidence-based guidelines for PCOS. Furthermore, the article went into detail about the diagnosis of PCOS in adults together with the challenges faced in diagnosing adolescent girls. We have found a lack of age-specific guidelines that is a consequence of insufficient scientific investigations. In addition, the causal links, both genetic and environmental, have been summarized with a brief insight into the pathogenesis of PCOS. Finally, the current state of the treatment is looked at, and the new options with considerable potential have been discussed.
The incentive to work with PCOS came from the understanding that a certain percentage of women are still being misdiagnosed or left undiagnosed due to unawareness and misunderstanding. While working on this article, it was made clear that many countries, especially Bangladesh, are yet to take PCOS seriously. This was demonstrated through the lack of research in these geographical regions. Thus, we believe that putting forth the actual situation about the condition would help narrow the chasm in recognition and pave the way for better answers.
Diagnosis of PCOS
Introduction to different diagnostic criteria.
PCOS is a recurrent endocrinopathy prevalent in approximately 8%–13% (varying across different populations) of women in the reproductive age bracket. 6 – 9 Despite its frequency, guidance regarding implementing the diagnostic procedures for detecting PCOS is relatively obscure and inconsistent among health professionals. As a result, up to 70% of these women are known to remain undiagnosed. 8 It further adds to the problem of unestablished etiology or origin of PCOS. However, over the past decades, the characteristic traits observed in women with PCOS have been analyzed, and eventually resulted in the development of three diagnostic criteria based on the hallmarks of PCOS.
The three distinctive features of this endocrinologic condition include ovulatory dysfunction, hyperandrogenism, and PCOM in accordance with the consensus-based international guidelines. 10 – 12 In addition, PCOS usually manifests in the form of hirsutism, oligo-anovulation, amenorrhea or irregular menstrual cycle, and infertility. 13 Because of the heterogenic characteristics, three classifications of diagnostic criteria were established over time. The criteria are listed as follows:
- 1. National Institute of Health (NIH) criteria . It was established in 1990 based on the agreement of a veteran panel and the first kind of diagnostic criteria to be ever generated. The NIH criteria authorized two definite features to be representative of PCOS: (a) signs of hyperandrogenism (clinical or biochemical) and (b) oligo-anovulation or oligomenorrhea. 10 This criterion was later revised in 2012, where the third determinant, PCOM, was integrated to conform to the Rotterdam criteria. 14
- • Phenotype A—Hyperandrogenism + Ovulatory Dysfunction + PCOM
- • Phenotype B—Hyperandrogenism + Ovulatory Dysfunction
- • Phenotype C—Hyperandrogenism + PCOM
- • Phenotype D—Ovulatory Dysfunction + PCOM
The Rotterdam criteria are widely used by gynecologists, obstetricians, and other healthcare personnel; it was also adopted by the 2018 International PCOS guideline and other guidelines. 15 , 16 Furthermore, the criteria were also suggested by the NIH evidence-based methodology PCOS workshop held in 2012, alongside phenotype identification in all researches. 14
- 3. AE-PCOS Criteria . It was put forward in 2006 by the Androgen Excess and PCOS Society. The third criteria suggested the presence of hyperandrogenism along with any one or two of the remaining determinants (ovulatory dysfunction and/or PCOM) for PCOS diagnosis. 6
In short, the three diagnostic criteria present the identification and quantification of the classical features of PCOS (hyperandrogenism, ovulatory dysfunction, and PCOM) for a definitive diagnosis of the condition. However, it is worth noting that diagnosis through any one of the above-mentioned three criteria will only be conclusive of the condition provided that other endocrine disorders such as hyperprolactinemia, thyroid disease, Non-classical Congenital Adrenal Hyperplasia (NCAH), Cushing’s syndrome/disease, hypogonadotropic hypogonadism or androgen producing tumors which exhibit similar manifestations (clinical/biochemical/morphological) as that of PCOS are ruled out. 14 , 15 For instance, NCAH that manifests in hirsutism or irregular menstruation can be tested by measuring 17-hydroxyprogesterone (17 OHP) levels with an additional ACTH stimulation test in borderline cases. 17 Similarly, hyperprolactinemia can be detected if a prolactin level exceeding the threshold of 500 μg/L is found, exhibiting galactorrhea symptoms and irregular periods. 18 In addition, thyroid diseases can be ruled out by calculating the levels of the thyroid-stimulating hormone (TSH). 14 On the other hand, Cushing’s syndrome is a relatively severe condition that displays obesity, high blood pressure, and amenorrhea features. In addition, it is associated with over secretion of cortisol. Thus, an overnight dexamethasone suppression test or midnight salivary cortisol test will assist in distinguishing this condition from PCOS. 19
Diagnostic features in adults
Data from a recent meta-analysis and systematic review revealed a clear picture of the overall prevalence of PCOS based on the three available diagnostic features where ovulatory dysfunction, hirsutism, biochemical hyperandrogenism, and PCOM were found to be in 12%, 13%, 11%, and 28% of women, respectively. 9 The major diagnostic features observed in women with PCOS in a spectrum of degrees are discussed in the succeeding parts of the article.
Ovulatory dysfunction
A staggering proportion of approximately 75% of PCOS individuals is known to have ovulatory dysfunction. 20 It is described as a state of irregular menstrual cycle. 14 In a standard ovulation cycle, menstruation begins by the 24th/25th day. 21 In an adult, irregular menstruation may be indicated by a cycle consisting of <21 or >35 days, or less than eight menstrual cycles per year in a few cases where the gynecologic age is relatively higher. 16 , 22 Continued irregular menstruation indicates anovulation or oligo-anovulation, which can later aggravate a PCOS condition. 14
Contrastingly, regular menstruation reflecting normal ovulatory cycles has also been noticed in women with PCOS. 23 The phenomenon is known as subclinical ovulatory dysfunction and may be explained by hyperandrogenism. This poses a challenge in the diagnosis of PCOS. 24 On the other hand, serum progesterone level above 5 ng/mL (during days 21–24 of the cycle or the luteal phase of the menstrual cycle) may function as a confirmatory test for ovulation in women with PCOM or hirsutism. 25
Hyperandrogenism
Excessive serum androgen level is another salient feature of PCOS, as stated by the Rotterdam criteria. A significant proportion (about 60%–100%) of PCOS-afflicted women are likely to be suffering from hyperandrogenism (clinical and/or biochemical). 15 Hyperandrogenism in PCOS women may be assessed by their clinical signs or biochemical tests.
Clinical hyperandrogenism
Clinical hyperandrogenism observed in the form of hirsutism, acne, or alopecia usually represents low-to-average levels of androgen excess: 26
- Hirsutism appears more frequently than the remaining two in about 80% of people with hyperandrogenism. It refers to the visually detectable growth of terminal hair (hair that can grow beyond 5 mm, if untroubled). 25 The extent of hirsutism is evaluated via the modified Ferriman–Gallwey (mFG) score. 27 This involves visual evaluation of the body’s nine areas (chin, chest, upper lips, upper arms, thighs, upper and lower abdomen, and back) with a score ranging from 0 to 4. 28 , 29 The scores are then presented in the form of a graphical image. The state of hirsutism is conclusive if the total mFG score is ranged within ⩾4–6; this range was extended to a score of eight to adjust for variation in ethnicities. 30 , 31
- Comedonal acne is a widespread issue among women, especially adolescent girls. It was estimated that around 40% of the acne incidence could be traced back to a susceptible PCOS condition. 32 Although acne can be linked with biochemical hyperandrogenism, there is no specialized measuring tool to assess this condition as of now. 26
- Alopecia is the least common feature among the three. Only 22% of women displaying male-like hair loss were discovered during PCOS diagnosis. 32 Many factors other than hyperandrogenemia may be associated with alopecia. The Ludwig scale is a measurement tool for hair loss around the scalp that rates the degree of hair loss from grades I to III upon visual assessment. 1
The subjective variability, racial/ethnic differences, and the existence of a condition named idiopathic hirsutism (hirsutism devoid of hyperandrogenism) play a significant role in determining the clinical signs of PCOS diagnosis and thus require more well-defined data. 33
Biochemical hyperandrogenism
When the clinical signs of hyperandrogenism are obscure, women are assessed for signs of biochemical hyperandrogenism. 12 This diagnostic criterion relies on one of the characteristic traits of PCOS involving elevated serum androgen levels. About 60%–80% of women with PCOS are known to exhibit features of biochemical hyperandrogenism. 6 According to the evidence-based recommendation, the condition can be detected through measurement of total testosterone (TT), free androgen index (FAI), calculated free testosterone (fT), and/or calculated bioavailable testosterone. 12 Previous data suggest that serum-free testosterone is the most sensitive parameter for detecting biochemical hyperandrogenism compared to the other parameters such as total testosterone, dehydroepiandrosterone sulfate (DHEAS), or androstenedione (A4). The latter two have been observed to be elevated in women with PCOS and are particularly useful when a high testosterone level is not detected. Furthermore, DHEAS and A4 facilitate the exclusion of other hyperandrogenic conditions. 15 , 26 Then again, FAI is another indirect means of evaluating the free testosterone level; it is the ratio of the total testosterone and Sex Hormone Binding Globulin (SHBG) multiplied by 100. 34
Total circulating or free testosterone levels can be measured using high-quality assays like extraction/chromatography immunoassays, liquid chromatography-mass spectrometry (LCMS) or mass spectrometry. Other automated direct assays include enzyme-linked immune sorbent assay (ELISA), chemiluminescence assay (CLIA), and radioimmunoassay (RIA). However, these assays exhibit reduced sensitivity and therefore provide imprecise results. 35 – 37 Furthermore, cut-off values vary widely within laboratories and with the method used. Normal thresholds may be derived from a healthy population of women. 12 To conclude, the lack of cut-off values based on evidence, the choice of androgen to test, and the consensus regarding the use of assessment techniques form the major areas of uncertainty in the evaluation of biochemical hyperandrogenism.
PCOM is recognized as the most widely used feature in the diagnosis of PCOS. It was first introduced in the Rotterdam criteria as the third feature of PCOS in 2003. 11 However, this diagnostic criterion is debatable due to the lack of homogeneity in the results regarding its implementation, and its non-applicability for females above the gynecological age of 8 years. Therefore, it has been deemed a non-essential feature in the presence of the remaining two criteria. 15
The ovulation process is ceased due to follicular arrest in adult women with PCOS. The minute follicles resemble cyst-like structures on transvaginal ultrasonography. 38 – 42 Initially, as authorized in the Rotterdam criteria, the cut-off value for identifying a polycystic morphology was a value of 12 or more Follicle Number Per Ovary (FNPO), measuring between the size of 2–9 mm or, an ovary with a volume of 10 cm 3 on a transvaginal scan. 26 The FNPO value, a key parameter, was later updated in conformity with the technological advancements that enabled more magnified imaging. When using a high-resolution transducer frequency of 8 MHz or more, FNPO value of 20 or more of the same-sized follicles (2–9 mm) and an ovary volume of 10 cm 3 for adult women was recommended by an international evidence-based PCOS guideline consensus held in 2018. 15 The ovarian volume plays a significant role when it is challenging to determine FNPO/Antral Follicle Count (AFC) due to technical complications in imaging, as it is in the case of transabdominal ultrasound. 26
Transvaginal ultrasound is the recommended approach for examining FNPO suggestive of a polycystic ovary. However, it is to be used only in sexually active women. 12 Automatic antral follicle count under 3D scan has shown more accurate results than the 2D grid system method; the information regarding this is still sparse and so is not yet recommended for routine purposes. 43 – 49 Other ultrasound metrics include ovarian stroma and ovarian blood flow; studies concerning these parameters are minimal and hence no cut-off values are available for clinical use. 12
Anti-Mullerian hormone as an alternative diagnostic feature for PCOS
Considering the ambiguity about the efficacy of ultrasound as a diagnostic tool Anti-Mullerian Hormone (AMH) has been suggested as an alternative for detecting PCOM. 15 An upward trend of AMH levels in correlation with the number of antral follicles in PCOS was observed in women with PCOS. It is because AMH is produced by the granulosa cells in ovarian follicles (namely pre-antral and antral), which are elevated in PCOS. 50 – 52 Despite its potential as a valuable diagnostic tool, AMH is still not authorized as a part of routine PCOS diagnosis owing to the inadequate standardization of cut-off values and heterogeneity among the studies 12 , 53 .
Diagnostic challenges in adolescents
Much of the already existing gray areas in PCOS diagnosis for females of all ages can be attributed to its unspecified etiology heterogeneity, the lack of evidence-based cut-off values for the diagnostic features, and the unavailability of clearly defined, universal technology for the most accurate results. 54 Furthermore, a distinct set of diagnostic criteria for adolescents is essential since the existing guidelines mostly comply with features relevant to adults (cystic acne, irregular menstruation, and PCOM). Applying these guidelines for adolescents may lead to over- or under-diagnosis since the manifestation of these features in young girls is also a result of normal pubertal development stemming from an underdeveloped hypothalamic–pituitary–ovarian (HPO) axis. 55 At the outset, the adolescence period has been defined to be the age frame within 10 and 19 years by the World Health Organization. Alternatively, young women within the gynecological age of 8 years were also considered for PCOS studies aimed toward adolescents. 56
A conclusive PCOS condition cannot be diagnosed without the concurrent existence of both menstrual irregularity and hyperandrogenism in PCOS. It is also essential to acknowledge a state of “at-risk” category to be followed up by further age-specific diagnosis for PCOS as a means to avoid over or under-diagnosis of young women. 56 , 57
Problems with defining ovulatory dysfunction in adolescence
Diagnosis of PCOS has been recommended to women with persistent irregular menstruation; however, defining irregular menstruation as reflective of ovulatory dysfunction in adolescents is much of a challenge in itself. 58 Definition of irregular menstruation following the gynecological age is tabulated in Figure 1 . 56

Definitions of irregular menstruation according to gynecologic age.
Irregular menstruation is common in young adults after menarche, which is common in the first 2 years, but it can extend as long as 5 years post-menarche. Therefore, the presence of this physiological event cannot be considered a prerequisite for PCOS diagnosis until 2 years succeeding menarche. 58 – 61 However, continued oligomenorrhea even after the 2-year threshold following menarche indicates an “at-risk” status of PCOS in young adults. 55
Determining concurrent anovulation in adolescents is yet another challenge since about 85% of the cycles are known to be anovulatory in the first year following menarche, which shows a downward trend with the number of years post-menarche being 59% and 25% in the second and third year, respectively. 60 In addition, anovulation can be confirmed by serum progesterone level measurement as like in adults. 14 Finally, the variation in age of menarche among females further complicates the assessment and identification of ovulatory dysfunction. 62
Adolescent physiological aspects mimicking hyperandrogenic conditions
As mentioned previously, besides persistent irregular menstruation, androgen excess is a valuable indication of PCOS in adolescents, which may present itself as visible clinical signs (hirsutism, severe acne, and/or rarely alopecia) or elevated serum androgen level.
Acne is a common condition in adolescents and therefore not a definitive diagnostic criterion for PCOS unless accompanied by other features. However, this may indicate hyperandrogenism if the degree of acne ranges from moderate to severe and is not responsive toward topical dermatologic therapy. 58 , 63
Alopecia in adolescents is still not well understood
Although hirsutism has been linked with hyperandrogenism when in association with menstrual irregularity, 64 the presence of various confounding factors such as genetic and ethnic variations deem it to be a less prominent feature in diagnosing a hyperandrogenic status. 29 , 65 , 66 Moreover, the modified Ferriman–Gallwey scoring system may not accurately assess the degree of hirsutism in adolescents since it was structured using data from a population of adult, particularly Caucasian women, which may not apply to women from other ethnicities. 67 – 69
Assessment of biochemical hyperandrogenism has its own set of complications due to lack of standardization, technical difficulties, interference with other steroid hormones, and effect of SHBG on testosterone level, all of which are irrespective of a woman’s age. Despite the physiological impact of puberty leading to a rise in testosterone levels, the same determinants (free and/or total testosterone) are used to gauge androgen excess in adolescents. These methods are limited by the lack of specifically designed studies for adolescents and well-adjusted thresholds.
Controversies regarding PCOM in adolescents
According to the International guidelines on PCOM, transabdominal ultrasound is not recommended for use as a diagnostic criterion until at least 8 years post-menarche, mainly due to the high prevalence of characteristic follicular increase in young adults and an enlarged ovarian volume during this period. 12 , 70 – 73 In addition, the implementation of adult thresholds adjusted for the transvaginal route may lead to over-diagnosis of PCOM in adolescents; hence, PCOM in adolescents is not a reliable diagnosis of PCOS. 74
Risks associated with PCOS
PCOS has been associated with the potential risk of cardiovascular and cerebrovascular events, type 2 diabetes mellitus (T2D), impaired glucose tolerance (IGT), pregnancy-linked complications, gestational diabetes, venous thromboembolism, and endometrial cancer. 1 Many of these metabolic and reproductive conditions stem out from an intrinsic feature of PCOS-insulin resistance (IR). 75 – 77
Cardiometabolic events
Cardiometabolic impacts of PCOS described below are primarily linked with dysglycemia resulting from the insulin-resistant characteristic of PCOS. Insulin resistance or consequential hyperinsulinemia in turn is a state heavily influenced by the hyperandrogenic mechanism of the PCOS pathophysiology. 77 Despite the paucity of comparative studies concerning cardiovascular disease (CVD) risk factors with and without PCOS, it is noteworthy that the cardiometabolic conditions are prominent in PCOS, which pose a risk of developing CVDs. According to clinical consensus recommendation by the PCOS guideline group (2018), the manifestation of the cardiometabolic risk factors such as impaired glucose tolerance (IGT), dyslipidemia, hypertension, smoking, obesity, other metabolic syndromes, and a sedentary lifestyle in PCOS women allocates them in the vulnerable category. 15
According to past meta-analyses, the prevalence of IGT and T2D in PCOS-affected women has been observed to be independent of, but made worse with body mass index (BMI). It can be traced back to the correlation of PCOS with dysglycemia, which refers to the aberrant blood glucose level. Thus, estimation of the glycemic status of women with PCOS has been recommended by the International Evidence-based Guideline (2018) at a frequency of 1–3 years depending on the presence of other diabetic confounding factors. In addition to that, screening for T2D has been suggested by all consensus recommendations (Endocrine Society, International Evidence-based Guideline in Australia, as well as Androgen Excess and PCOS society). 61 , 78 However, the screening method is still undecided among Oral Glucose Tolerance Test (OGTT), fasting glucose, and HbA1c test. 15
Dyslipidemia
Dyslipidemia is a recurring CVD risk factor identified in women with PCOS. A significant proportion of women (70%) with PCOS were known to exhibit dyslipidemia in a past report. 79 Furthermore, a meta-analysis of 30 studies demonstrated higher levels of lipids in women with PCOS (age < 45 years), particularly high-density lipoprotein (HDL), low-density lipoprotein (LDL), non-HDL-C, LDL-C, and triglyceride (TG). Moreover, TG and HDL-C were substantially higher in the obese stratum indicating a possible link of PCOS with obesity. 80 , 81 Therefore, the latest guidelines suggest women of all ages are diagnosed to undertake a lipid profile. 12
Hypertension
The association between hypertension and PCOS is somewhat complex and influenced by many other factors. The inconsistency between the studies necessitates the requirement for more investigation. 67 , 82 – 84 However, the recent international evidence-based guideline recommends annual blood pressure measurement considering the significance of hypertension in cardiovascular events. 12
Despite its frequency in women with PCOS, there is surprisingly no solid evidence of their causal relationship. However, obesity has been linked to some of the severe PCOS manifestations, including CVD. 85 – 87 Therefore, regular monitoring of body weight has been suggested by the most recent guideline. 12
Other risks
Sympathetic nervous system dysfunction, chronic inflammation, oxidative stress, and vitamin D deficiency are emerging risk factors of PCOS, paving the path for further research. 77
The combined effect of a low number of studies and the relatively young population of women restricts the development of a concrete relationship between PCOS and CVD and therefore calling for more research. Nonetheless, the importance of screening for CVDs in PCOS women has been acknowledged. Quantitative research on the clinical manifestations of CVD is insufficient despite researches based on the sub-clinical CVD. 15 , 77
Fertility-related complications in women with PCOS
PCOS comes with lifelong repercussions for women, as stated earlier. Gestational complications associated with PCOS are gradually being recognized, some of which include preeclampsia, gestational diabetes, pregnancy-induced hypertension, and even miscarriage. 88 It is estimated that much of the pregnancy-related inconveniences are partly ramifications of the already existing metabolic and endocrine effects of PCOS in women well ahead of pregnancy, such as hyperandrogenism and increased BMI. 89 Manifestation of gestational diabetes mellitus (GDM) in expecting women with relatively high weight was observed to be more alarming than their counterparts of lower weight. However, it is worth noting some of these complications are also influenced by other independent factors such as age, obesity, ongoing fertility treatment, and ethnicity. 88 Apart from this, several studies have also reported atypical newborn anthropometrics in children of women with PCOS. 90 – 92
Although screening for GDM and hypertension in women with PCOS (pre-conception and antenatal) has been mentioned in the recommended guidelines, other avenues of pre-conception and antenatal screening for PCOS are still underdeveloped as they are not supported by enough evidence. 15 , 88
Endometrial cancer
Malignancies associated with PCOS are rather indirect and stem from PCOS-induced infertility in women. Of these, endometrial cancer has been acknowledged to have an association with PCOS. 93 Although its occurrence is multifactorial and influenced by other morbidities (T2D, obesity, infertility, and the administered PCOS treatment methods), it has been shown to rise by 2–6 times in women with PCOS. This may be attributed to the anovulatory cycles where the endometrium is exposed to a continuous flux of estrogen. 94 – 96 Despite the correlation between the two, routine screening for endometrial cancer has not yet been recommended. However, awareness on this issue is encouraged. 15
OSA is a chronic sleeping disorder accompanied by disruptive upper airway function and consequential hypoxia and erratic sleeping pattern. 97 It has also been linked with modified heart rate, sympathetic activity, and altered blood pressure, ultimately extending toward more severe outcomes such as CVD and hypertension. 98 – 100 A positive correlation between women with PCOS and OSA was demonstrated through several systematic reviews and meta-analyses. 101 The evaluation of this condition is based on qualitative analysis and a screening tool involving the Berlin Questionnaire. As of present, treatment for OSA only involves the management of distinctive patient symptoms without therapeutic remedy of the linked metabolic diseases. 12
Etiology of PCOS
The current literature emphasizes the role of genetics in PCOS. Many genes have been said to directly or indirectly contribute to the progression of the disease. But to date, no penetrant gene has been identified. 102 Studies conducted in multiple families show low penetrance linked with hormonal/environmental factors or other co-variants. Many studies have suggested that PCOS is a polygenic, multifactorial disorder. Single genes, gene–gene interactions, and gene–environment interactions have been reported to pave the way for the development of the disease. 102 This part of the article will review the current genetic understanding of the disease and some of the environmental determinants explored later in the article.
Understanding the roles of these genes better helps to look at some aspects of PCOS pathogenesis, the ovaries, and hormonal metabolism. The ovaries are the primary reproductive organ that releases eggs meant to be fertilized by sperms. They also produce estrogen and progesterone, which help regulate the monthly menstrual cycle, and tiny amounts of the male hormone testosterone, one of the five kinds of androgen. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are gonadotropin hormones released by the pituitary gland in response to gonadotropin-releasing hormone (GnRH) secretion by the hypothalamus. These two hormones control ovulation; FSH primarily stimulates the growth of follicles into proper eggs while LH triggers the release of these eggs. Their hormonal interplay in the body is illustrated in Figure 2 . PCOS is a syndrome (or a group of symptoms) that interferes with ovaries and ovulation, in brief. PCOS predominantly has three features: irregular/missed periods, high androgen levels, and cysts, which are fluid-filled sacs in the ovaries. These sacs are essentially immature follicles that never see ovulation. Thus, the lack of ovulation disturbs the hormonal harmony in the body. On top of this, raised androgen levels disrupt the monthly cycles. The underlying justification behind upsetting hormonal balance has been pointed toward genetic alterations, environmental determinants, and epistatic changes.

The hormonal cycle in the female body illustrated with the positive and negative feedback mechanisms. The diagram on the left shows a state prior to ovulation and the right after ovulation.
Biosynthesis of hormones in the ovary in brief
In a secondary follicle, thecal and granulosa cells work in conjunction to produce estrogen, progesterone, and testosterone. The process has been outlined in Figure 3 . There are five types of androgens: dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), androstenedione, and testosterone. LH and FSH secreted by the pituitary gland activate these cells. The thecal cells express LH receptors for LH to bind. Granulosa cells, on the other hand, bind with FSH. When activated by LH, thecal cells increase their absorption of LDLs from the bloodstream. The cholesterol is then used in the synthesis of steroids, like progesterone. Progesterone is then enzymatically converted in a series of steps into androgens. Due to the lack of aromatase enzymes, thecal cells are unable to produce estrogen independently. Thus, the androgens diffuse into the blood and granulosa cells, where it successfully gets converted into estrogen via aromatase. This estrogen later enters the blood. The hypothalamus is stimulated in a positive feedback mechanism; consequently, the characteristic LH surge in the menstrual cycle is seen.

The biosynthesis of androgen and estrogen inside the ovary.
The granulosa cells also have LH receptors but cannot pick up LDLs from the blood; LDLs cannot easily move past the basal membrane. When the follicle ruptures during ovulation, the membrane is destroyed enabling LDL absorption and progesterone production. However, the cells do lack the enzymes needed to convert progesterone into androgens. Thus, the majority of the progesterone diffuses into the blood, which explains the rapid rise in its level post-ovulation. After ovulation, both cells produce progesterone and, to a lesser extent, androgens.
Cholesterol is the precursor of all steroid hormones classified into three categories: glucocorticoids, mineralocorticoids, and gonadocorticoids (or sex hormones). Sex steroids are mainly androgens, estrogens, and progesterone. All steroid hormones are hydrophobic and require a protein carrier when transported in the blood. These are albumin, corticosteroid-binding globulin, and sex hormone–binding globulin. Cholesterol is a 27-carbon compound that undergoes a multi-step process and gets shortened and hydroxylated eventually. The series of conversions is shown briefly in Figure 4 . These enzymes (e.g. cytochrome p450 members) are involved here that are targeted by studies; polymorphisms in their coding region can ultimately affect hormonal metabolism and lead to hyperandrogenism. The idea is that some defect in the hormonal pathway causes the classic characteristics of PCOS; these avenues are probable areas for research. Numerous studies on the relationship between gene polymorphisms and PCOS have been conducted. Some of the significant ones have been discussed later in the review.

Summary of steroidogenesis with the end-products shown.
While ovaries are generally considered the primary source of androgens, the adrenal glands also contribute. Increased adrenal androgens (DHEA and DHEAS) are consistent with 20%–30% of the PCOS population 103 —a phenomenon called adrenal hyperandrogenism .
Hormonal association in PCOS
Hormones play a crucial role in the normal functioning of the ovary and the regulation of menstruation. If hormonal disturbances persist, the ovary’s function is interrupted, leading to the formation of a cyst inside of its sac. 104 PCOS patients exhibit an imbalance in levels of GnRH, FSH, LH, androgen, and prolactin. 105 The progression of PCOS and its severity rises with an increasing level of insulin and testosterone. Hyperinsulinemia is known to affect ovarian theca cells inflating androgen concentration. 106 Then again, elevated androgen levels trigger visceral adipose tissue (VAT), that is, responsible for the production of free fatty acids (FFA), which in turn elicits insulin resistance. 107 Due to insulin resistance and its consequent outcome of elevated insulin levels, androgen levels rise, which leads to anovulation. 7
To support the hormonal association with PCOS, a cross-sectional study in Pakistan examined healthy and affected women. Blood samples were drawn from individuals, and hormonal analysis was performed using immunoradiometric assay and radioimmunoassay. Their findings stated that FSH, LH, prolactin levels, and BMI were higher in PCOS cases. The current diagnosis of PCOS involves looking at FSH, LH, and androgen levels. 108 We know that raised LH levels result in higher androgen levels, which gives rise to PCOS, among other reasons.
The genetic connection
There are several genes involved in the etiology of PCOS. At present, there are three databases manually curated and published: PCOSKB R2 (2020), PCOSBase (2017), and PCOSDB (2016). 109 – 111 PCOSDB had been inaccessible at the time of writing. The three databases are compared in Table 1 .
A brief comparison of the three PCOS databases published so far.
| Attributes | PCOSKB | PCOSBase | PCOSDB |
|---|---|---|---|
| 1. Official name | PolyCystic Ovary Syndrome KnowledgeBase | PolyCystic Ovary Syndrome Base | PolyCystic Ovary Syndrome Database |
| 2. Database URL | |||
| 3. Overall content | 533 genes, 145 SNPs, 29 miRNAs, 1150 pathways, and 1237 PCOS-associated diseases Also included are more 4023 genes identified from microarray expression studies on PCOS | 8185 PCOS-related proteins, 7936 domains, 1004 pathways, 1928 PCOS-associated diseases, 29 disease classes, and 91 tissues | 208 genes, 427 molecular alterations including detailed annotations, 46 associated phenotypes |
PCOS: polycystic ovary syndrome; SNP: single nucleotide polymorphism.
As per PCOSKB R2 , 241 genes and 114 SNPs are closely involved in PCOS. 109 Changes like polymorphisms negatively affect the transcriptional activity of the gene, ultimately leading to PCOS. Now, the genes suitable for PCOS study are those that code for receptors of hormones like androgen, LH, FSH, and even, leptin. 112 Genes, namely, AR, CAPN10, FTO, follicle-stimulating hormone receptor (FSHR), cytochrome family P450, and insulin genes, have been widely discussed.
This review has discussed some genes commonly involved in ovarian and adrenal steroidogenesis, gonadotropin action and regulation, insulin action and secretion, and a few notables. Table 2 details the research on these groups of genes.
A rundown on the associations of different polymorphisms with PCOS found by most recent studies.
| SL. | Category | Gene | Year | Sample Size and ethnicity/location | Findings/Conclusion | Key methods | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Involved in ovarian and adrenal steroidogenesis | CYP11A | 2014 | 267 cases versus 275 controls “South Indian” | Fifteen different alleles were identified with repeats ranging from 2 to 16. Repeats greater than 8 were thrice more likely to be susceptible to PCOS and have been found comparatively more in the patients. CYP11A1 repeat polymorphism is likely to be a potential molecular marker for PCOS diagnosis. | DNA extracted from blood and genotyped by PCR-PAGE | Reddy et al. | |
| 2 | 2014 | 1236 cases versus 1306 controls “Asian and Caucasian” Argentina, China, Greece, India, Spain, United Kingdom | Positive association between CYP11A1 repeat polymorphism and PCOS. | Meta-analysis of nine studies published between 2000 and 2010 | Yu et al. | |||
| 3 | 2014 | 1571 cases versus 1918 controls “Asian and Caucasian” China, India, Greece, Spain, Turkey, United Kingdom, United States | CYP11A1 microsatellite repeat polymorphism (along with CYP1A1 MspI) showed significant association with PCOS risk in the Caucasian population. | Meta-analysis of 13 studies published between 2000 and 2010 | Shen et al. | |||
| 4 | 2012 | 314 cases versus 314 controls “Chinese” | SNP rs4077582 in CYP11A1 was found to be strongly associated with PCOS susceptibility. No association was observed in rs11632698. | PCR-RFLP | Assessed the association of SNPs rs4077582 and rs11632698 in CYP11A1 with PCOS | Zhang et al. | ||
| 5 | CYP21 | 2013 | 197 patients | The study looked into 14 molecular defects of the CYP21A2 gene and concluded that its contribution to PCOS is unsubstantiated. | Allele-specific PCR | The study investigated the contribution of CYP21A2 heterozygous mutations to PCOS pathogenesis | Settas et al. | |
| 6 | 2010 | 50 cases versus 60 controls “Italian” | The data suggested a lack of association between CYP21 V281L polymorphism and PCOS. | PCR-RFLP | Pucci et al. | |||
| 7 | 2005 | 114 cases versus 95 controls “Non-Hispanic White” | CYP21 mutations were found to play a limited role in the development of PCOS. | Prospective case-control study | Witchel et al. | |||
| 8 | 2000 | n < 50 | CYP21 V281L mutations seemingly manifested PCOS-like phenotype. | Witchel and Aston | ||||
| 9 | CYP17 | 2021 | 394 cases versus 306 controls “Kashmiri” | Mutant genotype is associated with hyperandrogenism. | PCR-RFLP | T/C polymorphism in 5′UTR of CYP17 gene was analyzed to find its connection to hyperandrogenism and PCOS | Ashraf et al. | |
| 10 | 2021 | 204 cases versus 100 controls “Pakistani” | rs743572 polymorphism is significantly associated with PCOS. | PCR-RFLP | 5′UTR region (MspA1) of CYP17 gene was analyzed | Munawar Lone et al. | ||
| 11 | 2019 | 50 cases versus 109 controls “Kurdish” West Iran | Data suggested a positive link of CYP17 T-34C polymorphism with PCOS risk. | PCR-RFLP; chemiluminescent method for hormone measurements | Rahimi and Mohammadi | |||
| 12 | 2018 | 250 cases versus 250 controls North India | Data suggested that − 34T > C polymorphism in CYP17A1 is associated with PCOS in North Indian females. | PCR-RFLP; lipid profile via biochemical analyzer | Kaur et al. | |||
| 13 | 2012 | 287 cases versus 187 controls “Caucasian” | The gene has been suggested as a non-major risk factor. | SNP genotyping and haplotype determination | Four SNPs of CYP17 analyzed | Chua et al. | ||
| 14 | CYP19 | 2020 | 204 cases versus 100 controls “Pakistani” | The polymorphism, rs2414096 (genotype GA) of the CYP 19 gene was considerably associated with PCOS vulnerability. | PCR-RFLP | The study looked at both CYP17 and CYP19 genes | Munawar Lone et al. | |
| 15 | 2019 | 50 cases versus 109 controls “Kurdish” West Iran | No statistical significance was found in the association of CYP19A1 with PCOS risk. | PCR-RFLP; chemiluminescent method for hormone measurements | Rahimi and Mohammadi | |||
| 16 | 2018 | 250 cases versus 250 controls North India | Variations of CYP19A1 were not statistically relevant with PCOS. | Kaur et al. | ||||
| 17 | 2017 | 70 cases versus 70 controls Iran | The study concluded that SNP rs2414096 in CYP19 is likely to play a role in developing PCOS in Iranian women. | PCR-RFLP; enzyme digestion with HSP92II | Mehdizadeh et al. | |||
| 18 | 2014 | 62 cases versus 60 controls | Polymorphism of rs2414096 in CYP19 was found to be associated with the pathogenesis of PCOS. | PCR-RFLP; statistical analysis by SPSS | Hemimi et al. | |||
| 19 | Involved in steroid hormone effects | AR | 2013 | 114 cases versus 1409 controls Diverse ethnicities | CAG variants in the AR gene were unassociated with PCOS risk while they may be related to the T levels in PCOS. | Meta-analysis of 11 studies published between 2000 and 2012 | Zhang et al. | |
| 20 | SHBG | 2020 | 1660 cases versus 1312 controls | rs6259 and rs727428 polymorphisms in SHBG are not associated with PCOS susceptibility. | Meta-analysis of seven studies published between 2007 and 2019 | Liao and Cao | ||
| 21 | Involved in insulin secretion and action | CAPN10 | 2017 | 169 cases versus 169 controls South India | No association of rs2975766 and rs7607759 with PCOS. | RT-PCR | The study looked at other gene polymorphisms too | Thangavelu et al. |
| 22 | 2014 | 127 cases versus 150 controls Tunisia | UCSNP-43 (rs3792267), UCSNP-19 (rs3842570), and UCSNP-63 (rs5030952) were investigated along with their haplotypes. No significant association, except one with obese PCOS subjects, was found. | PCR-RFLP | Ben Salem et al. | |||
| 23 | 2014 | 668 cases versus 200 controls “Caucasian Greek” | There was no correlation of CAPN 10 polymorphism (UCSNP-43) with the incidence of PCOS. Additionally, the gene polymorphism could not be associated with any biochemical, clinical, hormonal, or ovarian features of PCOS. | Primer extension; MALDI-TOF mass spectrometry | Anastasia et al. | |||
| 24 | 2013 | 2123 cases versus 3612 controls | Nine common SNPs were examined. UCSNP 19/63/44 is likely to be associated with increased PCOS risk among Asians. No statistically significant association with UCSNP-22, UCSNP-43, UCSNP-45, UCSNP-56, UCSNP-58, and UCSNP-110 polymorphisms. | Meta-analysis of 14 studies published between 2002 and 2013 | The review is comprehensive and helpful | Shen et al. | ||
| 25 | 2009 | 88 cases “Brazilian” | Data provide evidence that UCSNP-43 may play a role in PCOS while UCSNP-19 and UCSNP-63 remained unassociated with phenotypic traits in PCOS. | Wiltgen et al. | ||||
| 26 | 2008 | 50 cases versus 70 controls “Chilean” | Data suggests a contribution of UCSNP-43 polymorphism to PCOS in Chilean women. | PCR-RFLP | The study looked at UCSNP-19 and UCSNP-63 as well | Márquezet al. | ||
| 27 | IRS | 2016 | 2975 cases versus 3011 controls “Asian and Caucasian” | The findings suggested that IRS-1 Gly972Arg polymorphism be associated with PCOS in the Caucasian ethnicity, and IRS-2 Gly1057Asp polymorphism correlated with PCOS in the Asians. | A meta-analysis of 28 studies published between 2001 and 2014 | Shi et al. | ||
| 28 | 2013 | 150 cases versus 175 controls Croatia | Data did not support an association between Gly792Arg IRS-1 (along with VNTR INS, C/T INSR) polymorphisms and PCOS. Nor did it find any correlation with insulin resistance in Croatian women with PCOS. | Skrgatić et al. | ||||
| 29 | INSR | 2016 | 2975 cases versus 3011 controls “Asian and Caucasian” | The INSR polymorphism, His 1058 C/T, was not found to be associated with PCOS development. | A meta-analysis of 28 studies published between 2001 and 2014 | Shi et al. | ||
| 30 | 2015 | 17,460 cases versus 23,845 controls | The meta-analytical data suggested no significant correlation between rs1799817/rs2059806 SNPs and PCOS susceptibility. On the other hand, it was concluded that rs2059807 could be a promising SNP involved in PCOS susceptibility. | A meta-analysis of 12 studies published between 1994 and 2013 | Feng et al. | |||
| 31 | Involved in gonadotropin action and regulation | AMH | 2020 | 383 cases versus 433 controls “Chinese” | Fifteen rare, but known AMH variants were identified, along with seven novel heterozygous variants. Researchers conclude that AMH can play a role in PCOS development. | Sanger sequencing | Qin et al. | |
| 32 | 2019 | 608 case versus 142 controls | The AMH signaling cascade was deduced as a key player in PCOS etiology. Variants have been identified. | Targeted sequencing | Regions of AMH and AMHR2 were looked at | Gorsic et al. | ||
| 33 | 2017 | 643 case versus 153 controls | Rare genetic variants of AMH related to PCOS were identified. | Targeted sequencing | Replication of whole-genome sequencing | Gorsic et al. | ||
| 34 | 2017 | 2042 cases versus 1071 controls | Meta-analytical data showed no association of AMH (or AMHR2) with a heightened risk of PCOS. | A meta-analysis of five studies published between 2002 and 2013 | Wang et al. | |||
| 35 | FSHR | 2021 | 130 cases Iran | FSHR polymorphisms Ala307Thr and Asn680Ser were concluded to be statistically associated with PCOS women. | PCR followed by sequencing | Seyed Abutorabi et al. | ||
| 36 | 2020 | 1882 cases versus 708 controls “Chinese” | rs2300441 was found to be a primary contributor. | – | GWAS | Yan et al. | ||
| 37 | 2018 | 93 cases versus 52 controls “Kurdish” Northern Iraq | Significant differences were found in FSH and LH levels in PCOS patients with different genotypes of Ala307Thr polymorphism. No relationship was established between polymorphism and PCOS. | PCR-RFLP; Eam1105I enzymatic digestion | Baban et al. | |||
| 38 | 2017 | 377 women versus 388 controls “Korean” | Findings suggested a significant association between FSHR gene polymorphisms (Thr307Ala or Asn680Ser) and PCOS. | RT-PCR | Kim et al. | |||
| 39 | 2014 | 1760 cases versus 4521 controls “Asian and Caucasian” China, Italy, Korea, Netherlands, Turkey, United Kingdom | Results showed no association between Thr307Ala and Asn680Ser polymorphisms of FSHR with PCOS susceptibility. | A meta-analysis of 10 studies published between 1999 and 2013 | Chen et al. | |||
| 40 | 2014 | 215 cases versus 205 controls “Han Chinese” North China | The Ala307Thr and Ser680Asn polymorphisms of FSHR are not related to PCOS in Han ethnic Chinese women. | PCR-RFLP; direct sequencing | Another study in North China with slightly more PCOS cases has found similar results | Wu et al. | ||
| 41 | LHCGR | 2015 | 203 cases versus 211 controls “Bahraini Arab” | The first study suggested an association of LHCGR polymorphisms (rs7371084 and rs4953616) with PCOS. The study also added a strong association of FSHR (rs11692782). | RT-PCR | Almawi et al. | ||
| 42 | Other genes | FTO | 2019 | 55 cases versus 110 controls “Srilankan” | FTO gene rs9939609 polymorphism was significantly more common among PCOS subjects. | Allele-specific real-time quantitative PCR (AS-qPCR) | Branavan et al. |
PCR: polymerase chain reaction; PCOS: polycystic ovary syndrome; SNP: single nucleotide polymorphism; GWAS: genome-wide association studies; PAGE: polyacrylamide gel electrophoresis; RFLP: restriction fragment length polymorphism; RT: reverse transcription.
Red text means a negative association or no association, the orange text implies a weak link to PCOS, and the green text indicates a strong correlation with PCOS.
Table 2 focuses on some of the recent and unique studies that have been carried out. The genes discussed are merely a fraction of the entire set of genes that are likely to play a role in PCOS pathogenesis. However, it begs the question of what makes a gene suitable or a target for investigation. For example, cytochromes P450 are a superfamily of enzymes that play a significant role in steroid conversion; it aids in converting androgen into estrogen. Any defect in this pathway will cease the conversion. 152 The human genome includes 18 CYP families, each having a sub-family of its own. 153 The CYP families are CYP1-8, CYP11, CYP17, CYP19-21, CYP24, CYP26-27, CYP39, CYP46, and CP51. The aromatase genes frequently reported in PCOS databases are CYP11A1, CYP11B2, CYP17A1, CYP19A1, CYP1A1, CYP21A2, and CYP3A7. 154 Thus, any abnormality in this gene family can potentially lead to PCOS. Aside from the studies condensed in the table, similar investigations have taken place earlier, most of which have been included in the meta-analytical reviews mentioned. Similarly, elevated androgen levels have been commonly seen in PCOS cases. Thus, the genes that are usually targeted are somehow connected with androgen, its receptor, or its metabolism. Any defect or polymorphism in their coding regions could lead to an explanation for the increased androgen levels. Examples of these genes are CYP1A1, CYP11A, CYP17, CAPN10, INSR, SHBG, and so on. The genes summarized in Table 3 , when studied, will reveal some form of a connection that could potentially pave the way for PCOS pathogenesis. In this way, possible avenues for genes to be involved in the defective mechanism of PCOS are identified and assessed. Genetic links can be sought anywhere as long as it is relevant to PCOS; the genes that code for enzymes involved in the metabolism of different hormones, the genes responsible for insulin action, and so on. Insulin is a key player in androgen production by the theca cells. Like LH, a higher level of insulin enhances androgen synthesis.
A list of common endocrine-disrupting chemicals (EDCs) accompanied with their uses.
| Endocrine disruptor | Use |
|---|---|
| Bisphenol A (BPA) | Epoxy resins are found in many plastic products, including food storage containers. |
| Dioxins | A by-product of herbicide manufacture and paper bleaching, released during the burning of waste and wildfires. |
| Parabens | Cosmetics, personal care products. |
| Per- and polyfluoroalkyl chemicals (PFAS) | Non-stick cookware, waterproof clothing, food packaging. |
| Phthalates | Cosmetics, children’s toys, food packaging. |
| Polybrominated diphenyl ethers (PBDE) | Flame retardants. |
| Polychlorinated biphenyls (PCB) | Electrical equipment like transformers, hydraulic fluids, lubricants, etc. |
| Triclosan | Anti-microbial and personal care products. |
In conclusion, the databases mentioned earlier can be a useful starting point in finding susceptible genes. The pattern in studies that aim to establish an association between different gene polymorphisms and PCOS is that every study narrows its subjects to a specific geographical location or ethnicity, collects blood samples, and analyzes the DNA in whichever way is feasible. This process led to many individual studies with inconsistent findings; some suggest a genetic link to PCOS, while others disagree with the same conclusion. Numerous studies have been carried out to date to find novel polymorphisms or support existing data. Systematic reviews and meta-analyses come in very useful too.
In addition, strong emphasis on the outcomes of GWAS has been placed. However, in the end, all papers demand large-scale studies to be undertaken. Often these researches look at multiple genes together. Perhaps, other than looking at the genes and how they may correlate with PCOS or subjects’ biochemical profile, researchers can start looking at the environmental risk factors the subjects could have been exposed to simultaneously. PCOS is said to be a multifactorial, polygenic complex disease. To fully elucidate its etiology, all elements in play should be investigated as much as possible.
Environmental determinants of PCOS
We have already discussed the genetic susceptibility associated with PCOS. With this in mind, it is likely that the environment is an active player in the expression of PCOS-related genes. 155 , 156 Some evidence already supports the fact that environmental toxins play a role in disrupting reproductive health. But the research linking these to the development of PCOS is very limited. Furthermore, these environmental risk factors can eventually trigger or aggravate PCOS pathology. 157 Therefore, this section of the review has briefly discussed the environmental determinants, especially the endocrine disruptors potentially involved in the etiology and modulation of PCOS.
Prenatal exposure
Individuals can be exposed to environmental risks during prenatal or postnatal periods of life. For example, intrauterine exposure to excess androgens/glucocorticoids at critical phases of fetal development may lead to PCOS symptoms and determine phenotypic expression in adulthood, according to experimental studies. 156 One way to explain this would be that intrauterine growth restriction (IUGR) can cause increased prenatal exposure of androgens and glucocorticoids, which could possibly induce PCOS programming in the fetus. 156 , 158 Of course, it goes without saying that studies concerning prenatal exposure have their implementation-related difficulties.
Exposure after birth and in adulthood
Evidence to investigate potential prenatal risk factors for humans is lacking. However, an increasing amount of research is being carried out to study the effects of postnatal exposure. Obesity and low physical activity are harmful lifestyle factors that are targeted in disease management. Obesity has been found to exacerbate the metabolic and ovulatory dysfunction in PCOS. 159 , 160 On the other side, weight loss restores ovulation and improves hyperandrogenism. 161 , 162 Moreover, phenotypical variations between ethnicities suggest that cultural factors play a more substantial role in the metabolic consequences than previously thought.
One such postnatal exposure is environmental toxins. Environmental toxins are chemical pollutants present in the environment that enter living organisms via inhalation, ingestion, or absorption through skin/mucous membranes, ultimately having a detrimental impact on them. 163 An emerging body of evidence points to the lasting effects of environmental toxins on human reproductive health. 164 – 166 Common pollutants include mercury, lead, pesticides, chlorofluorocarbons (CFCs), and so forth. Nevertheless, when it comes to PCOS, a specific group of chemicals known as endocrine-disrupting chemicals (EDCs) have gained particular interest and are the main focus in this review section. They have been proposed in their etiology as they can interfere with the hormone system. Some compounds have been described in Table 3 and are accompanied by examples of their uses in our daily lives. It has been estimated that of all synthetic chemicals, about 1000 of them are likely to exhibit endocrine-acting properties; 167 these compounds can be categorized under groups such as phthalates, xenoestrogens, and so forth. The compounds are a heterogenic group of molecules that interfere with steroid hormone synthesis and interact with hormone receptors. 168 As a consequence of their lipophilic structure, they tend to bio-accumulate in the adipose tissue. Thus, humans being at the end of the food chain become the most exposed to these toxins. Apart from adipose tissue, endocrine disruptors have been detected in amniotic fluid, 169 milk, 170 serum, 171 and urine. 172
BPA or Bisphenol A is an endocrine disruptor. BPA is produced globally in abundance; production exceeds 6 billion pounds every year. 173 It is a xenoestrogen, a chemical that mimics natural estrogen, owing to its phenolic structure, enabling it to bind to estrogen receptors (ER). Higher serum level of BPA has been found among PCOS women compared with non-PCOS women. 174 Animal studies show an association between neonatal BPA exposure and PCOS-like symptoms. 175 To make matters worse, BPA directly stimulates the synthesis of androgens in ovarian theca-interstitial cells. 176 Furthermore, a correlation between testosterone and BPA levels has been seen in the serum of women with PCOS. 174 Data from rat studies have shown that BPA can increase testosterone production in theca-interstitial cells and decrease estradiol formation in granulosa cells. These effects can be explained via some form of upregulation induced by BPA on the key genes involved in ovarian steroidogenesis—CYP17A1, CYP11A1 177 and downregulation of CYP19A1. 178 BPA is also known to interact with sex hormone–binding globulin, whose gene is another PCOS candidate gene. 179
A study in 2019 focusing on adolescents looked at BPA levels in 62 girls with PCOS and 33 control subjects in the age range, 12–18 years. 180 High-performance liquid chromatography was employed to measure urinary BPA concentrations. The adolescent patients demonstrated markedly greater BPA levels (case: 15.89 μg/g versus control: 7.30 μg/g creatinine). A similar study using serum BPA from adolescents has found similar results too. 181 On the contrary, a study focusing on several EDCs failed to establish an association of urinary BPA level with PCOS in relatively older women (18–45 years). 182 There are many other studies available linking specific groups of EDC with PCOS. For instance, two case-control studies delved into finding the correlation between the concentrations of EDCs and PCOS. 182 , 183 Both studies revealed a significantly higher serum level of per-fluorinated compounds in PCOS women as compared to control subjects. It is to be noted no causal relationship was proven. Similar to the genetic links, the ultimate findings of the studies are inconsistent. Although it is true EDCs negatively impact the reproductive health of humans, the mechanism as to how these chemicals upset the hormonal balance or interfere with their receptors is yet to be elucidated. A few common ones are listed in Table 3 . 167 , 184 , 185
Treatment options for PCOS
The remedy for this endocrinopathy has been on the lookout ever since its gravity was recognized. There is no permanent cure for this particular disorder, so far. Treatment is adjusted as per an individual’s need to tackle the symptoms and allow the patient to live a less cumbersome life. Below are listed some possible medications suggested for PCOS patients.
Current therapies
For decades, metformin has been used to induce ovulation and fight against insulin resistance, a salient feature detected in women battling PCOS. Metformin belongs to a class of drugs called biguanide and is mostly prescribed to individuals with T2D. Through various experimental evaluations conducted over the years, metformin has proven to be beneficial by increasing the overall glucose uptake in the body, leading to improved insulin sensitivity, reduction in serum androgen level, and proper regulation of the menstrual cycle. 186 , 187 . However, its mode of action remains unclear when used exclusively or in combination with other drugs while handling complications like infertility or the clinical live birth rate. According to a cohort study conducted by a hospital in Riyadh, Saudi Arabia, it was notified that metformin did not demonstrate much significant result when it is used as a co-treatment for improving the pregnancy rate in women seeking in vitro fertilization (IVF). 188 However, based on another systematic analysis conducted, 189 metformin was found to be successful in eradicating the risk of ovarian hyperstimulation syndrome for a pregnant woman. Still, nonetheless, it had no association with the clinical live birth rate. On the other hand, Sun et al. 190 reported that linking metformin with drugs like clomiphene citrate was better at reforming infertility and ovulation rate, but at the same time, Kar 191 found no difference in their combined effect. All these evidences imply that the potency of this drug is debatable and requires thoughtful systematic analysis to procure better results.
Spironolactone
The most notable effects of spironolactone involve the reduction of androgen level, improvement of hirsutism, and acne when used as a form of treatment. 192 An aldosterone antagonist predominantly used as a diuretic, 193 the basic concept behind its complex mode of action involves blocking androgenic receptors, partly obstructing adrenal steroidogenesis, and blocking 5α reductase, thereby increasing the level of SHBG (sex hormone–binding globulin) protein. 194 Unlike metformin, the dosage needs to be considered and supplemented precisely to avoid health issues; this is why spironolactone is suggested to be taken with an oral contraceptive while avoiding pregnancy so as not to promote complications like feminization of male fetus. 194 , 195 Other side effects found to be involved with high doses were hypokalemia and menstrual disturbances. 196 According to a study conducted by C Sabbadin et al. 194 regarding the effects of spironolactone on estradiol levels and intermenstrual bleeding on 30 individuals with normal BMI, it was concluded that certain individuals did exhibit intermenstrual bleeding as a form of side effect due to lower estradiol levels and endometrial thickness. But then again, it was also detected that those individuals who displayed this effect had pre-treatment estradiol values lesser than those who did not face this issue. For this reason, this particular proposition requires further assessment in order to provide confirmatory evidence. Based on another study conducted by Zulian et al., 197 this drug significantly improved glucose and metabolic lipid profile when experimented on 25 PCOS patients over 12 months. Therefore, this medication still continues to be prescribed when it comes down to tackling hormonal imbalance and disease management.
Some of the other commonly prescribed drugs are listed in Table 4 concerning the metabolic problems they target, their dosages, and their side effects.
Treatment options for various symptoms listed along with the administration dosage and side effects.
| Metabolic syndrome | Treatment | Dosage | Side effect | Ref. |
|---|---|---|---|---|
| Insulin resistance | Metformin | 500 mg (starting) Over the course of 1–2 weeks | Nausea, bloating | Lashen |
| Menstrual irregularity | Oral contraceptive pill | 20–35µg | Weight gain | Nader , Domecq et al. |
| Hirsutism | Cyproterone acetate | 50–100 mg (alone) Ethinylestradiol (combined) 20–50 µg | Headaches, breast tenderness | Nader |
| Infertility | Clomiphene citrate | 50–150 mg for 5 days | Nausea, mood swings | Nader , Practice Committee of the American Society for Reproductive Medicine |
| Hyperandrogenism | Spironolactone, flutamide | Spironolactone 100 mg Flutamide 500 mg + OCP | Spironolactone: Fatigue, menstrual irregularity (in high dosages) Flutamide: Hepatotoxicity (if not maintained properly) | Nader , Domecq et al. |
Newly emerging therapies
There are various therapies in consideration with the hopes of being implemented in the near future.
Statin is an inhibitor and performs its function by impeding 3-hydroxy-3-methylglutaryl coenzyme reductase (HMG-CoA), a rate-determining enzyme involved in cholesterol synthesis, thereby halting its conversion to mevalonate. Atorvastatin specifically has shown significant outcomes when it comes to the reduction of insulin resistance and hyperandrogenism in a 12-week study. The study showed increased levels of vitamin D in PCOS women when compared to control women; headaches were seen as side effects. However, another study conducted in 2013 validated that while atorvastatin improved lipid profile and chronic inflammation, it did not do much in the case of insulin sensitivity. Despite its promising result, more clinical studies need to be conducted in order to determine its efficacy. 202 , 203
Surgical method
Procedures like ovarian drilling and bariatric surgery have been considered for treating PCOS despite the risks of reduced ovarian cells and decreased fertility these methods carry. 193 Even though laparoscopic ovary drilling has improved ovulation and reduced androgens, various clinical trials conducted over the years were somewhat inconsistent. They failed to provide concrete evidence to sustain it as a form of treatment. On the other hand, bariatric surgery does promote excessive weight loss for obese individuals leading to improved ovulation and reduced risk of T2D. 204 , 205
The most commonly described forms of inositol are myo-inositol and d -chiro-inositol. These sugar molecules function as a messenger that mostly up-regulates glucose intake and synthesis. Myo-inositol has been used as a supplement in PCOS women and has proven to improve insulin sensitivity and menstrual cycles and cause lesser gastric issues than metformin. However, a Cochrane Systematic Review could not display any promising role of inositol when tested on 1472 sub-fertile PCOS females. 202 , 203 , 205
Few studies are backing up the fact that vitamin D deficiency plays a role in insulin resistance, thereby being connected to the pathogenesis of PCOS. When a dosage was given, it did ameliorate insulin resistance; 195 nonetheless, its role remains contradictory based on many RCTs (randomized controlled trials) conducted previously. For example, according to a double-blind, randomized, placebo-controlled trial executed by Trummer et al. 206 at a medical university in Austria, no significant results were achieved in attesting the effects of vitamin D on different metabolic parameters. While on the other hand, a similar trial conducted by Gupta et al. 207 showed vitamin D supplements enhance the menstrual cycle, ovulatory dysfunction, and fasting blood sugar level.
Gut microbiota
There have been widespread speculations regarding the alterations in gut microbiota being related to the metabolic syndromes of PCOS or vice versa. There are some key features that have been reported to provide a better understanding of this hypothesized linkage.
Studies have come forward with contradictory results regarding the imbalance between gut microbial diversities (α, β) caused by gut microbiota partaking in the regulation of sex hormones leading to its dysbiosis or circulating sex hormones alone alters the gut microbiota.
Other than being observed in obese patients, PCOS also gives rise to the aspect of gene encoding pro-inflammatory cytokines like TNF-α (tumor necrosis factor) and IL-6 (interleukin 6) being associated with the triggering of the immune system and causing intestinal permeability has been pinpointed, that leads to the elevation of LPS (lipopolysaccharide) which ends up causing inflammation by pooling the endotoxin in the blood circulation hindering the metabolic system ultimately leading to ovarian inflammation too. This particular theory has been related to the imbalance of microbial composition. 208
Probiotics stood out and were chosen as a form of treatment to deal with gut microbial-related issues based on the benefits it comes along with. It has displayed anti-inflammatory activities and healthy regulation of gut flora. Based on randomized trials conducted, it has also been shown to alleviate insulin resistance overall, keeping the lipid profile balanced. According to a study initiated by Jing Xue et al., 209 the efficacy of inulin (probiotic) and metformin was assessed by targeting gut microbiota in PCOS mice. The result did provide promising evidence when it came down to reducing pro-inflammatory cytokines through a surge of anti-inflammatory cytokines, in turn decreasing ovarian inflammation. 208 , 209
PCOS looks different for everyone to some extent. It is currently incurable and continues way beyond the child-bearing age or post-menopause. Research points toward substantial genetic implications, but the dots are yet to be connected to give us the complete picture. It is likely that once etiological grounds are explicated, the diagnostics, treatment, and disease management will be subject to change dramatically. It had come a long way since 1935, when American gynecologists Irvin F Stein, Sr and Michael L Leventhal first officially described it. With advancements in diagnostic technology, it has been easier to treat patients, yet genetic, ethnic, and so on, variations pose difficulty in instituting universal “laws” of the disease. In spite of progress, accounts of dissatisfaction related to diagnosis are still significant. The gaps in the field of PCOS diagnosis can be attributed to a plethora of causes, including the heterogeneity of the condition itself, discrepant use of the diagnostic criteria and tools, vagueness in the assessment of the salient features, and due to lack of clarity in adolescent diagnosis. Knowledge around these discrepancies and a multidisciplinary intervention must be adapted to reduce delayed and poor PCOS diagnosis in women. It is also worth mentioning that all the guidelines developed to date are predominantly based upon consensus veteran opinion, clearly indicating the need to generate evidence-based data. The development of PCOS has a strong genetic component. Results from twin studies and familial clustering ground a strong genetic basis for PCOS; having a mother or a sister with PCOS increases the risk of developing PCOS by 30%–50%. Following the analysis of the studies conducted on different gene polymorphisms, it can be said that some of these polymorphisms could potentially serve as biomarkers for the diagnosis and prognosis of PCOS. Given that many studies link genes with PCOS pathogenesis, it is essential that this research be extrapolated into projects with much larger sample sizes. PCOS phenotype does vary with ethnicity, geographical region, and probably socioeconomic status. Thus, this would further add to the complex polygenic nature of PCOS. The importance of GWAS is already established in discovering a new locus for susceptibility. The next step would be to link the genetic factors in question with other determinants and the patient history to establish whether PCOS is indeed a polygenic disease and what genes can truly be called key triggers.
Researchers are continually trying to dig deep to get to the root cause behind this complex metabolic syndrome. With new discoveries flow in tougher challenges, and it all comes down to selecting an effective solution through various trials and errors. The therapies mentioned above do give assuring outcomes, but large-scale, properly structured and funded trials/studies need to be carried out so that this particular disorder can be demystified even more while taking contributive factors like ethnicity, environmental exposures, familial history, and more into account. Keeping the diverse outcomes of PCOS in mind, it can be suggested that a team comprising of a physician, a gynecologist, an endocrinologist, and a reproductive medicine specialist will help these patients manage their lives much better.

Declarations
Ethics approval and consent to participate: An ethics statement is not applicable because this study is a review and is based exclusively on published literature.
Consent for publication: Not applicable.
Author contribution(s): Hiya Islam: Conceptualization; Writing – original draft; Writing – review & editing.
Yushe Nazrul Islam: Formal analysis; Writing – original draft.
Fahim Kabir Monjurul Haque: Conceptualization; Supervision; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
Guarantor: None.
Endocrine Abstracts
- Issues/Conferences
- Our Services
ECE2017 Eposter Presentations: Reproductive Endocrinology Female Reproduction (62 abstracts)
A case of polycystic ovary syndrome (PCOS)
Kristina kljajic babic , dubravka majic milotic , kristijan peros & lea smircic duvnjak.
Clinical Hospital Merkur, Zagreb, Croatia.
: Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women of reproductive age. It’s a heterogeneous functional disorder of unclear etiology. The features of PCOS are disorders of ovulation, androgen excess, polycystic ovaries; it’s associated with presence of associated risk factors for cardiovascular disease (obesity, glucose intolerance, dyslipidemia). The diagnosis of PCOS is made using the Rotterdam 2003 criteria. 23yo female patient was evaluated for oligomenorrhea. At the age of 17, she consulted for the first time her gynecologist because of irregluar menstrual cycles since the menarche (age 14) and excessive hair growth. A diagnosis of PCOS was made and oral contraceptives (OC) were introduced in therapy. After 1.5 yrs, OC were excluded because of undetectable levels of LH and FSH. Without them, the menstrual cycle length was 40–120 days. Examination revealed high BMI 25; normal BP; excessive hair on chin, forearms and lower abdomen; no striae, no acanthosis nigricans, normal thyroid. High levels of insulin, LH, total testosterone, androstendione and low levels of SHBG and progesterone in the luteal phase were found. An oral glucose tolerance test, fasting lipid profile and concentrations of TSH, prolactin, 17OHP were normal. A pelvic ultrasound confirmed polycystic ovaries. The clinical and lab. tests were consistent with PCOS. Therapy with life style changes (weight reduction) and metformin was started (500 mg bid). After 10 months of treatment, she lost 8 kg, menstrual cycles were regular (26–28 days), concentrations of LH, testosterone, progesterone and insulin were normal. The patients with PCOS are treated according to their symptoms, risks and desire for pregnancy. The OCs are the mainstay of pharmacologic therapy for women with PCOS for managing hyperandrogenism and menstrual dysfunction. The case has showed impact of the OC on the pituitary-ovarian suprresion. There are relatively few publications examining the effect of the OC on ovarian function in women and it’s less clear whether the pituitary–ovarian suppression induced by the OCs has any impact on functional ovarian reserve, so we need further evaluation. Although, the use of metformin in the treatment of PCOS is off-label, in this case, metformin had showed as safe and effective. Benefit was made on oligomenorrhea, fertility and obesity.
19th European Congress of Endocrinology
Lisbon, Portugal 20 May 2017 - 23 May 2017
Browse other volumes
Article tools
My recent searches, my recently viewed abstracts, kljajic babic kristina, majic milotic dubravka, peros kristijan, smircic duvnjak lea.

Endocrine Abstracts ISSN 1470-3947 (print) | ISSN 1479-6848 (online) © Bioscientifica 2024 | Privacy policy | Cookie settings
BiosciAbstracts
Bioscientifica Abstracts is the gateway to a series of products that provide a permanent, citable record of abstracts for biomedical and life science conferences.
Case-based learning: management of polycystic ovary syndrome
As the most common incurable hormonal condition affecting women of reproductive age, polycystic ovary syndrome can lead to infertility, anxiety and cardiovascular disease if it is not managed appropriately.
DR P. MARAZZI/SCIENCE PHOTO LIBRARY / The Pharmaceutical Journal
Polycystic ovary syndrome (PCOS) develops during the early pubertal years and is the most common endocrinopathy (hormonal condition) affecting women of reproductive age [1] , [2] . In the UK, around one in ten women are affected by PCOS [3] .
Prevalence of the syndrome varies according to diagnostic consensus used, with estimates ranging from 6% (using the National Institutes of Health consensus) to 18% (using the Rotterdam consensus) of reproductive-aged women of different ethinicity [4] . One systematic review and meta-analysis suggested the incidence of PCOS is lowest in Chinese women (5.6%) and Caucasian women (5.5%), followed by Middle Eastern women (6.1%) and black women (7.4%) [4] .
Pharmacists can provide advice to patients on how to take medicines to treat the symptoms of PCOS, as well as counsel concerned patients and patients who are trying to conceive. This article describes the management options available, including three worked case studies.
Causes and pathophysiology
The exact aetiology is unknown, but PCOS often runs in families and appears to be the result of environmental and genetic factors [5] . Other factors thought to affect the pathophysiology and phenotype of PCOS include:
- Insulin secretion/sensitivity;
- Lack of exercise and activity;
- Persistent hormone imbalance [6] .
However, not all factors affect all women, resulting in different associated pathologies (see Figure 1). For example, androgen excess can lead to infertility, owing to numerous cysts in the ovaries (see Figure 2), type 2 diabetes mellitus (T2DM) and cardiovascular disease [7] , [8] , [9] .
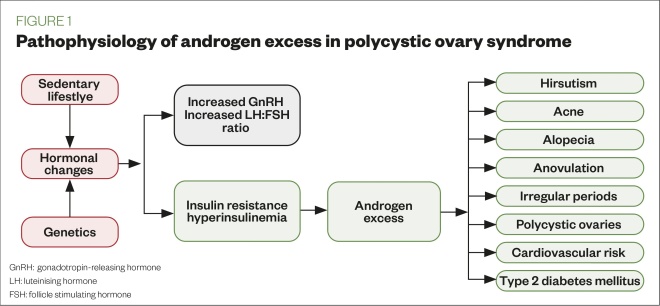
Figure 1: Pathophysiology of androgen excess in polycystic ovary syndrome
Source: Mclean
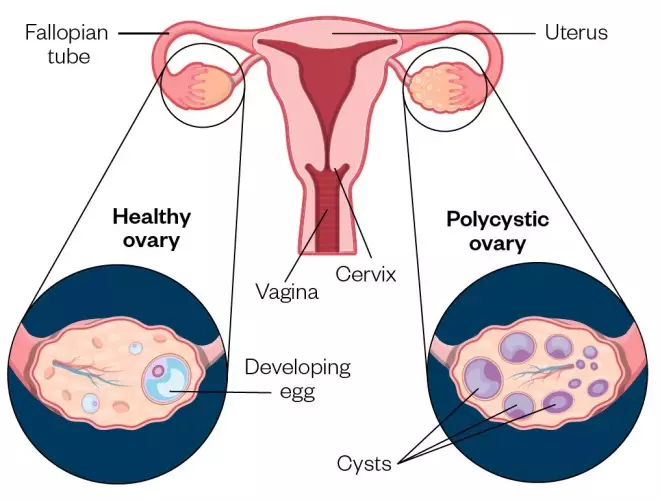
Figure 2: A normal ovary compared with a polycystic ovary. The polycystic ovary has many fluid-filled sacs (follicles) that surround the eggs
Source: Mclean/Shutterstock.com
These typically develop soon after menarche and can vary from woman to woman. They include:
- Menstrual disturbances — oligomenorrhea (infrequent menstruation), amenorrhea (the absence of menstruation) and prolonged erratic menstrual bleeding;
- Obesity — weight gain, being overweight and difficulty losing weight;
- Hirsutism — thick, excessive hair growth on the face, neck and body (see Figure 3);
- Alopecia and/or thinning of hair on the head (see Figure 3);
- Acne as a result of hyperandrogenism;
- Acanthosis nigricans — thick, pigmented skin over the nape of neck, axilla, underarms, inner thigh and groin, which occurs as a result of insulin resistance (see Figure 3) [10] , [11] , [12] , [13] .
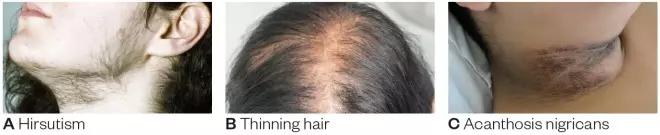
Figure 3: Symptoms commonly seen in polycystic ovary syndrome: (a) hirsutism; (b) thinning hair/alopecia and (c) acanthosis nigricans
Source: Science Photo Library/Shutterstock.com
The symptoms of PCOS can affect self-esteem; therefore, women with PCOS may also have mental health conditions, such as low mood, anxiety and depression [14] .
There are no standardised diagnostic criteria to identify PCOS, but it can be diagnosed if two of the following three criteria are met, provided all other causes of menstrual disturbance and hyperandrogenism are excluded [15] :
- Infrequent or no menstruation;
- Hyperandrogenism (i.e. acne, hirsutism);
- Polycystic ovarian morphology (as seen on an ultrasound scan) [16] .
Physical appearance, blood pressure checks, weight control and blood tests are commonly assessed in initial appointments to confirm if the patient can be referred for an ultrasound scan.
Before receiving a PCOS diagnosis, women may have visited their community pharmacist for reasons relating to the condition (e.g. advice regarding acne), while some may have continually visited their GP until obtaining a referral to a gynaecologist. Therefore, diagnosis can be a lengthy and distressing time for the patient.
Complications
PCOS is the underlying cause in 75% of women with infertility owing to anovulation [16] . Although women with PCOS may conceive, they are more at risk of pregnancy complications (e.g. preeclampsia, hypertension) than women without PCOS [16] . Women with PCOS who are planning a pregnancy should be offered a 75g oral glucose tolerance test. This test should be repeated at 24–28 weeks gestation [16] . Women with PCOS who experience fertility problems should be referred to a specialist [17] .
Owing to the abnormal hormone levels observed in PCOS, metabolic disease (i.e. glucose intolerance, T2DM or dyslipidaemia) and an increased cardiovascular risk are more common in women with the condition [18] , [19] . Women with PCOS are also three times more likely to develop endometrial cancer compared with women without PCOS [16] .
Lifestyle changes
The first-line management options for PCOS include regular exercise (e.g. 150 minutes of brisk walking per week), good hydration, weight control and following a healthy eating plan (e.g. high protein, low carbohydrate and low glycaemic load diets) to aid weight loss and improve insulin sensitivity [20] , [21] . Research has shown that weight loss of 5–10% of initial body weight in women who are obese can significantly improve symptoms of PCOS and may improve the chance of pregnancy [21] .
Pharmacological management
Combined oral contraceptives
A combined oral contraceptive (COC) pill can be prescribed to help:
- Control androgen excess (excess hair growth, acne);
- Regulate periods and painful periods.
COCs are contraindicated in patients with risk factors, such as venous thromboembolism. Treatment effectiveness should be assessed after a minimum of six months [16] .
Common side effects tend to disappear after treatment has become established; however, it is common for patients to experience breast tenderness, bloating, altered mood and weight increase [22] . Patients experiencing these symptoms should be assessed by a specialist.
Medroxyprogesterone
For women with infrequent or no menstruation, medroxyprogesterone can be prescribed at 10mg for 14 days to induce bleeding, with a follow-up ultrasound undertaken to assess for endometrial thickening. Depending on the result of the ultrasound, patients may be continued on medroxyprogesterone 10mg for 14 days or started on the COC or hormonal coil with levonorgestrel. Women with PCOS who are amenorrhoeic are not oestrogen deficient and do not need osteoporosis prophylaxis when taking medroxyprogesterone [16] .
For women with ovulatory dysfunction who are trying to conceive, specialists may prescribe clomifene 50mg once-daily. The treatment is for five days starting on day five of the menstrual cycle; although, if the patient has not had recent uterine bleeding, it may be started at any time. If ovulation has not occurred after the first course of therapy, a second course of clomifene 100mg once-daily for five days can be prescribed. The summary of product characteristics (SmPC) contraindicates clomifene in patients with liver disease, abnormal uterine bleeding and ovarian cysts (except those owing to PCOS) [23] . Long-term cyclical therapy, defined as beyond a total of six cycles, is not recommended [23] .
Adverse effects appear to be dose-related, occurring more frequently at a higher dose. Ovarian enlargement, hot flushes, abdominal-pelvic discomfort, nausea and vomiting, breast discomfort, visual symptoms, headache and intermenstrual spotting have been commonly reported in the SmPC [23] .
Although metformin is only licensed in T2DM, specialists may use it unlicensed in adolescents with PCOS to palliate symptoms and in clomifene-resistant patients who decide to opt for in vitro fertilisation.
In PCOS, long-term metformin treatment may increase ovulation, improve menstrual cyclicity and reduce serum androgen levels, as well as decrease the risk of ovarian hyperstimulation syndrome [24] , [25] . The dose has not been fully established and varies between 1g and 2g per day, and there is no standard duration of treatment. Furthermore, metformin is contraindicated in patients with renal or hepatic insufficiency. Side effects include gastrointestinal disorders and taste disturbance [26] .
Excess hair growth and acne
These symptoms can be managed with the COC pill, topical creams/gels and laser hair removal. Spironolactone is used as an unlicensed product for acne vulgaris owing to its antiandrogen-acting properties [27] , [28] . Other antiandrogens, such as finasteride and flutamide, can be used for hirsutism [29] . Unlicensed treatments are prescribed by dermatologists and require close monitoring of efficacy and side effects; therefore, these should be reserved as a last option.
Case studies
Case study 1: a teenager with suspected polycystic ovary syndrome*.
A regular patient comes to the pharmacy with her daughter, Nadia, who is aged 12 years and is experiencing long and painful periods, as well as mild acne.
Advice and recommendations
Nadia should be advised to record the regularity and duration of her periods, as well as a description of the pain (i.e. duration and intensity). If she feels more tired than normal, this could possibly indicate anaemia.
Explain that mild acne is normal owing to hormonal changes, but it is important to follow a healthy lifestyle (i.e. maintain a balanced diet, keep hydrated and exercise regularly), as well as avoiding picking spots to help maintain good skincare.
Advise Nadia’s mother to give her 500mg of paracetamol up to four times daily and ibuprofen 200–400mg, as recommended in the summary of product characteristics, to control the pain during her period. However, if both analgesics are taken and symptoms persist, she should visit the GP for further assessment.
Diagnosis of polycystic ovary syndrome (PCOS) in adolescents should include a thorough family history, exclusion of other causes of hyperandrogenism (e.g. Cushing’s syndrome) and appropriate laboratory tests. The scarcity of controlled clinical trials makes pharmacological management of PCOS in this patient group difficult. Therapeutic options include lifestyle intervention, oral contraceptive pills and insulin sensitisers. Long-term follow-up is also needed to determine the effectiveness of these approaches in changing the natural history of the reproductive and metabolic outcomes, without causing undue harm [30] .
Case study 2: a woman with polycystic ovary syndrome who is trying to conceive*
Sheila is a 35-year-old patient who would like advice about natural remedies to aid conception. She stopped taking the contraceptive pill a year ago, which had been prescribed to control symptoms of polycystic ovary syndrome (PCOS). However, the symptoms, which included weight gain, have come back and she has gained more weight owing to her anxiety. She has been referred to a fertility clinic, but her appointment is in four months. Sheila does not drink alcohol and is not taking any medicines, but she does smoke occasionally.
Listen Sheila’s concerns and provide advice where possible. For example, reassure her that losing weight can increase fertility. Explain that one of the requirements for patients to be eligible to commence assisted reproduction is to have a body mass index of 19–30; being outside of this range is likely to reduce the success of assisted reproduction procedures [17] . Weight loss can be achieved by following a healthy diet and increasing amount of exercise. She should also be referred to smoking cessation services.
Refer Sheila to her GP so they can assess if she requires an oral glucose tolerance test, as well as to evaluate her anxiety [16] .
Advise her to start taking folic acid 400 micrograms daily or any other vitamin supplement that contains this in order to prepare the body during pre-conception , as well as other pre-pregnancy advice such as ensuring her vaccinations are up-to-date [31] .
Furthermore, signpost the patient to support groups for women with PCOS, such as PCOS Challenge and Verity .
Case study 3: a woman with polycystic ovary syndrome who would like information about managing symptoms*
Johanna, aged 25 years, comes to the pharmacy asking for information about how to manage the hair growth and acne on her face.
Johanna explains that she has polycystic ovary syndrome and started taking the combined oral contraceptive pill three months ago. She is worried that although the treatment has helped with most of her symptoms (e.g. her periods are more regular and less painful), the acne on her face and the hair on her chin are still present. The patient confirms there is no family history of cardiovascular disease and she has recently joined a running club to help her maintain a fit and healthy lifestyle.
Since Johanna has only been taking the pill for three months and has seen an improvement in her symptoms, she does not need to go to her GP for referral to a dermatologist. To see the full benefit of the pill, up to six months of treatment is needed; therefore, Johanna should be reassured that it might take another three months for the full effects of treatment to be achieved. She should also be advised to maintain a balanced diet and continue with her running club.
The patient can be advised to buy over-the-counter benzoyl peroxide as a 5% gel or as a 10% wash for her acne. For better efficacy, she should apply it to her face using a cleanser and sun protection moisturiser rather than applying to individual lesions. The treatment can be completed for six weeks and be assessed thereafter [32] . Johanna should also be advised that non-comedogenic make up is available.
If after six months of treatment, Johanna still has acne and facial hair, she should be referred to her GP for a prescription for topical agents for acne, such as retinoids/antibiotics, or a topical cream for facial hirsutism. However, it is important to advise her that a common side effect of facial hirsutism cream is acne; therefore, hair removal may be a good option for this patient [33] . Laser removal of facial hair may be available on the NHS in some parts of the UK [34] .
*All case studies are fictional
About the author
Veronica Chorro-Mari is specialist pharmacist for women and children services, Barts Health NHS Trust, UK
How to have effective consultations on contraception in pharmacy
What benefits do long-acting reversible contraceptives offer compared with other available methods?
Community pharmacists can use this summary of the available devices to address misconceptions & provide effective counselling.
Content supported by Bayer
[1] Apter D, Bützow T, Laughlin GA & Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab 1994;79(1):119–125. doi: 10.1210/jcem.79.1.8027216
[2] Conway G, Dewaily D, Diamanti-Kandarakis E et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol 2014;171(4):1–29. doi: 10.1530/EJE-14-025
[3] March WA, Moore VM, Willson KJ et al . The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25(2):544–551. doi: 10.1093/humrep/dep399
[4] Ding T, Hardiman PJ, Petersen I et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget 2017;8(56):96351–96358. doi: 10.18632/oncotarget.19180
[5] Barthelmess E & Naz RK. Polycystic ovary syndrome: current status and future perspective. Front Biosci (Elite Ed) 2014;6:104–119. PMID: 24389146
[6] Joshi M. Polycystic ovary syndrome. International Journal of Scientific & Engineering Research 2014;5(12):514–521.
[7] Ajmal N, Khan SZ & Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol X 2019;3:100060. doi: 100060/j.eurox.2019.10.1016
[8] Legro RS. Type 2 diabetes and polycystic ovary syndrome. Fertil Steril 2006;86(Supp 1):S16–S17. doi: 10.1016/j.fertnstert.2006.04.010
[9] Dokras A. Cardiovascular disease risk in women with PCOS. Steroids 2013;78(8):773–776. doi: 10.1016/j.steroids.2013.04.009
[10] Farquhar C. Introduction and history of polycystic ovary syndrome . 2nd ed. Cambridge: Cambridge University Press; 2007.
[11] Lim SS, Davies MJ, Norman RJ & Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Human Reproduction Update 2012;18(6):618–637. doi: 10.1093/humupd/dms030
[12] Rosenfield RL & Lucky AW. Acne, hirsutism and alopecia in adolescent girls: clinical expressions of androgen excess. Endocrinology and metabolism clinics in North America 1993;22(3):507–532. doi: 10.1016/S0889-8529(18)30148-8
[13] Kumarendran B, O’Reilly MW, Manolopoulos KN et al. Polycystic ovary syndrome, androgen excess, and the risk of non-alcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. PLoS 2018;15(3):e1002542. doi: 10.1371/j.eurox. 1002542
[14] Kerchner A, Lester W, Stuart SP et al. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: a longitudinal study. Fertil Steril 2009;91(1):207–212. doi: 101016/j.fertnstert.2007.11.022
[15] Dewailly D. Diagnostic criteria for PCOS: is there a need for a rethink? Best Pract Res Clin Obstet Gynaecol 2016;37:5–11. doi: 10.1016/j.bpobgyn.2016.03.009
[16] National Institute for Health and Care Excellence. Polycystic ovary syndrome. Clinical Knowledge Summary. 2018. Available at: https://cks.nice.org.uk/polycystic-ovary-syndrome (accessed March 2020)
[17] National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. Clinical guideline [CG156]. 2017. Available at: https://www.nice.org.uk/guidance/cg156 (accessed March 2020)
[18] Wang ET, Calderon-Margalit R, Cedars MI et al . Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynaecol 2011;117(1):6–13. doi: 10.1097/AOG.0b013e31820209bb
[19] Peigne M & Dewailly D. Long term complications of polycystic ovary syndrome. Ann Endocrinol 2014;75(4):194–199. doi: 10.1016/j.ando.2014.07.111
[20] NHS. Exercise. 2019. Available at: https://www.nhs.uk/live-well/exercise/#what-counts-as-moderate-aerobic-activity/ (accessed March 2020)
[21] Teede H, Deeks A & Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. doi: 10.1186/1741-7015-8-41
[22] Electronic medicines compendium. Yasmin®, Microgynon 30®, Mycrogynon 30ED®. Available at: https://www.medicines.org.uk/emc/product/1607 (accessed March 2020)
[23] Electronic medicines compendium. Clomid 50mg tablets. 2019. Available at: https://www.medicines.org.uk/emc/product/961/smpc (accessed March 2020)
[24] Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med 2008;358(1):47–54. doi: 10.1056/NEJMct0707092
[25] Tso L O, Costello MF, Albuquerque LE et al. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2014;18(11):CD006105. doi: 10.1002/14651858.CD006105.pub3
[26] Electronic medicines compendium. Competact 15mg/850mg film-coated tablets. 2018. Available at: https://www.medicines.org.uk/emc/product/236/smpc (accessed March 2020)
[27] Kim GK & Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol 2012;5(3):37–50. PMID: 22468178
[28] Charny JW, Choi JK & James WD. Spironolactone for the treatment of acne in women, a retrospective study of 110 patients. Int J Womens Dermatol 2017;3(2):111–115. doi: 10.1016/j/ijwd.2016.12.002
[29] Moghetti P, Tosi F, Tosti A et al . Comparison of spironolactone, flutamide, and finasteride efficacy in the treatment of hirsutism: a randomized, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2000;85(1):89–94. doi: 10.1210/jcem.85.1.6245
[30] Warren-Ulanch J & Arslanian S. Treatment of PCOS in adolescence. Best Pract Res Clin Endocrinol Metab 2006;20(2):311–330. doi: 10.1016/j.beem.2006.02.002
[31] NHS. Trying to get pregnant. 2018. Available at: https://www.nhs.uk/conditions/pregnancy-and-baby/getting-pregnant/ (accessed March 2020)
[32] Tucker R. Case-based learning; acne vulgaris. Pharm J 2019;302(7926):359–363. doi: 10.1211/PJ.2019.20206226
[33] Electronic medicines compendium. Vaniqa®. 2017. Available at: https://www.medicines.org.uk/emc (accessed March 2020)
[34] NHS. Polycystic ovary syndrome. 2019. Available at: https://www.nhs.uk/conditions/polycystic-ovary-syndrome-pcos/ (accessed March 2020)
You might also be interested in…
New estimates for breast cancer risk associated with HRT
Change of guidance needed for management of missed miscarriage, researchers say
Half of pharmacists are not discussing risks of valproate with patients
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Rehabilitation Care of Women with PCOS: A Case Study

2020, Clinical Case Reports
Background: The most widely recognized endocrine problem is Polycystic Ovary Syndrome (PCOS) that shows during puberty with menstrual irregularities, acne and hirsutism. The fundamental imperfections in PCOS are still unclear, so insulin resistance and metabolic syndrome are most common in both healthy weight and as well as in obese patients. Numerous treatments are offered for women related to PCOS including weight loss, gonadotropins, metformin, DASH diet, letrozole and laparoscopic ovarian diathermy. Case presentation: This case report 35-years-old female presented for evaluation of obesity, menstrual irregularity and amenorrhea, and had no received any kind of hormonal treatment. Luteinizing hormone and serum testosterone level were elevated, while follicle stimulating hormone and estradiol level was normal. Conclusion and Outcome: Metformin and DASH diet was best treatment choice for PCOS patient that requires further examination before to being suggested on a long term basis a...
Related Papers
https://ijshr.com/IJSHR_Vol.5_Issue.3_July2020/IJSHR_Abstract.0032.html
International Journal of Science and Healthcare Research (IJSHR)
Polycystic ovary syndrome (PCOS) is a multifaceted condition portrayed by constant anovulation and excess ovarian activity, as opposed to different reasons for anovulation that include ovarian lethargy or essential inadequacy. Ongoing examinations showed that PCOS is related with second rate ceaseless aggravation and that women with PCOS are at expanded danger of non-alcoholic fatty liver disease. The inflammatory and metabolic disturbances related with PCOS are clarified to some extent by the conjunction of insulin resistance and obesity however are additionally filled by the androgen abundance. New bits of knowledge into the guideline of hormones and cytokines in muscle and fat tissue bolster the idea that PCOS is a foundational condition. The therapeutic arrangement ought to be customized to the patient phenotype, complaints, and reproductive desire. Of note, the aromatase inhibitor letrozole is by all accounts more powerful than the reference medicate clomiphene citrate to treat barrenness due to PCOS. Fundamental administration by a multidisciplinary team may help the patients to adhere to lifestyle interventions and in this way lessen body adiposity and recuperate their metabolic and reproductive health.
International Journal of Research and Development in Pharmacy & Life Sciences
Shruti Shangloo
Current Women s Health Reviews
Chandana Pyne
: Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder affecting women worldwide. It refers to a condition that often has ‘poly’ liquid containing sacks around ovaries. It affects reproductive-aged females, giving rise to menstrual and related reproductive issues. PCOS is marked by hormonal imbalance, often resulting in hyperandrogenism. Women with PCOS might experience abnormal insulin activity and complications such as acne, mood swings, hirsutism, obesity, and infertility. The disease is linked with severe clinical ailments such as type 2 diabetes (T2DM), cardiovascular diseases (CVDs), and cancer. A faulty lifestyle, neuroendocrine factors, genetic causes, and androgen exposures often cause PCOS. The approach of society towards physiological problems such as PCOS in women is that it must be under the veil that is the ultimate barrier to the early diagnosis of PCOS. Thus, this review summarizes the causes, symptoms, pathophysiology, diagnosis, and possible treatment (medical, herbal, and lifestyle improvement, acupuncture, and bariatric surgery) related to PCOS.
International Journal of Advanced Research (IJAR)
IJAR Indexing
Polycystic Ovarian Syndrome (PCOS) is hyteregenous, multisystem with Ovarianendocrinopathy in woman of reproductive age with ovarian expression of various metabolic disturbances. The prevalance of PCOS is general population has been estimated to be 5 ? 10%. The classical presentation is characterized by features of anovultion, amenorrhea, oligomenorrhea or irregular cycles in combination with signs of androgen excess, acne, hersuitism or alopecia. It is associated frequently with insulin resistance. It is the commonest cause of infertility due to anovultion. PCOD affects 5 ? 10%. Of reproductive age woman rising till 15% in woman with infertility. In this paper we will discuss the role of diet in the management of PCOS.
Human Reproduction
E. Renard , S. Clouet
Naresh Gill
Vivek Mahalwar
Polycystic ovary syndrome (PCOS), characterized by chronic anovulation and hyperandrogenism, is common in women of childbearing age. Most of these women also have insulin resistance, and insulin sensitizing agents-metformin and the thiazolidinediones-can restore ovulation and often fertility. Treatment of hirsutism and depression are important components of therapy. The increased risk for uterine cancer because of unopposed estrogen can be managed with progestin therapy. Women with PCOS are also at greater risk for both type 2 diabetes and cardiovascular disease.
Archives of Gynecology and Obstetrics
Erica Repaci , Francesca Allieri
Scholar Science Journals
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder characterized by chronic anovulation, hyperandrogenemia, hyperinsulinemia and insulin resistance. It is the most common endocrine disorder in women of reproductive age with an enigmatic pathophysiology. The current proposed diagnostic criteria for PCOS include a number of disorders with similar phenotypes but radically different etiologies. Since there is no universally accepted clinical definition for PCOS identification, clinical associations and assessment of treatment is delayed. It is the most frequently encountered endocrine disturbance in women of reproductive age with prevalence from 5% to 10% affecting all ethnic groups, and it is not only a reproductive disorder but a metabolic one. Recognition of this syndrome makes management of symptoms such as acne, hirsutism, infertility and also reduces the development of type 2diabetes mellitus (T2DM) subsequent strokes and myocardial infarction much easier. Once a diagnosis is made, a thorough investigation of the propensity to developT2DM, noting features of the MS and markers of risk factors for cardio vascular disease (CVD), should be made in both obese and non-obese women. Studies of PCOS in India carried out in convenience samples reported a prevalence of 3.7% to 22.5%, with 9.13% to 36% prevalence in adolescents only. The wide variation in prevalence might be due to diagnostic criteria practiced, limitations in diagnosis, heterogeneous presentation of symptoms, age groups, and ethnic populations studied. This article will discuss the clinical presentation, risk assessment and management of polycystic ovary syndrome.
Pediomaternal Nursing Journal
Ni Ketut Alit Armini
Introduction: Polycystic ovarian syndrome (PCOS) is characterized by infrequent or absent ovulation as well as elevated levels of androgens and insulin (hyperinsulinaemia). The purpose of this study was to determine the efficacy of endocrine treatment in improving reproductive and metabolic outcomes in women with PCOS.Methods: We searched the following databases from inception to Maret 2020: PubMed, Proquest, ScienceDirect, Scopus and CINAHL. We investigated at metformin, clomiphene citrate, metformin plus clomiphene citrate, D-chiro-inositol, statins, and resveratrol as treatments. We compared them to each other, as well as to a placebo or no therapy. The quality of the evidence ranged from extremely low to moderate. The risks of bias (poor reporting of technique and inadequate outcome data), imprecision, and inconsistency were the limitations.Results: Although the evidence quality was low, our latest evaluation indicated that metformin alone may be superior to placebo for live bir...
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Fertility and Sterility
Richard K Miller
Asian Journal of Healthy and Science
Rini Aryani , tia dinanti
Pakistan Journal of Medical and Health Sciences
Muhammad Inzamam
European Journal of Obstetrics & Gynecology and Reproductive Biology
Aarti Sharma
Journal of Enam Medical College
mimi parvin
Dr Purushothama R E D D Y Kudumula
How do we Manage Obese PCOS Infertile Patients”.
Kulvinder K O C H A R Kaur
BIRDEM Medical Journal
dr.milton barua
European journal of endocrinology
Dorte Glintborg
Didier Dewailly , Michel Pugeat
The Australian and New Zealand Journal of Obstetrics and Gynaecology
Journal of Education, Health and Sport
Aleksandra Wójcik
Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi
Liliana Chelaru
International Journal of Scientific Research in Science, Engineering and Technology
International Journal of Scientific Research in Science, Engineering and Technology IJSRSET
Dr yogendra singh bhadoriya
Middle East Fertility Society Journal
Rubina Merchant
International journal of women's health
Aboubakr Elnashar
Clinical Endocrinology
Moustafa Gadalla
Sonia Malik
Ayman S Dawood
Annals of the New York Academy of Sciences
Valentina Pontello
HUMAN REPRODUCTION
Didier Dewailly , Joop Laven
BMC Endocrine Disorders
Christie Bennett
Stephen Franks
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 04 July 2024
Systematic review and meta-analysis of birth outcomes in women with polycystic ovary syndrome
- Mahnaz Bahri Khomami ORCID: orcid.org/0000-0002-5955-1283 1 ,
- Somayeh Hashemi 2 ,
- Soulmaz Shorakae 1 ,
- Cheryce L. Harrison 1 , 3 ,
- Terhi T. Piltonen ORCID: orcid.org/0000-0002-9921-7300 4 ,
- Daniela Romualdi ORCID: orcid.org/0000-0002-5236-2586 5 ,
- Chau Thien Tay 1 ,
- Aya Mousa 1 ,
- Eszter Vanky 6 , 7 &
- Helena J. Teede ORCID: orcid.org/0000-0001-7609-577X 1 , 3
Nature Communications volume 15 , Article number: 5592 ( 2024 ) Cite this article
446 Accesses
17 Altmetric
Metrics details
- Endocrine reproductive disorders
- Risk factors
It is unclear whether polycystic ovary syndrome (PCOS) is an independent risk factor for adverse birth outcomes in the offspring of affected women. Here, we investigate the association of PCOS with birth outcomes in the offspring of women with PCOS overall and by potential confounders. This systematic review and meta-analysis included 73 studies and 92,881 offspring of women with and without PCOS from inception until 13 th July 2022. We report that mothers with PCOS are younger and have higher body mass index (BMI) around conception and have greater gestational weight gain. The odds of preterm birth, fetal growth restriction and low birth weight are higher and mean birthweight is lower in PCOS of which a lower mean birthweight and a higher small for gestational age are probably independent of BMI. This work informed the recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome, emphasizing that PCOS status should be captured at pregnancy to identify risk and improve birth outcomes in the offspring.
Similar content being viewed by others
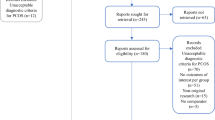

Systematic review and meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome

Increased risk of abortion after frozen-thawed embryo transfer in women with polycystic ovary syndrome phenotypes A and D
Overweight and obesity determined by body mass index criteria for Asian populations adversely affect assisted reproductive outcomes among Chinese women with polycystic ovary syndrome
Introduction.
Polycystic ovary syndrome (PCOS) impacts ~13% of women during their reproductive years 1 and is diagnosed by oligo/anovulation, clinical and/or biochemical hyperandrogenism, and/or polycystic ovary morphology on ultrasound or elevated anti-Mullerian hormone levels 2 . Women with PCOS are more likely to have anovulatory infertility and undergo fertility treatments, including assisted reproductive technology (ART), to conceive. They are also at a higher risk of obesity 3 , insulin resistance 4 , diabetes, hypertension, depression, anxiety and poor quality of life 5 .
Similar risk factors are seen in pregnant women with PCOS, including higher BMI and gestational weight gain and an increased likelihood of developing gestational diabetes mellitus (GDM) and hypertensive disorders 6 , 7 , 8 , 9 . These factors contribute to worsened birth outcomes in offspring 10 , 11 . Meta-analyses have demonstrated higher preterm birth 7 , 8 , 9 , 12 , 13 , admission to neonatal intensive care units (NICUs) 7 , 13 and perinatal mortality 7 , 9 and lower mean birthweight 7 , 12 , 13 in offspring of women with PCOS, compared with offspring of women without PCOS, while fetal growth restriction 9 , 12 , respiratory distress syndrome 9 and neonatal malformations 7 , 9 appear similar. However, there are inconsistent reports for weight-related indices of offspring of women with and without PCOS (e.g. small for gestational age is reported to be similar 7 , 9 , 12 or higher 8 in offspring of women with PCOS). Other key gaps include disparate data regarding the impact of maternal characteristics such as age, BMI, and ART on relevant outcomes, substantial clinical heterogeneity in pooled analyses for multiple outcomes, and insufficient studies precluding subgroup analyses or multivariate or univariate meta-regression 12 .
A diagnosis of PCOS can take over 2 years 14 , with delays being associated with lower socioeconomic status 15 , more severe obesity and higher infertility, hypertension, and dysglycemia, compared with non-PCOS 16 , 17 , all of which adversely impact offspring outcomes. Despite its important implications for pregnancy and offspring health and potential opportunities for improved identification, monitoring and prevention, PCOS is poorly captured in pregnancy and is not widely recognized as a risk factor for adverse birth outcomes 18 .
To inform pregnancy recommendations in the upcoming International Evidence‐based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2023 , we updated our prior systematic review, meta-analysis, and meta-regression to determine the association of PCOS with birth outcomes in offspring of women with and without PCOS. Using a larger number of studies and a broader dataset of participants, we aimed to address the existing evidence gaps regarding the small number of studies per outcome, sensitivity, and risk factor.
The literature search identified 4595 articles published since 2017, of which 28 studies were included in the meta-analysis after full-text review. Agreements between the reviewers in the title and abstract screening and full-text screening were considered good and excellent, with kappa values of 0.72 and 0.94, respectively 19 . There were 53 studies in the 2017 meta-analysis, of which 45 were eligible in the current review (after excluding studies with PCOS diagnosis by self-report or ICD). Combining the new ( n = 28) and previous ( n = 45) articles, a total of 73 articles were included in the present systematic review (Fig. 1 ). Further details are delineated in the Technical Report for the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 20 .

This figure illustrates the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram detailing the study selection process. The diagram includes the number of records identified, screened, assessed for eligibility, and included in the meta-analysis.
The outcomes were reported in 15,070 offsprings of women with PCOS and 77,811 offsprings of women without PCOS (Supplementary Table 1 ). Thirty-one studies were conducted in Asia, 24 in Europe, 15 in America, one in Australia and New Zealand, and two in Africa. One study recruited women with multiple pregnancies 21 . Five studies reported outcomes in women who took metformin after conception 22 , 23 , 24 , 25 , 26 and one in women who had conceived after bariatric surgery 27 . Fourteen studies reported outcomes in post-ART pregnancies 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 and four reported outcomes in pregnancies with GDM in women with and without PCOS 42 , 43 , 44 , 45 . Thirteen studies had a high-quality design 28 , 38 , 43 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 . Twenty studies matched women with and without PCOS for age 24 , 25 , 29 , 38 , 43 , 46 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 and 14 for age and BMI 38 , 43 , 47 , 49 , 50 , 52 , 53 , 54 , 57 , 58 , 59 , 60 , 61 , 62 . No publication bias was found for any of the outcomes. The certainty of evidence for the outcomes was very low to moderate, mainly due to a high risk of bias, serious inconsistency, and serious indirectness.
Overall, 50 studies reported age 21 , 23 , 24 , 25 , 26 , 27 , 29 , 33 , 34 , 36 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 50 , 51 , 53 , 54 , 55 , 56 , 58 , 59 , 60 , 61 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , and 45 studies reported BMI 21 , 23 , 24 , 26 , 27 , 29 , 34 , 36 , 38 , 40 , 41 , 43 , 44 , 45 , 46 , 47 , 50 , 51 , 52 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 84 , either at preconception or at early pregnancy. Women with PCOS were younger (MD: −0.47 years; 95% CI: −0.72, −0.21) and had a higher BMI (1.82 kg/m 2 ; 1.42, 2.22), compared with women without PCOS. Sensitivity analysis showed that after that exclusion of studies in which women were taking metformin after conception 23 , 24 , 26 , or conceived after bariatric surgery 27 , lower age (−0.52 years; −0.78, −0.25) and higher BMI (1.71 kg/m 2 ; 1.30, 2.12) in PCOS remained significant. Fifteen studies reported gestational weight gain 33 , 43 , 45 , 46 , 50 , 56 , 58 , 59 , 63 , 64 , 67 , 68 , 69 , 70 , 84 . Women with PCOS had higher gestational weight gain compared with women without PCOS (1.06 kg; 0.07, 2.05). Table 1 shows the effect sizes for outcomes of interest on pooled and sensitivity analyses. Forest and cumulative plots, funnel plots (Supplementary Figs. 1a – 7c ), and Egger’s test results are provided in the Supplementary information.
Preterm birth
Fifty-six studies reported preterm birth 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 48 , 49 , 51 , 52 , 53 , 54 , 55 , 56 , 58 , 59 , 60 , 63 , 64 , 65 , 66 , 67 , 68 , 71 , 74 , 76 , 77 , 78 , 79 , 82 , 85 , 86 , 87 , 88 , 89 , 90 , 91 ; three were excluded from meta-analysis because of overlapping participants 22 , 53 , 62 , leaving 13,213 offsprings of women with PCOS and 68,830 offsprings of women without, PCOS. Preterm birth was defined as birth prior to 37 weeks of pregnancy in 18 studies 21 , 27 , 29 , 31 , 36 , 37 , 42 , 45 , 48 , 55 , 59 , 60 , 65 , 68 , 74 , 82 , 89 , 90 , birth prior to 37 weeks of pregnancy after exclusion of indicated deliveries 79 , 22–37 weeks in two 33 , 46 , 24–37 weeks in one 38 28–37 weeks in two 41 , 76 , 32–37 weeks in one 34 , and self-reported in one study 28 . The remaining 28 studies did not provide a definition. The odds of preterm birth were comparatively higher in the offspring of women with PCOS (OR: 1.53; 95% CI: 1.33, 1.75). Sensitivity analysis showed that, after the exclusion of studies in which women were taking metformin after conception 23 , 24 , 25 , 26 or conceived after bariatric surgery 27 , preterm birth remained higher in PCOS (1.57; 1.36, 1.81). Cumulative meta-analysis suggested that the odds of preterm birth in offspring born to women with and without PCOS did not substantially change over time. The higher odds of preterm birth were retained in post-ART pregnancies 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , prospective 35 , 43 , 45 , 48 , 49 , 51 , 52 , 58 , 60 , 68 , 77 , 86 , 88 , 89 , 91 , 92 and high-quality studies 28 , 38 , 43 , 46 , 49 , 51 , 52 , 54 , 55 , but not in pregnancies with GDM 42 , 43 , 44 , 45 . In 12 studies, women with and without PCOS were matched for age 29 , 38 , 43 , 46 , 49 , 52 , 54 , 55 , 56 , 58 , 59 , 60 and in nine for age and BMI 38 , 43 , 49 , 52 , 54 , 55 , 58 , 59 , 60 ; PCOS was associated with increased odds of preterm birth in age-matched studies, but not in age- and BMI-matched studies.
Birthweight
Forty-five studies reported birthweight in 8997 offsprings of women with PCOS and 58,106 offsprings of women without PCOS 21 , 22 , 24 , 25 , 26 , 27 , 29 , 31 , 33 , 34 , 36 , 37 , 42 , 43 , 44 , 46 , 47 , 50 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 77 , 79 , 83 , 84 , 86 , 93 , 94 . The offspring of women with PCOS had a comparatively lower mean birthweight (MD: −57.87 g; 95% CI: −97.57, −18.17). Sensitivity analysis after exclusion of studies in women taking metformin after conception 22 , 24 , 25 , 26 or who conceived after bariatric surgery 27 , showed that mean birthweight remained lower in PCOS (−56.02 g; −97.87, −14.16). Cumulative meta-analysis suggested a diminishing magnitude in the MD of birthweight in offspring born to women with and without PCOS did not substantially change over time. The lower mean birthweight was retained in prospective 43 , 47 , 53 , 58 , 60 , 61 , 68 , 69 , 70 , 72 , 73 , 77 , 86 , 92 , 93 and high-quality studies 43 , 46 , 47 , 50 , 53 , 54 , 55 , but not in post-ART pregnancies 29 , 31 , 33 , 34 , 36 , 37 , 95 and in pregnancies with GDM 42 , 43 , 44 . In 14 studies, women with and without PCOS were matched for age 29 , 43 , 46 , 47 , 50 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , and in 11, for age and BMI 43 , 47 , 50 , 53 , 54 , 55 , 57 , 58 , 59 , 60 , 61 ; PCOS was associated with lower mean birthweight in both age-matched and age- and BMI-matched studies.
Fetal growth restriction
Thirteen studies reported fetal growth restriction in 2123 offsprings of women with PCOS and 8301 offsprings of women without PCOS 29 , 48 , 51 , 52 , 54 , 60 , 62 , 67 , 77 , 80 , 82 , 91 , 96 . Two studies were excluded from the meta-analysis because of overlapping participants 62 , 96 . Fetal growth restriction was defined as restriction in fetal growth recognized by a minimum of two ultrasound assessments in one study 48 , estimated fetal weight below the 10th percentile for gestational age in one study 82 , estimated fetal weight or fetal abdominal circumference below the 10th percentile for gestational age in one study 60 , estimated fetal growth indices below the 10th percentile for gestational age in the Chinese population in one study 51 , and estimated fetal weight below 1.5 SD in the Japanese population in one study 54 . The remaining six studies did not provide a definition. The odds of fetal growth restriction were higher in the offspring of women with PCOS compared with the offspring of women without PCOS (OR: 1.84; 95% CI: 1.09, 3.10). There were no studies in which women were taking metformin after conception or conceived after bariatric surgery. Convergence could not be achieved during tau 2 estimation in random-effects cumulative meta-analysis. The higher odds of fetal growth restriction were retained in prospective studies 51 , 53 , 60 , 62 , 77 , 91 but were not retained in post-ART pregnancies 29 and high-quality studies 51 , 52 , 54 . No studies reported fetal growth restriction in pregnancies with GDM. In four studies, women with and without PCOS were matched for age- 29 , 52 , 54 , 60 and in three for age and BMI 52 , 54 , 60 ; PCOS was associated with fetal growth restriction neither in age-matched studies nor in age- and BMI-matched studies.
Low birth weight
Fifteen studies reported low birth weight in 7079 offsprings of women with PCOS and 42,848 offsprings of women without PCOS 21 , 27 , 30 , 33 , 34 , 36 , 37 , 39 , 40 , 55 , 64 , 68 , 74 , 76 , 78 . Low birth weight was defined as birthweight below 2500 g at the 37th week of pregnancy in three studies 30 , 34 , 68 , and birthweight below 2500 g in nine studies 21 , 27 , 33 , 36 , 37 , 40 , 55 , 74 , 76 . The remaining three studies did not provide a definition. The odds of low birth weight were higher in the offspring of women with PCOS compared with the offspring of women without PCOS (OR: 1.28; 95% CI: 1.04, 1.59). Sensitivity analysis showed that after the exclusion of one study after bariatric surgery 27 , low birth weight remained higher in PCOS (1.27; 1.03, 1.57). Cumulative meta-analysis suggested that the odds of low birth weight in offspring born to women with and without PCOS did not substantially change over time; however, one study published before 2006 contributed to heterogeneity. The higher odds of low birth weight were retained in post-ART pregnancies 30 , 33 , 34 , 36 , 37 , 39 , 40 , but not in prospective 68 and high-quality studies 55 . No studies reported low birth weight in pregnancies with GDM. There were no studies where women with and without PCOS were matched for age and/or BMI.
Small for gestational age
Twenty-seven studies reported small gestational age in 3989 offsprings of women with PCOS and 25,129 offsprings of women without PCOS 21 , 25 , 30 , 33 , 37 , 42 , 48 , 52 , 55 , 58 , 62 , 69 , 70 , 71 , 73 , 74 , 75 , 80 , 87 , 88 , 89 , 96 . Two studies were excluded from the meta-analysis because of overlapping participants 62 , 96 . Small for gestational age was defined as birthweight below the 5th percentile for gestational age in two studies 58 , 69 , birthweight below the 10th percentile for gestational age in 14 studies 25 , 29 , 30 , 33 , 42 , 55 , 59 , 70 , 73 , 74 , 75 , 80 , 87 , 89 , birthweight below the 10th percentile for gestational age and sex in the Chinese population in one study 37 , birthweight below the 22nd percentile for gestational age and sex in two studies 21 , 88 , and fetal indices below the 5th percentile for gestational age in the white population in one study 48 . The remaining nine studies did not provide a definition. The odds of small for gestational age were similar in offspring of women with and without PCOS (OR: 1.11; 95% CI: 0.87, 1.41), including in sensitivity analysis after excluding one study in which women were taking metformin after conception 25 (1.13; 0.88, 1.45). Cumulative meta-analysis suggested a diminishing magnitude in the odds of small for gestational age in offspring born to women with and without PCOS over time. The odds of small for gestational age were lower in offspring of women with PCOS in post-ART pregnancies 30 , 33 , 37 and prospective studies 43 , 49 , 52 , 58 , 62 , 69 , 70 , 72 , 73 , 86 , 88 , 89 , but not in high-quality studies 43 , 49 , 53 , 55 and in pregnancies with GDM ( P > 0.05). Two studies reported small for gestational age, the odds of which were similar in the offspring of women with and without PCOS (1.23; 0.52, 2.93). In five studies, women with and without PCOS were matched for both age 43 , 49 , 53 , 58 , 59 and BMI 43 , 49 , 53 , 58 , 59 ; PCOS was associated with increased odds of small for gestational age in these studies.
Twenty-three studies reported macrosomia in 9078 offsprings of women with PCOS and 53,411 offsprings of women without PCOS 22 , 30 , 33 , 34 , 36 , 37 , 40 , 42 , 43 , 44 , 45 , 51 , 55 , 60 , 64 , 65 , 67 , 68 , 74 , 76 , 78 , 82 , 94 . Macrosomia was defined as birthweight above 4000 g in nine studies 22 , 33 , 36 , 37 , 40 , 42 , 45 , 55 , 82 , birthweight equal to or above 4000 g in three studies 51 , 74 , 76 , birthweight above 4000 g at the 37th week of pregnancy in three studies 30 , 34 , 68 , birthweight above 4000 g or above the 95th percentile for gestational age in one study 68 , and birthweight above 4500 g in one study 65 . The remaining six studies did not provide a definition. The odds of macrosomia were similar in the offspring of women with and without PCOS (OR: 1.13; 95% CI: 0.95, 1.35; I 2 : 61.0%), and this was unchanged in sensitivity analysis after the exclusion of one study in which women were taking metformin after conception 22 (1.16; 0.97, 1.39). Cumulative meta-analysis suggested that the odds of macrosomia in offspring born to women with and without PCOS did not substantially change over time. The odds of macrosomia remained similar in post-ART pregnancies 30 , 33 , 34 , 36 , 37 , 40 , prospective 43 , 45 , 51 , 60 , 68 , and high-quality studies 43 , 51 , 55 , and in pregnancies with GDM 42 , 43 , 44 , 45 . In two studies, women with and without PCOS were matched for both age and BMI 43 , 60 ; PCOS was not associated with macrosomia in these studies.
Large for gestational age
Twenty-five studies reported large for gestational age in 1029 offsprings of women with PCOS and 10,052 offsprings of women without PCOS 25 , 30 , 37 , 42 , 43 , 48 , 49 , 53 , 55 , 58 , 59 , 62 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 77 , 80 , 86 , 88 , 89 , 96 . Two studies were excluded from the meta-analysis because of overlapping participants 62 , 96 . Large for gestational age was defined as birthweight above the 90th percentile for gestational age in fourteen studies 25 , 30 , 42 , 55 , 58 , 59 , 69 , 70 , 73 , 74 , 75 , 80 , 87 , 89 , birthweight above the 90th percentile for gestational age and sex in the Chinese population in one study 37 , and fetal indices above the 95th percentile for gestational age in the white population in one study 48 . The remaining seven studies did not provide a definition. The odds of being large for gestational age were similar in the offspring of women with and without PCOS (OR: 1.14; 95% CI: 0.98, 1.33). Odds of large for gestational age remained similar in sensitivity analysis excluding a study in which women were taking metformin after conception 25 (1.16; 0.99, 1.35), and in post-ART pregnancies, prospective 43 , 48 , 49 , 53 , 58 , 69 , 70 , 72 , 73 , 77 , 86 , 88 , 89 and high-quality studies 43 , 49 , 53 , 55 and in pregnancies with GDM 42 , 43 . Cumulative meta-analysis suggested a diminishing magnitude in the odds of large for gestational age in offspring born to women with and without PCOS over time. In five studies, women with and without PCOS were matched for both age and BMI 43 , 49 , 53 , 58 , 59 ; PCOS was not associated with large for gestational age in these studies.
Meta-regression
Significant heterogeneity ( I 2 > 50%) was observed for studies reporting preterm birth, birthweight, low birth weight, small for gestational age and macrosomia. Where reported (Table 2 ), maternal age was lower in PCOS for preterm birth and birthweight; gestational weight gain was higher in PCOS for birthweight; and BMI was higher in PCOS for preterm birth, birthweight, low birth weight, small for gestational age and macrosomia. Variations in age, BMI, and gestational weight gain were not associated with increased odds/lower MD for any of the outcomes on meta-regression.
In this systematic review and meta-analysis of 73 published articles in 92,881 offsprings of women with and without PCOS, women with PCOS were younger in age and had higher BMI around conception and higher gestational weight gain. The offspring of women with PCOS were more likely to be born preterm and have lower mean birthweight, low birth weight, and fetal growth restriction. Preterm birth and low birth weight remained higher in the offspring of women with PCOS in post-ART pregnancies. Higher preterm birth and lower mean birthweight also remained associated with maternal PCOS in both prospective and high-quality studies. In subgroup meta-analyses of studies matched for maternal age or BMI, PCOS was associated with lower mean birthweight and higher small for gestational age but was not associated with fetal growth restriction. Given the higher risks of adverse offspring health outcomes in PCOS, there is a clear need for greater PCOS recognition and identification and for fetal monitoring to facilitate prevention, especially in post-ART pregnancies and pregnancies with higher maternal BMI. This has led to new recommendations in the International PCOS Guideline that PCOS status should be identified during pregnancy to provide appropriate monitoring and support.
Advanced maternal age at pregnancy is associated with higher preterm birth and fetal growth restriction in offspring as well as higher obesity, infertility, pregnancy complications, and chronic diseases in women 97 , which further exacerbate birth outcomes in offspring 11 . We found that mothers with PCOS had a higher BMI around conception and had higher gestational weight gain, despite being slightly younger than mothers without PCOS. These have been assessed and consistently reported in just one 12 of the five 7 , 8 , 9 , 13 previous systematic reviews. While meta-analyses of study-level aggregate data do not allow for evaluation of confounding effects or independent associations, exploring subsets of studies matched for, or limited to, confounding variables, as well as adjusting for confounders, may provide an indication of the impact of confounders. Here, in the majority of sensitivity analyses, the odds of adverse birth outcomes increased in the offspring of women with PCOS in age- or BMI-matched studies and decreased in post-ART pregnancies, with variable changes in the direction of effects. The odds of adverse birth outcomes were similar in the offspring of women with and without PCOS in pregnancies with GDM. However, our sensitivity analyses lacked power due to the small number of studies per analysis and/or moderate to substantial heterogeneity 98 . On meta-regression, we did not find a confounding effect from variations in age, BMI, or gestational weight gain across studies in odds ratios. This was in contrast with our findings in the sensitivity analysis for preterm birth, where the increased odds appeared to be related to maternal BMI. An individual patient data meta-analysis may be able to distinguish different characteristics between pregnant women and whether they mediate the association of PCOS with adverse birth outcomes in offspring.
Globally, 10.6% of offspring are born preterm, with a high burden of morbidity in both mothers and offspring, as well as an increased neonatal mortality 99 . Maternal low socioeconomic status, age and BMI extremes, poor diet, overdistension of the uterus, pregnancy complications, chronic disease, and increased inflammatory markers are risk factors for preterm birth 100 . Here, we found that the offspring of women with PCOS have 53–57% higher odds of preterm birth, independent of time and ART. In both prospective and high-quality studies, the association of PCOS with preterm birth was retained. While these findings are consistent with our prior systematic review 12 , here, we found for the first time that the higher odds of preterm birth were in offspring of younger women with PCOS 100 . Women with PCOS have chronic inflammation that is independent of but worsened by obesity 101 . In early pregnancy, a strong mobilization of inflammatory and other cytokines has been reported in PCOS, persisting throughout pregnancy, indicating a more activated immune status 83 . Women with PCOS also have higher weight gain 102 , and higher GDM and hypertensive disorders of pregnancy independent of, but worsened by, aging and obesity 6 ; thus, our finding of preterm birth being reported in offspring of women with PCOS at a younger age and with a higher BMI, bodes potentially higher odds of preterm birth in offspring of an older population of women with PCOS.
Worldwide, ~14.6% of offspring are born at low birth weight with significant short- and long-term sequelae 103 . Maternal age extremes, multiple pregnancies, pregnancy complications and chronic diseases, infections and poor diet are risk factors for a lower birthweight. We found that the offspring of women with PCOS have a 41.52 g lower mean birthweight and 28% higher odds of low birth weight, regardless of sex and gestational age at birth. Overlapping measures are fetal growth restriction and small for gestational age. While fetal growth restriction reflects poor intrauterine growth mostly resulting from placental insufficiency, small for gestational age, which takes fetal gestational age and sex into account, does not indicate the cause of the finding (i.e. genetic/epigenetic variations, insufficient placenta, inadequate nutrition) 104 . We found that, while the offspring of women with PCOS had higher odds of fetal growth restriction, the odds of small for gestational age were similar to the offspring of women without PCOS at birth. These disparate findings could be due to the timing, diagnostic measurements used, and accuracy of ultrasonographic assessments of offspring growth 105 .
Macrosomia, with a prevalence of 5–15%, is associated with maternal age, obesity, gestational weight gain, GDM, and induced and operative delivery 106 . We found that the offspring of women with and without PCOS had similar odds of macrosomia and large for gestational age. While our findings confirm the results of our previous systematic review 12 , this contrasts with our findings for large for gestational age, which was similar here but higher in PCOS in the prior systematic review 12 . Given that women with PCOS usually have higher mean BMI gestational weight gain and rates of GDM than their non-PCOS counterparts 6 , higher odds of macrosomia and large for gestational age in offspring of women with PCOS were expected. Our current findings on similar odds of macrosomia and large for gestational age in offspring of women with and without PCOS, despite higher BMI, weight gain, and GDM as known risk factors, could be related to opposing effects of the mechanisms driving fetal growth restriction and low birth weight in PCOS, including placental insufficiency 104 . Over time, the odds of small for gestational age diminished in PCOS. This could be due to later studies including younger women with PCOS 107 who are less likely to have chronic conditions 108 .
The strengths of this systematic review and meta-analysis include a large number of observational studies and participants across five continents and the exclusion of less reliable PCOS diagnoses. The inclusion of a reliable diagnosis minimizes inaccuracies due to recall bias or misinterpretation of the diagnosis and the inherent variability in diagnostic criteria across medical organizations 109 , 110 . For the first time, we reported mean age and BMI, corresponding to each outcome of interest. We performed sensitivity and cumulative analyses to examine the potential impact of confounders and performed meta-regression to assess the impact of age, BMI, and gestational weight gain where substantial heterogeneity was observed, a pioneering approach from our previous systematic review. Certainty of evidence was assessed for individual outcomes using the GRADE approach. Limitations should also be noted. A limitation of this review is the inclusion of studies that were published in English, which contributes to potential language bias since English studies are more likely to demonstrate positive findings. Approximately 82% of the included studies had a moderate to high risk of bias, mainly due to high confounding bias (53.4%), followed by high selection bias (38.35%). However, sensitivity analyses of high-quality studies confirmed the overall findings. The majority of studies did not report gestational weight gain, and many had unclear or inconsistent definitions for some of the outcomes. A small number of studies were available for subgroup analyses by age-matched or BMI-matched cohorts, post-ART pregnancies, pregnancies with GDM, or high-quality studies. As this is a meta-analysis of aggregate level data, we were unable to account for confounders in our analyses and could only explore this in studies matched by age, BMI, or limited to a post-ART population or pregnancies with GDM.
In this systematic review and meta-analysis, we found that offspring of women with PCOS are more likely to have fetal growth restriction and be born preterm, with low birth weight. This systematic review and meta-analysis of 92,881 infants of women with and without PCOS directly inform the 2023 International Evidence‐based PCOS Guideline recommendation to consider PCOS as a risk factor for adverse birth outcomes in preconception and antenatal guidelines to facilitate screening, proper monitoring, and timely intervention. Other risk factors, such as age, BMI, and ART, should also be considered in women with PCOS. The recommendations outlined in the PCOS guideline hold the potential to influence offspring born to 17 million pregnancies with PCOS annually across the globe.
Additional research, by means of individual patient data meta-analyses with enough statistical power, is warranted to further clarify the impact of PCOS features and associated conditions on birth outcomes in the offspring.
Search strategy and selection criteria
This systematic review and meta-analysis represents an updated version of previously published systematic reviews 6 , 12 and follows the Meta‐Analyses and Systematic Reviews of Observational Studies (MOOSE) guidelines 111 . Given that the majority of the studies on birth outcomes in prospectively identified PCOS come from selected populations with a high-risk profile 112 , we prioritized the overall quality of the studies over the distinction between retrospective and prospective study designs. The protocol for the original review was prospectively registered in PROSPERO (CRD 42017067147). Methods and findings of the previous search, which included publications up to the 4 April 2017, were previously published 6 , 12 . For this update, the search was limited to English language studies and updated (M.B.K.) from 2017 to 13 July 2022 through Medline, Medline in‐process, and other non‐indexed citations, EMBASE, and all EBM reviews, including Cochrane Database of Systematic Reviews, Cochrane Clinical Answers, Cochrane Central Register of Controlled Trials, American College of Physicians Journal Club, Cochrane Methodology Register, Health Technology Assessments, The Database of Abstracts of Reviews of Effectiveness and the National Health Service Economic Evaluation Database. Bibliographies of relevant systematic reviews and meta-analyses were searched to identify any additional studies. The search incorporated a broader range of outcomes and was distributed across two systematic reviews and meta-analyses.
Eligible studies included studies reporting observational data on preterm birth, birthweight, fetal growth restriction, low birth weight, small for gestational age, macrosomia, and large for gestational age in offspring of both women with and without PCOS. Our search was limited to studies published in English. A protocol amendment was made to only include studies where the PCOS diagnosis met the Rotterdam criteria 110 . Birth outcomes were accepted based on the definitions provided in the primary studies.
Studies published in languages other than English, case reports, case series, editorials, scoping, and narrative reviews were excluded. Additionally, we excluded studies using self-reported or International Classification of Diseases (ICD) for PCOS diagnosis, and studies not reporting the outcomes of interest in the two groups of PCOS versus non-PCOS.
Two reviewers (M.B.K., S.H., or S.S.) independently screened studies by titles and abstracts and reviewed full texts for eligibility. Discrepancies were discussed and resolved through consensus or arbitration between reviewers. Studies identified through the new search were combined with those published before April 2017. Studies not meeting the new criteria needed for PCOS diagnosis were excluded (i.e. studies captured in the 2017 reviews 6 , 12 that used self-report or ICD for PCOS diagnosis were excluded from the current review).
Data analysis
Data extraction and quality appraisal were independently performed by the same reviewers. Using an a priori researcher‐developed data extraction form, data were extracted from each study. These included the author, year of publication, study design, study location, participant characteristics, and frequency/mean and standard deviation of outcomes per group. Participant characteristics included maternal age, BMI, mode of conception, existing medical conditions, and medications used during pregnancy.
When participants were overlapping between multiple publications for the same outcome, data from the study with the largest sample size were included in the meta-analysis.
Risk of bias was independently assessed by two reviewers (M.B.K., S.H., or S.S.) using the Newcastle–Ottawa Scale (NOS) for non‐randomized studies for selection, comparability, and outcome ascertainment 113 . Studies were considered high-quality if they scored at least three stars in selection, one star in comparability, and two stars in outcome ascertainment. Studies with two stars in selection, a minimum of one star in comparability, and two stars in outcome ascertainment were considered fair-quality. Studies meeting neither of these two thresholds were considered low-quality.
We performed random-effects meta-analyses to generate pooled effect estimates, reported as the mean differences (MDs) or odds ratios (ORs) with 95% confidence intervals (CIs), for the association between PCOS status and pregnancy outcomes. Between-study heterogeneity was assessed using the I ² statistic; I ² above 50% was deemed substantial heterogeneity 114 .
Sensitivity analyses were performed excluding studies in which women were taking metformin after conception or conceived after bariatric surgery. Prior research indicates that metformin intake in pregnancy 115 and conception post bariatric surgery 116 may impact birth outcomes in offspring. Cumulative random-effects meta‐analyses based on Hedges’s adjusted g and its 95% confidence intervals (95% CI) 117 were performed to explore the effect of time (publication year) on the association of PCOS with each outcome of interest. Publication bias was assessed using funnel plots. We assessed the certainty of evidence for each outcome according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system 118 using Gradepro software 119 .
Sensitivity analyses of the outcomes by conception with ART (in vitro fertilization, in vitro maturation intracytoplasmic sperm injection, zygote intrafallopian transfer, and gamete intro-fallopian transfer), pregnancies with GDM, and prospective and high-quality studies as well as by age- and BMI-matched designs were also performed after exclusion of studies in which women were taking metformin after conception or conceived after bariatric surgery.
Restricted maximum likelihood (REML)‐based random effects meta‐regression 120 was performed to explore the effects of variations in maternal age, BMI, and gestational weight gain on each outcome of interest if there were at least 10 studies per coefficient. Effect sizes of age, BMI, and gestational weight gain for individual studies were used in meta‐regression analyses. The percentage of between-study variance explained by the model (tau 2 ) was estimated using Knapp–Hartung modification. Normal distributions for mean values were checked using skewness‐kurtosis tests. As age and BMI were not both significant ( p < 0.01) for any of the outcomes on univariate meta-regression analyses, multivariable meta-regression could not be performed. Statistical significance was defined as two-sided p < 0.05. All statistical analyses were performed using Stata version 17 (StataCorp, 14 College Station, TX, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data that support the findings of this study have been deposited in https://data.mendeley.com/datasets/npy96r94p2/1 ( https://doi.org/10.17632/npy96r94p2.1 ). Source data are provided with this paper.
Bozdag, G., Mumusoglu, S., Zengin, D., Karabulut, E. & Yildiz, B. O. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31 , 2841–2855 (2016).
Article PubMed Google Scholar
Teede, H. J. et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 108 , 2447–2469 (2023).
Article PubMed PubMed Central Google Scholar
Lim, S. S., Davies, M. J., Norman, R. J. & Moran, L. J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 18 , 618–637 (2012).
Article CAS PubMed Google Scholar
Lim, S. S., Norman, R. J., Davies, M. J. & Moran, L. J. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes. Rev. 14 , 95–109 (2013).
Yin, X., Ji, Y., Chan, C. L. W. & Chan, C. H. Y. The mental health of women with polycystic ovary syndrome: a systematic review and meta-analysis. Arch. Women’s Ment. Health 24 , 11–27 (2021).
Article Google Scholar
Bahri Khomami, M. et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—a systematic review, meta‐analysis, and meta‐regression. Obes. Rev. 20 , 659–674 (2019).
Boomsma, C. M. et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 12 , 673–683 (2006).
Kjerulff, L. E., Sanchez-Ramos, L. & Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am. J. Obstet. Gynecol. 204 , 558.e551–556 (2011).
Yu, H. F., Chen, H. S., Rao, D. P. & Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 95 , e4863 (2016).
Goldstein, R. F. et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 317 , 2207–2225 (2017).
Berger, H. et al. Impact of diabetes, obesity and hypertension on preterm birth: population-based study. PLoS ONE 15 , e0228743 (2020).
Article CAS PubMed PubMed Central Google Scholar
Bahri Khomami, M. et al. The role of maternal obesity in infant outcomes in polycystic ovary syndrome—a systematic review, meta‐analysis, and meta‐regression. Obes. Rev. 20 , 842–858 (2019).
Qin, J. Z. et al. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 11 , 56 (2013).
Gibson-Helm, M., Teede, H., Dunaif, A. & Dokras, A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102 , 604–612 (2017).
PubMed Google Scholar
Ding, T., Baio, G., Hardiman, P. J., Petersen, I. & Sammon, C. Diagnosis and management of polycystic ovary syndrome in the UK (2004–2014): a retrospective cohort study. BMJ Open 6 , e012461 (2016).
Fernandez, R. C. et al. Diagnosis delayed: health profile differences between women with undiagnosed polycystic ovary syndrome and those with a clinical diagnosis by age 35 years. Hum. Reprod. 36 , 2275–2284 (2021).
Kim, C. et al. Factors associated with self-report of polycystic ovary syndrome in the Coronary Artery Risk Development in Young Adults study (CARDIA). BMC Women’s Health 23 , 248 (2023).
Piltonen, T. T. et al. Awareness of polycystic ovary syndrome among obstetrician-gynecologists and endocrinologists in Northern Europe. PLoS ONE 14 , e0226074 (2019).
Orwin, R. G. Evaluating coding decisions. In The handbook of research synthesis (eds. Cooper, H. & Hedges, L. V.) pp. 139–162 (Russell Sage Foundation, 1994).
Mousa, A., Tay, C. & Teede, H. Technical Report for the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome (Monash University, 2023).
Jonsdottir, F. et al. Obstetrical complications in dichorionic twin pregnancies in women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 96 , 1453–1459 (2017).
Glueck, C. J. et al. Metformin, pre-eclampsia, and pregnancy outcomes in women with polycystic ovary syndrome. Diabet. Med. 21 , 829–836 (2004).
Glueck, C. J. et al. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum. Reprod. 19 , 1323–1330 (2004).
Kovo, M. et al. Neonatal outcome in polycystic ovarian syndrome patients treated with metformin during pregnancy. J. Matern. Fetal Neonatal Med. 19 , 415–419 (2006).
Bolton, S., Cleary, B., Walsh, J., Dempsey, E. & Turner, M. J. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur. J. Pediatr. 168 , 203–206 (2009).
De Leo, V., Musacchio, M. C., Piomboni, P., Di Sabatino, A. & Morgante, G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 157 , 63–66 (2011).
Benito, E. et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J. Clin. Endocrinol. Metab. 105 , 01 (2020).
Dokras, A. et al. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstet. Gynecol. 108 , 61–69 (2006).
Wan, H. L., Hui, P. W., Li, H. W. & Ng, E. H. Obstetric outcomes in women with polycystic ovary syndrome and isolated polycystic ovaries undergoing in vitro fertilization: a retrospective cohort analysis. J. Matern. Fetal Neonatal Med. 28 , 475–478 (2015).
Sterling, L. et al. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil. Steril. 105 , 791–797.e792 (2016).
Chen, X., Kong, L., Piltonen, T. T., Gissler, M. & Lavebratt, C. Association of polycystic ovary syndrome or anovulatory infertility with offspring psychiatric and mild neurodevelopmental disorders: a Finnish population-based cohort study. Hum. Reprod. 35 , 2336–2347 (2020).
Liu, L. et al. Effect of pregravid obesity on perinatal outcomes in singleton pregnancies following in vitro fertilization and the weight-loss goals to reduce the risks of poor pregnancy outcomes: a retrospective cohort study. PLoS ONE 15 , e0227766 (2020).
Abdulkhalikova, D. et al. Perinatal outcome of in vitro fertilization pregnancies in women with polycystic ovary syndrome by pregravid BMI. J. Perinat. Med. 49 , 514–519 (2021).
Cai, H. et al. Early and late pregnancy loss in women with polycystic ovary syndrome undergoing IVF/ICSI treatment: a retrospective cohort analysis of 21 820 pregnancies. BJOG 128 , 1160–1169 (2021).
Gongadashetti, K., Gupta, P., Dada, R. & Malhotra, N. Follicular fluid oxidative stress biomarkers and art outcomes in PCOS women undergoing in vitro fertilization: a cross-sectional study. Int. J. Reprod. BioMed. 19 , 449–456 (2021).
CAS PubMed PubMed Central Google Scholar
Hu, S., Xu, B., Long, R. & Jin, L. The effect of polycystic ovary syndrome without hyperandrogenism on pregnancy-related outcomes: a retrospective cohort study. BJOG 128 , 1003–1010 (2021).
Lin, J., Guo, H., Wang, B., Chen, Q. & Zhu, Q. Neonatal outcomes in women with polycystic ovary syndrome after frozen-thawed embryo transfer. Fertil. Steril. 115 , 447–454 (2021).
Wu, P. F., Li, R. Z., Zhang, W., Su, Z. Z. & Lin, Y. H. Polycystic ovary syndrome is not associated with offspring birth weight: a Mendelian Randomization Study. Biomed. Environ. Sci. 34 , 170–174 (2021).
Zhu, J. et al. A comprehensive evaluation of progestin-primed ovarian stimulation protocol in patients with or without PCOS undergoing in vitro fertilization. Reprod. Biol. 21 , 100540 (2021).
Ni, Z. et al. Adverse effects of polycystic ovarian syndrome on pregnancy outcomes in women with frozen-thawed embryo transfer: propensity score-matched study. Front. Endocrinol. 13 , 878853 (2022).
Wang, Q. et al. Association of polycystic ovary syndrome phenotypes with adverse pregnancy outcomes after in-vitro fertilization/intracytoplasmic sperm injection. Front. Endocrinol. 13 , 889029 (2022).
Li, G., Fan, L., Zhang, L., Zhang, W. & Huang, X. Metabolic parameters and perinatal outcomes of gestational diabetes mellitus in women with polycystic ovary syndrome. J. Perinat. Med. 38 , 141–146 (2010); erratum 38 , 343 (2010).
Palomba, S. et al. The risk of a persistent glucose metabolism impairment after gestational diabetes mellitus is increased in patients with polycystic ovary syndrome. Diabetes Care 35 , 861–867 (2012).
Foroozanfard, F., Moosavi, S. G., Mansouri, F. & Bazarganipour, F. Obstetric and neonatal outcome in PCOS with gestational diabetes mellitus. J. Fam. Reprod. Health 8 , 7–12 (2014).
Google Scholar
Aktun, H. L., Yorgunlar, B., Acet, M., Aygun, B. K. & Karaca, N. The effects of polycystic ovary syndrome on gestational diabetes mellitus. Gynecol. Endocrinol. 32 , 139–142 (2016).
Haakova, L. et al. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum. Reprod. 18 , 1438–1441 (2003).
Falbo, A. et al. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): relationships with adverse outcomes. J. Ovarian Res. 3 , (no pagination) (2010).
Palomba, S. et al. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil. Steril. 94 , 1805–1811 (2010).
Nouh, A. A. & Shalaby, S. M. The predictive value of uterine blood flow in detecting the risk of adverse pregnancy outcome in patients with polycystic ovary syndrome. Middle East Fertil. Soc. J. 16 , 284–290 (2011).
Mehrabian, F. & Kelishadi, R. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J. Res. Med. Sci. 17 , 207–211 (2012).
Wang, Y. et al. Risks for gestational diabetes mellitus and pregnancy-induced hypertension are increased in polycystic ovary syndrome. Biomed. Res. Int. 2013 , 182582 (2013).
Article ADS PubMed PubMed Central Google Scholar
Palomba, S. et al. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J. Clin. Endocrinol. Metab. 99 , 2942–2951 (2014).
Palomba, S. et al. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. Steroids 88 , 36–43 (2014).
Sawada, M. et al. Pregnancy complications and glucose intolerance in women with polycystic ovary syndrome. Endocr. J. 62 , 1017–1023 (2015).
Zheng, W. et al. Early pregnancy metabolic factors associated with gestational diabetes mellitus in normal-weight women with polycystic ovary syndrome: a two-phase cohort study. Diabetol. Metab. Syndr. 11 , (no pagination) (2019).
Fridstrom, M., Nisell, H., Sjoblom, P. & Hillensjo, T. Are women with polycystic ovary syndrome at an increased risk of pregnancy-induced hypertension and/or preeclampsia? Hypertens Pregnancy 18 , 73–80 (1999).
Vollenhoven, B., Clark, S., Kovacs, G., Burger, H. & Healy, D. Prevalence of gestational diabetes mellitus in polycystic ovarian syndrome (PCOS) patients pregnant after ovulation induction with gonadotrophins. Aust. N. Z. J. Obstet. Gynaecol. 40 , 54–58 (2000).
Sir-Petermann, T. et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum. Reprod. 20 , 2122–2126 (2005).
Reyes-Munoz, E. et al. The risk of gestational diabetes mellitus among Mexican women with a history of infertility and polycystic ovary syndrome. Fertil. Steril. 97 , 1467–1471 (2012).
Elkholi, D. G. E. Y. & Nagy, H. M. The effects of adipocytokines on the endocrino-metabolic features and obstetric outcome in pregnant obese women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 19 , 293–302 (2014).
Diboun, I. et al. Metabolomic profiling of pregnancies with polycystic ovary syndrome identifies a unique metabolic signature and potential predictive biomarkers of low birth weight. Front. Endocrinol. (Lausanne) 12 , 638727 (2021).
Palomba, S. et al. Uterine blood flow in pregnant patients with polycystic ovary syndrome: relationships with clinical outcomes. BJOG 117 , 711–721 (2010).
Lesser, K. B. & Garcia, F. A. Association between polycystic ovary syndrome and glucose intolerance during pregnancy. J. Matern. Fetal Med. 6 , 303–307 (1997).
CAS PubMed Google Scholar
Urman, B., Sarac, E., Dogan, L. & Gurgan, T. Pregnancy in infertile PCOD patients. Complications and outcome. J. Reprod. Med. 42 , 501–505 (1997).
Mikola, M., Hiilesmaa, V., Halttunen, M., Suhonen, L. & Tiitinen, A. Obstetric outcome in women with polycystic ovarian syndrome. Hum. Reprod. 16 , 226–229 (2001).
Bjercke, S. et al. Impact of insulin resistance on pregnancy complications and outcome in women with polycystic ovary syndrome. Gynecol. Obstet. Investig. 54 , 94–98 (2002).
Article CAS Google Scholar
Turhan, N. O., Seckin, N. C., Aybar, F. & Inegol, I. Assessment of glucose tolerance and pregnancy outcome of polycystic ovary patients. Int. J. Gynaecol. Obstet. 81 , 163–168 (2003).
Al-Ojaimi, E. H. Pregnancy outcomes after laparoscopic ovarian drilling in women with polycystic ovarian syndrome. Saudi Med. J. 27 , 519–525 (2006).
Maliqueo, M. et al. Metabolic parameters in cord blood of newborns of women with polycystic ovary syndrome. Fertil. Steril. 92 , 277–282 (2009).
Anderson, H. et al. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J. Clin. Endocrinol. Metab. 95 , 2180–2186 (2010).
Dmitrovic, R., Katcher, H. I., Kunselman, A. R. & Legro, R. S. Continuous glucose monitoring during pregnancy in women with polycystic ovary syndrome. Obstet. Gynecol. 118 , 878–885 (2011).
Boutzios, G. et al. Polycystic ovary syndrome offspring display increased oxidative stress markers comparable to gestational diabetes offspring. Fertil. Steril. 99 , 943–950 (2013).
Koster, M. P. et al. Placental characteristics in women with polycystic ovary syndrome. Hum. Reprod. 30 , 2829–2837 (2015).
Xiao, Q., Cui, Y. Y., Lu, J., Zhang, G. Z. & Zeng, F. L. Risk for gestational diabetes mellitus and adverse birth outcomes in Chinese women with polycystic ovary syndrome. Int. J. Endocrinol. 2016 , 5787104 (2016).
Kent, J. et al. Gestational weight gain in women with polycystic ovary syndrome: a controlled study. J. Clin. Endocrinol. Metab. 103 , 4315–4323 (2018).
Li, Y. et al. Comparing the risk of adverse pregnancy outcomes of Chinese patients with polycystic ovary syndrome with and without antiandrogenic pretreatment. Fertil. Steril. 109 , 720–727 (2018).
Foroozanfard, F. et al. Comparing pregnancy, childbirth, and neonatal outcomes in women with different phenotypes of polycystic ovary syndrome and healthy women: a prospective cohort study. Gynecol. Endocrinol. 36 , 61–65 (2020).
Jiang, L. et al. Associations between sex hormone levels and autistic traits in infertile patients with polycystic ovary syndrome and their offspring. Front. Endocrinol. 12 , 789395 (2021).
Kaing, A. et al. Highly elevated level of antiMullerian hormone associated with preterm delivery in polycystic ovary syndrome patients who underwent ovulation induction. Fertil. Steril. 115 , 438–446 (2021).
Kollmann, M. et al. Article vitamin D concentrations at term do not differ in newborns and their mothers with and without polycystic ovary syndrome. J. Clin. Med. 10 , 1–9 (2021).
Liu, Y. et al. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet. 38 , 471–477 (2021).
Liu, Q., Wang, J., Xu, Q., Kong, L. & Wang, J. A retrospective cohort study of obstetric complications and birth outcomes in women with polycystic ovarian syndrome. J. Obstet. Gynaecol. 42 , 574–579 (2022).
Stokkeland LMT et al. Changes in serum cytokines throughout pregnancy in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 107 , 39–52 (2022).
Wang, Y. et al. Development of 1–2 years offspring born to mothers with polycystic ovary syndrome. J. Coll. Physicians Surg. Pak. 31 , 1186–1190 (2021).
Yamamoto, M. et al. Risk of preterm delivery in non-diabetic women with polycystic ovarian syndrome. J. Perinatol. 32 , 770–776 (2012).
Naver, K. V. et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG 121 , 575–581 (2014).
Kollmann, M. et al. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum. Reprod. 30 , 2396–2403 (2015).
Mumm, H. et al. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet. Gynecol. Scand. 94 , 204–211 (2015).
de Wilde, M. A. et al. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil. Steril. 108 , 333–340 (2017).
Liu, S. et al. Pregnancy outcomes of women with polycystic ovary syndrome for the first in vitro fertilization treatment: a retrospective cohort study with 7678 patients. Front. Endocrinol. 11 , 575337 (2020).
Tobiasz, A. M., Duncan, J. R., Detti, L. & Mari, G. Lack of fetal insulin resistance in maternal polycystic ovary syndrome. Reprod. Sci. 27 , 1253–1258 (2020).
Chen, M. et al. Systematic oxidative stress is not associated with live birth rate in young non-obese patients with polycystic ovarian syndrome undergoing assisted reproduction cycles: aA prospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 253 , 154–161 (2020).
Diamant, Y. Z., Rimon, E. & Evron, S. High incidence of preeclamptic toxemia in patients with polycystic ovarian disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 14 , 199–204 (1982).
Wortsman, J., de Angeles, S., Futterweit, W., Singh, K. B. & Kaufmann, R. C. Gestational diabetes and neonatal macrosomia in the polycystic ovary syndrome. J. Reprod. Med. 36 , 659–661 (1991).
Tu, M. et al. Effect of lncRNA MALAT1 on the granulosa cell proliferation and pregnancy outcome in patients with PCOS. Front Endocrinol. 13 , 825431 (2022).
Kollmann, M. et al. Androgen and anti-Mullerian hormone concentrations at term in newborns and their mothers with and without polycystic ovary syndrome. J. Clin. Med. 8 , (no pagination) (2019).
Fall, C. H. et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration). Lancet Glob. Health 3 , e366–e377 (2015).
Harrer, M., Cuijpers, P., Furukawa, T. A. & Ebert, D. D. Doing Meta-Analysis With R: A Hands-On Guide , 1st edn (Chapman & Hall/CRC Press, 2021).
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health 7 , e37–e46 (2019).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371 , 75–84 (2008).
Aboeldalyl, S. et al. The role of chronic inflammation in polycystic ovarian syndrome-a systematic review and meta-analysis. Int. J. Mol. Sci. 22 , 2734 (2021).
Teede, H. J. et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring) 21 , 1526–1532 (2013).
Blencowe, H. et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob. Health 7 , e849–e860 (2019).
Schlaudecker, E. P. et al. Small for gestational age: case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine 35 , 6518–6528 (2017).
Ciobanu, A., Khan, N., Syngelaki, A., Akolekar, R. & Nicolaides, K. H. Routine ultrasound at 32 vs 36 weeks’ gestation: prediction of small-for-gestational-age neonates. Ultrasound Obstet. Gynecol. 53 , 761–768 (2019).
Koyanagi, A. et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet 381 , 476–483 (2013).
Funai, E. F. et al. Risk factors for pre-eclampsia in nulliparous and parous women: the Jerusalem Perinatal Study. Paediatr. Perinat. Epidemiol. 19 , 59–68 (2005).
Ananth, C. V. et al. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension 74 , 1089–1095 (2019).
Herrett, E. et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int. J. Epidemiol. 44 , 827–836 (2015).
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81 , 19–25 (2004).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283 , 2008–2012 (2000).
Bahri Khomami, M. et al. Clinical management of pregnancy in women with polycystic ovary syndrome: an expert opinion. Clin. Endocrinol. 97 , 227–236 (2022).
Wells, G. A. et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2014).
Deeks, J. J. H. J. & Altman, D. G. (eds). Analysing Data and Undertaking Meta-analyses Ch. 10. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (eds Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J. & Welch, V. A.) (Cochrane, 2022).
Brand, K. M. G. et al. Metformin in pregnancy and risk of adverse long-term outcomes: a register-based cohort study. BMJ Open Diabetes Res. Care 10 , e002363 (2022).
Kominiarek, M. A. Preparing for and managing a pregnancy after bariatric surgery. Semin. Perinatol. 35 , 356–361 (2011).
Hedges, L. V. & Olkin, I. Statistical Methods for Meta-analysis (Academic Press, 2014).
Guyatt, G. H. et al. Going from evidence to recommendations. BMJ 336 , 1049–1051 (2008).
The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations (The GRADE Working Group, 2013).
Seide, S. E., Röver, C. & Friede, T. Likelihood-based random-effects meta-analysis with few studies: empirical and simulation studies. BMC Med. Res. Methodol. 19 , 16 (2019).
Download references
Acknowledgements
We extend our gratitude to the colleagues who contributed to the initial systematic review published in 2019. While these collaborators were not directly involved in this update, their contributions to the foundational work have been instrumental in shaping the direction and scope of the current study. This work is supported by the Australian National Health and Medical Research Council (NHMRC) funded Centre for Research Excellence in Women’s Health in Reproductive Life (CRE-WHiRL) [APP#1171592] (H.J.T.). The funder of the study had no role in study design, data extraction, data analysis, data interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Author information
Authors and affiliations.
Faculty of Medicine, Nursing and Health Sciences, Monash Centre for Health Research and Implementation, Monash University, Melbourne, Australia
Mahnaz Bahri Khomami, Soulmaz Shorakae, Cheryce L. Harrison, Chau Thien Tay, Aya Mousa & Helena J. Teede
Brock University, St. Catharines, ON, Canada
Somayeh Hashemi
Endocrinology and Diabetes Units, Monash Health, Melbourne, VIC, Australia
Cheryce L. Harrison & Helena J. Teede
Department of Obstetrics and Gynecology, Research Unit of Clinical Medicine, Medical Research Center, Oulu University Hospital, University of Oulu, Oulu, Finland
Terhi T. Piltonen
Department of Woman and Child Health and Public Health, Woman Health Area, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy
Daniela Romualdi
Department of Clinical and Molecular Medicine, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, Trondheim, Norway
Eszter Vanky
Department of Obstetrics and Gynecology, St. Olav’s Hospital, Trondheim University Hospital, Trondheim, Norway
You can also search for this author in PubMed Google Scholar
Contributions
M.B.K. contributed to the concept and design, and performed the systematic search and statistical analysis. M.B.K., S.S., and S.H. performed screening, data extraction, quality appraisal, and drafting of the paper. H.J.T. obtained funding and led the overarching guideline process. C.T.T. and A.M. were evidence synthesis leads for the guidelines included in this study. E.V. was the clinical expert lead in the guideline committee. M.B.K., S.S., S.H., C.H.L., T.T.P., D.R., C.T.T., H.J.T., E.V., and A.M. contributed to the concept and design and provided substantial contributions to the drafting of the work, including critical revision for important intellectual content. M.B.K., S.S., S.H., C.H.L., T.T.P., D.R., C.T.T., H.J.T., E.V., and A.M. had full access to the data, were responsible for study integrity, and approved the final version of the manuscript for publication.
Corresponding author
Correspondence to Mahnaz Bahri Khomami .
Ethics declarations
Competing interests.
T.P. is supported by Novo Nordisk and Sigrid Jusélius Foundation. E.V. is supported by Novo Nordisk and Merck as a lecturer and advisor for clinical studies. H.J.T. and A.M. are supported by NHMRC fellowships [APP#2009326 and APP#1161871, respectively]. The remaining authors declare no competing interests.
Inclusion and ethics
All researchers involved in the study are listed as authors or acknowledged, as appropriate.
Peer review
Peer review information.
Nature Communications thanks Dan Zhang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bahri Khomami, M., Hashemi, S., Shorakae, S. et al. Systematic review and meta-analysis of birth outcomes in women with polycystic ovary syndrome. Nat Commun 15 , 5592 (2024). https://doi.org/10.1038/s41467-024-49752-6
Download citation
Received : 03 December 2023
Accepted : 18 June 2024
Published : 04 July 2024
DOI : https://doi.org/10.1038/s41467-024-49752-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Education
I would like to explain to you about your diagnosis of Polycystic Ovary Syndrome along with some of the symptoms you are experiencing.
What is Polycystic Ovary Syndrome? Polycystic ovary syndrome (PCOS) is a hormonal disorder common among women of reproductive age. Women with PCOS may experience infrequent or prolonged menstrual cycles, an excess level of male hormone androgen, which can cause excessive facial and body hair (hirsutism) and occasionally severe acne or male pattern baldness. The exact cause of PCOS is unknown, certain genes are thought to be linked to PCOS along with hereditary gene traits. This can cause the weight gain along with excess hair growth, acne, and your delayed menstrual cycle.
How do I know if I need to be concerned with this? Qualities that play a role in PCOS can include excess insulin, a hormone which is produced to use sugar as your primary energy source. Normally insulin is a hormone made by your pancreas and controls your blood glucose levels in your body. With this disease, your body cells can become resistant to insulin and this can interfere with the way insulin controls your blood sugar levels. With insulin resistance, your bodies insulin is less likely to be able to lower your extra blood sugar levels. The extra insulin may increase androgen hormone production which can make ovulation more difficult. With the extra male androgen hormone, you may notice more facial or body hair that mimics male patterns, along with more acne. Complications that can arise with PCOS is infertility, along with problems during pregnancy such as gestational diabetes, pregnancy-induced high blood pressure, miscarriages or premature births. Other complications with PCOS can include obesity, type 2 diabetes, metabolic disorder, sleep apnea, depression, anxiety, and eating disorders. Obesity complicates PCOS and makes your symptoms worse.
How is it diagnosed? There are several tests to aid in the diagnosis of PCOS. They include a pelvic exam and blood test to monitor your hormones, lipid panels and glucose tolerance levels along with an ultrasound of your ovaries which looks for enlargement and cysts. Having you monitor your menstrual periods and any weight changes, acne or excess hair growth can be important for your doctor to review.
What is the treatment, can it be managed ? The treatment for PCOS focus on managing your individual concerns, such as having excessive hair growth, acne, obesity or infertility. Management recommends weight loss and exercise which can improve PCOS and the symptoms of infertility. Your doctor may recommend medications to regulate your menstrual cycle, help you ovulate and reduce excessive hair growth for help with managing the symptoms of PCOS.
Inside Levels
- Metabolic Basics
- Weight Loss
Women's Health
- Mental Health
Metabolic Health

| The Explainer
Using continuous glucose monitoring in people with PCOS
Jennifer Chesak
Reviewed By
Dr. Ami Kapadia
Updated: 07/10/2024
Published: 07/10/2024
Polycystic ovary syndrome (PCOS) is a complex metabolic and reproductive disorder affecting millions of women. Although symptoms vary by person, PCOS involves some combination of hyperandrogenism, ovulatory dysfunction, and polycystic ovaries on imaging. The condition can be painful and cause a host of symptoms and complications that affect quality of life.
Symptoms and associated conditions include acne , hirsutism (male-pattern facial and body hair distribution in women), obesity , Type 2 diabetes , non-alcoholic fatty liver disease , menstrual cycle dysfunction including anovulation (not ovulating), and depression and anxiety . PCOS is also a leading cause of infertility .
Somewhere between 6% and 21% of women globally have PCOS, making it one of the most common endocrine conditions affecting people assigned female at birth in their reproductive years. However, the World Health Organization reports that up to 70% of people with PCOS may be undiagnosed.
PCOS is deeply connected to metabolic health. The hormone dysregulation that occurs in PCOS impacts insulin, a core metabolic hormone, and the relationship is bidirectional: poor metabolic health can worsen PCOS, and PCOS can lead to metabolic dysfunction.
Because of these connections, continuous glucose monitoring (CGM) has been used frequently in PCOS research over the past decade. It’s a tool that can help those with PCOS keep their glucose (blood sugar) levels more stable, which can help manage and reduce PCOS symptoms .
The Link Between PCOS and Insulin Resistance
Although PCOS has different phenotypes, insulin resistance is a common characteristic among all phenotypes. Up to 95% of people with PCOS also have insulin resistance and hyperinsulinemia (high insulin levels), according to a review in the Journal of Ovarian Research .
Insulin is a hormone produced in the pancreas. When glucose enters the bloodstream, the pancreas releases insulin, which signals cells to uptake glucose. Frequent blood sugar spikes and chronically high blood sugar lead to high insulin levels, which can make cells resistant to insulin’s signaling, known as insulin resistance. The result is high blood sugar and high insulin levels.
The high insulin levels caused by insulin resistance stimulate the ovaries’ theca cells, which have insulin receptors, to produce more androgens (e.g., testosterone). Hyperandrogenism (high androgen levels) is another major characteristic of PCOS. Insulin can also encourage the production of more theca cells, which can cause even higher androgen levels.
High androgen levels drive the symptoms of PCOS , but high androgens can also further exacerbate insulin resistance, creating a vicious cycle. Androgens can also cause a redistribution of fat to the belly. An increase in fat cells can further worsen insulin resistance.
Elevated blood sugar levels, insulin resistance and PCOS are all intertwined. Keeping blood sugar more stable often helps people with PCOS manage and mitigate symptoms, including acne , obesity , and infertility .
Continuous Glucose Monitoring (CGM) Technology
CGM allows people to monitor their glucose levels in real time to see how their diet and habits impact their blood sugar. Users place a small sensor, about the diameter of a quarter, on their body, usually on their upper arm. The sensor has a tiny filament that painlessly enters the interstitial fluid just under the skin, where it measures glucose. This sensor sends a signal to their smartphone or handheld monitoring device, where they can read their glucose levels.
Bio-individuality means the same foods can affect people differently. Lifestyle factors, like sleep , stress , and physical activity , also affect our glucose levels. That’s why the real-time feedback of a CGM is the best way to connect your lifestyle choices with your blood sugar patterns, and identify the foods and habits that keep your blood sugar stable.
If you have PCOS, maintaining stable blood sugar with the help of a CGM can reduce surges of insulin that contribute to insulin resistance, and ultimately help manage symptoms.
Case Studies and Research Findings
We don’t yet have large-scale studies on the use of CGM for people with PCOS. However, clinical trials are underway. Smaller, older studies show the potential benefit of CGM use.
One study from 2012 , for example, involved 28 women with PCOS and hyperandrogenism and 25 with PCOS but without hyperandrogenism. All participants wore a CGM for 72 hours. The researchers found that the participants with hyperandrogenism had higher minimum and mean glucose readings than those with normal androgen levels. The researchers concluded that CGM may help identify people with PCOS who are at risk for developing Type 2 diabetes.
Other studies show how keeping blood sugar more stable by eating a metabolically friendly diet can benefit people with PCOS.
- One 2013 study featuring 21 people with PCOS demonstrated that a low-glycemic index diet for 12 weeks improved insulin sensitivity in people with PCOS. Such a diet prioritizes foods that won’t rapidly spike blood sugar.
- A 2019 study of 28 people with PCOS and 34 without it demonstrated that a six-month low-glycemic diet produced a significant reduction in total testosteron e in women with PCOS. Participants with PCOS also experienced an increase in sex hormone binding globulin (SHBG). Low SHBG can further contribute to high androgen levels, exacerbating PCOS symptoms . The participants with PCOS on the low-glycemic index diet also saw improvements in acne and hirsutism issues, and 80% experienced an improvement in menstrual regularity.
- A 2020 study put 14 people with PCOS who were also overweight on a ketogenic Mediterranean diet for three months. They experienced a significant decrease in glucose and insulin levels and improvements in insulin sensitivity. Their triglycerides and cholesterol levels also improved, their testosterone levels decreased, and their SHBG increased.
Although we need more studies to show how CGM may help people with PCOS, some women are taking charge of their health and using one. These Levels members shared their PCOS and CGM success stories:
- Abby O’Connor uses CGM to keep tabs on her nutrition, improve energy, and ease PCOS symptoms.
- Natalie Ellis , the cofounder of BossBabe, is using CGM to root out sneaky, seemingly healthy foods that can spike blood sugar and to prepare her body for starting a family.
- Edie Horstman was able to get her PCOS in remission and used a CGM to help create strategies for maintaining stable blood sugar and improve her sleep.
Practical Tips for Using CGM to Manage PCOS
To get a CGM in the United States, you’ll need a prescription from a doctor. In Europe and Canada, you do not. CGMs are traditionally used by people with diabetes. However, you can still ask your physician for a prescription, even if you do not have diabetes. You can also work with Levels or other companies.
With Levels, you’ll answer a questionnaire, which a licensed telehealth physician will review. If you’re eligible, the physician will write you a prescription for CGM. The prescription will be filled by a partnering pharmacy and shipped to your door. Your kit will come with instructions for getting started, such as how to apply the sensor and access your readings. Levels members can also get CGMs without a prescription by participating in our IRB-approved study , which looks to uncover glucose patterns in people without diabetes.
Once you start using your CGM, taking baby steps can be helpful. For example, for the first week, you may just want to practice getting readings and logging your meals and activities without changing your diet. This will give you a chance to see how the usual foods you eat and your usual lifestyle habits are affecting your glucose levels. Then in the second or third week, try implementing different strategies to reduce blood sugar spikes and to keep your blood sugar in the target range as much as possible. The Levels app will provide helpful feedback and insights.
Here are a few simple strategies for optimizing blood sugar control if you have PCOS:
- Dress your carbs. Pairing carbohydrates with healthy fats, protein , fiber , or a combination of these can help slow glucose absorption into your bloodstream. You may also want to experiment with eating the carbs on your plate last .
- Walk after a meal. Going for a walk after eating , ideally up to 30 minutes, can help blunt a blood sugar spike.
- Avoid ultra-processed foods. Stick to whole, nutrient-dense foods as much as possible. Ultra-processed foods are loaded with chemical additives, added sugar, sodium, and more. Their consumption is linked to insulin resistance . Additionally, many ultra-processed foods come in packaging that has endocrine-disrupting chemicals that could further exacerbate PCOS symptoms .
- Build muscle. Muscle tissue is more metabolically healthy than fat tissue. Resistance training improves blood sugar control and helps with body recomposition , which is a reduction in fat mass and an increase in muscle mass.
- Up your protein intake. Higher protein diets have been shown to reduce insulin resistance and insulin levels. Protein is also crucial for building and preserving muscle.
CGM can help people with PCOS monitor their glucose levels and develop nutrition and activity strategies for keeping their blood sugar more stable. In turn, more stable blood sugar could help increase insulin sensitivity and potentially reduce symptoms of PCOS, prevent the development of other chronic conditions like Type 2 diabetes, and help improve menstrual cycle function, which could have benefits for fertility.
More research is needed with clinical trials to show how CGM may be of benefit to people with PCOS, but clinical trials are in the works. Meanwhile, CGM is a non-medication, non-invasive tool that has shown promise in helping and empowering those with the condition.
Interested in trying CGM to manage your metabolic health?
Levels, the health tech company behind this blog, helps people improve their metabolic health by showing how food and lifestyle impact your blood sugar, using continuous glucose monitoring (CGM), along with an app that offers personalized guidance and helps you build healthy habits. Click here to learn more about Levels.
Get updates, new articles, exclusive discounts, and more

Ultimate Guide
The ultimate guide to PCOS and metabolic health
Polycystic ovarian syndrome (PCOS) is a condition affecting fertility, weight, and more—and it's intimately tied to metabolic health.
Monica Karpinski
May 26, 2020
Azure Grant, PhD
The Explainer
The link between PCOS and insulin resistance
PCOS is a metabolic disorder that is intricately tied to insulin resistance. Here's how the two conditions influence each other.
January 6, 2023
Anjali Dsouza, MD

What diet is best for PCOS?
Eating a metabolically friendly diet can have a significant impact on PCOS symptoms. Here's what you need to know.
February 15, 2023

Should people with PCOS consider inositol as a treatment?
This supplement may help stabilize hormone and glucose regulation to improve fertility and alleviate other PCOS symptoms.
Grace Newjik
May 23, 2023

The Latest From Levels

Metabolic Markers
What is the oral glucose tolerance test, and who should get one?
This blood test determines whether your body can use and store glucose normally, a key indicator of metabolic health or dysfunction. Here’s what you need to know and how one doctor uses the test in her practice.
July 3, 2024

About Levels
How to lower the cost of Levels
We know cost can be a barrier to try Levels, but you can get the benefits of Levels for less money, and improve your health for no money at all.
The Levels Team
July 1, 2024
4 mins read

Levels Grocery List
The 10 best low-sugar protein bars
When done right, this convenient snack can fend off hunger while keeping blood sugar stable. Here’s how to shop for them, and 10 of our favorites.
Sharon Liao
June 26, 2024
Zoë Atlas, MPH, RDN

Why food logging matters to metabolic health and how Levels can help
What you eat is your most critical health input, and using Levels’ simple food logging can unlock insights and strategies to reach your goals faster.
June 21, 2024
5 mins read
Sign up for the Levels Newsletter
- Search Menu
- Sign in through your institution
Endocrine Reviews
Endocrinology, journal of the endocrine society.
- The Journal of Clinical Endocrinology & Metabolism
JCEM Case Reports
- Molecular Endocrinology (Archives)
- Author Guidelines
- Author Resources
- Submission Sites
- Why Publish with the Endocrine Society?
- Thematic Issues
- Basic Science Collections of Endocrinology
- About the Endocrine Society
- Editorial Boards
- Reviewer Resources
- Rights & Permissions
- Conferences
- ENDO Meeting Abstracts
- Other Publications
- Become a Member
- Member Access
- Terms and Conditions
- Advertising and Corporate Services
- Reprints, ePrints, Supplements
- Journals on Oxford Academic
- Books on Oxford Academic
An Endocrine Society Thematic Issue: Women’s Health 2024
Read our special collection of journal articles focused on women’s health research! Curation of the collection, based on articles published in 2023 and 2024, was guided by Altmetric Attention Scores, article downloads, and Featured Article designations.
In Journal of the Endocrine Society , Ahmad and coauthors report favorable results from a small study on radiofrequency ablation of cervical metastases of papillary thyroid cancer. Dumesic et al. describe a study of cortisol and testosterone levels in patients with polycystic ovary syndrome (PCOS), finding that reduced cortisol may protect against preferential accumulation of fat in the abdomen in normal-weight women with PCOS and normal serum 11-oxyandrogens. Khalid and colleagues use a meta-analysis to conclude that a short-term ketogenic diet can improve the hormonal imbalances associated with PCOS.
In JCEM Case Reports , Iioka and colleagues describe the case of a pregnant woman with Cushing syndrome whose delivery was complicated by placental abruption and who presented a month later with compression fractures. Walfish and associates discuss a case of hyperthyroidism induced by a molar pregnancy. Bhatia et al. report on an instance of spontaneous remission of generalized postpartum lipodystrophy.
In Endocrine Reviews , Marquadt et al. survey “the ever-growing recent evidence of epigenetic contributions to the pathophysiology of endometriosis” and its relevance to the development of new therapies. Mauro and colleagues review findings that call into question the standard narrative that progesterone is protective in ovarian cancer. Eng and associates examine facts supporting the existence of a “non-PCOS female obesity-related secondary hypogonadism.”
In Endocrinology , a mini-review by Flaherty and coauthors summarizes “current understanding of the epidemiological associations between ovarian hormones and lobular breast cancer,” highlighting mechanistic insights. Bae and associates , in another mini-review, discuss the dissemination of circulating tumor cells that seed metastases in prostate and breast cancer but also offer the possibility of early detection. Hancock et al. review cutting-edge studies on estrogen receptor alpha structural and transcriptional relationships that are being harnessed to produce new therapies for breast cancer patients who carry specific mutations.
JCEM carries a report by Retnakaran and Shah indicating that the glucose challenge test can predict future diabetes in pregnant women. Motevalizadeh and associates provide evidence that parity is associated with worse early-pregnancy insulin resistance, an effect aggravated in women who are overweight. And Frey and colleagues describe how parathyroidectomy significantly improved bone mineral density and remodeling biomarkers in women with osteopenia.
Radiofrequency Ablation of Cervical Thyroid Cancer Metastases—Experience of Endocrinology Practices in the United States

Interplay of Cortisol, Testosterone, and Abdominal Fat Mass in Normal-weight Women With Polycystic Ovary Syndrome

Effects of Ketogenic Diet on Reproductive Hormones in Women With Polycystic Ovary Syndrome

A Rare Case of Placental Abruption and Postpartum Compression Fractures in Pregnancy With Cushing Syndrome

Molar Pregnancy–Induced Hyperthyroidism: The Importance of Early Recognition and Timely Preoperative Management

Spontaneous Remission of Acquired Generalized Lipodystrophy Presenting in the Postpartum Period

Epigenetic Dysregulation in Endometriosis: Implications for Pathophysiology and Therapeutics

Reevaluating the Role of Progesterone in Ovarian Cancer: Is Progesterone Always Protective?

Obesity-Related Hypogonadism in Women

Is There a Special Role for Ovarian Hormones in the Pathogenesis of Lobular Carcinoma?

Dissemination of Circulating Tumor Cells in Breast and Prostate Cancer: Implications for Early Detection

Estrogen Receptor Alpha Mutations, Truncations, Heterodimers, and Therapies

THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
The glucose challenge test in pregnancy identifies future risk of diabetes.

Women with gestational diabetes (GDM) have an increased future risk of type 2 diabetes but, in practice, their recommended postpartum glucose tolerance testing is often missed or substituted with measurement of A1c instead.
Association of Parity With Insulin Resistance Early in Pregnant Women: ECLIPSES Study

Parathyroidectomy Improves Bone Density in Women With Primary Hyperparathyroidism and Preoperative Osteopenia

Interested in Publishing your Women's Health Research?
Discover the benefits of publishing your work with the high-profile, highly cited, and widely read journals of the Endocrine Society.
- Journals Career Network
Affiliations
- Copyright © 2024
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Cyp21a2 intron 2 genetic variants might be associated with the clinical characteristics of women with pcos.

Share and Cite
Robeva, R.; Andonova, S.; Todorov, T.; Feyzullova, A.; Elenkova, A.; Kirilov, G.; Savov, A.; Zacharieva, S.; Todorova, A. CYP21A2 Intron 2 Genetic Variants Might Be Associated with the Clinical Characteristics of Women with PCOS. Biomedicines 2024 , 12 , 1528. https://doi.org/10.3390/biomedicines12071528
Robeva R, Andonova S, Todorov T, Feyzullova A, Elenkova A, Kirilov G, Savov A, Zacharieva S, Todorova A. CYP21A2 Intron 2 Genetic Variants Might Be Associated with the Clinical Characteristics of Women with PCOS. Biomedicines . 2024; 12(7):1528. https://doi.org/10.3390/biomedicines12071528
Robeva, Ralitsa, Silvia Andonova, Tihomir Todorov, Aylin Feyzullova, Atanaska Elenkova, Georgi Kirilov, Alexey Savov, Sabina Zacharieva, and Albena Todorova. 2024. "CYP21A2 Intron 2 Genetic Variants Might Be Associated with the Clinical Characteristics of Women with PCOS" Biomedicines 12, no. 7: 1528. https://doi.org/10.3390/biomedicines12071528
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
Dr. Suzanne Byrd Department of Biological Sciences. Polycystic Ovarian Syndrome (PCOS) is a physiological disorder that causes many negative. effects involving a variety of systems in the body, such as the endocrine, metabolic, psychological, and reproductive systems. This paper will explore the complex mechanisms.
Polycystic ovary syndrome (PCOS) is a highly prevalent disease seen in women of reproductive age, and yet a majority of cases go undiagnosed. This autobiographical case report describes a young doctor's experience with PCOS and attempts to highlight the significance of a missed diagnosis. In addition to the endocrine system, PCOS affects the ...
Patient Info. CM is a 25 yo Hispanic woman, who was referred to the OBGYN department by her PCP after complaining of not having a period for the last six months. She also has been trying to conceive for the last year and a half without success. She has noticed a significant weight gain (60 pounds) over the last few months or so and was ...
CASE HISTORY: The patient is a fourteen-year-old female who presented to the clinic for bilateral hip and lumbar back pain. She stated that the pain has been present for approximately seven months and described it as a deep ache in the low back and both hips anteriorly. The patient said she plays a variety of sports but denies any specific event that could contribute to her pain. She stated ...
Materials and Methods This is a prospective case control study, 550 women recruited (aged 20-40 years), in which 450 PCOS women recruited according to the Rotterdam criteria and 100 non-PCOS ...
Abstract and Figures. Introduction: Polycystic ovarian syndrome (PCOS) is also known as polycystic ovarian disorder (POD). The disease is mainly metabolic (hyper androgenic) one. It usually ...
Polycystic ovary syndrome (PCOS) is the major endocrine related disorder in young age women. Physical appearance, menstrual irregularity as well as infertility are considered as a sole cause of mental distress affecting health-related quality of life (HRQOL). This prospective case-control study was conducted among 100 PCOS and 200 healthy control cases attending tertiary care set up of AIIMS ...
case of PCOS [11]. Many genes participated in etiology of this syndrome but this is not fully investigated yet but the study shows that abnormality of genes in case of PCOS mostly affects the pathways of signal transduction which controls the steroidogenesis (formation of steroids) [12], insulin action [22] and secretion, gonadotropin action
Polycystic o vary syndrome (PCOS) is one of most common female endocrine d isorder t hat affects 6-15% of the female population. Women wit h. PCOS have a hormonal imbalance and metabolism pro ...
Introduction. Polycystic Ovary Syndrome (PCOS) is a multifactorial disorder caused by interactions between genetic, environmental and intrauterine factors. 1 Various studies have reported the prevalence of this syndrome in adolescence as 9% to 15%. 2 - 4 Based on the available evidence, metabolic, inflammatory, oxidative, emotional and psychological stress is an important part of PCOS. 5 ...
PCOS is a multifactorial disorder in which diagnosis, treatment and management is very difficult as its causes and symptoms vary from ... In this report we present a case study of a 20-year-old female diagnosed with PCOS at an early age. The elevated random blood ... ( Pdf, E-pub, Full Text, Audio) • Unceasing customer service Track the below ...
Numerous treatments are offered for women related to PCOS including weight loss, gonadotropins, metformin, DASH diet, letrozole and laparoscopic ovarian diathermy. Case presentation: This case report 35-years-old female presented for evaluation of obesity, menstrual irregularity and amenorrhea, and had no received any kind of hormonal treatment.
A similar study using serum BPA from adolescents has found similar results too. 181 On the contrary, a study focusing on several EDCs failed to establish an association of urinary BPA level with PCOS in relatively older women (18-45 years). 182 There are many other studies available linking specific groups of EDC with PCOS. For instance, two ...
The features of PCOS are disorders of ovulation, androgen excess, polycystic ovaries; it's associated with presence of associated risk factors for cardiovascular disease (obesity, glucose intolerance, dyslipidemia). The diagnosis of PCOS is made using the Rotterdam 2003 criteria. 23yo female patient was evaluated for oligomenorrhea.
Welcome to LSBU Open Research : LSBU Open Research
Case study 2: a woman with polycystic ovary syndrome who is trying to conceive*. Sheila is a 35-year-old patient who would like advice about natural remedies to aid conception. She stopped taking the contraceptive pill a year ago, which had been prescribed to control symptoms of polycystic ovary syndrome (PCOS).
Polycystic ovary syndrome (PCOS) is one of the most common female endocrine disorders. It is recognized by the presence of enlarged ovaries with multiple small cysts and a hypervascularized, androgen secreting stroma. This syndrome is characterized by menstrual abnormalities, infertility, obesity, excess hair growth, acanthosis nigricans and acne.
This article will discuss the clinical presentation, risk assessment and management of polycystic ovary syndrome. Download Free PDF. View PDF. Clinical Case Reports Case Report Volume 10:11, 2020 ISSN: 2165-7920 Open Access Rehabilitation Care of Women with PCOS: A Case Study Hafiza Madiha Jaffar*, Shehnai Basharat, Tara Khursheed, Faiza ...
Abstract and Figures. Polycystic ovary syndrome (PCOS), which affects 5-20% of women in their reproductive age, is the most common endocrinopa-thy affecting women worldwide. It is a condition ...
This systematic review and meta-analysis provides evidence that polycystic ovary syndrome (PCOS) is a risk factor for adverse birth outcomes such as preterm birth, fetal growth restriction and low ...
The cause of PCOS is unknown, although some scientists believe people may be able to inherit the condition. PCOS may put women at risk for diabetes, heart disease, and cancer of the uterus. About 30% of women with PCOS have a problem processing blood sugar called glucose intolerance. This is a major risk factor for adult-onset diabetes.
Polycystic ovary syndrome (PCOS) is a hormonal disorder common among women of reproductive age. Women with PCOS may experience infrequent or prolonged menstrual cycles, an excess level of male hormone androgen, which can cause excessive facial and body hair (hirsutism) and occasionally severe acne or male pattern baldness.
Case Studies and Research Findings. We don't yet have large-scale studies on the use of CGM for people with PCOS. However, clinical trials are underway. Smaller, older studies show the potential benefit of CGM use. One study from 2012, for example, involved 28 women with PCOS and hyperandrogenism and 25 with PCOS but without hyperandrogenism ...
Based on the PICOS framework, we established the following inclusion criteria for the studies: 1) reporting hepcidin means and standard deviations (SDs), or providing sufficient information to calculate these parameters, for both PCOS patients and healthy controls and 2) having an observational design (e.g. cohort, case-control or cross ...
Abstract. Polycystic Ovarian Syndrome (PCOS) is one of the most common problems affecting women. PCOS can affect menstrual cycle, fertility, and hormone level as well as appearance including acne ...
Khalid and colleagues use a meta-analysis to conclude that a short-term ketogenic diet can improve the hormonal imbalances associated with PCOS. In JCEM Case Reports, Iioka and colleagues describe the case of a pregnant woman with Cushing syndrome whose delivery was complicated by placental abruption and who presented a month later with ...
Aims: Pathogenic variants in the CYP21A2 gene are related to the classic and non-classic forms of congenital adrenal hyperplasia (CAH). However, the role of CAH carrier status in the clinical presentation of polycystic ovarian syndrome (PCOS) is still unclear. Moreover, the possible associations of different CYP21A2 gene polymorphisms with metabolic and reproductive abnormalities in PCOS have ...
Here we report a case of an adult woman with PCOS, who has been treated with metformin (1.5 year) and DASH diet (3 months). Case Report. In August 2020, a 35-year-old female presented for ...