Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 05 May 2021

COVID research: a year of scientific milestones
For just over a year of the COVID-19 pandemic, Nature highlighted key papers and preprints to help readers keep up with the flood of coronavirus research. Those highlights are below. For continued coverage of important COVID-19 developments, go to Nature’s news section .
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-020-00502-w
Reprints and permissions
- Medical research
- Epidemiology

How anti-obesity drugs cause nausea: finding offers hope for better drugs
News 10 JUL 24
Repeated plague infections across six generations of Neolithic Farmers
Article 10 JUL 24

Bionic leg moves like a natural limb — without conscious thought
News 01 JUL 24
Alzheimer’s plaques and tangles revealed by 3D microscopy
News & Views 10 JUL 24
A liver immune rheostat regulates CD8 T cell immunity in chronic HBV infection
Vaccines save lives: how can uptake be increased?
Editorial 09 JUL 24
If bird flu sparks a human pandemic, your past immunity could help
News 09 JUL 24
Pathogenicity and transmissibility of bovine H5N1 influenza virus
Article 08 JUL 24
Southeast University Future Technology Institute Recruitment Notice
Professor openings in mechanical engineering, control science and engineering, and integrating emerging interdisciplinary majors
Nanjing, Jiangsu (CN)
Southeast University
Neuroscience Research Assistant/Tech - Manhattan Weill Cornell Medical College
We are seeking motivated and enthusiastic research tech applicants to work on autism mouse models and brain organoids.
New York City, New York (US)
Weill Cornell Medical College
Postdoctoral Fellowship - Graph Database Developer
Postdoctoral Fellowship - Graph Database Developer Organization National Library of Medicine, National Institutes of Health, Bethesda, MD and surro...
Bethesda, Maryland
National Institutes of Health/National Library of Medicine
Postdoctoral Fellow - Boyi Gan lab
New postdoctoral positions are open in a cancer research laboratory located within The University of Texas MD Anderson Cancer Center. The lab curre...
Houston, Texas (US)
The University of Texas MD Anderson Cancer Center - Experimental Radiation Oncology
Senior Research Associates x 3 – Bioinformatician Team
The Genomics and Bioinformatics Core (GBC) within the Institute of Metabolic Science – Metabolic Research Laboratories at the Clinical School, Univers
Cambridge, Cambridgeshire
University of Cambridge
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Introduction
- Conclusions
- Article Information
eTable 1. Point Prevalences of Long COVID-19 Among Individuals Testing Positive for COVID-19 by Antigen Test or PCR, and Among All Surveyed US Adults, Weighted to Reflect US Adult Population
eFigure 1. Logistic Regression Model for Development of Long COVID-19 Among Individuals Testing Positive for COVID-19 by Antigen Test or PCR, With Age by Decade
eTable 2. Long COVID-19 Symptom Frequencies by Predominant Variant at Time of Initial Illness
eTable 3. Characteristics of Individuals Who Tested Positive for COVID-19 by Antigen Test or PCR at Least Two Months Prior to Survey Date, Who Did or Did Not Experience Long COVID-19 Symptoms by More Stringent Definition
eFigure 2. Logistic Regression Model for Development of Long COVID-19 Among Individuals Testing Positive for COVID-19 by Antigen Test or PCR, With Age by Decade, Limited to Individuals With Index Infection January 2021 or Later
eFigure 3. Logistic Regression Model for Development of More Stringently Defined Long COVID-19 Among Individuals Testing Positive for COVID-19 by Antigen Test or PCR
eFigure 4. Logistic Regression Model for Development of Long COVID-19 by More Stringent Definition Among Individuals Testing Positive for COVID-19 by Antigen Test or PCR, Including Predominant Variant and Vaccination Status
- Long COVID Linked With Unemployment in New Analysis JAMA News From the JAMA Network March 7, 2023 This Medical News article discusses new research on the association between long COVID and employment status. Melissa Suran, PhD, MSJ
- Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection JAMA Original Investigation June 13, 2023 This study aims to develop a definition of postacute sequelae of SARS-CoV-2 infection (PASC) based on self-reported symptoms and describe PASC frequencies across cohorts, vaccination status, and number of infections using a cohort of adults with and without SARS-CoV-2 infection. Tanayott Thaweethai, PhD; Sarah E. Jolley, MD, MS; Elizabeth W. Karlson, MD, MS; Emily B. Levitan, ScD; Bruce Levy, MD; Grace A. McComsey, MD; Lisa McCorkell, MPP; Girish N. Nadkarni, MD, MPH; Sairam Parthasarathy, MD; Upinder Singh, MD; Tiffany A. Walker, MD; Caitlin A. Selvaggi, MS; Daniel J. Shinnick, MS; Carolin C. M. Schulte, PhD; Rachel Atchley-Challenner, PhD; Leora I. Horwitz, MD; Andrea S. Foulkes, ScD; RECOVER Consortium Authors; RECOVER Consortium; Judith A. Aberg; Natalie L. Adolphi; Shreya Ahirwar; Shifa Ahmed; Neera Ahuja; Masanori Aikawa; Almary Akerlundh; Teresa T. Akintonwa; Aseel Al-Jadiri; Natalya Alekhina; Heather A. Algren; Akram N. Alshawabkeh; Nariman Ammar; Adit Anand; Brett R. Anderson; Lisa Aponte-Soto; Judy L. Aschner; Mary M. Atha; Andrew M. Atz; Robin L. Aupperle; Mirna Ayache; Eduardo Azziz-Baumgartner; L. C. Bailey; Fiona C. Baker; Venkataraman Balaraman; Jennifer A. Bandy; Dithi Banerjee; Deanna M. Barch; James M. Bardes; Jackson Barlocker; R. G. Barr; Arielle Baskin-Sommers; Sanjib Basu; Tracy A. Battaglia; Leah Baucom; Carmen J. Beamon; Casey L. Beaty; Noam D. Beckmann; Jasmine A. Berry; Nahid Bhadelia; Daksh Bhargava; Sultana Bhuiyan; Jiang Bian; Christian Bime; James M. Bjork; Lora J. Black; Catherine A. Blish; Jason P. Block; Amanda Bogie; Dawn Bolliger; William Bonaventura; Seuli Bose-Brill; Mary B. Bower; Andrew D. Boyd; Jerusha Boyineni; Steven B. Bradfute; Carolyn T. Bramante; M. D. Brannock; J. D. Bremner; Shari B. Brosnahan; Natalie C. Buchbinder; Elliott Bueler; Irina A. Buhimschi; Hulya Bukulmez; H. T. Bunnell; John B. Buse; Elizabeth A. Calhoun; Tingyi Cao; Michael D. Carrithers; Thomas W. Carton; Abigail Case; B.J. Casey; Faye Victoria C. Casimero; Lauren Castro; Teresa Cato; Patricia Ceger; Connie L. Cerullo; Linda Chang; Arunee A. Chang; Praneeth Chebrolu; Yong Chen; Li Qing Chen; Benjamin K. Chen; David Chestek; Robert F. Chew; Deena J. Chisolm; Dominic C. Chow; Maryanne R. Chrisant; Dimitri A. Christakis; Christopher G. Chute; Mine S. Cicek; Cheryl R. Clark; Duncan B. Clark; Geoffrey D. Clarke; Katharine N. Clouser; Thomas J. Connors; Judith A. Cook; Krista Coombs; Carlos Cordon-Cardo; Julie L. Costello; Lesley Cottrell; Kelly Cowan; Lindsay G. Cowell; Savannah Cranford; Jamie Cronin; Mollie R. Cummins; Hannah L. Curry; Viren D'Sa; Sean G. Dabney; Casey L. Daniel; Mirella Dapretto; Dawood Darbar; Paul M. Darden; Raktima Dasgupta; Soham Dasgupta; Felicia Davis Blakley; Katherine Dea; Sara J. Deakyne Davies; Lauren A. Decker; Ralph A. DeFronzo; Walter Dehority; Amelia N. Deitchman; James del Alcazar; Phoebe Del Boccio; Carlos del Rio; Marina Del Rios; Julie A. DeLisa; Sean C. Deoni; Maya Z. Diaz; John D. Dickinson; Audrey Dionne; Kathleen R. Diviak; Sarah E. Donohue; Michael J. Downey; Allen J. Dozor; Benard P. Dreyer; Kirsten B. Dummer; Matthew S. Durstenfeld; Mark S. Dworkin; Sherrie L. Edmonds; Matthew D. Elias; Jamie Elifritz; Evan Ellingworth; Amy J. Elliott; Angela M. Ellison; Mike L. Enger; Joaquin M. Espinosa; Shari Esquenazi-Karonika; Michael D. Evans; Danielle N. Evans; Julio C. Facelli; Camila S. Fang; E. Vincent S. Faustino; Maria E. Fayad Lara; Candace H. Feldman; Alexander G. Fiks; Rebecca Fineman; Aloke V. Finn; Melinda S. Fischer; Megan L. Fitzgerald; Valerie J. Flaherman; Thomas J. Flotte; Daniel Forsha; Meghan R. Fortune; John J. Foxe; Nicole Franks; Michael B. Freedman; Catherine E. Freeland; Naomi P. Friedman; Greta Fry; Margot Gage Witvliet; Emily J. Gallagher; Richard Gallagher; Hugh Garavan; Sunanda M. Gaur; Dylan G. Gee; Maria Laura Gennaro; Lynn B. Gerald; Saikat B. Ghosh; Joseph T. Giacino; Andrew T. Girvin; Stephanie L. Godfrey; Mark P. Goldberg; Steven N. Goodman; Howard S. Gordon; Ramkiran Gouripeddi; Paige Graham; Joey P. Granger; Kevin M. Gray; Tina Greimes; Rachel S. Gross; Nicholas Guthe; Evan Gutter; Stephanie Haasnoot; Emily C. Hadley; Melissa A. Haendel; Stephanie Hafner; Katia C. Halabi; Patrick C. Hanley; Ashraf S. Harahsheh; Michelle S. Harkins; Kimberly L. Hartwig; Keren Hasbani; Sharon Hasek; Kristine S. Hauser; Andrew C. Heath; Camden L. Hebson; Mary M. Heitzeg; Monica Hendrickson; Timothy J. Henrich; Alfonso C. Hernandez-Romieu; Christina M. Hester; Miranda Higginbotham; Sophia Hill; Kathryn Hirabayashi; On Ho; Laura Hobart-Porter; M. C. Hoffman; Maryann Holtcamp; Travis K. Hong; Benjamin D. Horne; Carol R. Horowitz; Daniel S. Hsia; Harvey Hsu; Priscilla Y. Hsue; Matthew Huentelman; Bryan E. Huerta; Jared D. Huling; Kathy Hummel; William G. Iacono; Alejandra L. Ibanez; Carmel Ibeawuchi; Katherine Irby; Nahed Ismail; Joanna Jacobus; Vanessa L. Jacoby; Leonard A. Jason; Vidhi Javia; Kyle J. Jennette; Terry L. Jernigan; Sihang Jiang; Naimin Jing; Jace D. Johnny; Nadia Johnson; Brandi Johnson; Brandon Johnson; Pei-Ni Jone; Suzanne E. Judd; Joy J. Juskowich; Edmond K. Kabagambe; David C. Kaelber; Diane G. Kanjilal; Mayank M. Kansal; Tyler J. Kasmarcak; Daniel Kaufman; Rika Kawakami; Dean L. Kellogg; Denise A. Kent; Deepshikha Kewlani; Dhruv Khullar; Keri S. Kim; Arthur Y. Kim; Patricia A. Kinser; Lawrence C. Kleinman; Elizabeth B. Klerman; Matthew J. Kluko; Stacey Knight; Misaki Kobayashi; Karla J. Kopp; Michael Koropsak; Jessica S. Kosut; Ellen Kraig; Amanda Krausert; Ana C. Krieger; Hema Krishna; Aparna Krishnamoorthy; Sankaran S. Krishnan; James P. Lahs; Angela R. Laird; Victoria Laleau; Michelle F. Lamendola-Essel; Jeremy Landeo-Gutierrez; Sean M. Lang; Christine L. Larson; James P. Lash; Jessica Lasky-Su; Gregory Laynor; Simon Lee; Grace M. Lee; F. E. Lee; Matthew A. Lee; Peter J. Leese; R. C. Lefebvre; Angelica J. Levreault; Kennedy C. Lewis; Lu Li; Peter Paul C. Lim; Janet Y. Lin; Krista M. Lisdahl; Matthew B. Litvack; Xiaokang Liu; Jennifer Lloyd; Jennifer K. Logue; Johanna J. Loomba; Vitaly Lorman; Yiwen Lu; Katelyn R. Ludwig; Allison M. Lutz; Jeri Mack; Abeer M. Mahmoud; Cheryl L. Maier; Shahdi K. Malakooti; Sergey Malchenko; Gail L. Mallett; Gabrielle Maranga; Megan W. Martel; Susanne P. Martin-Herz; Maria Martinez-Lage; Christine Maughan; Cammeo Mauntel-Medici; Heidi T. May; Kenneth H. Mayer; Diego R. Mazzotti; Caitryn M. McCallum; Brian W. McCrindle; Russell J. McCulloh; Dylan McDonald; Stephanie McInnis; Julie A. McMurry; Asuncion Mejias; Jonathan Melamed; Martha Menchaca; Alan L. Mendelsohn; Lucio Miele; Mitchell G. Miglis; Cris Milne; Joshua D. Milner; Roger A. Mitchell; Murray A. Mittleman; Sindhu Mohandas; Jonathan G. Monteiro; Vanessa Monzon; David J. Moreno; Lerraughn M. Morgan; Dmitry Morozyuk; Keith E. Morse; Erick Moyneur; Praveen C. Mudumbi; Eva M. Müller-Oehring; Naoko Muramatsu; Hugh Musick; Kshema Nagavedu; Erica R. Nahin; Anoop M. Nambiar; Benjamin H. Natelson; Michael C. Neale; Manette Ness-Cochinwala; Jane W. Newburger; Lisa T. Newman; Amber N. Nguyen; Lauren Nichols; Sheila M. Nolan; Seth Noland; Richard M. Novak; George T. O'Connor; John J. O'Keefe; Princess U. Ogbogu; Carlos R. Oliveira; Matthew E. Oster; Robert F. Padera; Henry H. Paik; Nathan M. Pajor; Huaqin Helen Pan; Deepti Pant; Andrew Partridge; Payal B. Patel; Saaya Patel; Khushboo Patel; Martin P. Paulus; Ronald M. Payne; Ann Pearman; Myriam Peralta-Carcelen; Nicolas L. Perez; Emily R. Pfaff; De-Ann M. Pillers; Chloe E. Pitsch; Neil H. Pliskin; Michael A. Portman; Radu Postelnicu; Jennifer S. Potter; Bellur S. Prabhakar; Priya Prahalad; Bharati Prasad; Barbara Predki; Alexander J. Preiss; Heather M. Prendergast; Davin K. Quinn; Yuri Quintana; Dustin J. Rabideau; Jonathan M. Radosta; Jeffrey Radwell; Hengameh Raissy; Ramaswamy Ramchandran; Isabelle Randall; Suchitra Rao; Amy Rapkiewicz; Sonja A. Rasmussen; Hanieh Razzaghi; Candida J. Rebello; Paulina A. Rebolledo; Neha V. Reddy; Unma M. Reddy; Cara Reedy; Jalees Rehman; R. R. Reichard; Kayleigh M. Reid; Jane E. Reusch; Kyung E. Rhee; Mary B. Rice; John-Ross R. Rizzo; Nadia R. Roan; Polly Robarts; Timothy R. Roberts; Nitza Rochez; Kathleen E. Rodgers; Colin M. Rogerson; Maria E. Romero; Johana M. Rosas; Erika B. Rosenzweig; Russell L. Rothman; Nadine G. Rouphael; Mark W. Russell; Melissa Rutherfoord; Arash A. Sabati; Samer B. Sader; Marzieh Salehi; Amy L. Salisbury; Barbara A. Sampson; Yamuna Sanil; Alice I. Sato; Sharon H. Saydah; Michael S. Schechter; Edward J. Schenck; Katherine I. Schlepphorst; Julia Schuchard; Tina L. Schuh; Jennifer A. Sculley; Alan C. Seifert; Anisha K. Sekar; Rangaraj Selvarangan; Sudha Seshadri; Howard D. Sesso; Dimpy P. Shah; Divya Shakti; Nancy L. Shapiro; Suchetha Sharma; Kumar Sharma; Shubhi Sharma; Kavita Sharma; Gwendolyn Shaw; Eyal Shemesh; Yishan Shen; Elizabeth Shenkman; Stephanie Shiau; Michelle Siciliano; Aylin Simsir; Veronica E. Smith; Jessica N. Snowden; Isaac H. Solomon; Leslie A. Spikes; Lindsay M. Squeglia; Shubhika Srivastava; Mary L. St. Jean; Kenneth A. Stapleford; Cheryl R. Stein; Michelle D. Stevenson; Sarah A. Stewart de Ramirez; Lauren E. Stiles; Aryeh Stock; Melissa S. Stockwell; James R. Stone; Jeran Stratford; Til Stürmer; Vignesh Subbian; Jun Sun; Mehul S. Suthar; Ashley Sylvera; David M. Systrom; Jacqueline Szmuszkovicz; Maria M. Talavera-Barber; Kelan G. Tantisira; Nancy Tartt; Tracy Terlinde; Ronald J. Teufel 2nd; Deepika Thacker; Mansi Thakrar; Stephen N. Thibodeau; Gelise L. Thomas; Moriah E. Thomason; Jiayi Tong; Robert Torres; Jessica Traenkner; Robin Tragus; Joel D. Trinity; Jena S. Tronieri; Andrea B. Troxel; Jennifer Truong; Dongngan T. Truong; Joel Tsevat; Mmekom M. Udosen; Kristen Unterberger; Paul J. Utz; Viola Vaccarino; Brittany B. Vallejos; Terry L. Vanden Hoek; Nita Vangeepuram; Jay K. Varma; Suzanne D. Vernon; Crystal M. Vidal; Sara Vivensi; Adam S. Vohra; Laura K. Wagner; Fei Wang; David Warburton; Megan R. Warner; Rebecca L. Watkins; Sara E. Watson; Ryan Webb; Ryan M. Weeks; Mark G. Weiner; Alan Werzberger; Shelby C. West; Jordan C. Weyer; Jennifer L. Wheeler; Kenneth J. Wilkins; Natasha J. Williams; Charles T. Williams; Erika S. Wimberly; Terra J. Winter; Scott C. Woller; Rachel Wong; Jeremy P. Wood; John C. Wood; Marion J. J. Wood; Qiong Wu; Yinglin Xia; Jie Xu; Samuel Yang; H. S. Yin; Hsiang S. Yin; Yun Jae Yoo; Chloe E. Young; Natalie M. Young; Chengxi Zang; William T. Zempsky; Bingyu Zhang; Yongkang Zhang; Dazheng Zhang; Emily Zimmerman; John Andrefsky I; Jeanne M Marrazzo; Jennifer Dixon; Lisa Gale; Phoebe Maholovich; Praveen Sudhindra; Tiffany Thompson; Elyce Sheehan; Alisha Parada; Kiirk Knowlton; Jeffrey L. Anderson; Marjorie McIntyre; Sean McCandless; Sarah Montoya; Debra Davis; Eric Spanier; Thomas Wodushek; Ron Sokol; Yvonne Maldonado; Karen Jacobson; Xiaolin "Kathleen" Jia; Jake Scott; Orlando Quintero; Francois Haddad; Hannah Valantine; Roham Zamanian; Divya Pathak; Jeanette Boyce; Francesca Facco; Sarah Hankle; John A. Vargo IV; Donna Campbell; Donna Armstrong; Madison Mann; Nicole Burrell; Anna Bartholomew; William A. Grobman; Barbara Cackovic; Baylee Klopfenstein; Samantha Weigand; E. Kaye Snow; Kathleen Fennig; M. Sean Esplin; Denise Lamb; Amanda Nelsen; Jocelyn Phipers; Lauren Fischer; Olivia Docter; Jeanette Brown; Angelica DeMartino; Donna Allard; Emily Miller; Sabine Z. Bousleiman; Megan M. Loffredo; Ashley Vanneman; Imene Beche; Rosalyn Chan-Akeley; Luis D. Pacheco; Jennfier D. DeVolder; Ashley Salazar; Lisa Thibodeaux; Jennifer Cornwell; Amelia A. Nounes; Eugenia Sweet; Abigail Pierse; Brittany Desantis; Parmjit Gill-Jones; David N. Hackney; Suneet P. Chauhan; Felecia Ortiz; Jenifer Treadway; Juanita Rugerio; Kelly Clark; Molly Leatherland; Sally Timlin; Chelsea Grinnan; Jennifer Ferrara; Michelle Kominiarek; Dequana Jones; Trista Reynolds; Katherine M Kearns; Eleanor Saffian; Mariana Karasti; Chrsitinia Pizzi; Anna Filipczak; Emily Long; Megan Mitchell; Katia J. Barrett; Celia Mullowney; George A. Macones; George A. Alba, MD; Radica Alicic, MD; Natasha Altman, MD; Khamal Anglin, MD, MPH; Urania Argueta, BS; Hassan Ashktorab, PhD; Gaston Baslet, MD; Ingrid V. Bassett, MD, MPH; Lucinda Bateman, MD; Brahmchetna Bedi, PhD; Shamik Bhattacharyya, MD, MS; Marie-Abele Bind, PhD; Andra L. Blomkalns, MD, MBA; Hector Bonilla, MD; Hassan Brim, PhD; Patricia A. Bush, MS, EdD; Mario Castro, MD, MPH; James Chan, MA; Alexander W. Charney, MD, PhD; Peter Chen, MD; Lori B. Chibnik, PhD, MPH; Helen Y. Chu, MD, MPH; Rebecca G. Clifton, PhD; Maged M. Costantine, MD; Sushma K. Cribbs, MD, MSc; Sylvia I. Davila Nieves, MS; Steven G. Deeks, MD; Alexandria Duven, RN; Ivette F. Emery, PhD; Nathan Erdmann, MD, PhD; Kristine M. Erlandson, MD, MS; Kacey C. Ernst, PhD, MPH; Rachael Farah-Abraham, PhD; Cheryl E. Farner, MSN; Elen M. Feuerriegel, PhD; Judes Fleurimont, MPH; Vivian Fonseca, MD; Nicholas Franko, BS; Vivian Gainer, MS; Jennifer C. Gander, PhD; Edward M. Gardner, MD; Linda N. Geng, MD, PhD; Kelly S. Gibson, MD; Minjoung Go, MD, MPH; Jason D. Goldman, MD, MPH; Halle Grebe, BS; Frank L. Greenway, MD; Mounira Habli, MD; John Hafner, MD, MPH; Jenny E. Han, MD, MS; Keith A. Hanson, MD, PhD; James Heath, PhD; Carla Hernandez, RN; Rachel Hess, MD, MS; Sally L. Hodder, MD; Matthew K. Hoffman, MD, MPH; Susan E. Hoover, MD, PhD; Beatrice Huang, BA; Brenna L. Hughes, MD; Prasanna Jagannathan, MD; Janice John, MS, MHCDS; Michael R. Jordan, MD; Stuart D. Katz, MD, MS; Elizabeth S. Kaufman, MD; John D. Kelly, MD; Sara W. Kelly, PhD, MPH; Megan M. Kemp, BA; John P. Kirwan, PhD; Jonathan D. Klein, MD, MPH; Kenneth S. Knox, MD; Jerry A. Krishnan, MD, PhD; Andre Kumar, MD; Adeyinka O. Laiyemo, MD; Allison A. Lambert, MD; Margaret Lanca, PhD; Joyce K. Lee-Iannotti, MD; Brian P. Logarbo, MD, MS; Michele T. Longo, MD; Carlos A. Luciano, MD; Karen Lutrick, PhD; Jason H. Maley, MD, MS; Gail Mallett, MS; Jai G. Marathe, MD, MBBS; Vincent Marconi, MD; Gailen D. Marshall, MD, PhD, MS; Christopher F. Martin, MBA; Yuri Matusov, MD; Alem Mehari, MD; Hector Mendez-Figueroa, MD; Robin Mermelstein, PhD; Torri D. Metz, MD, MS; Richard Morse, BA; Jarrod Mosier, MD; Christian Mouchati, MD; Janet Mullington, PhD; Shawn N. Murphy, MD, PhD; Robert B. Neuman, MD; Janko Z. Nikolich, MD, PhD; Ighovwerha Ofotokun, MD; Elizabeth Ojemakinde, MD, MPH; Anna Palatnik, MD; Kristy Palomares, MD, PhD; Tanyalak Parimon, MD; Samuel Parry, MD; Jan E. Patterson, MD; Thomas F. Patterson, MD; Rachel E. Patzer, PhD, MPH; Michael J. Peluso, MD; Priscilla Pemu, MD, MS; Christian M. Pettker, MD; Beth A. Plunkett, MD, MPH; Kristen Pogreba-Brown, PhD; Athena Poppas, MD; John G. Quigley, MD; Uma Reddy, MD; Rebecca Reece, MD; Harrison Reeder, PhD; W. B. Reeves, MD; Eric M. Reiman, MD; Franz Rischard, DO, MSc; Jonathan Rosand, MD, MS; Dwight J. Rouse, MD; Adam Ruff, BS; George Saade, MD; Grecio J. Sandoval, PhD; Jorge L. Santana, MD; Shannon M. Schlater, MS; Frank C. Sciurba, MD; Fitzgerald Shepherd, MD; Zaki A. Sherif, PhD; Hyagriv Simhan, MD; Nora G. Singer, MD; Daniel W. Skupski, MD; Amber Sowles, RN, BSN; Jeffrey A. Sparks, MD, MMSc; Fatima I. Sukhera, MD; Barbara S. Taylor, MD; Larissa Teunis, MPA; Robert J. Thomas, MD; John M. Thorp, MD, MS; Paul Thuluvath, MD; Amberly Ticotsky, MPH, RN; Alan T. Tita, MD, PhD; Katherine R. Tuttle, MD; Alfredo E. Urdaneta, MD; Daisy Valdivieso, BS; Timothy M. VanWagoner, PhD; Andrew Vasey, MD; Monica Verduzco-Gutierrez, MD; Zachary S. Wallace, MD; Honorine D. Ward, MD; David E. Warren, PhD; Steven J. Weiner, MS; Shelley Welch, MS; Sidney W. Whiteheart, PhD; Zanthia Wiley, MD; Juan P. Wisnivesky, MD, DrPH; Lynn M. Yee, MD; Sokratis Zisis, MD
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
- CME & MOC
Perlis RH , Santillana M , Ognyanova K, et al. Prevalence and Correlates of Long COVID Symptoms Among US Adults. JAMA Netw Open. 2022;5(10):e2238804. doi:10.1001/jamanetworkopen.2022.38804
Manage citations:
© 2024
- Permissions
Prevalence and Correlates of Long COVID Symptoms Among US Adults
- 1 Department of Psychiatry, Massachusetts General Hospital, Boston
- 2 Department of Psychiatry, Harvard Medical School, Boston, Massachusetts
- 3 Department of Political Science, Northeastern University, Boston, Massachusetts
- 4 Department of Communication, School of Communication and Information, Rutgers University, New Brunswick, New Jersey
- 5 John F. Kennedy School of Government and Department of Government, Harvard University, Cambridge, Massachusetts
- 6 Department of Political Science, University of Pennsylvania, Philadelphia
- 7 Department of Political Science, Northwestern University, Evanston, Illinois
- News From the JAMA Network Long COVID Linked With Unemployment in New Analysis Melissa Suran, PhD, MSJ JAMA
- Original Investigation Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection Tanayott Thaweethai, PhD; Sarah E. Jolley, MD, MS; Elizabeth W. Karlson, MD, MS; Emily B. Levitan, ScD; Bruce Levy, MD; Grace A. McComsey, MD; Lisa McCorkell, MPP; Girish N. Nadkarni, MD, MPH; Sairam Parthasarathy, MD; Upinder Singh, MD; Tiffany A. Walker, MD; Caitlin A. Selvaggi, MS; Daniel J. Shinnick, MS; Carolin C. M. Schulte, PhD; Rachel Atchley-Challenner, PhD; Leora I. Horwitz, MD; Andrea S. Foulkes, ScD; RECOVER Consortium Authors; RECOVER Consortium; Judith A. Aberg; Natalie L. Adolphi; Shreya Ahirwar; Shifa Ahmed; Neera Ahuja; Masanori Aikawa; Almary Akerlundh; Teresa T. Akintonwa; Aseel Al-Jadiri; Natalya Alekhina; Heather A. Algren; Akram N. Alshawabkeh; Nariman Ammar; Adit Anand; Brett R. Anderson; Lisa Aponte-Soto; Judy L. Aschner; Mary M. Atha; Andrew M. Atz; Robin L. Aupperle; Mirna Ayache; Eduardo Azziz-Baumgartner; L. C. Bailey; Fiona C. Baker; Venkataraman Balaraman; Jennifer A. Bandy; Dithi Banerjee; Deanna M. Barch; James M. Bardes; Jackson Barlocker; R. G. Barr; Arielle Baskin-Sommers; Sanjib Basu; Tracy A. Battaglia; Leah Baucom; Carmen J. Beamon; Casey L. Beaty; Noam D. Beckmann; Jasmine A. Berry; Nahid Bhadelia; Daksh Bhargava; Sultana Bhuiyan; Jiang Bian; Christian Bime; James M. Bjork; Lora J. Black; Catherine A. Blish; Jason P. Block; Amanda Bogie; Dawn Bolliger; William Bonaventura; Seuli Bose-Brill; Mary B. Bower; Andrew D. Boyd; Jerusha Boyineni; Steven B. Bradfute; Carolyn T. Bramante; M. D. Brannock; J. D. Bremner; Shari B. Brosnahan; Natalie C. Buchbinder; Elliott Bueler; Irina A. Buhimschi; Hulya Bukulmez; H. T. Bunnell; John B. Buse; Elizabeth A. Calhoun; Tingyi Cao; Michael D. Carrithers; Thomas W. Carton; Abigail Case; B.J. Casey; Faye Victoria C. Casimero; Lauren Castro; Teresa Cato; Patricia Ceger; Connie L. Cerullo; Linda Chang; Arunee A. Chang; Praneeth Chebrolu; Yong Chen; Li Qing Chen; Benjamin K. Chen; David Chestek; Robert F. Chew; Deena J. Chisolm; Dominic C. Chow; Maryanne R. Chrisant; Dimitri A. Christakis; Christopher G. Chute; Mine S. Cicek; Cheryl R. Clark; Duncan B. Clark; Geoffrey D. Clarke; Katharine N. Clouser; Thomas J. Connors; Judith A. Cook; Krista Coombs; Carlos Cordon-Cardo; Julie L. Costello; Lesley Cottrell; Kelly Cowan; Lindsay G. Cowell; Savannah Cranford; Jamie Cronin; Mollie R. Cummins; Hannah L. Curry; Viren D'Sa; Sean G. Dabney; Casey L. Daniel; Mirella Dapretto; Dawood Darbar; Paul M. Darden; Raktima Dasgupta; Soham Dasgupta; Felicia Davis Blakley; Katherine Dea; Sara J. Deakyne Davies; Lauren A. Decker; Ralph A. DeFronzo; Walter Dehority; Amelia N. Deitchman; James del Alcazar; Phoebe Del Boccio; Carlos del Rio; Marina Del Rios; Julie A. DeLisa; Sean C. Deoni; Maya Z. Diaz; John D. Dickinson; Audrey Dionne; Kathleen R. Diviak; Sarah E. Donohue; Michael J. Downey; Allen J. Dozor; Benard P. Dreyer; Kirsten B. Dummer; Matthew S. Durstenfeld; Mark S. Dworkin; Sherrie L. Edmonds; Matthew D. Elias; Jamie Elifritz; Evan Ellingworth; Amy J. Elliott; Angela M. Ellison; Mike L. Enger; Joaquin M. Espinosa; Shari Esquenazi-Karonika; Michael D. Evans; Danielle N. Evans; Julio C. Facelli; Camila S. Fang; E. Vincent S. Faustino; Maria E. Fayad Lara; Candace H. Feldman; Alexander G. Fiks; Rebecca Fineman; Aloke V. Finn; Melinda S. Fischer; Megan L. Fitzgerald; Valerie J. Flaherman; Thomas J. Flotte; Daniel Forsha; Meghan R. Fortune; John J. Foxe; Nicole Franks; Michael B. Freedman; Catherine E. Freeland; Naomi P. Friedman; Greta Fry; Margot Gage Witvliet; Emily J. Gallagher; Richard Gallagher; Hugh Garavan; Sunanda M. Gaur; Dylan G. Gee; Maria Laura Gennaro; Lynn B. Gerald; Saikat B. Ghosh; Joseph T. Giacino; Andrew T. Girvin; Stephanie L. Godfrey; Mark P. Goldberg; Steven N. Goodman; Howard S. Gordon; Ramkiran Gouripeddi; Paige Graham; Joey P. Granger; Kevin M. Gray; Tina Greimes; Rachel S. Gross; Nicholas Guthe; Evan Gutter; Stephanie Haasnoot; Emily C. Hadley; Melissa A. Haendel; Stephanie Hafner; Katia C. Halabi; Patrick C. Hanley; Ashraf S. Harahsheh; Michelle S. Harkins; Kimberly L. Hartwig; Keren Hasbani; Sharon Hasek; Kristine S. Hauser; Andrew C. Heath; Camden L. Hebson; Mary M. Heitzeg; Monica Hendrickson; Timothy J. Henrich; Alfonso C. Hernandez-Romieu; Christina M. Hester; Miranda Higginbotham; Sophia Hill; Kathryn Hirabayashi; On Ho; Laura Hobart-Porter; M. C. Hoffman; Maryann Holtcamp; Travis K. Hong; Benjamin D. Horne; Carol R. Horowitz; Daniel S. Hsia; Harvey Hsu; Priscilla Y. Hsue; Matthew Huentelman; Bryan E. Huerta; Jared D. Huling; Kathy Hummel; William G. Iacono; Alejandra L. Ibanez; Carmel Ibeawuchi; Katherine Irby; Nahed Ismail; Joanna Jacobus; Vanessa L. Jacoby; Leonard A. Jason; Vidhi Javia; Kyle J. Jennette; Terry L. Jernigan; Sihang Jiang; Naimin Jing; Jace D. Johnny; Nadia Johnson; Brandi Johnson; Brandon Johnson; Pei-Ni Jone; Suzanne E. Judd; Joy J. Juskowich; Edmond K. Kabagambe; David C. Kaelber; Diane G. Kanjilal; Mayank M. Kansal; Tyler J. Kasmarcak; Daniel Kaufman; Rika Kawakami; Dean L. Kellogg; Denise A. Kent; Deepshikha Kewlani; Dhruv Khullar; Keri S. Kim; Arthur Y. Kim; Patricia A. Kinser; Lawrence C. Kleinman; Elizabeth B. Klerman; Matthew J. Kluko; Stacey Knight; Misaki Kobayashi; Karla J. Kopp; Michael Koropsak; Jessica S. Kosut; Ellen Kraig; Amanda Krausert; Ana C. Krieger; Hema Krishna; Aparna Krishnamoorthy; Sankaran S. Krishnan; James P. Lahs; Angela R. Laird; Victoria Laleau; Michelle F. Lamendola-Essel; Jeremy Landeo-Gutierrez; Sean M. Lang; Christine L. Larson; James P. Lash; Jessica Lasky-Su; Gregory Laynor; Simon Lee; Grace M. Lee; F. E. Lee; Matthew A. Lee; Peter J. Leese; R. C. Lefebvre; Angelica J. Levreault; Kennedy C. Lewis; Lu Li; Peter Paul C. Lim; Janet Y. Lin; Krista M. Lisdahl; Matthew B. Litvack; Xiaokang Liu; Jennifer Lloyd; Jennifer K. Logue; Johanna J. Loomba; Vitaly Lorman; Yiwen Lu; Katelyn R. Ludwig; Allison M. Lutz; Jeri Mack; Abeer M. Mahmoud; Cheryl L. Maier; Shahdi K. Malakooti; Sergey Malchenko; Gail L. Mallett; Gabrielle Maranga; Megan W. Martel; Susanne P. Martin-Herz; Maria Martinez-Lage; Christine Maughan; Cammeo Mauntel-Medici; Heidi T. May; Kenneth H. Mayer; Diego R. Mazzotti; Caitryn M. McCallum; Brian W. McCrindle; Russell J. McCulloh; Dylan McDonald; Stephanie McInnis; Julie A. McMurry; Asuncion Mejias; Jonathan Melamed; Martha Menchaca; Alan L. Mendelsohn; Lucio Miele; Mitchell G. Miglis; Cris Milne; Joshua D. Milner; Roger A. Mitchell; Murray A. Mittleman; Sindhu Mohandas; Jonathan G. Monteiro; Vanessa Monzon; David J. Moreno; Lerraughn M. Morgan; Dmitry Morozyuk; Keith E. Morse; Erick Moyneur; Praveen C. Mudumbi; Eva M. Müller-Oehring; Naoko Muramatsu; Hugh Musick; Kshema Nagavedu; Erica R. Nahin; Anoop M. Nambiar; Benjamin H. Natelson; Michael C. Neale; Manette Ness-Cochinwala; Jane W. Newburger; Lisa T. Newman; Amber N. Nguyen; Lauren Nichols; Sheila M. Nolan; Seth Noland; Richard M. Novak; George T. O'Connor; John J. O'Keefe; Princess U. Ogbogu; Carlos R. Oliveira; Matthew E. Oster; Robert F. Padera; Henry H. Paik; Nathan M. Pajor; Huaqin Helen Pan; Deepti Pant; Andrew Partridge; Payal B. Patel; Saaya Patel; Khushboo Patel; Martin P. Paulus; Ronald M. Payne; Ann Pearman; Myriam Peralta-Carcelen; Nicolas L. Perez; Emily R. Pfaff; De-Ann M. Pillers; Chloe E. Pitsch; Neil H. Pliskin; Michael A. Portman; Radu Postelnicu; Jennifer S. Potter; Bellur S. Prabhakar; Priya Prahalad; Bharati Prasad; Barbara Predki; Alexander J. Preiss; Heather M. Prendergast; Davin K. Quinn; Yuri Quintana; Dustin J. Rabideau; Jonathan M. Radosta; Jeffrey Radwell; Hengameh Raissy; Ramaswamy Ramchandran; Isabelle Randall; Suchitra Rao; Amy Rapkiewicz; Sonja A. Rasmussen; Hanieh Razzaghi; Candida J. Rebello; Paulina A. Rebolledo; Neha V. Reddy; Unma M. Reddy; Cara Reedy; Jalees Rehman; R. R. Reichard; Kayleigh M. Reid; Jane E. Reusch; Kyung E. Rhee; Mary B. Rice; John-Ross R. Rizzo; Nadia R. Roan; Polly Robarts; Timothy R. Roberts; Nitza Rochez; Kathleen E. Rodgers; Colin M. Rogerson; Maria E. Romero; Johana M. Rosas; Erika B. Rosenzweig; Russell L. Rothman; Nadine G. Rouphael; Mark W. Russell; Melissa Rutherfoord; Arash A. Sabati; Samer B. Sader; Marzieh Salehi; Amy L. Salisbury; Barbara A. Sampson; Yamuna Sanil; Alice I. Sato; Sharon H. Saydah; Michael S. Schechter; Edward J. Schenck; Katherine I. Schlepphorst; Julia Schuchard; Tina L. Schuh; Jennifer A. Sculley; Alan C. Seifert; Anisha K. Sekar; Rangaraj Selvarangan; Sudha Seshadri; Howard D. Sesso; Dimpy P. Shah; Divya Shakti; Nancy L. Shapiro; Suchetha Sharma; Kumar Sharma; Shubhi Sharma; Kavita Sharma; Gwendolyn Shaw; Eyal Shemesh; Yishan Shen; Elizabeth Shenkman; Stephanie Shiau; Michelle Siciliano; Aylin Simsir; Veronica E. Smith; Jessica N. Snowden; Isaac H. Solomon; Leslie A. Spikes; Lindsay M. Squeglia; Shubhika Srivastava; Mary L. St. Jean; Kenneth A. Stapleford; Cheryl R. Stein; Michelle D. Stevenson; Sarah A. Stewart de Ramirez; Lauren E. Stiles; Aryeh Stock; Melissa S. Stockwell; James R. Stone; Jeran Stratford; Til Stürmer; Vignesh Subbian; Jun Sun; Mehul S. Suthar; Ashley Sylvera; David M. Systrom; Jacqueline Szmuszkovicz; Maria M. Talavera-Barber; Kelan G. Tantisira; Nancy Tartt; Tracy Terlinde; Ronald J. Teufel 2nd; Deepika Thacker; Mansi Thakrar; Stephen N. Thibodeau; Gelise L. Thomas; Moriah E. Thomason; Jiayi Tong; Robert Torres; Jessica Traenkner; Robin Tragus; Joel D. Trinity; Jena S. Tronieri; Andrea B. Troxel; Jennifer Truong; Dongngan T. Truong; Joel Tsevat; Mmekom M. Udosen; Kristen Unterberger; Paul J. Utz; Viola Vaccarino; Brittany B. Vallejos; Terry L. Vanden Hoek; Nita Vangeepuram; Jay K. Varma; Suzanne D. Vernon; Crystal M. Vidal; Sara Vivensi; Adam S. Vohra; Laura K. Wagner; Fei Wang; David Warburton; Megan R. Warner; Rebecca L. Watkins; Sara E. Watson; Ryan Webb; Ryan M. Weeks; Mark G. Weiner; Alan Werzberger; Shelby C. West; Jordan C. Weyer; Jennifer L. Wheeler; Kenneth J. Wilkins; Natasha J. Williams; Charles T. Williams; Erika S. Wimberly; Terra J. Winter; Scott C. Woller; Rachel Wong; Jeremy P. Wood; John C. Wood; Marion J. J. Wood; Qiong Wu; Yinglin Xia; Jie Xu; Samuel Yang; H. S. Yin; Hsiang S. Yin; Yun Jae Yoo; Chloe E. Young; Natalie M. Young; Chengxi Zang; William T. Zempsky; Bingyu Zhang; Yongkang Zhang; Dazheng Zhang; Emily Zimmerman; John Andrefsky I; Jeanne M Marrazzo; Jennifer Dixon; Lisa Gale; Phoebe Maholovich; Praveen Sudhindra; Tiffany Thompson; Elyce Sheehan; Alisha Parada; Kiirk Knowlton; Jeffrey L. Anderson; Marjorie McIntyre; Sean McCandless; Sarah Montoya; Debra Davis; Eric Spanier; Thomas Wodushek; Ron Sokol; Yvonne Maldonado; Karen Jacobson; Xiaolin "Kathleen" Jia; Jake Scott; Orlando Quintero; Francois Haddad; Hannah Valantine; Roham Zamanian; Divya Pathak; Jeanette Boyce; Francesca Facco; Sarah Hankle; John A. Vargo IV; Donna Campbell; Donna Armstrong; Madison Mann; Nicole Burrell; Anna Bartholomew; William A. Grobman; Barbara Cackovic; Baylee Klopfenstein; Samantha Weigand; E. Kaye Snow; Kathleen Fennig; M. Sean Esplin; Denise Lamb; Amanda Nelsen; Jocelyn Phipers; Lauren Fischer; Olivia Docter; Jeanette Brown; Angelica DeMartino; Donna Allard; Emily Miller; Sabine Z. Bousleiman; Megan M. Loffredo; Ashley Vanneman; Imene Beche; Rosalyn Chan-Akeley; Luis D. Pacheco; Jennfier D. DeVolder; Ashley Salazar; Lisa Thibodeaux; Jennifer Cornwell; Amelia A. Nounes; Eugenia Sweet; Abigail Pierse; Brittany Desantis; Parmjit Gill-Jones; David N. Hackney; Suneet P. Chauhan; Felecia Ortiz; Jenifer Treadway; Juanita Rugerio; Kelly Clark; Molly Leatherland; Sally Timlin; Chelsea Grinnan; Jennifer Ferrara; Michelle Kominiarek; Dequana Jones; Trista Reynolds; Katherine M Kearns; Eleanor Saffian; Mariana Karasti; Chrsitinia Pizzi; Anna Filipczak; Emily Long; Megan Mitchell; Katia J. Barrett; Celia Mullowney; George A. Macones; George A. Alba, MD; Radica Alicic, MD; Natasha Altman, MD; Khamal Anglin, MD, MPH; Urania Argueta, BS; Hassan Ashktorab, PhD; Gaston Baslet, MD; Ingrid V. Bassett, MD, MPH; Lucinda Bateman, MD; Brahmchetna Bedi, PhD; Shamik Bhattacharyya, MD, MS; Marie-Abele Bind, PhD; Andra L. Blomkalns, MD, MBA; Hector Bonilla, MD; Hassan Brim, PhD; Patricia A. Bush, MS, EdD; Mario Castro, MD, MPH; James Chan, MA; Alexander W. Charney, MD, PhD; Peter Chen, MD; Lori B. Chibnik, PhD, MPH; Helen Y. Chu, MD, MPH; Rebecca G. Clifton, PhD; Maged M. Costantine, MD; Sushma K. Cribbs, MD, MSc; Sylvia I. Davila Nieves, MS; Steven G. Deeks, MD; Alexandria Duven, RN; Ivette F. Emery, PhD; Nathan Erdmann, MD, PhD; Kristine M. Erlandson, MD, MS; Kacey C. Ernst, PhD, MPH; Rachael Farah-Abraham, PhD; Cheryl E. Farner, MSN; Elen M. Feuerriegel, PhD; Judes Fleurimont, MPH; Vivian Fonseca, MD; Nicholas Franko, BS; Vivian Gainer, MS; Jennifer C. Gander, PhD; Edward M. Gardner, MD; Linda N. Geng, MD, PhD; Kelly S. Gibson, MD; Minjoung Go, MD, MPH; Jason D. Goldman, MD, MPH; Halle Grebe, BS; Frank L. Greenway, MD; Mounira Habli, MD; John Hafner, MD, MPH; Jenny E. Han, MD, MS; Keith A. Hanson, MD, PhD; James Heath, PhD; Carla Hernandez, RN; Rachel Hess, MD, MS; Sally L. Hodder, MD; Matthew K. Hoffman, MD, MPH; Susan E. Hoover, MD, PhD; Beatrice Huang, BA; Brenna L. Hughes, MD; Prasanna Jagannathan, MD; Janice John, MS, MHCDS; Michael R. Jordan, MD; Stuart D. Katz, MD, MS; Elizabeth S. Kaufman, MD; John D. Kelly, MD; Sara W. Kelly, PhD, MPH; Megan M. Kemp, BA; John P. Kirwan, PhD; Jonathan D. Klein, MD, MPH; Kenneth S. Knox, MD; Jerry A. Krishnan, MD, PhD; Andre Kumar, MD; Adeyinka O. Laiyemo, MD; Allison A. Lambert, MD; Margaret Lanca, PhD; Joyce K. Lee-Iannotti, MD; Brian P. Logarbo, MD, MS; Michele T. Longo, MD; Carlos A. Luciano, MD; Karen Lutrick, PhD; Jason H. Maley, MD, MS; Gail Mallett, MS; Jai G. Marathe, MD, MBBS; Vincent Marconi, MD; Gailen D. Marshall, MD, PhD, MS; Christopher F. Martin, MBA; Yuri Matusov, MD; Alem Mehari, MD; Hector Mendez-Figueroa, MD; Robin Mermelstein, PhD; Torri D. Metz, MD, MS; Richard Morse, BA; Jarrod Mosier, MD; Christian Mouchati, MD; Janet Mullington, PhD; Shawn N. Murphy, MD, PhD; Robert B. Neuman, MD; Janko Z. Nikolich, MD, PhD; Ighovwerha Ofotokun, MD; Elizabeth Ojemakinde, MD, MPH; Anna Palatnik, MD; Kristy Palomares, MD, PhD; Tanyalak Parimon, MD; Samuel Parry, MD; Jan E. Patterson, MD; Thomas F. Patterson, MD; Rachel E. Patzer, PhD, MPH; Michael J. Peluso, MD; Priscilla Pemu, MD, MS; Christian M. Pettker, MD; Beth A. Plunkett, MD, MPH; Kristen Pogreba-Brown, PhD; Athena Poppas, MD; John G. Quigley, MD; Uma Reddy, MD; Rebecca Reece, MD; Harrison Reeder, PhD; W. B. Reeves, MD; Eric M. Reiman, MD; Franz Rischard, DO, MSc; Jonathan Rosand, MD, MS; Dwight J. Rouse, MD; Adam Ruff, BS; George Saade, MD; Grecio J. Sandoval, PhD; Jorge L. Santana, MD; Shannon M. Schlater, MS; Frank C. Sciurba, MD; Fitzgerald Shepherd, MD; Zaki A. Sherif, PhD; Hyagriv Simhan, MD; Nora G. Singer, MD; Daniel W. Skupski, MD; Amber Sowles, RN, BSN; Jeffrey A. Sparks, MD, MMSc; Fatima I. Sukhera, MD; Barbara S. Taylor, MD; Larissa Teunis, MPA; Robert J. Thomas, MD; John M. Thorp, MD, MS; Paul Thuluvath, MD; Amberly Ticotsky, MPH, RN; Alan T. Tita, MD, PhD; Katherine R. Tuttle, MD; Alfredo E. Urdaneta, MD; Daisy Valdivieso, BS; Timothy M. VanWagoner, PhD; Andrew Vasey, MD; Monica Verduzco-Gutierrez, MD; Zachary S. Wallace, MD; Honorine D. Ward, MD; David E. Warren, PhD; Steven J. Weiner, MS; Shelley Welch, MS; Sidney W. Whiteheart, PhD; Zanthia Wiley, MD; Juan P. Wisnivesky, MD, DrPH; Lynn M. Yee, MD; Sokratis Zisis, MD JAMA
Question How common are COVID-19 symptoms lasting longer than 2 months, also known as long COVID , among adults in the United States, and which adults are most likely to experience long COVID?
Findings In this cross-sectional study of more than 16 000 individuals, 15% of US adults with a prior positive COVID-19 test reported current symptoms of long COVID. Those who completed a primary vaccination series prior to infection were less likely to report long COVID symptoms.
Meaning This study suggests that long COVID is prevalent and that the risk varies among individual subgroups in the United States; vaccination may reduce this risk.
Importance Persistence of COVID-19 symptoms beyond 2 months, or long COVID, is increasingly recognized as a common sequela of acute infection.
Objectives To estimate the prevalence of and sociodemographic factors associated with long COVID and to identify whether the predominant variant at the time of infection and prior vaccination status are associated with differential risk.
Design, Setting, and Participants This cross-sectional study comprised 8 waves of a nonprobability internet survey conducted between February 5, 2021, and July 6, 2022, among individuals aged 18 years or older, inclusive of all 50 states and the District of Columbia.
Main Outcomes and Measures Long COVID, defined as reporting continued COVID-19 symptoms beyond 2 months after the initial month of symptoms, among individuals with self-reported positive results of a polymerase chain reaction test or antigen test.
Results The 16 091 survey respondents reporting test-confirmed COVID-19 illness at least 2 months prior had a mean age of 40.5 (15.2) years; 10 075 (62.6%) were women, and 6016 (37.4%) were men; 817 (5.1%) were Asian, 1826 (11.3%) were Black, 1546 (9.6%) were Hispanic, and 11 425 (71.0%) were White. From this cohort, 2359 individuals (14.7%) reported continued COVID-19 symptoms more than 2 months after acute illness. Reweighted to reflect national sociodemographic distributions, these individuals represented 13.9% of those who had tested positive for COVID-19, or 1.7% of US adults. In logistic regression models, older age per decade above 40 years (adjusted odds ratio [OR], 1.15; 95% CI, 1.12-1.19) and female gender (adjusted OR, 1.91; 95% CI, 1.73-2.13) were associated with greater risk of persistence of long COVID; individuals with a graduate education vs high school or less (adjusted OR, 0.67; 95% CI, 0.56-0.79) and urban vs rural residence (adjusted OR, 0.74; 95% CI, 0.64-0.86) were less likely to report persistence of long COVID. Compared with ancestral COVID-19, infection during periods when the Epsilon variant (OR, 0.81; 95% CI, 0.69-0.95) or the Omicron variant (OR, 0.77; 95% CI, 0.64-0.92) predominated in the US was associated with diminished likelihood of long COVID. Completion of the primary vaccine series prior to acute illness was associated with diminished risk for long COVID (OR, 0.72; 95% CI, 0.60-0.86).
Conclusions and Relevance This study suggests that long COVID is prevalent and associated with female gender and older age, while risk may be diminished by completion of primary vaccination series prior to infection.
For a subset of individuals with acute COVID-19 disease, symptoms may persist beyond 1 month, with some patients reporting symptoms at least 6 months later. 1 Initially referred to as postacute sequelae of COVID-19 or post–COVID-19 syndrome , 2 this phenomenon is now more commonly described as long COVID . 3 The World Health Organization 4 defined long COVID as generally occurring 3 months from the onset of COVID-19 with symptoms that last for at least 2 months.
Prevalence estimates for long COVID vary widely, in part because of variability in the definition and sampling frame. A self-report symptom tracking study among 4182 individuals found rates of symptomatic persistence of 13.3% at 1 month and 4.5% at 2 months. 5 In a United Kingdom COVID-19–focused survey, among 20 000 individuals with a positive SARS-CoV-2 test result, 13.7% reported symptom persistence at 12 weeks based on a single survey question. 6
Two studies have used administrative claims or electronic health records to examine long COVID symptoms among samples not limited to inpatients. One investigation using administrative data from the Veterans Affairs health system in the United States confirmed that a range of symptoms affecting multiple organ systems was common, among them respiratory, metabolic, cardiovascular, gastrointestinal, and neuropsychiatric diagnoses, 7 but did not report prevalence of the syndromes per se. More recently, a Centers for Disease Control and Prevention investigation using commercial electronic health record data found that 1 in 5 individuals aged 18 to 64 years and up to 1 in 4 individuals aged 65 years or older experienced new onset of a disease identified using a diagnostic code that could be associated with COVID-19 at or beyond 30 days from onset 8 ; however, that study did not otherwise account for age, gender, or a range of other confounding features. A key limitation in both of these studies is the reliance on coded diagnoses, which may miss individual symptoms that do not contribute to a medical encounter or are not coded as part of an encounter; a study using natural language processing to identify such symptoms of long COVID reported prevalences of 10% to 15%. 9
Numerous aspects of long COVID remain poorly understood, with reviews suggesting that this phenomenon may actually reflect multiple different syndromes. 3 , 10 In particular, it is not known which individuals will experience full recovery and which individuals will experience persistence of symptoms. One such concern is whether disadvantaged groups, such as individuals from racial and ethnic minority groups or socioeconomically disadvantaged groups, may have a disproportionately high prevalence of long COVID because they have experienced a disproportionately high burden of acute infection. 11 If high-risk individuals could be identified, it might be possible to develop strategies to mitigate or prevent symptom persistence, prompting calls for increased emphasis on investigation of postacute sequelae of COVID-19. 2 , 12 A prior self-report study identified older age and female gender as correlates of greater risk for persistent COVID-19 symptoms 13 ; associations with gender were further supported in a UK survey. 6
A particular correlate of interest has been the role of prior vaccination and long COVID risk (ie, the extent to which so-called breakthrough infections might be associated with differential risk). In a recent reanalysis of Veterans Health Administration data, 14 including more than 30 000 previously vaccinated individuals who experienced breakthrough infection, the risk of long COVID was modestly but statistically significantly diminished. A corresponding secondary analysis of a complementary study drew on self-report from more than 1 million users of a UK COVID-19 symptom tracking application, including approximately 8400 individuals who experienced COVID-19 infection after at least 1 vaccine dose 15 ; that study found marked protection after a second vaccine dose but no significant protection from an initial dose.
To better characterize symptom persistence with a broader and less select sampling frame, we used data from a multiwave US survey that included questions about COVID-19 encompassing 50 states and the District of Columbia. The survey did not focus only on COVID-19, yielding less likelihood of selection bias than more focused surveys of COVID-19 persistence in which participants opt in. We aimed to characterize the prevalence of long COVID in the general US population, to identify sociodemographic features associated with persistence of symptoms for at least 2 months after onset, and to estimate the protective association, if any, of vaccination.
We included data collected from 8 waves of the COVID States Project, a large-scale internet survey conducted for an academic consortium approximately every 6 weeks between February 5, 2021, and July 6, 2022, inclusive of all 50 states and the District of Columbia. Survey participants were individuals aged 18 years or older who resided in the United States. The nonprobability 16 sampling method has previously been validated in similar contexts as a substantially lower-cost alternative to traditional survey approaches. 17 , 18 The survey applied representative quotas to balance age, gender, race and ethnicity, and geographic distribution. Survey participants provided signed informed consent online prior to survey access. Because data were deidentified, the study was determined to be exempt by the institutional review board of Harvard University. This study followed the American Association for Public Opinion Research ( AAPOR ) reporting guideline. 19
All respondents were asked if they had received a positive COVID-19 test result, which did not distinguish between polymerase chain reaction test or antigen test, and in which month they received this result. Those who reported any positive diagnosis were further asked whether their symptoms had resolved; for those who identified continued symptoms, they were asked to complete a checklist of commonly reported symptoms. Month of first and second vaccination, where applicable, was also identified via a checklist. All sociodemographic variables were collected by self-report. Data on race and ethnicity were obtained from 5 US Census categories to confirm representativeness of the US population, with categories analyzed and reported in accordance with a recent medical publication guidance statement. 20
We adapted the World Health Organization 4 definition of long COVID, including all individuals whose survey start date was more than 2 months after the month in which they initially identified a positive COVID-19 test result and defining casees as reporting continued symptoms at the time of the survey. (A planned sensitivity analysis applied a stricter definition, excluding individuals who said that ongoing symptoms did not affect their life [answering “not at all” regarding effect], consistent with the World Health Organization reference to symptoms that “generally have an impact on everyday functioning,” 4 and those for whom loss of smell was the only reported symptom). Vaccination prior to illness was defined by comparing the month of vaccination with the first identified month of illness. For purposes of primary analysis, completion of the primary vaccination series was defined as 2 vaccinations occurring prior to the first month of illness, or a single vaccination when the Ad.26.COV2.S vaccine (Janssen) was identified in response to the question, “Which COVID-19 vaccine did you receive?” The predominant US viral variant at the time of infection was derived on the basis of CoVariants analysis of GISAID (Global Initiative on Sharing Avian Influenza Data) data 21 indicating 50% or more typed variants reflecting a given variant. For participants who responded to more than 1 survey wave, the most recent survey was included.
We applied multiple logistic regression in R, version 4.0 (R Project for Statistical Computing) 22 to examine the association of persistence of symptoms with sociodemographic features, and then we extended these models to include terms for vaccination status and predominant variant at month of infection. Post hoc analysis also examined the association of age by decade to detect possible nonlinear associations. To generate population-weighted estimates of prevalence either among those with a prior positive COVID-19 test result or the adult US population as a whole regardless of COVID-19 status, survey results from all survey respondents were reweighted with interlocking national weights for age, gender, race and ethnicity, educational level, urbanicity (urban, suburban, or rural), and region, based on the 2019 US Census American Community Survey. 23 All P values were from 2-sided tests and results were deemed statistically significant at P < .05
Without reweighting the survey sample, the 16 091 survey respondents reporting test-confirmed COVID-19 illness at least 2 months prior had a mean age of 40.5 (15.2) years; 10 075 (62.6%) were women, and 6016 (37.4%) were men; 817 (5.1%) were Asian, 1826 (11.3%) were Black, 1546 (9.6%) were Hispanic, and 11 425 (71.0%) were White. From this cohort, 2359 individuals (14.7%) reported continued COVID-19 symptoms more than 2 months after acute illness.
Table 1 summarizes additional characteristics of the resulting cohort, by presence or absence of persistent symptoms. When the cohort was restricted to the 12 441 individuals who tested positive for COVID-19 at least 6 months previously, 1843 (14.8%) reported continued COVID-19 symptoms. Of the 7462 individuals who tested positive at least 12 months previously, 1135 (15.2%) reported continued symptoms.
We then reweighted the sample to reflect national sociodemographic distributions, enabling estimates of national point prevalences. Individuals meeting criteria for long COVID represented 13.9% of those who had tested positive for COVID-19 (10.1% of men and 17.9% of women), including 12.6% of Asian adults, 9.7% of Black adults, 15.3% of Hispanic adults, and 15.5% of White adults. In reweighted analysis including all survey participants (eTable 1 in the Supplement ), to estimate the proportion of the US adult population who met criteria for current long COVID (ie, point prevalence), these individuals represented 1.7% of US adults; this included 1.3% of men, 2.0% of women, 0.7% of Asian adults, 1.0% of Black adults, 2.0% of Hispanic adults, and 1.8% of White adults.
In logistic regression models including sociodemographic features ( Figure 1 ), older age per decade above 40 years (adjusted odds ratio [OR], 1.15; 95% CI, 1.12-1.19) and female gender (adjusted OR, 1.91; 95% CI, 1.73-2.13) were associated with greater risk of persistence; individuals with a graduate education vs high school or less (adjusted OR, 0.67; 95% CI, 0.56-0.79) and urban vs rural residence (adjusted OR, 0.74; 95% CI, 0.64-0.86) were less likely to report persistence. In light of conflicting results from prior investigations, we also examined nonlinear associations of age (eFigure 1 in the Supplement ), with maximal liability observed in the age group of 50 to 59 years—compared with the reference group aged 18 to 29 years, the adjusted OR was 2.38 (95% CI, 1.92-2.98).
Table 2 summarizes individual symptoms most commonly reported by survey participants. Fatigue was most common (1232 of 2359 [52.2%]), followed by loss of smell (1031 of 2359 [43.7%]), “brain fog” (952 of 2359 [40.4%]), and shortness of breath (937 of 2359 [39.7%]); 1079 of 2359 participants (45.7%) reported either poor memory or brain fog. Frequencies of individual symptoms differed significantly by gender: women were significantly more likely than men to report loss of smell (832 of 1795 [46.4%] vs 199 of 564 [35.3%]; P < .001), cognitive symptoms (874 of 1795 [48.7%] vs 205 of 564 [36.3%]; P < .001), anxiety (552 of 1795 [30.8%] vs 126 of 564 [22.3%]; P < .001), and sleep disruption (581 of 1795 [32.4%] vs 127 of 564 [22.5%]; P < .001). In exploratory analysis, symptom frequencies were generally similar by predominant variant at time of initial illness (eTable 2 in the Supplement ), with the exception that anosmia was less frequently reported for infections when the Omicron variant was the predominant variant (Omicron variant, 83 of 246 [33.7%]; Alpha variant, 59 of 147 [40.1%]; Delta variant, 210 of 416 [50.5%]; P < .001). In population-weighted estimates, 0.7% (95% CI, 0.7%-0.8%) of sampled US adults reported cognitive symptoms; this sample included 6.1% (95% CI, 5.7%-6.6%) of those with a prior positive COVID-19 test result.
We next examined the association of predominant variant at time of infection and of vaccination prior to acute illness with risk for long COVID. Compared with ancestral COVID-19, infection during periods when the Epsilon variant (OR, 0.81; 95% CI, 0.69-0.95) or the Omicron variant (OR, 0.77; 95% CI, 0.64-0.92) predominated in the US was associated with diminished likelihood of long COVID ( Figure 2 ). Completion of the primary vaccine series prior to acute illness was associated with diminished risk for long COVID (OR, 0.72; 95% CI, 0.60-0.86). However, partial vaccination (ie, a single vaccination from a 2-vaccine series) was not associated with significant reduction in risk in fully adjusted models (OR, 0.93; 95% CI, 0.69-1.25). A sensitivity analysis excluding infection prior to January 2021, to exclude secular trends or biases arising from inclusion of infection before vaccination was more widely available, yielded similar results (for completion of primary vaccination: OR, 0.73; 95% CI, 0.60-0.88; for partial vaccination: OR, 0.94; 95% CI, 0.69-1.25) (eFigure 2 in the Supplement ).
In a sensitivity analysis, when individuals who identified the effect of ongoing symptoms as “not at all” or their only symptom as loss of smell were excluded (n = 354), 2005 of 16 091 participants (12.5%; 12.0% with US population weighting) met diagnostic criteria (eTable 3 in the Supplement ). Regression models yielded similar results for sociodemographic features (eFigure 3 in the Supplement ) and greater numeric magnitude of benefit associated with prior vaccination (eFigure 4 in the Supplement ) (for complete vaccination: OR, 0.69; 95% CI, 0.57-0.84).
In this cross-sectional study of a cohort of 16 091 adults surveyed between February 2021 and July 2022 in all 50 states in the US and the District of Columbia, we estimated that 14.7% of those who reported a positive COVID-19 test result more than 2 months previously continued to describe symptoms that they associated with acute infection, or 13.9% after reweighting to reflect the US adult population. These point prevalence estimates were similar when the cohort was restricted to those whose acute illness was 6 and 12 months in the past.
Our results are broadly similar to those previously reported among nonhospitalized cohorts. In a study using app-based symptom recording for 4182 patients with COVID-19, only 4.5% reported symptoms for more than 8 weeks, broadly similar to our results—as in the inpatient cohorts, fatigue was among the most common symptoms, along with dyspnea and headache. 13 Our results are consistent with that app-based study in identifying age and female gender as factors associated with risk, even though our sampling frame is markedly different. Our design more closely resembles that of a very large-scale UK survey, which found the greatest risk for persistence among female respondents and younger respondents. 6 More broadly, the differences among these 3 studies, which all used self-report, may reflect differences in ascertainment and question design and may indicate the importance of multiple convergent methods to characterize long COVID. The associations with income, educational level, and race and ethnicity that we identified in our sample highlight the importance of considering these features in understanding the differential longer-term, as well as shorter-term, outcomes of infection.
Two recent studies directly examined the question of protection afforded by prior vaccination, using different designs. One study examined more than 33 000 previously vaccinated individuals with breakthrough COVID-19 infection from Veterans Affairs electronic health records. 14 The Veterans Affairs population may not fully reflect the general adult population in the US, and coded clinical data may be less sensitive to symptoms than narrative notes or patient-reported symptoms. 9 Still, despite these differences, the approximately 24% reduction in odds of long COVID that we observed after a single vaccination (approximately 33% when applying a stricter definition of long COVID) does approximate the 15% reduction in hazard of long COVID in that study. 14
A complementary study drew on self-report from more than 1 million users of a UK COVID-19 symptom app, including approximately 8400 users who experienced COVID-19 infection after at least 1 vaccine dose. 15 In that study, no apparent protective association of an initial vaccine dose with symptoms beyond 28 days was detected (OR, 1.03; 95% CI, 0.85-1.24), consistent with our findings after a single dose, although marked protection was observed after a second dose (OR, 0.51; 95% CI, 0.32-0.82).
This study has some limitations. First, because this study used preempaneled respondents in a nonprobability design, we cannot reliably calculate the response rate; as such, nonresponse bias cannot be estimated. However, in other domains, these nonprobability surveys have closely mirrored results from more traditional designs, 24 and prior work with this survey found results that closely approximate estimates obtained using other methods, including probability polls and administrative data. 25 , 26 Furthermore, our cross-sectional design does not allow for a more precise estimate of symptom persistence and relies on participant recall in some cases nearly 1 year after initial illness. In particular, we cannot exclude the possibility that some individuals who previously experienced long COVID symptoms had recovered by the time of the survey, although the stability of our estimates when samples were restricted to greater follow-up periods since acute infection suggests that this is less likely. Misclassification of individuals who previously had long COVID but recovered at the time of the survey should bias our results toward smaller estimates of effect, such that any associations we identify may actually represent conservative estimates. Conversely, absent measures of symptom frequency among individuals without prior COVID-19, we cannot estimate the extent to which apparent long COVID symptoms would be identified as a consequence of other illnesses. Lacking detailed assessment of respondents’ medical history, we also cannot examine the associations of comorbid medical illness or acuity of acute illness with risk for long COVID, which could explain some of the observed associations. Finally, we relied on self-report of symptoms rather than objective physiological or cognitive measures. As such, our results must be seen as complementing, rather than replacing, analyses using administrative claims 14 or electronic health records. Prospective studies will be necessary to confirm our results; the National Institutes of Health RECOVER (Researching COVID to Enhance Recovery) study, for example, will be valuable in providing systematic and objective measurement of sequelae. 27
Despite these limitations, we also emphasize the strengths of this systematic assessment, namely, that by design it should be more representative than other single-cohort studies because it captures individuals drawn from every state. Moreover, because the survey is not specifically aimed at individuals with COVID-19 or symptom persistence, it may be less biased toward those with a greater interest in long-term symptoms than (for example) symptom tracking applications. 13 That is, because recruitment materials did not specify COVID-19 or persistence, our results are less likely to reflect individuals with greater interest in COVID-19 persistence. At the other extreme, our approach is less likely to overestimate prevalence than investigations based solely on artifacts of clinical care.
A key question for further investigation will be the differences by race and ethnicity in the prevalence of long COVID that we observed, even after accounting for a range of sociodemographic correlates. These differences cannot be explained by a lack of access to COVID-19 testing because our outcome definition was contigent on obtaining such a test. The finding that greater educational levels, greater income, and urban vs rural setting are associated with diminished long COVID risk highlights the importance of accounting for nonbiological associations in understanding this phenomenon, a limitation of prior investigations. Finally, the suggestion that rates of long COVID may vary by predominant variant at time of infection also merits further investigation because it may help to inform efforts to understand the mechanisms underlying the development of this syndrome.
In aggregate, the results of this cross-sectional study provide an estimate of the mean point prevalence of long COVID in a large, representative population sample of individuals in the United States, complementing studies using administrative claims, electronic health records, or COVID-19–focused self-report apps and surveys. They support the potential protective association of vaccination in reducing but not eliminating long COVID risk. If confirmed in prospective studies, these results may facilitate risk stratification, with a goal of early intervention to minimize the effect of long COVID, and could contribute to efforts to prevent this syndrome altogether.
Accepted for Publication: September 12, 2022.
Published: October 27, 2022. doi:10.1001/jamanetworkopen.2022.38804
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2022 Perlis RH et al. JAMA Network Open .
Corresponding Author: Roy H. Perlis, MD, MSc, Massachusetts General Hospital, 185 Cambridge St, 6th Floor, Boston, MA 02114 ( [email protected] ).
Author Contributions : Dr Perlis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Perlis, Safarpour, Druckman, Lazer.
Acquisition, analysis, or interpretation of data: Perlis, Santillana, Ognyanova, Safarpour, Lunz Trujillo, Simonson, Green, Quintana, Baum, Lazer.
Drafting of the manuscript: Perlis, Lazer.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Perlis, Santillana, Green.
Obtained funding: Ognyanova, Druckman, Baum, Lazer.
Administrative, technical, or material support: Perlis, Lunz Trujillo, Simonson, Quintana, Druckman, Lazer.
Supervision: Quintana.
Conflict of Interest Disclosures: Dr Perlis reported receiving personal fees from Burrage Capital, Genomind, Psy Therapeutics, Takeda, and Circular Genomics outside the submitted work. Dr Lazer reported receiving grants from the National Science Foundation during the conduct of the study. No other disclosures were reported.
Funding/Support: The survey was supported in part by the National Science Foundation (Drs Ognyanova, Druckman, Baum, and Lazer).
Role of the Funder/Sponsor: The National Science Foundation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: Dr Perlis is associate editor of JAMA Network Open , but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
SYSTEMATIC REVIEW article
Potential limitations in systematic review studies assessing the effect of the main intervention for treatment/therapy of covid-19 patients: an overview.

- 1 Knowledge Utilization Research Centre, Tehran University of Medical Sciences, Tehran, Iran
- 2 Health Policy and Management Research Center, Department of Health Management and Economics, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 3 Social Determinants of Health Research Center, Birjand University of Medical Sciences, Birjand, Iran
Background: Although several studies have assessed the safety, efficacy, and effectiveness of interventions in treating the COVID-19, many of them have limitations that can have an immense impact on their results. This study aims to assess the potential limitations in systematic reviews (SRs) that evaluate the effect of interventions on the treatment of the COVID-19.
Methods: PubMed, Scopus, and Web of Sciences (WOS) databases were searched from inception to January 1, 2022. All systematic reviews investigated the effectiveness, efficacy, safety, and outcome of the main intervention (Favipiravir, Remdesivir, Hydroxychloroquine, Ivermectin, Lopinavir/Ritonavir, or Tocilizumab) for the treatment of COVID-19 patients and reported the potential limitations of the included studies. We assessed the quality of the included studies using the Quality Assessment Tool (QAT) for review articles. We conducted a content analysis and prepared a narrative summary of the limitations.
Results: Forty-six studies were included in this review. Ninety one percent of the included studies scored as strong quality and the remaining (9%) as moderate quality. Only 29.7% of the included systematic reviews have a registered protocol. 26% of the included studies mentioned a funding statement. The main limitations of the included studies were categorized in 10 domains: sample size, heterogeneity, follow-up, treatment, including studies, design, definitions, synthesis, quality, and search.
Conclusion: Various limitations have been reported in all the included studies. Indeed, the existence of limitations in studies can affect their results, therefore, identifying these limitations can help researchers design better studies. As a result, stronger studies with more reliable results will be reported and disseminated. Further research on COVID-19 SRs is essential to improve research quality and also, efficiency among scientists across the world.
The COVID-19 pandemic began in early 2020 with major health consequences ( 1 ). According to live data from Worldometer website, the total number of coronavirus cases and the number of deaths so far is 595,494,252 and 6,455,301, respectively (Tue, 16 Aug 2022). Numerous studies have assessed the effects of the different interventions on the treatment of the COVID-19 patients ( 2 – 6 ). These studies differ in many ways, including the type of treatment, follow-up time, study design, patient type, and disease severity, each of which can have a positive or negative effect on the results of these studies ( 7 ).
As the global community eagerly awaits credible scientific solutions for this pandemic, researchers and scientists are under much pressure to identify effective therapeutic and preventive strategies for COVID-19. Also, there are many unknowns, and the massive demand for evidence on the treatment of a novel disease such as COVID-19 may be unintentionally affecting studies’ design and conduct. Furthermore, it may inadvertently affect the peer-review and publication process, leading to significant methodology gaps and overall lower quality evidence on COVID-19. These gaps lead to less-informative studies, loss of precious time, and valuable resources ( 8 ).
With the growth of evidence in this area ( 9 ), there is a need for studies that report the results of these individual studies in general. Systematic reviews objectively summarize large amounts of information, identifying gaps in medical research, and identifying beneficial or harmful interventions which will be useful for clinicians, researchers, and even for public and policymakers. The value of a systematic review depends on what was done, what was found, and the clarity of reporting ( 10 ). The results of a systematic review are influenced by the quality of the primary studies included. Methodologically, poor studies tend to exaggerate the overall estimate of treatment effect and may lead to incorrect inferences ( 11 ).
While a need to disseminate information to the medical community and general public was paramount, concerns have been raised regarding the scientific rigor, quality, and limitations in published reports which may potentially effect on the systematic reviews and meta-analysis results ( 1 ). In this study, we aim to identify the potential limitations in systematic reviews that evaluated the effect of interventions on the treatment of the COVID-19 which can help to improve and make the result of studies more accurate in the future.
Methodology
Protocol and registration.
We conducted this overview based on Smith et al. guideline for conducting a systematic review of systematic reviews of healthcare interventions ( 12 ). We also followed the PRISMA guideline for reporting the methods and results of this study ( 13 ).
Eligibility criteria
All systematic reviews with available full text and in EN languages investigated the effectiveness, efficacy, safety, and outcome of the main intervention (Favipiravir, Remdesivir, Hydroxychloroquine, Ivermectin, Lopinavir/Ritonavir, or Tocilizumab) for treatment of COVID-19 patients and reported the potential limitation of the study were included.
We exclude articles that are full-text not available or used other treatment options than mentioned drugs. For example, acupuncture or traditional medicine, or supplement therapy. Preprint and without peer review articles also was excluded.
Information sources and search strategy
We searched PubMed, Scopus, and Web of Sciences (WOS) databases from inception to January 1, 2022, for the keywords COVID-19, “SARS-CoV-2,” “novel coronavirus,” “systematic review,” OR limitation in the title, abstract, or main text of the published article. There was no limitation regarding time or language. We also conducted a manual search in Google Scholar for potential missing articles. In addition to database searches, we screened reference lists of included studies after screening records were retrieved via databases and also contacted the corresponding authors of the included studies. The full search strategy for all databases is presented in Supplementary Table 1 .
Selection process
After the search was completed, all retrieved records were imported in EndNote, version X7, and duplicate removed. Two independent reviewers (HA, MM) screened the records based on the title, abstract, and full text. For increasing the agreement between reviewers we piloted a set of 30 studies before the screening. Discrepancies at this stage were resolved by consensus with a third reviewer (MA-Z).
Data collection process and data items
Two independent reviewers (HA, MM) extracted the data. We designed a data extraction table for this study, which was piloted by two reviewers (5 studies). we extracted the following data: first author name, corresponding authors name and email, Publication year, number of authors, study design, number of included studies in each included systematic review, investigated drug, country, language limitation, time of the search, number of the investigated outcome, sample size, limitations, funding statement, mean age, gender (%), protocol and registration information. Discrepancies at this stage were resolved by consensus with a third reviewer (MA-Z).
Quality appraisal
Two reviewers (HA and MM) independently assessed the quality of the included studies. We assessed the quality of the included studies using the Quality Assessment Tool (QAT) for review articles developed by healthevidence.org , which was piloted by two reviewers (5 studies) including ten quality criteria. A final review quality rating for each review is assigned: strong (8–10/10), moderate (5–7/10), or weak (1–4/10). Any discrepancies were resolved upon consultation with a third reviewer (MA-Z).
QAT tool available at: https://www.healthevidence.org/our-appraisal-tools.aspx .
Synthesis of results
For data synthesis, we prepared a table summarizing systematic review information. We also used graphs for presenting some information. We then conducted a content analysis and prepared a narrative summary of the limitations. Two authors (HA, MM) read and reread the results reported in published articles to extract limitations. The coding frame and final categories were developed by 3 authors (HA, MM, and MA-Z) using these data.
Study selection
A total of 773 records were retrieved from the database search. After removing duplicates, 525 records were screened by title, abstract, and full-text based on eligibility criteria, of which 46 studies were included in the final review ( 14 – 58 , 59 ). Twenty-seven studies were excluded after Full-text screening. The reasons for exclusion were as follows: Protocol (5 records), Preprint (6 records), Full-text not available after contacting the corresponding author (2 records), Not reporting limitation (5 records), and not investigating our target intervention (9 records). The PRISMA flow diagram for the complete study selection process is presented in Figure 1 .
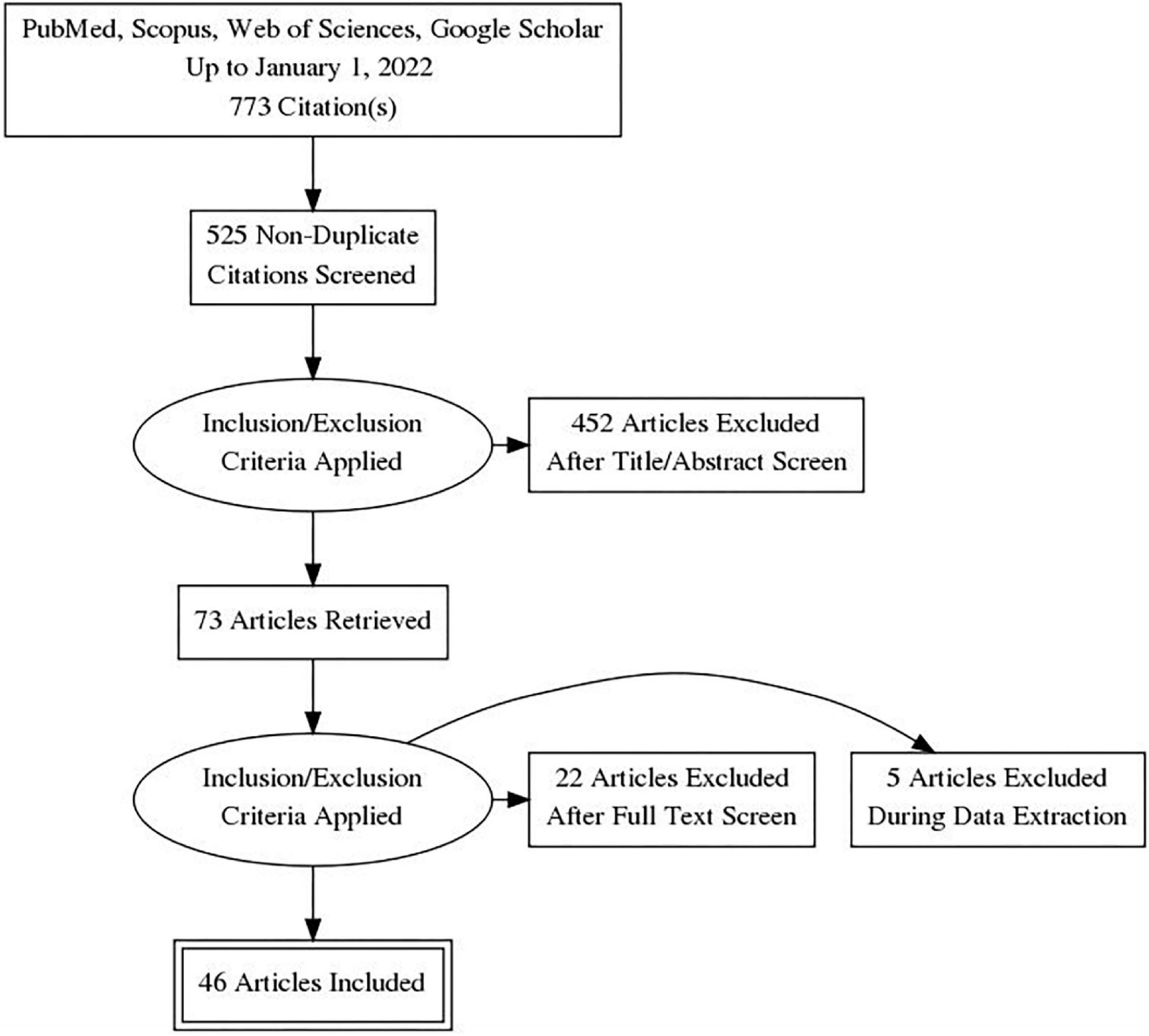
Figure 1. PRISMA flow diagram.
Study characteristics
The number of authors of the included systematic reviews varied between 3 and 58 people. Most studies were from Asia (46%), America (31%), and Europe (17%). Also, by country, most studies were reported from the United States and India ( Figure 2 ). 80.4% of the included systematic review conducted a meta-analysis. The number of included studies in the included systematic reviews varied between 2 and 136. Only 29.7% of the included systematic reviews have a registered protocol. Also, 26% of the included systematic reviews mentioned a funding statement. More details about the characteristics of included systematic reviews are presented in Table 1 . The most studied drug in the included studies was Remdesivir (17.37%) ( Figure 3 ).
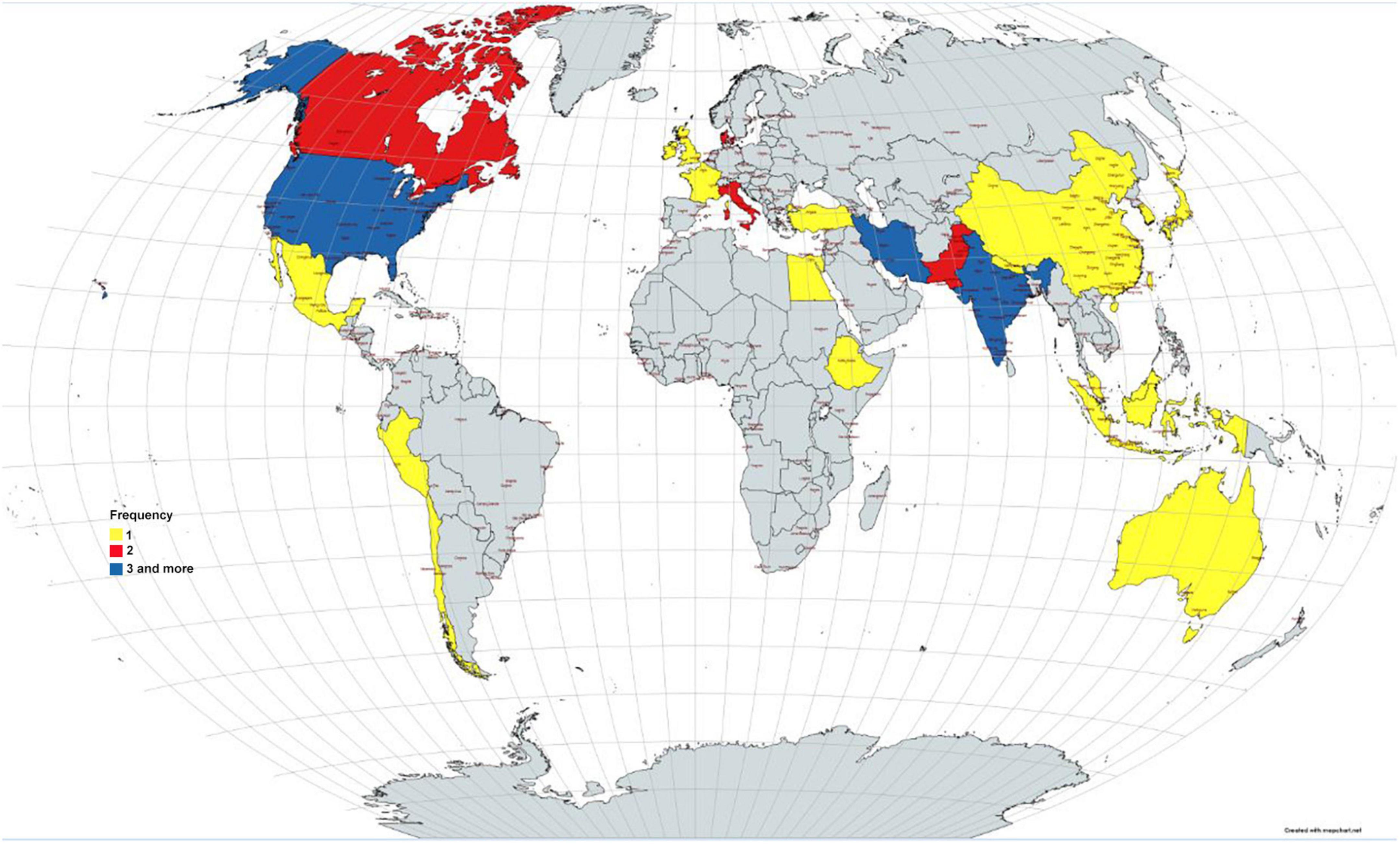
Figure 2. Distribution of the included studies by countries.
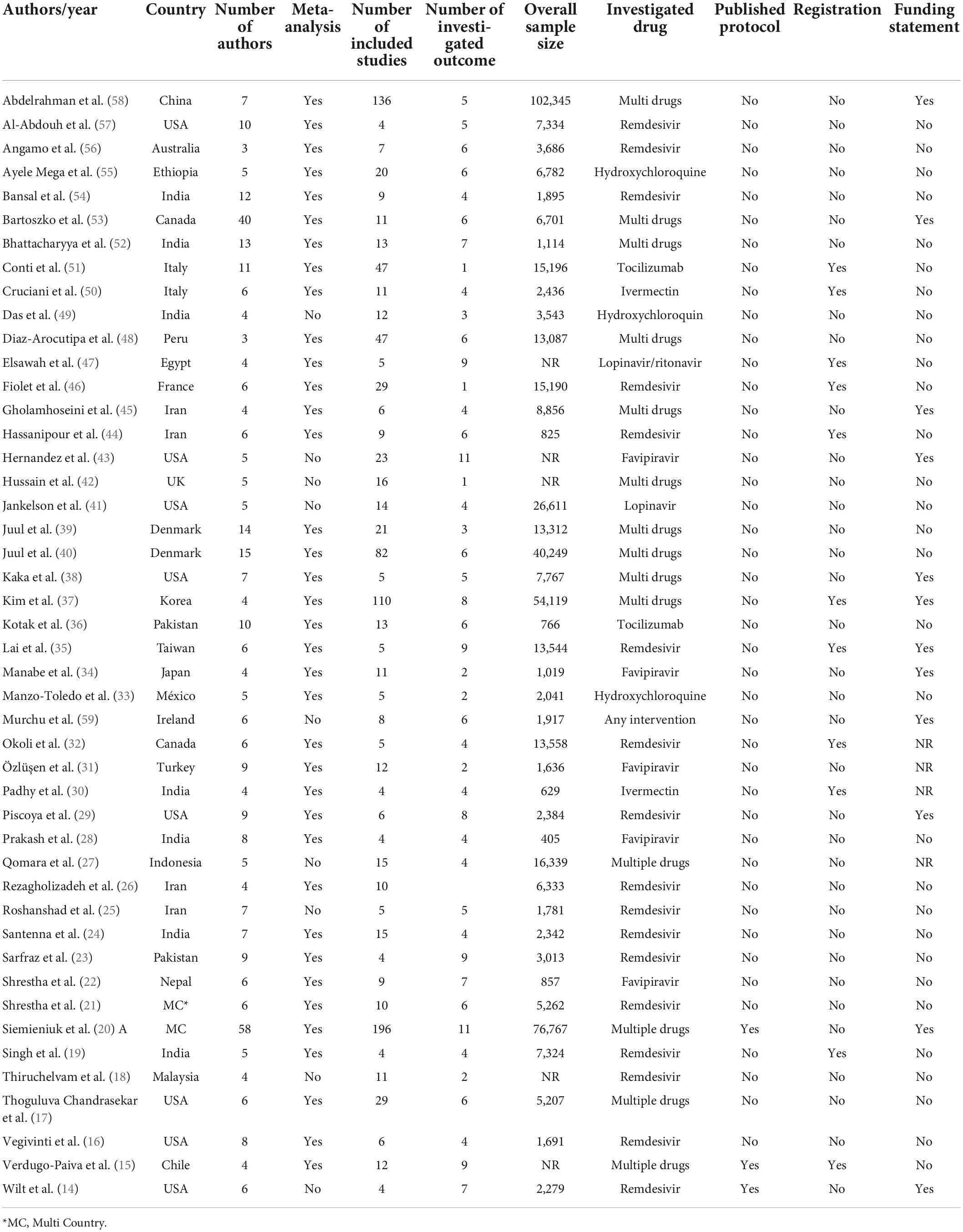
Table 1. Summary characteristics of the included studies.
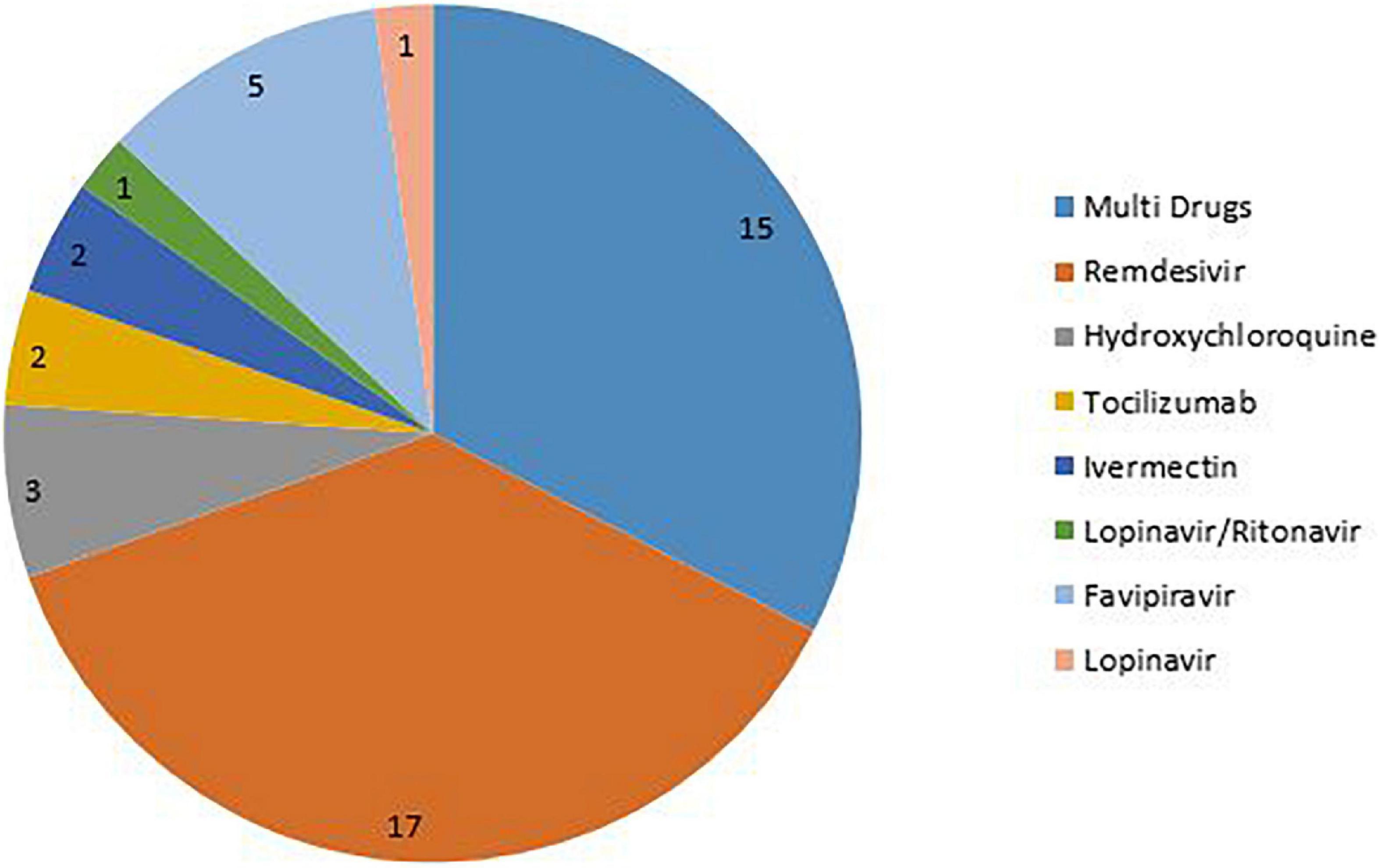
Figure 3. Distribution of the drugs in the included studies.
The overall mean quality score of the included studies was 9.5. Overall, 91% of the included studies were scored as strong quality and the remaining (9%) as moderate quality. The overall scores ranged between 7 and 10. About 74% of the included studies had a score of 10, 11% had a score of 9, 6% had a score of 8, and the remaining (9%) had a score of 7 ( Figure 4 ) (for more details about items on the QAT checklist see Supplementary Table 2 ).
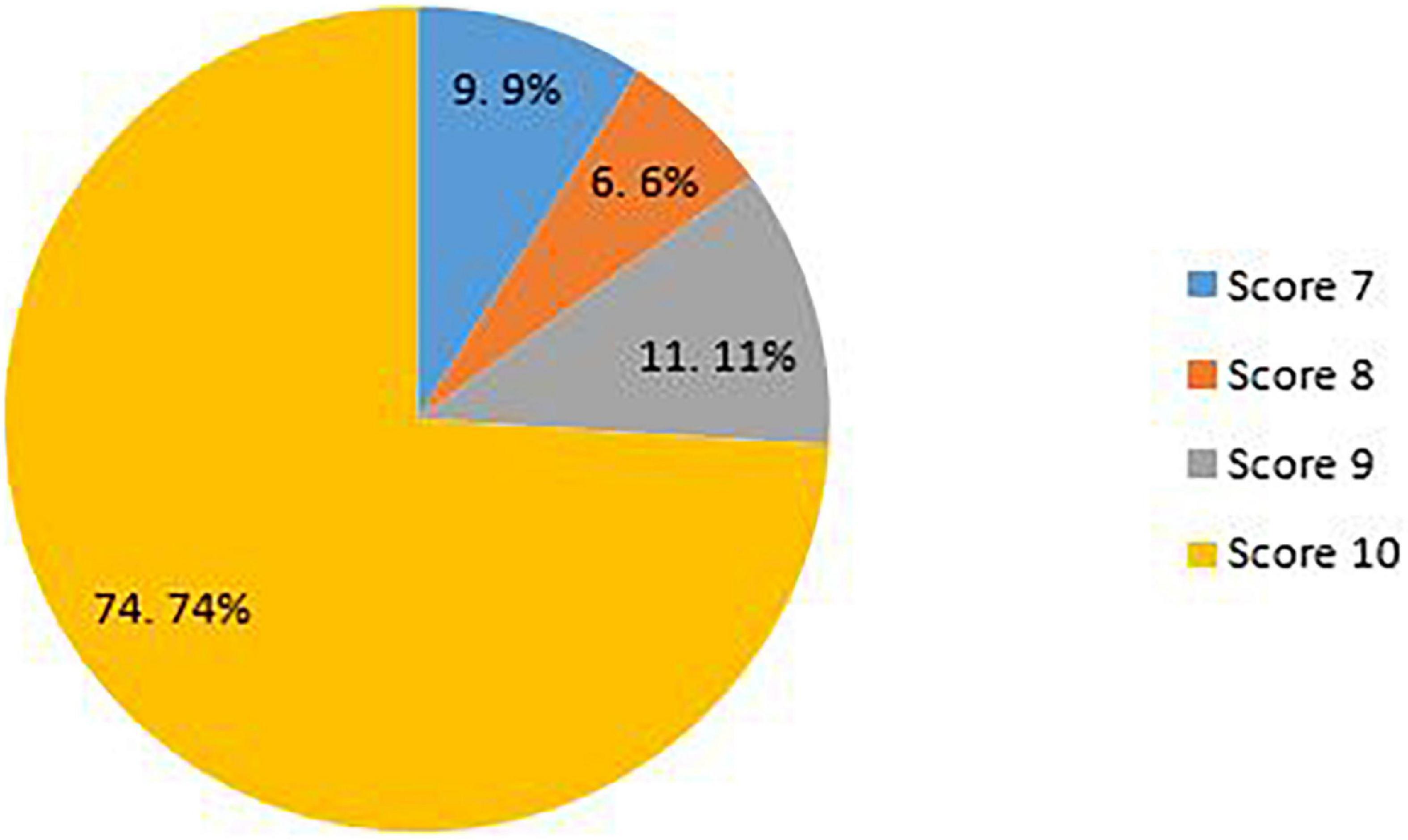
Figure 4. Quality scores of the included studies.
Results of synthesis
Potential limitations of the included studies.
Various studies have listed different limitations for the studies, some related to how the systematic review was conducted and some related to the studies included in these systematic reviews. The main limitations of the included studies were categorized in 10 domains: Heterogeneity (4 sub-categories), sample size (2 sub-categories), follow-up (2 sub-categories), treatment (7 sub-categories), included studies (4 sub-categories), design (10sub-categories), definitions (3 sub-categories), synthesis (4 sub-categories), quality (2 sub-categories), and search (4 sub-categories). The highest frequencies reported in the included studies related to the heterogeneity in sample population, small sample size, and database searches.
Heterogeneity in studies has been reported for a variety of reasons, including differences in the sample population regarding age, gender, ethnicity, and racial groups; different level of disease severity in the included patients; different control group; and difference in the investigated outcome. Treatment-related limitations were mostly related to differences regarding the administration of drug, dose, duration of treatment, and different standard protocols and guidelines. Also, there are differences related to discontinuation, combination therapy, and supportive care which obscure the effect of the main treatment.
The studies had several design shortcomings. Many studies have suffered from a lack of randomization, placebo, blinding, and comparator arm. Selection bias, and publication bias, confounding bias were also reported in the studies. Also, different strategies regarding search were another limitation. Different databases, using pre-print and un-published data, limitations on language, and missing some eligible studies were the important limitation in this regard.
More details about the potential limitations of the included systematic reviews are presented in Table 2 .
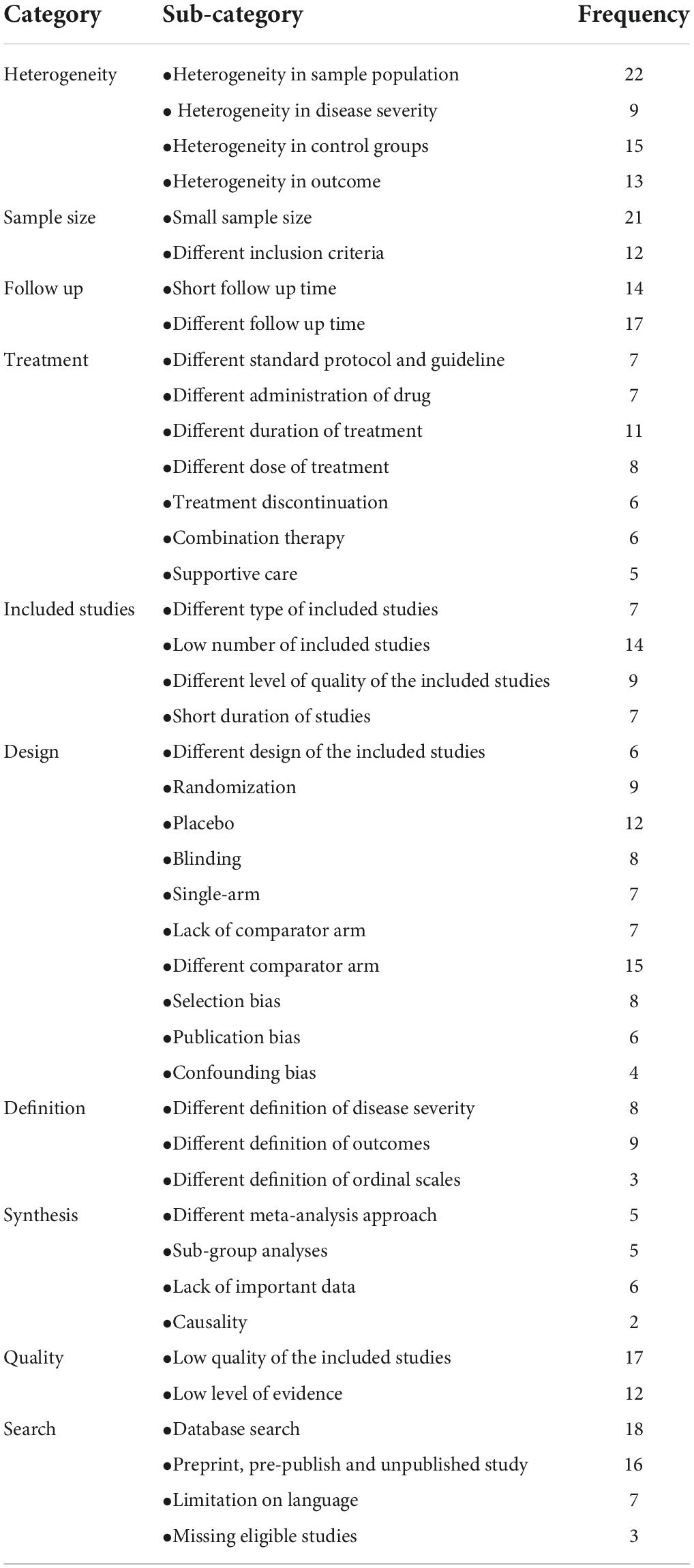
Table 2. Potential limitation of the included studies.
With the spread of the COVID-19 pandemic and has many consequences, the need arose to conduct studies and disseminate their findings ( 1 ).
Systematic reviews are a valuable resource in academia and practice. Well-done systematic reviews, which include but are not limited to meta-analyses, offer an efficient way to evaluate a large amount of information for decision-makers in areas of research, policy, and patient care. Systematic reviews can help us know what we know about a topic, and what is not yet known, often to a greater extent than the findings of a single study ( 60 – 64 ). Systemic review studies on the safety and efficacy of COVID-19 have grown in numbers. Regarding the growing number of studies and rapid publication time, there are concerns about accuracy, quality, and limitations. Richard et al. performed a systematic review to evaluate the methodological quality of currently available COVID-19 studies compared to historical controls. This research showed that COVID-19 clinical studies have a shorter time to publication and have lower methodological quality scores than control studies in the same journal ( 1 ). We tried to identify the potential limitations of COVID-19 systematic reviews which can improve and make the result of studies more accurate.
The current review examined 46 systematic reviews and all of them were conducted on COVID-19 patients. These studies differ in many aspects, including the type of treatment, follow-up time, study design, patient type, and disease severity. Most of them (80.4%) have conducted meta-analyses. Overall, 91% of the included studies were scored as strong quality, and the rest of them were moderate. The number of studies in the included systematic reviews ranged from 2 to 136.
In this study, we classified the reported limitations into 10 categories and 42 sub-categories. Heterogeneity, sample size, follow-up, treatment, including studies, design, definitions, synthesis, quality, and search are identified as the main limitation of included studies. These limitations were attributed to the included systematic reviews or due to primary studies in these systematic reviews. Among all the limitations, sample population, sample size, and database search were found to be the most-mentioned limitations with frequencies of 22, 21, and 18 in the studies, respectively. It seems that a greater number of limitations could be due to primary studies in the systematic reviews including heterogeneity, small sample size, short follow-up time, and low quality of included studies. Limitations in systematic review studies result from selection of studies, choice of relevant outcomes, methods of analysis, interpretation of heterogeneity, and generalization, application of results, and proper search ( 65 ).
Heterogeneity contains four subcategories including differences in the sample population, differences in disease severity of patients, different control group, and different measured outcomes. Differences in the sample population in terms of age, gender, race, and comorbidities in the participants are the most reported limitation. Heterogeneity across the studies may affect the study results ( 65 ). For instance, pooling the data of the original articles would be highly difficult due to heterogeneity in the study design and reported outcomes ( 25 ), and heterogeneity in disease severity could affect the treatment output.
The small sample size is the second most frequently reported limitation in 21 studies. The number of participants in the included studies was small which could decrease the power of the studies, furthermore comparing the interventions regarding the efficacy would not be incontrovertible. Therefore, the findings need to be interpreted with caution.
Database search is another important item that belongs to the search category and is reported in 18 studies. It may potentially limit access to eligible trials for inclusion and miss some data.
Treatment-related limitations are mostly associated with differences regarding the administration of drugs, dose, duration of treatment, and different standard protocols and guidelines. The lack of uniform guidelines for administering additional treatments and providing supportive care for COVID-19 patients in clinical trials may lead to inaccurate and unreliable outcomes. These limitations can generate confounding bias ( 36 ). Also, there are other items belonging to this category such as differences related to discontinuation, combination therapy, and supportive care which obscure the effect of the main treatment.
In addition to the above-mentioned limitations, different follow-up times, low quality of the included studies, pre-publish and unpublished studies, different comparator arms, and heterogeneity in control groups are the other highly reported items. The lack of a comparison/control group can limit the validity of the meta-analysis.
As mentioned, although systematic reviews are considered the gold standard of evidence for clinical decision-making, one should keep in mind that meta-analyses should neither be a replacement for well-designed large-scale randomized studies nor a justification for conducting small underpowered studies ( 65 ). As other studies reported, the quality of the methodology and reporting of present COVID-19 SR is far from optimal. In addition, Differences in disease definition and heterogeneity in studies are important factors influencing the results of these studies. Following existing guidelines and proper study design can be one of the factors reducing the limitations of these studies ( 66 , 67 ). Taken together, poor designs and various limitations of the studies render them ineffective in gaging the full extent of its safety and efficacy and thus warrant further research into the use of interventions in COVID-19 patient treatment. Our study further highlighted the importance of conducting quality studies so that the results can be trusted with more certainty.
Implications for future research
Our results can be used as a guide for designing and reporting the future studies in this field. Undoubtedly, awareness of the limitations of articles in this field can reduce bias and on the other hand increase the power of studies. Considering these issues helps researchers to report studies in a more integrated way, which can also help readers to better understand the results of studies and prevent the repetition of errors and mistakes or limitations reported in previous studies. It is recommended that researchers interested in research related to COVID-19, as well as those interested in investigating the effectiveness of treatments for this disease, must consider the points mentioned in this study when designing, implementing, and reporting their studies. In addition, respected researchers can design similar studies for other fields related to this disease and report their results. Designing such studies can greatly contribute to evidence-based decision making.
Strengths and limitations
Although this study is an overview, and the quality appraisal is optional, but the quality of the articles has been evaluated in it, which is one of the strong points of the study. Also, we conducted this overview based on Smith et al. guideline for conducting a systematic review of systematic reviews and report the results of this study based on PRISMA guideline. All the steps of this study were done by two independent reviewers, which reduces errors and increases the power of the study. There are many potential limitations to this overview. First, a literature search was conducted in the three major electronic databases, Scopus, Pubmed, and WOS, but no other databases were searched, as was the “gray” literature. Therefore, additional relevant studies might have been missed. Second, we included all systematic reviews with available full text and in EN languages investigated the effectiveness, efficacy, safety, and outcome of the main intervention (Favipiravir, Remdesivir, Hydroxychloroquine, Ivermectin, Lopinavir/Ritonavir, or Tocilizumab) for treatment of COVID-19 patients. However, there are other interventions for the treatment of this disease, which can be investigated in other studies, but due to the small number of them, they were not included in this study and only the main interventions were used. Third, we excluded articles published in preprint databases due to lack of peer review.
Data availability statement
The original contributions presented in this study are included in the article/ Supplementary material , further inquiries can be directed to the corresponding author.
Author contributions
MA-Z: idea, design, analyses, writing the first draft, and revising. MM and HA: data extraction, quality appraisal, writing the first draft, and revising. All authors read and approved the final draft before submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.966632/full#supplementary-material
1. Jung RG, Di Santo P, Clifford C, Prosperi-Porta G, Skanes S, Hung A, et al. Methodological quality of COVID-19 clinical research. Nat Commun. (2021) 12:1–10. doi: 10.1038/s41467-021-21220-5
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Ansarin K, Tolouian R, Ardalan M, Taghizadieh A, Varshochi M, Teimouri S, et al. Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: a randomized clinical trial. Bioimpacts. (2020) 10:209. doi: 10.34172/bi.2020.27
3. Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. (2020) 64:e01897–20. doi: 10.1128/AAC.01897-20
4. Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Internal Med. (2021) 181:24–31. doi: 10.1001/jamainternmed.2020.6615
5. Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.22.20040758
CrossRef Full Text | Google Scholar
6. Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. (2020) 64:e01061–20. doi: 10.1128/AAC.01061-20
7. Barceló MA, Saez M. Methodological limitations in studies assessing the effects of environmental and socioeconomic variables on the spread of COVID-19: a systematic review. Environ Sci Eur. (2021) 33:1–18. doi: 10.1186/s12302-021-00550-7
8. Alexander PE, Debono VB, Mammen MJ, Iorio A, Aryal K, Deng D, et al. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol. (2020) 123:120–6. doi: 10.1016/j.jclinepi.2020.04.016
9. Spinner CD, Gottlieb RL, Criner GJ, López JRA, Cattelan AM, Viladomiu AS, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. (2020) 324:1048–57. doi: 10.1001/jama.2020.16349
10. Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. (2013) 2:9. doi: 10.4103/2249-4863.109934
11. Khan KS, Daya S, Jadad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Arch Internal Med. (1996) 156:661–6. doi: 10.1001/archinte.156.6.661
12. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. (2011) 11:15. doi: 10.1186/1471-2288-11-15
13. Page ME, McKenzie J, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. (2021) 134:103–12. doi: 10.1016/j.jclinepi.2021.02.003
14. Wilt TJ, Kaka AS, MacDonald R, Greer N, Obley A, Duan-Porter W. Remdesivir for adults with COVID-19 : a living systematic review for American College of Physicians Practice Points. Ann Internal Med. (2021) 174:209–20. doi: 10.7326/M20-5752
15. Verdugo-Paiva F, Izcovich A, Ragusa M, Rada G. Lopinavir-ritonavir for COVID-19: a living systematic review. Medwave. (2020) 20:e7967. doi: 10.5867/medwave.2020.06.7966
16. Vegivinti CTR, Pederson JM, Saravu K, Gupta N, Barrett A, Davis AR, et al. Remdesivir therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann Med Surg. (2021) 62:43–8. doi: 10.1016/j.amsu.2020.12.051
17. Thoguluva Chandrasekar V, Venkatesalu B, Patel HK, Spadaccini M, Manteuffel J, Ramesh M. Systematic review and meta-analysis of effectiveness of treatment options against SARS-CoV-2 infection. J Med Virol. (2021) 93:775–85. doi: 10.1002/jmv.26302
18. Thiruchelvam K, Kow CS, Hadi MA, Hasan SS. The use of remdesivir for the management of patients with moderate-to-severe COVID-19: a systematic review. Exp Rev Anti Infect Ther. (2021) 20:211–29. doi: 10.1080/14787210.2021.1949984
19. Singh S, Khera D, Chugh A, Khera PS, Chugh VK. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. BMJ Open. (2021) 11:e048416. doi: 10.1136/bmjopen-2020-048416
20. Siemieniuk RAC, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Pardo-Hernandez H, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. (2020) 370:m2980.
Google Scholar
21. Shrestha DB, Budhathoki P, Rawal E, Raut S, Khadka S. Remdesivir: a potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci. (2021) 264:118663. doi: 10.1016/j.lfs.2020.118663
22. Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. (2020) 17:141. doi: 10.1186/s12985-020-01412-z
23. Sarfraz A, Sarfraz Z, Marcos Sanchez-Gonzalez JM, Michel G, Frontela O, Posada J, et al. Randomized controlled trials of remdesivir in hospitalized coronavirus disease 2019 patients: a meta-analysis. Turk J Emerg Med. (2021) 21:43. doi: 10.4103/2452-2473.309139
24. Santenna C, Vidyasagar K, Amarneni KC, Ghanta SN, Sadasivam B, Pathan S, et al. The safety, tolerability and mortality reduction efficacy of remdesivir; based on randomized clinical trials, observational and case studies reported safety outcomes: an updated systematic review and meta-analysis. Therap Adv Drug Saf. (2021) 12:20420986211042517. doi: 10.1177/20420986211042517
25. Roshanshad A, Kamalipour A, Ashraf MA, Roshanshad R, Jafari S, Nazemi P, et al. The efficacy of remdesivir in coronavirus disease 2019 (COVID-19): a systematic review. Iran J Microbiol. (2020) 12:376. doi: 10.18502/ijm.v12i5.4597
26. Rezagholizadeh A, Khiali S, Sarbakhsh P, Entezari-Maleki T. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur J Pharmacol. (2021) 897:173926. doi: 10.1016/j.ejphar.2021.173926
27. Qomara WF, Primanissa DN, Amalia SH, Purwadi FV, Zakiyah N. Effectiveness of remdesivir, lopinavir/ritonavir, and favipiravir for COVID-19 treatment: a systematic review. Int J Gen Med. (2021) 14:8557–71. doi: 10.2147/IJGM.S332458
28. Prakash A, Singh H, Kaur H, Semwal A, Sarma P, Bhattacharyya A, et al. Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients. Indian J Pharmacol. (2020) 52:414–21. doi: 10.4103/ijp.ijp_998_20
29. Piscoya A, Ng-Sueng LF, Parra del Riego A, Cerna-Viacava R, Pasupuleti V, Roman YM, et al. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PLoS One. (2020) 15:e0243705. doi: 10.1371/journal.pone.0243705
30. Padhy BM, Mohanty RR, Das S, Meher BR. Therapeutic potential of ivermectin as add on treatment in COVID 19: a systematic review and meta-analysis. J Pharm Pharmaceut Sci. (2020) 23:462–9. doi: 10.18433/jpps31457
31. Özlüşen B, Kozan ş, Akcan RE, Kalender M, Yaprak D, Peltek ÝB, et al. Effectiveness of favipiravir in COVID-19: a live systematic review. Eur J Clin Microbiol Infect Dis. (2021) 40:2575–83. doi: 10.1007/s10096-021-04307-1
32. Okoli GN, Rabbani R, Copstein L, Al-Juboori A, Askin N, Abou-Setta AM. Remdesivir for coronavirus disease 2019 (COVID-19): a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Infect Dis. (2021) 53:691–9. doi: 10.1080/23744235.2021.1923799
33. Manzo-Toledo A, Torres-Rosas R, Mendieta-Zerón H, Arriaga-Pizano L, Argueta-Figueroa L. Hydroxychloroquine in the treatment of covid-19 disease: a systematic review and meta-analysis. Med J Indonesia. (2021) 30:20–32. doi: 10.13181/mji.oa.205012
34. Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. (2021) 21:489. doi: 10.1186/s12879-021-06164-x
35. Lai C-C, Chen C-H, Wang C-Y, Chen K-H, Wang Y-H, Hsueh P-R. Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. J Antimicrob Chemother. (2021) 76:1962–8. doi: 10.1093/jac/dkab093
36. Kotak S, Khatri M, Malik M, Malik M, Hassan W, Amjad A, et al. Use of tocilizumab in COVID-19: a systematic review and meta-analysis of current evidence. Cureus. (2020) 12:e10869. doi: 10.7759/cureus.10869
37. Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med. (2020) 17:e1003501. doi: 10.1371/journal.pmed.1003501
38. Kaka AS, MacDonald R, Greer N, Vela K, Duan-Porter W, Obley A, et al. Major update: remdesivir for adults with COVID-19 : a living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann Internal Med. (2021) 174:663–72. doi: 10.7326/M20-8148
39. Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS Med. (2020) 17:e1003293. doi: 10.1371/journal.pmed.1003293
40. Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS One. (2021) 16:e0248132. doi: 10.1371/journal.pone.0248132
41. Jankelson L, Karam G, Becker ML, Chinitz LA, Tsai MC. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm. (2020) 17:1472–9. doi: 10.1016/j.hrthm.2020.05.008
42. Hussain N, Yoganathan A, Hewage S, Alom S, Harky A. The effect of antivirals on COVID-19: a systematic review. Expert Rev Anti Infect Ther. (2021) 19:473–86. doi: 10.1080/14787210.2021.1823832
43. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Internal Med. (2020) 173:287–96. doi: 10.7326/M20-2496
44. Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. (2021) 11:11022. doi: 10.1038/s41598-021-90551-6
45. Gholamhoseini MT, Yazdi-Feyzabadi V, Goudarzi R, Mehrolhassani MH. Safety and efficacy of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. J Pharm Pharmaceut Sci. (2021) 24:237–45. doi: 10.18433/jpps31870
46. Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. (2021) 27:19–27. doi: 10.1016/j.cmi.2020.08.022
47. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol. (2021) 31:e2187. doi: 10.1002/rmv.2187
48. Diaz-Arocutipa C, Brañez-Condorena A, Hernandez AV. QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. (2021) 30:694–706. doi: 10.1002/pds.5234
49. Das S, Bhowmick S, Tiwari S, Sen S. An updated systematic review of the therapeutic role of hydroxychloroquine in coronavirus disease-19 (COVID-19). Clin Drug Invest. (2020) 40:591–601. doi: 10.1007/s40261-020-00927-1
50. Cruciani M, Pati I, Masiello F, Malena M, Pupella S, De Angelis V. Ivermectin for prophylaxis and treatment of COVID-19: a systematic review and meta-analysis. Diagnostics. (2021) 11:1645. doi: 10.3390/diagnostics11091645
51. Conti V, Corbi G, Sellitto C, Sabbatino F, Maci C, Bertini N, et al. Effect of tocilizumab in reducing the mortality rate in covid-19 patients: a systematic review with meta-analysis. J Pers Med. (2021) 11:628. doi: 10.3390/jpm11070628
52. Bhattacharyya A, Kumar S, Sarma P, Kaur H, Prajapat M, Shekhar N, et al. Safety and efficacy of lopinavir/ritonavir combination in COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Indian J Pharmacol. (2020) 52:313–23.
PubMed Abstract | Google Scholar
53. Bartoszko JJ, Siemieniuk RAC, Kum E, Qasim A, Zeraatkar D, Ge L, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. (2021) 373:n949. doi: 10.1136/bmj.n949
54. Bansal V, Mahapure KS, Bhurwal A, Gupta I, Hassanain S, Makadia J, et al. Mortality benefit of remdesivir in COVID-19: a systematic review and meta-analysis. Front Med. (2021) 7:606429. doi: 10.3389/fmed.2020.606429
55. Ayele Mega T, Feyissa TM, Dessalegn Bosho D, Kumela Goro K, Zeleke Negera G. The outcome of hydroxychloroquine in patients treated for COVID-19: systematic review and meta-analysis. Can Respir J. (2020) 2020:4312519. doi: 10.1155/2020/4312519
56. Angamo MT, Mohammed MA, Peterson GM. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. (2021) 50:27–41. doi: 10.1007/s15010-021-01671-0
57. Al-Abdouh A, Bizanti A, Barbarawi M, Jabri A, Kumar A, Fashanu OE, et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Contemp Clin Trials. (2021) 101:106272. doi: 10.1016/j.cct.2021.106272
58. Abdelrahman Z, Liu Q, Jiang S, Li M, Sun Q, Zhang Y, et al. Evaluation of the current therapeutic approaches for COVID-19: a systematic review and a meta-analysis. Front Pharmacol. (2021) 12:607408. doi: 10.3389/fphar.2021.607408
59. O Murchu E, Spillane S, Byrne P, O’Neill M, Harrington P, Ryan M. Interventions in an ambulatory setting to prevent progression to severe disease in patients with COVID-19: a systematic review. Ann. Pharmacother. (2021) 56:309–18.
60. Baker KA, Weeks SM. An overview of systematic review. J Perianesth Nurs. (2014) 29:454–8. doi: 10.1016/j.jopan.2014.07.002
61. Leucht S, Kissling W, Davis J. How to read and understand and use systematic reviews and meta-analyses. Acta Psychiatr Scand. (2009) 119:443–50. doi: 10.1111/j.1600-0447.2009.01388.x
62. Mulrow CD. Systematic reviews: rationale for systematic reviews. BMJ. (1994) 309:597–9. doi: 10.1136/bmj.309.6954.597
63. Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Internal Med. (1997) 126:376–80. doi: 10.7326/0003-4819-126-5-199703010-00006
64. Owens JK. Systematic reviews: brief overview of methods, limitations, and resources. Nurse Author Ed. (2021) 31:69–72. doi: 10.1111/nae2.28
65. Bartolucci AA, Hillegass WB. Overview, strengths, and limitations of systematic reviews and meta-analyses. In: F Chiappelli editor. Evidence-Based Practice: Toward Optimizing Clinical Outcomes. Berlin: Springer (2010). p. 17–33. doi: 10.1007/978-3-642-05025-1_2
66. Wurth R, Hajdenberg M, Barrera FJ, Shekhar S, Copacino CE, Moreno-Peña PJ, et al. Scoping review of COVID-19-related systematic reviews and meta-analyses: can we really have confidence in their results? Postgrad Med J. (2022) 98:372–9. doi: 10.1136/postgradmedj-2020-139392
67. Nothacker J, Stadelmaier J, Siemens W, Meerpohl JJ, Schmucker C. Characteristics of registered and published systematic reviews focusing on the prevention of COVID-19: a meta-research study. BMJ Open. (2022) 12:e060255. doi: 10.1136/bmjopen-2021-060255
Keywords : COVID-19, systematic review, limitations, intervention, treatment
Citation: Mohseni M, Ameri H and Arab-Zozani M (2022) Potential limitations in systematic review studies assessing the effect of the main intervention for treatment/therapy of COVID-19 patients: An overview. Front. Med. 9:966632. doi: 10.3389/fmed.2022.966632
Received: 11 June 2022; Accepted: 30 August 2022; Published: 15 September 2022.
Reviewed by:
Copyright © 2022 Mohseni, Ameri and Arab-Zozani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morteza Arab-Zozani, [email protected] ; orcid.org/0000-0001-7223-6707
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The challenges arising from the COVID-19 pandemic and the way people deal with them. A qualitative longitudinal study
Contributed equally to this work with: Dominika Maison, Diana Jaworska, Dominika Adamczyk, Daria Affeltowicz
Roles Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing
Affiliation Faculty of Psychology, University of Warsaw, Warsaw, Poland
Roles Formal analysis, Investigation, Writing – original draft, Writing – review & editing
Roles Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Roles Conceptualization, Formal analysis, Investigation, Methodology
- Dominika Maison,
- Diana Jaworska,
- Dominika Adamczyk,
- Daria Affeltowicz

- Published: October 11, 2021
- https://doi.org/10.1371/journal.pone.0258133
- Peer Review
- Reader Comments
The conducted qualitative research was aimed at capturing the biggest challenges related to the beginning of the COVID-19 pandemic. The interviews were carried out in March-June (five stages of the research) and in October (the 6 th stage of the research). A total of 115 in-depth individual interviews were conducted online with 20 respondents, in 6 stages. The results of the analysis showed that for all respondents the greatest challenges and the source of the greatest suffering were: a) limitation of direct contact with people; b) restrictions on movement and travel; c) necessary changes in active lifestyle; d) boredom and monotony; and e) uncertainty about the future.
Citation: Maison D, Jaworska D, Adamczyk D, Affeltowicz D (2021) The challenges arising from the COVID-19 pandemic and the way people deal with them. A qualitative longitudinal study. PLoS ONE 16(10): e0258133. https://doi.org/10.1371/journal.pone.0258133
Editor: Shah Md Atiqul Haq, Shahjalal University of Science and Technology, BANGLADESH
Received: April 6, 2021; Accepted: September 18, 2021; Published: October 11, 2021
Copyright: © 2021 Maison et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files ( S1 Dataset ).
Funding: This work was supported by the Faculty of Psychology, University of Warsaw, Poland from the funds awarded by the Ministry of Science and Higher Education in the form of a subsidy for the maintenance and development of research potential in 2020 (501-D125-01-1250000). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The coronavirus disease (COVID-19), discovered in December 2019 in China, has reached the level of a pandemic and, till June 2021, it has affected more than 171 million people worldwide and caused more than 3.5 million deaths all over the world [ 1 ]. The COVID-19 pandemic as a major health crisis has caught the attention of many researchers, which has led to the creation of a broad quantitative picture of human behavior during the coronavirus outbreak [ 2 – 4 ]. What has been established so far is, among others, the psychological symptoms that can occur as a result of lockdown [ 2 ], and the most common coping strategies [ 5 ]. However, what we still miss is an in-depth understanding of the changes in the ways of coping with challenges over different stages of the pandemic. In the following study, we used a longitudinal qualitative method to investigate the challenges during the different waves of the coronavirus pandemic as well as the coping mechanisms accompanying them.
In Poland, the first patient was diagnosed with COVID-19 on the 4 th March 2020. Since then, the number of confirmed cases has grown to more than 2.8 million and the number of deaths to more than 73,000 (June 2021) [ 1 ]. From mid-March 2020, the Polish government, similarly to many other countries, began to introduce a number of restrictions to limit the spread of the virus. These restrictions had been changing from week to week, causing diverse reactions in people [ 6 ]. It needs to be noted that the reactions to such a dynamic situation cannot be covered by a single study. Therefore, in our study we used qualitative longitudinal research in order to monitor changes in people’s emotions, attitudes, and behavior. So far, few longitudinal studies have been carried out that investigated the various issues related to the COVID-19 pandemic; however, all of them were quantitative [ 7 – 10 ]. The qualitative approach (and especially the use of enabling and projective techniques) allows for an in-depth exploration of respondents’ reactions that goes beyond respondents’ declarations and captures what they are less aware of or even unconscious of. This study consisted of six stages of interviews that were conducted at key moments for the development of the pandemic situation in Poland. The first stage of the study was carried out at the moment of the most severe lockdown and the biggest restrictions (March 2020) and was focused on exploration how did people react to the new uncertain situation. The second stage of the study was conducted at the time when restrictions were extended and the obligation to cover the mouth and nose everywhere outside the household were introduced (middle of April 2020) and was focused at the way how did people deal with the lack of family gatherings over Easter. The third stage of the study was conducted at the moment of announcing the four stages of lifting the restrictions (April 2020) and was focused on people’s reaction to an emerging vision of getting back to normalcy. The fourth stage of the study was carried out, after the introduction of the second stage of lifting the restrictions: shopping malls, hotels, and cultural institutions were gradually being opened (May 2020). The fifth stage of the study was conducted after all four stages of restriction lifting were in place (June 2020). Only the obligation to cover the mouth and nose in public spaces, an order to maintain social distance, as well as the functioning of public places under a sanitary regime were still in effect. During those 5 stages coping strategies with the changes in restrictions were explored. The sixth and last stage of the study was a return to the respondents after a longer break, at the turn of October and November 2020, when the number of coronavirus cases in Poland began to increase rapidly and the media declared “the second wave of the pandemic”. It was the moment when the restrictions were gradually being reintroduced. A full description of the changes occurring in Poland at the time of the study can be found in S1 Table .
The following study is the first qualitative longitudinal study investigating how people cope with the challenges arising from the COVID-19 pandemic at its different stages. The study, although conducted in Poland, shows the universal psychological relations between the challenges posed by the pandemic (and, even more, the restrictions resulting from the pandemic, which were very similar across different countries, not only European) and the ways of dealing with them.
Literature review
The COVID-19 pandemic has led to a global health crisis with severe economic [ 11 ], social [ 3 ], and psychological consequences [ 4 ]. Despite the fact that there were multiple crises in recent years, such as natural disasters, economic crises, and even epidemics, the coronavirus pandemic is the first in 100 years to severely affect the entire world. The economic effects of the COVID-19 pandemic concern an impending global recession caused by the lockdown of non-essential industries and the disruption of production and supply chains [ 11 ]. Social consequences may be visible in many areas, such as the rise in family violence [ 3 ], the ineffectiveness of remote education, and increased food insecurity among impoverished families due to school closures [ 12 ]. According to some experts, the psychological consequences of COVID-19 are the ones that may persist for the longest and lead to a global mental health crisis [ 13 ]. The coronavirus outbreak is generating increased depressive symptoms, stress, anxiety, insomnia, denial, fear, and anger all over the world [ 2 , 14 ]. The economic, social, and psychological problems that people are currently facing are the consequences of novel challenges that have been posed by the pandemic.
The coronavirus outbreak is a novel, uncharted situation that has shaken the world and completely changed the everyday lives of many individuals. Due to the social distancing policy, many people have switched to remote work—in Poland, almost 75% of white-collar workers were fully or partially working from home from mid-March until the end of May 2020 [ 15 ]. School closures and remote learning imposed a new obligation on parents of supervising education, especially with younger children [ 16 ]. What is more, the government order of self-isolation forced people to spend almost all their time at home and limit or completely abandon human encounters. In addition, the deteriorating economic situation was the cause of financial hardship for many people. All these difficulties and challenges arose in the aura of a new, contagious disease with unexplored, long-lasting health effects and not fully known infectivity and lethality [ 17 ]. Dealing with the situation was not facilitated by the phenomenon of global misinformation, called by some experts as the “infodemic”, which may be defined as an overabundance of information that makes it difficult for people to find trustworthy sources and reliable guidance [ 18 ]. Studies have shown that people have multiple ways of reacting to a crisis: from radical and even violent practices, towards individual solutions and depression [ 19 ]. Not only the challenges arising from the COVID-19 pandemic but also the ways of reacting to it and coping with it are issues of paramount importance that are worth investigating.
The reactions to unusual crisis situations may be dependent on dispositional factors, such as trait anxiety or perceived control [ 20 , 21 ]. A study on reactions to Hurricane Hugo has shown that people with higher trait anxiety are more likely to develop posttraumatic symptoms following a natural disaster [ 20 ]. Moreover, lack of perceived control was shown to be positively related to the level of distress during an earthquake in Turkey [ 21 ]. According to some researchers, the COVID-19 crisis and natural disasters have much in common, as the emotions and behavior they cause are based on the same primal human emotion—fear [ 22 ]. Both pandemics and natural disasters disrupt people’s everyday lives and may have severe economic, social and psychological consequences [ 23 ]. However, despite many similarities to natural disasters, COVID-19 is a unique situation—only in 2020, the current pandemic has taken more lives than the world’s combined natural disasters in any of the past twenty years [ 24 ]. It needs to be noted that natural disasters may pose different challenges than health crises and for this reason, they may provoke disparate reactions [ 25 ]. Research on the reactions to former epidemics has shown that avoidance and safety behaviors, such as avoiding going out, visiting crowded places, and visiting hospitals, are widespread at such times [ 26 ]. When it comes to the ways of dealing with the current COVID-19 pandemic, a substantial part of the quantitative research on this issue focuses on coping mechanisms. Studies have shown that the most prevalent coping strategies are highly problem-focused [ 5 ]. Most people tend to listen to expert advice and behave calmly and appropriately in the face of the coronavirus outbreak [ 5 ]. Problem-focused coping is particularly characteristic of healthcare professionals. A study on Chinese nurses has shown that the closer the problem is to the person and the more fear it evokes, the more problem-focused coping strategy is used to deal with it [ 27 ]. On the other hand, a negative coping style that entails risky or aggressive behaviors, such as drug or alcohol use, is also used to deal with the challenges arising from the COVID-19 pandemic [ 28 ]. The factors that are correlated with negative coping include coronavirus anxiety, impairment, and suicidal ideation [ 28 ]. It is worth emphasizing that social support is a very important component of dealing with crises [ 29 ].
Scientists have attempted to systematize the reactions to difficult and unusual situations. One such concept is the “3 Cs” model created by Reich [ 30 ]. It accounts for the general rules of resilience in situations of stress caused by crises, such as natural disasters. The 3 Cs stand for: control (a belief that personal resources can be accessed to achieve valued goals), coherence (the human desire to make meaning of the world), and connectedness (the need for human contact and support) [ 30 ]. Polizzi and colleagues [ 22 ] reviewed this model from the perspective of the current COVID-19 pandemic. The authors claim that natural disasters and COVID-19 pandemic have much in common and therefore, the principles of resilience in natural disaster situations can also be used in the situation of the current pandemic [ 22 ]. They propose a set of coping behaviors that could be useful in times of the coronavirus outbreak, which include control (e.g., planning activities for each day, getting adequate sleep, limiting exposure to the news, and helping others), coherence (e.g., mindfulness and developing a coherent narrative on the event), and connectedness (e.g., establishing new relationships and caring for existing social bonds) [ 22 ].
Current study
The issue of the challenges arising from the current COVID-19 pandemic and the ways of coping with them is complex and many feelings accompanying these experiences may be unconscious and difficult to verbalize. Therefore, in order to explore and understand it deeply, qualitative methodology was applied. Although there were few qualitative studies on the reaction to the pandemic [e.g., 31 – 33 ], they did not capture the perception of the challenges and their changes that arise as the pandemic develops. Since the situation with the COVID-19 pandemic is very dynamic, the reactions to the various restrictions, orders or bans are evolving. Therefore, it was decided to conduct a qualitative longitudinal study with multiple interviews with the same respondents [ 34 ].
The study investigates the challenges arising from the current pandemic and the way people deal with them. The main aim of the project was to capture people’s reactions to the unusual and unexpected situation of the COVID-19 pandemic. Therefore, the project was largely exploratory in nature. Interviews with the participants at different stages of the epidemic allowed us to see a wide spectrum of problems and ways of dealing with them. The conducted study had three main research questions:
- What are the biggest challenges connected to the COVID-19 pandemic and the resulting restrictions?
- How are people dealing with the pandemic challenges?
- What are the ways of coping with the restrictions resulting from a pandemic change as it continues and develops (perspective of first 6 months)?
The study was approved by the institutional review board of the Faculty of Psychology University of Warsaw, Poland. All participants were provided written and oral information about the study, which included that participation was voluntary, that it was possible to withdraw without any consequences at any time, and the precautions that would be taken to protect data confidentiality. Informed consent was obtained from all participants. To ensure confidentiality, quotes are presented only with gender, age, and family status.
The study was based on qualitative methodology: individual in-depth interviews, s which are the appropriate to approach a new and unknown and multithreaded topic which, at the beginning of 2020, was the COVID-19 pandemic. Due to the need to observe respondents’ reactions to the dynamically changing situation of the COVID-19 pandemic, longitudinal study was used where the moderator met on-line with the same respondent several times, at specific time intervals. A longitudinal study was used to capture the changes in opinions, emotions, and behaviors of the respondents resulting from the changes in the external circumstances (qualitative in-depth interview tracking–[ 34 ]).
The study took place from the end of March to October 2020. Due to the epidemiological situation in the country interviews took place online, using the Google Meets online video platform. The audio was recorded and then transcribed. Before taking part in the project, the respondents were informed about the purpose of the study, its course, and the fact that participation in the project is voluntary, and that they will be able to withdraw from participation at any time. The respondents were not paid for taking part in the project.
Participants.
In total, 115 interviews were conducted with 20 participants (6 interviews with the majority of respondents). Two participants (number 11 and 19, S2 Table ) dropped out of the last two interviews, and one (number 6) dropped out of the last interview. The study was based on a purposive sample and the respondents differed in gender, age, education, family status, and work situation (see S2 Table ). In addition to demographic criteria intended to ensure that the sample was as diverse as possible, an additional criterion was to have a permanent Internet connection and a computer capable of online video interviewing. Study participants were recruited using the snowball method. They were distant acquaintances of acquaintances of individuals involved in the study. None of the moderators knew their interviewees personally.
A total of 10 men and 10 women participated in the study; their age range was: 25–55; the majority had higher education (17 respondents), they were people with different professions and work status, and different family status (singles, couples without children, and families with children). Such diversity of respondents allowed us to obtain information from different life perspectives. A full description of characteristics of study participants can be found in S2 Table .
Each interview took 2 hours on average, which gives around 240 hours of interviews. Subsequent interviews with the same respondents conducted at different intervals resulted from the dynamics of the development of the pandemic and the restrictions introduced in Poland by the government.
The interviews scenario took a semi-structured form. This allowed interviewers freely modify the questions and topics depending on the dynamics of the conversation and adapt the subject matter of the interviews not only to the research purposes but also to the needs of a given respondent. The interview guides were modified from week to week, taking into account the development of the epidemiological situation, while at the same time maintaining certain constant parts that were repeated in each interview. The main parts of the interview topic guide consisted of: (a) experiences from the time of previous interviews: thoughts, feeling, fears, and hopes; (b) everyday life—organization of the day, work, free time, shopping, and eating, etc.; (c) changes—what had changed in the life of the respondent from the time of the last interview; (d) ways of coping with the situation; and (e) media—reception of information appearing in the media. Additionally, in each interview there were specific parts, such as the reactions to the beginning of the pandemic in the first interview or the reaction to the specific restrictions that were introduced.
The interviews were conducted by 5 female interviewers with experience in moderating qualitative interviews, all with a psychological background. After each series of interviews, all the members of the research teams took part in debriefing sessions, which consisted of discussing the information obtained from each respondent, exchanging general conclusions, deciding about the topics for the following interview stage, and adjusting them to the pandemic situation in the country.
Data analysis.
All the interviews were transcribed in Polish by the moderators and then double-checked (each moderator transcribed the interviews of another moderator, and then the interviewer checked the accuracy of the transcription). The whole process of analysis was conducted on the material in Polish (the native language of the authors of the study and respondents). The final page count of the transcript is approximately 1800 pages of text. The results presented below are only a portion of the total data collected during the interviews. While there are about 250 pages of the transcription directly related to the topic of the article, due to the fact that the interview was partly free-form, some themes merge with others and it is not possible to determine the exact number of pages devoted exclusively to analysis related to the topic of the article. Full dataset can be found in S1 Dataset .
Data was then processed into thematic analysis, which is defined as a method of developing qualitative data consisting of the identification, analysis, and description of the thematic areas [ 35 ]. In this type of analysis, a thematic unit is treated as an element related to the research problem that includes an important aspect of data. An important advantage of thematic analysis is its flexibility, which allows for the adoption of the most appropriate research strategy to the phenomenon under analysis. An inductive approach was used to avoid conceptual tunnel vision. Extracting themes from the raw data using an inductive approach precludes the researcher from imposing a predetermined outcome.
As a first step, each moderator reviewed the transcripts of the interviews they had conducted. Each transcript was thematically coded individually from this point during the second and the third reading. In the next step, one of the researchers reviewed the codes extracted by the other members of the research team. Then she made initial interpretations by generating themes that captured the essence of the previously identified codes. The researcher created a list of common themes present in all of the interviews. In the next step, the extracted themes were discussed again with all the moderators conducting the coding in order to achieve consistency. This collaborative process was repeated several times during the analysis. Here, further superordinate (challenges of COVID-19 pandemic) and subordinate (ways of dealing with challenges) themes were created, often by collapsing others together, and each theme listed under a superordinate and subordinate category was checked to ensure they were accurately represented. Through this process of repeated analysis and discussion of emerging themes, it was possible to agree on the final themes that are described below.
Main challenges of the COVID-19 pandemic.
Challenge 1 –limitation of direct contact with people . The first major challenge of the pandemic was that direct contact with other people was significantly reduced. The lockdown forced many people to work from home and limit contact not only with friends but also with close family (parents, children, and siblings). Limiting contact with other people was a big challenge for most of our respondents, especially those who were living alone and for those who previously led an active social life. Depending on their earlier lifestyle profile, for some, the bigger problem was the limitation of contact with the family, for others with friends, and for still others with co-workers.
I think that because I can’t meet up with anyone and that I’m not in a relationship , I miss having sex , and I think it will become even more difficult because it will be increasingly hard to meet anyone . (5 . 3_ M_39_single) . The number In the brackets at the end of the quotes marks the respondent’s number (according to Table 1 ) and the stage of the interview (after the dash), further is information about gender (F/M), age of the respondent and family status. Linguistic errors in the quotes reflect the spoken language of the respondents.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0258133.t001
Changes over time . Over the course of the 6 months of the study, an evolution in the attitudes to the restriction of face-to-face contact could be seen: from full acceptance, to later questioning its rationale. Initially (March and April), almost all the respondents understood the reasons for the isolation and were compliant. At the beginning, people were afraid of the unknown COVID-19. They were concerned that the tragic situation from Italy, which was intensively covered in the media, could repeat itself in Poland (stage 1–2 of the study). However, with time, the isolation started to bother them more and more, and they started to look for solutions to bypass the isolation guidelines (stage 3–4), both real (simply meeting each other) and mental (treating isolation only as a guideline and not as an order, perceiving the family as being less threatening than acquaintances or strangers in a store). The turning point was the long May weekend that, due to two public holidays (1 st and 3 rd May), has for many years been used as an opportunity to go away with family or friends. Many people broke their voluntary isolation during that time encouraged by information about the coming loosening of restrictions.
During the summer (stage 5 of the survey), practically no one was fully compliant with the isolation recommendations anymore. At that time, a growing familiarity could be observed with COVID-19 and an increasing tendency to talk about it as “one of many diseases”, and to convince oneself that one is not at risk and that COVID-19 is no more threatening than other viruses. Only a small group of people consciously failed to comply with the restrictions of contact with others from the very beginning of the pandemic. This behavior was mostly observed among people who were generally less anxious and less afraid of COVID-19.
I’ve had enough. I’ve had it with sitting at home. Okay, there’s some kind of virus, it’s as though it’s out there somewhere; it’s like I know 2 people who were infected but they’re still alive, nothing bad has happened to anyone. It’s just a tiny portion of people who are dying. And is it really such a tragedy that we have to be locked up at home? Surely there’s an alternative agenda there? (17.4_F_35_Adult and child)
Ways of dealing . In the initial phase, when almost everyone accepted this restriction and submitted to it, the use of communication platforms for social meetings increased (see Ways of dealing with challenges in Table 1 ) . Meetings on communication platforms were seen as an equivalent of the previous face-to-face contact and were often even accompanied by eating or drinking alcohol together. However, over time (at around stage 4–5 of the study) people began to feel that such contact was an insufficient substitute for face-to-face meetings and interest in online meetings began to wane. During this time, however, an interesting phenomenon could be seen, namely, that for many people the family was seen as a safer environment than friends, and definitely safer than strangers. The belief was that family members would be honest about being sick, while strangers not necessarily, and—on an unconscious level—the feeling was that the “family is safe”, and the “family can’t hurt them”.
When it became clear that online communication is an insufficient substitute for face-to-face contacts, people started to meet up in real life. However, a change in many behaviors associated with meeting people is clearly visible, e.g.: refraining from shaking hands, refraining from cheek kissing to greet one another, and keeping a distance during a conversation.
I can’t really say that I could ‘feel’ Good Friday or Holy Saturday. On Sunday, we had breakfast together with my husband’s family and his sister. We were in three different places but we connected over Skype. Later, at noon, we had some coffee with my parents, also over Skype. It’s obvious though that this doesn’t replace face-to-face contact but it’s always some form of conversation. (9.3_F_25_Couple, no children)
Challenge 2 –restrictions on movement and travel . In contrast to the restrictions on contact with other people, the restrictions on movement and the closing of borders were perceived more negatively and posed bigger challenges for some people (especially those who used to do a lot of travelling). In this case, it was less clear why these regulations were introduced (especially travel restrictions within the country). Moreover, travel restrictions, particularly in the case of international travels, were associated with a limitation of civil liberties. The limitation (or complete ban) on travelling abroad in the Polish situation evoked additional connotations with the communist times, that is, with the fact that there was no freedom of movement for Polish citizens (associations with totalitarianism and dictatorship). Interestingly, the lack of acceptance of this restriction was also manifested by people who did not travel much. Thus, it was not just a question of restricting travelling abroad but more of restricting the potential opportunity (“even if I’m not planning on going anywhere, I know I still can”).
Limitations on travelling around the country were particularly negatively felt by families with children, where parents believe that regular exercise and outings are necessary for the proper development of their children. For parents, it was problematic to accept the prohibition of leaving the house and going to the playground (which remained closed until mid-May). Being outdoors was perceived as important for maintaining immunity (exercise as part of a healthy lifestyle), therefore, people could not understand the reason underlying this restriction and, as a consequence, often did not accept it.
I was really bothered by the very awareness that I can’t just jump in my car or get on a plane whenever I want and go wherever I want. It’s not something that I have to do on a daily basis but freedom of movement and travelling are very important for me. (14.2_M_55_Two adults and children)
Changes over time . The travel and movement limitations, although objectively less severe for most people, aroused much greater anger than the restrictions on social contact. This was probably due to a greater sense of misunderstanding as to why these rules were being introduced in the first place. Moreover, they were often communicated inconsistently and chaotically (e.g., a ban on entering forests was introduced while, at the same time, shopping malls remained open and masses were allowed to attend church services). This anger grew over time—from interview to interview, the respondents’ irritation and lack of acceptance of this was evident (culminating in the 3 rd -4 th stage of the study). The limitation of mobility was also often associated with negative consequences for both health and the economy. Many people are convinced that being in the open air (especially accompanied by physical activity) strengthens immunity, therefore, limiting such activity may have negative health consequences. Some respondents pointed out that restricting travelling, the use of hotels and restaurants, especially during the holiday season, will have serious consequences for the existence of the tourism industry.
I can’t say I completely agree with these limitations because it’s treating everything selectively. It’s like the shopping mall is closed, I can’t buy any shoes but I can go to a home improvement store and buy some wallpaper for myself. So I don’t see the difference between encountering people in a home improvement store and a shopping mall. (18.2_F_48_Two adults and children)
Ways of dealing . Since the restriction of movement and travel was more often associated with pleasure-related behaviors than with activities necessary for living, the compensations for these restrictions were usually also from the area of hedonistic behaviors. In the statements of our respondents, terms such as “indulging” or “rewarding oneself” appeared, and behaviors such as throwing small parties at home, buying better alcohol, sweets, and new clothes were observed. There were also increased shopping behaviors related to hobbies (sometimes hobbies that could not be pursued at the given time)–a kind of “post-pandemic” shopping spree (e.g., a new bike or new skis).
Again, the reaction to this restriction also depended on the level of fear of the COVID-19 disease. People who were more afraid of being infected accepted these restrictions more easily as it gave them the feeling that they were doing something constructive to protect themselves from the infection. Conversely, people with less fears and concerns were more likely to rebel and break these bans and guidelines.
Another way of dealing with this challenge was making plans for interesting travel destinations for the post-pandemic period. This was especially salient in respondents with an active lifestyle in the past and especially visible during the 5 th stage of the study.
Today was the first day when I went to the store (due to being in quarantine after returning from abroad). I spent loads of money but I normally would have never spent so much on myself. I bought sweets and confectionery for Easter time, some Easter chocolates, too. I thought I’d do some more baking so I also bought some ingredients to do this. (1.2_ F_25_single)
Challenge 3 –necessary change in active lifestyle . Many of the limitations related to COVID-19 were a challenge for people with an active lifestyle who would regularly go to the cinema, theater, and gym, use restaurants, and do a lot of travelling. For those people, the time of the COVID constraints has brought about huge changes in their lifestyle. Most of their activities were drastically restricted overnight and they suddenly became domesticated by force, especially when it was additionally accompanied by a transition to remote work.
Compulsory spending time at home also had serious consequences for people with school-aged children who had to confront themselves with the distance learning situation of their children. The second challenge for families with children was also finding (or helping find) activities for their children to do in their free time without leaving the house.
I would love to go to a restaurant somewhere. We order food from the restaurant at least once a week, but I’d love to go to the restaurant. Spending time there is a different way of functioning. It is enjoyable and that is what I miss. I would also go to the cinema, to the theater. (13.3_M_46_Two adults and child.)
Changes over time . The nuisance of restrictions connected to an active lifestyle depended on the level of restrictions in place at a given time and the extent to which a given activity could be replaced by an alternative. Moreover, the response to these restrictions depended more on the individual differences in lifestyle rather than on the stage of the interview (except for the very beginning, when the changes in lifestyle and everyday activities were very sudden).
I miss that these restaurants are not open . And it’s not even that I would like to eat something specific . It is in all of this that I miss such freedom the most . It bothers me that I have no freedom . And I am able to get used to it , I can cook at home , I can order from home . But I just wish I had a choice . (2 . 6_F_27_single ).
Ways of dealing . In the initial phase of the pandemic (March-April—stage 1–3 of the study), when most people were afraid of the coronavirus, the acceptance of the restrictions was high. At the same time, efforts were made to find activities that could replace existing ones. Going to the gym was replaced by online exercise, and going to the cinema or theater by intensive use of streaming platforms. In the subsequent stages of the study, however, the respondents’ fatigue with these “substitutes” was noticeable. It was then that more irritation and greater non-acceptance of certain restrictions began to appear. On the other hand, the changes or restrictions introduced during the later stages of the pandemic were less sudden than the initial ones, so they were often easier to get used to.
I bought a small bike and even before that we ordered some resistance bands to work out at home, which replace certain gym equipment and devices. […] I’m considering learning a language. From the other online things, my girlfriend is having yoga classes, for instance. (7.2_M_28_Couple, no children)
Challenge 4 –boredom , monotony . As has already been shown, for many people, the beginning of the pandemic was a huge change in lifestyle, an absence of activities, and a resulting slowdown. It was sometimes associated with a feeling of weariness, monotony, and even of boredom, especially for people who worked remotely, whose days began to be similar to each other and whose working time merged with free time, weekdays with the weekends, and free time could not be filled with previous activities.
In some way, boredom. I can’t concentrate on what I’m reading. I’m trying to motivate myself to do such things as learning a language because I have so much time on my hands, or to do exercises. I don’t have this balance that I’m actually doing something for myself, like reading, working out, but also that I’m meeting up with friends. This balance has gone, so I’ve started to get bored with many things. Yesterday I felt that I was bored and something should start happening. (…) After some time, this lack of events and meetings leads to such immense boredom. (1.5_F_25_single)
Changes over time . The feeling of monotony and boredom was especially visible in stage 1 and 2 of the study when the lockdown was most restrictive and people were knocked out of their daily routines. As the pandemic continued, boredom was often replaced by irritation in some, and by stagnation in others (visible in stages 3 and 4 of the study) while, at the same time, enthusiasm for taking up new activities was waning. As most people were realizing that the pandemic was not going to end any time soon, a gradual adaptation to the new lifestyle (slower and less active) and the special pandemic demands (especially seen in stage 5 and 6 of the study) could be observed.
But I see that people around me , in fact , both family and friends , are slowly beginning to prepare themselves for more frequent stays at home . So actually more remote work , maybe everything will not be closed and we will not be locked in four walls , but this tendency towards isolation or self-isolation , such a deliberate one , appears . I guess we are used to the fact that it has to be this way . (15 . 6_M_43_Two adults and child) .
Ways of dealing . The answer to the monotony of everyday life and to finding different ways of separating work from free time was to stick to certain rituals, such as “getting dressed for work”, even when work was only by a computer at home or, if possible, setting a fixed meal time when the whole family would gather together. For some, the time of the beginning of the pandemic was treated as an extra vacation. This was especially true of people who could not carry out their work during the time of the most severe restrictions (e.g., hairdressers and doctors). For them, provided that they believed that everything would return to normal and that they would soon go back to work, a “vacation mode” was activated wherein they would sleep longer, watch a lot of movies, read books, and generally do pleasant things for which they previously had no time and which they could now enjoy without feeling guilty. Another way of dealing with the monotony and transition to a slower lifestyle was taking up various activities for which there was no time before, such as baking bread at home and cooking fancy dishes.
I generally do have a set schedule. I begin work at eight. Well, and what’s changed is that I can get up last minute, switch the computer on and be practically making my breakfast and coffee during this time. I do some work and then print out some materials for my younger daughter. You know, I have work till four, I keep on going up to the computer and checking my emails. (19.1_F_39_Two adults and children)
Challenge 5 –uncertainty about the future . Despite the difficulties arising from the circumstances and limitations described above, it seems that psychologically, the greatest challenge during a pandemic is the uncertainty of what will happen next. There was a lot of contradictory information in the media that caused a sense of confusion and heightened the feeling of anxiety.
I’m less bothered about the changes that were put in place and more about this concern about what will happen in the future. Right now, it’s like there’s these mood swings. […] Based on what’s going on, this will somehow affect every one of us. And that’s what I’m afraid of. The fact that someone will not survive and I have no way of knowing who this could be—whether it will be me or anyone else, or my dad, if somehow the coronavirus will sneak its way into our home. I simply don’t know. I’m simply afraid of this. (10.1_F_55_Couple, no children)
Changes over time . In the first phase of the pandemic (interviews 1–3), most people felt a strong sense of not being in control of the situation and of their own lives. Not only did the consequences of the pandemic include a change in lifestyle but also, very often, the suspension of plans altogether. In addition, many people felt a strong fear of the future, about what would happen, and even a sense of threat to their own or their loved ones’ lives. Gradually (interview 4), alongside anxiety, anger began to emerge about not knowing what would happen next. At the beginning of the summer (stage 5 of the study), most people had a hope of the pandemic soon ending. It was a period of easing restrictions and of opening up the economy. Life was starting to look more and more like it did before the pandemic, fleetingly giving an illusion that the end of the pandemic was “in sight” and the vision of a return to normal life. Unfortunately, autumn showed that more waves of the pandemic were approaching. In the interviews of the 6 th stage of the study, we could see more and more confusion and uncertainty, a loss of hope, and often a manifestation of disagreement with the restrictions that were introduced.
This is making me sad and angry. More angry, in fact. […] I don’t know what I should do. Up until now, there was nothing like this. Up until now, I was pretty certain of what I was doing in all the decisions I was making. (14.4_M_55_Two adults and children)
Ways of dealing . People reacted differently to the described feeling of insecurity. In order to reduce the emerging fears, some people searched (sometimes even compulsively) for any information that could help them “take control” of the situation. These people searched various sources, for example, information on the number of infected persons and the number of deaths. This knowledge gave them the illusion of control and helped them to somewhat reduce the anxiety evoked by the pandemic. The behavior of this group was often accompanied by very strict adherence to all guidelines and restrictions (e.g., frequent hand sanitization, wearing a face mask, and avoiding contact with others). This behavior increased the sense of control over the situation in these people.
A completely opposite strategy to reducing the feeling of uncertainty which we also observed in some respondents was cutting off information in the media about the scale of the disease and the resulting restrictions. These people, unable to keep up with the changing information and often inconsistent messages, in order to maintain cognitive coherence tried to cut off the media as much as possible, assuming that even if something really significant had happened, they would still find out.
I want to keep up to date with the current affairs. Even if it is an hour a day. How is the pandemic situation developing—is it increasing or decreasing. There’s a bit of propaganda there because I know that when they’re saying that they have the situation under control, they can’t control it anyway. Anyhow, it still has a somewhat calming effect that it’s dying down over here and that things aren’t that bad. And, apart from this, I listen to the news concerning restrictions, what we can and can’t do. (3.1_F_54_single)
Discussion and conclusions
The results of our study showed that the five greatest challenges resulting from the COVID-19 pandemic are: limitations of direct contact with people, restrictions on movement and travel, change in active lifestyle, boredom and monotony, and finally uncertainty about the future. As we can see the spectrum of problems resulting from the pandemic is very wide and some of them have an impact on everyday functioning and lifestyle, some other influence psychological functioning and well-being. Moreover, different people deal with these problems differently and different changes in everyday life are challenging for them. The first challenge of the pandemic COVID-19 problem is the consequence of the limitation of direct contact with others. This regulation has very strong psychological consequences in the sense of loneliness and lack of closeness. Initially, people tried to deal with this limitation through the use of internet communicators. It turned out, however, that this form of contact for the majority of people was definitely insufficient and feelings of deprivation quickly increased. As much data from psychological literature shows, contact with others can have great psychological healing properties [e.g., 29 ]. The need for closeness is a natural need in times of crisis and catastrophes [ 30 ]. Unfortunately, during the COVID-19 pandemic, the ability to meet this need was severely limited by regulations. This led to many people having serious problems with maintaining a good psychological condition.
Another troubling limitation found in our study were the restrictions on movement and travel, and the associated restrictions of most activities, which caused a huge change in lifestyle for many people. As shown in previous studies, travel and diverse leisure activities are important predictors of greater well-being [ 36 ]. Moreover, COVID-19 pandemic movement restrictions may be perceived by some people as a threat to human rights [ 37 ], which can contribute to people’s reluctance to accept lockdown rules.
The problem with accepting these restrictions was also related to the lack of understanding of the reasons behind them. Just as the limitation in contact with other people seemed understandable, the limitations related to physical activity and mobility were less so. Because of these limitations many people lost a sense of understanding of the rules and restrictions being imposed. Inconsistent communication in the media—called by some researchers the ‘infodemic’ [ 18 ], as well as discordant recommendations in different countries, causing an increasing sense of confusion in people.
Another huge challenge posed by the current pandemic is the feeling of uncertainty about the future. This feeling is caused by constant changes in the rules concerning daily functioning during the pandemic and what is prohibited and what is allowed. People lose their sense of being in control of the situation. From the psychological point of view, a long-lasting experience of lack of control can cause so-called learned helplessness, a permanent feeling of having no influence over the situation and no possibility of changing it [ 38 ], which can even result in depression and lower mental and physical wellbeing [ 39 ]. Control over live and the feeling that people have an influence on what happens in their lives is one of the basic rules of crisis situation resilience [ 30 ]. Unfortunately, also in this area, people have huge deficits caused by the pandemic. The obtained results are coherent with previous studies regarding the strategies harnessed to cope with the pandemic [e.g., 5 , 10 , 28 , 33 ]. For example, some studies showed that seeking social support is one of the most common strategies used to deal with the coronavirus pandemic [ 33 , 40 ]. Other ways to deal with this situation include distraction, active coping, and a positive appraisal of the situation [ 41 ]. Furthermore, research has shown that simple coping behaviors such as a healthy diet, not reading too much COVID-19 news, following a daily routine, and spending time outdoors may be protective factors against anxiety and depressive symptoms in times of the coronavirus pandemic [ 41 ].
This study showed that the acceptance of various limitations, and especially the feeling of discomfort associated with them, depended on the person’s earlier lifestyle. The more active and socializing a person was, the more restrictions were burdensome for him/her. The second factor, more of a psychological nature, was the fear of developing COVID-19. In this case, people who were more afraid of getting sick were more likely to submit to the imposed restrictions that, paradoxically, did not reduce their anxiety, and sometimes even heightened it.
Limitations of the study.
While the study shows interesting results, it also has some limitations. The purpose of the study was primarily to capture the first response to problems resulting from a pandemic, and as such its design is not ideal. First, the study participants are not diverse as much as would be desirable. They are mostly college-educated and relatively well off, which may influence how they perceive the pandemic situation. Furthermore, the recruitment was done by searching among the further acquaintances of the people involved in the study, so there is a risk that all the people interviewed come from a similar background. It would be necessary to conduct a study that also describes the reaction of people who are already in a more difficult life situation before the pandemic starts.
Moreover, it would also be worthwhile to pay attention to the interviewers themselves. All of the moderators were female, and although gender effects on the quality of the interviews and differences between the establishment of relationships between women and men were not observed during the debriefing process, the topic of gender effects on the results of qualitative research is frequently addressed in the literature [ 42 , 43 ]. Although the researchers approached the process with reflexivity and self-criticism at all stages, it would have seemed important to involve male moderators in the study to capture any differences in relationship dynamics.
Practical implications.
The study presented has many practical implications. Decision-makers in the state can analyze the COVID-19 pandemic crisis in a way that avoids a critical situation involving other infectious diseases in the future. The results of our study showing the most disruptive effects of the pandemic on people can serve as a basis for developing strategies to deal with the effects of the crisis so that it does not translate into a deterioration of the public’s mental health in the future.
The results of our study can also provide guidance on how to communicate information about restrictions in the future so that they are accepted and respected (for example by giving rational explanations of the reasons for introducing particular restrictions). In addition, the results of our study can also be a source of guidance on how to deal with the limitations that may arise in a recurrent COVID-19 pandemic, as well as other emergencies that could come.
The analysis of the results showed that the COVID-19 pandemic, and especially the lockdown periods, are a particular challenge for many people due to reduced social contact. On the other hand, it is social contacts that are at the same time a way of a smoother transition of crises. This knowledge should prompt decision-makers to devise ways to ensure pandemic safety without drastically limiting social contacts and to create solutions that give people a sense of control (instead of depriving it of). Providing such solutions can reduce the psychological problems associated with a pandemic and help people to cope better with it.
Conclusions
As more and more is said about the fact that the COVID-19 pandemic may not end soon and that we are likely to face more waves of this disease and related lockdowns, it is very important to understand how the different restrictions are perceived, what difficulties they cause and what are the biggest challenges resulting from them. For example, an important element of accepting the restrictions is understanding their sources, i.e., what they result from, what they are supposed to prevent, and what consequences they have for the fight against the pandemic. Moreover, we observed that the more incomprehensible the order was, the more it provoked to break it. This means that not only medical treatment is extremely important in an effective fight against a pandemic, but also appropriate communication.
The results of our study showed also that certain restrictions cause emotional deficits (e.g., loneliness, loss of sense of control) and, consequently, may cause serious problems with psychological functioning. From this perspective, it seems extremely important to understand which restrictions are causing emotional problems and how they can be dealt with in order to reduce the psychological discomfort associated with them.
Supporting information
S1 table. a full description of the changes occurring in poland at the time of the study..
https://doi.org/10.1371/journal.pone.0258133.s001
S2 Table. Characteristics of study participants.
https://doi.org/10.1371/journal.pone.0258133.s002
S1 Dataset. Transcriptions from the interviews.
https://doi.org/10.1371/journal.pone.0258133.s003
- 1. JHU CSEE. COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020 [cited 2021 Jun 1]. https://github.com/CSSEGISandData/COVID-19#covid-19-data-repository-by-the-center-for-systems-science-and-engineering-csse-at-johns-hopkins-university .
- View Article
- PubMed/NCBI
- Google Scholar
- 5. Gerhold L. COVID-19: Risk perception and coping strategies. Results from a survey in Germany. PsyArXiv [Preprint]. 2020 Mar 25.
- 6. Nowakowska K. Rok z koronawirusem: Od paniki, przez luz, do fatalizmu. [A year with coronavirus—from panic through chill to fatalism]. Dziennik Gazeta Prawna [Internet]. 2021 Mar 4 [cited 2021 Jun 1]. https://www.gazetaprawna.pl/wiadomosci/kraj/artykuly/8111858,szczepienia-maseczki-rok-z-koronawirusem-zycie-codzienne.html
- 11. Fernandes N. Economic effects of coronavirus outbreak (COVID-19) on the world economy. IESE Business School Working Paper No. WP-1240-E. 2020 Mar 23.
- 15. ARC. Praca z domu w polskim wydaniu—badanie na zlecenie Gumtree.pl we współpracy z Randstat Polska [The Polish way of working from home—a study commissioned by Gumtree.pl in collaboration with Randstat Polska]. 2020 [cited 2021 Jun 1]. https://www.randstad.pl/strefa-pracownika/centrum-prasowe/badanie-gumtreepl-we-wspolpracy-z-randstad-potrafimy-sie-zorganizowac-choc-czasem-lubimy-sobie-poprzeszkadzac-praca-z-domu-w-polskim-wydaniu/
- 16. Sierpowska, I. O edukacji w czasie pandemii [On Education during pandemic]. Centrum Prasowe SWPS [Internet]. 2020 Sep 8. [cited 2021 Jun 1]. https://www.swps.pl/centrum-prasowe/informacje-prasowe/22390-o-edukacji-w-czasie-pandemii-2?dt=1622540060078
- 17. Polish Academy of Sciences. Understanding COVID-19. Report by the COVID-19 team at the President of the Polish Academy of Sciences. 2020 Sep 14. [Cited 2021 Jun 1]. https://informacje.pan.pl/images/2020/opracowanie-covid19-14-09-2020/ZrozumiecCovid19_opracowanie_PAN.pdf
- 25. Brown, K. The pandemic is not a natural disaster. The New Yorker [Internet]. 2020 Apr 13 [cited 2021 Jun 1]. https://www.newyorker.com/culture/annals-of-inquiry/the-pandemic-is-not-a-natural-disaster .
- 34. Maison D. Qualitative marketing research. Understanding consumer behaviour. London: Routledge; 2019.
- 36. Argyle M. Causes and correlates of happiness. In: Kahneman D, Diener E, Schwarz N, editors. Well-Being: The Foundations of Hedonic Psychology. New York: Russell Sage Foundation; 1999. p. 353–373.
- Open access
- Published: 10 September 2021
Methodological limitations in studies assessing the effects of environmental and socioeconomic variables on the spread of COVID-19: a systematic review
- Maria A. Barceló 1 , 2 &
- Marc Saez ORCID: orcid.org/0000-0003-1882-0157 1 , 2
Environmental Sciences Europe volume 33 , Article number: 108 ( 2021 ) Cite this article
10k Accesses
10 Altmetric
Metrics details
While numerous studies have assessed the effects of environmental (meteorological variables and air pollutants) and socioeconomic variables on the spread of the COVID-19 pandemic, many of them, however, have significant methodological limitations and errors that could call their results into question. Our main objective in this paper is to assess the methodological limitations in studies that evaluated the effects of environmental and socioeconomic variables on the spread of COVID-19.
We carried out a systematic review by conducting searches in the online databases PubMed, Web of Science and Scopus up to December 31, 2020. We first excluded those studies that did not deal with SAR-CoV-2 or COVID-19, preprints, comments, opinion or purely narrative papers, reviews and systematic literature reviews. Among the eligible full-text articles, we then excluded articles that were purely descriptive and those that did not include any type of regression model. We evaluated the risk of bias in six domains: confounding bias, control for population, control of spatial and/or temporal dependence, control of non-linearities, measurement errors and statistical model. Of the 5631 abstracts initially identified, we were left with 132 studies on which to carry out the qualitative synthesis. Of the 132 eligible studies, we evaluated 63.64% of the studies as high risk of bias, 19.70% as moderate risk of bias and 16.67% as low risk of bias.
Conclusions
All the studies we have reviewed, to a greater or lesser extent, have methodological limitations. These limitations prevent conclusions being drawn concerning the effects environmental (meteorological and air pollutants) and socioeconomic variables have had on COVID-19 outcomes. However, we dare to argue that the effects of these variables, if they exist, would be indirect, based on their relationship with social contact.
Numerous studies have assessed the effects of environmental and socioeconomic variables on the spread of the COVID-19 pandemic. Most of them have addressed the influence meteorological variables have, although there are also quite a few that have considered the effects of air pollutants and socioeconomic variables. Those which assessed the effects of meteorological variables were the first to appear, specifically between the last week of March and the first week of April 2020. In other words, very close to COVID-19 being officially declared a global pandemic (11 March 2020) [ 1 ]. Later, there were those which evaluated the effects of air pollutants, the first of which appeared between the end of April and the first week of May 2020. Finally, the last ones to appear were those related to socioeconomic variables; the first of which was mid-May 2020.
The studies differ in their outcomes (new and cumulative cases, mortality, reproductive number, etc.), study populations (the world, countries, regions, cities), confounders as well as in the way of controlling for them, and in the modelling strategies adopted. However, with the exception of socioeconomic variables, several systematic reviews attempting to synthesize the evidence have already been published.
For instance, with regard to meteorological variables, Mecenas et al. carried out a bibliographic search until the end of March 2020 [ 2 ]. In reviewing 17 studies (most of them preprints), they found that warm wet climates seemed to reduce the spread of COVID-19. However, the role of temperature and humidity on the spread of the virus was very moderate, since these variables alone could not explain most of the variability in the disease’s transmission. Smit et al., in a systematic review carried out in July 2020 (that is, of studies that used data from the first wave), critically evaluated 42 articles published in scientific journals and 80 preprints [ 3 ]. They concluded that the evidence suggested that either there was no modulating effect of the summer weather conditions (i.e., high temperature and low humidity reduce the transmission rate of the virus) or, along the same lines as Mecenas et al., if it did exist, it was weak. Smit et al. also found similar results for other meteorological variables, such as ultraviolet radiation and wind speed [ 3 ]. McClymont and Hu discussed 23 articles with moderate or high ratings (out a total of 86 eligible peer-reviewed articles) published until October 1 (also contemplating only the first wave) [ 4 ], and found that temperature and humidity were associated with COVID-19 incidence. However, while the decrease in temperature was associated with increases in incidence, in the variations in humidity the results were mixed (positive and negative associations were found). They also found that wind speed and rainfall results were not consistent across studies [ 4 ].
In relation to air pollutants, Copat et al. carried out a systematic review of 15 studies (13 articles and 2 preprints) published between April 2020 and July 6th, 2020 [ 5 ]. They found a consistent association between some air pollutants (fine particles, PM 2.5 with a diameter of 2.5 microns (μm) or less, and nitrogen dioxide, NO 2 , and with a less extent coarse particles, PM 10, with a diameter of 10 μm or less) and a higher incidence and mortality from COVID-19. They pointed out, however, that there were important limitations for any direct comparison of the results and that more studies were needed to strengthen scientific evidence. Malecki et al. carried out a systematic review of 19 studies, published through to October 31, 2020, that assessed the association of particulate matter (i.e., PM 10 and PM 2.5 ) pollution and the spread of SARS-CoV-2 [ 6 ]. They pointed out that although there were suggestions that particulate matter (PM) played a role in the spread of SARS-CoV-2, PM concentration alone cannot be effective in spreading the COVID-19 disease, and that other meteorological and environmental variables were also involved.
Until today (June 2021), no peer-reviewed systematic reviews have been published concerning the influence socioeconomic variables have on the spread of the pandemic. However, let us advance some of our results here by noting that in ecological studies the results were not conclusive. In some, especially those carried out in the United States, the areas with greater economic deprivation had a higher incidence and also a higher mortality. That said, in others no association was found, or deprivation was even found to be a protective factor. What was consistently observed was the fact that the higher the population density was, the greater incidence and mortality were. In individual studies, however, individuals with lower incomes or from more disadvantaged groups were at greater risk of hospitalization and death.
Nevertheless, all the reviews state that many of the studies have significant methodological limitations and errors that could bring their results into question. Our main objective here is to assess the methodological limitations in the studies that evaluated the effects environmental and socioeconomic variables have had on the spread of COVID-19. Furthermore, we discuss the results of those studies that were, in fact, able to control those very limitations.
Systematic review
The protocol for this review is registered in the Prospective Register of Systematic Reviews (PROSPERO 2020 CRD42020201540). In the review process, we followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) protocols [ 7 ]. The literature search, study selection, data extraction, and quality assessment were performed by each of us independently. In case of any discrepancy between us, we all reached an agreement on the final decision.
By combining the keyword ‘COVID-19’ with the keywords ‘temperature’, ‘(meteorological variables)’, ‘(air pollutants)’, ‘(environmental variables)’, and ‘(socioeconomic variables)’, through the Boolean connector ‘AND’ we conducted a search in the online databases PubMed, Web of Science and Scopus, up to December 31, 2020. We did not impose any language restrictions, nor did we contact any author for additional information.
All the articles retrieved underwent an initial title and abstract screening, where any duplicates were discarded, followed by a full-text screening for eligible abstracts. We made a first exclusion of those studies that did not deal with SARS-CoV-2 or COVID-19, preprints (non-peer-reviewed articles), comments, opinion or purely narrative papers, reviews and systematic literature reviews (Fig. 1 ). Among the eligible full-text articles, we made a second exclusion of those articles that were purely descriptive (including only plots or maps, etc.) and those that did not include any type of regression model (those that only included the analysis of correlations, for example).
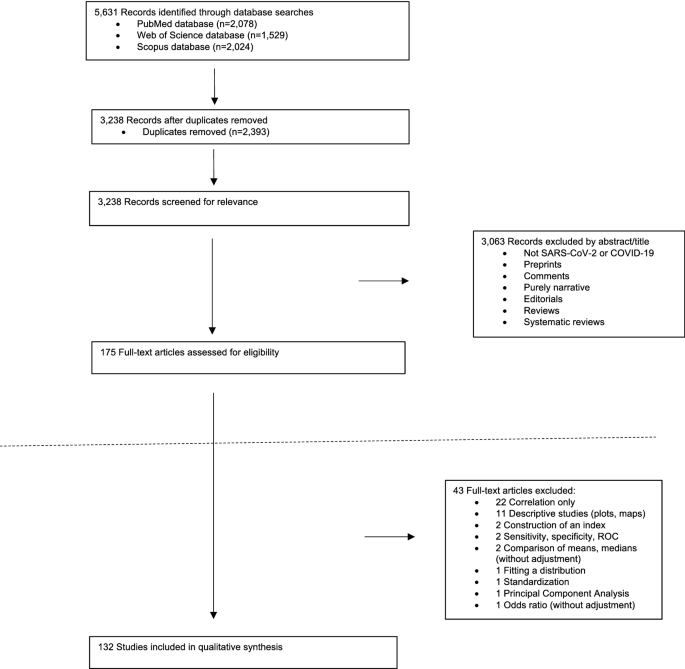
Flow-chart of the study selection process
We extracted the following data from the articles included in the qualitative analysis: first author, study population, study period, outcome, explanatory variables, covariates, the statistical method (including the model specification and the methods to control the confounding), and the study findings.
Methodological limitations
The usual assessment tools for observational studies were not entirely suitable for assessing the risk of bias of the studies we reviewed. We preferred to adapt the tool proposed by Parmar et al. [ 8 ] who, in turn, adapted the Newcastle–Ottawa scale [ 9 ] and the RTI item bank [ 10 ]. Specifically, we used six domains: two from Parmar et al. [ 8 ]—confounding bias and measurement errors in the outcome and/or in the exposure variables; one based on the dimension ‘unobserved confounding’ in Saez et al. [ 11 ]—control of the spatial and/or the temporal dependence; and three that we added ex novo in this paper—control for the population, statistical model, and control of non-linearities.
In each study, each of the six domains were rated as: 1—low risk of bias, 2—moderate risk of bias, or 3—high risk of bias) (Table 1 ). For the overall rating of each study, we evaluated it as 'strong' (low risk of bias) if, at most, one of the six domains was rated as high risk of bias (i.e., a rating of 3), 'moderate' (moderate risk of bias) if up to two domains were rated as weak, or 'weak' (high risk of bias) if three or more domains were rated as high risk of bias. For the rating of both the domains and the studies, we rely on Parmar et al. [ 8 ].
Three of the six dimensions corresponded to the specification error known as omission of relevant variables: confounding bias, control of the population and control of the spatial and/or of the temporal dependence. This specification error leads to biased and inconsistent estimators (that is, the estimators biased even asymptotically, i.e., when the number of observations is very high) and, in addition, the variances of the estimators are also misleading [ 12 ]. In any case, the inference of those studies that do not control for this error is highly compromised.
Confounding bias
None of the studies included all possible confounders, especially if the studies were ecological (as most of them were). However, as regards the spread of COVID-19, there is a confounder that, at a minimum, must be controlled for, namely, social contact.
The main route of transmission for COVID-19 is through the direct or indirect contact with an infected subject via the small droplets that occur when they cough or sneeze [ 13 ]. Thus, this contact must be controlled for in the models, even if indirectly. The control, although partial, can be carried out through mobility or, much more indirectly, through socioeconomic variables. In general, greater mobility implies greater levels of contact. Likewise, areas with high population densities are known to have greater social contact. Furthermore, some occupations present a greater risk, particularly those that were less able to switch to teleworking and, therefore, require greater mobility and the resulting higher level of social contact.
Unobserved confounding (i.e., residual confounding) including, for example, random effects that capture heterogeneity, should also be controlled for. In other words, unobserved variables specific to the unit of analysis (area or individual) that could influence the risk of, in this case, the spread of the COVID-19.
We scored this domain with a 3 if the confounding was not controlled for by any method, with a 2 if the observed confounding was controlled for with a moderate number of confounders (up to two maximum), in particular mobility or socioeconomic variables, or with a 1 if the observed confounding was controlled for with a large number of confounders (more than two) and/or unobserved confounding was also controlled for.
Control of the population
Perhaps the main relevant variable that should not be omitted by any study is that of population at risk, either in the study area (in ecological studies) or in the area in which the subject resides (in individual studies). It is evident that both incidence and mortality, as well as other outcomes (hospitalizations, ICU admissions, etc.), depend both on the population of the area under study and on the age structure of that population.
Population control can be carried out in various ways: using rates, including the population or the expected value of the outcome in each area under study in the model as an offset, or controlling, as covariates, the size of the population or its structure (for example, percentage of population aged 65 years or more).
A control of the population can also be achieved by including population density (i.e., the number of people per unit of area, usually per square kilometre) as a covariate. However, it is possible that, in this case, control would only be partial. On one hand, an area with a higher population density does not always have more population than another, but it depends, logically, on its surface. On the other hand, population density could be capturing other socioeconomic variables.
This domain was scored with a 3 if the population was not controlled for by any method, a 2 if the population was controlled for by only including population density as a covariate, or a 1 if the population was controlled for, in addition to including the population density by other additional method.
Control of the spatial and/or of the temporal dependence
Several studies analyze, as outcome, cumulative cases and cumulative deaths. Many others, however, use a temporal design. This is a design, where both the outcome and its possible explanatory variables, as well as the covariates, are measured in the form of time series. Time series are observed with a certain periodicity, usually regular (for example, daily) over a given period of time.
In this case there is temporal dependency. The outcome observations are not independent but are related, so their future behavior is predictable. In general, this dependence can be long or short term. A long-term dependency, or trend, could be defined as a movement or tendency in the data. As is known, in the case of COVID-19 there have been between two and four waves, depending on the country. That is, long-term swings have occurred. Periods in which the outcome values are persistently high, followed by others in which the values have been low. Short-term dependency, also called serial autocorrelation, refers to the relationship of the values of an outcome on, for example, a given day with the values of the previous days, especially with those of the preceding day.
Most studies use a spatial or spatio-temporal design. In other words, they observe the outcome in different geographical areas, and sometimes over time. When a spatial design is available, it is important to distinguish two sources of variation. In the first place, the most important source is usually the so-called 'spatial dependence' and is a consequence of the correlation of the spatial unit with neighboring spatial units, generally those that are geographically contiguous. In this way, the risks (for example, of transmission) of contiguous or nearby areas are more similar than the risks of spatially distant areas. Part of this dependency is not really a structural dependency but is mainly due to the existence of uncontrolled variables, that is, not included in the analysis. Meanwhile, the second source, the existence of spatially independent and unrelated variation called ‘spatial heterogeneity’, must be assumed. This is a consequence of the existence of unobserved variables without spatial structure that could influence risk [ 14 ].
The temporal and the spatial dependence must be controlled for, because, otherwise, in the best of cases, the variances of the estimators will be misleading (when the outcome is a continuous variable, normally distributed, and least squares methods are used for the inference) and in most cases, not only will the variances be biased, but the estimators will also be biased (when the outcome is not a continuous variable, not normally distributed, and least squares methods cannot be used) [ 12 ].
In some studies, the control of temporal or spatial dependence is not applicable. Thus, in studies with a time series design but in which a very short period of time is analyzed, it does not make sense to control for temporal dependence. Likewise, in those studies with a spatial (or spatio-temporal) design but that analyze very spatially distant territories (for example, several countries in the world) it does not make sense to control for the spatial dependence.
We scored this domain with a 3 when neither temporal nor spatial dependency was controlled and should have been; a 2 when the control was partial, controlling only one dependency and not controlling the other; and a 1 when they were controlled.
Control of non-linearities
Along with the omission of relevant variables, the error in the functional form constitutes the most important specification error. The relationships between environmental variables and COVID-19 outcomes are not usually linear. Thus, for example, in Fig. 2 , we show the smoothed curves for the relationship between the daily temperature and the daily levels of nitrogen dioxide (NO 2 ) and the daily number of cases for Spain in the period between January 1, 2020 and April 14, 2021. Specifically, we draw the estimated curves in a generalized additive model in which we use smoothing splines with a quasi-likelihood Poisson link, i.e., taking into account over-dispersion.
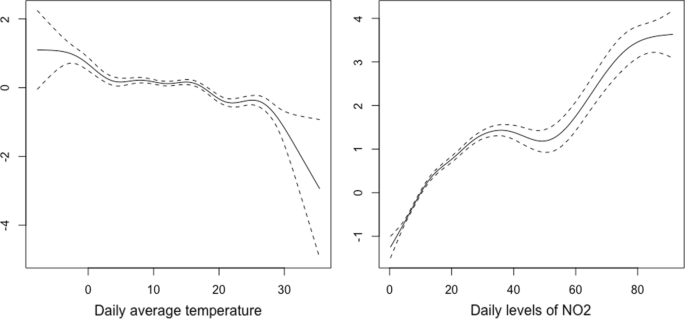
. Environmental data [ 81 , 82 ]
Smoothed curves for the relationships between daily temperature and daily levels of nitrogen dioxide and the number of daily cases of COVID-19. Spain, January 1, 2020 to April 14, 2021. The data were obtained from: [ 16 ]
As can be seen, in none of the cases was the relationship linear. These non-linearities must be controlled in the models, because, otherwise, as when relevant variables are omitted, the estimators will be inconsistent and their variances misleading.
We scored this dimension with a 3 if non-linearities were not controlled for (again, when applicable) or a 1 if they had been controlled.
Measurement errors
Measurement errors (also known as misclassification) can occur in both the response variable and in the exposure variables.
The definition of the response variable can vary in space and time, even within the same country, leading to differential misclassification. In Spain, for example, the Catalan government, on the one hand, defined a death from COVID-19 as being a positive result on some test (PCR or fast test) or symptoms presented at some point which a health professional subsequently classified as a possible case, but the individual did not have a diagnostic test with a positive result [ 15 ], whereas on the other hand, the Spanish government, defined a death from COVID-19 as being someone who presented a positive PCR result [ 16 ], thus providing significantly lower figures. This misclassification continued until May 21, 2020, when the Government of Spain adopted the same definition as the Government of Catalonia [ 17 ].
However, the measurement errors in the response variable are not attributable to the investigators, although they should certainly discuss them if appropriate. Furthermore, fortunately, when measurement errors occur in the dependent variable, the estimators remain consistent, although they are not efficient [ 12 ], that is, not very precise, thus leading to wider confidence intervals than if there had been no measurement errors.
There is, however, an important problem if measurement errors occur in the explanatory variables (exposure or covariates). If the explanatory variables are measured with error, the estimators will be inconsistent [ 12 ].
Even in studies at the individual level, the exposure variables and, obviously, the contextual variables (for example, the socioeconomic ones) are not observed at the individual level, but are aggregated at the level of the area under study. Nevertheless, not all residents in the area under study are actually exposed to the same mean values of the explanatory variables, which leads to a measurement error. If the misclassification is non-differential (over time and over space within the area under study) and, furthermore, if the between-area variability of the variable measured with error is much greater than the within-area variability of such variable, that is, that the area under study is not very heterogeneous (for example, because it is a small area), then the effect of the measurement error on the estimator consistency may be negligible [ 18 ]. This is what happens in the case of contextual socioeconomic variables as long as the area under study is not very large.
In the exposure variables (both air pollutants and meteorological variables), however, there is differential misclassification, because the exposure exhibits spatial variation across the area under study. If the spatial structure (i.e., spatial dependence) of the data is ignored, the estimators will be biased and inconsistent [ 19 ]. Many studies use the measurements observed in the area under study to estimate, by means of point estimators, exposure levels for that entire area. The estimators most widely used are the arithmetic mean of the values of the exposure, observed in several monitoring or meteorological stations in the area, and sometimes the inverse-distance weighted average of these values.
This measurement error in the exposure variables must be controlled for, either explicitly incorporating the spatial dependence, in the ecological studies, or by correcting the misalignment between the locations of the observation points of the exposure variables and that locations of the individuals, in the studies at the individual level.
In studies with an ecological spatial design, the 'modifiable areal unit problem' (MAUP) occurs [ 20 ]. The MAUP is a consequence either because areas of different sizes are added (scale effect) and/or because of the way the area is divided (zoning effect) [ 21 ]. In either case, it is a potential source of bias. For example, Wang and Di found that the association between nitrogen dioxide (NO 2 ) and COVID-19 deaths varies when the data is aggregated at different levels: a risk factor when the area is smaller (aggregation of districts and cities) and a protective factor at the province level [ 22 ]. Similarly, we also found a positive association between NO 2 and deaths as a consequence of COVID-19 at the level of a county-like area [ 17 ] and no association at a lower level of aggregation [ 23 ].
When using a temporal design, the ‘modifiable temporal unit problem’ (MTUP) [ 24 ] also occurs, whereby the results depend on the way data are temporally aggregated [ 21 ]. Furthermore, in this type of design, temporal misalignment can occur. In other words, the relationship between exposure and the occurrence of COVID-19 outcome is not contemporary, but rather is distributed over time as a consequence of the incubation period of COVID-19 and due to the diagnostic delays of the outcome. This temporal misalignment must be controlled by including lags, for example.
We scored this dimension with a 3 if measurement errors in the exposure variables are not controlled at all, a 2 if they are only partially controlled (not including lags, for example) or the areas under study are very large (countries, for example) and a 1 if they have been controlled for.
Statistical model
Many of the studies, even though the response variable is a count data, used regression models with normally distributed errors (linear regression models, generalized linear and additive models with Gaussian link, etc.). Using this type of models leads to biased results, unless the number of counts is very large. However, this was not the case in most studies.
Some studies did not model the counts but rather the rates, dividing the dependent variable by the size of the population. However, since the numerator, being a count data, is actually distributed following a Poisson distribution, the variance is proportional to the mean, so it is not constant, leading to heteroscedasticity (i.e., overdispersion). This must be controlled for, otherwise, the variances of the estimators are misleading.
To illustrate the effects on the results of erroneously using a regression model with normally distributed errors, we used the data in Filippini et al. [ 25 ]. Their objective was to investigate the link between the transmission of SARS-CoV-2 infection and long-term exposure to NO 2 in the provinces of three regions of Northern Italy (Lombardia, Venetto and Emilia Romagna), between March 8 and April 5, 2020 ( n = 84). Using their data, we first estimated a linear regression model including, as a dependent variable, the number of new daily SARS-CoV-2 positive cases (count data variable). We found that long-term NO 2 levels to which the inhabitants of the provinces of the Italian regions studied had been exposed to be positively associated with the total number of cases that occurred in the period considered. Specifically, for every 1 μg/m 3 increase in the NO 2 levels, the number of cases increased by 18.478 for the entire period (95% confidence interval, 95% CI 10.285–27.210). However, the residuals of the model were not normally distributed (Fig. 3 ). We then modelled the rates (cases per 100,000 inhabitants) using a linear regression model, although we did not control for heteroscedasticity. For every 1 μg/m 3 increase in NO 2 , the number of cases increased by 1.207 cases per 100,000 inhabitants (95% CI 0.050–2.364). However, the residuals presented a clear heteroscedasticity behavior (the scatter plot of the residuals against the adjusted values did not present a constant dispersion, i.e., variance), and furthermore, they were not normally distributed (Fig. 3 ). When we estimated a generalized Poisson model, in which we took into account the over-dispersion, and in which we included the population size as an offset, we could not reject the null hypothesis that the parameter associated with the long-term exposure of NO 2 was equal to zero (95% CI: − 0.004, 0.001).
Residual analysis of the linear regression models relating the transmission of SARS-CoV-2 infection and long-term exposure to NO 2 in the provinces of three regions of Northern Italy (Lombardia, Venetto and Emilia Romagna), between March 8 and April 5, 2020. a Response variable: new daily SARS-CoV-2 positive cases. b Response variable: new daily SARS-CoV-2 positive cases per 100,000 habs. The data were obtained from: [ 25 ]
We scored this dimension with a 3 when the outcome was a count data and regression models with normally distributed error were used. We also scored a 3 when rates were modelled but heteroscedasticity was not controlled for. Meanwhile, we scored a 2 if rates were modelled and heteroscedasticity was controlled, and a 1 if models for count data response variables were used (Poisson regression, negative binomial regression, etc.).
Figure 1 shows a flowchart of the review process. Of the 5631 abstracts initially identified, and after excluding duplicates, we were left with 3238 studies. From these we excluded 3063 studies that did not refer to SARS-CoV-2 or COVID-19, preprints, comments, those purely narrative studies, editorials and reviews and systematic reviews, thus leaving us with 175 eligible studies. As we said, we excluded 43 studies that were purely descriptive and those that did not include any type of regression model (Additional file 1 : Table S1). In the end we were left with 132 studies with which to carry out the qualitative synthesis (Additional file 1 : Tables S2 and S3).
Of the 132 studies, 92 referred to meteorological variables, 40 to socioeconomic variables and 34 to air pollutants. Seventy-one of the studies referred only to meteorological variables, 21 only to socioeconomic variables and 16 only to air pollutants. Of the 92 studies that referred to meteorological variables, 16 also considered air pollutants, 14 meteorological variables and socioeconomic variables. Four of the studies referring to air pollutants also referred to socioeconomic variables but not to meteorological variables. Nine referred to meteorological variables and socioeconomic variables but not to air pollutants. Finally, five studies considered meteorological variables, air pollutants and socioeconomic variables (Additional file 1 : Figure S1).
Of the 132 studies finally selected, 124 used an ecological design and nine an individual design. Most ecological studies considered different regions (states, regions, provinces, counties, cities, etc.) within the same country as study populations (71 studies). This is followed by those that considered countries or cities in the world (34 studies) and, finally, those that considered individual cities or smaller areas (19 studies). Seven of the eight studies with an individual design, analyzed the influence of socioeconomic variables, while only two considered socioeconomic variables and air pollutants.
Most of the studies (129 out of 132) analyzed data referring up to August 1, 2020 (i.e., only considering the first wave). In fact, only three consider the first two waves of the pandemic.
Table 2 shows the evaluation of the studies included in the qualitative synthesis. Of the 132 eligible studies, we evaluated 63.64% (84 of 132) as weak (high risk of bias), 19.70% (26 of 132) as moderate (moderate risk of bias) and 16.67% (22 of 132) as strong (low risk of bias). Only four studies did not have any dimension scored with a 3 (high risk of bias) [ 17 , 26 , 27 , 28 ].
In decreasing order of the studies that considered socioeconomic variables, 62.50% (25 of 40 studies) were evaluated as moderate (15 studies, 37.50%) or strong (10, 25.00%). Of the 34 which considered air pollutants, 41.18% (14 studies) were evaluated as moderate (9 studies, 26.47%) or strong (5 studies, 14.71%). Finally, of the 92 studies that considered meteorological variables, 25.00% (23 studies) were evaluated as moderate (11 studies, 11.96%) or strong (12 studies, 13.04%).
However, in the case of studies that consider socioeconomic variables, it should be noted that the high risk of bias could be underestimated. As is known, socioeconomic variables are contextual variables measured at an ecological level in a geographic area and invariant over time. Their influence on COVID-19, if any, is highly unlikely to be non-linear. Consequently, in many cases this dimension was not evaluated.
The dimension in which we evaluated more studies with a high risk of bias was that of measurement errors (90 of 132 studies, 68.19%), followed by the control of the spatial and temporal dependence dimension (80 studies, 60.61%) and of the statistical model (77 studies, 58.33%) and control of non-linearities (73 studies, 55.30%) dimensions. The dimensions with fewer studies with a high risk of bias were confounding bias (47 studies, 35.61%) and control of the population (53 studies, 40.15%).
Findings from studies assessed as moderate or strong
In relation to the studies that considered meteorological variables, the ones that we evaluated as moderate or strong [ 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ] have not consistently found an attenuating effect of meteorological variables. That is, they have not found that high temperature and low humidity were associated with lower incidence or mortality from COVID-19. In seven of 22 studies, temperature was either positively associated or not statistically associated with incidence [ 27 , 30 , 34 , 35 , 44 ], transmission (reproductive number) [ 46 ] and mortality [ 28 ] (four out of 11 studies were assessed as strong and another three out of 11 studies assessed as moderate). Among the studies that found an attenuating effect, five (three evaluated as strong [ 31 , 38 , 40 ] and one as moderate [ 41 ]) did not include lags and, therefore, assumed that the effect of the meteorological variables was contemporaneous. The studies that did include lags were evaluated with high risk of bias in some dimension. In particular, control of non-linearities [ 32 , 39 , 42 , 45 , 48 ], confounding bias [ 37 , 43 , 48 ], and measurement errors [ 37 , 42 , 47 ], followed by control of population [ 29 , 36 ] and control of spatial and/or temporal dependence [ 33 , 39 ]. Interestingly, Xie et al. [ 27 ], whose units of analysis were 122 Chinese cities, (a study that we evaluated as strong and did not have any dimension evaluated as high risk), points out that there is no evidence supporting that case counts of COVID-19 could decline when the weather becomes warmer.
There was very little evidence in relation to other meteorological variables such as wind speed (only two strong [ 33 , 49 ] and one moderate [ 34 ] study analyzed it and found a negative association between wind speed and incidence); cloud percentage [ 29 ] or solar radiation [ 42 ] (both evaluated as moderate and with contradictory results: higher percentage of cloud was associated with higher incidence, while no association was found with solar radiation); or precipitation (considered in only one strong study that found a significant negative association with incidence [ 31 ]).
Greater consistency was found in the association between greater exposure to levels of air pollution, especially long-term exposure, and an increase in COVID-19 outcomes, both in ecological [ 17 , 28 , 29 , 44 , 48 , 49 , 50 , 51 , 54 , 55 , 56 ] and individual studies [ 52 , 53 ]. The areas that were most exposed to air pollution were those with the highest incidence (new daily cases, new positive tests, and cumulative cases) [ 17 , 29 , 44 , 48 , 49 , 53 , 54 ] and the highest mortality [ 17 , 28 , 29 , 49 , 50 , 51 , 52 , 55 , 56 ] from COVID-19. This result occurs, above all, for fine particles, PM 2.5 [ 28 , 44 , 50 , 51 , 52 , 53 , 54 , 55 , 56 ], but also for ozone, O 3 [ 29 , 49 , 50 ], coarse particles, PM 10 [ 17 , 50 ], nitrogen dioxide, NO 2 [ 17 , 50 ], benzene [ 55 ] and for an air quality index [ 48 ]. In Saez et al. [ 17 ] (which we evaluated as strong) as in Adhikari et al. [ 29 ] and Rodríguez-Villamizar et al. [ 56 ] (these last two evaluated as moderate), some of the pollutants were not found to be associated with mortality (PM 10 in Saez et al., O 3 in Adhikari et al., PM 2.5 in Rodríguez-Villamizar et al.).
In relation to studies that considered socioeconomic variables, as we said, we must distinguish between the findings of ecological [ 17 , 28 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 ] and individual studies [ 53 , 67 , 68 , 69 , 70 , 71 , 72 ]. In the ecological studies, there was no consistent association between socioeconomic contextual variables and COVID-19 outcomes. In just over half of the studies, the socioeconomic variables were risk factors and in the rest they were either protective factors or no statistically significant association was found. Even in some studies, such as Saez et al. [ 17 ] or Wu X et al. [ 28 ] (both of which we evaluated as strong and did not have any dimension evaluated with high risk of bias), apparently contradictory results were found. Thus, in Saez et al. [ 17 ], whose unit of analysis were small areas (counties and health zones, some made up of census tracts, others by municipalities) in Catalonia, Spain, the higher the percentage of poor housing in the small area and the more economically deprived the area was, the greater the risk of a positive result and death. Conversely, the higher the unemployment rate and the percentage of foreigners in the small area, the lower the risk of a positive result and death. In Wu et al. [ 28 ], whose units of analysis were US counties, while percent of the adult population with less than high school education and percent of Black residents, both in the county, were found to be positively associated with the number of deaths in the county, the median household income, the percentage of owner-occupied housing and, marginally, the median house value were also found positively associated. Meanwhile, others, such as the percentage of people in the county in poverty, were not found to be statistically significant associated.
More consistency has been found in relation to population density. In the areas with a higher population density, there was a higher incidence, a higher number of positives, a higher transmission (measured by the reproductive number) and a higher number of deaths than in others less densely populated areas. In Wu et al. [ 28 ], however, the higher the population density, the lower the risk of mortality (although statistical significance only occurs in the fourth quintile).
Of the seven individual studies that we evaluated as moderate or strong, five found an association between both individual socioeconomic status (income, non-white ethnicity—especially Blacks-, lower educational attainment, being an immigrant from a low- or middle-income country) and contextual (income of the area, where the subject resided, residing in a neighborhood with financial insecurity) and various COVID-19 outcomes (positive tests, hospital admissions and deaths). We did, however, find one exception. In Price-Haywood et al. (a study that we evaluated as strong), whose study population was the Ochsner Health facility in New Orleans, Louisiana, USA, Black race was not associated with higher in-hospital mortality than white race, after adjustment for differences in sociodemographic and clinical characteristics on admission [ 70 ].
Our results, both with regard to the methodological limitations that we found in the review and the results of the studies that control them, were similar to those of other reviews. Regarding the methodological limitations, we will refer, in order of publication, to two reviews (not systematic): one that considered air pollutants [ 73 ] and the other meteorological variables [ 74 ]. Villeneuve and Goldberg review six studies on COVID-19 (only two were peer-reviewed) and two on SARS, published up to May 2020 [ 74 ]. Hunter Kerr et al. review 43 studies (23 of them peer-reviewed), published in 2020 [ 74 ]. Both reviews found, as we did, that all studies have methodological limitations in one way or another. Almost all the methodological limitations that we have pointed out here were also considered in these two reviews. There are, however, some differences. Hunter Kerr et al. did not consider choosing a statistical model with normally distributed errors [ 74 ] as a limitation. Villeneuve and Goldberg, for their part, did not consider the error of the functional form (i.e., control of non-linearities), at least directly, inasmuch as they do so indirectly by pointing out, as a limitation, the inadequate evaluation of effect modification [ 73 ]. In contrast, Villeneuve and Goldberg point out, as the most important error, possible cross-level bias in ecological studies.
Regarding the influence of environmental variables (meteorological and air pollutants) in COVID-19 outcomes, the findings of the studies evaluated as moderate or strong in our review, coincided with the findings of the other reviews (both systematic and non-systematic).
We cannot conclude that there was an attenuating effect of weather conditions on the spread of the COVID-19 pandemic. In addition to the fact that, as mentioned, we did not find a systematic behaviour in the reviewed studies, so the attenuation shown by some of them could actually be a consequence of an inadequate adjustment. Thus, on the one hand, the study period of all the studies reviewed by the systematic reviews of Mecenas et al. [ 2 ], Smit et al. [ 3 ] and McClymont and Hu [ 4 ] as well as by the Hunter Kerr et al.’s review [ 74 ], corresponded to the first wave. The same occurs with most of the studies in our review (all except three). However, with a single exception [ 45 ], none of the studies controlled for non-pharmaceutical interventions either as containment or suppression strategies undertaken in that period. Thus, in this case, the reduction in the spread of the pandemic as temperature increased and humidity decreased, could have been confounded by the effects of lockdowns and other restrictions. Although Tobías and Molina [ 45 ] controlled for the effects of lockdown (and also those of seasonality as a consequence of weekends), they did not adjust for other confounders. Consequently, and perhaps for this reason, they found a significant effect only in the contemporary association (the same day) between an increase in temperature and a reduction in the incidence rate. We believe that, if they exist, the effect of meteorological variables on the spread of COVID-19 would be indirect. In the spring–summer of 2020, better weather conditions (higher temperature, lower relative humidity, lower wind speed, etc.) and a relaxation of restrictions, led to greater mobility and, therefore, greater social contact that, in turn, led to an increase in transmission and, consequently, in incidence. This was what happened, for example, in Spain during the second wave (which began in August 2020) [ 23 ].
The results of all reviews, including ours, suggest that there is an association between exposure to air pollutants (particularly in the long term but also in the short term) and COVID-19 outcomes. In fact, two hypotheses have been suggested that would explain this association. First, some studies have proposed that air particulate matter can operate as a virus carrier, promoting the spread of the SARS-CoV-2 [ 74 , 75 , 76 ]. It should be noted, however, that these studies were either not eligible as they used only correlation analysis to test their hypothesis [ 75 ] or they were eligible but were assessed as a high risk of bias [ 76 ].
A second hypothesis has been proposed which suggests there could be potential biological mechanisms that may explain the association between air pollutants and respiratory viral infections. According to this, the effects of exposure to air pollutants would occur not so much on transmission or incidence but on the worsening of the disease (hospitalization, ICU admissions, mortality). Exposure exacerbates the severity of COVID-19 infection symptoms and worsens the prognosis of COVID-19 patients [ 73 ]. In this sense, Wu X et al. [ 28 ] argue that long-term exposure to PM 2.5 could cause alveolar angiotensin-converting enzyme 2 (ACE-2) receptor overexpression and impairs host defences [ 77 ]. This could cause a more severe form of COVID-19 in ACE-2—depleted lungs, increasing the likelihood of poor outcomes, including death [ 78 ]. We, however, believe that air pollutants have actually been surrogates of other variables, such as the mobility of residents and several socioeconomic conditions (high population density, poor housing, use of public transport, occupations in which it is not possible to telecommute, etc.) that facilitate social contact [ 17 ]. In fact, Dey and Dominici, in a very recent editorial commenting on the study by Wu et al. [ 28 ], and of which Dominici is a co-author, point out that the health risks of some racial subgroups are spiraling as they have higher levels of exposure to air pollutants, hence being more susceptible to mortality from COVID-19 [ 79 ]. We do not deny that exposure to air pollutants had an independent effect on, above all, the worsening of the disease among those diagnosed with COVID-19. However, we are convinced that this effect cannot be observed using an ecological design.
As we noted, we have found a consistency in the effects of socioeconomic variables on COVID-19 outcomes only in individual studies and in indicators also at the individual level (ethnicity—particularly being Black—education, etc.). We believe that the effect, if it exists, would be indirect. Poorer socioeconomic conditions would be associated, on the one hand, with greater social contact, which would affect the transmission of the virus and the incidence of COVID-19 and, on the other, with a greater number of comorbidities and greater difficulties in accessing health care which would affect a poorer prognosis of the disease. Furthermore, poorer socio-economic conditions could be related both to a differential exposure to air pollution and to a differential susceptibility to its effects (i.e., modification of the effect) [ 80 ].
In short, a large part of the methodological problems that we have encountered and, therefore, of the uncertainty in the findings, are the consequence of using an ecological design. In this sense, we could not agree more with Hunter Kerr et al. [ 74 ], who recommend, as an epidemiological design, a longitudinal study with individual-level data, in which those diagnosed with COVID-19 would be followed through time.
Our study may have three limitations. First, some studies published during 2020 may have escaped us. That said, this is unlikely, since, as of January 2021, we have been regularly reviewing PubMed and periodically reviewing the other databases. Nevertheless, it is not impossible that a study may have eluded us. Second, both the information extraction and the quality control we carried out could have some subjectivity. We have tried to minimize this as much as possible.
Finally, as we noted, the rating of both the domains and the studies are based on Parmar et al. [ 8 ], with the only difference being that in Parmar et al., an overall rating of strong was given if none of its domains was rated as weak. In our case, this assignment seemed too restrictive. In fact, applying this criterion would imply that only one of the studies could be rated as strong. In our case, we observed some biases that were not contemplated in Parmar et al., such as the lack of control of the population and of the spatial and/or temporal dependence, the non-control of non-linearity and the inappropriate use of statistical models. In our case, the probability that at least one of these biases occurred was very high. In any case, we admit that there could be some degree of arbitrariness in the assignment of the overall rating to one category or another.
All the studies we reviewed have methodological limitations to a greater or lesser extent. Even those that we have evaluated as strong (16.67% of the studies reviewed) and, among them, those in which we did not evaluate any dimension as having a high risk of bias (4 studies), have the limitation of using an ecological epidemiological design or, in any case, either of measuring the exposure in an ecological way (exposure misclassification). These limitations prevent conclusions about the effects of environmental (meteorological and air pollutants) and socioeconomic variables on COVID-19 outcomes being drawn. However, we dare to argue that the effects of these variables, if they exist, would be indirect, based on their relationship with social contact. In any case, the estimation of these independent effects requires the use of an individual design and the control of the methodological limitations explained in this work. Among them, an estimate of individual exposure free of biases (non-differential misclassification, non-existence of spatial–temporal misalignment, etc.).
Availability of data and materials
All the studies, as well as the code to make the figures, can be requested from the corresponding author ([email protected]).
Abbreviations
Novel coronavirus disease
Fine particles with a diameter of 2.5 microns (μm) or less
Nitrogen dioxide
Coarse particles with a diameter of 10 μm or less
Severe acute respiratory syndrome coronavirus 2
Particulate matter
Prospective Register of Systematic Reviews
Preferred reporting items for systematic reviews and meta-analysis
Item Bank for Assessment of Risk of Bias and Precision for Observational Studies of Interventions or Exposures
Polymerase chain reaction
Modifiable areal unit problem
Modifiable temporal unit problem
World Health Organization (WHO) Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 . Accessed 2 Apr 2021.
Mecenas P, Bastos RTDRM, Vallinoto ACR, Normando D (2020) Effects of temperatura and humidity on the spread of COVID-19: a systematic review. PLoS ONE 15(9):e0238339. https://doi.org/10.1371/journal.pone0238339
Article CAS Google Scholar
Smit AJ, Fitchett JM, Engelbrecht FA, Scholes RJ, Dzhivhuho G, Sweijd NA (2020) Winter is coming: a southern hemisphere perspective of the environmental drivers of SARS-CoV-2 and the potential seasonality of COVID-19. Int J Environ Res Public Health 17:5634. https://doi.org/10.3390/ijerph17165634
McClymont H, Hu W (2021) Weather variability and COVID-19 transmission: a review of recent research. Int J Environ Res Public Health 18(2):396. https://doi.org/10.3390/ijerph18020396
Copat C, Cristaldi A, Fiore M, Grasso A, Zuccarello P, Signorelli SS, Conti GO, Ferrante M (2020) The role of air pollution (PM and NO 2 ) in COVID-19 spread and lethality: a systematic review. Environ Res 191:110129. https://doi.org/10.1016/j.envres.2020.110129
Maleki M, Anvari E, Hopke PK, Noorimotlagh Z, Mirzaee SA (2021) An updated systematic review on the association between atmospheric particulate matter pollution and prevalence of SARS-CoV-2. Environ Res 195:110898. https://doi.org/10.1016/j.envres.2021.110898
Page MJ, McKenzie JR, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartssoon A, Lalu MM, Li T, Lode EW, Mayo-Wilson E, McDonald S, McGuinness LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Article Google Scholar
Parmar D, Stavropoulou C, Ioannidis JP (2016) Health outcomes during the 2008 financial crisis in Europe: systematic literature review. BMJ 354:i4588. https://doi.org/10.1136/bmj.i4588
Wells GA, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 3 Apr 2021
Viswanathan M, Berkman ND (2012) Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol 65:163–178. https://doi.org/10.1016/j.jclinepi.2011.05.008
Saez M, Barceló MA, Saurina C, Cabrera A, Daponte A (2019) Evaluation of the biases in the studies that assess the effects of the Great Recession on health. A systematic review. Int J Environ Res Public Health 16(14):2479. https://doi.org/10.3390/ijerph16142479
Greene WH (2018) Econometric analysis, chapter 5, 8th edn. Pearson, Boston, London
Google Scholar
Domingo JL, Marquès M, Rovira J (2020) Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. a review. Environ Res 188:109861. https://doi.org/10.1016/j.envres.2020.109861
Barceló MA, Saez M, Cano-Serral G, Martínez-Beneito MA, Martínez JM, Borrell C, Ocaña-Riola R, Montoya I, Calvo M, López-Abente G, Rodríguez-Sanz M, Toro S, Alcalá JT, Saurina C, Sánchez-Villegas P, Figueiras A (2008) Methods to smooth mortality indicators: application to analysis of inequalities in mortality in Spanish cities (the MEDEA Project) [in Spanish]. Gac Sanit 22(6):596–608. https://doi.org/10.1016/s0213-9111(08)75362-7
Open Government. Generalitat de Catalunya. Open data and COVID-19. http://governobert.gencat.cat/en/dades_obertes/dades-obertes-covid-19/index.html . Accessed 1 May 2021.
Secretaría General de Sanidad. Dirección General de Salud Pública, Calidad e Innovación. Ministerio de Sanidad. Gobierno de España. [in Spanish]. https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov-China/situacionActual.htm . Accessed 1 May 2021.
Saez M, Tobias A, Barceló MA (2020) Effects of long-term exposure to air pollutants on the spatial spread of COVID-19 in Catalonia. Spain Environ Res 191:110177. https://doi.org/10.1016/j.envres.2020.110177
Elliott P, Savitz DA (2008) Design issues in small-area studies of environment and health. Environ Health Perspect 116(8):1098–1104. https://doi.org/10.1289/ehp.10817
Wannemuehler K, Lyles R, Waller L, Hoekstra R, Klein M, Tolbert P (2009) A conditional expectation approach for associating ambient air pollutant exposures with health outcomes. Environmetrics 20(7):877–894. https://doi.org/10.1002/env.978
Openshaw S (1984) Concepts and techniques in modern geography no. 38: the modifiable areal unit problem. Geo Books, Norwich
Helbich M, Mute Browning MHE, Kwan MP (2021) Time to address the spatiotemporal uncertainties in COVID-19 research: concerns and challenges. Sci Total Environ 764:142866. https://doi.org/10.1016/j.scitotenv.2020.142866
Wang Y, Di Q (2020) Modifiable areal unit problem and environmental factors of COVID-19 outbreak. Sci Total Environ 740:139984. https://doi.org/10.1016/j.scitotenv.2020.139984
Ribas V, Miralles F, Rey O, Rafael X, Subías P, Torrent M, Vicens JA, Saez M, Barceló MA, Ponce-de-León M, Valencia A, Arenas A, Saura P (2021) Big Data i Intel·ligència Artificial per a la prevenció d’epidèmies. Monitoratge i predicció per a la detecció primerenca de brots epidemics [in Catalan]. Barcelona: Generalitat de Catalunya.
Cheng T, Adepeju M (2014) Modifiable temporal unit problem (MTUP) and its effect on space-time cluster detection. PLoS ONE 9:e100465. https://doi.org/10.1371/journal.pone.0100465
Filippini T, Rothman KJ, Goffi A, Ferrari F, Maffeis G, Orsini N, Vinceti M (2020) Satellite-detected trophospheric nitrogen dioxide and spreadd of SARS-CoV-2 infection in Northern Italy. Sci Total Environ 739:140278. https://doi.org/10.1016/j.scitotenv.2020.140278
DiMaggio C, Klein M, Berry C, Frangos S (2020) Black/African American Communities are at highest risk of COVID-19: spatial modeling of New York City ZIP Code-level testing results. Ann Epidemiol 51:7–13. https://doi.org/10.1016/j.annepidem.2020.08.012
Xie J, Zhu Y (2020) Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci Total Environ 724:138201. https://doi.org/10.1016/j.scitotenv.2020.138201
Wu X, Nethery RC, Sabath MB, Braun D, Dominici F (2020) Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci Adv 6(45):eabd4049. https://doi.org/10.1126/sciadv.abd4049
Adhikari A, Yin J (2020) Short-term effects of ambient ozone, PM 2.5 , and meteorological factors on COVID-19 confirmed cases and deaths in Queens, New York. Int J Environ Res Public Health 17(11):4047. https://doi.org/10.3390/ijerph17114047
Briz-Redón A, Serrano-Aroca A (2020) A spatio-temporal analysis for exploring the effect of temperature on COVID-19 early evolution in Spain. Sci Total Environ 728:138811. https://doi.org/10.1016/j.scitotenv.2020.138811
Chien LC, Chen LW (2020) Meteorological impacts on the incidence of COVID-19 in the U.S. Stoch Environ Res Risk Assess. 4:1–6. https://doi.org/10.1007/s00477-020-01835-8
Fu S, Wang B, Zhou J, Xu X, Liu J, Ma U, Li L, He X, Li S, Niu J, Luo B, Zhang K (2021) Meteorological factors, governmental responses and COVID-19: evidence from four European countries. Environ Res 194:110596. https://doi.org/10.1016/j.envres.2020.110596 ( Epub 2020 Dec 9 )
Guo C, Bo Y, Changqing L, Li HB, Zeng Y, Zhang Y, Hossain S, Chan JWM, Yeung DW, Kwok KO, Wong SYS, Lau AKH, Lao XQ (2021) Meteorolgical factors and COVID-19 incidence in 190 countries: An observational study. Sci Total Environ 757:143783. https://doi.org/10.1016/j.scitotenv.2020.143783 ( Epub 2020 Nov 23 )
Islam N, Bukhari Q, Jameel Y, Shabnam S, Erzurumluoglu AM, Siddique MA, Massaro JM, D’Agostino RB (2021) COVID-19 and climatic factors: a global analysis. Environ Res 193:110355. https://doi.org/10.1016/j.envres.2020.110355 ( Epub 2020 Oct 28 )
Jüni P, Rothenbühler M, Bobos P, Thorpe KE, da Costa BR, Fisman DN, Slutsky AS, Gesink D (2020) impact of climate and public health interventions on the COVID-19 pandemic: a prospective cohort study. CMAJ 192(21):E566–E573. https://doi.org/10.1503/cmaj.200920
Liu J, Zhou J, Yao J, Zhang X, Li L, Xiaocheng X, He W, Wang B, Fu S, Niu T, Yan J, Shi Y, Ren X, Niu J, Zhu W, Li S, Luo B, Zhang L (2020) Impact of meterological factors on the COVID-19 transmission: a multi-ciy study in China. Sci Total Environ 726:138513. https://doi.org/10.1016/j.scitotenv.2020.138513
Ma Y, Zhao Y, Liu J, He X, Wang B, Fu S, Yan J, Niu J, Zhou J, Luo B (2020) Effects of temperature variation and humididty on the death of COVID-19 in Wuhan, China. Sci Total Environ 724:138226. https://doi.org/10.1016/j.scitotenv.2020.138226
Meyer A, Sadler R, Faverjon C, Cameron AR, Bannister-Tyrrell M (2020) Evidence that higher temperature are associated with a marginally lower incidence of COVID-19 cases. Front Public Health 8:367. https://doi.org/10.3389/fpubh.2020.00367
Pequeno P, Mendel B, Rosa C, Bosholn M, Souza JL, Baccaro F, Barbosa R, Magnusson W (2020) Air transportation, population density and temperature predict the spread of COVID-19 in Brazil. PeerJ 8:e9322. https://doi.org/10.7717/peerj.9322
Prata DN, Rodrigues W, Bermejo PH (2020) Temperature significantly changes COVID-19 transmission in (sub)tropical cities of Brazil. Sci Total Environ 729:138862. https://doi.org/10.1016/j.scitotenv.2020.138862
Qi H, Xiao S, Shi R, Ward MP, Chen Y, Tu W, Su Q, Wang W, Wang X, Zhang Z (2020) COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Sci Total Environ 728:138778. https://doi.org/10.1016/j.scitotenv.2020.138778
Runkle JD, Sugg MM, Leeper RD, Rao Y, Matthews JL, Rennie JJ (2020) Short-term effects of specific humidity and temperature on COVID-19 morbidity in select US cities. Sci Total Environ 740:140093. https://doi.org/10.1016/j.scitotenv.2020.140093
Shi P, Dong Y, Yan H, Zhao C, Li X, Liu W, He M, Tang S, Xi S (2020) Impact of temperature on the dynamics of the COVID-19 outbreak in China. Sci Total Environ 728:138890. https://doi.org/10.1016/j.scitotenv.2020.138890
Stieb DM, Evans GJ, To TM, Brook JR, Burnett RT (2020) An ecological analysis of long-term exposure to PM 2.5 and incidence of COVID-19 in Canadian health regions. Environ Res 191:110052. https://doi.org/10.1016/j.envres.2020.110052
Tobías A, Molina T (2020) Is temperature reducing the transmission of COVID-19? Environ Res 186:109553. https://doi.org/10.1016/j.envres.2020.109553
Wang Q, Zhao Y, Zhang Y, Qiu J, Li J, Yan N, Li N, Zhang J, Tian D, Sha X, Jing J, Yang C, Wang K, Xu R, Zhang Y, Yang H, Zhao S, Zhao Y (2021) Could the ambient higher temperature decrease the transmissibility of COVID-19 in China? Environ Res 193:110576. https://doi.org/10.1016/j.envres.2020.110576 ( Epub 2020 Dec 3 )
Wu Y, Jing W, Liu J, Ma Q, Yuan J, Wang Y, Du M, Liu M (2020) Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci Total Environ 729:139051. https://doi.org/10.1016/j.scitotenv.2020.139051
Xu H, Yan C, Fu Q, Xiao K, Yu Y, Han D, Wang W, Cheng J (2020) Possible environmental effects on the spread of COVID-19 in China. Sci Total Environ 731:139211. https://doi.org/10.1016/j.scitotenv.2020.139211
Coccia M (2021) How do low wind speeds and high levels of air pollution support the spread of COVID-19? Atmos Pollut Res 12(1):437–445. https://doi.org/10.1016/j.apr.2020.10.002 ( Epub 2020 Oct 7 )
Liang D, Shi L, Zhao J, Liu P, Sarnat JA, Gao S, Schwartz J, Liu Y, Ebelt ST, Scovronick N, Chang HH (2020) Urban air pollution may enhace COVID-19 case-fatality and mortality rates in the United States. Innovation (NY) 1(3):100047. https://doi.org/10.1016/j.xinn.2020.100047
Pozzer A, Dominici F, Haines A, Witt C, Münzel T, Lelieveld J (2020) Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc Res 116(14):2247–2253. https://doi.org/10.1093/cvr/cvaa288
López-Feldman A, Heres D, Márquez-Padilla F (2021) Air pollution exposure and COVID-19: a look at mortality in Mexico City using individual-level data. Sci Total Environ 756:143929. https://doi.org/10.1016/j.scitotenv.2020.143929 ( Epub 2020 Nov 26 )
Chadeau-Hyam M, Bodinier B, Elliott J, Whitaker MD, Tzoulaki I, Vermeulen R, Kelly-Irving M, Delpierre C, Elliott P (2020) Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK biobank data. Int J Epidemiol 49(5):1454–1467. https://doi.org/10.1093/ije/dyaa134
Chakrabarty RK, Beeler P, Liu P, Gooswami S, Harvey RD, Pervez S, van Donkelaar A, Martin RV (2021) Ambient PM 2.5 exposure and rapid spread of COVID-19 in the United States. Sci Total Environ 760:143391. https://doi.org/10.1016/j.scitotenv.2020.143391 ( Epub 2020 Nov 9 )
Luo Y, Yan j, McClure S, (2021) Distribution of the environmental and socioeconomic risk factors on COVID-19 death rate across continental USA: a spatial nonlinear analysis. Environ Sci Pollut Res Int 28(6):6587–6599. https://doi.org/10.1007/s11356-020-10962-2 ( Epub 2020 Oct 1 )
Rodríguez-Villamizar L, Belalcázar-Ceróon LC, Fernández-Niño JA, Marín-Pineda DM, Rojas-Sánchez O, Acuña-Merchán LA, Ramírez-García N, Mangones-Matos SC, Vargas-González JN, Herrera-Torres J, Agudelo-Castañeda DM, Piñeros-Jiménez JG, Rojas-Roa NY, Herrera-Galindo M (2021) Air pollution, sociodemographic and health conditions effects on COVID-19 mortality in Colombia: an ecological study. Sci Total Environ 756:144020. https://doi.org/10.1016/j.scitotenv.2020.144020 ( Epub 2020 Nov 26 )
Chaudhry R, Dranitsaris G, Mubashir T, Bartoszko J, Riazi S (2020) A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine 25:100464. https://doi.org/10.1016/j.eclinm.2020.100464
Kaiser JC, Stathopoulos GT (2020) Socioeconomic correlates of SARS-CoV-2 and influenza H1N1 outbreaks. Eur Respir J 56(3):2001400. https://doi.org/10.1183/13993003.01400-2020
Lamb MR, Kandula S, Shaman J (2021) Differential COVID-19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses 15(2):209–217. https://doi.org/10.1111/irv.12816 ( Epub 2020 Oct 14 )
Madhav KC, Oral E, Straif-Bourgeois S, Rung AL, Peters ES (2020) The effect of area deprivation on COVID-19 risk in Lousiana. PLoS ONE 15(12):e0243028. https://doi.org/10.1371/journal.pone.0243028
Plümper T, Neumayer E (2020) The pandemic predominantly hits poor neighboourhoods? SARS-CoV-2 infections and COVID-19 fatalities in German districts. Eur J Public Health 30(6):1176–1180. https://doi.org/10.1093/eurpub/ckaa168
Richmond HL, Tome J, Rochani H, Fung CH, Shah GH, Schwind JS (2020) The use of penalized regression analysis to identify county-level demographic and socioeconomic variables predictive of increased COVID-19 cumulative case rates in the state of Georgia. Int J Environ Res Public Health 17(21):8036. https://doi.org/10.3390/ijerph17218036
Rubin D, Huang J, Fisher BT, Gasparrini A, Tam V, Song L, Wang X, Kaufman J, Fitzpatrick K, Jain A, Griffis H, Cramer K, Morris J, Tasian G (2020) Association of social distancing, population density, and temperature with the instantaneous reproduction number of SARS-CoV-2 in counties across the United States. JAMA Netw Open 3(7):e2016099. https://doi.org/10.1001/jamanetworkopen.2020.16099
Sannigrahi S, Pilla F, Basu B, Basu AS, Molter A (2020) Examining the association between socio-demographic composition and COVID-29 fatalities in the European region using spatial regression approach. Sustain Cities Soc 62:102418. https://doi.org/10.1016/j.scs.2020.102418
Scarpone C, Brinkmann ST, Große T, Sonnenwald D, Fuchs M, Byron WB (2020) A multimethod approach for county-scale geospatial analysis of emerging infectious diseases: a cross-sectional case study of COVID-19 incidence in Germany. Int J Health Geogr 19(1):32. https://doi.org/10.1186/s12942-020-00225-1
You H, Wu X, Guo X (2020) Distribution of COVID-19 morbidity rate in association with social and economic factors in Wuhan, China: Implications for urban development. Int J Environ Res Public Health 17(10):3417. https://doi.org/10.3390/ijerph17103417
Azar KMJ, Shen Z, Romanelli RJ, Lockhart SH, Smits K, Robinson S, Brown S, Pressman AR (2020) Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood) 39(7):1253–1262. https://doi.org/10.1377/hlthaff.2020.00598
Drefahl S, Wallace M, Mussino E, Aradhya S, Kolk M, Brandén M, Malmberg B, Andersson G (2020) A population-based cohort study of socio-demographic risk factors for COVID-19 deaths in Sweden. Nat Commun 11(1):5097. https://doi.org/10.1038/s41467-020-18926-3
Marcielde Souza W, Fletcher Buss L, da Silva Candido D, Carrera JP, Li S, Zarebski AE, Moraes Pereira RH, Prete CA, de Souza-Santos AA, Parag KV, Belotti MC, Vincenti-González MF, Messina J, da Silva Sales FC, Dos Santos Andrade P, Heloiz Nascimento V, Ghilardi F, Abade L, Gutiérrez B, Kraemer MUG, Braga CKV, Santana Aguiar R, Alexander N, Mayaud P, Brady OJ, Marcilio I, Gouveia N, Li G, Tami A, Barbosade Oliveira S, Gomes Porto VB, Ganem F, Ferreirade Almeida WA, Sutile Tardetti Fantinato FF, Marques Macário E, Kleberde Oliveiira W, Nogueiira ML, Pybus OG, Wu CH, Croda J, Sabino EC, Rodrigues Faria N (2020) Epidemiological and clinical characteristics of the COVID-10 epidemic in Brazil. Nat Hum Behav 4(8):856–865. https://doi.org/10.1038/s41562-020-0928-4
Price-Haywood EG, Burton H, Fort D, Seoane L (2020) Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med 382(26):2534–2543. https://doi.org/10.1056/NEJMsa2011686
Rozenfeld Y, Beam J, Maier H, Haggerson W, Boudreau K, Carlson J, Medows R (2020) A model of disparities: risk factors associated with COVID-19 infection. Int J Equity Health. 19(1):126. https://doi.org/10.1186/s12939-020-01242-z
Zakery R, Bendayan R, Ashworth M, Bean DM, Dodhia H, Durbaba S, O’Gallagher K, Palmmer C, Curcin V, Aitken E, Bernal W, Barker RD, Norton S, Gulliford M, Teo JTH, Galloway J, Dobson RJB, Shah AM (2020) A case-control and cohort study to determine the relationship between ethnic background and severe COVID-19. EClinicalMedicine 28:100574. https://doi.org/10.1016/j.eclinm.2020.100574
Villeneuve PJ, Goldberg MS (2020) Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ Health Perspect 128(9):95001. https://doi.org/10.1289/EHP7411
Hunter Kerr G, Badr HS, Gardner LM, Pérez-Saez J, Zaitchik BF (2021) Associations between meteorology and COVID-19 in early studies: Inconsistencies, uncertainties, and recommendations. One Health 12:100225. https://doi.org/10.1016/j.onehlt.2021.100225
Bashir MF, Ma BB, Komal B, Bashir MA, Tan D, Bashir M (2020) Correlation between climate indicators and COVID-19 pandemic in New York, USA. Sci Total Environ 728:138835. https://doi.org/10.1016/j.scitotenv.2020.138835
Fattorini D, Regoli F (2020) Role of the chronic air pollution levels in the COVID-19 oubreak risk in Italy. Environ Pollut 264:114732. https://doi.org/10.1016/j.envpol.2020.114732
Miyashita L, Foley G, Semple S, Grigg J (2020) Traffic-derived particulate matter and angiotensin-converting enzyme 2 expression in human airway epithelial cells. bioRxiv. https://doi.org/10.1101/2020.05.15.097501v2
Frontera A, Cianfanelli L, Vlachos K, Landoni G, Cremona G (2020) Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J Infect 81:255–259. https://doi.org/10.1016/j.jinf.2020.05.031
Dey T, Dominici F (2021) COVID-19, ari pollution, and racial inequity: Connecting the dots. Chem Res Toxicol 34(3):669–671. https://doi.org/10.1021/acs.chemrestox.0c00432
Saez M, López-Casasnovas G (2019) Assessing the effects on health inequalities of differential exposure and differential susceptibility of air pollution and environmental noise in Barcelona, 2007–2014. Int J Environ Res Public Health 16(18):3470. https://doi.org/10.3390/ijerph16183470
Martorell-Marugán J, Villatoro-García JA, et al. DatAC: a visual analytics platform to explore climate and air quality indicators associated with the COVID-19 pandemic in Spain. Sci Total Environ 2020; 750: 141424. https://doi.org/10.1016/j.scitotenv.2020.141424
DatAC: Data against COVID-19 [in Spanish]. https://covid19.genyo.es . Accessed 23 Apr 2021.
Download references
Acknowledgements
This study was carried out within the ‘Cohort-Real World Data’ subprogram of CIBER of Epidemiology and Public Health (CIBERESP).
This work was partially financed by the SUPERA COVID19 Fund from SAUN: Santander Universidades, CRUE and CSIC, and by the COVID-19 Competitive Grant Program from Pfizer Global Medical Grants. The funding sources did not participate in the design or conduct of the study, the collection, management, analysis, or interpretation of the data, or the preparation, review, or approval of the manuscript.
Author information
Authors and affiliations.
Research Group On Statistics, Econometrics and Health (GRECS), and CIBER of Epidemiology and Public Health (CIBERESP), University of Girona, Carrer de la Universitat de Girona 10, Campus de Montilivi, 17003, Girona, Spain
Maria A. Barceló & Marc Saez
CIBER of Epidemiology and Public Health (CIBERESP), Madrid, Spain
You can also search for this author in PubMed Google Scholar
Contributions
MS had the original idea for the paper. MS designed the study. The bibliographic search and the writing of the introduction were carried out by MS and MAB. The methods were chosen and performed by all authors. MAB created the tables and figures. All authors wrote the results and the discussion. The writing and final editing was done by all authors. All authors reviewed and approved the manuscript.
Corresponding author
Correspondence to Marc Saez .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interest.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1.
. List of studies excluded. Table S2 . Studies included in the qualitative synthesis Table S3 . List of studies included in the qualitative synthesis. Figure S1. Number of studies by type of explanatory variable analyzed
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Barceló, M.A., Saez, M. Methodological limitations in studies assessing the effects of environmental and socioeconomic variables on the spread of COVID-19: a systematic review. Environ Sci Eur 33 , 108 (2021). https://doi.org/10.1186/s12302-021-00550-7
Download citation
Received : 26 June 2021
Accepted : 03 September 2021
Published : 10 September 2021
DOI : https://doi.org/10.1186/s12302-021-00550-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Environmental (meteorological and air pollutants) variables
- Socioeconomic variables
- Social contacts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Methodological limitations in studies assessing the effects of environmental and socioeconomic variables on the spread of COVID-19: a systematic review
Affiliations.
- 1 Research Group On Statistics, Econometrics and Health (GRECS), and CIBER of Epidemiology and Public Health (CIBERESP), University of Girona, Carrer de la Universitat de Girona 10, Campus de Montilivi, 17003 Girona, Spain.
- 2 CIBER of Epidemiology and Public Health (CIBERESP), Madrid, Spain.
- PMID: 34522574
- PMCID: PMC8432444
- DOI: 10.1186/s12302-021-00550-7
Background: While numerous studies have assessed the effects of environmental (meteorological variables and air pollutants) and socioeconomic variables on the spread of the COVID-19 pandemic, many of them, however, have significant methodological limitations and errors that could call their results into question. Our main objective in this paper is to assess the methodological limitations in studies that evaluated the effects of environmental and socioeconomic variables on the spread of COVID-19.
Main body: We carried out a systematic review by conducting searches in the online databases PubMed, Web of Science and Scopus up to December 31, 2020. We first excluded those studies that did not deal with SAR-CoV-2 or COVID-19, preprints, comments, opinion or purely narrative papers, reviews and systematic literature reviews. Among the eligible full-text articles, we then excluded articles that were purely descriptive and those that did not include any type of regression model. We evaluated the risk of bias in six domains: confounding bias, control for population, control of spatial and/or temporal dependence, control of non-linearities, measurement errors and statistical model. Of the 5631 abstracts initially identified, we were left with 132 studies on which to carry out the qualitative synthesis. Of the 132 eligible studies, we evaluated 63.64% of the studies as high risk of bias, 19.70% as moderate risk of bias and 16.67% as low risk of bias.
Conclusions: All the studies we have reviewed, to a greater or lesser extent, have methodological limitations. These limitations prevent conclusions being drawn concerning the effects environmental (meteorological and air pollutants) and socioeconomic variables have had on COVID-19 outcomes. However, we dare to argue that the effects of these variables, if they exist, would be indirect, based on their relationship with social contact.
Supplementary information: The online version contains supplementary material available at 10.1186/s12302-021-00550-7.
Keywords: COVID-19; Environmental (meteorological and air pollutants) variables; Social contacts; Socioeconomic variables.
© The Author(s) 2021.
PubMed Disclaimer
Conflict of interest statement
Competing interestThe authors declare that they have no competing interests.
Flow-chart of the study selection…
Flow-chart of the study selection process
Smoothed curves for the relationships…
Smoothed curves for the relationships between daily temperature and daily levels of nitrogen…
Residual analysis of the linear…
Residual analysis of the linear regression models relating the transmission of SARS-CoV-2 infection…
Similar articles
- Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia. Osborne SR, Alston LV, Bolton KA, Whelan J, Reeve E, Wong Shee A, Browne J, Walker T, Versace VL, Allender S, Nichols M, Backholer K, Goodwin N, Lewis S, Dalton H, Prael G, Curtin M, Brooks R, Verdon S, Crockett J, Hodgins G, Walsh S, Lyle DM, Thompson SC, Browne LJ, Knight S, Pit SW, Jones M, Gillam MH, Leach MJ, Gonzalez-Chica DA, Muyambi K, Eshetie T, Tran K, May E, Lieschke G, Parker V, Smith A, Hayes C, Dunlop AJ, Rajappa H, White R, Oakley P, Holliday S. Osborne SR, et al. Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
- Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence. Baldacci S, Gorini F, Santoro M, Pierini A, Minichilli F, Bianchi F. Baldacci S, et al. Epidemiol Prev. 2018 May-Aug;42(3-4 Suppl 1):1-34. doi: 10.19191/EP18.3-4.S1.P001.057. Epidemiol Prev. 2018. PMID: 30066535 Review. English.
- Response to letter to the editor from Dr Rahman Shiri: The challenging topic of suicide across occupational groups. Niedhammer I, Milner A, Witt K, Klingelschmidt J, Khireddine-Medouni I, Alexopoulos EC, Toivanen S, Chastang JF, LaMontagne AD. Niedhammer I, et al. Scand J Work Environ Health. 2018 Jan 1;44(1):108-110. doi: 10.5271/sjweh.3698. Epub 2017 Dec 8. Scand J Work Environ Health. 2018. PMID: 29218357
- Association between pacifier use and breast-feeding, sudden infant death syndrome, infection and dental malocclusion. Callaghan A, Kendall G, Lock C, Mahony A, Payne J, Verrier L. Callaghan A, et al. JBI Libr Syst Rev. 2005;3(6):1-33. doi: 10.11124/01938924-200503060-00001. JBI Libr Syst Rev. 2005. PMID: 27819973
- A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Sutcliffe P, Connock M, Shyangdan D, Court R, Kandala NB, Clarke A. Sutcliffe P, et al. Health Technol Assess. 2013 Sep;17(42):1-274. doi: 10.3310/hta17420. Health Technol Assess. 2013. PMID: 24070110 Free PMC article. Review.
- Systematic review of empiric studies on lockdowns, workplace closures, and other non-pharmaceutical interventions in non-healthcare workplaces during the initial year of the COVID-19 pandemic: benefits and selected unintended consequences. Ahmed F, Shafer L, Malla P, Hopkins R, Moreland S, Zviedrite N, Uzicanin A. Ahmed F, et al. BMC Public Health. 2024 Mar 22;24(1):884. doi: 10.1186/s12889-024-18377-1. BMC Public Health. 2024. PMID: 38519891 Free PMC article.
- Combining aggregate and individual-level data to estimate individual-level associations between air pollution and COVID-19 mortality in the United States. Woodward SM, Mork D, Wu X, Hou Z, Braun D, Dominici F. Woodward SM, et al. PLOS Glob Public Health. 2023 Aug 2;3(8):e0002178. doi: 10.1371/journal.pgph.0002178. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37531330 Free PMC article.
- Neighborhood Socioeconomic Characteristics Associated with the COVID-19 Incidence in Elementary School Children: An Ecological Study in Osaka City, Japan. Oishi K, Mori T, Nakaya T, Ishii K. Oishi K, et al. Children (Basel). 2023 Apr 30;10(5):822. doi: 10.3390/children10050822. Children (Basel). 2023. PMID: 37238370 Free PMC article.
- Uncovering COVID-19 infection determinants in Portugal: towards an evidence-based spatial susceptibility index to support epidemiological containment policies. Alves A, da Costa NM, Morgado P, da Costa EM. Alves A, et al. Int J Health Geogr. 2023 Apr 6;22(1):8. doi: 10.1186/s12942-023-00329-4. Int J Health Geogr. 2023. PMID: 37024965 Free PMC article.
- The influence of meteorological factors on COVID-19 spread in Italy during the first and second wave. Balboni E, Filippini T, Rothman KJ, Costanzini S, Bellino S, Pezzotti P, Brusaferro S, Ferrari F, Orsini N, Teggi S, Vinceti M. Balboni E, et al. Environ Res. 2023 Jul 1;228:115796. doi: 10.1016/j.envres.2023.115796. Epub 2023 Apr 3. Environ Res. 2023. PMID: 37019296 Free PMC article.
- World Health Organization (WHO) Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/director-general/speeches/detail/who-director-genera... . Accessed 2 Apr 2021.
- Mecenas P, Bastos RTDRM, Vallinoto ACR, Normando D. Effects of temperatura and humidity on the spread of COVID-19: a systematic review. PLoS ONE. 2020;15(9):e0238339. doi: 10.1371/journal.pone0238339. - DOI - PMC - PubMed
- Smit AJ, Fitchett JM, Engelbrecht FA, Scholes RJ, Dzhivhuho G, Sweijd NA. Winter is coming: a southern hemisphere perspective of the environmental drivers of SARS-CoV-2 and the potential seasonality of COVID-19. Int J Environ Res Public Health. 2020;17:5634. doi: 10.3390/ijerph17165634. - DOI - PMC - PubMed
- McClymont H, Hu W. Weather variability and COVID-19 transmission: a review of recent research. Int J Environ Res Public Health. 2021;18(2):396. doi: 10.3390/ijerph18020396. - DOI - PMC - PubMed
- Copat C, Cristaldi A, Fiore M, Grasso A, Zuccarello P, Signorelli SS, Conti GO, Ferrante M. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: a systematic review. Environ Res. 2020;191:110129. doi: 10.1016/j.envres.2020.110129. - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 04 July 2024
Characteristics and outcomes of patients with COVID-19 at high risk of disease progression receiving sotrovimab, oral antivirals, or no treatment: a retrospective cohort study
- Myriam Drysdale ORCID: orcid.org/0000-0002-8994-2816 1 ,
- Holly Tibble ORCID: orcid.org/0000-0001-7169-4087 2 ,
- Vishal Patel ORCID: orcid.org/0000-0002-1517-4148 1 ,
- Daniel C. Gibbons ORCID: orcid.org/0000-0002-3769-9535 1 ,
- Emily J. Lloyd 1 ,
- William Kerr 3 ,
- Calum Macdonald ORCID: orcid.org/0000-0001-7857-9188 2 ,
- Helen J. Birch ORCID: orcid.org/0000-0002-4924-4810 1 &
- Aziz Sheikh ORCID: orcid.org/0000-0001-7022-3056 2
BMC Infectious Diseases volume 24 , Article number: 670 ( 2024 ) Cite this article
190 Accesses
2 Altmetric
Metrics details
The clinical benefit of coronavirus disease 2019 (COVID-19) treatments against new circulating variants remains unclear. We sought to describe characteristics and clinical outcomes of highest risk patients with COVID-19 receiving early COVID-19 treatments in Scotland.
Retrospective cohort study of non-hospitalized patients diagnosed with COVID-19 from December 1, 2021–October 25, 2022, using Scottish administrative health data. We included adult patients who met ≥ 1 of the National Health Service highest risk criteria for early COVID-19 treatment and received outpatient treatment with sotrovimab, nirmatrelvir/ritonavir or molnupiravir, or no early COVID-19 treatment. Index date was defined as the earliest of COVID-19 diagnosis or early COVID-19 treatment. Baseline characteristics and acute clinical outcomes in the 28 days following index were reported. Values of ≤ 5 were suppressed.
In total, 2548 patients were included (492: sotrovimab, 276: nirmatrelvir/ritonavir, 71: molnupiravir, and 1709: eligible highest risk untreated). Patients aged ≥ 75 years accounted for 6.9% ( n = 34/492), 21.0% ( n = 58/276), 16.9% ( n = 12/71) and 13.2% ( n = 225/1709) of the cohorts, respectively. Advanced renal disease was reported in 6.7% ( n = 33/492) of sotrovimab-treated and 4.7% ( n = 81/1709) of untreated patients, and ≤ 5 nirmatrelvir/ritonavir-treated and molnupiravir-treated patients. All-cause hospitalizations were experienced by 5.3% ( n = 25/476) of sotrovimab-treated patients, 6.9% ( n = 12/175) of nirmatrelvir/ritonavir-treated patients, ≤ 5 (suppressed number) molnupiravir-treated patients and 13.3% ( n = 216/1622) of untreated patients. There were no deaths in the treated cohorts; mortality was 4.3% ( n = 70/1622) among untreated patients.
Conclusions
Sotrovimab was often used by patients who were aged < 75 years. Among patients receiving early COVID-19 treatment, proportions of 28-day all-cause hospitalization and death were low.
Peer Review reports
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, as caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared by the World Health Organization in March 2020 [ 1 ]. Older individuals, immunocompromised patients or those with comorbidities such as cancer, diabetes, advanced renal disease or cardiovascular disease are at increased risk of developing severe COVID-19, which may result in hospitalization or death [ 2 , 3 ].
In the UK, early COVID-19 treatment with either antivirals (e.g., nirmatrelvir/ritonavir, molnupiravir, remdesivir) or monoclonal antibodies (mAbs; e.g., casirivimab/imdevimab, sotrovimab) has been recommended for people with “highest risk” conditions. At the time of the study, examples of these conditions included solid cancer, advanced renal and liver disease, and human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) [ 4 ]. The emergence of the pandemic threatened to overwhelm healthcare systems in the UK and around the world. Strategies to minimize hospitalizations due to COVID-19 were key to the public health policy response, and hospitalization was an important outcome in the pivotal clinical trials of COVID-19 therapies [ 5 , 6 , 7 ].
Sotrovimab is a dual-action engineered human immunoglobulin G1κ mAb derived from the parental mAb S309, a potent neutralizing mAb directed against the spike protein of SARS-CoV-2 [ 8 , 9 , 10 , 11 ]. In the COVID-19 Monoclonal antibody Efficacy Trial-Intent to Care Early (COMET-ICE) randomized clinical trial (NCT04545060), sotrovimab (500 mg intravenous dose) was shown to significantly reduce the relative risk of all-cause > 24-h hospitalization or death by 79% compared with placebo in high-risk patients with mild-to-moderate COVID-19 [ 5 ]. In December 2021, sotrovimab received conditional marketing authorization in the UK for use in symptomatic adults and adolescents (aged ≥ 12 years and weighing ≥ 40 kg) with acute COVID-19 who did not require supplemental oxygen and were deemed at increased risk of progression to severe COVID-19 [ 12 ].
Two oral antivirals, molnupiravir and nirmatrelvir/ritonavir, have been shown to reduce the risk of progression to severe COVID-19 compared with placebo among high-risk patients with mild-to-moderate COVID-19 [ 6 , 7 , 13 ]. In the MOVe-OUT trial (NCT04575597), participants receiving molnupiravir had a lower risk of hospitalization or death through day 29 compared with the placebo group (6.8% vs 9.7%; difference, 3.0 percentage points; 95% CI, − 5.9 to − 0.1) [ 7 ]. In the EPIC-HR trial (NCT04960202), nirmatrelvir/ritonavir significantly reduced the incidence of hospitalization or death by day 28 compared with placebo (difference, 5.81 percentage points; 95% CI, –7.78 to –3.84; relative risk reduction, 88.9%) [ 6 ]. Molnupiravir and nirmatrelvir/ritonavir received conditional marketing authorization in November 2021 and December 2021, respectively, for use in patients with COVID-19 who were deemed at increased risk of disease progression [ 14 , 15 ].
During the study period (December 2021–October 2022), National Health Service (NHS) guidelines recommended sotrovimab and nirmatrelvir/ritonavir as first-line treatment options, and molnupiravir as a third-line option [ 16 ]. It should be noted that nirmatrelvir/ritonavir may be contraindicated for certain highest-risk patients, including those with advanced renal disease or receiving ritonavir-containing medication for HIV/AIDS [ 17 ]. These guidelines apply to all parts of the UK, including Scotland [ 18 ].
Here, we describe real-world use of early COVID-19 treatments (including patient characteristics and clinical outcomes) for the management of non-hospitalized patients with COVID-19 at highest risk of developing severe disease in Scotland.
Study design and data source
This retrospective cohort study followed STROBE and RECORD reporting guidelines. We used data from administrative health datasets managed by Public Health Scotland and National Records of Scotland, linked and pseudonymized by the electronic Data Research and Innovation Service.
The study cohort was drawn from the Scottish general practitioner-registered population living within six health boards (i.e. Ayrshire & Arran, Dumfries & Galloway, Forth Valley, Greater Glasgow & Clyde, Lanarkshire and Lothian) that used the Hospital Electronic Prescribing and Medicines Administration (HEPMA) system for recording administration and prescription of COVID-19 therapies.
The index date (Day 1) was defined as the earliest date of a confirmed COVID-19 diagnosis (via reverse transcriptase polymerase chain reaction [RT-PCR] or lateral flow test [LFT]), or treatment date during the study period. Patients were followed up for 28 days (Day 28) from index (defined as the acute period), during which patient outcomes were evaluated. We included patients diagnosed with COVID-19 from December 1, 2021–October 25, 2022. The baseline period was defined as the 2 years prior to index for secondary care events and 1 year prior to index for general practitioner prescriptions.
Study population
Non-hospitalized patients (defined as having treatment for COVID-19 initiated in an outpatient or community setting) were eligible for inclusion if they were aged ≥ 18 years on the index date; had a COVID-19 diagnosis/positive SARS-CoV-2 RT-PCR or LFT; lived within 1 of the 6 geographical zones attached to a Scottish health board that used the HEPMA prescribing system; met ≥ 1 of the NHS highest-risk conditions criteria for receiving early treatment with sotrovimab, nirmatrelvir/ritonavir or molnupiravir (as defined by the presence of diagnosis codes) (Table 1 ); and received outpatient treatment with sotrovimab, nirmatrelvir/ritonavir, or molnupiravir, or received no early COVID-19 treatment.
At the time of study, the NHS highest-risk criteria were Down’s syndrome, solid cancer, hematologic diseases (including cancers), advanced renal disease, advanced liver disease, immune-mediated inflammatory disease (IMID), immune deficiencies, HIV/AIDS, solid-organ and stem-cell transplant recipients, and rare neurologic conditions [ 4 ]. These highest-risk criteria were evaluated during the baseline period.
Patients were excluded if they received more than one COVID-19 treatment (sotrovimab, nirmatrelvir/ritonavir, molnupiravir, or remdesivir) in an outpatient setting during the acute period. Patients were also excluded if they received remdesivir as an early treatment in an outpatient setting, or initiated any COVID-19 treatment while in an inpatient setting (defined as overnight admission on the day of or prior to treatment, and discharge after the day of treatment). These latter two criteria were intended to ensure that only non-hospitalized patients with mild-to-moderate COVID-19 were included.
Study outcomes
The primary outcomes of this study were the proportions of patients with all-cause and COVID-19-related hospitalizations during the acute period (28 days following index). COVID-19-related hospitalizations were defined as any non-elective hospital visit for which COVID-19 was listed in the primary diagnosis field (among patients in whom the hospitalization episode was complete, i.e. discharge had occurred and clinical coding was complete).
Secondary outcomes included the number of all-cause and COVID-19-related inpatient hospitalization days, the proportion of patients with a critical care admission as part of hospitalization, the proportion of patients requiring non-invasive ventilation and mechanical ventilation, and the proportion of deaths during the acute period.
For all outcomes, patients were excluded if there was less than 45 days between the index diagnosis and data extraction censoring dates (regardless of whether they died in this interval) for the outcomes data (October 6, 2022). Forty-five days is the period recommended by Public Health Scotland to account for a reporting lag in the datasets used.
Patient characteristics were also recorded, including age, sex, COVID-19 vaccination status, and comorbidity history. Cohorts were described in relation to “highest-risk” conditions that made patients eligible for early treatment with sotrovimab, nirmatrelvir/ritonavir, or molnupiravir, as mentioned above. Additionally, the cohorts were described in relation to other “high-risk” conditions that may predispose patients to severe COVID-19 outcomes (Table 1 ).
Outcomes were reported for the following cohorts: Cohort 1, patients receiving early treatment with sotrovimab; Cohort 2, patients receiving early treatment with nirmatrelvir/ritonavir; Cohort 3, patients receiving early treatment with molnupiravir; and Cohort 4, patients at highest risk who received no early COVID-19 treatment.
A subgroup analysis was also conducted. We described 28-day COVID-19-related hospitalization among sotrovimab-treated patients (Cohort 1) and those without any early COVID-19 treatments (Cohort 4) during the periods of Omicron BA.1 (December 1, 2021–February 28, 2022), Omicron BA.2 (March 1–May 31, 2022) and Omicron BA.5 (June 1–September 30, 2022) subvariant predominance in the UK (Fig. 1 ) [ 19 ]. These analyses were not performed for patients treated with nirmatrelvir/ritonavir or molnupiravir due to small sample sizes. Due to low sequencing rates, periods of most prevalent circulating variants were used as a proxy for the infecting variant.

SARS-CoV-2 variant predominance from February 2021 to September 2022. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 45. 2022. Reprinted from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1115071/Technical-Briefing-45-9September2022.pdf [ 19 ]. Contains public sector information licensed under the Open Government Licence v3.0: https://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/ . Accessed 17 Aug 2023. SARS-CoV-2 , severe acute respiratory syndrome coronavirus 2
Data analysis
Continuous variables (e.g. age) were summarized using mean, standard deviation, median, interquartile range, and range. Categorical variables (e.g. sex) were described using frequencies and percentages. Due to the study’s information governance and data suppression rules, counts of between 0 and 5 were suppressed and are reported as n = ≤ 5 throughout (unless they were structural zeros caused by inclusion/exclusion criteria). This suppression did not apply to mortality data.
Patient demographics and baseline characteristics
Following application of the eligibility criteria, demographics and baseline characteristics were available for 2548 patients, including 492 patients treated with sotrovimab, 276 patients treated with nirmatrelvir/ritonavir, 71 patients treated with molnupiravir, and 1709 eligible highest-risk untreated patients (Fig. 2 ). Baseline characteristics are reported in Table 2 .

Flow diagram of patient cohort inclusion/exclusion criteria. COVID-19, coronavirus disease 2019; HEPMA, Hospital Electronic Prescribing and Medicines Administration; PCR, polymerase chain reaction
Patients aged ≥ 75 years accounted for 6.9% ( n = 34/492) of the sotrovimab-treated cohort, 21.0% ( n = 58/276) of the nirmatrelvir/ritonavir-treated cohort, 16.9% ( n = 12/71) of the molnupiravir-treated cohort, and 13.2% ( n = 225/1709) of untreated patients.
A high proportion of patients receiving an early COVID-19 treatment did not have an identifiable highest-risk condition in the database (71.7% for sotrovimab [ n = 353/492], 85.1% for nirmatrelvir/ritonavir [ n = 235/276], 85.9% for molnupiravir [ n = 61/71], and 0% for untreated patients [resulting from the cohort’s inclusion criteria]). A high percentage of untreated patients had solid cancer (29.3%, n = 501/1709) and IMID (56.4%, n = 964/1709), while comparatively lower percentages were reported for other highest-risk comorbidities among this cohort (Table 2 ). In the treated cohorts, most patients did not have data available on highest-risk comorbidities, and low proportions of such conditions were therefore reported. Among sotrovimab-treated patients, 9.1% ( n = 45/492) had solid cancer and 6.3% ( n = 31/492) had IMID. Solid cancer was the most frequently reported highest-risk condition for nirmatrelvir/ritonavir-treated patients (5.8%, n = 16/276), and each of the highest-risk comorbidities were reported in five or fewer molnupiravir-treated patients. Advanced renal disease was reported for 6.7% ( n = 33/492) of sotrovimab-treated patients and 4.7% ( n = 81/1709) of untreated patients. Five or fewer patients treated with nirmatrelvir/ritonavir ( n = ≤ 5/276) and molnupiravir ( n = ≤ 5/71) had advanced renal disease. Due to the high level of missing data, the proportions of patients with highest-risk comorbidities could not be compared between cohorts.
Acute period outcomes
In total, 8.5% ( n = 216/2548) of patients did not have sufficient observation time (45 days, including after death, from index to data extraction date) to assess acute period outcomes (sotrovimab: n = 16/492 [3.3%]; nirmatrelvir/ritonavir: n = 101/276 [36.6%]; molnupiravir: n = 12/71 [16.9%]; untreated: n = 87/1709 [5.1%]). Fewer than five patients with insufficient observation time died.
The percentage of patients who experienced an all-cause hospitalization was 5.3% ( n = 25/476) for sotrovimab-treated patients and 6.9% ( n = 12/175) for nirmatrelvir/ritonavir-treated patients (Table 3 ). Five or fewer molnupiravir-treated patients ( n = ≤ 5/59) experienced an all-cause hospitalization. For untreated patients, the percentage of all-cause hospitalizations was 13.3% ( n = 216/1622). The median (interquartile range) number of days in hospital due to any cause during the acute period was 5.0 (2–9) for sotrovimab-treated patients, 3.5 (2–9.5) for patients treated with nirmatrelvir/ritonavir, and 6.0 (3–14) for untreated patients. Median length of stay could not be reported for molnupiravir due to low patient numbers. Five or fewer patients in all cohorts required non-invasive ventilation or mechanical ventilation. Critical care admissions were experienced by five or fewer patients in each of the treated cohorts and 10 (0.6%) untreated patients.
Five or fewer patients treated with each of sotrovimab, nirmatrelvir/ritonavir, and molnupiravir experienced COVID-19-related hospitalizations during the acute period (Table 3 ). For untreated patients, the percentage of COVID-19-related hospitalizations was 3.0% ( n = 48/1622). Five or fewer patients in all cohorts required non-invasive ventilation or mechanical ventilation, or experienced critical care admission.
There were no deaths within 28 days of index for patients treated with sotrovimab, nirmatrelvir/ritonavir or molnupiravir. Mortality was 4.3% ( n = 70/1622) in untreated patients, of whom 13 patients (18.6%) had COVID-19 as the primary cause (Table 3 ).
COVID-19-related hospitalizations during BA.1, BA.2, and BA.5 predominance
During BA.1 predominance, 283 patients were treated with sotrovimab and 721 received no treatment (Table 4 ). Similar to the overall analysis, five or fewer sotrovimab-treated patients ( n = ≤ 5/283) experienced a COVID-19-related hospitalization. The percentage for untreated patients was 3.1% ( n = 22/721).
During BA.2 predominance, 144 patients were treated with sotrovimab and 625 received no treatment (Table 4 ). Five or fewer sotrovimab-treated patients ( n = ≤ 5/144) and 2.2% (n = 14/625) of untreated patients had COVID-19-related hospitalizations.
During BA.5 predominance, 49 patients were treated with sotrovimab and 276 received no treatment (Table 4 ). Five or fewer sotrovimab-treated patients (n = ≤ 5/49) and 4.3% ( n = 12/276) of untreated patients had COVID-19-related hospitalizations.
This study described the characteristics and severe clinical outcomes of non-hospitalized patients who received early COVID-19 treatment in Scotland from December 1, 2021–October 25, 2022, or those who were likely eligible but did not receive treatment. Our findings indicate that sotrovimab was used among patients who were slightly younger (6.9% were aged ≥ 75 years vs 21.0% for nirmatrelvir/ritonavir and 16.9% for molnupiravir). We also found that patients who received early COVID-19 treatment with sotrovimab or antivirals experienced low levels of 28-day hospitalization. Low levels of all-cause death were also observed.
The observed characteristics of sotrovimab-treated patients differed to those described in a similar real-world study conducted in another part of the UK [ 20 ]. In a retrospective cohort study of non-hospitalized patients who received early treatment for, or were diagnosed with, COVID-19 in Northwest London from December 1, 2021–May 31, 2022, Patel et al. reported descriptive results for patients treated with sotrovimab, nirmatrelvir/ritonavir, or molnupiravir. Sotrovimab was found to be used most often among older patients with multiple comorbidities that increased their risk of severe COVID-19, such as advanced renal disease [ 20 ]. In the present study, we found that sotrovimab-treated patients were younger than the other treated cohort. Of note, we observed a particularly high number of untreated patients with IMID (56.4%). This suggests that the effective eligibility criteria may have been narrower than the definitions used herein, with certain IMIDs associated with higher risk and real-world treatment allocation reflecting this. Alternatively, it may indicate genuine clinical differences between the treated and untreated cohorts.
We found that the proportion of all-cause hospitalizations was low for patients treated with sotrovimab and antivirals. These results are supported by other real-world studies. Patel et al. reported low all-cause hospitalizations among patients treated with sotrovimab, nirmatrelvir/ritonavir, and molnupiravir, with low hospitalization rates for sotrovimab being consistent among patients with advanced renal disease, those aged 18–64 years and ≥ 65 years, and across periods of Omicron BA.1, BA.2, and BA.5 predominance (through July 2022) [ 20 ]. Our results for the overall cohort are also similar to those of a recent retrospective cohort study of patients presumed to be treated with sotrovimab in England, where 4.6% of patients experienced an all-cause hospitalization [ 21 ].
A recent publication investigated outcomes among patients on kidney replacement therapy in Scotland and England using OpenSAFELY and UK Renal Registry data. Among this population, 1.1% ( n = 21/1852) of sotrovimab-treated patients experienced 28-day COVID-19-related hospitalization or death compared with 3.3% ( n = 17/515) of molnupiravir-treated patients [ 22 ]. In an analysis of sotrovimab vs molnupiravir in England (conducted from December 2021 to February 2022), 0.1% ( n = 32/3331) of sotrovimab-treated patients and 2.0% ( n = 55/2689) of molnupiravir-treated patients experienced 28-day COVID-19-related hospitalization or death [ 23 ]. In a US study conducted during Delta and early Omicron variant predominance, 2.67% ( n = 418/15,633) of sotrovimab-treated patients experienced 30-day all-cause hospitalization and 0.08% ( n = 13/15,633) died, compared with 5.57% ( n = 84,307/1,514,868) and 0.54% ( n = 8167/1,514,868), respectively, of patients who did not receive mAb treatment [ 24 ]. In a further study conducted from February to October 2022, very similar proportions of patients treated with sotrovimab and nirmatrelvir/ritonavir experienced 28-day COVID-19-related hospitalization or death [ 25 ]. In another recent study, the OpenSAFELY platform was used to emulate target trials to estimate the effectiveness of sotrovimab vs no treatment during BA.1 and BA.2 predominance. Estimated hazard ratios for 28-day COVID-19-related hospitalization or death were 0.76 (95% confidence interval [CI]: 0.66–0.89) during BA.1 and 0.92 (95% CI: 0.79–1.06) during BA.2 for sotrovimab vs no treatment [ 26 ].
We previously assessed the uptake of mAbs and antiviral therapies in Scotland, and discussed whether these treatments were used as recommended [ 27 ]. We reported that only around half of eligible patients received a mAb/antiviral for COVID-19, but the vast majority of patients who received treatment did so within the recommended timeframe. In this study, we also found that some eligible patients (based on COVID-19 diagnosis and high-risk comorbidities) were untreated; the reasons for this were not ascertained as part of this study but are of interest for future research.
We also found that five or fewer sotrovimab-treated patients experienced COVID-19-related hospitalizations across periods of BA.1, BA.2 and BA.5 predominance. Due to data suppression rules and small patient numbers (particularly with decreasing sotrovimab use during BA.5), interpretation of these results is challenging, particularly during BA.2 and BA.5 predominance. The risk of ecologic bias should be noted, as we did not have access to sequencing data to confirm each patient’s variant.
It should be noted that these descriptive results require confirmation with formal statistical testing adjusting for confounding factors. In the current study, formal comparison was not considered appropriate for several reasons. Due to the relatively small sample size for some groups, and low numbers of events observed (resulting in suppressed numbers), it was highly unlikely that there would be sufficient power to show statistical significance. More importantly, despite it being an inclusion criterion, for a high number of patients in the treatment groups (> 70%) their highest-risk conditions could not be identified in the database; this meant it was impossible to adequately adjust for potential confounders.
This study has several limitations. Firstly, we present descriptive analyses with no adjustment for differences in patient characteristics between cohorts, meaning that results may be subject to bias and confounding. Further, the limited sample size (particularly for molnupiravir) led to frequent suppression of small values. We did not collect data on COVID-19 severity and symptoms at disease onset, and therefore cannot confirm the cohorts were comparable in this regard. It is possible that the sotrovimab-treated cohort had mild-to-moderate COVID-19, while some untreated patients may have been asymptomatic, mildly symptomatic, or improving. In addition, 85% of the treated cohort had no identified RT-PCR or (registered) LFT in the 40 days prior to treatment, whereas the untreated cohort were required to have a positive test recorded. These “untested” treated patients were likely to have tested at home and not reported their results. There were limited sequencing data available for included patients, and dominance period for Omicron subvariant was used as a surrogate; hence, there is potential for misclassification of the infecting variant. Also, comorbidities were identified using inpatient admissions and procedures data; conditions treated in specialist departments (including maternity and renal wards) or in primary care could not, therefore, be determined, which may partially explain the level of missing data observed. Finally, there was an imbalance across study cohorts in the proportion of patients from each group who could not be included for analysis due to insufficient observation time (< 45 days); in particular, more than one-third of those in the nirmatrelvir/ritonavir group had an insufficient observation period, which may have impacted our findings. Patients with observation time < 45 days were excluded from the main analysis even if they died; this accounted for less than five patients, and their inclusion in a sensitivity analysis had no impact on study results (data not shown).
Our findings indicate that among patients who received early COVID-19 treatment with sotrovimab or antivirals in Scotland, low proportions experienced all-cause hospitalizations and death within 28 days of treatment. Sotrovimab was observed to be frequently utilized in patients aged below 75 years old in Scotland. Most treated patients had missing data for their high/highest-risk status and conditions, which reduced the feasibility of conducting a comparative effectiveness analysis to assess the impact of sotrovimab in preventing severe COVID-19 among this population.
Availability of data and materials
Data for this study (study ID: 2223–0033) are held by the National Services Scotland electronic Data Research and Innovation Service in the National Safe Haven. Restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data would be made available from a reasonable request to [email protected].
Abbreviations
Acquired immune deficiency syndrome
Confidence interval
COVID-19 Monoclonal antibody Efficacy Trial-Intent to Care Early
Coronavirus disease 2019
Hospital Electronic Prescribing and Medicines Administration
Human immunodeficiency virus
Immune-mediated inflammatory disease
Interquartile range
Lateral flow test
- Monoclonal antibody
National Health Service
Not reported
Polymerase chain reaction
Reverse transcriptase polymerase chain reaction
Severe acute respiratory syndrome coronavirus 2
Standard deviation
World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/europe/emergencies/situations/covid-19 . Accessed 17 Aug 2023.
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855.
Article CAS PubMed PubMed Central Google Scholar
Hippisley-Cox J, Khunti K, Sheikh A, Nguyen-Van-Tam JS, Coupland CAC. QCovid 4 - predicting risk of death or hospitalisation from COVID-19 in adults testing positive for SARS-CoV-2 infection during the Omicron wave in England. medRxiv. 2022; https://doi.org/10.1101/2022.08.13.22278733 .
Department of Health & Social Care. Defining the highest-risk clinical subgroups upon community infection with SARS-CoV-2 when considering the use of neutralising monoclonal antibodies (nMABs) and antiviral drugs: independent advisory group report. https://www.gov.uk/government/publications/higher-risk-patients-eligible-for-covid-19-treatments-independent-advisory-group-report/defining-the-highest-risk-clinical-subgroups-upon-community-infection-with-sars-cov-2-when-considering-the-use-of-neutralising-monoclonal-antibodies . Accessed 17 Aug 2023.
Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Rodrigues Falci D, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–46.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–408.
Article CAS PubMed Google Scholar
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20.
Article PubMed Google Scholar
Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15: e1002493.
Article PubMed PubMed Central Google Scholar
Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–5.
Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–5.
Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid M, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2022; https://doi.org/10.1101/2021.03.09.434607 .
Medicines & Healthcare products Regulatory Agency. Summary of product characteristics for Xevudy. https://www.gov.uk/government/publications/regulatory-approval-of-xevudy-sotrovimab/summary-of-product-characteristics-for-xevudy . Accessed 17 Aug 2023.
Hung YP, Lee JC, Chiu CW, Lee CC, Tsai PJ, Hsu IL, et al. Oral nirmatrelvir/ritonavir therapy for COVID-19: the dawn in the dark? Antibiotics (Basel). 2022;11:220.
Medicines & Healthcare products Regulatory Agency. Summary of product characteristics for Paxlovid. https://www.gov.uk/government/publications/regulatory-approval-of-paxlovid/summary-of-product-characteristics-for-paxlovid . Accessed 17 Aug 2023.
Medicines & Healthcare products Regulatory Agency. Last updated 19/10/22 - summary of product characteristics for Lagevrio. https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir/summary-of-product-characteristics-for-lagevrio . Accessed 17 Aug 2023.
Department of Health & Social Care. Interim clinical commissioning policy: antivirals or neutralising monoclonal antibodies for non-hospitalised patients with COVID-19 (Version 6). https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=104044 . Accessed 17 Aug 2023.
National Health Service. Who can and cannot take Paxlovid. https://www.nhs.uk/medicines/paxlovid/who-can-and-cannot-take-paxlovid/#:~:text=severe%20liver%20disease,have%20a%20weakened%20immune%20system . Accessed 17 Aug 2023.
Scottish Medicines Consortium. Sotrovimab (Xevudy). https://www.scottishmedicines.org.uk/medicines-advice/sotrovimab-xevudy-full-smc2555/ . Accessed 17 Aug 2023.
UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 45. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1115071/Technical-Briefing-45-9September2022.pdf . Accessed 17 Aug 2023.
Patel V, Yarwood MJ, Levick B, Gibbons DC, Drysdale M, Kerr W, et al. Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England. medRxiv. 2022; https://doi.org/10.1101/2022.11.28.22282808 .
Patel V, Levick B, Boult S, Gibbons DC, Drysdale M, Lloyd EJ, et al. Characteristics and outcomes of COVID-19 patients presumed to be treated with sotrovimab in NHS hospitals in England. medRxiv. 2023; https://doi.org/10.1101/2023.02.08.23285654 .
The OpenSAFELY Collaborative, Zheng B, Campbell J, Carr EJ, Tazare J, Nab L, et al. Comparative effectiveness of sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients on kidney replacement therapy: observational cohort study using the OpenSAFELY-UKRR linked platform and SRR database. medRxiv. 2022; https://doi.org/10.1101/2022.12.02.22283049 .
Zheng B, Green ACA, Tazare J, Curtis HJ, Fisher L, Nab L, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ. 2022;379: e071932.
Cheng MM, Reyes C, Satram S, Birch H, Gibbons DC, Drysdale M, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States. medRxiv. 2022; https://doi.org/10.1101/2022.09.07.22279497 .
Zheng B, Tazare J, Nab L, Mehrkar A, MacKenna B, Goldacre B, et al. Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform. medRxiv. 2023; https://doi.org/10.1101/2023.01.20.23284849 .
The OpenSAFELY collaborative, Tazare J, Nab L, Zheng B, Hulme WJ, Green ACA, et al. Effectiveness of sotrovimab and molnupiravir in community settings in England across the Omicron BA.1 and BA.2 sublineages: emulated target trials using the OpenSAFELY platform. medRxiv. 2023; https://doi.org/10.1101/2023.05.12.23289914 .
Tibble H, Mueller T, Proud E, Hall E, Kurdi A, Robertson C, et al. Uptake of monoclonal antibodies and antiviral therapies for COVID-19 in Scotland. Lancet. 2023;401:101–2.
Download references
Acknowledgements
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables, grammatical editing and referencing) was provided by Kathryn Wardle of Apollo, OPEN Health Communications, in accordance with Good Publication Practice (GPP) guidelines ( www.ismpp.org/gpp-2022 ), and was funded by GSK and Vir Biotechnology, Inc.
This study was funded by GSK in collaboration with Vir Biotechnology, Inc (study number: 219082).
Author information
Authors and affiliations.
Value Evidence and Outcomes, GSK House, 980 Great West Road, Brentford, Middlesex, TW89GS, UK
Myriam Drysdale, Vishal Patel, Daniel C. Gibbons, Emily J. Lloyd & Helen J. Birch
Usher Institute, University of Edinburgh, Scotland, UK
Holly Tibble, Calum Macdonald & Aziz Sheikh
Global Medical Affairs, GSK, Brentford, UK
William Kerr
You can also search for this author in PubMed Google Scholar
Contributions
MD, HT, VP, EJL, WK, HJB, and AS were involved in the study design, conception, and execution. HT, DCG, and CM took part in the acquisition and analysis of data. All authors were involved in data interpretation; drafting, revising, or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript.
Corresponding author
Correspondence to Myriam Drysdale .
Ethics declarations
Ethics approval and consent to participate.
This study received data access and processing approval from the Public Benefit and Privacy Panel for Health and Social Care, NHS Scotland on November 9, 2022. We complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred as part of this study; therefore, informed consent, ethics committee, or IRB approval were not required, as determined by the NHS Health Research Authority Research Ethics Committee decision tool ( https://www.hra-decisiontools.org.uk/ethics/ ). Study results were reported in tabular form and aggregate analyses that omitted subject identification. Publications, reports or any other research outputs do not include subject identifiers and low patient numbers were suppressed.
Consent for publication
Not applicable.
Competing interests
MD, DCG, EJL, WK, and HJB and are employees of, and/or shareholders in, GSK. VP was an employee of GSK at the time of the study and is now an employee of KVM Analytics. HT, CM, and AS are employees of the University of Edinburgh, which received funding from GSK and Vir Biotechnology, Inc. to conduct the study. AS has also served on a number of Scottish Government and UK Government COVID-19 advisory groups, all of which have been unremunerated.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vishal Patel at the time of study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Drysdale, M., Tibble, H., Patel, V. et al. Characteristics and outcomes of patients with COVID-19 at high risk of disease progression receiving sotrovimab, oral antivirals, or no treatment: a retrospective cohort study. BMC Infect Dis 24 , 670 (2024). https://doi.org/10.1186/s12879-024-09576-7
Download citation
Received : 23 August 2023
Accepted : 27 June 2024
Published : 04 July 2024
DOI : https://doi.org/10.1186/s12879-024-09576-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Molnupiravir
- Nirmatrelvir/ritonavir
- Omicron BA.1
- Omicron BA.2
- Omicron BA.5
- Oral antivirals
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
- Open access
- Published: 25 January 2023
Limitations of COVID-19 testing and case data for evidence-informed health policy and practice
- Elizabeth Alvarez ORCID: orcid.org/0000-0003-2333-0144 1 ,
- Iwona A. Bielska 1 ,
- Stephanie Hopkins 1 ,
- Ahmed A. Belal 1 ,
- Donna M. Goldstein 2 ,
- Jean Slick 3 ,
- Sureka Pavalagantharajah 4 ,
- Anna Wynfield 2 ,
- Shruthi Dakey 5 ,
- Marie-Carmel Gedeon 6 ,
- Edris Alam 7 &
- Katrina Bouzanis 8
Health Research Policy and Systems volume 21 , Article number: 11 ( 2023 ) Cite this article
8852 Accesses
6 Altmetric
Metrics details
Coronavirus disease 2019 (COVID-19) became a pandemic within a matter of months. Analysing the first year of the pandemic, data and surveillance gaps have subsequently surfaced. Yet, policy decisions and public trust in their country’s strategies in combating COVID-19 rely on case numbers, death numbers and other unfamiliar metrics. There are many limitations on COVID-19 case counts internationally, which make cross-country comparisons of raw data and policy responses difficult.
Purpose and conclusions
This paper presents and describes steps in the testing and reporting process, with examples from a number of countries of barriers encountered in each step, all of which create an undercount of COVID-19 cases. This work raises factors to consider in COVID-19 data and provides recommendations to inform the current situation with COVID-19 as well as issues to be aware of in future pandemics.
Peer Review reports
Since the emergence of coronavirus disease 2019 (COVID-19) in Wuhan, China, the world has faced serious data issues, ranging from a lack of transparency on the emergence, spread and nature of the virus to an absence of grounded comparative analyses, with temporal differences considered, about emerging social and economic challenges [ 1 , 2 ]. Most critically, scientists have lacked data to conduct analyses on non-pharmaceutical interventions (NPIs), including policies and strategies that governments have engaged to mitigate the situation, and how these have varied across regions, presumably affecting both short- and long-term outcomes [ 1 , 2 ].
Out of all the strategies implemented to date, physical distancing policies have emerged as one of the more effective NPIs to battle COVID-19 [ 3 , 4 ]. While physical distancing policies have been the mainstay in the battle against COVID-19, there has been a call to understand which forms of physical distancing policies are effective so that targeted and less disruptive measures can be taken in further waves of this pandemic and future pandemics [ 1 , 2 , 5 , 6 ]. The best time to institute physical distancing policies and what happens when and how they are eased remain unclear. There are many aspects of distancing, such as recommendations for maintaining a physical distance in public, banning group gatherings (the maximum number and where they take place), or complete lockdowns, that complicate their assessment. Timing and synergies of policies and sociodemographic and political factors play a role in the effectiveness of these policies [ 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. Some hypothesized sociodemographic factors for increased exposure and severity of COVID-19 include living in a long-term care facility or being institutionalized, age (older), gender (mixed findings), having comorbidities (including high blood pressure, diabetes, obesity, immunocompromised status, tobacco smoking) and social vulnerabilities including race or ethnicity. Also relevant is the carrying capacity and infrastructure of health systems. These factors pose challenges for comparison among countries. Comparison is a prime requisite for evaluating the effectiveness of implementation of various policies between countries. Policymakers and the public have been using metrics such as number of cases, number of deaths and testing capacity to make policy or programme decisions or to decide whether to trust the actions of their governments, respectively.
An international team of researchers has been collecting data on physical distancing policies and contextual factors, such as health and political systems and demographics, to expedite knowledge translation (which means applying high-quality research evidence to processes of decision making) on the effect of policies and their influence on the epidemiology of COVID-19 [ 14 , 15 , 16 ]. Through this work, we identified gaps in the accuracy of reported numbers of COVID-19 cases and deaths, which make cross-country comparisons of the raw data, indexes using the raw data, and policy outcomes challenging [ 7 , 17 ]. While the work of this team is ongoing, this paper limits the findings from the inception of the pandemic to the end of 2020. It is important to understand the limitations of available COVID-19 data in order to properly inform decision making, especially at the outset as a novel infectious disease. This paper focuses on the testing and reporting cycle (Fig. 1 ) and provides examples from a number of countries of possible barriers leading to inaccurate data on reported COVID-19 cases. It also describes other cross-cutting implications of COVID-19 data for policy, practice and research, including reported deaths, missing information, implementation of policy, and unpredictable population behaviour. Furthermore, it calls into question analyses performed to date, which do not account for a number of known data gaps.

COVID-19 testing and reporting cycle. *The icons in this figure are in the public domain (Creative Commons CC0 1.0 Universal Public Domain) and were obtained from Wikimedia Commons at: https://commons.wikimedia.org/wiki/File:Medical_Library_-_The_Noun_Project.svg ; https://commons.wikimedia.org/wiki/File:Home_(85251)_-_The_Noun_Project.svg ; https://commons.wikimedia.org/wiki/File:Laboratory_-_The_Noun_Project.svg ; https://commons.wikimedia.org/wiki/File:Noun_project_1063.svg ; https://commons.wikimedia.org/wiki/File:Analysis_-_The_Noun_Project.svg
It is important to note that Fig. 1 only represents the testing and reporting cycle, which leads to counting of cases, and it does not include COVID-19 contact tracing and case management; however, we recognize that testing, contact tracing and case management are intricately linked to each other in the spread of COVID-19 [ 18 , 19 ]. As ‘Our World in Data’ states, “Without testing there is no data.” [ 20 ]. Understanding the links between testing, data and action underlies country responses to the pandemic. Ultimately, this work serves to provide a basis to improve pandemic planning, surveillance and reporting systems, and communications.
In Fig. 1 , the first level of testing is at the healthcare recipient level (Sect. “ Healthcare recipient level ”), followed by sample collection and processing (Sect. “ Sample collection and processing ”) and surveillance and reporting (Sect. “ Surveillance and reporting ”). Each level will be further explained below and examples provided as to potential or actual barriers at each level. These descriptions are not exhaustive, and nuanced understanding of the context will be needed to evaluate these steps and potential barriers in different settings.
Healthcare recipient level
Testing starts with individuals getting tested. There may be times when it is predetermined who gets tested and when, such as health workers getting tested prior to starting work in a long-term care facility or travellers returning from overseas [ 21 , 22 ]. However, most individuals are tested in the community, where a number of steps predicate individuals’ decisions to seek out testing. First, case definition, testing criteria and referral for testing influence our understanding of what the disease entity is and whether people are encouraged or discouraged to get tested. Given the novel status of COVID-19, there were challenges at the onset of this pandemic in establishing a working case definition. In China, arguably the leader in COVID-19 knowledge at the time, the case definition for reporting changed over time and between places [ 23 ]. These definitions were not always consistent with one another. Between 22 January and 12 February 2020, China’s National Health Commission had revised the COVID-19 outbreak response guidelines at least six times, resulting in significant differences in the daily counts due to changes over time in the definition of a case [ 24 ]. Adding to the uncertainty, the World Health Organization did not publish case definition guidelines until 16 April 2020, long after many countries had created their own working case definitions [ 25 ]. Although changes in methodology are expected as we learn more about the disease and as new variants emerge, these changes have implications for case counts [ 23 , 25 ]. Yet, communication does need to be flexible during a crisis. For example, little was known about asymptomatic COVID-19 spread at the beginning of the pandemic. As more evidence was garnered on this topic, information about precautions and testing criteria needed to be flexible to keep up with what was known [ 26 ].
Not only have case definitions changed over time, criteria for testing have changed over time and across jurisdictions on the basis of a number of factors, such as better understanding of the disease process, availability and capacity for testing, and national and local strategies for addressing the pandemic [ 27 ]. In some places, testing criteria were narrow, which discouraged people from getting tested because they did not fit the criteria. In its early response, Canada only tested symptomatic people returning from specific countries known to have high numbers of cases of COVID-19 [ 18 ]. Given that there were no treatments and media reported that the hospitals were overwhelmed, people were also discouraged from seeking medical attention unless they warranted hospitalization. If people were feeling unwell, but not needing to be on a ventilator, testing might not have been deemed necessary. Shifting testing criteria and differences in referral channels for testing, such as going through public health or needing a physician referral versus self-referrals, could create additional barriers.
Depending on the testing strategy, whether based on specific criteria or population-based, will make a difference for number of COVID-19 cases identified. Changes in criteria for testing sometimes led to increased demand without a corresponding increase in the availability of testing resources, which then led to delays in accessing tests [ 28 ]. Additionally, as different sectors, such as schools, resumed in-person activity, there was an increased demand for testing within certain population groups. Again, testing capacity could not always keep up with demand, leading, in some instances, to further limitations of who could be tested to prioritize resources for testing [ 27 ].
In the case that an individual has a choice to get tested, once a person is determined to be eligible for testing, that person has to decide whether or not to get tested, following a decisional process for getting tested, which can be affected by factors such as the availability of education and decision supports, health literacy, health status, trade-offs between knowing their results and potential economic and social consequences, health system complexity, and personal costs, such as time and out-of-pocket expenses [ 29 , 30 ]. Availability of education and decision support is needed for people to understand that there is a pandemic, what that means, how it might impact them, how and where to get tested (if available) and why getting tested is important for them or their loved ones. This relies on accurate and timely information, which is discussed in more detail in Sect. “ Governance and knowledge translation ”.
Furthermore, health literacy can involve a general understanding of factors that affect health or it can be specific to a disease entity, such as the virus that causes COVID-19. Health status can decrease the number of people seeking testing if they have mild symptoms and decide it is not worthwhile to seek testing or care, or they may not fit the testing criteria. On the other hand, some people with severe symptoms may not have the physical resources to go to a testing centre.
Of course, even individual-level factors are affected by broader systematic determinants of health. As the gravity of the pandemic took hold, jurisdictions began implementing more robust isolation policies to prevent the spread of COVID-19. These policies included self-isolation or a quarantine period for those who tested positive or who had come in contact with a known case. In many countries, governments provided economic relief to support people who were unable to work [ 31 , 32 ]. However, in countries such as Brazil and Mexico there were limited social safety nets, and in many other countries such as the USA, COVID-19 exposed gaps in these nets [ 33 , 34 ]. This created an economic barrier for people to access testing, as a positive test would force them to stay home without adequate financial means to survive. On 28 April 2020, the French Prime Minister, Edouard Philippe, urged the population of France to “protect-test-isolate”; meanwhile, containment measures generated a “disaffiliation process” among migrants and asylum seekers. Absence of work, isolation from French society, and fear of being checked by the police brought individuals into a “disaffiliation zone” marked by social non-existence, in a context of global health crisis [ 35 ].
Health systems themselves created a barrier to testing through their slow response to testing requests, causing some individuals to abandon testing [ 36 ]. In some countries, testing was expensive and not offered in the poorest communities [ 37 ]. For those travelling, mandatory testing, with varying requirements between different countries and potential out-of-pocket costs, increased the complexity of getting tested. Furthermore, competing crises may have lowered the number of people seeking testing due to other, more immediate, priorities, such as floods or wildfires [ 38 , 39 , 40 , 41 ].
Sample collection and processing
Once a person decides to seek testing, tests must be available and accessible and there must also be sufficient test processing centres. While these factors are often lumped together, it is important to distinguish these two steps in the testing cycle as they often require different structural and/or operational components.
Tests and testing sites
For an individual to get tested, there must be availability of testing sites and accessibility to these testing sites. Testing sites may include already available clinic, hospital or community sites, or assessment centres which are created for the purpose of testing. Having separate assessment centres can ease conflicting burdens on already overwhelmed health systems, and they can allow for efficiency in the process of testing and in keeping potentially infectious individuals separate from those who are seen for other ailments. Not only do testing sites have to be available, they have to be accessible. Times of operation, parking and other accessibility considerations are important. Testing sites can be centralized in one or several locations, where people have to find transportation to the sites, or can be mobile sites, which can increase access to those in rural/remote areas or those with mobility or transportation issues. Drive-thru testing has been showcased in countries such as South Korea [ 42 ]. However, limitations also exist with drive-thru sites for those who do not own a vehicle, or those who have to drive long distances or endure long wait times [ 43 ]. In areas with poor health system infrastructure, lack of access can exacerbate inequities in testing.
Operational components include the need for adequate human resources and testing supplies. In Ontario, Canada, assessment centres were slow to set up and there was a lack of swabs and other testing supplies [ 18 ]. In France, laboratories struggled to keep up with testing demands due to delays in receiving chemicals and testing kits produced abroad, given France’s reliance on global supply chains [ 44 ]. Bangladesh had a very limited number of case testing capacity in the beginning of the outbreak. The country conducted fewer than 3000 tests in the first four weeks of the outbreak between 8 March and 5 April 2020 for its 164 million population as well as 155,898 overseas passengers, some arriving from hard-hit countries such as Italy, allowing for community transmission [ 45 ].
The method of specimen collection and specimen management for processing are also important considerations. Specimen collection has varied between contexts and over time [ 46 ]. Nasopharyngeal, nasal and throat swabs have been used in community settings. Saliva tests and blood samples, mainly for hospitalized patients, are other methods of obtaining specimens. Each of these testing modalities has different properties, but none is 100% sensitive or able to pick up all positive cases of COVID-19. There are reports of very ill patients testing negative on multiple occasions on nasopharyngeal samples but subsequently testing positive from lung samples [ 47 , 48 ]. Specimen management requires the proper labelling, storage and transportation of samples from the testing site to the laboratory for processing.
Laboratories
Laboratory preparedness and laboratory capacity played crucial roles in COVID-19 testing globally [ 27 , 49 ]. Issues with this preparedness and capacity, along with lack of testing supplies, resulted in “lack of testing” as a prime factor for not having accurate numbers of COVID-19 cases, especially at the beginning of the pandemic. Laboratory capacity includes human resources and specimen processing supplies, often called the testing kits, which require specific reagents and equipment. Over time, countries with low laboratory preparedness focused on improving their testing capacities [ 49 ]. Since the start of the pandemic, Germany was touted as testing widely and therefore having a robust ability to contact trace in order to find people who may transmit the virus causing COVID-19. However, other countries struggled to get testing in place. In the USA, initial tests developed were invalid, which delayed the ability to distribute and complete tests [ 50 ]. This was further exacerbated by bureaucratic/institutional red tape which centralized testing to the Centers for Disease Control and Prevention (CDC) and prohibited local public health and commercial laboratories from developing or administering more effective tests [ 51 ]. Supply chain management issues for swabs, transport media and reagents slowed down early testing in multiple countries [ 27 ].
Once testing methods have been established, there are a number of tests available for COVID-19 [ 52 ]. Test properties include the sensitivity and specificity of a test, among others, and these can vary by test. Therefore, the type of test used can also influence case counts. Recent studies have highlighted the need to validate laboratory tests and share the results during a pandemic. Evidence from a study in Alberta, Canada suggested that variations in test sensitivity for the virus causing COVID-19, particularly earlier in a pandemic, can result in “an undercounting of cases by nearly a factor of two” (p. 398) [ 53 ]. With rapid tests and home-approved testing kits available during the course of the pandemic, testing properties can vary even more greatly [ 52 , 54 , 55 ].
Surveillance and reporting
Once individuals have been tested and the results are processed, surveillance and reporting systems must be in place to communicate that information back to individuals, public health officials or others involved in case management or treatment, and to politicians and other stakeholders to act on this information and prevent further spread.
Data systems
Data management refers to the inputting and tracking of data. However, because of the need to quickly and accurately inform the public and decision makers in the time of a crisis, coordination of information technology is needed to align all the various data management systems within a jurisdiction and internationally. For example, each hospital system, clinic or laboratory may have separate electronic medical record or data management systems. Not many countries maintain a common database system for COVID-19-related management (testing, response, etc.). Even if database management systems are in place, lack of trained professionals, serious lags in updating data, challenges with interdepartmental coordination among various task force members, and new innovations such as artificial intelligence, health tracking apps, telemedicine and big data, which are suddenly in place, can lead to disrupted transparency. An exception is China, which developed a highly responsive national notifiable disease reporting system (NNDRS) in the aftermath of severe acute respiratory syndrome (SARS) [ 56 , 57 ]. The United Nations Department of Economic and Social Affairs statistics division launched a common website for improving the data capacities of countries [ 58 ]. This information has to be further coordinated to create larger and more robust surveillance and notification systems. Robust surveillance systems help decision makers know what is happening locally or how a disease is moving through populations. Notification systems are needed for sharing information between the testing site, laboratory and public health or local health agencies for case management and contact tracing and for letting people know their test results in a timely manner to help prevent further spread. The COVID Tracking Project has highlighted many discrepancies in USA reporting and surveillance, demonstrating unreliability of the data [ 59 ]. For example, hospitals were required to change how COVID-19 data were relayed to the federal government, and the switch from reporting through the CDC to the Health and Human Services (HHS) system resulted in misreporting of data and administrative lags across several states. Countries’ national-level CDCs collect information from state and local sources. The time lag can hence be one of the reasons for misleading the overall comprehensive pandemic impact. Lastly, with rapid, point-of-care and home tests available, keeping track of positive cases may be even more difficult, and COVID-19 case counts could be even further artificially decreased [ 60 ]. These tests could make contact tracing even more difficult if there is a lack of disclosure from the user end. It is important to note that, while there are many available sites for international COVID-19 data comparisons, including John’s Hopkins COVID-19 Dashboard [ 61 ], Worldometer [ 62 ], Our World in Data [ 20 ] and the World Health Organization (WHO) COVID-19 Dashboard [ 63 ], these all rely on locally-acquired data for their reporting, and therefore fall into and potentially augment the same fallacies discussed in this paper.
Governance and knowledge translation
Even with robust surveillance and notification systems, transparency and accountability are important for informing decision makers and the public. Decision makers need to know what the health and laboratory systems are finding so that evidence-informed policy and practice decisions can be made for the public good. At the same time, trust in government and government responses rely in part on perceived transparency of government by the public [ 64 , 65 ]. Accountability spans all through the spectrum discussed in the testing and reporting cycle, in a whole-of-society approach. Individuals are accountable for knowing when to get tested, getting tested and following public health guidelines and other policies. The public health and healthcare systems are accountable for planning testing and sharing information. Decision makers are accountable for transparency in sharing information, communicating appropriately with the public and relevant stakeholders, and making decisions for those they represent. In parts of Russia, there were two separate reports for those who died from COVID-19 and those who were positive but died from other causes [ 25 ]. In Florida, state officials instructed medical examiners to remove causes of death in their lists [ 66 ]. In China, despite having a highly responsive national data surveillance and reporting system, at the beginning of the pandemic, cases were only reported to the system once they had been approved by local members of government who only allowed cases with a direct connection to the original source of the outbreak, the seafood market, to be recorded [ 67 ].
Political will has been shown to be a barrier or facilitator in the fight against COVID-19. Examples of good leadership and political will can be found in places like New Zealand, where decisions were made early on, implemented, supported and continued to be informed by emerging evidence, or as described, following “science and empathy”[ 68 ]. Poor leadership has also come through clearly during this pandemic. Tanzania, Iran, the USA, Brazil and Egypt are only a handful of countries demonstrating the impact of political will on the course of the pandemic, in some cases resulting from subversion and corruption. Communication in these countries was often not transparent or mixed, and accountability for the lack of decision making or poor decision making was limited or non-existent in the pandemic’s outset. Tanzania stopped reporting cases due to political optics [ 30 , 69 ]. Iran’s Health Ministry reported 14,405 deaths due to COVID-19 through July 2020, which was a significant discrepancy from the 42,000 deaths recorded through government records [ 70 ]. The number of cases was also almost double those reported, 451,024 as compared with 278,827. One main reason for releasing underestimated information about the cases was considered to be upcoming parliamentary elections [ 70 , 71 ]. The former president of the USA, Donald Trump, often flouted public health and healthcare expert advice [ 72 ]. The Washington Post reported that Brazil was testing 12 times fewer people than Iran and 32 times fewer people than the USA, and hospitalized patients and some healthcare professionals were not tested in an effort to lower the case numbers [ 73 ]. Hiding numbers of deaths from COVID-19, whether intentionally or inadvertently, shored up far-right supporters of Brazil’s President Bolsonaro at a time when he was facing possible charges of impeachment for corruption and helped bolster the President’s messaging that the pandemic was under control. This further enabled a large swath of the population to call for less strict rules around COVID-19 and a quick reopening of the economy. Similarly, in July 2020, it was reported that at least eight doctors and six journalists had been arrested because they criticized the Egyptian government’s response to the pandemic [ 74 ].
Lastly, communication and information dissemination link to every piece of this process. Why, when and how people seek testing, how and where to set up testing sites, supply chain management, setting up and managing data systems, and policy decision making all work in a cycle. Good communication between systems and dissemination of information to the public and relevant stakeholders is imperative during a crisis, such as the COVID-19 pandemic. The amount of information available and rapid change in information creates an infodemic problem. ‘Infodemic’ is a term used by the WHO in the context of COVID-19 and refers to informational problems, such as misinformation and fake news, that accompany the pandemic [ 75 ]. Addressing the infodemic issue was highlighted as one of the prominent factors needed to improve future global mitigation efforts [ 76 ]. A report published in the second week of April 2020 by the Reuters Institute for the Study of Journalism at the University of Oxford found that roughly one-third of social media users across the USA, as well as Argentina, Germany, South Korea, Spain and the UK, reported seeing false or misleading information about COVID-19 [ 77 ]. The presidents of Brazil and the USA were themselves sources of misinformation, as they were seen in public without masks and touting the benefits of hydroxychloroquine after it was largely known that harms outweighed benefits of its use [ 72 , 78 , 79 ]. Having clear public health communications, from trusted sources, and breaking down silos between systems could be helpful in combating ever-changing information during a pandemic.
Other implications of COVID-19 data for policy, practice and research
There are several cross-cutting issues separate, but related, to the testing and reporting cycle which arose during this work. These issues also affect COVID-19 case counts and optimal timing of policies: how deaths are reported, missing information, implementation of policies, and unpredictable population behaviour.
Reported deaths
Deaths from COVID-19 tend to occur weeks after infection; therefore, assessments of policy changes using death counts need to account for this timing. However, reported death counts from COVID-19 carry many similar limitations given lack of testing for those who are deceased, attributing cause of death to COVID-19-related complications, processes for declaring deaths and causes of deaths, and lack of transparency [ 80 ]. In Brazil, hospitalized patients were not being tested, and deaths were attributed to respiratory ailments [ 73 ]. Further, COVID-19 deaths from the City of Rio de Janeiro’s dashboard were blacked out for 4 days in May (22–26 May 2020) [ 81 ]. When the dashboard was restarted, the death count was artificially lowered by changing the cause of death from COVID-19 to its comorbidities. Additional changes included requiring a confirmed COVID-19 test at the time of death in order for the death certificate to list COVID-19 as the cause; however, the results of the test often came after the death certificates were issued [ 81 ]. In Italy, the reverse occurred where only those in hospital were counted as COVID-19 deaths, while many people died at home or in care homes without being tested [ 82 , 83 ]. In Ireland, early discrepancies in reported deaths were noted between official government figures and an increase in deaths noted on the website Rip.ie, which has served as a public forum disclosing deaths and wake information in line with Irish funeral traditions. Information from this forum was used to re-assess mortality and in some cases aid epidemiological modelling [ 84 ].
Missing information
Given the lack of access to treatments at the beginning of the pandemic, understanding who was at highest risk of obtaining or dying from COVID-19 was important to know in order to develop appropriate policies that balanced health with social and economic impacts of the pandemic. Early data showed a sex and age gradient for COVID-19 cases and deaths. However, not all countries report data by sex and/or age. Race/ethnicity and sociodemographic findings were not collected or reported early in the pandemic [ 85 ]. France has been criticized for laws which prohibit the collection of race and ethnicity data, since they lack data which demonstrate whether certain groups are overrepresented in COVID-19 cases and deaths [ 86 , 87 ]. Another aspect of missing data early in the pandemic was that of asymptomatic spread. Due to limited testing early in the pandemic, asymptomatic cases were not picked up. Population-based studies are being conducted to better understand the role of asymptomatic and pre-symptomatic spread of COVID-19 in different population groups, such as children [ 26 ].
Implementation of policy
Population-level strategies since the start of the pandemic and reported findings in the literature go hand and hand. Cause and effect are difficult to attribute. For example, early literature looking at the role of children on the spread of COVID-19 found that children played a small role. This was to be expected given that many schools around the world closed, and children would not be exposed through transportation and workplaces as adults would be. Therefore, family spread would naturally flow from adults to children given these circumstances. In addition, many places were not testing mild to asymptomatic cases, which were more commonly found in children. Publications early on related to the few severe COVID-19 cases in children or to school-related cases in places that had low community transmission rates of COVID-19 and were following public health guidelines [ 88 ]. Limitations of these data have been described, yet findings have been used to justify specific policies in places that were dissimilar, with expected results ensuing, such as an increase in community transmission and school closures due to COVID-19 infections [ 89 ]. Therefore, it is even more important to understand the context of policies before applying them to various jurisdictions.
Unpredictable population behaviour
There is a difference between stated policy, implementation and enforcement. To understand which policies worked to combat COVID-19, it is important to consider the level of compliance with stated policies. Some people may follow recommended approaches for protective actions while others may not comply and see these recommendations as problematic [ 90 ]. For example, people may change their behaviours in anticipation of an announced change; for example, individuals may start working from home even before it is enforced or if it is never officially mandated, or people may go on a shopping spree prior to known closures [ 91 , 92 ]. Of course, people’s behaviours may also be dependent on a disconnect between policy messages at different levels of government and exacerbated by rapid updates in a fast-moving pandemic of unknown properties and the associated information overload. Therefore, communication management and clarity are of utmost importance during a crisis.
Discussion and recommendations
The need for cross-country comparison is necessary for understanding the effectiveness of policies in various countries. Policy decisions are being made and judged on the basis of case numbers, deaths and testing, among others. Understanding the steps and barriers in testing and reporting data related to COVID-19 case numbers can help address the limitations of data to strengthen these systems for future pandemics and can also help in the interpretation of findings across jurisdictions. Robust and timely public health measures are needed to decrease the health, social and economic ramifications of the pandemic. Even with available vaccines, it will still take time to have sufficient population coverage internationally.
There are a few assumptions considered in this paper. First, we assume that the reported numbers for each country are not inflated. There could be some cases that are counted more than once if repeated tests are taken and the person continues to test positive. Most data do not disclose how often this occurs, but it is likely not a significant issue for population reports, at least at from the beginning of the pandemic [ 20 ]. Next, ideally COVID-19 case counts are accurate. This is the assumption that is made by policymakers and the public in judging their decisions and their outcomes. We argue that the reported COVID-19 data are likely an undercount of actual cases. The reasons are highlighted in this paper.
Future global discussions will continue around who is most affected by COVID-19 and how to best prepare for pandemics, among others. COVID-19 case and death counts will be used in determining successful approaches. It is important to understand the context of COVID-19 data in these discussions, especially with respect to other global indicators that may look to COVID-19 data, such as the Sustainable Development Goals (SDGs) through improvement of early warning, risk reduction and management of national and global health risks [ 93 ]. Specifically, SDG 3 (good health and wellbeing) with an emphasis on highlighting the lacunas in informed data tying policy and epidemiology, SDG 10 to reduce inequalities within and among countries, and SDG 16 (peace, justice and strong institutions) with a goal to build effective and accountable institutions at all levels. This research also contributes to the Sendai Framework for Disaster Risk Reduction, specifically priority 2, strengthening disaster risk governance to manage disaster risk, and priority 3, investing in disaster risk reduction for resilience [ 94 ]. Unfortunately, there is little published on good governance in reporting systems during COVID-19, and our findings in this area are limited to media and news sources. Future research could focus on this critical aspect.
Decision makers could consider the following overarching recommendations, contextualized to their individual jurisdictions (i.e. regional, country, province, territory, state), to evaluate the testing and reporting cycle and improve accuracy and comparability of COVID-19 data:
Understand barriers to accurate testing and reporting —This paper lays out the steps in the testing and reporting process and components of these steps. Barriers are described at each of these steps, and examples are provided.
Address barriers to testing and reporting —Understanding barriers in the testing and reporting process can uncover facilitators. Each setting will deal with different barriers. Ultimately, political will, capacity building and robust information systems will be needed to address any of these barriers.
Transparency and accountability for surveillance and reporting —Any attempt to assign causality to these policies must take into account the timing and quality of surveillance data. Data quality issues, such as completeness, accuracy, timeliness, reliability, relevance and consistency, are important for surveillance and reporting [ 95 , 96 ].
Invest in health system strengthening, including surveillance and all-hazards emergency response plans —COVID-19, as this and past pandemics have shown, is not just a health issue, and instead requires community, health systems, social systems and policy approaches to mitigate its effects. Preparing for infectious disease outbreaks and other crises needs to incorporate all-hazards emergency response plans in order to have all the necessary resources in place at the time of the events.
Identify promising communication strategies —Research is needed to understand how messages conveyed at all stages of a pandemic are received and understood at the micro-level and used by the public [ 97 ]. Development of communication strategies aimed at promoting good understanding of information may defer inappropriate behaviours.
Invest in research to further understand data reporting systems and policy strategies and implementation . Research could compare global COVID-19 data reporting platforms mentioned in this article to see from where they obtained their raw data to further understand data reporting accuracy and comparability of data over time and whether any limitations of data were noted. Further research could address what policy and implementation strategies worked in a variety of settings to strengthen future recommendations for emerging pandemics.
The use and effectiveness of government responses, specifically pertaining to physical distancing policies in the COVID-19 pandemic, has been evolving constantly. Testing is a measure of response performance and becomes a focal point during an infectious disease pandemic as all countries are faced with a similar situation. COVID-19 represents a unique opportunity to evaluate and measure success by countries to control its spread and address social and economic impacts of interventions. Understanding limitations of COVID-19 case counts by addressing factors related to testing and reporting will strengthen country responses to this and future pandemics and increase the reliability of knowledge gained by cross-country comparisons. Alarmingly, with COVID-19 having asymptomatic spread, lack of testing can discredit the efforts of an entire community, not to say an entire population.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Centers for Disease Control and Prevention
Coronavirus disease 2019
Health and Human Services
National notifiable disease reporting system
Non-pharmaceutical interventions
Severe acute respiratory syndrome
Sustainable Development Goals
World Health Organization
Pueyo T. Coronavirus: the hammer and the dance. Medium. 2020. https://medium.com/@tomaspueyo/coronavirus-the-hammer-and-the-dance-be9337092b56 . Accessed 5 Sep 2020.
Ferguson N, Laydon D, Nedjati-Gilani G, et al. Report 9—impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. WHO Collaborating Centre for Infectious Disease Modelling; MRC Centre for Global Infectious Disease Analysis; Abdul Latif Jameel Institute for Disease and Emergency Analytics; Imperial College London, UK; 2020. http://www.imperial.ac.uk/medicine/departments/school-public-health/infectious-disease-epidemiology/mrc-global-infectious-disease-analysis/covid-19/report-9-impact-of-npis-on-covid-19/ . Accessed 14 Dec 2020.
Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–87.
Article CAS Google Scholar
Islam N, Sharp SJ, Chowell G, Shabnam S, Kawachi I, Lacey B, et al. Physical distancing interventions and incidence of coronavirus disease 2019: natural experiment in 149 countries. BMJ. 2020;15(370): m2743.
Article Google Scholar
WHO. Archived: WHO Timeline—COVID-19. https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 . Accessed 14 Dec 2020.
Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). WHO; 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf . Accessed 7 May 2020.
Ho S. Breaking down the COVID-19 numbers: should we be comparing countries? CTV News. 2020. https://www.ctvnews.ca/health/coronavirus/breaking-down-the-covid-19-numbers-should-we-be-comparing-countries-1.4874552 . Accessed 14 Dec 2020.
D’Adamo H, Yoshikawa T, Ouslander JG. Coronavirus Disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J Am Geriatr Soc. 2020;68(5):912–7.
Kluge HHP. Statement—older people are at highest risk from COVID-19, but all must act to prevent community spread. WHO, Regional Office for Europe. 2020. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/statements/statement-older-people-are-at-highest-risk-from-covid-19,-but-all-must-act-to-prevent-community-spread . Accessed 14 Dec 2020.
Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020. https://doi.org/10.3389/fpubh.2020.00152/full .
Canadian Institutes of Health Research. Why sex and gender need to be considered in COVID-19 research—CIHR. Government of Canada. 2020. https://cihr-irsc.gc.ca/e/51939.html . Accessed 14 Dec 2020.
Gaynor TS, Wilson ME. Social vulnerability and equity: the disproportionate impact of COVID-19. Public Adm Rev. 2020;80(5):832–8.
Karaye IM, Horney JA. The impact of social vulnerability on COVID-19 in the US: an analysis of spatially varying relationships. Am J Prev Med. 2020;59(3):317–25.
Alvarez E. Policy frameworks and impacts on the epidemiology of COVID-19. CONVERGE. 2020. https://converge.colorado.edu/resources/covid-19/working-groups/issues-impacts-recovery/policy-frameworks-and-impacts-on-the-epidemiology-of-covid-19 . Accessed 14 Dec 2020.
Alvarez E. Physical distancing policies and their effect on the epidemiology of COVID-19: a multi-national comparative study. World Pandemic Research Network. 2020. https://wprn.org/item/457852 . Accessed 14 Dec 2020.
COVID-19 Policies & Epidemiology Research Project. Home. https://covid19-policies.healthsci.mcmaster.ca/ . Accessed 20 Oct 2020.
CDC. Coronavirus Disease 2019 (COVID-19)—Transmission. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/faq-surveillance.html . Accessed 2 Sep 2020.
Crowe K. Why it’s so difficult to get tested for COVID-19 in Canada | CBC News. CBC News. 2020. https://www.cbc.ca/news/health/covid-testing-shortages-1.5503926 . Accessed 5 Sep 2020.
Confused about COVID-19 testing guidelines? Find out if you should get tested | CBC News. CBC News. 2020. https://www.cbc.ca/news/canada/toronto/covid-19-testing-ontario-1.5737683 . Accessed 14 Dec 2020.
Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, Macdonald B, et al. Coronavirus (COVID-19) Testing—our world in data. Our world in data. n.d. https://ourworldindata.org/coronavirus-testing . Accessed 14 Dec 2020.
Fox C. Workers at long-term care homes in COVID-19 hot spots will now be required to get tested weekly. CP24. 2020. https://www.cp24.com/news/workers-at-long-term-care-homes-in-covid-19-hot-spots-will-now-be-required-to-get-tested-weekly-1.5194380?cache=%3FclipId%3D89926%3FautoPlay%3DtrueSC . Accessed 8 Jan 2021.
Mzezewa T. Travel and coronavirus testing: your questions answered. The New York Times. 2020. https://www.nytimes.com/article/virus-covid-travel-questions.html . Accessed 8 Jan 2021.
Tsang TK, Wu P, Lin Y, Lau EHY, Leung GM, Cowling BJ. Effect of changing case definitions for COVID-19 on the epidemic curve and transmission parameters in mainland China: a modelling study. Lancet Public Health. 2020;5(5):e289–96.
Feuer W. “Confusion breeds distrust:” China keeps changing how it counts coronavirus cases. CNBC. 2020. https://www.cnbc.com/2020/02/26/confusion-breeds-distrust-china-keeps-changing-how-it-counts-coronavirus-cases.html . Accessed 14 Dec 2020.
Sauer P, Gershkovich E. Russia is boasting about low coronavirus deaths. The numbers are deceiving. The Moscow Times. 2020. https://www.themoscowtimes.com/2020/05/08/russia-is-boasting-about-low-coronavirus-deaths-the-numbers-are-deceiving-a70220 . Accessed 14 Dec 2020.
Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. N Engl J Med. 2020;382(22):2158–60.
Pabbaraju K, Wong AA, Douesnard M, Ma R, Gill K, Dieu P, et al. A public health laboratory response to the pandemic. J Clin Microbiol. 2020;58(8):e01110–20.
Sherriff-Scott I. Ontario is changing how it tests for COVID. Here’s what we know so far. iPolitics. 2020. https://ipolitics.ca/2020/10/05/ontario-is-changing-how-it-tests-for-covid-heres-what-we-know-so-far/ . Accessed 16 Dec 2020.
Cousins S. Bangladesh’s COVID-19 testing criticised. Lancet. 2020;396(10251):591.
Maclean R. COVID-19 outbreak in Nigeria is just one of Africa’s alarming hot spots. The New York Times. 2020. https://www.nytimes.com/2020/05/17/world/africa/coronavirus-kano-nigeria-hotspot.html . Accessed 30 Oct 2020.
Government of Singapore. Budget 2020. 2020. http://www.gov.sg/features/budget2020 . Accessed 8 Jan 2021.
Hapuarachchi P. President announces relief measures to the people amid COVID-19. 2020. https://www.newsfirst.lk/2020/03/23/president-grants-concessions-for-the-people-amid-covid-19/ . Accessed 8 Jan 2021.
Rauls L. COVID-19 is exposing the holes in Latin America’s safety nets. Americas Quarterly. 2020. https://americasquarterly.org/article/covid-19-is-exposing-the-holes-in-latin-americas-safety-nets/ . Accessed 8 Jan 2021.
Shafer P, Avila CJ. 4 ways COVID-19 has exposed gaps in the US social safety net. The Conversation. 2020. http://theconversation.com/4-ways-covid-19-has-exposed-gaps-in-the-us-social-safety-net-138233 . Accessed 16 Dec 2021.
Carillon S, Gosselin A, Coulibaly K, Ridde V, Desgrées du Loû A. Immigrants facing COVID 19 containment in France: an ordinary hardship of disaffiliation. J Migr Health. 2020;1–2: 100032.
Charbonneau D. Ottawa residents frustrated with multi-day wait for COVID-19 test results. CTV News. 2020. https://ottawa.ctvnews.ca/ottawa-residents-frustrated-with-multi-day-wait-for-covid-19-test-results-1.5122365 . Accessed 14 Dec 2020.
Faust L, Zimmer AJ, Kohli M, Saha S, Boffa J, Bayot ML, et al. SARS-CoV-2 testing in low- and middle-income countries: availability and affordability in the private health sector. Microbes Infect. 2020;22(10):511–4.
FloodList. India—over 60 dead after more rain and floods hit Telangana—FloodList. 2020. http://floodlist.com/asia/india-floods-telangana-october-2020 . Accessed 15 Dec 2020.
Al Jazeera. India: cyclone Nivar makes landfall bringing rains, flood | Weather News. 2020. https://www.aljazeera.com/news/2020/11/26/cyclone-nivar-makes-landfall-bringing-rains-flood . Accessed 15 Dec 2020.
Niles N, Hauck G, Aretakis R, Shannon J. Tropical storm sally: hurricane watch, evacuations for New Orleans; storm expected to strengthen. USA TODAY. 2020. https://www.usatoday.com/story/news/nation/2020/09/12/tropical-storm-update-florida-gulf-path-paulette-path-bermuda/5778321002/ . Accessed 15 Dec 2020.
Flaccus G, Cline S. Wildfires raging across Oregon, Washington may cause historic destruction: officials. Global News. 2020. https://globalnews.ca/news/7325865/oregon-washington-wildfires/ . Accessed 15 Dec 2020.
Watson I, Jeong S. South Korea pioneers coronavirus drive-through testing station—CNN. CNN . 2020. https://www.cnn.com/2020/03/02/asia/coronavirus-drive-through-south-korea-hnk-intl/index.html . Accessed 15 Dec 2020.
Herhalt C. “This is torture”: Ontario mom waits 6.5 hours for COVID-19 test. CTV News. 2020. https://toronto.ctvnews.ca/this-is-torture-ontario-mom-waits-6-5-hours-for-covid-19-test-1.5109045 . Accessed 15 Dec 2020.
Lough R. French labs show how global supply bottlenecks thwart effort to ramp up testing. Reuters. 2020. https://www.reuters.com/article/us-health-coronavirus-france-reagents-idINKCN26G2EP . Accessed 8 Jan 2021.
Huq S, Biswas RK. COVID-19 in Bangladesh: data deficiency to delayed decision. J Glob Health. 2020;10(1): 010342.
Alberta Precision Laboratories. Major changes in COVID-19 specimen collection recommendations. 2020. https://www.albertahealthservices.ca/assets/wf/lab/wf-lab-bulletin-major-changes-in-covid-19-specimen-collection-recommendations.pdf .
Ramos KJ, Kapnadak SG, Collins BF, Pottinger PS, Wall R, Mays JA, et al. Detection of SARS-CoV-2 by bronchoscopy after negative nasopharyngeal testing: stay vigilant for COVID-19. Respir Med Case Rep. 2020;30: 101120.
Google Scholar
Chen LD, Li H, Ye YM, Wu Z, Huang YP, Zhang WL, et al. A COVID-19 patient with multiple negative results for PCR assays outside Wuhan, China: a case report. BMC Infect Dis. 2020;20(1):517.
Gupta N, Potdar V, Praharaj I, Giri S, Sapkal G, Yadav P, et al. Laboratory preparedness for SARS-CoV-2 testing in India: harnessing a network of virus research & diagnostic laboratories. Indian J Med Res. 2020;151(2 & 3):216–25.
CAS Google Scholar
Johnson CY, McGinley L. What went wrong with the coronavirus tests in the U.S. Washington Post. https://www.washingtonpost.com/health/what-went-wrong-with-the-coronavirus-tests/2020/03/07/915f5dea-5d82-11ea-b29b-9db42f7803a7_story.html . Accessed 5 Sep 2020.
Resnick B, Scott D. America’s shamefully slow coronavirus testing threatens all of us . Vox. 2020. https://www.vox.com/science-and-health/2020/3/12/21175034/coronavirus-covid-19-testing-usa . Accessed 14 Dec 2020.
Health Canada. Authorized medical devices for uses related to COVID-19: List of authorized testing devices. AEM. 2020. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/authorized/list.html . Accessed 15 Dec 2020.
Burstyn I, Goldstein ND, Gustafson P. It can be dangerous to take epidemic curves of COVID-19 at face value. Can J Public Health. 2020;111(3):397–400.
Bachelet VC. Do we know the diagnostic properties of the tests used in COVID-19? A rapid review of recently published literature. Medwave. 2020;20(3): e7890.
Mina MJ, Parker R, Larremore DB. Rethinking COVID-19 test sensitivity— a strategy for containment. N Engl J Med. 2020;383(22): e120.
Jia P, Yang S. China needs a national intelligent syndromic surveillance system. Nat Med. 2020;26(7):990–990.
Jia P, Yang S. Early warning of epidemics: towards a national intelligent syndromic surveillance system (NISSS) in China. BMJ Glob Health. 2020;5(10): e002925.
United Nations Department of Economic and Social Affairs. Statistics, COVID-19 response. https://covid-19-response.unstatshub.org/https:/covid-19-response.unstatshub.org/ . Accessed 20 Oct 2022.
The Atlantic Monthly Group. The COVID tracking project. The COVID tracking project. https://covidtracking.com/ . Accessed 21 Oct 2021.
Erdman SL. FDA authorizes first rapid COVID-19 self-testing kit for at-home diagnosis. CNN. https://www.cnn.com/2020/11/18/health/covid-home-self-test/index.html . Accessed 15 Dec 2020.
Johns Hopkins Coronavirus Resource Center. COVID-19 Map. https://coronavirus.jhu.edu/map.html . Accessed 20 Oct 2022.
Worldometer. COVID Live—Coronavirus Statistics. https://www.worldometers.info/coronavirus/ . Accessed 20 Oct 2022.
World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int . Accessed 20 Oct 2022.
Enria L, Waterlow N, Rogers NT, Brindle H, Lal S, Eggo RM, et al. Trust and transparency in times of crisis: results from an online survey during the first wave (April 2020) of the COVID-19 epidemic in the UK. PLoS ONE. 2021;16(2): e0239247.
OECD. Transparency, communication and trust: The role of public communication in responding to the wave of disinformation about the new Coronavirus. OECD. https://www.oecd.org/coronavirus/policy-responses/transparency-communication-and-trust-the-role-of-public-communication-in-responding-to-the-wave-of-disinformation-about-the-new-coronavirus-bef7ad6e/ . Accessed 21 Oct 2022.
McGrory K, Wollington R. Florida medical examiners were releasing coronavirus death data. The state made them stop. Tampa Bay Times. 2020. https://www.tampabay.com/news/health/2020/04/29/florida-medical-examiners-were-releasing-coronavirus-death-data-the-state-made-them-stop/ . Accessed 14 Dec 2020.
Myers SL. China created a fail-safe system to track contagions. It failed. The New York Times. 2020. https://www.nytimes.com/2020/03/29/world/asia/coronavirus-china.html . Accessed 16 Dec 2020.
Coronavirus: how New Zealand relied on science and empathy—BBC News. BBC News. 2020. https://www.bbc.com/news/world-asia-52344299 . Accessed 15 Dec 2020.
Wasike A. Tanzanian president claims “country free of COVID-19”. Anadolu Agency. 2020. https://www.aa.com.tr/en/africa/tanzanian-president-claims-country-free-of-covid-19/1869961 . Accessed 30 Oct 2020.
Coronavirus: Iran cover-up of deaths revealed by data leak. BBC News. 2020. https://www.bbc.com/news/world-middle-east-53598965 . Accessed 15 Dec 2020.
Hosseini K. Is Iran covering up its outbreak? BBC News. 2020. https://www.bbc.com/news/world-middle-east-51930856 . Accessed 15 Dec 2020.
Pazzanesse C. Calculating possible fallout of Trump’s face mask remarks. Harvard Gazette. 2020. https://news.harvard.edu/gazette/story/2020/10/possible-fallout-from-trumps-dismissal-of-face-masks/ . Accessed 15 Dec 2020.
McCoy T, Traiano H. Limits on coronavirus testing in Brazil are hiding the true dimensions of Latin America’s largest outbreak. Washington Post. https://www.washingtonpost.com/world/the_americas/coronavirus-brazil-testing-bolsonaro-cemetery-gravedigger/2020/04/22/fe757ee4-83cc-11ea-878a-86477a724bdb_story.html . Accessed 14 Dec 2020.
Dyer O. Covid-19: at least eight doctors in Egypt arrested for criticising government response. BMJ. 2020;370: m2850.
Joint statement by WHO, UN, UNICEF, UNDP, UNESCO, UNAIDS, ITU, UN Global Pulse, and IFRC. Managing the COVID-19 infodemic: Promoting healthy behaviours and mitigating the harm from misinformation and disinformation. WHO. https://www.who.int/news/item/23-09-2020-managing-the-covid-19-infodemic-promoting-healthy-behaviours-and-mitigating-the-harm-from-misinformation-and-disinformation . Accessed 30 Oct 2020.
Hua J, Shaw R. Corona Virus (COVID-19) “Infodemic” and emerging issues through a data lens: the case of China. Int J Environ Res Public Health. 2020;17(7):2309.
Nielsen RK, Fletcher R, Newman N, Brennen JS, Howard PN. Navigating the ‘Infodemic’: how people in six countries access and rate news and information about coronavirus. Reuters Institute for the Study of Journalism as part of the Oxford Martin Programme on Misinformation, Science and Media, a three-year research collaboration between the Reuters Institute, the Oxford Internet Institute, and the Oxford Martin School; 2020.
Gittleson B, Phelps J, Cathey L. Trump doubles down on defense of hydroxychloroquine to treat COVID-19 despite efficacy concerns. ABC News. 2020. https://abcnews.go.com/Politics/trump-doubles-defense-hydroxychloroquine-treat-covid-19-efficacy/story?id=72039824 . Accessed 15 Dec 2020.
De Sousa M, Biller D. Brazil’s president, infected with virus, touts malaria drug. CTVNews. 2020. https://www.ctvnews.ca/world/brazil-s-president-infected-with-virus-touts-malaria-drug-1.5015608 . Accessed 15 Dec 2020.
COVID-19 INED. Key issues. Ined—Institut national d’études démographiques. n.d. https://dc-covid.site.ined.fr/en/presentation/ . Accessed 14 Dec 2020.
Lima T. Following Data Blackout, Rio City Government’s “Updated” Covid-19 dashboard excludes 40% of deaths. RioOnWatch. 2020. https://www.rioonwatch.org/?p=59883 . Accessed 15 Dec 2020.
Ciminelli G, Garcia-Mandicó S. COVID-19 in Italy: an analysis of death registry data. J Public Health Oxf Engl. 2020;42(4):723–30.
Italy’s coronavirus death toll is likely much higher: “Most deaths simply aren’t counted.” CBS News . 2020. https://www.cbsnews.com/news/italy-coronavirus-deaths-likely-much-higher/ . Accessed 15 Dec 2020.
McCarthy G, Dempsey R, Parnell A, MacCarron. Just a “bad flu”? How death notices debunk that Covid-19 myth. 2020. https://www.rte.ie/brainstorm/2020/0928/1167878-covid-19-rip-death-notices-bad-flu-excess-mortality/ . Accessed 10 Jan 2021.
Osman L. Canada still only considering gathering race-based COVID-19 data. CTV News . 2020. https://www.ctvnews.ca/health/coronavirus/canada-still-only-considering-gathering-race-based-covid-19-data-1.4927648 . Accessed 15 Dec 2020.
Gilbert J, Keane D. How French law makes minorities invisible. The Independent . 2016. https://www.independent.co.uk/news/world/politics/how-french-law-makes-minorities-invisible-a7416656.html . Accessed 15 Dec 2020.
Timsit A. France’s data collection rules obscure the racial disparities of COVID-19. Quartz. 2020. https://qz.com/1864274/france-doesnt-track-how-race-affects-covid-19-outcomes/ . Accessed 15 Dec 2020.
Vogel L. Have we misjudged the role of children in spreading COVID-19? CMAJ. 2020;192(38):E1102–3.
Aguilar B. TDSB closes six more schools because of COVID-19. Toronto. 2020. https://toronto.ctvnews.ca/tdsb-closes-six-more-schools-because-of-covid-19-1.5229597 . Accessed 15 Dec 2020.
Kleitman S, Fullerton DJ, Zhang LM, Blanchard MD, Lee J, Stankov L, et al. To comply or not comply? A latent profile analysis of behaviours and attitudes during the COVID-19 pandemic. PLoS ONE. 2021;16(7): e0255268.
Savage M. Could the Swedish lifestyle help fight coronavirus? BBC . 2020. https://www.bbc.com/worklife/article/20200328-how-to-self-isolate-what-we-can-learn-from-sweden . Accessed 15 Dec 2020.
Green K. Worried about possible lockdown, Alberta shoppers flood the stores. CTV News . 2020. https://calgary.ctvnews.ca/worried-about-possible-lockdown-alberta-shoppers-flood-the-stores-1.5201682 . Accessed 15 Dec 2020.
Department of Economic and Social Affairs. The 17 GOALS | sustainable development. United Nations. n.d. https://sdgs.un.org/goals . Accessed 14 Dec 2020.
United Nations Office for Disaster Risk Reduction. Sendai Framework for Disaster Risk Reduction 2015–2030. 2015. https://www.undrr.org/publication/sendai-framework-disaster-risk-reduction-2015-2030 . Accessed 14 Dec 2020.
Chen H, Hailey D, Wang N, Yu P. A review of data quality assessment methods for public health information systems. Int J Environ Res Public Health. 2014;11(5):5170–207.
Rajan D, Koch K, Rohrer K, Bajnoczki C, Socha A, Voss M, et al. Governance of the Covid-19 response: a call for more inclusive and transparent decision-making. BMJ Glob Health. 2020;5(5):e002655.
Sherbrooke U de. Study by the Université de Sherbrooke on the psychosocial impacts of the pandemic. https://www.newswire.ca/news-releases/study-by-the-universite-de-sherbrooke-on-the-psychosocial-impacts-of-the-pandemic-886218205.html . Accessed 30 Jan 2021.
Download references
Acknowledgements
The authors acknowledge Dr. Neil Abernethy’s contributions to the COVID-19 Policies and Epidemiology Working Group.
No funding was received for this manuscript.
Author information
Authors and affiliations.
Department of Health Research Methods, Evidence and Impact, McMaster University, CRL 2nd Floor, 1280 Main Street West, Hamilton, ON, L8S4K1, Canada
Elizabeth Alvarez, Iwona A. Bielska, Stephanie Hopkins & Ahmed A. Belal
Department of Anthropology, University of Colorado Boulder, Boulder, CO, USA
Donna M. Goldstein & Anna Wynfield
Disaster and Emergency Management, Royal Roads University, British Columbia, Canada
School of Medicine, McMaster University, Hamilton, ON, Canada
Sureka Pavalagantharajah
Department of Architecture and Planning, Visvesvaraya National Institute of Technology, Nagpur, India
Shruthi Dakey
University Integrated Health Center of the Nord-de-l’île de Montréal (CIUSSS NIM), Montréal, QC, Canada
Marie-Carmel Gedeon
Faculty of Resilience, Rabdan Academy, Abu Dhabi, United Arab Emirates
Department of Global Health, McMaster University, Hamilton, ON, Canada
Katrina Bouzanis
You can also search for this author in PubMed Google Scholar
Contributions
E.A. drafted the manuscript, and all other authors contributed to the content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Elizabeth Alvarez .
Ethics declarations
Ethics approval and consent to participate.
Not applicable as publicly available information was used.
Consent for publication
Not applicable as no individual-level data are used in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Alvarez, E., Bielska, I.A., Hopkins, S. et al. Limitations of COVID-19 testing and case data for evidence-informed health policy and practice. Health Res Policy Sys 21 , 11 (2023). https://doi.org/10.1186/s12961-023-00963-1
Download citation
Received : 27 March 2021
Accepted : 15 January 2023
Published : 25 January 2023
DOI : https://doi.org/10.1186/s12961-023-00963-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiology
- Physical distancing policy
Health Research Policy and Systems
ISSN: 1478-4505
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
COVID-19 Limitations Unique Opportunity for Researchers to Decrease Digital Divide
Researchers need to develop new ways to reach rural participants.
- by Karen Nikos-Rose
- April 29, 2020

The COVID-19 shelter-in-place orders and other limitations could offer researchers the chance to use technology to decrease the digital divide and disparities in academic research, suggests a University of California, Davis, professor in a new commentary.
“While I know many of my colleagues are frustrated with this pause in clinical research, it is actually a unique opportunity,” said Leigh Ann Simmons, chair of the Department of Human Ecology, whose research interests include increased equity in health care delivery and chronic disease prevention in rural areas. “People who live in rural areas are often left out of clinical trials that can benefit them, partly because they are not near large medical centers,” she said. This includes migrant workers, farmers and the general public who live in outlying areas.
She is co-author of the commentary , “Navigating Nonessential Research Trials During COVID 19: The Push We Needed for Using Digital Technology to Increase Access for Rural Participants?” published in The Journal of Rural Health earlier this month. Co-author is Devon Noonan, a researcher at Duke University.
Simmons said some research in which research subjects have to be contacted personally for interviews, testing or surveys has stopped since social distancing went into effect. This is a mistake, she said. “If we think creatively we can extend our reach.”
“We need to stop and think,” said Simmons, who is herself currently engaged in two rural health prevention studies that are being conducted solely using remote strategies. “How can we do our work remotely? Is there a way to get our data without human contact? And if we go this route, how can we include people who may not usually participate in our studies?”
It is well known, the authors said in their paper, that rural populations experience significant health disparities, especially in rates of common chronic diseases such as heart disease, diabetes, cancer and the associated health behaviors such as diet, physical activity, and tobacco and other substance use. “These disparities are in part due to rural residents’ lack of access to, knowledge about, and participation in clinical trials,” they said.
Participation in such trials is made more difficult in these areas too by lack of good internet access. Simmons said this could be augmented by researchers using community centers or regional facilities, or other community partners, to enable access for those in the study. Regional facilities could also be used to help with data and sample collections.
Further, state departments of heath “could replicate the partnership that the California Department of Education initiated with Google to distribute mobile hotspots to areas without broadband access so that K-12 education could continue amid school closures associated with shelter-in-place orders,” the authors suggest.
“Moving to remote clinical trials is not without its challenges, especially for studies that are well underway,” she emphasizes. “Importantly, the steps we take now to continue nonessential research remotely may provide the evidence we need to ensure that future studies target these hard-to-reach populations for study inclusion.”
Establishing remote access to clinical trials will serve to not only decrease rural clinical trial disparities, the authors said, but also to promote rural health equity into the next decade and beyond.

Media Resources
Karen Nikos-Rose, News and Media Relations, 530-219-5472, [email protected]
Primary Category
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Health Sci Rep
- v.6(8); 2023 Aug
- PMC10404843
Challenges and solutions in clinical research during the COVID‐19 pandemic: A narrative review
Mahin nomali.
1 Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran Iran
Neda Mehrdad
2 Diabetes Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran Iran
3 Nursing and Midwifery Care Research Center, Health Management Research Institute, Iran University of Medical Sciences, Tehran Iran
Mohammad Eghbal Heidari
4 Students' Scientific Research Center, School of Nursing and Midwifery, Tehran University of Medical Sciences, Tehran Iran
Aryan Ayati
5 Tehran Heart Center, Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Tehran Iran
Amirhossein Yadegar
6 Endocrinology and Metabolism Research Center (EMRC), Vali‐Asr Hospital, Tehran University of Medical Sciences, Tehran Iran
Moloud Payab
7 Non‐Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran Iran
Alireza Olyaeemanesh
8 National Institute of Health Research, Tehran University of Medical Sciences, Tehran Iran
9 Health Equity Research Center (HERC), Tehran University of Medical Sciences (TUMS), Tehran Iran
Bagher Larijani
10 Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran Iran
Associated Data
All data associated with the article is available upon request.
Background and Aims
The COVID‐19 pandemic has presented significant challenges to clinical research, necessitating the adoption of innovative and remote methods to conduct studies. This study aimed to investigate these challenges and propose solutions for conducting clinical research during the pandemic.
A narrative review was conducted (approval ID: IR.AMS.REC.1401.029), utilizing keyword searches in PubMed and Web of Science (WOS) citation index expanded (SCI‐EXPANDED) from January 2020 to January 2023. Keywords included COVID‐19, clinical research, barriers, obstacles, facilitators and enablers.
Out of 2508 records retrieved, 43 studies were reviewed, providing valuable insights into the challenges and corresponding solutions for conducting clinical research during the COVID‐19 pandemic. The identified challenges were categorized into four main groups: issues related to researchers or investigators, issues related to participants and ethical concerns, administrative issues, and issues related to research implementation. To address these challenges, multiple strategies were proposed, including remote monitoring through phone or video visits, online data collection and interviews to minimize in‐person contact, development of virtual platforms for participant interaction and questionnaire completion, consideration of financial incentives, adherence to essential criteria such as inclusion and exclusion parameters, participant compensation, and risk assessment for vulnerable patients.
The COVID‐19 pandemic has significantly impacted clinical research, requiring the adaptation and enhancement of existing research structures. Although remote methods and electronic equipment have limitations, they hold promise as effective solutions during this challenging period.
1. INTRODUCTION
The COVID‐19 disease has caused millions of deaths around the world. The most common symptoms of the infection were respiratory problems, which caused the disease to spread rapidly. 1 At the beginning of the pandemic, measures such as quarantine, social distancing, rapid tracking of patients and restriction of presence in closed spaces, and crowding were carried out to control the disease. 2 In the absence of definitive treatment and effective vaccines, these measures were effective to some extent but negatively affected society. 3 The COVID‐19 pandemic changed many aspects of human life worldwide, causing adverse social and economic consequences that will persist for years. 4 , 5
Research is a critical aspect of responding to public health emergencies. Research efforts from various groups were focused on the origin of the COVID‐19 disease and management strategies, including drugs and vaccines through numerous clinical trials. 6 Health scientists were confronting COVID‐19 and solving its complications with investigations, research, and clinical trials. Many researchers were faced with significant challenges due to the spread of COVID‐19. These issues include the unwillingness of volunteers and research participants, reductions in research funds due to shifting toward the treatment and hospitalization of the affected, and emerging difficulties in traveling and field investigations. Furthermore, stress and concerns about COVID‐19 were shared among the participants, volunteers, research team, and scientists. 7
Field investigations are necessary for many studies, requiring the presence of scientists in the clinical environment. Furthermore, the presence of participants and volunteers is essential for many researchers. Ethical problems such as obtaining consent to participate in the research, explaining the purpose of the study, follow‐ups, and referring vulnerable patients during the study were negatively affected by the pandemic. 8 Due to the rapid spread of the COVID‐19 disease and its high mortality, there was an urgent need for research on medications, vaccines, improving diagnostic tools, and medical management, which was a challenge for scientists. 9 , 10
The COVID‐19 pandemic has significantly impacted various clinical research methods, particularly clinical trials, which are crucial in evaluating the safety and efficacy of new medical treatments. Many aspects of clinical trials, including patient recruitment, obtaining informed consent, and implementing interventions, were traditionally conducted in person by the research team. However, due to the pandemic, these processes have been disrupted, and alternative methods have had to be developed to ensure the continuity of clinical research while prioritizing the safety of participants and researchers. 11 The COVID‐19 pandemic also affected clinical research about infectious diseases as well as other medical fields, including cancer, chronic diseases, obstetrics, and gynecology. 12 , 13
The COVID‐19 pandemic led to the discontinuation of several significant health studies. This was primarily due to a lack of effective preparation for such a crisis and inadequate guidance for clinical researchers on addressing research challenges by utilizing various strategies rather than halting research in clinical settings.
As a result, many researchers were not equipped to adapt to the new circumstances and continue their studies safely and effectively. However, it is crucial to note that innovative solutions have emerged in response to these challenges, such as remote data collection and telemedicine, which have enabled researchers to continue their work while ensuring the safety of study participants and staff. Going forward, it is essential to prioritize preparedness for potential crises and provide clear guidance to researchers to ensure the continuity of critical health studies during challenging times.
Previous reviews have examined the challenges of conducting research during the COVID‐19 pandemic. However, these reviews are limited to clinical trials, research sponsors, or particular diseases. 7 , 14 A comprehensive review that examines all aspects of conducting clinical research during the pandemic is currently lacking, yet it is crucial for future crises.
Therefore, we aimed to comprehensively review the challenges faced worldwide in conducting clinical research during the COVID‐19 pandemic. We will also identify solutions to these challenges to aid researchers and policymakers in facilitating clinical research during future crises. By conducting this review, we hope to provide a comprehensive understanding of the impact of the pandemic on clinical research and contribute to the development of strategies to mitigate its effects on research activities.
2.1. Study design
This study was a narrative review approved by the ethical committee of the Iranian Academy of Medical Sciences (IAMS) (REC approval ID: IR.AMS.REC.1401.029).
2.2. Search strategy and information sources
We searched PubMed and Web of Science (WOS) citation index expanded (SCI‐EXPANDED) on January 4, 2023, to identify related articles published between January 2020 to January 2023. We developed the search strategy for the PubMed database and modified it for the WOS database (Table 1 ). The search strategy was applied without any limitation on data and languages. Manual searching in key journals for relevant articles was conducted after the initial search of databases, and the reference list of included articles was checked for possible related studies (Figure S1 ).
Search strategies used to retrieve related documents.
| Search syntax in database |
| (covid‐19 OR Sars‐cov‐2 OR coronavirus OR Cov‐19 OR 2019‐ncov OR covid19 OR “covid‐19 pandemic”) AND (“clinical research” [tiab] OR “clinical study” [tiab] OR “clinical trial*“ [tiab]) AND (barriers[tiab] OR obstacles[tiab] OR challenges[tiab] OR difficulties[tiab] OR facilitators[tiab] OR enablers[tiab]) AND 2019/01/01:2023/01/01[dp] |
| Search syntax in citation index expanded (SCI‐EXPANDED) |
| (ALL = (covid‐19 OR sars‐cov‐2 OR coronavirus OR Cov‐19 OR 2019‐ncov OR covid19 OR “covid‐19 pandemic”) AND TS = (“clinical research” OR “clinical study” OR “clinical trial*“) AND TS = (barriers OR obstacles OR challenges OR difficulties OR facilitators OR enablers) AND PY = (2019‐2023)) |
2.3. Study selection process
Two authors reviewed and screened all retrieved documents independently (MN, MH) based on titles and abstracts according to the eligibility criteria. Afterward, the full texts of the selected studies were assessed. Any disagreements were resolved by the third author (NM).
The inclusion criteria consisted of studies conducted during the COVID‐19 pandemic examining challenges related to conducting research regardless of their study design. There is no limitation on the type of studies. Articles without abstract, full‐text, or sufficient relevant data were excluded.
2.4. Data extraction
The list of data extraction included the first author's name, publication year, study design, and study country or setting. Information about the challenges and related solutions was extracted and reviewed from the included articles narratively. To categorize challenges, we used expert opinion.
A total of 2508 records were retrieved through electronic databases. After removing duplicates and screening based on title and abstract, the full texts of 156 studies were assessed, and 43 studies were included in the qualitative synthesis (Figure S1 ).
All the studies were conducted during the COVID‐19 pandemic and evaluated the challenges of conducting research in this era. The characteristics of the included studies are provided in Table 2 .
Characteristics of included studies.
| Author | Year | Location | Study type | Main findings |
|---|---|---|---|---|
| Collier, Erin K | 2020 | USA | Editorial | Investigators must conduct clinical trials in a safe, compliant, and ethnically appropriate manner. |
| de Miguel, Maria | 2020 | Spain | Editorial | Individualizing patient management while guaranteeing their safety and adherence to the study protocol, implementing specialized staff contingency plans, and maintaining sponsor and contract research organization (CRO) alignment are some of the critical problems for the research's long‐term success. |
| Ellen Townsend | 2020 | United Kingdom | Editorial | To be deemed ethical, all research must have a high benefit‐to‐risk ratio. |
| Neeta Kantamneni | 2020 | USA | research agenda | More research is needed on individuals experiencing increased discrimination or workload due to the pandemic. |
| Michelle I. Cardel | 2020 | USA | Quantitative/qualitative cross‐sectional survey | COVID‐19 has four effects on research experiences: transition, remote intervention delivery, ability to adhere to program goals and interest in research involvement |
| Celeste Cagnazzo | 2020 | Italy | Editorial | The quest to quickly uncover answers to health concerns and therapeutic ideas in the specialized clinical research domain has underlined the necessity for efficient, timely, ethically right research. |
| Adam Palayew | 2020 | Canada | Editorial | Rapid publication ensures that new evidence is shared on time, particularly during a fast‐moving health crisis such as the COVID‐19 pandemic. Nonetheless, there are steps that the scientific community can and should take to prevent the accelerated pace of COVID‐19 publishing from weakening the evidence base. |
| Hamasaki, Toshimitsu | 2020 | USA | Editorial | The COVID‐19 pandemic poses huge hurdles, particularly to medical research, as these disruptions have wreaked havoc on ongoing and planned clinical studies. |
| M Wolkewitz | 2020 | Germany | Editorial | Numerous methodological challenges are associated with producing, gathering, analyzing, reporting, and publishing data in the compressed timeframes required during a pandemic. |
| Padala, Prasad R | 2020 | USA | Perspective | The risk‐benefit ratio of conducting, rescheduling, or canceling each research visit is determined by the principal investigator (PI). To ultimately decide on the course of action, the PI should examine the ethical principles of research, local/national advice, the community danger of the pandemic in their region, staffing strain, and the risk involved to each participant. |
| M Akacha | 2020 | Switzerland | Editorial | The COVID‐19 pandemic and associated measures may also impact the types, incidence, severity, and duration of adverse events (AEs) recorded for experimental therapies and in the control group. |
| Sarah J Richardson | 2020 | UK | Commentary | COVID‐19 research must be inclusive, particularly involving elderly living with frailty, cognitive impairment, or multimorbidity. Non‐COVID‐19‐related research for older people remains critical and must not be neglected in the rush to study the pandemic. Significant changes are required in designing and delivering research for older people in a world where movement and face‐to‐face contact are restricted. |
| Xitao Ma | 2020 | China | Commentary | Ethical review‐related regulations must be updated, and a unified supervision system for the overall ethical review committee is required. |
| B.E. Bierer | 2020 | USA | Editorial | During COVID‐19, clinical research was halted or terminated due to urgent patient care needs, and clinical trials focused on treating and preventing coronavirus infection were prioritized over studies focused on other diseases. Electronic data collection and cloud computing; And obligations to share protocols, consents, and data should be applied to rigorous research methods in the service of public health |
| Shahmir H Ali | 2020 | USA | Web‐based survey | It is vital to use social media recruitment for the rapid collecting of survey data related to rapidly emerging health problems, such as COVID‐19. |
| Leonardo Tamariz | 2021 | USA | Editorial | Appropriate scientific review of protocols, Devoting special research ethics committees to Covid‐19‐related research, Using alternate members and consultants, and facilitating the conduct of sound scientific and ethical research are the steps that are effective in reducing the pressure of research ethics committees. |
| Mohan, Sumit | 2021 | USA | Editorial | Process flexibility for staff and research participants may be the catalyst needed to make sustainable improvements to our research processes, roles, and goals. |
| Shields, Charlotte N | 2021 | USA | Survey study | The most significant barriers to follow‐up included fear of COVID‐19, wait times, and costs. |
| Jay J H Park | 2021 | Canada | Editorial‐Series | The COVID‐19 pandemic has re‐emphasized the importance of well‐designed randomized clinical trials and highlighted the need for large‐scale clinical trials structured according to a master protocol in a coordinated and collaborative manner. |
| Caputo, Eduardo L | 2021 | Brazil | Editorial | During the COVID‐19 pandemic, at the same time as there are changes in the format and conduct of research, the process of conducting research should not be interrupted. |
| Susanne Röhr | 2021 | Germany | Perspective Study | There is a need for special statistical programs to conduct research during the COVID‐19 pandemic. |
| MeeLee Tom | 2021 | USA | Perspective Study | Strong communication and constant commitment, combined with technical capabilities for remote work, visits, and study medicine distribution, were critical to the effective retention of study participants and resumption of enrolment. |
| Anderson, Melanie | 2021 | USA | Perspective study | COVID‐19 has significantly impacted clinical studies, and there is a need to change the format of different phases of clinical trials. |
| TL Loucks | 2021 | USA | special communication | virtual visits and digital approaches are ways to facilitate research conduction. |
| Perrine Janiaud | 2021 | Switzerland | Survey | Collaborative efforts such as consortiums of trials prospectively planning to pool their results4 and adaptive platform trials such as the RECOVERY trial5 are promising approaches to provide reliable and timely evidence. |
| B Hensen | 2021 | UK | Commentary | Remote data collection is one of the strategies to continue conducting public health research. |
| Stephanie Tremblay | 2021 | Canada | Commentary | Conducting interviews in qualitative studies in the era of COVID‐19 is considered a fundamental challenge, and remote methods can, to some extent, continue qualitative research. |
| Abhinav Bassi | 2022 | Australia | Editorial | Remote methods, the necessity of prioritizing research, and improving research infrastructures are among the critical measures in solving the challenges of conducting research. |
| Jenail Mobaraka | 2022 | USA | Editorial | Conducting in‐person data collection during a pandemic would place participants and researchers at risk of infection. Therefore, adjusting and compromising the study's goals, design, and methodology to address the new subjective conditions of all actors involved are crucial protective measures. |
| Catherine A. Sewell | 2022 | USA | Special Report | increased collaboration among stakeholders (federal agencies, industry, academia, and patients and patient advocates) can support progress in conducting research. |
| Walshe C | 2022 | UK | Multinational Survey | Consideration must be given to widening the volunteer base away from those most vulnerable to COVID‐19. |
| Jon Salmanton‐García | 2022 | Germany | Epidemiological Study | The VACCELERATE Volunteer Registry is an active single‐entry point for European residents interested in participating in COVID‐19 clinical trials. |
| Donna A. Santillan | 2022 | USA | Report | Adaptability is essential for network site maintenance. Constant intra‐ and inter‐institutional contact were necessary to manage the rapidly shifting rules for starting and continuing research during the epidemic. |
| RM Haynes | 2022 | Canada | Point of view | The use of technology can prevent the challenges of conducting research in the era of COVID‐19. |
| LA Simmons | 2022 | USA | Study design | Remote clinical trials have the ability to not only boost representation and reduce participant travel and study visit hardship but also introduce implementation and participant retention problems. |
| Rashmi K. Sharma | 2022 | USA | Editorial | During COVID‐19, the use of technology and virtual platforms is critical in doing research. |
| Diallo, Alpha | 2022 | France | Commentary | Effective medical teamwork is critical in responding to epidemics/pandemics. Regulatory, legal, and financial barriers have dramatically delayed clinical trial efficiency, which is untenable during an active epidemic. Adaptive, large‐scale clinical studies during pandemics should be regarded as a key countermeasure, and regulatory approval should be expedited in accordance with the situation's urgency. This applies to non‐emergencies as well as multicenter clinical trials in general. |
| Daniel Munblit | 2022 | UK | Editorial | There is an urgent need to refine and standardize outcome metrics for this significant patient group for clinical services and research, as well as to enable data comparison and pooling. |
| Ricardo Almeida‐Magana | 2022 | UK | Editorial | During the COVID‐19 pandemic, remote e‐Consent‐based recruiting was vital for trial continuation. |
| Gianna McMillan | 2022 | USA | Editorial | Innovative trial designs, such as basket and umbrella studies, designs that use external data sources, multi‐stage seamless trials, and preplanned data sharing amongst larger trials, are required in pandemic situations. |
| Micah A. Skeens | 2022 | USA | Survey | Social media recruiting reduced traditional time and engagement hurdles for participants while also avoiding social and physical distancing requirements imposed by the pandemic, allowing for real‐time assessment of the pandemic's effects on families. |
| Kellie Pertl | 2023 | USA | Cohort Study | Researchers had to move from in‐person to virtual recruitment tactics to reach and engage potential study participants during the COVID‐19 epidemic. During a pandemic, virtual recruitment looks less efficient and has hampered efforts to achieve recruitment goals. |
| Theresa Burgess | 2023 | South Africa | Qualitative exploration | REC members recognized numerous substantial ethical issues and problems in examining COVID‐19‐related research. While RECs are resilient and adaptive, weariness among reviewers and REC members was a big concern. |
The challenges included “issues related to researchers or investigators, issues related to participants and ethical concerns, administrative issues (i.e., research ethics committee [REC] or institutional review board [IRB] approval), and issues related to research conduction,” which are reviewed in the following sections.
3.1. Issues related to researchers or investigators
The increasing pressure of the pandemic on healthcare systems caused an extensive change in the employee workflow, increasing the duties of research personnel. 53 Research nurses had to work as clinical nurses in labor, delivery, and postpartum units. The pressure of researching clinical staff was significantly reduced, allowing them to respond to the patient's needs. 39 The research staffs were concerned about the risk of COVID‐19. Exposure to the disease during face‐to‐face meetings increased the chance of infection. Reducing the number of in‐person meetings and planning them through video conferences were necessary to reduce the risk of disease transmission. Also, appropriate personal protective equipments were mandatory to address these concerns. 7 , 12 Although the COVID‐19 limited medical students' classes and their presence in the hospital, many remained at their workplaces and engaged in their clinical and scientific activities. 54 At some institutions, medical students and residents played a more prominent role in screening, consenting, and enrolling clinical research participants. Tasks that were generally performed by research personnel. For example, residents performed research activities for high‐priority research regarding public health concerns such as COVID‐19 instead of research staff. 9 The participation of medical students in research varied in different countries and was related to the policies of each country and university. 55 The activity of medical students may be necessary and temporary in some critical times, such as the COVID‐19 pandemic. 54
International research and collaboration among researchers can enhance global knowledge and awareness. However, differences in goals, research priorities, and pandemic conditions can hinder cooperation. Despite these challenges, international researchers can collaborate on shared topics such as preventing disease spread, treatments, and vaccines, including different phases of clinical studies 40 (Table 1 ).
3.2. Issues related to participants and ethical concerns
During the COVID‐19 pandemic, study participant presence was a significant research limitation due to quarantine, social distancing, travel restrictions, and participant concerns. Many participants withdrew from studies due to infection fears, while high‐risk populations, such as infants, the elderly, and pregnant women, were still needed for research purposes. 41
Pandemic circumstances caused additional burdens on the health system, including the psychological pressure on researchers to provide a solution for the pandemic. Therefore, The process of project approvals should be revised in terms of speed, prioritization, and the presence of experts in the ethical approach. The research hypotheses may not be investigated in time if they are not approved and started at the right time. Also, prioritizing critical issues related to health in the ethics committees should be considered due to the crisis conditions. 6 During the Ebola epidemic, for example, clinical trials proceeded non‐stop. Research design and conduction should differ from traditional approaches during infectious disease outbreaks. 7
The main ethical challenges that organizations should investigate were obtaining informed consent and addressing ethical issues according to the study design and human interventions. 7 Another ethical issue that organizations should consider was the participants' interest in participating in research. For any research to be considered ethical, its benefits should be higher than its risks. Moreover, COVID‐19 has psychological effects on individuals. Thus, studies on mental health, depression, suicide, and self‐harm had to be carefully considered. In high‐risk projects, the purpose of the research, the stakeholders, and how it can be implemented should be apparent. Ethics committees worldwide must consider these fundamental issues and examine them seriously. 17 , 56
The expansion of online methods created favorable flexibility against COVID‐19 restrictions, such as obtaining consent electronically, responding online to ethical issues, and creating a platform for employees to handle research files remotely and outside work and office hours. 7 Some of the remote qualitative methods that were utilized included online or phone‐based interviews and focus group discussions, audio‐diary forms, photovoice (use of photography to capture lived experiences), video documenting, documentary analysis of social media (e.g., Facebook and WhatsApp groups, YouTube comments or podcasts) and auto‐ethnography. Remote quantitative methods included mobile phone surveys implemented using: interactive voice response (IVR), short messaging service (SMS), or computer‐assisted telephone interviews (CATI) and self‐completed online questionnaires shared via email or social media platforms. These methods were not new, with telephone and postal surveys used in higher‐income countries, yet their use became essential during the COVID‐19 era to support data collection directly from individuals and populations. Using technology in conducting studies was different in each country, and it depended on the national policies regarding the use of technology in health‐related research. 37 A study by Megana found that remote e‐consent‐based recruitment was crucial for trial continuity during the COVID‐19 pandemic. This method adheres to ethical and regulatory guidelines for informed consent while minimizing face‐to‐face interactions that increase COVID‐19 transmission risk. Patients provided positive feedback on using these platforms. 49
Ensuring participant safety and privacy are critical ethical considerations in clinical studies. 15 , 28 Accountability, tracking, and follow‐up before and after interventions must be prioritized to continue trials. Confidentiality of patient data and the secure delivery of investigational treatments from trial sites are essential. Participants must also be provided with instructions for properly storing and using investigational drugs. 16 , 29
Result by Shields et al. 30 showed that fear of COVID‐19 was a major barrier to follow‐ups. This fear included patients who felt unsafe exposing themselves or their family members or a patient's family member feeling unsafe exposing the patient. The next most commonly reported barriers were long waiting times and financial costs.
Informed consent is a common and fundamental part of any clinical research. It is usually provided by paper forms that explain the purpose of the study, the procedures, and possible adverse effects and are signed by the participants. 28 , 57 A virtual electronic consent form is an alternative approach to traditional written forms. 16 Considering the risk of infection transmission during pandemics, consent can be acquired electronically. Verbal consent for quarantined patients can be obtained first in the presence of a witness, followed by written consent when participants are released from quarantine. Thus, institutions should allocate the necessary resources to develop an appropriate consent form. Facilitating communication between participants, researchers, and institutions can help better collaboration between participants. 57
Many studies are conducted on healthy community populations, and some projects are carried out on sensitive populations and high‐risk patients. Various studies are conducted in the suburbs and villages. These populations are essential in many ways, including dangerous risk factors such as obesity, unemployment, health considerations, and community health. However, the COVID‐19 pandemic prevented these individuals from participating in studies, and effective incentives were needed to encourage participation. 58 , 59 On the other hand, research on sensitive populations was considered dangerous. Addicts, sex workers, and the homeless did not follow many health protocols. Many lived in the same room with several people and did not practice social distancing. These cases could cause the spread of the coronavirus to the researchers and others, endangering the health of the participants and study operators. 18 , 58
Another effective way to attract participants is through financial incentives. Allocating the necessary funding for these incentives is a task that health organizations should notice. Providing essential funding, creating financial incentives, and paying attention to the participants' health can facilitate active participation in the research. 58 A study by Basel showed that statistically significant increases were seen in participants' consent rates and responses when offered even small monetary value incentives. These findings suggest that incentives may be used to reduce the rate of recruitment failure and subsequent study termination 60 (Table 3 ).
Challenges and solutions in clinical research during the COVID‐19 pandemic.
| Challenges | Solutions | |
|---|---|---|
| Issues related to researchers or investigators |
|
|
| Issues related to participants and ethical concerns |
|
|
| Administrative issues |
|
|
| Issues related to research conduction |
| visits
|
3.3. Administrative issues (REC or IRB approval)
3.3.1. rapid review of research.
Thousands of clinical trials were registered in the first few months of the pandemic, facing ethics committees with a high load of studies. A thorough review was necessary to prevent high‐risk and low‐benefit treatments on patients. IRBs had to prioritize specific issues such as inclusion or exclusion criteria, participant compensation, and risk assessments for vulnerable patients to facilitate rapid research review and management of time (Table 3 ).
3.3.2. Ethical issues after IRBs
Despite ethical review board approval, many studies deviated from their protocols due to circumstances during the study. To ensure transparency and efficiency, modifications to pre‐study documents, consent forms, study entry reports, conflict of interest, sponsorship, and side effects had to be reported to the ethics committee. This allowed for transparency and ensures that changes are made appropriately. 15 , 39
3.3.3. Structure and process of IRB
Ethics committees faced the challenge of requiring a multidisciplinary team of experts in virology, infectious diseases, pharmaceuticals, and public health for quick and accurate document review. 28 To address this, committees should prioritize investigator‐initiated trials from a public health perspective and expedite the review of academic trials that address important questions. Regulatory approval processes should be streamlined, redundancies in research design approval processes eliminated, and urgent public health trials facilitated. Experts in different fields can review these indicators. 39 To expedite the approval of interventional studies, having only one national ethics committee review and approve studies is recommended. This approval should be accepted throughout the country without needing re‐approval by another hospital or city's ethics committee 20 (Table 3 ).
3.4. Issues related to research conduction
3.4.1. clinical trials.
Limited access to healthcare facilities and resources significantly impacted research during the COVID‐19 pandemic. Quarantine restrictions affected adherence to clinical study protocols, making it challenging to conduct studies, document procedures, and report adverse events and safety evaluations. This prevented the implementation of numerous clinical studies. Risk assessment was necessary to consider current risks and disadvantages when starting a new study or recruiting trial participants. 12 , 42
The pandemic significantly impacted clinical trials, particularly in cases where patient follow‐ups and randomization were halted, leading to economic losses. Many unnecessary experiments were stopped to prioritize the research with a greater benefit‐to‐harm ratio. 21
Ethically speaking, exposing trial participants to risk is unacceptable if the study is not designed to provide valid results. Therefore, rigorous methodology should be implemented, including randomization, blinding, and placebo use, to enhance scientific validity and societal value. However, in severe epidemics, insisting on randomization can create a conflict between individual health and societal interests, precluding patients' autonomy in choosing their therapy. 66 In a clinical trial conducted during the Ebola epidemic, Perez et al. recommended prioritizing individual patient interests over the reliability of trial methodology when faced with a high risk of death. In a pandemic scenario, a high number of seriously ill patients presenting simultaneously with a high mortality rate make it ethically unacceptable to randomly allocate patients from the same family or location to receive or not receive an experimental drug. Additionally, critically ill patients may find the randomization procedure difficult to understand. 67 It would be unethical and impractical to conduct a randomized controlled trial (RCT) that asks patients or family members to consent to standard care when a potentially beneficial therapy is available. In the LOTUS China, an open‐label RCT, 31 patients' families (8.6%) did not provide consent. For the Ebola trial, investigators conducted one group open‐label non‐randomized trial, where all patients received Favipiravir with standardized care. The investigators used historical mortality data to define efficacy endpoints and a target mortality threshold a priori, which was valuable in deciding whether to stop or continue the trial and guide data analysis and interpretation. This approach could improve the utility of efficacy information from non‐randomized trials. The World Health Organization (WHO) has planned SOLIDARITY, a large global trial of four drugs—Remdesivir, Chloroquine and Hydroxychloroquine, Lopinavir‐Ritonavir, and lopinavir‐ritonavir plus interferon‐beta. Its simple design allowed physicians to recruit confirmed COVID‐19 cases after obtaining informed consent and administer any of the four available drugs as per randomization by the WHO. 68 , 69 , 70
Patient enrolment
One of the problems in conducting research during the COVID‐19 pandemic was patient enrolment. VACCELERATE Volunteer Registry was one of the systems that facilitated the enrolment of patients into studies. VACCELERATE is a comprehensive and coordinated database for conducting and enrolling volunteers for Phase II and Phase III clinical trials. Moreover, this registry can also be expanded to test vaccines on humans in future health emergencies. 43
The pandemic limitations urged new measures for retaining study participants and registering new participants. Strong communication and commitment to participants, creating technological capabilities for teleworking, visits, and delivery of study medication are essential in effectively retaining study participants and recruiting new participants during the COVID‐19 pandemic. 34 , 35 , 52 Facilitating remote patient visits, motivation to perform procedures at the patient's home, permission to use healthcare facilities, direct distribution of the medicine to the patient's home by site personnel or sponsors, and extension of reimbursement to patients and caregivers are solutions that can facilitate the process of clinical studies in pandemic crisis. 20 , 36 , 45 , 63 Online platforms and social media were among the most practical strategies to reduce the imposed limitations. 29 Simmons et al. 46 replaced all in‐person parts of their clinical study using two key technology platforms: Study Pages (Yuzu Labs Public Benefit Corporation, 2022) and Pattern Health (Durham, NC). Recruitment and screening, consent, enrollment, randomization, data collection, blinding, adherence, and retention were performed with these platforms.
While recruiting study subjects can be difficult in typical circumstances, the COVID‐19 pandemic posed additional obstacles for individuals and children seeking to participate in pediatric nursing research. Skeens' study found that using social media to recruit a sample of parent‐child dyads during the COVID‐19 pandemic was an innovative technique. 51 In addition, an original web‐based survey determined that social media was a successful and efficient technique for gathering data on COVID‐19 in a short period of time. 27
Faster research dissemination
In response to COVID‐19, the research community has rapidly adopted a new way of disseminating research. However, unfortunately, the way in which research is being conducted has not changed. There has been an unprecedented surge of COVID‐19‐related preprints and peer‐reviewed publications. While preprint servers and faster peer review processes have clear merits, such as quicker dissemination of results, informing policies, and speeding up the R&D process for COVID‐19 therapeutics and vaccines, the quality of COVID‐19 research has been largely subpar. Many preprints, which are not peer‐reviewed, were rushed to dissemination without sufficient oversight, leading to potential inaccuracies and false claims. 31
Employing virtual platforms
Limited face‐to‐face interactions during the pandemic significantly reduced the number of research visits, and study evaluations, requiring most research visits to be conducted remotely or via phone or video calls. For example, in drug effectiveness studies, by editing the protocols, the study medications could be mailed to the participants instead of in‐person deliveries. 7 , 15
The lack of experience regarding virtual platforms to implement clinical studies also affected the results. Lack of face‐to‐face communication, the reduction of interpersonal interaction between the researcher and the participants, and the accuracy of the acquired information were among the limitations that could cause bias in clinical studies. 39
Each remote data collection method has its advantages and disadvantages that determine its feasibility and acceptability in certain settings. For example, when considering a mobile phone survey, IVR and SMS surveys are more affordable than CATI, but require participants to have high literacy levels. CATI, on the other hand, allows for the inclusion of individuals regardless of their literacy level and provides opportunities for researchers to encourage participation and clarify questions. In low‐ and middle‐income countries, where mobile phone ownership is widespread but access to smartphones and the internet is limited, mobile phone methods are more commonly used and are the focus of this commentary. However, few experts interviewed had implemented or planned online strategies due to their limited reach in certain low‐ and middle‐income countries. Some exceptions include online surveys designed for specific target groups, such as members of established professional associations and university students. 37
3.4.2. Epidemiologic studies
Epidemiological studies, like other studies, have been affected by COVID‐19. During recruitment and longitudinal assessments, epidemiologic studies are susceptible to refusals and losses of follow‐up. In face‐to‐face data collection, researchers adopt strategies such as changing the interviewer or contacting the participant on different days/times to mitigate this issue. However, researchers cannot pinpoint the number of people reached by internet‐based approaches. While some social media platforms, such as Instagram, allow publishers to see how many people were reached by posted advertisements, others, like WhatsApp, do not. Thus, it is not possible to calculate refusal/loss rates. However, sample size calculations should consider a certain percentage of losses and refusals. Therefore, sample size calculations should be conducted before data collection begins, and researchers should devise a recruitment strategy that allows them to reach the previously defined sample. 32
3.4.3. Data analysis
One of the essential component of clinical studies is statistical models methods. 22 Statistical methods are necessary to prevent or minimize the risk of bias, a common threat in clinical and epidemiological studies. Obtaining appropriate clinical information from patients with COVID‐19 in the city of Wuhan was only possible by the epidemiological data. Data Integration and cleaning from large multicenter hospitals are critical and require complex data management. Artificial intelligence (AI) and deep learning algorithms can be crucial in dealing with these challenges. AI and machine‐learning solutions could have a significant impact on fighting the disease. For instance, machine learning techniques have been used intensively in studying different conditions regarding protein analysis, forecasting, prediction, and paving the way towards vaccines and antivirals. An example of such a disease is the seasonal Flu. From this perspective, many AI approaches (including disease forecasting, surveillance, expected peak, and spread models) have been proposed and developed for several diseases, including the seasonal flu, which is relatively similar in its symptoms to COVID‐19. 64
There was also a need for an international committee of statistical experts to decide on statistical methods during the COVID‐19 pandemic. 33 , 71 Additional measures were needed besides the usual strategies for conducting a clinical trial to deal with the mentioned challenges in a pandemic. The conditions of participation, measures needed to prevent infection, and the possibility of withdrawing from the study should be available before making decisions for participants at increased risk of infection. 7 , 24
3.4.4. Research protocols and guidelines during a pandemic
During a pandemic, data security, patient satisfaction, and ethical statements, which are necessary in non‐pandemic situations, can be considered bureaucratic obstacles. However, rapid access to clinical data during epidemic circumstances requires special handling of these matters, which should be discussed nationally. 23 Another statistical challenge during the COVID‐19 pandemic was that many clinical studies were not implemented according to written protocols due to the inability to blind, obtain a high sample size, and randomize. Therefore, statistical methods must be adapted to pandemic conditions. Data should be collected and analyzed in a standardized way, and statisticians are encouraged to develop appropriate analytical strategies for data collected from standardized protocols such as ISARIC and LEOSS. Rapid and valid information flow and reporting are crucial during a pandemic, and long‐lasting reporting guidelines may do more harm than good. Specific reporting guidelines are needed for pandemic settings. 23
Another challenge was related to studies started before the pandemic that were affected by COVID‐19. Challenges included discontinuation of medication, withdrawal of a significant number of participants, deaths due to COVID‐19, and changes in study arms, which were not foreseen and affect study designs. Changing and updating the study protocol, continuing the investigation, and performing sensitivity analysis for missing data can be suitable solutions. 25
3.4.5. High‐risk populations
Research on the elderly population with chronic diseases posed another challenge. To prevent disruptions in research implementation for this population, patient registry systems, improved interactions with other institutions associated with the elderly, and improved study participation conditions such as transportation, health, and safety are necessary. 43 COVID‐19 has also posed one of the biggest challenges for non‐COVID‐19 research on older people. The pandemic has made research challenging to conduct in practice and diverted the time and resources of investigators, funders, regulators, and delivery teams away from non‐COVID‐19 research. Survey data from the British Association of Stroke Physicians showed that most UK stroke research projects had been halted, and all responding sites had seen a substantial decrease in stroke research activity. The economic shock delivered by the pandemic is likely to lead to significant cuts to public and charity budgets worldwide, and it is unclear to what extent this will affect medical research. Even if medical research budgets are preserved, COVID‐19‐related research will likely compete with non‐COVID‐19 research for funding. 26
Funding and financial sponsorship were other prominent issues during the COVID‐19 pandemic. Most funds were devoted to the treatment of patients and protective measures, leading to financial challenges that need to be resolved by organizations and institutions during crises and pandemics. To address this issue, a top‐down decision‐making mechanism was established in the European Union, where adequate funding was quickly provided through the Horizon Europe and ERA4Health budgets. 48
4. DISCUSSION
4.1. main findings.
In this study, we reviewed fundamental challenges in conducting clinical research in the era of COVID‐19 pandemic. Individuals, communities, and societies are facing severe social, physical, and emotional challenges during the COVID‐19 pandemic. Decisions about conduct research using remote methods should consider the research burden and the risks associated with COVID‐19 to study participants.
4.2. Comaprison with previous studies
Remote data collection requires much effort from the study participants, who may need to use their own resources, such as a phone, internet access, and identifying a private space to participate in the study. On the other hand, remote methods may be preferable for study participants and eliminate the time and opportunity costs associated with travel to study sites. As with any research, the potential risks must be weighed against the benefits and the ethical imperative to continue the research to produce evidence useful for public health. 37 Original studies also showed that remote data collection was an effective way to deal with the restrictions created by COVID‐19. Also, original studies determined that using technology like social media was an effective strategy for conducting research. 27 , 51
Key challenges in remote data collection encompass gathering diverse experiences in qualitative research, obtaining a representative sampling frame of the target population in quantitative research, and reaching out to more accessible populations. 63 , 72 While some of these challenges also exist in face‐to‐face research, the limited ability to personally recruit participants, whether at home, in a clinic, or any other locations, along with the reliance on mobile phones for recruitment, poses a specific challenge. This necessitates the exploration of alternative sampling methods for qualitative research, including purposive, snowball, and convenience sampling.
Purposive sampling aims to ensure diversity by considering key factors that are theorized to influence the experience. Recruitment can be facilitated through community‐based organizations, influential community leaders, neighborhood health committees, or established networks. Snowball sampling can be an effective approach for qualitative research; however, it is crucial to involve several initial participants who can then recruit others from within their own networks to achieve the desired diversity. 73 , 74 These sampling methods can also be used in quantitative research. Snowball sampling may be useful for online surveys shared via email or social media platforms, 75 and a convenience sample can be employed through online social networking platforms.
Verbal consent (via phone or voice note) or written consent (via email, WhatsApp, or SMS) is accepted by some ethics committees because written informed consent becomes challenging or impossible during a pandemic. For mobile phone‐based research with adolescents, which requires parental consent, additional challenges arise in verifying the participant's age to determine the adolescent consent. Parents' satisfaction should be examined in line with adolescent satisfaction. For these reasons, verbal consent may be preferred over written consent, which can be recorded or performed in conjunction with written consent. Concise and simple language is required to convey complete information remotely while maintaining the strict ethical standards of face‐to‐face research. Consent should always be documented appropriately while protecting patient information and confidentiality. Documentation can take the form of a list of participants, stored on a password‐protected computer, who have consented to participate in various study components, which can also serve as a record for audit purposes. 37
The privacy and safety of participants are crucial considerations when conducting research. In face‐to‐face studies, it is the responsibility of the researcher to establish and ensure privacy, and data collection must be halted if privacy is compromised. However, the onus is placed on the study participant in remote research to maintain their privacy. Nonetheless, establishing privacy can be challenging when participants share living spaces and have limited access to private areas and time. This becomes particularly significant in studies that examine sensitive topics like gender‐based violence, where compromised privacy can have harmful consequences. 76
To address this issue, it is essential to inform participants about the potentially sensitive nature of the study at the beginning of data collection and encourage them to seek a private space. Strategies such as incorporating “passwords” or “exit buttons” can be implemented to mitigate risks. These mechanisms allow participants to verbally state or click on an option to indicate a breach of privacy. 76 IVR and online surveys allow participants to complete surveys at a time and place of their choice, enabling them to establish privacy more effectively. Furthermore, these surveys can include a question asking respondents whether they completed the survey in private or in the presence of someone else, such as their child, parent/guardian, or friend. 37
Data protection, including end‐to‐end encryption of phone calls and the security of platforms used to deliver online surveys and interview transcripts, is an additional privacy and confidentiality issue that needs to be addressed. 77 In addition, researchers have the duty of care and should carefully consider safeguarding issues, particularly where COVID‐19 has affected the availability of support services. Information about online or telephone services must be available during the consent process. Specific referral protocols should be established, interviewers should be notified if certain responses may trigger automatic referrals, and follow‐up should be provided if safeguarding issues arise. As part of this protocol, researchers must establish a system to regularly check that these services remain operational. 42
4.3. Strengths and limitations
This is the first review to study both the challenges and solutions of conducting clinical research during the COVID‐19 pandemic, providing a practical guide for researchers and policymakers in future similar pandemic conditions. However, this study had some limitations. We had to rely on primary studies, as there was not enough information about the challenges of conducting studies in all types of research. Additionally, the majority of the studies discussed in this article were in the form of editorials, highlighting the need for more rigorous studies to investigate the subject matter further.
Nevertheless, this study has proposed effective solutions that policymakers can consider for implementation in the context of decision‐making for addressing the ongoing pandemic and future crises. Although WHO has declared the end of the COVID‐19 pandemic, 65 this review can still provide valuable information to achieve structured guidelines for researchers in future crises.
5. CONCLUSION
The study findings revealed significant challenges associated with conducting research during the COVID‐19 era. These challenges span various stages, ranging from research inception and study approval to patient enrollment and data analysis. Existing solutions must be adapted to the prevailing circumstances, highlighting the importance of enhancing the underlying research infrastructure to ensure continuity during times of crisis and pandemics. Numerous studies have proposed remote methods and electronic equipment as viable approaches to conduct research. However, the successful implementation of these methods relies on the availability of adequate infrastructure and adherence to country‐specific national and university policies.
AUTHOR CONTRIBUTIONS
Mahin Nomali : Data curation; Formal analysis; Investigation; Validation; Writing—original draft. Neda Mehrdad : Conceptualization; Investigation; Supervision; Validation. Mohammad Eghbal Heidari : Data curation; Investigation; Writing—original draft. Aryan Ayati : Writing—original draft; Writing—review & editing. Amirhossein Yadegar : Writing—review & editing. Moloud Payab : Supervision; Validation. Alireza Olyaeemanesh : Data curation; Investigation. Bagher Larijani : Conceptualization; Project administration; Supervision; Validation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICAL STATEMENT
All the authors declare that the present work has been carried out per the Journal's Practice Guidelines on Publishing Ethics and has been performed ethically and responsibly, with no research misconduct. The article has not been previously published and is not currently submitted elsewhere. The study proposal was passed by the ethical committee of the Iranian Academy of Medical Sciences (IAMS) (ID: IR.AMS.REC.1401.029).
TRANSPARENCY STATEMENT
The lead author Bagher Larijani affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We would like to thank the Iranian Academy of Medical Sciences for approving the study protocol and for the financial support, which make this review possible. This study was supported financially by the Iranian Academy of Medical Sciences (IAMS).
Nomali M, Mehrdad N, Heidari ME, et al. Challenges and solutions in clinical research during the COVID‐19 pandemic: a narrative review . Health Sci Rep . 2023; 6 :e1482. 10.1002/hsr2.1482 [ CrossRef ] [ Google Scholar ]
Mahin Nomali and Neda Mehrdad equally contributed as co‐first authors.
DATA AVAILABILITY STATEMENT
- Systematic Review
- Open access
- Published: 07 July 2024
Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis
- Marcel Ottiger 1 ,
- Iris Poppele 1 ,
- Naveen Sperling 1 ,
- Torsten Schlesinger 1 &
- Katrin Müller 1
BMC Public Health volume 24 , Article number: 1811 ( 2024 ) Cite this article
612 Accesses
369 Altmetric
Metrics details
In addition to several sequelae of post-COVID-19, individuals also experience significant limitations in work ability, resulting in negative consequences for the return-to-work (RTW) process. This systematic review and meta-analysis were conducted to assess the impact of post-COVID-19 on work ability and RTW of individuals previously infected with SARS-CoV-2.
Studies on the work ability and RTW of patients with post-COVID-19 (more than 12 weeks after an acute SARS-CoV-2 infection) were regarded eligible for inclusion. Systematic search of literature was performed up to March 2023 using five databases (MEDLINE, EMBASE, CINAHL, CENTRAL and WHO COVID 19). Study selection followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) Statement. A meta-analysis estimated the overall success rate of RTW. The risk of bias of the included studies was evaluated with the Newcastle Ottawa Scale (NOS).
19 relevant studies, published between 2021 and 2023, were included in the systematic review, involving 21.155 patients from 14 different countries. The findings indicate that a significant proportion of individuals with post-COVID-19 experience persistent symptoms and functional impairments, with fatigue being the most prominent symptom. These persistent symptoms can have a considerable (negative) impact on individuals’ physical and psychological capacity to participate in work-related activities, leading to lower work ability and increased absenteeism. The RTW for post-COVID-19 patients is complex, with approximately 60.9% of patients successfully returning to work after 12 or more weeks following SARS-CoV-2 infection. Among those who successfully returning to work, a considerable number need modifications in their work duties or hours to cope with residual impairments. Factors such as workplace accommodations, supportive policies, and occupational rehabilitation programs play a crucial role in facilitating successful RTW.
Conclusions
The systematic review underscores the substantial impact of post-COVID-19 on work-related outcomes. The implications of this research highlight the need for healthcare providers, employers, and policymakers to collaborate in creating inclusive work environments and implementing tailored rehabilitation programs to support individuals recovering from post-COVID-19. Further research should focus on long-term follow-up studies with mixed methods to gain a more comprehensive understanding of the long-term consequences of post-COVID-19 on work ability and RTW outcomes.
PROSPERO registration number
CRD42023385436.
Peer Review reports
Workplaces were generally a high-risk setting for virus transmission of SARS-CoV-2 due to interpersonal contacts with colleagues, clients or patients [ 1 , 2 ]. Reuter et al. [ 3 ] conducted a study involving more than 100.000 workers across diverse occupational segments such as medical healthcare, as well as business management, and observed an incidence rate of 3.7 infections per 1.000 workers. SARS-CoV-2 infections were higher in essential (180 infections among 33.458 workers) workers compared with workers in non-essential (224 infections among 75.502 workers) occupations (incidence rate ratio 1.95) [ 3 ]. Particularly, healthcare workers were more likely to be affected by COVID-19 (coronavirus disease 2019), compared with other professions [ 4 , 5 , 6 ]. In Germany until October 2023, 350.045 cases of COVID-19 were recognized as occupational diseases (BK) with BK-No. 3101. Furthermore, 26.698 recognized cases of COVID-19 were recorded as work-related accidents (according to the German Social Accident Insurance) [ 7 ]. Acute infection with SARS-CoV-2 can lead to several persistent symptoms [ 8 , 9 , 10 ]. According to the NICE (National Institute for Health and Care Excellence) guideline, persistent signs and symptoms after an acute SARS-CoV-2 infection, which continue for more than 12 weeks and cannot be explained by an alternative diagnosis, are classified as post-COVID-19 [ 11 ].
In population-based cohort studies, the prevalence of ongoing post-COVID-19 symptoms was estimated to be around 6% depending on, for example, virus variants, study design and study population [ 12 , 13 , 14 ]. A pooled analysis of data from 22 countries defined three main post-COVID-19 symptom clusters: persistent fatigue, cognitive problems and ongoing respiratory problems [ 12 ]. This study showed, that 6.2% of over one million individuals, who had symptomatic SARS-CoV-2 infection, experienced at least 1 of the 3 symptom clusters [ 12 ]. In a systematic review, including 70 studies of working age adults, the most frequently reported long-/post-COVID-19 symptoms were fatigue (92%), shortness of breath (82%), muscle pain (44%), and joint pain (35%) [ 15 ]. Moreover, findings from systematic reviews and meta-analyses indicated, that the prevalence of post-COVID-19 symptoms in adults, who were hospitalized due to COVID-19, was substantially higher, than in cases with mild or asymptomatic courses of the disease [ 16 , 17 ]. Studies revealed that both physical as well as neuropsychological limitations can persist for several months (4–24 months) after acute SARS-CoV-2 infection [ 18 , 19 , 20 , 21 , 22 ]. Especially in the case of post-infectious fatigue, a symptom that affected a large number of post-COVID-19 patients, results on long-term courses of other viral and non-viral infectious diseases (e.g., SARS virus, Q-fever, Lyme disease) indicated the risk of chronification [ 23 ].
Patients also reported severe limitations in their ability to work with negative consequences on the RTW process. A systematic review concluded that long- and post-COVID-19 symptoms are increasing problems in occupational medicine, because they influence the RTW-process and quality of life of workers previously hospitalized with SARS-CoV-2 [ 24 ]. Even a mild SARS-CoV-2 infection can result in a significant reduction in work capacity [ 25 ]. Work ability is a multidimensional concept of various factors that enable employees to successfully complete the work tasks [ 26 ]. The interaction of both personal resources (health, psychophysical performance, professional competence, values and attitudes) as well as work requirements (e.g., work conditions) for maintaining individual work ability takes place in the concept of the “House of Workability” [ 27 ]. Previous research showed that a variety of factors influence the work ability of people with long-term diseases, such as work demands, age, gender, comorbidities and somatic complaints [ 28 ]. In addition, poor work ability was also associated with early retirement [ 29 ], a factor that had significant consequences for both the labor market (fewer skilled workers) and the economy (low productivity) [ 30 ]. As we delved into the concept of RTW, this comprehensive understanding became crucial. Importantly, the RTW process hinged on the restoration of work ability, emphasizing the need for employees to recover their physical and mental capacity to work before entering the occupational reintegration phase. Previous studies showed that improved work ability influenced the RTW positively [ 31 , 32 ]. This relation emphasized the interdependence of work ability and the subsequent RTW, which refers to the process of reintegration into the workforce after an extended period of absence, whether due to illness, injury, or other reasons. Accordingly, various factors both at individual (e.g., socioeconomic status, expectations, psychological recourses) as well organizational level (e.g., workplace factors, RTW coordination) can determine successful RTW after an injury or illness [ 33 ]. In the case of post-COVID-19 patients, the RTW process might be complicated by a range of factors, such as ongoing symptoms, and long-term effects of the disease [ 34 ]. Aben et al. [ 35 ] observed variations in the RTW duration influenced by different virus variants. The duration was found to be longest when the alpha variant was predominant and became progressively shorter with the emergence of the delta and omicron variants [ 35 ]. As highlighted by the meta-analysis of Kamdar et al. [ 36 ], conducted before the COVID-19 pandemic, RTW after critical illness is often delayed (36% at 1–3 months, 60% at 12 months) and accompanied by worsening employment status and performance (e.g., fewer work hours).
While the long-term physical and psychological effects of COVID-19 are meanwhile well documented, the potential occupational impact remains to be explored. Understanding the work ability and RTW of patients with post-COVID-19 is crucial for healthcare professionals, occupational health professionals, employers, and policymakers in adapting or developing strategies and interventions to support the recovery and reintegration of these patients into the workforce and to ensure their social participation. It is particularly pivotal for mitigating potential increases in occupational disability and early retirements, thereby playing an essential role in minimizing broader impacts on the labor market and the economy. However, there is currently limited research available on this topic. A systematic review of the available evidence on work ability and RTW of patients with post-COVID-19 is therefore needed to provide a comprehensive analysis of the existing literature and to identify gaps in knowledge and research needs that guide future research. The aim of this systematic review and meta-analysis is to synthesize the evidence on work ability and RTW of patients with post-COVID-19. Specifically, the review will address the following research question:
What is the impact of post-COVID-19 on work ability and the RTW process of patients previously infected with SARS-CoV-2?
By addressing and bridging the gaps in knowledge and synthesizing the available evidence, this systematic review will contribute to systematize the growing body of literature on the post-COVID-19 population and support efforts to mitigate the long-term effects of the pandemic on the global workforce.
This systematic review of the literature was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [ 37 ]. To ensure transparency and reproducibility, the review adheres to the PRISMA checklist, which can be found in Appendix 1 in the supplementary materials. The protocol was registered in the International Prospective Register for Systematic Reviews (PROSPERO) database (Registration number: CRD42023385436).
Search strategy
The PRISMA Statement suggest performing the search across multiple databases; therefore, we have chosen five databases due to their relevance in the medical field. A comprehensive search in MEDLINE (via EBSCO), EMBASE (via Ovid), CINAHL (via EBSCO), Cochrane Central Register of Controlled Trials (CENTRAL) and WHO COVID 19 was conducted from January 2020 until December 2022 to encompass the period since the onset of the COVID-19 pandemic. The search in the five databases was repeated in March 2023 to identify further studies with longer analysis periods. The literature search strategy used MeSH terms (Medical Subject Headings) and text words associated with post-COVID-19 and work ability or RTW. MeSH terms used to perform the search in MEDLINE, were the following:
(“coronavirus” OR “covid-19” OR “sars-cov-2” OR “coronavirus infections” OR “betacoronavirus”) AND (“workplace” OR “return to work” OR “absenteeism” OR “occupational health” OR “work performance” OR “work capacity evaluation” OR “sick leave”).
SIGN search filters were used to identify randomized trials and observational studies in the MEDLINE, CINAHL and EMBASE databases [ 38 ]. In addition, secondary searches in other sources, such as Google Scholar and medRxiv were also carried out, to retrieve relevant publications that were not found with the database search. To ensure that the literature was comprehensive, the reference lists of the included studies or relevant reviews identified through the search, were scanned. Furthermore, a search was carried out in the German Register for Clinical Studies (DRKS) and the International Clinical Trials Registry Platform (ICTRP) search portal of the WHO to identify ongoing, discontinued and completed studies. The full search strategy is presented in Appendix 2 . The literature search was conducted independently by two reviewers.
Eligibility criteria
Studies on the work ability and RTW of patients with post-COVID-19 were regarded eligible for inclusion if the following criteria were fulfilled: (1) population: patients with persistent signs and symptoms more than 12 weeks after an acute SARS-CoV-2 infection; (2) intervention: SARS-CoV-2 infection (diagnosed by RT-PCR, suspected, self-report). Comparison was not applicable due to the aim of the performed review; (3) outcomes: the primary outcome measures of the systematic review were work ability and RTW of patients with post-COVID-19. Since work ability and RTW can be measured in different ways, several methods for outcome collection were accepted for this review, including for example interviews; and (4) following types of studies: interventional studies (e.g., randomised clinical trials) and observational studies (e.g., prospective cohort studies).
The exclusion criteria were as follows: (1) studies involving subjects without SARS-CoV-2 infection; (2) case series, case reports, pilot studies, unpublished data, editorials, news articles, commentaries, studies not involving humans and systematic reviews; and (3) studies published before 2020. This review was restricted to articles written in English or German language.
Study selection
The screening of articles was carried out in two stages [ 39 ]. After duplicates were removed, two persons independently screened the titles and abstracts of references retrieved from the searches and categorized them as relevant, not relevant, or possibly relevant. Studies that clearly did not align with the research objectives, such as those unrelated to COVID-19 work outcomes, were excluded. Studies that were categorized as relevant or potentially relevant by at least one reviewer underwent a full-text-screening. In this second stage the full-text versions of eligible articles were evaluated by both reviewers regarding the inclusion criteria independently. Articles were included if they met the predefined criteria. Any disagreement for inclusion was resolved through discussion between the reviewers and, if necessary, via consultation of the supervising researcher. The screening process was facilitated using Rayyan [ 40 ], which allowed blinding in each step of the process. The selection process is documented in the PRISMA flowchart (Fig. 1 ).

PRISMA flowchart of the article selection process
Data extraction
At the end of the filtering, the extracted features were recorded using a pre-designed data table in Excel. The review authors extracted data from each eligible study. The study team extracted bibliographic data (author, title, year, location and study design), population (age, gender distribution, occupation, comorbidities/risk factors, acute COVID-19 severity), sample size, duration of follow-up, outcome measurements, main results, post-COVID-19 symptoms and results on RTW/ work ability.
Statistical analysis
A random effects proportional meta-analysis [ 41 ] was used to compute a pooled estimate of the RTW rate and respective 95% confidence intervals (CIs). When conducting a meta-analysis on prevalence and encountering heterogeneity in prevalence estimates across studies, it is recommended to employ a random effects model [ 42 ]. This is because a fixed effects model may yield misleading outcomes when significant heterogeneity is present, which is the case in current study. The analysis was performed using the DerSimonian and Laird random-effect [ 43 ]. The statistical heterogeneity between the studies was determined by the τ 2 and I 2 . I 2 indexes of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively [ 44 ]. Also, the corresponding sample sizes were considered. Events classified the number of individuals who successfully achieved RTW, while Total represented the overall number of individuals, from which RTW rates could be derived. Publication bias was evaluated through a visual inspection of funnel plots. Statistical analysis was conducted using R [ 45 ] and RStudio (version 4.3.0) [ 46 ] and statistical significance was set at p < 0.05. The forest plot was generated using the “tidyverse” [ 47 ], “meta” [ 48 ], and “metafor” [ 49 ] packages in R. When conducting the meta-analysis, studies that did not provide data for analysing RTW rates were excluded from the analysis. This ensured that only studies with comprehensive RTW rates were included in the meta-analysis. No subgroup meta-analysis could be performed due to the to the limited number of studies providing data on RTW. A narrative, qualitative summary of the work ability of patients with post-COVID-19 was carried out and is presented in text and table form to summarise and explain the characteristics and findings of the included studies.

Meta-analysis of RTW of post-COVID-19 patients
Risk of bias in individual studies
The risk of bias of the included cohort studies was evaluated with the Newcastle Ottawa Scale (NOS) [ 63 ], modified for cohort and cross-sectional studies [ 22 ]. The NOS’s utilizes a star system. The cohort tool assigns a maximum of 9 stars in 3 domains: (1) selection of study groups (max. 4 stars), (2) comparability (max. 2 stars), and (3) ascertainment of outcome (max. 3 stars). In the cross-sectional tool a maximum of 9 stars for quality assessment across the same 3 domains can be attained: (1) selection of study groups (max. 5 stars), (2) comparability (max. 2 stars), and (3) ascertainment of outcome (max. 2 stars). A higher score indicates a higher quality of the study. The total score could be categorized into three groups: low quality (0–4 stars), moderate quality (5–6 stars), high quality (7–9 stars). The risk of bias was assessed independently by two review authors and results were corroborated, with discrepancies resolved through discussion. Modified NOS’s (see Appendix 4 ) and methodological quality rankings for each study type are provided.
Search results and study selection
The comprehensive search identified a total of 5.967 articles across the five databases (MEDLINE, EMBASE, CINAHL, CENTRAL and WHO COVID 19) and 8 additional records through other sources (see Fig. 1 ). After duplicates were removed, 4.625 references remained for the initial screening by title and abstract. This screening resulted in a total of 4.571 excluded articles. The remaining 54 articles were screened by full text, of which 35 did not meet the inclusion criteria with following reasons: wrong study population ( n = 13), wrong outcome ( n = 10), no full-text available ( n = 6), wrong follow-up time ( n = 2), wrong study design ( n = 2), duplicate ( n = 1) and wrong language ( n = 1). 19 studies met the inclusion criteria and were included in the systematic review [ 18 , 25 , 50 - 62 , 64 - 67 ].
Study characteristics
The characteristics of the 19 studies included are reported in Table 2 . The studies were published between 2021 and 2023. Regarding study design, 12 studies corresponded to cohort studies and 7 to cross-sectional studies. The overall population included 21.155 patients (65.3% female; 34.7% male). Study sample sizes ranged from 42 to 11.955 patients with post-COVID-19 (mean: 1.113). Most studies included middle aged participants (on average 49.15 years of age). The mean follow-up time was 11 months (range 3 months – 24 months). In all, 14 studies included patients treated in the hospital (range: 6.5 − 100%), 8 studies patients treated in intensive care units (ICU) (range: 1.2 − 28.5%) during acute SARS-CoV-2 infection, 3 studies included only non-hospitalized patients and 2 studies did not provide information. The studies were conducted in 14 different countries, of which 5 were implemented in Germany, 3 in Sweden, 2 each in Italy and the United Kingdom and 1 each in Brazil, Canada, Spain, Switzerland, Australia, Denmark and France.
Impact of post-COVID-19 on work ability
Out of the 19 studies included in the review, 15 of them providing data on the impact of post-COVID-19 on work ability. The following analysis summarizes the key findings from each study regarding work status, sick leave, work ability, and limitations in work duties/hours and taking into account different follow-up periods (see Table 1 ).
Follow-up less than 12 months
Many post-COVID-19 patients experienced a prolonged recovery period after COVID-19, leading to temporary or long-term work limitations. Five studies had a follow-up time less than 12 months after the acute SARS-CoV-2 infection. The studies revealed that even individuals with mild or moderate acute SARS-CoV-2 infection required an extended period to recover their pre-illness work capacity. In the study by Davis et al. [ 25 ] 957 (27.3%) participants were able to maintain the same working hours as before the onset of infection. 817 (23.3%) patients were not working 3–7 months after the SARS-CoV-2 infection. Additionally, 1.598 (49.3%) participants were working reduced hours, suggesting some limitations in their work capacity. The cross-sectional study conducted by Nielsen et al. [ 54 ] with a follow-up of approximately 8 months reported a higher percentage of participants working the same hours as before the SARS-CoV-2 infection. Specifically, 39.4% of the participants in the study were able to continue working to the same extent as they did prior to the infection, while 215 out of 401 (53.6%) were on sick leave (84/215 full-time sick leave; 131/215 part-time sick leave). The national registry-based study by Westerlind et al. [ 51 ] involved 11.955 patients (follow-up: 4 months) and reported that 1.592 (13.3%) were on full-time sick leave for post-COVID-19. Kedor et al. [ 53 ] consisted a cross-sectional study of 42 post-COVID-19 patients with a follow-up of 6 months and highlighted that participants had a median Bell disability score of 40 (post-COVID/ME/CFS-group) and 50 (post-COVID/non-ME/CFS-group) indicating limited work ability (reduced working hours or inability to work). Kupferschmitt et al. [ 50 ] and Rutsch et al. [ 52 ] are two studies that evaluated work ability following rehabilitation. Kupferschmitt et al. [ 50 ] examined a sub-sample of 51 post-COVID-19 patients. Out of the 51 individuals assessed, 28 (54.9%) were unable to work on admission. At the time of discharge, 18 participants (35.3%) showed an ability to work at least 6 h per day. Additionally, 6 patients (11.8%) underwent gradual reintegration. Prior to admission, a significant proportion of participants (43.1%) were on sick leave for over 6 months, 13.7% for 3–6 months, and 41% for less than 3 months. 2.0% were not employable. In the study conducted by Rutsch et al. [ 52 ], the rehabilitation took place 5 months after SARS-CoV-2 infection. The study reported that 32% of participants experienced restoration of their work ability after rehabilitation. The mean Work Ability Score (WAS) of the Work Ability Index (WAI) was 4 on a scale of 0–10, indicating some limitations in work capacity. 41% perceive their work ability as permanently at risk. Among the participants, 88 out of 178 (49%) were on sick leave, with an average duration of 21 weeks.
Follow-up between 12 months and 18 months
In 15 studies that provided data on the impact of post-COVID-19 on work ability, there were 6 studies with a follow-up period between 12 months and 18 months. In the study of Buonsenso et al. [ 55 ] with a sample size of 154 participants, the majority (85.7%) maintained the same occupational status as before COVID-19. However, 22 patients (14.3%) experienced a change in their work status with following reasons: sick leave ( n = 7), loss of job due to ill health ( n = 3), shortening of working hours ( n = 3), fired ( n = 1), different reasons ( n = 7). Kisiel et al. [ 56 ] included 158 post-COVID-19 patients followed up for 12 months. Among the 158 participants, 35% were on sick leave during the follow-up period, with an average duration of 8.1 weeks. Patients with persistent symptoms at the 12-month follow-up reported a decrease in work ability. With a follow-up of 13 months, Diem et al. [ 57 ] highlighted that 168 patients (62.7%) were unable to work, and the average sick leave duration was 26.6 weeks. There was a significant association between inability to work and symptoms such as fatigue, pain, and sleep disturbances. Müller et al. [ 58 ] performed a study on a total of 127 patients, and had a median time between SARS-CoV-2 infection and beginning of rehabilitation of 408.81 days. Among the participants, 90 (72.5%) were unable to work after rehabilitation. The majority of patients reported poor (69.8%), 29.3% moderate, and only 0.9% good work ability measured by the WAI. WAI-scores (scale 7–49) before (Median (Mdn): 24.75) and after (Mdn: 24.75) rehabilitation did not show significant changes. The study by Peters et al. [ 59 ] involved a large sample of 1.406 post-COVID-19 patients. The WAS-scores (scale 0–10) decreased from 9.3 before COVID-19 to 6.8 at the time of the survey, indicating a decline in work ability over time. The authors showed that the work ability was significant different between patients with symptoms > 3 months and patients without symptoms. Sansone et al. [ 60 ] conducted a study with 247 participants who were followed up for 15 months. The findings reveal that participants with symptoms lasting 200 or more days (Mean (M): 4.5 ± 1.44) had significantly lower mean work ability-scores (scale 1–6) compared to those with symptoms lasting less than 200 days (M: 5.18 ± 1.08; p < 0,001).
Follow-up more than 18 months
Some individuals experienced a prolonged recovery period after COVID-19, leading to long-term work limitations. Three studies had a follow-up time of 15 months and longer. Delgado-Alonso et al. [ 61 ] involved 77 participants who were followed up for an average of 20.71 months. Out of the participants, 38 (49.4%) were working, while 39 (50.6%) were not working. Among those who were currently not working, 36 (92.3%) were on sick leave. A portion of the participants (16%) reported reduced working hours, and 23% required job adaptation (e.g., more breaks, telework, cognitive aids, or a position change). Factors contributing to work disability include higher levels of fatigue, and lower cognitive performance. The cohort study by van Wambeke et al. [ 62 ] included 45 participants who were followed up for 22 months. Among these participants, 18 (40%) patients were working full-time, 3 (6%) working 60-70% of the time, 8 (18%) working half-time and the remaining individuals (36%) did not RTW. Among the mentioned studies, Wahlgren et al. [ 18 ] conducted the longest follow-up period with 24 months. At the 4-month follow-up, the majority (69.1%) of the participants were working, while a smaller proportion (23.4%) were on sick leave. At the 24-month follow-up, a similar percentage (66 out of 94 patients) were working, and a smaller proportion (16 out of 94 patients; 16%) were on sick leave.
Impact of post-COVID-19 on return-to-work
The meta-analysis included eight studies that examined the RTW outcomes of patients previously infected with SARS-CoV-2 (Fig. 2 ). The random-effects meta-analysis estimated a pooled proportion of 0.609 (95% CI: 0.458–0.751), indicating that approximately 60.9% of post-COVID-19 patients were able to successfully RTW 12 or more weeks following the SARS-CoV-2 infection. In the Forest Plot, the dashed line represents the aggregated average RTW rate across all studies. Studies to the right of this line tend to indicate higher RTW rates, while those to the left suggest lower rates. Among the individual studies, Hodgson et al. [ 67 ] had the highest weight (13.1%) and reported a proportion of 0.886 (95% CI: 0.813–0.938), suggesting a high likelihood of successful RTW. The remaining studies had weights ranging from 11.5 to 13.0% and reported proportions ranging from 0.414 to 0.833. Heterogeneity analysis yielded an I 2 index of 92% and a τ 2 of 0,042 with p < 0.01, indicating substantial variability and inconsistency. Visual inspection of funnel plot asymmetry for the RTW meta-analysis did not suggest the presence of publication bias (Appendix 5 ), and the Peters’ regression test (intercept = 1.111; standard error (SE) = 0.147; p = 0.146) was not statistically significant.
Factors influencing the work ability and return-to-work of post-COVID-19 patients
Based on the information provided from various studies, several influencing factors for work ability and RTW of post-COVID-19 patients could be identified. The duration between symptom onset and the beginning of rehabilitation or treatment influences the likelihood of returning to work [ 65 ]. Early intervention and rehabilitation improve the chances of returning to work. Job adaptations and modified duties, such as reduced working hours, tasks with lower physical or mental strain, telework or flextime can positively affect work ability and facilitate the RTW [ 25 , 65 ]. Economic factors and financial needs can force post-COVID-19 patients to continue working or RTW sooner despite ongoing symptoms [ 25 ]. An individuals’ work ability can be significantly impacted by various psychological factors, among which include high levels of fatigue, depressive symptoms, and reduced cognitive performance. These factors are closely linked with diminished work capacity and overall effectiveness in the workplace [ 61 ]. In addition, Diem et al. [ 57 ] reported, that inability to work is commonly reported alongside symptoms such as fatigue, sleep disturbances, and pain. These symptoms act as significant barriers for post-COVID-19 patients, impeding their ability to engage in work-related activities and having a negative impact on overall performance and productivity. The presence of fatigue is also associated with a lower likelihood of returning to previous work hours [ 66 ]. Additionally, certain demographic and health-related factors have been associated with higher odds of not returning to work after SARS-CoV-2 infection. According to the study conducted by Westerlind et al. [ 51 ], factors such as older age, being male, having a history of sick leave before contracting COVID-19, and having received inpatient care are all associated with an increased probability of not returning to work. Overall, the influencing factors for work ability and RTW of post-COVID-19 patients are diverse and can vary between individuals, interacting in complex ways to determine work outcomes.
Post-COVID-19 symptoms
Studies [ 61 , 62 , 63 , 64 , 65 , 66 ] have underscored the impact of post-COVID-19 symptoms on an individual’s work ability and RTW. Therefore, it is crucial to outline the prevalent post-COVID-19 symptoms reported in the included studies. 13 studies investigated self-reported post-COVID-19 symptoms in COVID-19 patients 12 or more weeks following diagnosis. The studies reported on a wide range of post-COVID-19 symptoms experienced by patients. Appendix 3 presents the five most commonly reported symptoms in the included studies, along with their respective prevalence rates. The prevalence of post-COVID-19 symptoms varied across studies, with estimates ranging from 12.2 to 100% of individuals who had recovered from the acute phase of the illness. Fatigue was the most commonly reported symptom, with prevalence rates exceeding 80% in many studies (mean prevalence: 72.9%). Other frequently reported symptoms included neurocognitive disorders such as concentration impairment, dizziness or memory problems. Estimates of neurocognitive symptoms prevalence ranged from 14 to 92% (mean prevalence: 59.5%). Most of the studies also reported physical ailments such as weakness, muscle pain or exercise intolerance with prevalence rates between 13% and 100% (mean prevalence: 56.2%). Other frequently reported symptoms included shortness of breath, headache, and sleep disturbances. Long-term follow-up studies indicated that patients with post-COVID-19 continued to experience symptoms for up to two years after the initial infection [ 18 , 60 , 61 , 62 ]. For a comprehensive understanding of the full range of post-COVID-19 symptoms, it is recommended to refer to the original studies included in this systematic review.
Risk of bias
Of the 19 studies, more than half were assessed to be of moderate quality ( n = 10). Five studies were considered to be of high quality, and the remaining studies ( n = 4) were considered to be of poor quality. Taken together, the NOS rating of the component studies was moderate, evidenced by mean scores of 6.2 for cohort studies and 4.8 for cross-sectional studies. The NOS quality assessment results for cohort studies are summarized in Table 3 , and quality assessment results for cross-sectional studies are summarized in Table 4 .
Within the cohort studies ( n = 12), nearly all studies scoring a star for being either truly or somewhat representative of the average target population. Common methodological limitations were the failure to include a non-exposed group in cohort studies, and to ascertain whether outcomes were present prior to SARS-CoV-2 infection. The exposure (COVID-19) was usually measured using either objective measurement (e.g., polymerase chain reaction (PCR) test) or clinical judgment. Within cross-sectional studies ( n = 7), all but two studies had somewhat representative or truly representative samples (with selection bias). However, nonresponse characteristics (with non-response/self-selection bias); and a sample size justification were not provided or poorly described in all of the cross-sectional studies. 5 out of 7 studies used validated measurement tools.
Comparability
Cohort studies controlled for confounders in 12 of the 13 studies. However, only two studies controlled for age, sex, and an additional factor required to score two stars. One study scored zero stars, as it used unadjusted analyses. In the cross-sectional studies, 5 studies used adjusted analyses.
Within the cohort studies, all studies used a validated objective assessment tool (e.g., WAI) or a structured/systematic interview conducted by a trained healthcare/research professional and were followed up after a sufficient duration (3 months). The follow-up cohort rate was inadequate in 3 studies, as no description of differences in responders and non-responders was provided, or less than 80% responded. Within the cross-sectional studies, 3 studies used a validated objective assessment tool or a structured/systematic interview conducted by a trained healthcare/research professional; therefore, they scored a star. 3 studies scored zero stars, as they used self-reported work ability measurements. All of the cross-sectional studies were considered to have used appropriate and clearly described statistical tests.
The present systematic review aimed to assess the impact of post-COVID-19 on work ability and the RTW of patients previously infected with SARS-CoV-2. Through a comprehensive analysis of the available literature, we have identified several key findings that shed light on the long-term consequences of COVID-19 on individuals’ ability to work and their journey back to the workforce. The comprehensive search and rigorous study selection process resulted in the inclusion of 19 relevant studies published between 2021 and 2023, involving a diverse population of 21.155 patients from 14 different countries.
Work ability and return-to-work
An essential determinant of a sustainable RTW is the perceived work ability, which is more independent of the patient’s specific context compared to the aspects of returning to work [ 68 , 69 ]. The impact of post-COVID-19 on work ability was assessed in 15 of the 19 included studies. The findings varied depending on the follow-up period. In studies with a follow-up period of less than 12 months, it was found that many post-COVID-19 patients experienced a prolonged recovery period, resulting in temporary or long-term work limitations. A significant proportion of patients were not working or were working reduced hours (range between 13.3% and 54.9%). Even those with mild or moderate acute SARS-CoV-2 infection required extended periods to regain their pre-illness work capacity [ 25 ]. This indicates that a significant proportion of patients face challenges in resuming their work responsibilities. However, some participants were able to maintain their pre-illness work capacity. Also, in studies with a follow-up period between 12 months and 18 months, a decline in work ability over time was observed. The percentage of patients unable to work or on sick leave was relatively high in these studies (14.3 − 67.7%). Work ability scores decreased compared to pre-COVID-19 levels, indicating limitations in work capacity. For studies with a follow-up period of more than 18 months, some individuals experienced long-term work limitations. The percentage of patients working varied across studies, with a range of 40.0–70.2%. However, it is important to note that these patients may also experience a decline in work ability, resulting in reduced work productivity. Lemhöfer et al. [ 70 ] demonstrated that more than half of the post-COVID-19 patients who were able to work experienced impairments in the physical sum score of Health-Related Quality of Life (HRQoL), resulting in reduced productivity. In this context, the concept of presenteeism gains relevance.
Presenteeism involves individuals continuing to work despite being unwell, and exerting extra efforts to manage job demands, which can exacerbate health problems [ 71 ]. The estimated costs of having a sick employee could potentially be higher than the costs of their actual absence, due to lower productivity and if illnesses become worse and chronic as a result which is associated with longer periods of absence from work [ 30 , 72 ]. This becomes especially notable as a substantial proportion of participants in this systematic review remained on sick leave or needed job adaptation for 15 months or longer after the SARS-CoV-2 infection.
Fatigue remained a prevalent symptom even in the long term, which could significantly impact an individual’s ability to perform daily work tasks and maintain productivity. Especially in the case of post-infectious fatigue, a symptom that affects a considerable number of post-COVID-19 patients, results concerning the long-term course of other infectious diseases (e.g., SARS virus, Q-fever, Lyme disease ) indicate the risk of chronicity [ 23 ]. Other frequently reported symptoms, such as neurocognitive disorders, physical ailments, and sleep disturbances also contributed to work limitations and challenges in returning to work [ 57 , 61 ]. Delgado-Alonso et al. [ 61 ] confirmed, that there was a wide variability of influencing post-COVID-19 symptoms on work ability among the participants. Similarly, results from Pauwels et al. [ 34 ] and Sanchez-Ramirez et al. [ 20 ] indicated, that the impact on work ability and RTW for patients with long- and post-COVID-19 is complex and varies due to the different symptomatology, disease severity during the acute infection and age. Rehabilitation can play a central role in restoring the ability to work after a SARS-CoV-2 infection [ 73 ]. According to national [ 74 , 75 ] and international guidelines [ 76 ], a specific post-COVID-19 rehabilitation program is recommended to contribute to the preservation and restoration of biopsychosocial health and work ability. Positive rehabilitation effects have been demonstrated for both physical and mental health in patients with post-COVID-19 [ 77 , 78 ]. After regaining work ability through rehabilitation and ongoing aftercare, the process of occupational reintegration is essential.
The RTW of post-COVID-19 patients is complex and multifaceted. The meta-analysis estimated that approximately 60.9% of post-COVID-19 patients were able to successfully RTW 12 or more weeks following the SARS-CoV-2 infection. This finding highlights the importance of understanding the long-term impacts of COVID-19 on individuals’ ability to return to their pre-infection work status. However, there was substantial heterogeneity among the studies. This suggests that differences in methodologies such as study populations, follow-up durations, and other factors might have contributed to the variability in RTW outcomes observed across the studies.
Moreover, it is crucial to recognize that this prevalence figure represents just the endpoint of a much more complex process of RTW. RTW is not a straightforward, linear process. It often involves multiple stages, including gradual reintegration, adaptations, and even job changes [ 34 ]. The coexistence of comorbidities alongside post-COVID-19 can increase the complexity of the RTW process [ 51 ]. Additionally, psychosocial factors like anxiety, depression and stress can have a significant impact, leading to delays, problems, or even making it necessary to change jobs [ 79 ]. Individual differences in resilience, coping strategies and self-efficacy are key factors in how they handle the disease and navigate the RTW process [ 80 , 81 ]. Some may adapt more effectively, while others may face challenges.
The findings in this systematic review reveal that environmental and organisational factors such as the availability of workplace accommodations, supportive policies, and occupational rehabilitation programs play a crucial role in facilitating successful RTW. Workplaces that offer flexible work arrangements, including modified duties, reduced working hours and remote work options were positively related with the reintegration process [ 25 , 65 ]. This is particularly relevant for healthcare workers who face high work demands and workplace stress [ 82 ]. Therefore, the possibilities for adjusting workplace conditions within the company should be given more emphasis, especially to facilitate RTW for post-COVID-19 patients with extended periods of work disability. Moreover, collaboration between healthcare providers, employers, and employees was emphasized as crucial in developing personalized RTW plans tailored to individuals’ specific needs and capabilities [ 83 ]. In order to facilitate the RTW of patients with post-COVID-19, it is necessary to develop a long-term strategy [ 83 ]. Strategies for returning to work after SARS-CoV-2- infection may be similar to programs already developed for chronic diseases [ 84 , 85 , 86 ]. It is important to recognize that the RTW for COVID-19 survivors may be influenced by a multitude of factors, including the severity of the infection, the presence of long-term symptoms, individual resources such as resilience or self-efficacy, the socio-economic context and the nature of their occupation. Therefore, a comprehensive understanding of these factors is necessary to facilitate the successful reintegration of COVID-19 survivors into the workforce. This is in line with results by Pauwels et al. [ 34 ] indicated, that the impact on return to the workplace for patients with long-COVID and post-COVID-19 is complex and varies due to the different symptomatology. Economic aspects such as continued wage payment during illness must also be taken into consideration. The financial losses resulting from extended absences are not sustainable for some patients in the long term and can lead to psychological disorders, e.g., depression disruption of financial wellbeing up to existential fears [ 87 ]. The risk of not achieving a successful RTW increases significantly with the duration of absence. Approximately 50% of individuals are unable to RTW after a sick leave of six months [ 88 ]. In the study by Wahlgren et al. [ 18 ], it was also found that out of the 22 patients who were on sick leave four months after the SARS-CoV-2 infection, only 11 patients managed to achieve a RTW after 24 months. The process of returning to work itself can contribute significantly to the recovery from SARS-CoV-2 infection. When individuals successfully adapt the requirements of their workplace to accommodate their existing limitations, employment can serve as an effective way to improve overall performance and reduce mental stress [ 89 , 90 ]. It’s important to mention that aftercare and support or self-help groups also play an important role in assisting individuals during their RTW process. They take care of the medical, emotional as well as mental and social aspects of the individuals and can create a sense of togetherness and social inclusion.
The systematic review highlights that a significant proportion of individuals who have recovered from COVID-19 experience persistent symptoms and functional impairments that can impact their work ability. Identifying and presenting these symptoms not only provided a clear insight into the challenges individuals face but also contributes to developing interventions and support measures to reduce the long-term effects of COVID-19 on work ability. Neurocognitive disorders (e.g., concentration impairment or dizziness), physical ailments (e.g., weakness or exercise intolerance), shortness of breath, headache, and sleep disturbances were commonly reported symptoms among these individuals. Fatigue emerged as the most prominent symptom, with prevalence rates exceeding 80% in many studies. The occurrence of fatigue is consistent with systematic reviews on post-COVID-19 [ 8 , 22 ], indicating that persistent fatigue is a common and debilitating symptom for many individuals recovering from SARS-CoV-2 infection. According to a previous study, 40% of SARS survivors experienced chronic fatigue for an average duration of 41 months following the infection [ 91 ].
Persistent symptoms can have a significant impact on the physical and psychological capacity of post-COVID-19 patients to participate in work-related activities, resulting in lower work ability and increased sick leave. Residual impairments lasting months after the acute SARS-CoV-2 infection could also explain why some of the people returning to work required modifications in their work duties or hours. This is supported by Böckermann et al. [ 92 ], who demonstrated that poor health status is linked with a higher rate of unemployment. Lemhöfer et al. [ 70 ] already revealed, that 38% of the patients were unable to work and showed impairments in physical and mental health 3–12 months after the SARS-CoV-2 infection. Especially cognitive and physical limitations, as well as existing fatigue symptoms, were associated with reduced work capacity [ 93 , 94 ]. Similar associations were also demonstrated in studies included in this systematic review [ 57 , 61 ].
It’s important to note that these findings are based on the available studies, and individual experiences may vary. Additionally, the long-term effects of COVID-19 on work ability are still being researched, and further studies may provide additional insights.
Implications
The results of this systematic review and meta-analysis generate a lot of implications for healthcare providers, occupational health professionals, employers, and policymakers. It highlights the need for a comprehensive understanding of the post-COVID-19 symptoms and their impact on work ability. Healthcare professionals should be aware of the potential long-term consequences of COVID-19 and consider appropriate rehabilitation as well as aftercare and support services to help individuals RTW. Early identification of post-COVID-19 symptoms and timely interventions may significantly improve the work ability, overall well-being of patients and the successful reintegration into the workplace. Brehon et al. [ 65 ] showed, that patients with a shorter time between the onset of SARS-CoV-2 infection and admission to a rehabilitation program had a higher likelihood of RTW. It is important to note that the long-term effects of COVID-19 on work ability and the RTW process are not limited to physical symptoms alone. The systematic review highlights the significant impact of neurocognitive health challenges, such as concentration impairment, dizziness or memory problems, on individuals’ ability to work and return to the workforce. Therefore, comprehensive support systems encompassing both physical as well as neurocognitive health interventions under consideration of individual load limitations are crucial for optimizing work outcomes in post-COVID-19 patients. Rehabilitation plays a central role in restoring workability and reintegrating into the professional routine after SARS-CoV-2 infection [ 73 ]. In accordance with national [ 74 , 75 ] and international guidelines [ 76 ], a specifically designed post-COVID-19 rehabilitation aims to contribute to the preservation and recovery of biopsychosocial health and work capability within the rehabilitation management of long-/post-COVID-19 patients.
Employers may need to implement flexible work arrangements, offering support services and provide reasonable accommodations for employees recovering from SARS-CoV-2 infection to facilitate their RTW and foster a more supportive and inclusive work environment. The possibilities of adapting working conditions within the company should be given more attention, especially to enable an optimal reintegration into the profession and achieve a RTW for post-COVID-19 patients with prolonged work disability periods [ 65 , 83 ]. Employers should also be educated about the potential challenges faced by post-COVID-19 patients and the importance of providing appropriate resources and support to aid in their recovery and successful RTW. Policy initiatives could focus on ensuring that post-COVID-19 patients have access to necessary healthcare and rehabilitation services, and protections against discrimination in the workplace due to COVID-19 related symptoms. Furthermore, continuous monitoring of the potential increase in the number of people opting for early retirement due to post-COVID-19 is crucial in the upcoming years, as reduced work ability serves as a predictor for such decisions [ 29 ].
Limitations
While the systematic review provides valuable insights into the impact of post-COVID-19 on work ability and the RTW process, there are some limitations to consider. The available literature is still evolving, and the studies included in this review vary in their methodologies, leading to heterogeneity, and thus, difficulties of comparison. Additionally, there is a scarcity of long-term follow-up studies, making it challenging to ascertain the impact of post-COVID-19 on work ability and RTW. Future research should focus on longer follow-up periods to better understand how post-COVID-19 symptoms change over time and affect people’s ability to work. Large and long-term cohort studies incorporating mixed methods, encompassing both qualitative as well as quantitative approaches, are essential for gaining comprehensive insights into the long-term consequences of COVID-19. These studies allow a more differentiated understanding of the multifaceted impacts of the disease on individuals’ work ability and RTW and should aim for diverse representation in terms of age, gender, severity of acute COVID, occupation, and geographical locations. Supplementing quantitative approaches with qualitative research can offer a better understanding of the lived experiences and challenges faced by individuals recovering from post-COVID-19. Previous research has descriptively analysed work ability and RTW as a secondary parameter over mostly short periods of time. No articles were found that analysed the impact of post-COVID-19 on work ability and RTW as their primary objective. Data about work ability and RTW was collected from different questionnaires (Bell Disability Scale, World Health Organization Disability Assessment Schedule, Work ability index), open questions, online surveys or work ability scales. The studies primarily relied on self-reported work ability, which may introduce biases and problems such social desirability. Moreover, there were no articles found describing a validated screening tool for post-COVID-19. Employing standardized assessment tools for work ability and RTW outcomes will enhance the reliability and comparability of findings across studies.
There is heterogeneity across the studies with respect to the selection of participants, the assessment of outcomes, follow-up periods and sample sizes in almost all the studies which may influence the generalizability of the results of this study. Despite these limitations, the authors maintain that this systematic review significantly addresses the knowledge gap regarding the impact of post-COVID-19 on work ability and RTW.
The risk of bias assessment indicated that more than half of the included studies were of moderate quality. Common limitations included the failure to include a non-exposed group in cohort studies and inadequate control for confounders. In cross-sectional studies, nonresponse characteristics and sample size justifications were often poorly described. The Quality Assessment of the included studies using the Newcastle-Ottawa Scale has revealed certain consistency problems and its reliability relies on the expertise of the operator [ 95 , 96 ]. Consequently, if conducted by a different research group, the quality assessment might have yielded varying results.
Another notable constraint of this systematic review is the restriction to articles published only in English or German. This might result in the exclusion of relevant studies published in other languages, limiting the reviews’ overall comprehensiveness.
The majority of studies included hospitalized patients, potentially impacting the generalizability of our findings to the broader population of individuals with post-COVID-19. This focus might introduce bias due to the higher prevalence of comorbidities among hospitalized patients [ 97 ]. However, previous studies demonstrated that individuals, even with mild SARS-CoV-2 infections, developed post-COVID-19 symptoms [ 98 , 99 ], underscoring the importance of considering varying disease severities. To improve our understanding, future studies should include more non-hospitalized individuals, providing a more balanced perspective on the post-COVID-19 landscape.
In addition to the previously mentioned limitations, one crucial aspect that most of the included studies overlooked is the consideration of the specific COVID-19 variants by which the patients were infected. This oversight prevents us from comprehensively understanding the potential differential effects of various variants on individuals’ ability to RTW after recovering from SARS-CoV-2 infection. Notably, most studies with a large sample size were conducted in the early stages of the pandemic when the alpha and delta variants were predominant. However, the study by Aben et al. [ 35 ] provided the insight that later virus variants (e.g., Omicron) demonstrated a shorter duration between infection and RTW. This crucial finding does not receive adequate attention within the context of this systematic review.
Unanswered questions
In addition to detailing a number of methodological considerations, this review has also highlighted gaps in the literature. The following unanswered questions will help focus future research:
How does the individual recovery of employees’ ability to work with post-COVID-19 look like?
How do processes of occupational reintegration post-COVID-19 employees look like, and which (promoting and hindering) factors are relevant?
What problems arise during the recovery process to the ability to work, and how do employees deal with such problems? What coping strategies do they develop and apply?
What influence do physical and psychological resources have on the ability to work of employees with post-COVID-19?
What role does the social as well as the workplace environment (considering the broader context of the International Classification of Functioning, Disability, and Health) play in the recovery of employees’ ability to work?
How do demographic factors impact the ability to work of individuals with post-COVID-19, and what tailored interventions can be developed to address specific needs based on demographic diversity?
What are the different subtypes of post-COVID-19, and how can a deeper understanding of the various clinical manifestations post-COVID-19 enhance the effectiveness of RTW interventions?
To what extent do specific interventions such as rehabilitation programmes, aftercare and support services for employees with post-COVID-19 result in improvements of ability to work and faster RTW?
This systematic review and meta-analysis provide valuable insights into the impact of post-COVID-19 on work ability and the RTW. The findings underscore the need for comprehensive support for individuals recovering from SARS-CoV-2 infection to improve their work capacity and overall quality of life. However, the influence of post-COVID-19 on the working-age population seems to be substantial, and it is expected to result in enduring strains on economic and healthcare systems. Policymakers, healthcare providers, occupational health professionals and employers need to collaborate to create inclusive work environments and implement tailored rehabilitation and aftercare programs that improve the work ability of post-COVID-19 patients in long-term. Future research should focus on long-term follow-up studies with mixed methods (qualitative and quantitative) to gain a more comprehensive understanding of the trajectory of post-COVID-19 symptoms and their impact on work outcomes and to identify effective interventions to facilitate the RTW for affected individuals.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
Six-minute walking distance 6MWD
Confidence intervals
Coronavirus disease 2019
German Register for Clinical Studies
Health-Related Quality of Life
International Clinical Trials Registry Platform
Intensive care units
Interquartile range
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
International Prospective Register for Systematic Reviews
- Return-to-work
Polymerase chain reaction
Medical Subject Headings
National Institute for Health and Care Excellence
Newcastle Ottawa Scale
Standard deviation
Standard error
Work ability index
Work ability score
Khalatbari-Soltani S, Cumming RC, Delpierre C, Kelly-Irving M. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J Epidemiol Community Health. 2020;74(8):620. https://doi.org/10.1136/jech-2020-214297
Article PubMed Google Scholar
Burdorf A, Porru F, Rugulies R. The COVID-19 pandemic: one year later - an occupational perspective. Scand J Work Environ Health. 2021;47(4):245–7. https://doi.org/10.5271/sjweh.3956
Reuter M, Rigó M, Formazin M, Liebers F, Latza U, Castell S, et al. Occupation and SARS-CoV-2 infection risk among 108 960 workers during the first pandemic wave in Germany. Scand J Work Environ Health. 2022;48(6):446–56. https://doi.org/10.5271/sjweh.4037
Article PubMed PubMed Central Google Scholar
Alshamrani MM, El-Saed A, Al Zunitan M, Almulhem R, Almohrij S. Risk of COVID-19 morbidity and mortality among healthcare workers working in a large Tertiary Care Hospital. Int J Infect Dis. 2021;109:238–43. https://doi.org/10.1016/j.ijid.2021.07.009
Article CAS PubMed PubMed Central Google Scholar
Wachtler B, Neuhauser H, Haller S, Grabka MM, Zinn S, Schaade L, et al. The risk of infection with SARS-CoV-2 among Healthcare workers during the pandemic. Dtsch Arztebl Int. 2021;118(49):842–3. https://doi.org/10.3238/arztebl.m2021.0376
Ferland L, Carvalho C, Gomes Dias J, Lamb F, Adlhoch C, Suetens C, et al. Risk of hospitalization and death for healthcare workers with COVID-19 in nine European countries, January 2020-January 2021. J Hosp Infect. 2022;119:170–4. https://doi.org/10.1016/j.jhin.2021.10.015
Deutsche Gesetzliche Unfallversicherung (DGUV). Berufskrankheiten und Arbeitsunfälle im Zusammenhang mit COVID-19. 2023. https://www.dguv.de/medien/inhalt/mediencenter/hintergrund/covid/dguv_zahlen_covid.pdf . Accessed: 15.11.2023.
Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(5):657–66. https://doi.org/10.1016/j.cmi.2022.01.014
Almas T, Malik J, Alsubai AK, Jawad Zaidi SM, Iqbal R, Khan K, et al. Post-acute COVID-19 syndrome and its prolonged effects: an updated systematic review. Ann Med Surg (Lond). 2022;80:103995. https://doi.org/10.1016/j.amsu.2022.103995
Yuan N, Lv ZH, Sun CR, Wen YY, Tao TY, Qian D, et al. Post-acute COVID-19 symptom risk in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Front Public Health. 2023;11:1112383. https://doi.org/10.3389/fpubh.2023.1112383
Sivan M, Taylor S. NICE guideline on long covid. Br Med J Publishing Group. 2020;371. https://doi.org/10.1136/bmj.m4938
Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, Ballouz T, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–15. https://doi.org/10.1001/jama.2022.18931
Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13(1):3528. https://doi.org/10.1038/s41467-022-30836-0
Peter RS, Nieters A, Kräusslich HG, Brockmann SO, Göpel S, Kindle G, et al. Post-acute sequelae of covid-19 six to 12 months after infection: population based study. BMJ. 2022;379:e071050. https://doi.org/10.1136/bmj-2022-071050
Kokolevich ZM, Crowe M, Mendez D, Biros E, Reznik JE. Most common long COVID physical symptoms in working age adults who experienced mild COVID-19 infection: a scoping review. Healthc [Internet]. 2022;10(12). https://doi.org/10.3390/healthcare10122577
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of Post-coronavirus Disease 2019 (COVID-19) Condition or Long COVID: a Meta-analysis and systematic review. J Infect Dis. 2022;226(9):1593–607. https://doi.org/10.1093/infdis/jiac136
Article CAS PubMed Google Scholar
O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of long covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine. 2023;55. https://doi.org/10.1016/j.eclinm.2022.101762
Wahlgren C, Forsberg G, Divanoglou A, Östholm Balkhed Å, Niward K, Berg S, et al. Two-year follow-up of patients with post-COVID-19 condition in Sweden: a prospective cohort study. Lancet Reg Health Eur. 2023;100595. https://doi.org/10.1016/j.lanepe.2023.100595
Liao T, Meng D, Xiong L, Wu S, Yang L, Wang S, et al. Long-Term effects of COVID-19 on Health Care workers 1-Year Post-discharge in Wuhan. Infect Dis Ther. 2022;11(1):145–63. https://doi.org/10.1007/s40121-021-00553-0
Sanchez-Ramirez DC, Normand K, Zhaoyun Y, Torres-Castro R. Long-term impact of COVID-19: a systematic review of the literature and Meta-analysis. Biomedicines. 2021;9(8). https://doi.org/10.3390/biomedicines9080900
Morioka S, Tsuzuki S, Maruki T, Terada M, Miyazato Y, Kutsuna S, et al. Epidemiology of post-COVID conditions beyond 1 year: a cross-sectional study. Public Health. 2023;216:39–44. https://doi.org/10.1016/j.puhe.2023.01.008
Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. https://doi.org/10.1016/j.bbi.2021.12.020
Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue: Biomed Health Behav. 2020;8(2):61–9. https://doi.org/10.1080/21641846.2020.1778227
Article Google Scholar
Gualano MR, Rossi MF, Borrelli I, Santoro PE, Amantea C, Daniele A, et al. Returning to work and the impact of post COVID-19 condition: a systematic review. Work. 2022;73(2):405–13. https://doi.org/10.3233/wor-220103
Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. https://doi.org/10.1016/j.eclinm.2021.101019
Ilmarinen J, Tempel J. Arbeitsfähigkeit 2010 - was können wir tun, damit sie gesund bleiben? Hamburg: VSA-; 2002.
Google Scholar
Ilmarinen J. Towards a longer worklife! Ageing and the quality of Worklife in the European Union. Z für Arbeits- und Organisationspsychologie A&O. 2006;52. https://doi.org/10.1026/0932-4089.52.1.47
Slebus FG, Kuijer PP, Willems JH, Sluiter JK, Frings-Dresen MH. Prognostic factors for work ability in sicklisted employees with chronic diseases. Occup Environ Med. 2007;64(12):814–9. https://doi.org/10.1136/oem.2006.031807
Lundin A, Kjellberg K, Leijon O, Punnett L, Hemmingsson T. The Association between Self-assessed future work ability and long-term sickness absence, disability pension and unemployment in a General Working Population: a 7-Year Follow-Up study. J Occup Rehabil. 2016;26(2):195–203. https://doi.org/10.1007/s10926-015-9603-4
Gandjour A, Long COVID. Costs for the German economy and health care and pension system. BMC Health Serv Res. 2023;23(1):641. https://doi.org/10.1186/s12913-023-09601-6
Rashid M, Heiden M, Nilsson A, Kristofferzon ML. Do work ability and life satisfaction matter for return to work? Predictive ability of the work ability index and life satisfaction questionnaire among women with long-term musculoskeletal pain. BMC Public Health. 2021;21(1):584. https://doi.org/10.1186/s12889-021-10510-8
Sviridova O, Michaelson P. Predictors for return to work after multimodal rehabilitation in persons with persistent musculoskeletal pain. Edorium J Disabil Rehabilitation. 2018;4:4. https://doi.org/10.5348/100038D05SO2018OA
Cancelliere C, Donovan J, Stochkendahl MJ, Biscardi M, Ammendolia C, Myburgh C, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Th. 2016;24(1):32. https://doi.org/10.1186/s12998-016-0113-z
Pauwels S, Boets I, Polli A, Mylle G, De Raeve H, Godderis L. Return to work after long COVID: Evidence at 8th March 2021. 2021. https://www.hse.gov.uk/research/assets/docs/return-to-work-after-long-covid.pdf . Accessed: 16.11.2023.
Aben B, Kok RN, de Wind A. Return-to-work rates and predictors of absence duration after COVID-19 over the course of the pandemic. Scand J Work Environ Health. 2023;49(3):182–92. https://doi.org/10.5271/sjweh.4077
Kamdar BB, Suri R, Suchyta MR, Digrande KF, Sherwood KD, Colantuoni E, et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 2020;75(1):17. https://doi.org/10.1136/thoraxjnl-2019-213803
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
Scottish Intercollegiate Guidelines Network (SIGN). Search filters. 2021. https://www.sign.ac.uk/what-we-do/methodology/search-filters/ . Accessed: 15.11.2023.
Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Metzendorf M-I et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editorsOctober. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated 2023). Cochrane, 2023.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Reviews. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4
Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21(1):189. https://doi.org/10.1186/s12874-021-01381-z
Wang K-S, Liu X. Statistical methods in the meta-analysis of prevalence of human diseases. J Biostatistics Epidemiol. 2016;2(1). doi.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557
R Core Team. R: A Language and Environment for Statistical Computing. 2023. https://www.r-project.org/ . Accessed: 21.11.2023.
Posit team. RStudio: Integrated Development Environment for R. 2023. http://www.posit.co/ . Accessed: 21.11.2023.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43). https://doi.org/10.21105/joss.01686
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. https://doi.org/10.1136/ebmental-2019-300117
Viechtbauer W. Conducting Meta-analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. https://doi.org/10.18637/jss.v036.i03
Kupferschmitt A, Langheim E, Tüter H, Etzrodt F, Loew TH, Köllner V. First results from post-COVID inpatient rehabilitation. Front Rehabil Sci. 2023;3:1093871. https://doi.org/10.3389/fresc.2022.1093871
Westerlind E, Palstam A, Sunnerhagen KS, Persson HC. Patterns and predictors of sick leave after Covid-19 and long Covid in a national Swedish cohort. BMC Public Health. 2021;21(1):1023. https://doi.org/10.1186/s12889-021-11013-2
Rutsch M, Frommhold J, Buhr-Schinner H, Gross T, Schüller PO, Deck R. Pneumologische Rehabilitation bei Long Covid – Gesundheitliche Veränderungen am Ende der stationären Rehabilitationsmaßnahme. Rehabilitation (Stuttg). 2023(EFirst). https://doi.org/10.1055/a-1964-7401
Kedor C, Freitag H, Meyer-Arndt L, Wittke K, Hanitsch LG, Zoller T, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13(1):5104. https://doi.org/10.1038/s41467-022-32507-6
Nielsen TB, Leth S, Pedersen M, Harbo HD, Nielsen CV, Laursen CH, et al. Mental fatigue, activities of Daily Living, Sick Leave and Functional Status among patients with long COVID: a cross-sectional study. Int J Environ Res Public Health. 2022;19(22). https://doi.org/10.3390/ijerph192214739
Buonsenso D, Gualano MR, Rossi MF, Valz Gris A, Sisti LG, Borrelli I, et al. Post-acute COVID-19 sequelae in a Working Population at one year Follow-Up: a wide range of impacts from an Italian sample. Int J Environ Res Public Health. 2022;19(17). https://doi.org/10.3390/ijerph191711093
Kisiel MA, Janols H, Nordqvist T, Bergquist J, Hagfeldt S, Malinovschi A, et al. Predictors of post-COVID-19 and the impact of persistent symptoms in non-hospitalized patients 12 months after COVID-19, with a focus on work ability. Ups J Med Sci. 2022;127. https://doi.org/10.48101/ujms.v127.8794
Diem L, Schwarzwald A, Friedli C, Hammer H, Gomes-Fregolente L, Warncke J, et al. Multidimensional phenotyping of the post-COVID-19 syndrome: a Swiss survey study. CNS Neurosci Ther. 2022;28(12):1953–63. https://doi.org/10.1111/cns.13938
Müller K, Poppele I, Ottiger M, Zwingmann K, Berger I, Thomas A, et al. Impact of Rehabilitation on Physical and Neuropsychological Health of patients who Acquired COVID-19 in the Workplace. Int J Environ Res Public Health. 2023;20(2). https://doi.org/10.3390/ijerph20021468
Peters C, Dulon M, Westermann C, Kozak A, Nienhaus A. Long-Term effects of COVID-19 on Workers in Health and Social Services in Germany. Int J Environ Res Public Health. 2022;19(12). https://doi.org/10.3390/ijerph19126983
Sansone D, Tassinari A, Valentinotti R, Kontogiannis D, Ronchese F, Centonze S, et al. Persistence of symptoms 15 months since COVID-19 diagnosis: prevalence, risk factors and residual work ability. Life. 2023;13(1). https://doi.org/10.3390/life13010097
Delgado-Alonso C, Cuevas C, Oliver-Mas S, Diez-Cirarda M, Delgado-Alvarez A, Gil-Moreno MJ, et al. Fatigue and cognitive dysfunction are Associated with Occupational Status in Post-COVID Syndrome. Int J Environ Res Public Health. 2022;19(20). https://doi.org/10.3390/ijerph192013368
Van Wambeke E, Bezler C, Kasprowicz AM, Charles AL, Andres E, Geny B. Two-years Follow-Up of symptoms and return to work in Complex Post-COVID-19 patients. J Clin Med. 2023;12(3). https://doi.org/10.3390/jcm12030741
Wells G, Shea B, O’Connell J. The Newcastle-Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta-analyses. 2014. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed: 16.11.2023.
Amorim CEN, Gomes VLT, Cristelli MP, Viana LA, de Luca Correa H, Lima GBB, et al. High prevalence of Long-COVID among kidney transplant recipients: a longitudinal cohort study. Transplantation. 2022;106(12):2408–15. https://doi.org/10.1097/tp.0000000000004359
Brehon K, Niemelainen R, Hall M, Bostick GP, Brown CA, Wieler M, et al. Return-to-work following Occupational Rehabilitation for Long COVID: descriptive cohort study. JMIR Rehabil Assist Technol. 2022;9(3):e39883. https://doi.org/10.2196/39883
Harvey-Dunstan TC, Jenkins AR, Gupta A, Hall IP, Bolton CE. Patient-related outcomes in patients referred to a respiratory clinic with persisting symptoms following non-hospitalised COVID-19. Chron Respir Dis. 2022;19:14799731211069391. https://doi.org/10.1177/14799731211069391
Hodgson CL, Higgins AM, Bailey MJ, Mather AM, Beach L, Bellomo R, et al. The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care. 2021;25(1):382. https://doi.org/10.1186/s13054-021-03794-0
Gragnano A, Negrini A, Miglioretti M, Corbière M. Common psychosocial factors Predicting Return to Work after Common Mental disorders, Cardiovascular diseases, and cancers: a review of Reviews supporting a Cross-disease Approach. J Occup Rehabil. 2018;28(2):215–31. https://doi.org/10.1007/s10926-017-9714-1
Lamore K, Dubois T, Rothe U, Leonardi M, Girard I, Manuwald U, et al. Return to work interventions for Cancer survivors: a systematic review and a Methodological Critique. Int J Environ Res Public Health. 2019;16(8). https://doi.org/10.3390/ijerph16081343
Lemhöfer C, Sturm C, Loudovici-Krug D, Guntenbrunner C, Bülow M, Reuken P, et al. Quality of life and ability to work of patients with Post-COVID syndrome in relation to the number of existing symptoms and the duration since infection up to 12 months: a cross-sectional study. Qual Life Res. 2023;1–12. https://doi.org/10.1007/s11136-023-03369-2
Kinman G. Sickness presenteeism at work: prevalence, costs and management. Br Med Bull. 2019;129(1):69–78. https://doi.org/10.1093/bmb/ldy043
Kigozi J, Jowett S, Lewis M, Barton P, Coast J. The estimation and inclusion of Presenteeism costs in Applied Economic evaluation: a systematic review. Value Health. 2017;20(3):496–506. https://doi.org/10.1016/j.jval.2016.12.006
Asaba E, Sy M, Pineda RC, Aldrich R, Anzai T, Bontje P, et al. Return to work after COVID-19: an international perspective. World Federation Occup Therapists Bull. 2022;1–11. https://doi.org/10.1080/14473828.2022.2045819
Koczulla AR, Ankermann T, Behrends U, Berlit P, Böing S, Brinkmann F et al. S1-Leitlinie „Post-COVID/Long-COVID. 2022. https://register.awmf.org/assets/guidelines/020-027l_S1_Post_COVID_Long_COVID_2022-08.pdf . Accessed: 01.11.2023.
Kluge S, Rabe KF. S3-Leitlinie Empfehlungen zur stationären Therapie von Patienten mit COVID-19 – Living Guideline. 2022. https://register.awmf.org/assets/guidelines/113-001LGl_S3_Empfehlungen-zur-stationaeren-Therapie-von-Patienten-mit-COVID-19_2022-09_1.pdf . Accessed: 01.11.2023.
WHO. Clinical management of COVID-19: living guideline, 13 January 2023. 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1 . Accessed: 01.11.2023.
Bailly M, Pélissier L, Coudeyre E, Evrard B, Bingula R, Rochette C, et al. Systematic review of COVID-19-Related physical activity-based rehabilitations: benefits to be confirmed by more robust methodological approaches. Int J Environ Res Public Health. 2022;19(15). https://doi.org/10.3390/ijerph19159025
Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient Pulmonary Rehabilitation in patients with long COVID improves Exercise Capacity, Functional Status, Dyspnea, fatigue, and Quality of Life. Respiration. 2022;101(6):593–601. https://doi.org/10.1159/000522118
Stevelink SAM, Mark KM, Fear NT, Hotopf M, Chalder T. Chronic fatigue syndrome and occupational status: a retrospective longitudinal study. Occup Med (Lond). 2022;72(3):177–83. https://doi.org/10.1093/occmed/kqab170
Macía P, Barranco M, Gorbeña S, Álvarez-Fuentes E, Iraurgi I. Resilience and coping strategies in relation to mental health outcomes in people with cancer. PLoS ONE. 2021;16(5):e0252075. https://doi.org/10.1371/journal.pone.0252075
Aarestad SH, Harris A, Einarsen SV, Gjengedal RGH, Osnes K, Hannisdal M, et al. Exposure to bullying behaviours, resilience, and return to work self-efficacy in patients on or at risk of sick leave. Ind Health. 2021;59(3):180–92. https://doi.org/10.2486/indhealth.2020-0064
Young KP, Kolcz DL, O’Sullivan DM, Ferrand J, Fried J, Robinson K. Health Care Workers’ Mental Health and Quality of Life during COVID-19: results from a mid-pandemic, National Survey. Psychiatr Serv. 2021;72(2):122–8. https://doi.org/10.1176/appi.ps.202000424
Lunt J, Hemming S, Burton K, Elander J, Baraniak A. What workers can tell us about post-COVID workability. Occup Med (Lond). 2022. https://doi.org/10.1093/occmed/kqac086
Vooijs M, Leensen MC, Hoving JL, Wind H, Frings-Dresen MH. Interventions to enhance work participation of workers with a chronic disease: a systematic review of reviews. Occup Environ Med. 2015;72(11):820–6. https://doi.org/10.1136/oemed-2015-103062
Wegrzynek PA, Wainwright E, Ravalier J. Return to work interventions for chronic pain: a systematic review. Occup Med (Lond). 2020;70(4):268–77. https://doi.org/10.1093/occmed/kqaa066
Popa AE, Bejenaru A, Mitrea EC, Morandau F, Pogan L. Return to work after chronic disease: a theoretical framework for understanding the worker-employer dynamic. Chronic Illn. 2022;17423953221117852. https://doi.org/10.1177/17423953221117852
Wong J, Kudla A, Pham T, Ezeife N, Crown D, Capraro P, et al. Lessons learned by Rehabilitation Counselors and Physicians in Services to COVID-19 Long-Haulers: a qualitative study. Rehabilitation Couns Bull. 2021;66(1):25–35. https://doi.org/10.1177/00343552211060014
Blank L, Peters J, Pickvance S, Wilford J, Macdonald E. A systematic review of the factors which predict return to work for people suffering episodes of poor mental health. J Occup Rehabil. 2008;18(1):27–34. https://doi.org/10.1007/s10926-008-9121-8
Karanika-Murray M, Biron C. The health-performance framework of presenteeism: towards understanding an adaptive behaviour. Hum Relat. 2019;73(2):242–61. https://doi.org/10.1177/0018726719827081
Tan W, Hao F, McIntyre RS, Jiang L, Jiang X, Zhang L, et al. Is returning to work during the COVID-19 pandemic stressful? A study on immediate mental health status and psychoneuroimmunity prevention measures of Chinese workforce. Brain Behav Immun. 2020;87:84–92. https://doi.org/10.1016/j.bbi.2020.04.055
Lam MH, Wing YK, Yu MW, Leung CM, Ma RC, Kong AP, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–7. https://doi.org/10.1001/archinternmed.2009.384
Böckerman P, Ilmakunnas P. Unemployment and self-assessed health: evidence from panel data. Health Econ. 2009;18(2):161–79. https://doi.org/10.1002/hec.1361
Haller J, Kocalevent RD, Nienhaus A, Peters C, Bergelt C, Koch-Gromus U. Persistent fatigue symptoms following COVID-19 infection in healthcare workers: risk factors and impact on quality of life. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022;65(4):471–80. https://doi.org/10.1007/s00103-022-03511-4
Hasenoehrl T, Palma S, Huber DFX, Kastl S, Steiner M, Jordakieva G, et al. Post-COVID: effects of physical exercise on functional status and work ability in health care personnel. Disabil Rehabil. 2022;1–7. https://doi.org/10.1080/09638288.2022.2111467
Hartling L, Hamm M, Milne A, Vandermeer B, Santaguida PL, Ansari M, et al. Validity and Inter-rater Reliability Testing of Quality Assessment Instruments. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012.
Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. https://doi.org/10.1186/1471-2288-14-45
Pérez-González A, Araújo-Ameijeiras A, Fernández-Villar A, Crespo M, Poveda E. Long COVID in hospitalized and non-hospitalized patients in a large cohort in Northwest Spain, a prospective cohort study. Sci Rep. 2022;12(1):3369. https://doi.org/10.1038/s41598-022-07414-x
Reme BA, Gjesvik J, Magnusson K. Predictors of the post-COVID condition following mild SARS-CoV-2 infection. Nat Commun. 2023;14(1):5839. https://doi.org/10.1038/s41467-023-41541-x
Lund LC, Hallas J, Nielsen H, Koch A, Mogensen SH, Brun NC, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021;21(10):1373–82. https://doi.org/10.1016/s1473-3099(21)00211-5
Download references
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Institute of Human Movement Science and Health, Faculty of Behavioral and Social Sciences, Chemnitz University of Technology, 09107, Chemnitz, Germany
Marcel Ottiger, Iris Poppele, Naveen Sperling, Torsten Schlesinger & Katrin Müller
You can also search for this author in PubMed Google Scholar
Contributions
Concept and design: K.M. and M.O. Search strategy: M.O., I.P. and K.M. Screening of titles/abstracts and full texts: M.O., I.P. and N.S. Critical review of screening process: I.P. and K.M. Acquisition of data: M.O, I.P., N.S. and K.M. Assessing risk of bias: M.O., N.S. Statistical analysis and interpretation of data: M.O., I.P. and K.M. Drafting of the manuscript: M.O., I.P. and K.M. Critical revision of the manuscript: M.O., I.P., N.S., T.S. and K.M. Supervision: K.M. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Marcel Ottiger .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ottiger, M., Poppele, I., Sperling, N. et al. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health 24 , 1811 (2024). https://doi.org/10.1186/s12889-024-19328-6
Download citation
Received : 26 November 2023
Accepted : 01 July 2024
Published : 07 July 2024
DOI : https://doi.org/10.1186/s12889-024-19328-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Post-COVID-19
- Work ability
- Occupational status
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 8—August 2024
Research Letter
Infective sars-cov-2 in skull sawdust at autopsy, finland.
Suggested citation for this article
We assessed the distribution of SARS-CoV-2 at autopsy in 22 deceased persons with confirmed COVID-19. SARS-CoV-2 was found by PCR (2/22, 9.1%) and by culture (1/22, 4.5%) in skull sawdust, suggesting that live virus is present in tissues postmortem, including bone. Occupational exposure risk is low with appropriate personal protective equipment.
Autopsies afford simultaneous access to all tissues and body compartments. The unique opportunity for extensive sampling during autopsy enables several research questions to be addressed. Early in the COVID-19 pandemic autopsies were rare, mainly because of presumed transmission risk and shortage of personal protective equipment (PPE), and suspicions that autopsies might be of limited value ( 1 , 2 ).
Autopsies pose an occupational infectious hazard to the personnel involved in a pathogen-dependent manner. For example, Mycobacterium tuberculosis deserves particular attention as a major cause of airborne infections in autopsies that puts pathologists at a 100–200-fold risk for infection compared with the general public ( 3 ). Viable SARS-CoV-2 has been detected in tissues for prolonged periods after death from COVID-19 ( 4 ). However, to our knowledge, no confirmed occupational cases of COVID-19 transmitted at autopsies have been reported.
Protection against aerosols remains a challenge in autopsy settings. Bone sawing is a major source of aerosols that can carry pathogens. Sawing of the skull is a standard procedure in every routine autopsy to enable access to the brain. SARS-CoV-2 has previously been documented in bone tissues in 2 reported cases, neither of which were in the skull ( 5 ). Here, we present results of SARS-CoV-2 analyses from 22 deceased persons with PCR-confirmed COVID-19 and detail our experience of managing the occupational hazards associated with COVID-19 autopsies.
Our study belongs to the Clin_COVID-19 master study approved by the Helsinki University Hospital Ethics committee (approval no. HUS/1238/2020). All autopsies were clinical (nonforensic) and conducted in compliance with research laws and regulations in Finland, after consent from the next of kin.
The postmortem examinations were conducted in the pathology department of the HUS Diagnostic Center in Meilahti, Helsinki, Finland. The series comprised 22 PCR-confirmed cases (any positive airway sample from nasopharynx, bronchi, lungs, tonsils, sclera, or airway-associated cervical or parabronchial lymph nodes) of SARS-CoV-2 identified during 2021–2022 that had skull sawdust sampled during autopsy. Testing was carried out in the pathology and virology laboratories by using accredited and previously published methods ( 6 ) ( Appendix ). All autopsies encompassed a neuropathological examination and a collection of swabs/fresh tissues from airway, nonairway, and central nervous system (CNS) categories. In addition, swab samples were collected from skull sawdust and the contaminated autopsy table with the organ block. Each tissue was sampled with separate sterile equipment. PCR-positive samples were cultured using VeroE6 cells to assess for infective SARS-CoV-2.
We detected SARS-CoV-2 by reverse transcription PCR in 22/22 (100%) airway, 10/22 (45.5%) nonairway, 0/22 CNS, 2/22 (9.1%) skull sawdust, and 13/22 (59.1%) autopsy table samples ( Table ). The virus was culturable in 13/22 (59.1%) airway, 2/22 (9.1%) nonairway, 1/22 (4.5%) skull sawdust, and 3/22 (13.6%) autopsy table samples.
Among the personnel present at COVID-19 autopsy procedures, no cases of COVID-19 resulting from occupational exposure were identified. Serologic screening results of all persons involved in COVID-19 autopsies (n = 5) in June 2020 were negative, and none showed PCR positivity when tested during symptoms.
Our findings revealed that SARS-CoV-2 was detectable by PCR in 9.1% and by viral culture in 4.5% of skull sawdust samples, suggesting the presence of live virus and a risk, although low, of infective viruses becoming aerosolized. We could not identify previous work examining cranial sawdust for the presence of pathogens, but our results align with a previous study showing SARS-CoV-2 PCR positivity for 4.5% of goggles and no masks tested after autopsy ( 7 ).
The sample size for our study was limited but represents a consecutive and nonselected series of cases at a single institution. We did not directly assess aerosols, but given that bone sawing is the only high-energy technique used, and considering the findings from a previous study ( 7 ), the presence of concomitant other sources of infective aerosols in the autopsy room is unlikely. The personnel present during COVID-19 autopsies were not systematically tested, but symptomatic persons were extensively PCR tested for SARS-CoV-2 during the study period (2020–2022). In addition, skull sawdust samples might not consist solely of bone and could contain adjacent tissues because of anatomy, particularly the frontal sinus, which is lined with respiratory epithelium. Skullcap sawing has the potential to generate infective aerosols, but in our experience, general autopsy safety measures are effective. The absence of positive findings in our CNS samples give confidence in the sterility of our sampling technique, thereby making other sources of contamination in the skull sawdust samples less likely.
Pandemic preparedness should encompass plans for early, rapid autopsies to acquire vital data at the onset. General safety measures appear adequate for most pathogens encountered during autopsy, including SARS-CoV-2 ( 3 ). However, early testing for pathogens in skull sawdust, along with other tissues, could prove beneficial in further assessing the risk for occupational infections resulting from autopsies during future pandemics.
Dr. Kantonen is a certified pathologist and medical doctor performing research at the University of Helsinki, Finland. His research interests focus on the use of autopsies for medical research.
Acknowledgments
We thank Mira Utriainen and Leena Palmunen for their excellent work on PCR and viral culture in the BioSafety Level 3 laboratory and personnel at Helsinki University Central Hospital Diagnostic Center and the University of Helsinki for excellent technical assistance.
Funding was a provided by a DeLaval COVID donation, the Juho Vainio Foundation, and a Fazer COVID donation.
- Ledford H . Autopsy slowdown hinders quest to determine how coronavirus kills. Nature . 2020 . DOI PubMed Google Scholar
- Fineschi V , Aprile A , Aquila I , Arcangeli M , Asmundo A , Bacci M , et al. ; Scientific Society of Hospital Legal Medicine of the National Health System (COMLAS) ; Italian Society of Anatomical Pathology and Cytology (SIAPEC) . Management of the corpse with suspect, probable or confirmed COVID-19 respiratory infection - Italian interim recommendations for personnel potentially exposed to material from corpses, including body fluids, in morgue structures and during autopsy practice. Pathologica . 2020 ; 112 : 64 – 77 . PubMed Google Scholar
- Kritselis M , Remick DG . Universal precautions provide appropriate protection during autopsies of patients with infectious diseases. Am J Pathol . 2020 ; 190 : 2180 – 4 . DOI PubMed Google Scholar
- Plenzig S , Bojkova D , Held H , Berger A , Holz F , Cinatl J , et al. Infectivity of deceased COVID-19 patients. Int J Legal Med . 2021 ; 135 : 2055 – 60 . DOI PubMed Google Scholar
- Jurek T , Rorat M , Szleszkowski Ł , Tokarski M , Pielka I , Małodobra-Mazur M . SARS-CoV-2 viral RNA is detected in the bone marrow in post-mortem samples using RT-LAMP. Diagnostics (Basel) . 2022 ; 12 : 515 . DOI PubMed Google Scholar
- Corman VM , Landt O , Kaiser M , Molenkamp R , Meijer A , Chu DK , et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill . 2020 ; 25 : 2000045 . DOI PubMed Google Scholar
- Brandner JM , Boor P , Borcherding L , Edler C , Gerber S , Heinemann A , et al. Contamination of personal protective equipment during COVID-19 autopsies. Virchows Arch . 2022 ; 480 : 519 – 28 . DOI PubMed Google Scholar
- Table . SARS-CoV-2 distribution among cohort of 22 autopsied deceased persons with COVID-19 who had skull sawdust sampling, Finland
Suggested citation for this article : Kantonen JN, Kuivanen S, Smura T, Puttonen H, Kekäläinen E, Sajantila A, et al. Infective SARS-CoV-2 in skull sawdust at autopsy, Finland. Emerg Infect Dis. 2024 Jul [ date cited ]. https://doi.org/10.3201/eid3008.240145
DOI: 10.3201/eid3008.240145
Original Publication Date: July 02, 2024
1 Current affiliation: Charité-Universitätsmedizin Berlin, Berlin, Germany.
2 These senior authors contributed equally to this article.
Table of Contents – Volume 30, Number 8—August 2024
| EID Search Options |
|---|
| – Search articles by author and/or keyword. |
| – Search articles by the topic country. |
| – Search articles by article type and issue. |
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jonas N. Kantonen, University of Helsinki, Haartmaninkatu 3 C 323, 00290 Helsinki, Finland
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Metric Details
Article views: 12301.
Data is collected weekly and does not include downloads and attachments. View data is from .
What is the Altmetric Attention Score?
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Global contribution of statistical control charts to epidemiology monitoring: A 23-year analysis with optimized EWMA real-life application on COVID-19
Waqas, Muhammad PhD a,b ; Xu, Song Hua PhD c,d ; Usman Aslam, Muhammad PhD a ; Hussain, Sajid PhD a ; Shahzad, Khurram MS e,f ; Masengo, Gilbert PhD g,*
a Department of Statistics, School of Mathematics and Statistics, Xian Jiaotong University, Xian, China
b Department of Statistics, University of WAH, Pakistan
c Department of Health Management & Institute of Medical Artificial Intelligence, The Second Affiliated Hospital, Xi’an Jiaotong University, Xian, China
d Yale University, New Haven, CT
e SysReforms International, Department Health Monitoring, Pakistan
f Monitoring and Evaluation Department, Chemonics International Inc., Islamabad, Pakistan
g Department of Mechanical Engineering, Rwanda Polytechnic/Integrated Polytechnic Regional College Karongi, Kigali, Rwanda.
Received: 23 April 2024 / Received in final form: 15 May 2024 / Accepted: 10 June 2024
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Waqas M, Xu SH, Usman Aslam M, Hussain S, Shahzad K, Masengo G. Global contribution of statistical control charts to epidemiology monitoring: A 23-year analysis with optimized EWMA real-life application on COVID-19. Medicine 2024;103:27(e38766).
* Correspondence: Gilbert Masengo, Department of Mechanical Engineering, Rwanda Polytechnic/Integrated Polytechnic Regional College Karongi, Kigali, Rwanda (e-mail: [email protected] ).
This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY) , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Control charts help epidemiologists and healthcare professionals monitor disease incidence and prevalence in real time, preventing outbreaks and health emergencies. However, there remains a notable gap in the comprehensive exploration and application of these techniques, particularly in the context of monitoring and managing disease outbreaks. This study analyses and categorizes worldwide control chart applications from 2000 to 2023 in outbreak monitoring in over 20 countries, focusing on corona-virus (COVID-19), and chooses optimal control charts for monitoring US COVID-19 death waves from February 2020 to December 2023. The systematic literature review analyzes available 35 articles, categorizing data by year, variable, country, study type, and chart design. A selected optimal chart is applied to monitor COVID-19 death patterns and waves in the USA. Control chart adoption in epidemiology monitoring increased during the COVID-19 pandemic, with annual patterns showing a rise in 2021 to 2023 (18%, 36%, 41%). Important variables from 2000 to 2019 include influenza counts, Salmonella cases, and infection rates, while COVID-19 studies focus more on cases, infection rates, symptoms, and deaths. Among 22 countries, the USA (29%) is the top applier of control charts. The monitoring of USA COVID-19 deaths reveals 8 waves with varying severity W 5 ( 1899 ) > W 3 ( 1881 ) > W 4 ( 1393 ) > W 1 ( 1036 ) > W 2 ( 853 ) > W 6 ( 478 ) > W 7 ( 140 ) > W 8 . The W 8 associated with the JN.1 variant, highlights ongoing challenges. This study emphasizes the significance of control charts in outbreak monitoring for early disease diagnosis and intervention. Control charts help healthcare workers manage epidemics using data-driven methods, improving public health. COVID-19 mortality analysis emphasizes their importance, encouraging worldwide use.
1. Introduction
Epidemiology underpins public health and healthcare administration. This field focuses on identifying disease burdens, determining priorities, assessing cause-and-effect correlations, developing solutions, monitoring, and evaluating healthcare. [ 1 ] Devastating pandemics have occurred throughout history such as the Plague, Spanish Flu, HIV, and Ebola causing deaths, political government collapse, and financial and psychosocial hardships. We have seen immense anguish, mortality, and instability in everyday life for more than 4 years during the corona-virus (COVID-19) pandemic. [ 2 ] Human populations could be severely burdened by emerging viruses in terms of death, morbidity, and economic costs. The examination of case data collected during an outbreak has historically been used to monitor the spread of infectious illnesses and aid in their containment. [ 3 ]
Control charts aid in the early detection of pandemics by tracking the number of cases and deaths of a certain illness over time; any significant increase in the number of cases and deaths might indicate an outbreak. Control charts assist in monitoring process reliability and efficacy over time and discovering notable changes or trends that may indicate the need for intervention. They could serve as indicators of the occurrence of infectious illnesses. [ 4–9 ] Moreover, disease monitoring allowed researchers to ascertain seasonal or cyclical patterns in the incidence of the condition as well as if it was growing or decreasing. The effectiveness of an intervention in reducing the number of incidents before and after implementation may be ascertained by intervention evaluation. The quality of medical facilities may be monitored and enhanced using quality improvement techniques. [ 10–13 ]
Worldwide, respiratory syncytial virus is a major cause of illness and death in young children. [ 14 ] Due to prolonged suboptimal viral exposure, immunologically vulnerable older children had more symptomatic respiratory syncytial virus infections and hospitalizations in 2021 to 22 and 2022 to 23 in British Columbia. [ 15 ] Acute COVID-19 was the main trait linked with high severity and death in Pakistani children. [ 16 ] Numerous academics have examined data on COVID-19 hospitalizations and deaths throughout different waves; such as COVID-19 deaths in the Western Pacific, [ 17 ] COVID-19 among the top 5 causes of deaths in Australia, [ 18 ] and COVID-19 cases and deaths in the USA. [ 19–22 ] Furthermore, in the context of COVID-19 death monitoring by using control charts following studies proposed different charts and identified death patterns. [ 23–27 ]
Medical information determines control chart classification. There may be continuous, count, or attribute-based data. For instance, attribute control charts provide data with limited values, such as disease presence or absence. Many settings may employ the P, NP, and U control charts. Event-counting data, such as the number of unfavorable occurrences, may be utilized with count data control charts like the C and Histogram charts. Continuous data control charts display statistics like blood pressure, heart rate, and body temperature within a set range. Additionally, there are 2 scenarios: individual moving range control charts and MR X, R, and S. Future, plans include creating control charts for time series data, such as monthly patient satisfaction ratings or daily hospital admissions. Additionally, run charts and time-weighted control charts also exist. [ 28–33 ] Rising trend in machine learning techniques in healthcare quality monitoring is observed. [ 34–37 ] Choosing the right control chart for the data may prevent inaccurate inferences regarding a healthcare process’s stability.
2. Research gap and study significance
How did the world’s most prosperous country, ranked first in its ability to respond to pandemics according to the Global Health Security Index, see more than 1·2 million people die from COVID-19 and have one of the highest rates of death per capita in the world. The importance of evidence-based monitoring and decision-making is emphasized by the study. [ 38 ] This study addresses a notable gap in understanding the evolving landscape of control charts in epidemiology monitoring. While there are systematic reviews on the topic of control chart applications in healthcare in general, they frequently do not provide a comprehensive view of control chart applications in epidemiology. Furthermore, many applications contributed to monitoring COVID-19 variables during 2019 to 2023, but an analysis of how those studies contributed overall is also lacking. Furthermore, a debate and the need for a comprehensive analysis of all-time applications were felt to be fulfilled for the selection and application of optimum control charts [ 23 ] and their appropriateness in monitoring epidemiological phenomena. This article aims to contribute to the body of knowledge by thoroughly examining the applications of control charts in epidemiological surveillance and global trends in the past 2 decades.
Monitoring gaps impose a burden on the health sector. During an extensive review, we discovered that while the USA is the largest user of control charts in all of healthcare and epidemiology, Shewhart charts are used 90% of the time. Other types of charts that suit better than Shewhart charts should be introduced for monitoring. According to data sources (United States COVID-19 Coronavirus Statistics from Worldometer [worldometers.info]) and ( https://covid.cdc.gov/covid-data-tracker/#datatracker-home ) the number of cases and deaths are still significant, and there is a significant positive correlation exists between number of cases and deaths in the USA situation. [ 23 ] Since, December 9, 2023, the JN.1 variant has surged, the number of cases rising again. The goal of this study is to extend the use of optimal control charts in epidemiology to monitor variations in COVID-19 deaths to better understand recent pandemic mortality patterns. The application of an appropriate statistical process monitoring technique for distinct deaths in the USA in 8 phases, including pregrowth, and postgrowth, is an essential aspect of the current study.
3. Methodology
3.1. method 1, 3.1.1. systematic review.
Employing the guidelines and methods for the systematic evaluation of QI interventions [ 28 ] and Johan, Jonas, Jakob, Jesper, Cheryl, Karin Pukk, and Mats, [ 39 ] an extensive search was conducted to uncover materials published between 2000 and 2023 about establishing control charts in the epidemiology. SCOPUS, PubMed, Web of Science, and Google Scholar, databases are used to identify research that examines control charts in monitoring any outbreaks. The search terms included “SPC,” “Control chart,” “Epidemiology,” “COVID-19,” “Application,” and “Outbreaks” etc. Because the emphasis was on locating studies published in academic and professional journals, master’s and doctorate dissertations were eliminated.
3.1.2. Selection of studies and data collection
The title and abstract were reviewed to determine if the paper matched the inclusion criteria. A control chart application in the epidemiology department is the inclusion standard. The required articles were read carefully under the instruction of. [ 40 ] Articles that did not match the requirements were discarded. Title, authors, year of publication, location, inclusion criteria, research aims, results, output variables, journal, unit of analysis, study setting and level, and statistical process control (SPC) chart data period were entered in an Excel sheet. Visual statistics in graphs showed country and publication year. SPC in any epidemic research objectives, outcomes, limits, and benefits were qualitatively analyzed in the review.
We approached 2 SPC experts, 1 of whom is the most prominent in the healthcare monitoring engaged with Chemonics Inc (Islamabad, Pakistan). The other is a specialist in SPC application outside of healthcare, to review an earlier draft of this manuscript to improve our review through investigator triangulation. [ 41 ] Their feedback improved our data synthesis and reduced findings.
In this evaluation, research papers must apply control charts to epidemiology departments. After reviewing abstracts, 90 of 139 articles were deleted as irrelevant, review papers, tutorials, or used in psychiatric or other health departments. From the remaining 49 research papers, after carefully reading in the full-text form a certain number of 35 studies (14 other outbreaks, 21 COVID-19 studies) are chosen for comprehensive examination and review, see Figure S1, Supplemental Digital Content, https://links.lww.com/MD/N101 .
3.2. Method 2
3.2.1. data and settings.
The Epidemic Intelligence from Open Sources initiative, which is led by the World Health Organization, signifies an unparalleled coalition of various public health stakeholders from around the globe. The daily COVID-19 deaths in the USA are obtained from the portal, https://portal.who.int/report/eios-covid19-counts . The data spans the periods of February 2020 to December 2020 to 2023. A thorough examination revealed that an optimal.
Exponentially weighted moving average (EWMA) control chart outperformed both the Shewhart and cumulative sum charts in monitoring deaths in the USA. Following the conclusions given in, [ 23 ] rather than reproducing the aforementioned methodology, we chose the EWMA chart to extend COVID-19 death wave monitoring in the USA. This decision was influenced by the EWMA chart’s superior performance when compared to cumulative sum, which has known limits, [ 42 ] and Shewhart (X-bar, R and C) charts, which performed poorly for the USA situation. The mathematical structure of the EWMA chart is provided in the Method 2, Supplemental Digital Content, https://links.lww.com/MD/N101 . We estimated process parameters using wave-1 data. Simulations were run with a range of L values using these estimates. L is used when the average run length is 370, indicating the expected number of observations before a signal is identified. The targeted degree of sensitivity for detecting process changes was L = 2.87 to ensure that the control chart successfully recognizes any substantial deviations from expected behavior. The following tools; such as draw.io, Microsoft Excel 16, Origin 9.0, Minitab 21, and R 4.3.2 Language, were utilized in the analysis, graphic work, and simulations of this study.
4. Results and discussion
4.1. stage 1.
After analyzing all 35 studies, it was determined that, overall, between 2000 and 2023, the majority of attention was still focused on the recent pandemic, which accounted for 60% of control chart applications between 2020 and 2023, while the other outbreaks received 40% of applications between 2000 and 2019. A detailed summary of each article is presented, see Table 1 and Figures 1–3 . Epidemiology control chart applications vary by country, demonstrating worldwide infectious disease surveillance methodologies. The US leads epidemiologically with 29% of control chart applications, followed by Australia at 5%, Brazil, Indonesia, China, and Thailand at 5% each. Control charts are common in the US, indicating their use in epidemiological monitoring. Switzerland, France, the UK, Senegal, Pakistan, Iraq, Jordan, the UAE, Nigeria, KSA, Qatar, Iran, Italy, Malaysia, and Taiwan account for different percentages in Europe, the Middle East, and Asia. The distribution of control chart applications changes for COVID-19 monitoring. The US accounts for 19% of control chart applications. The other countries-specific findings also highlight the necessity for specialized epidemiological monitoring systems to address pandemic problems in varied locales, see Figure 2C .
| Outbreak | Study | Chart | Year | Monitored variables | Category | Country |
|---|---|---|---|---|---|---|
| Influenza, , infection and other outbreaks | Gustafson ] | p, run, and XMR chart | 2000 | SIR | Retrospective study | USA |
| Quesenberry ] | Q chart | Infection’s rate | ||||
| Hanslik et al ] | U chart | 2001 | Number of patients | Longitudinal study | France | |
| Arantes et al ] | p chart | 2003 | Per thousand nosocomial infection patients per day | Brazil | ||
| Grant and Kim ] | XMR chart | 2007 | Infection control consultancy visits and duration | Retrospective study | USA | |
| Curran et al ] | p chart | 2008 | Percentage of errors | UK | ||
| Harbarth et al ] | Run chart | Per day MRSA infections per thousand | Switzerland | |||
| Limaye et al ] | G, U, and CUSUM chart | Number of infections associated with hospitals | Longitudinal study | USA | ||
| Chimka ] | Regression chart | 2009 | Number of influenza cases | |||
| Sparks et al ] | CUSUM and EWMA chart | 2010 | Daily visit count | Australia | ||
| Gomes et al ] | Shewhart, EWMA, CUSUM chart | 2011 | Nosocomial infections | Retrospective study | Brazil | |
| Zhou et al ] | EST model | 2014 | Number of infection points | China | ||
| Wiemken et al ] | p chart | 2017 | Hand hygiene complaints | USA | ||
| Vanli and Giroux ] | CUSUM chart | 2022 | Count of cases | Longitudinal study | ||
| COVID-19 | Yuyun Hidayat and Titi ] | t-chart and I-MR chart | 2020 | COVID-19 positive cases | Retrospective study | Indonesia |
| Mbaye et al ] | p chart | 2021 | Positive COVID cases per day | Senegal | ||
| Mahmood et al ] | EWMA and c chart | Deaths due to the COVID-19 infection | Pakistan | |||
| Inkelas et al ] | I and c chart | Daily reported COVID cases | USA | |||
| Yupaporn and Rapin ] | EWMA chart | Routine alerts on total COVID cases | Thailand | |||
| Mustafa and Jabir ] | KPCI and KNN chart | Number of COVID-infected cases | Iraq | |||
| Arafah ] | Laney p’, EWMA chart | 2022 | COVID infection rate | Jordan | ||
| Karoon et al ] | EEWMA chart | COVID-19 cases | Longitudinal and retrospective study | Thailand | ||
| Sanmugam and Abdul ] | Shewhart chart | COVID-19 cases | Longitudinal study | Malaysia | ||
| Fernandez et al ] | X-bar chart | COVID-19 cases, surgery prepping time | Retrospective study | USA | ||
| Barone and Chakhunashvili ] | Individual chart | 2023 | COVID-19 cases | Longitudinal and retrospective study | Italy | |
| Faisal Shah and Khan ] | ṼSQ chart | COVID-19 incubation period | Retrospective study | China | ||
| Alkhatib et al ] | Np chart | Infection rate | Longitudinal study | UAE | ||
| Adekeye and Adekeye ] | Gamma CUSUM chart | Number of deaths | Nigeria | |||
| Waqas et al ] | Shewhart and EWMA chart | Reproduction number, number of cases and deaths | USA | |||
| Boone et al ] | MEWMA chart | SEIRD | Qatar | |||
| Imro’ah et al ] | I-MR and ARIMA chart | Vaccinate rate | Indonesia | |||
| Alamri et al ] | MEWMA chart | SEIRD model | KSA | |||
| Elhambakhsh et al ] | Hoteling T-square chart | COVID symptoms | Iran | |||
| Cheema et al ] | GLM based chart | COVID patients | Retrospective study | Pakistan | ||
| Wang ] | X-bar chart | COVID-19 cases | Taiwan |

4.2. Overall interesting inferences
Following intriguing patterns shed light on the diverse strategies employed in epidemiological studies, the specificity in COVID-19 monitoring, and the collaborative global effort to tackle the unprecedented challenges presented by infectious diseases.
4.2.1. USA dominance in the standard control chart
A striking observation is the dominance of standard control charts in the USA, with 90% of applications utilizing charts other than EWMA. This suggests a strong reliance on traditional control chart methodologies, pointing toward a preference for well-established and widely accepted techniques in the country’s epidemiological studies. Other side, this also provides a space for the application of other control charts in epidemiolocal phenomena.
4.2.2. Global adoption of control charts for diverse variables
The diverse range of variables studied using control charts reflects a global trend. Countries such as France, Brazil, Switzerland, China, and the UK employ various control charts for monitoring infection-related variables, showcasing the adaptability of control charts across different nations and epidemiological contexts.
4.2.3. Specificity in COVID-19 monitoring techniques
In COVID-19 studies, there is an efficient approach with specific control charts tailored for distinct variables related to the pandemic. This includes the use of charts such as EWMA & c chart for deaths, p chart for positive COVID cases per day, and Laney p and EWMA chart for COVID infection rate. These tailored approaches emphasize the need for precision in monitoring the unique characteristics of the ongoing global health crisis.
4.2.4. Global collaboration in COVID-19 research
The inclusion of multiple countries in COVID-19 studies, such as the USA, UK, China, Italy, Switzerland, Thailand, Iraq, Jordan, Senegal, Pakistan, and more, suggests a collaborative effort in understanding and combatting the pandemic. This international collaboration underscores the collective response to a global health crisis, leveraging diverse methodologies and chart types to gain comprehensive insights into the impact of COVID-19.
4.2.5. Longitudinal studies for COVID-19 impact
The use of longitudinal studies for COVID-19 variables, such as COVID-19 cases, deaths, reproduction numbers, and surgery prepping time, indicates a keen interest in understanding the long-term impact and trends associated with the pandemic. This longitudinal perspective is crucial for formulating effective strategies for managing and mitigating the ongoing challenges posed by COVID-19.
4.3. Stage 2
4.3.1. usa covid-19 deaths monitoring.
The timeline analysis from 2020 to December 2023, see Figure 4 shows how COVID-19 waves are dynamic and are affected by several variables over time, covering average deaths, number of days, and severe waves, see Table 2 . Seasonal fluctuations, adaption to novel varieties, and vaccination campaigns are all important factors in determining how the pandemic develops. Effective public health initiatives and well-informed decision-making depend on an understanding of these temporal trends.
| Time | Mortality waves | Days | Missing | Mean | SE mean | SD | Sum | Minimum | Q1 | Median | Maximum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| February–June 2020 | Initial period deaths | 123 | 0 | 1036 | 70.7 | 784.1 | 127,430 | 0.0 | 379 | 870 | 2624 |
| July–September 2020 | Second wave | 92 | 0 | 853 | 32.6 | 312.9 | 78,517 | 258.0 | 555 | 920 | 1532 |
| October 2020–March 2021 | Third wave (vacc. rollout) | 182 | 0 | 1881 | 79.7 | 1075.4 | 342,395 | 384.0 | 992 | 1600 | 4408 |
| April–July 2021 | Spring/summer | 122 | 0 | 501 | 30.4 | 335.9 | 61,139 | 40.0 | 286 | 445 | 2598 |
| August–November 2021 | Fourth wave (delta variant) | 122 | 0 | 1393 | 78.0 | 861.2 | 169,972 | 140.0 | 567 | 1335 | 3487 |
| December 2021–February 2022 | Fifth wave (Omicron variant) | 90 | 0 | 1899 | 115 | 1092 | 170,889 | 225 | 810 | 1909 | 4185 |
| March 2022–February 2023 | Sixth wave | 363 | 1 | 478 | 22.9 | 436.5 | 173,650 | 1.0 | 82 | 407 | 2097 |
| March–November 2023 | Seventh wave | 285 | 0 | 140 | 11.4 | 193.1 | 39,970 | 0.0 | 14 | 65 | 1370 |

4.3.1.1. Emerging phase
The initial stages of the pandemic were symbolized by the first wave, which ran from February to June 2020. Its 123-day lifespan and mean fatalities value of 1036 per day, upper control limit (UCL) = 1169, and peak touch to 2624 deaths in a say demonstrated the early difficulties in comprehending and controlling the new coronavirus, see Figure 5A .

4.3.1.2. Second wave
The mean daily mortality rate dropped to 853.4, UCL = 1020 during the second wave, but 1532 deaths in a single day hit drums, which ran from July to September 2020. This wave, which persisted for 92 days, represented a period of ongoing adjustment and reaction to the changing epidemic, see Figure 5B .
4.3.1.3. Vaccination rollout-third wave
The third wave, which coincided with the vaccine program, lasted 182 days, maximum number of deaths in a single day of 4408, and ran from October 2020 to March 2021. This wave, which had a mean mortality value of 1881.3, UCL = 2224 demonstrated how vaccination campaigns had a positive effect in reducing the virus’s severity, see Figure 5C .
4.3.1.4. Spring/summer wave
The 2021 spring and summer wave had a mean fatalities value of 501.1, UCL = 645, and lasted 122 days. Seasonal changes and ongoing efforts to control the epidemic were evident during this time, see Figure 5D .
4.3.1.5. Fourth wave (delta variant)
The delta variant’s fourth wave had a mean mortality value of 1393.2 per day, UCL = 1804 for 122 days. This wave demonstrated the difficulties brought about by newly developing varieties and the necessity of continuous modification in public health interventions, see Figure 5E .
4.3.1.6. Omicron variant-fifth wave
The Omicron variant-related fifth wave had a significant mean mortality value of 1899 per day, UCL = 2422 and 4185 in a single day, for 90 days. This time frame brought attention to the possible increased severity or transmissibility linked to new variations, see Figure 5F .
4.3.1.7. Sixth wave
With a duration of 363 days, the sixth wave showed a mean death value of 478.4 per day and UCL = 736. This prolonged duration indicated that the pandemic management was still challenging, either because of novel varieties or shifting dynamics, see Figure 5G .
4.3.1.8. The seventh wave
The previous seventh wave, which lasted 285 days, showed a significantly lower mean daily mortality rate of 140.2 and UCL = 243.7. This most recent wave suggests that efforts to contain the virus are still being made, perhaps with better therapies or public health initiatives, see Figure 5H .
4.3.1.9. Eightieth wave; JN.1 variant
Starting from December 9, 2023, a new surge in COVID-19 cases was noticed due to the JN.1 variant. The cases are rising daily which will positively be correlated to an increased number of deaths, see Figure 5I .
Since the beginning of COVID-19, many national and international platforms have begun to collect and manage daily cases, including the number of infected, recovered, and deaths. The data used in this study were nearly complete and efficiently handled. During the analysis, 1 missing value was discovered in the sixth wave data and replaced by the weakly moving average value. The third, fifth, and sixth waves remain the most severe in a total number of deaths more than 686,934. A detailed analysis improves decision-making and reaction strategy formulation for healthcare authorities in pandemic control measures. [ 24 ] Rather than only using UCLs, we also applied the EWMA chart lower control limit (LCL). As UCL tells alarming situation, LCL shows a safer situation. Data below the LCL are also evident that the COVID-19 pandemic may be a postgrowth period as mortality has dropped. Positive scores below the LCL imply epidemic decline. The EWMA chart’s LCL can help public health professionals analyze and monitor current conditions. Lockdowns, isolations, vaccines, and other COVID-19 prevention methods are compared to the LCL. By regularly monitoring data points below the LCL, researchers may analyze these indicators and public health strategies to limit infection and reduce deaths.
5. Strength and limitations
This study, which spans 23 years and includes 35 studies, provides a robust and extensive analysis of control chart applications in epidemiology. It provides a comprehensive and global perspective by incorporating data from various countries such as the USA, Australia, Brazil, and China, enhancing the generalizability of findings. Notably, the study focuses on COVID-19, in particular, providing detailed insights into its impact on control chart applications.
While this study has strengths, it faces limitations. Variability in data availability and reporting among selected studies may restrict reliance on existing literature. The study’s endpoint in 2023 might overlook emerging trends. The dominance of data from certain countries could impact the broader applicability of findings. Further validation and exploration of the EWMA methodology are needed to assess its effectiveness beyond the USA. These limitations should be considered when interpreting the findings.
6. Key takeaways for academia and health experts
First, the study emphasizes the adaptability of traditional control charts to many infectious diseases beyond COVID-19. As epidemiological studies improve precision and efficiency, researchers should incorporate new technologies like machine learning, deep learning, and artificial intelligence (AI) based charts. Despite the growing importance of technology, AI-based charts are scarce, suggesting an area for exploration and development. The study emphasizes longitudinal and retrospective approaches to capture infectious disease trends’ dynamic nature. Health experts say the findings emphasize the need for sophisticated and variable-specific COVID-19 monitoring using tailored control charts for pandemic variables. International epidemiology research requires shared methods and insights to improve pandemic preparedness and response. The study offers actionable insights that can guide future research and suggests that AI may improve epidemiological surveillance.
7. Conclusion
In conclusion, this thorough study provides a sophisticated knowledge of epidemiology’s expanding control chart applications, with a focus on the extraordinary COVID-19 pandemic difficulties. According to the study control chart applications increased significantly, peaking during the pandemic, with 60% of studies dedicated to this vital era. The rise in applications in 2021 and 2022 emphasizes the need for control charts to monitor and analyze the global health situation.
Influenza counts, Salmonella cases, infection rates, and COVID-19-specific measures remain prominent topics in epidemiological discussions. Control charts’ broad approach to infectious diseases is shown by their applicability to varied epidemiological scenarios and the variety of variables addressed. International collaboration is evident in epidemiological control chart applications, with the US as the leading country. The rise of COVID-19 charts and their varying use across nations highlight the need for pandemic-specific monitoring tools and research collaborations. The study’s second part monitored the 8 COVID-19 death waves in the US. The essential findings from each wave are highlighted for health professionals and policymakers to delve deeply into the outcomes of decisions made over these years. These dynamics demonstrate the effects of vaccination efforts, seasonal variations, and novel mutations. As the virus mutated to JN.1 a sudden spike in cases started which translated into the increased number of deaths. The measures taken before the sixth, fifth, and third waves should be examined and debated to avoid future catastrophes.
The chart is suggested for future applications in other healthcare departments. This study demonstrates to academics, policymakers, and public health practitioners that infectious disease management requires personalized tactics and continual monitoring. The complex dynamics of COVID-19 fatalities waves highlight the necessity for adaptive public health strategies. The suggested tools improve epidemiological monitoring with specialized distributions, adding to global infectious disease control efforts.
Acknowledgments
The authors thank the Institute of Medical Artificial Intelligence, the Second Affiliated Hospital, and School of Mathematics and Statistics Xi’an Jiaotong University, Xi’an China, and the University of WAH for offering research facilities.
Author contributions
Conceptualization: Muhammad Waqas.
Data curation: Muhammad Waqas.
Formal analysis: Muhammad Waqas.
Methodology: Muhammad Waqas.
Visualization: Muhammad Waqas.
Writing—original draft: Muhammad Waqas.
Supervision: Song Hua Xu.
Writing—review & editing: Muhammad Usman Aslam, Sajid Hussain, Gilbert Masengo.
Validation: Khurram Shahzad.
Abbreviations:
- Cited Here |
- Google Scholar
applications; control charts; COVID-19; epidemiology; global; healthcare; monitoring
Supplemental Digital Content
- MD_2024_06_14_MASENGO_MD-D-24-04456_SDC1.pdf; [PDF] (943 KB)
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Evaluation of menstrual irregularities following covid-19 infection or..., evaluating private hospital performance before and during covid-19 in china, using the alluvial diagram to display variable characteristics for covid-19....
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
‘Visionary’ study finds inflammation, evidence of Covid virus years after infection
By Isabella Cueto July 3, 2024

R emember when we thought Covid was a two-week illness? So does Michael Peluso, assistant professor of medicine at the University of California, San Francisco.
He recalls the rush to study acute Covid infection, and the crush of resulting papers. But Peluso, an HIV researcher, knew what his team excelled at: following people over the long term.
advertisement
So they adapted their HIV research infrastructure to study Covid patients. The LIINC program, short for “Long-term Impact of Infection with Novel Coronavirus,” started in San Francisco at the very beginning of the pandemic. By April 2020, the team was already seeing patients come in with lingering illness and effects of Covid — in those early days still unnamed and unpublicized as long Covid. They planned to follow people’s progress for three months after they were infected with the virus.
By the fall, the investigators had rewritten their plans. Some people’s symptoms were so persistent, Peluso realized they had to follow patients for longer. Research published Wednesday in Science Translational Medicine builds on years of that data. In some cases, the team followed patients up to 900 days, making it one of the longest studies of long Covid (most studies launched in 2021 or 2022, including the NIH-funded RECOVER program).
Investigators found long-lasting immune activation months and even years after infection. And, even more concerning, they report what looked like lingering SARS-CoV-2 virus in participants’ guts. Even those who’d had Covid but no continuing symptoms had different results than those who’d never been infected.
Related: Listen: Why Long Covid can feel scarier than a gun to the head
The team’s big idea — hypothesizing in early 2020 that, contrary to the popular narrative, Covid would last in the body — was “visionary,” long Covid researcher Ziyad Al-Aly said. “A lot of people don’t think like that.” Al-Aly was not involved with the study, but has published other long-term studies of Covid patients. He is chief of research and development at the VA Saint Louis Healthcare System.
The research makes use of novel technology developed by the paper’s senior authors, Henry Vanbrocklin, professor in the department of radiology at UCSF, and associate professor of medicine Timothy Henrich. They figured out in the last several years they could use an antibody that bound to HIV’s code protein as a guide to see viral reservoirs. The HIV antibody, labeled with radioactive isotopes, could be tracked with imaging as it moved through the body and migrated to infected tissues.
There were no antibodies to latch onto early in the coronavirus pandemic. Vanbrocklin instead used a chemical agent, called F-AraG, that binds to activated T cells — immune cells that flood into infected tissues. They injected F-AraG into patients, and into a scan they went.
Tissues full of activated T cells glowed in the resulting image. Researchers found more glowing sites of immune activation in people who had been infected with Covid than in those who had not, including: the brain stem, spinal cord, cardiopulmonary tissues, bone marrow, upper pharynx, chest lymph nodes, and gut wall.
In people with long Covid symptoms, like brain fog and fatigue, the study found the gut wall and spinal cord lit up more than in other participants. People with continuing pulmonary symptoms showed greater immune activation in their lungs. Gut biopsies in five participants revealed what appears to be persistent virus, said Peluso, who is part of the LongCovid Research Consortium of the PolyBio Research Foundation (which helped fund the study).
Related: ‘Concern is real’ about long Covid’s impact on Americans and disability claims, report says
“The data are striking,” said Akiko Iwasaki, a professor of immunobiology and long Covid researcher at Yale University. Iwasaki was not involved in the study but is also part of PolyBio’s long Covid research group.
Researchers used pre-pandemic scans as a control group, “the cleanest comparison that there is, before anybody on the planet could’ve possibly had this virus,” Peluso said. There were 30 participants in total (24 who’d had Covid, and six controls). Uninfected participants showed some T cell activation, but it showed up in parts of the body that help clear inflammation, like the kidney and liver. In the post-Covid group, immune activation was widespread, even in those who report that they are back to their normal health.
The data don’t explain what exactly T cells are reacting to. As Iwasaki noted, activated T cells can be responding to persistent SARS-CoV-2 antigens or autoantigens found in people with autoimmune disease. The immune response could also be to antigens coming from other pathogens, like the common Epstein-Barr Virus. This piece requires more study, she said.
In the gut, the researchers found what they think is RNA that encodes the virus’s signature spike protein. Other studies have found similar pieces of virus in autopsies, or within a couple of months after infection. Peluso’s work suggests the virus may stay in the body much longer — up to years after infection.
The researchers don’t know if what they’re seeing is “fossilized” leftover virus or active, productive virus. But they found double-stranded RNA in the guts of some patients who underwent biopsy. That should technically only be there if a virus is still alive, going through its life cycle, Peluso said.
Related: Long Covid research gets a big-time funding boost
Scientists and patient advocates have been suspicious for a while of the gut reservoir post-Covid. This new data may add fuel to the idea that SARS-CoV-2 stays in some people’s guts for a long time and could actually be driving long Covid. Or, on the other hand, it could mean our immune response is failing to clear the virus and leaving behind little pieces (which might not be harmful). There are still a lot of questions, Peluso admitted. But the paper undermines the paradigm that declares Covid infection disappears after two weeks, and long Covid is just residual damage.
The findings also suggest a need for more aggressive evaluation of immunomodulating therapies, and treatments that target leftover virus.
Most researchers hunting for a long Covid biomarker have turned to the blood or small pieces of tissue as surrogates for what’s happening inside a patient. With the new imaging technique, Peluso and his team can see a full person on their screen — a patient’s phantom figure and gauzy organs covered in splotches of light. “It’s really striking,” he said. “‘Oh, my goodness, this is happening in someone’s spinal cord, or their GI tract, or their heart wall, or their lungs.’”
For patients like Ezra Spier, a member of the LIINC cohort who’s had imaging done after the period captured in this latest study, the experience was validating. Finally, the life-changing experience of long Covid had become visible. “ I can now see with my own eyes the kind of dysfunction going on throughout my own body,” said Spier, who created a website for long Covid patients to more easily find clinical trials near them.
Most participants had been infected with a pre-Omicron variant of the virus, and one person had repeat infections throughout the study period. Two participants had been hospitalized during their initial bout of Covid, but neither one received intensive care. A half-dozen patients in the study reported zero long Covid symptoms, but still showed elevated levels of immune activation.
Related: Could long Covid’s signs of immune dysregulation in the blood lead to a diagnostic test?
The paper does not explain what the sites of infection mean for symptoms, and immune activation in a particular organ doesn’t correspond to symptoms (for example, a gut full of T cells doesn’t necessarily match with GI problems). More studies are needed to figure out what the glowing spots mean for patients’ experience of long Covid.
And the scans don’t work as a diagnostic. In other words, patients shouldn’t rush to San Francisco (Peluso’s group only accepts study participants from the area). The imaging technique isn’t available to the general public, either. F-AraG is still being studied in this context.
But Peluso and Vanbrocklin said imaging could be a major tool in figuring out long Covid. They’ve expanded their research program to do imaging on about 50 additional patients. They are also scanning people before and after they receive different long Covid clinical trial interventions to see if there’s a change in immune activity.
About the Author Reprints
Isabella cueto.
Chronic Disease Reporter
Isabella Cueto covers the leading causes of death and disability: chronic diseases. Her focus includes autoimmune conditions and diseases of the lungs, kidneys, liver (and more). She writes about intriguing research, the promises and pitfalls of treatment, and what can be done about the burden of disease.
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

STAT Plus: Pharmalittle: We’re reading about biopharma layoffs, Purdue bankruptcy talks, and more

uniQure shares soar on Huntington’s data

STAT Plus: Troubled for-profit chains are stealthily operating dozens of psychiatric hospitals under nonprofits’ names

STAT Plus: Ascension is racing to unload hospitals as execs work to stem losses

STAT Plus: Pulling back the curtain on psychiatric hospital joint ventures

Flawed Autopsy ‘Review’ Revives Unsupported Claims of COVID-19 Vaccine Harm, Censorship
By Jessica McDonald
Posted on July 5, 2024
SciCheck Digest
COVID-19 vaccination is generally very safe, and except for extremely rare cases, there is no evidence that it contributes to death. Social media posts about a now-published, but faulty review of autopsy reports, however, are repeating an unfounded claim from last summer that “74% of sudden deaths are shown to be due to the COVID-19 vaccine.”
More than half a billion doses of COVID-19 vaccines have now been administered in the U.S. and only a few, very rare, safety concerns have emerged. The vast majority of people experience only minor, temporary side effects such as pain at the injection site, fatigue, headache, or muscle pain — or no side effects at all. As the Centers for Disease Control and Prevention has said , these vaccines “have undergone and will continue to undergo the most intensive safety monitoring in U.S. history.”
A small number of severe allergic reactions known as anaphylaxis, which are expected with any vaccine, have occurred with the authorized and approved COVID-19 vaccines. Fortunately, these reactions are rare, typically occur within minutes of inoculation and can be treated. Approximately 5 per million people vaccinated have experienced anaphylaxis after a COVID-19 vaccine, according to the CDC.
To make sure serious allergic reactions can be identified and treated, all people receiving a vaccine should be observed for 15 minutes after getting a shot, and anyone who has experienced anaphylaxis or had any kind of immediate allergic reaction to any vaccine or injection in the past should be monitored for a half hour. People who have had a serious allergic reaction to a previous dose or one of the vaccine ingredients should not be immunized. Also, those who shouldn’t receive one type of COVID-19 vaccine should be monitored for 30 minutes after receiving a different type of vaccine.
There is evidence that the Pfizer/BioNTech and Moderna mRNA vaccines may rarely cause inflammation of the heart muscle (myocarditis) or of the surrounding lining (pericarditis), particularly in male adolescents and young adults .
Based on data collected through August 2021, the reporting rates of either condition in the U.S. are highest in males 16 to 17 years old after the second dose (105.9 cases per million doses of the Pfizer/BioNTech vaccine), followed by 12- to 15-year-old males (70.7 cases per million). The rate for 18- to 24-year-old males was 52.4 cases and 56.3 cases per million doses of Pfizer/BioNTech and Moderna vaccines, respectively.
Health officials have emphasized that vaccine-related myocarditis and pericarditis cases are rare and the benefits of vaccination still outweigh the risks. Early evidence suggests these myocarditis cases are less severe than typical ones. The CDC has also noted that most patients who were treated “responded well to medicine and rest and felt better quickly.”
The Johnson & Johnson vaccine has been linked to an increased risk of rare blood clots combined with low levels of blood platelets, especially in women ages 30 to 49 . Early symptoms of the condition, which is known as thrombosis with thrombocytopenia syndrome, or TTS, can appear as late as three weeks after vaccination and include severe or persistent headaches or blurred vision, leg swelling, and easy bruising or tiny blood spots under the skin outside of the injection site.
According to the CDC, TTS has occurred in around 4 people per million doses administered. As of early April , the syndrome has been confirmed in 60 cases, including nine deaths, after more than 18.6 million doses of the J&J vaccine. Although TTS remains rare, because of the availability of mRNA vaccines, which are not associated with this serious side effect, the FDA on May 5 limited authorized use of the J&J vaccine to adults who either couldn’t get one of the other authorized or approved COVID-19 vaccines because of medical or access reasons, or only wanted a J&J vaccine for protection against the disease. Several months earlier, on Dec. 16, 2021 , the CDC had recommended the Pfizer/BioNTech and Moderna shots over J&J’s.
The J&J vaccine has also been linked to an increased risk of Guillain-Barré Syndrome, a rare disorder in which the immune system attacks nerve cells. Most people who develop GBS fully recover, although some have permanent nerve damage and the condition can be fatal.
Safety surveillance data suggest that compared with the mRNA vaccines, which have not been linked to GBS, the J&J vaccine is associated with 15.5 additional GBS cases per million doses of vaccine in the three weeks following vaccination. Most reported cases following J&J vaccination have occurred in men 50 years old and older.
Link to this
Last July, an unpublished paper authored by several physicians known for spreading COVID-19 misinformation briefly appeared on a preprint server hosted by the prestigious British medical journal the Lancet.

The paper claimed to have reviewed autopsy reports and found — in the opinion of three of its authors — that 73.9% of the selected deaths were “directly due to or significantly contributed to by COVID-19 vaccination.” Those conclusions, however, were often contrary to the original scientists’ determinations. Moreover, abundant evidence contradicts the suggestion that the COVID-19 vaccines are frequently killing people.
The preprint repository quickly removed the manuscript because, it said, “the study’s conclusions are not supported by the study methodology,” and indicated that the preprint had violated its screening criteria.
Social media soon flooded with posts highlighting the purported findings and alleging censorship, with many falsely stating that the paper had been published in the Lancet.
Multiple scientists and fact checkers detailed numerous problems with the preprint and the resulting social media posts. As Dr. Jonathan Laxton, an assistant professor of medicine at the University of Manitoba who frequently debunks misinformation online, wrote at the time on Twitter, “this is not a conspiracy, the paper was literally biased hot garbage and the Lancet was right to remove it.”
Despite these efforts, the same claims are back this summer after the paper was published in the journal Forensic Science International on June 21. Capitalizing on the paper’s now-published status, numerous posts are once again spreading the review’s supposed findings and realleging censorship.
“Largest autopsy series in the world. Censored by what was the most reputable peer reviewed journal,” reads one popular Instagram post. “74% of the 325 Suddenly Died Autopsies point the cause to the dart,” it added, using coded language to refer to the COVID-19 vaccines.
Another post , from Dr. Sherri Tenpenny , an osteopathic physician in Ohio known for her opposition to vaccines and her false claim that the COVID-19 vaccines magnetize people, also repeated the falsehood that the paper had been previously published in the Lancet.
“Bottom line results: 74% of sudden deaths are shown to be due to the COVID-19 vaccine,” the post went on to say. “This paper is a game changer. Sadly, it was censored for ONE YEAR. Just think of all the lives that could have been saved.”
As we’ve explained before , publication in a peer reviewed journal does not necessarily mean a paper is accurate or trustworthy, although the process can improve manuscripts and weed out bad science. In this case, the published paper is highly similar to the previously criticized manuscript. Experts say its conclusions are unreliable and misleading.
“The vast majority of these cases do not show a causal, but coincidental, effect,” wrote Marc Veldhoen, an immunologist at the Instituto de Medicina Molecular João Lobo Antunes in Portugal, in a thread on X, addressing the paper’s central claim. “This certainly does not apply to the general population!”
When asked about the published paper, Dr. Cristina Cattaneo, co-editor-in-chief of Forensic Science International, told us the journal was “currently looking into the matter.”
Problematic ‘Review’
For their “ review ,” the authors searched the medical literature for published autopsy studies related to any kind of COVID-19 vaccination. After excluding duplicates and studies without deaths, autopsies, or vaccination status information, the authors were left with 44 studies comprising 325 autopsies. Three of the authors then reviewed the described cases and decided for themselves if the deaths were vaccine-related; if at least two agreed, the death was counted as being attributable to COVID-19 vaccination.
In the end, the authors thought 240, or nearly 74%, of the reviewed autopsies were vaccine-related (rounded to one decimal, 240 out of 325 is actually 73.8%, not 73.9% as reported in the paper). Among these deaths, 46.3% occurred after a Sinovac vaccine, 30.1% after a Pfizer/BioNTech vaccine, 14.6% after an AstraZeneca vaccine, 7.5% after a Moderna vaccine and 1.3% after a Johnson & Johnson vaccine.
As others have pointed out before, there’s reason to suspect that the authors may have been biased in their determinations. All three adjudicators, including Dr. Peter McCullough , are well known for spreading COVID-19 misinformation. Dr. William Makis, a Canadian radiologist, has previously claimed , without evidence, that 80 Canadian doctors died from COVID-19 vaccines. The only pathologist, Dr. Roger Hodkinson, incorrectly claimed in 2020 that COVID-19 was a “hoax” and “just a bad flu.”
Hodkinson and McCullough, along with five other authors, are also affiliated with and have a financial interest in The Wellness Company, a supplement and telehealth company that sells unproven treatments , including for purported protection against vaccines.
Perhaps most tellingly, the scientists who conducted many of the autopsy studies came to opposite conclusions than the review authors. Of the 240 cases, for example, 105 come from a single paper in Colombia, whose authors found “[n]o relation between the cause of death and vaccination.”
Similarly, the review authors counted 24 of 28 autopsies from a study from Singapore as vaccine-related, even though the original authors identified “no definite causative relationship” to mRNA vaccines.
The authors of a German study also attributed 13 of 18 autopsy deaths to preexisting diseases, but the review authors decided 16 cases were vaccine-related.
In a LinkedIn post debunking the preprint, Dr. Mathijs Binkhorst , a Dutch pediatrician, went back to each cited paper, and found that of the 325 autopsies and one heart necropsy the review authors said were vaccine-related, only 31, or 9.5%, were likely related and 28, or 8.6%, were possibly related. The rest — 267, or 81.9% — were unlikely, uncertainly, or not related to vaccination.
In other words, even among a set of studies that is more likely to identify some vaccine involvement, less than a fifth of deaths were possibly or likely vaccine-related.
Even if the authors aren’t biased, this type of study is not able to provide information on how frequently COVID-19 vaccination leads to death, and whether the risks outweigh the benefits.
“They only looked at ‘published autopsy and necropsy reports relating to COVID-19 vaccination,’” Veldhoen said of the published study on X. “If you look only at autopsies of those related (in time) with drugX: X-involvement is then a high proportion of all cases.”
Indeed, as Binkhorst noted, the autopsy reports come from 14 countries that collectively administered some 2.2 billion vaccine doses. If the COVID-19 vaccines truly were as dangerous as the review authors contend, this would be evident in other data sources — but it’s not.
Vaccine safety surveillance systems and other studies from across the globe have found that serious side effects can occur, but they are rare.
The Johnson & Johnson and AstraZeneca vaccines, for example, can in very rare cases cause a dangerous and sometimes fatal blood clotting condition combined with low blood platelets.
Rarely, the mRNA COVID-19 vaccines from Moderna and Pfizer/BioNTech have caused inflammation of the heart muscle or surrounding tissue, known as myocarditis or pericarditis. In almost all cases, however, those conditions are not deadly.
There is no evidence that COVID-19 vaccination increases the risk of death and has led to excess deaths or a large number of deaths. Instead, a wealth of data supports the notion that COVID-19 vaccines protect against severe disease and death from COVID-19. The flawed autopsy “review” doesn’t change this.
Roley, Gwen. “ Misinformation swirls around unpublished paper on Covid-19 vaccine risks .” AFP. 14 Jul 2023.
Hulscher, Nicolas et al. “ A Systematic REVIEW of Autopsy findings in deaths after covid-19 vaccination .” Forensic Science International. Available online 21 Jun 2024.
Binkhorst, Mathijs. “ McCullough’s misinformation .” LinkedIn post. Archived 4 Sep 2023.
Laxton, Jonathan (@dr_jon_l). “ McCullough et al attempted upload a preprint to the Lancet server, and it was removed because it was hot garbage. However, I feel going through this paper for you guys will help you spot dodgy science … ” X. 6 Jul 2023.
Payne, Ed. “ Fact Check: A ‘Lancet Study’ Does NOT Show COVID Vaccine Caused 74% Of Deaths In Sample — Lancet Rejected Paper And Its Methods .” Lead Stories. 7 Jul 2023.
Carballo-Carbajal, Iria. “ Flawed preprint based on autopsies inadequate to demonstrate that COVID-19 vaccines caused 74% of those deaths .” Health Feedback. 31 Jul 2023.
Jaramillo, Catalina. “ Review Article By Misinformation Spreaders Misleads About mRNA COVID-19 Vaccines .” FactCheck.org. 16 Feb 2024.
Veldhoen, Marc (@Marc_Veld). “ Does ‘We found that 73.9% of deaths were directly due to or significantly contributed to by COVID-19 vaccination.’ Hold? No. The vast majority of these cases do not show a causal, but coincidental, effect. This certainly does not apply to the general population! ” X. 22 Jun 2024.
Cattaneo, Cristina. Co-Editor-in-Chief, Forensic Science International. Email to FactCheck.org. 26 Jun 2024.
“ No evidence that 80 Canadian doctors died from COVID vaccinations .” Reuters Fact Check. 22 Dec 2022.
Lajka, Arijeta. “ Pathologist falsely claims COVID-19 is a hoax, no worse than the flu .” AP. 2 Dec 2020.
Yandell, Kate. “ Posts Push Unproven ‘Spike Protein Detoxification’ Regimen .” FactCheck.org. 21 Sep 2023.
Chaves, Juan José et al. “ A postmortem study of patients vaccinated for SARS-CoV-2 in Colombia .” Revista Española de Patología. 31 Oct 2022.
Yeo, Audrey et al. “ Post COVID-19 vaccine deaths – Singapore’s early experience .” Forensic Science International. 19 Jan 2022.
Schneider, Julia et al. “ Postmortem investigation of fatalities following vaccination with COVID-19 vaccines .” International Journal of Legal Medicine. 30 Sep 2021.
Yandell, Kate. “ Study Largely Confirms Known, Rare COVID-19 Vaccine Side Effects .” FactCheck.org. 27 Feb 2024.
“ Selected Adverse Events Reported after COVID-19 Vaccination .” CDC. Accessed 5 Jul 2024.
“ COVID-19 vaccines: key facts .” European Medicines Agency. Accessed 5 Jul 2024.
Robertson, Lori. “ A Guide to Johnson & Johnson’s COVID-19 Vaccine .” FactCheck.org. 27 Feb 2021.
Lai, Francisco Tsz Tsun et al. “ Prognosis of Myocarditis Developing After mRNA COVID-19 Vaccination Compared With Viral Myocarditis .” Journal of the American College of Cardiology. 5 Dec 2022.
Yandell, Kate. “ No Evidence Excess Deaths Linked to Vaccines, Contrary to Claims Online .” FactCheck.org. 17 Apr 2023.
McDonald, Jessica. “ Flawed Analysis of New Zealand Data Doesn’t Show COVID-19 Vaccines Killed Millions .” FactCheck.org. 15 Dec 2023.

IMAGES
COMMENTS
Coronavirus disease 2019 (COVID-19) became a pandemic within a matter of months. Analysing the first year of the pandemic, data and surveillance gaps have subsequently surfaced. Yet, policy decisions and public trust in their country's strategies in combating COVID-19 rely on case numbers, death numbers and other unfamiliar metrics.
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
As a study case, a dataset of 373 CXR images, 139 of which were COVID-19 infected, collected from open sources, was used for diagnosis with deep learning approaches of COVID-19. The use of EfficientNet, an up-to-date and robust deep learning model for education, offers the possibility to become infected with an accuracy of 94.7%.
Consider that nearly all COVID-19 RCTs study the disease in hospital - an important setting, but only a small proportion of COVID-19 patients - and balance that with what can be learned from large observational research in terms of monitoring spread, evaluating symptom severity and understanding disease progression among community-dwelling ...
The present study demonstrates comparative differences in methodological quality scores between COVID-19 literature and historical control articles. Overall, the accelerated publication of COVID ...
Our study may have three limitations. First, some studies published during 2020 may have escaped us. ... Time to address the spatiotemporal uncertainties in COVID-19 research: concerns and challenges. Sci Total Environ. 2021; 764:142866. doi: 10.1016/j.scitotenv.2020.142866. [PMC free article] [Google Scholar] 22. Wang Y, Di Q. Modifiable areal ...
The body's antibody defence against the coronavirus remains strong for at least three months after infection, according to a study of more than 100 people who mostly had mild to moderate COVID-19.
The COVID-19 lockdown halted research collection during the 2-year follow-up visits (ages 11 to 12 years), but eventually resumed. As some youths already underwent their 2-year visits prior to lockdown, this allowed for a natural experiment-like design to compare prepandemic and intrapandemic groups. ... However, this study has limitations. The ...
This cross-sectional study of US adults estimates the prevalence of and sociodemographic factors associated with COVID-19 symptoms lasting beyond 2 [Skip to Navigation] ... This study followed the American Association for Public Opinion Research ... This study has some limitations. First, because this study used preempaneled respondents in a ...
Further research on COVID-19 SRs is essential to improve research quality and also, efficiency among scientists across the world. Background. The COVID-19 pandemic began in early 2020 ... In this study, we classified the reported limitations into 10 categories and 42 sub-categories. Heterogeneity, sample size, follow-up, treatment, including ...
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on ...
The conducted study had three main research questions: ... Many of the limitations related to COVID-19 were a challenge for people with an active lifestyle who would regularly go to the cinema, theater, and gym, use restaurants, and do a lot of travelling. For those people, the time of the COVID constraints has brought about huge changes in ...
Background: Although several studies have assessed the safety, efficacy, and effectiveness of interventions in treating the COVID-19, many of them have limitations that can have an immense impact on their results. This study aims to assess the potential limitations in systematic reviews (SRs) that evaluate the effect of interventions on the treatment of the COVID-19.
While numerous studies have assessed the effects of environmental (meteorological variables and air pollutants) and socioeconomic variables on the spread of the COVID-19 pandemic, many of them, however, have significant methodological limitations and errors that could call their results into question. Our main objective in this paper is to assess the methodological limitations in studies that ...
Background: While numerous studies have assessed the effects of environmental (meteorological variables and air pollutants) and socioeconomic variables on the spread of the COVID-19 pandemic, many of them, however, have significant methodological limitations and errors that could call their results into question. Our main objective in this paper is to assess the methodological limitations in ...
The clinical benefit of coronavirus disease 2019 (COVID-19) treatments against new circulating variants remains unclear. We sought to describe characteristics and clinical outcomes of highest risk patients with COVID-19 receiving early COVID-19 treatments in Scotland. Retrospective cohort study of non-hospitalized patients diagnosed with COVID-19 from December 1, 2021-October 25, 2022, using ...
Background Coronavirus disease 2019 (COVID-19) became a pandemic within a matter of months. Analysing the first year of the pandemic, data and surveillance gaps have subsequently surfaced. Yet, policy decisions and public trust in their country's strategies in combating COVID-19 rely on case numbers, death numbers and other unfamiliar metrics. There are many limitations on COVID-19 case ...
Several pieces of research have let us understand the life experiences and trajectories of these individuals, the challenges that they have faced, and the interventions conducted with them to address this inequality. Nonetheless, the research methodologies in those studies prove short in a situation such as the current COVID-19 pandemic.
The study followed the communicative methodology of research (Gómez González, 2019), which has been recognized for its success in achieving social impact, also when applied in research with vulnerable groups (Plaja Viñas, 2019). In the communicative methodology, researchers share scientific knowledge about the reality under study, and the ...
Researchers say now is the time to decrease digital divide in academic research. (Getty Images) The COVID-19 shelter-in-place orders and other limitations could offer researchers the chance to use technology to decrease the digital divide and disparities in academic research, suggests a University of California, Davis, professor in a new ...
Strengths and limitations. This is the first review to study both the challenges and solutions of conducting clinical research during the COVID‐19 pandemic, providing a practical guide for researchers and policymakers in future similar pandemic conditions. However, this study had some limitations.
in the wake of COVID-19, including unemployment, mental illness, and addiction; and, (3) the importance of moderating factors (e.g., age, race and ethnicity, gender, personality, family status, and culture) for which there are likely to be disparate COVID-19 impacts.
This study was designed to capture the lived experiences of palliative care inpatients during the COVID-19 pandemic. To date, research has largely focused on the perspectives of family members and healthcare providers of patients ... Study strengths and limitations. This study is unique in providing first-hand insights into the lived experience ...
Background In addition to several sequelae of post-COVID-19, individuals also experience significant limitations in work ability, resulting in negative consequences for the return-to-work (RTW) process. This systematic review and meta-analysis were conducted to assess the impact of post-COVID-19 on work ability and RTW of individuals previously infected with SARS-CoV-2. Methods Studies on the ...
The personnel present during COVID-19 autopsies were not systematically tested, but symptomatic persons were extensively PCR tested for SARS-CoV-2 during the study period (2020-2022). In addition, skull sawdust samples might not consist solely of bone and could contain adjacent tissues because of anatomy, particularly the frontal sinus, which ...
Global collaboration in COVID-19 research. The inclusion of multiple countries in COVID-19 studies, such as the USA, UK, China, Italy, Switzerland, Thailand, Iraq, Jordan, Senegal, Pakistan, and more, suggests a collaborative effort in understanding and combatting the pandemic. ... Strength and limitations. This study, which spans 23 years and ...
So they adapted their HIV research infrastructure to study Covid patients. The LIINC program, short for "Long-term Impact of Infection with Novel Coronavirus," started in San Francisco at the ...
More than half a billion doses of COVID-19 vaccines have now been administered in the U.S. and only a few, very rare, safety concerns have emerged. The vast majority of people experience only ...
As part of the UK COVID-19 Human Challenge study, led by Imperial College London, 36 healthy adult volunteers without previous history of COVID-19 were administered SARS-CoV-2 virus through the nose.
During the research, the work address, work phone, and e-mail address of the researcher were given to the participants, so they could reach the researcher with any questions or concerns. ... Limitations. The fact that the study was conducted at a single center is considered a limitation. Therefore, the results cannot be generalized to other ...