An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Materials (Basel)


Synthesis and Applications of Graphene Oxide
Associated data.
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Thanks to the unique properties of graphite oxides and graphene oxide (GO), this material has become one of the most promising materials that are widely studied. Graphene oxide is not only a precursor for the synthesis of thermally or chemically reduced graphene: researchers revealed a huge amount of unique optical, electronic, and chemical properties of graphene oxide for many different applications. In this review, we focus on the structure and characterization of GO, graphene derivatives prepared from GO and GO applications. We describe GO utilization in environmental applications, medical and biological applications, freestanding membranes, and various composite systems.
1. Introduction
Graphene is one of the most studied materials in the world; thanks to its unique properties, it was called a “material of the future” [ 1 ]. Graphene consists only of carbon atoms where every carbon atom is attached to three other carbon atoms with sp 2 hybridized orbitals making a honeycomb lattice [ 2 ]. Graphene’s rare properties make it a very promising material for a huge variety of applications, including field-effect transistors (FETs), gas and biomolecules sensors, transparent conductive films (TCFs), and graphene batteries [ 3 , 4 , 5 , 6 , 7 ].
Graphene oxide (GO) is a layered carbon structure with oxygen-containing functional groups (=O, -OH, -O-, -COOH) attached to both sides of the layer as well as the edges of the plane [ 8 ]. As with any 2D carbon material, GO can also have either single layer or multilayer structure. A structure with one layer is graphene oxide; two layers of graphene oxide are referred to as a two-layered GO. GO with more than two layers and less than five layers is called few-layered graphene oxide, GO with five to ten layers is called multilayered GO, and material with eleven or more layers is called graphite oxide [ 9 ]. GO can be synthesized by the oxidation of graphite into graphite oxide followed by the exfoliation of this graphite oxide into GO. The properties of the material are strongly dependent on the synthesizing method, which influences the resulting number and type of oxygen-containing groups in the formed GO. In contrary to graphene, GO is hydrophilic, and it is hence relatively simple to prepare a water- or organic solvent-based suspensions. Highly oxidized forms of GO are electric insulators with a bandgap of approximately 2.2 eV.
Due to the presence of various oxygen functionalities on the surface of GO, GO can be used as a starting material for the synthesis of graphene derivatives such as fluorographene, bromographene, graphane, and many others. On the other hand, by thermal or chemical reduction of GO, thermally or chemically reduced graphene can be prepared (see Figure 1 ). Interestingly, GO can also be used for advanced applications such as for drug delivery, in high-temperature materials, or in construction materials. There are still some remaining issues that can be improved and studied more intensively. It is very important to develop novel methods of environmentally friendly low-cost large-scale synthesis of GO. In this review, we tried to summarize available knowledge about GO structure, synthesis and characterization of GO, GO functionalization, and selected GO applications.

Scheme of preparation and utilization of GO (colors of atoms: grey—carbon, red—oxygen, yellow—fluorine, white—hydrogen).
2. Structure of GO
Over the years, the structure of GO was studied in detail using several instrumental techniques: annular dark-field imaging, 13 C and 1 H NMR, ultra-high-resolution transmission electron microscopy, X-ray diffraction, and many others [ 10 , 11 , 12 , 13 ]. Despite the number of attempts to reveal the structure of GO, a number of possible structural models exist with no unambiguous one. The main reason for this is the complexity of the material and the originality of every sample with variable stoichiometry [ 14 ]. Simplistically, GO is a monolayer sheet of graphite containing hydroxyl, carboxyl, and epoxy oxygen groups on its basal plane and edges, resulting in a mixture of sp 2 and sp 3 hybridized carbon atoms [ 15 ].
Many models of GO have been developed based on a number of analyses and theoretical simulations. The first model was suggested by Hofmann and Rudolf [ 16 ] in 1939, where a lot of epoxy groups were distributed randomly across the graphite monolayer. Then, in 1946, Ruess [ 17 ] updated the model by incorporating hydroxyl groups and alternating sp 2 hybridized carbons with those revealing sp 3 hybridization. In 1969, Scholz and Boem [ 18 ] suggested a less organized structure with double bonds C=C and periodically recurring C-C single bonds in the carbon layers that are corrugated with hydroxyls and carbonyls, without ether oxygen. Later, in 1994, Nakajima and Matsuo [ 19 ] proposed a model that resembled graphite intercalation compound. Then, in 1998, Lerf and Klinowski [ 11 ] created a model (LK model) that contains two different kinds of regions: regions with six-membered aliphatic rings and regions with nonoxidized benzene aromatic rings (see Figure 2 ). The size of the two regions is dependent on the level of material oxidation. The model is composed mainly of aromatic bodies, epoxide groups, and double bonds. Wrinkling in the monolayer is caused by the slightly distorted tetrahedral configuration of hydroxyl groups attached to carbon atoms. The oxygen functional groups are attached to the monolayer of carbon above and below, creating two layers of oxygen atoms with variable concentrations composed mainly of epoxide and hydroxyl groups that are very close to each other. All of the oxygen functionalities, aromatic bodies, and oxidized rings are distributed randomly across the carbon monolayer. The acidity of GO can be explained by the oxygen groups that are attached to the edges of the lattice, which are hydroxyl and carboxyl groups. This LK model has become one of the most acceptable and used models for moderately oxidized GO.

Lerf–Klinowski model of graphene oxide.
After the discovery of graphene, researchers worldwide started to focus on GO and other derivatives. In 2006, Szábó and Dékány examined previous models by a number of analyses and suggested a model without carboxylic acids composed of two main regions: corrugated hexane ribbons occupied with quinones and ketones, and translinked cyclohexane chairs with 1,3-epoxide and tertiary alcohols. In 2013, Dimiev, Alemany, and Tour [ 20 ] proposed a dynamical structural model (DSM) that describes the development of various carbon structures with attached water, contrary to the static LK model. Recently, Liu et al. [ 21 ] experimentally observed the evidence of the C=O bonds on the edge of the carbon monolayer, confirming parts of the earlier models, especially the LK model [ 22 ]. Real GO also includes some defects, such as topological defects (pentagons, heptagons, octagons, etc.), adatoms, vacancies, and adsorbed impurities.
By using concentrated acids for oxidation, low-molecular-weight fragments are produced, known as oxidation debris [ 23 , 24 ]. Oxidation debris is a mixture of highly oxidized polyaromatic fragments adsorbed on the poorly oxidized GO platelets by π–π stacking, hydrogen bonding, and van der Walls interactions [ 25 ]. The amount of the oxidation debris is strongly influenced by the reaction time of graphite and concentrated acids [ 26 , 27 ]. To wash away those fragments, a base washing is needed [ 27 , 28 , 29 ]. The pure GO without the oxidation debris presents an oxidation level that is similar to chemically reduced GO [ 30 ].
3. Conventional Routes of GO Synthesis
The first attempt to synthesize graphite oxide was performed in 1859 by British chemist B. C. Brodie who investigated the reactivity of flake graphite [ 31 , 32 ]. It is a chlorate route, where potassium chlorate is used as an oxidizing agent. Benjamin Brodie treated graphite with a number of strong oxidizing agents for the first time to decode its structure. In the experiment, he treated graphite in a mixture of potassium chlorate and fuming nitric acid at 60 °C for 4 days (Brodie’s graphene oxide (BR-GO)). He performed multiple oxidative treatments one after another (4–7) and the resulting composition of carbon, oxygen, and hydrogen was estimated as C 11 H 4 O 5 (corresponds to C/O ratio 2.2) [ 32 ]. The product was found to be soluble in pure water, while it tended to flocculate in a more acidic environment. Brodie named the product “graphic acid” because it had a slight reaction with litmus paper. Another chlorate route is the Staudenmaier method [ 33 ]. Later, L. Staudenmaier modified Brodie’s method by adjusting the way the chlorate was added and also adding sulfuric acid into the mixture (ST-GO—Staudenmaier’s graphene oxide). Potassium chlorate was added in small portions into the mixture in order to eliminate the danger of explosive by-products and heat evolution. The increased acidic environment caused a decrease in terms of reaction time. The obtained material has very similar properties to BR-GO. In 1937, Hofmann used potassium chlorate and nonfuming nitric acid to synthesize Hofmann’s graphene oxide (HO-GO) with lower oxygen content (C/O ratio 2.5). It was found that the concentration of nitric acid highly influences the level of oxidation of the resulting graphite oxide or graphene oxide [ 34 ]. The lower the concentration of nitric acid, the higher level of oxidation of graphene oxide.
The most used and effective method of all time is one of the permanganate methods, the Hummers method [ 35 ] created by Hummers and Offeman. It is a relatively fast conventional method used for the synthesis of GO. In this method, the reaction mixture is composed of an excess of potassium permanganate, sulfuric acid, and a small amount of sodium nitrate. The reaction time ranges between 8 and 12 h. This route is much safer because it avoids the creation of explosive ClO 2 . At the end of the reaction, the excess of the potassium permanganate is neutralized with a diluted solution of H 2 O 2 . The product of the Hummers’ method (Hummers’ graphene oxide (HU-GO)) has a very similar C/O ratio (2.25) to the C/O ratio of BR-GO (2.2). Unfortunately, this method is not environment-friendly, because of NO x that evolves during the reaction. There are several modified Hummers methods, including nitrate-free [ 36 , 37 , 38 ], two-step [ 36 , 37 ], co-oxidant [ 38 ], and low- and room-temperature [ 39 , 40 ] methods. Then, in 2010, Tour developed his own method, Tour’s method, which is described below.
4. Modern Ways of GO Synthesis
There are several ways to prepare graphite oxide/graphene oxide. The most common way is to use an oxidizing agent in an acidic environment. Other methods are electrochemical and microbial.
In 2010, a novel method was developed. Tour’s method [ 41 ] (Tour’s graphene oxide (TO-GO)) also falls under permanganate methods. In this procedure, phosphoric acid is mixed with sulfuric acid in the ratio 1:9 and potassium permanganate and graphite added in the ratio 6:1 in an ice bath ( Figure 3 A). The mixture is then heated at 50 °C and stirred for 12 h ( Figure 3 B). After cooling down, the mixture is poured onto ice ( Figure 3 C). Finally, 30% H 2 O 2 is added in order to remove the excess of potassium permanganate ( Figure 3 D). Phosphoric acid works as a dispersive and etching agent, as well as a stabilizer of the oxidation process, which makes the synthesis of GO safe. This route produces a higher yield of GO with a higher level of oxidation and a more regular structure.

Photographs describing preparation process of GO by Tour’s method: ( A ) before addition of potassium permanganate; ( B ) after oxidation; ( C ) after pouring on ice; ( D ) after addition of H 2 O 2.
Besides permanganate and chlorate methods, there are more modern ways to oxidize graphite in order to prepare GO, including the use of potassium chromate in combination with perchloric or nitric acid [ 42 ] or under the Jones conditions [ 43 , 44 ]. Alternatively, less toxic potassium ferrate in sulfuric acid [ 45 ] can be applied for GO preparation. Contrary to these results, another study evidences why it is not possible to prepare GO by using potassium ferrate [ 46 ]. Moreover, graphite can be oxidized in water with H 2 O 2 at 50 °C by Fe(VI) [ 47 ] or at 110 °C with benzoyl peroxide [ 48 ]. Let us note that GO prepared by chemical routes often displays a highly damaged structure due to the harsh acidic conditions of the synthesis as well as the presence of impurities. Such characteristics are indeed far from being optimal for electronics applications. Even though chemical, especially chlorate and permanganate, ways of preparation provide GO with poor electrical properties, the exploration is not at its end yet. For example, in 2017, Jankovský et al. modified Tour’s method and suggested that the shortened reaction time (from 12 h to 1 h) has no significant impact on the resulting material [ 49 ]. In 2018, Ranjan et al. [ 50 ] also modified Tour’s method and proposed the oxidation process of graphite flakes in permanganate (ratio 1:6) in a mixture of sulfuric and phosphoric acids (ratio 9:1) heated at 65 °C for 12 h. All of the chemicals were precooled at 5 °C.
Apart from the chemical routes, electrochemical synthesis represents another approach to GO synthesis that might be the key to large-scale production. Electrochemical production is more eco-friendly than chemical production due to reusing the electrolyte multiple times and minimal washing of the utensils [ 51 , 52 ]. The better quality of electrochemical GO (EGO), in contrast to standard procedures, can be explained by the use of aqueous electrolytes and no need for oxidizing agents, hence avoiding impurities [ 52 ]. Moreover, thanks to the variety of experimental setups, the level of oxidation and density of defects can be controlled.
Interestingly, the usage of biological systems to oxidize graphitic materials is very important to obtain eco-friendly graphene oxide. However, after the microbial cultivation, graphite is not homogeneously oxidized. Acidithiobacillus ferrooxidans or Pseudomonas have been tested [ 53 ] as oxidizing bacteria.
5. Derivatives of GO
Derivatives of graphene oxide are materials based on GO as a starting material. These involve graphene acid (GAF), a highly oxidized GO exhibiting a composition close to [C 1 (COOH) 1 ] n ; chemically reduced graphene oxide (CRG) and thermally reduced graphene oxide (TRG), which are reduced forms of GO with some remaining oxygen functionalities left in the structure; and fluorographene, a fluorinated form of graphene with composition [C 1 F 1 ] n (see Figure 1 ). The functionalization of graphene oxide is possible thanks to the presence of oxygen functionalities, unlike in other carbon nanomaterials.
For a closer inspection of the essential characteristics of those derivatives, we performed transmission electron microscopy ( Figure 4 left), scanning electron microscopy ( Figure 4 right), and energy-dispersive spectroscopy ( Figure 5 ) to study the surface and composition of the samples. The first micrographs in Figure 4 of GAF show a wrinkled structure, where the flakes of GAF are connected together forming a foil-like structure. Next, micrographs of [C 1 F 1 ] n show small flakes of fluorographene which consist of multiple wrinkled sheets. Whereas micrographs of CRG do not show such wrinkled structure of multiple sheets, the last micrographs of TRG show small flakes of multiple-sheet structure which are highly wrinkled. According to EDS (see Figure 5 ), all samples consist of carbon, oxygen, and sulfur except for fluorographene which consists of carbon, oxygen, and fluorine.

TEM ( left ) and SEM ( right ) micrographs of graphene acid (GAF), fluorographene (C 1 F 1 ), chemically reduced graphene (CRG), and thermally reduced graphene (TRG).

TEM and EDS micrographs of graphene acid (GAF), fluorographene (C 1 F 1 ), chemically reduced graphene (CRG), and thermally reduced graphene (TRG).
Graphene acid (GA) is a graphene derivative with a composition close to [C 1 (COOH) 1 ] n . The synthesis of such material consists of two consecutive oxidation steps of graphite. After the first oxidation by the Tour method, GO is obtained and further used as a starting material for the second oxidation. The second oxidation runs according to the Tour method as well [ 54 ]. Further oxidation leads to a total decomposition of GA (oxidation to CO 2 ). Another possible way to synthesize GA is by acidic hydrolysis of cyanographene (graphene–nitrile) by 20% HNO 3 [ 55 ].
GO is mostly used for the production of graphene (reduced graphene oxide (rGO)) by chemical (chemically reduced graphene (CRG)) or thermal (thermally reduced graphene (TRG)) reduction. Reduced graphene oxide can be used in electronic devices, energy storage devices, (bio)sensors, biomedical applications, supercapacitors, membranes, catalysts, and water purification. As an electronic device, rGO is used in field-effect transistors (FETs) as chemical sensors and biosensors [ 56 , 57 , 58 , 59 ]. rGO was also used in light-emitting diodes (LEDs) as a transparent electrode [ 60 , 61 ]. Thanks to the extreme surface area of rGO, the material is used as an electrode in double-layered capacitors, batteries, fuel cells, and solar cells [ 62 , 63 ]. Energy storage capacity and cycle stability of Li-ion battery devices can be enhanced using Fe 3 O 4 on rGO anode rather than pure Fe 3 O 4 or Fe 2 O 3 [ 64 ]. Stacked sheets of GO have nanocapillaries between individual sheets, which are closed by chemical reduction of GO, creating a material that is impermeable to liquids, gases, and even strong chemicals. Corrosive acids can be stored in glass or copper containers that are covered inside with such graphene paint [ 65 , 66 ]. In order to improve shelf life in medical infrastructure, graphene-coated plastic films may be used [ 67 ].
Graphene oxide can be reduced thermally simply by applying heat, a process called thermal annealing reduction. Firstly, the exfoliation of GO occurs during rapid heating, where gases such as CO 2 , CO, and H 2 O are released from the sample [ 68 , 69 , 70 ]. During the rapid increase in temperature, the oxygen-containing groups transform into gases mentioned above that generate huge pressure between the stacked layers of graphene oxide. At approximately 300 °C, the pressure reaches ~40 MPa, and it is increased to ~130 MPa if the temperature is raised to 1000 °C [ 71 ]. In fact, a pressure as low as 2.5 MPa is enough to separate two stacked layers of GO, as predicted by the evaluation of the Hamaker constant [ 71 ]. The originated platelets can be referred to as graphene or TRG because the elevated temperature causes decomposition of oxygen-containing functional groups which leads to the exfoliation of the material. This could be a good strategy for bulk rGO synthesis; however, this route yields only small-sized and wrinkled graphene sheets [ 68 , 72 ]. This effect is caused by the removal of carbon atoms during the transformation of oxygen-containing groups into the above-mentioned gases, which splits wide sheets of graphene oxide into small-sized graphene sheets [ 73 , 74 ]. High temperatures of the thermal reduction lead to the emissions of highly toxic volatile organic hydrocarbons [ 75 ]. Another way to fabricate reduced graphene oxide is by a liquid-phase exfoliation in an inert atmosphere. This route is highly affected by the temperature of GO reduction [ 6 , 68 , 76 , 77 ]. At a temperature lower than 500 °C, the C/O ratio is not higher than 7, while when the temperature reaches 750 °C, the C/O ratio is very likely to be higher than 13. In addition to this effect, there is also a great importance of the used annealing atmosphere. Annealing reduction of GO can be carried out in vacuum [ 6 ], inert [ 77 ], or reducing atmosphere [ 69 , 77 , 78 , 79 ].
The first option to reduce GO chemically is using the reducing agent at room temperature or at slightly elevated temperature. It is an easy and cheap way for the mass production of graphene in comparison to the thermal reduction route. Hydrazine was the first chemical compound used for the reduction of GO even before the discovery of graphene [ 80 ]. Stankovich et al. reported the preparation of chemically derived graphene using hydrazine [ 81 , 82 ]. Apart from hydrazine, its derivatives such as hydrazine hydrate and dimethylhydrazine can be also used to reduce GO [ 83 ]. The reduction is achieved by adding the liquid reagents to a GO aqueous suspension, where the graphene-based nanosheets are agglomerated due to the increased hydrophobicity. Another great chemical reducing reagent is ascorbic acid (vitamin C), which is considered to be an ideal hydrazine substitute [ 84 ]. The resulting material has a very similar C/O ratio as the one reduced by hydrazine, but vitamin C has a great advantage of nontoxicity. Moreover, the colloid state reduction of vitamin C does not bring about a product agglomeration, which is helpful for further applications. In addition, using Ar + ion irradiation of GO foils creates highly conductive graphene papers [ 85 ].
Fluorographene is fluorinated graphene with stoichiometry [C 1 F 1 ] n [ 86 , 87 ]. As with every graphene/graphene oxide derivative, it has extraordinary electronic, optical, physical, and chemical properties that make it one of the thinnest insulators with a wide electronic gap [ 88 ]. The preparation of such a material can be divided into two strategies. The first one is based on the exfoliation of bulk graphitic materials containing fluorine atoms, while the second strategy relies on the fluorination of graphene or graphene oxide with fluorinating agents. Exfoliation can be performed in a liquid phase or mechanically. In the liquid-phase exfoliation, a medium is used to weaken the van der Waals interactions between the layers, resulting in single- or few-layer fluorographene [ 89 ]. For the first time, sulfolane was used and the mixture of the solvent and bulk graphite fluoride was sonicated for 1 h at 50 °C [ 90 ]. Isopropanol [ 91 ], ethanol [ 92 ], acetonitrile [ 93 ], and chloroform [ 94 ] can be mentioned as other reported solvents for the exfoliation of bulk graphite fluoride. Another strategy to prepare fluorographene is to combine graphene with fluorination agents such as xenon difluoride in a reactor [ 95 ]. The process can be initiated by exposing the reactants to temperature, irradiation, or pressure. Fluorographite and fluorographene can be used as a precursor for the synthesis of highly hydrogenated graphene (graphane) [ 96 ].
6. Typically Used Analytical Methods for GO
In order to provide typical analytical results of GO, a sample of GO was prepared by modified Tour’s method [ 49 ] and analyzed by SEM, EDS, and TEM. Usually, Raman spectroscopy, XPS, XRD, XRF, EA, EDS, AFM, and STA-MS are also used for the characterization.
Transmission electron microscopy (TEM), energy-dispersive spectroscopy (EDS), and scanning electron microscopy (SEM) were used to study the surface of GO. The first micrographs in Figure 6 A of TO-GO show wrinkled sheets of graphene oxide by both TEM and SEM methods. According to EDS (see Figure 6 B), the sample consists of carbon, oxygen, and sulfur. Let us note that the hydrogen is not visible on EDS.

TEM ( left ) and SEM ( right ) micrographs ( A ) and EDS micrographs ( B ) of GO.
The composition of the sample can be determined via X-ray fluorescence (XRF). The composition of GO depends on the level of oxidation of the sample. Generally, graphene oxide is composed of carbon, oxygen, and hydrogen, but there are very often other impurities from the starting materials, such as sulfur, chlorine, nitrogen, manganese, and potassium [ 97 ]. Manganese and potassium are contaminants that remain from the starting reactants (potassium permanganate), and chlorine remains from hydrochloric acid that is usually used in order to wash away the contaminants.
The composition of TO-GO can be also investigated by elemental analysis (EA) [ 98 ]. The results can also prove the presence of impurities such as sulfur and nitrogen, depending on the used synthesis method. Sulfur and nitrogen remain from the sulfuric acid and nitric acid, respectively, that are used as starting chemicals to synthesize GO. EA is a suitable method to determine hydrogen content; however, the determination of oxygen is not precise due to the indirect calculation.
Raman spectroscopy is a powerful tool for the determination of defect rate. Using Raman spectroscopy, two local maxima around 1350 cm −1 and 1600 cm −1 , respectively, are usually registered. The first local maximum is called the D band and shows a quasitetrahedral coordination with sp 3 carbon hybridization (irregularities such as functional groups). The other local maximum is called the G band and detects a planar arrangement with sp 2 hybridization (regular graphene lattice). The D/G ratio, calculated from the intensity of peaks, shows the level of oxidation of the sample and should be around 1.00 [ 99 ].
X-ray diffraction is used to determine the interlayer distance between GO layers. Whereas pure graphite has (002) reflection at 26.3° that corresponds to the interlayer distance of 3.342 Å [ 100 ], the (002) reflection for GO can be found around 10.0–12.0° [ 101 , 102 , 103 , 104 , 105 ], indicating an interlayer distance of around 7.4–9.0 Å. This significant increase in the interlayer distance of pristine graphite and graphene oxide is caused due to oxygen functional groups attached to the carbon layer.
GO may be analyzed by X-ray photoelectron spectroscopy (XPS) to determine the composition of the sample surface and more interestingly to determine the ratio of individual functional groups. Two local maxima are usually detected around ~284.4 eV for the C1s peak and around ~532.4 eV for the O1s peak. Maxima for N1s, S2s, and S2p peaks might also be found, showing the contamination of the sample by sulfur and nitrogen. The composition is very variable and depends on the level of oxidation of the sample [ 106 ]. The deconvolution of the C1s peak can be used in order to quantify individual bonds that are present in the samples, for a sample of deconvoluted C1s peak. From the C1s peak, six kinds of bonds can be distinguished: C–C at ~284.5 eV; C=C at ~285.2 eV; C-O at ~286.2 eV; C=O at ~287.8 eV, O–C=O at ~289.0 eV; and π–π* interaction at ~291.0 eV. From the O1s peak, four kinds of bonds with the following binding energy may be identified: O-C=O at ~531.2 eV, C=O at ~ 532.5 eV, C-OH at ~533.3 eV, and C-O-C at ~534.0 eV [ 107 ].
With simultaneous thermal analysis in an inert atmosphere, the temperature of exfoliation is investigated. The literature claims that the temperature of the exfoliation of graphene oxide is around 200 °C [ 108 ]. The obtained value is dependent on the heating rate and other conditions. The process of exfoliation, where stacked layers of graphene oxide are divided into individual reduced graphene oxide sheets, is accompanied by the formation of gases; gases detected by mass spectrometer were H 2 O, CO, and CO 2 .
Atomic force microscopy (AFM) can be employed in order to study the thickness of the GO sample, therefore investigating the number of layers. It is known from the literature that a monolayered sample of GO has a thickness of 0.8–1.2 nm [ 109 , 110 ]. Shenghua Lv et al. reported AFM results of graphene oxide nanosheets with a sample thickness of less than 7.7 nm (see Figure 7 ).

Typical AFM image of GO ( a ); surface patterns from AFM in positions 1, 2, and 3 ( b ) [ 111 ] (with the approval of MDPI Materials ).
7. Applications of GO
7.1. environmental applications of go.
One of the biggest threats to the environment is air pollution caused by the industrial release of harmful gases such as CO 2 , CO, NO 2 , and NH 3 . Thanks to the oxygen groups decorating the basal planes and the edges, GO is capable of covalent or noncovalent interactions with various molecules. GO can be employed in catalysis for converting polluting gases during industrial processing. The elimination of such harmful gases can be performed by capturing and storing gases, catalyst reactions of gas conversion, or direct utilization [ 112 ]. Apart from gas pollution, water pollution also represents a very huge environmental problem. The approach of GO application in this area can be divided into two paths: pollutant adsorption and conversion. The main water pollutants are heavy metal ions and organic dyes; they strongly threaten humans, aquatic life, animals, and plants.
7.1.1. Removal of Toxic Gases
The functional groups of few-layered GO composites exhibit unique adsorption behavior towards CO 2 [ 113 , 114 , 115 , 116 ]. Laminar GO structures were assembled having fast and selective channels for gas separation with excellent preferential CO 2 permeation performance [ 117 ]. Multi-permselective mixed matrix membranes and other mixed matrix membranes for efficient separation of CO 2 were developed, enhancing the diffusivity selectivity, solubility selectivity, and reactive selectivity [ 118 , 119 , 120 , 121 , 122 ]. In addition, GO-based composites have a unique ammonia adsorption capability [ 123 , 124 , 125 , 126 , 127 ]. Moreover, other harmful gases such as acetone [ 128 , 129 ], formaldehyde [ 130 ], H 2 S [ 131 , 132 , 133 ], SO 2 [ 134 , 135 , 136 ], and NO x [ 137 , 138 ] can be adsorbed by GO-based composites.
7.1.2. Conversion of CO 2
Thanks to the unique electronic properties, GO-based composites exhibit superior photocatalyst abilities for CO 2 conversion [ 139 , 140 ]. Hsu et al. [ 141 ] reported the photocatalytic conversion of carbon dioxide to hydrocarbons or their derivatives such as methanol for possible simultaneous CO 2 reduction and solar energy harvesting.
7.1.3. Water Purification
GO exhibits high adsorption ability towards Cd(II), Co(II), Au(III), Pd(II), Ga(III), and Pt(IV) [ 142 , 143 , 144 ]. Researchers Klímová et al. explored the adsorption ability of GO towards the whole periodic table. Adsorption ability mainly depends on the synthesizing method [ 145 ]. Few-layered graphene oxide nanosheets show a very high affinity towards Pb(II) ions, with a sorption capacity of about 842 mg g −1 at 293 K [ 146 ]. On the other hand, the adsorption capacity of Cu 2+ ions is very low, even with oxygen groups on GO acting as active sites [ 147 ]. With the assistance of organic compounds, GO can provide more feasible anchoring sites for heavy metal ions [ 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 ]. Additionally, graphene oxide provides the ability to adsorb other harmful water pollutants—organic dyes [ 156 , 157 , 158 , 159 ]. Molla et al. [ 160 ] reported that the selectivity of positive dye methylene blue and rhodamine B was rapid (within 15 min) with the efficiencies of 97% and 88%, respectively, whereas the negative dye, methyl orange, was not absorbed.
7.2. Medical and Biological Applications of GO
The first possible application in this field is GO-based biosensors. GO-based biosensors rely on their preferred interaction with single-strand DNA (ssDNA) rather than double-strand DNA (dsDNA). This effect is caused by the effective hiding of nucleo-bases in dsDNA in a helical structure, which prevents GO from direct interaction with nucleo-bases [ 161 , 162 , 163 ].
Another interesting application is gene delivery, which is a promising way to treat genetic disorders, including cancer. The therapy uses gene vectors protecting DNA from nuclease degradation. GO sheets have been covered by polyethyleneimine (PEI) as a surface modifier for gene delivery into the cells. The delivery runs through complexation by electrostatic interaction and covalent conjugation for the loading of plasmid DNA (pDNA) [ 161 , 164 ].
Small-molecule drug delivery seems to be another promising medical application of GO. Small molecules of drugs can be attached to a GO surface using pH-sensitive linkers. A complex of doxorubicin and GO (DOX-GO) shows a release of DOX from GO dependent on pH due to higher solubility of DOX at low pH [ 165 ]. Moreover, cancer-targeting was successfully manifested as a codelivery of camptothecin (CPT) using folic acid conjugated nano-GO (FA-NGO) [ 166 ].
7.3. GO Membranes
GO membranes may be used as ionic and molecular sieves or for selective gas transport. GO membranes were first introduced to the world by Nair et al. [ 167 ]. It was reported that a membrane of pure graphene oxide can block everything except for water vapor (see Figure 8 A). Nair et al. claimed that a GO membrane allows only water vapor to pass through ( Figure 8 B), while ethanol and other alcohol molecules are blocked from passing through. The membrane can be prepared by vacuum filtration or by spraying a suspension of GO on a solid surface and then etching away the membrane from the surface. Others reported a study exploring the dependence of gases that pass through on the number of layers of GO. In other words, the selective diffusion of gases can be accomplished by the regulation of gas flow pores and channels by various stacking strategies [ 113 ].

Membrane system scheme ( A ); schematic of possible water transport mechanism ( B ).
As a reaction to Nair’s paper, Sun et al. [ 168 ] demonstrated a selective ion penetration through the GO membrane. They reported that salts of heavy metals and organic pollutants take a much longer time to permeate than sodium salts that pass through quite freely. The reason for this observation is that the salts of metals pass through capillaries in the membrane, where the heavy metal ions create coordination between the GO membrane and those ions that block the permeation. In 2014, Gao et al. [ 169 ] proposed the use of an ozonated GO membrane, having more oxygen functional groups, to improve proton conductivity in fuel cell applications at higher humidity. The water surface was used as a template for assembling the GO film on it by using the amphiphilicity of the GO [ 170 ].
7.4. High-Temperature Materials and GO
Graphene and graphene oxide are very promising materials for the reinforcement and general enhancement of mechanical properties of high-temperature materials. Some researchers studied the effects of graphene oxide on high-temperature materials such as metal alloys and ceramics. The mechanical resistance can be significantly improved by only 1 vol.% of GO. High energy ball milling was used to disperse graphene oxide powder in an aluminum (AlMg5) alloy matrix. Hot pressing was used to densify the obtained material [ 171 ]. More frequently, the reinforcement of high-temperature ceramics or graphene oxide/reduced graphene oxide coatings has been found to achieve better corrosion resistance. Spark plasma sintered Si 3 N 4 ceramic matrix was enriched by multilayered graphene or graphene oxide to study the influence of the addition on mechanical, tribological, and electrical properties. The addition of multilayered graphene caused higher hardness, modulus, and bending strength in comparison to graphene oxide addition. However, the addition of graphene oxide and multilayered graphene resulted in lower mechanical properties but better electrical and tribological properties [ 172 ].
7.5. Building Materials and GO
Ordinary Portland cement (OPC) is one of the most used materials in the field of civil engineering thanks to the thirst for urbanization. The concrete is produced by mixing aggregates, binder (OPC), and water for hydration. Concrete has its advantages, such as unique compressive strength, as well as disadvantages, including poor crack formation resistance or low tensile strength [ 173 ]. Researchers have attempted many times to enhance the properties of cement-based materials by admixtures [ 174 , 175 , 176 ], fibers [ 177 , 178 ], and supplementary cementitious materials [ 179 , 180 ]. In more recent studies, newly produced nanomaterials such as nano-titanium oxide, nano-silica, nano-iron oxide, carbon nanotubes, and graphene oxide have been incorporated into the cement-like structures to enhance the mechanical properties of such materials. Such nanoparticles are able to fill even the smallest pores in the cement, providing a compact structure. Since GO is a two-dimensional material, it offers a large surface area for C-S-H nucleation [ 181 , 182 ]. The large surface area and the presence of functional groups make GO a highly reactive material. Mechanical properties of graphene are degraded by functionalization, meaning that GO shows lower elastic modulus and tensile strength than graphene. However, GO’s tensile strength and elastic modulus are still superior to those of cement—adding GO to cement-like materials enhances the mechanical properties of such building materials. Introducing small amounts of GO (0.05 wt.%) increases the flexural strength by 40–60% and compressive strength by 15–33% [ 182 ].
Magnesium oxychloride cements (MOCs) are promising alternatives to Portland cement. Let us note that OPC production is connected to high emissions of CO 2 during manufacturing [ 183 ]. An alternative building material that can reduce the impact of CO 2 during the carbonation is magnesium oxychloride cement (also known as Sorel cement) [ 184 ]. In order to enhance its flexural and compressive strength, carbonaceous nanomaterials, such as graphene, graphene oxide, or graphite oxide, can be added to the mixture (see Figure 9 ) [ 97 ]. Even the very poor water resistance can be improved by the addition of graphene [ 185 ].

SEM micrograph of MOC-GO composite.
8. Conclusions
In this article, the history, synthesis, properties, and application of graphene oxide were reviewed. There are many GO derivatives, including graphene acid, fluorographene, and graphene oxide reduced by thermal or chemical reduction (TRG or CRG). Graphene oxide as well as its derivatives have plenty of various applications. Thanks to the oxygen functional groups on the edges and basal plane, GO can be used as a solution for environmental problems such as excess of CO 2 and toxic gases such as ammonia, acetone, formaldehyde, H 2 S, SO 2, and NO x . There is also a possibility to convert CO 2 by photocatalytic reaction using GO-based composites. Organic dyes and inorganic heavy metal ions in water represent another worldwide environmental issue that can be solved by using GO. There are also medical applications such as gene delivery, which is a very promising way of treating genetic disorders; drug delivery for targeting cancer; and GO-based biosensors. GO-based membranes can be employed as molecular and ionic sieves or for selective gas transport. In high-temperature materials, GO works mainly as an additive used for reinforcement and general enhancement of mechanical properties. Employing GO into building materials such as ordinary Portland cement or magnesium oxychloride can enhance their flexural and compressive strength as well as MOC’s poor water resistance.
Author Contributions
Conceptualization, O.J. and D.S.; writing—original draft preparation, A.J., Z.S., D.S. and O.J.; visualization, A.J.; supervision, O.J. All authors have read and agreed to the published version of the manuscript.
This research was funded by the CZECH SCIENCE FOUNDATION, grant number 20-01866S. This work was also supported by the grant of Specific University Research—Grant MSMT No. 20-SVV/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Graphene gets cleaned up
Graphene has been called "the wonder material of the 21st century." Since its discovery in 2004, the material -- a single layer of carbon atoms -- has been touted for its host of unique properties, which include ultra-high electrical conductivity and remarkable tensile strength. It has the potential to transform electronics, energy storage, sensors, biomedical devices, and more. But graphene has had a dirty little secret: it's dirty.
Now, engineers at Columbia University and colleagues at the University of Montreal and the National Institute of Standards and Technology are poised to clean things up with an oxygen-free chemical vapor deposition (OF-CVD) method that can create high-quality graphene samples at scale. Their work, published May 29 in Nature, directly demonstrates how trace oxygen affects the growth rate of graphene and identifies the link between oxygen and graphene quality for the first time.
"We show that eliminating virtually all oxygen from the growth process is the key to achieving reproducible, high-quality CVD graphene synthesis," said senior author James Hone, Wang Fong-Jen Professor of Mechanical Engineering at Columbia Engineering. "This is a milestone towards large-scale production of graphene."
Graphene has historically been synthesized in one of two ways. There's the "scotch-tape" method, in which individual layers are peeled from a bulk sample of graphite (the same material you'll find in pencil lead) using household tape. Such exfoliated samples can be quite clean and free from impurities that would otherwise interfere with graphene's desirable properties. However, they tend to be too small -- just a few tens of micrometers across-for industrial-scale applications and, thus, better suited for lab research.
To move from lab explorations to real-world applications, researchers developed a method to synthesize large-area graphene about 15 years ago. This process, known as CVD growth, passes a carbon-containing gas, such as methane, over a copper surface at a temperature high enough (about 1000 °C) that the methane breaks apart and the carbon atoms rearrange to form a single honeycomb-shaped layer of graphene. CVD growth can be scaled up to create graphene samples that are centimeters or even meters in size. However, despite years of effort from research groups around the world, CVD-synthesized samples have suffered from problems with reproducibility and variable quality.
The issue was oxygen. In prior publications, co-authors Richard Martel and Pierre Levesque from Montreal had shown that trace amounts of oxygen can slow the growth process and even etch the graphene away. So, about six years ago, Christopher DiMarco, GSAS'19, designed and built a CVD growth system in which the amount of oxygen introduced during the deposition process could be carefully controlled.
Current PhD students Xingzhou Yan and Jacob Amontree continued DiMarco's work and further improved the growth system. They found that when trace oxygen was eliminated, CVD growth was much faster -- and gave the same results every time. They also studied the kinetics of oxygen-free CVD graphene growth and found that a simple model could predict growth rate over a range of different parameters, including gas pressure and temperature.
The quality of the OF-CVD-grown samples proved virtually identical to that of exfoliated graphene. In collaboration with colleagues in Columbia's physics department, their graphene displayed striking evidence for the fractional quantum Hall effect under magnetic fields, a quantum phenomenon that had previously only been observed in ultrahigh-quality, two-dimensional electrical systems.
From here, the team plans to develop a method to cleanly transfer their high-quality graphene from the metal growth catalyst to other functional substrates such as silicon -- the final piece of the puzzle to take full advantage of this wonder material.
"We both became fascinated by graphene and its potential as undergraduates," Amontree and Yan said. "We conducted countless experiments and synthesized thousands of samples over the past four years of our PhDs. Seeing this study finally come to fruition is a dream come true."
Additional Information:
The work was initiated by Mechanical Engineering PhD student Christopher DiMarco and continued by current PhD students Xingzhou Yan and Jacob Amontree, who have spent the past four years modifying the system and conducting the experiments that are shown in the paper. The work was supervised by Prof. James Hone (Mechanical Engineering) and co-led by Prof. Katayun Barmak (APAM), with Prof. Abhay Pasupathy and Prof. Cory Dean (Physics) providing key contributions. Postdoc Madisen Holbrook (Physics) imaged the graphene atomic lattice, and PhD students Christian Cupo and Zhiying Wang (MECE) helped with data analysis and measurements. Physics PhD students Jordan Pack, Dihao Sun, and Adam Biachhi performed key electrical measurements.
Richard Martel and Pierre Levesque at the University of Montreal helped guide the research and test the reproducibility of the results. Dr. Angela Hight-Walker's team at the National Institute for Standards and Technology, in particular Dr. Tehseen Adel and Dr. Charlezetta Wilson-Stokes, characterized the graphene.
- Spintronics
- Materials Science
- Engineering and Construction
- Organic Chemistry
- Energy Technology
- Quantum Physics
- Carbon dioxide
- Electron microscope
- Quantum tunnelling
- Carbohydrate
Story Source:
Materials provided by Columbia University School of Engineering and Applied Science . Original written by Ellen Neff. Note: Content may be edited for style and length.
Journal Reference :
- Jacob Amontree, Xingzhou Yan, Christopher S. DiMarco, Pierre L. Levesque, Tehseen Adel, Jordan Pack, Madisen Holbrook, Christian Cupo, Zhiying Wang, Dihao Sun, Adam J. Biacchi, Charlezetta E. Wilson-Stokes, Kenji Watanabe, Takashi Taniguchi, Cory R. Dean, Angela R. Hight Walker, Katayun Barmak, Richard Martel, James Hone. Reproducible graphene synthesis by oxygen-free chemical vapour deposition . Nature , 2024; DOI: 10.1038/s41586-024-07454-5
Cite This Page :
Explore More
- Younger Classmates Diagnosed With ADHD
- Upending Theory of Milky Way Formation
- Black Holes a Byproduct of Dark Matter?
- Marine Cyanobacteria Can Communicate
- 'Tweezer-Like' Bionic Tools Feel Right
- Odd Planet-Forming Disks Around Low-Mass Stars
- Toward Blood Stem Cell Self-Renewal
- Restored Hearing and Speech in Kids Born Deaf
- Babies and AI Both Learn Key Foundation Models
- Myelination May Drive Drug Addiction
Trending Topics
Strange & offbeat.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (44,166,405 articles, preprints and more)
- Full text links
Composite of graphene oxide from rice husks with copper nanoparticles immobilized: synthesis and application in catalytic dye degradation
Preprint from Research Square , 04 Jun 2024 https://doi.org/10.21203/rs.3.rs-4461351/v1 PPR: PPR862194
Preprint v1
Preprint version history
- Version 1 [04 Jun 2024]
Abstract
Graphene and its derivatives, such as graphene oxide, have a wide range of applications in industry, especially in electronics, electrode construction, catalyst in electro- and photocatalytic reactions, etc. This work presents results from the synthesis of graphene oxide sheets (GOs) from rice husks ash and its modification by incorporating copper nanoparticles. Rice husks, a low-value waste product generated in large quantities, were thermally treated to obtain a mixture of natural carbons with silica. This carbonaceous material was then reacted with potassium hydroxide to produce GOs. The GOs were modified using an impregnation and reduction process to immobilize copper metal nanoparticles onto their surface and obtain graphene oxide with CuO nanoparticles in their surface (GOs-CuO). The synthesized composites were characterized by FTIR, SEM, BET, XRD, and AFM, demonstrating that the formed structure is composed of graphene with predominantly copper oxide nanoparticles adsorbed on its surface. The band gap for the synthesized structures was determined by finding a significant decrease in the band gap of graphene oxide when copper nanoparticles are incorporated. Catalytic capacities of synthetized samples were tested in the decomposition reaction of pollutants, using Rhodamine B (RhB) as a model molecule due to its environmental persistence and toxicity. Both GOs and GOs-CuO effectively degraded RhB, with GOs-CuO demonstrating a 8-fold faster kinetic rate, highlighting its potential for pollutant remediation applications.
Full text links
Read article at publisher's site: https://doi.org/10.21203/rs.3.rs-4461351/v1
Europe PMC is part of the ELIXIR infrastructure
Straightforward microwave-assisted synthesis of ultrasmall gold nanoparticles acnhored on reduced graphene oxide for enhanced antibacterial application
- Open access
- Published: 06 June 2024
- Volume 6 , article number 311 , ( 2024 )
Cite this article
You have full access to this open access article

- Pandji Zamzami Fathurrohman 1 ,
- Eko Sri Kunarti 1 ,
- Nastiti Wijayanti 2 &
- Sri Juari Santosa 1
Graphene derivative materials, such as graphene oxide (GO) and reduced graphene oxide (rGO), have garnered significant attention from scientists for over two decades due to their distinctive characteristics and versatile applications across various fields, particularly in biomedical applications. Incorporating gold nanoparticles (AuNPs) into rGO sheets as rGO-Au nanocomposites further enhances its performance in biomedical applications. This study presents a rapid and efficient method for synthesizing ultrasmall AuNPs anchored on reduced graphene oxide (rGO-Au) using microwave irradiation and ascorbic acid. The optimum microwave treatment was 4 min, ensuring the highest GO reduction degree. Structural characterization by TEM reveals a distinctive architecture with ultrasmall AuNPs (average size of 2.2 nm) distributed on the rGO sheets. Interestingly, while the synthesized rGO-Au did not exhibit any antibacterial activities against both Escherichia coli and Staphylococcus aureus in disk diffusion assays, it demonstrated bacteriostatic effect at remarkably low concentrations when assessed by optical density measurement. The effective concentration of rGO-Au to inhibit E. coli growth was determined to be 2.5 ppm, while for S. aureus , it was 5 ppm, resulting in growth inhibition of 53.1% and 50.0%, respectively. These findings provide a straightforward synthesis route for rGO-Au nanocomposites and underscore the importance of AuNPs’ size and quantity in modulating antibacterial properties.
Article Highlights
Microwave irradiation influenced the reduction degree of GO and the size of AuNPs in the rGO-Au nanocomposite.
The antibacterial properties of rGO-Au were determined by both the size and quantity of AuNPs present.
The mechanism of bacteria inhibition by rGO-Au mainly occurs through direct contact with the sharp edges of graphene sheets and wrapping around bacteria.
Avoid common mistakes on your manuscript.
1 Introduction
Since the discovery of graphene in 2003, the number of researches about the two-dimensional based materials/devices have steadily increased in various scientific, technological and industrial fields [ 1 , 2 , 3 , 4 ]. Graphene is a two-dimensional carbon material which exhibits remarkable properties such as high electrical conductivity, mechanical strength, and thermal stability [ 5 , 6 ]. Graphene oxide (GO) and reduced graphene oxide (rGO) are chemically modified graphene that become very popular in laboratories due to the challenges and scalability issues producing pristine graphene [ 3 , 7 ]. GO is obtained from graphite oxidation, introducing oxygen functional groups such as hydroxyl, carbonyl, carboxyl and epoxy groups on the surface [ 8 , 9 ]. Removing the oxygen functional group in GO through a reduction process will produce rGO [ 10 , 11 ]. This reduction process recovers sp 2 carbon structure and improves the electronical properties of rGO. However, due to the limited amount of oxygen functional groups in rGO, it becomes less hydrophilic and difficult to disperse in water compared to GO [ 1 , 5 ].
Theoretically, rGO has larger surface area than GO because of its surface's flatness like a pristine graphene [ 12 ]. This large surface area makes rGO very suitable as a scaffold for attaching nanoparticles and creating nanocomposites. Adding nanoparticles such as gold nanoparticles (AuNPs) into the surface of graphene materials could enhance their optical, electrical, and biomedical properties [ 13 , 14 , 15 , 16 , 17 ]. The existing conventional method for synthesizing rGO was time-consuming and involved hazardous reducing agents [ 18 , 19 , 20 ]. For this reason, microwave irradiation could be an alternative method, inducing thermal heating through molecular vibration and aligning with the electromagnetic field charge. This method offers a more energy-efficient approach and significantly reduces synthesis time [ 21 ]. Additionally, the hazardous reducing agent can be substituted with ascorbic acid, a green reducing agent that simultaneously acts as a capping agent in nanoparticle synthesis [ 22 , 23 , 24 ]. While depositing AuNPs on rGO surface as rGO-Au nanocomposite, the size of AuNPs influences the activity of that material, such as for antioxidant, antibacterial agent, and catalyst [ 21 , 23 , 25 ]. Smaller AuNPs are preferable because they provide a larger surface area to interact and contact with other materials [ 26 ]. Furthermore, synthesizing small AuNPs < 10 nm requires a stabilizer [ 20 , 27 ]. Hence, we propose a straightforward and rapid approach employing microwave irradiation and ascorbic acid for synthesizing ultrasmall AuNPs anchored on rGO as rGO-Au nanocomposite. This study also explores the impact of microwave irradiation time on the reduction degree of rGO-Au nanocomposites and AuNPs size.
GO and its derivatives have been recognized for their antibacterial properties [ 28 , 29 ]. Modifying the surface of GO or rGO with metal nanoparticles amplifies their antibacterial activity [ 30 , 31 ]. Notably, rGO-Au nanocomposites have demonstrated superior antibacterial efficacy compared to GO [ 32 , 33 ]. Nevertheless, in prior research, the AuNPs’ size was more than 10 nm [ 32 , 33 ]. Notably, smaller AuNPs could enhance their biological activities due to the larger surface area [ 26 ]. Thus, we want to investigate the influence of ultrasmall AuNPs size in the rGO-Au nanocomposite on its antibacterial activity against E. coli and S. aureus . To the best of our knowledge, this is the first time rGO-Au nanocomposite with an average size of AuNPs < 5 nm has been tested for antibacterial assays. We also conducted a comparative analysis of two different antibacterial assay methods to provide a more comprehensive understanding, particularly concerning the inconsistency in antibacterial activity assessment that has been reported [ 34 , 35 ].
2 Material and methods
2.1 materials.
Graphite, potassium permanganate (KMnO 4 ), gold standard solution (HAuCl 4 ) 1000 ppm, hydrogen peroxide and ascorbic acid from Merck were used as received. The deionized (DI) water used was from Onemed. H 2 SO 4 96% and HCl 37% were purchased from Mallinckrodt. Mueller Hinton Broth (MHB) and agarose media were obtained from Himedia. Two standard isolates of bacteria, E. coli and S. aureus from Microbiology Laboratory, Faculty of Biology, Gadjah Mada University were used in the study.
2.2 Synthesis of graphene oxide
The synthesis of GO was carried out using the Hummer method described by Chen [ 36 ] with some modification. Initially, 2 g of graphite was introduced into 50 mL of concentrated H 2 SO 4 and stirred for 25 min in an ice bath. While vigorously stirring, 10 g of KMnO 4 was slowly added to maintain the temperature below 20 °C. The temperature was then raised to 40 °C with continuous agitation and maintained for 2 h. Subsequently, 100 mL of DI water was gradually added, and the solution was stirred at 80 °C for 20 min. To halt the reaction, 300 mL of DI water and 1.4 mL of H 2 O 2 were added. The resulting suspension was left overnight. The mixture was filtered using vacuum filtration and washed with 200 mL of 1.2 M HCl and 200 mL of ethanol. The obtained solid was washed twice with DI water and centrifuged for 15 min at 7000 rpm. Finally, the resulting GO was dried in an oven at 60 °C for 6 h.
2.3 Synthesis of rGO-Au nanocomposites
The synthesis of rGO-Au was conducted using the assistance of microwave irradiation. Initially, 25 mg of the synthesized GO was dispersed in 50 mL of DI water using sonication for 1.5 h. Three milliliters of HAuCl 4 solution (500 ppm) were added to 50 mL of GO suspension (0.5 mg/mL) and stirred for 30 min. Afterwards, 87.5 mg of ascorbic acid was added to the GO suspension (3.5 times amount of GO) [ 37 ] and stirred for 10 min. The pH was then adjusted to 10 using NaOH 1 M. The mixture was positioned in the center of the microwave oven (Electrolux EMS2348X, frequency of 2450 MHz) for microwave irradiation at 800 W, conducted for 1–5 cycles. Each cycle comprises 1 min of microwave irradiation followed by a 30-s resting period. This process resulted in samples labeled as rGO-Au 1 min, rGO-Au 2 min, rGO-Au 3 min, rGO-Au 4 min, and rGO-Au 5 min. Subsequently, the solutions were cooled to room temperature. For comparison, one sample was not irradiated with microwaves, but it was only stirred for 10 min and labelled as rGO-Au NM. Next, the solutions were washed with deionized water and centrifuged at 4000 rpm for 20 min twice. Finally, the precipitated samples were freeze-dried to obtain solid rGO-Au.
2.4 Antibacterial assay by disk diffusion method
The antibacterial assay involved two species of human pathogenic bacteria, E. coli (gram-negative) and S. aureus (gram-positive). Initially, the bacteria were inoculated in sterile nutrient broth media and allowed to incubate for 24 h at 37 °C. Following incubation, the bacterial suspension underwent serial dilution until reaching a concentration of 10 5 cfu/mL. Subsequently, 100 µL of this diluted suspension was carefully applied to the surface of sterile agar media in a petri dish. A sterile cotton swab was then employed to ensure the even distribution of bacteria. On filter paper, 100 µL of GO, rGO-Au 4 min suspension (100 ppm), ciprofloxacin (positive control), and water (negative control) were methodically dripped. This loaded filter paper was then positioned on the petri dish containing agar media and the previously inoculated bacteria. The entire assembly was subjected to incubation at 37 °C for 24 h. Finally, the zones of inhibition of the samples were measured.
2.5 Antibacterial assay by OD measurement
To investigate impact of the synthesized nanocomposites on bacterial growth, optical density (OD) measurements were performed at 595 nm. S. aureus and E. coli bacteria underwent inoculation in Mueller Hinton Broth (MHB) media for 24 h at 37 °C. The bacterial suspension was then diluted to a concentration of 10 5 cfu/mL, determined by OD measurements. Following this, 100 µL suspensions of GO and rGO-Au were added to a 96-well plate, alongside MHB media, achieving a final concentration of 10 ppm. The prepared bacteria were then introduced into each well, and the plate was incubated at 37 °C. Subsequently, OD values were measured with an ELISA reader at 8, 10, 12, 14, 16, and 18 h. Sterile water served as the negative control, and due to the coloration of the nanocomposite suspensions, MHB media, and the nanocomposites suspension were also prepared to normalize OD values. The percentage bacterial growth inhibition was calculated as: Growth inhibition % = 100 × (OD negative control −OD sample normalized )/(OD negative control ).
Using the same method, rGO-Au at various concentrations (2.5, 5, 10, 25, 50 ppm) was also evaluated to determine the effective concentration for antibacterial activity. The incubation time was extended to 24 h.
2.6 Characterization
The synthesized nanocomposites were characterized by Attenuated Total Reflection–Fourier Transform Infrared (ATR-FTIR, Shimadzu FTIR-8201 PC) to determine the functional groups. UV–Vis spectroscopy (Shimadzu 1800) also used to observed material electronic properties at 100 ppm concentration. Raman spectra were collected by Spectrometer QEP03513 using excitation wavelength of 532 nm. The crystallinity of nanocomposites was determined by X-ray Diffraction pattern (D8 Advance Bruker) on silicon substrate. Scanning electron microscope-energy dispersive x-ray spectroscopy (SEM–EDX, SM-6510LA) was used to analyze materials morphology and interaction with bacteria. The powder samples for SEM–EDX were placed on carbon tape substrate. The bacteria and rGO-Au were incubated 24 h, fixated with glutaraldehyde and hydrated gradually with ethanol (50–100%) before observed with SEM. Transmission electron microscope (TEM, JEOL JEM-1400) 120 kV, high resolution transmission electron microscope (HRTEM, Tecnai G2 20 S-TWIN) 200 kV were used to observe, distribution and size of AuNPs.
3 Results and discussion
3.1 uv–vis analysis.
The UV–Vis spectra in Fig. 1 shows the successful of GO reduction. GO spectra shows maximum absorption peak at 230 due to π → π* transition of C=C bonds and a shoulder peak at 304 nm which attributed to n → π* electron transition of C=O bonds [ 29 ]. Ascorbic acid and microwave irradiation in rGO-Au (1–5 min) synthesis clearly reduced GO to rGO, which is indicated by the disappearance of GO peak at 230 nm and a new peak of rGO at 262 nm appears [ 22 ]. However, the spectra of rGO-Au NM neither exhibit the rGO peak nor the GO peak due to the incomplete reduction of GO sheets. The structure of rGO-Au NM lies between GO and rGO, making it challenging to observe the electron transition using UV–Vis. This result suggests a high degree of reduction in GO requires not only the presence of ascorbic acid as reductant but also a significant heat, such as that generated by microwave irradiation [ 38 ].
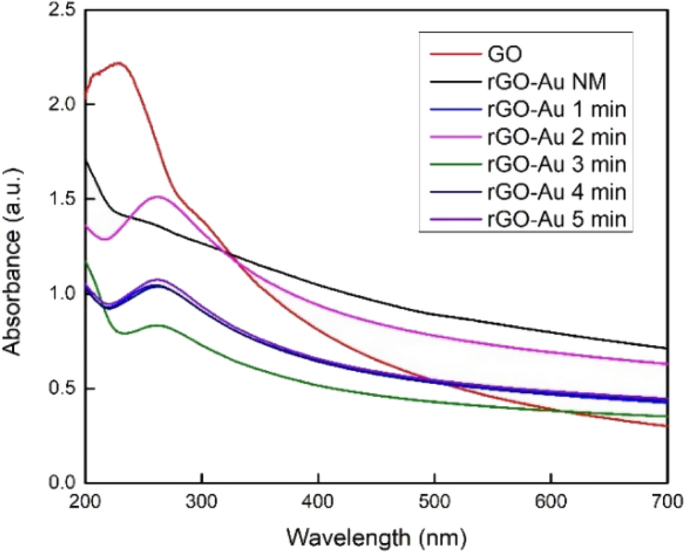
UV–Vis spectra of synthesized materials
The UV–Vis spectra in Fig. 1 did not exhibit the typical plasmon resonance peak of AuNPs, which typically appears around 500–550 nm [ 39 ]. The damping effect presumably caused this phenomenon due to the size of AuNPs is very small [ 40 , 41 ]. The highly conductive rGO surface is also probably channeling away the electrons of AuNPs and weakening the plasmon oscillation [ 42 ]. Some previous study also reported that depositing AuNPs on graphene sheets could drastically weaken and even eliminate the AuNPs peak [ 20 , 43 , 44 , 45 ]. Therefore, the absence of the AuNPs peak in this UV–Vis spectra does not necessarily mean that AuNPs are not formed on the rGO surface.
3.2 XRD analysis
Different forms of crystallinity were observed among GO, rGO, and AuNPs using XRD as illustrated in Fig. 2 . The unmodified GO exhibits a diffraction line at 2θ = 10.26°, attributed to the (001) plane [ 38 ]. After the reduction process, GO peak disappears and a new peak of rGO arises at 2θ = 25.04° for the (002) plane. This peak shift in rGO due to the removal of oxygen functional groups in GO, causing the interlayer space to shrink from 8.62 to 3.55 Å. In contrast, in the rGO-Au NM nanocomposite, the GO peak does not entirely vanish due to a partial reduction process; instead, it shifts to 10.97° (Fig. 2 b). De Silva et al. [ 22 ] also reported a similar diffraction pattern in the reduction process of GO. Extended microwave treatment leads to a more pronounced reduction of GO sheets and increased rGO crystallinity, as indicated by the heightened peak at around 25° 2θ. However, extending the microwave irradiation to 5 min still gives similar peaks and patterns to those observed at 4 min. The reduction process likely reaches its maximum after 4 min s
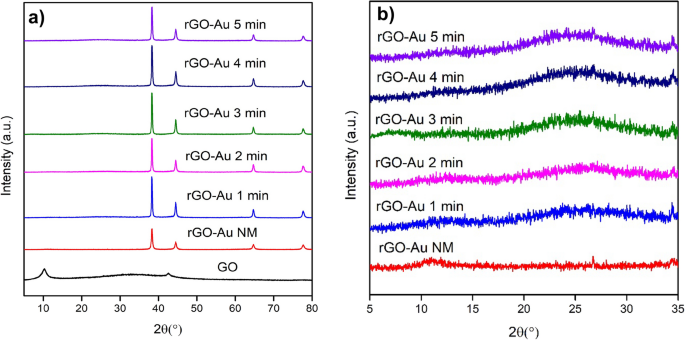
Diffraction patterns of GO and rGO-Au nanocomposites ( a ), along with a zoomed-in diffraction pattern specifically focusing on rGO-Au ( b )
Furthermore, the modification of the graphene surface with AuNPs introduces a new diffraction pattern for rGO-Au materials at 2θ = 38.28°, 44.51°, 64.73°, and 77.67°, corresponding to (111), (200), (220), and (311) planes of Au, respectively (Fig. 2 a). This pattern match with JCPDS 4–784 data of Au and it confirms the presence of AuNPs on rGO surface.
3.3 Attenuated total reflection–infrared (ATR-IR) analysis
ATR-IR spectroscopy was used to investigate the changes in functional groups within both GO and rGO-Au nanocomposites. Figure 3 shows that GO exhibits several characteristic IR absorption bands which similar to synthesized rGO-Au such as C=C stretching vibration of aromatic group at ~ 1620 cm −1 and C–O stretching vibration at 1422, 1224 and 1055 cm −1 as carboxyl, epoxy and alkoxy group, respectively [ 20 , 33 ]. However, ascorbic acid and microwave irradiation effectively remove oxygen functional groups in the synthesized rGO-Au materials, leading to a significant decrease intensity of all C–O peaks compared to GO. The GO reduction process also clearly observed at ~ 2800–3600 cm −1 and ~ 1700 cm −1 region in IR spectra. The broad peak around 2800–3600 cm −1 for both GO and rGO-Au materials in Fig. 3 is attributed to the O–H stretching vibration of hydroxyl groups and adsorbed water molecules [ 46 ]. This peak gradually diminishes with extended microwave irradiation, particularly evident in rGO-Au after 5 min irradiation, where the peak almost disappears due to the high degree of reduction. The reduction of GO to rGO-Au changes the material's characteristics from hydrophilic to hydrophobic [ 38 ], resulting in a significant decrease in adsorbed water molecules, as also reflected in 2800–3600 cm −1 region of the IR spectra. Additionally, the C=O stretching vibration of the carbonyl group in GO was initially observed at 1719 cm −1 (Fig. 3 ) and it vanishes in rGO-Au spectra due to the removal of carbonyl group in reduction process [ 46 ].
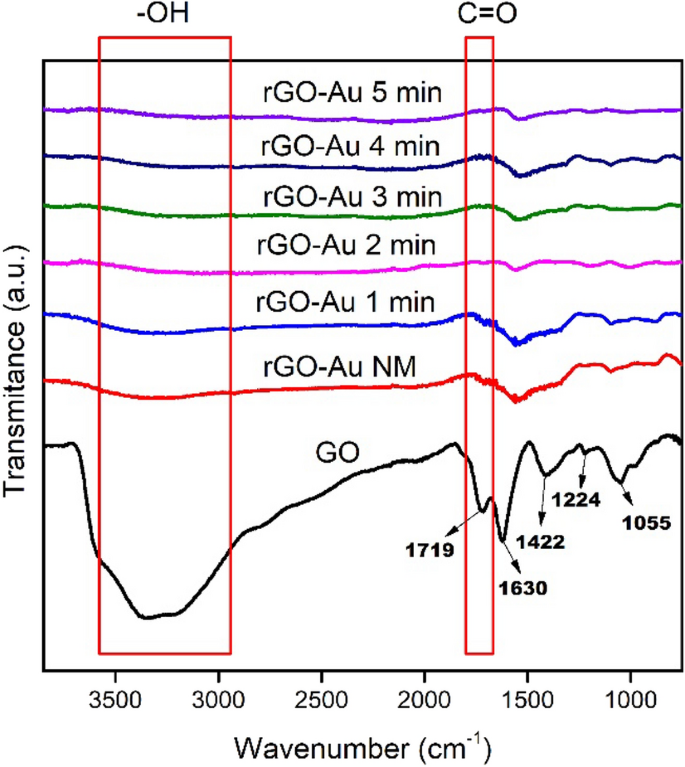
ATR-IR spectra of GO and rGO-Au nanocomposites
3.4 Elemental analysis by SEM–EDX
The percentage amounts of each element present in the synthesized materials were determined by SEM–EDX. As shown in Table 1 , the reduction of GO to rGO-Au decrease the oxygen content significantly. This data aligns with earlier ATR-IR analysis and further clarifies the successful of GO reduction using ascorbic acid and microwave heating. The lower oxygen content in rGO-Au NM (29.54%) compared to GO (38.22%) also can be attributed to the presence of ascorbic acid, which known as reducing agent and could partially reduce the GO sheets [ 22 ]. Increasing the duration of microwave irradiation clearly enhances the reduction degree of rGO, resulting in a change in the C/O atomic ratio from 1.62 to 4.02. It appears that the optimum microwave irradiation time for achieving a significant reduction degree is 4 min (C/O = 4.11), which is consistent with the previous XRD analysis.
The amount of Au impregnated in rGO surface is also mentioned in Table 1 . Interestingly, microwave irradiation lowers the Au content on the rGO surface. This phenomenon can be ascribed to the heat generated during microwave treatment, imparting high kinetic energy to molecules [ 47 ]. The heightened kinetic energy may induce collisions between gold ions (Au 3+ ) and the rGO sheets, resulting in the dislodgment of some AuNPs from the surface. In contrast, rGO-Au NM has more oxygen functional groups that can retain Au strongly through electrostatic interaction and coordination by complexing during nucleation process [ 48 ]. However, microwave irradiation time is not correlated to Au content in rGO surface. The presence of sodium metal residues, as indicated in the EDX results (Table 1 ), is attributed to impurities introduced during the pH adjustment of the reaction using NaOH. Further detailed characterization analysis and antibacterial application will be focused on the rGO-Au 4 min, as it exhibits the highest reduction degree and Au content.

3.5 Raman analysis
Raman spectroscopy is very useful to characterize carbon material structure [ 24 ]. Figure 4 shows sharp peaks at 1350 and 1590 cm −1 for both GO and rGO-Au 4 min were attributed to D band and G band, respectively. The G band represent sp 2 carbon domain vibration while D band correspond to structural defect [ 49 , 50 ]. In Fig. 4 the intensity of D band in both materials about the same as G band peak, indicating huge defect in the structure. The ID/IG ratio of GO found to be 0.92 while rGO-Au 4 min 1.04 suggesting that reduction process slightly increase the defect in graphene plane. The number of layers in synthesized graphene materials can be confirmed by 2D band around 2700 cm −1 [ 51 ]. Single-layer graphene produces sharp and intense peak 2D band region while the multilayer graphene sheets have broader peak [ 51 , 52 ]. The weak intensity and broad 2D peak observed around ~ 2700 cm −1 for GO and rGO-Au 4 min represent the presence of multilayer rGO sheets.
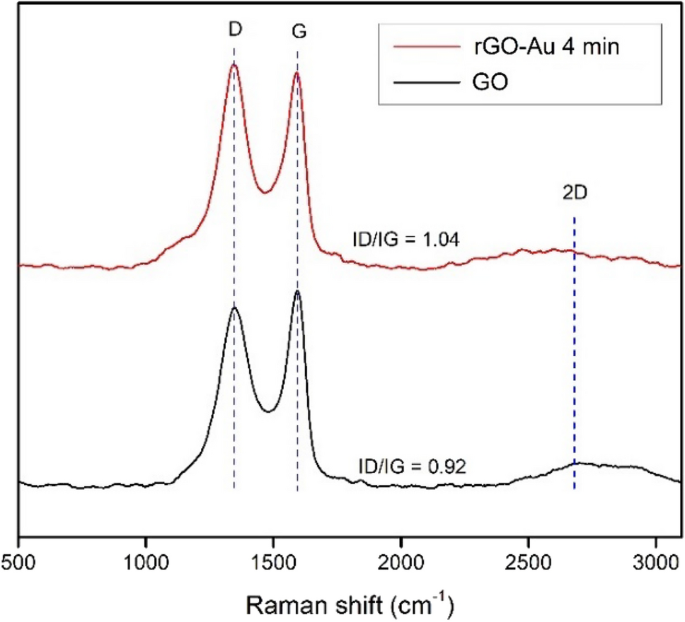
Raman spectra of GO and rGO-Au 4 min
3.6 Morphology analysis by scanning electron microscopy (SEM)
The SEM images depict the stacked layers' structure in both GO and rGO-Au materials (Fig. 5 ) which consistent with raman analysis. Notably, the removal of oxygen-containing groups in the reduction process results in a flatter surface of rGO-Au nanocomposites (Fig. 5 b-d) than GO (Fig. 5 a). The SEM images also confirms that AuNPs successfully anchored on rGO sheets (bright spots in Fig. 5 b-d). This AuNPs deposition is based on electrostatic interaction between the negatively charged rGO sheets and positively charged Au(III) ions [ 5 , 47 ]. In the absence of microwave heating, AuNPs shapes appear disordered, and agglomeration occurs, as shown in Fig. 5 b. On the other hand, when subjected to microwave irradiation, a more ordered arrangement with spherical shapes is observed in rGO-Au (Fig. 5 c and 5d). The shape and distribution of AuNPs also exhibit uniformity in both rGO-Au 2 min and rGO-Au 4 min, accompanied by a reduction in particle size. The SEM–EDX mapping image in Fig. S1 also reveals an even distribution of AuNPs on the graphene sheets of the rGO-Au 4 min nanocomposite. Apparently, in this case, ascorbic acid also plays a significant role as a capping agent, preventing the agglomeration of AuNPs and contributing to the uniformity [ 23 , 53 ]. Compared to previous research, without ascorbic acid, AuNPs exhibit various shapes, and the reduction of the GO does not occur, although the materials were subjected to microwave irradiation [ 54 ]. This suggests the effectiveness of ascorbic acid combining with microwave treatment in enhancing the structural characteristics of the rGO-Au nanocomposite.

SEM images of GO ( a ), rGO-Au NM ( b ), rGO-Au 2 min ( c ) and rGO-Au 4 min ( d )
3.7 TEM analysis and synthesis mechanism
TEM analysis was conducted to investigate the particle size of AuNPs within the rGO-Au nanocomposite as shown in Fig. 6 . AuNPs is confirmed in TEM images as a dark spots in the graphene sheet (pointed by the arrow) due to high electron density of gold material [ 55 ]. In the absence of microwave irradiation, AuNPs in the rGO-Au NM nanocomposite exhibit agglomeration (Fig. 6 b) and the average size is 26.4 nm. In contrast, under microwave radiation, a significant decrease in AuNPs size is observed. Both rGO-Au 2 min and rGO-Au 4 min exhibit very small AuNPs size (< 5 nm) (Fig. 6 c and 6d). The size of AuNPs was hardly seen in regular TEM. To validate this finding, TEM analysis was taken three times with different suspension of rGO-Au 4 min and the images were remained same without any agglomeration or larger particle of AuNPs observed. However, this result appears to contradict the larger size of AuNPs observed in previous SEM images (Fig. 5 c and 5d). This discrepancy can be attributed to the SEM analysis being performed on solid samples that were not dispersed under sonication, leading to AuNPs agglomeration. Moreover, regular SEM is also unable to observe very small nanoparticles. The SEM–EDX mapping image (Fig. S1 ) also found that many AuNPs were spread on graphene sheets but remain unseen in SEM images.
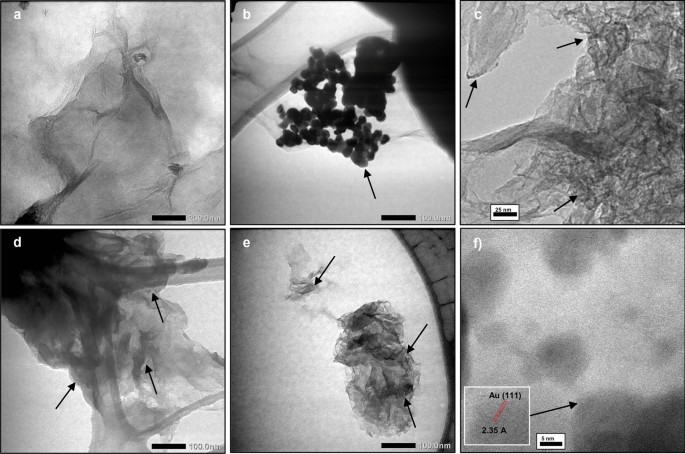
TEM images of GO ( a ), rGO-Au NM ( b ), rGO-Au 2 min ( d ), rGO-Au 4 min ( e ) and HRTEM images of rGO-Au 4 min ( c , f )
High-resolution TEM analysis is necessary to accurately measure the size of AuNPs in rGO-Au 4 min nanocomposite. Figure 6 e reveals that the average size of AuNPs is 2.2 nm on the rGO surface. The lattice spacing of 2.35 Å corresponds to (111) plane of AuNPs [ 56 ] is observed in Fig. 6 f. To provide a comprehensive comparison, Table 2 summarizes various methods and findings from previous research on the fabrication of GO/rGO-Au. Previously, to synthesize AuNPs under 5 nm size, separate capping agents like cetyltrimethylammonium bromide (CTAB) and polyvinylpyrrolidone (PVP) were needed, in addition to the reducing agent [ 20 , 27 ]. Relying on ascorbic acid alone was insufficient to produce ultrasmall gold nanoparticles [ 36 ]. Thus, our findings highlight the microwave-assisted synthesis method's simplicity, effectiveness, and rapidity in anchoring ultrasmall gold nanoparticles on the rGO surface using only ascorbic acid.
The key benefit of microwave-assisted synthesis is the quick heating of the reaction, allowing for faster synthesis. Conventional heating relies on conduction and convection heat transfer, which is very slow and creates a thermal gradient in the reaction vessel. On the contrary, microwave irradiation provides much faster energy transfer directly to the materials, creating uniform and rapid heating throughout the entire reaction vessel [ 47 , 60 ]. Figure 7 illustrates how microwave irradiation affects rGO-Au synthesis in this work. Microwave irradiation directly supplies energy to gold ions in the solution and functional groups on the rGO surface through electromagnetic waves. The ions and functional groups oscillate to align with the electromagnetic field, leading to vigorous vibration and collisions [ 47 , 61 ]. These dynamics might prevent AuNPs from agglomerating during the nucleation process, contributing to a more controlled and uniform synthesis. In this scenario, ascorbic acid also plays a critical role as a reducing and capping agent. Without it, the reduction of GO will not occur, and the size of AuNPs become larger [ 54 ]. Apparently, microwave heating serves not merely as an alternative heating method but potentially provides a different reaction mechanism in nanoparticle synthesis, resulting in different nanostructured materials. Although microwave synthesis method provides fast synthesis and distinct nanomaterial properties, scale up to industrial level could be very challenging. The depth of microwave radiation penetration is very limited (only few centimeters), thus large vessel in batch production will be ineffective [ 62 ].
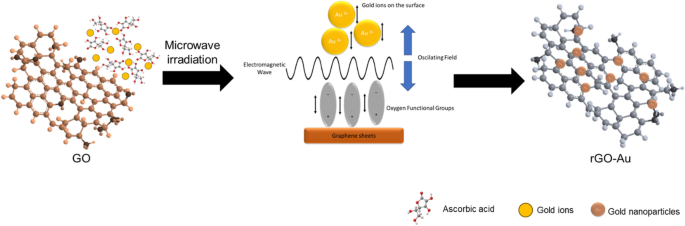
Schematic representation of microwave-assisted synthesis of rGO-Au nanocomposites
3.8 Antibacterial properties
For the following discussion, the rGO-Au nanocomposite, subjected to 4 min of microwave treatment, was employed for the antibacterial properties assay and will be referred to as rGO-Au. Antibacterial assay by disk diffusion method did not reveal any observed antibacterial activity using GO and rGO-Au at 100 ppm against human pathogens E. coli and S. aureus, as demonstrated in Fig. S2. Positive control ciprofloxacin is the only substance that shows antibacterial activity. In this case, it seems that the lack diffusion of GO and rGO-Au prevent these materials to spread in agar media. Some researcher also reported that graphene-based materials demonstrated no inhibition zone with the disk diffusion method [ 63 , 64 ]. However, this result contradicts Shalini’s [ 65 ] and Kadiyala’s [ 33 ] findings where GO, GO-Au and rGO-Au nanocomposite demonstrate > 10 mm inhibition zone in disk diffusion method against E. coli and S. aureus [ 65 ]. This inconsistency may be attributed to differences GO characteristic [ 35 ]. In Shalini's experiment [ 65 ], a distinct GO synthesis method was used, which may resuliting GO that exhibits better dispersibility in water and agar media. Improved dispersibility of GO can maximize the contact between the material and bacteria [ 66 ].
On the other hand, the OD assay demonstrates that both GO and rGO-Au has bacteriostatic effect that suppress E. coli and S. aureus growth at a low concentration of 10 ppm (see Fig. 8 a and 8b). By comparing OD of control and treated bacteria suspension, the relative growth inhibition ability of GO and rGO-Au can be determined. However, the synthesized nanocomposites did not kill the bacteria completely, explaining the lack of observed antibacterial activity in the disk diffusion method. These results suggest that the disk diffusion method is inadequate for accurately evaluating the antibacterial properties of graphene-based materials, which may demonstrate bacteriostatic activity rather than bactericidal effects.
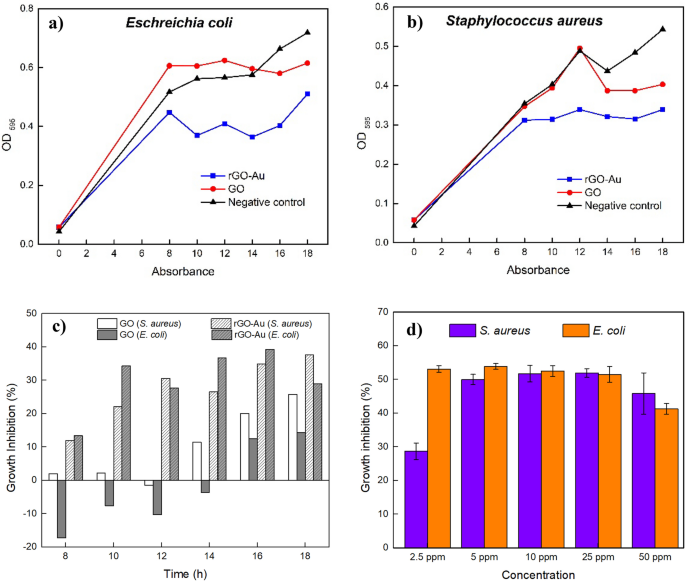
Bacterial growth of E. coli ( a ) and S. aureus b under rGO-Au exposure. Comparison of bacterial growth inhibition by GO and rGO-Au, determined by comparing OD values with the negative control ( c ). Antibacterial activity of rGO-Au in various concentration ( d )
As illustrated in Fig. 8 a and 8b, the bacterial number increased with prolonged incubation time, as observed in the negative control's OD increase. From the beginning rGO-Au showed higher bacterial growth inhibition than GO for both E. coli and S. aureus . Interestingly, GO enhanced E. coli growth in the beginning, and finally inhibit the bacterial growth 14 h incubation. The bacterial growth enhancement by GO due to the GO itself could act as framework for bacterial to attach and proliferate [ 63 ]. After 18 h incubation rGO-Au nanocomposite exhibited superior bacteriostatic activity compared to unmodified GO, inhibiting 29.0% of E. coli and 37.6% of S. aureus growth, whereas GO only inhibited 14.3% of E. coli and 25.8% of S. aureus growth (Fig. 8 c).
The presence of AuNPs on the rGO-Au nanocomposite clearly enhances bacteriostatic ability, suggesting the crucial role of AuNPs in the observed effects. rGO-Au was found to be more effective than GO in inhibiting bacterial growth against E. coli and S. aureus . After monitoring the bacterial growth at intervals, rGO-Au was retested at various concentrations to determine its effectiveness. Figure 8 d illustrates that rGO-Au displayed 53.1% inhibition on E. coli at just 2.5 ppm concentration, while S. aureus showed 28.7% inhibition. The optimal concentrations of rGO-Au were determined to be 2.5 ppm for E. coli and 5 ppm for S. aureus . At these concentrations, bacterial growth was suppressed by 53.1% and 50.0%, respectively. These number indicate that the synthesized rGO-Au was not an antibacterial agent, but has good potential as a bacteriostat since it could inhibit half of bacterial growth. The synthesized rGO-Au nanocomposite demonstrated greater efficacy against E. coli , a Gram-negative strain, than the Gram-positive strain S. aureus . Gram-negative bacteria like E. coli have a thinner peptidoglycan layer in their cell wall, making them more prone to damage. On the other hand, a higher concentration of rGO-Au is required to kill Gram-positive bacteria such as S. aureus because the cell wall structure is thicker [ 26 , 64 ].
In general, increasing rGO-Au concentration above 5 ppm did not result in a significant difference and could even slightly decrease the antibacterial activity at 50 ppm concentration (Fig. 9 d). The growth inhibition stagnancy of E. coli and S. aureus was likely due to the limited amount of gold on rGO sheets. Compared to other studies (as depicted in Table 3 ), the GO-AuNPs and rGO-AuNPs showed high antibacterial activity at around 40–60 ppm, but AuNPs content was around 2%, more than three times AuNPs amount in our rGO-Au nanocomposite. Although AuNPs are crucial for enhancing graphene-based material antibacterial properties, their amount should be considered and optimized. The AuNPs content of 0.62% in the rGO-Au material proved insufficient to completely kill all E. coli and S. aureus bacteria even at 50 ppm concentration. Furthermore, the ineffectiveness of high concentrations of rGO-Au in our study can be attributed to agglomeration and precipitation occurring as the concentration of rGO-Au increases. Despite the minimal amount of AuNPs content, our rGO-Au exhibits remarkable effectiveness even at low concentrations. Table 3 shows that the synthesized rGO-Au with very low Au content at a concentration of 2.5 ppm exhibits comparable bacteriostatic performance to bare AuNPs with a particle size of 20–40 nm against E. coli . The tiny size of the AuNPs attached to the rGO sheets, could be the reason behind its efficacy by providing a larger surface area for direct contact and interaction with bacteria [ 67 ]. In summary, AuNPs’ size and quantity on graphene based-materials have a vital role in bacterial growth inhibition. Nevertheless, silver nanoparticles, as well-known antibacterial nanomaterials, completely inhibit bacterial growth of E. coli and S. aureus at a concentration of 1 ppm [ 68 ], surpassing the antibacterial properties of rGO-Au nanocomposites.
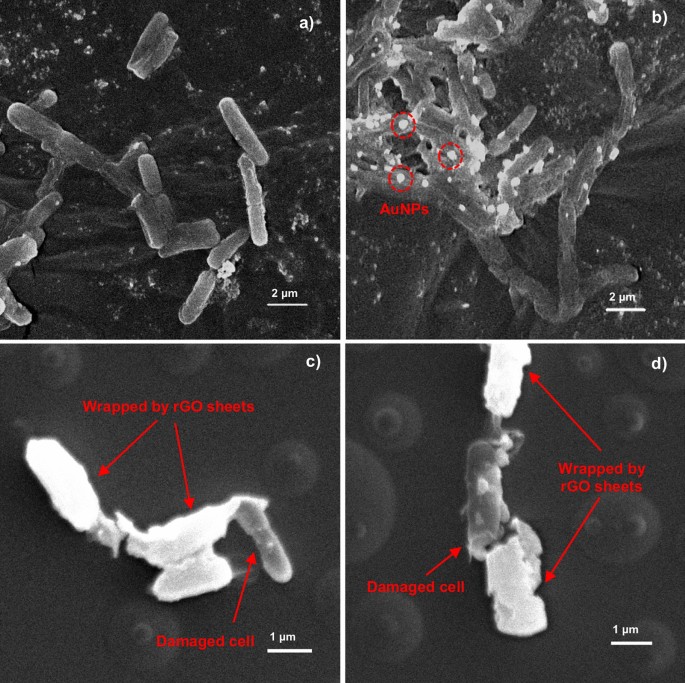
SEM images of normal E. coli bacteria ( a ) and treated E. coli with rGO-Au ( b – d )
The known mechanisms involved in the antibacterial activity of nanomaterials such as graphene-based materials are physical direct interaction of extremely sharp edges of nanomaterials with cell wall membrane [ 70 ], reactive oxygen species (ROS) generation even in dark condition [ 71 , 72 ], wrapping the cells within the aggregated nanomaterials for bacteria [ 73 ] and other motile cells [ 74 ], oxidative stress [ 28 ], interruption in the glycolysis process of the cells [ 75 ], DNA damaging [ 76 ], metal ion release [ 77 ] and contribution in generation and explosion of nanobubbles [ 78 ]. Antibacterial properties of synthesized rGO-Au Au mainly through the direct contact and wrapping mechanism. The direct contact and bacteria wrapping mechanism was observed in Fig. 9 . However, the carbon tape substrate makes rGO sheets challenging to observe. Therefore, the substrate was changed to a glass substrate, and the SEM images reveal that rGO sheets wrapped the bacteria (Fig. 9 c and 9d). Due to the high conductivity of rGO sheets and AuNPs, they appear very bright under the SEM microscope compared to the bacterial cell. SEM images display that AuNPs in rGO-Au attached to E. coli cell walls (Fig. 9 b). Apparently, the morphology of E. coli was also damaged after incubation with the rGO-Au nanocomposite. Direct contact of bacteria with the sharp edges of rGO surfaces rupture the bacteria membrane, eventually resulting in bacterial death [ 28 ]. The rGO sheets also wrap the bacterial cells, preventing nutrients intake to the cells and leading to growth inhibition [ 73 , 79 ]. Furthermore, the presence of AuNPs on the rGO-Au nanocomposite enhances antibacterial properties by interacting with bacterial cell walls and cytoplasm. Bacteria cell walls contain phospholipid bilayers, creating negatively charged cell surfaces surface [ 80 ]. This characteristic makes AuNPs easily bind with the cell wall through electrostatic interaction and potentially penetrating the inner cell. It also possible that rGO-Au damaging the DNA due to AuNPs exhibit an affinity for binding with thiol groups in DNA molecules and enzymes in the cytoplasm, causing disruptions in bacterial metabolic processes that eventually lead to bacterial death [ 26 ]. Hence, this study demonstrated the synergistic effect between rGO and AuNPs could effectively inhibit bacterial growth at remarkably low concentrations.
4 Conclusion
In conclusion, the microwave-assisted synthesis of rGO-Au using ascorbic acid proves to be a promising method for rapid and efficient production of nanocomposites with ultrasmall size of AuNPs. Microwave heating might offer a unique reaction mechanism, preventing agglomeration during the nucleation of AuNPs through molecular vibration and collisions induced by electromagnetic waves. The optimal microwave irradiation time was found to be 4 min, ensuring a high reduction degree of rGO-Au. Characterization of the synthesized nanocomposite clarifies the successful reduction of GO to rGO, showcasing a uniform distribution of ultrasmall AuNPs with the average size 2.2 nm on the rGO surface. This synthesized nanocomposite displays remarkable bacteriostatic activity at very low concentration despite the limited quantity of AuNPs. Compared to disk diffusion method, the OD method more suitable to observed antibacterial properties in graphene-based materials. The inhibition of bacterial growth is highly influenced by both the size and quantity of AuNPs present in graphene-based materials. These findings pave the way for further exploration in nanomaterials for biomedical applications such as developing new antibiotics and antimicrobial coatings. Although the microwave heating provides advantages in nanomaterial synthesis, the scalability of this method presents challenges. Further research is necessary to optimize AuNPs quantities in graphene-based materials while maintaining their ultrasmall size. It could make rGO-Au nanocomposite as a remarkable antibacterial agent in very low concentration.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Liang J, Liu J, Guo L, Wang W, Wang C, Gao W, et al. CO 2 hydrogenation over Fe–Co bimetallic catalysts with tunable selectivity through a graphene fencing approach. Nat Commun. 2024;15:512. https://doi.org/10.1038/s41467-024-44763-9 .
Article Google Scholar
Akhavan O, Saadati M, Jannesari M. Graphene jet nanomotors in remote controllable self-propulsion swimmers in pure water. Nano Lett. 2016;16:5619–30. https://doi.org/10.1021/acs.nanolett.6b02175 .
Safian MT, Umar K, Mohamad Ibrahim MN. Synthesis and scalability of graphene and its derivatives: a journey towards sustainable and commercial material. J Clean Prod. 2021;318:128603. https://doi.org/10.1016/j.jclepro.2021.128603 .
Mayes M, Farahmand F, Grossnickle M, Lohmann M, Aldosary M, Li J, et al. Mapping the intrinsic photocurrent streamlines through micromagnetic heterostructure devices. Proc Natl Acad Sci. 2023;120:e2221815120. https://doi.org/10.1073/pnas.2221815120 .
Dasari TP, Danielle S, Asok M, Paul KD. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano Micro Lett. 2018;10:1–34. https://doi.org/10.1007/s40820-018-0206-4 .
Mohan VB, Lau K, Hui D, Bhattacharyya D. Graphene-based materials and their composites: a review on production, applications and product limitations. Compos Part B Eng. 2018;142:200–20. https://doi.org/10.1016/j.compositesb.2018.01.013 .
Smith AT, LaChance AM, Zeng S, Liu B, Sun L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater Sci. 2019;1:31–47. https://doi.org/10.1016/j.nanoms.2019.02.004 .
You S, Luzan SM, Szabó T, Talyzin AV. Effect of synthesis method on solvation and exfoliation of graphite oxide. Carbon. 2013;52:171–80. https://doi.org/10.1016/j.carbon.2012.09.018 .
Akhavan O, Kalaee M, Alavi ZS, Ghiasi SMA, Esfandiar A. Increasing the antioxidant activity of green tea polyphenols in the presence of iron for the reduction of graphene oxide. Carbon. 2012;50:3015–25. https://doi.org/10.1016/j.carbon.2012.02.087 .
Guex LG, Sacchi B, Peuvot KF, Andersson RL, Pourrahimi AM, Ström V, et al. Experimental review: chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale. 2017;9:9562–71. https://doi.org/10.1039/C7NR02943H .
Akhavan O. The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon. 2010;48:509–19. https://doi.org/10.1016/j.carbon.2009.09.069 .
Goncalves G, Marques PAAP, Granadeiro CM, Nogueira HIS, Singh MK, Grácio J. Surface modification of graphene nanosheets with gold nanoparticles: the role of oxygen moieties at graphene surface on gold nucleation and growth. Chem Mater. 2009;21:4796–802. https://doi.org/10.1021/cm901052s .
Hernández-Sánchez D, Villabona-Leal G, Saucedo-Orozco I, Bracamonte V, Pérez E, Bittencourt C, et al. Highly stable graphene oxide-gold nanoparticle platforms for biosensing applications. Phys Chem Chem Phys. 2018;20:1685–92. https://doi.org/10.1039/c7cp04817c .
Song J, Xu L, Xing R, Li Q, Zhou C, Liu D, et al. Synthesis of au/graphene oxide composites for selective and sensitive electrochemical detection of ascorbic acid. Sci Rep. 2014;4:1–7. https://doi.org/10.1038/srep07515 .
Khalil I, Julkapli NM, Yehye WA, Basirun WJ, Bhargava SK. Graphene-gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced Raman scattering biosensor. Materials (Basel). 2016;9:406. https://doi.org/10.3390/ma9060406 .
Thangamuthu M, Hsieh KY, Kumar PV, Chen G-Y. Graphene- and graphene oxide-based nanocomposite platforms for electrochemical biosensing applications. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20122975 .
Assali A, Akhavan O, Adeli M, Razzazan S, Dinarvand R, Zanganeh S, et al. Multifunctional core-shell nanoplatforms (gold@graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomed Nanotechnol Biol Med. 2018;14:1891–903. https://doi.org/10.1016/j.nano.2018.05.016 .
Zhang Z, Chen H, Xing C, Guo M, Xu F, Wang X, et al. Sodium citrate: A universal reducing agent for reduction/decoration of graphene oxide with au nanoparticles. Nano Res. 2011;4:599–611. https://doi.org/10.1007/s12274-011-0116-y .
Park S, An J, Potts JR, Velamakanni A, Murali S, Ruoff RS. Hydrazine-reduction of graphite- and graphene oxide. Carbon. 2011;49:3019–23. https://doi.org/10.1016/j.carbon.2011.02.071 .
Zhuo Q, Ma Y, Gao J, Zhang P, Xia Y, Tian Y, et al. Facile synthesis of graphene/metal nanoparticle composites via self-catalysis reduction at room temperature. Inorg Chem. 2013;52:3141–7. https://doi.org/10.1021/ic302608g .
Xie X, Zhou Y, Huang K. Advances in microwave-assisted production of reduced graphene oxide. Front Chem. 2019;7:1–11. https://doi.org/10.3389/fchem.2019.00355 .
De Silva KKH, Huang H-H, Yoshimura M. Progress of reduction of graphene oxide by ascorbic acid. Appl Surf Sci. 2018;447:338–46. https://doi.org/10.1016/j.apsusc.2018.03.243 .
D’souza SL, Pati RK, Kailasa SK. Ascorbic acid functionalized gold nanoparticles as a probe for colorimetric and visual read-out determination of dichlorvos in environmental samples. Anal Methods. 2014;6:9007–14. https://doi.org/10.1039/C4AY01004C .
Kang H-H, Oh S-G. Synthesis of L-ascorbic acid derivative including 3-aminopropane phosphoric acid as a novel whitening agent. Bull Korean Chem Soc. 2003;24:1169–71. https://doi.org/10.5012/bkcs.2003.24.8.1169 .
Qiu Y, Wang Z, Owens ACE, Kulaots I, Chen Y, Kane AB, et al. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale. 2014;6:11744–55. https://doi.org/10.1039/C4NR03275F .
Shamaila S, Zafar N, Riaz S, Sharif R, Nazir J, Naseem S. Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials. 2016. https://doi.org/10.3390/nano6040071 .
Iliut M, Leordean C, Canpean V, Teodorescu C-M, Astilean S. A new green, ascorbic acid-assisted method for versatile synthesis of Au–graphene hybrids as efficient surface-enhanced Raman scattering platforms. J Mater Chem C. 2013;1:4094–104. https://doi.org/10.1039/C3TC30177J .
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5:6971–80. https://doi.org/10.1021/nn202451x .
Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim J-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa . Int J Nanomed. 2012;7:5901–14. https://doi.org/10.2147/IJN.S37397 .
Tang J, Chen Q, Xu L, Zhang S, Feng L, Cheng L, et al. Graphene oxide-silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl Mater Interfaces. 2013;5:3867–74. https://doi.org/10.1021/am4005495 .
Sandhya PK, Jose J, Sreekala MS, Padmanabhan M, Kalarikkal N, Thomas S. Reduced graphene oxide and ZnO decorated graphene for biomedical applications. Ceram Int. 2018;44:15092–8. https://doi.org/10.1016/j.ceramint.2018.05.143 .
Kurmarayuni CM, Chandu B, Yangalasetty LP, Gali SJ, Khandapu BMK, Bollikolla HB. Studies on the antioxidant and antibacterial activities of in situ green synthesized graphene-gold nanocomposite. ChemistrySelect. 2021;6:11832–7. https://doi.org/10.1002/slct.202103236 .
Kadiyala NK, Mandal BK, Ranjan S, Dasgupta N. Bioinspired gold nanoparticles decorated reduced graphene oxide nanocomposite using Syzygium cumini seed extract: evaluation of its biological applications. Mater Sci Eng C. 2018;93:191–205. https://doi.org/10.1016/j.msec.2018.07.075 .
Hegab HM, ElMekawy A, Zou L, Mulcahy D, Saint CP, Ginic-Markovic M. The controversial antibacterial activity of graphene-based materials. Carbon. 2016;105:362–76. https://doi.org/10.1016/j.carbon.2016.04.046 .
Barbolina I, Woods CR, Lozano N, Kostarelos K, Novoselov KS, Roberts IS. Purity of graphene oxide determines its antibacterial activity. 2D Mater. 2016;3:25025. https://doi.org/10.1088/2053-1583/3/2/025025 .
Chen J, Yao B, Li C, Shi G. An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon. 2013;64:225–9. https://doi.org/10.1016/j.carbon.2013.07.055 .
Cobos M, González B, Fernández MJ, Fernández MD. Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int J Biol Macromol. 2018;114:599–613. https://doi.org/10.1016/j.ijbiomac.2018.03.129 .
De Silva KKH, Huang H-H, Joshi RK, Yoshimura M. Chemical reduction of graphene oxide using green reductants. Carbon. 2017;119:190–9. https://doi.org/10.1016/j.carbon.2017.04.025 .
Johnson SR, Evans SD, Mahon SW, Ulman A. Alkanethiol molecules containing an aromatic moiety self-assembled onto gold clusters. Langmuir. 1997;13:51–7. https://doi.org/10.1021/la9607520 .
Yonezawa T, Kunitake T. Practical preparation of anionic mercapto ligand-stabilized gold nanoparticles and their immobilization. Colloids Surfaces A Physicochem Eng Asp. 1999;149:193–9. https://doi.org/10.1016/S0927-7757(98)00309-4 .
Varnavski O, Ramakrishna G, Kim J, Lee D, Goodson T. Critical size for the observation of quantum confinement in optically excited gold clusters. J Am Chem Soc. 2010;132:16–7. https://doi.org/10.1021/ja907984r .
Tepe S. Angular Light Scattering of Gold Nanoparticles on Graphene Oxide. Gothenburg: Chalmers University of Technology; 2016.
Google Scholar
Pan H, Low S, Weerasuriya N, Shon Y-S. Graphene oxide-promoted reshaping and coarsening of gold nanorods and nanoparticles. ACS Appl Mater Interfaces. 2015;7:3406–13. https://doi.org/10.1021/am508801e .
Zedan AF, Moussa S, Terner J, Atkinson G, El-Shall MS. Ultrasmall gold nanoparticles anchored to graphene and enhanced photothermal effects by laser irradiation of gold nanostructures in graphene oxide solutions. ACS Nano. 2013;7:627–36. https://doi.org/10.1021/nn304775h .
Pham TA, Choi BC, Lim KT, Jeong YT. A simple approach for immobilization of gold nanoparticles on graphene oxide sheets by covalent bonding. Appl Surf Sci. 2011;257:3350–7. https://doi.org/10.1016/j.apsusc.2010.11.023 .
Xu C, Shi X, Ji A, Shi L, Zhou C, Cui Y. Fabrication and characteristics of reduced graphene oxide produced with different green reductants. PLoS ONE. 2015;10:e0144842.
Kumar A, Kuang Y, Liang Z, Sun X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: a review. Mater Today Nano. 2020;11:100076. https://doi.org/10.1016/j.mtnano.2020.100076 .
Liu L, Liu S, Zhang Q, Li C, Bao C, Liu X, et al. Adsorption of Au(III), Pd(II), and Pt(IV) from aqueous solution onto graphene oxide. J Chem Eng Data. 2013;58:209–16. https://doi.org/10.1021/je300551c .
Xu Z, Gao H, Guoxin H. Solution-based synthesis and characterization of a silver nanoparticle–graphene hybrid film. Carbon. 2011;49:4731–8. https://doi.org/10.1016/j.carbon.2011.06.078 .
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, et al. Raman spectrum of graphene and graphene layers. Phys Rev Lett. 2006;97:187401. https://doi.org/10.1103/PhysRevLett.97.187401 .
Calizo I, Balandin AA, Bao W, Miao F, Lau CN. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett. 2007;7:2645–9. https://doi.org/10.1021/nl071033g .
Akhavan O. Bacteriorhodopsin as a superior substitute for hydrazine in chemical reduction of single-layer graphene oxide sheets. Carbon. 2015;81:158–66. https://doi.org/10.1016/j.carbon.2014.09.044 .
Malassis L, Dreyfus R, Murphy RJ, Hough LA, Donnio B, Murray CB. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016;6:33092–100. https://doi.org/10.1039/C6RA00194G .
Jasuja K, Linn J, Melton S, Berry V. Microwave-reduced uncapped metal nanoparticles on graphene: tuning catalytic, electrical, and Raman properties. J Phys Chem Lett. 2010;1:1853–60. https://doi.org/10.1021/jz100580x .
Peng S, McMahon J, Schatz G, Gray S, Sun Y. Reversing the size-dependence of surface plasmon resonances. Proc Natl Acad Sci USA. 2010;107:14530–4. https://doi.org/10.1073/pnas.1007524107 .
Chen J, Cui X, Wang Q, Wang H, Zheng X, Liu C, et al. One-pot photochemical synthesis of ultrathin Au nanocrystals on co-reduced graphene oxide and its application. J Colloid Interface Sci. 2012;383:140–7. https://doi.org/10.1016/j.jcis.2012.06.007 .
Hu H, Wang X, Xu C, Wang J, Wan L, Zhang M, et al. Microwave-assisted synthesis of graphene nanosheets-gold nanocomposites with enhancing electrochemical response. Fuller Nanotub Carbon Nanostructures. 2012;20:31–40. https://doi.org/10.1080/1536383X.2010.533307 .
Vinodgopal K, Neppolian B, Lightcap IV, Grieser F, Ashokkumar M, Kamat PV. Sonolytic design of graphene−Au nanocomposites. Simultaneous and sequential reduction of graphene oxide and Au(III). J Phys Chem Lett. 2010;1:1987–93. https://doi.org/10.1021/jz1006093 .
Tuz Johra F, Jung W-G. Low temperature synthesis of RGO-Au nanocomposite with apparently reduced time and its application as a chemical sensor. Appl Surf Sci. 2016;362:169–75. https://doi.org/10.1016/j.apsusc.2015.11.145 .
Bilecka I, Niederberger M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale. 2010;2:1358–74. https://doi.org/10.1039/B9NR00377K .
Collins MJ Jr. Future trends in microwave synthesis. Future Med Chem. 2010;2:151–5. https://doi.org/10.4155/fmc.09.133 .
La Hoz AD, Alcázar J, Carrillo J, Herrero MA, Muñoz JDM, Prieto P, et al. Reproducibility and scalability of microwave-assisted reactions. In: Chandra U, editor., et al., Microwave heating, Chapter 7. IntechOpen: Rijeka; 2011.
Ashry N, Bahgy H, Mohamed A, Alsubhi N, Alrefai G, Binothman N, et al. Evaluation of graphene oxide, chitosan, and their complex as antibacterial agents and anticancer apoptotic effect on HeLa cell line. Front Microbiol. 2022;13:922324.
Bhaisare M. MALDI MS analysis, disk diffusion and optical density measurements for the antimicrobial effect of zinc oxide nanorods integrated in graphene Q1 oxide nanostructures. Biomater Sci. 2015. https://doi.org/10.1039/c5bm00342c .
Shalini A, Priya K, Kothai S, Pandian K, Anbalagan G, Jaisankar V. Synthesis and characterisation of graphene oxide decorated gold nano particles and their application towards antibacterial activity. Chem Pap. 2022;76:6861–7. https://doi.org/10.1007/s11696-022-02375-x .
Liu S, Hu M, Zeng TH, Wu R, Jiang R, Wei J, et al. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir. 2012;28:12364–72. https://doi.org/10.1021/la3023908 .
Lin C, Tao K, Hua D, Ma Z, Zhou S. Size effect of gold nanoparticles in catalytic reduction of p -Nitrophenol with NaBH4. Molecules. 2013;18:12609–20. https://doi.org/10.3390/molecules181012609 .
Li W-R, Sun T-L, Zhou S-L, Ma Y-K, Shi Q-S, Xie X-B, et al. A comparative analysis of antibacterial activity, dynamics, and effects of silver ions and silver nanoparticles against four bacterial strains. Int Biodeterior Biodegrad. 2017;123:304–10. https://doi.org/10.1016/j.ibiod.2017.07.015 .
Dat NM, Cong CQ, Phuc NM, Dat NT, Huong LM, Tai LT, et al. Facile phytosynthesis of gold nanoparticles-doped graphene oxide using Mangifera indica leaf extract: characterization, antibacterial activity, and catalytic reduction of organic dyes. Mater Today Sustain. 2022;19:100216. https://doi.org/10.1016/j.mtsust.2022.100216 .
Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–6. https://doi.org/10.1021/nn101390x .
Dutta T, Sarkar R, Pakhira B, Ghosh S, Sarkar R, Barui A, et al. ROS generation by reduced graphene oxide (rGO) induced by visible light showing antibacterial activity: comparison with graphene oxide (GO). RSC Adv. 2015;5:80192–5. https://doi.org/10.1039/C5RA14061G .
Lakshmi Prasanna V, Vijayaraghavan R. Insight into the mechanism of antibacterial activity of ZnO: surface defects mediated reactive oxygen species even in the dark. Langmuir. 2015;31:9155–62. https://doi.org/10.1021/acs.langmuir.5b02266 .
Akhavan O, Ghaderi E, Esfandiar A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J Phys Chem B. 2011;115:6279–88. https://doi.org/10.1021/jp200686k .
Hashemi E, Akhavan O, Shamsara M, Rahighi R, Esfandiar A, Tayefeh AR. Cyto and genotoxicities of graphene oxide and reduced graphene oxide sheets on spermatozoa. RSC Adv. 2014;4:27213–23. https://doi.org/10.1039/C4RA01047G .
Akhavan O, Ghaderi E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon. 2012;50:1853–60. https://doi.org/10.1016/j.carbon.2011.12.035 .
Kumar A, Pandey AK, Singh SS, Shanker R, Dhawan A. Engineered ZnO and TiO 2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli . Free Radic Biol Med. 2011;51:1872–81. https://doi.org/10.1016/j.freeradbiomed.2011.08.025 .
Wang Y-W, Cao A, Jiang Y, Zhang X, Liu J-H, Liu Y, et al. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl Mater Interfaces. 2014;6:2791–8. https://doi.org/10.1021/am4053317 .
Jannesari M, Akhavan O, Madaah Hosseini HR, Bakhshi B. Oxygen-rich graphene/ZnO 2 -Ag nanoframeworks with pH-switchable Catalase/peroxidase activity as O 2 nanobubble-self generator for bacterial inactivation. J Colloid Interface Sci. 2023;637:237–50. https://doi.org/10.1016/j.jcis.2023.01.079 .
Zhang T, Tremblay P-L. Graphene: An antibacterial agent or a promoter of bacterial proliferation? Iscience. 2020;23:101787. https://doi.org/10.1016/j.isci.2020.101787 .
Białas N, Sokolova V, van der Meer SB, Knuschke T, Ruks T, Klein K, et al. Bacteria ( E. coli ) take up ultrasmall gold nanoparticles (2 nm) as shown by different optical microscopic techniques (CLSM, SIM, STORM). Nano Sel. 2022;3:1407–20. https://doi.org/10.1002/nano.202200049 .
Download references
Acknowledgements
The authors express gratitude to the Ministry of Research and Higher Education of the Republic of Indonesia for providing full funding for this research under the Master to Doctoral Education Program for Excellent Students (PMDSU) program with contract No. 2197/UN1/DITLIT/Dit-Lit/PT.01.03/2023; May 8, 2023.
This work was supported by the Ministry of Research and Higher Education of the Republic of Indonesia (Grant No. 2197/UN1/DITLIT/Dit-Lit/PT.01.03/2023; May 8, 2023).
Author information
Authors and affiliations.
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara, Yogyakarta, 55281, Indonesia
Pandji Zamzami Fathurrohman, Eko Sri Kunarti & Sri Juari Santosa
Department of Tropical Biology, Faculty of Biology, Universitas Gadjah Mada, Sekip Utara, Yogyakarta, 55281, Indonesia
Nastiti Wijayanti
You can also search for this author in PubMed Google Scholar
Contributions
Pandji Zamzami Fathurrohman: Methodology, Data curation, Writing – original draft. Eko Sri Kunarti: Validation, Supervision. Nastiti Wijayanti: Conceptualization, Validation, Supervision. Sri Juari Santosa: Conceptualization, Project Administration, Writing – review & editing, Supervision. All authors reviewed the manuscript.
Corresponding author
Correspondence to Sri Juari Santosa .
Ethics declarations
Competing interests.
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 4064 kb)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Fathurrohman, P.Z., Kunarti, E.S., Wijayanti, N. et al. Straightforward microwave-assisted synthesis of ultrasmall gold nanoparticles acnhored on reduced graphene oxide for enhanced antibacterial application. Discov Appl Sci 6 , 311 (2024). https://doi.org/10.1007/s42452-024-06002-0
Download citation
Received : 01 April 2024
Accepted : 27 May 2024
Published : 06 June 2024
DOI : https://doi.org/10.1007/s42452-024-06002-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Graphene oxide
- Gold nanoparticles
- Microwave synthesis
- Antibacterial
- Ascorbic acid
Advertisement
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 23 April 2020
From graphene oxide towards aminated graphene: facile synthesis, its structure and electronic properties
- Maxim K. Rabchinskii 1 ,
- Sergei A. Ryzhkov 1 ,
- Demid A. Kirilenko 1 , 2 ,
- Nikolay V. Ulin 1 ,
- Marina V. Baidakova 1 ,
- Vladimir V. Shnitov 1 ,
- Sergei I. Pavlov 1 ,
- Ratibor G. Chumakov 3 ,
- Dina Yu. Stolyarova 3 ,
- Nadezhda A. Besedina 4 ,
- Aleksandr V. Shvidchenko 1 ,
- Dmitrii V. Potorochin 2 , 6 , 7 ,
- Friedrich Roth 6 ,
- Dmitry A. Smirnov 8 ,
- Maksim V. Gudkov 5 ,
- Maria Brzhezinskaya 9 ,
- Oleg I. Lebedev 10 ,
- Valery P. Melnikov 5 &
- Pavel N. Brunkov 1 , 2
Scientific Reports volume 10 , Article number: 6902 ( 2020 ) Cite this article
14k Accesses
120 Citations
7 Altmetric
Metrics details
- Electronic properties and devices
- Synthesis of graphene
In this paper we present a facile method for the synthesis of aminated graphene derivative through simultaneous reduction and amination of graphene oxide via two-step liquid phase treatment with hydrobromic acid and ammonia solution in mild conditions. The amination degree of the obtained aminated reduced graphene oxide is of about 4 at.%, whereas C/O ratio is up to 8.8 as determined by means of X-ray photoelectron spectroscopy. The chemical reactivity of the introduced amine groups is further verified by successful test covalent bonding of the obtained aminated graphene with 3-Chlorobenzoyl chloride. The morphological features and electronic properties, namely conductivity, valence band structure and work function are studied as well, illustrating the influence of amine groups on graphene structure and physical properties. Particularly, the increase of the electrical conductivity, reduction of the work function value and tendency to form wrinkled and corrugated graphene layers are observed in the aminated graphene derivative compared to the pristine reduced graphene oxide. As obtained aminated graphene could be used for photovoltaic, biosensing and catalysis application as well as a starting material for further chemical modifications.
Similar content being viewed by others
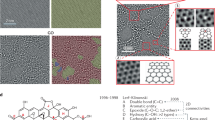
Controlling covalent chemistry on graphene oxide
Stepwise reduction of graphene oxide and studies on defect-controlled physical properties
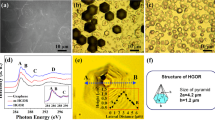
Revealing the Improved Catalytic Properties of Modified Graphene-like Structures
Introduction.
In the past years derivatization of graphene has become one of the central topics in the field of nanocarbon materials studies 1 , 2 . Significant efforts are being made to obtain graphene derivatives covalently modified by various functional groups, containing oxygen, nitrogen, sulfur, halogens and other elements 3 , 4 . As a result, the family of functionalized graphenes has grown dramatically during recent years 5 . Such excitement for the graphene functionalization is a result of wide opportunities in tailoring its physical and chemical properties which are being opened by adding certain type and number of organic moieties onto either graphene basal plane or its edges. Graphene derivatization allows to tune material electrical resistivity, luminescence properties, and optical transmittance, open and vary band gap what is of high interest for electronic, optoelectronic and electrochemical applications 6 , 7 , 8 . The addition of chemically reactive moieties, such as carboxyls, amides or amines 9 , 10 modifies graphene reactivity, surface energy and surface chemistry, substantially improving the performance of graphene-based catalysts, gas sensors, and biosensors 11 , 12 , 13 . And last but not least, functionalization supports the successful dispersion of graphene in organic solvents, which the main issue in processing the graphene-based materials 14 .
Besides the most known graphene derivatives, graphene oxide (GO) and reduced graphene oxide (rGO) 15 , 16 , 17 , 18 , as well as fluorographene and graphane 19 , 20 , amino-functionalized graphene is another derivative also being extensively studied nowadays. Primary amines represent attractive functionalities that enable an easy graphene grafting through amide coupling or so-called “click” reactions. Such an approach makes possible to covalently functionalize graphene with a large variety of biomolecules, particularly DNA strands and aptamers, as well as with carboxylated forms of carbon nanotubes or fullerenes 21 . Moreover, as amine is proved to be an electron-withdrawing group the functionalization of graphene with amines modify its electronic structure, in particular, enhance conductivity and provide controllable work function engineering 22 . As a net result, aminated graphene is regarded as a promising material for various applications in photovoltaic, gas sensing and biosensing, drug delivery and composite formation 12 , 23 , 24 , 25 .
Various strategies for amine functionalization of graphene are currently used. For instance, Baraket et al . 11 have demonstrated successful graphene grafting with about 9 at.% of primary amines using electron beam produced Ar/NH 3 plasma. Zhang et al . 24 have also reported the formation of amino-functionalized graphene via Hoffman rearrangement using graphene oxide as a starting material with amine content of around 4 at.%. However, the applied procedure involves several stages, requires hazardous reagents and works only on the edges of graphene flakes. The hydrothermal approach is widely used for graphene functionalization and, particularly, for nitrogen doping of graphene oxide via reaction with ammonia, melamine, etc 26 , 27 , 28 . However, all these reactions entail the use of autoclave operating at rather high temperatures (up to 195 °C) and, therefore, mostly provide incorporation of such nitrogen-containing heterocycles as pyrroles and pyridines, than the formation of amines. Recently, one-pot graphene oxide amination and reduction via Leuckart reaction, which involves the conversion of a carbonyl group of an aldehyde or a ketone into the amine group, was reported by Aguilar-Bolados et al . 29 . Although the proposed method is simple and easy operational its efficiency is noticeably limited by localization of the formed amines on the edges of GO platelets. Additionally, the as-synthesized GO commonly contains a rather small amount of carbonyls (around 2–3 at.%) and the increase of their content requires additional GO processing, for instance, via liquid-phase partial reduction 30 .
Apparently, the direct substitution of GO basal plane groups (hydroxyl and epoxide ones) by amine groups with the simultaneous restoration of the sp 2 -conjugated graphene network is the most attractive and effective way to obtain amino-functionalized graphene. This cannot be done straightforwardly in mild conditions; however, one solution is to use an additional step of GO reductive bromination. Earlier it was demonstrated that GO treatment with bromine solutions or hydrobromic acid results in graphene oxide reduction and functionalization by bromine with as high as ~5 at.% bromine concentration 5 , 31 , 32 Considering the high reactivity of bromine moieties, especially for substitution reactions, one can further easily obtain aminated graphene by treating prepared brominated graphene with ammonia.
This paper reports for the first time a scalable and facile approach for the formation of aminated graphene (rGO-Am) through two-step GO treatment with hydrobromic acid and ammonia solution in mild conditions. The effect of bromine and amine functionalization on morphology and electronic characteristics of graphene is discussed as well, providing further insights into the tuning of graphene physical and chemical properties via its derivatization.
Results and Discussion
Chemical composition analysis.
X-ray photoelectron spectroscopy (XPS) was used to determine the chemical composition of the initial graphene oxide (GO), brominated graphene (rGO-Br) and aminated graphene (rGO-Am). Figure 1a presents the survey XPS spectra. The survey spectra of the initial GO contain only C1s and O1s peaks, at ~284.7 eV and ~532.6 eV, respectively, indicating the absence of any impurities. The features related to Si near 100 eV (Si2p) and 151 eV (Si2) are due to the signal from Si substrate, underlying the studied sample. After the bromination procedure, Br3d peak at ~69 eV and Br3p doublet around ~182 and 189 eV appear, thereby confirming the presence of bromine moieties in rGO-Br sample. Considering the relevant atomic sensitivity factors, the atomic concentration of bromine calculated from the survey spectrum and was determined to be ~5.3 at.%. To analyze whether the revealed bromine is covalently bonded to graphene or just physisorbed to it the high-resolution Br3d spectrum was further measured and deconvoluted (Fig. 1b ). The set of six peaks combined into three doublets are resolved in the obtained spectrum: doublet at 67.8 (Br3d 5/2 ) and 68.8 eV (Br3d 3/2 ) is related to free Br − 31 , 32 , two analogous doublets at 70.0 and 71.0 eV, and at 72.7 and 73.8 eV, respectively, correspond to bromine atoms covalently bonded to carbon 31 , 32 , 33 and to oxygen 34 . The relative areas of these doublets are easily determined and are, ~23.8% for free Br − , 74.9% for C-Br, and 1.3% for O-Br. Accordingly, the concentration of C-Br species appears to be about ~3.96 at.%, what is comparable and even higher than the values obtained by other researchers 31 , 32 , 33 .
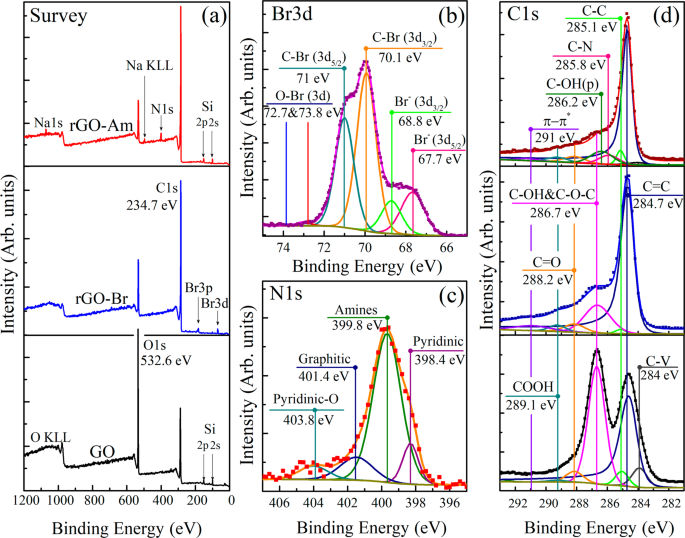
( a ) X-ray photoelectron survey spectra of the initial GO, rGO-Br and rGO-Am. ( b ) High-resolution Br3d XPS spectrum of rGO-Br. ( c ) High-resolution N1s XPS of rGO-Am. ( d ) High-resolution C1s spectra of the initial GO and modified rGOs. For clarity, C1s spectrum and their fits are vertically offset from the fitting components. The C1s spectra were fitted by Shirley background and a set of one asymmetric Doniach-Sunjic function (DS) and five symmetric Gaussian−Lorentzian product functions (Gaussian by 70% and Lorentzian by 30%) (GL(30)), while the O1s spectra were fitted by only the GL(30) functions whose number varied from 3 to 5.
The subsequent rGO-Br treatment with the ammonia solution resulted in the complete elimination of Br3d and Br3p lines from the XPS survey spectrum and the expected appearance of N1s signal at ~400 eV. This confirms the successful substitution of bromine species by nitrogen functionalities which concentration in rGO-Am was determined to be around 5.5 at.%. The higher nitrogen content in comparison to bromine is probably due to additional incorporation of nitrogen occurring via reductive amination of the retained oxygen-containing groups in ammonia enviroment 35 , 36 . The curve fitting of the obtained high-resolution N1s spectrum (Fig. 1c ) demonstrates the presence of four bands positioned at 398.4, 399.8, 401.4 and 403.8 eV and respectively corresponding to pyridines, amines, graphitic nitrogen and pyridine-N-oxide 36 , 37 . As seen from the spectrum, amine functionality with its peak area percentage of 72% appears to be a dominant type of nitrogen species, while the other ones, pyridine, graphitic nitrogen or pyridine-N-oxide, demonstrate much less relative content, not exceeding 10%. The data of NK edge XAS technique also confirms, although only qualitatively, the presence of significant amounts of amines and pyridines in the as-obtained material (Supplementary Fig. S1 ). The successful amination is additionally indicated by the means of Fourier-transform infrared spectroscopy (FTIR) (Supplementary Fig. S2 ). After the GO treatment, the characteristic absorption bands at 2970–3700 cm −1 , 1365 cm −1 , 1220 cm −1 and 980 cm −1 related to the interlayer water and oxygen functionalities diminish 38 . At the same time, new doublet at 3422 cm −1 and 3306 cm −1 along with peaks at 1560 cm −1 , 1260 cm −1 and 795 cm −1 corresponding to N-H stretching of primary amines, N-H bending of primary amines, C-N stretching and N-H wag, respectively 39 , 40 , appear and become dominant. The additional bands at 1404 cm −1 2920 cm −1 attributed to C-H/C-H 2 vibrations are due to the isopropyl alcohol molecules retained after the sample preparation.
Graphene oxide bromination and subsequent amination are accompanied by the elimination of oxygen-containing groups what is implied by significant diminishing of O1s peak in the rGO-Am survey spectra. This fact is further emphasized by the detailed peak-fitting analysis of the high-resolution C1s core level spectra (Fig. 1d ) in which seven distinct peaks are resolved. Three peaks centered at 283.9 eV, 284.7 eV, and 285.1 eV are respectively related to the vacancy defects of graphene lattice (peak C−V) 41 , sp 2 -bonded carbons of aromatic domains (C=C) and carbon atoms with the bonds distorted due to attachment of functional groups at a neighboring atom (C-C) 9 , 41 , 42 , 43 . The C=C peak exhibits asymmetric shape due to excitonic screening in sp 2 -conjugated graphene network of aromatic domains observed not only in graphite or graphene C1s XPS spectra but as well in the same spectra of highly reduced graphene oxide obtained by its high-temperature annealing (rGO-HT) (Supplementary Fig. S3 ) 43 , 44 . The other three peaks located at 286.7, 288.2 and 289.1 eV correspond to hydroxyl and epoxide (C-OH&C-O-C), carbonyl (C=O), and carboxyl (COOH) groups, respectively 42 , 45 . The last resolved peak at ~290.2 eV corresponds to π − π* shakeup satellite of the peak C=C. Quantitative analysis of the deconvoluted C1s XPS spectra (Table 1 ) demonstrates that initial GO has a rather high degree of oxidation with C/O ratio of 1.95. After the GO bromination, the concentration of its basal plane groups significantly reduces and the overall C/O ratio rises up to 4.18. As shown by Zheng, J. et al . 33 , the peak at 286.2 eV clearly observed in the XPS of brominated graphene may be assigned to the C–Br bonds. Thus, the similar broad feature observed in the C1s XPS spectrum of rGO-Br at 286.6 eV might be attributed to the sum of C–Br and C-OH&C-O-C peaks, suggesting even lower oxygen-groups content. Assuming this fact and taking into account the aforementioned bromine concentration of 3.4 at.% we obtain that the recalculated C/O ratio becomes equal to 5.02.
After the amination step, this ratio demonstrates further growth reaching for the rGO-Am the value of 8.85, due to the additional elimination of oxygen-containing groups and by their substitution with amines. The noted value of the C/O ratio is close to that obtained for rGOs prepared by chemical reduction using common reducing agents, namely, hydrazine, benzylamine, various alcohols and sodium borohydride 18 , 46 . The high reduction degree of rGO-Am is also emphasized by the results of UV-Vis spectroscopy (Supplementary Fig. S4 ), demonstrating restoration of graphene conjugated structure. The accurate deconvolution of the rGO-Am C 1 s spectrum also revealed the appearance of the C-N peak centered at 285.8 eV 29 , 47 , which overlaps with the peak near 286.2 eV, corresponding to the phenol groups (C-OH(p)). These oxygen species are known to be highly stable to elimination via various reduction techniques 9 , 30 retaining even after GO thermal reduction with the presence of the peaks located at 286.1–286.3 eV and 533.4–533.6 eV in the C 1 s and O 1 s XPS spectra, respectively.
Study of the morphological features
The structural features of the obtained brominated and aminated graphenes were further studied by the means of different techniques such as atomic force microscopy (AFM), X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM) and Raman spectroscopy. Representative bright field low magnification TEM images (Fig. 2 ) demonstrate the morphology of the initial GO, rGO-Br, and rGO-Am. No rips or nanoscale holes are observed in the initial GO, indicating its defect-free structure on the nanoscale level (Fig. 2a ). The corresponding hexagonal ED patterns formed by the set of sharp spots confirmed the monolayer nature of the studied GO since characteristic intensity ratio of different spot groups, what is further verified by AFM images and XRD patterns (Supplementary Fig. S5 ). After the bromination procedure rGO-Br continues to exhibit lamellar defect-free structure and monolayer platelets can be distinguished in the sample (Fig. 2b ). Well-preserved crystalline structure with the long-range order up to tens of nanometers retains after amination as well. However, rGO-Am demonstrates a tendency to scroll and wrinkle of initially flat graphene monolayer platelets leading to the formation of local multilayer areas distributed within single rGO-Am platelet. This is evident from the TEM image (Fig. 2c ) and the corresponding ED pattern. ED pattern consists of distinguishable hexagonal diffraction patterns rotated with respect to each other (Fig. 2c ). Diffraction spot intensities corresponding to the adjacent sheet areas in these diffraction patterns significantly differ from each other because of the different surface areas falling into the selective aperture of the microscope. At the same time, the intensities would be almost identical in the case of a bi- or trilayer sheets because one sheet lying under another has the same area within the aperture.

TEM images and corresponding selective area electron diffraction (SAED) patterns of ( a ) the initial GO, ( b ) rGO-Br, ( c ) rGO-Am.
More comprehensive information on the morphology of the initial GO and functionalized graphenes was obtained by the means of the new approach developed by Kirilenko et al . 48 based on the analysis of electron diffraction tilt series to determine the graphene nanorelief. The slope of the diffraction spot intensity dependence on the reciprocal space applicate square variation measured as g 2 corresponds to the average square of the graphene sheet corrugation amplitude (more details are in Supporting Information and at the reference). Applying this method, we have found that functionalization leads to some increase of the corrugation amplitude (Fig. 3a ) that results from local structural distortions caused by the bonded species. As seen from this figure, even though the concentration of functional groups on the basal plane in the case of rGO-Br and rGO-Am is substantially lower than that in the initial GO, out-of-plane distortion of the graphene layer in the modified graphenes is even higher (0.19 nm and 0.18 for brominated and aminated graphenes, respectively, which slightly exceed 0.16 nm value for the initial GO). This fact can be explained in terms of compensation of graphene net bonds distortion in GO by the opposite orientation of adjacent hydroxyl and epoxide groups with respect to the graphene net 49 . At the same time in rGO-Br and rGO-Am bromine and amine moieties are located separately and thus result in significant out of plane dislocation of carbon atoms and corrugation of graphene net.
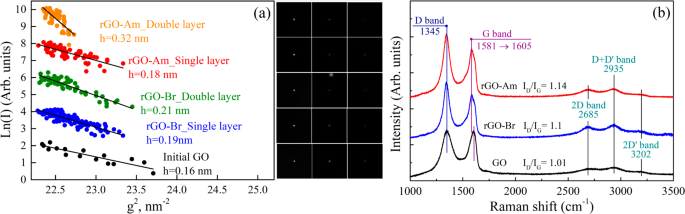
( a ) (100) diffraction spot intensity logarithm versus reciprocal space applicate. The corresponding slopes are related to the average square of the sheet corrugation amplitude. The plots were vertically offset for clarity. Inset – the corresponding electron diffraction refluxes. ( b ) Raman spectra of the GO, rGO-Br and rGO-Am samples recorded using a 532-nm laser.
The case of double-layered structures appears to be of even more interest. In general, when graphene and graphene oxide layers are stacked with each other they become flatter and the measured average corrugation amplitude significantly decreases 50 , 51 . In opposite, the studied functionalized graphenes show different behavior. The stacking of the sheets is obstructed by the functional groups what, in turn, leads to the formation of multiple knolls. As seen in Fig. 3a , in rGO-Br these knolls have the height of 0.21 nm, whereas in rGO-Am this value is 1.5 times higher and is determined to be 0.32 nm. Seemingly, knoll height should be defined by a molecular size of a functional group. However, C-Br and C-NH 2 molecular sizes are almost the same, 0.194 and 0.197 nm respectively. We assume that higher knoll height is related to stronger electrostatic repulsion between the amine group and second graphene layer, resulting in its stronger bending and larger knoll height.
Figure 3b presents the Raman spectra of the initial GO and modified graphenes. Two major bands are commonly observed in graphene-related materials: G band at 1580 cm −1 originating from the in-plane stretching of the graphene lattice and D band at 1345 cm −1 caused by lattice disorder, particularly distortion of carbon bonds and corrugation of graphene net, as well as GO-rGO platelets edges 52 , 53 . In the GO Raman spectrum G band is broadened and shifted from 1580 cm −1 to 1605 cm −1 due to oxidation of the graphene net. At the same time, in the case of rGO-Br and rGO-Am two peaks at 1580 cm −1 and 1605 cm −1 are simultaneously presented in the Raman spectra, indicating restoration of sp 2 -conjugated graphene network along with the presence of localized areas functionalized with bromine or amine moieties. Functionalization also results in a slight rise of D band intensity, with the increase of I D /I G relation from ~1 for the initial GO to 1.12 and 1.17 for the rGO-Br and rGO-Am, respectively. Considering both the aforementioned absence of observable defects and results of the electron diffraction studies the indicated rise of the D band is likely to originate from the distortion of the graphene network due to the provided covalent grafting. Both rGO-Am and rGO-Br also present three second order bands of medium intensity: 2D band (2685 cm −1 ), D + D′ band (2935 cm −1 ) and 2D′ band (3202 cm −1 ). The appearance of these bands is related to the interaction of the incorporated bromine and amine moieties in the double resonant processes that involve two phonons and was observed earlier for the aminated graphene 24 .
The morphology of the obtained aminated graphenes was also studied by scanning electron microscopy (SEM) at various scales. Regardless of the solvent type used during the deposition process, which was varied from the polar ones (isopropyl alcohol) to non-polar solvents (trichloromethane and tetrachloromethane), rGO-Am platelets display a wrinkled and twisted structure (Fig. 4a ). This results in the reduction of the π-π* interlayer stacking and leads to the formation of films, exhibiting irregular porous network structure (Fig. 4b ). The morphological features of rGO-Am films also appear in NK edge XAS spectra, in which the absence of angular dependence of π*-resonances is observed, asserting isotropic nature of the studied sample (Supplementary Fig. S6 ).
SEM images of rGO-Am ( a ) individual platelets, ( b ) multilayer film deposited on the Si wafer and ( c ) aerogel obtained by lyophilization of rGO-Am dioxane suspension.
To further facilitate the porous network arrangement lyophilization of rGO-Am dioxane suspension was carried out, resulting in the formation of aminated graphene aerogel (Fig. 4c ). The Brunauer-Emmett-Teller (BET) specific surface area of the formed aerogel determined by N 2 adsorption experiments has been measured to be ~265 m 2 g −1 . This value is lower than those achieved in formation of the structured GO and rGO aerogels 54 , but still sufficiently high for rGO-Am applications in the catalysis and adsorption of metallic or dye pollutants 55 .
It is worth noting that despite the rGO-Am films contain many voids, cracks, and ripples due to the corrugation of the graphene layer, SEM studies of the arrays of individual rGO-Am platelets reveal that the applied modification procedure does not lead to tearing and reduction of the lateral size of GO platelets. The obtained SEM images demonstrate (Supplementary Fig. S7 ) that the rGO-Am individual platelets are of the same scale as the initial GO platelets (10–40 μm) and the size distributions of these materials are almost equal.
Chemical reactivity of the aminated graphene
The formation of primary amines via the applied procedure and their chemical reactivity were further analyzed by a chemical test based on the reaction between rGO-Am and 3-Chlorobenzoyl chloride. This organic compound carries acyl chloride functional group, -COCl, known to react readily with primary amines forming covalent bond: so-called amide coupling 56 . This reaction commonly applied for covalent modification of amine-containing materials with species and nanocarbon structures, carrying carboxyl groups (-COOH), which can be transformed to acyl chloride moieties by the treatment with thionyl chloride (SOCl 2 ), phosphorus trichloride (PCl 3 ), or phosphorus pentachloride (PCl 5 ) 57 , 58 . Thus, the chosen test reaction allows to demonstrate the feasibility of rGO-Am covalent grafting with various biomolecules, dyes, functionalized nanocarbon materials, etc.
To check whether the modification of rGO-Am by 3-Chlorobenzoyl chloride successfully proceeded the series of additional XPS measurements were performed. Figure 5a demonstrates the XPS survey spectra of the rGO-Am sample prior to and after the test reaction (rGO-Am-Benz). Thanks to the presence of second chlorine, which is not taking part in the amide coupling, the successful covalent bonding between the aminated graphene and 3-Chlorobenzoyl chloride is unambiguously seen from the appearing of Cl2s peak at ~272 eV and Cl2p doublet around ~200 and 202 eV. This is additionally verified by high-resolution Cl2p spectrum (Fig. 5b ), where peaks at 200.4 eV (2p 3/2 ) and 201.9 eV (2p 1/2 ) related to the C-Cl bonds 59 are observed. The chlorine concentration calculated from the XPS survey spectrum is ~2.8 at.%. Since as it was earlier estimated the concentration of amine groups is ~4 at.%, the efficiency of the performed amide coupling appears to be about 74% determined as a relation between the concentration of bonded chlorine and the presented amines. Besides the XPS results, rGO-Am covalent grafting by the 3-Chlorobenzoyl chloride is also indicated by the obtained FTIR spectrum (Supplementary Fig. S8 ), where a set of characteristic bands at 740 cm −1 , 1170 cm −1 , 1565 cm −1 , 1660 cm −1 and 3220 cm −1 related to the amide bonding arise. Thus, the obtained results confirm the presence of chemically reactive primary amines. Additionally, the thermal stability of the aminated graphene was analyzed, as amines are known to easily convert in pyridines and pyrroles upon heating 36 . During the purification from the unreacted 3-Chlorobenzoyl chloride, the samples were heated up to 150 °C for 3 hours. The deconvolution and further analysis of the then-obtained N1s spectra (Fig. 5c ) demonstrate that the suggested modification of rGO-Am samples does not significantly affect their chemical composition and relative concentration of the amine groups still remains to be ~72%, while the concentrations of pyridines and graphitic nitrogen appear to be about 13% and 15%, respectively.
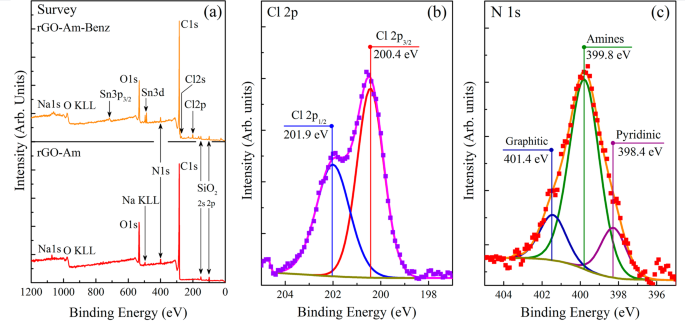
( a ) XPS survey spectra of the rGO-Am films prior to and after test covalent modification with 3-Chlorobenzoyl chloride (rGO-Am-Benz). High-resolution ( b ) Cl2p and ( c ) N1s XPS spectra of rGO-Am-Benz sample.
The chemical reactivity of the rGO-Am was also verified by the test reaction of its influence on the CuCl oxidation to CuCl 2 . 200 mg of CuCl was dispersed in 20 ml of 1 M HCl and the obtained solution was divided into two parts. The first one was left stirring in the air and the second half of the solution was mixed with 50 mg of the aminated graphene and rigorously stirred for two hours. In the HCl medium CuCl is unstable and is known to transform into CuCl 2 with the presence of Cu 2 + and Cl − ions in the mixture. Depending on the concentration of Cl − ions the obtained solution is either yellow (Cl − to Cu 2+ is about 2:1), green (high concentration of the Cl − ions) or blue (low concentration of Cl- ions). Amines from the rGO-Am are weak bases, which should interact with the Cl − ions and, thus, reduce their content in the solution, leading to the blue color of the resulting solution. Indeed, this effect we observed in the case of the mixture with the addition of rGO-Am (Supplementary Fig. 9 ) with the retention of the CuCl + HCl solution without rGO-Am green. The observed blue color of the resulting solution can also be related to the hydrolysis of the amine groups from the rGO-Am in the aqueous medium and formation of a tetraamine copper hydroxide complex, which also induces the blue colouring of the mixture. Thus, the performed reaction additionally demonstrates the presence of chemically active amines in the obtained rGO-Am material.
Electronic properties
Besides changing of graphene chemical reactivity the presence of amines, analogously to other nitrogen species, substantially affects the electronic properties of graphene 22 , 60 , 61 . Particularly, it is expected that the rGO-Am samples due to their n-doping 61 can demonstrate a noticeable increase in the conductivity as compared to the samples of pristine rGO with the same reduction degree (Supplementary Fig. S2 ). Figure 6 presents the voltage (V) versus current (I) characteristics plot of rGO-Br, rGO-Am and rGO-HT films. The V vs. I data show a linear behavior for all the samples, confirming the good Ohmic contact between the film and electrodes. The sheet resistance and electrical conductivity calculated considering the films geometry and averaged over several measurements are summarized in Table 2 . As seen, rGO-Am shows 2 times higher conductivity than that in pristine rGO-HT, 270 S/m and 134 S/m, respectively. This confirms the N-doping effect from the amine groups. At the same time, rGO-Br conductivity is almost 4 times lower due to a lower degree of graphene basal plane reduction.

I-V curves of rGO-Br, rGO-Am and rGO-HT samples.
To explore the effect of the functionalization with amine groups on the electronic structure of graphene, valence band spectra of the initial GO, rGO-HT, and rGO-Am using a photon at 130 eV were measured (Fig. 7 ). All the spectra are dominated by a broad band centered at ∼ 7.6 eV commonly attributed to the 2p-σ electron states of graphene net 62 . Additionally, broad features at 26–28 eV, corresponding to σ electronic states in carboxyls and carbonyls are presented, indicating their presence in all samples what coincides with XPS data. At the same time, significant differences are observed in the range 0–6 eV. In the initial GO spectrum noticeable peak at ~5.5 eV corresponding to 2p π-σ overlap states, related to C-O bonds formation 63 . This assignment is justified by the observed diminishing of this feature in the valence band spectra of both rGO-Am and rGO-HT, where most part of the oxygen-containing functional groups are eliminated. Furthermore, close to zero density of states (DOS) is observed between Fermi level and ~2.2 eV in the case of GO, indicating the presence of band gap of ~4.5 eV which is in a good agreement with the published data 64 . On the contrary, both rGO-HT and rGO-Am demonstrate non-zero DOS in the region from 3 to 0 eV as a result of the increase of C2p-π electron content due to enlargement of the π -conjugated polyaromatic sp 2 -domains during the GO reduction. In rGO-HT VB spectrum peak at ~3.2 eV corresponding to C 2p-π states is more distinguishable compared to the one in rGO-Am suggesting the more complete restoration of the delocalized π-conjugated system. Note, that this difference can be also attributed to the corrugated nature of rGO-Am lattice which distortion also influences the number of 2p-π electronic states. Despite this similarity between rGO-Am and rGO-HT valence band spectra, several bands related to the introduction of the nitrogen species appear in the former one. Particularly, the bands at ~5.0 eV and ~7.3 eV, corresponding to electronic states of N lone pair and the delocalized C–N π bonds can be distinguished in the rGO-Am valence band spectra 65 , 66 . Moreover, slight changes in the density of states near 2.3 eV arise due to the states related to C–C and C–N π bonds, existing in a planar sp 2 -graphene structure 67 . Finally, a broad peak centered between 18 and 20 eV can be observed after amination. While studying N-doped HOPG Favaro et al . 68 have discovered the appearance of such a feature in the VB spectra of HOPG upon its N implantation and assumed that it originates from the presence of the –CN groups. Considering the absence of this peak in the spectrum of the rGO-HT sample and following Favaro et al . we assume that it is related to the electronic states arising due to the incorporated amines.
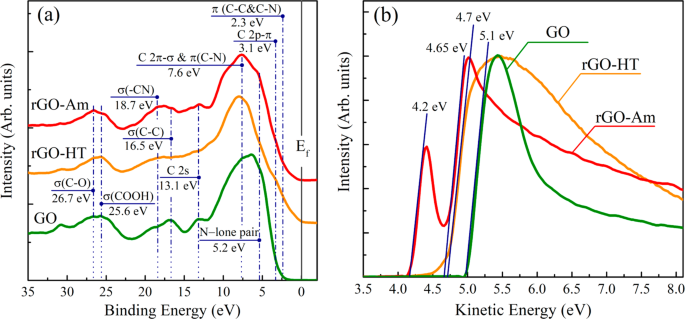
( a ) Valence band spectra and ( b ) secondary electron energy cutoff spectra of the initial GO, rGO-Am and rGO-HT. E f line corresponds to the position of Fermi level. The Fermi level position is referred at binding energy 0 eV. All the spectra were aligned considering the energy shift due to charging effect.
The effect of the amine functionalization on the graphene work function (WF) has also been studied (Fig. 7 ). The work functions of GO, rGO-HT and rGO-Am samples were determined using a standard approach based on subsequent measuring of the valence band and corresponding secondary electrons (SE) cut-off spectra (Fig. 7a,b , respectively). In this case, the value of work function, usually denoted as eΦ m , can be calculated using the following equation: eΦ m = hν – (E F – E SEC ), where hν = 130 eV is the photons energy, E F and E SEC are the positions of Fermi level and cut-off threshold both represented in the kinetic energy scale 69 . The obtained in such a way eΦ m values are represented in the Fig. 7b and equal respectively to ~4.2 eV for rGO-Am, to ~4.7 for rGO-HT and to ~5.1 eV for initial GO. It is worth noting that the value of the rGO-HT work function (~4.7 eV) is in a very good consistency with the literature data 70 , 71 . On the contrary, the value of the work function of initial GO (~5.1 eV) appears to be somewhat higher the typical values (~4.9 eV) obtained by other groups 71 , 72 , probably due to the higher degree of graphene oxidation in our case.
Interestingly, the SE spectrum of rGO-Am demonstrates the presence of two slopes indicating that the surface of this sample consists of two types of domains. The work function of first type domains has the value of ~4.2 eV, while for second type domains it becomes very close to the work function of the rGO-HT sample (~4.65 eV). This fact points out the substantial local decrease of the rGO-Am work function reaching the value of ~0.5 eV (see Fig. 7b ). The primary amines located along the edges and wrinkles of graphene platelets are the electron-donating moieties, and, as that, have to cause some decrease in the rGO work function 72 , 73 . Hence, the observed decrease in rGO-Am work function may be attributed to the contribution of the amines as well as pyridines, which both form a noticeable amount of rGO domains with the lower value of eФ m .
In summary, the approach for scalable production of the aminated graphene from graphene oxide is developed. The proposed method leads to the reduction of graphene and subsequent incorporation of up to 4 at.% of amines with low content of other nitrogen species (pyridines and graphitic N) as indicated by XPS and XAS data. Note, that the amination efficiency can be further enhanced by increasing Br concentration via modification of the bromination method. Both TEM and SEM studies revealed that due to the presence of amines and elimination of oxygen functional groups, the aminated graphene exhibits complicated morphology with a tendency to form wrinkled and corrugated structure. This facilitates the formation of the porous films and aerogels from the obtained aminated graphene, making it perspective for electrocatalyst and sensing applications. The use of the aminated graphene in biosensing applications, particularly aptasensors manufacturing, is further enhanced by its chemical reactivity through amide coupling what is emphasized by the successful covalent linking of 3-Chlorobenzoyl chloride to the obtained material. In addition, the amination increases the conductivity of graphene layers and alters its valence band structure and work function that is of interest not only for optoelectronic and photovoltaic applications but also for the study properties of variously functionalized graphenes.
GO synthesis and chemical modification
Graphene oxide was synthesized by the Hummers method 74 . Graphite powder (4 g) was oxidized by using concentrated H 2 SO 4 , KMnO 4 and 30% H 2 O 2 solution. No nitrates were used to prevent nitrogen doping of graphene oxide during the synthesis. The rest of the GO preparation procedure is analogous to that described in our previous work 30 and its main steps are as follows. The resulting mixture was centrifuged at 3500 rpm for 1 hour, and the supernatant was decanted away. The remaining material was additionally centrifuged at 1500 rpm for 10 min to obtain aqueous GO suspension as a supernatant. Sonication was excluded throughout the whole process to prevent damaging of graphene oxide flakes and obtain suspensions with GO flakes lateral size up to 100 μm.
Graphene oxide bromination was performed as follows. The GO aqueous suspension (0.05 wt.%) was centrifugated (18186 g, 15 minutes), the supernatant was decanted away. After that, HBr acid (46%, Sigma-Aldrich) was added to the sediment and suspension was intensively shacked for 60 seconds. The described procedure was repeated three times and the finally obtained suspension was stirred using magnetic stirrer during 20 hours in closed flask. The obtained brominated graphene was copiously washed with centrifugation (18186 g, 25 minutes) and rinsing the obtained sediment with organic solvent by mechanical stirrer (5 minutes stirring). This procedure was repeated 5 times. During the first 2 cycles chloroform was used as a solvent and in the last 3 cycles it was changed to the isopropyl alcohol.
Amination was carried out by centrifugation (18186 g, 15 minutes) of rGO-Br isopropyl alcohol suspension (0.05 wt.%), decanting the supernatant away and rinsing the sediment by a saturated solution of ammonia in isopropyl alcohol. The described procedure was repeated 3 times. The washing procedure is the same as in the case of rGO-Br synthesis.
Covalent modification of rGO-Am by 3-Chlorobenzoyl chloride was performed as follows. rGO-Am was filtered using a glass filter and 3 times washed with acetonitrile. Afterwards, 50 mg of rGO-Am was dispersed in 10 ml of acetonitrile and 100 μl of triethanolamine and 50 μl of 3-Chlorobenzoyl chloride were added while stirring. The obtained suspension was further stirred for one hour in a closed flask. The as-prepared suspension was filtered using glass filter with subsequent washing using acetonitrile, deionized water, ethyl alcohol, and chloroform.
Characterization of the initial GO and the obtained modified graphenes
A set of characterization techniques was exploited similarly to the used in the aforementioned work 30 with some extensions concerning the study of the aminated and brominated materials as follows. Measurements using X-ray photoelectron spectroscopy (XPS) were made using Thermo Fisher ESCALAB 250Xi XPS system equipped with an Al Kα X-ray source providing 1486.6 eV line. Calibration of the spectra was performed using the Au 4f7/2 line at 84.0 eV as a reference. Effect of surface charging of low-conducting GO surface was treated by aligning of XPS spectra according to the C1s line at 284.6 eV of a conductive rGO-HT (see Supplementary Fig. S2 ). CasaXPS software was used for quantification and fitting of the XPS spectra. Nonlinear least-squares routine was used for the χ2 minimization. Shimadzu-2450 spectrophotometer was used for the UV-vis absorption spectra acquisition from the studied samples. Fourier transform infrared spectroscopy was performed on the Infralum-08 FTIR spectrometer equipped with the attenuation of total reflectance attachment. Horiba Jobin-Yvon LabRam HR800 apparatus equipped with a Laser Quantum Torus 532-nm laser having 50 mW of the output power was used for Raman spectroscopy. The excitation source was attenuated with an optical density 1 filter condensed by a 20x lens to a 30 µm spot. The light power at the sample was 0.11 mW. The set of five Raman spectra were obtained in different spots of the studied samples and further averaged to provide reliable data. Measurements of electrical conductivity in the GO, rGO-Br and rGO-Am samples were performed using a two-electrode system. Thin films of the studied material were obtained by casting a drop of the corresponding suspension onto quartz substrates with two comb 80 nm thick Au electrodes with 500 µm separation prepared on them. The electrode comb included 8 electrode bar pairs (Supplementary Fig. S10 ).
N-K edge X-ray absorption spectra (XAS), Valence band spectra and Work function spectra were recorded at the Russian-German beamline of electron storage ring BESSY-II (Helmholtz-Zentrum Berlin) using the beamline ultrahigh vacuum experimental station 75 . The XAS measurements were performed in the total electron yield (TEY) mode realized by sweeping the incident photon energy and simultaneously recording the sample drain current. The thus-obtained TEY XAS spectra were then subjected to appropriate normalization and smoothing.
Structural characterization was mainly performed analogously to that presented in our previous work 30 with some differences as can be seen in the following. X-ray diffraction (XRD) technique was implemented using Bruker Smart Apex Duo set-up equipped with a CuKα source and an Apex 2D detector. Sample for these studies was prepared by the fixation of a material portion on a cactus needle end by a nitrocellulose lacquer. Then a series of diffraction patterns were acquired at various incidence angles (of the X-ray beam incidence on the detector), and the obtained 2D data was recalculated to the means of 2θ scan. DIFFRAC.EVA (Bruker Cor.) software was used for the obtained diffraction curves analysis based on the data from Powder Diffraction File ICCD PDF-2 release [JCPDS-International Centre for Diffraction Data ( http://www.icdd.com )]. The transmission electron microscopy including electron diffraction (ED) studies were performed on a JEM ARM200F cold FEG probe and image aberration corrected electron microscope equipped with a large solid-angle CENTURIO EDX detector, Gatan GIF QUANTUM, and ORIUS CCD camera and Jeol JEM-2100F microscope. TEM sample was prepared by wetting a TEM-grid with carbon lacey film in a diluted water dispersion of the studied material. Jeol JSM-7001F microscope was used for SEM studies. Langmuir−Blodgett method was exploited for monolayer films formation in a way described elsewhere 76 . AFM images were obtained using Veeco Dimension 3100 atomic force. Operation in tapping mode using RTESP probes was used for surface morphology and thickness of the rGO-Am films determination.
Specific surface area was measured by Brunauer-Emmett-Teller (BET) method. Immediately prior to the measurements, the sample of rGO-Am was preheated to 180 °C and kept at this temperature for an hour in a vacuum to remove any products that could be adsorbed on the highly developed surface of the sample.
Sturala, J., Luxa, J., Pumera, M. & Sofer, Z. Chemistry of Graphene Derivatives: Synthesis, Applications, and Perspectives. Chem. Eur. J. 24 , 5992–6006 (2018).
Article CAS PubMed Google Scholar
Dasari, B. L., Nouri, J. M., Brabazon, D. & Naher, S. Graphene and derivatives – Synthesis techniques, properties and their energy applications. Energy 140 , 766–778 (2017).
Article CAS Google Scholar
Xu, Y. & Shi, G. Assembly of chemically modified graphene: methods and applications. J. Mater. Chem. 21 , 3311–3323 (2011).
Eigler, S. & Hirsch, A. Chemistry with Graphene and Graphene Oxide – Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 53 , 2–21 (2014).
Karlicky, F., Datta, K. K. R., Otyepka, M. & Zboril, R. Halogenated Graphenes: Rapidly Growing Family of Graphene Derivative. ACS Nano 7 , 6434–6464 (2013).
Rummeli, M. H. et al . Graphene: Piecing it Together. Adv. Mater. 23 , 4471–4490 (2011).
Article PubMed CAS Google Scholar
Hunt, A., Kurmaev, E. Z. & Moewes, A. Band gap engineering of graphene oxide by chemical modification. Carbon 75 , 366–371 (2014).
Deka, M. J., Dutta, A. & Chowdhury, D. Tuning the wettability and photoluminescence of graphene quantum dots via covalent modification. New J. Chem. 42 , 355–362 (2018).
Rabchinskii, M. K. et al . Nanoscale perforation of graphene oxide during photoreduction process in the argon atmosphere. J. Phys. Chem. C 120 , 28261–28269 (2016).
Kulia, T. et al . Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 57 , 1061–1105 (2012).
Baraket, M. et al . Aminated graphene for DNA attachment produced via plasma functionalization. Appl. Phys. Lett. 100 , 233123 (2012).
Article ADS CAS Google Scholar
Suvarnaphaet, P. & Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 17 , 2161 (2017).
Article CAS PubMed Central Google Scholar
Kang, M.-A. et al . Highly sensitive and wearable gas sensors consisting of chemically functionalized graphene oxide assembled on cotton yarn. RSC Adv. 8 , 11991–11996 (2018).
Article CAS PubMed PubMed Central Google Scholar
Hernandez, Y. et al . High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol. 3 , 563–568 (2008).
Article ADS CAS PubMed Google Scholar
Zhan, D. et al . Electronic structure of graphite oxide and thermally reduced graphite oxide. Carbon 49 , 1362–1366 (2011).
Dreyer, D. R., Park, S., Bielawski, C. W. & Ruoff, R. S. The chemistry of graphene oxide. Chem. Soc. Rev. 39 , 228–240 (2010).
Dong, L., Yang, J., Chhowalla, M. & Loh, K. P. Synthesis and reduction of large sized graphene oxide sheets. Chem. Soc. Rev. 46 , 7306–7316 (2017).
Chua, C. K. & Pumera, M. Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem. Soc. Rev. 43 , 291–312 (2014).
Mazanek, V. et al . Tuning of fluorine content in graphene: towards large-scale production of stoichiometric fluorographene. Nanoscale 7 , 13646–13655 (2015).
Zhou, C. et al . Graphene’s cousin: the present and future of graphane. Nanoscale Res. Lett. 9 , 26 (2014).
Article PubMed PubMed Central CAS Google Scholar
Semenov, K. N. et al . Carboxylated fullerenes: Physico-chemical properties and potential applications. Prog. in Solid State Chem. 47-48 , 19–36 (2017).
Marsden, A. J. et al . Effect of oxygen and nitrogen functionalization on the physical and electronic structure of graphene. Nano Res. 8 , 2620–2635 (2015).
Valentini, L. et al . Use of butylamine modified graphene sheets in polymer solar cells. J. Mater. Chem. 20 , 995–1000 (2010).
Zhang, W. et al . Preparation of amino-functionalized graphene oxide by Hoffman rearrangement and its performances on polyacrylate coating latex. Prog. Org. Coat. 94 , 9–17 (2016).
Krasteva, N. et al . Aminated Graphene Oxide as a Potential New Therapy for Colorectal Cancer. Oxid. Med. Cell Longev. 2019 , 3738980 (2019).
Chen, P. et al . Hydrothermal synthesis of macroscopic nitrogen-doped graphene hydrogels for ultrafast supercapacitor. Nano Energy 2 , 249–256 (2013).
Long, D. et al . Preparation of Nitrogen-Doped Graphene Sheets by a Combined Chemical and Hydrothermal Reduction of Graphene Oxide. Langmuir 26 , 16096–16102 (2010).
Guo, H.-L., Su, P., Kang, X. & Ning, S.-K. Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J. Mater. Chem. A 1 , 2248–2255 (2013).
Aguilar-Bolados, H. et al . Facile and Scalable One-step Method for Amination of Graphene Using Leuckart Reaction. Chem. Mater. 29 , 6698–6705 (2017).
Rabchinskii, M. et al . Facile reduction of graphene oxide suspensions and films using glass wafers. Sci. Rep. 8 , 14154 (2018).
Article ADS PubMed PubMed Central CAS Google Scholar
Li, Y. et al . Synthesis of partially hydrogenated graphene and brominated graphene. J. Mater. Chem. 22 , 15021–15024 (2012).
Jankovsky, O. et al . Towards graphene bromide: bromination of graphite oxide. Nanoscale 6 , 6065 (2014).
Zheng, J. et al . Production of Graphite Chloride and Bromide Using Microwave Sparks. Sci. Rep. 2 , 662 (2012).
Batra, N., Vandana, Praveen, K., Srivastava, S. K. & Singh, P. K. X-ray photoelectron spectroscopic study of silicon surface passivation in alcoholic iodine and bromine solutions. J. Renew. Sustain. Energy. 6 , 013121 (2014).
Tao, H. et al . N-doping of graphene oxide at low temperature for oxygen reduction reaction. Chem. Commun. 53 , 873–876 (2017).
Schultz, B. J. et al . X-ray absorption spectroscopy studies of electronic structure recovery and nitrogen local structure upon thermal reduction of graphene oxide in an ammonia environment. RSC Adv. 4 , 634–644 (2014).
Jansen, R. J. J. & van Bekkum, H. XPS of nitrogen-containing functional groups on activated carbon. Carbon 33 , 1021–1027 (1995).
Acik, M. et al . Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat. Mater. 9 , 840–845 (2010).
Wang, X. & Gilham, J. K. Competitive Primary Amine/ Epoxy and Secondary Amine/ Epoxy Reactions: Effect on the Isothermal Time-to-Vitrify. J. Appl. Polym. Sci. 43 , 2267–2277 (1991).
Qin, H. et al . Chemical Amination via Cycloaddition of Graphene for Use in a Glucose Sensor. J. Nanosci. Nanotechnol. 16 , 5034–5037 (2016).
Blume, R. et al . The influence of intercalated oxygen on the properties of graphene on polycrystalline Cu under various environmental conditions. Phys. Chem. Chem. Phys. 16 , 25989–26003 (2014).
Fan, X. B. et al . Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation. Adv. Mater. 20 , 4490–4493 (2008).
Darmstadt, H. & Roy, C. Surface spectroscopic study of basic sites on carbon blacks. Carbon 41 , 2662–2665 (2003).
Cheung, T. T. P. X‐ray photoemission of carbon: Lineshape analysis and application to studies of coals. J. Appl. Phys. 53 , 6857–6862 (1982).
Perera, S. D. et al . Alkaline deoxygenated graphene oxide for supercapacitor applications: An effective green alternative for chemically reduced graphene. J. Power Sources 215 , 1–10 (2012).
Guex, L. G. et al . Experimental review: chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 9 , 9562–9571 (2017).
Wang, B. et al . Chemical amination of graphene oxides and their extraordinary properties in the detection of lead ions. Nanoscale 3 , 5059 (2011).
Kirilenko, D. A. & Brunkov, P. N. Measuring the height-to-height correlation function of corrugation in suspended graphene. Ultramicroscopy 165 , 1–7 (2016).
Boukhvalov, D. W. & Katsnelson, M. I. Modeling of Graphite Oxide. J. Am. Chem. Soc. 130 , 10697–10701 (2008).
Kirilenko, D. A., Dideykin, A. T. & van Tendeloo, G. Measuring the corrugation amplitude of suspended and supported graphene. Phys. Rev. B. 84 , 235417 (2011).
Thomsen, J. et al . Suppression of intrinsic roughness in encapsulated graphene. Phys. Rev. B 96 , 014101 (2017).
Article ADS Google Scholar
Kudin, K. N. et al . Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8 , 36 (2008).
Sreeprasad, T. S., Samal, A. K. & Pradeep, T. Tellurium Nanowire-Induced Room Temperature Conversion of Graphite Oxide to Leaf-like Graphenic Structures. J. Phys. Chem. C 113 , 1727–1737 (2009).
Wang, C. et al . Freeze-Casting Produces a Graphene Oxide Aerogel with a Radial and Centrosymmetric Structure. ACS Nano 12 , 5816–5825 (2018).
Liu, Y. et al . Aminated Graphene Oxide Impregnated with Photocatalytic Polyoxometalate for Efficient Adsorption of Dye Pollutants and Its Facile and Complete Photoregeneration. Small 13 , 1603174 (2017).
Dunetz, J. R., Magano, J. & Weisenburger, G. A. Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals. Org. Process Res. Dev. 20 , 140–177 (2016).
Eitan, A., Jiang, K., Dukes, D., Andrews, R. & Schadler, L. S. Surface Modification of Multiwalled Carbon Nanotubes: Toward the Tailoring of the Interface in Polymer Composites. Chem. Mater. 15 , 3198–3201 (2003).
Yan, W., Seifermann, S. M., Pierrat, P. & Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 13 , 25–54 (2015).
Li, B. et al . Photochemical Chlorination of Graphene. ACS Nano 5 , 5957–5961 (2011).
Usachov, D. et al . Nitrogen-Doped Graphene: Efficient Growth, Structure, and Electronic Properties. Nano Lett. 11 , 5401–5407 (2011).
Granzier-Nakajima, T., Fujisawa, K., Anil, V., Terrones, M. & Yeh, Y.-T. Controlling Nitrogen Doping in Graphene with Atomic Precision: Synthesis and Characterization. Nanomaterials 9 , 425 (2019).
Bianconi, A., Hagstrom, S. B. M. & Bachrach, R. Z. Photoemission studies of graphite high-energy conduction-band and valence-band states using soft-x-ray synchrotron radiation excitation. Phys. Rev. B 16 , 5543 (1977).
Sutar, D. S., Singh, G. & Botcha, V. D. Electronic structure of graphene oxide and reduced graphene oxide monolayers. Appl. Phys. Lett. 101 , 103103 (2012).
Hsu, H.-C. et al . Graphene oxide as a promising photocatalyst for CO 2 to methanol conversion. Nanoscale 5 , 262–268 (2013).
Hammer, P., Lacerda, R. G., Droppa, R. Jr. & Alvarez, F. Comparative study on the bonding structure of hydrogenated and hydrogen free carbon nitride films with high N content. Diamond Relat. Mater. 9 , 577–581 (2000).
Luo, Z. et al . Pyridinic N doped graphene: synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 21 , 8038 (2011).
Chen, Z. Y., Zhao, J. P., Yano, T. & Ooie, T. Valence band electronic structure of carbon nitride from x-ray photoelectron spectroscopy. J. Appl. Phys. 92 , 281 (2002).
Favaro, M. et al . A synchrotron-based spectroscopic study of the electronic structure of N-doped HOPG and PdY/N-doped HOPG. Surf. Sci. 646 , 132–139 (2016).
Helander, M. G., Greiner, M. T., Wang, Z. B. & Lu, Z. H. Pitfalls in measuring work function using photoelectron spectroscopy. Appl. Surf. Sci. 256 , 2602–2605 (2010).
Kang, B., Lim, S., Lee, W. H., Jo, S. B. & Cho, K. Work-Function-Tuned Reduced Graphene Oxide via Direct Surface Functionalization as Source/Drain Electrodes in Bottom-Contact Organic Transistors. Adv. Mater. 25 , 5856–5862 (2013).
Naito, K., Yoshinaga, N., Matake, S. & Akasaka, Y. Work-function decrease of transparent conducting films composed of hydrazine-reduced graphene oxide and silver nanowire stacked layers by electrochemical treatment. Synthetic Metals 195 , 260–265 (2014).
Becerril, H. A. et al . Fabrication and Evaluation of Solution-Processed Reduced Graphene Oxide Electrodes for p- and n-Channel Bottom-Contact Organic Thin-Film Transistors. ACS Nano 4 , 6343 (2010).
Ji, S. et al . Work function engineering of graphene oxide via covalent functionalization for organic field-effect transistors. Appl. Surf. Sci. 419 , 252–258 (2017).
Hummers, W. S. & Offeman, R. E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 80 , 1339–1339 (1958).
Molodtsov, S. L. et al . High-resolution Russian-German beamline at BESSY. Appl. Phys. A 94 , 501–505 (2009).
Aleksenskii, A. E. et al . Single layer graphene oxide films on a silicon surface. Tech. Phys. 58 , 1614–1618 (2013).
Download references
Author information
Authors and affiliations.
Ioffe Institute, 26 Politekhnicheskaya, 194021, Saint Petersburg, Russia
Maxim K. Rabchinskii, Sergei A. Ryzhkov, Demid A. Kirilenko, Nikolay V. Ulin, Marina V. Baidakova, Vladimir V. Shnitov, Sergei I. Pavlov, Aleksandr V. Shvidchenko & Pavel N. Brunkov
ITMO University, 49 Kronverksky Pr., 197101, Saint Petersburg, Russia
Demid A. Kirilenko, Dmitrii V. Potorochin & Pavel N. Brunkov
NRC “Kurchatov Institute”, 1 Akademika Kurchatova pl., 123182, Moscow, Russia
Ratibor G. Chumakov & Dina Yu. Stolyarova
St. Petersburg Academic University, Khlopin St. 8/3, 194021, Saint Petersburg, Russia
Nadezhda A. Besedina
Semenov Institute of Chemical Physics of Russian Academy of Sciences, Kosygina St., 4, 119991, Moscow, Russia
Maksim V. Gudkov & Valery P. Melnikov
Technische Universität Bergakademie Freiberg, Akademiestraße 6, 09599, Freiberg, Germany
Dmitrii V. Potorochin & Friedrich Roth
Deutsches Elektronen-Synchrotron DESY, 85 Notkestraße, Hamburg, D-22607, Germany
Dmitrii V. Potorochin
Institut fur Festkorper und Materialphysik, Technische Universitat Dresden, Dresden, Germany
Dmitry A. Smirnov
Helmholtz-Zentrum Berlin für Materialien und Energie, Hahn-Meitner-Platz 1, 14109, Berlin, Germany
Maria Brzhezinskaya
Laboratoire CRISMAT, ENSICAEN UMR6508, 6 Bd Maréchal Juin, Cedex 4, Caen, 14050, France
Oleg I. Lebedev
You can also search for this author in PubMed Google Scholar
Contributions
M.K.R. primarily designed the study and supervised the experimental research. M.K.R., S.A.R., A.V.S. and N.V.U. performed the chemical reduction, modification of GO, carried out test reactions and prepared samples for the whole series of measurements. M.V.G. and V.P.M. synthesized graphene oxide suspensions and performed the specific surface area measurements. M.K.R. carried out measurements of UV-Vis spectra. M.V.B., O.I.L. and D.A.K. obtained and processed TEM images and SAED patterns. S.I.P. obtained SEM images and performed elemental analysis. N.A.B. carried out measurements of Raman spectra and performed XRD measurements. V.V.S., D. Yu.S., D.V.P., F.R., M.B., R.G.C. and D.A.S. obtained and evaluated XPS, XAS, VB and WF spectra. S.I.P., M.V.B. and P.N.B. performed sheet resistance and electrical conductivity measurements. P.N.B. obtained AFM images. M.K.R., V.V.S. and D.A.K. co-wrote the manuscript with input from P.N.B, N.V.U. and D.Yu.S. All authors discussed the results and commented on the manuscript.
Corresponding author
Correspondence to Demid A. Kirilenko .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Rabchinskii, M.K., Ryzhkov, S.A., Kirilenko, D.A. et al. From graphene oxide towards aminated graphene: facile synthesis, its structure and electronic properties. Sci Rep 10 , 6902 (2020). https://doi.org/10.1038/s41598-020-63935-3
Download citation
Received : 21 August 2019
Accepted : 01 April 2020
Published : 23 April 2020
DOI : https://doi.org/10.1038/s41598-020-63935-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spatial decoupling of bromide-mediated process boosts propylene oxide electrosynthesis.
- Mingfang Chi
Nature Communications (2024)
Composites containing resins and carbon nano-onions as efficient porous carbon materials for supercapacitors
- Gabriela Siemiaszko
- Joanna Breczko
- Marta E. Plonska-Brzezinska
Scientific Reports (2023)
Applications of graphene-based tungsten oxide nanocomposites: a review
- Mehr-Un Nisa
- Nimra Nadeem
- Imran Shahid
Journal of Nanostructure in Chemistry (2023)
Fabrication of Multifunctional PA6 Composite Fibers Based on a Synergistic Effect of Ionic Liquid Modified Carbon Nanofillers
- Yonghuan Zhao
Fibers and Polymers (2023)
Surface reaction for efficient and stable inverted perovskite solar cells
- Jinhui Tong
Nature (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Abstract. Thanks to the unique properties of graphite oxides and graphene oxide (GO), this material has become one of the most promising materials that are widely studied. Graphene oxide is not only a precursor for the synthesis of thermally or chemically reduced graphene: researchers revealed a huge amount of unique optical, electronic, and ...
An improved method for the preparation of graphene oxide (GO) is described. Currently, Hummers' method (KMnO 4, NaNO 3, H 2 SO 4) is the most common method used for preparing graphene oxide. We have found that excluding the NaNO 3, increasing the amount of KMnO 4, and performing the reaction in a 9:1 mixture of H 2 SO 4 /H 3 PO 4 improves the ...
1. Introduction. Graphene oxide (GO) is the oxidized analogy of graphene, recognized as the only intermediate or precursor for obtaining the latter in large scale, [1] since the English chemist, sir Brodie first reported about the oxidation of graphite centuries ago [2].About thirty years ago, the term graphene was officially claimed to define the single atom-thin carbon layer of graphite [3 ...
The earliest synthesis of graphene oxide (GO) was achieved by chemist B. Brodie at the University of Oxford, in 1859 4 — over a century before the discovery of modern carbon materials such as ...
The overall synthesis route can be modified to fit an individual researcher's needs. For instance, it is worth noting that the size and shape of the carbon source will determine the size and shape of the resulting graphene oxide via a modified Hummer's method [10].Typically, this means that the average diameter of the graphite powders used in synthesis will determine the average lateral ...
Graphene oxide is highly desired for printing electronics, catalysis, energy storage, separation membranes, biomedicine, and composites. However, the present synthesis methods depend on the ...
The synthesis of graphene has a sincerely lower cost t h an the afor e m entioned c a rbon- based n a nom a terials. The rese arch com m unit y show ed incre a sed int e rest for in vest ig a -
Here is an overview of the synthesis, properties, and applications of graphene and related materials (primarily, graphite oxide and its colloidal suspensions and materials made from them), from a materials science perspective.
Graphene oxide (GO) is a water soluble carbon material in general, suitable for applications in electronics, the environment, and biomedicine. GO is produced by oxidation of abundantly available graphite, turning black graphite into water-dispersible single layers of functionalized graphene-related materials Chemistry of 2D materials: graphene and beyond Recent Review Articles
A number of top-down strategies for the synthesis of graphene, including exfoliation and chemical reduction of graphene oxide, are discussed. A few bottom-up synthesis methods for graphene are also covered, including thermal chemical vapor deposition, plasma-enhanced chemical vapor deposition, thermal decomposition of silicon, unzipping of ...
Metrics. As an important precursor and derivate of graphene, graphene oxide (GO) has received wide attention in recent years. However, the synthesis of GO in an economical and efficient way ...
Actually, ultrasonic treatment is an important stage in the synthesis of graphene oxide [80-82], since the duration and conditions of ultrasonic treatment change the fractional composition of the processed material; in particular, a decrease in the size of graphene oxide grains is observed already in the first 15 min.
graphite oxide comprising these groups have been reported so far in the literature.36-39 GO can be considered as a precursor for graphene synthesis by either chemical, thermal or microwave reductionprocesses.GOconsistsofa1Dsingle-layerofgraphite oxide and is usually produced by the chemical treatment of
One of the common techniques of graphene synthesis is the thermal decomposition of silicon carbide (SiC). ... exhibits an absorption peak of monolayer graphene oxide at around 230 nm, whereas pristine graphene exhibit its absorption peak between 250 and 270 nm [83]. The number of layers and graphene thickness can be studied by ultraviolet ...
Graphene oxide is single or few atomic layers of graphene attached to oxygen-containing groups. It is a flexible material and is prepared by the energetic oxidation of graphite. Graphene oxide has a lot of potential due to easy synthesis, cost-effectiveness and scope for mass scale production. It is one of the important materials for the ...
A review. The chem. of graphene oxide is discussed in this crit. review. Particular emphasis is directed toward the synthesis of graphene oxide, as well as its structure. Graphene oxide as a substrate for a variety of chem. transformations, including its redn. to graphene-like materials, is also discussed.
Abstract. A low cost, non-explosive process for the synthesis of graphene oxide (GO) is demonstrated. Using suitable choice of reaction parameters including temperature and time, this recipe does ...
Reproducible graphene synthesis by oxygen-free chemical vapour ... 2024 — Researchers have found a new way to synthesize graphene oxide which has significantly fewer defects compared to ...
Experimental procedure 2.1 Preparation of graphene oxide by a modified hummer’s method In typical procedure, graphene oxide (GO) was produced using modified hummers method from pure graphite powder. In this method, 27 ml of sulfuric acid (H2SO4) and 3 ml of phosphoric acid (H3PO4) (volume ratio 9:1) were mixed and stirred for several ...
For this purpose, the synthesis of graphene oxide (GO) and silane-grafted GO followed by chemical reduction of particles was conducted to prepare rGOs with different surface chemistry. Particles were characterized by Fourier transform infrared spectroscopy, Raman spectroscopy, x-ray diffraction, scanning electron microscopy, and energy ...
As frontier materials, graphene oxide (GO) and graphene have penetrated almost all research areas and advanced numerous technologies in sensing, electronics, energy storage, catalysis, water treatment, advanced composites, biomedical, and more. However, the affordable large-scale synthesis of high-quality GO and graphene remains a significant challenge that negatively affects its ...
Graphite, diamonds, graphene oxide (GO), fullerenes, carbon nanotubes (CNTs), carbon dots, carbon quantum dots, and graphene quantum dots (GQDs) are some of the conventional allotropes of carbon-based nanomaterials that have gathered prominence due to their different applications in the energy, environmental, and healthcare sectors owing to ...
This Comment discusses relevant issues for industrial-scale graphene synthesis, one of the critical aspects for the future graphene industry. ... (non-oxidized), graphene oxide (GO) or reduced GO ...
This work presents results from the synthesis of graphene oxide sheets (GOs) from rice husks ash and its modification by incorporating copper nanoparticles. Rice husks, a low-value waste product generated in large quantities, were thermally treated to obtain a mixture of natural carbons with silica. This carbonaceous material was then reacted ...
Thermal reduction of GO/graphite oxide via annealing is among the most widely used methods for the synthesis of graphene/rGO. During thermal reduction, GO is exfoliated into fewer graphene sheets by rapidly heating process GO at a high temperature without or with an inert/reducing atmosphere (slightly reducing) or under vacuum conditions [216 ...
Improving fracture toughness, electrical conductivity, and biocompatibility has consistently presented challenges in the development of artificial bone replacement materials. This paper presents a new strategy for creating high-performance, multifunctional composite ceramic materials by doping graphene oxide (GO), which is known to induce osteoblast differentiation and enhance cell adhesion ...
Graphene derivative materials, such as graphene oxide (GO) and reduced graphene oxide (rGO), have garnered significant attention from scientists for over two decades due to their distinctive characteristics and versatile applications across various fields, particularly in biomedical applications. Incorporating gold nanoparticles (AuNPs) into rGO sheets as rGO-Au nanocomposites further enhances ...
The wet chemical synthesis of pure 2D materials such as graphene and MoS 2 surged in the early 21st century 28,29, and more recently, doped 2D materials, nanocomposites, and their heterostructures ...
DOI: 10.1016/j.matlet.2024.136724 Corpus ID: 270055237; Synthesis and photocatalytic properties of Cerium(IV) oxide graphene oxide composites @article{Wang2024SynthesisAP, title={Synthesis and photocatalytic properties of Cerium(IV) oxide graphene oxide composites}, author={WeiE Wang and Huili Ren and Siqi Huang and Jianyan Li and Yuguang Lv and Libo Du}, journal={Materials Letters}, year ...
In this paper we present a facile method for the synthesis of aminated graphene derivative through simultaneous reduction and amination of graphene oxide via two-step liquid phase treatment with ...