- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs


Chapter 5: 10 Real Cases on Acute Heart Failure Syndrome: Diagnosis, Management, and Follow-Up
Swathi Roy; Gayathri Kamalakkannan
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Case review, case discussion.
- Full Chapter
- Supplementary Content
Case 1: Diagnosis and Management of New-Onset Heart Failure With Reduced Ejection Fraction
A 54-year-old woman presented to the telemetry floor with shortness of breath (SOB) for 4 months that progressed to an extent that she was unable to perform daily activities. She also used 3 pillows to sleep and often woke up from sleep due to difficulty catching her breath. Her medical history included hypertension, dyslipidemia, diabetes mellitus, and history of triple bypass surgery 4 years ago. Her current home medications included aspirin, atorvastatin, amlodipine, and metformin. No significant social or family history was noted. Her vital signs were stable. Physical examination showed bilateral diffuse crackles in lungs, elevated jugular venous pressure, and 2+ pitting lower extremity edema. ECG showed normal sinus rhythm with left ventricular hypertrophy. Chest x-ray showed vascular congestion. Laboratory results showed a pro-B-type natriuretic peptide (pro-BNP) level of 874 pg/mL and troponin level of 0.22 ng/mL. Thyroid panel was normal. An echocardiogram demonstrated systolic dysfunction, mild mitral regurgitation, a dilated left atrium, and an ejection fraction (EF) of 33%. How would you manage this case?
In this case, a patient with known history of coronary artery disease presented with worsening of shortness of breath with lower extremity edema and jugular venous distension along with crackles in the lung. The sign and symptoms along with labs and imaging findings point to diagnosis of heart failure with reduced EF (HFrEF). She should be treated with diuretics and guideline-directed medical therapy for congestive heart failure (CHF). Telemetry monitoring for arrythmia should be performed, especially with structural heart disease. Electrolyte and urine output monitoring should be continued.
In the initial evaluation of patients who present with signs and symptoms of heart failure, pro-BNP level measurement may be used as both a diagnostic and prognostic tool. Based on left ventricular EF (LVEF), heart failure is classified into heart failure with preserved EF (HFpEF) if LVEF is >50%, HFrEF if LVEF is <40%, and heart failure with mid-range EF (HFmEF) if LVEF is 40% to 50%. All patients with symptomatic heart failure should be started on an angiotensin-converting enzyme (ACE) inhibitor (or angiotensin receptor blocker if ACE inhibitor is not tolerated) and β-blocker, as appropriate. In addition, in patients with New York Heart Association functional classes II through IV, an aldosterone antagonist should be prescribed. In African American patients, hydralazine and nitrates should be added. Recent recommendations also recommend starting an angiotensin receptor-neprilysin inhibitor (ARNI) in patients who are symptomatic on ACE inhibitors.
Get Free Access Through Your Institution
Pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
- Search Menu
- Sign in through your institution
- Author videos
- ESC Content Collections
- Supplements
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- About European Heart Journal Supplements
- About the European Society of Cardiology
- ESC Publications
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, case presentation.
- < Previous
Clinical case: heart failure and ischaemic heart disease
- Article contents
- Figures & tables
- Supplementary Data
Giuseppe M C Rosano, Clinical case: heart failure and ischaemic heart disease, European Heart Journal Supplements , Volume 21, Issue Supplement_C, April 2019, Pages C42–C44, https://doi.org/10.1093/eurheartj/suz046
- Permissions Icon Permissions
Patients with ischaemic heart disease that develop heart failure should be treated as per appropriate European Society of Cardiology/Heart Failure Association (ESC/HFA) guidelines.
Glucose control in diabetic patients with heart failure should be more lenient that in patients without cardiovascular disease.
Optimization of cardiac metabolism and control of heart rate should be a priority for the treatment of angina in patients with heart failure of ischaemic origin.
This clinical case refers to an 83-year-old man with moderate chronic obstructive pulmonary disease and shows that implementation of appropriate medical therapy according to the European Society of Cardiology/Heart Failure Association (ESC/HFA) guidelines improves symptoms and quality of life. 1 The case also illustrates that optimization of glucose metabolism with a more lenient glucose control was most probably important in improving the overall clinical status and functional capacity.
The patient has family history of coronary artery disease as his brother had suffered an acute myocardial infarction (AMI) at the age of 64 and his sister had received coronary artery by-pass. He also has a 14-year diagnosis of arterial hypertension, and he is diabetic on oral glucose-lowering agents since 12 years. He smokes 30 cigarettes per day since childhood.
In February 2009, after 2 weeks of angina for moderate efforts, he suffered an acute anterior myocardial infarction. He presented late (after 14 h since symptom onset) at the hospital where he had been treated conservatively and had been discharged on medical therapy: Atenolol 50 mg o.d., Amlodipine 2.5 mg o.d., Aspirin 100 mg o.d., Atorvastatin 20 mg o.d., Metformin 500 mg tds, Gliclazide 30 mg o.d., Salmeterol 50, and Fluticasone 500 mg oral inhalers.
Four weeks after discharge, he underwent a planned electrocardiogram (ECG) stress test that documented silent effort-induced ST-segment depression (1.5 mm in V4–V6) at 50 W.
He underwent a coronary angiography (June 2009) and left ventriculography that showed a not dilated left ventricle with apical dyskinesia, normal left ventricular ejection fraction (LVEF, 52%); occlusion of proximal LAD, 60% stenosis of circumflex (CX), and 60% stenosis of distal right coronary artery (RCA). An attempt to cross the occluded left anterior descending (LAD) was unsuccessful.
He was therefore discharged on medical therapy with: Atenolol 50 mg o.d., Atorvastatin 20 mg o.d., Amlodipine 2.5 mg o.d., Perindopril 4 mg o.d., oral isosorbide mono-nitrate (ISMN) 60 mg o.d., Aspirin 100 mg o.d., metformin 850 mg tds, Gliclazide 30 mg o.d., Salmeterol 50 mcg, and Fluticasone 500 mcg b.i.d. oral inhalers.
He had been well for a few months but in March 2010 he started to complain of retrosternal constriction associated to dyspnoea for moderate efforts (New York Heart Association (NYHA) II–III, Canadian Class II).
For this reason, he was prescribed a second coronary angiography that showed progression of atherosclerosis with 80% stenosis on the circumflex (after the I obtuse marginal branch) and distal RCA. The LAD was still occluded.
After consultation with the heart team, CABG was avoided because surgical the risk was deemed too high and the patient underwent palliative percutaneous coronary intervention (PCI) of CX and RCA. It was again attempted to cross the occlusion on the LAD. But this attempt was, again, unsuccessful. Collateral circulation from posterior interventricular artery (PDL) to the LAD was found. The pre-PCI echocardiogram documented moderate left ventricular dysfunction (EF 38%), the pre-discharge echocardiogram documented a LVEF of 34%. Because of the reduced LVEF, atenolol was changed for Bisoprolol (5 mg o.d.).
At follow-up visit in December 2012, the clinical status and the haemodynamic conditions had deteriorated. He complained of worsening effort-induced dyspnoea/angina that now occurred for less than a flight of stairs (NYHA III). On clinical examination clear signs of worsening heart failure were detected ( Table 1 ). His medical therapy was modified to: Bisoprolol 5 mg o.d., Atorvastatin 20 mg o.d., Amlodipine 2.5 mg o.d., Perindopil 5 mg o.d., ISMN 60 mg o.d., Aspirin 100 mg o.d., Metformin 500 mg tds, Furosemide 50 mg o.d., Gliclazide 30 mg o.d., Salmeterol 50 mcg oral inhaler, and Fluticasone 500 mcg oral inhaler. A stress perfusion cardiac scintigraphy was requested and revealed dilated ventricles with LVEF 19%, fixed apical perfusion defect and reversible perfusion defect of the antero-septal wall (ischaemic burden <10%, Figure 1 ). He was admitted, and an ICD was implanted.
Clinical parameters during follow-up visits
| . | December 2012 . | March 2013 . | September 2013 . | January 2014 . | January 2015 . |
|---|---|---|---|---|---|
| Weight (kg) | 72 | 71 | 74 | 70 | 68 |
| Height (cm) | 170 | 170 | 170 | 170 | 170 |
| BMI | 24.9 | 24.9 | 25.1 | 24.9 | 24.8 |
| JVP | +2 cm H O | +2 cm H O | +2 cm H O | Normal | Normal |
| Oedema | Bilateral oedema up to mid shins | Bilateral pretibial oedema (2+) | Bilateral pretibial oedema (3+) | No pedal oedema | No pedal oedema |
| Blood pressure (mmHg) | 115/80 | 115/75 | 110/60 | 110/70 | 112/68 |
| Pulse (bpm) | 88 | 86 | 92 | 68 | 56 |
| Auscultation | |||||
| Heart | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex | Systolic murmur 4/6 at apex |
| Lungs | Bilateral fine basilar crackles | Bilateral fine basilar crackles | Bilateral fine basilar and mid lung crackles | Clear | Clear |
| Laboratory findings | |||||
| FPG (mg/dL) | 100 | 98 | 96 | 106 | 112 |
| HbA1c (%) | 6.8 | 6.7 | 6.6 | 7 | 7.3 |
| Plasma creatinine (mg/dL) | 1.1 | 1.2 | 1.5 | 1.1 | 1.2 |
| Triglycerides | 118 mg/dL | NA | NA | 107 mg/dL | 114 mg/dL |
| Total cholesterol | 146 mg/dL | NA | NA | 142 mg/dL | 148 mg/dL |
| LDL-C | 68 mg/dL | NA | NA | 64 mg/dL | 68 mg/dL |
| HDL-C | 51 mg/dL | NA | NA | 48 mg/dL | 54 mg/dL |
| BNP | NA | 862 | 1670 | 276 | 244 |
| LVEF | 19 | 20 | 32 | 32 | |
| . | December 2012 . | March 2013 . | September 2013 . | January 2014 . | January 2015 . |
|---|---|---|---|---|---|
| Weight (kg) | 72 | 71 | 74 | 70 | 68 |
| Height (cm) | 170 | 170 | 170 | 170 | 170 |
| BMI | 24.9 | 24.9 | 25.1 | 24.9 | 24.8 |
| JVP | +2 cm H O | +2 cm H O | +2 cm H O | Normal | Normal |
| Oedema | Bilateral oedema up to mid shins | Bilateral pretibial oedema (2+) | Bilateral pretibial oedema (3+) | No pedal oedema | No pedal oedema |
| Blood pressure (mmHg) | 115/80 | 115/75 | 110/60 | 110/70 | 112/68 |
| Pulse (bpm) | 88 | 86 | 92 | 68 | 56 |
| Auscultation | |||||
| Heart | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex | Systolic murmur 4/6 at apex |
| Lungs | Bilateral fine basilar crackles | Bilateral fine basilar crackles | Bilateral fine basilar and mid lung crackles | Clear | Clear |
| Laboratory findings | |||||
| FPG (mg/dL) | 100 | 98 | 96 | 106 | 112 |
| HbA1c (%) | 6.8 | 6.7 | 6.6 | 7 | 7.3 |
| Plasma creatinine (mg/dL) | 1.1 | 1.2 | 1.5 | 1.1 | 1.2 |
| Triglycerides | 118 mg/dL | NA | NA | 107 mg/dL | 114 mg/dL |
| Total cholesterol | 146 mg/dL | NA | NA | 142 mg/dL | 148 mg/dL |
| LDL-C | 68 mg/dL | NA | NA | 64 mg/dL | 68 mg/dL |
| HDL-C | 51 mg/dL | NA | NA | 48 mg/dL | 54 mg/dL |
| BNP | NA | 862 | 1670 | 276 | 244 |
| LVEF | 19 | 20 | 32 | 32 | |

Myocardial perfusion scintigraphy and left ventriculography showing dilated left ventricle with left ventricular ejection fraction 19%. Reversible perfusion defects on the antero-septal wall and fixed apical perfusion defect.
In March 2013, he felt slightly better but still complained of effort-induced dyspnoea/angina (NYHA III, Table 1 ). Medical therapy was updated with bisoprolol changed with Nebivolol 5 mg o.d. and perindopril changed to Enalapril 10 mg b.i.d. The switch from bisoprolol to nebivolol was undertaken because of the better tolerability and outcome data with nebivolol in elderly patients with heart failure. Perindopril was switched to enalapril because the first one has no indication for the treatment of heart failure.
In September 2013, the clinical conditions were unchanged, he still complained of effort-induced dyspnoea/angina (NYHA III) and did not notice any change in his exercise capacity. His BNP was 1670. He was referred for a 3-month cycle of cardiac rehabilitation during which his medical therapy was changed to: Nebivolol 5 mg o.d., Ivabradine 5 mg b.i.d., uptitrated in October to 7.5 b.i.d., Trimetazidine 20 mg tds, Furosemide 50 mg, Metolazone 5 mg o.d., K-canrenoate 50 mg, Enalapril 10 mg b.i.d., Clopidogrel 75 mg o.d., Atorvastatin 40 mg o.d., Metformin 500 mg b.i.d., Salmeterol 50 mcg oral inhaler, and Fluticasone 500 mcg oral inhaler.
At the follow-up visit in January 2014, he felt much better and had symptomatically, he no longer complained of angina, nor dyspnoea (NYHA Class II, Table 1 ). Trimetazidine was added because of its benefits in heart failure patients of ischaemic origin and because of its effect on functional capacity. Ivabradine was added to reduce heart rate since it was felt that increasing nebivolol, that was already titrated to an effective dose, would have had led to hypotension.
He missed his follow-up visits in June and October 2014 because he was feeling well and he had decided to spend some time at his house in the south of Italy. In January and June 2015, he was well, asymptomatic (NYHA I–II) and able to attend his daily activities. He did not complain of angina nor dyspnoea and reported no limitations in his daily activities. Unfortunately, in November 2015 he was hit by a moped while on the zebra crossing in Rome and he later died in hospital as a consequence of the trauma.
This case highlights the need of optimizing both the heart failure and the anti-anginal medications in patients with heart failure of ischaemic origin. This patient has improved dramatically after the up-titration of diuretics, the control of heart rate with nebivolol and ivabradine and the additional use of trimetazidine. 1–3 All these drugs have contributed to improve the clinical status together with a more lenient control of glucose metabolism. 4 This is another crucial point to take into account in diabetic patients, especially if elderly, with heart failure in whom aggressive glucose control is detrimental for their functional capacity and long-term prognosis. 5
IRCCS San Raffaele - Ricerca corrente Ministero della Salute 2018.
Conflict of interest : none declared. The authors didn’t receive any financial support in terms of honorarium by Servier for the supplement articles.
Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JG , Coats AJ , Falk V , González-Juanatey JR , Harjola VP , Jankowska EA , Jessup M , Linde C , Nihoyannopoulos P , Parissis JT , Pieske B , Riley JP , Rosano GM , Ruilope LM , Ruschitzka F , Rutten FH , van der Meer P ; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC . Eur J Heart Fail 2016 ; 18 : 891 – 975 .
Google Scholar
Rosano GM , Vitale C. Metabolic modulation of cardiac metabolism in heart failure . Card Fail Rev 2018 ; 4 : 99 – 103 .
Vitale C , Ilaria S , Rosano GM. Pharmacological interventions effective in improving exercise capacity in heart failure . Card Fail Rev 2018 ; 4 : 1 – 27 .
Seferović PM , Petrie MC , Filippatos GS , Anker SD , Rosano G , Bauersachs J , Paulus WJ , Komajda M , Cosentino F , de Boer RA , Farmakis D , Doehner W , Lambrinou E , Lopatin Y , Piepoli MF , Theodorakis MJ , Wiggers H , Lekakis J , Mebazaa A , Mamas MA , Tschöpe C , Hoes AW , Seferović JP , Logue J , McDonagh T , Riley JP , Milinković I , Polovina M , van Veldhuisen DJ , Lainscak M , Maggioni AP , Ruschitzka F , McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology . Eur J Heart Fail 2018 ; 20 : 853 – 872 .
Vitale C , Spoletini I , Rosano GM. Frailty in heart failure: implications for management . Card Fail Rev 2018 ; 4 : 104 – 106 .
- myocardial ischemia
- cardiac rehabilitation
- heart failure
- older adult
| Month: | Total Views: |
|---|---|
| April 2019 | 719 |
| May 2019 | 146 |
| June 2019 | 86 |
| July 2019 | 98 |
| August 2019 | 112 |
| September 2019 | 144 |
| October 2019 | 281 |
| November 2019 | 263 |
| December 2019 | 229 |
| January 2020 | 254 |
| February 2020 | 303 |
| March 2020 | 271 |
| April 2020 | 380 |
| May 2020 | 372 |
| June 2020 | 427 |
| July 2020 | 320 |
| August 2020 | 307 |
| September 2020 | 376 |
| October 2020 | 553 |
| November 2020 | 459 |
| December 2020 | 473 |
| January 2021 | 305 |
| February 2021 | 424 |
| March 2021 | 479 |
| April 2021 | 445 |
| May 2021 | 251 |
| June 2021 | 307 |
| July 2021 | 228 |
| August 2021 | 248 |
| September 2021 | 383 |
| October 2021 | 414 |
| November 2021 | 442 |
| December 2021 | 367 |
| January 2022 | 276 |
| February 2022 | 354 |
| March 2022 | 537 |
| April 2022 | 373 |
| May 2022 | 451 |
| June 2022 | 253 |
| July 2022 | 161 |
| August 2022 | 208 |
| September 2022 | 281 |
| October 2022 | 424 |
| November 2022 | 568 |
| December 2022 | 442 |
| January 2023 | 305 |
| February 2023 | 357 |
| March 2023 | 533 |
| April 2023 | 474 |
| May 2023 | 403 |
| June 2023 | 235 |
| July 2023 | 242 |
| August 2023 | 230 |
| September 2023 | 339 |
| October 2023 | 456 |
| November 2023 | 477 |
| December 2023 | 262 |
| January 2024 | 226 |
| February 2024 | 283 |
| March 2024 | 282 |
| April 2024 | 400 |
| May 2024 | 298 |
| June 2024 | 97 |
Email alerts
More on this topic, related articles in pubmed, citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1554-2815
- Print ISSN 1520-765X
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Survival analysis of heart failure patients: A case study
Roles Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing
Affiliation Department of Statistics, Government College University, Faisalabad, Pakistan
Roles Data curation, Formal analysis, Methodology, Writing – original draft
Roles Conceptualization, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Roles Formal analysis, Investigation, Methodology, Validation
Roles Methodology, Software, Visualization, Writing – review & editing
- Tanvir Ahmad,
- Assia Munir,
- Sajjad Haider Bhatti,
- Muhammad Aftab,
- Muhammad Ali Raza

- Published: July 20, 2017
- https://doi.org/10.1371/journal.pone.0181001
- Reader Comments
This study was focused on survival analysis of heart failure patients who were admitted to Institute of Cardiology and Allied hospital Faisalabad-Pakistan during April-December (2015). All the patients were aged 40 years or above, having left ventricular systolic dysfunction, belonging to NYHA class III and IV. Cox regression was used to model mortality considering age, ejection fraction, serum creatinine, serum sodium, anemia, platelets, creatinine phosphokinase, blood pressure, gender, diabetes and smoking status as potentially contributing for mortality. Kaplan Meier plot was used to study the general pattern of survival which showed high intensity of mortality in the initial days and then a gradual increase up to the end of study. Martingale residuals were used to assess functional form of variables. Results were validated computing calibration slope and discrimination ability of model via bootstrapping. For graphical prediction of survival probability, a nomogram was constructed. Age, renal dysfunction, blood pressure, ejection fraction and anemia were found as significant risk factors for mortality among heart failure patients.
Citation: Ahmad T, Munir A, Bhatti SH, Aftab M, Raza MA (2017) Survival analysis of heart failure patients: A case study. PLoS ONE 12(7): e0181001. https://doi.org/10.1371/journal.pone.0181001
Editor: Chiara Lazzeri, Azienda Ospedaliero Universitaria Careggi, ITALY
Received: February 26, 2017; Accepted: June 23, 2017; Published: July 20, 2017
Copyright: © 2017 Ahmad et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Heart failure is the state in which muscles in the heart wall get fade and enlarge, limiting heart pumping of blood. The ventricles of heart can get inflexible and do not fill properly between beats. With the passage of time heart fails in fulfilling the proper demand of blood in body and as a consequence person starts feeling difficulty in breathing.
The main reason behind heart failure include coronary heart disease , diabetes , high blood pressure and other diseases like HIV, alcohol abuse or cocaine, thyroid disorders, excess of vitamin E in body, radiation or chemotherapy, etc. As stated by WHO [ 1 ] Cardiovascular Heart Disease (CHD) is now top reason causing 31% of deaths globally. Pakistan is also included in the list of countries where prevalence of CHD is increasing significantly. According to report by Al-Shifa hospital [ 2 ], 33% of Pakistani population above 45 has hypertension, 25% of patients over 45 years suffer diabetes mellitus, and CHD deaths in Pakistan has reached about 200,000 per year i.e. 410/100,000 of the population). All this results in increased prevalence of heart failure. Rate of heart failure patients in Pakistan is estimated to be 110 per million [ 1 ]. Rising stress of economic and social issues in the modern era, greasy food with little exercise results towards increased prevalence of heart failure in Pakistan.
Despite of this alarming situation, Pillai and Ganapathi [ 3 ] have reported that there are no reliable estimates of heart failure incidence and prevalence in this region while they are required due to poor and oily diet, lack of exercise and poor health care policies in this region. There are some projections based on prevalence data only from western countries.
In addition to relative scarcity of studies focusing on heart failure in this region, the present study has specific importance in the Pakistani context, as diet patterns in Pakistan are different with other the countries of South Asia like India, Bangladesh, Nepal and Sri Lanka.
The main objective of this study is to estimate death rates due to heart failure and to investigate its link with some major risk factors by choosing Faisalabad (third most populous city of Pakistan) as study area.
Detail of data
Current study is based on 299 patients of heart failure comprising of 105 women and 194 men. All the patients were more than 40 years old, having left ventricular systolic dysfunction and falling in NYHA class III and IV. Follow up time was 4–285 days with an average of 130 days. Disease was diagnosed by cardiac echo report or notes written by physician. Age, serum sodium, serum creatinine, gender, smoking, Blood Pressure (BP), Ejection Fraction (EF), anemia, platelets, Creatinine Phosphokinase (CPK) and diabetes were considered as potential variables explaining mortality caused by CHD. Age, serum sodium and CPK are continuous variables whereas EF, serum creatinine and platelets were taken as categorical variables. EF was divided into three levels (i.e. EF≤30, 30<EF≤45 and EF>45) and platelets was also divided into three level on the basis of quartiles. Serum creatinine greater than its normal level (1.5) is an indicator of renal dysfunction. Its effect on mortality was studied as creatinine >1.5 vs ≤1.5. Anemia in patients was assessed by their haematocrit level. Following McClellan et al. [ 4 ] the patients with haematocrit less than 36 (minimum normal level of haematocrit) were taken as anemic. The information related to risk factors were taken from blood reports while smoking status and blood pressure were taken from physician’s notes.
The study was approved by Institutional Review Board of Government College University, Faisalabad-Pakistan and the principles of Helsinki Declaration were followed. Informed consent was taken by the patients from whom the information on required characteristics were collected/accessed.
Statistical techniques
Due to the presence of censored data, survival analysis was used to estimate the survival and mortality rates. Kaplan & Meier [ 5 ] product limit estimator was used to make comparisons between survival rates at different levels explanatory variables. Cox regression as presented by Collett [ 6 ] was used to develop a model that can link the hazard of death for an individual with one or more explanatory variables and test the significance of these variables.
For determining the functional form of any particular independent variable following Fitrianto & Jiin [ 7 ] and Gillespie [ 8 ], plot of Martingale residuals versus different values (or levels) of a variable were used. The functional form of CPK was not linear therefore it was log transformed.
Following Pavlou et al. [ 9 ] model validation was assessed by bootstrapping [ 10 – 12 ] with 200 bootstrap replications. Internal validation of model was further checked by calculating calibration slope [ 13 ] for the average linear predictor. The calibration slope helped in estimating the ability of model for survival probability prediction. Discriminating ability of model was assessed by ROC curve [ 14 ]. A nomogram [ 15 ] was also built to predict the survival probabilities graphically.
Up to end of follow-up period, 96 (32%) patients died due to CHD. Table 1 , presents different baseline characteristics of dead and censored patients at the end of follow up period.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0181001.t001
The results of Cox regression model are presented in Table 2 . As Cox regression is semi parametric model, hence estimate of intercept (baseline hazard) was not provided by model fitting. According to Cox model, age was most significant variable.
https://doi.org/10.1371/journal.pone.0181001.t002
Coefficient concerning age indicated that chances of death due to CHD increase with growing age. Hazard of death due to CHD increases by 4% for every additional year of age. EF was another significant factor, hazard rate among patients with EF ≤30 was 67% and 59% higher as compared to the patients with 30<EF≤45 and EF≥45 respectively. In Fig 1(a) , Kaplan Meier survival curve was constructed for each level of EF. It is obvious that survival for EF ≤30 was lower than other two levels. Moreover, relatively small difference between the survival of patients with 30<EF<45 and EF≥45 can be observed. Serum creatinine was significant with p-value = 0.0026. It means death hazard gets more than double for unit increase in Serum creatinine. Serum sodium was significant with p-value = 0.0052 and its one unit (meq/L) increase decreases the hazard by 6%. Anemia was significant variable with p-value = 0.0096 and an anemic patient had 76% more chances of death as compared to non-anemic patient. According to results in Table 2 , gender, smoking, diabetes, CPK and platelets were found to be non-significant.
https://doi.org/10.1371/journal.pone.0181001.g001
Ejection fraction is an important measurement of how well one’s heart is pumping and is used to help classify heart failure and guide treatment. The EF is also found to be significant correlate of deaths among heart failure patients from Cox regression for present sample. Keeping its importance in view, EF is further analyzed through baseline characteristics ( Table 3 ) and Kaplan Meier curves ( Fig 1(a) ) which shows similar pattern as presented in Cox regression results.
https://doi.org/10.1371/journal.pone.0181001.t003
In Fig 1(b) , Kaplan Meier survival curves were constructed for both genders showed almost identical survival pattern.
Model validation
For model validation, calibration slope and ROC curve are developed from 200 bootstrapped samples. Calibration slope was equal to 0.96, which showed that model was not over fitted and predictions made by this model would neither be overestimated nor under estimated.
Discrimination ability was checked by ROC curve in Fig 2(a) . Area under the curve (AUC) was 0.81 at time of 250 days and 0.77 at time of 50 days thus it can be interpreted that the model was able to correctly recognize the event of death for 81% and 77% patients within 250 and 50 days respectively. It shows that discrimination ability of Cox model is higher at longer follow up time. The reason of this difference may be due to the violation of constant effect assumption of EF which is evident in Fig 2(b) which displays that effect of EF increases with the passage of time. As EF is highly significant for mortality (see Table 2 ), hence with passage of time model’s discrimination ability increases.
https://doi.org/10.1371/journal.pone.0181001.g002
Nomogram for prediction
Calibration slope and discrimination ability suggested that Cox model is able to predict probability of survival and hazard sufficiently. On the basis of these results, nomogram is presented in Fig 3 to provide the graphical predictions of probability after assigning different points to each independent variable with respect to their significance. Sum of these points provides an estimate of probability of survival.
https://doi.org/10.1371/journal.pone.0181001.g003
For example, an 80 year old non-smoker female diabetic patient with high blood pressure, EF = 40, haematocrit = 35, sodium = 140, creatinine = 5.2, platelets = 300 thousands and CPK = 3000 have points equal to 50+0+8+3+14+56+50+12+40+10+20 = 263 and probability of her survival is 0.60. The Cox model used for constructing this nomogram was fitted on original values of variables.
The statistical analysis identified age, EF, creatinine, sodium, anemia and BP as the significant variables affecting the likelihood of mortality among heart failure patients. Most of studies [ 16 – 17 ] supported the male gender as predictor of mortality among heart failure patients. However, like Román et al. [ 18 ] in this study odd ratio of men/women is not significant. With respect to significance and importance of variables the findings of the present study are more in lines with Rahimi et al. [ 19 ]. The results are found to be similar with other international studies like [ 20 – 23 ].
The findings that seem surprising are non-significance of smoking and diabetes. However, similar results concerning diabetes and smoking have been reported in other studies [ 24 – 25 ] as well. The reason behind may be smoking and diabetes are basically causes of heart problem at initial stages. We were only concerned with patients of NYHA class III and IV which are advanced stages of heart failure. Up to these stages, these factors (diabetes and smoking) may probably be controlled by medications and hence these factors do not have significant effect on deaths due to heart failure in class III and IV.
Performance of model was checked using calibration slope and ROC curve. Both concluded in adequacy of model for prediction. ROC curves were also used to discuss the goodness of model with respect to time. Nomogram was used to find the probability of survival by graphical method. It was observed that fall of survival probability was almost same for Kaplan Meier plot and nomogram.
It can be concluded that growing age, renal dysfunction (having serum creatinine greater than its normal level 1.5), high BP (higher than normal range), higher level of anaemia and lower values of ejection fraction (EF) are the key factors contributing towards increased risk of mortality among heart failure patients. Increased level of serum sodium can reduce the odds of death. No significant differences were found due to smoking status, diabetes and gender of patients.
Supporting information
S1 data. data_minimal..
https://doi.org/10.1371/journal.pone.0181001.s001
- 1. WHO. Fact sheet on CVDs. Global Hearts. World Health Organization. 2016.
- 2. Al-Shifa IH. Cardiac Diseases in Pakistan [Internet]. 2016 [cited 15 Jun 2017]. http://www.shifa.com.pk/chronic-disease-pakistan/
- View Article
- PubMed/NCBI
- Google Scholar
- 6. Collett D. Modelling Survival Data in Medical Research. 2nd ed. Taylor & Francis; 2003.
- 8. Gillespie B. Checking Assumptions in the Cox Proportional Hazards Regression Model. Midwest SAS Users Group (MWSUG). 2006.
- 11. Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman and Hall, New York; 1993.
- 15. Yang D. Build Prognostic Nomograms for Risk Assessment Using SAS. Proceedings of SAS Global Forum 2013. 2013.
Case Study: Heart Failure Exacerbation Due to an Often Overlooked Cause
— shows importance of using wide differential when investigating hf.
by Kate Kneisel , Contributing Writer, MedPage Today

"Medical Journeys" is a set of clinical resources reviewed by physicians, meant for the medical team as well as the patients they serve. Each episode of this journey through a disease state contains both a physician guide and a downloadable/printable patient resource. "Medical Journeys" chart a path each step of the way for physicians and patients and provide continual resources and support, as the caregiver team navigates the course of a disease.
This month: A noteworthy case study
Why has a 64-year-old man become increasingly short of breath over the past 2 weeks? That's what Sandra K. Rabat, DO, of A.T. Still University School of Osteopathic Medicine in Mesa, Arizona, and colleagues needed to determine, as they reported in Cureus .
The patient's medical history included a diagnosis of congestive heart failure and coronary artery disease in 2014, after stenting of his left anterior descending artery and right coronary artery. He also had high blood pressure and stage III chronic kidney disease (CKD) when he presented to the hospital for assessment after 2 weeks of worsening dyspnea.
The patient told clinicians he became winded even after a few steps, and that at night, he needed to prop himself up on three pillows to improve his breathing. He also had fluid retention in his lower legs, feet, and ankles that lasted all day, and continued to be worsening.
He said he was not aware of anything that might have exacerbated his shortness of breath, and that he did not use oxygen therapy or inhalers at home. He admitted that he was not consistent about taking his prescribed medications – carvedilol, lisinopril, furosemide, atorvastatin, and clopidogrel – and that that he sometimes forgot them entirely.
His family history was significant for premature coronary artery disease and the sudden cardiac death of his grandfather at age 49.
On questioning, he reported feeling that his heart beat was very rapid, but had no other observations. He said he did not use illicit drugs, smoke, or drink alcohol. Social determinants of the patient's health included experiencing homelessness, and he had very little social or family support.
Initial examination found that he was in a hypertensive emergency. His blood pressure was 220/110 mmHg and oxygen saturation was 84% oxygen on room air. Significant lab test findings included a creatinine level that was increased to 2.4 mg/dL from his baseline of 1.7 mg/dL. Troponins were 12,333 pg/ml and brain natriuretic peptide (BNP) was 1,431 pg/ml.
Clinicians noted the complexities of interpreting cardiac troponin levels and BNP in the setting of CKD. However, they said, "the magnitude of elevation of the troponins and BNP was very concerning for another process within the myocardium rather than being a false-positive elevation from CKD alone."
EKG findings included the following:
- Prolonged QTC interval
- Left-axis deviation
- Non-specific ST-T changes
- No ST-segment elevations
Chest x-ray showed that the patient had cardiomegaly with pulmonary edema. Given the high troponin levels and EKG results, the team ruled out ST-segment elevation myocardial infarction (STEMI) as a diagnosis in favor of non-ST-segment elevation myocardial infarction (NSTEMI).
In the emergency department, the patient was started on one dose of clonidine, nasal cannula oxygen, and heparin drip, and later also received hydralazine as needed for systolic blood pressure that exceeded 160 mmHg. In light of his elevated BNP levels and chest x-ray findings, the patient was admitted for acute exacerbation of congestive heart failure. Clinicians started the patient on aggressive diuresis with IV furosemide and accelerated his cardiac workup.
The workup for pulmonary embolus was unremarkable, given the patient's negative venous duplex and V/Q scan, the case authors noted. "Transthoracic echocardiogram revealed significant findings, including an estimated ejection fraction of 10% with moderate mitral regurgitation and moderate tricuspid regurgitation, a dilated right ventricle with severely impaired systolic function, and grade three diastolic dysfunction with restrictive filling."
The team noted that a previous echocardiogram performed about 2 years earlier showed that the patient's estimated ejection fraction had been 60% with preserved left ventricular systolic function. Because of the severity of his left ventricular dysfunction, and dilation of the left ventricle, the patient received a portable external cardiac defibrillator.
An ultrasound of his abdomen revealed bilateral renal atrophy with diffusely increased echogenicity bilaterally, which is indicative of CKD. Because he was in volume overload, clinicians continued his diuresis and closely monitored his creatinine levels.
After interval improvement of his kidney function, the patient underwent cardiac catheterization, which indicated "nonobstructive coronary artery disease and severe pulmonary hypertension."
Right heart hemodynamics revealed a mean pulmonary capillary wedge pressure of 40 mmHg, mean pulmonary artery pressure of 60 mmHg, and mean right atrial (RA) pressure of 32 mmHg, the case authors reported, noting that this ruled out nonischemic cardiomyopathy as a cause of the patient's acute decompensation.
Following the cardiac catheterization, the team discontinued diuretic treatment. The patient was started on dobutamine infusion at 5 mcg/kg/min, and the dose was titrated to achieve a minimum mean arterial pressure of 65 mmHg. He began taking isosorbide mononitrate and hydralazine, and continued with carvedilol.
Diuretic therapy with torsemide was reinstated. Treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor neprilysin inhibitor was contraindicated, due to the patient's medical status: acute kidney injury in the presence of CKD stage III and a glomerular filtration rate of less than 30 ml/min/1.73 m 2 .
Efforts to wean the patient off dobutamine, however, failed when his kidney function worsened to a creatinine level of 2.7 mg/dL, which the authors noted confirmed a need for inotropic support. When his kidney function improved, they started the patient on milrinone infusion with close monitoring, based on evidence of decompensated heart failure with low cardiac output and signs of end-organ hypoperfusion.
The objective was to combine milrinone infusion with standard heart failure therapy, including a beta-blocker, as tolerated. "The benefit of using milrinone over dobutamine in this patient's case is that milrinone, a phosphodiesterase inhibitor, will not antagonize a beta-blocker like dobutamine," the authors explained.
Because dobutamine's action is partly related to beta-1 and beta-2 adrenergic receptors, concomitant beta-blocker therapy would likely reduce the hemodynamic response to treatment, the team speculated. The patient was scheduled for a cardiac MRI, possibly to be followed by endomyocardial biopsy.
This proved to be unnecessary, however, when the test result came back as "positive for Coxsackie B viral antibody immunoglobulin G (IgG), indicating chronic viral infection," Rabat and co-authors said.
"This case highlights how viruses continue to be an underappreciated cause of heart failure. In fact, viral myocarditis is an underdiagnosed cause of acute heart failure and chronic dilated cardiomyopathy," as is iron deficiency anemia , the authors wrote.
Cardiomyopathy – which is associated with muscle or electrical dysfunction of the heart – is defined by the American Heart Association as a heterogeneous group of diseases of the myocardium, usually with inappropriate ventricular hypertrophy or dilatation.
Noting that viral myocarditis is often overlooked due to its varied presentation, Rabat and co-authors urged clinicians not to underestimate the substantial cardiovascular risks associated with a large spectrum of viral infections, some of which can lead to significant deterioration in decompensated patients.
"Coxsackie B virus is one of the most common causes of viral myocarditis and is responsible for 10-20% of all myocarditis and dilated cardiomyopathy cases," the case authors said. Parvovirus B19, adenovirus, Epstein-Barr virus, HIV, and COVID-19 have also been reported to cause myocarditis.
Viral myocarditis may go undiagnosed due to the wide variety of presentations, which can range from dyspnea to more aggressive symptoms suggestive of acute coronary syndrome. One review noted that among more than 3,000 patients with suspected acute or chronic myocarditis, dyspnea was found in 72%, chest pain in 32%, and arrhythmias in 18%.
"Myocarditis generally results from cardiotropic viral infection followed by active inflammatory destruction of the myocardium," the case authors stated. After the initial acute symptoms of viral myocarditis, the viral infection may either clear completely, persist, or "lead to a persistent auto-immune-mediated inflammatory process with long-term symptoms of heart failure."
A persistent viral infection of the myocardium can result in a progressive deterioration of left ventricular ejection fraction (LVEF), which likely explains the current patient's decline in LVEF from 60% to 10% over less than 2 years, Rabat and co-authors noted.
Despite being considered the diagnostic gold standard for acute or chronic inflammatory heart disease, endomyocardial biopsy is used infrequently because of the perception of associated risks and the absence of a widely accepted and sensitive histologic standard.
Endomyocardial biopsies may be complemented with use of liquid biopsy to monitor circulating biomarkers, including microRNAs (miRNAs), which have also demonstrated excellent diagnostic capability, the team noted. In fact, in a recent study , expression levels of miRNAs differentiated between patients with viral myocarditis, inflammatory cardiomyopathy, and healthy donors with a specificity of over 95%.
"However, further studies would be needed to elevate the routine use of miRNA-panel in addition to further guidelines to help optimize the management of this disease," the case authors wrote, noting that current guidelines advise optimal use of heart failure medications to manage symptoms.
Rabat and co-authors noted that the COVID-19 pandemic has brought to light a global sensitivity to viral infections. The pathogenesis of viral myocarditis in heart failure remains poorly understood and represents a significant global public health issue. The team urged clinicians investigating heart failure to maintain a wide index of suspicion and be aware "that even chronic Coxsackie B viral infection can cause an acute presentation of heart failure."
Read previous installments of this series:
Part 1: Heart Failure: A Look at Low Ejection Fraction
Part 2: Exploring Heart Failure With Preserved Ejection Fraction
Part 3: Heart Failure With Reduced Ejection Fraction: Diagnosis and Evaluation
Part 4: Case Study: Lightheadedness, Fatigue in Man With Hypertension
Part 5: Heart Failure With Preserved Ejection Fraction: Diagnosis and Evaluation
Part 6: Heart Failure Medical Management
Part 7: Managing Heart Failure Comorbidities
![case study heart failure author['full_name']](https://clf1.medpagetoday.com/media/images/author/KKneisel_188.jpg)
Kate Kneisel is a freelance medical journalist based in Belleville, Ontario.
Disclosures
The authors reported no conflicts of interest.
Primary Source
Source Reference: Rabat S K, et al "A case report on an underappreciated cause of heart failure: Chronic viral myocarditis" Cureus 2022; DOI: 10.7759/cureus.27253.
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
This case study involves a 76 year old female named Mary Lou Poppins, who presented to the ED accompanied by her son. She called her son after having symptoms of shortness of breath and confusion. Her past medical history includes hypertension, hyperlipidemia, coronary artery disease, and she was an everyday smoker for 30 years. She reports her home medications are lisinopril, simvastatin, and baby aspirin. Her current lifestyle includes: being a widow of six years, she lives alone, she walks her dog everyday, she drives to her knitting group three days a week, she makes dinner for her grandchildren once a week, she attempts to eat healthy but admits to consuming salty and high fat foods, and she insists on being very independent.
Mary Lou Poppins initial vitals in the emergency department includes a blood pressure of 138/70, heart rate of 108. respiratory rate of 26, temperature 98.9 degrees fahrenheit, and oxygen saturation of 84%. Her initial assessment included alert and oriented to person and place, dyspnea, inspiratory crackles in bilateral lungs, and a cough with pink frothy sputum. Her labs and diagnostics resulted in a BNP of 740 pg/ml, an echocardiogram showing an ejection fraction of 35%, an ECG that read sinus tachycardia, and a chest x-ray that confirmed pulmonary edema.
The Emergency Department physician diagnosed Mary Lou Poppins with left-sided heart failure. The orders included: supplemental oxygen titrated to keep saturation >93%, furosemide IV, enoxaparin subq, and metoprolol PO. Nursing Interventions included: monitoring oxygen saturation, adjusting oxygen route and dosage according to orders, assessing mentation and confusion, obtaining IV access, reassessing vitals, administering medications, and keeping the head of the bed elevated greater than 45 degrees. She was admitted to the telemetry unit for further stabilization, fluid balance monitoring, and oxygen monitoring.
On day one of hospital admission, Mary Lou Poppins required 4L of oxygen via nasal cannula in order to maintain the goal saturation of >93%. Upon assessment, it was determined that she was oriented to person and place. Auscultation of the lungs revealed bilateral crackles throughout, requiring collaboration with respiratory therapy once in the morning, and once in the afternoon. Physical therapy worked with the patient, but she was only able to ambulate for 100 feet. During ambulation, the patient had a decrease of oxygen saturation and dyspnea, requiring her oxygen to be increased to 6L. At the end of the day, strict intake and output monitoring showed an intake of 1200 mL of fluids, with an urinary output of 2L.
On day two of admission, Mary Lou began demonstrating signs of improvement. She only required 2 L of oxygen via nasal cannula with diminished crackles heard upon auscultation. Morning weight showed a weight loss of 1.3 lbs and the patient was oriented to person, place, and sequence of events. During physical therapy, she was able to ambulate 300 feet without required increased oxygen support. Daily fluid intake was 1400 mL with a urinary output of 1900 mL.
On the third and final day of admission, Mary Lou was AOx4 and did not require any type of oxygen support. When physical therapy arrived, the patient was able to ambulate 500 feet, which was close to her pre-hospital status. When the doctor arrived, the patient informed him that she felt so much better and felt confident going home. The doctor placed orders for discharge.
Upon discharge and throughout the patient’s hospital stay, Mary Lou Poppins was educated regarding the disease process of heart failure; symptoms to monitor for and report to her doctor; the importance of daily monitoring of weight, blood pressure, and heart rate; and the importance of adhering to a diet and exercise regime. Education was also provided regarding her medications and the importance of strictly adhering to them in order to prevent exacerbations of heart failure. Smoking cessation was also included in her plan of care. The patient received an informational packet regarding her treatment plan, symptoms to monitor for, and when to call her physician. Upon discharge, the patient was instructed to schedule a follow up appointment with her cardiologist for continued management of her care.
The patient was put in contact with a home health agency to help manage her care. The home health nurse will help to reinforce the information provided to the patient, assess the patient’s home and modify it to meet her physical limitations, and help to create a plan to meet daily dietary and exercise requirements. Regular follow-up appointments were stressed to Mary Lou Poppins in order to assess the progression of her disease. It will be important to monitor her lab values to also assess her disease progression and for any potential side effects associated with her medications. Repeat echocardiograms will be necessary to monitor her ejection fraction; if it does not improve with the treatment plan, an implanted cardiac defibrillator may be necessary to prevent cardiac death.
Open-Ended Questions
- What were the clinical manifestations that Mary Lou Poppins presented with in the ED that suggested the new onset of CHF?
- What factors most likely contributed to the onset of CHF?
- What patient education should Mary Lou Poppins receive on discharge in regards to managing her CHF?
Nursing Case Studies by and for Student Nurses Copyright © by jaimehannans is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book

Create Free Account or
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Cardiovascular Care Team
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
- View All Cardiology Updates
- Earn Credit
- View the Education Catalog
- ACC Anywhere: The Cardiology Video Library
- CardioSource Plus for Institutions and Practices
- ECG Drill and Practice
- Heart Songs
- Nuclear Cardiology
- Online Courses
- Collaborative Maintenance Pathway (CMP)
- Understanding MOC
- Image and Slide Gallery
- Annual Scientific Session and Related Events
- Chapter Meetings
- Live Meetings
- Live Meetings - International
- Webinars - Live
- Webinars - OnDemand
- Certificates and Certifications
- ACC Accreditation Services
- ACC Quality Improvement for Institutions Program
- CardioSmart
- National Cardiovascular Data Registry (NCDR)
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Cardiovascular Buyers Guide
- Clinical Solutions
- Clinician Well-Being Portal
- Diversity and Inclusion
- Infographics
- Innovation Program
- Mobile and Web Apps
Feature | Advancing Health Equity For Heart Failure Patients: A Case Study for Team-Based Care and Innovation
Cardiology Magazine

Caring for vulnerable populations presents some unique challenges. It also offers immense opportunities for innovation to effect positive change for many lives.
To achieve health care parity, emphasis should be placed on addressing not only the medical needs but also the adverse social determinants of health that often serve as barriers to care.
We describe here the contributions of the Grady Heart Failure Program to decreasing health disparities and addressing the needs of our patients through a comprehensive and multidisciplinary approach.

Grady Health System serves as the safety net hospital for residents of metro Atlanta and its environs, providing care for the underserved in partnership with Emory and Morehouse Schools of Medicine. The geographic area served by Grady comprises predominantly underrepresented minority population groups who call Grady their medical home.
The Grady Heart Failure Program (GHFP) is a unique and innovative multidisciplinary program with an overarching goal of promoting health equity by improving the quality of care and outcomes of vulnerable patients with HF.
Our program provides a broad range of inpatient and outpatient services. Established in March 2011, the Grady Heart Failure Clinic provides comprehensive outpatient care for patients with HF through coordinated systems of care.
The clinic model focuses on four pillars of management:
- optimizing guideline-directed medical therapy (GDMT)
- patient education
- improving process and access to care, and
- addressing social determinants of health (SDOH)
Integrated in this model are facilitating follow-up in the cardiology and primary care clinics, cardiovascular risk factor management, depression screening and a personalized care approach that focuses on patient autonomy/empowerment skills such as adherence to therapy, dietary modifications, physical activity and daily weight self-assessment.
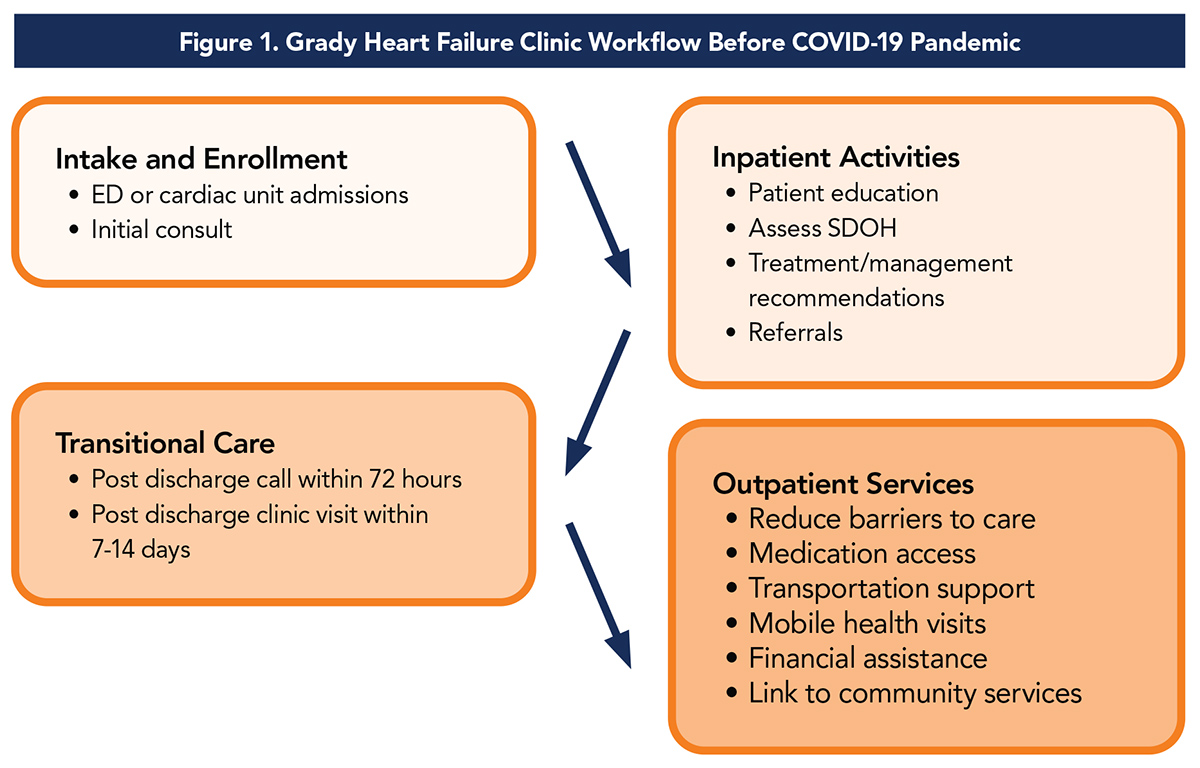
Our multidisciplinary team consists of eight full-time and several part-time advanced practice providers (APPs) in collaboration with cardiologists, a community health worker, nurse care coordinator, pharmacist and quality/process improvement personnel (Table 1).
Core Program Components Addressing Barriers to Health Equity
The core components of the GHFP that address barriers to health equity include a 30-day supply of HF medications at hospital discharge, hospital-based financial assistance, connection to community resources by a dedicated community health worker, rideshare support for patients who lack means of transportation to or from clinic appointments, provision of medically tailored meals post discharge, and mobile health home visits for patients unable to leave their residence due to mobility problems or caregiver responsibilities.
Program Innovation
We have developed several innovative programs specifically tailored to meet the unique needs of our patients and improve quality of care. The routine assessment of SDOH is incorporated into our EMR – a care management tool that has an HF-specific component, completed during the initial consultation by the APP.
With the aid of a community grant to support and expand our partnerships, we created education initiatives for patients and their caregivers that included support to overcome identified barriers to care such as low health literacy, financial strain, lack of insurance and lack of transportation.
Remote blood pressure and weight monitoring using a telehealth program was initiated for patients at high risk for recurrent admissions. Although the initial grant funding has ended, we continue to offer remote patient monitoring, free weight scales and blood pressure monitors, and a 30-day supply of medications to our patients through support from the health system and partnerships with national and community organizations.
We provide rideshare support for patients to attend clinic appointments. Our nurse care coordinator and community health worker also manage other innovative services and partnerships: community partnerships such as Open Hand (prepares and delivers healthy meals to patients), Home Instead (provides in-home care and support with common activities of daily living) and mobile integrated health (MIH) home visits.
The Grady MIH Team conducts home visits to patients identified as high-risk for hospital readmissions within 30 days post discharge. Working in close collaboration with the GHFP team, they assess vital signs, assess home safety, perform medication reconciliation and optimization of HF therapy, and administer appropriate point of care tests and intravenous diuretics if needed. Our partnership with the MIH Team has evolved significantly during the COVID-19 pandemic.
Impact of Our Program on Patients With HF

We perform an average of 140 inpatient and 25 clinical decision unit consults each month. We have completed >1,700 inpatient consults in the past year. The HF clinic consists of nine half-day clinic sessions plus four half-day clinic sessions at a neighborhood health center.
A combined census of approximately 600 patients are seen in clinic each month, with >5,000 patient visits in the past year with a staff of five APPs. Over 6,500 unique patients have received care at the Grady HF clinic since its inception in 2011.
By addressing socioeconomic barriers to care, the program has reduced 30-day readmission rates. For FY 2019, the 30-day all-cause readmission rate dropped to 18.5%, compared with 24.9% at inception, and a 30-day HF-related readmission rate of 10.7%.
The program's goal is to reach 85% of eligible patients within three days of initial discharge. To date, we have surpassed that goal, contacting 94.3% of patients by telephone within three days of hospital discharge and scheduling follow-up clinic visits within seven to 14 days post discharge for more than 97% of our patients.
Awards and Recognition
In recognition of the outstanding service provided by the GHFP, the National Association of Public Hospitals and Health Systems (now America's Essential Hospitals) presented our team with the Gage Award for improving quality in vulnerable populations.
The GHFP actively participates in national quality improvement initiatives such as the Get with the Guidelines and Target-Heart Failure programs of the American Heart Association (AHA). Since 2015, the GHFP has received annual recognition for our participation. We are recipients of the highest form of recognition, "Gold Plus Target-Heart Failure Honor Roll" Award, since 2018. This is an advanced level of recognition that acknowledges hospitals for consistent compliance with quality improvement measures outlined in the AHA secondary prevention guidelines for the treatment of HF patients.
The Centers for Disease Control and Prevention (CDC) recognized the GHFP as a promising program that advances health equity, reduces health disparities and addresses the SDOH related to heart disease. We were selected to participate in a rigorous evaluation process with feedback from experts on how to continually improve our processes and practices. Results from this evaluation showed that our patients had significantly fewer readmissions and reduced length of stay, with estimated net savings to the health system of $899,059 for 348 annual averted readmissions.
These national recognitions and awards are a testament to the dedication and passion of our multidisciplinary team to improve outcomes of the patients we serve.
Adapting HF Care in the Time of COVID-19
The advent of the novel SARS-CoV-2 virus in 2019 has led to significant changes in the delivery of health care. Accordingly, we modified our clinic workflow to deliver continuous and effective care to our patients during this period. Prioritizing the safety of our patients while recognizing the unique needs of our population has been our primary goal.
Despite the challenges we faced, we continue to provide our patients with access to quality HF care safely, thus promoting health equity during this COVID-19 pandemic, a time that has magnified the effects of health disparities.
The Heart Failure Society of America recently issued a statement, providing guidance on virtual visits, emphasizing the need for patient risk stratification, the importance of having a virtual visit workflow and the role of multidisciplinary HF clinicians such as APPs, physicians, pharmacists and licensed social workers. 1
We developed new protocols, which included virtual visits, MIH home visits and remote patient monitoring.
Preliminary comparison of HF clinic visits in the early COVID-19 a seven-week period (March 23-May 9) in 2020 vs. 2019 showed we had only 24 face-to-face visits, vs. 677 in 2019. This reflects a shift from no virtual visits in 2019 to 679 telephone virtual visits and 13 video virtual visits in 2020, along with 122 MIH home visits (vs. none in 2019).
Here are some key changes we made to address disparities during this COVID-19 era:
- Provision of free weight scales and blood pressure monitors to patients prior to hospital discharge or during MIH visits (traditionally given at follow-up clinic visits)
- Access to medications: patients receive their medications from the Grady Pharmacy by mail at no cost
- Access to Care: nurse care coordinator, community health worker and APPs increased communication with our patients through telehealth (decreasing transportation needs)
- MIH Home Visits: in-home follow-up visits for concerns noted during virtual visit. See Figure 2 for more detail.
The success of the Grady Heart Failure Program has truly demonstrated the importance of multidisciplinary team-based care and innovative approaches in managing adverse SDOH and reducing disparities in care for vulnerable patients with HF, as outlined in a recent scientific statement from the AHA on SDOH in this population. 2
Since the Program was established nearly a decade ago, we have achieved consistency in the quality of care despite challenges we face in our safety net health system.
Although the COVID-19 pandemic has highlighted significant disparities in health care and intensified the impact of adverse SDOH, we made swift modifications to our current care delivery process to mitigate its effects and ensure that both medical and nonmedical needs of our HF patients are met.
Our ongoing quest remains achieving health equity for our patients through patient-centered activities.
Our passion to ensure equitable access to high-quality medical care for the underserved gives credibility to the saying, "Atlanta can't live without Grady."
Tweet #CardiologyMag

This article was authored by Modele Ogunniyi, MD, MPH, FACC , associate professor, Emory University School of Medicine and associate medical director, Grady Heart Failure Program; Yetunde Fatade, MD, MPH , PGY 2 Resident, J. Willis Hurst Internal Medicine Residency, Emory University School of Medicine; and Andrea Cafarelli, FNP-BC , advanced practice provider, Faith Works-Fleming, FNP-BC , advanced practice provider, and Diane Wirth, ANP-BC, CACP , manager, all with the Grady Heart Failure Program.
Clinical Topics: Cardiovascular Care Team, COVID-19 Hub, Heart Failure and Cardiomyopathies, Acute Heart Failure
Keywords: ACC Publications, Cardiology Magazine, Patient Readmission, Patient Discharge, Pharmacists, Caregivers, Medication Reconciliation, Diuretics, COVID-19, Safety-net Providers, House Calls, Community Health Workers, Inpatients, Blood Pressure, Pregnancy, Heart Failure
You must be logged in to save to your library.

The Latest From Cardiology
Read the entire October issue by clicking the links below!
Guest Editorial | NCDR: Taking the Lead in the Quest For Optimal Cardiovascular Care
Cover Story | Caught Between Two Worlds: Cardiovascular Care in American Indians and Alaska Natives
The Pulse of ACC | Call For Applications for CardioSmart Editor; ACC and Butterfly Join Forces; More
Feature | Atrial Fibrillation: What's New From ESC Congress Science?
Perspective | Health Equity: An Academic Approach For Research and Change
Feature | ACC 2020 Legislative Conference: A Virtual Opportunity to Engage, Advocate and Influence
Information Graphic Feature | Fall COVID-19 Recommendations
Innovative Endovascular Therapies For Iliofemoral Disease
The Wearable Cardioverter Defibrillator: A Life (Vest) of Controversy
Business of Medicine | Taking Care of Patients' Hearts During Cancer Treatment: Collaborative Oncology and Cardiovascular Care
Flu Vaccine More Critical Than Ever
Heart House Roundtable Convenes Stakeholders on HFrEF
JACC in a Flash
Journal Wrap
Number Check | Quality in Health Care
PAD Treatment Options: Staying on Point in Patient Discussions
Feature Interview | Reducing Health Disparities, Diversifying Physician Workforce Key to Achieving Quality Improvement
Innovation at ACC | Automated Data Abstraction Utilizing Artificial Intelligence and Natural Language Processing
Training and Education: New Strategies For New Times
Just One More | ACC's Taking the Latest Science Global
JACC Journals on ACC.org
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- Diabetes and Cardiometabolic Disease
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Pulmonary Hypertension and Venous Thromboembolism
Latest in Cardiology
Education and meetings.
- Online Learning Catalog
- Products and Resources
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- Accreditation Services
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Contact Member Care
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Case studies in heart failure
Affiliation.
- 1 Hickory Cardiology Associates, 1771 Tate Blvd. SE, Suite 201, Hickory, NC 28602, USA. [email protected]
- PMID: 14717398
- DOI: 10.1016/s0899-5885(02)00088-6
This article presents four case studies of patients with heart failure and the rationale for optimal treatment in each case.
PubMed Disclaimer
Similar articles
- Carvedilol: use in chronic heart failure. Doughty RN, White HD. Doughty RN, et al. Expert Rev Cardiovasc Ther. 2007 Jan;5(1):21-31. doi: 10.1586/14779072.5.1.21. Expert Rev Cardiovasc Ther. 2007. PMID: 17187454 Review.
- A clinical pharmacologist's response to Dr Milton Packer's perspective on the results of the COMET trial. Shepherd AM. Shepherd AM. J Card Fail. 2003 Dec;9(6):454-7. doi: 10.1016/j.cardfail.2003.08.005. J Card Fail. 2003. PMID: 14966784 Review. No abstract available.
- Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. Metra M, Nodari S, D'Aloia A, Muneretto C, Robertson AD, Bristow MR, Dei Cas L. Metra M, et al. J Am Coll Cardiol. 2002 Oct 2;40(7):1248-58. doi: 10.1016/s0735-1097(02)02134-4. J Am Coll Cardiol. 2002. PMID: 12383572 Clinical Trial.
- Effectiveness of carvedilol alone versus carvedilol + pimobendan for severe congestive heart failure. For the Keio Interhospital Cardiology Study (KICS) Group. Yoshikawa T, Baba A, Suzuki M, Yokozuka H, Okada Y, Nagami K, Takahashi T, Mitamura H, Ogawa S. Yoshikawa T, et al. Am J Cardiol. 2000 Jun 15;85(12):1495-7; A7. doi: 10.1016/s0002-9149(00)00803-1. Am J Cardiol. 2000. PMID: 10856401 Clinical Trial. No abstract available.
- Beta-blockade in heart failure: a comparison of carvedilol with metoprolol. Sanderson JE, Chan SK, Yip G, Yeung LY, Chan KW, Raymond K, Woo KS. Sanderson JE, et al. J Am Coll Cardiol. 1999 Nov 1;34(5):1522-8. doi: 10.1016/s0735-1097(99)00367-8. J Am Coll Cardiol. 1999. PMID: 10551702 Clinical Trial.
Publication types
- Search in MeSH
Related information
- PubChem Compound
- PubChem Compound (MeSH Keyword)
- PubChem Substance
LinkOut - more resources
Full text sources.
- Elsevier Science
- W.B. Saunders
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Find a Doctor
- Patients & Visitors
- ER Wait Times
- For Medical Professionals
- Piedmont MyChart

- Toggle navigation --> Back to Piedmont.org
- Piedmont Healthcare
Learning Center
- Education and Resources
- Women's Heart Support Network
- Heart Failure Tools and Resources
Case Study: Acute Heart Failure in a 20-year-old Patient
At Piedmont Heart’s Napa Valley Cardiology Conference, Dr. David Dean presents a challenging case of acute heart failure in a 20-year-old patient. Hear Piedmont’s unusual approach to therapy and tips for success from Dr. Dean, surgical director of Piedmont’s Samsky Advanced Heart Failure Center.
Download the Piedmont Now app
- Indoor Hospital Navigation
- Find & Save Physicians
- Online Scheduling
Download the app today!
Ongoing and Future Clinical Trials of Pharmacotherapy for Heart Failure
- Review Article
- Published: 22 June 2024
Cite this article

- Taha Mansoor ORCID: orcid.org/0000-0002-0447-7186 1 ,
- Subaina N. Khalid 2 ,
- Muhammad Ibraiz Bilal 3 ,
- Sardar Hassan Ijaz 4 ,
- Marat Fudim 5 , 6 ,
- Stephen J. Greene 5 , 6 ,
- Haider J. Warraich 7 ,
- Vijay Nambi 8 , 9 ,
- Salim S. Virani 8 , 10 ,
- Gregg C. Fonarow 11 ,
- Dmitry Abramov 12 &
- Abdul Mannan Khan Minhas 8
54 Accesses
8 Altmetric
Explore all metrics
Increasing knowledge of the processes leading to heart failure (HF) has allowed significant developments in therapies for HF over the past few decades. Despite the evolution of HF treatment, it still places a large burden on patients and health care systems across the world.
We used clinicaltrials.gov to gather information about clinical trials as of August 2023 studying pharmacotherapy for HF. We included interventional trials that were “ active, not recruiting ”, “ recruiting ”, or looking for participants but “ not yet recruiting ”. In total, 119 studies met our criteria of ongoing clinical trials studying novel as well as currently approved HF pharmacotherapies. The major interventions were novel medications/already approved medications for other diseases 29 % (34 trials), sodium-glucose co-transporter inhibitors 21 % (25 trials), angiotensin receptor blocker-neprilysin inhibitors 10 % (12 trials), diuretics 14 % (17 trials) and mineralocorticoid receptor antagonists 5 % (6 trials). Ongoing research will aid in reducing the impact of HF and we summarize clinical trials leading the way to better HF treatment in this review.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Effect of pharmacological treatment on outcomes of heart failure with preserved ejection fraction: an updated systematic review and network meta-analysis of randomized controlled trials

SGLT-2 Inhibitors on Top of Current Pharmacological Treatments for Heart Failure: A Comparative Review on Outcomes and Cost Effectiveness

Update on the Impact of Comorbidities on the Efficacy and Safety of Heart Failure Medications
Ugene E, Raunwald B. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. https://doi.org/10.1056/NEJM199711063371906 .
Article Google Scholar
Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128:1421–34.
Article CAS PubMed Google Scholar
Sayed A, Abramov D, Fonarow GC, Mamas MA, Kobo O, Butler J, et al. Reversals in the decline of heart failure mortality in the US, 1999–2021. JAMA Cardiol. 2024;9:585.
Article PubMed Google Scholar
Bozkurt B, Ahmad T, Alexander KM, Baker WL, Bosak K, Breathett K, et al. Heart failure epidemiology and outcomes statistics: a report of the heart failure society of America. J Card Fail. 2023;29:1412–51.
Article PubMed PubMed Central Google Scholar
Carluccio E, Dini FL, Bitto R, Ciccarelli M, Correale M, D’Agostino A, et al. Benefit from sacubitril/valsartan is associated with hemodynamic improvement in heart failure with reduced ejection fraction: an echocardiographic study. Int J Cardiol. 2022;350:62–8.
Donal E, L’official G, Kosmala W. New guidelines for managing chronic heart failure patients and new needs in echocardiography. Int J Cardiol. 2022;353:71–2.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:E895-1032.
PubMed Google Scholar
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. https://doi.org/10.1056/NEJMoa2022190 .
Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81:1810–34.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. https://doi.org/10.1056/NEJMoa1911303 .
Miller AB. Aldosterone antagonism in heart failure. Vasc Health Risk Manag. 2007;3:605.
PubMed PubMed Central Google Scholar
Whittaker A, Kragh ÅM, Hartleib-Geschwindner J, Albayaty M, Backlund A, Greasley PJ, et al. Safety, tolerability, and pharmacokinetics of the mineralocorticoid receptor modulator AZD9977 in healthy men: a phase i multiple ascending dose study. Clin Transl Sci. 2020;13:275–83.
Gupta R, Testani J, Collins S. Diuretic resistance in heart failure. Curr Heart Fail Rep. 2019;16:57–66.
Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387:1185–95.
Felker GM. New decongestion strategies in an evolving heart failure landscape. N Engl J Med. 2022;387:1231–3. https://doi.org/10.1056/NEJMe2209997 .
Halatchev IG, Wu WC, Heidenreich PA, Djukic E, Balasubramanian S, Ohlms KB, et al. Inpatient versus outpatient intravenous diuresis for the acute exacerbation of chronic heart failure. Int J Cardiol Heart Vasc. 2021;36: 100860.
Skali H, Dwyer EM, Goldstein R, Haigney M, Krone R, Kukin M, et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT-CRT. Eur J Heart Fail. 2014;16:560–5.
Jiang WL, Gu HB, Zhang YF, Xia QQ, Qi J, Chen JC. Vitamin D supplementation in the treatment of chronic heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol. 2016;39:56–61.
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–9.
Coratelli P, Petrarulo F, Buongiorno E, Giannattasio M, Antonelli G, Amerio A. Improvement in left ventricular function during treatment of hemodialysis patients with 25-OHD3. Contrib Nephrol. 1984;41:433–7.
Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–41.
Article CAS PubMed PubMed Central Google Scholar
Banerjee S, Mwangi JG, Stanley TK, Mitra R, Ebong EE. Regeneration and assessment of the endothelial glycocalyx to address cardiovascular disease. Ind Eng Chem Res. 2021;60:17328–47.
Article CAS Google Scholar
Fukaya E, Gloviczki ML. An introduction to endothelial glycocalyx-what is it and why does it matter?
The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. https://pubmed.ncbi.nlm.nih.gov/9036306/ . Cited 22 Apr 2024.
Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–8.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–31.
Benes J, Kotrc M, Kroupova K, Wohlfahrt P, Kovar J, Franekova J, et al. Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF). Sci Rep. 2022;12: 13038.
Jyothirmayi GN, Soni BJ, Masurekar M, Lyons M, Regan TJ. Effects of metformin on collagen glycation and diastolic dysfunction in diabetic myocardium. J Cardiovasc Pharmacol Therap. 1998;3:319–26. https://doi.org/10.1177/107424849800300407 .
Halabi A, Sen J, Huynh Q, Marwick TH. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol. 2020;19:01100.
Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J. 2006;70:1–7.
Woessner MN, Neil C, Saner NJ, Goodman CA, McIlvenna LC, De Zevallos JO, et al. Effect of inorganic nitrate on exercise capacity, mitochondria respiration, and vascular function in heart failure with reduced ejection fraction. J Appl Physiol. 2020;128:1355–64.
Hirai DM, Zelt JT, Jones JH, Castanhas LG, Bentley RF, Earle W, et al. Dietary nitrate supplementation and exercise tolerance in patients with heart failure with reduced ejection fraction. Am J Physiol Regul Integr Comp Physiol. 2017;312:R13-22.
Kansakar U, Varzideh F, Jankauskas SS, Gambardella J, Trimarco B, Santulli G. Advances in the understanding of excitation-contraction coupling: the pulsing quest for drugs against heart failure and arrhythmias. Eur Heart J Cardiovasc Pharmacother. 2021;7: e91.
PW A, B P, KJ A, J E, AF H, J B, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382. https://pubmed.ncbi.nlm.nih.gov/32222134/ . Cited 22 Apr 2024.
Barratt J, Andric B, Tataradze A, Schömig M, Reusch M, Valluri U, et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, open-label, active-controlled study (DOLOMITES). Nephrol Dial Transplant. 2021;36:1616–28.
Verdino P, Lee SL, Cooper FN, Cottle SR, Grealish PF, Hu CC, et al. Development of a long-acting relaxin analogue, LY3540378, for treatment of chronic heart failure. Br J Pharmacol. 2023;180:1965–80.
Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T, et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J. 2021;42:192.
Nieminen M, Fruhwald S, Heart LH-, and lung, 2013 undefined. Levosimendan: current data, clinical use and future development. ncbi.nlm.nih.govMS Nieminen, S Fruhwald, LMA Heunks, PK Suominen, AC Gordon, M Kivikko, P PolleselloHeart, lung and vessels, 2013 ncbi.nlm.nih.gov [Internet]. [cited 2024 Apr 22]; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3868185/ . Cited 22 Apr 2024.
Kida K, Miyajima I, Suzuki N, Greenberg BH, Akashi YJ. Nutritional management of heart failure. J Cardiol. 2023;81:283–91.
Abdin A, Anker SD, Butler J, Coats AJS, Kindermann I, Lainscak M, et al. “Time is prognosis” in heart failure: time-to-treatment initiation as a modifiable risk factor. ESC Heart Fail. 2021;8:4444–53.
Greene SJ, Bauersachs J, Brugts JJ, Ezekowitz JA, Lam CSP, Lund LH, et al. Worsening heart failure: nomenclature, epidemiology, and future directions: JACC review topic of the week. J Am Coll Cardiol. 2023;81:413–24.
Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5:632.
Ferhatbegović L, Mršić D, Macić-Džanković A. The benefits of GLP1 receptors in cardiovascular diseases. Front Clin Diabetes Health care. 2023;4: 1293926.
Download references
Author information
Authors and affiliations.
Department of Internal Medicine, Western Michigan University Homer Stryker M.D. School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008, USA
Taha Mansoor
Department of Internal Medicine, SUNY Upstate Medical University, Syracruse, NY, USA
Subaina N. Khalid
Department of Internal Medicine, Allegheny Health Network, Pittsburgh, PA, USA
Muhammad Ibraiz Bilal
Division of Cardiology, Hartford Hospital, Hartford, CT, USA
Sardar Hassan Ijaz
Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA
Marat Fudim & Stephen J. Greene
Department of Medicine, Duke University School of Medicine, Durham, NC, USA
Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Haider J. Warraich
Section of Cardiovascular Research, Baylor College of Medicine, Houston, TX, USA
Vijay Nambi, Salim S. Virani & Abdul Mannan Khan Minhas
Michael E. DeBakey, Veterans Affair Medical Center, Houston, TX, USA
Vijay Nambi
Department of Medicine, Aga Khan University, Karachi, Pakistan
Salim S. Virani
Division of Cardiology, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Gregg C. Fonarow
Division of Cardiology, Department of Medicine, Loma Linda University Health, Loma Linda, CA, USA
Dmitry Abramov
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Taha Mansoor .
Ethics declarations
Not applicable.

Conflict of Interest
Dr. Fonarow reports consulting for Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene has received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association (#929502), National Heart Lung and Blood Institute, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Merck, Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim/ Lilly, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, scPharmaecuticals, and Sanofi; serves as a consultant for Amgen, Bayer, Bristol Myers Squibb, Corteria Pharmaceuticals, CSL Vifor, Lexicon Pharmaceuticals, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, Urovant Pharmaceuticals; and has received speaker fees from Bayer, Boehringer Ingelheim, Cytokinetics, Lexicon, and Roche Diagnostics. Dr. Dmitry Abramov has received speaker fees from Bayer and AstraZeneca. Dr. Virani: Grant support from the Department of Veterans Affairs, NIH, Tahir and Jooma Family. Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org- ended June 2021). No relationships with industry to disclose.
Availability of data and materials
Clinicaltrials.gov.
Ethical approval
Author contributions.
Data extraction, writing: TM. Writing: SNK, MIB. Reviewing and editing: SHI, MF, SJG, HJW, VN, SSV, GCF, DA. Conceptualizing, reviewing, editing, and supervision: AMKM.
Consent for participation
Consent for publication, code availability, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Mansoor, T., Khalid, S.N., Bilal, M.I. et al. Ongoing and Future Clinical Trials of Pharmacotherapy for Heart Failure. Am J Cardiovasc Drugs (2024). https://doi.org/10.1007/s40256-024-00658-0
Download citation
Accepted : 30 May 2024
Published : 22 June 2024
DOI : https://doi.org/10.1007/s40256-024-00658-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Congestive heart failure.
Ahmad Malik ; Daniel Brito ; Sarosh Vaqar ; Lovely Chhabra .
Affiliations
Last Update: November 5, 2023 .
- Continuing Education Activity
Congestive heart failure (CHF) is a complex clinical syndrome characterized by inefficient myocardial performance, resulting in compromised blood supply to the body. CHF results from any disorder that impairs ventricular filling or ejection of blood to the systemic circulation. Patients usually present with fatigue and dyspnea, reduced exercise tolerance, and systemic or pulmonary congestion. The etiology of HF is variable and extensive. A comprehensive assessment is required when evaluating a patient with HF. The general management aims at relieving systemic and pulmonary congestion and stabilization of hemodynamic status, regardless of the cause. This activity reviews the evaluation and management of congestive heart failure and highlights the role of the healthcare team in improving care for patients with this condition.
- Apply the staging and classification systems of heart failure.
- Assess and monitor patients with heart failure for signs of decompensation, fluid retention, and response to treatment.
- Select appropriate diagnostic tests, like echocardiography and biomarker assays, to aid in heart failure diagnosis and monitoring.
- Collaborate with multidisciplinary healthcare teams, including cardiologists, nurses, and pharmacists, to ensure coordinated and comprehensive care for heart failure patients.
- Introduction
Congestive heart failure (CHF), as defined by the American College of Cardiology (ACC) and the American Heart Association (AHA), is "a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood.” Ischemic heart disease is the leading cause of death worldwide and also the leading cause of CHF. CHF is a common disorder worldwide with a high morbidity and mortality rate. With an estimated prevalence of 26 million people worldwide, CHF contributes to increased healthcare costs, reduces functional capacity, and significantly affects quality of life. It is imperative to diagnose and effectively treat the disease to prevent recurrent hospitalizations, decrease morbidity and mortality, and enhance patient outcomes. [1]
The etiology of heart failure (HF) is variable and extensive. The general management aims at relieving systemic and pulmonary congestion and stabilization of hemodynamic status, regardless of the cause. The treatment of HF requires a multifaceted approach involving patient education, optimal medication administration, and decreasing acute exacerbations.
Left ventricle ejection fraction (LV EF) is used to classify HF. [1]
- HF with reduced ejection fraction (HFrEF): LV EF ≤ 40%
- HF with mildly reduced ejection fraction: LV EF 41% - 49% and evidence of HF (elevated cardiac biomarkers or elevated filling pressures)
- HF with preserved ejection fraction (HFpEF): LV EF ≥ 50% and evidence of HF (elevated cardiac biomarkers or elevated filling pressures)
- HF with improved ejection fraction: LV EF >40%, with previously documented LV EF ≤ 40%
Patients with HFpEF have traditionally been underdiagnosed but comprise between 44% and 72% of CHF cases. On echocardiogram (echo), LV EF ≥ 50% with evidence of impaired diastolic function. The most significant risk factor is hypertension (HTN), and other risk factors include older age, female sex, and diabetes. [2]
The ACC and the AHA together classify HF by stages, with the first 2 stages being asymptomatic and the second 2 being classified by severity of symptoms.
ACC/AHA Heart Failure Stages
- Stage A: At risk for HF. No symptoms, structural heart disease, or evidence of elevated cardiac biomarkers, but risk factors are present. Risk factors include hypertension, diabetes, metabolic syndrome, cardiotoxic medications, or having a genetic variant for cardiomyopathy.
- Stage B: Pre-HF. Patients have no signs or symptoms of HF but have structural heart disease, evidence of elevated filling pressures (by invasive or noninvasive assessment), or persistently elevated cardiomarkers in the absence of other reasons for elevated markers, like chronic kidney disease or myocarditis.
- Stage C: Patients with structural heart disease and current or past history of HF symptoms.
- Stage D: Patients with refractory symptoms that interfere with daily life or recurrent hospitalization despite targeted guideline-directed medical therapy.
The New York Heart Association Functional Classification is used for patients with symptoms of HF. This system is subjectively determined by clinicians and is widely used in clinical practice to direct therapy.
New York Heart Association Functional Classification
Based on symptoms, the patients can be classified using the New York Heart Association (NYHA) functional classification as follows: [3]
- Class I: Symptom onset with more than ordinary level of activity
- Class II: Symptom onset with an ordinary level of activity
- Class III: Symptom onset with minimal activity
- Class IIIa: No dyspnea at rest
- Class IIIb: Recent onset of dyspnea at rest
- Class IV: Symptoms at rest
There are many etiologies of CHF, and coronary artery disease (CAD) causing ischemic heart disease is the most common cause. Every attempt should be made to identify causative factors to help guide treatment strategies. The etiologies can be broadly classified as intrinsic heart disease and pathologies that are infiltrative, congenital, valvular, myocarditis-related, high-output failure, and secondary to systemic disease. [2] [4] These classifications have significant overlap. The 4 most common etiologies responsible for about two-thirds of CHF cases are ischemic heart disease, chronic obstructive pulmonary disease (COPD), hypertensive heart disease, and rheumatic heart disease. Higher-income countries have higher rates of ischemic heart disease and COPD; lower-income countries have higher rates of hypertensive heart disease, cardiomyopathy, rheumatic heart disease, and myocarditis.
Ischemic heart disease is by far the most common cause of CHF worldwide. Ischemia leads to a lack of blood flow to heart muscles, reducing the EF. Incidence is increasing in developing countries as they adopt a more Western diet and lifestyle, and improved medical care decreases the infectious burden in these countries (myocarditis is often infection-related.)
Valvular heart disease is another common intrinsic heart condition that can cause CHF. Rheumatic heart disease is the most common cause of valvular heart disease in children and young adults worldwide. It is caused by an immune response to group A Streptococcus and primarily causes mitral and aortic stenosis. [5] The most common overall cause of valvular disease is age-related degeneration, and the aortic valve is the most commonly affected valve. Women are more likely to experience mitral valve rheumatic heart disease or mitral valve prolapse, while men are more likely to suffer from aortic valve diseases such as regurgitation or stenosis. Endocarditis is also more common in men.
Hypertension causes CHF even in the absence of CAD or ischemic heart disease. High blood pressure causes mechanical stress by increased afterload and neurohormonal changes that increase ventricular mass. [2] HTN is also strongly associated with other comorbidities for CHF development, and aggressively treating hypertension is shown to lower the incidence of CHF. [2]
Cardiomyopathy is a heterogeneous group of diseases characterized by enlarged ventricles with impaired function not related to secondary causes such as ischemic heart disease, valvular heart disease, hypertension, or congenital heart disease. The most common types of cardiomyopathies are hypertrophic, dilated, restrictive, arrhythmogenic right ventricular, and left ventricular noncompaction. [6] In addition to CHF, cardiomyopathy can present as arrhythmia or sudden cardiac death, further compelling the identification of underlying disorders. Many of these conditions have a genetic basis, and a detailed family history of sudden cardiac death, especially in first-degree relatives older than 35 years, should be taken. There are over 50 identified genes contributing to the development of dilated cardiomyopathy alone. Genetic determinants have variable phenotypic expression, and many nongenetic factors also affect the clinical symptoms. Some of these factors include diabetes, toxic exposure, or pregnancy. Fabry disease is a rare glycogen storage disease that can cause CHF symptoms through a hypertrophic cardiomyopathy pattern. [2] [6]
Inflammatory cardiomyopathy is defined by myocarditis along with ventricular remodeling and cardiac dysfunction. The most common cause is viral infection. Other etiologies are bacterial, fungal, or protozoal infections; toxic substances or drugs; and immune-mediated diseases. Chagas disease is caused by Trypanosoma cruzi, which is endemic in Latin America and commonly causes myocarditis, cardiomyopathy, and CHF. Other viral causes of myocarditis and inflammatory cardiomyopathy include adenoviruses, enteroviruses, herpes virus 6, Epstein-Barr virus, and cytomegalovirus. Viruses can also activate autoimmune myocarditis, including HIV, hepatitis C virus, influenzas A and B, and coronaviruses (including COVID-19). When associated with CHF, these conditions tend to have a poor prognosis. [7]
Infiltrative cardiomyopathies cause a restrictive cardiomyopathy pattern (simar to the genetically determined restrictive cardiomyopathy variant), which is notable for normal ventricular systolic function, but with diastolic dysfunction and restrictive filling dynamics of the LV and RV. This is often associated with a high E/A ratio showing increased early filling and delayed late filling. [6] [8]
Cardiac amyloidosis results from misfolded protein deposits in the heart; this leads to cardiomyocyte separation, cellular toxicity, and tissue stiffness. Patients are preload dependent and are prone to symptomatic hypotension. Currently, tamifidis is the only medication known to prevent cardiac amyloidosis. It prevents, but does not reverse, amyloid deposition. Its high cost is also a limiting factor. [1] [9] [1]
Sarcoidosis is an acquired cardiomyopathy that presents with conduction defects and arrhythmias due to granuloma formation. The most common cardiac manifestation is CHF. Caution must be used when treating with beta-blockers due to the associated conduction abnormalities.
Cardiac hemochromatosis is present in 15% to 20% of patients with hereditary hemochromatosis. This condition initially presents with a restrictive pattern but develops into biventricular systolic dysfunction. [8] Patients with restrictive cardiomyopathy physiology can develop hypotension when treated with traditional CHF medications due to preload dependence, so caution should be used to avoid systemic hypoperfusion. [10]
Takotsubo or stress-induced cardiomyopathy (colloquially broken-heart syndrome) is an underrecognized cause of CHF, which causes transient left-ventricular wall abnormalities that are not localized to a specific vascular territory. It has several proposed pathophysiologic mechanisms, including coronary vasospasm, microcirculatory dysfunction, and increased activation of the sympathetic nervous system. This condition is treated with medications typical for CHF with the addition of antithrombotic medications in certain clinical situations with wall motion abnormalities. Recognized cases increased significantly during the COVID-19 epidemic. [11] [12] [13] [12]
Peripartum cardiomyopathy is a significant cause of maternal mortality. During pregnancy, cardiac output is increased by 20% to 30% due to increased heart rate and stroke volume. It presents with CHF due to LV systolic dysfunction during late pregnancy, postpartum, or up to several months after delivery. There is likely an underlying genetic component, and it is more common in women with advanced maternal age, Black race, and multifetal pregnancies. If wall motion abnormalities are present, anticoagulation is essential due to the hypercoagulable state caused by pregnancy. Recovery is variable by global region and inversely correlates with lowered EF. [14]
Obesity is a leading cause of CHF in patients younger than 40 years, according to the "Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity" (the CHARM study). The "obesity paradox" described elsewhere has significant study flaws and is derived from older data. It is thought that up to 10% of CHF cases are attributable to obesity alone. Patients with obesity are more likely to have HFpEF, possibly secondary to adipose-produced cytokines such as IL-1b, IL-8, and TNFα. Adipose tissue also degrades natriuretic peptides. [15] [16] [17]
Tachycardia and arrhythmia can induce a low-output CHF state. There is usually dilation of all cardiac chambers, and there is preservation or thinning of biventricular wall thickness. Electrophysiologic changes, including prologued duration and decreased amplitude of action potentials in the myocytes, accompany this. All of these factors induce the typical neurohormonal response causing CHF. With rate control, these changes are often reversible due to myocardial hibernation. [18]
Thyrotoxicosis is a rare cause of HF despite initiating a hyperdynamic circulatory state. This may be partially due to activation of the renin-angiotensin-aldosterone axis, causing sodium and water retention, as well as upregulation of erythropoietin-stimulating agent, both of which will cause increased blood volume. Sustained tachycardia with or without atrial fibrillation can also cause CHF. [19]
High-output cardiac failure can be associated with thiamine deficiency, which is a rare condition found primarily among patients who are elderly, homeless, or have alcohol abuse disorder. Thiamine deficiency causes decreased ATP production with an accumulation of adenosine, which causes systemic vasodilation. This leads to lowered systemic vascular resistance and increased cardiac output. This evolves to weakened myocardium and decreased EF. Diuretic use can also cause urinary thiamine loss, further compounding the situation. [20] [21] Other common causes of high-output cardiac failure are obesity, liver disease, and arteriovenous shunts. The causative physiologic changes are decreased afterload (ie, systemic vascular resistance) and increased metabolism. These can often present with preserved EF, pulmonary congestion, increased filling pressures, and elevated natriuretic peptides. [22] [23]
- Epidemiology
The global magnitude of the disease cannot be accurately assessed given the significant differences in geographical distribution, assessment methods, lack of imaging modalities, and non-adherence to the uniform staging and diagnosis of the disease. Approximately 1.2 million hospitalizations were due to CHF in 2017, with an increase in the percentage of patients with HFpEF compared to HFrEF. [1]
By some reports, the incidence rate has plateaued; however, the prevalence increases as more patients receive therapy. This has not translated to improved quality of life or a decrease in the number of hospitalizations for patients with CHF. According to the Global Health Data Exchange registry, the current worldwide prevalence of CHF is 64.34 million cases. This translates to 9.91 million years lost due to disability (YLDs) and 346.17 billion US dollars in healthcare expenditure. [24]
Age is a major determinant of HF. Regardless of the cause or the definition used to classify patients with HF, the prevalence of HF increases steeply with age. The Framingham Heart Study showed CHF prevalence to be 8 per 1000 males aged 50 to 59 years, with an increase to 66 per 1000 males aged 80 to 89. [25] The incidence of HF in men doubles with each 10-year age increase after the age of 65, whereas in women, for the same age cohort, the incidence triples. Men have higher rates of heart disease and CHF than women worldwide. [26] [2]
The global registry also notes a predilection for a race with a 25% higher prevalence of HF in Black patients than in White patients. HF is still the primary cause of hospitalization in the elderly population and accounts for 8.5% of cardiovascular-related deaths in the United States. [26]
International statistics regarding the epidemiology of HF are similar. The incidence increases dramatically with age, metabolic risk factors, and a sedentary lifestyle. Ischemic cardiomyopathy and hypertension are significant causes of HF in developing countries. [27] A notable difference based on a review of small cohort studies from these nations is a higher prevalence of isolated right HF. The theoretical cause of this is thought to be due to the higher prevalence of tuberculous, pericardial, and lung diseases. There is a lack of robust data to verify these claims.
- Pathophysiology
HF is a progressive disease. Any acute insult to cardiac structure or acute alteration secondary to genetic mutation, cardiac tissue infiltration, ischemia, valvular heart disease, myocarditis, or acute myocardial injury may initiate the compensatory mechanism, which, once exhausted, results in maladaptation.
In the initial stages of CHF, several compensatory mechanisms attempt to maintain cardiac output and meet the systemic demands. The chronic activation of the sympathetic nervous system results in reduced beta-receptor responsiveness and adrenaline stores. This results in changes in myocyte regeneration, myocardial hypertrophy, and myocardial hypercontractility. [28] The increased sympathetic drive also results in the activation of the renin-angiotensin-aldosterone system (RAAS) system, systemic vasoconstriction, and sodium retention. [28] [29]
A decrease in cardiac output and increased sympathetic drive stimulate the RAAS, leading to increased salt and water retention, along with increased vasoconstriction. This further fuels the maladaptive mechanisms in the heart and causes progressive HF. In addition, the RAAS system releases angiotensin II, which has been shown to increase myocardial cellular hypertrophy and interstitial fibrosis, contributing to myocardial remodeling. [3]
A decrease in cardiac output stimulates the neuroendocrine system with a release of epinephrine, norepinephrine, endothelin-1 (ET-1), and vasopressin. These mediators cause vasoconstriction, leading to increased afterload. There is an increase in cyclic adenosine monophosphate (cAMP), which causes an increase in cytosolic calcium in the myocytes. This increases myocardial contractility and further prevents myocardial relaxation. Increased afterload and myocardial contractility with impaired myocardial relaxation increase myocardial oxygen demand. This paradoxical need for increased cardiac output to meet myocardial demand eventually leads to myocardial cell death and apoptosis. As apoptosis continues, a decrease in cardiac output with increased demand leads to a perpetuating cycle of increased neurohumoral stimulation and maladaptive hemodynamic and myocardial responses. [29] The loss of myocytes decreases EF (cardiac contractility), which leads to incomplete LV emptying. Increased LV volume and pressure cause pulmonary congestion. [30]
Renal hypoperfusion causes the release of antidiuretic hormone (ADH), further potentiating sodium and water retention. Increased central venous and intraabdominal pressure causes reduced renal blood flow, further decreasing GFR. [31]
Decompensated CHF is characterized by peripheral vasoconstriction and increased preload delivery to the overburdened heart. The natriuretic peptides BNP and ANP are secreted but are ineffective in counteracting the excess sodium and water retention. [31]
Neprilysin is an enzyme that breaks down several hormones, including BNP, ANP, and bradykinin; it targets several novel therapeutics. It is always used with an angiotensin receptor blocker because it increases angiotensin II levels, and when administered with an ACE inhibitor, it causes significant angioedema. [32] [33]
Causes of CHF are split about equally between HFrEF and HFpEF but require different treatment plans. In HFpEF, there is a decrease in myocardial relaxation and an increase in the stiffness of the ventricle due to an increase in ventricular afterload. This perpetuates a similar maladaptive hemodynamic compensation and leads to progressive HF. Patients with HFpEF tend to be older, female, and hypertensive. Atrial fibrillation and anemia are also more likely co-occurring conditions. There is some evidence that the prognosis is worse than those with HFrEF. It is possible that appropriate targets have not been identified for optimal therapeutic interventions. [34] [35]
- History and Physical
The diagnosis and classification of HF are primarily based on the presence and severity of symptoms and physical exam findings. It is imperative to obtain a detailed history of symptoms, underlying medical conditions, and functional capacity to treat the patient adequately.
Acute CHF presents primarily with signs of congestion and may also present with organ hypoperfusion or cardiogenic shock. [36] The most commonly reported symptom is shortness of breath. This must be further classified as exertional, positional (orthopnea), and whether acute or chronic. Other commonly reported symptoms of CHF include chest pain, anorexia, and exertional fatigue. Anorexia is due to hepatic congestion, bowel edema, and reduced blood flow to splanchnic circulation. Some patients may present with a recumbent cough due to orthopnea. Patients may also experience abdominal discomfort due to hepatic congestion or ascites. Patients with arrhythmias can present with palpitations, presyncope, or syncope.
Another symptom that increases morbidity is edema, especially of the lower extremities. This can limit mobility and balance; total body water and weight increases of > 20 lbs are not uncommon.
While patients with acute HF present with overt respiratory distress, orthopnea, and paroxysmal nocturnal dyspnea, patients with chronic heart failure tend to curtail their physical activity; hence, symptoms may be obscured. It is essential to identify triggers of acute decompensation such as recent infection, noncompliance with cardiac medications, use of NSAIDs, or increased salt intake.
Physical Examination
The examination findings vary with the stage and acuity of the disease. Patients may have isolated symptoms of left-sided HF, right-sided HF, or combined.
General physical examination: The general appearance of patients with severe CHF or those with acutely decompensated HF includes anxiety, diaphoresis, tachycardia, and tachypnea. Patients with chronic decompensated HF can appear cachexic. On chest examination, the classical finding of pulmonary rales translates to heart failure of moderate-to-severe intensity. Wheezing may be present in acute decompensated heart failure. As the severity of pulmonary congestion increases, frothy and blood-tinged sputum may be seen. It is important to note that the absence of rales does not exclude pulmonary congestion. Jugular venous distention is another classical finding that must be assessed in all patients with HF. In patients with elevated left-sided filling pressures, hepatojugular reflux (sustained increase in JVP of >4 cm after applying pressure over the liver with the patient lying at a 45° angle) is often seen.
Patients with Stage D HF may show signs of poor perfusion, such as hypotension, reduced capillary refill, cold extremities, poor mentation, and reduced urine output. There may be pulsus alternans (an alternating weak and strong pulse), suggestive of severe ventricular dysfunction. The pulse can be irregular in the presence of atrial fibrillation or ectopic beats. Some degree of peripheral edema is present with most HF. [37] Weight gain is another method for assessing volume retention, and precise daily weights can be a useful monitoring tool.
Precordial findings in patients with HF include an S3 gallop, or displaced apex beat (dilated heart). There may be murmurs of associated valvular lesions such as the pansystolic murmur of mitral regurgitation or tricuspid regurgitation, systolic ejection murmur of aortic stenosis, or early diastolic murmur of aortic regurgitation. Patients with pulmonary hypertension may have palpable or loud P2 or parasternal heave. Patients with congenital heart disease may also have associated clubbing, cyanosis, and splitting of the second heart sound.
An S3 gallop is the most significant and early finding associated with HF. [38] Patients with hypertensive heart disease may have an S4 or loud A2. Patients with HF with preserved EF may have an S4 gallop related to ventricular noncompliance.
The commonly used Framingham Diagnostic Criteria for Heart Failure require the presence of 2 major criteria or 1 major and 2 minor criteria to make the diagnosis. This clinical diagnostic tool is highly sensitive for the diagnosis of HF but has a relatively low specificity. The Framingham Diagnostic criteria are as follows: [37]
Major Criteria
- Acute pulmonary edema
- Cardiomegaly
- Hepatojugular reflex
- Neck vein distention
- Paroxysmal nocturnal dyspnea or orthopnea
- Pulmonary rales
- Third heart sound (S3 Gallop)
Minor Criteria
- Ankle edema
- Dyspnea on exertion
- Hepatomegaly
- Nocturnal cough
- Pleural effusion
- Tachycardia (heart rate greater than 120 beats per minute)
A comprehensive assessment is required when evaluating a patient with HF. This includes a complete blood picture, iron profile, renal profile, and liver profile. After the basic metabolic and blood panel, patients require further investigations, depending on the etiology and clinical stage. [1]
A CBC may suggest anemia or leukocytosis suggestive of an infection triggering CHF.
A complete renal profile is necessary for all patients with HF. It indicates the degree of renal injury associated with HF and guides medication choice. It is essential to know baseline renal function before the patient is started on medications, including renin-angiotensin-aldosterone (RAAS) inhibitors, sodium-glucose transporter-2 (SGLT-2) inhibitors, or diuretics. Serum sodium level has prognostic value as a predictor of mortality in patients with chronic HF. "The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure" (OPTIME-CHF) trial demonstrated a significantly increased risk of in-hospital mortality as well as 30-day mortality in patients with HF who presented with hyponatremia. [39]
A liver profile is usually performed. Hepatic congestion secondary to HF may result in elevated gamma-glutamyl transferase levels, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). [40]
Urine studies can be useful in diagnosis. If amyloidosis is suspected, urine and serum electrophoresis and monoclonal light chain assays should be performed. If clinical suspicion is high despite negative testing for light chains, bone scintigraphy can be performed. [1]
Serum B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-ProBNP) levels can aid in differentiating cardiac from noncardiac causes of dyspnea in patients with ambiguous presentations. BNP is an independent predictor of increased left ventricular end-diastolic pressure, and it is used for assessing mortality risk in patients with HF. BNP levels correlate with NYHA classification, and the utility is primarily used as a marker to assess treatment efficacy. NT-ProBNP is the chemically inert N-terminal fragment of BNP and has a longer half-life. The ratio of NT-ProBNP/BNP varies depending on underlying comorbidities and may be a useful tool in the future. [41] In patients with a clear clinical presentation of HF, natriuretic peptides should not be used to drive treatment plans. It is important to remember that BNP and NT-ProBNP levels can be elevated in patients with renal dysfunction, atrial fibrillation, and older patients. Conversely, BNP levels can be falsely low in patients with obesity, hypothyroidism, and advanced HF (due to myocardial fibrosis).
Troponin-I or T suggests ongoing myocardial injury when persistently elevated and predicts adverse outcomes and mortality.
An electrocardiogram may show evidence of prior infarction, chamber enlargement, intraventricular conduction delay, or arrhythmia. It may also give clues to specific etiologies. A low voltage and pseudo infarction pattern of ECG is seen in cardiac amyloidosis. An epsilon wave is seen in ARVC. ECG also suggests the presence of ventricular desynchrony, with a QRS duration of more than 120 msec, predicting the patient's response to device therapy for HF.
Chest radiographs are used to assess the degree of pulmonary congestion and cardiac contour (to determine the presence of cardiomegaly). Findings indicative of CHF on chest radiographs include enlarged cardiac silhouette, edema at the lung bases, and vascular congestion. In florid HF, Kerley B lines may be seen on chest radiographs. The absence of these findings in patients with a suggestive clinical presentation does not rule out CHF. [37]
Echocardiography is the initial choice of modality in patients with suspected HF and is an easily available bedside tool. Echocardiography quantifies right and left ventricular function, denotes structural abnormalities in cardiac chambers and valves, and helps visualize the presence of focal wall motion abnormalities. However, in patients with severe obesity, pregnancy, or mechanical ventilation, it may be challenging to obtain adequate acoustic windows. Transesophageal echocardiography (TEE) is an alternative for these patients. Adequate rate control in patients with tachyarrhythmias is necessary to obtain adequate echocardiographic images. [37]
Cardiac catheterization is often required for diagnosing ischemic cardiomyopathy and can be useful for accurately evaluating intracardiac pressures such as left ventricular end-diastolic pressure or pulmonary artery pressures.
Computed tomography may be used for the assessment of coronary artery disease in a young patient with ventricular dysfunction (older patients are likely to have baseline calcifications). It may also be used in patients with congenital heart diseases causing HF. Cardiac CT may help with the detection of tumors causing HF. CT may also be used for the evaluation of stent patency and graft evaluation.
SPECT-Myocardial Perfusion Imaging helps define the presence of ischemia in patients with newly diagnosed left ventricular dysfunction and not undergoing coronary angiography. It is particularly useful for assessing CAD in patients with no history of ischemia but elevated troponin. ECG-gated myocardial perfusion imaging is used to evaluate LV EF, regional wall motion, and regional wall thickening. EF measurement with this study may be affected in patients with an irregular heart rate, low count density, and extracardiac radiotracer uptake. ECG-gated images are also useful in recognizing artifactual defects seen on SPECT imaging, such as breast tissue and diaphragmatic attenuation. [42]
Cardiac magnetic resonance imaging has evolved as an essential tool when a discrepancy exists between the clinical stage of the disease and echocardiographic findings. It helps with the precise evaluation of volume, chamber sizes, and ventricular function. It also assesses the stage of valvular heart disease in detail. Cardiac MRI also helps with the evaluation of complex congenital heart diseases. The tool can also be used for noninvasive assessment of conditions such as myocarditis, dilated cardiomyopathy, infiltrative cardiomyopathy, or arrhythmogenic right ventricular dysplasia. [43]
Radionuclide multiple-gated acquisition (MUGA) scan is a reliable imaging technique for evaluating EF and is used in patients when there is a disparity of EF measurements from other studies. [42]
Noninvasive stress imaging includes stress echocardiography, stress cardiac MRI, and SPECT imaging. These studies can be used to assess the benefit of coronary revascularization in patients with ischemic cardiomyopathy.
Genetic testing is indicated for identifying genetic variants causing cardiomyopathies, such as Titin, laminin A or C, myosin heavy chain, and cardiac troponin-T mutations. [44]
- Treatment / Management
The goal of therapy for chronic CHF is to improve symptoms and quality of life, decrease hospitalizations, and improve cardiac mortality. The goal of pharmacologic therapy is to control symptoms and to initiate and escalate drugs that reduce mortality and morbidity in HF. [1]
Management for the respective stages of HF is outlined by the American College of Cardiology and the American Heart Association. [1]
For Stage A (At-Risk for HF)
- In patients with hypertension, guideline-directed medical therapy (GDMT) should be used for the management of hypertension.
- In patients with type 2 diabetes, SGLT-2 inhibitors are indicated to reduce HF hospitalizations.
- Lifestyle modifications such as healthy eating, physical activity, maintaining a normal weight, and avoidance of smoking are indicated.
- The use of prognostication scores is recommended in patients with HF to estimate the risk of future HF events. [45] Examples include the Framingham Heart Failure Risk Score (1999), Health ABC Heart Failure Score (2008), ARIC Risk Score (2012), and PCP-HF score (2019).
- There should be optimal management of cardiovascular diseases in patients known to have coronary artery disease.
- Patients at risk for HF due to exposure to cardiotoxic medications (eg, chemotherapy) should be managed with a multidisciplinary approach.
- Natriuretic peptide screening and periodic evaluation are recommended.
For Stage B (Pre-HF)
Management of Stage B is focused on preventing clinical HF and reducing mortality and adverse cardiovascular events.
- For patients with LV EF ≤40%, ACEi should be used to prevent clinical HF and for mortality reduction.
- For patients with LV EF ≤ 40% and evidence of prior or recent acute coronary syndrome or myocardial infarction, the use of a statin and beta-blocker is recommended for reduction of mortality, CHF, and reducing adverse cardiovascular events.
- For patients with LV EF ≤ 30% and receiving optimal medical therapy, with NYHA-class I and an expectation of meaningful survival of more than 1 year, a primary prevention ICD is recommended.
- Beta-blockers are recommended for patients with LV EF ≤ 40%, irrespective of the etiology, to prevent symptomatic HF.
- For patients with LV EF ≤ 50%, the use of thiazolidinediones and non-dihydropyridine calcium channel blockers increases the risk of adverse outcomes and HF hospitalizations, so should be avoided.
- Valve repair, replacement, or interventions have associated guidelines for asymptomatic valvular heart disease.
- Patients with congenital heart disease also have associated guidelines.
For Stage C (HF)
- Multidisciplinary management is indicated for improving self-care and mortality of patients with HF.
- Patient education and social support are required for optimal management.
- Vaccination against respiratory illnesses is effective in reducing mortality.
- It is reasonable to screen patients for frailty, depression, low literacy, low social support, and resource and transport logistics during healthcare encounters.
- A low-sodium diet is recommended.
- Exercise training is effective in improving functional class and quality of life.
- For patients with congestion, diuretics improve symptoms and reduce HF progression.
- A thiazide diuretic (such as metolazone) should be added only to patients who do not respond well to a moderate or high dose of loop diuretics.
- For patients with HFrEF, an ARNi is recommended to reduce mortality and morbidity. ARNi should not be given to patients who are intolerant of ACEi, and an ARB should be substituted. For patients not able to take an ARNi due to economic factors, the use of an ACEi or ARB is indicated. ARNi should not be used within 36 hours of the last dose of ACEi. For patients tolerating ACEi/ARB well, switching to ARNi is recommended, with a high economic value. As with ACEi, ARNi should not be given to patients with a history of angioedema.
- For patients with HFrEF, the use of the beta-blockers carvedilol, bisoprolol, or sustained-release metoprolol is effective in reducing mortality and hospitalization.
- For patients with HFrEF, NYHA class II-IV, an eGFR of more than 30 mL/min/1.73 m2 and a serum potassium of less than 5.0 mEq/L, the use of MRA is recommended. For patients with a serum potassium of more than 5.0 mEq/L, the use of MRA is harmful.
- For patients with HFrEF, the use of SGLT-2 inhibitors is recommended to reduce mortality and HF hospitalization, irrespective of the diabetes status.
- For African American patients with HFrEF and NYHA class III-IV, who are already receiving optimal medical therapy (OMT), the addition of a combination of hydralazine and nitrate is recommended to reduce morbidity and mortality. This is of high economic value.
- For patients with HFrEF and intolerant to RAASi or in whom RAASi is contraindicated due to renal insufficiency, the use of a combination of hydralazine and nitrate might be effective.
- It is recommended to titrate medications aggressively to achieve desired outcomes. This can be done as frequently as 1-2 weeks as tolerated.
- Ivabradine can be useful in patients on OMT with and heart rate of more than 70 bpm, providing mortality benefits, and reducing HF hospitalization.
- Digoxin may be considered in symptomatic patients with sinus rhythm despite adequate goal-directed therapy to reduce the all-cause rate of hospitalizations, but its role is limited.
- In patients with HFrEF and recent HF, an oral soluble guanylate cyclase stimulator (Vericiguat) might be useful in reducing mortality and HF hospitalization. Vericiguat is a soluble guanylate cyclase stimulator that stimulates the intracellular receptor for endogenous NO, which is a potent vasodilator. It also improves cardiac contractility. [46] [47]
- An implantable cardioverter-defibrillator (ICD) is indicated for primary prevention of sudden cardiac death in patients with HF who have an LVEF of less than or equal to 35% and an NYHA functional class of II to III while on goal-directed medical therapy. It is also indicated if a patient has NYHA functional class I and an EF of less than or equal to 30% on adequate medical therapy.
- Cardiac resynchronization therapy (CRT) with biventricular pacing is recommended in patients with HFrEF and an NYHA functional class of II to III or ambulatory class IV with an LVEF less than or equal to 35%, QRS duration ≥ 150 msec, and sinus rhythm with left bundle branch block (LBBB) morphology. It can also be considered in non-LBBB morphology and QRS ≥ 150 msec.
- Revascularization is indicated in selected patients with coronary artery disease and HFrEF while on GDMT.
- Valvular heart disease interventions such as transcatheter edge-to-edge mitral valve repair or mitral valve surgery might be beneficial for patients with HF and on GDMT.
For Stage D (Advanced HF)
- Referral to an HF specialist is indicated.
- It is reasonable to utilize inotropic support and device therapy in patients awaiting mechanical cardiac support or transplant. Inotropic support alone can be used in patients not eligible for a transplant or mechanical cardiac support.
- Mechanical cardiac support such as a durable left ventricle assist device (LVAD) or ECMO can be beneficial as a bridge to transplant.
- For highly selected patients, cardiac transplant is indicated to improve survival and quality of life.
- Goals of care should be decided by shared decision-making. This includes considering comorbid conditions, frailty, and socio-economic support. Palliative care should be offered as indicated after shared decision-making.
- Differential Diagnosis
Diseases that may present with clinical features of volume overload or dyspnea are in the differential for HF. These include acute renal failure, acute respiratory distress syndrome, cirrhosis, pulmonary fibrosis, nephrotic syndrome, and pulmonary embolism.
According to the Centers for Disease Control and Prevention (CDC), in December 2015, the rate of HF-related deaths decreased from 103.1 deaths per 100,000 population in 2000 to 89.5 in 2009 but subsequently increased to 96.9 in 2014. The report noted that the trend correlates with a shift from coronary heart disease as the underlying cause of HF deaths to metabolic diseases and other noncardiac causes of HF, such as obesity, diabetes, malignancies, chronic pulmonary diseases, and renal disease. The mortality rate following hospitalization for HF is estimated at around 10% at 30 days, 22% at 1 year, and 42% at 5 years. This can increase to greater than 50% for patients with stage D HF. [48]
The Ottawa Heart Failure Risk Score is a useful tool for determining prognosis in patients presenting to the emergency department with HF. [49] This score is used to determine the 14-day mortality risk, hospital readmission, and acute coronary syndrome to help arrive at safe disposition planning. Patients with a score of 0 are considered low risk. A score of 1 to 2 is considered moderate risk, a score of 3-4 is considered high risk, and a score of 5 or higher is considered very high risk. The scoring criteria are as follows:
One point for each of the following:
- History of stroke or transient ischemic attack
- Oxygen saturation less than 90%
- Heart rate greater than 110 bpm on the 3-minute walk test
- Acute ischemic ECG changes
- An NT-ProBNP level of greater than 5000 ng/L
Two points for each of the following:
- Prior history of mechanical ventilation for respiratory distress
- Heart rate greater than 110 bpm on presentation
- Blood urea nitrogen (BUN) greater than 33.6 mg/dL (12 mmol/L)
- Serum bicarbonate greater level than 35 mg/d
- Complications
Complications of CHF include:
- Reduced quality of life
- Arrhythmia and sudden cardiac death
- Cardiac cachexia
- Cardiorenal disease
- Liver dysfunction
- Functional valvular insufficiencies (such as functional MR or TR)
- Mural thrombi and risk of thromboembolism (brain, kidney, lung, major limb vessels)
- Recurrent hospitalizations and nosocomial infection
- Consultations
The consultation type depends on the disease stage and the intended management strategy. Commonly consulted specialists include HF specialists, the cardiac transplant team for stage D CHF, cardiam imaging radiologists, cardiac rehabilitation, dieticians, and, if aligned with patient preference, palliative care (also for class D).
- Deterrence and Patient Education
Risk factor reduction and aggressive management of comorbid conditions are crucial to reducing morbidity and mortality associated with HF. In addition to compliance with medications, patients need guidance on self-monitoring of symptoms of HF and avoiding the triggers of HF. These strategies can help prevent the development of HF in patients at high risk for the disease and slow the progression in those who are already diagnosed with it. Patient education is necessary to facilitate self-care and compliance. Close supervision, including surveillance by the patient and family, home-based visits, telephone support, and remote monitoring, is recommended. Socio-economic support is pivotal in the appropriate management of the disease. [1] Patients require close clinical follow-up for assessing volume status, effects of drug therapy, and escalation of care as indicated.
- Enhancing Healthcare Team Outcomes
HF is a complex clinical syndrome with high morbidity and mortality. HF requires a multifaceted treatment approach, including patient education, pharmacologic management, and surgical interventions to optimize clinical outcomes. Specialty-trained HF nurses are an essential component of the multidisciplinary team in educating patients on the importance of lifestyle modifications and medical compliance to help improve morbidity and mortality. Educating patients on symptom assessment and weight management is essential to prevent HF exacerbations and hospital admissions. The HF-trained social worker and case manager can help evaluate the patient in community settings or in-home visits to help the patient adhere to the lifestyle modifications. Clinical pharmacists assist medical providers by reviewing patient medication lists and decreasing potential adverse drug-drug interactions. Primary care medical providers and cardiologists must coordinate care to minimize any adverse outcomes of medical therapy and prevent the progression of this disease. A collaborative interprofessional team can significantly improve the quality of life for patients with HF and decrease mortality.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Congestive Heart Failure Contributed by S Bhimji, MD
Disclosure: Ahmad Malik declares no relevant financial relationships with ineligible companies.
Disclosure: Daniel Brito declares no relevant financial relationships with ineligible companies.
Disclosure: Sarosh Vaqar declares no relevant financial relationships with ineligible companies.
Disclosure: Lovely Chhabra declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Malik A, Brito D, Vaqar S, et al. Congestive Heart Failure. [Updated 2023 Nov 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Heart Failure With Preserved Ejection Fraction (HFpEF). [StatPearls. 2024] Heart Failure With Preserved Ejection Fraction (HFpEF). Golla MSG, Shams P. StatPearls. 2024 Jan
- Heart Failure and Ejection Fraction. [StatPearls. 2024] Heart Failure and Ejection Fraction. Golla MSG, Hajouli S, Ludhwani D. StatPearls. 2024 Jan
- Biventricular pacing (cardiac resynchronization therapy): an evidence-based analysis. [Ont Health Technol Assess Ser....] Biventricular pacing (cardiac resynchronization therapy): an evidence-based analysis. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2005; 5(13):1-60. Epub 2005 Sep 1.
- Review Combination Use of Ivabradine with Sacubitril/Valsartan: A Review of Clinical Effectiveness and Guidelines [ 2020] Review Combination Use of Ivabradine with Sacubitril/Valsartan: A Review of Clinical Effectiveness and Guidelines Pohar R, MacDougall D. 2020 Feb 13
- Review Heart Failure With Preserved Ejection Fraction: A Review. [JAMA. 2023] Review Heart Failure With Preserved Ejection Fraction: A Review. Redfield MM, Borlaug BA. JAMA. 2023 Mar 14; 329(10):827-838.
Recent Activity
- Congestive Heart Failure - StatPearls Congestive Heart Failure - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Open access
- Published: 27 June 2024
Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review
- Hyunjoo Kim 1 , 2 ,
- Bomi Hong 3 ,
- Sanghee Kim 4 ,
- Seok-Min Kang 5 &
- Jeongok Park ORCID: orcid.org/0000-0003-4978-817X 4
Systematic Reviews volume 13 , Article number: 167 ( 2024 ) Cite this article
86 Accesses
Metrics details
Chemotherapy-related cardiotoxicity is a significant concern because it is a major cause of morbidity. This study aimed to provide in-depth information on the symptoms of chemotherapy-related cardiotoxicity (CRCT) by exploring literature that concurrently reports the types and symptoms of CRCT in patients with breast cancer.
A scoping review was performed according to an a priori protocol using the Joanna Briggs Institute’s guidelines. The participants were patients with breast cancer. The concept was the literature of specifically reported symptoms directly matched with CRCT and the literature, in English, from 2010, and the context was open. The search strategy included four keywords: “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” All types of research designs were included; however, studies involving patients with other cancer types, animal subjects, and symptoms not directly related to CRCT were excluded. Data were extracted and presented including tables and figures.
A total of 29 articles were included in the study, consisting of 23 case reports, 4 retrospective studies, and 2 prospective studies. There were no restrictions on the participants’ sex; however, all of them were women, except for one case report. The most used chemotherapy regimens were trastuzumab, capecitabine, and doxorubicin or epirubicin. The primary CRCT identified were myocardial dysfunction and heart failure, followed by coronary artery disease, pulmonary hypertension, and other conditions. Major tests used to diagnose CRCT include echocardiography, electrocardiography, serum cardiac enzymes, coronary angiography, computed tomography, and magnetic resonance imaging. In all case reports, CRCT was diagnosed through an incidental checkup according to the patient’s symptom presentation; however, only 10 of these studies showed a baseline checkup before chemotherapy. The five most common CRCT symptoms were dyspnea, chest pain, peripheral edema, fatigue, and palpitations, which were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Dyspnea with trastuzumab treatment and chest pain with capecitabine treatment were particularly characteristic. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days.
Conclusions
This scoping review allowed data mapping according to the study design and chemotherapy regimens. Cardiac assessments for CRCT diagnosis were performed according to the patient’s symptoms. There were approximately five types of typical CRCT symptoms, and the timing of symptom occurrence varied. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Peer Review reports
Breast cancer is currently the most common cancer worldwide. Its incidence and mortality rates in East Asia in 2020 accounted for 24% and 20% of the global rates, respectively, and these rates are expected to continue increasing until 2040 [ 1 ]. In the USA, since the mid-2000s, the incidence rate of breast cancer has been increasing by 0.5% annually, while the mortality rate has been decreasing by 1% per year from 2011 to 2020 [ 2 ]. Despite the improved long-term survival rate in patients with breast cancer due to the development of chemotherapy, the literature has highlighted that cardiotoxicity, a cardiac problem caused by chemotherapy, could be a significant cause of death among these patients [ 3 ]. Chemotherapy-related cardiotoxicity (CRCT) can interfere with cancer treatment and progress to congestive heart failure during or after chemotherapy [ 4 ], potentially lowering the survival rate and quality of life of patients with cancer [ 5 ].
The term cardiotoxicity was first used in the 1970s to describe cardiac complications resulting from chemotherapy regimens, such as anthracyclines and 5-fluorouracil. The early definition of cardiotoxicity centered around heart failure, but the current definition is broad and still imprecise [ 6 ]. The 2022 guidelines on cardio-oncology from the European Society of Cardiology (ESC) define cardiotoxicity as including cardiac dysfunction, myocarditis, vascular toxicity, arterial hypertension, and cardiac arrhythmias. Some of these definitions reflect the symptoms. For example, cardiac dysfunction, which accounts for 48% of cardiotoxicity in patients with cancer, is divided into asymptomatic and symptomatic cardiac dysfunction. Asymptomatic cardiac dysfunction is defined based on left ventricular ejection fraction (LVEF), myocardial global longitudinal strain, and cardiac biomarkers. Symptomatic cardiac dysfunction indicates heart failure and presents with ankle swelling, breathlessness, and fatigue [ 7 ]. The ESC guidelines for heart failure present more than 20 types of symptoms [ 8 ]; however, to the best of our knowledge, few studies have been conducted to determine which heart failure symptoms and their characteristics are associated with CRCT in patients with breast cancer. Similarly, there is a lack of information related to vascular toxicity such as myocardial infarction [ 7 ].
Professional societies in cardiology and oncology have proposed guidelines for the prevention and management of cardiotoxicity in patients with cancer. According to the American Society of Clinical Oncology and the ESC, it is recommended to identify high-risk patients, comprehensively evaluate clinical signs and symptoms associated with CRCT, and conduct cardiac evaluations before, during, and after chemotherapy [ 7 , 9 , 10 ]. In addition, guidelines for patients with cancer, including those for breast cancer survivorship care, emphasize that patients should be aware of the potential risk of CRCT and report symptoms, such as fatigue or shortness of breath to their healthcare providers [ 7 , 11 , 12 ]. Although these guidelines encompass cardiac monitoring as well as symptom observation, many studies have focused solely on objective diagnostic tests, such as echocardiography, cardiac magnetic resonance, and cardiac biomarkers [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ], which means that there is little interest in CRCT symptoms in patients under breast cancer care.
This lack of interest in CRCT symptoms may be related to the absence of a specific symptom assessment tool for CRCT. Symptom monitoring of CRCT in patients with breast cancer was conducted through patient interviews and reported using the appropriate terminology [ 23 ]. In terms of interviews, patients with cancer experienced the burden of expressing symptoms between cardiovascular problems and cancer treatment. Qualitative research on patients with cancer indicates that these patients experience a daily battle to distinguish the symptoms they experience during chemotherapy [ 24 ]. To reduce the burden of identifying CRCT symptoms, it is crucial to educate patients with breast cancer undergoing chemotherapy about these symptoms. To report cardiotoxicity, healthcare providers in oncology can use a dictionary of terms called the Common Terminology Criteria for Adverse Events (CTCAE) for reporting adverse events in patients with cancer [ 25 ]. Patients can also use Patient-Reported Outcome (PRO), which allows unfiltered reporting of symptoms directly to the clinical database [ 26 ]. PRO consists of 78 symptomatic adverse events out of approximately 1,000 types of CTCAE [ 27 ]. Basch et al. suggested that PRO could enable healthcare providers to identify patient symptoms before they worsen, thereby improving the overall survival rate of patients with metastatic cancer [ 28 ]. This finding implies that symptoms can provide valuable clues for enhancing the timeliness and accuracy of clinical assessments of CRCT [ 29 ]. Therefore, it is necessary to explore the scope of research focusing on CRCT symptoms for prevention and early detection of CRCT in patients with breast cancer. The detailed research questions are as follows:
What are the general characteristics of the studies related to CRCT in patients with breast cancer?
What diagnostic tools and monitoring practices are used to detect CRCT?
What are the characteristics and progression of symptoms associated with CRCT?
A scoping review is a research method for synthesizing evidence that involves mapping the scope of evidence on a particular topic [ 30 ]. It aims to clarify key concepts and definitions, identify key characteristics of factors related to a concept, and highlight gaps or areas for further research [ 30 ]. This study used a scoping review methodology based on the Joanna Briggs Institute (JBI) framework. The JBI methodology, refined from the framework initially developed by Arksey and O’Malley [ 31 ], involves developing a research question, establishing detailed inclusion and exclusion criteria, and selecting and analyzing literature accordingly [ 32 ]. In contrast to systematic reviews, scoping reviews can encompass a variety of study designs and are particularly suitable when the topic has not been extensively studied [ 33 ]; hence, the decision was made to conduct a scoping review.
Development of a scoping review protocol
To conduct this review, an a priori scoping review protocol was developed to enhance transparency and increase the usefulness and reliability of the results. The protocol included the title, objective, review questions, introduction, eligibility criteria, participants, concept, context, types of evidence source, methods, search strategy, source of evidence selection, data extraction, data analysis and presentation, and deviation from the protocol [ 34 ] (Supplementary File 1).
Eligibility criteria
A participant-concept-context (PCC) framework was constructed based on the following research criteria. The participants were patients with breast cancer. The concept was that studies that specifically reported symptoms directly matched to CRCT in patients with breast cancer and the literature, published in English since 2010, in line with the year the CRCT guidelines were announced by the Cardio-Oncology Society. The context was open. We included all types of research designs. The exclusion criteria were studies that included patients with other types of cancer, involved animal subjects, and reported symptoms not directly related to CRCT.
Search strategy
The keywords consisted of “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” The keywords for “cardiotoxicity” were constructed according to the clinical cardiotoxicity report and ESC guidelines [ 7 , 35 ]. The keywords for “symptoms” included 40 specific symptoms of arrhythmia, heart failure, and cardiac problems [ 36 , 37 ] (Supplementary Table 1). We used PubMed, Embase, and CINAHL.
Source of evidence selection
Duplicate studies were removed using EndNote 21. The titles and abstracts were then reviewed according to the inclusion criteria, the primary literature was selected, and the final literature was selected through a full-text review. Any disagreements were resolved through discussions between the investigators.
Data extraction
The data from the literature included the general characteristics of the study, as well as information on the patients, chemotherapy, cardiotoxicity, and symptoms. The general characteristics of the study included author, publication year, country of origin, study design; patient information including sample size, sex, age, cancer type, and cancer stage; chemotherapy information including chemotherapy regimen; cardiotoxicity information including type of cardiotoxicity, diagnostic tests, and times of assessment; and symptom information including type of symptom, characteristics of symptom worsening or improvement, onset time, progression time, and time to symptom improvement. Information on whether to receive chemotherapy after the diagnosis of cardiotoxicity was explored.
Data analysis and presentation
The contents of the included studies were divided into three categories: (1) general characteristics, which encompassed study designs, patients, and medications; (2) type of CRCT and cardiac assessment for CRCT; and (3) characteristics and progression of the symptoms associated with CRCT. CRCT symptom-related data are presented in tables and figures.
In total, 487 studies were identified through database searches, and 116 duplicates were subsequently removed. After reviewing the titles and abstracts, we excluded 197 studies in which participants had cancers other than breast cancer, no symptoms, or symptom-related expressions. Of the remaining 174 studies, 146 were excluded after full-text review. Among the excluded studies, 79 were mainly clinical trials that the symptoms were not directly related to CRCT, 62 did not report specific symptoms, four were in the wrong population, and one was unavailable for full-text review. An additional study was included after a review of references, bringing the final count to 29 studies included in the analysis (Fig. 1 ).

Preferred reporting items for systematic reviews flowchart
General characteristics of studies including designs, sex and age, chemotherapy regimen, and CRCT criteria
Table 1 presents the general characteristics of the studies included in this review. The majority of these studies were published in the USA ( n =14), with Japan ( n =3), and Romania ( n =2) following. The study designs primarily consisted of case reports ( n =23), retrospective studies ( n =4), and prospective studies ( n =2).
All case reports involved female patients, except for one involving a male patient. Five quantitative studies did not specify or limit the sex of the participants, and one retrospective study included only female patients. In terms of cancer stage, the majority of studies involved patients with advanced breast cancer ( n =13), while a smaller number involved patients with early-stage breast cancer ( n =4). Twelve studies did not specify the cancer stage. Approximately 20 types of chemotherapy regimens are currently in use. Trastuzumab, which is a human epidermal growth factor receptor 2 (HER2) blocker, was mentioned in the majority of studies ( n =8), followed by capecitabine (an antimetabolite) ( n =7), and doxorubicin or epirubicin (anthracycline-based chemotherapy) ( n =6). Current chemotherapy and previous treatment methods were described together, with the exception of eight studies. Six quantitative studies defined the CRCT criteria, five of which were based on decreased LVEF and one of which was based on significant cardiac symptoms and/or electrocardiogram changes. Twenty-three case reports described the cardiovascular diagnosis as CRCT.
Diagnostic tools and monitoring practice for CRCT
Table 2 displays the types of CRCT, diagnostic tools, and times of cardiac assessment according to chemotherapy regimens. The most prevalent CRCT were myocardial dysfunction and heart failure, identified in 12 case studies, respectively. This was followed by coronary artery disease, represented in 8 case studies, pulmonary hypertension in 2 case studies, and a single case study of periaortitis. The most used test for diagnosing CRCT was echocardiography ( n =22), followed by EKG ( n =20), various types of cardiac enzymes ( n =16), coronary angiography (CAG, n =12), computed tomography ( n =6), and magnetic resonance imaging (MRI, n =4). Regarding the CRCT symptom assessment tools, the CTCAE was used in two studies, the New York Heart Association classification for heart failure in two studies, the dyspnea assessment scale in one study, and symptoms of cardiac origin, which consisted of chest pain, dyspnea, and palpitations in one study.
Regarding the times of cardiac evaluation, two studies performed regular cardiac checkups including before, during, and after chemotherapy. There were 10 case studies and six quantitative studies describing cardiac function testing before chemotherapy, of which seven studies performed regular cardiac screening tests and two studies mentioned cardiac screening even after the completion of chemotherapy. The frequency of regular checkups varied from every 3 months to every two to four cycles. In all case reports ( n =23), CRCT were diagnosed through incidental checkups based on patients’ symptom presentation, and in most cases, several tests were performed subsequentially for CRCT diagnosis. In one case study, cardiac evaluation was conducted 3 days after the patient’s initial symptom presentation, when the symptoms became more severe.
Characteristics and progression of symptoms associated with CRCT
Table 3 shows the descriptive scope of the CRCT-related symptoms according to the chemotherapy regimens used in the included studies. The mapping factors included initial symptoms, symptom onset or severity, symptom progression, medical management, and CRCT results. One of the most frequent symptoms associated with CRCT was dyspnea, which was discussed in 19 studies and described as difficulty in breathing, shortness of breath, or New York Heart Association (NYHA) class II or III. When dyspnea appeared as the initial symptom of CRCT, the symptom progression was worsening in eight case studies and persistent in two cases. Chest pain was described in 12 studies as a symptom characterized by a squeezing, tingling, burning, tightened, or atypical feeling that was relieved by rest and exacerbated by exertion. Other symptoms included peripheral edema ( n =6), fatigue ( n =5), and palpitation ( n =2). The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool.
The symptoms could be categorized based on the type of chemotherapy regimens used. In the case studies involving anthracycline-based regimen and HER2 blockers, dyspnea was the most frequently observed symptom ( n =7), followed by peripheral edema ( n =2), and chest pain or discomfort ( n =2). In case studies where antimetabolites were used, specifically capecitabine, chest pain was a common and prominent symptom. This chest pain typically manifested between 1 and 7 days after drug administration and persisted until treatment. Notably, four out of seven patients reported this symptom on the first day of chemotherapy, according to the case reports. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days. Figure 2 shows the progression of symptoms in case studies, detailing the time of symptom onset, the date of symptom reporting, and the date of treatment completion following the use of chemotherapy. The studies that did not specify any of the dates of symptom onset, reporting, and completion of treatment were excluded from the figure.

Figure 3 shows symptoms according to the main types of chemotherapy regimens reported in case studies. Dyspnea with trastuzumab and chest pain with capecitabine are particularly characteristic. A retrospective study included in this scoping review reported that chest pain was the most common symptom associated with capecitabine, followed by dyspnea and palpitation [ 40 ]. Furthermore, peripheral edema was primarily observed with anthracycline, alkylating, and HER2 blockers, while fatigue was noted with various anticancer drugs, irrespective of the type of chemotherapy regimen.

Ongoing chemotherapy was discontinued after CRCT was detected in 20 case studies. When patients presented symptoms indicative of CRCT, the majority were promptly hospitalized for further evaluation, medication, or interventional treatment. The majority of studies indicated the initiation of cardiac medication ( n =21), with three case studies involving coronary intervention and two involving treatment with wearable devices. Most management procedures were conducted in a general ward or an intensive care unit.
In most case studies, symptoms improved following cardiac treatment, with either complete or partial recovery of LVEF observed in 19 instances. However, a few studies reported a poor prognosis, including two instances of death. LVEF recovered in most patients within 6 months when treated with an anthracycline-based regimen and HER2 blockers (Fig. 2 ). A retrospective study reported that the rates of complete or partial recovery of CRCT following treatment with doxorubicin-based chemotherapy and trastuzumab were 42.9% and 86.1%, respectively [ 39 ]. Another retrospective study noted that the recovery time of CRCT when treated with HER2 blockers increased in correlation with the severity of the NYHA class, ranging from 8 to 80 weeks [ 38 ]. In the case of the antimetabolite capecitabine, all patients recovered within a day to a week, except one patient who did not recover.
This scoping review was conducted to explore the scope of studies focusing on CRCT symptoms, including the general characteristics of the studies, diagnostic tools, monitoring practices related to detecting CRCT, and the characteristics and progression of symptoms associated with CRCT. The primary findings of this review were as follows: (1) common symptoms related to CRCT and differences in symptoms according to the chemotherapy regimens used were identified; (2) the symptoms reported by the patient served as clues to suspect a specific type of CRCT; and (3) regular monitoring practices for CRCT prevention and detection were insufficient.
First, the current study identified common symptoms such as dyspnea, chest pain, peripheral edema, fatigue, and palpitation associated with CRCT, as well as variations in symptoms depending on the chemotherapy regimen used in patients with breast cancer. Among these symptoms, dyspnea, edema, and chest pain were frequently observed in patients receiving anthracycline-based and/or HER2 blocker drugs. These symptoms, which are associated with heart failure, appeared later compared to those observed with capecitabine, as depicted in Fig. 2 . This may be due to the known impact of anthracycline-based and/or HER2 blocker regimens on cardiomyocytes and other cells, leading to myocardial damage [ 42 ]. Therefore, the symptoms are related to heart failure, potentially resulting from the impairment of ventricular filling or ejection in patients undergoing treatment with these regimens [ 43 ].
In a similar vein, Attin et al. (2022) documented the occurrence of symptoms such as lower extremity edema, chest pain, difficulty breathing, and fatigue before the diagnosis of CRCT in women undergoing breast cancer treatment. They conducted a retrospective and longitudinal investigation of the symptoms, signs, and cardiac tests of 15 patients who experienced CRCT, using their electronic medical records. In their study, cardiotoxicity was defined by an echocardiogram or MRI showing a decrease in LVEF of 5 to 10%, with a specialist’s confirmation note. They compared the number of symptom occurrences during the first half of the year with those during the second half of the year prior to the diagnosis of cardiotoxicity. Specifically, the frequency of lower-extremity edema significantly increased from three occurrences in the first half of the year to 17 occurrences in the second half of the year. The frequency of symptoms for dyspnea and chest pain also increased from 10 and 8 times, respectively, to 16 and 14 times in the second half of the year. While there was limited information on the doses or timing of chemotherapy, 87% of the patients received the same chemotherapy regimens, namely anthracyclines and/or HER2 blockers [ 44 ]. This suggests that the increase in symptom occurrence over time may be related to the accumulation of anthracycline and the duration of anti-HER2 therapy [ 45 ].
Salyer et al. (2019) conducted a study on the prevalent symptoms of heart failure and their clustering. They identified three symptom clusters: sickness behavior, gastrointestinal disturbance, and discomfort of illness. Notably, dyspnea, edema, and pain were grouped into the discomfort of illness cluster, which aligns with the symptoms we observed in patients treated with anthracyclines and/or HER2 blockers [ 46 ]. Therefore, it is crucial for patients undergoing treatment with anthracyclines and/or HER2 blockers to be vigilant for symptoms such as dyspnea, edema, or chest pain, as these are indicative of heart failure.
Chest pain caused by vasospasm was a predominant symptom in patients taking antimetabolite regimens such as oral capecitabine, and it manifested as the following types of cardiotoxicities: vasospasm-related arrhythmia, myocardial disease, and ischemia [ 47 ]. Vasospasm can be triggered by endothelial dysfunction, hypersensitive vascular smooth muscle, reactive oxidative stress, or chemotherapy regimens [ 48 , 49 ]. According to previous studies, in patients using antimetabolite drugs such as 5-fluorouracil or capecitabine, chest pain was usually reported to occur from several hours to 72 hours after the first administration [ 47 , 50 , 51 , 52 , 53 ]. To detect chemotherapy-related coronary vasospasm in the early stage, it is recommended to carefully monitor typical or atypical symptoms of chest pain and EKG monitoring during drug infusion [ 54 ]. Muco et al. (2022) reported severe outcomes resulting from delayed management of vasospastic angina symptoms. The patient’s cardiac evaluation was performed 3 days after the onset of symptoms, and unfortunately, she did not recover from brain damage caused by coronary vasospastic sequelae. The authors stressed the importance of medical teams recognizing the symptoms of CRCT through vigilant monitoring and patient education [ 55 ].
As seen in the symptoms of CRCT caused by heart failure and vasospasm, careful observation of symptoms and conducting appropriate tests are crucial to prevent cardiotoxicity and minimize damage. These characteristics of CRCT and the associated symptoms related to chemotherapy regimens can provide crucial educational content for healthcare providers and patients preparing for chemotherapy. In addition, CRCT and symptom progression according to chemotherapy regimens could be used to formulate research questions for future systematic reviews.
Second, the preventive management of CRCT necessitates adherence to recommended guidelines. The 2022 ESC guidelines on cardio-oncology have updated the classification of CRCT and the monitoring protocols based on the chemotherapy regimens used [ 7 ]. The CRCT identified in the current study aligns with the drug toxicity outlined in the 2022 ESC guidelines. These guidelines advocate for regular cardiac monitoring before, during, and after chemotherapy to prevent and manage CRCT induced by anthracycline and HER2 blockers [ 7 , 12 ]. In this scoping review, two of 23 records described cardiac monitoring before, during, and after chemotherapy. An Australian multicenter study revealed that 59% of patients were referred to a cardiologist before CRCT occurred, but only 15% of patients diagnosed with CRCT had consulted a cardiologist before chemotherapy [ 41 ]. Given the declining mortality rates among cancer patients, managing CRCT requires a collaborative approach between oncology and cardiology to minimize mortality and morbidity in patients with breast cancer undergoing chemotherapy [ 7 ]. Therefore, it remains crucial to emphasize adherence to cardiac monitoring guidelines and foster cooperation between oncology and cardiology.
Additionally, symptom assessment is important for the early detection of patients with CRCT. The studies included in the current scoping review assessed whether patients’ symptoms could detect CRCT using interviews with patients, the New York Heart Association classification, a dyspnea assessment scale, and CTCAE tools. The United States National Cancer Institute recommends that healthcare providers use CTCAE and patients with cancer use PRO to report adverse events, including symptoms. CTCAE is a broad and comprehensive terminology that encompasses adverse events related to cancer treatment, has been used since the 1980s [ 25 ], and is not specialized in cardiotoxicity. Additionally, a discrepancy between CTCAE and PRO discovered that healthcare providers often underestimate both the incidence and duration of symptoms compared to the patients [ 56 , 57 , 58 ]. Specifically, healthcare providers tend to report symptom severity as lower than that reported by patients. For instance, there are notable discrepancies between healthcare providers and patients when reporting severe or very severe symptoms of fatigue, dyspnea, and limb edema in patients with early-stage breast cancer undergoing chemotherapy. The reported rates were 8% and 22% for fatigue, 0% and 4% for dyspnea, and 0% and 5% for limb edema, from healthcare providers and patients, respectively. Therefore, it is necessary to develop a user-friendly questionnaire to assess the various symptoms of CRCT.
Finally, we found that once CRCT was confirmed, cardiac treatment was promptly initiated and chemotherapy was frequently halted until CRCT resolution. A Delphi study on the use of anthracycline and trastuzumab proposed altering the treatment schedule or discontinuing treatment until there was an improvement in LVEF [ 59 ]. However, the professional societies did not provide definitive recommendations regarding continuing or ceasing ongoing chemotherapy. Instead, they suggested that the decision to continue or discontinue ongoing chemotherapy should be made based on the patient’s potential risks and benefits [ 60 ]. For example, Polk et al. (2016) reported that out of 22 patients with CRCT resulting from capecitabine, six continued medications with or without cardiac treatment; some of these patients experienced the same symptoms, while others did not exhibit significant symptoms [ 40 ]. Further research is required to explore the continuation or discontinuation of chemotherapy when CRCT is confirmed.
This study has some limitations. First, although we did not restrict the patients’ sex when reviewing the literature, most patients, except for one, were female. This may be related to the lower incidence of breast cancer in men. Second, although this scoping review mapped CRCT symptoms according to chemotherapy regimens, including anthracycline-based drugs, HER2 blockers, and antimetabolites, it did not cover cardiotoxicity related to other types of chemotherapy regimens. Thus, exploring the symptoms by focusing on expanded chemotherapy regimens and cardiovascular toxic diseases will assist in overcoming this limitation. Third, of the 29 studies, 23 were case reports with some grey literature, which may be justified by the nature of scoping reviews that allow for inclusion irrespective of the data source [ 61 ] and the study type. Experimental or observational clinical studies use objective criteria, such as diagnostic tests to generate primary evidence. However, case reports have led to new medical discoveries regarding the prevention and treatment of diseases [ 62 ]. Given the nature of case reports, specific symptoms that could provide clues for evaluating CRCT in patients with breast cancer are most often found in these reports. We incorporated grey literature to gather more comprehensive information on CRCT-related symptoms. However, to mitigate the potential issue of unverified quality in grey literature, we initially organized 16 studies from peer-reviewed literature and subsequently incorporated the grey literature into our findings. This approach helped to clarify the results of the peer-reviewed literature, particularly the types of chemotherapy regimens [ 63 ]. Finally, regarding the literature selection criteria, we examined articles written in English and published since 2010, the year the cardio-oncology guidelines were announced, thereby excluding articles published before 2010.
This scoping review allowed data mapping according to the study design and chemotherapy regimens. The key messages included a type of CRCT, cardiac assessment, and in-depth information regarding the CRCT symptoms. There were approximately five typical CRCT symptoms, including dyspnea, chest pain, peripheral edema, fatigue, and palpitations, and the timing of symptom occurrence varied. The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: global statistics for 2020 and 2040. The Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Article PubMed Google Scholar
Agha A, Wang X, Wang M, Lehrer EJ, Horn SR, Rosenberg JC, Trifiletti DM, Diaz R, Louie AV, Zaorsky NG. Long-term risk of death from heart disease among breast cancer patients. Front Cardiovasc Med. 2022;9: 784409.
Oikawa M, Ishida T, Takeishi Y. Cancer therapeutics-related cardiovascular dysfunction: Basic mechanisms and clinical manifestation. J Cardiol. 2023;81(3):253–9.
Piepoli MF, Adamo M, Barison A, Bestetti RB, Biegus J, Böhm M, Butler J, Carapetis J, Ceconi C, Chioncel O, et al. Preventing heart failure: a position paper of the Heart Failure Association in collaboration with the European Association of Preventive Cardiology. Eur J Heart Fail. 2022;24(1):143–68.
Chung R, Ghosh AK, Banerjee A: Cardiotoxicity: precision medicine with imprecise definitions. In., vol. 5: Archives of Disease in childhood; 2018: e000774.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). European Heart Journal - Cardiovascular Imaging. 2022;23(10):e333–465.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O et al: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022, 24(1):4-131.
Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2017;13(4):270–5.
Lanza O, Ferrera A, Reale S, Solfanelli G, Petrungaro M, Tini Melato G, Volpe M, Battistoni A: New insights on the toxicity on heart and vessels of breast cancer therapies. Med Sci (Basel) 2022, 10(2).
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43–73.
Lee GA, Aktaa S, Baker E, Gale CP, Yaseen IF, Gulati G, Asteggiano R, Szmit S, Cohen-Solal A, Abdin A, et al. European Society of Cardiology quality indicators for the prevention and management of cancer therapy-related cardiovascular toxicity in cancer treatment. Eur Heart J Qual Care Clin Outcomes. 2022;9(1):1–7.
Alexandraki A, Papageorgiou E, Zacharia M, Keramida K, Papakonstantinou A, Cipolla CM, Tsekoura D, Naka K, Mazzocco K, Mauri D et al: New insights in the era of clinical biomarkers as potential predictors of systemic therapy-induced cardiotoxicity in women with breast cancer: a systematic review. Cancers (Basel) 2023, 15(13).
Di Lisi D, Manno G, Madaudo C, Filorizzo C, Intravaia RCM, Galassi AR, Incorvaia L, Russo A, Novo G: Chemotherapy-related cardiac dysfunction: the usefulness of myocardial work indices. Int J Cardiovasc Imaging 2023.
Kar J, Cohen MV, McQuiston SA, Malozzi CM. Can global longitudinal strain (GLS) with magnetic resonance prognosticate early cancer therapy-related cardiac dysfunction (CTRCD) in breast cancer patients, a prospective study? Magn Reson Imaging. 2023;97:68–81.
Article CAS PubMed Google Scholar
Lim A, Jang H, Jeon M, Fadol AP, Kim S. Cancer treatment-related cardiac dysfunction in breast cancer survivors: a retrospective descriptive study using electronic health records from a Korean tertiary hospital. Eur J Oncol Nurs. 2022;59: 102163.
Liu W, Li W, Li H, Li Z, Zhao P, Guo Z, Liu C, Sun L, Wang Z. Two-dimensional speckle tracking echocardiography help identify breast cancer therapeutics-related cardiac dysfunction. BMC Cardiovasc Disord. 2022;22(1):548.
Article CAS PubMed PubMed Central Google Scholar
Mauro C, Capone V, Cocchia R, Cademartiri F, Riccardi F, Arcopinto M, Alshahid M, Anwar K, Carafa M, Carbone A et al: Cardiovascular side effects of anthracyclines and HER2 inhibitors among patients with breast cancer: a multidisciplinary stepwise approach for prevention, early detection, and treatment. J Clin Med 2023, 12(6).
Okushi Y, Saijo Y, Yamada H, Toba H, Zheng R, Seno H, Takahashi T, Ise T, Yamaguchi K, Yagi S et al: Effectiveness of surveillance by echocardiography for cancer therapeutics-related cardiac dysfunction of patients with breast cancer. J Cardiol 2023.
Ositelu K, Trevino A, Tong A, Chen MH, Akhter N: Challenges in cardiovascular imaging in women with breast cancer. Curr Cardiol Rep 2023.
Terui Y, Sugimura K, Ota H, Tada H, Nochioka K, Sato H, Katsuta Y, Fujiwara J, Harada-Shoji N, Sato-Tadano A, et al. Usefulness of cardiac magnetic resonance for early detection of cancer therapeutics-related cardiac dysfunction in breast cancer patients. Int J Cardiol. 2023;371:472–9.
Thavendiranathan P, Shalmon T, Fan CS, Houbois C, Amir E, Thevakumaran Y, Somerset E, Malowany JM, Urzua-Fresno C, Yip P, et al. Comprehensive cardiovascular magnetic resonance tissue characterization and cardiotoxicity in women with breast cancer. JAMA Cardiol. 2023;8(6):524–34.
Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–7.
White J, Byles J, Williams T, Untaru R, Ngo DTM, Sverdlov AL. Early access to a cardio-oncology clinic in an Australian context: a qualitative exploration of patient experiences. Cardiooncology. 2022;8(1):14.
PubMed PubMed Central Google Scholar
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P: CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003, 13(3):176-181.
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP et al: Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014, 106(9).
Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book. 2016;36:67–73.
Article Google Scholar
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–8.
Liu L, Suo T, Shen Y, Geng C, Song Z, Liu F, Wang J, Xie Y, Zhang Y, Tang T, et al. Clinicians versus patients subjective adverse events assessment: based on patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res. 2020;29(11):3009–15.
Munn Z, Pollock D, Khalil H, Alexander L, Mclnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evidence Synthesis. 2022;20(4):950–2.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32.
Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil H: Chapter 11: scoping reviews (2020 version). In: JBI Manual for Evidence Synthesis. edn. Edited by Aromataris E MZ: JBI; 2020.
Munn Z, Peters MD, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC medical research methodology. 2018;18:1–7.
Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, Pollock D, Tricco AC, Munn Z. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20(4):953–68.
Bohdan M, Kowalczys A, Mickiewicz A, Gruchala M, Lewicka E: Cancer therapy-related cardiovascular complications in clinical practice: current perspectives. J Clin Med 2021, 10(8).
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63.
Bozkurt B, Coats A, Tsutsui H: Universal definition and classification of heart failure. J Card Fail 2021.
Aldiab A. Cardiotoxicity with adjuvant trastuzumab use in breast cancer: a single institution»s experience. J Saudi Heart Assoc. 2010;22(3):133–6.
Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–21.
Polk A, Shahmarvand N, Vistisen K, Vaage-Nilsen M, Larsen FO, Schou M, Nielsen DL: Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 2016, 6(10).
Clark RA, Marin TS, McCarthy AL, Bradley J, Grover S, Peters R, Karapetis CS, Atherton JJ, Koczwara B. Cardiotoxicity after cancer treatment: a process map of the patient treatment journey. Cardiooncology. 2019;5:14.
Anjos M, Fontes-Oliveira M, Costa VM, Santos M, Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021;280: 119760.
Malik A, Brito D, Vaqar S, Chhabra L: Congestive heart failure. In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2023.
Attin M, Reifenstein K, Mehta S, Arcoleo K, Lin CD, Storozynsky E. Reported signs, symptoms, and diagnostic tests before cardiotoxicity among women with breast cancer: a pilot study. J Cardiovasc Nurs. 2022;37(2):104–11.
Huang P, Dai S, Ye Z, Liu Y, Chen Z, Zheng Y, Shao X, Lei L, Wang X. Long-term tolerance and cardiac function in breast cancer patients receiving trastuzumab therapy. Oncotarget. 2017;8(2):2069–75.
Salyer J, Flattery M, Lyon DE. Heart failure symptom clusters and quality of life. Heart Lung. 2019;48(5):366–72.
Padegimas A, Carver JR. How to diagnose and manage patients with fluoropyrimidine-induced chest pain: a single center approach. JACC CardioOncol. 2020;2(4):650–4.
Sheth MA, Widmer RJ, Dandapantula HK. Pathobiology and evolving therapies of coronary artery vasospasm. Proc (Bayl Univ Med Cent). 2021;34(3):352–60.
PubMed Google Scholar
Hokimoto S, Kaikita K, Yasuda S, Tsujita K, Ishihara M, Matoba T, Matsuzawa Y, Mitsutake Y, Mitani Y, Murohara T, et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. J Cardiol. 2023;82(4):293–341.
Kanduri J, More LA, Godishala A, Asnani A. Fluoropyrimidine-associated cardiotoxicity. Cardiol Clin. 2019;37(4):399–405.
Garbis K, Rafiee MJ, Luu J. 5-fluorouracil-induced coronary vasospasm: a cardiovascular magnetic resonance imaging case report. Glob Cardiol Sci Pract. 2023;2023(3): e202316.
Dyhl-Polk A, Vaage-Nilsen M, Schou M, Vistisen KK, Lund CM, Kümler T, Appel JM, Nielsen DL. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020;59(4):475–83.
Becker K, Erckenbrecht JF, Häussinger D, Fueling T. Cardiotoxicity of the antiprolif erative compound fluorouracil. Drugs. 1999;57(4):475–84.
Lestuzzi C, Vaccher E, Talamini R, Lleshi A, Meneguzzo N, Viel E, Scalone S, Tartuferi L, Buonadonna A, Ejiofor L, Schmoll HJ. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol. 2014;25(5):1059–64.
Muco E, Patail H, Shaik A, McMahon S. Capecitabine-associated coronary vasospasm and cardiac arrest. Cureus. 2022;14(8): e28184.
Montemurro F, Mittica G, Cagnazzo C, Longo V, Berchialla P, Solinas G, Culotta P, Martinello R, Foresto M, Gallizioli S, et al. Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncol. 2016;2(4):445–52.
Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y, Basch E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–76.
Galizia D, Milani A, Geuna E, Martinello R, Cagnazzo C, Foresto M, Longo V, Berchialla P, Solinas G, Calori A, et al. Self-evaluation of duration of adjuvant chemotherapy side effects in breast cancer patients: a prospective study. Cancer Med. 2018;7(9):4339–44.
Gavila J, Seguí M, Calvo L, López T, Alonso JJ, Farto M. Sánchez-de la Rosa R: Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol. 2017;19(1):91–104.
Leong DP, Lenihan DJ. Clinical practice guidelines in cardio-oncology. Heart Fail Clin. 2022;18(3):489–501.
Munn Z, Pollock D, Khalil H, Alexander L, McLnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evid Synth. 2022;20(4):950–2.
Li YR, Jia Z, Zhu H. Understanding the value of case reports and studies in the context of clinical research, research design and evidence-based practice. J Case Reports and Studies. 2013;1(2):1–4.
Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003;52(4):256–61.
Download references
Acknowledgements
The authors thank Nawon Kim, a librarian at the Yonsei University Medical Library, for building search terms and guiding the database searches.
This research is supported by the Brain Korea 21 FOUR Project founded by the National Research Foundation (NRF) of Korea, Yonsei University College of Nursing.
Author information
Authors and affiliations.
Graduate School, College of Nursing, Yonsei University, 50 Yonsei-ro, Seoul, South Korea
Hyunjoo Kim
Severance Cardiovascular Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Brain Korea 21 FOUR Project, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Mo-Im Kim Nursing Research Institute, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Sanghee Kim & Jeongok Park
Department of Internal Medicine, Division of Cardiology, Heart Failure Center, Severance Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Seok-Min Kang
You can also search for this author in PubMed Google Scholar
Contributions
HK, BH, SK, and JP contributed to the study conception and design. The literature search and record screening were performed by HK and BH under the supervision of JP. Material preparation, data collection, and analysis were performed by HK, BH, and JP. The first draft of the manuscript was written by HK and JP commented on each updated version of the manuscript. The tables and figures were prepared by BH under the instruction of JP. SK helped to interpret the data and provided critical feedback on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jeongok Park .
Ethics declarations
Ethics approval and consent to participate.
This study was exempted from ethical approval by the Yonsei University Institute Review Board, and consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kim, H., Hong, B., Kim, S. et al. Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review. Syst Rev 13 , 167 (2024). https://doi.org/10.1186/s13643-024-02588-z
Download citation
Received : 25 December 2023
Accepted : 14 June 2024
Published : 27 June 2024
DOI : https://doi.org/10.1186/s13643-024-02588-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Chemotherapy
- Cardiotoxicity
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Introduction
- Conclusions
- Article Information
ADHD indicates attention-deficit/hyperactivity disorder; CVD, cardiovascular disease.
a Controls were derived from the same base cohort as the cases; thus, a case with a later date of CVD diagnosis could potentially serve as a control for another case in the study.
Crude odds ratios (ORs) were based on cases and controls matched on age, sex, and calendar time. Adjusted ORs (AORs) were based on cases and controls matched on age, sex, and calendar time and adjusted for country of birth, educational level, somatic comorbidities (type 2 diabetes, obesity, dyslipidemia, and sleep disorders), and psychiatric comorbidities (anxiety disorders, autism spectrum disorder, bipolar disorder, conduct disorder, depressive disorder, eating disorders, intellectual disability, personality disorders, schizophrenia, and substance use disorders).
The solid lines represent the adjusted odds ratios, and the shaded areas represent the 95% CIs. In restricted cubic splines analysis, knots were placed at the 10th, 50th, and 90th percentiles of ADHD medication use.
eTable 1. International Classification of Diseases (ICD) Codes from the Swedish National Inpatient Register
eTable 2. Type of Cardiovascular Disease in Cases
eTable 3. Risk of CVD Associated With ADHD Medication Use Across Different Average Defined Daily Doses
eTable 4. Risk of CVD Associated With Cumulative Duration of Use of Different Types of ADHD Medications
eTable 5. Sensitivity Analyses of CVD Risk Associated With Cumulative Use of ADHD Medications, Based On Different Cohort, Exposure, and Outcome Definitions
eFigure. Risk of CVD Associated With Cumulative Use of ADHD Medications, Stratified by Sex
Data Sharing Statement
- Long-Term ADHD Medications and Cardiovascular Disease Risk JAMA Medical News in Brief December 26, 2023 Emily Harris
- Long-Term Cardiovascular Effects of Medications for ADHD—Balancing Benefits and Risks of Treatment JAMA Psychiatry Editorial February 1, 2024 Samuele Cortese, MD, PhD; Cristiano Fava, MD, PhD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Zhang L , Li L , Andell P, et al. Attention-Deficit/Hyperactivity Disorder Medications and Long-Term Risk of Cardiovascular Diseases. JAMA Psychiatry. 2024;81(2):178–187. doi:10.1001/jamapsychiatry.2023.4294
Manage citations:
© 2024
- Permissions
Attention-Deficit/Hyperactivity Disorder Medications and Long-Term Risk of Cardiovascular Diseases
- 1 Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
- 2 Unit of Cardiology, Heart and Vascular Division, Department of Medicine, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden
- 3 School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
- 4 Department of Applied Health Science, School of Public Health, Indiana University, Bloomington
- 5 Department of Psychological and Brain Sciences, Indiana University, Bloomington
- Editorial Long-Term Cardiovascular Effects of Medications for ADHD—Balancing Benefits and Risks of Treatment Samuele Cortese, MD, PhD; Cristiano Fava, MD, PhD JAMA Psychiatry
- Medical News in Brief Long-Term ADHD Medications and Cardiovascular Disease Risk Emily Harris JAMA
Question Is long-term use of attention-deficit/hyperactivity disorder (ADHD) medication associated with an increased risk of cardiovascular disease (CVD)?
Findings In this case-control study of 278 027 individuals in Sweden aged 6 to 64 years who had an incident ADHD diagnosis or ADHD medication dispensation, longer cumulative duration of ADHD medication use was associated with an increased risk of CVD, particularly hypertension and arterial disease, compared with nonuse.
Meaning Findings of this study suggest that long-term exposure to ADHD medications was associated with an increased risk of CVD; therefore, the potential risks and benefits of long-term ADHD medication use should be carefully weighed.
Importance Use of attention-deficit/hyperactivity disorder (ADHD) medications has increased substantially over the past decades. However, the potential risk of cardiovascular disease (CVD) associated with long-term ADHD medication use remains unclear.
Objective To assess the association between long-term use of ADHD medication and the risk of CVD.
Design, Setting, and Participants This case-control study included individuals in Sweden aged 6 to 64 years who received an incident diagnosis of ADHD or ADHD medication dispensation between January 1, 2007, and December 31, 2020. Data on ADHD and CVD diagnoses and ADHD medication dispensation were obtained from the Swedish National Inpatient Register and the Swedish Prescribed Drug Register, respectively. Cases included individuals with ADHD and an incident CVD diagnosis (ischemic heart diseases, cerebrovascular diseases, hypertension, heart failure, arrhythmias, thromboembolic disease, arterial disease, and other forms of heart disease). Incidence density sampling was used to match cases with up to 5 controls without CVD based on age, sex, and calendar time. Cases and controls had the same duration of follow-up.
Exposure Cumulative duration of ADHD medication use up to 14 years.
Main Outcomes and Measures The primary outcome was incident CVD. The association between CVD and cumulative duration of ADHD medication use was measured using adjusted odds ratios (AORs) with 95% CIs.
Results Of 278 027 individuals with ADHD aged 6 to 64 years, 10 388 with CVD were identified (median [IQR] age, 34.6 [20.0-45.7] years; 6154 males [59.2%]) and matched with 51 672 control participants without CVD (median [IQR] age, 34.6 [19.8-45.6] years; 30 601 males [59.2%]). Median (IQR) follow-up time in both groups was 4.1 (1.9-6.8) years. Longer cumulative duration of ADHD medication use was associated with an increased risk of CVD compared with nonuse (0 to ≤1 year: AOR, 0.99 [95% CI, 0.93-1.06]; 1 to ≤2 years: AOR, 1.09 [95% CI, 1.01-1.18]; 2 to ≤3 years: AOR, 1.15 [95% CI, 1.05-1.25]; 3 to ≤5 years: AOR, 1.27 [95% CI, 1.17-1.39]; and >5 years: AOR, 1.23 [95% CI, 1.12-1.36]). Longer cumulative ADHD medication use was associated with an increased risk of hypertension (eg, 3 to ≤5 years: AOR, 1.72 [95% CI, 1.51-1.97] and >5 years: AOR, 1.80 [95% CI, 1.55-2.08]) and arterial disease (eg, 3 to ≤5 years: AOR, 1.65 [95% CI, 1.11-2.45] and >5 years: AOR, 1.49 [95% CI, 0.96-2.32]). Across the 14-year follow-up, each 1-year increase of ADHD medication use was associated with a 4% increased risk of CVD (AOR, 1.04 [95% CI, 1.03-1.05]), with a larger increase in risk in the first 3 years of cumulative use (AOR, 1.08 [95% CI, 1.04-1.11]) and stable risk over the remaining follow-up. Similar patterns were observed in children and youth (aged <25 years) and adults (aged ≥25 years).
Conclusions and Relevance This case-control study found that long-term exposure to ADHD medications was associated with an increased risk of CVDs, especially hypertension and arterial disease. These findings highlight the importance of carefully weighing potential benefits and risks when making treatment decisions about long-term ADHD medication use. Clinicians should regularly and consistently monitor cardiovascular signs and symptoms throughout the course of treatment.
Attention-deficit/hyperactivity disorder (ADHD) is a common psychiatric disorder characterized by developmentally inappropriate inattentiveness, impulsivity, and hyperactivity. 1 , 2 Pharmacological therapy, including both stimulants and nonstimulants, is recommended as the first-line treatment for ADHD in many countries. 1 , 3 The use of ADHD medication has increased greatly in both children and adults during the past decades. 4 Although the effectiveness of ADHD medications has been demonstrated in randomized clinical trials (RCTs) and other studies, 5 , 6 concerns remain regarding their potential cardiovascular safety. 7 Meta-analyses of RCTs have reported increases in heart rate and blood pressure associated with both stimulant and nonstimulant ADHD medications. 5 , 7 - 9
As RCTs typically evaluate short-term effects (average treatment duration of 75 days), 7 it remains uncertain whether and to what extent the increases in blood pressure and heart rate associated with ADHD medication lead to clinically significant cardiovascular disease (CVD) over time. Longitudinal observational studies 10 - 12 examining the association between ADHD medication use and serious cardiovascular outcomes have emerged in recent years, but the findings have been mixed. A meta-analysis 13 of observational studies found no statistically significant association between ADHD medication and risk of CVD. However, the possibility of a modest risk increase cannot be ruled out due to several methodological limitations in these studies, including confounding by indication, immortal time bias, and prevalent user bias. Additionally, most of these studies had an average follow-up time of no more than 2 years. 13 , 14 Thus, evidence regarding the long-term cardiovascular risk of ADHD medication use is still lacking.
Examining the long-term cardiovascular risk associated with ADHD medicine use is clinically important given that individuals with a diagnosis of ADHD, regardless of whether they receive treatment, face an elevated risk of CVD. 15 Additionally, a substantial proportion of young individuals with ADHD continues to have impairing symptoms in adulthood, 16 necessitating prolonged use of ADHD medication. Notably, studies have indicated a rising trend in the long-term use of ADHD medications, with approximately half of individuals using ADHD medication for over 5 years. 17 Furthermore, evidence is lacking regarding how cardiovascular risk may vary based on factors such as type of CVD, type of ADHD medication, age, and sex. 13 Therefore, there is a need for long-term follow-up studies to address these knowledge gaps and provide a more comprehensive understanding of the cardiovascular risks associated with ADHD medication use. This information is also crucial from a public health perspective, particularly due to the increasing number of individuals receiving ADHD medications worldwide. 4
This study aimed to assess the association between cumulative use of ADHD medication up to 14 years and the risk of CVD by using nationwide health registers in Sweden. We hypothesized that longer cumulative use of ADHD medication would be associated with increased CVD risk. In addition, we aimed to examine whether the associations differ across types of ADHD medication, types of CVD, sex, and age groups.
We used data from several Swedish nationwide registers linked through unique personal identification numbers. 18 Diagnoses were obtained from the National Inpatient Register, 19 which contains data on inpatient diagnoses since 1973 and outpatient diagnoses since 2001. Information on prescribed medications was retrieved from the Swedish Prescribed Drug Register, which contains all dispensed medications in Sweden since July 2005 and includes information on drug identity based on the Anatomical Therapeutic Chemical (ATC) classification, 20 dispensing dates, and free-text medication prescriptions. Socioeconomic factors were obtained from the Longitudinal Integrated Database for Health Insurance and Labour Market studies. 21 Information on death was retrieved from the Swedish Cause of Death Register, 22 which contains information on all deaths since 1952. The study was approved by the Swedish Ethical Review Authority. Informed patient consent is not required for register-based studies in Sweden. The study followed the Reporting of Studies Conducted Using Observational Routinely Collected Health Data–Pharmacoepidemiological Research ( RECORD-PE ) guideline. 23
We conducted a nested case-control study of all individuals residing in Sweden aged 6 to 64 years who received an incident diagnosis of ADHD or ADHD medication dispensation 15 between January 1, 2007, and December 31, 2020. The diagnosis of ADHD ( International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ ICD-10 ] code F90) was identified from the National Inpatient Register. Incident ADHD medication dispensation was identified from the Swedish Prescribed Drug Register and was defined as a dispensation after at least 18 months without any ADHD medication dispensation. 24 Baseline (ie, cohort entry) was defined as the date of incident ADHD diagnosis or ADHD medication dispensation, whichever came first. Individuals with ADHD medication prescriptions for indications other than ADHD 25 and individuals who emigrated, died, or had a history of CVD before baseline were excluded from the study. The cohort was followed until the case index date (ie, the date of CVD diagnosis), death, migration, or the study end date (December 31, 2020), whichever came first.
Within the study cohort, we identified cases as individuals with an incident diagnosis of any CVD (including ischemic heart diseases, cerebrovascular diseases, hypertension, heart failure, arrhythmias, thromboembolic disease, arterial disease, and other forms of heart disease; eTable 1 in Supplement 1 ) during follow-up. For each case, the date of their CVD diagnosis was assigned as the index date. Using incidence density sampling, 26 up to 5 controls without CVD were randomly selected for each case from the base cohort of individuals with ADHD. The matching criteria included age, sex, and calendar time, ensuring that cases and controls had the same duration of follow-up from baseline to index date. Controls were eligible for inclusion if they were alive, living in Sweden, and free of CVD at the time when their matched case received a diagnosis of CVD, with the index date set as the date of CVD diagnosis of the matched case ( Figure 1 ). Controls were derived from the same base cohort as the cases. Thus, a case with a later date of CVD diagnosis could potentially serve as a control for another case in the study. 26
The main exposure was cumulative duration of ADHD medication use, which included all ADHD medications approved in Sweden during the study period, including stimulants (methylphenidate [ATC code N06BA04], amphetamine [ATC code N06BA01], dexamphetamine [ATC code N06BA02], and lisdexamfetamine [ATC code N06BA12]) as well as nonstimulants (atomoxetine [ATC code N06BA09] and guanfacine [ATC code C02AC02]). Duration of ADHD medication use was derived from a validated algorithm that estimates treatment duration from free text in prescription records. 25 The cumulative duration of ADHD medication use was calculated by summing all days covered by ADHD medication between baseline and 3 months prior to the index date. The last 3 months before the index date were excluded to reduce reverse causation, as clinicians’ perception of potential cardiovascular risks may influence ADHD medication prescription. This time window was chosen because routine psychiatric practice in Sweden limits a prescription to a maximum 3 months at a time. 27 Individuals with follow-up of less than 3 months were excluded.
We conducted conditional logistic regression analyses to estimate odds ratios (ORs) for the associations between cumulative durations of ADHD medication use and incident CVD. Crude ORs were adjusted for all matching variables (age, sex, and calendar time) by design. Adjusted ORs (AORs) were additionally controlled for country of birth (Sweden vs other), highest educational level (primary or lower secondary, upper secondary, postsecondary or postgraduate, or unknown; individuals aged <16 years were included as a separate category), and diagnoses of somatic (type 2 diabetes, obesity, dyslipidemia, and sleep disorders) and psychiatric comorbidities (anxiety disorders, autism spectrum disorder, bipolar disorder, conduct disorder, depressive disorder, eating disorders, intellectual disability, personality disorders, schizophrenia, and substance use disorders; eTable 1 in Supplement 1 ) before baseline. The association between cumulative ADHD medication use and incident CVD was assessed using both continuous and categorical measures (no ADHD medication use, 0 to ≤1, 1 to ≤2, 2 to ≤3, 3 to ≤5, and >5 years). To capture potential nonlinear associations, we used restricted cubic splines to examine ADHD medication use as a continuous measure throughout follow-up. 28 The associations were examined in the full sample and stratified by age at baseline, that is, children or youth (<25 years old) and adults (≥25 years old). Furthermore, to evaluate the association with dosage of ADHD medication, we estimated the risk of CVD associated with each 1-year increase in use of ADHD medication across different dosage groups categorized by the average defined daily dose (DDD; for instance, 1 DDD of methylphenidate equals 30 mg) during follow-up. 29
In subgroup analyses, we examined the associations between ADHD medication use and specific CVDs, including arrhythmias, arterial disease, cerebrovascular disease, heart failure, hypertension, ischemic heart disease, and thromboembolic disease (eTable 1 in Supplement 1 ). Additionally, we investigated the associations with CVD risk for the most commonly prescribed ADHD medications in Sweden, ie, methylphenidate, lisdexamfetamine, and atomoxetine, while adjusting for other ADHD medication use. We also examined sex-specific associations.
To further examine the robustness of our findings, we conducted 4 sensitivity analyses. First, we restricted the sample to ever users of ADHD medication to reduce unmeasured confounding between ADHD medication users and nonusers. Second, we assessed ADHD medication exposure over the entire follow-up period without excluding the 3 months prior to the index date. Third, to capture fatal cardiovascular events, we additionally included death by CVD in the outcome definition. Finally, we constructed a conditional logistic regression model that adjusted for propensity scores of ADHD medication use. Data management was performed using SAS, version 9.4 (SAS Institute Inc) and all analyses were performed using R, version 4.2.3 (R Foundation for Statistical Computing).
The study cohort consisted of 278 027 individuals with ADHD aged 6 to 64 years. The incidence rate of CVD was 7.34 per 1000 person-years. After applying exclusion criteria and matching, the analysis included 10 388 cases (median [IQR] age at baseline, 34.6 (20.0-45.7) years; 6154 males [59.2%] and 4234 females [40.8%]) and 51 672 matched controls (median [IQR] age at baseline, 34.6 [19.8-45.6] years; 30 601 males [59.2%] and 21 071 females [40.8%]) ( Figure 1 and Table 1 ). Median (IQR) follow-up in both groups was 4.1 (1.9-6.8) years. Among the controls, 3363 had received a CVD diagnosis after their index dates. The most common types of CVD in cases were hypertension (4210 cases [40.5%]) and arrhythmias (1310 cases [12.6%]; eTable 2 in Supplement 1 ). Table 1 presents the sociodemographic information and somatic and psychiatric comorbidities in cases and controls. In general, cases had higher rates of somatic and psychiatric comorbidities and a lower level of educational attainment compared with controls.
A similar proportion of cases (83.9%) and controls (83.5%) used ADHD medication during follow-up, with methylphenidate being the most commonly dispensed type, followed by atomoxetine and lisdexamfetamine. Longer cumulative duration of ADHD medication use was associated with an increased risk of CVD compared with nonuse (0 to ≤1 year: AOR, 0.99 [95% CI, 0.93-1.06]; 1 to ≤2 years: AOR, 1.09 [95% CI, 1.01-1.18]; 2 to ≤3 years: AOR, 1.15 [95% CI, 1.05-1.25]; 3 to ≤5 years: AOR, 1.27 [95% CI, 1.17-1.39]; and >5 years: AOR, 1.23 [95% CI, 1.12-1.36]) ( Figure 2 ). The restricted cubic spline model suggested a nonlinear association, with the AORs increasing rapidly for the first 3 cumulative years of ADHD medication use and then becoming stable thereafter ( Figure 3 ). Throughout the entire follow-up, each 1-year increase in the use of ADHD medication was associated with a 4% increased risk of CVD (AOR, 1.04 [95% CI, 1.03-1.05]), and the corresponding increase for the first 3 years was 8% (AOR, 1.08 [95% CI, 1.04-1.11]). We observed similar results when examining children or youth and adults separately ( Figure 2 ). The restricted cubic spline model suggested a similar nonlinear association, with higher AORs in children or youth than in adults, but the 95% CIs largely overlapped ( Figure 3 ). Furthermore, similar associations were observed for females and males (eFigure in Supplement 1 ). The dosage analysis showed that the risk of CVD associated with each 1 year of ADHD medication use increased with higher average DDDs. The risk was found to be statistically significant only among individuals with a mean dose of at least 1.5 times the DDD (eTable 3 in Supplement 1 ). For example, among individuals with a mean DDD of 1.5 to 2 or less (eg, for methylphenidate, 45 to ≤60 mg), each 1-year increase in ADHD medication use was associated with a 4% increased risk of CVD (AOR, 1.04 [95% CI, 1.02-1.05]). Among individuals with a mean DDD >2 (eg, for methylphenidate >60 mg), each 1-year increase in ADHD medication use was associated with 5% increased risk of CVD (AOR, 1.05 [95% CI, 1.03-1.06]).
When examining the risk for specific CVDs, we found that long-term use of ADHD medication (compared with no use) was associated with an increased risk of hypertension (AOR, 1.72 [95% CI, 1.51-1.97] for 3 to ≤5 years; AOR, 1.80 [95% CI 1.55-2.08] for >5 years) ( Table 2 ), as well as arterial disease (AOR, 1.65 [95% CI, 1.11-2.45] for 3 to ≤5 years; AOR, 1.49 [95% CI 0.96-2.32] for >5 years). However, we did not observe any statistically significant increased risk for arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease ( Table 2 ). Furthermore, long-term use of methylphenidate (compared with no use) was associated with an increased risk of CVD (AOR, 1.20 [95% CI, 1.10-1.31] for 3 to ≤5 years; AOR, 1.19 [95% CI, 1.08-1.31]) for >5 years; eTable 4 in Supplement 1 ). Compared with no use, lisdexamfetamine was also associated with an elevated risk of CVD (AOR, 1.23 [95% CI, 1.05-1.44] for 2 to ≤3 years; AOR, 1.17 [95% CI, 0.98-1.40] for >3 years), while the AOR for atomoxetine use was significant only for the first year of use (1.07 [95% CI 1.01-1.13]; eTable 4 in Supplement 1 ).
In sensitivity analyses, we observed a similar pattern of estimates when the analysis was restricted to ever users of ADHD medications. Significantly increased risk of CVD was found when comparing ADHD medication use for 1 year or less with use for 3 to 5 or less years (AOR, 1.28 (95% CI, 1.18-1.38) or for use for more than 5 years (AOR, 1.24 [95% CI, 1.13-1.36]) (eTable 5 in Supplement 1 ). When assessing ADHD medication use across the entire follow-up period, and compared with no use, the pattern of estimates was similar to the main analysis (3 to ≤5 years: AOR, 1.28 [95% CI, 1.18-1.39]; >5 years: AOR, 1.25 [95% CI, 1.14-1.37]) (eTable 5 in Supplement 1 ). The analysis that included cardiovascular death as a combined outcome also had results similar to the main analysis. Moreover, when adjusting for propensity scores of ADHD medication use, the findings remained consistent (eTable 5 in Supplement 1 ).
This large, nested case-control study found an increased risk of incident CVD associated with long-term ADHD medication use, and the risk increased with increasing duration of ADHD medication use. This association was statistically significant both for children and youth and for adults, as well as for females and males. The primary contributors to the association between long-term ADHD medication use and CVD risk was an increased risk of hypertension and arterial disease. Increased risk was also associated with stimulant medication use.
We found individuals with long-term ADHD medication use had an increased risk of incident CVD in a dose-response manner in the first 3 years of cumulative ADHD medication use. To our knowledge, few previous studies have investigated the association between long-term ADHD medication use and the risk of CVD with follow-up of more than 2 years. 13 The only 2 prior studies with long-term follow-up (median, 9.5 and 7.9 years 30 , 31 ) found an average 2-fold and 3-fold increased risk of CVD with ADHD medication use compared with nonuse during the study period, yet 1 of the studies 30 included only children, and participants in the other study 31 were not the general population of individuals with ADHD (including those with ADHD and long QT syndrome). Furthermore, both studies were subject to prevalent user bias. Results from the current study suggest that the CVD risk associated with ADHD medication use (23% increased risk for >5 years of ADHD medication use compared with nonuse) is lower than previously reported. 30 , 31 Furthermore, we observed that the increased risk stabilized after the first several years of medication use and persisted throughout the 14-year follow-up period.
The association between ADHD medication use and CVD was significant for hypertension and arterial disease, while no significant association was observed with other types of cardiovascular events. To our knowledge, only 1 previous study 12 has examined the association between ADHD medication use and clinically diagnosed hypertension, and it found an increased risk, although the increase was not statistically significant. Furthermore, increased blood pressure associated with ADHD medication use has been well documented. 7 , 9 One study 32 found that blood pressure was mainly elevated during the daytime, suggesting that the cardiovascular system may recover at night. However, the cross-sectional nature of that study cannot preclude a long-term risk of clinically diagnosed hypertension associated with ADHD medication use. We also identified an increased risk for arterial disease. To date, no previous study has explored the association between ADHD medication use and arterial disease. A few studies have reported that ADHD medication may be associated with changes in serum lipid profiles, but the results were not consistent. 33 , 34 Further research is needed on the potential implications of ADHD medications for individuals’ lipid profiles. We did not observe any association between ADHD medication use and the risk of arrhythmias. A recent systematic review of observational studies of ADHD medication use reported an elevated risk of arrhythmias, but the finding was not statistically significant. 13 A review of RCTs also found that the use of stimulants was associated with an average increase in heart rate of 5.7 beats/min, 9 but no evidence of prolonged QT interval or tachycardia was found based on electrocardiograms. 7 Additionally, it is worth noting that some individuals receiving ADHD medications might be prescribed antiarrhythmic β-blockers to alleviate palpitation symptoms, thus potentially attenuating an association between ADHD medications and arrhythmias. Nevertheless, the absence of an association between ADHD medication use and clinically diagnosed arrhythmias in the present study does not rule out an increased risk for mild arrhythmias or subclinical symptoms, as palpitations and sinus tachycardia are not routinely coded as arrhythmia diagnoses. Further research is necessary to replicate our findings.
Regarding types of ADHD medication, findings of the present study suggest that increasing cumulative durations of methylphenidate and lisdexamfetamine use were associated with incident CVD, while the associations for atomoxetine were statistically significant only for the first year of use. Previous RCTs have reported increased blood pressure and heart rate with methylphenidate, lisdexamfetamine, and atomoxetine, 5 , 35 , 36 but the mechanisms behind these adverse effects are still a topic of debate; there might be differences in cardiovascular adverse effects in stimulants vs nonstimulants. 37
We found that the association between cumulative duration of ADHD medication use and CVD was similar in females and males. Previous investigations exploring sex-specific association found higher point estimates in females, although the differences were not statistically significant. 13 Research has indicated that females diagnosed with ADHD may demonstrate different comorbidity patterns and potentially have different responses to stimulant medications compared with males. 38 - 40 Therefore, additional studies are needed to explore and better understand the potential sex-specific differences in cardiovascular responses to ADHD medications.
A strength of this study is that data on ADHD medication prescriptions and CVD diagnoses were recorded prospectively, so the results were not affected by recall bias. The findings should, however, be interpreted in the context of several limitations. First, our approach for identification of patients with CVD was based on recorded diagnoses and there could be under ascertainment of cardiovascular diagnoses in the registers used. This means that some controls may have had undiagnosed CVD that did not yet require medical care, which would tend to underestimate associations between ADHD medication use and CVD. Second, exposure misclassification may have occurred if patients did not take their medication as prescribed. This misclassification, if nondifferential, would tend to reduce ORs such that the estimates we observed were conservative. Third, while we accounted for a wide range of potential confounding variables, considering the observational nature of the study and the possibility of residual confounding, we could not prove causality. It is possible that the association observed might have been affected by time-varying confounders. For example, other psychotropic medications and lifestyle factors could have affected both ADHD medication use and the occurrence of cardiovascular events. 41 , 42 Confounding by ADHD severity is also a potential factor to consider, as individuals with more severe ADHD symptoms may have more comorbidities and a less healthy lifestyle, which could affect the risk of CVD. Fourth, the study did not examine the risk of CVD among individuals with preexisting CVD. Individuals with preexisting CVD represent a distinct clinical group that requires careful monitoring; thus, evaluating the risk among them necessitates a different study design that carefully considers the potential impact of prior knowledge and periodic monitoring. Finally, the results by type of ADHD medication and type of CVD need to be replicated by studies with larger sample sizes.
The results of this population-based case-control study with a longitudinal follow-up of 14 years suggested that long-term use of ADHD medication was associated with an increased risk of CVD, especially hypertension and arterial disease, and the risk was higher for stimulant medications. These findings highlight the importance of carefully weighing potential benefits and risks when making treatment decisions on long-term ADHD medication use. Clinicians should be vigilant in monitoring patients, particularly among those receiving higher doses, and consistently assess signs and symptoms of CVD throughout the course of treatment. Monitoring becomes even more crucial considering the increasing number of individuals engaging in long-term use of ADHD medication.
Accepted for Publication: August 29, 2023.
Published Online: November 22, 2023. doi:10.1001/jamapsychiatry.2023.4294
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Zhang L et al. JAMA Psychiatry .
Corresponding Authors: Zheng Chang, PhD ( [email protected] ) and Le Zhang, PhD ( [email protected] ), Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Nobels väg 12A, 171 65 Stockholm, Sweden.
Author Contributions: Dr Zhang and Prof Chang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zhang, Johnell, Larsson, Chang.
Acquisition, analysis, or interpretation of data: Zhang, Li, Andell, Garcia-Argibay, Quinn, D'Onofrio, Brikell, Kuja-Halkola, Lichtenstein, Johnell, Chang.
Drafting of the manuscript: Zhang.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Zhang, Li.
Obtained funding: Larsson, Chang.
Administrative, technical, or material support: Garcia-Argibay, D'Onofrio, Kuja-Halkola, Lichtenstein, Chang.
Supervision: Andell, Lichtenstein, Johnell, Larsson, Chang.
Conflict of Interest Disclosures: Dr Larsson reported receiving grants from Takeda Pharmaceuticals and personal fees from Takeda Pharmaceuticals, Evolan, and Medici Medical Ltd outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by grants from the Swedish Research Council for Health, Working Life, and Welfare (2019-01172 and 2022-01111) (Dr Chang) and the European Union’s Horizon 2020 research and innovation program under grant agreement 965381 (Dr Larsson).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
The Daily Show Fan Page

Explore the latest interviews, correspondent coverage, best-of moments and more from The Daily Show.
Extended Interviews

The Daily Show Tickets
Attend a Live Taping
Find out how you can see The Daily Show live and in-person as a member of the studio audience.
Best of Jon Stewart

The Weekly Show with Jon Stewart
New Episodes Thursdays
Jon Stewart and special guests tackle complex issues.
Powerful Politicos

The Daily Show Shop
Great Things Are in Store
Become the proud owner of exclusive gear, including clothing, drinkware and must-have accessories.
About The Daily Show

COMMENTS
Heart failure is a systemic and complex clinical syndrome, ... Prevalence studies estimate that 23 million individuals worldwide have heart failure and that 2 million new cases are diagnosed annually. ... The patient in this case presented with progressive dyspnea triggered by less than ordinary activities, lower-extremity edema, and ascites ...
On examination, the temperature was 36.4°C, the heart rate 103 beats per minute, the blood pressure 79/51 mm Hg, the respiratory rate 30 breaths per minute, and the oxygen saturation 99% while ...
In this case, a patient with known history of coronary artery disease presented with worsening of shortness of breath with lower extremity edema and jugular venous distension along with crackles in the lung. The sign and symptoms along with labs and imaging findings point to diagnosis of heart failure with reduced EF (HFrEF).
Presentation of Case. Dr. Amy A. Sarma: A 54-year-old man was evaluated at this hospital because of new heart failure. One month before this evaluation, a nonproductive cough developed after the ...
Heart Failure Patient Case Quizzes. Unrecognized HFpEF in a Type 2 DM Patient - Reducing CV and HF Risk ; Untreated HFrEF/Ischemic Cardiomyopathy in Type 2 DM - How to Optimize Medical Therapy to Improve Heart Failure Outcomes ; Restrictive Cardiomyopathies Series: Advanced HF Therapies in ATTR Cardiac Amyloidosis (Certified Patient Case Study)
A 67-year-old woman sought emergency medical care due to prolonged chest pain. In April 2009 the patient had prolonged chest pain and at that time she sought medical care. She was admitted at the hospital and diagnosed with myocardial infarction. The patient had hypertension, diabetes mellitus, dyslipidemia and was a smoker.
Dr. Amy A. Sarma: A 54-year-old man was evaluated at this hospital because of new heart failure. One month before this evaluation, a nonproductive cough developed after the patient took a business ...
Less is known about the definition and management of diastolic dysfunction. The following case studies have been chosen to illustrate the basis for therapeutic management of systolic heart failure and outline the remaining gaps in knowledge, of which there are several. The issues apply across the spectrum of patients seen in clinical practice.
Introduction. This clinical case refers to an 83-year-old man with moderate chronic obstructive pulmonary disease and shows that implementation of appropriate medical therapy according to the European Society of Cardiology/Heart Failure Association (ESC/HFA) guidelines improves symptoms and quality of life. 1 The case also illustrates that optimization of glucose metabolism with a more lenient ...
Heart failure patients presenting to primary care clinics often have multiple, complex comorbidities. Several different disease processes and treatment options may need to be considered simultaneously in the setting of acute on chronic exacerbation of symptoms. This...
Each JACC Patient Care Pathway is an immersive, multimedia case report depicting the integration of cross-specialty decision making supported by evidence-based medicine within a single patient journey. ... (Exercise Training in Diastolic Heart Failure) pilot study". J Am Coll Cardiol 2011;58:1780-1791. View Article Google Scholar; 102.
About this book. This guide provides a clear and concise overview of heart failure for primary care clinicians. Written by two nurse practitioners for nurses, nurse practitioners, physician assistants, medical students, and pharmacists, it is uniquely designed to bridge the gap between cardiology and primary care.
This study was focused on survival analysis of heart failure patients who were admitted to Institute of Cardiology and Allied hospital Faisalabad-Pakistan during April-December (2015). All the patients were aged 40 years or above, having left ventricular systolic dysfunction, belonging to NYHA class III and IV. Cox regression was used to model mortality considering age, ejection fraction ...
Discussion. "This case highlights how viruses continue to be an underappreciated cause of heart failure. In fact, viral myocarditis is an underdiagnosed cause of acute heart failure and chronic ...
This case study involves a 76 year old female named Mary Lou Poppins, who presented to the ED accompanied by her son. ... Mary Lou Poppins was educated regarding the disease process of heart failure; symptoms to monitor for and report to her doctor; the importance of daily monitoring of weight, blood pressure, and heart rate; and the importance ...
Advanced Heart Failure Case Study. Advanced Heart Failure Case Study. Patient Case History A 65 y/o man with chronic systolic heart failure comes to the office with progressive heart failure symptoms (dyspnea, fatigue) and a 10# unintentional weight loss. He is having more difficulty carrying on ADLs. He also has nocturnal dyspnea.
Introduction. This case study maps the journey of a patient and his family following a diagnosis of chronic heart failure secondary to left ventricular systolic dysfunction. It outlines the personal, medical, nursing, and social needs, and highlights the wider implications of heart failure and its progression.
This article was authored by Modele Ogunniyi, MD, MPH, FACC, associate professor, Emory University School of Medicine and associate medical director, Grady Heart Failure Program; Yetunde Fatade, MD, MPH, PGY 2 Resident, J. Willis Hurst Internal Medicine Residency, Emory University School of Medicine; and Andrea Cafarelli, FNP-BC, advanced ...
This article presents four case studies of patients with heart failure and the rationale for optimal treatment in each case. Case studies in heart failure Crit Care Nurs Clin North Am. 2003 Dec;15(4):519-23, x. doi: 10.1016/s0899-5885(02)00088-6. Authors Sara Paul 1 ...
Case Study: Acute Heart Failure in a 20-year-old Patient. At Piedmont Heart's Napa Valley Cardiology Conference, Dr. David Dean presents a challenging case of acute heart failure in a 20-year-old patient. Hear Piedmont's unusual approach to therapy and tips for success from Dr. Dean, surgical director of Piedmont's Samsky Advanced Heart ...
Presentation of Case. Dr. Jacqueline B. Henson (Medicine): A 54-year-old man was evaluated at this hospital after cardiac arrest associated with ventricular fibrillation. The patient had been in ...
Heart failure (HF) is a grave public health problem and has an enormous impact on health care utilization and the cost of health care. An aging population has contributed to an increase in the prevalence of HF patients and candidates for recurrent hospitalization [1,2,3].As of 2020, 6.7 million adults in the USA had HF, which contributed to 415,922 deaths in the USA in 2020 [].
Congestive heart failure (CHF), as defined by the American College of Cardiology (ACC) and the American Heart Association (AHA), is "a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood." Ischemic heart disease is the leading cause of death worldwide and also the leading cause of CHF. CHF is a common disorder ...
Study with Quizlet and memorize flashcards containing terms like Knowing that the client has two risk factors that cannot be modified, which intervention is most important for the nurse to include in the client's plan of care?, Which assessment finding provides the earliest indication that the client is experiencing right-sided heart failure?, Based on the electrocardiogram rhythm strip, what ...
The most prevalent CRCT were myocardial dysfunction and heart failure, identified in 12 case studies, respectively. This was followed by coronary artery disease, represented in 8 case studies, pulmonary hypertension in 2 case studies, and a single case study of periaortitis.
Within the study cohort, we identified cases as individuals with an incident diagnosis of any CVD (including ischemic heart diseases, cerebrovascular diseases, hypertension, heart failure, arrhythmias, thromboembolic disease, arterial disease, and other forms of heart disease; eTable 1 in Supplement 1) during follow-up. For each case, the date ...
The source for The Daily Show fans, with episodes hosted by Jon Stewart, Ronny Chieng, Jordan Klepper, Dulcé Sloan and more, plus interviews, highlights and The Weekly Show podcast.