February 17, 2021

COVID Vaccines Are Safe and Effective—What the Research Says
As more coronavirus vaccines are rolled out, researchers are learning about the extent and nature of side effects
By Ariana Remmel & Nature magazine
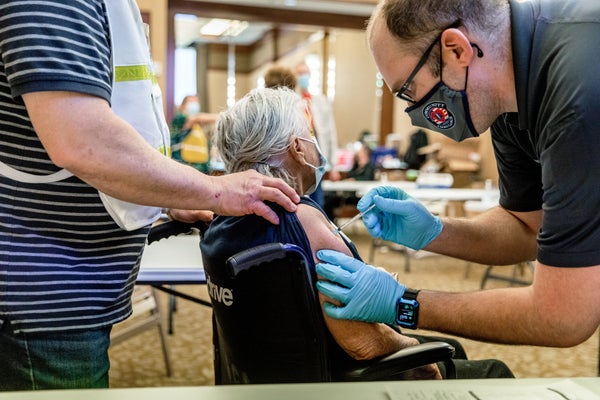
A healthcare worker administers a dose of the Pfizer-BioNTech Covid-19 vaccine at the Sun City Anthem Community Center vaccination site in Henderson, Nevada, U.S., on Thursday, Feb. 11, 2021.
Roger Kisby Getty Images
As people around the world receive COVID-19 vaccines, reports of temporary side effects such as headaches and fevers are rolling in. Much of this was expected—clinical-trial data for the vaccines authorized so far suggested as much. But now that millions of people are vaccinated, compared with the thousands enrolled in early studies, reports of some rare, allergic reactions are surfacing, and questions are arising about whether any deaths are linked to the shots.
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly coronavirus. Nature looks at what scientists are learning about the frequency and nature of side effects as huge numbers of people report their reactions to physicians and through safety-monitoring systems, such as smartphone apps.
How many people experience common side effects from COVID-19 vaccines?
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
For the two available messenger RNA (mRNA) vaccines—one made by Moderna at Cambridge, Massachusetts, and the other developed through a collaboration between Pfizer in New York City and BioNTech in Mainz, Germany—a significant portion of people experience non-serious reactions, such as injection-site pain, headache and fatigue. These vaccines deliver bits of RNA that code for coronavirus proteins, which the body mounts a response against.
According to data from the US Vaccine Adverse Event Reporting System (VAERS), about 372 out of every million administered doses of the mRNA vaccines lead to a non-serious reaction report. This number is lower than would be expected from clinical-trial data, which indicated that at least 80% of people would experience injection-site pain. Researchers running trials monitor patients closely and record every reaction. VAERS, meanwhile, relies on health-care workers and vaccinated individuals to self-report side effects.
So far, reactions to the mRNA vaccines are similar. These vaccines are administered in a two-dose regimen: the first shot triggers an immune reaction, and the second is a ‘booster’ that strengthens the body’s ability to fight the coronavirus. For the Pfizer–BioNTech vaccine, which has been in use longer than the Moderna vaccine and therefore has generated more data, side effects increase with the second dose.
In the United Kingdom, three million doses of another vaccine, developed by the University of Oxford and pharmaceutical firm AstraZeneca, have been doled out. This vaccine, which also requires a two-dose regimen, contains a inactivated cold-causing adenovirus with genetic instructions for making coronavirus proteins to trigger immunity. According to UK safety-monitoring system the Yellow Card Scheme, about 4,000 doses out of every million administered lead to adverse reactions. Again, clinical-trial data suggest that a higher frequency is more accurate: around 50% of participants had injection-site pain, headache or fatigue, according to data reported to the European Medicines Agency (EMA).
Few people have received a second dose of the Oxford–AstraZeneca vaccine because the United Kingdom used its supplies to administer a first dose to as many people as possible, but clinical-trial data presented to the EMA suggest that side effects of the second shot are milder than those caused by the first.
Safety data for shots rolling out in other parts of the world, such as the COVID-19 vaccines in China, are harder to come by. Preliminary data from clinical trials of the adenovirus-based Sputnik V vaccine in Russia suggest its most common side effects include flu-like symptoms and injection-site reactions.
How does that compare with side effects from an annual flu shot?
At least for the mRNA vaccines, physicians are seeing more side effects than for flu shots, says Helen Chu, an infectious-disease specialist at the University of Washington School of Medicine in Seattle, who directs the Seattle Flu Study. In clinical trials for the Pfizer–BioNTech vaccine, for instance, 75% of participants reported a ‘systemic reaction’, such as headache, fever or chills. In a clinical trial for the common influenza vaccine Flubok Quadravalent, around 34% of participants aged 18–49 had a systemic reaction. Side effects were even less frequent in study participants who were at least 50 years old.
Chu says the mRNA COVID-19 vaccines generate a particularly strong immune response that increases the risk of side effects, although this also means that the vaccines are working. She notes that her second dose of the Pfizer–BioNTech vaccine made her ill. “I got the vaccine, and 6 hours later, I had chills, a high fever, muscle aches and I went to bed for 24 hours,” she says. “Then by 36 hours later, it was totally over and I was back to normal.” But Chu would rather be temporarily ill from a vaccine than deal with COVID-19, “a potentially mortal disease that could kill me”, she says.
Have investigations linked any deaths to a COVID-19 vaccine?
Although some have questioned whether the vaccines have led to deaths, none have been directly attributed to a COVID-19 jab. After 33 elderly care-home residents in Norway died within 6 days of receiving the Pfizer–BioNTech vaccine, investigations by both the Norwegian Medicines Agency and the World Health Organization concluded that these deaths were in line with normal death rates in this age group and that the vaccine is still safe for older people. India's Ministry of Health and Family Welfare reported 27 deaths in the country, but none of these have been linked directly to a COVID-19 vaccine either.
It is “extremely difficult” to definitively link a death to the vaccine itself, says Hilda Bastian, a writer and scientist who specializes in validating evidence-based health claims. That is partially because the deaths reported so far have occurred days or weeks after an injection, making it hard to rule out other circumstances. Another reason is that, right now, clinicians are prioritizing vaccines largely for a population of older people with underlying health conditions. Most of those who have died after vaccination have been in this group, according to reports from the United Kingdom and the United States .
What do researchers know about the rare, but severe, allergic reactions to the vaccines?
The Moderna vaccine elicits about three anaphylactic reactions per million doses administered, and the Pfizer–BioNTech vaccine triggers five reactions per million doses, according to VAERS data . This is a higher rate than most other vaccines—including annual flu shots, which trigger anaphylaxis for only one out of every million doses administered. For the Oxford–AstraZeneca vaccine, 30 cases of anaphylaxis have been confirmed overall so far, out of a little more than 3 million administered doses. Vaccine specialists expect that these rates might change as more shots are administered.
Although some people have required hospitalization, all have fully recovered. Public-health officials advise people with a history of allergies to any of the vaccines’ ingredients not to get a COVID-19 jab.
Unlike COVID-19, anaphylaxis is treatable with drugs such as epinephrine if caught quickly, says Paul Offit, a vaccine and infectious-disease specialist at the Children’s Hospital of Philadelphia in Pennsylvania, who participated in the US Food and Drug Administration advisory-committee meetings that led the agency to authorize both mRNA vaccines. “I wish that SARS-CoV-2 could be immediately treated with a shot of epinephrine!” he says.
Most of the people who experienced anaphylaxis had reacted to other substances before: about 80% of people who reacted to the Pfizer–BioNTech vaccine, and 86% to the Moderna vaccine, had a history of allergies, according to the US Centers for Disease Control and Prevention.
The specific cause of the anaphylactic reactions remains unknown, but the US National Institute of Allergy and Infectious Diseases told Nature in an e-mail that the agency has designed a clinical trial to determine the underlying mechanism, but did not specify when the trial would begin.
What could be causing the allergic reactions?
Some researchers have had their eye on polyethylene glycol (PEG) as the anaphylaxis-causing agent in the mRNA vaccines. The Moderna and Pfizer–BioNTech vaccines use hollow lipid nanoparticles to store and then deliver their mRNA payload to cells. PEG is linked to the lipids in these particles and, under normal circumstances, helps them to sneak by the immune system. Although PEG-linked molecules are found in a variety of products, such as laxatives and gout medicines, they have been known to cause allergic reactions.
Follow-up studies in people who experienced anaphylaxis could help to determine whether PEG is the culprit, says Samuel Lai, a pharmaco-engineer at the University of North Carolina at Chapel Hill. If blood samples from these people contain anti-PEG antibodies, it could be an indicator, says Lai, but it is as yet unclear how long these proteins remain in the bloodstream after anaphylaxis.
Vaccines that don’t use PEG—such as the not-yet-authorized shot from Johnson & Johnson, which also uses an adenovirus to trigger immunity to the coronavirus—might be a way to vaccinate people with a sensitivity to the polymer, he adds.
Because mRNA vaccines have shown such promise, Ulrich Schubert, a polymer scientist at the University of Jena in Germany, thinks now is the time to invest in developing vaccine-compatible polymers that don’t cause allergic reactions. At the German Research Foundation-funded collaborative research center PolyTarget, where Schubert works, these studies are already in progress. “If we want to be ready for the next pandemic—which will come—we have to start now,” he says.
This article is reproduced with permission and was first published on February 16 2021.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS EXPLAINER
- 16 February 2021
COVID vaccines and safety: what the research says
- Ariana Remmel
You can also search for this author in PubMed Google Scholar
As people around the world receive COVID-19 vaccines, reports of temporary side effects such as headaches and fevers are rolling in. Much of this was expected — clinical-trial data for the vaccines authorized so far suggested as much. But now that millions of people are vaccinated, compared with the thousands enrolled in early studies, reports of some rare, allergic reactions are surfacing, and questions are arising about whether any deaths are linked to the shots.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 590 , 538-540 (2021)
doi: https://doi.org/10.1038/d41586-021-00290-x
With reporting from Smriti Mallapaty and Amy Maxmen.
Logunov, D. Y. et al. Lancet https://doi.org/10.1016/S0140-6736(21)00234-8 (2021).
Article Google Scholar
McNeil, M. M. et al. J. Allergy Clin. Immunol . 2 , T37–T52 (2016).
Article PubMed Google Scholar
Yang, Q. et al. Anal. Chem . 88 , 11804–11812 (2016).
Download references
Reprints and permissions
Related Articles

- Public health
- Health care

Bird flu could become a human pandemic. How are countries preparing?
News 12 JUL 24
Vaccines save lives: how can uptake be increased?
Editorial 09 JUL 24

Combined COVID–flu vaccines are coming: Moderna jab clears major test
News 28 JUN 24

Spike deep mutational scanning helps predict success of SARS-CoV-2 clades
Article 03 JUL 24
First encounter with SARS-CoV-2: immune portraits of COVID susceptibility
News & Views 19 JUN 24

These period pads solidify blood to prevent leaks
News 10 JUL 24
If bird flu sparks a human pandemic, your past immunity could help
News 09 JUL 24
Postdoctoral Associate- Translational Research
Houston, Texas (US)
Baylor College of Medicine (BCM)
2024 Recruitment notice Shenzhen Institute of Synthetic Biology: Shenzhen, China
The wide-ranging expertise drawing from technical, engineering or science professions...
Shenzhen,China
Shenzhen Institute of Synthetic Biology
Tenure-Track Assistant Professor, Associate Professor, and Professor
Westlake Center for Genome Editing seeks exceptional scholars in the many areas.
Westlake Center for Genome Editing, Westlake University
Postdoctral Researcher for microbial electron transfer study
Elucidation and engineering of biological electron transfer in biofilm for medical and environmental applications.
NIMS is at Tsukuba (Ibaraki) by Tokyo (JP)
National Institute for Materials Science (NIMS)
DIRECTOR AT THE FUNDACIÓ CENTRE DE REGULACIÓ GENÒMICA
The Institute The Centre for Genomic Regulation (CRG) is an international biomedical research institute of excellence, based in Barcelona, Spain, w...
Barcelona (Localidad), Cataluña (ES)
CRG-Centre for Genomic Regulation
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
How Pfizer Makes Its Covid-19 Vaccine
By Emma Cott , Elliot deBruyn and Jonathan Corum April 28, 2021
- Share full article
Inside this facility in Chesterfield, Missouri, trillions of bacteria are producing tiny loops of DNA containing coronavirus genes — the raw material for the Pfizer-BioNTech vaccine .
It’s the start of a complex manufacturing and testing process that takes 60 days and involves Pfizer facilities in three states. The result will be millions of doses of the vaccine, frozen and ready to ship.
Pull DNA from Cold Storage
A scientist removes vials of DNA from the master cell bank, the source of every batch of Pfizer’s Covid-19 vaccine. The vials are kept at –150°C (–238°F) or below, and contain small rings of DNA called plasmids.

Coronavirus
spike protein
spike proteins

Each plasmid contains a coronavirus gene, the genetic instructions for a human cell to build coronavirus proteins and trigger an immune response to the virus.
Scientists thaw the plasmids and modify a batch of E. coli bacteria to take the plasmids inside their cells.

A single vial can eventually produce up to 50 million doses of the vaccine.
Grow the Cells
The vial of modified bacteria is swirled into a flask of amber-colored growth medium, a sterile and warm environment that encourages the bacteria to multiply.

Multiplying

Ferment the Mixture
The bacteria are allowed to grow overnight and then moved into a large fermenter that contains up to 300 liters of a nutrient broth.

The bacterial broth spends four days in the fermenter, multiplying every 20 minutes and making trillions of copies of the DNA plasmids.
Harvest and Purify the DNA
When the fermentation is complete, scientists add chemicals to break open the bacteria and release the plasmids from their enclosing cells.

The mixture is then purified to remove the bacteria and leave only the plasmids.
Test for Quality
The plasmids are tested for purity, and compared against previous samples to confirm that the coronavirus gene sequence has not changed.

for comparison

Cut the Plasmids
If the plasmids pass the quality checks, proteins called enzymes are added to the mixture. The enzymes cut the circular plasmids and separate the coronavirus genes into straight segments, a process called linearization that takes about two days.

cutting the

Filter the DNA
Any remaining bacteria or plasmid fragments are filtered out, leaving one-liter bottles of purified DNA.

The DNA sequences are tested again, and will serve as templates for the next stage of the process. Each bottle of DNA will produce about 1.5 million doses of the vaccine.
The Chesterfield facility is Pfizer’s only source of plasmids for its Covid-19 vaccine. But finishing the vaccine requires several more steps in two other facilities.
Freeze, Pack and Ship
Each bottle of DNA is frozen, bagged, sealed and packed with a small monitor that will record its temperature in transit.
Up to 48 bottles are packed in a container with enough dry ice to keep them frozen at –20°C (–4°F) . The containers are locked to prevent tampering and shipped to a Pfizer research and manufacturing facility in Andover, Mass.
The Andover plant will process the DNA into messenger RNA, or mRNA, the active ingredient of the Pfizer-BioNTech vaccine.

Chesterfield

Other bottles are flown to BioNTech facilities in Mainz, Germany, where they are processed for Europe and other markets.
Transcribe the DNA into mRNA
Inside the Andover facility, yellow walls mark the mRNA suite. Five bottles of DNA are thawed for a day, then mixed with the building blocks of messenger RNA.
Over several hours, enzymes pry open the DNA templates and transcribe them into strands of mRNA. The finished vaccine will carry the mRNA into human cells, which will read the coronavirus gene and begin producing coronavirus proteins.

Transcribing
DNA into mRNA

The mixture is moved into a holding tank, then filtered to remove any unwanted DNA, enzymes or other impurities. Each batch will eventually yield up to 7.5 million doses of the vaccine.
Test the mRNA
The Pfizer-BioNTech vaccine was the first mRNA vaccine to be authorized for emergency use in people.
Analytical scientists repeatedly test the filtered mRNA to verify its purity and confirm the genetic sequence is correct.

The result is 10 bags of coronavirus mRNA. Each bag holds 16 liters and represents the raw material for about 750,000 doses of the vaccine.
Freeze, Pack and Ship (Again)
The bags of mRNA are frozen to –20°C (–4°F) and shipped to a Pfizer facility in Kalamazoo, Mich., where they will be processed into the finished vaccine and packaged in vials. Samples are also sent back to Pfizer’s Chesterfield facility, where they are tested again.

The Andover plant can produce two batches of mRNA a week, each about 10 bags. The facility made its first test batch last July, and recently doubled its mRNA capacity by adding a second suite.
A parallel process in Mainz, Germany, processes DNA from the Chesterfield facility and sends bags of filtered mRNA to Puurs, Belgium.
Prepare the mRNA
The Kalamazoo facility receives the bags of mRNA, keeps them frozen until needed and then thaws enough to produce 3.6 million doses of the vaccine, or 600,000 vials.
The thawed mRNA is mixed with water in preparation for making the vaccine.
Prepare the Lipids
In a separate process, scientists prepare the oily lipids that will protect the mRNA and help it enter human cells.
The lipids are measured out and mixed with ethanol, which will eventually be removed from the finished vaccine.

Assemble the mRNA Vaccine
A rack of 16 pumps precisely controls the flow of the mRNA and lipid solutions, then mixes them together to create lipid nanoparticles.

Lipids enveloping
nanoparticles

When the lipids come into contact with the naked strands of mRNA, electric charge pulls them together in a nanosecond. The mRNA is enveloped in several layers of lipids, forming an oily, protective vaccine particle.
Synchronizing eight pairs of pumps is not an ideal solution, but Pfizer engineers chose to scale up existing technology instead of trying to build a larger, unproven type of precision mixing device.
The newly made vaccine is filtered to remove the ethanol, concentrated and filtered again to remove any impurities, and finally sterilized.
Prepare the Vials
Hundreds of thousands of empty vials are washed and heat sterilized.
A set of 13 cameras performs a high-speed visual inspection, taking more than 100 photographs of each vial. Any vials with cracks, chips or other imperfections are removed from the line.
A separate machine puts each vial under vacuum to confirm it doesn’t leak.
Rush to Fill the Vials
The flow of vials is narrowed to a single-file line. Machines inject 0.45 ml of a concentrated vaccine solution into each vial, enough for six doses after dilution. The vials are sealed with foil and capped with purple lids, at a pace of up to 575 vials per minute. (The footage above shows a test run, with empty vials.)

The vaccine is chilled but warms up quickly during the bottling process, and the mRNA will deteriorate if left unfrozen for too long. Kalamazoo has limited time, about 46 hours, to get the liquid vaccine into vials and then into deep freeze.
Package, Freeze and Test
The filled vials are inspected again, and then labeled and packed into “pizza boxes,” small plastic trays that hold 195 vials each.
The trays are bundled in stacks of five and loaded into one of 350 industrial freezers. Each freezer holds 300 trays.
It takes a couple of days for the vaccine to reach the –70°C (–94°F) required for long-term storage, and each freezer is tested to ensure that every shelf can maintain that ultracold temperature.
Once frozen, the vials of vaccine are held for four weeks of testing. Samples are sent back to the Andover facility that produced the mRNA, and to the Chesterfield site that provided the DNA templates.

Pfizer currently operates on a 60-day timeline from start to finish, and more than half of that time is dedicated to testing.
Pack and Ship the Finished Vaccine
After weeks of testing, the vaccine is ready to ship. Workers pull trays from the freezers and pack them in shipping boxes with temperature and location sensors. The minimum order is one tray of 195 vials, and a box holds up to five trays.
Each box contains 45 pounds of dry ice — so much that Pfizer’s Kalamazoo facility now makes dry ice on site. Pfizer is also evaluating different formulations of the vaccine, including freeze-dried and ready-to-use versions that would not require ultracold storage.
Commercial production of the vaccine began in September. As of April 22, the plant had delivered more than 150 million vaccine doses to the United States. Pfizer expects to deliver 220 million doses by the end of May, and 300 million by mid-July.
THE LAST STEP
Administer the vaccine.
Some 141 million people in the United States — more than half of the nation’s adults — have received at least one dose of a Covid-19 vaccine. More than a billion doses have been administered worldwide.
The City of Los Angeles hosts a mass vaccination site at Dodger Stadium, above. On Feb. 5, health care workers gave thousands of shots of the Moderna vaccine , which also uses mRNA to build immunity. (Moderna declined to provide filming access to their facilities.)
The single-dose Johnson & Johnson vaccine uses an adenovirus to carry DNA into human cells. A facility in Baltimore run by Emergent BioSolutions had to throw out up to 15 million doses of Johnson & Johnson’s vaccine because of possible contamination.
A Vaccine for Variants
Many of the coronavirus variants now in circulation have key mutations in their spike proteins that help the virus bind more tightly to human cells or evade some kinds of antibodies.
Pfizer and BioNTech are developing and testing new versions of their vaccine against recent variants, and might eventually alter their genetic recipe to mass-produce Covid-19 vaccines that target specific variants.

To do that, Pfizer would go back to where their vaccine production began, to the master cell bank in Chesterfield that keeps rings of DNA in deep freeze.
A new batch of DNA carrying modified coronavirus genes could eventually produce a slightly different vaccine, one that encourages the immune system to better recognize recent coronavirus mutations.
Chesterfield video by Elliot deBruyn. Dodger Stadium video by Luisa Conlon. Video editing by Meg Felling. Cinematography directed by Jonah M. Kessel. Additional video production by Alexandra Eaton and Sarah Kerr.
Andover and Kalamazoo video provided by Pfizer, with some barcodes and other identifying information obscured.
Advertisement
Jump to navigation
- Bahasa Malaysia

What are the benefits and risks of vaccines for preventing COVID-19?
Key messages
– Most vaccines reduce, or probably reduce, the number of people who get COVID-19 disease and severe COVID-19 disease.
– Many vaccines likely increase number of people experiencing events such as fever or headache compared to placebo (sham vaccine that contains no medicine but looks identical to the vaccine being tested). This is expected because these events are mainly due to the body's response to the vaccine; they are usually mild and short-term.
– Many vaccines have little or no difference in the incidence of serious adverse events compared to placebo.
– There is insufficient evidence to determine whether there was a difference between the vaccine and placebo in terms of death because the numbers of deaths were low in the trials.
– Most trials assessed vaccine efficacy over a short time, and did not evaluate efficacy to the COVID variants of concern.
What is SARS-CoV-2 and COVID-19?
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is the virus that causes COVID-19 disease. Not everyone infected with SARS-CoV-2 will develop symptoms of COVID-19. Symptoms can be mild (e.g. fever and headaches) to life-threatening (e.g. difficulty breathing), or death.
How do vaccines prevent COVID-19?
While vaccines work slightly differently, they all prepare the body's immune system to prevent people from getting infected with SARS-CoV-2 or, if they do get infected, to prevent severe disease.
What did we want to find out?
We wanted to find out how well each vaccine works in reducing SARS-CoV-2 infection, COVID-19 disease with symptoms, severe COVID-19 disease, and total number of deaths (including any death, not only those related to COVID-19).
We wanted to find out about serious adverse events that might require hospitalization, be life-threatening, or both; systemic reactogenicity events (immediate short-term reactions to vaccines mainly due to immunological responses; e.g. fever, headache, body aches, fatigue); and any adverse events (which include non-serious adverse events).
What did we do?
We searched for studies that examined any COVID-19 vaccine compared to placebo, no vaccine, or another COVID-19 vaccine.
We selected only randomized trials (a study design that provides the most robust evidence because they evaluate interventions under ideal conditions among participants assigned by chance to one of two or more groups). We compared and summarized the results of the studies, and rated our confidence in the evidence based on factors such as how the study was conducted.
What did we find?
We found 41 worldwide studies involving 433,838 people assessing 12 different vaccines. Thirty-five studies included only healthy people who had never had COVID-19. Thirty-six studies included only adults, two only adolescents, two children and adolescents, and one included adolescents and adults. Three studied people with weakened immune systems, and none studied pregnant women.
Most cases assessed results less than six months after the primary vaccination. Most received co-funding from academic institutions and pharmaceutical companies. Most studies compared a COVID-19 vaccine with placebo. Five evaluated the addition of a 'mix and match' booster dose.
Main results
We report below results for three main outcomes and for 10 World Health Organization (WHO)-approved vaccines (for the remaining outcomes and vaccines, see main text). There is insufficient evidence regarding deaths between vaccines and placebo (mainly because the number of deaths was low), except for the Janssen vaccine, which probably reduces the risk of all-cause deaths.
People with symptoms
The Pfizer, Moderna, AstraZeneca, Sinopharm-Beijing, and Bharat vaccines produce a large reduction in the number of people with symptomatic COVID-19.
The Janssen vaccine reduces the number of people with symptomatic COVID-19.
The Novavax vaccine probably has a large reduction in the number of people with symptomatic COVID-19.
There is insufficient evidence to determine whether CoronaVac vaccine affects the number of people with symptomatic COVID-19 because results differed between the two studies (one involved only healthcare workers with a higher risk of exposure).
Severe disease
The Pfizer, Moderna, Janssen, and Bharat vaccines produce a large reduction in the number of people with severe disease.
There is insufficient evidence about CoronaVac vaccine on severe disease because results differed between the two studies (one involved only healthcare workers with a higher risk of exposure).
Serious adverse events
For the Pfizer, CoronaVac, Sinopharm-Beijing, and Novavax vaccines, there is insufficient evidence to determine whether there was a difference between the vaccine and placebo mainly because the number of serious adverse events was low.
Moderna, AstraZeneca, Janssen, and Bharat vaccines probably result in no or little difference in the number of serious adverse events.
What are the limitations of the evidence?
Most studies assessed the vaccine for a short time after injection, and it is unclear if and how vaccine protection wanes over time. Due to the exclusion criteria of COVID-19 vaccine trials, results cannot be generalized to pregnant women, people with a history of SARS-CoV-2 infection, or people with weakened immune systems. More research is needed comparing vaccines and vaccine schedules, and effectiveness and safety in specific populations and outcomes (e.g. preventing long COVID-19). Further, most studies were conducted before the emergence of variants of concerns.
How up to date is this evidence?
The evidence is up to date to November 2021. This is a living systematic review. Our results are available and updated bi-weekly on the COVID-NMA platform at covid-nma.com.
Compared to placebo, most vaccines reduce, or likely reduce, the proportion of participants with confirmed symptomatic COVID-19, and for some, there is high-certainty evidence that they reduce severe or critical disease. There is probably little or no difference between most vaccines and placebo for serious adverse events. Over 300 registered RCTs are evaluating the efficacy of COVID-19 vaccines, and this review is updated regularly on the COVID-NMA platform ( covid-nma.com ).
Implications for practice
Due to the trial exclusions, these results cannot be generalized to pregnant women, individuals with a history of SARS-CoV-2 infection, or immunocompromized people. Most trials had a short follow-up and were conducted before the emergence of variants of concern.
Implications for research
Future research should evaluate the long-term effect of vaccines, compare different vaccines and vaccine schedules, assess vaccine efficacy and safety in specific populations, and include outcomes such as preventing long COVID-19. Ongoing evaluation of vaccine efficacy and effectiveness against emerging variants of concern is also vital.
Different forms of vaccines have been developed to prevent the SARS-CoV-2 virus and subsequent COVID-19 disease. Several are in widespread use globally.
To assess the efficacy and safety of COVID-19 vaccines (as a full primary vaccination series or a booster dose) against SARS-CoV-2.
We searched the Cochrane COVID-19 Study Register and the COVID-19 L·OVE platform (last search date 5 November 2021). We also searched the WHO International Clinical Trials Registry Platform, regulatory agency websites, and Retraction Watch.
We included randomized controlled trials (RCTs) comparing COVID-19 vaccines to placebo, no vaccine, other active vaccines, or other vaccine schedules.
We used standard Cochrane methods. We used GRADE to assess the certainty of evidence for all except immunogenicity outcomes.
We synthesized data for each vaccine separately and presented summary effect estimates with 95% confidence intervals (CIs).
We included and analyzed 41 RCTs assessing 12 different vaccines, including homologous and heterologous vaccine schedules and the effect of booster doses. Thirty-two RCTs were multicentre and five were multinational. The sample sizes of RCTs were 60 to 44,325 participants. Participants were aged: 18 years or older in 36 RCTs; 12 years or older in one RCT; 12 to 17 years in two RCTs; and three to 17 years in two RCTs. Twenty-nine RCTs provided results for individuals aged over 60 years, and three RCTs included immunocompromized patients. No trials included pregnant women. Sixteen RCTs had two-month follow-up or less, 20 RCTs had two to six months, and five RCTs had greater than six to 12 months or less. Eighteen reports were based on preplanned interim analyses.
Overall risk of bias was low for all outcomes in eight RCTs, while 33 had concerns for at least one outcome.
We identified 343 registered RCTs with results not yet available.
This abstract reports results for the critical outcomes of confirmed symptomatic COVID-19, severe and critical COVID-19, and serious adverse events only for the 10 WHO-approved vaccines. For remaining outcomes and vaccines, see main text. The evidence for mortality was generally sparse and of low or very low certainty for all WHO-approved vaccines, except AD26.COV2.S (Janssen), which probably reduces the risk of all-cause mortality (risk ratio (RR) 0.25, 95% CI 0.09 to 0.67; 1 RCT, 43,783 participants; high-certainty evidence).
Confirmed symptomatic COVID-19
High-certainty evidence found that BNT162b2 (BioNtech/Fosun Pharma/Pfizer), mRNA-1273 (ModernaTx), ChAdOx1 (Oxford/AstraZeneca), Ad26.COV2.S, BBIBP-CorV (Sinopharm-Beijing), and BBV152 (Bharat Biotect) reduce the incidence of symptomatic COVID-19 compared to placebo (vaccine efficacy (VE): BNT162b2: 97.84%, 95% CI 44.25% to 99.92%; 2 RCTs, 44,077 participants; mRNA-1273: 93.20%, 95% CI 91.06% to 94.83%; 2 RCTs, 31,632 participants; ChAdOx1: 70.23%, 95% CI 62.10% to 76.62%; 2 RCTs, 43,390 participants; Ad26.COV2.S: 66.90%, 95% CI 59.10% to 73.40%; 1 RCT, 39,058 participants; BBIBP-CorV: 78.10%, 95% CI 64.80% to 86.30%; 1 RCT, 25,463 participants; BBV152: 77.80%, 95% CI 65.20% to 86.40%; 1 RCT, 16,973 participants).
Moderate-certainty evidence found that NVX-CoV2373 (Novavax) probably reduces the incidence of symptomatic COVID-19 compared to placebo (VE 82.91%, 95% CI 50.49% to 94.10%; 3 RCTs, 42,175 participants).
There is low-certainty evidence for CoronaVac (Sinovac) for this outcome (VE 69.81%, 95% CI 12.27% to 89.61%; 2 RCTs, 19,852 participants).
Severe or critical COVID-19
High-certainty evidence found that BNT162b2, mRNA-1273, Ad26.COV2.S, and BBV152 result in a large reduction in incidence of severe or critical disease due to COVID-19 compared to placebo (VE: BNT162b2: 95.70%, 95% CI 73.90% to 99.90%; 1 RCT, 46,077 participants; mRNA-1273: 98.20%, 95% CI 92.80% to 99.60%; 1 RCT, 28,451 participants; AD26.COV2.S: 76.30%, 95% CI 57.90% to 87.50%; 1 RCT, 39,058 participants; BBV152: 93.40%, 95% CI 57.10% to 99.80%; 1 RCT, 16,976 participants).
Moderate-certainty evidence found that NVX-CoV2373 probably reduces the incidence of severe or critical COVID-19 (VE 100.00%, 95% CI 86.99% to 100.00%; 1 RCT, 25,452 participants).
Two trials reported high efficacy of CoronaVac for severe or critical disease with wide CIs, but these results could not be pooled.
Serious adverse events (SAEs)
mRNA-1273, ChAdOx1 (Oxford-AstraZeneca)/SII-ChAdOx1 (Serum Institute of India), Ad26.COV2.S, and BBV152 probably result in little or no difference in SAEs compared to placebo (RR: mRNA-1273: 0.92, 95% CI 0.78 to 1.08; 2 RCTs, 34,072 participants; ChAdOx1/SII-ChAdOx1: 0.88, 95% CI 0.72 to 1.07; 7 RCTs, 58,182 participants; Ad26.COV2.S: 0.92, 95% CI 0.69 to 1.22; 1 RCT, 43,783 participants); BBV152: 0.65, 95% CI 0.43 to 0.97; 1 RCT, 25,928 participants). In each of these, the likely absolute difference in effects was fewer than 5/1000 participants.
Evidence for SAEs is uncertain for BNT162b2, CoronaVac, BBIBP-CorV, and NVX-CoV2373 compared to placebo (RR: BNT162b2: 1.30, 95% CI 0.55 to 3.07; 2 RCTs, 46,107 participants; CoronaVac: 0.97, 95% CI 0.62 to 1.51; 4 RCTs, 23,139 participants; BBIBP-CorV: 0.76, 95% CI 0.54 to 1.06; 1 RCT, 26,924 participants; NVX-CoV2373: 0.92, 95% CI 0.74 to 1.14; 4 RCTs, 38,802 participants).
For the evaluation of heterologous schedules, booster doses, and efficacy against variants of concern, see main text of review.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Safety of COVID-19 Vaccines
On June 27, 2024, the CDC Director adopted the ACIP’s recommendations for use of 2024–2025 COVID-19 vaccines in people ages 6 months and older as approved or authorized by FDA. The 2024–2025 vaccines are expected to be available in fall 2024. This page will be updated at that time to align with the new recommendations. Learn more: www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html
What You Need to Know
- COVID-19 vaccines are safe and effective .
- During the COVID-19 pandemic , hundreds of m illions of people in the United States received COVID-19 vaccines under the most intense safety monitoring in U . S . history.
- CDC, the U.S. Food and Drug Administration (FDA), and other federal agencies continue to monitor the safety of the updated COVID-19 vaccines and will share information with the public as it becomes available.
- CDC recommends everyone ages 6 months and older get an updated COVID-19 vaccine to protect against serious illness.
Everyone 6 months and older should receive a COVID-19 Vaccine
COVID-19 vaccines are safe and effective . The safety of COVID-19 vaccines has been rigorously monitored and evaluated since their emergency use authorization (EUA) in December 2020. T he updated mRNA COVID-19 vaccines f or 2023-2024 are manufactured using a similar process to the previous vaccines .
Learn more about EUAs in this video.
As with all vaccines authorized or approved for use in the U.S., CDC , FDA and other federal agencies will continue to closely monitor the safety of the updated 2023-2024 COVID-19 vaccines using multiple vaccine safety monitoring systems and will share information with the public as it becomes available.
Common Side Effects
Some people have side effects after COVID-19 vaccin ation , while others might have no side effects. Side effects tend to be mild , such as soreness at the injection site and fever , and should go away within a few days. Learn more about common side effects after COVID-19 vaccination .
Adverse Events (Health Problems) Are Rare
In rare cases, people have experienced mo re significant adverse events after COVID-19 vaccination. Any health problem that happens after vaccination is considered an adverse event. An adverse event can be caused by the vaccine or can be caused by a coincidental event not related to the vaccine, such as an unrelated fever, that happened following vaccination.
Benefits of COVID-19 Vaccination Outweigh the Risks
COVID-19 vaccination continues to be the best way to protect against serious illness. The benefits of COVID-19 vaccination outweigh the known and potential risks. CDC, the U.S. Food and Drug Administration (FDA), and other federal agencies continue to monitor the safety of the updated COVID-19 vaccines and will share information with the public as it becomes available.
Have you experienced a side effect following COVID-19 vaccination?
Please report it to VAERS . In addition, enrolling yourself or your dependent in V-safe allows you to easily report to CDC how you are feeling after getting a COVID-19 vaccine.
More Information
- ACIP COVID-19 Vaccine Safety Technical (VaST) Work Group Reports
- COVID-19 Vaccine Safety Publications
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 vaccine: A 2021 analysis of perceptions on vaccine safety and promise in a U.S. sample
Roles Conceptualization, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Global Health, Indiana University School of Medicine, Indianapolis, Indiana, United States of America
Roles Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing
Affiliation Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia, United States of America
Roles Investigation, Methodology, Validation, Visualization, Writing – review & editing
Affiliation Department of Psychiatry, Harvard Medical School, Boston, Massachusetts, United States of America
Roles Data curation, Formal analysis, Software, Visualization, Writing – review & editing
Affiliation Department of Biostatistics and Health Data Science, Indiana University School of Medicine, Indianapolis, Indiana, United States of America
Roles Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing
Affiliation Department of Global Health, Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, Indiana, United States of America
- Vitalis C. Osuji,
- Eric M. Galante,
- David Mischoulon,
- James E. Slaven,
- Gerardo Maupome

- Published: May 19, 2022
- https://doi.org/10.1371/journal.pone.0268784
- Reader Comments
Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine.
Study participants in the U.S. (79% female, median age group 46–60 years) were recruited through an online Qualtrics survey distributed as a Facebook advertisement from 3/19/21–4/30/21. We assumed that every participant is at risk of COVID-19 infection and should be able to get the vaccine with proper access. Bivariate and multivariable models were performed. Collinearity between variables was assessed.
A total of 2,626 responses were generated and 2,259 were included in data analysis. According to our multivariate model analysis, vaccines were perceived as safe by those who had or planned to obtain full vaccination (adjusted odds ratio (aOR) (95% confidence interval) = 40.0 (19.0, 84.2); p< 0.0001) and those who indicated trust in science (aOR = 10.5 (5.1, 21.8); p< 0.0001); vaccines were perceived as not safe by those who self-identified as Republicans vs. self-identified Democrats (aOR = 0.2 (0.1, 0.5); p = 0.0020) and those with high school or lower education (aOR = 0.2 (0.1, 0.4); p = 0.0007). Similarly, according to our multivariate model analysis, the following groups were most likely to reject vaccination based on belief in vaccinations: those with lower income (aOR = 0.8 (0.6, 0.9); p = 0.0106), those who do not know anyone who had been vaccinated (aOR = 0.1 (0.1, 0.4); p< 0.0001), those who are unwilling to get vaccinated even if family and friends had done so (aOR = 0.1 (<0.1, 0.2); p< 0.0001), those who did not trust science (aOR < 0.1 (<0.1, 0.1); p< 0.0001), those who believe that vaccination was unnecessary if others had already been vaccinated (aOR = 2.8 (1.5, 5.1); p = 0.0007), and those who indicate refusal to vaccinate to help others (aOR = 0.1 (0.1, 0.2); p< 0.0001). An alpha of p<0.05 was used for all tests.
Level of education and partisanship, but not race/ethnicity, were the most likely factors associated with vaccine hesitancy or likelihood to vaccinate. Also, low vaccination rates among underrepresented minorities may be due to distrust for healthcare industries. Population sub-groups less likely to be vaccinated and/or receptive to vaccines should be targeted for vaccine education and incentives.
Citation: Osuji VC, Galante EM, Mischoulon D, Slaven JE, Maupome G (2022) COVID-19 vaccine: A 2021 analysis of perceptions on vaccine safety and promise in a U.S. sample. PLoS ONE 17(5): e0268784. https://doi.org/10.1371/journal.pone.0268784
Editor: Weijing He, University of Texas Health Science Center at San Antonio, UNITED STATES
Received: July 26, 2021; Accepted: May 8, 2022; Published: May 19, 2022
Copyright: © 2022 Osuji et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
In early 2020, the SARS-CoV-2 (COVID-19) pandemic unmasked the many flaws that healthcare systems faced worldwide. While some of these issues were difficult to predict, such as the feasibility of pandemic response protocols or federal government regulations to be activated [ 1 ], other healthcare issues were to be expected, especially in the United States. For example, disparities in healthcare treatment and outcomes derived from different socioeconomic factors. Studies published in 2020 showed that the pandemic had much higher infection rates in minority populations such as Black and Hispanic/Latinx compared to their white counterparts; American Indians/ Alaska Natives (AI/ ANs), Black and Hispanic/Latinx communities also experienced significantly higher mortality rates [ 2 , 3 ]. The Centers for Disease Control and Prevention (CDC) released information relating social determinants of health to poorer COVID-19 outcomes, stating that “factors such as discrimination, neighborhood and physical environment, housing, occupation, education, income, and wealth gaps put some racial and ethnic minority groups at increased risk of severe illness from COVID-19, including death” [ 4 ]. Many factors play a role in disparities relevant to the COVID-19 pandemic. These include limited access to health services, education, and transportation, which tend to affect more severely communities of color and people of low socioeconomic status [ 5 ].
Just under one year after the first identification of COVID-19 in China [ 6 , 7 ], the PfizerBioNTech and Moderna COVID-19 vaccines were approved by the US Food and Drug Administration (FDA) under Emergency Use Authorization [ 8 , 9 ]. Ultimately, the Pfizer vaccine was fully approved as of August 23 rd , 2021. These vaccines represented a major milestone in vaccine production history, as no other vaccine had ever been created so rapidly with such positive results [ 10 ]. Although mistrust of vaccines is not uncommon in American culture, hesitation regarding the COVID-19 vaccines may be among the strongest yet [ 11 ]. Despite substantial evidence-based research supporting the vaccines’ safety and efficacy, there are lay public concerns regarding the vaccine rollout. For instance, an analysis [ 12 ] from March 2021 in individuals getting vaccines showed that white Americans were receiving vaccinations at a rate two times that of Black Americans, and the gap for Hispanic/Latinx was even larger. The rationale behind these gaps between racial/ethnic groups remains uncertain and highlights the importance of characterizing the factors and mechanisms underlying potential associations amongst demographic and socioeconomic groups.
With the current vaccines showing 95% efficacy, the estimated percentage of Americans needing vaccination to reach herd immunity ranges from 60 to 72% [ 13 ]. However, according to a November 2020 survey [ 14 ], 40% of Americans said that they will “definitely not” or “probably not” get the COVID-19 vaccine when it becomes available to them. Therefore, more needs to be done to bolster interest and trust in the vaccines. While companies and governmental organizations attempt to convey the necessary strategies to ease vaccine uncertainty and hesitation, a large segment of the lay public remains skeptical. As of May 2021, there were state-level COVID-19 vaccine incentives developed to increase vaccination rates across the United States. Irrespective of these incentives, only 48.6% of the US population was fully vaccinated as of July 2021, while 56% had received at least one dose [ 15 ]. Given these data, reasons surrounding vaccination hesitancy needed to be further explored. We aimed to expand current knowledge about COVID-19 vaccine perceptions through a characterization of sociocultural, socioeconomic, and demographic features in the context of opinions about receiving a COVID-19 vaccine. The objectives of the present survey were to establish:
- What segments of the population believe the COVID-19 vaccines to be safe?
- What are the perceived barriers to obtaining the COVID-19 vaccine—for self and others?
- Is there an association between individual sociocultural characteristics and either acceptance or rejection of the vaccine?
- Is there an association between individual demographic characteristics and either acceptance or rejection of the vaccine?
Materials and methods
This research project was granted IRB approval by Indiana University (protocol #10670).
Data collection was done using an online survey distributed to the general public, and our methodology followed criteria from the CHERRIES checklist [ 16 ]. The survey was created using Qualtrics and piloted with 15 respondents. Based on responses and feedback from our iterative process to pilot the survey, questions were added, rephrased, or deleted. The final survey had 37 questions, with 1–6 questions per page. Question format included 28 multiple choices, with the remainder as yes/no questions. Both English and Spanish versions of the survey were available. A description of the ethical approval, anonymity, and data utilization was provided and acknowledged at the beginning of the survey. Personal information was not required, and participants were offered the option to enter an email address if they wished to participate in an optional raffle draw for five $20 Walmart gift cards. All data were stored in a secure password protected website, to which only study investigators had access. A completeness check prior to submission was not implemented, but a forced response feature on Qualtrics was used for all questions except those involving zip code and email address, to ensure that no significant questions were left unanswered. A link to the final version of the survey was posted to a Facebook page created for the study, and Facebook advertisements were used to promote the study. The survey was made available on March 19 th , 2021 and was closed on April 30 th , 2021. The final data collection survey is available as an attachment ( S1 File ).
This was a survey open to every Facebook user in the United States, based on the assumption that every adult was at risk of COVID-19 infection and should theoretically be able to get the vaccine. We limited responses to people stating they were at least 18-years old and able to read, understand, and agree to the terms of the online survey. Bivariate associations were evaluated using Mantel-Haenszel chi-square tests for questions where one or both variables had ordered categorical responses, and Pearson chi-square tests if both variables had nominal categories. Multivariable models were also performed, using an a priori p-value cut point of 0.20 for inclusion in the model. Collinearity between variables was assessed, leading to the exclusion of several variables from each multivariable model, retaining those based on statistical analysis and the team’s clinical experience. For ease of analysis, race was grouped into 2 categories: white and underrepresented minority. Low income was categorized based on respondents who indicated making less than $40,000 in annual income. The final level of significance for these multivariable models was set at p < 0.05.
All analytic assumptions were verified, and the analyses were performed using SAS/STAT software ® v9.4 [ 17 ].
A total of 2,626 responses were obtained. Based on a total of 3,743 potential participants who clicked our survey link on Facebook, our completion rate was 70.2%. Following data cleaning and exclusion of incomplete responses, a total of 2,259 responses were evaluable.
As outlined in Table 1 , most participants were under 60 years of age (61.5%; median age in the 46–60 years group), female (79.2%) and white (89.6%). Most had never been employed in the healthcare field (63.4%), some were employed full time (44.5%), many had at least some college education (93.1%), about half were affiliated with the Democratic party (54.7%), and many lived within family households (75.7%).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0268784.t001
To determine what groups perceived the vaccine as safe, bivariate and multivariable models were created. Table 2 shows that subjects who perceived the vaccination as being safe were more likely to have already obtained their second dose or planned on getting it (we allowed for single shot vaccines in our analyses) (97% vs. 12%; p< 0.0001), did not have a prior health condition (98% vs. 86%; p< 0.0001), trusted science (97.1% vs. 21%; p< 0.0001)/vaccines (97% vs. 17%; p< 0.0001)/doctors (97% vs. 21%; p< 0.0001), believed in the effectiveness of hand washing (94% vs. 88%; p = 0.0056)/social distancing (96% vs. 59%; p< 0.0001)/wearing a mask (95% vs. 43%; p< 0.0001), were female (88% vs. 66%; p = 0.0005), were white (90% vs. 82%; p = 0.0063), had higher levels of education (94% vs. 79%; p< 0.0001), and identified as Democrats (58% vs. 7%; p< 0.0001). In the multivariate model, subjects who were still independently associated with the perception of the vaccines being safe were those more likely to have received their second dose (or planned on it) (p< 0.0001), who trusted science (p< 0.0001), had higher levels of education (p = 0.0007), or were Democrats (p = 0.0020).
https://doi.org/10.1371/journal.pone.0268784.t002
To determine what groups were likely to perceive the most barriers to vaccination, bivariate and multivariable models were created ( Table 3 ). By analyzing subjects who were actively seeking vaccination versus those who were not, we found the former were more likely to have had their second dose (or were likely to get it) (92% vs. 20%; p< 0.0001), did not have a prior health condition (94% vs. 85%; p = 0.0283), trusted science (96% vs. 34%; p< 0.0001)/vaccines (95% vs. 31%; p< 0.0001)/doctors (93% vs. 35%; p< 0.0001), believed in the effectiveness of social distancing (91% vs. 68%; p< 0.0001)/wearing a mask (97% vs. 52%; p< 0.0001), were younger (p< 0.0001), were not male (72% vs. 68%; p = 0.0326), were an under-represented minority (40% vs. 23%; p = 0.0043), had a higher median income ($56,000 vs. $49,000; p = 0.0053), or were Democrats (48% vs. 12%; p< 0.0001). In the multivariate model, subjects that were still independently associated with actively seeking a vaccination were those with their second dose already received (or planned on it) (p< 0.0001) and who trusted in science (p = 0.0006).
https://doi.org/10.1371/journal.pone.0268784.t003
Data for the final two objectives were aggregated and analyzed together ( Table 4 ). For those who “do not believe in vaccines”, the variables more likely associated with such outcome included not having a high-risk medical condition (42% vs. 53%; p = 0.0111), not knowing someone who is vaccinated (87% vs. 98%; p< 0.0001), not trusting vaccines (21% vs. 97%; p< 0.0001)/science (26% vs. 97%; p< 0.0001)/doctors (28% vs. 97%; p< 0.0001), not believing in the effectiveness of hand washing (90% vs. 94%; p = 0.0410)/ social distancing (65% vs. 96%; p< 0.0001)/wearing a mask (51% vs. 94%; p< 0.0001), not receiving an annual flu shot (21% vs. 83%; p< 0.0001), thinking there is no need if others have been vaccinated (58% vs. 8%; p< 0.0001), and not wanting to get vaccinated to help others (27% vs. 96%; p< 0.0001). In the multivariate model, subjects that were still independently associated with not believing in vaccines did not know someone who was vaccinated (p< 0.0001), did not trust science (p< 0.0001), believed vaccination is unnecessary if others were vaccinated (p = 0.0007), and would not get vaccinated to help others (p< 0.0001).
https://doi.org/10.1371/journal.pone.0268784.t004
Additionally, the variables associated with subjects who “do not believe in vaccines” included not getting vaccinated even if friends and family had been vaccinated (26% vs. 89%; p< 0.0001), being male (30% vs. 19%; p = 0.0053), being an underrepresented minority (25% vs. 9%; p< 0.0001), not being employed full time (65% vs. 55%; p = 0.0260), having a lower median income ($ 49, 000 vs. $51, 000; p = 0.0020), having lower levels of educational attainment (21% vs. 6%; p< 0.0001), and not being a Democrat (89% vs. 43%; p< 0.0001). In the multivariate model, subjects who were still independently associated with not believing in vaccines were those not getting vaccinated even if friends and family had done so (p< 0.0001), and having a lower median income (p = 0.0106).
Our study is not the first to examine the relationship between various demographics and vaccine hesitancy. Kini and colleagues explored 39 studies regarding demographics of vaccine acceptance and hesitation. Their systematic review suggests that vaccine acceptance increases with age and is higher for males and white individuals [ 18 ]. While our study reports different significant findings (see below), this is likely attributed to the context and sample of the studies, along with possible confounding variables as discussed later. Our results pertain to the time when data were collected: given the long and haphazard evolution of the pandemic and associated perceptions, the relevance of our results must be contextualized to the time and the stage of the pandemic. Our data show some disparities in perception and opinions regarding the COVID-19 vaccines based on the following key variables: age, race, income, educational level, underlying health conditions, and political partisanship. Participants who had received the first of two doses of the COVID-19 vaccine at the time of our study may already have been convinced of the safety of the vaccines. Additionally, during the early stages of vaccine promotion, there was emphasis from the CDC on possible worsening of underlying pulmonary, cardiac, and other health conditions, such as chronic obstructive pulmonary disease, heart failure, and asthma [ 19 ]. This could explain why individuals with underlying health conditions were likely to regard the vaccines as protective and safe.
Our results also showed that those who identifies as white, compared to members of underrepresented minorities, were more likely to consider the vaccine as safe. Based on an assumption of a positive correlation between perceiving the vaccine as safe and actually getting the vaccine, the CDC has shown that as of July 4 th , 2021, of those who had received at least one dose of the vaccine, 59% were white, 9% were black, 16% were Hispanic/Latinx, and 6% were Asian Americans [ 20 ]. However, it is unclear whether such disparity is affected by the communities in which vaccines are most readily available, or if such disparity in fact represents an individual decision due to distrust that might exist between underrepresented minorities and the healthcare industry. As such, it is vital to review past literature as it pertains to recent findings during the pandemic. Regarding vaccine hesitancy of underrepresented minorities, there has been clear evidence of disparities in healthcare treatment for Black and white patients. Davidio et al reviewed multiple papers that describe physician perceptions and treatment of Black vs. white patients with clear significance regarding the negative handling of Black patients [ 21 ]. Armstrong et al point out that experience of discrimination was strongly associated with healthcare system distrust (HCSD) in their study comparing African American and white survey respondents [ 22 ]. Additionally, Balasuriya et al explored factors associated with COVID-19 acceptance and access among Black and Latinx communities, and identified the pervasive mistreatment of Black and Latinx communities, rooted in structural racism, to be a key influence on vaccine acceptance [ 23 ]. Results such as this provide a strong basis to argue why underrepresented minorities may have been less eager to seek out vaccinations. Regarding vaccine hesitancy and political affiliation, other studies corroborate these results. In one study, it was found that US Republican counties consistently had lower general vaccination rates than Democratic counties [ 24 ]. In a polling done by Kaiser Family Foundation in May 2020, it was found that Republicans were less likely to report wearing masks, social distancing or getting vaccinated against COVID-19 [ 25 ].
Level of education has a strong effect on willingness to receive a COVID-19 vaccine: having a college degree has been associated with a 43% increase in likelihood of getting the vaccine [ 26 ]. Assuming the likelihood of obtaining the COVID-19 vaccine is positively correlated with perception that the vaccine is safe, it is worthwhile posing the question whether level of education outweighs other effects of race, gender, political affiliation, and underlying health conditions. Delay in COVID-19 vaccination notwithstanding (earlier in 2021 when our data were collected), the CDC has pointed to a divide in communities based on political party affiliation. To ultimately determine the prime factors in safety perception, we conducted a multivariable analysis and found that the following groups were most likely to perceive vaccines as being safe: 99.3% Democrats (vs. 86.0% Republicans, specifically) and 93.1% with higher educational attainment (vs. 6.8% with high school level specifically). It is important to correlate these results with previous studies that examined similar topics. Regarding results about education impacting vaccine rates, previous studies would support this. Suryadevara and colleagues collaborated with their county health department to educate high-risk, resource-poor families regarding vaccination concerns. Their results showed a drastic increase for general vaccine completion and annual influenza vaccine rates [ 27 ]. Another study showed that when providing low-literacy educational materials to resource-poor families regarding the pneumococcal vaccine, the test group was four times more likely to discuss the vaccination in appointments and five times more likely to receive the vaccine than control group [ 28 ]. Even more recent studies with COVID-19 support our findings. For instance, a recent study indicated that lack of high school education positively correlates with increased vaccine hesitancy and decreased vaccination levels [ 29 ].
Our multivariable model outcome also suggests that race and ethnicity are not necessarily the primary determinants of vaccine hesitancy and likelihood of vaccination, because low vaccination rates among underrepresented populations may be explained by the historical distrust within some members of underrepresented minorities toward health care organizations and providers, as well as suspicion about clinical research studies, in view of past atrocities such as the Tuskegee Syphilis experiment [ 30 ], or similar experiments with STD infections in Guatemala [ 31 ]. Our multivariable results support this possibility by indicating those being potential factors in rejecting the COVID-19 vaccine. Specifically, after adjusting for variables, one of the groups found to be independently associated and most likely to reject vaccination according to socioeconomic and demographic factors were individuals with lower income. Considering that low-income populations usually consist of groups that identify as underrepresented minorities [ 32 ], slow rates of vaccination in these groups might reflect individual distrust of health care providers. However, this finding does not rule out the possibility of low distributions in low-income locations (e.g., rural), which could be a barrier by itself for vaccination opportunities. As pointed out by DeMaria-Ghalili and colleagues, “health inequalities are most acute among those living in rural and low resourced areas of the state, and among underrepresented populations (particularly Black/African American and Latino), who lack access to health care, experience digital divide, and face persistent local healthcare workforce shortages.” The report further discusses that people in areas of lower socio-economic status or fewer resources (usually rural areas) have a more difficult time scheduling and going to appointments for vaccinations, noting “pharmacy deserts” to be an issue in having access to appropriate healthcare resources such as vaccines [ 33 ]. Economic precarity and poor technological advancements may be obstacles to both registering for and getting the vaccine, possibly associated with sparse information among low-income populations [ 34 ]. Therefore, to bolster vaccination, efforts should be made to target groups who are most likely to encounter barriers to COVID-19 vaccination, through governmental incentives, including free childcare and rides to vaccination sites, lottery tickets or cash vouchers, complimentary food and drinks at the vaccination sites, and tax credit [ 35 ], rather than privately offered incentives that may vary greatly throughout the country.
Our successful recruitment for this survey was helped by the ever-increasing prevalence of social media in peoples’ lives. This highlights the need for proper, scientific-based information regarding the pandemic to reach the lay public before opinions appear on social media newsfeeds. On the other hand, only 2.1% of our sample thought that social media sites were reliable sources for vaccine information. While this would appear to suggest limited influence of social media with regard to COVID vaccines, we have to interpret this with caution in view of a small, self-selected sample that may not reflect the U.S. population as a whole. While some individuals may have legitimate reasons for declining vaccination, e.g. allergies to some ingredients in the preparation or other medical contraindications, misperceptions about vaccines as presented by some members of the media can lead to vaccine refusal for inappropriate reasons [ 36 ]. Therefore, it is important to disseminate the scientific basis for vaccines whenever possible. Negative press about variant viruses and the possibility of ineffective vaccines lead to further public distrust of the otherwise monumental feat of creating and distributing the COVID-19 vaccines [ 37 ]. Education of the public is essential for the continued success of vaccination efforts in general. As an example, in one study [ 38 ], Human Papilloma Virus (HPV) vaccine education sessions were held for parents, healthcare and school staff who had little knowledge regarding HPV vaccines. After the sessions, results showed that over 90% of respondents felt vaccine education was important and 85–97% were supportive of school-based vaccine clinics. In another study on flu vaccination during pregnancy [ 39 ], pregnant women refused flu vaccines due to likely susceptibility to influenza and concerns for vaccine safety. The study intervention was a brief educational video by the CDC, which addressed vaccination health beliefs in a clear and easy to understand format. The primary outcome was receipt of the flu vaccine on the next prenatal visit, and suggested that appropriate education and reassurance were influential in vaccination. We must do the same for the COVID-19 vaccine, seeing that our findings suggest that educational attainment is one of the two most important factors that determine the likelihood that one will perceive the vaccine as safe and be likely to accept vaccination. Given that an overwhelming majority of our respondents indicated that they considered doctors, nurses, and other healthcare workers as reliable sources of vaccination information, it is imperative to begin incorporating COVID-19 vaccine questions and education during health care visits. Moreover, training healthcare professionals in cultural competency, defined as “the ability of individuals and systems to work or respond effectively across cultures in a way that acknowledges and respects the culture of the person or organization being served” [ 40 ] would help them navigate this conversation with knowledge and transparency to promote mutual trust and possibly increased likelihood of vaccination [ 41 , 42 ]. Unfortunately, cultural competency training is still limited in medical schools and residency programs [ 43 ], and broader implementation is needed. This will be critical for engaging minority/underrepresented groups, though we acknowledge that these groups may have general difficulties accessing any medical care and this in turn may contribute to lower vaccination rates. Some respondents chose “no access” as a reason for not receiving the vaccine. The term “no access” is admittedly broad and could have included decreased vaccination distribution to impoverished neighborhoods, or it could mean that individuals do not know where to go to get their vaccine. We kept our questionnaire concise so as not to overburden respondents, and consequently could not necessarily qualify the specific reasons for perception about no or limited access. Further investigation is needed to characterize the specific obstacles experienced by people seeking the vaccination. As health literacy regarding the still relatively new COVID-19 pandemic remains a challenge [ 44 ], our present survey can hopefully act as a compass to inform providers on the underlying rationale that their patients have for being skeptical about vaccines or medical advice.
In addition, we need steps to encourage the population to get vaccinated irrespective of political affiliation. Per our findings, those who identify as Democrats are more likely to perceive the vaccine as being safe. Partisanship and vaccination status continue to play a role in both U.S. vaccination efforts and the government’s response to the pandemic in general. Other studies have shown similar results [ 45 ], where 65% of Democrats and 51% of vaccinated adults say that the surge in COVID cases makes them angry at people who have not gotten a vaccine, while 59% of Republicans and 56% of unvaccinated adults say that the federal government should be blamed. Our study shows that Republicans less likely to become vaccinated trust information that comes directly from their health care team, more than information that originates from the government. Therefore, ensuring that all personnel on the health care team are culturally competent to facilitate conversations brought on by patients regarding the COVID-19 vaccine will be instrumental in ensuring vaccination acceptance across spectra. Finally, incentives must be focused on core groups that we believe are more likely to reject the vaccine. These include underrepresented minorities, people with lower educational level, those who identify as young, males, and those with high risk underlying medical conditions.
Our study has limitations, especially regarding data collection. Given the current pandemic and difficulty with in-person survey distribution, it was decided that an online distribution would be preferable, based on the assumption that every individual is at risk of contracting the virus and becoming affected by the pandemic. We used Facebook due to its wide reach. However, we recognize that not everyone has access to computers or Facebook, so this survey may favor those of higher socioeconomic status. Likewise, we did not seek parity since the sample was largely one of convenience, based on who responded to the questionnaire. Although forced responses were used for our survey to ensure completion and prevent answers, we could not determine other potential factors that may have caused incomplete responses in cases where respondents were allowed to select up to three options, e.g. for trusted sources of information. Obstacles to completion might have included feeling pressed for time, concerns about privacy in view of the open nature of social media, or rejection based on personally held political views. This could result in a self-selection bias due to differences between respondents and non-respondents, therefore skewing the findings. For example, many participants were white, female, and/or Democrat voters, which is not representative of the U.S. population per se and could bias the results in favor of opting for vaccination, perhaps due to stronger belief in vaccines. Obviously, given the enormous number of Facebook users in the U.S., and the fact that users are allowed to protect their privacy by restricting access to personal data (including by omitting it in their profiles), it would be difficult to assess the “typical” Facebook user in the context of these factors. Along those lines, about 87% of respondents were already vaccinated, which suggests that most considered the benefits greater than the risks. This may therefore result in under-reporting and under-characterizing negative views of the vaccine that we sought to capture in the survey. Another limitation of this study is that it only represents a snapshot in time of opinions of COVID-19 vaccine perceptions, which can be fluid. Because the vaccine data are rapidly changing and information provided to the public may evolve as days progress, our results can only be applicable to this specific point in time. Ideally, the present study should be repeated in the future to ascertain trends over time. From a methodological standpoint, future studies should focus on obtaining a wider and more diverse set of respondents, including individuals that do not have access to computers or Facebook. One feasible alternative could be the distribution of both online and paper surveys to the same group of respondents during the same wave of data collection, thus allowing for estimation of changes across strategies for survey contact.
While our findings are in line with some existing perspectives in the field, as they relate to the role of socioeconomic factors [ 26 , 32 ], educational influence [ 38 , 39 ], and partisanship [ 45 ], we have contributed a more robust and elaborate perception of the U.S population on COVID vaccines, while identifying specific groups at risk for not getting a vaccine. In conclusion, level of education and partisanship, but not race/ethnicity, were the most likely factors associated with vaccine hesitancy or likelihood to vaccinate. This suggests that improved education, not just about vaccines per se, but with regard to formal schooling in general, may be at the heart of promoting greater acceptability of vaccination. Likewise, low vaccination rates among underrepresented minorities may be due to distrust for healthcare industries, but further research is needed to fully characterize the relative contributions of low access vs. distrust. Many white people and many with a Republican party affiliation also expressed reluctance about vaccination, suggesting that mistrust of the healthcare industry, vaccinations in general, and/or the government is not limited to minorities and/or economically challenged populations. Regardless, population sub-groups less likely to be vaccinated and/or receptive to vaccines should be targeted for vaccine education and incentives, and outcomes of these interventions need to be closely studied for determination of efficacy.
Supporting information
S1 file. qualtrics survey questionnaire..
https://doi.org/10.1371/journal.pone.0268784.s001
S1 Data. Inclusion criteria.
https://doi.org/10.1371/journal.pone.0268784.s002
Acknowledgments
The authors would like to thank our collaborators at Qualtrics and Facebook for helping facilitate the successful completion of this study.
- View Article
- PubMed/NCBI
- Google Scholar
- 4. Centers for Disease Control and Prevention (CDC): COVID-19 Racial and Ethnic Health Disparities . https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/index.html . Accessed on 10 th , Dec 2020.
- 5. Johnson, Akilah: Lack of Health Services and Transportation Impede Access to Vaccine in Communities of Color . https://www.washingtonpost.com/health/2021/02/13/covid-racial-ethnic-disparities/ . Accessed on 13 th , Feb 2021.
- 8. U.S Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine?s_cid=11700:covid%20vaccine%20fda%20approval:sem.ga:p:RG:GM:gen:PTN:FY22 Accessed on 11th, December, 2020.
- 9. U.S Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid Accessed on 18 th , December 2020
- 12. The New York Times: Pandemics Racial Disparities Persist in Vaccine Rollout . https://www.nytimes.com/interactive/2021/03/05/us/vaccine-racial-disparities.html?campaign_id=37&emc=edit_rr_20210306&instance_id=27792&nl=race%2Frelated®i_id=76131559&segment_id=52913&te=1&user_id=4fc6fb065b9e912a39a1a6408b1e81c2 . Accessed on 5 th , March, 2021.
- 14. Pew Research Center. https://www.pewresearch.org/science/2020/12/03/intent-toget-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-processincreases Accessed on 3 rd , December, 2020.
- 15. Mayo Clinic: US COVID-19 Vaccine Tracker. https://www.mayoclinic.org/coronavirus-covid-19/vaccine-tracker Accessed on: 17 th , July 2021.
- 17. All result output were created using SAS. Copyright © 2021 SAS Institute Inc., Cary, NC, USA.
- 19. Centers for Disease Control: Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19 . https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Accessed on: 18 th , October 2021.
- 20. Centers for Disease Control: Latest Data on COVID-19 vaccination by Race/ Ethnicity . https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/ Accessed on: 18 th , October 2021.
- 25. Hamel, L., Kearney, A., Kirzinger, A., Lopes, L., Munana, C., Brodie, M. KFF Health Tracking Poll — May 2020 . Kaiser Family Foundation . https://www.kff.org/coronavirus-covid-19/report/kff-health-tracking-poll-may-2020/ Accessed on 19 th , January 2022.
- 26. Thomas, K., Darling, J. Education is now a Bigger Factor than Race in Desire for COVID-19 Vaccine . https://healthpolicy.usc.edu/evidence-base/education-is-now-a-bigger-factor-than-race-in-desire-for-covid-19-vaccine/ Accessed on 17 th , July 2021.
- 30. Ada McVean. McGill. 40 Years of Human Experimentation in America : The Tuskegee Study . https://www.mcgill.ca/oss/article/history/40-years-human-experimentation-america-tuskegee-study Accessed on 19th January, 2022.
- 31. Amy Gutmann. Presidential Commission for the Study of Bioethical Issues. “Ethically Impossible” STD Research in Guatemala from 1946 to 1948. https://bioethicsarchive.georgetown.edu/pcsbi/node/654.html Accessed on 19 th January, 2022.
- 32. Simms, M. The Urban Institute. Racial and Ethnic Disparities among low-income Families . https://www.urban.org/sites/default/files/publication/32976/411936-racial-and-ethnic-disparities-among-low-income-families.pdf Accessed on 18th October, 2021
- 33. DiMaria-Ghalili, R., Foreshaw Rouse, A., Coates, M., Hathaway, Z., Hirsch, J., Wetzel, S., et al. Disrupting Disparities in Pennsylvania: Retooling for Geographic, Racial and Ethnic Growth [White paper]. https://aarp-states.brightspotcdn.com/6f/b6/de161f3a4a63a23e811693d90b68/aarp-drexel-pennsylvania-disrupting-disparities-design-0421-final.pdf Accessed on 19th January, 2022.
- 35. Devon Delfino. Incentives for CoVID-19 Vaccination : Food , Cash , & Other Perks . https://www.goodrx.com/health-topic/vaccines/covid-19-vaccination-incentives Accessed on 20th, January, 2022.
- 37. The Atlantic. 5 Pandemic Mistakes We Keep Repeating . https://www.theatlantic.com/ideas/archive/2021/02/how-public-health-messaging-backfired/618147/ Accessed on 26 th , February 2021.
Essay on Coronavirus Vaccine
500+ words essay on coronavirus vaccine.
The Coronavirus has infected millions of people so far all over the world. In addition to that, millions of people have lost their lives to it. Ever since the outbreak, researchers all over the world have been working constantly to develop vaccines that will work effectively against the virus. We will take a look at the Coronavirus vaccine that is present today. Vaccines have the ability to save people’s lives. Developing the vaccine for Coronavirus was a huge step to end the pandemic.


Working of Coronavirus Vaccine
As Coronavirus caused a lot of confusion and fear amongst people, it is natural people were not aware of how the vaccine works. To begin with, a vaccine will work by mimicking an infectious agent.
The agent can be viruses, bacteria or any other microorganisms. They carry the potential of causing disease. When it mimics that, our immune system learns how to respond against it rapidly and efficiently.
As per the traditional methods, vaccines have managed to do this as they introduce a weakened form of an infectious agent. It enables our immune system to basically build its memory.
As a result, our immune system can then identify it quickly and fight against it before it gets the chance to harm us or make us ill. Similarly, some of the coronavirus vaccines have been made like that.
On the other hand, there are other coronavirus vaccines that researchers have developed by making use of new approaches. We refer to them as messenger RNA or mRNA vaccines.
Over here, they do not introduce antigens in our bodies. Instead, mRNA vaccines give the genetic code our body needs to enable our immune system for producing the antigen itself.
For several years, researchers have been studying mRNA vaccine technology. Thus, they do not contain any live virus and also do not interfere with the human DNA .
Get the huge list of more than 500 Essay Topics and Ideas
Safety of Coronavirus Vaccine
While the vaccines are being developed at a fast pace, they also require rigorous testing. The tests are done in clinical trials to ensure that they meet the benchmarks for the safety and efficiency of international standards.
When they meet the standards, then only can they get the go-ahead from WHO and national regulatory agencies. UNICEF has said that it will attain and supply only those vaccines that meet the WHO guidelines and have met the regulatory approval.
As of now, the vaccine doses are limited in number. Thus, the healthcare workers, first responders, people over the age of 75 and residents of long-term care facilities will receive the first doses.
After that, everyone will be able to get it once more of them are available. To get the vaccine, a person may require to pay a fee. However, some government institutions are providing it free of cost.
In order to get the vaccine, one must check with their local and state health departments on a regular basis. When they get the chance, they must get the dose right away.
The Coronavirus outbreak has challenged the whole world. Constantly, the experts and authorities are working to develop the vaccines. Therefore, we can also do our bit and adopt preventive measures to limit the spread of this disease. The major goal is to get the vaccine to everyone so that we can go on and about with our normal lives.
FAQ on Essay on Coronavirus Vaccine
Question 1: What are some common side effects of the Coronavirus vaccine?
Answer 1: The most common side effect includes a sore arm, fever , headache, and fatigue. However, not to worry, side effects are good in this case. They indicate that your vaccine is starting to work as it triggers your immune system.
Question 2: When do Coronavirus vaccine side effects kick in?
Answer 2: Usually, most of the side effects start to kick in within the first 3 days after you get your vaccine. Moreover, they will last up to 1 to 2 days only.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Top 100 cited research on COVID-19 vaccines: A bibliometric analysis and evidence mapping
Affiliations.
- 1 Child Rehabilitation Department, Gansu Rehabilitation Center Hospital, Lanzhou, Gansu, China.
- 2 Evidence-Based Medicine Center, Lanzhou University, Lanzhou, Gansu, China.
- 3 School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu, China.
- 4 School of Nursing, Gansu University of Chinese Medicine, Lanzhou, Gansu, China.
- 5 College of Traditional Chinese Medicine, Gansu Health Vocational College, Lanzhou, Gansu, China.
- PMID: 38977415
- PMCID: PMC11232646
- DOI: 10.1080/21645515.2024.2370605
The outbreak of the COVID-19 has seriously affected the whole society, and vaccines were the most effective means to contain the epidemic. This paper aims to determine the top 100 articles cited most frequently in COVID-19 vaccines and to analyze the research status and hot spots in this field through bibliometrics, to provide a reference for future research. We conducted a comprehensive search of the Web of Science Core Collection database on November 29, 2023, and identified the top 100 articles by ranking them from highest to lowest citation frequency. In addition, we analyzed the year of publication, citation, author, country, institution, journal, and keywords with Microsoft Excel 2019 and VOSviewer 1.6.18. Research focused on vaccine immunogenicity and safety, vaccine hesitancy, and vaccination intention.
Keywords: COVID-19 vaccine; Vosviewer; bibliometric analysis; research topic.
PubMed Disclaimer
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Comparison of each WoS category’s…
Comparison of each WoS category’s average number of citations with average citations of…
Collaborative network and cluster distribution…
Collaborative network and cluster distribution of authors in the top 100 articles on…
Collaborative network and cluster distribution of institutions in the top 100 articles on…
Country analysis. (a) Corresponding Author’s…
Country analysis. (a) Corresponding Author’s Country (Where MCP represents the number of coauthored…
Keyword analysis. (a) Density map…
Keyword analysis. (a) Density map of main keywords; (b) Clustering distribution of keywords…
- Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, Bedhiafi T, Raza A, Al-Zaidan L, Mohsen MO, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54(1):524–10. doi:10.1080/07853890.2022.2031274. - DOI - PMC - PubMed
- Meng X, Deng Y, Dai Z, Meng Z.. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5):102581. doi:10.1016/j.amjoto.2020.102581. - DOI - PMC - PubMed
- Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, Ng LPW, Wong YKE, Pei XM, Li MJW, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):877–88. doi:10.1080/14787210.2021.1863146. - DOI - PubMed
- Yang FW, Pang B, Jin XY, Chen Z, Pang W, Liu Q, Zhang J, Zhang B. Post COVID-19 burden: focus on the short-term condition. Acupunct Herb Med. 2022;2(3):139–42. doi:10.1097/HM9.0000000000000036. - DOI - PMC - PubMed
- Campos DMO, Fulco UL, de Oliveira CBS, Oliveira JIN. SARS-CoV-2 virus infection: targets and antiviral pharmacological strategies. J Evid Based Med. 2020;13(4):255–60. doi:10.1111/jebm.12414. - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding, linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
- Taylor & Francis
Other Literature Sources
- figshare - Data
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Introduction
- Conclusions
- Article Information
The reports to the Vaccine Adverse Event Reporting System met the case definition of myocarditis (reported cases). Among individuals older than 40 years of age, there were no more than 8 reports of myocarditis for any individual age after receiving either vaccine. For the BNT162b2 vaccine, there were 114 246 837 first vaccination doses and 95 532 396 second vaccination doses; and for the mRNA-1273 vaccine, there were 78 158 611 and 66 163 001, respectively. The y-axis range differs between panels A and B.
The reports to the Vaccine Adverse Event Reporting System met the case definition of myocarditis (reported cases). Among recipients of either vaccine, there were only 13 reports or less of myocarditis beyond 10 days for any individual time from vaccination to symptom onset. The y-axis range differs between panels A and B.
A, For the BNT162b2 vaccine, there were 138 reported cases of myocarditis with known date for symptom onset and dose after 114 246 837 first vaccination doses and 888 reported cases after 95 532 396 second vaccination doses.
B, For the mRNA-1273 vaccine, there were 116 reported cases of myocarditis with known date for symptom onset and dose after 78 158 611 first vaccination doses and 311 reported cases after 66 163 001 second vaccination doses.
eMethods. Medical Dictionary for Regulatory Activities Preferred Terms, Definitions of Myocarditis and Pericarditis, Myocarditis medical review form
eFigure. Flow diagram of cases of myocarditis and pericarditis reported to Vaccine Adverse Event Reporting System (VAERS) after receiving mRNA-based COVID-19 vaccine, United States, December 14, 2020-August 31, 2021.
eTable 1. Characteristics of all myocarditis cases reported to Vaccine Adverse Event Reporting System (VAERS) after mRNA-based COVID-19 vaccination, United States, December 14, 2020–August 31, 2021.
eTable 2. Characteristics of all pericarditis cases reported to Vaccine Adverse Event Reporting System (VAERS) after mRNA-based COVID-19 vaccination, United States, December 14, 2020–August 31, 2021.
eTable 3. Characteristics of myocarditis cases reported to Vaccine Adverse Event Reporting System after mRNA-based COVID-19 vaccination by case definition status.
- Myocarditis and Pericarditis After Vaccination for COVID-19 JAMA Research Letter September 28, 2021 This study investigates the incidence of myocarditis and pericarditis emergency department or inpatient hospital encounters before COVID-19 vaccine availability (January 2019–January 2021) and during a COVID-19 vaccination period (February-May 2021) in a large US health care system. George A. Diaz, MD; Guilford T. Parsons, MD, MS; Sara K. Gering, BS, BSN; Audrey R. Meier, MPH; Ian V. Hutchinson, PhD, DSc; Ari Robicsek, MD
- Myocarditis Following a Third BNT162b2 Vaccination Dose in Military Recruits in Israel JAMA Research Letter April 26, 2022 This study assessed whether a third vaccine dose was associated with the risk of myocarditis among military personnel in Israel. Limor Friedensohn, MD; Dan Levin, MD; Maggie Fadlon-Derai, MHA; Liron Gershovitz, MD; Noam Fink, MD; Elon Glassberg, MD; Barak Gordon, MD
- Myocarditis Cases After mRNA-Based COVID-19 Vaccination in the US—Reply JAMA Comment & Response May 24, 2022 Matthew E. Oster, MD, MPH; David K. Shay, MD, MPH; Tom T. Shimabukuro, MD, MPH, MBA
- Myocarditis Cases After mRNA-Based COVID-19 Vaccination in the US JAMA Comment & Response May 24, 2022 Sheila R. Weiss, PhD
- JAMA Network Articles of the Year 2022 JAMA Medical News & Perspectives December 27, 2022 This Medical News article is our annual roundup of the top-viewed articles from all JAMA Network journals. Melissa Suran, PhD, MSJ
- Diagnosis and Treatment of Acute Myocarditis—A Review JAMA Review April 4, 2023 This Review summarizes current evidence regarding the diagnosis and treatment of acute myocarditis. Enrico Ammirati, MD, PhD; Javid J. Moslehi, MD
- Patient Information: Acute Myocarditis JAMA JAMA Patient Page August 8, 2023 This JAMA Patient Page describes acute myocarditis and its symptoms, causes, diagnosis, and treatment. Kristin Walter, MD, MS
- Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military JAMA Cardiology Brief Report October 1, 2021 This case series describes myocarditis presenting after COVID-19 vaccination within the Military Health System. Jay Montgomery, MD; Margaret Ryan, MD, MPH; Renata Engler, MD; Donna Hoffman, MSN; Bruce McClenathan, MD; Limone Collins, MD; David Loran, DNP; David Hrncir, MD; Kelsie Herring, MD; Michael Platzer, MD; Nehkonti Adams, MD; Aliye Sanou, MD; Leslie T. Cooper Jr, MD
- Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination JAMA Cardiology Brief Report October 1, 2021 This study describes 4 patients who presented with acute myocarditis after mRNA COVID-19 vaccination. Han W. Kim, MD; Elizabeth R. Jenista, PhD; David C. Wendell, PhD; Clerio F. Azevedo, MD; Michael J. Campbell, MD; Stephen N. Darty, BS; Michele A. Parker, MS; Raymond J. Kim, MD
- Association of Myocarditis With BNT162b2 Vaccination in Children JAMA Cardiology Brief Report December 1, 2021 This case series reviews comprehensive cardiac imaging in children with myocarditis after COVID-19 vaccine. Audrey Dionne, MD; Francesca Sperotto, MD; Stephanie Chamberlain; Annette L. Baker, MSN, CPNP; Andrew J. Powell, MD; Ashwin Prakash, MD; Daniel A. Castellanos, MD; Susan F. Saleeb, MD; Sarah D. de Ferranti, MD, MPH; Jane W. Newburger, MD, MPH; Kevin G. Friedman, MD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
- CME & MOC
Oster ME , Shay DK , Su JR, et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi:10.1001/jama.2021.24110
Manage citations:
© 2024
- Permissions
Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021
- 1 US Centers for Disease Control and Prevention, Atlanta, Georgia
- 2 School of Medicine, Emory University, Atlanta, Georgia
- 3 Children’s Healthcare of Atlanta, Atlanta, Georgia
- 4 Vanderbilt University Medical Center, Nashville, Tennessee
- 5 Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio
- 6 Boston Medical Center, Boston, Massachusetts
- 7 Duke University, Durham, North Carolina
- 8 US Food and Drug Administration, Silver Spring, Maryland
- Research Letter Myocarditis and Pericarditis After Vaccination for COVID-19 George A. Diaz, MD; Guilford T. Parsons, MD, MS; Sara K. Gering, BS, BSN; Audrey R. Meier, MPH; Ian V. Hutchinson, PhD, DSc; Ari Robicsek, MD JAMA
- Research Letter Myocarditis Following a Third BNT162b2 Vaccination Dose in Military Recruits in Israel Limor Friedensohn, MD; Dan Levin, MD; Maggie Fadlon-Derai, MHA; Liron Gershovitz, MD; Noam Fink, MD; Elon Glassberg, MD; Barak Gordon, MD JAMA
- Comment & Response Myocarditis Cases After mRNA-Based COVID-19 Vaccination in the US—Reply Matthew E. Oster, MD, MPH; David K. Shay, MD, MPH; Tom T. Shimabukuro, MD, MPH, MBA JAMA
- Comment & Response Myocarditis Cases After mRNA-Based COVID-19 Vaccination in the US Sheila R. Weiss, PhD JAMA
- Medical News & Perspectives JAMA Network Articles of the Year 2022 Melissa Suran, PhD, MSJ JAMA
- Review Diagnosis and Treatment of Acute Myocarditis—A Review Enrico Ammirati, MD, PhD; Javid J. Moslehi, MD JAMA
- JAMA Patient Page Patient Information: Acute Myocarditis Kristin Walter, MD, MS JAMA
- Brief Report Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military Jay Montgomery, MD; Margaret Ryan, MD, MPH; Renata Engler, MD; Donna Hoffman, MSN; Bruce McClenathan, MD; Limone Collins, MD; David Loran, DNP; David Hrncir, MD; Kelsie Herring, MD; Michael Platzer, MD; Nehkonti Adams, MD; Aliye Sanou, MD; Leslie T. Cooper Jr, MD JAMA Cardiology
- Brief Report Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination Han W. Kim, MD; Elizabeth R. Jenista, PhD; David C. Wendell, PhD; Clerio F. Azevedo, MD; Michael J. Campbell, MD; Stephen N. Darty, BS; Michele A. Parker, MS; Raymond J. Kim, MD JAMA Cardiology
- Brief Report Association of Myocarditis With BNT162b2 Vaccination in Children Audrey Dionne, MD; Francesca Sperotto, MD; Stephanie Chamberlain; Annette L. Baker, MSN, CPNP; Andrew J. Powell, MD; Ashwin Prakash, MD; Daniel A. Castellanos, MD; Susan F. Saleeb, MD; Sarah D. de Ferranti, MD, MPH; Jane W. Newburger, MD, MPH; Kevin G. Friedman, MD JAMA Cardiology
Question What is the risk of myocarditis after mRNA-based COVID-19 vaccination in the US?
Findings In this descriptive study of 1626 cases of myocarditis in a national passive reporting system, the crude reporting rates within 7 days after vaccination exceeded the expected rates across multiple age and sex strata. The rates of myocarditis cases were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively).
Meaning Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men.
Importance Vaccination against COVID-19 provides clear public health benefits, but vaccination also carries potential risks. The risks and outcomes of myocarditis after COVID-19 vaccination are unclear.
Objective To describe reports of myocarditis and the reporting rates after mRNA-based COVID-19 vaccination in the US.
Design, Setting, and Participants Descriptive study of reports of myocarditis to the Vaccine Adverse Event Reporting System (VAERS) that occurred after mRNA-based COVID-19 vaccine administration between December 2020 and August 2021 in 192 405 448 individuals older than 12 years of age in the US; data were processed by VAERS as of September 30, 2021.
Exposures Vaccination with BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna).
Main Outcomes and Measures Reports of myocarditis to VAERS were adjudicated and summarized for all age groups. Crude reporting rates were calculated across age and sex strata. Expected rates of myocarditis by age and sex were calculated using 2017-2019 claims data. For persons younger than 30 years of age, medical record reviews and clinician interviews were conducted to describe clinical presentation, diagnostic test results, treatment, and early outcomes.
Results Among 192 405 448 persons receiving a total of 354 100 845 mRNA-based COVID-19 vaccines during the study period, there were 1991 reports of myocarditis to VAERS and 1626 of these reports met the case definition of myocarditis. Of those with myocarditis, the median age was 21 years (IQR, 16-31 years) and the median time to symptom onset was 2 days (IQR, 1-3 days). Males comprised 82% of the myocarditis cases for whom sex was reported. The crude reporting rates for cases of myocarditis within 7 days after COVID-19 vaccination exceeded the expected rates of myocarditis across multiple age and sex strata. The rates of myocarditis were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively). There were 826 cases of myocarditis among those younger than 30 years of age who had detailed clinical information available; of these cases, 792 of 809 (98%) had elevated troponin levels, 569 of 794 (72%) had abnormal electrocardiogram results, and 223 of 312 (72%) had abnormal cardiac magnetic resonance imaging results. Approximately 96% of persons (784/813) were hospitalized and 87% (577/661) of these had resolution of presenting symptoms by hospital discharge. The most common treatment was nonsteroidal anti-inflammatory drugs (589/676; 87%).
Conclusions and Relevance Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men. This risk should be considered in the context of the benefits of COVID-19 vaccination.
Myocarditis is an inflammatory condition of the heart muscle that has a bimodal peak incidence during infancy and adolescence or young adulthood. 1 - 4 The clinical presentation and course of myocarditis is variable, with some patients not requiring treatment and others experiencing severe heart failure that requires subsequent heart transplantation or leads to death. 5 Onset of myocarditis typically follows an inciting process, often a viral illness; however, no antecedent cause is identified in many cases. 6 It has been hypothesized that vaccination can serve as a trigger for myocarditis; however, only the smallpox vaccine has previously been causally associated with myocarditis based on reports among US military personnel, with cases typically occurring 7 to 12 days after vaccination. 7
With the implementation of a large-scale, national COVID-19 vaccination program starting in December 2020, the US Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration began monitoring for a number of adverse events of special interest, including myocarditis and pericarditis, in the Vaccine Adverse Event Reporting System (VAERS), a long-standing national spontaneous reporting (passive surveillance) system. 8 As the reports of myocarditis after COVID-19 vaccination were reported to VAERS, the Clinical Immunization Safety Assessment Project, 9 a collaboration between the CDC and medical research centers, which includes physicians treating infectious diseases and other specialists (eg, cardiologists), consulted on several of the cases. In addition, reports from several countries raised concerns that mRNA-based COVID-19 vaccines may be associated with acute myocarditis. 10 - 15
Given this concern, the aims were to describe reports and confirmed cases of myocarditis initially reported to VAERS after mRNA-based COVID-19 vaccination and to provide estimates of the risk of myocarditis after mRNA-based COVID-19 vaccination based on age, sex, and vaccine type.
VAERS is a US spontaneous reporting (passive surveillance) system that functions as an early warning system for potential vaccine adverse events. 8 Co-administered by the CDC and the US Food and Drug Administration, VAERS accepts reports of all adverse events after vaccination from patients, parents, clinicians, vaccine manufacturers, and others regardless of whether the events could plausibly be associated with receipt of the vaccine. Reports to VAERS include information about the vaccinated person, the vaccine or vaccines administered, and the adverse events experienced by the vaccinated person. The reports to VAERS are then reviewed by third-party professional coders who have been trained in the assignment of Medical Dictionary for Regulatory Activities preferred terms. 16 The coders then assign appropriate terms based on the information available in the reports.
This activity was reviewed by the CDC and was conducted to be consistent with applicable federal law and CDC policy. The activities herein were confirmed to be nonresearch under the Common Rule in accordance with institutional procedures and therefore were not subject to institutional review board requirements. Informed consent was not obtained for this secondary use of existing information; see 45 CFR part 46.102(l)(2), 21 CFR part 56, 42 USC §241(d), 5 USC §552a, and 44 USC §3501 et seq.
The exposure of concern was vaccination with one of the mRNA-based COVID-19 vaccines: the BNT162b2 vaccine (Pfizer-BioNTech) or the mRNA-1273 vaccine (Moderna). During the analytic period, persons aged 12 years or older were eligible for the BNT162b2 vaccine and persons aged 18 years or older were eligible for the mRNA-1273 vaccine. The number of COVID-19 vaccine doses administered during the analytic period was obtained through the CDC’s COVID-19 Data Tracker. 17
The primary outcome was the occurrence of myocarditis and the secondary outcome was pericarditis. Reports to VAERS with these outcomes were initially characterized using the Medical Dictionary for Regulatory Activities preferred terms of myocarditis or pericarditis (specific terms are listed in the eMethods in the Supplement ). After initial review of reports of myocarditis to VAERS and review of the patient’s medical records (when available), the reports were further reviewed by CDC physicians and public health professionals to verify that they met the CDC’s case definition for probable or confirmed myocarditis (descriptions previously published and included in the eMethods in the Supplement ). 18 The CDC’s case definition of probable myocarditis requires the presence of new concerning symptoms, abnormal cardiac test results, and no other identifiable cause of the symptoms and findings. Confirmed cases of myocarditis further require histopathological confirmation of myocarditis or cardiac magnetic resonance imaging (MRI) findings consistent with myocarditis.
Deaths were included only if the individual had met the case definition for confirmed myocarditis and there was no other identifiable cause of death. Individual cases not involving death were included only if the person had met the case definition for probable myocarditis or confirmed myocarditis.
We characterized reports of myocarditis or pericarditis after COVID-19 vaccination that met the CDC’s case definition and were received by VAERS between December 14, 2020 (when COVID-19 vaccines were first publicly available in the US), and August 31, 2021, by age, sex, race, ethnicity, and vaccine type; data were processed by VAERS as of September 30, 2021. Race and ethnicity were optional fixed categories available by self-identification at the time of vaccination or by the individual filing a VAERS report. Race and ethnicity were included to provide the most complete baseline description possible for individual reports; however, further analyses were not stratified by race and ethnicity due to the high percentage of missing data. Reports of pericarditis with evidence of potential myocardial involvement were included in the review of reports of myocarditis. The eFigure in the Supplement outlines the categorization of the reports of myocarditis and pericarditis reviewed.
Further analyses were conducted only for myocarditis because of the preponderance of those reports to VAERS, in Clinical Immunization Safety Assessment Project consultations, and in published articles. 10 - 12 , 19 - 21 Crude reporting rates for myocarditis during a 7-day risk interval were calculated using the number of reports of myocarditis to VAERS per million doses of COVID-19 vaccine administered during the analytic period and stratified by age, sex, vaccination dose (first, second, or unknown), and vaccine type. Expected rates of myocarditis by age and sex were calculated using 2017-2019 data from the IBM MarketScan Commercial Research Database. This database contains individual-level, deidentified, inpatient and outpatient medical and prescription drug claims, and enrollment information submitted to IBM Watson Health by large employers and health plans. The data were accessed using version 4.0 of the IBM MarketScan Treatment Pathways analytic platform. Age- and sex-specific rates were calculated by determining the number of individuals with myocarditis ( International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes B33.20, B33.22, B33.24, I40.0, I40.1, I40.8, I40.9, or I51.4) 22 identified during an inpatient encounter in 2017-2019 relative to the number of individuals of similar age and sex who were continually enrolled during the year in which the myocarditis-related hospitalization occurred; individuals with any diagnosis of myocarditis prior to that year were excluded. Given the limitations of the IBM MarketScan Commercial Research Database to capture enrollees aged 65 years or older, an expected rate for myocarditis was not calculated for this population. A 95% CI was calculated using Poisson distribution in SAS version 9.4 (SAS Institute Inc) for each expected rate of myocarditis and for each observed rate in a strata with at least 1 case.
In cases of probable or confirmed myocarditis among those younger than 30 years of age, their clinical course was then summarized to the extent possible based on medical review and clinician interviews. This clinical course included presenting symptoms, diagnostic test results, treatment, and early outcomes (abstraction form appears in the eMethods in the Supplement ). 23
When applicable, missing data were delineated in the results or the numbers with complete data were listed. No assumptions or imputations were made regarding missing data. Any percentages that were calculated included only those cases of myocarditis with adequate data to calculate the percentages.
Between December 14, 2020, and August 31, 2021, 192 405 448 individuals older than 12 years of age received a total of 354 100 845 mRNA-based COVID-19 vaccines. VAERS received 1991 reports of myocarditis (391 of which also included pericarditis) after receipt of at least 1 dose of mRNA-based COVID-19 vaccine (eTable 1 in the Supplement ) and 684 reports of pericarditis without the presence of myocarditis (eTable 2 in the Supplement ).
Of the 1991 reports of myocarditis, 1626 met the CDC’s case definition for probable or confirmed myocarditis ( Table 1 ). There were 208 reports that did not meet the CDC’s case definition for myocarditis and 157 reports that required more information to perform adjudication (eTable 3 in the Supplement ). Of the 1626 reports that met the CDC’s case definition for myocarditis, 1195 (73%) were younger than 30 years of age, 543 (33%) were younger than 18 years of age, and the median age was 21 years (IQR, 16-31 years) ( Figure 1 ). Of the reports of myocarditis with dose information, 82% (1265/1538) occurred after the second vaccination dose. Of those with a reported dose and time to symptom onset, the median time from vaccination to symptom onset was 3 days (IQR, 1-8 days) after the first vaccination dose and 74% (187/254) of myocarditis events occurred within 7 days. After the second vaccination dose, the median time to symptom onset was 2 days (IQR, 1-3 days) and 90% (1081/1199) of myocarditis events occurred within 7 days ( Figure 2 ).
Males comprised 82% (1334/1625) of the cases of myocarditis for whom sex was reported. The largest proportions of cases of myocarditis were among White persons (non-Hispanic or ethnicity not reported; 69% [914/1330]) and Hispanic persons (of all races; 17% [228/1330]). Among persons younger than 30 years of age, there were no confirmed cases of myocarditis in those who died after mRNA-based COVID-19 vaccination without another identifiable cause and there was 1 probable case of myocarditis but there was insufficient information available for a thorough investigation. At the time of data review, there were 2 reports of death in persons younger than 30 years of age with potential myocarditis that remain under investigation and are not included in the case counts.
Symptom onset of myocarditis was within 7 days after vaccination for 947 reports of individuals who received the BNT162b2 vaccine and for 382 reports of individuals who received the mRNA-1273 vaccine. The rates of myocarditis varied by vaccine type, sex, age, and first or second vaccination dose ( Table 2 ). The reporting rates of myocarditis were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.73 [95% CI, 61.68-81.11] per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.86 [95% CI, 91.65-122.27] per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.43 [95% CI, 45.56-60.33] per million doses of the BNT162b2 vaccine and 56.31 [95% CI, 47.08-67.34] per million doses of the mRNA-1273 vaccine). The lower estimate of the 95% CI for reporting rates of myocarditis in adolescent males and young men exceeded the upper bound of the expected rates after the first vaccination dose with the BNT162b2 vaccine in those aged 12 to 24 years, after the second vaccination dose with the BNT162b2 vaccine in those aged 12 to 49 years, after the first vaccination dose with the mRNA-1273 vaccine in those aged 18 to 39 years, and after the second vaccination dose with the mRNA-1273 vaccine in those aged 18 to 49 years.
The reporting rates of myocarditis in females were lower than those in males across all age strata younger than 50 years of age. The reporting rates of myocarditis were highest after the second vaccination dose in adolescent females aged 12 to 15 years (6.35 [95% CI, 4.05-9.96] per million doses of the BNT162b2 vaccine), in adolescent females aged 16 to 17 years (10.98 [95% CI, 7.16-16.84] per million doses of the BNT162b2 vaccine), in young women aged 18 to 24 years (6.87 [95% CI, 4.27-11.05] per million doses of the mRNA-1273 vaccine), and in women aged 25 to 29 years (8.22 [95% CI, 5.03-13.41] per million doses of the mRNA-1273 vaccine). The lower estimate of the 95% CI for reporting rates of myocarditis in females exceeded the upper bound of the expected rates after the second vaccination dose with the BNT162b2 vaccine in those aged 12 to 29 years and after the second vaccination dose with the mRNA-1273 vaccine in those aged 18 to 29 years.
Among the 1372 reports of myocarditis in persons younger than 30 years of age, 1305 were able to be adjudicated, with 92% (1195/1305) meeting the CDC’s case definition. Of these, chart abstractions or medical interviews were completed for 69% (826/1195) ( Table 3 ). The symptoms commonly reported in the verified cases of myocarditis in persons younger than 30 years of age included chest pain, pressure, or discomfort (727/817; 89%) and dyspnea or shortness of breath (242/817; 30%). Troponin levels were elevated in 98% (792/809) of the cases of myocarditis. The electrocardiogram result was abnormal in 72% (569/794) of cases of myocarditis. Of the patients who had received a cardiac MRI, 72% (223/312) had abnormal findings consistent with myocarditis. The echocardiogram results were available for 721 cases of myocarditis; of these, 84 (12%) demonstrated a notable decreased left ventricular ejection fraction (<50%). Among the 676 cases for whom treatment data were available, 589 (87%) received nonsteroidal anti-inflammatory drugs. Intravenous immunoglobulin and glucocorticoids were each used in 12% of the cases of myocarditis (78/676 and 81/676, respectively). Intensive therapies such as vasoactive medications (12 cases of myocarditis) and intubation or mechanical ventilation (2 cases) were rare. There were no verified cases of myocarditis requiring a heart transplant, extracorporeal membrane oxygenation, or a ventricular assist device. Of the 96% (784/813) of cases of myocarditis who were hospitalized, 98% (747/762) were discharged from the hospital at time of review. In 87% (577/661) of discharged cases of myocarditis, there was resolution of the presenting symptoms by hospital discharge.
In this review of reports to VAERS between December 2020 and August 2021, myocarditis was identified as a rare but serious adverse event that can occur after mRNA-based COVID-19 vaccination, particularly in adolescent males and young men. However, this increased risk must be weighed against the benefits of COVID-19 vaccination. 18
Compared with cases of non–vaccine-associated myocarditis, the reports of myocarditis to VAERS after mRNA-based COVID-19 vaccination were similar in demographic characteristics but different in their acute clinical course. First, the greater frequency noted among vaccine recipients aged 12 to 29 years vs those aged 30 years or older was similar to the age distribution seen in typical cases of myocarditis. 2 , 4 This pattern may explain why cases of myocarditis were not discovered until months after initial Emergency Use Authorization of the vaccines in the US (ie, until the vaccines were widely available to younger persons). Second, the sex distribution in cases of myocarditis after COVID-19 vaccination was similar to that seen in typical cases of myocarditis; there is a strong male predominance for both conditions. 2 , 4
However, the onset of myocarditis symptoms after exposure to a potential immunological trigger was shorter for COVID-19 vaccine–associated cases of myocarditis than is typical for myocarditis cases diagnosed after a viral illness. 24 - 26 Cases of myocarditis reported after COVID-19 vaccination were typically diagnosed within days of vaccination, whereas cases of typical viral myocarditis can often have indolent courses with symptoms sometimes present for weeks to months after a trigger if the cause is ever identified. 1 The major presenting symptoms appeared to resolve faster in cases of myocarditis after COVID-19 vaccination than in typical viral cases of myocarditis. Even though almost all individuals with cases of myocarditis were hospitalized and clinically monitored, they typically experienced symptomatic recovery after receiving only pain management. In contrast, typical viral cases of myocarditis can have a more variable clinical course. For example, up to 6% of typical viral myocarditis cases in adolescents require a heart transplant or result in mortality. 27
In the current study, the initial evaluation and treatment of COVID-19 vaccine–associated myocarditis cases was similar to that of typical myocarditis cases. 28 - 31 Initial evaluation usually included measurement of troponin level, electrocardiography, and echocardiography. 1 Cardiac MRI was often used for diagnostic purposes and also for possible prognostic purposes. 32 , 33 Supportive care was a mainstay of treatment, with specific cardiac or intensive care therapies as indicated by the patient’s clinical status.
Long-term outcome data are not yet available for COVID-19 vaccine–associated myocarditis cases. The CDC has started active follow-up surveillance in adolescents and young adults to assess the health and functional status and cardiac outcomes at 3 to 6 months in probable and confirmed cases of myocarditis reported to VAERS after COVID-19 vaccination. 34 For patients with myocarditis, the American Heart Association and the American College of Cardiology guidelines advise that patients should be instructed to refrain from competitive sports for 3 to 6 months, and that documentation of a normal electrocardiogram result, ambulatory rhythm monitoring, and an exercise test should be obtained prior to resumption of sports. 35 The use of cardiac MRI is unclear, but it may be useful in evaluating the progression or resolution of myocarditis in those with abnormalities on the baseline cardiac MRI. 36 Further doses of mRNA-based COVID-19 vaccines should be deferred, but may be considered in select circumstances. 37
This study has several limitations. First, although clinicians are required to report serious adverse events after COVID-19 vaccination, including all events leading to hospitalization, VAERS is a passive reporting system. As such, the reports of myocarditis to VAERS may be incomplete, and the quality of the information reported is variable. Missing data for sex, vaccination dose number, and race and ethnicity were not uncommon in the reports received; history of prior SARS-CoV-2 infection also was not known. Furthermore, as a passive system, VAERS data are subject to reporting biases in that both underreporting and overreporting are possible. 38 Given the high verification rate of reports of myocarditis to VAERS after mRNA-based COVID-19 vaccination, underreporting is more likely. Therefore, the actual rates of myocarditis per million doses of vaccine are likely higher than estimated.
Second, efforts by CDC investigators to obtain medical records or interview physicians were not always successful despite the special allowance for sharing information with the CDC under the Health Insurance Portability and Accountability Act of 1996. 39 This challenge limited the ability to perform case adjudication and complete investigations for some reports of myocarditis, although efforts are still ongoing when feasible.
Third, the data from vaccination administration were limited to what is reported to the CDC and thus may be incomplete, particularly with regard to demographics.
Fourth, calculation of expected rates from the IBM MarketScan Commercial Research Database relied on administrative data via the use of ICD-10 codes and there was no opportunity for clinical review. Furthermore, these data had limited information regarding the Medicare population; thus expected rates for those older than 65 years of age were not calculated. However, it is expected that the rates in those older than 65 years of age would not be higher than the rates in those aged 50 to 64 years. 4
Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men. This risk should be considered in the context of the benefits of COVID-19 vaccination.
Corresponding Author: Matthew E. Oster, MD, MPH, US Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333 ( [email protected] ).
Correction: This article was corrected March 21, 2022, to change “pericarditis” to “myocarditis” in the first row, first column of eTable 1 in the Supplement.
Accepted for Publication: December 16, 2021.
Author Contributions: Drs Oster and Su had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Oster, Shay, Su, Creech, Edwards, Dendy, Schlaudecker, Woo, Shimabukuro.
Acquisition, analysis, or interpretation of data: Oster, Shay, Su, Gee, Creech, Broder, Edwards, Soslow, Schlaudecker, Lang, Barnett, Ruberg, Smith, Campbell, Lopes, Sperling, Baumblatt, Thompson, Marquez, Strid, Woo, Pugsley, Reagan-Steiner, DeStefano, Shimabukuro.
Drafting of the manuscript: Oster, Shay, Su, Gee, Creech, Marquez, Strid, Woo, Shimabukuro.
Critical revision of the manuscript for important intellectual content: Oster, Shay, Su, Creech, Broder, Edwards, Soslow, Dendy, Schlaudecker, Lang, Barnett, Ruberg, Smith, Campbell, Lopes, Sperling, Baumblatt, Thompson, Pugsley, Reagan-Steiner, DeStefano, Shimabukuro.
Statistical analysis: Oster, Su, Marquez, Strid, Woo, Shimabukuro.
Obtained funding: Edwards, DeStefano.
Administrative, technical, or material support: Oster, Gee, Creech, Broder, Edwards, Soslow, Schlaudecker, Smith, Baumblatt, Thompson, Reagan-Steiner, DeStefano.
Supervision: Su, Edwards, Soslow, Dendy, Schlaudecker, Campbell, Sperling, DeStefano, Shimabukuro.
Conflict of Interest Disclosures: Dr Creech reported receiving grants from the National Institutes of Health for the Moderna and Janssen clinical trials and receiving personal fees from Astellas and Horizon. Dr Edwards reported receiving grants from the National Institutes of Health; receiving personal fees from BioNet, IBM, X-4 Pharma, Seqirus, Roche, Pfizer, Merck, Moderna, and Sanofi; and receiving compensation for being the associate editor of Clinical Infectious Diseases . Dr Soslow reported receiving personal fees from Esperare. Dr Schlaudecker reported receiving grants from Pfizer and receiving personal fees from Sanofi Pasteur. Drs Barnett, Ruberg, and Smith reported receiving grants from Pfizer. Dr Lopes reported receiving personal fees from Bayer, Boehringer Ingleheim, Bristol Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi and receiving grants from Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi. No other disclosures were reported.
Funding/Support: This work was supported by contracts 200-2012-53709 (Boston Medical Center), 200-2012-53661 (Cincinnati Children’s Hospital Medical Center), 200-2012-53663 (Duke University), and 200-2012-50430 (Vanderbilt University Medical Center) with the US Centers for Disease Control and Prevention (CDC) Clinical Immunization Safety Assessment Project.
Role of the Funder/Sponsor: The CDC provided funding via the Clinical Immunization Safety Assessment Project to Drs Creech, Edwards, Soslow, Dendy, Schlaudecker, Lang, Barnett, Ruberg, Smith, Campbell, and Lopes. The authors affiliated with the CDC along with the other coauthors conducted the investigations; performed collection, management, analysis, and interpretation of the data; were involved in the preparation, review, and approval of the manuscript; and made the decision to submit the manuscript for publication.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC or the US Food and Drug Administration. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC or the US Food and Drug Administration.
Additional Contributions: We thank the following CDC staff who contributed to this article without compensation outside their normal salaries (in alphabetical order and contribution specified in parenthesis at end of each list of names): Nickolas Agathis, MD, MPH, Stephen R. Benoit, MD, MPH, Beau B. Bruce, MD, PhD, Abigail L. Carlson, MD, MPH, Meredith G. Dixon, MD, Jonathan Duffy, MD, MPH, Charles Duke, MD, MPH, Charles Edge, MSN, MS, Robyn Neblett Fanfair, MD, MPH, Nathan W. Furukawa, MD, MPH, Gavin Grant, MD, MPH, Grace Marx, MD, MPH, Maureen J. Miller, MD, MPH, Pedro Moro, MD, MPH, Meredith Oakley, DVM, MPH, Kia Padgett, MPH, BSN, RN, Janice Perez-Padilla, MPH, BSN, RN, Robert Perry, MD, MPH, Nimia Reyes, MD, MPH, Ernest E. Smith, MD, MPH&TM, David Sniadack, MD, MPH, Pamela Tucker, MD, Edward C. Weiss, MD, MPH, Erin Whitehouse, PhD, MPH, RN, Pascale M. Wortley, MD, MPH, and Rachael Zacks, MD (for clinical investigations and interviews); Amelia Jazwa, MSPH, Tara Johnson, MPH, MS, and Jamila Shields, MPH (for project coordination); Charles Licata, PhD, and Bicheng Zhang, MS (for data acquisition and organization); Charles E. Rose, PhD (for statistical consultation); and Scott D. Grosse, PhD (for calculation of expected rates of myocarditis). We also thank the clinical staff who cared for these patients and reported the adverse events to the Vaccine Adverse Event Reporting System.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Should you get the updated COVID-19 vaccine? See current guidelines from CDC.
As cases of COVID-19 are on the rise and with a new variant of the disease emerging this summer, the Centers for Disease Control and Prevention (CDC) is recommending updated vaccines ahead of the fall and winter virus season.
"Make a plan now for you and your family to get both updated flu and COVID vaccines this fall, ahead of the respiratory virus season," CDC Director Dr. Mandy Cohen said in a statement Thursday.
The CDC is tracking the growth of multiple variants of COVID-19, including the KP.3 frontrunner and the rising LB.1 , the newest variant circulating in the U.S. There was a 1.4% increase in test positivity as of June 22, according to data collected by the agency.
Here's what to know about the state of COVID-19 in the U.S., and the CDC's latest vaccine guidance.
COVID-19 cases increasing this summer: Insight into the uptick
Should you get the updated COVID-19 vaccine?
The CDC recommends for everyone ages 6 months and older, with some exceptions, receive an updated 2024-2025 COVID-19 vaccine to protect against the disease, regardless whether or not you have previously been vaccinated against the virus.
Should you get the updated flu vaccine?
The agency also recommends the updated 2024-205 flu vaccines.
Most people only need one dose of flu vaccine each season, and September and October remain the best times for most people to be vaccinated.
Can you get the COVID and flu vaccine at the same time?
The CDC says it is safe to receive both the COVID-19 and the flu vaccines at the same visit.
What are the current COVID variants?
For a two-week period starting on June 9 and ending on June 22, the CDC's Nowcast data tracker showed the projections of COVID-19 variants, with the KP.3 variant accounting for 33.1% of positive infections, the KP.2 variant at 20.8% and the new variant LB.1 at 17.5% of infections.
The JN.1 variant accounted for only 1.6% of positive infections, according to the data.
What is the LB.1 variant?
The LB.1 variant is the newest COVID-19 variant that is circulating in the United States.
With the information that the CDC has available right now there’s no indication that the LB.1 variant poses a serious risk.
“There is currently no evidence that LB.1 causes more severe disease," CDC Spokesperson, Dave Daigle, previously told USA TODAY.
What are the current symptoms of COVID-19?
There are a wide range of symptoms that could point to a COVID-19 infection, and may appear 2-14 days after exposure to the virus. Symptoms can include:
- Fever or chills
- Shortness of breath or difficulty breathing
- Muscle or body aches
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
The CDC said you should seek medical attention if you have the following symptoms:
- Trouble breathing
- Persistent pain or pressure in the chest
- New confusion
- Inability to wake or stay awake
- Pale, gray or blue-colored skin, lips, or nail beds
How do COVID and flu symptoms compare?
The flu and COVID-19 share some of the same signs, but flu symptoms will come on suddenly, the CDC says. People who have the flu often feel some or all of the following symptoms:
- Fever or feeling feverish/chills
- Runny or stuffy nose
Some people may have vomiting or diarrhea, although the CDC says this is more common in children than in adults.
What is the CDC's recommended isolation period for COVID-19?
The CDC's updated respiratory virus guidance recommends that people stay home and away from others until at least 24 hours after there is no fever and their symptoms are getting better overall. This is a change from the previous guidance, which recommended a minimum isolation period of five days for COVID-19.
Instead, the CDC urges an added precaution over the next five days and using prevention strategies, including:
- Taking steps for cleaner air
- Enhancing hygiene practices
- Wearing a well-fitting mask
- Keeping distance from others
- Getting tested for respiratory viruses
Contributing: Ahjané Forbes, USA TODAY.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Researchers study differences in attitudes toward Covid-19 vaccines between women and men in Africa
Press contact :.

Previous image Next image
While many studies over the past several years have examined people’s access to and attitudes toward Covid-19 vaccines, few studies in sub-Saharan Africa have looked at whether there were differences in vaccination rates and intention between men and women. In a new study appearing in the journal Frontiers in Global Women’s Health , researchers found that while women and men self-reported similar Covid-19 vaccination rates in 2022, unvaccinated men expressed more intention to get vaccinated than unvaccinated women.
Women tend to have better health-seeking behaviors than men overall. However, most studies relating to Covid-19 vaccination have found that intention has been lower among women. “We wondered whether this would hold true at the uptake level,” says Rawlance Ndejjo, a leader of the new study and an assistant lecturer in the Department of Disease Control and Environmental Health at Makerere University.
The comparable vaccination rates between men and women in the study is “a good thing to see,” adds Lula Chen, research director at MIT Governance Lab (GOV/LAB) and a co-author of the new study. “There wasn’t anything gendered about how [the vaccine] was being advertised or who was actually getting access to it.”
Women’s lower intention to vaccinate seemed to be driven by concerns about vaccine safety, suggesting that providing factual information about vaccine safety from trusted sources, like the Ministry of Health, could increase uptake.
The work is a collaboration between scholars from the MIT GOV/LAB, Makerere University’s School of Public Health in Uganda, University of Kinshasa’s School of Public Health in the Democratic Republic of the Congo (DRC), University of Ibadan’s College of Medicine in Nigeria, and Cheikh Anta Diop University in Senegal.
Studying vaccine availability and uptake in sub-Saharan Africa
The authors’ collaboration began in 2021 with research into Covid-19 vaccination rates, people’s willingness to get vaccinated, and how people’s trust in different authorities shaped attitudes toward vaccines in Uganda, the DRC, Senegal, and Nigeria. A survey in Uganda found that people who received information about Covid-19 from health workers were more likely to be vaccinated, stressing the important role people who work in the health-care system can play in vaccination efforts.
Work from other scientists has found that women were less likely to accept Covid-19 vaccines than men, and that in low- and middle-income countries, women also may be less likely to get vaccinated against Covid-19 and less likely to intend to get vaccinated, possibly due to factors including lower levels of education, work obligations, and domestic care obligations.
Previous studies in sub-Saharan Africa that focused on differences between men and women with intention and willingness to vaccinate were inconclusive, Ndejjo says. “You would hardly find actual studies on uptake of the vaccines,” he adds. For the new paper, the researchers aimed to dig into uptake.
People who trust the government and health officials were more likely to get vaccinated
The researchers relied on phone survey data collected from adults in the four countries between March and July 2022. The surveys asked people about whether they’d been vaccinated and whether those who were unvaccinated intended to get vaccinated, as well as their attitudes toward Covid-19, their trust in different authorities, demographic information, and more.
Overall, 48.5 percent of men said they had been vaccinated, compared to 47.9 percent of women. Trust in authorities seemed to play a role in people’s decision to vaccinate — receiving information from health workers about Covid-19 and higher trust in the Ministry of Health were both correlated with getting vaccinated for men, whereas higher trust in the government was correlated with vaccine uptake in women.
Lower interest in vaccines among women seemed related to safety concerns
A smaller percentage of unvaccinated women (54 percent) said they intended to get vaccinated, compared to 63.4 percent of men. More unvaccinated women said they had concerns about the vaccine’s safety than unvaccinated men, which could be driving their lower intention.
The researchers also found that unvaccinated women and men over 40 had similar levels of intention to get vaccinated — lower intention in women under 40 may have driven the difference between men and women. Younger women could have concerns about vaccines related to pregnancy, Chen says. If this is the case, the research suggests that officials need to provide additional reassurance to pregnant people about vaccine safety, she adds.
Trust in authorities also contributed to people’s intention to vaccinate. Trust in the Ministry of Health was tied to higher intention to vaccinate for both men and women. Men with more trust in the World Health Organization were also more likely to intend to vaccinate.
“There’s a need to deal with a lot of the myths and misconceptions that exist,” Ndejjo says, as well as ensure that people’s concerns related to vaccine safety and effectiveness are addressed. Officials need “to work with trusted sources of information to bridge some of the gaps that we observe,” he adds. People need to be supported in their decision-making so they can make the best decisions for their health.
“This research highlights linkages between citizen trust in government, their willingness to get vaccines, and, importantly, the differences between men and women on this issue — differences that policymakers will need to understand in order to design more targeted, gender-specific public health interventions,” says study co-author Lily L. Tsai, who is MIT GOV/LAB’s director and founder and the Ford Professor of Political Science at MIT.
This project was funded by the Bill & Melinda Gates Foundation.
Share this news article on:
Related links.
- MIT Governance Lab
- Department of Political Science
Related Topics
- Public health
- Political science
- Health sciences and technology
- Race and gender
- School of Humanities Arts and Social Sciences
Related Articles

Championing health workers to lead vaccination efforts in Uganda
Study finds lockdowns effective at reducing travel in Sierra Leone

Informing Covid-19 preparedness in Sierra Leone
Previous item Next item
More MIT News

Professor Emeritus John Vander Sande, microscopist, entrepreneur, and admired mentor, dies at 80
Read full story →

Polina Anikeeva named head of the Department of Materials Science and Engineering

MIT OpenCourseWare “changed how I think about teaching and what a university is”

Study reveals how an anesthesia drug induces unconsciousness

Q&A: Helping young readers explore curiosity about rocks through discovery and play

Q&A: What past environmental success can teach us about solving the climate crisis
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Questions and answers /
Coronavirus disease (COVID-19): Vaccine research and development
Reviewed and current on 10 August 2021.
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety.
Vaccines go through various phases of development and testing – there are usually three phases to clinical trials, with the last one designed to assess the ability of the product to protect against disease, which is called efficacy. All phases assess safety. The last phase, phase III, are usually conducted in a large number of people, often 10’s of thousands. After that, the vaccine needs to go through a review by the national regulatory authority, who will decide if the vaccine is safe and effective enough to be put on the market, and a policy committee, who will decide how the vaccine should be used.
In the past, vaccines have been developed through a series of consecutive steps that can take many years. Now, given the urgent need for COVID-19 vaccines, unprecedented financial investments and scientific collaborations are changing how vaccines are developed. This means that some of the steps in the research and development process have been happening in parallel, while still maintaining strict clinical and safety standards. For example, some clinical trials are evaluating multiple vaccines at the same time. It is the scale of the financial and political commitments to the development of a vaccine that has allowed this accelerated development to take place. However, this does not make the studies any less rigorous.
The more vaccines in development the more opportunities there are for success.
Any longer-term safety assessment will be conducted through continued follow up of the clinical trial participants, as well as through specific studies and general pharmacovigilance of those being vaccinated in the roll out. This represents standard practise for all newly authorized vaccines.
In a regular vaccine study, one group of volunteers at risk for a disease is given an experimental vaccine, and another group is not; researchers monitor both groups over time and compare outcomes to see if the vaccine is safe and effective.
In a human challenge vaccine study, healthy volunteers are given an experimental vaccine, and then deliberately exposed to the organism causing the disease to see if the vaccine works. Some scientists believe that this approach could accelerate COVID-19 vaccine development, in part because it would require far fewer volunteers than a typical study.
However, there are important ethical considerations that must be addressed – particularly for a new disease like COVID-19, which we do not yet fully understand and are still learning how to treat; it may be difficult for the medical community and potential volunteers to properly estimate the potential risks of participating in a COVID-19 human challenge study. For more information, see this WHO publication on the ethics of COVID-19 human challenge studies .
Small (phase I) safety studies of COVID-19 vaccines should enroll healthy adult volunteers. Larger (phase II and III) studies should include volunteers that reflect the populations for whom the vaccines are intended. This means enrolling people from diverse geographic areas, racial and ethnic backgrounds, genders, and ages, as well as those with underlying health conditions that put them at higher risk for COVID-19. Including these groups in clinical trials is the only way to make sure that a vaccine will be safe and effective for everyone who needs it.
Opportunities to volunteer for a COVID-19 vaccine trial vary from country to country. If you are interested in volunteering, check with local health officials or research institutions or email [email protected] for more information about vaccine trials.
The push for a COVID-19 vaccine
Vaccines explained
Related Q&As:
Vaccines and immunization: What is vaccination?
Coronavirus disease (COVID-19): Vaccines
Coronavirus disease (COVID-19): COVID-19 Vaccine access and allocation
More From Forbes
Summer 2024 covid-19 surge occurring with flirt variants taking over.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Data suggests that the U.S. has been experiencing a Covid-19 Summer surge since early June. Here ... [+] people visit The Edge observation deck for New York City's 48th annual Macy's 4th of July fireworks on July 4, 2024. (Photo by Noam Galai/Getty Images)
You could say that the U.S. is more than flirting with yet another Covid-19 surge. Chances are that a surge has already been occurring for at least a month—since early June. And the “FLiRT” variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been front-and-center of this surge.
Now, the U.S. still doesn’t have a comprehensive surveillance system that can catch surges before they happen or soon after they start happening even though it’s been over four and a half years since the the SARS-CoV-2 first surged in the U.S. Plus, nowadays, you don’t see the public health alerts about Covid-19 surges that you saw during the first three years of the pandemic. So, these days if you want to know whether Covid-19 is surging, you have to rely on checking Covid-19-related emergency room visits, hospitalizations and deaths to see if a surge has already been occurring with an emphasis on the words “has already been.”
Indeed, emergency room visits due to Covid-19 from June 16 through June 22—that’s two weeks ago—were up 23.3% from the previous week, according to data posted on the Centers for Disease and Control and Prevention . Also, Covid-19-related hospitalizations during the week of June 9 through 15 were 13.3% higher than they were than the week before. Recall that it can take a week or two after someone gets infected to develop symptoms severe enough to require an emergency room visit or a hospitalization. All of this suggests that Covid-19 cases were rising throughout most of June.
It shouldn’t be too surprising that yet another Covid-19 Summer surge has been happening. Many political and business leaders are not advocating for Covid-19 precautions such as face mask use and indoor air filtration and purification. And take a wild guess as to what may happen to a virus that’s still spreading and mutating when you don’t really do much to prevent its spread. The answer is not that it will go away on its own. Moreover, it’s been about 10 months since the last Covid-19 vaccine update was rolled out last fall, and protection offered by the vaccine tends to wane significantly after four to six months.
Then there are the “FLiRT” variants, a new group of SARS-CoV-2 omicron variants that have two key mutations in their spike proteins. If you recall, the spike proteins are what makes the virus looks like a spiky ball and help the virus latch on to your cells to then invade them. The name FLiRT is derived from the actual amino acid changes that result from the pair of mutations: a switch of a phenylalanine (F) for a leucine (L) at position 456 and arginine (R) for threonine (T) at position 346 in the spike protein.
Best High-Yield Savings Accounts Of 2024
Best 5% interest savings accounts of 2024.
These FLiRTs are descendants of the JN.1 variant that was dominant in the United States earlier this year and encompass a group of variants with names that begin with the letters JN and KP. In the first week of June, KP.3 accounted for an estimated 33.1% of SARS-CoV-2 infections in the U.S., KP.2 for an estimated 20.8% and KP.1.1 for an estimated 9%. And these percentages have been growing, which is not surprising since preliminary data has suggested that the R e —the effective reproduction number—for KP.2 may be 1.22 times higher than the R e for JN.1.
Whenever new variants emerge, the big question is whether they will be able to evade the existing protection that you may have from vaccination or previous Covid-19 infections. Well, the two mutations in the FLiRT variants do affect important locations in the spike protein—namely where antibodies against the virus typically bind. Nevertheless, so far, there’s no indication that vaccination will not be effective against the FLiRT variants. But—and this is big “but,” one cannot lie—more data are needed to determine how effective vaccination will be against the FLiRT variants.
Covid-19 is less of a concern now than it was in the earlier days of the pandemic. Your immune system is probably more used to the spike protein and the virus now. You are likely less likely to get hospitalized and suffer more severe consequences when infected with SARS-CoV-2. But the risks of more severe outcomes are still there. There is still a significant chance of suffering long Covid. Therefore, it is a good idea to maintain appropriate precautions such as making sure that indoor locations are well-ventilated and wearing a face mask when you may come into close sustained contact with others who may be infected. And a summer surge does increase the latter possibility.

- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.

IMAGES
VIDEO
COMMENTS
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
Amid the staggering amount of suffering and death during this historic pandemic of COVID-19, a remarkable success story stands out. The development of several highly efficacious vaccines against a previously unknown viral pathogen, severe acute ...
The COVID-19 pandemic has led the world to undertake the largest vaccination campaign in human history. In record time, unprecedented scientific and governmental efforts have resulted in the acquisition of immunizers utilizing different technologies (nucleotide ...
However, some viral vector or mRNA vaccines may be associated with complications like thrombocytopenia and myocarditis, raising concerns about the safety of these COVID-19 vaccines.
Since the outbreak of the COVID-19 pandemic, there has been a rapid expansion in vaccine research focusing on exploiting the novel discoveries on the pathophysiology, genomics, and molecular biology of the severe acute respiratory syndrome coronavirus ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
Conclusions Most of the COVID-19 vaccines appear to be effective and safe. Double-dose vaccination is recommended. However, more research is needed to investigate the long-term efficacy and safety of the vaccines and the influence of dose, age, and production process on the protective efficacy.
This page answers the most frequently asked questions about COVID-19 vaccines and vaccine safety. If the information you are looking for is not here, check out our related links on the right-hand side of the page.
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still ...
It is clear that coronavirus vaccines are safe and effective, but as more are rolled out, researchers are learning about the extent and nature of side effects.
How Pfizer Makes Its Covid-19 Vaccine. By Emma Cott , Elliot deBruyn and Jonathan Corum April 28, 2021. Inside this facility in Chesterfield, Missouri, trillions of bacteria are producing tiny ...
COVID-19 vaccines. The development and roll-out of COVID-19 vaccines have been fundamental in saving lives and protecting people from severe disease and death —especially those most at-risk and vulnerable to COVID-19, protecting health systems, and reducing widescale social disruption. WHO in the Western Pacific continues to work with ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
There are many benefits of getting vaccinated against COVID-19. Prevents serious illness: COVID-19 vaccines available in the United States are safe and effective at protecting people from getting seriously ill, being hospitalized, and dying. A safer way to build protection: Getting a COVID-19 vaccine is a safer, more reliable way to build ...
Vaccines are a critical tool in the battle against COVID-19, and getting vaccinated is one of the best ways to protect yourself and others from COVID-19.
What are the benefits and risks of vaccines for preventing COVID-19? Key messages. - Most vaccines reduce, or probably reduce, the number of people who get COVID-19 disease and severe COVID-19 disease. - Many vaccines likely increase number of people experiencing events such as fever or headache compared to placebo (sham vaccine that ...
How COVID-19 Vaccines Work. COVID-19 vaccines help our bodies develop immunity to the virus that causes COVID-19 without us having to get the illness. Different types of vaccines work in different ways to offer protection. But with all types of vaccines, the body is left with a supply of "memory" T-lymphocytes as well as B-lymphocytes that ...
Safety of COVID-19 Vaccines. On June 27, 2024, the CDC Director adopted the ACIP's recommendations for use of 2024-2025 COVID-19 vaccines in people ages 6 months and older as approved or authorized by FDA. The 2024-2025 vaccines are expected to be available in fall 2024. This page will be updated at that time to align with the new ...
Background Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine. Methods Study participants in the U.S. (79% female, median age group 46-60 years) were ...
FAQ on Essay on Coronavirus Vaccine. Question 1: What are some common side effects of the Coronavirus vaccine? Answer 1: The most common side effect includes a sore arm, fever, headache, and fatigue. However, not to worry, side effects are good in this case.
The outbreak of the COVID-19 has seriously affected the whole society, and vaccines were the most effective means to contain the epidemic. This paper aims to determine the top 100 articles cited most frequently in COVID-19 vaccines and to analyze the research status and hot spots in this field through bibliometrics, to provide a reference for future research.
COVID‑19 vaccination is safe for people who are pregnant or are breastfeeding. As of 1 May 2024, 13.58 billion doses of COVID‑19 vaccines have been administered worldwide, based on official reports from national public health agencies.
A COVID-19 vaccine appointment is another opportunity to get your child caught up on all of their recommended vaccines. Can I get the COVID-19 vaccine if I'm pregnant, lactating or planning to become pregnant? Yes, data show that COVID-19 vaccines are safe during pregnancy.
This descriptive study compares the effect of mRNA-based COVID-19 vaccination with BNT162b2 (Pfizer-BioNTech) vs mRNA-1273 (Moderna) on the reported cases of myocarditis in the US after each vaccination dose.
As cases of COVID-19 are on the rise and with a new variant of the disease emerging this summer, the Centers for Disease Control and Prevention (CDC) is recommending updated vaccines ahead of the ...
Studying vaccine availability and uptake in sub-Saharan Africa. The authors' collaboration began in 2021 with research into Covid-19 vaccination rates, people's willingness to get vaccinated, and how people's trust in different authorities shaped attitudes toward vaccines in Uganda, the DRC, Senegal, and Nigeria.
In a human challenge vaccine study, healthy volunteers are given an experimental vaccine, and then deliberately exposed to the organism causing the disease to see if the vaccine works. Some scientists believe that this approach could accelerate COVID-19 vaccine development, in part because it would require far fewer volunteers than a typical study.
To study COVID-19 vaccination status in kidney transplant recipients (KTRs), reasons for incomplete vaccination and the clinical impact of vaccination on patient outcomes. Methods. A single-centre retrospective analysis of KTR (n = 543) conducted between 1970 and December 2022. Data included baseline demographics, number of vaccinations, reason ...
CDC ER and hospitalization data suggests that a Covid-19 summer surge has been occurring in the U.S. since early June, as the FLiRT variants KP.3 and KP.2 spread.
The difficulty of selecting a vaccine strain of virus was on display during the planning for 2024-2025 COVID-19 vaccines. Find out what happened and what it means for this year's updated versions.