- Share full article
Advertisement
Supported by

A Cure for Type 1 Diabetes? For One Man, It Seems to Have Worked.
A new treatment using stem cells that produce insulin has surprised experts and given them hope for the 1.5 million Americans living with the disease.

By Gina Kolata
Brian Shelton’s life was ruled by Type 1 diabetes.
When his blood sugar plummeted, he would lose consciousness without warning. He crashed his motorcycle into a wall. He passed out in a customer’s yard while delivering mail. Following that episode, his supervisor told him to retire, after a quarter century in the Postal Service. He was 57.
His ex-wife, Cindy Shelton, took him into her home in Elyria, Ohio. “I was afraid to leave him alone all day,” she said.
Early this year, she spotted a call for people with Type 1 diabetes to participate in a clinical trial by Vertex Pharmaceuticals. The company was testing a treatment developed over decades by a scientist who vowed to find a cure after his baby son and then his teenage daughter got the devastating disease.
Mr. Shelton was the first patient. On June 29, he got an infusion of cells, grown from stem cells but just like the insulin-producing pancreas cells his body lacked.
Now his body automatically controls its insulin and blood sugar levels.
Mr. Shelton, now 64, may be the first person cured of the disease with a new treatment that has experts daring to hope that help may be coming for many of the 1.5 million Americans suffering from Type 1 diabetes.
“It’s a whole new life,” Mr. Shelton said. “It’s like a miracle.”
Diabetes experts were astonished but urged caution. The study is continuing and will take five years, involving 17 people with severe cases of Type 1 diabetes. It is not intended as a treatment for the more common Type 2 diabetes.
“We’ve been looking for something like this to happen literally for decades,” said Dr. Irl Hirsch, a diabetes expert at the University of Washington who was not involved in the research. He wants to see the result, not yet published in a peer-reviewed journal, replicated in many more people. He also wants to know if there will be unanticipated adverse effects and if the cells will last for a lifetime or if the treatment would have to be repeated.
But, he said, “bottom line, it is an amazing result.”
Dr. Peter Butler, a diabetes expert at U.C.L.A. who also was not involved with the research, agreed while offering the same caveats.
“It is a remarkable result,” Dr. Butler said. “To be able to reverse diabetes by giving them back the cells they are missing is comparable to the miracle when insulin was first available 100 years ago.”
And it all started with the 30-year quest of a Harvard University biologist, Doug Melton.
‘A Terrible, Terrible Disease’
Dr. Melton had never thought much about diabetes until 1991 when his 6-month-old baby boy, Sam, began shaking, vomiting and panting.
“He was so sick, and the pediatrician didn’t know what it was,” Dr. Melton said. He and his wife Gail O’Keefe rushed their baby to Boston Children’s Hospital. Sam’s urine was brimming with sugar — a sign of diabetes.
The disease, which occurs when the body’s immune system destroys the insulin-secreting islet cells of the pancreas, often starts around age 13 or 14. Unlike the more common and milder Type 2 diabetes, Type 1 is quickly lethal unless patients get injections of insulin. No one spontaneously gets better.
“It’s a terrible, terrible disease,” said Dr. Butler at U.C.L.A.
Patients are at risk of going blind — diabetes is the leading cause of blindness in this country. It is also the leading cause of kidney failure. People with Type 1 diabetes are at risk of having their legs amputated and of death in the night because their blood sugar plummets during sleep. Diabetes greatly increases their likelihood of having a heart attack or stroke. It weakens the immune system — one of Dr. Butler’s fully vaccinated diabetes patients recently died from Covid-19.
Added to the burden of the disease is the high cost of insulin, whose price has risen each year.
The only cure that has ever worked is a pancreas transplant or a transplant of the insulin-producing cell clusters of the pancreas, known as islet cells, from an organ donor’s pancreas. But a shortage of organs makes such an approach an impossibility for the vast majority with the disease.
“Even if we were in utopia, we would never have enough pancreases,” said Dr. Ali Naji, a transplant surgeon at the University of Pennsylvania who pioneered islet cell transplants and is now a principal investigator for the trial that treated Mr. Shelton.
For Dr. Melton and Ms. O’Keefe, caring for an infant with the disease was terrifying. Ms. O’Keefe had to prick Sam’s fingers and feet to check his blood sugar four times a day. Then she had to inject him with insulin. For a baby that young, insulin was not even sold in the proper dose. His parents had to dilute it.
“Gail said to me, ‘If I’m doing this you have to figure out this damn disease,’” Dr. Melton recalled. In time, their daughter Emma, four years older than Sam, would develop the disease too, when she was 14.
Dr. Melton had been studying frog development but abandoned that work, determined to find a cure for diabetes. He turned to embryonic stem cells, which have the potential to become any cell in the body. His goal was to turn them into islet cells to treat patients.
One problem was the source of the cells — they came from unused fertilized eggs from a fertility clinic. But in August 2001, President George W. Bush barred using federal money for research with human embryos. Dr. Melton had to sever his stem cell lab from everything else at Harvard. He got private funding from the Howard Hughes Medical Institute, Harvard and philanthropists to set up a completely separate lab with an accountant who kept all its expenses separate, down to the light bulbs.
Over the 20 years it took the lab of 15 or so people to successfully convert stem cells into islet cells, Dr. Melton estimates the project cost about $50 million.
The challenge was to figure out what sequence of chemical messages would turn stem cells into insulin-secreting islet cells. The work involved unraveling normal pancreatic development, figuring out how islets are made in the pancreas and conducting endless experiments to steer embryonic stem cells to becoming islets. It was slow going.
After years when nothing worked, a small team of researchers, including Felicia Pagliuca, a postdoctoral researcher, was in the lab one night in 2014, doing one more experiment.
“We weren’t very optimistic,” she said. They had put a dye into the liquid where the stem cells were growing. The liquid would turn blue if the cells made insulin.
Her husband had already called asking when was she coming home. Then she saw a faint blue tinge that got darker and darker. She and the others were ecstatic. For the first time, they had made functioning pancreatic islet cells from embryonic stem cells.
The lab celebrated with a little party and a cake. Then they had bright blue wool caps made for themselves with five circles colored red, yellow, green, blue and purple to represent the stages the stem cells had to pass through to become functioning islet cells. They’d always hoped for purple but had until then kept getting stuck at green.
The next step for Dr. Melton, knowing he’d need more resources to make a drug that could get to market, was starting a company.
Moments of Truth
His company Semma was founded in 2014, a mix of Sam and Emma’s names.
One challenge was to figure out how to grow islet cells in large quantities with a method others could repeat. That took five years.
The company, led by Bastiano Sanna, a cell and gene therapy expert, tested its cells in mice and rats, showing they functioned well and cured diabetes in rodents.
At that point, the next step — a clinical trial in patients — needed a large, well financed and experienced company with hundreds of employees. Everything had to be done to the exacting standards of the Food and Drug Administration — thousands of pages of documents prepared, and clinical trials planned.
Chance intervened. In April 2019, at a meeting at Massachusetts General Hospital, Dr. Melton ran into a former colleague, Dr. David Altshuler, who had been a professor of genetics and medicine at Harvard and the deputy director of the Broad Institute. Over lunch, Dr. Altshuler, who had become the chief scientific officer at Vertex Pharmaceuticals, asked Dr. Melton what was new.
Dr. Melton took out a small glass vial with a bright purple pellet at the bottom.
“These are islet cells that we made at Semma,” he told Dr. Altshuler.
Vertex focuses on human diseases whose biology is understood. “I think there might be an opportunity,” Dr. Altshuler told him.
Meetings followed and eight weeks later, Vertex acquired Semma for $950 million. With the acquisition, Dr. Sanna became an executive vice president at Vertex.
The company will not announce a price for its diabetes treatment until it is approved. But it is likely to be expensive. Like other companies, Vertex has enraged patients with high prices for drugs that are difficult and expensive to make.
Vertex’s challenge was to make sure the production process worked every time and that the cells would be safe if injected into patients. Employees working under scrupulously sterile conditions monitored vessels of solutions containing nutrients and biochemical signals where stem cells were turning into islet cells.
Less than two years after Semma was acquired, the F.D.A. allowed Vertex to begin a clinical trial with Mr. Shelton as its initial patient.
Like patients who get pancreas transplants, Mr. Shelton has to take drugs that suppress his immune system. He says they cause him no side effects, and he finds them far less onerous or risky than constantly monitoring his blood sugar and taking insulin. He will have to continue taking them to prevent his body from rejecting the infused cells.
But Dr. John Buse, a diabetes expert at the University of North Carolina who has no connection to Vertex, said the immunosuppression gives him pause. “We need to carefully evaluate the trade-off between the burdens of diabetes and the potential complications from immunosuppressive medications.”
Mr. Shelton’s treatment, known as an early phase safety trial, called for careful follow-up and required starting with half the dose that would be used later in the trial, noted Dr. James Markmann, Mr. Shelton’s surgeon at Mass General who is working with Vertex on the trial. No one expected the cells to function so well, he said.
“The result is so striking,” Dr. Markmann said, “It’s a real leap forward for the field.”
Last month, Vertex was ready to reveal the results to Dr. Melton. He did not expect much.
“I was prepared to give them a pep talk,” he said.
Dr. Melton, normally a calm man, was jittery during what felt like a moment of truth. He had spent decades and all of his passion on this project. By the end of the Vertex team’s presentation, a huge smile broke out on his face; the data were for real.
He left Vertex and went home for dinner with Sam, Emma and Ms. O’Keefe. When they sat down to eat, Dr. Melton told them the results.
“Let’s just say there were a lot of tears and hugs.”
For Mr. Shelton the moment of truth came a few days after the procedure, when he left the hospital. He measured his blood sugar. It was perfect. He and Ms. Shelton had a meal. His blood sugar remained in the normal range.
Mr. Shelton wept when he saw the measurement.
“The only thing I can say is ‘thank you.’”
Gina Kolata writes about science and medicine. She has twice been a Pulitzer Prize finalist and is the author of six books, including “Mercies in Disguise: A Story of Hope, a Family's Genetic Destiny, and The Science That Saved Them.” More about Gina Kolata
What to Know About Diabetes
Diabetes, a condition in which the body has trouble regulating blood sugar, is increasingly common among americans..
Over 37 million Americans have some form of diabetes. Scientists say that medical care won’t be enough to halt the spread of the disease: Sweeping societal changes are needed .
Insulin resistance can be a precursor to diabetes and pre-diabetes. Here is what to know about the condition and how to know if you have it .
For people with Type 1 diabetes, which often strikes in adolescence, staying healthy can be exhausting . A treatment that can delay the disease’s onset offers some hope .
People who regularly eat red meat may have a higher risk of Type 2 diabetes later in life , according to a new study. Those who often consume processed meats have an even greater risk.
Healthy practices can delay and prevent Type 2 diabetes. Something as simple as going for a 15-minute walk after a meal could help ward off the disease.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 16 October 2020
Risk factors for type 1 diabetes, including environmental, behavioural and gut microbial factors: a case–control study
- Deborah Traversi 1 , 8 ,
- Ivana Rabbone 2 , 7 ,
- Giacomo Scaioli 1 , 8 ,
- Camilla Vallini 2 ,
- Giulia Carletto 1 , 8 ,
- Irene Racca 1 ,
- Ugo Ala 5 ,
- Marilena Durazzo 4 ,
- Alessandro Collo 4 , 6 ,
- Arianna Ferro 4 ,
- Deborah Carrera 3 ,
- Silvia Savastio 3 ,
- Francesco Cadario 3 ,
- Roberta Siliquini 1 , 8 &
- Franco Cerutti 1 , 2
Scientific Reports volume 10 , Article number: 17566 ( 2020 ) Cite this article
7016 Accesses
15 Citations
28 Altmetric
Metrics details
- Microbiology
- Molecular biology
- Risk factors
Type 1 diabetes (T1D) is a common autoimmune disease that is characterized by insufficient insulin production. The onset of T1D is the result of gene-environment interactions. Sociodemographic and behavioural factors may contribute to T1D, and the gut microbiota is proposed to be a driving factor of T1D. An integrated preventive strategy for T1D is not available at present. This case–control study attempted to estimate the exposure linked to T1D to identify significant risk factors for healthy children. Forty children with T1D and 56 healthy controls were included in this study. Anthropometric, socio-economic, nutritional, behavioural, and clinical data were collected. Faecal bacteria were investigated by molecular methods. The findings showed, in multivariable model, that the risk factors for T1D include higher Firmicutes levels (OR 7.30; IC 2.26–23.54) and higher carbohydrate intake (OR 1.03; IC 1.01–1.05), whereas having a greater amount of Bifidobacterium in the gut (OR 0.13; IC 0.05 – 0.34) was a protective factor for T1D. These findings may facilitate the development of preventive strategies for T1D, such as performing genetic screening, characterizing the gut microbiota, and managing nutritional and social factors.
Similar content being viewed by others

Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome

Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes

Diet, lifestyle and gut microbiota composition among Malaysian women with gestational diabetes mellitus: a prospective cohort study
Introduction.
Type 1 diabetes (T1D) is a multifactor disease caused by β-cell destruction (which is mostly immune-mediated) and absolute insulin deficiency. At present, the management of T1D has been improved, but the disease remains incurable. T1D onset is most common in childhood. T1D represents approximately 5–10% of all diabetes diagnoses 1 . Between 70 and 90% of T1D patients at diagnosis exhibit evidence of an immune-mediated process with β-cell autoantibodies. T1D onset is preceded by a preclinical period that lasts approximately 3 years, in which autoantibodies appear in the circulatory system 2 . Immune destruction of the β-cells can be detected by the evaluation of some haematic markers 3 . The disease has strong HLA associations, which explain nearly half of the genetic disease predisposition, while the remainder is due to other genetic polymorphisms 3 , 4 .
Analysis of genetic disease susceptibility suggests that there is a greater risk of T1D development when the father is affected by the disease than when the mother is affected 5 . On the other hand, there is evidence that a critical role is played by non-genetic factors, including both environmental and host-related factors, which are considered to play decisive roles in the disease process, leading to the manifestation of clinical T1D 6 .
The worldwide incidence of T1D in the age group of 0–15 years varies considerably by region (from 0.5 to 60 per 100,000 children), and the yearly increase ranges from 0.6% to 9.3%. In Europe, the percentage of cases in the age group of 0–15 years will rise by 70% 7 . In the Piedmont region, up to 2013, there were approximately 8,000 cases in this age group with an incidence of 27 new diagnoses per 100,000 8 . Migrant populations tend to show an incidence of diabetes similar to that of most host populations; therefore, a higher T1D incidence in migrant children was observed in Europe 6 , 9 , 10 . Such a pronounced increase in incidence cannot be attributable to genetic factors alone. Other major risk factors may include the environment, Western lifestyle and nutrition 10 . Other diseases with immune involvement, such as allergies, exhibit a similar trend, suggesting an inductor role for exogenous factors regarding the increased predisposition to autoimmunity 11 . Preventive measures to reduce the incidence of T1D have not been defined to date. Various factors seem to be involved in modulating the incidence of T1D, including birth delivery mode, feeding, birth weight, infections (especially viral), dietary behaviour, and pharmaceutical use (especially antibiotics). Such factors may contribute to T1D development during the early disease stage 12 ; however, compared with genetic factors, environmental factors are less well characterized 13 . β- Cell vulnerability to stress factors has been discussed as the basis of the overload hypothesis 14 . Associations among the microbiome, metabolome, and T1D were shown, highlighting a host-microbiota role in the onset of the disease 12 , 15 . The origin of the disease process was suspected to be gut microbiota dysbiosis (imbalances in the composition and function of intestinal microbes) associated with altered gut permeability and a major vulnerability of the immune system 6 . Accordingly, evidence obtained from both animal models and human studies suggests that the gut microbiota and the immune system interact closely, emphasizing the role of the intestinal microbiota in the maturation and development of immune functions 16 . Recently, mycobiome-bacteriome interactions, as well as intestinal virome and islet autoimmunity, were hypothesized to be drivers of dysbiosis 17 . Several studies have specifically investigated microbiota composition in children with T1D 18 , 19 , 20 , but the results have not been consistent. Interestingly, most studies are in agreement regarding the reduced microbial diversity observed in subjects with T1D compared with controls; moreover, the microbiota structure in T1D subjects was found to be different from that of control subjects 21 , 22 . To date, a typical T1D-associated microbiota has not been identified 23 , 24 , 25 , 26 . The research also determined that T1D clinical management could be improved by in-depth analysis of the partial remission phase 27 ; however, preventive measures are limited and generally focus only on genetic susceptibility 28 and general population screening for islet autoimmunity 29 . The development of an integrated prediction strategy could be useful for increasing early diagnosis while avoiding onset complications by identifying children at risk of T1D to place under observation and, in the future, to treat with preventive methods 10 .
The aim of this study is to identify environmental, behavioural, and microbial risk factors of T1D onset to develop an integrated T1D preventive management strategy that is suitable for paediatricians in the Piedmont region.
Subject description and origin factor analysis
To analyse the origin factor, the study population was subdivided by the children's origins (Italian and migrant, 69 and 27 children, respectively). An analysis of the socio-demographic and behavioural factors examined in the study showed many differences between Italian and migrant children, while other variables appear to be quite homogeneous (Table 1 ). In the studied cohort, migrant status did not produce a significant increase in T1D onset.
Approximately 79% of the children in the cohort had siblings; approximately 40% of the included children lived with a pet in the house, and more than 65% of the children took antibiotics during the first two years of life. The residency zone was notably different between Italians and migrants: the percentage of migrant children living in urban sites was higher but not significant following the adjusted model. Regular sports activities seem to be practised more by Italian children than by migrant children (73.5% vs 51.8%, p = 0.054). A total of 77.9% of Italian children and 55.6% of migrant children were subjected to regular health check-ups (p = 0.017). A significant difference was confirmed for the ages of the migrant mother and father (Table 1 ), meanly 6 years and 4 years younger respectively at recruitment, respect the Italians (p = 0.017 and p = 0.0425). The analysis of eating habits and nutritional intake revealed that the majority of the children were breastfed. Moreover, the weaning age was 6 months, as recommended. Migrant children showed higher total carbohydrate intake (+ 12%, p = 0.044) and simple carbohydrate intake (+ 24%, p = 0.0045). Moreover, among migrants, the children tended to access food by themselves and to consume meals alone. The percentage of migrant children who ate meals while watching TV was higher but not significant. Finally, the one-course meal was more frequent in migrant families (ratio 1:3, p = 0.006).
The analysis of microbiota and bioindicator species displayed no significant differences between Italian and migrant children: the qRT-PCR measurements showed a trend of greater value for the total bacteria (both for the experimental design with and without probe), Bacteroides and M. smithii (both using 16S rDNA and nifH) in migrant children. The DGGE profile and dendrogram analysis did not show a different clustering pattern based on the origin, and the migrant group showed a trend towards greater α-diversity of the faecal microbiota profiles (Shannon index + 5%). Additionally, the α-diversity analyses in next generation sequencing (NGS) showed a difference in taxonomic units (OTUs), i.e., there were more OTUs in migrants than in Italians, but the difference was not significant, though it was close to the limit of significance (p = 0.057). Furthermore, the phylogenetic diversity index (Faith PD) suggested that the origin of the subjects could influence the structure of the microbial community. Although the overall number of OTUs did not change significantly, the phylogenetic distance of the individual OTUs was greater in the migrant group than in the Italian group, as the OTUs occupied a broader ecological niche in the migrant group.
T1D risk factors
Previous results indicated that being a migrant child in the Piedmont region is not a significant risk factor for T1D onset 30 . Table 2 shows single logistic regressions performed to estimate the impact of the different variables on the outcome. Notably, the analysis of socio-demographic, behavioural, and nutritional determinants revealed that having parents with at least a high school certificate seems to be a protective factor for T1D onset, even if not significant after adjusted comparisons.
High total caloric intake, as well as high protein intake and consumption of total carbohydrates, are associated with only a slightly increased risk of T1D onset.
The DGGE gel and the results of the cluster analysis are shown in Fig. 1 . The Pearson similarity clustering showed macro beta-diversity differences between the T1D patients and healthy children, with the main division being in two different clusters.
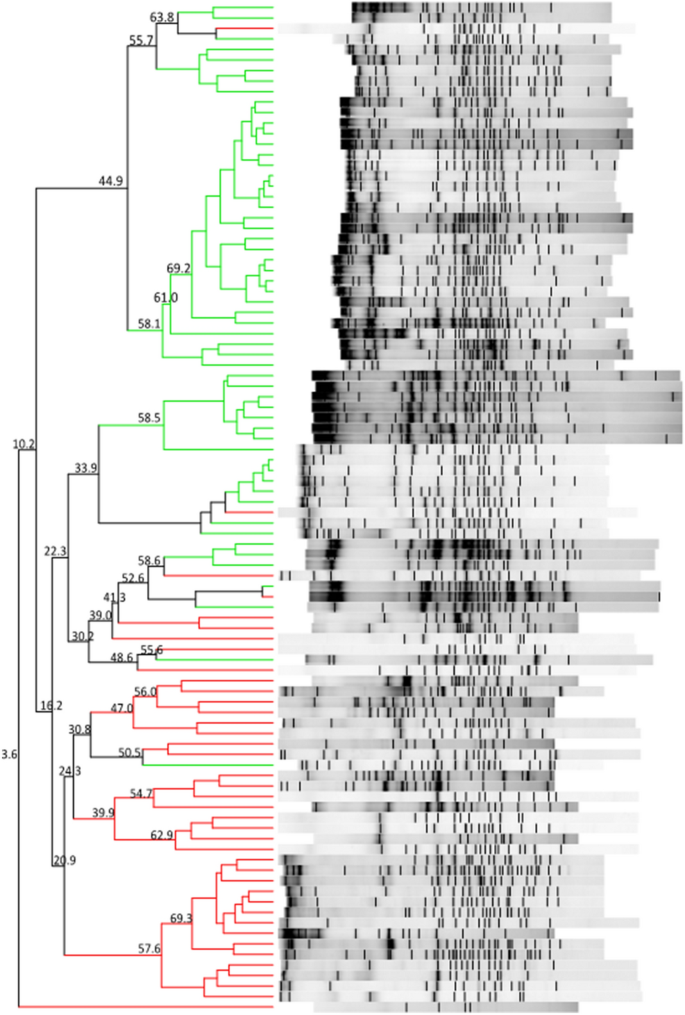
DGGE banding patterns and the results of the analysis in which the Pearson coefficient (numbers reported near the nodes) was used for measuring similarity in banding patterns. The cluster identifies T1D patients (red lines) and healthy children (green lines).
Firmicutes and Bacteroidetes followed by Proteobacteria and Actinobacteria (Table 3 ) predominantly composed the gut microbiota of all children. In the children with diabetes, an increase in the levels of three members of Bacteroidetes ( Alistipes senegalensis , Bacteroides timonensis , and Barnesiella intestinihominis ) and three members of Firmicutes ( Christensenella timonensis ,
Ruminococcus bromii , and Urmitella timonensis ) was observed by sequencing.
Furthermore, other notable results were obtained by NGS analyses. The taxonomic analysis revealed that the gut microbiota of the study participants was composed of nine relevant phyla: Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia, Euryarchaeota, Tenericutes, Cyanobacteria, and an unclassified phylum.
Moreover, beta-diversity analyses were carried out to highlight the differences among the samples based on the structures of their microbial communities. The weighted UniFrac metric showed that the samples were not subdivided into clusters. The intragroup and intergroup distances were comparable, and there was no separation between the clusters. These findings were confirmed by the Permanova test. Finally, analyses of the differential abundance were performed to compare the increase or decrease in the abundance of one or more bacteria in the case and control groups. DeSeq2 showed 48 significantly abundant OTUs (p < 0.001). The most abundant OTU was Rikenellaceae followed by Prevotellaceae ( Prevotella copri ), Barnesiellaceae , Lachnospiraceae, and Ruminococcaceae ( Ruminococcus bromii ), which were significantly more abundant in children with diabetes.
The difference in the results observed between methods is an interesting discussion point. The methods are characterized by different sensitivities; they represent different molecular perspectives regarding the faecal microbiota. When a method with a higher sensibility is used (NGS), a flattening effect is possible. On the other hand, the major abundance of such genera as Ruminococcu s was confirmed by different microbiota study methods, which is in keeping with the qRT-PCR results. A group of 23 samples showed different clusterization compared to the others (Fig. 2 , left). This small group was not different from the main group regarding any characteristics. The only significant difference was observed for the M. smithii presence and the A. muciniphila levels, both of which were higher in the separated group (Fig. 2 , right). A. muciniphila was proposed as a probiotic 31 , while M. smithii has been characterized as the most abundant methanogen in the gut 32 .
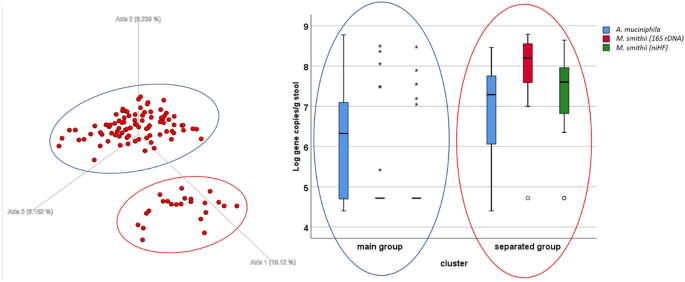
Left-Unweighted UniFrac graph of the NGS results. There are two identifiable groups: the blue circle (main group) and the red circle (separated group). No experimental hypothesis was confirmed for the cluster definition. On the Right: box plot of the qRT-PCR results for some microbiological targets ( Akkermansia muciniphila and Methanobrevibacter smithii ), the difference between the groups is significant (t-test p < 0.05).
The qRT-PCR gut microbiota analysis indicated significant differences among T1D patients and healthy children (Table 2 ). The logistic regression analysis showed that the increase in the Margalef index was associated with a decrease in the likelihood of disease onset (OR 0.20; 95% CI 0.09–0.46, p = 0.000). Increased Firmicutes levels and decreased Bacteroidetes levels were significant risk factors for T1D (OR 7.49; 95% CI 3.25–17.28, p = 0.0001; OR 0.28; 95% CI 0.15–0.51 p = 0.0001, respectively). Moreover, Bifidobacterium spp. was a protective factor for T1D onset (OR 0.20; 95% CI 0.10–0.38, p = 0.0001).
The multivariable analysis produced a R 2 = 0.6259 (p < 0.001). After adjusting for confounding factors, the likelihood of having diabetes is significantly higher in those with higher amount of Firmicutes, lower amount of Bifidobacterium spp and a higher amount of total carbohydrate intake (Table 4 ).
T1D is an important disease that affects health with onset primarily occurring in childhood. At present, there is no cure for this disease, and only disease management is possible. The disease burden of T1D is immense, especially considering the number of years of life lost due to disability but also the years of life lost due to premature death. The life expectancy for T1D patients is approximately 16 years shorter than that of the comparable healthy population 33 . Even if relevant risk factors are known, to date, such scientific determinants do not include a screening programme for preventive purposes. Of course, preventive action must be considered as a systematic process that focuses on the main risk factors to identify children at higher risk of T1D and to suggest efficacious preventive treatments. In the study, the main T1D onset risk factors seem to be identifiable in the composition of the microbiota and, in particular, the microbiota α-diversity, Firmicutes and Bacteroidetes levels and their ratio, as well as the Bifidobacterium level. Similar evidence was obtained by other studies, which observed both higher Bacteroidetes in T1D patients 34 , 35 and less abundant anti-inflammatory genera in children with multiple islet autoantibodies 36 . Reduced microbial diversity appears to become significant between seroconversion and overt T1D 15 . A significant difference in the Bifidobacterium level was observed in different studies, including both a small cohort of autoimmune children 37 , 38 and a larger population associated with such protective factors as breastfeeding 21 . At the genus level, a significant difference in, for example, Blautia (increased in patients), was observed 39 ; however, in other studies, different single species ( Bacteroides ovatus ) seem to be more abundant in patients than in the controls 18 . However, prior studies suggest the presence of duodenal mucosa abnormalities in the inflammatory profile for T1D patients 22 , 40 and on the T1D-related changes in the gut microbiota, even if proving the causality of these factors has remained challenging 21 .
The characterization of the microbiota is rapidly evolving. Traditional methods that are not as sensitive as PCR-DGGE are still suitable, while NGS methods are expanding. Sophisticated whole-genome sequencing methods integrated with metabolomics and proteomics have been proposed. However, the large amount of data, being affected by multiple confounding factors, has not had a clear impact on T1D prevention strategies. The development of a simple method to describe microbiota modulation using validated biomarkers, which could serve as a rapid screening test, may be warranted.
Another risk factor is the occurrence of stress due to a traumatic or emotional experience. This stress seems to be able to affect the autoimmunity process. Therefore, particular attention could be paid to such risk factors for T1D risk in children.
A high education level of one or both parents could be also protective, suggesting that socioeconomic factors affect the T1D risk. Other factors, identified as significant risk modulators among behavioural and nutritional factors, had minor effects.
The study has some potential limitations, including susceptibility to bias in recollection about exposure and reverse causality. The exposure recollection could be biased, but this issue can be less influential at the onset, as in this study. Moreover, recruitment at the onset guarantees a temporal coherence of the exposure with respect to the disease onset.
T1D is one of the most frequently diagnosed diseases in children; however, it is not a high-incidence disease. The prospective inclusion of a large number of healthy children, which is needed for the observation of enough cases, requires a very long time of observation. Moreover, a restricted age range was necessary in children for the rapid changes in behaviour and microbiota. This requirement resulted in an additional included subject restriction. On the other hand, the study of multifactorial diseases with poorly understood pathogenic pathways is imperative, even if it is at risk for obtaining less conclusive evidence. Of course, such a study alone could not elucidate the causation process, but the evidence obtained could be important for the selection of higher-risk subpopulations, planning of future research, and improving prevention.
Identification of a higher-risk subpopulation is strictly relevant for the subsequent validation of an efficient preventive screening to be produced with a prospective method. Of course, the pathogenesis of type 1 diabetes has not been fully elucidated to date; however, in this study, various factors (associated with both the disease and the microbiota composition) were included, such as the origin of the children, the age of the mother, the age of breastfeeding and the age of weaning. Other possible confounding factors not included in our analysis are viral infections, particularly enteroviruses, and preterm birth; however, there was no clear consensus regarding these novel factors at the beginning of the study.
Concerning the microbiota, the knowledge is still incomplete, and various factors can interact to produce a T1D risk modulation that is not explainable at present. Moreover, the results obtained using different techniques were also dissimilar (for example, clusterization due to β-diversity analysis). This finding is likely due to the different sensitivities of the applied methods 41 . Furthermore, even if the time between the symptom comparison and the diagnosis is very short, there is a danger of biased estimates due to reverse causality.
In conclusion, this study confirmed that T1D onset risk is modulated by compositional changes in the gut microbiota and that such evidence must be employed to devise preventive measure. The results showed that the gut microbial indicators found in children with T1D differ from those found in healthy children. These findings also pave the way for new research attempting to develop strategies to control T1D development by modifying the gut microbiota. However, a better knowledge of gut microbial composition associated with the development of T1D must be obtained to choose the best treatment 10 , 42 , 43 , 44 , 45 .
In brief, direct or indirect manipulations of the intestinal microbiome may provide effective measures for preventing or delaying the disease process leading to the manifestation of clinical T1D. At present, a preventive strategy could be developed that includes the main genetic and microbiome risk factors. Then, this strategy could be applied to healthy children to reduce the burden of T1D.
Study design and participants
The case–control study began in January 2016 46 and ended in September 2018 (case–control phase of clinicaltrial.gov Protocol ID: G12114000080001). The work was conducted following the STROBE Statement for a case–control study. The activity is bicentric and includes the two main paediatric hospitals in the Piedmont region (located in Torino and Novara), which cover the clinical management for cases of T1D in the region. The ethics committees of the two hospitals approved the research activities during 2015 (“Comitato etico interaziendale A.O.U. Ordine Mauriziano di Torino ASLTO1” with record number 0117120 and “Comitato etico Interaziendale A.O.U. “Maggiore della Carità” ASL BI, NO, VCO” record number 631/CE).
The recruitment included 40 paediatric patients with T1D (cases) and 56 healthy children (controls), who were comparable in terms of age, gender, and ethnicity to avoid bias. The included subjects represent the most convenient sample possible. The inclusion criteria were age (5–10 years), normal weight, and residence in Piedmont. Exclusion criteria were celiac disease, chronic disease diagnosis, eating disorders, active infections, use of antibiotics and/or probiotics and/or any other medical treatment that influences intestinal microbiota during the 3 months before recruitment and children with parents of mixed origins (Italian and migrant) for the exclusion of important confounding factors due to genetic and cultural mixed backgrounds 19 .
The T1D children were integrated into the study at disease onset, with hyperglycaemia, with or without ketoacidosis, polyuria symptoms, a high value of glycated haemoglobin (HbA1c > 42 mmol/mol) and T1D-specific autoantibody positivity. Healthy children were contacted by paediatricians in the territory of the acute care system. The guardians of the enlisting children read, understood, and then signed informed consent forms following the declaration of Helsinki. A module is prepared for parents, children, and mature children 47 . All the following methods were carried out following relevant guidelines and regulations when available. A questionnaire was given to the parents containing items and questions to retrieve data on the family contest with particular regards to emotive stressors, such as mourning or separation, anthropometrics, and socio-demographic, nutritional, and behavioural information.
Anthropometric and nutritional data included weight, height, body mass index (BMI), food frequency based on 24-h recall and a food frequency questionnaire (FFQ), neonatal feeding, and age of weaning. The anthropometric parameters (weight and height) were measured according to standard recommendations. The BMI values were interpreted according to the WHO criterion. The 24-h recall technique reconstructed the meals and food intake on a recent "typical" day, estimating the bromatological inputs according to a food composition database for epidemiological studies in Italy (BDA). The FFQ, developed for the study, focused on the consumption of certain food categories (those containing sugars, fibre, omega-3, calcium, vitamin D, condiments, and cereals) and eating habits (e.g., alone or with adults, in front of the TV).
Twenty-eight percent of the involved population is migrants (both parents not Italian). Such data are consistent with the percentage of newborns from non-Italian mothers, which is approximately 30% in northern Italy 48 . The migrant group included children coming mainly from northern Africa and Eastern Europe. The migration involved the parents and sometimes the children; on average, the included children as migrants were residents in Italy for less than 5 years. At the end of recruitment, no significant differences were observed between the case and control groups for age, sex composition, and origins (criteria for pairing) or for height, weight, and BMI (T-test, p > 0.05) (Table 5 ).
Sample collection and DNA extraction
A kit for stool collection was delivered to each study participant following a validated procedure 49 , 50 and using a Fecotainer device (Tag Hemi VOF, Netherlands). Faecal samples were homogenized within 24 h in the laboratory, and five 2 g aliquots were stored at − 80 °C until DNA isolation was performed. Total DNA extractions from the stool samples were performed using the QiaAmp PowerFecal DNA Kit (QIAGEN, Hilden, Germany). The nucleic acids were quantified using a NanoQuant Plate (TECAN Trading AG, Switzerland), which allows quantification using a spectrophotometer read at 260 nm. The spectrophotometer used was the TECAN Infinite 200 PRO, and the software was i-Control (version 1.11.10). The extracted DNA concentrations ranged from 1.1–155.5 ng/μl (mean 41.35 ± 38.70 ng/μL). Samples were stored at –20 °C until molecular analysis was performed.
The PCR products for denaturing gradient gel electrophoresis (DGGE) were obtained by amplifying the bacterial 16S rRNA genes following a marker gene analysis approach 51 . The primer pairs were 357F-GC and 518R (Table 6 ) 52 . All PCRs were performed with the T100 Bio-Rad Thermocycler in a 25-μl reaction volume containing 1X Master Mix (166–5009, Bio-Rad, Berkeley, CA, USA), 0.02 bovine serum albumin (BSA), 0.4 μM of each primer, and 2 μl of DNA diluted 1:10 in sterile DNase-treated water. DGGE was carried out using a DCode System (Bio-Rad) with a 30–50% denaturing gradient of formamide and urea 53 . Electrophoresis ran at 200 V for 5 h at 60 °C in 1X TAE buffer. Gels were stained for 30 min with SYBR Green I nucleic acid gel stain (10.000X in DMSO, S9430, Sigma-Aldrich, USA) and were visualized using the D-Code XR apparatus from Bio-Rad. Then, DGGE bands were excised, incubated overnight at − 20 °C, washed, and crushed in 20 μl of molecular-grade water. The supernatant (2 μl) was used as a template and reamplified, as previously described, without BSA and using modified linker-PCR bacterial primers (357F-GC; 518R-AT-M13) (Table 6 ) 19 , 52 , 54 , 55 , 56 , 57 , 58 , 59 , 60 . The obtained PCR products were sequenced with Sanger sequencing (Genechron-Ylichron S.r.l.). The sequence similarities were obtained by the National Centre for Biotechnology Information (NCBI) database using nucleotide Basic Local Alignment Search Tool (BLASTn) analysis.
High-throughput DNA sequencing and analysis were conducted by BMR Genomics s s.r.l. The V3-V4 region of 16S rDNA was amplified using the MiSeq 300PEPro341F and Pro805R primer pair 6 . The sample reads were above 12*10 6 . The reaction mixture (25 μl) contained 3–10 ng/μl genomic DNA, Taq Platinum HiFi (Invitrogen, Carlsbad, CA), and 10 μM of each primer. The PCR conditions for amplification of DNA were as follows: 94 °C for 1 min (1X), 94 °C for 30 s, 55 °C for 30 s, 68 °C for 45 s (25X), and 68 °C for 7 min (1X). PCR products were purified through Agencourt XP 0.8X Magnetic Beads and amplified shortly with the Index Nextera XT. The amplicons were normalized with SequalPrep (Thermo Fisher) and multiplexed. The pool was purified with Agencourt XP 1X Magnetic Beads, loaded onto MiSeq, and sequenced with the V3 chemistry-300PE strategy.
Starting from the extracted DNA, the following microbial targets were quantified by qRT-PCR using a CFX Touch Real-Time PCR Detection System (Bio-Rad-Hercules, CA) and CFX Manager (3.1 Software): total Bacteria, Bacteroidetes, Bacteroides spp., Firmicutes, Bifidobacterium spp., Akkermansia muciniphila, and Methanobrevibacter smithii . Total bacteria and M. smithii were detected following two reaction designs. For M. smithii , the analysis was performed using as target both the 16S rDNA and then a specific functional gene ( nifH ). For total bacteria, quantification was carried out using a protocol with or without a probe. For the determination of total bacteria (method without probe), Bacteroidetes, Bacteroides spp., Firmicutes, Bifidobacterium spp. and Akkermansia muciniphila , 2 µl of 1:10 extracted DNA was added to a reaction mixture consisting of 10 µl Sso Advance SYBR Green Supermix (172–5261, Bio-Rad), 0.5 µl each of the forward and reverse primers (10 µM final concentration) and 7 µl of ultrapure water in a 20 µl final reaction volume. The reaction conditions were set as follows: 95 °C for 3 min (1X), 95 °C for 10 s, and 59 °C for 15 s (57 °C for Bacteroidetes spp. and 60 °C for Firmicutes), 72 °C for 10 s (39X), 65 °C for 31 s, 65 °C for 5 s + 0.5 °C/cycle, ramp 0.5 °C/s (60X). Moreover, for the determinations of M. smithii and total bacteria (method with probe), the reaction was as follows. Two microlitres of 1:10 extracted DNA was added to a reaction mixture consisting of 10 µl IQ Multiplex PowerMix (Bio-Rad-Hercules, CA), 0.2 µl of the molecular probe (10 µM), 0.5 µl each of the forward and reverse primers (10 µM final concentration) and 6.8 µl of ultrapure water in a 20 µl final reaction volume. The reaction conditions were 95 °C for 3 min (1X), 95 °C for 10 s, 59 °C for 15 s, 72 °C for 15 s (39X), and 72 °C for 5 min. Standard curves were produced with serial six-fold dilutions of genomic DNA from the microorganism target, provided by ATCC (Manassas, Virginia, USA) or DSMZ (Braunschweig, Germany). All PCR tests were carried out in triplicate. Table 6 provides detailed information regarding oligonucleotide sequences and genomic standards 19 , 54 , 55 , 56 , 57 , 58 , 59 , 60 . The PCR efficiencies were always between 90 and 110%. To confirm the amplification of each target, gel electrophoresis was performed on 2% agarose gels.
Data elaboration and statistical analyses
The statistical analysis was performed using STATA version 11.0. Moreover, the data on the included T1D patients and healthy controls were elaborated to highlight the likelihood of having diabetes. A descriptive analysis of the variables was conducted. The data were reported as absolute numbers and percentages for categorical variables and as means and standard deviations for continuous variables. Moreover, the subjects were divided by individual origins into two groups: Italian and migrant, considering the origin of the children and their families, to show differences in the distribution of disease determinants and to assess whether being a migrant could be associated with T1D onset. Differences between Italian and migrant children were assessed using the χ 2 test with Fisher’s correction for categorical variables and Student’s t-test for continuous variables. Univariable logistic regression was then performed to estimate the impact of sociodemographic, nutritional, and microbiota-related variables on the outcome. These associations were expressed as odds ratios (OR) at a 95% confidence interval (CI). Moreover, the adjusted p-value for multiple comparisons was calculated using the Benjamini and Hochberg false discovery rate method. We conducted multivariable analyses including various variables (age, gender, Firmicutes, Bifidobacterium spp ., and total carbohydrate intake) and the risk of type 1 diabetes using logistic regression models. The Spearman rank-order correlation coefficient was also determined to assess the relationships between variables. A p-value p < 0.05 was considered significant for all analyses.
The DGGE gel analysis was performed with Bionumerics 7.2. The hierarchical classification was performed with a UPGMA system (1% tolerance and optimization level) and Pearson correlation. Simpson's diversity index, Shannon’s index, and Margalef index were calculated for each DGGE profile to evaluate alpha diversity.
NGS bioinformatics analysis was performed with the software pipeline Qiime2. The reads were cleaned up by the primers using the software Cutadapt (version 2018.8.0) and processed with the software DADA2. The sequences were trimmed at the 3′ end (forward: 270 bp; reverse 260 bp), filtered by quality, and merged with default values. Subsequently, the sequences were elaborated to obtain unique sequences. In this phase, the chimaeras (denoised-paired) are also eliminated. The sequences were clustered against unique sequences at 99% similarity. The taxonomies of both GreenGenes (version 13–8) and Silva (version 132) were assigned to the OTU sequences. Alpha-diversity analyses were performed on all samples using the observed OTUs, Shannon, Pielou's evenness, and Faith PD indices, and for each index, the Kruskal–Wallis test was used to verify the significance of the comparisons between samples. Beta-diversity analyses were performed on all samples using the Bray–Curtis, Jaccard, and UniFrac metrics (weighted and unweighted). Multivariable statistical analyses were performed using the PERMANOVA, Adonis, and ANOSIM tests; instead, the analysis of the differential abundance was based on the packages of R (MetagenomeSeq, DeSeq2, and ANCOM).
Data availability
The database includes human data that are available upon reasonable request.
National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2017. Estimates of Diabetes and Its Burden in the United States. CDC (2017).
Mikael Knip, Md, P. et al. Prediction of Type 1 Diabetes in the General Population. Diabetes Care 33 , 1206–1212 (2010).
American Diabetes Association. Standard medical care in diabetes - 2018. Diabetes Care 41 , 1–159 (2018).
Article Google Scholar
Pociot, F. & Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet 387 , 2331–2339 (2016).
Article CAS PubMed Google Scholar
Turtinen, M. et al. Characteristics of familial type 1 diabetes : effects of the relationship to the affected family member on phenotype and genotype at diagnosis. (2019).
Knip, M., Luopajärvi, K. & Härkönen, T. Early life origin of type 1 diabetes. Semin. Immunopathol. 39 , 653–667 (2017).
World Health Organization. Global Report on Diabetes . (2016).
Bruno, G. Il registro diabete Piemonte. Ital. Heal. Policy Br. 1–8 (2016).
Regnell, S. E. & Lernmark, Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 60 , 1370–1381 (2017).
Knip, M. & Honkanen, J. Modulation of type 1 diabetes risk by the intestinal microbiome. Curr. Diab. Rep. 17 , 4–11 (2017).
Article CAS Google Scholar
Bach, J.-F. & Chatenoud, L. The hygiene hypothesis : an explanation for the increased frequency of insulin. Cold Sping Harb. Perpect. Med. 2 , a007799 (2012).
Google Scholar
Zununi Vahed, S., Moghaddas Sani, H., Rahbar Saadat, Y., Barzegari, A. & Omidi, Y. Type 1 diabetes: through the lens of human genome and metagenome interplay. Biomed. Pharmacother. 104 , 332–342 (2018).
Butalia, S., Kaplan, G. G., Khokhar, B. & Rabi, D. M. Environmental risk factors and type 1 diabetes: past, present, and future. Can. J. Diabetes 40 , 586–593 (2016).
Article PubMed Google Scholar
Ilonen, J., Lempainen, J. & Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 15 , 635–650 (2019).
Siljander, H., Honkanen, J. & Knip, M. Microbiome and type 1 diabetes. EBioMedicine 46 , 512–521 (2019).
Hooper, L. V., Littman, D. R., Macpherson, A. J. & Program, M. P. Interactions between the microbiota and the immune system. 336 , 1268–1273 (2012).
CAS Google Scholar
Davis-richardson, A. G. & Triplett, E. W. On the role of gut bacteria and infant diet in the development of autoimmunity for type 1 diabetes. Reply to Hänninen ALM and Toivonen RK [ letter ]. 2197–2198 (2015). doi: https://doi.org/10.1007/s00125-015-3701-x
Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5 , 82–91 (2011).
Murri, M. et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children : a case-control study. 1–12 (2013).
Mejìa-Leòn, M. E., Petrosino, J. F., Ajami, N. J., Domìnguez-Bello, M. G. & Calderòn de la Barca, M. Fecal microbiota imbalance in Mexican children with type 1 diabetes. 4 , 1–5 (2013).
Stewart, C. J. et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562 , 583–588 (2018).
Article ADS CAS PubMed Google Scholar
Vatanen, T. et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562 , 589–594 (2018).
De Goffau, M. C. et al. Fecal Microbiota Composition Differs Between Children With Beta-Cell Autoimmunity and Those Without. Diabetes 62 , 1238–1244 (2013).
Article PubMed CAS Google Scholar
Davis-Richardson, A. G. et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 5 , 1–11 (2014).
Kostic, A. D. et al. The Dynamics of the Human Infant Gut Microbiome in Development and in Progression toward Type 1 Diabetes. Cell Host Microbe 17 , 260–273 (2015).
Article MathSciNet CAS PubMed Google Scholar
Kemppainen, K. M. et al. Early childhood gut microbiomes show strong geographic differences among subjects at high risk for type 1 diabetes. Diabetes Care 38 , 329–332 (2015).
Zhong, T. et al. The remission phase in type 1 diabetes: changing epidemiology, definitions and emerging immuno-metabolic mechanisms. Diabetes Metab. Res. Rev. https://doi.org/10.1002/dmrr.3207 (2019).
Winkler, C. et al. Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials—GPPAD-02 study design and first results. Pediatr. Diabetes https://doi.org/10.1111/pedi.12870 (2019).
Ziegler, A.-G. et al. Screening for asymptomatic β-cell autoimmunity in young children No Title. Lancet Child Adolesc. Heal. May , 288–290 (2019).
Rabbone, I. et al. Microbiota, epidemiological and nutritional factors related to ketoacidosis at the onset of type 1 diabetes. Acta Diabetol. https://doi.org/10.1007/s00592-020-01555-z (2020).
Cani, P. D. Human gut microbiome: hopes, threats and promises. Gut 67 , 1716–1725 (2018).
Dridi, B., Raoult, D. & Drancourt, M. Archaea as emerging organisms in complex human microbiomes. Anaerobe 17 , 56–63 (2011).
Rawshani, A. et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392 , 477–486 (2018).
Mejía-León, M. E., Petrosino, J. F., Ajami, N. J., Domínguez-Bello, M. G. & De La Barca, A. M. C. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 4 , 1–5 (2014).
Alkanani, A. K. et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64 , 3510–3520 (2015).
Harbison, J. E. et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: a prospective cohort study. Pediatr. Diabetes 20 , 574–583 (2019).
CAS PubMed Google Scholar
Maffeis, C. et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. 700–709 (2016). doi: https://doi.org/10.1002/dmrr
Murri, M. et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. 1–12 (2013). doi: https://doi.org/10.1002/dmrr
Qi, C. J. et al. Imbalance of fecal microbiota at newly diagnosed type 1 diabetes in Chinese Children. 129 , 1298–1304 (2016).
Pellegrini, S. et al. Duodenal mucosa of patients with type 1 diabetes shows distinctive inflammatory profile and microbiota. J. Clin. Endocrinol. Metab. 102 , 1468–1477 (2017).
Putignani, L., Del Chierico, F., Petrucca, A., Vernocchi, P. & Dallapiccola, B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr. Res. 76 , 2–10 (2014).
Regueiro, L. et al. Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 167 , 581–589 (2012).
Uusitalo, U. et al. Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study. JAMA Pediatr. 33612 , 1–9 (2015).
Panigrahi, P. Probiotics and prebiotics in neonatal necrotizing enterocolitis: New opportunities for translational research. Pathophysiology 21 , 35–46 (2014).
Brüssow, H. Biome engineering-2020. Microb. Biotechnol. 9 , 553–563 (2016).
Traversi, D. et al. Gut microbiota diversity and T1DM onset: Preliminary data of a case-control study. Hum. Microbiome J. 5–6 , 11–13 (2017).
World Health Organization. ICF Parental Consent-clinicalstudies. (2018).
Ministero della Salute. Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita - Anno 2015 . (2018).
Franzosa, E. A. et al. Relating the metatranscriptome and metagenome of the human gut. PNAS 111 , E2329–E2338 (2014).
IHMS Consortium. IHMS-SOP 02 V2: Standard Operating Procedure for Fecal Samples Self ‐ Collection Laboratory Analysis Handled Within 4 To 24 Hours . (2015).
Knight, R. et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 16 , (2018).
Muyzer, G., Waal, E. C. D. E. & Uitierlinden, A. G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59 , 695–700 (1993).
Article CAS PubMed PubMed Central Google Scholar
Webster, N. S. & Negri, A. P. Site-specific variation in Antarctic marine biofilms established on artificial surfaces. Environ. Microbiol. 8 , 1177–1190 (2006).
Dridi, B., Henry, M., El Khechine, A., Raoult, D. & Drancourt, M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS ONE 4 , e7063 (2009).
Article ADS PubMed CAS Google Scholar
Dao, M. C. et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity : relationship with gut microbiome richness and ecology. Gut Microbiota 65 , 426–436 (2016).
Guo, X. et al. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47 , 367–373 (2008).
Johnston, C., Ufnar, J. A., Griffith, J. F., Gooch, J. A. & Stewart, J. R. A real-time qPCR assay for the detection of the nifH gene of Methanobrevibacter smithii, a potential indicator of sewage pollution. J. Appl. Microbiol. 109 , 1946–1956 (2010).
Matsuki, T. et al. Quantitative PCR with 16S primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70 , 167–173 (2004).
Nakayama, T. & Oishi, K. Influence of coffee ( Coffea arabica ) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol. Lett. 343 , 161–168 (2013).
Takahashi, S., Tomita, J., Nishioka, K., Hisada, T. & Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 9 , 1–9 (2014).
Download references
Acknowledgements
The authors are grateful to the Italian Ministry of Health (RF-2011-02350617), the University of the Study of Torino and the Città della salute e e della scienza di Torino and the Hospital “Maggiore della Carità" di Novara for co-funding this project. Moreover, the authors wish to thank dr. Barbara Di Stefano (Sanitary Direction AOU Novara) and Mrs Rim Maatoug, Mrs Shpresa Xheka, and Mrs Daniela Elena Zelinschi (cultural intermediaries) at Novara Hospital for the translation of the questionnaire for migrant people. Finally, the authors make a special acknowledgement to the participant children and their families.
Author information
Authors and affiliations.
Department of Public Health and Pediatrics, University of Turin, Piazza Polonia 94, 10126, Torino, Italy
Deborah Traversi, Giacomo Scaioli, Giulia Carletto, Irene Racca, Roberta Siliquini & Franco Cerutti
S.S.V.D. Endocrinology and Diabetology, O.I.R.M., Azienda Ospedaliera Città Della Salute E Della Scienza, Turin, Italy
Ivana Rabbone, Camilla Vallini & Franco Cerutti
Paediatric Endocrinology, Azienda Ospedaliero Universitaria Maggiore Della Carità - Novara, Novara, Italy
Deborah Carrera, Silvia Savastio & Francesco Cadario
S.C.U. Medicina Interna 3, Azienda Ospedaliera Città Della Salute e Della Scienza Di Torino, Torino, Italy
Marilena Durazzo, Alessandro Collo & Arianna Ferro
Department of Veterinary Sciences, University of Turin, Torino, Italy
Dietetic and Clinical Nutrition Department, Azienda Ospedaliero Universitaria Maggiore Della Carità, Novara, Italy
Alessandro Collo
Department of Health Science, University of Eastern Piedmont Amadeo Avogadro - Azienda Ospedaliero Universitaria Maggiore Della Carità - Novara, Novara, Italy
Ivana Rabbone
Department of Public Health and Pediatrics, Hygiene Unit, University of the Study of Turin, via Santena 5 bis, 10126, Torino, Italy
Deborah Traversi, Giacomo Scaioli, Giulia Carletto & Roberta Siliquini
You can also search for this author in PubMed Google Scholar
Contributions
F.C. and R.S. coordinate the work. F.C., I.R., R.S., D.T.: design the work. F.C., I.R., S.S., and F.C.: patient inclusion and questionnaire administration. C.V., D.C.: clinical data collection, Torino and Novara, respectively. I.R.: patient sample collection and transport, questionnaire elaboration. D.T., G.C.: sample processing and extraction, molecular analysis. G.S., U.A., D.T. : statistical analysis and bioinformatics. M.D., A.C., A.F.: nutritional data elaboration. G.C., G.S.: drafted the work. F.C., I.R., R.S., M.D.: revised the work. D.T.: substantively revised the work.
Corresponding author
Correspondence to Deborah Traversi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Traversi, D., Rabbone, I., Scaioli, G. et al. Risk factors for type 1 diabetes, including environmental, behavioural and gut microbial factors: a case–control study. Sci Rep 10 , 17566 (2020). https://doi.org/10.1038/s41598-020-74678-6
Download citation
Received : 22 January 2020
Accepted : 30 September 2020
Published : 16 October 2020
DOI : https://doi.org/10.1038/s41598-020-74678-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Microbiota e patologie endocrino-metaboliche.
- Valentina Antoniotti
- Marina Caputo
- Flavia Prodam
L'Endocrinologo (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Diabetes Care for Children & Young People
Vol:05 | No:01
Children and young people’s diabetes care: Case study
- 12 Jul 2016
This case study demonstrates the physical and psychological difficulties faced by many young people with type 1 diabetes. Over the year following her diagnosis, Max had a deterioration in glycaemic control despite reporting that little had changed in her management. Detailed assessment revealed a number of psychosocial factors that were preventing her from achieving good control. However, working with her multidisciplinary team, she was able to address these issues and improve her blood glucose levels. This article outlines these issues and the action plan that Max and her diabetes team drew up to overcome them.
Share this article + Add to reading list – Remove from reading list ↓ Download pdf
This case study represents the challenges and issues, both physical and psychological, faced by a young person with type 1 diabetes and the support given by her diabetes multidisciplinary team (MDT). Implications for practice are addressed using current evidence-based research. The names of the child and family have been anonymised to protect their identity.
Case study Max (a pseudonym) is a 17-year-old girl who was diagnosed with type 1 diabetes 4 years ago at the age of 13 years. She and her mother were shocked and upset by the diagnosis, and both felt its management would be too great a task to take on by themselves.
Max is an only child and lives with her mother, a single parent. She attends the local state comprehensive school and is popular with her peer group. Her mother was very involved in her care and diabetes management from the onset. Despite this, her diabetes control deteriorated over time ( Table 1 ). In October 2012, her HbA 1c was 56 mmol/mol (7.3%); however, over the next year, this increased to 84 mmol/mol (9.8%) in July 2013. She found it difficult to count the carbohydrate portions in her food and her injections were hurting much more than when she was first diagnosed. She also expressed a fear of hypoglycaemia and of “looking stupid” in front of her friends.
Max and her MDT discussed treatment options to improve her glycaemic control. She refused insulin pump therapy but agreed to a blood glucose monitor and bolus advisor to assist with her regimen of multiple daily insulin injections (MDI). She is now using the bolus advisor confidently and has had regular one-to-one sessions with a psychologist. She is having fewer hypoglycaemic episodes and her HbA 1c has improved; in January 2016 it was 69 mmol/mol (8.5%) and in April 2016 it was 58 mmol/mol (7.5%).
Discussion Diagnosis Max and her mother were extremely shocked and upset by the diagnosis of type 1 diabetes and the potential severity of the condition and intense management required. Both felt it would be too great a task to take on by themselves.
Kübler-Ross and Kessler (2005) suggested that a diagnosis of diabetes is a life-changing event comparable to the experience of loss, and that children and families will often go through the five stages of grief defined by Kübler-Ross (1970) and outlined in Box 1 . They use this as a coping strategy to enable them to eventually acknowledge the condition. However, many families never reach the fifth stage of acceptance and many will fluctuate between the stages.
Although Max and her mum did accept the diagnosis eventually, at times both of them reverted to the earlier stages of grief. The diabetes MDT supported the family from diagnosis and will continue to support them throughout their time within the paediatric diabetes service, through the transition period with both paediatric and young people’s teams, until discharged to adult diabetes care.
The diabetes MDT was established after the Best Practice Tariff was introduced in 2012. It consists of doctors, nurses, dietitians, a psychologist and a personal assistant. It is well recognised that the MDT needs to work together in close cooperation to achieve good practice, and this can be strengthened by using written protocols, guidelines and targets (Brink, 2010). Logic would suggest that centres with MDTs and the same approaches and treatment regimens would have similar outcomes, yet the Hvidøre Childhood Diabetes Study Group has shown this is not the case (de Beaufort et al, 2013). In terms of glycaemic control, there were notable differences in patient outcomes across 21 diabetes clinics, all of which were committed to MDT-based practice. Although factors such as age, type of insulin regimen and socioeconomic status were shown to have some influence over specific outcomes, they did not explain the apparent differences between these clinics.
Family/social history Max is an only child and lives with her mother, a single parent. East et al (2006) suggested that rapid social change over the past 20 years has seen a marked increase in the number of mother-headed single-parent families. Max attends the local state comprehensive school, where she is generally doing well. She is popular with her peer group. La Greca et al (1995) suggested that peer relationships are important in diabetes management, as children and young people (CYP) may receive considerable emotional support from their friends. However, on occasions, Max’s peer relationships have had a counterproductive effect on her, and she feels she is different from her friends as the only one who has diabetes. This at times affects her self-esteem and impacts her diabetes control.
Max’s mother was very involved in her care and diabetes management from the onset. Anderson and Brackett (2005) suggested that parents typically take on most of the responsibility for management of diabetes when children are young or newly diagnosed.
Deterioration in diabetes control Max’s diabetes control had deteriorated since her diagnosis ( Table 1 ). In October 2012, her HbA 1c was 56 mmol/mol (7.3%), which indicated a good level of diabetes control and a reduced risk of diabetes complications, as suggested by the DCCT (Diabetes Control and Complications Trial; DCCT Research Group, 1994). At her subsequent diabetes clinic appointments up to July 2013, she reported that “nothing had really changed,” except she “didn’t have time to think about her diabetes,” although she felt guilty because she knew she could make herself ill and her mum would get upset. She stated that it was hard counting the carbohydrate portions in her food and her injections were hurting much more than when she was first diagnosed. Her height and weight remained static.
Diabetes care is greatly influenced by psychosocial factors when they obstruct people’s ability to manage their diabetes and achieve good metabolic control. A team-based approach to addressing an individual’s ability to cope is critical (Kent et al, 2010). It is important for healthcare professionals to be aware of how CYP think at the different stages of their development, as their understanding of illness and chronic health conditions is often greater than that of their peers. Jean Piaget (1896–1980) investigated cognitive processes in children, calling them “schemas”. By the time children reach around 12 years of age, they can describe illness in terms of non-functioning or malfunctioning of an internal organ or process. Later in development they can appreciate that a person’s thoughts or feelings can affect the way the body functions, which demonstrates an awareness of psychological factors (Taylor et al, 1999).
Spear (2013) proposed that we can begin to understand how young people with type 1 diabetes think, feel and behave if we consider the cognitive and biological changes that occur during adolescence. Glasper and Richardson (2005) suggested there is now a growing awareness that CYP are able to make their own decisions if given information in an age-appropriate manner. Gillick competence identifies children aged under 16 years as having the capacity to consent to their own treatment if they understand the consequences (NSPCC, 2016).
Butler et al (2007) suggest that adolescence is a time of upheaval when young people have to deal with the influence of peers, school life and developing their own identity, as well as all the physiological changes that occur. Young people with type 1 diabetes have the added responsibility of developing autonomy regarding the self-management of their condition. Hanas (2006) suggests that parents should continue to take part in their child’s diabetes care into adolescence and not hand the responsibility to the young person too early. Snoek and Skinner (2002) suggest that intensive self-management of diabetes is complex and time-consuming, and creates a significant psychosocial burden on children and their families.
There are significant challenges for CYP to engage in effective diabetes self-management. Several of these were identified with Max and her mother:
- Deterioration in diabetes control.
- Difficulty with carbohydrate counting.
- Insulin omission.
- Fear of hypoglycaemia.
- Painful injections.
Action plan An action plan was discussed between Max and the MDT. As she was on an MDI regimen (a long-acting insulin at bedtime and rapid-acting insulin with meals), a bolus advisor/blood glucose monitor was demonstrated and discussed with her and her mum. Max felt she would be able to use this to help eliminate the calculations which, although she was capable of doing them, she often lacked time to do so. With further discussion, Max said she was “scared of getting it wrong and having a hypo”. Insulin pump therapy was discussed but she did not want to “have a device attached to my body because it would remind me all the time that I have diabetes”. Insulin pump therapy is recommended as a treatment option for adults and children over 12 years of age with type 1 diabetes whose HbA 1c levels remain above 69 mmol/mol (8.5%) on MDI therapy despite a high level of care (NICE, 2015a).
The National Service Framework standard 3 (Department of Health, 2001) recommends empowering people with diabetes and encourages them and their carers to gain the knowledge and skills to be partners in decision-making, and giving them more personal control over the day-to-day management of their diabetes, ensuring the best possible quality of life. However, if a diabetes management plan is discussed in partnership with a (Gillick-competent) young person but they elect not to comply with the plan despite full awareness of the implications of their actions, then the diabetes team should support them whilst trying to encourage them to maintain the treatment plan. This can be very difficult and frustrating at times, as a healthcare professional is an advocate for the patient, and promotion of the best interests of the patient is paramount.
Psychology involvement Max was reviewed by the psychologist to assess her psychological health and wellbeing. The psychologist used the Wellbeing in Diabetes questionnaire (available from the Yorkshire and Humber Paediatric Diabetes Network) to assess her and identify an optimal plan of care.
The psychology sessions were focussed on her issues around the following:
- Worry about deterioration in control.
- The consequences of insulin omission.
Max had a series of one-to-one appointments and some joint sessions with the paediatric diabetes specialist nurse and/or dietitian, so this linked into other team members’ specialities.
Carbohydrate counting and use of a bolus advisor The dietitian assessed Max and her mother’s ability to carbohydrate count using a calculator, food diagrams and portion sizes, and both of them were able to demonstrate competency in this task. Garg et al (2008) have shown that the use of automated bolus advisors is safe and effective in reducing postprandial glucose excursions and improving overall glycaemic control. However, this can only be true if the bolus advisor is being used correctly and is confirmed as such by comparing blood glucose and HbA 1c results before and after initiation of the bolus advisor, and observing the patient using the device to ensure it is being used safely and correctly.
Barnard and Parkin (2012) propose that, as long as safety and lifestyle are taken into consideration, advanced technology will benefit CYP, as inaccurate bolus calculation can lead to persistent poor diabetes control. These tools can help with removing the burden of such complex maths and have the potential to significantly improve glycaemic control.
Insulin omission and fear of hypoglycaemia Max also expressed her fear of hypoglycaemia and of “looking stupid” in front of her friends. She admitted to missing some of her injections, especially at school. Wild et al (2007) suggest that a debilitating fear of hypoglycaemia can result in poor adherence to insulin regimens and subsequent poor metabolic control. Crow et al (1998) describe the deliberate omission or reduced administration of insulin, which results in hyperglycaemia and subsequent rapid reduction in body weight. Type 1 diabetes predisposes a person to a high BMI. Adolescent girls and adult women with type 1 diabetes generally have higher BMI values than their peers without the condition (Domargård et al, 1999). Affenito et al (1998) observed that insulin misuse was the most common method of weight control used by young women with type 1 diabetes. However, Max’s weight remained stable and there was no clinical indication that she was missing insulin to lose weight; rather, it was her fear of hypoglycaemia that drove her to omitting insulin at school. With the use of the bolus calculator, she was reassured about her calculations for insulin-to-carbohydrate ratios, but it was reinforced with her that the device would only work efficiently if she used it correctly with each meal.
Painful injections Max also highlighted that her injections were now more painful than when she was first diagnosed, and this was causing her distress each time she had to inject. Injection technique was discussed with her and demonstrated using an injection model, and her injection technique was observed and appeared satisfactory. She was using 5-mm insulin needles and so was switched to 4-mm needles, as recommended by Forum for Injection Technique (2015) guidelines.
Appropriate technique when giving injections is key to optimal blood glucose control; however, evidence suggests that injection technique is often imperfect. Studies by Strauss et al (2002) and Frid et al (2010) revealed disturbing practices in relation to injection technique, with little improvement over the years. Current diabetes guidelines do not include detailed advice on injection technique, and only the guidance on type 2 diabetes in adults (NICE, 2015b) makes any reference to providing education about injectable devices for people with diabetes. However, the older Quality Standard for diabetes in adults (NICE, 2011) recommends a structured programme of education, including injection site selection and care (Diggle, 2014).
Conclusion The issues and concerns this young girl had were identified and addressed by the diabetes MDT. She was assessed by several members of the team, and a credible, evidence-based action plan was put into place to assist her and her mother to manage her diabetes at this difficult time. Max is now using the bolus advisor confidently and having fewer hypoglycaemic episodes, and her HbA 1c has improved. She prefers using the 4-mm injection pen needles, although she remains hesitant when giving injections; she will still not consider insulin pump therapy. Her one-to-one sessions with the psychologist have now ceased, but she is aware she can access a psychologist at clinic on request, or if the MDT assesses that her psychological health has deteriorated.
When a child in a family develops a chronic condition such as type 1 diabetes, effective communication is vitally important to address issues with the family at the earliest stage so that problems can be discussed and, hopefully, resolved before they escalate out of control. Upon reflection, the team could have become more intensely involved at an earlier stage to prevent Max’s diabetes management issues and stop her HbA 1c from reaching such a high level. Furthermore, the new NICE (2015a) guideline has set the target HbA 1c at ≤48 mmol/mol (6.5%), so there is still some work to be done. However, the outcome of this case appears to be favourable at present.
Affenito SG, Rodriguez NR, Backstrand JR et al (1998) Insulin misuse by women with type 1 diabetes mellitus complicated by eating disorders does not favorably change body weight, body composition, or body fat distribution. J Am Diet Assoc 98 : 686–8 Anderson BJ, Brackett J (2005) Diabetes in children. In: Snoek FJ, Skinner TC (eds). Psychology in Diabetes Care (2nd edition). John Wiley & Sons, Chichester Barnard K, Parkin C (2012) Can automated bolus advisors help alleviate the burden of complex maths and lead to optimised diabetes health outcomes? Diabetes Care for Children & Young People 1 : 6–9 Brink SJ (2010) Pediatric and adolescent multidisciplinary diabetes team care. Pediatr Diabetes 11 : 289–91 Butler JM, Skinner M, Gelfand D et al (2007) Maternal parenting style and adjustment in adolescents with type I diabetes. J Pediatr Psychol 32 : 1227–37 Crow SJ, Keel PK, Kendall D (1998) Eating disorders and insulin-dependent diabetes mellitus. Psychosomatics 39 : 233–43 de Beaufort CE, Lange K, Swift PG et al (2013) Metabolic outcomes in young children with type 1 diabetes differ between treatment centers: the Hvidoere Study in Young Children 2009. Pediatr Diabetes 14 : 422–8 Department of Health (2001) National Service Framework: Diabetes . DH, London. Available at: http://bit.ly/18OpAzL (accessed 24.02.16) Diabetes Control and Complications Trial Research Group (1994) Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 125 : 177–88 Diggle J (2014) Are you FIT for purpose? The importance of getting injection technique right . Journal of Diabetes Nursing 18 : 50–7 Domargård A, Särnblad S, Kroon M et al (1999) Increased prevalence of overweight in adolescent girls with type 1 diabetes mellitus. Acta Paediatr 88 : 1223–8 East L, Jackson D, O’Brien L (2006) Father absence and adolescent development: a review of the literature. J Child Health Care 10 : 283–95 Forum for Injection Technique (2015) The UK Injection Technique Recommendations (3rd edition). Available at: http://bit.ly/1QeZU2E (accessed 24.02.16) Frid A, Hirsch L, Gaspar R et al (2010) The Third Injection Technique Workshop in Athens (TITAN). Diabetes Metab 36 (Suppl 2): 19–29 Garg SK, Bookout TR, McFann KK et al (2008) Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software. Diabetes Technol Ther 10 : 369–75 Glasper EA, Richardson J (2005) A Textbook of Children’s and Young People’s Nursing . Churchill Livingston, London Hanas R (2006) Type 1 Diabetes in Children, Adolescents and Young Adults (3rd edition). Class Publishing, London: 329, 349–50 Kent D, Haas L, Randal D et al (2010) Healthy coping: issues and implications in diabetes education and care. Popul Health Manag 13 : 227–33 Kübler-Ross E (1970) On Death and Dying: What the Dying Have to Teach Doctors, Nurses, Clergy and Their Own Families . Tavistock Publications, London Kübler-Ross E, Kessler D (2005) On Grief and Grieving: Finding the Meaning of Grief Through the Five Stages of Loss . Simon & Schuster UK, London La Greca AM, Auslander WF, Greco P et al (1995) I get by with a little help from my family and friends: adolescents’ support for diabetes care. J Pediatr Psychol 20 : 449–76 NICE (2011) Diabetes in adults (QS6). NICE, London. Available at: www.nice.org.uk/guidance/qs6 (accessed 24.02.16) NICE (2015a) Diabetes (type 1 and type 2) in children and young people: diagnosis and management (NG18). NICE, London. Available at: www.nice.org.uk/guidance/ng18 (accessed 24.02.16) NICE (2015b) Type 2 diabetes in adults: management (NG28). NICE, London. Available at: www.nice.org.uk/guidance/ng28 (accessed 24.02.16) NSPCC (2016) A Child’s Legal Rights: Gillick Competency and Fraser Guidelines . NSPCC, London. Available at: http://bit.ly/1Tj6DcF (accessed 24.02.16) Snoek FJ, Skinner TC (2002) Psychological counselling in problematic diabetes: does it help? Diabet Med 19 : 265–73 Spear LP (2013) Adolescent neurodevelopment. J Adolesc Health 52 (Suppl 2): 7–13 Strauss K, De Gols H, Hannat I et al (2002) A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Practical Diabetes International 19 : 71–76 Taylor J, Müller D, Wattley L, Harris P (1999) The development of children’s understanding. In: Nursing Children: Psychology, Research and Practice . Stanley Thornes, Cheltenham Wild D, von Maltzahn R, Brohan E et al (2007) A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 68 : 10–5
Do youth workers have a role in improving diabetes transition services?
Cgm for children and young people with type 1 diabetes: nice criteria and effects of decision fatigue and alarm fatigue , improving paediatric diabetes in england: areas of focus, delays in accessing continuous glucose monitoring in people with type 1 diabetes, celebrating may ng: the woman behind the obe, fiona campbell awarded an obe for services to paediatric diabetes, diabetes transition: a time to act.

Can the involvement of youth workers improve diabetes care for young people transitioning to adult diabetes services?

The impact of decision fatigue and alarm fatigue in children and young people using continuous glucose monitoring

NHSEI National Clinical Lead for Diabetes in Children and Young People, Fulya Mehta, outlines the areas of focus for improving paediatric diabetes care.
16 Nov 2022

NICE guidance urges local trusts to improve processes and advocate for CGM use in children and young people.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Diabetes & Primary Care
- Journal of Diabetes Nursing
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
Type 1 diabetes mellitus in childhood: a matched case control study in Lancashire and Cumbria, UK
Affiliation.
- 1 AstraZeneca Pharmaceuticals, Macclesfield, UK.
- PMID: 15317611
- DOI: 10.1111/j.1464-5491.2004.01282.x
Aims: The aim of the study was to identify environmental risk factors for insulin-dependent diabetes mellitus (Type 1 DM) in childhood.
Methods: A matched case-control study of Type 1 DM conducted in Lancashire and Cumbria, UK, using a structured interview. Cases (n=196, participation rate 83%) were children under 16 years of age diagnosed prior to October 1998 and attending diabetic clinics. Controls (n=381) were healthy children from the community matched by gender and by age (within a few days of birth). The data were analysed by logistic regression using the technique of Breslow and Day for matched case control studies.
Results: The multivariate regression model showed that the following factors were significantly associated with the risk of developing Type 1 DM (odds ratio, 95% confidence intervals): sharing a room with a sibling (0.458, 0.290-0.721), social contact with other children when aged 6-11 months (0.439, 0.256-0.752), consumption of sugary food (0.080, 0.024-0.261), parental insulin dependent diabetes mellitus (10.651, 3.086-36.761), maternal thyroid disease (4.861, 1.681-14.058), consuming more than one pint of milk per day prior to school entry (0.498, 0.310-0.802), maternal smoking during pregnancy (0.373, 0.218-0.636), a father with no academic qualifications (0.504, 0.278-0.913), maternal age at time of birth (0.900, 0.854-0.948), maternal infections in pregnancy (2.453, 1.011-5.948), other maternal illnesses or conditions in pregnancy (2.007, 1.139-3.535), belonging to an Asian family (0.104, 0.028-0.394), and regular contact with pets and other animals (0.552, 0.309-0.987).
Conclusion: Many of the results are consistent with the hygiene hypothesis which links improved living standards with decreased exposure to microorganisms and increased risk of immune mediated disease in childhood. These findings challenge the idea that improved hygiene acts exclusively through a Th2 mechanism leading to atopic disease as Type 1 DM is mediated by a Th1 reaction. The association with maternal smoking could be due to recall bias but a causal link cannot be excluded with confidence.
- Animals, Domestic
- Case-Control Studies
- Child, Preschool
- Diabetes Mellitus, Type 1 / ethnology
- Diabetes Mellitus, Type 1 / etiology*
- Dietary Carbohydrates / administration & dosage
- Family Health
- Infant Formula / administration & dosage
- Pregnancy Complications
- Risk Factors
- Smoking / adverse effects
- Social Environment
- Socioeconomic Factors
- Typhoid-Paratyphoid Vaccines / administration & dosage
- Dietary Carbohydrates
- Typhoid-Paratyphoid Vaccines
- Campus Directory
- Current Students
- Faculty & Staff

Type 1 Diabetes - Case Summary
1. Insulin-Dependent Diabetes Mellitus (IDDM), also categorized as Type I diabetes, is an autoimmune disease of children in which the body's immune system attacks the insulin-producing beta cells on the pancreas. It is theorized that this immune response may be triggered during a viral infection in those with a genetic predisposition to the disease.
2. The symptoms of undiagnosed diabetes include excessive hunger and thirst, weight loss, frequent urination, and fatigue.
3. The diagnoses of diabetes, in this case, was made by the endocrinologist based upon elevated levels of glucose in the blood and urine. Ali was not seen until her disease had progressed to diabetic ketoacidosis. DKA was diagnosed based on the arterial blood gas results showing a pH of less than 7.3, large amounts of ketones in the urine, elevated potassium, and physical symptoms such as rapid heart rate and rapid breathing.
4. The goal of treatment of Type I diabetes is to regulate the patient's blood glucose so that it does not rise too high (hyperglycemia) or drop too low (hypoglycemia). This is done by insulin injections, a controlled diet, and exercise. Insulin pumps function much more like a normal pancreas and are being used increasingly more. Because of the complexity of operating insulin pumps, they are generally not recommended until the teenage years.
5. There is no cure for Type I Diabetes Mellitus, except for a pancreas transplant. Because of the highly volatile nature of the pancreas, a transplant is generally done as a last resort. Studies have now proven that diabetics who remain in tight control may prevent or slow the start of diabetic complications.
6. There is no known prevention of Type I diabetes. Animal research and small studies in people have indicated that type 1 diabetes can be delayed in those at high risk for the disease by regular, small doses of insulin. This is currently under study.
7. Healthcare workers depend on each other in treating a diabetic patient. Laboratory personnel report critical laboratory values including blood chemistry, CBC, and urinalysis results. The respiratory therapist monitors arterial blood gasses in the diabetic patient in DKA. Diabetic educator nurses have a responsibility to not only show the patient how to give injections but to educate the diabetic about all aspects of their disease. It should be noted that in many cases staff nurses may work to educate patients about diabetes. The diabetics overall control relies heavily on their diet. The dietician plays a key role in developing a meal plan and educating the patient about the importance of diet. Social changes and adjustments by the patient and family are facilitated by the medical social worker. The endocrinologist has the ultimate responsibility for the diabetics health. He prescribes insulin based on the patient's diet and lifestyle. He must be ready to make key decisions for the day to day health of the diabetic as well as for life-threatening complications.
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Case report article, case report: insulin-dependent diabetes mellitus and diabetic keto-acidosis in a child with covid-19.

- 1 Division of Pediatric Infectious Diseases, Department of Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
- 2 Division of Critical Care Medicine, Department of Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
- 3 Department of Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
During the COVID pandemic, a surge in pediatric Type 1 Diabetes Mellitus (T1DM) cases appears to be occurring, potentially due to the presence of autoantibody-induced immune dysregulation triggered by COVID-19. We describe one such case in a previously healthy 7-year-old with asymptomatic COVID-19 presenting with a high nasopharyngeal SARS CoV-2 virus load, detectable COVID-19 IgG antibodies, diabetic keto-acidosis and islet cell autoantibodies. COVID-19 is not a trivial disease in children and adolescents and can lead to lifelong sequelae such as T1DM. Raising awareness about a possible association between COVID-19 and T1DM in children is critical.
Introduction
During the COVID-19 pandemic, the number of cases of T1DM in youth spiked, with evidence suggesting an association between both conditions ( 1 , 2 ). Studies have long implicated viruses, particularly respiratory infections, as potential triggers of T1DM in children and young adults ( 3 ). In a large prospective pediatric study, a temporal association was noted between respiratory infections and development of autoantibodies against insulin-producing pancreatic beta islet cells ( 3 ). Following a surge in COVID-19 cases, a prospective registry demonstrated a significant increase in pediatric diabetic ketoacidosis (DKA) diagnoses ( 1 ). Between March to May 2020, 532 children in Germany were diagnosed with T1DM, with 45% presenting with DKA. The incidence of DKA in children was nearly double of that reported in the prior year (24.5%) with the risk of DKA in 2020 being 1.85 times higher than in the 2 prior years (2.75 times higher in children <6 years of age as compared to 2019) ( 1 ). In the U.K., investigators reported an 80% increase in the number of cases of T1DM in children as compared to prior years ( 2 ). The reason for higher rates of DKA in youth could be multi-factorial and related to delayed medical care ( 4 ). Findings, however, parallel what was observed in adults with COVID-19 ( 5 ).
Patient Information
A 7-year-old previously healthy Hispanic male with no pre-existing co-morbidities presented to the UCLA emergency department with progressive anorexia and a 10-pound weight loss over 3 weeks in August 2020. Three days prior to presentation, the patient complained of acutely worsened anorexia with polydipsia, abdominal pain, nausea, and headache ( Table 1 ). Both the patient and his mother denied any prior or concurrent presence of fever, cough, nasal congestion, shortness of breath, diarrhea, or dysuria. There was no history of recent illnesses or sick contacts. The child lived in a multigenerational family household in south Los Angeles, including both grandparents who worked as school janitors, his mother who was studying for her degree remotely, a young adolescent cousin and a 13-year-old healthy sister. No one in the household reported recent illnesses and the family history was overall unremarkable.

Table 1 . Timeline.
Clinical Findings
In the emergency department, the patient's initial vital signs were a temperature of 36.8°C, heart rate of 131 beats per minute (BPM), blood pressure of 114/75 mmHg, respiratory rate of 37 breaths per minute and an oxygen saturation of 99% on room air. His weight was 25 kg, height 121.9 cm, and BMI was at the 75% percentile for age. On initial examination, he was drowsy but arousable. His exam was notable for dry mucous membranes, reduced skin turgor, tachypnea, clear lungs clear to auscultation, and soft, non-distended, non-tender abdomen. While he appeared fatigued, he had a non-focal neurological exam and responded appropriately to questions.
Diagnostic Assessment
An initial point-of-care blood glucose was 470 mg/dL. A complete blood count (CBC) showed a normal white blood cell (WBC) count of 6.69 × 10 E 3 /uL, with an absolute neutrophil count (ANC) of 4,650 cells/uL, as well as a mild lymphopenia (1,420 lymphocytes/μL) ( Table 2 ). The urinalysis was significant for a specific gravity of 1.024, 2+ ketones, 3+ glucose, 2+ protein. Chest X-ray did not show any pulmonary pathology. Clinical and laboratory findings were consistent with diabetic ketoacidosis (DKA). In the emergency department, the patient received a 10 mL/kg normal saline bolus and started on continuous insulin infusion. He was then transferred to the pediatric intensive care unit (PICU) for further care.
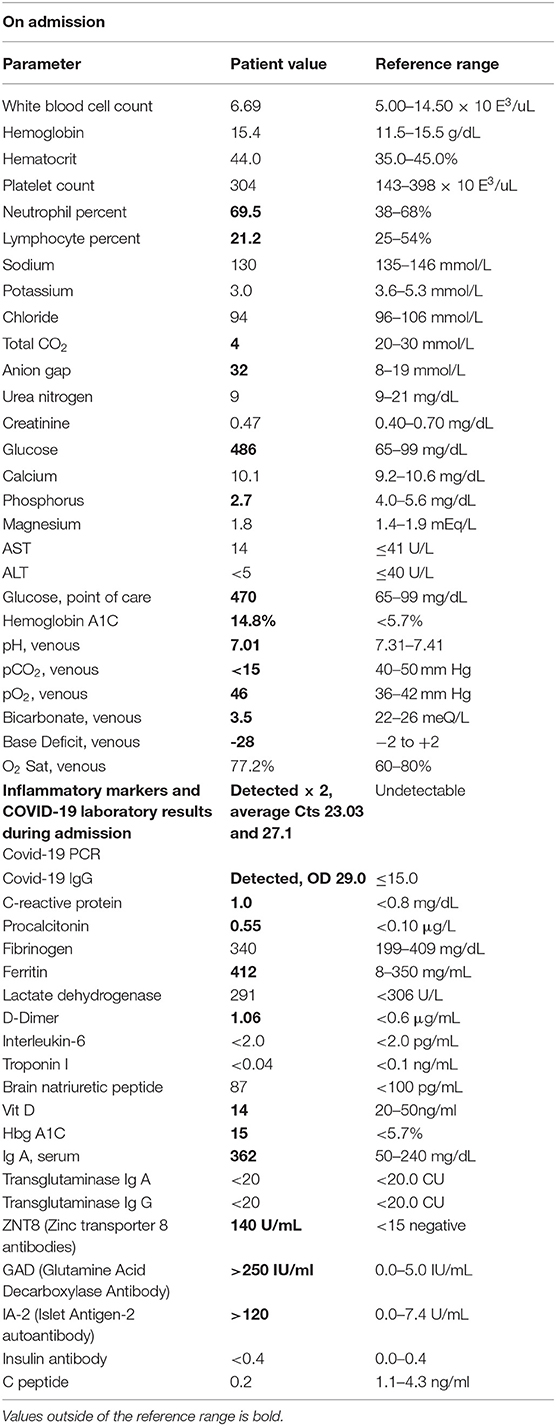
Table 2 . Laboratory values on and during admission with abnormal results highlighted.
On arrival to the PICU, the patient was persistently tachycardic, with heart rates ranging from 100–140 BPM. He required a total of 40 mL/kg of normal saline fluid boluses over 24 h. An electrocardiogram was obtained, which revealed sinus tachycardia with incomplete right bundle branch block. His heart rate improved to 90–100 beats per minute over the next 2 days. A repeat CBC following hydration 24 h later showed a WBC count of 15.27 × 10 E 3 /μL with an ANC of 12,740 cells/μL, persistent lymphopenia (1,430 lymphocytes/μL) and 70 immature granulocytes/μL, hemoglobin of 16.1 g/dL, hematocrit of 46%, platelet count of 362 × 10 E 3 /μL. As the DKA was resolving on the two-bag system, the patient's hypokalemia, hypomagnesemia, hypocalcemia, and hypophosphatemia were repleted as appropriate.
On admission to the hospital, as per current hospital policy, a nasopharyngeal swab was tested for the presence of SARS-CoV-2 RNA using the Thermo Fisher TaqPath assay (Thermo Fisher Scientific, Waltham, MA). This returned positive the next morning, with cycle threshold (Ct) values of 23.25 for ORF1ab target, 23.12 for the N target, and 22.73 for the S target. A repeat upper respiratory specimen obtained 3 days later and evaluated using the BDMax assay (Beckon Dickinson and Company, Franklin Lakes, NJ) also returned positive, with Ct values of 26.7 for the N1 target and 27.5 for the N2 target. LIAISON ® SARS-CoV-2 IgG assay targeting the spike protein (S1/S2) (Diasorin S.p.A., Saluggia (VC)—Italy) was also positive on admission (optical density of 29.0; positive ≥ 15.0, range 400 units/mL. The patient never had any fever, diarrhea, nor any respiratory symptoms during the admission. Additional laboratory evaluations for inflammatory markers potentially associated with COVID-19 Multi-Inflammatory Syndrome in Children (MIS-C) are shown in Table 2 . Except for very mildly elevated procalcitonin, C-reactive protein, D-Dimer and ferritin levels, inflammatory markers were normal. Markers of type 1 diabetes, however, were grossly abnormal, with an islet antigen 2 (IA-2) autoantibody of >120.0 U/ml and the glutamic acid decarboxylase antibody >250.0 IU/ml. Hemoglobin A1C was elevated at 14.8%.
The patient remained on a continuous insulin infusion and the two-bag system for 2 days and then was switched to a subcutaneous insulin regimen once the acidosis resolved. Adequate glucose control on the new subcutaneous insulin regimen was achieved and he was discharged after a 4-day admission.
Therapeutic Intervention (Plan of Treatment)
The patient responded well to usual DKA protocol, with somewhat interesting features of high insulin requirements and low potassium. After stabilization of the metabolic acidosis, a subcutaneous insulin regimen was started per endocrinology with the patient transferred to the pediatric ward. He continued on a Lantus insulin regimen for basal coverage with carb correction. A sliding scale with Humolog was initiated. Additional laboratory studies including IgA, IA-2 Ab, insulin Ab, transglutaminase Ab panel, ICA-512-HgbA1c, transglutaminase abs panel GAD 65, anti-insulin antibodies, c-peptide, and Zinc Transporter 8 were ordered ( Table 2 ).
Both the patient and his mother received diabetes education for home regimens. He required electrolyte correction and was discharged home with oral potassium supplementation and 2,000 IU vit D per dietary recommendations. A repeat Covid PCR test was stil positive on 8/24. The patient was discharged home to quarantine with mother for 14 days from the first positive SARS CoV-2 PCR test.
Follow-up, Expected, and Actual Outcomes
The child was seen in the endocrine clinic 1 day after discharge and again 1 and 3 weeks after discharge, being found to have acceptable blood glucose levels. He was following a carbohydrate-controlled diet fairly well and thus no changes were made during the diabetic nutrition follow-up visits. The expected outcome for new onset T1DM is well-controlled DM care and dietary modification, however, we do not have enough information regarding the long term outcomes of simultaneous T1DM and Covid-19 infection in children.
Our pediatric patient illustrates the typical pattern of T1DM in children during the COVID-19 pandemic and differs from that of a published report in a 19 year old with new onset DKA following COVID-19 ( 6 ) as our patient had a classic presentation of autoantibody-mediated T1DM. The timing of SARS-CoV-2 infection in our patient coincided with development of indolent symptoms of diabetes, particularly anorexia and weight loss. Although T1DM is most commonly diagnosed in childhood, it is a relatively rare disease, occurring in about 1.5 in 1,000 children ( 7 ). COVID-19 is also infrequently identified in children as compared to adults, with pediatric cases mainly diagnosed during pandemic surges ( 7 ). For this reason, the magnitude of the association between T1DM, DKA, and COVID-19 in youth is difficult to quantify, but is, nonetheless, apparent. In a report of U.S. children hospitalized with COVID-19, 2.7% had a history of chronic diabetes, and 2.9% developed DKA during their hospital stay ( 8 ). Whether SARS CoV-2 itself or deferred medical care are responsible for a higher presentation of DKA cases in younger populations has been a matter of debate ( 4 , 9 ).
ACE2 is expressed in multiple organs, including exocrine and endocrine tissues of the pancreas. SARS-CoV, responsible for the epidemic of 2003, was shown to bind to ACE2 receptors through its spike protein, similarly to SARS-CoV-2 ( 10 ). Diabetes has been recognized as a risk factor for increased COVID-19 morbidity and mortality in adults since the onset of the SARS-CoV-2 pandemic ( 11 ). More recently, data from adults suggest that COVID-19 may lead to worse outcomes in patients with pre-established diabetes, and may trigger diabetic ketoacidosis ( 12 ). New-onset diabetes during the course of COVID-19 infection is recognized in both adults and children, with a small number of case reports described to date ( 2 , 13 ). A study of SARS patients with diabetes strongly suggested that the localization of ACE2 expression in the endocrine part of the pancreas allowed SARS coronavirus to enter and damage pancreatic islets, leading to acute diabetes ( 10 ). Both SARS-CoV and SARS-CoV-2 have been reported to trigger transient insulin resistance and hyperglycemia ( 10 , 11 ).
A report of a 19 year-old male with autoantibody negative Type 1 diabetes mellitus (T1DM) following COVID-19 infection acquired following exposure to symptomatic parents highlights the important consideration of whether SARS-CoV-2 infection may directly damage pancreatic islet cells abundantly expressing ACE2 viral receptors ( 6 ). Another possibility is that immune dysregulation during the course of COVID-19 disease may induce development of autoantibodies against pancreatic beta cells. Potentially both circumstances may occur in parallel, with infection of cells expressing ACE2 receptors triggering a dysregulated humoral immune response resulting in the death of pancreatic islet cells. Deferred medical care which discourages patients and parents of children to seek help during lockdown situations may likely contribute to more patients with new onset T1DM present in DKA ( 9 , 14 ). Decline in pediatric medical care occurs COVID-19 pandemic, where for example childhood immunization programs have suffered despite best efforts ( 15 ). In the case of our patient, symptoms went unrecognized for nearly 3 weeks, and upon diagnosis, off-scale levels of autoantibodies were present, a common finding in T1DM. Both mechanisms of pathogenesis and the underlying issues associated with pandemic situations and unavailability of hospital beds are likely leading to an unprecedented number of DKA cases in youth with COVID-19. Table 3 summarizes current pediatric studies on the topic to date.
Table 3 . Reports of DKA in children and youth during the COVID-19 pandemic.
Although SARS CoV-2 infection of pancreatic beta cells has not been yet demonstrated, there is enough evidence of direct viral damage leading to organ failure in different body compartments, as in the case of COVID-19 myocarditis ( 16 ). Cases of pancreatitis in patients with COVID-19 have been reported in both adults and children ( 17 , 18 ). It is difficult to discern which cases of new onset T1DM are due to direct viral damage, and which are due to immune dysregulation induced by COVID-19. While DM is a risk factor for severe COVID-19, SARS-CoV-2 infection also triggers T1DM, a bidirectional relationship shown to occur in adults and now increasingly demonstrable in youth. It is critical that awareness regarding this specific complication of SARS-CoV-2 infection in children be heightened, not only to enable early identification of DM, but also to counteract the belief that COVID-19 poses no threat to young patients.
In summary, our intent with this case report was to raise awareness among pediatricians about the potential for a large increase in the number of COVID-19 associated T1DM and DKA cases in children and youth following pandemic surges. Other institutions might be witnessing the same phenomenon and through this publication we wished to share this unique presentation of COVID-19 in children. Because the number of COVID-19 cases have sky-rocketed in recent months globally (December 2020/ January 2021), it is very likely that we will be seeing a very sharp rise in the number of T1DM and DKA events in children exposed to the virus through their family members. These children often require admission to critical care, and it is very important to recognize this potential complication of this condition in pediatric populations.
Patient Perspective
Despite the difficult circumstances, the patient and his family are adapting to the diagnosis of T1DM and mother and child are now heavily engaged with our institution's pediatric diabetes clinic. The child continues to be closely monitored with bi-monthly in person and telehealth visits. Family support through pediatric diabetes networks has been instrumental.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author/s.
Ethics Statement
The patient's mother provided informed consent for the publication of this case report.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.628810/full#supplementary-material
1. Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. (2020) 324:801–4. doi: 10.1001/jama.2020.13445
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, et al. New-onset Type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diab Care. (2020) 43:e170–e1. doi: 10.2337/dc20-1551
3. Lönnrot M, Lynch KF, Elding Larsson H, Lernmark Å, Rewers MJ, Törn C, et al. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. (2017) 60:1931–40. doi: 10.1007/s00125-017-4365-5
4. Tittel SR, Rosenbauer J, Kamrath C, Ziegler J, Reschke F, Hammersen J, et al. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. (2020) 43:e172–e3. doi: 10.2337/dc20-1633
5. Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-onset diabetes in Covid-19. N Engl J Med. (2020) 383:789–90. doi: 10.1056/NEJMc2018688
CrossRef Full Text | Google Scholar
6. Hollstein T, Schulte DM, Schulz J, Glück A, Ziegler AG, Bonifacio E, et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. (2020) 2:1021–4. doi: 10.1038/s42255-020-00281-8
7. Rush T, McGeary M, Sicignano N, Buryk MA. A plateau in new onset type 1 diabetes: Incidence of pediatric diabetes in the United States Military Health System. Pediatr Diabetes. (2018) 19:917–22. doi: 10.1111/pedi.12659
8. Kim L, Whitaker M, O'Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1081–88. doi: 10.15585/mmwr.mm6932e3
9. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A, Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Has COVID-19 delayed the diagnosis and worsened the presentation of Type 1 diabetes in children? Diab Care . (2020) 43:2870–2. doi: 10.2337/dc20-1321
10. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. (2010) 47:193–9. doi: 10.1007/s00592-009-0109-4
11. Abdi A, Jalilian M, Sarbarzeh PA, Vlaisavljevic Z. Diabetes and COVID-19: a systematic review on the current evidences. Diab Res Clin Pract. (2020) 166:108347. doi: 10.1016/j.diabres.2020.108347
12. Goldman N, Fink D, Cai J, Lee YN, Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diab Res Clin Pract. (2020) 166:108291. doi: 10.1016/j.diabres.2020.108291
13. Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: A report of two cases and review of literature. Diabetes Metab Syndr. (2020) 14:1459–62. doi: 10.1016/j.dsx.2020.07.050
14. DiMeglio LA, Albanese-O'Neill A, Muñoz CE, Maahs DM. COVID-19 and children with diabetes-updates, unknowns, and next steps: first, do no extrapolation. Diabetes Care. (2020) 43:2631–4. doi: 10.2337/dci20-0044
15. Khan A, Bibi A, Sheraz Khan K, Raza Butt A, Alvi HA, Zahra Naqvi A, Mushtaq S, et al. Routine pediatric vaccination in Pakistan during COVID-19: how can healthcare professionals help? Front Pediatr. (2020) 10:613433. doi: 10.3389/fped.2020.613433
16. Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvêa M, Viu Degaspare N, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. (2020) 4:790–4. doi: 10.1016/S2352-4642(20)30257-1
17. Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, Trindade AJ, et al. Prevalence, risk factors, and outcomes of hospitalized patients with COVID-19 presenting as acute pancreatitis. Gastroenterology. (2020) 159:2226–8.e2. doi: 10.1053/j.gastro.2020.08.044
18. Stevens JP, Brownell JN, Freeman AJ, Bashaw H. COVID-19-Associated multisystem inflammatory syndrome in children presenting as acute pancreatitis. J Pediatr Gastroenterol Nutr. (2020) 71:669–71. doi: 10.1097/MPG.0000000000002860
Keywords: COVID-19, children, type 1 diabetes mellitus (T1DM), diabetic keto-acidosis (DKA), SARS CoV-2, pediatric COVID-19
Citation: Nielsen-Saines K, Li E, Olivera AM, Martin-Blais R and Bulut Y (2021) Case Report: Insulin-Dependent Diabetes Mellitus and Diabetic Keto-Acidosis in a Child With COVID-19. Front. Pediatr. 9:628810. doi: 10.3389/fped.2021.628810
Received: 13 November 2020; Accepted: 12 January 2021; Published: 10 February 2021.
Reviewed by:
Copyright © 2021 Nielsen-Saines, Li, Olivera, Martin-Blais and Bulut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erica Li, ericali@mednet.ucla.edu

- Previous Article
- Next Article
Case Presentation
Case study: a patient with uncontrolled type 2 diabetes and complex comorbidities whose diabetes care is managed by an advanced practice nurse.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Geralyn Spollett; Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse. Diabetes Spectr 1 January 2003; 16 (1): 32–36. https://doi.org/10.2337/diaspect.16.1.32
Download citation file:
- Ris (Zotero)
- Reference Manager
The specialized role of nursing in the care and education of people with diabetes has been in existence for more than 30 years. Diabetes education carried out by nurses has moved beyond the hospital bedside into a variety of health care settings. Among the disciplines involved in diabetes education, nursing has played a pivotal role in the diabetes team management concept. This was well illustrated in the Diabetes Control and Complications Trial (DCCT) by the effectiveness of nurse managers in coordinating and delivering diabetes self-management education. These nurse managers not only performed administrative tasks crucial to the outcomes of the DCCT, but also participated directly in patient care. 1
The emergence and subsequent growth of advanced practice in nursing during the past 20 years has expanded the direct care component, incorporating aspects of both nursing and medical care while maintaining the teaching and counseling roles. Both the clinical nurse specialist (CNS) and nurse practitioner (NP) models, when applied to chronic disease management, create enhanced patient-provider relationships in which self-care education and counseling is provided within the context of disease state management. Clement 2 commented in a review of diabetes self-management education issues that unless ongoing management is part of an education program, knowledge may increase but most clinical outcomes only minimally improve. Advanced practice nurses by the very nature of their scope of practice effectively combine both education and management into their delivery of care.
Operating beyond the role of educator, advanced practice nurses holistically assess patients’ needs with the understanding of patients’ primary role in the improvement and maintenance of their own health and wellness. In conducting assessments, advanced practice nurses carefully explore patients’ medical history and perform focused physical exams. At the completion of assessments, advanced practice nurses, in conjunction with patients, identify management goals and determine appropriate plans of care. A review of patients’ self-care management skills and application/adaptation to lifestyle is incorporated in initial histories, physical exams, and plans of care.
Many advanced practice nurses (NPs, CNSs, nurse midwives, and nurse anesthetists) may prescribe and adjust medication through prescriptive authority granted to them by their state nursing regulatory body. Currently, all 50 states have some form of prescriptive authority for advanced practice nurses. 3 The ability to prescribe and adjust medication is a valuable asset in caring for individuals with diabetes. It is a crucial component in the care of people with type 1 diabetes, and it becomes increasingly important in the care of patients with type 2 diabetes who have a constellation of comorbidities, all of which must be managed for successful disease outcomes.
Many studies have documented the effectiveness of advanced practice nurses in managing common primary care issues. 4 NP care has been associated with a high level of satisfaction among health services consumers. In diabetes, the role of advanced practice nurses has significantly contributed to improved outcomes in the management of type 2 diabetes, 5 in specialized diabetes foot care programs, 6 in the management of diabetes in pregnancy, 7 and in the care of pediatric type 1 diabetic patients and their parents. 8 , 9 Furthermore, NPs have also been effective providers of diabetes care among disadvantaged urban African-American patients. 10 Primary management of these patients by NPs led to improved metabolic control regardless of whether weight loss was achieved.
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes.
A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes. Although he was diagnosed in 1997, he had symptoms indicating hyperglycemia for 2 years before diagnosis. He had fasting blood glucose records indicating values of 118–127 mg/dl, which were described to him as indicative of “borderline diabetes.” He also remembered past episodes of nocturia associated with large pasta meals and Italian pastries. At the time of initial diagnosis, he was advised to lose weight (“at least 10 lb.”), but no further action was taken.
Referred by his family physician to the diabetes specialty clinic, A.B. presents with recent weight gain, suboptimal diabetes control, and foot pain. He has been trying to lose weight and increase his exercise for the past 6 months without success. He had been started on glyburide (Diabeta), 2.5 mg every morning, but had stopped taking it because of dizziness, often accompanied by sweating and a feeling of mild agitation, in the late afternoon.
A.B. also takes atorvastatin (Lipitor), 10 mg daily, for hypercholesterolemia (elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides). He has tolerated this medication and adheres to the daily schedule. During the past 6 months, he has also taken chromium picolinate, gymnema sylvestre, and a “pancreas elixir” in an attempt to improve his diabetes control. He stopped these supplements when he did not see any positive results.
He does not test his blood glucose levels at home and expresses doubt that this procedure would help him improve his diabetes control. “What would knowing the numbers do for me?,” he asks. “The doctor already knows the sugars are high.”
A.B. states that he has “never been sick a day in my life.” He recently sold his business and has become very active in a variety of volunteer organizations. He lives with his wife of 48 years and has two married children. Although both his mother and father had type 2 diabetes, A.B. has limited knowledge regarding diabetes self-care management and states that he does not understand why he has diabetes since he never eats sugar. In the past, his wife has encouraged him to treat his diabetes with herbal remedies and weight-loss supplements, and she frequently scans the Internet for the latest diabetes remedies.
During the past year, A.B. has gained 22 lb. Since retiring, he has been more physically active, playing golf once a week and gardening, but he has been unable to lose more than 2–3 lb. He has never seen a dietitian and has not been instructed in self-monitoring of blood glucose (SMBG).
A.B.’s diet history reveals excessive carbohydrate intake in the form of bread and pasta. His normal dinners consist of 2 cups of cooked pasta with homemade sauce and three to four slices of Italian bread. During the day, he often has “a slice or two” of bread with butter or olive oil. He also eats eight to ten pieces of fresh fruit per day at meals and as snacks. He prefers chicken and fish, but it is usually served with a tomato or cream sauce accompanied by pasta. His wife has offered to make him plain grilled meats, but he finds them “tasteless.” He drinks 8 oz. of red wine with dinner each evening. He stopped smoking more than 10 years ago, he reports, “when the cost of cigarettes topped a buck-fifty.”
The medical documents that A.B. brings to this appointment indicate that his hemoglobin A 1c (A1C) has never been <8%. His blood pressure has been measured at 150/70, 148/92, and 166/88 mmHg on separate occasions during the past year at the local senior center screening clinic. Although he was told that his blood pressure was “up a little,” he was not aware of the need to keep his blood pressure ≤130/80 mmHg for both cardiovascular and renal health. 11
A.B. has never had a foot exam as part of his primary care exams, nor has he been instructed in preventive foot care. However, his medical records also indicate that he has had no surgeries or hospitalizations, his immunizations are up to date, and, in general, he has been remarkably healthy for many years.
Physical Exam
A physical examination reveals the following:
Weight: 178 lb; height: 5′2″; body mass index (BMI): 32.6 kg/m 2
Fasting capillary glucose: 166 mg/dl
Blood pressure: lying, right arm 154/96 mmHg; sitting, right arm 140/90 mmHg
Pulse: 88 bpm; respirations 20 per minute
Eyes: corrective lenses, pupils equal and reactive to light and accommodation, Fundi-clear, no arteriolovenous nicking, no retinopathy
Thyroid: nonpalpable
Lungs: clear to auscultation
Heart: Rate and rhythm regular, no murmurs or gallops
Vascular assessment: no carotid bruits; femoral, popliteal, and dorsalis pedis pulses 2+ bilaterally
Neurological assessment: diminished vibratory sense to the forefoot, absent ankle reflexes, monofilament (5.07 Semmes-Weinstein) felt only above the ankle
Lab Results
Results of laboratory tests (drawn 5 days before the office visit) are as follows:
Glucose (fasting): 178 mg/dl (normal range: 65–109 mg/dl)
Creatinine: 1.0 mg/dl (normal range: 0.5–1.4 mg/dl)
Blood urea nitrogen: 18 mg/dl (normal range: 7–30 mg/dl)
Sodium: 141 mg/dl (normal range: 135–146 mg/dl)
Potassium: 4.3 mg/dl (normal range: 3.5–5.3 mg/dl)
Lipid panel
• Total cholesterol: 162 mg/dl (normal: <200 mg/dl)
• HDL cholesterol: 43 mg/dl (normal: ≥40 mg/dl)
• LDL cholesterol (calculated): 84 mg/dl (normal: <100 mg/dl)
• Triglycerides: 177 mg/dl (normal: <150 mg/dl)
• Cholesterol-to-HDL ratio: 3.8 (normal: <5.0)
AST: 14 IU/l (normal: 0–40 IU/l)
ALT: 19 IU/l (normal: 5–40 IU/l)
Alkaline phosphotase: 56 IU/l (normal: 35–125 IU/l)
A1C: 8.1% (normal: 4–6%)
Urine microalbumin: 45 mg (normal: <30 mg)
Based on A.B.’s medical history, records, physical exam, and lab results, he is assessed as follows:
Uncontrolled type 2 diabetes (A1C >7%)
Obesity (BMI 32.4 kg/m 2 )
Hyperlipidemia (controlled with atorvastatin)
Peripheral neuropathy (distal and symmetrical by exam)
Hypertension (by previous chart data and exam)
Elevated urine microalbumin level
Self-care management/lifestyle deficits
• Limited exercise
• High carbohydrate intake
• No SMBG program
Poor understanding of diabetes
A.B. presented with uncontrolled type 2 diabetes and a complex set of comorbidities, all of which needed treatment. The first task of the NP who provided his care was to select the most pressing health care issues and prioritize his medical care to address them. Although A.B. stated that his need to lose weight was his chief reason for seeking diabetes specialty care, his elevated glucose levels and his hypertension also needed to be addressed at the initial visit.
The patient and his wife agreed that a referral to a dietitian was their first priority. A.B. acknowledged that he had little dietary information to help him achieve weight loss and that his current weight was unhealthy and “embarrassing.” He recognized that his glucose control was affected by large portions of bread and pasta and agreed to start improving dietary control by reducing his portion size by one-third during the week before his dietary consultation. Weight loss would also be an important first step in reducing his blood pressure.
The NP contacted the registered dietitian (RD) by telephone and referred the patient for a medical nutrition therapy assessment with a focus on weight loss and improved diabetes control. A.B.’s appointment was scheduled for the following week. The RD requested that during the intervening week, the patient keep a food journal recording his food intake at meals and snacks. She asked that the patient also try to estimate portion sizes.
Although his physical activity had increased since his retirement, it was fairly sporadic and weather-dependent. After further discussion, he realized that a week or more would often pass without any significant form of exercise and that most of his exercise was seasonal. Whatever weight he had lost during the summer was regained in the winter, when he was again quite sedentary.
A.B.’s wife suggested that the two of them could walk each morning after breakfast. She also felt that a treadmill at home would be the best solution for getting sufficient exercise in inclement weather. After a short discussion about the positive effect exercise can have on glucose control, the patient and his wife agreed to walk 15–20 minutes each day between 9:00 and 10:00 a.m.
A first-line medication for this patient had to be targeted to improving glucose control without contributing to weight gain. Thiazolidinediones (i.e., rosiglitizone [Avandia] or pioglitizone [Actos]) effectively address insulin resistance but have been associated with weight gain. 12 A sulfonylurea or meglitinide (i.e., repaglinide [Prandin]) can reduce postprandial elevations caused by increased carbohydrate intake, but they are also associated with some weight gain. 12 When glyburide was previously prescribed, the patient exhibited signs and symptoms of hypoglycemia (unconfirmed by SMBG). α-Glucosidase inhibitors (i.e., acarbose [Precose]) can help with postprandial hyperglycemia rise by blunting the effect of the entry of carbohydrate-related glucose into the system. However, acarbose requires slow titration, has multiple gastrointestinal (GI) side effects, and reduces A1C by only 0.5–0.9%. 13 Acarbose may be considered as a second-line therapy for A.B. but would not fully address his elevated A1C results. Metformin (Glucophage), which reduces hepatic glucose production and improves insulin resistance, is not associated with hypoglycemia and can lower A1C results by 1%. Although GI side effects can occur, they are usually self-limiting and can be further reduced by slow titration to dose efficacy. 14
After reviewing these options and discussing the need for improved glycemic control, the NP prescribed metformin, 500 mg twice a day. Possible GI side effects and the need to avoid alcohol were of concern to A.B., but he agreed that medication was necessary and that metformin was his best option. The NP advised him to take the medication with food to reduce GI side effects.
The NP also discussed with the patient a titration schedule that increased the dosage to 1,000 mg twice a day over a 4-week period. She wrote out this plan, including a date and time for telephone contact and medication evaluation, and gave it to the patient.
During the visit, A.B. and his wife learned to use a glucose meter that features a simple two-step procedure. The patient agreed to use the meter twice a day, at breakfast and dinner, while the metformin dose was being titrated. He understood the need for glucose readings to guide the choice of medication and to evaluate the effects of his dietary changes, but he felt that it would not be “a forever thing.”
The NP reviewed glycemic goals with the patient and his wife and assisted them in deciding on initial short-term goals for weight loss, exercise, and medication. Glucose monitoring would serve as a guide and assist the patient in modifying his lifestyle.
A.B. drew the line at starting an antihypertensive medication—the angiotensin-converting enzyme (ACE) inhibitor enalapril (Vasotec), 5 mg daily. He stated that one new medication at a time was enough and that “too many medications would make a sick man out of me.” His perception of the state of his health as being represented by the number of medications prescribed for him gave the advanced practice nurse an important insight into the patient’s health belief system. The patient’s wife also believed that a “natural solution” was better than medication for treating blood pressure.
Although the use of an ACE inhibitor was indicated both by the level of hypertension and by the presence of microalbuminuria, the decision to wait until the next office visit to further evaluate the need for antihypertensive medication afforded the patient and his wife time to consider the importance of adding this pharmacotherapy. They were quite willing to read any materials that addressed the prevention of diabetes complications. However, both the patient and his wife voiced a strong desire to focus their energies on changes in food and physical activity. The NP expressed support for their decision. Because A.B. was obese, weight loss would be beneficial for many of his health issues.
Because he has a sedentary lifestyle, is >35 years old, has hypertension and peripheral neuropathy, and is being treated for hypercholestrolemia, the NP performed an electrocardiogram in the office and referred the patient for an exercise tolerance test. 11 In doing this, the NP acknowledged and respected the mutually set goals, but also provided appropriate pre-exercise screening for the patient’s protection and safety.
In her role as diabetes educator, the NP taught A.B. and his wife the importance of foot care, demonstrating to the patient his inability to feel the light touch of the monofilament. She explained that the loss of protective sensation from peripheral neuropathy means that he will need to be more vigilant in checking his feet for any skin lesions caused by poorly fitting footwear worn during exercise.
At the conclusion of the visit, the NP assured A.B. that she would share the plan of care they had developed with his primary care physician, collaborating with him and discussing the findings of any diagnostic tests and procedures. She would also work in partnership with the RD to reinforce medical nutrition therapies and improve his glucose control. In this way, the NP would facilitate the continuity of care and keep vital pathways of communication open.
Advanced practice nurses are ideally suited to play an integral role in the education and medical management of people with diabetes. 15 The combination of clinical skills and expertise in teaching and counseling enhances the delivery of care in a manner that is both cost-reducing and effective. Inherent in the role of advanced practice nurses is the understanding of shared responsibility for health care outcomes. This partnering of nurse with patient not only improves care but strengthens the patient’s role as self-manager.
Geralyn Spollett, MSN, C-ANP, CDE, is associate director and an adult nurse practitioner at the Yale Diabetes Center, Department of Endocrinology and Metabolism, at Yale University in New Haven, Conn. She is an associate editor of Diabetes Spectrum.
Note of disclosure: Ms. Spollett has received honoraria for speaking engagements from Novo Nordisk Pharmaceuticals, Inc., and Aventis and has been a paid consultant for Aventis. Both companies produce products and devices for the treatment of diabetes.
Email alerts
- Advanced Practice Care: Advanced Practice Care in Diabetes: Epilogue
- Advanced Practice Care: Advanced Practice Care in Diabetes: Preface
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
- Insulin, Medicines, & Other Diabetes Treatments
- Español
Insulin, Medicines, & Other Diabetes Treatments
On this page:
What medicines might I take for diabetes?
What type of diabetes do i have, what are the different types of insulin, what are the different ways to take insulin, what oral medicines treat type 2 diabetes, what other injectable medicines treat diabetes, what should i know about side effects of diabetes medicines.
- What questions should I ask about my diabetes medicines?
Do I have other treatment options for my diabetes?
Clinical trials for insulin, medicines, & other diabetes treatments.
Taking insulin or other diabetes medicines is often part of treating diabetes. In addition to making healthy food and beverage choices, getting physical activity, getting enough sleep, and managing stress, medicines can help you manage the disease. Some other treatment options are also available.
The medicine you take depends on the type of diabetes you have and how well the medicine controls your blood glucose levels, also called blood sugar levels. Other factors, such as any other health conditions you may have, medication costs, your insurance coverage and copays, access to care, and your lifestyle, may affect what diabetes medicine you take.
Type 1 diabetes
If you have type 1 diabetes , you must take insulin because your pancreas does not make it. You will need to take insulin several times during the day, including when you eat and drink, to control your blood glucose level.
There are different ways to take insulin . You can use a needle and syringe , an insulin pen , or an insulin pump . An artificial pancreas —also called an automated insulin delivery system—may be another option for some people.
Type 2 diabetes
Some people with type 2 diabetes can control their blood glucose level by making lifestyle changes. These lifestyle changes include consuming healthy meals and beverages, limiting calories if they have overweight or obesity , and getting physical activity.
Many people with type 2 diabetes need to take diabetes medicines as well. These medicines may include diabetes pills or medicines you inject, such as insulin. Over time, you may need more than one diabetes medicine to control your blood glucose level. Even if you do not take insulin, you may need it at special times, such as if you are pregnant or if you are in the hospital for treatment.
Gestational diabetes
If you have gestational diabetes , you can manage your blood glucose level by following a healthy eating plan and doing a moderate-intensity physical activity, such as brisk walking for 150 minutes, each week. If consuming healthy food and beverages and getting regular physical activity aren’t enough to keep your blood glucose level in your target range, a doctor will work with you and may recommend you take insulin. Insulin is safe to take while you are pregnant.
No matter what type of diabetes you have, taking diabetes medicines every day can feel like a burden sometimes. New medications and improved delivery systems can help make it easier to manage your blood glucose levels. Talk with your doctor to find out which medications and delivery systems will work best for you and fit into your lifestyle.
Several types of insulin are available. Each type starts to work at a different speed, known as “onset,” and its effects last a different length of time, known as “duration.” Most types of insulin reach a peak, which is when they have the strongest effect. After the peak, the effects of the insulin wear off over the next few hours or so. Table 1 lists the different types of insulin, how fast they start to work, when they peak, and how long they last.
Table 1. Types of insulin and how they work 1,2
Another type of insulin, called premixed insulin, is a combination of insulins listed in Table 1. Premixed insulin starts to work in 15 to 60 minutes and can last from 10 to 16 hours. The peak time varies depending on which insulins are mixed.
Your doctor will work with you to review your medication options. Talk with your doctor about your activity level, what you eat and drink, how well you manage your blood glucose levels, your age and lifestyle, and how long your body takes to absorb insulin.
Follow your doctor’s advice on when and how to take your insulin. If you're worried about the cost, talk with your doctor. Some types of insulin cost more than others. You can also find resources to get financial help for diabetes care .
The way you take insulin may depend on your lifestyle, insurance plan, and preferences. Talk with your doctor about the options and which one is best for you. Most people with diabetes take insulin using a needle and syringe, insulin pen, or insulin pump. Inhalers and insulin jet injectors are less common ways to take insulin. Artificial pancreas systems are now approved by the U.S. Food and Drug Administration (FDA). Talk with your doctor to see if an artificial pancreas is an option for you.
Needle and syringe
You can give yourself insulin shots using a needle and syringe . You draw up your dose of insulin from the vial—or bottle—through the needle into the syringe. Insulin works fastest when you inject it in your belly, but your doctor may recommend alternating the spot where you inject it. Injecting insulin in the same spot repeatedly could cause the tissue to harden, making it harder to take shots in that area over time. Other spots you can inject insulin include your thigh, buttocks, or upper arm, but it may take longer for the insulin to work from those areas. Some people with diabetes who take insulin need 2 to 4 shots a day to reach their blood glucose targets. Others can take a single shot. Injection aids can help you give yourself the shots.

An insulin pen looks like a writing pen but has a needle for its point. Some insulin pens come filled with insulin and are disposable. Others have room for an insulin cartridge that you insert and replace after use. Many people find insulin pens easier to use, but they cost more than needles and syringes. You may want to consider using an insulin pen if you find it hard to fill the syringe while holding the vial or cannot read the markings on the syringe. Different pen types have features that can help with your injections. Some reusable pens have a memory function, which can recall dose amounts and timing. Other types of “connected” insulin pens can be programmed to calculate insulin doses and provide downloadable data reports, which can help you and your doctor adjust your insulin doses.

An insulin pump is a small machine that gives you steady doses of insulin throughout the day. You wear one type of pump outside your body on a belt or in a pocket or pouch. The insulin pump connects to a small plastic tube and a very small needle. You insert the plastic tube with a needle under your skin, then take out the needle. The plastic tube will stay inserted for several days while attached to the insulin pump. The machine pumps insulin through the tube into your body 24 hours a day and can be programmed to give you more or less insulin based on your needs. You can also give yourself doses of insulin through the pump at mealtimes.
Another type of pump has no tubes. This pump attaches directly to your skin with a self-adhesive pad and is controlled by a hand-held device. The plastic tube and pump device are changed every several days.

Another way to take insulin is by breathing powdered insulin into your mouth from an inhaler device. The insulin goes into your lungs and moves quickly into your blood. You may want to use an insulin inhaler to avoid using needles. Inhaled insulin is only for adults with type 1 or type 2 diabetes. Taking insulin with an inhaler is less common than using a needle and syringe.
Jet injector
A jet injector is a device that sends a fine spray of insulin into the skin at high pressure instead of using a needle to deliver the insulin. It is used less commonly than a needle and syringe or a pen.
Artificial pancreas
An artificial pancreas is a system of three devices that work together to mimic how a healthy pancreas controls blood glucose in the body. A continuous glucose monitor (CGM) tracks blood glucose levels every few minutes using a small sensor inserted under the skin that is held in place with an adhesive pad. The CGM wirelessly sends the information to a program on a smartphone or an insulin infusion pump. The program calculates how much insulin you need. The insulin infusion pump will adjust how much insulin is given from minute to minute to help keep your blood glucose level in your target range. An artificial pancreas is mainly used to help people with type 1 diabetes.
You may need to take medicines to manage your type 2 diabetes, in addition to consuming healthy foods and beverages and being physically active. You can take many diabetes medicines by mouth. These medicines are called oral medicines.
Most people with type 2 diabetes start with metformin pills. Metformin also comes as a liquid. Metformin helps your liver make less glucose and helps your body use insulin better. This drug may help you lose a small amount of weight.
Other oral medicines act in different ways to lower blood glucose levels. Combining two or three kinds of diabetes medicines can lower blood glucose levels better than taking just one medicine.
Read about different kinds of diabetes medicines (PDF, 2.8 MB) from the FDA.
If you have type 1 diabetes, your doctor may recommend you take other medicines, in addition to insulin, to help control your blood glucose. Some of these medicines work to slow how fast food and beverages move through your stomach . These medicines also slow down how quickly and how high your blood glucose levels rise after eating. Other medicines work to block certain hormones in your digestive system that raise blood glucose levels after meals or help the kidneys to remove more glucose from your blood.
Besides insulin, other types of injected medicines (PDF, 2.8 MB) are available that will keep your blood glucose level from rising too high after you eat or drink. These medicines, known as glucagon-like peptide-1 (GLP-1) receptor agonists, 3 may make you feel less hungry and help you lose some weight. GLP-1 medicines are not substitutes for insulin.
Side effects are problems that result from taking a medicine. Some diabetes medicines can cause hypoglycemia , also called low blood glucose, if you don’t balance your medicines with food and activity.
Ask your doctor whether your diabetes medicine can cause hypoglycemia or other side effects, such as upset stomach and weight gain. Aim to take your diabetes medicines as your doctor instructs you, to help prevent side effects and diabetes problems.
If medicines and lifestyle changes are not enough to manage your diabetes, there are other treatments that might help you. These treatments include weight-loss (bariatric) surgery for certain people with type 1 or type 2 diabetes, or pancreatic islet transplantation for some people with type 1 diabetes.
Weight-loss surgery
Weight-loss surgery are operations that help you lose weight by making changes to your digestive system. Weight-loss surgery is also called bariatric or metabolic surgery.
This type of surgery may help some people who have obesity and type 2 diabetes lose a large amount of weight and bring their blood glucose levels back to a healthy range. How long the improved response lasts can vary by patient, type of weight-loss surgery, and the amount of weight the person lost. Other factors include how long a person had diabetes and whether the person used insulin. Some people with type 2 diabetes may no longer need to use diabetes medicines after weight-loss surgery . 4
Researchers are studying whether weight-loss surgery can help control blood glucose levels in people with type 1 diabetes who have obesity. 5
Pancreatic islet transplantation
Pancreatic islet transplantation is an experimental treatment for people with type 1 diabetes who have trouble controlling their blood glucose levels. Pancreatic islets are clusters of cells in the pancreas that make the hormone insulin. In type 1 diabetes, the body’s immune system attacks these cells. A pancreatic islet transplantation replaces destroyed islets with new islets from organ donors. The new islets make and release insulin. Because researchers are still studying pancreatic islet transplantation , the procedure is only available to people enrolled in research studies.
The NIDDK conducts and supports clinical trials in many diseases and conditions, including diabetes. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for insulin, medicines, and other diabetes treatments?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help health care professionals and researchers learn more about disease and improve health care for people in the future.
Find out if clinical trials are right for you .
Researchers are studying many aspects of diabetes medicines, including
- new types of insulin
- the most effective times to take diabetes medicines
- new types of monitoring devices and delivery systems
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical trials for insulin, medicines, and other diabetes treatments are looking for participants?
You can view a filtered list of clinical studies on insulin, medicines, and other diabetes treatments covered in this health topic that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the National Institutes of Health does not review these studies and cannot ensure they are safe. Always talk with your health care provider before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank Stuart A. Weinzimer, M.D., Yale University School of Medicine

The Price of Uncertainty: Unveiling the Impact of Unexpected Type 1 Diabetes Diagnoses
Receiving a type 1 diabetes (T1D) diagnosis can be life-altering, bringing with it many visible and invisible costs.
Findings from a new survey commissioned by Sanofi, called “The Cost of Not Knowing,” have shed light on the profound impact of receiving a T1D diagnosis without prior warning. The findings, which were announced on June 5, 2024, highlight the social, emotional, and financial costs associated with unexpected diagnoses while also outlining a pathway to empowerment via early screening and education. Autoantibody screening is a blood test that looks for diabetes-related autoantibodies to see if someone is at risk for developing T1D.
What if There Was More Time to Prepare for a T1D Diagnosis?
“The Cost of Not Knowing” explores the impact of a T1D diagnosis on caregivers of children under the age of 18 who also have the condition, as well as adults with T1D over the age of 18.
The aim of the survey research was to examine in more detail the apparent and invisible consequences of an unexpected T1D diagnosis, and to determine if early detection using autoantibody screening could have mitigated these effects.
By taking the step to have ourselves, our children, or our family members screened for T1D, we may give ourselves more time to prepare and plan for the future. It’s important to talk to your doctor about screening.
Key Survey Findings from The Cost of Not Knowing:
The heavy weight of hindsight: caregivers and adults wish they had known sooner.
“The Cost of Not Knowing” reveals a poignant truth: most people with T1D carry the heavy burden of regret, and wish they had known about the risks sooner.
- 68% of adults with T1D who were not screened say they regret not having an autoantibody test to determine their risk of developing the disease.
- 93% of caregivers say they wish they had learned about their loved one’s risk of developing T1D prior to their diagnosis.
The Far-Reaching Impact: Emotional, Social, and Financial Costs of Unexpected Diagnoses
The toll of an unexpected T1D diagnosis extends far beyond the physical, affecting emotional well-being, social lives, and financial stability.
- Emotional: 64% of adults with T1D said their emotional health declined upon diagnosis. 61% who experienced feelings such as sadness, fear, anger, or uncertainty believe knowing their risk sooner would have diminished these feelings.
- Social: 87% of caregivers and 71% of adults with T1D gave up some of their interests or future plans when they learned of their diagnosis.
- Financial: 50% of adults with T1D spent at least $5,000 on emergency care for their symptoms before or during diagnosis, with nearly 1 in 4 (24%) spending at least $10,000.
Empowering Lives: The Transformative Potential of Early Screening
Imagine a world where individuals and families facing T1D had the power of an early diagnosis on their side. This survey research sheds light on the profound difference early screening could make.
- 79% of adults with T1D and 83% of caregivers changed how they lived their life when they first learned of their/their loved one’s T1D diagnosis because they felt overwhelmed and underprepared.
- 34% say they might have felt more in control of their health
- 28% say they may have had more time to prepare
- 20% say they may have avoided the stress that comes with not knowing.
These results reveal the striking potential benefits of early screening. Another startling discovery is that 64% of adults with T1D who underwent autoantibody screening reported their emotional health improved when they received their diagnosis. When these adults were diagnosed:
- Only 26% of them felt fearful.
- Only 10% felt uncertain about the future.
In contrast, among those who did not have the autoantibody test for T1D:
- 40% felt fearful
- 28% felt uncertain about the future.
The Invisible Barriers: Access Barriers Prevent Making Autoantibody Screening Common Practice
Despite these clear impacts, these findings also expose both a lack of awareness and perceived barriers preventing access to early T1D screening.
- Only 14% of adults with T1D say they were screened with an autoantibody test prior to diagnosis to see if they were at risk of developing the disease.
- Furthermore, among those who did not screen—or whose loved one did not screen—72% of adults with T1D—and 66% of caregivers—report that they were unaware that a T1D autoantibody test was even available.
- Among those who were aware, 84% of adults with T1D and 78% of caregivers say that barriers prevented them/their loved one from screening, including insurance (40% and 21%) and screening not being offered by their healthcare provider (28% & 35%).
It’s clear that we must work to remove barriers to screening access and prioritize widespread education. No one should have to face a life-altering diagnosis unprepared.
A Resounding Call for Change: Nearly All Adults with T1D and Caregivers Recommend Early Screening
These results point to an urgent need for action, with nearly all adults with T1D (92%) and caregivers (96%) recommending early autoantibody testing to friends and family to better understand their risk of developing T1D.
Anyone can develop T1D at any time, with about 90% of those diagnosed having no family history. By advocating for change, championing early screening, and connecting those at risk with resources like Beyond Type 1, we can transform the T1D journey from one of fear and isolation to one of empowerment and support.
No one should have to experience the price of uncertainty. Through early autoantibody screening, we can help ensure that no one has to face this life-altering diagnosis unprepared.
Take action today – talk to your doctor about autoantibody screening and become an advocate.
Learn more about “The Cost of Not Knowing” below.

Background & Methodology
The Cost of Not Knowing research, from Beyond Type 1, was commissioned by Sanofi and conducted by Wakefield Research (www.wakefieldresearch.com).
The research was conducted via two survey instruments—one among 1,000 US adults with type 1 diabetes and a second among 1,000 US caregivers to children under the age of 18 with type 1 diabetes, between March 15th and March 29th, 2024, using an email invitation and an online survey.
The Isolated Caregiver—A Mom’s Story -
9 bad diabetes photos -, diabetes isn’t even that bad -, life with type 1—a photo essay -, what is type 2 diabetes -.

- TERMS OF USE
- 400 CONCAR DR.
- SAN MATEO, CA 94402

- BEYOND TYPE 1 BEYOND TYPE 1 EN ESPAÑOL
- IN ENGLISH EN ESPANOL BEYOND TYPE 1 AUF DEUTSCH BEYOND TYPE 1 IN ITALIANO BEYOND TYPE 1 EN FRANÇAIS BEYOND TYPE 1 IN HET NEDERLANDS BEYOND TYPE 1 PÅ SVENSKA BEYOND TYPE 1 EM PORTUGUÊS BEYOND TYPE 1 بالعربية

- BEYOND TYPE 2 BEYOND TYPE 2 EN ESPAÑOL IN ENGLISH EN ESPANOL BEYOND TYPE 2 AUF DEUTSCH BEYOND TYPE 2 en Français BEYOND TYPE 2 in Italiano BEYOND TYPE 2 Canada (French) BEYOND TYPE 2 Canada (English)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Health Promot Perspect
- v.10(2); 2020
Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis
Majid mobasseri.
1 Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran

Masoud Shirmohammadi
2 Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Tarlan Amiri
3 Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
Nafiseh Vahed
4 Emergency Medicine Research Team, Tabriz University of Medical Sciences, Tabriz, Iran
5 Research Center for Evidence-Based Medicine, Iranian EBM Centre: A Joanna Briggs Institute Affiliated Group, Tabriz University of Medical Sciences, Tabriz, Iran
Hossein Hosseini Fard
Morteza ghojazadeh, associated data.
Supplementary file 1 contains search strategy.
Background: Diabetes is referred to a group of diseases characterized by high glucose levels in blood. It is caused by a deficiency in the production or function of insulin or both, which can occur because of different reasons, resulting in protein and lipid metabolic disorders. The aim of this study was to systematically review the prevalence and incidence of type 1 diabetes in the world.
Methods: A systematic search of resources was conducted to investigate the prevalence and incidence of type 1 diabetes in the world. The databases of Medline (via PubMed and Ovid),ProQuest, Scopus, and Web of Science from January 1980 to September 2019 were searched to locate English articles. The located articles were screened in multiple levels of title, abstract,and full-text and final studies that met the inclusion criteria were retrieved and included in the study.
Results: From 1202 located articles, 193 studies were included in this systematic review. The results of meta-analysis showed that the incidence of type 1 diabetes was 15 per 100,000 people and the prevalence was 9.5% (95% CI: 0.07 to 0.12) in the world, which was statistically significant.
Conclusion: According to the results, the incidence and prevalence of type 1 diabetes are increasing in the world. As a result, insulin will be difficult to access and afford, especially in underdeveloped and developing countries.
Introduction
Diabetes is referred to a group of diseases characterized by high glucose levels in blood. It is caused by a deficiency in the production or function of insulin or both, which can occur because of different reasons, resulting in protein and lipid metabolic disorders. 1 The long-term effects of hypoglycemia are tissue and organ damage. 2
Symptoms of diabetes include polyuria, thirst, vision disorders, and weight loss. In some cases there are more severe forms of diabetic ketoacidosis and hyperosmolar that may lead to stupor and coma. But most symptoms are not severe, which may cause damage or even failure of different organs in the long run and lead to irreparable injuries such as blindness, amputation, stroke and eventually death. Previously, type 1 diabetes was called insulin-dependent diabetes and it could happen at any age but is most common in children and young people. 3
People with type 1 diabetes are not able to produce enough insulin. This type constitutes about 5%–10% of all cases of diabetes. In this type, the cellular destruction of beta cells occurs in the pancreas. In type 1 diabetes, the pancreas does not release any insulin. Since there is no epidemiologically accurate information on the prevalence and incidence of type 1 diabetes in the world and in the region, therefore, the present study was designed and implemented as a systematic review and meta-analysis, because of geopolitical map of the policy on the prevention and treatment of this disease can be done better.
Materials and Methods
In this systematic review and meta-analysis, a systematic search of resources was conducted by a librarian (N.V.) to investigate the prevalence and incidence of type 1 diabetes (condition) in the people (population) of the world (context). The PICO of study based on the JBI protocol as CoCoPop for prevalence and incidence studies.
Data sources and search strategy
The databases of Medline via (PubMed, Ovid), Embase, Scopus, Web of Science from January 1980 to September 2019 were searched to locate English articles. Also, SID, Magiran, and Barakat databases were searched for Persian studies. The grey literature and ongoing studies were searched using the following: OpenGrey, Google Scholar and for thesis and dissertations ProQuest and studies presented at conferences were also searched. Also, experts and professionals on this subject were reached and their opinions were gathered for information on published and unpublished studies. The search was performed using MESH and free keywords. The keywords selected for the search were: “type 1 diabetes”, “prevalence”, and “incidence” with this search strategy: (((“Diabetes Mellitus, Type 1”[Mesh]) OR ((((((((((((((((((((IDDM[Title/Abstract]) OR T1DM[Title/Abstract]) OR “Type 1 Diabetes”[Title/Abstract]) OR “Autoimmune Diabetes”[Title/Abstract]) OR “Juvenile Onset Diabetes”[Title/Abstract]) OR “Juvenile-Onset Diabetes”[Title/Abstract]) OR “Brittle Diabetes Mellitus”[Title/Abstract]) OR “brittle diabetes”[Title/Abstract]) OR “diabetes mellitus type 1”[Title/Abstract]) OR “diabetes mellitus type I”[Title/Abstract]) OR “diabetes type 1”[Title/Abstract]) OR “diabetes type I”[Title/Abstract]) OR “early onset diabetes mellitus”[Title/Abstract]) OR “insulin dependent diabetes”[Title/Abstract]) OR “juvenile diabetes”[Title/Abstract]) OR “juvenile diabetes mellitus”[Title/Abstract]) OR “type I diabetes”[Title/Abstract]) OR “type I diabetes mellitus”[Title/Abstract]) OR “Insulin Dependent Diabetes Mellitus”[Title/Abstract]) OR “Insulin-Dependent Diabetes Mellitus”[Title/Abstract]))) AND ((((“Prevalence”[Mesh]) OR ((Prevalence[Title/Abstract]) OR Prevalences[Title/Abstract]))) OR ((“Incidence”[Mesh]) OR ((Incidence[Title/Abstract]) OR Incidences[Title/Abstract]))). The complete search strategy of Medline and Embase is in Supplementary file 1.
Inclusion and exclusion criteria
Inclusioncriteria for selecting studies include: 1. Articles published between 1980 and 2019; 2. Articles published in English and Persian. The exclusion criteria were: 1. Studies with no reported sample size; 2. Studies that had low quality; 3. Studies that were published before 1990.
Study selection
The located articles were screened in multiple levels of title, abstract, and full-text and final studies that met the inclusion criteria were retrieved and included in the study. The studies were critically appraised by 2 subject specialists and low-quality studies were excluded. In cases of disagreements between two experts (M.M. and M.S.) at each stage of selection and appraisal, third person opinion was used.
Quality appraisal
Articles were evaluated using the STROBE checklist. In this checklist, the minimum score was 2 and the maximum was 4. Finally, articles that received a score of 4 on checklist questions were included in the research, 128 articles earned 4 score, 46 articles earned 3 score and 19 articles earned 2 score and finally their data were extracted to perform the meta-analysis.
Data extraction and quality assessment
The information extracted from the articles were entered in the extraction form. Extracted data included: first author, year of publication, country of study, sample size, and incidence of diabetes in the studies.
Statistical analysis
Statistical analysis was performed using CMA v.2.0 software and P value less than 0.05 was considered as significant. The binomial distribution was used to calculate the variance. Weighted mean was used to combine the prevalence rate of different studies. Meta-analysis was used to obtain the incidence of type 1 diabetes. The heterogeneity between studies was assessed by Cochran (Q) and I 2 statistics, which expressed the percentage of variation between studies. Random effects model was used to calculate the overall and pooled effect size.
Search results and study characteristics
In a systematic search of sources, 65 765 articles were identified. A total of 58 239 articles were duplicates, and 7107 were excluded after reviewing the title and abstract of the articles. After reviewing the full-text articles, 49 articles were excluded. Finally, 193 studies were included in the systematic review and meta-analysis. Figure 1 shows the identified and retrieved articles in the study. Tables Tables1, 1 , ,2 2 and and3 3 show the specifications of the articles that were studied.

Flow chart of systematic review.
Prevalence and incidence of type 1 diabetes in Asia
Prevalence and incidence of type 1 diabetes were extracted from meta-analysis studies. In type 1 diabetes incidence, the heterogeneity between studies in the meta-analysis was significant (Q = 50.51; df = 16; P < 0.001; I 2 = 68.33), but in the prevalence of diabetes 1, the heterogeneity was not significant (Q = 5220; df = 6; P < 0.001; I 2 = 99.88). The incidence of type 1 diabetes in Asia was 15 per 100 000 population, which was statistically significant (Incidence = 0.015, 95% CI = 0.010 to 0.021, P < 0.001), and the prevalence of type 1 diabetes was 6.9 per 10 000 people, which was statistically significant (Prevalence = 0.069, 95% CI = 0.020 to 0.214, P < 0.001). Figures 2A and 2B show the forest plot of prevalence and incidence of type 1 diabetes in Asia.

(A) Incidence and (B) prevalence of type 1 diabetes in Asia.
Prevalence and incidence of type 1 diabetes in Africa
Prevalence and incidence of type 1 diabetes were extracted from meta-analysis studies. In type 1 diabetes incidence, the heterogeneity between studies in the meta-analysis was not significant (Q = 23.79; df = 6; P < 0.001; I 2 = 74.78) and in the prevalence of diabetes 1, the heterogeneity was not significant too, (Q = 4.4; df = 1; P < 0.001; I 2 = 77.27). The incidence of type 1 diabetes in Africa was 8 per 100 000 population, which was statistically significant (Incidence = 0.008, 95% CI = 0.003 to 0.021 P < 0.001), and the prevalence of type 1 diabetes was 3.5 per 10 000 people, which was not statistically significant (prevalence = 0.035, 95% CI: 0.022 to 0.055, P < 0.001). Figures 3A and 3B show the forest plot of prevalence and incidence of type 1 diabetes in Africa.

(A) Incidence and (B) prevalence of type 1 diabetes in Africa.
Prevalence and incidence of type 1 diabetes in Europe
Prevalence and incidence of type 1 diabetes were extracted from meta-analysis studies. In type 1 diabetes incidence, the heterogeneity between studies in the meta-analysis was significant (Q = 895.56, df = 96, P < 0.001, I 2 = 89.28) but in the prevalence of diabetes 1, the heterogeneity was not significant, (Q = 5792.85, df = 15, P < 0.001, I 2 = 99.74). The incidence of type 1 diabetes in Europe was 15 per 100 000 population, which was statistically significant (Incidence = 0.015, 95% CI = 0.013 to 0.018, P < 0.001), and the prevalence of type 1 diabetes was 12.2 per 10 000 people, which was statistically significant (Prevalence = 0.122, 95% CI = 0.085 to 0.171, P < 0.001). Figures 4 and and5 5 show the forest plot of prevalence and incidence of type 1 diabetes in Europe.

Incidence of type 1 diabetes in Europe.

Prevalence of type 1 diabetes in Europe.
Prevalence and incidence of type 1 diabetes in America
Prevalence and incidence of type 1 diabetes were extracted from meta-analysis studies. In type 1 diabetes incidence, the heterogeneity between studies in the meta-analysis was significant (Q = 18.88, df = 16, P = 0.27, I 2 = 15.28) and in the prevalence of diabetes 1, the heterogeneity was significant too, (Q = 1120.79, df = 7, P < 0.001, I 2 = 99.38). The incidence of type 1 diabetes in America was 20 per 100 000 population, which was statistically significant (Incidence = 0.020, 95% CI = 0.010 to 0.021, P < 0.001), and the prevalence of type 1 diabetes was 12.2 per 10 000 people, which was statistically significant (Prevalence = 0.093, 95% CI = 0.063 to 0.137, P < 0.001). Figures 6A and 6B show the forest plot of prevalence and incidence of type 1 diabetes in America. A sensitivity analysis was done for Incidence of type 1 diabetes in America based on excluding studies with too wide CIs. Sensitivity analysis’s results show that the incidence of type 1 diabetes in America is 19 per 100 000 population, which is statistically significant (Incidence = 0.019, 95% CI = 0.016 to 0.022, P < 0.001).

(A) Incidence and (B) prevalence of type 1 diabetes in America.
Prevalence and incidence of type 1 diabetes in the world
Prevalence and incidence of type 1 diabetes were extracted from meta-analysis studies. In type 1 diabetes incidence, the heterogeneity between studies in the meta-analysis was significant (Q = 1020.30, df = 137, P < 0.001, I 2 = 86.57) and in the prevalence of diabetes 1, the heterogeneity was significant too, (Q = 14760.32, df = 32, P < 0.001, I 2 = 99.78). The incidence of type 1 diabetes in world was 15 per 100 000 population, which was statistically significant (Incidence = 0.015, 95% CI = 0.013 to 0.017, P < 0.001), and the prevalence of type 1 diabetes was 9.5 per 10 000 people, which was statistically significant (prevalence = 0.095, 95% CI = 0.070 to 0.128, P < 0.001). Figure 7 shows the forest plot of prevalence and incidence of type 1 diabetes in the world.

Prevalence of type 1 diabetes in the world.
Publication bias
In order to assess the publication bias, Eggers Regression test was used. Based on the results, the population bias between studies was not significant (t-value = 1.26, df 93, P = 0.21).
Meta-Regression
Meta-regression was used to determine the effect of time on type 1 diabetes incidence. The results showed that the incidence of type 1 diabetes has increased over time. The meta-regression plot is shown in Figure 8 .

The meta-regression plot.
The global trend of increasing prevalence of type 1 diabetes, with multiple etiologies, operates through multiple mechanisms. In the present study, data were extracted from 193 articles between 1990 and 2019. The results showed that the incidence of type 1 diabetes in continental subgroups (Asia, Africa, Europe, and America) was 15 per 100 000, 8 per 100 000, 15 per 100 000 and 20 per 100, respectively. Also, the global prevalence of continental subtypes of type 1 diabetes in the above regions was, 6.9 per 10 000, 3.5 per 10 000, and 12.2 per 10 000, respectively.
Relative differences between obtained results and previous statistics may be due to different research time periods and new global population status. Especially in recent years (social, political and economic migration), the changing global climate coupled with new policies and sanctions that have led to poorer middle-income and low-income countries. 212
The pathogenesis of type 2 diabetes is known, which is associated with different genes and the involvement of multiple factors. Type 2 diabetes can be prevented and treated by removing or reducing these factors. Most of the warnings of national and international health bodies and diabetes associations are based on lifestyle changes and stress reduction that can prevent diabetes. 213
But in type 1 diabetes, that make up 5 to 15 percent of diabetics and often involve children, Prevention ways have not yet been defined. However, screening of type 1 diabetes in prone families in relation to autoantibodies has recently been proposed. Also, clinical studies on the prevention of type 1 diabetes have been conducted. 214
If one foot was amputated every 30 seconds, today it’s every 15 seconds. Need for dialysis equipment will increase. The CCU and ICU beds will be full of stroke and myocardial infarction patients. The population of the blind increases and unfortunately, new, effective, and less complicated treatments become more expensive. 215
The disease shows a significant increase in glucose and possibly DKA. These patients definitely need insulin due to the pathogenesis of insulin deficiency. Manufacturing and production of insulin (traditional insulins and analog insulins) and insulin pumps, despite being inexpensive in producing countries, is shipped to low- and middle-income countries for high prices which is a major problem for the managing of type 1 diabetes patients. Certainly, uncontrolled hyperglycemia in type 1 diabetic patients will make all the problems more severe. 216
Limitations
One of the limitations of the study was the poor quality of some articles and, despite a careful search, the lack of access to some of the full text of the published articles.
According to the results, the incidence and prevalence of type 1 diabetes are increasing in the world. As a result, insulin will be difficult to access and afford, especially in underdeveloped and developing countries. Thus, warnings about this can help international organizations and countries to plan for preventive measures.
Ethical approval
This research was approved by the Local Ethics Committee with No. 61701.
Competing interests
The authors declare that they have no competing interests.
This article was supported by the Research Center for Evidence-Based Medicine, and the Research Vice-Chancellor of Tabriz University of Medical Sciences.
Authors’ contributions
Concept: MM. Study design: MSH and TA. Systematic search: NV. Critical reviews: MM and TA. Data extraction: MSH and MGH. Data analysis: MGH and HHF. Writing: NV, TA and MM. All authors had primary responsibility for the final content of the manuscript and read and approved the final manuscript.
Acknowledgments
Special thanks to the Research Vice-Chancellor of Tabriz University of Medical Sciences for financial support for this study.
Supplementary Materials
Advertisement
Impact of polygenic risk score for triglyceride trajectory and diabetic complications in subjects with type 2 diabetes based on large electronic medical record data from Taiwan: a case control study
- Original Article
- Published: 25 May 2024
Cite this article

- W.-L. Liao 1 , 2 ,
- Y.-C. Huang 3 , 4 ,
- Y.-W. Chang 1 , 2 , 4 ,
- C.-F. Cheng 5 ,
- T.-Y. Liu 6 ,
- H.-F. Lu 6 ,
- H.-L. Chen 5 na1 &
- F.-J. Tsai 3 , 4 , 7 , 8 na1
87 Accesses
1 Altmetric
Explore all metrics
The prevalence of diabetic dyslipidemia has gradually increased worldwide and individuals with hypertriglyceridemia often have a high polygenic burden of triglyceride (TG)-increasing variants. However, the contribution of genetic variants to dyslipidemia in patients with type 2 diabetes (T2D) remains limited. Therefore, in this study, we aimed to investigate the genetic characteristics of longitudinal changes in TG levels among patients with T2D and summarize the genetic effects of polygenic risk score (PRS) on TG trajectory and risk of diabetic complications.
We conducted a case–control study. A total of 11,312 patients with T2D with longitudinal TG and genetic data were identified from a large hospital database in Taiwan. We then performed a genome-wide association study and calculated the relative PRS.
In total, 21 single-nucleotide polymorphisms (SNPs) related to TG trajectory were identified and yielded an area under the receiver operating characteristic curve (ROC) of 0.712 for high TG trajectory risk among Taiwanese patients with T2D. A cumulative genetic effect was observed for high TG trajectory, even when considering the adherence of a lipid-lowering agent in stratified analysis. An increased PRS increases high TG trajectory risk in a logistic regression model (odds ratio = 1.55; 95% confidence interval [CI] = 1.31–1.83 in the validation cohort). The TG-specific PRS was associated with the risk of diabetic microvascular complications, including diabetic retinopathy and nephropathy (with hazard ratios of 1.11 [95% CI = 1.01–1.21, P = 0.027] and 1.05 [95% CI = 1.01–1.1, P = 0.018], respectively).
Conclusions
This study may contribute to the identification of patients with T2D who are at risk of abnormal TG levels and diabetic microvascular complications using polygenic information.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Association of APOE genotype with lipid profiles and type 2 diabetes mellitus in a Korean population

High genetic burden of type 2 diabetes can promote the high prevalence of disease: a longitudinal cohort study in Iran
Polygenic risk scores predict diabetes complications and their response to intensive blood pressure and glucose control
Data availability.
The GWAS summary statistics that support the findings of this study are available upon request from the corresponding author. However, raw data (even de-identified) are not publicly available under current CMUH policy as they contain information that could compromise research participant privacy.
Abbreviations
Areas under the ROC curve
Confidence interval
China medical university hospital
Clinical research platform
Cardiovascular disease
Diabetic nephropathy
Diabetic retinopathy
Electronic medical record
Group-based trajectory modeling
- Genome-wide association study
High-density lipoprotein
Low-density lipoprotein
Principal component
Proportion of days covered
- Polygenic risk score
Receiver operating characteristic
Standard deviation
Single-nucleotide polymorphisms
- Type 2 diabetes
- Triglyceride
Khan MAB et al (2020) Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health 10(1):107–111
Article PubMed PubMed Central Google Scholar
Li Y et al (2018) The prevalence and risk factors of dyslipidemia in different diabetic progression stages among middle-aged and elderly populations in China. PLoS One 13(10):e0205709
Harding JL et al (2019) Global trends in diabetes complications: a review of current evidence. Diabetologia 62(1):3–16
Article PubMed Google Scholar
Bin Saleh FS et al (2022) Prevalence and regulation of dyslipidemia among adults with Type 2 diabetes from three primary health care centers in Riyadh. Cureus 14(8):e27573
PubMed PubMed Central Google Scholar
Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 5(3):150–159
CAS PubMed Google Scholar
Tsai CW et al (2019) Longitudinal lipid trends and adverse outcomes in patients with CKD: a 13-year observational cohort study. J Lipid Res 60(3):648–660
Article CAS PubMed PubMed Central Google Scholar
Gong L et al (2021) High concentrations of triglycerides are associated with diabetic kidney disease in new-onset type 2 diabetes in China: findings from the China cardiometabolic disease and cancer cohort (4C) study. Diabetes Obes Metab 23(11):2551–2560
Kuo SC et al (2015) Association between comorbidities and dementia in diabetes mellitus patients: population-based retrospective cohort study. J Diabetes Complications 29(8):1071–1076
Spracklen CN et al (2020) Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature 582(7811):240–245
Vujkovic M et al (2020) Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 52(7):680–691
Sakaue S et al (2021) A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet 53(10):1415–1424
Article CAS PubMed Google Scholar
Tam CHT et al (2021) Development of genome-wide polygenic risk scores for lipid traits and clinical applications for dyslipidemia, subclinical atherosclerosis, and diabetes cardiovascular complications among East Asians. Genome Med. https://doi.org/10.1186/s13073-021-00831-z
Hegele RA et al (2014) The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2(8):655–666
Tam CHT et al (2021) Development of genome-wide polygenic risk scores for lipid traits and clinical applications for dyslipidemia, subclinical atherosclerosis, and diabetes cardiovascular complications among East Asians. Genome Med 13(1):29
Chiang HY et al (2021) Electronic medical record-based deep data cleaning and phenotyping improve the diagnostic validity and mortality assessment of infective endocarditis: medical big data initiative of CMUH. Biomedicine (Taipei) 11(3):59–67
Liu TY et al (2021) Comparison of multiple imputation algorithms and verification using whole-genome sequencing in the CMUH genetic biobank. Biomedicine (Taipei) 11(4):57–65
Liang HY, Lo Y-C, Chiang HY, Chen MF, Kuo CC (2020) Validation and comparison of the 2003 and 2016 diastolic functional assessments for cardiovascular mortality in a large single-center cohort. J Am Soc Echocardiogr 33(4):469–480
Shen WC, Chiang H, Chen PS, Lin YT, Kuo CC, Wu PY (2022) Risk of all-cause mortality, cardiovascular mortality, and cancer mortality in patients with bullous pemphigoid: an 11-year retrospective matched cohort study. JAMA Dermatol 158(2):9
Article Google Scholar
Chiang HY et al (2021) Association between preoperative blood glucose level and hospital length of stay for patients undergoing appendectomy or laparoscopic cholecystectomy. Diabetes Care 44(1):107–115
Nagin DS et al (2018) Group-based multi-trajectory modeling. Stat Methods Med Res 27(7):2015–2023
Nagin DS, Odgers CL (2010) Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6:109–138
Liao WL et al (2022) Analysis of HLA variants and Graves’ disease and its comorbidities using a high resolution imputation system to examine electronic medical health records. Front Endocrinol (Lausanne) 13:842673
Wei CY et al (2021) Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom Med 6(1):10
Delaneau O, Zagury JF, Marchini J (2013) Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 10(1):5–6
Howie B et al (2012) Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet 44(8):955–959
Sudmant PH et al (2015) An integrated map of structural variation in 2,504 human genomes. Nature 526(7571):75–81
Chang CC et al (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:7
Choi SW, O’Reilly PF (2019) PRSice-2: polygenic risk score software for biobank-scale data. Gigasci. https://doi.org/10.1093/gigascience/giz082
D, N. Pharmacy quality alliance adherence measures . https://www.pqaalliance.org/adherence-measures
Purcell S et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575
Spracklen CN et al (2017) Association analyses of East Asian individuals and trans-ancestry analyses with European individuals reveal new loci associated with cholesterol and triglyceride levels. Hum Mol Genet 26(9):1770–1784
Chou W, Chen W, Shen CY (2022) A common variant in 11q23.3 associated with hyperlipidemia is mediated by the binding and regulation of GATA4. NPJ Genom Med 7(1):4
Jurado-Camacho PA et al (2022) Exome sequencing data analysis and a case-control study in Mexican population reveals lipid trait associations of new and known genetic variants in dyslipidemia-associated loci. Front Genet 13:807381
Read RW et al (2021) Genome-wide identification of rare and common variants driving triglyceride levels in a nevada population. Front Genet 12:639418
Lin E et al (2016) Association and interaction of APOA5, BUD13, CETP, LIPA and health-related behavior with metabolic syndrome in a Taiwanese population. Sci Rep 6:36830
Kong X et al (2015) Genetic variants associated with lipid profiles in chinese patients with type 2 diabetes. PLoS ONE 10(8):e0135145
Santoro N et al (2021) Genome-wide association study of lipid traits in youth with type 2 diabetes. J Endocr Soc. https://doi.org/10.1210/jendso/bvab139
Lu X et al (2016) Genetic susceptibility to lipid levels and lipid change over time and risk of incident hyperlipidemia in Chinese populations. Circ Cardiovasc Genet 9(1):37–44
Dron JS et al (2019) Severe hypertriglyceridemia is primarily polygenic. J Clin Lipidol 13(1):80–88
Trinder M, Francis GA, Brunham LR (2020) Association of monogenic vs polygenic hypercholesterolemia with risk of atherosclerotic cardiovascular disease. JAMA Cardiol 5(4):390–399
Isaacs A et al (2013) Risk scores of common genetic variants for lipid levels influence atherosclerosis and incident coronary heart disease. Arterioscler Thromb Vasc Biol 33(9):2233–2239
Zheng D et al (2019) Association between triglyceride level and glycemic control among insulin-treated patients with type 2 diabetes. J Clin Endocrinol Metab 104(4):1211–1220
Hsiung CN et al (2020) The causal relationship of circulating triglyceride and glycated hemoglobin: a mendelian randomization study. J Clin Endocrinol Metab 105(3):908–919
Chen YH et al (2015) Effects of sulfonylureas on lipids in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. J Evid Based Med 8(3):134–148
Download references
Acknowledgements
We thank the iHi CRP and iHi Genomics from the Big Data Center of China Medical University Hospital for data exploration, administrative and statistical analytic support, and providing Manhattan and Q-Q plots.
This study was supported by China Medical University and China Medical University Hospital (CMU110-MF-71, DMR-113-092, and DMR-112-071), and the National Science and Technology Council (NSTC 112-2314-B-039-041-MY2). The sponsors had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
H.-L. Chen and F.-J. Tsai contributed equally.
Authors and Affiliations
Graduate Institute of Integrated Medicine, College of Chinese Medicine, China Medical University, Taichung, 40402, Taiwan
W.-L. Liao & Y.-W. Chang
Center for Personalized Medicine, China Medical University Hospital, Taichung, 40447, Taiwan
School of Chinese Medicine, China Medical University, Taichung, 40402, Taiwan
Y.-C. Huang & F.-J. Tsai
Genetic Center, Department of Medical Research, China Medical University Hospital, Taichung, 40447, Taiwan
Y.-C. Huang, Y.-W. Chang & F.-J. Tsai
Big Data Center, China Medical University Hospital, Taichung, 40447, Taiwan
C.-F. Cheng & H.-L. Chen
Million-Person Precision Medicine Initiative, Department of Medical Research, China Medical University Hospital, Taichung, 40447, Taiwan
T.-Y. Liu & H.-F. Lu
Division of Medical Genetics, China Medical University Children’s Hospital, Taichung, 40447, Taiwan
Department of Biotechnology and Bioinformatics, Asia University, Taichung, 413305, Taiwan
You can also search for this author in PubMed Google Scholar
Contributions
WLL conception and design of the study. WLL, YCH, YWC, CFC, TYL, and HFL acquisition of data, or analysis and interpretation of data. WLL wrote the main manuscript text. WLL, CFC, HLC, and FJT revised it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Corresponding author
Correspondence to F.-J. Tsai .
Ethics declarations
Conflict of interest.
The authors declare that they have no competing interests.
Ethical approval and consent to participate
This study was approved by the ethics committee of the Institutional Review Board of CMUH (CMUH109-REC1-003; CMUH105-REC3-068; CMUH111-REC3-138).
Consent for publication
Not applicable.
Informed consent
Participants gave informed consent to participate in the study before taking part.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 478 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Liao, WL., Huang, YC., Chang, YW. et al. Impact of polygenic risk score for triglyceride trajectory and diabetic complications in subjects with type 2 diabetes based on large electronic medical record data from Taiwan: a case control study. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02397-0
Download citation
Received : 16 January 2024
Accepted : 15 May 2024
Published : 25 May 2024
DOI : https://doi.org/10.1007/s40618-024-02397-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 05 June 2024
Genetic polymorphisms of inflammatory and bone metabolism related proteins in a population with dental implants of the Basque Country. A case-control study
- Irene Lafuente-Ibáñez-de-Mendoza ORCID: orcid.org/0000-0001-7704-4122 1 , 2 ,
- Xabier Marichalar-Mendia ORCID: orcid.org/0000-0001-5948-9443 1 , 3 , 4 ,
- Amaia Setién-Olarra ORCID: orcid.org/0000-0002-7481-3990 1 , 3 ,
- Ana María García-de-la-Fuente ORCID: orcid.org/0000-0003-4043-1449 1 , 2 ,
- Rafael Martínez-Conde-Llamosas 2 &
- José Manuel Aguirre-Urizar ORCID: orcid.org/0000-0002-2652-0755 1 , 2
BMC Oral Health volume 24 , Article number: 659 ( 2024 ) Cite this article
Metrics details
Peri-implantitis (PI) is a frequent inflammatory disorder characterised by progressive loss of the supporting bone. Not all patients with recognised risk factors develop PI. The aim of this study is to evaluate the presence of single nucleotide polymorphisms (SNP) of inflammatory and bone metabolism related proteins in a population treated with dental implants from the Basque Country (Spain).
We included 80 patients with diagnosis of PI and 81 patients without PI, 91 women and 70 men, with a mean age of 60.90 years. SNPs of BMP-4, BRINP3, CD14, FGF-3, FGF-10, GBP-1, IL-1α, IL-1β, IL-10, LTF, OPG and RANKL proteins were selected. We performed a univariate and bivariate analysis using IBM SPSS® v.28 statistical software.
Presence of SNPs GBP1 rs7911 ( p = 0.041) and BRINP3 rs1935881 ( p = 0.012) was significantly more common in patients with PI. Patients with PI who smoked (> 10 cig/day) showed a higher presence of OPG rs2073617 SNP ( p = 0.034). Also, BMP-4 rs17563 ( p = 0.018) and FGF-3 rs1893047 ( p = 0.014) SNPs were more frequent in patients with PI and Type II diabetes mellitus.
Conclusions
Our findings suggest that PI could be favoured by an alteration in the osseointegration of dental implants, based on an abnormal immunological response to peri-implant infection in patients from the Basque Country (Spain).
Peer Review reports
Introduction
The first cases of peri-implantitis (PI) were described as inflammatory reactions leading to bone loss around a functioning dental implant [ 1 ]. Currently, PI is considered a peri-implant disease characterised by inflammation of the peri-implant mucosa and progressive loss of the supporting bone [ 2 ]. It is an infectious disease with a multifactorial nature, affecting up to 50% of patients with dental implants [ 2 ].
The main risk factors for developing PI are: (1) previous clinical history of periodontitis, and (2) poor plaque control and/or maintenance therapy [ 3 ]. However, other factors such as smoking and Type II diabetes mellitus may also be related [ 2 ]. Patients with periodontal background show higher probing depth (> 6 mm) and marginal bone loss (> 3 mm) numbers than those without periodontitis; as well as a higher rate of conversion from peri-implant mucositis to peri-implantitis (31%) (Roccuzzo et al., 2010; Roccuzzo et al., 2012). Furthermore, presence of active periodontitis during dental implant therapy is a 4- to 7-fold risk factor for developing PI (Máximo et al., 2008; Koldsland et al., 2010; Koldsland et al., 2011; Casado et al., 2013; Renvert et al., 2014; de Araujo Nobre et al., 2015; Daubert et al., 2015; Dalago et al., 2017). Active control of dental implants allows assessment of bacterial plaque accumulation, as well as changes in clinical data. Thus, maintenance therapy is key in the management of this disease, for both patient monitoring and primary prevention (Aguirre-Zorzano et al., 2015). Up to a 14-fold increased risk of PI has been reported in patients not attending a supervised treatment, with an incidence of PI of 44% compared to 18% in patients following active control therapy (Ferreira et al., 2006; Rokn et al., 2017).
Given that not all individuals with this background develop PI [ 2 , 4 ], a facilitating genetic susceptibility could explain the development this disorder in some individuals [ 5 ].
Genetic polymorphisms are variations at a particular point in the DNA sequence, which occur in more than 1% of the population [ 6 ]. Single nucleotide polymorphisms (SNP) are the most frequent genetic polymorphisms [ 6 ]. The detection of these alterations can be used to identify the genes and proteins involved in a specific disease; thus, their study allows population-based genetic predisposition analysis [ 7 ].
The most studied SNPs in the context of PI belong to inflammatory interleukins IL-1β, IL-1α, and IL-10 [ 8 , 9 , 10 , 11 ] and proteins related to bone metabolism BMP-4, BRINP3, CD14, FGF, LTF, OPG and RANKL [ 12 , 13 , 14 , 15 ]. Since PI is an inflammation-based disorder that causes bone loss, these mutated genes could trigger an abnormal inflammatory response and/or a reduced peri-implant osseointegration, which lead to peri-implantitis. These inflammatory and bone-related molecules may work as effective diagnostic tools or personalized treatment approaches as potential biomarkers of PI for individuals at higher genetic risk.
Sadly, so far, only the C/C genotype of IL-1β (-511) has been statistically linked to PI [ 16 ]. Although a highly suggestive avenue of study, the true implication of genetic susceptibility and the existence of specific SNPs in relation to the development of PI has not yet been demonstrated. We believe the reason behind these poor results is due to the methodology of the studies being heterogeneous, differences in diagnostic evaluation, population heterogeneity, and exclusion of some important parameters like history of periodontitis and tobacco consumption.
In this regard, there is little research on the association between PI and single nucleotide polymorphisms in the Spanish population (García-Delaney et al., 2007), which makes necessary to perform larger studies, with well-selected samples and an updated and specific methodological design. The objective of this study is, therefore, to perform a genetic study in a population from the Basque Country (Spain) treated with dental implants, with and without PI, to determine the SNP profile of inflammatory and bone metabolism related proteins.
Study design and participants
A retrospective study was carried out at the Periodontology and Osseointegration Unit of the Dental Clinic Service of the University of the Basque Country and the Centro Odontológico Médico Quirúrgico. Because of the unpredictable nature of PI and its onset, the methodology of case-control studies ensures sample size reach, as PI is sometimes has a long latency. Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test, 78 cases and 78 controls were needed to recognize as statistically significant odds ratios greater than or equal to 3.
This project matched STROBE guidelines and was approved by the University of the Basque Country/EHU Research Ethics Committee (CEISH: M10/2016/057, CEIAB/2016/180). The data extracted from this study were physically stored in an anonymised form with a code assigned to each patient in the computer of the head of research. A back-up copy in a hard disk/usb format was also made.
Patients were selected on their dental visit, upon those who underwent implant maintenance therapy. We approached 161 individuals in total: 80 patients with PI (case group, CAG) and 81 patients without PI (control group, COG). These corresponded to 91 women (56.50%) and 70 men (43.50%), whose mean age was 60.90 ± 10.22 (range: 31–86). All participants signed an informed consent form at the baseline before participating in the study. Two experienced and calibrated specialists in oral surgery and periodontology, who followed the same maintenance therapy for patients with dental implants and were blinded to the case/control of patients made the clinical and radiographic assessment (AMG, RMC) in order to enhance transparency.
Case participants were diagnosed with PI on at least one dental implant [ 3 ]: (1) Evidence of visual inflammatory changes in the peri-implant soft tissues combined with bleeding on probing and/or suppuration, (2) Increasing probing pocket depths as compared to measurements obtained at placement of the supra-structure, (3) Progressive bone loss in relation to the radiographic bone level assessment at 1 year following the delivery of the implant-supported prosthetics reconstruction, and (4) In the absence of initial radiographs and probing depths, radiographic evidence of bone level ≥ 3 mm and/or probing depths ≥ 6 mm in conjunction with profuse bleeding represents peri-implantitis. Control participants only had healthy implants without PI.
Inclusion criteria for patients were: (1) being over 18 years old, (2) having at least one functional dental implant for a minimum of 1 year, and (3) being enrolled in a supportive program therapy protocol [ 17 ]. Exclusion criteria were: (1) having received periodontal-tissue healing, antibiotics and/or bone metabolism related drugs in the last 6 months prior to genetic sampling, (2) having cement-retained implant-supported dental restorations, and (3) having been surgically treated for PI in the last 6 months prior to genetic sampling.
Clinical and radiographic analysis
We gathered the following clinical features of the participants: age (years), gender (female, male), follow-up time (months), smoking habit (more or less than 10 cigarettes per day), alcohol consumption (number of alcoholic units per week) and systemic diseases. Individuals who have quitted smoking for more than 10 years were considered former smokers. History of periodontitis (aggressive, chronic) [ 18 ] was also collected.
We registered the following dental implant data: number, location, probing depth (PD), bleeding on probing (BOP), suppuration on probing, and marginal bone loss (MBL) (through periapical intraoral radiographs with parallel technique at the time of genetic sampling). In case of more than one dental implant with PI, the implant with the highest PD and MBL was considered for the analyses.
If any clinical and/or radiographic data was missing at the baseline of the study, this information was obtained at future monthly maintenance follow-ups.
DNA isolation and genotyping analysis
Samples for the genetic analysis were taken by vigorous scraping of the buccal mucosa with a Rovers Orcellex® nylon brush (Lekstraat, The Netherlands), which was later placed in 2.5 ml of sterile miliQ water and stored at -20 °C [ 19 ].
Sample thawing was performed in a 37ºC bath with agitation. We used a standard Qiagen® kit (Redwood City, USA) for the DNA extraction, taking 1.5mL of the oral scraping sample. DNA quantification was made with a ThermoScientific NanoDrop™ spectrophotometer (Massachusetts, USA) (260/280 or 260/230 ratios). We obtained the DNA via fluorometric analysis, at concentrations 2.5–250 ng/uL.
For the genotyping analysis, specific target amplifications of the genomic regions of the SNPs were performed, then 96.96 JUNO™ chips were loaded (IFC - integrated fluidic circuits - controller), and finally allele-specific RT-PCR for each SNP and sample were made.
For the selection of the specifics SNPs, a systematic review of the literature was carried out [ 5 ] as well as an in-depth study on the presence of SNPs in the Caucasian population of the Basque County. only those related to the inflammatory response and bone metabolism made the final cut. Finally, we analysed the following polymorphisms with the Fluidigm SNP Genotyping Analysis® Software (San Francisco, USA): BMP-4 (rs17563), BRINP3 (rs1935881), CD14 (rs2569190), FGF-3 (rs1893047), FGF10 (rs900379), GBP-1 (rs7911), IL-1α (rs1800795), IL-1β (rs16944), IL-10 (rs1800896), LTF (rs1126477), OPG (rs2073617), RANKL (rs9533156). Biomark Data Collection™ software (San Francisco, USA) was used for data collection.
Two blinded biologist to the case/control status of the individuals (XMM, ASO), made all the genetic study process.
Statistical analysis
First, we performed a univariate description with frequencies and percentages (qualitative variables), as well as means, standard deviations and ranges (quantitative variables). Secondly, a bivariate analysis was implemented: Chi-square test (categorical variables), Student’s t-test (quantitative and categorical variables) or Mann Whitney U-test (quantitative with abnormal distribution and categorical variable). For multivariate analysis, a logistic regression model was used. It was considered statistically significant when p < 0.05. We used IBM SPSS® v.28 statistical software.
In addition, Hardy-Weinberg Equilibrium (HWE) analysis was stablished using the Chi-square test. Hardy-Weinberg equilibrium describes a state in which allele and genotypic frequencies in a population remain constant from one generation to the next, under certain ideal conditions, such as the absence of natural selection, migration, mutation and genetic drift. None of the polymorphisms showed an imbalance.
A blinded biostatistician, who was blind to the case/control nature of the participants (XMM) carried the statistical analysis.
Patient population
The main clinical data of the study groups are shown in Table 1 . Clinical follow-up of the patients was longer in the CAG (mean: 7.45 ± 3.19 months; range: 2–13) ( p < 0.01). The mean time between implant placement and PI diagnosis was 4.61 ± 2.5 years (range: 1–10).
Overall, 39.10% of participants (n: 63) had a history of periodontal disease, this number being higher in the CAG (42.50%) ( p = 0.503). There were more former smokers in the COG (49.40%) ( p < 0.05), and more heavy current smokers (> 10 cig/day) in the CAG (21.30%) ( p = 0.009) (Table 1 ).
Arterial hypertension was the most frequent medical pathology in both study groups (29.20%), followed by hypercholesterolemia (15.50%), depression (8.10%), hypothyroidism (7.50%), bronchial asthma (6.80%), Type II diabetes mellitus (6.20%) and cardiovascular disease (6.20%) (Table 1 ).
Clinical and radiographic outcomes
The 161 individuals included in the study had a total of 799 dental implants, out of which 60.20% were located in the mandible, 39.80% in the upper maxilla ( p = 0.676), 72.23% in the posterior sector and 27.76% in the anterior sector ( p = 0.713). Table 2 shows the clinical data of the 229 implants with PI (28.66%) and the 570 without PI.
At the time of diagnosis, the implants with PI had a mean PD of 5.13 ± 1.25 mm (range: 2–9) and a mean MBL of 5.58 ± 1.13 mm (range: 3–10). Also, 56.30% (n: 129) PI cases had BOP and 14% showed signs of suppuration (n: 32). All these differences were statistically significant ( p < 0.001) (Table 2 ).
Genetic analysis
The results of the genetic analysis are displayed in Tables 3 and 4 . All SNPs were consistent with HWE (Table 3 ). The overall comparative analysis showed that only GBP1 rs7911 ( p = 0.041) and BRINP3 rs1935881 were significantly more common in patients with PI ( p = 0.012) (Table 3 ), but none of them significantly increased the risk of developing PI (Table 5 ).
When analysing the study groups in relation to risk factors, OPG rs2073617 ( p = 0.034) was more frequent in patients with PI who smoked more than 10 cig/day, and BMP-4 rs17563 ( p = 0.018) or FGF-3 rs1893047 ( p = 0.014) in patients with PI and Type II diabetes mellitus (Table 4 ). We did not find any association between patients with history of periodontal disease and the included SNP.
Peri-implant disease is currently one of the most clinically relevant oral disorders [ 3 ]. It is usually diagnosed 2–3 years after the implant surgery and appears in up to 20% of dental implants [ 20 ]. In our study, the mean time from implant placement to clinical diagnosis of PI was 4.61 + 2.50 years, similar to other studies (Lindhe et al. 2008; Lang et al. 2011; Derks et al. 2016). As expected, the clinical follow-up time was longer in the case group, which highlights the need for lifelong follow-up of patients with dental implants.
The clinical features of our patients were similar to those of previous studies and the overall Spanish population [ 21 ]. Smoking is considered a clear inducer of MBL (Rinke et al. 2011), as it directly inhibits osteoblastic and angiogenic proliferation by nicotine, and indirectly suppresses calcium absorption and the production of PTH, OPG and vitamin D (Sgolastra et al. 2015). In our study we were able to recognise a significant association between the presence of PI and the consumption of more than 10 cigarettes/day (0 = 0.01). However, there are studies where this relationship has not been recognised (Aguirre-Zorzano et al. 2015; Dalago et al. 2017). We think that these differences could be due to the lack of homogeneity in defining tobacco consumer’sand the number of cigarettes they smoke.
In relation to the medical history of the patients, we did not observe an association between peri-implant disease and having a systemic disease. As expected, the most prevalent condition in both study groups was hypertension, given the age of our study population. Diabetes mellitus is a well-knows pathology that may be linked to the development of PI (Ferreira et al. 2006). However, most studies that analyse its involvement do not collect important data such as the patients’ glycaemic level (Taylor and Borgnakke 2008), or do not dissociate it from other cofactors, such as smoking or a history of periodontitis (Daubert et al. 2015). In our study we did not recognise any association with this disease, similar to other authors (Roos-Jansaker et al. 2006; Máximo et al. 2008; Costa et al. 2012; Marrone et al. 2013; Renvert et al. 2014; Derks et al. 2016; Rokn et al. 2017). Like Genco et al. (2013), we believe that further studies are needed to properly assess this possible link. Findings of association between PI and other systemic diseases, such as cardiovascular disease, rheumatoid arthritis, osteoporosis, hypothyroidism, depression or liver disease, are also not yet conclusive (Koldsland et al. 2011; Maximo et al. 2008; Renvert et al. 2014; Wang et al. 2022; Strooker et al. 2022).
History of periodontitis and tobacco consumption are two important risk factors for PI [ 22 ]. Patients with history of periodontal disease show higher rates of PD, MBL and an increased risk of PI when not following a maintenance therapy [ 4 , 23 ]. Furthermore, active periodontitis during dental implant treatment favours PI development [ 24 , 25 ]. The rigorous maintenance therapy followed by our patients may explain why we did not recognise any link between PI and history of periodontitis, as obtainted by other authors [ 26 , 27 ]. It is known that tobacco use inhibits osteoblastic and vascular proliferation, suppresses calcium absorption and reduces PTH, OPG and vitamin D production, therefore inducing bone loss [ 28 ]. In this regard, heavy tobacco consumption (> 10 cig/day) was strongly associated to our patients with PI, as demonstrated in other studies [ 2 , 25 ].
At the time of clinical diagnosis, the mean PD of the implants with PI of our case group 5.13 + 1.3 mm, with a mean MBL of 5.55 + 1.1 mm and higher number of implants (56.20%) presenting BOP. These data match previous studies (Karoussis et al. 2003; Ferreira et al. 2006; Máximo et al. 2008; Costa et al. 2012; Marrone et al. 2013; de Araujo Nobre et al. 2015; Dalago et al. 2017; Schwarz et al. 2018). In addition, the lack of significant difference in implant location suggests its potential non-impact on peri-implantitis development.
In recent years, there has been a growing interest in recognizing genetic factors related to the development of PI to mirror the case with periodontal disease [ 5 , 29 ]. Previously, some researchers have suggested the association of IL-1β (-511) to MBL > 0.5 mm in Chinese population with peri-implant disease [ 30 , 31 ]. Although we know MBL can be the first sign of PI, the final diagnosis must be confirmed by other clinical parameters (BOP, suppuration, increased PD) [ 2 ]. The absence on this relation matches the results obtained by other authors [ 8 , 9 , 32 ], which were also performed with similar and more up-to-date diagnostic criteria of PI.
IL-10 is another inflammatory cytokine that plays an important role in bone remodelling by reducing IL-1 and MMP synthesis, enhancing osteoblastic differentiation and inhibiting osteoclastic action [ 33 ]. Neither us nor previous studies have been able to recognise its association with PI [ 9 , 11 ].
Osteoclastic activity is modulated by CD14 and RANKL (Massey and Flanagan, 1999). CD14 protein regulates the differentiation of monocytes into osteoclasts, while RANKL induces osteoclastogenesis in mature osteoclasts (Sørensen et al. 2007). Work on SNPs to date in Serbian and German populations has not found a link between CD14 (-159) and the development of PI (Rakic et al. 2015; Petkovic-Curcin et al. 2017). Similarly, no association of RANKL (-438) has been observed in Iranian and Brazilian populations (Kadkhodazadeh et al. 2013; Ribeiro et al. 2017; Reis et al. 2020). These results coincide with those obtained in our study in the population of the Basque Country [ 11 , 12 , 15 , 34 , 35 , 36 , 37 , 38 ].
Another protein involved in bone remodelling is LTF, a transferrin glycoprotein that is present in salivary secretion and inflammatory neutrophilic granules (Naot et al. 2005). Under physiological conditions, LTF is a salivary antimicrobial and immunomodulatory defence factor against bacterial infections (including peri-implantopathogens) that also stimulates osteoblastic proliferation and differentiation to produce new bone matrix, while inhibiting osteoclastic action by stimulating the binding of OPG to RANK (Naot et al. 2005). Similar to us, only one group of Brazilian authors has studied the LTF SNP (rs1126477), without having recognised a positive association with the appearance of PI (Doetzer et al. 2015) [ 11 , 12 , 15 , 34 , 35 , 36 , 37 , 38 ].
GBP1 (interferon-induced guanylate-binding protein 1) is a GTPase expressed in T lymphocytes and endothelial cells [ 39 ]. This enzyme regulates the maturation of intracellular pathogen-infected autophagosomes and the macrophage cell response to PAMPs [ 39 ]. GBP1 is known to play an important role in cell-autonomous immunity against intracellular pathogens (Tietzel et al. 2009; Zhu et al. 2013) and it is also implicated in chronic active Epstein–Barr virus infection and inflammatory response suppression [ 40 ]. There are still no studies that have analysed the SNP of GBP1 in relation to PI, except our own. Nonetheless, we believe that, given that many important peri-implantopathogens involved in PI are intracellular, such as Porphyromonas gingivalis, Tanarella forsythia, Actinomyces. actinomycetemcomitans, Treponema denticola , etc., a mutation of GBP1 could lead to an ineffective inflammatory response against peri-implant pathogens involved in the IP process.
Bone morphogenetic proteins (BMP) like BRINP3 (BMP/Retinoic Acid Inducible Neural Specific 3), are a set of 15 osteoinductive proteins from the transforming growth factor-beta (TGF-β) superfamily located in osteoprogenitor cells [ 41 ]. As in our analysis ( p = 0.012), similar findings have also been found in Brazilian population [ 42 ], which demonstrate that BRINP3 mutation rs1935881 is significantly more common in patients with PI. Since the role of these proteins is to regulate osteoblastic differentiation of pluripotent cells, that is, bone regeneration and remodelling, individuals with this SNP could have a disrupted osseointegration response after dental implant placement, promoting the development of PI.
BRINP3 protein is a bone morphogenetic protein belonging to the TGFβ family, a superfamily of proteins with bone neoformation-inducing and connective capacity (Kawano et al. 2004). BMPs are a set of 15 osteoinductive proteins of osteoprogenitor cells, which regulate osteoblast differentiation during bone remodelling and promote bone regeneration (Anderson et al. 2000). They also stimulate the differentiation of pluripotent cells into different cell lines: adipose tissue, cartilage and bone. Only one group of authors (Casado et al. 2015) has recognised the existence of an association between the presence of BRINP3 rs1935881 and the development of IP in the Brazilian population. In view of our concordant results, we consider that this polymorphism could condition an alteration in the peri-implant osseointegration process that favours the appearance of IP.
Osteoprotegerin (OPG) is an osteoblastic molecule that works as RANK receptor antagonist, inhibiting bone resorption [ 43 ]. Different authors [ 14 , 44 , 45 ] have shown a relationship between OPG SNP (-1181) and PI in Chinese, Brazilian and Iranian populations. In our study, this SNP was also more statistically frequent in patients with PI who smoked more than 10 cig/day. It is known that smoker individuals have a lower production and serum levels of OPG than non-smokers probably due to the action of nicotine at the osteoblastic level [ 46 , 47 ]. This, together with the presence of OPG mutation, could explain the biological plausibility between these two processes and why heavy smokers have a higher risk for PI and higher MBL.
A significant association of BMP-4 rs17563 and FGF-3 rs1893047 to patients with PI and Type II diabetes mellitus was also identified in our analysis. These results are similar to those previously obtained by Coelho et al. [ 13 ] in a Brazilian population. BMP/FGF protein axis has been reported as an important element in the osseointegration of dental implants, actively involved in bone regeneration and angiogenesis [ 48 , 49 ]. It is known that genetic mutations of BMP-4 may be associated with decreased bone density in postmenopausal women and reduced adipocyte glucose uptake, thus inducing insulin resistance [ 50 , 51 ]. Specifically, BMP4 reduces glucose uptake by adipocytes and has an antagonistic effect on insulin signalling, inducing resistance (Ahrens et al. 1993; Bowers & Lane, 2007; Chattopadhyay et al. 2017). These latter functions would explain the recognised association between the presence of the BMP4 rs17563 SNP in diabetic patients with PI (Perera et al. 2019), as we have been able to recognise in our study.
On the other hand, FGF-3 protein regulates the growth of mature pancreatic islets; so, its modification could potentially lead to alterations in insulin secretion [ 52 ]. A poor function of both proteins due to these SNPs might explain the increased risk of PI in patients with Type II diabetes mellitus. Only Coelho et al. (2016) have demonstrated the existence of a significant association between BMP4 and FGF3 SNPs and the development of PI amongst Brazilian patients, but their status in relation to the development of diabetes remains unknown to us. Furthermore, we should point to the small number of patients with diabetes mellitus included in our study, so further studies are needed to reinforce our results.
Considering all this, it is important to note that after performing regression analysis, and similar to all the studies to date on the relationship between SNPs and peri-implant disease with updated diagnostic criteria, none of the SNPs initially associated with the presence of PI (GBP1, BRINP3, OPG, BMP4, FGF3) increased the risk of PI. This forces us to interpret our findings with caution and point to a non-genetic susceptibility nature of PI.
To conclude, the limitations inherent in our study should be considered when interpreting the findings. Among these, the main one is that causality cannot be explained with this type of sample, because of its case-control nature. It is noteworthy that, although we did address many potential confounders, certain residual confounding variables were not taken into account, such dietary habits, use of hormone replacement therapy and levels of inflammatory markers. The omission of these factors may have contributed to result variability, and their exclusion could have affected the generalizability of our results. Despite these limitations, we believe this study provides a valuable contribution to the field.
Our aim in the future is to search for associations between the included SNPs (and/or more) and PI and its risk factors. We are currently working to acknowledge the link between inflammatory and bone metabolism SNPs and patients with PI who have history of periodontal disease, diabetes mellitus and poor plaque control and/or lack of maintenance therapy.
In summary, the overall genetic features of Basque patients match those found by previous authors. This study shows that individuals with dental implant therapy and peri-implantitis from the Basque Country (Spain) under maintenance therapy program do not have a specific genotype of proinflammatory proteins. Nevertheless, our findings light up the current genetic understanding of peri-implantitis in the sense that, patients with PI could have a particular genotype of GBP1 and BRINP3 proteins, which could favour a modified osseointegration, due to an abnormal immunological response to periodontopathogens. The mutations of OPG, BMP-4 and FGF-3 in patients with PI who are heavy smokers or diabetics could explain why these two conditions are risk factors for peri-implantitis.
Although the current results do not stablish preventive strategies or personalized treatments for patients with PI, individuals with a higher risk for this disease could be genetically assessed. Further genetic susceptibility studies are needed in different populations to assess the true role of the SNPs involved in the pathogenesis of this frequent oral disease, in order to stablish preventive programmes and accurate therapies.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
Bone morphogenetic protein
Bleeding on probing
BMP/Retinoic Acid Inducible Neural Specific 3
Cluster of differentiation 14
Deoxyribonucleic acid
Fibroblastic growth factor
Interferon-induced guanylate-binding protein
Interleukin
Lactoferrin
Marginal bone loss
Matrix metalloproteinase
Osteoprotegerin
Parathyroid hormone
Receptor Activator for Nuclear Factor κ B Ligand
Real time polymerase chain reaction
Single nucleotide polymorphism
Supportive program therapy
Tumor growth factor
Albrektsson T, Isidor F. Consensus report of session IV. In: Lang NP, Karring T, editors. Proceedings of the 1st European Workshop on Periodontology. Chicago: Quintessence; 1994. pp. 365–369.
Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018;45(20):S246–66.
PubMed Google Scholar
Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, Peri‐implant mucositis, and peri‐implantitis: case definitions and diagnostic considerations. J Clin Periodontol. 2018;45(20):S278–85.
Ferreira SD, Silva GL, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006;33(12):929–35.
Article CAS PubMed Google Scholar
de Lafuente-Ibáñez I, Setien-Olarra A, Aguirre-Urizar JM, Marichalar-Mendia X. Role of proinflammatory mutations in peri-implantitis: systematic review and meta-analysis. Int J Implant Dent. 2022;8(1):1–9.
Google Scholar
Thorisson GA, Smith AV, Krishnan L, Stein LD. The international HapMap project web site. Genome Res. 2005;15(11):1592–3.
Article CAS PubMed PubMed Central Google Scholar
Landegren U, Nilsson M, Kwok PY. Reading bitsof genetic information: methods for single-nucleotide polymor-phism analysis. Genome Res. 1998;8(8):769–76.
Laine ML, Leonhardt Å, Roos-Jansåker AM, Peña AS, Van Winkelhof AJ, Winkel EG, Renvert S. IL-1RN gene polymorphism is associated with periimplantitis. Clin Oral Implants Res. 2006;17(4):380–5.
Article PubMed Google Scholar
Melo RF, Lopes BM, Shibli JA, Marcantonio Junior E, Marcantonio RAC, Galli GMT. Interleukin-1β and interleukin-6 expression and gene polymorphisms in subjects with peri-implant disease. Clin Implants Dent Relat Res. 2012;14(6):905–14.
Article Google Scholar
García-Delaney C, Sánchez-Garcés MA, Figueiredo R, Sánchez-Torres A, Gay-Escoda C. Clinical significance of interleukin-1 genotype in smoking patients as a predictor of peri-implantitis: a case-control study. Med Oral Patol Oral Cir Bucal. 2015;20(6):e737–43.
Article PubMed PubMed Central Google Scholar
Petkovic-Curcin A, Zeljic K, Cikota-Aleksic B, Dakovic D, Tatic Z, Magic Z. Association of cytokine gene polymorphism with peri-implantitis risk. Int J Oral Maxillofac Implant. 2017;32(5):e241–8.
Rakic M, Petkovic-Curcin A, Struillou X, Matic S, Stamatovic N, Vojvodic D. CD14 and TNFα single nucleotide polymorphisms are candidates for genetic biomarkers of peri-implantitis. Clin Oral Inv. 2015;19(4):791–801.
Coelho RB, Gonçalves Junior R, Villas-Boas RDM, Bonato LL, Quinelato V, Pinheiro ADR, et al. Haplotypes in BMP4 and FGF genes increase the risk of peri-implantitis. Braz Dent J. 2016;27(4):367–74.
Zhou J, Zhao Y. Osteoprotegerin gene (OPG) Polymorphisms associated with peri-implantitis susceptibility in a Chinese Han Population. Med Sci Monit. 2016;22:4271–6.
Ribeiro R, Melo R, Tortamano Neto P, Vajgel A, Souza PR, et al. Polymorphisms of Il-10 (-1082) and RANKL (-438) genes and the failure of dental implants. Int J Dent. 2017;28:3901368. https://doi.org/10.1155/2017/3901368
Article CAS Google Scholar
Del Valle AE, López-Vicente J, Martínez-Conde R, Aguirre-Zorzano LA. Current understanding of genetic polymorphisms as biomarkers for risk of biological complications in implantology. J Clin Exp Dent. 2018;10(10):e1029–39.
Lang NP, Wilson TG, Corbet EF. Biological complications with dental implants: their prevention, diagnosis and treatment. Clin Oral Implant Res. 2000;11(1):146–55.
Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6.
Marichalar-Mendia X, Acha-Sagredo A, Rodriguez-Tojo MJ, Rey-Barja N, Hernando-Rodriguez M, Aguirregaviria, et al. Alcohol-dehydrogenase (ADH1B) Arg48His polymorphism in Basque Country patients with oral and laryngeal cancer: preliminary study. Anticancer Res. 2011;31(2):677–80.
CAS PubMed Google Scholar
Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implant Res. 2012;23(6):67–76.
Rodrigo D, Sanz-Sánchez I, Figuero E, Llodrá JC, Bravo M, Caffesse RG, et al. Prevalence and risk indicators of peri‐implant diseases in Spain. J Clin Periodontol. 2018;45(12):1510–20.
Polymeri A, Loos BG, Aronovich S, Steigmann L, Inglehart MR. Risk factors, diagnosis, and treatment of peri-implantitis: a cross‐cultural comparison of US and European periodontists’ considerations. J Periodontol. 2022;93(4):481–92.
Roccuzzo M, Bonino F, Aglietta M, Dalmasso P. Ten-year results of a three arms prospective cohort study on implants in periodontally compromised patients. Part 2: clinical results. Clin Oral Implants Res. 2012;23(4):389–95.
Koldsland OC, Scheie AA, Aass AM. The association between selected risk indicators and severity of peri-implantitis using mixed model analyses. J Clin Periodontol. 2011;38(3):285–92.
de Araujo Nobre M, Mano Azul A, Rocha E, Malo P. Risk factors of peri-implant pathology. Eur J Oral Sci. 2015;123(3):131–9.
Marrone A, Lasserre J, Bercy P, Brecx MC. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin Oral Implant Res. 2013;24(8):934–40.
Canullo L, Tallarico M, Radovanovic S, Delibasic B, Covani U, Rakic M. Distinguishing predictive profiles for patient-based risk assessment and diagnostics of plaque induced, surgically and prosthetically triggered peri‐implantitis. Clin Oral Implants Res. 2016;27(10):1243–50.
Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Smoking and the risk of peri-implantitis. A systematic review and meta‐analysis. Clin Oral Implant Res. 2015;26(4):e62–7.
Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontol 2000. 2007;43(1):102–32.
Lin YH, Huang P, Lu X, Guan DH, Man Y, Wei N, Gong P. The relationship between IL-1 gene polymorphism and marginal bone loss around dental implants. J Oral Maxillofac Surg. 2007;65(11):2340–4.
Shimpuku H, Nosaka Y, Kawamura T, Tachi Y, Shinohara M, Ohura K. Genetic polymorphisms of the interleukin-1 gene and early marginal bone loss around endosseous dental implants. Clin Oral Implants Res. 2003;14(4):423–9.
Cardoso JM, Ribeiro AC, Palos C, Proença L, Noronha S, Alves RC. Association between IL-1A and IL-1B gene polymorphisms with Peri-implantitis in a Portuguese population—a pilot study. PeerJ. 2022;10:e13729. https://doi.org/10.7717/peerj.13729
Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S, et al. Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. BioMed Res Int. 2014;2014:16. https://doi.org/10.1155/2014/284836
Reis MBL, Arid J, Flores EKB, Cruz GV, Marañón-Vásquez GA, Souza LKFD, et al. Association between genetic polymorphisms in RANK, RANKL and OPG and Peri-implant diseases in patients from the Amazon Region. Braz Dent J. 2020;31(1):63–8.
Naot D, Grey A, Reid IR, Cornish J. Lactoferrin–a novel bone growth factor. Clin Med Res. 2005;3(2):93–101.
Sørensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, Dziegiel MH, et al. Characterization of osteoclasts derived from CD14 + monocytes isolated from peripheral blood. J Bone Min Met. 2007;25(1):36–45.
Kadkhodazadeh M, Ebadian AR, Gholami GA, Khosravi A, Tabari ZA. Analysis of RANKL gene polymorphism (rs9533156 and rs2277438) in Iranian patients with chronic periodontitis and periim-plantitis. Arch Oral Biol. 2013;58(5):530–6.
Doetzer AD, Schlipf N, Alvim-Pereira F, Alvim‐Pereira CC, Werneck R, Riess O, et al. Lactotransferrin gene (LTF) polymorphisms and dental implant loss: a case‐control association study. Clin Implant Dent Rel Res. 2015;17:e550–61.
Honkala AT, Tailor D, Malhotra SV. Guanylate-binding protein 1: an emerging target in inflammation and cancer. Front Immunol. 2020;10:3139.
Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332(6030):717–21.
Kawano H, Nakatani T, Mori T, Ueno S, Fukaya M et al. (2004). Identification and characterization of novel developmentally regulated neural-specific proteins, BRINP family. Mol Brain Res. 2004;125(1–2):60–75.
Casado PL, Aguiar DP, Costa LC, Fonseca MA, Vieira TC, Alvim-Pereira CC, et al. Different contribution of BRINP3 gene in chronic periodontitis and peri-implantitis: a cross-sectional study. BMC Oral Health. 2013;5:1–10.
Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Cur Rev Musculoskel Med. 2009;2(1):56–64.
Kadkhodazadeh M, Alizadeh Tabari Z, Ardakani MRT, Ebadian AR, Brook A. Analysis of osteoprotegerin (OPG) gene polymorphism in Iranian patients with chronic periodontitis and peri-implantitis. A cross-sectional study. Eur J Oral Implant. 2012;5(4):381–8.
Xu M, Zhang C, Han Y, Yue Z, Shu C, Hou J. Association between osteoprotegerin rs2073618 polymorphism and peri-implantitis susceptibility: a meta-analysis. BMC Oral Health. 2022;22(1):598. https://doi.org/10.1186/s12903-022-02657-6
Lappin DF, Sherrabeh S, Jenkins WM, Macpherson LM. Effect of smoking on serum RANKL and OPG in sex, age and clinically matched supportive-therapy periodontitis patients. J Clin Periodontol. 2007;34(4):271–7.
Liu D, Xu JK, Figliomeni L, Pavlos NJ, Rogers M, Tan A, et al. Expression of RANKL and OPG mRNA in periodontal disease: possible involvement in bone destruction. Int J Mol Med. 2002;11(1):17–22.
CAS Google Scholar
Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene. 2003;316:23–32.
Guimarães JM, Guimarães IC, Duarte ME, Vieira ME, Vieira T, Vianna VF, et al. Polymorphisms in BMP4 and FGFR1 gene are associated with fracture non-union. J Orthop Res. 2013;31(12):1971–9.
Ramesh BL, Wilson SG, Dick IM, Islam FM, Devine A, Prince RL. Bone mass effects of a BMP4 gene polymorphism in postmenopausal women. Bone. 2005;36(3):555–61.
Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007;6(4):385–9.
Arnaud-Dabernat S, Kritzik M, Kayali AG, Zhang YQ, Liu G, Ungles C, et al. FGFR3 is a negative regulator of the expansion of pancreatic epithelial cells. Diabetes. 2007;56(1):96–106.
Download references
Acknowledgements
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and affiliations.
Research Group: GIU21/042, University of the Basque Country (UPV/EHU), Leioa, Spain
Irene Lafuente-Ibáñez-de-Mendoza, Xabier Marichalar-Mendia, Amaia Setién-Olarra, Ana María García-de-la-Fuente & José Manuel Aguirre-Urizar
Department of Stomatology, University of the Basque Country (UPV/EHU), Barrio Sarriena s/n, Leioa, 48940, Spain
Irene Lafuente-Ibáñez-de-Mendoza, Ana María García-de-la-Fuente, Rafael Martínez-Conde-Llamosas & José Manuel Aguirre-Urizar
Department of Nursery I, Barrio Sarriena s/n, Leioa, 48940, Bizkaia, Spain
Xabier Marichalar-Mendia & Amaia Setién-Olarra
Biobizkaia Health Research Institute, Barakaldo, Spain
Xabier Marichalar-Mendia
You can also search for this author in PubMed Google Scholar
Contributions
JMAU and AMG conceived the ideas; ILIM, RFM collected the data; XMM and ASO analyzed the data; and JMAU and ILIM led the writing.
Corresponding author
Correspondence to Xabier Marichalar-Mendia .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the UPV/EHU Research Ethics Committee (CEISH: M10/2016/057, CEIAB/2016/180). All participants signed an informed consent form before participating in the study.
Consent for publication
All participants gave their consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lafuente-Ibáñez-de-Mendoza, I., Marichalar-Mendia, X., Setién-Olarra, A. et al. Genetic polymorphisms of inflammatory and bone metabolism related proteins in a population with dental implants of the Basque Country. A case-control study. BMC Oral Health 24 , 659 (2024). https://doi.org/10.1186/s12903-024-04319-1
Download citation
Received : 03 January 2024
Accepted : 02 May 2024
Published : 05 June 2024
DOI : https://doi.org/10.1186/s12903-024-04319-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Peri-implantitis
- Single nucleotide polymorphisms
- Case-control studies
- Inflammation
- Bone metabolism
BMC Oral Health
ISSN: 1472-6831
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Claudia Shwide-Slavin; Case Study: A Patient With Type 1 Diabetes Who Transitions to Insulin Pump Therapy by Working With an Advanced Practice Dietitian. Diabetes Spectr 1 January 2003; 16 (1): ... B.C. is a 51-year-old white man who was diagnosed with type 1 diabetes 21 years ago. He believes that his diabetes has been fairly well controlled ...
PRESENTATION OF CASE. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit.
All data generated or analyzed during this study are included in this published article. ... (DKA), hence, pediatricians should be aware of its diagnosis and management. Keywords: case report, diabetes mellitus, hyperglycemic hyperosmolar syndrome (HHS ... First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic ...
A 59-year-old woman with type 1 diabetes and a 2-year history of cognitive decline presented with obtundation. There was diffuse, symmetric hypointensity in the brain on T2-weighted images and abno...
To the Editor: Most patients with new-onset type 1 diabetes have substantial intact beta-cell reserve. 1 Thus, we analyzed the efficacy of semaglutide, an agonist of glucagon-like peptide 1 (GLP-1 ...
Tight glycemic control in type 1 diabetes mellitus patients is associated with the risk of hypoglycemia. Diabetic patients are forced to change their lifestyle to adjust to the disease condition and survive it. ... The outcomes of this case study were that in case of T1DM the physician should not be very aggressive except during the first 2 ...
The study is continuing and will take five years, involving 17 people with severe cases of Type 1 diabetes. It is not intended as a treatment for the more common Type 2 diabetes.
Describe the physiologic consequences of diabetes mellitus. Outline the importance of maintaining glycemic control in reducing the risk of diabetic complications. Detail specific clinical applications of insulin therapy to achieve both basal and meal-related glycemic control. Manage a patient's glycemic status by continuously refining the ...
The burden of type 1 diabetes in 2021 is vast and is expected to increase rapidly, especially in resource-limited countries. Most incident and prevalent cases are adults. The substantial missing prevalence highlights the premature mortality of type 1 diabetes and an opportunity to save and extend lives of people with type 1 diabetes. Our new model, which will be made publicly available as the ...
Type 1 diabetes (T1D) is a common autoimmune disease that is characterized by insufficient insulin production. ... This case-control study attempted to estimate the exposure linked to T1D to ...
This case study represents the challenges and issues, both physical and psychological, faced by a young person with type 1 diabetes and the support given by her diabetes multidisciplinary team (MDT). Implications for practice are addressed using current evidence-based research. The names of the child and family have been anonymised to protect ...
Case Study: Hyperglycemia, concern for diabetic ketoacidosis, and type 1 diabetes History of present illness The patient is a 36-year-old man who has had type 1 diabetes for 15 years.
Rationale: Fulminant type 1 diabetes mellitus (FT1DM) is a new subtype of type 1 diabetes mellitus that was first proposed by the Japanese scholar Imagawa in 2000. In the 2 patient cases described in this study, gastrointestinal symptoms were the first symptoms reported, and the initial blood glucose levels were very high.
The most recent National Health and Nutrition Examination Survey noted that 30% of adolescents are now overweight, 2 and there has been a commensurate rise in the number of cases of type 2 diabetes found in adolescents. 3 Recently, in a cohort of obese adolescents, 20% were noted to have impaired glucose tolerance, and 4% had undiagnosed type 2 ...
Type 1 diabetes accounts for approximately 6% of all cases of diabetes in adults (≥18 years of age) in the United States, 4 and 80% of these cases are diagnosed before the patient is 20 years of ...
Aims: The aim of the study was to identify environmental risk factors for insulin-dependent diabetes mellitus (Type 1 DM) in childhood. Methods: A matched case-control study of Type 1 DM conducted in Lancashire and Cumbria, UK, using a structured interview. Cases (n=196, participation rate 83%) were children under 16 years of age diagnosed prior to October 1998 and attending diabetic clinics.
The diagnoses of diabetes, in this case, was made by the endocrinologist based upon elevated levels of glucose in the blood and urine. ... Animal research and small studies in people have indicated that type 1 diabetes can be delayed in those at high risk for the disease by regular, small doses of insulin. This is currently under study. 7 ...
During the COVID pandemic, a surge in pediatric Type 1 Diabetes Mellitus (T1DM) cases appears to be occurring, potentially due to the presence of autoantibody-induced immune dysregulation triggered by COVID-19. We describe one such case in a previously healthy 7-year-old with asymptomatic COVID-19 presenting with a high nasopharyngeal SARS CoV-2 virus load, detectable COVID-19 IgG antibodies ...
Type 1 diabetes (T1D) is a multifactor disease caused by β-cell destruction (which is mostly immune-mediated) and absolute insulin deficiency. At present, the management of T1D has been improved, but the disease remains incurable. ... The case-control study began in January 2016 46 and ended in September 2018 (case-control phase of ...
Geralyn Spollett; Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse. Diabetes Spectr 1 January 2003; 16 (1): ... It is a crucial component in the care of people with type 1 diabetes, and it becomes increasingly important in the care of patients with ...
Type 1 Diabetes (T1D) is a chronic health condition (CHC) with a prevalence of 3.9 per 10 000 and incidence of 20 per 100,000 among adults in the United States (Mobasseri et al., 2020). ... The authors appreciate the thirty-one college students living with Type 1 Diabetes for participating in this study. Additionally, the authors would like to ...
The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes ... If so does the Use additional code note apply to the case study No Is there an. document. 181106 Asian 30 Lecture.docx. notes. Chapter 5 Journal Entry 17.docx. Define1.docx111.docx ...
The presence of metabolic syndrome is significantly more likely to be present in those with type 2 diabetes than in patients diagnosed with LADA or type 1 diabetes. 7 This patient in this case was ...
The patient was diagnosed with type 1 diabetes mellitus supported by a low C peptide level of 43 (370-1470 pmol/L) and an antiglutamic acid decarboxylase (GAD) antibody titre of 4.7 (ref. 0-1.0). ... In the case report presented here, the patient was newly diagnosed with diabetes with a short duration of symptoms of the disease. ...
If you have type 1 diabetes, your doctor may recommend you take other medicines, in addition to insulin, to help control your blood glucose. ... Always talk with your health care provider before you participate in a clinical study. References [1] Types of insulin. Centers for Disease Control and Prevention. Updated March 25, 2021. Accessed ...
Receiving a type 1 diabetes diagnosis can be life-altering, bringing with it many visible and invisible costs. Sanofi commissioned ground-breaking research called "The Cost of Not Knowing" to shed light on the profound impact of receiving a type 1 diabetes (T1D) diagnosis without prior warning.The findings, which were published on June 5, 2024, highlight the social, emotional, and ...
Watch #yasetenam ((((LIVE))))) on Asempa 94.7 FM with Nana Yaw Sarfoh.....join us now #AsempaFM #yasetenam
A ten-year (1989-1998) perspective study of the incidence of type 1 diabetes in the district of Catania (Sicily) in a 0-14 year age group. J Endocrinol Invest. 2002 ... Charkaluk ML, Czernichow P, Lévy-Marchal C. Incidence data of childhood-onset type I diabetes in France during 1988-1997: the case for a shift toward younger age at onset. ...
Globally, an estimated 462 million individuals (6.3% of the world's population) are affected by type 2 diabetes (T2D). Statistical forecasting based on data from 1990-2017 showed that global diabetes prevalence may increase to 7100 cases per 100,000 individuals by 2030 and to 7900 per 100,000 by 2040 [].More than 50% of patients with T2D have dyslipidemia, and the prevalence of diabetic ...
When analysing the study groups in relation to risk factors, OPG rs2073617 (p = 0.034) was more frequent in patients with PI who smoked more than 10 cig/day, and BMP-4 rs17563 (p = 0.018) or FGF-3 rs1893047 (p = 0.014) in patients with PI and Type II diabetes mellitus (Table 4). We did not find any association between patients with history of ...