
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

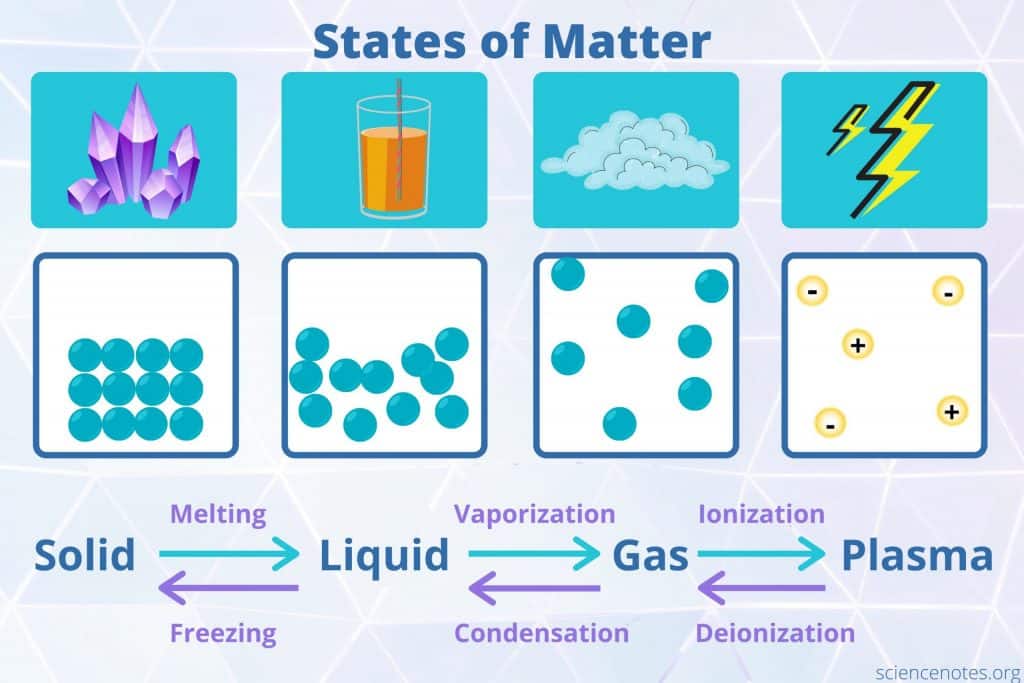
States of Matter

States of matter are forms in which matter exists. The four states of matter observed in everyday life are solids , liquids , gases , and plasma . Other states of matter also exist, although they require special conditions. Here is a look at the states of matter, their properties, and the names of phase transitions between them.
What Is a State of Matter?
Matter is anything that has mass and takes up space. It consists of subatomic particles, atoms, ions , and compounds . Sometimes these particles are tightly bound and close together, while other times particles are loosely connected and widely separated. States of matter describe the qualities displayed by matter. Basically, the state of matter of a substance depends on how much energy its particles have.
We can change the energy of matter by altering its temperature or pressure, causing matter to transition from one state to another. But, when matter changes state, its chemical identity remains the same. So, if you take ice, melt it, and then boil it, its state of matter changes, but it’s always water.
List of the States of Matter
The four fundamental states of matter are solids, liquids, gases, and plasma. But, scientists are discovering new states of matter that exist under extreme conditions.
A solid is a state of matter with a defined shape and volume . Atoms, ions, and molecules in a solid pack tightly together and may form crystals. Examples of solids include rocks, ice, diamond, and wood.
A liquid is a state of matter with a defined volume, but no defined shape. In other words, liquids take the shape of their container. Particles in a liquid have more energy than in a solid, so they are further apart and less organized (more random). Examples of liquids include water, juice, and vegetable oil.
A gas is a state of matter lacking either a defined volume or defined shape. Like a liquid, a gas takes the shape of a container. Unlike a liquid, a gas easily expands or contracts to fill the entire volume of the container. Particles in a gas have more energy than in solids or liquids. They tend to be further apart and move more randomly than in a liquid. Examples of gases include air, water vapor, and helium.
Plasma is a state of matter similar to a gas, except all of the particles carry an electrical charge. Also, plasma tends to exist at very low pressure, so the particles are even further apart than in a gas. Plasma can consist of ions, electrons, or protons. Examples of plasma include lightning, the aurora, the Sun, and the inside of a neon sign.
Bose-Einstein Condensate
Bose-Einstein condensate (BEC) is sometimes called the fifth state of matter. In Bose-Einstein condensate, atoms and ions stop behaving as separate particles and collapse into a single quantum state that can be described using a single wavefunction. This state of matter was verified experimentally in 1995 by Eric Cornell and Carl Wieman. Bose-Einstein condensate is “colder” than an ordinary solid and may form very near absolute zero .
A superfluid is a second liquid state formed by some types of matter. A superfluid displays zero viscosity . In other words, it has no resistance to flow. Superfluidity was observed for helium in 1937. Because it could flow without friction, superfluid helium climbed the walls of its container and dripped over the sides. Like Bose-Einstein condensate, superfluidity occurs near absolute zero.
Fermionic Condensate
A fermionic condensate is a state of matter similar to a Bose-Einstein condensate, except it consists of fermions, such as quarks and leptons. Normally, the Pauli exclusion principle forbids fermions from entering the same quantum state. In a fermionic condensate, a pair of fermions behaves as a boson, allowing multiple pairs to enter the same quantum state.
Rydberg Matter
Rydberg matter is a type of plasma formed when excited ions condense. You can think of it as dusty plasma. So far, it occurs in the elements hydrogen, potassium, nitrogen, and cesium. This type of matter consists mainly of small hexagonal planar clusters. Scientists make Rydberg matter in a lab or observe it in the upper atmosphere of planets and in clouds in space.
Photonic Matter
Photonic matter is the state of matter formed when photons interact with a gas in such a way that the photons have apparent mass and can interact with each other. Photons with apparent mass can even form photonic “molecules.”
Color-Glass Condensate
Color-glass condensate is a state of matter proposed to exist when atomic nuclei travel near the speed of liquid. Because of their speed, the nucleus appears compressed along its direction of motion. This causes the gluons of the nucleus to appear as a sort of wall or region of increased density.
Other States of Matter
There are other proposed states of matter, including quark matter, degenerate matter, dropleton, quantum Hall state, superglass, supersolid, and string-net liquid.
Phase Transitions Between States of Matter

Changes in temperature and pressure causes matter to change from one state to another. This change is called a phase transition or phase change . Examples of phase transitions including the melting of ice (a solid) into water (a liquid) and the boiling of water into water vapor (a gas). Here are the names of the phase transitions between solids, liquids, gases, and plasma:
- Melting : Phase transition from solid to liquid.
- Freezing : Phase transition from liquid to solid.
- Vaporization : Phase transition from liquid to gas.
- Condensation : Phase transition from gas to liquid.
- Sublimation : Phase transition from solid to gas.
- Deposition : Phase transition from gas to solid.
- Ionization : Phase transition from gas to plasma.
- De-ionization or recombination : Phase transition from plasma to gas.
- Goodstein, D.L. (1985). States of Matter . Dover Phoenix. ISBN 978-0-486-49506-4.
- Murthy, G.; et al. (1997). “Superfluids and Supersolids on Frustrated Two-Dimensional Lattices”. Physical Review B . 55 (5): 3104. doi: 10.1103/PhysRevB.55.3104
- Sutton, A.P. (1993). Electronic Structure of Materials . Oxford Science Publications. ISBN 978-0-19-851754-2.
- Wahab, M.A. (2005). Solid State Physics: Structure and Properties of Materials . Alpha Science. ISBN 978-1-84265-218-3.
- White, F. (2003). Fluid Mechanics . McGraw-Hill. ISBN 978-0-07-240217-9.
Related Posts
States of Matter: Kinetic molecular theory and phase transitions
by Anthony Carpi, Ph.D.
Listen to this reading
Did you know that solids, liquids, and gases are not the only states of matter? Among others are plasmas, which have such high energy that molecules are ripped apart. And Bose-Einstein Condensates, seen for the first time in 1995, are a weird state of matter that can actually trap light.
As a young child, I remember staring in wonder at a pot of boiling water. Searching for an explanation for the bubbles that formed, I believed for a time that the motion of the hot water drew air down into the pot, which then bubbled back to the surface . Little did I know that what was happening was even more magical than I imagined – the bubbles were not air, but actually water in the form of a gas .

The different states of matter have long confused people. The ancient Greeks were the first to identify three classes (what we now call states) of matter based on their observations of water. But these same Greeks, in particular the philosopher Thales (624 – 545 BCE), incorrectly suggested that since water could exist as a solid , liquid , or even a gas under natural conditions, it must be the single principal element in the universe from which all other substances are made. We now know that water is not the fundamental substance of the universe; in fact, it is not even an element.
- Kinetic Molecular Theory
To understand the different states in which matter can exist, we need to understand something called the Kinetic Molecular Theory of Matter . Kinetic Molecular Theory has many parts, but we will introduce just a few here. One of the basic concepts of the theory states that atoms and molecules possess an energy of motion that we perceive as temperature. In other words, atoms and molecules are constantly moving, and we measure the energy of these movements as the temperature of the substance. The more energy a substance has, the more molecular movement there will be, and the higher the perceived temperature will be. An important point that follows this is that the amount of energy that atoms and molecules have (and thus the amount of movement) influences their interaction with each other. Unlike simple billiard balls, many atoms and molecules are attracted to each other as a result of various intermolecular forces such as hydrogen bonds , van der Waals forces , and others. Atoms and molecules that have relatively small amounts of energy (and movement) will interact strongly with each other, while those that have relatively high energy will interact only slightly, if even at all, with others.
Comprehension Checkpoint
- Energy and states of matter
How does this produce different states of matter? Atoms that have low energy interact strongly and tend to "lock" in place with respect to other atoms. Thus, collectively, these atoms form a hard substance, what we call a solid . Atoms that possess high energy will move past each other freely, flying about a room, and forming what we call a gas . As it turns out, there are several known states of matter ; a few of them are detailed below.

Solids are formed when the attractive forces between individual molecules are greater than the energy causing them to move apart. Individual molecules are locked in position near each other, and cannot move past one another. The atoms or molecules of solids remain in motion. However, that motion is limited to vibrational energy; individual molecules stay fixed in place and vibrate next to each other. As the temperature of a solid is increased, the amount of vibration increases, but the solid retains its shape and volume because the molecules are locked in place relative to each other. To view an example of this, click on the animation below which shows the molecular structure of ice crystals .

Liquids are formed when the energy (usually in the form of heat) of a system is increased and the rigid structure of the solid state is broken down. In liquids , molecules can move past one another and bump into other molecules; however, they remain relatively close to each other like solids. Often in liquids , intermolecular forces (such as the hydrogen bonds shown in the animation below) pull molecules together and are quickly broken. As the temperature of a liquid is increased, the amount of movement of individual molecules increases. As a result, liquids can "flow" to take the shape of their container but they cannot be easily compressed because the molecules are already close together. Thus, liquids have an undefined shape, but a defined volume . In the example animation below, we see that liquid water is made up of molecules that can freely move past one another, yet remain relatively close in distance to each other.

Gases are formed when the energy in the system exceeds all of the attractive forces between molecules . Thus gas molecules have little interaction with each other beyond occasionally bumping into one another. In the gas state, molecules move quickly and are free to move in any direction, spreading out long distances. As the temperature of a gas increases, the amount of movement of individual molecules increases. Gases expand to fill their containers and have low density . Because individual molecules are widely separated and can move around easily in the gas state, gases can be compressed easily and they have an undefined shape.
Solids, liquids , and gases are the most common states of matter that exist on our planet. If you would like to compare the three states to one another, click on the comparison animation below. Note the differences in molecular motion of water molecules in these three states.

Plasmas are hot, ionized gases. Plasmas are formed under conditions of extremely high energy , so high, in fact, that molecules are ripped apart and only free atoms exist. More astounding, plasmas have so much energy that the outer electrons are actually ripped off of individual atoms, thus forming a gas of highly energetic, charged ions . Because the atoms in plasma exist as charged ions, plasmas behave differently than gases, thus representing a fourth state of matter . Plasmas can be commonly seen simply by looking upward; the high energy conditions that exist in stars such as our sun force individual atoms into the plasma state.
- Other states of matter
As we have seen, increasing energy leads to more molecular motion. Conversely, decreasing energy results in less molecular motion. As a result, one prediction of Kinetic Molecular Theory is that if we continue to decrease the energy (measured as temperature) of a substance, we will reach a point at which all molecular motion stops. The temperature at which molecular motion stops is called absolute zero and has been calculated to be -273.15 degrees Celsius. While scientists have cooled substances to temperatures close to absolute zero , they have never actually reached absolute zero. The difficulty with observing a substance at absolute zero is that to "see" the substance, light is needed, and light itself transfers energy to the substance, thus raising the temperature. Despite these challenges, scientists have recently observed a fifth state of matter that only exists at temperatures very close to absolute zero.
Bose-Einstein Condensates represent a fifth state of matter only seen for the first time in 1995. The state is named after Satyendra Nath Bose and Albert Einstein who predicted its existence in the 1920s. B-E condensates are gaseous superfluids cooled to temperatures very near absolute zero . In this weird state, all the atoms of the condensate attain the same quantum-mechanical state and can flow past one another without friction . Even more strangely, B-E condensates can actually "trap" light , releasing it when the state breaks down.
Several other less common states of matter have also either been described or actually seen. Some of these states include liquid crystals , fermionic condensates , superfluids, supersolids, and the aptly named strange matter . To read more about these phases, see "Phase" in our Resources for this module.
- Phase transitions
The transformation of one state of matter into another state is called a phase transition . The more common phase transitions even have names; for example, the terms melting and freezing describe phase transitions between the solid and liquid state, and the terms evaporation and condensation describe transitions between the liquid and gas state.
Phase transitions occur at very precise points, when the energy (measured as temperature) of a substance in a given state exceeds that allowed in the state. For example, liquid water can exist at a range of temperatures. Cold drinking water may be around 4ºC. Hot shower water has more energy and thus may be around 40ºC. However, at 100°C under normal conditions, water will begin to undergo a phase transition into the gas phase. At this point, energy introduced into the liquid will not go into increasing the temperature; it will be used to send molecules of water into the gas state. Thus, no matter how high the flame is on the stove, a pot of boiling water will remain at 100ºC until all of the water has undergone transition to the gas phase. The excess energy introduced by a high flame will accelerate the liquid-to-gas transition; it will not change the temperature. The heat curve below illustrates the corresponding changes in energy (shown in calories) and temperature of water as it undergoes a phase transition between the liquid and gas states.
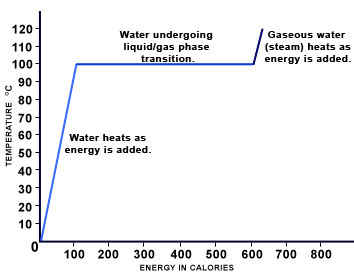
As can be seen in the graph above, as we move from left to right, the temperature of liquid water increases as energy (heat) is introduced. At 100ºC, water begins to undergo a phase transition and the temperature remains constant even as energy is added (the flat part of the graph). The energy that is introduced during this period goes toward breaking intermolecular forces so that individual water molecules can "escape" into the gas state. Finally, once the transition is complete, if further energy is added to the system , the heat of the gaseous water, or steam, will increase.
This same process can be seen in reverse if we simply look at the graph above starting on the right side and moving left. As steam is cooled, the movement of gaseous water molecules and thus temperature will decrease. When the gas reaches 100ºC, more energy will be lost from the system as the attractive forces between molecules re-form; however, the temperature remains constant during the transition (the flat part of the graph). Finally, when condensation is complete, the temperature of the liquid will begin to fall as energy is withdrawn.
- Phase transitions and our world
Phase transitions are an important part of the world around us. For example, the energy withdrawn when perspiration evaporates from the surface of your skin allows your body to correctly regulate its temperature during hot days. Phase transitions play an important part in geology, influencing mineral formation and possibly even earthquakes . And who can ignore the phase transition that occurs at about -3ºC, when cream, perhaps with a few strawberries or chocolate chunks, begins to form solid ice cream?
Now we understand what is happening in a pot of boiling water. The energy (heat) introduced at the bottom of the pot causes a localized phase transition of liquid water to the gaseous state. Because gases are less dense than liquids , these localized phase transitions form pockets (or bubbles) of gas , which rise to the surface of the pot and burst. But nature is often more magical than our imagination. Despite all that we know about the states of matter and phase transitions, we still cannot predict where the individual bubbles will form in a pot of boiling water.
Table of Contents
Activate glossary term highlighting to easily identify key terms within the module. Once highlighted, you can click on these terms to view their definitions.
Activate NGSS annotations to easily identify NGSS standards within the module. Once highlighted, you can click on them to view these standards.

- Sign in / Register
- Administration
- Edit profile

The PhET website does not support your browser. We recommend using the latest version of Chrome, Firefox, Safari, or Edge.
- Random article
- Teaching guide
- Privacy & cookies

States of matter
by Chris Woodford . Last updated: July 26, 2023.
What makes something solid, liquid, or gas?
Inside matter, changing from one state to another, the kinetic theory of matter, what is absolute zero, why are solids, liquids, and gases so different.
Photo: Solid properties: All these things are solids, but they have very different physical properties. Steel (can, left) is strong, resists heat well, conducts electricity, and melts at a high temperature. Rubber (tennis ball, top) is stiff, stretchy, melts at a low temperature, and doesn't conduct electricity. Plastic (bottle, right) is fairly strong, squashes when you push it but doesn't usually return to its original shape, melts at a low temperature, and doesn't conduct electricity or heat. Artificial sponge (top right) is similar to rubber but much weaker and less durable.
What about plasma?
Photo: A plasma "sphere" is a completely sealed glass bowl (or, in this case, cylinder) containing hot, ionized gas produced with the help of electricity . If you place your hands on the glass, they attract the free electrons in the plasma, so it swirls about in response to your touch! Photo by John Suits courtesy of US Navy and Wikimedia Commons .
Are there any other states of matter?
If you liked this article..., don't want to read our articles try listening instead, find out more, on this website.
- Refrigerators
For younger readers
- States of Matter (Science in a Flash) by Georgia Amson-Bradshaw. Hachette, 2020. A visually engaging, 32-page introduction for ages 8–11, which includes highlighted cool facts, activities, and quizzes.
- The Solid Truth about States of Matter with Max Axiom, Super Scientist by Agnieszka Jòzefina Biskup. Capstone, 2019. A 32-page, graphic-style introduction (with a linked app) for ages 9–12.
- Experiments with Solids, Liquids, and Gases by Christine Taylor-Butler. Scholastic, 2011. A hands-on exploration of states of matter, suitable for ages 8–10; grades 3–5.
- Many Kinds of Matter (A Look at Solids, Liquids, and Gases) by Jennifer Boothroyd. Lerner, 2010. A very simple 32-page outline for ages 6–9, grades 1–3. It covers the basic states and changing between them, with clear, real-world examples.
- States of Matter (Why Chemistry Matters) by Lynnette Brent. Crabtree, 2009. Also designed for ages 8–10, grades 3–5, this book covers the basics, then goes on to look at why matter has properties like elasticity.
For older readers
- Six Easy Pieces by Richard P. Feynman. Penguin, 1998. These excerpts from the famous Feynman lectures are hardly "easy," unless you already understand them from elsewhere. However, chapter 1 is a simple introduction to solids, liquids, and gases from an atomic point of view.
- Absolute Zero and the Conquest of Cold by Tom Shachtman. Houghton Mifflin Harcourt, 2000. The scientific quest for colder than colder!
- States of Matter by David L. Goodstein. Courier Dover, 2014 (reissue of the 1985 edition). A more detailed book about statistical physics, thermodynamics, and kinetic theory for undergraduates.
Text copyright © Chris Woodford 2009, 2021. All rights reserved. Full copyright notice and terms of use .
Rate this page
Tell your friends, cite this page, more to explore on our website....
- Get the book
- Send feedback

States of Matter
Core concept – states of matter.
In this tutorial, you will learn about the four main states of matter (solid, liquid, gas, and plasma), as well as some intermediate states of matter , by reading about their properties, applications, and examples.
Topics Covered in Other Articles
- Properties of Solids, Liquids, and Gases
- Quantum Numbers
- Phase Diagram
- Physical vs. Chemical Properties
- Law of Conservation of Matter
- Heat of Fusion
- Bose-Einstein Condensate
Vocabulary for States of Matter
- Magnetic field – a region around a magnet or an electric current that describes the magnetic influence on moving electric charges, currents, and magnetic materials. A moving electric charge in a magnetic field experiences a force perpendicular to its velocity.
- Matter – anything that has mass and occupies space; it constitutes atoms and compounds, which compose physical and chemical properties. For more in-depth information on the concept of matter, check out this article !
- Phase and Matter are occasionally used as synonyms; however, it is possible to form several phases that are in the same state of matter (such as how solids can have different crystalline structures).
What are the States of Matter?
The states of matter refer to the physical forms that matter can take. There are three main states of matter: solid, liquid, and gas. The state of a substance depends on its temperature and pressure. For example, at room temperature and pressure, water is a liquid. But if the water is heated to a high enough temperature, it will become a gas (steam), and if it is cooled to a low enough temperature, it will become a solid (ice). The behavior of matter also changes depending on its state. Solids have a fixed shape and volume, while liquids have a fixed volume but can take the shape of their container, and gases have neither a fixed shape nor a fixed volume. The properties of a substance, such as its density, conductivity, and viscosity, also vary depending on its state of matter.
There are four main states of matter if you include plasma. Additionally, there are many intermediate states, many of which only exist under extreme conditions; in total, there are twenty! Due to their difference in properties being their distinguishing factors, let’s go over some of the states of matter below:
In the solid state, particles tightly pack together in a fixed arrangement. Due to the strong forces holding them together, the particles of a solid are only able to move back and forth in small vibrations. In other words, they stay in their fixed positions. As a result, solids have the lowest kinetic energy of all the states of matter.
The particles of the compound bind in either an organized, geometric lattice or a random, unstructured shape. The materials present and the conditions in which a solid is created dictate whether it will be a crystalline or amorphous solid. Usually, when conditions are steady (such as slow and gradual cooling/heating), the particles have a chance to align uniformly. However, when there are extreme and rapid temperature changes, an indefinitely shaped solid will most likely be the result.
Solids have a definite shape and volume, meaning they have a fixed position and will not conform to the shape of their container. Because their particles are so densely packed, solids tend to have a high density and are hard to compress further without the use of great external force.

Classes of Solids
Whether or not we realize it, there are so many types of solids all around us—from table salt to a wooden chair! Since the types of forces and bonding between particles can vary, there are different classes of solids. These classes include metals, minerals, (glass) ceramics, organic molecules, composite materials, semiconductors, nanomaterials, and biomaterials. Due to their different force interactions, these categories of solids have different physical and chemical properties. These properties include elasticity, conductivity, light transmittance, plasticity, and more.
In the liquid state, particles flow around each other. They are more loosely packed than a solid. Due to the weaker forces holding their particles together, liquids conform to the shape of their container. However, the interaction is strong enough to keep the particles attracted to each other. As a result, liquids are incompressible. This means that liquids have a fixed volume (no matter the shape of their container) as long as the temperature and pressure are held constant. Since there is more particle movement than within a solid, liquids have a higher kinetic energy value.
Solids, when heated past their melting point, can absorb thermal energy, which gets the particles moving. Once enough energy enters the system to weaken the forces keeping them fixed, the particles move more as they transition into the liquid state. Some properties to look at when researching liquids are buoyancy, surface tension , fluidity, and density .
In the gaseous state, the particles have even more freedom to move than in a liquid. Here, the particles can move in random directions without attracting each other. The molecules have enough kinetic energy that the intermolecular forces holding them together are negligible, which is the reasoning behind their amount of movement. Like liquids, gases do not have a definite shape; therefore, they also conform to the shape of their container. However, unlike liquids, gases are compressible—they do not have a fixed volume. This means that the gas particles will spread out to fill the container they are in. Because of the distance between gas particles, it is common for a colorless gas to be invisible to the human eye. This is why we have ways to detect gases, such as carbon monoxide detectors!
Due to the properties of this state of matter, it can be difficult to mathematically analyze gases. This is why there is the Ideal Gas Law , which sets up conditions for how a gas should act under ‘perfect’ conditions. There are also different mathematical relationships that set up conditions for the behavior of gases such as Boyle’s Law , Charles’ Law , Gay-Lussac’s Law , Henry’s Law , the Combined Gas Law , and Avogadro’s Law.

Pure Gases vs Gas Mixture
A pure gas can be made up of individual atoms (e.g., noble gases like neon and argon), elemental molecules with a single type of atom (e.g., diatomic gases like O 2 and N 2 ), or compound molecules with multiple types of atoms (e.g., carbon dioxide). A gas mixture, on the other hand, contains a variety of pure gases. A common example of a gas mixture is the air in the earth’s atmosphere. The earth’s atmosphere contains nitrogen, oxygen, argon, and several other gases.
This lesser-known state of matter is a subset of gases. Similar to gases, plasmas do not have a definite shape or volume and have lower density. Again, this means that the particles conform to both the shape and volume of the container in which they are held. However, while gases are made up of molecules with a net charge of zero, plasmas are made up of charged particles. They consist of a freely moving sea of electrons with positively charged nuclei “floating about”. As a result, plasmas can conduct electrical charges and interact with other electromagnetic forces.
A plasma can become a gas in one of two ways. First, being exposed to a big voltage difference (equal or greater than a charge difference of 2) will strip the electrical charge and ionize it, giving it the neutral charge of a gas. Second, by exposing the plasma to high-temperature conditions, the electrons leave the atoms, resulting in free electrons. Since only some electrons are free, this is called partially ionized plasma. In some extreme conditions, it can be assumed that all electrons are free; this is called fully ionized plasma.

Examples of Plasmas
Plasma comprises approximately 99% of the universe. It glows in the form of stars, nebulas, and auroras. In addition, a layer of the Earth’s atmosphere known as the ionosphere is considered to be a plasma. Bolts of lightning in the sky and neon signs in city streets are other examples of plasma.
Video Tutorial on States of Matter
Please enjoy our animated lecture on Phases and States of Matter: The Law of Conservation of Mass.
Bose-Einstein Condensate (BEC)
In 1995, scientists demonstrated a man-made state of matter: Bose-Einstein condensate. It is a group of atoms cooled to near absolute zero (-273.15°C). At this temperature, the atoms do not have free energy to move relative to each other. Therefore, they begin to coalesce into a single quantum state and become identical, behaving as a single atom. Bose-Einstein condensates play a major role in the development of energy-efficient lasers and ultrafast optical switches.

Color Glass Condensate
This type of matter has a theory to it! It supposedly exists inside atomic nuclei when they collide while traveling close to the speed of light. In association with Einstein’s theory of relativity, a high-energy nucleus can appear compressed; as a result, the gluons within the nucleus appear as a wall traveling at the speed of light. The gluon wall’s density increases. The saturated gluon matter is known as the Color Glass Condensate. This state of matter is important because it has been proposed as a universal form of matter to analyze and describe properties of high-energy, strongly interacting particles.
States of Matter in different conditions
Standard temperature and pressure.
At standard temperature and pressure (STP), most substances exist as gases, but under different conditions, they can take on different states. For example, at low temperatures and high pressures, some substances can exist as a solid, liquid, or gas simultaneously, in a state known as a triple point . Other substances can exist as a solid and a gas at the same time, in a state known as a sublimation . The properties of a substance, such as its density, conductivity, and viscosity, also vary depending on its state of matter. In high school or college-level chemistry, students learn about the different states of matter and how to predict and explain the phase changes between them.
Temperature
The states of matter change when the temperature or pressure of a substance changes. For example, when a substance is heated, the energy of its molecules increases. Increased energy causes them to move faster and farther apart. This can lead to a change in the state of the substance, such as a solid melting to become a liquid or a liquid boiling to become a gas. Similarly, when a substance cools, the energy of its molecules decreases, causing them to move slower and closer together. This can lead to a change in the state of the substance, such as a liquid freezing to become a solid or a gas condensing to become a liquid.
The pressure of a substance can also affect its state of matter. For example, increasing the pressure on a gas can cause it to condense and become a liquid. While decreasing the pressure on a liquid can cause it to vaporize and become a gas. These changes in temperature and pressure come from a variety of factors, such as the addition or removal of heat, changes in the volume of the substance, or the presence of other substances that can affect its state.
Further Reading
- Mixtures vs. Compounds
- What is a Solution
- Mole & Avogadro’s Number
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Unit 6: States of matter and intermolecular forces
About this unit.
This unit is part of the Chemistry library. Browse videos, articles, and exercises by topic.
States of matter
- States of matter (Opens a modal)
- States of matter follow-up (Opens a modal)
- Specific heat and latent heat of fusion and vaporization (Opens a modal)
- Specific heat, heat of fusion and vaporization example (Opens a modal)
- Chilling water problem (Opens a modal)
- Change of state example (Opens a modal)
- Vapor pressure (Opens a modal)
- Phase diagrams (Opens a modal)
- Representing solids, liquids, and gases using particulate models (Opens a modal)
- Crystalline and amorphous polymers (Opens a modal)
- Representing alloys using particulate models (Opens a modal)
- Structure of metals and alloys 4 questions Practice
- Solids, liquids, and gases 4 questions Practice
Introduction to intermolecular forces
- London dispersion forces (Opens a modal)
- Dipole–dipole forces (Opens a modal)
- Hydrogen bonding (Opens a modal)
- Ion–dipole forces (Opens a modal)
- Intermolecular forces and vapor pressure (Opens a modal)
- Solubility and intermolecular forces (Opens a modal)
- Surface tension (Opens a modal)
- Capillary action and why we see a meniscus (Opens a modal)
- Boiling points of organic compounds (Opens a modal)
- Boiling point comparison: AP Chemistry multiple choice (Opens a modal)
- Solubility of organic compounds (Opens a modal)
- 2015 AP Chemistry free response 2f (Opens a modal)
- Intermolecular forces 4 questions Practice
- Intermolecular forces and properties of liquids 4 questions Practice
- Solubility 4 questions Practice
Mixtures and solutions
- Types of mixtures (Opens a modal)
- Molarity (Opens a modal)
- Dilution (Opens a modal)
- Representing solutions using particulate models (Opens a modal)
- Boiling point elevation and freezing point depression (Opens a modal)
- Molarity calculations 4 questions Practice
- Solutions and mixtures 4 questions Practice
- Representations of solutions 4 questions Practice
Separating mixtures and solutions
- Distillation (Opens a modal)
- Distillation curves (Opens a modal)
- Thin-layer chromatography (TLC) (Opens a modal)
- Calculating retention factors for TLC (Opens a modal)
- Column chromatography (Opens a modal)
- Separation of solutions and mixtures chromatography 4 questions Practice
- My Wishlist

Section 4: States of Matter
Matter is made up of tiny particles called atoms. When these atoms come together, they form molecules. The combination of atoms and molecules in different ways forms three types of matter: solids, liquids, and gases. Kinetic theory explains how particles in matter behave. For example, all matter is composed of particles; particles are in constant, random motion, and particles collide with each other and the walls of their containers.
A solid is matter that has a definite shape and definite volume. In a solid, particles are closely packed together in a geometric arrangement. Like a solid, a liquid has a definite shape. However, a liquid will take the shape of the container it is poured into, characterizing its shape as indefinite. The particles in a liquid have more space and can slide past each other. Gas is a state of matter that has no definite shape or volume. The particles move fast and are spread out evenly in a container. Plasma is often considered the fourth state of matter. Plasma is a high-temperature gas with positively and negatively charged particles.
The state of a sample of matter depends on the temperature. Temperature is related to the average kinetic energy of an object’s atoms or molecules. Thermal expansion is the tendency of matter to change its shape in response to a change in temperature. For example, a substance will increase in size when the temperature increases and contract when cooled. The exception to this rule is that water expands when it’s cooled.
- What is kinetic theory?
- Compare a solid to a gas.
- The state of a sample of matter depends on what?
Click here to go back to the Table of Contents
States of matter: Definition and phases of change
The four fundamental states of matter are solid, liquid, gas and plasma, but there others, such as Bose-Einstein condensates and time crystals, that are man-made.

Solids, liquids and gas
Bose-einstein condensate, new states of matter.
- Change in state
Additional resources
The phrase five states of matter is a term to describe everything that makes up the "stuff" in the universe — anything that takes up space and has mass is matter. But that phrase is actually outdated, as there are many more states of matter than that. Four of these occur naturally, while others are only made fleetingly in the lab, under extreme conditions.
All matter is made up of atoms , which are in turn made up of protons, neutrons and electrons.
Atoms come together to form molecules, which are the building blocks for all types of matter, according to Washington State University . Both atoms and molecules are held together by a form of potential energy called chemical energy , according to the U.S. Energy Information Administration.
Related: How many atoms are in the observable universe?
The four natural states of matter are: Solids, liquids, gases and plasma. Bose-Einstein condensate s, however, are only made in the lab. Other exotic states of matter can also be manufactured under extreme conditions in a lab, such as fermionic condensates and time crystals . There's even a strange type of matter, known as a chain-melted state, that stably exists as both a solid and liquid at once .
In a solid , particles are packed tightly together so they don't move much. The electrons of each atom are constantly in motion, so the atoms have a small vibration, but they are fixed in their position. Because of this, particles in a solid have very low kinetic energy.
Solids have a definite shape, as well as mass and volume, and do not conform to the shape of the container in which they are placed. Solids also have a high density, meaning that the particles are tightly packed together.
In a liquid , the particles are more loosely packed than in a solid and are able to flow around each other, giving the liquid an indefinite shape. Therefore, the liquid will conform to the shape of its container.
Much like solids, liquids (most of which have a lower density than solids) are incredibly difficult to compress.
In a gas , the particles have a great deal of space between them and have high kinetic energy. A gas has no definite shape or volume. If unconfined, the particles of a gas will spread out indefinitely; if confined, the gas will expand to fill its container. When a gas is put under pressure by reducing the volume of the container, the space between particles is reduced and the gas is compressed, according to NASA's Glenn Research Center.
Plasma is not a common state of matter here on Earth, but it may be the most common state of matter in the universe, according to the Jefferson Laboratory . Stars like the sun are essentially superheated balls of plasma.
Plasma consists of highly charged particles with extremely high kinetic energy. The noble gases (helium, neon, argon, krypton, xenon and radon) are often used to make glowing signs by using electricity to ionize them to the plasma state.
A BEC was first created by scientists in 1995. Using a combination of lasers and magnets , Eric Cornell and Carl Weiman, scientists at the Joint Institute for Lab Astrophysics (JILA) in Boulder, Colorado, cooled a sample of rubidium to within a few degrees of absolute zero. At this extremely low temperature, molecular motion comes very close to stopping. Since there is almost no kinetic energy being transferred from one atom to another, the atoms begin to clump together. There are no longer thousands of separate atoms, just one "super atom."
BECs are used to study quantum mechanics on a macroscopic level. Light appears to slow down as it passes through a BEC, allowing scientists to study the particle/wave paradox. A BEC also has many of the properties of a superfluid , or a fluid that flows without friction . BECs are also used to simulate conditions that might exist in black holes.
Many other states of matter have been created under extreme or exotic conditions. For instance, in May 2023, scientists created a " bosonic correlated insulator ," or a symmetric crystalline state with a neutral charge. In 2021, scientists smooshed water to ultrahigh pressures and blasted it with lasers to create " superionic ice ," or a strange new form of H20 similar to a solid oxygen lattice sitting in an ocean of floating hydrogen atoms. That same year, research published in the journal PNAS revealed that during the transformation between the state of liquid and solid, glass becomes a new state of matter referred to as liquid glass.
On a microscopic level, liquid glass is somewhere between a solid and a gel-like substance called a colloid — a mixture of particles that are larger than a single atom or molecule. When a substance transforms from a liquid to a solid, molecules are arranged in a crystalline structure — for glass, this doesn’t happen and particles are frozen in place before crystallisation occurs. The particles in liquid glass — however -are more flexible than solid glass, but can not rotate, according to the researchers.
"Our experiments provide the kind of evidence for the interplay between critical fluctuations and glassy arrest that the scientific community has been after for quite some time," senior author of the study and Professor of Soft Condensed Matter Theory at the University of Konstanz Matthias Fuchs, said in a statement .
Related: How do you weigh an atom?

Time crystals are a form of matter that were first proposed in 2012 by Nobel-prize winning physicist Frank Wilczek. Time crystals are made in the lab and have the ability to cycle between two states of energy without ever losing energy. Because they don't reach equilibrium or a steady state, they are able to dodge the second law of thermodynamics , which states that the disorder, or entropy, of a closed system, always increases.
Time crystals were created in a lab in 2017 and in 2021, Google announced that it had made a time crystal in a quantum computer , and that the crystal had lasted for 100 seconds before the ephemeral state disintegrated.
Fermionic condensates are another type of lab-made matter. A sister phase to the BEC, fermionic condensates were first created in 2004 , according to NASA. Fermionic condensates are superfluids, meaning they can flow with no viscosity. Unlike BECs, they are made up of fermions, a type of matter that includes protons, neutrons and electrons with odd atomic numbers. Fermions normally like to be alone, but to create this matter phase, scientists have to coax them to pair up.
To do this, scientists make the matter very, very cold. In the first experiment to demonstrate this oddball phase, described in a 2003 study in the journal Physical Review Letters , scientists at JILA in Boulder, Colorado cooled a cloud of half a million potassium-40 atoms to less than a millionth of a degree above absolute zero, then applied a magnetic field to them. This forced the potassium atoms to pair up, creating a state akin to superconductivity that occurs in electron pairs.
How states of matter change
Adding or removing energy from matter causes a physical change as matter moves from one state to another. For example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). And removing energy from liquid water causes it to become ice (a solid). Physical changes can also be caused by motion and pressure, according to the Abridged Science for High School Students by H.Messel.
Melting and freezing
When heat is applied to a solid, its particles begin to vibrate faster and move farther apart. When the substance reaches a certain combination of temperature and pressure, its melting point , the solid will begin to melt and turn into a liquid.

When two states of matter, such as solid and liquid, are at the equilibrium temperature and pressure, additional heat added into the system will not cause the overall temperature of the substance to increase until the entire sample reaches the same physical state, according to Encyclopaedia Britannica . For example, when you put ice into a glass of water and leave it out at room temperature, the ice and water will eventually come to the same temperature. As the ice melts from heat coming from the water, it will remain at 32 degrees Fahrenheit (0 degrees Celsius) until the entire ice cube melts before continuing to warm.
When heat is removed from a liquid, its particles slow down and begin to settle in one location within the substance. When the substance reaches a cool enough temperature at a certain pressure, the freezing point, the liquid becomes a solid.
Sublimation
When a solid is converted directly into a gas without going through a liquid phase, the process is known as sublimation. This may occur either when the temperature of the sample is rapidly increased beyond the boiling point (flash vaporization) or when a substance is "freeze-dried" by cooling it under vacuum conditions so that the water in the substance undergoes sublimation and is removed from the sample, according to the U.S. Geological Survey . A few volatile substances will undergo sublimation at room temperature and pressure, such as frozen carbon dioxide, or dry ice .

Vaporization
Vaporization is the conversion of a liquid to a gas and can occur through either evaporation or boiling , according to Encyclopaedia Britannica.
Because the particles of a liquid are in constant motion, they frequently collide with each other. Each collision also causes energy to be transferred, and when enough energy is transferred to particles near the surface they may be knocked completely away from the sample as free gas particles. Liquids cool as they evaporate because the energy transferred to surface molecules, which causes their escape, gets carried away with them.
Liquid boils when enough heat is added to a liquid to cause vapor bubbles to form below the surface. This boiling point is the temperature and pressure at which a liquid becomes a gas.
Condensation and deposition
Condensation occurs when a gas loses energy and comes together to form a liquid, according to the U.S. Geological Survey. For example, water vapor condenses into liquid water, known as its dew point .
Deposition occurs when a gas transforms directly into a solid, without going through the liquid phase. Water vapor becomes ice or frost when the air touching a solid, such as a blade of grass, is cooler than the rest of the air.
- Watch: Creation of a Bose-Einstein condensate , from the National Institute of Standards and Technology. Learn where the matter in the universe came from , from Cornell University's Ask an Astronomer.Read more about matter, elements and atoms , from Khan Academy.
This article was updated on Oct. 20, 2022 by Tia Ghose.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Does honey ever go bad?
What is the world's most dangerous chemical?
Mysterious Maya underground structure unearthed in Mexico
Most Popular
- 2 What causes you to get a 'stitch in your side'?
- 3 Newly discovered asteroid larger than the Great Pyramid of Giza will zoom between Earth and the moon on Saturday
- 4 China opens Chang'e 6 return capsule containing samples from moon's far side
- 5 Neanderthals cared for 6-year-old with Down syndrome, fossil find reveals
- 2 Newly discovered asteroid larger than the Great Pyramid of Giza will zoom between Earth and the moon on Saturday
- 3 2,000 years ago, a bridge in Switzerland collapsed on top of Celtic sacrifice victims, new study suggests
- 4 Self-healing 'living skin' can make robots more humanlike — and it looks just as creepy as you'd expect
- 5 Tasselled wobbegong: The master of disguise that can eat a shark almost as big as itself
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Collections
- Sustainability in chemistry
- Simple rules
- Teacher well-being hub
- Women in chemistry
- Global science
- Escape room activities
- Decolonising chemistry teaching
- Teaching science skills
- Get the print issue
- RSC Education

- More navigation items
How to teach states of matter and particle theory

- No comments
David Paterson suggests ideas, activities and resources for your classroom
Source: © Getty Images
How does the knowledge of the fundamental states of matter help us make better ice cream ?
Simply mixing cream, sugar and egg yolk and sticking it in the freezer isn’t enough. Careful control of the mixing and freezing process leads to a material with all three states of matter – a foam and an oil-in-water emulsion. The taste of ice cream comes partially from your taste buds on your tongue, but also due to evaporation of volatile compounds reaching your smell receptors. Ice cream really does taste better on a warm day!

In your class
Download integrated instructions (as MS Powerpoint or pdf ) and technician and teacher notes (as MS Word or pdf ) for a microscale diffusion practical.
What students need to know

Source: © Science Photo Library
Primary school will have introduced students to solids, liquids and gases; now it’s time to link these states to the microscopic world of the particle
Students will have studied the types and properties of matter during primary education. They will have investigated various different materials, classifying them as solids, liquids and gases. They will have looked at reversible reactions, including freezing/melting and boiling/condensing. These macroscopic observations and explanations need to be linked to the microscopic world of the particle using ‘particle theory’.
The particle (or kinetic) theory of matter can be summarised as:
- All matter is formed of tiny particles.
- The particles are constantly randomly moving about.
- The particles can be arranged regularly or randomly.
- The particles are held together by weak or strong forces.
- As temperature increases, the particles move faster.
There is a strong overlap between this area in chemistry and physics. Look at where it is scheduled in your school’s schemes of work to see whether you will be teaching it for the first time, or consolidating the knowledge.
Ideas for your classroom
Hands-on demonstrations, simulations and practical work provide lots of opportunities for your students to understand the dynamic nature of particles. Check with your physics department to see if they have a kinetic theory model (eg SciChem XPG050011; Timstar SI68190). These use ball bearings to model the particles. A vibrating base ‘energises’ the particles. By increasing the vibration speed, you can model solid, liquid and gas.
Place a few drops of a perfume on a watch glass and ask the students to raise their hands when they can smell it
Freely available online simulations, such as the pHET States of matter , allow you to demonstrate the effects of heating and cooling substances. Students can see state changes, and relate this to movement and arrangement of the particles. Consider allowing the students to investigate the simulations for themselves, either in class or for homework; this worksheet could be suitable. An alternative paper-based activity is Particle model cards from the Royal Society of Chemistry.
Your understanding of using models can be enhanced with the RSC CPD course Developing and using models .
Diffusion of particles provides many useful contexts to help students make the link between the macroscopic world and the sub-microscopic . Start with simple demonstrations such as placing a few drops of a perfume on a watch glass and ask the students to raise their hands when they can smell it. They should observe a wave of hands going up as the fragrance particles spread from the watch glass.
This can be followed with diffusion of nitrogen dioxide in gas jars . In this demonstration, NO 2 is synthesised by mixing copper turnings and concentrated nitric acid in one gas jar . A second air-filled jar is then placed over the top, and the NO 2 allowed to diffuse into it. Your students will observe the dark brown vapour moving into the upper air-filled jar. Ask them to consolidate their observations with particle diagrams showing the before, during and after. They should annotate the movement and mixing of nitrogen dioxide and air particles.
A commonly used demonstration is soaking mineral wool separately in concentrated hydrochloric acid and concentrated ammonia solution, and placing these at either end of a large glass tube. However this can be fiddly to set up and requires a fume hood.
A good alternative is a microscale diffusion practical, with all the chemistry happening on a plastic sheet under a petri dish. Students first make up an array of iodide/starch drops on a plastic sheet. Second, they mix a couple of drops of acid and bleach to synthesise a small volume of chlorine. Finally, the whole arrangement is covered in a petri dish to contain the chlorine and allow it to diffuse to the iodide/starch drops. Your students will observe the drops darkening as the iodine is displaced from solution and forms the blue/black iodine-starch complex. They can set up, carry out and clear away the apparatus inside 15 minutes, leaving plenty of time for discussions and explanations. Download the instructions and a template practical sheet below.
Common misconceptions
‘Space between particles is filled with air’ – a common misconception
Misconceptions in this topic are common and can be deep-seated. This isn’t surprising given the abstract ideas we are trying to help students grasp. Use the Best Evidence in Science Teaching project resources to help expose these misconceptions – the Topic 1, Key concept 1 Substance will be useful for this topic.
Common misconceptions include:
- Matter is continuous, rather than being formed from discrete particles – everyday experience reinforces this – solids ‘feel’ solid and liquids stay together in drops even if they are sprayed. Discussing gases can be a helpful way in to particles, but as they ‘disappear’, many students think gases aren’t properly there.
- Space between particles is filled with air – even when students accept the idea of matter being made of particles, the space between particles can still be considered as air. Everyday life again creates a false analogy with solid and liquid objects being separated from each other by the air we breathe.
- Forces are responsible for all particle movement – this links with a common misconception from physics, whereby force is considered required for all movements. Students struggle with the idea that a particle keeps moving once it starts. Everyday experiences, such as a hockey ball rolling to a stop once hit, create this false analogy.
- Particles change state – eg when ice melts it is the ice particles melting to form water particles, rather than the substance changing state due to the change in energy and position of the particles relative to each other.
- Atoms and cells are about the same size – this isn’t helped by chemists and biologists using ‘nucleus’ to describe the centre of our respective fundamental building blocks. Discussing relative scales of objects can help – eg a human cell is 100,000x smaller than a human, while an atom is 100,000x smaller than a human cell. Videos such as Powers of ten can also help.
Formative assessment
As the kinetic theory is one of the key models underpinning much of chemistry, discuss it regularly, and give the students plenty of opportunities to create artefacts to help them express and consolidate their understanding of the model. Drawing posters showing the particle arrangement of solids, liquids and gases, surrounded by notations of the properties is useful. Success criteria could be displayed for the students to peer assess each other’s work, identifying areas for improvement. If you have a visualiser available, display high-quality examples to model the expectations for the class.
The RSC Assessment for Learning suite of resources contains a useful document discussing many example of substances in different states . You may need to select carefully, as there are examples of compounds and mixtures included, along with particles shown as molecules. However, identifying early on that particles are being modelled as a simple circle is useful in the general discussion of use of models in learning about science.
Progression to 14–16
The structure of matter at the sub-microscopic level, and the forces between particles, underlies the explanation of macroscopic properties. During 11–14, students gain some appreciation of the nature of the particles (atoms, ions, molecules). At 14–16, you will expand on how these different particles are formed and how this affects the nature of the forces between them. Students will need to identify the similarities and differences between ionic, covalent and metallic bonding, and weak intermolecular forces.
Another key area is reaction kinetics – collision theory is predicated on an understanding that particles collide, and the models used at this level are very reminiscent of the particle model.
Finally, there is strong overlap between chemistry and physics in this area of study. Changes of state are discussed in much greater depth in physics, looking at the internal energy of substances, delineating kinetic and potential energies. The two plateaus on the heating/cooling curves of substances are much more explicable when this has been learned.
Take-home points
- Kinetic theory describes the position and movement of particles in substances.
- Students commonly equate the size of atoms with that of cells – take time to help them visualise the orders of magnitude involved.
- Make use of simple demonstrations and practical work to help your students link between the macroscopic and sub-microscopic.
- Kinetic theory is built on throughout 14–16, and allows structure and bonding to be explicable.
David Paterson is a chemistry/physics teacher at Aldenham School, Elstree. He tweets @dave2004b
Microscale diffusion integrated instructions
Teacher technician notes microscale diffusion.

More David Paterson

Enhance students’ learning and development with digital resources

Use AI to successfully assess students’ understanding
Everything you need to teach energetics at 14–16
- Misconceptions
- Properties of matter
Related articles

Particle diagrams | Structure strip | 14–16
By Kristy Turner
Support learners to describe and evaluate the particle model for solids, liquids and gases with this writing activity

Cosmetics, technical services chemist
Sharlotte makes environmentally friendly beauty products

Illustrate polymer properties with a self-siphoning solution
2024-04-22T05:38:00Z By Declan Fleming
Demonstrate the tubeless siphon with poly(ethylene glycol) and highlight the polymer’s viscoelasticity to your 11–16 learners
No comments yet
Only registered users can comment on this article..


How to teach titration post-16
2024-07-08T05:32:00Z By Jo Haywood
Tips for teaching practical titration techniques and the underlying theory

Everything you need to introduce alkenes
2024-06-04T08:22:00Z By Dan Beech
Help your 14–16 learners to master the fundamentals of the reactions of alkenes with these ideas and activities

How to teach polymers at post-16
2024-05-28T06:57:00Z By Martin Bluemel
Teaching strategies and resources to help learners master polymers and overcome misconceptions
- Contributors
- Print issue
- Email alerts
Site powered by Webvision Cloud
Introduction to the Particle Theory of Matter
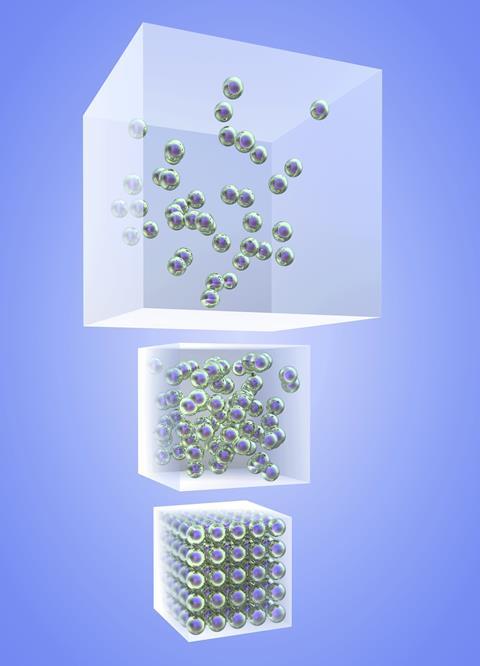
Cube overflowing with spheres representing particles (akinbostanci, iStockphoto)
How does this align with my curriculum?
| Grade | Course | Topic |
|---|
Share on: facebook X/Twitter LinkedIn Pinterest
Learn about how the Particle Theory helps us understand matter.
Particle Theory of Matter
Matter is anything that has mass and takes up space. It is a general name we call all the physical things around us. Matter includes things so tiny humans can’t see them with their eyes.
The Particle Theory of Matter is a scientific model . A scientific model is a way of illustrating ideas, objects and processes so they’re easier to understand. Scientists use models to explain things that can’t be seen without special equipment. One of these things is an individual atom.
The Particle Theory of Matter helps us think about how matter behaves. It also helps us explain why different matter has different properties. It includes these key ideas:
- All matter is made of tiny particles . These particles are either individual atoms, or groups of atoms called molecules .
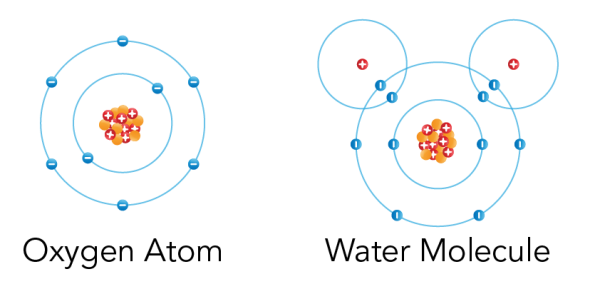
Shown is a colour diagram of two different atoms with blue spheres shown along concentric rings around a clump of red and orange spheres at the centre. The atom on the left is labelled "Oxygen Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Eight blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom on the right is labelled "Water Molecule." The main part of this diagram has the same amount of spheres and circles as the first. But there are two additional red spheres with plus signs. These are in the top left and right corners. Each one is surrounded by an additional blue circle with one blue sphere with a minus sign. These overlap the edges of the larger circle below.
Did you know? Any particle smaller than an atom is called a subatomic particle . Protons, neutrons and electrons are all subatomic particles.
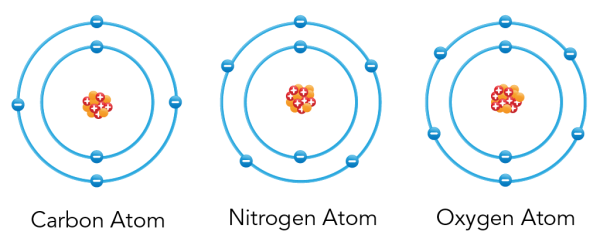
Shown is a colour diagram of three different atoms with blue spheres shown along concentric rings around a clump of red and orange spheres at the centre. The atom on the left is labelled "Carbon Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Six blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom in the middle is labelled "Nitrogen Atom." This has a clump of orange spheres and red spheres with plus signs at the centre. Seven blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom on the right is labelled "Oxygen Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Eight blue spheres with minus signs are neatly arranged along two concentric blue circles around it.
- Particles are attracted to each other by forces. In some kinds of matter, like a diamond, this force is very strong. In other kinds of matter, like orange juice, the force is weaker.
- Particles of matter have spaces between them. In a gas , there are large spaces between them. In a liquid they are closer together. In a solid , the particles are packed so close they can hardly move.
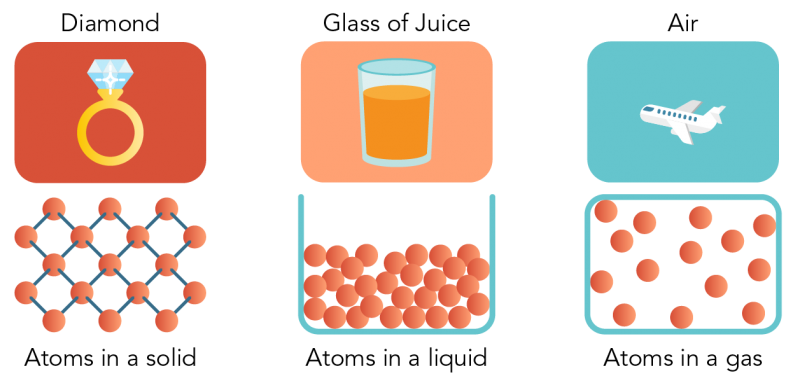
Shown is a colour illustration of three different kinds of matter above diagrams of their atoms. Starting on the left, the first illustration is labelled "Diamond." It shows a gold ring topped with a large, clear gem on a red background. Below, atoms are represented as pink spheres connected by small blue sticks. These are arranged in a neat diamond pattern, like a chain link fence. Text below reads "Atoms in a solid." In the middle is an illustration labelled "Glass of Juice." It shows a clear glass of orange juice against a pink background. Below, atoms are piled together, filling the bottom of an open blue box shape that resembles the glass. Some spheres appear to be touching each other, but there is space between others. Text below reads "Atoms in a liquid." On the right is an illustration labelled "Air." This shows a white airplane flying in a blue sky. Below, atoms float loosely in a blue rectangle, with lots of space between them. Text below reads "Atoms in a gas."
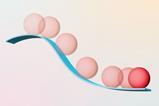
- Particles are always moving at any temperature above -273.15 degrees Celsius. But the human eye can’t see them move.
Did you know? -273.15 degrees Celsius is also 0 kelvin (0 K). This temperature is called absolute zero .
- The faster particles move, the warmer they get. So, the molecules in hot water are moving faster than the ones in cold water.

Shown is a colour diagram illustrating the speed and temperature of particles along a scale from colder to warmer. A thick horizontal stripe runs across the bottom edge. This is blue on the left side, and gradually changes to purple, then pink on the right. The left end is labelled "Slower" on top, and "Colder" below. The right end is labelled "Faster" on top, and "Warmer" below. Three black circles are spaced out along the scale. Each one contains round, coloured circles to represent particles. These have translucent tails that indicate the speed of their movement. The left circle has blue particles with short, wide tails. These match the blue, colder end of the scale. The middle circle has purple particles with longer, narrower tails. These match the middle part of the scale. The right circle has pink particles with even longer, narrower tails. These match the pink, warmer end of the scale.
Living Space
Explore the optimal environmental conditions for human life. How do you think your classroom conditions compare to those on the International Space Station? Free project for grades 6-9 students.
The Particle Theory of Matter Quiz See how well you know the particle theory. Try this short quiz by Science Source.
What's Matter? This video (3:30 min) by Crash Course Kids explains three states of matter: Solid, Liquid, and Gas. There is also a quick experiment you can do at home to prove that air is matter.
GCSE Physics - Particle Theory & States of Matter #25 (2020) This video (4:33 min.) from Cognito covers particle theory, how substances change from one state to another, and the idea that density doesn't change when substances change state.
BBC Bitesize. (n. d.) Kinetic particle theory - Kinetic particle theory and state changes - GCSE Physics (Single Science) Revision - Other .
EduMedia. (n. d.) Understanding Matter and Energy, Pure Substances and Mixtures
Petersen, D. (2019 March 26). How to teach states of matter and particle theory - Royal Society of Chemistry
Related Topics
What Are the States of Matter?
Solids, Liquids, Gases, and Plasma
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Matter occurs in four states: solids, liquids, gases , and plasma . Often the state of matter of a substance may be changed by adding or removing heat energy from it. For example, the addition of heat can melt ice into liquid water and turn that water into steam.
Key Takeaways: States of Matter
- Matter has mass and takes up space.
- The four main states of matter are solids, liquids, gases, and plasma.
- Under exceptional conditions, other states of matter also exist.
- A solid has a definite shape and volume. A liquid has a definite volume, but takes the shape of its container. A gas lacks either a defined shape or volume. Plasma is similar to a gas in that its particles are very far apart, but a gas is electrically neutral and plasma has a charge.
What Is a State of Matter?
The word "matter" refers to everything in the universe that has mass and takes up space. All matter is made up of atoms of elements. Sometimes atoms bond together closely, while at other times they are scattered widely.
States of matter are generally described on the basis of qualities that can be seen or felt. Matter that feels hard and maintains a fixed shape is called a solid; matter that feels wet and maintains its volume but not its shape is called a liquid. Matter that can change both shape and volume is called a gas.
Some introductory chemistry texts name solids, liquids, and gases as the three states of matter, but higher level texts recognize plasma as the fourth state of matter. Like a gas, plasma can change its volume and shape, but unlike a gas, it can also change its electrical charge.
The same element, compound, or solution can behave very differently depending on its state of matter. For example, solid water ( ice ) feels hard and cold while liquid water is wet and mobile. It's important to note, however, that water is a very unusual type of matter: rather than shrinking when it forms a crystalline structure, it actually expands.
A solid has a definite shape and volume because the molecules that make up the solid are packed closely together and move slowly. Solids are often crystalline; examples of crystalline solids include table salt, sugar, diamonds, and many other minerals. Solids are sometimes formed when liquids or gases are cooled; ice is an example of a cooled liquid which has become solid. Other examples of solids include wood, metal, and rock at room temperature.
A liquid has a definite volume but takes the shape of its container. Examples of liquids include water and oil. Gases may liquefy when they cool, as is the case with water vapor. This occurs as the molecules in the gas slow down and lose energy. Solids may liquefy when they heat up; molten lava is an example of solid rock which has liquefied as a result of intense heat.
A gas has neither a definite volume nor a definite shape. Some gases can be seen and felt, while others are intangible for human beings. Examples of gases are air, oxygen , and helium . Earth's atmosphere is made up of gases including nitrogen , oxygen, and carbon dioxide.
Plasma has neither a definite volume nor a definite shape. Plasma is often seen in ionized gases, but it is distinct from a gas because it possesses unique properties. Free electrical charges (not bound to atoms or ions) cause the plasma to be electrically conductive. The plasma may be formed by heating and ionizing a gas. Examples of plasma include stars, lightning, fluorescent lights, and neon signs.
Other States of Matter
Scientists are discovering new states of matter all the time! Some argue that there are five states of matter, or even six. In addition to the four main states of matter, other states of matter include superfluid, Bose-Einstein condensate, fermionic condensate, Rydberg molecules, quantum Hall state, photonic matter, and dropleton.
- Goodstein, D.L. (1985). States of Matter . Dover Phoenix. ISBN 978-0-486-49506-4.
- Murthy, G.; et al. (1997). "Superfluids and Supersolids on Frustrated Two-Dimensional Lattices". Physical Review B . 55 (5): 3104. doi:10.1103/PhysRevB.55.3104
- Sutton, A.P. (1993). Electronic Structure of Materials . Oxford Science Publications. ISBN 978-0-19-851754-2.
- Wahab, M.A. (2005). Solid State Physics: Structure and Properties of Materials . Alpha Science. ISBN 978-1-84265-218-3.
- Examples of Solids, Liquids, and Gases
- A to Z Chemistry Dictionary
- The Difference Between Homogeneous and Heterogeneous Mixtures
- How to Calculate Density of a Gas
- Examples of Physical Changes and Chemical Changes
- 10 Examples of Heterogeneous and Homogeneous Mixtures
- State of Matter Definition
- What Is the Definition of a Solid?
- Liquid Definition in Chemistry
- The Difference Between a Phase and State of Matter
- Gas Definition and Examples in Chemistry
- List of Phase Changes Between States of Matter
- Is Glass a Liquid or a Solid?
- Plasma Definition in Chemistry and Physics
- What Is Plasma Used For, and What Is It Made Of?
- Phase Definition and Examples

- Games & Quizzes
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction
Kinetic-molecular picture
Intermolecular separation and average speed, mean-free path and collision rate.
- Molecular sizes
- Summary of numerical magnitudes
- Free-molecule gas
- Continuity of gaseous and liquid states
- Ideal gas equation of state
- Internal energy
- Heat conduction
- Thermal diffusion
- Thermal transpiration
- Thermal conductivity
- Diffusion and thermal diffusion
- Boltzmann equation
- Equation of state
- Transport properties

Our editors will review what you’ve submitted and determine whether to revise the article.
- LiveScience - Properties of Matter: Gases
- UEN Digital Press with Pressbooks - Properties of Gases
- Chemistry LibreTexts Library - Gases
- gas - Student Encyclopedia (Ages 11 and up)
- Table Of Contents

gas , one of the three fundamental states of matter, with distinctly different properties from the liquid and solid states.
The remarkable feature of gases is that they appear to have no structure at all. They have neither a definite size nor shape, whereas ordinary solids have both a definite size and a definite shape, and liquids have a definite size, or volume, even though they adapt their shape to that of the container in which they are placed. Gases will completely fill any closed container; their properties depend on the volume of a container but not on its shape.
Gases nevertheless do have a structure of sorts on a molecular scale. They consist of a vast number of molecules moving chaotically in all directions and colliding with one another and with the walls of their container. Beyond this, there is no structure—the molecules are distributed essentially randomly in space, traveling in arbitrary directions at speeds that are distributed randomly about an average determined by the gas temperature . The pressure exerted by a gas is the result of the innumerable impacts of the molecules on the container walls and appears steady to human senses because so many collisions occur each second on all sections of the walls. More subtle properties such as heat conductivity , viscosity (resistance to flow), and diffusion are attributed to the molecules themselves carrying the mechanical quantities of energy , momentum , and mass, respectively. These are called transport properties, and the rate of transport is dominated by the collisions between molecules, which force their trajectories into tortuous shapes. The molecular collisions are in turn controlled by the forces between the molecules and are described by the laws of mechanics.
Thus, gases are treated as a large collection of tiny particles subject to the laws of physics . Their properties are attributed primarily to the motion of the molecules and can be explained by the kinetic theory of gases . It is not obvious that this should be the case, and for many years a static picture of gases was instead espoused , in which the pressure, for instance, was attributed to repulsive forces between essentially stationary particles pushing on the container walls. How the kinetic-molecular picture finally came to be universally accepted is a fascinating piece of scientific history and is discussed briefly below in the section Kinetic theory of gases . Any theory of gas behaviour based on this kinetic model must also be a statistical one because of the enormous numbers of particles involved. The kinetic theory of gases is now a classical part of statistical physics and is indeed a sort of miniature display case for many of the fundamental concepts and methods of science . Such important modern concepts as distribution functions, cross sections, microscopic reversibility, and time-reversal invariance have their historical roots in kinetic theory, as does the entire atomistic view of matter.

Numerical magnitudes
When considering various physical phenomena, it is helpful for one to have some idea of the numerical magnitudes involved. In particular, there are several characteristics whose values should be known, at least within an order of magnitude (a factor of 10), in order for one to obtain a clear idea of the nature of gaseous molecules. These features include the size, average speed, and intermolecular separation at ordinary temperatures and pressures. In addition, other important considerations are how many collisions a typical molecule makes in one second under these conditions and how far such a typical molecule travels before colliding with another molecule. It has been established that molecules have sizes on the order of a few angstrom units (1 Å = 10 −8 centimetre [cm]) and that there are about 6 × 10 23 molecules in one mole , which is defined as the amount of a substance whose mass in grams is equal to its molecular weight (e.g., 1 mole of water, H 2 O , is 18.0152 grams). With this knowledge, one could calculate at least some of the gas values. It is interesting to see how the answers could be estimated from simple observations and then to compare the results to the accepted values that are based on more precise measurements and theories.
One of the easiest properties to work out is the average distance between molecules compared to their diameter; water will be used here for this purpose. Consider 1 gram of H 2 O at 100° C and atmospheric pressure , which are the normal boiling point conditions. The liquid occupies a volume of 1.04 cubic centimetres (cm 3 ); once converted to steam it occupies a volume of 1.67 × 10 3 cm 3 . Thus, the average volume occupied by one molecule in the gas is larger than the corresponding volume occupied in the liquid by a factor of 1.67 × 10 3 /1.04, or about 1,600. Since volume varies as the cube of distance, the ratio of the mean separation distance in the gas to that in the liquid is roughly equal to the cube root of 1,600, or about 12. If the molecules in the liquid are considered to be touching each other, the ratio of the intermolecular separation to the molecular diameter in ordinary gases is on the order of 10 under ordinary conditions. It should be noted that the actual separation and diameter cannot be determined in this way; only their ratio can be calculated.
It is also relatively simple to estimate the average speed of gas molecules. Consider a sound wave in a gas, which is just the propagation of a small pressure disturbance. If pressure is attributed to molecular impacts on a test surface, then surely a pressure disturbance cannot travel faster than the molecules themselves. In other words, the average molecular speed in a gas should be somewhat greater than the speed of sound in the gas. The speed of sound in air at ordinary temperatures is about 330 metres per second (m/s), so the molecular speed will be estimated here to be somewhat greater, say, about 5 × 10 4 centimetres per second (cm/s). This value depends on the particular gas and the temperature, but it will be sufficient for the kind of estimates sought here.
The average molecular speed, along with an observed rate of the diffusion of gases, can be used to estimate the length and tortuosity of the path traveled by a typical molecule. If a bottle of ammonia is opened in a closed room, at least a few minutes pass before the ammonia can be detected at a distance of just one metre. (Ammonia, NH 3 , is a gas; the familiar bottle of “ammonia” typically seen is actually a solution of the gas in water.) Yet, if the ammonia molecules traveled directly to an observer at a speed somewhat faster than that of sound, the odour should be detectable in only a few milliseconds. The explanation for the discrepancy is that the ammonia molecules collide with many air molecules, and their paths are greatly distorted as a result. For a quantitative estimate of the diffusion time, a more controlled system must be considered, because even gentle stray air currents in a closed room greatly speed up the spreading of the ammonia. To eliminate the effect of such air currents, a closed tube—say, a glass tube one centimetre in diameter and one metre in length—can be used. A small amount of ammonia gas is released at one end, and both ends are then closed. In order to measure how long it takes for the ammonia to travel to the other end, a piece of moist red litmus paper might be used as a detector; it will turn blue when the ammonia reaches it. This process takes quite a long time—about several hours—because diffusion occurs at such a slow rate. In this case, the time will be taken to be approximately 3 hours, or roughly 10 4 seconds (s). During this time interval, a typical ammonia molecule actually travels a distance of (5 × 10 4 cm/s)(10 4 s) = 5 × 10 8 cm = 5,000 kilometres (km), roughly the distance across the United States . In other words, such a molecule travels a total distance of five million metres in order to progress a net distance of only one metre.
The solution to a basic statistical problem can be used to estimate the number of collisions such a typical diffusing molecule experienced ( N ) and the average distance traveled between collisions ( l ), called the mean free path . The product of N and l must equal the total distance traveled—i.e., N l = 5 × 10 8 cm. This distance can be thought of as a chain 5,000 km long, made up of N links, each of length l . The statistical question then is as follows: If such a chain is randomly jumbled, how far apart will its ends be on the average? This end-to-end distance corresponds to the length of the diffusion tube (one metre). This is a venerable statistical problem that recurs in many applications. One of the more vivid ways of illustrating the concept is known as the “ drunkard’s walk .” In this scenario a drunkard takes steps of length l but, because of inebriation, takes them in random directions. After N steps, how far will he be from his starting point? The answer is that his progress is proportional not to N but to N 1/2 . For example, if the drunkard takes four steps, each of length l , he will end up at a distance of 2 l from his starting point. Gas molecules move in three dimensions, whereas the drunkard moves in two dimensions; however, the result is the same. Thus, the square root of N multiplied by the length of the mean free path equals the length of the diffusion tube: N 1/2 l = 10 2 cm. From the equations for N l and N 1/2 l , it can readily be calculated that N = 2.5 × 10 13 collisions and l = 2.0 × 10 -5 cm. The mean time between collisions, τ, is found by dividing the time of the diffusion experiment by the number of collisions during that time: τ = (10 4 )/(2.5 × 10 13 ) = 4 × 10 -10 seconds between collisions, corresponding to a collision frequency of 2.5 × 10 9 collisions per second. It is thus understandable that gases appear to be continuous fluids on ordinary scales of time and distance.
States of Matter
- States Of Matter: Solid, Liquid, And Gas
What is Matter in Chemistry?
As discovered by scientists,
The matter is made up of very tiny particles and these particles are so small that we cannot see them with naked eyes.
It has been observed that matter exists in nature in different forms. Some substances are rigid and have a fixed shape like wood and stone; some substances can flow and take the shape of their container like water, while there are forms of matter that do not have definite shape or size such as air.
Table of Contents
- Matter Definition
Recommended Videos
Solid definition, liquid definition.
- Gas Definition
Bose-Einstein Condensates
- Frequently Asked Questions – FAQs
Matter can be classified into different categories based on the physical properties exhibited by them and the states in which they exist; these are called states of matter.
Following are the basic three states of matter :
Apart from the above mentioned three, there are 2 more states of matter which we do not see in our everyday life. They are Plasma & Bose-einstein condensate .
Changes in the characteristics of matter related with external influences such as pressure and temperature separate states of matter. A discontinuity in one of those qualities frequently distinguishes states: rising the temperature of ice, for example, generates a discontinuity at 0 °C (32 °F) as energy flows into a phase transition rather than temperature rise.
Matter Definition Chemistry
Chemistry is the study of the composition of matter and its transformation. Another term often considered synonymous with matter is substance, but a substance has a more limited definition in chemistry. Chemistry deals with the study of behaviour of – matter Chemistry is concerned with the – Composition, structure and properties of matter and the phenomenon which occurs when different kinds of matter undergo changes.
Matter theory covers the changing ideas and systems that were used to describe and explain the material world. A large part of matter theory was based on a theory of the elements.
Matter In Our Surroundings – States of Matter

- In solids, particles are tightly or closely packed.
- The gaps between the particles are tiny and hence it is tough to compress them.
- Solid has a fixed shape and volume.
- Due to its rigid nature, particles in solid can only vibrate about their mean position and cannot move.
- Force of attraction between particles is adamant.
- The rate of diffusion in solids is very low.
- An example of solids: solid ice, sugar, rock, wood, etc.

- In a liquid state of matter, particles are less tightly packed as compared to solids.
- Liquids take the shape of the container in which they are kept.
- Liquids are difficult to compress as particles have less space between them to move.
- Liquids have fixed volume but no fixed shape.
- The rate of diffusion in liquids is higher than that of solids.
- Force of attraction between the particles is weaker than solids.
- Example of a liquid state of matter: water, milk, blood, coffee, etc.
Gas Definition
- In gases, particles are far apart from each other.
- Force of attraction between the particles is negligible, and they can move freely.
- Gases have neither a fixed volume nor a fixed shape.
- The gaseous state has the highest compressibility as compared to solids and liquids.
- The rate is diffusion is higher than solids and liquids.
- The kinetic energy of particles is higher than in solids and liquids.
- An example of gases: air, helium , nitrogen, oxygen, carbon dioxide, etc.
- Plasma is a not so generally seen form of matter. Plasma consists of particles with extremely high kinetic energy. Electricity is used to ionize noble gases and make glowing signs, which is essentially plasma.
- Superheated forms of plasma are what stars are.
- Discovered in 1995, Bose-Einstein condensates were made with the help of the advancements in technology.
- Carl Weiman and Eric Cornell cooled a sample of rubidium with the help of magnets and lasers to within a few degrees of absolute zero.
- At the said temperature, the motion of the molecules becomes negligible. As this brings down the kinetic energy, the atoms no longer stay separate, but they begin to clump together. As the atoms join together they form a super-atom.
- Light slows down as it passes through a BEC helping scientists to study more about the nature of light as a wave and particle.
- BEC’s also show properties of a superfluid which implies, that it flows without friction.
Related Videos
Ideal gas equation & its applications.

Kinetic Theory of Gases

Frequently Asked Questions – FAQs
What are the three common states of matter.
The common thing among the three states of matter is-they consist of tiny, small particles. They have a specific mass and can take up space. There is a volume in these three states. In these three states ‘atoms have the strength of attractions between them.
Can matter be created?
In addition, the first law of thermodynamics does not state that matter can not be created or destroyed, but rather that the total amount of energy in a closed system can not be created or destroyed, although it can be modified from one form to another.
Is matter created or destroyed?
There is a scientific law called the Mass Conservation Law, which Antoine Lavoisier discovered in 1785. It states in its most compact form: matter is not created or destroyed. The universe’s total mass and energy is constant.
Is matter-energy?
The mass of these three particles is less than a neutron’s mass, so each of them still gets some energy. So the same thing is really power and matter. Fully interchangeable. So in a way, all facets of the same thing are energy, matter, space and time.
What is Einstein’s theory of relativity?
In 1905, Albert Einstein determined that for all non-accelerating observers, the laws of physics were the same and that the speed of light in a vacuum was independent of all observers ‘ movement. This was the special relativity theory.
To know more about the states of matter, properties of matter and more, register with BYJU’S and download our app.
- Change of states of matter

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
It is very helpful 👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍
this vdieo is very nice and we will learn intersting
Thank you So Much it helped me very much in my school Project awesome information. Thank you very much The video is informative.
It is very good aap i can give a practice for it
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

We need to consider alternatives to dark matter that better explain cosmological observations
Adjunct professor, Physics, L’Université d’Ottawa/University of Ottawa
Disclosure statement
Rajendra Gupta receives unconditional research funding from Macronix Research Corporation. He is affiliated with the American Physical Society, Royal Astronomical Society, European Astronomical Society, and CASCA.
University of Ottawa provides funding as a founding partner of The Conversation CA-FR.
University of Ottawa provides funding as a member of The Conversation CA.
View all partners
Do constants of nature — the numbers that determine how things behave, like the speed of light — change over time as the universe expands ? Does light get a little tired travelling vast cosmic distances? It was believed that dark matter and dark energy explained these cosmological phenomena, but recent research indicates that our universe has been expanding without dark matter or dark energy.
Doing away with dark matter and dark energy resolves the “ impossible early galaxy problem ,” that arises when trying to account for galaxies that do not adhere to expectations regarding to size and age. Finding an alternative to dark matter and energy that complies with existing cosmological observations, including galaxy distribution , is possible.
Read more: How old is the universe exactly? A new theory suggests that it's been around for twice as long as believed
- Dark matter
Dark matter is a hypothetical form of matter that does not interact with ordinary matter in any way except through gravity. It was proposed as a theoretical way to explain our astrophysical and cosmological observations. Ordinary matter can travel through the dark matter without any resistance and vice versa.
In space, gravitational pull determines the speed at which an object orbits. A higher speed than expected from surrounding orbiting objects is attributed to the existence and gravitational pull of dark matter .
The gravitational pull of dark matter can also bend light rays, causing a gravitational lensing effect just like normal matter. This allows for the measurement of dark matter in the object causing the bending, such as in galaxies and clusters of galaxies.
The most robust support for the existence of dark matter comes from the tiny variations observed in the cosmic microwave background radiation (remnant radiation from the big bang), measured with increasingly high precision .
Another argument for the existence of dark matter is that large-scale structures of the universe, such as galaxies, would not be able to form without the dark matter within the limited age of the universe.

Alternative theories
There are alternatives to dark matter that account for many astrophysical observations. The oldest and most popular theory is modified Newtonian dynamic (MoND), which suggests that the Newtonian inverse square law of gravitational attraction force is a simplified version of a complete force that becomes perceptible only at very large distances when Newtonian force becomes negligible.
Another alternative is a version of MoND that includes Einstein’s relativistic effects and explains observations where MoND is limited, such as cosmic microwave background radiation . Then there is the proposed theory of retarded gravity that also claims to comply with such observations.
Astronomers are surprised to learn that many observations show a complete absence of dark matter or dark matter-deficient structures . This leads one to question its existence.
One then has to find an explanation of what might have created the problem, such as tidal forces exerted by the passing of nearby galaxies stripping away dark matter . Even the mass of the Milky Way has recently been determined to be much smaller than expected from cosmology .
Does dark matter exist?
Recent discoveries create doubt around the existence of dark matter. Despite extensive research and billions of dollars in investment, there has been no direct detection of any dark matter.
The dark energy theory negates the gravitational pull of matter, causing the universe to expand faster with time, as observed. The interrelated variation of constants of nature, dubbed covarying coupling constants (CCC), achieve the same effect by weakening the gravitational pull and other forces of nature with time, eliminating the need for dark energy.
Combined with the tired light (TL) effect which posits that light slows down as a result of energy loss, such a cosmological model has no room for dark matter . The CCC approach could also replace the dark energy-like constant considered responsible for the extremely rapid expansion of the universe following the Big Bang, called inflation .
The age of the universe is determined from the historical expansion rate of the universe, and can vary depending on the model used for the expansion. Measuring the redshift of exploding stars, called supernovae type 1a, and their observed brightness can determine the expansion rate.
Redshift is the lowering of spectral line frequencies depending on the recessional speed of the emitting object, similar to the frequency of a receding ambulance siren. By allowing the redshift due to the tired light effect to coexist with the expansion redshift, the universe’s expansion rate is reduced, and age of the universe increases.

This new model predicts the universe is older than we think it is — 26.7 billion years in the CCC cosmology compared to 13.8 in the standard cosmology — and allows galaxies and their clusters to form without dark matter. The increase in the age of the universe in early times when structures started forming was up to 100 times larger in the new model.
The absence of dark matter that reduces the gravitational force and increases the time for collapsing the matter to form structures is greatly overcompensated by increased age in the CCC model.
Slowing time
The expansion of the universe causes time to appear slowed down when observing distant galaxies . The CCC+TL model complies with observations showing a time dilation effect that appears to slow down the clock in distant objects.
Emerging criticisms of the CCC+TL model rely on flawed hypotheses, such as the deficiencies presented by the tired light concept, or incorrect analyses, including redshift analysis of cosmic microwave background temperatures . A single free parameter in the CCC cosmology determines the variation of all constants that asymptotically approach their respective constant values. As in the standard cosmology, CCC cosmology has only two free parameters. Adding tired light to CCC does not require any additional free parameter.
The standard cosmology model requires dark matter to fit observations, such as accounting for redshift when measuring the brightness of supernovae. Dark matter is also used to explain physical processes such as galaxy rotation curves, galaxy clusters or gravitational lensing. Using CCC+TL cosmology means that we must seriously consider alternative physical processes to account for astrophysical observations that had previously been attributed to dark matter.
- Dark energy
- Cosmic microwave background
- Time dilation

Project Manager

Management Information Systems & Analytics – Limited Term Contract

Publications Manager

Audience Insight Officer

Director, Student Administration
Help | Advanced Search
Nuclear Theory
Title: neutron matter from local chiral eft interactions at large cutoffs.
Abstract: Neutron matter is an important many-body system that provides valuable constraints for the equation of state (EOS) of neutron stars. Neutron-matter calculations employing chiral effective field theory (EFT) interactions have been extensively used for this purpose. Among the various many-body methods, quantum Monte Carlo (QMC) methods stand out due to their nonperturbative nature and the achievable precision. However, QMC methods require local interactions as input, which leads to the appearance of stronger regulator artifacts as compared to non-local interactions. To circumvent this, we employ large-cutoff interactions derived within chiral EFT (400 MeV $\leq \Lambda_c \leq$ 700 MeV) for studies of pure neutron matter. These interactions have been adjusted to nucleon-nucleon scattering phase shifts, the triton binding energy, as well as the triton beta-decay half life. We find that regulator artifacts significantly decrease with increasing cutoff, leading to a significant reduction of uncertainties in the neutron-matter EOS. We discuss implications for the symmetry energy and demonstrate how our new calculations lead to a reduction in the theoretical uncertainty of predicted neutron-star radii by up to 30% for low-mass stars.
| Comments: | 7 pages, 3 figures |
| Subjects: | Nuclear Theory (nucl-th); High Energy Astrophysical Phenomena (astro-ph.HE) |
| Report number: | LA-UR-23-32163 |
| Cite as: | [nucl-th] |
| (or [nucl-th] for this version) |
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- INSPIRE HEP
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
Physical Review B
Covering condensed matter and materials physics.
- Collections
- Editorial Team
Perturbation theory analysis of the strain-dependent superconducting phase diagram for Sr 2 RuO 4
J. j. deisz, phys. rev. b 110 , 014509 – published 11 july 2024.
- No Citing Articles
- INTRODUCTION
- MODEL AND NUMERICAL METHODS
- RESULTS WITHOUT STRAIN
- RESULTS WITH STRAIN
- ACKNOWLEDGMENTS
Previously, it was shown that a superconducting state dominated by singlet d x 2 − y 2 intraband pairs emerges from the fluctuation exchange approximation (FLEX) applied to a realistic model for Sr 2 RuO 4 , a result that is increasingly aligned with experimental data. Here we apply FLEX to model the strain-dependent phase diagram of Sr 2 RuO 4 and show that we are able to reproduce its unusual features. This adds weight to the argument that a predominantly d x 2 − y 2 singlet pairing state represents a reasonable starting point for describing the superconducting properties of Sr 2 RuO 4 .
- Received 25 July 2023
- Revised 13 June 2024
- Accepted 1 July 2024
DOI: https://doi.org/10.1103/PhysRevB.110.014509
©2024 American Physical Society
Physics Subject Headings (PhySH)
- Research Areas
Authors & Affiliations
- Department of Physics, California Lutheran University , Thousand Oaks, California 91360, USA
Article Text (Subscription Required)
References (subscription required).
Vol. 110, Iss. 1 — 1 July 2024
Access Options
- Buy Article »
- Log in with individual APS Journal Account »
- Log in with a username/password provided by your institution »
- Get access through a U.S. public or high school library »

Authorization Required
Other options.
- Buy Article »
- Find an Institution with the Article »
Download & Share
Calculated FLEX results for T c vs dimensionless coupling strength parameter, g o . While experimental quasiparticle renormalizations are most accurately represented with g o ∼ 0.67 , a g o value near 0.4 is more consistent with the observed T c for Sr 2 RuO 4 .
Quasiparticle energies E qp vs momentum k / π along the cuts Γ − M x and Γ − M y . In the unstrained case, η = 0 , these cuts are degenerate, i.e., x = y . In the strained case, η = 0.002 , excitations along the x ( y ) are lower (higher) in energy in comparison the unstrained case, with the van Hove singularity at M x driven toward the Fermi level where E qp = 0 .
(a) Quasiparticle energy at the van Hove singularity E vH vs strain parameter η at various coupling strengths g o , and (b) E vH normalized by its zero strain value vs normalized strain parameter, η / η vH = ɛ x x / ɛ vH . Stronger coupling leads to a downward dynamical renormalization of E vH ( η ) and, consequently, the critical strain value η vH , where E vH = 0 , is reduced as well. However, E vH vs η follows the same trends at all couplings as observed in the scaled plot.
Superconducting order parameter m p vs temperature T for several strain parameters at a coupling strength g o = 0.40 for which η vH ∼ 0.06 . The unstrained result, η = 0.00 , shows a typical second order transition at T c ∼ 9.5 K . For the intermediate strain value of η = 0.04 < η vH an elevated T c ∼ 11.5 K is observed and smooth, single-phase behavior for T < T c . At η = 0.06 ∼ η vH , a maximum T c ∼ 15.8 K is obtained and single phase behavior persists below the transition. The subsequent curve for η = 0.07 > η vH , shows a dramatic reduction in both T c and the overall strength of the order parameter.
Relative superconducting transition temperature T c ( η ) / T c ( η = 0 ) vs normalized strain η / η vH for different coupling strengths g o . For all g o , T c drops off rapidly for η / η vH > 1 , consistent with what is observed for Sr 2 RuO 4 . For g o = 0.40 , which corresponds to T c ( η = 0 ) ∼ 9.5 K , we observe a pronounced peak in T c at or near η / η vH ∼ 1 in alignment with experimental results for Sr 2 RuO 4 . Thus, when the coupling strength is set to values consistent with the low T c values observed in Sr 2 RuO 4 , FLEX-based three-band Hubbard model results accurately represent the unusual strain-dependent phase diagram for Sr 2 RuO 4 .
Sign up to receive regular email alerts from Physical Review B
- Forgot your username/password?
- Create an account
Article Lookup
Paste a citation or doi, enter a citation.
The attack on Donald Trump unleashes a flood of misinformation
The left thinks the shooting was just a performance; the right sees an inside job. will the truth matter.

“A merican politics has often been an arena for angry minds,” the political scientist Richard Hofstadter wrote 60 years ago in his classic essay, “The Paranoid Style in American Politics”. Yet across many eras, this discourse of “heated exaggeration, suspicion and conspiratorial fantasy” festered largely on the fringe. Then the internet made the fringe accessible to everyone, amplifying dissonance and disinformation.
In the initial hours after the shocking assassination attempt against Donald Trump on Saturday evening, meme-makers and influencers on the left and right came to fast agreement about one thing: the shooting must have been orchestrated. Some on the left described it as a false-flag operation staged to make Mr Trump look invincible and bolster his election prospects. They pointed to the way Mr Trump paused to pose for photos with his fist in the air and blood streaking down his cheek as evidence that the attack must have been choreographed by the candidate’s own image-makers.
The right’s citizen-pundits—and even some elected office-holders—reckoned that the attempt to kill Mr Trump looked like an inside job. Within minutes of the shooting, media mogul Elon Musk endorsed Mr Trump and later suggested to his 190m followers that the failure of the Secret Service to stop the shooter may have been “deliberate”. Mike Collins, a Republican congressman from Georgia, asserted that “Joe Biden sent the orders.”
Conspiracy theories about a shocking institutional failure often take hold when an alternative explanation—incompetence—is unsatisfying. Mr Trump’s attacker, identified by the FBI as a 20-year-old Pennsylvania man named Thomas Matthew Crooks, apparently managed to mount a nearby roof with an AR -15-style rifle in hand without being stopped by the police. This might be judged as a stunning failure of professional practice by the Secret Service; President Joe Biden has promised an independent review of what went wrong. In right-wing media, however, the failure to stop the shooter is presented as evidence of deliberate intrigue. Rumours spread quickly on Instagram that Alejandro Mayorkas, the Secretary of Homeland Security, who has been villainised by Republicans for his handling of the southern border, rebuffed the Trump campaign’s requests for better protection.
Such views are not confined to Reddit obsessives pounding their keyboards late at night; they are commonplace among ordinary MAGA Republicans. Sandra Chase, a Republican delegate from Brooklyn who is attending this week’s Republican National Convention in Milwaukee, says she thinks the assassination attempt was an inside job and that the FBI will cover up the real crime. She and like-minded sceptics are likely to have plenty to talk about as they debate their theories in the days and weeks ahead. On Sunday, the FBI reported that Mr Crooks appears to have acted alone, a finding that was unaccompanied by the release of evidence to back it up.
Ms Chase said she belongs to a chat group that is concerned about another angle: that diversity mandates within the Secret Service forced the organisation to hire lots of ineffective women, which exacerbated the danger faced by Mr Trump. Photos from the attack that show female agents who are smaller than Mr Trump rushing to provide cover for him have been transformed into misogynistic memes across social-media platforms.
On the evidence available so far, Mr Crooks’s motives and political views are largely a mystery. News outlets reported that he was registered as a Republican but gave a $15 donation to a liberal political action committee, the Progressive Turnout Project, in January 2021. He apparently grew up in middle-class circumstances and made little impression on schoolmates.
The online far right has already invented a profile of him that fits their worldview, however. Minutes after authorities released the shooter’s identity, myriad fake Instagram accounts appeared under his name with invented biographies that describe Mr Crooks as transgender, Jewish and a Black Lives Matter supporter. They and other influencers cited a comment Mr Biden reportedly made on a private call to donors last week: “We’re done talking about the debate, it’s time to put Trump in a bullseye.”
Disinformation experts expect such narratives will continue to spread, even as details of the shooter’s motives and background become clearer. New information is unlikely to change the minds of those who already believe that one party or the other planned the assassination attempt. “The defining feature of conspiracy theories is that they explain everything, so they’re pretty resistant to individual details,” says Jonathan Stray of the University of California, Berkeley.
How much does such widespread confusion matter? To date, American democracy has somehow lurched through its crises, despite the enduring persuasiveness of Hofstadter’s observations. This week may seem more worrisome than usual because of the violence inflicted on Mr Trump and the normalisation of discourse about such radical action online. Some angry progressives are lamenting that the bullet grazed Mr Trump’s ear, missing his skull by millimetres. Destiny, a leftist social-media commentator, told his 250,000 X followers that Mr Trump and his supporters will “reap what they sow, and I’m here to watch the harvest”. Meanwhile, on the right, a post in a Florida Proud Boys Telegram channel depicts Mr Trump with blazing red eyes above a banner headline: “THIS IS WAR!!!” ■
Stay on top of American politics with The US in brief , our daily newsletter with fast analysis of the most important electoral stories, and Checks and Balance , a weekly note from our Lexington columnist that examines the state of American democracy and the issues that matter to voters.
Explore more
More from united states.

What was the motive of Trump’s would-be assassin?
A frenzied media hunt is under way

Donald Trump survives an assassination attempt
The shooting is a dark turn in an already chaotic presidential campaign

Gretchen Whitmer would like to be America’s first woman president
Could abortion rights and “fixing the damn roads” take Michigan’s governor to the White House?
The disorganisation of the Democratic rebels against Joe Biden
Why the party is failing to mount a concerted push to replace its nominee
Biden survives his “big boy” press conference
His performance wasn’t perfect and the Democratic Party rebellion is far from over
The Republicans’ policy platform previews the coming campaign
Social conservatives and fiscal hawks will be disappointed. Opponents of immigration will not
Nuclear-matter saturation and symmetry energy within Δ -full chiral effective field theory
- Jiang, W. G.
- Forssén, C.
Nuclear saturation and the symmetry energy are key properties of low-energy nuclear physics that depend on fine details of the nuclear interaction. The equation of state around saturation is also an important anchor for extrapolations to higher densities and studies of neutron stars. Here we develop a unified statistical framework that uses realistic nuclear forces to link the theoretical modeling of finite nuclei and infinite nuclear matter. We construct fast and accurate emulators for nuclear-matter observables and employ an iterative history-matching approach to explore and reduce the enormous parameter domain of Δ -full chiral interactions. We perform rigorous uncertainty quantification and find that model calibration including 16 O observables gives saturation predictions that are more precise than those that only use few-body data.
- Nuclear Theory
- Election 2024
- Entertainment
- Photography
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- Auto Racing
- 2024 Paris Olympic Games
- Movie reviews
- Book reviews
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
In the wake of Trump’s attempted assassination, investigators search for clues around the motive
Donald Trump’s campaign says he is “fine” after being whisked off the stage at a rally in Butler, Pennsylvania, after what law enforcement officials are treating as an apparent assassination attempt.
A campaign rally site for Republican presidential candidate former President Donald Trump is empty and littered with debris Saturday, July 13, 2024, in Butler, Pa. (AP Photo/Evan Vucci)
- Copy Link copied
Republican presidential candidate former President Donald Trump is helped off the stage by U.S. Secret Service agents at a campaign event in Butler, Pa., on Saturday, July 13, 2024. (AP Photo/Gene J. Puskar)
This June 3, 2022 still image taken from video provided by the Bethel Park School District shows student Thomas Matthew Crooks in the 2022 Bethel Park High School Commencement in Bethel Park, Pa. (The Bethel Park School District via AP)
Law enforcement block a street in Bethel Park, Pa., that they say is near a residence of Thomas Matthew Crooks, the suspected shooter of former President Donald Trump, Sunday, July 14, 2024. (AP Photo/Joshua A. Bickel)
This 2021 photo provided by Bethel Park School District shows student Thomas Matthew Crooks who graduated from Bethel Park High School with the Class of 2022, in Bethel Park, Pa. Crooks was identified by the FBI as the shooter involved in an assassination attempt of former President Donald Trump at a campaign rally on Saturday, July 13, 2024, in Butler, Pa. (Bethel Park School District via AP)
WASHINGTON (AP) — The 20-year-old man who tried to assassinate former President Donald Trump first came to law enforcement’s attention at Saturday’s rally when spectators noticed him acting strangely outside the campaign event. The tip sparked a frantic search but officers were unable to find him before he managed to get on a roof, where he opened fire.
In the wake of the shooting that killed one spectator, investigators were hunting for any clues about what may have drove Thomas Matthew Crooks, of Bethel Park, Pennsylvania, to carry out the shocking attack. The FBI said they were investigating it as a potential act of domestic terrorism , but the absence of a clear ideological motive by the man shot dead by the Secret Service led conspiracy theories to flourish.
“I urge everyone — everyone, please, don’t make assumptions about his motives or his affiliations,” President Joe Biden said in remarks Sunday from the White House . “Let the FBI do their job, and their partner agencies do their job. I’ve instructed that this investigation be thorough and swift.”
The FBI said it believes Crooks, who had bomb-making materials in the car he drove to the rally, acted alone. Investigators have found no threatening comments on social media accounts or ideological positions that could help explain what led him to target Trump before the Secret Service rushed the presumptive Republican presidential nominee off the stage, his face smeared with blood.
Trump said on social media the upper part of his right ear was pierced in the shooting, but advisers said he was “great spirits” ahead of his arrival Sunday in Milwaukee for the Republican National Convention. Two spectators were critically injured, while a former fire chief from the area, Corey Comperatore was killed. Pennsylvania’s governor said Comperatore, 50, died a hero by diving onto his family to protect them.
Relatives of Crooks didn’t respond to numerous messages from The Associated Press. His father, Matthew Crooks, told CNN late Saturday that he was trying to figure out “what the hell is going on” but wouldn’t speak about his son until after he talked to law enforcement. An FBI official told reporters that Crooks’ family is cooperating with investigators.
Several rallygoers reported to local officers that Crooks was acting suspiciously and pacing near the magnetometers, according to a law enforcement official who spoke on the condition of anonymity because they were not authorized to discuss the investigation. Officers were then told Crooks was climbing a ladder, the official said. Officers searched for him but were unable to find him before he made it to the roof, the official added.
Butler County Sheriff Michael Slupe told the AP that a local officer climbed to the roof and encountered Crooks, who saw the officer and turned toward him just before the officer dropped down to safety. Slupe said the officer couldn’t have wielded his own gun under the circumstances. The officer retreated down the ladder, and Crooks quickly took a shot toward Trump, and that’s when Secret Service snipers shot him, according to two officials who spoke to AP on condition of anonymity to discuss an ongoing investigation.
FBI officials said Sunday that they were combing Crooks’ background and social media activities while working to get access to his phone. The chatting app Discord, a social media platform popular with people playing online games, said Crooks appears to have had an account but used it rarely and not in the last several months. There’s no evidence he used his account to promote violence or discuss his political views, a Discord spokesperson said.
Crooks’ political leanings were not immediately clear. Records show Crooks was registered as a Republican voter in Pennsylvania, but federal campaign finance reports also show he gave $15 to a progressive political action committee on Jan. 20, 2021, the day Biden was sworn into office.
Crooks graduated from Bethel Park High School in 2022. In a video of the school’s graduation ceremony posted online, Crooks can be seen crossing the stage to receive his diploma, appearing slight of build and wearing glasses. The school district said it will cooperate fully with investigators. His senior year, Crooks was among several students given an award for math and science, according to a Tribune-Review story at the time.
Crooks tried out for the school’s rifle team but was turned away because he was a bad shooter, said Frederick Mach, a current captain of the team who was a few years behind Crooks at the school.
Jason Kohler, who said he attended the same high school but did not share any classes with Crooks, said Crooks was bullied at school and sat alone at lunch time. Other students mocked him for the clothes he wore, which included hunting outfits, Kohler said.
“He was bullied almost every day,” Kohler told reporters. “He was just a outcast, and you know how kids are nowadays.”
Crooks worked at a nursing home as a dietary aide, a job that generally involves food preparation. Marcie Grimm, the administrator of Bethel Park Skilled Nursing and Rehabilitation, said in a statement she was “shocked and saddened to learn of his involvement.” Grimm added that Crooks had a clean background check when he was hired.
What to know :
- Timeline of events : How the assassination attempt on former President Donald Trump unfolded.
- On the suspect : What we know about the 20-year-old man who tried to assassinate Trump .
- Motive still not known : The FBI said that it had not yet determined a motive , but the agency believed that the shooter acted alone.
- Biden’s response : The president appealed for “unity” and said he was ordering an independent security review.
- A “man of conviction” : Victim Corey Comperatore, a former fire chief, used his body to shield his family from gunfire.
A blockade had been set up Sunday preventing traffic near Crooks’ house, which is in an enclave of modest brick houses in the hills outside Pittsburgh and about an hour’s drive from the site of the Trump rally. Police cars were stationed at an intersection near the house and officers were seen walking through the neighborhood.
Crooks used an AR-style rifle, which authorities said they believe was purchased by his father. Kevin Rojek, FBI special agent in charge in Pittsburgh, said that investigators do not yet know if he took the gun without his father’s permission.
A video posted to social media and geolocated by AP shows Crooks wearing a gray t-shirt with a black American flag on the right arm lying motionless on the roof of a manufacturing plant just north of the Butler Farm Show grounds where Trump’s rally was held.
The roof where Crooks lay was less than 150 meters (164 yards) from where Trump was speaking, a distance from which a decent marksman could reasonably hit a human-sized target. That is a distance at which U.S. Army recruits must hit a scaled human-sized silhouette to qualify with the M-16 rifle.
Images of Crooks’ body reviewed by AP show he appears to have been wearing a T-shirt from Demolition Ranch, a popular YouTube channel that regularly posts videos of its creator firing off handguns and assault rifles at targets that include human mannequins.
Matt Carriker, the Texas-based creator of Demolition Ranch, did not respond to a phone message or email on Sunday, but posted a photo of Crooks’ bloody corpse wearing his brand’s T-shirt on social media with the comment “What the hell.”
Mustian reported from New York and Balsamo reported from Chicago. Associated Press writers Marc Levy in Harrisburg, Pa., Julie Smyth, Lindsey Bahr and Joshua Bickel in Bethel Park, Michael R. Sisak and Randy Herschaft in New York, Michael Kunzelman in Silver Spring, Md., and Colleen Long and Eric Tucker in Washington contributed to this report.


IMAGES
VIDEO
COMMENTS
Explore the basics of states of matter with interactive simulations in a game-like environment on PhET.
State of matter. In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose-Einstein ...
States of Matter
Watch this video to learn about the different states of matter and how they change with temperature and pressure. Khan Academy offers free, world-class education for anyone, anywhere.
Animated tutorial video explaining the three states of matter for kids: solids, liquids and gases, in terms of particle theory. Suitable for year 7-9 high sc...
Learn about the states of matter. Learn the four main states of matter, other states, and phase transitions between them.
There are many states of matter beyond solids, liquids, and gases, including plasmas, condensates, superfluids, supersolids, and strange matter. This module introduces Kinetic Molecular Theory, which explains how the energy of atoms and molecules results in different states of matter. The module also explains the process of phase transitions in matter.
Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases.
An easy-to-understand introduction to the three main states of matter - solids, liquids, and gases.
States Of Matter - Solids, Liquids & Gases | Properties of Matter | Chemistry | FuseSchool Learn the basics about the three well-known States of Matter - solids, liquids and gases.
The states of matter refer to the physical forms that matter can take. There are three main states of matter: solid, liquid, and gas. The state of a substance depends on its temperature and pressure. For example, at room temperature and pressure, water is a liquid. But if the water is heated to a high enough temperature, it will become a gas ...
Learn about the different states of matter and how intermolecular forces affect them. Explore videos, articles, and exercises by topic in the Chemistry library.
Matter is made up of tiny particles called atoms. When these atoms come together, they form molecules. The combination of atoms and molecules in different ways forms three types of matter: solids, liquids, and gases. Kinetic theory explains how particles in matter behave. For example, all matter is composed of particles; particles are in constant, random motion, and particles collide with each ...
The four fundamental states of matter are solid, liquid, gas and plasma, but there others, such as Bose-Einstein condensates and time crystals, that are man-made.
States of Matter Another way that we can describe the properties of matter is the state (also called phase). The amount of energy in molecules of matter determines the state of matter. Matter can exist in one of several different states, including a gas, liquid, or solid state.
The structure of matter at the sub-microscopic level, and the forces between particles, underlies the explanation of macroscopic properties. During 11-14, students gain some appreciation of the nature of the particles (atoms, ions, molecules). At 14-16, you will expand on how these different particles are formed and how this affects the ...
Particle Theory of Matter. Matter is anything that has mass and takes up space. It is a general name we call all the physical things around us. Matter includes things so tiny humans can't see them with their eyes. The Particle Theory of Matter is a scientific model. A scientific model is a way of illustrating ideas, objects and processes so ...
List of states of matter Matter organizes into various phases or states of matter depending on its constituents and external factors like pressure and temperature. In common temperatures and pressures, atoms form the three classical states of matter: solid, liquid and gas. Complex molecules can also form various mesophases such as liquid crystals, which are intermediate between the liquid and ...
Solids, liquids, gases, and plasma are all states of matter. Learn how scientists distinguish among states of matter and how to recognize each.
matter, material substance that constitutes the observable universe and, together with energy, forms the basis of all objective phenomena. At the most fundamental level, matter is composed of elementary particles known as quarks and leptons (the class of elementary particles that includes electrons ). Quarks combine into protons and neutrons ...
The modern atomic theory, proposed about 1803 by the English chemist John Dalton (Figure 2.1.4 2.1. 4 ), is a fundamental concept that states that all elements are composed of atoms. Previously, an atom was defined as the smallest part of an element that maintains the identity of that element.
Any theory of gas behaviour based on this kinetic model must also be a statistical one because of the enormous numbers of particles involved. The kinetic theory of gases is now a classical part of statistical physics and is indeed a sort of miniature display case for many of the fundamental concepts and methods of science.
States of Matter - The matter is classified into solids, liquids, and gases in termed physical classification of matter. Solid, liquids and gas are the three states of matter. Visit BYJU'S to learn more about it.
Cosmology does not need dark matter or dark energy in an expanding universe that allows the constants of nature to evolve, and light loses energy as it travels vast distances.
Density Functional Theory for Matter under Extreme Conditions. This project focuses on the advancement, implementation, and use of finite-temperature density functional theory , especially in orbital-free form, to achieve simulations (ab initio molecular dynamics, path integral MD, and beyond to approximate quantum dynamics) of matter under extreme conditions.
Neutron matter is an important many-body system that provides valuable constraints for the equation of state (EOS) of neutron stars. Neutron-matter calculations employing chiral effective field theory (EFT) interactions have been extensively used for this purpose. Among the various many-body methods, quantum Monte Carlo (QMC) methods stand out due to their nonperturbative nature and the ...
Previously, it was shown that a superconducting state dominated by singlet d x 2 − y 2 intraband pairs emerges from the fluctuation exchange approximation (FLEX) applied to a realistic model for Sr 2 RuO 4, a result that is increasingly aligned with experimental data.Here we apply FLEX to model the strain-dependent phase diagram of Sr 2 RuO 4 and show that we are able to reproduce its ...
The left thinks the shooting was just a performance; the right sees an inside job. Will the truth matter?
Nuclear saturation and the symmetry energy are key properties of low-energy nuclear physics that depend on fine details of the nuclear interaction. The equation of state around saturation is also an important anchor for extrapolations to higher densities and studies of neutron stars. Here we develop a unified statistical framework that uses realistic nuclear forces to link the theoretical ...
Investigators are hunting for any clues about what may have driven Thomas Matthew Crooks to try to assassinate former President Donald Trump. The FBI said Sunday it was investigating the assassination attempt as a potential act of domestic terrorism.