

New to Climate Change?
Hydrogen is the lightest chemical element and the most abundant chemical substance in the universe. Using fossil fuels or clean electricity, we can produce hydrogen gas, which can be stored, transported, and burned to provide power. Unlike most fuels, hydrogen does not produce the greenhouse gas carbon dioxide (CO 2 ) when burned: instead, it yields water. This means that burning hydrogen fuel does not contribute to climate change.
The versatility of hydrogen fuel creates many opportunities to replace fossil fuels in different parts of our economy. It can provide long-term energy storage for the electric power sector, fuel for heavy duty transportation, and heat for industrial processes requiring high temperatures, like steel or concrete production. Today, hydrogen is mainly used in the petrochemical, food processing, and fertilizer industries , and in cars with hydrogen fuel cells. Countries such as Japan are exploring its use in public transportation .
For the climate, not all hydrogen is created equal
Because pure hydrogen is so rare on Earth, the hydrogen we use must be produced from other compounds. However, hydrogen production can have a large environmental impact depending on how it is produced. Today, close to 95 percent of hydrogen production is from fossil fuels like natural gas and coal. As a result, we emit 830 million tonnes of CO 2 each year to produce 74 million tonnes of hydrogen.
A wide range of processes can be used to produce hydrogen, including:
- thermochemical processes like “reforming” natural gas or coal or “gasifying” biomass;
- electrolytic processes that split water into hydrogen and oxygen using electricity;
- photolytic processes that split water into hydrogen and oxygen using sunlight;
- biological processes using algae or bacteria.
Among these options, there are two ways to move toward cleaner hydrogen production that are cheap enough to pursue on a large scale in the near future. One is to combine fossil fuel-based hydrogen production with carbon capture and storage . The second is to use water electrolysis powered by electricity from low-carbon sources, such as renewable energy or nuclear power .
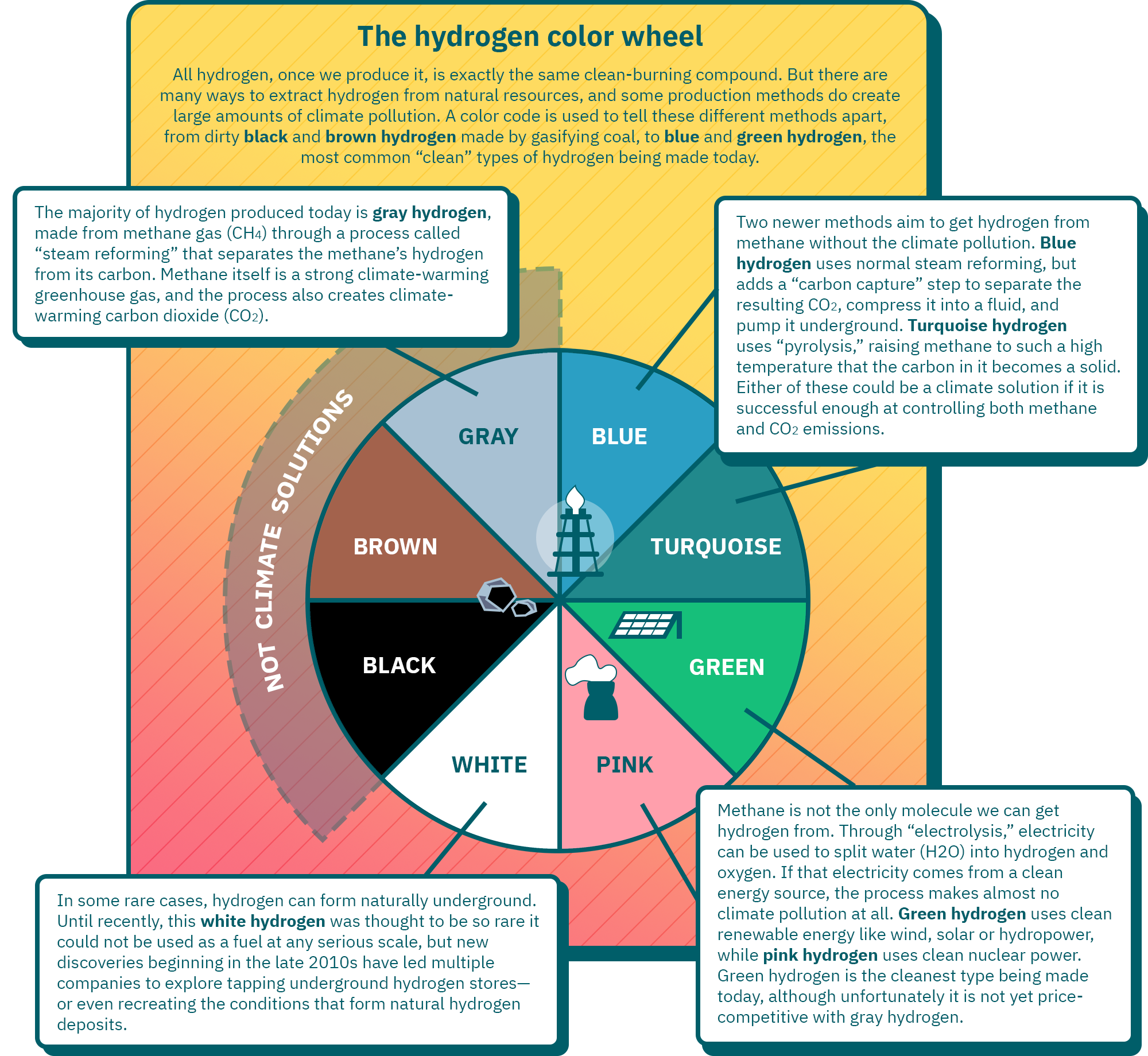
| Type of hydrogen | Description | Could it be a climate solution? |
|---|---|---|
| Black hydrogen | Black hydrogen is made by gasifying anthracite (or "black") coal. | No |
| Brown hydrogen | Black hydrogen is made by gasifying lignite (or "brown") coal. | No |
| Gray hydrogen | The majority of hydrogen produced today is gray hydrogen, made from methane gas (CH ) through a process called “steam reforming” that separates the methane’s hydrogen from its carbon. Methane itself is a strong climate-warming greenhouse gas, and the process also creates climate-warming carbon dioxide (CO ). | No |
| Blue hydrogen | Blue hydrogen, like gray hydrogen, uses steam reforming of methane gas, but adds a “carbon capture” step to separate the resulting CO , compress it into a fluid, and pump it underground. | Yes, if it is successful enough at controlling both methane and CO emissions |
| Turquoise hydrogen | Turquoise hydrogen uses “pyrolysis,” raising methane gas to such a high temperature that the carbon in it becomes a solid. | Yes, if it is successful enough at controlling methane emissions |
| Green hydrogen | Through “electrolysis,” electricity can be used to split water (H O) into hydrogen and oxygen. If that electricity comes from a clean energy source, the process makes almost no climate pollution at all. Green hydrogen uses clean renewable energy like wind, solar or hydropower. | Yes |
| Pink hydrogen | Pink hydrogen, like green hydrogen, uses electrolysis of water, but the electricity is supplied with clean nuclear power. | Yes |
| White hydrogen | In some rare cases, hydrogen can form naturally underground. Until recently, this white hydrogen was thought to be so rare it could not be used as a fuel at any serious scale, but new discoveries beginning in the late 2010s have led multiple companies to explore tapping underground hydrogen stores—or even recreating the conditions that form natural hydrogen deposits. | Yes |
Breathing new life into an old idea
Hydrogen has been in the public imagination since the 1870s, when Jules Verne wrote that “water will be the coal of the future” in his novel The Mysterious Island. However, interest in hydrogen has changed over time. The idea of a “hydrogen economy” was first introduced in the 1970s, to describe using hydrogen as a fuel for the transportation sector at a time when oil prices were rising quickly. Now, the attraction of the hydrogen economy is as a tool for fighting climate change, by replacing fossil fuels in some of the hardest parts of our economy to decarbonize. A key barrier that needs to be overcome to support this transition is the lack of hydrogen infrastructure such as hydrogen pipeline networks, widespread production facilities, and hydrogen fueling stations.
Updated May 23, 2024.

More Resources for Learning
Keep exploring.
With more Explainers from our library:

Renewable Energy

Mining and Metals
Mit climate news in your inbox.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 July 2024
Technological evolution of large-scale blue hydrogen production toward the U.S. Hydrogen Energy Earthshot
- Wanying Wu ORCID: orcid.org/0009-0003-6278-252X 1 ,
- Haibo Zhai ORCID: orcid.org/0000-0001-8585-9559 1 , 2 , 3 &
- Eugene Holubnyak 2
Nature Communications volume 15 , Article number: 5684 ( 2024 ) Cite this article
175 Accesses
Metrics details
- Energy policy
- Environmental impact
Hydrogen potentially has a crucial role in the U.S. transition to a net-zero emissions economy. Learning from large-scale hydrogen projects will boost technological evolution and innovation toward the U.S. Hydrogen Energy Earthshot. We apply experience curves to estimate the evolving costs of blue hydrogen production and to further examine the economic effect on technological evolution of the Inflation Reduction Act’s tax credits for carbon sequestration and clean hydrogen. Learning-by-doing alone can decrease the production cost of blue hydrogen. Without tax incentives, however, it is hard for blue hydrogen production to reach the cost target of $1/kg H 2 . Here we show that the breakeven cumulative production capacity required for gas-based blue hydrogen to reach the $1/kg H 2 target highly depends on tax credit, natural gas price, inflation rate, and learning rates. We make recommendations for hydrogen hub development and for accelerating technological progress toward the Hydrogen Energy Earthshot.
Similar content being viewed by others
Endogenous learning for green hydrogen in a sector-coupled energy model for Europe
A unified European hydrogen infrastructure planning to support the rapid scale-up of hydrogen production
A cost comparison of various hourly-reliable and net-zero hydrogen production pathways in the United States
Introduction.
Clean hydrogen has the potential to help achieve 10% economy-wide emissions reductions by 2050 relative to 2005, promote energy security and resilience, and develop a new economy in the United States 1 . In 2030, the hydrogen economy could create about 100,000 net new jobs for the development of new capital projects and clean hydrogen infrastructure 2 . The U.S. Bipartisan Infrastructure Law has appropriated $9.5 billion for clean hydrogen for the U.S. Department of Energy (DOE) 1 . Both zero- and low-carbon hydrogen production technologies are key options in a diverse toolbox enabling the transition to a sustainable and equitable clean energy future 1 . In October 2023, the U.S. DOE announced $7 billion to launch seven Regional Clean Hydrogen Hubs across the nation 3 . Some regional hubs will use water and natural gas as the feedstock for renewable-powered electrolysis and steam methane reforming (SMR) with carbon capture and storage (CCS) to produce clean hydrogen, which is also called green and blue hydrogen in practice, respectively. Blue hydrogen is often viewed as a near-term bridge to a zero-carbon hydrogen economy. Given potential high methane leakage, however, there are scientific debates on the competitiveness of blue hydrogen 4 , 5 , which makes a serious call for methane abatement.
The U.S. is making significant efforts to accelerate progress through historic investments and additional policies and incentives for clean hydrogen 1 . The U.S. National Clean Hydrogen Strategy and Roadmap has outlined strategic pathways for annual clean hydrogen production of 10 million metric tons (MMT) by 2030, 20 MMT by 2040, and 50 MMT by 2050 in the U.S. 1 . Although renewable-powered green hydrogen has much less carbon emissions than blue hydrogen, the current cost of green hydrogen production can be several times higher 6 , 7 , as shown later. Fossil fuel-based hydrogen production with CCS or blue hydrogen is among a portfolio of pathways for clean hydrogen. In 2021, the U.S. DOE launched the Energy Earthshots Initiative that aims to accelerate breakthroughs of more abundant, affordable, and reliable clean energy solutions by 2030 8 . To catalyze technological innovation and scale in clean hydrogen, this initiative included a hydrogen shot that aims to decrease the cost of clean hydrogen to $1 per 1 kilogram in 1 decade 8 , which is called the Hydrogen Energy Earthshot.
The Inflation Reduction Act (IRA) of 2022 provides a set of tax credits to stimulate the deployment of clean hydrogen technologies 9 . The IRA contains two incentive provisions: a new Section 45 V Tax Credit for clean hydrogen production and an enhanced Section 45Q Tax Credit for carbon sequestration 9 . The 45 V tax credit is available for hydrogen projects with life cycle greenhouse gas (GHG) emissions of less than 4.0 kilograms of carbon dioxide (CO 2 ) equivalent per kilogram of hydrogen during the 10-year period and ranges from $0.6 to 3.0 per kilogram of hydrogen. The production credit varies with the level of life cycle GHG emissions. In addition, the IRA has enhanced the 45Q tax credit to $85 per metric ton of CO 2 stored in saline reservoirs and $60 per metric ton of CO 2 used for enhanced oil recovery (EOR) or other industrial applications for up to twelve years. The sequestration threshold required for eligible CCS projects has been lowered to 18,750 metric tons of CO 2 per year for power plants and 12,500 metric tons per year for other facilities. These tax incentives would facilitate large-scale blue hydrogen production.
In 2020, global hydrogen production reached 90 million metric tons per annum (MMTA), of which 72 MMTA were pure hydrogen 10 , while the U.S. hydrogen production reached about 10 MMTA 11 . Globally, SMR and coal gasification without carbon abatement accounted for 74% and 24% of the pure hydrogen production 10 , respectively. Similarly, they accounted for 99% of the U.S. hydrogen production in 2020, of which 95% was made by SMR 11 . SMR and coal gasification can be coupled with CCS to produce low-carbon hydrogen. However, less than 1% of the global hydrogen is produced currently from fossil fuel resources with CCS 12 . Table 1 summarizes fossil fuel-based blue hydrogen projects installed around the world. Globally, these projects produced 0.7 MMTA of blue hydrogen from gas, coal, and oil resources and captured 10 MMTA of CO 2 in 2020 10 . In the U.S., the blue hydrogen projects produced 0.23 MMTA of hydrogen in 2021 10 .
The U.S. DOE’s National Energy Technology Laboratory (NETL) has provided a comparative assessment of the performance and cost of state-of-the-art fossil fuel-based hydrogen production technologies, including reforming and gasification without and with CCS 7 . The levelized cost of hydrogen production without carbon abatement ranges roughly from $1.1 to 2.6 per kilogram of hydrogen and varies with feedstock type or production technology. The addition of CCS for low-carbon hydrogen increases the levelized cost by more than 50% for the reforming production and 20% for the gasification production 7 . The largest contributor to the levelized cost is the fuel cost for the reforming production, whereas it is the capital cost for the gasification production 7 .
The production process of blue hydrogen often involves multiple subsystems, such as SMR and CCS. Future costs of individual subsystems will likely decline as a result of learning-by-doing as they scale up. However, the progress in learning may vary by subsystem. Schoots et al. 13 analyzed hydrogen cost data observed during the period of 1940 to 2007 and found that the learning rate of SMR, defined as the fractional reduction in cost for each doubling of cumulative installed capacity, is 11 ± 6% in the investment, but there is no cost reduction in the overall production cost, which implies a zero-learning rate in operating and maintenance (O&M) costs. Rubin et al. 14 and IEAGHG 15 reported that the learning rate of gasification is 14% for capital cost and 12% for O&M cost. CCS is an essential technology for blue hydrogen production. Rubin et al. 14 reported that the learning rate of a carbon capture system is 11% for capital cost and 22% for O&M cost. Deployment of large-scale CCS projects will decrease the future cost of carbon capture for advanced technologies 16 . Although a large number of learning curve studies have been done in the past years, almost no breakthroughs have been made in estimating the learning rates for SMR, gasification, and CCS beyond the two pioneering studies by Schoots et al. 13 and Rubin et al. 14 . Recent studies frequently adopted the learning rates from the two pioneering studies for a variety of applications 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 . However, few studies provide a thorough assessment of the overall technological learning of blue hydrogen produced from fossil fuel resources and examine the potential effect of tax incentives on technological evolution.
Projections of technological evolution are crucial to research and development in advanced technology, market analysis and forecast, investment analysis, resource planning, and decision- and policy-making. The major objectives of this study are to estimate the future evolving costs of major blue hydrogen production pathways from large-scale deployment, including state-of-the-art SMR and coal gasification with CCS, and to examine the economic effect on the technological evolution of the IRA’s tax credits for clean hydrogen. This study evaluates both the 45Q and 45 V tax credits and compares their economic role in promoting blue hydrogen production toward the Hydrogen Energy Earthshot. This study demonstrates how learning-by-doing will lower the cost of blue hydrogen production in the future and how much capacity of blue hydrogen projects should be installed to reach a cost target. This study offers an outlook for large-scale blue hydrogen production from abated fossil resources and reveals its potential policy-driven evolutionary trends, including their dependence on key factors.
This study first characterizes greenhouse gas emissions and costs of commercial technologies for blue hydrogen production and then develops technological learning and diffusion models to assess the future costs and evolutionary trajectories of blue hydrogen production without and with tax incentives toward the U.S. Hydrogen Energy Earthshot. A series of parametric analyses are further performed to reveal the dependence of the overall hydrogen production cost on key factors, such as fuel price, carbon capture cost uncertainties, learning rates, and inflation rate.
Current blue hydrogen production
This study adopts state-of-the-art reforming and gasification technologies as a point of reference to explore the evolutionary trends of blue hydrogen production driven by learning-by-doing. The current performance and cost estimates of these technologies are obtained from the recent NETL study 7 . The majority of hydrogen produced in the U.S. is made via steam methane reforming (SMR). In addition, the cost of blue hydrogen produced by SMR with carbon capture and storage (CCS) is similar to that by autothermal reforming with CCS, but the on-site and life-cycle emissions from the SMR process are less 7 . This study, therefore, focuses on SMR with CCS for gas-based blue hydrogen production. In the meantime, an oxygen-blown, entrained-flow Shell-type gasifier is employed with CCS for coal-based blue hydrogen production 7 . Supplementary Table 1 in Supplementary Note 1 summarizes the major techno-economic parameters and assumptions made for blue hydrogen production plants using natural gas and coal resources as the feedstocks, which include the project book lifetime, capacity factor, hourly production capacity, fuel price, and fixed charge rate. In addition, the land and water footprints per unit of hydrogen produced by these plants and the amounts of CO 2 sequestration are also reported in Supplementary Table 2 . Blue hydrogen plants produce high-purity hydrogen (99.9 vol.%) at the pressure of 6.48 MPa and transport the captured CO 2 at the pressure of 15.3 MPa for storage in saline reservoirs, which are typical design conditions. This study reports the cost results in 2018 U.S. real dollars unless otherwise noted.
For the given assumptions, the total levelized cost of hydrogen (LCOH) is $1.64/kg H 2 for SMR with CCS and $3.09/kg H 2 for coal gasification with CCS. In comparison, the plant LCOH is 88.4% higher for gasification production than the reforming production, which indicates that the integration of SMR with CCS is much more competitive for blue hydrogen. In addition, the on-site stack CO 2 emissions from hydrogen production by SMR with CCS are 0.4 kg CO 2 /kg H 2 , which is much less than that (1.4 kg CO 2 /kg H 2 ) from gasification with CCS 7 . In addition to the stack CO 2 emissions, there may be fugitive GHG emissions from various sources at an SMR production plant, mainly from the piping equipment and fittings 26 . However, fugitive GHG emissions are about 0.05% of the stack GHG emissions 26 , which indicates that plant methane leakage is not a serious issue.
A hydrogen production plant is decomposed into major subsystems. The components included in each of the subsystems defined for gas- and coal-based production plants are reported in Supplementary Tables 3 and 4 , respectively. Figure 1 a, b show the distribution by subsystem in the plant LCOH for the two production plants, respectively. The contributions of individual subsystems to the overall production cost are different. Given the gas price of $4.2/GJ, SMR and associated fuel consumption collectively account for 65.9% of the plant LCOH for gas-based production. Please note that at the gas-based hydrogen plant with CCS, the fuel combustion unit generates thermal energy for not only SMR but also the carbon capture process’s solvent regeneration. Given the coal price of $57.3/metric ton, gasification and associated fuel consumption collectively account for 52.1% of the coal-based production. Thus, the progress of individual subsystems in learning will have a different effect on the overall production cost in the future. In addition, the fuel costs account for 50.0% and 14.2% of the plant LCOH for the gas- and coal-based production cases, respectively. Natural gas price is a key factor influencing gas-based blue hydrogen production.

a Distribution of initial levelized cost for gas-based H 2 production. b Distribution of initial levelized cost for coal-based H 2 production. c Learning curves for coal-based and gas-based H 2 production capital and operating and maintenance (O&M) costs without tax incentives. d Learning curves for overall levelized cost of coal-based and gas-based H 2 production without and with tax incentives. e Future cost reductions for coal-based and gas-based H 2 production with tax incentives.
Currently, hydrogen is mainly produced by SMR without CCS in the U.S., which is often called gray hydrogen. Compared to it, the blue hydrogen production by SMR with CCS can decrease the stack CO 2 emission intensity by 96% but increase the LCOH by 55% 7 . The resulting CO 2 avoidance cost by blue hydrogen is $65 per metric ton of CO 2 . In contrast, the green hydrogen production by polymer electrolyte membrane electrolyzers almost has no stack CO 2 emissions but a high LCOH value ranging from $3.0 − 7.5/kg H 2 27 . The resulting CO 2 avoidance cost by green hydrogen relative to gray hydrogen varies from $212 − 689 per metric ton of CO 2 , which is much higher than that by blue hydrogen. Obviously, there are tradeoffs in CO 2 avoidance cost and emission savings between the blue and green hydrogen production pathways. The details of emission and cost data and CO 2 avoidance cost estimation are available in Supplementary Note 2 . Please note that the choice of a reference case affects the CO 2 avoidance cost.
Future costs of blue hydrogen production without and with tax credits
A blue hydrogen production plant consists of numerous subsystems. However, the maturity status of individual subsystems and their initial installed capacity are different. As a result, learning rates and initial installed capacity vary by subsystem. Thus, a component-based learning curve model is employed to construct a plant-level learning curve based on individual subsystems’ learning rates and initial installed capacity. In addition, the technological learning is evaluated in terms of the cumulative installed capacity of blue hydrogen instead of the number of new hydrogen production plants. To characterize the evolving costs of blue hydrogen produced from natural gas and coal resources in the future, this study first constructs learning curves for the total as-spent capital (TASC) and total operating and maintenance (TOM) cost of individual subsystems at each plant and then establishes the learning curve of the plant LCOH as a function of cumulative production capacity. To construct a learning curve for either the TASC or the TOM, initial installed capacity, initial cost, and learning rate have to first be determined. As discussed above, the initial TASC and TOM of individual subsystems are derived from the NETL study 7 and summarized in Supplementary Tables 6 – 10 in Supplementary Note 3 and Supplementary Tables 11 and 12 in Supplementary Note 4 . The initial installed capacity (Supplementary Table 13 ) and learning rates of individual subsystems are collected mainly from numerous well-established studies and summarized in Table 2 , in which bracketed values indicate uncertain ranges related to the base values. Both the initial installed capacity and learning rates vary significantly by subsystem. There are also high uncertainties in learning rates for both capital and O&M costs.
Blue hydrogen production without tax credits. At a global scale, the initial installed capacity of hydrogen production in 2021 was estimated to be 0.31 MMTA for gas-based blue hydrogen and 0.15 MMTA for coal-based blue hydrogen 12 , 28 . Figure 1c shows the learning curves for levelized capital and O&M costs and plant LCOH for fossil-based hydrogen production. A comparison between different cost categories over a range of cumulative production capacity indicates that the overall levelized cost of blue hydrogen will still be affected largely by TOM in the future, especially for gas-based production. In addition, a comparison between the two methods implies that SMR with CCS would continue to be more economically competitive for blue hydrogen production than gasification with CCS.
The annual demand for clean hydrogen produced from renewable and decarbonized fossil resources in the U.S. may reach 10 million metric tons of hydrogen per year by 2030 1 . As shown in Fig. 1c , the costs decline via incremental improvements to current technologies when the cumulative production capacity increases. When it reaches 10 MMTA, the capital and O&M costs decrease by 20.0% and 8.3% from the current levels for gas-based production, respectively. There are similar cost reductions for coal-based production. As a result, the plant LCOH decreases to $1.46/kg H 2 by 10.7% for the gas-based production and $2.75/kg H 2 by 10.9% for the coal-based production after 10 MMTA of hydrogen production capacity. Although experience learned from large-scale deployed projects will help to lower the future costs of blue hydrogen production, it is hard for both reforming and gasification technologies with CCS to reach the cost target of $1/kg H 2 .
The plant LCOH trends are affected largely by key subsystems’ capital and O&M learning rates, along with feedstock prices. For gas-based production, SMR and CCS are the key subsystems that dominate the plant LCOH, as discussed above. However, as shown in Table 2 , there are no reductions gained in O&M costs from deploying SMR (including associated components), pressure swing adsorption (PSA) for hydrogen purification, and CO 2 compression. For the given natural gas price of $4.2/GJ, therefore, it is difficult to reach the cost target of $1/kg H 2 , even when the cumulative production capacity goes beyond 10 MMTA.
Blue hydrogen production with tax credits. Both the 45Q and 45 V tax credits are to promote investment in clean hydrogen technologies and then lower the cost of hydrogen production. For blue hydrogen projects with CCS, however, a taxpayer cannot simultaneously claim both 45 V and 45Q tax credits during a given period. To claim either the 45Q tax credit or the 45 V tax credit, facilities must be placed in service before January 1 st , 2033 29 . The credit is available for such qualified facilities for a period. The period of credit availability is common to eligible facilities, regardless of their start-of-service time.
In this study, it is assumed that the captured CO 2 is stored in saline reservoirs, which earns a carbon-sequestration credit of $85 per metric ton of CO 2 for 12 years. As mentioned earlier, the 45 V tax credit depends on the life cycle emissions of hydrogen production, which include greenhouse gas emissions from plant stacks, fuel supply, electric power supply, and CO 2 sequestration or management. The life cycle emissions were estimated by the NETL to range from 3.1 to 8.9 kg CO 2 -eq/kg H 2 for the gas-based blue hydrogen in the 90% confidence interval between the 5 th and 95 th percentile values and from 3.4 to 8.9 kg CO 2 -eq/kg H 2 for the coal-based blue hydrogen, which is driven mainly by the uncertainty in fuel supply 7 . The largest contributor among the multiple stages to the life cycle emissions is the fuel supply 7 . The median estimate of life cycle emissions is 4.6 kg CO 2 -eq/kg H 2 for the gas-based blue hydrogen and 4.1 kg CO 2 -eq/kg H 2 for the coal-based blue hydrogen 7 , which is close to the threshold value of 4.0 kg CO 2 -eq/kg H 2 required to claim the minimum tax credit for clean hydrogen. Blue hydrogen projects have a fair possibility of earning a 45 V tax credit. Thus, the production tax credit for hydrogen projects is assumed to be $0.6 per kilogram of H 2 for 10 years. This assumption is optimistic for blue hydrogen in this study. However, there is no 45 V tax credit if the life cycle emissions of specific blue hydrogen projects are more than 4.0 kg CO 2 -eq/kg H 2. See Supplementary Note 5 for additional information about life cycle emissions and tax credits. Figure 1d shows the learning curves for the plant LCOH for the gas- and coal-based hydrogen production with tax incentives.
Tax incentives lower the plant LCOH of hydrogen production. When the cumulative production capacity reaches 10 MMTA, the overall cost of hydrogen produced by SMR with CCS declines to $1.14 and $1.26 per kilogram of hydrogen produced with the 45Q and 45 V tax credits, respectively. They are 22.2% and 13.7% less than the LCOH without tax incentives, respectively. There are similar cost reductions with gasification-based production. These results indicate that the 45Q tax credit provides more economic incentives for blue hydrogen projects than the 45 V tax credit.
Tax incentives decrease the time-related learning experience necessary to reach a cost target. However, Fig. 1d shows that with either a hydrogen production tax credit or a carbon-sequestration tax credit, it is still hard for coal gasification with CCS to produce blue hydrogen at a cost of $1/kg H 2 . In contrast, with the carbon-sequestration tax credit claimed for hydrogen projects, the cost of blue hydrogen produced by SMR with CCS approximates the Hydrogen Energy Earthshot, as shown in Fig. 1d .
Learning-by-doing will reduce the cost of hydrogen production for coal- and gas-based blue hydrogen. Figure 1e shows the cost reduction by subsystem and by the 45Q tax credit when the cumulative installed capacity of blue hydrogen reaches 10 MMTA. For blue hydrogen produced from both coal and gas resources, the overall cost reduction will be driven largely by the carbon-sequestration tax credit and the improvement in carbon capture. In contrast, other subsystems, such as SMR and PSA, will make limited contributions because they are mature technologies and have no or limited reductions from an additional 10 MMTA deployment in their future costs. These results indicate the importance of continued support from both public and private sectors for CCS-related research, development, and demonstration programs at federal and state levels.
Time-based diffusion of blue hydrogen production
It is helpful for hydrogen energy planning to explore if certain production capacity and cost targets can be achieved by 2030. A new study reports the cumulative installed capacity of low-carbon hydrogen production over time-based on globally announced, planned, and committed projects through 2030 30 . A diffusion-of-innovation model was established based on the current and future low-carbon hydrogen capacities through 2030 to explore the time-based diffusion of gas-based blue hydrogen over a long-term planning horizon through 2050.
Figure 2a shows the cumulative installed capacity estimates for global low-carbon hydrogen production over time. The gas-based blue hydrogen capacity accounts for 49% of the total low-carbon hydrogen capacity given in Table 1 and is estimated to be 90% in 2030 in terms of the International Energy Agency’s hydrogen project databases 28 , 31 . Given the changing shares over time, Fig. 2a also shows a range of cumulative installed capacity for gas-based blue hydrogen in a particular year. The cumulative installed capacity of the global gas-based blue hydrogen may range from 6 to 12 MMTA in 2030, which implies that it would be hard for the blue hydrogen production by SMR with CCS alone in the U.S. to reach 10 MMTA in 2030.
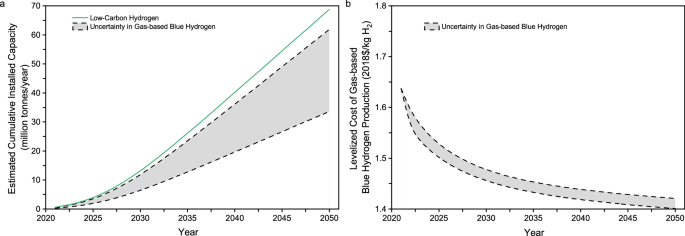
a Diffusion of cumulative installed capacity. b Time-based learning curves of blue hydrogen production cost.
Figure 1c shows the overall plant LCOH as a function of cumulative installed capacity for gas-based blue hydrogen, whereas Fig. 2a shows the cumulative installed capacity over time. Combining them together, Fig. 2b shows the overall plant LCOH of gas-based blue hydrogen production without tax credit over time. The result shown in Fig. 2b implies that for the fuel price and learning rates given in the base case, it would also be difficult for gas-based blue hydrogen to reach the ambitious cost target of $1/kg H 2 by 2030 in normal scenarios without aggressive incentives and game-changing technologies.
Sensitivity of blue hydrogen production cost to key factors
Massive deployment of hydrogen projects will lower future costs for clean hydrogen production. Tax incentives for clean hydrogen will further decrease production costs and accelerate the technological evolution toward the Hydrogen Energy Earthshot. However, it is hard for coal gasification with CCS to reach the cost target of $1/kg H 2 for clean hydrogen. In contrast, the tax-incentivized production for blue hydrogen by SMR with CCS has the potential to reach the Hydrogen Energy Earthshot. Blue hydrogen projects announced in the U.S. will mainly employ gas-based reformation technologies with CCS 31 . The future production costs and their evolutionary trends are affected by natural gas price, carbon removal system cost, and learning rates in capital and O&M costs, as well as inflation when the cost is estimated in nominal dollars. In the U.S., natural gas prices are highly volatile. There are also high uncertainties in learning rates for many subsystems, which are shown in Table 2 . The sensitivity analysis, therefore, is performed for the gas-based blue hydrogen with a focus on natural gas price, carbon removal system cost uncertainties, learning rates, and inflation rate. In each parametric analysis, other parameters were kept at the base case values given in Table 2 and Supplementary Tables 1 , 15 , and 16 unless otherwise noted.
Effect of natural gas price. For blue hydrogen produced by SMR with CCS, the feedstock cost accounts for 50.0% of the plant LCOH, with an assumed natural gas price of $4.2/GJ. In the past years from 2017 to 2022, the annual average Henry Hub gas prices ranged from $1.9/GJ to $6.1/GJ 32 . Thus, it is necessary to examine the economic benefit of low gas prices for blue hydrogen production. Figure 3 shows the effect of natural gas prices on the plant LCOH for hydrogen production without and with tax incentives. Obviously, the plant LCOH is highly sensitive to the natural gas price. Cheap natural gas resources help SMR-CCS decrease the cumulative production capacity necessary to reach the Hydrogen Energy Earthshot.
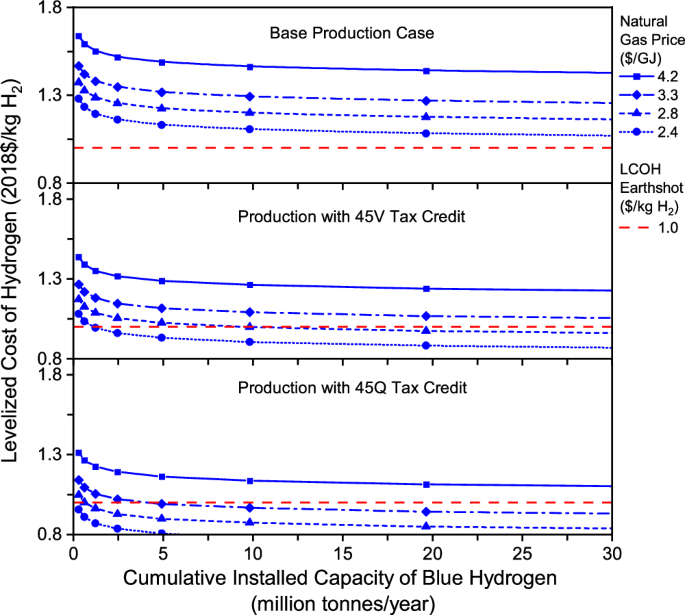
Effect of natural gas price on future levelized cost of gas-based blue hydrogen production in three cases including base production case, production with a 45V tax credit, and production with a 45Q tax credit.
When the 45Q tax credit is claimed for hydrogen projects, the cumulative production capacity necessary to reach the cost target of $1/kg H 2 is 4.9 and 0.6 MMTA if the gas price declines to $3.3/GJ and $2.8/GJ, respectively. As shown in Fig. 3 , the initial plant LCOH is already less than $1/kg H 2 if the natural gas price is $2.4/GJ. When the 45 V tax credit is claimed, the cumulative production capacity should reach 9.8 and 1.2 MMTA to achieve the cost target when the gas price declines to $2.8/GJ and $2.4/GJ, respectively. Without tax incentives like 45Q and 45 V and increased learning rates, however, it is still difficult for this production method, even with cheap natural gas resources to reach the Hydrogen Energy Earthshot.
It is also worth noting that the gas-based hydrogen industry may have a sizable effect on the natural gas markets in the U.S., depending on the scale of blue hydrogen production in the future. For example, the production of 10 MMTA hydrogen by SMR with CCS would consume 1.9 billion GJ of natural gas per year, which is equivalent to about 17% of the national industrial natural gas consumption in 2022 33 .
Effect of carbon removal system cost uncertainties. There are uncertainties in the process and project contingencies of two CO 2 removal systems employed for producing low-carbon hydrogen from natural gas resources. Such uncertainties affect the TASC and LCOH of a hydrogen production plant. The process contingency depends on the maturity level of a technology, whereas the project contingency depends on the availability of site-specific project details. In the base case, the process contingency is 18% of the bare erected cost (BEC) for the Cansolv system and 0% for the MDEA system, while the project contingency is 25% of the sum of BEC, engineering, construction management, home office and fees, and process contingency and 25% for the Cansolv unit and 20% for the MDEA unit 7 . A parametric analysis is then conducted to reveal the collective impacts of uncertain processes and project contingencies, which take into account low and high contingencies. In the low contingencies scenario, the process contingency is 10% for the Cansolv system and 0% for the MDEA system, while the project contingency is 10% for both the CO 2 removal systems. In the high contingencies scenario, the process contingency is 40% for both the CO 2 removal systems, while the project contingency is 30% for both the CO 2 removal systems 34 .
As shown in Fig. 4 , the uncertainties in carbon removal system cost estimates have a sizable effect on the hydrogen production plant’s TASC. As a result, the plant LCOH varies from $1.45 to 1.48/kg H 2 at the cumulative installed capacity of 10 MMTA. To reach the cost of $1.46/kg H 2 , the cumulative installed capacity requirements vary from 7 to 16 MMTA. These results imply that cost uncertainties in carbon removal systems may result in pronounced variations in the estimation of the cumulative installed capacity necessary to reach a cost target.
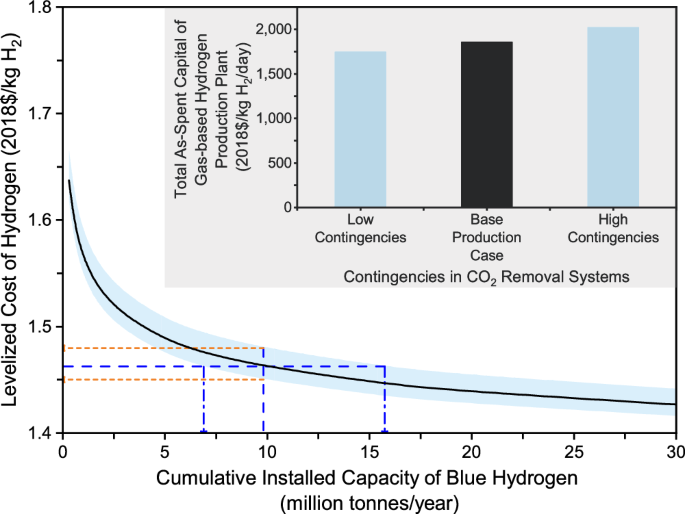
The light blue shading area represents the uncertainties in the levelized cost of hydrogen production, which are driven by the uncertain cost estimates of carbon removal systems. The orange dash lines represent that the levelized cost of hydrogen varies from $1.45 to 1.48/kg H 2 at the cumulative installed capacity of 10 MMTA, while the blue dash lines represent that to reach the cost of $1.46/kg H 2 , the cumulative installed capacity requirements vary from 7 to 16 MMTA.
Effect of learning rates. Learning rates directly drive future cost trends. In particular, the O&M learning rates of SMR, PSA, and CO 2 compression largely influence the pace of cost reductions toward the Hydrogen Energy Earthshot as their costs and associated fuel or electricity consumption collectively dominate the plant LCOH. In the base case, the O&M learning rates are zero for the three subsystems. However, Table 2 shows that there are uncertainties in learning rates, which can vary by 50% or more relative to the base values for some subsystems. Given such high uncertainties, it is important to examine the sensitivity of future cost trends to learning rates.
Additional scenarios are explored to examine the effect on the overall LCOH of increases in both the capital and O&M learning rates of individual subsystems with an emphasis on the increased O&M learning rates for SMR, PSA, and CO 2 compression. In these scenarios, the capital and O&M learning rates are elevated for individual subsystems to be 25% and 50% higher than the base values, except for SMR, PSA, and CO 2 compression. The O&M learning rates are increased for the three subsystems to 5% and 10% on an absolute basis. Figure 5 shows the sensitivity of the plant LCOH to the increased learning rates.
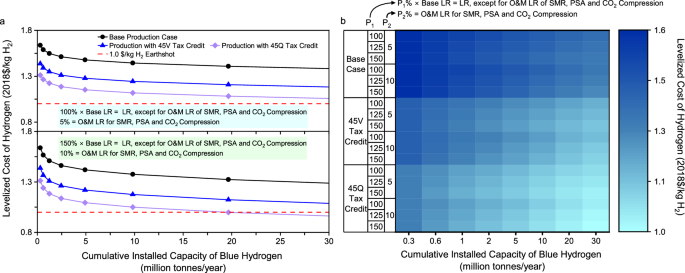
a LCOH under two boundary scenarios of learning rates. b LCOH under the range of 100%–150% time-based learning rates, except for O&M cost learning rates, which are equal to 5%–10% for SMR, PSA, and CO 2 compression. Note to Fig. 5: P 1 means a percentage relative to the base learning rate, whereas P 2 means the learning rate on an absolute basis. Note to abbreviations: CCS means carbon capture and storage; LCOH means levelized cost of hydrogen; LR means learning rate; O&M means operating and maintenance; PSA means pressure swing adsorption; and SMR means steam methane reforming.
The increases in learning rates, especially the O&M learning rates of SMR, PSA, and CO 2 compression, obviously lower the cumulative production capacity necessary to reach a cost target. The results shown in Fig. 5, however, also imply that without tax incentives for clean hydrogen, it would still be challenging for blue hydrogen produced from expensive natural gas resources to reach the Hydrogen Energy Earthshot by 2030, even if the progress in learning is to accelerate substantially. If the O&M learning rates of SMR, PSA, and CO 2 compression reach more than 5%, massive deployment of blue hydrogen projects claimed with 45Q tax incentives can decrease the plant LCOH to $1/kg H 2 . Figure 5a, b show that with an O&M learning rate of 10% for the three subsystems, the breakeven cumulative production capacity is 20 MMTA or more, which is also affected by other subsystems’ learning rates.
Effect of inflation rate. In general, this study estimates the cost of hydrogen production in real dollars. When the cost is estimated in nominal dollars, however, both the initial and future LCOH estimates vary with the inflation rate as it affects the discount rate, fixed charge rate, and levelization factor. A parametric analysis was further performed for the inflation rate to quantify its effect on the evolving cost of gas-based blue hydrogen production toward the Hydrogen Energy Earthshot. Figure 6 shows the learning curves of blue hydrogen production with inflation. Figure 6a , b show that at a given level of cumulative installed capacity, the LCOH in nominal dollars increases when the inflation rate increases from 1% to 3%. As a result, blue hydrogen production may not reach the cost target of $1/kg H 2 for both scenarios without and with a 45Q tax credit even when the cumulative installed capacity reaches 30 MMTA. Figure 6c further shows that with an inflation rate of 3%, the future LCOH may get close to the cost target when cheap natural gas resources are used as the feedstock to produce blue hydrogen with a cumulative installed capacity of up to 30 MMTA.
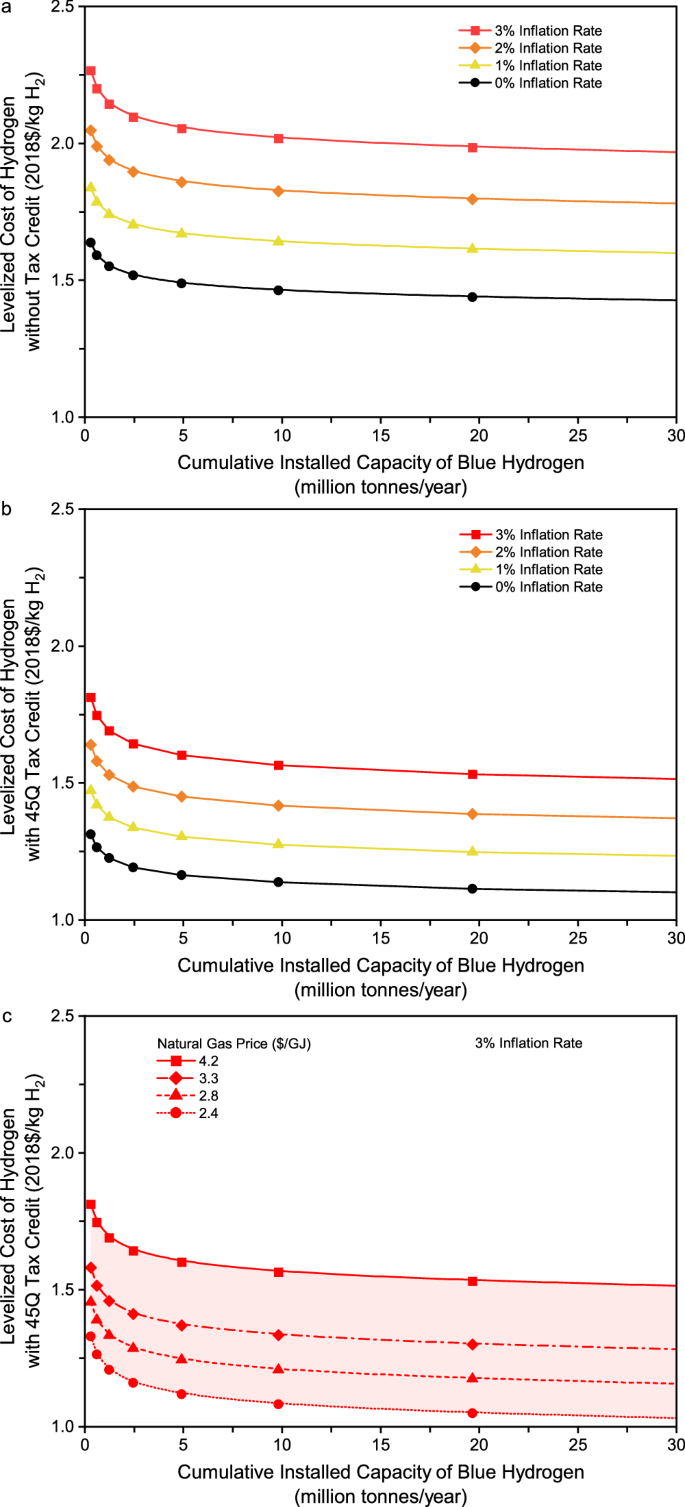
a Levelized cost of hydrogen production with a gas price of $4.2/GJ and without a 45Q tax credit. b Levelized cost of hydrogen production with a gas price of $4.2/GJ and 45Q tax credit. c Levelized cost of hydrogen production with a 3% inflation rate and 45Q tax credit.
Figure 6a, b also compare the learning curves of blue hydrogen production between the two scenarios without and with inflation. As shown in Fig. 6a for the scenario without a 45Q tax credit, the reduction in hydrogen production cost from deploying the cumulative installed capacity of 10 MMTA can be offset by an inflation rate of 1%. There is a similar result at the cumulative installed capacity of 5 MMTA for the scenario with a 45Q tax credit, as shown in Fig. 6b . All these results imply that inflation would remarkably raise challenges for blue hydrogen production to reach the Hydrogen Energy Earthshot in the near future.
This study reveals opportunities and challenges while creating the new hydrogen economy. The capex learning rates of green hydrogen production are 9% and 13% for alkaline electrolysis and polymer electrolyte membrane electrolysis, respectively 35 , 36 , which are similar to those for SMR and PSA. However, the cost competitiveness of green hydrogen relative to blue hydrogen in the future remains an open question as the future production cost also depends on initial installed capacity, renewable electricity cost, tax credit, and other factors 27 , 36 , 37 , 38 . Experience learned from the deployment of blue hydrogen projects will be helpful in lowering future costs of hydrogen production by both SMR and gasification with CCS. In comparison between the two hydrogen production methods, SMR with CCS will continue to be more economically competitive. For the given estimates of learning rates for SMR and CCS, the overall cost of blue hydrogen production without tax incentives can be decreased by 10.7% from the current level to $1.46/kg H 2 when the cumulative production capacity reaches 10 MMTA. In addition to the learning rates, the overall LCOH is also affected significantly by natural gas price. Without tax incentives, however, it is still hard for SMR with CCS to reach the Hydrogen Energy Earthshot even when natural gas resources are cheap, which implies an urgent need for radical innovation in technology. With tax incentives, the breakeven cumulative installed production capacity, which is required for SMR with CCS to reach the cost target of $1/kg H 2 , highly depends on the gas price and can be much less than 10 MMTA when gas prices are not more than $3.3/GJ. In contrast, when natural gas resources are not cheap, the breakeven cumulative production capacity required for tax-incentivized SMR with CCS is at a level of 20 MMTA or more even if the learning rates for capital and O&M costs are increased remarkably. In a short summary of these findings, tax credit, natural gas price, and learning rates are the most significant factors that collectively determine the breakeven cumulative production capacity required for SMR with CCS to reach the Hydrogen Energy Earthshot. However, inflation can remarkably elevate challenges for blue hydrogen to reach the cost target.
The global production capacity of low-carbon hydrogen will reach 12.3 MMTA by 2030 based on the announced, planned, and committed projects 30 . The low-carbon hydrogen capacity in North America will reach 6.8 MMTA by 2030 30 . However, only 1.8 MMTA 30 and 1.5 MMTA 2 of the announced projects in North America and the U.S. have reached the final investment decision (FID), mainly because many announced projects have not yet secured financing and nailed down contracted offtake 1 , 2 . The hesitancy to long-term, scaled contracts is influenced by numerous factors, such as lack of price certainty, unavailability and reliability of large-scale hydrogen supply, near-term policy implementation uncertainty, and long-term political uncertainty 1 , 2 . For blue hydrogen projects, enhancements in tax credits for carbon sequestration can improve the economics of hydrogen production. For example, an extension of the 45Q tax credit period from the current 12 years to 18 years would significantly reduce the cumulative installed capacity required for gas-based blue hydrogen projects to reach the Hydrogen Energy Earthshot, as demonstrated in Supplementary Fig. 3 . Extending the period of the 45Q tax credit for blue hydrogen projects can be considered an option to secure financing and promote long-term offtake.
Production of blue hydrogen requires fossil fuel resources, water, and land. For illustrative purposes, Supplementary Fig. 4 shows the annual natural resource requirements for SMR with CCS as a function of cumulative hydrogen production capacity based on the plant design and performance given in Supplementary Table 1 . The hydrogen production of 10 MMTA by SMR with CCS would annually require 1.9 billion GJ of natural gas resources, withdraw 0.30 km 3 of water resources, and occupy 25.1 km 2 of land resources. Deployment of large-scale hydrogen projects toward the Hydrogen Energy Earthshot will pronouncedly affect multiple natural resources and local resource planning. In addition to the production cost, massive blue hydrogen production should also be planned in the context of resource sustainability.
Tax incentives for either clean hydrogen production or carbon sequestration accelerate technological learning to reduce the time-related cumulative production capacity necessary to reach a cost target for hydrogen production and in turn, lower natural resource consumption and environmental impacts. In comparison between the two types of tax credits, blue hydrogen projects gain more economic value from the 45Q tax credit than the 45 V tax credit. For post-combustion CCS, enhancing the CO 2 removal efficiency from 90% to 99% or more can achieve net-zero emissions while increasing the CO 2 avoidance cost by only 3–13% or less 39 . Thus, deep CCS for 99% CO 2 capture can be considered for blue hydrogen production to nearly fully remove site emissions and in the meantime, receive increased economic benefits from the 45Q tax credit.
Radical innovation in technology and systems integration is urgently needed to reach the Hydrogen Energy Earthshot by 2030. In addition to deep CCS, advanced hydrogen technologies, such as thermal and catalytic pyrolysis of natural gas, dry reforming of methane, and chemical looping, should be researched, developed, and demonstrated (RD&D) for buying down learning curves 40 . Autothermal reforming and partial oxidation can be considered an option for greenfield investment in blue hydrogen 41 . There is a strong need for collaborative support and joint efforts on RD&D from both the public and private sectors. However, it may be still challenging for advanced hydrogen technologies to reach the cost target by 2030. Improvements in hydrogen technology alone may not be enough to reach the cost target of $1/kg H 2 42 . Cost reduction pathways beyond technology advancement for clean hydrogen production should also be explored extensively. As SMR without carbon abatement is the most widely-used process for hydrogen production now, retrofitting CCS to existing SMR facilities can substantially decrease the investment and then the overall production cost for blue hydrogen. While building new production plants to meet the significantly increasing demand for clean hydrogen, retrofit of CCS or deep CCS is a cost reduction option for blue hydrogen in the near term. In addition, advances in solvent regeneration for CCS or deep CCS can lower the energy penalty to improve economic viability 43 . By-product sales, CO 2 valuation, hydrogen production integration with other energy systems, and optimal siting of production plants are the additional options for cost reductions toward the Hydrogen Energy Earthshot 42 .
Competing strategies and supportive policy and regulatory actions should be made rapidly on both the hydrogen demand and supply sides at both federal and state levels in alignment with the innovation expansion. A variety of high-level strategies are needed on the demand side to promote the widespread use of low-carbon hydrogen in industrial, transportation, and power sectors and then establish large-sale markets for low-carbon hydrogen 1 , 2 . To jumpstart a clean hydrogen economy, a cluster approach can be employed on the supply side to establish regional production-transportation-demand networks by co-locating feedstock supply, hydrogen production, and carbon sequestration with multiple end-users and by utilizing existing infrastructure, such as pipeline infrastructure for natural gas, CO 2 , and H 2 transportation and geological reservoirs for CO 2 storage. To scale the regional hydrogen economy, secured investments in hydrogen production and supporting infrastructure are required with funding from both public and private sectors, plus subsidies and tax incentives. In addition, deploying large hydrogen production plants instead of small ones can improve engineering economics at a plant level. Given the important role of CCS in producing competitive blue hydrogen, continued support for large-scale demonstration projects should be boosted in the near term to reduce the CCS cost and its uncertainty. Investments in blue hydrogen should be prioritized to lock down sufficient financial resources for the most competitive technologies in the near term. Economic and policy incentives can be tailored with emphasis on gas-based blue hydrogen to catalyze its widespread deployment and technological evolution because of the pronounced cost advantage relative to coal-based blue hydrogen. Extending the 45Q tax credit from the current 12-year period to a longer period for gas-based blue hydrogen projects would remarkably lower the time-related cumulative installed capacity necessary to reach the Hydrogen Energy Earthshot. In addition to the enhanced tax incentives, emission trading can be another driver to facilitate low-carbon technology deployment and then accelerate technological evolution 44 .
Although the future cost of blue hydrogen is expected to decrease from cumulative experience in conjunction with tax incentives, blue hydrogen may not be economically competitive with gray hydrogen in the near term. To jumpstart a clean hydrogen economy, therefore, a carrot-and-stick policy approach can be employed to stimulate low-carbon hydrogen deployment and constrain gray hydrogen production from unabated fossil resources.
Numerous tax-incentivized blue hydrogen projects with cheap feedstocks will be needed at a total capacity of roughly several million metric tons of hydrogen per year to reach the Hydrogen Energy Earthshot, which highly depends on the several key factors discussed above. However, the current deployment of SMR with CCS for blue hydrogen production is lagging far behind estimates of what is required. Regional hydrogen hubs will be needed across the country to produce clean hydrogen at the targeted cost level. Large-scale blue hydrogen production will consume multiple types of natural resources, such as fossil, water, and land resources, and geological reservoirs and transportation infrastructure for CO 2 storage, and affect local management and planning of these natural resources. The availability and price of these natural resources vary by region or location in the country. Thus, siting blue hydrogen production plants should take into account the co-location and co-availability of these natural resources. In contrast, green hydrogen would ideally be sited in regions with high penetration of solar and wind energy.
Several caveats accompany this study. A single-factor top-down model using constant learning rates is applied to characterize technological evolving trends in the future. However, it is a simplified representation of future cost trends for large-scale new technologies or process designs 38 . Technological evolution is projected for blue hydrogen based on the empirical experience of continuing improvements to current technologies, which is similar to other learning curve studies on CCS 14 , 16 , 38 . However, technology innovation may progress in a complex pattern instead. Sensitivity analysis is performed to explore the potential consequences of uncertain learning rates but without the quantification of the likelihood of the outcomes due to a lack of sufficient empirical data. If new game-changing technologies were to be created and deployed widely, however, the resulting learning could be accelerated substantially to result in greater cost reductions or less cumulative production capacity requirements to reach the Hydrogen Energy Earthshot than those estimated in this study.
Last but not least, it is very important to prevent upstream methane emissions while reforming natural gas with CCS to produce blue hydrogen. Methane leakage along the natural gas supply chain can jeopardize the role of natural gas in the energy transition to a low-carbon or net-zero future, even when CCS is deployed 45 , 46 . A high methane leakage rate of 3.5% or more can elevate the blue hydrogen’s carbon footprint and make it uncompetitive or even unviable in a hydrogen economy 4 . To reduce or avoid the risks of committing to high-emitting blue hydrogen, stringent standards and regulations should be imposed to limit methane leakage and promote the deployment of the best available technologies for methane abatement 46 .
This study applies empirical learning curves to characterize the evolving costs of blue hydrogen production without and with future tax incentives. The section presents a diffusion-of-innovation model, a component-based learning curve model, and an economic metric for technology evaluation and then discusses the sources of data used for the model formulation and cost estimation.
Diffusion-of-innovation model
The diffusion of innovation describes how a new technology would spread over time. An S-shaped curve is often used to measure the diffusion over time, in which the adoption rate increases during the early stage, reaches a maximum level at the point of inflection, and decreases until the diffusion curve saturates 47 . To estimate the annual installed capacity of low-carbon hydrogen over time, the S-shaped diffusion function is employed 48 , 49 :
Where cc t is the annual installed capacity of low-carbon hydrogen in a particular year “ t ” (million metric tons per annum, MMTA); cc sat is the saturation level of annual installed capacity (MMTA); cc 0 is the initial annual installed capacity in the start year (MMTA); r is the growth rate (fraction); and t is a particular year after the start period. The function coefficients are estimated by regression based on current and future low-carbon hydrogen production capacities through 2030 12 , 28 , 30 . Additional details about the regression and diffusion function are available in Supplementary Note 7 . Once the annual installed capacity in future years is determined, the cumulative annual installed capacity can be estimated as a function of time.
Component-based learning curve model
A learning curve represents an empirical relationship between production unit cost and cumulative production capacity over time. The improved performance gained from time-related experience translates to lower the cost of hydrogen production. The one-factor learning curve model is used widely to characterize cost trends of new technologies or systems 14 , 16 , 38 . In this model, the future cost of a technology is estimated as a function of cumulative installed production capacity 38 :
Where C is the unit cost at the cumulative installed capacity; x is the ratio of cumulative installed capacity relative to initial capacity; a is the constant unit cost at the initial installed capacity; and b is the constant learning parameter. The fractional reduction in cost from each doubling of cumulative installed capacity is defined as the learning rate and is calculated as 38 :
Where LR is the learning rate, and factor 2 b is the progress ratio. The cost of interest can be either the total capital cost or the total operating and maintenance (O&M) cost. To construct a learning curve for a technology with respect to its either total capital cost or total O&M cost, three types of model parameters have to be specified, including the initial cost, initial installed capacity, and learning rate. Capital and O&M learning rates can be estimated using empirical data for mature technologies or an analogous approach for advanced technologies. For example, the learning rates for SMR were derived from its historical installed capacity and cost data 13 , whereas the learning rates for post-combustion carbon capture were estimated by referring to those of post-combustion flue-gas desulfurization as they are technically analogous 14 , 38 . The data collected for these parameters are discussed later.
A hydrogen production plant involves multiple technologies or subsystems, which have different values regarding the three parameters defining a learning curve. At blue hydrogen production, individual subsystems lie at different levels of technological maturity. Learning rates and initial installed capacity count on maturity level and then vary by subsystem. For example, at a gas-based blue production plant, SMR and PSA are mature subsystems, whereas carbon capture has not been deployed widely, though it is commercially available. As a result, the O&M learning rates are zero for SMR and PSA but 22% for carbon capture. Thus, a component-based learning curve model is applied to estimate the total cost of hydrogen production at a certain level of cumulative installed capacity as the sum of individual subsystem costs 38 :
Where C is the total cost per plant at the cumulative installed capacity; a i is the initial cost of subsystem “i” to produce the first unit; b i is the learning parameter for subsystem “i”; LR i is the learning rate of subsystem “i”; and Num is the number of subsystems at a plant. The gas- and coal-based production plants are shown in Supplementary Note 1 and Supplementary Figs. 1 and 2 are decomposed into five subsystems and nine subsystems, respectively. The individual subsystems are reported in detail for the two plants in Supplementary Tables 3 and 4 , respectively.
Cost metric for technology evaluation
The component-based learning curve model is applied to project the future total capital cost and total O&M cost of individual subsystems and an overall plant as a function of cumulative installed capacity and then estimate the overall cost of hydrogen production for a given cumulative installed capacity. The cost metric considered for technology evaluation is the levelized cost of hydrogen and is estimated in real dollars 7 , 34 .
Where the subscript “R” means the real dollars; LCOH R is the levelized cost of hydrogen of a blue hydrogen production plant ($/kg H 2 ); LCC R is the levelized capital cost ($/kg H 2 ); LOM R is the non-fuel levelized operating and maintenance cost ($/kg H 2 ); LFP R is the levelized fuel price ($/kg H 2 ); TASC R is the total as-spent capital of a blue hydrogen production plant ($); FCR R is the fixed charge rate (fraction/year); CF is the plant capacity factor (%); AH is the total annual hours (8760 h); KG H2 is the hourly hydrogen production rate (kg H 2 /hour); OM R is the total non-fuel operating and maintenance (O&M) cost ($/year), including both the fixed and non-fuel variable O&M costs; FC R is the natural gas cost ($/GJ) or coal cost ($/metric ton); FR is the hourly natural gas flow rate (GJ/hour) or coal flow rate (metric ton/hour). When estimating the LCOH in real dollars, the FCR has to be determined using Eq. ( 10 ) to ( 13 ), which varies with the project book lifetime and a panel of financial variables 34 :
Where the superscript “nonfuel” represents the non-fuel component; CRF R is the capital recovery factor (fraction/year); ETR is the effective tax rate (%); PV plant is the present value of tax depreciation expense of a blue hydrogen project (fraction/year); ATWACC R is the after-tax weighted average cost of capital (%); BL is the project book lifetime (year); d n is the tax depreciation fraction in a year (n) (fraction); m is the number of years of depreciation (year); PC equity is the percent of equity (%); PC debt is the percent of debt (%); ROE R is the real rate of return on equity (%); kd R is the real rate of cost of debt (%). The financial parameters and their data sources are detailed in Supplementary Table 17 and the resulting FCR is available in Supplementary Table 18 .
Inflation affects discount rate, ATWACC, FCR, and other factors. To evaluate the effect of inflation on the cost of blue hydrogen production, the LCOH is estimated in nominal dollars. The detailed estimation of the LCOH in nominal dollars is reported in Supplementary Note 8 .
When either a clean-hydrogen production tax credit or a carbon-sequestration tax credit is considered for a blue hydrogen project, the levelized tax credit (LTC) and the levelized cost of hydrogen with a tax credit are estimated in real dollars using Equation ( 14a ) or Equation ( 14b ) and Equation ( 15 ), respectively:
Where LCOH R,TC is the levelized cost of hydrogen with a tax credit ($/kg H 2 ); LTC R,45V and LTC R,45Q are the levelized tax credit of Sections 45 V and 45Q over the book lifetime of a blue hydrogen project, respectively ($/kg H 2 ); AHP H2 is the annual hydrogen production (kg H 2 /year); ACS CO2 is the annual CO 2 sequestration amount (metric ton CO 2 /year); BL is the book lifetime of a hydrogen project (years); CP 45V is the 45 V credit period (years); CP 45Q is the 45Q credit period (years); TC 45V is the 45V bonus rate ($/kg H 2 ), which depends on the life cycle GHG emissions (“well-to-gate” emissions); and TC 45Q is the 45Q bonus rate ($/metric ton CO 2 ).
Data sources
To construct a learning curve, initial installed capacity, initial cost, and learning rate must be specified. As discussed below, the data for these parameters are collected from various sources for individual subsystems.
The initial TASC and total O&M cost of individual subsystems are derived from the NETL’s recent study on state-of-the-art commercial technologies for blue hydrogen production 7 . The NETL study provides the estimates of total plant cost for individual processes or systems, as well as the owner’s cost and the TASC and O&M costs for the overall plant. The cost breakdowns are applied to the NETL’s plant-level cost estimates to come up with the initial TASC and total O&M cost for individual subsystems. The cost allocation methods and relevant cost estimates are detailed in Supplementary Notes 3 to 4 and Supplementary Tables 6 to 12 .
The initial installed capacity of individual subsystems is estimated based on the current global installed capacity unless noted otherwise, which implies conservative projections of technological learning. The installed capacity is measured in equivalent gigawatts of thermal energy. When the installed capacity is measured in electric power capacity, it is converted to equivalent gigawatts of thermal energy with an assumed thermal efficiency of 40%. Thermal energy capacity is further converted to the mass-based production capacity of hydrogen in terms of its higher heating value. The data of initial installed capacity are mainly derived or collected from the International Energy Agency’s Global Hydrogen Review 12 , 28 , a highly-cited learning curve article 14 , and the Global Syngas Technology Council syngas database 50 . Additional details of initial installed capacity are available in Supplementary Note 4 and Supplementary Table 13 .
The data on capital and O&M cost learning rates are mainly collected from three learning curve studies on reforming hydrogen production and power generation with CCS, respectively 13 , 14 , 15 . Additional learning rate data come from other studies 16 , 51 , 52 .
Data availability
The source data generated in this study have been deposited in the Figshare database: [ https://doi.org/10.6084/m9.figshare.23821926 ]. The data can also be obtained by contact with the corresponding author.
U.S. Department of Energy. U.S. national clean hydrogen strategy and roadmap . https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/us-national-clean-hydrogen-strategy-roadmap.pdf (2023).
U.S. Department of Energy. Pathways to commercial liftoff: Clean hydrogen . https://liftoff.energy.gov/wp-content/uploads/2023/03/20230320-Liftoff-Clean-H2-vPUB.pdf (2023).
U.S. Department of Energy. Biden-Harris Administration Announces $7 Billion For America’s First Clean Hydrogen Hubs, Driving Clean Manufacturing and Delivering New Economic Opportunities Nationwide . https://www.energy.gov/articles/biden-harris-administration-announces-7-billion-americas-first-clean-hydrogen-hubs-driving (2023).
Howarth, R. W. & Jacobson, M. Z. How green is blue hydrogen? Energy Sci. Eng. 9 , 1676–1687 (2021).
Article CAS Google Scholar
Romano, M. C. et al. Comment on “How green is blue hydrogen?”. Energy Sci. Eng. 10 , 1944–1954 (2022).
Article Google Scholar
International Renewable Energy Agency. Making the breakthrough: Green hydrogen policies and technology costs . ISBN 978-92-9260-314-4 (2021).
National Energy Technology Laboratory. Comparison of commercial, state-of-the-art, fossil-based hydrogen production technologies . Report No. DOE/NETL-2022/3241 (2022).
U.S. Department of Energy. Energy Earthshots: Hydrogen . Report No. DOE/EE-2362 (2021).
Congressional Research Service. H.R.5376 - Inflation Reduction Act of 2022 . https://www.congress.gov/bill/117th-congress/house-bill/5376/text (2022).
International Energy Agency. Global hydrogen review 2021 . https://iea.blob.core.windows.net/assets/5bd46d7b-906a-4429-abda-e9c507a62341/GlobalHydrogenReview2021.pdf (2021).
U.S. Department of Energy. Hydrogen strategy enabling a low-carbon economy . https://www.energy.gov/sites/prod/files/2020/07/f76/USDOE_FE_Hydrogen_Strategy_July2020.pdf (2020).
International Energy Agency. Global hydrogen review 2022 . https://iea.blob.core.windows.net/assets/c5bc75b1-9e4d-460d-9056-6e8e626a11c4/GlobalHydrogenReview2022.pdf (2022).
Schoots, K., Ferioli, F., Kramer, G. J. & Van der Zwaan, B. C. C. Learning curves for hydrogen production technology: An assessment of observed cost reductions. Int. J. Hydrog. Energy 33 , 2630–2645 (2008).
Article ADS CAS Google Scholar
Rubin, E. S., Yeh, S., Antes, M., Berkenpas, M. & Davison, J. Use of experience curves to estimate the future cost of power plants with CO 2 capture. Int. J. Greenh. Gas. Control 1 , 188–197 (2007).
International Energy Agency Greenhouse Gas R&D Programme. Estimating future trends in the cost of CO 2 capture technologies . Report No. 2006/6 (2006).
Zhai, H. Advanced membranes and learning scale required for cost-effective post-combustion carbon capture. iScience 13 , 440–451 (2019).
Article ADS CAS PubMed PubMed Central Google Scholar
Böhm, H., Zauner, A., Rosenfeld, D. C. & Tichler, R. Projecting cost development for future large-scale power-to-gas implementations by scaling effects. Appl. Energy 264 , 114780 (2020).
Fan, J. L., Li, Z., Li, K. & Zhang, X. Modelling plant-level abatement costs and effects of incentive policies for coal-fired power generation retrofitted with CCUS. Energy Policy 165 , 112959 (2022).
Fan, J. L., Xu, M., Yang, L., Zhang, X. & Li, F. How can carbon capture utilization and storage be incentivized in China? A perspective based on the 45Q tax credit provisions. Energy Policy 132 , 1229–1240 (2019).
George, J. F., Müller, V. P., Winkler, J. & Ragwitz, M. Is blue hydrogen a bridging technology? The limits of a CO 2 price and the role of state-induced price components for green hydrogen production in Germany. Energy Policy 167 , 113072 (2022).
Kang, J. N. et al. The prospects of carbon capture and storage in China’s power sector under the 2 °C target: A component-based learning curve approach. Int. J. Greenh. Gas. Control 101 , 103149 (2020).
Lee, H., Lee, J. & Koo, Y. Economic impacts of carbon capture and storage on the steel industry - A hybrid energy system model incorporating technological change. Appl. Energy 317 , 119208 (2022).
Malhotra, A. & Schmidt, T. S. Accelerating low-carbon innovation. Joule 4 , 2259–2267 (2020).
Nicodemus, J. H. Technological learning and the future of solar H 2 : A component learning comparison of solar thermochemical cycles and electrolysis with solar PV. Energy Policy 120 , 100–109 (2018).
Yang, L., Xu, M., Yang, Y., Fan, J. & Zhang, X. Comparison of subsidy schemes for carbon capture utilization and storage (CCUS) investment based on real option approach: Evidence from China. Appl. Energy 255 , 113828 (2019).
Alhamdani, Y. A., Hassim, M. H., Ng, R. T. & Hurme, M. The estimation of fugitive gas emissions from hydrogen production by natural gas steam reforming. Int. J. Hydrog. Energy 42 , 9342–9351 (2017).
Peterson, D., Vickers, J. & DeSantis, D. Hydrogen Production Cost from PEM Electrolysis-2019 . Prepared for Department of Energy. https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/19009_h2_production_cost_pem_electrolysis_2019.pdf?Status=Master (2020).
International Energy Agency. Hydrogen projects database . https://www.iea.org/reports/hydrogen-projects-database (2021).
The White House. Building a clean energy economy: A guidebook to the Inflation Reduction Act’s investments in clean energy and climate action . January 2023 Version 2. https://www.whitehouse.gov/wp-content/uploads/2022/12/Inflation-Reduction-Act-Guidebook.pdf (2023).
Hydrogen Councial and McKinsey & Company. Hydrogen insights 2023 . https://hydrogencouncil.com/wp-content/uploads/2023/05/Hydrogen-Insights-2023.pdf (2023).
International Energy Agency. Hydrogen projects database . https://www.iea.org/reports/hydrogen-projects-database (2023).
U.S. Energy Information Administration. Henry hub natural gas spot price . https://www.eia.gov/dnav/ng/hist/rngwhhdA.htm (2023).
U.S. Energy Information Administration. Natural Gas Explained. Use of Natural Gas . https://www.eia.gov/energyexplained/natural-gas/use-of-natural-gas.php (2023).
National Energy Technology Laboratory. Quality guidelines for energy system studies: Cost estimation methodology for NETL assessments of power plant performance . Report No. NETL-PUB-22580 (2021).
Hydrogen Council. Path to hydrogen competitiveness a cost perspective . https://hydrogencouncil.com/wp-content/uploads/2020/01/Path-to-Hydrogen-Competitiveness_Full-Study-1.pdf (2020).
International Renewable Energy Agency. Green hydrogen cost reduction: Scaling up electrolysers to meet the 1.5 °C Climate Goal . ISBN 978-92-9260-295-6 (2020).
International Renewable Energy Agency. Hydrogen: A renewable energy perspective . ISBN 978-92-9260-151-5 (2019).
Rubin, E. S., Azevedo, I. M., Jaramillo, P. & Yeh, S. A review of learning rates for electricity supply technologies. Energy Policy 86 , 198–218 (2015).
Zhai, H. & Rubin, E. S. It is time to invest in 99% CO 2 capture. Environ. Sci. Technol. 56 , 9829–9831 (2022).
Article ADS CAS PubMed Google Scholar
Bistline, J. E. Roadmaps to net-zero emissions systems: Emerging insights and modeling challenges. Joule 5 , 2551–2563 (2021).
AlHumaidan, F. S., Halabi, M. A., Rana, M. S. & Vinoba, M. Blue hydrogen: Current status and future technologies. Energy Convers. Manag. 283 , 116840 (2023).
National Energy Technology Laboratory. Strategies for achieving the DOE hydrogen shot goal: Thermal conversion approaches . Report No. DOE/NETL-2023/3824 (2023).
Ji, T. et al. Microwave-accelerated regeneration of a non-aqueous slurry for energy-efficient carbon sequestration. Mater. Today Sustain. 19 , 100168 (2022).
Talati, S., Zhai, H. & Morgan, M. G. Viability of carbon capture and sequestration retrofits for existing coal-fired power plants under an emission trading scheme. Environ. Sci. Technol. 50 , 12567–12574 (2016).
Achakulwisut, P. et al. Global fossil fuel reduction pathways under different climate mitigation strategies and ambitions. Nat. Commun. 14 , 5425 (2023).
Shirizadeh, B. et al. The impact of methane leakage on the role of natural gas in the European energy transition. Nat. Commun. 14 , 5756 (2023).
Fleiter, T. & Plötz, P. In Encyclopedia of Energy, Natural Resource, and Environmental Economics (ed Shogren, J. F.) 1, 63–73 (Elsevier, London, 2013).
Geroski, P. A. Models of technology diffusion. Res. Policy 29 , 603–625 (2000).
Schmidt, O., Hawkes, A., Gambhir, A. & Staffell, I. The future cost of electrical energy storage based on experience rates. Nat. Energy 2 , 1–8 (2017).
Higman, C. GSTC syngas database: 2017 update . Gasification & Syngas Technologies Conference (2017).
National Energy Technology Laboratory. Quality guidelines for energy system studies: Technology learning curve (FOAK to NOAK) . Report No. DOE/NETL-341/081213 (2013).
Industrial Economics, Inc. and E.H. Pechan & Associates, Inc. Direct cost estimates for the Clean Air Act second section 812 prospective analysis . Report No. EP-D-04-006 (2011).
Download references
Acknowledgements
This study is supported by the University of Wyoming’s School of Energy Resources. The authors greatly acknowledge Holly Krutka, Scott Quillinan, Edward Rubin, Zitao Wu, and Landen Fuller for assistance with this study. The views and opinions of this article are those of the authors alone and do not necessarily state or reflect those of the United States Government or any agency thereof.
Author information
Authors and affiliations.
College of Engineering and Physical Sciences, University of Wyoming, Laramie, WY, USA
Wanying Wu & Haibo Zhai
School of Energy Resources, University of Wyoming, Laramie, WY, USA
Haibo Zhai & Eugene Holubnyak
Department of Engineering and Public Policy, Carnegie Mellon University, Pittsburgh, PA, USA
You can also search for this author in PubMed Google Scholar
Contributions
H.Z. conceived and guided the research; W.W. performed the experiments; W.W. and H.Z. did the analysis; E.H. contributed to the discussion; H.Z. and W.W. wrote the original manuscript; and all the authors reviewed and edited the manuscript.
Corresponding author
Correspondence to Haibo Zhai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Fiona Beck, Michel Noussan, and Philipp Verpoort for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wu, W., Zhai, H. & Holubnyak, E. Technological evolution of large-scale blue hydrogen production toward the U.S. Hydrogen Energy Earthshot. Nat Commun 15 , 5684 (2024). https://doi.org/10.1038/s41467-024-50090-w
Download citation
Received : 02 August 2023
Accepted : 27 June 2024
Published : 06 July 2024
DOI : https://doi.org/10.1038/s41467-024-50090-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
Advertisement
Hydrogen production, storage, utilisation and environmental impacts: a review
- Open access
- Published: 06 October 2021
- Volume 20 , pages 153–188, ( 2022 )
Cite this article
You have full access to this open access article

- Ahmed I. Osman ORCID: orcid.org/0000-0003-2788-7839 1 ,
- Neha Mehta 1 , 2 ,
- Ahmed M. Elgarahy 3 , 4 ,
- Mahmoud Hefny 5 , 6 ,
- Amer Al-Hinai 7 ,
- Ala’a H. Al-Muhtaseb 8 &
- David W. Rooney 1
82k Accesses
19 Altmetric
Explore all metrics
A Correction to this article was published on 31 March 2022
This article has been updated
Dihydrogen (H 2 ), commonly named ‘hydrogen’, is increasingly recognised as a clean and reliable energy vector for decarbonisation and defossilisation by various sectors. The global hydrogen demand is projected to increase from 70 million tonnes in 2019 to 120 million tonnes by 2024. Hydrogen development should also meet the seventh goal of ‘affordable and clean energy’ of the United Nations. Here we review hydrogen production and life cycle analysis, hydrogen geological storage and hydrogen utilisation. Hydrogen is produced by water electrolysis, steam methane reforming, methane pyrolysis and coal gasification. We compare the environmental impact of hydrogen production routes by life cycle analysis. Hydrogen is used in power systems, transportation, hydrocarbon and ammonia production, and metallugical industries. Overall, combining electrolysis-generated hydrogen with hydrogen storage in underground porous media such as geological reservoirs and salt caverns is well suited for shifting excess off-peak energy to meet dispatchable on-peak demand.
Similar content being viewed by others
Social, environmental, and economic consequences of integrating renewable energies in the electricity sector: a review

Methanol fuel production, utilization, and techno-economy: a review
A review of water electrolysis–based systems for hydrogen production using hybrid/solar/wind energy systems
Avoid common mistakes on your manuscript.
Introduction
The continual growth and rapid urbanisation of the world population and economy have resulted in an enormous increase in energy need, urging the switch from fossil-based fuels into alternative clean renewables (Dawood et al. 2020 ). Consequently, global decarbonisation in the transportation, industry and electricity generation sectors is crucially needed to mitigate anthropogenic climate change (Fawzy et al. 2020 ; Osman et al. 2021a ). In this context, there has been a growing interest from scholars and industries with versatile production routes. There is abundant availability of renewable sources used in hydrogen production; however, the variable and intermittent nature of these resources is the major challenge in the transition towards a hydrogen economy. Hence, this calls for technical accommodation, especially for balancing variable renewable supply, i.e. solar, wind and others, and varying energy demand. Furthermore, cost-effective production methods, policies, research and development and hydrogen infrastructure development are areas that need more investigation when transitioning towards the hydrogen economy.
More than 100 current and planned hydrogen production technologies are reported to date, with over 80% of those technologies are focused on the steam conversion of fossil fuels and 70% of them are based on natural gas steam reforming. However, in order to minimise carbon footprint emissions, a wider range of hydrogen extraction processes, such as methane pyrolysis and seawater electrolysis using alternative energy sources, must be addressed. All hydrogen production routes are highlighted in Fig. 1 .
Hydrogen production routes, including renewables, fossil fuels and nuclear, with hydrogen being produced in power plants, pharmaceutical applications, synthetic fuels or their upgrades in transportation, ammonia synthesis, metal production or chemical industry applications
Hydrogen is the most abundant element in the universe, and due to its reactivity, it only exists on earth in compounds such as water and organic materials. It is an odourless, flammable and colourless gas, which is leading to its safety concern, especially if a leak is not detected and gas collects in a confined area; it can ultimately ignite and causes explosions. Furthermore, metal hydrogen embrittlement is an issue as it could damage pipelines and containers due to its small molecular size; thus, it escapes through materials. The higher heating value (HHV) of hydrogen is 141.8 MJ/kg at 298 K, and the lower heating value is 120 MJ/kg at the same temperature. This is significantly higher than that of most fuels such as gasoline with a value of 44 MJ/kg at 298 K. However, liquid hydrogen has a lower energy density by volume than hydrocarbon fuels such as gasoline by a factor of four with a density of 8 MJ/l versus density of 32 MJ/l. While hydrogen gas has a high energy density by weight but a low energy density by volume compared to hydrocarbons, it requires a larger tank to store. For example, as opposed to liquified natural gas, liquified hydrogen contains 2.4 times the energy but takes 2.8 times the volume to store. At the same time, the low temperature for liquified hydrogen storage at ambient pressure and a temperature of −253 °C raises quite a few risks. When exposed, it can cause cold burns; furthermore, leakage can result in a combination of liquefied air and hydrogen, resulting in an explosive mixture or the formation of flammable or explosive conduits (Atilhan et al. 2021 ; El-Halwagi et al. 2020 ).
Like electricity, hydrogen is an energy carrier and not an energy source; using it to store renewable energies instead of being wasted when not in use is crucial since it is storable, utilisable and transportable (Parra et al. 2019 ; Abe et al. 2019 ).
Hydrogen cleanness and colour coding
Dawood et al. (Dawood et al. 2020 ) reported the four main stages in hydrogen economy: production, storage, safety and utilisation, where hydrogen purification and compression (subsystems) need to be considered along with the life cycle assessment (LCA) when selecting the production method for hydrogen. Hydrogen cleanness level is described in the literature with many colour coding: mainly green, blue and grey, which relies only on the production route, i.e. hydrogen origin, and fails to assess the deep cleanness of the produced hydrogen (Merzian and Bridges 2019 ), for instance: (1) Grey hydrogen is produced using fossil fuels such as natural gas, one tonne of hydrogen produced in this way is responsible for 10 tonnes of carbon dioxide (Dvoynikov et al. 2021 ), as shown in Fig. 2 ; (2) blue hydrogen is produced from fossil fuels like grey hydrogen but with combination of carbon capture and storage to mitigate emissions; (3) green hydrogen is typically produced from 100% renewable sources such as wind or solar energies with lower carbon footprint; (4) brown hydrogen is produced from gasification of coal-based fuel; and (5) turquoise hydrogen is produced from the thermal decomposition of natural gas, i.e. methane pyrolysis or cracking by spitting methane into hydrogen and carbon at a temperature range from 600 to 1200–1400 °C (Dvoynikov et al. 2021 ). This process produces black carbon (soot) as a by-product instead of carbon oxide emissions in the grey hydrogen, allowing for the sequestration of carbon emissions in the form of solid carbon. However, carbon stability in this black soot is critical for long-term carbon sequestration, along with the utilisation of renewable energy sources in the high-temperature process to achieve carbon neutrality. Interestingly, hydrogen could be produced with a negative carbon footprint via biogas pyrolysis.
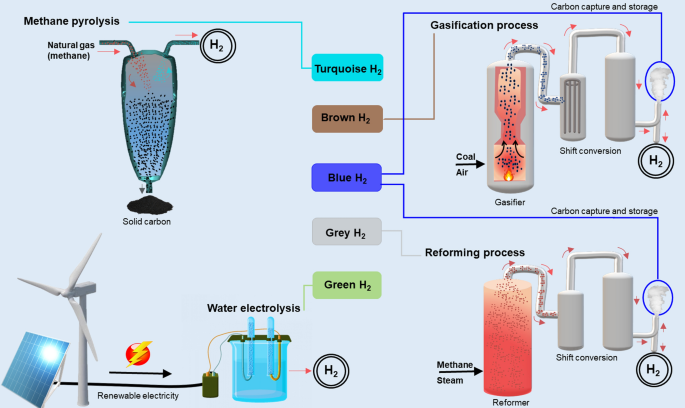
Hydrogen colour coding for various manufacturing processes. Green hydrogen is produced using renewable energy sources such as solar or wind energy, followed by water electrolysis. Grey and brown hydrogen are produced by methane steam reforming and coal gasification, respectively, and when combined with carbon capture and storage, blue hydrogen is produced. Turquoise hydrogen is produced through the pyrolysis of methane, with solid carbon as a by-product
However, this colour coding is not precise as it assumes that green hydrogen always has low-carbon emission than blue or grey hydrogen, which is not applicable in all cases. Blue hydrogen, for example, is regarded as less safe than green hydrogen, even though it releases no carbon at the point of use or during the entire process, while green hydrogen may do. For instance, bioenergy feedstocks such as biomass emit greenhouse gas emissions such as CH 4 , SO x , NO x and CO 2 during their growth or thermochemical conversions. Furthermore, the carbon capture and storage technique used in the blue hydrogen reduces toxic emissions significantly. The manufacture of photovoltaic panels as renewable energy technology also has a significant carbon footprint and generates various types of waste, liquid and gaseous by-products that are hazardous to the environment. Starting from the extraction of quartz and other materials used to manufacture solar panels, this is coupled with the carbon and sulphur emission in the energy-intensive process when producing metallurgical silicon. Moreover, the solar panel has a 30-year lifespan, and then, it must be handled as a particular waste at its end of life.
A recent LCA study compared environmental impacts for steam methane reforming with water electrolysis using wind, solar photovoltaic, hydropower, solar thermal and biomass gasification as energy sources (Al-Qahtani et al. 2021 ). It was concluded that among all the technologies evaluated, solar photovoltaic electrolysis had the most damaging environmental implications because of the significant acidification potential in the photovoltaic panel production phase and the relatively poor efficiency of photovoltaic systems.
Thus, measuring the emitted greenhouse gas emissions accurately in the entire production process along with the life cycle of the equipment used is crucial. This is required to determine how green is the green hydrogen and how blue is the blue hydrogen. A recently proposed model for improved hydrogen colour coding consisted of a hydrogen cleanness index followed by the number of depth levels (Han et al. 2021 ). For instance, 80 green-4 means hydrogen is produced via renewable resources; however, it is not a zero-emission process, only 80% green, due to emissions related to the process. The number after the colour, which in this case is 4, indicates that greenhouse gas emissions (CO 2-e ) linked with the purification during the production route have been considered. This model still requires much more analysis to decide the start and end of the continuum thresholds for each colour, as well as the evaluation depth levels and related weight for each level.
Hydrogen production routes
According to the International Energy Agency (IEA), green hydrogen could help reduce our carbon footprint if major challenges such as infrastructure, logistics, cost-effective manufacturing methods and safety are overcome. Globally, hydrogen is responsible for about 843 metric tonnes of CO 2 emissions per annum, equal to the combined total emissions of the UK and Indonesia (IEA 2019 ). The global hydrogen demand is projected to increase from 70 million tonnes in 2019 to 120 million tonnes by 2024 (Global hydrogen market insights 2020 ; Atilhan et al. 2021 ; Safari and Dincer 2020 ). In 2025, the largest global green hydrogen plant will be built, with a capacity of 237,250 tonnes per annum, i.e. 650 tonnes/day hydrogen output through electrolysis and 4 gigawatts of renewable energy from wind, solar and storage.
A wide range of resources is available for hydrogen production, mainly fossil-based and renewable fuels (Dawood et al. 2020 ; Saithong et al. 2019 ; Osman et al. 2020 a). The former is the more mature and most common used industrially as it is a cost-effective method that deploys cracking or reforming fossil-based fuels. In 2016, hydrogen production globally was about 85 million tonnes used in petroleum, metal industry, fertiliser, food processing, semiconductor production, power plants and generations (Chen and Hsu 2019 ; El-Emam and Özcan 2019 ; Acar and Dincer 2019 ).
There are many ways to extract hydrogen from hydrogen-containing materials, either hydrocarbon or non-hydrocarbon, such as photonic, electric, chemical, bioenergy, heat and a combination of those methods together (Abe et al. 2019 ; El-Emam and Özcan, 2019 ; Osman et al. 2020 b). Table 1 shows different hydrogen production routes with different energy sources, technology readiness level (TRL) and their % energy efficiency.
Advances and challenges in water electrolysis
Water is typically purified and then sent to an electrolyser, which produces hydrogen and oxygen. The hydrogen is then dried, purified and compressed from a 10.3 to 413.7 bar pressure, and then stored in a tank. Although the electrolysis pathway offers a 100% renewable route for hydrogen production, it represents less than 5% of worldwide hydrogen production (Han et al. 2021 ). Despite this low percentage contribution, water electrolysis is gaining momentum for various reasons such as zero-carbon emissions, the absence of unwanted by-products such as sulphates, carbon oxides and nitrogen oxides, and high hydrogen purity. The cost of producing hydrogen through electrolysis would be reduced by approximately 70% over the next decade, allowing for the widespread adoption of a green hydrogen production approach.
By 2040, the worldwide market for hydrogen electrolysers is expected to have grown by 1000-fold. Aurora Energy Research predicted that about 213.5 gigawatts of projects will be completed over the next 19 years; this compares to an estimated 200 megawatt that is currently in service. They reported that 85 per cent of anticipated projects are in Europe, with Germany accounting for 23 per cent of expected global electrolyser capacity. The European Union has already set a goal of 40 gigawatts of electrolyser capability by 2030 (Research, 2021). If all this power is available, it will supply up to 32 million tons of hydrogen per year, which is already half of the currently demanded hydrogen. In a 1.5-degree climate change mitigation scenario, meeting 24% of energy demand with hydrogen will necessitate massive amounts of additional renewable electricity generation. To power electrolysers in this scenario, approximately 31,320 terawatt-hours of electricity would be required, i.e. more than is currently produced globally from all sources combined (BNEF 2020 ). Besides, an investment of more than $11 trillion in manufacturing, storage and transportation infrastructure would be required.
Proton exchange membrane (PEM) along with alkaline anion exchange membrane (AEM) and concentrated potassium hydroxide solution KOH are the most common techniques used in low-temperature water electrolysis. The key benefit of alkaline anion exchange membrane electrolysis over other methods is lower cost since no platinum group metals are used as catalysts herein. The main challenge, however, is the low rate of hydrogen production and the instability of the alkaline method owing to its susceptibility to pressure drop (Dvoynikov et al. 2021 ; Yu et al. 2019 ). A typical electrolysis system consists of two metal electrodes, an anode and a cathode, separated by a membrane and immersed in an electrolyte solution (Zhu et al. 2019 ). As an electric current flows through the solution, oxygen and hydrogen bubbles rise above the anode and cathode, respectively. Both electrodes are typically coated with a catalyst to reduce the amount of energy needed to liberate hydrogen from water.
However, large amounts of freshwater would be needed to generate hydrogen, and these supplies are already depleted worldwide; thus, the utilisation of seawater will be an option to overcome this issue. However, seawater utilisation in hydrogen production is associated with challenges such as the corrosion of chloride ions in seawater to the anode metal. Hung et al. reported a solution to this issue by designing the anode material as a porous nickel foam pan collector coated with an active and inexpensive nickel and iron catalyst, which showed strong conductivity and corrosion resistance. It is worth noting that, while using freshwater is more expensive than using seawater, the cost of water usually accounts for less than 2% of the total cost of hydrogen production via electrolysis (Milani et al. 2020 ). The affordability and accessibility of freshwater is one side of the coin, while inexpensive and sustainable green energy alternatives are the other, and the proximity of these two supplies, i.e. renewable energy and freshwater, does not always coincide. The main areas that need further investigation in water electrolysis are reducing the capital cost of electrolysis technology, finding water resources and increasing efficiency.
According to the recent literature summarised in Table 1 , membrane reactor technology is increasingly being recognised as an encouraging route to expand clean hydrogen production paths from hydrocarbons and hydrogen purification. At least 99.8% can be achieved without any gas purification using a proton exchange membrane analyser (Jorschick et al. 2021 ).
Recently, it was reported for Australia that the levelised cost of hydrogen (LCOH) for steam methane reforming could reach a cost of $(1.88–2.30)/kg H 2 and $(2.02–2.47)/kg H 2 for coal gasification production routes. In comparison, the LCOH via electrolysis technologies costs between $4.78 and $5.84/kg H 2 for alkaline electrolysis and $6.08–7.43/kgH 2 for proton exchange membrane technologies (Milani et al. 2020 ).
When using partial methane oxidation for hydrogen production via synthesis gas, the average cost is 1.33 euros/kg H 2 , while the cost of large-scale H 2 processing ranges between 1 and 1.5 euro/kg H 2 (Dvoynikov et al. 2021 ). It is important to note that the economic viability of using natural gas or related petroleum gas for hydrogen production should be seen in the light of transportation systems or the direct use of hydrogen on-site of the gas or oil plant.
In terms of blue hydrogen, carbon capture and utilisation lower greenhouse gas emissions but raise the overall production cost. Chemical looping reforming, for instance, has a comparatively short life cycle, global warming potential and low fossil fuel intake. Nevertheless, adding carbon capture and liquefaction process units raises the expense of the steam methane reforming by 18% and autothermal reforming processes by 2% (Atilhan et al. 2021 ). The process of liquefying hydrogen absorbs approximately 30% of the energy content of hydrogen. Additionally, keeping liquified hydrogen under one atmospheric pressure and at a low temperature of −253 °C is difficult. Furthermore, evaporation and leakage can occur even with robust insulation, losing typically 1 per cent of the stored volume per day (Atilhan et al. 2021 ).
Biomass gasification
Biomass gasification is seen as one of the most feasible, sustainable and potentially carbon-neutral alternatives to generate hydrogen (Saidi et al. 2020 ). Since biomass is a renewable feedstock that absorbs atmospheric carbon dioxide during growth, it has a much lower net CO 2 footprint than fossil-based fuels. However, the economic feasibility of hydrogen output from biomass must be closely related to the availability and affordability of raw materials in the local area. The biomass physicochemical properties, distribution and hydrogen rate are the main attributes of the supply materials. Since biomass feedstocks vary widely in structural composition and shape, all of these characteristics must be taken into account when combining the feedstock with the appropriate conversion technology (Srivastava et al. 2020 ).
Consequently, moisture, energy and ash contents are the core criteria for evaluating biomass utilisation in this route. The hydrogen yield from biomass is comparatively poor since the hydrogen content of biomass is roughly 5.9 wt% compared to 25 wt% for methane (natural gas), and the energy content is also low due to high oxygen content within the biomass of 40%. Thus, techno-economic studies backed by adequate life cycle assessment evaluation are crucial in this matter. Since biomass has a lower density, transportation and storage costs for either biomass feedstock or the produced hydrogen should be well justified in terms of economies of scale. In certain ways, these characteristics would make it impossible for biomass-based hydrogen production to compete with common natural gas such as steam methane reforming method unless new regulatory frameworks such as carbon tax favour competitively sustainable hydrogen production routes.
Biomass gasification, like coal, is the most practical process for biomass feedstocks because it produces the best yield at high temperatures, generally, 500–1400 °C, where the overall reaction is presented in Eq. 1 . Interestingly, the integration between biomass gasification and carbon capture and storage can potentially lead to an overall negative carbon footprint.
Advances and challenges in fossil-based hydrogen production route
The breakdown of the long-chain hydrocarbon via gasification, reforming or pyrolysis reaction routes is required for hydrogen production from fossil-based feedstocks. The primary product in the reforming reaction is the synthesis gas (a mixture of H 2 and CO), followed by H 2 separation via autothermal reforming, steam methane reforming, partial oxidation or membrane reforming. Another well-known method that is commonly used in hydrogen production is the gasification of fossil fuels, such as coal gasification (Milani et al. 2020 ).
Al-Qahtani et al. evaluated and compared the most common hydrogen generation routes on a monetary basis, such as steam methane reforming, coal or biomass gasification, methane pyrolysis with or without carbon capture and storage technology. Besides, the hydrogen production from the water via electrolysis derived from solar or nuclear energy were also assessed. They reported that, at the moment, steam methane reforming with carbon capture and storage appeared to be the most viable alternative (Al-Qahtani et al. 2021 ).
Steam methane reforming and methane pyrolysis
The primary feedstock for steam methane reforming is natural gas, predominantly methane mixed with other hydrocarbons and carbon dioxide (Osman 2020 ) Natural gas and steam reaction occur in a two-step reaction, as shown in Eq. 2 at high temperatures, followed by an interaction between the carbon monoxide and the produced hydrogen along with the unreacted natural gas. Following that, more steam is supplied to react with carbon monoxide in a water–gas shift reaction (WGSR), as shown in Eq. 3 , to recover further hydrogen and convert carbon monoxide into carbon dioxide. The entire process efficiency is around 76% (Al-Qahtani et al. 2021 ). The entire process releases a significant amount of carbon dioxide emissions, which may be decreased by installing carbon capture and storage technology, removing and separating the flue gases from the product stream. Following that, an amine solvent such as monoethanolamine absorbs about 90% of the carbon dioxide emission, and then, the processed flue gas stream is released into the environment. Afterwards, carbon dioxide is thermally desorbed and compressed to 110 bars for storage. The integration between steam methane reforming and carbon capture and storage (SMR + CCS) technologies has an energy efficiency of 68 per cent, owing mostly to the energy necessary to regenerate the monoethanolamine and the power required for compression. After the WGSR, hydrogen is further purified to 99.99 per cent in both situations, with or without carbon capture and storage, in a pressure swing adsorption unit, which is also utilised in the gasification technology such as coal or biomass gasification routes.
Regarding methane pyrolysis at high temperatures, thermally or catalytically, the processes degrade hydrocarbons into hydrogen and solid carbon, as shown in Eq. 4 . Because there is no oxygen in the process, no carbon oxides are generated, possibly removing the requirement for subsequent processing stages such as the WGSR and lowering the capital and operating expenditures compared to steam methane reforming (Al-Qahtani et al. 2021 ). The greater H 2 content in the product gas stream has the potential to reduce downstream clean-up operations significantly. The cost of methane pyrolysis is heavily influenced by the natural gas prices, processing method and solid carbon by-product.
Coal gasification
During the coal gasification process at high temperatures ranging from 800 to 1300 °C and 30–70 bar pressures, coal is partially oxidised in oxygen or air atmosphere into synthesis gas, as shown in Eq. 5 . The synthesis gas is typically composed of carbon monoxide and dioxide, hydrogen and unreacted methane, where the WGSR process (Eq. 3 ) enriches the syngas further to recover additional hydrogen. Thus, combining Eqs. 3 and 5 will lead to the overall reaction as in Eq. 6 . Coal gasification is less efficient than steam methane reforming with 55%, although it has a larger single-train capacity.
Bibliometric analysis
Key research studies were identified to summarise state of the art and discover knowledge gaps in the hydrogen production and LCA research arenas. The advanced search tool for publications from the Web of Science was used for this study, using the terms ‘Hydrogen production’ AND ‘ Life cycle assessment ’ as inputs. The results were manually scanned, and 24 most complete and relevant studies published from 2019 to 2021 were selected for review in the present study.
- Life cycle assessment
Life cycle assessment (LCA) is recognised as a comprehensive tool to evaluate environmental impacts associated with products and processes. There are many hydrogen production methods, such as steam methane reforming, electrochemical routes through water electrolysis using renewable power sources, thermochemical pathways involving renewable feedstock as the hydrogen carrier and biological processes (Valente et al. 2021 ; Owgi et al. 2021 ). However, environmental sustainability based on LCA remains one of the key requirements for selecting these processes for hydrogen production (Falcone et al. 2021 ). This is because policymakers need to adopt transformative solutions based on robust data and evidence-based research to identify processes that go beyond a one-fits-all approach.
To this end, we reviewed 24 LCA studies published from 2019 to 2021 on hydrogen production and life cycle assessment (Table 2 ). The four main stages defined by ISO 14040 and IS0 14,044 for conducting LCA are: (1) goal and scope definition, (2) life cycle inventory analysis, (3) environmental impacts assessment and (4) life cycle interpretation (Al-Muhtaseb et al. 2021 ).
Goal and scope of the life cycle assessment
The first stage of LCA consists of defining a goal and the scope of the study. This stage determines whether a study would be attributional or consequential, what functional unit will be considered to evaluate environmental impacts and the extent of the system boundary. This is an important initial step as the questions to be answered determine the results and associated policy implications.
Types of life cycle assessment: attributional and consequential
Life cycle assessment studies can be broadly classified into two categories: (1) Attributional LCA incorporates immediate physical flows such as raw materials, energy and emissions involved across the life cycle of a product (Jeswani et al. 2020 ), and (2) consequential LCA accounts for how physical flows can change as a consequence of an increase or decrease in demand for the product system under study (Earles and Halog 2011 ). It includes unit processes inside and outside the product's immediate system boundaries; therefore, consequential LCA studies are more suited for policy decisions. However, as LCA for hydrogen production remains at an embryonic stage, attributional studies are more commonly found. Nevertheless, both attributional and consequential approaches were considered for the purpose of this study.
Functional unit
In LCA, the functional unit is a measure of the purpose of the studied system, and it provides a reference by which the inputs and outputs can be related. This enables the comparison of two essentially different systems. The definition of the functional unit is intricately linked to the goal of an LCA study. It was observed that ~ 42% of the reviewed studies used ‘kg of hydrogen produced’ as the functional unit (Fig. 3 ). While some studies provided results considering hydrogen as an energy carrier and therefore recorded functional unit as ‘energy produced in MJ or kWh’. Very few studies reported ‘distance travelled in km’ as a functional unit when hydrogen was utilised as fuel for vehicles. The choice of different functional units for the same product, i.e. hydrogen, shows the challenges associated with comparing LCA models.
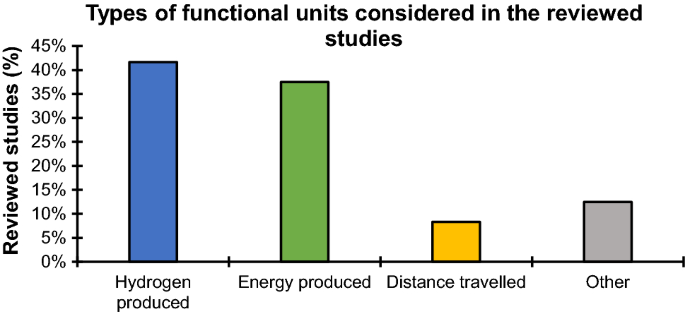
Types of functional units used in the life cycle assessment studies reviewed in the present work (N = 24)
System boundary
In LCA, the system boundary definition profoundly impacts the materials, processes and emissions considered for evaluation. As such, system boundary limits can also considerably influence the calculation of environmental impacts (Collotta et al. 2019 ). The two commonly studied kinds of system boundary for hydrogen production are ‘cradle-to-gate’ or ‘well-to-pump’ that includes processes only until production and ‘cradle-to-grave’ or ‘well-to-wheel’, which incorporates emissions during end use as well.
The generalised system boundary used for conducting the LCA of hydrogen production and consumption includes: (1) raw materials and primary energy sources such as natural gas, coal, biomass, nuclear energy and water; (2) the hydrogen production processes, for instance, water electrolysis and thermochemical processes. Some processes may also consider hydrogen purification as a subsystem to the production; (3) storage of hydrogen in underground caves or compressed tanks; (4) transportation of hydrogen in liquified or compressed gaseous form using trucks and tube trailers or pipelines; (5) emissions during end use such as by hydrogen trains or generation of power using hydrogen; and (6) finally, waste treatment processes from these systems such as emissions to land, air and water (Fig. 4 ).
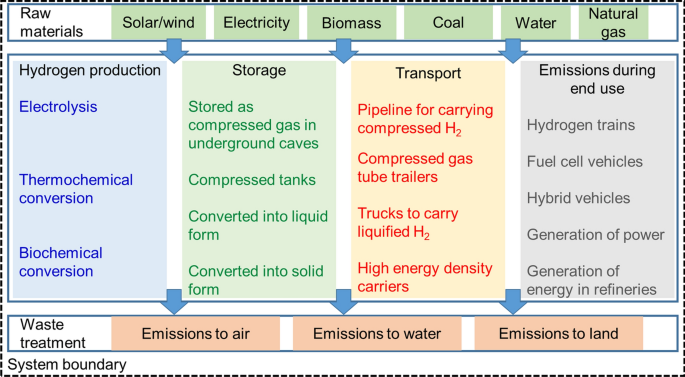
Generalised system boundary used for conducting life cycle assessment of hydrogen production and consumption. This includes various raw materials such as solar, wind, biomass, coal, water and natural gas
During the review, we observed that studies employed an array of processes and limits in system boundary for conducting LCA of hydrogen production and consumption (Table 3 ). There were only a handful of studies that considered emissions during the use phase. However, given the increasing interest in using hydrogen as a clean energy carrier, it is important to consider the emissions during the use phase and conduct LCAs that present ‘well-to-wheel’ estimates.
Allocation approaches
The allocation approach refers to both ‘partitioning’ and system expansion/substitution method. The allocation approach has been identified to significantly control the values obtained for environmental impacts (Finnveden et al. 2009 ). Allocation approaches are required because the life cycle of a product can consist of many multifunctional processes. Therefore, it is imperative to allocate the environmental impacts between the different coproducts generated by the same process in a justified manner.
Life cycle inventory analysis
Life cycle inventory analysis includes data collation for all the inputs and outputs for processes within the system boundary. In general, the more the processes included in the system boundary, the more complex, challenging and cumbersome is the inventory analysis. This also explains the fact that many studies did not include all the processes ranging from raw material acquisition to end-of-life management (summarised in Table 3 ). The two different kinds of data to be collected for an LCA study are: (1) foreground data for foreground systems which includes primary data that can be easily modified or improved and (2) background data for background systems typically comes from Life Cycle Inventory databases (Silva et al. 2020 ). Background systems support the foreground systems. Table 2 details the databases/data sources incorporated in LCA studies on hydrogen production such as Ecoinvent, expert communications, Greenhouse gases, Regulated Emissions and Energy use in Transportation.
Environmental impacts assessment
Midpoint and endpoint indicators.
Global warming potential due to emissions of greenhouse gases and depletion of fossil fuels was the centre of the attention in the environmental indicators for hydrogen production, with 100% of the studies accounting for either of these two categories (Table 4 ). More than half (54%) of the reviewed studies computed environmental impacts in categories that go beyond global warming potential and net energy use/performance. These environmental impacts included but were not limited to acidification, eutrophication, abiotic depletion, marine, freshwater and terrestrial ecotoxicity, and human toxicity.
Global warming potential expressed as kg CO 2 equivalent relates to greenhouse gas emissions; abiotic depletion recorded in kg Sb equivalent is linked to depletion of minerals, peat and clay; acidification reported in kg SO 2 equivalent is due to the emission of acidifying substances; eutrophication measured as kg PO 4 3− equivalent is due to release of nutrients; particulate matter formation calculated as PM 2.5/PM 10 equivalent relates to the emission of PM 2.5 (particulate matter with ≤ 2.5 µm in diameter) and/or PM10 (particulate matter with ≤ 10 µm in diameter). Photochemical oxidation (commonly called as ‘summer smog’) occurs in stagnant air, in the presence of pollutants such as NO x , non-methane VOCs and others. Ozone layer depletion evaluates the global loss of ozone gas caused by trichlorofluoromethane (CFC-11) of the same mass. Land use calculated in m 2 is categorised as the transformation of urban, agricultural and natural land. Damage to terrestrial, freshwater and marine ecosystems is measured by ecotoxicity potential. Finally, human toxicity is caused due to the potential human health impacts of carcinogenic and non-carcinogenic pollutants.
The midpoint categories are aggregated to present results as endpoint categories such as human health, damage to ecosystem quality in the form of loss of species and resources depletion (Osman et al. 2021b ). It is argued that the environmental impacts should be presented as midpoint categories to prevent oversimplification or misinterpretation of environmental impacts (Kalbar et al. 2017 ). This is because endpoint indicators entail weighting of impacts. Evidently, only one study was identified that presented environmental impacts for both midpoint and endpoint indicators (Ozturk and Dincer 2019 ).
Uncertainty and sensitivity analysis
Uncertainty arises in LCA studies due to sparse and imprecise nature of the available data and model assumptions (Cherubini et al. 2018 ). It is, therefore, imperative to consider and compute these uncertainties quantitatively to reach transparent, robust and trustworthy decisions.
There has been a vast development on the methods to imbibe these uncertainties in LCA models such as parameter variation and scenario analysis, classical statistical theory (e.g. probability distributions and tests of hypothesis); Monte Carlo simulations, bootstrapping and other sampling approaches; nonparametric statistics, Bayesian analysis, fuzzy theory; and the use of qualitative uncertainty methods (Finnveden et al. 2009 ).
This review recorded that 67% of the studies used scenario analysis to account for parameter uncertainty (Fig. 5 ). Together with comparative studies mentioned in (Table 2 ) and scenario analysis in Fig. 5 , this value reaches 96%, i.e. all but one study performed comparative and/or scenario analysis (Cvetković et al. 2021 ). This can be attributed to the dearth of the data and the serious effort required to conduct an LCA of biohydrogen production via anaerobic digestion (Cvetković et al. 2021 ). Furthermore, it was noted that 8% of the studies employed Monte Carlo simulations to propagate parameter uncertainties in the model.
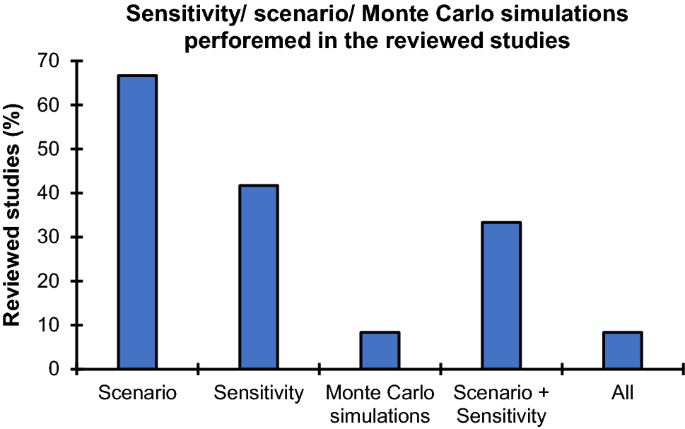
Details of the scenario, sensitivity and Monte Carlo simulations (to propagate uncertainty) conducted in the reviewed studies (N = 24). Scenario analysis was conducted in 67% of the reviewed studies
Sensitivity analysis is conducted to distinguish processes in the hydrogen production chain that contribute to the burdensome environmental footprints. Relatedly, if environmental impacts are to be minimised, these will be the processes where future research should focus on (Al-Muhtaseb et al. 2021 ). 42% of the studies reviewed here conducted sensitivity analysis.
Interpretation of results
This stage of the LCA includes making interpretations, drawing conclusions and distinguishing the processes that can be improved to increase the environmental feasibility of the system. This stage could also involve presenting and communicating results to stakeholders. Table 5 summarises key findings from the reviewed studies.
Key findings and recommendations for future life cycle assessment studies
Life cycle assessment is a complex tool that sits at the interface between science, engineering and policy. Despite this inherent complexity, it is recognised as a comprehensive tool to evaluate environmental impacts associated with products and processes. We reviewed LCA studies published from 2019 to 2021. This section draws recommendations for policymakers to create a sustainable hydrogen economy and LCA practitioners to conduct future studies.
During the review, no two LCA studies were identified to be similar. Differences in the geographical and temporal span, functional units and system boundaries considered, and environmental impact categories were reported. Therefore, it is recommended that the policymakers pay heed to the modelled processes and extent of the system boundary for making decisions for creating a sustainable hydrogen economy.
Most of the studies did not encompass processes, inputs and outputs for ‘cradle-to-grave’ LCA analysis. Thus, future studies should conduct ‘cradle-to-grave’ evaluation for robust decision-making.
About 54% of the reviewed studies computed environmental impacts in categories that go beyond global warming potential and depletion of fossil fuels. It is crucial to assess environmental impacts in more categories. Otherwise, there can be the issue of burden shifting, where hydrogen production processes are developed to mitigate climate change and energy security, however, leading to severe environmental and human health impacts such as acidification, eutrophication and human toxicity.
Finally, focusing on production pathways, only eight studies were identified that computed environmental impacts for biohydrogen, showing that there is a considerable knowledge gap in production processes utilising bio-based feedstocks.
Hydrogen underground storage
There are ambitious goals of the Paris agreement for climate change to be met than ever by 2050. However, the continuous increase in carbon dioxide (CO 2 ) emission generated by the use and storage of fossil fuels has created a clear demand for alternative sources of clean and renewable energy (Ochedi et al. 2021 ). Solar and wind energy, however, provide intermittent and volatile power sources (as shown in Fig. 6 ) that are requiring backup solutions and/or energy storage at scales comparable to their power generation capacity (i.e. longer-term TWh storage solutions). In particular, some industrial sectors are hard to be decarbonised. To help balance the energy supply and demand, a capability of various energy storage technologies, with a dynamic combination of daily, weekly and seasonal storage, can reduce CO 2 emissions per unit of energy provided.
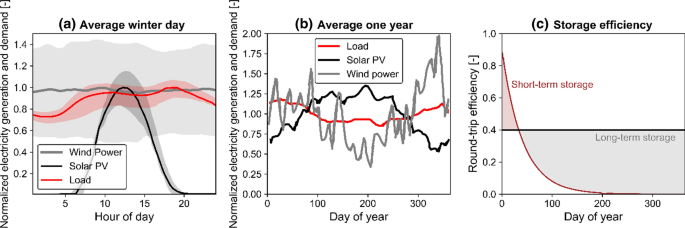
[A] Diurnal time series shows the matching of load, wind and solar of a typical day during the winter season for Europe with 15th and 85th percentiles for each average day time series. [B] Annual time series of weekly averages illustrate the seasonal correlation (i.e. excess/shortage) of load, wind and solar. Electricity generation and demand normalised over the corresponding average value. [C] Schematic round-trip efficiency for a short-term (e.g. battery, brown line) and long-term (e.g. power to hydrogen, black line) storage technology. The figures were adapted from (2017) and (Gabrielli et al., 2020 )
To date, the technical feasibility and economic attractiveness for developing large-scale, lithium-ion-based and seasonal energy storage batteries can be challenging to be implemented and provide an energy supply during high demand times. Such shortfall can be eliminated by storing the excess renewable energy chemically—in the form of hydrogen—in the subsurface aquifers, salt caverns and/or exhausted hydrocarbon reservoirs in the so-called Underground Seasonal Hydrogen Storage (USHS). The usage of hydrogen as an energy carrier can be a promising solution for clean energy because of its non-toxicity, high specific energy and non-CO 2 emission after combustion. The challenge is to find hydrogen storage materials with high capacity. USHS, therefore, can be one of the most promising solutions for offsetting seasonal mismatch between energy generation and demand (Fig. 6 ), firstly for medium- and long-term storage while increasing contribution to low-carbon energy supply. Despite the vast opportunity provided by USHS, maturity still is considered low, with several uncertainties and challenges (Heinemann et al. 2021 ).
Hydrogen-based economy requires a large gas transport infrastructure. It has been suggested that existing natural gas pipe networks could be used to transport hydrogen (Melaina et al. 2013 ; Panfilov 2016 ). The gases would be transported as a mixture and separated afterwards. Some methods for separating mixtures of methane and hydrogen, particularly gas membrane separation, appear promising (Ockwig and Nenoff 2007 ).
Geologically, underground formations are suitable for storing hydrogen, which may then be used as a carrier of chemical energy produced in times of surplus energy production, stored for several months and ultimately retrieved for re-electrification when it is needed most (Bauer et al. 2013 ; Bauer et al. 2017 ). As an illustration of the possible storage potential, a system volumetric capacity (i.e. the Net Energy Density) of hydrogen-based flow battery stores approximately 2.7 kWh/L (NREL) of electrolyte, and hence, an exhausted million-barrel oil field would hold > 3 TWh of electricity. This is equivalent to 30 weeks’ output from a large offshore wind farm which is far more than is needed to eliminate the intermittency issues associated with such a facility. Hence, it was proved that only a few offshore gas fields are required to store enough energy as hydrogen to balance the entire seasonal demand for UK domestic heating (Mouli-Castillo et al. 2021 ).
Thermophysical properties of hydrogen
After hydrogen is produced at the surface from one of the technologies, it must be transported to a seasonal storage facility in a liquid or gas phase. Moreover, hydrogen can also be stored on the surfaces of solids (i.e. by adsorption) or within solids (i.e. by absorption) (El-Eskandarany 2020 ). During the loading cycle, where the power demand is at a peak, hydrogen can be easily re-converted for electrical generation.
Hydrogen can be considered as an ideal gas that may occur in various states over a wide temperature range and even at high pressures. Here, the thermophysical properties of hydrogen at the conditions relevant to the underground hydrogen storage were provided. One of its most important thermophysical characteristics is its low density, making it necessary for any practical application to compress the hydrogen or liquefy it. At intended storage depths, the density and dynamic viscosity of hydrogen are iteratively calculated using equation of state (EOS) and following (Span et al. 2020 ). Primarily, the hydrogen density (kg/m 3 ) mainly increases with increasing pressure while dynamic viscosities (μPa.s) significantly increase with increasing temperature, as shown in Fig. 7 . At low temperatures of − 262 °C, hydrogen is solid with a density of 70.6 kg/m 3 . At higher temperatures, hydrogen is a gas with a small density of 0.089 kg/m 3 at 0 °C and at a pressure of 1 bar. The extent of hydrogen's liquid state can be presented as a narrow zone between the triple and critical points, with a density of 70.8 kg/m 3 at − 253 °C.
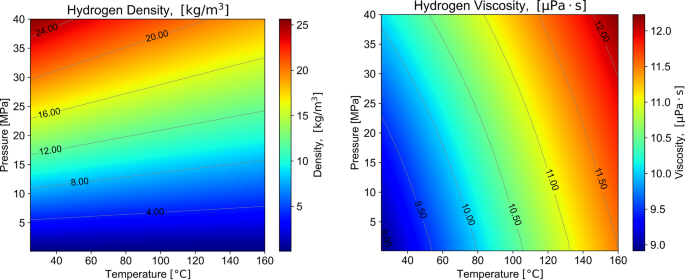
(left) Density [kg/m 3 ], (right) dynamic viscosity [µPa.s] of hydrogen at representative P–T conditions which are typical for Underground Hydrogen Storage system. The calculations were carried out by the authors, using the fundamental properties of Hydrogen as an ideal gas. By the time pressure of > 35 MPa is reached, a deviation of 15% from the real values is expected
Three potential technologies for hydrogen storage, therefore, can be considered according to combinations of pressure and temperature relevant to the storage conditions (Table 6 ):
Cryo-compressed hydrogen storage (CcH 2 ) and liquid hydrogen (LH 2 ) storage: storage of hydrogen as a liquid requires cryogenic temperatures because the boiling point of hydrogen at one-atmosphere pressure is − 253 °C with a density of close to 71 kg/m 3 . These properties make storing hydrogen under standard atmospheric pressure and temperature extremely difficult due to the high cost and safety issues. Whereas other gases can be liquefied around the standard temperature of 20 ºC, this is unfortunately practically impossible for hydrogen. Therefore, hydrogen needs compression into cryogenic vessels that can be pressurised to 25–35 MPa. Accordingly, the size of liquid hydrogen requires larger tanks reaching about three times larger than the currently used gasoline tank (El-Eskandarany, 2020 ).
For pressure ranges between 5 and 30 MPa and temperature between 25 and 130 °C, hydrogen can safely be stored as a gas in underground geological formations. For USHS, hydrogen must be transported to a wellhead for underground storage. The hydrogen must then be compressed to be injected at sufficient pressure to enter the geological formation at the in situ pressure and temperature. Different potential geological storage sites for USHS are shown in Fig. 10 and will be discussed in more detail in the following sections.
Additionally, pressurised hydrogen gas takes a great deal of volume compared with, for example, gasoline with equal energy content—about 30 times bigger volume at 10 MPa gas pressure (El-Eskandarany 2020 ). USHS basically implies the reduction of the enormous volume of hydrogen gas due to the reservoir pressure gradient (Fig. 8 ). One kilogram of hydrogen in ambient temperature and at atmospheric pressure occupies a volume of 11 m 3 .
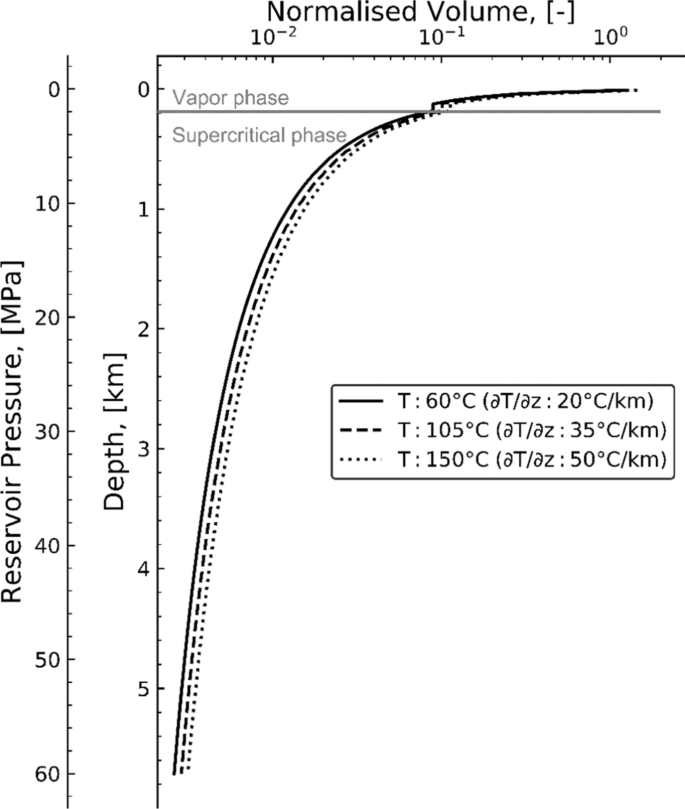
Normalised volume of hydrogen at the pressure–temperature (over the range of geothermal gradients) conditions plotted as a function of depth. Grey horizontal line at 800 m marks the minimum depth recommended for hydrogen injection, where it can be found as a supercritical phase at pressure and temperature conditions relevant for USHS (above 1.3 MPa)
Fluid dynamics of hydrogen in a brine-saturated porous medium
In the context of the USHS system, the cyclic injection of hydrogen into (and possible retrieval from) a brine-filled permeable formation is part of multi-phase flow problems that have been studied extensively (Hashemi et al. 2021 ; Liebscher et al. 2016 ). In this case, a two-phase hydrogen–brine system is immiscible—the fluids are separated by a capillary interface. Likewise, the CO 2 geological storage, an important first approximation to the behaviour of the hydrogen–brine system, is found via applying a group of dimensionless ratios and solubility (and hence its mobility) that analyse the dynamics of two-phase immiscible flow systems (Ringrose et al. 2021 ). Viscous/capillary ( N vc ) and gravity/viscous ( N gv ) ratios are, respectively, the characteristic time ratios for fluid to flow in the transverse direction due to capillary and gravity forces to that in the horizontal direction due to viscous forces using the assumption of (Zhou et al. 1997 ). The two fluids here are assumed to be vertically segregate due to the gravity and density difference. Both ratios can be formulated in Eqs. 7 and 8 as follows:
where u x is the total flow velocity in the horizontal (x) direction, ∆x and ∆z are the system dimensions, μ nw is the viscosity of the non-wetting phase (hydrogen), k av is the average permeability, ∆ρ is fluid density difference, g is the acceleration due to gravity and (dP c /dS w ) is the capillary pressure gradient as a function of wetting-phase saturation.
Around the injection/production wellbore, viscous-dominated conditions are expected to occur due to the high-pressure gradient (Ringrose et al. 2021 ). However, within the reservoir and away from the injection/production wellbore region, gravity-dominated conditions are expected to occur. Such ratios, therefore, can be used to expect the fluid dynamic behaviour of the hydrogen-brine flow system and determine which factors are likely to be most critical, particularly when assessing large-scale macroscopic fluid flow, where the capillary and gravity forces become important enough to be not neglected.
Another important factor for USHS is the solubility of hydrogen in the resident formation fluid (water/brine). Therefore, forecasting the phase equilibria (solubility of hydrogen in brine and water content in the hydrogen-rich phase) under the geological storage conditions (i.e. at different temperatures, pressure and molality) is necessary for the study of hydrogen mobility and reactivity, as well as the control, monitoring and optimisation of the storage. Based on new experimental datasets, Chabab et al. developed predictive models to estimate the water content in the hydrogen-rich phase and precisely capture the salting-out effect on hydrogen solubility (Fig. 9 ) (Chabab et al. 2020 ).
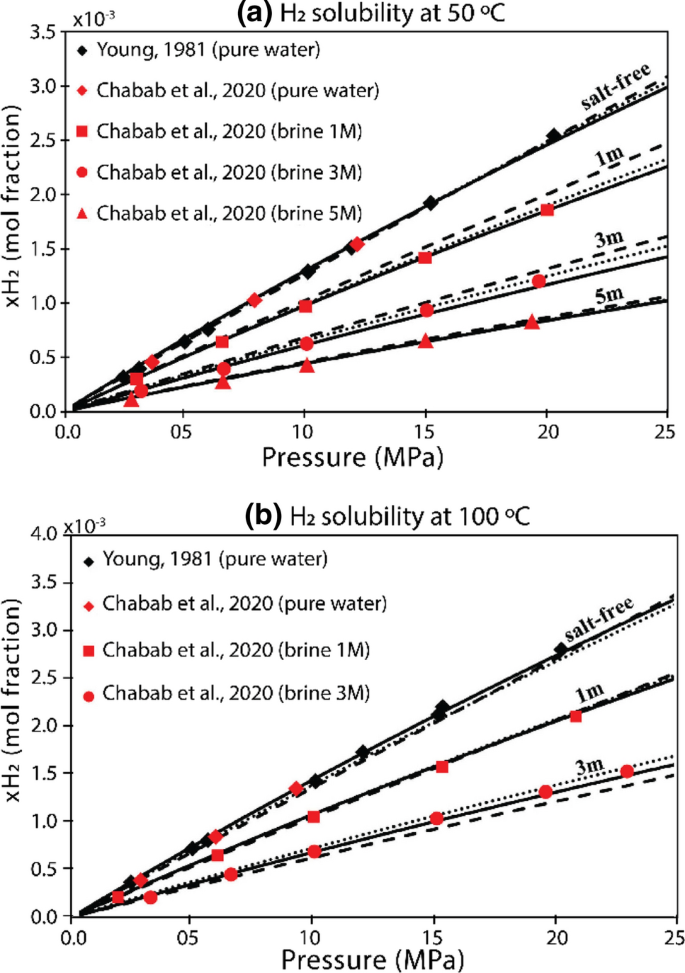
Solubility of hydrogen in pure water as well as the brine of different molalities (up to 5 M), as a function of pressure (up to 25 MPa), and at the temperature of 50ºC [a] and 100ºC [b]. The symbols represent experimental results from the literature (Chabab et al., 2020 ). The solid, dotted and dashed lines represent the hydrogen solubilities calculated by the e-PR-CPA, SW and geochemical models, respectively. The figure is modified from Chabab et al., ( 2020 )
Large-scale hydrogen geological storage
A promising solution to help balances the energy supply from renewable intermittent sources and demand is hydrogen as an energy carrier for clean energy and must be accompanied by energy storage systems. The benefits of using hydrogen are because of its non-toxicity, high specific energy and non-CO 2 emission after combustion. However, the challenge is to find hydrogen storage materials with high capacity. Large-scale underground storage of natural gas has been practised successfully for many decades, with a global total of 413 billion standard cubic metres (BSCM) of natural gas storage accommodated in depleted gas fields (80%), underground aquifers (12%), and engineered salt caverns (8%) (Perry 2005 ), as shown in Fig. 10 . Here, these types of underground hydrogen storage systems have been considered (Lord et al. 2014 ; Panfilov 2010 ).
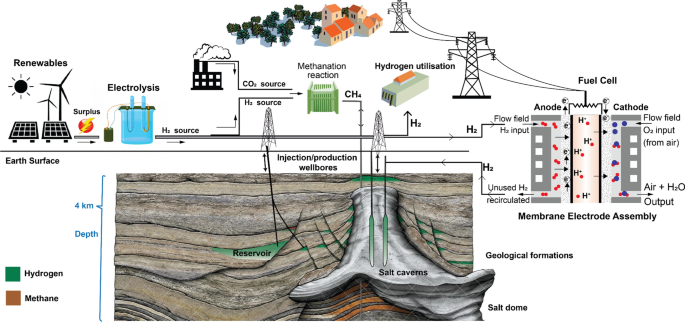
Schematic diagram of different processes which are associated with hydrogen production using electrolysis, seasonal storage in geological formations and/or salt caverns, utilisation for ammonia production and re-electrification of hydrogen using fuel cells. The figure shows different potential storage mediums for the hydrogen in the underground geological formations: reservoir/aquifer and salt caverns. The dimensions are not to scale
Depleted hydrocarbon reservoirs
More often than not, depleted hydrocarbon reservoirs are appealing targets for USHS because of their storage capacity, proven seal, previous knowledge of reservoirs characterisation and existing infrastructure (i.e. natural gas pipeline network). Nevertheless, various physical, chemical and microbial processes are associated with USHS in hydrocarbon reservoirs (Heinemann et al. 2021 ) (summarised in Fig. 10 ).
While one can transfer know-how and technology from underground natural gas storage and underground carbon storage, some of the challenges USHS faces are peculiar. In both compressed gas and liquid forms, the low density of hydrogen makes the seasonal storage of hydrogen in porous media (and possible retrieval) problematic. With a mass–density ratio of less than 0.01 compared to water for most relevant subsurface storage conditions, H 2 is very light. Consequently, an H 2 plume would experience strong buoyancy forces (i.e. the stronger the buoyancy forces, the higher the potential for hydrogen leakage), and water upconing towards the extraction borehole may occur (Heinemann et al. 2021 ; Sainz-Garcia et al. 2017 ).
This limitation is felt most strongly during the hydrogen retrieval from the subsurface. The gas saturation around the production well required to keep a gas well flowing is of major concern since it will impact and reduce the production and ultimately will kill the well. The thinner the hydrogen plume will be, the lower gas saturation and the higher accumulation of resident formation brine in the downhole. Therefore, the dynamics of the USHS system require a wellbore model capable of describing/predicting the conditions (pressure and temperature) in the extraction borehole as the fluid(s) flow up (or the liquid accumulation at the bottom of) the borehole.
Water upconing is the change in the hydrogen–water contact profile due to drawdown pressures. This phenomenon can be seen as the name implies: a cone of water formed below the perforations. One way to avoid upconing during H 2 production is the use of a cushion gas (Kim et al. 2015 ; Oldenburg 2003 ), usually a cheaper and denser gas like nitrogen (N 2 ), which helps prevent water flooding of the gas plume when H 2 is being produced. This concept is well known in underground natural gas storage and has previously been proposed for USHS (Cao et al. 2020 ).
Additionally, it is important to note that USHS involves cyclic hydrogen injection (i.e. during power surplus) into and withdrawal (i.e. during energy demand) from the geological formations, where changes in the reservoir pressure may induce fatigue in the caprock and lowering the fracturing pressure at which hydrogen commences to leak through a seal rock. Therefore, assessing the sealing capacity to hydrogen (or hydrogen column height) will be crucial to keeping the risk of the potential upward leakage of hydrogen through the sealing caprock at a minimum. Seal rocks have fine pore and pore throat sizes that, in turn, generate hydraulically tight low-permeability caprocks with high capillary threshold pressures. High threshold pressures, together with wettability and interfacial tension (IFT) properties, determine the final column height that a seal can hold, thereby affecting the ultimate reservoir storage volumes. Compared to the underground natural gas storage, higher capillary entry pressures are expected to occur for hydrogen due to its higher interfacial tension (Hassanpouryouzband et al. 2021 ; Naylor et al. 2011 ). Therefore, hydrogen can be stored at a higher pressure in the reservoir than methane, with a reduced risk of geomechanical failure.
On the hydrogen injection into a storage reservoir, a very small fraction of hydrogen will dissolve into the formation fluids (Chabab et al. 2020 ), and water vapour may contaminate the hydrogen phase due to chemical disequilibrium. Hydrogen losses through diffusion need to be considered, as the diffusion ability of hydrogen is several times more than that of CO 2 and methane, to such an extent that hydrogen can travel between the structures of ice-like crystals (Hassanpouryouzband et al. 2020 ).
In order to show the influence of the large density difference (Fig. 11 ) between the injected gas (hydrogen) and the resident formation fluid (brine) on the hydrogen plume migration during the seasonal storage period, we numerically simulate the injection of 10-ton kg of hydrogen over 10 days and its storage for 35 days. We used the numerical simulator PorousFlow Module, open-source software for solving parallel tightly coupled nonlinear THM processes in porous media (Wilkins et al. 2021 ; Wilkins et al. 2020 ). It is based on the MOOSE framework (Gaston et al. 2009 ) and its internal architecture relies on state-of-the-art libraries for finite element analysis (Kirk et al. 2006 ) and nonlinear iterative algebraic solvers (Balay et al. 2019 ). The simulation results are shown in Fig. 11 . It is shown from the simulation standpoint that the leakage rate of hydrogen is going to be the biggest challenge due to the very high mobility of hydrogen, the small molecule size, the high dispersion rate and the large density difference between the hydrogen and brine. Therefore, a proper tightness assessment of the caprock above the reservoir is required to prove its effectiveness for any possible hydrogen leakage. In addition, we propose expressly storing H 2 /CH 4 gas mixtures to improve the density contrast with the water. The mixed gas can, upon demand, then be extracted and transported in the same natural gas pipelines.
Hydrogen–brine displacement in an idealised 2D horizontal cross section (i.e. geological storage formation). The injection wellbore is located at the left-hand side of the simulated domain. The subfigures are showing only the first 50 m horizontal distance from the injection well with 10 × horizontal exaggeration. The horizontal exaggeration is 10x. [A] the reservoir is fully saturated with brine (i.e. before the hydrogen injection start). The migration of the hydrogen phase after [B] 9 days, [C] 23 days, [D] 36 days and [E] 45 days
Subsurface microorganisms, including methanogens, sulphate reducers, homoacetogenic bacteria and iron(iii) reducers can make use of H 2 as an electron donor, which may lead to an unwanted accumulation of biomass in the vicinity of the injection borehole and/or loss of H 2 (Ganzer et al. 2013 ; Hagemann et al. 2015 a). The local rate of the biochemical reactions depends on the number of the particular microorganism (Hagemann et al. 2015 b). Hence, an important problem for the modelling of USHS is the description of microbial growth and decay functions. Microbial conversion of hydrogen can only occur if the hydrogen is in the aqueous phase. A mixture of hydrogen with another gas means it will have a lower partial pressure and hence lower solubility in water. It was stated that if the temperature of the formation is higher than 122ºC or the salinity is higher than 5 M NaCl, the hydrogenotrophic microbial activity becomes highly unlikely (Thaysen and Katriona 2020 ). Hence, if a storage reservoir is hot enough, one can combine hydrogen storage with CO 2 , since methanogenic microbial activity will be limited by the temperature constraint. Further, a high-pressure environment is toxic for some microorganisms.
Considering the deep depleted gas-condensate reservoirs, the risks are minimised here due to the presence of well-defined geological traps related to previously formed gas reservoirs. Unfortunately, the risk of migration from the target storage formation cannot be eliminated completely, particularly due to the re-pressurisation and change of the stresses and the long-term well integrity issues of the casing and cement.
Salt caverns
Another underground storage medium, which could be used under certain conditions and locations, is the usage of salts caverns as high-pressure gas storage facilities (Fig. 10 ) (Gabrielli et al. 2020 ; Hassanpouryouzband et al. 2021 ; Pudlo et al. 2013 ; Foh et al. 1979 ). Based on energy storage capacity (GWh) and discharge timescale, storing hydrogen in salt caverns can afford utility-scale, long-duration energy storage to meet the market need to shift excess off-peak energy to meet dispatchable on-peak demand. Salt caverns can hold substantial promise due to the self-sealing nature of the salt and the ability to customise the size and often shape of the caverns (Lord et al. 2014 ). However, the inaccessibility of the salt caverns in the area where hydrogen production is can be a limiting factor.
Salt caverns can be artificially constructed in the salt formation (or salt dome) by injecting water through an access wellbore, dissolving the salt and generating large volumes of brine in the so-called solution mining process. This process is associated with retrieving a large quantity of brine which requires disposal in an eco-environmental way. Finding suitable disposal repositories for brine disposal can be economically problematic due to higher costs for constructing longer pipelines which eventually may slow down or even hinder the permitting process. During the hydrogen withdrawing from the caverns under constant pressure, part of this saturated brine can be injected into the caverns to maintain the caverns' pressure and stability. Cushion gas, therefore, is not needed under these operating conditions (Foh et al. 1979 ; Taylor et al. 1986 ).
Compared to depleted oil and gas reservoirs, the key advantages for storing hydrogen in salt caverns are: (1) salt surrounding the caverns is highly impermeable and virtually leakproof where the only possibility for gas loss is escaped through leaky wells (Lord et al. 2014 ). (2) Salt does not react with hydrogen (Bünger et al. 2016 ). (3) Withdrawal of ‘discharge’ of hydrogen is highly flexible in rate, duration and volume with lower cushion gas requirements to avoid rock breakage. (4) Caverns are a mature, financeable storage technology that has been successfully used to store compressed gases for over 75 years with possible extensions for USHS.
The city of Kiel’s public utility, as an illustration, has been storing town gas with a hydrogen content of 60–65% in a gas cavern with a geometric volume of about 32,000 m 3 and a pressure of 8–16 MPa at a depth of 1330 m since 1971 (Kruck et al. 2013 ; Carpetis, 1988 ) estimated the hydrogen storage capacity for cavern volume of 500,000 m 3 and a casing shoe depth of 1000 m a pressure range of 180 to 60 bar is suitable of 4.0 Mio kg hydrogen (47 Mio m 3 (st)) and a cushion gas of 2.2 Mio kg (26 Mio m 3 (st)). For an economic prospect, the total installed costs, including wellbore drilling, compressors and gas treatment, were estimated to be about € 100 million (Michalski et al., 2017 ). Compared to energy storage in Li-ion batteries with a cost of 100 €/kWh, USHS in salt caverns offers a significant cost reduction potential in the total investment cost by a factor of 100.
Storage of hydrogen in the form of methane (natural gas) may be a preferable alternative for overcoming the storage problems associated with storing pure hydrogen in geological formations. When there is a surplus of renewable energy in the summer, hydrogen can be produced through water electrolysis. Furthermore, when this hydrogen and carbon dioxide combine in the methanation reaction, methane is produced, which can then be stored in a geological reservoir for winter use. This could be accomplished through a methane reforming reaction followed by using a fuel cell to generate electricity that can be fed into the power grid.
In short, hydrogen storage in a geological medium can offer a viable option for utility-scale, long-duration energy storage, allowing the hydrogen economy to grow to the size necessary to achieve net-zero emissions by 2050. While the operational experience of storing town gas in salt caverns provides considerable proof of its viability and operational best practice, full-scale deployment of USHS has yet to be evaluated for any associated risks and public acceptance of viewpoints, similar to the potential for induced seismicity.
- Hydrogen utilisation
Fuel and power systems
Globally, the heat generated from domestic as well as industrial activities contributes by 33 and 50% of the carbon dioxide emissions and universal energy consumption rate, respectively (Dodds et al. 2015 ). The majority of gaseous emitted by the conventional burning process of natural gas are implicated in numerous environmental contamination issues (i.e. greenhouse gaseous emissions). The primary source of carbon dioxide emissions was energy consumption, with a global emissions rate of 33.1 gigatonnes in 2018, mainly resulting from the burning of fossil fuels. Contrarily, applying hydrogen gas as an alternative fuel to natural gas has proved to be an efficient pathway to reduce greenhouse gaseous emissions. Once it is generated from renewable energy sources, as shown in Fig. 1 , it can directly participate in the decarbonisation process in the energy sector thanks to its reacting nature, whether combusted or utilised in the fuel cell. The hydrogen is currently produced by conventional (non-renewable sources) of 18%, 30% and 48% from coal, heavy oil/naphtha and natural gas, respectively, which was negatively responsible for releasing about million 560 tonnes of carbon dioxide per year (Lui et al. 2020 ).
Moreover, given the costly natural gas employed throughout the power-producing framework (i.e. requires a huge area to store), hydrogen appears to be a viable option as a fuel feeding to gas turbines (Bicer and Khalid 2020 ). The utilisation of hydrogen in the central heating system instead of natural gas offers numerous merits: comparable operational activity and an increased heat generation rate with minimal harmful emissions (Dodds et al. 2015 ). Several factors, such as the Wobbe index, should be considered before forwarding hydrogen to various appliances. Generally, Wobbe index values differ considering the chemical composition of the gas. The Wobbe index number of pure hydrogen is about 48 MJ/m 3 ; it falls within the permissible natural gas integrity extent for the vast majority of burners (Zachariah-Wolff et al. 2007 ). Supplying the operating system with a fuel beyond the Wobbe index band can negatively result in some operational problems (i.e. incomplete combustion and burner overheating). Clearly, attributing to the hydrogen's higher combustion velocity compared with the natural gas fuel, advanced burners with specialised technical specifications must be operated with hydrogen as a fuel feed stream.
Furthermore, the overabundant electricity generated from power facilities can be transformed into hydrogen, which can be either directed to the existing natural system (direct consumption) or chemically converted into chemicals used in different industrial aspects (Collet et al. 2017 ). Besides, hydrogen can be used individually in the aerospace industry or in combination with oxygen as propellants. The mentioned liquid mixture (oxygen and liquid) generates a large amount of energy and makes it more suitable for space applications. Because of releasing water during hydrogen combustion, in addition to its high efficacy compared with gasoline, these characters qualify it to be employed as an automotive fuel (Gurz et al. 2017 ).
Hydrogen employment in power systems
Hydrogen is enormously used to store and transport energy in a variety of power applications, typically illustrated in Fig. 1 and discussed as follows (Parra et al. 2019 ):
Storing of energy and auxiliary services
Given the hydrogen's high storing efficacy, hydrogen-based energy storage has gained traction for storing energy over a medium/long term and in auxiliary services in the last decades. It can meet energy storage requirements over a broad timescales to avoid any defect (shortage) that may occur between the product and the demand (required) of energy (Al Shaqsi et al. 2020 ). Recently, renewable energy production has grown rapidly; however, certain renewable energy supplies are sporadic and seasonally dependent. As a result, the produced renewable energy should be stored in a dependable form that is resistant to the fluctuation in those energy sources (Mehrjerdi et al. 2019 ). In particular, the most popular types of energy storage are: (1) power-to-power, (2) power-to-heat and (3) power-to-gas (Widera 2020 ). Hydrogen, in comparison, has a large energy storing capacity, a great storing time and flexibility. It has the ability to reduce energy volatility and absorb the surplus of energy production. Practically, it can deal with the economic and seasonal variations issues. Hydrogen can exceptionally balance between the resultant and required energies by storing the surplus energy when the production rate exceeds the required one as well as in times at which the electricity's price is minimal and reuse it in the opposite cases. Contrarily, hydrogen can be forwarded to generate electricity in the high energy demand.
Moreover, the storing capacity of hydrogen is higher than batteries, as it may range to weeks or months, unlike batteries that may extend (limited) for hours (Bocklisch 2016 ). Otherwise, hydrogen can be subjected to transform renewable resources to produce energy during different climatic conditions in different seasons. The storage capacity of hydrogen is estimated to reach up to megawatt-hours (1000 Kilowatts hours), even terawatts-hours, which is considered a high value by considering that of batteries (i.e. kilowatts hours). A slew of hydrogen power storage plants has been commenced worldwide, showing the technology's potency for the large scale. Examples of power plants established to produce and store hydrogen are Underground Sun Storage, Orsted and SoCalGas in Austria, Denmark and USA, respectively (Home | SoCalGas, https://www.socalgas.com ).
In the Underground Sun Storage, the energy derived from wind and solar renewable resources is stored beneath the earth's surface. Referring to the difficult storing of the produced energy from renewable resources, the rest released power in reprocessed into hydrogen via electrolysis process and conserved for the futuristic challenges. The findings of the plant outlines revealed that it has the efficiency to equilibrate the basic energy requirements in line with the various seasonal variations. Other projects were established to face the shortage between the system supply and demand. Orsted plant was designed to operate the electrolysers by subjecting the oversupply of energy generated from wind farms to them. Another project launched by SoCalGas on campus succeeded in directly converting the produced hydrogen from the solar electric system into methane inside a bioreactor.
Besides, hydrogen is hugely accounted as an assistant tool for providing the energy sector (grid) with the necessary services such as frequency maintenance and voltage strengthening via electrolysers and fuel cells (Bird et al. 2016 ). In the HAEOLUS facility (Haeolus. https://www.haeolus.eu/ ), the oversupply of wind generation is directly fed into an electrolyser to generate hydrogen, which is subsequently forwarded into fuel cells to be used later for various purposes (utilities, data transmittance, systems controlling and others) (Larscheid et al. 2018 ). Another form of energy storage can be achieved by regulating the grid frequency near its normal value (50–60 Hz) by injecting or consuming energy in a coordinated manner to maintain the gap between the product and the required power. Numerous regulation reserves have been installed in different European grid systems. Commonly, frequent containment and restoration reserves have been used to handle the frequencies through the distributed control systems. The first mentioned controlling scenario supplies a steady feed stream in case of occurring a sudden corruption in frequency in a very short period, whereas the latter can tolerate a longer corruption beyond the 30 s. The twice services can be attained via electrolysers and fuel cells by incrementing or decreasing their power setpoints related to frequency signals (Alshehri et al. 2019 ).
Besides, hydrogen-based equipment can contribute to voltage support by adjusting their power factor to meet the local voltage support requirements, which can be accomplished using inverter or rectifier monitoring systems (Alshehri et al. 2019 ). Some troubles such as blackout can occur in power plants, which was conventionally faced using a diesel Genset. The use of fuel cells may have the advantage to realise this scope given its no emissions and noiseless nature. These studies imply the profitability of hydrogen scaling up in the power sector.

Power-to-gas
Power-to-gas is a process in which electrical energy is used to generate a combustible gas. Since hydrogen is thought to be a combustible gas with a large power density, power-to-hydrogen technologies are increasing (Eveloy and Gebreegziabher 2018 ). Because of the combustibility nature of hydrogen, it has been inserted into gas applications. The hydrogen generated from the electrolyser can be converted into methane by the methanation process, which is either pumped to the natural gas grid operating system or stored to achieve the financial budget for the energy market (Gondal 2019 ). By the literature, numerous pilot projects have been commenced worldwide with the highest establishment rate of 85% in Europe, followed by the USA and Japan (Thema et al. 2019 ). Among different European countries, Germany constructed a power-to-gas plant with a maximum production capacity of (40–100 megawatts) to be directed for industrial purposes, and it will pump in the natural gas grid operating system from 2022 (Romeo et al. 2020 ).
Furthermore, several power-to-gas infrastructures have been installed in the regions rich in solar and wind renewable resources. A realistic study is displayed in the HAEOLUS project (north of Norway). Chiefly, its core idea was based on using 2.5 Megawatts proton exchange membrane electrolyser to transform the produced wind power generated from wind farms into hydrogen, which can be consumed in various aspects. HyCAUNAIS project displays the viability of running a resilient power to gas facility in conjunction with the methanation approach by equipping a nominal 1 megawatts electrolysis area to produce hydrogen, which was methanated and inserted into natural gas grid operating system or combined with biomethane generation area from landfill biogas (HYCAUNAIS – Storengy – Europe en BFC. https://www.europe-bfc.eu/beneficiaire/hycaunais-storengy/ ).
Lately, fuel cells have gained worldwide attention as efficient and environmentally friendly energy generators. Practically, they are integrated electrochemical devices widely used to convert the delivered chemical energy into its electrical counterpart via redox reactions (Yuan et al. 2021 ). Regarding their efficacy for energy generation, they can be served as energy carriers. Fuel cells are composed of two electrodes (i.e. anode and cathode) separated by electrolytes responsible for the migration of ions between electrodes (Ogawa et al. 2018 ). There are numerous types of fuel cells such as alkaline fuel cell, direct carbon fuel cell, direct methanol fuel cell, microbial fuel cells, molten carbonate fuel cells, phosphoric acid fuel cell, proton exchange membrane fuel cells and solid acid fuel cells.
Table 7 displays different types of fuel cells with their operational conditions and efficiency%. During system operation, hydrogen is passed to the anode while oxygen is passed to the cathode. At the anode, the hydrogen molecules are split into protons and electrons by a catalyst. The positive hydrogen particles can pass through the membrane to the cathode side, but the negative cannot. However, electrons change their path by being forced to the circuit and generating electric current. At the cathode, the hydrogen protons, electrons and oxygen combine to produce a water molecule which is the end product of this reaction. Among different types of fuels (i.e. hydrocarbons and chemical hydrides), applying hydrogen in fuel cells is eco-friendly because it does not expel any pollutants (Psoma and Sattler 2002 ). It works within low temperatures ranges comparing with the internal combustion engine. As mentioned before, the end product of the hydrogen-based fuel cell is water, whereas the end products of diesel/natural gas-based fuel cells are carbon dioxide and greenhouse gases (Xu et al. 2021 ). The main differences between fuel cells and traditional batteries are presented as follow: (1) operational mode of fuel cells is mostly like the traditional batteries, but the latter requires an electrical powering to run, (2) batteries can store hydrogen, unlike fuel cells that can provide a continuous electricity supply wherever hydrogen (fuel) and oxygen (oxidising agent) are available from outside sources. In addition to the mentioned differences, the batteries electrodes are steadily consumed during their extended usage, which entirely differs (not found) in the fuel cells (Spingler et al. 2017 ; Aydın et al. 2018 ).
Co-generation and tri-generation distribution systems
Interestingly, fuel cells can be employed to optimise the efficiency of different power systems and reduce the overall production cost of these processes in several aspects, including co-generation systems (i.e. heat + power/cold + power) or tri-generation systems (i.e. cold + heat + power). Co-generation is the sequential generation of two different forms of beneficial energy from a primary single source (fuel cells). In that case, the electricity generated from fuel cells is used to meet the electrical demand, and the released heat is directed towards the heating activities. As a result, total efficiency will be about 95%. Systematically, co-generation fuel cell systems consist of different components, including fuel processors, power suppliers, heat recovery unit, energy (thermal/electrochemical) storage unit, control devices, additional apparatus (i.e. pumps) and stack. Commercially, a large number of facilities have been launched to improve the performance of co-generation systems. Different co-generation projects were erected around the world. In Japan, the plant installed by the ENE-FARM project (300,000 units/2018) simultaneously supplied the home with electricity and heat necessary for daily activities by using proton exchange membrane fuel cells ranged from 0.3 to 1 kilowatt. Initially, liquefied petroleum gas feedstock streams are fed into a reformer, where they are converted into hydrogen, which is further combined with oxygen inside the fuel cells to produce water, electricity and heat used later for various residential purposes (Yue et al. 2021 ). Recently, the manufacturing of micro-co-generation fuel cells has grown in Europe. Besides, more than 1000 micro-combined heat and power fuel cells were launched in 10 European countries between 2012 and 2017. The primary European plant for a micro-co-generation fuel cell was the ENE. Field project (ene.field. http://enefield.eu/ ). An LCA study was successfully performed for the mentioned project, and simply it revealed that co-generation fuel cell was environmentally in nature compared with other gas boilers and heat pumps strategies considering its less greenhouse gaseous emissions. PACE was another project, firstly started in 216, whereas about 2800 of combined heat and power fuel cells are fabricated. Briefly, the overall development in the electrical efficiency through the two inspected projects were 60 and 95%, respectively (Home - PACE Pathway to a competitive European fuel cell micro-cogeneration market. https://pace-energy.eu/ ).
Tri-generation strategy is an improved strategy of co-generation in which a single primary source achieves the required cooling by thermally driven equipment. The working principle of heat pumps mainly stands on producing cooling from a thermal source. Typically, this can be achieved by using condenser and evaporator types of equipment. The gas released from absorbent/adsorbent is cooled down in the condenser and converted into a liquid by releasing its heat (refrigeration process). Then, the cooled down fluid continues to an evaporator, whereas it is evaporated by losing its contained heat. Significantly, the tri-generation fuel cells simultaneously reduce carbon emissions and enhance energy efficacy (Yue et al. 2021 ). Fong and Lee ( 2014 ) reported that employing a 593 kilowatts solid oxide fuel cell and absorption chillers, the carbon emissions were notably decreased by about 50% with an increase in the energy efficacy up to 75% (Fong and Lee 2014 ). A simulated 339 kilowatts solid oxide fuel cell combined with a combustor and a heat recuperation system proficiently recovered about 267 kilowatts of heat with an efficacy of 84%. Besides, they announced that 339 kilowatts solid oxide fuel cells provided with an absorption chiller generated about 303.6 kilowatts of cold with an efficacy of 89% (Yu et al. 2011 ).
Transportation sector
Compared with conventional battery-powered powertrains, vehicles based on hydrogen fuel (hydrogen-fuelled vehicles) represent a promising solution to surpass them. Globally, the sales rate of hydrogen-fuelled vehicles is anticipated to be 3% and enhanced up to 36% in 2030 and 2050, respectively (Path to Hydrogen Competitiveness: A Cost Perspective - Hydrogen Council. https://hydrogencouncil.com/en/ ). Currently, innumerable vehicles companies are developing their operating system to be hydrogen-based, attributing to its dependability and quality. Toyota has evolved Mirai fuel cell vehicles by using proton exchange membrane fuel cells with a volume power density and maximum power productivity of 3.1 km/L and 144 kilowatts, respectively. The hydrogen-fuelled vehicles can be driven by different forms of hydrogen (i.e. liquid and compressed). The compressed (high pressurised) hydrogen is the most appropriate form in the vehicles storage system of Clarity and NEXO; hydrogen-based fuel cell vehicles developed by Honda and Hyundai companies, respectively. At the same time, liquid hydrogen operates Hydrogen 7 vehicle improved by BMW company (Yue et al. 2021 ). Moreover, regional multi-unit trains powered by hydrogen have been entered into service in Europe and are projected to gain more economic benefits. Approximately 30% of presently employed diesel fleets may be phased out in the future (Study on the use of Fuel Cells and Hydrogen in the Railway Environment - Shift2Rail. https://shift2rail.org/publications/study-on-the-use-of-fuel-cells-and-hydrogen-in-the-railway-environment/ ).
Among different modes of transportation, the aviation division is regarded as the fastest transportation mode with anticipated annual growth in air traffic. The most common aircraft fuel is kerosene. Various aviation fuels often display a set of specifications, such as resistance to corrosion and severe temperature changes (Tzanetis et al. 2017 ). It is worth noting that petroleum accounts for the majority of the fuel used in the aviation sector. To improve energy preservation and reduce the negative environmental effects of fossil fuels, alternative, less harmful fuels such as liquid hydrogen are developed and thought to be eco-friendly. Table 8 presents some variations in the physicochemical properties between hydrogen and kerosene fuels. Refrigerated hydrogen fuel can be potentially better than kerosene as aviation fuel. It emits fewer greenhouse gaseous emissions and is easily produced from a variety of sources. Aside from that, the operating hydrogen-fuelled aircraft is characterised by minimal maintenance costs, long lifetime engines, high energy content and better combustion.
Furthermore, some constraints may arise during hydrogen utilisation as aviation fuel, such as depressed ignition energy, high flammability and the possibility of unburned traces forming that promotes metal embrittlement. Furthermore, the hydrogen admission with the onboard technology instead of inserting into the grid commercially allows its manufacturing companies to resell it (Nanda et al. 2017 ). The National Renewable Energy Laboratory manifested that the hydrogen cost in the mentioned case ranges from 3 to 10 USD/Kg, while the most traded hydrogen cost is about 13.99 USD/Kg. To sum up, liquid hydrogen presents admirable efficacy as an aviation fuel for reducing greenhouse gaseous emissions, resulting in a significant improvement in air quality. Furthermore, by using hydrogen-based aviation fuels, over-reliance on traditional fuels could be decreased. The total cost of aircraft powered by liquid hydrogen is predominately associated with the cost of production and storage technologies (Eichman et al. 2012 ).
Recently, the global navigation movement in terms of maritime shipping has become increasingly important in the movement of different types of goods worldwide, which is in line with tremendous industrial progress in various fields. Unfortunately, this, in turn, led to an increase in the consumption of conventional fuels (i.e. diesel and heavy fuels). Regrettably, the pollution created by ships significantly implicates about 2.5% of the universal greenhouse gaseous emissions. Furthermore, bunkering activities broadly contribute to the leakage of heavy fuels in the aquatic environment, consequently posing a threat to the ecosystem. It was announced that carbon dioxide emissions associated with shipping activities release about 3.3% of the global emissions (Vogler and Sattler 2016 ). Other gaseous emissions such as nitrogen oxide and sulphur oxide are also associated with shipping activities. Accordingly, the maritime industry seeks more environmentally alternative fuels than conventional ones to overcome these obstacles (Prussi et al. 2021 ). Numerous suitable substitutes in different states, gas (i.e. hydrogen, propane) and liquid (i.e. bio-oil, methanol and ethanol) are used to compensate for the usage of traditional fuels (Al-Enazi et al. 2021 ; Abou Rjeily et al. 2021 ). Among them, hydrogen can be employed in maritime activities in two routes: (1) internal combustion engines and (2) fuel cells (Banawan et al. 2010 ). Relatively, fuel cells meet the energy requirements needed by ships sailing for long distances travelling and supply the ancillary energy requirements of larger ships in contrast to the other battery-powered ones. Numerous studies have been conducted to assess the feasibility of using hydrogen in maritime activities. Deniz and Zincir ( 2016 ) stated that hydrogen had a durable, safe and bunker capability criterion, qualifying as a favourable fuel for shipping. Although they reported that liquefied natural gas has the preference to be used as an alternative fuel, they recommended more research studies on utilising hydrogen as an effective alternative fuel (Deniz and Zincir 2016 ).
Production of hydrocarbon fuels
Production of hydrocarbon fuels via fischer–trospch pathway.
Syngas (synthesis gas), a mixture of carbon monoxide and hydrogen, is a product of different thermochemical conversion processes (i.e. pyrolysis, gasification and others) and can be utilised by two scenarios: (1) direct fuel or (2) transformed into transportation fuels via Fischer–Trospch synthesis process and syngas fermentation (Wainaina et al. 2018 ). The two strategies are categorised as gas-to-liquid transformation strategies that can generate hydrocarbon fuels and alcohols based on syngas feedstock stream (Gruber et al. 2019 ). Normally, the Fischer–Trospch strategy (exothermic) operates at 200–350 °C and 1.5–4 MPa for reaction temperature and pressure, respectively (Okolie et al. 2019 ). Majorly, it comprises three main stages: (1) syngas production, (2) syngas treatment and (3) transforming into hydrocarbon fuels associated with their upgrading. Besides the production process of transportation fuel, other valuable products (i.e. paraffin, naphtha and others) can be produced. Significantly, the as-produced green fuels based on the Fischer–Trospch process have numerous advantages over petroleum-based fuels. They have excellent burning characters, elevated smoking points and free of heavy contaminants. The physicochemical properties of resultant fuels depend heavily on reaction conditions (i.e. reactor type, heating rate, residence time and others) (Sun et al. 2017 ). The given equations from (Eqs. 9 , 10 , 11 , 12 , 13 , 14 and 15 ) explicates the synthesis of different products (i.e. alkanes, alkenes, oxygenated products, methanol, ethanol and dimethyl ether via the Fischer–Trospch process by participating in hydrogen. The hydrogen/carbon monoxide ratio is a critical controlling parameter in the Fischer–Trospch synthesis process (Bermudez and Fidalgo 2016 ). Different types of catalysts (i.e. copper-based catalysts) can be used to optimise the yield of the Fischer–Trospch process.
Synthesis of alkanes:
Synthesis of alkenes:
Synthesis of alcohols:
Synthesis of carbonyl:
Synthesis of ethanol:
Synthesis of methanol:
A ratio of H 2 / CO of 2:1 is preferable for the synthesis of hydrocarbon fuels via water—gas shift reaction as given in Eq. 15 :
Dimethyl ether is admirable commonly realised as an efficient alternate for diesel fuel (Kim and Park 2016 ). Distinctly, numerous physicochemical features characterise liquefied petroleum gas, such as anti-corrosive, anti-carcinogenic, less nitrogen oxide and carbon monoxide emissions during its burning, less engine noise and high cetane number (Dincer and Bicer, 2020 ). In general, dimethyl ether can be produced by (1) direct route (combined single step of methanol synthesis and dehydration) or (2) indirect route (separated methanol synthesis and dehydration steps) as shown in Eqs. ( 16 , 17 and 18 ) (Gogate, 2018 ):
Direct route (single step):
Indirect route (two steps):
Dehydration of methanol:
Production of hydrocarbon fuels via Syngas fermentation pathway
Syngas fermentation (biorefining) pathway is regarded as the interconnection between the biochemical and thermochemical scenarios (Thi et al. 2020 ). It produces value-added products (i.e. alcohols) from syngas by flexibly employing several groups of microorganisms at different reaction temperatures of 37–40 °C and 55–90 °C for mesophilic (i.e. Clostridium autoethanogenum ) and thermophilic (i.e. Moorella thermoacetica ), respectively. During the process, the feedstock of syngas can be simply converted into alcohols (i.e. ethanol) via two subsequent stages via (1) producing acetyl coenzyme A and then (2) its transformation into ethanol. Other alcohols and chemicals (i.e. acetate, butanol and formate) can be synthesised by acetogenic bacteria (Park et al. 2017 ). Regarding several operational advantages characterised to syngas fermentation such as (1) no necessity for using costly pretreatment step, (2) process' versatility with different biomass composition, (3) independent on the hydrogen/carbon monoxide ratio in the feedstock upstream, (4) high selectivity of as-used microorganisms and (5) moderate (ambient) working parameters with no necessity for catalysts usage or its poising trouble, they support it over the Fischer–Trospch process. However, there are some operational challenges such as (1) weak solubility of the gas in the liquid state, (2) complicated bioreactor design, (3) existence of impurities and (4) low yield of production. Briefly, integration between different thermochemical, biochemical and hydrothermal routes can effectively compensate for the shortage of individual techniques and maximise productivity (Rigueto et al. 2020 ).
Refining of crude oil and petroleum products
Commercially, hydrogen is conceived as an upgrading (improving) agent for crude oil products and petroleum distillates in terms of hydrocracking and hydroprocessing and processes. The hydrocracking process is defined as treating heavier hydrocarbons with hydrogen to simultaneously split them into lighter derivatives and enhance the hydrogen/carbon ratio (El-Sawy et al. 2020 ). In hydroprocessing, various heteroatoms such as nitrogen, sulphur, oxygen and heavy metals are majorly captured from petroleum products via different treatment processes named: hydrodenitrogenation (Dasgupta and Atta 2020 ), hydrodesulphurisation (Han et al. 2018 ), hydrodeoxygenation (Yfanti and Lemonidou 2020 ) and hydrodemetallisation (Rana et al. 2020 ), respectively, as displayed in Eqs. ( 19 – 21 ).
Hydrodenitrogenation:
Hydrodesulphurisation:
Hydrodeoxygenation:
This can be achieved by reacting the upstream feedstock (heavy oils and petroleum products) with hydrogen through catalytic reaction, resulting in removing these contaminants and saturating the aromatics (C–C) bonds. The elimination process of these contaminates from feedstocks directly contributes to fuel upgrading because they deactivate the as-used catalysts due to their adsorption on the surfaces of the catalyst (blocking of active catalyst sites). Recently, the appeal for inserting hydrogen in hydroprocessing has been increasingly growing (Al Obaidi et al. 2018 ). From the environmental point of view, the key cause of this pattern is the combination of strict environmental legislation governing gaseous greenhouse emissions and other particulate contaminants, as well as product quality specifications. Generally, numerous upgrading techniques are directed to improve the physicochemical properties of heavy oils by decreasing their viscosity and carbon/hydrogen ratio at the same time (Misra et al. 2017 ).
Production of ammonia
Ammonia is deemed one of the essential chemicals largely employed in industrial fertiliser activities with huge global production rates. The biggest ammonia production plant has projected to achieve a daily capacity rate of 3300 metric tons (Brightling 2018 ). Broadly, ammonia can be introduced as fertiliser in the agriculture sector. Additionally, it is provided to various industries such as polymers processing, explosives, refrigerant, pharmaceuticals, gas sensors and fuel cells. The ammonia synthesis process is promoted by the catalytic reaction between hydrogen and nitrogen elements through the Haber process (Arora et al. 2018 ). It is performed in the as-designed reactor under operating conditions of 20–30 Mpa and 300–500 °C for pressure and temperature, respectively, using KOH-promoted finely divided iron catalysts with the required energy of 2.5 EJ (Tolod et al. 2019 ).
Moreover, the hydrogen addressed to the ammonia synthesis process is primarily derived from steam gas reforming, which is not regarded as environmentally friendly. Accordingly, there is an increasing interest in other green and sustainable ammonia synthesis pathways, such as electrochemical hydrogen manufacturing techniques and photocatalytic nitrogen fixation (artificial photocatalysis). The distinctiveness of the electrochemical ammonia synthesis pathways routes is controlled by the employed energy sources. Hydrogen can be generated from water employing an electrolysis process using renewable green sources (i.e. wind and solar energy) and, hence, reduce harmful greenhouse gaseous emissions (Bicer and Dincer 2017 ).
Metallurgical industries
Generally, hydrogen can produce oxy-hydrogen flames in industrial metallurgical activities and act as a reducing agent to obtain metals from their ores. During the oxy-hydrogen flames synthesis process (exothermic reaction), hydrogen is allowed to react with oxygen at very high temperatures (3000 °C) to produce oxy-hydrogen flames, used later for cutting and welding working on non-ferrous metals (Polverino et al. 2019 ). Otherwise, hydrogen is reliably characterised by its high ability to recover (reduce) metals from the aqueous solutions of their salts (hydrogen reduction). The metals may be powdered for later metallurgical usage or incorporated into a composite material. Chemically, hydrogen can interact with the elements of periodic tables in three ways: (1) ionic bond formation between the elements of Ia and IIa groups, (2) interstitial solid solution between the elements of VIa, VIIa and VIII groups and (3) metallic bond between elements of IIIa, Iva and Va groups. Additionally, the electrostatic shielding phenomenon can be generated by attributing the hydrogen's capability to capture free electrons and the self-trapping of metals. Likewise, the small particle size of hydrogen effectively facilitates the process of metal–hydrogen interaction (Agrawal et al. 2006 ).
To ensure the long-term production of clean and green hydrogen, it is crucial to conduct a critical assessment of various production routes and their environmental impacts, as well as seasonal storage and utilisation options. Hydrogen is produced from either fossil-based or renewable feedstocks; however, each route has advantages and disadvantages. The current hydrogen colour coding is imprecise, assuming that green hydrogen always has lower carbon emissions than blue or grey hydrogen, which is not always accurate.
Water electrolysis is gaining momentum; however, meeting 24% of energy demand with hydrogen in a 1.5-degree scenario of climate change mitigation will necessitate massive amounts of additional renewable electricity generation. In this scenario, approximately 31,320 terawatt-hours of electricity would be required to power electrolysers, which is more than is currently produced globally from all sources combined. Furthermore, > $11 trillion in manufacturing, storage and transportation infrastructure would be needed. The affordability and accessibility of freshwater is one side of the coin, and the proximity of these two supplies, i.e. renewable energy and freshwater, is the other. Water electrolysis research priorities include lowering the capital cost of electrolysis technology, locating water resources, find utilisation routes for the produced oxygen and increasing the efficiency of the process.
In terms of biomass gasification, the economic feasibility of producing hydrogen from biomass must be closely related to the availability and affordability of raw materials in the surrounding area. The main characteristics of the supply materials are the biomass physicochemical properties, distribution and hydrogen rate. Because biomass feedstocks differ greatly in structural composition and shape, all of these factors must be considered when combining the feedstock with the appropriate conversion technology. In conclusion, there are challenges associated with the most common hydrogen generation routes, such as steam methane reforming, water electrolysis, coal or biomass gasification, methane pyrolysis with or without carbon capture and storage technology.
To understand advances in evaluating environmental impacts due to hydrogen production, we performed an intensive critical review of 24 life cycle assessment studies published from 2019 to 2021, including methods and findings. The important methodological approaches and key findings observed were:
No two life cycle assessment studies were identified to be similar. There were differences in the geographical and temporal span, functional units and system boundaries considered, and environmental impact categories assessed. Therefore, it is recommended that the policymakers pay heed to the modelled processes and extent of the system boundary for making decisions for creating a sustainable hydrogen economy.
Many life cycle assessment studies did not encompass processes, inputs and outputs for ‘cradle-to-grave’ analysis. Thus, future research should pay more attention to ‘cradle-to-grave’ evaluation for robust decision-making.
In addition to the global warming potential and depletion of fossil fuels, environmental impacts in more categories for hydrogen production processes must be evaluated.
Furthermore, large-scale energy storage is key in securing the energy supply chain for the next energy transition using electrolysis-generated hydrogen. The Underground Seasonal Hydrogen Storage (USHS) holds great potential to overcome the natural temporal fluctuations inherent in renewable energy production at the scale required to achieve net-zero by 2050. The selection of geological porous media for USHS should be based on a comprehensive geological investigation that includes an assessment of their utility on both a basin and regional scale, fluid flow behaviour of hydrogen in brine-saturated subsurface reservoirs, an assessment of storage capacity, the safety of long-term storage, geochemical and biological reactions triggered by hydrogen injection, the geomechanical response of the subsurface to hydrogen storage and other measures. The discussed procedures can lead to informed decision-making in terms of forecasting best-operating strategies and ensuring safe and efficient hydrogen storage installation. Further research to integrate the theoretical studies with existing experimental USHS trials is required to minimise the uncertainty that might be associated with the feasibility of large-scale hydrogen storage. Finally, blending the need with the various utilisation routes such as fuel production, ammonia production, metallurgical industries and power systems is crucial in the hydrogen economy.
Change history
31 march 2022.
A Correction to this paper has been published: https://doi.org/10.1007/s10311-022-01432-x
Abbreviations
Carbon capture storage and utilisation
Levelised cost of hydrogen
Water–gas shift reaction
Underground Seasonal Hydrogen Storage
Proton exchange membrane fuel cells
Phosphoric acid fuel cells
Solid oxide fuel cells
Molten carbonate fuel cells
Direct methanol fuel cells
Abe JO et al (2019) Hydrogen energy, economy and storage: review and recommendation. Int J Hydrog Energy. 44:15072–15086. https://doi.org/10.1016/j.ijhydene.2019.04.068
Article CAS Google Scholar
Abou Rjeily M et al (2021) Pyrolysis-catalytic upgrading of bio-oil and pyrolysis-catalytic steam reforming of biogas: a review. Environ Chem Lett 19:2825–2872. https://doi.org/10.1007/s10311-021-01190-2
Acar C, Dincer I (2019) Review and evaluation of hydrogen production options for better environment. J Clean Product. 218:835–849. https://doi.org/10.1016/j.jclepro.2019.02.046
Agrawal A et al (2006) A comprehensive review on the hydro metallurgical process for the production of nickel and copper powders by hydrogen reduction. Mater Res Bull 41:879–892. https://doi.org/10.1016/j.materresbull.2005.09.028
Al Obaidi Y et al (2018) Hydrodearomatization of distillates and heavy naphtha over a precious metal hydrogenation catalyst and the determination of low aromatic content. Ind Eng Chem Res 57:12029–12035. https://doi.org/10.1021/acs.iecr.8b02909
Al Shaqsi AZ et al (2020) Review of energy storage services, applications, limitations, and benefits. Energy Rep 6:288–306. https://doi.org/10.1016/j.egyr.2020.07.028
Article Google Scholar
Al-Enazi A et al (2021) A review of cleaner alternative fuels for maritime transportation. Energy Rep 7:1962–1985. https://doi.org/10.1016/j.egyr.2021.03.036
Al-Muhtaseb AAH et al (2021) Circular economy approach of enhanced bifunctional catalytic system of CaO/CeO2 for biodiesel production from waste loquat seed oil with life cycle assessment study. Energy Convers Manage 236:114040. https://doi.org/10.1016/j.enconman.2021.114040
Al-Qahtani A et al (2021) Uncovering the true cost of hydrogen production routes using life cycle monetisation. Appl Energy. 281:115958. https://doi.org/10.1016/j.apenergy.2020.115958
Alanne K, Cao S (2019) An overview of the concept and technology of ubiquitous energy. Appl Energy. 238:284–302. https://doi.org/10.1016/j.apenergy.2019.01.100
Alshehri F et al (2019) Modelling and evaluation of PEM hydrogen technologies for frequency ancillary services in future multi-energy sustainable power systems. Heliyon 5:e01396–e01396. https://doi.org/10.1016/j.heliyon.2019.e01396
Alviani VN et al (2021) Local initiative hydrogen production by utilization of aluminum waste materials and natural acidic hot-spring water. Appl Energy 293:116909. https://doi.org/10.1016/j.apenergy.2021.116909
Argonne National Laboratory, 2019. https://ora.ox.ac.uk/objects/uuid:fa2b9e7c-1c58-429c-90fd-f780a3c3dc7d
Arora P et al (2018) Remote, small-scale, ‘greener’ routes of ammonia production. J Clean Prod 199:177–192. https://doi.org/10.1016/j.jclepro.2018.06.130
Atilhan S et al (2021) Green hydrogen as an alternative fuel for the shipping industry. Current Opin Chem Eng. 31:100668. https://doi.org/10.1016/j.coche.2020.100668
Aydın Ö et al (2018) Mass transport limitation in inlet periphery of fuel cells: studied on a planar solid oxide fuel cell. Int J Hydrog Energy 43:17420–17430. https://doi.org/10.1016/j.ijhydene.2018.07.030
BNEF, Hydrogen Economy Outlook, Key messages, March 30, 2020, https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf , accessed on 20–5–2021. 2020, https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf
Balay S, Abhyankar S, Adams M, Brown J, Brune P, Buschelman K, Dalcin L, Dener A, Eijkhout V, Gropp W, Karpeyev D, Kaushik D, Knepley M, MAY D, Curfman McInnes L, Mills R, Munson T, Rupp K, Sanan P, Smith B, Zampini S, Zhang H, Zhang H (2019) Computer, computational, and statistical sciences division, PETSc users manual, Argonne National Laboratory Argonne National Laboratory. https://ora.ox.ac.uk/objects/uuid:fa2b9e7c-1c58-429c-90fd-f780a3c3dc7d
Banawan AA et al. (2010) Environmental and economical benefits of changing from marine diesel oil to natural-gas fuel for short-voyage high-power passenger ships. Proceedings of the Institution of Mechanical Engineers Part M: Journal of Engineering for the Maritime Environment. 224 , 103-113. https://doi.org/10.1243/14750902JEME181
Bareiß K et al (2019) Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl Energy 237:862–872. https://doi.org/10.1016/j.apenergy.2019.01.001
Bauer S et al (2013) Impacts of the use of the geological subsurface for energy storage: an investigation concept. Environ Earth Sci 70:3935–3943. https://doi.org/10.1007/s12665-013-2883-0
Bauer S et al (2017) Subsurface energy storage: geological storage of renewable energy—capacities, induced effects and implications. Environ Earth Sci 76:695–695. https://doi.org/10.1007/s12665-017-7007-9
Bermudez JM, Fidalgo B (2016) Production of bio-syngas and bio-hydrogen via gasification Handbook of biofuels production. Elsevier Inc, Hoboken, pp 431–494. https://doi.org/10.1016/B978-0-08-100455-5.00015-1
Book Google Scholar
Bicer Y, Dincer I (2017) Assessment of a sustainable electrochemical ammonia production system using photoelectrochemically produced hydrogen under concentrated sunlight. ACS Sustain Chem Eng 5:8035–8043. https://doi.org/10.1021/acssuschemeng.7b01638
Bicer Y, Khalid F (2020) Life cycle environmental impact comparison of solid oxide fuel cells fueled by natural gas, hydrogen, ammonia and methanol for combined heat and power generation. Int J Hydrog Energy 45:3670–3685. https://doi.org/10.1016/j.ijhydene.2018.11.122
Bird L et al (2016) Wind and solar energy curtailment: a review of international experience. Renew Sustain Energy Rev 65:577–586. https://doi.org/10.1016/j.rser.2016.06.082
Bocklisch T (2016) Hybrid energy storage approach for renewable energy applications. J Energy Storage 8:311–319. https://doi.org/10.1016/j.est.2016.01.004
Booto GK et al (2021) Comparative life cycle assessment of heavy-duty drivetrains: a Norwegian study case. Transp Res Part d: Transp Environ 95:102836. https://doi.org/10.1016/j.trd.2021.102836
Brightling J (2018) Ammonia and the fertiliser industry: the development of ammonia at Billingham. Johns Matthey Technol Rev 62:32–47. https://doi.org/10.1595/205651318X696341
Bui M et al (2021) Delivering carbon negative electricity, heat and hydrogen with BECCS – Comparing the options. Int J Hydrog Energy 46:15298–15321. https://doi.org/10.1016/j.ijhydene.2021.02.042
Bünger U et al (2016) Large-scale underground storage of hydrogen for the grid integration of renewable energy and other applications. Compend Hydrog Energy. https://doi.org/10.1016/B978-1-78242-364-5.00007-5
Cao C et al (2020) Utilization of CO2 as cushion gas for depleted gas reservoir transformed gas storage reservoir. Energies 13:576–576. https://doi.org/10.3390/EN13030576
Carpetis C (1988) Storage, transport and distribution of hydrogen. Hydrog Energy Carrier. https://doi.org/10.1007/978-3-642-61561-0_10
Chabab S et al (2020) Measurements and predictive models of high-pressure H2 solubility in brine (H2O+NaCl) for underground hydrogen storage application. Int J Hydrog Energy 45:32206–32220. https://doi.org/10.1016/j.ijhydene.2020.08.192
Chen J et al (2019) System development and environmental performance analysis of a solar-driven supercritical water gasification pilot plant for hydrogen production using life cycle assessment approach. Energy Convers Manage 184:60–73. https://doi.org/10.1016/j.enconman.2019.01.041
Chen Y-T, Hsu C-W (2019) The key factors affecting the strategy planning of Taiwan’s hydrogen economy. Int J Hydrog Energy. 44:3290–3305. https://doi.org/10.1016/j.ijhydene.2018.07.159
Cheng J et al (2019) Improving fermentative hydrogen and methane production from an algal bloom through hydrothermal/steam acid pretreatment. Int J Hydrog Energy. 44:5812–5820. https://doi.org/10.1016/j.ijhydene.2019.01.046
Cherubini E et al (2018) Uncertainty in LCA case study due to allocation approaches and life cycle impact assessment methods. Int J Life Cycle Assess 23:2055–2070. https://doi.org/10.1007/s11367-017-1432-6
Collet P et al (2017) Techno-economic and life cycle assessment of methane production via biogas upgrading and power to gas technology. Appl Energy 192:282–295. https://doi.org/10.1016/j.apenergy.2016.08.181
Collotta M et al (2019) Critical indicators of sustainability for biofuels: an analysis through a life cycle sustainabilty assessment perspective. Renew Sustain Energy Rev 115:109358. https://doi.org/10.1016/j.rser.2019.109358
Contreras A et al (1997) Hydrogen as aviation fuel: a comparison with hydrocarbon fuels. Int J Hydrog Energy 22:1053–1060. https://doi.org/10.1016/s0360-3199(97)00008-6
Cortés A et al (2019) Environmental implications of biohydrogen based energy production from steam reforming of alcoholic waste. Ind Crops Prod 138:111465. https://doi.org/10.1016/j.indcrop.2019.111465
Cvetković SM et al (2021) Life Cycle Energy Assessment of biohydrogen production via biogas steam reforming: case study of biogas plant on a farm in Serbia. Int J Hydrog Energy 46:14130–14137. https://doi.org/10.1016/j.ijhydene.2021.01.181
Dasgupta S, Atta A (2020) Computational insights on intensification of hydrodenitrogenation in a trickle bed reactor using periodic flow modulation. Chem Eng Process-Process Intensif 157:108135–108135. https://doi.org/10.1016/j.cep.2020.108135
Dawood F et al (2020) Hydrogen production for energy: an overview. Int J Hydrog Energy. 45:3847–3869. https://doi.org/10.1016/j.ijhydene.2019.12.059
Deniz C, Zincir B (2016) Environmental and economical assessment of alternative marine fuels. J Clean Prod 113:438–449. https://doi.org/10.1016/j.jclepro.2015.11.089
Desantes JM et al (2020) Comparative global warming impact and NOX emissions of conventional and hydrogen automotive propulsion systems. Energy Convers Manage 221:113137. https://doi.org/10.1016/j.enconman.2020.113137
Dincer I, Bicer Y (2020) Enhanced dimensions of integrated energy systems for environment and sustainability Integrated energy systems for multigeneration. Elsevier, Hoboken, pp 403–440. https://doi.org/10.1016/b978-0-12-809943-8.00007-8
Dodds PE et al (2015) Hydrogen and fuel cell technologies for heating: a review. Int J Hydrog Energy 40:2065–2083. https://doi.org/10.1016/j.ijhydene.2014.11.059
Dvoynikov M et al (2021) New concepts of hydrogen production and storage in arctic region. Resources. https://doi.org/10.3390/resources10010003
EIA - U.S. Battery Storage Market Trends. https://www.eia.gov/analysis/studies/electricity/batterystorage/
Earles JM, Halog A (2011) Consequential life cycle assessment: a review. Int J Life Cycle Assess 16:445–453. https://doi.org/10.1007/s11367-011-0275-9
Edwards PP et al (2008) Hydrogen and fuel cells: towards a sustainable energy future. Energy Policy 36:4356–4362. https://doi.org/10.1016/j.enpol.2008.09.036
Eichman, J., et al., Economic Assessment of Hydrogen Technologies Participating in California Electricity Markets. 2012, www.nrel.gov/publications .
El-Emam RS, Özcan H (2019) Comprehensive review on the techno-economics of sustainable large-scale clean hydrogen production. J Clean Product. 220:593–609. https://doi.org/10.1016/j.jclepro.2019.01.309
El-Eskandarany MS (2020) Solid-state hydrogen storage nanomaterials for fuel cell applications. Mech Alloy. https://doi.org/10.1016/B978-0-12-818180-5.00009-1
El-Halwagi MM et al (2020) Disaster-Resilient design of manufacturing facilities through process integration: principal strategies, perspectives, and research challenges. Front Sustain. https://doi.org/10.3389/frsus.2020.595961
El-Sawy MS et al (2020) Co-hydroprocessing and hydrocracking of alternative feed mixture (vacuum gas oil/waste lubricating oil/waste cooking oil) with the aim of producing high quality fuels. Fuel 269:117437–117437. https://doi.org/10.1016/j.fuel.2020.117437
El-Shafie M, Kambara S, Hayakawa Y (2019) Hydrogen production technologies overview. J Power Energy Eng 7:107–154. https://doi.org/10.4236/jpee.2019.71007
Eveloy V, Gebreegziabher T (2018) A review of projected power-to-gas deployment scenarios. Energies. https://doi.org/10.3390/en11071824
Falcone PM et al (2021) Hydrogen economy and sustainable development goals: Review and policy insights. Current Opin Green Sustain Chem 31:100506. https://doi.org/10.1016/j.cogsc.2021.100506
Fawzy S et al (2020) Strategies for mitigation of climate change: a review. Environ Chem Lett 18:2069–2094. https://doi.org/10.1007/s10311-020-01059-w
Fernández-Dacosta C et al (2019) Potential and challenges of low-carbon energy options: comparative assessment of alternative fuels for the transport sector. Appl Energy 236:590–606. https://doi.org/10.1016/j.apenergy.2018.11.055
Finnveden G et al (2009) Recent developments in life cycle assessment. J Environ Manage 91:1–21. https://doi.org/10.1016/j.jenvman.2009.06.018
Foh S et al. (1979) Underground hydrogen storage. Final report. [Salt caverns, excavated caverns, aquifers and depleted fields]. https://doi.org/10.2172/6536941
Fong KF, Lee CK (2014) Investigation on zero grid-electricity design strategies of solid oxide fuel cell trigeneration system for high-rise building in hot and humid climate. Appl Energy 114:426–433. https://doi.org/10.1016/j.apenergy.2013.10.001
Gabrielli P et al (2020) Seasonal energy storage for zero-emissions multi-energy systems via underground hydrogen storage. Renew Sustain Energy Rev 121:109629–109629. https://doi.org/10.1016/j.rser.2019.109629
Ganzer L et al (2013) The H2STORE project - Experimental and numerical simulation approach to investigate processes in underground hydrogen reservoir storage European association of geoscientists and engineers. EAGE, Netherlands, pp 679–687. https://doi.org/10.2118/164936-ms
Gaston D et al (2009) MOOSE: a parallel computational framework for coupled systems of nonlinear equations. Nucl Eng Des 239:1768–1778. https://doi.org/10.1016/J.NUCENGDES.2009.05.021
Global hydrogen market insights, 2020–2024 by production process, end-user, generation system and region. Focus Catal. 2020(5):2. https://doi.org/10.1016/j.focat.2020.04.005
Gogate MR (2018) The direct dimethyl ether (DME) synthesis process from syngas I. Process feasibility and chemical synergy in one-step LPDMEtm process. Petrol Sci Technol 36:547–554. https://doi.org/10.1080/10916466.2018.1428628
Gondal IA (2019) Hydrogen integration in power-to-gas networks. Int J Hydrog Energy 44:1803–1815. https://doi.org/10.1016/j.ijhydene.2018.11.164
Gruber H et al (2019) Fischer-Tropsch products from biomass-derived syngas and renewable hydrogen. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-019-00459-5
Gurz M et al (2017) The meeting of hydrogen and automotive: a review. Int J Hydrog Energy 42:23334–23346. https://doi.org/10.1016/j.ijhydene.2017.02.124
Hagemann B et al (2015) Mathematical modeling of unstable transport in underground hydrogen storage. Environ Earth Sci 73:6891–6898. https://doi.org/10.1007/s12665-015-4414-7
Hagemann B et al (2015) Hydrogenization of underground storage of natural gas. Comput Geosci 20(3):595–606. https://doi.org/10.1007/S10596-015-9515-6
Han Z et al (2018) Novel application of MgH2/MoS2 hydrogen storage materials to thiophene hydrodesulfurization: a combined experimental and theoretical case study. Mater Des 158:213–223. https://doi.org/10.1016/j.matdes.2018.08.036
Han W-B et al (2021) Directly sputtered nickel electrodes for alkaline water electrolysis. Electrochimica Acta. 386:138458. https://doi.org/10.1016/j.electacta.2021.138458
Hashemi L et al (2021) Pore-scale modelling and sensitivity analyses of hydrogen-brine multiphase flow in geological porous media. Sci Rep 11:8348–8348. https://doi.org/10.1038/s41598-021-87490-7
Hassanpouryouzband A et al (2020) Gas hydrates in sustainable chemistry. Chem Soc Rev 49:5225–5309. https://doi.org/10.1039/C8CS00989A
Hassanpouryouzband A et al (2021) Offshore geological storage of hydrogen: is this our best option to achieve net-zero? ACS Energy Lett 6:2181–2186. https://doi.org/10.1021/ACSENERGYLETT.1C00845
Heinemann N et al (2021) Enabling large-scale hydrogen storage in porous media – the scientific challenges. Energy Environ Sci 14:853–864. https://doi.org/10.1039/d0ee03536j
IEA (2019), The Future of hydrogen, IEA, Paris. https://www.iea.org/reports/the-future-of-hydrogen , Accessed on 20 May 2021
Jeswani HK et al. (2020) Environmental sustainability of biofuels: a review. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 476 , 20200351. https://doi.org/10.1098/rspa.2020.0351
Jorschick H et al (2021) Hydrogenation of aromatic and heteroaromatic compounds – a key process for future logistics of green hydrogen using liquid organic hydrogen carrier systems. Sustain Energy Fuels 5:1311–1346. https://doi.org/10.1039/D0SE01369B
Kalbar PP et al (2017) Weighting and aggregation in life cycle assessment: do present aggregated single scores provide correct decision support? J Ind Ecol 21:1591–1600. https://doi.org/10.1111/jiec.12520
Karaca AE et al (2020) Life cycle assessment study on nuclear based sustainable hydrogen production options. Int J Hydrog Energy 45:22148–22159. https://doi.org/10.1016/j.ijhydene.2020.06.030
Kerscher F et al (2021) Low-carbon hydrogen production via electron beam plasma methane pyrolysis: techno-economic analysis and carbon footprint assessment. Int J Hydrogen Energy 46:19897–19912. https://doi.org/10.1016/j.ijhydene.2021.03.114
Kim J et al (2015) Comparison of nitrogen and carbon dioxide as cushion gas for underground gas storage reservoir. Geosyst Eng 18:163–167. https://doi.org/10.1080/12269328.2015.1031916
Kim H-S et al (2021) Life cycle assessment of molten carbonate fuel cell system for power plants. J Clean Prod 302:126911. https://doi.org/10.1016/j.jclepro.2021.126911
Kim HJ, Park SH (2016) Optimization study on exhaust emissions and fuel consumption in a dimethyl ether (DME) fueled diesel engine. Fuel 182:541–549. https://doi.org/10.1016/j.fuel.2016.06.001
Kirk BS et al (2006) libMesh : a C++ library for parallel adaptive mesh refinement/coarsening simulations. Eng Comput 22:237–254. https://doi.org/10.1007/S00366-006-0049-3
Kruck, O., et al., 2013. Assessment of the potential, the actors and relevant business cases for large scale and seasonal storage of renewable electricity by hydrogen underground storage in Europe. KBB Undergr. Technol. GmbH.
Larscheid P et al (2018) Potential of new business models for grid integrated water electrolysis. Renew Energy 125:599–608. https://doi.org/10.1016/j.renene.2018.02.074
Li G et al (2019) Life cycle assessment of coal direct chemical looping hydrogen generation with Fe2O3 oxygen carrier. J Clean Prod 239:118118. https://doi.org/10.1016/j.jclepro.2019.118118
Li H et al (2021) Technology selection for hydrogen production in China by integrating emergy into life cycle sustainability assessment. J Clean Prod 294:126303. https://doi.org/10.1016/j.jclepro.2021.126303
Liebscher A et al (2016) Geologic storage of hydrogen - fundamentals processing and projects hydrogen science and engineering: materials processes systems and technology. Wiley, USA, pp 629–658. https://doi.org/10.1002/9783527674268.ch26
Liu F et al (2021) Deployment of fuel cell vehicles in China: Greenhouse gas emission reductions from converting the heavy-duty truck fleet from diesel and natural gas to hydrogen. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2021.02.198
Logan KG et al (2020) Electric and hydrogen rail: Potential contribution to net zero in the UK. Transp Res Part d: Transp Environ 87:102523. https://doi.org/10.1016/j.trd.2020.102523
Lord AS et al (2014) Geologic storage of hydrogen: scaling up to meet city transportation demands. Int J Hydrog Energy 39:15570–15582. https://doi.org/10.1016/j.ijhydene.2014.07.121
Lui J et al (2020) A critical review on the principles, applications, and challenges of waste-to-hydrogen technologies. Renew Sustain Energy Rev 134:110365–110365. https://doi.org/10.1016/j.rser.2020.110365
Mah AXY et al (2019) Review of hydrogen economy in Malaysia and its way forward. Int J Hydrog Energy. 44:5661–5675. https://doi.org/10.1016/j.ijhydene.2019.01.077
Mehrjerdi H et al (2019) Daily-seasonal operation in net-zero energy building powered by hybrid renewable energies and hydrogen storage systems. Energy Convers Manage 201:112156–112156. https://doi.org/10.1016/j.enconman.2019.112156
Melaina M et al (2013) Blending hydrogen into natural gas pipeline networks: a review of key issues. Contract 303:275–3000. https://doi.org/10.2172/1068610
Merzian, R., Bridges, T., 2019. Hydrogen and climate: Trojan horse or golden goose? The Australia Institute, https://apo.org.au/node/230061
Michalski J et al (2017) Hydrogen generation by electrolysis and storage in salt caverns: potentials, economics and systems aspects with regard to the German energy transition. Int J Hydrog Energy. 42:13427–13443. https://doi.org/10.1016/j.ijhydene.2017.02.102
Milani D et al (2020) Renewable-powered hydrogen economy from Australia’s perspective. Int J Hydrog Energy. 45:24125–24145. https://doi.org/10.1016/j.ijhydene.2020.06.041
Misra P et al (2017) Denitrogenation and desulfurization of model diesel fuel using functionalized polymer: charge transfer complex formation and adsorption isotherm study. Chem Eng J 325:176–187. https://doi.org/10.1016/j.cej.2017.05.033
Mouli-Castillo J et al (2021) Mapping geological hydrogen storage capacity and regional heating demands: an applied UK case study. Appl Energy 283:116348–116348. https://doi.org/10.1016/j.apenergy.2020.116348
Nanda S et al (2017) Advancements and confinements in hydrogen production technologies Bioenergy systems for the future. Elsevier Inc, Hoboken, pp 373–418
Google Scholar
Naylor M et al (2011) Calculation of CO2 column heights in depleted gas fields from known pre-production gas column heights. Mar Pet Geol 28:1083–1093. https://doi.org/10.1016/J.MARPETGEO.2010.10.005
Ochedi FO et al (2021) Carbon dioxide capture using liquid absorption methods: a review. Environ Chem Lett 19:77–109. https://doi.org/10.1007/s10311-020-01093-8
Ockwig NW, Nenoff TM (2007) Membranes for hydrogen separation. Chem Rev 107:4078–4110. https://doi.org/10.1021/cr0501792
Ogawa T et al (2018) Comprehensive analysis of trends and emerging technologies in all types of fuel cells based on a computational method. Sustainability. https://doi.org/10.3390/su10020458
Okolie JA et al (2019) Supercritical water gasification of biomass: a state-of-the-art review of process parameters, reaction mechanisms and catalysis. Sustain Energy Fuels 3:578–598. https://doi.org/10.1039/c8se00565f
Oldenburg CM (2003) Carbon dioxide as cushion gas for natural gas storage. Energy Fuels 17:240–246. https://doi.org/10.1021/ef020162b
Osman AI et al (2020) Exploring the photocatalytic hydrogen production potential of titania doped with alumina derived from foil waste. Int J Hydrog Energy. 45:34494–34502. https://doi.org/10.1016/j.ijhydene.2020.02.065
Osman AI et al (2020) Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00965-x
Osman AI (2020) Catalytic hydrogen production from methane partial oxidation: mechanism and kinetic study. Chem Eng Technol. 43:641–648. https://doi.org/10.1002/ceat.201900339
Osman AI et al (2021a) Recent advances in carbon capture storage and utilisation technologies: a review. Environ Chem Lett 19:797–849. https://doi.org/10.1007/s10311-020-01133-3
Osman AI et al (2021b) Conversion of biomass to biofuels and life cycle assessment: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-021-01273-0
Owgi AHK et al (2021) Catalytic systems for enhanced carbon dioxide reforming of methane: a review. Environ Chem Lett 19:2157–2183. https://doi.org/10.1007/s10311-020-01164-w
Ozturk M, Dincer I (2019) Comparative environmental impact assessment of various fuels and solar heat for a combined cycle. Int J Hydrog Energy 44:5043–5053. https://doi.org/10.1016/j.ijhydene.2019.01.003
Panfilov M (2010) Underground storage of hydrogen. In situ self-organisation and methane generation. Transp Porous Media 85:841–865. https://doi.org/10.1007/s11242-010-9595-7
Panfilov M (2016) Underground and pipeline hydrogen storage Compendium of hydrogen energy. Elsevier, Hoboken, pp 91–115
Park S et al (2017) Acetate-assisted increase of butyrate production by Eubacterium limosum KIST612 during carbon monoxide fermentation. Biores Technol 245:560–566. https://doi.org/10.1016/j.biortech.2017.08.132
Parra D et al (2019) A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew Sustain Energy Rev. 101:279–294. https://doi.org/10.1016/j.rser.2018.11.010
Perry KF (2005) Natural gas storage industry experience and technology: potential application to CO2 geological storage. Carbon Dioxide Capture Storage Deep Geol Form. https://doi.org/10.1016/B978-008044570-0/50135-5
Pinsky R et al (2020) Comparative review of hydrogen production technologies for nuclear hybrid energy systems. Prog Nuclear Energy 123:103317. https://doi.org/10.1016/j.pnucene.2020.103317
Polverino P et al (2019) Study of the energetic needs for the on-board production of Oxy-Hydrogen as fuel additive in internal combustion engines. Energy Convers Manage 179:114–131. https://doi.org/10.1016/j.enconman.2018.09.082
Prussi M et al (2021) Potential and limiting factors in the use of alternative fuels in the European maritime sector. J Clean Prod 291:125849–125849. https://doi.org/10.1016/j.jclepro.2021.125849
Psoma A, Sattler G (2002) Fuel cell systems for submarines: from the first idea to serial production. J Power Sour 106:381–383. https://doi.org/10.1016/S0378-7753(01)01044-8
Pudlo D et al (2013) The H2STORE project: Hydrogen underground storage-A feasible way in storing electrical power in geological media? Springer, Berlin, pp 395–412. https://doi.org/10.1007/978-3-642-37849-2_31
Rana MS et al (2020) Synthesis of large pore carbon-alumina supported catalysts for hydrodemetallization. Catal Today 353:204–212. https://doi.org/10.1016/j.cattod.2019.07.009
Reaño RL (2020) Assessment of environmental impact and energy performance of rice husk utilization in various biohydrogen production pathways. Biores Technol 299:122590. https://doi.org/10.1016/j.biortech.2019.122590
Reaño RL, Halog A (2020) Analysis of carbon footprint and energy performance of biohydrogen production through gasification of different waste agricultural biomass from the Philippines. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01151-9
Research, A. E., Companies are developing over 200 gw of hydrogen electrolyser projects globally, 85% of which are in europe, https://auroraer.com/media/companies-are-developing-over-200-gw-of-hydrogen-electrolyser-projects-globally-85-of-which-are-in-europe/ , accessed on 20–5–2021. 2021. https://auroraer.com/media/companies-are-developing-over-200-gw-of-hydrogen-electrolyser-projects-globally-85-of-which-are-in-europe/
Rigueto CVT et al (2020) Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol Eng 150:105817. https://doi.org/10.1016/j.ecoleng.2020.105817
Ringrose PS et al (2021) Storage of carbon dioxide in saline aquifers: physicochemical processes, key constraints, and scale-up potential. Annu Rev Chem Biomol Eng 12:471–494. https://doi.org/10.1146/annurev-chembioeng-093020-091447
Romeo LM et al (2020) Review of power-to-X demonstration projects in Europe. Front Energy Res. https://doi.org/10.3389/fenrg.2020.00191
Sadeghi S et al (2020) Comparative economic and life cycle assessment of solar-based hydrogen production for oil and gas industries. Energy 208:118347. https://doi.org/10.1016/j.energy.2020.118347
Safari F, Dincer I (2020) A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers Manag. 205:112182. https://doi.org/10.1016/j.enconman.2019.112182
Saidi M et al (2020) Hydrogen production from waste gasification followed by membrane filtration: a review. Environ Chem Lett 18:1529–1556. https://doi.org/10.1007/s10311-020-01030-9
Sainz-Garcia A et al (2017) Assessment of feasible strategies for seasonal underground hydrogen storage in a saline aquifer. Int J Hydrog Energy 42:16657–16666. https://doi.org/10.1016/j.ijhydene.2017.05.076
Saithong N et al (2019) Thermodynamic analysis of the novel chemical looping process for two-grade hydrogen production with CO2 capture. Energy Convers Manag. 180:325–337. https://doi.org/10.1016/j.enconman.2018.11.003
Sako N et al (2021) Techno-economic and life cycle analyses of battery-assisted hydrogen production systems from photovoltaic power. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.126809
Sanchez N et al (2021) Technical and environmental analysis on the power production from residual biomass using hydrogen as energy vector. Renew Energy 175:825–839. https://doi.org/10.1016/j.renene.2021.04.145
Siddiqui O, Dincer I (2019) A well to pump life cycle environmental impact assessment of some hydrogen production routes. Int J Hydrog Energy 44:5773–5786. https://doi.org/10.1016/j.ijhydene.2019.01.118
Silva FB et al (2020) Primary data priorities for the life cycle inventory of construction products: focus on foreground processes. Int J Life Cycle Assess 25:980–997. https://doi.org/10.1007/s11367-020-01762-4
Span R et al. (2020) TREND. Thermodynamic Reference and Engineering Data 5.0. Lehrstuhl für Thermodynamik, Ruhr-Universität Bochum
Spingler FB et al (2017) Investigating fuel-cell transport limitations using hydrogen limiting current. Int J Hydrog Energy 42:13960–13969. https://doi.org/10.1016/j.ijhydene.2017.01.036
Srivastava RK et al (2020) Biofuels, biodiesel and biohydrogen production using bioprocesses. A Rev Environ Chem Lett 18:1049–1072. https://doi.org/10.1007/s10311-020-00999-7
Sun Y et al (2017) Fischer-Trospch synthesis using iron-based catalyst in a microchannel reactor: hybrid lump kinetic with ANNs/RSM. Chem Eng Process 122:181–189. https://doi.org/10.1016/j.cep.2017.10.005
Taylor JB et al (1986) Technical and economic assessment of methods for the storage of large quantities of hydrogen. Int J Hydrog Energy 11:5–22. https://doi.org/10.1016/0360-3199(86)90104-7
Thaysen EM, McMahon S, Strobel G, Butler I, Ngwenya B, Heinemann N, Wilkinson M, Hassanpouryouzband A, McDermott C, Edlmann K (2020) Estimating microbial hydrogen consumption in hydrogen storage in porous media as a basis for site selection. https://doi.org/10.31223/X5HC7H
Thema M et al (2019) Power-to-Gas: electrolysis and methanation status review. Renew Sustain Energy Rev 112:775–787. https://doi.org/10.1016/j.rser.2019.06.030
Thi HN et al (2020) Medium compositions for the improvement of productivity in syngas fermentation with clostridium autoethanogenum. Biotechnol Bioprocess Eng 25:493–501. https://doi.org/10.1007/s12257-019-0428-4
Tolod KR et al (2019) Visible light-driven catalysts for water oxidation towards solar fuel biorefineries horizons in sustainable industrial chemistry and catalysis. Elsevier Inc, Hoboken, pp 65–84. https://doi.org/10.1016/B978-0-444-64127-4.00004-5
Tzanetis KF et al (2017) Analysis of biomass hydrothermal liquefaction and biocrude-oil upgrading for renewable jet fuel production: the impact of reaction conditions on production costs and GHG emissions performance. Renew Energy 113:1388–1398. https://doi.org/10.1016/j.renene.2017.06.104
U.S. wind projects, https://us.orsted.com/wind-projects# . https://us.orsted.com/wind-projects#
Valente A et al (2019) Life cycle sustainability assessment of hydrogen from biomass gasification: a comparison with conventional hydrogen. Int J Hydrog Energy 44:21193–21203. https://doi.org/10.1016/j.ijhydene.2019.01.105
Valente A et al (2021) Harmonised carbon and energy footprints of fossil hydrogen. Int J Hydrog Energy 46:17587–17594. https://doi.org/10.1016/j.ijhydene.2020.03.074
Vogler F, Sattler G (2016) Hydrogen-fueled marine transportation Compendium of hydrogen energy. Elsevier, Hoboken, pp 35–65. https://doi.org/10.1016/b978-1-78242-364-5.00003-8
Wainaina S et al (2018) Biochemicals from food waste and recalcitrant biomass via syngas fermentation: a review. Biores Technol 248:113–121. https://doi.org/10.1016/j.biortech.2017.06.075
Widera B (2020) Renewable hydrogen implementations for combined energy storage, transportation and stationary applications. Thermal Sci Eng Prog 16:100460–100460. https://doi.org/10.1016/j.tsep.2019.100460
Wilberforce T et al (2016) Advances in stationary and portable fuel cell applications. Int J Hydrog Energy 41:16509–16522. https://doi.org/10.1016/j.ijhydene.2016.02.057
Wilkins A et al (2020) PorousFlow: a multiphysics simulation code for coupled problems in porous media. J Open Sour Softw 5:2176–2176. https://doi.org/10.21105/joss.02176
Wilkins A et al (2021) An open-source multiphysics simulation code for coupled problems in porous media. Comput Geosci 154:104820–104820. https://doi.org/10.1016/J.CAGEO.2021.104820
Xu Z et al (2021) New insights in light-assisted microbial fuel cells for wastewater treatment and power generation: a win-win cooperation. J Power Sour 501:230000–230000. https://doi.org/10.1016/j.jpowsour.2021.230000
Yfanti VL, Lemonidou AA (2020) Effect of hydrogen donor on glycerol hydrodeoxygenation to 1,2-propanediol. Catal Today 355:727–736. https://doi.org/10.1016/j.cattod.2019.04.080
Yu Z et al (2011) Investigation on performance of an integrated solid oxide fuel cell and absorption chiller tri-generation system. Int J Hydrog Energy 36:12561–12573. https://doi.org/10.1016/j.ijhydene.2011.06.147
Yu L et al (2019) Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat Commun 10:5106. https://doi.org/10.1038/s41467-019-13092-7
Yuan XZ et al (2021) A review of functions, attributes, properties and measurements for the quality control of proton exchange membrane fuel cell components. J Power Sour 491:229540–229540. https://doi.org/10.1016/j.jpowsour.2021.229540
Yue M et al (2021) Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew Sustain Energy Rev 146:111180–111180. https://doi.org/10.1016/j.rser.2021.111180
Zachariah-Wolff JL et al (2007) From natural gas to hydrogen via the Wobbe index: the role of standardized gateways in sustainable infrastructure transitions. Int J Hydrog Energy 32:1235–1245. https://doi.org/10.1016/j.ijhydene.2006.07.024
Zhou D et al (1997) Scaling of multiphase flow in simple heterogeneous porous media. SPE Reserv Eng 12:173–178. https://doi.org/10.2118/27833-PA
Zhu C et al (2019) Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl Catal B: Environ. 242:178–185. https://doi.org/10.1016/j.apcatb.2018.09.096
Download references
Acknowledgements
The authors would like to thank OQ Oman for their generous financial support (project code: CR/DVC/SERC/19/01). The authors would also like to acknowledge the support of the Sustainable Energy Research Centre at Sultan Qaboos University. Ahmed Osman and David Rooney wish to acknowledge the support of The Bryden Centre project (Project ID VA5048). The Bryden Centre project is supported by the European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB). Neha Mehta acknowledges funding from the Centre for Advanced Sustainable Energy (CASE). CASE is funded through Invest NI’s Competence Centre Programme and aims to transform the sustainable energy sector through business research.
Author information
Authors and affiliations.
School of Chemistry and Chemical Engineering, Queen’s University Belfast, Belfast, BT9 5AG, Northern Ireland, UK
Ahmed I. Osman, Neha Mehta & David W. Rooney
The Centre for Advanced Sustainable Energy, David Keir Building, Queen’s University Belfast, Stranmillis Road, Belfast, BT9 5AG, Northern Ireland, UK
Environmental Science Department, Faculty of Science, Port Said University, Port Said, Egypt
Ahmed M. Elgarahy
Egyptian Propylene and Polypropylene Company (EPPC), Port-Said, Egypt
Department of Earth Sciences, Geothermal Energy and Geofluids, ETH Zurich, Switzerland
Mahmoud Hefny
Geology Department, South Valley University, Qena, Egypt
Department of Electrical and Computer Engineering, College of Engineering, Sultan Qaboos University, Muscat, Oman
Amer Al-Hinai
Department of Petroleum and Chemical Engineering, College of Engineering, Sultan Qaboos University, Muscat, Oman
Ala’a H. Al-Muhtaseb
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Ahmed I. Osman , Amer Al-Hinai or Ala’a H. Al-Muhtaseb .
Ethics declarations
Conflict of interest.
The authors declare that no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Osman, A.I., Mehta, N., Elgarahy, A.M. et al. Hydrogen production, storage, utilisation and environmental impacts: a review. Environ Chem Lett 20 , 153–188 (2022). https://doi.org/10.1007/s10311-021-01322-8
Download citation
Received : 14 August 2021
Accepted : 07 September 2021
Published : 06 October 2021
Issue Date : February 2022
DOI : https://doi.org/10.1007/s10311-021-01322-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hydrogen production
- Hydrogen storage
- Climate change
- Find a journal
- Publish with us
- Track your research
/
Green hydrogen can be stored in a liquid form. Wolfgang Kumm/picture-alliance/dpa/AP Images
Green Hydrogen: Could It Be Key to a Carbon-Free Economy?
Green hydrogen, which uses renewable energy to produce hydrogen from water, is taking off around the globe. Its boosters say the fuel could play an important role in decarbonizing hard-to-electrify sectors of the economy, such as long-haul trucking, aviation, and heavy manufacturing.
By Jim Robbins • November 5, 2020
Saudi Arabia is constructing a futuristic city in the desert on the Red Sea called Neom. The $500 billion city — complete with flying taxis and robotic domestic help — is being built from scratch and will be home to a million people. And what energy product will be used both to power this city and sell to the world? Not oil. The Saudis are going big on something called green hydrogen — a carbon-free fuel made from water by using renewably produced electricity to split hydrogen molecules from oxygen molecules.
This summer, a large U.S. gas company, Air Products & Chemicals, announced that as part of Neom it has been building a green hydrogen plant in Saudi Arabia for the last four years. The plant is powered by 4 gigawatts from wind and solar projects that sprawl across the desert. It claims to be the world’s largest green hydrogen project — and more Saudi plants are on the drawing board.
Green hydrogen? The Saudis aren’t alone in believing it’s the next big thing in the energy future. While the fuel is barely on the radar in the United States, around the world a green hydrogen rush is underway, and many companies, investors, governments, and environmentalists believe it is an energy source that could help end the reign of fossil fuels and slow the world’s warming trajectory.
“It is very promising,” said Rachel Fakhry, an energy analyst for the Natural Resources Defense Council. Experts like Fakhry say that while wind and solar energy can provide the electricity to power homes and electric cars, green hydrogen could be an ideal power source for energy-intensive industries like concrete and steel manufacturing, as well as parts of the transportation sector that are more difficult to electrify. “The last 15 percent of the economy is hard to clean up — aviation, shipping, manufacturing, long-distance trucking,” Fakhry said in an interview. “Green hydrogen can do that.”
Germany has allocated the largest share of its clean energy stimulus funds to green hydrogen.
Europe, which has an economy that is saddled with high energy prices and is heavily dependent on Russian natural gas, is embracing green hydrogen by providing funding for construction of electrolysis plants and other hydrogen infrastructure. Germany has allocated the largest share of its clean energy stimulus funds to green hydrogen. “It is the missing part of the puzzle to a fully decarbonized economy,” the European Commission wrote in a July strategy document.
Hydrogen’s potential as a fuel source has been touted for decades, but the technology has never gotten off the ground on a sizeable scale — and with good reason, according to skeptics. They argue that widespread adoption of green hydrogen technologies has faced serious obstacles, most notably that hydrogen fuels need renewable energy to be green, which will require a massive expansion of renewable generation to power the electrolysis plants that split water into hydrogen and oxygen. Green hydrogen is also hard to store and transport without a pipeline. And right now in some places, such as the U.S., hydrogen is a lot more expensive than other fuels such as natural gas.
While it has advantages, says Michael Liebreich, a Bloomberg New Energy Finance analyst in the United Kingdom and a green hydrogen skeptic, “it displays an equally impressive list of disadvantages.”
“It does not occur in nature so it requires energy to separate,” Liebreich wrote in a pair of recent essays for BloombergNEF. “Its storage requires compression to 700 times atmospheric pressure, refrigeration to 253 degrees Celsius… It carries one quarter the energy per unit volume of natural gas… It can embrittle metal; it escapes through the tiniest leaks and yes, it really is explosive.”
In spite of these problems, Liebreich wrote, green hydrogen still “holds a vice-like grip over the imaginations of techno-optimists.”
Green hydrogen is produced using renewable energy, making it a CO2-free source of fuel. SGN
Ben Gallagher, an energy analyst at Wood McKenzie who studies green hydrogen, said the fuel is so new that its future remains unclear. “No one has any true idea what is going on here,” he said. “It’s speculation at this point. Right now it’s difficult to view this as the new oil. However, it could make up an important part of the overall fuel mix.”
Hydrogen is the most abundant chemical in the universe. Two atoms of hydrogen paired with an atom of oxygen creates water. Alone, though, hydrogen is an odorless and tasteless gas, and highly combustible. Hydrogen derived from methane — usually from natural gas, but also coal and biomass — was pioneered in World War II by Germany, which has no petroleum deposits. But CO2 is emitted in manufacturing hydrogen from methane and so it’s not climate friendly; hydrogen manufactured this way is known as gray hydrogen.
Green is the new kid on the hydrogen block, and because it’s manufactured with renewable energy, it’s CO2-free. Moreover, using renewable energy to create the fuel can help solve the problem of intermittency that plagues wind and solar power, and so it is essentially efficient storage. When demand for renewables is low, during the spring and fall, excess electricity can be used to power the electrolysis that is needed to split hydrogen and oxygen molecules. Then the hydrogen can be stored or sent down a pipeline.
Such advantages are fueling growing interest in global green hydrogen. Across Europe, the Middle East, and Asia, more countries and companies are embracing this high-quality fuel. The U.S. lags behind because other forms of energy, such as natural gas, are much cheaper, but several new projects are getting underway, including a green hydrogen power plant in Utah that will replace two aging coal-fired plants and produce electricity for southern California.
The Middle East, with the world’s cheapest wind and solar power, is angling to be a major player in green hydrogen.
In Japan, a new green hydrogen plant, one of the world’s largest, just opened near Fukishima — an intentionally symbolic location given the plant’s proximity to the site of the 2011 nuclear disaster. It will be used to power fuel cells, both in vehicles and at stationary sites. An energy consortium in Australia just announced plans to build a project called the Asian Renewable Energy Hub in Pilbara that would use 1,743 large wind turbines and 30 square miles of solar panels to run a 26-gigawatt electrolysis factory that would create green hydrogen to send to Singapore.
As Europe intensifies its decarbonization drive, it, too, is betting big on the fuel. The European Union just drafted a strategy for a large-scale green hydrogen expansion, though it hasn’t been officially adopted yet. But in its $550-billion clean energy plan, the EU is including funds for new green hydrogen electrolyzers and transport and storage technology for the fuel. “Large-scale deployment of clean hydrogen at a fast pace is key for the EU to achieve its high climate ambitions,” the European Commission wrote.
The Middle East, which has the world’s cheapest wind and solar power, is angling to be a major player in green hydrogen. “Saudi Arabia has ridiculously low-cost renewable power,” said Thomas Koch Blank, leader of the Rocky Mountain Institute’s Breakthrough Technology Program. “The sun is shining pretty reliably every day and the wind is blowing pretty reliably every night. It’s hard to beat.”
BloombergNEF estimates that to generate enough green hydrogen to meet a quarter of the world’s energy needs would take more electricity than the world generates now from all sources and an investment of $11 trillion in production and storage. That’s why the focus for now is on the 15 percent of the economy with energy needs not easily supplied by wind and solar power, such as heavy manufacturing, long-distance trucking, and fuel for cargo ships and aircraft.
The Fukushima Hydrogen Energy Research Field (FH2R), a green hydrogen facility that can generate as much as 1,200 normal meter cubed (Nm3) of hydrogen per hour, opened in Japan in March. Toshiba ESS
The energy density of green hydrogen is three times that of jet fuel, making it a promising zero-emissions technology for aircraft. But Airbus, the European airplane manufacturer, recently released a statement saying that significant problems need to be overcome, including safely storing hydrogen on aircraft, the lack of a hydrogen infrastructure at airports, and cost. Experts say that new technologies will be needed to solve these problems. Nevertheless, Airbus believes green hydrogen will play an important role in decarbonizing air transport.
“Cost-competitive green hydrogen and cross-industry partnerships will be mandatory to bring zero-emission flying to reality,” said Glen Llewellyn, vice president of Zero Emission Aircraft for Airbus. Hydrogen-powered aircraft could be flying by 2035, he said.
In the U.S., where energy prices are low, green hydrogen costs about three times as much as natural gas, though that price doesn’t factor in the environmental damage caused by fossil fuels. The price of green hydrogen is falling, however. In 10 years, green hydrogen is expected to be comparable in cost to natural gas in the United States.
A major driver of green hydrogen development in the U.S. is California’s aggressive push toward a carbon-neutral future. The Los Angeles Department of Water and Power, for example, is helping fund the construction of the green hydrogen-fueled power plant in Utah. It’s scheduled to go online in 2025.
A company called SGH2 recently announced it would build a large facility to produce green hydrogen in southern California. Instead of using electrolysis, though, it will use waste gasification, which heats many types of waste to high temperatures that reduce them to their molecular compounds. Those molecules then bind with hydrogen, and SGH2 claims it can make green hydrogen more cheaply than using electrolysis.
California officials see green hydrogen as an alternative to fossil fuels for diesel vehicles.
California officials also see green hydrogen as an alternative to fossil fuels for diesel vehicles. The state passed a Low Carbon Fuel Standard in 2009 to promote electric vehicles and hydrogen vehicles. Last month, a group of heavy-duty vehicle and energy industry officials formed the Western States Hydrogen Alliance to press for rapid deployment of hydrogen fuel cell technology and infrastructure to replace diesel trucks, buses, locomotives, and aircraft.
“Hydrogen fuel cells will power the future of zero-emission mobility in these heavy-duty, hard-to-electrify sectors,” said Roxana Bekemohammadi, executive director of the Western States Hydrogen Alliance. “That fact is indisputable. This new alliance exists to ensure government and industry can work efficiently together to accelerate the coming of this revolution.”
Earlier this year, the U.S. Department of Energy announced a $100 million investment to help develop large, affordable electrolyzers and to create new fuel cell technologies for long-haul trucks.
In Australia, the University of New South Wales, in partnership with a global engineering firm, GHD, has created a home-based system called LAVO that uses solar energy to generate and store green hydrogen in home systems. The hydrogen is converted back into electricity as needed.
All these developments, says Blank of the Rocky Mountain Instiute, are “really good news. Green hydrogen has high potential to address many of the things that keep people awake at night because the climate change problem seems unsolvable.”
Related Articles
Can a california oilfield be retrofitted to store solar energy.
By Stephen Robert Miller
In a Dammed and Diked Mekong, a Push to Restore the Flow
By Stefan Lovgren
Will New Leader End Progress in Saving Indonesia’s Forests?
By Fred Pearce
More From E360
The race to save glacial ice records before they melt away, turning brownfields to blooming meadows, with the help of fungi, to foil a deadly pest, scientists aim for a beetle-resistant ash tree, could the global boom in greenhouses help cool the planet, biodiversity, in north macedonia, an ancient lake faces modern threats, how a ‘citizen map’ is helping brazil prepare for next big flood, a key court ruling could weaken u.s. environmental protections, as ‘zombie’ deer disease spreads, scientists look for answers, despite criticism, the last of the rattlesnake roundups hang on, pollution paradox: how cleaning up smog drives ocean warming, how an el niño-driven drought brought hunger to southern africa.
Essay on Hydrogen Energy | Types | Renewable Energy | Energy Management
Here is an essay on ‘Hydrogen Energy’ for class 8, 9, 10, 11 and 12. Find paragraphs, long and short essays on ‘Hydrogen Energy’ especially written for school and college students.
Essay on Hydrogen Energy
Essay Contents:
- Essay on the Limitations of Hydrogen Energy
Essay # 1. Introduction to Hydrogen Energy:
ADVERTISEMENTS:
Electrical energy is the most convenient form of energy because it can be easily controlled, transported and converted into heat and work at very high efficiencies. The only shortcoming of electrical energy is that it cannot be stored in large quantities. Alternative energy of future is hydrogen energy which can also be easily stored in addition to other qualities of electrical energy.
But is highly inflammable and special handling precautions are needed during its production, transportation, storage and utilization. Hydrogen is a secondary fuel that is produced by utilizing energy from a primary source. Water and solar energy are freely and abundantly available in nature on earth. Hydrogen can be produced from water by using solar energy. All plants and hydrocarbons (fossil fuels) are sources of hydrogen.
Hydrogen can be stored as gas underground or in high pressure cylinders. Also it can be stored in liquid form at low temperatures. There are a number of metals and alloys which form solid hydrides with hydrogen. The hydrogen can be easily recovered by heating metal hydrides. Thus metal hydrides provide a possible means for hydrogen storage in solid form. Hydrogen can be similarly transported as gas through pipes or storage cylinders or as liquid hydrogen or as metal hydrides.
Hydrogen has the highest energy content per unit mass. Its burning process is non-polluting and it can be used in fuel cells to produce both electricity and useful heat. It can be used as a fuel directly in gas turbines or spark-ignition engines. It can be used as motor vehicle fuel in urban transportation where air pollution problems are critical.
Its specific energy content is almost three times that of hydrocarbon fuels. Therefore it can be directly used as aircraft fuel for air transport. Hydrogen has been used as a fuel for space craft’s. A H 2 – O 2 fuel cell liberates energy and also water as sole material product for the use of space craft passengers.
The simplest practical way to obtain hydrogen from water is its electrolysis using electricity. The latter can be generated from removable energy sources like solar energy, wind energy, geothermal energy.
Hydrogen has huge market. However, enormous capital investment is required for its production, distribution and storage. Special design precautions are needed for the safe operation of equipment and systems. This is presenting the essential obstacle in the quick introduction of hydrogen.
Essay # 2. Utilization of Hydrogen Energy:
Hydrogen has the following properties which make it an attractive alternative energy source:
1. At room temperature and pressure, hydrogen is a light gas. Its density is only 1/14th of that of air and 1/9th that of natural gas.
2. At atmospheric pressure, hydrogen can be liquefied at – 253°C. The liquid hydrogen has a specific gravity of 0.07 which is 1/10th that of gasoline.
3. The standard heating value of hydrogen gas is 12.1 MJ/m 3 compared with 38.3 MJ/m 3 for natural gas.
4. The heating value of liquid hydrogen is 120 MJ/kg or 8400 MJ/m 3 as compared to 44MJ/kg or 32000 MJ/m 3 of aviation petrol. The specific energy of hydrogen liquid is superior to gasoline on mass basis but inferior on volume basis.
5. The flame speed of hydrogen when burning in air is much greater than for natural gas.
6. The ignition energy to initiate combustion is less for hydrogen than for natural gas.
7. Detonation can occur between hydrogen-air mixture between 18 and 59 percent. The internal combustion engine on hydrogen fuel can work from very rich (excess fuel) to very lean (excess air) mixture. The adjustment of air fuel ratio is less critical than for gasoline engine.
8. Mixture of hydrogen and air are combustible over a wide range of composition. The flammability limits are from 4 to 74 percent by volume of hydrogen in air at ordinary temperatures.
9. The combustion of hydrogen with oxygen from air results in release of energy and water as by-product.
10. The burning process of hydrogen is pollution free.
The possible areas of use of hydrogen in the near future are as follows:
1. Production of Useful Heat:
(i) In a high-temperature combustion of hydrogen with oxygen or air.
(ii) In a low-temperature flameless catalytic combustion with extremely low NO x emission.
2. Power Generation:
(i) In reactors with direct steam generation.
(ii) In high-temperature and membrane fuel cells.
3. Cogeneration of Heat and Electricity:
(i) In internal combustion engine based cogeneration plants.
(ii) In combined-cycle power plants.
4. Automotive and Aircraft Fuel:
(i) Environmentally friendly fuel for motor vehicles.
(ii) Aircraft fuel.
5. Energy Storage:
(a) Compressed hydrogen gas storage under high pressure.
(ii) Metal hydrides.
6. Synthesis of Fuels:
(i) Raw material to produce methanol, ammonia or hydrocarbon using carbon dioxide or nitrogen from air.
(ii) Raw material for manufacture of gaseous fuels.
Essay # 3. Hydrogen Energy for Air and Surface Transport:
1. Jet Fuel:
The high energy density 33.3 kWh/kg of liquid hydrogen against 12.7 kWh/kg of conventional jet fuel is the main advantage in air transportation where hydrogen energy can be used. Although volume of liquid hydrogen would be greater than regular fuel but this could be accommodated on a large aircraft.
The cold liquid hydrogen can also be used directly or indirectly for cooling engine and airframe surfaces of high speed aircrafts. Liquid hydrogen may be the only practical fuel for hypersonic aircraft when developed. Because of smaller total weight, it may be possible to achieve shorter take-off runs, steeper climbing path, smaller engine thrust and less noise production. `
The favorable diffusion properties and high thermal conductivity of hydrogen help to use shorter combustion chambers. Wide range of ignition for H 2 – O 2 mixtures (5% to 75%) by volume of hydrogen helps better control of engine operation especially under part load conditions and reduction in NO x emission.
The heat required to vaporize hydrogen for the engines can be obtained from certain outer skin of wings and fuselage. This helps to cool the boundary layer. The laminar boundary layer so developed helps to reduce drag and fuel consumption.
The main problems are economic production of hydrogen, infrastructure for fueling of aircrafts and sitting of bulky hydrogen tanks in the aircraft body.
2. Road Vehicles:
The use of hydrogen fuel in engines of automobiles, buses, trucks and farm machinery can help conserve petroleum products and reduce atmospheric pollution. A mixture of hydrogen gas and air of constant ratio is introduced into the manifold. The engine speed and power are controlled by varying the quality of mixture entering the cylinder with the help of a throttle valve.
In another design, hydrogen gas under pressure can be directly injected into the engine cylinder through a valve and air is admitted through another intake valve. The spark advance has to be retarded because of higher speed of flame of hydrogen in air. The engine emission will not contain carbon monoxide and hydrocarbon because the only product of combustion is H 2 O. In order to control NO x emission, the cylinder exhaust containing H 2 O is injected to reduce combustion temperature.
Storage of hydrogen as compressed gas or metal hydrides for the vehicles is a dis-advance against hydrogen fuel because of lesser energy density per unit volume and the weight of metal hydrides is also excessive. The best way to use hydrogen as vehicle fuel is the use of fuel cells. The electricity generated in the fuel cells operating with hydrogen fuel can be utilized to operate electric motors for propelling the vehicle.
Essay # 4. Power Generation Using Hydro Energy:
1. Central Power Plants:
The plants working on natural gas can be changed to hydrogen-fired plants without significant technological changes. These plants may be combined-cycle power plants with gas and steam turbines or cogeneration plants with gas turbine and internal combustion engines. Hydrogen-fired plants compared to gas-fired plants have lower capital cost and higher efficiency.
Hydrogen fuel has higher combustion velocity and flame temperature as compared to natural gas. Higher combustion velocity results in unstable combustion and higher flame temperature leads to higher No x emissions.
2. Autonomous Power Plants:
Hydrogen fuelled fuel cells can be used for domestic power generation as well as industrial power generation. Alkaline fuel cells can be used for producing electricity from pure hydrogen and oxygen. Fuel cells with acid electrolytes can be operated with impure hydrogen and hydrocarbons. High temperature fuel cells with molten carbonate electrolytes operating at 600°C are advanced generation fuel cells for electricity and heat production in cogeneration plants.
Essay # 5. Miscellaneous Applications of Hydro Energy:
Hydrogen can be used for domestic cooking replacing LPG. The burner design has to be changed with bigger holes and air supply system to take care of greater flame speed and low specific energy per unit volume when hydrogen is used as fuel.
Hydrogen can be usefully used in radiant space heating with flameless combustion on a catalytic surface. In this case combustion temperature is low with negligible NO x formation.
Hydrogen has many advantages over industrial gases for production of heat and other uses.
Essay # 6. Hydrogen Storage and Distribution:
There are five principle methods that have been considered for hydrogen storage:
1. Compressed gas storage. Hydrogen is conveniently stored for many applications in higher pressure cylinders. The method is rather expensive and bulky.
2. Liquid storage as cryogenic storage in vacuum insulated or super insulted storage tanks. The liquid hydrogen fuel used as rocket propellant in the space programme is stored in large tanks.
3. Line packs system where it is allowed to vary the pressure in the transmission and distribution system.
4. Underground storage of hydrogen gas in depleted oil and gas fields or in aquifer systems. This is the cheapest way to store large amounts of hydrogen for subsequent distribution.
5. Storage as metal hydrides in chemically bound form. A number of metals and alloys form solid compounds by direct reaction with hydrogen gas. The metal hydrides can be transported in solid form. When the hydride is heated, hydrogen is released for use.
Hydrogen Transportation:
1. Long distance hydrogen gas transmission pipelines of lengths greater than 90 km must be supplied with booster compressors. Therefore, the cost of transmitting hydrogen by pipelines must include the cost of piping, compressor and power consumption by compressors. Another problem of hydrogen transmission is hydrogen embrittlement of the pipeline materials.
2. Hydrogen in bulk can be transported and distributed as the liquid in double-walled insulated tanks. Distribution of liquid hydrogen by pipelines, jacketed with liquid nitrogen can also be considered.
3. Hydrogen can also be transported as a solid metal hydride. The main drawback is the heavy weight of hydride relative to its hydrogen yield.
Safety Precautions:
Hydrogen is highly inflammable and explosive and can lead to fire and serious accidents. The production, storage and distribution of hydrogen require special precautions.
1. The system should be designed to withstand the explosion pressures.
2. The system should be designed to withstand pressure surges.
3. Proper exposition-relief system must be provided.
4. Flame traps, flame suppressors, explosion-relief devices and rapid-closing devices must be used.
5. The design, manufacture, storage should follow Petroleum Act.
Essay # 7. Hydrogen Production:
1. From Fossil Fuels:
The conventional hydrogen production processes are shown in Fig. 17.1.

A. Natural Gas/Naptha:
1. The natural gas or naptha is reformed with steam at 900°C to produce a mixture of gases by the following reaction:
Hydrogen is produced by electrolytic dissociation of water.

By Hiroko Tabuchi
It is seen by many as the clean energy of the future. Billions of dollars from the bipartisan infrastructure bill have been teed up to fund it.
But a new peer-reviewed study on the climate effects of hydrogen, the most abundant substance in the universe, casts doubt on its role in tackling the greenhouse gas emissions that are the driver of catastrophic global warming .
The main stumbling block: Most hydrogen used today is extracted from natural gas in a process that requires a lot of energy and emits vast amounts of carbon dioxide. Producing natural gas also releases methane, a particularly potent greenhouse gas.
And while the natural gas industry has proposed capturing that carbon dioxide — creating what it promotes as emissions-free, “blue” hydrogen — even that fuel still emits more across its entire supply chain than simply burning natural gas, according to the paper, published Thursday in the Energy Science & Engineering journal by researchers from Cornell and Stanford Universities.
“To call it a zero-emissions fuel is totally wrong,” said Robert W. Howarth, a biogeochemist and ecosystem scientist at Cornell and the study’s lead author. “What we found is that it’s not even a low-emissions fuel, either.”
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Home — Essay Samples — Science — Hydrogen — Hydrogen as a Form of Energy
Hydrogen as a Form of Energy
- Categories: Hydrogen
About this sample

Words: 431 |
Published: Jan 4, 2019
Words: 431 | Page: 1 | 3 min read

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr. Heisenberg
Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
2 pages / 717 words
2 pages / 738 words
2 pages / 976 words
2 pages / 1003 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Hydrogen
Hydrogen peroxide (H2O2) is a common chemical compound with versatile applications in fields ranging from medicine to industrial processes. One of the fundamental chemical characteristics of hydrogen peroxide is its tendency to [...]
Space exploration has long captured the human imagination, and as we set our sights on distant celestial bodies, the need for innovative and sustainable solutions becomes increasingly critical. Hydrogen, often referred to as the [...]
Purpose: The purpose of the lab is to learn the functions of enzymes, especially in chemical reactions, and to determine if, over time, enzymes activity changes in rate. More specifically, the lab was performed to learn the use [...]
Basic uses include fertilizer manufacturing, mineral processing, wastewater processing, oil rening, and chemical synthesis. Sulphuric acid is used for a wide range of purposes both domestically and industrially. Sulphuric acid [...]
Fabrication and Designing Process: The material allows for considerable flexibility is term of design. FRP can be field –fabricated using simple carpenter’s tool’s with carbon and diamond tip blades. No torches [...]
A mixed combination in which solute particles are greater than molecules or ions on the other hand cannot be seen by nude eye is called colloidal solution. An familiar mixture of two substances, one of which, called the [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
- Search Menu
- Sign in through your institution
- Advanced Articles
- Editor's Choice
- Author Guidelines
- Publish with us
- Submission Site
- Open Access
- Self-Archiving Policy
- About Clean Energy
- About the National Institute of Clean and Low-Carbon Energy
- Editorial Board
- Instructions for Reviewers
- Advertising & Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, 1 microbial electrochemical cells, 2 the potential role of human cell lines in hydrogen generation, 3 electron-transfer mechanisms, 4 the perspective of hydrogen production using human cells, acknowledgements, authors’ contributions, conflict of interest statement, data availability.
- < Previous
Could hydrogen gas be produced using human cells?
- Article contents
- Figures & tables
- Supplementary Data
Tunc Catal, Could hydrogen gas be produced using human cells?, Clean Energy , Volume 8, Issue 4, August 2024, Pages 34–39, https://doi.org/10.1093/ce/zkae034
- Permissions Icon Permissions
Although fossil fuels are widely used to meet energy needs, intensive research has been carried out in recent years on hydrogen production from renewable sources due to their decrease over time and environmental pollution concerns. Biofuel cell technology is one of the promising current technologies. It has been proven that various microorganisms produce energy through their natural metabolism, and that energy production is produced in biofuel cells by exoelectrogenic microorganisms that can transfer electrons to an electrode surface. Although it has been stated that employing human cells to generate energy is feasible, it is unknown whether doing so would enable the production of hydrogen. Within the scope of this perspective article, the issue of hydrogen production in bioelectrolysis cells using human cells will be discussed for the first time. Optimizing hydrogen production in bioelectrolysis cells using human cells is important in terms of contributing to hydrogen technologies. Within the scope of the article, promising human cell lines for hydrogen production are emphasized and hydrogen production potentials in bioelectrolysis cells using these cell lines are discussed. In conclusion, some human cells can be used for hydrogen gas production in bioelectrolysis cells due to their bioelectrochemical and metabolic properties.

Today, fossil fuels such as coal, natural gas and oil can produce highly efficient power to meet the energy needs of vehicle engines, electronic devices and people [ 1 ]. Fossil fuels are not sustainable energy sources, however, because of their limited supply. As a result, in order to expand modern industrial civilization and replace fossil fuels, renewable and sustainable fuels are required [ 2 , 3 ]. Different technologies have been reported to prevent the damage caused to the green ecosystem by the ever-increasing energy crisis. One of the promising technologies for the recovery of our resources is fuel cell technology. Because hydrogen fuel has so many natural benefits, including a zero carbon impact, it is a contender to replace fossil fuels. Various waste materials and renewable energy sources can produce hydrogen [ 4 ]. Numerous mechanisms, including thermal, chemical, electrochemical, photochemical and biological ones, can transform the comparatively large energy content of biomass into other forms [ 5 , 6 ]. Fuel cells are devices that directly convert chemical energy into electrical energy [ 5 ]. Fuels such as hydrogen, carbon dioxide, methane, propane and methanol can be used to produce electrical energy in fuel cells [ 7 ]. Although these devices are an environmentally friendly technology, they also have limitations. For example, the difficulty in processing gaseous fuel is the need to keep the fuel gas (hydrogen, oxygen, etc.) as a liquid in a specially made cylinder at very low temperature and high pressure, and the catalysts required for electrode reactions are quite costly. Additionally, the electrolytes used in fuel cells are extremely caustic and pose a number of practical problems [ 8 ]. A new approach that could contribute to the increasing energy need around the world could be the use of human cells in fuel cells for hydrogen generation. Before examining the details of this approach, the principles of microbial electrochemical cell technology, which is an important biological application example, need to be discussed.
Microbial fuel cells can operate under milder environmental conditions. Additionally, operations can be carried out more easily due to the biological nature of the microorganisms. While waste materials are treated biologically in this process, it is possible to simultaneously produce energy [ 9 ]. Microbial fuel and electrolysis cells are current technologies that have been actively studied, especially since 2008, and they are used for energy production in various environmental applications. With this technology, it is possible to directly convert biological energy into electrical or hydrogen energy [ 10 , 11 ]. There are two main subgroups of biofuel cells: (i) microbial fuel cells and (ii) enzyme fuel cells. A subtype of microbial fuel cells is microbial electrolysis cells, which can produce hydrogen from organic substrates. Research on hydrogen production in fuel cells has increased significantly due to its potential to revolutionize power sources. Electricity production with microorganisms was first demonstrated in 1911 [ 12 ] but remarkable increases in power production have been shown in recent years. Although microbial fuel cells have various configurations such as single-chamber [ 11 ] and double-chamber [ 13 ], research is being conducted on their use as cylindrical [ 14 ], soil microbial fuel cells [ 15 ] or sensors [ 16 ]. Various electrode materials have been studied to increase energy production efficiency, and performance improvement studies continue with new approaches to increase surface area and electron transfer [ 17 ]. Another important parameter is that carbon sources and complex compounds have been proposed as substrates [ 18 , 19 ]. Characterization research is important for the broad application of this technology to promote optimal microbial ecology [ 20 ], especially in mixed culture studies. Hydrogen production studies with microbial electrolysis cells [ 21 ], which differ from microbial fuel cells in terms of their working principle, have significant potential to meet the need for clean energy. While these technologies can play an important role in the field of energy, especially electricity and hydrogen, they also have versatile properties as an alternative for cleaning wastewater.
Hydrogen offers an important opportunity to obtain continuous and clean power [ 22 ]. Although potentially very useful, it is insufficient to meet the increasing need for renewable energy production due to weak participation from the industry. Fuel cells primarily use hydrogen produced from hydrocarbon sources. However, there is an abundance of renewable carbon-based resources in the world that can be produced naturally or industrially. The fact that biofuel cells can produce hydrogen or electricity from readily available renewable sources offers the opportunity to contribute significantly to the world’s energy needs. Microbial electrochemical cells contribute to the energy needs in the world by producing electrical power or hydrogen through microbial metabolism [ 23 ]. Microorganisms adhere to the anode, while the cathode contains an electrocatalyst for oxygen reduction. Electrons and protons released by the breakdown of carbon sources such as glucose are used in the electricity production process.
There has been a remarkable increase in power generation and coulombic efficiency in microbial fuel cells over the past 10 years. It has been reported that microbial fuel cells can achieve a power density of 1 kW/m 3 by loading wastewater at a rate of 0.1–10 kg of chemical oxygen demand per cubic metre [ 10 ]. For this reason, intense research is being carried out in the field of microbial fuel cell technology.
In particular, the topic of hydrogen production using microbial electrolysis cells is of interest, as there is a global effort to use hydrogen as an energy source. Microbial electrolysis is an effective biological analogue of chemical electrolysis—much like a microbial fuel cell is to a chemical fuel cell. In microbial electrolysis cells, an organic substrate is microbially oxidized to produce protons and the protons are transferred to the cathode to be reduced to hydrogen gas. The main issue in hydrogen production using microbial electrolysis cells is the ability to produce higher current density at lower voltage levels for cost-effective operation. One of the main factors affecting the operation is ensuring high-efficiency hydrogen production. With an application potential of 0.6 V, a hydrogen production rate of 2.3 m 3 /day/m 3 was achieved using single-chamber microbial electrolysis cells. 10 The current density has been reported as 9.3 A/m 2 and 75% coulombic efficiency, and it has been suggested that this system has the potential to increase hydrogen production [ 24 ].
It has been found that various microorganisms produce hydrogen through their own natural metabolism and hydrogen production has been produced by biofuel cells using exoelectrogenic microorganisms that can transfer electrons to an electrode surface. However, the fact that the prokaryotic cells used in these technologies can be pathogenic and affected by various environmental factors such as temperature requires the investigation of alternative approaches for more stable energy production against external variables. These approaches also include the investigation of different human cell lines. In a previously completed study, the human SH-SY5Y cell line was shown to produce electric current and transfer electrons to the electrode surface, and its power production was found to be comparable to that of biofuel fuel cells operated using bacteria [ 25 ]. In order to further develop the bio-based hydrogen production system, there is also a need for studies to investigate the hydrogen production mechanism and determining actors. Cancer cells are a better possibility to begin studying the hydrogen production process, as they engage metabolic reactions and are appropriate candidates due to their energy metabolism. In the future, a bio-based energy production device or a tiny electrical device implanted may be powered by bio-based energy sources. Bacteria have disadvantages for hydrogen production, especially when applied as pure culture or mixed culture in wastewater treatment systems. These disadvantages are the reservations about the use of bacteria in situations that directly concern human health. In particular, the issue of hydrogen production by cancer cells is important, as it will provide basic information for the development of a new bio-based energy production device in the future. The electron transport chain of eukaryotic cells contains the chemical conversion of nicotinamide adenine dinucleotide hydrogen (NADH) into nicotinamide adenine dinucleotide (NAD + ), which is utilized by microbes to generate electricity in microbial fuel cells in bio-based energy production. In this way, protons and electrons are obtained from the chemical reaction of NADH to NAD + . At the end of the electron transport chain, adenosine triphosphate (ATP) is used as a reservoir for biological energy released in the form of protons and electrons. Ninety percent of the overall production is represented by the ATP produced. Both the cytoplasm and the mitochondria are sites of the metabolic response in eukaryotic cells; however, the mitochondria produce a disproportionate number of protons and electrons. It is expected that mitochondria will participate in electricity production in microbial fuel cells.
According to the endosymbiotic theory, mitochondria in cells originated from the endosymbiosis of prokaryotic cells. Therefore, it is not surprising from an evolutionary perspective that there are similarities in the outer membrane porins of bacteria and mitochondria. It has been shown in bacteria that electron flow occurs from the cell to the cell surface by reducing insoluble substrates in the external environment [ 10 ]. Proteins in the membrane play an important role in this process. Direct electron transfer from microorganisms and mammalian cells to electrodes has been confirmed by using cyclic voltammetry methods [ 25 , 26 ]. Electrons from the bacteria are transferred to the anode surface, and protons are transferred to the cathode part and combine with electrons to form water. Various research results indicate a close relationship between electron leakage and proton leakage in mammalian cells. When electrons from the respiratory chain leave before oxygen is reduced to water at cytochrome c oxidase, they cause electron leakage. Instead, these electrons combine with oxygen to generate superoxide (O 2 •− ) [ 27 ]. Electron leakage in cytochrome c without reducing oxygen to water leads to the formation of superoxide—the main reactive oxygen species (ROS) in cells [ 26 , 28 ]. The level of ROS in cells affects the rate of proton leakage, and proton leakage reduces the level of ROS [ 26 , 29 ]. There are many unknowns about the exact mechanism of electron transfer from cells in biofuel cells. Electrons are produced in cells by various metabolic processes and electron leakage plays a critical role in electrical generation [ 30 ]. Electrons and protons produced as a result of metabolic reactions in both the cytosol and mitochondria of the eukaryotic cell can be used to produce electricity. The main reason for considering cancer cells for hydrogen production is that proton leak is significantly promoted in cancer cells and uncoupling proteins such as UCP2 are highly synthesized due to high expression [ 31 ]. Fig. 1 shows a proposed model for hydrogen production using human cell lines.

A proposed model for generating hydrogen using human cell lines (created with BioRender.com)
In the process of ATP synthesis in cells, electrons coming from the breakdown of the substrate flow through complexes in the electron transport chain, while protons are accumulated on one side of the membrane, creating potential energy [ 32 ]. These protons accumulated on one side of the membrane, creating a proton and electrochemical gradient, are used in ATP synthesis with the help of ATP synthase and, in aerobic respiration, these electrons are reduced to water by oxygen, which is the final electron acceptor. However, protons can return to the matrix independently of ATP synthase. The total proton leak of a mitochondrion can be considered the result of two processes: the uncontrolled basal leak and the inducible leak that is caused by certain inner membrane proteins in the mitochondria [ 27 ]. Also, electron leakage that may occur in cytochrome c in the electron transport chain without reducing oxygen to water reduces ATP production efficiency and creates superoxide—a large ROS in cells [ 33 ]. In such a case, the cell tries to suppress these ROS by proton leakage in order to compensate for this situation. In cells, this proton leak is carried out by uncoupling proteins (UCPs) and the increased ROS due to electron leak are suppressed by the increased proton leak, and thus the cell can be protected from the degenerative effects of free radicals. The uncoupling proteins are transporters found in the inner membrane of the mitochondria that facilitate the controlled release of the proton gradient produced by the respiratory chain for purposes such as thermogenesis, redox balance maintenance and reduced ROS production [ 34 ]. Numerous physiological processes are carried out by mitochondrial UCPs, including non-shivering thermogenesis (UCP1), glucose-stimulated insulin release, satiety signalling (UCP2) and muscle fuel consumption (UCP3). UCPs mediate these tasks by assisting protons in returning to the matrix [ 35 ]. Neuronal uncoupling proteins (UCP4 and UCP5) reduce the build-up of ROS in mitochondria and have anti-apoptotic and antioxidant properties [ 36 ]. Previous studies have shown the existence of at least two types of electric currents with different properties. These include UCP-2-mediated and non-UCP-2-mediated electrical current [ 26 ]. UCP-2-mediated electrical current has been shown to have a higher ROS reduction effect compared with non-UCP-2-mediated electrical current. The fact that UCP-2-mediated electrical current was observed to be higher in cancer cells has been suggested to contribute to the drug resistance of cancer cells. Understanding the mechanism of UCP-2-mediated current in cancer cells could lead to the development of drugs that target this specific pathway. By inhibiting UCP-2 function, these drugs could potentially decrease the electrical current and make cancer cells more susceptible to existing chemotherapy drugs [ 37 ]. Therapies that target UCP-2 alongside traditional chemotherapy could be explored. By combining these approaches, treatment efficacy might be improved while reducing resistance.
In light of all this information, it is expected that hydrogen gas production is carried out by cancer cells. Transferring electrons from cells that are not anaerobic in nature to an electrode surface and measuring this are difficult. However, removing oxygen from biofuel cells is necessary and makes it difficult for aerobic eukaryotic cells to remain alive for long periods of time. Cancer cells are the most suitable cells, especially for investigating mitochondrial electron-transfer mechanisms, due to their different energy metabolism. It is possible to investigate the role of mitochondria in the hydrogen production of living cancer cells and the hydrogen production efficiencies of different cancer lines. Practically, bioelectrolysis cells could combine the metabolic activity of cancer cells with a small electrical input to efficiently generate hydrogen gas from substrates. This method offers a renewable and potentially sustainable way to produce clean fuel [ 38 ]. For this purpose, various human cells can be used for hydrogen production research because some cell lines are rich in hydrogen pumps and suitable for scale-up studies. This research could contribute to the development of bioelectrolysis cells, future volume expansion studies and also the development of bio-based energy generators. It may be the first time that practical information can be obtained, as it provides insight into the determination of the hydrogen production potential of cancer cells. There are studies in the literature on the production of hydrogen from waste materials and renewable resources using microbial electrolysis cells and prokaryotic cells. In this context, hydrogen gas production potentials in bioelectrolysis cells can be investigated using human cell lines, including cancer cells. Gas-containing cancer therapeutics have attracted great attention in recent years due to their high therapeutic efficacy and biosafety. Research is ongoing on the development of stimuli-responsive gas-releasing molecules and gas nanogenerators for cancer bioimaging, targeted and controlled gas therapy, and gas-sensitive synergistic therapy. Particularly oxygen, carbon monoxide, nitric oxide and hydrogen gas are known to have anticancer effects [ 39 ]. The basic scientific knowledge that can be obtained through research on this subject can be added to the literature by providing the basic scientific knowledge required for bio-based energy production devices.
In mammalian cells, it has been shown by using SH-SY5Y human neuroblastoma cells that electrons and protons coming from the degradation of organic substrates are possible, as well as electron transfer to the electrode surface in biofuel cells [ 25 ]. However, no study has been found so far on hydrogen production using cancer cells in bioelectrolysis cells. Electricity can be produced in biofuel cells with human cells under optimum growth conditions in a carbon dioxide incubator at 37°C [ 25 , 26 ]. As adhesive cells were used in previous studies, carbon cloth was used as an electrode and it was shown that the cells could form a biofilm structure. Therefore, it may be possible to use similar conditions or perhaps modified conditions in hydrogen production studies with human cells. Osteoclasts, as bone-resorbing cells, have high levels of proton pumps to create an acidic environment around the bone surface [ 40 , 41 ]. In order to maintain proper acid–base balance in the body and regulate blood pressure, kidney proximal tubule cells use proton pumps [ 42 ]. Cells exposed to acidic environments might have more proton pumps to regulate internal pH [ 43 ]. Previous studies have shown that UCP2-mediated proton leak increases proton transfer and electricity production in cancer and healthy cells. In this regard, it can be said that hydrogen production using human cells may be possible and a new research field in the area of biofuel cells may emerge in terms of both basic science and technology. In this way, bio-based energy production devices based on hydrogen production can be developed. In addition, the relationship between hydrogen production potentials and electroactive biofilm properties and hydrogen production can be understood using bioelectrolysis cell technology, which will be the successor of microbial electrolysis cell technology. Microbial electrolysis cells are promising for sustainable hydrogen production but their performance is still in the early stages of development. Efficiency can be significantly affected by the use of organic matter as a fuel [ 44 ] and the efficiency of hydrogen production can be affected by several factors such as pH, temperature and applied voltage [ 45 ]. There are also limitations such as their sensitivity to methanogenesis and the need for expensive materials such as membranes and electrodes [ 21 , 46 ]. Considering that the energy values produced by microorganisms have increased at a remarkable level [ 47 ], it can be said that hydrogen production using human cells may be possible in the coming years and can be used for various purposes, including biomedical technologies.
Not applicable.
Writing the original manuscript (TC)
The author declared that there are no conflicts of interest.
Ortiz-Martínez VM , Salar-García MJ , de los Ríos AP et al. . Developments in microbial fuel cell modeling . Chem Eng J , 2015 , 271 : 50 – 60 . https://doi.org/10.1016/j.cej.2015.02.076
Google Scholar
Obileke K , Onyeaka H , Meyer EL et al. . Microbial fuel cells, a renewable energy technology for bio-electricity generation: a mini-review . Electrochem Commun , 2021 , 125 : 107003 . https://doi.org/10.1016/j.elecom.2021.107003
Nguyen AT , Ng KH , Kumar PS et al. . Sustainable hydrogen production and CO 2 mitigation from acetic acid dry reforming over Ni/Al2O3 catalyst . Int J Hydrog Energy , 2024 . https://doi.org/10.1016/j.ijhydene.2024.02.179
Ellabban O , Abu-Rub H , Blaabjerg F. Renewable energy resources: current status . Renew Sust Energ Rev , 2014 , 39 : 748 – 764 . https://doi.org/10.1016/j.rser.2014.07.113
Abdelkareem MA , Elsaid K , Wilberforce T et al. . Environmental aspects of fuel cells: a review . Sci Total Environ , 2021 , 752 : 141803 . https://doi.org/10.1016/j.scitotenv.2020.141803
Qureshi F , Yusuf M , Tahir M et al. . Renewable hydrogen production via biological and thermochemical routes: nanomaterials, economic analysis and challenges . Process Safety and Environmental Protection , 2023 , 179 : 68 – 88 . https://doi.org/10.1016/j.psep.2023.07.075
Nabgan W , Alqaraghuli H , Owgi AHK et al. . A review on the design of nanostructure-based materials for photoelectrochemical hydrogen generation from wastewater: bibliometric analysis, mechanisms, prospective, and challenges . Int J Hydrog Energy , 2024 , 52 : 622 – 663 . https://doi.org/10.1016/j.ijhydene.2023.05.152
Thomas JM , Edwards PP , Dobson PJ et al. . Decarbonising energy: the developing international activity in hydrogen technologies and fuel cells . J Energy Chem , 2020 , 51 : 405 – 415 . https://doi.org/10.1016/j.jechem.2020.03.087
Munoz-Cupa C , Hu Y , Xu C et al. . An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production . Sci Total Environ , 2021 , 754 : 142429 . https://doi.org/10.1016/j.scitotenv.2020.142429
Rabaey K , Boon N , Höfte M et al. . Microbial phenazine production enhances electron transfer in biofuel cells . Environ Sci Technol , 2005 , 39 : 3401 – 3408 . https://doi.org/10.1021/es048563o
Catal T , Li K , Bermek H et al. . Electricity production from twelve monosaccharides using microbial fuel cells . J Power Sour , 2008 , 175 : 196 – 200 . https://doi.org/10.1016/j.jpowsour.2007.09.083
Potter MC. Electrical effects accompanying the decomposition of organic compounds . Proc R Soc London Ser B Biol Sc , 1911 , 84 : 260 . https://doi.org/10.1098/rspb.1911.0073
Qiu B , Hu Y , Tang C et al. . Simultaneous mineralization of 2-anilinophenylacetate and denitrification by Ru/Fe modified biocathode double-chamber microbial fuel cell . Sci Total Environ , 2021 , 792 : 148446 . https://doi.org/10.1016/j.scitotenv.2021.148446
Zhang M , Ma Z , Zhao N et al. . Increased power generation from cylindrical microbial fuel cell inoculated with P. aeruginosa . Biosens Bioelectron , 2019 , 141 : 111394 . https://doi.org/10.1016/j.bios.2019.111394
Yoon Y , Kim B , Cho M. Mineral transformation of poorly crystalline ferrihydrite to hematite and goethite facilitated by an acclimated microbial consortium in electrodes of soil microbial fuel cells . Sci Total Environ , 2023 , 902 : 166414 . https://doi.org/10.1016/j.scitotenv.2023.166414
Varshney A , Sharma L , Pandit C et al. . Microbial fuel cell-based biosensors and applications . Appl Biochem Biotechnol , 2023 , 195 : 3508 – 3531 . https://doi.org/10.1007/s12010-023-04397-x
Yashiro Y , Yamamoto M , Muneta Y et al. . Comparative studies on electrodes for rumen bacteria microbial fuel cells . Sensors (Basel, Switzerland) , 2023 , 23 : 4162 . https://doi.org/10.3390/s23084162
Catal T , Fan Y , Li K et al. . Utilization of mixed monosaccharides for power generation in microbial fuel cells . J Chem Technol Biotechnol , 2011 , 86 : 570 – 574 . https://doi.org/10.1002/jctb.2554
Yan X , Zhu MJ. Enhanced bioelectricity generation in thermophilic microbial fuel cell with lignocellulose as an electron donor by resazurin-mediated electron transfer . Bioresour Technol , 2023 , 388 : 129764 . https://doi.org/10.1016/j.biortech.2023.129764
Paitier A , Godain A , Lyon D et al. . Microbial fuel cell anodic microbial population dynamics during MFC start-up . Biosens Bioelectron , 2017 , 92 : 357 – 363 . https://doi.org/10.1016/j.bios.2016.10.096
Catal T , Lesnik KL , Liu H. Suppression of methanogenesis for hydrogen production in single-chamber microbial electrolysis cells using various antibiotics . Bioresour Technol , 2015 , 187 : 77 – 83 . https://doi.org/10.1016/j.biortech.2015.03.099
Qureshi F , Yusuf M , Ibrahim H et al. . Contemporary avenues of the hydrogen industry: opportunities and challenges in the eco-friendly approach . Environ Res , 2023b , 229 : 115963 . https://doi.org/10.1016/j.envres.2023.115963
Catal T , Pasaoglu E , Akagunduz D et al. . Enhanced hydrogen production by mevastatin in microbial electrolysis cells . Int J Energy Res , 2021 , 45 : 13990 – 13998 . https://doi.org/10.1002/er.6669
Hu H , Fan Y , Liu H. Hydrogen production in single-chamber tubular microbial electrolysis cells using non-precious-metal catalysts . Int J Hydrog Energy , 2009 , 34 : 8535 – 8542 . https://doi.org/10.1016/j.ijhydene.2009.08.011
Atasever-Arslan B , Akdogan E , Cebeci FC et al. . Bioelectricity generation using human neuronal-like cells in single chamber biofuel cells . J Clean Prod , 2020 , 271 : 122505 . https://doi.org/10.1016/j.jclepro.2020.122505
Wang R , MoYung CK , Zhang MH et al. . UCP2- and non-UCP2-mediated electric current in eukaryotic cells exhibits different properties . Environ Sci Pollut Res Int , 2015 , 22 : 19618 – 19631 . https://doi.org/10.1007/s11356-015-5155-6
Jastroch M , Divakaruni AS , Mookerjee S et al. . Mitochondrial proton and electron leaks . Essays Biochem , 2010 , 47 : 53 – 67 . https://doi.org/10.1042/bse0470053
Brand MD , Affourtit C , Esteves TC et al. . Mitochondrial superoxide production, biological effects, and activation of uncoupling proteins . Free Radic Biol Med , 2004 , 37 : 755 – 767 . https://doi.org/10.1016/j.freeradbiomed.2004.05.034
Bevilacqua L , Ramsey JJ , Hagopian K et al. . Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria . Am J Physiol Endocrinol Metab , 2005 , 289 : E429 – E438 . https://doi.org/10.1152/ajpendo.00435.2004
Brutinel ED , Gralnick JA. Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella . Appl Microbiol Biotechnol , 2012 , 93 : 41 – 48 . https://doi.org/10.1007/s00253-011-3653-0
Ayyasamy V , Owens KM , Desouki MM et al. . Cellular model of warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by Genipin . PLoS One , 2011 , 6 : e24792 . https://doi.org/10.1371/journal.pone.0024792
Mitchell P , Moyle J. Chemiosmotic hypothesis of oxidative phosphorylation . Nature , 1967 , 213 : 137 – 139 . https://doi.org/10.1038/213137a0
Brand MD. Mitochondrial proton and electron leaks essays in biochemistry . Essays Biochem , 2010 , 47 : 53 – 67 . https://doi.org/10.1042/bse0470053
Ledesma A , de Lacoba MG , Rial E. The mitochondrial uncoupling proteins . Genome Biol , 2002 , 3 : REVIEWS3015.1 . https://doi.org/10.1186/gb-2002-3-12-reviews3015
Hirschenson J , Melgar-Bermudez E , Mailloux RJ. The uncoupling proteins: a systematic review on the mechanism used in the prevention of oxidative stress . Antioxidants (Basel) , 2022 , 11 : 322 . https://doi.org/10.3390/antiox11020322
Jamwal S , Blackburn JK , Elsworth JD. Age-associated sex difference in the expression of mitochondria-based redox sensitive proteins and effect of pioglitazone in nonhuman primate brain . Biol Sex Differ , 2023 , 14 : 65 . https://doi.org/10.1186/s13293-023-00551-6
Luby A , Alves-Guerra MC. UCP2 as a cancer target through energy metabolism and oxidative stress control . Int J Mol Sci , 2022 , 23 : 15077 . https://doi.org/10.3390/ijms232315077
Kilinc B , Akagunduz D , Ozdemir M et al. . Hydrogen production using cocaine metabolite in microbial electrolysis cells . 3 Biotech , 2023 , 13 : 382 . https://doi.org/10.1007/s13205-023-03805-7
Chen L , Zhou SF , Su L et al. . Gas-mediated cancer bioimaging and therapy . ACS Nano , 2019 , 13 : 10887 – 10917 . https://doi.org/10.1021/acsnano.9b04954
Ribet ABP , Ng PY , Pavlos NJ. Membrane transport proteins in osteoclasts: the ins and outs . Front Cell Dev Biol , 2021 , 9 : 644986 . https://doi.org/10.3389/fcell.2021.644986
El Sayed SA , Nezwek TA , Varacallo M. Physiology, Bone. [Updated 2022 Oct 19] . In: StatPearls [Internet] . Treasure Island, FL : StatPearls Publishing , 2024 . https://www.ncbi.nlm.nih.gov/books/NBK441968/
Google Preview
Boron WF , Boulapep EL. Medical Physiology . 2nd edn. Philadelphia : Saunders , 2012 , 1052 .
Tafech A , Jacquet P , Beaujean C et al. . Characterization of the intracellular acidity regulation of brain tumor cells and consequences for therapeutic optimization of temozolomide . Biology (Basel) , 2023 , 12 : 1221 . https://doi.org/10.3390/biology12091221
Catal T. Comparison of various carbohydrates for hydrogen production in microbial electrolysis cells . Biotechnol Biotechnol Equip , 2015 , 30 : 75 – 80 . https://doi.org/10.1080/13102818.2015.1081078
Cheng D , Ngo HH , Guo W et al. . Impact factors and novel strategies for improving biohydrogen production in microbial electrolysis cells . Bioresour Technol , 2022 , 346 : 126588 . https://doi.org/10.1016/j.biortech.2021.126588
Rousseau R , Etcheverry L , Roubaud E et al. . Microbial electrolysis cell (MEC): strengths, weaknesses and research needs from electrochemical engineering standpoint . Appl Energy , 2020 , 257 : 113938 . https://doi.org/10.1016/j.apenergy.2019.113938
Fan Y , Janicek A , Liu H. Stable and high voltage and power output of CEA-MFCs internally connected in series (iCiS-MFC) . Eur Chem Biotech J , 2024 , 1 : 47 – 57 . https://doi.org/10.62063/ecb-17
| Month: | Total Views: |
|---|---|
| July 2024 | 169 |
Email alerts
Citing articles via.
- Advertising and Corporate Services
Affiliations
- Online ISSN 2515-396X
- Print ISSN 2515-4230
- Copyright © 2024 National Institute of Clean-and-Low-Carbon Energy
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- DOI: 10.1016/j.renene.2024.120911
- Corpus ID: 271035006
A comprehensive review of green hydrogen energy systems
- Fanourios Kourougianni , A. Arsalis , +4 authors G. E. Georghiou
- Published in Renewable Energy 1 July 2024
- Environmental Science, Engineering
362 References
Strategies for life cycle impact reduction of green hydrogen production – influence of electrolyser value chain design, low platinum fuel cell as enabler for the hydrogen fuel cell vehicle, design and performance evaluation of power system for unmanned ship based on proton exchange membrane fuel cell, a feasibility study of green hydrogen liquefaction for hydrogen refueling station: multi-criteria based integrative assessment, multi-criteria decision-making for techno-economic and environmentally sustainable decentralized hybrid power and green hydrogen cogeneration system, techno-economic analysis for design and management of international green hydrogen supply chain under uncertainty: an integrated temporal planning approach, a review on the research progress and application of compressed hydrogen in the marine hydrogen fuel cell power system, off-grid vs. grid-based: techno-economic assessment of a power-to-liquid plant combining solid-oxide electrolysis and fischer-tropsch synthesis, thermo-economic performance maps of green hydrogen production via water electrolysis powered by ranges of solar and wind energies, experimental evaluation of a solid oxide fuel cell system exposed to inclinations and accelerations by ship motions, related papers.
Showing 1 through 3 of 0 Related Papers
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
Berkleley Lab
Office of Deputy Lab Director for Research
Geologic Hydrogen: A New Source of Carbon-Free Fuel for the World, New Opportunities for the Lab
Published on June 26, 2024 by Ruby Barcklay.

Berkeley Lab’s strategic research themes describe research programs and projects at the Lab that could make a significant contribution to the world, while also potentially providing growth opportunities for research at the Lab and for science. The two Lab research projects recently funded by ARPA-E to develop geologic hydrogen as a new source of carbon-free fuel are such projects, relating to the strategic research themes “discovering materials, chemical processes, and biological systems for energy and the environment” and “dramatically accelerating clean energy technologies.”
The Earth and Environmental Sciences Area (EESA)’s senior scientist Ben Gilbert and research scientist Mengsu Hu, both with the Energy Geosciences Division (EGD), have just embarked on two-year projects that aim to address significant challenges involved in stimulating and extracting hydrogen from rocks in Earth’s subsurface before this promising source of low-carbon energy can achieve widespread use. Ben is trying to understand the chemical mechanisms responsible for producing geologic hydrogen and then investigating ways to accelerate this process, while Mengsu’s research explores seismically safe ways to create fractures in rock, stimulate geologic hydrogen production, and ultimately transport the hydrogen back to Earth’s surface.
Ben explained: “When certain rocks encounter water, they can undergo mineralogical and chemical reduction or oxidation changes spontaneously. In a process called ‘serpentinization,’ iron-rich rock from the oceanic crust is moved up through tectonic processes, and when it meets with water, iron is released while water is reduced to hydrogen. As discovered in Mali and other places , this geologic hydrogen could be naturally formed and trapped in reservoirs. This could well be a new significant source of fuel and has led to hydrogen prospecting by energy companies.”
ARPA-E’s newly funded projects take exploring the potential of geologic hydrogen beyond locating and extracting trapped geologic hydrogen, towards the possibility that the hydrogen can actually be produced intentionally, by drilling and flowing water into rock and then transporting the hydrogen to the surface for collection.
Achieving economic hydrogen production will require new knowledge about the hydrogen generation process. “We don’t know enough about these chemical reactions that happen during serpentinization–[a process which causes the rock to release iron],” said Ben. “How does iron lose electrons and reduce water or protons to hydrogen? Does it happen at mineral surfaces? Is the process catalyzed by other metals? These are just some of the material science, chemistry, and Earth science questions that we need to answer. And, these natural processes take many years. Once we have a better understanding of these mechanisms, we can look for tools to accelerate the process,” he continued.
Ben’s project team uses quantum chemistry simulations and experiments to show that these reactions can be understood, predicted, and controlled in the laboratory and, ultimately, the field. Using rock samples from field sites in Montana and elsewhere, Mengsu’s team will deploy multiscale numerical modeling, laboratory tests, and field characterization to develop and test their technology, which aims to extract hydrogen safely and economically at a commercial scale.
“Our goal is to develop novel cyclic injection technology for the commercial extraction of geologic hydrogen without inducing harmful seismicity,” she said. “We will develop predictive understanding and technology for adaptive control of the chemo-mechanical processes associated with serpentinization. These capabilities will allow us to increase the permeability of the rocks, thus allowing access of water to drive the hydrogen generation reactions and then to extract the hydrogen without inducing seismicity.”
Lab Capabilities Apply to Geologic Hydrogen Research
Mengsu and Ben noted that the ARPA-E funded projects are already utilizing a range of Berkeley Lab capabilities, but the opportunities for the geologic hydrogen research teams to collaborate with others at the Lab will likely expand.
Said Ben, “Many ideas and capabilities in the project were enabled in the Basic Energy Sciences geoscience project at Berkeley Lab. And to study iron redox chemistry, we plan to use the Advanced Light Source (ALS). But there are also opportunities to leverage the Lab’s high-performance computing capabilities, and to work with electron microscopy experts and facilities at the Molecular Foundry.”
Said Mengsu, “This field is rich with opportunities for earth scientists and geoscientists, but there are also many research questions that require other expertise. For example, research into the transportation and storage of hydrogen will be important, and future geologic hydrogen work would likely be integrated with California’s hydrogen hub (called the Alliance for Renewable Clean Hydrogen Energy Systems or ARCHES) and other hydrogen work at the Lab.
Newsha Ajami, EESA’s Chief Strategic Development Officer, said that if and when enhanced geologic hydrogen comes to pass, there could also be research into community impact, the potential impact of geologic hydrogen on natural resources such as water, and potential unintended consequences, as well as work partnering with communities on land use issues,” she said. An example of research at Berkeley Lab that includes community impact work is the study of the possibility of a direct air capture hub while prioritizing the needs of surrounding communities .
“As we explore and transition to alternative energy sources, we will need to consider not just how to produce these fuels but also how to address demand and supply considerations, and the potential impact on communities. This means that scientists and researchers who don’t conventionally collaborate will be coming together to work with communities directly and answer some of their place-based questions. Enabling this collaboration is important,” Newsha continued.
Mengsu notes that in the course of their research, her team is making use of existing laboratory and computational capabilities that were built at the Lab and also building new capabilities that may also be useful elsewhere. For example, Mengsu’s team is building new modeling capabilities that conduct chemo-mechanical analysis to understand the mechanisms of geological hydrogen stimulation by creating fractures and introducing hot water to drive the reactions, all without inducing harmful seismicity. In addition, software for conducting smaller-scale modeling studies ( CrunchFlow ) will be combined with the TOUGH family of codes to analyze induced seismicity and the transport of hydrogen at reservoir scale. These software applications could potentially be applied in a wide range of settings for geologic hydrogen production and storage.
The Potential of Geologic Hydrogen Research
“Geologic hydrogen research is a new field that holds a lot of potential. The ability to extract hydrogen from rocks, if it works, could be a game-changer,” said Mengsu.
Mengsu is hopeful that if the current research projects are successful, more funding could be available, from government agencies as well as from industry. “Even before the research started, I was already contacted by mining companies and venture capital firms. They are staying close to the research on this project. And this space is rich with research questions; I can already see different follow-on research questions to explore after these projects are completed,” she said.
Mengsu notes that Berkeley Lab, with two projects under its belt, would be in a good position to work on follow-on projects.
Ben agrees. “Ultimately, this relatively new field of research into mineral processes to understand redox, and to apply it to energy challenges, could be a significant opportunity for science. And with our X-ray, electron microscopy, and high-performance computing facilities as well as our growing expertise in geologic hydrogen, Berkeley Lab has a lot to offer.”
As Ben pointed out, new sources of energy don’t come around very often. “The last new potentially significant energy resource introduced to the world was nuclear fusion . Hydrogen from the earth is potentially a significant new energy resource and one that is carbon-free. Even more broadly, beyond geologic hydrogen, there is the potential to manipulate minerals in ways that are important for energy and the environment. I’m excited to do this kind of work at Berkeley Lab, which offers so many contacts and people and resources to help move this field forward,” he said.
For more information:
“Energy Department Awards $20 Million for Research into Carbon-Free Geologic Hydrogen,” Forbes magazine, Feb. 8, 2024
“Two EGD Project Teams Selected to Receive Funding to Explore Potential of Geologic Hydrogen Energy,” EESA website, Feb. 12, 2024
Berkeley Lab hydrogen research , Berkeley Lab website
- Announcements
- Research News
- Uncategorized
Was this page useful?

Analysis on the feasibility of hydrogen fuel engine based on engine operation process
- Ruan, Siyuan
In recent years, the heightened focus on combating climate change and environmental pollution has underscored the importance of alternative energy sources, with hydrogen fuel emerging as a prime candidate due to its clean, efficient, and renewable attributes. This study investigates the potential amalgamation of hydrogen fuel technologies with innovative three-stroke engines, aligning with the objectives of dual carbon initiatives. Initially, the distinct characteristics of hydrogen fuel, including its pollution-free nature and high efficiency are examined, highlighting promising prospects in the transportation sector. Subsequently, the benefits and challenges of three-stroke engines are analyzed, exploring their possible integration with hydrogen energy systems, especially in the realms of aviation and rotor engines. Furthermore, this research delves into the critical role of hydrogen energy within the framework of the dual carbon program, emphasizing its transformative potential in high carbon emission industries such as the steel and chemical sectors. In conclusion, the study accentuates the central role of hydrogen energy in spearheading a future low-carbon economy and sustainable development, advocating for its substantial inclusion in upcoming environmental conservation endeavors.
- ELECTRONICS AND POWER SYSTEMS
No Sources Found

Hydrogen For Energy Types Are Getting More And More Angry
Over the past year or so, as the hydrogen for energy hype bubble has started to leak badly, a clear indicator of the end times for hydrogen energy proponents has started to emerge, anger and hostility. Why is this a clear indicator?
Let’s cast our minds back eight years. Sometime before then I’d created the above continuum of climate change denial positions, from utter and mind-boggling refusal to accept reality all the way to the tiny category of people who thought it would be worse than it is going to be. As I noted at the time, Climate Change Deniers Are Getting Angrier & Here’s Why .
As I observed, it was all about cognitive dissonance . What’s that?
In psychology, cognitive dissonance is the mental stress or discomfort experienced by an individual who holds two or more contradictory beliefs, ideas, or values at the same time, performs an action that is contradictory to one or more beliefs, ideas, or values, or is confronted by new information that conflicts with existing beliefs, ideas, or values.
Yes, when people are bombarded by data, facts and logic which contradict their biases, they feel uncomfortable. They are stressed. If they don’t resolve this by embracing it, considering the facts and logic and improving their positions to accommodate reality, then it builds up. That was happening more and more to climate change deniers a decade ago and they were lashing out enough for it to be obvious even to me and I’m kind of oblivious to most of that kind of thing.
This is a resonant time to be bringing up the anger of denialists. Famed climate scientist Michael Mann, who I had the privilege to spend a bit of time with recording a CleanTechnica podcast a few years ago and who quoted one of my whatever-you-call-tweets-now in a recent book, is suffering through PTSD relapse in a court room as his deeply idiotic slanderers cross-examine him or shift uneasily in their slime-filled seats. Mann and his family suffered a lot of stress and anguish from the unthinking anger of climate change denialists — and people who should know better — and he decided to sue the worst of them for defamation.
Yes, you heard me right, one of the defendants, Mark Steyn, is defending himself, which means he has a fool for a lawyer and a jackass for a client, as if there were any doubt about him being either. He had no choice, one assumes, because his lawyers abandoned him in 2021.
As a reminder, Steyn and his fellow idiot defendant Rand Simberg — a man who denies saying things he’s already said under oath and who continues to malign under oath experts who he didn’t even bother to look up — asserted stridently, regularly and often that the hockey stick of of temperatures that Mann first published was a hoax and false while it has been established to be completely accurate and valid by something like two dozen separate studies . This is about as well established as science gets. It’s not even a model, it’s just reporting on reality.
Mann was one of the early victims of the anger of denialists. He felt it long before I noticed it and now a dozen years later his vicious detractors are showing once again their colors in court. It’s not a pleasing palette.
What does this have to do with hydrogen for energy types?

Well, over the past 25 years, people who thought hydrogen was the bee’s knees, like climate change deniers, have been forced off position after position. To be clear, these are people who accepted the reality of climate change and were usually sincerely trying to do something about it. This is not synonymous with climate change denial except where it is. I’ll get to that.
Let’s step through this abbreviated infographic briefly. Around 2000, it was possible with only slightly rose-colored glasses to think that hydrogen was going to be the energy source for everything everywhere all the time. Jeremy Rifkin, author, economist, STEM illiterate and advisor to power brokers on both sides of the Atlantic certainly did and helped make it a policy on two continents. To be clear, 25 years ago there wasn’t really an alternative and even the few people who realized how absurdly costly this was going to be grimaced and swallowed it. Unchecked climate change would be worse than the cost of a hydrogen economy.
But every time it’s been tried out or serious spreadsheet jockeys have plied their data scalpels since, it’s been failing compared to alternatives. Batteries, grid ties and biofuels have been kicking hydrogen to the curb in every energy domain where rational total cost of ownership assessment studies have been performed. Every time the systems boundary includes alternatives the analysis shows that hydrogen is a mug’s game.
And it’s getting worse and worse. Long held assumptions about different aspects of cost cases are falling apart due to little things like data, evidence and math. The Boston Consulting Group published a gloomy assessment saying that the consensus of €3 per kilogram green hydrogen in 2030 — a consensus shared among STEM illiterate fantasists and hydrogen for energy advocates, if that’s not a redundancy — wasn’t going to be achieved and that more realistic costs would be €5 to €8.80. They didn’t say that the upper end of that range was more likely, but it is.
So much for fantasies of US$1.40 hydrogen that think tanks like the International Council on Clean Transportation included in their publications. That’s so far off of reality that it’s difficult to imagine what their analyst was thinking.
The International Energy Agency published an e-fuels for transportation assessment late in 2023 which had some eye widening findings, although not for me or anyone who has spent any time doing realistic cost workups in the space. They found that in the absolutely best case scenarios synthetic fuels made from green hydrogen would cost four to six times as much as current fuels. That’s far above the assumption of hydrogen for energy fantasists who thought that they would be cost competitive with fossil fuels, never mind biofuels.
And this month the Transportation & Environment research and policy advocacy group in Europe reported that 25 of 25 synthetic kerosene for aviation proposals failed to reach final investment decision because they couldn’t find any airlines willing to pay ten times the cost of current fuels.
BCG again reported, albeit without realizing what they were reporting, that only 0.2% of hydrogen proposals by volume had reached operation, an infinitesimal level. BNEF reported that only 13% of hydrogen deals they were tracking that had reached final investment decision had off takers and that only 10% of those were firm. The International Energy Agency reported that only 7% of the theoretically required hydrogen supplies for 2030 were likely to be present.
Into this swirling mix of painful reality I’ve been casting my own meager offerings. In recent months I’ve been assessing the International Council on Clean Transportation’s reports and finding that they diverged quite substantially from reality and quality control after 2020 as they tried to find a way to justify hydrogen energy pathways being cheaper than they could possibly be. I have been publishing on green hydrogen and synthetic fuel pathways for maritime shipping and aviation compared to obvious alternatives and saying the bleedingly obvious, that hydrogen and synthetic fuels are so economically uncompetitive that they won’t be used.
I’ve been assessing the poorly boundaried assessments of HVDC vs hydrogen pipelines that fail many tests of basic systems engineering. I’ve been digging through the tragicomic history of hydrogen transportation fleets , where the pattern is so obvious it’s become a pantomime. I spent time with the decarbonization lead for A.P. Moller Maersk division APM Terminal’s decarbonization lead talking about why hydrogen makes no economic sense for transporation .
I’ve been observing and occasionally commenting on the UK attempts to shove an explosive round peg into the square hole of home heating with its hydrogen village trials, when now 54 independent studies make it clear that hydrogen has exactly no role to play in residential or commercial buildings.
More recently, I was asked to assist in quality reviewing a European total cost of ownership study of multiple pathways to road freight decarbonization. As a result, I started poking at assumptions around hydrogen vehicle and refueling station maintenance costs. I found that instead of being only as expensive as internal combustion vehicles or even cheaper, hydrogen buses in California were 50% more expensive than diesel buses to maintain even after years in service and twice as expensive as battery electric buses. And I found that hydrogen refueling stations were costing what an at least somewhat validated methodology found to be 30% of capital expenditures per year to maintain instead of 3% or 4% as assumed by total cost of ownership studies.
And so, back to the thread. Across this broad engagement where sanity and rational thought is breaking through about 25 years of of hydrogen for energy inertia, I’ve been seeing a lot of anger directed in a variety of directions. A lot of knees have been hitting noses while attached to jerks. Whatever twitter is called these days is filled with even more frothy diatribes. LinkedIn has been seeing its share as well.
My acquaintance Tom Baxter, chemical engineer Senior Lecturer at University of Aberdeen and generally delightful bearded Scotsman was accused of being a bitter troll by a UK gas utility CEO. The same CEO blocked me after a single comment suggesting that the firm needed to be considering strategic distribution network downsizin g.
A major manufacturer’s hydrogen lead snapped at me in a professional thread for, you know, pointing out relevant but inconvenient truths. A major cleantech think tank’s hydrogen lead kept poking me on social media until I dropped a 13,000 word critique of his team’s positions in his lap. Comments on my articles and on LinkedIn have filled up with aggrieved souls fighting for their dream of hydrogen in their cars, their stoves, their furnaces and in their water. Etc, etc, etc.
All of this has been playing out on other people’s threads. I’ve observed hydrogen “Ambassadors” whining about basic data and logic. I’ve seen chemical engineers with decades of hydrogen experience described as ignorant haters.
It’s taken a lot of blocking and muting to bring the aggravated dyspepsia quotient in my various feeds down to merely a dull roar. The cognitive dissonance of the hydrogen for energy crowd is growing daily and for running the numbers and pointing out other people who are running the numbers I’m getting a bit of grief, while others are getting more grief.
Nothing like what Michael Mann experienced thankfully, but it’s in the same context, people not accepting reality and lashing out in anger. You would think that hydrogen for energy advocates would realize that these were terrible optics, not to mention dumb as a box of oiled velveteen hammers, but no, they are too busy whacking their septum with their patella to notice that other people are looking at them as if they had three eyes.
Earlier in this piece I said that all of this hydrogen for energy advocacy was not synonymous with climate change denial except where it is . It’s time to lean into that just slightly. Hydrogen for energy plans are slowing the required transition to actually economically sensible, practical and rapid decarbonization. They are distracting legislators, policy makers, entrepreneurs and investors from getting on with what will inevitably win in the end. Governmental money continues to be hurled into the hydrogen power paper shredder on multiple continents, money that could be funding wind farms, solar farms, transmission, storage and electric vehicles.
Hydrogen for energy advocates and fans need to step back and realize that their anger is their subconscious telling them that they are wrong whether they want to accept it or not. They need to take a break. They need to update their facts to match reality. They need to emerge from their bubbles of increasingly frantic hopium and engage more fully with people outside of them. They need to put their energy and talents to the actual solution space. That’s what their anger is really telling them and they need to listen to it.
Latest CleanTechnica.TV Videos

CleanTechnica's Comment Policy
Share this story!
Michael barnard.
is a climate futurist, strategist and author. He spends his time projecting scenarios for decarbonization 40-80 years into the future. He assists multi-billion dollar investment funds and firms, executives, Boards and startups to pick wisely today. He is founder and Chief Strategist of TFIE Strategy Inc and a member of the Advisory Board of electric aviation startup FLIMAX. He hosts the Redefining Energy - Tech podcast (https://shorturl.at/tuEF5) , a part of the award-winning Redefining Energy team.
Michael Barnard has 758 posts and counting. See all posts by Michael Barnard
Germany could import up to 100 TWh of green hydrogen via pipelines by 2035, study shows
- Medium Text

Sign up here.
Reporting by Riham Alkousaa Editing by Tomasz Janowski
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Thomson Reuters
Riham Alkousaa is the energy and climate change correspondent for Reuters in Germany, covering Europe’s biggest economy's green transition and Europe’s energy crisis. Alkousaa is a Columbia University Journalism School graduate and has 10 years of experience as a journalist covering Europe’s refugee crisis and the Syrian civil war for publications such Der Spiegel Magazine, USA Today and the Washington Times. Alkousaa was on two teams that won Reuters Journalist of the year awards in 2022 for her coverage of Europe’s energy crisis and the Ukraine war. She has also won the Foreign Press Association Award in 2017 in New York and the White House Correspondent Association Scholarship that year.

Business Chevron

Disney to add new ship in Tokyo to expanding cruise business
Walt Disney unveiled plans on Tuesday to launch a new cruise ship that will set sail from Tokyo starting in fiscal 2028, adding a ninth vessel to the brand's growing fleet.

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Journal Menu
- Hydrogen Home
- Aims & Scope
- Editorial Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Article Processing Charge
- Indexing & Archiving
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Editorial Office
Journal Browser
- arrow_forward_ios Forthcoming issue arrow_forward_ios Current issue
- Vol. 5 (2024)
- Vol. 4 (2023)
- Vol. 3 (2022)
- Vol. 2 (2021)
- Vol. 1 (2020)
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
Hydrogen Energy Technologies
- Print Special Issue Flyer
Special Issue Editors
Special issue information.
- Published Papers
A special issue of Hydrogen (ISSN 2673-4141).
Deadline for manuscript submissions: closed (30 June 2023) | Viewed by 7740
Share This Special Issue

Dear Colleagues,
Hydrogen has an important potential to replace fossil fuel-based energy infrastructure due to its cleanliness, unlimited supply, and higher energy content per unit mass. It can provide storage options for renewable resources, and when combined with emerging decarbonization technologies, can accelerate the process of scaling up clean and renewable energy. Several technologies have evolved through the years, for hydrogen production/storage and utilization, while at the same time, hydrogen energy still face a number of technical barriers that must be overcome. This Special Issue aims to collect original research articles and comprehensive reviews focusing on hydrogen production, storage, transport, appliacations, and utilization technologies.
Prof. Dr. Wei Wang Prof. Dr. Yunfei Bu Dr. Huayang Zhang Guest Editors
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website . Once you are registered, click here to go to the submission form . Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Hydrogen is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1000 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
- hydrogen technologies
- solar hydrogen
- hydrogen production
- techniques for hydrogen storage
- hydrogen powered energy systems
Published Papers (3 papers)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
This paper is devoted to treating hydrogen powered energy systems as a whole and analysing the role of hydrogen in the energy systems. As hydrogen has become an important intermediary for the energy transition and it can be produced from renewable energy sources, re-electrified to provide electricity and heat, as well as stored for future use, key technologies including water electrolysis ...
Hydrogen is an energy carrier that can transform our fossil-fuel dependent economy into a hydrogen economy, which can provide an emissions-free transportation fuel. Literature reviews and independent research were the main methods of research. Hydrogen storage and transport are issues of intense research due to hydrogen's characteristic low ...
Green hydrogenis defined as hydrogen produced by splitting water into hydrogen and oxygen using renewable electricity. This is a very different pathway compared to both grey and blue. Grey hydrogen is traditionally produced from methane (CH4), split with steam into CO2 - the main culprit for climate change - and H2, hydrogen.
Introduction. Nowadays, the technology of renewable-energy-powered green hydrogen production is one method that is increasingly being regarded as an approach to lower emissions of greenhouse gases (GHGs) and environmental pollution in the transition towards worldwide decarbonization [1, 2].However, there is a societal realization that fossil fuels are not zero-carbon, which leads to ...
Hydrogen is a clean energy carrier that can play an important role in the global energy transition. Its sourcing is critical. Green hydrogen from renewable sources is a near-zero carbon production route. Important synergies exist between accelerated deployment of renewable energy and hydrogen production and use.
Hydrogen is the lightest chemical element and the most abundant chemical substance in the universe. Using fossil fuels or clean electricity, we can produce hydrogen gas, which can be stored, transported, and burned to provide power. Unlike most fuels, hydrogen does not produce the greenhouse gas carbon dioxide (CO 2) when burned: instead, it ...
As a clean and versatile energy carrier, green hydrogen offers a range of benefits that make it a vital component in our quest to decarbonize the global economy. •. Tackling climate change: green hydrogen is produced through the electrolysis of water using renewable energy sources, such as solar, wind, or hydropower.
The gas-based blue hydrogen capacity accounts for 49% of the total low-carbon hydrogen capacity given in Table 1 and is estimated to be 90% in 2030 in terms of the International Energy Agency's ...
Green hydrogen energy is a natural substitute for fuel-based energy, ... The top 5 papers under OA are further analyzed, as seen in Table 3. Remarkably, the publications in 2018 and 2020 have achieved a high TC/Y ranging from 39 to 120 in a short span of 3 years. Table 2.
Hydrogen is emerging as a new energy vector outside of its traditional role and gaining more recognition internationally as a viable fuel route. This review paper offers a crisp analysis of the most recent developments in hydrogen production techniques using conventional and renewable energy sources, in addition to key challenges in the production of Hydrogen. Among the most potential ...
Dihydrogen (H2), commonly named 'hydrogen', is increasingly recognised as a clean and reliable energy vector for decarbonisation and defossilisation by various sectors. The global hydrogen demand is projected to increase from 70 million tonnes in 2019 to 120 million tonnes by 2024. Hydrogen development should also meet the seventh goal of 'affordable and clean energy' of the United ...
Global demand for primary energy rises by 1.3% each year to 2040, with an increasing demand for energy services as a consequence of the global economic growth, the increase in the population, and advances in technology. In this sense, fossil fuels (oil, natural gas, and coal) have been widely used for energy production and are projected to remain the dominant energy source until at least 2050 ...
The global issue of climate change caused by humans and its inextricable linkage to our present and future energy demand presents the biggest challenge facing our globe. Hydrogen has been introduced as a new renewable energy resource. It is envisaged to be a crucial vector in the vast low-carbon transition to mitigate climate change, minimize oil reliance, reinforce energy security, solve the ...
GH is hydrogen fuel that is produced using renewable energy rather than fossil fuels. Every year, about 100 million of tons of hydrogen are generated for a variety of commercial applications ...
Green hydrogen, which uses renewable energy to produce hydrogen from water, is taking off around the globe. Its boosters say the fuel could play an important role in decarbonizing hard-to-electrify sectors of the economy, such as long-haul trucking, aviation, and heavy manufacturing. By Jim Robbins • November 5, 2020.
The role of hydrogen as a clean energy source is a promising but also a contentious issue. The global energy production is currently characterized by an unprecedented shift to renewable energy sources (RES) and their technologies. However, the local and environmental benefits of such RES-based technologies show a wide variety of technological maturity, with a common mismatch to local RES ...
Essay # 3. Hydrogen Energy for Air and Surface Transport: 1. Jet Fuel: The high energy density 33.3 kWh/kg of liquid hydrogen against 12.7 kWh/kg of conventional jet fuel is the main advantage in air transportation where hydrogen energy can be used. Although volume of liquid hydrogen would be greater than regular fuel but this could be ...
The global interest in green hydrogen as a sustainable energy for socioeconomic growth and development is gaining much attention. South Africa, being recognized as a water-stressed country with ...
A McKinsey & Company report co-authored with industry estimated that the hydrogen economy could generate $140 billion in annual revenue by 2030 and support 700,000 jobs. The study also projected ...
Hydrogen is an environmentally friendly energy carrier. As fuel for fuel cells, there is no emission other than clean water. Fuel cells convert hydrogen and oxygen electrochemically to water, and in this process electricity is generated. Hydrogen is also a flexible energy carrier that can be used in combustion engines with minimal emissions.
Making hydrogen from electricity that dirty is worse than simply making it from fossil fuels, according to an April 2023 analysis from Energy Innovation, a clean energy think tank.
In particular, the topic of hydrogen production using microbial electrolysis cells is of interest, as there is a global effort to use hydrogen as an energy source. Microbial electrolysis is an effective biological analogue of chemical electrolysis—much like a microbial fuel cell is to a chemical fuel cell.
4. Applications of hydrogen energy. The positioning of hydrogen energy storage in the power system is different from electrochemical energy storage, mainly in the role of long-cycle, cross-seasonal, large-scale, in the power system "source-grid-load" has a rich application scenario, as shown in Fig. 11.
DOI: 10.1016/j.renene.2024.120911 Corpus ID: 271035006; A comprehensive review of green hydrogen energy systems @article{Kourougianni2024ACR, title={A comprehensive review of green hydrogen energy systems}, author={Fanourios Kourougianni and Alexandros Arsalis and Andreas V. Olympios and Georgios Yiasoumas and Charalampos Konstantinou and Panos Papanastasiou and George E. Georghiou}, journal ...
left to right: Mengsu Hu, Ben Gilbert. Berkeley Lab's strategic research themes describe research programs and projects at the Lab that could make a significant contribution to the world, while also potentially providing growth opportunities for research at the Lab and for science. The two Lab research projects recently funded by ARPA-E to develop geologic hydrogen as a new source of carbon ...
A little more than a decade ago, hydrogen fuel cell vehicles appeared poised to challenge hybrid and battery-powered electric vehicles as a green alternative to gasoline-powered cars and trucks ...
In recent years, the heightened focus on combating climate change and environmental pollution has underscored the importance of alternative energy sources, with hydrogen fuel emerging as a prime candidate due to its clean, efficient, and renewable attributes. This study investigates the potential amalgamation of hydrogen fuel technologies with innovative three-stroke engines, aligning with the ...
Hydrogen for energy plans are slowing the required transition to actually economically sensible, practical and rapid decarbonization. They are distracting legislators, policy makers, entrepreneurs ...
Produced using solar and wind power, green hydrogen is a pillar of Germany's planned energy transition, The study by Berlin-based Agora Energiewende and Agora Industry think tanks said that by ...
Hydrogen is an international peer-reviewed open access quarterly journal published by MDPI. Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1000 CHF (Swiss Francs). Submitted papers should be well formatted and use good English.