Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 September 2022

Antioxidant, cytotoxic, and antibacterial activities of Clitoria ternatea flower extracts and anthocyanin-rich fraction
- Ethel Jeyaseela Jeyaraj 1 ,
- Yau Yan Lim 1 &
- Wee Sim Choo 1
Scientific Reports volume 12 , Article number: 14890 ( 2022 ) Cite this article
11k Accesses
25 Citations
Metrics details
- Biochemistry
- Drug discovery
Clitoria ternatea flower is a traditional medicinal herb that has been used as a natural food colourant. As there are limited studies on investigating the bioactivities of the anthocyanin-rich fraction of Clitoria ternatea flower, this study aimed to determine an efficient column chromatography method to obtain the anthocyanin-rich fraction from this flower and characterise its composition, antioxidant, antibacterial, and cytotoxic activities. Amberlite XAD-16 column chromatography was more efficient in enriching the total anthocyanin content (TAC) of the fraction with the highest TAC to total phenolic content (TPC) ratio of 1:6 than that using C18-OPN. A total of 11 ternatin anthocyanins were characterised in the anthocyanin-rich fraction by LC–MS analysis. The antioxidant activity of the anthocyanin-rich fraction was more potent in the chemical-based assay with an IC 50 value of 0.86 ± 0.07 mg/mL using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay than cellular antioxidant assay using RAW 264.7 macrophages. In vitro cytotoxicity assay using human embryonic kidney HEK-293 cell line showed the anthocyanin-rich fraction to be more toxic than the crude extracts. The anthocyanin-rich fraction had more potent antibacterial activity than the crude extracts against Bacillus cereus , Bacillus subtilis , and Escherichia coli . The anthocyanin-rich fraction of C. ternatea has the potential to be used and developed as a functional food ingredient or nutraceutical agent.
Similar content being viewed by others

Effects and safety of Psilocybe cubensis and Panaeolus cyanescens magic mushroom extracts on endothelin-1-induced hypertrophy and cell injury in cardiomyocytes
Antibacterial apple cider vinegar eradicates methicillin resistant Staphylococcus aureus and resistant Escherichia coli
Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria
Introduction.
Anthocyanins are classified under the flavonoid group of polyphenol compounds, which gives rise to the red and blue colours of vegetables, fruits, flowers, and leaves 1 . Anthocyanins are present in plants as a glycoside in which the anthocyanidin is bound to a sugar group, with glucose, galactose, rhamnose, xylose, or arabinose bound to an aglycon 2 . The six major types of anthocyanidin which occur widely in plants are cyanidin, delphinidin, petunidin, peonidin, pelargonidin, and malvidin 3 . Various studies have shown anthocyanins to have a wide range of biological activities such as antimicrobial, antioxidant, cardiovascular protection, and anticancer activities 4 , 5 , 6 .
Clitoria ternatea flower (butterfly pea), a member of the Fabaceae family has a vivid blue colour which is widely used as a natural food colourant (e.g. in rice cakes, tea, snacks, and sweet desserts), traditional medicine as well as an ornamental plant 7 , 8 , 9 . C. ternatea plant is widely distributed in India, the Philippines, other Asian countries, and South and Central America 7 . The flowers are mainly composed of flavonols (quercetin, myricetin, and kaempferol derivatives) and anthocyanins (ternatin A1-A3, B1-B4, C1-C4, and D1-D3) 10 . The six major anthocyanins in C. ternatea flowers are ternatin A1, A2, B1, B2, D1, and D2 which are based on delphinidins. These are triacylated anthocyanins that showed relatively higher stability compared with nonacylated and monoacylated anthocyanins 7 , 11 . The crude flower extract has been shown to have various therapeutic potentials such as antidiabetic, antioxidant, and antimicrobial activities 12 , 13 , 14 . However, it is not known if these activities are contributed by the flavonols or anthocyanins.
Preliminary processing of plant extracts is essential for the enrichment and purification of active compounds. Column sorbents such as RP-C18, Toyopearl, Sephadex LH-20, Amberlite XAD-7, and Amberlite XAD-16 have been employed for the separation and enrichment of various phytochemicals. They are known for their unique adsorption properties in which some of these resins have been successfully used for the fractionation and purification of anthocyanins 15 . The use of preparative high-performance liquid chromatography (HPLC) for the separation of active compounds on a large scale is rather expensive and inconvenient 16 . Several studies investigated the potential of C. ternatea flower extract for several bioactivities. However, most of these studies only used a particular solvent for extraction from the raw material without further purification steps in which there are high chances of various other phytochemicals being present in the crude extract. Most of those studies also did not further investigate, determine or characterise the anthocyanins that are present in their extracts.
Column chromatography has been commonly employed for the purification of anthocyanins. Amberlite XAD-16 is known to be a non-ionic macroreticular resin that adsorbs and releases ionic species through hydrophobic and polar interactions whereas C18-OPN, the external surface of the silica gel is coated with a hydrophilic group which makes the reversed-phase open column chromatography possible with 100% water. Apart from that, Amberlite XAD-16 also has a larger surface area and pore size, but smaller particle size compared to C18-OPN. Thus, the objective of this study was to compare the efficiency in separating the anthocyanins of C. ternatea flowers using these two open column chromatography methods (C18-OPN and Amberlite XAD-16) followed by the characterisation of the anthocyanins. The anthocyanin-rich fraction of the flower has also not been explored for its antibacterial potential, cytotoxic activity as well as antioxidant potential in a cell-based assay for its ability to attenuate ROS production. RAW264.7 murine macrophage cell line has been used in many studies to determine the anti-oxidative and anti-inflammatory potential of compounds by observing the release of various cytokines, interleukins as well as reactive oxygen species (ROS) 17 . The generation of excess free radicals (e.g. ROS) induces oxidative stress in cells which can damage cells, and long-term oxidative stress leads to the generation of various chronic diseases. This macrophage cell line is increasingly being used as an approach to determine the antioxidant potential of various bioactive compounds of natural origin and was thus used in the current study 18 . Subsequently, the potential of the anthocyanin-rich fraction of C. ternatea flowers was also determined and compared to the crude extracts for its antibacterial activity against various Gram-positive and Gram-negative bacterial strains and its cytotoxic activity was also determined on a normal human cell line model, human embryonic kidney HEK-293 cell line.
Results and discussion
Characterisation and identification of anthocyanins by lc–ms in c. ternatea anthocyanin-rich fraction using c18-opn and amberlite xad-16 column.
There is limited natural blue food colourant available commercially. The blue colour of C. ternatea flowers is attributed to its anthocyanins which have been mainly characterised and termed as ternatins. These anthocyanins are polyacylated derivatives of delphinidin 3,3′, 5′-triglucoside. The ternatins which are polyacylated with p-coumaroyl groups contribute to the colour of the anthocyanins to the bluish region. The high stability of the anthocyanins is due to the polyacylation at the 3′ position which also contributes to the stable blue colouration 19 . In our previous study comparing the effectiveness of solvent or water extraction methods, 50% ethanol was found to be the best for solvent extraction method while the condition of 50 °C for 1 h was the best for the water extraction method. Both extracts had similar phytochemical content 20 . The crude solvent extract (50% ethanol) of C. ternatea flowers was used in this study to determine a column chromatography method that was more effective to isolate the anthocyanins. Previous studies have either used Amberlite XAD7HP resin or C-18 Sep-Pak cartridges for the partial purification of C. ternatea flower anthocyanins. The study utilising the Amberlite XAD7HP resin characterised ternatins B2 or B3, B4, D2 and some delphinidin derivatives while the ternatin B2, B3, B4, C2, D1, D3 and some delphinidin derivatives were characterised in the study utilising C-18 Sep-Pak cartridges. However, it is not known which method is superior or more effective for the purification of C. ternatea flower anthocyanins 21 , 22 , 23 . The volume of sample which can be loaded onto the C-18 Sep-Pak cartridges is rather small and would not be convenient thus exploring a larger scale method for anthocyanin purification would be beneficial. In this study, the crude solvent extract (50% ethanol) of C. ternatea flowers was subjected, separately, to column chromatography methods utilising two resins with distinct properties (Table 1 ) to determine its efficiency for the partial purification of anthocyanins. The extract was semi-purified using C18-OPN or Amberlite XAD-16 open column chromatography to remove phenolic acids and flavonols. Ethyl acetate facilitated the removal of flavonols while acidified water assisted in the removal of phenolic acids and sugars 24 . To determine an open column chromatography method with better efficiency to obtain a semi-purified anthocyanin-rich fraction, the extraction yield, TAC and TPC were determined and compared to the crude extract (Table 2 ).
In terms of extraction yield, there was no significant difference in both column chromatography methods. As for TAC, both column chromatography methods were equally potent in separating anthocyanins as they had significantly higher values compared to the crude extract in which the TAC values were almost 5 times higher than that in the crude extract. As for TPC, both column chromatography methods were significantly higher than the crude extract. The TPC of the C18-OPN anthocyanin-rich fraction was significantly higher than semi-purified using Amberlite XAD-16 (Table 2 ). In terms of enriching TAC, the crude extract showed the lowest ratio of TAC:TPC (1:9). The anthocyanin-rich fraction of Amberlite XAD-16 showed a higher ratio of TAC:TPC (1:6) compared to that fractionated using C18-OPN (1:7) which indicates that the anthocyanins were much more enriched in the former method. The comparison of the ratio of TAC to TPC is used as a quantitative method to relate to the increase of anthocyanins in the overall content. Resins with a higher surface area and larger average pore diameter have shown to have better adsorption and desorption capacities for anthocyanins 15 . This is supported by the open column chromatography method utilising Amberlite XAD-16 to be more effective than C18-OPN. A higher TPC value indicates that more flavonols, phenolic acids, or other compounds may have been retained. The anthocyanin-rich fraction obtained via Amberlite XAD-16 was selected for further investigation as it had a higher overall enrichment of anthocyanins and was subjected to LC–MS analysis to characterise the anthocyanins that were present in the fraction.
Results from LC–MS analysis (Table 3 ) show the tentative anthocyanins identified in the anthocyanin-rich fraction of C. ternatea flowers obtained through Amberlite XAD-16 column chromatography based on literature 22 , 23 , 25 , 26 . The anthocyanins of C. ternatea are based on delphinidin and are polyacylated. The structure of the anthocyanins was characterised as malonylated delphinidin 3,3′,5′-triglucosides having 3′,5′-side chains with alternative D-glucose (G) and p-coumaric acid (C) units 7 . Compound 1 with [M + H] + m/z 1491.3587 and identified fragments at m/z 1021.2399 [M-2G-2C] + , and 773.2185 [M-malonate-G-C] + was identified as ternatin C2. Compound 2 with [M + H] + m/z 1329.3152 and identified fragments at m/z 1021.2403 [M-G-C] + , 788.4357 [M—malonate-G-2C] + , 611.1727 [M-malonate-2G-C] + , and 465.1190 [M-malonate-2G-2C] + was identified as ternatin B4. Compound 3 with [M + H] + m/z 1167.2703 and identified fragments at m/z 1021.2398 [M-C] + , and 859.1952 [M-G-C] + was identified as ternatin D3. Compound 4 with [M + H] + m/z 1637.3876 and identified fragment at m/z 1329.3138 [M-G-C] + was identified as ternatin B3. Compound 5 with [M + H] + m/z 1637.3904 and identified fragments at m/z 1329.3144 [M-G-C] + , 1167.2699 [M-2G-C] + , and 1021.2398 [M-2G-2C] + was identified as ternatin B2. Compound 6 with [M + H] + m/z 1167.2703 and identified fragment at m/z 1021.2398 [M-C] + was identified as ternatin D3 isomer. Compound 7 with [M + H] + m/z 1329.3150 and identified fragment at m/z 1167.2694 [M-G] + was identified as ternatin C1. Compound 8 with [M + H] + m/z 1946.4632 and identified fragment at m/z 1167.2691 [M-3G-2C] + was identified as ternatin B1. Compound 9 with [M + H] + m/z 1475.3456 and identified fragment at m/z 1167.2702 [M-G-C] + was identified as ternatin D2. Compound 10 with [M + H] + m/z 1167.2703 and identified fragment at m/z 859.1950 [M-G-C] + was identified as ternatin D3 isomer. Compound 11 with [M + H] + m/z 1783.4192 and identified fragment at m/z 1167.2699 [M-2G-2C] + was identified as ternatin D1. Ternatin B2, ternatin D1, and ternatin D2 have [G-C-G-C and G-C], [G-C-G-C and G-C-G-C], and [G-C-G-C and G-C-G] linked at positions 3′ and 5′ in the structure of delphinidin, respectively, and were found to be the most abundant anthocyanin present having a peak area of 23.93, 20.44, and 20.03%, while ternatin D3 and its isomers having [G-C and G-C] linked at positions 3′ and 5′ in the structure of delphinidin were the least abundant in the anthocyanin-rich fraction (Table 3 ). The anthocyanin composition obtained in this study is in accordance with the findings of another study using the crude extract of C. ternatea flowers 10 . A similar profile of anthocyanin composition was also detected in our previous study in the crude extract of the flowers 20 .
Antioxidant activity of anthocyanin-rich fraction from C. ternatea flower
2,2-diphenyl-1-picrylhydrazyl radical (DPPH) and ferric reducing power (FRP) antioxidant assays (chemical-based) were performed to assess the antioxidant activity of C. ternatea flower anthocyanin-rich fraction. In the DPPH assay, the radical scavenging activity of the anthocyanin-rich fraction was found to have an IC 50 value of 0.86 ± 0.07 mg/mL. The result indicated that the fraction was more potent than the crude extracts (IC 50 value of solvent extract = 1.24 ± 0.05 and 1.18 ± 0.07 mg/mL for the water extract) as reported by our previous study 20 . According to the results for FRP, C. ternatea flower anthocyanin-rich fraction was found to have 34.5 mg gallic acid equivalent/g extract which was more potent than the crude extracts as reported previously 20 . Most of the previous studies have determined the antioxidant activity of C. ternatea flower solvent or water extracts only in which the IC 50 values ranged from 0.08 to 4 mg/mL in DPPH assay 12 , 27 . Other studies have reported the anthocyanins of other fruits to be more potent than its crude extracts. A particular study compared the antioxidant activity of crude mulberry extract and its anthocyanin-rich extract. The anthocyanin-rich extract was found to have higher antioxidant activity compared to the crude extract in the DPPH assay 28 . A similar pattern was also obtained in another study where the anthocyanin-rich blackberry extract had higher antioxidant activity than the crude extract in the ORAC assay 29 . The anthocyanins in these studies differed structurally in that they were based on cyanidin derivatives with aglycone functional groups in mulberry fruit while the anthocyanins of blackberry fruit were mainly composed of cyanidin-3-glucoside (90%) which differed from the anthocyanins in C. ternatea flower which are based on delphinidin derivatives and are in the triacylated form 7 . Previous studies suggested the antioxidant activity of C. ternatea flowers was contributed by the presence of various flavonols and anthocyanins 30 .
Chemical antioxidant assays are conventionally used to measure antioxidant activity. However, these assays bear no similarity to biological systems 31 . Most studies on the antioxidant activity of C. ternatea flower extracts done so far are based on chemical assays. Cellular antioxidant activity (CAA) assay was performed in this study as it can address issues faced using chemical antioxidant assays being bioavailability, metabolism, and uptake of the antioxidant compounds in the biological system 32 , 33 . The cytotoxicity of the anthocyanin-rich fraction (39.1–2500 µg/mL) was evaluated using MTA assay on RAW264.7 cells (Fig. 1 ) at 24 h to determine the non-toxic concentration to be used in the CAA assay to ensure the concentration range used and the antioxidant activity observed is not due to cell toxicity or death.

Viability of RAW264.7 cells treated with different concentrations (µg/mL) of C. ternatea flower anthocyanin-rich fraction at 24 h. Values are means ( n = 3) ± standard deviations. Values with different letters are significantly different ( p < 0.05) in cell viability. Cells lysed by 1% Triton X-100 were used as the positive control, while the untreated cells were assayed as the negative control.
The anthocyanin-rich fraction of C. ternatea flower was found to be non-toxic up to 156.3 µg/mL against RAW264.7 cells and was thus selected as the highest concentration to be used in the CAA assay. RAW264.7 macrophage cells were used as a research model, and oxidative stress was induced by 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH) for the generation of ROS. The ability of the extract to reduce the extent of AAPH-generated free radicals in RAW264.7 cells is shown in Fig. 2 . The anthocyanin-rich fraction showed weak antioxidant activity at 78.1 µg/mL in which the inhibition of ROS production was about 20% and was also less potent than the positive control quercetin at 20 µg/mL (Fig. 2 ). The crude extracts of C. ternatea flower had more potent cellular antioxidant activity (75–80% inhibition at 156.3 µg/mL) than the anthocyanin-rich fraction in this study 20 . The crude extract is known to be mainly composed of flavonols and anthocyanins which may have acted synergistically to exert the antioxidant activity 10 . Although the anthocyanin-rich fraction was shown to have better antioxidant activity than the crude extracts in the chemical-based assays (DPPH and FRP), this effect was not able to be observed in the cellular antioxidant assay. However, it should be noted that the concentration of extracts in the chemical assay was much higher to achieve the IC 50 values.

ROS production of AAPH-induced oxidation of DCFH to DCF in RAW264.7 cells treated with C. ternatea flower anthocyanin-rich fraction. Different letters indicate significant differences in ROS production at p < 0.05. The negative control was cells treated with DCFH-DA and AAPH without plant extract while the positive control was cells treated with DCFH-DA and AAPH with quercetin.
The concentration which showed an effect in chemical assays could not be translated to the cell-based assay and other factors are involved as well such as the bioavailability and uptake of compounds by the cell. The anthocyanins present in different fruits, flowers, and vegetables have different structures and thus have different pharmacological outcomes which explain the difference in the trend observed 2 . A study found the anthocyanin-enriched extract of blackberry with a greater ability to suppress free radical generation than the crude extract in human intestinal (INT-407) cells 29 . However, it should be noted that various factors could have affected the outcome between both assays such as the difference in the cell line, treatment time, and also the composition of anthocyanins in both extracts where cyanidin-3-glucoside was the major anthocyanin in blackberry extract and is in an unacylated form with a molecular weight of 484.8 29 . The anthocyanins of C. ternatea known as ternatins (based on delphinidins) are triacylated and the molecular weight ranged from around 1168 to 1946 (Table 3 ). Although the acylated anthocyanins have been known to have several advantages over the nonacylated forms such as high stability and resistance to changes in heat, light, and pH, it is unknown for its characteristics in cellular uptake due to the nature of the functional groups present as well as it being a rather large molecule compared to cyanidin-3-glucoside. The anthocyanins of C. ternatea (ternatins) being large molecules are highly acylated with the structure of delphinidin 3,3′,5′-triglucoside in which the 3′- and 5′-glucoses are acylated with variable lengths of p-coumaric acid–glucose side chains 34 . C. ternatea anthocyanins are also highly hydrophilic in nature which may have affected the cellular uptake of these compounds to effectively prevent oxidation as it may affect its penetration across cell membranes which are hydrophobic 35 , 36 .
Cytotoxic activity of water extract, solvent extract, and the anthocyanin-rich fraction of C. ternatea flower in HEK-293 cell line
MTA assay was performed to assess the cytotoxic activity of C. ternatea flower crude solvent extract (50% ethanol), crude water extract (50 °C for 1 h), and anthocyanin-rich fraction in HEK-293 cell line (isolated from the kidney of a human embryo) at 24 h (Fig. 3 ) HEK-293 cells are considered as a normal human cell line model (being representative of human cells) which has been routinely used to evaluate the cytotoxicity of compounds 37 . Percentages of cell viability above 80% are considered non-cytotoxic; within 80–60% weak; 60–40% moderate and below 40% strong cytotoxicity respectively which is in accordance with ISO 10,993–5 in vitro test for cytotoxicity 38 . The water and solvent extracts were found to be non-toxic from 19.5 to 156.3 µg/mL while the anthocyanin-rich fraction was found to be non-toxic at the concentration range from 19.5 to 78.1 µg/mL. The water and solvent extracts displayed strong cytotoxic activity at 312.5 µg/mL while it was 156.3 µg/mL for the anthocyanin-rich fraction. The anthocyanin-rich fraction displayed higher cytotoxic activity compared to the water and solvent extracts. Previous studies investigated mostly the cytotoxic activity of water and solvent extracts of C. ternatea flower on various cancer cell lines such as MCF-7 (hormone-dependent breast cancer cell line), K562 (human leukemia cells), and SKBR (human breast carcinoma) cell lines which displayed IC 50 (concentration of test agent that causes 50% cell death) values ranging from 27.2 to 68.2 µg/mL 39 . Another study found the cytotoxicity of the solvent extracts against Dalton’s lymphoma ascites (DLA) cells with IC 50 values of 36 µg/mL and 57 µg/mL 40 . The water and solvent extracts of C. ternatea flowers were found to be cytotoxic to various cancer cell lines below 100 µg/mL but were not toxic up to 100 µg/mL on normal human foreskin fibroblast (Hs27) cell line. However, the cytotoxic concentration on the normal cell line was not determined as the highest concentration tested was 100 µg/mL. It could only be concluded that the extracts were more toxic to cancer cell lines compared to a normal cell line 41 . These studies suggested that the cytotoxic activity of the extracts could be contributed by the presence of flavonoids.

Viability of HEK-293 cells treated with different concentrations (µg/mL) of C. ternatea flower crude solvent extract, crude water extract, and anthocyanin-rich fraction at 24 h. Values are means ( n = 3) ± standard deviations. Values with different letters are significantly different ( p < 0.05) in cell viability. Cells lysed by 1% Triton X-100 were used as the positive control, while the untreated cells were assayed as the negative control.
There are no cytotoxic studies done so far for the anthocyanin-rich fraction. A particular study investigated the cytotoxic activity of the crude extract, anthocyanin fraction, phenolic fraction, and organic acid fraction of specialty potatoes on PC-3 (androgen-independent) and LNCaP (androgen-dependent) human prostate carcinoma cell lines. The anthocyanin fraction was found to be the most active component of the potato extracts for inhibition of LNCaP and PC-3 cell proliferation 42 . C. ternatea flower extract is composed mainly of various flavonols (quercetin, kaempferol, and myricetin) and anthocyanins 7 . Although anthocyanins are the main components of the anthocyanin-rich fraction, the minor presence of other flavonols may have contributed to the cytotoxic effect as well. Apart from anthocyanins, studies have also shown flavonols such as quercetin and kaempferol to possess cytotoxic activity 43 . As the anthocyanin-rich fraction of C. ternatea had a higher TPC value than that of crude extract (Table 2 ), this may be the reason that the anthocyanin-rich fraction possesses higher cytotoxic activity.
Several studies investigating various other bioactivities of C. ternatea flower extract determined the in vivo toxicity of the extract in mice or rats 14 , 44 . An acute toxicity study (14 days) was done using albino Wistar rats which were treated orally with 50% ethanol extract of C. ternatea flowers at 2000 mg/kg body weight. The treatment group showed no signs of mortality or abnormality and there was no significant difference in the hematological values compared to the control untreated group which indicates no acute toxicity of C. ternatea flower extracts up to 2000 mg/kg 44 . There are no in vivo studies done for the anthocyanin-rich fraction of C. ternatea so far. Although the crude extracts of C. ternatea flower displayed toxicity at doses above 156.3 µg/mL against HEK-293 cells, the findings from the acute toxicity study in rats show the extract to be rather safe for consumption 44 Further in vivo studies are necessary to determine the toxic effects of anthocyanin-rich fraction besides considering the effects of other factors such as stability and bioavailability of the compounds present.
Antibacterial activity of water extract, solvent extract, and the anthocyanin-rich fraction of C. ternatea flower
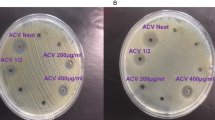
The agar dilution method (ADM) was used to determine the minimum inhibitory concentration (MIC) values of C. ternatea flower crude extracts and the anthocyanin-rich fraction. The test concentration ranged from 0.16 to 40 mg/mL (Table 4 ). ADM was used to test the extracts instead of broth microdilution assay due to the colour of the C. ternatea flower extracts that interfered with the detection of microbial growth in the broth. The crude water and solvent extracts of C. ternatea flower were found to have activity against B. cereus and B. subtilis with a MIC value of 10 mg/mL while the anthocyanin-rich fraction was found to be more potent against these strains with a MIC value of 0.63 mg/mL. The C. ternatea anthocyanin-rich fraction but not the crude extracts was found to have activity against the Gram-negative bacteria E. coli with a MIC value of 10 mg/mL. The MIC values of chloramphenicol were similar to those reported in the literature showing the validity of the tests 45 , 46 .
Most of the antibacterial studies done previously for C. ternatea flower extract utilised the disc diffusion assay in which the zone of inhibition ranged from 7 to 26 mm on various bacterial species 47 , 48 . A study reported that the MIC range of C. ternatea flower crude extract was 1.25–10 mg/mL against E. coli , K. pneumonia , and P. aeruginosa which were isolated from patients 47 . However, in this current study, the activity was not achieved most probably due to the difference in the strains used. The findings obtained in the current study found the crude extracts to have activity against B. cereus and B. subtilis . Another study reported activity against both of these strains (isolated from contaminated food samples) with a MIC value of 25 mg/mL which was higher than obtained in the current study 48 . This is the first study to report the antibacterial activity by comparing the crude extracts and anthocyanin-rich fraction utilising the agar dilution method. The higher antimicrobial activity achieved by the anthocyanin-rich fraction was most likely caused by the higher anthocyanin content compared to the crude extracts which had a higher content of flavonols. Our previous study investigated the flavonol and anthocyanin-rich fraction for antibiofilm activity against P. aeruginosa . The anthocyanin-rich fraction but not the flavonol fraction was responsible for the potent antibiofilm activity where the biofilm formation by four P. aeruginosa strains was significantly reduced (minimum biofilm inhibitory concentration ranging between 0.625 and 5.0 mg/mL). The anthocyanin-rich fraction also significantly reduced bacterial attachment to the polystyrene surface by 1.1 log CFU/cm 2 based on SEM analysis 49 . These findings may suggest the antibacterial activity of the anthocyanin-rich fraction of C. ternatea is due to the anthocyanins and not flavonols but this requires further investigation.
There was no clear trend observed for the anthocyanin-rich fraction against the Gram-positive or Gram-negative bacteria. However, it displayed activity against B. cereus , B. subtilis , and E. coli (Table 4 ). Previous studies have shown the antibacterial potential of anthocyanin-rich fractions of other fruits or flowers such as hibiscus or blueberry which were mainly composed of anthocyanins based on cyanidin, delphinidin, petunidin, and malvidin derivatives. These different anthocyanin derivatives may have acted synergistically for the antibacterial activity of these anthocyanin-rich fractions against bacterial strains such as E. coli , B. cereus , and P. aeruginosa 50 , 51 . The effect of the compounds in previous studies was found to affect bacterial cell membrane structure leading to the inactivation of crucial enzymes, affecting gene expression, and impairment of the metabolism of bacteria which may affect their growth and reproduction. The anthocyanins were found to have affected the tricarboxylic acid cycle (TCA) cycle which is one of the main ways for cells to gain energy and also the common metabolic pathway for oxidation of sugar, fats, and protein. Disruption of the TCA cycle leads to weakened cellular respiration and inadequate energy supply leading to death 52 , 53 .
The anthocyanins of C. ternatea were ternatin anthocyanins based on delphinidins 7 and these different ternatins may have acted synergistically to achieve the antibacterial effect. The findings suggest a possibility of the anthocyanins being responsible for the observed antibacterial activity. Many antibacterial studies of antibiotics and various other natural compounds have shown the compounds to have higher activity against Gram-positive bacterial strains than the Gram-negative bacterial strains which is mainly attributed to the morphology of the Gram-positive bacterial strains which are known to be void of the outer membrane known to be present in Gram-negative bacterial strains making them more resistant to antibacterial agents 54 . However, the findings obtained in this study are rather interesting as the flower extracts and enriched anthocyanin fraction were shown to have activity only against certain Gram-positive ( B. subtilis and B. cereus ) and Gram-negative ( E. coli ) bacterial strains but not on the other strains. Studies have reported the structural similarities of certain proteins observed in both E. coli (FtsW and RodA) and B. subtilis (spoVE) which are known to function in cell division, cell elongation, and spore formation respectively 55 . Similarities were also found in the mreB proteins of E. coli and B. subtilis important for the structural maintenance of the cells 56 . We can speculate that the crude flower extracts and anthocyanin enriched fraction may have exerted a unique mechanism of antibacterial activity with a potential target of a specific region which may be present in these 3 bacterial strains due to their similarities. However, further studies (e.g. molecular docking and gene expression studies) are required to facilitate and understand the underlying mechanism for the antibacterial effect and its potential against bacteria virulence.
Conclusions
This study demonstrated that the anthocyanin-rich fraction of C. ternatea flowers was successfully obtained using C18-OPN and Amberlite XAD-16 open column chromatography methods. The Amberlite XAD-16 method was found to be the superior method as it efficiently enhanced the TAC compared to the TPC. The anthocyanins were successfully characterised and their composition in the anthocyanin-rich fraction was obtained by LC–MS analysis. The anthocyanin-rich fraction had more potent antioxidant activity in the chemical-based assays over a cellular assay. It was also found to have higher cytotoxic and antibacterial activity compared to the crude extracts. In future studies, the use of techniques such as preparative high-performance liquid chromatography (HPLC) for the isolation of active compounds is recommended to determine the compound/s responsible for the observed activity. It is worthwhile to investigate further the anthocyanin-rich fraction of C. ternatea as it has the potential to be used and developed as a functional food ingredient or nutraceutical agent.
Plant samples
Freshly harvested Clitoria ternatea cv. Double Blue flowers were obtained from a plant nursery in Subang Jaya, Malaysia. Only the petals of fresh C . ternatea flowers were used in the extraction process. The petals were cut into smaller pieces (approximately 0.5 × 0.3 cm) before usage.
Declaration statement
We declare that the collection of plant material is in accordance with relevant institutional, national, and international guidelines and legislation.
Extraction of samples
The fresh flower sample material (50 g) was extracted in 1 L of 50% ethanol with constant shaking for 3 h at room temperature (25 °C) for the solvent extract while it was extracted in 1 L of distilled water with constant shaking for 1 h at 50 °C in a water bath for the water extract. The solution was then vacuum-filtered and the marc was discarded. The solution was concentrated under vacuum at 45 °C using a rotary evaporator followed by freeze-drying at −80 °C. The freeze-dried extracts were kept at − 80 °C until further analysis. Chromatography columns [S24/29, 25 (D) x 300 mm (L)] with sintered glass disc (porosity 0) and stopcock with PTFE key were used for the open column chromatography as described below. Both C18-OPN and Amberlite XAD-16 adsorbents were immersed overnight in methanol before loading into the columns (¾ of column height).
C18-OPN column chromatography
The fractionation of the crude extract was carried out with some modifications using C18-OPN column chromatography 21 . Briefly, 5 g of freeze-dried crude extract was dissolved in 10 mL of water and adjusted to pH 7.0 with 5 N NaOH. A total of 10 mL of extract was loaded in Cosmosil C18-OPN column (Nacalai, San Diego, USA) previously conditioned to pH 7.0 with 0.5 L of 100% methanol and 1 L of nanopure water (pH 7.0). The neutral phenolics were absorbed in the column, whereas the phenolic acids were not. The column was washed with 1 L of water (pH 7.0) to remove the phenolic acids. The column was then adjusted to pH 2.0 with acidified water. The flavonols were eluted using 1 L of 100% ethyl acetate and the anthocyanins using 0.5 L of 100% methanol. The methanol fraction (containing anthocyanins) was concentrated under vacuum at 45 °C in a rotary evaporator followed by freeze-drying at − 80 °C. The freeze-dried extracts were kept at − 80 °C until further analysis.
Amberlite XAD-16 column chromatography
The fractionation of crude extract was carried out according to a previous study 57 . The extracts (5 g of freeze-dried crude extract) were first dissolved in distilled water (100 mL) and adjusted to pH 2. They were then partitioned with 100 mL of ethyl acetate in triplicate to remove the flavonols. The aqueous phase (containing anthocyanins) was concentrated under reduced pressure at 37 °C in a rotary evaporator. The sample was then subjected to further purification using Amberlite XAD-16 column chromatography. Briefly, the column was rinsed with 1 L of purified water and then activated with 0.5 L of 2% aqueous sodium hydroxide solution. After rinsing with purified water, the material was conditioned to pH 3 by washing with 1 L of acidified water. The concentrated sample (10 mL) was loaded in the column and rinsed with 0.3 L of acidified water (pH 3) at a flow rate of 10 mL/min to remove the phenolic acids. Then, the anthocyanins were eluted with acidified methanol [95:5, methanol/acidified water (pH 2), v/v]. The methanol fraction (containing anthocyanins) was concentrated under vacuum at 45 °C in a rotary evaporator followed by freeze-drying at − 80 °C. The freeze-dried extracts were kept at − 80 °C until further analysis.
Liquid chromatography-mass spectrometry (LC–MS) analysis
LC–MS analysis was done for the characterisation of anthocyanins with minor modifications 21 . Individual compounds were identified based on retention time, and mass-to-charge ratio using LC–MS. 1290 Infinity LC system coupled to 6520 Accurate-Mass Q-TOF mass spectrometer with a dual ESI source (Agilent, Santa Clara, USA) using Zorbax Eclipse XDB-C18, Narrow-Bore 2.1 × 150 mm, 3.5 microns (Agilent, Santa Clara, USA) and a guard column of the same chemistry was used for the chromatographic separations. A mass range of 500–2000 m/z was used for MS detection in positive mode. The elution gradients were performed with acetonitrile/methanol (1:1), formic acid (0.5:99.5, v/v) (phase A) and formic acid/water (0.5:99.5, v/v) (phase B). The applied elution conditions were as follows: 0–2 min, 2% A; 98% B; 3–5 min, 5% A, 95% B; 5–30 min, 20% A, 80% B; 30–72 min, 35% A, 65% B; 72–83 min, 100% A, 0% B; 83–85 min, isocratic, 100% A; 87–90 min, 2% A, 98% B to return to the starting condition. Nitrogen was used as desolvation gas, at 300 °C and a flow rate of 60 L/h, and He gas was used as damping gas, declustering potential 40 of eV; collision energy 5 eV; collision cell entrance potential 10 eV.
Measurement of total phenolic content (TPC)
The extract was determined for total phenolic content (TPC) using the Folin-Ciocalteu method with minor modifications 58 . The freeze-dried extract was dissolved in distilled water to a concentration of 1 mg/mL. Gallic acid was used as the standard and a calibration curve was established (0 – 1000 mg/mL). The extract or gallic acid (0.5 mL) was added to 2.5 mL Folin-Ciocalteu reagent (tenfold diluted with distilled water) and mixed thoroughly for 3 min. Sodium carbonate (2 mL, 7.5% w/v) was added to the mixture and the mixture was allowed to stand for 30 min at room temperature. The absorbance of the mixture was measured using a Perkin Elmer Lambda 25 UV–VIS spectrophotometer (Norwalk, USA) at 760 nm. TPC was expressed as mg gallic acid equivalent/g dry weight of extract (mg GAE/g extract).
Measurement of total anthocyanin content (TAC)
The pH differential method was used to analyze the total anthocyanin content (TAC) 59 . The absorption of the samples in pH 1 buffer (potassium chloride, 0.025 M) and pH 4.5 buffer (sodium acetate, 0.4 M) was measured using a Perkin Elmer Lambda 25 UV–VIS spectrophotometer (Norwalk, USA) at 520 and 700 nm. The anthocyanin concentration was expressed as cyanidin-3-glucoside equivalents, and calculated as follows:
where A = [(A 520 −A 700 ) at pH 1.0]–[(A 520 −A 700 ) at pH 4.5], MW is the molecular weight of cyanidin-3-glucoside (449.2), ε is the molar absorptivity (26,900), and DF is the dilution factor. The total anthocyanin content was expressed as mg cyanidin-3-glucoside equivalents/g dry weight of extract (mg CGE/g extract).
DPPH free radical scavenging activity
DPPH assay with minor modifications was carried out for free radical scavenging (FRS) analysis 58 . One mL of DPPH (5.9 mg per 100 mL in 100% methanol) was added to 500 µL samples (triplicate) of different dilutions. Samples were left to stand for 30 min in dark at room temperature followed by an absorbance measurement at 517 nm. The radical scavenging activity was calculated using the equation below:
A C = negative control absorbance (without sample); A S = sample absorbance ; IC 50 , the concentration of the extract required to destroy 50% of the DPPH was determined.
Ferric reducing power
The potassium ferricyanide-ferric chloride method was used to determine the ferric reducing power of C. ternatea flower extracts 58 . Different dilutions of 400 µL samples were added to 1 mL phosphate buffer (0.2 M, pH 6.6) and 1 mL potassium ferricyanide (1% w/v) to determine the ferric reducing power (FRP) activity. The mixture was left to stand for 20 min at 50 °C followed by the addition of 1 mL of trichloroacetic acid (10% w/v). The mixture was separated into aliquots of 1 mL and diluted with 1 mL of water. Then, 200 µL of ferric chloride (0.1% w/v) was added to the mixture. The mixture was kept in the dark for 30 min followed by an absorbance measurement at 700 nm. Gallic acid (GA) was used as the standard and FRP activity was expressed as mg GAE/g.
Cell culture
HEK-293 (human embryonic kidney) cells and RAW264.7 (mouse macrophage) cells were purchased from the American Type Culture Collection (Manassas, Virginia, USA). HEK-293 cells were grown as monolayer culture in Roswell Park Memorial Institute (RPMI) medium while RAW264.7 cells were grown in Dulbecco’s modified Eagle’s medium–high glucose (DMEM-HG) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 µg/mL) incubated at 37 °C in an atmosphere of 5% CO 2 -95% air mixture.
Microculture tetrazolium assay (MTA)
MTA assay was used to assess cell viability 60 . Cells were seeded on 96-well plates at 5000 cells per well for HEK-293 cells and at 10,000 cells/well for RAW264.7 cells. The cells were incubated at 37 °C in 5% CO 2 for 24 h. The cells were treated with the extracts and the anthocyanin-rich fraction and incubated further at 37 °C in 5% CO 2 for 24 h. Triton X-100 (1%) solution was used as the positive control. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) reagent (final concentration: 0.5 mg/mL) was added to the cells after treatment and incubated for 4 h at 37 °C. The medium was discarded and 0.1 mL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals formed. The absorbance was read at 570 nm in a microplate reader and cell viability was calculated.
Cellular antioxidant activity (CAA) assay
RAW 264.7 cells were used to determine the CAA of C. ternatea flower anthocyanin-rich fraction 61 . The basis of this antioxidant assay involves the cellular uptake of 2′-7′dichlorofluorescin diacetate (DCFH-DA) which is a non-fluorescent probe followed by hydrolyzation of intracellular esterase to form dichlorofluorescein (DCFH). The non-fluorescent substrate is oxidized by the peroxyl radicals generated from 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH), producing a fluorescent product (dichlorofluorescein, DCF). The antioxidants within the cell act to quench the free radical and reduce fluorescence intensity, thus indicating modulation of intracellular oxidation. RAW 264.7 cells were seeded into a 96-well plate (5 × 10 4 cells/well) and incubated for 24 h. The medium was removed and the wells were washed with phosphate-buffered saline (PBS). The cells were treated for 1 h with 100 µL of extracts mixed with 20 µM DCFH-DA dissolved in medium with the absence of FBS in triplicates. Quercetin was used as the positive control (20 µg/mL). The extracts and DCFH-DA mixture were removed from the wells after treatment and were then washed with 100 µL of PBS. Then 100 µL of 1 mM AAPH in PBS was added to the wells. Fluorescence reading was measured every 5 min for 1 h in a microplate reader at 37 °C with emission at 535 nm and excitation at 485 nm. Each plate had triplicate wells of negative control wells (cells treated with DCFH-DA and AAPH without plant extract), and blank wells (cells treated with DCFH-DA, without AAPH and plant extract). Blank subtraction was done by subtraction of final fluorescence values with initial fluorescence values. The antioxidant activity of the sample was calculated with the formula:
Antimicrobial test
The antimicrobial activity of C. ternatea flower crude extracts and the anthocyanin-rich fraction was tested against nine Gram-positive [ Staphylococcus aureus (ATCC 6538P, ATCC 29,213), Methicillin-resistant S. aureus (ATCC 700,699, ATCC 33,591, ATCC 43,300), Enterococcus faecalis (ATCC 29,212, ATCC700802) Bacillus cereus (ATCC 14,579), B. subtilis (ATCC 8188)] and eight Gram-negative [ Pseudomonas aeruginosa (ATCC 10,145, ATCC BAA-47, BAA-2110, ATCC 27,853, ATCC 9027), Shigella flexneri (ATCC 12,022), Salmonella typhimurium (ATCC 14,028), Escherichia coli (ATCC 25,922)] laboratory control bacterial strains obtained from the American Type Culture Collection (ATCC, Virginia, U.S.A.). The bacterial strains were stored at − 80 °C supplemented with glycerol (25% v/v). The antimicrobial activity was evaluated by determining the minimum inhibitory concentration (MIC) using the agar dilution method described by the Clinical and Laboratory Standard Institute 62 . The extracts were filtered through a membrane filter (0.20 μm) followed by serial dilution at 0.16–40 mg/mL (final concentration) and added to molten Mueller–Hinton agar that has been allowed to equilibrate in a water bath at 45 °C. The agar and extract solution was mixed thoroughly and poured into Petri dishes and allowed to solidify at room temperature. Chloramphenicol (0.001–0.5 mg/mL) was used as the positive control, whereas the negative control was bacterial suspension alone (no plant extract). The inoculum was prepared by making a direct broth suspension of isolated colonies selected from a 24 h agar plate. The suspension was adjusted to achieve turbidity equivalent to a 0.5 McFarland standard, which is equivalent to about 1 × 10 8 colony-forming units (CFU)/mL. The 0.5 McFarland suspension was diluted 1:10 in sterile broth to obtain a concentration of 10 7 CFU/mL. Two µL of bacterial suspension was delivered and the final inoculum on the agar was approximately 10 4 CFU per spot. The inoculated plates were allowed to stand at room temperature for 30 min to allow the spots to be absorbed into the agar. The plates were inverted and incubated at 37 °C for 24 h. The MIC was recorded as the lowest concentration of extract that completely inhibits the growth of the bacteria.
Statistical analysis
All experiments were carried out in independent triplicates. The results were expressed as the mean value ± standard deviation. The data obtained were analysed using one-way ANOVA followed by post-hoc Tukey’s test and significance was set at p < 0.05 using SPSS 23 software (New York, USA).
Kong, J. M., Chia, L. S., Goh, N. K., Chia, T. F. & Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 64 , 923–933 (2003).
Article CAS PubMed Google Scholar
Pojer, E., Mattivi, F., Johnson, D. & Stockley, C. S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 12 , 483–508 (2013).
Choo, W. S. Fruit pigment changes during ripening. In: Encyclopedia of food chemistry , (eds. Melton, L., Shahidi, F. & Varelis, P.) 117–123 (Elsevier, 2018).
Zhang, Y., Seeram, N. P., Lee, R., Feng, L. & Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 56 , 670–675 (2008).
Lacombe, A., Wu, V. C. H., Tyler, S. & Edwards, K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int. J. Food Microbiol. 139 , 102–107 (2010).
Ziberna, L. et al. Acute cardioprotective and cardiotoxic effects of bilberry anthocyanins in ischemia-reperfusion injury: Beyond concentration-dependent antioxidant activity. Cardiovasc. Toxicol. 10 , 283–294 (2010).
Mukherjee, P. K., Kumar, V., Kumar, N. S. & Heinrich, M. The Ayurvedic medicine Clitoria ternatea -From traditional use to scientific assessment. J. Ethnopharmacol. 120 , 291–301 (2008).
Article PubMed Google Scholar
Chusak, C., Henry, C. J., Chantarasinlapin, P., Techasukthavorn, V. & Adisakwattana, S. Influence of Clitoria ternatea flower extract on the in vitro enzymatic digestibility of starch and its application in bread. Foods 7 , 102 (2018).
Article PubMed Central CAS Google Scholar
Lijon, M. B., Meghla, N. S., Jahedi, E., Rahman, M. A. & Hossain, I. Phytochemistry and pharmacological activities of Clitoria ternatea . Int. J. Nat. Soc. Sci. 4 , 1–10 (2017).
Google Scholar
Kazuma, K., Noda, N. & Suzuki, M. Flavonoid composition related to petal color in different lines of Clitoria ternatea . Phytochemistry 64 , 1133–1139 (2003).
Vidana Gamage, G. C., Lim, Y. Y. & Choo, W. S. Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. J. Food Sci. Technol. 59 , 831–845 (2022).
Kamkaen, N. & Wilkinson, J. M. The antioxidant activity of Clitoria ternatea flower petal extracts and eye gel. Phytother. Res. 23 , 1624–1625 (2009).
Pahune, B., Niranjane, K., Danao, K., Bodhe, M. & Rokade, V. Anti-microbial activity of Clitoria ternatea L flower extract and use as a natural indicator in acid base titration. J. Nat. Prod. Plant Resour. 3 , 48–51 (2013).
Rajamanickam, M., Kalaivanan, P. & Sivagnanam, I. Evaluation of anti-oxidant and anti-diabetic activity of flower extract of Clitoria ternatea L. J. Appl. Pharm. Sci. 5 , 131–138 (2015).
Article CAS Google Scholar
Yang, Y., Yuan, X., Xu, Y. & Yu, Z. Purification of anthocyanins from extracts of red raspberry using macroporous resin. Int. J. Food Prop. 18 , 1046–1058 (2015).
Shoji, T., Yanagida, A. & Kanda, T. Gel permeation chromatography of anthocyanin pigments from rose cider and red wine. J. Agric. Food Chem. 47 , 2885–2890 (1999).
Lin, X. et al. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 14 , e0216711 (2019).
Article CAS PubMed PubMed Central Google Scholar
Heffernan, S. et al. Blue whiting protein hydrolysates exhibit antioxidant and immunomodulatory activities in stimulated murine RAW264.7 cells. Appl. Sci. 11 , 9762 (2021).
Article Google Scholar
Vidana Gamage, G. C., Lim, Y. Y. & Choo, W. S. Anthocyanins from Clitoria ternatea flower: Biosynthesis, extraction, stability, antioxidant activity, and applications. Front. Plant Sci. 12 , 792303 (2021).
Article PubMed PubMed Central Google Scholar
Jeyaraj, E. J., Lim, Y. Y. & Choo, W. S. Effect of organic solvents and water extraction on the phytochemical profile and antioxidant activity of Clitoria ternatea flowers. ACS Food Sci. Technol. 1 , 1567–1577 (2021).
Nair, V., Bang, W. Y., Schreckinger, E., Andarwulan, N. & Cisneros-Zevallos, L. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea ( Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 63 , 6355–6365 (2015).
Vuong, T. T., Srivichai, S. & Hongsprabhas, P. Effect of sugar and CaCl 2 concentration on fluorescence quenching characteristics of whey proteins by delphinidin derivatives from butterfly pea flower. Agric. Nat. Resour. 55 , 569–578 (2021).
Escher, G. B., Wen, M., Zhang, L., Rosso, N. D. & Granato, D. Phenolic composition by UHPLC-Q-TOF-MS/MS and stability of anthocyanins from Clitoria ternatea L (butterfly pea) blue petals. Food Chem. 331 , 127341 (2020).
Tenore, G. C., Novellino, E. & Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya ( Hylocereus polyrhizus ) extracts. J. Funct. Foods 4 , 129–136 (2012).
Terahara, N. et al. Five new anthocyanins, ternatins A3, B4, B3, B2, and D2, from Clitoria ternatea flowers. J. Nat. Prod. 59 , 139–144 (1996).
Terahara, N. et al. Eight new anthocyanins, ternatins C1–C5 and D3 and preternatins A3 and C4 from young Clitoria ternatea flowers. J. Nat. Prod. 61 , 1361–1367 (1998).
Bhalke, R. D. & Anarthe, S. J. Antinociceptive and antioxidant activity of various parts of Clitoria ternatea (Fabaceae). J. Pharm. Res. 8 , 30–34 (2009).
Du, Q. Z., Zheng, J. F. & Xu, Y. Composition of anthocyanins in mulberry and their antioxidant activity. J. Food Compos. Anal. 21 , 390–395 (2008).
Elisia, I., Hu, C., Popovich, D. G. & Kitts, D. D. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chem. 101 , 1052–1058 (2007).
Jeyaraj, E. J., Lim, Y. Y. & Choo, W. S. Extraction methods of butterfly pea ( Clitoria ternatea ) flower and biological activities of its phytochemicals. J. Food Sci. Technol. 58 , 2054–2067 (2020).
Article PubMed PubMed Central CAS Google Scholar
Gengatharan, A., Dykes, G. A. & Choo, W. S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 64 , 645–649 (2015).
Wolfe, K. L. & Liu, R. H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 55 , 8896–8907 (2007).
López-Alarcón, C. & Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta. 763 , 1–10 (2013).
Article PubMed CAS Google Scholar
Terahara, N., Saito, N., Honda, T., Toki, K. & Osajima, Y. Acylated anthocyanins of Clitoria ternatea flowers and their acyl moieties. Phytochemistry 29 , 949–953 (1990).
Prochiantz, A. Getting hydrophilic compounds into cells: Lessons from homeopeptides. Curr. Opin. Neurobiol. 6 , 629–634 (1996).
Zhao, C. L. et al. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 214 , 119–128 (2017).
Ma, Z. et al. Establishment and validation of an in vitro screening method for traditional Chinese medicine-induced nephrotoxicity. Evid. Based Complement. Alternat. Med. 2018 , 2461915 (2018).
López-García, J., Lehocký, M., Humpolíček, P. & Sáha, P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 5 , 43–57 (2014).
Shivaprakash, P., Balaji, K. S., Chandrashekara, K. T., Rangappa, K. S. & Jayarama, S. Induction of apoptosis in MCF-7 cells by methanolic extract of Clitoria ternatea L. Int J. Appl. Biol. Pharm. 6 , 80–86 (2015).
CAS Google Scholar
Kumar, B. S. & Bhat, K. I. In-vitro cytotoxic activity studies of Clitoria ternatea linn flower extracts. Int. J. Pharma. Sci. Rev. Res. 6 , 120–121 (2011).
Neda, G. D., Rabeta, M. S. & Ong, M. T. Chemical composition and anti-proliferative properties of flowers of Clitoria ternatea . Int. Food Res. J. 20 , 1229–1234 (2013).
Reddivari, L., Vanamala, J., Chintharlapalli, S., Safe, S. H. & Miller, J. C. Jr. Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis 28 , 2227–2235 (2007).
Matsuo, M., Sasaki, N., Saga, K. & Kaneko, T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 28 , 253–259 (2005).
Srichaikul, B. Ultrasonication extraction, bioactivity, antioxidant activity, total flavonoid, total phenolic and antioxidant of Clitoria ternatea linn flower extract for anti-aging drinks. Pharmacogn. Mag. 14 , 322 (2018).
Yong, Y. Y., Dykes, G., Lee, S. M. & Choo, W. S. Comparative study of betacyanin profile and antimicrobial activity of red pitahaya ( Hylocereus polyrhizus ) and red spinach ( Amaranthus dubius ). Plant Foods Hum. Nutr. 72 , 41–47 (2017).
Yong, Y. Y., Dykes, G., Lee, S. M. & Choo, W. S. Effect of refrigerated storage on betacyanin composition, antibacterial activity of red pitahaya (Hylocereus polyrhizus) and cytotoxicity evaluation of betacyanin rich extract on normal human cell lines. LWT Food Sci. Technol. 91 , 491–497 (2018).
Uma, B., Prabhakar, K. & Rajendran, S. Phytochemical analysis and antimicrobial activity of Clitoria ternatea Linn against extended spectrum beta lactamase producing enteric and urinary pathogens. Asian J. Pharm. Clin. Res. 2 , 94–96 (2009).
Leong, C. R. et al. Anthocyanins from Clitoria ternatea attenuate food-borne Penicillium expansum and its potential application as food biopreservative. Nat. Prod. Sci. 23 , 125–131 (2017).
Jeyaraj, E. J., Nathan, S., Lim, Y. Y. & Choo, W. S. Antibiofilm properties of Clitoria ternatea flower anthocyanin-rich fraction towards Pseudomonas aeruginosa . Access Microbiol. 4 , 000343 (2022).
Jabeur, I. et al. Hibiscus sabdariffa L as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 100 , 717–723 (2017).
Cerezo, A. B. et al. Anthocyanins in blueberries grown in hot climate exert strong antioxidant activity and may be effective against urinary tract bacteria. Antioxidants 9 , 478 (2020).
Article CAS PubMed Central Google Scholar
Salaheen, S., Peng, M., Joo, J., Teramoto, H. & Biswas, D. Eradication and sensitization of methicillin resistant Staphylococcus aureus to methicillin with bioactive extracts of berry pomace. Front. Microbiol. 8 , 253 (2017).
Sun, X. H. et al. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 94 , 155–161 (2018).
Breijyeh, Z., Jubeh, B. & Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 25 , 1340 (2020).
Ikeda, et al. Structural similarity among Escherichia coli FtsW and RodA proteins and Bacillus subtilis SpoVE protein, which function in cell division, cell elongation, and spore formation, respectively. J. Bacteriol 171 , 6375–6378 (1989).
Abhayawardhane, Y. & Stewart, G. C. Bacillus subtilis possesses a second determinant with extensive sequence similarity to the Escherichia coli mreB morphogene. J. Bacteriol. 177 , 765–773 (1995).
Chang, Y. J., Pong, L. Y., Hassan, S. S. & Choo, W. S. Antiviral activity of betacyanins from red pitahaya ( Hylocereus polyrhizus ) and red spinach ( Amaranthus dubius ) against dengue virus type 2 (GenBank accession no. MH488959). Access Microbiol. 2 (2020).
Chong, K. L. & Lim, Y. Y. Effects of drying on the antioxidant properties of herbal tea from selected vitex species. J. Food Qual. 35 , 51–59 (2012).
Lee, J., Durst, R. W., Wrolstad, R. E. & Collaborators. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J. AOAC Int. 88 , 1269–1278 (2005).
Sylvester, P. W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol. Biol. 716 , 157–168 (2011).
Loh, Z. H. & Lim, Y. Y. Drying effects on antioxidant activity, enzyme activity, and phytochemicals of avocado ( Persea americana ) leaves. J. Food Process Preserv. 42 , e13667 (2018).
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th edn. CLSI document M07-A9, Wayne, PA (2012).
Download references
Acknowledgements
We gratefully acknowledge the School of Science, Monash University Malaysia for funding this work.
Author information
Authors and affiliations.
School of Science, Monash University Malaysia, Jalan Lagoon Selatan, 47500, Bandar Sunway, Selangor, Malaysia
Ethel Jeyaseela Jeyaraj, Yau Yan Lim & Wee Sim Choo
You can also search for this author in PubMed Google Scholar
Contributions
E.J.J. wrote the manuscript and performed the experiments. Y.Y.L. and W.S.C. supported the experiments and preparation of the manuscript. W.S.C. acted as the corresponding author, supervising the overall research and the manuscript preparation.
Corresponding author
Correspondence to Wee Sim Choo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jeyaraj, E.J., Lim, Y.Y. & Choo, W.S. Antioxidant, cytotoxic, and antibacterial activities of Clitoria ternatea flower extracts and anthocyanin-rich fraction. Sci Rep 12 , 14890 (2022). https://doi.org/10.1038/s41598-022-19146-z
Download citation
Received : 17 December 2021
Accepted : 24 August 2022
Published : 01 September 2022
DOI : https://doi.org/10.1038/s41598-022-19146-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Optimization of biomass-to-water ratio and glycerol content to develop antioxidant- enriched bioplastics from whole seaweed biomass of kappaphycus sp..
- Eunice Lua Hanry
- Noumie Surugau
Journal of Applied Phycology (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
REVIEW article
Anthocyanins from clitoria ternatea flower: biosynthesis, extraction, stability, antioxidant activity, and applications.

- School of Science, Monash University Malaysia, Subang Jaya, Malaysia
Clitoria ternatea plant is commonly grown as an ornamental plant and possesses great medicinal value. Its flower is edible and also known as blue pea or butterfly pea flower. The unique feature of anthocyanins present in blue pea flowers is the high abundance of polyacylated anthocyanins known as ternatins. Ternatins are polyacylated derivatives of delphinidin 3,3′,5′-triglucoside. This review covers the biosynthesis, extraction, stability, antioxidant activity, and applications of anthocyanins from Clitoria ternatea flower. Hot water extraction of dried or fresh petals of blue pea flower could be employed successfully to extract anthocyanins from blue pea flower for food application. Blue pea flower anthocyanins showed good thermal and storage stability, but less photostability. Blue pea flower anthocyanins also showed an intense blue colour in acidic pH between pH 3.2 to pH 5.2. Blue pea flower anthocyanin extracts demonstrate significant in vitro and cellular antioxidant activities. Blue pea flower anthocyanins could be used as a blue food colourant in acidic and neutral foods. The incorporation of blue pea flower anthocyanins in food increased the functional properties of food such as antioxidant and antimicrobial properties. Blue pea flower anthocyanins have also been used in intelligent packaging. A comparison of blue pea flower anthocyanins with two other natural blue colouring agents used in the food industry, spirulina or phycocyanin and genipin-derived pigments is also covered. Anthocyanins from blue pea flowers are promising natural blue food colouring agent.
– Blue pea flower contains high amount of blue colour anthocyanins.
– Blue pea flower contains polyacylated anthocyanins called ternatins.
– Blue pea anthocyanins demonstrate good thermal and storage stability.
– Anthocyanins from blue pea flower is a good alternative to spirulina and genipin.
Introduction
Food colourants play an important role in food industry altering or conferring colours to food to increase the customer attractiveness and sensory acceptability ( Lin et al., 2018 ). Food colourants are classified as artificial and natural, based on their origin. Artificial food colours are chemicals which originate from coal tar derivatives, and most of them contain an azo group ( Dilrukshi et al., 2019 ). Considering artificial blue colours, Brilliant Blue FCF (E133, FD&C Blue No. 1) and Indigo Carmine/Indigotine (E132, FD&C blue No. 2) are approved as food colours in the European Union and the United States. Patent Blue V (E131) is authorised as a food additive in the European Union (Directive 94/36/EC; US Food and Drug Administration ( FDA, 2015 ). Artificial blue colourants are used in various types of food. A study done in the Iranian market found that Brilliant blue colourant was commonly found in edible ices, jelly, fruit drink powder, chocolate/ice cream powder, soft drink, syrup, and candy ( Asadnejad et al., 2018 ). Natural food colours consist of pigments such as anthocyanins, carotenoids, chlorophyll etc. that are extracted from mainly plants and micro-organisms ( Sen et al., 2019 ). The demand for food products with natural colouring agents has increased since consumption of synthetic colourings are believed to cause allergies, food intolerance, hyperactivity, irritability and sleep disorders in children ( Feketea and Tsabouri, 2017 ). Pigments giving red, orange and yellow hues are widely available but only a few sources are available giving blue colour. Anthocyanins are present in fruits giving rise to blue colour ( Choo, 2019 ). Apart from anthocyanins, commonly used blue colourants in the food industry are spirulina/phycocyanin (a protein extracted from cyanobacteria Spirulina platensis , from eukaryote algae such as Rhodophytes and Cryptophytes and Galdieria sulphuraria , a unicellular rhodophyte) and the blue colour pigments produced by the reaction between primary amines and genipin (a colourless iridoid from monoterpenes class extracted from fruits of both Genipa americana and Gardenia jasminoides Ellis) ( Landim Neves et al., 2021 ). Both spirulina and the blue pigment derived from genipin possess both advantages and disadvantages, that are unique to them. For example, phycocyanin is stable in the presence of citric acid, sugar, and soluble in warm or cold water but being a protein, it tends to get denatured in elevated temperature, low pH and is highly unstable under light ( Gustiningtyas et al., 2020 ). Genipin-derived pigments show good thermal, photostability but become unstable in the presence of ascorbic acid. From an industrial perspective, the extraction procedures to obtain blue pigments from both spirulina and genipin are complicated. The extraction of spirulina involves the chemical breakdown of cell walls of respective organisms and the production of genipin-derived pigments involves a synthesis step from its precursor ( Buchweitz, 2016 ). Therefore, new sources of natural blue colour are needed.
Anthocyanins are the largest group of water-soluble pigments belonging to flavonoids, a subclass of the polyphenol family, contributing to the attractive orange, red, purple, violet, and blue colours of fruits, vegetables, and flowers ( Jing and Giusti, 2007 ). More than 700 anthocyanins have been identified in nature and they play a vital role in pollination and protecting plant cells from ultra-violet (UV) radiation ( Salehi et al., 2020 ). Anthocyanins are glycosides of anthocyanidins. Pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin are the most common anthocyanidins in the plant kingdom ( Choo, 2019 ). Colour of the anthocyanins depend on the pH of the solution because the structure of the anthocyanins alters depending on the pH of the surrounding medium ( Khoo et al., 2017 ). Anthocyanins have been used in traditional medicine and for colouring food since ancient times. The therapeutic effects of anthocyanins are mainly attributed to their antioxidant activities ( Khoo et al., 2017 ). The structure of anthocyanins allows anthocyanins to display direct antioxidant activity toward radicals in two mechanisms named: hydrogen atom transfer (HAT) and single electron transfer (SET). In both mechanisms, the anthocyanin becomes a free radical itself, but it is more stable and the oxidative damage from the initial free radical is prevented ( Garcia and Blesso, 2021 ). Anthocyanins have demonstrated several other health benefits such as antibacterial, antiproliferative, hypoglycaemic etc. ( Yoon et al., 2018 ; Li et al., 2019 ; Yue et al., 2019 ). Therefore, the application of anthocyanins as food colourants should be encouraged to deliver these health benefits to consumers. Generally, anthocyanins are well known for their unstable nature since the stability of anthocyanins is influenced by factors such as chemical structure, pH, temperature, light, presence of oxygen, solvent, the presence of co-pigments, metal ions, and enzymes ( Vidana Gamage et al., 2021a ). Blue colour anthocyanins are generally found in blue colour flowers and fruits. Clitoria ternatea flower is one anthocyanin source containing stable blue colour polyacylated anthocyanins ( Abidin et al., 2019 ; Thuy et al., 2021 ). The presence of polyacylated anthocyanins, metal ions, other phenolic compounds and the resulting co-pigmentation effect may assist to form more stable and intense blue colours ( Yoshida et al., 2009 ).
Clitoria ternatea L. commonly known as butterfly pea or blue pea is a perennial leguminous herb belonging to family Fabaceae having several beneficial agricultural and medical applications, such as fodder, nitrogen-fixing crop, an eco-friendly insecticide ( Oguis et al., 2019 ), food colouring ( Pham et al., 2019 ), and in traditional medicine for disorders such as anasarca and ascites ( Lakshan et al., 2019 ). It is commonly grown as an ornamental plant and is also used for revegetation ( Kosai et al., 2015 ). Blue pea plants are distributed in several countries all over the world such as Thailand, Malaysia, Kenya, Australia, the United States, Sri Lanka, Brazil, Cuba, Sudan etc. ( Havananda and Luengwilai, 2019 ). Blue pea flower is being eaten as vegetables in Southeast Asia ( Leong et al., 2017 ) and blue pea flower extract has been used in desserts and beverages in Southeast Asian countries such as Malaysia and Thailand ( Pasukamonset et al., 2017 ). Polyacylated derivatives of delphinidin 3,3′,5′-triglucoside, named “ternatins” are the major anthocyanins present in blue pea flower ( Terahara et al., 1990 ; Vidana Gamage et al., 2021b ). All ternatins carry the basic structure of delphinidin-3, 3′, 5′-triglucoside. A series of 15 ternatins A1-A3, B1-B4, C1-C5 and D1-D3 have been discovered so far ( Nair et al., 2015 ; Jeyaraj et al., 2020 ). This review focus on the biosynthesis, extraction, stability, antioxidant activity and applications of anthocyanins from blue pea flower. Specifically, the potential of using blue pea flower anthocyanins as a natural blue food colouring agent is also covered.
Blue Pea Flower Anthocyanins
Clitoria ternatea L./blue pea flower ( Figure 1 ) is a rich source of polyacylated anthocyanins and their higher stability compared with non-acylated anthocyanins provide the advantage to be used as a natural food colouring agent ( Buchweitz et al., 2012 ; Marpaung et al., 2019 ). Like all anthocyanins, the colour of blue pea flower anthocyanin extract also changes with pH. At pH lower than 3.2 the red colour exists, from pH 3.2 to 5.2 the colour changes from violet to blue, from pH 5.2 until pH 8.2 light blue colour exists and from pH 8.2 to pH 10.2 the colour changes from light blue to dark green colour ( Escher et al., 2020a ). This colour change could be explained by the structural alteration occurring in anthocyanin molecules along with the change in H + and OH – concentration in the medium. The red colour is attributed to the presence of flavylium ion, blue colour to the presence of the neutral quinoidal base and the green colour to ionic chalcone ( Liu et al., 2014 ). In non-acylated anthocyanins, flavylium ion transforms to colourless carbinol pseudo base when pH increases. But in blue pea flower anthocyanins, the presence of acyl groups prevents the hydrolysis of flavylium ion to less stable carbinol pseudo base form and instead form the blue colour quinoidal that possess less sensitivity to pH changes in mildly acidic or neutral medium ( Bridle and Timberlake, 1997 ). Therefore, blue pea flower anthocyanins could be used as a blue colouring agent in acidic and neutral food systems. Figure 2 shows the structural alteration of delphinidin-3-glucoside with increasing pH and formation of blue colour in blue pea flower anthocyanins.

Figure 1. Blue pea flower ( Clitoria ternatea ).
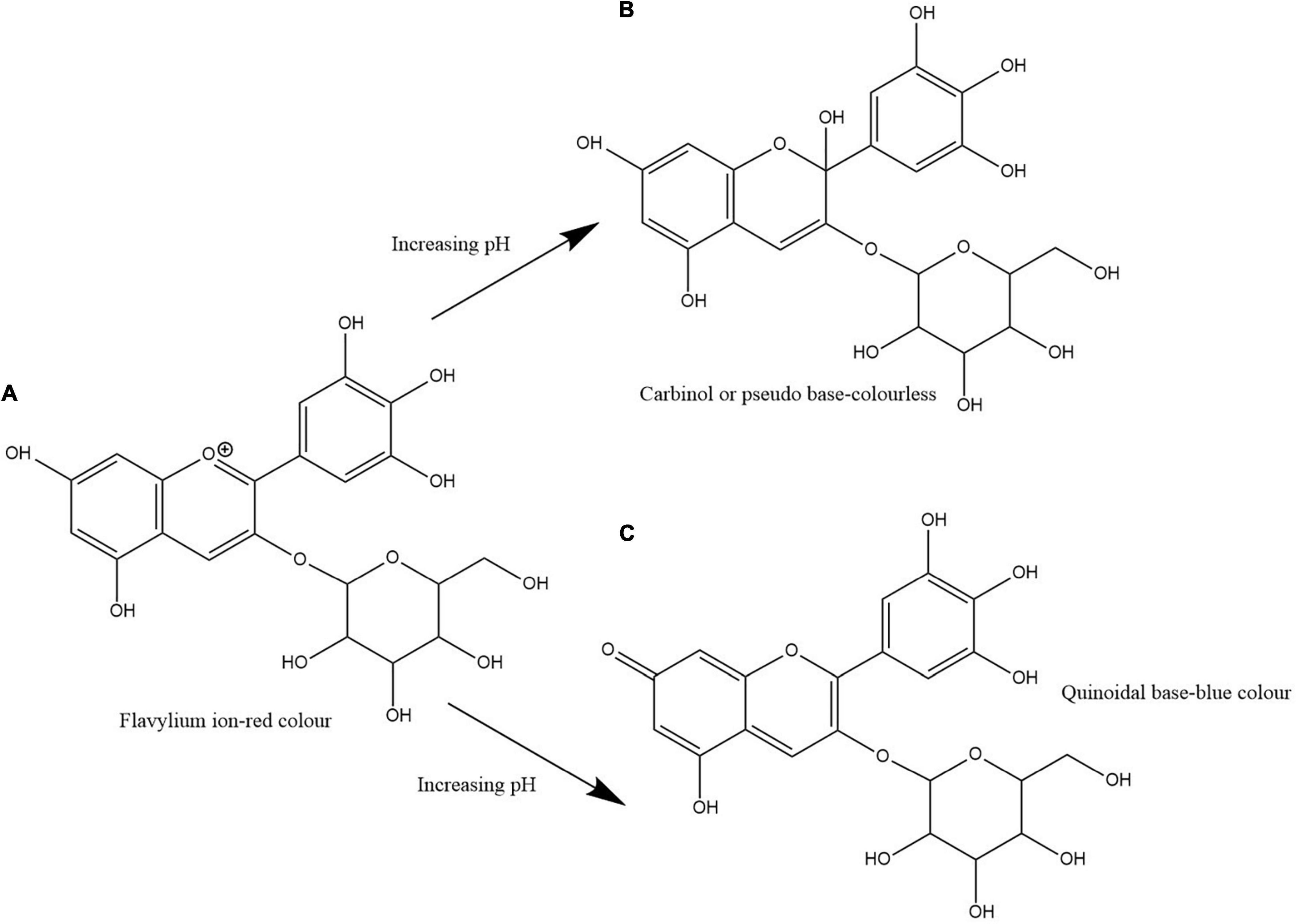
Figure 2. Structural change of delphinidin-3-glucoside with increasing pH. Path (A,B) shows the transformation of flavylium ion to carbinol or pseudo base. Path (A,C) shows the transformation of flavylium ion to quinoidal base. Path (A,C) shows the structural alteration responsible for the blue colour formation in blue pea flower anthocyanins.
Biosynthesis of Ternatins
The anthocyanin biosynthetic pathway is an extension of the general flavonoid pathway ( Tanaka et al., 2009 ). Anthocyanin biosynthesis pathway is an elucidated metabolic pathway involving enzymes such as chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H), flavonoid 3′,5′-hydroxylase (F3′5′H), dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS), glycosyltransferase (GT), and acyltransferase (AT). Anthocyanins are synthesised in the cytoplasm of the cell and then transported to the vacuole. The vacuolar transportation of anthocyanins takes place in several pathways that include endoplasmic reticulum-derived vesicles and a tonoplast-bound glutathione S-transferase-like transporter ( Collings, 2019 ). However, acylation of anthocyanins, catalysed by acyltransferases (ATs) and the final modification of anthocyanins occurs after being transported to the vacuole ( Lu et al., 2021 ).
Figure 3 shows the proposed ternatin synthesis pathway drawn based on the studies done by Kogawa et al. (2007a) and Tanaka et al. (2009) . In the biosynthesis of ternatins, first delphinidin-3- O -β-glucoside is formed and then it is modified by further glucosylation and acylation. For the synthesis of delphinidin-3- O -β-glucoside, 4-coumaroyl-CoA and malonyl-CoA act as precursors. Synthesis of the naringenin chalcone from the two precursors is mediated by CHS. CHS is the initial key enzyme of flavonoid biosynthesis. Then, naringenin chalcone is isomerised by CHI to naringenin. Naringenin is then converted to dihydrokaempferol in the presence F3H. Dihydrokaempferol is then converted to dihydromyricetin by F3′5′H. Both F3′H and F3′5′H are the enzymes responsible for the diversification of anthocyanins by determining their B-ring hydroxylation pattern and consequently the colour of the anthocyanins ( Liu et al., 2018 ). Therefore, F3′5′H directly contribute to the blue colour anthocyanins in blue pea flower, since increased hydroxylation of the B-ring shifts the anthocyanin colour toward blue ( Togami et al., 2006 ). Next, dihydromyricetin is converted into colourless leucodelphinidin mediated by DFR and subsequently to delphinidin by ANS. According to Kogawa et al. (2007a) , a glucosyl group is added to delphinidin by anthocyanin 3- O -glucosyltransferase (3GT) to form delphinidin 3- O -β-glucoside. Kogawa et al. (2007a) stated that other glucosyl groups are added to the B-ring of delphinidin 3- O -β-glucoside, only after malonylation of delphinidin 3- O -β-glucoside. Accordingly, delphinidin 3- O -β-glucoside is malonylated in the presence of anthocyanidin 3- O -glucoside 6″- O -malonyltransferase (A6″MaT). Then, two glucose molecules are added to delphinidin 3- O -(6″- O -malonyl)-β-glucoside, first to 3′ position followed by 5′ position ( Kazuma et al., 2004 ). This glycosylation is mediated by anthocyanin 3′,5′- O -glucosyltransferase (UA3′5′GT) in two subsequent steps ( Kogawa et al., 2007b ). The molecule is now referred to as delphinidin 3- O -(6″- O -malonyl)-β-glucoside-3′,5′-di- O -β-glucoside and can be called as ternatin C5. Ternatin C5 is the simplest ternatin. Other 14 ternatins are synthesised by adding acyl and glucosyl groups to ternatin C5 in the presence of acyltransferases (ATs) and glucosyltransferases (GTs). Acyltransferases (AT) also plays a major role resulting in the blue colour and the stability of ternatins, because polyacylation of ternatins with p-coumaroyl groups results in a shift of colour of anthocyanins to bluish region due to intramolecular co-pigmentation among acyl moieties and between acyl moieties and anthocyanin chromophore ( Honda and Saito, 2002 ). Furthermore, polyacylation at the 3′ position of anthocyanins results in stable blue colouration ( Lu et al., 2021 ). This is the main reason for the high stability of ternatins because most ternatins are polyacylated at the 3′ position. Therefore, when studying the ternatin biosynthesis pathway, hydroxylation, glycosylation and acylation can be considered as the most important steps that are responsible for synthesising stable blue colour anthocyanins in blue pea petals.
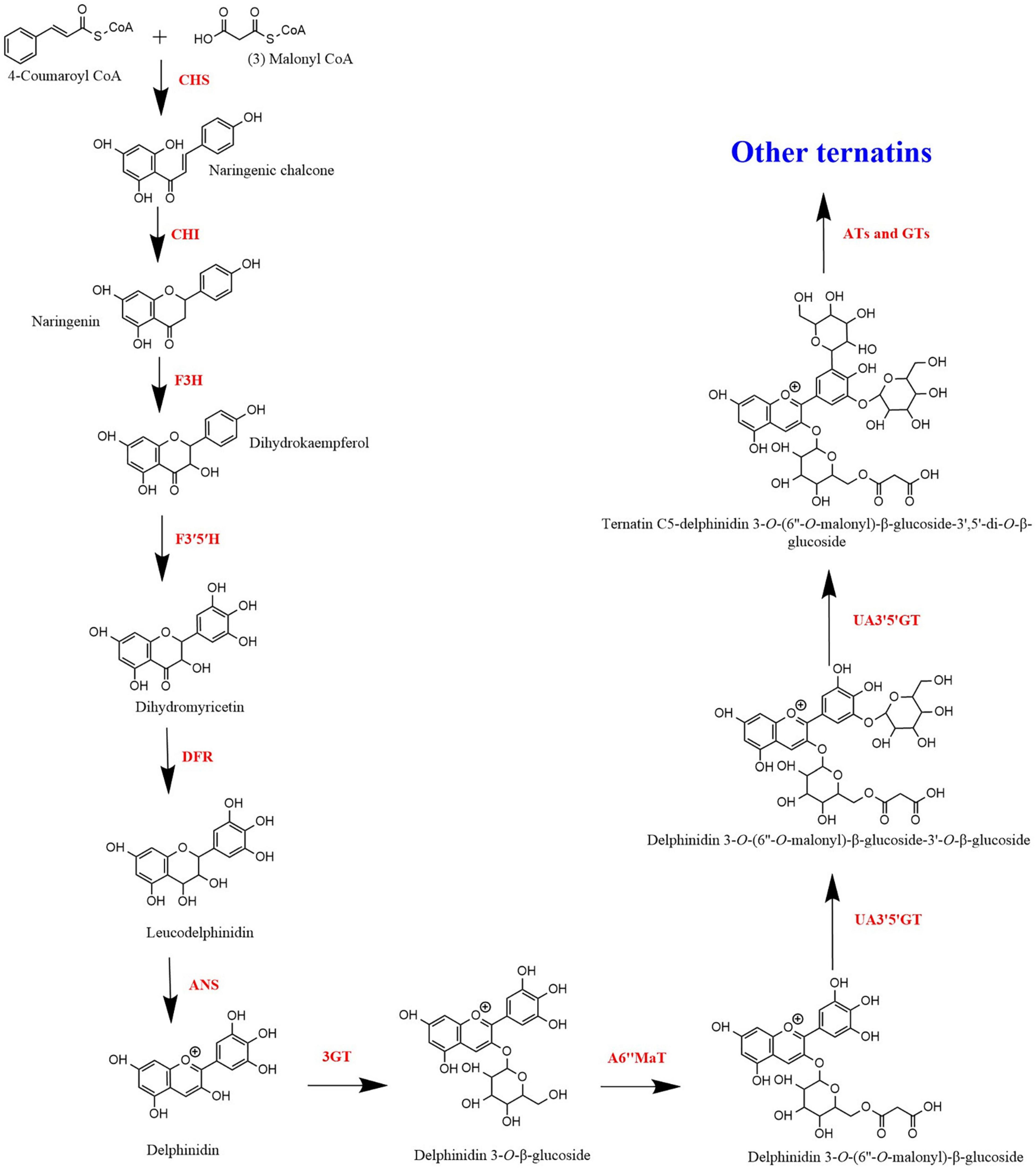
Figure 3. Biosynthesis of ternatins.
Extraction of Blue Pea Flower Anthocyanins
Extraction is the first important step in the recovery of active ingredients from plant materials ( Jeyaraj et al., 2020 ). The purpose of selecting a suitable extraction method is to obtain the maximum yield with a high concentration of target compounds. Since anthocyanins are sensitive to heat, light, acids and alkalis, selecting a suitable extraction method to get the maximum amount of anthocyanins without degradation is critical ( Chandrasekhar et al., 2012 ; Jeyaraj et al., 2020 ).
Considering conventional solvent extraction methods, type of solvent, substrate: solvent ratio, extraction temperature, extraction time and soaking time may affect the extraction yield and total anthocyanin content (TAC) of a blue pea flower anthocyanin extract ( Rocha et al., 2020 ). Selection of solvent should be done based on the application of the anthocyanin extract. Therefore, when anthocyanins from blue pea flowers are extracted for food application, the use of hazardous organic solvents should be avoided ( Khoo et al., 2017 ; Chemat et al., 2019 ). Several studies have used hydro alcoholic extraction [i.e., 37% ethanol ( Jaafar et al., 2020 ), 50% ethanol ( Pham et al., 2019 ), 50% methanol ( Shen et al., 2019 )] to extract anthocyanins from blue pea flower. However, FDA (2018) has categorised methanol as a class 2 solvent having inherent toxicity and ethanol as a class 3 solvent that should be limited by good manufacturing practices (GMP) and other quality-based requirements. Distilled water is the best solvent for extracting anthocyanins for food applications because water could be considered as a non-toxic, non-flammable, and inexpensive green solvent ( Chemat et al., 2019 ). Therefore, this review focuses only on the extraction of anthocyanins from blue pea flowers with water. Table 1 shows previous studies that carried out extraction of anthocyanins from blue pea flowers using water as the solvent.
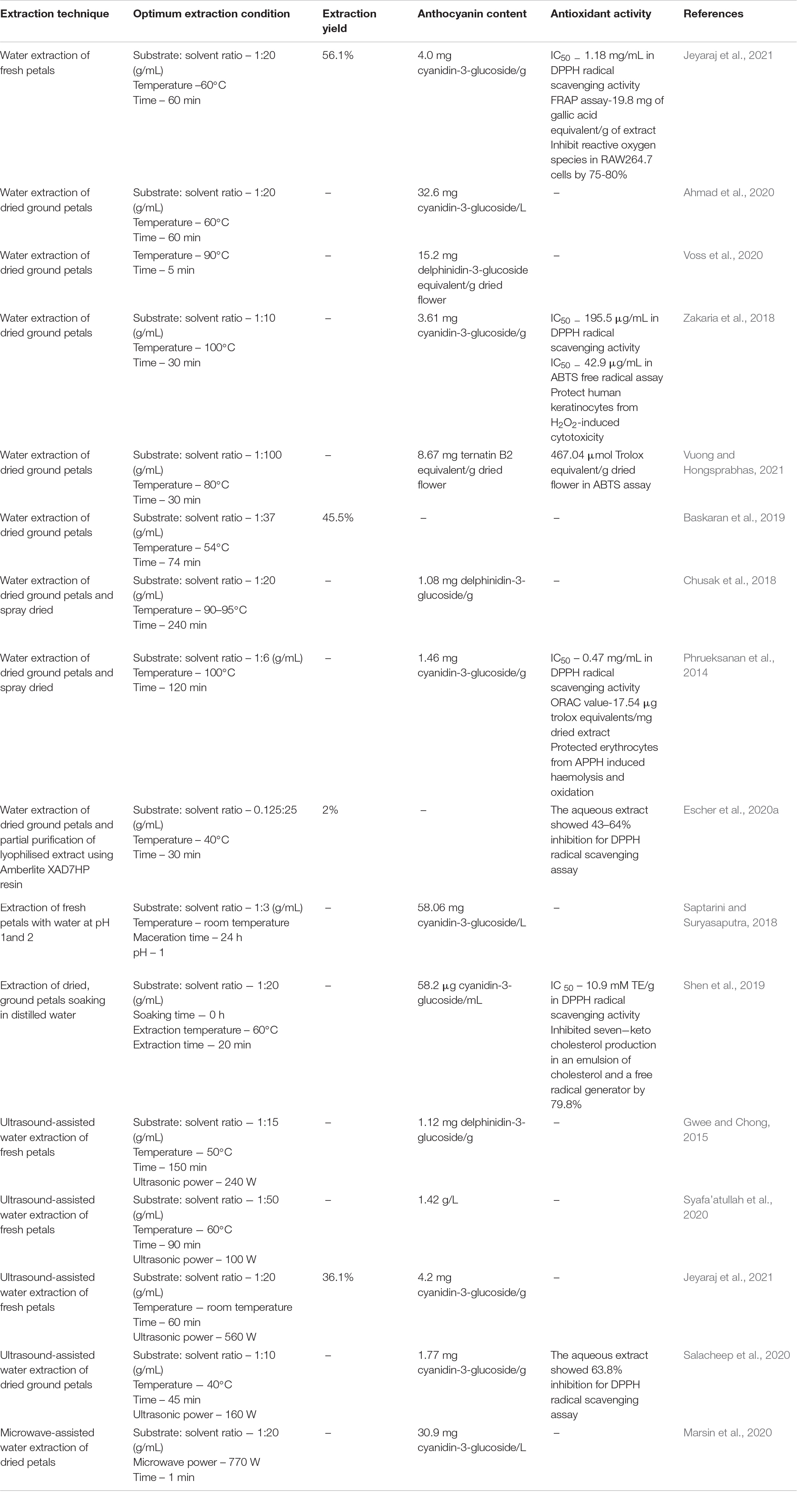
Table 1. Previous studies on extraction of anthocyanins from blue pea flower using water and the antioxidant activity of extracts.
Saptarini and Suryasaputra (2018) showed that the anthocyanin content of blue pea flower anthocyanin extract that was extracted using water at pH 1 was higher than that of pH 2. This shows that the pH of water used for extraction can affect the TAC of the blue pea flower anthocyanin extract. Kang et al. (2021) suggested that the reason for obtaining a higher extraction efficiency when using more acidic solvents is the higher stability shown by the anthocyanins in acidic medium. Some studies use a soaking step before extracting anthocyanins. However, Shen et al. (2019) showed that soaking is not necessary when extracting anthocyanins from blue pea flowers using water. Soaking significantly reduced the TAC of the blue pea flower anthocyanin extract from 58.2 to 39.9 μg cyanidin-3-glucoside/mL when the petals were soaked in water for 24 h but there was no significant difference in the TAC of the blue pea flower anthocyanin extract when soaking was carried out for 6 and 12 h. The reason for the reduction of TAC due to soaking could be attributed to the increase of hydrolysis of anthocyanins when more water molecules are available in the matrix ( Matsufuji et al., 2007 ; Marpaung et al., 2017 ). Therefore, extraction of anthocyanins from blue pea flower with water could be done without a soaking step that gives an advantage in time factor during the extraction process.
Considering substrate: solvent ratio, 1:20 (g/mL) was reported by Chusak et al. (2018) and Ahmad et al. (2020) as the best ratio for extraction of anthocyanins from blue pea flower ( Table 1 ). The volume of water used for extraction is an important factor because less solvent would not extract a sufficient amount of anthocyanins from blue pea petals and excess water requires more energy for evaporation. Temperature around 50–60°C and extraction time around 20–60 min could be considered as the most suitable extraction temperature and time for extraction of anthocyanins from blue pea flower ( Table 1 ) because higher temperature and long extraction time may result in deterioration of anthocyanins ( Loypimai et al., 2016 ; Aprodu et al., 2020 ). High temperature and long extraction durations are disadvantageous in terms of energy consumption. Extraction conditions also affect the antioxidant activity of the blue pea flower anthocyanin extract. Shen et al. (2019) showed that the antioxidant activity of a blue pea flower anthocyanin extract was dependent on the solvent used for extraction and the soaking time upon extraction. However, there was no significant difference in the 2,2diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of blue pea flower anthocyanin extract that was extracted using distilled water without soaking (10.9 mM Trolox equivalents (TE)/g dry basis) and after soaking for 6 h (11.7 mM TE/g dry basis). Soaking in water up to 24 h significantly reduced the antioxidant activity of blue pea flower anthocyanin extracts (9.45 mM TE/g dry basis) extracted by distilled water ( Shen et al., 2019 ). Furthermore, the antioxidant activity of blue pea flower anthocyanin extract that was extracted with 50% methanol (12.2 mM TE/g dry basis) was not significantly different in DPPH radical scavenging activity compared to blue pea flower anthocyanin extract that was extracted using distilled water (10.9 mM TE/g dry basis) at 0 h soaking. Similarly, Jeyaraj et al. (2021) also found that there was no significant difference in the antioxidant activity of the blue pea flower anthocyanin extracts obtained from water extraction and 50% ethanol extraction measured using DPPH and ferric reducing antioxidant power (FRAP) assays. This suggests that blue pea flower anthocyanins can be extracted using distilled water and used as a natural blue colouring agent with high antioxidant activity. Therefore, when extracting anthocyanins from blue pea flowers for food applications, hot water extraction with short extraction time is preferred.
Conventional solvent extraction techniques consume more solvents, time, thermal energy and are associated with several disadvantages. To overcome the shortcomings of conventional solvent extraction and to increase extraction efficiency, several non-conventional extraction techniques were explored. Ultrasonication is one such technique. In ultrasound-assisted extraction, acoustic cavitation causes molecular movement of solvent and sample results in the breakdown of plant cell walls and membranes and facilitate their movement to surrounding solvent ( Chemat et al., 2011 ). One study employed ultrasound-assisted water extraction of blue pea flower anthocyanins and showed that ultrasound-assisted water extraction yielded 246.48% better anthocyanin extract yield compared with the conventional ethanolic extraction. According to that study, the TAC of the blue pea flower extract (1.126 mg delphinidin-3- O -glucoside equivalent/g) obtained by ultrasound-assisted water extraction was higher than that (0.325 mg delphinidin-3- O -glucoside equivalent/g) obtained by conventional ethanolic extraction ( Gwee and Chong, 2015 ). Marsin et al. (2020) applied microwave-assisted extraction to extract blue pea flower anthocyanins, but the TAC of the blue pea flower anthocyanin extract obtained from microwave-assisted extraction did not show a higher value compared to the TAC of the blue pea flower anthocyanin extract obtained from conventional hot water extraction in the study by Ahmad et al. (2020) ( Table 1 ). Therefore, the use of ultrasound-assisted extraction is promising to extract blue pea flower anthocyanins for food applications. When extracting anthocyanins from blue pea flower petals using water, other bioactive compounds that are soluble in water are also extracted together. Therefore, those compounds may also contribute to the antioxidant property of the anthocyanin extract and greater extraction efficiency could result in a higher antioxidant activity of the extract ( Jeyaraj et al., 2020 ). The blue pea flower anthocyanin extract obtained by ultrasound-assisted extraction demonstrated 47.21% more DPPH radical scavenging activity compared to the blue pea flower anthocyanin extract obtained from conventional ethanolic extraction ( Gwee and Chong, 2015 ). Further studies need to be carried on investigating the effect of other novel extraction technologies such as high-pressure processing, sub-critical water extraction etc. to extract blue pea flower anthocyanins.
Stability of Anthocyanins From Blue Pea Flower
The stability of anthocyanins is highly affected by pH, temperature, light, metal ions in media etc. thus their application in food products is limited ( Vidana Gamage et al., 2021a ). Therefore, if anthocyanins are used as food colourants, those anthocyanins should possess reasonable thermal, photo and storage stabilities. This is because during food processing the food materials undergo several heat processes such as pasteurisation or sterilisation where else during storage, food must withstand storage conditions and photo stress ( Charurungsipong et al., 2020 ). Several studies have investigated the stability of anthocyanins from blue pea flowers concerning their thermal, storage and photo stabilities.
Temperatures above 50°C cause partial or complete degradation of anthocyanins from natural sources and this results in a reduction of colour intensity ( Escher et al., 2020a ). Anthocyanins from blue pea flower demonstrate good heat stability in acidic pH. Blue pea flower anthocyanins at pH 3.6 and 5.4 showed stability at 60 and 70°C. The absorbance remained unchanged for 360 min but when the temperature was increased from 70 to 100°C, the degradation constant significantly increased. At pH 3.6 the degradation rate constant increased from 5.57 × 10 –4 to 3.41 × 10 –3 min –1 , when temperature increased from 80 to 100°C and at pH 5.4 the degradation rate constant increased from 5.33 × 10 –4 to 3.32 × 10 –3 min –1 when temperature increased from 80 to 100°C ( Escher et al., 2020a ). Another study supports the above finding that the degradation rate of blue pea flower anthocyanins increases when the temperature is above 70°C ( Lee et al., 2011 ).
One study investigated the degradation of anthocyanins from blue pea flowers at 28, 60, and 90°C. The degradation half-lives at each temperature were 8.63, 5.16, and 3.75 min, respectively. This shows how the degradation rate increased (half-life reduced) with increasing temperature. This study also showed that the addition of catechin as a co-pigment to the anthocyanin extract of blue pea flower at pH 3.5 increased the half-life of anthocyanins from blue pea flower to 8.39 at 90°C ( Charurungsipong et al., 2020 ). Catechin molecules also have a similar structure as anthocyanins where each molecule possesses two benzene rings and one dihydropyran heterocycle. A co-pigment complex is formed between catechin and ternatins by intermolecular co-pigmentation either as an interlock complex or parallel complex ( Charurungsipong et al., 2020 ). The presence of several aromatic acyl groups in ternatins may increase the chance of forming several co-pigment complexes with the available co-pigments. Generally, most food products fall into the pH range of pH 3.6 to 5.4 and the pasteurising temperature also ranges between 60 and 70°C (Low-Temperature Long Time (LTLT) – 62.5°C for 30 min and High-Temperature Short Time (HTST) – 72°C for 15 seconds) ( Ranieri et al., 2009 ). This stability makes anthocyanins from blue pea flowers suitable as a natural food colour in functional food. Another study investigated the storage stability of anthocyanins from blue pea flowers with different temperatures in terms of colour stability index (absorbance on sampling day/absorbance of day 0). This study showed that at frozen and refrigerated conditions the colour stability index of anthocyanins from blue pea flower reduced only by 0.2 points after 30 days of storage. At temperatures above 25°C, the stability of blue pea flower anthocyanins reduced significantly. Their colour stability index reduced by 0.8 points during 20 days of storage at 25°C. The storage stability of anthocyanins from blue pea flowers was significantly low when stored at temperatures above 50°C and the colour stability index reduced by 0.9 points in 15 days of storage at 50°C ( Ab Rashid et al., 2021 ). Another study showed that, when anthocyanin extract from blue pea flower was stored at 5°C, 80% of initial anthocyanins were retained after 30 days and the residual colour remained almost unchanged for about one year and the stability reduced within a week when stored between 25 and 37°C but at 45°C again the anthocyanins from blue pea flower demonstrated good stability for more than 10 days ( Lee et al., 2011 ). This could be due to the activation of enzymes that cause anthocyanin degradation since 37°C is the optimum temperature for enzyme activity ( Daniel and Danson, 2013 ). Therefore, it is recommended to use anthocyanins from blue pea flowers for food stored in cold conditions (e.g., frozen desserts, yoghurt, cool drinks) and to store food containing anthocyanins from blue pea flowers in the freezer, refrigerator or temperatures below 25°C.
Anthocyanins from blue pea flowers are less stable to photo stress compared to thermal stress ( Mahmad and Taha, 2018 ). When anthocyanins from blue pea flowers were exposed to the direct light of a white fluorescent lamp (20 W) at 32°C, the anthocyanins from blue pea flowers degraded at a higher rate compared with anthocyanins kept covered from light. Blue pea flower anthocyanins at pH 3.6 had a 34.4% retention percentage when exposed to light whereas the covered anthocyanins had a 90.1% retention percentage after equal periods. Blue pea flower anthocyanins at pH 5.4 had a 48.3% retention percentage when exposed to light whereas the covered anthocyanins had a 94.6% retention percentage after equal periods ( Escher et al., 2020a ). Anthocyanins from blue pea flower demonstrated higher photostability at pH 5.4 compared with 3.6 but in both cases, the degradation rate looks high. But microencapsulation of anthocyanins from blue pea flowers was able to increase the photostability of blue pea flower anthocyanins ( Marsin et al., 2020 ; Ab Rashid et al., 2021 ). Marsin et al. (2020) optimised the microwave-assisted encapsulation of blue pea flower anthocyanins and obtained an encapsulation efficiency of 73.24% with 40% (w/w) maltodextrin at 770 W microwave power for 7 min. Ab Rashid et al. (2021) reported an encapsulation efficiency of 87.3% using spray drying as the encapsulation technique and 20% (w/w) maltodextrin as the carrier agent. When microencapsulated anthocyanins from blue pea flower with maltodextrin and control blue pea flower anthocyanins were exposed to light at 25°C and pH 5.5, microencapsulated anthocyanins from blue pea flower showed a significantly higher colour stability index (colour stability index – 0.83) compared to the control anthocyanin extract from blue pea flower (colour stability index – 0.57) after 21 days ( Ab Rashid et al., 2021 ). Therefore, when manufacturing food with blue pea flower anthocyanins as a food colourant, it is recommended to avoid using transparent packaging material to protect anthocyanins from direct light exposure or use an encapsulation technique.
The good thermal stability up to 70°C, the storage stability at 25°C and the intense blue colour demonstrated at pH 3.6 to 5.4, makes anthocyanins from blue pea flowers, suitable to be used as a blue colour food colourant. Table 2 shows a comparison of extraction yield, thermal stability, photostability, antioxidant activity and aggregate formation in acidic beverages of natural blue colouring agents used in the food industry: phycocyanin, genipin-derived pigments and anthocyanins from blue pea flowers. The extraction yield on dry basis of blue pea flower anthocyanin extract was higher than that of phycocyanin and genipin ( Table 2 ). One possible reason for obtaining a lower extraction yield could be the presence of structures such as hard cell walls and fibre in the sources of phycocyanin and genipin that hinders the extraction ( Buchweitz, 2016 ). Considering pH stability, blue pea flower anthocyanins demonstrated higher stability in acidic pH compared to phycocyanin and genipin-derived pigments.
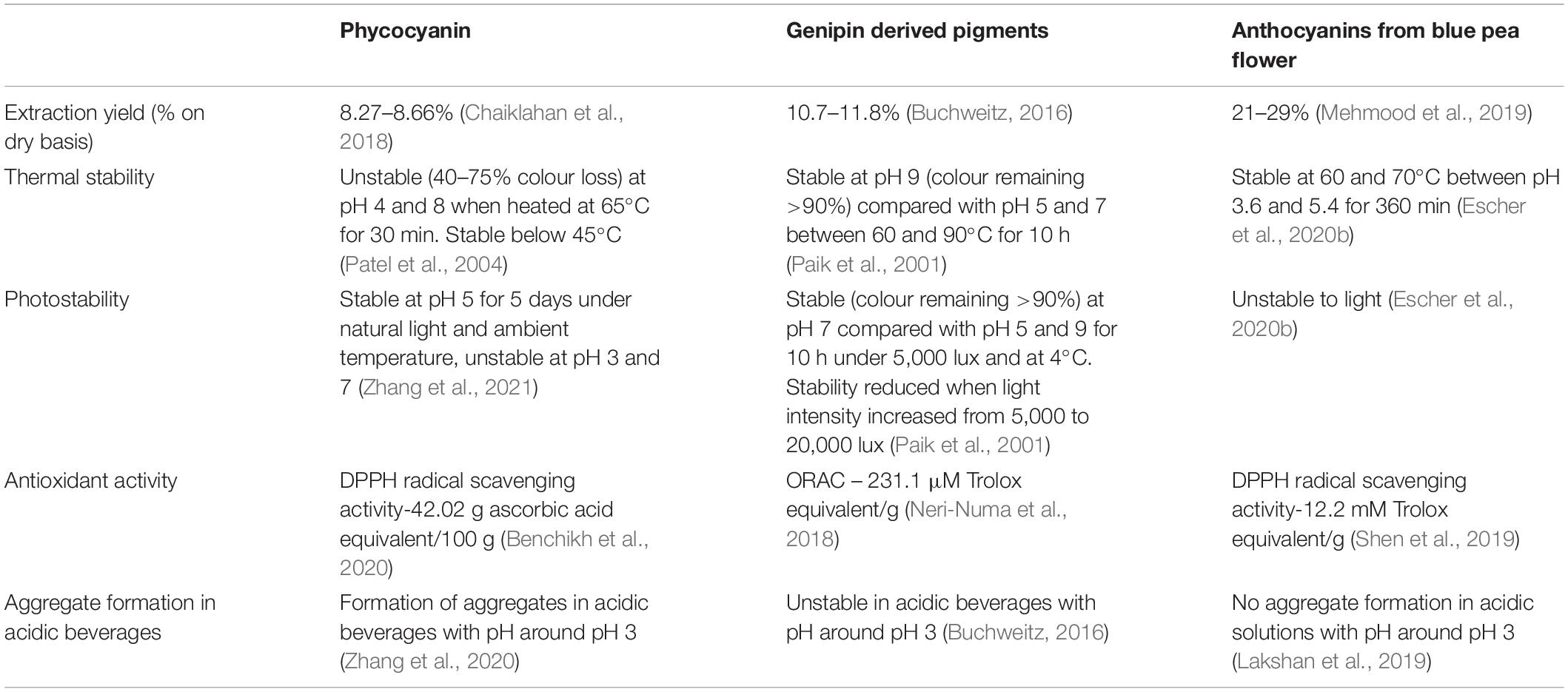
Table 2. Comparison of common blue food colourants with anthocyanins from blue pea flower.
Comparing the thermal stability of phycocyanin, genipin-derived pigments and anthocyanins of blue pea flower ( Table 2 ), phycocyanin was less stable at low-temperature long time pasteurisation conditions (at 63°C for 30 min) but Chaiklahan et al. (2012) reported that the colour loss of phycocyanin in high-temperature short-time pasteurisation (71°C for 15 s) was negligible. The thermal stability of genipin-derived pigments was higher in the alkaline medium compared with acidic and neutral media, but most of the blue coloured food products consist of an acidic pH ( Asadnejad et al., 2018 ). Contrastingly, blue pea flower anthocyanins showed stability in acidic pH under pasteurising conditions (63°C for 30 min). Considering photostability, both phycocyanin and blue pea flower anthocyanins showed unstable nature against photo stress compared to genipin-derived pigments ( Table 2 ). Therefore, special attention should be paid when using blue pea flower anthocyanins or genipin-derived pigments as a food colour to avoid photodegradation. Both phycocyanin and genipin-derived pigments were not stable in the acidic medium whereas the blue pea flower anthocyanins demonstrated high stability in acidic pH around pH 3 to 5. Products such as soft drinks, fruit drinks and jelly mainly contain artificial blue colourants belonging to this pH range ( Asadnejad et al., 2018 ). This could be considered as a major advantage of using blue pea flower anthocyanins as a blue colourant over phycocyanin and genipin-derived pigments. All three blue colourants showed antioxidant activities, but a direct comparison cannot be made as they were determined by different antioxidant assays.
Antioxidant Activity of Blue Pea Flower Anthocyanins
The antioxidant property is the ability to donate hydrogen atoms or electrons to free radicals and displace free radicals, thus preventing the damage caused by the free radicals ( Tan and Lim, 2015 ). Anthocyanins demonstrate both in vivo and in vitro antioxidant activity ( Migliorini et al., 2019 ; Vidana Gamage et al., 2021a ). It is believed that blue pea flower anthocyanins could prevent cardiovascular and neurological diseases, cancer and diabetes, due to their antioxidant capabilities ( Cazarolli et al., 2009 ; Shen et al., 2019 ). The toxicological safety of using blue pea flower extracts have been prove from some studies. An aqueous extract of blue pea flower petals showed no cytotoxicity in human fibroblast (IMR90) cells (LC 50 > 900 μg/mL) and showed a protective effect in human erythrocytes and inhibited the oxidation of pBR322 plasmid DNA ( Mehmood et al., 2019 ; Escher et al., 2020b ). A water extract of blue pea flower was non-toxic up to 625 μg/mL on RAW264.7 cells ( Jeyaraj et al., 2021 ). The in vitro antioxidant properties displayed by water extracts of blue pea flower anthocyanins are shown in Table 1 .
In one study, it was found that blue pea flower anthocyanin extract demonstrated significant antioxidant activity against DPPH and peroxyl radicals. Using DPPH assay, the IC 50 (concentration of the antioxidants needed to decrease the initial free radical concentration by 50%) of the blue pea flower anthocyanin extract (0.47 mg/mL) was significantly higher compared to that of ascorbic acid (0.002 mg/mL) ( Phrueksanan et al., 2014 ). Similarly, in the study done by Zakaria et al. (2018) , the anthocyanin extract from blue pea flower showed significant scavenging activity against DPPH and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging assays ( Table 1 ) and the IC 50 values of the Trolox standard for both DPPH (IC 50 – 3.32 μg/mL) and ABTS assays (IC 50 – 6.51 μg/mL) were significantly lower compared to blue pea flower anthocyanin extract. This indicates that ascorbic acid and Trolox possess higher antioxidant activity compared with the blue pea flower anthocyanin extract.
Shen et al. (2019) also studied how the antioxidant property of blue pea flower anthocyanins prevent lipid oxidation. Lipid oxidation occurs when free radicals are available in the surrounding medium. A dose of 6 mg/mL blue pea flower anthocyanins extracted by distilled water was able to inhibit 7-keto cholesterol production in an emulsion of cholesterol and a free radical generator by 79.8% after 48 h of treatment ( Shen et al., 2019 ). 7-keto cholesterol is formed from the cholesterol-free radical chain reaction through 7-hydroxyperoxycholesterol (7-OOH) dehydration or 7-hydroxycholesterol (7-OH) dehydrogenation ( Xu et al., 2005 ). Therefore 7-keto cholesterol is used to measure the extent of lipid oxidation. Furthermore, the inhibition of lipid oxidation could be explained by the antioxidant activity of blue pea flower anthocyanins against free radicals. In another study, Jeyaraj et al. (2021) investigated the antioxidant activity of a water extract of blue pea petals, based on the ability of the blue pea flower extract to reduce the extent of 2,2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH)-generated free radicals in RAW264.7 cells (mouse macrophage cells). It was found that the water extract with a concentration of 156.3 μg/mL blue pea flower extract demonstrated 75-80% inhibition against AAPH-generated free radical formation. Likewise, Phrueksanan et al. (2014) found that anthocyanin-rich extract of blue pea petals could protect erythrocytes from AAPH-induced haemolysis and oxidative damage. It was found that an anthocyanin-rich extract of blue pea petals (400 μg/mL) could reduce membrane lipid peroxidation, protein carbonyl group formation and prevent the reduction of glutathione concentration in APPH induced haemolysis ( Phrueksanan et al., 2014 ). Furthermore, Zakaria et al. (2018) showed that a blue pea flower anthocyanin extract could protect human keratinocytes from H 2 O 2 -induced cytotoxicity and UV-induced mtDNA damage in human keratinocytes. The cellular antioxidant activity of blue pea flower extract is clearly demonstrated in these studies. Overall, the results of in vitro antioxidant assays should not be interpreted directly as the antioxidant property inside the body of a living being ( Gengatharan et al., 2015 ; Jeyaraj et al., 2020 ). Therefore, the antioxidant properties of blue pea flower anthocyanins in living systems should be investigated.
A major cause of non-communicable diseases is hyperglycaemia ( Kim and Oh, 2013 ). Increased serum glucose levels after a meal could create several complications such as the production of mitochondrial reactive oxygen species (ROS) that can deplete antioxidant enzymes in serum. Studies have found that bioactive compounds such as anthocyanins could inhibit the action of carbohydrate digestive enzymes such as pancreatic α-amylase and intestinal α-glucosidase consequently reducing postprandial hyperglycaemia ( McDougall et al., 2005 ). One study investigated the effect of ingestion of blue pea flower anthocyanins with or without sucrose on the glucose level and antioxidant capacity of the serum of humans. Eighteen healthy men between 18 and 40 years were selected and administered with different sucrose, water and blue pea flower anthocyanin extract treatments (1 or 2 g of blue pea flower anthocyanin extract + 400 mL water, 50 g sucrose + 400 mL water, 1 or 2 g of blue pea flower anthocyanin extract + 50 g sucrose + 400 mL water) after 12 h fasting period. Subjects who were administered with 1 and 2 g of blue pea flower anthocyanin extract with 400 mL of distilled water did not show any change in serum glucose level. It was observed that the subjects who ingested sucrose and water, had a rapid increase in plasma glucose level approximately by 75 mg/dL after 30 min of administration and fell back to normal level within 90 min. Subjects who were administered with 1 or 2 g of blue pea flower anthocyanins with 50 g sucrose and 400 mL distilled water demonstrated an increase of serum glucose level only by 60 mg/dL within 30 min of ingestion but reduced significantly within 60 min and came to normal level in 90 min. The postprandial plasma glucose concentration after 30 and 60 min of ingestion was significantly lower ( p < 0.05) in the sucrose treatment with blue pea flower anthocyanins when compared to the sucrose treatment with water. Plasma insulin level increased in sucrose only treatment, but plasma insulin level did not change significantly with blue pea flower anthocyanins treatment. When sucrose was administered with blue pea flower anthocyanin extract the rise of plasma insulin level was significantly suppressed after 60 min of administration. The plasma antioxidant activity measured by FRAP and ABTS assays increased in all treatments, but subjects treated with blue pea flower anthocyanins with or without sucrose demonstrated a significantly higher plasma antioxidant activity. Ingestion of blue pea flower anthocyanin extract with or without sucrose showed reduced levels of plasma malondialdehyde (MDA) during the postprandial period that indicating a low level of lipid peroxidation. The plasma thiol concentration which is an indicator of plasma antioxidant defence mechanism reduced significantly within 30 min after ingestion of sucrose but administration of blue pea flower anthocyanin extract with or without sucrose increased the plasma thiol level indicating the strengthening of plasma antioxidant defence mechanism by blue pea flower anthocyanins ( Chusak et al., 2018 ). Therefore, blue pea flower anthocyanins could reduce the serum glycaemic index, MDA level and increase the plasma antioxidant level during the postprandial period. The application of blue pea flowers anthocyanins in food as a food colourant or functional food ingredient may provide these health benefits to consumers.
Applications of Anthocyanins From Blue Pea Flower
Several studies have been done on the application of blue pea flower anthocyanins in many areas such as developing dye-sensitised solar cells, pharmaceuticals etc. ( Gokilamani et al., 2013 ; Nair et al., 2015 ). This review focuses on the current research on the application of anthocyanins from blue pea flowers in the food industry. Several studies have been carried out on the application of anthocyanins from blue pea flowers as a natural food colouring agent and those food demonstrate antioxidant activity and bio-preservative properties ( Table 3 ). A study was carried out to develop a functional beverage using blue pea flower extract, stevia extract and lime. Out of the three formulations screened from preliminary tests, the most acceptable beverage selected from the sensory evaluation had the combination of the above three constituents in ratio 983.25:1.75:15 in manufacturing 1 L of the beverage. The beverage developed with the above-mentioned combination of constituents were again tested with a sensory evaluation using a 9-point hedonic scale and for all the attributes (colour, sweetness, lime flavour, aroma and overall acceptability), a median score of 7 was obtained indicating moderate likeness. The antioxidant property of the functional beverage measured with DPPH, ABTS, FRAP and oxygen radical absorbance capacity (ORAC) assays are shown in Table 3 . The total phenolic content of the functional beverage was 85.5 mg gallic acid equivalent/L. The beverage could be preserved at room temperature for 28 days without using any preservatives. When the pH of the beverage was adjusted between pH 2 to pH 4 to investigate the variation of colour, intense blue colour was observed between pH 3.5 and pH 4 ( Lakshan et al., 2019 ). The reason for preserving the ability of blue pea flower extract incorporated functional beverage could be explained by the anti-microbial effect demonstrated by blue pea flower anthocyanins ( Yanti et al., 2018 ). Therefore, blue pea flower anthocyanins can be used as a blue colourant in beverages having acidic pH between 3 to 4. Similar work was done by Marpaung et al. (2020) in which a crystallised functional drink powder was prepared with an anthocyanin-rich blue pea flower extract and supersaturated sugar solution and citric acid. The best formulation was selected using an sensory evaluation with a 9-hedonic scale and the formulation with 58 g sucrose, 0.46 g citric acid and 80 g of blue pea flower extract in 250 mL distilled water was selected as the most acceptable drink for colour, aroma, taste and overall acceptability. The initial TAC of the powder was reduced by 50% within 28 days when stored at 27°C in dark and the half-life of the anthocyanins was further reduced at high storage temperature. Interestingly, the initial antioxidant capacity (35–40% scavenging in DPPH radical scavenging activity) of the powder remained almost unchanged during the storage period of four weeks. Therefore, it would be better to use anthocyanins from blue pea flowers as a natural colouring agent for functional beverages with shorter shelf life.
Table 3. Application of blue pea flower anthocyanins as a natural colouring agent and their antioxidant/bioactivity.
Sutakwa et al. (2021) investigated the antioxidant activity of yoghurts prepared with 10% (v/v) blue pea flower anthocyanin extracts. Five different types of milk (liquid skim milk, ultra-heat treated (UHT) milk, pasteurised milk, UHT milk with skim milk, and pasteurised milk with skim milk) were used to prepare the yoghurt. The antioxidant activity of yoghurt samples coloured with blue pea flower anthocyanins was significantly higher compared to the control samples and yoghurts prepared with skim milk showed the highest antioxidant activity (437.04 ppm measured by DPPH radical scavenging activity calculated using a standard linear equation with the butylated hydroxytoluene (BHT) as a standard curve). These studies evidently showed that the antioxidant activity of food increases when anthocyanins from blue pea flowers are used as a food colourant.
Thanh et al. (2020) investigated the application of blue pea flower anthocyanin extract in cupcakes. After baking at 170°C for 20 min, only 41.8% of the initial anthocyanin content was retained in the cupcake. The blue colour of the blue pea flower anthocyanin extract turned to greenish colour due to the pH change that happened in the dough. However, in the sensory evaluation, it was found that the aroma, colour, flavour and overall acceptability of the cupcakes with blue pea flower anthocyanins were higher compared with the control samples. The reason for the lower retention percentage could be attributed to the thermal deterioration of anthocyanins at high baking temperatures.
Studies show that the protection of anthocyanins with wall material by microencapsulation could enhance the stability of anthocyanins ( Marsin et al., 2020 ). Therefore, the application of encapsulated blue pea flower anthocyanins in functional food provides a solution to enhance their stability in functional food. The application of encapsulated blue pea flower anthocyanins as a food colourant in baked food products (muffins) was studied ( Ab Rashid et al., 2021 ). This study reported that the bacterial load on muffins with blue pea flower anthocyanins was significantly lower compared with the control sample. Therefore, blue pea flower anthocyanins have played two roles: one as a natural colouring agent and the other as a bio-preservative in this baked food product. This bio-preservative action of blue pea flower anthocyanins is supported by the study of Leong et al. (2017) where the anthocyanins from blue pea flower showed anti-fungal properties against the food-borne Penicillium expansum conidia. Nikijuluw and Andarwulan (2013) applied blue pea flower anthocyanin extracts to colour a yoghurt drink and rice. When the blue pea flower anthocyanin extract was added to yoghurt drink (pH 4.5) at a concentration of 3.37 × 10 –5 mg anthocyanin/mL and rice (pH 7) at a concentration of 1.6 × 10 –4 mg anthocyanin/g, the yoghurt drink has a purplish-blue colour and the rice had a dark blue colour. In this study, Brilliant Blue, a synthetic colourant was also added to yoghurt drink at a concentration of 3.13 × 10 –4 mg/mL and to rice at a concentration of 3.2 × 10 –4 mg/g and the colour of yoghurt drink and rice were green-blue and light blue, respectively ( Nikijuluw and Andarwulan, 2013 ). Even though this study compared the stability of blue pea flower anthocyanin extract and Brilliant Blue, they did not investigate the stability of the colours after applying them in the food systems. It would be useful if a comparison was done in food systems to determine the colour stability and the bioactivity after adding the colourants.
The use of a colourimetric indicator in intelligent packaging has been used as a freshness indicator of perishable food products such as fish and meat. Colourimetric indicators provide real-time information on the freshness of the food material based on the pH dependant colour change ( Priyadarshi et al., 2021 ). Microbial action on fish and meat produces chemical compounds such as amines that cause a pH change ( Fletcher et al., 2018 ). Generally used chemical reagents as colourimetric indicators in intelligent packaging contain synthetic chemical compounds such as bromophenol blue and chlorophenol red are not safe to use for food packaging due to the possibility of migration of these compounds to food and their possible harmful effects on human health ( Zhang et al., 2014 ; Poyatos-Racionero et al., 2018 ). Therefore, the invention of safer alternative reagents for this purpose is desired. The pH dependant colour variation of anthocyanins could be used to develop these kinds of intelligent packaging systems.
Several studies have been done on the application of anthocyanins from blue pea flowers as a colour indicator in intelligent packaging ( Ahmad et al., 2020 ; Netramai et al., 2020 ; Salacheep et al., 2020 ; Singh et al., 2021 ). One study used anthocyanins from blue pea flowers in an intelligent packaging system incorporated in a film made with distilled water and 5% (w/v) sago powder ( Ahmad et al., 2020 ). It was observed the colour of the packaging film changed from blue to green in 24 h when the chicken sample was kept at room temperature but the chicken sample kept in frozen condition did not alter the colour of the indicator even after 48 h ( Ahmad et al., 2020 ). This study reported that the colour change of anthocyanin extract from blue pea flower at varying pH was more distinct compared with those of anthocyanin extracts from hibiscus, purple sweet potato and red yeast rice. Another study used anthocyanin extract from blue pea flowers as a colour indicator in gelatin film ( Rawdkuen et al., 2020 ). A packaging film was made with gelatin, glycerol and anthocyanin extract from blue pea flower. The initial pH of gelatin film was 6 and the colour was blue. When pH reduced to 4, the film turned to violet and when pH increased to pH 8 the film turned to green. The incorporation of anthocyanin extract from the blue pea flower slightly reduced the tensile strength and water vapour permeability of the gelatin film but significantly increased the antioxidant capacity of the gelatin film ( Rawdkuen et al., 2020 ). Another study used a gelatin film with blue pea flower anthocyanins as a colour indicating package to monitor fish freshness. Initially, the packaging film was dark bluish-purple at the beginning and turned to bluish-green when the fish was kept at room temperature for 24 h ( Salacheep et al., 2020 ). Another study used anthocyanin extract from blue pea flowers in a freshness monitoring packaging for prawns made with starch and TiO 2 . The colour of the colour indicator changed from pink to green when prawns were stored at 4°C for 6 days ( Mary et al., 2020 ). Similarly, Wu et al. (2021) developed an intelligent packaging film using gellan gum adding blue pea flower anthocyanin extract to monitor the freshness of shrimp. The colour of the film changed distinctly from blue to green when the shrimp were kept at 25°C for 24 h. The pH of shrimp changed from pH 5 to pH 8 within 24 h due to the production of nitrogenous compounds produced during spoilage. Therefore, blue pea flower anthocyanin extracts can be used to develop intelligent packaging systems to monitor the freshness of seafood as well.
In addition to freshness monitoring systems of animal products, Singh et al. (2021) used anthocyanins of blue pea flowers to develop an intelligent film to monitor the freshness of beverages. Anthocyanin extracts from Clitoria ternatea and Carissa carandas were incorporated into chitosan-poly (vinyl alcohol) films separately and used to monitor the freshness of milk and fresh orange juice. It was observed that incorporation of anthocyanin extracts did not significantly alter the physical properties of the film and the film with Clitoria ternatea anthocyanin extract was more pH-sensitive in terms of colour change compared with the film with the anthocyanin extract of Carissa carandas . The film with Clitoria ternatea anthocyanin extract showed a distinct change in colour for both milk and juice stored at 25°C after 72 h where the pH of milk changed from 6.2 to 4.1 and the pH of juice changed from 4.2 to 3.4 ( Singh et al., 2021 ). Therefore, anthocyanins from blue pea flowers could be considered as a promising colour indicator for intelligent packaging.
Conclusion and Future Perspective of Research
Clitoria ternatea or blue pea flower is an edible flower with medicinal and ornamental value. The blue pea flower is a rich source of polyacylated anthocyanins demonstrating higher stability than non-acylated anthocyanins. Blue pea flower anthocyanins demonstrate an intense and stable blue colour in acidic medium which facilitate their application in acidic food systems as a blue food colouring agent. The cellular and in vitro antioxidant activities of blue pea flower anthocyanins show their potential application in functional foods. Several studies have been conducted on investigating the application of blue pea flower anthocyanins as a food colourant in bakery products, yoghurt and functional beverages. Further research should be carried out on the application of blue pea flower anthocyanins in other food systems. Studies have shown antioxidant and antimicrobial activities of blue pea flower anthocyanins in different applications. Further research could be carried out to investigate the bioavailability and other functional properties of blue pea flower anthocyanins. Since there are limited blue food colourants available, blue pea flower anthocyanins will be a good alternative to be used as a natural blue food colouring agent.
Author Contributions
GCVG: responsible for writing and editing the manuscript. YYL: responsible for reviewing and editing the manuscript. WSC: responsible for the conceptualisation, funding acquisition, and reviewing and editing of the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by the School of Science, Monash University, Malaysia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ab Rashid, S., Tong, W. Y., Leong, C. R., Ghazali, N. M. A., Abu Taher, M., Ahmad, N., et al. (2021). Anthocyanin microcapsule from Clitoria ternatea : Potential bio-preservative and blue colorant for baked food products. Arab. J. Sci. Eng. 46, 65–72. doi: 10.1007/s13369-020-04716-y
CrossRef Full Text | Google Scholar
Abidin, Z. H. Z., Manah, N. S. A., Hadi, A. N., Saugi, N. S., Fuad, F. A., Mazni, N. A., et al. (2019). The colour stability of natural blue dye extracted from Clitoria ternatea L. in poly(acrylamide-co-acrylic acid) coating film. Pigment Resin Technol. 48, 265–271. doi: 10.1108/prt-12-2017-0106
Ahmad, A. N., Lim, S. A., and Navaranjan, N. (2020). Development of sago (Metroxylon sagu)-based colorimetric indicator incorporated with butterfly pea ( Clitoria ternatea ) anthocyanin for intelligent food packaging. J. Food Saf. 40:9. doi: 10.1111/jfs.12807
Aprodu, I., Milea, S. A., Enachi, E., Râpeanu, G., Bahrim, G. E., and Stǎnciuc, N. (2020). Thermal degradation kinetics of anthocyanins extracted from purple maize flour extract and the effect of heating on selected biological functionality. Foods 9:1593. doi: 10.3390/foods9111593
PubMed Abstract | CrossRef Full Text | Google Scholar
Asadnejad, S., Nabizadeh, R., Nazarinia, A., Jahed, G. R., and Alimohammadi, M. (2018). Data on prevalence of additive colors in local food and beverage products, Tehran, Iran. Data Brief 19, 2104–2108. doi: 10.1016/j.dib.2018.07.001
Baskaran, A., Mudalib, S. K. A., and Izirwan, I. (2019). Optimization of aqueous extraction of blue dye from butterfly pea flower. J. Phys. 1358:012001. doi: 10.1088/1742-6596/1358/1/012001
Benchikh, Y., Filali, A., and Rebai, S. (2020). Modeling and optimizing the phycocyanins extraction from Arthrospira platensis ( Spirulina ) algae and preliminary supplementation assays in soft beverage as natural colorants and antioxidants. J. Food Process. Preserv. 45:e15170. doi: 10.1111/jfpp.15170
Bridle, P., and Timberlake, C. (1997). Anthocyanins as natural food colours-selected aspects. Food Chem. 58, 103–109. doi: 10.1016/S0308-8146(96)00222-1
Buchweitz, M. (2016). “Natural solutions for blue colors in food,” in The Handbook on Natural Pigments in Food and Beverages , eds R. Carle and R. M. Schweiggert (Oxford: Woodhead Publishing), 355–384. doi: 10.1016/B978-0-08-100371-8.00017-8
Buchweitz, M., Nagel, A., Carle, R., and Kammerer, D. R. (2012). Characterisation of sugar beet pectin fractions providing enhanced stability of anthocyanin-based natural blue food colourants. Food Chem . 132, 1971–1979. doi: 10.1016/j.foodchem.2011.12.034
Cazarolli, L. H., Folador, P., Pizzolatti, M. G., and Silva, F. R. M. B. (2009). Signaling pathways of kaempferol-3-neohesperidoside in glycogen synthesis in rat soleus muscle. Biochimie 91, 843–849. doi: 10.1016/j.biochi.2009.04.004
Chaiklahan, R., Chirasuwan, N., and Bunnag, B. (2012). Stability of phycocyanin extracted from Spirulina sp.: influence of temperature, pH and preservatives. Process Biochem . 47, 659–664. doi: 10.1016/j.procbio.2012.01.010
Chaiklahan, R., Chirasuwan, N., Loha, V., Tia, S., and Bunnag, B. (2018). Stepwise extraction of high-value chemicals from Arthrospira (Spirulina) and an economic feasibility study. Biotechnol. Rep . 20:e00280. doi: 10.1016/j.btre.2018.e00280
Chandrasekhar, J., Madhusudhan, M. C., and Raghavarao, K. S. M. S. (2012). Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod. Process. 90, 615–623. doi: 10.1016/j.fbp.2012.07.004
Charurungsipong, P., Tangduangdee, C., Amornraksa, S., Asavasanti, S., and Lin, J. (2020). Improvement of anthocyanin stability in butterfly pea flower extract by co-pigmentation with catechin. E3S web of conferences, 2020. EDP Sci. 141:03008. doi: 10.1051/e3sconf/202014103008
Chemat, F., Abert Vian, M., Ravi, H. K., Khadhraoui, B., Hilali, S., Perino, S., et al. (2019). Review of alternative solvents for green extraction of food and natural products: panorama, principles, applications and prospects. Molecules 24:3007. doi: 10.3390/molecules24163007
Chemat, F., Huma, Z. E., and Khan, M. K. (2011). Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason. Sonochem . 18, 813–835. doi: 10.1016/j.ultsonch.2010.11.023
Choo, W. S. (2019). “Fruit pigment changes during ripening,” in The Encyclopedia of Food Chemistry , eds L. Melton, F. Shahidi, and P. Varelis (Elsevier: The Netherlands), 17–123.
Google Scholar
Chusak, C., Thilavech, T., Henry, C. J., and Adisakwattana, S. (2018). Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: a randomized crossover trial. BMC Complement. Altern. Med. 18:11. doi: 10.1186/s12906-017-2075-7
Collings, D. A. (2019). Anthocyanin in the vacuole of red onion epidermal cells quenches other fluorescent molecules. Plants 8:596. doi: 10.3390/plants8120596
Daniel, R. M., and Danson, M. J. (2013). Temperature and the catalytic activity of enzymes: a fresh understanding. FEBS Lett. 587, 2738–2743. doi: 10.1016/j.febslet.2013.06.027
Dilrukshi, P. G. T., Munasinghe, H., Silva, A. B. G., and De Silva, P. G. S. M. (2019). Identification of synthetic food colours in selected confectioneries and beverages in jaffna District, Sri Lanka. J. Food Qual . 2019:453169. doi: 10.1155/2019/7453169
Escher, G. B., Marques, M. B., do Carmo, M. A. V., Azevedo, L., Furtado, M. M., Sant’Ana, A. S., et al. (2020b). Clitoria ternatea L. Petal bioactive compounds display antioxidant, antihemolytic and antihypertensive effects, inhibit α-amylase and α-glucosidase activities and reduce human LDL cholesterol and DNA induced oxidation. Food Res. Int. 128:108763. doi: 10.1016/j.foodres.2019.108763
Escher, G. B., Wen, M., Zhang, L., Rosso, N. D., and Granato, D. (2020a). Phenolic composition by UHPLC-Q-TOF-MS/MS and stability of anthocyanins from Clitoria ternatea L. (butterfly pea) blue petals. Food Chem . 331:127341. doi: 10.1016/j.foodchem.2020.127341
FDA (2015). Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. Silver Spring, MD: FDA.
FDA (2018). Q3C–Tables and List Guidance for Industry. Available online at: www.fda.gov/regulatory-information/search-fda-guidance-documents/q3c-tables-and-list-rev-4 (accessed September 8, 2021)
Feketea, G., and Tsabouri, S. (2017). Common food colorants and allergic reactions in children: myth or reality? Food Chem . 230, 578–588. doi: 10.1016/j.foodchem.2017.03.043
Fletcher, B., Mullane, K., Platts, P., Todd, E., Power, A., Roberts, J., et al. (2018). Advances in meat spoilage detection: a short focus on rapid methods and technologies. CYTA J. Food 16, 1037–1044. doi: 10.1080/19476337.2018.1525432
Garcia, C., and Blesso, C. N. (2021). Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 172, 152–166. doi: 10.1016/j.freeradbiomed.2021.05.040
Gengatharan, A., Dykes, G. A., and Choo, W. S. (2015). Betalains: natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 64, 645–649. doi: 10.1016/j.lwt.2015.06.052
Gokilamani, N., Muthukumarasamy, N., Thambidurai, M., Ranjitha, A., and Velauthapillai, D. (2013). Utilization of natural anthocyanin pigments as photosensitizers for dye-sensitized solar cells. J. Sol Gel Sci. Technol. 66, 212–219. doi: 10.1007/s10971-013-2994-9
Gustiningtyas, A., Setyaningsih, I., Hardiningtyas, S. D., and Susila, A. A. R. (2020). Improvement stability of phycocyanin from Spirulina platensis encapsulated by water soluble chitosan nanoparticles. IOP Conf. Ser. 414:012005. doi: 10.1088/1755-1315/414/1/012005
Gwee, X. F., and Chong, F. C. (2015). Ultrasonic extraction of anthocyanin from Clitoria ternatea flowers using response surface methodology. Nat. Prod. Res. 29, 1485–1487. doi: 10.1080/14786419.2015.1027892
Havananda, T., and Luengwilai, K. (2019). Variation in floral antioxidant activities and phytochemical properties among butterfly pea ( Clitoria ternatea L.) germplasm. Genet. Resour. Crop Evol. 66, 645–658. doi: 10.1007/s10722-018-00738-6
Honda, T., and Saito, N. (2002). Recent progress in the chemistry of polyacylated anthocyanins as flower color pigments. Heterocycles 56, 633–692.
Jaafar, N. F., Ramli, M. E., and Salleh, R. M. (2020). Optimum extraction condition of Clitorea ternatea flower on antioxidant activities, total phenolic, total flavonoid and total anthocyanin contents. Trop. Life Sci. Res. 31, 1–17. doi: 10.21315/tlsr2020.31.2.1
Jeyaraj, E. J., Lim, Y. Y., and Choo, W. S. (2020). Extraction methods of butterfly pea ( Clitoria ternatea ) flower and biological activities of its phytochemicals. J. Food Sci. Technol. 58, 2054–2067. doi: 10.1007/s13197-020-04745-3
Jeyaraj, E. J., Lim, Y. Y., and Choo, W. S. (2021). Effect of organic solvents and water extraction on the phytochemical profile and antioxidant activity of Clitoria ternatea flowers. ACS Food Sci. Technol 1, 1567–1577. doi: 10.1021/acsfoodscitech.1c00168
Jing, P., and Giusti, M. M. (2007). Effects of extraction conditions on improving the yield and quality of an anthocyanin-rich purple corn ( Zea mays L.) color extract. J. Food Sci. 72, C363–C368. doi: 10.1111/j.1750-3841.2007.00441.x
Kang, H. J., Ko, M. J., and Chung, M. S. (2021). Anthocyanin structure and pH dependent extraction characteristics from blueberries ( Vaccinium corymbosum ) and chokeberries ( Aronia melanocarpa ) in Subcritical Water State. Foods 10:527. doi: 10.3390/foods10030527
Kazuma, K., Kogawa, K., Noda, N., Kato, N., and Suzuki, M. (2004). Identification of delphinidin 3- O -(6″- O -malonyl)-beta-glucoside-3′- O -beta-glucoside, a postulated intermediate in the biosynthesis of ternatin C5 in the blue petals of Clitoria ternatea (butterfly pea). Chem. Biodivers. 1, 1762–1770. doi: 10.1002/cbdv.200490132
Khoo, H. E., Azlan, A., Tang, S. T., and Lim, S. M. (2017). Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 61:1361779. doi: 10.1080/16546628.2017.1361779
Kim, H. C., and Oh, S. M. (2013). Noncommunicable diseases: current status of major modifiable risk factors in Korea. J. Prev. Med. Public Health 46:165.
Kogawa, K., Kazuma, K., Kato, N., Noda, N., and Suzuki, M. (2007a). Biosynthesis of malonylated flavonoid glycosides on the basis of malonyltransferase activity in the petals of Clitoria ternatea . J. Plant Physiol . 164, 886–894. doi: 10.1016/j.jplph.2006.05.006
Kogawa, K., Kato, N., Kazuma, K., Noda, N., and Suzuki, M. (2007b). Purification and characterization of UDP-glucose: anthocyanin 3′,5′-O-glucosyltransferase from Clitoria ternatea . Planta 226, 1501–1509. doi: 10.1007/s00425-007-0584-1
Kosai, P., Sirisidthi, K., Jiraungkoorskul, K., and Jiraungkoorskul, W. (2015). Review on ethnomedicinal uses of memory boosting herb, butterfly pea, Clitoria ternatea . J. Nat. Remedies 15, 71–76.
Lakshan, S. A. T., Jayanath, N. Y., Abeysekera, W. P. K. M., and Abeysekera, W. K. S. M. (2019). A commercial potential blue pea ( Clitoria ternatea L.) flower extract incorporated beverage having functional properties. Evid. Based Complement. Alternat. Med. 2019:2916914. doi: 10.1155/2019/2916914
Landim Neves, M. I., Silva, E. K., and Meireles, M. A. A. (2021). Natural blue food colorants: consumer acceptance, current alternatives, trends, challenges, and future strategies. Trends Food Sci. Technol . 112, 163–173. doi: 10.1016/j.tifs.2021.03.023
Lee, P. M., Abdullah, R., and Hung, L. K. (2011). “Thermal degradation of blue anthocyanin extract of Clitoria ternatea flower,” in Biotechnology and Food Service , ed. L. Xuan (Singaore: International Assocation of Computer Science & Information Technology Press-IACSIT Press), 49–53.
Leong, C. R., Kamarul Azizi, M. A., Taher, M. A., Wahidin, S., Lee, K. C., Tan, W. N., et al. (2017). Anthocyanins from Clitoria ternatea attenuate food-borne Penicillium expansum and its potential application as food biopreservative. Nat. Prod. Sci . 23, 125–131. doi: 10.20307/nps.2017.23.2.125
Li, N., Li, J., Hao, J. Y., Zhang, M., Yin, J. J., Geng, J. T., et al. (2019). Bilberry anthocyanin improves the serum cholesterol in aging perimenopausal rats via the estrogen receptor signaling pathway. Food Funct. 10, 3430–3438. doi: 10.1039/c9fo00639g
Lin, W. S., He, P. H., Chau, C. F., Liou, B. K., Li, S., and Pan, M. H. (2018). The feasibility study of natural pigments as food colorants and seasonings pigments safety on dried tofu coloring. Food Sci. Hum. Wellness 7, 220–228. doi: 10.1016/j.fshw.2018.09.002
Liu, S., Fu, Y., and Nian, S. (2014). Buffering colour fluctuation of purple sweet potato anthocyanins to acidity variation by surfactants. Food Chem . 162, 16–21. doi: 10.1016/j.foodchem.2014.04.029
Liu, Y., Tikunov, Y., Schouten, R. E., Marcelis, L. F. M., Visser, R. G. F., and Bovy, A. (2018). Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: a Review. Front. Chem. 6:52. doi: 10.3389/fchem.2018.00052
Loypimai, P., Moongngarm, A., and Chottanom, P. (2016). Thermal and pH degradation kinetics of anthocyanins in natural food colorant prepared from black rice bran. J. Food Sci. Technol. 53, 461–470. doi: 10.1007/s13197-015-2002-1
Lu, C., Li, Y., Cui, Y., Ren, J., Qi, F., Qu, J., et al. (2021). Isolation and functional analysis of genes involved in polyacylated anthocyanin biosynthesis in blue Senecio cruentus . Front. Plant Sci. 12:640746. doi: 10.3389/fpls.2021.640746
Mahmad, N., and Taha, R. M. (2018). Effects of pH, UV-B radiation and NaCl on anthocyanin stability from vivid blue petals of Clitoria ternatea L., a potential natural colourant from legume crop. Pigment Resin Technol. 47, 507–510. doi: 10.1108/prt-11-2016-0106
Marpaung, A. M., Andarwulan, N., Hariyadi, P., and Faridah, D. N. (2017). The colour degradation of anthocyanin-rich extract from butterfly pea ( Clitoria ternatea L.) petal in various solvents at pH 7. Nat. Prod. Res. 31, 2273–2280. doi: 10.1080/14786419.2017.1303689
Marpaung, A. M., Andarwulan, N., Hariyadi, P., and Faridah, D. N. (2019). The difference in colour shifting of Clitoria ternate a L. Flower extract at pH 1, 4, and 7 during storage. Curr. Nutr. Food Sci. 15, 694–699. doi: 10.2174/1573401314666180503152636
Marpaung, A. M., Lee, M., and Kartawiria, I. S. (2020). The development of butterfly pea ( Clitoria ternatea ) flower powder drink by co-crystallization. Indone Food Sci. Technol. J. 3, 34–37. doi: 10.22437/ifstj.v3i2.10185
Marsin, A. M., Jusoh, Y. M. M., Abang, D. N., Zaidel, Z. H., Yusof, A. H. M., and Muhamad, I. I. (2020). Microwave-assisted encapsulation of blue pea flower ( Clitoria ternatea ) colourant: maltodextrin concentration, power, and time. Chem. Eng. Trans. 78, 199–204. doi: 10.3303/CET2078034
Mary, S. K., Koshy, R. R., Daniel, J., Koshy, J. T., Pothen, L. A., and Thomas, S. (2020). Development of starch based intelligent films by incorporating anthocyanins of butterfly pea flower and TiO 2 and their applicability as freshness sensors for prawns during storage. RSC Adv . 10, 39822–39830. doi: 10.1039/D0RA05986B
Matsufuji, H., Kido, H., Misawa, H., Yaguchi, J., Otsuki, T., Chino, M., et al. (2007). Stability to light, heat, and hydrogen peroxide at different pH values and DPPH radical scavenging activity of acylated anthocyanins from red radish extract. J. Agric. Food Chem . 55, 3692–3701. doi: 10.1021/jf063598o
McDougall, G. J., Shpiro, F., Dobson, P., Smith, P., Blake, A., and Stewart, D. (2005). Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem . 53, 2760–2766. doi: 10.1021/jf0489926
Mehmood, A., Ishaq, M., Zhao, L., Yaqoob, S., Safdar, B., Nadeem, M., et al. (2019). Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower ( Clitoria ternatea L.). Ultrason. Sonochem. 51, 12–19. doi: 10.1016/j.ultsonch.2018.10.013
Migliorini, A. A., Piroski, C. S., Daniel, T. G., Cruz, T. M., Escher, G. B., Vieira do Carmo, M. A., et al. (2019). Red chicory ( Cichorium intybus ) extract rich in anthocyanins: chemical stability, antioxidant activity, and antiproliferative activity in vitro. J. Food Sci. 84, 990–1001. doi: 10.1111/1750-3841.14506
Nair, V., Bang, W. Y., Schreckinger, E., Andarwulan, N., and Cisneros-Zevallos, L. (2015). Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea ( Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem . 63:6355. doi: 10.1021/acs.jafc.5b00928
Neri-Numa, I. A., Angolini, C. F. F., Bicas, J. L., Ruiz, A., and Pastore, G. M. (2018). Iridoid blue-based pigments of Genipa americana L. (Rubiaceae) extract: influence of pH and temperature on color stability and antioxidant capacity during in vitro simulated digestion. Food Chem . 263, 300–306. doi: 10.1016/j.foodchem.2018.05.001
Netramai, S., Kijchavengkul, T., Kham-Ngam, C., Sirinupong, P., Kwanmuang, S., Samsudin, H., et al. (2020). “Development of colorimetric film with butterfly pea ( Clitoria ternatea L.) extract for application in intelligent packaging,” in Proceedings of the 22 nd Food Innovation Asia Conference 2020 (FIAC 2020) , (Bangkok).
Nikijuluw, C., and Andarwulan, N. (2013). Color Characteristic of Butterfly Pea (Clitoria ternatea L.) Anthocyanin Extracts and Brilliant Blue. Bogor: Bogor Agriculture University.
Oguis, G. K., Gilding, E. K., Jackson, M. A., and Craik, D. J. (2019). Butterfly pea ( Clitoria ternatea ), a cyclotide-bearing plant with applications in agriculture and medicine. Front. Plant. Sci. 10:23. doi: 10.3389/fpls.2019.00645
Paik, Y. S., Lee, C. M., Cho, M. H., and Hahn, T. R. (2001). Physical stability of the blue pigments formed from geniposide of gardenia fruits: effects of pH, temperature, and light. J. Agric. Food Chem . 49, 430–432. doi: 10.1021/jf000978f
Pasukamonset, P., Kwon, O., and Adisakwattana, S. (2017). Oxidative stability of cooked pork patties incorporated with Clitoria ternatea extract (Blue pea flower petal) during refrigerated storage. J. Food Process Preserv. 41:10. doi: 10.1111/jfpp.12751
Patel, A., Pawar, R., Mishra, S., Sonawane, S., and Ghosh, P. K. (2004). Kinetic studies on thermal denaturation of C-phycocyanin. Ind. J. Biochem. Biophys . 41, 254–257.
Pham, T. N., Nguyen, D. C., Lam, T. D., Van Thinh, P., Le, X. T., Nguyen, D. V. V., et al. (2019). “Extraction of anthocyanins from Butterfly pea ( Clitoria ternatea L. Flowers) in Southern Vietnam: response surface modeling for optimization of the operation conditions,” in Proceedings of the 2018 the 6 th International Conference on Mechanical Engineering, IOP Conference Series: Materials Science and Civil Engineering , (Bristol: Iop Publishing Ltd).
Phrueksanan, W., Yibchok-anun, S., and Adisakwattana, S. (2014). Protection of Clitoria ternatea flower petal extract against free radical-induced hemolysis and oxidative damage in canine erythrocytes. Res. Vet. Sci . 97, 357–363. doi: 10.1016/j.rvsc.2014.08.010
Poyatos-Racionero, E., Ros-Lis, J. V., Vivancos, J. L., and Martínez-Máñez, R. (2018). Recent advances on intelligent packaging as tools to reduce food waste. J. Clean Prod . 172, 3398–3409. doi: 10.1016/j.jclepro.2017.11.075
Priyadarshi, R., Ezati, P., and Rhim, J. W. (2021). Recent advances in intelligent food packaging applications using natural food colorants. ACS Food Sci. Technol . 1, 124–138. doi: 10.1021/acsfoodscitech.0c00039
Ranieri, M. L., Huck, J. R., Sonnen, M., Barbano, D. M., and Boor, K. J. (2009). High temperature, short time pasteurization temperatures inversely affect bacterial numbers during refrigerated storage of pasteurized fluid milk. J. Dairy Sci. 92, 4823–4832. doi: 10.3168/jds.2009-2144
Rawdkuen, S., Faseha, A., Benjakul, S., and Kaewprachu, P. (2020). Application of anthocyanin as a color indicator in gelatin films. Food Biosci . 36:100603. doi: 10.1016/j.fbio.2020.100603
Rocha, R., Pinela, J., Abreu, R. M. V., Añibarro-Ortega, M., Pires, T. C. S. P., Saldanha, A. L., et al. (2020). Extraction of anthocyanins from red raspberry for natural food colorants development: processes optimization and in vitro bioactivity. Processes 8:1447. doi: 10.3390/pr8111447
Salacheep, S., Kasemsiri, P., Pongsa, U., Okhawilai, M., Chindaprasirt, P., and Hiziroglu, S. (2020). Optimization of ultrasound-assisted extraction of anthocyanins and bioactive compounds from butterfly pea petals using Taguchi method and Grey relational analysis. J. Food Sci. Technol. 57, 3720–3730. doi: 10.1007/s13197-020-04404-7
Salehi, B., Sharifi-Rad, J., Cappellini, F., Reiner, Ž., Zorzan, D., Imran, M., et al. (2020). The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Front. Pharmacol. 11:1300. doi: 10.3389/fphar.2020.01300
Saptarini, N. M., and Suryasaputra, D. (2018). Total anthocyanins content in various extract of butterfly pea (C litoria ternatea linn) flower. Res. J. Pharm. Biol. Chem. Sci . 9, 185–188.
Sen, T., Barrow, C. J., and Deshmukh, S. K. (2019). Microbial pigments in the food industry—challenges and the way forward. Front. Nutr. 6:7. doi: 10.3389/fnut.2019.00007
Shen, Y., Ardoin, R., Osorio, L. F., Cardona, J., López Prado, A. S., Osorio, L. F., et al. (2019). Effects of different solvents on total phenolic and total anthocyanin contents of Clitoria ternatea L. petal and their anti-cholesterol oxidation capabilities. Int. J. Food Sci. Technol. 54:424431. doi: 10.1111/ijfs.13953
Singh, S., Nwabor, O. F., Syukri, D. M., and Voravuthikunchai, S. P. (2021). Chitosan-poly(vinyl alcohol) intelligent films fortified with anthocyanins isolated from Clitoria ternatea and Carissa carandas for monitoring beverage freshness. Int. J. Biol. Macromol. 182, 1015–1025. doi: 10.1016/j.ijbiomac.2021.04.027
Sutakwa, A., Nadia, L. S., and Suharman, S. (2021). Addition of blue pea flower ( Clitoria ternatea L.) extract increase antioxidant activity in yogurt from various types of milk. J. Agercolere 3, 31–37. doi: 10.37195/jac.v3i1.123
Syafa’atullah, A. Q., Amira, A., Hidayati, S., and Mahfud, M. (2020). Anthocyanin from butterfly pea flowers ( Clitoria ternatea ) by ultrasonic-assisted extraction. AIP Conf. Proc. 2237:020069. doi: 10.1063/5.0005289
Tan, J. B. L., and Lim, Y. Y. (2015). Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem . 172, 814–822. doi: 10.1016/j.foodchem.2014.09.141
Tanaka, Y., Brugliera, F., and Chandler, S. (2009). Recent progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 10, 5350–5369.
Terahara, N., Saito, N., Honda, T., Toki, K., and Osajima, Y. (1990). Acylated anthocyanins of Clitoria ternatea flowers and their acyl moieties. Phytochemistry 29, 949–953. doi: 10.1016/0031-9422(90)80053-J
Thanh, V. T., Tran, N. Y. T., Linh, N. T. V., Vy, T. A., and Truc, T. T. (2020). Application of anthocyanin natural colors from butterfly pea ( Clitoria ternatea L.) extracts to cupcake. IOP Conf. Ser. 736:062014. doi: 10.1088/1757-899x/736/6/062014
Thuy, N. M., Minh, V. Q., Ben, T. C., Thi Nguyen, M. T., Ha, H. T. N., and Tai, N. V. (2021). Identification of anthocyanin compounds in butterfly pea flowers ( Clitoria ternatea L.) by ultra performance liquid chromatography/ultraviolet coupled to mass spectrometry. Molecules 26:4539. doi: 10.3390/molecules26154539
Togami, J., Tamura, M., Ishiguro, K., Hirose, C., Okuhara, H., Ueyama, Y., et al. (2006). Molecular characterization of the flavonoid biosynthesis of Verbena hybrida and the functional analysis of verbena and Clitoria ternatea F3′ 5′H genes in transgenic verbena. Plant Biotechnol . 23, 5–11. doi: 10.5511/plantbiotechnology.23.5
Vidana Gamage, G. C., Lim, Y. Y., and Choo, W. S. (2021a). Black goji berry anthocyanins: extraction, stability, health benefits, and applications. ACS Food Sci. Technol . 1, 1360–1370. doi: 10.1021/acsfoodscitech.1c00203
Vidana Gamage, G. C., Lim, Y. Y., and Choo, W. S. (2021b). Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. J. Food Sci. Technol. doi: 10.1007/s13197-021-05054-z
Voss, D. M., Grouge, S. M., and Giusti, M. M. (2020). Comparison of Hot Water and Acetone Extraction Methods on Anthocyanin Content and Color Characteristics of Butterfly Pea Flower Extracts. Columbus, OH: CFAES.
Vuong, T. T., and Hongsprabhas, P. (2021). Influences of pH on binding mechanisms of anthocyanins from butterfly pea flower ( Clitoria ternatea ) with whey powder and whey protein isolate. Cogent Food Agric . 7:1889098. doi: 10.1080/23311932.2021.1889098
Wu, L. T., Tsai, I. L., Ho, Y. C., Hang, Y. H., Lin, C., Tsai, M. L., et al. (2021). Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym . 254:117410. doi: 10.1016/j.carbpol.2020.117410
Xu, Z., Zhang, T., Prinyawiwatkul, W., and Godber, J. S. (2005). Capabilities of different cooking oils in prevention of cholesterol oxidation during heating. J. Am. Oil Chem. Soc . 82, 243–248. doi: 10.1007/s11746-005-1062-9
Yanti, Y., Setiawan, T., and Lay, B. W. (2018). Antibacterial, antibiofilm and quorum sensing inhibitory activities of Clitoria ternatea anthocyanin against Streptococcus mutans. Int. J. Infect. Dis. 73, 143–144. doi: 10.1016/j.ijid.2018.04.3739
Yoon, B. I., Bae, W. J., Choi, Y. S., Kim, S. J., Ha, U. S., Hong, S. H., et al. (2018). Anti-inflammatory and antimicrobial effects of anthocyanin extracted from black soybean on chronic bacterial prostatitis rat model. Chin. J. Integr. Med . 24, 621–626. doi: 10.1007/s11655-013-1547-y
Yoshida, K., Mori, M., and Kondo, T. (2009). Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat. Prod. Rep. 26, 884–915. doi: 10.1039/b800165k
Yue, E., Tuguzbaeva, G., Chen, X., Qin, Y., Li, A., Sun, X., et al. (2019). Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine 56, 286–294. doi: 10.1016/j.phymed.2018.09.223
Zakaria, N. N. A., Okello, E. J., Howes, M. J., Birch-Machin, M. A., and Bowman, A. (2018). In vitro protective effects of an aqueous extract of Clitoria ternatea L. Flower against hydrogen peroxide-induced cytotoxicity and UV-induced mtDNA damage in human keratinocytes. Phytother. Res. 32, 1064–1072. doi: 10.1002/ptr.6045
Zhang, X., Lu, S., and Chen, X. (2014). A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sens. Actuators B 198, 268–273. doi: 10.1016/j.snb.2014.02.094
Zhang, Z., Cho, S. E., Dadmohammadi, Y., Li, Y., and Abbaspourrad, A. (2021). Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocoll. 110:106055. doi: 10.1016/j.foodhyd.2020.106055
Zhang, Z., Li, Y., and Abbaspourrad, A. (2020). Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein-phycocyanin interactions. Food Hydrocoll. 105:105747. doi: 10.1016/j.foodhyd.2020.105747
Keywords : blue pea, blue colourant, delphinidin, functional food, genipin, phycocyanin, spirulina, ternatin
Citation: Vidana Gamage GC, Lim YY and Choo WS (2021) Anthocyanins From Clitoria ternatea Flower: Biosynthesis, Extraction, Stability, Antioxidant Activity, and Applications. Front. Plant Sci. 12:792303. doi: 10.3389/fpls.2021.792303
Received: 10 October 2021; Accepted: 25 November 2021; Published: 17 December 2021.
Reviewed by:
Copyright © 2021 Vidana Gamage, Lim and Choo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wee Sim Choo, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
To read this content please select one of the options below:
Please note you do not have access to teaching notes, inatural blue dye from clitoria ternatea : extraction and analysis methods.
Research Journal of Textile and Apparel
ISSN : 1560-6074
Article publication date: 1 May 2012
An increased environmental awareness of health hazards caused by synthetic dyes has led to revival of natural dyes. Dyes obtained from natural sources have emerged as important substitutes for synthetic dyes. Color-yielding plants such as butterfly pea flowers have been used to study color extraction and can be used as dyeing material for coloring industry. Analytical studies, such as UV–VIS spectrophotometer analysis and dye concentration analysis, are performed on extracted dye using flower petals. In this study, aqueous extractions of dye from flowers were carried out in the following conditions to obtain optimization characterization: time of extraction (30-180 minutes), temperature (60-90°C), amount of flowers (0.1-2gm) and pH (2-10). It was observed that dye concentration increases gradually at higher temperature and for longer time.
- Butterfly Pea
- Aqueous Extraction
- Analytical Method
- Spectrophotometer Analysis
Sinha, K. , Das (Saha), P. and Datta, S. (2012), "INatural Blue Dye from Clitoria Ternatea : Extraction and Analysis Methods", Research Journal of Textile and Apparel , Vol. 16 No. 2, pp. 34-38. https://doi.org/10.1108/RJTA-16-02-2012-B003
Emerald Group Publishing Limited
Copyright © 2012 Emerald Group Publishing Limited
Related articles
We’re listening — tell us what you think, something didn’t work….
Report bugs here
All feedback is valuable
Please share your general feedback
Join us on our journey
Platform update page.
Visit emeraldpublishing.com/platformupdate to discover the latest news and updates
Questions & More Information
Answers to the most commonly asked questions here
- Privacy Policy
- Login/Register
Search form

FFTC Agricultural Policy Platform (FFTC-AP)
- Policy Articles
- Policy-Related News
- Accepted Manuscripts
- About E-Journal
- External Links
You are here
Production of tea from the flower of blue ternate (clitoria ternatea linn.): a new social enterprise for blue ternate growers in the municipality of batad, iloilo, philippines.

The study determines the nutrient values, safety, and market acceptability of the flower of Blue Ternate (Clitoria ternatea L. ) tea specifically by an identified and target tea user in terms of health and wellness benefits, nutritional values, hygiene and safety. This study used descriptive method of research using the laboratory tests results of the Blue Ternate dried flower to determine the nutrient values and a survey questionnaire for its market acceptability. The respondents were sixty (60) potential tea users taken by purposive sampling. Data were statistically processed and analyzed using frequency count and percentages. Nutritional values of Blue Ternate Tea included the nutritional and proximate analysis limited to the priority macronutrients: fiber, protein, fat and ash; the caloric content was based on the laboratory test results per grams and per sample of the tea by the Philippines’ Department of Science and Technology (DOST). Laboratory test results showed that the tea product was hygienic and safe for consumption. Microbiological analysis and evaluation shown no indications of pathogen entry and tea produced was in adherence to current practices and standard and ingredient acceptability as indicated by the laboratory test results of Aerobic Plate Count (APC), Yeast and Mold Count (YMC), Escherichia coli (E. coli) and Staphylococcus aureus. In terms of market acceptability, results of the survey revealed that the Blue Ternate tea was hygienically prepared, safe to be consumed and had high level of market acceptability. Economically, it will bring about future source of rural livelihood for people to be involved in the propagation and collection of the raw materials and future cultivation. Employment opportunities are envisioned for those who will be involved in product development and packaging of Blue Ternate Tea. The mass propagation and sustainable production of the tea derived from Blue Ternate will ultimately address the program of the present government on Sustainable Development Goals (SDGs) for health security, poverty alleviation and reduction platform. This project was anchored on the platform of The Philippine Development Plan (PDP) 2017-2022 for Agriculture, Forestry and Fishery Sector. Mass production of tea from this indigenous plant promotes job generation to the Filipino people and inclusive growth through the development of micro, small and medium enterprises in consonance to RA 10644 otherwise known as the “Go Negosyo Act” of 2013.
Keywords: Blue ternate ( Clitoria ternatea L.), nutrient values, market acceptability, health and wellness, safety, tea production, economic profitability
INTRODUCTION
Blue Ternate also branded as Butterfly Pea and scientifically known as Clitoria ternatea is now getting well-known in the Philippines because of its combined health benefits and unique aesthetic value. The following are agronomic information in propagating Blue Ternate pea plant: (1) Soil Adaptation. Blue Ternate is well adapted to grow in wide range of soil types (in between pH range 5.5-8.9) from deep alluvial to sandy including calcareous soils. It extremely well adapted to heavy clay alkaline soils, and especially on clay soils but also grow well in moderate fertile soils. Clitoria ternatea likes a rich, moist soil (peat moss: part sand or perlite 2:1:1) therefore the soil should be evenly moist at all times for well growth; (2) Water Requirement . It requires approximately 400mm of rainfall but also performs well under irrigation areas and grows from drier to the fairly drought tolerant areas. Due to the nature of Blue Ternate , it cannot tolerate prolonged inundation or water logging but can tolerate short term flooding; (3) Favorable Temperature and Sunlight. It needs moderate temperature down to 25 0 C but not suited to locations with frequent frosts or severe frosts, but it stands up well in hot summer temperature and having low frost tolerance. It is moderately shade tolerant but can normally grow in full sunlight; (4) Fertilizer Requirement. Blue Ternate is normally grown in soil containing phosphorus (P) and sulfur(S) which may be required as fertilizers if sown in the infertile soils; and (5) Propagation. The Blue Ternate pods contain around 20% of hard seed according to the seasonal conditions in where it is produced and grow rapidly in warm-moist weather. It is harvested manually by hands and is propagated from seed or by cuttings. The seeds of Blue Ternate are covered by hard seed coats therefore do not germinate or imbibe water, but when stored for 6 months 15-20% germination can be obtained. The used of hot water, sulfuric acid (H 2 SO 4) , potassium hydroxide and soaking in 100mg/L solution of Sodium cyanide (NaCN) has also improved germination and early plant growth while mechanical scarification increased germination of 6-month-old seed from 30% to 71%. This perennial climber blooms the whole year by the used of trellising. It is expected that the plant can bloom in 6 weeks. It has a twining fine stem about 0.5-3m long. The leaves are pinnate, with 5-7 elliptic to lanceolate lengths, 3-5cm long. Pods are flat, linear, beaked, 6-12cm long, 0.7-1.2mm wide and slightly pubescent with up to 10 seeds. The seeds are olive, brown or black in color, often mottled, 4.5-7mm long and 3-4mm wide (Manju Latu Zingare et al., 2009).
Plantation Area
The seeds and other materials that were used during propagation were provided by the Farmers Information and Technology Services (FITS) Center under the umbrella of Agricultural Training Institute (ATI-ROF6) Regional Field Office 6 of the Department of Agriculture. Furthermore, this wonder herb is grown abundantly under College’ Techno-Demo Farm propagated by the Agriculture students in the School of Agriculture. For technology dissemination, the Office of Research, Development and Extension (RDE) Services of the Northern Iloilo Polytechnic State College (NIPSC) Batad Campus took a major role. The propagation of Blue Ternate herb was extended to the community to cater future source of livelihood to those who are involved in large and sustainable scale production. Employment opportunities are envisioned for those who will be involved in product development and packaging of Blue Ternate Tea for value adding.
Health Benefits of Blue Ternate Tea
Tea is the most widely consumed beverage in the world next to water (Knite, 2014). The trend and foreseen demand for drinks with helpful benefits make an opportune time to uncover another “tea flavor”. Blue Ternate ( Clitoria ternatea L.) is a potential source of phytochemicals and hormones with nutritional and helpful benefits. The flower has the power to heal and offer beneficial enzymes needed by the body that other plants cannot provide. There are various benefits that can be derived from the flower of Butterfly Pea: It has natural antioxidants, helps improve blood circulation, helps prevent hair loss and graying. It also cleanses blood, improves night vision, revitalizes skin and hair (Dizon, 2014). Butterfly pea herb has been proven as a safe herb and this has not any major side effect or mortality. It is used in enhancing memory and reducing stress. The flower extract of the plant has been proven scientifically to protect against free radical and is full of antioxidants. Furthermore, the levels of some non-enzymic antioxidant namely ascorbic acid, reduced glutathione and total carotenoids were estimated in flowers of Butterfly Pea. (Venkateshwaran, 2015).
The Key Players of Blue Ternate Flower Tea in the Global Market
The key players operating in the global Blue Ternate flower tea market are Siam Industries International, Yumchaa, Manila Superfoods, Morning Farm, healthy Organic, Woodland Foods, Chaidee Factory Co., Ltd., Bluechai, Longevity Warehouse, and Tea Forte. In 2016, Seven Tea One launched its Blue Ternate flower tea commercial products in South East Asia. The company uses a sustainable urban farming concept to target growing ethical and healthy food and beverage consumer base of the region. In 2018, Starbucks Asia launched a special edition cold brew beverage for the spring season based on the Blue Ternate flower tea. The Blue Ternate flower tea was chosen both for its aesthetic value and cooling effect. Geographies chosen for the Blue Ternate flower tea infused cold brew drink are Malaysia, Philippines, Thailand, Cambodia, Taiwan, Vietnam, and other major South East Asian countries. Developed regions like North America and Europe is anticipated to form a major consuming region in terms of value and volume of global Blue Ternate flower tea market. However, Asia Pacific region is expected to form a dominant market for the butterfly pea flower tea resulted in traditional consumption and well-established market in the region. Latin America is expected to form a substantial volume demand for the Blue Ternate flower tea resulted in its growing healthy food trend in the region. The Middle East and Africa to witness overall slower growth dynamics resulted from its unorganized retail sector [1] .
From RDE-based research to business opportunities for micro-entrepreneurs
Due to this overwhelming turn-out in terms of the market acceptability of Blue Ternate tea in the global scale, and because of the increasing demand among the healthy-pursue tea users in the Philippines and considering the health benefits of Blue Ternate tea, the researchers were motivated to conduct another technological research that would determine the nutrient values and market acceptability of the tea produced from the flower of Blue Ternate in the local settings. Owing to greater nutritional value, the social enterprise of the Blue Ternate flower tea is expected to form strong consumer base in the herbal tea market. Increasing per capita food spending and expanding retail sector is expected to help Blue Ternate flower tea producer to gain traction quickly in developing regions of the Asia Pacific and Latin America as a premium offering. Organic Blue Ternate flower tea segment is anticipated to experience faster growth rates in the global Blue Ternate flower tea market. In the Philippines, the mass propagation and sustainable production of the tea derived from Blue Ternate will ultimately address the program of the present government on Sustainable Development Goals (SDGs) for health security, poverty alleviation and reduction platform by enhancing micro, small and medium enterprises (MSMEs) to venture and invest in this new lucrative business. Blue Ternate flower tea was identified to be one of the key segments under the herbal tea market which has a competitive nutritional profile and a key competitor in the key herbal tea types. Millennial and baby boomers are identified as the key consumer base for the Blue Ternate flower tea market which is expected to be targeted by the key players in the market through various marketing approach. To increase know-how of the target markets about traditional based food, the products is posted and advertise in various internet online publications and social media platforms such as Facebook, Instagram, and YouTube channel.
OBJECTIVES OF THE STUDY
The study determined the nutrient values, safety, and market acceptability of the flower of Blue Ternate ( Clitoria ternatea L.) tea specifically by an identified and target tea user in terms of health and wellness benefits, nutritional values, hygiene and safety.
Specifically, it aimed to answer the following questions:
- What are the nutritional values derived from the powdered tea of the flowers of Blue Ternate?
- Is powdered tea derived from the flower of Blue Ternate hygienically safe for consumption?
- What is the level of market acceptability of powdered tea based on the assessment result obtained from potential tea consumers?
RESEARCH METHODOLOGY
This study used descriptive method of research using the laboratory tests results of the Blue Ternate dried flowers to determine the nutrient values and a survey questionnaire for its market acceptability. The respondents were 60 potential tea users taken by purposive sampling. Data were statistically processed and analyzed using frequency count and percentages. Nutritional values of Blue Ternate Tea included the nutritional and proximate analysis limited to the priority macronutrients: fiber, protein, fat and ash; the caloric content was based on the laboratory test results per grams and per sample of the tea by the Department of Science and Technology (DOST). The procedural design in the preparation of the materials in the production of tea from Blue Ternate is illustrated in Figure 1.
RESULTS AND DISCUSSION
Nutrient values of Blue Ternate
Results of the laboratory tests revealed that in terms of nutritional value, Blue Ternate tea had a considerable amount of nutrients that could supplement the daily diet. Nutrients available per 3 g serving size, were total fat (0.69%); crude protein (6.43%), carbohydrate (18.8%) and ash/traces of minerals (1.40%). Energy available in blue ternate was 107.3 kcal per serving.
MICROBIOLOGICAL TEST RESULTS OF BLUE TERNATE TEA
Laboratory test results showed that the tea product was hygienic and safe for consumption. Microbiological analysis and evaluation showed no indications that there is any pathogen entry and tea produced was in adherence to current practices and standard as indicated by the laboratory test results of Aerobic Plate Count (APC), Yeast and Mold Count (YMC), Escherichia coli ( E. coli ) and Staphylococcus aureus .
MARKET ACCEPTABILITY OF BLUE TERNATE TEA
Results showed that of the 60 potential tea users 17 or 23.33%, positively accepted Blue ternate tea as naturally produced tea with no preservatives, 21 or 35% agreed that tea product has plenty of health benefits, 11 or 18.33% agreed that the tea product suited their taste and 18.33% agreed that the tea product has attractive color, pleasant odor, and is convenient to use if it is readily available in the market. Results further showed that potential tea users had indicated positive response towards using of Blue ternate tea once it would be readily available in the market in convenient packaging.
Blue ternate tea had shown good indicators of marketability because of its nutritional value, as indicated in the laboratory test results and microbiological tests. Overall results showed that the Blue Ternate tea has positive market acceptability. If we are to base in the context of promotional marketing, it is considered that the viability of producing Blue Ternate tea have a high market segment in terms of consumers (tea users), price (economical), tea product (high value for health and wellness). Furthermore, this wonder herb can be abundantly grown under NIPSC Batad condition. In terms of economic profitability, it will cater future source of livelihood to those who are involved in large and sustainable propagation and production. Employment opportunities are envisioned for those who will be involved in product development and packaging of Blue Ternate Tea.
Blue Ternate tea social enterprise in support of RA 10644 of the Philippine law
The Philippines enacted into law Republic Act (RA) 10644, otherwise known as an act promoting job generation and inclusive growth through the development of micro, small and medium enterprises or the “Go Negosyo Act” of 2013 in support to the Filipino people directly engaging or to those who are planning to engage in micro, small and medium enterprises (MSMEs) like the production of tea from an indigenous plant locally and abundantly grown in the Municipality of Batad, Iloilo. In this law, it is hereby declared, the policy of the State to foster national development, promote inclusive growth, and reduce poverty by encouraging the establishment of micro, small and medium enterprises that facilitate local job creation, production and trade in the country. MSMEs increase income for poor households and build both business equity and personal assets over a period of time. To this end, the State shall develop plans and initiate means to ease the constraints on the establishment of MSMEs in order to rationalize the existing bureaucratic regulations, providing greater incentives and benefits to MSMEs, and strengthening the Micro, Small and Medium Enterprise Development (MSMED) Council (RA 10644, section 2).
The mass propagation and sustainable production of the tea derived from Blue Ternate will ultimately address the program of the present government on Sustainable Development Goals (SDGs) for health security, poverty alleviation and reduction platform. This project was anchored on the platform of The Philippine Development Plan (PDP) 2017-2022 for Agriculture, Forestry and Fishery Sector. The development agenda was crafted in-line with the President’s 10-point Socio-economic Agenda pursuant to Executive Order No. 5, s. 2016, including: (1) a prosperous, predominantly middle-class society where no one is poor; (2) a healthy and resilient society; (3) a smart and innovative society; and (4) a high trust society.
To promote ease of doing business and access to services for this particular business enterprise for MSMEs within its jurisdiction, the production, processing and product development of Blue Ternate tea has gain merit of acceptance from the Intellectual Property Office of the Philippines (IPOPHL) by virtue of the issuance of Certificate of Utility Model Registration Number: 2-2019 000256 and Trademark Registration Number: 508581 which can be helpful for product recognition and for its business operations in the future upon the approval of the Bureau of Food and Drug Administration (BFAD) for public use and consumption.
CONCLUSIONS
Blue Ternate tea contains nutritive and medicinal values as indicated in its health and wellness benefits based on laboratory test results and market acceptability of tea consumers. The tea product is hygienic and safe for consumption as revealed in the results of the microbiological analysis and product evaluation tests. The tea produced from the flower of Blue Ternate is positively accepted and has good market potential due its health and wellness benefits.
The wonder herb can be abundantly grown under NIPSC Batad condition. Large scale production of this tea product will ensure future source of livelihood to our local communities by virtue of the establishment of micro, small and medium enterprises that facilitate local job creation, production and trade in the country in consonance to RA 10644 or the Go Negosyo Act of 2013. The mass propagation and sustainable production of the tea derived from Blue Ternate will ultimately address the program of the present government on Sustainable Development Goals (SDGs) for health security, poverty alleviation and reduction platform especially for those who are living in the countryside.
RECOMMENDATIONS
Product development, packaging and value adding for commercialization of Blue Ternate tea is highly recommended. Mass production of this product will boost backyard growers to start a small-scale business of selling plants’ vegetative parts for propagation (including seeds/seedlings and flowers) aside from tea products. Having stated the health and wellness benefits of Blue Ternate, the researchers also recommend the proliferation of this herb in the backyards of every household for the consumption of the family.
Further researches are encouraged to use other parts of this herbal plant such as leaves, stem, fruits and roots to further develop its nutritive potential and medicinal benefits.
Department of Health-Bureau of Food and Drugs. DOH (2004) Administrative Order No. 153 S. 2004: Revised Guidelines on Current Good manufacturing Practice in Manufacturing, Packing, Repacking, or Holding Food. Retrieved from http://www.doh.gov.ph
Department of Health - Bureau of Food and Drugs (DOH-BFAD). (1999). Memorandum Circular No. 02, S. 1999: Amendment to BFAD M.C. No. 25, s. 1992 Otherwise Known as “Additional Labelling Requirement for Food Supplements”. Retrieved from http://www.doh.gov.ph
Department of Health-Bureau of Food and Drugs. DOH-BFAD (2004) Bureau Circular Order No.0-4 S. 2004: Guidelines for the Assessment of Microbiological Quality of Processed Foods. Retrieved from http:// www.doh.gov.ph
Department of Health-Bureau of Food and Drugs. DOH-BFAD (2005) Bureau Circular Order No. 16 S. 2005: Adopting the 2002 recommended energy and nutrient intake as the new dietary standard Retrieved from http://www.doh.gov.ph
Dizon,R., 2014. The Extentionalist’s Lense: The Healing Wonders of Blue Flower ( www.ati . da. gov. ph)
Gupta GK, Chahal J and Bhatia M Clitoria ternatea (L.): Old and new aspects. Journal of Pharmacy Research, 3: 2610-2614, 2010
Kshetrimayum, B. Medicinal and its Therapeutic uses, A Review on Clitoria ternatea Linn.; Chemistry and Pharmacology, OMICS International; page 374 and 376’. 2017
Manju Lata Zingare, 2009: total Phenolic Compound and Scavenging Activity in Clitoria ternatea and Vitex negundolinn.
Malik J. Karan M, VasishtK Nootropic, anxiolytic and CNS- depressant studies on different plant sources of shankhpushpi. Pharm Biol., 2011
NeelammaG, Swamy BD and Dhamodaran P. Pharmacognostical, Phytochemical and pharmacological perspective of Clitoria ternatea L. International Journal of Current Trends in Pharmaceutical Research, 4: 159-165., 2016
Properties of Flowers of Clitoria ternatea retrieved via www.ifrj.upm.edu
Rabeta, M. S. and Nabil, Z., (2012). Total Phenolic Compound and Scavenging Activity of Clitoria ternatea”
Sarumathy K, Rajan MSD, Vijay,T and Jayakanthi, J. (2011) Evaluation of phytoconstituents, nephro- protective and antioxidant activities of Clitoria ternatea. Journal of Applied Pharmaceutical Science, 1: 164-172., 2011
Sethiya, NK, An update on Shankpushpi, a cognition- boosting Ayurvedic medicine Zhong Xi Yi Jie He XueBAo., 2009
Taur DJ, and Patil R.Y. (2011) Evaluation of antiasthmatic activity of Clitoria ternatea L. roots .J Ethnopharmacol. Retrieved via www.
Dizon,R., 2014. The Extentionalist’s Lense: The Healing Wonders of Blue Flower ( www.ati . da. gov. ph) Available from: https://www.researchgate.net/ publication/286463760_Clitoria_ternatea_Linn_An _
Neda, G. D., Rabeta, M. S. and Ong, M. T.,International Food Research Journal 20(3): 1229-1234 (2013), Chemical composition and anti-proliferative properties of flowers of Clitoria ternatea( www.ifrj.upm.edu.my )
Venkateshwaran, R, (2015). Medicinal Uses of Butterfly Pea retrieved via www.wildturmeric.com
[1] www.openpr.com/news/1872981/butterfly-pea-flower-tea-market-business-analysis
- Please login to bookmark this post
- 102102 read

You may also like

Policies in Vietnam’s Tea Industry
Development of good agricultural practices (gaps) models for tea, rice and vegetables in vietnam, introduction of technology on verification of oolong tea’s product origin.
An Experimental Study on Effects of Blue Ternatea in Alleviating Anxiety and Stress among Jose Rizal University’s College Students
Literature review, 2021, 11 pages, grade: 1.8, bachelor of science in psychology aldrin puerto et al. (author).
Abstract or Introduction
Stress can cause significant consequences in our health alongside with coping anxiety. It has become widely known problems in the society nowadays more especially of pandemic that also introduces the implementation of virtual classes to students. Students in this matter are affected not only for the finances to go on learning but also anxiety and stress. Level of stress and anxiety can increase if stimulated by some factors. The fundamental reason for this proposal is that findings could help in resolving different psychological conditions specifically stress and anxiety of an individual person. It is believe that stress and anxiety could further develop in to more serious mental condition like depression. And so, we the researchers conducted this study to examine the effects of Blue Ternatea in alleviating stress and anxiety condition. Furthermore, positive results of this study could lead into new scientific basis and understandings on how to help people with these kind of mental conditions and it could also influence clinical practices and public health policies
- No comments yet.

Similar texts

José Rizal und der Unabhängigkeitskampf der Filipinos bis 1896

Stress during studies. Mental stress and illness among students in face-to-fa...

Allgemeingültige Wirksamkeit von Coping Mechanismen gegen Stress bei Studiere...

Effects of temporary examination stress on biochemical parameters in academic...

The Search for Identity in Leslie Feinberg's "Stone Butch Blues"

Work Stress. Why Organisations should focus on it and provide effective examples

The Effects of Nutrition on Student’s Academic Performance among Secondary Sc...

A Cross sectional Study of Contextual Causes of Demotivation among Algerian U...

Cyberbullying among University Students in Nigeria

Fear of Death Among University Students. A Study

Factors Responsible for Aggressive Behaviour Among Students in Anambra State ...

Effects of New Business Models on Organizations in the Field of Student Tutoring
Effects of Task-based Language Teaching on the Students' Motivation with ...

The Factors Influencing International Students' Choice of Working in Taiw...

Determinants of Strategic Choice among Universities in Kenya

Driver misconduct, traffic congestion and the effectiveness of urban transpor...

The Effect of Japan's Structural Difficulties in the 1990s on the Japanes...

Effect of N-Management Practices and different organic Sources on Growth and ...

Effectiveness of the economic sanctions imposed by the EU on Russia since Mar...
Upload papers
Your term paper / thesis:
- Publication as eBook and book - High royalties for the sales - Completely free - with ISBN - It only takes five minutes - Every paper finds readers
Publish now - it's free

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Plant Sci
Butterfly Pea ( Clitoria ternatea ), a Cyclotide-Bearing Plant With Applications in Agriculture and Medicine
The perennial leguminous herb Clitoria ternatea (butterfly pea) has attracted significant interest based on its agricultural and medical applications, which range from use as a fodder and nitrogen fixing crop, to applications in food coloring and cosmetics, traditional medicine and as a source of an eco-friendly insecticide. In this article we provide a broad multidisciplinary review that includes descriptions of the physical appearance, distribution, taxonomy, habitat, growth and propagation, phytochemical composition and applications of this plant. Notable amongst its repertoire of chemical components are anthocyanins which give C. ternatea flowers their characteristic blue color, and cyclotides, ultra-stable macrocyclic peptides that are present in all tissues of this plant. The latter are potent insecticidal molecules and are implicated as the bioactive agents in a plant extract used commercially as an insecticide. We include a description of the genetic origin of these peptides, which interestingly involve the co-option of an ancestral albumin gene to produce the cyclotide precursor protein. The biosynthesis step in which the cyclic peptide backbone is formed involves an asparaginyl endopeptidase, of which in C. ternatea is known as butelase-1. This enzyme is highly efficient in peptide ligation and has been the focus of many recent studies on peptide ligation and cyclization for biotechnological applications. The article concludes with some suggestions for future studies on this plant, including the need to explore possible synergies between the various peptidic and non-peptidic phytochemicals.
Introduction
Clitoria ternatea , commonly known as butterfly pea, is a perennial herbaceous plant from the Fabaceae family. It has recently attracted a lot of interest as it has potential applications both in modern medicine and agriculture, and as a source of natural food colorants and antioxidants. C. ternatea has long been cultivated as a forage and fodder crop, and early studies assessed the plant for these purposes ( Reid and Sinclair, 1980 ; Barro and Ribeiro, 1983 ; Hall, 1985 ). Numerous field trials in Queensland, Australia, eventually led to the registry of C. ternatea cv. ‘Milgarra’ ( Oram, 1992 ), the only cultivar in Australia that was released for grazing purposes ( Conway and Doughton, 2005 ). Additionally, C. ternatea has been widely used in traditional medicine, particularly as a supplement to enhance cognitive functions and alleviate symptoms of numerous ailments including fever, inflammation, pain, and diabetes ( Mukherjee et al., 2008 ).
In as early as the 1950s, studies on C. ternatea sought to elucidate its pharmacological activities, phytochemical composition and active constituents ( Grindley et al., 1954 ; Piala et al., 1962 ; Kulshreshtha and Khare, 1967 ; Morita et al., 1976 ). The novel C. ternatea anthocyanins termed “ternatins” which render C. ternatea flowers with their vivid blue color, were first isolated in 1985 ( Saito et al., 1985 ). Following further isolation and structural characterization of numerous other ternatins, the ternatin biosynthetic pathway was postulated a decade later ( Terahara et al., 1998 ). In 2003, comparison of C. ternatea lines bearing different floral colors provided insights into the role of acylation on C. ternatea floral color determination ( Kazuma et al., 2003a ). The abundance of these unique anthocyanins alongside other secondary metabolites in C. ternatea makes the plant an ideal source of natural additives that can enhance the appearance and nutritive values of consumer products ( Pasukamonset et al., 2016 , 2017 , 2018 ; Siti Azima et al., 2017 ). Although a number of recent studies has endeavored to elucidate the pharmacological activities of C. ternatea ( Adhikary et al., 2017 ; Kavitha, 2018 ; Singh et al., 2018 ), the contribution of individual extract components on any bioactivity measured remains unknown.
Figure 1 summarizes some of the key agricultural and biochemical studies conducted on C. ternatea from the 1950s to the present, providing a convenient timeline of discoveries. The corresponding references to the key studies and milestones are listed in Table 1 . In recent years, the small circular defense molecules called cyclotides, in C. ternatea ( Nguyen et al., 2011 ; Poth et al., 2011a , b ; Nguyen et al., 2014 ) have fueled scientific innovations that may have impact in modern agriculture, biotechnology and medicine. In 2017, Sero-X ® , a cyclotide-containing eco-friendly pesticide made from extracts of C. ternatea , was approved for commercial use in Australia 1 . In addition, the C. ternatea cyclotide processing enzyme, butelase-1, which is the fastest ligase known to date and is capable of ligating peptides across a vast range of sizes (26 to >200 residues), can potentially be used in the large scale synthesis of macrocycle libraries and peptide-based pharmaceuticals ( Nguyen et al., 2014 , 2015 ).

Timeline of the key studies and milestones on Clitoria ternatea research from the 1950s to the present. The biological (blue) and biochemical (purple) studies pursued from the 1950s to early 1970s characterized the properties of roots and seeds. Toward the end of the 1970s, researchers began to isolate and characterize the phytochemical compounds from C. ternatea . Ternatins, the anthocyanins that render C. ternatea its vivid blue color, were first isolated in 1985; and the structure of the largest of the ternatins, ternatin A1, was characterized in 1989. Further isolation and characterization of the ternatins in C. ternatea led to the elucidation of the ternatin biosynthetic pathway in 1998. Parallel to the studies that characterized the phytochemical composition of C. ternatea , were agricultural studies that evaluated C. ternatea as a forage and fodder crop. A series of field studies in Queensland, Australia lead the development and eventual release of the C. ternatea Milgarra cultivar in 1991. From 2001 to the present, studies have been determining the pharmacological activities and biological activities of C. ternatea extracts. In 2011, cyclotides, the circular insecticidal molecules which can also be used as scaffolds for peptide-based therapeutics, were discovered in C. ternatea. While cyclotides had previously been characterized in other angiosperm species, C. ternatea is to date, the only legume that is known to produce them. In 2014, butelase-1, the ligase that facilitates cyclization in C. ternatea cyclotides, was discovered and characterized. Cyclotides and the auxiliary enzymes, have applications both in modern medicine and agriculture. In 2017, Sero-X ® an eco-friendly insecticide made from C. ternatea extracts was registered for commercial use in Australia.
Milestones in Clitoria ternatea studies.
Plant Description
Clitoria ternatea produces pentamerous zygomorphic pea-shaped flowers with a tubular calyx consisting of five sepals which are fused about two thirds of their length. The showy corollae consists of five free petals, with one large and rounded banner, two wrinkled wings which are often half the length of the banner and two white keels which aid in protecting the floral organs ( Cobley, 1956 ; Biyoshi and Geetha, 2012 ) ( Figure 2A ). The corollae are most often dark blue in color but may also occur in white and various blue and white shades in between ( Morris, 2009 ; Biyoshi and Geetha, 2012 ). The diadelphous C. ternatea stamens consist of 10 filaments where nine are fused and one is free lying ( Cobley, 1956 ; Biyoshi and Geetha, 2012 ). Attached to each filament is a pollen-bearing white anther, which consists of four lobes ( Cobley, 1956 ; Pullaiah, 2000 ). C. ternatea produces a monocarpellary ovary bearing ten ovules ( Pullaiah, 2000 ; Biyoshi and Geetha, 2012 ). Surmounting this is a long and thick style with a bent tip ( Cobley, 1956 ; Biyoshi and Geetha, 2012 ). C. ternatea pods are narrow and flattened with pointy tips, and they typically contain around 10 seeds ( Cobley, 1956 ) ( Figure 2B ). The seeds contain palmitic acid (19%), stearic acid (10%), oleic acid (51-52%), linoleic acid (17%) and linolenic acid (4%) ( Grindley et al., 1954 ; Joshi et al., 1981 ). The caloric content of the seed is reported to be around 500 cal/100 g ( Joshi et al., 1981 ). C. ternatea produces pinnate compound leaves that are obovate and entire with emarginate tips ( Taur et al., 2010 ) ( Figure 2C ). The epidermis on both leaf surfaces consist of a single layer of cells protected by a thick cuticle and with trichome outgrowths ( Taur et al., 2010 ). A layer of palisade cells, lignified xylem and paracytic stomata lie underneath the upper epidermis ( Taur et al., 2010 ). C. ternatea produces an extensive deep-root system, which enables the plant to survive up to 7–8 months of drought ( Cobley, 1956 ). The roots also produce large nodules for nitrogen fixation ( Cobley, 1956 ) ( Figure 2D ).

Clitoria ternatea (A) flower, (B) pods, (C) leaves, and (D) roots with nodules. The C. ternatea flower consists of the stamen (st), pistil (p), sepals (sp), and corollae. The corollae consist of five petals: one banner (b), two wings (w) and two keels (k). C. ternatea has pinnate compound leaves, flat and pointed pods and roots that produce nodules (n).
Taxonomy, Geographic Distribution and Habitat
The genus Clitoria occurs in tropical and subtropical environments across the globe. The number of subfamilial taxa remains unclear, and as in the case of Clitoria , the descriptions of species and citations of type specimens are noted as being incomplete or incorrect according to Fantz (1977) . Thus, it is difficult to estimate species richness of the genus. Within Clitoria , three subgenera have been described and held as valid according to the monograph of Clitoria . Across all three subgenera, Fantz retains 58 species as valid, with numerous lower classifications of varieties and subspecies ( Fantz, 1977 ).
Clitoria ternatea is the holotype of Clitoria subgenus Clitoria, and represents the archetypical Clitoria . The etymology of the specific name is postulated to be from the island of Ternate in the Indonesian archipelago because it is from specimens from that location that Linnaeus produced the specific description. Ternate is not in the Indian Ocean but is instead in the Molucca Sea and in eastern Indonesia, lending ambiguity to the native range of the species. The distribution of all other taxa in subgenus Clitoria is restricted to Southern and Eastern Africa, India, Madagascar, and other islands of the Western Indian Ocean ( Figure 3 ). The exact geographic origin of C. ternatea is thus difficult to determine, but we may infer from the center of diversity for subgenus Clitoria, that C. ternatea arose in or around the Indian Ocean and not the Pacific Ocean or South China Sea where it has been in use as a food coloring historically ( Fantz, 1977 ; Staples, 1992 ). It is also entirely possible that the taxon we know as C. ternatea is an ancient hybrid of one or more members of the subgenus Clitoria that had subsequently been introduced to Southeast Asia. Testing of this synthetic origin hypothesis would require large scale genetics work on C. ternatea and related taxa like Clitoria biflora , C. kaessneri , C. lasciva , and C. heterophylla . Regardless of the specific geographical origin and evolutionary history of C. ternatea , the present day distribution of naturalized populations of C. ternatea is pantropical, as facilitated by key characteristics of the species: tolerance to drought conditions, non-reliance on specific pollinators because of self-pollination, and nitrogen fixation capability ( Cobley, 1956 ; Staples, 1992 ; Conway et al., 2001 ). It is also possible to cultivate and maintain populations in subtropical regions (ex. Wee Waa NSW, located at -30.2, 149.433333).

Distribution of Clitoria subgenus Clitoria species adapted from Fantz, 1977 . Points of occurrence are approximate. Map data from Openstreetmap.org . Symbols represent: ◼ C. biflora , □ C. heterophylla , Δ C. kaessneri , • C. lasciva , + C. ternatea .
The habitat of C. ternatea is open mesic forest or shrub land (personal observations of authors and records in the Australasian Virtual Herbrarium 2 ). In Australia, the authors note that populations of C. ternatea occur in tropical regions in open areas where sunlight is plentiful due to a sparse canopy and in areas near where fresh water would collect such as the border of wetlands, small gullies, or at the base of rocky hillsides. When present, the plants are often vigorous and smother other vegetation.
Growth and Propagation
Germination and establishment of C. ternatea is most favorable when the temperature is between 24–32°C, and when seeds are sown in moist soil at 2.5–5 cm deep and 20–30 cm apart ( McDonald, 2002 ; Conway, 2005 ). Although C. ternatea can withstand arid conditions ( Cobley, 1956 ), the plant grows best with ample moisture and rainfall (650–1250 mm) and when the temperature reaches 27°C or higher ( Conway and Collins, 2005 ). Like most tropical legumes, C. ternatea is susceptible to frost damage ( Conway and Collins, 2005 ). However, it can retain its leaves for as long as 7 days, and its woody parts typically recover ( Conway and Collins, 2005 ).
Despite its hardy features, one of the impediments in propagating C. ternatea is its low seed germination rate. This problem has long been recognized as evident in a study conducted in 1967 ( Mullick and Chatterji, 1967 ). The study showed that freshly harvested C. ternatea would not imbibe water and germinate ( Mullick and Chatterji, 1967 ). On the other hand, storing the seeds for another 6 months promoted germination in 15–20% of the seeds ( Mullick and Chatterji, 1967 ). Chemical scarification by means of soaking the seeds in boiling water or sulfuric acid was also found to promote C. ternatea seed germination ( Cruz et al., 1995 ) where soaking the seeds in concentrated sulfuric acid for at least 10 min resulted in a reported 100% seed germination rate ( Patel et al., 2016 ).
In vitro propagation can circumvent the unreliably low seed germination rate in C. ternatea. It can also be an alternative method for conserving and mass propagating C. ternatea lines with superior qualities. In 1968, a study determined the effects of adding ascochitine on the growth of C. ternatea embryos ( Lakshmanan and Padmanabhan, 1968 ). That study reported that 60% of the embryos produced callus in both the upper and lower hypocotyl when 5–10 ppm ascochitine was added to the culture media. Numerous studies have since been conducted from 1990 to 2016 to determine the optimal plant hormone concentrations, basal media types and explant types for C. ternatea in vitro propagation ( Table 2 ).
Summary of published Clitoria ternatea in vitro propagation studies.
With the optimal hormone concentrations supplemented in the basal medium, callus production was observed from mature C. ternatea embryos, leaf and root explants obtained from aseptic seedlings ( Lakshmanan and Dhanalakshmi, 1990 ; Shahzad et al., 2007 ; Mohamed and Taha, 2011 ). In some instances, prolonged explant maintenance in the same callus induction medium led to embryoid production ( Lakshmanan and Dhanalakshmi, 1990 ). Recently, a study described a protocol to produce encapsulated embryogenic callus from leaf explants using the optimal hormone concentrations and 3% sodium alginate ( Mahmad et al., 2016 ). The study reported that more than 50% of the encapsulated explants stored at 4°C for 90 days survived ( Mahmad et al., 2016 ). Studies showed that shoots can be regenerated from callus ( Shahzad et al., 2007 ; Mahmad et al., 2016 ). Alternatively, shoots can also be induced and proliferated directly from different explant types such as isolated shoot buds ( Lakshmanan and Dhanalakshmi, 1990 ), axillary buds ( Mhaskar et al., 2011 ), shoot tips ( Pandeya et al., 2010 ), leaf ( Mohamed and Taha, 2011 ), and root ( Shahzad et al., 2007 ) from aseptic seedlings, cotyledonary nodes ( Pandeya et al., 2010 ; Mukhtar et al., 2012 ) and nodal explants ( Rout, 2005 ; Pandeya et al., 2010 ; Ismail et al., 2012 ; Mukhtar et al., 2012 ). These in vitro grown C. ternatea shoots when subsequently placed in a medium supplemented with the optimal auxin concentrations produced roots in vitro ( Lakshmanan and Dhanalakshmi, 1990 ; Rout, 2005 ; Shahzad et al., 2007 ; Mhaskar et al., 2011 ; Mohamed and Taha, 2011 ; Ismail et al., 2012 ; Mukhtar et al., 2012 ). Nevertheless, ex vitro root production was observed when elongated shoots were soaked in a concentrated auxin solution ( Pandeya et al., 2010 ).
Moreover, a study has described propagation of C. ternatea via hairy root cultures ( Swain et al., 2012b ). Using the wild-type Agrobacterium rhizogenes strain A4T with the optimal culture conditions, a transformation frequency of as high as 85.8% was observed ( Swain et al., 2012b ). Compared to roots obtained from outdoor grown plants, C. ternatea hairy root cultures produced fourfold the amount of taraxerol, an anticancer triterpenoid compound that is naturally produced in C. ternatea roots ( Swain et al., 2012a ).
Historical and Current Applications
Agriculture, fodder and forage crop.
Clitoria ternatea has long been cultivated as a forage crop ( Cobley, 1956 ), with yields reaching 17–29 tons/ha of palatable hay for cattle ( Barro and Ribeiro, 1983 ; Abdelhamid and Gabr, 1993 ). This yield is on par with the established forage crop, alfalfa ( Medicago sativa ), and can potentially replace it in warm areas with low rainfall ( Barro and Ribeiro, 1983 ). In Australia, C. ternatea has been cultivated predominantly in Queensland, due to its adaptability in the arid regions and persistence in heavy-textured farm lands ( Hall, 1985 ). In 1991, the Queensland Department of Primary Industries, released the C. ternatea cv. ‘Milgarra’ mainly for grazing purposes ( Oram, 1992 ). Milgarra is a composite of 21 introduced and naturalized C. ternatea lines that were grown for over three generations ( Oram, 1992 ). As it is a composite cultivar, phenotypic variations are commonly observed in the field ( Conway and Doughton, 2005 ).
Timing of harvest has been demonstrated to be important for maximizing dry matter content and digestibility of C. ternatea hay, with 45 days shown to be optimal ( Mahala et al., 2012 ). Further increases in dry matter content have been reported if C. ternatea is pruned every 42 days at 20 cm ( Colina et al., 1997 ), with dry matter yields of 1122 kg/ha reported. Compared to other legumes, animal feeds prepared from C. ternatea have consistently lower acid detergent fiber content. This low amount of acid detergent fiber increases energy density of the feed, and retains a high nitrogen content ( Jones et al., 2000 ). Thus, feeds made from this plant have favorable nutritional characteristics compared to other legume forages. C. ternatea is also a great source of carotenoids with the carotenoid content of a 6-month old hay reaching 600 mg/kg dry matter ( Barro and Ribeiro, 1983 ).
Nitrogen Fixation and Improvement of Soil Nutrient Content
Clitoria ternatea roots produce large round nodules ( Cobley, 1956 ) ( Figure 2D ) known to house nitrogen-fixing bacteria, making the plant ideal for use in a crop rotation system. As early as the 1970s, studies were conducted to assess the nitrogen-fixing capacity of C. ternatea ( Oblisami, 1974 ; De Souza et al., 1996 ). Nodulation was shown to be more favorably induced with a soil moisture content of around 25–45% with a light duration of 11–14 h and an intensity of 11–17 W/m 2 ( Habish and Mahdi, 1983 ). Supplementing the soil with sulfur was also demonstrated as beneficial for nodule formation ( Zaroug and Munns, 1980a ). Several studies have reported the benefits of C. ternatea to soil health ( De Souza et al., 1996 ; Dwivedi and Kumar, 2001 ; Kamh et al., 2002 ; Alderete-Chavez et al., 2011 ). Field trials conducted in Mexico reported that at 180 days post planting of C. ternatea , the organic matter, N, P, and K content of the soil all increased significantly ( Alderete-Chavez et al., 2011 ). A similar study conducted in India reported that intercropping C. ternatea with the fodder crop Setaria sphacelata enriched the N content of the soil to an estimated 39.8 kg/ha ( Dwivedi and Kumar, 2001 ). The results suggest that intercropping C. ternatea may potentially lead to a shorter fallow period requirement ( Njunie et al., 2004 ).
When considering crop rotations, it is important to determine the cross nodulation capacity of nitrogen fixing Rhizobium species. One study showed that the Rhizobium species isolated from C. ternatea , cow pea and soybean are more compatible to each other than other legume species ( Oblisami, 1974 ), while cross inoculation of Rhizobium sp. from C. ternatea and the legume species, Phaseolus vulgaris , M. sativa , and Pisum sativum , produced no nodules ( Oblisami, 1974 ). These studies provide insights as to which legume species, when planted together with C. ternatea , are more likely to form nodules and thereby yield the most soil benefits. Another early study showed that the symbiotic efficiencies measured, based on C. ternatea dry matter yield, varied depending on the Rhizobium sp. strains tested ( Zaroug and Munns, 1980b ). A more recent study reported the isolation and identification of 11 rhizobial strains from C. ternatea grown in Thailand ( Duangkhet et al., 2018 ). The 16s rDNA phylogenetic analysis revealed that ten of these isolates were Bradyrhizobium elkanii strains while the remaining isolate was a Bradyrhizobium japonicum strain. These C. ternatea B. elkanii strains were shown to promote better plant growth and induce higher nitrogen-fixing capacity than B. elkanii strains isolated from soybean ( Duangkhet et al., 2018 ).
The popular use of C. ternatea in traditional medicine has stimulated researchers to elucidate the pharmacological activities of extracts obtained from various C. ternatea tissues. Numerous animal studies have reported that the extracts exhibit diuretic, nootropic, antiasthmatic, anti-inflammatory, analgesic, antipyretic, antidiabetic, antilipidemic, anti-arthritic, antioxidant, and wound healing properties. The results of the animal and in in vitro studies are summarized in Tables 3 and and4, 4 , respectively. Although these combined studies claim that C. ternatea extracts showcase a diverse range of pharmacological properties, many of these studies are preliminary and require more thorough investigation. In many instances the authors have attributed the extract activities to the presence of flavonols and anthocyanins, however, attempts to isolate and test individual components are limited. Indeed several components in C. ternatea extracts could be acting synergistically. For instance, cyclotides which have been reported to have immunosuppressive properties may contribute ( Gründemann et al., 2012 , 2013 ; Thell et al., 2016 ), as could the abundance of delphinidins ( Sogo et al., 2015 ; Tani et al., 2017 ; Harada et al., 2018 ).
Animal studies and clinical trial demonstrating the pharmacological activities of Clitoria ternatea extracts.
In vitro studies demonstrating the pharmacological properties of Clitoria ternatea extract.
Nootropic Activity
Several studies have reported improvement in cognitive performance when C. ternatea extracts were administered to experimental animals ( Taranalli and Cheeramkuzhy, 2000 ; Rai et al., 2001 ; Jain et al., 2003 ). In one study, rats orally dosed with ethanolic extracts derived from C. ternatea roots or aerial tissues were shown to attenuate electric shock-induced amnesia better than the controls ( Taranalli and Cheeramkuzhy, 2000 ). In a separate study, 7-day old neonatal rats orally dosed with aqueous C. ternatea root extract also showed improved memory retention and enhanced spatial learning performance 48 h and 30 days post treatment ( Rai et al., 2001 ). Further investigations revealed that the brains of treated rats contained a significantly higher acetylcholine content than the controls ( Taranalli and Cheeramkuzhy, 2000 ; Rai et al., 2002 ). A more recent study of the effects of C. ternatea leaf extracts on diabetic-induced cognitive decline showed that the acetylcholinesterase activity, total nitric oxide levels and lipid peroxide levels all significantly decreased upon treatment, whilst the catalase, superoxide dismutase and glutathione levels all significantly increased ( Talpate et al., 2014 ). Another recent study showed that rats fed for 60 days with “medhya rasayana,” a mixture of crushed C. ternatea and jaggery (1:1), exhibited significant reduction in autophagy in the brain ( Raghu et al., 2017 ). The treated and the control rats also differentially expressed genes implicated in autophagy regulation, nucleotide excision repair, homologous recombination, etc. The study suggested that C. ternatea protects the brain by affecting the autophagy directed pathway ( Raghu et al., 2017 ).
Anti-inflammatory, Analgesic, and Antipyretic Activity
Extracts of C. ternatea roots and leaves have been reported to demonstrate anti-inflammatory, analgesic, and antipyretic activities ( Devi et al., 2003 ; Parimaladevi et al., 2004 ; Bhatia et al., 2014 ; Singh et al., 2018 ). Oral administration of the methanolic root extracts and ethanolic floral extracts of C. ternatea was reported to significantly inhibit carrageenin-induced rat paw oedema and acetic acid-induced vascular permeability in rats ( Devi et al., 2003 ; Singh et al., 2018 ). Results with an oral dosage of 400 mg extract per kg body weight were on par with a 20 mg/kg oral dosage of diclofenac sodium ( Devi et al., 2003 ), a non-steroidal anti-inflammatory drug. In an antipyretic study, oral administration of C. ternatea methanolic root extracts significantly reduced the body temperature of Wistar rats that had yeast-induced elevated body temperature ( Parimaladevi et al., 2004 ). This antipyretic activity of the extract was found to be comparable to paracetamol ( Parimaladevi et al., 2004 ). More recently, C. ternatea leaf extracts have been implicated for use as an analgesic ( Bhatia et al., 2014 ). In this study the established rat tail flick pain assay was used to determine the effects of pre-treatment with both ethanolic and petroleum C. ternatea extracts. A positive analgesic effect of C. ternatea leaf extracts was reported, comparable to diclofenac sodium (10 mg/kg) 1 h post treatment ( Bhatia et al., 2014 ).
Antidiabetic Activity
Recently, C. ternatea leaf extracts have shown potential for use as an antidiabetic ( Chusak et al., 2018b ; Kavitha, 2018 ). Wistar rats orally dosed with 400 mg C. ternatea ethanolic leaf extract per kg of body weight per day for 28 days, had significantly lower levels of blood glucose, insulin, glycosylated hemoglobin, urea and creatinine than the diabetic control. Furthermore, the levels of liver enzymes (serum glutamate oxalate transaminase, serum glutamate pyruvate transaminase, lactate dehydrogenase, and alkaline phosphatase) in treated rats were lower than the diabetic control rats and were comparable to the normal control rats ( Kavitha, 2018 ). More recent studies have focused on the effects of C. ternatea extracts on the glycemic response and antioxidant capacity in humans ( Chusak et al., 2018b ). A small scale clinical trial involving 15 healthy males revealed that when either 1 or 2 g of C. ternatea extract was ingested together with 50 g sucrose the resulting plasma glucose and insulin levels were suppressed ( Chusak et al., 2018b ). Furthermore the postprandial plasma antioxidant capacities of the subjects were also enhanced upon extract consumption.
Antioxidant Activity
The antioxidant properties of C. ternatea extracts are well documented ( Phrueksanan et al., 2014 ; Sushma et al., 2015 ). One study demonstrated that C. ternatea extracts could protect canine erythrocytes from hemolysis and oxidative damage induced by 2,2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH) ( Phrueksanan et al., 2014 ). Compared to the AAPH control, erythrocytes treated with 400 μg/mL of the C. ternatea extract had significantly lower levels of AAPH-induced lipid peroxidation and protein oxidation, and significantly higher levels of glutathione ( Phrueksanan et al., 2014 ). In another study the antioxidant properties within a C. ternatea extract facilitated the production of magnesium oxide nanoparticles, materials which are increasingly being utilized for biomedical applications ( Sushma et al., 2015 ).
Pesticidal Activities
The anthelmintic and insecticidal activities, and the antimicrobial activities of C. ternatea extracts and several isolated protein and peptide components are summarized in Tables 5 and and6, 6 , respectively. These biological activities presumably evolved for host-defense purposes but can have potential applications both in agriculture and medicine. Further details on these activities are described in the following sections.
Anthelmintic and insecticidal activities of Clitoria ternatea .
Antimicrobial activities of Clitoria ternatea .
Anthelmintic Activity
The anthelmintic properties of C. ternatea have been reported in several studies ( Hasan and Jain, 1985 ; Khadatkar et al., 2008 ; Salhan et al., 2011 ; Kumari and Devi, 2013 ; Gilding et al., 2015 ) ( Table 5 ). Characterization of 27 homozygous C. ternatea lines showed that individual lines displayed different degrees of resistance against the parasitic root-knot nematode, Meloidogyne incognita ( Hasan and Jain, 1985 ). The methanolic extract of C. ternatea was also found to inhibit 93% of M. incognita eggs from hatching ( Kumari and Devi, 2013 ). In another study that utilized the model organism, Caenorhabditis elegans , C. ternatea extracts were found to effectively kill nematode larvae, with the root extracts showing greater lethality than the leaf extracts ( Gilding et al., 2015 ). Two studies also reported C. ternatea activities against annelids ( Khadatkar et al., 2008 ; Salhan et al., 2011 ). Using Pheretima posthuma as a test worm, one study showed that the ethanolic C. ternatea extract (50 mg/mL) caused significantly higher mortality rate and incidence of worm paralysis than piperazine citrate, a commonly used drug for controlling parasitic worms ( Khadatkar et al., 2008 ). Similarly, using Eisenia foetida as a test worm, another study showed that the ethanolic and aqueous C. ternatea extract induced worm paralysis and mortality at 100 mg/mL ( Salhan et al., 2011 ). However, compared to the commonly used antiparasitic drug levamisole, the rate of worm paralysis and death was significantly slower in the C. ternatea extracts ( Salhan et al., 2011 ).
Insecticidal Activity
Proteins and peptides isolated from C. ternatea are reported to exhibit insecticidal properties ( Kelemu et al., 2004 ; Poth et al., 2011a ) ( Table 5 ). One study reported 100% larval mortality when 1% w/w and 5% w/w of the purified C. ternatea protein (20 kDa), finotin, was applied to the bruchids Acanthoscelides obtectus and Zabrotes subfasciatus , respectively ( Kelemu et al., 2004 ). Another study showed that when the C. ternatea cyclotide, Cter M, was incorporated in the diet of the lepidopteran species Helicoverpa armigera , larval growth retardation was observed in a dose dependent manner ( Poth et al., 2011a ). Larval mortality was observed at 1 μmol CterM peptide g -1 diet ( Poth et al., 2011a ).
Expanding on the initial findings of Poth et al. (2011a) , additional studies have reported pesticidal activities of cyclotide extracts from C. ternatea ( Gilding et al., 2015 ; Mensah et al., 2015 ) ( Table 5 ). Gilding et al. (2015) showed that C. ternatea extracts permeabilized insect-like membrane lipids, with the shoot extracts exhibiting the greatest potency (0.31 μg/mL LC 50 ). Another study reported that application of oil-based C. ternatea mixture (1–2% v/v) to transgenic and conventional cotton crops, resulted in Helicoverpa spp. larval mortality and reduced oviposition and larval feeding ( Mensah et al., 2015 ). Detrimental effects of the extract against beneficial insects were not observed ( Mensah et al., 2015 ), suggesting that C. ternatea extracts could provide the basis for eco-friendly natural insecticides.
Antimicrobial Activity
The antimicrobial properties of proteins isolated from C. ternatea have previously been described ( Kelemu et al., 2004 ; Ajesh and Sreejith, 2014 ) ( Table 6 ). The C. ternatea 20 kDa protein finotin demonstrated inhibitory activities over a wide range of plant fungal pathogens ( Kelemu et al., 2004 ). Finotin also exhibited activities against the plant bacterial pathogen Xanthomonas axonopodis ( Kelemu et al., 2004 ). Another study reported isolation of a 14.3 kDa protein from C. ternatea seeds ( Ajesh and Sreejith, 2014 ) that exhibited activities against the human fungal pathogens, Cryptococcus spp. and Candida spp., and against a number of mold fungi ( Ajesh and Sreejith, 2014 ). Studies also reported the antimicrobial properties of C. ternatea cyclotides against Gram-negative, but not Gram-positive, bacteria ( Nguyen et al., 2011 , 2016b ).
Ethanol extract of C. ternatea outdoor grown leaves and calli inhibited the growth of the bacterial species Staphylococcus spp., Streptococcus spp., Enterococcus faecalis , and Bacillus spp. ( Shahid et al., 2009 ). On the other hand, the antibacterial activities of the calli aqueous extract were only limited to Bacillus spp. and Streptococcus pyogenes; and activity of the leaf aqueous extract was limited to Bacillus spp. ( Shahid et al., 2009 ). Furthermore, a recent study reported that the ultrasound-assisted aqueous extract of C. ternatea leaves and petals inhibited the growth of Staphylococcus aureus ( Anthika et al., 2015 ). C. ternatea petals extracted for 30 min using ultrasound yielded the highest anthocyanin content and also displayed the highest antibacterial activity ( Anthika et al., 2015 ).
The antifungal properties of C. ternatea have also been reported ( Kamilla et al., 2009 ; Das and Chatterjee, 2014 ) ( Table 6 ). Growth of the mold fungus Aspergillus niger was inhibited at a minimum inhibitory concentration of 0.8 mg/mL of the methanolic C. ternatea leaf extract ( Kamilla et al., 2009 ). Scanning electron microscopy images from the study revealed that addition of the extract lead to conidial and hyphal collapse and distortion which is likely due to cell wall disruption ( Kamilla et al., 2009 ). Another study reported that the 50% aqueous-ethanolic C. ternatea leaf extract inhibited the growth of Fusarium oxysporum and promoted the activities of amylase, protease and dehydrogenase in P. sativum seeds, enzymes that otherwise had low activities during F. oxysporum infestation ( Das and Chatterjee, 2014 ).
Phytochemical Composition
Non-proteinaceous components.
As early as 1967, a study reported that C. ternatea seeds contain flavonol glycosides as well as phenolic aglycones, cinnamic acid, and a range of other compounds ( Kulshreshtha and Khare, 1967 ). Nearly two decades later, Saito et al. (1985) reported the isolation of five C. ternatea flavonols, namely kaempferol, kaempferol 3-glucoside, kaempferol 3-robinobioside-7-rhamnoside, quercetin, and quercetin 3-glucoside ( Saito et al., 1985 ). Subsequent studies reported the isolation of flavonol glycosides from C. ternatea leaves ( Morita et al., 1976 ) and flowers ( Kazuma et al., 2003a , b ). With some exceptions, the identified flavonol glycosides were found in all C. ternatea lines bearing different floral colors (blue, mauve and white) ( Kazuma et al., 2003a ). For instance, myricetin 3-(2″-rhamnosyl-6″-malonyl)glucoside, myricetin 3-rutinoside and myricetin 3-glucoside were not detected in the C. ternatea line bearing mauve petals ( Kazuma et al., 2003a ). The flavonols isolated from C. ternatea are summarized in Table 7 .
Flavonol and anthocyanin content of Clitoria ternatea .
Anthocyanins
In 1985, six acylated anthocyanins were isolated from blue C. ternatea flowers that were all derivatives of delphinidin 3,3′,5′-triglucoside ( Saito et al., 1985 ). The chemical properties of the acylated C. ternatea delphinidins, which were named ternatins, were further elucidated in subsequent studies ( Terahara et al., 1989a , 1990a , b ). In 1989, the structure of the largest isolated blue anthocyanin, ternatin A1, was determined ( Terahara et al., 1989a ). The study also showed that not only was ternatin A1 the largest, it was also one of the most stable in neutral solution ( Terahara et al., 1989a ). The structure of ternatins A2 ( Terahara et al., 1990c ), B1 ( Kondo et al., 1990 ), B2 ( Terahara et al., 1996 ), D1 ( Terahara et al., 1989b ), and D2 ( Terahara et al., 1996 ) were elucidated shortly after.
Subsequent studies isolated and determined the structures of several other novel ternatins isolated from C. ternatea : ternatins A3, B3–B4, C1–C5, D3, and preternatins A3 and C4 ( Terahara et al., 1996 , 1998 ) ( Table 7 ). Terahara et al. (1998) observed that lower molecular weight ternatins are more abundant in young flowers while higher molecular weight ternatins are more prevalent in mature flowers. The authors proposed that ternatin A1 is the final compound, and the other ternatins are intermediate products ( Terahara et al., 1998 ). Starting with ternatin C5, production of ternatin A1 can be achieved via four p- coumaric acid acylation steps and four glucosylation steps ( Terahara et al., 1998 ). The biosynthetic pathway of ternatin A1 is summarized in Figure 4 ( Terahara et al., 1998 ). The key enzymatic steps and the biosynthetic pathway to produce ternatin C5 from delphinidin was elucidated in 2004 ( Kazuma et al., 2004 ).

Proposed ternatin biosynthetic pathway. Adapted from Terahara et al. (1998) . Beginning with ternatin C5 (PubChem CID 10843319), ternatin A1 (PubChem CID 16173494) can be produced through the addition of four p- coumaroyl (C) and four glucosyl moieties (G) at the 3′ sidechain (in blue) and 5′ sidechain (in orange). The other ternatins are products of the intermediate steps.
A 2003 study compared the anthocyanin contents of C. ternatea lines bearing different floral colors ( Kazuma et al., 2003a ). The study showed that white C. ternatea flowers do not produce anthocyanins. Furthermore, unique to the mauve C. ternatea flowers, is the accumulation delphinidins lacking the 3′ and 5′ (polyacelated) glucosyl group substitutions ( Kazuma et al., 2003a ). The study concluded that glucosylation of delphinidins at these positions are crucial to the production of C. ternatea flowers ( Kazuma et al., 2003a ).
Other Non-proteinaceous Components
The pentacyclic triterpenoids, taraxerol and taraxerone, were isolated from C. ternatea roots in the 1960s ( Banerjee and Chakravarti, 1963 , 1964 ). Realizing the potential of C. ternatea as a source of taraxerol, in 2008, a method was developed for the routine quantification of the content in C. ternatea extracts of this medicinal compound ( Kumar et al., 2008 ). In 2012, in vitro propagated hairy root cultures were sought as alternative to in vivo grown roots as source of taraxerol ( Swain et al., 2012a ). In 2016, in addition to taraxerol, novel norneolignans, clitorienolactones A-C, were isolated from C. ternatea roots ( Vasisht et al., 2016 ). C. ternatea floral extracts also contain other types of flavonoids, including rutin (flavone), epicatechin (flavanol) and other polyphenolic acids (gallic acid, protocatechuic acid, and chlorogenic acid) ( Siti Azima et al., 2017 ).
Proteinaceous Components
In general, there has traditionally been a greater focus in phytochemical studies on the non-protein components of plants and C. ternatea is no exception. However, over the last decade, with advances in nucleic acid sequencing and mass spectroscopic peptide and protein characterization techniques there is now much more focus on proteinaceous components, particularly in the characterization of peptides and proteins implicated in plant defense. Of the known C. ternatea phytochemical components implicated in defense, a class of peptides known as cyclotides is particularly noteworthy ( Nguyen et al., 2011 ; Poth et al., 2011a , b ). These peptides mature into cyclic molecules of ∼30 aa from linear precursors through an enzymatic transpeptidation reaction of the peptide backbone. Cyclotides contain three disulfide bonds that form a knot ( Figure 5A ), similar to configurations seen in linear knottins cataloged across diverse taxa ( Gelly et al., 2004 ). Together, the cyclic and knotted nature of cyclotides makes them highly stable in conditions that would otherwise facilitate peptide degradation ( Craik et al., 1999 ). Unlike linear knottins, which are found across multiple kingdoms of life, cyclotides are restricted to relatively few taxa in Viridiplantae, namely the dicotyledon angiosperms ( Gruber et al., 2008 ). Searching all Viridiplantae sequences for cyclotides using the widely distributed program tblastn, has highlighted the restriction of cyclotides and linear non-cyclotide-like sequences to a handful of plant families discussed below ( Altschul et al., 1990 ).

Graphical representations of C . ternatea cyclotide structure and diversity. (A) Topological depiction of Cter M displaying the position and threading of disulfide bonds. (B) Diversity of residues at non-cystine positions of 74 sequence defined C . ternatea cyclotides. (C) Loop length diversity and disulfide connectivity map of C . ternatea cyclotides.
Despite reports of cyclotide-like sequences in the Poaceae, none of the described sequences have been shown to exist as cyclic molecules in planta , thus failing the definition of the term cyclotide. The taxonomic distribution of cyclotides is often disjointed in a taxonomic group; for instance the taxonomically sparse occurrence of cyclotides observed in the Rubiaceae ( Gruber et al., 2008 ; Koehbach et al., 2013 ) contrasts with the ubiquitous occurrence of cyclotides in all species tested far-off the Violaceae family ( Burman et al., 2015 ; Göransson et al., 2015 ). Within the Fabaceae, C. ternatea is the only family member in which cyclotides have been observed despite examination of diverse Fabaceae, including other species of Clitoria ( Gilding et al., 2015 ). Cyclotides are therefore one of the most interesting proteinaceous components of C. ternatea . That they are processed from genetically encoded precursor proteins opens opportunities for detecting them either or both as nucleic acid or peptide sequences.
Gene and Transcript Characterization
RNA-seq experiments to define the transcripts that encode for cyclotides have been performed by several groups. The resulting transcriptomes have allowed the cataloging of at least 74 cyclotide sequences ( Table 8 ) which exhibit high levels of diversity at loops intervening the conserved Cys residues ( Figure 5B,C ) ( Gilding et al., 2015 ; Nguyen et al., 2016b ). All of the precursors observed have singleton cyclotide domains similar to that observed in Petunia x hybrida (Solanaceae), whereas cyclotide precursors from the Cucurbitaceae, Rubiaceae, and Violaceae families possess multiple cyclotide domains ( Table 9 ) ( Felizmenio-Quimio et al., 2001 ; Mylne et al., 2011 ; Poth et al., 2011a ; Koehbach and Gruber, 2015 ; Park et al., 2017 ). Unlike precursors from the Cucurbitaceae, Rubiaceae, Solanaceae, and Violaceae, C. ternatea cyclotides are encoded in albumin-1 genes ( Poth et al., 2011a ).
Cyclotides in Clitoria ternatea .
Characteristics of cyclotide gene precursors.
Pea-like albumin-1 genes are restricted to the tribe Faboideae, as evidenced by the lack of hits when albumin-1 prepropeptide sequences from P. sativum are used as queries in a tblastn search on all sequences exclusive of the Faboideae. The canonical albumin-1 gene structure in all taxa examined thus far consists of a signal peptide followed immediately by a b-chain peptide domain with a knottin fold, a short intervening sequence, and a ∼54 aa a-chain domain ( Figure 6 , Cter M precursor). Typical functions ascribed to the albumin-1 gene family include protein storage and defense through the potentially toxic b-chain. Their function as a toxin is exemplified in the Pisum sativum albumin-1 b-chain (Pa1b), a peptide that effectively kills weevils and select insects through inhibitory activity of insect vacuolar proton pumps ( Jouvensal et al., 2003 ; Chouabe et al., 2011 ).

Structure of a Fabaceae albumin-1 cyclotide prepropeptide exemplified by Cter M. The linear Cter M precursor consists of the endoplasmic reticulum (ER) signal (red), the cyclotide domain (blue) which replaces the typical Fabaceae albumin-1 b-chain, the a-chain (yellow) and the C-terminal interlinker region (green). A specialized asparaginyl endopeptidease (AEP, butelase-1 (PDB code: 6DHI) effects head-to-tail cyclization of the Cter M domain of the precursor in planta and results in a mature CterM cyclotide (PDB code: 2LAM).
Interestingly, the loops between the cystine residues are similar in size and in some cases residue composition between C. ternatea cyclotides and other albumin-1 b-chains ( Gilding et al., 2015 ). This observation implies that the development of cyclotide domains from progenitor albumin-1 b-chains would have involved adaptation of the b-chain into a cyclotide domain structure. A further necessary adaptation to facilitate cyclization is the acquisition of an Asp or Asn residue at the C-terminus of the cyclotide domain. These specific residues are required for cyclization by asparaginyl endopeptideases (AEPs) through a transpeptidation reaction between the C-terminal Asp/Asn residue and the N-terminal residue ( Figure 6 ) ( Nguyen et al., 2014 ; Harris et al., 2015 ).
In C. ternatea , all transcripts encoding albumin-1 a-chain domains contain a cyclotide domain in place of what would otherwise be the b-chain domain. The complete transition of this region in C. ternatea albumin-1 genes to cyclotide domains implies canonical b-chains were disfavoured in the evolutionary history of C. ternatea ( Gilding et al., 2015 ). The albumin-1 gene family members of C. ternatea are ∼74 in number, whereas albumin-1 gene families from the other genome-sequenced Faboideae, Glycine , Medicago , and Phaseolus , are 3, 33, and 17 in number respectively ( Goodstein et al., 2012 ; Gilding et al., 2015 ; Nguyen et al., 2016b ). This observation on albumin-1 gene expansion in C. ternatea further supports the hypothesis that cyclotide domains exhibit qualities and functions that increase fitness.
Transcriptomic expression analysis of various C. ternatea organs illustrates the partitioning of cyclotide expression to certain organs for some precursors, while other precursors are expressed constitutively throughout the examined organs ( Gilding et al., 2015 ). As a class of defense molecules, it is logical that some would be preferentially expressed to target specific threats that different organs may face. Other albumin-1 genes are expressed at a notable level throughout the whole plant. The precursor for Cter M is an example of a cyclotide that is expressed constitutively, so much so that transcripts encoding Cter M are upward of ninefold higher than the rubisco small subunit in shoots ( Gilding et al., 2015 ). Clearly, the plant is investing large amounts of resources to produce these transcripts and the resulting peptides.
Peptide Characterization
Clitoria ternatea cyclotides generally have Gly residues at the proto- N-terminus and Asn residues at the proto- C-terminus of the cyclotide domain within the precursor proteins, similar to the case from other plant families. By contrast with the conserved terminal residues, the intervening backbone loops between the conserved Cys residues tend to be variable in size and sequence. Some of the biophysical properties of C. ternatea cyclotides deviate notably from peptides of other cyclotide-producing plant families. For example, Cter 13 contains eight Arg residues that confer a predicted charge of +7 and pI of 10, well above that predicted for MCoTI-I, which contains four Arg residues, from the Cucurbit Momordica cochinchinensis ( Felizmenio-Quimio et al., 2001 ; Mylne et al., 2012 ). The more highly charged and high-pI cyclotides of C. ternatea are preferentially expressed in organs that encounter challenges from the soil, namely the roots and seeds of the plant. Cyclotide extracts from roots, compared to leaves, exhibit increased toxicity against the juvenile L1 stage of the model nematode C. elegans , whereas adults and late stage juveniles were not affected ( Gilding et al., 2015 ). The high charge of these potentially nematicidal peptides in on trend with other described nematicidal peptides ( Liu et al., 2011 ). Further study is required to test for specific activity of organ-specific cyclotides against organisms.
Cyclotide sequences observed in aerial tissue typically have lower predicted charges and pI values than cyclotides in soil-contacting tissues. Cyclotide extracts of these aerial tissues exhibit a different MALDI-MS profile compared to other plant parts and greater propensity to bind to insect-mimetic plasma membranes. This implies that the aerially-expressed cyclotides are targeting insects ( Gilding et al., 2015 ).
The cyclotide diversity of C. ternatea is further increased by post-translational modifications (PTM). Serra et al. (2016) described the first observations of hexosylation and methylation of cyclotides through enzymatic digests and MS techniques, with the estimated cyclotide diversity conferred by primary sequence and PTM diversity numbering in the hundreds. What the function of these post-translational modifications may be remains to be defined. Modifications of amino acid side chains reported in cyclotides include oxidation (Met and Trp), methylation, deamination (common at C-terminal Asn to Asp), hexosylation, dehydration, and hydroxylation (select Pro residues) ( Plan et al., 2007 ; Serra et al., 2016 ).
Biosynthetic Auxiliary Enzymes
Cyclotide transcripts of C. ternatea encode for a signal peptide that immediately precedes the N-terminal residue of the cyclotide domain ( Poth et al., 2011a ; Gilding et al., 2015 ; Nguyen et al., 2016b ). The current model for C. ternatea cyclotide biosynthesis mimics that of other cyclotide producing species and begins with the signal peptide inducing the docking of the ribosome-transcript complex with the rough endoplasmic reticulum (ER) ( Conlan and Anderson, 2011 ; Göransson et al., 2015 ). Unique to C. ternatea cyclotide precursors is that the signal peptide cleavage alone releases the N-terminus of the cyclotide domain, thus no other N-terminal processing proteases are required. Following this, it is postulated that folding of the cyclotide domain begins, presumably aided by protein disulfide isomerases (PDIs), as the propeptide is held within the ER. From there the folded propeptide is transported via vesicles to the Golgi, and later to prevacuolar or vacuolar compartments. Somewhere during this transport pathway the propeptide encounters a specific type of AEP that catalyzes the simultaneous cyclization and cleavage of the cyclotide domain from the precursor ( Göransson et al., 2015 ; Jackson et al., 2018 ). Post-translational modifications are possibly acquired along the maturation pathway but are poorly defined and thus need further investigation ( Serra et al., 2016 ).
Protein disulfide isomerases
The disulfide knot of cyclotides must be formed from the oxidation of the six cysteine residues in a specific order. Incorrect connectivity may result in the precursor not being able to be cyclized and flagged as a faulty molecule needing destruction. In all cyclotide producing taxa, the specific in vivo physical of genetic interactions of PDI family members and cyclotide precursors is not known. In vitro evidence for PDI involvement is known from a PDI cloned in the Rubiaceae plant, Oldenlandia affinis , however, under the conditions tested the isolated PDI was not as efficient as using an isopropanol buffer to effect proper disulfide bond formation ( Gruber et al., 2007 ). A systematic in vivo examination of C. ternatea PDIs discovered in the transcriptome is hindered by the lack of reverse genetic resources in C. ternatea .
Asparaginyl endopeptidases
Asparaginyl endopeptidases (AEPs), like most proteases, are known primarily for their function in peptide bond hydrolysis ( Yamada et al., 2005 ), thus a proposed role for peptide bond creation for a selection of AEPs is particularly intriguing. The first direct evidence for this came about through work by the Tam group ( Nguyen et al., 2014 ), who set out to identify the peptide ligase responsible for the maturation of cyclotides in C. ternatea . Through activity-guided protein-fractionation studies, the researchers identified a single C. ternatea AEP isoform (termed butelase-1) that was highly efficient in intermolecular peptide cyclization. Since the discovery of butelase-1 in 2014, several other AEP peptide ligases have been identified from cyclotide producing plant species, including OaAEP1 b from O. affinis ( Harris et al., 2015 ), PxAEP3b ( Petunia x hybrida ) ( Jackson et al., 2018 ), and HeAEP3 ( Hybanthus enneaspermus ) ( Jackson et al., 2018 ). Through bioinformatic and functional testing the structural features that differentiate AEP ligases from proteases are beginning to emerge. Specifically, plant AEPs that function as transpeptidase-preferring enzymes in vivo have been shown to possess specific markers in their protein sequence, most notably one termed the Marker for Ligase Activity (MLA) ( Jackson et al., 2018 ).
Subsequent work by Gilding et al. defined the expression levels of butelase-1 (referred to as CtAEP1) and the full length sequence of butelase-2/CtAEP2, CtAEP3, and CtAEP5 via RNA-seq ( Gilding et al., 2015 ). In contrast, a total of six butelases were described by Nguyen et al. (2014) , with assembled sequences for butelase-4 and -6 not showing any homology to any of the CtAEPs described by Gilding et al. (2015) . It might be the case that there is natural AEP isoform variation amongst C. ternatea accessions, or that differences in data assembly conditions, or choice of tissue RNA sampled between the studies of Nguyen et al. (2014) and Gilding et al. (2015) are responsible for this apparent discrepancy. Importantly, of all six AEPs, only butelase-1 has been shown to prefer transpeptidation over proteolysis.
Next Generation Applications
In this section we describe recent applications of C. ternatea components in biotechnological, agricultural and pharmaceutical industries.
Butelase-1 has proven to be a very versatile molecular tool for a range of in vitro peptide engineering applications ( Nguyen et al., 2016a , c ; Bi et al., 2017 ). When compared to other characterized AEP ligases, butelase-1 displays superior reaction kinetics. Despite this, one obvious limitation for end-user uptake is that a recombinant production system is yet to be established ( Nguyen et al., 2014 ). In lieu of this, a detailed protocol for the purification of butelase-1 from C. ternatea seed pods is available ( Nguyen et al., 2016c ), but is restricted to those with access to the source material and protein purification expertise. It remains unclear if butelase-1 has evolved superior structural features over other AEP ligases or that its greater catalytic efficiency is a by-product of purifying source activated enzyme.
Butelase-1 Mediated Intramolecular Peptide/Protein Cyclization
Tools to enable backbone cyclization of peptides have garnered considerable interest from the pharmaceutical industry as a means to provide proteolytically stable peptide therapeutics ( Craik et al., 2012 ). In this regard, butelase-1 has been demonstrated as a highly versatile enzyme, cyclizing a range of diverse peptides, including cysteine rich cyclotides, conotoxins (e.g., MrIA) and sunflower trypsin inhibitors (SFTI-1) ( Nguyen et al., 2014 ). Additionally a wide range of non-cysteine containing peptides have been cyclized, including human apelin, galanin, neuromedin U and salusin ( Nguyen et al., 2015 ). In all cases, the substrate requirements for cyclization include the introduction of, if not already present, an Asn residue at the peptide ligation point, which must be linked to the C-terminal tailing residues of His-Val. These tailing residues, which are subsequently cleaved off and are not incorporated into the final cyclized product have been shown to be essential for butelase-1 cyclization efficiency ( Nguyen et al., 2014 ). At the substrates N-terminus, requirements are flexible at the P1’ position, with all residues accepted apart from Pro. However, at the P2’ position more stringent requirements exist, with Cys, Ile, Leu, and Val all preferred ( Nguyen et al., 2014 ). Together these requirements mean that most peptides, require at least some modifications of the termini residues to allow butelase-1 mediated cyclization. When these substrate requirements are met, butelase-1 has remarkably catalytic efficiency, with substrate to enzyme ratios of 100 ∼ 1000:1 commonly used, with typical cyclization reactions completed within 5 ∼ 30 min ( Nguyen et al., 2015 ).
The benefits of backbone cyclization are not limited to small peptides, with the thermal and proteolytic stability of a number of larger proteins also improved by backbone cyclization. Like smaller peptides, these proteins must first be engineered to include optimal flanking residues for butelase-1 activity, with specific consideration given to the proximity of N and C residues. Where termini are not held close enough together, considerations should be given to include appropriate sized linker sequences. Using butelase-1, three different recombinantly produced proteins have been successfully cyclized, including green fluorescent protein (GFP), interleukin-1 receptor antagonist (IL-1Ra) and human growth hormone (somatropin) ( Nguyen et al., 2015 ). In all cases butelase-1 (0.1uM) and target protein (25 μM) were incubated together with cyclization essentially complete within 15 min. In the case of IL-1Ra, backbone cyclization was shown to increase the thermostability of the protein, without affecting biological activity ( Nguyen et al., 2015 ).
Butelase-1 Mediated Intermolecular Peptide Bond Formation
Butelase-1 has additionally shown great potential for the selective labeling of proteins by intermolecular peptide bond formation. Here, butelase-1 recognizes the required NHV motif engineered into a protein of interest and initiates ligation of incoming intermolecular nucleophiles, provided that substrate requirements are met. In this way a protein of interest can be labeled with any number of functional cargoes. Site specific labeling of proteins has applications for elucidating cellular pathways, defining protein–protein interactions and for the development of innovative medical imaging approaches and therapeutics ( Falck and Müller, 2018 ; Harmand et al., 2018 ). One additional promising application is the site specific labeling of surface proteins of live bacteria ( Bi et al., 2017 ). To accomplish this, the authors engineered an NHV motif to the C-terminus of the anchoring protein OmpA of Escherichia coli . Upon incubation of live cells with butelase-1, a range of cargo molecules were able to be successfully linked to the engineered bacterial surface protein OmpA. These included a fluorescein probe, useful for cellular tracking of pathogen response, and a tumor associated monoglycosylated peptide, which provided a proof of concept for delivering post translationally modified antigens as live bacteria vaccines.
Insecticidal Applications of C. ternatea Peptide Extracts
Conventional pesticides have for decades been of paramount importance in sustaining agricultural productivity under an ever-increasing population burden. However, many traditional pesticides are increasingly becoming disfavored due to off-target toxicities and human health concerns. These concerns, together with increasing incidences of insects developing resistance mechanisms necessitates the discovery or engineering of novel pesticides with new modes of action ( Perry et al., 2011 ). Recently an organic ethanolic extract prepared from C. ternatea vegetative tissue has shown promising insecticidal activity against a wide range of crop pests 3 . The extract, termed Sero-X ® has thus far been registered in Australia for applications in cotton and macadamia, with further applications pending both in Australia and overseas. Although the exact mode of action of this ethanolic extract remains to be determined, it is likely in part to be due to the high concentrations of C. ternatea cyclotides present ( Poth et al., 2011a , b ; Gilding et al., 2015 ). The prototypic C. ternatea cyclotide Cter M is indeed enriched in the Sero-X ® extract and when tested in isolation, displays lethality against cotton budworm ( H. armigera ) ( Poth et al., 2011a ). Like other cyclotides, such as kalata B1 from O. affinis , the predicted mode of action is through insect cell membrane disruption ( Poth et al., 2011a ; Craik, 2012 ), but it remains unclear if other non-proteinaceous components present in the Sero-X ® extract play a synergistic role. Importantly, Sero-X ® displays no toxicity to tested rodents or bee pollinators, and is considered non-hazardous according to the Globally Harmonized System of Classification and labeling of Chemicals.
Food Colorants/Consumer Products
Butterfly pea flowers can range from white to intense blue to shades in between. This coloring largely stems from the anthocyanin content and degree of aromatic acylation ( Kazuma et al., 2003a ). The deep blue pigment of C. ternatea has been particularly popular in Asia, where flower petals are used to color teas, deserts and clothes. More recently, C. ternatea flower extracts have been used to create vibrant blue alcoholic gins 4 , which change color depending on the pH, such as occurs on mixing with tonic water or lime. Specifically, the deep blue color of C. ternatea flowers is a particularly sought after alternative to synthetic blue food colorants which have become increasingly disfavored due to health concerns ( Nigg et al., 2011 ). Studies reported that addition of C. ternatea extracts increased the polyphenolic and antioxidant contents of sponge cakes ( Pasukamonset et al., 2018 ), enhanced the oxidative stability of cooked pork patties ( Pasukamonset et al., 2017 ) and reduced the predicted glycemic index of flour ( Chusak et al., 2018a ). Microencapsulation using alginate prevented the degradation and enhanced the retainment of the antioxidant activities of C. ternatea polyphenolic extracts post gastrointestinal digestion ( Pasukamonset et al., 2016 ). Currently there exists no commercial scale production of C. ternatea for anthocyanins, with harvesting of plant material at large-scale not likely to be economically feasible. However, recent advances in engineering plant cell suspension cultures with anthocyanin regulatory pathway genes offers an alternative approach ( Appelhagen et al., 2018 ).
Conclusion and Future Outlook
Here we have attempted to provide a comprehensive and multidisciplinary account of the diverse properties and applications of C. ternatea and its constituent molecules. The plant is readily grown in a range of habitats and there is wide opportunity for it to be used for rotational cropping to aid in soil nitrogen regeneration, as a fodder crop for cattle, or as source of novel phytochemicals. There are already a host of cosmetic and food colorants on the market and the first C. ternatea based insecticide (Sero-X ® ) is also approved and being used for insect control on cotton and macadamia nut crops. The butelase-1 enzyme derived from C. ternatea pods is also creating a lot of interest as a biotechnological tool for peptide ligation and cyclization.
We anticipate that the success of products (including enzymes, extracts, and purified phytochemicals) deriving from C. ternatea will encourage more research on this plant and stimulate further discoveries that might lead to second and third generation products. For example, so far only a small fraction of the more than 70 cyclotides in this plant have been tested for pesticidal activity and there may be components in this cocktail of cyclotides that are significantly more potent as pesticides than what is currently known. Further work is needed to understand the biotic and abiotic factors that modulate the production of individual cyclotides in this plant and to understand possible synergies between different cyclotides and between cyclotides and non-cyclotide components.
We also anticipate that there will be more studies in the future on pharmaceutical applications of C. ternatea components. The ability to harvest large amounts of plant material means that one of the limitations encountered in many natural product research and commercialization (i.e., lack of source material) is not a factor for C. ternatea . While the multitude of medicinal applications reported so far from various C. ternatea preparations are impressive, we caution that many of these are one-off studies that have yet to be independently validated by groups other than the original reporting group. It is to be expected that the claims for the various bioactivities of plant extracts need to be tested with rigorous controls to establish the efficacy of the plant components. Furthermore, very few of the cyclotides in C. ternatea have been screened for medicinal applications and we feel this would be a useful exercise for future studies. Likewise, none of the C. ternatea cyclotides have yet been used as molecular grafting frameworks to introduce new desired pharmaceutical activities as has been done for cyclotides from other plants such as kalata B1 or MCoTI-II. With these suggestions for future work on this fascinating plant we feel that many more exciting discoveries are on the horizon.
Author Contributions
DC and GO conceived and planned the framework for this article. All authors contributed to the writing and editing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Innovate Ag Pty. Ltd for the financial support through an Australian Research Council (ARC) Linkage grant (LP130100550). DC is an Australian Research Council Australian Laureate Fellow (FL150100146).
1 https://innovate-ag.com.au/
2 https://avh.chah.org.au/
3 https://innovate-ag.com.au/
4 https://www.inkgin.com/
- Abdelhamid A. M., Gabr A. A. (1993). The evaluation of new sources of fodder ( Clitoria and Phillipesara ) under Egyptian conditions. Arch. Anim. Nutr. 44 85–93. 10.1080/17450399309386060 [ CrossRef ] [ Google Scholar ]
- Adhikary R., Sultana S., Bishayi B. (2017). Clitoria ternatea flower petals: effect on TNFR1 neutralization via downregulation of synovial matrix metalloproteases. J. Ethnopharmacol. 210 209–222. 10.1016/j.jep.2017.08.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ajesh K., Sreejith K. (2014). A novel antifungal protein with lysozyme-like activity from seeds of Clitoria ternatea . Appl. Biochem. Biotechnol. 173 682–693. 10.1007/s12010-014-0880-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alderete-Chavez A., Guerra-Santos J. J., Cruz-Landero De la N., Brito R., Gelabert R., Endanu E., et al. (2011). Evaluation of Clitoria ternatea L. in relation with fertility in tropical soils. J. Appl. Sci. 11 1044–1048. 10.3923/jas.2011.1044.1048 [ CrossRef ] [ Google Scholar ]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 403–410. 10.1016/S0022-2836(05)80360-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anthika B., Kusumocahyo S. P., Sutanto H. (2015). Ultrasonic approach in Clitoria ternatea (butterfly pea) extraction in water and extract sterilization by ultrafiltration for eye drop active ingredient. Procedia Chem. 16 237–244. 10.1016/j.proche.2015.12.046 [ CrossRef ] [ Google Scholar ]
- Appelhagen I., Wulff-Vester A. K., Wendell M., Hvoslef-Eide A.-K., Russell J., Oertel A., et al. (2018). Colour bio-factories: towards scale-up production of anthocyanins in plant cell cultures. Metab. Eng. 48 218–232. 10.1016/j.ymben.2018.06.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banerjee S. K., Chakravarti R. N. (1963). Taraxerol from Clitoria ternatea Linn. Bull. Calcutta Sch. Trop. Med. 11 106–107. [ PubMed ] [ Google Scholar ]
- Banerjee S. K., Chakravarti R. N. (1964). Taraxerone from Clitoria ternatea Linn. Bull. Calcutta Sch. Trop. Med. 12 : 23 . [ PubMed ] [ Google Scholar ]
- Barro C., Ribeiro A. (1983). The study of Clitoria ternatea L. hay as a forage alternative in tropical countries. Evolution of the chemical composition at four different growth stages. J. Sci. Food Agric. 34 780–782. 10.1002/jsfa.2740340803 [ CrossRef ] [ Google Scholar ]
- Bhatia M., Chahal J., Gupta S. (2014). Analgesic and anti-inflammatory activities of Clitoria ternatea Linn. leaves extract on rat model. Int. J. Pharm. Sci. Res. 5 600–606. 10.13040/IJPSR.0975-8232.5(2).600-06 [ CrossRef ] [ Google Scholar ]
- Bi X., Yin J., Nguyen G. K. T., Rao C., Halim N. B. A., Hemu X., et al. (2017). Enzymatic engineering of live bacterial cell surfaces using butelase 1. Angew. Chem. 56 7822–7825. 10.1002/anie.201703317 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Biyoshi A. K., Geetha K. A. (2012). Polymorphism in flower colour and petal type in Aparajita ( Clitoria ternatea ). Open Access J. Med. Aromat. Plants 3 12–14. [ Google Scholar ]
- Burman R., Yeshak M. Y., Larsson S., Craik D. J., Rosengren K. J., Göransson U. (2015). Distribution of circular proteins in plants: large-scale mapping of cyclotides in the Violaceae. Front. Plant Sci. 6 : 855 . 10.3389/fpls.2015.00855 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chouabe C., Eyraud V., Da Silva P., Rahioui I., Royer C., Soulage C., et al. (2011). New mode of action for a knottin protein bioinsecticide: pea albumin 1 subunit b (PA1b) is the first peptidic inhibitor of V-ATPase. J. Biol. Chem. 286 36291–36296. 10.1074/jbc.M111.281055 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chusak C., Henry C., Chantarasinlapin P., Techasukthavorn V., Adisakwattana S. (2018a). Influence of Clitoria ternatea flower extract on the in vitro enzymatic digestibility of starch and its application in bread. Foods 7 : 102 . 10.3390/foods7070102 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chusak C., Thilavech T., Henry C. J., Adisakwattana S. (2018b). Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: a randomized crossover trial. BMC Complement. Altern. Med. 18 : 6 . 10.1186/s12906-017-2075-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cobley L. S. (1956). An Introduction to the Botany of Tropical Crops. Bristol: Western Printing Services LTD. [ Google Scholar ]
- Colina V., Clavero T., Razz R., Castro C. (1997). Effect of defoliation on biomass production of Clitoria ternatea L. Cuban J. Agric. Sci. 31 113–117. [ Google Scholar ]
- Conlan B. F., Anderson M. A. (2011). Circular micro-proteins and mechanisms of cyclization. Curr. Pharm. Des. 17 4318–4328. 10.2174/138161211798999410 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Conway M. (2005). “Planting and establishment,” in The Butterfly Pea Book: a Guide to Establishing and Managing Butterfly Pea Pastures in Central Queensland , eds Collins R., Grundy T. (Brisbane: Department of Primary Industries and Fisheries; ), 19–27. [ Google Scholar ]
- Conway M., Collins R. (2005). “Climate and soils,” in The Butterfly Pea Book: a Guide to Establishing and Managing Butterfly Pea Pastures in Central Queensland , eds Collins R., Grundy T. (Brisbane: Department of Primary Industries and Fisheries; ), 16–18. [ Google Scholar ]
- Conway M., Doughton J. (2005). “Introduction,” in The Butterfly Pea Book: a Guide to Establishing and Managing Butterfly Pea Pastures in Central Queensland , eds Collins R., Grundy T. (Brisbane: Department of Primary Industries and Fisheries; ), 6–9. [ Google Scholar ]
- Conway M. J., McCosker K., Osten V., Coaker S., Pengelly B. C. (2001). “Butterfly pea - A legume success story in cropping lands of Central Queensland,” in Proceedings of the 10th Australian Agronomy Conference , eds Rowe B., Mendham N., Donaghy D. (Hobart: The Regional Institute; ). [ Google Scholar ]
- Craik D. J. (2012). Host-defense activities of cyclotides. Toxins 4 139–156. 10.3390/toxins4020139 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Craik D. J., Daly N. L., Bond T., Waine C. (1999). Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 294 1327–1336. 10.1006/jmbi.1999.3383 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Craik D. J., Swedberg J. E., Mylne J. S., Cemazar M. (2012). Cyclotides as a basis for drug design. Expert Opin. Drug Discov. 7 179–194. 10.1517/17460441.2012.661554 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cruz M. S. D., Perez-Urria E., Martin L., Avalos A., Vicente C. (1995). Factors affecting germination of Canavalia brasiliensis , Leucaena leucocephala , Clitoria ternatea and Calopogonium mucunoides seeds. Seed Sci. Technol. 23 447–454. [ Google Scholar ]
- Das N., Chatterjee P. (2014). Antifungal effect of Clitoria ternatea leaf extract on seeds of Pisum sativum in relation to the activities of some enzymes. Int. J. Res. Ayurveda Pharm. 5 99–101. 10.7897/2277-4343.05120 [ CrossRef ] [ Google Scholar ]
- De Souza E. S., Burity H. A., Oliveira J. D., Figueiredo M. D. B., DeLyra M. D. C. P. (1996). N2-fixation and growth of the calopogonium ( Calopogonium mucunoides Desv.) and of the Clitoria ( Clitoria ternatea L.) after sucessive cuts R. Soc. Bras. Zootec. 25 1036–1048. [ Google Scholar ]
- Devi B. P., Boominathan R., Mandal S. C. (2003). Anti-inflammatory, analgesic and antipyretic properties of Clitoria ternatea root. Fitoterapia 74 345–349. 10.1016/S0367-326X(03)00057-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duangkhet M., Chikoti Y., Thepsukhon A., Thapanapongworakul P., Chungopast S., Tajima S., et al. (2018). Isolation and characterization of rhizobia from nodules of Clitoria ternatea in Thailand. Plant Biotechnol. 35 123–129. 10.5511/plantbiotechnology.18.0402a [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dwivedi G. K., Kumar D. (2001). Nitrogen economy, dry matter production and seed production potential of Setaria sphacelata by intercropping of pasture legumes. J. Agron. Crop Sci. 182 121–126. 10.1046/j.1439-037x.1999.00275.x [ CrossRef ] [ Google Scholar ]
- Falck G., Müller K. (2018). Enzyme-based labeling strategies for antibody-drug conjugates and antibody mimetics. Antibodies 7 : 4 10.3390/antib7010004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fantz P. R. (1977). A Monograph of the Genus Clitoria (Leguminosae: Glycineae). Doctoral dissertation, University of Florida, Gainesville, FL. [ Google Scholar ]
- Felizmenio-Quimio M. E., Daly N. L., Craik D. J. (2001). Circular proteins in plants: solution structure of a novel macrocyclic trypsin inhibitor from Momordica cochinchinensis . J. Biol. Chem. 276 22875–22882. 10.1074/jbc.M101666200 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gelly J.-C., Gracy J., Kaas Q., Le-Nguyen D., Heitz A., Chiche L. (2004). The KNOTTIN website and database: a new information system dedicated to the knottin scaffold. Nucleic Acids Res. 32 D156–D159. 10.1093/nar/gkh015 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gilding E. K., Jackson M. A., Poth A. G., Henriques S. T., Prentis P. J., Mahatmanto T., et al. (2015). Gene coevolution and regulation lock cyclic plant defence peptides to their targets. New Phytol. 210 717–730. 10.1111/nph.13789 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goodstein D. M., Shu S., Howson R., Neupane R., Hayes R. D., Fazo J., et al. (2012). Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40 D1178–D1186. 10.1093/nar/gkr944 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Göransson U., Malik S., Slazak B. (2015). “Cyclotides in the Violaceae,” in Advances in Botanical Research , ed. Craik D. J. (Cambridge, MA: Academic Press; ), 15–49. 10.1016/bs.abr.2015.09.001 [ CrossRef ] [ Google Scholar ]
- Grindley D. N., Burden E. H. W. J., Akour A. A. (1954). The seed oils of Clitoria ternatea and Entada phaseoloides . J. Sci. Food Agric. 5 278–280. 10.1002/jsfa.2740050605 [ CrossRef ] [ Google Scholar ]
- Gruber C. W., Cemažar M., Clark R. J., Horibe T., Renda R. F., Anderson M. A., et al. (2007). A novel plant protein-disulfide isomerase involved in the oxidative folding of cystine knot defense proteins. J. Biol. Chem. 282 20435–20446. 10.1074/jbc.M700018200 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gruber C. W., Elliott A. G., Ireland D. C., Delprete P. G., Dessein S., Göransson U., et al. (2008). Distribution and evolution of circular miniproteins in flowering plants. Plant Cell 20 2471–2483. 10.1105/tpc.108.062331 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gründemann C., Koehbach J., Huber R., Gruber C. (2012). Do plant cyclotides have potential as immunosuppressant peptides? J. Nat. Prod. 75 167–174. 10.1021/np200722w [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gründemann C., Unutmaz D., Thell K., Lengen K., Garcia-Käufer M., Huang Y.-H., et al. (2013). Cyclotides suppress human T-lymphocyte proliferation by an interleukin 2-dependent mechanism. PLoS One 8 : 68016 . 10.1371/journal.pone.0068016 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Habish H., Mahdi A. (1983). Nodulation of legumes in the Sudan. IV. Effects of soil moisture, light and temperature on nodulation of Clitoria ternatea . East Afr. Agric. For. J. 44 229–236. 10.1080/00128325.1979.11663013 [ CrossRef ] [ Google Scholar ]
- Hall T. J. (1985). Adaptation and agronomy of Clitoria ternatea L. in Northern Australia. Trop. Grasslands 19 156–163. [ Google Scholar ]
- Harada G., Onoue S., Inoue C., Hanada S., Katakura Y. (2018). Delphinidin-3-glucoside suppresses lipid accumulation in HepG2 cells. Cytotechnology 70 1707–1712. 10.1007/s10616-018-0246-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harmand T. J., Bousbaine D., Chan A., Zhang X., Liu D. R., Tam J. P., et al. (2018). One-pot dual labeling of IgG 1 and preparation of C-to-C fusion proteins through a combination of sortase A and butelase 1. Bioconjug. Chem. 29 3245–3249. 10.1021/acs.bioconjchem.8b00563 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harris K. S., Durek T., Kaas Q., Poth A. G., Gilding E. K., Conlan B. F., et al. (2015). Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase. Nat. Commun. 6 : 10199 . 10.1038/ncomms10199 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hasan N., Jain R. K. (1985). Preliminary assessment of the response of Clitoria ternatea lines to the root-knot nematode, Meloidogyne incognita . Nematologica 31 236–238. 10.1163/187529285X00319 [ CrossRef ] [ Google Scholar ]
- Ismail N., Rani U., Batra A. (2012). High frequency in vitro shoot regeneration of Clitoria ternatea L. affected by different cultural conditions. Indian J. Biotechnol. 11 210–214. [ Google Scholar ]
- Jackson M. A., Gilding E. K., Shafee T., Harris K. S., Kaas Q., Poon S., et al. (2018). Molecular basis for the production of cyclic peptides by plant asparaginyl endopeptidases. Nat. Commun. 9 : 2411 . 10.1038/s41467-018-04669-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jain N. N., Ohal C. C., Shroff S. K., Bhutada R. H., Somani R. S., Kasture V. S., et al. (2003). Clitoria ternatea and the CNS. Pharmacol. Biochem. Behav. 75 529–536. 10.1016/S0091-3057(03)00130-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jones R. M., Bishop H. G., Clem R. L., Conway M. J., Cook B. G., Moore K., et al. (2000). Measurements of nutritive value of a range of tropical legumes and their use in legume evaluation. Trop. Grasslands 34 78–90. [ Google Scholar ]
- Joshi S. S., Shrivastava R. K., Shrivastava D. K. (1981). Chemical examination of Clitoria ternatea seeds. J. Am. Oil Chem. Soc. 58 714–715. [ Google Scholar ]
- Jouvensal L., Quillien L., Ferrasson E., Rahbé Y., Guéguen J., Vovelle F. (2003). PA1b, an insecticidal protein extracted from pea seeds ( Pisum sativum ): 1 H-2-D NMR study and molecular modeling. Biochemistry 42 11915–11923. 10.1021/bi034803l [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kamh M., Abdou M., Chude V., Wiesler F., Horst W. J. (2002). Mobilization of phosphorus contributes to positive rotational effects of leguminous cover crops on maize grown on soils from northern Nigeria. J. Plant Nutr. Soil Sci. 165 566–572. 10.1002/1522-2624(200210)165:5<566::aid-jpln566>3.0.co;2-o [ CrossRef ] [ Google Scholar ]
- Kamilla L., Mansor S. M., Ramanathan S., Sasidharan S. (2009). Effects of Clitoria ternatea leaf extract on growth and morphogenesis of Aspergillus niger . Microsc. Microanal. 15 366–372. 10.1017/S1431927609090783 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kavitha R. (2018). Biochemical studies on the effect of ethanolic extracts of Trichosanthes dioica and Clitoria ternatea in streptozotocin induced male Wistar rats. Int. J. Pharm. Sci. Res. 9 4682–4689. 10.13040/ijpsr.0975-8232.9(11).4682-89 [ CrossRef ] [ Google Scholar ]
- Kazuma K., Kogawa K., Noda N., Kato N., Suzuki M. (2004). Identification of delphinidin 3-O-(6″-O-malonyl)-β-glucoside-3′-O-β-glucoside, a postulated intermediate in the biosynthesis of ternatin C5 in the blue petals of Clitoria ternatea (Butterfly Pea). Chem. Biodivers. 1 1762–1770. 10.1002/cbdv.200490132 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kazuma K., Noda N., Suzuki M. (2003a). Flavonoid composition related to petal color in different lines of Clitoria ternatea . Phytochemistry 64 1133–1139. 10.1016/S0031-9422(03)00504-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kazuma K., Noda N., Suzuki M. (2003b). Malonylated flavonol glycosides from the petals of Clitoria ternatea . Phytochemistry 62 229–237. 10.1016/S0031-9422(02)00486-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kelemu S., Cardona C., Segura G. (2004). Antimicrobial and insecticidal protein isolated from seeds of Clitoria ternatea , a tropical forage legume. Plant Physiol. Biochem. 42 867–873. 10.1016/j.plaphy.2004.10.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khadatkar S. N., Manwar J., Bhajipale N. S. (2008). In-vitro anthelmintic activity of root of Clitoria ternatea Linn. Pharmacogn. Mag. 4 148–150. [ Google Scholar ]
- Koehbach J., Attah A. F., Berger A., Hellinger R., Kutchan T. M., Carpenter E. J., et al. (2013). Cyclotide discovery in Gentianales revisited—identification and characterization of cyclic cystine-knot peptides and their phylogenetic distribution in Rubiaceae plants. Biopolymers 100 438–452. 10.1002/bip.22328 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koehbach J., Gruber C. W. (2015). “Cyclotides in the Rubiaceae,” in Advances in Botanical Research , ed. Craik D. J. (Cambridge, MA: Academic Press; ), 51–78. 10.1016/bs.abr.2015.09.002 [ CrossRef ] [ Google Scholar ]
- Kondo T., Ueda M., Goto T. (1990). Structure of ternatin B1, a pentaacylated anthocyanin substituted on the B-ring asymmetrically with two long chains. Tetrahedron 46 4749–4756. 10.1016/S0040-4020(01)85593-9 [ CrossRef ] [ Google Scholar ]
- Kulshreshtha D. K., Khare M. P. (1967). Chemical investigation of the seeds of Clitoria ternatea ‘Linn’. Curr. Sci. 36 124–125. [ Google Scholar ]
- Kumar V., Mukherjee K., Kumar S., Mal M., Mukherjee P. K. (2008). Validation of HPTLC method for the analysis of taraxerol in Clitoria ternatea . Phytochem. Anal. 19 244–250. 10.1002/pca.1042 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumari N. V., Devi M. L. (2013). Effect of some indigenous plant extracts on the inhibition of egg hatching of nematode Meloidogyne incognita Chitwood infesting mulberry. HortFlora Res. Spec. 2 35–39. [ Google Scholar ]
- Lakshmanan K. K., Dhanalakshmi S. (1990). Callus, organogenesis and plantlet formation in tissue cultures of Clitoria ternatea . Ann. Bot. 66 451–455. 10.1093/oxfordjournals.aob.a088047 [ CrossRef ] [ Google Scholar ]
- Lakshmanan M., Padmanabhan D. (1968). Effect of ascochitine on the in vitro growth of embryos of Clitoria Ternatea L. Curr. Sci. 37 321–322. [ Google Scholar ]
- Liu R., Mu L., Liu H., Wei L., Yan T., Chen M., et al. (2011). Two antimicrobial and nematicidal peptides derived from sequences encoded Picea sitchensis . J. Pept. Sci. 17 627–631. 10.1002/psc.1380 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mahala A. G., Amasiab S. O., Yousif M. A., Elsadig A. (2012). Effect of Plant age on DM yield and nutritive value of some leguminous plants ( Cyamopsis tetragonoloba , Lablab purpureus and Clitoria ( Clitoria ternatea ). Int. Res. J. Agric. Sci. Soil Sci. 2 502–508. [ Google Scholar ]
- Mahmad N., Taha R. M., Othman R., Elias H., Saleh A. (2016). Encapsulated embryogenic callus of Clitoria ternatea L. for regeneration and conservation. Int. J. Environ. Sci. Dev. 7 363–367. 10.7763/ijesd.2016.v7.801 [ CrossRef ] [ Google Scholar ]
- Maity N., Nema N., Sarkar B., Mukherjee P. (2012). Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Indian J. Pharmacol. 44 584–587. 10.4103/0253-7613.100381 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McDonald C. K. (2002). Germination response to temperature in tropical and subtropical pasture legumes. 1. Constant temperature. Aust. J. Exp. Agric. 42 407–419. [ Google Scholar ]
- Mensah R., Leach D., Young A., Watts N., Glennie P. (2015). Development of Clitoria ternatea as a biopesticide for cotton pest management: assessment of product effect on Helicoverpa spp. and their natural enemies. Entomol. Exp. Appl. 154 131–145. 10.1111/eea.12263 [ CrossRef ] [ Google Scholar ]
- Mhaskar A., Krishnan P., Vishwakarma K., Maheshwari V. (2011). In vitro regeneration of Clitoria ternatea L. through axillary bud culture. Int. J. Pharmacol. Biol. Sci. 5 17–23. [ Google Scholar ]
- Mohamed N., Taha R. M. (2011). Plant regeneration of Clitoria ternatea from leaf explants cultured in vitro. J. Food Agric. Environ. 9 268–270. [ Google Scholar ]
- Morita N., Ariwasa M., Nagase M., Hsu H.-Y., Chen Y.-P. (1976). Studies on the constituents of formosan leguminosae: the constituents in the leaves of Clitoria ternatea L. Yakugaku Zasshi 97 649–653. 10.1248/yakushi1947.97.6_649 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morris J. B. (2009). Characterization of butterfly pea ( Clitoria ternatea L.) accessions for morphology, phenology, reproduction and potential nutraceutical, pharmaceutical trait utilization. Genet. Resour. Crop Evol. 56 421–427. 10.1007/s10722-008-9376-0 [ CrossRef ] [ Google Scholar ]
- Mukherjee P. K., Kumar V., Kumar N. S., Heinrich M. (2008). The Ayurvedic medicine Clitoria ternatea -from traditional use to scientific assessment. J. Ethnopharmacol. 120 291–301. 10.1016/j.jep.2008.09.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mukhtar S., Ahmad N., Khan M. I., Anis M., Aref I. M. (2012). Influencing micropropagation in Clitoria ternatea L. through the manipulation of TDZ levels and use of different explant types. Physiol. Mol. Biol. Plants 18 381–386. 10.1007/s12298-012-0136-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ] Retracted
- Mullick P., Chatterji U. N. (1967). Eco-physiological studies on seed germination: germination experiments with the seeds of Clitoria ternatea Linn. Trop. Ecol. 8 116–125. [ Google Scholar ]
- Mylne J. S., Chan L. Y., Chanson A. H., Daly N. L., Schaefer H., Bailey T. L., et al. (2012). Cyclic peptides arising by evolutionary parallelism via asparaginyl-endopeptidase-mediated biosynthesis. Plant Cell 24 2765–2778. 10.1105/tpc.112.099085 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mylne J. S., Colgrave M. L., Daly N. L., Chanson A. H., Elliott A. G., McCallum E. J., et al. (2011). Albumins and their processing machinery are hijacked for cyclic peptides in sunflower. Nat. Chem. Biol. 7 257–259. 10.1038/nchembio.542 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen G. K., Kam A., Loo S., Jansson A. E., Pan L. X., Tam J. P. (2015). Butelase 1: a versatile ligase for peptide and protein macrocyclization. J. Am. Chem. Soc. 137 15398–15401. 10.1021/jacs.5b11014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen G. K., Wang S., Qiu Y., Hemu X., Lian Y., Tam J. P. (2014). Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat. Chem. Biol. 10 732–738. 10.1038/nchembio.1586 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen G. K. T., Hemu X., Quek J. P., Tam J. P. (2016a). Butelase-mediated macrocyclization of d-amino-acid-containing peptides. Angew. Chem. 55 12802–12806. 10.1002/anie.201607188 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen K. N., Nguyen G. K., Nguyen P. Q., Ang K. H., Dedon P. C., Tam J. P. (2016b). Immunostimulating and gram-negative-specific antibacterial cyclotides from the butterfly pea ( Clitoria ternatea ). FEBS J. 283 2067–2090. 10.1111/febs.13720 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen G. K. T., Qiu Y., Cao Y., Hemu X., Liu C.-F., Tam J. P. (2016c). Butelase-mediated cyclization and ligation of peptides and proteins. Nat. Protoc. 11 1977–1988. 10.1038/nprot.2016.118 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen G. K. T., Zhang S., Ngan T. K. N., Phuong Q. T. N., Chiu M. S., Hardjojo A., et al. (2011). Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family. J. Biol. Chem. 286 24275–24287. 10.1074/jbc.M111.229922 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nigg J., Lewis K., Edinger T., Falk M. (2011). Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, sestriction diet, and synthetic food color additives. J. Am. Acad. Child Adolesc. Psychiatry 51 86–97. 10.1016/j.jaac.2011.10.015 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Njunie M. N., Wagger M. G., Luna-Orea P. (2004). Residue decomposition and nutrient release dynamics from two tropical forage legumes in a Kenyan environment. Agron. J. 96 1073–1081. 10.2134/agronj2004.1073 [ CrossRef ] [ Google Scholar ]
- Oblisami G. (1974). Studies on the rhizobium and nodulation pattern in a forage legume Clitoria ternatea . Proc. Indian Natl. Sci. Acad. B Biol. Sci. 40 618–623. [ Google Scholar ]
- Oram R. N. (1992). Register of Australian herbage plant cultivars. Aust. J. Exp. Agric. 32 547–548. [ Google Scholar ]
- Pandeya K., Tiwari K. N., Singh J., Dubey D., Prakash Verma J. (2010). In vitro propagation of Clitoria ternatea L.: a rare medicinal plant. J. Med. Plants Res. 4 664–668. 10.5897/jmpr09.418 [ CrossRef ] [ Google Scholar ]
- Parimaladevi B., Boominathan R., Mandal S. C. (2004). Evaluation of antipyretic potential of Clitoria ternatea L. extract in rats. Phytomedicine 11 323–326. 10.1078/0944711041495191 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Park S., Yoo K.-O., Marcussen T., Backlund A., Jacobsson E., Rosengren K. J., et al. (2017). Cyclotide evolution: insights from the analyses of their precursor sequences, structures and distribution in violets ( Viola ). Front. Plant Sci. 8 : 2058 . 10.3389/fpls.2017.02058 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pasukamonset P., Kwon O., Adisakwattana S. (2016). Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 61 772–779. 10.1016/j.foodhyd.2016.06.039 [ CrossRef ] [ Google Scholar ]
- Pasukamonset P., Kwon O., Adisakwattana S. (2017). Oxidative stability of cooked pork patties incorporated with Clitoria ternatea extract (blue pea flower petal) during refrigerated storage. J. Food Process. Preserv. 41 : e12751 10.1111/jfpp.12751 [ CrossRef ] [ Google Scholar ]
- Pasukamonset P., Pumalee T., Sanguansuk N., Chumyen C., Wongvasu P., Adisakwattana S., et al. (2018). Physicochemical, antioxidant and sensory characteristics of sponge cakes fortified with Clitoria ternatea extract. J. Food Sci. Technol. 55 2881–2889. 10.1007/s13197-018-3204-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patel J., Pillai V., Sharma A., Dholakiya B., Gajbhiye N., Saravanan R. (2016). Effect of seed treatment on germination and flavonoids diversity in accessions of butterfly pea ( Clitoria ternatea ). Indian J. Agric. Sci. 86 1553–1558. [ Google Scholar ]
- Perry T., Batterham P., Daborn P. J. (2011). The biology of insecticidal activity and resistance. Insect Biochem. Mol. Biol. 41 411–422. 10.1016/j.ibmb.2011.03.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phrueksanan W., Yibchok-anun S., Adisakwattana S. (2014). Protection of Clitoria ternatea flower petal extract against free radical-induced hemolysis and oxidative damage in canine erythrocytes. Res. Vet. Sci. 97 357–363. 10.1016/j.rvsc.2014.08.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Piala J. J., Madissoo H., Rubin B. (1962). Diuretic activity of roots of Clitoria ternatea L. in dogs. Experientia 18 : 89 . 10.1007/BF02138275 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Plan M. R. R., Göransson U., Clark R. J., Daly N. L., Colgrave M. L., Craik D. J. (2007). The cyclotide fingerprint in Oldenlandia affinis : elucidation of chemically modified, linear and novel macrocyclic peptides. ChemBioChem 8 1001–1011. 10.1002/cbic.200700097 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Poth A. G., Colgrave M. L., Lyons R. E., Daly N. L., Craik D. J. (2011a). Discovery of an unusual biosynthetic origin for circular proteins in legumes. Proc. Natl. Acad. Sci. U.S.A. 108 10127–10132. 10.1073/pnas.1103660108 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Poth A. G., Colgrave M. L., Philip R., Kerenga B., Daly N. L., Anderson M. A., et al. (2011b). Discovery of cyclotides in the Fabaceae plant family provides new insights into the cyclization, evolution, and distribution of circular proteins. ACS Chem. Biol. 6 345–355. 10.1021/cb100388j [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pullaiah T. (2000). Embryology of Clitoria ternatea (Fabaceae). Plant Biosyst. 134 39–43. 10.1080/11263500012331350325 [ CrossRef ] [ Google Scholar ]
- Raghu K. S., Shamprasad B. R., Kabekkodu S. P., Paladhi P., Joshi M. B., Valiathan M. S., et al. (2017). Age dependent neuroprotective effects of medhya rasayana prepared from Clitoria ternatea Linn. in stress induced rat brain. J. Ethnopharmacol. 197 173–183. 10.1016/j.jep.2016.07.068 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rai K., Murthy D. S., Karanth K., Rao M. (2001). Clitoria ternatea (Linn) root extract treatment during growth spurt period enhances learning and memory in rats. Indian J. Physiol. Pharmacol. 45 305–313. [ PubMed ] [ Google Scholar ]
- Rai K. S., Murthy K. D., Karanth K. S., Nalini K., Rao M. S., Srinivasan K. K. (2002). Clitoria ternatea root extract enhances acetylcholine content in rat hippocampus. Fitoterapia 73 685–689. 10.1016/S0367-326X(02)00249-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rai K. S., Murthy K. D., Rao M. S., Karanth K. S. (2005). Altered dendritic arborization of amygdala neurons in young adult rats orally intubated with Clitorea ternatea aqueous root extract. Phytother. Res. 19 592–598. 10.1002/ptr.1657 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reid R., Sinclair D. F. (1980). An evaluation of C. ternatea for forage and grain production. Genet. Resour. Commun. 1 1–8. [ Google Scholar ]
- Rout G. R. (2005). Micropropagation of Clitoria ternatea Linn. (Fabaceae)— An important medicinal plant. In Vitro Cell. Dev. Biol. Plant 41 516–519. 10.1079/IVP2005675 [ CrossRef ] [ Google Scholar ]
- Saito N., Abe K., Honda T., Timberlake C. F., Bridle P. (1985). Acylated delphinidin glucosides and flavonols from Clitoria ternatea . Phytochemistry 24 1583–1586. [ Google Scholar ]
- Salhan M., Kumar B., Tiwari P., Sharma P., Sandhar H. K., Gautam M. (2011). Comparative anthelmintic activity of aqueous and ethanolic leaf extracts of Clitoria ternatea . Int. J. Drug Dev. Res. 3 68–69. [ Google Scholar ]
- Serra A., Hemu X., Nguyen G. K., Nguyen N. T., Sze S. K., Tam J. P. (2016). A high-throughput peptidomic strategy to decipher the molecular diversity of cyclic cysteine-rich peptides. Sci. Rep. 6 : 23005 . 10.1038/srep23005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shahid M., Shahzad A., Anis M. (2009). Antibacterial potential of the extracts derived from leaves and in vitro raised calli of medicinal plants Pterocarpus marsupium Roxb ., Clitoria ternatea L., and Sanseveiria cylindrica Bojer ex Hook. Orient. Pharm. Exp. Med. 9 174–181. 10.3742/OPEM.2009.9.2.174 [ CrossRef ] [ Google Scholar ]
- Shahzad A., Faisal M., Anis M. (2007). Micropropagation through excised root culture of Clitoria ternatea and comparison between in vitro regenerated plants and seedlings. Ann. Appl. Biol. 150 341–349. 10.1111/j.1744-7348.2007.00132.x [ CrossRef ] [ Google Scholar ]
- Singh N. K., Garabadu D., Sharma P., Shrivastava S. K., Mishra P. (2018). Anti-allergy and anti-tussive activity of Clitoria ternatea L. in experimental animals. J. Ethnopharmacol. 224 15–26. 10.1016/j.jep.2018.05.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siti Azima A. M., Noriham A., Manshoor N. (2017). Phenolics, antioxidants and color properties of aqueous pigmented plant extracts: Ardisia colorata var. elliptica , Clitoria ternatea , Garcinia mangostana and Syzygium cumini . J. Funct. Foods 38 232–241. 10.1016/j.jff.2017.09.018 [ CrossRef ] [ Google Scholar ]
- Sogo T., Terahara N., Hisanaga A., Kumamoto T., Yamashiro T., Wu S., et al. (2015). Anti-inflammatory activity and molecular mechanism of delphinidin 3-sambubioside, a Hibiscus anthocyanin. BioFactors 41 58–65. 10.1002/biof.1201 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Solanki Y. B., Jain S. M. (2010). Antihyperlipidemic activity of Clitoria ternatea and Vigna mungo in rats. Pharm. Biol. 48 915–923. 10.3109/13880200903406147 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Staples I. B. (1992). “ Clitoria ternatea L,” in Plant Resources of Southeast Asia , eds Mannetje L. T., Jones R. M. (Wageningen: Pudoc Scientific Publishers; ). [ Google Scholar ]
- Sushma N. J., Prathyusha D., Swathi G., Madhavi T., Raju B. D. P., Mallikarjuna K., et al. (2015). Facile approach to synthesize magnesium oxide nanoparticles by using Clitoria ternatea -characterization and in vitro antioxidant studies. Appl. Nanosci. 6 437–444. 10.1007/s13204-015-0455-1 [ CrossRef ] [ Google Scholar ]
- Swain S. S., Rout K. K., Chand P. K. (2012a). Production of triterpenoid anti-cancer compound taraxerol in Agrobacterium -transformed root cultures of butterfly pea ( Clitoria ternatea L.). Appl. Biochem. Biotechnol. 168 487–503. 10.1007/s12010-012-9791-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Swain S. S., Sahu L., Pal A., Barik D. P., Pradhan C., Chand P. K. (2012b). Hairy root cultures of butterfly pea ( Clitoria ternatea L.): Agrobacterium x plant factors influencing transformation. World J. Microbiol. Biotechnol. 28 729–739. 10.1007/s11274-011-0869-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Talpate K., Bhosale U., Zambare M., Somani R. (2014). Neuroprotective and nootropic activity of Clitorea ternatea Linn.(Fabaceae) leaves on diabetes induced cognitive decline in experimental animals. J. Pharm. Bioallied Sci. 6 48–55. 10.4103/0975-7406.124317 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tani T., Nishikawa S., Kato M., Tsuda T. (2017). Delphinidin 3-rutinoside-rich blackcurrant extract ameliorates glucose tolerance by increasing the release of glucagon-like peptide-1 secretion. Food Sci. Nutr. 5 929–933. 10.1002/fsn3.478 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taranalli A. D., Cheeramkuzhy T. C. (2000). Influence of Clitoria ternatea extracts on memory and central cholinergic activity in rats. Pharm. Biol. 38 51–56. 10.1076/1388-0209(200001)3811-BFT051 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taur D. J., Patil R. Y. (2011). Evaluation of antiasthmatic activity of Clitoria ternatea L. roots. J. Ethnopharmacol. 136 374–376. 10.1016/j.jep.2011.04.064 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taur D. J., Taware S. B., Patil R. N., Patil R. Y., Kharya M. D. (2010). Pharmacognostical and preliminary phytochemical evaluation of Clitoria ternatea leaves. Pharmacogn. J. 2 260–265. 10.1016/S0975-3575(10)80114-2 [ CrossRef ] [ Google Scholar ]
- Terahara N., Oda M., Matsui T., Osajima Y., Saito N., Toki K., et al. (1996). Five new anthocyanins, ternatins A3, B4, B3, B2, and D2, from Clitoria ternatea flowers. J. Nat. Prod. 59 139–144. 10.1021/np960050a [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Terahara N., Saito N., Honda T., Toki K., Osajima Y. (1989a). Structure of ternatin A1, the largest ternatin in the major blue anthocyanins from Clitoria ternatea flowers. Tetrahedron Lett. 31 2921–2924. 10.1016/0040-4039(90)80185-O [ CrossRef ] [ Google Scholar ]
- Terahara N., Saito N., Honda T., Toki K., Osajima Y. (1989b). Structure of ternatin D1, an acylated anthocyanin from Clitoria ternatea flowers. Tetrahedron Lett. 30 5305–5308. 10.1016/S0040-4039(01)93771-2 [ CrossRef ] [ Google Scholar ]
- Terahara N., Saito N., Honda T., Toki K., Osajima Y. (1990a). Acylated anthocyanins of Clitoria ternatea flowers and their acyl moieties. Phytochemistry 29 949–953. 10.1016/0031-9422(90)80053-J [ CrossRef ] [ Google Scholar ]
- Terahara N., Saito N., Honda T., Toki K., Osajima Y. (1990b). Further structural elucidation of the anthocyanin, deacylternatin, from Clitoria ternatea . Phytochemistry 29 3686–3687. 10.1016/0031-9422(90)85308-3 [ CrossRef ] [ Google Scholar ]
- Terahara N., Saito N., Honda T., Toki K., Osajima Y. (1990c). Structure of ternatin A2, one of Clitoria ternatea flower anthocyanins having the unsymmetrical side chains. Heterocycles 31 1773–1776. 10.3987/COM-90-5544 [ CrossRef ] [ Google Scholar ]
- Terahara N., Toki K., Saito N., Honda T., Matsui T., Osajima Y. (1998). Eight new anthocyanins, ternatins C1-C5 and D3 and preternatins A3 and C4 from young Clitoria ternatea flowers. J. Nat. Prod. 61 1361–1367. 10.1021/np980160c [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thell K., Hellinger R., Sahin E., Michenthaler P., Gold-Binder M., Haider T., et al. (2016). Oral activity of a nature-derived cyclic peptide for the treatment of multiple sclerosis. Proc. Natl.Acad. Sci. U.S.A. 113 3960–3965. 10.1073/pnas.1519960113 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vasisht K., Dhobi M., Khullar S., Mandal S. K., Karan M. (2016). Norneolignans from the roots of Clitoria ternatea L. Tetrahedron Lett. 57 1758–1762. 10.1016/j.tetlet.2016.03.024 [ CrossRef ] [ Google Scholar ]
- Yamada K., Shimada T., Nishimura M., Hara-Nishimura I. (2005). A VPE family supporting various vacuolar functions in plants. Physiol. Plant 123 369–375. 10.1111/j.1399-3054.2005.00464.x [ CrossRef ] [ Google Scholar ]
- Zaroug M. G., Munns D. N. (1980a). Effects of phosphorus and sulfur nutrition on soluble sugars and growth in Clitoria ternatea L. Plant Soil 55 243–250. 10.1007/BF02181804 [ CrossRef ] [ Google Scholar ]
- Zaroug M. G., Munns D. N. (1980b). Screening strains of Rhizobium for the tropical legumes Clitoria ternatea and Vigna trilobata in soils of different pH. Trop. Grasslands 14 28–33. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
In 1985, six acylated anthocyanins were isolated from blue C. ternatea flowers that were all derivatives of delphinidin 3,3′,5′-triglucoside (Saito et al., 1985). The chemical properties of the acylated C. ternatea delphinidins, which were named ternatins, were further elucidated in subsequent studies (Terahara et al., 1989a, 1990a,b).
Clitoria ternatea flower (butterfly pea), a member of the Fabaceae family has a vivid blue colour which is widely used as a natural food colourant (e.g. in rice cakes, tea, snacks, and sweet ...
Clitoria ternatea plant is classified in the kingdom Plantae, phylum Tracheophyta, class of Magnoliopsida and a family of Fabaceae (Jamil et al. 2018). Clitoria ternatea is a perennial climber (2-3 m in height) and is known by its common name as butterfly pea or blue pea flower (Mukherjee et al. 2008).
The Clitoria ternatea (butterfly pea) has gained a lot of attention due to its role in traditional medicine, food coloring, cosmetics, fodder, and as a source of an environmentally friendly insecticide, among other agricultural and medical applications [].There are various names for this perennial leguminous plant in the Fabaceae family, including Blue Bell Vine, Asian Pigeon Wings, Cordofan ...
described in this thesis. Clitoria Ternatea Linn (Blue Ternate) ... blossoms of ternatea with their striking blue coloring, first distinguished in 1985 according to Saito. The ternatin ...
Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: a randomized crossover trial. BMC Complementary and Alternative Medicine 18:6. Makasana, J., B.Z. Dholakiya, N.A. Gajbhiye, and S. Raju, 2017. Extractive determination of bioactive flavonoids from butterfly pea (Clitoria ternatea ...
C. ternatea L contains abundant concentrations of both quercetin and kaempferol and their respective derivatives (Santana et al., 2021). This study, therefore, aims to evaluate the antioxidant activity of Clitoria ternatea (blue pea) flowers by determining its total phenolic content and free radical scavenging activity.
Clitoria ternatea commonly also called Clitoria, blue-pea, kordofan. pea (Sudan), cunha (Brazil or pokindong (Philippines) is a vigorous, summer. growing, legume of old world origin. Clitoria L ...
Blue butterfly pea (Clitoria ternatea L.) flower (BPF) is an underutilized plant known for several health benefits. BPF can be used for increasing consumer demand for healthy foods by replacing ...
The butterfly pea flower (Clitoria ternatea L.) (BPF) has a high anthocyanin content, which can be incorporated into polymer-based films to produce intelligent packaging for real-time food freshness indicators. The objective of this work was to systematically review the polymer characteristics used as BPF extract carriers and their application on various food products as intelligent packaging ...
Clitoria ternatea L./blue pea flower is a rich source of polyacylated anthocyanins and their higher stability compared with non-acylated anthocyanins provide the advantage to be used as a natural food colouring agent (Buchweitz et al., 2012; Marpaung et al., 2019). Like all anthocyanins, the colour of blue pea flower anthocyanin extract also ...
The anthocyanins are blue in the petals and acylated based on delphinidin, known as ternatins isolated from C. ternatea, which are ternatin A1-A3, B1-B4, C1-C4, and D1-D3 [21,23,32,33,34,35]. Another study reported that minor delphinidin glycosides and the preternatins A3 and C4 were isolated from the young C. ternatea [ 34 ].
Abstract. Also known as Asian Pigeon Wings, Blue Bell Vine, Blue Pea, Cordofan Pea and Darwin pea, 'Butterfly Pea' (Clitoria ternatea) is an amazing brain boosting herb native to tropical equatorial Asia.A traditional Chinese and Ayurvedic medicine, Clitoria Ternatea has been consumed for centuries as a memory enhancer, brain booster, anti-stress and calmative agent.
In this study, aqueous extractions of dye from flowers were carried out in the following conditions to obtain optimization characterization: time of extraction (30-180 minutes), temperature (60-90°C), amount of flowers (0.1-2gm) and pH (2-10). It was observed that dye concentration increases gradually at higher temperature and for longer time.
ABSTRACT. The study determines the nutrient values, safety, and market acceptability of the flower of Blue Ternate (Clitoria ternatea L.) tea specifically by an identified and target tea user in terms of health and wellness benefits, nutritional values, hygiene and safety.This study used descriptive method of research using the laboratory tests results of the Blue Ternate dried flower to ...
An Experimental Study on Effects of Blue Ternatea in Alleviating Anxiety and Stress among Jose Rizal University's College Students Course Psychology - Experimental Psychology Grade 1.8 ... Your term paper / thesis: - Publication as eBook and book - High royalties for the sales - Completely free - with ISBN
The perennial leguminous herb Clitoria ternatea (butterfly pea) has attracted significant interest based on its agricultural and medical applications, which range from use as a fodder and nitrogen fixing crop, to applications in food coloring and cosmetics, traditional medicine and as a source of an eco-friendly insecticide. In this article we provide a broad multidisciplinary review that ...
THE ACCEPTABILITY OF BLUE TERNATE (Clitoria ternatea) FLOWER EXTRACT AS HIGHLIGHTER INK CHAPTER 1 Introduction of the Study The ink used in a highlighter pen plays an important role in emphasizing the important points in a reading passage or article.Teachers, students, or ordinary people use highlighter pen to find key points and keywords when they are studying, also used to organize information.
The study determines the nutrient values, safety, and market acceptability of the flower of Blue Ternate (Clitoria ternatea L.) tea specifically by an identified and target tea user in terms of ...
Clitoria ternatea, commonly known as Asian pigeonwings, bluebellvine, blue pea, butterfly pea, cordofan pea or Darwin pea, is a plant species belonging to the family Fabaceae, ... The blue colour of C. ternatea is a result of various anthocyanins, most importantly ternatins - polyacylated derivatives of delphinidin 3,3', 5'-triglucoside (Da-T).
Clitoria ternatea Linn Fabaceae which is also known as bunga telang in Malaysia, contains many useful functions leading to various activities such as blue food colorant, a cure for cancer and used for treatment from poisonous bite. Demand on the natural blue colorant is increasing due to the health issues from the usage of blue synthetics colorant.
Blue butterfly pea (Clitoria ternatea L.) flower (BPF) is an underutilized plant known for several health benefits. BPF can be used for increasing consumer demand for healthy foods by replacing ...
Apple was well-known for its ability to create "Blue Oceans" during the Steve Jobs era, but it currently appears that the company struggles to differentiate and create brand new multi-billion ...
The shape of owers of the Clitoria plant is a re ection of its genus name. The owers of. this plant resemble in shape with human female clitoris, hence the Latin name of the genus. "Clitoria ...