- Patient Care & Health Information
- Tests & Procedures
A urinalysis is a test of your urine. It's used to detect and manage a wide range of disorders, such as urinary tract infections, kidney disease and diabetes.
A urinalysis involves checking the appearance, concentration and content of urine. For example, a urinary tract infection can make urine look cloudy instead of clear. Increased levels of protein in urine can be a sign of kidney disease.
Unusual urinalysis results often require more testing to find the source of the problem.

Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Why it's done
A urinalysis is a common test that's done for several reasons:
- To check your overall health. A urinalysis might be part of a routine medical exam, pregnancy checkup or pre-surgery preparation. Or it might be used to screen for a variety of disorders, such as diabetes, kidney disease or liver disease, when you're admitted to a hospital.
- To diagnose a medical condition. A urinalysis might be requested if you have abdominal pain, back pain, frequent or painful urination, blood in your urine, or other urinary problems. A urinalysis can help diagnose the cause of these signs and symptoms.
- To monitor a medical condition. If you've been diagnosed with a medical condition, such as kidney disease or a urinary tract infection, your doctor might recommend testing your urine regularly to monitor your condition and treatment.
Other tests, such as pregnancy testing and drug screenings, might rely on a urine sample, but these tests look for substances that aren't included in a typical urinalysis.
More Information
- Acute kidney injury
- Amyloidosis
- Amyotrophic lateral sclerosis (ALS)
- Anaphylaxis
- Anorexia nervosa
- Anterior vaginal prolapse (cystocele)
- Appendicitis
- Bed-wetting
- Binge-eating disorder
- Bipolar disorder
- Bladder stones
- Blood in urine (hematuria)
- Bulimia nervosa
- Carcinoid tumors
- Castleman disease
- Chlamydia trachomatis
- Chronic daily headaches
- Chronic kidney disease
- Congenital adrenal hyperplasia
- Cyclothymia (cyclothymic disorder)
- Dehydration
- Diabetes insipidus
- Diabetic ketoacidosis
- Epididymitis
- Erectile dysfunction
- Febrile seizure
- Granulomatosis with polyangiitis
- Heat exhaustion
- Henoch-Schonlein purpura
- High blood pressure in children
- Histoplasmosis
- Horner syndrome
- Hyperparathyroidism
- Hyponatremia
- Hypoparathyroidism
- IgA nephropathy (Berger disease)
- Infant jaundice
- Infant reflux
- Interstitial cystitis
- Kidney cancer
- Kidney infection
- Listeria infection
- Nephrotic syndrome
- Non-Hodgkin's lymphoma
- Overactive bladder
- Pelvic inflammatory disease (PID)
- Pheochromocytoma
- Pituitary tumors
- Postpartum preeclampsia
- Post-vasectomy pain syndrome
- Prostatitis
- Retrograde ejaculation
- Rett syndrome
- Scrotal masses
- Secondary hypertension
- Sexually transmitted diseases (STDs)
- Sjogren's syndrome
- Stress incontinence
- Sweating and body odor
- Testicular torsion
- Toxic shock syndrome
- Trichomoniasis
- Type 1 diabetes
- Urine color
- Vaginal atrophy
- Vesicoureteral reflux
- Wilms tumor
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
How you prepare
If you're having only a urinalysis, you can eat and drink before the test. If you're having other tests, you might need to fast before the test. Your health care provider will give you specific instructions.
Many drugs, including nonprescription medications and supplements, can affect the results of a urinalysis. Before a urinalysis, tell your doctor about medications, vitamins or other supplements you take.
What you can expect
You might collect a urine sample at home or at your health care provider's office. Providers typically give out containers for urine samples. You might be asked to collect the sample at home first thing in the morning, when your urine is more concentrated.
You might be instructed to collect the sample midstream, using a clean-catch method. This method involves the following steps:
- Cleanse the urinary opening. Women should spread the labia and clean from front to back. Men should wipe the tip of the penis.
- Begin to urinate into the toilet.
- Pass the collection container into your urine stream.
- Urinate at least 1 to 2 ounces (30 to 60 milliliters) into the collection container.
- Finish urinating into the toilet.
- Deliver the sample as directed by your health care provider.
- If you can't deliver the sample to the designated area within 60 minutes of collection, refrigerate the sample, unless your provider has told you otherwise.
In some cases, if needed, your provider can insert a thin, flexible tube (catheter) through the urinary tract opening and into the bladder to collect the urine sample.
The urine sample is sent to a lab for analysis. You can return to your usual activities immediately.
For a urinalysis, your urine sample is evaluated in three ways: visual exam, dipstick test and microscopic exam.
Visual exam
A lab technician examines the urine's appearance. Urine is typically clear. Cloudiness or an unusual odor can indicate a problem, such as an infection. Protein in urine can make it appear foamy.
Blood in the urine can make it look red or brown. Urine color can be influenced by what you've just eaten or by certain drugs you're taking. For example, beets or rhubarb might tint your urine red.
Dipstick test
A dipstick — a thin, plastic stick with strips of chemicals on it — is placed in the urine. The chemical strips change color if certain substances are present or if their levels are above typical levels. A dipstick test checks for:
- Acidity (pH). The pH level indicates the amount of acid in urine. The pH level might indicate a kidney or urinary tract disorder.
- Concentration. A measure of concentration shows how concentrated the particles are in your urine. A higher than normal concentration often is a result of not drinking enough fluids.
- Protein. Low levels of protein in urine are typical. Small increases in protein in urine usually aren't a cause for concern, but larger amounts might indicate a kidney problem.
- Sugar. The amount of sugar (glucose) in urine is typically too low to be detected. Any detection of sugar on this test usually calls for follow-up testing for diabetes.
- Ketones. As with sugar, any amount of ketones detected in your urine could be a sign of diabetes and requires follow-up testing.
- Bilirubin. Bilirubin is a product of red blood cell breakdown. Usually, bilirubin is carried in the blood and passes into your liver, where it's removed and becomes part of bile. Bilirubin in your urine might indicate liver damage or disease.
- Evidence of infection. Either nitrites or leukocyte esterase — a product of white blood cells — in your urine might indicate a urinary tract infection.
- Blood. Blood in your urine requires additional testing. It may be a sign of kidney damage, infection, kidney or bladder stones, kidney or bladder cancer, or blood disorders.
Microscopic exam
Sometimes performed as part of a urinalysis, this test involves viewing drops of concentrated urine — urine that's been spun in a machine — under a microscope. If any of the following levels are above average, you might need more tests:
- White blood cells (leukocytes) might be a sign of an infection.
- Red blood cells (erythrocytes) might be a sign of kidney disease, a blood disorder or another underlying medical condition, such as bladder cancer.
- Bacteria, yeast or parasites can indicate an infection.
- Casts — tube-shaped proteins — can be a result of kidney disorders.
- Crystals that form from chemicals in urine might be a sign of kidney stones.
A urinalysis alone usually doesn't provide a definite diagnosis. Depending on the reason your provider recommended this test, you might need follow-up for unusual results. Evaluation of the urinalysis results with other tests can help your provider determine next steps.
Getting standard test results from a urinalysis doesn't guarantee that you're not ill. It might be too early to detect disease or your urine could be too diluted. Tell your provider if you still have signs and symptoms.
For specifics about what your urinalysis results mean, talk with your health care provider.
- Lab Tests Online. Urinalysis. https://labtestsonline.org/tests/urinalysis. Accessed Aug. 14, 2021.
- What is a urinalysis (also called a "urine test")? National Kidney Foundation. https://www.kidney.org/atoz/content/what-urinalysis. Accessed Aug. 14, 2021.
- Evaluation of the renal patient. Merck Manual Professional Version. https://www.merckmanuals.com/professional/genitourinary-disorders/approach-to-the-genitourinary-patient/evaluation-of-the-renal-patient?query=urinalysis#v1152664. Accessed Aug. 14, 2021.
- Wald R. Urinalysis in the diagnosis of kidney disease. https://www.uptodate.com/contents/search. Accessed Aug. 14, 2021.
- Benign prostatic hyperplasia (BPH)
- Cushing syndrome
- Delayed ejaculation
- Hemolytic uremic syndrome (HUS)
- Kidney stones
- Reye's syndrome
- Urinary incontinence
- Doctors & Departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Double your impact on fighting cancer
Make a gift before July 31 and it can go twice as far to fight cancer.
You are here
Urinalysis (urine test).
A urinalysis is a simple test that looks at a small sample of your urine. It can help find problems that need treatment, including infections or kidney problems. It can also help find serious diseases in the early stages, like kidney disease , diabetes , or liver disease. A urinalysis is also called a “urine test.”
A urine test can include three parts:
- Visual exam. The urine will be looked at for color and clearness. Blood may make urine look red or the color of tea or cola. An infection may make urine look cloudy. Foamy urine can be a sign of kidney problems.
- Microscopic exam. A small amount of urine will be looked at under a microscope to check for things that do not belong in normal urine that cannot be seen with the naked eye, including red blood cells, white blood cells (or pus cells), bacteria (germs), or crystals (which are formed from chemicals in the urine and may eventually get bigger and become kidney stones).
- Acidity (pH) is a measure of the amount of acid in the urine. A pH that is above normal may be a sign of kidney stones, urinary infections, kidney problems, or other disorders.
- Protein is an important building block in the body. Everyone has protein in their blood. But it should only be in your blood, not your urine. Your kidneys play a role in this process. Healthy kidneys remove waste products and extra water from your blood, but leave behind the things your body needs, like protein. When kidneys are injured, protein leaks into your urine. Having protein in your urine suggests that your kidney's filtering units are damaged by kidney disease.
- Glucose (sugar) is usually a sign of diabetes.
- White blood cells (pus cells) are signs of infection.
- Bilirubin is a waste product from the breakdown of old red blood cells. It is normally removed from the blood by the liver. Its presence in the urine may be a sign of liver disease.
- Blood can It can be a sign of an infection, a kidney problem, certain medicines, or even heavy exercise. Finding blood in the urine requires further testing. It does not mean you have a serious medical problem.
A urinalysis can help to detect many diseases before you feel symptoms. Finding and treating a problem early can help keep serious diseases from getting worse.
For More Information
Urinalysis and Kidney Disease: What You Need to Know
Related content
Tests to Check Your Kidney Health
Urinary Incontinence
Urinary Tract Infections
Save this content:
Share this content:
Is this content helpful?
Back to top:

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
GENERAL URINE EXAMINATION (URINE ANALYSIS)
Published by Imogene McDonald Modified over 6 years ago
Similar presentations
Presentation on theme: "GENERAL URINE EXAMINATION (URINE ANALYSIS)"— Presentation transcript:

Routine urine analysis
Portland Community College

Urinalysis Belinda Jim, MD July 17, Urinalysis Major noninvasive tool Provide information about disease severity, though not always a direct relationship.

Abnormal constituents of urine Proteinuria (protein in urine); Small mwt proteins (such as peptide hormones,Insulin glucagon, growth hormone) can appear.
Urine Testing.

Practical # 3: Microscopic Examination of Urine

Dalia kamal Eldien Mohammed. Urine examination A. PHYSICAL CHARACTERISTICS OF URINE The physical characteristics of urine include observations and measurements.

UA Problem Solving Questions

Urine analysis.
Usually done on the mid stream urine Fresh voided urine The container is clean and sterile (for culture ) The sample must be tested within 1hr.
Urinalysis.
Unit #5A – Clinical Laboratory Testing - Urinalysis

Medical Physiology Lab.

Urinalysis Report Interpretation

Performing the Urinalysis

URINALYSIS Finding the Clues Hidden in Urine

Week 7: Intro to UA Urinalysis Renal anatomy and physiology Nephron anatomy Urine collection and preservation Physical properties of urine Color Appearance.

1 URINALYSIS 2 UA Casts 3 Cast Formation Urinary casts are formed only in the distal convoluted tubule (DCT) or the collecting duct (distal nephron).
About project
© 2024 SlidePlayer.com Inc. All rights reserved.
- Login / FREE TRIAL

‘Nursing is on the front line in times of war and elections’
STEVE FORD, EDITOR
- You are here: Continence
Urinalysis: how to interpret results
07 June, 2016 By Ann Yates
Urinalysis is an important screening and diagnostic tool, but health professionals must know how to perform the test and interpret results correctly for it to be beneficial. The article comes with a self-assessment enabling you to test your knowledge after reading it
Analysing an individual’s urine can be a useful way of detecting or ruling out some diseases and infections. Urinalysis can be undertaken in many ways, one of which is using a reagent stick. To be effective, the test must be performed properly and the results interpreted correctly. This article gives an overview of the most important aspects of this investigation, highlighting signs to look for and what they may mean.
Citation: Yates A (2016) Urinalysis: how to interpret results. Nursing Times ; Online issue 2, 1-3.
Author: Ann Yates is director of continence services, Cardiff and Vale University Health Board.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here
- Assess your knowledge and gain CPD evidence by taking the NT Self-assessment test
- Click here for more NT Self-assessment articles
Introduction
Urine testing or urinalysis is a valuable tool to screen an patient and diagnose their health status. It provides valuable information about hydration , renal and urinary tracts, liver disease, diabetes mellitus and urinary-tract infections . Urine is formed in the kidneys and, through glomerular filtration, tubular reabsorption and tubular secretion, is how the body gets rid of its natural waste products (Marieb and Hoehn, 2010). Urinalysis is easy to undertake but results must be interpreted correctly.
Types of analysis
There are different ways of analysing urine and for different reasons, namely:
- 24-hour collection: patient voids into toilet, then all urine is collected for the next 24 hours. As the body chemistry alters constantly, this is used to measure substances, such as steroids, white cells, electrolytes or determine urine osmolarity (Tortora and Derrickson, 2009);
- First-morning specimen: first specimen of morning (or eight hours after recumbent position). Best sample for pregnancy testing;
- Fasting specimen: the second voided specimen after a period of fasting;
- Mid-stream urine (MSU): used to obtain urine for bacterial culture. First and last part of urine stream is voided into the toilet to avoid contaminating the specimen with organisms presenting on the skin;
- Random specimen: for chemical or microscopic examination, a randomly collected specimen suitable for most screening purposes;
- Catheter specimen of urine: collected for bacteriological examination if a patient’s symptoms suggest the presence of a UTI. The sampling technique used for collection is important (Baillie and Arrowsmith, 2005).
This article focuses on random specimen and MSU samples, and analysis using dipstick reagent strips.
Patient assessment/preparation
Urinalysis can potentially identify the presence of life-changing conditions, such as diabetes and renal disease. If abnormalities are detected, the individual may need further investigations, so they should be appropriately counselled to understand the implications before providing a sample. This has to be balanced against harm that could be caused by a missed diagnosis if urinalysis is not done.
Approximately 50ml of urine is required for urinalysis. Adults and children who are continent and can empty their bladder should either provide a random sample or be advised to provide an MSU sample. They should be mobile and dextrous enough to be able to do this, and be instructed in the technique to prevent contamination from hands or the genital area. Specific cleaning of the genital area seems not to affect contamination rates (Mousseau, 2001), but may be appropriate when personal hygiene is poor or faecal contamination is apparent.
Box 1 outlines the routine observations when undertaking urinalysis. The properties listed should be considered in line with clinical presentation, fluid intake and urine output. Before testing the urine using a reagent dipstick strip, the observations listed should be completed. The following factors can also affect results:
- Use a fresh sample of urine (preferably less than 4 hours old or in line with the reagent strip manufacturer’s instructions to obtain accurate results. Bilirubin and urobilinogen are relatively unstable compounds when left in light or at room temperature;
- Exposure of unpreserved urine to room temperature for a period of time can change pH and increase micro-organisms. If it cannot be tested immediately, the sample needs to be stored in line with the reagent strip manufacturer’s instructions or at 2-4°C and then brought to room temperature (15-20°C) before testing;
- Bacterial growth of contaminated organisms may produce positive blood reactions;
- Urine high in alkaline can show false positive results for protein;
- Presence of glucose may reduce pH;
- Presence of urea-splitting organisms may cause urine to become more alkaline (Dougherty and Lister, 2015).
Box 1. Routine observation of urine
This usually ranges from pale straw to deep amber, depending on concentration (Steggall, 2007).
- Dark urine: may indicate dehydration
- Brown/green or strong yellow: may indicate presence of bilirubin
- Green: may indicate presence of pseudomonas infection or excretion of cytotoxic drugs, such as mitomycin
- Bright red/red-brown: may indicate presence of blood (haematuria). Menstruation should be ruled out in females
Certain food or drugs may also influence colour; beetroot can produce a pinkish shade and rifampicin can turn urine orange/red.
This is usually referred to as clear, slightly cloudy, cloudy or turbid.
Substances that can cause cloudiness but are not harmful include mucus, sperm, prostatic fluid and skin cells. Other substances that make urine cloudy are white/red blood cells, pus or bacteria that need attention. Frothy urine signifies protein in the urine.
Freshly voided urine may have a slight but inoffensive smell.
- Fishy smell/ammonia: may indicate urinary infection
- “Pear drop” or acetone smell: may indicate presence of ketones, as in diabetic ketoacidosis
- Some strongly flavoured foods can also produce an odour, eg asparagus
Standard urine-test analysis
Many chemical reagent strips are available and differ between manufacturers. All detect a wide range of substances that can be identified in urine. The tests available include those for substances that are:
- Produced by the body and naturally found in urine;
- Produced by the body and not usually present in the urine;
- Not normally found in the body.
The following test paddles are commonly featured on reagent strips: blood; bilirubin; urobilinogen; nitrite; leucocytes (white blood cells); protein; ketones; glucose; pH (a measure of how acidic or alkaline urine is); and specific gravity (relative density). It is important that the professional undertaking the test understands the manufacturer’s guidance before using the strip. Box 2 outlines the steps that should be followed when performing the urinalysis.
Box 2. Urinalysis using chemical reagent strips
- Explain procedure to patient and gain consent
- Comply with infection-prevention principles: wash hands, use protective equipment
- Check expiry date on reagent-strip container and make sure it has been stored in line with the manufacturer’s recommendations
- Advise patient how to collect a fresh sample, preferably a mid-stream sample if possible, as stored urine can give false results
- Remove reagent dipstick from container, taking care to touch only the plastic handle; replace lid immediately
- Observe urine for colour and clarity, then fully immerse reagent stick, so all reagent areas are covered. Hold for approximately two seconds. Remove strip from urine and tap on absorbent paper or against inside of urine container to remove excess urine
- Wait for manufacturer’s recommended time to elapse, holding strip in horizontal position to prevent interaction between adjacent test pads
- Compare reagent strip against colour reference guide on outside of container (Fig 1, attached)
- If sample is not being sent to a laboratory for further investigations, dispose of urine, used strip, urine container and gloves, following local policy, and wash hands
- Document results, and inform doctor and patient; take appropriate action as required
Significance of findings
Urine tests are frequently done in various settings, so it is vital that professionals understand how to interpret the common findings displayed on reagent strips and what they mean. This section will discuss each of the paddles identified on the strip.
Urine does not normally contain blood detected by reagent strips. Blood in the urine is known as haematuria and can be subclassified as follows:
- Macroscopic: large volumes of blood in the urine, which takes on a rose or dark colour, especially if left to stand;
- Microscopic: undetectable to the naked eye; reagent strips or a microscope are needed to identify it.
Blood can enter urine via damage to the filtration barrier in the kidneys that normally prevents blood from entering the urine or because of an abnormality to the structures that usually drain urine from the kidneys, store urine (bladder) or transport urine outside (urethra) (Bryant and Catto, 2008). Blood in the urine can be indicative of kidney disease; inflammatory lesions of the urinary tract (infection or cancer); renal damage; or kidney/renal stones.
It can also indicate a blood-clotting disorder or be a side-effect of anticoagulant drugs. Health professionals should also remember that urine can be contaminated with menstrual blood. Goddard et al (2010) highlighted that in most patients investigated for haematuria, no real presence of an underlying cause could be found and the haematuria was put down to a benign cause. However, as serious conditions cannot be identified unless investigated, it is important that haematuria is appropriately investigated unless a sensible reason, such as menstruation, can be identified.
Bilirubin and urobilinogen
Bilirubin is a chemical produced when red blood cells are broken down. It is transported in the blood to the liver, where it is processed and excreted into the gut as a constituent of bile. In the gut, bacteria acts on the bilirubin to transform it into urobiligen. It is usual for urine to contain urobiligen but not bilirubin. Bilirubin in the urine may be an indicator of a breakdown of red blood cells. It may not be effectively removed by the liver, which may suggest liver disease or a problem with drainage of bile into the gut, such as gall stones.
Nitrites are not usually found in urine and are associated with the presence of bacteria that can convert nitrate into nitrite. The presence of nitrites can be suggestive of a UTI but clinical presentation of symptoms should also be taken into account. The absence of nitrites, however, does not always rule out the presence of a UTI; Devillé et al (2004) identified that in approximately 50% of urine samples containing bacteria, the nitrites test was negative.
Leucocytes (white blood cells)
In urine, leucocytes are usually associated with a urinary infection but sometimes may indicate a more severe renal problem (Steggall, 2007). When white blood cells are present in the urine, patients are said to have pyuria (pus in the urine). To establish the cause, a clean-catch urine sample should be examined under a microscope, cultured to see what bacteria grows and tested for sensitivity to establish antibiotic treatment. Where no bacterial cells are detected, the patient is said to have sterile pyuria; this can occur in tuberculosis and inflammatory disease of the kidneys (Higgins, 2007).
In a healthy person, urine does not contain a level of protein that is detectable on a urine reagent strip. This is due to the protein molecules being too large to pass through the glomerular filtration barrier. When protein can pass through this barrier, it is known as proteinuria. Proteinuria can be caused by many things, such as damage or disease to the glomerular filtration barrier; hypertension; kidney damage; diabetes mellitus; and pre-eclampsia (Mulryan, 2011). Specific investigations will be required to detect the cause of proteinuria.
These are chemicals that are formed during the abnormal breakdown of fat and are not normal constituents of urine. Breakdown of fat may result from prolonged vomiting, fasting or starvation; individuals on a diet or who present with diarrhoea and vomiting may have a positive result. Ketones can also be present in the urine of people with poorly controlled diabetes. This can make the blood more acidic and is known as diabetic ketoacidosis; it should be reviewed urgently by a doctor. Some medications, such as captopril, may also produce a false positive result (Steggall, 2007).
Glucose in the urine (glycosuria) can occur in pregnancy or patients taking corticosteroids. It may also be indicative of diabetes mellitus but is not a normal constituent of urine. Although glycosuria is an indication of endocrine abnormality, it is not diagnostic and further investigation, such as fasting blood tests, may be required.
This is a measure of acidity or alkalinity in urine. All urine will give a pH reading on analysis and it is usually slightly acidic. A range of 5.0-8.0 is considered normal (Higgins, 2007). Acidic urine may indicate formation of urinary stones, while alkaline urine may indicate a UTI with certain types of bacteria, such as Proteus mirabilis, Klebsiella or Pseudomonas (Higgins, 2007). However, pH is also affected by diet; a high protein intake can give rise to acidic urine, whereas a high intake of dairy products or vegetables can give rise to alkaline urine. UTIs and medication can also result in alkaline urine. Results should be interpreted in conjunction with an individual’s specific presentation.
Specific gravity (SG) (relative density)
Urine can range from very diluted to very concentrated; its density is measured against pure water at room temperature and pressure. Specific gravity identifies the hydration of an individual – a well-hydrated person will have diluted urine whereas someone who is dehydrated will present with concentrated urine. The normal range of specific gravity is 1.001-1.035.
Diluted urine could occur in an individual who has high fluid intake; diabetes insipidus; hypercalcaemia; endocrine disorders, such as kidney disease; or failed to produce anti-diuretic hormone.
Concentrated urine can be the result of dehydration. When assessing specific gravity, environmental factors such as temperatures should be taken into account.
Urinalysis using a dipstick reagent strip is an effective screening tool to assess the health status of an individual and detect some diseases and infections. It is important that professionals understand methods for collecting urine, limit the risk of contamination by using reagent strips correctly and accurately interpret results.
- Urinary dipstick reagent strips are a quick, effective screening aid to urinalysis
- Nursing staff should understand the importance of examining urine for colour, clarity and odour before undertaking dipstick analysis
- Urine can be collected in different ways to limit contamination
- Nursing staff should be able to carry out the procedure correctly and accurately interpret the results
- Different components of the reagent strip have different clinical implications

- After reading this article, test your knowledge with NT Self-assessment. If you score 80% or more, you can download a personalised certificate and store in your NT Portfolio as evidence of CPD for revalidation
- Take the NT Self-assessment for this article
Related files
080616_urinalysis-how-to-interpret-results.pdf, fig 1 colour guide.pdf.
- Add to Bookmarks
Tagged with: Assessment skills: continence Newly qualified nurses: practical procedures
Related articles

Digital removal of faeces to enhance bowel care for neurogenic conditions
This article discusses digital removal of faeces and how nurses can improve bowel care delivery for neurogenic patients.

Supporting continence care for people living at home with dementia
This article describes the development of the DemCon website, which provides continence resources for health professionals and family carers.

Improving bowel management in adults with intellectual disability
A bowel support service created by learning disability nurses has improved constipation management in adults with intellectual disability. This project won the Continence Promotion and Care category in the 2023 Nursing Times Awards.

The environmental footprint of catheters
In reality, studies show that the usage of a urinary catheter which is made from more sustainable material, could reduce the user’s carbon footprint by 20 kg CO2e annually.
Great read. Thanks for sharing.
I need to make assessment and get CPD
I read this as a refresher, as I have moved from an area where patients are routinely catheterised and urine is measured hourly. I wanted to revisit and see what could be used in current practice, there were only one or 2 points to change current practice but it’s good to refresh
Many thanks for your feedback. It is great that you could update practice as a result of reading the article.
Where do I locate the CPD hours for the self-assessment courses?
Have your say
Sign in or Register a new account to join the discussion.

VICTORIA J. SHARP, MD, DANIEL K. LEE, MD, AND ERIC J. ASKELAND, MD
A more recent article on office-based urinalysis is available.
Am Fam Physician. 2014;90(8):542-547
Author disclosure: No relevant financial affiliations.
Urinalysis is useful in diagnosing systemic and genitourinary conditions. In patients with suspected microscopic hematuria, urine dipstick testing may suggest the presence of blood, but results should be confirmed with a microscopic examination. In the absence of obvious causes, the evaluation of microscopic hematuria should include renal function testing, urinary tract imaging, and cystoscopy. In a patient with a ureteral stent, urinalysis alone cannot establish the diagnosis of urinary tract infection. Plain radiography of the kidneys, ureters, and bladder can identify a stent and is preferred over computed tomography. Asymptomatic bacteriuria is the isolation of bacteria in an appropriately collected urine specimen obtained from a person without symptoms of a urinary tract infection. Treatment of asymptomatic bacteriuria is not recommended in nonpregnant adults, including those with prolonged urinary catheter use.
Urinalysis with microscopy has proven to be an invaluable tool for the clinician. Urine dipstick testing and microscopy are useful for the diagnosis of several genitourinary and systemic conditions. 1 , 2 In 2005, a comprehensive review of urinalysis was published in this journal. 3 This article presents a series of case scenarios that illustrate how primary care physicians can utilize the urinalysis in common clinical situations.
| Microscopic confirmation of a positive urine dipstick test is required to diagnose microscopic hematuria. | C | |
| The initial evaluation of patients with asymptomatic microscopic hematuria should include renal function testing, urinary tract imaging, and cystoscopy. | C | |
| Computed tomographic urography is the preferred imaging modality for the evaluation of patients with asymptomatic microscopic hematuria. | C | , |
| Treatment of asymptomatic bacteriuria is not recommended in nonpregnant adults, including those with prolonged urinary catheter use. | C |
Microscopic Hematuria: Case 1
Microscopic hematuria is common and has a broad differential diagnosis, ranging from completely benign causes to potentially invasive malignancy. Causes of hematuria can be classified as glomerular, renal, or urologic 3 – 5 ( Table 1 6 ) . The prevalence of asymptomatic microscopic hematuria varies among populations from 0.18% to 16.1%. 4 The American Urological Association (AUA) defines asymptomatic microscopic hematuria as three or more red blood cells per high-power field in a properly collected specimen in the absence of obvious causes such as infection, menstruation, vigorous exercise, medical renal disease, viral illness, trauma, or a recent urologic procedure. 5 Microscopic confirmation of a positive dipstick test for microscopic hematuria is required. 5 , 7
| Familial causes | |
| Fabry disease | |
| Hereditary nephritis (Alport syndrome) | |
| Nail-patella syndrome | |
| Thin basement membrane nephropathy | |
| Primary glomerulonephritis | |
| Focal segmental glomerulosclerosis | |
| Goodpasture syndrome | |
| Henoch-Schönlein purpura | |
| Immunoglobulin A nephropathy (Berger disease) | |
| Mesangial proliferative glomerulonephritis | |
| Postinfectious glomerulonephritis | |
| Rapidly progressive glomerulonephritis | |
| Secondary glomerulonephritis | |
| Hemolytic uremic syndrome | |
| Systemic lupus nephritis | |
| Thrombotic thrombocytopenic purpura | |
| Vasculitis | |
| Polycystic kidney disease | |
| Renal artery embolism | |
| Renal papillary necrosis | |
| Renal vein thrombosis | |
| Sickle cell disease or trait | |
| Arteriovenous malformation | |
| Hypercalciuria | |
| Hyperuricosuria | |
| Loin pain–hematuria syndrome | |
| Malignant hypertension | |
| Medullary sponge kidney | |
| Benign prostatic hyperplasia | |
| Cancer (kidney, ureteral, bladder, prostate, or urethral) | |
| Cystitis/pyelonephritis | |
| Nephrolithiasis | |
| Prostatitis | |
| infection | |
| Tuberculosis | |
| Drugs (e.g., nonsteroidal anti-inflammatory drugs, heparin, warfarin [Coumadin], cyclophosphamide) | |
| Trauma (e.g., contact sports, running, Foley catheter) | |
DIAGNOSTIC APPROACH
Case 1: microscopic hematuria.
A 58-year-old truck driver with a 30-year history of smoking one pack of cigarettes per day presents for a physical examination. He reports increased frequency of urination and nocturia, but does not have gross hematuria. Physical examination reveals an enlarged prostate. Results of his urinalysis with microscopy are shown in Table 2 .
Based on this patient's history, symptoms, and urinalysis findings, which one of the following is the most appropriate next step?
A. Repeat urinalysis in six months.
B. Obtain blood urea nitrogen and creatinine levels, perform computed tomographic urography, and refer for cystoscopy.
C. Treat with an antibiotic and repeat the urinalysis with microscopy.
D. Inform him that his enlarged prostate is causing microscopic hematuria, and that he can follow up as needed.
E. Perform urine cytology to evaluate for bladder cancer.
The correct answer is B .
| Color | Pale yellow | — |
| Clarity | Clear | — |
| pH | 6.5 | — |
| Specific gravity | 1.010 | — |
| Glucose | Negative | Negative |
| Blood | 1+ | Negative |
| Ketones | Negative | Negative |
| Protein | Negative | Negative |
| Urobilinogen | Negative | Negative |
| Bilirubin | Negative | Negative |
| Leukocyte esterase | Negative | Negative |
| Nitrite | Negative | Negative |
| White blood cells | 1 per high-power field | 0 to 5 per high-power field |
| Red blood cells | 7 per high-power field | 0 to 4 per high-power field |
| Squamous epithelial cells | None | None |
For the patient in case 1 , because of his age, clinical history, and lack of other clear causes, the most appropriate course of action is to obtain blood urea nitrogen and creatinine levels, perform computed tomographic urography, and refer the patient for cystoscopy. 5 An algorithm for diagnosis, evaluation, and follow-up of patients with asymptomatic microscopic hematuria is presented in Figure 1 . 5 The AUA does not recommend repeating urinalysis with microscopy before the workup, especially in patients who smoke, because tobacco use is a risk factor for urothelial cancer ( Table 3 ) . 5
| Analgesic abuse |
| Exposure to chemicals or dyes (benzenes or aromatic amines) |
| History of chronic indwelling foreign body |
| History of chronic urinary tract infection |
| History of exposure to carcinogenic agents or chemotherapy (e.g., alkylating agents) |
| History of gross hematuria |
| History of irritative voiding symptoms |
| History of pelvic irradiation |
| History of urologic disorder or disease |
| Male sex |
| Older than 35 years |
| Smoking (past or current) |
A previous article in American Family Physician reviewed the American College of Radiology's Appropriateness Criteria for radiologic evaluation of microscopic hematuria. 8 Computed tomographic urography is the preferred imaging modality for the evaluation of patients with asymptomatic microscopic hematuria. 5 , 8 It has three phases that can detect various causes of hematuria. The non–contrast-enhanced phase is optimal for detecting stones in the urinary tract; the nephrographic phase is useful for detecting renal masses, such as renal cell carcinoma; and the delayed phase outlines the collecting system of the urinary tract and can help detect urothelial malignancies of the upper urinary tract. 9 Although the delayed phase can detect some bladder masses, it should not replace cystoscopy in the evaluation for bladder malignancy. 9 After a negative microscopic hematuria workup, the patient should continue to be followed with yearly urinalysis until at least two consecutive normal results are obtained. 5
In patients with microscopic hematuria, repeating urinalysis in six months or treating empirically with antibiotics could delay treatment of potentially curable diseases. It is unwise to assume that benign prostatic hyperplasia is the explanation for hematuria, particularly because patients with this condition typically have risk factors for malignancy. Although urine cytology is typically part of the urologic workup, it should be performed at the time of cystoscopy; the AUA does not recommend urine cytology as the initial test. 5
Dysuria and Flank Pain After Lithotripsy: Case 2
After ureteroscopy with lithotripsy, a ureteral stent is often placed to maintain adequate urinary drainage. 10 The stent has one coil that lies in the bladder and another that lies in the renal pelvis. Patients with ureteral stents may experience urinary frequency, urgency, dysuria, flank pain, and hematuria. 10 They may have dull flank pain that becomes sharp with voiding. This phenomenon occurs because the ureteral stent bypasses the normal nonrefluxing uretero-vesical junction, resulting in transmission of pressure to the renal pelvis with voiding. Approximately 80% of patients with a ureteral stent experience stent-related pain that affects their daily activities. 11
POTENTIALLY MISLEADING URINALYSIS
Case 2: dysuria and flank pain after lithotripsy.
A 33-year-old woman with a history of nephrolithiasis presents with a four-week history of urinary frequency, urgency, urge incontinence, and dysuria. She recently had ureteroscopy with lithotripsy of a 9-mm obstructing left ureteral stone; she does not know if a ureteral stent was placed. She has constant dull left flank pain that becomes sharp with voiding. Results of her urinalysis with microscopy are shown in Table 4 .
A. Treat with three days of ciprofloxacin (Cipro), and tailor further antibiotic therapy according to culture results.
B. Treat with 14 days of ciprofloxacin, and tailor further antibiotic therapy according to culture results.
C. Obtain a urine culture and perform plain radiography of the kidneys, ureters, and bladder.
D. Perform a 24-hour urine collection for a metabolic stone workup.
E. Perform computed tomography.
The correct answer is C .
| Color | Yellow | — |
| Clarity | Clear | — |
| pH | 6.0 | — |
| Specific gravity | 1.010 | — |
| Glucose | Negative | Negative |
| Blood | 2+ | Negative |
| Ketones | Negative | Negative |
| Protein | Negative | Negative |
| Urobilinogen | Negative | Negative |
| Bilirubin | Negative | Negative |
| Leukocyte esterase | 2+ | Negative |
| Nitrite | Negative | Negative |
| White blood cells | 15 per high-power field | 0 to 5 per high-power field |
| Red blood cells | 6 per high-power field | 0 to 4 per high-power field |
| Squamous epithelial cells | None | None |
The presence of a ureteral stent causes mucosal irritation and inflammation; thus, findings of leukocyte esterase with white and red blood cells are not diagnostic for urinary tract infection, and a urine culture is required. In this setting, plain radiography of the kidneys, ureters, and bladder would be useful to determine the presence of a stent. If a primary care physician identifies a neglected ureteral stent, prompt urologic referral is indicated for removal. Retained ureteral stents may become encrusted, and resultant stone formation may lead to obstruction. 10
Flank discomfort and recent history of urinary tract manipulation suggest that this is not an uncomplicated urinary tract infection; therefore, a three-day course of antibiotics is inadequate. Although flank pain and urinalysis suggest possible pyelonephritis, this patient should not be treated for simple pyelonephritis in the absence of radiography to identify a stent. A metabolic stone workup may be useful for prevention of future kidney stones, but it is not indicated in the acute setting. Finally, although computed tomography would detect a ureteral stent, it is not preferred over radiography because it exposes the patient to unnecessary radiation. Typically, microscopic hematuria requires follow-up to ensure that there is not an underlying treatable etiology. In this case , the patient's recent ureteroscopy with lithotripsy is likely the etiology.
Urinalysis in a Patient Performing Clean Intermittent Catheterization: Case 3
Case 3: urinalysis in a patient performing clean intermittent catheterization.
A 49-year-old man who has a history of neurogenic bladder due to a spinal cord injury and who performs clean intermittent catheterization visits your clinic for evaluation. He reports that he often has strong-smelling urine, but has no dysuria, urge incontinence, fever, or suprapubic pain. Results of his urinalysis with microscopy are shown in Table 5 .
A. Inform the patient that he has a urinary tract infection, obtain a urine culture, and treat with antibiotics.
B. Refer him to a urologist for evaluation of a complicated urinary tract infection.
C. Perform computed tomography of the abdomen and pelvis to evaluate for kidney or bladder stones.
D. Inform him that no treatment is needed.
E. Obtain a serum creatinine level to evaluate for chronic kidney disease.
The correct answer is D .
| Color | Dark yellow | — |
| Clarity | Turbid | — |
| pH | 7.0 | — |
| Specific gravity | 1.010 | — |
| Glucose | Negative | Negative |
| Blood | Negative | Negative |
| Ketones | Negative | Negative |
| Protein | Negative | Negative |
| Urobilinogen | Negative | Negative |
| Bilirubin | Negative | Negative |
| Leukocyte esterase | Positive | Negative |
| Nitrite | Positive | Negative |
| White blood cells | 20 per high-power field | 0 to 5 per high-power field |
| Red blood cells | 2 per high-power field | 0 to 4 per high-power field |
| Squamous epithelial cells | None | None |
| Bacteria | Many | — |
Although the urinalysis results are consistent with a urinary tract infection, the clinical history suggests asymptomatic bacteriuria. Asymptomatic bacteriuria is the isolation of bacteria in an appropriately collected urine specimen obtained from a person without symptoms of a urinary tract infection. 12 The presence of bacteria in the urine after prolonged catheterization has been well described; one study of 605 consecutive weekly urine specimens from 20 chronically catheterized patients found that 98% contained high concentrations of bacteria, and 77% were polymicrobial. 13
Similar results have been reported in patients who perform clean intermittent catheterization; another study of 1,413 urine cultures obtained from 407 patients undergoing clean intermittent catheterization found that 50.6% contained bacteria. 14 Guidelines from the Infectious Diseases Society of America recommend against treatment of asymptomatic bacteriuria in nonpregnant patients with spinal cord injury who are undergoing clean intermittent catheterization or in those using a chronic indwelling catheter. 12
In the absence of symptoms of a urinary tract infection or nephrolithiasis, there is no need to culture the urine, treat with antibiotics, refer to a urologist, or perform imaging of the abdomen and pelvis. There is no reason to suspect acute kidney injury in this setting; thus, measurement of the serum creatinine level is also unnecessary.
Data Sources : Literature searches were performed in PubMed using the terms urinalysis review, urinalysis interpretation, microscopic hematuria, CT urogram, urinary crystals, indwelling ureteral stent, asymptomatic bacteriuria, and bacteriuria with catheterization. Guidelines from the American Urological Association were also reviewed. Search dates: October 2012 and June 2013.
Wu X. Urinalysis: a review of methods and procedures. Crit Care Nurs Clin North Am. 2010;22(1):121-128.
Hardy PE. Urinalysis interpretation. Neonatal Netw. 2010;29(1):45-49.
Simerville JA, Maxted WC, Pahira JJ. Urinalysis: a comprehensive review [published correction appears in Am Fam Physician . 2006;74(7):1096]. Am Fam Physician. 2005;71(6):1153-1162.
Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348(23):2330-2338.
American Urological Association. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults. http://www.auanet.org/education/asymptomatic-microhematuria.cfm . Accessed June 6, 2014.
Ahmed Z, Lee J. Asymptomatic urinary abnormalities. Hematuria and proteinuria. Med Clin North Am. 1997;81(3):641-652.
Rao PK, Jones JS. How to evaluate ‘dipstick hematuria’: what to do before you refer. Cleve Clin J Med. 2008;75(3):227-233.
Choyke PL. Radiologic evaluation of hematuria: guidelines from the American College of Radiology's Appropriateness Criteria. Am Fam Physician. 2008;78(3):347-352.
Sadow CA, Wheeler SC, Kim J, Ohno-Machado L, Silverman SG. Positive predictive value of CT urography in the evaluation of upper tract urothelial cancer. AJR Am J Roentgenol. 2010;195(5):W337-W343.
Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: a systematic review. J Urol. 2008;179(2):424-430.
Joshi HB, Stainthorpe A, MacDonagh RP, Keeley FX, Timoney AG, Barry MJ. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169(3):1065-1069.
Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults [published correction appears in Clin Infect Dis . 2005;40(10):1556]. Clin Infect Dis. 2005;40(5):643-654.
Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723.
Bakke A, Digranes A. Bacteriuria in patients treated with clean intermittent catheterization. Scand J Infect Dis. 1991;23(5):577-582.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2014 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

Urine Microscopic Examination
urine Microscopic examination is a laboratory test used to examine the physical, chemical, and microscopic characteristics of urine. The test helps to detect any abnormalities in the urine, which may indicate underlying health conditions.
Urine Microscopic Examination is a laboratory test used to examine the physical, chemical, and microscopic characteristics of urine. The test helps to detect any abnormalities in the urine, which may indicate underlying health conditions. The procedure involves analyzing a small sample of urine under a microscope, which allows the technician to view the cells, bacteria, crystals, and other substances present in the urine.
Explanation of Urine Microscopic Examination:
Urine microscopic examination is a laboratory test that analyzes a small sample of urine under a microscope to evaluate the physical, chemical, and microscopic characteristics of the urine. It helps to diagnose various health conditions, such as urinary tract infections, kidney disease, and bladder cancer. The test involves analyzing the color, clarity, pH, and specific gravity of the urine and identifying the type and number of cells, bacteria, and other substances present in the urine. Abnormal findings may indicate an underlying health condition.
Purpose of the Test:
Here are some of the purposes of urine microscopic examination:
- Diagnose Urinary Tract Infections (UTIs): The test is used to identify bacteria, white blood cells, and other substances that indicate the presence of a UTI.
- Diagnose Kidney Diseases: The presence of red blood cells, white blood cells, and other substances in the urine can indicate kidney damage or disease.
- Monitor Kidney Function: The test can be used to monitor the progress of kidney disease and assess the effectiveness of treatment.
- Screen for Bladder Cancer: Abnormal cells in the urine may indicate the presence of bladder cancer.
- Evaluate Kidney Stones: The presence of crystals in the urine can indicate the formation of kidney stones.
- Diagnose Metabolic Disorders: The presence of glucose, proteins, and other substances in the urine can indicate metabolic disorders such as diabetes or proteinuria.
- Monitor Pregnancy: The test can be used to monitor the health of pregnant women and identify potential complications such as preeclampsia.
- Evaluate Autoimmune Diseases: The presence of antibodies in the urine can indicate autoimmune diseases such as lupus or glomerulonephritis.
Overall, urine microscopic examination is a versatile and useful diagnostic tool that can provide important information about the health of the urinary system and the presence of various health conditions.
Why get Tested:
Here are some reasons why urine microscopic examination may be ordered by a healthcare provider:
- To diagnose a urinary tract infection (UTI)
- To diagnose kidney disease
- To monitor kidney function
- To screen for bladder cancer
- To evaluate kidney stones
- To diagnose metabolic disorders, such as diabetes or proteinuria
- To monitor the health of pregnant women and identify potential complications
- To evaluate autoimmune diseases, such as lupus or glomerulonephritis
- To monitor the effectiveness of treatment for various health conditions
- To assess the risk of kidney damage from certain medications or medical conditions
- To evaluate the health of the urinary system in people with chronic conditions, such as hypertension or diabetes
- To identify potential kidney problems in people with a family history of kidney disease
- To assess the risk of developing kidney disease in people with risk factors, such as high blood pressure or diabetes.
Overall, urine microscopic examination is a useful diagnostic tool that can provide valuable information about the health of the urinary system and the presence of various health conditions. It can help healthcare providers make an accurate diagnosis, monitor the progress of treatment, and identify potential complications.
When to get Tested:
Here are some situations where a healthcare provider may order a urine microscopic examination:
- If a person is experiencing symptoms of a urinary tract infection (UTI), such as painful urination, frequent urination, or blood in the urine.
- If a person is experiencing symptoms of kidney disease, such as swelling in the legs, fatigue, or high blood pressure.
- If a person is being monitored for kidney disease, to assess the progress of the disease or the effectiveness of treatment.
- If a person has risk factors for kidney disease, such as high blood pressure, diabetes, or a family history of kidney disease.
- If a person is being monitored for bladder cancer, to assess the effectiveness of treatment or to screen for recurrence.
- If a person has a history of kidney stones or is experiencing symptoms of kidney stones, such as pain in the back or side.
- If a person is pregnant and needs to be monitored for potential complications, such as preeclampsia.
- If a person is being evaluated for an autoimmune disease, such as lupus or glomerulonephritis.
- If a person is being monitored for the effects of certain medications on the kidneys.
- If a person is being evaluated for a metabolic disorder, such as diabetes or proteinuria.
Overall, a urine microscopic examination may be ordered in various situations where a healthcare provider needs to assess the health of the urinary system or diagnose certain health conditions.
Sample Required for Microscopic Examination:
The sample required for a urine microscopic examination is a midstream clean-catch urine sample. Here are the steps for collecting this type of sample:
- Wash your hands thoroughly with soap and water.
- Clean your genital area with a cleansing wipe or towelette provided by your healthcare provider. Wipe from front to back to avoid contaminating the sample with bacteria from the anus.
- Start to urinate into the toilet bowl.
- After a few seconds, place a sterile collection cup under the stream of urine. Be careful not to touch the inside of the cup with your hands.
- Collect about 30 to 60 milliliters of urine, or the amount specified by your healthcare provider.
- Remove the cup from the stream of urine and finish urinating into the toilet bowl.
- Replace the lid on the collection cup.
- Label the collection cup with your name, date of birth, and the date and time of the collection.
- Deliver the sample to the laboratory or healthcare provider as soon as possible. If you can’t deliver it immediately, store it in the refrigerator until you can.
Overall, collecting a clean-catch urine sample is important to ensure that the sample is not contaminated with bacteria from the genital area, which could affect the accuracy of the results of the microscopic examination.
Type of urine samples:
- Random sample : This is a diluted urine sample and may give an inaccurate interpretation of patient health. But is best to do microscopy to evaluate WBC or RBC.
- First Morning sample : This is the best sample for microscopy and urine analysis. This is the concentrated urine because of urine remained throughout the night in the urinary bladder. This will contains an increased concentration of analytes and cellular elements. Urine must have remained in the bladder for 8 hours is considered as the first-morning sample.
- Urine for sugar (Postprandial 2 hours) : Postprandial 2 hours sample collected after 2 hours of high carbohydrate diet.
- Midstream clean catch urine : This sample is needed for the culture and sensitivity of urinary infection. The patient is advised to clean the urethra, then discard the first few mL of urine. Now midstream of the urine is collected in the sterile container.
- In this case, discard the first urine and note the time.
- Now collect urine in the container for 24 hours and put the last sample in the container.
- Refrigerate the sample.
- This 24 hours samples are needed for measuring urea, creatinine, sodium, potassium, glucose, and catecholamines.
- Suprapubic collection of the urine sample : This is done in the patients who cannot be catheterized and the sample is needed for culture. This sample is collected by the needle.
- Catheter collection of urine : This is done by patients who are bedridden and can not urinate.
- Pediatric urine sample : In infants, special collection bags are made adherent around the urethra. Then urine is transferred to a container.
Microscopic Examination tests:
The following are some of the tests that may be included in a urine microscopic examination:
- Urine sediment analysis: This test involves examining a urine sample under a microscope to look for cells, bacteria, crystals, and other substances that may indicate a urinary tract infection or other health condition.
- Red blood cell (RBC) count: This test measures the number of red blood cells in a urine sample, which may be elevated in cases of kidney stones, bladder cancer, or other urinary tract disorders.
- White blood cell (WBC) count : This test measures the number of white blood cells in a urine sample, which may be elevated in cases of urinary tract infections or other inflammatory conditions.
- Epithelial cell count: This test measures the number of epithelial cells (cells from the lining of the urinary tract) in a urine sample, which may be elevated in cases of bladder cancer or other urinary tract disorders.
- Bacteria and yeast culture: This test involves growing bacteria or yeast from a urine sample to identify the specific type of microorganism causing an infection.
- Crystals analysis: This test involves identifying crystals that may be present in a urine sample, which can provide information about the pH and composition of the urine and may indicate the presence of kidney stones or other urinary tract disorders.
- Casts examination: This test involves examining a urine sample under a microscope to identify casts (structures made of proteins or other substances), which may be present in cases of kidney disease or other urinary tract disorders.
Overall, the specific tests included in a urine microscopic examination may vary depending on the healthcare provider’s reason for ordering the test and the patient’s individual health situation.
Significance of Urine Microscopic Examination:
The significance of a urine microscopic examination includes:
- Detecting Urinary Tract Infections: A urine microscopic examination is used to detect the presence of bacteria, white blood cells, and other indicators of a urinary tract infection.
- Evaluating Kidney Function: The test can help to identify the presence of casts, red blood cells, and other substances in the urine, which can be useful in evaluating kidney function.
- Diagnosing Certain Kidney Disorders: Urine sediment analysis can detect the presence of proteins, casts, and other substances that may indicate certain kidney disorders, such as glomerulonephritis.
- Monitoring Certain Medical Conditions: The test can be useful in monitoring certain medical conditions, such as diabetes, which can affect kidney function and urine composition.
- Detecting Pregnancy: A urine microscopic examination can detect the presence of hCG, a hormone produced during pregnancy, which can confirm the presence of a pregnancy.
- Assessing Urinary Tract Stones: The test can detect the presence of crystals, which may indicate the formation of urinary tract stones.
Overall, a urine microscopic examination is a valuable diagnostic tool that can provide important information about a person’s urinary tract health and kidney function. The test can be used to detect and diagnose a variety of medical conditions, monitor treatment progress, and help healthcare providers make informed treatment decisions.

Normal Urine Findings:
Here is a table of normal urine findings:
| Pale or yellow | |
| Clear | |
| Mildly aromatic | |
| 1200 to 2000 ml/24 hours | |
| 5 to 7 | |
| 1.001 to 1.035 | |
| 0 to 5 / HPF | |
| ≤ 3 / HPF | |
| Negative Rarely 2 to 3 RBCs/HPF | |
| ≤2 to 5 HPF Male = 1 to 2 /HPF Female = 0 to 5 /HPF | |
| ≤ 15 to 20 / HPF | |
| Negative | |
| Negative | |
| Negative | |
| Random sample = Negative 24 hours sample = 1 to 15 mg/dL | |
| Urine = Negative | |
| Negative (o to 0.02 mg/dL) | |
| Negative Random sample= <1 mg/dL 2-hour sample = <1 mg /2 hours 24- hours sample = 0.5 to 4.0 mg/dL | |
| Negative | |
| 10 to 100 mg /24 hours | |
| Quantitative = negative Urine 24 hours sample: Adult male = 1 to 14 mg/dL Adult female = 3 to 10 mg/dL Child <10 years = 1 to 10 mg/dL | |
| Normal diet = 100 to 300 mg/24 hours Low-calcium diet = 50 to 150 mg/24 hours(Another source = 0.3 g/24 hours) | |
| average 10 g /24 hours Patient with moderate to severe salt depletion = <10 mmol/L or <20 mmol/L /24 hours(Another source = 15.0 g/24 hours) | |
| Adult = 40 to 220 meq/24 hoursChild = 41 to 115 meq/24 hours | |
| Adult = 25 to 125 meq/ 24 hours Child = 10 to 60 meq/24 hours(Another source = 3.3 g/24 hours) | |
| 75 to 150 mg/24 hours(Another source = 0.1 g/24 hours) | |
| Negative | |
| Male = 20 to 28 mg/Kg/24 hours Female = 15 to 21 mg/Kg/24 hours(Another source = 1.5 g/24 hours) | |
| 5 to 15 g/24 hours | |
| 7 to 20 g/24 hours | |
| 10 to 35 g/24 hours(Another source = 25.0 to 35.0 g/24 hours) | |
| With normal diet = 250 to 750 mg/24 hours With purine-free diet = <400 mg/24 hours With high-purine diet = <1000 mg/24 hours(Another source = 0.4 to 1.0 g/24 hours) | |
| 0.2 to 4.0 mg/24 hours | |
| Adult = 110 to 250 meq/24 hours Child: <6 years = 15 to 40 meq/24 hours Child: 10 to 16 years = 64 to 176 meq/24 hours |
Advantages and Limitations of the test:
Advantages of urine microscopic examination include:
- Non-invasive: Urine collection is a non-invasive procedure that can be easily performed in a healthcare setting.
- Wide Range of Applications: Urine microscopic examination can be used to detect a variety of medical conditions, including urinary tract infections, kidney disease, and pregnancy.
- Quick Results: Results of the test are usually available within 24-48 hours, allowing for prompt diagnosis and treatment.
- Cost-effective: Compared to other diagnostic tests, urine microscopic examination is relatively inexpensive.
- Useful in Monitoring Treatment: The test can be used to monitor the effectiveness of treatment for urinary tract infections, kidney disease, and other medical conditions.
Limitations of urine microscopic examination include:
- False Negative Results: The test may fail to detect certain medical conditions, particularly in cases of early-stage disease or low bacterial counts.
- False Positive Results: The test may produce false positive results in cases where there is contamination of the urine sample or the presence of non-pathogenic bacteria.
- Limited Specificity: The test may not provide specific information about the type of bacteria or other microorganisms causing an infection.
- Limited Diagnostic Value for Some Conditions: The test may not be helpful in diagnosing certain medical conditions, such as some forms of kidney disease.
- Variable Accuracy: The accuracy of the test may be affected by a variety of factors, including the timing of urine collection, the storage and handling of the sample, and the expertise of the healthcare provider performing the test.
Conclusion:
In conclusion, a urine microscopic examination is a useful diagnostic tool that provides important information about a person’s urinary tract health and kidney function. The test is non-invasive, cost-effective, and has a wide range of applications, including the detection and diagnosis of urinary tract infections, kidney disease, and pregnancy. However, it is important to keep in mind the limitations of the test, such as the possibility of false positive or negative results and limited diagnostic value for certain conditions. Healthcare providers must carefully consider the results of a urine microscopic examination in conjunction with other clinical data to make an accurate diagnosis and develop an effective treatment plan for their patients.
Home | Blog | About Us | Contact Us | Disclaimer
Lab Tests Guide Founder
The website covers a wide range of lab tests, including blood tests, urine tests, stool tests, and imaging tests such as X-rays and CT scans. It also provides information about different health conditions and diseases, as well as tips for maintaining good health.
It's important to note that while labtestsguide.com may provide valuable information about lab tests and their interpretation, it's always best to consult with a healthcare professional if you have any concerns or questions about your lab results. We can provide personalized guidance and advice based on your individual health status and medical history.
Similar Posts

Red Blood Cells
MicroBiology MCQs with Answer and Explnation | Chapter 17
Urine albumin.

Microbiology MCQs with Answer and Explnation | Chapter 8
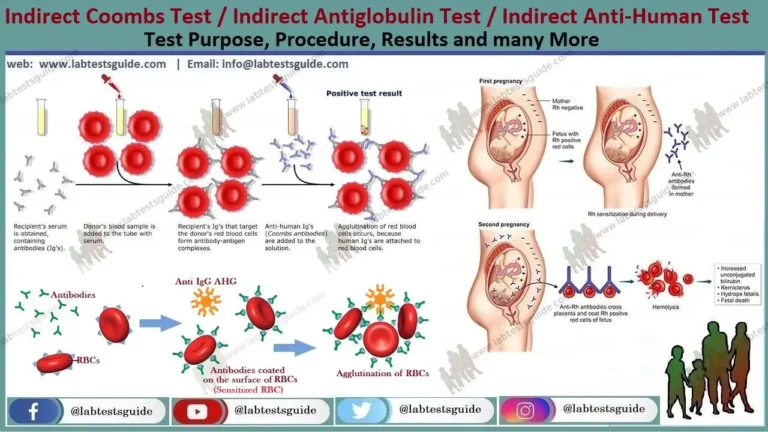
Indirect Antiglobulin Test
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Urine Test – Types, Procedure, Preparation, Results Interpretation
Most people have been asked to submit the urine sample for testing when they visit a health institution. The sample is used to analyze various urine test types that can help the doctors diagnose disease or monitor a patient’s progress . Urine elimination in the body is important as it helps maintain the body water balance as well as remove toxic substances produced during the metabolism process.
What Does A Urine Test Show?
The smell and the color of the urine sample can be an indication of something wrong. Adults should the urinalysis periodically to ensure that their health is intact. A urine test might result in different scenarios. Even before the test, there are some factors that indicate the patient is not okay.
- Having dark urine indicates there is less water intake or a problem with the kidneys while flaky and cloudy urine indicates the presence of a urinary tract infection .
- In some cases, the urine can be reddish indicating that there might be blood. To avoid speculations and to know further, it is essential to take a urine test strip which shows either a person is suffering from diabetes or urinary tract infection.
- Persistent occurrence of protein in urine tests is an early indicator of a chronic kidney disease while the glucose elements indicate signs of potential diabetes.
- The white blood cells in the urine indicate the presence of urinary tract infection. Undertaking a urine test is important as it helps the doctors to know exactly what they are dealing with and offer the suitable solutions.
The urine test types for people who are already suffering are done in intervals to monitor their progress especially those suffering from chronic kidney disease . The urine test show if the given treatment is reducing the protein in the urine or not.
Urine Test Types
There are various urine test types that are performed for various reasons in the quest to determine what is wrong with a patient. It may be part of a regular physical routine check
1. Urinalysis
A complete urinalysis is done in the laboratory and performed on people who are about to go for surgery, having been admitted to the hospital or whose different urine results are abnormal. It is used to either determine or monitor bleeding in the urinary system, urinary tract infection , diabetes, kidney disease, bladder stones and blood disease.
2. Rapid Urine Test
It is the easiest way to test urine and involves dipping the urine test strips in the urine samples. Diabetic people use this test to check their sugar levels. It can also be performed ion people with acute lower abdomen pains, backaches, blood in the urine, stomachache and persistent painful urination. The rapid urine test detects the following
- Glucose, Nitrate, Protein, Ketone in Urine
- White Blood Cells in Urine
- Red Blood Cells in Urine
- Bilirubin in Urine
3. Pregnancy Test
Urine for pregnancy test determines whether a woman has conceived or not. The test is done like a rapid test, eight to ten days after a late menstrual date for accurate results. The test should not be done too soon when a woman is on medication or having taken a lot of fluid just before the test to avoid altering the results.
4. Urine Culture Test
The urine test is done in the laboratory to determine the presence of fungi or bacteria. If they are found, the right kind of antibiotics is then administered for treatment.
5. 24 Hour Urine Collection Test
This test is used to determine the presence of proteins, salt, metabolic products, hormones among other substances are excreted in the body. The urine is collected in 24-hour duration and stored in the refrigerator during that period.
How to Prepare for A Urine Test?
It is important to take normal meals when going for a urine test. It is also essential to take plenty of water. Let your physician know about any type of medicine, supplements or substance you have been using as some o them might alter the urine test results . Also, for women, let the doctor know if menstruating.
What is The Procedure of The Urine Test?
The health institution issues a container where the urine sample is collected. One can use the clean-catch collection method which involves washing up the urinary opening area first. One can then urinate in the toilet first then a few ounces in the container and finishing up in the toilet. The sample is handed over to the doctor for testing.
In some cases, the doctor might use a catheter which is inserted into the bladder through the urethra. The procedure can be discomforting causing mild pains. For the testing, the doctor can use either a visual exam, dipstick test or microscopic exam when conducting the urine test.
Urine Test Results and Interpretation
When the results are abnormal, the doctor determines the next step based on various factors. Previous diagnosis with urinary tract problems, kidney issues or any other related condition may result in further tests. It is vital to identify the occurrence of the abnormal contents of the urine.
Having high level proteins in urine indicates there are underlying issues that can result in kidney disease. Such conditions include leukemia, lupus , heart conditions , hypertension , sickle cell anemia and rheumatoid arthritis. Also, a high level of glucose in the urine can indicate the presence of diabetes .
Urine test pus cells indicate that the urinary tract has major issues. Most of the times it can either be a bladder infection or kidney infection . If there is the presence of too much pus cells in the urine test, then it means the patient has an abscess near the kidney, sexually transmitted disease , kidney infection or urinary tract infection. The condition can also be an indication of stones in the urinary passage.
It is important to have a regular checkup where the doctor takes different urine test types to determine your general health. Some of the diagnosed conditions don’t show the normal signs and symptoms thus the importance of carrying out the tests.
Share with friends
You might also like.

Sensorineural Hearing Loss: Causes, Symptoms, Diagnosis and Treatment
Acute Respiratory Distress Syndrome (ARDS): Symptoms & Treatments

International Normalized Ratio (INR) Blood Test

Baker’s Cyst Behind the Knee: Symptoms, Treatment, and Pictures
Ovarian Hyperstimulation Syndrome: Symptoms, Causes & Treatment

What Is the Most Common Female Factor in Infertility Cases?

- Upload Ppt Presentation
- Upload Pdf Presentation
- Upload Infographics
- Related Template
Weight Loss Roux En Y

Hairstyle Hair Salon
Esophageal Cancer
Gastrointestinal Organ System
Otitis Media Eustachian
Health a to z.
- Eye Disease
- Heart Attack
- Medications
iCliniq Cope
For doctors and hospitals
For faster experience, download our app
Ask a Doctor Online Now
Urine Test Results: Normal And Abnormal
A urine test can reveal a lot about a person’s health. It gives information about kidney health and any infection that could be present in the body.
Dr. Glady Ann Thomas
Medically reviewed by
Dr. Madhav Tiwari
Introduction
There will only be people given a urine sample for testing at least once. A urine sample can give away a lot of information regarding an individual's health. A single sample can be used for a variety of tests. It can help to diagnose a condition or the progress of a condition. The removal of urine from the body is essential for the body's normal functioning. For example, it controls the body's water balance and eliminates materials that accumulate during metabolic processes but are no longer required by the body. Urine tests can be used to identify metabolic disorders like diabetes or liver disease, as well as disorders of the urinary system.
What Can a Urine Test Reveal?
Simple findings like the color, odor, and density can provide various information regarding the body. More detailed tests provide more information about the functioning of the body. Electrolytes and minerals are used by the various body systems for their proper functioning. Some systems require more nutrients, while others require less. For instance, potassium is required more for the proper functioning of the heart, while the muscles require more calcium and magnesium. The nutrient required by one part might not be required by another. If accumulated in a part where not required, it can cause harmful effects. Any deviation from the normal range of these elements can result in significant symptoms. Hence, maintaining a balance is essential. The color, odor, and amount of the urine can give information if there is something wrong with the normal functioning of the body. For example, dark-colored urine can indicate dehydration or improper functioning of the kidney. A cloudy urine sample can be indicative of an infection.
What Are the Different Types of Urine Tests?
Urine can be tested by various methods. It can include:
Visual Examination: The urine is observed for its color and odor. Normal urine is a clear liquid that is pale yellow in color. Any change in color or appearance can indicate an infection or a metabolic disorder.
Microscopic Examination: The urine sample is observed under the microscope for any abnormal cells. Any abnormal findings like abnormal cells, crystals, bacteria, etc can show the presence of an infection or a metabolic condition.
Dipstick Test: A reactive strip of paper that changes color when specific compounds are present is used in a dipstick test. These tests can be customized for a variety of purposes, but they are frequently used to monitor levels of protein or glucose, hormones , pregnancy, or drug consumption.
Creatinine to Albumin Ratio: This test measures the level of albumin (the body's main protein type) to creatinine (a waste product produced by the muscles). The quantity of these two compounds that enter the urine can reveal a lot about the functioning of the kidneys. It also reveals the kidneys' efficiency in removing toxins from the body.
Microalbumin Test : It is a more comprehensive test than the creatinine-to-albumin ratio test. The presence of smaller albumin particles in the urine can be indicative of a more general problem. For instance, trace amounts of these can be found in the case of diabetes and hypertensive patients.
Urine Culture : It examines the urine for any bacteria or growth of any micro-organism for a few days. It helps to prescribe the accurate antibiotics required for an infection.
24-hour Urine Test : An individual's urine is collected in different containers for 24 hours and stored in a cool place for evaluation. The healthcare provider can use the information gathered from this collection as a tool in the diagnosis of diseases like diabetes, lupus, and high blood pressure.
What Are the Normal Results in a Urine Test?
Most substances in the body, including salt and blood, have estimated normal values. Problems can arise from having too much or too little of anything. The patient is generally healthy if the test results are within the normal range. The normal range is as follows:
Color - Yellow (light or pale to dark or deep amber).
Clarity or turbidity - Clear or cloudy.
pH – 4.5-8.
Specific gravity - 1.005-1.025.
Glucose - Less than 130 mg/d.
Ketones - None.
Nitrites - Negative.
Leukocyte esterase - Negative.
Bilirubin - Negative.
Urobilirubin - Small amount (0.5-1 mg/dL).
Blood - less than 3 RBCs.
Protein - less than 150 mg/d.
RBCs - less than 2 RBCs/hpf (high power field).
WBCs - less than 2 to 5 WBCs/hpf.
Squamous epithelial cells - less than 15 to 20 squamous epithelial cells/hpf.
Casts - 0 to 5 hyaline casts/lpf.
Crystals - Occasionally.
Bacteria - None.
Yeast - None.
What Are the Abnormal Results in a Urine Test?
Urine test results that are not within the normal range can indicate various reasons. Every value has a high and a low that might be beneficial or horrifying. The ideal range may vary depending on the age, medical problems, and other factors for certain electrolytes.
A dark-colored urine can indicate internal bleeding or a liver condition. If the urine is foul-smelling, it can indicate an infection. In diabetic ketoacidosis, the urine will have a fruity smell. Variations in the specific gravity can show that the kidney over filters or under filters the urine.
High levels of glucose in the urine can be indicative of diabetes. The presence of ketones in urine is abnormal and can reveal the presence of diabetes.
The presence of proteins or albumin in the blood may point to kidney disease . A bleeding disorder, or an abnormality in the functioning of the liver, is seen in the presence of bilirubin in the urine. An increased amylase level may indicate pancreatitis (inflammation of the pancreas) or some other pancreatic disorder. The presence of a hormone called human chorionic gonadotropin (hCG) can be indicative of a pregnancy.
Urine tests might reveal essential details about a person's health based on whether the findings are within or outside of the normal range. The mere presence of some compounds, like protein, in the urine may indicate red flags and warrant further testing. Urine tests are less effective when the patient is dehydrated or has very diluted urine (they have drunk too much water). In addition, they may not be effective in identifying early disease.
In brief: Understanding urine tests
https://www.ncbi.nlm.nih.gov/books/NBK279350/
Urinalysis (urine test)
https://www.kidney.org/atoz/content/what-urinalysis

General Surgery
Rate this article
Source Article Arrow Most popular articles

Ovulation and Safe Period: What is the Safe Period to Have Sex?

Dr. Sabita Laskar

Unwanted 72 - Uses, Dosage, Side Effects, Drug Warnings, and Precautions

Dr. Divya Banu M

Jelqing - Penis Enlargement Exercise

Dr. Ramchandra Lamba

Dolo 650 MG Tablet

Dr. Anshul Varshney

I-Pill - Uses, Dosage, Side Effects, Drug Warnings, and Precautions

Dr. Vasantha. K. S
Ask your health query to a doctor online
*guaranteed answer within 4 hours
Disclaimer: No content published on this website is intended to be a substitute for professional medical diagnosis, advice or treatment by a trained physician. Seek advice from your physician or other qualified healthcare providers with questions you may have regarding your symptoms and medical condition for a complete medical diagnosis. Do not delay or disregard seeking professional medical advice because of something you have read on this website. Read our Editorial Process to know how we create content for health articles and queries.
This website uses cookies to ensure you get the best experience on our website. iCliniq privacy policy

- Health A to Z
- Alternative Medicine
- Blood Disorders
- Bone and Joint
- Cardiovascular Diseases
- Child Health
- Coronavirus
- Dental Care
- Digestive System
- Disabilities
- Drug Center
- Ear, Nose, and Throat
- Environmental Health
- Exercise And Fitness
- First Aid and Emergencies
- General Health
- Health Technology
- Hearing Loss
- Hypertension
- Infectious Disease
- LGBTQ Health
- Liver Health
- Men's Health
- Mental Health
- Pain Management
- Public Health
- Pulmonology
- Senior Health
- Sexual Health
- Skin Health
- Women's Health
- News For Medical Professionals
- Our Products
- Consumer News
- Physician's Briefing
- HealthDay TV
- Wellness Library
- HealthDay Living
- Conference Coverage
- Custom Products
- Health Writing
- Health Editing
- Video Production
- Medical Review
New Urine Test Shows Promise in the Early Detection and Prevention of Cervical Cancer
A new, non-invasive urine test may help detect cervical changes that can lead to cancer if left untreated.
The test looks for proteins produced by a cancer-causing strain of the human papilloma virus called HPV16.
Researchers tested it on women with different stages of cervical intraepithelial neoplasia (CIN), a precursor to cervical cancer.
They say it successfully detected the proteins in 80% of participants with stage 1 disease, 71% with stage 2 and 38% with stage 3.
These findings suggest the HPV16 protein may play a more critical role in the early stages of cancer formation.
The lead author says, “This new method holds great promise for early detection and prevention of cervical cancer.” And it would be more accessible and less invasive than the traditional Pap smear.
He hopes further testing will lead to its widespread use in clinical settings.
In 2022, approximately 660,000 new cases of cervical cancer were diagnosed worldwide.
Source: Microorganisms
Related Stories

- Introduction
- Conclusions
- Article Information
A, Discovery and nomination of candidate biomarkers for the multiplex urinary panel. Biomarker discovery was performed using RNA sequencing data from 220 benign prostates, 71 with cancers of grade group 1, and 484 with cancers of grade group 2 or greater available through the Cancer Genome Atlas, the Genotype-Tissue Expression portal, and the University of Michigan. A total of 72 markers met predefined criteria. Of these, quantitative polymerase chain reaction probes could not be successfully designed for 19, and 9 genes were highly cross-correlated, resulting in exclusion from the candidate panel. The remaining 44 transcripts meeting nomination criteria were supplemented with 10 curated genes to yield a 54-gene candidate panel. B, Development of the optimal 18-gene model for high-grade cancer. To avoid multicollinearity in regression models, highly correlated variables were identified and removed with a stepwise procedure. We assessed 3 model-building approaches: (1) logistic regression with stepwise feature selection, (2) logistic regression with recursive feature elimination, and (3) regularized logistic regression with elastic net. Performance of each model-building approach was quantified as the area under the receiver operating characteristic curve on repeated cross-validation (10-fold cross-validation repeated 3 times) with upsampling of the minor class to yield balanced classes. Elastic net modeling yielded the highest median area under the curve and was used for development. Using an ensemble approach, the development set was randomly divided into 4 partitions, and the model yielding the highest area under the curve was identified for each partition. This approach was repeated 10 times with different random seeds, yielding 40 elastic net models in total. For each candidate gene, the frequency of model inclusion and importance to high-grade prostate cancer detection was tabulated across models. Based on analysis of optimal feature size and technical features of the OpenArray platform (Thermo Fisher Scientific), the 17 biomarkers providing optimal discriminative accuracy for prostate cancer of grade group 2 or greater were included with standard clinical variables and the normalization gene KLK3 in the MyProstateScore 2.0 model (without prostate volume) and MyProstateScore 2.0 Plus model (with prostate volume). Models were calibrated and internally cross-validated prior to external validation.
A, Decision curve analysis plots for net clinical benefit of prebiopsy testing with prostate-specific antigen (PSA) alone, the Prostate Cancer Prevention Trial risk calculator, Prostate Health Index (PHI), derived multiplex 2-gene model, derived multiplex 3-gene model, MyProstateScore (MPS), MPS2, and MPS2+ compared with baseline approaches of biopsy all or biopsy none. The threshold probability (x-axis) reflects how the patient and clinician value potential clinical outcomes. For example, a threshold probability of 5% applies to patients that would choose to pursue biopsy if their risk of high-grade cancer is 5% or higher. For high-grade prostate cancer, a 5% threshold probability represents a risk-averse population, such as younger men with a long life expectancy. At a practice level, this implies that the clinician would be willing to perform as many as 20 biopsies to detect an additional high-grade cancer. At the other end of the spectrum, a threshold probability of 20% applies to patients that would choose to pursue biopsy only if their risk of high-grade cancer was 20% or greater. Such a population strongly values avoiding biopsy and is willing to accept a higher risk of delayed detection of high-grade cancer. The unit of net benefit (y-axis) is true positives. A net benefit of 0.15 is equivalent to an approach in which 15 patients per 100 are directed to biopsy based on use of the test, and all 15 patients are found to have high-grade cancer. As illustrated in the figure, the MPS2 and MPS2+ models provided the highest net benefit across the range of clinically pertinent threshold probabilities (5% to 20%). B, Decision curve analysis plots illustrating the net reduction in biopsies performed per 100 patients without missing a single diagnosis of cancer of grade group 2 or greater based on prebiopsy testing with PSA alone, the Prostate Cancer Prevention Trial risk calculator, PHI, the derived multiplex 2-gene model, the derived multiplex 3-gene model, MPS, MPS2, and MPS2+ compared with a baseline approach of biopsying all patients. The MPS2 and MPS2+ models provided the largest net reduction in biopsies performed across clinically pertinent threshold probabilities.
eTable 1. RNAseq-Based Marker Nomination Criteria
eTable 2. Indications for Prostate Biopsy in the External Validation Cohort
eTable 3. Frequency of Inclusion and Cumulative Importance of the 17 Most Informative Markers Across 40 Elastic Net Models Assessed in Development
eTable 4. Model Coefficients for the MPS2 and MPS2+ Models
eTable 5. Characteristics of the NCI EDRN External Validation Population Stratified by Previous Biopsy Status
eTable 6. Performance Measure Calculations for High-Grade Cancer using PSA Alone, Prostate Cancer Prevention Trial Risk Calculator, Prostate Health Index (PHI), Derived Multiplex 2-Gene and 3-Gene Models, MyProstateScore (MPS), MPS2, and MPS2 Plus Prostate Volume (MPS2+) in the EDRN Validation Cohort
eTable 7. Clinical Performance of High Sensitivity MPS2 Threshold Values in the Initial Biopsy Subpopulation of the External Validation Population
eTable 8. Clinical Performance of High Sensitivity MPS2 Threshold Values in the Repeat Biopsy Subpopulation of the External Validation Population
eTable 9. Characteristics of the Secondary Development Cohort for Patients Undergoing Initial Biopsy at U-M
eTable 10. Clinical Performance of Secondary Initial Biopsy (iMPS2) Models in the Initial Biopsy Subpopulation of the External Validation Cohort (n = 496)
eTable 11. Model Coefficients from MPS2 Redevelopment Using Maximum Grade Group From All Biopsy and Surgical Specimens
eFigure 1. Heatmap Illustrating Differential Expression of RNAseq Target Genes
eFigure 2. Box and Dot Plots of Gene Expression in Benign, Low-Grade Cancer, and High-Grade Cancer Tissue in the Discovery Set
eFigure 3. Box and Dot Plots of Gene Expression in Benign vs Prostate Cancer Tissue in the Discovery Set
eFigure 4. Flow Diagram of the University of Michigan Development Cohort
eFigure 5. Postcalibration Curves of MPS2 and MPS2+ in the Development Cohort
eFigure 6. MPS2 and MPS2+ Cross-Validated Area Under the ROC Curves for High-Grade Prostate Cancer in the Development Cohort
eFigure 7. Flow Diagram of the NCI EDRN External Validation Cohort
eFigure 8. MPS2 Values by Biopsy Pathology in the External Validation Cohort
eFigure 9. MPS2 and MPS2+ Area Under the ROC Curves for High-Grade Prostate Cancer in the External Validation Cohort
eFigure 10. Postcalibration Curves of iMPS2 and iMPS2+ in the External Validation Cohort
eReferences.
eTable. Characterization of 58 724 Gene Targets Assessed by RNAseq
eTable. Characterization of the 54-Marker Candidate Panel
Group Information. EDRN-PCA3 Study Group
Data Sharing Statement
- Urine-Based Test Could Help Identify Aggressive Prostate Cancer JAMA Medical News in Brief June 11, 2024 Emily Harris
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Tosoian JJ , Zhang Y , Xiao L, et al. Development and Validation of an 18-Gene Urine Test for High-Grade Prostate Cancer. JAMA Oncol. 2024;10(6):726–736. doi:10.1001/jamaoncol.2024.0455
Manage citations:
© 2024
- Permissions
Development and Validation of an 18-Gene Urine Test for High-Grade Prostate Cancer
- 1 Department of Urology, Vanderbilt University Medical Center, Nashville, Tennessee
- 2 Vanderbilt-Ingram Cancer Center, Nashville, Tennessee
- 3 Department of Pathology, University of Michigan, Ann Arbor
- 4 Department of Biostatistics, Fred Hutchinson Cancer Research Center, Seattle, Washington
- 5 Departments of Pathology and Urology, Johns Hopkins University School of Medicine, Baltimore, Maryland
- 6 Department of Urology, Northwestern University Feinberg School of Medicine, Chicago, Illinois
- 7 Department of Urology, University of Michigan, Ann Arbor
- 8 Strata Oncology, Ann Arbor, Michigan
- 9 Division of Cancer Prevention, National Institutes of Health, Bethesda, Maryland
- 10 Department of Urology, Emory University, Atlanta, Georgia
- 11 Howard Hughes Medical Institute, Chevy Chase, Maryland
- Medical News in Brief Urine-Based Test Could Help Identify Aggressive Prostate Cancer Emily Harris JAMA
Question Can a new 18-gene urinary test for high-grade prostate cancer (ie, grade group [GG] 2 or greater) improve prostate-specific antigen (PSA) screening outcomes relative to existing biomarker tests?
Findings In this diagnostic study including 761 men in the development cohort and 743 men in the validation cohort, novel cancer-specific and high-grade cancer-specific genes were identified from RNA sequencing data and optimally modeled in a development cohort, yielding an 18-gene test for high-grade prostate cancer. Applying a testing approach with 95% sensitivity for high-grade prostate cancer to an external validation population, use of the 18-gene test would have reduced the number of unnecessary biopsies performed relative to current guideline-endorsed tests.
Meaning The new 18-gene prostate cancer test may reduce more burdensome additional testing (eg, imaging and biopsy) while maintaining highly sensitive detection of high-grade cancer in patients undergoing PSA screening.
Importance Benefits of prostate cancer (PCa) screening with prostate-specific antigen (PSA) alone are largely offset by excess negative biopsies and overdetection of indolent cancers resulting from the poor specificity of PSA for high-grade PCa (ie, grade group [GG] 2 or greater).
Objective To develop a multiplex urinary panel for high-grade PCa and validate its external performance relative to current guideline-endorsed biomarkers.
Design, Setting, and Participants RNA sequencing analysis of 58 724 genes identified 54 markers of PCa, including 17 markers uniquely overexpressed by high-grade cancers. Gene expression and clinical factors were modeled in a new urinary test for high-grade PCa (MyProstateScore 2.0 [MPS2]). Optimal models were developed in parallel without prostate volume (MPS2) and with prostate volume (MPS2+). The locked models underwent blinded external validation in a prospective National Cancer Institute trial cohort. Data were collected from January 2008 to December 2020, and data were analyzed from November 2022 to November 2023.
Exposure Protocolized blood and urine collection and transrectal ultrasound-guided systematic prostate biopsy.
Main Outcomes and Measures Multiple biomarker tests were assessed in the validation cohort, including serum PSA alone, the Prostate Cancer Prevention Trial risk calculator, and the Prostate Health Index (PHI) as well as derived multiplex 2-gene and 3-gene models, the original 2-gene MPS test, and the 18-gene MPS2 models. Under a testing approach with 95% sensitivity for PCa of GG 2 or greater, measures of diagnostic accuracy and clinical consequences of testing were calculated. Cancers of GG 3 or greater were assessed secondarily.
Results Of 761 men included in the development cohort, the median (IQR) age was 63 (58-68) years, and the median (IQR) PSA level was 5.6 (4.6-7.2) ng/mL; of 743 men included in the validation cohort, the median (IQR) age was 62 (57-68) years, and the median (IQR) PSA level was 5.6 (4.1-8.0) ng/mL. In the validation cohort, 151 (20.3%) had high-grade PCa on biopsy. Area under the receiver operating characteristic curve values were 0.60 using PSA alone, 0.66 using the risk calculator, 0.77 using PHI, 0.76 using the derived multiplex 2-gene model, 0.72 using the derived multiplex 3-gene model, and 0.74 using the original MPS model compared with 0.81 using the MPS2 model and 0.82 using the MPS2+ model. At 95% sensitivity, the MPS2 model would have reduced unnecessary biopsies performed in the initial biopsy population (range for other tests, 15% to 30%; range for MPS2, 35% to 42%) and repeat biopsy population (range for other tests, 9% to 21%; range for MPS2, 46% to 51%). Across pertinent subgroups, the MPS2 models had negative predictive values of 95% to 99% for cancers of GG 2 or greater and of 99% for cancers of GG 3 or greater.
Conclusions and Relevance In this study, a new 18-gene PCa test had higher diagnostic accuracy for high-grade PCa relative to existing biomarker tests. Clinically, use of this test would have meaningfully reduced unnecessary biopsies performed while maintaining highly sensitive detection of high-grade cancers. These data support use of this new PCa biomarker test in patients with elevated PSA levels to reduce the potential harms of PCa screening while preserving its long-term benefits.
Prostate cancer (PCa) remains the most commonly diagnosed malignancy and a leading cause of cancer death worldwide. 1 The European Randomized Study of Screening for PCa and Göteborg Randomized Prostate Cancer Screening trial showed significant reductions in cancer mortality for men participating in prostate-specific antigen (PSA)–based screening. 2 , 3 At the same time, these studies confirmed that PSA screening leads to unnecessary invasive biopsies in men without cancer and frequent overdiagnosis of low-grade, indolent cancers (grade group [GG] 1). 4 In response to this, current clinical guidelines offer that men with an elevated PSA level undergo multiparametric magnetic resonance imaging (mpMRI), if available, or biomarker testing for risk stratification prior to biopsy. 5 , 6
Indeed, use of prostate mpMRI with targeted biopsy has improved detection of clinically significant, high-grade cancer (ie, cancer of GG 2 or greater) in men with tumors visible on mpMRI. 7 While these data support prebiopsy mpMRI in patients requiring biopsy, the use of negative findings on mpMRI to rule out high-grade cancers in men with elevated PSA levels is not well supported. Population-level data spanning academic and community settings reveal a negative predictive value (NPV) of only 77% for high-grade cancers, 8 and subjective interpretation of mpMRI is highly problematic, with NPVs as low as 63% by site and 40% among radiologists. 9 , 10 Thus, even following negative findings on mpMRI, its limited sensitivity merits biopsy in a substantial proportion of men. Moreover, there are practical reasons mpMRI may not be feasible for populationwide use after PSA, including its resource burden and limited availability in the community setting. 11 , 12
Objective, noninvasive biomarker tests could be a more practical option. Current National Comprehensive Cancer Network (NCCN) guidelines offer 6 blood-based and urine-based biomarker tests, each including 3 or fewer markers of PCa (ie, cancer of any grade). 5 While consistently outperforming PSA alone, 13 these assays have not evolved to reflect current understanding of PCa biology. For one, given the minimal metastatic potential of low-grade cancers, contemporary practice is focused on detecting high-grade cancers, while reducing overdiagnosis of low-grade disease. 5 Thus, assays based solely on markers associated with cancer of any grade have limited biologic specificity for high-grade cancers. Moreover, assays including only 2 to 3 biomarkers simply cannot capture the multitude of diverse molecular pathways driving lethal disease. 14 , 15
We hypothesized that augmenting the prior generation of cancer-associated biomarkers with novel molecules selectively expressed by high-grade, aggressive cancers would improve testing accuracy. Leveraging multi-institutional transcriptomic data, 14 , 16 , 17 we identified novel genes specifically overexpressed by high-grade cancers. We then adopted multiplex polymerase chain reaction (PCR)–based technology to evaluate 54 candidate markers in a development cohort, deriving an optimal 18-gene assay for standard clinical use. Finally, we performed blinded external validation of the new assay, including direct comparison with currently endorsed biomarker tests.
Institutional review board approval was obtained from the University of Michigan Institutional Review Board and at each site, and all participants provided written informed consent. This study followed the Standards for Reporting of Diagnostic Accuracy ( STARD ) reporting guideline. 18
The original MyProstateScore (MPS) test incorporates prostate cancer antigen 3 ( PCA3 ) and TMPRSS2:ERG gene fusion expression with serum PSA level to estimate risk of high-grade cancers and is endorsed by NCCN guidelines for prebiopsy risk stratification. 5 , 19 To derive a gene panel for high-grade cancers, we performed differential expression analysis of 58 724 genetic targets in multi-institutional RNA sequencing data ( Figure 1 ; eFigures 1 and 2 in Supplement 1 and the eTable in Supplement 2 ). A total of 72 genes met predefined nomination criteria for cancer (n = 50) or high-grade cancer (n = 22) (eTable 1 in Supplement 1 ). Removal of collinear genes and those without PCR primers resulted in 44 candidate markers (eFigures 1 to 3 in Supplement 1 ). These were supplemented with 10 previously described PCa-associated or reference genes, yielding a 54-gene candidate panel.
Prebiopsy urine has been prospectively collected at the University of Michigan Prostate Specialized Program of Research Excellence under a National Cancer Institute (NCI) Early Detection Research Network (EDRN) protocol approved by the University of Michigan Institutional Review Board since 2008. First-catch urine was obtained following digital rectal examination and was mixed with RNA stabilization buffer and frozen at −70 °C. 20 The development cohort included patients presenting for 12-core or greater prostate biopsy due to elevated PSA levels (3-10 ng/mL; to convert to micrograms per liter, multiply by 1) from 2008 to 2020. In accordance with guidelines, 5 we excluded patients with PCa. Based on proposed use of this test as a pre-mpMRI, prebiopsy test to rule out the need for mpMRI or biopsy, we excluded men with a history of prostate mpMRI and targeted biopsy.
OpenArray technology (Thermo Fisher Scientific) is a high-throughput real-time quantitative PCR (qPCR) method for rapid screening of multiple TaqMan assays. RNA isolation, extraction, and complementary DNA synthesis were performed (eFigure 4 in Supplement 1 ).
We assessed the 54-gene candidate panel using multiple model-building strategies ( Figure 1 ). Clinical factors consistently associated with PCa (age, race, digital rectal examination findings, PSA level, family history of prostate cancer, and prior negative biopsy) 21 were locked into models a priori. Because prostate volume improves predictive value 22 , 23 but is not available for all patients, we developed a second model including volume for use when volume is clinically available (ie, previous biopsy or mpMRI). Test outputs were standardized to represent the percentage likelihood of detecting high-grade cancers (0% to 100%). The optimal 18-gene model without prostate volume (MPS2) and with prostate volume (MPS2+) were calibrated (eFigure 5 in Supplement 1 ) to account for differences in outcome prevalence between cohorts, 21 , 24 , 25 and the calibrated models were locked for external validation. The MPS2 test is owned by LynxDx, which has obtained an exclusive license for commercialization from the University of Michigan.
The validation cohort consisted of patients in the prospective NCI EDRN PCA3 Evaluation Trial. This trial enrolled consecutive patients presenting for biopsy across 11 academic centers, primarily due to elevated PSA levels or abnormal digital rectal examination findings (eTable 2 in Supplement 1 ). 20 Patient race was self-reported via a questionnaire; selectable options included American Indian or Alaska Native, Asian, Black, Native Hawaiian or Other Pacific Islander, White, other, or unknown race. Black race was considered pertinent to this study based on the known association of Black race with PCa incidence, outcomes, and molecular subtypes. 26 Because such associations are not well established for other racial groups and because racial groups other than Black and White are frequently misclassified, 27 racial groups were categorized as Black or other race.
Deidentified urine specimens were shipped to the University of Michigan for OpenArray profiling. Laboratory procedures were conducted per the identical protocol used in development. We derived a multiplex 2-gene model ( HOXC6 and DLX1) and a multiplex 3-gene model ( PCA3 , ERG , and SPDEF) . These genes are measured in the commercially available SelectMDx and ExoDx Prostate Intelliscore (EPI) tests, respectively. The multiplex models considered herein were independently derived based on gene expression measured using the OpenArray qPCR platform and are not proposed to represent the commercial products. Serum PSA, free PSA, and [−2]proPSA were measured using the Access 2 Immunoassay System (Beckman Coulter) at the Johns Hopkins EDRN Laboratory.
Expression data and model coefficients were available to 2 investigators (C.X. and Y. Zheng) at the NCI EDRN for predefined validation. Locked model coefficients from development were used to generate outputs of the derived multiplex 2-gene model, derived multiplex 3-gene model, MPS2, and MPS2+. The original MPS was calculated using qPCR-based PCA3 and TMPRSS2:ERG scores 19 ; a subset of data were previously described. 28 Prostate Health Index (PHI) was calculated using the formula ([−2]proPSA/free PSA) × √(PSA).
Comparative analysis included PSA, the Prostate Cancer Prevention Trial risk calculator, 21 PHI, the derived multiplex 2-gene and 3-gene models, MPS, 19 MPS2, and MPS2+. The primary outcome was cancer of GG 2 or greater on biopsy; cancer of GG 3 or greater was secondarily assessed. Diagnostic potential was visualized by receiver operating characteristic (ROC) curves and quantified by area under the ROC curve (AUC) using R package pROC. 29 For the development cohort, mean AUC from repeated 10-fold cross-validation was reported. For the validation cohort, 95% CIs of AUCs were computed with 2000 stratified bootstrap.
We sought to illustrate test performance using a straightforward, clinically applicable approach. As described, 30 considering prevalence of high-grade cancers in testing populations (17% to 31%), 31 - 35 relative harms of false-negative and false-positive testing results, 36 and the proposed role of biomarkers for rule-out testing, 37 , 38 we assessed thresholds providing 95% sensitivity for high-grade cancer. Performance measures were calculated using the confusionMatrix function of R package caret. Given disparate risk profiles of initial and repeat biopsy populations, 39 - 41 analyses were carried out in each subpopulation.
Decision curve analysis (DCA) was used to quantify net benefit of each biomarker on the decision to undergo biopsy compared with (1) biopsying all patients and (2) biopsying no patients. 42 Considering a more than 20% risk of high-grade cancer justifies performing biopsy and a less than 5% risk justifies foregoing biopsy in most patients, 43 we assessed threshold probabilities spanning this clinically relevant range. DCA was performed using dca in the R package dcurves.
Statistical analyses were performed using R version 4.1.1 (The R Foundation). Two-tailed tests were used for all comparisons, and P values less than .05 were considered statistically significant.
Among 815 participants in the development cohort, qPCR yielded valid results in 761 (93.4%) (eFigure 4 in Supplement 1 ). The median (IQR) age was 63 (58-68) years, and the median (IQR) PSA level was 5.6 (4.6-7.2) ng/mL ( Table 1 ). On study biopsy, 293 men (38.5%) had cancer of GG 2 or greater. The contribution of candidate genes to model predictions was quantified across elastic net models ( Figure 1 ; eTable 3 in Supplement 1 ). The final MPS2 model included clinical variables and the 17 most informative markers, including 13 from the discovery analysis (4 high-grade cancer–specific genes [ APOC1 , B3GNT6 , NKAIN1 , and SCHLAP1 ] and 9 cancer-specific genes [ PCGEM1 , SPON2 , TRGV9 , PCA3 , OR51E2 , CAMKK2 , TFF3 , PCAT14 , and TMSB15A ]), 4 curated markers ( HOXC6 , ERG , TMPRSS2:ERG , and KLK4 ), plus the reference gene KLK3 (eTable in Supplement 3 ). Model coefficients were determined in the overall cohort (eTable 4 in Supplement 1 ). Calibration and internal cross-validation were performed (eFigures 5 and 6 in Supplement 1 ), and the MPS2 models were locked for external validation.
Of 813 patients in the validation cohort (eFigure 7 in Supplement 1 ), qPCR was successful in 743 (91.4%). The median (IQR) age was 62 (57-68) years, and the median (IQR) PSA level was 5.6 (4.1-8.0) ng/mL. A total of 95 men (12.8%) were Black and 648 (87.2%) were another race, and 247 men (33.2%) had a previous negative biopsy ( Table 1 ). On study biopsy, 151 men (20.3%) had high-grade PCa. Median (IQR) MPS2 values were significantly higher in men with cancer of GG 2 or greater (0.44 [0.23-0.69]) than in men with negative biopsies (0.08 [0.03-0.19]; P < .001) and in men with cancer of GG 1 (0.20 [0.08-0.43]; P < .001) ( Table 1 ; eFigure 8 in Supplement 1 ). Similarly, median (IQR) MPS2+ values were significantly higher in men with PCa of GG 2 or greater (0.54 [0.27-0.79]) relative to those with negative biopsies (0.08 [0.03-0.21]; P < .001) or those with cancer of GG 1 (0.25 [0.09-0.48]; P < .001). The AUC values for high-grade cancer were 0.60 (95% CI, 54.7-64.6) for PSA alone, 0.66 (95% CI, 61.1-70.7) for the Prostate Cancer Prevention Trial risk calculator, 0.77 (95% CI, 73.0-81.3) for PHI, 0.76 (95% CI, 71.9-80.3) for the derived multiplex 2-gene model, 0.72 (95% CI, 67.0-76.1) for the derived multiplex 3-gene model, and 0.74 (95% CI, 69.4-78.0) for MPS compared with 0.81 (95% CI, 76.9-84.6) for MPS2 and 0.82 (95% CI, 78.1-85.5) for MPS2+ (eFigure 9 in Supplement 1 ). The observed prevalence of high-grade cancer closely approximated MPS2 and MPS2+ predicted probabilities ( Figure 2 ), reflecting good calibration. Critically, the models were particularly well-calibrated for predicted probabilities less than 30%, which are most clinically pertinent.
We assessed clinical consequences of prebiopsy biomarker testing. At a 95% sensitivity testing threshold, the proportions of unnecessary biopsies that would have been avoided using each test were 11% for PSA alone, 20% for the Prostate Cancer Prevention Trial risk calculator, 26% for PHI, 27% for the derived multiplex 2-gene model, 17% for the derived multiplex 3-gene model, and 23% for MPS compared with 37% for MPS2 and 41% for MPS2+. Full performance measures and the estimated numbers of unnecessary biopsies avoided per 1000 patients are listed in Table 2 . Critically, MPS2 and MPS2+ each provided 99% sensitivity and 99% NPV for cancer of GG 3 or greater.
The initial biopsy population included 496 patients with a median (IQR) PSA level of 5.0 (3.8-6.6) ng/mL (eTable 5 in Supplement 1 ). On study biopsy, 133 (26.8%) had high-grade cancer. Using a 95% sensitivity threshold, the proportions of unnecessary biopsies avoided were 15% for PSA alone, 27% for the Prostate Cancer Prevention Trial risk calculator, 30% for PHI, 30% for the derived multiplex 2-gene model, 17% for the derived multiplex 3-gene model, and 27% for MPS compared with 35% for MPS2 ( Table 2 ; eTable 6 in Supplement 1 ). Although patients undergoing initial biopsy often may not have prostate volume available, use of MPS2+ would have avoided 42% of unnecessary biopsies. Performance of MPS2 models with and without clinical factors are provided by subpopulation in eTables 7 and 8 in Supplement 1 . An alternative initial biopsy model was developed in the initial biopsy population of the development cohort and similarly validated (eTables 9 and 10 and eFigure 10 in Supplement 1 ).
The repeat biopsy population included 247 men with median (IQR) PSA level of 7.2 (5.5-9.8) ng/mL, of which 18 (7.3%) were found to have high-grade cancer (eTable 5 in Supplement 1 ). At 95% sensitivity, the proportions of unnecessary biopsies that would have been avoided were 15% for PSA alone, 8.7% for PHI, 14% for the derived multiplex 2-gene model, 16% for the derived multiplex 3-gene model, 15% for MPS, 46% for MPS2, and 51% for MPS2+ ( Table 2 ). Accordingly, MPS2 testing would have avoided approximately one-half of unnecessary biopsies while maintaining detection of 94.4% of high-grade cancers.
DCA was used to evaluate the net benefit of biomarker testing relative to performing biopsy in all patients and performing no biopsies. Across the clinically pertinent threshold probabilities spanning 5% to 20%, use of the MPS2 models would have provided the highest net clinical benefit across all tests ( Figure 3 A). Expressing benefit as net reduction in unnecessary biopsies, use of the MPS2 models would have provided the greatest net reduction in unnecessary biopsies without failing to biopsy a single patient with high-grade cancer ( Figure 3 B).
Acknowledging the indolent nature of low-grade PCa, contemporary guidelines emphasize a narrowed diagnostic focus on high-grade cancers. 5 , 6 , 44 Existing biomarkers expressed by all PCa—including low-grade, indolent tumors—therefore offer limited potential to selectively detect high-grade disease. Translating sequencing-based discovery to an expandable qPCR platform, we developed and validated a new urinary test incorporating 17 markers of cancer, and—for the first time, to our knowledge—novel markers uniquely overexpressed by high-grade cancers relative to low-grade cancers. On validation, MPS2 testing with 95% sensitivity for high-grade cancer had 95% to 99% NPV and 35% to 51% specificity across subgroups. For individual patients, NPVs approaching 100% provide clear guidance for confident decision-making. For clinicians, uniform use of MPS2 could avoid unnecessary biopsies while preserving immediate detection of 95% of cancers of GG 2 or greater diagnosed using the biopsy all approach. Critically, MPS2 had 99% sensitivity and 99% NPV for cancers of GG 3 or greater, meaning the rare false-negative MPS2 results were almost uniformly more favorable cancers of GG 2 least likely to metastasize.
The 2023 NCCN guidelines for PCa early detection propose consideration of prebiopsy risk stratification with validated biomarker tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA. 5 These tests have consistently outperformed PSA alone, with aggregate data approximating 90% to 95% sensitivity and 30% to 40% specificity for high-grade cancer. 13 , 28 , 32 However, heterogeneity of published data and a lack of head-to-head comparisons have precluded recommendations of any particular testing approach. 45 Using an NCI cohort clinically indicated for biomarker testing, we directly compared the new 18-gene test for high-grade PCa with existing guideline-endorsed tests. Broadly, AUC values for MPS2 models were associated with meaningful improvement compared with currently available options. Using a testing approach with 95% sensitivity for high-grade cancer, MPS2 would have meaningfully reduced unnecessary biopsies performed relative to other tests. These data support use of MPS2 to mitigate the potential harms of screening while preserving its long-term benefits.
Patients with a prior negative biopsy pose a unique challenge. 40 Because most patients undergo initial biopsy due to PSA elevation, the value of repeated PSA testing is particularly limited in this population. 46 In one prior study, among 229 patients undergoing repeat biopsies, the EPI test provided 82% sensitivity and 27% specificity for high-grade cancer. 47 Among 268 patients undergoing repeat biopsies, MPS provided 100% sensitivity and 23% specificity. 48 In the current analysis including 247 patients, at 94.4% sensitivity, MPS2+ provided 51% specificity, compared with 8.7% with PHI, 14% with the derived multiplex 2-gene model, 16% with the derived multiplex 3-gene model, and 15% with MPS. While striking, these findings are plausible, as most current assays include PSA and PSA isoforms, underscoring a continued dependence on PSA. Second, existing assays measure 3 or fewer non-PSA markers. Given the multiple pathways driving lethal PCa, 14 , 15 it is difficult to conceive that most aggressive cancers would overexpress one of so few markers early in the disease course. By capturing 17 cancer-associated, PSA-independent markers, MPS2 provides roughly 5-fold the breadth of previous tests and offers promise of a new generation of biomarkers not reliant on PSA.
The ideal diagnostic test has been described as safe, accurate, available, and actionable and providing a favorable benefit-to-harm ratio. 49 , 50 While PSA alone offers favorable practical attributes, its lack of cancer specificity has driven the need for a complementary test to improve screening outcomes. 4 While prebiopsy mpMRI improves detection of high-grade cancer in men with positive findings on mpMRI, 7 , 51 data describing the use of negative mpMRI findings to rule out significant cancer merit concern. Findings from a statewide collaborative revealed an NPV of only 77% across diverse settings. 8 Even at experienced centers, subjective MRI interpretation yields significant variability, with NPVs as low as 63% at one center and 40% among individual radiologists. 9 , 10 Moreover, MRI bears an extensive time and resource burden, is not widely available in community settings, and is not an option for some patients, posing critical barriers to widespread use. 11 , 12 While a valuable component of the diagnostic armamentarium, practical limitations and suboptimal rule-out performance suggest MRI may be best used later in the diagnostic pathway, eg, to improve the yield of biopsy in men most likely to benefit from invasive testing.
The accuracy of MPS2 offers potential for straightforward application at the primary care level (ie, negative test rules out high-grade disease; positive test prompts specialist referral). For specialists, providing patients with early noninvasive molecular tumor data 52 - 55 could enable more informed, individualized cancer care. For example, in patients indicated for biopsy, the relationship of tumor subtypes with MRI visibility could help identify patients likely to benefit from prebiopsy mpMRI and those better served by immediate biopsy. 56 In men with PCa of GG 1, high-grade markers could signal the presence of occult aggressive tumors, while their absence could obviate the need for scheduled surveillance biopsies. 57 Finally, while biopsy and tissue-based assays rely on the specific tumor foci sampled, 58 , 59 urine provides a comprehensive assessment of prostatic gene expression—an ideal complement to mitigate the sampling limitations of biopsy.
The current study has limitations. For one, there was limited racial diversity in the study population. Thus, it is unclear how our findings could differ in Black men, and we are currently pursuing analyses to ensure optimal testing for all patients. Second, the reference standard was systematic biopsy, which is subject to undersampling that could increase NPV and decrease positive predictive value relative to surgical pathology. 60 - 62 Nonetheless, sampling misclassification would be expected to impact all tests equally, and we uniquely performed head-to-head comparison of MPS2 with existing biomarker tests. Furthermore, we repeated model development in patients with more definitive pathologic data (eg, radical prostatectomy), and prostatectomy-derived MPS2 models did not differ substantially (eTable 11 in Supplement 1 ). Notably, the current analysis used the Prostate Cancer Prevention Trial risk calculator due to its extensive validation and recognition by clinicians 63 ; other risk calculators could have performed differently. 64
We acknowledge the limitations of deriving molecular models developed on other platforms. Although the derived multiplex models capture the components of other commercially available tests, these models should not be interpreted as equivalent to the commercial assays, just as no conclusions can be drawn regarding biomarkers not assessed. Still, external comparison of a newly validated test with guideline-endorsed tests has not previously been performed, to our knowledge, and the 18-gene test would have yielded clinically meaningful improvement in accuracy for high-grade PCa relative to current testing options. While encouraging, these findings do not rule out disparate findings in additional cohorts. Moreover, the 95% sensitivity threshold is a single data point that, while illustrative and clinically applicable, may not be ideal for all populations; decision curves presented herein provide a greater breadth of information regarding utility. Finally, this study population was not suitable for comparing biomarkers with mpMRI, which remains a critical knowledge gap. We are currently conducting a prospective multicenter trial for this assessment. 65 Regardless, the externally validated performance of MPS2 supports its effectiveness in accurately ruling out the need for mpMRI and biopsy altogether. Additional studies are needed to corroborate these data and confirm the observed positive impact of MPS2 testing on longer-term outcomes.
In this study, within an external validation population referred for prostate biopsy, an 18-gene urinary test had higher diagnostic accuracy for high-grade PCa beyond currently available testing options. Clinically, use of this test would have safely avoided unnecessary additional testing with imaging or biopsy in 35% to 51% of patients while maintaining high sensitivity for high-grade cancers that stand to benefit from early detection. These findings suggest that use of the test in patients with elevated PSA levels can reduce the potential harms of prostate cancer screening while preserving its long-term benefits.
Accepted for Publication: December 6, 2023.
Published Online: April 18, 2024. doi:10.1001/jamaoncol.2024.0455
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Tosoian JJ et al. JAMA Oncology .
Corresponding Author: Arul M. Chinnaiyan, MD, PhD, Department of Pathology, University of Michigan, 1500 E Medical Center Dr, 5316 Rogel Cancer Center, Ann Arbor, MI 48109-0940 ( [email protected] ).
Author Contributions: Drs Y. Zheng and Chinnaiyan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Tosoian, Zhang, and Xiao serve as co–first authors. Drs Wei and Chinnaiyan serve as co–senior authors.
Concept and design: Tosoian, Xiao, Niknafs, Tomlins, Srivastava, Feng, Sanda, Y. Zheng, Chinnaiyan.
Acquisition, analysis, or interpretation of data: Tosoian, Zhang, Xiao, Xie, Samora, Niknafs, Chopra, Siddiqui, H. Zheng, Herron, Vaishampayan, Robinson, Arivoli, Trock, Ross, Morgan, Palapattu, Salami, Kunju, Sokoll, Chan, Sanda, Y. Zheng, Wei, Chinnaiyan.
Drafting of the manuscript: Tosoian, Zhang, Xiao, Xie, Samora, Niknafs, Trock, Chinnaiyan.
Critical review of the manuscript for important intellectual content: Tosoian, Xiao, Samora, Niknafs, Chopra, Siddiqui, H. Zheng, Herron, Vaishampayan, Robinson, Arivoli, Trock, Ross, Morgan, Palapattu, Salami, Kunju, Tomlins, Sokoll, Chan, Srivastava, Feng, Sanda, Y. Zheng, Wei, Chinnaiyan.
Statistical analysis: Zhang, Xie, Samora, Niknafs, Vaishampayan, Arivoli, Trock, Salami, Y. Zheng.
Obtained funding: Tosoian, Xiao, Niknafs, Tomlins, Chan, Feng, Sanda, Chinnaiyan.
Administrative, technical, or material support: Tosoian, Xiao, Samora, Chopra, Siddiqui, H. Zheng, Herron, Arivoli, Palapattu, Salami, Chan, Srivastava, Feng, Sanda, Wei.
Supervision: Tosoian, Morgan, Palapattu, Srivastava, Feng, Y. Zheng, Chinnaiyan.
Conflict of Interest Disclosures: Dr Tosoian reported personal fees from LynxDx and equity interest from LynxDx outside the submitted work; and has a patent for a novel multiplex urine test for high-grade prostate cancer pending. Dr Zhang reported personal fees from LynxDx outside the submitted work and has a patent for a novel multiplex urine test for high-grade prostate cancer pending. Dr Xiao reported grants from Prostate Cancer Foundation as well as personal fees from LynxDx during the conduct of the study; and has a patent for a novel multiplex urine test for high-grade prostate cancer pending. Dr Niknafs reported personal fees from LynxDx during the conduct of the study; personal fees from LynxDx outside the submitted work; and has a patent for use of some biomarkers as diagnostic tools issued. Dr Trock reported personal fees from Artera during the conduct of the study as well as personal fees from Myriad Genetics outside the submitted work. Dr Salami reported personal fees from Bayer and NRichDx during the conduct of the study. Dr Tomlins reported grants and personal fees from Astellas as well as equity interest from Strata Oncology outside the submitted work; and has a patent for ETS gene fusions in prostate cancer issued and licensed to LynxDx. Dr Sokoll reported grants from the National Institutes of Health during the conduct of the study. Dr Feng reported grants from the National Cancer Institute during the conduct of the study. Dr Chinnaiyan reported grants from the National Institutes of Health/National Cancer Institute, Prostate Cancer Foundation, and Howard Hughes Medical Institute; nonfinancial support from the American Cancer Society during the conduct of the study; and equity interest from LynxDx outside the submitted work; and has a patent for a novel multiplex urine test for high-grade prostate cancer pending. No other disclosures were reported.
Funding/Support: This work was funded by the Michigan-Vanderbilt Early Detection Research Network Biomarker Characterization Center (grant U2C CA271854) and the Early Detection Research Network Data Management and Coordinating Center (grant U24 CA086368). The Early Detection Research Network Data Management and Coordinating Center carried out analyses on the blinded validation cohort. Other sources of funding not involved in the design and conduct of the study included the Michigan Prostate Specialized Program of Research Excellence (grant P50 CA186786), National Cancer Institute Outstanding Investigator Award (Dr Chinnaiyan; grant R35 CA231996), Johns Hopkins University Biomarker Reference Laboratory (grant U24 CA115102), National Cancer Institute Early Detection Research Network Clinical Validation Center (grant U01 CA113913), Prostate Cancer Foundation Young Investigator Award (Drs Tosoian and Xiao), Prostate Cancer Foundation, Howard Hughes Medical Institute (Dr Chinnaiyan), and the American Cancer Society (Dr Chinnaiyan).
Role of the Funder/Sponsor: The Early Detection Research Network Data Management and Coordinating Center members had access to the blinded validation cohort analysis; the other funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: Members of the EDRN-PCA3 Study Group appear in Supplement 4 .
Meeting Presentation: These data were presented in abstract form at the American Urological Association 2023 Annual Meeting; April 28, 2023; Chicago, IL.
Previous Posting: This article was posted as a preprint on medRxiv.org.
Data Sharing Statement: See Supplement 5 .
Additional Contributions: We thank Stephanie Miner, PhD (Michigan Center for Translational Pathology, University of Michigan, Ann Arbor), for her help in the editing and file preparation of this study. Dr Miner was not compensated for her work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

URINALYSIS DIPSTICK TESTING
Aug 06, 2014
530 likes | 1.93k Views
URINALYSIS DIPSTICK TESTING. WellOne Primary Medical and Dental Care For Medical Clinical Staff. Click here to move on. WARNING. Conducting this test exposes the operator to potentially infectious material. Standard precautions, including glove use are required for this procedure.
Share Presentation
- direct sunlight replace cap
- abdominal pain
- medical clinical staff click
- perform hand
- clinitek status

Presentation Transcript
URINALYSIS DIPSTICK TESTING WellOne Primary Medical and Dental Care For Medical Clinical Staff Click here to move on
WARNING Conducting this test exposes the operator to potentially infectious material. Standard precautions, including glove use are required for this procedure. Click here to go back Click here to move on
Urine test strips: Storage and Stability Click here to view the previous slide Click here to move on Store strips in original container at room temperature (15-30 C) out of direct sunlight Replace cap immediately after removing a test strip Do not remove the desiccant from the bottle (small white package that helps to collect excess moistures)
Urine test strips: Storage and Stability Replace cap quickly Do Not expose strips to air Click here to view the previous slide Click here to move on
Considerations: Click here to view the previous slide Click here to move on As with all laboratory test, definitive diagnostic or therapeutic decisions should not be based on any single result or method. The urinalysis is a screening test and any positive, significant results should be confirmed with a laboratory specimen. Color changes must be read exactly at the indicated time. Substances that cause abnormal urine color, such as drugs containing azo dyes (e.g. Pyridium, Azo Gantrisin) and riboflavin, may affect the readability of reagent areas on urinalysis reagent strips. The color development on the reagent pad may be masked, or a color reaction may be produced on the pad that could be interpreted visually and/or instrumentally as a false positive.
Standing Order for Urinalysis for MA Staff: Click here to view the previous slide Click here to move on Collect and test urinalysis by dipstick on all children 3 years and older presenting for physical examination, all adult patients annually, and all patients with symptoms of urological involvement, i.e. Blood in urine, burning upon urination, frequent urination, odor of urine, pelvic or abdominal pain, vomiting, or confusion.
Processing the Specimen: General Steps Click here to view the previous slide Click here to move on • Identify patient using two patient identifiers (name and DOB) • Explain procedure to patient in culturally appropriate, age appropriate terms. • Perform hand hygiene before and after patient contact; before and after donning gloves.
Specimen Collection Click here to view the previous slide Click here to move on Have patient urinate in clean, labeled specimen (name and date of birth) container Test the specimen as soon as possible: refrigerate specimen if unable to test within two hours and let specimen return to room temperature before testing.
A freshly voided urine specimen is needed for the urinalysis. If the urine is more than _______ old, it must be refrigerated and allowed to _______________________ prior to testing. Click here to choose this answer Click here to choose this answer Click here to choose this answer Click here to view the previous slide 2 hours, chill on ice 2 hours, return to room temp. 3 hours, return to room temp.
The correct answer is….. 2 hours Return to room temp. Click here to view the previous slide Click here to move on A freshly voided urine specimen is needed for the urinalysis. If the urine is more than _______ old, it must be refrigerated and allowed to _______________________ prior to testing.
Processing the Specimen: Testing Procedure Click here to view the previous slide Click here to move on Inspect reagent bottle to ensure the reagent has not expired. Remove one strip from bottle and replace cap promptly. Inspect strip for any signs of deterioration prior to use. Completely immerse end of strip briefly into the freshly voided urine specimen and remove immediately. Tap edge of strip against edge of specimen container.
The test strip must be inserted into the urine and removed ___________. Click here to choose this answer Click here to choose this answer Click here to choose this answer Click here to view the previous slide in one minute in 20 seconds as quickly as possible
The correct answer is……. Click here to view the previous slide Click here to move on as quickly as possible
Processing the Specimen: Testing Procedure Click here to view the previous slide Click here to move on Hold strip in a horizontal position to prevent possible mixing of chemicals.
True or False: To avoid mixing of colors, the test strip should be held in a horizontal position after removing from the urine Click here to choose this answer Click here to choose this answer Click here to view the previous slide True False
The correct answer is true: Correct Way to hold strip Not Correct Way to hold strip Click here to view the previous slide Click here to move on Holding horizontally is correct. Holding vertically leads to mixing of chemicals and colors= invalid results
Processing the Specimen: Testing Procedure Click here to view the previous slide Click here to move on Compare color of dipped end of stick with color chart located on the bottle. a. read Glucose and Bilirubin at 30 seconds. b. read Ketone at 30 seconds. c. read Specific Gravity at 45 seconds. d. read PH, Protein, Urobilinogen, blood and nitrate at 60 seconds. e. read Leukocytes at 2 minutes. Dispose of strip in regular trash. Remove gloves and place in regular trash. Perform hand hygiene. Document results in patient's record.
Processing the Specimen: Testing Procedure Click here to view the previous slide Click here to move on Test results must be interpreted by comparing the color of the test pad to the color on the bottle for the specific substance EXACTLY at the time indicated on the bottle. Test results read at times other than that on the bottle are of NO DIAGNOSTIC VALUE
Just as you are half way done reading a test strip, a coworker approaches and begins talking with you. You are not sure how many seconds have gone by. At this point you must…. Click here to choose this answer Click here to choose this answer Click here to choose this answer Click here to view the previous slide Continue reading the test strip where you left off Wash your hands Discard the test strip and start over with a new strip
The correct answer is: Test results must be read exactly at the times indicated on the bottle. Click here to view the previous slide Click here to move on Discard the test strip and start over with a new strip
You are testing this specimen pictured below Click here to view the previous slide Click here to move on
At 60 seconds, you record the result as “trace” blood as indicated on the color chart You look again in about 90 seconds and notice the change in color to indicate “moderate” blood At this point you should………. Click here to view the previous slide Click here to move on
At this point, you should….. Click here to choose this answer Click here to choose this answer Click here to choose this answer Click here to view the previous slide Change the results on the result form to indicate moderate blood Record the results as trace blood but verbally notify the provider of finding of moderate blood Record the results as trace blood
The correct answer is….. NEVER INTERPRET RESULTS AFTER THE SPECIFIED TIME This result read after the specified time must be completely ignored an d not reported Click here to view the previous slide Click here to move on Change the results on the result form to indicate moderate blood Record the results as trace blood but verbally notify the provider of finding of moderate blood Record the results as trace blood
You have reached the end of this program….. Start Over Click here to go back and review previous slide Exit For more information, refer to the manufacturer package insert at: http://diagnostics.siemens.com/siemens/en_GLOBAL/gg_diag_FBAs/files/urinalysis_pdf/clinitek_status/clinitek_status_multistix_10.pdf Once you are confident that you are comfortable with all the materials presented, proceed to the Urinalysis Dipstick Testing post-test at http://www.classmarker.com/professional/ Your username is the first initial of your first name followed by your full last name. Your password is= nwhealth
- More by User

Urinalysis. Urinary System. Purpose. General evaluation of health Diagnosis of disease or disorders of the kidneys or urinary tract Diagnosis of other systemic disease that affect kidney function Monitoring of patients with diabetes
1.46k views • 26 slides

URINALYSIS. Collection and Preservation of Urine Urinalysis performed for two purposes Check for metabolic by-products Observe physical, chemical and microscopic characteristics Types of Specimens First morning urine specimen is preferred
1.36k views • 18 slides

URINALYSIS. The urinalysis is a fundamental test that should be performed in all urologic patients A complete urinalysis includes both chemical and microscopic analyses. . Reasons for inadequate urinalyses include. Improper collection, Failure to examine the specimen immediately,
2.59k views • 32 slides
URINALYSIS. UA Crystals. Brown or colorless granules Na or K Sediment in spin down tube may have brick red color. Amorphous Urates. Ca or Mg Found in Basic urine Dissolves in Acetic Acid. Amorphous Phosphates. Acid urine Yellowish Brown Ovals with pointed Ends. Uric Acid.
1.03k views • 52 slides

URINALYSIS. UA Casts.
1.5k views • 48 slides

Unit #5A – Clinical Laboratory Testing - Urinalysis
Unit #5A – Clinical Laboratory Testing - Urinalysis. Cecile Sanders, M.Ed., MLS(ASCP). Unit #5A – Clinical Laboratory Testing - Urinalysis. Urinary system is an excretory system Renal System (reproduced with permission from Baylor College of Medicine).
471 views • 16 slides

Urinalysis. Dr. A. Basu MD. Topic. Definition and normal urine Indication Collection Examination Physical Chemical Microscopical . Urinalysis : Definition. Qualitative and semi quantitative evaluation of renal products. Normal Urine = At a glance.
1.16k views • 49 slides

Urinalysis. Veterinary Assistant Program Middlesex Community College Michael Lavoie 12/17/12. Diaphragm. Base. Always carry a microscope with one hand holding the arm and one hand under the base . Ocular lens (Eyepiece). Body Tube. Nosepiece. Arm. Objectives. Stage. Stage Clips.
1.17k views • 64 slides

Urinalysis. By Elkhedir Elgorashi Lecturer Immunology M Sc, MLT, MT(MOH). Material Requirement. - Microscope - Centrifuge - Slides and cover slides - Centrifuge tubes 15 ml - Urine tube - Urine strips - Gloves. Urine strips. Procedure ( Sample preparation) .
1.43k views • 20 slides

URINALYSIS . Clinical Textbook for Veterinary Technicians 4 th Ed. Dennis M McCurnin Saunders. Urinalysis is a clinical laboratory procedure that should be performed as part of any minimum data base . However, it is often skipped. Tests should be run on fresh urine when possible.
2.44k views • 30 slides

Reference: Laboratory Procedures for Veterinary Technicians 5 th Ed. (Hendrix, Sirois ). Urinalysis. Label samples immediately after collection and perform u/a as soon as possible. Keep reagent strips and tablets in tightly sealed bottles; replace outdated reagents with fresh reagents.
1.4k views • 97 slides

Urinalysis. Prepared by Hamad ALAssaf [email protected]. Urine Examination. 1- Physical Examination 2- Chemical Examination 3- Microscopic Examination. URINE ANALYSIS Physical Examination. 1- Volume 2- Specific Gravity 3- Apperance 4- Color 5- Odor 6- Deposit
24.03k views • 40 slides

Aim To identify the purpose, method and value of testing urine. Urinalysis. Objectives. To identify the purpose of urinalysis To identify the benefits of urinalysis To identify the importance a clean sample of urine To identify normal and abnormal parameters in urinalysis.
1.29k views • 31 slides

Urinalysis. By: Adelle & Heidi. (UOIT Clinical Biochemistry). Definition. (Papaya Tablets, 2013). (The Free Dictionary, 2013). Urinalysis is the examination of a urine sample. Purpose. (BCM 101 Biochemistry, 2013). 1. Obtain simplified knowledge about routine urine analysis.
1.48k views • 13 slides
Urinalysis. Urinary System. PURPOSE. General evaluation of health – urine is filtered blood. Diagnosis of disease or disorders of the kidneys or urinary tract. Diagnosis of other systemic diseases that affect kidney function. Monitoring of patients with Diabetes.
2.91k views • 27 slides

URINALYSIS.
1.53k views • 116 slides
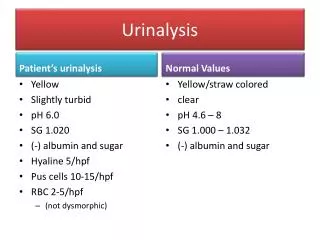
Urinalysis. Patient’s urinalysis. Normal Values. Yellow/straw colored clear pH 4.6 – 8 SG 1.000 – 1.032 (-) albumin and sugar. Yellow Slightly turbid pH 6.0 SG 1.020 (-) albumin and sugar Hyaline 5/hpf Pus cells 10-15/hpf RBC 2-5/hpf (not dysmorphic). Q5. Compute for Na deficit.
1.42k views • 3 slides

Urinalysis. pH = 4.6 – 8.0. Normal pH is within this range Average = 6. Specific Gravity = 1.003 – 1.035. Normal SG is within this range This is the “density” of the liquid. Water = 1.000 1.003 = dilute 1.035 = concentrated. Urochrome / urobilin.
684 views • 16 slides

Urinalysis. What is a urinalysis?. Urinalysis (UA) simply means analysis of urine. This is a very commonly ordered test which is performed in many clinical settings such as hospitals, clinics, emergency departments, and outpatient laboratories.
466 views • 32 slides

URINALYSIS. Click Here to Start the Lab. Instructor Terry Wiseth. WHAT DO I NEED TO HAND IN WITH THIS LAB When your are finished with this lab you will need to hand in the following for your lab report. Remember that all labs must have a cover sheet attached to the lab.
1.36k views • 116 slides

Urinalysis. Agriculture, Food, and Natural Resource Standards Addressed. AS.07.01. Design programs to prevent animal diseases, parasites and other disorders and ensure animal welfare. AS.07.01.01.a. Identify and summarize specific tools and technology used in animal health management.
861 views • 70 slides

URINALYSIS. Click Here to Start the Lab. WHAT DO I NEED TO HAND IN WITH THIS LAB When your are finished with this lab you will need to hand in your lab report. URINALYSIS LAB Click on the blackboard to view a larger blackboard for discussion. Urine Sample. Click Here to Continue.
1.37k views • 116 slides
| Advertisement |
Noninvasive urine test might help detect cervical cancer

A new urine test might help doctors more easily screen for cervical cancer, researchers report.
The test looks for proteins generated by a type of cancer-causing human papillomavirus, HPV 16. Advertisement
HPV strains 16 and 18 are responsible for nearly all cervical cancers, according to the National Cancer Institute.
- Women more likely to experience anxiety, depression after cardiac arrest
- Study: RSV vaccine safe in late pregnancy
- Air pollution linked to drop in live births among IVF patients
"Our new urine test can detect HPV16 E7 proteins, which are critical markers of cervical cancer risk, at extremely low levels," said lead researcher Etsuro Ito , a professor of biology at Waseda University in Japan. "This means that women may be able to screen for cervical cancer without the discomfort and inconvenience of a traditional Pap test."
The test detected E7 proteins in 80% of women with Stage 1 cervical intraepithelial neoplasia (CIN), a precursor to cervical cancer.
It also found the proteins in 71% of women with Stage 2 CIN and 38% of women with Stage 3 CIN, results show.
"We believe that the E7 oncoprotein is critical in the early stages of HPV-related cervical carcinogenesis and E7 may play a more significant role in the progression of CIN1 and CIN2 than in CIN3," Ito said in a university news release. Advertisement
The availability of a simple urine test could aid efforts to eradicate cervical cancer by eliminating barriers related to screening, researchers said.
"This new method holds great promise for early detection and prevention of cervical cancer," Ito said. "We are optimistic that further development and validation of this assay will lead to its widespread use in clinical settings."
The study was recently published in the journal Microorganisms .
More information
The National Cancer Institute has more on HPV and cancer .
Copyright © 2024 HealthDay. All rights reserved.

Latest Headlines

Trending Stories

This is a potential security issue, you are being redirected to https://csrc.nist.gov .
You have JavaScript disabled. This site requires JavaScript to be enabled for complete site functionality.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Tailored Health Tests for Physical Entropy Sources
Description, presented at, parent project, related topics.
Security and Privacy: random number generation

COMMENTS
Bilirubin urine test: Bilirubin is a yellowish pigment found in bile, a fluid produced by your liver. If you have bilirubin in your urine, it may indicate liver or bile duct issues. Nitrite urine test: A positive nitrite test result can indicate a urinary tract infection (UTI). However, not all bacteria are capable of converting nitrate (a ...
Urine analysis, also called urinalysis, is one of the oldest medical laboratory procedures. It involves physical, chemical, and microscopic examination of urine samples to evaluate health, diagnose diseases of the kidneys or urinary tract, and monitor certain medical conditions like diabetes. Proper collection and handling of urine specimens is ...
Fouchets Test/Harrison's spot test: PROCEDURE Place 5 ml of acidified urine in a test tube. Add 5ml of 10 % barium chloride. Mix and filter through filter paper. Let the paper dry. Add 1-2 drops of Fouchet's reagent to the ppt on filter paper. RESULT: A green color indicates the presence of bilirubin. 80.
The urine that is produced by the kidney is a by-product of some of the kidney's primary functions, which include: • Waste excretion (urea, creatinine, drug metabolites, sulfates, uric acid). ... urinalysis is a simple and noninvasive test that provides valuable information.
A urinalysis is a test of your urine. It's used to detect and manage a wide range of disorders, such as urinary tract infections, kidney disease and diabetes. A urinalysis involves checking the appearance, concentration and content of urine. For example, a urinary tract infection can make urine look cloudy instead of clear.
During download, if you can't get a presentation, the file might be deleted by the publisher. ... is mixed with the remaining drop of urine in the test tube and one drop is analyzed under a microscope . MICROSCOPIC URINALYSIS 1.Red Blood Cells( N < 3/HPF) Hematuria is the presence of abnormal numbers of red cells in urine due to: a. Glomerular ...
26. Test for detection of Glucose in urine BENEDICT TEST (1/2) Principal: When urine is boiled in Benedict solution, blue alkaline copper sulphate is reduced to red brown cuprous oxide if a reducing agent is present. Method: Take 5ml of Benedict's reagent in a test tube, add 8 drops of urine.
ketones. presence in urine is abnormal, may indicate diabetes. albumin. presence is abnormal, may indicate kidney disease. protein. presence is abnormal, may indicate kidney disease. bilirubin ...
Urinalysis. urinalysis (UA), also known as routine and microscopy (R&M), is an array of tests performed on urine. part of a urinalysis can be performed by using urine test strips, in which the test results can be read as color changes. Another method is light microscopy of urine samples.
A urinalysis is also called a "urine test.". A urine test can include three parts: Visual exam. The urine will be looked at for color and clearness. Blood may make urine look red or the color of tea or cola. An infection may make urine look cloudy. Foamy urine can be a sign of kidney problems. Microscopic exam.
3 Urinalysis A urinalysis (UA), also known as routine and microscopy (R&M), is an array of tests performed on urine. A part of a urinalysis can be performed by using urine test strips, in which the test results can be read as color changes. Another method is light microscopy of urine samples. - The first part of a urinalysis is direct visual ...
Urinalysis Interpretation. 11. Urinalysis Interpretation. Urinalysis and urine sediment exam is most helpful with fresh urine whether voided or collected from clamped foley catheter tubing and collected from valve with syringe (within 30-60 minutes). Foley catheters can introduce hematuria due to insertion and urethra/bladder trauma.
Urinalysis is an important screening and diagnostic tool, but health professionals must know how to perform the test and interpret results correctly for it to be beneficial. The article comes with a self-assessmentenabling you to test your knowledge after reading it. Abstract. Analysing an individual's urine can be a useful way of detecting ...
Urinalysis with microscopy has proven to be an invaluable tool for the clinician. Urine dipstick testing and microscopy are useful for the diagnosis of several genitourinary and systemic ...
During download, if you can't get a presentation, the file might be deleted by the publisher. ... This lab will allow the student to test their own urine for color, pH, specific gravity, glucose, and protein. An addition to the lab will allow studetns to learn how diet, drugs, and disease alter kidney function. 415 views • 12 slides.
Urine Microscopic Examination is a laboratory test used to examine the physical, chemical, and microscopic characteristics of urine. The test helps to detect any abnormalities in the urine, which may indicate underlying health conditions. The procedure involves analyzing a small sample of urine under a microscope, which allows the technician to ...
Presentation Transcript. Urine Analysis. Urine • Urine is formed in the kidneys, is a product of ultrafiltration of plasma by the renal glomeruli. Collection of Urine • Early morning sample-qualitative • Random sample- routine • Midstream sample-UTI • Post prandial sample-D.M • 24hrs sample-preservatives like Toulene, thymol ...
Most people have been asked to submit the urine sample for testing when they visit a health institution. The sample is used to analyze various urine test types that can help the doctors diagnose disease or monitor a patient's progress.Urine elimination in the body is important as it helps maintain the body water balance as well as remove toxic substances produced during the metabolism process.
This free Urine Analysis Medical PPT is royalty free and easy to use. Features of this free Urine Analysis Medical PowerPoint template: Instant download. Absolutely free for medical and healthcare professionals. Premium quality free medical PowerPoint templates. Mac and Keynote compatible.
30. • When heavy sugar is found in the urine test for acetone is done. • 1) Take 3 cm of ammonium Sulphate crystals in a test tube. • 2) Add equal amount of urine to it. • 3) Put one crystal of sodium nitroprusside. • 4) Close the test tube with Cork and shake the test tube. • 5) Add 1.5 cm of concentrate ammonia • 6) Read the ...
Urine Culture: It examines the urine for any bacteria or growth of any micro-organism for a few days. It helps to prescribe the accurate antibiotics required for an infection. 24-hour Urine Test: An individual's urine is collected in different containers for 24 hours and stored in a cool place for evaluation. The healthcare provider can use the ...
A new, non-invasive urine test may help detect cervical changes that can lead to cancer if left untreated.. The test looks for proteins produced by a cancer-causing strain of the human papilloma virus called HPV16. Researchers tested it on women with different stages of cervical intraepithelial neoplasia (CIN), a precursor to cervical cancer.
For example, ERG and PCA3 are monitored in one commercial urine test, and are represented as a subset of the biomarkers measured in the 18-gene urine test, MPS2. ... Meeting Presentation: These data were presented in abstract form at the American Urological Association 2023 Annual Meeting; April 28, 2023; Chicago, IL. Previous Posting: This ...
The ELISA test detected E7 proteins in 80% of women with CIN1, 71% with CIN2, and 38% with CIN3, suggesting that the presence of E7 oncoproteins correlates with lower-grade CIN lesions.
In April, researchers at the University of Michigan published results with a test that screens for prostate cancer in urine samples. Called the MyProstateScore 2.0 (MPS2) test, it looks for 18 ...
Remove one strip from bottle and replace cap promptly. Inspect strip for any signs of deterioration prior to use. Completely immerse end of strip briefly into the freshly voided urine specimen and remove immediately. Tap edge of strip against edge of specimen container. The test strip must be inserted into the urine and removed _____. Click ...
A new urine test might help doctors more easily screen for cervical cancer, researchers report. The test looks for proteins generated by a type of cancer-causing human papillomavirus, HPV 16.
A urine analysis involves macroscopic examination of properties like volume, color, odor, pH and specific gravity. Microscopic examination analyzes cellular elements and crystals in sediment. Chemical analysis tests for proteins, glucose, ketones, blood, and other substances. Abnormal results can indicate issues with the kidneys, urinary tract ...
Post-Transplant, JC Virus DNA, Real-Time PCR, Urine - JC Polyoma Virus is the cause of Progressive Multifocal Leukoencephalopathy (PML), a severe demyelinating disease of the central nervous system. PML is a particular concern for individuals infected with the human immunodeficiency virus. Quantification of JC virus DNA is based upon the real-time PCR amplification and detection of JCV genomic ...
NIST and BSI discussed tailored health tests for Physical Entropy Sources including the health test requirements for SP 800-90B and AIS 20/31 for physical TRNGs. . An official website of the United States government ... Presentations 2023. Presentation. Tailored Health Tests for Physical Entropy Sources. September 20, 2023. Share to Facebook ...