Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts

Research articles

Transcription and DNA replication collisions lead to large tandem duplications and expose targetable therapeutic vulnerabilities in cancer
Yang et al. show that transcription–replication collisions lead to large tandem duplications, which are frequent in female-enriched, upper gastrointestinal tract and prostate cancers and are associated with poor survival and mutations in specific genes, such as CDK12 .
- Michelle L. Badura
- Lixing Yang

Single-cell transcriptomic landscape deciphers olfactory neuroblastoma subtypes and intra-tumoral heterogeneity
Yu and colleagues present a single-cell transcriptomic landscape of olfactory neuroblastoma, characterizing different tumor subtypes and cancer cell responses to drug treatments.
- Jingyi Yang
- Xiaole Song
- Hongmeng Yu
The pro-oncogenic noncanonical activity of a RAS•GTP:RanGAP1 complex facilitates nuclear protein export
Tripathi et al. identify a noncanonical RAS•GTP activity that increases XPO1-dependent export of nuclear proteins into the cytoplasm. This depends on a RAS–RanGAP1 complex, and this export function contributes to RAS oncogenic activity.
- Brajendra K. Tripathi
- Nicole H. Hirsh
- Douglas R. Lowy

Recapitulating the adenoma–carcinoma sequence by selection of four spontaneous oncogenic mutations in mismatch-repair-deficient human colon organoids
Mizutani et al. use a model that recapitulates the colon adenoma–carcinoma transition through the sequential elimination of media factors leading to spontaneous accumulation of hotspot mutations of mismatch-repair-deficient tumors.
- Tomohiro Mizutani
- Matteo Boretto
- Hans Clevers

Global loss of promoter–enhancer connectivity and rebalancing of gene expression during early colorectal cancer carcinogenesis
Snyder and colleagues show global loss of promoter–enhancer connectivity during early colorectal carcinogenesis and the dependency of gene dysregulation during this process on the baseline of promoter–enhancer interaction in normal colonic epithelium.
- Michael P. Snyder
Multiomic analysis of familial adenomatous polyposis reveals molecular pathways associated with early tumorigenesis
Snyder and colleagues present a comprehensive multiomic atlas of normal mucosal, benign polyps and dysplastic polyps from six persons with familial adenomatous polyposis, comprising transcriptomic, proteomic, metabolomic and lipidomic datasets.
- Edward D. Esplin
- Casey Hanson

Differential chromatin accessibility and transcriptional dynamics define breast cancer subtypes and their lineages
Ding and colleagues use bulk, single-cell and single-nucleus multi-omics together with spatial transcriptomics and multiplex imaging of clinical tumor samples to characterize gene expression and chromatin accessibility of breast cancer lineages.
- Michael D. Iglesia
- Reyka G. Jayasinghe
Selenocysteine tRNA methylation promotes oxidative stress resistance in melanoma metastasis
Nease and colleagues demonstrate that the methyltransferase FTSJ1 mediates selenocysteine-specific tRNA methylation of selenoproteins, which protects melanoma cells against oxidative stress and promotes metastasis.
- Leona A. Nease
- Kellsey P. Church
- Elena Piskounova
Two distinct epithelial-to-mesenchymal transition programs control invasion and inflammation in segregated tumor cell populations
Youssef et al. study epithelial-to-mesenchymal transition (EMT) programs in breast cancer and identify two interdependent trajectories coexisting within tumors, with EMT transcription factors such as PRRX1 at their nexus.
- Khalil Kass Youssef
- Nitin Narwade
- M. Angela Nieto
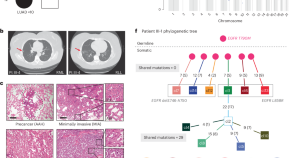
Developmental mosaicism underlying EGFR -mutant lung cancer presenting with multiple primary tumors
Burr et al. analyze multiple primary EGFR -mutant lung tumors from ten patients and define developmental mosaicism as a mechanism underlying multiple tumor presentation in this setting.
- Ignaty Leshchiner
- Daniel A. Haber
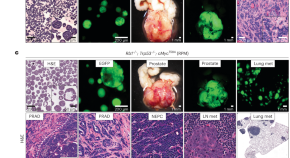
The neuroendocrine transition in prostate cancer is dynamic and dependent on ASCL1
Sawyers and colleagues describe an in vivo platform used to explore the dynamics and key factors of neuroendocrine lineage transformation. They find that Ascl1 depletion blocks plasticity and leads to cancer that is sensitive to castration.
- Rodrigo Romero
- Charles L. Sawyers
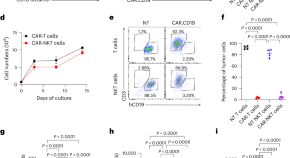
CAR-redirected natural killer T cells demonstrate superior antitumor activity to CAR-T cells through multimodal CD1d-dependent mechanisms
Dotti and colleagues show that chimeric antigen receptor (CAR) natural killer T cells have superior antitumor activity compared with CAR-T cells, mediated through the elimination of CD1d-expressing tumor-associated macrophages, activation of dendritic cells and promotion of endogenous T cell responses.
- Gianpietro Dotti
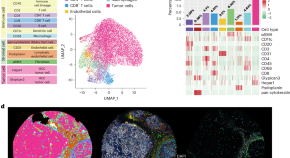
Spatial single-cell protein landscape reveals vimentin high macrophages as immune-suppressive in the microenvironment of hepatocellular carcinoma
Qui et al. perform co-detection by indexing profiling of 401 hepatocellular carcinoma patient samples and identify a role for vimentin high macrophages in instructing an immune-suppressive microenvironment by enhancing the suppressive activity of regulatory T cells via interleukin-1β.
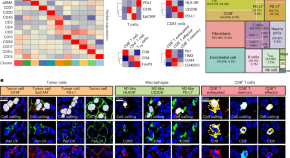
Spatial analysis reveals targetable macrophage-mediated mechanisms of immune evasion in hepatocellular carcinoma minimal residual disease
Dhanasekaran and colleagues study minimal residual disease in hepatocellular carcinoma using single-cell spatial transcriptomic and proteomic analysis and find a targetable role for immunosuppressive macrophages.
- Lea Lemaitre
- Nia Adeniji
- Renumathy Dhanasekaran
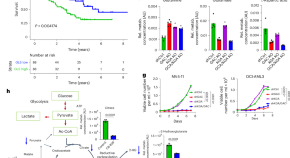
Glutaminase inhibition in combination with azacytidine in myelodysplastic syndromes: a phase 1b/2 clinical trial and correlative analyses
DiNardo et al. perform a phase 1b/2 clinical trial of telaglenastat (CB-839) in combination with azacytidine in persons with advanced myelodysplastic syndromes and report on the treatment safety and efficacy, including a definition of clinical responders.
- Courtney D. DiNardo
- Divij Verma
- Marina Konopleva
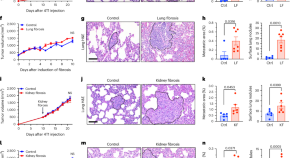
Vascular heterogeneity of tight junction Claudins guides organotropic metastasis
Kalluri and colleagues use mammary carcinoma models to study the causes of metastatic organotropism and find an organ-specific role for angiopoietin 2 in driving lung metastasis through the suppression of the tight junction protein Claudin 5.
- Xunian Zhou
- Valerie S. LeBleu
- Raghu Kalluri


ISB 2001 trispecific T cell engager shows strong tumor cytotoxicity and overcomes immune escape mechanisms of multiple myeloma cells
Perro and colleagues develop a CD3 + T cell engager co-targeting BCMA and CD38 to improve immunotherapy for multiple myeloma, demonstrate cytotoxicity in patient-derived samples and murine models and develop a quantitative systems pharmacology model.
- Laura Carretero-Iglesia
- Olivia J. Hall
- Maria Pihlgren
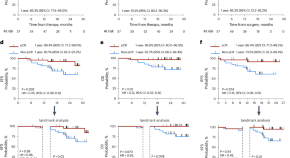
Neoadjuvant gemcitabine–cisplatin plus tislelizumab in persons with resectable muscle-invasive bladder cancer: a multicenter, single-arm, phase 2 trial
Li et al. perform a phase 2 single-arm clinical trial of neoadjuvant chemotherapy plus checkpoint blockade in participants with resectable muscle-invasive bladder cancer and conduct genomic and transcriptomic profiling to describe molecular subtypes.
- Wenlong Zhong
- Tianxin Lin
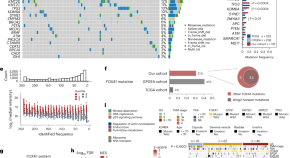
Integrative proteogenomic profiling of high-risk prostate cancer samples from Chinese patients indicates metabolic vulnerabilities and diagnostic biomarkers
Dong et al. present an integrative proteogenomic analysis of high-risk prostate cancer samples from a cohort of Chinese patients and highlight potential therapeutic vulnerabilities and diagnostic markers.
- Baijun Dong
A protein expression atlas on tissue samples and cell lines from cancer patients provides insights into tumor heterogeneity and dependencies
Liang and colleagues establish a high-quality protein expression resource for 8,000 The Cancer Genome Atlas patient samples and 900 Cancer Cell Line Encyclopedia cell lines for approximately 450 proteins, which they use to identify synthetic lethality pairs and metastasis markers.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
NIH-funded scientists uncover clues to precancer and tumor biology
- Posted: October 30, 2024
240-760-6600
The Human Tumor Atlas Network seeks to understand the molecular features of tumors and their microenvironments.
New insights from multiple studies provide critical information on how tumors develop, spread, and respond to treatments. The 10 studies from the Human Tumor Atlas Network (HTAN) , a National Institutes of Health (NIH)-funded Cancer Moonshot℠ initiative to construct three-dimensional maps of human tumors, will be published Oct. 31, 2024, across several Nature journals.
Several studies explore the role of the tumor microenvironment and the immune system in promoting the spread of cancer and its resistance to treatment. Three studies map the trajectory of precancerous colorectal tissues toward cancer by measuring the contributions of multiple molecular and cellular events. Multiple new HTAN papers describe the development of innovative single-cell technology and analysis platforms. An accompanying research briefing by W. Kimryn Rathmell, M.D., Ph.D., director of NIH’s National Cancer Institute (NCI), and Dinah Singer, Ph.D., NCI deputy director for scientific strategy and development, discusses the history, progress, and future of HTAN.
Launched in 2018, HTAN constructs three-dimensional maps of human tumors that capture their molecular features and surrounding microenvironments over time. The work is being done by teams of investigators from research institutions across the country using a variety of technologies and computational approaches to study tumors at the single-cell level. This comprehensive, publicly available resource aims to help researchers better understand the development and progression of cancer to inform its prevention and treatment. The first tumor atlas studies from this initiative were published in 2020 and 2021.
Shannon Hughes, Ph.D., Division of Cancer Biology, NCI; Sean Hanlon, Ph.D., Center for Strategic Scientific Initiatives, NCI; Sudhir Srivastava, Ph.D., M.P.H., Division of Cancer Prevention, NCI
The Studies
The collection of studies is available at https://www.nature.com/collections/fihchcjehc .
About the National Cancer Institute (NCI): NCI leads the National Cancer Program and NIH’s efforts to dramatically reduce the prevalence of cancer and improve the lives of cancer patients and their families, through research into prevention and cancer biology, the development of new interventions, and the training and mentoring of new researchers. For more information about cancer, please visit the NCI website at cancer.gov or call NCI’s contact center, the Cancer Information Service, at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit nih.gov .
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Recent developments in cancer research: Expectations for a new remedy
Qingjiang hu, yuta kasagi, masaki mori.
- Author information
- Article notes
- Copyright and License information
Correspondence , Koji Ando, Department of Surgery and Science, Kyushu University, 3‐1‐1 Maidashi, Higashi‐ku, Fukuoka, Japan. Email: [email protected]
Corresponding author.
Revised 2020 Dec 18; Received 2020 Oct 30; Accepted 2021 Jan 20; Collection date 2021 Jul.
This is an open access article under the terms of the http://creativecommons.org/licenses/by/4.0/ License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Cancer research has made remarkable progress and new discoveries are beginning to be made. For example, the discovery of immune checkpoint inhibition mechanisms in cancer cells has led to the development of immune checkpoint inhibitors that have benefited many cancer patients. In this review, we will introduce and describe the latest novel areas of cancer research: exosomes, microbiome, immunotherapy. and organoids. Exosomes research will lead to further understanding of the mechanisms governing cancer proliferation, invasion, and metastasis, as well as the development of cancer detection and therapeutic methods. Microbiome are important in understanding the disease. Immunotherapy is the fourth treatment in cancer therapy. Organoid biology will further develop with a goal of translating the research into personalized therapy. These research areas may result in the creation of new cancer treatments in the future.
Keywords: exosomes, immunotherapy, microbiome, organoid
Cancer research has made remarkable progress and new discoveries are beginning to be made. In this review, we will introduce and describe the latest novel areas of cancer research: exosomes, microbiomes, immunotherapy, and organoids.
1. INTRODUCTION
The cancer research field has developed significantly through use of new equipment and technology. One example of new technology is Next‐Generation Sequencing (NGS). Also known as high‐throughput sequencing, NGS is the catch‐all term used to describe a number of different modern nucleic acid sequencing technologies. These methods allow for much quicker and cheaper sequencing of DNA and RNA compared with the previously used Sanger sequencing, and as such have revolutionized the study of genomics and molecular biology. NGS also allows for easier detection of mutations in cancer samples, leading to development of many new agents that can be used to treat patients. For example, if the RAS gene status is detected as wild type in a colorectal cancer patient, then an anti‐EGFR antibody, such as cetuximab or panitumumab, can be used for treatment.
A liquid biopsy, also known as fluid biopsy or fluid phase biopsy, is the sampling and analysis of non‐solid biological tissue, primarily blood. 1 It is being used as a novel way to detect cancer. Like a traditional biopsy, this type of technique is mainly used as a diagnostic and monitoring tool for diseases, and also has the added benefit of being largely noninvasive. Therefore, liquid biopsies can be performed more frequently, allowing for better tracking of tumors and mutations over a duration of time. This technique may also be used to validate the effectiveness of a cancer treatment drug by taking multiple liquid biopsy samples in the span of a few weeks. It may also prove to be beneficial for monitoring relapse in patients after treatment.
Novel devices and drugs have also been developed and used for cancer treatment. For surgery procedures, robotic‐assisted laparoscopic surgery has evolved and made it possible to visualize the fine movement of the forceps in three dimensions. This method is now used in esophageal, gastric, and rectal cancer surgeries in Japan. 2 , 3 , 4
Recently, immunotherapy became an additional method for treating cancer patients. The discovery of the immune checkpoint by Dr Honjo led to the development of immune checkpoint inhibitors. 5 Despite these developments, gastrointestinal cancers are still a major problem in need of new treatment methods. In this review, we introduce and describe four new areas of cancer research that may contribute to cancer treatment in the future: exosomes, microbiome, immunotherapy, and organoids.
2. AN APPLICATION OF EXOSOME RESEARCH IN CANCER THERAPY
An exosome is a small particle that is secreted by cells. Its size can range from 50 to 150 nm and has a surface consisting of proteins and lipids that originate from the cell membrane. Additionally, proteins and nucleic acids, such as DNA, microRNAs, and mRNAs, can be found inside the exosome as its “cargo.” 6 Recently, many researchers have discovered that exosomes are involved in the mechanisms of various diseases. As mentioned above, various functional compounds, such as microRNAs, mRNAs, and proteins, can be contained within exosomes. 7 , 8 Many cells use secretion of exosomes to communicate with one another, and these exosomes can even reach distant cells. Cancer cells can also secrete exosomes that contain molecules beneficial to cancer growth. For example, microRNAs found in cancer exosomes can modulate gene expression to induce angiogenesis in the tumor microenvironment, which supports metastasis. 9 Exosomes released from cancer cells can also reportedly break the blood‐brain barrier, which makes it contribute to brain metastasis. 10 , 11 Cancer cells themselves are similarly affected by the exosomes secreted by the surrounding normal cells. 12 In one case, the exosomes secreted by bone marrow‐delivered mesenchymal stem cells can force cancer cells into a dormant state. 13 These dormant cancer cells become resistant to chemotherapy and are involved in long‐term disease recurrence. Thus, exosomes are deeply involved in cancer proliferation, invasion, and metastasis, as well as in the formation of the tumor microenvironment and pre‐metastatic niche. 13 Further research on cancer‐related exosomes is ongoing.
Knowledge of exosomes can be applied to cancer treatment. If the secretion of exosomes from cancer cells can be prevented, then signal transduction supporting the formation of the tumor microenvironment and pre‐metastatic niche can be blocked. Work focusing on the removal of cancer exosomes is now ongoing. 14
Exosomes can also be utilized for cancer diagnosis. Exosomes secreted by many cell types are found in various body fluids, such as blood and urine. Capturing and analyzing exosomes from cancer cells can be used to detect the presence of disease. 15 Obtaining blood or urine from patients is not very invasive or painful. Since many molecules, such as various proteins, DNA, and microRNAs, can be found in exosomes from normal cells, it is important to distinguish them from cancer‐related ones. If exosomes are to be used for cancer diagnosis, then specific biomarkers need to be discovered. Additionally, the development of a method to detect these exosomes must be done. Currently, exosome detection methods for exosomes abundantly found in the serum of colorectal and pancreatic cancer patients, as well as exosomes found in the urine of bladder cancer patients, are being developed. 16 , 17 Thus, further understanding of the mechanisms governing cancer proliferation, invasion, and metastasis, as well as the development of cancer detection and therapeutic methods, is significantly affected by exosome research.
3. MICROBIOME IN CANCER RESEARCH
A large number of microorganisms inhabit the human body. These microorganisms include bacteria, viruses, and fungi. Among them, bacteria have the most important relationship with the human body. Bacteria can live anywhere within the human body, including the digestive tract, respiratory system, and oral cavity. 18 , 19 , 20 In particular, bacteria in the digestive tract are rich in type and number, 21 with possibly 1000 types and more than 100 trillion individual bacterial cells present. 22 , 23 The overall population of various bacteria found in the human intestine is referred to as the “intestinal flora.” Recently, the terms “microbiota” or “microbiome” have also been widely used.
Recent advancements with NGS have led to a much more precise understanding of the intestinal microbiome. 24 The bacteria in the human microbiome mainly belong to four phyla: Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteri. Of these, Firmicutes and Bacteroidetes are the most dominant species. It is reported that microbiome vary depending on age and race. 25 , 26 Dysbiosis is a condition in which the diversity of the microbiome is reduced. Dysbiosis is reportedly involved in various diseases such as inflammatory bowel disease, colorectal cancer, obesity, diabetes, and allergic diseases. 27 , 28 , 29 For example, bacteria such as Atopobium parvulum and Actinomyces odontolyticus increase in number during the early stages of colorectal cancer (adenomas or intramucosal cancers) and decrease in number during cancer progression. 30 This suggests that a specific microbiome is associated with early stages of colorectal cancer development, which may be useful knowledge for early cancer detection.
Various studies have also been conducted to elucidate the relationship between the microbiome and the human immune system. 31 The IgA antibody, which is one of the most important elements in the intestinal immune system, is believed to play a role in the elimination of pathogens and maintenance of the intestinal environment. The IgA antibody recognizes, eliminates, and neutralizes pathogenic bacteria and toxins. It also maintains a symbiotic relationship by recognizing and binding to the normal microbiome of the host. 32 Mice lacking a microbiome have reduced production of the IgA antibody. A microbiome is required for IgA antibody differentiation. Recent studies have identified W27IgA antibodies that have the ability to bind to various bacteria. 33 Oral administration of a W27IgA antibody to enteritis model mice suppressed enteritis by altering the microbiome. This W27IgA antibody can recognize a part of the amino acid sequence of serine hydroxymethyl transferase, which is a metabolic enzyme involved in bacterial growth. The W27IgA antibody can suppress the growth of E coli by binding to them. However, the W27IgA antibody does not bind to bacteria that suppress enteritis, such as bifidobacteria and lactic acid bacteria. 33 Thus, the microbiome is deeply involved in human intestinal immunity. Recently, it is having been established that the microbiome is not only involved in intestinal immunity, but also in the systemic immune system.
As the analysis of the microbiome progresses, the pathophysiology of various diseases, such as cancers, and its relationship with the regulatory function of the human immune system will be further elucidated. It has been demonstrated that F nucleatum plays a role in the development and progression of colon adenomas and colorectal cancer. It is also related to lymph node metastases and distant metastasis. 34 , 35 Also, microbiome is associated with hepatocellular carcinoma. 36 Studying microbiome will give us some clue in the development and remedy for gastrointestinal cancers (Table 1 ).
Gastrointestinal cancer and their related microbiome
4. THE RISE OF IMMUNOTHERAPY IN CANCER TREATMENT
For many years, surgery, chemotherapy, and radiation therapy were the main methods of cancer treatment. In addition to these therapies, immunotherapy has recently attracted great attention worldwide (Table 2 ). 37 , 38 Under normal circumstances, a cancer antigen will activate the patient's immune system to attack the cancer cells. However, sometimes the immune system does not recognize the cancer cells as non‐self, or it simply fails to attack them. This can result in the development and progression of cancer.
Immune checkpoint inhibitors
Although therapies that activate the immune system against cancer cells have been studied for a long time, the use of the patient's own immune system for cancer treatment was not established. Recently, the effectiveness of both immune checkpoint inhibition therapy and chimeric antigen receptor (CAR)‐T cell therapy has proved to be promising. 39 , 40 Immunotherapy has moved to the forefront of cancer treatment strategies.
There are two major reasons why proving the efficacy of cancer immunotherapies was difficult for some time. First, cancer immunity is strongly suppressed. Signal transduction from immune checkpoint compounds, such as PD‐1 and CTLA4, strongly inhibits cytotoxic T cells (CTLs). 38 This checkpoint mechanism can prevent the immune system from attacking cancer cells. The development of immune checkpoint inhibitors has arisen from the discovery of this mechanism. Inhibition of immune checkpoint molecules with neutralizing antibodies can release the suppression of cancer‐specific CTLs, activate immunity, and promote cancer elimination. The effectiveness of immune checkpoint antibodies has been confirmed and clinically applied to many solid cancers such as melanoma, 41 lung cancer, 42 urothelial cancer, 43 gastric cancer, 44 and esophageal cancer. 45 In addition to PD‐1 and CTLA4, new immune checkpoint molecules, such as LAG3, TIGIT, and SIRPA, are also being actively studied. 46 , 47 , 48 Although this therapy is promising, the cancer cases who respond to these therapies are limited. This is because use of this therapy requires the presence of cancer‐specific CTLs in the patient's body. To maximize the therapeutic effect, it is desirable to select appropriate cases and develop useful biomarkers.
The second difficulty for immunotherapy is that T cells do not recognize specific cancer cell antigens and immune accelerators are too weak. One goal of CAR‐T cell therapy is to strengthen the immune accelerator by administering CTLs to the patient's body that recognize specific cancer cell‐specific antigens. A CAR is prepared by fusing a single chain Fv (scFv), derived from a monoclonal antibody that recognizes a specific antigen expressed by cancer cells, with CD3z and costimulatory molecules (CD28, 4‐1BB, and others). Next, the CAR is introduced to the T cells obtained from a cancer patient and CAR‐T cells are made. CAR‐T cells recognize the specific antigen of the cancer cells and are activated to damage these cells. CAR‐T cells recognize cancer‐specific antigens with high antibody specificity and attack the respective cancer cells with strong cytotoxic activity and high proliferative activity. CAR‐T therapy is effective in blood cancers such as B‐cell acute lymphoblastic leukemia and myeloma. 49 , 50 While CAR‐T cell therapy has a high therapeutic effect, a frequent and serious adverse event called cytokine release syndrome has been observed in some patients. 51 , 52 The development of a technique for suppressing the occurrence of cytokine release syndrome is anticipated. In addition, the development of CAR‐T cell therapies for solid tumors is ongoing.
Recently, there was new progress made in treating gastrointestinal cancer patients. For MSI‐H colorectal cancer, the combination therapy with nivolumab and ipilimumab was approved. From the nivolumab plus ipilimumab cohort of CheckMate‐142, progression‐free survival rates were 76% (9 months) and 71% (12 months); respective overall survival rates were 87% and 85% which were quite high. This new treatment will benefit MSI‐H colorectal cancer patients. 53
Thus, it is expected that further understanding of cancer immune mechanisms and the development of various immunotherapies will contribute to great progress in cancer treatment.
One problem for immunotherapy is that there is no certain predictive biomarker. It was thought that the expression of PD‐1 or PD‐L1 would predict the effect. However, this was not the case. To find a new biomarker, we assessed the cytolytic activity (CYT) score. The CYT score is a new index of cancer immunity calculated from the mRNA expression levels of GZMA and PRF1. We are now evaluating CYT score in gastric cancer patients (data not published). The development in the biomarker search will benefit many gastrointestinal cancer patients.
5. ADVANTAGES FOR USING ORGANOIDS IN CANCER RESEARCH
The three‐dimensional (3D) organoid system is a cell culture‐based, novel, and physiologically relevant biologic platform. 54 An organoid is a miniaturized and simplified version of an organ that is produced in vitro in 3D and shows realistic microanatomy. With only one to a few cells isolated from tissue or cultured cells as the starting material, organoids are grown and passaged in a basement membrane matrix, which contributes to their self‐renewal and differentiation capacities. 54 , 55 The technique used for growing organoids has rapidly improved since the early 2010s with the advent of the field of stem cell biology. The characteristics of stem, embryonic stem cells (ES cells), or induced pluripotent stem cells (iPS cells) that allow them to form an organoid in vitro are also found in multiple types of carcinoma tissues and cells. Therefore, cancer researchers have applied ES cells or iPS cells in their field. 56 , 57 , 58
Organoid formation generally requires culturing stem cells or their progenitor cells in 3D. 54 , 55 The morphological and functional characteristics of various types of carcinoma tissue have been recapitulated in organoids that were generated from single‐cell suspensions or cell aggregates. These suspensions or aggregates were isolated from murine and human tissues or cultured cells, as well as from cancer stem cells propagated in culture. The structures of the organoids show the potential of cancer stem cell self‐renewal, proliferation, and differentiation abilities, and also provide insights into the roles of molecular pathways and niche factors that are essential in cancer tissues. 56 , 57 , 59 , 60 , 61 , 62 The organoid system also has been utilized for studying multiple biological processes, including motility, stress response, cell‐cell communications, and cellular interactions that involve a variety of cell types such as fibroblasts, endothelial cells, and inflammatory cells. These interactions are mediated via cell surface molecules, extracellular matrix proteins, and receptors in the microenvironment under homeostatic and pathologic conditions.
Although the organoid system is a complex and not effortless procedure that requires specific media, supplements, and many tricky techniques, 58 , 63 application of this system has been extended to a variety of cell types from different carcinomas (colorectal, pancreatic, prostate, breast, ovary, and esophageal cancers). 56 , 57 , 59 , 60 , 61 An organoid is generally induced within a few days to weeks, and is faster and less costly than the murine xenograft assay. Furthermore, applying novel genetic manipulations (e.g. CRISPR‐Cas9) can be carried out in the organoid system. 64 , 65
Kasagi et al modified keratinocyte serum‐free medium to grow 3D organoids from endoscopic esophageal biopsies, immortalized human esophageal epithelial cells, and murine esophagi. Esophageal 3D organoids serve as a novel platform to investigate regulatory mechanisms in squamous epithelial homeostasis in the context of esophageal cancers. 64
We anticipate that many experimental results that utilize the organoid system will be published in the future.
The 3D organoid system has emerged in the past several years as a robust tool in basic research with the potential to be used for personalized medicine. 66 By passaging dissociated primary structures to generate secondary 3D organoids, this system can be performed using live tissue pieces obtained from biopsies, operative‐resected specimens, or even frozen tissues. This method has the potential to transform personalized therapy. For example, in the case of cancer recurrence, an effective chemotherapy can be selected by testing the chemotherapeutic sensitivity of cancer‐derived organoids from an individual patient's tissue stocks. In many cases, a patient's organoid accumulation is helpful for testing the sensitivity of novel therapeutic agents for treating carcinoma. 66 Hence, it appears that organoid biology will further develop with a goal of translating the research into personalized therapy.
6. SUMMARY AND FUTURE DIRECTIONS
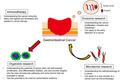
This review describes four new cancer‐related studies: exosomes, microbiome, immunotherapy, and organoids (Figure 1 ).
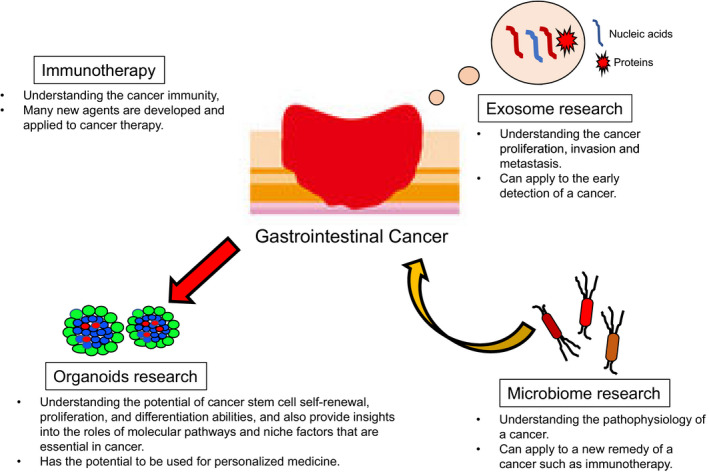
The summary of the four cancer research areas. In this figure the summary of the four cancer research areas is shown: exosome, microbiome, immunotherapy, and organoid research
Since exosomes are released in blood or urine, if the capturing system is established, it will be a less invasive test to diagnose cancer. In the present, the presence of circulating tumor DNA (ctDNA) is one of the tools to detect the minimal residual disease. However, since ctDNA is only DNA, it is difficult to spread to cancer research. In that respect, as exosomes include not only DNA but also other nucleic acids and proteins, this will be a new tool for cancer research such as the diagnosis of early cancer.
Microbiome may lead to improved cancer diagnosis and treatment. Detecting a specific microbiome in a gastrointestinal tract may predict a specific cancer. And changing microbiome in some way may result in preventing cancer development.
Organoids may help address the problem of drug resistance, and also lead to the development of personalized therapy. However, producing organoids takes time and testing the drug resistance may take more time. If we could overcome these problems, the research into organoids can contribute to overcoming cancer.
As shown in Table 3 , many new studies and findings are reported into this field of research. These four novel cancer research areas will make many contributions to the diagnosis and treatment of cancer.
Recent studies on exosome, microbiome, immunotherapy, and organoids
Conflict of Interest: All the authors have no conflict of interest to disclose.
ACKNOWLEDGMENTS
We thank Dr Hirofumi Hasuda and Dr Naomichi Koga for their help in preparing this manuscript. We also thank J. Iacona, PhD, from Edanz Group for editing a draft of this manuscript.
Ando K, Hu Q, Kasagi Y, Oki E, Mori M. Recent developments in cancer research: Expectations for a new remedy. Ann Gastroenterol Surg. 2021;5:419–426. 10.1002/ags3.12440
- 1. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer‐genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472–84. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Ishihara S, Otani K, Yasuda K, Nishikawa T, Tanaka J, Tanaka T, et al. Recent advances in robotic surgery for rectal cancer. Int J Clin Oncol. 2015;20(4):633–40. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Seto Y, Mori K, Aikou S. Robotic surgery for esophageal cancer: Merits and demerits. Ann Gastroenterol Surg. 2017;1(3):193–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Terashima M, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Miki Y, et al. Robotic surgery for gastric cancer. Gastric Cancer. 2015;18(3):449–57. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Villarroya‐Beltri C, Baixauli F, Gutierrez‐Vazquez C, Sanchez‐Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2(1):20424. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, et al. Exosome‐derived miR‐130a activates angiogenesis in gastric cancer by targeting C‐MYB in vascular endothelial cells. Mol Ther. 2018;26(10):2466–75. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] [ Retracted ]
- 10. Cai Q, Zhu A, Gong L. Exosomes of glioma cells deliver miR‐148a to promote proliferation and metastasis of glioblastoma via targeting CADM1. Bull Cancer. 2018;105(7–8):643–51. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Lu Y, Chen L, Li L, Cao Y. Exosomes derived from brain metastatic breast cancer cells destroy the blood‐brain barrier by carrying lncRNA GS1‐600G8.5. Biomed Res Int. 2020;2020:7461727. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R‐U, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Moloudizargari M, Asghari MH, Abdollahi M. Modifying exosome release in cancer therapy: How can it help? Pharmacol Res. 2018;134:246–56. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Li W, Li C, Zhou T, Liu X, Liu X, Li X, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16(1):145. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Bu H, He D, He X, Wang K. Exosomes: Isolation, analysis, and applications in cancer detection and therapy. ChemBioChem. 2019;20(4):451–61. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, et al. Ultra‐sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. D'Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451(Pt A):97–102. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Le Bars P, Matamoros S, Montassier E, Le Vacon F, Potel G, Soueidan A, et al. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Can J Microbiol. 2017;63(6):475–92. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15(5):259–70. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Davenport ER, Sanders JG, Song SJ, Amato KR, Clark AG, Knight R. The human microbiome in evolution. BMC Biol. 2017;15(1):127. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Ley RE, Gewirtz AT. Corralling colonic flagellated microbiota. N Engl J Med. 2016;375(1):85–7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Wang WL, Xu SY, Ren ZG, Tao L, Jiang JW, Zheng SS. Application of metagenomics in the human gut microbiome. World J Gastroenterol. 2015;21(3):803–14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Human Microbiome Project Consortium . A framework for human microbiome research. Nature. 2012;486(7402):215–21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Chen J, Ryu E, Hathcock M, Ballman K, Chia N, Olson JE, et al. Impact of demographics on human gut microbial diversity in a US Midwest population. PeerJ. 2016;4:e1514. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao J‐Z, et al. Age‐related changes in gut microbiota composition from newborn to centenarian: a cross‐sectional study. BMC Microbiol. 2016;16:90. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Gagniere J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22(2):501–18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Imhann F, Vich Vila A, Bonder MJ, Fu J, Gevers D, Visschedijk MC, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67(1):108–19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage‐specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968–76. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Pabst O, Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13(1):12–21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Okai S, Usui F, Yokota S, Hori‐i Y, Hasegawa M, Nakamura T, et al. High‐affinity monoclonal IgA regulates gut microbiota and prevents colitis in mice. Nat Microbiol. 2016;1(9):16103. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–88. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Mima K, Nakagawa S, Sawayama H, Ishimoto T, Imai K, Iwatsuki M, et al. The microbiome and hepatobiliary‐pancreatic cancers. Cancer Lett. 2017;402:9–15. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. McCune JS. Rapid advances in immunotherapy to treat cancer. Clin Pharmacol Ther. 2018;103(4):540–4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Zou F, Lu L, Liu J, Xia B, Zhang W, Hu Q, et al. Engineered triple inhibitory receptor resistance improves anti‐tumor CAR‐T cell performance via CD56. Nat Commun. 2019;10(1):4109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Larkin J, Chiarion‐Sileni V, Gonzalez R, Grob J‐J, Rutkowski P, Lao CD, et al. Five‐year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Hellmann MD, Paz‐Ares L, Bernabe Caro R, Zurawski B, Kim S‐W, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in advanced non‐small‐cell lung cancer. N Engl J Med. 2019;381(21):2020–31. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open‐label, two‐stage, multi‐arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Kang Y‐K, Boku N, Satoh T, Ryu M‐H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro‐oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Kato K, Cho BC, Takahashi M, Okada M, Lin C‐Y, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–17. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6(6):457–64. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Huang C‐T, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG‐3 in regulatory T cells. Immunity. 2004;21(4):503–13. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Yu X, Harden K, C Gonzalez L, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor‐modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Hosen N, Matsunaga Y, Hasegawa K, Matsuno H, Nakamura Y, Makita M, et al. The activated conformation of integrin beta7 is a novel multiple myeloma‐specific target for CAR T cell therapy. Nat Med. 2017;23(12):1436–43. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, et al. Lymphoma remissions caused by anti‐CD19 chimeric antigen receptor T cells are associated with high serum interleukin‐15 levels. J Clin Oncol. 2017;35(16):1803–13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Wei J, Liu Y, Wang C, Zhang Y, Tong C, Dai G, et al. The model of cytokine release syndrome in CAR T‐cell treatment for B‐cell non‐Hodgkin lymphoma. Signal Transduct Target Ther. 2020;5(1):134. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Overman MJ, Lonardi S, Wong KYM, Lenz H‐J, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair‐deficient/microsatellite instability‐high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Sato T, Clevers H. SnapShot: growing organoids from stem cells. Cell. 2015;161(7):1700–e1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–38. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Gao D, Vela I, Sboner A, Iaquinta P, Karthaus W, Gopalan A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176–87. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. van de Wetering M, Francies H, Francis J, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–45. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell‐ and patient‐derived tumor organoids. Nat Med. 2015;21(11):1364–71. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Kijima T, Nakagawa H, Shimonosono M, Chandramouleeswaran PM, Hara T, Sahu V, et al. Three‐dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol Gastroenterol Hepatol. 2019;7(1):73–91. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7(5):462–77. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, et al. Long‐term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141(5):1762–72. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Kasagi Y, Chandramouleeswaran PM, Whelan KA, Tanaka K, Giroux V, Sharma M, et al. The esophageal organoid system reveals functional interplay between notch and cytokines in reactive epithelial changes. Cell Mol Gastroenterol Hepatol. 2018;5(3):333–52. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Matano M, Date S, Shimokawa M, Takano AI, Fujii M, Ohta Y, et al. Modeling colorectal cancer using CRISPR‐Cas9‐mediated engineering of human intestinal organoids. Nat Med. 2015;21(3):256–62. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Fujii M, Shimokawa M, Date S, Takano AI, Matano M, Nanki K, et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18(6):827–38. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (447.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
