
- Sign in / Register
- Administration
- Edit profile

The PhET website does not support your browser. We recommend using the latest version of Chrome, Firefox, Safari, or Edge.
- Random article
- Teaching guide
- Privacy & cookies


States of matter
by Chris Woodford . Last updated: July 26, 2023.
What makes something solid, liquid, or gas?
Inside matter, changing from one state to another, the kinetic theory of matter, what is absolute zero, why are solids, liquids, and gases so different.
Photo: Solid properties: All these things are solids, but they have very different physical properties. Steel (can, left) is strong, resists heat well, conducts electricity, and melts at a high temperature. Rubber (tennis ball, top) is stiff, stretchy, melts at a low temperature, and doesn't conduct electricity. Plastic (bottle, right) is fairly strong, squashes when you push it but doesn't usually return to its original shape, melts at a low temperature, and doesn't conduct electricity or heat. Artificial sponge (top right) is similar to rubber but much weaker and less durable.
What about plasma?
Photo: A plasma "sphere" is a completely sealed glass bowl (or, in this case, cylinder) containing hot, ionized gas produced with the help of electricity . If you place your hands on the glass, they attract the free electrons in the plasma, so it swirls about in response to your touch! Photo by John Suits courtesy of US Navy and Wikimedia Commons .
Are there any other states of matter?
If you liked this article..., find out more, on this website.
- Refrigerators
For younger readers
- States of Matter (Science in a Flash) by Georgia Amson-Bradshaw. Hachette, 2020. A visually engaging, 32-page introduction for ages 8–11, which includes highlighted cool facts, activities, and quizzes.
- The Solid Truth about States of Matter with Max Axiom, Super Scientist by Agnieszka Jòzefina Biskup. Capstone, 2019. A 32-page, graphic-style introduction (with a linked app) for ages 9–12.
- Experiments with Solids, Liquids, and Gases by Christine Taylor-Butler. Scholastic, 2011. A hands-on exploration of states of matter, suitable for ages 8–10; grades 3–5.
- Many Kinds of Matter (A Look at Solids, Liquids, and Gases) by Jennifer Boothroyd. Lerner, 2010. A very simple 32-page outline for ages 6–9, grades 1–3. It covers the basic states and changing between them, with clear, real-world examples.
- States of Matter (Why Chemistry Matters) by Lynnette Brent. Crabtree, 2009. Also designed for ages 8–10, grades 3–5, this book covers the basics, then goes on to look at why matter has properties like elasticity.
For older readers
- Six Easy Pieces by Richard P. Feynman. Penguin, 1998. These excerpts from the famous Feynman lectures are hardly "easy," unless you already understand them from elsewhere. However, chapter 1 is a simple introduction to solids, liquids, and gases from an atomic point of view.
- Absolute Zero and the Conquest of Cold by Tom Shachtman. Houghton Mifflin Harcourt, 2000. The scientific quest for colder than colder!
- States of Matter by David L. Goodstein. Courier Dover, 2014 (reissue of the 1985 edition). A more detailed book about statistical physics, thermodynamics, and kinetic theory for undergraduates.
Text copyright © Chris Woodford 2009, 2021. All rights reserved. Full copyright notice and terms of use .
Rate this page
Tell your friends, cite this page, more to explore on our website....
- Get the book
- Send feedback
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Collections
- Sustainability in chemistry
- Simple rules
- Teacher well-being hub
- Women in chemistry
- Global science
- Escape room activities
- Decolonising chemistry teaching
- Teaching science skills
- Post-lockdown teaching support
- Get the print issue
- RSC Education

- More from navigation items
How to teach states of matter and particle theory

- No comments
David Paterson suggests ideas, activities and resources for your classroom
Source: © Getty Images
How does the knowledge of the fundamental states of matter help us make better ice cream ?
Simply mixing cream, sugar and egg yolk and sticking it in the freezer isn’t enough. Careful control of the mixing and freezing process leads to a material with all three states of matter – a foam and an oil-in-water emulsion. The taste of ice cream comes partially from your taste buds on your tongue, but also due to evaporation of volatile compounds reaching your smell receptors. Ice cream really does taste better on a warm day!

In your class
Download integrated instructions (as MS Powerpoint or pdf ) and technician and teacher notes (as MS Word or pdf ) for a microscale diffusion practical.
What students need to know

Source: © Science Photo Library
Primary school will have introduced students to solids, liquids and gases; now it’s time to link these states to the microscopic world of the particle
Students will have studied the types and properties of matter during primary education. They will have investigated various different materials, classifying them as solids, liquids and gases. They will have looked at reversible reactions, including freezing/melting and boiling/condensing. These macroscopic observations and explanations need to be linked to the microscopic world of the particle using ‘particle theory’.
The particle (or kinetic) theory of matter can be summarised as:
- All matter is formed of tiny particles.
- The particles are constantly randomly moving about.
- The particles can be arranged regularly or randomly.
- The particles are held together by weak or strong forces.
- As temperature increases, the particles move faster.
There is a strong overlap between this area in chemistry and physics. Look at where it is scheduled in your school’s schemes of work to see whether you will be teaching it for the first time, or consolidating the knowledge.
Ideas for your classroom
Hands-on demonstrations, simulations and practical work provide lots of opportunities for your students to understand the dynamic nature of particles. Check with your physics department to see if they have a kinetic theory model (eg SciChem XPG050011; Timstar SI68190). These use ball bearings to model the particles. A vibrating base ‘energises’ the particles. By increasing the vibration speed, you can model solid, liquid and gas.
Place a few drops of a perfume on a watch glass and ask the students to raise their hands when they can smell it
Freely available online simulations, such as the pHET States of matter , allow you to demonstrate the effects of heating and cooling substances. Students can see state changes, and relate this to movement and arrangement of the particles. Consider allowing the students to investigate the simulations for themselves, either in class or for homework; this worksheet could be suitable. An alternative paper-based activity is Particle model cards from the Royal Society of Chemistry.
Your understanding of using models can be enhanced with the RSC CPD course Developing and using models .
Diffusion of particles provides many useful contexts to help students make the link between the macroscopic world and the sub-microscopic . Start with simple demonstrations such as placing a few drops of a perfume on a watch glass and ask the students to raise their hands when they can smell it. They should observe a wave of hands going up as the fragrance particles spread from the watch glass.
This can be followed with diffusion of nitrogen dioxide in gas jars . In this demonstration, NO 2 is synthesised by mixing copper turnings and concentrated nitric acid in one gas jar . A second air-filled jar is then placed over the top, and the NO 2 allowed to diffuse into it. Your students will observe the dark brown vapour moving into the upper air-filled jar. Ask them to consolidate their observations with particle diagrams showing the before, during and after. They should annotate the movement and mixing of nitrogen dioxide and air particles.
A commonly used demonstration is soaking mineral wool separately in concentrated hydrochloric acid and concentrated ammonia solution, and placing these at either end of a large glass tube. However this can be fiddly to set up and requires a fume hood.
A good alternative is a microscale diffusion practical, with all the chemistry happening on a plastic sheet under a petri dish. Students first make up an array of iodide/starch drops on a plastic sheet. Second, they mix a couple of drops of acid and bleach to synthesise a small volume of chlorine. Finally, the whole arrangement is covered in a petri dish to contain the chlorine and allow it to diffuse to the iodide/starch drops. Your students will observe the drops darkening as the iodine is displaced from solution and forms the blue/black iodine-starch complex. They can set up, carry out and clear away the apparatus inside 15 minutes, leaving plenty of time for discussions and explanations. Download the instructions and a template practical sheet below.
Common misconceptions
‘Space between particles is filled with air’ – a common misconception
Misconceptions in this topic are common and can be deep-seated. This isn’t surprising given the abstract ideas we are trying to help students grasp. Use the Best Evidence in Science Teaching project resources to help expose these misconceptions – the Topic 1, Key concept 1 Substance will be useful for this topic.
Common misconceptions include:
- Matter is continuous, rather than being formed from discrete particles – everyday experience reinforces this – solids ‘feel’ solid and liquids stay together in drops even if they are sprayed. Discussing gases can be a helpful way in to particles, but as they ‘disappear’, many students think gases aren’t properly there.
- Space between particles is filled with air – even when students accept the idea of matter being made of particles, the space between particles can still be considered as air. Everyday life again creates a false analogy with solid and liquid objects being separated from each other by the air we breathe.
- Forces are responsible for all particle movement – this links with a common misconception from physics, whereby force is considered required for all movements. Students struggle with the idea that a particle keeps moving once it starts. Everyday experiences, such as a hockey ball rolling to a stop once hit, create this false analogy.
- Particles change state – eg when ice melts it is the ice particles melting to form water particles, rather than the substance changing state due to the change in energy and position of the particles relative to each other.
- Atoms and cells are about the same size – this isn’t helped by chemists and biologists using ‘nucleus’ to describe the centre of our respective fundamental building blocks. Discussing relative scales of objects can help – eg a human cell is 100,000x smaller than a human, while an atom is 100,000x smaller than a human cell. Videos such as Powers of ten can also help.
Formative assessment
As the kinetic theory is one of the key models underpinning much of chemistry, discuss it regularly, and give the students plenty of opportunities to create artefacts to help them express and consolidate their understanding of the model. Drawing posters showing the particle arrangement of solids, liquids and gases, surrounded by notations of the properties is useful. Success criteria could be displayed for the students to peer assess each other’s work, identifying areas for improvement. If you have a visualiser available, display high-quality examples to model the expectations for the class.
The RSC Assessment for Learning suite of resources contains a useful document discussing many example of substances in different states . You may need to select carefully, as there are examples of compounds and mixtures included, along with particles shown as molecules. However, identifying early on that particles are being modelled as a simple circle is useful in the general discussion of use of models in learning about science.
Progression to 14–16
The structure of matter at the sub-microscopic level, and the forces between particles, underlies the explanation of macroscopic properties. During 11–14, students gain some appreciation of the nature of the particles (atoms, ions, molecules). At 14–16, you will expand on how these different particles are formed and how this affects the nature of the forces between them. Students will need to identify the similarities and differences between ionic, covalent and metallic bonding, and weak intermolecular forces.
Another key area is reaction kinetics – collision theory is predicated on an understanding that particles collide, and the models used at this level are very reminiscent of the particle model.
Finally, there is strong overlap between chemistry and physics in this area of study. Changes of state are discussed in much greater depth in physics, looking at the internal energy of substances, delineating kinetic and potential energies. The two plateaus on the heating/cooling curves of substances are much more explicable when this has been learned.
Take-home points
- Kinetic theory describes the position and movement of particles in substances.
- Students commonly equate the size of atoms with that of cells – take time to help them visualise the orders of magnitude involved.
- Make use of simple demonstrations and practical work to help your students link between the macroscopic and sub-microscopic.
- Kinetic theory is built on throughout 14–16, and allows structure and bonding to be explicable.
David Paterson is a chemistry/physics teacher at Aldenham School, Elstree. He tweets @dave2004b
Microscale diffusion integrated instructions
Teacher technician notes microscale diffusion.

More from David Paterson

Use AI to successfully assess students’ understanding
Everything you need to teach energetics at 14–16

Banish misconceptions with digital whiteboards
- Misconceptions
- Properties of matter
Related articles

Particle diagrams | Structure strip | 14–16
By Kristy Turner
Support learners to describe and evaluate the particle model for solids, liquids and gases with this writing activity

Cosmetics, technical services chemist
Sharlotte makes environmentally friendly beauty products

Illustrate polymer properties with a self-siphoning solution
2024-04-22T05:38:00Z By Declan Fleming
Demonstrate the tubeless siphon with poly(ethylene glycol) and highlight the polymer’s viscoelasticity to your 11–16 learners
No comments yet
Only registered users can comment on this article., more from cpd.

How to teach extraction of metals at 14–16
2024-04-09T07:20:00Z By Niall Begley
Solidify learners’ understanding of extraction processes with these tips, misconception busters and teaching ideas

How to teach chromatography at post-16
2024-03-11T04:00:00Z By Andy Markwick
Everything you need to help your students master the fundamentals of this analytical technique
How to teach atmospheric chemistry at 14–16
2024-02-06T06:00:00Z By Martin Bluemel
Use these guiding questions to guarantee student understanding of this tricky topic
- Contributors
- Print issue
- Email alerts
Site powered by Webvision Cloud

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
States of Matter

States of matter are forms in which matter exists. The four states of matter observed in everyday life are solids , liquids , gases , and plasma . Other states of matter also exist, although they require special conditions. Here is a look at the states of matter, their properties, and the names of phase transitions between them.
What Is a State of Matter?
Matter is anything that has mass and takes up space. It consists of subatomic particles, atoms, ions , and compounds . Sometimes these particles are tightly bound and close together, while other times particles are loosely connected and widely separated. States of matter describe the qualities displayed by matter. Basically, the state of matter of a substance depends on how much energy its particles have.
We can change the energy of matter by altering its temperature or pressure, causing matter to transition from one state to another. But, when matter changes state, its chemical identity remains the same. So, if you take ice, melt it, and then boil it, its state of matter changes, but it’s always water.
List of the States of Matter
The four fundamental states of matter are solids, liquids, gases, and plasma. But, scientists are discovering new states of matter that exist under extreme conditions.
A solid is a state of matter with a defined shape and volume . Atoms, ions, and molecules in a solid pack tightly together and may form crystals. Examples of solids include rocks, ice, diamond, and wood.
A liquid is a state of matter with a defined volume, but no defined shape. In other words, liquids take the shape of their container. Particles in a liquid have more energy than in a solid, so they are further apart and less organized (more random). Examples of liquids include water, juice, and vegetable oil.
A gas is a state of matter lacking either a defined volume or defined shape. Like a liquid, a gas takes the shape of a container. Unlike a liquid, a gas easily expands or contracts to fill the entire volume of the container. Particles in a gas have more energy than in solids or liquids. They tend to be further apart and move more randomly than in a liquid. Examples of gases include air, water vapor, and helium.
Plasma is a state of matter similar to a gas, except all of the particles carry an electrical charge. Also, plasma tends to exist at very low pressure, so the particles are even further apart than in a gas. Plasma can consist of ions, electrons, or protons. Examples of plasma include lightning, the aurora, the Sun, and the inside of a neon sign.
Bose-Einstein Condensate
Bose-Einstein condensate (BEC) is sometimes called the fifth state of matter. In Bose-Einstein condensate, atoms and ions stop behaving as separate particles and collapse into a single quantum state that can be described using a single wavefunction. This state of matter was verified experimentally in 1995 by Eric Cornell and Carl Wieman. Bose-Einstein condensate is “colder” than an ordinary solid and may form very near absolute zero .
A superfluid is a second liquid state formed by some types of matter. A superfluid displays zero viscosity . In other words, it has no resistance to flow. Superfluidity was observed for helium in 1937. Because it could flow without friction, superfluid helium climbed the walls of its container and dripped over the sides. Like Bose-Einstein condensate, superfluidity occurs near absolute zero.
Fermionic Condensate
A fermionic condensate is a state of matter similar to a Bose-Einstein condensate, except it consists of fermions, such as quarks and leptons. Normally, the Pauli exclusion principle forbids fermions from entering the same quantum state. In a fermionic condensate, a pair of fermions behaves as a boson, allowing multiple pairs to enter the same quantum state.
Rydberg Matter
Rydberg matter is a type of plasma formed when excited ions condense. You can think of it as dusty plasma. So far, it occurs in the elements hydrogen, potassium, nitrogen, and cesium. This type of matter consists mainly of small hexagonal planar clusters. Scientists make Rydberg matter in a lab or observe it in the upper atmosphere of planets and in clouds in space.
Photonic Matter
Photonic matter is the state of matter formed when photons interact with a gas in such a way that the photons have apparent mass and can interact with each other. Photons with apparent mass can even form photonic “molecules.”
Color-Glass Condensate
Color-glass condensate is a state of matter proposed to exist when atomic nuclei travel near the speed of liquid. Because of their speed, the nucleus appears compressed along its direction of motion. This causes the gluons of the nucleus to appear as a sort of wall or region of increased density.
Other States of Matter
There are other proposed states of matter, including quark matter, degenerate matter, dropleton, quantum Hall state, superglass, supersolid, and string-net liquid.
Phase Transitions Between States of Matter

Changes in temperature and pressure causes matter to change from one state to another. This change is called a phase transition or phase change . Examples of phase transitions including the melting of ice (a solid) into water (a liquid) and the boiling of water into water vapor (a gas). Here are the names of the phase transitions between solids, liquids, gases, and plasma:
- Melting : Phase transition from solid to liquid.
- Freezing : Phase transition from liquid to solid.
- Vaporization : Phase transition from liquid to gas.
- Condensation : Phase transition from gas to liquid.
- Sublimation : Phase transition from solid to gas.
- Deposition : Phase transition from gas to solid.
- Ionization : Phase transition from gas to plasma.
- De-ionization or recombination : Phase transition from plasma to gas.
- Goodstein, D.L. (1985). States of Matter . Dover Phoenix. ISBN 978-0-486-49506-4.
- Murthy, G.; et al. (1997). “Superfluids and Supersolids on Frustrated Two-Dimensional Lattices”. Physical Review B . 55 (5): 3104. doi: 10.1103/PhysRevB.55.3104
- Sutton, A.P. (1993). Electronic Structure of Materials . Oxford Science Publications. ISBN 978-0-19-851754-2.
- Wahab, M.A. (2005). Solid State Physics: Structure and Properties of Materials . Alpha Science. ISBN 978-1-84265-218-3.
- White, F. (2003). Fluid Mechanics . McGraw-Hill. ISBN 978-0-07-240217-9.
Related Posts
States of Matter: Kinetic molecular theory and phase transitions
by Anthony Carpi, Ph.D.
Listen to this reading
Did you know that solids, liquids, and gases are not the only states of matter? Among others are plasmas, which have such high energy that molecules are ripped apart. And Bose-Einstein Condensates, seen for the first time in 1995, are a weird state of matter that can actually trap light.
As a young child, I remember staring in wonder at a pot of boiling water. Searching for an explanation for the bubbles that formed, I believed for a time that the motion of the hot water drew air down into the pot, which then bubbled back to the surface . Little did I know that what was happening was even more magical than I imagined – the bubbles were not air, but actually water in the form of a gas .

The different states of matter have long confused people. The ancient Greeks were the first to identify three classes (what we now call states) of matter based on their observations of water. But these same Greeks, in particular the philosopher Thales (624 – 545 BCE), incorrectly suggested that since water could exist as a solid , liquid , or even a gas under natural conditions, it must be the single principal element in the universe from which all other substances are made. We now know that water is not the fundamental substance of the universe; in fact, it is not even an element.
- Kinetic Molecular Theory
To understand the different states in which matter can exist, we need to understand something called the Kinetic Molecular Theory of Matter . Kinetic Molecular Theory has many parts, but we will introduce just a few here. One of the basic concepts of the theory states that atoms and molecules possess an energy of motion that we perceive as temperature. In other words, atoms and molecules are constantly moving, and we measure the energy of these movements as the temperature of the substance. The more energy a substance has, the more molecular movement there will be, and the higher the perceived temperature will be. An important point that follows this is that the amount of energy that atoms and molecules have (and thus the amount of movement) influences their interaction with each other. Unlike simple billiard balls, many atoms and molecules are attracted to each other as a result of various intermolecular forces such as hydrogen bonds , van der Waals forces , and others. Atoms and molecules that have relatively small amounts of energy (and movement) will interact strongly with each other, while those that have relatively high energy will interact only slightly, if even at all, with others.
Comprehension Checkpoint
- Energy and states of matter
How does this produce different states of matter? Atoms that have low energy interact strongly and tend to "lock" in place with respect to other atoms. Thus, collectively, these atoms form a hard substance, what we call a solid . Atoms that possess high energy will move past each other freely, flying about a room, and forming what we call a gas . As it turns out, there are several known states of matter ; a few of them are detailed below.

Solids are formed when the attractive forces between individual molecules are greater than the energy causing them to move apart. Individual molecules are locked in position near each other, and cannot move past one another. The atoms or molecules of solids remain in motion. However, that motion is limited to vibrational energy; individual molecules stay fixed in place and vibrate next to each other. As the temperature of a solid is increased, the amount of vibration increases, but the solid retains its shape and volume because the molecules are locked in place relative to each other. To view an example of this, click on the animation below which shows the molecular structure of ice crystals .

Liquids are formed when the energy (usually in the form of heat) of a system is increased and the rigid structure of the solid state is broken down. In liquids , molecules can move past one another and bump into other molecules; however, they remain relatively close to each other like solids. Often in liquids , intermolecular forces (such as the hydrogen bonds shown in the animation below) pull molecules together and are quickly broken. As the temperature of a liquid is increased, the amount of movement of individual molecules increases. As a result, liquids can "flow" to take the shape of their container but they cannot be easily compressed because the molecules are already close together. Thus, liquids have an undefined shape, but a defined volume . In the example animation below, we see that liquid water is made up of molecules that can freely move past one another, yet remain relatively close in distance to each other.

Gases are formed when the energy in the system exceeds all of the attractive forces between molecules . Thus gas molecules have little interaction with each other beyond occasionally bumping into one another. In the gas state, molecules move quickly and are free to move in any direction, spreading out long distances. As the temperature of a gas increases, the amount of movement of individual molecules increases. Gases expand to fill their containers and have low density . Because individual molecules are widely separated and can move around easily in the gas state, gases can be compressed easily and they have an undefined shape.
Solids, liquids , and gases are the most common states of matter that exist on our planet. If you would like to compare the three states to one another, click on the comparison animation below. Note the differences in molecular motion of water molecules in these three states.

Plasmas are hot, ionized gases. Plasmas are formed under conditions of extremely high energy , so high, in fact, that molecules are ripped apart and only free atoms exist. More astounding, plasmas have so much energy that the outer electrons are actually ripped off of individual atoms, thus forming a gas of highly energetic, charged ions . Because the atoms in plasma exist as charged ions, plasmas behave differently than gases, thus representing a fourth state of matter . Plasmas can be commonly seen simply by looking upward; the high energy conditions that exist in stars such as our sun force individual atoms into the plasma state.
- Other states of matter
As we have seen, increasing energy leads to more molecular motion. Conversely, decreasing energy results in less molecular motion. As a result, one prediction of Kinetic Molecular Theory is that if we continue to decrease the energy (measured as temperature) of a substance, we will reach a point at which all molecular motion stops. The temperature at which molecular motion stops is called absolute zero and has been calculated to be -273.15 degrees Celsius. While scientists have cooled substances to temperatures close to absolute zero , they have never actually reached absolute zero. The difficulty with observing a substance at absolute zero is that to "see" the substance, light is needed, and light itself transfers energy to the substance, thus raising the temperature. Despite these challenges, scientists have recently observed a fifth state of matter that only exists at temperatures very close to absolute zero.
Bose-Einstein Condensates represent a fifth state of matter only seen for the first time in 1995. The state is named after Satyendra Nath Bose and Albert Einstein who predicted its existence in the 1920s. B-E condensates are gaseous superfluids cooled to temperatures very near absolute zero . In this weird state, all the atoms of the condensate attain the same quantum-mechanical state and can flow past one another without friction . Even more strangely, B-E condensates can actually "trap" light , releasing it when the state breaks down.
Several other less common states of matter have also either been described or actually seen. Some of these states include liquid crystals , fermionic condensates , superfluids, supersolids, and the aptly named strange matter . To read more about these phases, see "Phase" in our Resources for this module.
- Phase transitions
The transformation of one state of matter into another state is called a phase transition . The more common phase transitions even have names; for example, the terms melting and freezing describe phase transitions between the solid and liquid state, and the terms evaporation and condensation describe transitions between the liquid and gas state.
Phase transitions occur at very precise points, when the energy (measured as temperature) of a substance in a given state exceeds that allowed in the state. For example, liquid water can exist at a range of temperatures. Cold drinking water may be around 4ºC. Hot shower water has more energy and thus may be around 40ºC. However, at 100°C under normal conditions, water will begin to undergo a phase transition into the gas phase. At this point, energy introduced into the liquid will not go into increasing the temperature; it will be used to send molecules of water into the gas state. Thus, no matter how high the flame is on the stove, a pot of boiling water will remain at 100ºC until all of the water has undergone transition to the gas phase. The excess energy introduced by a high flame will accelerate the liquid-to-gas transition; it will not change the temperature. The heat curve below illustrates the corresponding changes in energy (shown in calories) and temperature of water as it undergoes a phase transition between the liquid and gas states.
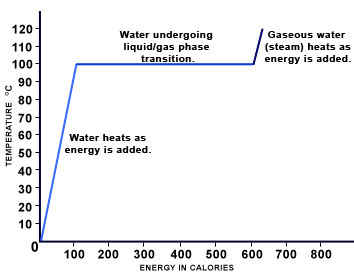
As can be seen in the graph above, as we move from left to right, the temperature of liquid water increases as energy (heat) is introduced. At 100ºC, water begins to undergo a phase transition and the temperature remains constant even as energy is added (the flat part of the graph). The energy that is introduced during this period goes toward breaking intermolecular forces so that individual water molecules can "escape" into the gas state. Finally, once the transition is complete, if further energy is added to the system , the heat of the gaseous water, or steam, will increase.
This same process can be seen in reverse if we simply look at the graph above starting on the right side and moving left. As steam is cooled, the movement of gaseous water molecules and thus temperature will decrease. When the gas reaches 100ºC, more energy will be lost from the system as the attractive forces between molecules re-form; however, the temperature remains constant during the transition (the flat part of the graph). Finally, when condensation is complete, the temperature of the liquid will begin to fall as energy is withdrawn.
- Phase transitions and our world
Phase transitions are an important part of the world around us. For example, the energy withdrawn when perspiration evaporates from the surface of your skin allows your body to correctly regulate its temperature during hot days. Phase transitions play an important part in geology, influencing mineral formation and possibly even earthquakes . And who can ignore the phase transition that occurs at about -3ºC, when cream, perhaps with a few strawberries or chocolate chunks, begins to form solid ice cream?
Now we understand what is happening in a pot of boiling water. The energy (heat) introduced at the bottom of the pot causes a localized phase transition of liquid water to the gaseous state. Because gases are less dense than liquids , these localized phase transitions form pockets (or bubbles) of gas , which rise to the surface of the pot and burst. But nature is often more magical than our imagination. Despite all that we know about the states of matter and phase transitions, we still cannot predict where the individual bubbles will form in a pot of boiling water.
Table of Contents
Activate glossary term highlighting to easily identify key terms within the module. Once highlighted, you can click on these terms to view their definitions.
Activate NGSS annotations to easily identify NGSS standards within the module. Once highlighted, you can click on them to view these standards.
Introduction to the Particle Theory of Matter
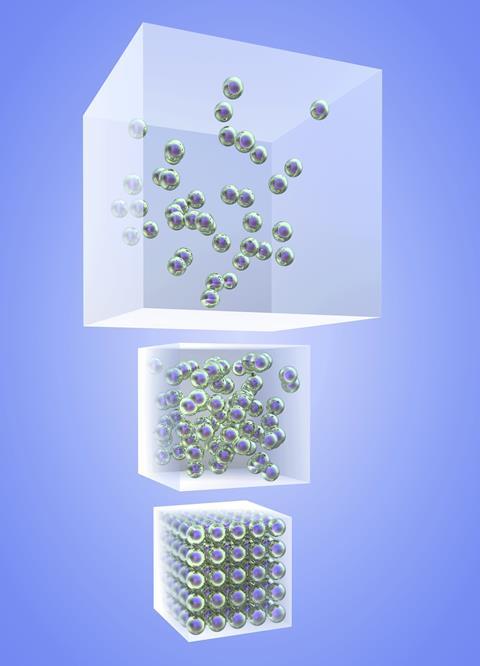
Cube overflowing with spheres representing particles (akinbostanci, iStockphoto)
How does this align with my curriculum?
Share on: facebook x/twitter linkedin pinterest.
Learn about how the Particle Theory helps us understand matter.
Particle Theory of Matter
Matter is anything that has mass and takes up space. It is a general name we call all the physical things around us. Matter includes things so tiny humans can’t see them with their eyes.
The Particle Theory of Matter is a scientific model . A scientific model is a way of illustrating ideas, objects and processes so they’re easier to understand. Scientists use models to explain things that can’t be seen without special equipment. One of these things is an individual atom.
The Particle Theory of Matter helps us think about how matter behaves. It also helps us explain why different matter has different properties. It includes these key ideas:
- All matter is made of tiny particles . These particles are either individual atoms, or groups of atoms called molecules .
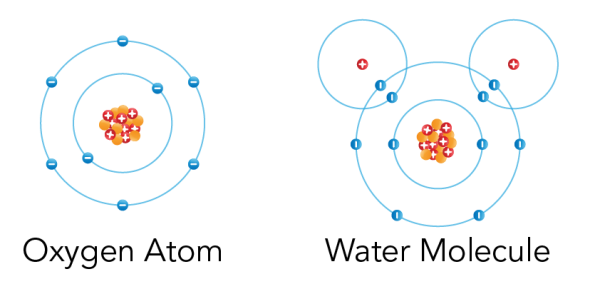
Shown is a colour diagram of two different atoms with blue spheres shown along concentric rings around a clump of red and orange spheres at the centre. The atom on the left is labelled "Oxygen Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Eight blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom on the right is labelled "Water Molecule." The main part of this diagram has the same amount of spheres and circles as the first. But there are two additional red spheres with plus signs. These are in the top left and right corners. Each one is surrounded by an additional blue circle with one blue sphere with a minus sign. These overlap the edges of the larger circle below.
Did you know? Any particle smaller than an atom is called a subatomic particle . Protons, neutrons and electrons are all subatomic particles.
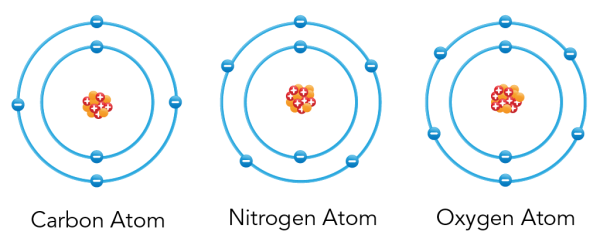
Shown is a colour diagram of three different atoms with blue spheres shown along concentric rings around a clump of red and orange spheres at the centre. The atom on the left is labelled "Carbon Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Six blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom in the middle is labelled "Nitrogen Atom." This has a clump of orange spheres and red spheres with plus signs at the centre. Seven blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom on the right is labelled "Oxygen Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Eight blue spheres with minus signs are neatly arranged along two concentric blue circles around it.
- Particles are attracted to each other by forces. In some kinds of matter, like a diamond, this force is very strong. In other kinds of matter, like orange juice, the force is weaker.
- Particles of matter have spaces between them. In a gas , there are large spaces between them. In a liquid they are closer together. In a solid , the particles are packed so close they can hardly move.
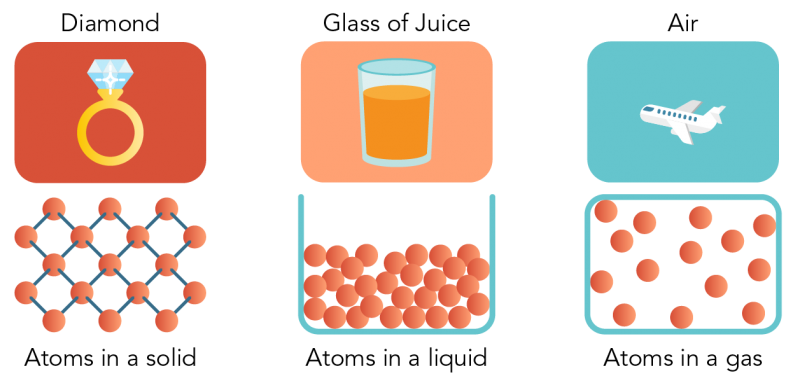
Shown is a colour illustration of three different kinds of matter above diagrams of their atoms. Starting on the left, the first illustration is labelled "Diamond." It shows a gold ring topped with a large, clear gem on a red background. Below, atoms are represented as pink spheres connected by small blue sticks. These are arranged in a neat diamond pattern, like a chain link fence. Text below reads "Atoms in a solid." In the middle is an illustration labelled "Glass of Juice." It shows a clear glass of orange juice against a pink background. Below, atoms are piled together, filling the bottom of an open blue box shape that resembles the glass. Some spheres appear to be touching each other, but there is space between others. Text below reads "Atoms in a liquid." On the right is an illustration labelled "Air." This shows a white airplane flying in a blue sky. Below, atoms float loosely in a blue rectangle, with lots of space between them. Text below reads "Atoms in a gas."
- Particles are always moving at any temperature above -273.15 degrees Celsius. But the human eye can’t see them move.
Did you know? -273.15 degrees Celsius is also 0 kelvin (0 K). This temperature is called absolute zero .
- The faster particles move, the warmer they get. So, the molecules in hot water are moving faster than the ones in cold water.

Shown is a colour diagram illustrating the speed and temperature of particles along a scale from colder to warmer. A thick horizontal stripe runs across the bottom edge. This is blue on the left side, and gradually changes to purple, then pink on the right. The left end is labelled "Slower" on top, and "Colder" below. The right end is labelled "Faster" on top, and "Warmer" below. Three black circles are spaced out along the scale. Each one contains round, coloured circles to represent particles. These have translucent tails that indicate the speed of their movement. The left circle has blue particles with short, wide tails. These match the blue, colder end of the scale. The middle circle has purple particles with longer, narrower tails. These match the middle part of the scale. The right circle has pink particles with even longer, narrower tails. These match the pink, warmer end of the scale.
Living Space
Explore the optimal environmental conditions for human life. How do you think your classroom conditions compare to those on the International Space Station? Free project for grades 6-9 students.
The Particle Theory of Matter Quiz See how well you know the particle theory. Try this short quiz by Science Source.
What's Matter? This video (3:30 min) by Crash Course Kids explains three states of matter: Solid, Liquid, and Gas. There is also a quick experiment you can do at home to prove that air is matter.
GCSE Physics - Particle Theory & States of Matter #25 (2020) This video (4:33 min.) from Cognito covers particle theory, how substances change from one state to another, and the idea that density doesn't change when substances change state.
BBC Bitesize. (n. d.) Kinetic particle theory - Kinetic particle theory and state changes - GCSE Physics (Single Science) Revision - Other .
EduMedia. (n. d.) Understanding Matter and Energy, Pure Substances and Mixtures
Petersen, D. (2019 March 26). How to teach states of matter and particle theory - Royal Society of Chemistry
Related Topics

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

6.6: De Broglie’s Matter Waves
- Last updated
- Save as PDF
- Page ID 4524

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section, you will be able to:
- Describe de Broglie’s hypothesis of matter waves
- Explain how the de Broglie’s hypothesis gives the rationale for the quantization of angular momentum in Bohr’s quantum theory of the hydrogen atom
- Describe the Davisson–Germer experiment
- Interpret de Broglie’s idea of matter waves and how they account for electron diffraction phenomena
Compton’s formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie’s hypothesis of matter waves . In 1926, De Broglie’s hypothesis, together with Bohr’s early quantum theory, led to the development of a new theory of wave quantum mechanics to describe the physics of atoms and subatomic particles. Quantum mechanics has paved the way for new engineering inventions and technologies, such as the laser and magnetic resonance imaging (MRI). These new technologies drive discoveries in other sciences such as biology and chemistry.
According to de Broglie’s hypothesis, massless photons as well as massive particles must satisfy one common set of relations that connect the energy \(E\) with the frequency \(f\), and the linear momentum \(p\) with the wavelength \(λ\). We have discussed these relations for photons in the context of Compton’s effect. We are recalling them now in a more general context. Any particle that has energy and momentum is a de Broglie wave of frequency \(f\) and wavelength \(\lambda\):
\[ E = h f \label{6.53} \]
\[ \lambda = \frac{h}{p} \label{6.54} \]
Here, \(E\) and \(p\) are, respectively, the relativistic energy and the momentum of a particle. De Broglie’s relations are usually expressed in terms of the wave vector \(\vec{k}\), \(k = 2 \pi / \lambda\), and the wave frequency \(\omega = 2 \pi f\), as we usually do for waves:
\begin{aligned} &E=\hbar \omega \label{6.55}\\ &\vec{p}=\hbar \vec{k} \label{6.56} \end{aligned}
Wave theory tells us that a wave carries its energy with the group velocity . For matter waves, this group velocity is the velocity \(u\) of the particle. Identifying the energy E and momentum p of a particle with its relativistic energy \(mc^2\) and its relativistic momentum \(mu\), respectively, it follows from de Broglie relations that matter waves satisfy the following relation:
\[ \lambda f =\frac{\omega}{k}=\frac{E / \hbar}{p / \hbar}=\frac{E}{p} = \frac{m c^{2}}{m u}=\frac{c^{2}}{u}=\frac{c}{\beta} \label{6.57} \]
where \(\beta = u/c\). When a particle is massless we have \(u=c\) and Equation \ref{6.57} becomes \(\lambda f = c\).
Example \(\PageIndex{1}\): How Long are de Broglie Matter Waves?
Calculate the de Broglie wavelength of:
- a 0.65-kg basketball thrown at a speed of 10 m/s,
- a nonrelativistic electron with a kinetic energy of 1.0 eV, and
- a relativistic electron with a kinetic energy of 108 keV.
We use Equation \ref{6.57} to find the de Broglie wavelength. When the problem involves a nonrelativistic object moving with a nonrelativistic speed u , such as in (a) when \(\beta=u / c \ll 1\), we use nonrelativistic momentum p . When the nonrelativistic approximation cannot be used, such as in (c), we must use the relativistic momentum \(p=m u=m_{0} \gamma u=E_{0} \gamma \beta/c\), where the rest mass energy of a particle is \(E_0 = m c^2 \) and \(\gamma\) is the Lorentz factor \(\gamma=1 / \sqrt{1-\beta^{2}}\). The total energy \(E\) of a particle is given by Equation \ref{6.53} and the kinetic energy is \(K=E-E_{0}=(\gamma-1) E_{0}\). When the kinetic energy is known, we can invert Equation 6.4.2 to find the momentum
\[ p=\sqrt{\left(E^{2}-E_{0}^{2}\right) / c^{2}}=\sqrt{K\left(K+2 E_{0}\right)} / c \nonumber \]
and substitute into Equation \ref{6.57} to obtain
\[ \lambda=\frac{h}{p}=\frac{h c}{\sqrt{K\left(K+2 E_{0}\right)}} \label{6.58} \]
Depending on the problem at hand, in this equation we can use the following values for hc :
\[ h c=\left(6.626 \times 10^{-34} \: \mathrm{J} \cdot \mathrm{s}\right)\left(2.998 \times 10^{8} \: \mathrm{m} / \mathrm{s}\right)=1.986 \times 10^{-25} \: \mathrm{J} \cdot \mathrm{m}=1.241 \: \mathrm{eV} \cdot \mu \mathrm{m} \nonumber \]
- For the basketball, the kinetic energy is \[ K=m u^{2} / 2=(0.65 \: \mathrm{kg})(10 \: \mathrm{m} / \mathrm{s})^{2} / 2=32.5 \: \mathrm{J} \nonumber \] and the rest mass energy is \[ E_{0}=m c^{2}=(0.65 \: \mathrm{kg})\left(2.998 \times 10^{8} \: \mathrm{m} / \mathrm{s}\right)^{2}=5.84 \times 10^{16} \: \mathrm{J} \nonumber \] We see that \(K /\left(K+E_{0}\right) \ll 1\) and use \(p=m u=(0.65 \: \mathrm{kg})(10 \: \mathrm{m} / \mathrm{s})=6.5 \: \mathrm{J} \cdot \mathrm{s} / \mathrm{m} \): \[ \lambda=\frac{h}{p}=\frac{6.626 \times 10^{-34} \: \mathrm{J} \cdot \mathrm{s}}{6.5 \: \mathrm{J} \cdot \mathrm{s} / \mathrm{m}}=1.02 \times 10^{-34} \: \mathrm{m} \nonumber \]
- For the nonrelativistic electron, \[ E_{0}=mc^{2}=\left(9.109 \times 10^{-31} \mathrm{kg}\right)\left(2.998 \times 10^{8} \mathrm{m} / \mathrm{s}\right)^{2}=511 \mathrm{keV} \nonumber \] and when \(K = 1.0 \: eV\), we have \(K/(K+E_0) = (1/512) \times 10^{-3} \ll 1\), so we can use the nonrelativistic formula. However, it is simpler here to use Equation \ref{6.58}: \[ \lambda=\frac{h}{p}=\frac{h c}{\sqrt{K\left(K+2 E_{0}\right)}}=\frac{1.241 \: \mathrm{eV} \cdot \mu \mathrm{m}}{\sqrt{(1.0 \: \mathrm{eV})[1.0 \: \mathrm{eV}+2(511 \: \mathrm{keV})]}}=1.23 \: \mathrm{nm} \nonumber \] If we use nonrelativistic momentum, we obtain the same result because 1 eV is much smaller than the rest mass of the electron.
- For a fast electron with \(K=108 \: keV\), relativistic effects cannot be neglected because its total energy is \(E = K = E_0 = 108 \: keV + 511 \: keV = 619 \: keV\) and \(K/E = 108/619\) is not negligible: \[ \lambda=\frac{h}{p}=\frac{h c}{\sqrt{K\left(K+2 E_{0}\right)}}=\frac{1.241 \: \mathrm{eV} \cdot \mu \mathrm{m}}{\sqrt{108 \: \mathrm{keV}[108 \: \mathrm{keV}+2(511 \: \mathrm{keV})]}}=3.55 \: \mathrm{pm} \nonumber \].
Significance
We see from these estimates that De Broglie’s wavelengths of macroscopic objects such as a ball are immeasurably small. Therefore, even if they exist, they are not detectable and do not affect the motion of macroscopic objects.
Exercise \(\PageIndex{1}\)
What is de Broglie’s wavelength of a nonrelativistic proton with a kinetic energy of 1.0 eV?
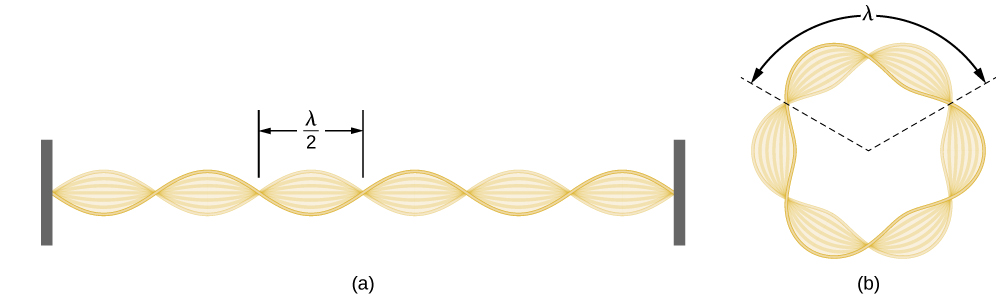
Using the concept of the electron matter wave, de Broglie provided a rationale for the quantization of the electron’s angular momentum in the hydrogen atom, which was postulated in Bohr’s quantum theory. The physical explanation for the first Bohr quantization condition comes naturally when we assume that an electron in a hydrogen atom behaves not like a particle but like a wave. To see it clearly, imagine a stretched guitar string that is clamped at both ends and vibrates in one of its normal modes. If the length of the string is l (Figure \(\PageIndex{1}\)), the wavelengths of these vibrations cannot be arbitrary but must be such that an integer k number of half-wavelengths \(\lambda/2\) fit exactly on the distance l between the ends. This is the condition \(l=k \lambda /2\) for a standing wave on a string. Now suppose that instead of having the string clamped at the walls, we bend its length into a circle and fasten its ends to each other. This produces a circular string that vibrates in normal modes, satisfying the same standing-wave condition, but the number of half-wavelengths must now be an even number \(k\), \(k=2n\), and the length l is now connected to the radius \(r_n\) of the circle. This means that the radii are not arbitrary but must satisfy the following standing-wave condition:
\[ 2 \pi r_{n}=2 n \frac{\lambda}{2} \label{6.59}. \]
If an electron in the n th Bohr orbit moves as a wave, by Equation \ref{6.59} its wavelength must be equal to \(\lambda = 2 \pi r_n / n\). Assuming that Equation \ref{6.58} is valid, the electron wave of this wavelength corresponds to the electron’s linear momentum, \(p = h/\lambda = nh / (2 \pi r_n) = n \hbar /r_n\). In a circular orbit, therefore, the electron’s angular momentum must be
\[ L_{n}=r_{n} p=r_{n} \frac{n \hbar}{r_{n}}=n \hbar \label{6.60} . \]
This equation is the first of Bohr’s quantization conditions, given by Equation 6.5.6 . Providing a physical explanation for Bohr’s quantization condition is a convincing theoretical argument for the existence of matter waves.
Example \(\PageIndex{2}\): The Electron Wave in the Ground State of Hydrogen
Find the de Broglie wavelength of an electron in the ground state of hydrogen.
We combine the first quantization condition in Equation \ref{6.60} with Equation 6.5.6 and use Equation 6.5.9 for the first Bohr radius with \(n = 1\).
When \(n=1\) and \(r_n = a_0 = 0.529 \: Å\), the Bohr quantization condition gives \(a_{0} p=1 \cdot \hbar \Rightarrow p=\hbar / a_{0}\). The electron wavelength is:
\[ \lambda=h / p = h / \hbar / a_{0} = 2 \pi a_{0} = 2 \pi(0.529 \: Å)=3.324 \: Å .\nonumber \]
We obtain the same result when we use Equation \ref{6.58} directly.
Exercise \(\PageIndex{2}\)
Find the de Broglie wavelength of an electron in the third excited state of hydrogen.
\(\lambda = 2 \pi n a_0 = 2 (3.324 \: Å) = 6.648 \: Å\)
Experimental confirmation of matter waves came in 1927 when C. Davisson and L. Germer performed a series of electron-scattering experiments that clearly showed that electrons do behave like waves. Davisson and Germer did not set up their experiment to confirm de Broglie’s hypothesis: The confirmation came as a byproduct of their routine experimental studies of metal surfaces under electron bombardment.
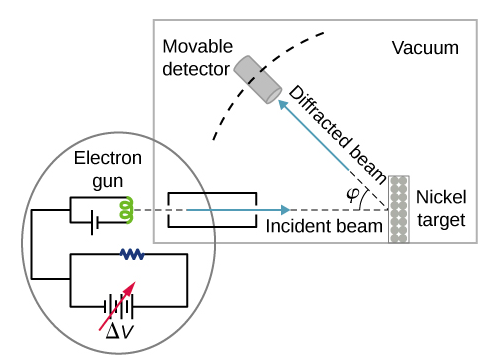
In the particular experiment that provided the very first evidence of electron waves (known today as the Davisson–Germer experiment ), they studied a surface of nickel. Their nickel sample was specially prepared in a high-temperature oven to change its usual polycrystalline structure to a form in which large single-crystal domains occupy the volume. Figure \(\PageIndex{2}\) shows the experimental setup. Thermal electrons are released from a heated element (usually made of tungsten) in the electron gun and accelerated through a potential difference ΔV, becoming a well-collimated beam of electrons produced by an electron gun. The kinetic energy \(K\) of the electrons is adjusted by selecting a value of the potential difference in the electron gun. This produces a beam of electrons with a set value of linear momentum, in accordance with the conservation of energy:
\[ e \Delta V=K=\frac{p^{2}}{2 m} \Rightarrow p=\sqrt{2 m e \Delta V} \label{6.61} \]
The electron beam is incident on the nickel sample in the direction normal to its surface. At the surface, it scatters in various directions. The intensity of the beam scattered in a selected direction φφ is measured by a highly sensitive detector. The detector’s angular position with respect to the direction of the incident beam can be varied from φ=0° to φ=90°. The entire setup is enclosed in a vacuum chamber to prevent electron collisions with air molecules, as such thermal collisions would change the electrons’ kinetic energy and are not desirable.
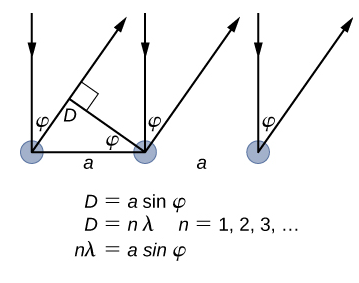
When the nickel target has a polycrystalline form with many randomly oriented microscopic crystals, the incident electrons scatter off its surface in various random directions. As a result, the intensity of the scattered electron beam is much the same in any direction, resembling a diffuse reflection of light from a porous surface. However, when the nickel target has a regular crystalline structure, the intensity of the scattered electron beam shows a clear maximum at a specific angle and the results show a clear diffraction pattern (see Figure \(\PageIndex{3}\)). Similar diffraction patterns formed by X-rays scattered by various crystalline solids were studied in 1912 by father-and-son physicists William H. Bragg and William L. Bragg. The Bragg law in X-ray crystallography provides a connection between the wavelength \(\lambda\) of the radiation incident on a crystalline lattice, the lattice spacing, and the position of the interference maximum in the diffracted radiation (see Diffraction ).
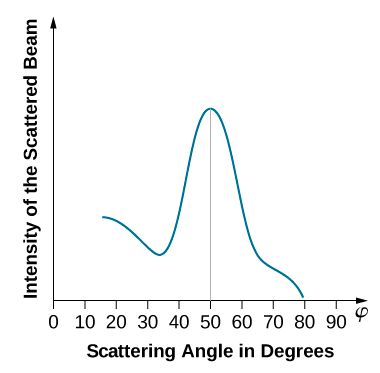
The lattice spacing of the Davisson–Germer target, determined with X-ray crystallography, was measured to be \(a=2.15 \: Å\). Unlike X-ray crystallography in which X-rays penetrate the sample, in the original Davisson–Germer experiment, only the surface atoms interact with the incident electron beam. For the surface diffraction, the maximum intensity of the reflected electron beam is observed for scattering angles that satisfy the condition nλ = a sin φ (see Figure \(\PageIndex{4}\)). The first-order maximum (for n=1) is measured at a scattering angle of φ≈50° at ΔV≈54 V, which gives the wavelength of the incident radiation as λ=(2.15 Å) sin 50° = 1.64 Å. On the other hand, a 54-V potential accelerates the incident electrons to kinetic energies of K = 54 eV. Their momentum, calculated from Equation \ref{6.61}, is \(p = 2.478 \times 10^{−5} \: eV \cdot s/m\). When we substitute this result in Equation \ref{6.58}, the de Broglie wavelength is obtained as
\[ \lambda=\frac{h}{p}=\frac{4.136 \times 10^{-15} \mathrm{eV} \cdot \mathrm{s}}{2.478 \times 10^{-5} \mathrm{eV} \cdot \mathrm{s} / \mathrm{m}}=1.67 \mathrm{Å} \label{6.62}. \]
The same result is obtained when we use K = 54eV in Equation \ref{6.61}. The proximity of this theoretical result to the Davisson–Germer experimental value of λ = 1.64 Å is a convincing argument for the existence of de Broglie matter waves.
Diffraction lines measured with low-energy electrons, such as those used in the Davisson–Germer experiment, are quite broad (Figure \(\PageIndex{3}\)) because the incident electrons are scattered only from the surface. The resolution of diffraction images greatly improves when a higher-energy electron beam passes through a thin metal foil. This occurs because the diffraction image is created by scattering off many crystalline planes inside the volume, and the maxima produced in scattering at Bragg angles are sharp (Figure \(\PageIndex{5}\)).

Since the work of Davisson and Germer, de Broglie’s hypothesis has been extensively tested with various experimental techniques, and the existence of de Broglie waves has been confirmed for numerous elementary particles. Neutrons have been used in scattering experiments to determine crystalline structures of solids from interference patterns formed by neutron matter waves. The neutron has zero charge and its mass is comparable with the mass of a positively charged proton. Both neutrons and protons can be seen as matter waves. Therefore, the property of being a matter wave is not specific to electrically charged particles but is true of all particles in motion. Matter waves of molecules as large as carbon \(C_{60}\) have been measured. All physical objects, small or large, have an associated matter wave as long as they remain in motion. The universal character of de Broglie matter waves is firmly established.
Example \(\PageIndex{3A}\): Neutron Scattering
Suppose that a neutron beam is used in a diffraction experiment on a typical crystalline solid. Estimate the kinetic energy of a neutron (in eV) in the neutron beam and compare it with kinetic energy of an ideal gas in equilibrium at room temperature.
We assume that a typical crystal spacing a is of the order of 1.0 Å. To observe a diffraction pattern on such a lattice, the neutron wavelength λ must be on the same order of magnitude as the lattice spacing. We use Equation \ref{6.61} to find the momentum p and kinetic energy K . To compare this energy with the energy \(E_T\) of ideal gas in equilibrium at room temperature \(T = 300 \, K\), we use the relation \(K = 3/2 k_BT\), where \(k_B = 8.62 \times 10^{-5}eV/K\) is the Boltzmann constant.
We evaluate pc to compare it with the neutron’s rest mass energy \(E_0 = 940 \, MeV\):
\[p = \frac{h}{\lambda} \Rightarrow pc = \frac{hc}{\lambda} = \frac{1.241 \times 10^{-6}eV \cdot m}{10^{-10}m} = 12.41 \, keV. \nonumber \]
We see that \(p^2c^2 << E_0^2\) and we can use the nonrelativistic kinetic energy:
\[K = \frac{p^2}{2m_n} = \frac{h^2}{2\lambda^2 m_n} = \frac{(6.63\times 10^{−34}J \cdot s)^2}{(2\times 10^{−20}m^2)(1.66 \times 10^{−27} kg)} = 1.32 \times 10^{−20} J = 82.7 \, meV. \nonumber \]
Kinetic energy of ideal gas in equilibrium at 300 K is:
\[K_T = \frac{3}{2}k_BT = \frac{3}{2} (8.62 \times 10^{-5}eV/K)(300 \, K) = 38.8 \, MeV. \nonumber \]
We see that these energies are of the same order of magnitude.
Neutrons with energies in this range, which is typical for an ideal gas at room temperature, are called “thermal neutrons.”
Example \(\PageIndex{3B}\): Wavelength of a Relativistic Proton
In a supercollider at CERN, protons can be accelerated to velocities of 0.75 c . What are their de Broglie wavelengths at this speed? What are their kinetic energies?
The rest mass energy of a proton is \(E_0 = m_0c^2 = (1.672 \times 10^{−27} kg)(2.998 \times 10^8m/s)^2 = 938 \, MeV\). When the proton’s velocity is known, we have β = 0.75 and \(\beta \gamma = 0.75 / \sqrt{1 - 0.75^2} = 1.714\). We obtain the wavelength λλ and kinetic energy K from relativistic relations.
\[\lambda = \frac{h}{p} = \frac{hc}{\beta \gamma E_0} = \frac{1.241 \, eV \cdot \mu m}{1.714 (938 \, MeV)} = 0.77 \, fm \nonumber \]
\[K = E_0(\gamma - 1) = 938 \, MeV (1 /\sqrt{1 - 0.75^2} - 1) = 480.1\, MeV \nonumber \]
Notice that because a proton is 1835 times more massive than an electron, if this experiment were performed with electrons, a simple rescaling of these results would give us the electron’s wavelength of (1835)0.77 fm = 1.4 pm and its kinetic energy of 480.1 MeV /1835 = 261.6 keV.
Exercise \(\PageIndex{3}\)
Find the de Broglie wavelength and kinetic energy of a free electron that travels at a speed of 0.75 c .
\(\lambda = 1.417 \, pm; \, K = 261.56 \, keV\)
- Atoms and Molecules

Particle Theory of Matter

What is the Particle Theory of Matter?
The particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in observable macroscopic properties (such as pressure, volume, and temperature). The theory can be used to explain why matter exists in different phases (solid, liquid, and gas), as well as how matter can change from one phase to the next.
Table of Contents
Postulates of particle theory of matter, the properties of matter.
- Frequently Asked Questions – FAQs
The postulates of the particle theory of matter are given as:
1. All matter is made up of tiny particles known as atoms.
2. Particles of matter are constantly in motion.
3. Particles of matter attract each other.
4. Particles of matter have spaces between them.
5. As temperature increases, particles of matter move faster.
6. Atoms of the same element are essentially identical and atoms of different elements are different.
All matter is made up of tiny particles known as atoms.
Individual atoms or groups of atoms known as molecules make up the particles. Atoms are the most fundamental and smallest part that can exist of an element. A molecule is when two or more atoms are chemically bonded together.

Particles of matter are constantly in motion.
In the case of solids, the particles vibrate at their own position.
Particles in liquids and gases move from one location to another.
For example –
1. We can smell the food being prepared in the kitchen from a far because the tiny particles of food vapour mix with the air and move in all directions continuously through the process of diffusion.
2. A few drops of ink (potassium permanganate) are evenly distributed in the water.

Particles of matter attract each other.
Particles of matter are attracted to one another by force known as the intermolecular force of attraction. The intermolecular force of attraction varies between the three states of matter.
It is greatest in solids, which is why they are strong and do not change their structure. It is very low in gases due to which it can be compressed.
The force of attraction is weaker in liquids than in solids but not as weak as in gases. Liquids are not compressible as gases.

Read more: Three States of Matter
Particles of matter have spaces between them.
In the case of solids, particles are very close to each other and have very little space in between them.
Particles in liquids and gasses are a little far from each other and have more space than solids. For example – Sugar or salt completely dissolves in water.

As temperature increases, particles of matter move faster.
When the temperature rises, the kinetic energy of the particles rises, and they begin to vibrate. As a result, they move quickly, weakening the forces of attraction between the particles. This can eventually result in a change in the state of matter.

Read more: Change in the States of Matter
Atoms of the same element are essentially identical, and Atoms of different elements are different.
Every atom of a given element, such as gold, is identical to every other atom of that element. The atoms of one element are distinct from those of all other elements. A sodium atom is not the same as a carbon atom. Although some elements have similar boiling points, melting points, and electronegativities, no two elements have exactly the same set of properties.
Frequently Asked Questions on Particle Theory of Matter
What does particle theory explain.
The particle theory explains-
- The properties of matter.
- What happens when matter undergoes physical changes such as melting, boiling, and evaporation.
Why is water an exception in the particle model of matter?
Water behaves differently than most of other substances because its particles are less densely packed in their solid-state (ice) than in their liquid state. This explains why ice floats.
What are the limitations of the particle theory of matter?
The particle theory does not take into account:
- The size and shape of particles
- The space between particles
- Furthermore, the particle theory does not account for the forces that exist between particles. In the gas state, for example, some weak forces of attraction remain between particles.
Why is the particle theory important?
The particle theory is useful for two reasons.
- First, it provides a reasonable explanation for the matter’s behaviour.
- Second, it conveys an important concept that matter particles are always in motion. As a result, the particle model can explain the properties of solids, liquids, and gases.
What is the energy involved in the motion of particles?
Kinetic energy is involved in the motion of particles. When the temperature rises, the kinetic energy of the particles increases thus, aking particles move/vibrate with a greater speed.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2.1: The Atomic Theory of Matter
- Last updated
- Save as PDF
- Page ID 21697
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Explain how all matter is composed of atoms.
- Describe the modern atomic theory.
Take some aluminum foil. Cut it in half. Now there are two smaller pieces of aluminum foil. Cut one of the pieces in half again. Cut one of those smaller pieces in half again. Continue cutting, making smaller and smaller pieces of aluminum foil. It should be obvious that the pieces are still aluminum foil; they are just becoming smaller and smaller. But how far can this exercise be taken, at least in theory? Can one continue cutting the aluminum foil into halves forever, making smaller and smaller pieces? Or is there some limit, some absolute smallest piece of aluminum foil? Thought experiments like this—and the conclusions based on them—were debated as far back as the fifth century BC .
John Dalton (1766-1844) is the scientist credited for proposing the atomic theory. The theory explains several concepts that are relevant in the observable world: the composition of a pure gold necklace, what makes the pure gold necklace different than a pure silver necklace, and what occurs when pure gold is mixed with pure copper. This section explains the theories that Dalton used as a basis for his theory: (1) Law of Conservation of Mass , (2) Law of Definite Proportions , and (3) Law of Multiple Proportions
Law 1: The Conservation of Mass
"Nothing comes from nothing" is an important idea in ancient Greek philosophy that argues that what exists now has always existed , since no new matter can come into existence where there was none before. Antoine Lavoisier (1743-1794) restated this principle for chemistry with the law of conservation of mass, which "means that the atoms of an object cannot be created or destroyed, but can be moved around and be changed into different particles." This law says that when a chemical reaction rearranges atoms into a new product, the mass of the reactants (chemicals before the chemical reaction) is the same as the mass of the products (the new chemicals made). More simply, whatever you do, you will still have the same amount of stuff (however, certain nuclear reactions like fusion and fission can convert a small part of the mass into energy.
The law of conservation of mass states that the total mass present before a chemical reaction is the same as the total mass present after the chemical reaction; in other words, mass is conserved . The law of conservation of mass was formulated by Lavoisier as a result of his combustion experiment, in which he observed that the mass of his original substance—a glass vessel, tin, and air—was equal to the mass of the produced substance—the glass vessel, “tin calx”, and the remaining air.
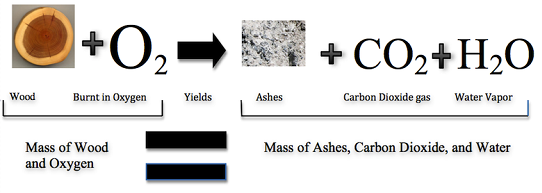
Historically, this was a difficult concept for scientists to grasp. If this law was true, then how could a large piece of wood be reduced to a small pile of ashes? The wood clearly has a greater mass than the ashes. From this observation scientists concluded that mass had been lost. However, Figure \(\PageIndex{1}\) shows that the burning of word does follow the law of conservation of mass. Scientists did not account for the gases that play a critical role in this reaction.
The law of conservation of mass states that the total mass present before a chemical reaction is the same as the total mass present after the chemical reaction.
Law 2: Definite Proportions
Joseph Proust (1754-1826) formulated the law of definite proportions (also called the Law of Constant Composition or Proust's Law ). This law states that if a compound is broken down into its constituent elements, the masses of the constituents will always have the same proportions, regardless of the quantity or source of the original substance. Joseph Proust based this law primarily on his experiments with basic copper carbonate. The illustration below depicts this law in action.
Law of Definite Proportions states that in a given type of chemical substance, the elements are always combined in the same proportions by mass.
The Law of Definite Proportions applies when elements are reacted together to form the same product. Therefore, while the Law of Definite Proportions can be used to compare two experiments in which hydrogen and oxygen react to form water, the Law of Definite Proportions can not be used to compare one experiment in which hydrogen and oxygen react to form water, and another experiment in which hydrogen and oxygen react to form hydrogen peroxide (peroxide is another material that can be made from hydrogen and oxygen).
Example \(\PageIndex{1}\): water
Oxygen makes up 88.8% of the mass of any sample of pure water, while hydrogen makes up the remaining 11.2% of the mass. You can get water by melting ice or snow, by condensing steam, from river, sea, pond, etc. It can be from different places: USA , UK , Australia, or anywhere. It can be made by chemical reactions like burning hydrogen in oxygen.
However, if the water is pure , it will always consist of 88.8 % oxygen by mass and 11.2 % hydrogen by mass, irrespective of its source or method of preparation.
Law 3: Multiple Proportions
Many combinations of elements can react to form more than one compound. In such cases, this law states that the weights of one element that combine with a fixed weight of another of these elements are integer multiples of one another. It's easy to say this, but please make sure that you understand how it works. Nitrogen forms a very large number of oxides, five of which are shown here.
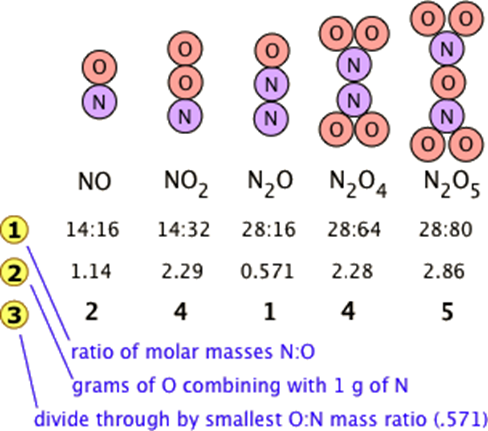
- Line is obtained by dividing the figures the previous line by the smallest O:N ratio in the line above, which is the one for N 2 O. Note that just as the law of multiple proportions says, the weight of oxygen that combines with unit weight of nitrogen work out to small integers.
- Of course we just as easily could have illustrated the law by considering the mass of nitrogen that combines with one gram of oxygen; it works both ways!
The law of multiple proportions states that if two elements form more than one compound between them, the masses of one element combined with a fixed mass of the second element form in ratios of small integers.
Example \(\PageIndex{2}\): Oxides of Carbon
Consider two separate compounds are formed by only carbon and oxygen. The first compound contains 42.9% carbon and 57.1% oxygen (by mass) and the second compound contains 27.3% carbon and 72.7% oxygen (again by mass). Is this consistent with the law of multiple proportions?
The Law of Multiple Proportions states that the masses of one element which combine with a fixed mass of the second element are in a ratio of whole numbers. Hence, the masses of oxygen in the two compounds that combine with a fixed mass of carbon should be in a whole-number ratio.
Thus for every 1 g of the first compound there are 0.57 g of oxygen and 0.429 g of carbon. The mass of oxygen per gram carbon is:
\[ \dfrac{0.571\; \text{g oxygen}}{0.429 \;\text{g carbon}} = 1.33\; \dfrac{\text{g oxygen}}{\text{g carbon}}\nonumber \]
Similarly, for 1 g of the second compound, there are 0.727 g oxygen and 0.273 g of carbon. The ration of mass of oxygen per gram of carbon is
\[ \dfrac{0.727\; \text{g oxygen}}{0.273 \;\text{g carbon}} = 2.66\; \dfrac{\text{g oxygen}}{\text{g carbon}}\nonumber \]
Dividing the mass of oxygen per g of carbon of the second compound:
\[\dfrac{2.66}{1.33} = 2\nonumber \]
Hence the masses of oxygen combine with carbon in a 2:1 ratio which s consistent with the Law of Multiple Proportions since they are whole numbers.
Dalton's Atomic Theory
The modern atomic theory, proposed about 1803 by the English chemist John Dalton (Figure \(\PageIndex{4}\)), is a fundamental concept that states that all elements are composed of atoms. Previously, an atom was defined as the smallest part of an element that maintains the identity of that element. Individual atoms are extremely small; even the largest atom has an approximate diameter of only 5.4 × 10 −10 m. With that size, it takes over 18 million of these atoms, lined up side by side, to equal the width of the human pinkie (about 1 cm).

Dalton’s ideas are called the modern atomic theory because the concept of atoms is very old. The Greek philosophers Leucippus and Democritus originally introduced atomic concepts in the fifth century BC. (The word atom comes from the Greek word atomos , which means “indivisible” or “uncuttable.”) Dalton had something that the ancient Greek philosophers didn’t have, however; he had experimental evidence, such as the formulas of simple chemicals and the behavior of gases. In the 150 years or so before Dalton, natural philosophy had been maturing into modern science, and the scientific method was being used to study nature. When Dalton announced a modern atomic theory, he was proposing a fundamental theory to describe many previous observations of the natural world; he was not just participating in a philosophical discussion.
Dalton's Theory was a powerful development as it explained the three laws of chemical combination (above) and recognized a workable distinction between the fundamental particle of an element (atom) and that of a compound (molecule). Six postulates are involved in Dalton's Atomic Theory:
- All matter consists of indivisible particles called atoms.
- Atoms of the same element are similar in shape and mass, but differ from the atoms of other elements.
- Atoms cannot be created or destroyed.
- Atoms of different elements may combine with each other in a fixed, simple, whole number ratios to form compound atoms.
- Atoms of same element can combine in more than one ratio to form two or more compounds.
- The atom is the smallest unit of matter that can take part in a chemical reaction.
In light of the current state of knowledge in the field of Chemistry, Dalton’s theory had a few drawbacks. According to Dalton’s postulates,
- The indivisibility of an atom was proved wrong: an atom can be further subdivided into protons, neutrons and electrons. However an atom is the smallest particle that takes part in chemical reactions.
- According to Dalton, the atoms of same element are similar in all respects. However, atoms of some elements vary in their masses and densities. These atoms of different masses are called isotopes. For example, chlorine has two isotopes with mass numbers 35 and 37.
- Dalton also claimed that atoms of different elements are different in all respects. This has been proven wrong in certain cases: argon and calcium atoms each have an same atomic mass (40 amu).
- According to Dalton, atoms of different elements combine in simple whole number ratios to form compounds. This is not observed in complex organic compounds like sugar (\(C_{12}H_{22}O_{11}\)).
- The theory fails to explain the existence of allotropes (different forms of pure elements); it does not account for differences in properties of charcoal, graphite, diamond.
Despite these drawbacks, the importance of Dalton’s theory should not be underestimated. He displayed exceptional insight into the nature of matter. and his ideas provided a framework that was later modified and expanded by other. Consequentiually, John Dalton is often considered to be the father of modern atomic theory.
Fundamental Experiments in Chemistry: Fundamental Experiments in Chemistry, YouTube(opens in new window) [youtu.be]
- Petrucci, Ralph, William Harwood, Geoffrey Herring, and Jeffry Madura. General Chemistry. 9th ed. Upper Saddle River, New Jersey: Pearson Prentince Hall, 2007
- Moore, John. Chemistry for Dummies. John Wiley & Sons Inc, 2002.
- Asimov, Isaac. A Short History of Chemistry. , CT .: Greenwood Press, 1965.
- Patterson, Elizabeth C. John Dalton and the Atomic Theory. Garden City, NY: Doubleday, 1970
- Myers, Richard. The Basics of Chemistry. Greenwood, 2003
- Demtröder, Wolfgang. Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics. 1st ed. Springer. 2002
This article explains the theories that Dalton used as a basis for his theory: (1) the Law of Conservation of Mass, (2) the Law of Constant Composition, (3) the Law of Multiple Proportions.

States of Matter Science Experiments

Are you using states of matter science experiments? Matter has three states; solid, liquid, and gas. Most students know and can identify the three states of matter in isolation. Still, to test their knowledge, they should experience hands on activities that allow them to see how the three states of matter interact with one another. If you do not include science experiments in your states of matter unit , your students miss this critical part of the scientific process. Including hands on activities does not have to be complicated. Read about five easy activities that you can incorporate into your states of matter unit.
Five Hands on Activities to Teach States of Matter
States of matter experiment #1 raisins dance :.
How much fun is it to dance? While it might be difficult to throw an upper elementary science dance party, making your science experiment dance doesn’t have to be as hard.
This activity requires three materials:
- Clear soda, such as Sprite
This simple states of matter experiment will allow students to see how solid, liquid, and gas substances react.
To perform the activity, fill the glass 3/4 full of the clear soda. Then, add the raisins. Watch what happens.
The science behind this experiment : Students will see the raisins “dancing” in the soda. The raisins will fall to the bottom of the glass and then float back up to the top. They will then fall again. What is happening in the carbonation gas from the soda adheres to the raisin. This causes the raisins to float to the top. Once the bubble pops at the surface, the raisin then falls to the bottom again.

States of Matter Experiment #2 Shaving Cream:
Shaving cream is a peculiar substance as it can be difficult to distinguish whether it is a solid or a liquid. This activity is also reasonably easy; however, it will take several days for students to fully process their observations and inferences .
Materials needed for this activity are:
- Shaving cream
- Paper towel
For this activity, you will put a blob of shaving cream on each student’s paper towel. Allow students to observe the shaving cream up close. If you have magnifying glasses, you may want students to use them to try to get as close a look as possible.
After students have had time to observe, allow them to hypothesize which state of matter they think shaving cream falls into.
The science behind the experiment : Shaving cream is a unique substance because its characteristics do not neatly fall into a solid, liquid, or gas category. When in a can, the shaving cream is a mixture of soap and water compressed as a gas. When the can is sprayed, the shaving cream is released as a solid, which eventually condenses to a liquid.
This activity is fun because it shows students how substances can change their state of matter over time. Even though the materials are simple, students love this activity.

States of Matter Experiment #3 Ice Cream in a Bag :
Who doesn’t love ice cream? No matter what time of year, this is a fun activity to show your students how temperature can affect the state of matter.
Materials needed :
- Vanilla extract
- Half and half
- Kosher or rock salt
- Ziploc bags
- Thermometer
Before the states of matter experiment, have a discussion with students about the characteristics of each substance and which state of matter it falls into. Combine the vanilla extract and half and half. Pour the mixture into a small Ziploc bag. Place the smaller bag into a larger one filled with ice and the Kosher or rock salt. Then SHAKE! Students can take turns shaking as this experiment can take up to ten minutes of shaking. After that time, the liquid should turn into a solid for a delicious treat.
The science behind this experiment : The salt lowers the temperature of the ice, which allows the half and half and vanilla to freeze. Be sure to use enough rock salt and ice, or the mixture will not freeze.
Grab the Ice Cream in a Bag activity for FREE by clicking the button below.
States of Matter Experiment #4 Air Balloons:
It can be challenging for students to understand that gases have mass and are spread out to fill their containers. This states of matter experiment will allow students to visualize this concept as the balloon fills up with carbon dioxide.
- One liter soda bottle
- Triple beam balance
Your science students will enjoy this simple states of matter experiment. All that needs to be done is to open the soda bottle cap and then place the balloon around its opening. Now, you will have to wait.
While you are waiting, indulge in a classroom conversation about the characteristics of states of matter.
The science behind this experiment : After about ten minutes, you should be able to observe the carbon dioxide gas fill the balloon.
To show students that the gas in the balloon has mass, place it on the triple beam balance to measure its mass. Compare this mass to an empty balloon to signify the difference.

States of Matter Experiment #5 Soapy States :
Teach students that states of matter are all around us. Pointing out real-life examples of a solid, liquid, or gas will help students make deeper connections to the content.
One everyday object that we can use to illustrate this point is soap. While it is super easy to distinguish that soap is solid, what happens when you put soap in the microwave?
- Two other brands of soap
- Bin of water
For this activity, students will observe differences between the different brands of soap. Placing the soaps into the water bin will allow students to see what the Ivory brand will float while the other brands sink.
The science behind this experiment : It is imperative to use Ivory soap as this is the only soap brand on the market that has air whipped into it. The air created little pockets of gas which allow this brand of soap to float in water.
It also allows this brand to expand when it is put into the microwave. When placed in the microwave for 30 seconds, gas pockets will expand, which creates an incredible visual for the students.
Your students will love these science activities to enhance your states of matter unit. Want these activities ready to go? Check them out here .

Need more ideas to teach your states of matter unit? Click the button below to learn how I teach this unit to my students.

- Read more about: Uncategorized
You might also like...

4 Engaging Strategies for Teaching Solar Eclipses

5 Interview Tips for Teachers

Earth’s Motions Project
On instagram, @ateachingmuse.
Please go to the Instagram Feed settings page to create a feed.
Join the Science Squad
Help | Advanced Search
Condensed Matter > Mesoscale and Nanoscale Physics
Title: theory of generalized landau levels and implication for non-abelian states.
Abstract: Quantum geometry is a fundamental concept to characterize the local properties of quantum states. It is recently demonstrated that saturating certain quantum geometric bounds allows a topological Chern band to share many essential features with the lowest Landau level, facilitating fractionalized phases in moiré flat bands. In this work, we systematically extend the consequence and universality of saturated geometric bounds to arbitrary Landau levels by introducing a set of single-particle states, which we term as ``generalized Landau levels''. These generalized Landau levels exhibit exactly quantized values of integrated trace of quantum metric determined by their corresponding Landau level indices, regardless of the nonuniformity of their quantum geometric quantities. We derive all geometric quantities for individual and multiple generalized Landau levels, discuss their relations, and understand them in light of the theory of holomorphic curves and moving frames. We further propose a model by superposing few generalized Landau levels which is supposed to capture a large portion of the single-particle Hilbert space of a generic Chern band analogous to the first Landau level. Using this model, we employ exact diagonalization to identify a single-particle geometric criterion for permitting the non-Abelian Moore-Read phase, which is potentially useful for future engineering of moiré materials and beyond. We use a double twisted bilayer graphene model with only adjacent layer hopping term to show the existence of first generalized Landau level type narrow band and zero-field Moore-Read state at the second magic angle which serves as a promising starting point for more detailed future studies. We expect that generalized Landau levels will serve as a systematic tool for analyzing topological Chern bands and fractionalized phases therein.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- INSPIRE HEP
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .

First proof that “plunging regions” exist around black holes in space
An international team led by researchers at Oxford University Physics have proved Einstein was correct about a key prediction concerning black holes. Using X-ray data to test Einstein’s theory of gravity, their study gives the first observational proof that a “plunging-region” exists around black holes: an area where matter stops circling the hole and instead falls straight in. Furthermore, the team found that this region exerts some of the strongest gravitational forces yet identified in the galaxy. The findings have been published in Monthly Notices of the Astronomical Society .
Einstein’s theory predicted that this final plunge would exist, but this is the first time we have been able to demonstrate it happening. Think of it like a river turning into a waterfall – hitherto, we have been looking at the river. This is our first sight of the waterfall. Lead author Dr Andrew Mummery , Oxford University Physics.
The new findings are part of wide-ranging investigations into outstanding mysteries around black holes by astrophysicists at Oxford University Physics. This study focused on smaller black holes relatively close to Earth, using X-ray data gathered from NASA’s space-based Nuclear Spectroscopic Telescope Array (NuSTAR) and Neutron star Interior Composition Explorer (NICER) telescopes. Later this year, a second Oxford team hopes to move closer to recording the first videos of larger, more distant black holes as part of a European initiative.
Unlike in Newton’s theory of gravity, Einstein’s theory states that sufficiently close to a black hole it is impossible for particles to safely follow circular orbits. Instead they rapidly “plunge” toward the black hole at close to the speed of light. The Oxford study assessed this region in depth for the first-time, using X-ray data to gain a better understanding of the force generated by black holes.
‘This is the first look at how plasma, peeled from the outer edge of a star, undergoes its final fall into the centre of a black hole, a process happening in a system around ten thousand light years away,’ said Dr Andrew Mummery , of Oxford University Physics, who led the study. ‘What is really exciting is that there are many black holes in the galaxy, and we now have a powerful new technique for using them to study the strongest known gravitational fields.’
‘Einstein’s theory predicted that this final plunge would exist, but this is the first time we have been able to demonstrate it happening,’ Dr Mummery continued. ‘Think of it like a river turning into a waterfall – hitherto, we have been looking at the river. This is our first sight of the waterfall.’
‘We believe this represents an exciting new development in the study of black holes, allowing us to investigate this final area around them. Only then can we fully understand the gravitational force,’ Mummery added. ‘This final plunge of plasma happens at the very edge of a black hole and shows matter responding to gravity in its strongest possible form.’

Debate between astrophysicists has been underway for many decades as to whether the so-called plunging region would be detectable. The Oxford team has spent the last couple of years developing models for it and, in the study just published, demonstrate its first confirmed detection found using X-ray telescopes and data from the International Space Station.
Whilst this study focuses on small black holes closer to Earth, a second study team from Oxford University Physics is part of a European initiative to build a new telescope, The Africa Millimetre Telescope , which would greatly enhance our ability to make direct images of black holes. Over 10 million Euro funding has already been secured, part of which will support several first PhDs in astrophysics for The University of Namibia, working closely with the Oxford Physics University team.
The new telescope is expected to enable observation, and filming, for the first time of large black holes at the centre of our own galaxy, as well as far beyond. As with the small black holes, large black holes are expected to have a so-called “event horizon”, dragging material from space toward their centre in a spiral as the black hole rotates. These represent almost unimaginable sources of energy and the team hope to observe – and film - them rotating for the first time.
The study “Continuum emission from within the plunging region of black hole discs” has been published in Monthly Notices of the Astronomical Society .
DISCOVER MORE
- Support Oxford's research
- Partner with Oxford on research
- Study at Oxford
- Research jobs at Oxford
You can view all news or browse by category

Is dark matter’s main rival theory dead? There’s bad news from the Cassini spacecraft and other recent tests
Postdoctoral Research Fellow in Astrophysics, University of St Andrews
Senior Research Fellow of Cosmology, University of Portsmouth
Disclosure statement
Indranil Banik receives funding from the Science and Technologies Facilities Council to test MOND using the dynamics of wide binary stars.
Harry Desmond does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Portsmouth and University of St Andrews provide funding as members of The Conversation UK.
View all partners
One of the biggest mysteries in astrophysics today is that the forces in galaxies do not seem to add up. Galaxies rotate much faster than predicted by applying Newton’s law of gravity to their visible matter, despite those laws working well everywhere in the Solar System.
To prevent galaxies from flying apart, some additional gravity is needed. This is why the idea of an invisible substance called dark matter was first proposed. But nobody has ever seen the stuff. And there are no particles in the hugely successful Standard Model of particle physics that could be the dark matter – it must be something quite exotic.
This has led to the rival idea that the galactic discrepancies are caused instead by a breakdown of Newton’s laws. The most successful such idea is known as Milgromian dynamics or Mond , proposed by Israeli physicist Mordehai Milgrom in 1982. But our recent research shows this theory is in trouble.
The main postulate of Mond is that gravity starts behaving differently to what Newton expected when it becomes very weak, as at the edges of galaxies. Mond is quite successful at predicting galaxy rotation without any dark matter, and it has a few other successes. But many of these can also be explained with dark matter, preserving Newton’s laws.
Read more: Dark matter: our review suggests it's time to ditch it in favour of a new theory of gravity
So how do we put Mond to a definitive test? We have been pursuing this for many years. The key is that Mond only changes the behaviour of gravity at low accelerations, not at a specific distance from an object. You’ll feel lower acceleration on the outskirts of any celestial object – a planet, star or galaxy – than when you are close to it. But it is the amount of acceleration, rather than the distance, that predicts where gravity should be stronger.
This means that, although Mond effects would typically kick in several thousand light years away from a galaxy, if we look at an individual star, the effects would become highly significant at a tenth of a light year. That is only a few thousand times larger than an astronomical unit (AU) – the distance between the Earth and the Sun. But weaker Mond effects should also be detectable at even smaller scales, such as in the outer Solar System.
This brings us to the Cassini mission , which orbited Saturn between 2004 and its final fiery crash into the planet in 2017. Saturn orbits the Sun at 10 AU. Due to a quirk of Mond, the gravity from the rest of our galaxy should cause Saturn’s orbit to deviate from the Newtonian expectation in a subtle way.

This can be tested by timing radio pulses between Earth and Cassini. Since Cassini was orbiting Saturn, this helped to measure the Earth-Saturn distance and allowed us to precisely track Saturn’s orbit. But Cassini did not find any anomaly of the kind expected in Mond. Newton still works well for Saturn.
One of us, Harry Desmond, recently published a study investigating the results in greater depth. Perhaps Mond would fit the Cassini data if we tweaked how we calculate galaxy masses from their brightness? That would affect how much of a boost to gravity Mond has to provide to fit models of galaxy rotation, and thus what we should expect for Saturn’s orbit.
Another uncertainty is the gravity from surrounding galaxies, which has a minor effect. But the study showed that, given how Mond would have to work to fit with models for galaxy rotation, it cannot also fit the Cassini radio tracking results – no matter how we tweak the calculations.
With the standard assumptions considered most likely by astronomers and allowing for a wide range of uncertainties, the chance of Mond matching the Cassini results is the same as a flipped coin landing heads up 59 times in a row. This is more than twice the “5 sigma” gold standard for a discovery in science, which corresponds to about 21 coin flips in a row.
More bad news for Mond
That’s not the only bad news for Mond. Another test is provided by wide binary stars – two stars that orbit a shared centre several thousand AU apart. Mond predicted that such stars should orbit around each other 20% faster than expected with Newton’s laws. But one of us, Indranil Banik, recently led a very detailed study that rules out this prediction. The chance of Mond being right given these results is the same as a fair coin landing heads up 190 times in a row.
Results from yet another team show that Mond also fails to explain small bodies in the distant outer Solar System. Comets coming in from out there have a much narrower distribution in energy than Mond predicts. These bodies also have orbits that are usually only slightly inclined to the plane that all the planets orbit close to. Mond would cause the inclinations to be much larger.
Newtonian gravity is strongly preferred over Mond on length scales below about a light year. But Mond also fails on scales larger than galaxies: it cannot explain the motions within galaxy clusters. Dark matter was first proposed by Fritz Zwicky in the 1930s to account for the random motions of galaxies within the Coma Cluster, which requires more gravity to hold it together than the visible mass can provide.
Mond cannot provide enough gravity either, at least in the central regions of galaxy clusters. But in their outskirts, Mond provides too much gravity. Assuming instead Newtonian gravity, with five times as much dark matter as normal matter, seems to provide a good fit to the data.
The standard dark matter model of cosmology isn’t perfect, however. There are things it struggles to explain , from the universe’s expansion rate to giant cosmic structures. So we may not yet have the perfect model. It seems dark matter is here to stay, but its nature may be different to what the Standard Model suggests. Or gravity may indeed be stronger than we think – but on very large scales only.
Ultimately though, Mond, as presently formulated, cannot be considered a viable alternative to dark matter any more. We may not like it, but the dark side still holds sway.
- Dark matter

Research Fellow

Senior Research Fellow - Women's Health Services

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health
Newfound 'glitch' in Einstein's relativity could rewrite the rules of the universe, study suggests
Einstein's theory of general relativity is our best description of the universe at large scales, but a new observation that reports a "glitch" in gravity around ancient structures could force it to be modified.

A strange "cosmic glitch" in gravity could explain the universe's weird behavior on the largest scales, researchers suggest.
First formulated by Albert Einstein in 1915, the theory of general relativity remains our best and most accurate understanding of how gravity works on medium to large scales.
Yet, zoom out even farther to view enormous groups of gravitationally bound galaxies interacting, and some inconsistencies appear to emerge. This suggests that gravity, which is theorized to be a constant across all times and scales, could actually become slightly weaker at cosmic distances.
In a study published March 20 in the Journal of Cosmology and Astroparticle Physics , researchers described this discrepancy as a "cosmic glitch," and they say their proposed fix for it could help us understand some of the universe's most enduring mysteries.
"[It's] like making a puzzle on the surface of a sphere, then laying the pieces on a flat table and trying to fit them together," study co-author Niayesh Afshordi , a professor of astrophysics at the University of Waterloo in Ontario, told Live Science. "At some point, the pieces on the table will not quite fit each other, because you are using the wrong framework.
Related: James Webb telescope confirms there is something seriously wrong with our understanding of the universe
"The glitch is the smoking gun for a fundamental violation of Einstein's equivalence principle (or Lorentz symmetry), which could point to radically different pictures for quantum gravity, the Big Bang , or black holes," Afshordi added.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Cosmos for concern
Einstein's theory of general relativity is remarkably good at describing the universe above quantum scales, and it has even predicted other aspects of our cosmos, including black holes , the gravitational lensing of light, gravitational waves, and the Big Bang.
Yet some discrepancies between theory and reality remain. First, attempts to scale down general relativity to describe how gravity operates on quantum scales transform its usually robust equations into incomprehensible nonsense.
Second, completing our current model of the universe required the introduction of two mysterious additions, known as dark matter and dark energy . Believed to make up most of the contents of the universe, these entities have never been directly detected and fail to explain why our cosmos is expanding at different speeds depending on where we look .
In response to these problems, the authors of the new paper came up with a simple suggestion: a tweak to Einstein's theory at different distance scales.
"The modification is very simple: We assume the universal constant of gravitation is different on cosmological scales, compared to smaller (like solar system or galactic) scales," Afshordi said. "We call this a cosmic glitch."
Afshordi said this tweak makes changes to patterns found in the cosmic microwave background — the leftover radiation produced 380,000 years after the Big Bang — and in the universe's structure and expansion. These adjustments are subtle, but the implication that the laws of gravity change over distance scales could be profound.
"We find evidence for the glitch: cosmic gravity is about 1% weaker than galactic/solar-system gravity," he added.
— Mysterious 'unparticles' may be pushing the universe apart, new theoretical study suggests
— 'It could be profound': How astronomer Wendy Freedman is trying to fix the universe
— James Webb telescope discovers oldest black hole in the universe
The researchers said the glitch's existence could be confirmed by next-generation galaxy surveys, including those performed with the European Space Agency 's Euclid space telescope , the Dark Energy Spectroscopic Instrument and the Simons Observatory . They say that these instruments should make measurements of the glitch four times more precise than is currently possible and, therefore, confirm or rule out their theory.
However, some scientists say a simple modification of Einstein's relativity might not be enough. In fact, it's possible that the discrepancies revealed by astronomical observations are hints that our understanding of the universe needs a complete rewrite.
"It's not that surprising that this new model is a slightly better fit to the data, but maybe that is telling us something," said Scott Dodelson , a professor of physics and the chair of the physics department at Carnegie Mellon University, who was not involved in the study.
"If so, it means we understand even less than we thought we did," he told Live Science. "My hunch is that instead of adding more new stuff, we need a new paradigm. But no one has come up with anything that makes any sense yet."
Ben Turner is a U.K. based staff writer at Live Science. He covers physics and astronomy, among other topics like tech and climate change. He graduated from University College London with a degree in particle physics before training as a journalist. When he's not writing, Ben enjoys reading literature, playing the guitar and embarrassing himself with chess.
Euclid space telescope reveals more than 300,000 new objects in 1st 24 hours of observations (photos)
Can a commercial airplane do a barrel roll?
Parasitic worms infect 6 after bear meat served at family reunion
Most Popular
- 2 Can a commercial airplane do a barrel roll?
- 3 The same genetic mutations behind gorillas' small penises may hinder fertility in men
- 4 Scientists grow diamonds from scratch in 15 minutes thanks to groundbreaking new process
- 5 Lost photos suggest Mars' mysterious moon Phobos may be a trapped comet in disguise
- 2 Scientists grow diamonds from scratch in 15 minutes thanks to groundbreaking new process
- 3 50,000-year-old Neanderthal bones harbor oldest-known human viruses
- 4 Scientists just discovered an enormous lithium reservoir under Pennsylvania
- 5 Ancient Mycenaean armor is so good, it protected users in an 11-hour battle simulation inspired by the Trojan War

Scientists prove that plunging regions exist around black holes in space
Scientists have proven one of Einstein’s theories, finding evidence that a plunging-region around black holes not only exists, but also exerts some of the strongest gravitational forces yet identified in the galaxy.
Einstein’s theory states that it is impossible for particles to safely follow circular orbits when close to a black hole.
Instead they rapidly plunge towards the object at close to the speed of light – giving the plunging region its name.
Einstein’s theory predicted that this final plunge would exist, but this is the first time we’ve been able to demonstrate it happening
Experts say the findings show matter responding to gravity in its “strongest possible form”.
The new study focused on this region in depth for the first time, with Oxford University Physics researchers using X-ray data to gain a better understanding of the force generated by black holes.
Dr Andrew Mummery, of Oxford University Physics, who led the study, said: “What’s really exciting is that there are many black holes in the galaxy, and we now have a powerful new technique for using them to study the strongest known gravitational fields.”
He added: “Einstein’s theory predicted that this final plunge would exist, but this is the first time we’ve been able to demonstrate it happening.
“Think of it like a river turning into a waterfall – hitherto, we have been looking at the river. This is our first sight of the waterfall.”
“We believe this represents an exciting new development in the study of black holes, allowing us to investigate this final area around them.
“Only then can we fully understand the gravitational force.
“This final plunge of plasma happens at the very edge of a black hole and shows matter responding to gravity in its strongest possible form.”
Researchers say that there has been much debate between astrophysicists for many decades as to whether the so-called plunging region would be detectable.
The Oxford team spent the last couple of years developing models for it and, in the study just published, demonstrate its first confirmed detection found using X-ray telescopes and data from the international space station.
The study, published in the Monthly Notices of the Astronomical Society, focused on smaller black holes relatively close to Earth , using X-ray data gathered from space-based telescopes.
Later this year, a second Oxford team hopes to move closer to filming first footage of larger, more distant black holes.
Register now for one of the Evening Standard’s newsletters. From a daily news briefing to Homes & Property insights, plus lifestyle, going out, offers and more. For the best stories in your inbox, click here .


IMAGES
VIDEO
COMMENTS
States of Matter - PhET Interactive Simulations
Describe the behavior of an ideal gas. The kinetic-molecular theory is a theory that explains the states of matter and is based on the idea that matter is composed of tiny particles that are always in motion. The theory helps explain observable properties and behaviors of solids, liquids, and gases. However, the theory is most easily understood ...
Learn about the basic states of matter and how they change with temperature and pressure in this interactive simulation. Experiment with different types of atoms and molecules and observe their behavior in different phases. Compare the properties of solids, liquids and gases and discover how they relate to the atomic bonding and motion.
How many states of matter there are depends on whom you ask and how they distinguish phases. This count could range anywhere from 5 or 6, to hundreds. But for a beginning chemistry student, there are only four that are important. Plasma is mainly important in understanding lightning and certain modern technologies.
Figure 2.1.1: States of Matter. All three containers contain a substance with the same mass, but the substances are in different states. In the left-hand container, the substance is a gas, which has spread to fill its container. It takes both the shape and volume of the container. In the middle container, the substance is a liquid, which has ...
2.2: Kinetic-Molecular Theory. The kinetic-molecular theory is a theory that explains the states of matter and is based on the idea that matter is composed of tiny particles that are always in motion. The theory helps explain observable properties and behaviors of solids, liquids, and gases. However, the theory is most easily understood as it ...
We recommend using the latest version of Chrome, Firefox, Safari, or Edge. Watch different types of molecules form a solid, liquid, or gas. Add or remove heat and watch the phase change. Change the temperature or volume of a container and see a pressure-temperature diagram respond in real time. Relate the interaction potential to the forces ...
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose-Einstein condensates and Fermionic ...
A hands-on exploration of states of matter, suitable for ages 8-10; grades 3-5. Many Kinds of Matter (A Look at Solids, Liquids, and Gases) by Jennifer Boothroyd. Lerner, 2010. A very simple 32-page outline for ages 6-9, grades 1-3. It covers the basic states and changing between them, with clear, real-world examples.
The particle (or kinetic) theory of matter can be summarised as: All matter is formed of tiny particles. The particles are constantly randomly moving about. The particles can be arranged regularly or randomly. The particles are held together by weak or strong forces. As temperature increases, the particles move faster.
The four main states of matter are solids, liquids, gases, and plasma. States of matter are forms in which matter exists. The four states of matter observed in everyday life are solids, liquids, gases, and plasma. Other states of matter also exist, although they require special conditions. Here is a look at the states of matter, their ...
III. The Kinetic-Molecular Theory of Matter A. The kinetic-molecular theory of matter is the theory used to explain the different states of matter. 1. Ancient Greeks proposed pieces of the theory over 2400 years ago. B. Generalizations that can be made based on this theory include: 1. Matter is composed of tiny particles called molecules. 2.
There are many states of matter beyond solids, liquids, and gases, including plasmas, condensates, superfluids, supersolids, and strange matter. This module introduces Kinetic Molecular Theory, which explains how the energy of atoms and molecules results in different states of matter. The module also explains the process of phase transitions in matter.
Particle Theory of Matter. Matter is anything that has mass and takes up space. It is a general name we call all the physical things around us. Matter includes things so tiny humans can't see them with their eyes. The Particle Theory of Matter is a scientific model. A scientific model is a way of illustrating ideas, objects and processes so ...
Chemistry. States of matter: Definition and phases of change. References. By Mary Bagley. last updated 6 October 2023. The four fundamental states of matter are solid, liquid, gas and plasma, but ...
The kinetic particle theory close kinetic theory The use of the arrangement and movement of particles to describe solids, liquids and gases. of matter close matter Sub-atomic particles and ...
Compton's formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie's hypothesis of matter waves.In 1926, De Broglie's hypothesis, together with Bohr's early ...
Theory and discovery Bose-Einstein condensates were first predicted theoretically in the 1920s by Satyendra Nath Bose (1894-1974), an Indian physicist who also discovered the subatomic particle ...
The postulates of the particle theory of matter are given as: 1. All matter is made up of tiny particles known as atoms. 2. Particles of matter are constantly in motion. 3. Particles of matter attract each other. 4. Particles of matter have spaces between them.
This article explains the theories that Dalton used as a basis for his theory: (1) the Law of Conservation of Mass, (2) the Law of Constant Composition, (3) the Law of Multiple Proportions. 2.1: The Atomic Theory of Matter is shared under a license and was authored, remixed, and/or curated by Stephen Lower & LibreTexts.
This simple states of matter experiment will allow students to see how solid, liquid, and gas substances react. To perform the activity, fill the glass 3/4 full of the clear soda. Then, add the raisins. Watch what happens. The science behind this experiment: Students will see the raisins "dancing" in the soda.
Theory of Generalized Landau Levels and Implication for non-Abelian States. Quantum geometry is a fundamental concept to characterize the local properties of quantum states. It is recently demonstrated that saturating certain quantum geometric bounds allows a topological Chern band to share many essential features with the lowest Landau level ...
Using X-ray data to test Einstein's theory of gravity, their study gives the first observational proof that a "plunging-region" exists around black holes: an area where matter stops circling the hole and instead falls straight in. Furthermore, the team found that this region exerts some of the strongest gravitational forces yet identified ...
Assuming instead Newtonian gravity, with five times as much dark matter as normal matter, seems to provide a good fit to the data. The standard dark matter model of cosmology isn't perfect, however.
Einstein's theory of general relativity is our best description of the universe at large scales, but a new observation that reports a "glitch" in gravity around ancient structures could force it ...
Release. May 7, 2024. ( 2024-05-07) -. present. ( present) Dark Matter is an American science fiction limited series created by Blake Crouch, based on his 2016 novel of the same name. It premiered on Apple TV+ on May 8, 2024, with two episodes. [1]
Einstein's theory states that it is impossible for particles to safely follow circular orbits when close to a black hole. Instead they rapidly plunge towards the object at close to the speed of ...
The Texas Board of Pardons has recommended a full pardon to Daniel Perry, a US Army sergeant who was sentenced to 25 years in prison after being convicted of murdering a protester at a Black Lives ...
The death of Iran's president will spark a high-stakes power struggle. Update (May 20th): This article was updated after Iranian state media confirmed Mr Raisi's death. T HREE YEARS ago, when ...