- Analytical Chemistry

Paper Chromatography
What is paper chromatography.
Chromatography technique that uses paper sheets or strips as the adsorbent being the stationary phase through which a solution is made to pass is called paper chromatography. It is an inexpensive method of separating dissolved chemical substances by their different migration rates across the sheets of paper. It is a powerful analytical tool that uses very small quantities of material. Paper chromatography was discovered by Synge and Martin in the year 1943.
Table of Contents
Paper chromatography principle, paper chromatography diagram, paper chromatography procedure, paper chromatography applications.
- Types of Paper Chromatography
- Frequently Asked Questions – FAQs
The principle involved can be partition chromatography or adsorption chromatography. Partition chromatography because the substances are partitioned or distributed between liquid phases. The two phases are water held in pores of the filter paper and the other phase is a mobile phase which passes through the paper. When the mobile phase moves, the separation of the mixture takes place. The compounds in the mixture separate themselves based on the differences in their affinity towards stationary and mobile phase solvents under the capillary action of pores in the paper. Adsorption chromatography between solid and liquid phases, wherein the solid surface of the paper is the stationary phase and the liquid phase is the mobile phase.
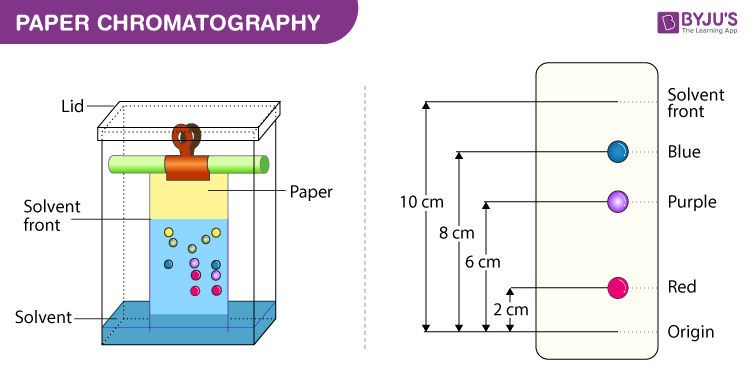
Below we have explained the procedure to conduct Paper Chromatography Experiment for easy understanding of students.
- Selecting a suitable type of development: It is decided based on the complexity of the solvent, paper, mixture, etc. Usually ascending type or radial paper chromatography is used as they are easy to perform. Also, it is easy to handle, the chromatogram obtained is faster and the process is less time-consuming.
- Selecting a suitable filter paper : Selection of filter paper is done based on the size of the pores and the sample quality.
- Prepare the sample: Sample preparation includes the dissolution of the sample in a suitable solvent (inert with the sample under analysis) used in making the mobile phase.
- Spot the sample on the paper: Samples should be spotted at a proper position on the paper by using a capillary tube.
- Chromatogram development: Chromatogram development is spotted by immersing the paper in the mobile phase. Due to the capillary action of paper, the mobile phase moves over the sample on the paper.
- Paper drying and compound detection : Once the chromatogram is developed, the paper is dried using an air drier. Also, detecting solution can be sprayed on the chromatogram developed paper and dried to identify the sample chromatogram spots.
There are various applications of paper chromatography . Some of the uses of Paper Chromatography in different fields are discussed below:
- To study the process of fermentation and ripening.
- To check the purity of pharmaceuticals.
- To inspect cosmetics.
- To detect the adulterants.
- To detect the contaminants in drinks and foods.
- To examine the reaction mixtures in biochemical laboratories.
- To determine dopes and drugs in humans and animals.
Types of paper chromatography:
- Ascending Paper Chromatography – The techniques goes with its name as the solvent moves in an upward direction.
- Descending Paper Chromatography – The movement of the flow of solvent due to gravitational pull and capillary action is downwards, hence the name descending paper chromatography.
- Ascending – Descending Paper Chromatography – In this version of paper chromatography, movement of solvent occurs in two directions after a particular point. Initially, the solvent travels upwards on the paper which is folded over a rod and after crossing the rod it continues with its travel in the downward direction.
- Radial or Circular Paper Chromatography – The sample is deposited at the centre of the circular filter paper. Once the spot is dried, the filter paper is tied horizontally on a Petri dish which contains the solvent.
- Two Dimensional Paper Chromatography – Substances which have the same r f values can be resolved with the help of two-dimensional paper chromatography.
Frequently Asked Questions – FAQs
What are the advantages of paper chromatography.
Paper Chromatography Has Many Benefits Simple and rapid Paper chromatography necessitates a minimal amount of quantitative material. Paper chromatography is less expensive than other chromatography methods. The paper chromatography method can identify both unknown inorganic and organic compounds. Paper chromatography takes up little space when compared to other analytical methods or equipment. Outstanding resolving power
Why water is not used in paper chromatography?
It is preferable to use a less polar solvent, such as ethanol, so that the non-polar compounds will travel up the paper while the polar compounds will stick to the paper, separating them.
What are the limitations of Paper Chromatography?
Limitations of Paper Chromatography are as follows- Paper chromatography cannot handle large amounts of sample. Paper chromatography is ineffective in quantitative analysis. Paper chromatography cannot separate complex mixtures. Less Accurate than HPLC or HPTLC
What is the importance of paper chromatography?
Paper chromatography has traditionally been used to analyse food colours in ice creams, sweets, drinks and beverages, jams and jellies. Only edible colours are permitted for use to ensure that no non-permitted colouring agents are added to the foods. This is where quantification and identification come into play.
Is paper chromatography partition or adsorption?
A type of partition chromatography is paper chromatography.
To learn more about the different types of paper chromatography from the experts, register with BYJU’S now!
Other important links:

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
It is so easy to understand by students Explained with applications also.
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Microbe Notes
Paper Chromatography- Definition, Types, Principle, Steps, Uses
Table of Contents
Interesting Science Videos
What is Paper Chromatography?
Paper chromatography (PC) is a type of planar chromatography whereby chromatography procedures are run on a specialized paper.
PC is considered to be the simplest and most widely used of the chromatographic techniques because of its applicability to isolation, identification, and quantitative determination of organic and inorganic compounds.
It was first introduced by German scientist Christian Friedrich Schonbein (1865).
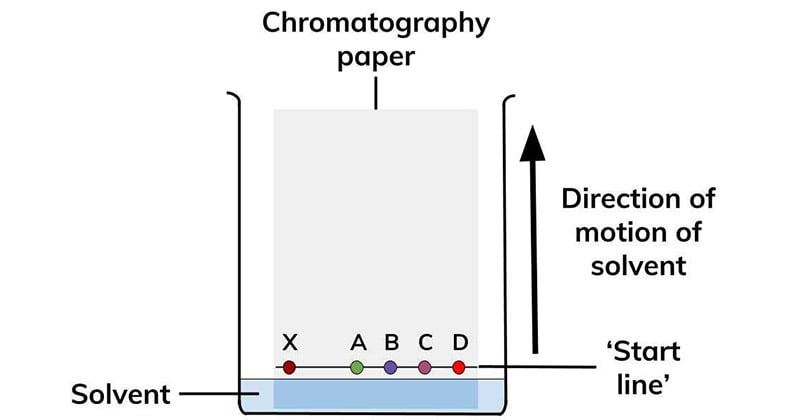
Types of Paper chromatography
Paper adsorption chromatography.
Paper impregnated with silica or alumina acts as adsorbent (stationary phase) and solvent as mobile phase.
Paper Partition Chromatography
Moisture / Water present in the pores of cellulose fibers present in filter paper acts as stationary phase & another mobile phase is used as solvent In general paper chromatography mostly refers to paper partition chromatography.
Principle of Paper chromatography
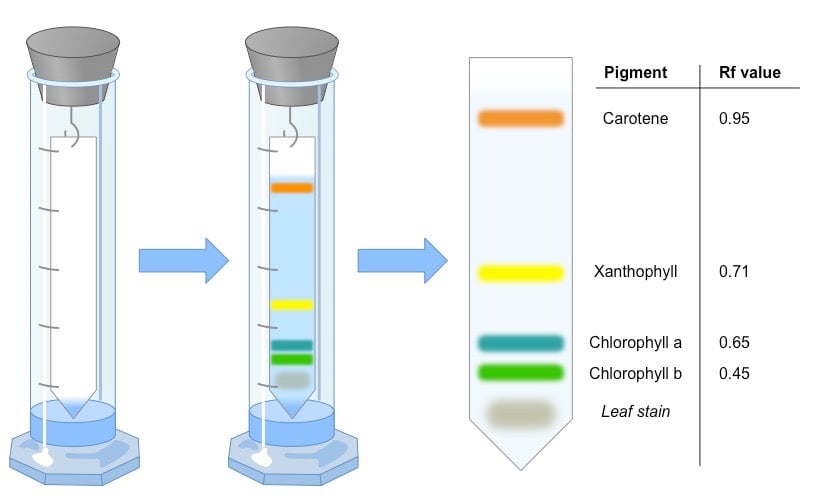
The principle of separation is mainly partition rather than adsorption. Substances are distributed between a stationary phase and a mobile phase. Cellulose layers in filter paper contain moisture which acts as a stationary phase. Organic solvents/buffers are used as mobile phase. The developing solution travels up the stationary phase carrying the sample with it. Components of the sample will separate readily according to how strongly they adsorb onto the stationary phase versus how readily they dissolve in the mobile phase.
Instrumentation of Paper chromatography
- Stationary phase & papers used
- Mobile phase
- Developing Chamber
- Detecting or Visualizing agents
1. STATIONARY PHASE AND PAPERS
- Whatman filter papers of different grades like No.1, No.2, No.3, No.4, No.20, No.40, No.42 etc
- In general the paper contains 98-99% of α-cellulose, 0.3 – 1% β -cellulose.
Other modified papers
- Acid or base washed filter paper
- Glass fiber type paper.
- Hydrophilic Papers – Papers modified with methanol, formamide, glycol, glycerol etc.
- Hydrophobic papers – acetylation of OH groups leads to hydrophobic nature, hence can be used for reverse phase chromatography.
- Impregnation of silica, alumna, or ion exchange resins can also be made.
2. PAPER CHROMATOGRAPHY MOBILE PHASE
- Pure solvents, buffer solutions or mixture of solvents can be used.
Hydrophilic mobile phase
- Isopropanol: ammonia:water 9:1:2
- Methanol : water 4:1
- N-butanol : glacial acetic acid : water 4:1:5
Hydrophobic mobile phases
- dimethyl ether: cyclohexane kerosene : 70% isopropanol
- The commonly employed solvents are the polar solvents, but the choice depends on the nature of the substance to be separated.
- If pure solvents do not give satisfactory separation, a mixture of solvents of suitable polarity may be applied.
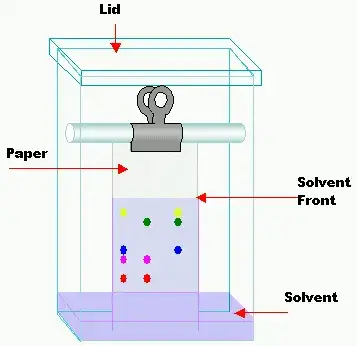
3. CHROMATOGRAPHIC CHAMBER
- The chromatographic chambers are made up of many materials like glass, plastic or stainless steel . Glass tanks are preferred most.
- They are available invarious dimensional size depending upon paper length and development type.
- The chamber atmosphere should be saturated with solvent vapor.
Steps in Paper Chromatography
In paper chromatography, the sample mixture is applied to a piece of filter paper, the edge of the paper is immersed in a solvent, and the solvent moves up the paper by capillary action. The basic steps include:
Selection of Solid Support
Fine quality cellulose paper with defined porosity, high resolution, negligible diffusion of the sample, and favoring good rate of movement of solvent.
Selection of Mobile Phase
Different combinations of organic and inorganic solvents may be used depending on the analyte.
Example. Butanol: Acetic acid: Water (12:3:5) is a suitable solvent for separating amino acids.
Saturation of Tank
The inner wall of the tank is wrapped with filter paper before the solvent is placed in the tank to achieve better resolution.
Sample Preparation and Loading
If the solid sample is used, it is dissolved in a suitable solvent. Sample (2-20ul) is added on the baseline as a spot using a micropipette and air dried to prevent the diffusion.
Development of the Chromatogram
Different types of development techniques can be used:
ASCENDING DEVELOPMENT
- Like conventional type, the solvent flows against gravity.
- The spots are kept at the bottom portion of paper and kept in a chamber with mobile phase solvent at the bottom.
DESCENDING TYPE
- This is carried out in a special chamber where the solvent holder is at the top.
- The spot is kept at the top and the solvent flows down the paper.
- In this method solvent moves from top to bottom so it is called descending chromatography.
ASCENDING – DESCENDING DEVELOPMENT
- A hybrid of above two techniques is called ascending-descending chromatography.
- Only length of separation increased, first ascending takes place followed by descending.
CIRCULAR / RADIAL DEVELOPMENT
- Spot is kept at the centre of a circular paper.
- The solvent flows through a wick at the centre & spreads in all directions uniformly.
Drying of Chromatogram
After the development, the solvent front is marked and left to dry in a dry cabinet or oven.
Colorless analytes were detected by staining with reagents such as iodine vapor, ninhydrin, etc.
Radiolabeled and fluorescently labeled analytes were detected by measuring radioactivity and fluorescence respectively.
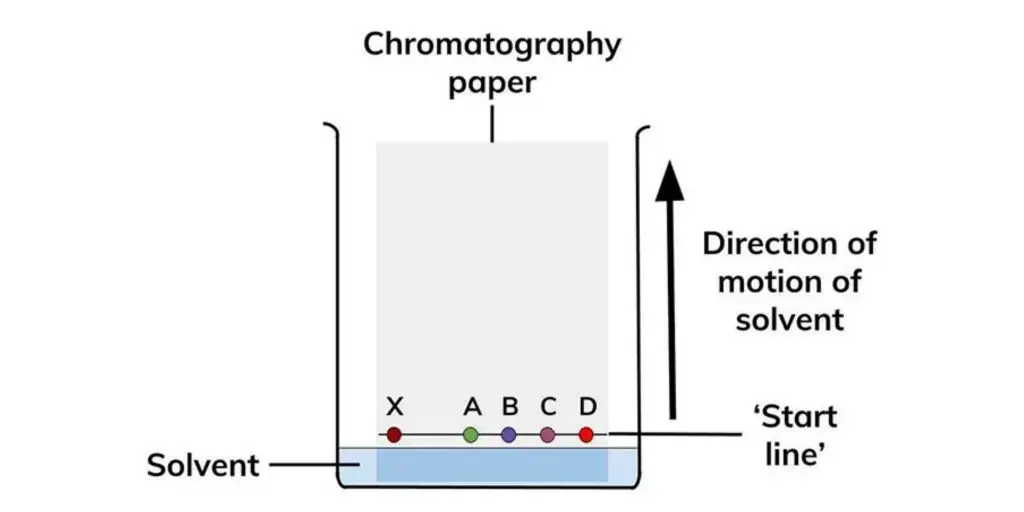
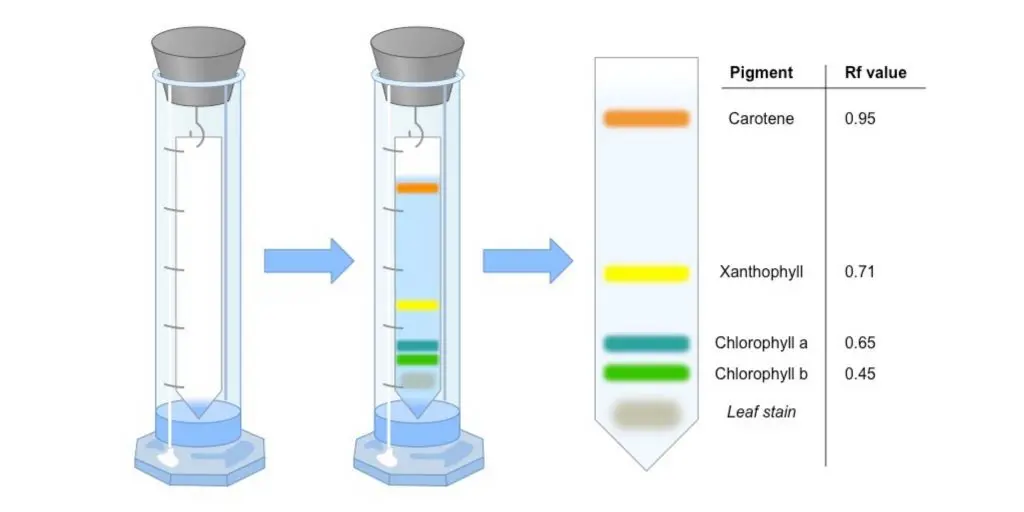
Some compounds in a mixture travel almost as far as the solvent does; some stay much closer to the baseline . The distance traveled relative to the solvent is a constant for a particular compound as long as other parameters such as the type of paper and the exact composition of the solvent are constant. The distance traveled relative to the solvent is called the Rf value.
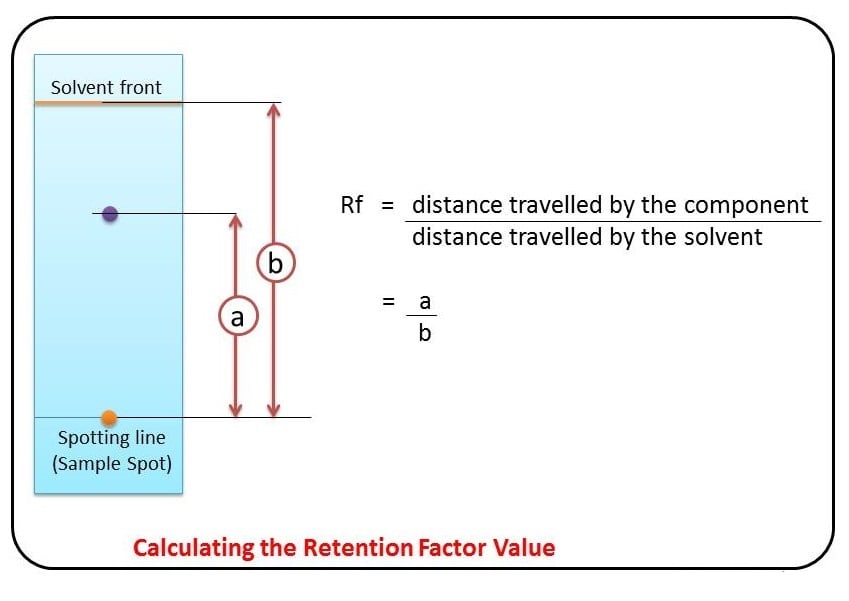
Thus, in order to obtain a measure of the extent of movement of a component in a paper chromatography experiment, “Rf value” is calculated for each separated component in the developed chromatogram. An Rf value is a number that is defined as the distance traveled by the component from the application point.
Applications of Paper Chromatography
- To check the control of purity of pharmaceuticals,
- For detection of adulterants,
- Detect the contaminants in foods and drinks,
- In the study of ripening and fermentation,
- For the detection of drugs and dopes in animals & humans
- In analysis of cosmetics
- Analysis of the reaction mixtures in biochemical labs.
Advantages of Paper Chromatography
- Paper Chromatography requires very less quantitative material.
- Paper Chromatography is cheaper compared to other chromatography methods.
- Both unknown inorganic as well as organic compounds can be identified by paper chromatography method.
- Paper chromatography does not occupy much space compared to other analytical methods or equipments.
- Excellent resolving power
Limitations of Paper Chromatography
- Large quantity of sample cannot be applied on paper chromatography.
- In quantitative analysis paper chromatography is not effective.
- Complex mixture cannot be separated by paper chromatography.
- Less Accurate compared to HPLC or HPTLC
- http://frndzzz.com/Advantages-and-Disadvantages-of-Paper-Chromatography
- https://www.slideshare.net/shaisejacob/paper-chromatography-pptnew?next_slideshow=1
- https://www.slideshare.net/shaisejacob/paper-chromatography-ppt-new
- https://www.biochemden.com/paper-chromatography/
- http://web.engr.oregonstate.edu/~rochefow/K-12%20Outreach%20Activities/Microfluidics%20&%20Pregnancy%20Test%20Kit%20Lab/paper%20chromatography_Chemguide.pdf
- https://pubs.acs.org/doi/abs/10.1021/ac60051a002
About Author
Sagar Aryal

6 thoughts on “Paper Chromatography- Definition, Types, Principle, Steps, Uses”
Examples of substances that can be effectively separated by paper chromatography is necessary.
I enjoy this write up, but the definition of Rf Values just mention distance traveled by the solute from the point from the point of application of the sample, how about the total distance traveled by the solvent? Some examples of how Rf value can be calculated is necessary.
Pls how is charging a disadvantage of filter paper in chromatography
Hi! I enjoyed reading it. Hmm just wanna know somepoints.
Why it is best to use the farthest distance traveled by a sugar if and when the solvent went over the paper and what is the purpose of heating the chromatographic paper after running the procedure?
This links are so much useful and it’s very helpful also….
Thanks for sharing important details like this. I enjoyed reading your site, and love to know the latest updates.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Paper Chromatography
How does paper chromatography work, stationary and mobile phases, paper chromatography experiment, applications.
Paper chromatography is a simple and cost-effective separation technique that separates and identifies different components in a mixture. [1-4]
In paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper. As the solvent moves up through capillary action, it carries along the individual components of the mixture at different rates based on their solubility and affinity for the stationary phase.
The principle behind paper chromatography lies in the differential partitioning of compounds between the stationary and mobile phases. The stationary phase typically consists of cellulose fibers embedded in filter paper or thin-layer chromatography plates. These fibers provide an adsorbent surface for compounds to interact with.
Understanding the mechanism behind paper chromatography requires knowledge of several key processes. [1-4]
The first process is capillary action, which refers to the ability of liquids to flow through narrow spaces against gravity. In paper chromatography, capillary action allows the solvent to move up the paper strip due to its attraction to the fibers in the paper. As the solvent moves up, it carries the solutes in the analyzed mixture. This migration of solutes is driven by two main mechanisms: adsorption and partitioning.
Adsorption occurs when solute molecules adhere to the fibers or other surfaces within the paper. It can be influenced by polarity and molecular size, with more polar or larger molecules having stronger interactions with the stationary phase.
Conversely, partitioning involves solute molecules distributing themselves between two immiscible phases – in this case, between the stationary phase (paper) and mobile phase (solvent). The extent of partitioning depends on factors such as solute polarity and affinity for either phase.
As solutes migrate up through capillary action, they may experience different degrees of adsorption and partitioning along their journey. This results in their separation based on their characteristics. By analyzing how far each component migrates on a chromatogram – a visual representation of separated components – scientists can determine properties such as retention factor (R f ) values and identify unknown substances based on known reference compounds.

Stationary and mobile phases play crucial roles in separating components of a mixture. [1-4]
The stationary phase refers to the absorbent material fixed on the chromatography paper. It can be made of cellulose or other materials with high absorbency. The stationary phase acts as a substrate for the sample mixture to interact with during separation.
On the other hand, the mobile phase is the solvent or liquid that moves through the stationary phase, carrying the sample components. The mobile phase must have good solubility with the components of interest. It should be able to flow easily through the paper.
As the mobile phase moves through the stationary phase, it interacts differently with each mixture component based on their solubility and affinity for both phases. This differential interaction leads to separation as different components travel at different rates along the paper.
Choosing an appropriate combination of stationary and mobile phases is important for effective separation in paper chromatography. Factors such as polarity, viscosity, and compatibility between phases must be considered to achieve optimal results.
Performing a paper chromatography experiment involves several essential steps to ensure accurate results. The process begins with preparing samples for paper chromatography, then spotting the sample on the paper strip, and finally, developing the chromatogram. [1-4]
Preparing the samples is crucial in obtaining reliable data. It involves selecting appropriate substances to analyze and ensuring they are suitable for chromatography. Samples can be liquid or solid and must be dissolved or crushed into a solution before application.
Next, spotting the sample on the paper strip is done carefully to ensure accurate separation. A small spot of the prepared sample is placed near one end of a designated area on the filter paper strip. It is essential to use a capillary tube or micropipette for precise and consistent application.
Once all samples are spotted on the filter paper strip, it is time for the development of the chromatogram. This step involves placing one end of the strip into a solvent traveling up through capillary action. The choice of solvent depends on factors such as solubility and desired separation distance.
As the solvent moves up through the filter paper strip, it carries different components in each sample. These components separate based on their affinity for stationary (filter paper) and mobile (solvent) phases. The separation occurs due to differences in molecular size, polarity, or other physical properties.
Throughout this process, it is important to maintain controlled conditions such as temperature and humidity to ensure reproducibility. Further analysis can be conducted once an optimal separation has been achieved, which can take several minutes or hours depending on various factors, including solvent choice and sample composition.
The diverse applications of paper chromatography across various fields are listed below. [1-4]
- It plays a crucial role in forensic analysis by separating and identifying different components in complex mixtures, such as blood or ink samples.
- Aids in the analysis of crime scene evidence, allowing forensic scientists to determine the presence of specific substances and identify potential suspects based on chromatographic patterns
- Enables the separation of different dyes used in food coloring, helping to ensure compliance with regulatory standards and quality control measures
- Determines the authenticity and safety of food products by identifying and quantifying specific components present in complex food matrices
- Separate and identify active ingredients, impurities, and by-products in pharmaceutical formulations.
- Chem.libretexts.org
- Swe.mit.edu
- Chemlab.truman.edu
Trending Topics
© 2024 ( Chemistry Learner )
Paper Chromatography – Principle, Types, Instrumentation, Steps
- AP Biology Note Podcast Free
- DeepDive into Cell Biology Podcast Free
- DeepDive into Biochemistry Podcast Free
What is Paper Chromatography?
Types of paper chromatography, principle of paper chromatography, 1. stationary phase & papers, 2. mobile phase, 3. chromatographic chamber, steps in paper chromatography, rf values – retention factors, applications of paper chromatography, advantages of paper chromatography, limitations of paper chromatography, what is paper chromatography, can paper chromatography be used for quantitative analysis, what are the different development techniques in paper chromatography, how can the components on a paper chromatogram be detected, how can i ensure the best results in paper chromatography, what are the limitations of paper chromatography, how is an rf value calculated in paper chromatography, how does paper chromatography work, what are the applications of paper chromatography, what are the advantages of paper chromatography.
- Paper chromatography is a sort of planar chromatography in which chromatographic techniques are carried out on specialized paper. Because of its capacity to separate, identify, and quantitatively quantify both organic and inorganic substances, it is largely considered as the simplest and most often used chromatographic method.
- Christian Friedrich Schonbein, a German physicist, pioneered the concept of paper chromatography in 1865. Originally, it was used to separate colored compounds or substances as an analytical procedure. It has, however, mostly evolved into a teaching tool, with alternative chromatography techniques, such as thin-layer chromatography (TLC), taking its place in laboratory applications.
- Three fundamental components comprise the paper chromatography setup. Through capillary action, the mobile phase, which is a solution, travels up to the stationary phase. The mobile phase is often a combination of non-polar organic solvents, whereas the stationary phase usually a polar inorganic solvent such as water. The paper serves as a support for the stationary phase (water) and has a cellulose network that binds polar water molecules in its void spaces. It is crucial to highlight that in paper chromatography, the stationary phase is less absorbent paper, as opposed to TLC, where the stationary phase is often a layer of adsorbent material such as silica gel or aluminum oxide.
- Two-dimensional chromatography is a form of paper chromatography that requires utilizing two solvents and rotating the paper by 90° in between. This approach is very effective for isolating complicated combinations of comparable polarity chemicals, such as amino acids .
- Paper chromatography uses paper sheets or strips as the stationary phase through which a solution is forced to flow. The solution’s dissolved chemical components segregate depending on their differing migration speeds over the paper sheets. This low-cost approach yields a powerful analytical tool while requiring only a little amount of ingredients.
- In general, paper chromatography is a useful technique for isolating and studying chemical compounds. While it has originally emerged as an instructional tool, its simplicity and adaptability make it a helpful approach in a variety of scientific domains.
There are two main types of paper chromatography: paper adsorption chromatography and paper partition chromatography.
- Paper Adsorption Chromatography : In paper adsorption chromatography, the stationary phase is created by impregnating the paper with silica or alumina. The adsorbent material, such as silica gel or alumina, is evenly distributed throughout the paper. The mobile phase, on the other hand, is a solvent that moves through the paper, carrying the analytes with it. As the mobile phase travels up the paper, the compounds in the sample are adsorbed onto the adsorbent material based on their affinity for it. The separation occurs as different compounds are adsorbed to different degrees, causing them to migrate at different rates up the paper. This type of paper chromatography is commonly used for separating mixtures of compounds based on their adsorption characteristics.
- Paper Partition Chromatography : In paper partition chromatography, the stationary phase is created by the moisture or water present in the pores of the cellulose fibers in the filter paper. The mobile phase is another solvent that is used to carry the analytes through the paper. In this technique, the separation is based on the partitioning of the analytes between the stationary phase (moisture in the paper) and the mobile phase (solvent). The different compounds in the sample distribute themselves between the two phases, with some being more soluble in the mobile phase and others having a greater affinity for the stationary phase. As the mobile phase moves up the paper, the analytes separate into distinct bands or spots based on their partitioning behavior. Paper partition chromatography is often referred to simply as paper chromatography, as it is the more commonly used type of paper chromatography.
Both paper adsorption chromatography and paper partition chromatography are valuable techniques for separating and analyzing mixtures of compounds. They rely on the principles of adsorption or partitioning to achieve separation, and the choice between the two depends on the specific requirements of the analysis and the properties of the compounds being studied.
The principle of paper chromatography is based on the distribution or partitioning of substances between a stationary phase and a mobile phase. In the case of paper chromatography, the cellulose fibers in the filter paper act as the stationary phase, while organic solvents or buffers are used as the mobile phase.
The separation occurs as the mobile phase travels up the stationary phase, carrying the sample with it. The components of the sample separate based on their affinity for the stationary phase and their solubility in the mobile phase. Substances that strongly adsorb onto the stationary phase will migrate more slowly, while those that readily dissolve in the mobile phase will move faster.
The principle involved in paper chromatography can be either partition chromatography or adsorption chromatography. In partition chromatography, the substances in the mixture are partitioned or distributed between liquid phases. The water held in the pores of the filter paper acts as one phase, while the mobile phase serves as the other. As the mobile phase moves, the separation of the mixture takes place. The compounds separate based on their different affinities for the stationary and mobile phase solvents under the capillary action within the pores of the paper.
On the other hand, adsorption chromatography involves the interaction between a solid surface (the paper) and a liquid phase (the mobile phase). In this case, the solid surface of the paper acts as the stationary phase, while the liquid phase serves as the mobile phase. The compounds in the mixture adsorb to different extents onto the solid surface, leading to their separation as the mobile phase moves.
In summary, the principle of paper chromatography relies on the distribution or partitioning of substances between a stationary phase (the cellulose fibers in the filter paper) and a mobile phase (organic solvents or buffers). The separation occurs based on the differences in affinity for the stationary and mobile phases, either through partition chromatography or adsorption chromatography.
Instrumentation of Paper chromatography
- Stationary phase & papers used
- Mobile phase
- Developing Chamber
- Detecting or Visualizing agents
The type of paper used in paper chromatography is critical since it dictates the properties of the stationary phase. The following papers are widely used in paper chromatography:
- Whatman filter papers: Whatman filter papers are available in a variety of grades, including No. 1, No. 2, No. 3, No. 4, No. 20, No. 40, No. 42, and so on. These sheets contain varying pore diameters and thicknesses, providing for greater adaptability in separating various chemicals.
- Modified papers: In addition to normal filter papers, there are modified papers made particularly for chromatographic purposes. These papers are subjected to various treatments or alterations in order to improve their qualities. Acid or base washed filter papers, for example, have been treated to eliminate contaminants that may interfere with the analysis. Glass fiber sheets are also utilized because they have better chemical resistance and stability.
- Hydrophilic papers: Hydrophilic papers are papers that have been treated with chemicals such as methanol, formamide, glycol, glycerol, or other hydrophilic compounds. These treatments increase the paper’s affinity for the mobile phase, allowing for improved analyte separation and migration.
- Hydrophobic papers: These papers have had the hydroxyl (OH) groups on the cellulose fibers acetylated. This process makes the paper hydrophobic, making it acceptable for reverse phase chromatography. The stationary phase in reverse phase chromatography is non-polar, whereas the mobile phase is polar, allowing for the separation of non-polar substances.
- Impregnated papers: Papers can also be impregnated with various chemicals to change their characteristics. Impregnating the paper matrix with silica, alumina, or ion exchange resins, for example. Depending on the intended application, these impregnations can give various selectivity and separation capabilities.
The stationary phase in paper chromatography is a complicated matrix of water and paper rather than just the paper itself. The cellulose fibers in the paper attach to water molecules in the surrounding air as well as moisture present during the production process. The combination of water and paper produces the stationary phase, which is important in compound separation because the mobile phase transports the sample through the paper.
The selection of stationary phase, which is represented by various types of papers and modifications, is critical in defining the separation properties and overall performance of paper chromatography.
In paper chromatography, the mobile phase is the solvent or mixture of solvents used to transport the mixture being studied through the stationary phase, which is the paper.
Depending on the nature of the chemicals to be separated, many types of mobile phases can be employed in paper chromatography. Mobile phases include the following:
- Hydrophilic mobile phases: Hydrophilic mobile phases are those that have a strong affinity for water. Isopropanol:ammonia:water (9:1:2), methanol:water (4:1), and n-butanol:glacial acetic acid:water (4:1:5) are examples of hydrophilic mobile phases. These solvent mixes can be used to separate polar substances.
- Hydrophobic mobile phases: These are nonpolar mobile phases that have a decreased affinity for water. Dimethyl ether:cyclohexane and kerosene:70% isopropanol are examples of hydrophobic mobile phases. Nonpolar chemicals are separated using these solvent mixes.
The mobile phase used is determined by the type of the compounds being examined. Polar solvents are often used, however the specific solvents or solvent mixes used are dictated by the polarity and properties of the chemicals to be separated.
If pure solvents do not offer acceptable separation, a polarity-appropriate combination of solvents can be utilized to maximize the separation. The solvent combination in the mobile phase can be changed to improve the resolution and separation of the components in the mixture.
The mobile phase, generally a nonpolar solvent, moves up the paper by capillary action in paper chromatography. As the mobile phase travels, it transports the various components of the mixture with it. Because the components of the mixture have different affinities for the mobile phase and the stationary phase (paper), they will separate and produce unique spots or bands on the paper.
Overall, the mobile phase selection is critical in paper chromatography for successful separation and analysis. It is chosen depending on the polarity and properties of the compounds under investigation, and the mobile phase aids in the movement and separation of the components on the paper.
- A chromatographic chamber is an important component in paper chromatography because it provides the conditions for the separation process to take place. Chromatographic chambers are commonly made of glass, plastic, or stainless steel, with glass tanks being the preferred material due to their clarity and chemical inertness.
- The dimensions and size of the chromatographic chamber might vary based on the length of the paper used and the development procedure used. Chambers are offered in a variety of sizes to accommodate different paper lengths and development procedures.
- The environment within the chromatographic chamber is an important factor. The solvent vapor in the chamber environment must be saturated. This is accomplished by depositing an appropriate amount of the mobile phase or solvent combination at the bottom of the chamber. The solvent evaporates and saturates the chamber atmosphere, resulting in a consistent and regulated condition for separation. Because of the saturated environment, the mobile phase moves equally and consistently across the paper, resulting in precise and reliable separations.
- The saturation of the chamber environment with solvent vapor is critical for effective and repeatable chromatographic separations. It keeps the paper wet throughout the procedure, allowing the analytes to migrate and preserving the connection between the stationary phase (paper) and the mobile phase (solvent). This regulated environment allows the components in the mixture to separate and distinct spots or bands to develop on the paper.
- To summarize, chromatographic chambers are essential in paper chromatography because they provide a controlled environment for the separation process. They are often made out of glass, plastic, or stainless steel. The chambers are available in a variety of sizes to fit varied paper lengths and development procedures. To provide uniform and dependable separations, the chamber environment must be saturated with solvent vapor. The saturated environment keeps the paper wet and allows analytes to migrate, resulting in effective chromatographic separations.
Paper chromatography involves several steps in order to separate and analyze a sample mixture effectively. The key steps in paper chromatography are as follows:
- Selection of Solid Support: High-quality cellulose paper with defined porosity and high resolution is chosen as the solid support. It should allow for good movement of the solvent while minimizing sample diffusion.
- Selection of Mobile Phase: The mobile phase, which is the solvent or solvent mixture, is selected based on the nature of the analyte being separated. Different combinations of organic and inorganic solvents can be used. For example, a suitable solvent for separating amino acids is butanol:acetic acid:water (12:3:5).
- Saturation of Tank: The inner wall of the chromatography tank is wrapped with filter paper to ensure better resolution. This pre-saturation of the tank helps maintain a uniform solvent atmosphere during the separation.
- Sample Preparation and Loading: If the sample is in solid form, it is dissolved in a suitable solvent. A small volume of the sample (2-20 μL) is applied as a spot on the baseline of the filter paper using a micropipette . The spot is air-dried to prevent diffusion.
- Ascending Development: The solvent flows against gravity in this procedure. The sample is put to the bottom of the paper, which is then placed in a chamber with the mobile phase solvent at the bottom. The components of the sample separate and migrate higher as the solvent travels up the paper via capillary action. This is the most popular and traditional paper chromatography method.
- Descending Development: This process is performed in a specific chamber with the solvent container at the top. The sample is put to the top of the paper, and the solvent is allowed to trickle down the paper. The solvent migrates from the top to the bottom, causing the components to separate and migrate downward. This is referred to as descending chromatography.
- Ascending-Descending Development: A combination of ascending and descending development. By executing both ascending and descending chromatography sequentially, the separation duration is enhanced. Initially, the solvent flows from the bottom to the top (ascending), then reverses direction and flows from the top to the bottom (descending). This approach enables longer separation distances and higher resolution.
- Circular/Radial Development: A circular paper is utilized in this procedure, and the sample spot is applied in the middle. The solvent is injected through a wick in the middle and spreads equally in all directions, resulting in a radial flow pattern. This approach is appropriate for assessing materials that require a wider separation range or when numerous components must be resolved at the same time.
- Drying of Chromatogram: After the development process, the solvent front is marked, and the chromatogram is left to dry in a dry cabinet or oven. Drying ensures that the separated components are immobilized on the paper.
- Detection : Once the chromatogram is dry, the separated components need to be visualized or detected. Colorless analytes can be detected by staining the chromatogram with reagents such as iodine vapor or ninhydrin. Radiolabeled or fluorescently labeled analytes can be detected by measuring radioactivity or fluorescence, respectively.
By following these steps, paper chromatography allows for the separation and analysis of different components within a sample mixture, providing valuable information about their composition and relative concentrations.
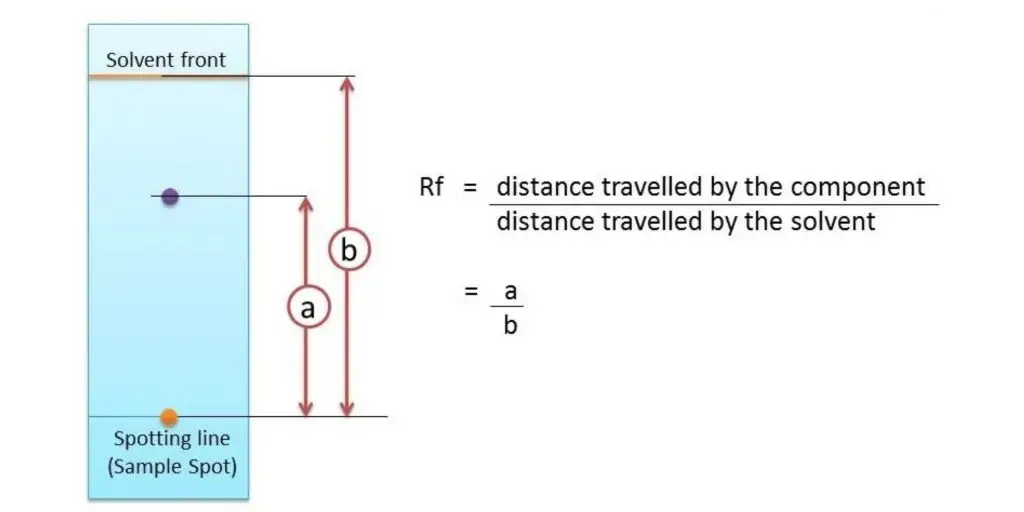
In paper chromatography, the Rf (retention factor) value is a significant parameter used to measure the extent of movement of a component in a chromatographic experiment. The Rf value is defined as the ratio of the distance traveled by the component from the application point to the distance traveled by the solvent front.
The Rf value is calculated by dividing the distance traveled by the component by the total distance traveled by the solvent. This value is a constant for a particular compound under specific conditions such as the type of paper and the composition of the solvent.
The Rf value provides valuable information about the relative affinity of a compound for the stationary phase and the mobile phase. It ranges between 0 and 1, with different values indicating different behaviors:
- If the Rf value is 0, it means the solute has no affinity for the mobile phase and remains immobilized in the stationary phase.
- If the Rf value is 1, the solute has no affinity for the stationary phase and travels with the solvent front.
For example, if a compound travels a distance of 9.9 cm and the solvent front travels a distance of 12.7 cm, the Rf value would be calculated as (9.9/12.7) = 0.779 or 0.78.
The Rf value is influenced by factors such as temperature and the choice of solvent. Different solvents can result in different Rf values for the same mixture of compounds. Therefore, by using various solvents, it is possible to obtain different Rf values and achieve better separation and identification of components.
Rf values are particularly useful because they serve as characteristic identifiers for components under specific experimental conditions. By comparing the Rf values obtained from an unknown sample with those in a database or reference standards, it becomes possible to identify the components present in the mixture.
In summary, Rf values play a crucial role in paper chromatography as they provide a quantitative measure of the relative migration of components. They allow for comparisons, identification, and characterization of compounds in a sample, aiding in the analysis and interpretation of chromatographic results.
Paper chromatography has several uses in a variety of industries. Among the most important uses are:
- Pharmaceutical purity control: Paper chromatography is used to assure the purity of pharmaceutical medications and the absence of impurities or contaminants.
- Adulterant detection: It is used to identify the presence of adulterants in a variety of commodities, including food, beverages , and cosmetics.
- Contaminant analysis in foods and drinks : Paper chromatography aids in the identification and quantification of pollutants in food and beverage items, assuring their safety and quality.
- Ripening and fermentation research: This method is used to investigate the ripening and fermentation processes in foods and drinks, offering insights on chemical changes and quality features.
- Detection of drugs and doping compounds: In sports and forensic sciences, paper chromatography is used to identify drugs and doping substances in biological samples such as urine or blood.
- Cosmetic analysis: It is used to determine the composition and purity of cosmetic items to ensure they fulfill regulatory criteria and are safe to use.
- Analysis of reaction mixtures in biochemical labs: Paper chromatography assists in the analysis of reaction mixtures in biochemical laboratories, allowing researchers to monitor the course of reactions and detect the presence of certain substances.
Furthermore, paper chromatography is used to analyze a wide range of chemicals such as amino acids, organic acids, alkaloids, polysaccharides, proteins and peptides, natural and synthetic colors, inorganic cations, and plant extracts.
In addition to these specific applications, paper chromatography has larger applications such as:
- Reaction monitoring: It allows for the monitoring of reaction progress by creating chromatograms at different time intervals and identifying the reactants.
- Isolation and purification : Paper chromatography aids in the isolation and purification of components from mixtures, allowing for further characterisation and analysis.
- Pharmaceutical R&D: It gives information on the creation of novel medication compounds, the completion of reactions, and the advancement of production procedures. It is a low-cost approach for detecting color fluctuations and monitoring active components in medication compositions.
- Forensics : In forensic investigation, paper chromatography is critical for the identification and comparison of drugs and their metabolites. It is very beneficial when working with tiny sample sizes.
- Food analysis: It aids with the examination of food colors in synthetic drinks, beverages, ice creams, and desserts, assuring compliance with legislation governing the use of approved edible colors.
Overall, paper chromatography is a flexible and effective analytical method that provides qualitative and quantitative information on substances and mixtures in a variety of businesses and research domains.
Paper chromatography has a number of features that make it a popular analytical method. These benefits include:
- Simplicity : Paper chromatography is a straightforward and easy procedure. It does not require any specialist equipment or training, making it accessible to a wide spectrum of consumers .
- Speed : Because this approach produces speedy findings, it allows for quick analysis and decision-making. In compared to other chromatographic procedures, the separation and identification of components on the paper occur rather fast.
- Minimal sample quantity required: Paper chromatography takes just a tiny amount of the sample for analysis. This approach can successfully examine even tiny levels of chemicals, saving significant resources.
- Cost-effective: Paper chromatography is a low-cost technology when compared to other chromatographic methods. Paper chromatography requires few supplies, such as filter paper, solvents, and developing chambers, making it an appealing alternative for research and regular analysis.
- Versatility : Paper chromatography is versatile in that it can detect both undiscovered inorganic and organic substances. It has a wide range of applications spanning multiple classes of substances and may be employed in a variety of industries such as pharmaceuticals, forensics, food analysis, and others.
- Space-efficient: Paper chromatography is space-efficient. Paper chromatography apparatus and setup are small, making it suited for laboratories with limited space.
- Excellent resolving power: Paper chromatography has very excellent resolving power and may separate components within a mixture. It can discriminate between closely related molecules and provide extensive information about the sample’s makeup.
Paper chromatography is an appealing alternative for qualitative and semi-quantitative analysis, screening tests, and early separations because of these benefits. Its ease of use, speed, low cost, and adaptability make it a vital tool in a variety of scientific fields and enterprises.
While paper chromatography has many advantages, it also has certain drawbacks that should be addressed. These constraints are as follows:
- Sample size limitation: Paper chromatography is not ideal for large-scale analysis since it requires a tiny amount of material. This might be a disadvantage when working with restricted or rare samples since it may not offer enough material for analysis or allow for repeated testing.
- Limited quantitative analysis: Paper chromatography is typically employed as a qualitative or semi-quantitative method with limited quantitative analysis. It is unsuitable for exact quantitative analysis, which requires accurate measurements and precise quantification of components. For quantitative analysis, other chromatographic methods such as high-performance liquid chromatography ( HPLC ) or high-performance thin-layer chromatography (HPTLC) are recommended.
- Inability to separate complicated mixes: Paper chromatography may fail to separate complex mixtures including several components with comparable characteristics. Paper chromatography has a lower resolving power than more modern chromatographic technologies. More advanced methods, such as column chromatography or gas chromatography , may be required for improved separation and identification of complex combinations.
- Lower accuracy: Paper chromatography is often thought to be less precise than methods such as HPLC or HPTLC. Visual identification and measurement of Rf values in paper chromatography might impose subjectivity and accuracy limits. For high-accuracy analysis, analytical techniques that produce more precise and accurate measurements, such as instrumental detection methods, are chosen.
When choosing a chromatographic technique for a given analysis, it is critical to keep these constraints in mind. While paper chromatography is useful for qualitative analysis and early separations, it may not be appropriate for all analytical demands, especially when better precision, accuracy, and quantitative analysis are required.
Paper chromatography is a technique used to separate and analyze the components of a mixture based on their differential migration through a paper or cellulose matrix. It relies on the principles of partition and adsorption chromatography.
Paper chromatography is not typically used for quantitative analysis due to limitations in accuracy and reproducibility. It is more commonly employed as a qualitative technique for separation, identification, and comparison of components in a mixture.
Different development techniques in paper chromatography include ascending development (solvent flows against gravity), descending development (solvent flows from top to bottom), ascending-descending development (a hybrid of ascending and descending techniques), and circular/radial development (solvent spreads uniformly in all directions from a central spot on a circular paper).
Components on a paper chromatogram can be detected using various methods. Colorless analytes can be visualized by treating the paper with reagents such as iodine vapor or ninhydrin, which produce color reactions. Radiolabeled or fluorescently labeled analytes can be detected by measuring radioactivity or fluorescence, respectively.
To achieve the best results in paper chromatography, it is important to carefully select the appropriate paper and mobile phase, ensure proper saturation of the chromatographic chamber with solvent vapor, apply the sample accurately and in small quantities, and optimize the development technique and conditions for the specific analyte or mixture being analyzed.
Some limitations of paper chromatography include the inability to handle large sample quantities, lack of effectiveness in quantitative analysis, difficulty in separating complex mixtures, and lower accuracy compared to more advanced chromatographic techniques like high-performance liquid chromatography (HPLC) or high-performance thin-layer chromatography (HPTLC).
The Rf (retention factor) value is calculated by dividing the distance traveled by a component from the application point to the distance traveled by the solvent front. It is a measure of the relative mobility of a component in the chromatogram and is constant under specific experimental conditions.
In paper chromatography, a sample mixture is applied to a piece of filter paper, and the edge of the paper is immersed in a solvent or mobile phase. The solvent moves up the paper through capillary action, carrying the components of the mixture with it. As the solvent moves, the components separate based on their affinity for the stationary phase (paper) and the mobile phase (solvent), resulting in distinct bands or spots on the paper known as chromatograms.
Paper chromatography has a wide range of applications, including analyzing the purity of pharmaceuticals, detecting adulterants in food and drinks, studying ripening and fermentation processes, identifying drugs and doping substances in humans and animals, analyzing cosmetics, and examining reaction mixtures in biochemical labs. It can also be used to analyze specific classes of compounds such as amino acids, alkaloids, polysaccharides, proteins, pigments, and plant extracts.
Paper chromatography offers several advantages, including its simplicity, rapidness, requirement of small sample quantities, low cost compared to other chromatography methods, ability to identify both organic and inorganic compounds, compact setup, and excellent resolving power.
- Skoog, D.A., West, D.M., Holler, F.J., Crouch, S.R. (2013). Fundamentals of Analytical Chemistry. Cengage Learning. Chapter 27: Paper Chromatography.
- Bhattacharya, S., Banerjee, R. (2018). Thin-Layer and Paper Chromatography: A Laboratory Handbook. CRC Press.
- Sherma, J., Fried, B. (2003). Handbook of Thin-Layer Chromatography. Marcel Dekker.
- Stahl, E. (1969). Thin-Layer Chromatography: A Laboratory Handbook. Springer.
- Smith, R.M. (1995). Introduction to Paper Chromatography. CRC Press.
Related Biology Study Notes
100 laboratory equipment names and uses, electron microscope – principle, types, parts, application, diagram, gas chromatography – definition, parts, principle, working, uses, autoclave validation methods – objective, procedure, result, simple microscope – definition, principle, parts, uses, autoclave – definition, principle, parts, operating procedure, uses, ph meter – principle, parts, types, procedure, calibration, application, examples, hplc – principle, instrumentation, types, uses, diagram, latest questions.
Start Asking Questions Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
We've detected that you are using AdBlock Plus or some other adblocking software which is preventing the page from fully loading.
We don't have any banner, Flash, animation, obnoxious sound, or popup ad. We do not implement these annoying types of ads!
We need money to operate the site, and almost all of it comes from our online advertising.
Please add biologynotesonline.com to your ad blocking whitelist or disable your adblocking software.
Essays on Chromatography

Whatman ® cellulose chromatography papers
3 mm blotting paper, diam. 25 mm
Synonym(s) :
Select a Size
Availability.
Available to ship on October 31, 2024 Details

About This Item
Recommended products.

WHA10426981
Whatman ® gel blotting papers, Grade GB005

WHA10426892
Whatman ® gel blotting paper, Grade GB003

WHA10427812

GE blotting paper

Sigma-Aldrich
Hh/Gli Antagonist, GANT61

WHA10016508
Whatman ® qualitative filter paper, Grade 1

cellulose membrane circles
non-sterile
pkg of 100 ea
manufacturer/tradename
Cytiva Whatman 1030-025 Whatman Article No. 28414095 (US reference)
130 mm/30 min speed
Looking for similar products? Visit Product Comparison Guide
Related Categories
Filter Paper
Description
General description, features and benefits.
- Pure cellulose produced entirely from the highest quality cotton linters with no additives of any kind. Ensures that no contamination will occur during the transfer steps.
- Manufactured and tested specifically for chromatographic and blotting techniques. This ensures the wicking capability and uniformity of capillary action that are important in obtaining clean and even transfers during blotting.
- Whatman 3MM Chr is considered the industry standard for blotting procedures.
- Convenient sizes available in sheets precisely cut to the most popular gel and transfer membrane sizes. Allows "out-of-the-box" usage and eliminates sheet-to-sheet variations.
Other Notes
Legal information, documentation, certificates of analysis (coa).
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Lot/Batch Number
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Visit the Document Library
Peer Reviewed Papers
Technical service.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others .
Research. Development. Production.
We are a leading supplier to the global Life Science industry with solutions and services for research, biotechnology development and production, and pharmaceutical drug therapy development and production.
© 2024 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.
Reproduction of any materials from the site is strictly forbidden without permission.
- English - EN
- Español - ES
Home — Essay Samples — Science — Chemistry — Chromatography
Essays on Chromatography
Separation of ink pigments using paper chromatography, gas chromatography (gc or glc), made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Tlc of Aqueous Extract of Carica Papaya
The use of ion chromatography in anions quantification, research in high-performance liquid chromatography, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Relevant topics
- Concentration
- Chemical Reaction
- Green Chemistry
- Mathematics in Everyday Life
- Stephen Hawking
- Engineering
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
We use cookies to enhance our website for you. Proceed if you agree to this policy or learn more about it.
- Essay Database >
- Essay Examples >
- Essays Topics >
- Essay on Chromatography
Example Of Essay On Chromatography
Type of paper: Essay
Topic: Chromatography , Testing , Separation , Identification , Mixture , Components , Amsterdam , Wixom
Published: 05/29/2023
ORDER PAPER LIKE THIS
Chromatography is the method for separating components from a mixture on the principle of the relative quantity of each element that is distributed between a moving fluid medium referred to as the mobile phase, and the immediate stationary phase. Chromatography is employed in the separation of various mixtures (Wixom and Charles, 103). It is used in the preparation of materials for purifying them. Chromatography has several uses in biological and chemical worlds. Chromatography is broadly applied in biochemical research to identify and separate chemicals or biological origin. This method is applied in the petroleum industry to determine and analyze the complex mixture of the hydrocarbons. Unlike other methods of separation such as distillation, decantation, extraction, et al., Chromatography can separate all the elements in a mixture without the need for an identity, number, or relative quantities of the components present. Chromatography is versatile; it deals with molecular species ranging from the size of viruses to the tiniest of all, hydrogen. The technique is applied to small or large quantities of the components. Some types of Chromatography identify elements present at attogram levels, making Chromatography an effective trace analytical method that is extensively applied in the identification of chlorinated pesticides in biology and environment. The method is used for the identification of therapeutically and narcotic drugs (Berezkin and Drugov, 302). Chromatography is used in forensic testing. It is used in the analyses of blood or samples of cloths in scenes of crime, arson verification, or even blood testing after death to identify alcohol levels, drug and poisonous compounds in the body (Betina, 221). The accuracy and precision of Chromatography in drug testing enhance identification of substances in the bloodstream. It is useful in testing for doping in athletes. In conclusion, Chromatography has a resolving power which is unequaled among other methods of separation.
Betina, Vladimír. Chromatography of Mycotoxins: Techniques and Applications. Amsterdam: Elsevier, 1993. Internet resource. Berezkin, V G, and Drugov. U.S. Gas Chromatography in Air Pollution Analysis. Amsterdam: Elsevier, 1991. Internet resource. Wixom, Robert L, and Charles W. Gehrke. Chromatography: A Science of Discovery. Hoboken, N.J: Wiley, 2010. Print.

Cite this page
Share with friends using:
Removal Request

Finished papers: 1672
This paper is created by writer with
ID 287174183
If you want your paper to be:
Well-researched, fact-checked, and accurate
Original, fresh, based on current data
Eloquently written and immaculately formatted
275 words = 1 page double-spaced

Get your papers done by pros!
Other Pages
Dancers essays, bimbos essays, bureaucracies essays, deters essays, delaney essays, contributing essays, darker essays, colonists essays, classmates essays, hartnell essays, checkup essays, budge essays, pawnee essays, haunted house essays, mobile home essays, revivalist essays, free research paper on research article commitment trust theory of relationship marketing, good article review about choson civil service exams, currency essay sample, good essay on the act of making things, free essay about alcoholism a curse, good example of research paper on black cowboys, good example of article review on concepts of childhood, critical prcis essay samples, free essay about external intrusion of the playstation network, effects of insurance coverage on the healthcare essay example, all roads lead to rome course work samples, global language essay example, good essay on reimbursement and pay for performance, sample business plan on taking initiative, free provide a hypothesis as to why shakespere would include three sonnets that are not essay sample, free learning from reflection summary course work example, essay on environmental scanning and industry analysis, strategic issues and problems case studies examples, free arts specific artist role on social activism essay example, conclusively the law guideline on the vehicle and other searches has limitations only if the suspicion is not valid a sample essay for inspiration mimicking, fast foods and obesity research paper to use for practical writing help, free statistics 2 for business essay example, e business and e commerce a sample essay for inspiration mimicking, king lear type to use as a writing model, good essay on strategic meetings management programs, competition law regime in uae free sample essay to follow, labor in china free sample essay to follow.
Password recovery email has been sent to [email protected]
Use your new password to log in
You are not register!
By clicking Register, you agree to our Terms of Service and that you have read our Privacy Policy .
Now you can download documents directly to your device!
Check your email! An email with your password has already been sent to you! Now you can download documents directly to your device.
or Use the QR code to Save this Paper to Your Phone
The sample is NOT original!
Short on a deadline?
Don't waste time. Get help with 11% off using code - GETWOWED
No, thanks! I'm fine with missing my deadline

IMAGES
VIDEO
COMMENTS
Space Efficiency: Paper chromatography requires less space than many other analytical techniques or instruments, making it suitable for use in limited laboratory settings. Disadvantages: 1. Limited Sample Capacity: Paper chromatography is not suitable for handling large quantities of samples, which can limit its use in certain applications. 2.
paper chromatography, in analytical chemistry, technique for separating dissolved chemical substances by taking advantage of their different rates of migration across sheets of paper. It is an inexpensive but powerful analytical tool that requires very small quantities of material. The method consists of applying the test solution or sample as ...
Paper Chromatography Principle. The principle involved can be partition chromatography or adsorption chromatography. Partition chromatography because the substances are partitioned or distributed between liquid phases. The two phases are water held in pores of the filter paper and the other phase is a mobile phase which passes through the paper.
Paper chromatography (PC) is a type of planar chromatography whereby chromatography procedures are run on a specialized paper. PC is considered to be the simplest and most widely used of the chromatographic techniques because of its applicability to isolation, identification, and quantitative determination of organic and inorganic compounds.
Paper chromatography is a simple and cost-effective separation technique that separates and identifies different components in a mixture. [1-4] Principle. In paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper.
Paper Chromatography. Paper Chromatography is a technique in chemistry that involves using a sheet of paper as the chromatographic bed, with water and cellulose acting as the stationary phase. It is used to separate and analyze different components of a sample solution by allowing a mobile phase to move through the paper.
Paper chromatography is a sort of planar chromatography in which chromatographic techniques are carried out on specialized paper. Because of its capacity to separate, identify, and quantitatively quantify both organic and inorganic substances, it is largely considered as the simplest and most often used chromatographic method.
Chromatography is a well known technique used for separation of compounds. Among all, chromatographic techniques, paper chromatography is a type of analytical tool which is used for separation of colored components. The principle involved may be separation and partition of components based on their affinity towards stationary phase. Literature survey reveals that paper chromatography has been ...
Paper chromatography is an analytical method used to separate coloured chemicals or substances. [1] It is now primarily used as a teaching tool, having been replaced in the laboratory by other chromatography methods such as thin-layer chromatography (TLC).. The setup has three components. The mobile phase is a solution that travels up the stationary phase by capillary action.
Paper chromatography is a technique used to separate and analyze a mixture. Simple paper chromatography, for example, can be used to separate a color mixture. The stationary phase is formed by the filter paper, which has a thin layer of water trapped on it. The solvent is referred to as the mobile phase or eluant.
Cheyenne Wilson 5.05 LAB REPORT CANDY CHROMATOGRAPHY BACKGROUND INFORMATION: Paper chromatography is a widely used method of separation. The lab will show the basic techniques of paper chromatography. In this lab, the separation of the dyes used in two different kinds of candy is performed.…. Free Essay: Introduction: In many cases in ...
Paper Chromatography Essay. Decent Essays. 346 Words; 2 Pages; Open Document. Results: The pigments that were obtained through the use of paper chromatography resulted in four distinct colors. The very goal of this study was to determine what pigments are present and what there absorbance is through the use of paper chromatography and a ...
Paper Type: 400 Words Essay Examples. Chromatography works on the principle that different impounds will have different solubility and adsorption to the two different phases between which are to be partitioned. Thin Layer Chromatography (TTL) is a solid-liquid technique. The mixture is observed when it is in two different phase; a solid ...
All chromatography techniques use two phases called the mobile phase and the stationary phase. In paper chromatography: The mobile phase is the solvent in which the sample molecules can move, which in paper chromatography is liquid e.g. water or ethanol. The stationary phase in paper chromatography is the actual chromatography paper itself.
General description. Grade 3MM Chr cellulose chromatography circles is a 0.34 mm paper for general chromatography and electrophoresis. Though widely used as a blotting paper, 3MM Chr is also used both in electrophoresis and for general chemistry. A medium thickness paper (0.34 mm) used extensively for general chromatography and electrophoresis.
7 pages / 3203 words. High-performance liquid chromatography is an analytical technique used to separate, identify, and quantify each component in a mixture. The liquid solvent containing the sample mixture passes through a column filled with a solid adsorbent material. Each component in the sample interacts differently with the adsorbent...
Chromatography is the method for separating components from a mixture on the principle of the relative quantity of each element that is distributed between a moving fluid medium referred to as the mobile phase, and the immediate stationary phase. Chromatography is employed in the separation of various mixtures (Wixom and Charles, 103).
This paper focuses on the first factor, seeking the best public policies for translating geroscience from the benchside to the bedside. It begins with a review of current geroscience policy, identifying the rules and institutions that help or hinder innovation in anti-aging healthcare. The paper then combines facts with theory to suggest ways ...