
Search for IQVIA clinical trials
Search for trials related to our patient communities, join one or more of our patient communities.
Learn about the conditions and related clinical research and hear from other patients.
- Alzheimer’s Disease
- Breast Cancer
- Lung Cancer
- Multiple Myeloma
- Multiple Sclerosis
- Nonalcoholic Steatohepatitis (NASH)
- Prostate Cancer
- Renal Cell Carcinoma
Browse trials by top conditions
- Allergies Trials
- Alzheimer's Disease Trials
- Anxiety Trials
- Arthritis Trials
- Asthma Trials
- Bipolar Disorder Trials
- Birth Control Trials
- Chest Pain Trials
- Cancer Trials
- Chronic Obstructive Pulmonary Disease (COPD) Trials
- Cardiovascular Diseases Trials
- Chronic Bronchitis Trials
- Chronic Pain Trials
- Crohn's Disease Trials
- Depression Trials
- Diabetes Trials
- Epilepsy Trials
- Fibromyalgia Trials
- Gastrointestinal Disorders Trials
- Gout Trials
- Heart Failure Trials
- High Blood Pressure Trials
- High Cholesterol Trials
- Inflammatory Bowel Disease (IBD) Trials
- Lupus Trials
- Headache Trials
- Multiple Myeloma Trials
- Multiple Sclerosis (MS) Trials
- Neuropathy Trials
- Nonalcoholic Steatohepatitis (NASH) Trials
- Osteoarthritis Trials
- Osteoporosis Trials
- Parkinson's Disease Trials
- Pediatrics and Adolescent Trials
- Pregnancy Trials
- Renal Cell Carcinoma Trials
- Restless Leg Syndrome Trials
- Smoking Trials
- Ulcerative Colitis Trials
- I prefer not to answer Trials
Featured trials
Study to evaluate the efficacy and safety of k-877....
A study to investigate the use of combination therapy with two investigational products for the...
Contraceptive Efficacy and Safety of NOMAC-E2 Comb...
The purpose of this study is evaluating Contraceptive Efficacy and Safety of NOMAC-E2 Combined Oral...

Study of Evobrutinib in Participants With RMS (evo...
The study is to evaluate the efficacy and safety of evobrutinib administered orally twice daily...
A Study to Investigate Efficacy and Safety of OG-6...
The purpose of this global Phase 2 study is to determine the efficacy, safety, and ...
A Phase 2 Study to Evaluate the Efficacy and Safet...
The purpose of this study is to evaluate the efficacy, safety, and tolerability of VIR-2482 ...
How can I find clinical trials near me?
Search this site for iqvia-managed clinical trials or visit clinicaltrials.gov for a listing of all clinical trials., you can also ask your doctor if they know of any suitable clinical trials..
We Value Your Privacy
We use cookies to allow our site to work properly, to personalize content and ads, to provide social media features and to analyze our traffic. Data may be shared with our partners involved in the delivery and/or personalization of ads elsewhere online as explained in “ Manage Cookie Preferences ” . Please click “ I Accept ” if you agree to our use of cookies, "Manage Cookie Preferences" to manage your preferences, or “ I Reject ” if you only agree to necessary cookies.
Manage Cookie Preferences
When you visit any website, it may store or retrieve information on your browser, mostly in the form of cookies. This information might be about you, your preferences or your device and is mostly used to make the site work as you expect it to. The information does not usually directly identify you, but it can give you a more personalized web experience. Because we respect your right to privacy, you can choose not to allow some types of cookies. Click on the different category headings to find out more and change our default settings. However, blocking some types of cookies may impact your experience of the site and the services we are able to offer.
Please visit our "Cookie Policy" to find out more about our cookie policy, how to block these cookies or contact information to reach out to us if you have any questions.
Cookies strictly necessary for essential website purposes and functions Cookies strictly necessary for essential website purposes and functions ALWAYS ACTIVE These cookies are strictly necessary for the proper operation of our site. They allow us to ensure the security and efficient delivery of our site. These cookies are needed for essential functions such as administering cookie preferences. Strictly necessary cookies cannot be switched off and they don ’ t store any personally identifiable information. View Cookies
These cookies are strictly necessary for the proper operation of our site. They allow us to ensure the security and efficient delivery of our site. These cookies are needed for essential functions such as administering cookie preferences. Strictly necessary cookies cannot be switched off and they don ’ t store any personally identifiable information.
| Name | Source | Duration | Purpose |
|---|---|---|---|
| BigIP | www.clinicalresearch.com | Until browser is closed | Used by hosting cluster to keep user site accesses on the same server in our cluster for the duration of user visit. |
| ASP.NET_SessionID | www.clinicalresearch.com | Until browser is closed | Allows the web application to store user-specific information on the server across pages within the site. |
| InitialParam | www.clinicalresearch.com | Until browser is closed | Its a dictionary cookie used to store vendor redirection information |
| shell#lang | www.clinicalresearch.com | Until browser is closed | Used to set the language of the site |
| AEC | www.google.com | 6 months | AEC cookies ensure that requests within a browsing session are made by the user, and not by other sites. These cookies prevent malicious sites from acting on behalf of a user without that user’s knowledge. |
| NID | 6 months | Google ReCaptcha stores a cookie to help detect whether the user is a robot or not (ensure security). | |
| _GRECAPTCHA | www.google.com | 6 months | To provide spam protection |
| AcceptCookie | www.clinicalresearch.com | 6 months | Saves user settings regarding the use of cookies on this website |
Analytics cookies and technologies Analytics cookies and technologies These cookies are used to collect information about how visitors use our site. We use the information to compile reports and to help us improve the site. The cookies collect information in an anonymous form, including the number of visitors to the site, where visitors have come to the site from and the pages they visited. View Cookies
These cookies are used to collect information about how visitors use our site. We use the information to compile reports and to help us improve the site. The cookies collect information in an anonymous form, including the number of visitors to the site, where visitors have come to the site from and the pages they visited.
| Name | Source | Duration | Purpose |
|---|---|---|---|
| SC_ANALYTICS_GLOBAL_COOKIE | www.clinicalresearch.com | 6 Months | Sitecore – visitor identification |
| _ga | .clinicalresearch.com | 6 Months | Google Analytics. Used to distinguish users. |
| 1P_JAR | .google.com | 30 days | Set by Google to display personalized advertisements on Google sites, based on recent searches and previous interactions. |
| _ga_GT3J5Y46L7 | .clinicalresearch.com | 6 Months | Google Analytics. Used to persist session state. |
| OTZ | .google.com | 30 days | This cookie is used to support Google’s advertising services. |
Investing in the next generation of medical and research pioneers
Masks strongly recommended but not required in maryland, starting immediately.
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.
Clinical trials study the safety and effectiveness of interventions and procedures on people’s health. Interventions may include medications, radiation, foods or behaviors, such as exercise. Usually, the treatments in clinical trials are studied in a laboratory and sometimes in animals before they are studied in humans. The goal of clinical trials is to find new and better ways of preventing, diagnosing and treating disease. They are used to test:
Drugs or medicines

New types of surgery

Medical devices

New ways of using current treatments

New ways of changing health behaviors

New ways to improve quality of life for sick patients

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story

Clinical Research
Clinical research is the key step in translating biomedical discoveries into new approaches to the diagnosis, treatment, and prevention of human illnesses.
In the last few decades, landmark developments in genetics, bioengineering, neuroscience, and molecular and structural biology have vastly increased our understanding of the causes of disease and raised new possibilities for treatment and prevention. However, there is a gap between the pace of scientific and technological advancements and the successful translation of this science into effective medical and health practices at the bedside, in the clinic, and in the community.
The AAMC believes that medical schools and major teaching hospitals — in partnership with the National Institutes of Health, other federal agencies, foundations, and the pharmaceutical and biotechnology industries — are uniquely responsible for providing both the institutional support and the rigorous training necessary to conduct high quality, hypothesis-driven clinical research and to nurture physician-scientists equipped to exploit scientific opportunities.
Publications and Presentations
- Principles for Protecting Integrity in the Conduct and Reporting of Clinical Trials : Outlines principles and standards to help guide institutions and their investigators in the analysis and reporting of clinical trials in which they participate. (Jan. 2006)
- Universal Use of Short and Readable Informed Consent Documents: How Do We Get There (PDF) : This report presents the findings of an expert panel convened to develop a strategy to foster the common usage of informed consent documents that are short and written in simple language, enabling potential subjects to make truly informed decisions about participating in research studies.
- Creating Informed Consent Documents That are Approachable, Readable, and Brief (PDF) : This July 31, 2007 presentation to the Health and Human Services Secretary's Advisory Committee on Human Research Protection highlights the findings and recommendations of an expert panel convened by the AAMC on May 30, 2007.
- Research & Technology
- Clinical Science
- Translational Research
- ITHS SharePoint
Search the Site
Connect with us, need help have a question.
Contact the Research Navigator
ITHS Email Updates

Clinical Research Centers
Northwest participant and clinical interactions network.
The Northwest Participant and Clinical Interactions (NW PCI) Network is a collaboration of regional clinical and translational research centers affiliated with medical centers, healthcare systems, and universities in the Washington, Wyoming, Alaska, Montana, and Idaho (WWAMI) region. NW PCI sites collaborate with investigators to conduct clinical research at centers across the region in rural and urban locations.
Available Services
Are you looking for regional sites for a clinical research study? Do you have an idea for a new patient-centered outcomes, or comparative effectiveness study? Are you interested in pulling together a multi-disciplinary team to co-develop your next grant application?
Through the NW PCI, we can help you create a partnership with clinical research centers in the WWAMI region, find collaborators, assemble a team, and provide guidance about working with regional sites.
NW PCI services include:
- Consultations regarding research ideas
- Development of collaborative teams
- Access to research tools and expertise for effective collaboration
- Assistance with grant development
NW PCI Network Capabilities
The NW PCI Network is comprised of 12 member institutions with experience and infrastructure to conduct many types of research, including phase I-IV clinical trials, social and behavioral interventions, and patient-centered outcomes and health services research. NW PCI sites are collectively capable of administering inpatient, outpatient, pediatric, and home-based protocols, and draw diverse patients from rural and urban settings in the WWAMI region.
Each NW PCI location has extensive research infrastructure including: investigators, research coordinators, financial and regulatory staff, investigational pharmacy services, and compliance programs. NW PCI locations also have committed champions who collaborate with the NW PCI to vet research ideas, link investigators with partners, and guide research proposals through the research systems at each site.
Work with the NW PCI Network
To work with the NW PCI, please contact us with your study idea. Ideas are accepted on an ongoing basis, please contact us with your study idea as early as possible.
NW PCI sites conduct in-depth feasibility assessments to 1) estimate the number eligible patients, 2) determine if the proposed budget will cover expenses, 3) assure alignment with clinical, patient, and institutional priorities, and 4) identify site investigators and key research personnel. Our team will assist you with developing your materials and facilitating connections with interested sites, but please plan to provide the following at least 8 weeks prior to your grant deadline:
- Abstract or Specific Aims page to describe the research question and importance of the study
- Study or protocol outline or synopsis with information about the role of NW PCI sites
- Draft site budget
- Number of research sites needed
- Study entry criteria, including inclusion and exclusion criteria, number of participants and expected recruitment rate
- Description of study funding, including link to the funding opportunity and grant deadline, if applicable
A typical timeline for collaborative development of grant applications with NW PCI sites includes the following activities before the grant submission deadline:
- Investigator contacts NW PCI Network
- Investigator develops research question, aims, protocol and budget, our team provides guidance and feedback on relevance and feasibility in NW PCI sites
- Investigator completes power and sample size estimations
- Our team works with the investigator to compile and tailor content for NW PCI sites
- NW PCI leadership reviews study packet
- Our team identifies prospective NW PCI sites and disseminates study packet to site champions
- Interested NW PCI sites complete feasibility assessments, including EHR data query
- Our team facilitates budget negotiation and refinement of the protocol between the investigator and NW PCI sites
- Our team drafts NW PCI site grant documents templates (e.g., budget, letter of support, scope of work) for investigator review and disseminates to NW PCI sites
- NW PCI sites complete grant documents, obtain required approvals and return final documents to the investigator
- Investigator integrates site content into application and completes final revisions
- Investigator completes submission and confirms submission and expected review and decision dates with NW PCI sites
Contact Us / Request Services
- Name * First Last
- Institution / Organization * Aids Healthcare Foundation Akron Children's Hospital Alaska Family Medicine Residency Alaska Native Medical Center Alaska Native Tribal Health Consortium Alere Wellbeing American Academy of Family Physicians National Research Network American Cancer Society American Indian Cancer Foundation American Indian or Alaska Native-Affiliated Entity or Tribe Applied Dexterity Arizona State University Asthma Inc Clinical Research Center Bastyr University Baylor College of Medicine Benaroya Research Institute at Virginia Mason Billings Clinic Biostrategy, LLC BJC Healthcare Bloodworks Northwest Research Institute Boise State University Boise Veterans Affairs Medical Center Boston Medical Center Bozeman Deaconess Hospital Brown University C3 Research Associates Cancer Research and Biostatistics (CRAB) Carleton College Case Western Reserve University Centers for Disease Control and Prevention Central Washington Family Medicine Central Washington Healthcare Partners Chief Andrew Isaac Health Center Children's Hospital Colorado Children's Hospital Los Angeles Children's Hospital of Philadelphia Children's Hospital of Pittsburgh Children's Hospital of Wisconsin Children's Hospitals and Clinics of Minnessota Children's Mercy Kansas City City of Seattle Clearwater Valley Hospital & St. Mary's Hospital Cleveland Clinic Clinical Development Solutions Columbia Basin Health Association Columbia Medical Associates Columbia University Community Health Associates of Spokane Community Health of Central Washington Cornell University Critical Access Hospital Network DARTNet Institute Deaconess & Valley Medical Centers Department of Health and Human Services Duke University East Pierce Family Medicine Emory University Entre Hermanos EpiGeneSys, Inc Esparza Plus Evergreen Treatment Services Fairbanks Memorial Hospital Family Medicine of Southwest Washington Family Medicine of Yakima Family Medicine Residency of Idaho Family Medicine Spokane Fielding Graduate University Fort Peck Health Promotion and Disease Prevention Fred Hutchinson Cancer Research Center Friends Research Institute Gonzaga University Group Health Hackensack University Medical Center Harborview Medical Center Harvard University Helen de Vos Children's Hospital Henry Ford Health System Idaho State University Immune Design Indiana University Purdue University Indianapolis Indiana University Health Center Institute for Systems Biology Institute of Translational Health Sciences Johns Hopkins Medicine Kadlec Health System Kaiser Permanente Center for Health Research Kidney Research Institute King County Kootenai Health LabKey Software Latino Community Fund Lincoln County Health Department Little Big Horn College Loma Linda University Lurie Children's Hospital Macalester College Makere University School of Public Health Massachusetts General Hospital Massachussetts Institute of Technology Mayo Clinic McLaughlin Research Institute Medical University of South Carolina MedImmune, Inc Medstar Research Memorial Healthcare System Memorial Physicians Metheor Theropeutics Corp Meyer's Primary Care Institute MGH Institute of Health Professions Micronutrient Initiative Migrant Clinicians Network Migrant Health Promotion Mille Lacs Band Minnesota State Department of Health Missula Indian Center MJ3 Industries Moffitt Cancer Center Montana BioScience Alliance Montana Cancer Consortium Montana Family Medicine Residency Montana Neurology Montana State University Montana State University Billings Montana State University Bozeman Montefiore Medical Center Mount Sinai Hospital MultiCare Health System Naahillahee Foundation National Congress of American Indians National Institutes of Health Nationwide Children's Native Action Network Nemours Foundation New York Medical College Nicklaus Children's Hospital Nimiipuu Tribal Health Northwest Association for Biomedical Research Northwest Asthma & Allergy Center Northwest Hospital and Medical Center Northwest Justice Project Northwest Kidney Centers Northwest Medical Specialties Northwest Nazarene University Northwest Regional Primary Care Association Northwestern Feinberg School of Medicine Northwestern University Norton Healthcare Notah Begay III Foundation Office of Minority Health-US DHHS Ohio State University Oregon Health Sciences University Oregon State University Oricula Orthopaedic Associates of Michigan Pacific Northwest Diabetes Research Institute Pacific Northwest National Laboratory Pacific Northwest University of Health Sciences Partners Healthcare Partners in Health and Research Development PATH Pediatrix Medical Group Perelman School of Medicine UPENN PharmaSmart Pocatello Family Medicine Portland Area Indian Health Board Providence - St. Peter Family Medicine Providence Health and Services Providence Medical Research Center Puget Sound Family Medicine Puget Sound Surgical Center Qualis Health Reed College Remedia Therapeutics RiverStone Health Rockwood Health System Rocky Mountain Laboratories Roswell Park Cancer Institute Sage Saint Alphonsus Health System Saint Patrick Hospital Salish Kootenai College Sea Mar Community Health Center Seattle Biomed Seattle Cancer Care Alliance Seattle Cancer Consortium Seattle Children's Hospital Seattle Indian Health Board Seattle Pacific University Seattle University South Puget Intertribal Planning Agency South Sound Care Foundation Southcentral Foundation Southeastern Idaho Public Health District Spokane Regional Health District St. Luke's Health System St. Peter Family Medicine - Providence St. Peter's Medical Group Stanford Children's Health Stanford University Swedish Family Medicine Residency - Cherry Hill Swedish Hospital and Medical Center Tacoma Family Medicine Tanana Chiefs Conference Texas Children's Hospital The Forsyth Institute The Native Project The State University of New York at Buffalo University of Alabama at Birmingham University of Alaska Anchorage University of Alaska Fairbanks University of Arkansas University of California at San Diego University of California at San Francisco University of Colorado Denver University of Florida University of Idaho University of Illinois Chicago University of Iowa University of Kansas Medical Center University of Kentucky University of Maryland University of Michigan University of Minnesota University of Montana University of Nevada University of New Mexico University of North Carolina University of Pennsylvania University of Pittsburgh University of Rochester Medical Center University of Texas Southwestern Medical Center University of Utah University of Vermont University of Washington University of Washington Family Medicine Northgate University of Wyoming University of Wyoming Family Medicine Casper US Department of Veteran's Affairs VA Medical Center Long Beach VA Puget Sound Valley Medical Center Vancouver Coastal Health Vanderbilt University Virginia Mason Medical Center Virginia Spine Research Institute Wake Forest Baptist Health Walden University Washington Dental Service Foundation Washington National Primate Research Center Washington State Board of Health Washington State Coalition For Language Access Washington State Department of Health Washington State University Washington University St. Louis Wellesley College Western Montana Family Medicine Residency White Sky Hope Center Wind River Tribal Health Wyoming Department of Health Yakima Valley Farm Workers Clinic Yakima Valley Memorial Hospital Yale University Zwitter Techonology -- Other --
- Other Institution / Organization *
- Department or Program *
- Email address *
Message / Question
- Attachments (optional) Drop files here or Select files Max. file size: 130 MB. empty to support CSS :empty selector. -->
- Name This field is for validation purposes and should be left unchanged.
- COVID Information
- Patient Care
- Referring Providers
- Price Transparency
- Employee Resources
Vanderbilt Clinical Research Center
The Vanderbilt Clinical Research Center is an inpatient and outpatient research facility staffed with personnel dedicated to conducting clinical research patient care. These resources are available to Vanderbilt University and Meharry Medical College clinical investigators and their study teams.
Medical Center North Unit (MCN)
Located on the 2nd floor of Medical Center North at S2400 MCN, this facility offers inpatient and outpatient services including:
- 12 patient rooms, specimen processing labs, and 2 private patient consult rooms
- 24/7 nursing care except for unit closure every other weekend
- Imaging, metabolic and exercise physiology services, sleep lab services, and scientific poster printing
Doctor’s Office Tower Unit (DOT)
The DOT outpatient services are located on the 9th floor of the Doctor’s Office Tower at 9103 DOT and are available:
Monday and Wednesday from 7:30 am to 6:00 pm
Tuesday, Thursday and Friday from 7:30 am to 4:00 pm
Services include:
- 5 patient rooms, pediatric specialty equipment, and specimen processing lab
- One large room to accommodate family participation, consent, consultations, neurocognitive testing, or survey-like interviews.
Contact Information
VICTR Clinical Research Center
Kevin Niswender, MD, PhD Medical Director
James M. Luther, MD, MSCI Associate Medical Director
Jennifer Adams, VICTR CRC Program Manager
Lana Howard, BSN, RN, CCRP Patient Care Services Manager
(615) 322-2312
Human Research Protections Program
Research Subject Advocate
(866) 224-8273 or (615) 322-2918 Option 2 for Participants
How To Find Clinical Trials Near You
Why participate in a clinical trial, how to find clinical trials, what to do once you have found a trial.
For some conditions, there is a go-to treatment. For others, there might be no available treatment, or existing treatments, such as chemotherapy, may produce intense side effects that result in further issues.
If you are a patient suffering from a disease for which there is no defined treatment on offer, then one of your options is to enroll in a clinical trial. Enrolling in a trial can benefit you and other future patients as you will contribute to research into your condition to help medical professionals develop the right treatment.
Alternatively, even if you do not have a medical condition, you could participate in clinical trials that recruit healthy people, such as trials for vaccines or those that need healthy control volunteers to help establish baselines.
Have you considered clinical trials for Clinical trials?
We make it easy for you to participate in a clinical trial for Clinical trials, and get access to the latest treatments not yet widely available - and be a part of finding a cure.
Clinical trials help with the development of new treatments for diseases or conditions. This could be in cases where there are currently no treatments available, or it could be to develop more effective treatments with fewer side effects.
There are numerous benefits for individuals who enroll in clinical trials, including more frequent health care check-ups (almost always free of charge), access to and monitoring by leading specialists, and, often, gaining a better understanding of your condition, which helps you to treat or manage it.
By participating in a clinical trial, 85% of trial participants¹ have felt they received better treatment than they otherwise would have.
If you are interested in enrolling in a clinical trial, keep in mind that not all trials are successful. Clinical trials can fail if the drug or device being trialed does not demonstrate efficacy. 57% of drugs² fail for this reason.
A common reason why a potentially effective treatment can fail during the trial stage is simply due to the trial sample size being too small. For this reason, it is incredibly important to recruit more volunteers to participate in clinical trials because a larger sample size ensures that treatment is adequately tested and generates better data about its effectiveness.
While there is always a risk that the drug or device being trialed does not result in the desired outcome, the potential benefits of enrolling in a clinical trial can still be useful for both you and the medical researchers, and you are making an important contribution to finding a treatment that does work.
Finally, another incentive to enroll in a clinical trial is that many offer a stipend to participants, varying from hundreds to thousands of dollars. Paid clinical trial opportunities are often available to healthy volunteers but can also be available to people seeking treatment for their condition. A trial can therefore help you offset the loss of income you might experience due to your illness.
The biggest obstacle for most patients in accessing clinical trials is simply finding out what trials are on offer. Additionally, not all trials are conveniently located, as you may have to travel. Some trials may have the funding to pay for your travel costs if you live far away, while others may not, so it is important to check the details of each trial.
There are a number of ways patients and volunteers can locate clinical trials in their local area.
Use an online service
An easy option is to sign up for a free online service that helps to match you with an appropriate clinical trial. As most clinical trial protocols are not written with patients in mind but instead are aimed at doctors, it can sometimes be hard to understand the jargon about what the trial involves and who is eligible. A free online service can simplify the process and save a lot of time for prospective trial participants.
Learn about using HealthMatch to find the right trial.
Ask your doctor
Your first port of call is typically to ask your doctor or another healthcare provider about whether they recommend you participate in any clinical trials, and if so, which ones. In any case, make sure to keep your doctor, such as your GP, in the loop. Your doctor can refer you to a clinical trial that you otherwise may not have access to.
Nurses are also an excellent source of knowledge, and many oncology nurses³ consider informing patients about trials to be part of their job.
It is a good idea to approach your doctor and other providers and let them know if you are interested in participating in clinical trials. Bear in mind that your doctor can’t know about every available trial. They may also not be able to refer you to the perfect trial, so you should not assume that a trial recommended by your doctor is automatically the right one. It is important to do your own research as well.
It is recommended that you have direct communication with the research team yourself when you are looking into a trial instead of just having your doctor handle all communication. It is important that you advocate for yourself with the trial team. However, it is good to start with your doctor in your search for a clinical trial as they can help you figure out whether it is worth looking into a specific study.
Check your local university or large hospital system
Clinical trials can be sponsored by various organizations, including drug manufacturers, governments, universities with teaching hospitals, or large hospital systems.
It's worth checking the trial organization’s website, which should have a list of the trials they are currently facilitating. Alternatively, you can call them and ask. The chances that they happen to have a relevant trial are relatively low, but it is often worth checking.
Some pharmaceutical companies may also list trials on their websites and have an online tool that matches you to a suitable trial. If you live near the NIH or another government organization, check their trial listings as well.
Use an online database
There are a number of public online databases of clinical trials. These databases allow you to search by a medical condition, treatment, other keywords, or a specific location to find trials best suited to your situation.
However, it can be tedious and time-consuming to search through tens of thousands of trials on these databases, and if you don't know which keywords to use, you might miss something useful. There is also a risk that you could end up applying for a trial you are not eligible for. Reviewing eligibility requirements with a healthcare provider can help avoid this, but it can be difficult to narrow down the list of potential trials yourself.
Talk to support and advocacy groups
Another option to match you with an appropriate clinical trial is to talk to support or advocacy groups for your condition. You could join Facebook groups for people with your condition or their family members, as it is possible that a member of the group is enrolled in, or knows of, a trial that might be suitable for you.
Generally, the larger the group, the higher your chance of finding out about a trial. As finding relevant clinical trials in your local area can be challenging, you may have a better chance of doing so if you join a local group.
If you have been searching for trials using the options above and have found some you are interested in, what should you do next?
Here are some steps to make sure that the trial you are looking at is a good fit for you:
Identify the sponsor.
Contact the trial administrator or principal investigator. Have a list of questions prepared to ask them about the trial. Clinical trials have complex written protocols, so going over them with a research team member can ensure you are eligible and improve your understanding of what the trial involves.
Talk to your doctor about the trial. Bring along a copy of the protocols and any other information so they can help you to make an informed decision about whether to enroll. Your doctor may spot something that means the trial is not a good match for you before you get too far into the process. If you are looking into several trials, your doctor can help you select the best one. It is important to make a special appointment with your doctor to do this, whether in person or via a video call.
Get a second opinion if you can. Talking to another doctor not connected to the trial can give you a broader picture of whether the trial is right for you.
Do your research into the type of clinical trial and whether the proposed treatment has been studied before or if it is being studied for other diseases.
Talk to friends and family members as they may offer insights you had not thought about.
Once you have decided to apply to a trial, contact the trial administrators again and seek guidance on the application process. Your doctor may also be able to help or may know someone who can assist with the paperwork.
Make sure that you are comfortable with the trial protocols and the amount of time and effort you will have to dedicate to it.
While it is always recommended that you ask your doctor, you can find a clinical trial through your own research. Online services can help match you with trials that may be right for you, and they let you take control over your condition and treatment.
Discover how HealthMatch is helping to simplify the process of finding and applying to the right clinical trial.
Global Public Attitudes About Clinical Research and Patient Experiences With Clinical Trials (2018)
Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review (2018)
The Role of Oncology Nurses in Discussing Clinical Trials (2018)
Share this story
Discover which clinical trials you are eligible for, do you want to know if there are any clinical trials you might be eligible for, have you been diagnosed with a medical condition, have you considered joining a clinical trial, related articles.
Clinical trials | Jun 2022
Explore more
Clinical trials.
Explore clinical trials for clinical trials and see those actively looking for patients near you.
Editor’s picks
Last updated: Apr 2022
Last updated: Mar 2022
Latest news
Last updated: Sep 2022
Last updated: Jun 2022
Professionals
If you work at a site, cro or sponsor, if you are a patient or caregiver, our top-rated products.
The CenterWatch Monthly Digital Subscription

The CRC’s Guide to Coordinating Clinical Research, Fourth Edition

Best Practices for Clinical Trial Site Management, Vol. 4
Latest news.

FDA Delivers Long-Anticipated Guidance on Diversity Action Plans

Sites May Be Underprepared for ICH E6 Revision, Survey Indicates

Lessons learned from oncology research: Put patients at the center and embrace new tools and strategies
Conferences and webinars, magi@home clinical research conference 2024, centerwatch webinar training pass, centerwatch in your inbox, sign up for centerwatch weekly now, books and management reports.

The PI’s Guide to Conducting Clinical Research, Third Edition

The CRA’s Guide to Monitoring Clinical Research, Sixth Edition

GCP Questions, FDA Answers, 2023 Edition

Best Practices for Clinical Trial Site Management

Online Books Library
Centerwatch company licenses.
- Success Stories

When you absolutely have to recruit patients on time
Home 5 Our Locations 5 Clinical Trials in the USA
Clinical Trials in the USA
For decades clinical trials in the USA have continuously been in high demand among pharmaceutical and biotechnological companies. The most prominent reasons for selecting the United States for clinical research are a large population pool, a developed network of experienced investigators, a supportive regulatory framework, and a vast market of drug consumption.
Accell’s team has decided to investigate what makes the USA such a favorable destination and the world’s principal hub for clinical research. So, we looked at the subject from different points of view varying from the USA’s geographical position, economic, and political status to its end-market advantages.
• Country’s overview
• USA health statistics & healthcare system overview
• Drug consumption market in the USA
• History of clinical trials in the USA
• USA regulatory landscape overview
• Current clinical trials situation in the USA
• Why partner with Accell for clinical research in the USA
Country’s overview
The United States of America is the third biggest country in the world, with the world’s most significant economic power measured in terms of gross domestic product (GDP). Politically, the USA is a federal republic with the federal government comprised of the three branches:
By 2019 estimates [ 1 ] , almost 330 million people live in the U.S., which makes it the third most populated country after China and India. The main characteristics of the U.S. population is its great racial, national, and ethnic diversity due to a global immigration phenomenon. Interestingly, the nine most populous U.S. states (California, Texas, Florida, New York, Pennsylvania, Illinois, Ohio, Georgia, North Carolina) contain slightly more than half of the total population . The largest state in terms of its population is California, with 39.6 million residents.
USA Health Statistics & Healthcare System Overview
According to the National Center of Health Statistics, life expectancy in the USA has recently increased from 77.8 (2006) to 78.6 (2016) years. Women still live longer (80.3 to 81.1 years) than men do (75.2 to 76.1 years) [ 2 ] ; nonetheless, the dominant causes of deaths remain the same for both sexes: heart disease, cancer, Chronic Lower Respiratory Disease, diabetes, stroke, Alzheimer’s disease, and unintentional injuries . Optimistically, for the past ten years (2006-2016), a steady decrease has been recorded in deaths from heart disease, stroke, cancer, and diabetes. However, the number of deaths from Alzheimer’s disease has significantly increased [ 3 ] .
The U.S. is famous for being the country with the best medical help worldwide. The country is home to such well-known hospitals as Mayo Clinic, Cleveland Clinic, Johns Hopkins Hospital, and Massachusetts General Hospital. It is essential to keep in mind that most of the healthcare facilities in the USA are private, and their services are pricey.
Healthcare insurance system in the USA
Since healthcare comes at a high cost, in the United States, citizens must have proper healthcare insurance. They usually obtain their health insurance through employment; however, it is possible to purchase insurance privately or through government-based programs, e.g., Medicare, Medicaid, Veterans Administration, or other military care.
Alternatively, some low-cost or free of charge healthcare services exist through non-profit organizations, charities, and publicly funded programs. The Patient Protection and Affordable Care Act (known as Obamacare) was signed in 2010. It aimed at the expansion of insurance coverage. The program had many opponents, and it still is under debate with parties being on both ‘for’ and ‘against’ sides.
The Organization for Economic Co-operation and Development (OECD) ranks the USA as the first country in the world by the spending of state funds for healthcare . In 2017, the expenditure was $10,206.5 per capita (or 17.1% of GDP). For comparison, Switzerland takes second place with 12.3% of GDP, and France comes third with 11.3% of GDP along with Germany with 11.2% of GDP. [ 4 ]
Despite prominent national spending on healthcare, the costs of medical services and lack of insurance coverage make Americans with lower incomes struggle to have adequate access to medical care. This situation is one of the reasons why these populations can get highly interested in participating in clinical trials and getting an opportunity to receive so much needed medical help.
Drug Consumption Market in the USA
The USA undoubtedly is the biggest pharmaceutical market in the world. By the second half of 2018, total pharmaceutical sales in the United States were estimated to reach about 464 billion U.S. dollars that year, which was 20 billion dollars more than the previous year [ 5 ] . Net medicine spending was $1,044 per person that is 0.9% (or $10) higher than in 2017. The primary factor that influenced the total net spending growth was the fact that patients were receiving existing branded drugs along with using newly launched ones.
- Currently, the most prominent players on the American pharmaceutical market are
- Pfizer Inc. with turnover of 25.32 billion USD;
- Johnson & Johnson (23.28 billion USD);
- Roche Holding AG (22.76 billion USD);
- AbbVie Inc. (USD 21.52 billion USD). [ 6 ]
Top-selling drugs are AbbVie’s Humira, Celgene’s Revlimid, Amgen’s Enbrel, and Roche’s Rituxan.
According to the Pharmaceutical Research and Manufacturers Association (PhRMA), U.S. firms conduct over half the world’s research and development (R&D) in pharmaceuticals (75 billion dollars) and hold the intellectual property rights on the newest medicines. [ 7 ] The total number of biotech and pharmaceutical companies in the USA surpasses 2,700 [ 8 ] .
History of Clinical Trials in the USA
Certain medical historians name the USA a cradle for the scientific approach to investigation of safety and efficacy of marketed drugs (as stated by Harry Marks, the author of “The Progress of Experiment: Science and Therapeutic Reform in the United States, 1900-1990”).
In 1905 American Medical Association (AMA), the largest association of physicians and medical students in the United States, founded in 1847, formed its own Council on Pharmacy and Chemistry with the primary goal to reveal safe and efficient drugs among placebos with big names. Since then, only medications approved by the Council could hold AMA’s seal and be advertised in AMA’s journal. To receive approval, manufacturers had to run a preclinical trial to prove an ingredient’s purity. After that, the Council on Pharmacy and Chemistry followed rudimentary efficacy and safety tests. In 1906 the Pure Food and Drugs Act proclaimed the Bureau of Chemistry as the official market regulator responsible for taking of adulterated and misbranded products.
In the 1920s, first multicenter trials were implemented in the USA to reduce possible mistakes of individual observers.
Later, in 1938, the Federal Food, Drug, and Cosmetic Act came in force . The Federal Act gave authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of food, drugs, medical devices, and cosmetics. The FDA itself is a federal agency founded in 1906 and responsible for protecting and promoting public health up to this day.
The 1938’s Act obliged drug manufacturers to submit safety data to FDA officials for evaluation before marketing. Soon New Drug Application rules were elaborated that required stating information about all performed preclinical and clinical research. Hence, a standardization process of preclinical and clinical tests of new drugs along with standards for clinical trial conduct started.
In the 1960’s, the Drug Amendments and investigational drug regulations obliged drug developers to run studies on animals before testing a drug on humans. Besides, controlled trials to prove the drug efficacy became necessary.
Since then, the FDA has developed a full package of standards and recommendations in the field of clinical trials and drug development. Recent FDA’s efforts concentrate on speeding up of more effective drugs appearance on the market and lowering of clinical trials costs. For instance, currently, the FDA works on the implementation of surrogate parameters in oncology studies and risk-based monitoring.
USA Regulatory Landscape Overview
In the USA, all food, drugs, cosmetics, and medical devices for both humans and animals, are regulated under the authority of the United States Food and Drug Administration, widely known as the FDA. Only after a review of the FDA and Institutional Review Boards (IRBs), clinical trials in humans can start. [ 9 ]
To initiate a clinical trial, a sponsor should submit to the FDA an Investigational New Drug (IND) Application with the content specified in the Code of Federal Regulations (CFR) 21, section 312, and should wait 30 days before the trial initiation. During this time, the FDA has an opportunity to review the documents and authorize the trial. Usually, a new drug application can be submitted when the drug successfully passes all three phases of clinical trials.
Given the fact that the USA’s pharmaceutical market is a desirable destination for many companies, it is worth noticing that clinical research does not have to be exclusively held within the United States to receive an FDA’s authorization . This particular notion can be beneficial to those companies, especially small or emerging ones with limited trial budgets, who want to optimize their expenditures and enter the end-market with their product faster.
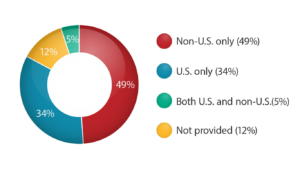
According to the clinicaltrials.gov information [ 10 ] , 58% of recruiting studies are “Non-U.S. only.” This number demonstrates clearly that almost 2/3 of all FDA-registered studies take place outside the United States.
Thus, to enter the USA market, you can hold a trial in other geographies. FDA registers the study results given the fact that everything was compliant with ICH-GCP and FDA’s regulations . Moreover, the FDA continually monitors the quality of clinical trials both in the USA and outside the country: the agency regularly conducts audits in various countries and publishes the findings. [ 11 ]
Current Clinical Trials Situation in the Country. Is it worth running clinical research in the USA?
In 2018 the United States was the top location for global clinical trials taking up 35% of the total number of geographies. Countries following the USA in the clinical research global arena were China (10%), Japan (5.2%), India (3.8%), and Germany (3.8%) rounding out the selection of the top five countries. According to the data on www.clinicaltrials.gov, by mid-2019, there are over 322,000 research studies registered worldwide where over 100,000 studies take place within the USA. These numbers mean that over 30% of all registered globally studies are held exclusively in the United States; however, the data shows that 49% of global studies happen strictly outside of the USA. [ 12 ]
Percentage of Registered Studies by Location
(in accordance with www.clinicaltrials.gov as of December 11, 2019)
Typically, only late phase (II and III) clinical trials require global coverage to make sure that they involve larger populations and a high number of both sites and patients. So, it makes perfect sense for many companies to run an early phase clinical research locally and preferably in the USA since most pharmaceutical and biotechnology firms are based in the region.
To facilitate and speed up recruitment, the most populated territories in the USA are of interest to pharmaceutical companies, biotechs, and clinical research organizations (CROs). The most clinical trial concentration is within such states as California, New York, and Texas , while Wyoming, Alaska, North Dakota, Hawaii, Maine, and Vermont remain mostly excluded from the competition with just a few studies listed. [ 13 ]
Number of Registered Clinical Trials in the USA
According to Accell’s internal data analytics system, in the second quarter of 2019, the majority of trials initiated in the United States was in oncology and central nervous system (CNS). With a noticeable gap, trials in infectious and cardiovascular diseases share the third place.
What makes the USA an attraction for sponsors is its robust regulatory system, developed investigators network, large population, the English language, and a vast potential of market’s drug consumption.
Pitfalls of running a clinical trial in the USA
Despite all benefits that running a clinical trial in the USA brings to a Sponsor, it is essential to know about certain complexities and risks involved in the process. First and foremost, the risk factor is patient recruitment and retention . The numbers are brutal: 48% percent of sites miss their enrollment targets [ 14 ] , and 80% of trials are delayed due to recruitment [ 15 ] .
The USA’s clinical research market is oversaturated, with various trials running simultaneously. Thus, there is a continually increasing competition for patients. It is a basic mathematics to count that the USA has one clinical trial for every 2,582 people. We can compare the data with Eastern Europe, where there is one clinical trial for every 6,975 or post-Soviet countries with a clinical trial ratio of one to 89,884 people. These numbers demonstrate that a “fight” for each patient is a severe endeavor in the States, especially given the fact that in the USA’s most medical practices are private with no central database of specific patients. So, each patient is searched for and enrolled on a case by case basis. Moreover, the recruitment is hugely dependent on the proactive work of an investigator. Yet, data shows that 27% of U.S. investigators fail to enroll any subjects in comparison with 19% of investigators elsewhere [ 16 ] .
Another factor influencing recruitment and, precisely, retention rate, is the domestic migration rate within the USA . Citizens face no cultural or nationally regulated barriers for moving from one state to another. Hence, there is a probability that a patient can easily drop out of a study if they move to a different location.
Finally, per-patient cost for clinical trials in the USA is high in comparison to other regions . Although in a certain way, logistics can be cheaper within the States than shipping drugs and bio-samples overseas, investigators’, sites’, and CROs’ services come at a substantial cost to a Sponsor. As an example, taking “1” as a total cost of a clinical trial in the USA, a Sponsor will have 0.5 in Germany, Brazil, or China, 0.41 in Russia, 0.39 in Poland, and 0.36 in India [ 17 ] .
To sum up, we could assume that the USA is generally a good location for a smaller Phase I trial with healthy volunteers or a limited number of needed for recruitment patients. However, for a more extensive Phase II and III trials, it would be a wise strategy to look for various clinical trial geographies options . In the meantime, it can be reasonable not to eliminate the USA, especially if a Sponsor wants to enter the U.S. market with their drug but reduce it just to a certain statistically required number of patients while concentrating the efforts on recruitment in other regions. At Accell, we highly recommend studying more closely such potent geographies for clinical research as Eastern Europe and post-Soviet states (if Caucasian population is statistically needed), India (primarily Indo-Aryan population), and China (Asian population).
Why selecting Accell as a research partner within and outside the USA?
Accell Clinical Research, LLC is an American based company founded in 2007 that delivers high-quality, full-service clinical research services for over a decade. While concentrating mostly on the Eastern European region for its numerous benefits, we have been conducting multicenter clinical trials with U.S. sites as well. Most of our Sponsors are small and medium-size American pharmaceutical and biotechnology companies with headquarters and primary R&D activities in the USA.
Depending on the stage of a trial and a required number of patients for recruitment, Accell suggests an optimal international strategy for a particular study to make sure a Sponsor gets involved with only well-performing and trusted investigators who work at high recruiting sites. Accell’s ultimate goal is to recruit patients on time and within a budget making each research project a cost-effective one.
Throughout the past decade, Accell participated in 11 global clinical trials with sites both within and outside the USA with American and European sponsors. Our vast experience in regulatory processes and comprehensive cooperation with U.S. partners renders us the right company to work with on the USA’s territory. Accell’s advantageous geographical positioning with offices and staff located in the USA, Europe, Russia, and Ukraine facilitates to share responsibilities between regions. It makes our model optimal, efficient, and cost-effective.
The article is written by Kirill Zhuravlev, MD, PhD (Medical Director) and Olga Nayanova (Business Development Associate). The article was updated on November 27, 2019.
References:
- Internet World Stats
- Centers for Disease Control and Prevention (1)
- Centers for Disease Control and Prevention (2) – Health, United States, 2017
- OECD.Stat – Health, Health Expenditures, and Financing
- Statista (1)
- Market Research Reports
- Statista (2) – U.S. number biotech companies 2012-2016
- U.S. Food and Drug Administration
- ClinicalTrials.gov – Locations of Recruiting Studies
- FDA – Clincial Investigator Inspection Search
- ClinicalTrials.gov – Types of Registered Studies
- ClinicalTrials.gov – Studies in the United States
- Tufts CSDD Impact Report, 2013, 15(1)
- Drugdevelopment-technology.com, 2012, Clinical Trial Delays: America’s Patient Recruitment Dilemma
- NCBI – The State of Clinical Research in the United States: An Overview
- NCBI – Challenges in Clinical Research
Our Locations
The top ten largest American cities with a population of over a million inhabitants are:
- New York City, NY – 8.6 million
- Los Angeles, CA – 4 million
- Chicago, IL – 2.6 million
- Houston, TX – 2.3 million
- Phoenix, AZ – 1.7 million
- Philadelphia, PA – 1.5 million
- San Antonio, TX – 1.5 million
- San Diego, CA – 1.4 million
- Dallas, TX – 1.3 million
- San Jose, CA – 1 million
discover Accell’s geography country by country

Non-EU 60 days for regulatory approval 9.5 million population

EU 60 days for regulatory approval 7.1 million population

EU 60 days for regulatory approval 4.1 million population

EU 60 days for regulatory approval 1.3 million population

Non-EU 28-56 days for regulatory approval 3.7 million population

EU 60 days for regulatory approval 9.8 million population

Non-EU 10 business days for regulatory approval | 17.8 million population

EU 60 days for regulatory approval 1.96 million population

EU 60 days for regulatory approval 2.8 million population

EU 60 days for regulatory approval 622 781 population

EU 75 days for regulatory approval 37.95 million population

EU 60 days for regulatory approval 19.71 million population

Non-EU 60 days for regulatory approval 144.3 million population

EU 60 days for regulatory approval 7 million population

Non-EU 60 days for regulatory approval 45 million population

Non-EU 60-90 days for regulatory approval 8.7 million population

North America 30-90 days for regulatory approval 327.2 million population
This Is Your Team. We Make Clinical Trials Happen.

One of the largest clinical research networks in North America
One of the largest clinical, research networks in, north america.
With sites across the US and Canada, chances are we’re in your neighborhood.
Featured News
Centricity Research acquires North Carolina-based Lucas Research, expanding its foothold across North America and its wide range of therapeutic area capabilities.
Join a paid clinical research study today.

FDA & Health Canada Drug Approvals
Therapeutic Areas

Help us end illness and change lives.
When you participate in a clinical study , you help us collect valuable information that aids in the development of new treatments and medications.
Even your favorite pain relievers, morning vitamins, and common antibiotics were made possible because of clinical research!
Explore our open studies and learn how you can get paid to participate.
Are you a Sponsor or CRO?


Could you be our next great team member?

Are you a physician?
Get our e-newsletter.
- Referring Physicians
USA Headquarters
800 Talbotton Road, Columbus, GA 31904
1.866.764 JOIN
1929 Bayview Avenue, Suite 106 Toronto, ON M4G 3E8
1.833.323 JOIN
Canadian headquarters.
Patient Outreach Center:
We’re going to The Alzheimer’s Association International Conference | July 28 – Aug 1 | Philadelphia, PA → Set up a Meeting
Advancing the development of new medical treatments through a flexible clinical trial network that reaches people everywhere.
Your source for site-based, hybrid and fully decentralized clinical trials.

IMAgine greater access to new treatments. We take a compassionate approach to clinical research services. Through patient-centric technology, we support a flexible trial model that allows us to set up additional patient outreach centers wherever and whenever they’re needed.
MORE INFORMATION
Sponsors & CRO

IMAgine site proximity, broad diversity and access to millions. We have a proven aptitude for enrolling qualified, diverse participants and providing sponsors and CROs with high-quality data, driving clarity in complex situations and accelerate the development of novel treatments.
The IMA Clinical Research Network
Full-service clinical trial sites, nationwide satellite sites.
with 10K+ Unique Weekly Visits
Patient Database
SITE PROXIMITY | PATIENT CENTRICITY BROAD DIVERSITY | ACCESS TO MILLIONS

Breaking the Stigma: The Importance of Open Conversations About Mental...

Advancing Diversity in Clinical Trials

Uncovering the Evolution of Diabetes Management

2023 Year In Review
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
- Department of Health and Human Services
- National Institutes of Health

NIH Clinical Center: There’s No Other Hospital Like It
The Clinical Center is a national resource that makes it possible to rapidly translate scientific observations and laboratory discoveries into new approaches for diagnosing, treating, and preventing disease. The NIH Clinical Center's vision is to lead the global effort in training today's investigators and discovering tomorrow's cures.
Improving for the Future
The Clinical Center is committed to improving the resources available to its patients and partners in research. The hospital is undergoing renovations and expanding capabilities to support patient outcomes and facilitate research.
In 2021, the Clinical Center opened a new Center for Cellular Engineering (CCE) facility to meet increasing demand for customized cellular-therapy products and services needed for personalized treatments. After a multi-year construction project, the redesigned Pharmacy will open in 2022 with a focus on safety and efficiency (link to Pharmacy story, once posted).
And on the horizon, the Clinical Center will add an eight-story wing that will house surgery, laboratory medicine and radiology and imaging sciences. These departments involve some of the most advanced and technology dependent programs supporting NIH's research. The new wing is anticipated to open in 2028.
History of Medical Milestones
At the NIH Clinical Center, clinical research participants—more than 500,000 since the hospital opened in 1953—are active partners in medical discovery, a partnership that has resulted in a long list of medical milestones , including development of chemotherapy for cancer; the first use of an immunotoxin to treat a malignancy (hairy cell leukemia); identification of the genes that cause kidney cancer, leading to the development of six new, targeted treatments for advanced kidney cancer; the demonstration that lithium helps depression; the first gene therapy; the first treatment of AIDS (with AZT); and the development of tests to detect AIDS/HIV and hepatitis viruses in blood, which led to a safer blood supply. Patients come from all 50 states and from around the world.
Currently, there are about 1,600 clinical research studies in progress at the NIH Clinical Center. About half are studies of the natural history of disease, especially rare diseases, which often are not studied anywhere else. What researchers learn by studying rare diseases often adds to the basic understanding of common diseases. Most other studies are clinical trials, which often are the first tests of new drugs and therapies in people. The clinical trials at the NIH Clinical Center are predominantly Phase I and Phase II, often first-in-human to test safety and efficacy.
Imagination and Collaboration of Specialists
Some 1,200 credentialed physicians, dentists, and PhD researchers; 620 nurses; and 450 allied health-care personnel work in patient care units and laboratories in numerous areas of clinical study. Specialists ' research at the NIH Clinical Center include: musculoskeletal and skin diseases; cancer; dental and craniofacial disorders; eye disorders; heart, lung, and blood diseases; infectious diseases; medical genetics; mental health; and neurological disorders.
This plethora of expertise under one roof allows patients to see specialists in one week that it would take months, if not years, to see in other settings. The collaborative environment of the NIH Clinical Center makes it possible for investigators to make referrals for immediate testing and confer with peers across research interests to come up with the best approach for diagnosing and treating patients. The freedoms that the NIH Clinical Center affords encourage clinician-scientists to stretch their imagination and pursue the ideas that may lead to a medical discovery.
The NIH Clinical Center recognizes that a special patient population requires a special team of nurses. In 2010, Nursing and Patient Care Services completed a four-year initiative to define the specialty of clinical research nursing. In addition to providing and coordinating clinical care, clinical research nurses have a central role in assuring participant safety, ongoing maintenance of informed consent, integrity of protocol implementation, accuracy of data collection, data recording and follow up. The step to formalize their specialty led a recommitment to the principles of primary nursing and application of those principles in the current practice environment for nurses.
Support of Patients and Caregivers
The NIH Clinical Center sees 10,000 new research participants a year. There are two types of research participants: patient volunteers and healthy volunteers. Patient volunteers are people with specific diseases or conditions who help medical investigators learn more about their condition or test new medications, procedures, or treatments. A healthy volunteer is a person with no known significant health problems who plays a vital role in research to test a new drug, device or intervention.
Acknowledging the importance of a patient's support system and comfort level, there are many programs in place to ease the clinical research process for both patients and their families.
Friends of Patients at the NIH is a nonprofit organization that provides patients in need emotional and financial support while they receive care at the NIH. This can include shelter near the NIH during treatment, help keeping up with housing costs at home, family and caregiver travel costs back and forth to the NIH, and minor quality of life activities to reduce stress on patients.
Pediatric patients and their families stay at The Children's Inn a 24-hours-a-day, seven-days-a-week, 365-days-a-year operation where kids can be kids for a while, instead of patients. Also to support children while at the NIH Clinical Center, there is a school teaching kindergarten through high school with a classroom and teachers who will go to the bedside.
For families and loved ones of adult patients, the Edmund J. Safra Family Lodge offers a home-like place of respite just steps away from the NIH Clinical Center, providing space for solitude, family meetings, and supportive fellowship.
Training for the Next Generation
Additionally, the NIH Clinical Center offers an extensive range of clinical research training to help prepare the next generation of clinician-scientists. The innovative curriculum includes courses in pharmacology, principles and practice of clinical research, and bioethics.
The NIH Clinical Center offers the Sabbatical in Clinical Research Management program for clinical investigators, health-care managers and administrators, and others who oversee clinical trials to learn about the foundational elements required to manage a clinical or translational research enterprise
Additionally, the NIH offers two programs-a collaboration between the Clinical Center, the Damon Runyon Cancer Research Foundation and the National Cancer Institute; as well as a partnership between the NIH and the Albert and Mary Lasker Foundation. These programs will bring external, early career investigators to the NIH Clinical Center for exposure to the hospital's unique resources.
The Clinical Center is part of the NIH's Intramural Research Scientific Program. The NIH is the medical research agency of the US government and part of the U.S. Department of Health and Human Services .
NOTE: PDF documents require the free Adobe Reader .
This page last updated on 05/20/2022
You are now leaving the NIH Clinical Center website.
This external link is provided for your convenience to offer additional information. The NIH Clinical Center is not responsible for the availability, content or accuracy of this external site.
The NIH Clinical Center does not endorse, authorize or guarantee the sponsors, information, products or services described or offered at this external site. You will be subject to the destination site’s privacy policy if you follow this link.
More information about the NIH Clinical Center Privacy and Disclaimer policy is available at https://www.cc.nih.gov/disclaimers.html
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
List of Central Labs in United States
Central labs in united states in alphabetical order.
Our Rochester, New York location is our headquarters and the site from which we serve clients throughout the United States and Canada. ACM is a regional leader in a full range of routine and esoteric testing services, backed by extensive laboratory f... View full profile
- United Kingdom
- United States
Our 40+ years of experience, innovative strategies, global capabilities, and expertise in early phase research make us faster than our competitors. That means you get key data sooner, enabling you to make earlier go/no-go decisions about your drug's... View full profile
- South Korea
- Switzerland
Central laboratory services. Every phase, every day. Cenetron was established in 1995 and has rapidly grown into an industry-leading central laboratory service provider, distinguishing itself as an innovative and responsive clinical trial partner. I... View full profile
For over 50 years, Cerba Research has evolved in one direction: toward better serving our customers. From corporate acquisitions to international expansions to everyday decision-making, one question guides our approach: Will this help us to better se... View full profile
- South Africa
We are one of the largest privately held clinical testing laboratories in the U.S. With dedicated facilities in North America, we perform hundreds of thousands of tests every day for clients large and small. Our staff of more than 700 associates work... View full profile
Results That Matter! Reliable, high quality laboratory data is pivotal to the success of clinical trials. It’s the RESULTS THAT MATTER. We are dedicated to providing the most cost effective and efficient testing solutions to pharmaceutical and biotec... View full profile
- Netherlands
At Frontage, we offer a wide range of central laboratory services. We provide a comprehensive range of laboratory testing, histology and pathology services, sample management and global distribution. Routine Laboratory Testing General Chemistry Gene... View full profile
ICON Central Laboratories is headquartered in Farmingdale, New York and provides global central laboratory services from wholly-owned facilities in New York, Dublin, Singapore and China. ICON Central Laboratories maintains a number of industry accred... View full profile
We are a central research laboratory in the area that offers a full scale test menu for anatomic pathology, clinical and specialty tests. Interlab was created with the mission to improve healthcare and advance the science of laboratory medicine. We c... View full profile
InVitro International is a pioneer in the development and application of proprietary non-animal testing alternatives for the determination of eye irritation, skin irritation and skin toxicity. The Company develops and markets in vitro assay kits and... View full profile
Leading the evolution in central laboratory services, LabConnect provides you with world-class capabilities through our network of laboratories and industry-leading support services, including routine and specialized laboratory testing, sample tracki... View full profile
Generating quality data starts with protocol- and visit-specific specimen collection kits that are accurately assembled and reach investigator sites on time. Global logistics experts monitor shipments to assure within stability delivery of specimens.... View full profile
Medpace provides customized, high-quality central laboratory services to pharmaceutical and biotech clinical development. For over 15 years Medpace’s central laboratory has been offering full-service global central laboratory support for Phase I-IV c... View full profile
Customized Services That Keep Your Trial in Motion. At MLM we take care of all laboratory aspects in your clinical trial. During study setup, we prepare all materials and documents and design your study and visit specific kits. Once our proprietary M... View full profile
Pharmaceutical development is evolving rapidly, and with it, the demand for high-quality centralized laboratory services continues to grow. Coupled with your need to access laboratory/patient data in real time and to make faster decisions about your... View full profile
Precision medicine clinical trials—those driven by biomarker data—pose a set of challenges unique from classical clinical trials. The complexity inherent in kit development, logistics, sample management, and data requires an approach to central lab... View full profile
Meeting the demands of today’s drug, device and diagnostics development environment requires a next generation service provider. When looking for a long-term central laboratory solution, it’s important to find a partner that can accommodate your need... View full profile
List of Clinical Research Labs by location
- Saudi Arabia
- United Arab Emirates
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, what is clinical research.
Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances.
This page last reviewed on September 29, 2016
Connect with Us
- More Social Media from NIH
.png/:/cr=t:0%25,l:0%25,w:100%25,h:100%25/rs=h:1000,cg:true)
Compassionate Care with Cutting-Edge Treatment
Learn about the benefits of volunteering!
.png/:/cr=t:0%25,l:1.37%25,w:97.27%25,h:97.27%25)
The Center for Clinical Research and Innovation (CCRI) at Spring Heights Hospital is focused on advancing therapies for patients through our affiliated providers and care sites. As part of a learning health system, we are unlocking insights into real-world data and clinical studies that lead to scientific breakthrough.
Who we serve
Looking to add or increase research activities in your clinical practice? We can integrate clinical research into your practice as seamlessly as possible.
Are you interested in participating in clinical research?
Clinical research can seem daunting. Here at CCRI, our team is ready to answer your questions.
See how our flexibility and depth of experience create new possibilities for you. We are motivated to conduct clinical research with integrity, honesty in reporting data and going the extra mile to ensure our quality.
what we offer
Improvement to research pipeline: quality and volume., decreased start-up timelines and overhead., increased efficiency through technology automation., expanded subject matter expertise and physician engagement., we bring clinical care to minority patients and other unique demographics who are underserved., affiliates and sponsors.
Sign up to hear from us.
Copyright © 2023 CCRIresearch - All Rights Reserved.
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.
- Department of Health and Human Services
- National Institutes of Health

The NIH Blood Bank Has Moved!
NIH Blood Bank has relocated to the new wing: North of Masur Auditorium towards the South Lobby, Magnuson Building.

There's no other hospital like it.
The NIH Clinical Center is the nation's largest hospital devoted entirely to clinical research.
Resources and Initiatives
COVID-19: Public health information from CDC . | Research information from NIH ( Español ). | NIH staff guidance on coronavirus ( NIH Only ).
Clinical and Safety Performance Metrics (1st Quarter 2024) The Clinical Center is pleased to post some key metrics that illustrate our strong commitment to patient and employee safety and continuous improvement.
You are now leaving the NIH Clinical Center website.
This external link is provided for your convenience to offer additional information. The NIH Clinical Center is not responsible for the availability, content or accuracy of this external site.
The NIH Clinical Center does not endorse, authorize or guarantee the sponsors, information, products or services described or offered at this external site. You will be subject to the destination site’s privacy policy if you follow this link.
More information about the NIH Clinical Center Privacy and Disclaimer policy is available at https://www.cc.nih.gov/disclaimers.html

Powerful Teamwork With Sponsors and CROs
- Fast, efficient study enrollments
- Meets or surpasses enrollment targets
- Active management in every phase
- Highly relevant, superior grade data
- Quality assurance in real time
OPERATIONAL CAPABILITIES
Electronic source.
- Automated data integration with study EDC
- Remote monitoring
- 21 CFE Part 11 compliant
- Real-time Quality Assurance with built-in edit checks
STIPEND CARDS
- Automated stipend payment processing that enforces ICF compliance
- Improved subject retention
EXPERIENCED RESEARCH STAFF
- Average staff experience of 4.5 years
- Rave reviews and awards for quality from various Sponsors and CROs
- Phase 1 to 4 research experience
SIX SIGMA PROJECT MANAGEMENT
- Drives on-time study startup
- A Check-n-balance approach that ensures the highest quality is maintained while maximizing study efficiency
- Solutions that are customized and applied to each study vs. standard fit-in-the-box solutions from competitors

Caring Innovators For Study Participation
You can play an important role in helping to advance medicine and healing, as a clinical study volunteer. 310 Clinical Research is accepting participants for new and ongoing studies that could lead to cures for conditions affecting you and others. Every study is FDA-governed and supervised by a caring, licensed physician. It’s easy to sign up now, through this website or a simple call to us. We’ll gladly guide you through the simple sign-up steps. Here’s what you can expect:
- Sign a simple consent form – withdraw at any time
- Easy requirements – no insurance required
- Receive a free physical exam and blood tests
- Earn money for your participation
- Play an active role in advancing medicine
Studies you may be interested in
- Contraceptive
- Flu Symptoms
- Heavy Menstrual Bleeding
- Post-COVID-19
- Respiratory Conditions
- Skin Conditions
[email protected]
Registration Form

EXCELLENCE IN ADVANCING MEDICINE
We are committed to groundbreaking research that can benefit our society for generations to come.

Research that makes an impact on Humanity
The mission of the Clinical Research Center of Florida is to support each Sponsor’s Research Trial by providing the highest standards of quality and fostering ethical conduct in research performed by our accomplished multi-specialty physicians.
We offer sponsors a state-of-the-art medical facility staffed by highly skilled investigators and support staff with a combined 100 years of clinical research expertise. We have demonstrated the utmost integrity in the conduct of our research activities in a way that has made sponsors proud to partner with us.

Participating in clinical studies can give you access to treatments that are not otherwise available. The knowledge gained from the study is a contribution to the collective research that helps pave the way to preventative and curative medicine. Without volunteers, progress in medicine would not be possible.
Our Areas of Focus

CNS / MENTAL HEALTH

With a number of positive outcomes, cancer studies have the potential to develop breakthrough treatments and bring hope to those afflicted

Research in pulmonary medicine and lung issues extends to a number of medical conditions that benefit from this important work.

INFECTIOUS DISEASE
Putting a stop to the spread of infectious diseases is a top priority on a global level. Our studies are a contribution to a worldwide effort.

Ophthalmology
Our expertise in eye diseases and disorders is extensive, with many successful clinical trials that resulted in positive outcomes for the participants.

Affecting both men and women, urinary tract conditions require highly-skilled staff to conduct the most effective clinical studies.

GASTROENTEROLOGY
The work we do with gastroenterology has the potential to improve the quality of life for those suffering from digestive and other related issues.

PHASE ONE TRIALS
Experience is vital to the efficacy of phase one trials, as it sets the blueprint for moving forward to effective treatment plans.

WOMEN'S HEALTH
As a core focus in our research facility, we recognize the significance and impact this work has to benefit the lives of women everywhere.
Meet Our Investigators

Akiva M. Daum, MD
Specialist in psychiatry.
Board Certified: American Board of Psychiatry

Allen Patterson, MD
Specialist in obstetrics & gynecology.
Board Certified: Obstetrics & Gynecology

Daniel Lindenberg, MD
Specialist in gastroenterology.
Board Certified: American Board of Internal Medicine; Gastroenterology

George P. Azar, Jr., MD
Specialist in pulmonology.
Board Certified: American Board of Internal Medicine; Pulmonary Medicine

Harold Rosen, MD
Board Certified: Gastroenterology; Internal Medicine

Roger Z. Samuel, MD

Romeo K. Fernandez, MD
Specialist in pediatric neurology.
Board Certified: Pediatric Neurology

Shani S. Reich, MD
Specialist in ophthalmology.
Board Certified: American Board of Ophthalmology

Steven C. Kester, MD
Specialist in urology.
Board Certified: American Board of Urology

Craig W. Herman, MD
Our research facility.

Our Research Center
We are an independent, research facility with a private practice specializing in a wide spectrum of medical conditions. Our Pompano Beach, Florida location houses cutting-edge research with some of the most experienced investigators and research staff in the nation. We are a preferred clinical research site with numerous sponsors and CROs, demonstrating the culture of quality found in our work and in the patients we recruit.
COORDINATORS
Acrp/socra certified personnel, percent successful subject retention, percent of studies meet enrollment, recent studies.
- CNS / Mental Health
Gastroenterology
Infectious disease.
- Phase 1 Trials
- Women's Health

Adolescent Postpartum Depression

Bladder Cancer
Depression after giving birth

Enlarged Prostate & Overactive Bladder

Irritable Bowel Syndrome with Diarrhea

Low Testosterone
Mild to Moderate Ulcerative Colitis

Pediatric Migraine

Prostate Cancer
Urinary Tract Infection (UTI)

Weight Gain with Schizophrenia
Trusted by the largest names in pharmaceuticals.

ENROLL TODAY!
- Name * First
- IBS with Diarrhea
- Enlarged Prostate & Overactive Bladder
- Postpartum Depression
- Weight gain Schizophrenia
- Date of Birth * Month Day Year
- How did you find us? Google Search LinkedIn Facebook Word of Mouth
- Additional Information
- I accept the privacy policy
- Phone This field is for validation purposes and should be left unchanged.
Proudly Giving Back To:

MAIN (954) 941-3330
RECRUITMENT (954) 941-3330
v Text CRCF to (954) 290-1049 To Join Our Enrollment System
Investigators

Akiva Daum, MD

Professionals

Aviva Malayev, APRN

Chaya Fellig, PA-C

Miriam Jeanie Schiller

Stainley William, MD

Patricia de la Cabada, MD

We are the Clinical Research Center of Florida. Learn more.
Our Facility

Cutting-edge research facility with experienced investigators.

Why participate in a Clinical Trial? Find out more info here.

Are you interested in being a part of a clinical trial Learn more!

Why participate in a Clinical Trial? Find out more info here.
Therapeutic Areas
CNS & Mental Health

Phase One Trials
Pulmonology
Women's Health
We are committed to excellence and driven by integrity.
Recent Studies
- Department of Health and Human Services
- National Institutes of Health

More Information
About clinical center, clinical trials and you, participate in a study, referring a patient.
About Clinical Research
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222
Nih Access Only. Your requested page cannot be open.
Human Subjects Office
Medical terms in lay language.
Please use these descriptions in place of medical jargon in consent documents, recruitment materials and other study documents. Note: These terms are not the only acceptable plain language alternatives for these vocabulary words.
This glossary of terms is derived from a list copyrighted by the University of Kentucky, Office of Research Integrity (1990).
For clinical research-specific definitions, see also the Clinical Research Glossary developed by the Multi-Regional Clinical Trials (MRCT) Center of Brigham and Women’s Hospital and Harvard and the Clinical Data Interchange Standards Consortium (CDISC) .
Alternative Lay Language for Medical Terms for use in Informed Consent Documents
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
ABDOMEN/ABDOMINAL body cavity below diaphragm that contains stomach, intestines, liver and other organs ABSORB take up fluids, take in ACIDOSIS condition when blood contains more acid than normal ACUITY clearness, keenness, esp. of vision and airways ACUTE new, recent, sudden, urgent ADENOPATHY swollen lymph nodes (glands) ADJUVANT helpful, assisting, aiding, supportive ADJUVANT TREATMENT added treatment (usually to a standard treatment) ANTIBIOTIC drug that kills bacteria and other germs ANTIMICROBIAL drug that kills bacteria and other germs ANTIRETROVIRAL drug that works against the growth of certain viruses ADVERSE EFFECT side effect, bad reaction, unwanted response ALLERGIC REACTION rash, hives, swelling, trouble breathing AMBULATE/AMBULATION/AMBULATORY walk, able to walk ANAPHYLAXIS serious, potentially life-threatening allergic reaction ANEMIA decreased red blood cells; low red cell blood count ANESTHETIC a drug or agent used to decrease the feeling of pain, or eliminate the feeling of pain by putting you to sleep ANGINA pain resulting from not enough blood flowing to the heart ANGINA PECTORIS pain resulting from not enough blood flowing to the heart ANOREXIA disorder in which person will not eat; lack of appetite ANTECUBITAL related to the inner side of the forearm ANTIBODY protein made in the body in response to foreign substance ANTICONVULSANT drug used to prevent seizures ANTILIPEMIC a drug that lowers fat levels in the blood ANTITUSSIVE a drug used to relieve coughing ARRHYTHMIA abnormal heartbeat; any change from the normal heartbeat ASPIRATION fluid entering the lungs, such as after vomiting ASSAY lab test ASSESS to learn about, measure, evaluate, look at ASTHMA lung disease associated with tightening of air passages, making breathing difficult ASYMPTOMATIC without symptoms AXILLA armpit
BENIGN not malignant, without serious consequences BID twice a day BINDING/BOUND carried by, to make stick together, transported BIOAVAILABILITY the extent to which a drug or other substance becomes available to the body BLOOD PROFILE series of blood tests BOLUS a large amount given all at once BONE MASS the amount of calcium and other minerals in a given amount of bone BRADYARRHYTHMIAS slow, irregular heartbeats BRADYCARDIA slow heartbeat BRONCHOSPASM breathing distress caused by narrowing of the airways
CARCINOGENIC cancer-causing CARCINOMA type of cancer CARDIAC related to the heart CARDIOVERSION return to normal heartbeat by electric shock CATHETER a tube for withdrawing or giving fluids CATHETER a tube placed near the spinal cord and used for anesthesia (indwelling epidural) during surgery CENTRAL NERVOUS SYSTEM (CNS) brain and spinal cord CEREBRAL TRAUMA damage to the brain CESSATION stopping CHD coronary heart disease CHEMOTHERAPY treatment of disease, usually cancer, by chemical agents CHRONIC continuing for a long time, ongoing CLINICAL pertaining to medical care CLINICAL TRIAL an experiment involving human subjects COMA unconscious state COMPLETE RESPONSE total disappearance of disease CONGENITAL present before birth CONJUNCTIVITIS redness and irritation of the thin membrane that covers the eye CONSOLIDATION PHASE treatment phase intended to make a remission permanent (follows induction phase) CONTROLLED TRIAL research study in which the experimental treatment or procedure is compared to a standard (control) treatment or procedure COOPERATIVE GROUP association of multiple institutions to perform clinical trials CORONARY related to the blood vessels that supply the heart, or to the heart itself CT SCAN (CAT) computerized series of x-rays (computerized tomography) CULTURE test for infection, or for organisms that could cause infection CUMULATIVE added together from the beginning CUTANEOUS relating to the skin CVA stroke (cerebrovascular accident)
DERMATOLOGIC pertaining to the skin DIASTOLIC lower number in a blood pressure reading DISTAL toward the end, away from the center of the body DIURETIC "water pill" or drug that causes increase in urination DOPPLER device using sound waves to diagnose or test DOUBLE BLIND study in which neither investigators nor subjects know what drug or treatment the subject is receiving DYSFUNCTION state of improper function DYSPLASIA abnormal cells
ECHOCARDIOGRAM sound wave test of the heart EDEMA excess fluid collecting in tissue EEG electric brain wave tracing (electroencephalogram) EFFICACY effectiveness ELECTROCARDIOGRAM electrical tracing of the heartbeat (ECG or EKG) ELECTROLYTE IMBALANCE an imbalance of minerals in the blood EMESIS vomiting EMPIRIC based on experience ENDOSCOPIC EXAMINATION viewing an internal part of the body with a lighted tube ENTERAL by way of the intestines EPIDURAL outside the spinal cord ERADICATE get rid of (such as disease) Page 2 of 7 EVALUATED, ASSESSED examined for a medical condition EXPEDITED REVIEW rapid review of a protocol by the IRB Chair without full committee approval, permitted with certain low-risk research studies EXTERNAL outside the body EXTRAVASATE to leak outside of a planned area, such as out of a blood vessel
FDA U.S. Food and Drug Administration, the branch of federal government that approves new drugs FIBROUS having many fibers, such as scar tissue FIBRILLATION irregular beat of the heart or other muscle
GENERAL ANESTHESIA pain prevention by giving drugs to cause loss of consciousness, as during surgery GESTATIONAL pertaining to pregnancy
HEMATOCRIT amount of red blood cells in the blood HEMATOMA a bruise, a black and blue mark HEMODYNAMIC MEASURING blood flow HEMOLYSIS breakdown in red blood cells HEPARIN LOCK needle placed in the arm with blood thinner to keep the blood from clotting HEPATOMA cancer or tumor of the liver HERITABLE DISEASE can be transmitted to one’s offspring, resulting in damage to future children HISTOPATHOLOGIC pertaining to the disease status of body tissues or cells HOLTER MONITOR a portable machine for recording heart beats HYPERCALCEMIA high blood calcium level HYPERKALEMIA high blood potassium level HYPERNATREMIA high blood sodium level HYPERTENSION high blood pressure HYPOCALCEMIA low blood calcium level HYPOKALEMIA low blood potassium level HYPONATREMIA low blood sodium level HYPOTENSION low blood pressure HYPOXEMIA a decrease of oxygen in the blood HYPOXIA a decrease of oxygen reaching body tissues HYSTERECTOMY surgical removal of the uterus, ovaries (female sex glands), or both uterus and ovaries
IATROGENIC caused by a physician or by treatment IDE investigational device exemption, the license to test an unapproved new medical device IDIOPATHIC of unknown cause IMMUNITY defense against, protection from IMMUNOGLOBIN a protein that makes antibodies IMMUNOSUPPRESSIVE drug which works against the body's immune (protective) response, often used in transplantation and diseases caused by immune system malfunction IMMUNOTHERAPY giving of drugs to help the body's immune (protective) system; usually used to destroy cancer cells IMPAIRED FUNCTION abnormal function IMPLANTED placed in the body IND investigational new drug, the license to test an unapproved new drug INDUCTION PHASE beginning phase or stage of a treatment INDURATION hardening INDWELLING remaining in a given location, such as a catheter INFARCT death of tissue due to lack of blood supply INFECTIOUS DISEASE transmitted from one person to the next INFLAMMATION swelling that is generally painful, red, and warm INFUSION slow injection of a substance into the body, usually into the blood by means of a catheter INGESTION eating; taking by mouth INTERFERON drug which acts against viruses; antiviral agent INTERMITTENT occurring (regularly or irregularly) between two time points; repeatedly stopping, then starting again INTERNAL within the body INTERIOR inside of the body INTRAMUSCULAR into the muscle; within the muscle INTRAPERITONEAL into the abdominal cavity INTRATHECAL into the spinal fluid INTRAVENOUS (IV) through the vein INTRAVESICAL in the bladder INTUBATE the placement of a tube into the airway INVASIVE PROCEDURE puncturing, opening, or cutting the skin INVESTIGATIONAL NEW DRUG (IND) a new drug that has not been approved by the FDA INVESTIGATIONAL METHOD a treatment method which has not been proven to be beneficial or has not been accepted as standard care ISCHEMIA decreased oxygen in a tissue (usually because of decreased blood flow)
LAPAROTOMY surgical procedure in which an incision is made in the abdominal wall to enable a doctor to look at the organs inside LESION wound or injury; a diseased patch of skin LETHARGY sleepiness, tiredness LEUKOPENIA low white blood cell count LIPID fat LIPID CONTENT fat content in the blood LIPID PROFILE (PANEL) fat and cholesterol levels in the blood LOCAL ANESTHESIA creation of insensitivity to pain in a small, local area of the body, usually by injection of numbing drugs LOCALIZED restricted to one area, limited to one area LUMEN the cavity of an organ or tube (e.g., blood vessel) LYMPHANGIOGRAPHY an x-ray of the lymph nodes or tissues after injecting dye into lymph vessels (e.g., in feet) LYMPHOCYTE a type of white blood cell important in immunity (protection) against infection LYMPHOMA a cancer of the lymph nodes (or tissues)
MALAISE a vague feeling of bodily discomfort, feeling badly MALFUNCTION condition in which something is not functioning properly MALIGNANCY cancer or other progressively enlarging and spreading tumor, usually fatal if not successfully treated MEDULLABLASTOMA a type of brain tumor MEGALOBLASTOSIS change in red blood cells METABOLIZE process of breaking down substances in the cells to obtain energy METASTASIS spread of cancer cells from one part of the body to another METRONIDAZOLE drug used to treat infections caused by parasites (invading organisms that take up living in the body) or other causes of anaerobic infection (not requiring oxygen to survive) MI myocardial infarction, heart attack MINIMAL slight MINIMIZE reduce as much as possible Page 4 of 7 MONITOR check on; keep track of; watch carefully MOBILITY ease of movement MORBIDITY undesired result or complication MORTALITY death MOTILITY the ability to move MRI magnetic resonance imaging, diagnostic pictures of the inside of the body, created using magnetic rather than x-ray energy MUCOSA, MUCOUS MEMBRANE moist lining of digestive, respiratory, reproductive, and urinary tracts MYALGIA muscle aches MYOCARDIAL pertaining to the heart muscle MYOCARDIAL INFARCTION heart attack
NASOGASTRIC TUBE placed in the nose, reaching to the stomach NCI the National Cancer Institute NECROSIS death of tissue NEOPLASIA/NEOPLASM tumor, may be benign or malignant NEUROBLASTOMA a cancer of nerve tissue NEUROLOGICAL pertaining to the nervous system NEUTROPENIA decrease in the main part of the white blood cells NIH the National Institutes of Health NONINVASIVE not breaking, cutting, or entering the skin NOSOCOMIAL acquired in the hospital
OCCLUSION closing; blockage; obstruction ONCOLOGY the study of tumors or cancer OPHTHALMIC pertaining to the eye OPTIMAL best, most favorable or desirable ORAL ADMINISTRATION by mouth ORTHOPEDIC pertaining to the bones OSTEOPETROSIS rare bone disorder characterized by dense bone OSTEOPOROSIS softening of the bones OVARIES female sex glands
PARENTERAL given by injection PATENCY condition of being open PATHOGENESIS development of a disease or unhealthy condition PERCUTANEOUS through the skin PERIPHERAL not central PER OS (PO) by mouth PHARMACOKINETICS the study of the way the body absorbs, distributes, and gets rid of a drug PHASE I first phase of study of a new drug in humans to determine action, safety, and proper dosing PHASE II second phase of study of a new drug in humans, intended to gather information about safety and effectiveness of the drug for certain uses PHASE III large-scale studies to confirm and expand information on safety and effectiveness of new drug for certain uses, and to study common side effects PHASE IV studies done after the drug is approved by the FDA, especially to compare it to standard care or to try it for new uses PHLEBITIS irritation or inflammation of the vein PLACEBO an inactive substance; a pill/liquid that contains no medicine PLACEBO EFFECT improvement seen with giving subjects a placebo, though it contains no active drug/treatment PLATELETS small particles in the blood that help with clotting POTENTIAL possible POTENTIATE increase or multiply the effect of a drug or toxin (poison) by giving another drug or toxin at the same time (sometimes an unintentional result) POTENTIATOR an agent that helps another agent work better PRENATAL before birth PROPHYLAXIS a drug given to prevent disease or infection PER OS (PO) by mouth PRN as needed PROGNOSIS outlook, probable outcomes PRONE lying on the stomach PROSPECTIVE STUDY following patients forward in time PROSTHESIS artificial part, most often limbs, such as arms or legs PROTOCOL plan of study PROXIMAL closer to the center of the body, away from the end PULMONARY pertaining to the lungs
QD every day; daily QID four times a day
RADIATION THERAPY x-ray or cobalt treatment RANDOM by chance (like the flip of a coin) RANDOMIZATION chance selection RBC red blood cell RECOMBINANT formation of new combinations of genes RECONSTITUTION putting back together the original parts or elements RECUR happen again REFRACTORY not responding to treatment REGENERATION re-growth of a structure or of lost tissue REGIMEN pattern of giving treatment RELAPSE the return of a disease REMISSION disappearance of evidence of cancer or other disease RENAL pertaining to the kidneys REPLICABLE possible to duplicate RESECT remove or cut out surgically RETROSPECTIVE STUDY looking back over past experience
SARCOMA a type of cancer SEDATIVE a drug to calm or make less anxious SEMINOMA a type of testicular cancer (found in the male sex glands) SEQUENTIALLY in a row, in order SOMNOLENCE sleepiness SPIROMETER an instrument to measure the amount of air taken into and exhaled from the lungs STAGING an evaluation of the extent of the disease STANDARD OF CARE a treatment plan that the majority of the medical community would accept as appropriate STENOSIS narrowing of a duct, tube, or one of the blood vessels in the heart STOMATITIS mouth sores, inflammation of the mouth STRATIFY arrange in groups for analysis of results (e.g., stratify by age, sex, etc.) STUPOR stunned state in which it is difficult to get a response or the attention of the subject SUBCLAVIAN under the collarbone SUBCUTANEOUS under the skin SUPINE lying on the back SUPPORTIVE CARE general medical care aimed at symptoms, not intended to improve or cure underlying disease SYMPTOMATIC having symptoms SYNDROME a condition characterized by a set of symptoms SYSTOLIC top number in blood pressure; pressure during active contraction of the heart
TERATOGENIC capable of causing malformations in a fetus (developing baby still inside the mother’s body) TESTES/TESTICLES male sex glands THROMBOSIS clotting THROMBUS blood clot TID three times a day TITRATION a method for deciding on the strength of a drug or solution; gradually increasing the dose T-LYMPHOCYTES type of white blood cells TOPICAL on the surface TOPICAL ANESTHETIC applied to a certain area of the skin and reducing pain only in the area to which applied TOXICITY side effects or undesirable effects of a drug or treatment TRANSDERMAL through the skin TRANSIENTLY temporarily TRAUMA injury; wound TREADMILL walking machine used to test heart function
UPTAKE absorbing and taking in of a substance by living tissue
VALVULOPLASTY plastic repair of a valve, especially a heart valve VARICES enlarged veins VASOSPASM narrowing of the blood vessels VECTOR a carrier that can transmit disease-causing microorganisms (germs and viruses) VENIPUNCTURE needle stick, blood draw, entering the skin with a needle VERTICAL TRANSMISSION spread of disease
WBC white blood cell

COMMENTS
ResearchMatch helps you find a clinical trial or research study near you, or across the country, by matching you with researchers from leading medical research institutions. Whether you are a healthy volunteer or have a health condition, ResearchMatch connects you to research opportunities so you can make a difference and advance scientific discoveries by participating in research studies ...
NIH conducts clinical research trials for many diseases and conditions, including cancer, Alzheimer's disease, allergy and infectious diseases, and neurological disorders. To search for other diseases and conditions, you can visit ClinicalTrials.gov. This is a searchable registry and results database of federally and privately supported ...
Name Source Duration Purpose; SC_ANALYTICS_GLOBAL_COOKIE: www.clinicalresearch.com: 6 Months: Sitecore - visitor identification: _ga.clinicalresearch.com
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Clinical Research. Clinical research is the key step in translating biomedical discoveries into new approaches to the diagnosis, treatment, and prevention of human illnesses. In the last few decades, landmark developments in genetics, bioengineering, neuroscience, and molecular and structural biology have vastly increased our understanding of ...
The Northwest Participant and Clinical Interactions (NW PCI) Network is a collaboration of regional clinical and translational research centers affiliated with medical centers, healthcare systems, and universities in the Washington, Wyoming, Alaska, Montana, and Idaho (WWAMI) region. NW PCI sites collaborate with investigators to conduct ...
VICTR Clinical Research Center. Kevin Niswender, MD, PhD Medical Director. James M. Luther, MD, MSCI Associate Medical Director. Jennifer Adams, VICTR CRC Program Manager. Lana Howard, BSN, RN, CCRP Patient Care Services Manager (615) 322-2312. Human Research Protections Program. Research Subject Advocate (866) 224-8273 or (615) 322-2918 Option ...
Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in ...
Use an online database. There are a number of public online databases of clinical trials. These databases allow you to search by a medical condition, treatment, other keywords, or a specific location to find trials best suited to your situation. However, it can be tedious and time-consuming to search through tens of thousands of trials on these ...
Learn More. CenterWatch provides a variety of clinical research products and services: including clinical trials and results, drug approvals, study grants, news and analysis, career and training opportunities for patients and professionals.
USA Health Statistics & Healthcare System Overview. According to the National Center of Health Statistics, life expectancy in the USA has recently increased from 77.8 (2006) to 78.6 (2016) years. Women still live longer (80.3 to 81.1 years) than men do (75.2 to 76.1 years) [2]; nonetheless, the dominant causes of deaths remain the same for both ...
To participate in a clinical trial or to learn more about research at Rochester General, contact us at (585) 922-3536. Please note that these trials are not for healthy volunteers. The studies we offer are for patients experiencing symptoms of the disease state addressed in the trial. Thank you for your interest in medical clinical research!
Help us end illness and change lives. When you participate in a clinical study, you help us collect valuable information that aids in the development of new treatments and medications. Even your favorite pain relievers, morning vitamins, and common antibiotics were made possible because of clinical research! Explore our open studies and learn ...
Patients. IMAgine greater access to new treatments. We take a compassionate approach to clinical research services. Through patient-centric technology, we support a flexible trial model that allows us to set up additional patient outreach centers wherever and whenever they're needed. MORE INFORMATION.
ResearchMatch helps you find a clinical trial or research study near you, or across the country, by matching you with researchers from leading medical research institutions. Whether you are a healthy volunteer or have a health condition, ResearchMatch connects you to research opportunities so you can make a difference and advance scientific discoveries by participating in research studies ...
The National Institutes of Health Clinical Center in Bethesda, Md., is the nation's largest hospital totally dedicated to clinical research.. The collaborations at the NIH Clinical Center represent an extraordinary opportunity to broaden research opportunities, stimulate research and ultimately improve public health.. In June 1990, The Children's Inn at NIH opened its doors to pediatric ...
InterLab Corp. We are a central research laboratory in the area that offers a full scale test menu for anatomic pathology, clinical and specialty tests. Interlab was created with the mission to improve healthcare and advance the science of laboratory medicine.
From a US national research authority. Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances. This page last reviewed on September 29, 2016.
Who We Are. The Center for Clinical Research and Innovation (CCRI) at Spring Heights Hospital is focused on advancing therapies for patients through our affiliated providers and care sites. As part of a learning health system, we are unlocking insights into real-world data and clinical studies that lead to scientific breakthrough.
Resources and Initiatives. COVID-19: Public health information from CDC . | Research information from NIH ( Español ). | NIH staff guidance on coronavirus ( NIH Only ). Clinical and Safety Performance Metrics (3rd Quarter 2023) The Clinical Center is pleased to post some key metrics that illustrate our strong commitment to patient and employee ...
Learn more about 310 Clinical Research, our stellar enrollment rates, quality data and wide range of medical specializations. Sponsors, CROs and participants alike can count on us for our highly professional manner and exceptional results. Call : 310-878-2636.
Our Research Center. We are an independent, research facility with a private practice specializing in a wide spectrum of medical conditions. Our Pompano Beach, Florida location houses cutting-edge research with some of the most experienced investigators and research staff in the nation. We are a preferred clinical research site with numerous ...
Posted: 29-Jun-24 Location: Grand Junction , Colorado Type: Full Time Salary: $120,000-$160,000/yr Categories:
Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in ...
CHRISTUS Health provides equal employment opportunities to all employees and applicants. All hiring and selections for job opportunities will be based on CHRISTUS Health's business needs and the qualifications, skills, relative abilities and performance of the candidates being considered, without regard to an individual's race, color, religion, sex, sexual orientation, pregnancy, national ...
Human Subjects Office / IRB Hardin Library, Suite 105A 600 Newton Rd Iowa City, IA 52242-1098. Voice: 319-335-6564 Fax: 319-335-7310