Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 January 2023

A student guide to writing a case report
- Maeve McAllister 1
BDJ Student volume 30 , pages 12–13 ( 2023 ) Cite this article
26 Accesses
Metrics details
As a student, it can be hard to know where to start when reading or writing a clinical case report either for university or out of special interest in a Journal. I have collated five top tips for writing an insightful and relevant case report.
A case report is a structured report of the clinical process of a patient's diagnostic pathway, including symptoms, signs, diagnosis, treatment planning (short and long term), clinical outcomes and follow-up. 1 Some of these case reports can sometimes have simple titles, to the more unusual, for example, 'Oral Tuberculosis', 'The escapee wisdom tooth', 'A difficult diagnosis'. They normally begin with the word 'Sir' and follow an introduction from this.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Guidelines To Writing a Clinical Case Report. Heart Views 2017; 18 , 104-105.
British Dental Journal. Case reports. Available online at: www.nature.com/bdj/articles?searchType=journalSearch&sort=PubDate&type=case-report&page=2 (accessed August 17, 2022).
Chate R, Chate C. Achenbach's syndrome. Br Dent J 2021; 231: 147.
Abdulgani A, Muhamad, A-H and Watted N. Dental case report for publication; step by step. J Dent Med Sci 2014; 3 : 94-100.
Download references
Author information
Authors and affiliations.
Queen´s University Belfast, Belfast, United Kingdom
Maeve McAllister
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Maeve McAllister .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
McAllister, M. A student guide to writing a case report. BDJ Student 30 , 12–13 (2023). https://doi.org/10.1038/s41406-023-0925-y
Download citation
Published : 30 January 2023
Issue Date : 30 January 2023
DOI : https://doi.org/10.1038/s41406-023-0925-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to write a medical...
How to write a medical case report
- Related content
- Peer review
- Seema Biswas , editor-in-chief, BMJ Case Reports, London, UK ,
- Oliver Jones , student editor, BMJ Case Reports, London, UK
Two BMJ Case Reports journal editors take you through the process
This article contains...
- Choosing the right patient
- Choosing the right message
- Before you begin - patient consent
- How to write your case report
- How to get published
During medical school, students often come across patients with a unique presentation, an unfamiliar response to treatment, or even an obscure disease. Writing a case report is an excellent way of documenting these findings for the wider medical community—sharing new knowledge that will lead to better and safer patient care.
For many medical students and junior doctors, a case report may be their first attempt at medical writing. A published case report will look impressive on your curriculum vitae, particularly if it is on a topic of your chosen specialty. Publication will be an advantage when applying for foundation year posts and specialty training, and many job applications have points allocated exclusively for publications in peer reviewed journals, including case reports.
The writing of a case report rests on skills that medical students acquire in their medical training, which they use throughout their postgraduate careers: these include history taking, interpretation of clinical signs and symptoms, interpretation of laboratory and imaging results, researching disease aetiology, reviewing medical evidence, and writing in a manner that clearly and effectively communicates with the reader.
If you are considering writing a case report, try to find a senior doctor who can be a supervising coauthor and help you decide whether you have a message worth writing about, that you have chosen the correct journal to submit to (considering the format that the journal requires), that the process is transparent and ethical at all times, and that your patient is not compromised in your writing. Indeed, try to include your patient in the process from the outset, and always gain consent.
A case report is the first line of medical evidence, and over time has become an important medium for sharing new findings (box 1). High quality case reports successfully bring together the various domains of medicine such as physiology, pathology, and anatomy. Using the patient as the focus, case reports provide a clinical “coat peg” on which to hang this knowledge.
Box 1: Notable case reports through the ages
Many case reports have changed the way clinicians view health and disease. For example, in 1861 the French surgeon Pierre Paul Broca reported the case of a dysphasic patient nicknamed “Tan”—owing to his inability to say any other words. After Tan’s death, Broca did an autopsy and discovered a syphilitic lesion in the frontal lobe of the brain, leading to the hypothesis of a speech centre in the brain—later known as Broca’s area. 1 Other notable case reports have documented the discovery of the Bence-Jones protein, 2 the first descriptions of Parkinson’s disease, 3 and AIDS. 4
Choosing the right patient
We can learn from all patients, but choose a patient from whom there is something new to learn. Search the literature and decide whether the topic you want to discuss, whether clinical or non-clinical (a radiological or microbiological finding, for example), has already been well discussed.
Your patient should ideally be someone who is not simply a willing participant in this process but someone who wants their story to be told to educate students, doctors, and other patients. Many journals have an option for patients to contribute to the manuscript.
Choosing the right message
Rare diseases are not in themselves a reason to write up a case, but unusual presentations of a common disease are important to communicate to the medical community. Early or subtle signs and symptoms that are easily missed are important for us to learn from. Indeed, the learning value of your case is the single most important factor in determining whether it is likely to be published.
Have in mind the journal that you want to submit your manuscript to before you begin to write. Your case and the message should fit with the style of the journal, whether a specialist journal, a case reports journal, or a journal that publishes case presentations in different formats. This may include question and answer formats, quizzes, or even interactive online educational formats useful for exam revision—for example, Endgames ( The BMJ ), Epilogue ( Archives of Disease in Childhood ), or Images ( New England Journal of Medicine ). These adapted formats are important, as most of these journals no longer accept case reports written in their traditional format.
Also, be careful in your claims about new diseases and new treatments. Case reports cannot make claims about the efficacy of novel treatments on the basis of individual cases and limited follow-up time. The most important message is a new or novel learning point—that is, the educational message.
Before you begin
Once you have chosen your patient and discussed with them what you would like to write, show them the case report so that they may give informed consent to your manuscript submission and familiarise themselves with the website.
It is important that a patient understands how their case will appear online or in print and that they truly give informed consent. You should do this under the supervision of the senior doctor who is the supervising coauthor of your manuscript; ideally, the senior doctor would obtain consent.
Writing the case report
Case presentation.
Begin with the case presentation (box 2): describe your encounter with the patient, their symptoms, and their signs. You should already have an idea what your take home messages will be. If the journal presentation of the case report allows, you can write these take home messages as bullet points (box 3).
Box 2: Case presentation
Acute pancreatitis and severe hypertriglyceridaemia masking unsuspected underlying diabetic ketoacidosis.
After 48 hours of anorexia, nausea, and non-bloody vomiting at home, the patient presented to her local hospital, where the diagnosis of moderate acute pancreatitis was made, based on an abdominal computed tomogram and ultrasound and serum chemistry. Ongoing symptoms, including left upper quadrant, 7/10 stabbing pain with generalised abdominal cramps, led to her transfer to the closest tertiary hospital for further management.
On admission to the tertiary hospital, the patient was treated as having uncomplicated pancreatitis. Immediate management included intravenous rehydration therapy, antiemetics, and narcotics for pain control with further orders for nothing to be ingested until the patient was re-evaluated. Initial assessment of the patient showed a temperature of 37.3ºC, heart rate 110 beats/min, blood pressure 126/68 mm Hg, respiratory rate 14 breaths/min, and oxygen saturation 98% on room air. She had a normal body habitus and was not in distress; however, she had a moderate amount of abdominal discomfort. Her physical examination showed no xanthalasmas or skin eruptions, nor was a fruity odour detected. Her gastrointestinal examination showed diffuse tenderness, with a soft, non-distended abdomen. Also, no organomegally was noted. Other than tachycardia, her cardiorespiratory examination was unremarkable with the notable absence of tachypnoea.
The patient was previously healthy without any medical history or surgical history. Her medication list was limited to the oral contraceptive pill (ethinyl oestradiol, norgestimate). The patient described only occasional social alcohol consumption (none within the last week) and no binge drinking or recreational drug use in the past. There were no recent surgeries, gastrointestinal endoscopic procedures, or abdominal trauma. She denied fever, chills, rigors, or recent unintended weight loss. There was no history of polyuria or polydipsia.
She did not have any prodromal abdominal symptoms There had been no similar episodes previously. There was no family history of dyslipidaemias, pancreatitis, or gallstones. Her family history was relevant for rectal carcinoma in her paternal grandfather and type 2 diabetes in her maternal grandmother. Six hours after her arrival at the tertiary hospital, and 12 hours from her first presentation and assessment at the local rural hospital, the patient began to decompensate with rapid progression of hypotension, tachycardia, and tachypnoea. The acute decompensation to hypotension and shock was assumed to be due to progression of the pancreatitis with potential infection complicating the pancreatitis. The patient was aggressively rehydrated and started on broad spectrum antibiotics. However, the hypotension failed to respond to fluid resuscitation and there was increased patient distress. She was urgently referred to the intensive care unit for supportive measures and management.
Aboulhosn K, Arnason T. Acute pancreatitis and severe hypertriglyceridaemia masking unsuspected underlying diabetic ketoacidosis. BMJ Case Rep 2013;2013, doi: 10.1136/bcr-2013-200431 .
Box 3: Learning points
Postpartum hellp syndrome and subcapsular liver haematoma.
Subcapsular liver haematoma is a potentially life threatening complication of severe pre-eclampsia and haemolysis, the breakdown of red blood cells; elevated liver enzymes; low platelet count syndrome.
The complication is rare but should be considered with severe upper abdominal pain in obstetric patients, especially in the presence of pre-eclampsia.
Real time ultrasound imaging of the liver is often diagnostic.
Messerschmidt L, Andersen LL, Sorensen MB. Postpartum HELLP syndrome and subcapsular liver haematoma. BMJ Case Rep 2014, doi: 10.1136/bcr-2013-202503 .
You should separate your case presentation section from the investigations and differential diagnoses. The key points to remember to include are your choice of investigations and how they helped you establish a working diagnosis (box 4).
Box 4: Investigations
Unilateral presentation of postpartum cardiomyopathy misdiagnosed as pneumonia.
On arriving at the emergency department, the patient had severe shortness of breath at rest 10 days after delivery. Her vital signs included an oral temperature of 36.7ºC, blood pressure 163/102 mmHg, pulse rate 146 beats/min, and oxygen saturation 88% in room air. Treatment with supplemental oxygen by mask yielded an increase in oxygen saturation to 95%. Her physical examination revealed no jugular venous distension, hepatic enlargement, or pedal oedema; heart sounds were fast and regular, with no evidence of murmurs or additional sounds. On lung auscultation bilateral crackles were present. Her laboratory analysis showed mild non-specific indicators of stress with a leucocyte count of 9.3×10 3 cells/mm 3 , haemoglobin value of 10.6 g/dL, and a platelet count of 791×10 3 cells/mm 3 . Her electrocardiogram was similar to the one obtained a day earlier showing T wave inversion in leads V4–V6; however, chest radiography showed a more bilateral presentation compared with the previous one showing both heart enlargement and pulmonary oedema. A chest computed tomography angiography performed to exclude pulmonary artery embolisation confirmed the presence of cardiomegaly and pulmonary oedema with bilateral effusions (fig 1). ⇓ An echocardiogram showed a diminished ejection fraction of 15-20% confirming the diagnosis of postpartum cardiomyopathy.
Amit BH, Marmor A, Hussein A. Unilateral presentation of postpartum cardiomyopathy misdiagnosed as pneumonia. BMJ Case Rep 2010, doi: 10.1136/bcr.05.2010.3039 .

Fig 1 Chest computed tomogram performed after deterioration showing heart enlargement, pulmonary oedema, and bilateral pleural effusions mainly on the right. From Amit BH et al. BMJ Case Rep 2010, doi: 10.1136/bcr.05.2010.3039 .
- Download figure
- Open in new tab
- Download powerpoint
Imagine that you are presenting at a grand round and have to explain your choices to your colleagues—this is essentially what you are doing as you write your case report. Do not simply list your differential diagnoses; describe how you worked through your list of differentials and how you established a final diagnosis.
Also, make sure you collect and include high quality and well annotated images that not only explain radiological findings but also show their importance in establishing your diagnosis.
Good quality annotated images

Fig 2 Craniocervical x ray film showing fusion of the posterior arch of C1 to the occiput. A fracture was not evident, but clinical suspicion prompted a computed tomography scan

Fig 3 Axial, left, and sagittal, right, computed tomography scans of the craniocervical junction at presentation showing fusion of the left occipital condyle with the lateral mass of C1 and a fracture involving both elements. The fracture is indicated by the arrowheads
Outcome and follow-up
The outcome and your follow-up of the patient are important. In both your case presentation and the section on patient outcome, you should describe what happened to your patient in terms of their specific symptoms, their general wellbeing, and their lifestyle and activity.
Some journals require you to write a summary of your case report. This usually has a word limit and appears in medical search engines, such as Pubmed/MEDLINE. It is the equivalent of the abstract of a research paper.
Ensure that your title is scientific and clinical. Cryptic and humorous titles translate poorly across a global audience and do not always accurately reflect the content of your case report. You may find that the word limit does not permit you to write all the detail you would want to include in the summary, but the background section allows you to do this. Try to make sure that the background section does not repeat the summary.
Publication process
Clinical videos and images are important alternatives or potential additions to clinical case reports which many journals encourage authors to submit. Again, prepare these in collaboration with clinical teachers or coauthors, who will help you annotate these images and point out important learning messages, and do this from the outset in the format of the journal that you have researched well and decided to submit your manuscript to.
All submitted case reports are usually sent for peer review. Reviewers are chosen according to their specialty and clinical or academic interests. Your choice of key words is therefore important as these are the basis for the assignment of reviewers. Keywords are also important for other authors doing literature searches who discover your case report and cite this in their own writing.
Decisions to accept, revise, or reject are based on editors’ and reviewers’ opinions together, and every attempt is made to ensure that criticism is constructive and useful.
Dependent on how quickly your manuscript is reviewed, you should receive a decision on your manuscript within three to six weeks of submission. Outright rejections for reasons such as the unsuitability of your manuscript for the particular journal and its audience, manuscripts in the wrong format, incomplete sections (especially the case presentation and differential diagnosis sections), and plagiarism tend to be prompt, and they would be easily avoided by following the steps above and choosing your patient, your topic, your journal, and your particular manuscript format well.
Rejections on the basis of the content of the case report tend to be at the peer review stage and may be a few weeks after submission. They could include reasons such as the lack of novelty or educational message, a poor literature search, or inconsistent clinical management. Again, this is avoidable by preparing well. It is unusual for a well thought out and well prepared manuscript to be rejected.
Autoformatting software, especially with references, may produce errors, so do double check these. Syntax errors, spelling mistakes, and poor grammar create a poor impression of an otherwise good case report. As always, first impressions matter, so be meticulous as you proofread your manuscript before you submit.
The entire process of publication depends on the number of revisions necessary and how quickly you submit a revised manuscript. For those of you aiming to submit in time to prepare for job applications, do take into account the time taken in the process of publication.
Further reading
1. BMJ Case Reports has produced a ‘‘How to’’ guide for completing case report submission: http://casereports.bmj.com/site/about/How_to_complete_full_cases_template.pdf .
2. BMJ Case Reports has produced a clinical case reports template which illustrates the important points in a manuscript and should help you in your writing: http://casereports.bmj.com/site/about/guidelines.xhtml .
3. Some journals recommend patient perspectives in the write up of a case report. An example is at http://casereports.bmj.com/content/2015/bcr-2014-208529.full?sid=bb53a333-2c59-453a-a9bf-5775edc0e5d7 .
Originally published as: Student BMJ 2016;24:h3731
Competing interests: SB and OJ are editors of BMJ Case Reports.
Provenance and peer review: Commissioned; not externally peer reviewed.
- ↵ Broca P. Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech). Bulletin de la Société Anatomique . 1861 ; 6 : 330 -57. OpenUrl
- ↵ Jones HB. On a new substance occurring in the urine of a patient with mollities ossium. Philosophical Transactions of the Royal Society of London . 1848 ; 138 : 55 -62. OpenUrl CrossRef
- ↵ Parkinson J. An essay on the shaking palsy, 1817. J Neuropsych Clin Neurosci 2002 ; 14 : 223 -6. OpenUrl CrossRef PubMed Web of Science
- ↵ Gottlieb GJ, Ragaz A, Vogel JV, et al. A preliminary communication on extensively disseminated kaposige sarcoma in a young homosexual man. Am J Dermatopath 1981 ; 3 : 111 . OpenUrl CrossRef PubMed Web of Science
Search the world's largest collection of clinical case reports
Browse case reports by:
Publish in BMJ Case Reports
Global health case reports.
These are case reports that focus on the causes of ill health, the social determinants of health and access to healthcare services, prevailing local and national issues that affect health and wellbeing, and the challenges in providing care to vulnerable populations or with limited resources.
Read the full collection now
Images in… :
31 July 2023
Unusual association of diseases/symptoms :
24 January 2024
14 July 2023
Obstetrics and gynaecology :
5 March 2024
Case report :
18 October 2023
Case Reports: Rare disease :
Case Reports by specialty
- Anaesthesia
- Dentistry and oral medicine
- Dermatology
- Emergency medicine
- Endocrinology
- General practice and family medicine
- Geriatric medicine
- Haematology
- Infectious diseases
- Obstetrics and gynaecology
- Ophthalmology
- Orthopaedics
- Paediatrics
- Respiratory medicine
- Rheumatology

Global Health Competition
Every year BMJ Case Reports selects authors of global health case reports to join our editorial team as a global health associate editor.
This is an opportunity to gain some editorial experience or join our team on research and educational projects. Students and graduates may apply.
Simply select Global Health Competition when you submit.
Latest Articles
Paediatrics :
4 June 2024
Endocrinology :
Ophthalmology :
Case Report: A Beginner’s Guide with Examples
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
A similar design involving a group of patients (with the similar problem) is referred to as case series.
Advantages of case reports
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
These events deserve to be reported since they might provide insights on some exceptions to general rules and theories in the field.
Case reports are great to get first impressions that can generate new hypotheses (e.g. detecting a potential side effect of a drug) or challenge existing ones (e.g. shedding the light on the possibility of a different biological mechanism of a disease).
In many of these cases, additional investigation is needed such as designing large observational studies or randomized experiments or even going back and mining data from previous research looking for evidence for theses hypotheses.
Limitations of case reports
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature.
This is because of:
- The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease
- Unmeasured Confounding caused by variables that influence both the exposure and the disease
A case report can have a powerful emotional effect (see examples of case reports below). This can lead to overrate the importance of the evidence provided by such case. In his book Against Empathy: The Case for Rational Compassion , Paul Bloom explains how a powerful story affects our emotions, can distort our judgement and even lead us to make bad moral choices.
When a case report describes a rare event it is important to remember that what we’re reading about is exceptional and most importantly resist generalizations especially because a case report is, by definition, a study where the sample is only 1 patient.
Selection bias is another issue as the cases in case reports are not chosen at random, therefore some members of the population may have a higher probability of being included in the study than others.
So, results from a case report cannot be representative of the entire population.
Because of these limitations, case reports have the lowest level of evidence compared to other study designs as represented in the evidence pyramid below:
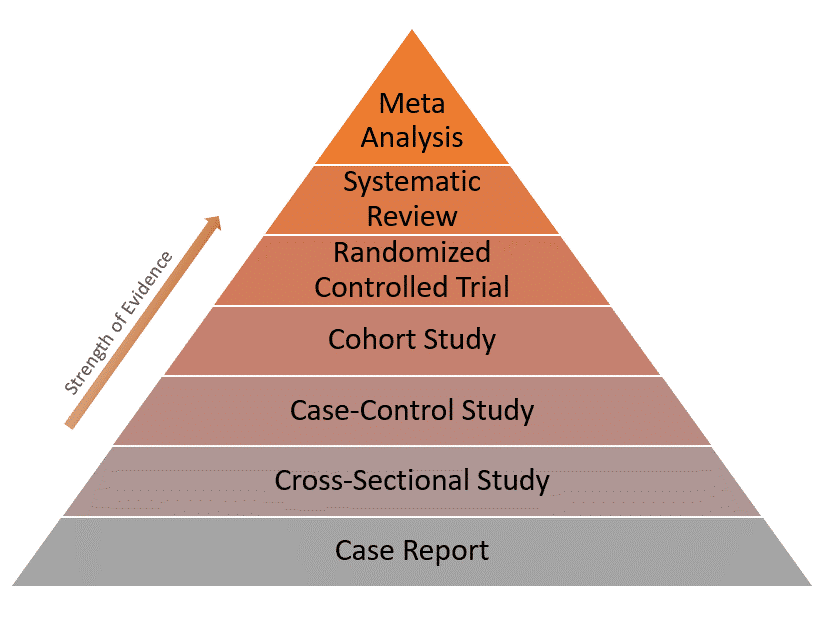
Real-world examples of case reports
Example 1: normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day.
This is the case of an old man with Alzheimer’s disease who has been eating 20-30 eggs every day for almost 15 years. [ Source ]
The man had an LDL-cholesterol level of only 142 mg/dL (3.68 mmol/L) and no significant clinical atherosclerosis (deposition of cholesterol in arterial walls)!
His body adapted by reducing the intestinal absorption of cholesterol, lowering the rate of its synthesis and increasing the rate of its conversion into bile acid.
This is indeed an unusual case of biological adaptation to a major change in dietary intake.
Example 2: Recovery from the passage of an iron bar through the head
This is an interesting case of a construction foreman named Phineas Gage. [ Source ]
In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe. He survived the event and the injury only affected 1 thing: His personality!
After the accident, Gage became profane, rough and disrespectful to the extent that he was no longer tolerable to people around him. So he lost his job and his family.
His case inspired further research that focused on the relationship between specific parts of the brain and personality.
- Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education . Cureus . 2017;9(8):e1546. Published 2017 Aug 7. doi:10.7759/cureus.1546.
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations . BMC Res Notes . 2014;7:264. Published 2014 Apr 23. doi:10.1186/1756-0500-7-264.
Further reading
- Case Report vs Cross-Sectional Study
- Cohort vs Cross-Sectional Study
- How to Identify Different Types of Cohort Studies?
- Matched Pairs Design
- Randomized Block Design
- Search Menu
- Sign in through your institution
- Volume 2024, Issue 5, May 2024 (In Progress)
- Volume 2024, Issue 4, April 2024
- Case of the Year
- MSF Case Reports
- Audiovestibular medicine
- Cardiology and cardiovascular systems
- Critical care medicine
- Dermatology
- Emergency medicine
- Endocrinology and metabolism
- Gastroenterology and hepatology
- Geriatrics and gerontology
- Haematology
- Infectious diseases and tropical medicine
- Medical ophthalmology
- Medical disorders in pregnancy
- Paediatrics
- Palliative medicine
- Pharmacology and pharmacy
- Radiology, nuclear medicine, and medical imaging
- Respiratory disorders
- Rheumatology
- Sexual and reproductive health
- Sports medicine
- Substance abuse
- Author Guidelines
- Submission Site
- Open Access
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Editor-in-Chief
Richard Watts
Executive Editors
Tamim Alsuliman
Aloysious Aravinthan
Amanda Goodwin
Eleana Ntatsaki
Vassilis Vassiliou
About Oxford Medical Case Reports
Oxford Medical Case Reports (OMCR) is an open access, peer-reviewed online journal publishing original and educationally valuable case reports that expand the field of medicine. The journal deposits all articles in PubMed Central (PMC) and is indexed in the Web of Science Core Collection.

Browse by specialty
Oxford Medical Case Reports publish insightful cases across all medical specialties.
Browse collections including nephrology , palliative care , and geriatric medicine .
Explore more

- Submit your case
Oxford Medical Case Reports publishes original and educationally valuable case reports across all medical specialties.
- Author guidelines

Case Reports From Humanitarian And Resource Limited Settings
Médecins Sans Frontières (MSF) is working with OMCR to encourage clinicians in low-income and/or emergency contexts to submit interesting case reports and series from the field.
Browse the case reports from humanitarian and low resource settings

Enhanced discoverability
Oxford Medical Case Reports deposits all cases in PubMed Central . Physicians and researchers can find your work through PubMed , helping you reach the widest possible audience.
The journal is also indexed in the Web of Science Core Collection .
Latest articles

Email alerts
Register to receive table of contents email alerts as soon as new issues of Oxford Medical Case Reports are published online.
Join us on Facebook and Twitter
Be the first to read the latest news and cases by joining the Oxford Medical Case Reports community on Facebook , or by following us on Twitter .
Publish with OMCR
Editor-in-Chief Dr Richard Watts explains the benefits of publishing with Oxford Medical Case Reports.

Test your knowledge
A 57 year-old man has chest pain, but what is the diagnosis? Answer multiple choice questions to find out.
Take the test
Related Titles
Affiliations
- Online ISSN 2053-8855
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Journal of Medical Case Reports
In the era of evidence-based practice, we need practice-based evidence. The basis of this evidence is the detailed information from the case reports of individual people which informs both our clinical research and our daily clinical care. Each case report published in this journal adds valuable new information to our medical knowledge. Prof Michael Kidd AO, Editor-in- Chief

Join the Editorial Board
We are recruiting Associate Editors to join our Editorial Board. Learn more about the role and how to apply here !

- Meet the Editors
Get to know the Editors behind Journal of Medical Case Reports !

megaflopp / Getty Images / iStock
Requirements for case reports submitted to JMCR
• Patient ethnicity must be included in the Abstract under the Case Presentation section.
• Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies .
Report of the Month
Superior mesenteric vein thrombosis due to covid-19 vaccination.
Vaccines have made a significant contribute to sowing the spread of the COVID-19 infection. However, side effects of the vaccination are beginning to appear, and one of which, thrombosis, is a particular problem as it can cuase serious complications. While cases of splanchnic venous thrombosis (SVT) after ChAdOx1 nCoV-19 vaccinations have been reported, cases of SVT mRNA-1273 vaccines are rare.
In this case report, clinicians describe a patient presenting with superior mesentric vein thrombosis following a COVID-19 vaccination, and examine the relationship between the mRNA-1273 vaccines and intestinal ischemia.
- Most accessed
Detection of novel PPP1R1B::STARD3 fusion transcript in acute myeloid leukemia: a case report
Authors: Elahe Dehghani Firouzabadi, Mohammed Allami, Eman Jassim Mohammed, Hossein Barzegar, Mahtab Dastpak, Reza Alemohammad, Vahid Moghimi, Reihaneh Alsadat Mahmoudian, Fatemeh Nasrabadi, Nahid Arghiani, Yohei Kitamura, Seyed Abolfazl Hosseini, Ali Ghasemi and Moein Farshchian
Sinonasal immunoglobulin G4-related disease: a case report of an atypical and rare entity
Authors: Faiq I. Gorial, Nabaa Ihsan Awadh, Shahlaa B. Ali, Sazan Abdulwahab Mirza and Murtadha Hussein Abbas
Case analysis of hepatotoxicity caused by vancomycin
Authors: Jiayao Wu and Yulu Zhou
Endovascular stenting using a sagittal sinus approach for sigmoid sinus wall dehiscence related to intractable pulsatile tinnitus: a case series
Authors: Luis Alberto Ortega-Porcayo, Guillermo Gonzalez-Garibay, Ángel Lee, Juan A. Ponce-Gómez, Victor Alcocer-Barradas, Samuel Romano-Feinholz and Marco Antonio Zenteno Castellanos
Surgical intervention of Lemierre’s syndrome: a case report and review of the literature
Authors: Yiqi Pan, Zhihong Shi, Bin Ye, Qian Da, Chaofu Wang, Yilin Shen and Mingliang Xiang
Most recent articles RSS
View all articles
An itchy erythematous papular skin rash as a possible early sign of COVID-19: a case report
Authors: Alice Serafini, Peter Konstantin Kurotschka, Mariabeatrice Bertolani and Silvia Riccomi
Red ear syndrome precipitated by a dietary trigger: a case report
Authors: Chung Chi Chan and Susmita Ghosh
How to choose the best journal for your case report
Authors: Richard A. Rison, Jennifer Kelly Shepphird and Michael R. Kidd
The Erratum to this article has been published in Journal of Medical Case Reports 2017 11 :287
COVID-19 with repeated positive test results for SARS-CoV-2 by PCR and then negative test results twice during intensive care: a case report
Authors: Masafumi Kanamoto, Masaru Tobe, Tomonori Takazawa and Shigeru Saito
Recurrent knee arthritis diagnosed as juvenile idiopathic arthritis with a 10-year asymptomatic period after arthroscopic synovectomy: a case report
Authors: Atsushi Teramoto, Kota Watanabe, Yuichiro Kii, Miki Kudo, Hidenori Otsubo, Takuro Wada and Toshihiko Yamashita
Most accessed articles RSS
A Guide to Writing and Using Case Reports
This thematic series, published in 2016, provides a valuable resource for clinicians who are considered producing a case report. It comprises of a special editorial series of guides on writing, reviewing and using case reports.

Aims and scope
Journal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports.
Case reports should show one of the following:
- Unreported or unusual side effects or adverse interactions involving medications
- Unexpected or unusual presentations of a disease
- New associations or variations in disease processes
- Presentations, diagnoses and/or management of new and emerging diseases
- An unexpected association between diseases or symptoms
- An unexpected event in the course of observing or treating a patient
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect
Suitable research articles include but are not limited to: N of 1 trials, meta-analyses of published case reports, research addressing the use of case reports and the prevalence or importance of case reporting in the medical literature and retrospective studies that include case-specific information (age, sex and ethnicity) for all patients.
Article accesses
Throughout 2022, articles were accessed from the journal website more than 4.17 million times; an average of over 11 ,400 accesses per day.
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
Peer Review Mentoring Scheme
The Editors at Journal of Medical Case Reports endorse peer review mentoring of early career researchers.
If you are a senior researcher or professor and supervise an early career researcher with the appropriate expertise, we invite you to co-write and mentor them through the peer review process. Find out how to express your interest in the scheme here .
Call for Papers
The Journal of Medical Case Reports is calling for submissions to our Collection on COVID-19 – a look at the past, present and future of the pandemic . Guest Edited by Dr. Jean Karl Soler, The Family Practice Malta, Malta

About the Editor-in-Chief
Professor Michael Kidd AO FAHMS is foundation Director of the Centre for Future Health Systems at the University of New South Wales in Sydney, Australia, and Professor of Global Primary Care and Future Health Systems with the Nuffield Department of Primary Care Health Sciences at the University of Oxford. During the COVID-19 pandemic, Prof Kidd was the Deputy Chief Medical Officer and Principal Medical Advisor with the Australian Government Department of Health and Aged Care, and Professor of Primary Care Reform at the Australian National University. He holds honorary appointments with the University of Toronto, the University of Melbourne, Flinders University, and the Murdoch Children's Research Institute, and is the Emeritus Director of the World Health Organization Collaborating Centre on Family Medicine and Primary Care. He is an elected Fellow of the Australian Academy of Health and Medical Sciences (FAHMS). In the 2023 King's Birthday Honours List he was made an Officer of the Order of Australia. Prof Kidd served as president of the World Organization of Family Doctors (WONCA) from 2013-2016, and as president of the Royal Australian College of General Practitioners from 2002-2006. He is the founder and Editor-in-Chief of the Journal of Medical Case Reports, the world's first PubMed-listed journal devoted to publishing case reports from all medical disciplines.
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 1.0 - 2-year Impact Factor 0.628 - SNIP (Source Normalized Impact per Paper) 0.284 - SJR (SCImago Journal Rank)
2023 Speed 33 days submission to first editorial decision for all manuscripts (Median) 148 days submission to accept (Median)
2023 Usage 4,048,208 downloads 2,745 Altmetric mentions
- More about our metrics

- Follow us on Twitter
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Clinical Trials
Clinical Study Reports (CSR): Types and Use Cases

Meredith Latino

The Clinical Study Report (CSR) is arguably the most important document emerging from a clinical trial
CSRs summarize a study’s data and outcomes to facilitate the evaluation of a drug’s therapeutic effectiveness. Unlike academic journal papers, in which methodological flaws may be glossed over, the CSR provides a detailed description of the study’s design and methodology, along with tables, figures, listings, and appendices that further elucidate the data.
These reports are used to fulfill regulatory requirements, substantiate labeling information, support marketing authorization, inform the development of promotional messages for commercialization, and more.
The information on clinical investigations should be submitted in specific formats based on a specific purpose:
A full CSR presents a comprehensive clinical and statistical description of a sponsor’s study conduct, along with efficacy and safety data.
- These reports are required by regulatory agencies, such as the FDA or European Medicines Agency (EMA), to support product approvals and the information contained within the product label.
Abbreviated CSR
An abbreviated CSR should be used for any study that is not intended to contribute to the evaluation of efficacy or provide definitive information on clinical pharmacology.
- These are used for studies not intended to contribute to the evaluation of product effectiveness or provide definitive information on clinical pharmacology. As their name implies, abbreviated CSRs usually contain abbreviated methods and efficacy sections, though they should include a comprehensive safety section.
Synoptic CSR
Use of synoptic CSRs are appropriate for any study that is not relevant to the evaluation of efficacy or clinical pharmacology.
- These documents should provide complete safety information to allow the reviewer to evaluate safety from the study. These CSRs are used, for example, in early general phase-1 safety-tolerance studies. (However, not for specific, required toxicity studies which ordinarily should be submitted as full reports.)
Supplemental CSR
Finally, supplemental CSRs can be written to provide additional detail to a full study report. These reports do not include all sections of the full CSR and typically include instances in the text referring to the main/full CSR.
Setting your study up for success
Across each use case, a well-constructed CSR plays a vital role in therapeutic development, representing the culmination of years of planning and effort to demonstrate safety, efficacy, and therapeutic value. As regulatory bodies are examining CSRs under increasingly high levels of scrutiny, it is essential that sponsors consider CSR in the early stages of clinical trial planning.
Learn more about Precision’s Medical Writing Services >
You may also like

Clinical Trials - Regulatory
Implications of EU-CTR Regulation 536/2014
- Nov 30, ‘21
Chris Ingram

Strategies to Elevate Your Study at Saturated Sites
- Feb 13, ‘24
Alexis Hobbins-White

Clinical Trials - Rare Diseases

Impact of COVID-19 on Rare Disease Clinical Research
- Jun 17, ‘22
Precision Experts
Business of Medicine | Understanding the Medical Malpractice Litigation Process
May 31, 2024
Cardiology Magazine
Few things in a clinician's life generates more stress and disruption than an allegation of professional malpractice. The litigation process and the uncertainty it creates may cause a clinician to experience feelings of anger, anxiety and depression, or even physical reactions such as insomnia or stress-related headaches. The first step in alleviating the uncertainty is to understand the litigation process and the defendant's role during each step. The following is an overview of the various phases of the medical malpractice litigation process a defendant may be involved in and recommended strategies to maximize a successful outcome.
Early Indicators of Possible Litigation
Records Request The earliest indication of a potential lawsuit may be a request for medical records from a patient or a plaintiff's attorney. Such a request, however, may simply be part of an attorney's investigation of an accident or workers' compensation claim, in which case the request is usually made in a letter with a signed authorization enclosed, or by subpoena or other court order.
If you suspect the request for medical records is related to a potential medical malpractice action, notify your professional liability carrier. Ask for assistance in determining the validity of the records request and how to manage it promptly. Importantly, be sure to obtain valid signed authorization before releasing any medical records.
Notice of a Claim If a patient pursues a claim, the patient's attorney may notify the clinician by letter. In some states, notice may be triggered by statutory requirements, such as a notice of intent to sue or pre-suit notice. Additionally, a number of jurisdictions currently require a medical liability or malpractice case be heard by a screening panel before trial. 1
Statute of Limitations
The statute of limitations defines the time frame within which a plaintiff must file a lawsuit, often one to three years from the date of the alleged injury. The terms of the statutes vary by state and may be different for adults, minors and adults who are not mentally competent. If your attorney believes the statute of limitations has expired, your attorney will file a motion with the court to dismiss the lawsuit.
For the statute of limitations in your state, contact your local medical society or professional liability insurance carrier.
Never Alter a Medical Record
Read More, Learn More
Learn more about the litigation process with these articles from The Doctors Company.
Click here to read about tips for health care professionals to cope with legislation.
Click here to read about key factors in a deposition.
Click here to read about strategies to help assist your defense.
Upon receiving notice that a malpractice suit is about to commence or has been filed, clinicians must ensure the safety and integrity of the patient's medical record. Any changes made to the record after learning of a lawsuit raise questions about the clinician's truthfulness, motives and the quality of the care. Many clinicians and defense counsel have been embarrassed during discovery proceedings to learn that an earlier copy of the record differs materially from the record provided after litigation commenced.
Forensic document experts are frequently called to testify that a paper record has been augmented or altered. In situations in which a clinician has an electronic health record (EHR), counsel will retain information technology experts to conduct a metadata audit. The audit provides a complete analysis of every keystroke (including additions, deletions and changes) and when the entries were made, by whom, and how long a document was open for review and revision. If experts discover that the record has been altered, it can also expose the practitioner to punitive damages and result in a licensing board investigation.
You've Been Served. Now What?
Don't panic! If you receive a summons and complaint (a lawsuit), notify your professional liability carrier. This type of legal document requires a formal response within a prescribed time limit. Failure to respond appropriately may jeopardize your defense or even possibly result in a default judgment against you. Your professional liability carrier will assign a defense attorney who specializes in medical malpractice litigation and will handle the case through resolution.
Pre-Trial Discovery
Attorneys for both parties engage in written and oral discovery to understand the nature and extent of the care provided, as well as the merits of the patient's allegations. During discovery, attorneys for the plaintiff and defense review all medical records and other relevant documents related to the case to fully evaluate the claim. Interrogatories and depositions are two important parts of the discovery process.
Interrogatories (Written Discovery) Interrogatories are written questions directed by one party to another party designed to further develop the facts or the legal and clinical foundation of a case. Interrogatories directed to health care professionals usually seek background information concerning the individual's education, training, professional experience and credentials.
Interrogatory responses are legally admissible in court, therefore it is imperative that you review your answers carefully with your defense attorney. Your attorney will assist you in preparing accurate and appropriate responses.
Depositions (Oral Discovery) A deposition is a discovery tool used in virtually all forms of civil, administrative and criminal litigation. It provides an opportunity for both parties to obtain material information, assist in developing strategies for trial, and formally preserve testimony for use later. Testimony obtained in a deposition frequently proves to be the single most important event of the pretrial process. It is almost always crucial to the outcome of a case.
Depositions are conducted under oath in a verbal question-and-answer format. They are always recorded, traditionally by a certified shorthand reporter, who then transcribes the exchanges into a verbatim document that the deponent is required to sign. With increasing regularity over the past decade, the testimony is also preserved by separate audio and video technologies.
Deposition Testimony In preparation for a deposition, your attorney will meet with you to explain the process, offer recommendations on demeanor and dress, provide valuable suggestions on pitfalls to avoid, and identify probable areas of questioning by the attorneys who will attend the deposition.
Your attorney will also advise you of the best approach to use in answering questions. Your responses should be brief, concise, and delivered in a calm and thoughtful manner. Avoid guessing when you are uncertain of the answer. It is preferable to respond, "I do not know" or "I do not recall."
During your deposition, your attorney may perceive that a question is ambiguous or subject to a legal objection. Allow your attorney to state the objection and consider the objection when formulating your answer. The objection may alert you to an ambiguity or hidden meaning that is not otherwise apparent. Your attorney can also instruct you to refrain from answering a question that the attorney believes is an effort to elicit information that is not legally discoverable.
Key factors to keep in mind:
- Tell the truth. Deponents must promise to "tell the truth, the whole truth, and nothing but the truth." Failure to comply with the oath may be considered perjury (often a felony) that is punishable and may result in fines, sanctions and even imprisonment.
- Answer only the question asked. Deponents should listen carefully, answer only the question asked and then stop talking. Volunteering extraneous information prolongs the proceeding and identifies potential new areas of inquiry that opposing counsel may not have previously considered. One classic tactic is for the attorney to pause, leaving dead air that tends to be uncomfortable and can lead the deponent to resume talking.
- Maintain respect. When providing deposition testimony, be well prepared in advance, appear on time and appropriately attired, always act professionally and courteously, stay focused, and respond to the questions directly and with respect.
Expert Witnesses The use of expert witnesses is critical to professional liability cases. Expert witnesses help to define the standard of care and determine if any deviations have occurred. Both the plaintiff and defendant retain experts – sometimes more than one – to provide opinions on issues of causation and damages. Considerations when retaining an expert witness include the expert's education, training and experience. It is also important to have an expert who is articulate and likely to be well received by a jury.
Dismissal, Settlement or Trial
The litigation process can be lengthy, typically lasting two to five years, and even longer in some jurisdictions. Be prepared for extended periods of inactivity. The legal process is inefficient and impossible to control. Flurries of activity are often followed by prolonged periods of inactivity. Depositions are often scheduled, canceled and rescheduled.
At some point, enough information will have been gathered during the pre-trial discovery process for an assessment to be made about whether the case is defensible through trial or settlement should be considered. These decisions will be made between you, your attorney and your insurance carrier.
It's possible, however, that the case may be dismissed during the discovery process if the plaintiff's attorney determines the case lacks the elements needed to recover damages. According to the National Practitioner Data Bank, between 80% and 90% of defensible claims are dismissed with no settlement. 2 Additionally, 96.9% of successful medical malpractice claims are settled out of court. 3 Thus, only a very small percentage of medical malpractice cases ever proceed to trial.
Take Care of Yourself
Participating in a lawsuit can be challenging, difficult and stressful. Remember you are not alone. Emotional reactions to litigation are normal and there are people and resources available to help.
- Continue to maintain a healthy lifestyle with proper diet and exercise.
- Share your feelings to help maintain positive psychological health.
- Seek professional assistance when feelings of anxiety and distress interfere with daily work and relationships.
- Stay engaged with the litigation process. Your attorney needs your expertise and partnership. Staying engaged also minimizes uncertainty and allows you to feel more in control.
- Know that you will get through this stressful time.
This article was authored by Richard F. Cahill, JD , vice president and associate general counsel, Debra Davidson, MJ, CPHRM, CPPS , senior patient safety risk manager, and Douglas McCullough, JD , vice president of claims, all with The Doctors Company, part of TDC Group, and Sunny Jhamnani, MD, FACC , partner at Tri-City Cardiology in Chandler, AZ.
The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each healthcare provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.
- Morton H. Medical Liability/Malpractice ADR and Screening Panels Statutes. National Conference of State Legislatures. Updated Aug. 10, 2021. Available here .
- U. S. Department of Health & Human Services. National Practitioner Data Bank. Data Analysis Tool. Available here .
- Rubin JB, Bishop TF. Characteristics of paid malpractice claims settled in and out of court in the USA: a retrospective analysis. BMJ Open 2013;3:e002985. doi:10.1136/bmjopen-2013-002985
Keywords: Cardiology Magazine, ACC Publications, Malpractice, Liability, Legal, Medical Records, Health Personnel, Lawyers
You must be logged in to save to your library.

Editors’ Corner | Refining Our Practice, Improving Patient Outcomes
Cover Story | Acute Coronary Syndromes: New Perspectives, New Data
Feature | JACC Family Series: Effect of Biological vs. Chronological Aging on CV Diseases
New in Clinical Documents | New ACC/AHA Guideline Focuses on Evaluation and Management of People With HCM
New in Clinical Documents | Management of Lower Extremity PAD Focus of New ACC/AHA Guideline
Focus on Heart Failure | Heart Failure Highlights From ACC.24
On the Move | Health Care Innovation Section Looks Ahead
Innovating Health Care | ACC.24 Innovation Pitch Challenge
Quality Improvement For Institutions | Advancing Quality CV Care Around the Globe
Quality Improvement For Institutions | ACC Accreditation Services: Leading Quality Improvement
Prioritizing Health | Lowering BP Without Drugs
Heart of Health Policy | CV Management Deep Dive: Navigating Ongoing Population and Workforce Trends
Heart of Health Policy | FTC Finalizes Ban on Noncompetes
The Pulse of ACC | JACC , The Lancet Align to Advance CV Health; Reset Your ACC Password; Dyad Leadership; More
Number Check | ACC Anywhere: Personalized Lifelong Learning
JACC in a Flash | RALES, EMPHASIS-HF; 6MWT Identifies ATTR-CA in High-Risk Patients; More
Just One More | Advocating For Women’s Health on Capitol Hill
JACC Journals on ACC.org
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Diversity and Inclusion
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
Latest in Cardiology
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
Education and Meetings
- Online Learning Catalog
- Understanding MOC
- Products and Resources
- Image and Slide Gallery
- Certificates and Certifications
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- CardioSmart
- Accreditation Services
- Clinical Solutions
- Clinician Well-Being Portal
- Mobile and Web Apps
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Contact Member Care
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.
The First Reported Foodborne Botulism Outbreak in Riyadh, Saudi Arabia: Lessons Learned
- Research Article
- Open access
- Published: 05 June 2024
Cite this article
You have full access to this open access article

- Nadeem Gul Dar 1 ,
- Sarah H. Alfaraj 1 ,
- Khulood Naser Alboqmy 1 ,
- Nazia Khanum 1 ,
- Faleh Alshakrah 1 ,
- Hassan Abdallah 1 ,
- Mohammad Hosni Badawi 1 ,
- Ohoud Mohammed Alharbi 1 ,
- Khadijh Ahmed Alshiekh 1 ,
- Abdullah M Alsallum 1 ,
- Ahmed Hassan Shrahili 1 ,
- Zeidan A Zeidan 2 ,
- Zaki Abdallah 3 ,
- Ahmed Ali Majrashi 4 &
- Ziad A. Memish 5 , 6 , 7
Botulism has not been previously reported in the Kingdom of Saudi Arabia. This rare and sometimes fatal foodborne illness is caused by neurotoxins and primarily results from consuming home-canned fruits, vegetables, dairy, and seafood products & it can lead to paralysis.
The purpose of this study was to evaluate the clinical features of patients who developed botulism in Riyadh in 2024 after consuming mayonnaise from a well-known local chain of restaurants in Riyadh, Saudi Arabia.
We conducted a retrospective analysis of medical records and interviewed patients or their attendants for all hospitalized cases of foodborne botulism at Riyadh First Health Cluster. For each patient, a standard case report form was completed, containing information on demographics, clinical aspects, botulinum test results, and type of exposure. Descriptive statistics were applied to assess the data. During the outbreak, nineteen patients with foodborne diseases were admitted to Riyadh First Health Cluster Hospitals. Following thorough physical examinations, botulism was suspected in each case.
Eight of the 19 suspected foodborne illness patients fully satisfied the botulism case definition requirements set forth by the Saudi Arabian Public Health Authority (Weqaya). Among these eight patients, 2 (25%) were male and 6 (75%) were female, with a mean age of 23.25 ± 9.29 years (range: 12–38 years). The incubation period for our patients was 36.25 ± 26.26 h. Notable symptoms included dysphagia in all eight patients (100%), dysarthria, generalized weakness, nausea and vomiting in seven patients (88%), diplopia in four patients (50%), and stomach discomfort in three patients (38%). Of the eight cases, six required intubation, one mimicked brain death, and two were stable. The presence of Clostridium botulinum spores as the cause of the outbreak was confirmed by detecting botulinum spores in contaminated food.
Diplopia and dysarthria were the most common early sign of botulism. Early manifestations may include respiratory symptoms without any musculoskeletal symptoms. or nausea, vomiting and disorientation.
Avoid common mistakes on your manuscript.
1 Introduction
In the Kingdom of Saudi Arabia, foodborne botulism has never been documented. Foodborne botulism is an uncommon yet deadly disease, affecting both humans and other vertebrates. The underlying cause is botulinum neurotoxin, which is considered one of the most lethal substances humans have ever encountered [ 1 , 2 ]. The predominant bacterium that produces this toxin is Clostridium botulinum, rarely strains of Clostridium baratii and Clostridium butyricum , a gram-positive bacillus, anaerobic, spore-forming that developed in harsh environments to promote survival. Normally, spores pass through healthy human digestive system without causing any disease, except for infants due to immature intestine and underdeveloped gut flora [ 3 , 4 ].
Traditionally, botulinum neurotoxins have been classified into at least seven serotypes, denoted by the letters A through G. Notably, a novel eighth serotype was identified in 2016 [ 5 , 6 ]. Among these, serotypes A, B, E, and more rarely F cause human disease. Serotype A is associated with the most severe clinical manifestations, with a higher proportion of patients requiring mechanical ventilation for respiratory support. On the other hand, serotype B typically manifests as a milder disease compared to serotype A [ 7 ].
Botulinum toxin affects human body with neuroparalytic illness characterized by flaccid paralysis of the motor and autonomic nerves that descend, starting with the cranial nerves. These symptoms can include dysphagia, muscle weakness, diplopia, ptosis, blurred vision, slurred speech, respiratory distress or failure, and ocular palsy. Despite the characteristic features of botulism, including symptoms that typically begin in the cranial nerves, consistent descending progression, symmetry, and absence of sensory nerve dysfunction, it is frequently misdiagnosed as Guillain-Barre syndrome, Miller-Fisher syndrome, myasthenia gravis, or other central nervous system diseases [ 1 , 2 , 7 ].
Human botulism comes in various forms, including foodborne, wound, infant, unclassified (adult intestinal colonization), iatrogenic (medical treatment complications), and inhalation botulism. Despite having different origins, all types of botulism present with a common clinical presentation that is typified by neuromuscular paralysis caused by the toxin. Particularly, ingesting preformed botulinum neurotoxin complexes found in contaminated food is the cause of foodborne botulism, which is the most common type [ 8 , 9 , 10 , 11 ].
Foodborne botulism exhibits a variable incubation period affected by toxin type and quantity digested, ranging from a minimum of 4 h to a maximum of 8 days after ingesting toxin-contaminated food. In most cases presentation occurs within a window of 12 to 36 h. Patients diagnosed with botulism are not considered contagious. Therefore, beyond standard precautions, no additional isolation measures are necessary [ 8 , 9 , 12 , 13 ]. The surveillance system for foodborne illness in Riyadh involves the systematic collection, analysis, and reporting of data on foodborne disease cases to detect outbreaks, identify sources of contamination, and implement public health interventions to prevent further spread. This system ensures timely monitoring and response to potential food safety threats, enhancing overall public health protection. To our knowledge, this is the first report to describe a clinicopathological outbreak of botulism involving multiple centers within Riyadh First Health Cluster (5 Hospitals with bed capacity of 2302). The aim of this study is to report on the clinical and epidemiological characteristics of the 19 individuals who were suspected and the 8 cases that met the case definition for botulism who presented to our health cluster in the recent food-borne outbreak affecting 75 cases in total in Riyadh, KSA.
2 Methodology
All patients referred to Riyadh First Health Cluster Hospitals between April 22–25, 2024, with gastrointestinal symptoms, respiratory distress, or descending paralysis after consuming mayonnaise from a popular Riyadh burger restaurant chain were admitted for assessment and care. The inclusion criteria, as provided by the Public Health Authority of Saudi Arabia, encompassed patients of both sexes and all ages who showed signs of respiratory distress, descending paralysis, or gastrointestinal distress within 72 h of consuming contaminated food. Exclusion criteria included individuals with pre-existing neurological disorders, a history of food allergies, or those who did not consume the contaminated food.
We conducted a retrospective analysis of medical records and interviewed patients, or their attendants hospitalized with foodborne botulism at Riyadh First Health Cluster. A standard case report form was completed for each patient, containing information on demographics, clinical aspects, botulinum test results, and type of exposure. Descriptive statistics were applied to assess the data.
Statistical analysis: Descriptive statistics, encompassing frequency distributions, were generated for all study variables using a recent statistical software package (Google sheets, Microsoft Excel).
Characteristics of a probable botulism case: In this study, a patient was deemed a likely case of botulism if they had a history of eating at a burger restaurant and showed symptoms of bilateral cranial nerve neuropathy, such as diplopia, impaired vision, dysphagia, bulbar paresthesia, and/or symmetrical weakness in peripheral muscles, as reported to the Saudi Public Health Authority.
Sample Gathering: Within 12 to 120 h of consuming contaminated food, biologic samples, such as feces, gastrointestinal contents, whole blood, and serum, were taken from each patient for laboratory investigation. The samples included:
50 g of stool samples in a suitable container.
25 mL of gastric contents, either vomitus or aspiration.
High rectal washout in cases where collecting stool samples was challenging.
15 mL serum sample (serum collection should occur prior to administering antitoxin).
Furthermore, food samples were gathered by the Saudi Arabian government’s official authority to verify food contamination.
Data gathering: A group of nurses, epidemiologists, and healthcare specialists with expertise in infection control collected the data. The procedure included clinical interviews and patient physical examinations. A designated healthcare provider was responsible for gathering thorough clinical data, interviewing, and examining each patient or patient attendant individually. Conducting interviews and examinations at the time of admission and during the patient’s hospital stay facilitated ongoing evaluations of symptoms and clinical progress. Additionally, history was collected from the patients’ relatives or friends who had eaten the same food but did not develop symptoms. These asymptomatic individuals were followed up by our public health staff to monitor for the development of any botulism-related symptoms.
3.1 Demographic Data
A total of eight confirmed botulism cases were reported between April 22–25, 2024, with the peak occurring on April 22 (seven cases). The mean age of admitted patients was 23.25 ± 9.29 years (range: 12–38 years). Of these patients, 2 (25%) were male, and 6 (75%) were female. All cases presented to the hospitals between 20 and 105 h after eating contaminated food, with a mean presentation time of 36.25 ± 26.26 h (Table 1 ). All eight cases had the same epidemiological link, having eaten from different branches of a well-known local burger restaurant chain in Riyadh. In addition to these eight cases, three accompanying individuals (family/friends) who ate the same food did not develop botulism-specific symptoms. One of the eight cases was a patient’s daughter who had the same food, experienced similar symptoms, required intubation, and had a negative test result for botulism.
The most commonly reported signs and symptoms at hospital admission included dysphagia in all eight patients (100%), dysarthria, generalized weakness, nausea and vomiting in seven patients (88%), diplopia in four patients (50%), and stomach discomfort in three patients (38%). (Table 2 ).
Outcome: Of the eight cases meeting the case definition, only one was laboratory-confirmed. Two cases were stable, six required intubations, with one of them being brain dead. All the cases eventually recovered, and no more deaths were reported in our study.
4 Discussion
This outbreak was traced to a small number of individuals who had consumed mayonnaise from a well-known group of a local chain of burger restaurant. Since serum samples were taken from patients at the time of admission and many patients were admitted to the hospital a few days after the onset of symptoms, the lengthy interval between the consumption of tainted food and the withdrawal of serum samples may account for these unfavorable outcomes. A number of variables, including the time and method of sample collection, the amount of toxin consumed, the rate at which the toxin enters the bloodstream, and the extravascular compartment’s uptake of the toxin, might lead to false-negative results in patients [ 14 ]. Additionally, it has been noted that a lot of clinical specimens had low toxin levels, which cannot show up for four days [ 15 ]. Furthermore, prior to the collection of samples for testing, all patients were administered anti-toxin, which explains the false negative result. The CDC recommends that serum specimens be obtained prior to administering botulism antitoxin because the antitoxin neutralizes the botulinum toxin, which can result in tests falsely indicating the absence of the toxin [ 16 ]. Thus, it can be said that clinical signs in patients who may have botulism are the most reliable indicator for a doctor to diagnose the disease rather than using laboratory markers.
Past medical and surgical history was taken to exclude neurological diseases, previous stroke, and other gastrointestinal problems. All patient’s symptomsstarted to appear after eating the meals from a specific restaurant. All the cases presented to the hospital from 20 h up to 5 days after ingestion of the contaminated food with a mean value of 36.25 ± 26.26 h. Systematic review, reported a median duration between exposure and symptom onset as approximately 1 day [ 17 , 18 ]. This difference is expected due to the long incubation period of foodborne botulism which would reach up to 8 days.
Botulism has specific clinical symptoms, but diagnosing it requires a high index of suspicion. Because the symptoms mostly affect the gastrointestinal tract and central nervous systems, this case series is similar to those that have been published elsewhere [ 19 , 20 , 21 , 22 ]. These specific symptoms with epidemiological link can be used for early recognition of foodborne botulism cases with further early botulinum antitoxin administration and other supportive care reduces the degree and severity of paralysis, sometimes preventing the development of respiratory compromise and reducing the length of time patients require mechanical ventilation and intensive care.
The fact that nearly 50% of suspected cases confirmed with botulism reflects the severity of clinical prodromes in persons with botulism. Out of the eight cases, two were stable, six required intubations, and one among 6 intubated is mimicking brain death. The attack rate is not 100%, as was shown in previous foodborne botulism outbreaks ( 23 – 24 ). In our investigation, only eight of the eleven participants who consumed the identical meal from the same local burger restaurant displayed signs of botulinum toxicity. The 73% attack rate among those who consumed it can be explained by unknown host characteristics that transmit resistance to the poison, an uneven distribution of the toxin in the meal, or a dose-response relationship. This exact attack rate for the cluster in Riyadh will depend on the detailed community public health outbreak investigation and the total number of cases detected and admitted to other hospitals in the Riyadh region.
Treatment in case of suspected cases of botulism should be initiated immediately to prevent complications. Primary treatment includes supportive in addition to ventilator care and antitoxin. Ideally antitoxin should be given as soon as possible within 24 h [ 25 ] without waiting for laboratory confirmation if case is meeting clinical and epidemiological criteria for botulism. Timely antitoxin bounds free toxin in blood and prevent development of symptoms [ 25 ], but in case it is delayed like in our study where patients present late to the hospital, wherein symptoms have already set in antitoxin can slow progress of disease and help in recovery by decreasing severity of illness. It has also known to avoid death [ 26 ].
5 Lessons Learned
The investigation of the botulism outbreak revealed several crucial lessons. Early detection and prompt diagnosis were essential for effective management, as recognizing characteristic symptoms like cranial nerve palsies and descending paralysis significantly improved patient outcomes. The case definition for identifying true cases of botulism is believed to be highly specific, accurately identifying individuals with the disease based on characteristic symptoms and laboratory confirmation. However, its sensitivity may be limited, potentially missing some true cases due to the variability of symptoms and delays in seeking medical care or diagnosis. Rapid administration of botulism antitoxin within the first 48 h of symptom onset played a key role in mitigating disease severity and preventing fatalities. Comprehensive assessment of symptoms, including gastrointestinal issues and neurological impairments, ensured that significant but less obvious symptoms like respiratory distress were not overlooked. Clinicians were advised to maintain a high index of suspicion for botulism, especially in patients with relevant clinical presentations and a history of consuming potentially contaminated food. Effective communication between healthcare providers, public health authorities, and the public was critical for disseminating information about the outbreak source and preventive measures. The importance of food safety through proper handling, preparation, and storage was underscored, highlighting the need for strict adherence to regulations and regular inspections of food establishments. The outbreak emphasized the necessity for healthcare facilities to have protocols for rapid response, including the availability of antitoxin and supportive care capabilities. Continuous education and training for healthcare providers on botulism identification and management were recommended to enhance preparedness and response. Applying these lessons can lead to more effective management of future outbreaks, reducing both the incidence and severity of botulism cases.
6 Study Limitation
Our study has certain limitations, including potential recall bias and inaccuracies in reported food consumption, as it relied on retrospective analysis. The small sample size further limits the generalizability of our findings. Additionally, some patients presented late to the hospital, which may have affected the outcomes and the comprehensiveness of the data collected. Blood samples were not obtained before administering the antitoxin, which complicates the assessment of toxin levels and the efficacy of the antitoxin treatment. Moreover, the results of the community outbreak investigation were not included in the analysis of the patients’ hospital admissions, which limits the context and understanding of the outbreak’s broader impact. These limitations highlight areas that could enhance foodborne botulism prevention, surveillance, and early detection. Improving public health education on recognizing early symptoms, ensuring timely presentation to healthcare facilities, and enhancing laboratory capabilities for toxin detection before treatment are essential steps. Additionally, a more robust surveillance system and comprehensive outbreak investigations could provide better data and inform more effective prevention strategies.
7 Conclusion
Our analysis revealed a range of symptoms upon hospital admission, including gastrointestinal complaints, cranial nerve palsies, extremity weakness, and respiratory distress. Patients exhibiting cranial nerve signs or symptoms should be assessed by clinicians for respiratory involvement and descending paralysis (i.e., proximal to distal), which may indicate botulism. Most patients arrived at the hospital within 48 h of symptom onset, and those requiring intubation typically received it within the first two days of hospitalization. The absence of fatalities in this patient cohort can be attributed to the prompt detection and treatment of botulism, which was linked to the consumption of contaminated mayonnaise from a well-known local burger establishment.
Data Availability
No datasets were generated or analysed during the current study.
Rawson AM, Dempster AW, Humphreys CM, Minton NP. Pathogenicity and virulence of Clostridium botulinum. Virulence. 2023;14(1):2205251. https://doi.org/10.1080/21505594.2023.2205251 .
Article CAS PubMed PubMed Central Google Scholar
Centers for Disease Control and Prevention (CDC). (2022, August 26). Botulism: Healthcare Professionals. https://www.cdc.gov/botulism/health-professional.html (02 May 2024,date last accessed).
Lund BM, Peck MW. Clostridium botulinum. In: Labbé RG, García S, editors. Guide to foodborne pathogens. 2nd ed. John Wiley & Sons, Ltd.; 2013. pp. 92–111.
Peck MW. Biology and genomic analysis of Clostridium botulinum. Adv Microb Physiol. 2009;55:183–265. https://doi.org/10.1016/S0065-2911(09)05503-9 .
Article CAS PubMed Google Scholar
Zhang S, Masuyer G, Zhang J, Shen Y, Lundin D, Henriksson L, Miyashita SI, Martínez-Carranza M, Dong M, Stenmark P. Identification and characterization of a novel botulinum neurotoxin. Nat Commun. 2017;8(1):14130. https://doi.org/10.1038/ncomms14130 .
Peck MW, Smith TJ, Anniballi F, Austin JW, Bano L, Bradshaw M, Cuervo P, Cheng LW, Derman Y, Dorner BG, Fisher A, Hill KK, Kalb SR, Korkeala H, Lindström M, Lista F, Lúquez C, Mazuet C, Pirazzini M, Singh BR, Stringer SC. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins(Basel). 2017;9(1):38. https://doi.org/10.3390/toxins9010038 . PMID: 28106761; PMCID: PMC5308270.
Sobel J. Botulism. Clin Infect Dis. 2005;41(8):1167–73. https://doi.org/10.1086/444507 .
Chaidoutis E, Keramydas D, Papalexis P, Migdanis A, Migdanis I, Lazaris AC, Kavantzas N. Foodborne botulism: a brief review of cases transmitted by cheese products (review). Biomed Rep. 2022;16(5):41. https://doi.org/10.3892/br.2022.1524 .
Lonati D, Schicchi A, Crevani M, Buscaglia E, Scaravaggi G, Maida F, Cirronis M, Petrolini VM, Locatelli CA. Foodborne botulism: clinical diagnosis and medical treatment. Toxins (Basel). 2020;12(8):509. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7472133/ .
Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of Botulism, 2021. MMWR Recommendations Rep. 2021;70RR–2:1–30. https://doi.org/10.15585/mmwr.rr7002a1 .
Article Google Scholar
Chatham-Stephens K, Fleck-Derderian S, Johnson SD, Sobel J, Rao AK, Meaney-Delman D. Clinical features of Foodborne and Wound Botulism: a systematic review of the literature, 1932–2015. Clin Infect Dis. 2017;66(Supplement1):S11–6. https://doi.org/10.1093/cid/cix811 .
Article PubMed Google Scholar
World Health Organization. (25. September 2023). Botulism. [Fact Sheet]. Retrieved May 4, 2024, from https://www.who.int/news-room/fact-sheets/detail/botulism .
Guidelines of. foodborne botulism (Clostridium botulinum), Version 1.2.
Sobel J. Diagnosis and treatment of botulism: a century later, clinical suspicion remains the cornerstone. Clin Infect Dis. 2009;48:1674–75.
Temeri et al. (2011) An Alternative In Vivo Method to Refine the Mouse Bioassay for Botulinum Toxin Detection.
Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of Botulism. MMWR Recomm Rep. 2021;70(2):1–30.
Article PubMed PubMed Central Google Scholar
Fleck-Derderian S, Shankar M, Rao AK, Chatham-Stephens K, Adjei S, Sobel J, et al. The epidemiology of foodborne botulism outbreaks: a systematic review. Clin Infect Dis. 2017;66(suppl1):S73. https://doi.org/10.1093/cid/cix846 . S81.DOI.
Gaware VM, Kotade KB, Dolas RT, Dhamak KB, Somawanshi SB, Nikam VK. Botulism foodborne disease: a review. J Chem Pharm Res. 2011;3(1):84–92.
Google Scholar
Okunromade O, Dalhat MM, Umar AM, et al. Emergency response to a cluster of suspected foodborne botulism in Abuja, Nigeria: challenges with diagnosis and treatment in a resource-poor setting. Pan Afr Med J. 2020;36:287.
PubMed PubMed Central Google Scholar
Viray M, Wamala J, Fagan R, et al. Outbreak of type A foodborne botulism at a boarding school. Uganda 2008 Epidemiol Infect. 2014;142(11):2297–301.
CAS PubMed Google Scholar
Kissani N, Moutawakkil S, Chakib A, et al. Le Botulisme alimentaire Au Maroc, a propos de 15 cas. Valeur diagnostique de l’electrophysiologie. Rev Neurol. 2009;165(12):1080–5.
Angulo FJ, Getz J, Taylor JP, et al. A large outbreak of botulism: the hazardous baked potato. J Infect Dis. 1998;178(1):172–7.
Townes JM, Cieslak PR, Hatheway CL, et al. An outbreak of type A botulism associated with a commercial cheese sauce. Ann Inter Med. 1996;125(7):558–63.
Article CAS Google Scholar
Varma JK, Katsitadze G, Moiscrafishvili M, et al. Signs and symptoms predictive of death in patients with foodborne botulism— Republic of Georgia, 1980–2002. Clin Infect Dis. 2004;39(3):357–62.
Botulism Jeremy Sobel Foodborne and Diarrheal Diseases Branch. Centers for Disease Control and Prevention, Atlanta, Georgia FOOD SAFETY Frederick J. Angulo, Section Editor Downloaded from https://academic.oup.com/cid/article/41/8/1167/379325 by guest on 30 April 2024.
Clinicopathological insights into. an outbreak of foodborne botulism in Hamadan, Iran, in 2023: A microbiological and laboratory findings Zohre Sadeghian1 and Fatemeh Torkaman Asadi2, SAGE Open Medicine Volume 11: 1– 8 © The Author(s) 2023.
Download references
Acknowledgements
No specific grant was obtained for this research from governmental, private, or nonprofit funding organizations.
Author information
Authors and affiliations.
Prevention and Control of Infection Administration, King Saud Medical city, Riyadh, Saudi Arabia
Nadeem Gul Dar, Sarah H. Alfaraj, Khulood Naser Alboqmy, Nazia Khanum, Faleh Alshakrah, Hassan Abdallah, Mohammad Hosni Badawi, Ohoud Mohammed Alharbi, Khadijh Ahmed Alshiekh, Abdullah M Alsallum & Ahmed Hassan Shrahili
King Salman Hospital, Riyadh, Saudi Arabia
Zeidan A Zeidan
Imam Abdulrahman Alfaisal Hospital, Riyadh, Saudi Arabia
Zaki Abdallah
Al Iman General Hospital, Riyadh, Saudi Arabia
Ahmed Ali Majrashi
Research and Innovation Center, King Saud Medical City, Riyadh, Saudi Arabia
Ziad A. Memish
College of Medicine, Al Faisal University, Riyadh, Saudi Arabia
Hubert Department of Global Health, Rollins School of Public Health, Emory, University, Atlanta, USA
You can also search for this author in PubMed Google Scholar
Contributions
NGD and SA conceived the idea of the manuscript. NGD, FA, NK and HA wrote the first draft of the manuscript. ZAM, NGD, commented and edited subsequent versions of the manuscript. All co-authors participated in data collection and analysis. All authors have reviewed and approved the final version of the manuscript submitted to the journal.
Corresponding author
Correspondence to Ziad A. Memish .
Ethics declarations
Ethics approval and consent to participate.
The Institutional Review Board issued their approval to the study (H1RI-12-May24-02). Strict confidentiality protocols were followed during data analysis to protect patient anonymity. The principal investigator was the only person with access to patient identifiers for research purposes.
Consent for Publication
All coauthors consented to the publication.
Competing Interests
The corresponding author is the EIC otherwise no other competing interest has been declared.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Gul Dar, N., Alfaraj, S.H., Alboqmy, K. et al. The First Reported Foodborne Botulism Outbreak in Riyadh, Saudi Arabia: Lessons Learned. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00255-z
Download citation
Received : 23 May 2024
Accepted : 29 May 2024
Published : 05 June 2024
DOI : https://doi.org/10.1007/s44197-024-00255-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Food Poisoning
- Saudi Arabia
- Outbreak Investigation
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Res Notes

The clinical case report: a review of its merits and limitations
Trygve nissen.
1 Department of Clinical Medicine, University of Tromsø, N-9038 Tromsø, Norway
2 Division of General Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
3 Division of Addictions and Specialized Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
The clinical case report has a long-standing tradition in the medical literature. While its scientific significance has become smaller as more advanced research methods have gained ground, case reports are still presented in many medical journals. Some scholars point to its limited value for medical progress, while others assert that the genre is undervalued. We aimed to present the various points of view regarding the merits and limitations of the case report genre. We searched Google Scholar, PubMed and select textbooks on epidemiology and medical research for articles and book-chapters discussing the merits and limitations of clinical case reports and case series.
The major merits of case reporting were these: Detecting novelties, generating hypotheses, pharmacovigilance, high applicability when other research designs are not possible to carry out, allowing emphasis on the narrative aspect (in-depth understanding), and educational value. The major limitations were: Lack of ability to generalize, no possibility to establish cause-effect relationship, danger of over-interpretation, publication bias, retrospective design, and distraction of reader when focusing on the unusual.
Conclusions
Despite having lost its central role in medical literature in the 20th century, the genre still appears popular. It is a valuable part of the various research methods, especially since it complements other approaches. Furthermore, it also contributes in areas of medicine that are not specifically research-related, e.g. as an educational tool. Revision of the case report genre has been attempted in order to integrate the biomedical model with the narrative approach, but without significant success. The future prospects of the case report could possibly be in new applications of the genre, i.e. exclusive case report databases available online, and open access for clinicians and researchers.
Throughout history the clinical case report and case report series have been integral components of medical literature [ 1 ]. The case report genre held a strong position until it was sidelined in the second half of the 20 th century [ 2 , 3 ]. New methodologies for research articles paved the way for evidence-based medicine. Editors had to make space for these research articles and at the same time signaled less enthusiasm for publishing case reports [ 4 ]. This spurred some heated debates in medical journals as readers were worried that the traditional case report was in jeopardy [ 5 , 6 ]. Those who welcomed the new trend with fewer case reports being published pointed mainly to their low quality and inclination to emphasize mere curiosa [ 7 - 9 ]. Some of the proponents of the genre claimed that the case report had been and still was indispensible for furthering medical knowledge and that it was unique in taking care of the detailed study of the individual patient as opposed to the new research methods with their “…nomothetic approach [taking] precedence…” [ 5 ]. Still, the case report got a low ranking on the evidence hierarchy. After a decline in popularity a new interest for the case report emerged, probably beginning in the late 1990s [ 2 ]. A peer-reviewed ‘Case reports’ section was introduced in the Lancet in 1995 [ 10 ]. In 2007, the first international, Pubmed-listed medical journal publishing only case reports was established [ 11 , 12 ]. In the following years, several similar journals, for the most part online and open-access, have been launched.
The present debate is not so much focused on whether case reporting is obsolete or not. Some of the discussions after the turn of the century have been about adapting the case report genre to new challenges. One example is the suggestion of incorporating the narrative, i.e. “… stressing the patient’s story”, in the case report [ 13 ]. The authors termed their initiative “The storied case report”. Their endeavor was not met with success. In analyzing the causes for this, they wondered if “… junior trainees find it too hard to determine what is relevant and senior trainees find it too hard to change their habits” [ 13 ]. A similar attempt was done when the editors of the Journal of Medical Case Reports in 2012 encouraged authors to include the patients’ perspectives by letting patients describe their own experiences [ 14 ].
Notwithstanding, we feel there is much to be gained from having an ongoing discussion highlighting the indications and contraindications for producing case reports. This can to some degree be facilitated by getting an understanding of the merits and limitations of the genre. The objective of this article is to present the merits and limitations of case reports and case series reports.
We adopted Taber’s Cyclopedic Medical Dictionary’s definition of the case report : “A formal summary of a unique patient and his or her illness, including the presenting signs and symptoms, diagnostic studies, treatment course and outcome” [ 15 ]. A case report consists of one or two cases, most often only one. The case series or case series report usually consists of three to ten cases [ 16 ]. (In the following we use the term case report to denote both case reports and case series report). Case reports are most often naturalistic and descriptive. Sometimes, however, they can be prospective and experimental.
As literature specifically dealing with the case report genre seemed harder to elicit from the databases than the vast amount of particular case reports, we performed iterative searches. We searched Google Scholar and PubMed using the search terms ‘case report(s)’, ‘case series’, ‘case series report(s)’, ‘case reporting’ in various combinations with ‘clinical’, ‘medical’, ‘anecdotal’, ‘methodology’, ‘review’, ‘overview’, ‘strengths’, ‘weaknesses’, ‘merits’, and ‘limitations’. Further references were identified by examining the literature found in the electronic searches. We also consulted major textbooks on epidemiology [ 17 , 18 ], some scholars of medical genres [ 19 , 20 ] and a monograph on case reporting by the epidemiologist M. Jenicek [ 16 ]. We delimited our review to the retrospective, naturalistic, and descriptive case report, also labeled the “traditional” or “classic” case report, and case series including such reports. Thus we excluded other types, such as the planned, qualitative case study approach [ 21 ] and simulated cases [ 22 - 24 ]. Finally, we extracted the relevant data and grouped the merits and limitations items in rank order with the items we judged to be the most important first.
New observations
The major advantage of case reporting is probably its ability to detect novelties [ 16 ]. It is the only way to present unusual, uncontrolled observations regarding symptoms, clinical findings, course of illness, complications of interventions, associations of diseases, side effects of drugs, etc. In short, anything that is rare or has never been observed previously might be important for the medical community and ought to be published. A case report might sensitize readers and thus facilitate detection of similar or identical cases.
Generating hypotheses
From a single, or preferably several single case reports or a case series, new hypotheses could be formulated. These could then be tested with formal research methods that are designed to refute or confirm the hypotheses, i.e. comparative (observational and experimental) studies.
There are numerous examples of new discoveries or major advancements in medicine that started with a case report or, in some cases, as humbly as a letter to the editor. The first concern from the medical community about the devastating side effect of thalidomide, i.e. the congenital abnormalities, appeared as a letter to the editor in the Lancet in 1961 [ 25 ]. Soon thereafter, several case reports and case series reports were published in various journals. Case reporting is thus indispensable in drug safety surveillance (pharmacovigilance) [ 26 ].
Sometimes significant advancements in knowledge have come not from what researchers were pursuing, but from “accidental discoveries”, i.e. by serendipity. The story of Alexander Fleming’s discovery of penicillin in 1928 is well known in the medical field [ 27 ]. Psychiatry has profited to a large degree from this mode of advancing medical science as many of the drugs used for mental disorders have been discovered serendipitously [ 27 ]. One notable example is the discovery of the effect of lithium on manic episodes in patients with manic-depressive disorder [ 28 ]. A more recent discovery is the successful treatment of infantile hemangiomas with systemic propranolol. This discovery was published, as a case series report, in the correspondence section in New England Journal of Medicine [ 29 ]. However, the evidence for the effect of this treatment is still preliminary, and several randomized trials are under way [ 30 , 31 ].
Clear and operational entities are prerequisites for doing medical research. Descriptions must come before understanding. Clinical observations that lead to new disorders being described are well suited for case reporting. The medical literature is replete with case-based articles describing new diseases and syndromes. One notable example is the first description of neurasthenia by G. Beard in Boston Medical and Surgical Journal in 1869 [ 32 ].
Researching rare disorders
For rare disorders randomized controlled trials (RCTs) can be impossible to run due to lack of patients to be enrolled. Research on drug treatment and other kinds of interventions must therefore be based on less rigorous methodologies, among them case series and case reports. This would be in accordance with the European Commission’s recommendation to its members to improve health care for those with rare disorders [ 33 ].
Solving ethical constraints
Case reporting can be valuable when ethical constraints prohibit experimental research. Take as an example the challenge of how to manage the side effects of accidental extravasation of cytotoxic drugs. As RCTs on humans seem unethical in this clinical situation the current guidelines rest on small observational studies, case reports and animal studies [ 34 ]. Or another example: Physical restraint is sometimes associated with sudden, unexpected death. The cause or causes for this are to some degree enigmatic, and it is hard to conceive of a controlled study that could be ethical [ 35 , 36 ]. Case reports and case series being “natural experiments” might be the only evidence available for guiding clinical practice.
In-depth narrative case studies
Case reporting can be a way of presenting research with an idiographic emphasis. As contrasted to nomothetic research, an idiographic approach aims at in-depth understanding of human phenomena, especially in the field of psychology and psychiatry. The objective is not generalizable knowledge, but an understanding of meaning and intentionality for an individual or individuals. Sigmund Freud’s case studies are relevant examples. This usage of case reports borders on qualitative research. Qualitative studies, although developed in the social sciences, have become a welcome contribution within health sciences in the last two decades.
Educational value
Clinical medical learning is to a large degree case-based. Typical case histories and vignettes are often presented in textbooks, in lectures, etc. Unusual observations presented as published case reports are important as part of doctors’ continuing medical education, especially as they demonstrate the diversity of manifestations both within and between medical diseases and syndromes [ 37 , 38 ]. Among the various medical texts, the case report is the only one that presents day-to-day clinical practice, clinicians’ diagnostic reasoning, disease management, and follow-up. We believe that some case reports that are written with the aim of contributing to medical knowledge turn out to be of most value educationally because the phenomena have already been described elsewhere. Other case reports are clearly primarily written for educational value [ 37 ]. Some journals have regular sections dedicated to educational case reports, e.g. The Case Records of the Massachusetts General Hospital in the New England Journal of Medicine and the Clinical Case Conference found in the American Journal of Psychiatry.
The cost of doing a case report is low compared to planned, formal studies. Most often the necessary work is probably done in the clinical setting without specific funding. Larger studies, for instance RCTs, will usually need an academic setting.
Fast publication
The time span from observation to publication can be much shorter than for other kinds of studies. This is obviously a great advantage as a case report can be an important alert to the medical community about a serious event. The unexpected side effects of the sedative-antinauseant thalidomide on newborn babies is a telling story. The drug had been prescribed during pregnancy to the babies’ mothers. After the first published observation of severe abnormalities in babies appeared as a letter to the editor of the Lancet in December 16 th , 1961 [ 25 ], several case reports and series followed [ 39 , 40 ]. It should be mentioned though that the drug company had announced on December 2 nd , 1961, i.e. two weeks before the letter from McBride [ 25 ], that it would withdraw the drug form the market immediately [ 41 ].
Flexible structure
Riaz Agha, editor of the International Journal of Surgery Case Reports suggests that the case report, with its less rigid structure is useful as it “… allows the surgeon(s) to discuss their diagnostic approach, the context, background, decision-making, reasoning and outcomes” [ 42 ]. Although the editor is commenting on the surgical case report, the argument can be applied for the whole field of clinical medicine. It should be mentioned though, that other commentators have argued for a more standardized, in effect more rigid, structure [ 43 ].
Clinical practice can be changed
Case reporting can lead to or contribute to a change in clinical practice. A drug might be withdrawn from the market. Or a relabeling might change the attitude to and treatment of a condition. During Word War I the shell shock syndrome was labeled and described thoroughly in several articles in the Lancet , the first of them appearing in February 1915 [ 44 ]. The author was the British captain and military doctor Charles S. Myers. Before his efforts to bring good care and treatment to afflicted soldiers there had been a common misconception that many of these dysfunctional soldiers were malingerers or cowards.
Exercise for novice researchers
The case report format is well suited for young doctors not yet trained as researchers. It can be an opportunity for a first exercise in authoring an article and a preparation for a scientific career [ 37 , 45 , 46 ].
Communication between the clinical and academic fields
Articles authored by clinicians can promote communication between practicing clinicians and academic researchers. Observations published can generate ideas and be a trigger for further studies. For instance, a case series consisting of several similar cases in a short period can make up the case-group for a case–control study [ 47 ]. Clinicians could do the observation and publish the case series while the case–control study could be left to the academics.
Entertainment
Some commentators find reading case reports fun. Although a rather weak argument in favor of case reporting, the value of being entertained should not be dismissed altogether. It might inspire physicians to spend more time browsing and reading scientific literature [ 48 ].
Studying the history of medicine
Finally, we present a note on a different and unintended aspect of the genre. The accumulated case reports from past eras are a rich resource for researching and understanding medical history [ 49 , 50 ]. A close study of old case reports can provide valuable information about how medicine has been practiced through the centuries [ 50 , 51 ].
Limitations
No epidemiological quantities.
As case reports are not chosen from representative population samples they cannot generate information on rates, ratios, incidences or prevalences. The case or cases being the numerator in the equation, has no denominator. However, if a case series report consists of a cluster of cases, it can signal an important and possibly causal association, e.g. an epidemic or a side effect of a newly marketed drug.
Causal inference not possible
Causality cannot be inferred from an uncontrolled observation. An association does not imply a cause-effect relationship. The observation or event in question could be a mere coincidence. This is a limitation shared by all the descriptive studies [ 47 ]. Take the thalidomide tragedy already mentioned as an example; Unusual events such as congenital malformations in some of the children born to mothers having taken a specific drug during pregnancy does not prove that the drug is the culprit. It is a mere hypothesis until further studies have either rejected or confirmed it. Cause-effect relationships require planned studies including control groups that to the extent possible control for chance, bias and confounders [ 52 ].
Generalization not possible
From the argument above, it follows that findings from case reports cannot be generalized. In order to generalize we need both a cause-effect relationship and a representative population for which the findings are valid. A single case report has neither. A case series, on the other hand, e.g. many “thalidomide babies” in a short time period, could strengthen the suspicion of a causal relationship, demanding further surveillance and research.
Publication bias could be a limiting factor. Journals in general favor positive-outcome findings [ 53 ]. One group of investigators studying case reports published in the Lancet found that only 5% of case reports and 10% of case series reported treatment failures [ 54 ]. A study of 435 case reports from the field of dentistry found that in 99.1%, the reports “…clearly [had] a positive outcome and the intervention was considered and described as successful by the authors” [ 55 ].
Overinterpretation
Overinterpretation or misinterpretation is the tendency or temptation to generalize when there is no justification for it. It has also been labeled “the anecdotal fallacy” [ 56 ]. This is not a shortcoming intrinsic to the method itself. Overinterpretation may be due to the phenomenon of case reports often having an emotional appeal on readers. The story implicitly makes a claim to truth. The reader might conclude prematurely that there is a causal connection. The phenomenon might be more clearly illustrated by the impact of the clinician’s load of personal cases on his or her practice. Here exemplified by a young doctor’s confession: “I often tell residents and medical students, ‘The only thing that actually changes practice is adverse anecdote.’” [ 57 ].
Emphasis on the rare
As case reporting often deals with the rare and atypical, it might divert the readers’ attention from common diseases and problems [ 58 ].
Confidentiality
Journals today require written informed consent from patients before publishing case reports. Both authors and publishers are responsible for securing confidentiality. A guarantee for full confidentiality is not always possible. Despite all possible measures taken to preserve confidentiality, sometimes the patient will be recognized by someone. This information should be given to the patient. An adequately informed patient might not consent to publication. In 1995 in an Editorial in the British Journal of Psychiatry one commentator, Isaac Marks, feared that written consent would discourage case reports being written [ 59 ]. Fortunately, judged form the large number of reports being published today, it seems unlikely that the demand for consent has impeded their publication.
Other methodological limitations
Case reports and series are written after the relevant event, i.e. the observation. Thus, the reports are produced retrospectively. The medical record might not contain all relevant data. Recall bias might prevent us from getting the necessary information from the patient or other informants such as family members and health professionals.
It has also been held against case reporting that it is subjective. The observer’s subjectivity might bias the quality and interpretation of the observation (i.e. information bias).
Finally, the falsification criterion within science, which is tested by repeating an experiment, cannot be applied for case reports. We cannot design another identical and uncontrolled observation. However, unplanned similar “experiments” of nature can be repeated. Several such observations can constitute a case series that represents stronger indicative evidence than the single case report.
The major advantages of case reporting are the ability to make new observations, generate hypotheses, accumulate scientific data about rare disorders, do in-depth narrative studies, and serve as a major educational tool. The method is deficient mainly in being unable to deliver quantitative data. Nor can it prove cause-effect relationship or allow generalizations. Furthermore, there is a risk of overinterpretation and publication bias.
The traditional case report does not fit easily into the qualitative-quantitative dichotomy of research methods. It certainly shares some characteristics with qualitative research [ 16 ], especially with regard to the idiographic, narrative perspective – the patient’s “interior world” [ 60 ] – that sometimes is attended to. Apart from “The storied case report” mentioned in the Background-section, other innovative modifications of the traditional case report have been tried: the “evidence-based case report” [ 61 ], the “interactive case report” [ 62 ] and the “integrated narrative and evidence based case report” [ 63 ]. These modifications of the format have not made a lasting impact on the way case reports in general are written today.
The method of case reporting is briefly dealt with in some textbooks on epidemiology [ 17 , 18 ]. Journals that welcome case reports often put more emphasis on style and design than on content in their ‘instruction to authors’ section [ 64 ]. As a consequence, Sorinola and coworkers argue for more consensus and more consistent guidance on writing case reports [ 64 ]. We feel that a satisfactory amount of guidance concerning both style and content now exists [ 12 , 16 , 65 , 66 ]. The latest contribution, “The CARE guidelines”, is an ambitious endeavor to improve completeness and transparency of reports [ 66 ]. These guidelines have included the “Patient perspective” as an item, apparently a bit half-heartedly as this item is placed after the Discussion section, thus not allowing this perspective to influence the Discussion and/or Conclusion section. We assume this is symptomatic of medicine’s problem with integrating the biomedical model with “narrative-based medicine”.
In recent years the medical community has taken an increased interest in case reports [ 2 ], especially after the surge of online, exclusive case report journals started in 2007 with the Journal of Medical Case Reports (which was the first international, Pubmed-listed medical journal publishing only case reports) as the first of this new brand. The climate of skepticism has been replaced by enthusiasm and demand for more case reports. A registry for case reports, Cases Database, was founded in 2012 [ 67 ]. On the condition that it succeeds in becoming a large, international database it could serve as a register being useful for clinicians at work as well as for medical research on various clinical issues. Assuming Pamela P. Powell’s assertion that “[a]lmost all practicing physicians eventually will encounter a case worthy of being reported” [ 60 ] is valid, there should be no shortage of potential cases waiting to be reported and filed in various databases, preferably online and open access.
Limitations of this review
There are several limitations to this study. It is a weakness that we have not been able to review all the relevant literature. The number of publications in some way related to case reports and case report series is enormous, and although we have attempted to identify those publications relevant for our purpose (i.e. those that describe the merits and limitations of the case report genre), we might have missed some. It was difficult to find good search terms for our objective. Still, after repeated electronic searches supplemented with manual searches in reference lists, we had a corpus of literature where essentially no new merits or limitations emerged.
As we point out above, the ranking of merits and limitations represents our subjective opinion and we acknowledge that others might rank the importance of the items differently.
The perspective on merits and limitations of case reporting has been strictly medical. As a consequence we have not analyzed or discussed the various non-medical factors affecting the publication of case reports in different medical journals [ 2 ]. For instance, case reports are cited less often than other kinds of medical research articles [ 68 ]. Thus they can lower a journal’s impact factor, potentially making the journal less attractive. This might lead some high-impact journals to publish few or no case reports, while other journals have chosen to specialize in this genre.
Before deciding on producing a case report or case series based on a particular patient or patients at hand, the observant clinician has to determine if the case report method is the appropriate article type. This review could hopefully assist in that judgment and perhaps be a stimulus to the continuing debate in the medical community on the value of case reporting.
Competing interests
The authors declare that there are no competing interests.
Authors’ contributions
TN contributed to the conception, drafting, and revision of the article. RW contributed to the conception, drafting, and revision of the article. Both authors approved the final manuscript.
Acknowledgements
There was no specific funding for this study.
- Cabán-Martinez AJ, Beltrán WF. Advancing medicine one note at a time: the educational value in clinical case reports. BMC Res Notes. 2012; 5 :293. doi: 10.1186/1756-0500-5-293. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The recent history of the clinical case report: a narrative review. JRSM Sh Rep. 2012; 3 :87. doi: 10.1258/shorts.2012.012046. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The history of the case report: a selective review. JRSM Open. 2014; 5 :4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G. Fare the well – the Editor’s last words. Br J Psychiatry. 2003; 182 :465–466. doi: 10.1192/bjp.182.6.465. [ CrossRef ] [ Google Scholar ]
- Williams DDR. In defence of case of the case report (Letter) Br J Psychiatry. 2004; 184 :84. doi: 10.1192/bjp.184.1.84. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Enoch MD. Case reports (Letter) Br J Psychiatry. 2005; 185 :169. [ PubMed ] [ Google Scholar ]
- Nahum AM. The clinical case report: “Pot boiler” or scientific literature? Head Neck Surg. 1979; 1 :291–292. doi: 10.1002/hed.2890010402. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doherty M. What value case reports? (Editorial) Ann Rheum Dis. 1994; 53 :1–2. doi: 10.1136/ard.53.1.1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Procopio M. Publication of case reports. Br J Psychiatry. 2005; 187 :91. [ PubMed ] [ Google Scholar ]
- Bignall J, Horton R. Learning from stories – Lancet’s case reports. Lancet. 1995; 346 :1256. [ PubMed ] [ Google Scholar ]
- Kidd M, Hubbard C. Introducing Journal of Medical Case Reports. J Med Case Rep. 2007; 1 :1. doi: 10.1186/1752-1947-1-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rison RA. A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes. J Med Case Reports. 2013; 7 :239. doi: 10.1186/1752-1947-7-239. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bayoumi AM, Kopplin PA. The storied case report. CMAJ. 2004; 171 :569–570. doi: 10.1503/cmaj.1031503. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kidd MR, Saltman D. Case reports at the vanguard of the 21 st century medicine (Editorial) J Med Case Rep. 2012; 6 :156. doi: 10.1186/1752-1947-6-156. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Venes D. Taber’s Cyclopedic Medical Dictionary. 21. Philadelphia: F.A. Davis Company; 2009. [ Google Scholar ]
- Jenicek M. Clinical case reporting in evidence-based medicine. Oxford: Butterworth Heinemann; 1999. [ Google Scholar ]
- Fletcher RH, Fletcher SW. Clinical epidemiology: The essentials. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [ Google Scholar ]
- Sackett DL, Brian Haynes R, Tugwell P. Clinical epidemiology. Boston: Little, Brown and Company; 1985. [ Google Scholar ]
- Berkencotter C. Patient tales. Case histories and the uses of narrative in psychiatry. Columbia: University of South Carolina Press; 2008. [ Google Scholar ]
- Hunter KM. Doctors’ stories. The narrative structure of medical knowledge. Princeton: Princeton University Press; 1991. [ Google Scholar ]
- Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach. BMC Med Res Methodol. 2011; 11 :100. doi: 10.1186/1471-2288-11-100. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Kvalvik AM, Hynnekleiv T. Attitudes to coercion at two Norwegian psychiatric units. Nord J Psychiatry. 2011; 65 :133–137. doi: 10.3109/08039488.2010.513068. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Myklebust LH, Bratlid T. Psychologists and coercion: decisions regarding involuntary psychiatric admission and treatment in a group of Norwegian psychologists. Nord J Psychiatry. 2007; 61 :433–437. doi: 10.1080/08039480701773139. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aaker E, Knudsen A, Wynn R, Lund A. General practitioners’ reactions to non-compliant patients. Scand J Prim Health Care. 2001; 19 :103–106. doi: 10.1080/028134301750235330. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McBride WG. Thalidomide and congenital abnormalities. (Letter) Lancet. 1961; 278 :1358. [ Google Scholar ]
- Chakra CAN, Pariente A, Pinet M, Nkeng L, Moore N, Moride Y. Case series in drug safety. A review to determine characteristics and quality. Drug Saf. 2010; 33 :1081–1088. doi: 10.2165/11539300-000000000-00000. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci. 2006; 8 :335–344. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949; 2 :349–352. [ PubMed ] [ Google Scholar ]
- Léauté-Labrèze C, de la Roque Dumas E, Hubiche T, Boralevi F. Propranolol for severe hemangiomas of infancy. (Letter to the editor) N Engl J Med. 2008; 358 :2649–2651. doi: 10.1056/NEJMc0708819. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fretheim A. Godt nok dokumentert? (Sufficiently documented?) (Editorial) Tidsskr Nor Legeforen. 2010; 130 :1806. [ PubMed ] [ Google Scholar ]
- U.S. National Institutes of Health. Studies found for the search 'propanolol and hemangiomas' in the ClinicalTrials.gov database. Accessed on 22 April, 2014 at: http://www.clinicaltrials.gov/ct2/results?term=propanolol+and+hemangiomas&Search=Search .
- Beard G. Neurasthenia, or nervous exhaustion. Boston Med Surg J. 1869; 80 :217–221. doi: 10.1056/NEJM186904290801301. [ CrossRef ] [ Google Scholar ]
- Taylor DM. Developing a strategy for the management of rare disorders. BMJ. 2012; 344 :e2417. doi: 10.1136/bmj.e2417. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bjånes TK. Lokale bivirkninger ved parenteral administrering av legemidler. (Localized side effects caused by parenteral administration of drugs) Tidsskr Nor Legeforen. 2011; 131 :472–474. [ PubMed ] [ Google Scholar ]
- Nissen T, Rørvik P, Haugslett L, Wynn R. Physical restraint and near death of a psychiatric patient. J Forensic Sci. 2013; 58 :259–262. doi: 10.1111/j.1556-4029.2012.02290.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R. Coercion in psychiatric care: clinical, legal, and ethical controversies. Int J Psychiatry Clin Pract. 2006; 10 :247–251. doi: 10.1080/13651500600650026. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lundh A, Christensen M, Jørgensen AW. International or national publication of case reports. Dan Med Bul. 2011; 58 :A4242. [ PubMed ] [ Google Scholar ]
- Grønli O, Wynn R. Normocalcemic hyperparathyroidism and treatment resistant depression. Psychosomatics. 2013; 54 :493–497. doi: 10.1016/j.psym.2012.10.008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ward SP. Thalidomide and congenital abnormalities. BMJ. 1962; 2 (5305):646–647. doi: 10.1136/bmj.2.5305.646. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coodin FJ, Uchida CM, Murphy CH. Phocomelia: report of three cases. CMAJ. 1962; 87 :735–739. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heyman DJ. Managing director of the Destillers Company Ltd. (Letter) Lancet. 1961; 278 :1262. [ Google Scholar ]
- Agha R, Rosin RD. Time for a new approach to case reports. (Editorial) Int J Surg Case Rep. 2010; 1 :1–3. doi: 10.1016/j.ijscr.2010.04.001. doi:10.1016/j.ijscr.2010.04.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aronson JA. Anecdotes as evidence. (Editorial) BMJ. 2003; 326 :1346. doi: 10.1136/bmj.326.7403.1346. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Myers CS. A contribution to the study of the shell shock. Being an account of three cases of loss of memory, vision, smell and taste, admitted into the Duchess of Westminster’s War Hospital, Le Touquet. Lancet. 1915; 185 :316–320. doi: 10.1016/S0140-6736(00)52916-X. [ CrossRef ] [ Google Scholar ]
- Morris BA. The importance of case reports. (Letter to the Editor) CMAJ. 1989; 141 :875–876. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rison RA. Neurology case reports: a call for all. J Med Case Rep. 2011; 5 :113. doi: 10.1186/1752-1947-5-113. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet. 2002; 359 :145–149. doi: 10.1016/S0140-6736(02)07373-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang S. Anecdotes in medicine - 15 years of Lancet case reports. Lancet. 2010; 376 :1448–1449. doi: 10.1016/S0140-6736(10)60624-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rose JC, Corn M. Dr. E. and other patients: new lessons from old case reports. J Hist Med Allied Sci. 1984; 39 :3–32. doi: 10.1093/jhmas/39.1.3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. Practice versus theory: tenth-century case histories from Islamic Middle East. Soc Hist Med. 2000; 13 :293–306. doi: 10.1093/shm/13.2.293. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. The case history in Medieval Islamic medical literature: Tajarib and Mujarrabat as source. Med Hist. 2010; 54 :195–214. doi: 10.1017/S0025727300006712. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 3. Philadelphia: Lippincott Williams & Wilkins; 2007. [ Google Scholar ]
- Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet. 1991; 337 :867–872. doi: 10.1016/0140-6736(91)90201-Y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Albrecht J, Meves A, Bigby M. Case reports and case series from the Lancet had significant impact on medical literature. J Clin Epidemiol. 2005; 58 :1227–1232. doi: 10.1016/j.jclinepi.2005.04.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oliveira GJ, Leles CR. Critical appraisal and positive outcome bias in case reports published in Brazilian dental journals. J Dent Educ. 2006; 70 :869–874. [ PubMed ] [ Google Scholar ]
- Charlton BG, Walston F. Individual case studies in clinical research. J Eval Clin Practice. 1998; 4 :147–155. doi: 10.1111/j.1365-2753.1998.tb00081.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stuebe AM. Level IV evidence – adverse anecdote and clinical practice. N Engl J Med. 2011; 365 :8–9. doi: 10.1056/NEJMp1102632. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffman JR. Rethinking case reports. West J Med. 1999; 170 :253–254. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G, Fahy T, Russell G, Healy D, Marks I, Tantam D, Dimond B. Case reports and confidentiality. (Editorial) Br J Psychiatry. 1995; 166 :555–558. doi: 10.1192/bjp.166.5.555. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Powell PP. The single-case report in medical literature: the ‘Elephant man’ serves as an exellent example. J Am Med Writers Assoc. 1990; 5 :9–12. [ Google Scholar ]
- Glasziou P. Evidence based case report: twenty year cough in a non-smoker. BMJ. 1998; 316 :1660–1661. doi: 10.1136/bmj.316.7145.1660. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harker N, Montgomery A, Fahey T. Interactive case report. Treating nausea and vomiting during pregnancy: case outcome. BMJ. 2004; 328 :276. doi: 10.1136/bmj.328.7434.276. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reis S, Hermoni D, Livingstone P, Borkan J. Integrated narrative and evidence based case report: case report of paroxysmal atrial fibrillation and anticoagulation. BMJ. 2002; 325 :1018–1020. doi: 10.1136/bmj.325.7371.1018. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sorinola O, Olufowobi O, Coomarasamy A, Khan KS. Instructions to authors for case reporting are limited: a review of a core journal list. BMC Med Educ. 2004; 4 :4. doi: 10.1186/1472-6920-4-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rossor MN. In: How to write a paper. 4. Hall GM, editor. London: Blackwell Publishing; 2008. How to write a case report; pp. 71–75. [ Google Scholar ]
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. CARE group. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep. 2013; 7 :223. doi: 10.1186/1752-1947-7-223. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BioMed Central. Cases Database. Accessed on 22 April, 2014 at: http://www.casesdatabase.com .
- Mason RA. The case report – an endangered species? (Editorial) Anesthesia. 2001; 56 :99–102. doi: 10.1046/j.1365-2044.2001.01919.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Skip to main content
- Keyboard shortcuts for audio player
Weekend Edition Sunday
- Latest Show
Sunday Puzzle
- Corrections
Listen to the lead story from this episode.
Politics chat: How voters are responding to Trump's felony conviction
by Ayesha Rascoe , Mara Liasson
The Americas
Mexico votes for a new president after a campaigning season plagued by violence.
by Eyder Peralta , Ayesha Rascoe
Middle East
Aid workers in gaza say nowhere is safe after israeli attacks on 'humanitarian zones'.
by Hadeel Al-Shalchi
Girls in the U.S. are getting their period earlier. Here's what parents should know
by Ayesha Rascoe , Maria Godoy
Bookstores have come under attack in Ukraine. But interest in reading is only growing
by Joanna Kakissis
25 years ago, Napster changed how we listen to music forever
by Ayesha Rascoe
What locals think of the proposal to build U.S.'s tallest building in Oklahoma City
by Graycen Wheeler

Sunday Puzzle NPR hide caption
Sunday Puzzle: Second in Line
by Will Shortz
Movie Interviews
A new animated film follows a lonely dog and his robot friend in new york city.
by Ayesha Rascoe , Matthew Schuerman , Andrew Craig
Conservative media sows doubt about the verdict in Trump's felony convictions
by Ayesha Rascoe , David Folkenflik
Supreme Court judge accused of bias towards Trump declines to recuse himself from case
by Ayesha Rascoe , Matthew Schuerman , Hiba Ahmad
Some states are adopting a new form of reading instruction to combat falling scores
by Juma Sei
A new movie tells the story of Kemba Smith Pradia, race and incarceration
Strange news, meet abby lampe, two-time champion of the cheese-wheel-chasing race, meet abby lampe, two-time champion of the chees-wheel-chasing race, 100 years ago, indigenous people were granted u.s. citizenship by law.
by Sandhya Dirks
The first professional women's hockey league in the U.S. has a winner
Music interviews, jon lampley, a veteran of stephen colbert's talk show, releases his debut album.
by D. Parvaz , Ayesha Rascoe , Ryan Benk
Searching for a song you heard between stories? We've retired music buttons on these pages. Learn more here.


IMAGES
VIDEO
COMMENTS
Informed consent in an ethical requirement for most studies involving humans, so before you start writing your case report, take a written consent from the patient as all journals require that you provide it at the time of manuscript submission. In case the patient is a minor, parental consent is required.
The clinical case report has been an integral part of medical literature throughout history. ... conduct further confirmatory quantitative experimental or observational study designs such as clinical trials or cohort studies. Despite that, case report provides the medical community with information which cannot be picked up by any other designs ...
The aim of this study was to get junior doctors to evaluate an online presentation as part of the process of developing a beginner's guide to writing a clinical case report. Materials and methods. In response to our previous studies an online presentation concerning how to write a clinical case report was provided for junior doctors.
First steps. Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline ...
Clinical Case Reports is very fortunate to be supported by many other journals published by Wiley, including a number of society-owned journals. These journals participate in the Manuscript Transfer Program by referring case reports and offering authors the option to have their paper, with any peer review reports, automatically transferred to Clinical Case Reports.
A case report is a structured report of the clinical process of a patient's diagnostic pathway, including symptoms, signs, diagnosis, treatment planning (short and long term), clinical outcomes ...
1 The following article was published in Oxford Medical Case Reports. Bottineau et al. A misleading appearance of a common diseases: tuberculosis with generalized lymphodenopathy - a Case Report." ... retrospective study in Zambia, TB was more commonly diagnosed among children with Kwashiorkor (47%) compared to Marasmus-Kwashiorkor (24% ...
Writing a case report is an excellent way of documenting these findings for the wider medical community—sharing new knowledge that will lead to better and safer patient care. For many medical students and junior doctors, a case report may be their first attempt at medical writing. A published case report will look impressive on your ...
This may be encountered either in rare diseases or conditions where treatment options are exhausted. Moreover, randomized trials report outcomes of a group and often do not inform about the individual patient. describes a few examples of case reports/case series which have had a remarkable impact on medical practice. Table 1:
A clinical case report or case study is a means of disseminating new knowledge gained from clinical practice. Medical practitioners often come across patient cases that are different or unusual such as a previously unknown condition, a complication of a known disease, an unusual side effect or adverse response to a mode of treatment, or a new ...
A journal publishing case reports in all medical disciplines, including general medicine, drug interaction and adverse reactions. The largest online collection of medical case reports. Validation period: 6/4/2024, 3:01:30 AM - 6/4/2024, 9:01:30 AM. Subscribe Login.
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient's history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. Crossref
16.1.2 Sample case report form (unique pages only).....28 16.1.3 List of IECs or IRBs (plus the name of the committee Chair if required ... The clinical study report described in this guideline is an "integrated" full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to
About Oxford Medical Case Reports. Oxford Medical Case Reports (OMCR) is an open access, peer-reviewed online journal publishing original and educationally valuable case reports that expand the field of medicine.The journal deposits all articles in PubMed Central (PMC) and is indexed in the Web of Science Core Collection.. Click here to find out more about the journal.
Structure Your Report. Once you have selected the journal of submission, carefully reread the author instructions to structure your submission. The JACC: Case Reports authors instructions suggest a specific structure for a clinical case: history of presentation, physical examination, past medical history, differential diagnosis, investigations, management (medical/interventions), discussion ...
Journal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports. Case reports should show one of the following: Unreported or unusual side effects or adverse interactions involving medications. Unexpected or unusual presentations of a disease.
The Clinical Study Report (CSR) is arguably the most important document emerging from a clinical trial. CSRs summarize a study's data and outcomes to facilitate the evaluation of a drug's therapeutic effectiveness. Unlike academic journal papers, in which methodological flaws may be glossed over, the CSR provides a detailed description of ...
1. The title accurately reflects the case. 2. The case involves an important area of health. 3. Where possible the case illustrates the use of an important clinical guideline or systematic review and if so, the report is clear about exactly which part of the review or guideline the case relates to. 4.
SAGE Open Medical Case Reports is a peer-reviewed, open access journal, which focusses on providing a publication home for short case reports and case series, which often do not find a place in traditional primary research journals, but provide key insights into real medical cases that are essential for physicians, and may ultimately help to improve patient outcomes.
Clinical Case Studies (CCS), peer-reviewed & published bi-monthly electronic only, is the only journal devoted entirely to innovative psychotherapy case studies & presents cases involving individual, couples, & family therapy.The easy-to-follow case presentation format allows you to learn how interesting & challenging cases were assessed & conceptualized, & how treatment followed such ...
Clinical Case Reports aims to improve global health outcomes by sharing clinical knowledge through the use of medical case reports, clinical images & procedural videos. Key Clinical Message In this case report, we describe the successful management of severe scrub typhus with pneumonia, sepsis, and multiple organ dysfunction in a pregnant woman ...
Medications ». Four medications received a conditional recommendation for use in the treatment of PTSD: sertraline, paroxetine, fluoxetine and venlafaxine. This website is for informational and educational purposes only. It does not render individual professional advice or endorse any particular treatment for any individuals.
Editorial. Introduction. Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition of additional knowledge ...
Interrogatories are written questions directed by one party to another party designed to further develop the facts or the legal and clinical foundation of a case. Interrogatories directed to health care professionals usually seek background information concerning the individual's education, training, professional experience and credentials.
For each patient, a standard case report form was completed, containing information on demographics, clinical aspects, botulinum test results, and type of exposure. ... The aim of this study is to report on the clinical and epidemiological characteristics of the 19 individuals who were suspected and the 8 cases that met the case definition for ...
The clinical case report has a long-standing tradition in the medical literature. While its scientific significance has become smaller as more advanced research methods have gained ground, case reports are still presented in many medical journals. ... A study of 435 case reports from the field of dentistry found that in 99.1%, the reports ...
Jon Lampley, a veteran of Stephen Colbert's talk show, releases his debut album. by D. Parvaz, Ayesha Rascoe, Ryan Benk. 7 min. Searching for a song you heard between stories?
Now, researchers hope to test their effectiveness in the U.S. May 30, 2024. AAMCNews. Women are changing the face of medicine in America. Data from the past 18 years show how women have driven growth in the supply of physicians and expanded their presence in some of the largest specialties. May 28, 2024.